Abstract
In the time since its discovery, phosducin’s functions have been intensively studied both in vivo and in vitro. Phosducin’s most important biochemical feature in in vitro studies is its binding to heterotrimeric G protein βγ-subunits. Data on phosducin’s in vivo relevance, however, have only recently been published but expand the range of biological actions, as shown both in animal models as well as in human studies. This review gives an overview of different aspects of phosducin biology ranging from structure, phylogeny of phosducin family members, posttranscriptional modification, biochemical features, localization and levels of expression to its physiological functions. Special emphasis will be placed on phosducin’s function in the regulation of blood pressure. In the second part of this article, findings concerning cardiovascular regulation and their clinical relevance will be discussed on the basis of recently published data from gene-targeted mouse models and human genetic studies.
Keywords: Phylogeny, Blood pressure, Cardiovascular regulation, Genetic studies, Mouse models, Human
Introduction
Phosducin was first described as an abundant phosphoprotein of the mammalian retina [1]. Starting from a mere “PKA-target”, terminology of the protein has changed with increasing knowledge about its size (initially identified as a 30K protein [1], later as the “33K protein”), its bovine amino acid sequence (“MEKA”), its function (“GIP”, G protein inhibitor protein) or its posttranslational modification and interaction partners (“phos-ducin”) [2–5]. Nowadays, it is solely referred to as phosducin, taking into account both its regulation by phosphorylation and its well-established binding partner, retinal G protein transducin. So far, phosducin’s proposed physiological function is mostly confined to the photopic system and its binding to βγ-subunits of heterotrimeric G proteins thereby modulating light-induced processes [4, 6, 7].
Phosducin family
Phosducin is highly conserved among mammalian species [3, 8, 9] (see Fig. 1 for sequence alignment). Phosducin proteins or homologues with stronger structural deviation have also been detected in a broad variety of non-mammalian eucaryotes, including teleost, ciliates, fungi and yeast, where similar functions (e.g. response to illumination, βγ-subunit binding) have been described [10–14]. Although, historically, several phosducin isoforms have been suggested to exist in mammals, it was finally established that they represent one and the same protein [3, 5]. However, with the discovery of more members of the phosducin family, a splice variant of human phosducin was discovered as a second phosducin transcript: phosducin-like orphan protein PhlOP1, which lacks the first 52 N-terminal amino acids [15]. Other isoforms that might have originated from phosducin gene duplication early in development are the phosducin-like proteins PhlP1 and 2 as well as phosducin-like orphan protein PhlOP2. Although structurally different from phosducin, some of them (PhlP1) have maintained Gβγ binding capacity (see Fig. 1 for sequence alignment). Most phosducin family members (PhlP1-3), however, are rather involved in protein folding by acting as co-chaperons with cytosolic chaperonin complex [16–19].
Fig. 1.
Sequence alignment of phosducin or phosducin-like orphan protein from different species. Sequences were aligned using ClustalW alignment tool available from EMBL–EBI (http://www.ebi.ac.uk/Tools/clustaw/index.html) [143]. Colour code marks degree of homology between species
Localisation
Phosducin was originally discovered in retinal photoreceptor cells but was soon suggested to be also expressed in the pineal gland [1, 2, 20–22]. Pineal gland neuroendocrine cells, pinealocytes, are embryologically related to photoreceptor cells of the retina. Both cell types share expression of the same photopic proteins that are most likely deduced from a common ancestral phototransduction system [23]. In the course of evolution, pinealocytes of more highly developed mammalian pineals have lost light sensitivity and adapted to neuroendocrine function, releasing the neurohormone melatonin during nighttime [24]. In a developmental study, expression of phosducin in murine wild-type photoreceptor cells has been shown to begin on postnatal day 6 (P6) and to increase until P18 [25]. Mouse strains with retinal degradation due to a point mutation in the rod-specific enzyme cGMP phosphodiesterase (rd mice) show a decrease in phosducin expression from day P10 onwards, in parallel with a loss of photoreceptors [25]. Expression of Gβ precedes phosducin expression in the pineal gland of rats, suggesting that phosducin might not be essential for norepinephrine (NE)-induced cAMP stimulation of pineal melatonin secretion [26]. The developmental profile of phosducin in rat pineal gland is similar to that of retina degradation in mice [26]. This raises the question whether phosducin might have only transient effects in early postnatal development and is negligible during adolescence and adulthood [27]. However, there is still insufficient evidence as to whether relatively lower expression levels of phosducin could have modulatory effects on NE-induced melatonin production.
Cell subtype specificity of phosducin expression in the retina is an ongoing debate. While some groups claim that phosducin is exclusively a rod protein, other studies demonstrate that phosducin is present both in retinal rod and cone photoreceptor cells in several species [5, 10, 28–31]. Moreover, even subcellular distribution of phosducin in rod photoreceptor cells of the rat is controversial. Based on immunohistochemical analyses, early studies claimed phosducin to be an outer segment protein [2, 6]. Later studies showed rather that phosducin is most abundantly expressed in the inner segment [21, 32–34] or that it accumulates at the photoreceptor synapse to enable modulation of synaptic signal transmission [34–37]. There is quite early evidence and, more recently, confirmation based on technologically advanced experiments that phosducin can translocate from the rod outer segments to the cytosol of inner photoreceptor segments upon illumination [34, 38]. In addition, phosducin has also been detected in nuclear fractions of bovine retinal homogenates [39]. Phosducin expression in cells or tissue other than photoreceptor cells and the pineal gland includes the sympathetic nervous system [40] and the liver [42], as well as the brain [41], kidneys, lungs, spleen and skeletal muscle [4].
Structure and stereochemistry
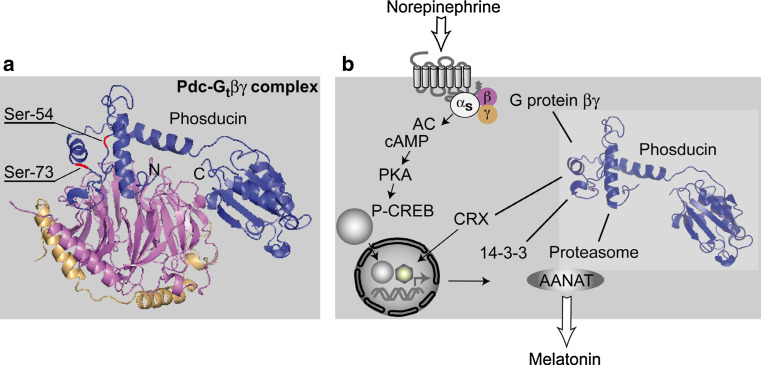
Mammalian full-length phosducin consists of 244–246 amino acids depending on the species. The molecular weight of bovine phosducin has been calculated as 28,185 Da. The weight of 33 kDa, identified electrophoretically on SDS gels, is possibly due to the loading of relatively low numbers of SDS molecules on the basis of phosducin’s acidity [5]. Rat phosducin was crystallised in its complex with Gtβγ by Gaudet and colleague [43], and its structure was identified by X-ray structure analysis [43]. Two non-interacting domains were identified by this approach, in the following named C- and N-terminal domain, respectively, [43–45]. Both domains enclose the βγ-subunit, forming large areas of interaction (see Fig. 2 for Gtβγ structure). The β-subunit beta-propeller is shielded from binding to the G protein α-subunit by phosducin’s N-terminal domain. Thereby, reassociation of heterotrimeric G protein subunits to allow for another cycle of G protein-coupled receptor (GPCR) activation is inhibited. In contrast, the C-terminal part of phosducin binds to the side of the complex and may encumber a γ-subunit prenylation site from serving as a lipophilic membrane anchor, and may thus facilitate translocation of the complex into the cytosol. The C-terminal domain binds Gtβγ with lower affinity than the N-terminal part [46–51]. Conformational changes in the latter will therefore influence binding to a greater extent.
Fig. 2.
a Structure of phosducin-Gtβγ complex in the retina as assessed after crystallography by X-ray analysis, phosducin (blue), G protein β-subunit (magenta), γ-subunit (beige) [43]. Phosducin’s N- and C-terminal domains are marked with N and C, respectively. Note the extensive interaction area between N-terminal phosducin and the top surface of the β-subunit. In the N-terminal domain, Ser-54 and Ser-73, as the two most important phosphorylation sites, are indicated in red. b Schematic illustration of phosducin’s proposed biochemical features and its putative binding partners in pinealocytes or other cells (βγ, CRX, 14-3-3ζ, proteasomal subunits) [56, 83, 84]
Posttranslational modification
Phosphorylation is well established to control phosducin activity. Ser-73 is one of the two main phosphorylation sites within the N-terminal domain of phosducin [52]. It is orientated away from the βγ-complex suggesting that loss of affinity upon phosphorylation is rather due to induction of conformational changes than to sterical disruption of interaction. This hypothesis is corroborated by the presence of positively charged amino acid residues in the close vicinity that may undergo conformational changes upon modification of Ser-73 with negatively charged phosphate. Moreover, the ectopic position of Ser-73 allows access of kinases even to Gtβγ-bound phosducin [43, 52, 53].
