Abstract
The balance between cellular proliferation and differentiation is a key aspect of development in multicellular organisms. Recent studies on Arabidopsis roots revealed distinct roles for different reactive oxygen species (ROS) in these processes. Modulation of the balance between ROS in proliferating cells and elongating cells is controlled at least in part at the transcriptional level. The effect of ROS on proliferation and differentiation is not specific for plants but appears to be conserved between prokaryotic and eukaryotic life forms. The ways in which ROS is received and how it affects cellular functioning is discussed from an evolutionary point of view. The different redox-sensing mechanisms that evolved ultimately result in the activation of gene regulatory networks that control cellular fate and decision-making. This review highlights the potential common origin of ROS sensing, indicating that organisms evolved similar strategies for utilizing ROS during development, and discusses ROS as an ancient universal developmental regulator.
Keywords: Evolution, Reactive oxygen species, Development
Introduction
The prevailing life forms today are aerobic organisms which rely on O2 for their energy production. Paradoxically, this life-essential gas is toxic in nature, and all aerobes including plants, humans or bacteria experience molecular damage when exposed to high concentrations of O2 [1]. Moreover, even under ambient O2 concentration, aerobic organisms suffer from continuous oxidative damage [2]. The ability to use O2 has greatly stimulated evolution of complex multicellular life forms [3], and is thought of as being a prerequisite to this evolutionary step [4, 5]. Still, in the anoxic deep sea, anaerobic multicellular metazoan life forms lacking mitochondria have recently been discovered [6], indicating that anaerobic metabolism does not preclude the development of complex life.
The evolution of the photosynthetic apparatus capable of splitting H2O into O2, protons and electrons was the main cause for the introduction of O2 into an anaerobic biosphere 2.2 billion years ago [7]. The release of O2 at that time put organisms under strong selection pressure to effectively develop new redox energy couples. Thus, organisms concomitantly evolved antioxidant strategies to mitigate the effects of highly toxic O2 derivates, i.e., superoxide, hydroxyl radical and hydrogen peroxide, commonly called reactive oxygen species (ROS) [8]. In the relative absence of O2 and under the strong reducing conditions that existed on the primordial Earth’s surface, ancient unicellular life forms relied on metabolic pathways that use electron acceptors such as CO2 and SO4 [9]. Oxygen (O2) is a much more effective electron acceptor resulting in the largest free energy release per electron transfer, yielding about fourfold more energy per molecule of oxidized glucose as compared to the most efficient anaerobic pathways [10]. Still, many enzymes involved in aerobic metabolism have remained O2/redox sensitive, which is especially the case for those containing iron-sulfur (Fe–S) clusters and/or cysteine residues [11–13]. In part, organisms have solved this problem by evolving efficient Fe–S cluster biogenesis machinery that allows for rapid reactivation of these enzymes during oxidative stress conditions [14]. Next to that, signaling and regulation processes can benefit from redox-sensitive proteins. Systematic analysis of the evolution of enzymes and the development of biochemical networks revealed that O2 is one of the most utilized molecules, superseding NAD and ATP [8], although it constitutes a major threat to cell viability by the inevitable generation of ROS. Reactive oxygen species are toxic in nature, however, in the past decade, it has become apparent that, in addition, they act as indispensable signaling molecules for numerous developmental and adaptation processes in plants, animals, bacteria and fungi [15–18].
Since the oxygenation of the atmosphere occurred at a time during which mainly unicellular organisms populated the Earth, the adaptation to oxidative stress has evolved alongside with organism complexity. ROS by-products are released from a whole palette of aerobic reactions, and therefore cells needed to be able to monitor and control these toxic products during the adaptation towards aerobic growth. The development of complex cells and multicellularity brought with it a requirement for more sophisticated signaling systems to enable communication between different organelles and cell types. In contrast to the classical animal and plant hormones, ROS might represent an ancient shared signal between kingdoms. If this is true, the ways in which ROS are received and through which they affect cellular functioning and development should partially be conserved at the mechanistic level. In this review, we aim to provide evidence for a conserved role of ROS during the development of bacteria, fungi, animals and plants.
Ancient oxygenic minimal signal transduction systems
A common theme for aerobic life forms is the evolution of various ROS-responsive systems. Although O2 is the most energetic electron acceptor, many unicellular organisms are only facultative aerobes. With some exceptions, eukaryotes depend on O2 respiration and organic carbon as fuel, whereas prokaryotes use a whole array of electron acceptors and energy sources [9]. Therefore, it is not surprising that eukaryotes have more sophisticated systems to not only signal but also actively generate ROS such as superoxide during signaling responses to a variety of environmental factors or developmental cues. The simplest signaling system involves the direct interaction of the signal with a transcriptional regulator, the so-called one-component system (Fig. 1). In fact, the best understood ROS-responsive transcription factors (TFs) in terms of their molecular mechanism are those from bacteria and fungi [19]. In Escherichia coli, the helix-turn-helix (HTH) OxyR TF is directly oxidized by hydrogen peroxide to form an intramolecular disulfide bond, resulting in the transcriptional activation of hydrogen peroxide-responsive genes [20, 21]. Similarly, the HTH OhrR transcriptional repressor in Bacillus subtilis is oxidized by peroxides at a single cysteine residue within its DNA binding domain releasing it from the target promoter. To date, the vast majority of ubiquitous so-called “single-Cys” and “two-Cys” redox sensors identified in bacteria are known or projected to essentially function in this way [22]. Bacteria also contain transcriptional superoxide sensors, with the E. coli HTH SoxR TF being the best characterized protein that is activated by oxidation of Fe–S clusters [23]. SoxR activates the expression of the gene SoxS in response to exposure to superoxide-generating agents which leads to increased expression of several antioxidant genes, e.g., superoxide dismutase and glucose-6-phosphate dehydrogenase, which provide reducing equivalents in the form of NADPH. The yeast bZIP domain containing TF AP-1-like transcription factor 1 (Yap1) represents the first H2O2-sensing mechanism in eukaryotes. Hydrogen peroxide activates the TF by oxidation of two cysteine residues resulting in the induction of antioxidant genes during oxidative stress [24]. The mammalian NF-E2-related factor 2 (Nrf2) bZIP TF is redox-regulated and a known inducer of antioxidant genes by directly binding to the antioxidant responsive element (ARE) in the promoter of target genes, which was originally described as the binding site for activator protein-1 (AP-1) [25, 26]. Activator protein-1 is a transcription factor complex that consists of either a Jun–Jun homodimer or a Jun–Fos heterodimer. Both forms induce the expression of a diverse range of genes involved in proliferation, differentiation and defense. Although no direct homologous of OxyR, SoxR or Yap1 proteins have been found in plants, the known redox sensing mechanisms are conserved between species albeit different molecular players are involved.
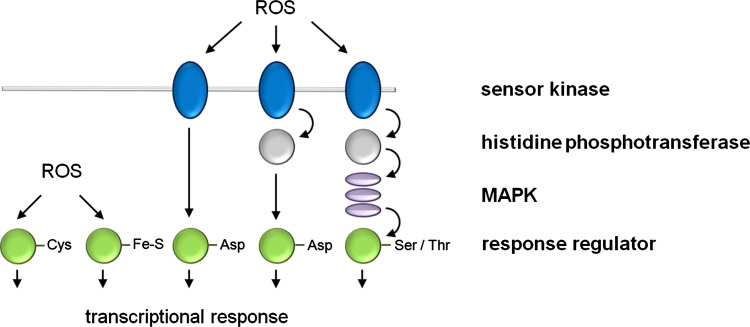
Fig. 1.
