Abstract
With the rapid rise in the emergence of bacterial strains resistant to multiple classes of antimicrobial agents, there is an urgent need to develop novel antimicrobial therapies to combat these pathogens. Cationic host defence peptides (HDPs) and synthetic derivatives termed innate defence regulators (IDRs) represent a promising alternative approach in the treatment of microbial-related diseases. Cationic HDPs (also termed antimicrobial peptides) have emerged from their origins as nature’s antibiotics and are widely distributed in organisms from insects to plants to mammals and non-mammalian vertebrates. Although their original and primary function was proposed to be direct antimicrobial activity against bacteria, fungi, parasites and/or viruses, cationic HDPs are becoming increasingly recognized as multifunctional mediators, with both antimicrobial activity and diverse immunomodulatory properties. Here we provide an overview of the antimicrobial and immunomodulatory activities of cationic HDPs, and discuss their potential application as beneficial therapeutics in overcoming infectious diseases.
Keywords: Host defence peptide, Antimicrobial, Immunomodulatory, Immunity, Infection, Therapeutics, Chemoattractant, Inflammation
Introduction
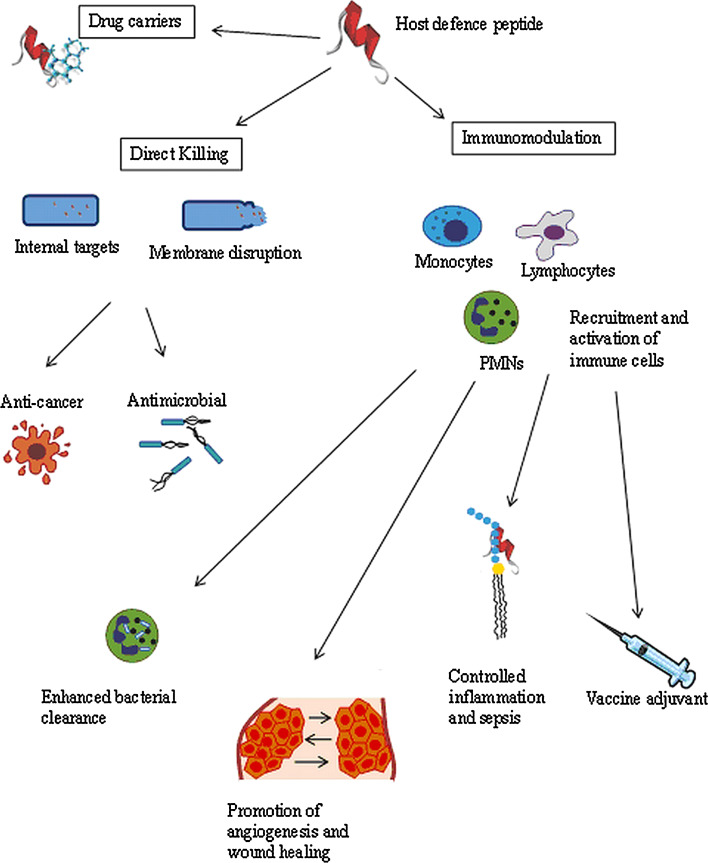
Cationic host defence peptides (HDPs) constitute a major component of the ancient, nonspecific innate defence system in most multicellular organisms, forming the first line of defence against invading microbes [12, 35, 36, 120]. Cationic HDPs are generally classified into four structural groups, with the first two classes being more predominant in Nature: α-helical, β-sheet, extended structures rich in certain amino acids and loop peptides. Despite the diversity in their sequences and structures, cationic HDPs are generally amphipathic, small (12–50 amino acids), have an overall net positive charge and have a high content of cationic and hydrophobic residues [32, 42, 120]. Early work on these defence molecules from insects, amphibians and mammalian phagocytes demonstrated that these molecules have antimicrobial activity against a variety of microbes [37, 39, 67]. More recently, it has become evident that these peptides exhibit a wide range of biological activities from direct killing of invading microbes to modulation of innate immune response and other biological responses of the host (Fig. 1) [38, 115, 116, 120]. Therefore, although they are often termed antimicrobial peptides (AMPs), they are perhaps more accurately termed host defence peptides (HDPs) to describe the breadth of their activities. Table 1 presents the immunomodulatory functions of cathelicidins and defensins. Moreover, synthetic variants of HDP, called innate defence regulator (IDR) peptides, show protective activity against bacterial infections mediated entirely through effects on the immunity of the host and independent of direct antimicrobial activity [19, 64].
Fig. 1.
Potential biological uses of HDPs. Many HDPs demonstrate both direct killing of cells and immunomodulatory properties, although not necessarily equally. Some HDPs have also been shown to carry conjugated molecules into a target cell
Table 1.
Immunomodulatory functions of mammalian cationic HDPs
| Peptide family | Species | Examples (structural class) | Collective functions (varies between individual peptides) |
|---|---|---|---|
| Cathelicidin | Human | LL-37 (α-helical) | Anti-endotoxin, induction of chemokines for neutrophils, monocytes, macrophages, mast cells and T lymphocytes, weakly directly chemotactic, selective inhibition of pro-inflammatory responses, wound healing, modulate angiogenesis, modulate apoptosis of epithelial cells (promote) and neutrophils (inhibit), increase histamine release, modulate dendritic cell differentiation and maturation, antimicrobial activity (generally weak), adjuvanticity |
| Murine | CRAMP (α-helical) | ||
| Bovine | BMAPs (α-helical) | ||
| Bactenecin (extended) | |||
| Indolicidin (extended WP rich) | |||
| Porcine | Protegrins (β-hairpin) | ||
| PR-39 (extended RP rich) | |||
| Prophenins (extended RP rich) | |||
| Defensin | Human, murine (only in the gut) but not bovine | α-Defensins | Anti-endotoxin, induction of chemokines for neutrophils, monocytes, macrophages, mast cells, dendritic cells and T lymphocytes, directly chemotactic, selective cytokine/chemokine induction, wound healing, antimicrobial activity including antibacterial, antiviral and antiparasitic (generally weak but high concentrations in phagocytes), adjuvanticity, inhibit complement activation, induction of cell death, increase collagen production |
| All mammals | β-Defensins | Anti-endotoxin, induction of chemokines, directly chemotactic, selective inhibition of pro-inflammatory responses, wound healing, antimicrobial activity including antiviral (generally weak with certain exceptions), adjuvanticity |
As a result of the multifunctional properties of cationic HDPs and the increasing bacterial resistance to conventional antibiotics, there are ample opportunities for developing them for use in the clinic as anti-infective and immunomodulatory therapeutics. Although there are very few HDPs currently in use in the market, many are progressing through clinical trials for the treatment of a host of disease conditions including microbial infections, organ failure, wound healing, diabetes and cancers. Because of the complex mechanisms of action and potential toxicities, most clinical trials have focussed on HDP treatment topically rather than systemically [19, 111].
Biological activities and clinical applications of HDPs
Cationic HDPs in immunity
Natural cationic HDPs are encoded by genes from many different organisms. In mammals HDPs are expressed in a variety of cell types including monocytes/macrophages, neutrophils, epithelial cells, keratinocytes and mast cells [12, 30, 67]. Expression of mature (biologically active) peptides requires proteolytic cleavage [109]. Dependent on the specific peptide, species and the tissue or cell type, some peptides may be constitutively expressed, such as human β-defensin-1 (hBD-1) in intestinal epithelial cells, whereas the expression of other peptides is induced by microbial signature molecules, inflammation or injury, such as with hBD-3 during inflammatory disorders such as Crohn’s disease [14, 112, 119]. The protective role of cationic HDPs such as cathelicidins and defensins in host immunity was implied by studies demonstrating that their enhanced expression in transgenic mice resulted in increased resistance to bacterial infections [1, 91]. Similarly the expression of these HDPs was also increased in various inflammatory human clinical conditions such as cystic fibrosis, bronchiolitis and psoriasis. It was also found that patients with specific granule deficiency syndrome lack α-defensins and suffer from severe and frequent bacterial infections. Similarly, patients with morbus Kostmann have a deficiency in the sole human cathelicidin LL-37 and typically suffer from severe and frequent oral infections [26, 87]. In rodent models, it was observed that mice lacking the endogenous cathelicidin CRAMP or β-defensin showed modestly increased susceptibility to streptococcal infections [77]. In contrast, administration of exogenous cathelicidin reduced bacterial load and prevented mortality when administered after bacterial challenge [25].
