Abstract
Autoreactive CD8+ regulatory T cells (Tregs) play important roles as modulators of immune responses against self, and numerical and functional defects in CD8+ Tregs have been linked to autoimmunity. Several subsets of CD8+ Tregs have been described. However, the origin of these T cells and how they participate in the natural progression of autoimmunity remain poorly defined. We discuss several lines of evidence suggesting that the autoimmune process itself promotes the development of autoregulatory CD8+ T cells. We posit that chronic autoantigenic exposure fosters the differentiation of non-pathogenic autoreactive CD8+ T cells into antigen-experienced, memory-like autoregulatory T cells, to generate a “negative feedback” regulatory loop capable of countering pathogenic autoreactive effectors. This hypothesis predicts that approaches capable of boosting autoregulatory T cell memory will be able to blunt autoimmunity without compromising systemic immunity.
Keywords: CD8+ regulatory T cells, Immune response, Autoimmunity, T cell memory, Memory-like phenotype
Introduction
As front-liners in the fight against foreign infectious agents, T cells are equipped with a wide repertoire of T cell receptors (TCRs) to ensure the recognition of microbe-derived epitopes present on self major histocompatibility complex molecules (MHC). However, the inherent danger of such a system lies in the possibility that some T cells will have the ability to recognize self peptide/MHC (pMHC) and mount an inappropriate autoimmune response. The immune system limits such a possibility through several mechanisms. First, it interferes with the development of autoreactive T cells in a process called negative selection whereby T cells bearing self-reactive TCRs with an inappropriately high affinity for self-pMHC complexes are deleted in the thymus through apoptosis. If negative selection should fail, however, and allow pathogenic, autoreactive T cells to exit into the periphery, these T cells face additional layers of restraints, including peripheral anergy induction and cellular suppression through the workings of a class of T cells with immune-regulatory properties—the regulatory T cells (Tregs).
The concept of a T cell being capable of suppressing immune responses was first noted in the 1970s, when Gershon et al. [1–3] observed transferable immune suppression mediated by lymphocytes from antigen-tolerized mice in a series of bone marrow chimera reconstitution experiments. Subsequent studies by Cantor et al. [4–6] identified T cells with suppressive activity residing in the CD8+ compartment. However, due to technical limitations, interests in studying Tregs waned and were only revived two decades later, with Sakaguchi’s [7] landmark discovery of the CD4+ CD25+ Tregs. Since then, researchers have been exploring various aspects of Tregs, leading to significant advancements in our understanding of Treg development and function, as well as of their relationship to the body in the context of infectious diseases, autoimmunity, transplantation, and cancer. One corollary of this exploration has been the opening up of numerous opportunities for therapeutic intervention to induce or expand Tregs to restore immune homeostasis.
Although research efforts in the past decade have mainly focused on CD4+ Tregs, it is now well established that Tregs exist within both the CD4+ and CD8+ compartments and constitute a heterogeneous class of cells with diverse mechanisms of action. Tregs that originate in the thymus are often referred to as “natural Tregs”, while those that arise in the periphery in response to antigenic stimulation are termed “adaptive/induced” Tregs [8]. The signals that give rise to natural Treg differentiation in the thymus remain largely uncharacterized, but studies using TCR transgenic models suggest that thymocytes commit to the Treg lineage after encountering self-antigen, presumably expressed on medullary thymic epithelial cells (mTECs) and presented by thymic dendritic cells (DCs) (reviewed in [12]) [9–11]. However, not all tissue-restricted self-antigens are expressed on mTECs, and Tregs with auto-specificities falling outside of the Aire-driven antigen repertoire are thought to be generated in the periphery, i.e., the so-called “adaptive/induced Tregs”.
With regards to CD4+ Tregs, the CD4+CD25+ FoxP3+ Treg subset is found in both the “natural” and “induced” compartments, whereas Tr1 cells and TH3 cells are considered to be “induced” Tregs that arise after chronic antigenic stimulation or in response to oral tolerance, respectively [13, 14]. A growing list of mechanisms is attributed to the suppressive action of CD4+FoxP3+ Tregs [15], but the mechanisms of immune regulation elaborated by the CD8+ Treg subsets remain less clear. In this review, we summarize current findings on Tregs in terms of their induction and mode of action, with a special focus on CD8+ Tregs and their induction in the face of chronic antigen exposure in the context of autoimmune diseases. Although, in principle, functional and phenotypic parallels could be drawn from subsets of CD4+ and CD8+ Tregs, enabling their categorization according to shared phenotypic and/or functional characteristics (i.e., according to similarities with CD4+ Tregs), for many of the subsets described herein there is no clear definition of mechanisms of action or phenotypes that enable such categorization without the danger of over-simplification. Accordingly, and with the aim to better understand how CD8+ Tregs arise and function, we broadly categorize the subsets characterized to date—without going into fine details regarding differences in the surface markers expressed—to detect important emerging trends.
CD8+ Tregs in autoimmune diseases
CD8+ T cells play dual roles in autoimmune diseases, both as pathogenic effectors and negative modulators [16–18]. Defects in the function and/or numbers of CD8+ Tregs have been correlated with the increased incidence and severity of autoimmune disorders [19–22], while the therapeutic induction and adoptive transfer of CD8+ Tregs were able to afford disease protection and amelioration [23–33].
Markers on CD8+ Tregs
Multiple subsets of CD8+ Tregs, both thymus derived and peripherally induced, have been described in both humans and mice. In both, no set of markers is present that unifies or distinguishes between the different reported subsets, and overlap between subsets described by different groups is not unlikely. Expression of specific Treg markers may differ based on the diverse origins from which these Tregs may have arisen in vivo and on the process of manipulation they have been subjected to prior to characterization. Adaptive/induced Tregs may be generated de novo by means of antigen-specific or polyclonal TCR stimulation, both in vivo and in vitro, under specific experimental conditions, or they may arise naturally but remain undetectable unless expanded by therapeutic means.
The antigenic specificities of CD8+ Tregs
Thymus- and peripherally derived CD8+ Tregs are polyclonal in nature, with undefined antigen specificity. A few laboratories use pMHC tetramer staining to identify disease-relevant Tregs, while others address the issue of antigen specificity by testing for antigen-specific activation and associated suppressive activity. Nonetheless, most functional studies of CD8+ or CD4+ Tregs often treat the population as a whole without filtering autoreactive Tregs away from non-autoreactive ones. In such instances, it becomes important to consider whether the suppressive effects are disease specific, and how that can be reconciled with the antigenic specificity or the polyclonality of the Treg population.
If these considerations are neglected, the following two questions cannot be addressed: does the transfer or expansion of polyclonal Tregs cure an autoimmune disease by inducing generalized immune suppression? And, if not, how is disease specificity achieved? Several case scenarios come to mind. First, a subset of Tregs may require antigen-specific activation but mediate suppression in an antigen-non-specific manner (e.g., through the secretion of soluble mediators). Second, in the opposite scenario, a polyclonal population with broad antigenic specificity (assuming that the majority is non-autoreactive) is able to mediate suppression upon transfer into a diseased host, in a cell–cell contact-dependent manner. In the first scenario, disease specificity could be achieved by the local effects of the secreted molecules on a third party population capable of orchestrating the local autoimmune response (e.g., antigen-presenting cells (APCs) in the draining lymph nodes or local endothelial cells). In the latter case, disease/antigen specificity could be achieved if few autoreactive Tregs within the polyclonal population go through antigen-specific activation before mediating cell contact-dependent suppression.
