Abstract
Type-2 diabetes mellitus (T2DM) is a disorder that is characterized by high blood glucose concentration in the context of insulin resistance and/or relative insulin deficiency. It causes metabolic changes that lead to the damage and functional impairment of organs and tissues resulting in increased morbidity and mortality. It is this form of diabetes whose prevalence is increasing at an alarming rate due to the ‘obesity epidemic’, as obesity is a key risk factor in the development of insulin resistance. However, the majority of individuals who have insulin resistance do not develop diabetes due to a compensatory increase in insulin secretion in response to an increase in insulin demand. This adaptive response is sustained by an increase in both β-cell function and mass. Importantly, there is increasing evidence that the Serine/Threonine kinase mammalian target of rapamycin (mTOR) plays a key role in the regulation of β-cell mass and therefore likely plays a critical role in β-cell adaptation. Therefore, the primary focus of this review is to summarize our current understanding of the role of mTOR in stimulating pancreatic β-cell mass and thus, in the prevention of type-2 diabetes.
Keywords: Type 2 diabetes, Obesity, β-cell mass, mTOR, TSC, Rheb, S6K, PKB, AMPK, RICTOR
An introduction to mTOR
Mammalian target of rapamycin (mTOR, also known as FRAP, RAFT or RAPT) is a highly conserved Ser/Thr protein kinase, which integrates nutrient availability with hormonal/growth factor signalling to regulate cell growth, proliferation, viability and function (for reviews, see [69, 142, 187]). It assembles into two biochemically and functionally distinct multi-component complexes termed mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Fig. 1), which phosphorylate different substrates, are differentially regulated and vary in their degree of sensitivity to the immunosuppressive drug rapamycin. mTORC1 is rapidly inhibited by rapamycin, which acts by binding in a complex with FKBP12 to the FKBP12-rapamycin binding domain of mTOR [23]. In contrast, mTORC2 is resistant to acute rapamycin treatment, although prolonged rapamycin treatment can inhibit mTORC2 in some cell types, probably due to the binding of rapamycin-FKBP12 to unbound mTOR and the inability of this complex to be incorporated into nascent mTORC2 [137].
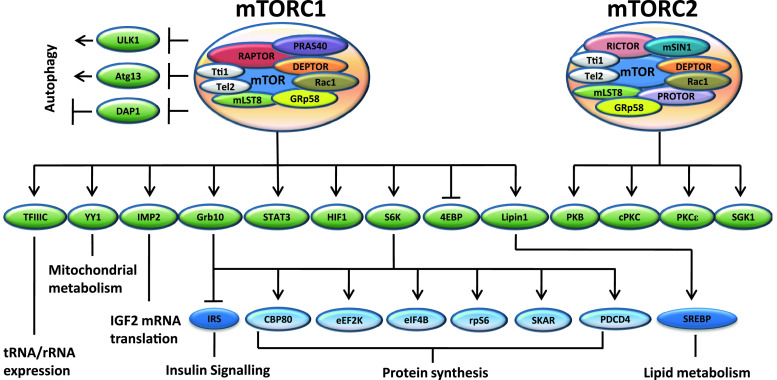
Fig. 1.
Downstream substrates regulated by the mTOR complexes. Both mTOR complexes are comprised of mTOR, Gβ-like protein mLST8 (for mammalian ortholog of lethal with sec 13, also known as GβL) [87], DEPTOR (for DEP domain-containing mTOR interacting protein) [118], GRp58 (for 58 KDa glucose-regulated protein, also referred to as ERp57) [124], Tti1 (Tel 2 interacting protein 1), Tel2 (telomere maintenance 2) [83] and Rac1 (Ras-related C3 botulinum toxin substrate 1) [132]. mTORC1 specific components include RAPTOR (for regulatory-associated protein of mTOR) [86] and PRAS40 (for Pro-rich Akt substrate of 40 kDa) [44, 112, 135, 166, 167]. mTORC2 specific components consist of RICTOR (for rapamycin-insensitive companion of TOR) [136], PROTOR (for protein observed with RICTOR) [114] and mSIN1 (mammalian stress activated protein kinase interacting protein 1) [45, 79, 179]. mTORC1 substrates include 4EBP (eukaryotic initiation factor 4E binding proteins) [9, 19] and S6K [ribosomal protein S6 (rpS6) kinase] [23, 28]. S6K phosphorylates rpS6 [6, 94], SKAR (S6K1 Aly/REF-like target) [126], eIF4B [125], PDCD4 (programmed cell death protein 4) [34], eEF2K [eukaryotic elongation factor 2 (eEF2) kinase] [17, 169], CBP80 (for 80 KDa nuclear cap-binding protein, also known as nuclear cap binding protein subunit 1 or NCBP1) [172] and insulin receptor substrates (IRS) [165]. The effect of mTORC1 on lipogenesis is mediated through the transcription factor SREBP (sterol regulatory element-binding protein) [38, 120], which has recently been shown to be controlled by mTORC1-mediated lipin1 nuclear import [119]. mTORC1 also upregulates the expression of genes implicated in mitochondrial metabolism through the YY1 (Ying Yang 1)-PGC1α (peroxisome proliferator-activated receptor gamma coactivator 1-α) transcription factor complex [30]. mTORC1 also increases the expression of Grb10 (growth factor receptor-bound protein 10), which inhibits insulin signalling via the inhibition of IRS [70, 182]. Other mTORC1 targets include TFIIIC (transcription factor 3C) [85], IMP2 (insulin-like growth factor 2 (IGF2) mRNA binding protein) [31], STAT3 (signal transducers and activator 3) [181] and HIF1 (hypoxia-induced factor 1) [74]. Another major function of mTORC1 is the regulation of autophagy. mTOR directly phosphorylates ULK1 and Atg13 and inhibits autophagosome formation [48, 68, 82], although it also inhibits DAP1, a negative regulator of autophagy which is activated under nutrient deprivation, via direct phosphorylation on Ser3 and Ser51 [92]. mTORC2 is responsible for the phosphorylation and activation of several AGC (for protein kinase A, G and C) kinases, including PKB [79, 138], SGK1 (serum/glucocorticoid-induced kinase 1) [50], conventional PKCs (cPKC) and PKCε (one of the novel PKCs or nPKCs) [42, 75, 136]
Mammalian target of rapamycin complex 1 (mTORC1)
mTORC1 is acutely activated by nutrients, growth factors and hormones (Fig. 2) and importantly, in the context of this review, has been shown to control both cell growth and proliferation of mammalian cells. This is mediated, at least in part, through the phosphorylation of its downstream targets eIF4E binding proteins (4EBPs) and the ribosomal protein S6 kinases-1 and -2 (S6K1/2), both of which impinge on the regulation of protein synthesis (Fig. 1).
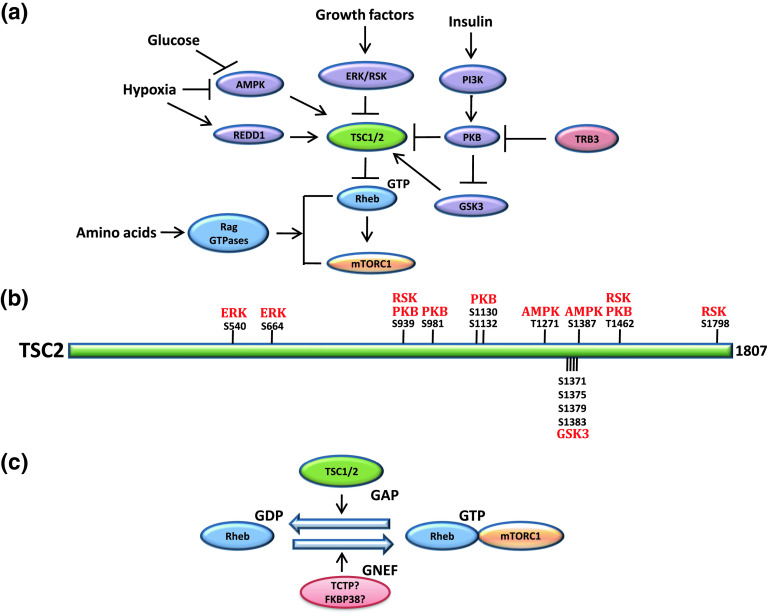
Fig. 2.