Antimicrobial agents
Cationic antimicrobial peptides (we use the abbreviation AMPs, as a subset of HDPs, when these peptides are studied solely for their direct antimicrobial activity) initially attracted attention as alternative antibiotic candidates due to their prospective potency (although most natural peptides have modest direct antimicrobial activities), rapid action, and broad spectrum of activity against Gram-negative and Gram-positive bacteria, viruses, fungi and parasites. In addition they exhibit multiple mechanisms of action and a consequent low potential to induce de novo resistance.
Initially cationic AMPs were believed to act only by disrupting the integrity of the bacterial membrane as described through one of four proposed models: barrel-stave [20], aggregate [114], carpet and toroidal pore [33, 63, 86, 113, 118], resulting in the formation of a transient channel, micellarization or dissolution of the membrane, or translocation across the membrane. Later studies demonstrated that the antimicrobial activity of many peptides was not limited to perforation of bacterial membranes, but rather they could translocate across the cytoplasmic membrane of bacteria to inhibit multiple internal targets including DNA/RNA synthesis, protein synthesis, cell wall synthesis, cell division, translation and protein folding [6, 24, 53, 82, 84, 102, 122]. An example of an AMP with multiple mechanisms of action is nisin, a lantibiotic produced by the bacterium Lactococcus lactis subsp. lactis. Nisin can inhibit bacterial growth through the formation of pores in the bacterial membranes. However, it also exhibits alternative mechanisms, such as binding to the peptidyloglycan precursor carrier lipid II, thereby inhibiting the synthesis and regeneration of the cell wall and, consequently, cell division [32, 90]. Regardless of their precise mode of action, the activities of antibacterial peptides are dependent on interaction with the bacterial cell membrane, in which the first step involves an electrostatic attraction between the cationic peptide and negatively charged components present on the outer bacterial envelope.
Cationic AMPs have also demonstrated an ability to confer protection against viral infections caused by a wide spectrum of viruses, including enveloped RNA and DNA viruses, feline calicivirus, and echovirus [3, 41, 43, 65, 85]. Some antiviral peptides act by targeting the viral envelope directly. For example, dermaseptin causes a direct inactivation of the HIV particle by direct interaction with the virus, destabilizing the viral membrane [59]. Other antiviral peptides work by targeting the viral adsorption and entry process. For example, polyphemusin analogue T22 binds to the chemokine receptor CXCR4 on T cells which serve as co-receptor for HIV-1 entry, thereby blocking entry of HIV strains that use this chemokine receptor [70, 104]. Still others impact on the viral growth cycle inside cells [43].
In recent years, an increasing number of antifungal peptides have been identified with new modes of action, including cell lysis, interference with cell wall synthesis, and inducing depolymerisation of actin cytoskeleton [16, 49]. For example, Lehrer et al. [54] showed that rabbit defensin NP-2 caused permeabilization of the yeast Candida albicans. Interestingly, in contrast to antiviral peptides where it appears to be nearly impossible to predict antiviral activity based on secondary structures of the peptide, antifungal peptides tend to be relatively rich in polar and neutral amino acids [43, 60].
Although substantial knowledge has accumulated on the antibacterial and antifungal properties of AMPs, far less is known regarding their activities against parasites. Magainin 2 was one of the first animal AMPs demonstrated to display antiparasitic activity, resulting in swelling and eventual bursting of Paramecium caudatum [121]. Since then, many additional AMPs and their synthetic derivatives were discovered to exhibit antiparasitic activities. For example, antiparasitic activity was demonstrated for the porcine cathelicidin PMAP-23 against both the eggs and adults of Caenorhabditis elegans [69]. More recently, Haines et al. [31] demonstrated that BMAP-18, a truncated form of BMAP-27 (bovine myeloid antimicrobial peptide 27), exhibited strong growth inhibitory activity against several species and life cycle stages of African trypanosomes, fish trypanosomes and Leishmania parasites in vitro. Studies have suggested that several AMPs exhibit antiparasitic modes of action resembling their antibacterial, antiviral or antifungal modes of action. For example, PMAP-23 exerts antinematodal and antifungal activities by disrupting the cell membrane via pore formation [51, 83]. However, structure–activity relationship studies revealed that the antiparasitic activities of AMPs may be dependent on certain peptide motifs present on these peptides that are different from those required for bacterial, viral, and fungal activities [43].
Clinical use of AMPs as antimicrobials
The discovery and subsequent use of antibiotics more than 60 years ago changed the course of human history by curing previously deadly diseases. Recently, however, an abundance of multidrug-resistant bacteria have emerged, yet very few new classes of antibiotics have been discovered. With the knowledge that AMPs can prevent infections in many organisms, it has been proposed that these peptides could form the basis of a new class of antimicrobials. To date, more than 1,000 natural cationic peptides with antimicrobial properties have been identified, often with broad-spectrum activity against Gram-negative and Gram-positive bacteria, viruses, protozoa, and/or fungi [62]. These peptides include those that display direct antimicrobial activity and those that stimulate the immune system to clear or prevent an infection, and have been considerably extended and improved through sequence modifications. Even so, as antibiotics AMPs have a mixed history. The cationic peptides polymyxin B and gramicidin S, produced by the bacteria Bacillus polymyxa and Bacillus brevis, respectively, have been used clinically for many years as topical over-the-counter medicines, whereas the peptide nisin, produced by L. lactis, has been used as a food preservative [34]. Nevertheless, clinical efficacy has been observed [34], e.g. with MX-226/Omeganan in the prevention of catheter-associated infections, but issues with clinical trial design and endpoints have precluded licensure to date. Other AMPs that have completed phase III clinical trials, including those targeted to the prevention of diabetic foot ulcers (pexiganan, from frog magainin), and the prevention of oral mucositis in radiation therapy patients (iseganan, from pig protegrin-1), have failed to achieve New Drug Application (NDA) approval, usually not because of lack of activity but rather an inability to demonstrate an advantage over existing therapeutics (i.e. non-equivalence).
To date the clinical trials performed have been restricted to topical applications and aimed at exploiting the direct antimicrobial activity of this class of drugs. Issues such as cost of goods, poor pharmacokinetics due to their susceptibility to body proteases and perhaps other clearance mechanisms, and unknown toxicity profiles have limited the potential systemic applications of AMPs. Nevertheless there do seem to be solutions developing for each of these issues. Regarding cost of goods increasingly practical recombinant DNA expression strategies are starting to impact on cost of goods [7, 55]; furthermore, the use of peptide array and advanced computational strategies [13] and addition of fatty acyl chains have led to much smaller broad-spectrum peptides. Susceptibility to proteases is being addressed through a variety of strategies including formulation, creation of peptidomimetics equivalent to AMPs and the use of d- or non-natural amino acids [81, 96, 103]. In the case of polymyxin, toxicity was dealt with through the creation of a prodrug (methane sulfonate derivative) and this as well as formulation offers great promise for AMPs in general.
There are several potential advantages to using AMPs as antimicrobial drugs over conventional antibiotics. They can be used as antibiotics alone, or in combination with other antimicrobials for a synergistic effect, as immunomodulatory/anti-inflammatory compounds, or as anti-endotoxin compounds. AMPs demonstrate broad-spectrum activity against bacteria like a subset of conventional antibiotics, but unlike these some AMPs also demonstrate antiviral and/or antifungal activities. Additionally, AMPs usually demonstrate less than twofold differences in their minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations, indicating that they are bactericidal rather than bacteriostatic, a highly attractive mode of action. Increasing evidence demonstrates that AMPs have multiple targets within the cell [11, 23]. As a result of their amphipathic nature, all AMPs interact directly with the cytoplasmic membrane of Gram-positive and Gram-negative bacteria, as well as eukaryotic microbes [43], leading to either membrane barrier disruption or to uptake and inhibition of intracellular targets. Some alternative targets include the cytoplasmic membrane permeability barrier, macromolecular synthesis, cell division, cell wall biosynthesis, macromolecular biosynthesis, and certain heat shock enzymes. This multiple targeting and interaction with fundamental physiological structures makes microbes less likely to develop resistance. It is also worth mentioning that at least two molecules developed as AMPs, Omeganan and hLF1-11, are now being tested in the clinic for their additional immunomodulatory activity (see “Cationic HDPs in immunity”).
