Abstract
Purpose
This study explored early (contrast discrimination) and intermediate (global form perception) visual processing in primary subtypes of glaucoma: primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). We aimed to understand early and intermediate visual processing in POAG and PACG, matched for similar visual field defect severity.
Methods
Early visual processing was measured using a contrast discrimination task described by Porkorny and Smith (1997), and intermediate processing using a global form perception task using glass pattern coherence thresholds. Thresholds were determined centrally and at a single midperipheral location (12.5°) in a quadrant without visual field defects. Controls were tested in corresponding quadrants to individuals with glaucoma.
Results
Sixty participants (20 POAG, 20 PACG, and 20 age-matched controls), aged 50 to 77 years, were included. Visual field defects were matched between POAG and PACG, with mean deviation values of −6.53 ± 4.46 (range: −1.5 to −16.85) dB and −6.2 ± 4.24 (range: −1.37 to −16.42) dB, respectively. Two-Way ANOVA revealed significant differences in thresholds between the glaucoma groups and the control group for both contrast discrimination and global form perception tasks, with higher thresholds in the glaucoma groups. Post hoc analyses showed no significant contrast discrimination difference between POAG and PACG, but POAG had significantly higher thresholds than PACG for form perception.
Conclusions
In form perception, POAG showed slightly worse performance than PACG, suggesting that individuals with POAG may experience more severe functional damage than PACG of similar visual field severity.
Keywords: POAG, PACG, contrast discrimination, form perception
Glaucoma, a progressive optic neuropathy, primarily affects the retinal ganglion cells and leads to visual field loss.1 Prior work shows that damage in glaucoma is not restricted to the retina, but causes neurodegenerative changes in visual and nonvisual centers of the brain, such as the lateral geniculate nucleus, visual cortex, and higher cortical areas.2–8 These changes in the visual pathway lead to functional deficits9 for contrast detection and discrimination tasks,10 increased motion coherence thresholds,11,12 increased form perception thresholds,11 impaired saccadic eye movements,13,14 impaired auditory processing,15 and impaired face perception in glaucoma patients,16 indicating functional deficits at early, intermediate, and later stages of the visual processing pathways. It is important to note that normal performance on these tasks not only relies on intact output from the retina, but also on normal cortical processing. Interestingly, participants with glaucoma have difficulty performing these tasks, even when the retinal damage is mild, such as when tested foveally or in the relatively intact areas of visual field.9,11,17 This strongly suggests that the impact of glaucoma extends beyond retinal input and involves cortical processing.
There are two major subtypes of primary glaucoma: primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). Prior studies of neurodegenerative changes and the resultant functional changes have been shown for POAG.9–12,14–16 However, it is equally important to understand upstream neurodegenerative changes and resultant functional changes in PACG. This point is particularly significant, because PACG is more prevalent in the South Asian population, which contributes 86% of the world's angle-closure glaucoma population.18,19 In India, various studies have estimated that the prevalence of PACG ranges from 0.24% to 7.24%, which is higher than the prevalence of POAG, which ranges from 1.62% to 3.51%.20
POAG and PACG may have distinct underlying causes and anatomical susceptibilities, resulting in distinct manifestations of visual damage to the visual pathways. Recent evidence suggests that PACG might exhibit a slower global rate of visual field progression compared with high-tension POAG and normal-tension glaucoma.21 The biomechanical responses of the optic nerve head to acute IOP elevation differ between POAG and PACG,22 and saccadic eye movement behavior also varies between these two subtypes.13 It has also been reported that individuals with POAG have a higher risk of developing dementia compared with those with PACG.23 These findings collectively suggest the presence of independent mechanisms between the two subtypes, with varying degrees of cortical visual function deficits between POAG and PACG. In this study, we aim to compare the severity of visual processing deficits for the same degree of visual field loss severity between POAG and PACG. Based on previous evidence, we hypothesize that, for the same level of visual field loss, the degree of damage to cortical visual processing may be less in PACG compared with POAG.