Schematic representation of signalling pathways that regulates mTORC1. a mTOR senses a wide range of upstream signals such as: amino acids availability, which modulates the activity of mTORC1 through Rag GTPases [88, 134]; glucose or oxygen levels through AMPK [AMP (5′-adenosine monophosphate) activated protein kinase] [78] and REDD1 (regulated in development and DNA damage responses 1) [18, 81, 149, 173]; growth factors, which activate MAPK (mitogen-activated protein kinase) and stimulate mTORC1 via ERK (extracellular signal-regulated kinases) and RSK (p90 ribosomal protein S6 kinase) [21, 22, 43, 101, 127, 128]; insulin via activation of PI3K (phosphoinositide 3-kinase), PKB (protein kinase B, also referred to as Akt) [52, 141], and inactivation of GSK3 (glycogen synthase kinase 3) [77] [n.b. the activity of PKB can be suppressed by TRB3 (mammalian homolog of Drosophila tribbles 3) [36]]. These pathways impinge on TSC1/2 (tuberous sclerosis complex 1/2), a GTPase activating protein (GAP) of the small G protein Rheb (Ras homolog enriched in brain), and GTP bound Rheb in turn activates mTORC1. b Sites of phosphorylation on TSC2 and their respective kinases, adapted from [73]. c The regulation of Rheb. TSC1/2 acts as a GAP for Rheb [139, 185], whereas TCTP (translationally controlled tumor protein) [71] and FKBP38 (FK506 binding protein 38) [4], have been proposed to act as a GNEF for Rheb
S6K and 4EBPs are recruited to mTOR via the mTORC1 specific component RAPTOR [64, 86]. mTORC1 phosphorylates S6K1 and S6K2 within their hydrophobic motif (HM), on Thr389 and Thr388, respectively, which is critical for their activation [115, 144]. Once activated, S6K1/2 can phosphorylate a number of proteins including: ribosomal protein S6 (rpS6) [6, 94], S6K1 Aly/REF-like target (SKAR) [126], eukaryotic initiation factor-4B (eIF4B) [125], programmed cell death protein 4 (PDCD4) [34], eukaryotic elongation factor 2 (eEF2) kinase (eEF2K) [17, 169], and 80KDa nuclear cap-binding protein (CBP80) [172] (Fig. 1). S6K1 can also phosphorylate IRS-1 on Ser307 [66] and Ser1101 [160], resulting in decreased insulin signalling. This acts as an important negative feedback mechanism in the regulation of mTORC1. Importantly, knock-out studies in Drosophila and mice have revealed that S6K1 plays an essential role in the regulation of cell size but not cell proliferation [105, 116, 144].
4EBPs, of which there are three isoforms (4EBP1, 4EBP2 and 4EBP3), act as repressors of cap-dependent translation by binding to and sequestering the mRNA cap binding protein eIF4E (for review, see [150, 171]). Upon phosphorylation of 4EBP by mTORC1, 4EBP is released from eIF4E [53], promoting the expression of highly cap-dependent proteins including the cell cycle proteins p27kip1, p21cip1/waf1, cyclin D1, D2, D3, E and A, leading to an increase in G1 to S cell cycle progression (for review, see [170]). Knock-out studies in Drosophila indicate that 4EBP regulates cell growth and proliferation [104]. However, results from mouse embryonic fibroblasts (MEFs) lacking 4EBP indicate that 4E-BPs only influences proliferation in mammalian cells [35].
mTORC1 is activated upon association with the small G-protein Rheb (Ras homolog enriched in brain), when the latter is in its GTP bound state [49, 139, 185] (Fig. 2). Rheb is in turn regulated through the activity of its GTPase-activating protein (GAP), the tuberous sclerosis complex 1 and 2 (TSC1/2) [49, 139, 185]. Importantly, growth factors, hormones and nutrients activate signalling pathways that lead to changes in the phosphorylation status of TSC2 (Fig. 2) and the inactivation of TSC1/2. This promotes the formation of Rheb-GTP and hence the activation of mTORC1. Although FKBP38 (FK506 binding protein 38) and TCTP (translationally controlled tumour protein) have been proposed to act as guanine nucleotide exchange factors (GNEF) for Rheb [4, 71], other studies have questioned this [164, 168].
Insulin, for example, can activate mTORC1 through the protein kinase B (PKB) (also known as Akt)-dependent phosphorylation of TSC2 on Ser939, Ser981, Ser1130, Ser1132 and Thr1462, which leads to the inactivation of TSC1/2 [32, 102, 121] (Fig. 2). PKB can also promote mTORC1 activation through the phosphorylation of mTORC1 specific component PRAS40 (for Pro-rich Akt substrate of 40 kDa) at Thr246 [93, 112, 135, 157, 166, 167], which results in its dissociation from mTORC1, thus preventing its inhibitory activity towards mTORC1 [112, 135, 157, 166, 167].
Growth factors can also activate mTORC1 via the inactivation of TSC1/2, but in this context, it is often via the ERK1/2 (extracellular signal-regulated kinase 1 and 2) and/or RSK (p90 ribosomal protein S6 kinase)-dependent phosphorylation of TSC2 at Ser540 and Ser664 [101], or Ser939, Thr1462 and Ser1798 [128], respectively (Fig. 2). In addition, both ERK and RSK are able to phosphorylate RAPTOR on sites that have been reported to promote the activation of mTORC1 [21, 22].
However, growth factor or hormonal activation of mTORC1 is dependent on the nutrient status of the cell. The presence of amino acids is an obligate requirement for the activation of mTORC1, regardless of stimuli. This amino acid “sensing” by mTORC1 occurs independently of TSC1/2 [148], but is dependent on a group of Ras-related small GTPases comprised of four proteins (Rag A, B, C and D) that form heterodimers [88, 134], which bind to a newly identified trimeric protein complex termed as “ragulator” [133]. These Rag GTPases are essential for the recruitment of mTORC1 to endosomal and lysosomal compartments, where it encounters Rheb-GTP and is activated by the latter. Importantly, these Rags are activated by amino acids [88, 134]. Other proteins, including hVps34 [homologue of vacuolar protein sorting 34, or class III PI3K (phosphoinositide 3-kinase)] [110], Ca2+/Calmodulin [61], MAP4K3 (mitogen-activated protein kinase kinase kinase kinase 3) [178] and IPMK (inositol polyphosphate multikinase) [89] have also been reported to impact on amino acids signalling to mTORC1. However, the exact mechanism by which amino acids are sensed and regulate mTORC1 is poorly understood and is the subject of intensive research.
mTORC1 also responds to changes in the energy status of the cell [33]. For example, glucose can stimulate mTORC1 through the inactivation of AMP (5′-adenosine monophosphate) activated protein kinase (AMPK) [78, 90], which responds to changes in the cellular AMP:ATP ratio (for a historical review, see [65]). AMPK suppresses mTORC1 activity via the phosphorylation of TSC2 on Thr1271 and Ser1387, which stimulates TSC1/2 GAP activity [78]. AMPK can also phosphorylate RAPTOR on sites that promote the inhibition of mTORC1 [62]. In addition, a decrease in the energy status of the cell can augment the expression of REDD1 (regulated in development and DNA damage responses 1) independently of AMPK and this has also been reported to activate TSC1/2 and inhibit mTORC1 [18, 149].