As with all new antimicrobials, the issue of resistance is a central theme in their development and clinical application. Although resistance has been demonstrated in vitro, this resistance is very modest compared to conventional antibiotics. For example, 30 passages of the bacterium Pseudomonas aeruginosa with sub-MIC concentrations of the aminoglycoside antibiotic gentamicin increased resistance by 190-fold [99], whereas under the same conditions with synthetic peptides the MIC increased only 2- to 4-fold [123]. There does not appear to be a general mechanism by which bacteria can become resistant to every single AMP [76]. It might be that mutations conferring resistance to AMPs are too metabolically expensive for maintenance. Indeed, microbe survival requires a balance between resisting direct microbial killing and innate immune clearance. Conversely the diversity of targets may prevent resistance because removal of one target by mutation still leaves other targets that can mediate cell killing. Interestingly, the microbicidal activity of AMPs is highly sensitive to the presence of divalent cations, serum, and anionic macromolecules such as glycosaminoglycans; yet many of these AMPs are still able to confer protection under these conditions [9]. In this regard, the immunomodulatory properties of AMPs—cell migration, survival, proliferation, induction of antimicrobial and immune mediators—are probably of more significance under physiological conditions.
Anticancer agents
Most cancer chemotherapy involves targeting rapidly dividing cells with a relatively small discrimination between neoplastic cells and normal proliferating cells. This results in side effects that range from nausea and vomiting to myelosuppression (bone marrow suppression) and thrombocytopenia (low platelet count). Dormant or slow-growing cancer cells respond poorly to chemotherapeutics. Furthermore, cancer cells frequently develop resistance to anticancer drugs by mechanisms that include overexpression of drug transporters and defects in apoptotic pathways [61]. Many HDPs, including bee venom melittin, tachyplesin II from horseshoe crab, and LL-37, have demonstrated an ability to kill cancer cells in vitro [94]; however, studies showing anticancer activity in vivo are limited because of the inactivation of peptides in serum (e.g. binding of peptides to serum components, proteolytic degradation). Interestingly, peptides composed of d-amino acids have demonstrated clear ability to prevent growth of human prostate carcinoma and human breast cancer xenographs in nude and SCID/NCr mice, respectively [79, 80].
It has been assumed that anticancer peptides are lytic and that they target fundamental differences between the membranes of cancer cells and normal cells. Many cancer cells carry a net negative charge on their membranes as a result of the overexpression of several anionic molecules, such as phosphatidylserine, O-glycosylated mucins, sialiated gangliosides, and heparin sulphates. Additionally, cancer cells often form microvilli which increase the surface area and hence the accessibility of the membrane to an anticancer drug. Normal host cells, in contrast, have membranes that are composed of more neutral zwitterionic phospholipids and sterols. In fact it is thought that the presence of cholesterol may protect normal cells from the action of cationic peptides [94].
Despite these observations, few mechanistic studies on the anticancer activities of HDPs have been performed and many HDPs are able to enter mammalian cells quite readily; indeed entry into cells and interaction with intracellular receptors are the major basis of their immunomodulatory properties. It is possible that peptides trigger apoptosis or other methods of programmed or unprogrammed cell death in such a receptor-mediated manner. In this regard it has been shown that LL-37 triggers apoptosis at pathological concentrations (25–50 μg/ml in epithelial cells) although the inverse happens in neutrophils [2]. Furthermore, certain HDPs have also been shown to target mitochondrial membranes. For example, buforin IIb, derived from histone H2A, was shown to traverse the plasma membranes of 62 human tumour cell lines without damaging them and induce mitochondrial-dependent apoptosis. Indeed buforin IIb was able to suppress the growth of tumours implanted into BALB/c mice [52]. Whether and how peptides might distinguish between normal and cancerous cells to induce apoptosis is unclear; nevertheless, physiological differences between cancer and normal cells could be exploited by researchers to design synthetic HDPs that can specifically target cancer cells with substantially lower toxicity to normal cells.
Immunomodulation
Virtually all cationic peptides have direct antimicrobial activity in vitro under the appropriate conditions, especially in situations where the peptides are tested at very high concentrations or in dilute medium [12, 32, 67]. Within the host, however, the direct antimicrobial activities of many cationic HDPs are often inhibited by the modest concentrations of peptides present and/or antagonism by physiological concentrations of monovalent and divalent cations, serum, and anionic macromolecules such as glycosaminoglycans [9]. Therefore, several cationic peptides that are described as antimicrobial peptides in fact probably do not work primarily in host defences by direct microbicidal action. For example, the human cathelicidin LL-37 added exogenously to mice can protect against Gram-positive bacterial infections but even at very high concentrations (100 μg/ml) does not reduce the bacterial load in tissue culture medium that contains physiologically relevant salt concentrations [9]. In contrast, at physiological concentrations of peptides, salt and serum, cationic HDPs such as LL-37, exhibit a wide range of alternative biological functions that do not target the pathogen directly, but rather selectively enhance and/or modulate host defence mechanisms to combat against microbial infections [12, 35, 44, 67]. The immunomodulatory activities of HDPs are extremely diverse and include stimulation of chemotaxis directly and/or through chemokine production, suppression of bacterial induced pro-inflammatory cytokine production, regulation of neutrophil and epithelial cell apoptosis, modulation of cellular differentiation pathways, modulation of dendritic cell activation and differentiation, and promotion of angiogenesis and wound healing.
The selective enhancement of innate immunity by cationic HDPs represents a novel approach to (adjunctive) anti-infective therapy that complements directly microbicidal compounds. Whereas the microbicidal activities of peptides are generally inhibited in the physiological environment of the host, HDPs are still able to exert their immunomodulatory action under these conditions [73]. Indeed potentially problematic issues related to direct cytotoxicity and haemolytic activities towards mammalian cells can be minimized by reducing the potential for membrane lytic activity [45, 95]. In contrast to other immunostimulatory treatments that are often associated with increased risk of pro-inflammatory tissue damage, the combination of both anti-infective and anti-inflammatory activities of HDPs reduces the risk for excessive inflammation [73]. Importantly, as the primary target of immunomodulatory peptides is the host, and effects on the pathogen are exerted indirectly via boosting the host’s immunity, the selective pressure for pathogen resistance to the drug is minimal [22].
Pro- and anti-inflammatory agents
There is now considerable evidence that cationic HDPs modify the nature of the inflammatory response, enhancing or reinforcing certain activities that would traditionally be considered pro-inflammatory, while suppressing the potent induction of pro-inflammatory cytokines by signature molecules from microbes. It is possible that their primary nature is to modulate inflammation, after its induction by bacterial signatures, and contribute to a transition to a more balanced inflammatory response after an initial potent cytokine response. In this regard cationic HDPs’ ability to enhance cellular recruitment, suppress pro-inflammatory cytokines and promote wound healing and the transition to adaptive immunity (adjuvant activity) would be critical.
A major factor in the mechanism of action of both natural HDPs and synthetic IDRs is their ability to selectively enhance particular pro-inflammatory responses, such as chemotaxis of leukocytes, induction of certain chemokines and cytokines, and, for some peptides like human LL-37, promoting histamine release [4, 74, 75]. HDPs induced or released by degranulation of phagocytes at sites of infection, can at higher concentrations (micromolar) act as direct chemoattractants for the cells of innate and adaptive immunity, and at lower concentrations (sub-micromolar), especially in the presence of certain host factors, stimulate the production and release of more potent chemokines [12, 44, 67]. Following pathogenic invasion of tissues, HDPs can thus directly or indirectly promote recruitment of effector cells such as neutrophils, monocytes/macrophages, immature dendritic cells, mast cells and T cells to the site of infection. For example, LL-37 attracts neutrophils, monocytes, T cells, and mast cells, using the G protein-coupled formylpeptide receptor-like 1 (FPRL1) [117].
The treatment of infections in seriously ill patients using current antibiotics frequently results in the release of endotoxins (e.g. lipopolysaccharide from the outer membrane of Gram-negative bacteria). The body produces a strong rapidly escalating inflammatory response to the released endotoxin followed by suppression of inflammation below baseline and an inability to defend against subsequent infections, even when the initial infection is cleared, and substantial deterioration of the patient’s condition, resulting in up to 200,000 deaths of North Americans annually. Hence, inflammatory responses triggered at the onset of infection are beneficial for combating pathogenesis. However, uncontrolled inflammation due to excessive pathogenic stimulus or breakdown of the coordinated inflammatory responses can lead to systemic inflammatory syndrome or sepsis.