For a long time cAMP-activated protein kinase A (PKA) was considered to be the only regulator of phosducin phosphorylation [53, 54]. Several newer studies indicate, however, that not only PKA but also Ca2+/calmodulin-dependent protein kinase II (CaMKII) is able to phosphorylate phosducin within consensus phosphorylation sequences at Ser-73 or Ser-54. More recent publications have identified additional phosphorylation sites within the phosducin molecule and provided a more detailed view of phosphorylation/dephosphorylation kinetics and the degree of phosphorylation-induced functional inhibition [45, 55–57]. Accordingly, phosphorylation by PKA at Ser-73 was shown to diminish binding affinity of phosducin for Gtβγ by only threefold [43, 53, 55]. CaMKII was also shown to phosphorylate Ser-73 but additionally Ser-6, Ser-36, Ser-54 and Ser-106. This reduced binding affinity up to 300-fold and may enable multiplication of signalling events, e.g. by virtue of binding to phosphoserine-binding protein 14-3-3ζ [56].
There is a general consensus that phosducin, which is especially highly expressed in retinal rod cells [54], plays an important role in adaptation to changes in ambient illumination [58]. In light-adapted rod photoreceptor cells, phosducin is unphosphorylated and tightly binds to βγ-subunits to sequester them to the cytosol [21]. It thereby prevents reassociation with the α-subunit and thus lowers signal amplification. During low levels of ambient light, phosducin is present in its phosphorylated state [2], which decreases affinity to transducin βγ [43, 53, 55]. Thereby, reassembly of transducin subunits to a heterotrimeric activatable G protein is favoured and maximal signal amplification occurs. Phosphorylation is thought to provide an on–off switch for phosducin’s function on βγ signal modulation. This tightly regulated light/dark response seems to be disrupted in the rd1 mouse model, where high levels of calcium and CaMKII lead to constitutively phosphorylated phosducin irrespective of illumination level [58, 59].
Similar regulation via phosphorylation can be seen in the pineal gland. In vitro culturing of pineal organs revealed rapid phosphorylation and increases in total phosducin amounts after adrenergic stimulation due to protein de novo synthesis [22]. Similar results were obtained from nocturnal pineal explants, in which illumination rapidly reduced phosphorylation [22]. In bovine retina, light-driven protein phosphatase 2A translocation provides a mechanism for efficient phosducin dephosphorylation [60].
On the posttranslational level, phosducin is also regulated by the small ubiquitin-related modifier SUMO. A consensus motif for SUMOylation is located in the N-terminal domain at Lys-33 [61]. In analogy to phosphorylation, SUMOylation was able to reduce Gβγ affinity, but could, additionally, protect phosducin from ubiquitination and subsequent proteasomal degradation.
Phosducin’s functions in the retina
It is common knowledge that in mammalian retinal photoreceptors photons induce intracellular signalling events that are eventually translated into an electrical stimulus at the photoreceptor-bipolar cell synapse, giving way to a neural response [62]. Steps of this canonical signalling cascade comprise the activation of the GPCR rhodopsin, dissociation of its G protein (transducin) subunits, α-subunit-induced stimulation of cGMP-specific phosphodiesterases (PDE) and the closure of cyclic nucleotide-gated channels in the photoreceptor outer segment with subsequent hyperpolarisation and reduction of synaptic transmission [63]. βγ-subunits that are released from activated transducin are hindered from reassembly with the α-subunit to form a functional heterotrimeric G protein by high affinity binding to unphosphorylated phosducin. As stimulation of PDE through GTP-bound transducin α-subunit is self-limiting due to auto-catalytic hydrolysis of GTP to GDP, sequestration of βγ by phosducin seems to provide a mechanism of light adaptation [64].
Moreover, phosducin might also influence GPCR signalling when preventing the βγ-mediated recruitment of GRKs to the activated receptor. It thereby impairs receptor phosphorylation and interferes with receptor internalization as shown for opioid receptors [65]. Whether this only occurs in cells over-expressing μ-opioid receptors and phosducin [66] or whether this is a general phenomenon in vivo remains elusive to date.
First in vivo evidence for relevance of phosducin in retinal signalling was provided by Sokolov et al. [34]. By applying a technique combining serial tangential sectioning of flat-mounted retinas with western blot analysis and co-immunoprecipitation experiments, this group could show the interaction of phosducin and its binding partner transducin throughout the rod photoreceptor cell on a 10-μm resolution basis. In dark-adapted rods (phosducin phosphorylated), transducin was found to be located in the outer segments whereas phosducin was almost equally distributed between inner and outer segments. Under conditions of illumination (phosducin dephosphorylated), transducin moved towards the inner segment and could be co-immunoprecipitated with phosducin along the entire cell [34]. This finding suggests that phosducin is involved in facilitating transducin translocation. Accordingly, in phosducin-deficient animals, translocation of transducin βγ-subunits was severely blunted along with a reduction in transducin expression levels. Inhibition of translocation was not observed for transducin α-subunits supporting the specificity of the phosducin-βγ interaction [34]. Movement of signalling proteins from the light-sensitive outer segment to different subcellular compartments is one of the key mechanisms to adapt vision to ever-changing conditions of ambient illumination. In rods, transducin translocation results in reduced light sensitivity, which prevents the saturation of rod photoresponses [67, 68] with subsequent blindness and limits energy consumption during largely cone-mediated daytime vision [68]. By interacting with transducin, phosducin can curb rod visual signalling.
However, single cell experiments based on suction electrode recordings have revealed that the above-mentioned effect on rod light adaptation is not influenced by the lack of phosducin under moderate light conditions [69]. The facilitation of transducin translocation seems rather to be a matter of light intensity playing a role in brightly illuminated environments. Yet, the gain of transduction—measured by the amplification constant—was diminished in Pdc −/− rods compared to wild-type cells in single cell recordings [69]. It has been suggested that, rather than adjusting the availability of transducin heterotrimer and thereby controlling light sensitivity, phosducin exerts longer-term regulatory functions allocating transducin expression levels.
Only recently, Herrmann et al. [37, 69] described phosducin to be a regulator of light sensitivity at the photoreceptor-to-ON-bipolar cell synapse rather than a mediator of phototransduction. By evaluating ERG b-wave responses in mice deficient in phosducin and wild-type animals, phosducin was shown to be important both for dark-adapted sensitivity of flash responses as well as for maintenance of b-wave sensitivity under sustained background illumination.
Given the fact that perturbances in the rhodopsin–transducin signalling events may lead to premature photoreceptor cell death and retinal degeneration [70], it seems conceivable that fine-tuning this signalling cascade through phosducin is essential.
Does phosducin have a role in human retinal disease?
The question as to the role of phosducin in the development of or predisposition to retinal disease has been addressed in humans and animals for a broad range of retinopathies. However, despite phosducin being one of the proteins with highest expression in retinal photoreceptors, not much is known about its role in retinal disease: phosducin has been excluded as a major cause for a number of retinopathies in humans, e.g. Usher syndrome II, retinopathia pigmentosa or Leber congenital amaurosis [71–75].
In a set of other retinal diseases or experimental disease settings, e.g. progressive rod-cone degeneration or experimental retinal detachment, alterations in phosducin expression levels or its posttranslational modifications have been observed, but there is no specific evidence of a causal link to the initiation of the disease [32, 59, 76, 77]. Several uveitopathogenic sites have, however, been identified within the phosducin molecule which were capable of eliciting experimental autoimmune uveitis [78–81]. Whether this is of relevance for human ocular disease, however, also remains elusive. Mouse models available today would be ideal to test the causal role of phosducin in retinal disease entities.
Other functions of phosducin
Although G protein βγ-binding and inhibition has been the most extensively studied function of phosducin [50, 82], several other biochemical features have been discussed in the literature. In 2000, Sheryl Craft’s group identified the phosducin C terminus as an intrinsic transcriptional activator in yeast [83]. In a yeast two-hybrid screen, two potential protein partners of phosducin were detected: SUG-1, a 26S proteasome subunit, and CRX, a retina- and pineal-specific transcription factor [83, 84]. Thus, direct (via phosducin’s C terminus) and indirect mechanisms (via CRX) of transcriptional control could be assigned to phosducin. The protein interaction with SUG-1 [85] makes an influence of phosducin on protein degradation plausible, but further investigation is needed to define to what extent this might be relevant in vivo.
Due to its βγ binding capacity, phosducin is widely used in experimental approaches to sequester βγ subunits and thereby block GPCR signalling [86–91].
Cardiovascular functions of phosducin
A study originally performed to decipher beneficial effects of βARKct versus another βγ-binding protein in heart failure provided evidence that in vivo adenoviral gene transfer of an N-terminally truncated version of the phosducin protein (nt-del-phosducin) was able to prevent rapid pacing-induced heart failure in rabbits without the need for resensitising β-adrenergic receptor signalling. Moreover, ex vivo gene transfer of nt-del-phosducin to both normal and failing cardiomyocytes resulted in positive inotropic effects [92].