Mammalian target of rapamycin complex 2 (mTORC2)
mTORC2 has originally been identified as a positive regulator of actin cytoskeletal organization, polarization and cell migration [60, 80, 100, 136]. The mechanism by which mTORC2 mediates these effects is not fully understood, but it is known that mTORC2 is able to phosphorylate the turn motif (TM) and the hydrophobic motif (HM) of several AGC (for protein kinase A, G and C) kinases, including PKB [79, 138], SGK1 (serum/glucocorticoid-induced kinase 1) [50], conventional PKCs (cPKC) and PKCε [42, 75, 136], resulting in their stabilisation and activation (Fig. 1). For example, in the case of PKB, mTORC2 associates with polysomes and phosphorylates nascent PKB on its TM at Thr450, which promotes the correct folding of PKB and hence its stability [111]. Then, upon an appropriate stimuli, the mature PKB is translocated to the plasma membrane where it is activated via the phosphorylation on Thr308 (Activation-loop) by PDK1 [2] and Ser473 (HM) by mTORC2 [138].
Initially it was thought that mTORC2 was not activated by hormones and growth factors. However, it has recently been demonstrated that mTORC2 activity towards PKB on S473 is acutely stimulated by insulin or serum via a PI3 kinase-dependent mechanism [47], yet the mechanism by which PI3 kinase activates mTORC2 is far from understood, and may just be through the PIP3-dependent translocation of PKB to mTORC2 at the plasma membrane. However, as TSC1/2 deletion inactivates mTORC2 [72], the effects of PI3 kinase could potentially be through TSC1/2. Interestingly, siRNA-mediated knock-down of proteins involved in ribosome maturation and formation, such as mNIP7 (mammalian nuclear import 7 homolog), Rpl7 (ribosomal protein L7) or Rps16 (ribosomal protein S16), also abrogates mTORC2 activity, indicating that ribosomes may bind to and promote mTORC2 activity [186].
mTOR and its role in the regulation of pancreatic β-cell mass
During the development of type-2 diabetes mellitus (T2DM), age-related body weight gain and the loss of insulin sensitivity (insulin resistance) leads to an increase in insulin demand, which is met by an increase in secretory output maintained though increased β-cell mass and function through a process referred to as β-cell compensation (for reviews see [99, 122]). It is the inability of pancreatic β-cell to adequately compensate for this increase in insulin demand which results in the development of impaired glucose tolerance and ultimately T2DM.
Augmentations in β-cell mass can be mediated through: (1) increased rate of neogenesis (i.e., the generation of new β-cells from ductal stem cells); (2) increased rate of differentiation of pancreatic precursor cells; (3) transdifferentiation of exocrine cells; (4) increased rate of replication (hyperplasia); (5) expansion of cell size (hypertrophy); and (6) decreased rates of cell death (apoptosis) (for reviews, see [1, 15, 16, 29]).
Many growth factors, hormones and nutrients have also been shown to play an important role in stimulating increases in β-cell mass and many, if not all, activate mTORC1. For example, glucose, a potent in vivo stimulator of β-cell mass in rodents [12, 14, 113, 158], acutely up-regulates mTORC1 activity in isolated rat islets and rodent β-cell lines [7, 56, 95]. Moreover, it has been demonstrated that, in vitro, glucose can stimulate β-cell proliferation [7, 95] and protein synthesis [55, 176], an important hypertrophic stimuli [151], via a rapamycin sensitive pathway. The mechanism by which glucose activates mTORC1 in islets and β-cell lines has been reported to be mediated via the autocrine action of insulin [176]. This is likely via the activation of PKB (El Sayed NM, Moore CE and Herbert TP, unpublished data) and the inactivation of AMPK [54]. However, it has recently been reported, in a β-cell line derived from insulin receptor knock-out mice, that glucose-stimulated mTORC1 activation is mediated via the MAPK pathway independently of PKB [7]. In the presence of glucose, the incretin hormone glucagon-like peptide-1 (GLP-1) is also a potent stimulator of β-cell mass in vivo [20, 153, 159, 177] and has been shown to potentiate glucose-stimulated mTORC1 activation in isolated islets and clonal cell lines via a PI3K dependent mechanism [95, 106]. Moreover, in vitro, GLP-1R agonists in the presence of glucose are able to enhance β-cell replication via an mTORC1-dependent pathway (Xie J and Herbert TP, unpublished data). Therefore, GLP1-stimulated β-cell replication in vivo is also likely to be mediated, at least in part, by the activation of mTORC1 [95].
Conversely, the inhibition of mTOR by rapamycin causes loss of β cell function and viability in pancreatic β-cell lines and murine and human islets [8, 41, 184], indicating that the maintenance of mTOR activity is critical for the integrity of the β-cell. It was assumed that these toxic effects of rapamycin on the β-cell were mediated by the inhibition of mTORC1. However, we have recently discovered that rapamycin also inhibits mTORC2 and that the toxic effects of rapamycin on β-cells are primarily mediated by the inhibition of mTORC2 (Barlow AD, Xie J and Herbert TP, unpublished data).
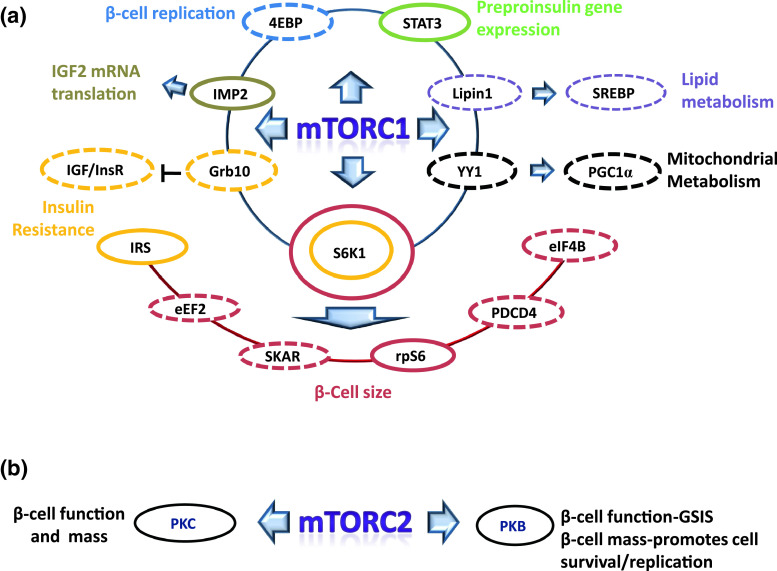
The roles of mTORC1 and 2 downstream targets in the regulation of pancreatic β-cell mass and function are summarized in Fig. 3.
Fig. 3.
Schematic representation of the involvement of protein targets downstream of mTORC1 and 2 in the control of pancreatic β-cell mass and function. Proteins circled in continuous line: function proved in β-cells; Proteins circled in dashed line: function reported in non β-cells, and yet to be studied in β-cells. a Effects of downstream targets of mTORC1 on β-cell function and mass. Using transgenic mouse models it has been shown that S6K1 [ribosomal protein S6 (rpS6) kinase 1] [116] and rpS6 [131] are crucial for the maintenance of β-cell size, although it remains a possibility that other S6K downstream targets, such as SKAR (S6K1 Aly/REF-like target) [126], eEF2 (eukaryotic elongation factor 2) [17, 169], PDCD4 (programmed cell death protein 4) [34] and eIF4B (eukaryotic initiation factor 4B) [125], also contribute to β-cell growth, presumably through their ability of regulating protein synthesis. Insulin resistance can be induced by the inhibition of IRS (insulin receptor substrate) resulted from the over-activation of S6K1 in β-cells [39]. Recently, it has also been reported that newly discovered mTORC1 substrate Grb10 (growth factor receptor-bound protein 10) negatively regulates insulin and IGF (insulin-like growth factor) signalling through the binding and inhibition of InsR (insulin receptor) and IGFR (IGF receptor) [70, 182], which may also contribute to insulin resistance. mTORC1-stimulated fat accumulation is driven by the activation of SREBP (sterol regulatory element-binding protein) [98, 145, 146] through the nuclear import of lipin1 [119]. It has been demonstrated in β-cells that mTORC1 directly phosphorylates IMP2 [insulin-like growth factor 2 (IGF2) mRNA binding protein] to promote IGF2 mRNA translation [31], and leptin-induced activation of STAT3 (signal transducers and activator 3) suppresses preproinsulin gene expression [96]. Furthermore, it can also be speculated that 4EBP [eukaryotic initiation factor 4E (eIF4E) binding proteins] controls β-cell proliferation, while YY1 (Ying Yang 1) and PGC1α (peroxisome proliferator-activated receptor gamma coactivator 1-α) [30] may play roles in β-cell mitochondrial metabolism. Circle colours represent: dark red β-cell size; yellow insulin resistance; black mitochondrial metabolism; tan IGF2 mRNA translation; blue β-cell replication; light green preproinsulin gene expression; purple lipid metabolism. b Downstream of mTORC2. Gu et al. [59] have demonstrated that mTORC2 is important in β-cell replication. In addition, mTORC2 is essential for the maintenance of β-cell viability and GSIS (Barlow AD, Xie J and Herbert TP, unpulished data). mTORC2 also controls the folding and protein stability of PKCα (protein kinase C α), PKCβ and PKCε [42, 75], which are known to play important roles in β-cell function and survival [13, 140]
Transgenic mouse models
Although in vitro studies have revealed important insights into the role and regulation of mTORC1 in β-cells, transgenic mouse models of upstream regulators and downstream effectors of mTOR have provided unequivocal evidence demonstrating that mTORC1/2 plays a critical role in the regulation of β-cell mass in vivo. What we consider to be the most pertinent examples are discussed below. However, we have also provided a comprehensive list of these transgenic mouse models and their phenotypes (see Table 1).