Although cationic HDPs have been demonstrated to induce expression of certain pro-inflammatory responses to boost host innate immunity, they have also been shown to offer the host protection against endotoxemia by selectively suppressing certain pro-inflammatory responses and stimulating the expression of particular anti-inflammatory genes. For example, LL-37 can selectively suppress pro-inflammatory responses, such as the induction by the Gram-negative signature lipopolysaccharide (LPS) or Gram-positive signature lipoteichoic acid (LTA) [72] of the transcription/production of potent pro-inflammatory cytokines like TNF-α and IL-6. They do this through a multiplicity of mechanisms including both extracellular neutralization of LPS and intracellular modulation of signalling pathways, including induction of anti-inflammatory cytokines like IL-10, and turn on of negative regulators of TNF-α production like A20/TNFAIP3 [67]. For example LL-37 has been demonstrated to significantly reduce endotoxin-induced pro-inflammatory cytokine responses and offer protection against endotoxemia in vivo [8, 25]. Thus, HDPs play a role in the delicate balancing and regulation of inflammatory responses [12, 44, 67]. The ability of HDPs to selectivity boost the host’s infection-resolving immunity while dampening endotoxin-induced pro-inflammatory responses results in a net anti-infective response without excessive, potentially harmful (septic) responses. Moreover, the endotoxin neutralizing effect of host HDPs might implicate that HDPs are not only involved in suppressing inflammation in the presence of pathogenic challenge, but also play a critical role in maintaining homeostasis by dampening the potential for induction of inflammation by commensals which contain the same conserved signature molecules, including LPS and LTA [12, 67].
We would argue that the immunomodulatory properties of HDPs are very exciting and have rejuvenated interest in the field for use as templates in drug design. The innate immunity modulatory properties of these peptides give them the potential to be developed as novel adjunctive therapeutics against microbial infection, because these HDPs do not target the pathogen directly but instead selectively modulate the host immune system. Therefore, the likelihood for development of resistance would appear to be very low. The field of immunomodulation started more than 200 years ago with Edward Jenner’s discovery that infection with cowpox could induce immunity to smallpox; however the use of HDPs as immunotherapeutics is very recent.
To date, only three synthetic cationic peptides (variants of HDPs) have progressed to phase III clinical trials and despite evidence of efficacy for two of them, none have been approved for clinical use. Various other synthetic HDPs are in preclinicals, or phase I or II clinical trials with applications as diverse as boosting immunity to combat infections, preventing endotoxic shock and enhancement of wound healing. Interestingly, several HDPs entered clinical trials as antimicrobials and subsequently demonstrated immunomodulatory activity, for example MX-226 has demonstrated activity in phase III clinical trials in suppressing inflammation due to rosacea, a non-infectious inflammatory skin condition; therefore it is quite likely that immunomodulatory activity plays a substantial role in any clinical benefit these drugs would demonstrate (see Table 2).
Table 2.
Novel synthetic cationic peptides and mimetics in clinical trials
| Drug | Company | Description | Medical target | Trial phase | Ref/Reg. # |
|---|---|---|---|---|---|
| IMX942 | Inimex | Synthetic cationic HDP from innate defence regulator peptide IDR1 and bactenecin | Nosocomial infections, febrile neutropenia | Ia | http://www.inimexpharma.com |
| hLF1-11 | AM Pharma | Cationic peptide, human lactoferricin fragment | Bacteremia and fungal infections in immunocompromised hematopoetic stem cell transplant recipients | I/II | NCT00509938 |
| Omiganan (MX-226/MBI-226) | Migenix | Synthetic cationic host defence peptide derived from indolicidin | Topical antiseptic, prevention of catheter infections, anti-inflammatory for acne vulgaris and papulopustular rosacea | III & II | NCT00027248, NCT00231153, NCT00608959 |
| Opebacan | Xoma | 21-mer peptide derivative of bactericidal/permeability-increasing protein | Endotoxemia in hematopoetic stem cell transplant recipients, | I/II | NCT00454155 |
| XOMA-629 | Xoma | 9-mer derivative of bactericidal permeability-increasing protein | Impetigo | IIa | http://www.xoma.com |
| Delmitide (RDP58) | Genzyme | Semisynthetic d-amino acid decapeptide from HLA class I B2702 | Inflammatory bowel disease | Post II | http://www.genzyme.com [107] |
| PAC-113 | Pacgen Biopharmaceuticals | Synthetic 12-mer from histatin | Antifungal | II | NCT00659971 |
| PMX-30063 | PolyMedix | Defensin structural mimetic, non-peptide, small molecule/copolymer | Antibiotic | Ib | http://www.polymedix.com |
| HB-1345 | Helix BioMedix | Lipohexapeptide | Acne | Pre-phase I | http://www.helixbiomedix.com |
| Pexiganan acetate (MSI-78) | MacroChem | Synthetic 22-mer from magainin | Topical antibiotic | III | NCT00563433, NCT00563394 |
| Iseganan (IB-367) | Ardea Biosciences | Synthetic 17-mer from protegrin-1 | Oral mucositis in radiation therapy patients | III | NCT00022373 |
| AP-214 | Action Pharma A/S | Synthetic derivative from HDP α-melanocyte-stimulating hormone fused to hexalysine at C-terminal | Sepsis and post-surgical organ failure | II | NCT00903604; [17] |
| CD-NP | Nile Therapeutics | Chimeric 37-mer derived from combination of two natriuretic peptides; modified to lack hemodynamic activity | Organ failure | II | NCT00482937; [58, 89] |
| Ghrelin | Miyazaki University, Japan; Papworth Hospital, UK | Endogenous HDP | Airway inflammation, chronic respiratory infection, cystic fibrosis | II | JPRN-UMIN000002599, JPRN-UMIN000001598, NCT00763477 |
| Heptapeptide-7 | Helix BioMedix | 7-mer from synthetic prototype HB-107 (itself from cecropin B) | Wound healing, skin regeneration | I | [21] |
| OP-145 | OctoPlus N.V. | Synthetic 24-mer from LL37 for binding LTA and LPS | Chronic bacterial otitis media | II | ISRCTN84220089 |
| Vasoactive intestinal peptide (VIP) | State University of New York, FDA OOPD | Endogenous HDP | Respiratory tract infections, sepsis | I | NCT00004494 |
| CZEN-002 | Zengen | Synthetic 8-mer from α-melanocyte-stimulating hormone | Vulvovaginal candidiasis | IIb | [29, 100] |
FDA US Food and Drug Administration, OOPD Office of Orphan Products Development
Wound healing agents
Wound and burn infections represent a common indication for the use of antimicrobial therapy. However, the growing bacterial resistance to available antibiotics and lack of alternative effective therapeutic approaches in wound and burn healing pose a huge problem in patient care, emphasizing the urgency to develop new approaches for the treatment of infected wounds. Owing to their ability to activate and mediate the innate and adaptive immune response in infection and inflammation, kill bacteria, especially topically, and promote wound healing, cationic HDPs appear to be promising candidates for new therapeutic approaches in wound healing.
The role of HDPs in wound healing is supported by the observation that HDPs, such as human cathelicidin (hCAP18/LL-37) and hBD-2 and -3, are highly expressed in epidermal keratinocytes in response to injury or infection of the skin [18, 98, 101]. In addition, treatment with exogenous hBD-3 led to enhanced re-epithelialization of wounds in a porcine model [40]. After wounding, growth factors, such as IGF-I and TGF-α, induce the expression of hCAP/LL-37. This cathelicidin then acts to activate epidermal cells and fibroblasts to form granulation tissue and serves as chemoattractants for wound healing macrophages and fibroblasts [27, 88, 97, 98]. In addition, HDPs have been demonstrated to stimulate the expression of growth factors and cytokines in epithelial cells and keratinocytes that are also important in wound healing. For example, LL-37 induces the secretion from keratinocytes of IL-18 [106], a cytokine that exhibits pleiotropic effects including promotion of angiogenesis and induction of IFN-γ [64]. Direct effects on angiogenesis were also demonstrated for LL-37. Koczulla et al. [48] demonstrated that LL-37 activated vessel growth in cultivated epithelial cells in a chorioallantoic membrane assay. Various studies have demonstrated a role for LL-37 in protection from invasive bacterial skin infections caused by leading human skin pathogens such as P. aeruginosa, Staphylococcus aureus, and group A Streptococcus [10, 18, 77, 78]. Importantly, omiganan demonstrated the ability to statistically significantly reduce the numbers of diverse skin bacteria when applied topically in advanced clinical trials. In mice the sole cathelicidin, CRAMP, appears to be important because compared to wild-type mice, mice with a deletion of the cathelicidin gene, cnlp, were more susceptible to pathogenic skin infection [10, 77].