In this study, our focus was on investigating the early and intermediate stages of visual processing. To assess early visual processing, we used the Pokorny and Smith steady and pulse pedestal contrast discrimination tasks, which assess function of the M and P pathways.17,24 For the evaluation of intermediate visual processing, we used a form perception task well-recognized for engaging the cortical extrastriate area V4 during normal visual processing.25
Methods
This study adhered to the principles of the Declaration of Helsinki. Ethical approval was obtained from the institutional ethics committee (IEC No: 1059/2019). Before their participation, all individuals provided informed consent. The study included 20 patients with POAG, 20 patients with PACG, and 20 control participants, all approximately age matched.
The diagnosis of POAG or PACG in glaucoma patients was conducted by experienced glaucoma specialists (V.P., with >20 years of experience, and N.K., >10 years of experience) at Kasturba Hospital, Manipal, India. Participants, including those with glaucoma and controls, were recruited from the outpatient department of the hospital's ophthalmology department. Additionally, 11 controls, comprising relatives and colleagues, were included after undergoing comprehensive eye examinations. All participants with glaucoma in this study were diagnosed initially and undergoing follow-up treatment by the same ophthalmologists involved in the study, except for two participants with PACG. These two individuals had initial diagnoses recorded elsewhere, but were subsequently reconfirmed and followed for further treatment by the ophthalmologist involved in this study.
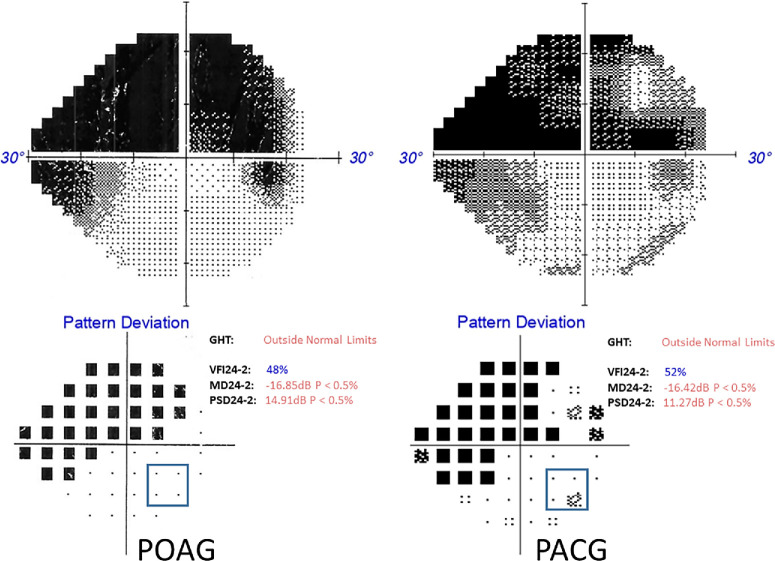
The diagnostic criteria for both POAG and PACG in our study adhere to standard and internationally accepted guidelines.26 The diagnosis of PACG and POAG relied on identifying characteristic optic nerve head changes indicative of glaucoma, such as rim thinning, notching, or nerve fiber defects, alongside visual field issues and elevated IOP (≥21 mm Hg). A PACG diagnosis specifically necessitated an occludable or closed angle (≥180°) with synechiae and evidence of glaucomatous disc damage. The ophthalmologists consistently applied these criteria throughout the study at the same hospital, ensuring accuracy and reliability in the diagnoses. All participants with glaucoma exhibited visual field loss, which was assessed using the Swedish Interactive Threshold Algorithm—standard with the Humphrey Field Analyzer or the Zippy Adaptive Threshold Algorithm—standard with the Henson 9000 Visual Field Analyzer, using the 24-2 or 30-2 test pattern. Participants with glaucoma had to meet specific inclusion criteria, which required at least one visual field quadrant to be relatively free from defects and mean deviation value less than -1dB in the eye to be tested. To define a quadrant as relatively free from visual field defects in the region of testing interest, the following pragmatic criteria were established to enable inclusion of participants with a wide range of defect severity: within the pattern deviation probability plot, only one of the four locations around the 12.5° eccentricity (our peripheral testing location, as described below) could exhibit a visual field defect. Furthermore, the affected location was not permitted to have a probability of less than 0.05 percent (i.e., P < 0.05% probability) (Fig. 1). To ensure comprehensive coverage and minimize potential variability, although the analysis of clinical visual field data was based on a single, recent field (see Supplementary File 1), we ensured that, within the pattern deviation plot, any of the four locations around the 12.5° eccentricity were not consistently affected (more than once within a year or the last three reports) in their previous records for participants who had multiple visual field reports. For participants recently diagnosed with glaucoma before the start of the experiment, the visual field test was conducted twice within a maximum interval of 3 months to ensure consistency.