Schematic overview of the one-component and two-component signal transduction paradigm. The simplest transduction system is the one-component system (left) which is mainly found in unicellular organisms and, e.g., includes the OxyR and SoxR TFs, depicted as response regulators (RR) whose cysteine residue or Fe–S cluster, respectively, is oxidized by ROS. In the classical two-component pathway (left), the HK domain of the sensor kinase autophosphorylates and subsequently transfers a phosphoryl group to the aspartate residue of the RR. The sensory domain connected to the HK domain varies widely to allow receiving the various signals. Phosphorelay systems (second from right) are a common extension of the minimal two-component signaling cascade which in plants is involved in cytokinin signaling. Perception of a stimulus activates autophosphorylation and the phosphoryl group is intramolecularly passed to a C-terminal receiver domain. Subsequently, an additional histidine phosphotransferase (HPT) shuttles the phosphoryl group from the hybrid kinase to a soluble RR protein. Alternatively, as in plant-specific ethylene signaling or in some other higher eukaryotic organisms (rats), the phosphorelay feeds forward onto the MAPK cascade (right), which subsequently activates an RR protein via Ser/Thr residues. Taken together, one- and two-component pathways enable cells to sense and respond to stimuli by inducing changes in transcription
Interestingly, in Arabidopsis thaliana, the redox-regulated NONEXPRESSOR OF PATHOGENESIS-RELATED PROTEINS 1 (NPR1) is present in an inactive oligomeric form established through intermolecular disulfide bonds which are released upon perturbation of the redox balance [27]. NPR1 functions as a transcriptional coregulator that, upon activation, localizes to the nucleus and interacts with members of the D group of Arabidopsis bZIP factors (TGA) [28]. Moreover, the TGA factors bind the oxidative stress and salicylic acid responsive as-1 cis-element found in promoters of glutathione transferases and other antioxidant genes [29]. Interestingly, both the mammalian ARE motif and the plant as-1 element share a TGAC core sequence which is the characteristic binding site for a subset of bZIP TFs [25, 29]. In addition, TFs of the plant-specific oxidative stress-responsive WRKY family can bind to a cis-element with a TGAC core motif [30]; however, no evidence for redox regulation of these TFs has so far been demonstrated [31].
Due to the simplicity of the one-component TFs, it is unlikely that they are sufficient to fully control gene expression in the highly compartmentalized eukaryotic cell. Especially for the transduction of signals from the extracellular to the intracellular space, other signaling cascades are required to act upstream of the transcription factors. Similar to WRKY proteins in plants, Fos and Jun in animal cells are targets of the mitogen-activated protein kinase (MAPK) cascade and are stabilized and activated by phosphorylation [32, 33].
Although the MAPK cascade is a well-defined integrator of ROS signals in plants, yeast and animals it is not present in prokaryotes [34–36]. In contrast, transmembrane signal transduction represents a common feature of all eukaryotic and prokaryotic cells [37]. As MAPK cascades act as mediators between sensor proteins and effector proteins, they likely evolved at a later stage in evolution. A more ancient evolutionary conserved signaling pathway is the two-component system which consists of a sensor histidine kinase (HK) and a response regulator (RR) with a DNA-binding domain (Fig. 1) [38]. In complex organisms, one or more phospho-relay system(s) and/or MAPK cascade(s) expanded the original HK-RR system, indicating that mainly the signaling mechanisms rather than individual proteins are conserved. In the case of phospho-relay, the HK autophosphorylates and transfers the phosphoryl group to an internal receiver domain rather than to a separate RR protein. Subsequently, the phosphoryl group is shuttled to a histidine phosphotransferase (HPT) and finally to a terminal RR protein (Fig. 1). The His–Asp phosphorelay systems were originally thought of as being exclusive to prokaryotes [39]; however, it was shown in the 1990s that fungi and plants also use these systems [40, 41]. Moreover, signaling in eukaryotes is not limited to His–Asp phosphotransfer as seen in the high-osmolarity glycerol (HOG) pathway in yeast (Saccharomyces cerevisiae and S. pombe), which forwards its signal to the MAPK cascade pathway [42]. The majority of bona fide two-component elements in plants play a central role in cytokinin signaling and involve HKs, HPTs and RRs [43]. The Arabidopsis genome encodes nine HKs, of which three, AHK2, AHK3 and AHK4, have been implicated in cytokinin signaling [44]. While the cytokinin receptors are located at the endoplasmic reticulum, AHK5 localizes to the plasma membrane and has been shown to be involved in H2O2-dependent closure of stomata [45]. Besides AHK5, ETHYLENE RESPONSE 1 (ETR1) contains an HK domain and functions in H2O2 signaling [46]. Though ETR1 does provide HK activity, such activity does not appear to be necessary for ethylene responses [46]. Two-component signaling systems, which in part also feed forward onto the MAPK cascade, have been confirmed as H2O2 sensors in yeast [47–50]. Here, the SLN1 transmembrane protein is part of a typical two-component signal transduction system that senses environmental changes leading to the activation of its HK domain. The phosphoryl group is transferred to a histidine residue in Ypd1p, a small cytoplasmic protein, and subsequently transferred to an aspartate residue in the receiver domain of the RR protein SSK1 [47]. Loss of the SLN1–SSK1 system results in H2O2 susceptibility (not other oxidants) that can be relieved by introducing ETR1, indicating conserved mechanisms for sensing hydrogen peroxide among yeast and plants [46]. Also, in cyanobacteria, HKs functioning as H2O2 sensors have been found [51]. In conclusion, the two-component system appears to be a conserved strategy for the perception of ROS in plants, fungi and bacteria. Animals lack HK two-component-like sensors, but nevertheless, histidine phosphatases, which are involved in the cytokinin signaling pathway in plants [44], and histidine–aspartate phosphorelay mechanisms as in E. coli [52] have been isolated in rats [53].
Interestingly, the potential plant ROS sensors overlap with known signaling pathways for phytohormones like salicylic acid, cytokinin and ethylene, suggesting that ROS is interconnected with these development-regulating hormones.
Role of ROS during cell proliferation and differentiation
During the evolution of multicellularity, the omnipresent oxygen radicals were potentially harnessed as second messengers coordinating cellular metabolism and growth [53]. The major advantage of redox sensing is the ability to respond rapidly and adequately to environmental stimuli and to integrate all cellular metabolic activities [54]. This is exemplified in the social amoeba Dictyostelium discoideum, which straddles the boundary between a unicellular and a multicellular organism. At the onset of its development shift from unicellular to multicellular, D. discoideum utilizes superoxide as a signaling intermediate [19]. Cellular decision-making, environmental sensing and cell-to-cell communication are key processes underlying pattern formation and development from microbes to plants and mammals. Note that the term “cellular decision-making” may be misleading, as the decisions actually occur at the level of gene regulatory networks such as the ones controlled by outputs of the one-component and two-component systems [55]. Cells merely provide microscopic meeting places for the real key players: genes connected into regulatory networks [56]. Cellular decision-making is frequently based on communication networks with multiple nested feedback loops. Studying the dynamics of the multiple feedback loops and their role in differentiation and development has revealed novel insights, as recently shown for plants and microbes [57–59]. Since the expression of many genes is affected by redox changes irrespective of the organism, ROS appear to have progressed as important inputs and outputs of cellular decision-making [60, 61].
One crucial step for life is reproduction, which in its most basic form is represented by a mother cell dividing into two daughter cells. In human cells, it has been found that very low levels of H2O2 or superoxide stimulate the proliferation of smooth muscle cells [62, 63], macrophages [64], and vascular endothelial cells [65], among others (Fig. 2). In addition, overexpression of catalase and/or superoxide dismutase, which lower H2O2 or superoxide levels, respectively, inhibit proliferation of vascular smooth muscle cells in response to epidermal growth factor (EGF), which is accompanied by a reduction in extracellular-signal-regulated kinase (ERK) 1 and 2 phosphorylation events [66, 67]. However, higher concentrations of hydrogen peroxide can temporarily arrest growth, followed by a transient adaptive response at the expression level for oxidant protection and DNA repair [68]. At even higher hydrogen peroxide concentrations, mammalian fibroblasts are not able to adapt; instead, they enter a state resembling cellular replicative senescence or die in an organized manner resulting in apoptosis [68]. In recent years, the implications of ROS on the cell cycle have been further refined by the discovery of a redox-dependent signaling pathway that controls cell cycle proteins [69]. Distribution of specific ROS acts as an important signal at the mRNA and protein levels during cell cycle progression in animal cells [70], both in healthy tissues and during disease [71]. In mammalian cells, a transient increase in cellular oxidant levels in the G1 phase [70] is required for entry into the S phase, which is also observed for plant cells [72]. Also, in yeast, a redox cycle that links the metabolic cycle with the budding phase has been discovered [73].
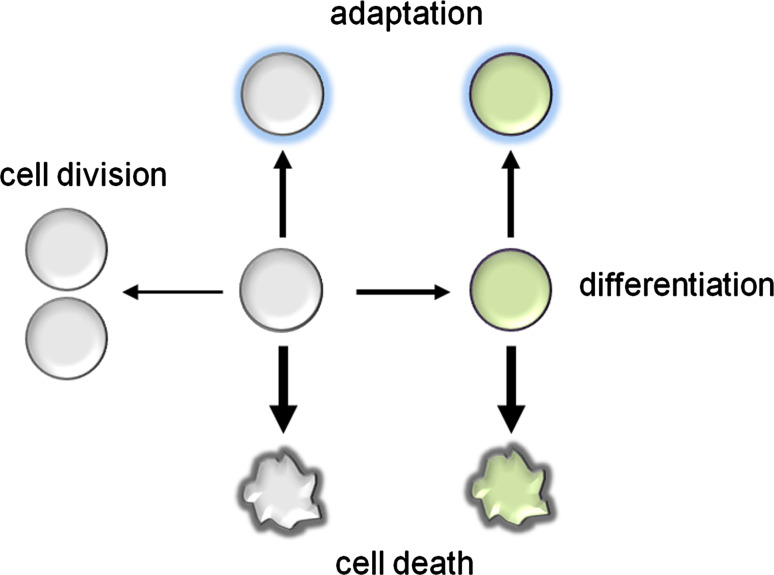
Fig. 2.