Vaccine adjuvants
Vaccination is the single most cost-effective method for controlling infectious diseases. Although vaccines based on subunit antigens are safer than live vaccines, they are often weak inducers of immune response. This could possibly be improved by addition of adjuvants. Adjuvants are components of vaccines that generally elicit little immunogenicity by themselves but when added to an antigen produce stronger immune responses than can be induced by the antigen alone [71]; they do this by stimulating innate immunity through one of three basic mechanisms: enhancing recruitment of immune cells to the site of immunization, promoting the activation of those cells and polarizing them to achieve the desired response (TH1 or TH2), and a more poorly described phenomenon known as the depot effect whereby the antigen is confined to a particular location permitting local innate and adaptive responses. Thus, the inclusion of appropriate adjuvants in vaccines helps to improve the efficacy of vaccines by improving the response both qualitatively and quantitatively and lowering the dose of antigen required to achieve effective immune responses and lasting immunological memory [64]. The ability of HDPs to modulate the innate immune system has made them promising candidates as vaccine adjuvants. Evidence for adjuvant activities of HDPs is based on various observations. Human neutrophil defensins have been shown to enhance both humoral and cell-mediated antigen-specific immune responses in murine models. For example, ovalbumin-specific immune responses were enhanced in mice when defensins were co-administered intranasally to C57/B1 mice [56]. Another group observed similar adjuvant activity of the defensins in an intraperitoneal injection of defensins with keyhole limpet hemocyanin and B cell lymphoma idiotype antigen into mice, and showed that IgG antibody responses were enhanced by the inclusion of defensins [105]. Furthermore, the same group showed that the inclusion of defensins significantly enhanced the resistance of immunized mice to subsequent tumour challenge [105]. More evidence to support HDPs as promising adjuvant candidates to enhance vaccine-specific immunity came from Biragyn et al. [5] who showed that DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fused to murine β-defensin 2 induced systemic and mucosal immune responses.
To co-formulate HDPs into novel vaccines, several issues need to be considered, including the quality and type of immune response, compatibility with the antigen, safety, stability and cost [71]. Dependent on the type of infection, a vaccine must be able to elicit an appropriately balanced TH1/TH2 response. Thus generally speaking, extracellular infections require a humoral (TH2) immune response for clearance, whereas intracellular infections required a cell-mediated (TH1) response [64]. Appropriate physical interactions between the antigen and adjuvant are important as adjuvants often act by increasing the uptake of antigens and in this regard it is worth noting that HDP peptides have the features of cell-penetrating peptides [124]. An ideal adjuvant should also help elicit a strong immune response, but not cause excessive harmful inflammation or other immunopathologies, a feature of most HDPs. Furthermore, as large percentages of vaccines are administered in developing countries, the production of the adjuvant must be relatively inexpensive. As adjuvants work to enhance the immunogenicity of the antigen, theoretically, the amount of antigen required for an effective immune response should be reduced and thus reduce production costs.
Recent research has also demonstrated that short peptide derivatives containing specific motifs for certain functions can behave similarly to the parent HDP and thereby reduce the cost to produce these derivatives [71]. For example, the effects on adaptive responses of synthetic IDR peptides (derived loosely from bovine peptides) in combination with CpG oligonucleotides, with or without the depot molecule polyphosphazene, were investigated. A bovine HDP, indolicidin, when co-administered with CpG and polyphosphazene, strongly enhanced antigen-specific cellular and humoral responses in cattle [50]. As well, a combination of CpG and the bactenecin derivative (IDR)-HH2 in a pertussis toxoid vaccine formulation dramatically enhanced the production of toxoid-specific TH1, TH2 and IgA antibodies in mice, and extraordinarily enhanced immunity in a single dose [47]. It was suggested that the peptide component likely aided in the recruitment of immune cells, and enhanced the activation of immune cells by CpG. In vitro studies by Davidson and colleagues [15] on human cathelicidin LL-37 indicated that peptides can influence the polarization, maturation and activation of dendritic cells.
Innate defence regulators
As the immunomodulatory role of natural cationic HDPs in the treatment of infectious agents is increasingly appreciated, synthetic peptides are being designed to selectively modulate the innate immune response to infection, without the potential deficits (mast cell degranulation and enhancement of apoptosis) demonstrated by certain HDPs. These synthetic innate defence regulators (IDRs) boost protective immunity against infection without direct antimicrobial action [19]. IDR-1, the sequence of which was based on the small bovine HDP bactenecin, completely lacked direct antimicrobial activity, but still conferred broad-spectrum protection in mouse models against systemic infections by multidrug-resistant bacteria including methicillin-resistant S. aureus and vancomycin-resistant Enterococcus and Gram-negative Salmonella [64, 95]. Evidence was provided that IDR-1 offers protection by selectively enhancing innate immunity in the host, e.g. through stimulation of chemokine production, while suppressing potentially harmful excessive inflammatory responses. IMX-942, which is based on IDR-1, has recently completed phase I clinical safety trials in patients with cancer chemotherapy-induced immune suppression (http://www.inimexpharma.com). More recently, an improved peptide IDR-1002, with no sequence similarity to IDR-1, was demonstrated to exhibit improved protection in mice challenged with S. aureus or Escherichia coli. Like IDR-1, the immunomodulatory peptide IDR-1002 offered protection, at least in part through induction of chemokines and leukocyte recruitment [73]. For both peptides, monocytes/macrophages (but not neutrophils or lymphocytes) were identified as the key cells mediating protection. Many details of mechanism were provided including the signal transduction pathways, transcription factors, downstream dysregulated genes and more recently the cellular receptors involved. Overall these results indicate that the peptides act intracellularly to selectively modulate signalling through inflammatory (innate immune) pathways.
Other immunomodulatory cationic HDPs that have demonstrated safety and efficacy in human clinical trials include MX-226, hLF1-11, OP-215 and RDP58 [107, 110]. Peptides MX-226 and hLF1-11 were originally developed as antimicrobial peptides but have also been demonstrated to have immunomodulatory activities [110]. MX-226 is formulated for topical delivery targeting inflammatory conditions such as severe acne and rosacea and has demonstrated efficacy in phase II clinical trials. hLF1-11, the N-terminal peptide of human lactoferrin, recently completed phase I clinical trials and has demonstrated efficacy as a systemic anti-infective via immunomodulatory effects [110]. OP-215, a synthetic 24 amino acid derivative of LL-37, recently completed phase II clinical trials and demonstrated efficacy and safety when applied topically in ear drops to chronic suppurative otitis media patients. Another cationic peptide that has demonstrated safety in clinical trials is RDP-58 derived from the heavy chain of HLA class I molecules. The mechanism of action of RDP-58 is proposed to be via suppression of the production of the pro-inflammatory cytokines such as TNF-α and IL-12, while maintaining other pro-inflammatory responses including the production of several other cytokines [107].
Drug carriers
A major consideration in the design and development of any drug is the method by which to deliver it to a target site. Even if a drug can be directly applied to a specific tissue, it often needs to cross the membrane and enter cells to exert its effects. Crossing this hydrophobic barrier is a major obstacle for many drugs, which are often soluble in aqueous solutions and therefore hydrophilic in nature. Short peptide sequences have been identified that can promote the transport of a wide variety of conjugated molecules across the membrane. These peptides have been collectively termed cell-penetrating peptides (CPPs), but share the same fundamental characteristics that make up HDPs; that is, they are short and cationic and often amphipathic. The mechanism by which the drugs are taken up seems to depend on the cargo. Two main uptake routes have been identified: endocytosis and translocation through the lipid bilayer. Both mechanisms depend on the interaction of the CPP with the membrane.