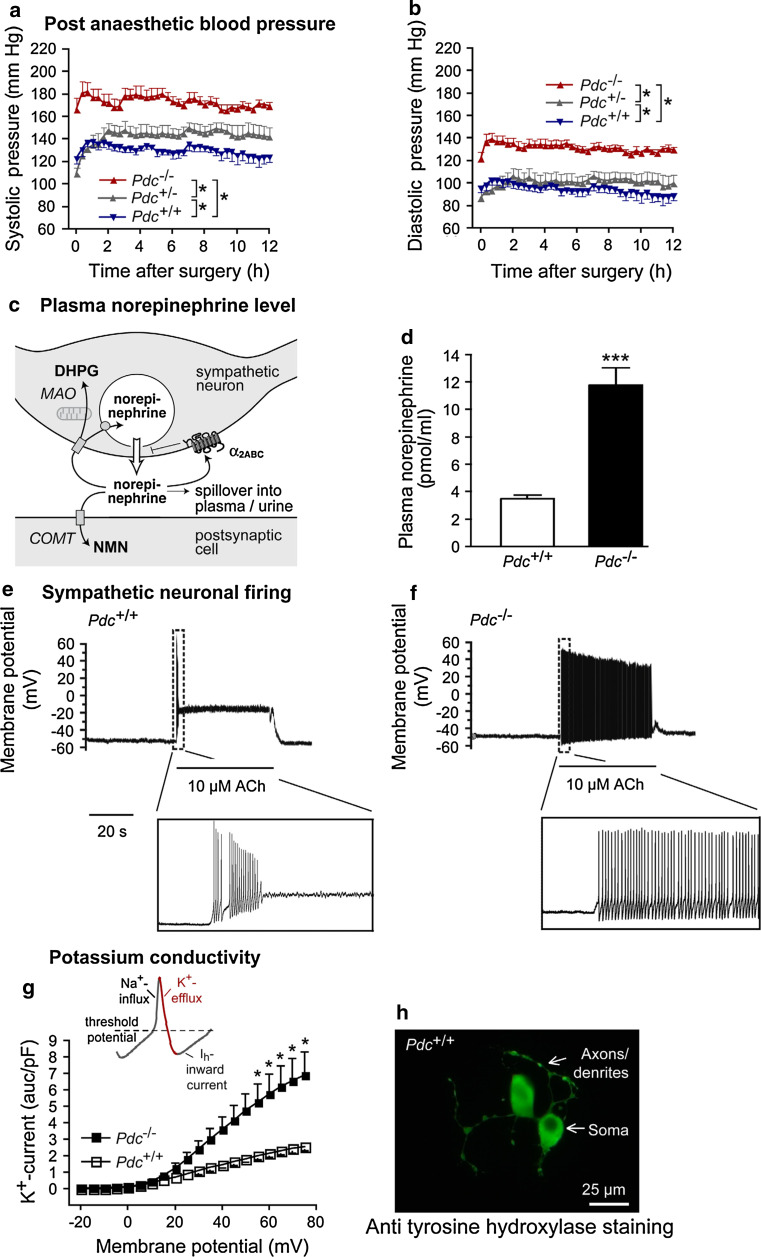
Further evidence for important cardiovascular functions of phosducin was only recently provided by targeted deletion of the phosducin gene in mice by our group [40, 93]. Mice deficient in phosducin (Pdc −/−) due to a deletion of exon 2 of the phosducin gene displayed elevated post-anaesthetic and stress-induced blood pressure (Fig. 3a, b). After recovery from surgery for at least 10 days, Pdc −/− mice presented with normal diurnal blood pressure rhythm but still displayed a hypertensive phenotype during nighttime measurements in comparison to control animals. Moreover, in a stress model of cage-switching, blood pressure responses were more pronounced in Pdc-deficient animals than in control mice [40]. From these results, the hypothesis originated that phosducin is a regulator of sympathetic tone [40].
Fig. 3.
Phosducin’s function in mice. a, b Post-operative systolic and diastolic blood pressure after surgery for implantation of telemetric blood pressure devices; *p < 0.05, n = 5–9 per genotype. c Schematic presentation of the fate of norepinephrine after its exocytosis into the synaptic cleft (DHPG dihydroxyphenylglycol, NMN normetanephrine, MAO monoaminoxidase, COMT catechol-O-methyltransferase). d Circulating plasma norepinephrine levels; ***p < 0.001, n = 6–9 per genotype. e, f Neuronal firing in isolated sympathetic neurons upon acetylcholine administration, representative traces of a wild-type and a knockout neuron are shown (ACh acetylcholine). g Potassium conductivity in sympathetic neurons depolarized by current injection; *p < 0.05, n = 5–8 per genotype. h Sympathetic neurons isolated from the superior cervical ganglion, cultured and stained with an antibody against tyrosine hydroxylase. (Figure reproduced with permission of the American Society for Clinical Investigation [40])
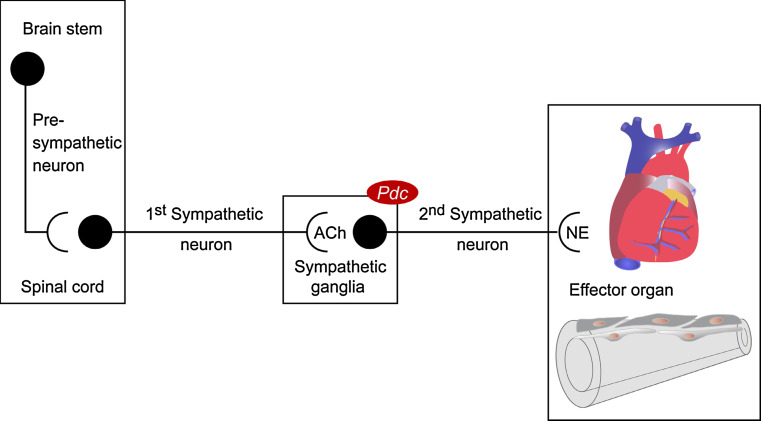
The peripheral sympathetic nervous system consists of two consecutive neurons. Synaptic transmission between these neurons occurs in sympathetic ganglia that line up paravertebrally to form the two sympathetic chains. Originating from the intermediolateral column of the spinal cord, preganglionic neurons integrate incoming signals from descending and sensory neurons and signal to postganglionic neurons via acetylcholine while postganglionic cells use NE for innervation of sympathetic effector organs (Fig. 4).
Fig. 4.
Overview of the sympathetic nervous system neuronal chain
Increased sympathetic activity in Pdc −/− mice was confirmed by measurement of catecholamine spillover into plasma and urine and determination of NE pre- and postsynaptic metabolite levels in sympathetically innervated organs (Fig. 3c). Plasma and nighttime urinary levels of NE as well as cardiac levels of dihydroxyphenylglycol (presynaptic NE metabolite) and normetanephrine (postsynaptic NE metabolite) were elevated upon Pdc deletion, suggesting enhanced catecholamine turnover in Pdc −/− mice (Fig. 3c, d). In contrast to α2A-adrenoceptor-deficient mice that suffer from catecholamine overflow due to the lack of presynaptic α2-adrenoceptor-mediated inhibition of transmitter release [94], deletion of the Pdc gene did not alter presynaptic regulation of NE exocytosis [40]. Further possible alterations in sympathetic functions were evaluated to explain the cardiovascular phenotype in phosducin-deficient mice, excluding (1) increased transmitter release upon a defined neuronal stimulus or (2) increased innervation density of target tissues [95]. While studying the second sympathetic neuron, increased firing of these neurons, which resulted in enhanced NE release, was identified to be the main cause of stress-dependent blood pressure dysregulation (Fig. 3e, f). By means of electrophysiological investigation of neurons isolated from the superior cervical sympathetic ganglion, it could be shown that altered action potential generation in Pdc −/− sympathetic neurons was originating from enhanced potassium conductivity while sodium and HCN channel currents appeared to be normal [40] (Fig. 3g). Increased potassium currents provided an explanation for quicker repolarisation of current-injected, depolarized cells.
There was no evidence for overactivity of either central pre-sympathetic neurons projecting to the intermediolateral column of the spinal cord or of preganglionic sympathetic neurons with pericarya in the spinal cord (Fig. 4). Both catecholamine levels in the brain stem and whole brain and neuronal activation on the level of spinal cord as assessed by c-fos immunostaining were similar in Pdc −/− and wild-type animals [40]. Most notably, Pdc mRNA could be detected in sympathetic ganglia but, for example, not in the heart or other cardiovascular tissues [40]. Blood pressure dysregulation in Pdc −/− mice could not be attributed to imbalanced function of the adrenal medulla as plasma and urinary levels of epinephrine, which accounts for up to 80% of adrenal medulla catecholamines, were not elevated.
It is well known that hypertension in humans can lead to end organ damage in the long run. Among these are endothelial dysfunction as well as vascular and cardiac hypertrophy, which may lead to secondary complications like myocardial infarction, heart failure or stroke [96–98]. Young phosducin-deficient animals displayed no signs of cardiac hypertrophy or endothelial dysfunction [40]. However, cardiac hypertrophy as well as pronounced vascular media thickening and endothelial dysfunction was present in animals of ages greater than 4 months. Moreover, significant alterations in foetal gene programme and adrenoceptor gene expression were found in the heart and in aortae: in cardiac tissue, mRNA levels of the β-myosin heavy chain gene were significantly upregulated as a sign of foetal gene activation probably due to pressure overload and/or direct enhancement of cardiac adrenoceptor stimulation. In aortae, we found downregulation of β1-, β2- and α1b-adrenoceptor mRNAs consistent with increased sympathetic tone [40].