Subsets of CD8+ Tregs
Qa-1-restricted CD8+ T cells in mice, HLA-E-restricted CD8+ T cells in humans
The regulatory role of MHC class Ib molecule Qa-1 in autoimmunity has been demonstrated in Qa-1-deficient mice, which display increased susceptibility to experimental autoimmune encephalomyelitis (EAE) induction and develop exaggerated CD4+ T cell responses upon viral infection and immunization with self peptides [34]. Evidence for the suppressive activity of Qa-1-restricted CD8+ T cells was first reported in a study that used TCR peptide vaccination to induce CD8+ T cell-dependent unresponsiveness [35]. It was later observed that Qa-1-restricted CD8+ Tregs can be induced by immunization with attenuated autoreactive CD4+ T cells. T cell vaccination (TCV) with myelin basic protein (MBP)-specific CD4+ T cells resulted in protection from the subsequent induction of EAE and the induction/expansion of a population of Qa-1-restricted CD8+ T cells [26] and that functional activation of this subset with anti-TCR monoclonal antibodies achieved the same effects [30]. In addition to EAE, TCV-induction of Qa-1-restricted CD8+ Tregs was shown to have beneficial effects in non-obese diabetic (NOD) mice (a murine model of autoimmune diabetes) as well as in a model of herpes simplex virus-1 (KOS strain)-induced herpes stromal keratitis [36]. Qa-1-restricted CD8+ Tregs were found to induce apoptotic deletion of pathogenic, activated CD4+ T cells [37, 38] and disease protection [39] in a Vβ-specific manner, as vaccination with three different Vβ8+ MBP-reactive CD4+ T cell clones protected the mice against disease induced by another MBP-reactive Vβ8+ CD4+ T cell clone, whereas vaccination with Vβ6+ MBP-reactive CD4+ T cell clones did not. It has recently been shown that the protective effects of Qa-1-restricted CD8+ Tregs require perforin and the presence of interferon-gamma (IFNγ) [37]. Qa-1-restricted Tregs cloned from TCR peptide-vaccinated mice have been further characterized with respect to the markers expressed, revealing that these cells are TCRαβ+, CD8αα+ and that they express interleukin (IL)-7R, CD25, CD122, CD28, and NKG2/CD94 [40]. Kim et al. [41] recently showed that Qa-1-restricted CD8+ T-cells specifically suppress follicular T-helper cells (TFH cells, expressing Qa-1) and that selective disruption of this interaction promotes systemic lupus erythematosus (SLE)-like disease in mice. In agreement with previous studies, the Qa-1-restricted CD8+ T-suppressor cells of Kim et al. [41] have a memory-like (CD44+ CD122+) phenotype, are IL-15 dependent, and suppress TFH cells via perforin. Chen et al. [42, 43] further proposed the avidity model of Qa-1-restricted CD8+ T cell-mediated regulation, which argues that Qa-1-restricted CD8+ T cells selectively delete intermediate-avidity autoreactive T cells by perceiving the differences in the expression level of Qa-1 in complex with a heat shock protein 60 signal peptide (hsp60sp)-derived epitope. CD4+ T cells with intermediate avidity express higher levels of hsp60sp/Qa-1, thus flagging them for deletion. This observation was recapitulated in human T1D patients who demonstrated defects in CD8+ T cell recognition of HLA-E/Hsp60sp [44]. HLA-E-restricted CD8+ regulatory T cells were also found in patients with multiple sclerosis (MS) treated with glatiramer acetate (GA; copaxone), an approved therapy for MS [23, 31, 45]. CD8+ T cell-mediated immune suppression was found to be defective in untreated MS patients as compared to healthy untreated controls [45], and treatment with GA might induce HLA-E-dependent, GA-specific cytotoxic responses with suppressive properties.
CD8+ CD122+ Tregs in mice
CD8+CD122+ Tregs are found in the periphery of naïve, unmanipulated mice. Although they constitute a naturally existing subset, the thymic origin of these cells has never been proven. Some reports suggest that CD8+CD122+ T cells may develop in the periphery [46, 47] in response to autoantigenic stimulation. Suzuki and co-workers [48] reported that IL-2/IL-15 receptor β-chain (CD122)-deficient mice demonstrate heightened immunopathology characterized by the spontaneous accumulation of activated T cells and the abnormal differentiation of B cells into antibody-secreting plasma cells. This phenotype of abnormal T and B cell activation could be corrected by adoptive transfer of bulk purified CD8+CD122+ T cells or by higher numbers of CD4+CD25+ T cells [49]. Mechanistically, CD8+CD122+ T cells down-regulate CD8+CD122− T cell-mediated responses in an APC-independent manner by secreting IL-10 [49, 50]. CD8+CD122+ T cell-mediated suppression in vitro can be blocked by neutralizing antibodies against CD28, CD80/86, but not by neutralizing antibodies against ICOS, CTLA-4, or PD-1, and CD8+CD122+ T cells isolated from CD28-deficient mice do not possess suppressive activity, suggesting the requirement of CD28:CD80/86 interaction for their functional activation [51]. In contrast, a later study showed that CD28 and PD-1 triggering promotes IL-10 secretion, suggesting that the suppressive activity of this Treg subset resides within its PD-1+ fraction. In fact, PD-1 blockade inhibits the suppressive function of CD8+CD122+PD-1+ T cells [52]. Accordingly, the role of PD-1 in CD8+CD122+ T cell-mediated suppression remains unclear. The protective role of CD8+CD122+ T cells has been documented in a number of allo- and autoimmune scenarios, including a murine model of Graves’ disease [53], EAE, a murine model of MS [54]), CD4+ T cell-induced colitis [55], and in skin allograft rejection [52]. In all of these cases, IL-10 neutralization largely reversed disease protection. With regards to the existence of a human counterpart, Suzuki’s group proposes that human CD8+CXCR3+ T cells are the functional equivalents of the murine CD8+CD122+ T cells [56] as they demonstrate suppressive activity in vitro in a similar fashion.
CD8+CD28−FoxP3+ T cells in mice and humans (antigen-/allospecific)
Allospecific CD8+CD28− T cells, which arise in mixed lymphocyte cultures of human peripheral blood mononuclear cells (PBMCs) in vitro, are able to suppress antigen-specific CD4+ T cell responses [57]. In addition, CD8+CD28− T cell lines cloned from humans can suppress autologous antigen-specific or xenoreactive CD4+ T cell responses in vitro [58–60]. In the literature, this CD8+CD28− T cell subset is often referred to as T suppressor (Ts); these cells express similar markers as CD4+CD25+ Tregs, including FoxP3, GITR, CTLA-4, CD25, CD103, CD62L, and 4-1BB [61]. Mechanistically, they are MHC class I-restricted and require direct contact with APCs to mediate their suppressive effects. Liu et al. showed that CD8+CD28− Tregs act by down-regulating co-stimulatory molecules on APCs, while in another study, in vitro alloantigen-primed CD8+CD28− Tregs were shown to up-regulate expression of the inhibitory receptors immunoglobulin-like transcript 3 (ILT3) and ILT4 on APCs [62] and endothelial cells [63], thereby rendering these APCs tolerogenic. Following this study, Kim-Schulze et al. [64] further showed that recombinant ILT3 could be used to generate allospecific CD8+ FoxP3+ Tregs from human mixed lymphocyte cultures.
A similar subset of CD8+CD28− T cells has been identified in mice. Najafian et al. [65] showed that depletion of CD8+ T cells prior to EAE induction with myelin oligodendrocyte glycoprotein (MOG) led to heightened disease progression, while adoptive transfer of in vitro-activated CD8+CD28− T cells prevented this phenotype. CD8+CD28− T cells were shown to suppress the proliferation of MOG-specific CD4+ T cells both in vitro and in vivo through a MHC class I-restricted, contact-dependent mechanism that required the presence of APCs. The CD8+CD28− T cell/APC interaction led to down-regulation of several co-stimulatory molecules on APCs, including CD80, CD86, and CD40. In another study, CD8+CD28− T cells were shown to exert regulatory activity in an animal model of myasthenia gravis (MG), an autoimmune disease characterized by antibody-mediated blockade of acetylcholine receptors (AchR) at neuromuscular junctions. Ben-David and coworkers [66] showed that altered peptide ligand therapy consisting of two tandemly fused myasthenogenic peptide analogs suppressed MG-associated autoimmunity through the induction of a group of CD8+CD28− cells that also expressed FoxP3. These CD8+CD28−FoxP3+ T cells were shown to produce immunosuppressive cytokines such as IL-10 and transforming growth factor-beta (TGF-β) and could transfer protection into MG-prone animals [66, 67].
Other classes of CD8+CD28− Tregs in humans
Other subsets of CD8+CD28− Tregs that lack FoxP3 expression have been identified. In the autoimmune setting, CD8+CD28−CD56+ T cell clones derived from synovial tissues of rheumatoid arthritis (RA) patients can blunt synovitis in severe combined immunodeficient (SCID) mice grafted with human synovial tissues [68], and CD8+CD28− T cells isolated from the peripheral blood or thyroid tissues of patients with Hashimoto thyroiditis and Grave’s disease, but not healthy individuals, demonstrated suppressive activity in vitro [69]. A decrease in the frequency of CD8+CD28− Tregs has been correlated to diseased states in T1D and relapsing–remitting MS patients [21, 70]. Work by Filaci et al. [71] showed that freshly isolated CD8+CD28− T cells from human PBMCs cannot suppress anti-CD3-driven T cell responses unless previously cultured/activated with autologous monocytes in the presence of IL-2 and granulocyte/macrophage colony stimulating factor (GM-CSF), suggesting that the CD8+CD28− T cells could be a precursor subset giving rise to Tregs upon activation. Defective function of the CD8+CD28−-derived Treg subset has also been correlated with diseased states, such as in patients with active SLE as compared to patients in remission or healthy controls [20] and in patients with chronic progressive MS as compared to remitting–relapsing MS and healthy controls [19]. In these settings, the in vitro-activated CD8+CD28− Tregs were found to suppress the disease state through an IL-6- (in the case of [20]) and IFNγ- [19, 20] dependent mechanism(s).