Figure 1.
An example visual field illustrating placement of the peripheral stimuli in POAG and PACG with severity approximately matched.
The severity of glaucomatous damage between the POAG and PACG groups was approximately matched with patients selected with similar spatial patterns of visual field defects and mean deviation value (Supplementary File 1). All POAG, PACG, and controls were free from other eye disease, apart from early cataract, or might have undergone cataract surgery and did not have systemic disease nor were taking any medications known to affect visual function at the time of recruitment. All participants had best corrected visual acuity of 6/9 or better and refractive error not more than 5 DS sphere and 2 DC. Those individuals who initially presented with PACG and subsequently developed POAG (combined mechanism glaucoma) at the time of recruitment were not included in the study.
All experiments were performed using a gamma corrected 32-inch LCD monitor Display++ (Cambridge Research Systems Ltd., Rochester, UK) with framerate of 120 Hz and resolution of 1920 × 1080 pixels. The monitor operated in a 10-bit mode, with a maximum brightness of 100 cd/m2. Stimuli for experiments were written in Psychopy 3.27 The monitor was placed at 57 cm from the observer. To stabilize the position of the participant, a chin and forehead rest were used.
Visual threshold estimates were determined monocularly and optically corrected for 57 cms (monitor distance), tested both centrally and at a single midperipheral location (12.5°) in a quadrant free from visual field defects.11,17 In all tasks, participants gave verbal responses, and the examiner, seated opposite the participant and unaware of stimulus presentation, recorded responses using key presses. The examiner also closely monitored the participant's fixation throughout the experiment. Participants underwent training sessions for each experiment, covering both central and peripheral locations, before the commencement of the experiments.
Early Visual Processing (Contrast Discrimination)
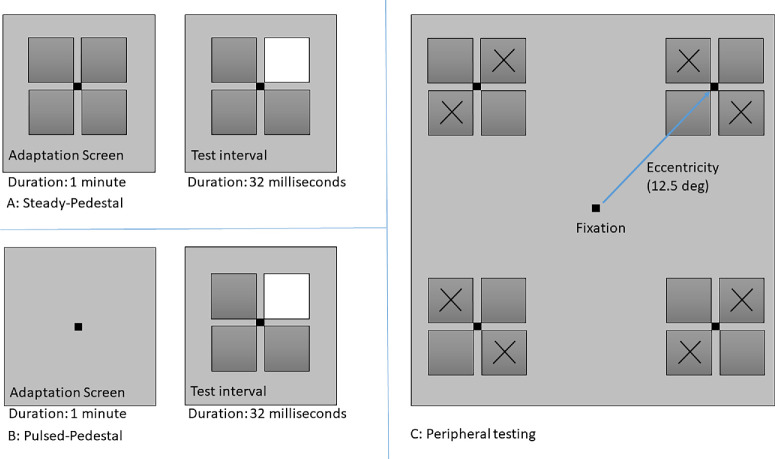
Steady pedestal and pulsed pedestal tasks (Fig. 2) as described by Pokorny and Smith24 were used to estimate contrast discrimination ability. A pedestal luminance of 24 cd/m2 was used; a previous study demonstrated this luminance to be effective to expose differences between glaucoma and control groups.17 Participants were tasked with identifying the square with the highest luminance (the odd-square out) during the test interval in both the steady and pulsed pedestal conditions, with one of the four squares briefly incrementing in luminance relative to the other three for approximately 32 ms (4 frames) (Fig. 2A).
Figure 2.
The contrast-discrimination stimuli. (A) Schematic representation of the steady pedestal task. (B) Schematic representation of the pulsed pedestal task. (C) Schematic of stimulus positioning for peripheral testing in the upper visual field.