Phosducin’s role in human essential hypertension
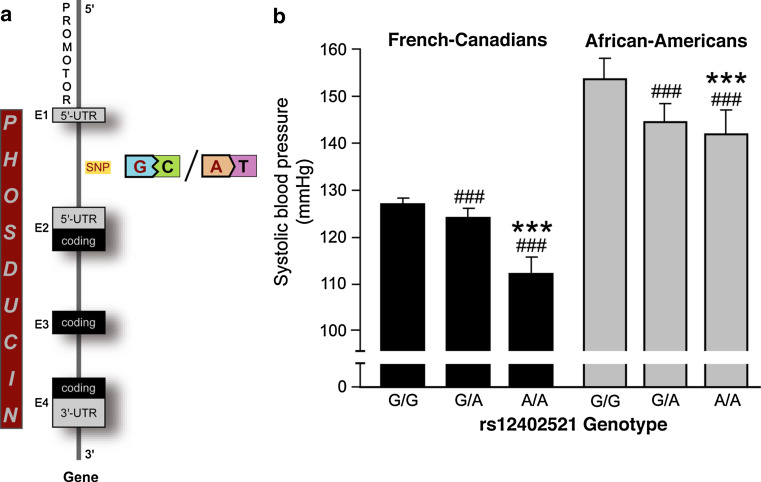
Given the result that phosducin is responsible for blood pressure regulation in mice, we sought to determine its relevance for human essential hypertension [40]. The human PDC gene (17.5 kb) has been mapped to chromosome 1q31.1, and it consists of four exons with the start codon in exon 2 [99, 100] (www.ensembl.org). Studies in humans using a candidate-gene approach revealed that single nucleotide polymorphisms (SNPs) within introns as well as 5′- and 3′-UTR of the human PDC gene were associated with stress-induced blood pressure phenotypes both in populations of French-Canadian and African-American origin [40].
Families examined in these studies were recruited from the Saguenay–Lac-St.-Jean region near Quebec or from Wilwaukee, respectively, if at least one sibling pair fulfilled the inclusion criteria such as essential hypertension, antihypertensive medication or dyslipidaemia [101]. Study participants were characterised thoroughly and their blood pressure was measured under baseline conditions as well as after a mathematics test or change in body position from supine to standing (“stress-induced”). In 48 unrelated individuals from each population, the phosducin gene was fully sequenced to determine haplotype diversity defined by specific SNP patterns. Forty-nine so far unknown SNPs within the PDC gene were identified and, together with SNPs for neighbouring genes, used for linkage analysis [102, 103]. In African-Americans, linkage analysis clearly showed equilibrium, i.e. the assumption that two alleles at different loci are independent from each other, between PDC haplotypes and SNPs within PTGS2. The gene for cyclooxygenase 2 (PTGS2) is located within 200 kb distance of the PDC gene and has previously been associated with blood pressure regulation [104, 105]. A certain PDC haplotype defined by a SNP located between exon 1 and 2 (rs12402521) influenced systolic blood pressure by more than 10 mmHg in range [40] (Fig. 5a). Individuals carrying two copies of the guanine allele at SNP rs12402521 had 12–15 mmHg higher blood pressure than those homozygous for the adenine allele [40], (Fig. 5b). Recent reports from genome wide association studies in a Scandinavian type 2 diabetes cohort and a British Caucasian population are perfectly in line with these findings [106, 107].
Fig. 5.
Phosducin’s function in essential hypertension. a Schematic illustration of the phosducin gene, the SNP rs12402521 is indicated between exon 1 and 2. b Average wake systolic blood pressure in French-Canadian and African-American populations according to the SNP rs12402521 genotype. ### p < 0.0001 versus G/G, ***p < 0.0001 versus G/A genotype. (Figure reproduced with permission of the American Society for Clinical Investigation [40])
The link between stress and essential hypertension was first established by Cannon and Folkow [108–110] and is meanwhile supported by accumulating evidence from numerous groups. It is well established that abnormal pressure responses to emotional stress (e.g. white-coat hypertension) can in the long run lead to the manifestation of hypertension [111, 112]. More recently, it has become a general consensus that hypertension is to a significant extent based on heritable features [101]. Genetic studies in humans, however, still face the difficulty of dealing with a conglomerate of genes each of which may only have moderate effects on blood pressure and which display genetic heterogeneity among different populations [101].
Therapeutic significance
Lewington and colleagues [113] previously demonstrated that an increase in systolic blood pressure by 10 mmHg increased the risk of cardiovascular disease such as ischaemic heart disease or stroke by 30 and 40%, respectively. Given the fact that in more than 90% of patients with hypertension the underlying cause is unknown, it seems reasonable to search for new candidates in blood pressure regulation. This may help to individualise anti-hypertensive therapy from a global approach to a more causal treatment [114]. However, recent studies have clearly demonstrated drawbacks in trying to identify a single candidate in a multifactorial disease like hypertension [115]. Moreover, once a molecule has finally proven to be involved in blood pressure regulation, the development of drugs which target that molecule is often an expensive and protracted process which does not necessarily lead to the entry of new antihypertensive drugs into the market [116].
There is no doubt that the activation of the sympathetic nervous system is an important pathophysiological feature in essential hypertension and its related cardiovascular morbidity and mortality [111, 117, 118]. In addition to increasing blood pressure, enhanced sympathetic activity per se may be a trigger for the aforementioned sudden cardiovascular events [119]. This nourishes the idea that anti-sympathetic treatment even of normotensive patients may be beneficial without necessarily lowering blood pressure [120, 121]. In particular, this might be true for individuals who have been identified to belong to a cardiovascular high-risk group, e.g. due to their genetic profile. However, anti-sympathetic drugs like centrally acting α2-adrenoceptor agonists or α1-adrenoceptor antagonists are not recommended as first-line agents in the treatment of essential hypertension in consequence of the lack of reduction of mortality compared to, e.g., ACE inhibitors or diuretics and due to their side-effect profile [122]. In view of the data from phosducin-deficient mice and the human studies, putting a brake on sympathetic activity may not only positively influence blood pressure it may also prevent the progression of other diseases that accelerate or deteriorate with blood pressure-independent sympathetic activation, e.g. chronic kidney disease or type 2 diabetes [123–125], or it may lower cardiovascular risk in the presence of further risk factors like obesity or family history of hypertension [126].
We and others suggest that targeting a molecule like phosducin to reduce sympathetic tone can potentially be superior to classical anti-sympathetic drugs for several reasons: (1) influencing the activity of phosducin may alter the release not only of NE but also of its cotransmitters neuropeptide Y and ATP, which potentiate pressure responses mediated by NE [127]; (2) as expression of Pdc is limited within the sympathetic nervous system regulation of adrenergic physiological processes by epinephrine may be unaffected; and (3) targeting phosducin may allow to selectively lower peak blood pressure under stress situations without affecting resting pressure levels. Whether phosducin may only influence “white-coat hypertension” or might also be relevant for masked hypertension, i.e. ambulatory or home hypertension, remains elusive [128].
Establishing phosducin as a new target molecule in the treatment of hypertension, however, still has to encounter a couple of challenges and to answer some open questions: is genetic testing for PDC tag SNPs enough to identify those patients who could profit from such a therapy? How high are the phosducin levels in those individuals carrying guanine or adenine alleles at the rs12402521 SNP? Can phosducin be targeted by a small molecule or would interference with its degradation be a better way to stabilise phosducin expression? How can therapy be made specific for the sympathetic nervous system without affecting retinal or pineal function?
Melatonin and hypertension
The release of the hormone melatonin from the pineal gland during darkness is activated by sympathetic neurons originating from the superior cervical ganglion [129, 130]. In addition, the circadian rhythm of melatonin secretion, generated by clock genes in the suprachiasmatic nucleus, is thought to be associated with blood pressure reduction (“dipping”) during nighttime. Accordingly, patients with hypertension whose blood pressure failed to dip during sleeping hours presented with blunted day–night differences in plasma melatonin levels [131]. It has been suggested but is still a matter of debate whether a non-dipping profile in hypertensive patients is associated with increased cardiovascular risk [132–135]. An experimental link between melatonin and hypertension, however, was established early based on the observation that surgical pinealectomy in rats was followed by a gradual and sustained increase in blood pressure [136] which could be suppressed by oral administration of melatonin [137, 138]. Melatonin was even seen as a potential endogenous anti-hypertensive hormone [139, 140]. Melatonin receptor-deficient animals that were examined within the context of our study did not display any signs of altered post-anaesthetic or stress-induced blood pressure [40]. This renders it unlikely that phosducin in the pineal gland can account for the observed hypertensive phenotype in mice. Nonetheless, it will be interesting and necessary to investigate whether melatonin levels are altered in Pdc −/− mice that develop hypertension during sympathetic activation.
Putative mechanisms of action
What is the nature of the mechanistic link between phosducin and blood pressure regulation? The current data indicate that control of sympathetic firing by phosducin is critical to prevent stress-induced hypertension. The following electrophysiological properties were identified in phosducin-deficient sympathetic ganglia: first, cultured postganglionic sympathetic neurons from phosducin-knockout mice responded to depolarisation and to stimulation by acetylcholine with higher firing rates than neurons from wild-type mice. Second, an outward current following depolarisation, which was most likely carried by potassium ions, was more pronounced in neurons from phosducin-deficient mice than in control sympathetic neurons. At present, one can only speculate how phosducin deficiency leads to these electrophysiological changes:
One possible mode of action could be ascribed to phosducin’s capacity to interact with G protein βγ-subunits. After activation of Gαi/o protein-coupled receptors, released βγ subunits inhibit voltage-gated calcium channels and activate G protein-coupled inwardly rectifying potassium (GIRK) channels. Phosducin would inhibit, whereas phosducin deficiency would potentiate, these processes. However, neither potentiation of inhibition of voltage-gated calcium channels nor potentiation of activation of GIRK channels is compatible with the sympathetic activation observed in the phosducin-knockout animals.