CD8+CD103+ Tregs in mice and humans (antigen-/allospecific)
CD103 (αEβ7 intergrin) is a receptor for E-cadherin on epithelial cells. In humans, CD103 expression is confined to <1% of the circulating memory T cells [72], while in mice CD103 is expressed at low levels on 40–60% of peripheral CD8+ T cells [73], mainly on those displaying a naïve phenotype [74]. However, CD103 expression is dramatically up-regulated on alloreactive CD8+ T cells upon entry into a renal graft site or on CD8+ T cells within the gut epithelium [75], in a TGFβ-dependent manner. CD103 thus represents a marker that characterizes CD8+ effector T cells in settings of renal transplant rejection and intestinal graft-versus-host disease [74–76]. Although alloantigen-induced CD8+CD103+ T cells were initially identified as an effector subset, Uss et al. [77] recently showed that these cells possess regulatory activity. The alloreactive CD8+CD103+ T cells in these studies arose by alloantigenic stimulation ex vivo and could not be induced by anti-CD3/CD28. CD8+CD103+ Tregs are mostly CD28+ and lack CD25, FoxP3, CTLA-4, LAG-3, and GITR, and their CD103 expression is augmented by the presence of TGFβ [73]. Although CD8+CD103+ T cells secrete IL-10 and TGFβ, these two cytokines do not seem to play a role in their suppressive activity. Instead, they suppress T-cell proliferation in mixed lymphocyte cultures in a manner that is dependent on cell–cell contact [78]. A different group identified CD8+CD103+ T cells as a regulatory subset after peptide vaccination in the presence of adjuvants and anti-4-1BB antibodies [79]. In this study, the autocrine action of IFNγ on the Tregs was shown to be necessary for potentiating their TGFβ-based suppressive activity [79]. CD8+CD103+, TGFβ-secreting T cells have also been shown to be protective in a model of TNF-driven chronic ileitis [80] and in a liver allograft setting [81]. The exact mechanism(s) of action of the CD8+CD103+ Tregs remain(s) to be determined.
HLA-G+ Tregs in humans
HLA-G is a non-classical HLA class Ib molecule with immunosuppressive function, as shown in its ability to suppress allospecific T cell proliferation and NK cell and cytolytic T lymphocyte (CTL)-mediated antigen-specific cytolysis [82–84]. HLA-G has recently been found to define a novel subset of Tregs in peripheral blood in healthy individuals, as well as at sites of inflammation. Feger et al. [85] noted HLA-G+ FoxP3− CD8+ or CD4+ T cells that are detectable in the human thymus and constitute on average 1.6 and 3.3% of the total CD4+ and CD8+ T cell population within human PBMCs, respectively. This HLA-G+ subset presents with an anergic phenotype in response to allogeneic and polyclonal stimuli and could suppress CD8 and CD4 proliferation in vitro in the absence of APCs by secreting HLA-G and TGFβ [85]. Comparison of cerebrospinal fluid (CSF) samples from MS patients versus healthy controls showed that the number of HLA-G+ CD4+ Tregs is elevated in MS patients, with notably higher numbers in MS patients in the acute phase of the disease compared to those in the stable phase. Similarly, HLA-G+ CD8+ T cells are elevated in muscle biopsies from patients with inflammatory myopathies, and the frequencies of this population correlate with the degree of cellular infiltrate in the muscle [85]. HLA-G+ T cells within the CSF of MS patients display a central memory phenotype (CD4+CD45RA−CD27+) with an up-regulation in the expression of ICOS. In addition, the preferential expression of CCR5 by these cells allows them to traffic across the blood–brain barrier and mediate suppression directly in the target tissue [86]. Taken together, these observations suggest that the HLA-G+ Treg population may expand on demand to modulate tissue inflammation. However, it remains to be determined whether this process is antigen driven or is elicited in response to non-antigen-specific inflammatory cues associated with disease.
CD8+CD25+ Tregs in mice and humans (antigen-/allospecific)
A subset of CD8+CD25+ T cells has been identified in the human thymus that suppresses allospecific T cell responses in a cell contact-dependent manner. These cells constitutively express surface TNFR2, CCR8, and intracellular CTLA-4 and, when activated with long-term anti-CD3/CD28 treatment in vitro, up-regulate surface expression of CTLA-4 and TGF-β1 [87]. These cells also carry FoxP3 transcripts, but only a few express FoxP3 protein.
Several other reports document the in vitro generation of CD8+CD25+ Treg clones. CD8+CD25+ Tregs have been generated by culturing human CD25− PBMCs with fibroblast-like bone marrow stromal cells [88], or with continuous antigen or mitogen stimulation [89, 90]. Mahic et al. [90] generated CD8+CD25+ FoxP3+ Tregs from CD8+CD25− precursors isolated from human peripheral blood. The CD25− precursor cells up-regulated CD25, FoxP3, GITR, CD28, and CTLA-4 after continuous stimulation with Staphylococcus enterotoxin B (SEB) or with anti-CD3/CD28-coated beads and, in parallel, acquired the expression of cytolytic molecules granzyme A, B and perforin. The CD8+CD25+ T cells generated in this manner suppressed SEB-stimulated CD25− PBMCs in a cell contact-dependent but CTLA-4-, IL-10- and TGF-β- independent manner [90]. Using a similar set-up, Joosten et al. showed that continuous in vitro antigenic stimulation of in vivo pre-primed human PBMCs led to the generation CD8+CD25+FoxP3+LAG-3+ Tregs that suppressed both antigen- and mitogen-driven T cell responses through a mechanism partially dependent on the secretion of CCL4. Compared to the Tregs described by Mahic et al., this subset is similar in terms of CD28, CTLA-4, and perforin expression, but it does not express granzyme B [89]. CD8+CD25+T cell clones with regulatory activity could also be generated by co-culturing human PBMCs with autologous lipopolysaccharide-activated DCs [91]. Similar to the previously described CD25+ subsets, these cells express CTLA-4 and FoxP3 and suppress in vitro CD4 responses in a cell contact-dependent, CTLA-4:CD86-dependent manner requiring the presence of APCs [91]. Of note, antigen-specific CD8+CD25+ Tregs (CTLA-4+, FoxP3+) with similar properties to those generated in vitro (described above) have also been described in diabetic patients receiving anti-CD3 treatment [92], as well as in MS patients (CTLA-4+, FoxP3+, CD122+, CD62L+) [93].
It is unclear whether the CD8+CD25+ T cells found in the human thymus are qualitatively similar to the T cell clones generated after continuous TCR stimulation, although FoxP3 expression is different between the thymus-derived T cells and the in vitro-generated clones.
In mice, a similar subset has been found in the thymus and periphery of MHC class II-deficient animals, expressing CD44, GITR, CD5, and LFA-1, and intracellular CTLA-4. CD8+CD25+ T cells from MHC class II-deficient mice suppressed anti-CD3-stimulated CD25− T cell responses in vitro in a contact-dependent, IL-10-independent manner. However, one may argue against the physiological relevance of this subset of cells as they may have arisen due to homeostatic proliferation in a lymphopenic environment (associated with the MHC class II deficiency) [94]. However, at least two other studies have documented the generation of CD8+CD25+FoxP3+ Tregs in mice. One study showed that co-culturing of mouse CD8+ T cells with LPS-stimulated splenocytes retrovirally transduced with a glutamate decarboxylase 65 (GAD)–immunoglobulin G (IgG) fusion construct can induce GAD-specific CD8+FoxP3+ T cells with suppressive activity [33]. An increase in CD8+FoxP3+ Tregs was also observed in the draining lymph nodes in a model of autoimmune intestinal inflammation employing ectopic expression of HA driven by the villin promoter and the HA-specific CL4 TCR transgene [95]. In this model, the HA-specific CD8+ T cells isolated from the mesenteric lymph nodes up-regulated the expression of CD103, LAG-3, CTLA-4, and CCL4 and were able to suppress antigen/APC-stimulated T cell responses in vitro through an undetermined mechanism [96].