Output of ROS on cellular proliferation and differentiation, depending on the strength and type of the signal. A transient increase in ROS levels stimulates cell division, while a higher level can result in cell differentiation. Even higher levels, as experienced during cellular stress, will result in growth inhibition and subsequent adaptation. However, when ROS levels further increase and cells can no longer adapt, they either go into a state resembling cellular replicative senescence or die in an organized manner resulting in cell death. The strength of the ROS signal is indicated by the weight of the arrow
The finding that ROS homeostasis regulates the transition from proliferation to differentiation has been nicely demonstrated by Benfey and co-workers in the root of the model plant species Arabidopsis thaliana (Fig. 2) [74]. Superoxide and H2O2 are differentially distributed within a plant root [75], while superoxide mainly accumulates in dividing and expanding cells of the meristem and H2O2 accumulates in the elongation zone; an overlap of both ROS types is observed within the so-called “transition zone”. The basic helix-loop-helix (bHLH) transcription factor UPBEAT1 (UPB1) shows increased expression in the root transition zone and directly represses the expression of peroxidase genes. Loss of UPB1 results in longer roots, while overexpression decreases root organ size. The elongation zone of upb1 mutant roots shows decreased and that of UPB1 overexpressor roots increased levels of H2O2. In contrast, superoxide increased in the elongation zone in upb1 roots and was reduced in plants overexpressing UPB1. Taken together, the position of the transition zone is determined by the coincidence of the gradients formed by H2O2 in the elongation zone (required for differentiation) and by superoxide in the meristem (to maintain cellular proliferation) [74]. The functional characterization of UPB1 revealed the first insight into the gene regulatory networks that link ROS signals with the developmental program in plants. Of note, ROS not only guide cell differentiation in plants: in Drosophila melanogaster, ROS prime hematopoietic progenitors for differentiation [76]. Multipotent hematopoietic progenitor cells show increased basal levels of ROS under physiological conditions, which are lowered upon differentiation. Decreasing ROS levels by scavengers retards their differentiation while treatment with moderate ROS levels triggers precocious differentiation into all three mature blood cell types found in D. melanogaster. Reactive oxygen species have long been known to induce apoptosis and negatively influence neuronal cell fate; intriguingly, they appear to be essential during neurogenesis [16]. Although the exact mechanisms involved in ROS-mediated neurogenesis remain unclear, it has been established that endogenously produced H2O2 is perceived by a MAPK pathway resulting in the activation of redox-sensitive TFs [16]. In the fungus Neurospora crassa, a hyperoxidant state occurs at the start of each cell differentiation step leading to conidia development [77]. The hyperoxidant state is defined as an unstable, transient state in which ROS surpass the antioxidant capacity of the cell [78]. It is known that, in several of the examples mentioned above, antioxidants inhibit development, which is consistent with the idea that ROS are required for the developmental process to occur. On the other hand, it is expected that a lack of antioxidant enzymes would result in higher ROS levels and precocious or enhanced cell differentiation.
The process of cell death induced by very high ROS levels is an active process in many species, and often part of their developmental and defense program (Fig. 2). Interestingly, the cell death response is conserved between unicellular and multicellular organisms, although its appearance comes in many flavors. In animals, the cell death types are divided into two categories: apoptosis and necrosis [79]. Discrimination between these two categories is based on the presence or absence of specific biochemical and molecular hallmarks, such as DNA laddering, cytochrome c release, caspase involvement, ATP depletion, cytoplasmic swelling, and loss of membrane integrity [80]. Next to eukaryotes, even bacteria harness ROS to undergo programmed death by activating the suicide module mazEF [81]. The reason for single cell organisms to commit suicide lies in the fact that most of them live in large colonies that may act as a multicellular organism [55]. Apoptosis-like elimination of defective cells in S. cerevisiae suggests that all unicellular life forms have evolved altruistic programmed death that serves a variety of useful functions [82]. In plants, the process of cell death is either passive (necrotic cell death) or active (programmed cell death), and it has been shown to be caused by high ROS levels during development [83].
Taken together, from the above reasoning, it becomes clear that, in unicellular and multicellular organisms, ROS appear to act in a similar manner on development. At a first glance, ROS seem to be rather unspecific regulators; however, to distinguish the message contained within the ROS signal four types of ‘information packaging’ evolved, in addition to the identity of the ROS involved, the intensity of the signal (burst), its duration, and the (sub)-cellular location are important.
Conservation of ROS producing and scavenging proteins and their role in development
As indicated above, ROS-employing signaling networks influencing development exist in distant species. To further explore this common theme, we highlight below several classes of ROS-related proteins that appear to be present in all kingdoms and to play essential roles during development.
Thioredoxins and glutaredoxins
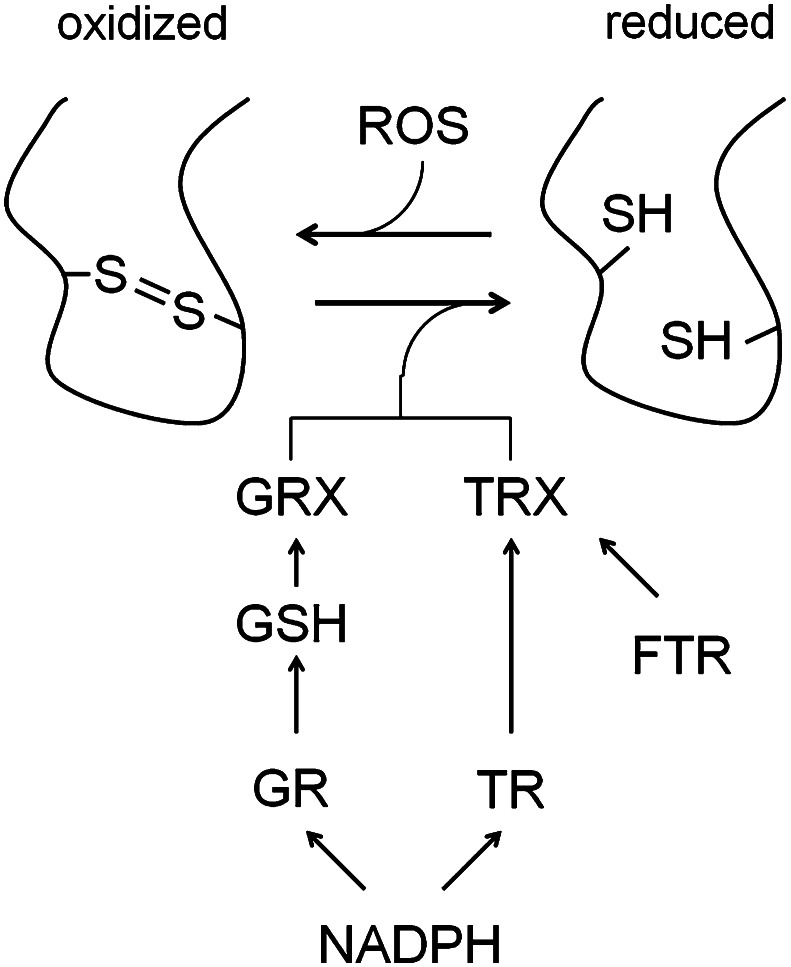
The thioredoxin (TRX) super family is characterized by a TRX fold and catalyzes oxidoreductase reactions by a dithiol/disulfide exchange mechanism involving two redox-active cysteine residues. Besides thioredoxins, the family of TRX fold proteins also includes glutaredoxins (GRXs); both types of proteins are considered as reductants and can be found in bacteria, fungi, mammals and plants [84]. The major function of TRXs and GRXs is to reduce disulfide bonds within target proteins [85]. Oxidized target proteins can be monomeric, homo- or hetero-dimeric and possibly glutathionylated proteins [84, 86]. Although TRXs and GRXs have similar structures and activities, they show little similarity regarding their amino acid sequence. Furthermore, GRXs are non-enzymatically reduced by glutathione (GSH), whereas TRXs are enzymatically reduced by TRX reductases (TR) (Fig. 3). E. coli encodes two TRX, three GRX and two GRX-like proteins, which are individually dispensable, whereas GRX4 is involved in Fe–S cluster assembly in E. coli and yeast [84, 87, 88]. Interestingly, lethality caused by simultaneous inactivation of TR and glutathione reductase in E. coli can be rescued by dithiothreitol treatment [89]. As mentioned before, the function of redox sensing is to adjust growth and development which in turn depend on metabolism. In E. coli, it was found that TRX and GRX proteins are involved in many housekeeping reactions, e.g., the synthesis of deoxyribonucleotides by activation of ribonucleotide reductase (RNR) [90] and sulfate assimilation by interacting with 3′-phosphoadenosine-5′-phosphosulfate reductase (PAPS) [91]. Furthermore, oxidative damage repair via methionine sulfoxide reductase (MSR), that rescues proteins whose methionine residues have been oxidized to the sulfoxide state (MetSO) [92], depends on TRX. Moreover, it was shown that plastidial MSR activity allows for maintaining vegetative growth of plants during environmental constraints through the preservation of photosynthetic antennae [93]. Furthermore, the OxyR one-component sensor described above is inactivated by TRXs and GRXs when oxidative stress is alleviating [94]. Yeast (S. cerevisiae and S. pombe) provides a similar set of TRX, GRX and GRX-like proteins as E. coli, but one member of each TRXs and GRXs is localized in mitochondria and the GSH-dependent system is essential [84].