Scavenging of βγ-subunits by nearby equimolar levels of phosducin was demonstrated in the retina [34, 68]. It has also been shown in cultured sympathetic neurons artificially expressing high levels of phosducin [141]. The concentration of phosducin in native sympathetic neurons is several orders of magnitude lower than in these tissues, which makes scavenging of βγ subunits unlikely. However, as it is unclear whether phosducin is equally distributed throughout the sympathetic neuron, we cannot fully rule out that phosducin and βγ-subunits are expressed at similar molar levels in distinct subcellular compartments of superior cervical ganglion cells so that βγ-sequestration might play a role in regulating channel activity.
It is possible that phosducin interferes with other potassium channels determining neuronal excitability: potassium channels contributing to resting membrane potential, and potassium channels participating in repolarisation after action potentials. How the activity of these channels is modified by phosducin is not known. An interference with βγ-subunits regulating the channels cannot be excluded. Phosducin-mediated regulation of expression [83, 85] and phosphorylation [142] of channel subunits are also possible.
In fact, further studies such as subcellular expression analysis or evaluation of phosducin’s phosphorylation status in sympathetic ganglia will be necessary to provide evidence for any of these putative functions. Finally, it cannot be ruled out that phosducin could adopt the prominent feature of some of its family members, i.e. acting as a co-chaperon assisting in protein folding.
Conclusion
Phosducin, a protein that has previously been detected in the retina and pineal gland, has proven to be an essential regulator of the cardiovascular system by controlling firing behaviour of sympathetic neurons and thereby modulating blood pressure. In phosducin-deficient mice, pronounced sympathetic activity could be attributed to increased potassium conductivity while the distinct interaction partner(s) in this process remain(s) elusive. A translational study in humans confirmed phosducin as a blood pressure gene especially in stress-induced blood pressure phenotypes. Oncoming studies need to specify phosducin’s validity as a drug target. One of the essential steps in this process will be to identify which of the proposed molecular interaction partners of phosducin are required for its biological function in the retina, pineal and sympathetic neuron. Identification of this molecular function will be an important prerequisite for establishment of a screening assay to identify small molecule compounds which modulate phosducin’s sympathetic function.
Acknowledgments
We thank Prof. Dr. Moritz Bünemann (Marburg) and Prof. Dr. Béla Szabó (Freiburg) for fruitful discussions on the manuscript. This study was supported by the Excellence Initiative of the German Federal and State Governments (EXC 294).
Abbreviations
- Pdc
Murine phosducin gene
- Pdc
Murine phosducin protein
- PDC
Human phosducin gene
- PDC
Human phosducin protein
- PhlOP
Human phosducin-like orphan protein
- PhlP
Human phosducin-like protein
- GPCR
G protein-coupled receptor
References
- 1.Lolley RN, Brown BM, Farber DB. Protein phosphorylation in rod outer segments from bovine retina: cyclic nucleotide-activated protein kinase and its endogenous substrate. Biochem Biophys Res Commun. 1977;78:572–578. doi: 10.1016/0006-291X(77)90217-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee RH, Brown BM, Lolley RN. Light-induced dephosphorylation of a 33K protein in rod outer segments of rat retina. Biochemistry. 1984;23:1972–1977. doi: 10.1021/bi00304a014. [DOI] [PubMed] [Google Scholar]
- 3.Kuo CH, Akiyama M, Miki N. Isolation of a novel retina-specific clone (MEKA cDNA) encoding a photoreceptor soluble protein. Brain Res Mol Brain Res. 1989;6:1–10. doi: 10.1016/0169-328X(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 4.Bauer PH, Muller S, Puzicha M, Pippig S, Obermaier B, Helmreich EJ, Lohse MJ. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992;358:73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee RH, Fowler A, McGinnis JF, Lolley RN, Craft CM. Amino acid and cDNA sequence of bovine phosducin, a soluble phosphoprotein from photoreceptor cells. J Biol Chem. 1990;265:15867–15873. [PubMed] [Google Scholar]
- 6.Lee RH, Lieberman B, Lolley RN. A novel complex from bovine visual cells of a 33,000-Dalton phosphoprotein with β and γ-transducin: purification and subunit structure. Biochemistry. 1987;26:3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- 7.Lee RH, Ting TD, Lieberman BS, Tobias DE, Lolley RN, Ho YK. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J Biol Chem. 1992;267:25104–25112. [PubMed] [Google Scholar]
- 8.Abe T, Nakabayashi H, Tamada H, Takagi T, Sakuragi S, Yamaki K, Shinohara T. Analysis of the human, bovine and rat 33-kDa proteins and cDNA in retina and pineal gland. Gene. 1990;91:209–215. doi: 10.1016/0378-1119(90)90090-E. [DOI] [PubMed] [Google Scholar]
- 9.Craft CM, Lolley RN, Seldin MF, Lee RH. Rat pineal gland phosducin: cDNA isolation, nucleotide sequence, and chromosomal assignment in the mouse. Genomics. 1991;10:400–409. doi: 10.1016/0888-7543(91)90325-9. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Hisatomi O, Satoh T, Tokunaga F. Identification of rod- and cone-specific phosducins in teleost retinas. FEBS Lett. 2001;502:117–121. doi: 10.1016/S0014-5793(01)02670-9. [DOI] [PubMed] [Google Scholar]
- 11.Fabczak H, Sobierajska K, Fabczak S. Identification of possible phosducins in the ciliate Blepharisma japonicum . Protist. 2004;155:181–192. doi: 10.1078/143446104774199583. [DOI] [PubMed] [Google Scholar]
- 12.Sobierajska K, Fabczak H, Fabczak S. Alterations of ciliate phosducin phosphorylation in Blepharisma japonicum cells. J Photochem Photobiol B. 2005;79:135–143. doi: 10.1016/j.jphotobiol.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Salamon JA, Acuna R, Dawe AL. Phosphorylation of phosducin-like protein BDM-1 by protein kinase 2 (CK2) is required for virulence and Gbeta subunit stability in the fungal plant pathogen Cryphonectria parasitica . Mol Microbiol. 2010;76:848–860. doi: 10.1111/j.1365-2958.2010.07053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanary PL, DiBello PR, Estrada P, Dohlman HG. Functional analysis of Plp1 and Plp2, two homologues of phosducin in yeast. J Biol Chem. 2000;275:18462–18469. doi: 10.1074/jbc.M002163200. [DOI] [PubMed] [Google Scholar]
- 15.Craft CM, Xu J, Slepak VZ, Zhan-Poe X, Zhu X, Brown B, Lolley RN. PhLPs and PhLOPs in the phosducin family of G βγ binding proteins. Biochemistry. 1998;37:15758–15772. doi: 10.1021/bi980921a. [DOI] [PubMed] [Google Scholar]
- 16.Lukov GL, Hu T, McLaughlin JN, Hamm HE, Willardson BM. Phosducin-like protein acts as a molecular chaperone for G protein βγ dimer assembly. EMBO J. 2005;24:1965–1975. doi: 10.1038/sj.emboj.7600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knol JC, Engel R, Blaauw M, Visser AJ, van Haastert PJ. The phosducin-like protein PhLP1 is essential for Gβγ dimer formation in Dictyostelium discoideum . Mol Cell Biol. 2005;25:8393–8400. doi: 10.1128/MCB.25.18.8393-8400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willardson BM, Howlett AC. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell Signal. 2007;19:2417–2427. doi: 10.1016/j.cellsig.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humrich J, Bermel C, Bunemann M, Harmark L, Frost R, Quitterer U, Lohse MJ. Phosducin-like protein regulates G-protein βγ folding by interaction with tailless complex polypeptide-1α: dephosphorylation or splicing of PhLP turns the switch toward regulation of Gβγ folding. J Biol Chem. 2005;280:20042–20050. doi: 10.1074/jbc.M409233200. [DOI] [PubMed] [Google Scholar]
- 20.Danner S, Lohse MJ. Phosducin is a ubiquitous G-protein regulator. Proc Natl Acad Sci USA. 1996;93:10145–10150. doi: 10.1073/pnas.93.19.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RH, Whelan JP, Lolley RN, McGinnis JF. The photoreceptor-specific 33 kDa phosphoprotein of mammalian retina: generation of monospecific antibodies and localization by immunocytochemistry. Exp Eye Res. 1988;46:829–840. doi: 10.1016/S0014-4835(88)80035-6. [DOI] [PubMed] [Google Scholar]