In the steady pedestal condition, a black fixation dot was presented at the center of the continuously displayed array of four squares against the background of 30 cd/m2. Participants adapted to these squares for 1 minute before the first presentation (first test interval). In the pulsed pedestal, the black fixation dot was presented against the background of 30 cd/m2. Participants were adapted to the background with the black fixation for 1 minute before testing: during the test interval, the four-square array was presented briefly for approximately 32 ms (4 frames), with one of the squares incremented in luminance relative to the other three (Fig. 2B).
The stimulus square size was 1° for foveal viewing and 1.73° when viewed peripherally.17 Because spatial judgments are more difficult in the periphery, to simplify the task slightly a two-alternative forced choice method was used with the stimuli projected along a diagonal meridian to ensure equal eccentricity of the stimuli presentation (Fig. 2C). A one-up, three-down staircase procedure was used to determine the luminance increment threshold for the detection of brighter square that converges at the approximate 79% correct response probability. The luminance increased or decreased with steps of 0.5 cd/m2 and the staircase terminated after six reversals with the average value of last four reversals taken as the threshold.
Intermediate Visual Processing (Global Form Perception)
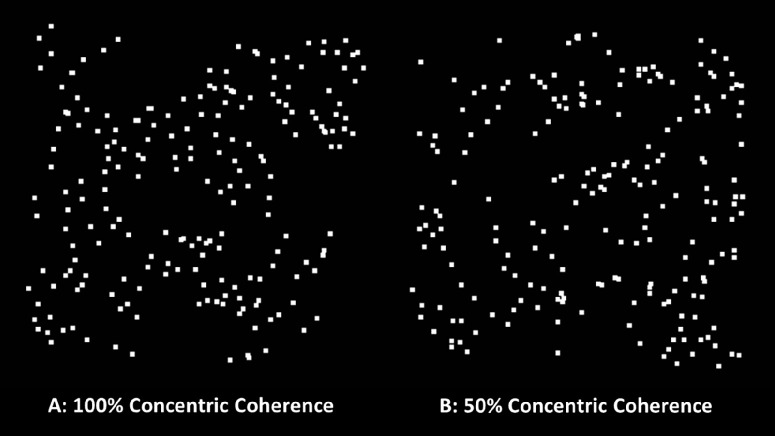
Glass patterns were used to measure global form perception.28 Glass patterns were constructed using paired white square dots (dot size, 8.69 minutes arc) according to a concentric rule within a 10deg square patch. Dots were presented on an 0.5 cd/m2 background at approximately 90% contrast. For the dots to form a concentric pattern, the orientation of the signal pairs were perpendicular to the center of the image (Figs. 3A, 3B). The coherence threshold was determined by randomly replacing a pair of signal dots with unpaired noise dots29; thus, the coherence is determined by the percentage of signal dots required to identify the pattern.
Figure 3.
Image showing (A) 100% and (B) 50% concentric glass pattern coherence used for global form perception task.
The global form perception threshold was determined using a two-interval forced choice method, where one of the two intervals displayed the concentric pattern and other displayed a random noise pattern made of paired and unpaired noise dots (presentation of the concentric pattern was randomly chosen on each presentation to be either first or second). The duration of the stimulus interval was approximately 400 ms11 (48 frames) with an interstimulus interval of 1 second (120 frames). Two adaptive staircases, using a one-up three-down method, were interleaved during the experiment. The staircases terminated after eight reversals. Initially, coherence was started at suprathreshold, both the staircase started at 100% coherence. For both staircases, the step size commenced at 20%, which was halved for next four reversals as 10%, 5%, 2%, and resulting in a final step size of 1%. The mean thresholds of last four reversals of each staircase were determined and the results of both the staircase were averaged to estimate the global form perception threshold.
Statistical analysis was conducted using Google Colaboratory (Python Version: 3.10.12). One-way ANOVA and unpaired t tests were performed to test for significant differences in the age of the groups, or the mean deviation of visual fields, or the years of suffering from glaucoma from their initial diagnosis. Two-way ANOVA was used, with eccentricity and groups as factors, to identify significant differences in thresholds between the groups for steady pedestal, pulsed pedestal, and form perception tasks separately. Post hoc testing using the Tukey honestly significant difference test was carried out, with a P value of less than 0.05 considered to be statistically significant.