Fig. 3.
Two common thiol redox pathways that set redox homeostasis in cells. Under oxidative conditions, disulfide bonds are formed within proteins that can either activate or inactivate them. The major pathways for reducing the disulfide bonds in proteins rely on thioredoxins (TRX) or glutaredoxins (GRX). GRXs are reduced non-enzymatically by glutathione (GSH), whereas TRXs are reduced enzymatically by TRX reductase (TR). Oxidized GSH is recycled by glutathione reductase (GR). Both, TR and GR use NADPH as a substrate during the reduction reactions, except for the plastidial TR proteins which rely on ferredoxin (FTR) instead of NADPH for reduction
Vertebrate genomes encode at least one cytosolic and one mitochondrial set of TRX, GRX and GRX-like proteins [84]. Similar to yeast and bacteria, TRX function is essential in animals as indicated by embryo lethality upon loss of TRX1 in mice [96]. In addition, some targets of the system are also conserved between the kingdoms, including RNR and MRS [97]. Peroxiredoxins (PRXs) represent another group of common target proteins potentially functioning as H2O2 sensors and scavengers which are themselves dependent on the TRX and/or GRX systems as discussed below [84, 95]. Furthermore, the activation of the AP-1 TFs (Fos and Jun) that bind and recognize the ARE element is partially regulated by this redox system [25, 98]. Finally, the TRX and GRX systems are conserved in plants but operate mechanistically different. First of all, the eukaryotic plant cell contains plastids and mitochondria, adding an additional layer of information processing to the system. The reduction of TRX in the cytosol and mitochondria of plant cells is performed by a bacterium-like NADPH-dependent TRX reductase (NTR), while in plastids the reduction is carried out by ferredoxin [99]. Plants have very complex thioredoxin systems, as revealed by sequencing of the Arabidopsis genome resulting in approximately 40 genes encoding putative TRX and TRX-related proteins. NADPH-dependent TRX reductases are key regulatory enzymes determining the redox state of the thioredoxin system. Arabidopsis encodes at least two NTRs (NTRA and NTRB). Surprisingly, the double knock-out mutant ntra ntrb is viable and fertile [100]. Thus, in contrast to the other biological systems, neither cytosolic nor mitochondrial NTRs are essential in plants. It has been shown that a glutathione-dependent pathway for reducing TRX is utilized in NTRA/B-deficient plants. Therefore, in contrast to bacteria and animals, in which the NTR/TRX and GSH/GRX systems operate separately, these pathways are interconnected in the cytosol of cells of land plants [84]. That GSH has a major role in plant development became evident after map-based cloning of the ROOT MERISTEMLESS 1 (RML1) gene, which encodes γ-glutamylcysteine synthetase (γ-ECS) that mediates the first step in glutathione biosynthesis [101]. Within the root meristem, the cell cycle gets blocked in the G1 phase, indicating a requirement for GSH during cell proliferation in plants. Later studies in both animals and plants demonstrated that GSH is specifically recruited into the nucleus during the early phase of cell proliferation to adjust whole-cell redox homeostasis [102, 103]. Crossing ntra ntrb with rml1 resulted in the complete inhibition of both shoot and root growth, indicating that at least NTR or the glutathione pathway is required for postembryonic activity in the apical meristem [100]. In the search for Arabidopsis mutants impaired in cell-to-cell transport via plasmodesmata (PD), the seedling lethal mutant gfp-arrested trafficking (gat1) was identified, which affects unloading of GFP from the phloem into the root meristem causing a plant size reduction [104]. GAT1 encodes the plastidial thioredoxin TRX-m3. Its effect on cellular redox status seems to play a role in the regulation of PD permeability, as indicated by wild-type plants subjected to oxidative conditions that phenocopied the gat1 trafficking defects. Most TRX proteins are specifically localized in plastids and encoded by nine small gene families in Arabidopsis: TRX-m, TRX-f, TRX-x, TRX-y, CDSP32, HCF164, WCRKC, APR, and Lilium with four, two, one, two, one, one, two, three, and five members, respectively [84]. So far, only TRX-o1 has been shown to be specific for mitochondria, while another ten members lacking a transit peptide are heterotrophically localized (h-type) [84]. The importance of plastidial TRXs lies in the fact that they regulate carbon metabolism during the diel cycle. To survive at night, chloroplasts derive energy from the breakdown of carbohydrates, in part via the oxidative pentose cycle [105]. During the day, glucose 6-phosphate dehydrogenase, the enzyme catalyzing the first step of the oxidative pentose phosphate pathway, is deactivated by TRXs [106]. Recently, it was demonstrated that chloroplastic TRXs are circadian regulated through the core clock, which might be related to their redox regulation of light-dependent protein targets [107]. Still, one of the most interesting proteins that depends on TRXs for its activity is the transcription factor PHAVOLUTA (PHV) which is a central regulator of plant development by controlling radial patterning [108, 109]. PHV only binds to DNA under reducing conditions. The protein contains a CxxxC motif that has been implicated as a redox-switch [110]. Worthy of note is the fact that the two cysteines involved in redox modulation are conserved in all members of the HD-Zip III family to which PHV belongs and which are known for their role in plant development [109]. Therefore, it would be of great interest to further analyze the redox regulation of this kind of TFs in relation to plant growth. The NPR1 activation through monomerization (see above) is achieved by the activity of TRX-h3 and TRX-h5 in the cytosol [111]. Thus, NPR1 represents a genuine target of the ROS sensors. The number of GRX genes is particularly high in plants, with at least 31 representatives in, e.g., Arabidopsis [112], which are tightly linked to the GSH network. GSH is a highly abundant antioxidant that can be present in millimolar concentrations in eukaryotic cells. A picture has emerged in the past that shows a role of GRXs in plant development. A screen for floral development mutants in Arabidopsis revealed a mutant defective in the initiation of petal primordia and subsequent development called roxy1 [113]. ROXY1 encodes a plant-specific CC-type GRX, 1 of the 21 members of the CC-type family [114]. Subsequently, the authors looked at the closest homologue of ROXY1, i.e., ROXY2, which does not cause an obvious developmental phenotype when knocked out. However, the double roxy1 roxy2 mutant is unable to develop normal anthers and lacks pollen [115]. The ROXY proteins hold another surprise: protein–protein interaction studies identified the TGA bZIP TFs as target proteins [116]. Interestingly, one of these interacting TGA factors is PERIANTHIA (PAN), which when mutated causes a characteristic transformation of tetrameric to pentameric flowers [117]. Moreover, PAN directly binds to the promoter of the MADS box transcription factor AGAMOUS (AG) to enhance its expression, indicating that redox-controlled growth has to be implicated in the ABC model of floral development [118]. TGA9 and TGA10 are two redundant factors that interact with ROXY1 and ROXY2. They play an essential role in anther development, but act downstream of the MADS-box-like TF SPOROCYTELESS/NOZZLE (SPL/NZZ) [119]. The cytosolically located class I GRX proteins are essential for plant development as indicated by the double mutant grxc1 grxc2, which is embryo lethal [120]. GRXS17 in Arabidopsis regulates polar auxin transport and thereby controls root development [121]. Root cells appeared to precociously enter differentiation, indicating that GRXS17 is required for cell cycle progression and proliferation. Next to that, loss of GRXS13 results in the accumulation of superoxide and reduced plant growth [122], indicating that the imbalance in redox homeostasis is directly translated into a growth phenotype. In conclusion, the cross-kingdom conserved GRX and TRX proteins seem to be encoded by highly specialized developmental genes in plants. This specialization became possible by the evolutionary establishment and diversification of the relative large number of family members. It will be of great interest in the coming years to identify and characterize the cellular targets regulated by each TRX and GRX.