- 22.Schaad NC, Shinohara T, Abe T, Klein DC. Photoneural control of the synthesis and phosphorylation of pineal MEKA (phosducin) Endocrinology. 1991;129:3289–3298. doi: 10.1210/endo-129-6-3289. [DOI] [PubMed] [Google Scholar]
- 23.Reig JA, Yu L, Klein DC. Pineal transduction. Adrenergic-cyclic AMP-dependent phosphorylation of cytoplasmic 33-kDa protein (MEKA) which binds βγ-complex of transducin. J Biol Chem. 1990;265:5816–5824. [PubMed] [Google Scholar]
- 24.Reiter RJ. The mammalian pineal gland: structure and function. Am J Anat. 1981;162:287–313. doi: 10.1002/aja.1001620402. [DOI] [PubMed] [Google Scholar]
- 25.Kuo CH, Watanabe Y, Yamagata K, Miki N. Developmental changes of MEKA protein and opsin in normal and rd mice. Brain Res Dev Brain Res. 1989;50:139–141. doi: 10.1016/0165-3806(89)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Babila T, Schaad NC, Simonds WF, Shinohara T, Klein DC. Development of MEKA (phosducin), G β, G γ and S-antigen in the rat pineal gland and retina. Brain Res. 1992;585:141–148. doi: 10.1016/0006-8993(92)91199-O. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PT, Williams RR, Reese BE. Developmental patterns of protein expression in photoreceptors implicate distinct environmental versus cell-intrinsic mechanisms. Vis Neurosci. 2001;18:157–168. doi: 10.1017/S0952523801181150. [DOI] [PubMed] [Google Scholar]
- 28.Lee RH, Lieberman BS, Lolley RN. Retinal accumulation of the phosducin/T βγ and transducin complexes in developing normal mice and in mice and dogs with inherited retinal degeneration. Exp Eye Res. 1990;51:325–333. doi: 10.1016/0014-4835(90)90029-T. [DOI] [PubMed] [Google Scholar]
- 29.Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe Y, Kawasaki K, Miki N, Kuo CH. Isolation and analysis of the human MEKA gene encoding a retina-specific protein. Biochem Biophys Res Commun. 1990;170:951–956. doi: 10.1016/0006-291X(90)92183-Z. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Hisatomi O, Yamamoto S, Tokunaga F. Distribution of rod- and cone-specific phosducins in retinas of non-mammalian vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:77–83. doi: 10.1016/S1096-4959(02)00109-4. [DOI] [PubMed] [Google Scholar]
- 32.Gropp KE, Huang JC, Aguirre GD. Differential expression of photoreceptor-specific proteins during disease and degeneration in the progressive rod-cone degeneration (prcd) retina. Exp Eye Res. 1997;64:875–886. doi: 10.1006/exer.1996.0257. [DOI] [PubMed] [Google Scholar]
- 33.Thulin CD, Howes K, Driscoll CD, Savage JR, Rand TA, Baehr W, Willardson BM. The immunolocalization and divergent roles of phosducin and phosducin-like protein in the retina. Mol Vis. 1999;5:40. [PubMed] [Google Scholar]
- 34.Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem. 2004;279:19149–19156. doi: 10.1074/jbc.M311058200. [DOI] [PubMed] [Google Scholar]
- 35.Nakano K, Chen J, Tarr GE, Yoshida T, Flynn JM, Bitensky MW. Rethinking the role of phosducin: light-regulated binding of phosducin to 14-3-3 in rod inner segments. Proc Natl Acad Sci USA. 2001;98:4693–4698. doi: 10.1073/pnas.071067198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis GP, Linberg KA, Geller SF, Guerin CJ, Fisher SK. Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544. [PubMed] [Google Scholar]
- 37.Herrmann R, Lobanova ES, Hammond T, Kessler C, Burns ME, Frishman LJ, Arshavsky VY. Phosducin regulates transmission at the photoreceptor-to-ON-bipolar cell synapse. J Neurosci. 2010;30:3239–3253. doi: 10.1523/JNEUROSCI.4775-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo CH, Miki N. Translocation of a photoreceptor-specific MEKA protein by light. Neurosci Lett. 1989;103:8–10. doi: 10.1016/0304-3940(89)90476-X. [DOI] [PubMed] [Google Scholar]
- 39.Margulis A, Dang L, Pulukuri S, Lee R, Sitaramayya A. Presence of phosducin in the nuclei of bovine retinal cells. Mol Vis. 2002;8:477–482. [PubMed] [Google Scholar]
- 40.Beetz N, Harrison MD, Brede M, Zong X, Urbanski MJ, Sietmann A, Kaufling J, Barrot M, Seeliger MW, Vieira-Coelho MA, Hamet P, Gaudet D, Seda O, Tremblay J, Kotchen TA, Kaldunski M, Nusing R, Szabo B, Jacob HJ, Cowley AW, Biel M, Stoll M, Lohse MJ, Broeckel U, Hein L. Phosducin influences sympathetic activity and prevents stress-induced hypertension in humans and mice. J Clin Invest. 2009;119:3597–3612. doi: 10.1172/JCI38433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sunayashiki-Kusuzaki K, Kikuchi T, Wawrousek EF, Shinohara T. Arrestin and phosducin are expressed in a small number of brain cells. Brain Res Mol Brain Res. 1997;52:112–120. doi: 10.1016/S0169-328X(97)00247-7. [DOI] [PubMed] [Google Scholar]
- 42.Kuo CH, Taira E, Takaha N, Sohma H, Akino T, Fukada Y, Sanada K, Miki N. Purification and characterization of three MEKA-like proteins in liver: association of a 94 kDa protein with beta gamma subunits of G-proteins. Biochem Biophys Res Commun. 1993;191:1097–1104. doi: 10.1006/bbrc.1993.1329. [DOI] [PubMed] [Google Scholar]
- 43.Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/S0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 44.LeVine H., 3rd Structural features of heterotrimeric G-protein-coupled receptors and their modulatory proteins. Mol Neurobiol. 1999;19:111–149. doi: 10.1007/BF02743657. [DOI] [PubMed] [Google Scholar]
- 45.Savage JR, McLaughlin JN, Skiba NP, Hamm HE, Willardson BM. Functional roles of the two domains of phosducin and phosducin-like protein. J Biol Chem. 2000;275:30399–30407. doi: 10.1074/jbc.M005120200. [DOI] [PubMed] [Google Scholar]
- 46.Hawes BE, Touhara K, Kurose H, Lefkowitz RJ, Inglese J. Determination of the G βγ-binding domain of phosducin. A regulatable modulator of G βγ signaling. J Biol Chem. 1994;269:29825–29830. [PubMed] [Google Scholar]
- 47.Xu J, Wu D, Slepak VZ, Simon MI. The N terminus of phosducin is involved in binding of beta gamma subunits of G protein. Proc Natl Acad Sci USA. 1995;92:2086–2090. doi: 10.1073/pnas.92.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka H, Kuo CH, Matsuda T, Fukada Y, Hayashi F, Ding Y, Irie Y, Miki N. MEKA/phosducin attenuates hydrophobicity of transducin βγ subunits without binding to farnesyl moiety. Biochem Biophys Res Commun. 1996;223:587–591. doi: 10.1006/bbrc.1996.0939. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka H, Iwami C, Kuo CH, Ding Y, Do E, Irie Y, Miki N. Analysis of the T beta gamma-binding domain of MEKA/phosducin. Neurochem Int. 1997;31:625–634. doi: 10.1016/S0197-0186(96)00053-8. [DOI] [PubMed] [Google Scholar]
- 50.Bluml K, Schnepp W, Schroder S, Beyermann M, Macias M, Oschkinat H, Lohse MJ. A small region in phosducin inhibits G-protein βγ-subunit function. EMBO J. 1997;16:4908–4915. doi: 10.1093/emboj/16.16.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroder S, Bluml K, Dees C, Lohse MJ. Identification of a C-terminal binding site for G-protein βγ-subunits in phosducin-like protein. FEBS Lett. 1997;401:243–246. doi: 10.1016/S0014-5793(96)01483-4. [DOI] [PubMed] [Google Scholar]
- 52.Gaudet R, Savage JR, McLaughlin JN, Willardson BM, Sigler PB. A molecular mechanism for the phosphorylation-dependent regulation of heterotrimeric G proteins by phosducin. Mol Cell. 1999;3:649–660. doi: 10.1016/S1097-2765(00)80358-5. [DOI] [PubMed] [Google Scholar]
- 53.Wilkins JF, Bitensky MW, Willardson BM. Regulation of the kinetics of phosducin phosphorylation in retinal rods. J Biol Chem. 1996;271:19232–19237. doi: 10.1074/jbc.271.6.3255. [DOI] [PubMed] [Google Scholar]
- 54.Lee RH, Brown BM, Lolley RN. Protein kinase A phosphorylates retinal phosducin on serine 73 in situ. J Biol Chem. 1990;265:15860–15866. [PubMed] [Google Scholar]
- 55.Lee BY, Thulin CD, Willardson BM. Site-specific phosphorylation of phosducin in intact retina. Dynamics of phosphorylation and effects on G protein βγ dimer binding. J Biol Chem. 2004;279:54008–54017. doi: 10.1074/jbc.M405669200. [DOI] [PubMed] [Google Scholar]
- 56.Thulin CD, Savage JR, McLaughlin JN, Truscott SM, Old WM, Ahn NG, Resing KA, Hamm HE, Bitensky MW, Willardson BM. Modulation of the G protein regulator phosducin by Ca2+/calmodulin-dependent protein kinase II phosphorylation and 14-3-3 protein binding. J Biol Chem. 2001;276:23805–23815. doi: 10.1074/jbc.M101482200. [DOI] [PubMed] [Google Scholar]
- 57.Chen F, Lee RH. Phosducin and βγ-transducin interaction I: effects of post-translational modifications. Biochem Biophys Res Commun. 1997;233:370–374. doi: 10.1006/bbrc.1997.6460. [DOI] [PubMed] [Google Scholar]