The two most discussed CPPs are TAT, derived from the trans-activating transcriptional activator (Tat) from human immunodeficiency virus 1 (HIV-1), and Penetratin, a 16-amino acid peptide derived from Drosophila transcriptional regulator antennapedia (Ant) [46, 93]. TAT has been conjugated to several molecules with promising in vivo results. Conjugation of β-galactosidase to TAT resulted in efficient penetration of the blood–brain barrier in mice, whereas immunization of dendritic cells (DCs) with TAT loaded with PTD-TRP2 resulted in complete protective immunity and inhibition of lung metastases in a 3-day tumour model. Administration of the anticancer agent methotrexate to Penetratin resulted in a fivefold increase in cytotoxicity in a breast cancer cell line. Penetratin is also currently sold as an in vitro transfection agent [93]. Intriguingly immunomodulatory peptides must be taken up into cells by mechanisms that appear reminiscent of the CPPs, in order to exert many of their immunomodulatory properties, including chemokine induction. Similarly the host defence peptide LL-37 is able to traverse into cells, which is required for chemokine induction [124], and demonstrates a cytosolic receptor [68]. It can also carry passenger molecules into the cell [92]. Mechanistically, LL-37 interacts with the cell surface and is found associated in the cytosol of host cells in tubulin-dependent endosomes, especially in the perinuclear region. All of these characteristics are analogous to those of CPPs.
Limitations and rational design
The immune system is a highly networked system of structures and processes that collectively protect an organism against disease. Inappropriate or excessive stimulation, or repression, of an immune response can result in the system being unable to return to homeostasis, leading to a cytokine storm or sepsis, respectively, both of which can lead to death. As a result of the intricacies of the immune response, immunomodulatory peptides introduced therapeutically may not exhibit a typical dose–response curve. Indeed, most clinical trials have investigated compounds as topical agents and consequently there are virtually no systemic toxicology or pharmacokinetic studies available for HDPs [64]. Owing to the important interaction of HDPs with cellular membranes including host cell membranes, cytotoxicity has been considered an important issue. However although the field often investigates red blood cell hemolysis as a surrogate for toxicity, peptides rarely demonstrate such cytotoxicity when these blood cells are present in their natural milieu, blood, and one must question the significance of lysis of blood cells suspended in phosphate-buffered saline. Although it has been suggested that many HDPs are membrane-active in prokaryotes, but much less so in eukaryotes, because of the absence of surface negatively charged lipids and strong transmembrane potentials and the presence of cholesterol in eukaryotic membranes [34], functionally important uptake of peptides into cells without cell lysis has been demonstrated for several peptides (see above). Thus a critical need in this field is a practical understanding of the toxic mechanisms associated with these peptides including both local cytotoxicities and systemic issues. Although it has been suggested that fundamental differences in the membrane composition of normal cells and cancer cells explain the cancer-selective toxicity of some peptides [94] it may be that selective induction of other mechanisms such as apoptosis are more important.
There are several other obstacles that hinder the development of HDPs as therapeutics. Natural peptides are substrates for proteases that abound in the body both in the blood and especially at inflamed or infected sites. It is thought that this might contribute to reduced effectiveness, although it is worth mentioning that immunomodulatory (IDR) peptides can still demonstrate systemic protection when given intravenously, even 48 h prior to the initiation of an infection or 6 h after an infection starts [95]. Peptide design can address lability to proteases by using one or more d-amino acids rather than l-amino acids, employing different backbones (peptidomimetics), chemically modifying protease-sensitive sites, or delivering the peptides in protective vehicles such as liposomes [34]. Another potential limitation is high cost. Chemical synthesis of peptides typically runs in the range of US $100–600 per gram, a cost that is extremely prohibitive to mass production for use as therapeutics [34], although competition is considerably driving down the cost [28] and experiences with Fuzeon, a 39 amino acid HIV drug, have helped to solve many of the attendant good manufacturing practice (GMP) delivery issues. Furthermore, as some peptides can act on growth factor receptors, peptides developed for wound healing will need to be screened for any potential to induce tumorigenesis [64]. Prior to their development as immunomodulators, preclinical testing will need to utilize human ex vivo systems such as whole blood or peripheral blood mononuclear cells (PBMCs) as well as in vivo animal models, because a synthetic peptide may have different effects on the immune system of different hosts.
There are only three HDPs that have progressed to phase III clinical trials, and none of them to date have achieved NDA approval for clinical use. Pexiganan completed two phase III clinical trials as an antimicrobial topically administered initially to treat impetigo (which turned out to be self-limiting in the face of good hygiene) and subsequently to treat infected diabetic foot ulcers. Despite being as effective as the oral administration of the antibiotic ofloxacin and with no development of resistance (unlike ofloxacin), the US Food and Drug Administration (FDA) did not approve this drug for medical use [34, 57]. Iseganan, used in a mouth wash, was found not to be any more effective against oral mucositis than standard good oral hygiene practices [108]. Omiganan has recently completed phase III trials and demonstrated statistically significant reductions in reducing catheter-associated infections, yet was dropped for development by its US partner [66]. Considering that all three of these peptides have hit major obstacles in their development, the introduction of HDPs as therapeutics will require significant improvements and innovations, although the seeds of these are evident as discussed above. In our opinion the major area of development in the future will likely be in the area of immunomodulatory peptides and these molecules will likely be developed as adjunctive strategies to support other treatments, including antibiotics rather than as stand-alone anti-infectives.
Conclusion
Naturally derived HDPs have demonstrated a multitude of influences on the human body and an ability to directly kill pathogens. As anti-infective agents, HDPs can act to directly kill pathogens or clear an infection by stimulating an appropriate immune response (e.g. recruitment of leukocytes, while suppressing excessive inflammation). Furthermore, HDPs are unlikely to promote microbial resistance and can suppress the potentially harmful inflammation that is often part of infection, two properties that make HDPs an exciting and novel approach to combating infections. The diverse immunomodulatory activities of HDPs illustrate the potential for their use in many other applications, including wound healing, vaccine adjuvants, anti-endotoxemia, and anticancer drugs.
Limitations in development and production have driven the field of HDPs as therapeutics away from natural peptides and towards the shorter, more stable synthetic IDRs. Many current limitations such as cost and stability are likely to be overcome in the near future as synthetic strategies and peptidomimetic technologies are refined. Although there are very few peptides in phase III clinical trials and in current clinical use, it seems very likely that HDPs and IDRs will be in use as therapeutics within the next few years.
Acknowledgments
We gratefully acknowledge financial support from Canadian Institutes for Health Research (CIHR). A.T.Y.Y. received studentships from Canadian Cystic Fibrosis Foundation (CCFF) and the Natural Sciences and Engineering Research Council of Canada (NSERC). R.E.W.H. holds a Canada Research Chair.