Results
The mean age of participants with POAG was 65.55 ± 6.66 years (range, 56–77 years), for participants with PACG was 66.5 ± 6.29 years (range, 52–75 years), and control participants was 61.45 ± 8.34 (range, 51–77 years). There was no significant difference in mean age between the groups, F(2,57) = 2.81, P = 0.068.
The visual field defects in the glaucoma groups ranged from early to advanced visual field loss. The mean deviation of POAG ranged from −1.5 to −16.85 dB, with a mean of −6.53 dB and SD of 4.46. Similarly, the mean deviation of PACG ranged from −1.37 to −16.42 dB, with a mean of −6.2 dB and SD of 4.24 dB (Supplementary File 1). There was no significance difference in mean deviation between the groups, t(36) = −0.24, P = 0.81. The average duration of glaucoma from the time of initial diagnosis was 5.15 ± 5.81 years (range, 0.08–17.00 years) for POAG and for participants with PACG was 6.55 ± 7.82 years (range, 0–27.25 years). There was no significance difference in the number of years each participant suffered from glaucoma after the initial diagnosis between the groups, t(36) = −0.65, P = 0.52. There were 8 pseudophakic and 12 phakic eyes in the POAG group, 7 pseudophakia and 13 phakic eyes in PACG group, and 2 pseudophakic and 18 phakic eyes in the control group. The number of phakic-to-pseudophakic eyes were approximately matched between the 2 glaucoma subtypes.
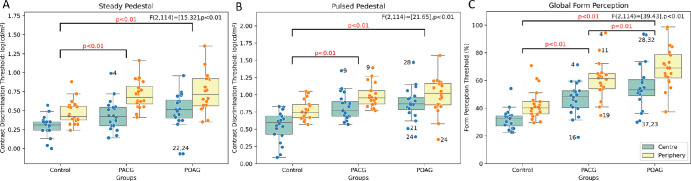
A two-way ANOVA showed significant elevation in contrast discrimination thresholds for peripheral locations compared with foveal viewing for all three groups for both steady, F(2,114) = [37.42], P < 0.01, and pulsed, F(2,114) = [20.7], P < 0.01, pedestal conditions (Figs. 4A, 4B). There was a significant difference between groups for both the steady pedestal, F(2,114) = [15.32], P < 0.01, and pulsed pedestal, F(2,114) = [21.65], P < 0.01, tasks, with glaucoma groups having higher thresholds than controls (Figs. 4A, 4B). Post hoc testing revealed that there was no significant difference (P > 0.05) in thresholds between POAG and PACG at either location, but there was a significant difference between controls and glaucoma groups. There was no significant interaction observed between the groups and eccentricities.
Figure 4.
(A–C) Boxplots showing the difference in the threshold between the groups and eccentricity of contrast discrimination tasks (early processing) and form perception tasks (Intermediate processing), displaying the median with 25th and 75th percentiles. Additionally, error bars extending from the boxes signify the range of variability in the data, and dots within the boxes represent individual thresholds of the participants. Note: The numbers printed near the outlier data points correspond to the participant number, where their age, severity, and duration since diagnosis can be found in Supplementary File 1.
Figure 4C shows the global form perception thresholds. A two-way ANOVA indicated significant differences between the groups, F(2,114) = 39.43, P < 0.01. Post hoc analysis revealed significant differences (P < 0.05) in thresholds between all three groups, with the POAG group exhibiting poorer performance than both controls and participants with PACG for both central and peripheral locations (Fig. 4C). Although there is a significant difference between the thresholds of central and peripheral locations (Fig. 4C), F(2,114) = 27.81, P < 0.01, there was no significant interaction observed between the groups and test location.
To explore whether age, lens condition (phakic versus pseudophakic), or duration since diagnosis may also influence the contrast discrimination and global form thresholds, we performed additional analyses (see Supplementary File 1). An analysis of covariance model with age, eccentricity, duration since diagnosis, and phakic/pseudophakic status as covariates was performed for each of the steady, pulse, and form perception tasks. Age did not attain statistical significance (P = 0.131) for form perception; however, age was a significant influencer of performance for both the steady (0.008; P < 0.004) and pulsed (0.007; P < 0.006) pedestal tasks. It is very well-established that contrast discrimination is affected by age30; however, important for our between-group comparisons, there was no significant difference in age between the three groups. Duration since diagnosis and phakic status were not significant influencers for either contrast discrimination or form perception tasks.