Table 1.
Phenotypes of selected transgenic mouse models
| Transgenic mice | β-cell mass | Metabolic parameters | Pancreas/Islet insulin content | Glucose in ITTi | Susceptibility to exp. diabetes | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Specificity | Size | Proliferation | Apoptosis | Total mass | Blood glucose | Blood Insulin | In vivo GSIS | |||||
| Fasted/Fed | OGTT/IGTT | Fasted/Fed | OGTT/IGTT | ||||||||||
| S6K1−/− | Global | ↓ | N/D | ↔ | ↓ (endocrine mass) | Fasted: ↔ | IGTT: ↑ | Fasted: ↓ | IGTT: ↓ | ↓ | ↓ | ↓ (HFD) | [116], [165] |
| S6KCARIP | β-cells | ↑ | ↑ | ↑ | ↔ | Fasted: ↓ | IGTT: ↓ | Fasted: ↑ | IGTT: ↑ | N/D | ↔ | N/D | [39] |
| rpS6P−/− | Global | ↓ | ↑ | N/D | ↔ | Fasted/Fed: ↔ | IGTT: ↑ | Fasted: ↓ | N/D | ↓ | ↓ | N/D | [131] |
| 4EBP1−/− | Global | N/D | N/D | N/D | N/D | Fasted/Fed: ↓ | N/D | Fed: ↔ | N/D | N/D | N/D | N/D | [162] |
| 4EBP1−/− 4EBP2−/− | Global | N/D | N/D | N/D | N/D | Fasted: ↔ | IGTT: ↔ | Fasted: ↑ | N/D | N/D | ↑ | ↑ (HFD) | [97] |
| Rheb (R3/R20a) | β-cells | ↑ (R3) | ↔ | ↔ | ↑ (R3) | Fed (R20): ↓ | OGTT and IGTT (R20): ↓ | Fed (R20): ↑ | OGTT and IGTT (R20): ↑ | N/D | N/D | ↓(R3) (STZ) | [63] |
| βTSC2−/−b | β-cells; 4–52 weeks | ↑ | ↑ | ↔ | ↑ (up to 52 weeks) | Fasted (8-52 weeks)/Fed (4-52 weeks): ↓ | IGTT (4,40 and 52 weeks): ↓ | Fasted/Fed (4 to 20 weeks): ↑ | IGTT (12 weeks): ↑ | N/D | N/D | N/D | [123] |
| β-cells; ≤35 weeks | ↑ (6 weeks) | N/D | ↔ (5 weeks) | ↑ (6 weeks) | Fed (4-32 weeks): ↓ | OGTT (8 weeks): ↓ | Fed (12 weeks): ↑ | OGTT/IGTT (8 weeks): ↑ | N/D | N/D | N/D | [143] | |
| β-cells; ≥35 weeks | ↑ (40 weeks) | N/D | ↑ (35 weeks) | ↓ (40 weeks) | Fed (36-48 weeks): ↑ | N/D | Fed (40-48 weeks): ↓ | N/D | |||||
| RIP-TSC1cKO | β-cells | ↑ | ↔ | ↔ | ↑ | Fed: 4 weeks:↓; 8 and 20 weeks: ↔; 12–16 weeks: ↑ | IGTT (4 weeks): ↓ | Fed (4-24 weeks): ↑ | IGTT (4 weeks): ↑ | ↑ | ↑ (≥8 weeks) | N/D | [107] |
| βLKB1KO | β-cells | ↑ | ↑ | N/D | ↑ | Fasted/fed: ↓ | IGTT: ↓ | Fasted/fed: ↑ | IGTT: ↑ | ↑ | ↔ | ↓ (HFD) | [46],[58],[155] |
| βAMPKDKO | β-cells | ↓ | ↑ | ↔ | ↔ | Fasted/fed: ↑ | IGTT: ↑ | Fasted/fed: ↓ | IGTT: ↓ | N/D | ↓ | ↓ (HFD) | [154] |
| βAMPK.CA (male)j | β-cells | ↓ | N/D | N/D | N/D | Fasted: ↔ | IGTT: ↑ (3 months) or ↔ (6 months) | Fasted: ↓ (3 months) | IGTT: ↓ (3 months) | N/D | ↔ | ↔ (HFD) | [154] |
| βPi3kr1 Pi3kr2DKO | β-cells | N/D | ↑ | ↑ | ↓ | ↔ | IGTT: ↑ | ↔ | IGTT: ↓ | N/D | ↔ | N/D | [84] |
| PTEN+/− | Global | N/D | N/D | N/D | ↔ | Fasted/fed: ↓ | IGTT: ↓ | Fasted: ↓ | IGTT: ↔ | ↔ | ↓ | N/D | [174] |
| RIPcre + Pten fl/fl | β-cells and hypothalamus | ↔ | ↑ | ↓ | ↑ | Fasted: ↓ | IGTT: ↔ | Fasted: ↔ | N/D | N/D | ↓ | ↓ (STZ) | [152] |
| ↑ | ↑ (not significant) | ↔ but ↓ if STZ treated | ↑ | Fasted: ↓ | IGTT: ↓ | Fasted: ↓ | IGTT: ↔ | ↑ | ↓ | ↓ (STZ) | [108] | ||
| βRicKOb | β-cells | ↔ | ↓ | ↔ | ↓ | Fed:↑ (12 and 16 weeks) | IGTT: ↑ | N/D | IGTT: ↓ | ↓ | ↔ | N/D | [59] |
| Akt1−/− | Global | N/D | N/D | ↑ (testes and thymus) | N/D | Fasted/fed: ↔ | OGTT/IGTT: ↔ | Fasted: ↔ | N/D | N/D | ↔ | N/D | [25], [27] |
| RIP-KdAkt | β-cells | ↔ | N/D | ↔ | ↔ | Fasted (4 month): ↔ | IGTT (6–8 weeks): ↔ | Fasted (4 months): ↔ | IGTT (6-8 weeks): ↔ | ↔ | N/D | ↑ (HFD) | [10] |
| Fed (4 month): ↑ | IGTT (6 months): ↑ | Fed (4 months): ↓ | IGTT (6 months): ↓ | ||||||||||
| Myr-Akt1c | β-cells | ↑ | ↔ | ↑ but ↓ if STZ treated | ↑ | Fasted/fed: ↓ | IGTT: ↓ | Fasted: ↑ | IGTT: ↑ | ↑ | ↔ | ↓ (STZ) in reference [163] | [3], [163] |
| ↑ | ↑ (5 weeks) | N/D | ↑ | Fasted/fed: ↔ | IGTT: ↓ | Fasted: ↑ | IGTT: ↑ | N/D | N/D | ↓ (STZ) | [11] | ||
| Myr-Akt1; S6K1−/−f | β-cellsf | ↔e | ↔e | N/D | N/D | Fasted/fed: ↔e | IGTT: ↑ | Fasted: ↓ | N/D | N/D | ↓e | N/D | [3] |
| Myr-Akt1; S6K1−/−; S6K2−/−f | β-cellsf | ↓e | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | [3] |
| Akt2−/−d | Global | N/D | N/D | N/D | ↑ | Fasted/fed: ↑ | OGTT: ↑ | ↑ | N/D | N/D | ↑ | N/D | [26] |
| N/D | N/D | ↑h (24 weeks) | ↓h (24 weeks) | Fasted/fed: ↑ | OGTT: ↑ (7 weeks) | ↑g; or ↓ after 8 weeksh | N/D | ↓h (24 weeks) | ↑ | N/D | [51] | ||
| N/D | N/D | N/D | N/D | N/D | OGTT: ↑ | N/D | N/D | N/D | ↑ | N/D | [37] | ||
| N/D | N/D | N/D | N/D | Fed: ↔ (6 months) | IGTT: ↑ (6 months) | Fed: ↑ (6 months) | IGTT: ↑ (6 months) | ↑ | ↔ | N/D | [24] | ||
↑, increased compared to wild-type; ↓, decreased compared to wild-type; ↔, similar to wild-type; exp., experimental; HFD, high fat diet; GSIS, glucose-stimulated insulin secretion; IGTT, intraperitoneal glucose tolerance test; N/D, no data; OGTT, oral glucose tolerance test; RIP, rat insulin promoter; STZ, streptozotocin
aRheb over-expression in mouse: R3 and R20 are two independent founder lines used in the study of [63]
bGlobal deletion of TSC2 or RICTOR causes embryonic lethality [147]
cMice expressing myristoylated (constitutively active) Akt1
dPhenotypes of other PKB isoform-combined knock-out mice (Akt1+/−Akt2−/−, Akt1−/−Akt2+/−, Akt2−/−Akt3−/−, Akt1+/−Akt2−/−Akt3−/−, Akt2−/−Akt3−/− and others) have also been described [24, 37, 180]. Description of other mouse models related to PKB pathway (e.g., RIP-IGF1, PTEN−/−, βGSK-3β−/−, and others) can be found in recent reviews [1, 40]
eAll parameters (↑, ↓ or ↔) are in comparison to Myr-Akt mice