Abbreviations
- AMP
Antimicrobial peptide
- BMAP-27
Bovine myeloid antimicrobial peptide 27
- CPP
Cell-penetrating peptide
- CRAMP
Cathelin-related antimicrobial peptide
- CXCR4
Chemokine receptor 4
- hBD-1
Human beta-defensin 1
- HDP
Host defence peptide
- hLF
Human lactoferrin
- IDR
Innate defence regulator
- IL-10
Interleukin 10
- PMAP-23
Porcine myeloid antimicrobial peptide 23
- LL37
Human cathelicidin (aka hCAP18)
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic acid
- MIC
Minimum inhibitory concentration
- MX-226
Migenix 226 (aka Omeganan)
- TAT
Trans-activating transcriptional factor (aka Tat)
- TNFα
Tumor necrosis factor alpha
- TNFAIP3
Tumor necrosis factor alpha-induced protein 3 (aka A20)
References
- 1.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defence by expression of a cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–6089. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, Haslett C, Simpson AJ, Hancock RE, Davidson DJ. The human cationic host defence peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastian A, Schafer H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–161. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 4.Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–953. [PubMed] [Google Scholar]
- 5.Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- 6.Boman HG, Agerberth B, Boman A. Mechanisms of action on Escherichia coli of cecropin-P1 and PR-39, 2 antibacterial peptides from pig intestine. Infect Immun. 1993;61:2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bommarius B, Jenssen H, Elliott M, Kindrachuk J, Pasupuleti M, Gieren H, Jaeger KE, Hancock REW, Kalman D. Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli . Peptides. 2010;31:1957–1965. doi: 10.1016/j.peptides.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowdish DME, Davidson DJ, Hancock REW. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 9.Bowdish DME, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock REW. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 10.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 12.Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Cherkasov A, Hilpert K, Jenssen H, Fjell CD, Waldbrook M, Mullaly SC, Volkmer R, Hancock REW. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem Biol. 2009;4:65–74. doi: 10.1021/cb800240j. [DOI] [PubMed] [Google Scholar]
- 14.Cunliffe RN, Mahida YR. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol. 2004;75:49–58. doi: 10.1189/jlb.0503249. [DOI] [PubMed] [Google Scholar]
- 15.Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, Hancock REW, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 16.de Lucca AJ, Walsh TJ. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrobial Agents Chemother. 1999;43:1–11. doi: 10.1128/aac.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi K, Hu X, Yuen PST, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TEN, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of α-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus . J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 19.Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenstein G, Lecar H. Electrically gated ionic channels in lipid bilayers. Q Rev Biophys. 1977;10:1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- 21.Falla TJ, Zhang L. Efficacy of hexapeptide-7 on menopausal skin. J Drugs Dermatol. 2010;9:49–54. [PubMed] [Google Scholar]
- 22.Finlay BB, Hancock REW. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich CL, Moyles D, Beveridge TJ, Hancock REW. Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob Agents Chemother. 2000;44:2086–2092. doi: 10.1128/aac.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrich CL, Rozek A, Patrzykat A, Hancock REW. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J Biol Chem. 2001;276:24015–24022. doi: 10.1074/jbc.M009691200. [DOI] [PubMed] [Google Scholar]
- 25.Fukumoto K, Nagaoka I, Yamataka A, Kobayashi H, Yanai T, Kato Y, Miyano T. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int. 2005;21:20–24. doi: 10.1007/s00383-004-1256-x. [DOI] [PubMed] [Google Scholar]
- 26.Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehrer RI. Microbicidal cytotoxic proteins of neutrophils are deficient in 2 disorders—Chediak–Higashi syndrome and specific granule deficiency. J Clin Invest. 1988;82:552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factor I and factor II expression in the healing wound. J Surg Res. 1992;52:389–394. doi: 10.1016/0022-4804(92)90121-f. [DOI] [PubMed] [Google Scholar]
- 28.Glaser V. Competition mounting in peptide market. GEN. 2009;29:38–41. [Google Scholar]
- 29.Grieco P, Rossi C, Colombo G, Gatti S, Novellino E, Lipton JM, Catania A. Novel alpha-melanocyte stimulating hormone peptide analogues with high candidacidal activity. J Med Chem. 2003;46:850–855. doi: 10.1021/jm0204338. [DOI] [PubMed] [Google Scholar]
- 30.Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Haines LR, Thomas JM, Jackson AM, Eyford BA, Razavi M, Watson CN, Gowen B, Hancock REW, Pearson TW. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl Trop Dis. 2009;3:e373. doi: 10.1371/journal.pntd.0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hale JDF, Hancock REW. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- 33.Hallock KJ, Lee DK, Ramamoorthy A. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys J. 2003;84:3052–3060. doi: 10.1016/S0006-3495(03)70031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancock REW, Sahl H. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 35.Hancock REW, Brown KL, Mookherjee N. Host defence peptides from invertebrates—emerging antimicrobial strategies. Immunobiology. 2006;211:315–322. doi: 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 37.Hancock REW, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 38.Hancock REW, Scott MG. The role of antimicrobial peptides in animal defences. Proc Natl Acad Sci U S A. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, Steinau H, Yao F, Onderdonk AB, Steinstraesser L, Eriksson E. Human beta-defensin-3 promotes wound heating in infected diabetic wounds. J Gene Med. 2009;11:220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 41.Horne WS, Wiethoff CM, Cui CL, Wilcoxen KM, Amorin M, Ghadiri MR, Nemerow GR. Antiviral cyclic d,l-alpha-peptides: targeting a general biochemical pathway in virus infections. Bioorg Med Chem. 2005;13:5145–5153. doi: 10.1016/j.bmc.2005.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol. 2004;11:963–967. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
- 43.Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenssen H, Hancock RE. Therapeutic potential of HDPs as immunomodulatory agents. Methods Mol Biol. 2010;618:329–347. doi: 10.1007/978-1-60761-594-1_20. [DOI] [PubMed] [Google Scholar]
- 45.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 46.Juliano RL, Alam R, Dixit V, Kang HM. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:324–335. doi: 10.1002/wnan.4. [DOI] [PubMed] [Google Scholar]
- 47.Kindrachuk J, Jenssen H, Elliott M, Townsend R, Nijnik A, Lee SF, Gerdts V, Babiuk LA, Halperin SA, Hancock REW. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine. 2009;27:4662–4671. doi: 10.1016/j.vaccine.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 48.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbruicker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koo JC, Lee B, Young ME, Koo SC, Cooper JA, Baek D, Lim CO, Lee SY, Yun DJ, Cho MJ. Pn-AMP1, a plant defence protein, induces actin depolarization in yeasts. Plant Cell Physiol. 2004;45:1669–1680. doi: 10.1093/pcp/pch189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacs-Nolan J, Mapletoft JW, Latimer L, Babiuk LA, den Hurk S. CpG oligonucleotide, host defense peptide and polyphosphazene act synergistically, inducing long-lasting, balanced immune responses in cattle. Vaccine. 2009;27:2048–2054. doi: 10.1016/j.vaccine.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 51.Lee DG, Kim PI, Park YK, Woo ER, Choi JS, Choi CH, Hahm KS. Design of novel peptide analogs with potent fungicidal activity, based on PMAP-23 antimicrobial peptide isolated from porcine myeloid. Biochem Biophys Res Commun. 2002;293:231–238. doi: 10.1016/S0006-291X(02)00222-X. [DOI] [PubMed] [Google Scholar]
- 52.Lee HS, Park CB, Kim JM, Jang SA, Park IY, Kim MS, Cho JH, Kim SC. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008;271:47–55. doi: 10.1016/j.canlet.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 53.Lehrer RI, Barton A, Daher KA, Harwig SSL, Ganz T, Selsted ME. Interaction of human defensins with Escherichia coli—mechanism of bactericidal activity. J Clin Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehrer RI, Szklarek D, Ganz T, Selsted ME. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985;49:207–211. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JF, Zhang J, Song R, Zhang JX, Shen Y, Zhang SQ. Production of a cytotoxic cationic antibacterial peptide in Escherichia coli using SUMO fusion partner. Appl Microbiol Biotechnol. 2009;84:383–388. doi: 10.1007/s00253-009-2109-2. [DOI] [PubMed] [Google Scholar]
- 56.Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci U S A. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipsky B, Holroyd K, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis. 2008;47:1537–1545. doi: 10.1086/593185. [DOI] [PubMed] [Google Scholar]
- 58.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorin C, Saidi H, Belaid A, Zairi A, Baleux F, Hocini H, Belec L, Hani K, Tangy F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology. 2005;334:264–275. doi: 10.1016/j.virol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Lustig F, Hoebeke J, Ostergren-Lunden G, Velge-Roussel F, Bondjers G, Olsson U, Ruetschi U, Fager G. Alternative splicing determines the binding of platelet-derived growth factor (PDGF-AA) to glycosaminoglycans. Biochemistry (NY) 1996;35:12077–12085. doi: 10.1021/bi960118l. [DOI] [PubMed] [Google Scholar]
- 61.Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Investig Drugs. 2006;15:933–946. doi: 10.1517/13543784.15.8.933. [DOI] [PubMed] [Google Scholar]