Discussion
In this study, our primary objective was to investigate early and intermediate visual processing in disease severity-matched POAG and PACG. We tested both centrally (foveally) and in the midperipheral region (12.5°), which was free from visual field defects. Early visual processing was assessed through a contrast discrimination task, revealing impaired visual processing in both glaucoma groups, with no significant difference observed between POAG and PACG. Intermediate visual processing was evaluated using a global form perception task. In contrast with the early processing tasks, the global form perception task demonstrated a significant difference between the two glaucoma groups, with POAG displaying higher thresholds than PACG (Fig. 4).
It is noteworthy that, in our study, the key focus is not the entire quadrant being defect free, but rather ensuring the specific area of interest within the quadrant is not significantly damaged in terms of standard visual field metrics. For our experiments, the stimuli were a 3.68° visual angle square patch for the steady/pulsed pedestal tasks, and a 10° visual angle square patch for the form perception task. Noting that the 24-2 test pattern has a 6° spacing, we decided on criteria that ensured that the area of interest would likely have near-normal sensitivity (all individual visual fields are shown in Supplementary File 1). At 10° of visual angle, the glass patterns do extend beyond the four locations used for inclusion, but do not quite extend to the next adjacent 24-2 test locations.
Both POAG and PACG are associated with optic nerve damage and vision loss.1 Despite both subtypes sharing the same primary site of insult, there may be differences in biomechanical insults between them.22 Recent studies have indicated varying degrees of cortical damage between POAG and PACG.13,21 Whereas protein misfolding and apoptosis are linked to POAG's neurodegenerative process, their role in PACG remains unclear.31,32 Individuals with POAG may experience more pronounced cognitive and visual functional decline compared with those with PACG.32 Investigations have revealed differences in brain structure and function between patients with POAG and patients with PACG.32 For instance, gray matter atrophy in cognitive processing regions has been observed in POAG,33 but it is unclear whether this phenomenon also occurs in patients with PACG. Additionally, differences in connectivity have been noted in visual memory, and working memory networks between these two glaucoma subtypes.32,33 There is some limited evidence suggesting that patients with POAG may exhibit more severe visual field loss and cognitive impairment than patients with PACG.32 Furthermore, POAG is associated with an increased risk of dementia; however, no such association has been established yet in PACG.23
Understanding functional impairments resulting from glaucoma aids in both recognizing the condition's progression and effectively addressing the behavioral impacts of visual impairment associated with glaucoma. In our study, both glaucoma groups demonstrated poor contrast discrimination performance in both the locations compared with their normal counterparts. Our study results also suggest that intermediate levels of perception can be affected, even in areas of visual field that are within normative limits. Additionally, POAG had worse thresholds compared with PACG. Although it is clear that having limited visual field can impact performance on more complex tasks, our study suggests that people may experience functional challenges even before their vision loss is categorized as severe. Intermediate form processing is important for recognizing objects and faces.25 Although not assessed directly here, our data are consistent with individuals with glaucoma having trouble recognizing faces,16,34 and that those with POAG might be more affected than those with PACG. However, more research is required for a comprehensive understanding on how glaucoma and its subtypes affects complex visual abilities.
Understanding neurodegenerative changes and associated functional changes in glaucoma subtypes has significant implications for evaluation of the effectiveness of future glaucoma treatment strategies. Novel therapeutic interventions, such as retinal ganglion cell transplantation and artificial retinal implants, should consider the potential variation in neurodegeneration within the posterior visual pathway, because it can be a confounding factor in assessing their efficacy.32,35,36 Evaluating the effectiveness of neuroprotective therapies on glaucomatous neurodegeneration within the brain will also be essential, and these therapies must take into account the varying levels of neurodegenerative changes between individuals and glaucoma subtypes before assessing their therapeutic efficacy.32
Our study's strength lies in its significant contribution toward understanding the functional deficits resulting from presumed cortical processing difficulties in both POAG and PACG, through the study of participants with matched severity of visual field defects. Although the precise mechanisms behind cortical degenerative changes in glaucoma remain unclear, the results may prompt additional research to evaluate the extent and cause of possible disparities in cortical degeneration between glaucoma subtypes and potential therapeutic strategies aimed at preventing cortical degeneration in patients with different types of glaucoma.