Thiol peroxidases; peroxiredoxins and glutathione peroxidases
As indicated above, TRX and GRX proteins serve as redox transmitters within the cellular thiol/disulfide redox network to control many cellular functions [123]. Peroxiredoxins (PRX) and glutathione peroxidases (GPX) act as thiol-dependent peroxidases with high affinity for peroxide to protect protein thiols from oxidation [124]. Peroxiredoxins have a much higher affinity to H2O2 than catalases providing the specificity required for H2O2 sensing and signaling [125]. Peroxiredoxins can react with H2O2 at low “signaling” concentrations before this molecule is intercepted and degraded by other systems, which fits the function of H2O2 receptors described for thiol peroxidases in lower eukaryotes [126]. Of the thiol-based peroxidases, the PRXs are conserved across all kingdoms while the related GPXs are confined to bacteria, fungi, protozoa, insects, and plants [125]. In fission yeast, the peroxiredoxin TPX1 is essential for growth under aerobic conditions, which can be attributed to its scavenging and signaling properties [127]. TPX1 is required for the H2O2-dependent activation of the bZIP TF PAP1 at mild H2O2 levels [128]. PAP1 temporarily enters the nucleus upon oxidative stress and negatively regulates growth, and returns to the cytoplasm once cells have adapted to the stress. At a higher concentration of H2O2, TPX1 activates the MAPK-dependent pathway controlled by Sty1 [129], revealing an elegant molecular switch in which H2O2 signaling is performed by PRXs in a concentration-dependent manner. Moreover, a recent transcriptomic study of yeast (S. cerevisiae) lacking all eight thiol PRXs resulted in the lack of a transcriptional response specifically towards H2O2 [130]. This study indicates that thiol peroxidases sense oxidative signals and transfer them to signaling proteins and regulate transcription. Moreover, the direct interaction of H2O2 with other cellular proteins is of only secondary importance in yeast. Whether the transcriptional response to H2O2 is also performed by PRX proteins in other organisms remains to be demonstrated. In bacteria, the deletion of the PRX gene ahpC results in shortening of the generation time [131]. One potential reason for a reduced generation time is that the mutant defective in ROS scavenging more rapidly accumulates DNA damage and thereby loses viability. A more rapid cycling might help to compensate for the negative effect on DNA integrity. Mammalian PRXs potentially have a role in H2O2 signaling by controlling the fluxes and concentration of hydrogen peroxide which is supported by effects of PRXs on downstream signaling of growth-factor tyrosine kinases and cytokinin receptors [132]. The mouse genome encodes six PRX genes which have been studied to some extent by knock-out analysis, mainly revealing a decreased oxidative stress tolerance and an early onset of several diseases like cancer, anemia and artery problems [125]. The regulation of transcription by PRXs is still poorly understood in animal systems, but redox signaling also takes place at physiological H2O2 fluxes similar to other systems, and altering PRXs levels affect these pathways [133]. In Arabidopsis, the PRX family is encoded by ten genes while the GPX family is represented by eight genes [124]. The PRX proteins are divided into six classes with different cellular locations. ATPER1 is specifically expressed during seed development and is regulated by ABA INSENSITIVE 3 (ABI3) to potentially control dormancy [134]. Furthermore, ATPER1 expression during early embryogenesis depends on an antioxidant-responsive promoter element (ARE). Of the four PRXII genes that encode cytosolically located enzymes, none has so far been functionally characterized. PRXIIB, C and D are each 162 amino acids long and exhibit highly similar amino acid sequences with 93–99 % identity, suggesting a potentially redundant role [124]. On the other hand, the PRXIIA protein has an additional F-box domain and thereby differs from its counterparts. PRXIIE is a plastidial protein which has been shown to be involved in nitrosylation and probably functions as a scavenger of nitric oxide in the plastid stroma [135]. Similarly, mitochondrial PRXIIF is involved in protein nitration; knock-out plants show reduced root length under oxidative stress conditions [136]. Furthermore, the authors suggested a potential role of PRXIIF in redox signaling in plant cells, since many mitochondrial and nuclear transcripts showed an altered abundance. In addition, the chloroplast contains another four PRX proteins of which two belong to the 2-Cys PRX class that is essential for chloroplast metabolism and plant biomass production [124, 137]. The other two are PRXQ proteins, which are atypical 2-Cys PRX forms that function as monomeric peroxidases with high reactivity towards H2O2 and lipid peroxides [124]. Single knock-out lines of PRXQ genes in Arabidopsis exhibit no clear phenotype, while RNAi targeting of both genes results in decreased oxidative stress tolerance and altered photosynthesis [124, 138]. So far, the function of PRX in plant development is still poorly analyzed and needs additional research efforts to unravel the redox regulation as shown in yeast. The glutathione peroxidases GPX1 and GPX7 represent two chloroplast localized proteins that modulate photooxidative stress tolerance and basal plant resistance [139]. Loss of function of both genes greatly affects leaf morphology in an adaxial-specific manner, suggesting that natural photooxidative stress might help guiding leaf growth. Interestingly, GPX3 has been shown to interact with the ABI1 and ABI2 Ser/Thr phosphatases and to have a role in drought stress tolerance and development [140]. Moreover, it was demonstrated that GPX3 inhibits the PP2C activity of ABI2 in a redox-dependent manner and thereby regulates ABA signaling in Arabidopsis. This indicates that, as in yeast, GPX-like enzymes might potentially act as peroxide sensors and redox transducers in plant cells. GPX5 is essential for female gametophyte development and causes a maternal defect [141]. While the other members of the Arabidopsis GPX family remain to be analyzed in more detail, it is tempting to state that in plants a redox-sensing and output network similar to that in yeast exists.
NADPH oxidase family
Whereas the TRX and PRX proteins constitute evolutionary conserved ROS sensors, ROS producing enzymes, including the specialized respiratory burst oxidase homolog (RBOH) or NADPH oxidase (NOX) enzymes, play a central role in ROS signaling and homeostasis [142]. NADPH oxidases are located within the plasma membrane where they generate a superoxide radical by using NADPH as an electron donor. At the time of evolution, during which the unique chemical/thermodynamic properties of O2 were harnessed for aerobic respiration, cells also adopted ROS for signaling and regulation [10]. The diversity and number of NOX proteins correlate with increasing organism complexity [143]. NOX enzymes are expressed in all multicellular eukaryotes, including fungi, plants, and animals, but not in unicellular prokaryotes or lower eukaryotes like, for instance, yeast. Humans and animals in most cases have seven NOX genes, while Arabidopsis has ten predicted members [143]. On the other hand, Caenorhabditis elegans, D. melanogaster and several fungi each express two members of this gene family. Numerous studies have pointed out that NOX genes play an essential role during development in all species so far examined. In plants, the first indication for a role of NOX genes in development derived from the identification of the rhd2 mutant lacking a functional RBOHC gene, which is essential for root hair cell expansion [144]. Similarly, NADPH oxidase activity is required for pollen tube growth [145]. Others like RBOHB, RBOHD and RBOHF appear to be involved in a multitude of processes, including pathogen response, systemic signaling, lignification and seed ripening [146]. However, due to functional redundancy, it is difficult to pinpoint specific functions for each of the ten RBOH proteins in Arabidopsis. One of the challenges is the identification of downstream signaling or effector proteins that mediate the ROS signal triggered by the RBOH proteins. In mammals, it has been put forward that PRX proteins are potentially involved in mediating the signaling downstream of NOX proteins [147].
Although ROS scavenging proteins like catalase and superoxide dismutase are highly conserved between kingdoms [148, 149], we have refrained from discussing their potential role in development here, since they do not appear to be as sensitive as, for example, PRX proteins to signal ROS [126]. However, the appearance of these proteins was certainly instrumental in protecting the cellular environment from the damaging potential of ROS and for successful embedding of mitochondria and chloroplasts into eukaryotic cells.