fMyr-Akt (beta-cell specific) and S6K1−/− (and S6K2−/−) (global deletion) mice were crossed to yield the Myr-Akt;S6K1−/− (and S6K2−/−) mice
gThese Akt2−/− mice was consistently hyperinsulinemic
hPlasma insulin levels from these Akt2−/− mice started to drop after 8 weeks, and they became hypoinsulinemic compared to their wild-type littermates after 24 weeks
iBlood glucose levels during insulin tolerance test (ITT) compared to wild-type animals
jFemale βAMPK.CA (expressing constitutively active AMPK in β-cells) and βAMPK.DN (expressing dominant negative AMPK in β-cells) mice displayed no abnormalities compared to wild-type in IGTT or ITT
Tuberous sclerosis complex 1 and 2 (TSC1/2)
TSC1/2 is an upstream negative regulator of mTORC1 [76, 156], and loss of TSC2 leads to the constitutive activation of mTORC1 [57, 156, 183]. Importantly, the generation of β-cell specific TSC2 knock-out mice (βTSC2−/−), from two independent groups, has revealed that TSC2 plays in important role in the regulation of β-cell size and mass [123, 143]. Rachdi et al. [123] reported that in 8 weeks old βTSC2−/− mice, β-cell mass is increased by over two-fold due to a doubling in β-cell size and proliferation and that this increase in β-cell mass is maintained for up to 52 weeks of age [123]. Shigeyama et al. [143] reported similar increases in β-cell size and mass in 6 weeks old βTSC2−/− mice but, in contrast to Rachdi et al. [123], β-cell mass had decreased dramatically (80% reduction compared to control) by 40 weeks of age likely due to an increase in apoptosis. This was accompanied by hypoinsulinemia and, as a consequence, hyperglycemia. The positive and negative effects of TSC2 knock-out on β-cell mass are likely mediated by mTORC1 as rapamycin treatment of young βTSC2−/− mice causes a reduction in β-cell mass, whereas rapamycin treatment (18–40 weeks) of Shigeyama et al.’s [143] βTSC2−/− mice resulted in the maintenance of β-cell mass and improved glyceamic control. The age-related decrease in β-cell mass observed in βTSC2−/− mice is likely due to the prolonged hyper-activation of mTORC1 resulting in feedback inhibition, possibly through S6K-dependent phosphorylation of IRS2.
β-cell specific TSC1 knock-out mice (RIP-TSC1cKO) also show an enhancement of β-cell size rather than number and this correlated with an augmentation in glucose stimulated insulin secretion (GSIS) in vivo and increased glucose clearance as determined by intravenous glucose tolerance test (IGTT) compared to WT mice [107]. As the observed enhancement of GSIS was eliminated by rapamycin treatment, the positive effects of TSC1 deletion are likely mediated by the activation of mTORC1.
Rheb
The over-expression of Rheb, the downstream target of the TSC1/2 complex, also results in the constitutive activation of mTORC1 [109, 148]. β-cell specific over-expression of Rheb in mice enhances β-cell mass by approximately 50% [63]. β-cell size was increased by up to 30%, whereas cell proliferation and cell viability were unaffected. This augmentation in β-cell mass correlates with improved glucose tolerance in oral glucose tolerance test (OGTT) and increased late phase GSIS in vivo compared to their wild-type littermates. These improvements in glucose tolerance and enhancement of GSIS were reversed upon rapamycin treatment, indicating that that these functional effects are likely mediated via the activation of mTORC1 [63].
Ribosomal protein S6 Kinase (S6K1/2) and ribosomal protein S6 (rpS6)
S6K1/2 are downstream targets of mTORC1 and S6K1 knock-out (S6K1−/−) mice have reduced β-cell mass due to a reduction in β-cell size (a 24% decrease in comparison with the WT) [116]. In addition, the islets from these mice had decreased islet insulin content and the amount of insulin secreted per cell in response to glucose was significantly reduced. Conversely, the over-expression of a constitutively active form of S6K1 in mouse β-cells (S6KCARIP) results in an increase in β-cell size by approximately 50% [39]. These reports indicate that S6K1 is a positive effector of β-cell size and function. The effects of S6K1 on β-cell size may be mediated through the phosphorylation of rpS6, as non-phosphorylatable rpS6 knock-in mice (rpS6p−/−) have smaller β-cells (a 35% decrease compared to WT) [131]. Yet, β-cell mass is unaffected due to a compensatory increase in β-cell number. Interestingly, β-cells from rpS6P−/− mice are smaller than those from S6K1−/− mice, indicating that other rpS6 kinases may be involved. However, there is no reduction in β-cell size or mass in S6K2 knock-out (S6K2−/−) mice [117], although S6K2 is considered to be the major in vivo rpS6 kinase [117]. It is therefore possible that the difference in cell size between rpS6P−/− and S6K1−/− mice is mediated by an alternative S6K such as p90 ribosomal S6 kinase (RSK) or protein kinase A (PKA) [106, 129]. Then again, the effects of rpS6 and S6K on β-cell size may occur via distinct mechanisms [130]. For example, the effects of S6K on β-cell size could be mediated by alternative S6K substrates. Intriguingly, the depletion of SKAR [126] has been shown to reduce cell size in other cell types. Therefore, it would be of interest to determine whether these S6K substrates also play a role in the regulation of β-cell size.