- 58.Willardson BM, Wilkins JF, Yoshida T, Bitensky MW. Regulation of phosducin phosphorylation in retinal rods by Ca2+/calmodulin-dependent adenylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1475–1479. doi: 10.1073/pnas.93.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauck SM, Ekstrom PA, Ahuja-Jensen P, Suppmann S, Paquet-Durand F, van Veen T, Ueffing M. Differential modification of phosducin protein in degenerating rd1 retina is associated with constitutively active Ca2+/calmodulin kinase II in rod outer segments. Mol Cell Proteomics. 2006;5:324–336. doi: 10.1074/mcp.M500217-MCP200. [DOI] [PubMed] [Google Scholar]
- 60.Brown BM, Carlson BL, Zhu X, Lolley RN, Craft CM. Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry. 2002;41:13526–13538. doi: 10.1021/bi0204490. [DOI] [PubMed] [Google Scholar]
- 61.Klenk C, Humrich J, Quitterer U, Lohse MJ. SUMO-1 controls the protein stability and the biological function of phosducin. J Biol Chem. 2006;281:8357–8364. doi: 10.1074/jbc.M513703200. [DOI] [PubMed] [Google Scholar]
- 62.Ripps H. Light to sight: milestones in phototransduction. FASEB J. 2010;24:970–975. doi: 10.1096/fj.10-0402ufm. [DOI] [PubMed] [Google Scholar]
- 63.Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 64.Jindrova H, Detwiler PB. Cyclic AMP has no effect on the generation, recovery, or background adaptation of light responses in functionally intact rod outer segments: with implications about the function of phosducin. Vis Neurosci. 2000;17:887–892. doi: 10.1017/S0952523800176072. [DOI] [PubMed] [Google Scholar]
- 65.Schulz R, Wehmeyer A, Murphy J, Schulz K. Phosducin, β-arrestin and opioid receptor migration. Eur J Pharmacol. 1999;375:349–357. doi: 10.1016/S0014-2999(99)00223-X. [DOI] [PubMed] [Google Scholar]
- 66.Schulz R, Wehmeyer A, Schulz K, Murphy J. Effect of phosducin on opioid receptor function. J Pharmacol Exp Ther. 1999;289:599–606. [PubMed] [Google Scholar]
- 67.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/S0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 68.Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW, Arshavsky VY. Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci. 2010;30:6815–6824. doi: 10.1523/JNEUROSCI.0613-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krispel CM, Sokolov M, Chen YM, Song H, Herrmann R, Arshavsky VY, Burns ME. Phosducin regulates the expression of transducin betagamma subunits in rod photoreceptors and does not contribute to phototransduction adaptation. J Gen Physiol. 2007;130:303–312. doi: 10.1085/jgp.200709812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brill E, Malanson KM, Radu RA, Boukharov NV, Wang Z, Chung HY, Lloyd MB, Bok D, Travis GH, Obin M, Lem J. A novel form of transducin-dependent retinal degeneration: accelerated retinal degeneration in the absence of rod transducin. Invest Ophthalmol Vis Sci. 2007;48:5445–5453. doi: 10.1167/iovs.06-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ara-Iwata F, Jacobson SG, Gass JD, Hotta Y, Fujiki K, Hayakawa M, Inana G. Analysis of phosducin as a candidate gene for retinopathies. Ophthalmic Genet. 1996;17:3–14. doi: 10.3109/13816819609057863. [DOI] [PubMed] [Google Scholar]
- 72.Williams H, Schachner M, Wang B, Kenwrick S. Radiation hybrid mapping of the genes for tenascin-R (TNR), phosducin (PDC), laminin C1 (LAMC1), and TAX in 1q25–q32. Genomics. 1997;46:165–166. doi: 10.1006/geno.1997.4994. [DOI] [PubMed] [Google Scholar]
- 73.van Soest S, te Nijenhuis S, van den Born LI, Bleeker-Wagemakers EM, Sharp E, Sandkuijl LA, Westerveld A, Bergen AA. Fine mapping of the autosomal recessive retinitis pigmentosa locus (RP12) on chromosome 1q; exclusion of the phosducin gene (PDC) Cytogenet Cell Genet. 1996;73:81–85. doi: 10.1159/000134313. [DOI] [PubMed] [Google Scholar]
- 74.Abe T, Kikuchi T, Chang T, Shinohara T. The sequence of the mouse phosducin-encoding gene and its 5′-flanking region. Gene. 1993;133:179–186. doi: 10.1016/0378-1119(93)90636-H. [DOI] [PubMed] [Google Scholar]
- 75.Nishiguchi KM, Berson EL, Dryja TP. Mutation screening of the phosducin gene PDC in patients with retinitis pigmentosa and allied diseases. Mol Vis. 2004;10:62–64. [PubMed] [Google Scholar]
- 76.Quin GG, Len AC, Billson FA, Gillies MC. Proteome map of normal rat retina and comparison with the proteome of diabetic rat retina: new insight in the pathogenesis of diabetic retinopathy. Proteomics. 2007;7:2636–2650. doi: 10.1002/pmic.200600486. [DOI] [PubMed] [Google Scholar]
- 77.Rex TS, Fariss RN, Lewis GP, Linberg KA, Sokal I, Fisher SK. A survey of molecular expression by photoreceptors after experimental retinal detachment. Invest Ophthalmol Vis Sci. 2002;43:1234–1247. [PubMed] [Google Scholar]
- 78.Abe T, Satoh N, Nakajima A, Koizumi T, Tamada M, Sakuragi S. Characterization of a potent uveitopathogenic site derived from rat phosducin. Exp Eye Res. 1997;65:703–710. doi: 10.1006/exer.1997.0379. [DOI] [PubMed] [Google Scholar]
- 79.Satoh N, Abe T, Nakajima A, Ohkoshi M, Koizumi T, Tamada H, Sakuragi S. Analysis of uveitogenic sites in phosducin molecule. Curr Eye Res. 1998;17:677–686. doi: 10.1080/02713689808951243. [DOI] [PubMed] [Google Scholar]
- 80.Wang M, Bai F, Pries M, Buus S, Prause JU, Nissen MH. Identification of MHC class I H-2 kb/Db-restricted immunogenic peptides derived from retinal proteins. Invest Ophthalmol Vis Sci. 2006;47:3939–3945. doi: 10.1167/iovs.06-0133. [DOI] [PubMed] [Google Scholar]
- 81.Dua HS, Lee RH, Lolley RN, Barrett JA, Abrams M, Forrester JV, Donoso LA. Induction of experimental autoimmune uveitis by the retinal photoreceptor cell protein, phosducin. Curr Eye Res. 1992;11(Suppl):107–111. doi: 10.3109/02713689208999519. [DOI] [PubMed] [Google Scholar]
- 82.Kuo CH, Taniura H, Watanabe Y, Fukada Y, Yoshizawa T, Miki N. Identification of a retina-specific MEKA protein as a 33K protein. Biochem Biophys Res Commun. 1989;162:1063–1068. doi: 10.1016/0006-291X(89)90781-X. [DOI] [PubMed] [Google Scholar]
- 83.Zhu X, Craft CM. The carboxyl terminal domain of phosducin functions as a transcriptional activator. Biochem Biophys Res Commun. 2000;270:504–509. doi: 10.1006/bbrc.2000.2414. [DOI] [PubMed] [Google Scholar]
- 84.Zhu X, Craft CM. Modulation of CRX transactivation activity by phosducin isoforms. Mol Cell Biol. 2000;20:5216–5226. doi: 10.1128/MCB.20.14.5216-5226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu X, Craft CM. Interaction of phosducin and phosducin isoforms with a 26S proteasomal subunit, SUG1. Mol Vis. 1998;4:13. [PubMed] [Google Scholar]
- 86.Preuss I, Kurig B, Nurnberg B, Orth JH, Aktories K. Pasteurella multocida toxin activates Gβγ dimers of heterotrimeric G proteins. Cell Signal. 2009;21:551–558. doi: 10.1016/j.cellsig.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Schulz R, Wehmeyer A, Schulz K. Opioid receptor types selectively cointernalize with G protein-coupled receptor kinases 2 and 3. J Pharmacol Exp Ther. 2002;300:376–384. doi: 10.1124/jpet.300.2.376. [DOI] [PubMed] [Google Scholar]
- 88.Dicker F, Quitterer U, Winstel R, Honold K, Lohse MJ. Phosphorylation-independent inhibition of parathyroid hormone receptor signaling by G protein-coupled receptor kinases. Proc Natl Acad Sci USA. 1999;96:5476–5481. doi: 10.1073/pnas.96.10.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DebBurman SK, Ptasienski J, Boetticher E, Lomasney JW, Benovic JL, Hosey MM. Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. J Biol Chem. 1995;270:5742–5747. doi: 10.1074/jbc.270.11.5742. [DOI] [PubMed] [Google Scholar]
- 90.Moore GD, Ayabe T, Visconti PE, Schultz RM, Kopf GS. Roles of heterotrimeric and monomeric G proteins in sperm-induced activation of mouse eggs. Development. 1994;120:3313–3323. doi: 10.1242/dev.120.11.3313. [DOI] [PubMed] [Google Scholar]
- 91.Jaffe LA, Gallo CJ, Lee RH, Ho YK, Jones TL. Oocyte maturation in starfish is mediated by the beta gamma-subunit complex of a G-protein. J Cell Biol. 1993;121:775–783. doi: 10.1083/jcb.121.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Laugwitz KL, Pinkernell K, Pragst I, Baumgartner C, Hoffmann E, Rosport K, Munch G, Moretti A, Humrich J, Lohse MJ, Ungerer M. Effects of two Gβγ proteins—N-terminally truncated phosducin and β-adrenergic receptor kinase C terminus (betaARKct)—in heart failure. Gene Ther. 2003;10:1354–1361. doi: 10.1038/sj.gt.3301995. [DOI] [PubMed] [Google Scholar]