In conclusion, both glaucoma subtypes perform more poorly than controls for contrast discrimination and form perception tasks in central and peripheral vision. Additionally, the POAG group demonstrated elevated thresholds relative to the PACG group for an intermediate form perception task, compared with tasks processed earlier in the visual pathways. Our findings suggest that people with POAG may experience more severe functional damage compared with those with PACG, despite having similar levels of visual field defect severity.
Supplementary Material
Acknowledgments
The authors thank Aswani Unikrishnan, a postgraduate student, Suharsha PVN, Junior Research Fellow, and Pooja Nandagopal, Junior Research Fellow from the Department of Optometry, Manipal Academy of Higher Education, for their support in the early stages of this research work.
SBG received Science and engineering Research Board, Statutory Body Established through an Act of Parliament: SERB Act 2008, Government of India (SERB/F/8740/2019-2020). SKK received the TMA PAI scholarship by Manipal Academy of Higher Education.
Disclosure: S.K. Karthikeyan, None; A.M. McKendrick, None; V.H. Pai, None; N.I.R. Kuzhuppilly, None; S.B. Ganeshrao, None
References
- 1. Stamper LR, Lieberman FM, Drake VM.. Becker-Shaffer's Diagnosis and Therapy of the Glaucomas. New York: Elsevier; 2009. [Google Scholar]
- 2. Frezzotti P, Giorgio A, Motolese I, et al.. Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS One. 2014; 9(8): e105931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta N, Greenberg G, De Tilly LN, Gray B, Polemidiotis M, Yücel YH.. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009; 93(1): 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta N, Yücel YH. What changes can we expect in the brain of glaucoma patients? Surv Ophthalmol. 2007; 52(6): 122–126. [DOI] [PubMed] [Google Scholar]
- 5. Gupta N, Yücel YH.. Brain changes in glaucoma. Eur J Ophthalmol. 2003; 13(3): 32–35. [DOI] [PubMed] [Google Scholar]
- 6. Weber AJ, Kaufman PL, Hubbard WC.. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998; 39(12): 2304–2320. [PubMed] [Google Scholar]
- 7. Yücel Y, Gupta N.. Glaucoma of the brain: a disease model for the study of transsynaptic neural degeneration. Prog Brain Res. 2008; 173(8): 465–478. [DOI] [PubMed] [Google Scholar]
- 8. Yücel Y, Zhang Q, Gupta N, Kaufman PL, Weinreb RN.. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000; 118(3): 378–384. [DOI] [PubMed] [Google Scholar]
- 9. McKendrick AM, Sampson GP, Walland MJ, Badcock DR.. Contrast sensitivity changes due to glaucoma and normal aging: low-spatial-frequency losses in both magnocellular and parvocellular pathways. Invest Ophthalmol Vis Sci. 2007; 48(5): 2115–2122. [DOI] [PubMed] [Google Scholar]
- 10. McKendrick AM, Sampson GP, Walland MJ, Badcock DR.. Impairments of contrast discrimination and contrast adaptation in glaucoma. Invest Ophthalmol Vis Sci. 2010; 51(2): 920–927. [DOI] [PubMed] [Google Scholar]
- 11. McKendrick AM, Badcock DR, Morgan WH.. The detection of both global motion and global form is disrupted in glaucoma. Invest Ophthalmol Vis Sci. 2005; 46(10): 3693–3701. [DOI] [PubMed] [Google Scholar]
- 12. Shabana N, Cornilleau Pérès V, Carkeet AK, Chew PT. Motion perception in glaucoma patients: a review. Surv Ophthalmol. 2003; 48(1): 92–106. [DOI] [PubMed] [Google Scholar]
- 13. Ballae Ganeshrao S, Jaleel A, Madicharla S, et al.. Comparison of saccadic eye movements among the high-tension glaucoma, primary angle-closure glaucoma, and normal-tension glaucoma. J Glaucoma. 2021; 30(3): e76–e82. [DOI] [PubMed] [Google Scholar]
- 14. Macaskill MR, Anderson TJ.. Eye movements in neurodegenerative diseases. Curr Opin Neurol. 2016; 29(1): 61–68. [DOI] [PubMed] [Google Scholar]
- 15. Rance G, Hare FO, Leary SO, et al.. Auditory processing deficits in individuals with primary open-angle glaucoma. Int J Audiol. 2012; 51: 10–15. [DOI] [PubMed] [Google Scholar]