Protein kinase B (PKB)
There are three isoforms of PKB: PKBα, PKBβ and PKBγ (also known as Akt1, Akt2 and Akt3, respectively). Although PKBα is the most abundant isoform in many mammalian tissues [25, 27], PKBβ is highly expressed in insulin responsive tissue [26, 51], whereas expression of PKBγ is restricted to the central nervous system and testis [91]. PKB, through its phosphorylation of TSC2 [76, 121] and PRAS40 [93, 112, 135, 157, 166, 167], is a well characterised positive regulator of mTORC1, and transgenic mice expressing constitutively active PKBα (Myr-Akt1) specifically in β-cells have increased β-cell mass, through an increase in β-cell size and proliferation, and an increase in function as manifested through increased high circulating insulin levels and improved glucose tolerance [3, 11, 163]. Interestingly, rapamycin treatment of Myr-Akt1 mice results in a decrease in β-cell proliferation and mass [5]. Yet, PKBα-dependent increase in β-cell size is unaffected by the deletion of S6K1 but is reduced by the deletion of both S6K1 and 2 [3], although β-cell size still remains bigger than that of their WT littermates [3]. This implies that other targets of PKBα are responsible for stimulating an increase in β-cell size. Indeed, the effects of rapamycin on β-cell mass in Myr-Akt1 mice is likely through the suppression of mTORC1-dependent activation of cdk4, and a reduction in cyclin D2 and D3 levels [5], possibly mediated by a decrease in cap-dependent translation via the hypophosphorylation of 4EBPs [9, 52]. Surprisingly, glucose homeostasis is maintained in PKBα knock-out (Akt1−/−) mice [27], and no significant changes in neither β-cell size nor number was observed in mice expressing kinase dead PKBα (RIP-KdAkt), which has a dominant-negative phenotype (although defects in insulin secretion were observed) [10]. However, kinase-dead PKBα reduces endogenous PKB activity by no more than 80%, and therefore it is conceivable that the remaining 20% of PKBα activity is sufficient to maintain β-cell mass [10]. Deletion of PKBβ (Akt2−/−) in mice resulted in a reduction in β-cell mass concomitant with an increase in the rate of apoptosis [51], although in a previous study Akt2−/− mice had enhanced β-cell mass (increase in both size and number), likely due to the development of insulin resistance and subsequent β-cell compensation [26]. In PKBγ KO mice, glucose homeostasis is unaffected yet effects on β-cell mass were not reported [161].
AMPK/LKB1
In clonal β-cell lines, an increase in energy status through, for example, an increase in glucose concentration can lead to the inactivation of AMPK and the activation of mTORC1 [54, 103], and increased glucose concentration in vivo is a potent stimulator of β-cell mass [16]. Yet, β-cell specific AMPK catalytic subunits (α1 and α2) knock-out mice (βAMPKdKO) exhibit no change in β-cell mass, although a 36% reduction in β-cell size and a two-fold increase in the rate of β-cell proliferation was observed [154]. Surprisingly, no increase in rpS6 phosphorylation (a marker of mTORC1 activation) was observed and, therefore, changes in β-cell size and proliferation in the βAMPKdKO mice may not be caused by altered mTORC1 signalling. In addition, these mice had abnormal glucose tolerance and enhanced in vivo GSIS. In contrast, and seemingly at odds with the results reported in βAMPKdKO mice, β-cell area was reduced in male transgenic mice over-expressing constitutively-active AMPK in β-cells (βAMPK.CA), as were circulating insulin levels, resulting in glucose intolerance [154]. However, no significant changes in β-cell number or size were observed in female βAMPK.CA mice or in β-cells expressing dominant-negative AMPK (βAMPKDKO) [154].
Liver kinase B1 (LKB1) is a kinase which can phosphorylate and activates AMPK [67, 175] and β-cell specific LKB1 knock-out (βLKB1KO) mice have increased β-cell mass due to increased β-cell size [46, 58, 155] and proliferation [46, 155]. Moreover, these mice have improved glucose tolerance because of enhanced in vivo GSIS [46, 58, 155]. However, Sun et al. [155] observed a diminished in vitro GSIS in their βLKB1KO model, which parallels a reduction in the levels of glucose transporter 2 (GLUT2) and ATP-sensitive potassium channel (KATP channel) subunit Kir6.2. The effects of LKB1 depletion, at least on β-cell mass, are likely through mTORC1, as mTORC1 was shown to be activated in the β-cells of these mice [46, 58, 155], and the effects of LKB1 knock-out on β-cell mass were reversed by rapamycin [46, 58]. The differences observed between βLKB1KO and βAMPKdKO suggest that LKB1 activates signalling pathways independent of its established role in the activation of AMPK.
RICTOR
β-cell specific knock-out of RICTOR (βRicKO), an essential component of the mTORC2 complex, in mice, results in a reduction in β-cell mass due to an impairment in proliferation [59]. However, no changes in β-cell size or the rate of cell death were detected. These mice also had decreased pancreatic insulin content, moderate hyperglycemia and glucose intolerance. In islets isolated from βRicKO mice, the phosphorylation of PKB at Ser473, a target for mTORC2 and an important site for PKB activation, was not surprisingly compromised. Moreover, this correlated with an increase in FoxO1 nuclear localisation, which is known to be inhibited by PKB-dependent phosphorylation on Ser473. However, the phosphorylation of PKB at Thr308, another important site for PKB activation and which is mediated by PDK1, was enhanced. Therefore, it is possible that the increase in the phosphorylation of PKB at Thr308 may compensate for the loss of Ser473 phosphorylation and that PKB activity towards specific subset of substrates is maintained. Indeed, no change in mTORC1 activity was detected in islets isolated from βRicKO mice. Interestingly, in β-cell specific Pten (phosphatase and tensin homolog, which inhibits the activation of PKB through promoting the dephosphorylation of PIP3 and thereby prevents plasma membrane translocation of PDK-1 and PKB) and RICTOR double knock-out mice (βPtenRicKO), Thr308 phosphorylation on PKB was dramatically enhanced and this correlated with an increase in β-cell size [59]. Therefore, the authors concluded that phosphorylation of PKB at Thr308 (by PDK-1) drives cell size, whereas the phosphorylation of PKB on Ser473 (by mTORC2) drives cell proliferation [59].
Concluding remarks
Transgenic mouse models have clearly demonstrated that mTORC1 is a positive regulator of β-cell mass through stimulating an increase in both β-cell proliferation and size. It has also been shown in vitro that physiological stimulators of β-cell mass, such as GLP-1 and glucose, activate mTORC1 and stimulate β-cell replication via a rapamycin sensitive mechanism [95]. It is also likely that, in vivo, all growth factors, hormones and nutrients that stimulate increases in β-cell mass also require the activation of mTORC1. However, the chronic activation of mTORC1 may in due course lead to a decrease in β-cell mass mediated by a potent negative feedback mechanism. Therefore, it is possible that chronic excess of nutrient availability, as seen in obesity, may ultimately result in decreased mTORC1 function and β-cell mass. The molecular mechanisms by which mTORC1 stimulates β-cell mass is not fully understood. Yet, it is likely that the effect of mTORC1 on β-cell size is primarily mediated by the activation of S6K1. Although the phosphorylation of rpS6, a substrate for S6K, has been implicated in increased β-cell size [131], it appears that S6K1 may stimulate β-cell size independently of rpS6. Therefore, S6K1-dependent increase in β-cell size may be mediated by the only known S6K1 specific substrate SKAR, which have been previously shown to be important in other cell types to regulate cell size [126]. How mTORC1 stimulates β-cell proliferation is unknown and clearly warrants further investigation. However, it may well be mediated by the phosphorylation of 4EBPs, which is known to stimulate cap-dependent translation and to increase the synthesis of proteins important in cell cycle progression [35]. Other substrates of mTORC1 may also play an important role in β-cell function such as the transcription factors STAT3 (signal transducers and activator 3) [181], which enhances proinsulin gene expression in clonal pancreatic β-cell lines and islets of Langerhans [96], or YY1 (Ying Yang 1), which stimulates mitochondrial gene expression and reduces oxygen consumption in respiration [30].
mTORC2 has also been shown to play a positive role in the regulation of β-cell mass through an increase in β-cell proliferation [59]. Surprisingly, although mTORC2 regulates the activity of PKB, a protein known to be critical in cell survival, β-cell viability is unaffected in RICTOR knock-out mice [59]. However, we have evidence that acute inactivation of mTORC2 causes loss of β-cell viability (Barlow AD, Xie J and Herbert TP, unpublished results).