CD8+CD45RO+ T cells in humans (allospecific)
A subset of CD8+ T cells displaying memory and regulatory phenotypes (expressing CD28+CD45RO+CCR7− and intracellular CTLA-4) has been identified in patients that received HLA-matched, minor histocompatibility antigen (HA-1)-mismatched renal transplants. In these patients, a regulatory population exhibiting low binding to the HA-1 tetramer was found to coexist with another pathogenic HA-1 tetramerhi effector population, both seemingly sustained by chronic antigenic stimulation mediated by HA-1 microchimerism in the DC population. The HA-1 tetramerlo subset suppresses both cognate delayed-type hypersensitivity responses or responses towards a third party antigen through IL-10, TGFβ, and CTLA-4-dependent mechanisms [97]. CD8+CD45RO+CCR7+ Tregs have also been found in patients with ovarian carcinoma and could be induced with tumor ascites plasmacytoid DCs [98]. These cells secrete IL-10 and are able to suppress tumor-antigen-driven T cell responses in vitro [98]. Allogeneic CD40 ligand-activated plasmacytoid DCs are also able to prime naïve CD8+ T cells into IL-10-producing CD8+ Tregs, although surface markers were not investigated in this study. This population suppresses naïve (but not effector) allospecific T cell responses in vitro in an IL-10-independent manner [99].
CD8+CD11c+ Tregs in mice (antigen specific)
CD8+CD11c+ Tregs represent yet another class of Tregs. CD8+ T cells have been shown to up-regulate CD11c as they acquire effector function after TCR stimulation in scenarios such as viral infections and graft-versus-host disease [100–102]. In mice, CD8+CD11c+ T cells can be detected in the periphery and constitute <3% of the peripheral T cell population, although they appear as naïve cells (based on their CD44 and CD62L profiles) and do not present appreciable suppressive activity in vitro [103]. These cells, however, do express significantly elevated levels of CD28 and CD103 relative to their CD11c-negative counterparts.
A study by Seo et al. [28], in which CD8+CD11c+ T cells were expanded following treatment with agonistic anti-4-1BB antibodies in an animal model of collagen-induced arthritis (CIA), showed that CD8+CD11c+ T cells require an additional activation signal to attain suppressive activity. In this study, the CD8+CD11c+ T cells were expanded in an antigen (collagen II, CII)-dependent manner and suppressed CII-specific CD4+ T cell responses and the development of CIA. The protective effect was linked to the production of IFNγ and the activity of indoleamine 2,3-deoxygenase; however, whether this was a direct result of CD8+CD11c+ Treg expansion was not demonstrated. In another study by Choi et al. [24], anti-4-1BB treatment resulted in the amelioration of interphotoreceptor retinoid binding protein-induced experimental autoimmune uveoretinitis (EAU), a CD4+ T cell-mediated autoimmune disease. Here, Choi et al. showed that anti-4-1BB expanded CD8+CD11c+ T cells that produced IFNγ but not those producing IL-4 or IL-10. Here, CD8+CD11c+ T cells appear to contribute to disease protection, as antibody depletion of CD8α+ cells partially neutralized the anti-4-1BB-mediated protective effects. In addition, inhibition of the IDO enzyme activity completely reversed anti-4-1BB-mediated disease protection, although a link between this systemic effect and the expanded CD8+CD11c+ T cells was not established, and the possibility of anti-4-1BB acting on other cell types was not excluded. A subsequent study by Vinay et al. [103] showed that CD8+CD11c+ T cells activated in culture with anti-4-1BB and antigen up-regulate the expression of markers such as CD103, CD122, and effector molecules Fas, perforin, and granzyme B. In the same study, CD8+CD11c+ T cells were shown to suppress CD4+ T cell responses in 4-1BB−/− hosts, and anti-4-1BB-expanded CD8+CD11c+ T cells isolated from mice with trinitrobenzene sulphonic acid-induced colitis were able to transfer disease protection to 4-1BB−/− animals [103]. Interestingly—and not inconsistent with the phenotype of up-regulated Fas, perforin, and granzyme B— activated CD8+CD11c+ T cells can also have an effector function, as demonstrated in a viral infection models [104, 105]. It would be of interest to explore the cytolytic activity of this subset of cells as another potential mechanism of immunoregulation.
Low-avidity, memory-like autoregulatory CD8+ T cells (antigen specific)
An imbalance between autoreactive pathogenic and regulatory forces underlies all autoimmune diseases. Autoreactive T cells, be they pathogenic or regulatory, recognize multiple autoantigens, of which only a fraction have been identified. To add to the complexity of such diseases, there exists a non-homogeneous population of T cells within each antigenic specificity that bind their cognate antigen/MHC with a spectrum of avidities. We previously proposed that, as spontaneous autoimmune diabetes progresses, autoreactive T cells undergo a process of ‘avidity maturation’, whereby the low-avidity, less pathogenic clonotypes are gradually out-competed and replaced by their higher avidity counterparts [106].
Using TCR-transgenic models, we found that, in the face of chronic antigenic exposure, naive high avidity CD8+ T cells are activated to become effector cells and die after fulfilling their cytotoxic function via activation-induced cell death. Low-avidity CD8+ T cells, on the other hand, are skewed towards an anergic, memory-like phenotype and accumulate in secondary lymphoid organs and the bone marrow [32]. Interestingly, NOD mice carrying a high-affinity autoreactive TCR transgene (17.4-NOD) develop accelerated autoimmune diabetes, while NOD mice carrying a low-affinity transgene of the same antigenic specificity (17.6-NOD) are nearly completely resistant to diabetes development [32, 107]. Upon further analysis of the low-affinity TCR-transgenic 17.6-NOD strain, we found that its diabetes resistance is mediated by the immunoregulatory activity of a population of low-avidity, memory-like CD8+ T cells. These CD8+ T cells are CD44hi, CD122+, express perforin and granzyme B, and proliferate (ex vivo) in response to the cytokines Il-2 and IL-15, even in the absence of antigen. Upon antigenic stimulation, they rapidly produce IFNγ but not IL-2 [32]. Importantly, antigen-specific activation of this subset of low-avidity autoreactive CD8+ T cells leads to APC-dependent down-regulation of other polyclonal, pancreatic beta cell-specific T cell responses through mechanism(s) involving perforin-mediated APC killing, IFNγ secretion, and IDO [32].
These observations led us to propose the following paradigm: that the autoimmune process seeks to limit itself through the workings of a negative feedback loop elaborated by low-avidity autoreactive T cells, which preferentially accumulate as memory-like suppressor cells in response to chronic autoantigenic stimulation and modulate self-antigen presentation by autoantigen-loaded APCs.
Building on this paradigm, we have recently discovered a novel immunotherapeutic approach that allows us to selectively expand these disease-generated, low-avidity, memory-like autoregulatory CD8+ T cells. We found that treatment of NOD mice with nanoparticles coated with disease-relevant pMHC complexes (pMHC–NP) effectively expanded cognate low-avidity, memory-like CD8+ T cells and abrogated disease [32]. Several lines of evidence further confirmed that the expanded CD8+ T cells are true memory cells: (1) treatment with nanoparticles coated with disease-irrelevant pMHC complexes did not lead to cognate T cell expansion, (2) treatment of diabetes-resistant strains (where there is no disease-associated memory) with disease-relevant pMHC-NP did not promote cognate T cell expansion, and (3) similar treatment of a mutant NOD strain lacking the endogenous T cell epitope (where there is no epitope-specific memory) with pMHC-NP failed to expand epitope-specific CD8+ T cells or blunt autoimmune diabetes [32].
Phenotypically similar to the TCR-transgenic low-avidity autoreactive CD8+ T cells, the expanded cells exhibit suppressive activity in vitro and in vivo through mechanisms involving perforin, IFNγ, and IDO. The low-avidity, memory-like CD8+ Tregs are FoxP3−,CD44hi, CD62Llow, CCR7−, and CD122+; however, unlike the CD8+CD122+ natural Tregs described above, this subset does not secrete IL-10 and it mediates suppression in an antigen-dependent and APC-dependent manner [32].
CD8+LAP+ Tregs in mice
It has been reported that LAP is expressed on approximately 3% of the splenic CD8+ T cell population in unmanipulated mice [108]. This subset is mostly FoxP3−, CD25− and secretes more IL-2, IFNγ, and TGFβ than their LAP− counterparts. Tested on a model of EAE induced with PLP139–151, CD8+LAP+ T cell transfer reduced disease severity in the relapse phase, in an IFNγ- and TGFβ-dependent manner [108]. The effect(s) of IFNγ on suppression might be mediated by a third party APC, as IFNγ receptor deficiency in neither the CD8+LAP+ suppressors nor the responder cells was found to affect suppression in vitro [108].
pCons-induced CD8+ Tregs in lupus (antigen specific)
The induction of regulatory CD8+ T cells in lupus-prone animals treated with a nucleosomal histone peptide has been documented [27]. The induced CD8+ T cell population suppressed antigen-driven T cell responses in vitro and autoantibody production and nephritis in vivo, in a TGFβ-dependent, contact-independent manner [27]. A similar subset has been described in a model of murine systemic lupus, where treatment with a tolerogenic anti-DNA IgG VH region-derived peptide called pConsensus (pCons) induced a population of CD8+ Tregs that reside in both CD28+ and CD28− subsets [109]. This subset requires antigen-specific activation in vitro and suppresses autoimmune antibody responses in a cell contact-independent, FoxP3−- and TGFβ-dependent manner. pCons-expanded CD8+ Tregs also display increased cytotoxic activity against B cells from nephritic, anti-DNA-positive mice, suggesting that this population acts by both suppressing helper T cells and killing pathogenic B cells [110].