Perspective
Compared with a decade ago, the opinion on ROS as a negative factor has been largely adjusted. It is not surprising that ROS evolved as a major signaling component in all aerobic life forms. Their co-appearance with the evolution of complex life has made them an integral part of the transcriptionally regulated developmental program. In all organisms discussed, ROS evoke a specific transcriptional response, which does not hold up for classical hormones which only function in specific kingdoms. Moreover, as depicted in the study on yeast PRX proteins [130], active ROS sensing mediates a transcriptional response rather than passive damaging of the cell. Amazingly, many parallels appear to exist in redox sensing strategies in the different organisms, which might be due to the conservation of ancient core redox sensor mechanisms. Just recently, it has been found that oxidation–reduction cycles of peroxiredoxin proteins constitute a universal marker for circadian rhythms in all domains of life [150]. Therefore, cellular time keeping appeared to have co-evolved with redox homeostasis and the oxygenation of the world.
Although it is now clear that ROS are conserved developmental regulators, many players that sense and execute the diverse transcriptional programs still need to be uncovered. Furthermore, in plants, ROS interact with virtually all phytohormone pathways; thus, from an evolutionary point of view, it would be interesting to determine when and how these pathways started to interact. For example, NPR1 is a redox- and salicylic acid-regulated transcriptional coregulator present in all higher plants; however, so far, no ortholog has been identified in algae. Still, algae do respond to salicylic acid treatment by upregulating alternative oxidase (AOX), as do higher plants [151]. Finally, further genetic and transcriptional studies are required to unravel in detail the functions of the many TRX, GRX, PRX and GPX proteins in plants, but also other organisms, to unveil how ROS have been integrated during aerobic development.
Contributor Information
Jos H. M. Schippers, FAX: +49-331-9772512, Email: schippers@mpimp-golm.mpg.de
Bernd Mueller-Roeber, Email: bmr@uni-potsdam.de.
References
- 1.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedges SB, Blair JE, Venturi ML, Shoe JL. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol Biol. 2004;4:2. doi: 10.1186/1471-2148-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dismukes GC, Klimov VV, Baranov SV, Kozlov YN, DasGupta J, Tyryshkin A. The origin of atmospheric oxygen on earth: the innovation of oxygenic photosynthesis. Proc Natl Acad Sci USA. 2001;98:2170–2175. doi: 10.1073/pnas.061514798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkowski PG. Evolution: tracing oxygen’s imprint on earth’s metabolic evolution. Science. 2006;311:1724–1725. doi: 10.1126/science.1125937. [DOI] [PubMed] [Google Scholar]
- 6.Danovaro R, Dell’Anno A, Pusceddu A, Gambi C, Heiner I, Kristensen RM. The first metazoa living in permanently anoxic conditions. BMC Biol. 2010;6(8):30. doi: 10.1186/1741-7007-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knoll AH. The geological consequences of evolution. Geobiology. 2003;1:3–14. [Google Scholar]
- 8.Raymond J, Segrè D. The effect of oxygen on biochemical networks and the evolution of complex life. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- 9.Nealson KH, Conrad PG. Life: past, present and future. Philos Trans R Soc Lond B. 1999;354:1923–1939. doi: 10.1098/rstb.1999.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thannickal VJ. Oxygen in the evolution of complex life and the price we pay. Am J Respir Cell Mol Biol. 2009;40:507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees DC, Howard JB. The interface between the biological and inorganic worlds: iron-sulfur metalloclusters. Science. 2003;300:929–931. doi: 10.1126/science.1083075. [DOI] [PubMed] [Google Scholar]
- 12.Outten FW. Iron-sulfur clusters as oxygen-responsive molecular switches. Nat Chem Biol. 2007;3:206–267. doi: 10.1038/nchembio0407-206. [DOI] [PubMed] [Google Scholar]
- 13.Schippers JHM, Nunes-Nesi A, Apetrei R, Hille J, Fernie AR, Dijkwel PP. The Arabidopsis onset of leaf death5 mutation of quinolinate synthase affects nicotinamide adenine dinucleotide biosynthesis and causes early ageing. Plant Cell. 2008;20:2909–2925. doi: 10.1105/tpc.107.056341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lill R. Function and biogenesis of iron–sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 15.Van Norman JM, Breakfield NW, Benfey PN. Intercellular communication during plant development. Plant Cell. 2011;23:855–864. doi: 10.1105/tpc.111.082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy KA, Sandiford SD, Skerjanc IS, Li SS. Reactive oxygen species and the neuronal fate. Cell Mol Life Sci. 2012;69:215–221. doi: 10.1007/s00018-011-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horke S, Witte I, Altenhöfer S, Wilgenbus P, Goldeck M, Förstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, Teiber JF. Paraoxonase 2 is down-regulated by the Pseudomonas aeruginosa quorumsensing signal N-(3-oxododecanoyl)-l-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J. 2009;426:73–83. doi: 10.1042/BJ20091414. [DOI] [PubMed] [Google Scholar]
- 18.Thön M, Al-Abdallah Q, Hortschansky P, Brakhage AA. The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J Biol Chem. 2007;282:27259–27269. doi: 10.1074/jbc.M704298200. [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield G, Pears C. Superoxide signalling required for multicellular development of Dictyostelium . J Cell Sci. 2003;116:3387–3397. doi: 10.1242/jcs.00649. [DOI] [PubMed] [Google Scholar]
- 20.Faulkner MJ, Ma Z, Fuangthong M, Helmann JD. Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. J Bacteriol. 2012;194:1226–1235. doi: 10.1128/JB.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 22.Giedroc DP. Hydrogen peroxide sensing in Bacillus subtilis: it is all about the (metallo) regulator. Mol Microbiol. 2009;73:1–4. doi: 10.1111/j.1365-2958.2009.06752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidalgo E, Ding H, Demple B. Redox signal transduction via iron-sulfur clusters in the SoxR transcription activator. Trends Biochem Sci. 1997;22:207–210. doi: 10.1016/s0968-0004(97)01068-2. [DOI] [PubMed] [Google Scholar]
- 24.Delaunay A, Isnard AD, Toledano MB. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy NM, Kleeberger SR, Yamamoto M, Kensler TW, Scollick C, Biswal S, Reddy SP. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol Genomics. 2007;32:74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 27.Mou Z, Fan W, Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 28.Fobert PR, Després C. Redox control of systemic acquired resistance. Curr Opin Plant Biol. 2005;8:378–382. doi: 10.1016/j.pbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Garretón V, Carpinelli J, Jordana X, Holuigue L. The as-1 promoter element is an oxidative stress-responsive element and salicylic acid activates it via oxidative species. Plant Physiol. 2002;130:1516–1526. doi: 10.1104/pp.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Ishihama N, Yoshioka H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr Opin Plant Biol. 2012 doi: 10.1016/j.pbi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Ransone LJ, Verma IM. Nuclear proto-oncogenes fos and jun . Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- 33.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 34.Tena G, Boudsocq M, Sheen J. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol. 2011;14:519–529. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyce KJ, Andrianopoulos A. Ste20-related kinases: effectors of signaling and morphogenesis in fungi. Trends Microbiol. 2011;19:400–410. doi: 10.1016/j.tim.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-0925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomason P, Kay R. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J Cell Sci. 2000;113:3141–3150. doi: 10.1242/jcs.113.18.3141. [DOI] [PubMed] [Google Scholar]
- 38.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci. 2001;26:369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson JS. Signal transduction schemes of bacteria. Cell. 1993;73:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 40.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 41.Yamada H, Hanaki N, Imamura A, Ueguchi C, Mizuno T. An Arabidopsis protein that interacts with the cytokinin-inducible response regulator, ARR4, implicated in the His–Asp phosphorylay signal transduction. FEBS Lett. 1998;436:76–80. doi: 10.1016/s0014-5793(98)01103-x. [DOI] [PubMed] [Google Scholar]
- 42.Wurgler-Murphy SM, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]
- 43.Schaller GE, Shiu SH, Armitage JP. Two-component systems and their co-option for eukaryotic signal transduction. Curr Biol. 2011;21:R320–R330. doi: 10.1016/j.cub.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 44.Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T. The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 2001;42:1017–10123. doi: 10.1093/pcp/pce127. [DOI] [PubMed] [Google Scholar]