In conclusion, both mTORC1 and 2 are required to maintain β-cell mass and function. Moreover, the activation of mTORC1 can stimulate increases in β-cell mass through an increase in both β-cell replication and an increase in cell size. Thus, the activation of mTORC1 may play an important role in β-cell compensation in response to an increase demand for insulin under conditions of insulin resistance and/or increased body mass. It is therefore possible that the activation of mTORC1 pharmacologically or using nutritional supplements may stimulate β-cell function and mass and therefore may be useful in the prevention and/or treatment of type 2 diabetes. On a cautionary note, it is worth mentioning that chronic nutritional activation of mTORC1 has been implicated in the development of insulin resistance [39, 160, 165], and that chronic activation of S6K1 can lead to its inactivation [39, 66, 160, 165] and a decline in both β-cell function and mass [39, 165]. However, there is no substantive evidence, as yet, that mTOR plays a role in β-cell dysfunction and death in the development of type 2 diabetes, although it is clearly worthy of investigation.
Acknowledgments
J.X. was supported by a CONACYT studentship awarded by the Mexican government (Scholarship No. 206710). T.P.H. was supported by a Welcome Trust project grant (WT081268MA). We thank Dr. Edith Gomez for a critical reading of this manuscript.
References
- 1.Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Alliouachene S, Tuttle RL, Boumard S, Lapointe T, Berissi S, Germain S, Jaubert F, Tosh D, Birnbaum MJ, Pende M. Constitutively active Akt1 expression in mouse pancreas requires S6 kinase 1 for insulinoma formation. J Clin Invest. 2008;118:3629–3638. doi: 10.1172/JCI35237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 5.Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem. 2009;284:7832–7842. doi: 10.1074/jbc.M807458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee P, Ahmad MF, Grove JR, Kozlosky C, Price DJ, Avruch J. Molecular structure of a major insulin/mitogen-activated 70-kDa S6 protein kinase. Proc Natl Acad Sci USA. 1990;87:8550–8554. doi: 10.1073/pnas.87.21.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartolome A, Guillen C, Benito M. Role of the TSC1-TSC2 complex in the integration of insulin and glucose signaling involved in pancreatic beta-cell proliferation. Endocrinology. 2010;151:3084–3094. doi: 10.1210/en.2010-0048. [DOI] [PubMed] [Google Scholar]
- 8.Bell E, Cao X, Moibi JA, Greene SR, Young R, Trucco M, Gao Z, Matschinsky FM, Deng S, Markman JF, Naji A, Wolf BA. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52:2731–2739. doi: 10.2337/diabetes.52.11.2731. [DOI] [PubMed] [Google Scholar]
- 9.Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard C, Thibault C, Berthault MF, Magnan C, Saulnier C, Portha B, Pralong WF, Penicaud L, Ktorza A. Pancreatic beta-cell regeneration after 48-h glucose infusion in mildly diabetic rats is not correlated with functional improvement. Diabetes. 1998;47:1058–1065. doi: 10.2337/diabetes.47.7.1058. [DOI] [PubMed] [Google Scholar]
- 13.Biden TJ, Schmitz-Peiffer C, Burchfield JG, Gurisik E, Cantley J, Mitchell CJ, Carpenter L. The diverse roles of protein kinase C in pancreatic beta-cell function. Biochem Soc Trans. 2008;36:916–919. doi: 10.1042/BST0360916. [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 17.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 20.Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 21.Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Zheng XF, Brown EJ, Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WS, Peng XD, Wang Y, Xu PZ, Chen ML, Luo Y, Jeon SM, Coleman K, Haschek WM, Bass J, Philipson LH, Hay N. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol. 2009;29:3151–3162. doi: 10.1128/MCB.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 28.Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 29.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 31.Dai N, Rapley J, Angel M, Yanik MF, Blower MD, Avruch J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011;25:1159–1172. doi: 10.1101/gad.2042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 33.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 34.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 35.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 37.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26:8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elghazi L, Balcazar N, Blandino-Rosano M, Cras-Meneur C, Fatrai S, Gould AP, Chi MM, Moley KH, Bernal-Mizrachi E. Decreased IRS signaling impairs beta-cell cycle progression and survival in transgenic mice overexpressing S6K in beta-cells. Diabetes. 2010;59:2390–2399. doi: 10.2337/db09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–251. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabian MC, Lakey JR, Rajotte RV, Kneteman NM. The efficacy and toxicity of rapamycin in murine islet transplantation. In vitro and in vivo studies. Transplantation. 1993;56:1137–1142. doi: 10.1097/00007890-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonseca BD, Alain T, Finestone LK, Huang BP, Rolfe M, Jiang T, Yao Z, Hernandez G, Bennett CF, Proud CG. Pharmacological and genetic evaluation of proposed roles of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK), extracellular signal-regulated kinase (ERK), and p90RSK in the control of mTORC1 protein signaling by phorbol esters. J Biol Chem. 2011;286:27111–27122. doi: 10.1074/jbc.M111.260794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 45.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 2009;10:285–295. doi: 10.1016/j.cmet.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganley IG, du Lam H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 51.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 55.Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J. 2008;410:485–493. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- 56.Gomez E, Powell ML, Greenman IC, Herbert TP. Glucose-stimulated protein synthesis in pancreatic beta-cells parallels an increase in the availability of the translational ternary complex (eIF2-GTP.Met-tRNAi) and the dephosphorylation of eIF2 alpha. J Biol Chem. 2004;279:53937–53946. doi: 10.1074/jbc.M408682200. [DOI] [PubMed] [Google Scholar]
- 57.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Jr, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 58.Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK, Stoeckert CJ, Jr, Meyuhas O, Seino S, Permutt MA, Piwnica-Worms H, Bardeesy N, Dor Y. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009;10:296–308. doi: 10.1016/j.cmet.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60:827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulati N, Karsy M, Albert L, Murali R, Jhanwar-Uniyal M. Involvement of mTORC1 and mTORC2 in regulation of glioblastoma multiforme growth and motility. Int J Oncol. 2009;35:731–740. doi: 10.3892/ijo_00000386. [DOI] [PubMed] [Google Scholar]
- 61.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, Nagata M, Yokono K. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes. 2009;58:1321–1332. doi: 10.2337/db08-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 65.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 66.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 72.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 77.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 78.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 79.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 80.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 81.Jin HO, Seo SK, Kim YS, Woo SH, Lee KH, Yi JY, Lee SJ, Choe TB, Lee JH, An S, Hong SI, Park IC. TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene. 2011;30:3792–3801. doi: 10.1038/onc.2011.102. [DOI] [PubMed] [Google Scholar]
- 82.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, Takehana K, Iemura S, Natsume T, Mizushima N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–20116. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaneko K, Ueki K, Takahashi N, Hashimoto S, Okamoto M, Awazawa M, Okazaki Y, Ohsugi M, Inabe K, Umehara T, Yoshida M, Kakei M, Kitamura T, Luo J, Kulkarni RN, Kahn CR, Kasai H, Cantley LC, Kadowaki T. Class IA phosphatidylinositol 3-kinase in pancreatic beta cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010;12:619–632. doi: 10.1016/j.cmet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kantidakis T, Ramsbottom BA, Birch JL, Dowding SN, White RJ. mTOR associates with TFIIIC, is found at tRNA and 5S rRNA genes, and targets their repressor Maf1. Proc Natl Acad Sci USA. 2010;107:11823–11828. doi: 10.1073/pnas.1005188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 87.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 88.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim S, Kim SF, Maag D, Maxwell MJ, Resnick AC, Juluri KR, Chakraborty A, Koldobskiy MA, Cha SH, Barrow R, Snowman AM, Snyder SH. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab. 2011;13:215–221. doi: 10.1016/j.cmet.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 91.Konishi H, Kuroda S, Tanaka M, Matsuzaki H, Ono Y, Kameyama K, Haga T, Kikkawa U. Molecular cloning and characterization of a new member of the RAC protein kinase family: association of the pleckstrin homology domain of three types of RAC protein kinase with protein kinase C subspecies and beta gamma subunits of G proteins. Biochem Biophys Res Commun. 1995;216:526–534. doi: 10.1006/bbrc.1995.2654. [DOI] [PubMed] [Google Scholar]