Tregs versus T memory cells
The Treg family is clearly heterogeneous and complex, as Tregs adopt multiple mechanisms (Fig. 1) and reside within multiple differentiation/maturation stages, ranging from natural, naïve-like, to antigen-experienced, central, or effector/memory-like T cells. This is true for both CD4+ and CD8+ Tregs, although in the CD4+ compartment, CD25 and FoxP3 are widely used markers that unify the “classical” CD4+ Tregs, while IL-10 is a signature of the CD25− Tr1 cells. For CD8+ Tregs, it is currently not possible to cluster all of the different subsets into a single major subpopulation of lymphocytes in which phenotypic and/or functional heterogeneity among cell types defined by different groups in different models can be ascribed to different stages of differentiation along a continuum. In other words, it is unclear whether some of the subsets that share phenotypic and functional markers belong to a single lymphocyte subset captured at different stages of differentiation. Nonetheless, and despite this heterogeneity, there are several lines of evidence supporting the hypothesis that many Treg subsets share a memory-like phenotype suggestive of prior antigenic encounters.
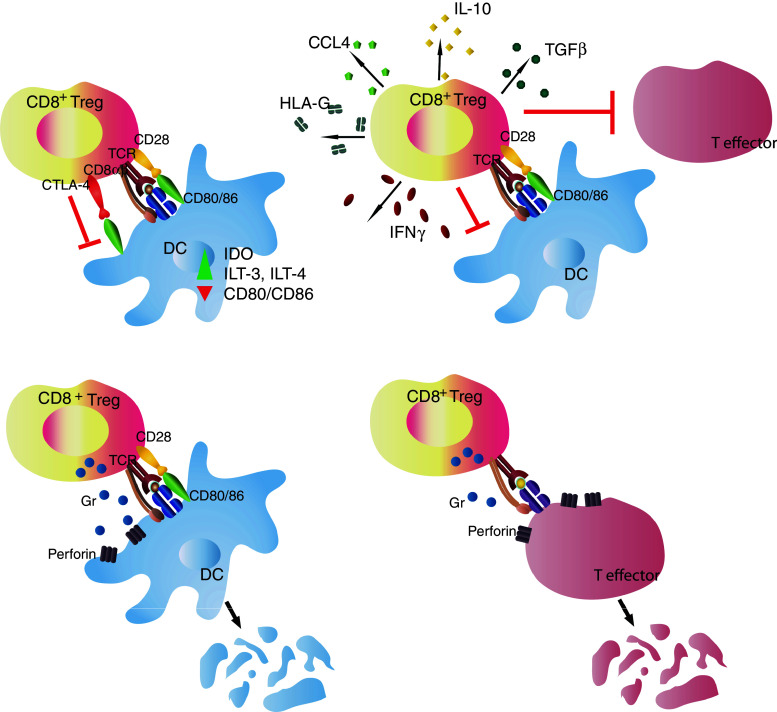
Fig. 1.
CD8+ T regulatory cells (Tregs) mediate immune suppression through various cell contact-dependent and independent mechanisms. a CD8+ Treg interacts with an autoantigen-loaded antigen-presenting cell (APC), leading to down-regulation of APC function, b CD8+ Treg secretes inhibitory cytokines and chemokines, and/or immuno-modulatory molecules, such as HLA-G, following antigen-specific activation, to down-regulate target effector cells in a cell contact-independent manner, c CD8+ Tregs kill antigen-loaded APCs as a means to down-regulate an autoimmune response; d CD8αα Tregs kill activated target CD4+ effector cells by sensing the hsp60sp presented on Qa-1
First, CD4+ and CD8+ Tregs subsets often express higher levels of activation markers, consistent with some sort of past antigenic experience. For example, the CD4+FoxP3+ Treg subset as well as certain CD8+ Treg subsets express the IL-2 receptor alpha chain (CD25) [87, 89–91, 93] and, in some instances, the beta chain (CD122) [32, 40, 49, 53, 103, 111]. CD44 expression is also up-regulated on Tregs [32, 61, 94, 112–114], and its level directly correlates with enhanced CD4+ Treg function [114]. In a recent discovery, we have conclusively demonstrated the requirement of autoantigen experience in the induction of the low-avidity autoreactive CD8+ Treg subset; expansion of cognate Tregs was only possible in animals expressing the endogenous epitope and only when there was an ongoing autoimmune response against that epitope (i.e., autoimmune inflammation) [32].
Second, consistent with the phenotype of antigen experience, several Treg subsets falling into both the “natural” and “induced” categories express markers that are normally found on memory cells. The Qa-1 restricted CD8αα+ Tregs, and the low-avidity memory-like autoregulatory Tregs described by us, for example, express receptors for IL-7 [32, 40], a key cytokine in memory T cell survival. Suzuki et al. [115] once commented that the CD8+CD122+ Treg subset is both regulatory and central memory-like (CD44hi, CD62L+), an observation that is echoed in a number of studies, including our own [32]. However, since CD122 is a marker of CD8+ T cell memory and the CD8+CD122+ subset was characterized as one population with undefined antigenic specificity, the CD122 marker alone can be considered to be insufficient to ascribe regulatory activity to this T cell subset. A recent report by Dai et al. [52] suggests that the expression of PD-1 differentiates Tregs from conventional, non-regulatory memory T cells within this subset. Similar to the CD8+CD122+ subset, the CD8+CD103+ Tregs described by Ho et al. [80] (CD44hiCD62L+) have features of central memory cells. In humans, the CD8+CD28− subset (CD62L+CD45RO+CD27+) [61, 116], the HLA-G+ CD4+ Tregs (CD45RA−, CD27+) [117], and the CD45RO+CCR7+ Tregs induced by tumor ascites plasmacytoid DCs all exhibit a central memory-like phenotype [98]. It is also noteworthy that several subsets of CD8+ Tregs express markers of effector function after activation in vitro and/or in vivo, coinciding with the acquisition of suppressive activity. For example, activation of CD8+ Tregs is often accompanied by the expression of cytolytic molecules, such as granzyme and perforin [32, 37, 103], although it is unclear whether the expressed effector molecules are required for suppression.
These considerations are not unique to the CD8+ Treg compartment. In the CD4+ compartment, the chemokine receptor CCR6, which is expressed on memory subsets [118] and on >30% of peripheral blood CD4+CD25+ Tregs in mice, differentiates naïve CD4+ Tregs from effector memory CD4+ Tregs (CD44hi, CD69hi, CD62L) [112]. This subset was also identified in humans [119]. Other markers, such as the αEβ7 integrin (CD103) in mice [120] and CD39 (an ectonucleotidase involved in adenosine synthesis) in humans [113, 121], have also been shown to delineate inflamed tissue-seeking, memory-like CD4+ Tregs. Moreover, human CD4+FoxP3+ Tregs displaying a memory phenotype (CD45RO+) rapidly self-renew and share the same TCR usage as the CD4+ memory pool, suggesting that some Tregs may derive from conventional memory T cells [122]. Functionally, the ability of memory T cells to rapidly respond to antigen encounters, as compared to naive T cells, is recapitulated in the IL-10-producing memory-like CD4+ Tregs [112, 123] and in the low-avidity memory-like autoregulatory CD8+ Tregs that promptly produce IFNγ upon antigen-specific stimulation and at a significantly faster tempo than their naïve high-avidity counterparts [32]. In light of these findings, it is tempting to speculate that Tregs with the memory phenotype are endowed with a superior ability to modulate autoimmune responses compared to their naïve-like Treg equivalents.
Third, subsets of CD4+ and CD8+ Tregs demonstrate a fitness which is superior to that of recently activated effector T cells in terms of their ability to withstand repeated encounters with their cognate antigens without undergoing activation-induced cell death; this is also a hallmark of conventional memory T cells. In some subsets, this may be achieved through the up-regulation of anti-apoptotic genes, such as Bcl2 and Bcl6 [25, 124, 125].