- 16. Glen FC, Crabb DP, Smith ND, Burton R, Garway-Heath DF.. Do patients with glaucoma have difficulty recognizing faces? Invest Ophthalmol Vis Sci. 2012; 53(7): 3629–3637. [DOI] [PubMed] [Google Scholar]
- 17. McKendrick AM, Badcock DR, Morgan WH.. Psychophysical measurement of neural adaptation abnormalities magnocellular and parvocellular pathways in glaucoma. Invest Ophthalmol Vis Sci. 2004; 45(6): 1846–1853. [DOI] [PubMed] [Google Scholar]
- 18. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 19. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90(3): 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. George R, Ve RS, Vijaya L.. Glaucoma in India: estimated burden of disease. J Glaucoma. 2010; 19(6): 391–397. [DOI] [PubMed] [Google Scholar]
- 21. Ganeshrao SB, Senthil S, Choudhari N, Durga SS, Garudadri CS.. Comparison of visual field progression rates among the high tension glaucoma, primary angle closure glaucoma, and normal tension glaucoma. Invest Ophthalmol Vis Sci. 2019; 60(4): 889–900. [DOI] [PubMed] [Google Scholar]
- 22. Tun TA, Atalay E, Baskaran M, et al.. Association of functional loss with the biomechanical response of the optic nerve head to acute transient intraocular pressure elevations. JAMA Ophthalmol. 2018; 136(2): 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Su CW, Lin CC, Kao C, Chen HY.. Association between glaucoma and the risk of dementia. Medicine (Baltimore). 2016; 95(7): e2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pokorny J, Smith VC.. Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. J Opt Soc Am A. 1997; 14(9): 2477–2486. [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson F, James TW, Wilson HR, Gati JS, Menon RS, Goodale MA.. An fMRI study of the selective activation of human extrastriate form vision areas by radial and concentric gratings. Curr Biol. 2000; 10(22): 1455–1458. [DOI] [PubMed] [Google Scholar]
- 26. Aung T, Crowston J.. Asia pacific glaucoma guidelines. Amsterdam: Kugler Publications; 2016. [Google Scholar]
- 27. Peirce J, Gray JR, Simpson S, et al.. PsychoPy2: experiments in behavior made easy. Behav Res Methods. 2019; 51(1): 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glass L. Moiré effect from random dots. Nature. 1969; 223(5206): 578–580. [DOI] [PubMed] [Google Scholar]
- 29. Wilson HR, Wilkinson F.. Detection of global structure in glass patterns: implications for form vision. Vision Res. 1998; 38(19): 2933–2947. [DOI] [PubMed] [Google Scholar]
- 30. Zhuang X, Tran T, Jin D, Philip R, Wu C. Aging effects on contrast sensitivity in visual pathways: a pilot study on flicker adaptation. PLoS One. 2022; 16(12): e0261927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wostyn P, Audenaert K, De Deyn PP.. Alzheimer's disease and glaucoma: is there a causal relationship? Br J Ophthalmol. 2009; 93(12): 1557–1559. [DOI] [PubMed] [Google Scholar]
- 32. Nuzzi R, Dallorto L, Rolle T.. Changes of visual pathway and brain connectivity in glaucoma: a systematic review. Front Neurosci. 2018; 12: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frezzotti P, Giorgio A, Motolese I, et al.. Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PLoS One. 2014; 9(8): e105931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glen FC, Smith ND, Crabb DP.. Saccadic eye movements and face recognition performance in patients with central glaucomatous visual field defects. Vision Res. 2013; 82: 42–51. [DOI] [PubMed] [Google Scholar]
- 35. Jutley G, Luk SM, Dehabadi MH, Cordeiro MF.. Management of glaucoma as a neurodegenerative disease. Neurodegener Dis Manag. 2017; 7(2): 157–172. [DOI] [PubMed] [Google Scholar]
- 36. Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL.. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun. 2016; 7: 10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.