- 45.Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, Grefen C, Cheung MK, Meixner AJ, Hooley R, Neill SJ, Hancock JT, Harter K. The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One. 2008;3(6):e2491. doi: 10.1371/journal.pone.0002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desikan R, Hancock JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ. A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol. 2005;137:831–834. doi: 10.1104/pp.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Hall AE, O’Malley R, Bleecker AB. Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA. 2003;100:352–357. doi: 10.1073/pnas.0237085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh KK. The Saccharomyces cerevisiae Sln1p–Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic Biol Med. 2000;29:1043–1050. doi: 10.1016/s0891-5849(00)00432-9. [DOI] [PubMed] [Google Scholar]
- 49.Buck V, Quinn J, Soto Pino T, Martin H, Saldanha J, Makino K, Morgan BA, Millar JB. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell. 2001;12:407–419. doi: 10.1091/mbc.12.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Day AM, Veal EA. Hydrogen peroxide-sensitive cysteines in the Sty1 MAPK regulate the transcriptional response to oxidative stress. J Biol Chem. 2010;285:7505–7516. doi: 10.1074/jbc.M109.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanesaki Y, Yamamoto H, Paithoonrangsarid K, Shoumskaya M, Suzuki I, Hayashi H, Murata N. Histidine kinases play important roles in the perception and signal transduction of hydrogen peroxide in the cyanobacterium, Synechocystis sp. PCC 6803. Plant J. 2007;49:313–324. doi: 10.1111/j.1365-313X.2006.02959.x. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara M, Mizuno T. The SixA phospho-histidine phosphatase modulates the ArcB phosphorelay signal transduction in Escherichia coli . FEBS Lett. 2000;470:118–124. doi: 10.1016/s0014-5793(00)01303-x. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Jiang YY, Kim KM, Qu G, Ji HF, Mittenthal JE, Zhang HY, Caetano-Anollés G. A universal molecular clock of protein folds and its power in tracing the early history of aerobic metabolism and planet oxygenation. Mol Biol Evol. 2011;1:567–582. doi: 10.1093/molbev/msq232. [DOI] [PubMed] [Google Scholar]
- 54.Oh JI, Kaplan S. Redox signaling: globalization of gene expression. EMBO J. 2000;19:4237–4247. doi: 10.1093/emboj/19.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balázsi G, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–925. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawkins R. The selfish gene (30th anniversary edition) New York: Oxford University Press; 2006. [Google Scholar]
- 57.Kushwah S, Jones AM, Laxmi A. Cytokinin interplay with ethylene, auxin, and glucose signaling controls Arabidopsis seedling root directional growth. Plant Physiol. 2011;156:1851–1866. doi: 10.1104/pp.111.175794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwari A, Balázsi G, Gennaro ML, Igoshin OA. The interplay of multiple feedback loops with post-translational kinetics results in bistability of mycobacterial stress response. Phys Biol. 2010;7:036005. doi: 10.1088/1478-3975/7/3/036005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray JC, Igoshin OA. Adaptable functionality of transcriptional feedback in bacterial two-component systems. PLoS Comput Biol. 2010;6:e1000676. doi: 10.1371/journal.pcbi.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vandenbroucke K, Robbens S, Vandepoele K, Inzé D, Van de Peer Y, Van Breusegem F. Hydrogen peroxide-induced gene expression across kingdoms: a comparative analysis. Mol Biol Evol. 2008;25:507–516. doi: 10.1093/molbev/msm276. [DOI] [PubMed] [Google Scholar]
- 61.Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152:1484–1500. doi: 10.1104/pp.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 63.Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arana L, Gangoiti P, Ouro A, Rivera IG, Ordoñez M, Trueba M, Lankalapalli RS, Bittman R, Gomez-Muñoz A. Generation of reactive oxygen species (ROS) is a key factor for stimulation of macrophage proliferation by ceramide 1-phosphate. Exp Cell Res. 2012;318:350–360. doi: 10.1016/j.yexcr.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Iruthayanathan M, O’Leary B, Paul G, Dillon JS. Hydrogen peroxide signaling mediates DHEA-induced vascular endothelial cell proliferation. Steroids. 2011;76:1483–1490. doi: 10.1016/j.steroids.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Brown MR, Miller FJ, Jr, Li WG, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, Weintraub NL. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res. 1999;85(6):524–533. doi: 10.1161/01.res.85.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi M, Yang H, Motley ED, Guo Z. Overexpression of Cu/Zn-superoxide dismutase and/or catalase in mice inhibits aorta smooth muscle cell proliferation. Am J Hypertens. 2004;17:450–456. doi: 10.1016/j.amjhyper.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 68.Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999;48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 69.Burch PM, Heintz NH. Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal. 2005;7:741–751. doi: 10.1089/ars.2005.7.741. [DOI] [PubMed] [Google Scholar]
- 70.Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–1109. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- 71.Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM. Common regulatory themes in meristem development and whole-plant homeostasis. Curr Opin Plant Biol. 2007;10:44–51. doi: 10.1016/j.pbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z, Odstrcil EA, Tu BP, McKnight SL. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity. Science. 2007;316:1916–1919. doi: 10.1126/science.1140958. [DOI] [PubMed] [Google Scholar]
- 74.Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 2007;174:332–341. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 76.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peraza L, Hansberg W. Neurospora crassa catalases, singlet oxygen and cell differentiation. Biol Chem. 2002;383:569–575. doi: 10.1515/BC.2002.058. [DOI] [PubMed] [Google Scholar]
- 78.Toledo I, Rangel P, Hansberg W. Redox imbalance at the start of each morphogenetic step of Neurospora crassa conidiation. Arch Biochem Biophys. 1995;319:519–524. doi: 10.1006/abbi.1995.1326. [DOI] [PubMed] [Google Scholar]
- 79.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pennell RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hazan R, Sat B, Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J Bacteriol. 2004;186:3663–3669. doi: 10.1128/JB.186.11.3663-3669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis K. Programmed death in bacteria. Microbiol Mol Biol Rev. 2000;64:503–514. doi: 10.1128/mmbr.64.3.503-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Doorn WG. Classes of programmed cell death in plants, compared to those in animals. J Exp Bot. 2011;62:4749–4761. doi: 10.1093/jxb/err196. [DOI] [PubMed] [Google Scholar]
- 84.Meyer Y, Belin C, Delorme-Hinoux V, Recihheld JP, Riondet C. Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, cross talks and functional significance. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4327. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 86.Zaffagnini M, Bedhomme M, Lemaire SD, Trost P. The emerging roles of protein glutathionylation in chloroplasts. Plant Sci. 2012;186:86–96. doi: 10.1016/j.plantsci.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Iwema T, Picciocchi A, Traore DA, Ferrer JL, Chauvat F, Jacquamet L. Structural basis for delivery of the intact [Fe2–S2] cluster by monothiol glutaredoxin. Biochemistry. 2009;48:6041–6043. doi: 10.1021/bi900440m. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Mapolelo DT, Dingra NN, Naik SG, Lees NS, Hoffman BM, Riggs-Gelasco PJ, Huynh BH, Johnson MK, Outten CE. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe–2S] cluster with cysteinyl and histidyl ligation. Biochemistry. 2009;48:9569–9581. doi: 10.1021/bi901182w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Faulkner MJ, Veeravalli K, Gon S, Georgiou G, Beckwith J. Functional plasticity of a peroxidase allows evolution of diverse disulfide-reducing pathways. Proc Natl Acad Sci USA. 2008;105:6735–6740. doi: 10.1073/pnas.0801986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boal AK, Cotruvo JA, Jr, Stubbe J, Rosenzweig AC. Structural basis for activation of class Ib ribonucleotide reductase. Science. 2010;329:1526–1530. doi: 10.1126/science.1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chartron J, Shiau C, Stout CD, Carroll KS. 3′-Phosphoadenosine-5′-phosphosulfate reductase in complex with thioredoxin: a structural snapshot in the catalytic cycle. Biochemistry. 2007;46:3942–3951. doi: 10.1021/bi700130e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moskovitz J, Oien DB. Protein carbonyl and the methionine sulfoxide reductase system. Antioxid Redox Signal. 2010;12:405–415. doi: 10.1089/ars.2009.2809. [DOI] [PubMed] [Google Scholar]
- 93.Laugier E, Tarrago L, Vieira Dos Santos C, Eymery F, Havaux M, Rey P. Arabidopsis thaliana plastidial methionine sulfoxide reductases B, MSRBs, account for most leaf peptide MSR activity and are essential for growth under environmental constraints through a role in the preservation of photosystem antennae. Plant J. 2010;61:271–282. doi: 10.1111/j.1365-313X.2009.04053.x. [DOI] [PubMed] [Google Scholar]
- 94.Kim SO, Merchant K, Nudelman R, Beyer WF, Jr, Keng T, DeAngelo J, Hausladen A, Stamler JS. OxyR: a molecular code for redox-related signaling. Cell. 2002;109:383–396. doi: 10.1016/s0092-8674(02)00723-7. [DOI] [PubMed] [Google Scholar]