And fourth, in at least some autoimmune disease states, disease-specific Tregs seem to expand and accumulate in situ upon disease onset [86, 111, 126–129]. In a study by Godebu et al. [123], in vitro-generated Tregs demonstrated self-antigen-driven propagation similar to that of memory-like Tregs when injected into NOD mice, in which a constant pressure exerted by the diabetes-associated autoantigens resulted in the selective expansion of certain clonotypes of Tregs with a skewing of Vβ TCR usage. We have shown, using the NOD mouse model, that antigen-specific expansion of low-avidity memory-like autoregulatory CD8+ Tregs with pMHC-coated nanoparticles is most efficient when given to animals at the onset of diabetes relative to young, non-diabetic animals [32], suggesting that the diabetogenic process fosters the generation of these memory-like Tregs. Interestingly, treatment of mice with the non-FcR-binding anti-CD3 monoclonal antibody blunts disease progression only when given post-diabetes onset, being largely ineffective when given to pre-diabetic animals [130]. A recent report describes an increase in autoreactive CD8+ T cells with an effector memory-like phenotype following anti-CD3 administration, although the suppressive activity of these cells was not assessed [131]. Chatenoud et al.’s [130] puzzling observation back in 1997 that anti-CD3 monoclonal antibody therapy only had anti-diabetogenic effects in newly diagnosed diabetic mice could thus be explained if we assume that anti-CD3 monoclonal antibody acts by expanding disease-generated memory Tregs, which may only reach the threshold required for effective therapeutic expansion as overt clinical disease approaches.
Concluding remarks
In summary, we observe that autoimmune disease-induced Tregs, be they CD4+ or CD8+, present a memory-like phenotype (in terms of the markers that are expressed, the dynamics of their activation, and their survival properties) that strongly suggests a history of antigenic experience. It is clear that disease-induced Tregs participate in a “negative feedback” regulatory loop that aims to hamper ongoing autoimmunity (see Fig. 2). We note that there is evidence for the existence of similar negative feedback processes in models of chronic infections and even cancer, presumably as an evolutionarily conserved mechanism to limit collateral damage resulting from chronic immune responses against persistent pathogen-derived antigens. In fact, a number of pathogens have evolved mechanisms to exploit these negative feedback mechanisms to evade host defence, and certain chronic, non-resolving infections have been associated with increased numbers of Tregs [132–135]. In autoimmunity and transplantation, on the other hand, the concept of disease- or allograft-driven negative feedback Treg loops is less acknowledged. In this review, based on available evidence, we propose that the autoimmune process auto-regulates itself through a process of feedback inhibition. In parallel to the autoantigen-driven activation and clonal expansion of pathogenic, autoreactive T cells, the autoimmune process would foster the generation of antigen-experienced, memory-like autoregulatory Tregs to limit the progression of autoimmunity. A schematic diagram of this feedback loop in relation to the autoantigen load and generation of autoreactive T effector cells is shown in Fig. 3. This class of Tregs would acquire a memory-like phenotype after continuous antigen exposure, much like non-autoimmune states of chronic antigen exposure (chronic infections and cancer) might generate pathogen- or tumor-specific Tregs that promote the chronicity of infection or the progression of cancer. It would therefore be reasonable to suspect that this type of memory-like autoregulatory T cells has evolved to abort bystander autoimmune processes arising in response to infection.
Fig. 2.
Autoreactive T regulatory cells (Tregs) participate in a negative feedback loop to dampen the autoimmune disease process. T regulatory memory arises in response to chronic antigenic stimulation [designated here as encounters with dendritic cells (DC), or other antigen-presenting cells presenting self-antigens] and exerts suppressive effects on the activation of pathogenic autoreactive T cells. AICD Activation-induced cell death
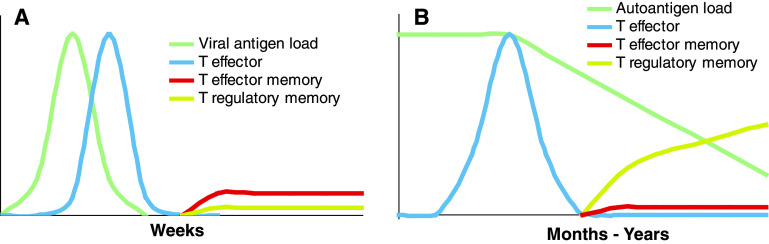
Fig. 3.
Dynamics of the generation of T-effector cells versus memory-like Tregs under acute versus chronic antigenic stimulation. In autoimmunity and viral infection (whether acute or chronic), exposure to self- and viral antigens, respectively, leads to T cell receptor stimulation of cognate T cells and T-effector cell generation. a In the case of an acute viral infection, T-effector cells promote viral antigen clearance, but this also leads to massive attrition of T-effector cells. The few survivors progressively give rise to the T-effector memory pool that will respond to subsequent antigen re-exposures. The prompt removal of antigen by the T-effectors hinders the formation of T-regulatory memory (from naive low-avidity precursors). b In autoimmunity (and, possibly, other instances in which there is chronic antigenic persistence, such as in chronic infection and/or cancer), chronic stimulation with antigen would promote the formation of T-regulatory memory cells (from naive low-avidity precursors). In contrast, chronic antigenic persistence would significantly compromise T-effector memory formation (from T-effector high-avidity clones, which would be constantly driven to undergo re-activation-induced cell death in response to repeated antigen encounters)
Acknowledgments
The work described here was funded by grants from the Canadian Institutes of Health Research (CIHR), the National and Engineering Research Council of Canada (NSERC), the Juvenile Diabetes Research Foundation (JDRF), the Diabetes Association (Foothills), and the Canadian Diabetes Association. S.T. and X.C.C. are supported by studentships from Alberta Innovates—Health Solutions (AIHS, formerly AHFMR) and the AXA Research Fund, respectively. P.S. is a Scientist of the AIHS and a JDRF Scholar. The JMDRC is supported by the Diabetes Association (Foothills).
References
- 1.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108(3):586–590. [PubMed] [Google Scholar]
- 2.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor H, Hugenberger J, McVay-Boudreau L, Eardley DD, Kemp J, Shen FW, Gershon RK. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978;148(4):871–877. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components, II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eardley DD, Hugenberger J, McVay-Boudreau L, Shen FW, Gershon RK, Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 8.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 9.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 10.Picca CC, Larkin J, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+CD25+ regulatory T-cell selection. Immunol Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 11.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettini ML, Vignali DA. Development of thymically derived natural regulatory T cells. Ann N Y Acad Sci. 2010;1183:1–12. doi: 10.1111/j.1749-6632.2009.05129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 14.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5(3):179–190. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 15.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goverman J, Perchellet A, Huseby ES. The role of CD8(+) T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy. 2005;4(2):239–245. doi: 10.2174/1568010053586264. [DOI] [PubMed] [Google Scholar]
- 17.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 18.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17(6):624–631. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95(6):2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, Setti M, Puppo F, Indiveri F. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166(10):6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 21.Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, Belobradkova J, Dite P, Michalek J. Numerical defects in CD8+ CD28- T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell Immunol. 2010;262(2):75–79. doi: 10.1016/j.cellimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Singh RP, Hahn BH, La Cava A. Tuning immune suppression in systemic autoimmunity with self-derived peptides. Inflamm Allergy Drug Targets. 2008;7(4):253–259. doi: 10.2174/187152808786848423. [DOI] [PubMed] [Google Scholar]
- 23.Biegler BW, Yan SX, Ortega SB, Tennakoon DK, Racke MK, Karandikar NJ. Glatiramer acetate (GA) therapy induces a focused, oligoclonal CD8+ T-cell repertoire in multiple sclerosis. J Neuroimmunol. 2006;180(1–2):159–171. doi: 10.1016/j.jneuroim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4–1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2, 3-dioxygenase-dependent mechanisms. Cytokine. 2006;34(5–6):233–242. doi: 10.1016/j.cyto.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol. 2005;175(11):7728–7737. doi: 10.4049/jimmunol.175.11.7728. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, Kashleva H, Xu LX, Forman J, Flaherty L, Pernis B, Braunstein NS, Chess L. T cell vaccination induces T cell receptor Vbeta-specific Qa-1-restricted regulatory CD8(+) T cells. Proc Natl Acad Sci USA. 1998;95(8):4533–4537. doi: 10.1073/pnas.95.8.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J Immunol. 2005;174(6):3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 28.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4–1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10(10):1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 29.Skaggs BJ, Singh RP, Hahn BH. Induction of immune tolerance by activation of CD8+ T suppressor/regulatory cells in lupus-prone mice. Hum Immunol. 2008;69(11):790–796. doi: 10.1016/j.humimm.2008.08.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Maricic I, Kumar V. Anti-TCR antibody treatment activates a novel population of nonintestinal CD8 alpha alpha + TCR alpha beta + regulatory T cells and prevents experimental autoimmune encephalomyelitis. J Immunol. 2007;178(10):6043–6050. doi: 10.4049/jimmunol.178.10.6043. [DOI] [PubMed] [Google Scholar]