- 62.Marr AK, Gooderham WJ, Hancock REW. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry (NY) 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- 64.Mayer ML, Easton DM, Hancock REW. Fine tuning host responses in the face of infection: emerging roles and clinical applications of host defense peptides. In: Wamg G, editor. Antimicrobial peptides: discovery, design and novel therapeutic strategies. Wallingford, UK: CABI; 2010. [Google Scholar]
- 65.McCann KB, Lee A, Wan J, Roginski H, Coventry MJ. The effect of bovine lactoferrin and lactoferricin B on the ability of feline calicivirus (a norovirus surrogate) and poliovirus to infect cell cultures. J Appl Microbiol. 2003;95:1026–1033. doi: 10.1046/j.1365-2672.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- 66.Migenix (2009) Migenix announces Omigard phase III clinical trial results. Available via http://www.migenix.com/prod_summaries.php. Accessed 19 Mar 2009
- 67.Mookherjee N, Hancock REW. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mookherjee N, Lippert DND, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, Pistolic J, Gardy J, Miri P, Naseer M, Foster LJ, Hancock REW. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–2696. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- 69.Mor A. Multifunctional host defense peptides: antiparasitic activities. FEBS J. 2009;276:6474–6482. doi: 10.1111/j.1742-4658.2009.07358.x. [DOI] [PubMed] [Google Scholar]
- 70.Murakami T, Nakajima T, Koyanagi N, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mutwiri G, Gerdts V, Lopez M, Babiuk LA. Innate immunity and new adjuvants. Rev Sci Tech. 2007;26:147–156. [PubMed] [Google Scholar]
- 72.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;16:41–47. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 73.Nijnik A, Madera L, Ma S, Waldbrook M, Elliott MR, Easton DM, Mayer ML, Mullaly SC, Kindrachuk J, Jenssen H, Hancock REW. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol. 2010;184:2539–2550. doi: 10.4049/jimmunol.0901813. [DOI] [PubMed] [Google Scholar]
- 74.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 77.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 78.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DYM. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 79.Papo N, Braunstein A, Eshhar Z, Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic d-,l-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 2004;64:5779–5786. doi: 10.1158/0008-5472.CAN-04-1438. [DOI] [PubMed] [Google Scholar]
- 80.Papo N, Seger D, Makovitzki A, Kalchenko V, Eshhar Z, Degani H, Shai Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defence-like lytic peptide. Cancer Res. 2006;66:5371–5378. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- 81.Papo N, Shai Y. Effect of drastic sequence alteration and d-amino acid incorporation on the membrane binding behavior of lytic peptides. Biochemistry (NY) 2004;43:6393–6403. doi: 10.1021/bi049944h. [DOI] [PubMed] [Google Scholar]
- 82.Park CB, Kim HS, Kim SC. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun. 1998;244:253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 83.Park Y, Jang SH, Lee DG, Hahm KS. Anti-nematodal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Caenorhabditis elegans . J Pept Sci. 2004;10:304–311. doi: 10.1002/psc.518. [DOI] [PubMed] [Google Scholar]
- 84.Patrzykat A, Friedrich CL, Zhang LJ, Mendoza V, Hancock REW. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli . Antimicrob Agents Chemother. 2002;46:605–614. doi: 10.1128/AAC.46.03.605-614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pietrantoni A, Ammendolia MG, Tinari A, Siciliano R, Valenti P, Superti F. Bovine lactoferrin peptidic fragments involved in inhibition of Echovirus 6 in vitro infection. Antiviral Res. 2006;69:98–106. doi: 10.1016/j.antiviral.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labelled analogs with phospholipid-membranes. Biochemistry (NY) 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 87.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 88.Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-alpha and other growth-factors in vivo—analysis by messenger-RNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 89.Rose RA. CD-NP, a chimeric natriuretic peptide for the treatment of heart failure. Curr Opin Investig Drugs. 2010;11:349–356. [PubMed] [Google Scholar]
- 90.Sahl HG, Pag U, Bonness S, Wagner S, Antcheva N, Tossi A. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005;77:466–475. doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 91.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 92.Sandgren S, Wittrup A, Cheng F, Jönsson M, Eklund E, Busch S, Belting M. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J Biol Chem. 2004;279:17951–17956. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 93.Sawant R, Torchilin V. Intracellular transduction using cell-penetrating peptides. Mol Biosyst. 2010;6:628–640. doi: 10.1039/b916297f. [DOI] [PubMed] [Google Scholar]
- 94.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 95.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill P, Yu JJ, Li Y, Donini O, Guarna MM, Finlay BB, North JR, Hancock REW. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 96.Shai Y, Oren Z. From “carpet” mechanism to de novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 97.Singer AJ, Clark RAF. Mechanisms of disease—cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu LD, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–5589. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 99.Steinberg D, Hurst M, Fujii C, Kung A, Ho J, Cheng F, Loury D, Fiddes J. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steinstraesser L, Kraneburg UM, Hirsch T, Kesting M, Steinau H, Jacobsen F, Al-Benna S. Host defense peptides as effector molecules of the innate immune response: a sledgehammer for drug resistance? Int J Mol Sci. 2009;10:3951–3970. doi: 10.3390/ijms10093951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steinstraesser L, Koehler T, Jacobsen F, Daigeler A, Goertz O, Langer S, Kesting M, Steinau H, Eriksson E, Hirsch T. Host defense peptides in wound healing. Mol Med. 2008;14:528–537. doi: 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subbalakshmi C, Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett. 1998;160:91–96. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 103.Svenson J, Stensen W, Brandsdal B, Haug BE, Monrad J, Svendsen JS. Antimicrobial peptides with stability toward tryptic degradation. Biochemistry (NY) 2008;47:3777–3788. doi: 10.1021/bi7019904. [DOI] [PubMed] [Google Scholar]
- 104.Tamamura H, Xu YO, Hattori T, Zhang XY, Arakaki R, Kanbara K, Omagari A, Otaka A, Ibuka T, Yamamoto N, Nakashima H, Fujii N. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem Biophys Res Commun. 1998;253:877–882. doi: 10.1006/bbrc.1998.9871. [DOI] [PubMed] [Google Scholar]
- 105.Tani K, Murphy WJ, Chertov O, Salcedo R, Koh CY, Utsunomiya I, Funakoshi S, Asai O, Herrmann SH, Wang JM, Kwak LW, Oppenheim JJ. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int Immunol. 2000;12:691–700. doi: 10.1093/intimm/12.5.691. [DOI] [PubMed] [Google Scholar]
- 106.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 107.Travis S, Yap LM, Hawkey C, Warren B, Lazarov M, Fong T, Tesi RJ, Group RIS. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm Bowel Dis. 2005;11:713–719. doi: 10.1097/01.mib.0000172807.26748.16. [DOI] [PubMed] [Google Scholar]
- 108.Trotti A, Garden A, Warde P, Symonds P, Langer C, Redman R, Pajak TF, Fleming TR, Henke M, Bourhis J, Rosenthal DI, Junor E, Cmelak A, Sheehan F, Pulliam J, Devitt-Risse P, Fuchs H, Chambers M, O’Sullivan B, Ang KK. A multinational, randomized phase III trial of iseganan HCl oral solution for reducing the severity of oral mucositis in patients receiving radiotherapy for head-and-neck malignancy. Int J Radiat Oncol Biol Phys. 2004;58:674–681. doi: 10.1016/S0360-3016(03)01627-4. [DOI] [PubMed] [Google Scholar]
- 109.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 110.van der Does AM, Bogaards SJP, Ravensbergen B, Beekhuizen H, van Dissel JT, Nibbering PH. Antimicrobial peptide hLF1–11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrobial Agents Chemother. 2010;54:811–816. doi: 10.1128/AAC.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Waldmann TA. Immunotherapy: past, present and future. Nat Med. 2003;9:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 112.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 113.Wildman KAH, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry (NY) 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 114.Wu MH, Maier E, Benz R, Hancock REW. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli . Biochemistry (NY) 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 115.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 116.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 117.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zasloff M. Inducing endogenous antimicrobial peptides to battle infections. Proc Natl Acad Sci U S A. 2006;103:8913–8914. doi: 10.1073/pnas.0603508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 121.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin—isolation, characterization of 2 active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang LJ, Rozek A, Hancock REW. Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem. 2001;276:35714–35722. doi: 10.1074/jbc.M104925200. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Parente J, Harris SM, Woods DE, Hancock REW, Falla TJ. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob Agents Chemother. 2005;49:2921–2927. doi: 10.1128/AAC.49.7.2921-2927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Oglęcka K, Sandgren S, Belting M, Esbjörner EK, Nordén B, Gräslund A. Dual functions of the human antimicrobial peptide LL-37—target membrane perturbation and host cell cargo delivery. Biochim Biophys Acta. 2010;1798:2201–2208. doi: 10.1016/j.bbamem.2009.12.011. [DOI] [PubMed] [Google Scholar]