- 93.Grassi G. Phosducin—a candidate gene for stress-dependent hypertension. J Clin Invest. 2009;119:3515–3518. doi: 10.1172/JCI41508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by α2-adrenoceptor subtypes. Mol Endocrinol. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- 95.Kiriazis H, Du XJ, Feng X, Hotchkin E, Marshall T, Finch S, Gao XM, Lambert G, Choate JK, Kaye DM. Preserved left ventricular structure and function in mice with cardiac sympathetic hyperinnervation. Am J Physiol Heart Circ Physiol. 2005;289:H1359–H1365. doi: 10.1152/ajpheart.01010.2004. [DOI] [PubMed] [Google Scholar]
- 96.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114:2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 97.Dzau VJ. Markers of malign across the cardiovascular continuum: interpretation and application. Circulation. 2004;109:IV1–IV2. doi: 10.1161/01.CIR.0000133445.78855.aa. [DOI] [PubMed] [Google Scholar]
- 98.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part II: clinical trial evidence (acute coronary syndromes through renal disease) and future directions. Circulation. 2006;114:2871–2891. doi: 10.1161/CIRCULATIONAHA.106.655761. [DOI] [PubMed] [Google Scholar]
- 99.Ding C, Li X, Griffin CA, Jabs EW, Hawkins AL, Levine MA. The gene for human phosducin (PDC), a soluble protein that binds G-protein beta gamma dimers, maps to 1q25–q31.1. Genomics. 1993;18:457–459. doi: 10.1006/geno.1993.1501. [DOI] [PubMed] [Google Scholar]
- 100.Sparkes RS, Lee RH, Shinohara T, Craft CM, Kojis T, Klisak I, Heinzmann C, Bateman JB. Assignment of the phosducin (PDC) gene to human chromosome 1q25–1q32.1 by somatic cell hybridization and in situ hybridization. Genomics. 1993;18:426–428. doi: 10.1006/geno.1993.1490. [DOI] [PubMed] [Google Scholar]
- 101.Kotchen TA, Broeckel U, Grim CE, Hamet P, Jacob H, Kaldunski ML, Kotchen JM, Schork NJ, Tonellato PJ, Cowley AW., Jr Identification of hypertension-related QTLs in African American sib pairs. Hypertension. 2002;40:634–639. doi: 10.1161/01.HYP.0000036400.79248.22. [DOI] [PubMed] [Google Scholar]
- 102.Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 104.Aw TJ, Haas SJ, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–496. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 105.Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol Renal Physiol. 2005;288:F1125–F1132. doi: 10.1152/ajprenal.00219.2004. [DOI] [PubMed] [Google Scholar]
- 106.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 107.Consortium TWTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cannon WB (1929) Bodily changes in pain, hunger, fear and rage. D. Appleton, New York
- 109.Folkow B, Heymans C, Neil E. Integrated aspects of cardiovascular regulation. Handbook of physiology Section 2. 1965;3:1787–1823. [Google Scholar]
- 110.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 111.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/S0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 112.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–485. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- 113.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 114.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 115.Charchar F, Zimmerli L, Tomaszewski M. The pressure of finding human hypertension genes: new tools, old dilemmas. J Hum Hypertens. 2008;22:821–828. doi: 10.1038/jhh.2008.67. [DOI] [PubMed] [Google Scholar]
- 116.Feig PU, Roy S, Cody RJ. Antihypertensive drug development: current challenges and future opportunities. J Am Soc Hypertens. 2010;4:163–173. doi: 10.1016/j.jash.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 117.Esler M. The sympathetic system in essential hypertension. Rev Port Cardiol. 2000;19(Suppl 2):II9–II14. [PubMed] [Google Scholar]
- 118.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35:S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 119.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 120.Izzo JL. Prehypertension: demographics, pathophysiology, and treatment. Curr Hypertens Rep. 2007;9:264–268. doi: 10.1007/s11906-007-0049-8. [DOI] [PubMed] [Google Scholar]
- 121.Nesbitt SD. Treatment options for prehypertension. Curr Opin Nephrol Hypertens. 2007;16:250–255. doi: 10.1097/MNH.0b013e3280c8eebe. [DOI] [PubMed] [Google Scholar]
- 122.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O’Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 123.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 124.Ritz E, Bakris G. World Kidney Day: hypertension and chronic kidney disease. Lancet. 2009;373:1157–1158. doi: 10.1016/S0140-6736(09)60355-X. [DOI] [PubMed] [Google Scholar]
- 125.Ritz E, Rump LC. Control of sympathetic activity—new insights; new therapeutic targets? Nephrol Dial Transplant. 2010;25:1048–1050. doi: 10.1093/ndt/gfq079. [DOI] [PubMed] [Google Scholar]
- 126.Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.CIR.0000019884.36724.D9. [DOI] [PubMed] [Google Scholar]
- 127.Vonend O, Okonek A, Stegbauer J, Habbel S, Quack I, Rump LC. Renovascular effects of sympathetic cotransmitters ATP and NPY are age-dependent in spontaneously hypertensive rats. Cardiovasc Res. 2005;66:345–352. doi: 10.1016/j.cardiores.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Kawano Y, Horio T, Matayoshi T, Kamide K. Masked hypertension: subtypes and target organ damage. Clin Exp Hypertens. 2008;30:289–296. doi: 10.1080/10641960802071026. [DOI] [PubMed] [Google Scholar]
- 129.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 130.Bowers CW, Dahm LM, Zigmond RE. The number and distribution of sympathetic neurons that innervate the rat pineal gland. Neuroscience. 1984;13:87–96. doi: 10.1016/0306-4522(84)90261-6. [DOI] [PubMed] [Google Scholar]
- 131.Zeman M, Dulkova K, Bada V, Herichova I. Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci. 2005;76:1795–1803. doi: 10.1016/j.lfs.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 132.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bombelli M, Cuspidi C, Facchetti R, Bolla G, Mancia G. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925–931. doi: 10.1161/HYPERTENSIONAHA.108.116368. [DOI] [PubMed] [Google Scholar]
- 133.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 134.Shimada K, Kawamoto A, Matsubayashi K, Nishinaga M, Kimura S, Ozawa T. Diurnal blood pressure variations and silent cerebrovascular damage in elderly patients with hypertension. J Hypertens. 1992;10:875–878. [PubMed] [Google Scholar]
- 135.Cuspidi C, Macca G, Sampieri L, Fusi V, Severgnini B, Michev I, Salerno M, Magrini F, Zanchetti A. Target organ damage and non-dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J Hypertens. 2001;19:1539–1545. doi: 10.1097/00004872-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 136.Zanoboni A, Zanoboni-Muciaccia W. Experimental hypertension in pinealectomized rats. Life Sci. 1967;6:2327–2331. doi: 10.1016/0024-3205(67)90043-4. [DOI] [PubMed] [Google Scholar]
- 137.Holmes SW, Sugden D. Proceedings: The effect of melatonin on pinealectomy-induced hypertension in the rat. Br J Pharmacol. 1976;56:360P–361P. [PMC free article] [PubMed] [Google Scholar]
- 138.Reiter RJ, Tan DX, Korkmaz A. The circadian melatonin rhythm and its modulation: possible impact on hypertension. J Hypertens Suppl. 2009;27:S17–S20. doi: 10.1097/01.hjh.0000358832.41181.bf. [DOI] [PubMed] [Google Scholar]
- 139.Paulis L, Simko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol Res. 2007;56:671–684. doi: 10.33549/physiolres.931236. [DOI] [PubMed] [Google Scholar]
- 140.Simko F, Paulis L. Melatonin as a potential antihypertensive treatment. J Pineal Res. 2007;42:319–322. doi: 10.1111/j.1600-079X.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 141.Partridge JG, Puhl HL, 3rd, Ikeda SR. Phosducin and phosducin-like protein attenuate G-protein-coupled receptor-mediated inhibition of voltage-gated calcium channels in rat sympathetic neurons. Mol Pharmacol. 2006;70:90–100. doi: 10.1124/mol.105.021394. [DOI] [PubMed] [Google Scholar]
- 142.Lee SY, Choi HK, Kim ST, Chung S, Park MK, Cho JH, Ho WK, Cho H. Cholesterol inhibits M-type K+ channels via protein kinase C-dependent phosphorylation in sympathetic neurons. J Biol Chem. 2010;285:10939–10950. doi: 10.1074/jbc.M109.048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sobierajska K, Fabczak H, Fabczak S. Phosducin interacts with the G-protein βγ-dimer of ciliate protozoan Blepharisma japonicum upon illumination. J Exp Biol. 2007;210:4213–4223. doi: 10.1242/jeb.005132. [DOI] [PubMed] [Google Scholar]