- 31.Tennakoon DK, Mehta RS, Ortega SB, Bhoj V, Racke MK, Karandikar NJ. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176(11):7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 32.Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32(4):568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Han G, Song L, Wang J, Chen G, Xu R, Yu M, Qian J, Shen B, Li Y. CD8+ regulatory T cells are responsible for GAD-IgG gene-transferred tolerance induction in NOD mice. Immunology. 2009;126(1):123–131. doi: 10.1111/j.1365-2567.2008.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5(5):516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 35.Gaur A, Ruberti G, Haspel R, Mayer JP, Fathman CG. Requirement for CD8+ cells in T cell receptor peptide-induced clonal unresponsiveness. Science. 1993;259(5091):91–94. doi: 10.1126/science.8418501. [DOI] [PubMed] [Google Scholar]
- 36.Panoutsakopoulou V, Huster KM, McCarty N, Feinberg E, Wang R, Wucherpfennig KW, Cantor H. Suppression of autoimmune disease after vaccination with autoreactive T cells that express Qa-1 peptide complexes. J Clin Invest. 2004;113(8):1218–1224. doi: 10.1172/JCI20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beeston T, Smith TR, Maricic I, Tang X, Kumar V. Involvement of IFN-gamma and perforin, but not Fas/FasL interactions in regulatory T cell-mediated suppression of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;229(1–2):91–97. doi: 10.1016/j.jneuroim.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule, Qa-1. Annu Rev Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 39.Madakamutil LT, Maricic I, Sercarz E, Kumar V. Regulatory T cells control autoimmunity in vivo by inducing apoptotic depletion of activated pathogenic lymphocytes. J Immunol. 2003;170(6):2985–2992. doi: 10.4049/jimmunol.170.6.2985. [DOI] [PubMed] [Google Scholar]
- 40.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston T, Jensen P, Kumar V. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha + TCRalphabeta + T cells. J Immunol. 2006;177(11):7645–7655. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467(7313):328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W, Zhang L, Liang B, Saenger Y, Li J, Chess L, Jiang H. Perceiving the avidity of T cell activation can be translated into peripheral T cell regulation. Proc Natl Acad Sci USA. 2007;104(51):20472–20477. doi: 10.1073/pnas.0709878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H. The Qa-1 dependent CD8+ T cell mediated regulatory pathway. Cell Mol Immunol. 2005;2(3):161–167. [PubMed] [Google Scholar]
- 44.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, Chess L. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120(10):3641–3650. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shmidt TE, Zhuchenko TD, Iakhno NN (2003) Glatiramer acetate (Copaxone) influence on different stages of multiple sclerosis pathogenesis. Zh Nevrol Psikhiatr Im S S Korsakova(Spec No 2):79–82 [PubMed]
- 46.Yamada H, Matsuzaki G, Iwamoto Y, Nomoto K. Unusual cytotoxic activities of thymus-independent, self-antigen-specific CD8(+) T cells. Int Immunol. 2000;12(12):1677–1683. doi: 10.1093/intimm/12.12.1677. [DOI] [PubMed] [Google Scholar]
- 47.Yamada H, Matsuzaki G, Chen Q, Iwamoto Y, Nomoto K. Reevaluation of the origin of CD44(high) “memory phenotype” CD8 T cells: comparison between memory CD8 T cells and thymus-independent CD8 T cells. Eur J Immunol. 2001;31(6):1917–1926. doi: 10.1002/1521-4141(200106)31:6<1917::aid-immu1917>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268(5216):1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 49.Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200(9):1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endharti AT, Rifa IM, Shi Z, Fukuoka Y, Nakahara Y, Kawamoto Y, Takeda K, Isobe K, Suzuki H. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175(11):7093–7097. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 51.Shi Z, Rifa'i M, Lee YH, Shiku H, Isobe K, Suzuki H. Importance of CD80/CD86-CD28 interactions in the recognition of target cells by CD8+CD122+ regulatory T cells. Immunology. 2008;124(1):121–128. doi: 10.1111/j.1365-2567.2007.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 53.Saitoh O, Abiru N, Nakahara M, Nagayama Y. CD8+CD122+ T cells, a newly identified regulatory T subset, negatively regulate Graves’ hyperthyroidism in a murine model. Endocrinology. 2007;148(12):6040–6046. doi: 10.1210/en.2007-0300. [DOI] [PubMed] [Google Scholar]
- 54.Lee YH, Ishida Y, Rifa'i M, Shi Z, Isobe K, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol. 2008;180(2):825–832. doi: 10.4049/jimmunol.180.2.825. [DOI] [PubMed] [Google Scholar]
- 55.Endharti AT, Okuno Y, Shi Z, Misawa N, Toyokuni S, Ito M, Isobe K, Suzuki H. CD8+CD122+ regulatory T Cells (Tregs) and CD4+ Tregs cooperatively prevent and cure CD4+ cell-induced colitis. J Immunol. 2011;186(1):41–52. doi: 10.4049/jimmunol.1000800. [DOI] [PubMed] [Google Scholar]
- 56.Shi Z, Okuno Y, Rifa'i M, Endharti AT, Akane K, Isobe K, Suzuki H. Human CD8+CXCR3+T cells have the same function as murine CD8+CD122+Treg. Eur J Immunol. 2009;39(8):2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z, Tugulea S, Cortesini R, Suciu-Foca N. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- T cells. Int Immunol. 1998;10(6):775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 58.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, Cortesini R, Suciu-Foca N. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28- regulatory T cells. J Immunol. 1998;161(10):5193–5202. [PubMed] [Google Scholar]
- 59.Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28- T cells. Transplantation. 2000;69(7):1304–1310. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- 60.Jiang S, Tugulea S, Pennesi G, Liu Z, Mulder A, Lederman S, Harris P, Cortesini R, Suciu-Foca N. Induction of MHC-class I restricted human suppressor T cells by peptide priming in vitro. Hum Immunol. 1998;59(11):690–699. doi: 10.1016/s0198-8859(98)00073-1. [DOI] [PubMed] [Google Scholar]
- 61.Scotto L, Naiyer AJ, Galluzzo S, Rossi P, Manavalan JS, Kim-Schulze S, Fang J, Favera RD, Cortesini R, Suciu-Foca N. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28- T suppressor cells. Hum Immunol. 2004;65(11):1297–1306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3(3):237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 63.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N. Alloantigen specific CD8+CD28- FOXP3+ T suppressor cells induce ILT3+ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16(8):1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 64.Kim-Schulze S, Scotto L, Vlad G, Piazza F, Lin H, Liu Z, Cortesini R, Suciu-Foca N. Recombinant Ig-like transcript 3-Fc modulates T cell responses via induction of Th anergy and differentiation of CD8+ T suppressor cells. J Immunol. 2006;176(5):2790–2798. doi: 10.4049/jimmunol.176.5.2790. [DOI] [PubMed] [Google Scholar]
- 65.Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, Clarkson MR, Sayegh MH, Khoury SJ. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112(7):1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28 regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci USA. 2007;104(44):17459–17464. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paas-Rozner M, Dayan M, Paas Y, Changeux JP, Wirguin I, Sela M, Mozes E. Oral administration of a dual analog of two myasthenogenic T cell epitopes down-regulates experimental autoimmune myasthenia gravis in mice. Proc Natl Acad Sci USA. 2000;97(5):2168–2173. doi: 10.1073/pnas.040554597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, Goronzy JJ, Weyand CM. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174(11):7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- 69.Filaci G, Rizzi M, Setti M, Fenoglio D, Fravega M, Basso M, Ansaldo G, Ceppa P, Borgonovo G, Murdaca G, Ferrera F, Picciotto A, Fiocca R, Torre G, Indiveri F. Non-antigen-specific CD8(+) T suppressor lymphocytes in diseases characterized by chronic immune responses and inflammation. Ann N Y Acad Sci. 2005;1050:115–123. doi: 10.1196/annals.1313.013. [DOI] [PubMed] [Google Scholar]
- 70.Crucian B, Dunne P, Friedman H, Ragsdale R, Pross S, Widen R. Alterations in levels of CD28-/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2(2):249–252. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, Setti M, Accolla RS, Puppo F, Indiveri F. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65(2):142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Picker LJ, Terstappen LW, Rott LS, Streeter PR, Stein H, Butcher EC. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990;145(10):3247–3255. [PubMed] [Google Scholar]
- 73.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+CTL. J Immunol. 1997;159(8):3748–3756. [PubMed] [Google Scholar]
- 74.Wang D, Yuan R, Feng Y, El-Asady R, Farber DL, Gress RE, Lucas PJ, Hadley GA. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J Immunol. 2004;172(1):214–221. doi: 10.4049/jimmunol.172.1.214. [DOI] [PubMed] [Google Scholar]
- 75.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201(10):1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hadley GA, Charandee C, Weir MR, Wang D, Bartlett ST, Drachenberg CB. CD103+CTL accumulate within the graft epithelium during clinical renal allograft rejection. Transplantation. 2001;72(9):1548–1555. doi: 10.1097/00007890-200111150-00013. [DOI] [PubMed] [Google Scholar]