- 95.Dietz KJ. Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal. 2011;15:1129–1159. doi: 10.1089/ars.2010.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 97.Neumann CA, Cao J. Manevich Y (2009) Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8:4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y, Wada T, Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dai S, Schwendtmayer C, Schürmann P, Ramaswamy S, Eklund H. Redox signaling in chloroplasts: cleavage of disulfides by an iron–sulfur cluster. Science. 2000;287:655–658. doi: 10.1126/science.287.5453.655. [DOI] [PubMed] [Google Scholar]
- 100.Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell. 2007;19:1851–1865. doi: 10.1105/tpc.107.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, May MJ, Sung ZR. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vivancos PD, Dong Y, Ziegler K, Markovic J, Pallardó FV, Pellny TK, Verrier PJ, Foyer CH. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 103.Markovic J, Borrás C, Ortega A, Sastre J, Viña J, Pallardó FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–22024. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 104.Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA. 2009;106:3615–3620. doi: 10.1073/pnas.0808717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kruger NJ, von Schaewen A. The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol. 2003;6:236–246. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 106.Wenderoth I, Scheibe R, von Schaewen A. Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J Biol Chem. 1997;272:26985–26990. doi: 10.1074/jbc.272.43.26985. [DOI] [PubMed] [Google Scholar]
- 107.Barajas-López Jde D, Serrato AJ, Cazalis R, Meyer Y, Chueca A, Reichheld JP, Sahrawy M. Circadian regulation of chloroplastic f and m thioredoxins through control of the CCA1 transcription factor. J Exp Bot. 2011;62:2039–2051. doi: 10.1093/jxb/erq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- 109.Comelli RN, Gonzalez DH. Conserved homeodomain cysteines confer redox sensitivity and influence the DNA binding properties of plant class III HD-Zip proteins. Arch Biochem Biophys. 2007;467:41–47. doi: 10.1016/j.abb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Williams JC, Sue C, Banting GS, Yang H, Glerum DM, Hendrickson WA, Schon EA. Crystal structure of human SCO1: implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J Biol Chem. 2005;280:15202–15211. doi: 10.1074/jbc.M410705200. [DOI] [PubMed] [Google Scholar]
- 111.Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. Plant immunity requires conformational changes (corrected) of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rouhier N, Gelhaye E, Jacquot JP. Plant glutaredoxins: still mysterious reducing systems. Cell Mol Life Sci. 2004;61:1266–1277. doi: 10.1007/s00018-004-3410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xing S, Rosso MG, Zachgo S. ROXY1, a member of the plant glutaredoxin family, is required for petal development in Arabidopsis thaliana . Development. 2005;132:1555–1565. doi: 10.1242/dev.01725. [DOI] [PubMed] [Google Scholar]
- 114.Reichheld JH, Riondet C, Delorme V, Vignols F, Meyer Y. Thioredoxins and glutaredoxins in development. Plant Science. 2010;178:420–423. [Google Scholar]
- 115.Xing S, Zachgo S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J. 2008;53:790–801. doi: 10.1111/j.1365-313X.2007.03375.x. [DOI] [PubMed] [Google Scholar]
- 116.Li S, Lauri A, Ziemann M, Busch A, Bhave M, Zachgo S. Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana . Plant Cell. 2009;21:429–441. doi: 10.1105/tpc.108.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chuang CF, Running MP, Williams RW, Meyerowitz EM. The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana . Genes Dev. 1999;13:334–344. doi: 10.1101/gad.13.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maier AT, Stehling-Sun S, Wollmann H, Demar M, Hong RL, Haubeiss S, Weigel D, Lohmann JU. Dual roles of the bZIP transcription factor PERIANTHIA in the control of floral architecture and homeotic gene expression. Development. 2009;136:1613–1620. doi: 10.1242/dev.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murmu J, Bush MJ, DeLong C, Li S, Xu M, Khan M, Malcolmson C, Fobert PR, Zachgo S, Hepworth SR. Arabidopsis basic leucine-zipper transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol. 2010;154:1492–1504. doi: 10.1104/pp.110.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Riondet C, Desouris JP, Montoya JG, Chartier Y, Meyer Y, Reichheld JP. A dicotyledon-specific glutaredoxin GRXC1 family with dimer-dependent redox regulation is functionally redundant with GRXC2. Plant Cell Environ. 2012;35:360–373. doi: 10.1111/j.1365-3040.2011.02355.x. [DOI] [PubMed] [Google Scholar]
- 121.Cheng NH, Liu JZ, Liu X, Wu Q, Thompson SM, Lin J, Chang J, Whitham SA, Park S, Cohen JD, Hirschi KD. Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem. 2011;286:20398–20406. doi: 10.1074/jbc.M110.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laporte D, Olate E, Salinas P, Salazar M, Jordana X, Holuigue L. Glutaredoxin GRXS13 plays a key role in protection against photooxidative stress in Arabidopsis . J Exp Bot. 2012;63:503–515. doi: 10.1093/jxb/err301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dietz KJ. Redox signal integration: from stimulus to networks and genes. Physiol Plant. 2008;133:459–468. doi: 10.1111/j.1399-3054.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 124.Dietz KJ. Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal. 2011;15:1129–1159. doi: 10.1089/ars.2010.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fourquet S, Huang ME, D’Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Signal. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- 126.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 127.Jara M, Vivancos AP, Calvo IA, Moldón A, Sansó M, Hidalgo E. The peroxiredoxin Tpx1 is essential as a H2O2 scavenger during aerobic growth in fission yeast. Mol Biol Cell. 2007;18:2288–2295. doi: 10.1091/mbc.E06-11-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayté J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J, Morgan BA. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Fomenko DE, Koc A, Agisheva N, Jacobsen M, Kaya A, Malinouski M, Rutherford JC, Siu KL, Jin DY, Winge DR, Gladyshev VN. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc Natl Acad Sci USA. 2011;108:2729–2734. doi: 10.1073/pnas.1010721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uhlich GA. KatP contributes to OxyR-regulated hydrogen peroxide resistance in Escherichia coli serotype O157: H7. Microbiology. 2009;155:3589–3598. doi: 10.1099/mic.0.031435-0. [DOI] [PubMed] [Google Scholar]
- 132.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 133.Brigelius-Flohé R, Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2338. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haslekås C, Grini PE, Nordgard SH, Thorstensen T, Viken MK, Nygaard V, Aalen RB. ABI3 mediates expression of the peroxiredoxin antioxidant AtPER1 gene and induction by oxidative stress. Plant Mol Biol. 2003;53:313–326. doi: 10.1023/b:plan.0000006937.21343.2a. [DOI] [PubMed] [Google Scholar]
- 135.Romero-Puertas MC, Laxa M, Mattè A, Zaninotto F, Finkemeier I, Jones AM, Perazzolli M, Vandelle E, Dietz KJ, Delledonne M. S-nitrosylation of peroxiredoxin II E promotes peroxynitrite-mediated tyrosine nitration. Plant Cell. 2007;19:4120–4130. doi: 10.1105/tpc.107.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Finkemeier I, Goodman M, Lamkemeyer P, Kandlbinder A, Sweetlove LJ, Dietz KJ. The mitochondrial type II peroxiredoxin F is essential for redox homeostasis and root growth of Arabidopsis thaliana under stress. J Biol Chem. 2005;280:12168–12180. doi: 10.1074/jbc.M413189200. [DOI] [PubMed] [Google Scholar]
- 137.Baier M, Noctor G, Foyer CH, Dietz KJ. Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiol. 2000;124:823–832. doi: 10.1104/pp.124.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lamkemeyer P, Laxa M, Collin V, Li W, Finkemeier I, Schöttler MA, Holtkamp V, Tognetti VB, Issakidis-Bourguet E, Kandlbinder A, Weis E, Miginiac-Maslow M, Dietz KJ. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 2006;45:968–981. doi: 10.1111/j.1365-313X.2006.02665.x. [DOI] [PubMed] [Google Scholar]
- 139.Chang CC, Slesak I, Jordá L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 2009;150:670–683. doi: 10.1104/pp.109.135566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pagnussat GC, Yu HJ, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie LF, Ye D, Sundaresan V. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis . Development. 2005;132:603–614. doi: 10.1242/dev.01595. [DOI] [PubMed] [Google Scholar]
- 142.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 143.Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;6(7):109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 145.Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 146.Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 147.Chen K, Craige SE, Keaney JF., Jr Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal. 2009;11:2467–2480. doi: 10.1089/ars.2009.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2011;10:1527–1548. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goyal A, Tolbert NE. Variations in the alternative oxidase in Chlamydomonas grown in air or high CO2 . Plant Physiol. 1989;89:958–962. doi: 10.1104/pp.89.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]