- 92.Koren I, Reem E, Kimchi A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr Biol. 2010;20:1093–1098. doi: 10.1016/j.cub.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 93.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 94.Kozma SC, Ferrari S, Bassand P, Siegmann M, Totty N, Thomas G. Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc Natl Acad Sci USA. 1990;87:7365–7369. doi: 10.1073/pnas.87.19.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53(Suppl 3):S225–S232. doi: 10.2337/diabetes.53.suppl_3.s225. [DOI] [PubMed] [Google Scholar]
- 96.Laubner K, Kieffer TJ, Lam NT, Niu X, Jakob F, Seufert J. Inhibition of preproinsulin gene expression by leptin induction of suppressor of cytokine signaling 3 in pancreatic beta-cells. Diabetes. 2005;54:3410–3417. doi: 10.2337/diabetes.54.12.3410. [DOI] [PubMed] [Google Scholar]
- 97.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 100.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 102.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 103.McDaniel ML, Marshall CA, Pappan KL, Kwon G. Metabolic and autocrine regulation of the mammalian target of rapamycin by pancreatic beta-cells. Diabetes. 2002;51:2877–2885. doi: 10.2337/diabetes.51.10.2877. [DOI] [PubMed] [Google Scholar]
- 104.Miron M, Verdu J, Lachance PE, Birnbaum MJ, Lasko PF, Sonenberg N. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat Cell Biol. 2001;3:596–601. doi: 10.1038/35078571. [DOI] [PubMed] [Google Scholar]
- 105.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 106.Moore CE, Xie J, Gomez E, Herbert TP. Identification of cAMP-dependent kinase as a third in vivo ribosomal protein S6 kinase in pancreatic beta-cells. J Mol Biol. 2009;389:480–494. doi: 10.1016/j.jmb.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 107.Mori H, Inoki K, Opland D, Muenzberg H, Villanueva EC, Faouzi M, Ikenoue T, Kwiatkowski D, Macdougald OA, Myers MG, Jr, Guan KL. Critical roles for the TSC-mTOR pathway in {beta}-cell function. Am J Physiol Endocrinol Metab. 2009;297:E1013–E1022. doi: 10.1152/ajpendo.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen KT, Tajmir P, Lin CH, Liadis N, Zhu XD, Eweida M, Tolasa-Karaman G, Cai F, Wang R, Kitamura T, Belsham DD, Wheeler MB, Suzuki A, Mak TW, Woo M. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol Cell Biol. 2006;26:4511–4518. doi: 10.1128/MCB.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nobukuni T, Kozma SC, Thomas G. hvps34, an ancient player, enters a growing game: mTOR Complex1/S6K1 signaling. Curr Opin Cell Biol. 2007;19:135–141. doi: 10.1016/j.ceb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 111.Oh WJ, Wu CC, Kim SJ, Facchinetti V, Julien LA, Finlan M, Roux PP, Su B, Jacinto E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010;29:3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paris M, Bernard-Kargar C, Berthault MF, Bouwens L, Ktorza A. Specific and combined effects of insulin and glucose on functional pancreatic beta-cell mass in vivo in adult rats. Endocrinology. 2003;144:2717–2727. doi: 10.1210/en.2002-221112. [DOI] [PubMed] [Google Scholar]
- 114.Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 117.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 122.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci USA. 2008;105:9250–9255. doi: 10.1073/pnas.0803047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramirez-Rangel I, Bracho-Valdes I, Vazquez-Macias A, Carretero-Ortega J, Reyes-Cruz G, Vazquez-Prado J. Regulation of mTORC1 complex assembly and signaling by GRp58/ERp57. Mol Cell Biol. 2011;31:1657–1671. doi: 10.1128/MCB.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Richardson CJ, Broenstrup M, Fingar DC, Julich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 127.Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2) Biochem J. 2005;388:973–984. doi: 10.1042/BJ20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 131.Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saci A, Cantley LC, Carpenter CL. Rac1 Regulates the Activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 136.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 137.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 138.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 139.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 140.Schmitz-Peiffer C, Biden TJ. Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes. 2008;57:1774–1783. doi: 10.2337/db07-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara SI, Matsuda T, Takeda A, Inoue T, Shibutani Y, Koyanagi M, Uchida T, Inoue M, Hino O, Kasuga M, Noda T. Biphasic response of pancreatic beta cell mass to ablation of TSC2 in mice. Mol Cell Biol. 2008;28:2971–2979. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 147.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 148.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 149.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: a potential role in the pathogenesis of vascular dysfunction in diabetes (review) Int J Mol Med. 2002;9:85–89. [PubMed] [Google Scholar]
- 152.Stiles BL, Kuralwalla-Martinez C, Guo W, Gregorian C, Wang Y, Tian J, Magnuson MA, Wu H. Selective deletion of Pten in pancreatic beta cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol. 2006;26:2772–2781. doi: 10.1128/MCB.26.7.2772-2781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 154.Sun G, Tarasov AI, McGinty J, McDonald A, da Silva Xavier G, Gorman T, Marley A, French PM, Parker H, Gribble F, Reimann F, Prendiville O, Carzaniga R, Viollet B, Leclerc I, Rutter GA. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia. 2010;53:924–936. doi: 10.1007/s00125-010-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun G, Tarasov AI, McGinty JA, French PM, McDonald A, Leclerc I, Rutter GA. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab. 2010;298:E1261–E1273. doi: 10.1152/ajpendo.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, Arrieumerlou C, Hall MN. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Topp BG, McArthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats. Diabetologia. 2004;47:1602–1610. doi: 10.1007/s00125-004-1493-5. [DOI] [PubMed] [Google Scholar]
- 159.Tourrel C, Bailbe D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50:1562–1570. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 160.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- 162.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 163.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 164.Uhlenbrock K, Weiwad M, Wetzker R, Fischer G, Wittinghofer A, Rubio I. Reassessment of the role of FKBP38 in the Rheb/mTORC1 pathway. FEBS Lett. 2009;583:965–970. doi: 10.1016/j.febslet.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 165.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 166.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 167.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 168.Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–267. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 171.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 172.Wilson KF, Wu WJ, Cerione RA. Cdc42 stimulates RNA splicing via the S6 kinase and a novel S6 kinase target, the nuclear cap-binding complex. J Biol Chem. 2000;275:37307–37310. doi: 10.1074/jbc.C000482200. [DOI] [PubMed] [Google Scholar]
- 173.Wolff NC, Vega-Rubin-de-Celis S, Xie XJ, Castrillon DH, Kabbani W, Brugarolas J. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol. 2011;31:1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wong JT, Kim PT, Peacock JW, Yau TY, Mui AL, Chung SW, Sossi V, Doudet D, Green D, Ruth TJ, Parsons R, Verchere CB, Ong CJ. Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia. 2007;50:395–403. doi: 10.1007/s00125-006-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 176.Xu G, Marshall CA, Lin TA, Kwon G, Munivenkatappa RB, Hill JR, Lawrence JC, Jr, McDaniel ML. Insulin mediates glucose-stimulated phosphorylation of PHAS-I by pancreatic beta cells. An insulin-receptor mechanism for autoregulation of protein synthesis by translation. J Biol Chem. 1998;273:4485–4491. doi: 10.1074/jbc.273.8.4485. [DOI] [PubMed] [Google Scholar]
- 177.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 178.Yan L, Mieulet V, Burgess D, Findlay GM, Sully K, Procter J, Goris J, Janssens V, Morrice NA, Lamb RF. PP2A T61 epsilon is an inhibitor of MAP4K3 in nutrient signaling to mTOR. Mol Cell. 2010;37:633–642. doi: 10.1016/j.molcel.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 179.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 182.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006;55:2429–2436. doi: 10.2337/db06-0173. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 186.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 187.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]