- 77.Uss E, Rowshani AT, Hooibrink B, Lardy NM, Lier RA, ten Berge IJ. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006;177(5):2775–2783. doi: 10.4049/jimmunol.177.5.2775. [DOI] [PubMed] [Google Scholar]
- 78.Koch SD, Uss E, Lier RA, ten Berge IJ. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol. 2008;69(11):737–744. doi: 10.1016/j.humimm.2008.08.281. [DOI] [PubMed] [Google Scholar]
- 79.Myers L, Croft M, Kwon BS, Mittler RS, Vella AT. Peptide-specific CD8 T regulatory cells use IFN-gamma to elaborate TGF-beta-based suppression. J Immunol. 2005;174(12):7625–7632. doi: 10.4049/jimmunol.174.12.7625. [DOI] [PubMed] [Google Scholar]
- 80.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180(4):2573–2580. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu L, Yu Y, Li G, Pu L, Zhang F, Zheng S, Wang X. CD8(+)CD103(+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9(5):546–548. doi: 10.1016/j.intimp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 82.Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J, Guillet JG, Carosella ED, Rouas-Freiss N. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11(8):1351–1356. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 83.Lila N, Rouas-Freiss N, Dausset J, Carpentier A, Carosella ED. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA. 2001;98(21):12150–12155. doi: 10.1073/pnas.201407398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riteau B, Menier C, Khalil-Daher I, Sedlik C, Dausset J, Rouas-Freiss N, Carosella ED. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol. 1999;43(2):203–211. doi: 10.1016/s0165-0378(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 85.Feger U, Tolosa E, Huang YH, Waschbisch A, Biedermann T, Melms A, Wiendl H. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110(2):568–577. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 86.Huang YH, Zozulya AL, Weidenfeller C, Metz I, Buck D, Toyka KV, Bruck W, Wiendl H. Specific central nervous system recruitment of HLA-G(+) regulatory T cells in multiple sclerosis. Ann Neurol. 2009;66(2):171–183. doi: 10.1002/ana.21705. [DOI] [PubMed] [Google Scholar]
- 87.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102(12):4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 88.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92(7):881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 89.Joosten SA, Meijgaarden KE, Savage ND, Boer T, Triebel F, Wal A, Heer E, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA. 2007;104(19):8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, Aandahl EM. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38(3):640–646. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 91.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35(10):2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 92.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115(10):2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Correale J, Villa A. Role of CD8 + CD25 + Foxp3 + regulatory T cells in multiple sclerosis. Ann Neurol. 2010;67(5):625–368. doi: 10.1002/ana.21944. [DOI] [PubMed] [Google Scholar]
- 94.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175(1):246–253. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 95.Westendorf AM, Fleissner D, Deppenmeier S, Gruber AD, Bruder D, Hansen W, Liblau R, Buer J. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology. 2006;131(2):510–524. doi: 10.1053/j.gastro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 96.Fleissner D, Hansen W, Geffers R, Buer J, Westendorf AM. Local induction of immunosuppressive CD8+ T cells in the gut-associated lymphoid tissues. PLoS One. 2010;5(10):e15373. doi: 10.1371/journal.pone.0015373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham WJ. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199(7):1017–1023. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65(12):5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 99.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195(6):695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huleatt JW, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154(11):5684–5693. [PubMed] [Google Scholar]
- 101.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163(8):4125–4132. [PubMed] [Google Scholar]
- 102.Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol. 2003;33(10):2736–2743. doi: 10.1002/eji.200324087. [DOI] [PubMed] [Google Scholar]
- 103.Vinay DS, Kim CH, Choi BK, Kwon BS. Origins and functional basis of regulatory CD11c+CD8+ T cells. Eur J Immunol. 2009;39(6):1552–1563. doi: 10.1002/eji.200839057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beyer M, Wang H, Peters N, Doths S, Koerner-Rettberg C, Openshaw PJ, Schwarze J. The beta2 integrin CD11c distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Respir Res. 2005;6:70. doi: 10.1186/1465-9921-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS. 4–1BB costimulation enhances HSV-1-specific CD8 + T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol. 2005;238(2):76–86. doi: 10.1016/j.cellimm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406(6797):739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 107.Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest. 2005;115(7):1879–1887. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen ML, Yan BS, Kozoriz D, Weiner HL. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur J Immunol. 2009;39(12):3423–3435. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178(12):7649–7657. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 110.Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB × NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol. 2008;180(4):2069–2080. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 111.York NR, Mendoza JP, Ortega SB, Benagh A, Tyler AF, Firan M, Karandikar NJ. Immune regulatory CNS-reactive CD8+ T cells in experimental autoimmune encephalomyelitis. J Autoimmun. 2010;35(1):33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105(7):2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9(10):2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu T, Soong L, Liu G, Konig R, Chopra AK. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+ Treg cells. Biol Direct. 2009;4:40. doi: 10.1186/1745-6150-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suzuki H, Shi Z, Okuno Y, Isobe K. Are CD8+CD122+ cells regulatory T cells or memory T cells? Hum Immunol. 2008;69(11):751–754. doi: 10.1016/j.humimm.2008.08.285. [DOI] [PubMed] [Google Scholar]
- 116.Colovai AI, Mirza M, Vlad G, Wang S, Ho E, Cortesini R, Suciu-Foca N. Regulatory CD8+CD28- T cells in heart transplant recipients. Hum Immunol. 2003;64(1):31–37. doi: 10.1016/s0198-8859(02)00742-5. [DOI] [PubMed] [Google Scholar]
- 117.Huang YH, Zozulya AL, Weidenfeller C, Schwab N, Wiendl H. T cell suppression by naturally occurring HLA-G-expressing regulatory CD4+ T cells is IL-10-dependent and reversible. J Leukoc Biol. 2009;86(2):273–281. doi: 10.1189/jlb.1008649. [DOI] [PubMed] [Google Scholar]
- 118.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162(1):186–194. [PubMed] [Google Scholar]
- 119.Rivino L, Gruarin P, Haringer B, Steinfelder S, Lozza L, Steckel B, Weick A, Sugliano E, Jarrossay D, Kuhl AA, Loddenkemper C, Abrignani S, Sallusto F, Lanzavecchia A, Geginat J. CCR6 is expressed on an IL-10-producing, autoreactive memory T cell population with context-dependent regulatory function. J Exp Med. 2010;207(3):565–577. doi: 10.1084/jem.20091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199(3):303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183(11):7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 122.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116(9):2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol. 2008;181(3):1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang CC, Vlad G, D'Agati VD, Liu Z, Zhang QY, Witkowski P, Torkamani AA, Stokes MB, Ho EK, Cortesini R, Suciu-Foca N. BCL6 is required for differentiation of Ig-like transcript 3-Fc-induced CD8 + T suppressor cells. J Immunol. 2010;185(10):5714–5722. doi: 10.4049/jimmunol.1001732. [DOI] [PubMed] [Google Scholar]
- 125.Vlad G, King J, Chang CC, Liu Z, Friedman RA, Torkamani AA, Suciu-Foca N. Gene profile analysis of CD8(+) ILT3-Fc induced T suppressor cells. Hum Immunol. 2011;72(2):107–114. doi: 10.1016/j.humimm.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 126.Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, de Jager W, Pugayung G, Giannoni F, Rijkers G, Albani S, Kuis W, Prakken B. CD4+CD25 bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172(10):6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 127.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baek HJ, Zhang L, Jarvis LB, Gaston JS. Increased IL-4 + CD8+ T cells in peripheral blood and autoreactive CD8+ T cell lines of patients with inflammatory arthritis. Rheumatology (Oxford) 2008;47(6):795–803. doi: 10.1093/rheumatology/ken089. [DOI] [PubMed] [Google Scholar]
- 129.Kao JK, Hsue YT, Lin CY. Role of new population of peripheral CD11c(+)CD8(+) T cells and CD4(+)CD25(+) regulatory T cells during acute and remission stages in rheumatoid arthritis patients. J Microbiol Immunol Infect. 2007;40(5):419–427. [PubMed] [Google Scholar]
- 130.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158(6):2947–2954. [PubMed] [Google Scholar]
- 131.Cernea S, Herold KC. Monitoring of antigen-specific CD8 T cells in patients with type 1 diabetes treated with antiCD3 monoclonal antibodies. Clin Immunol. 2010;134(2):121–129. doi: 10.1016/j.clim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008;69(11):771–775. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 133.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69(11):760–770. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 134.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 135.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]