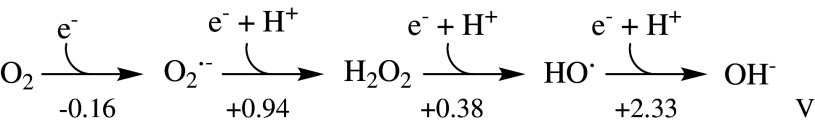Abstract
The so-called reactive oxygen species (ROS) are defined as oxygen-containing species that are more reactive than O2 itself, which include hydrogen peroxide and superoxide. Although these are quite stable, they may be converted in the presence of transition metal ions, such as Fe(II), to the highly reactive oxygen species (hROS). hROS may exist as free hydroxyl radicals (HO·), as bound (“crypto”) radicals or as Fe(IV)-oxo (ferryl) species and the somewhat less reactive, non-radical species, singlet oxygen. This review outlines the processes by which hROS may be formed, their damaging potential, and the evidence that they might have signaling functions. Since our understanding of the formation and actions of hROS depends on reliable procedures for their detection, particular attention is given to procedures for hROS detection and quantitation and their applicability to in vivo studies.
Keywords: Ferrous iron (Fe(II)), Fenton reaction, Hydroxyl radical, Microdialysis, Radical traps, Singlet oxygen
Introduction
Oxidative stress may be defined as an imbalance between the production of reactive oxygen species (ROS) and the ability of a biological system to detoxify the reactive intermediates and/or easily repair the resulting damage. The term ROS refers to oxygen-containing species that are more reactive than O2 itself. As shown in Scheme 1, it covers several redox states of oxygen, ranging from the relatively unreactive superoxide (O·−2) and H2O2 to the highly reactive oxygen species (hROS), which may exist as free hydroxyl radicals (HO·), as bound (“crypto”) radicals, or as Fe(IV)-oxo (ferryl) species [1–6]. There are also the nitrogen-containing ROS nitric oxide and peroxynitrite (RNS), which will not be discussed here but are reviewed in detail elsewhere [7, 8].
Scheme 1.
The redox states of oxygen with standard reduction potentials. The standard concentrations were taken as 1 M at a pH 7.0 (adapted from [9, 10])
The occurrence of oxidative stress has been implicated in numerous pathologies including neurodegenerative diseases, ischemic or traumatic brain injuries, cancer, diabetes, liver injury, and AIDS [2, 3, 11–17]. It has also been suggested that oxidative stress may play an important role in the ageing process [18–20].
Whereas the involvement of H2O2 and superoxide in a variety of cell-signaling processes is quite well established [see 21–24], the situation regarding hROS is less clear [see 25]. Problems in defining a role for hROS include difficulties in their detection, the exact nature of the species that might be involved, the lack of specificity of their formation, and their high non-specific reactivities.
The Fenton reaction and the nature of hROS
Many biochemical reactions are capable of forming H2O2 and superoxide but hROS are believed mainly to be generated in non-enzymic processes, including the breakdown of some xenobiotics, those mediated by transition metal cations, or generated by radiation. Although it is generally assumed that free hydroxyl radicals produced by the Fenton reaction, or Fenton-like processes involving other transition metals than iron, is the main pathway for initiating oxidative damage, this assumption cannot be considered as proven.
In its simplest form the Fenton reaction, in which ferrous iron catalyzes the formation of hydroxyl radicals from hydrogen peroxide can be written as a two-step process (Eqs. 1, 2):
 |
1 |
 |
2 |
where HOO· is the protonated form of superoxide, which is sometimes known as the hydroperoxyl or perhydroxyl radical. This has a pK a of about 4.8 and will therefore be largely in the superoxide form at physiological pH values (see Eq. 3) [see 26, 27]:
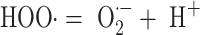 |
3 |
There has been a continuing debate about whether the product of the Fenton reaction or a Fenton-like process is the free hydroxyl radical, a bound “crypto” radical or a Fe(IV)-oxo species [4–6, 28–34]. Three possible alternatives to the first step of the Fenton reaction may be considered (see Eqs. 1a–c):
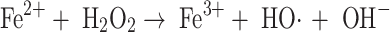 |
1a |
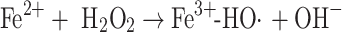 |
1b |
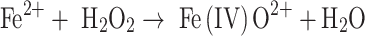 |
1c |
Thus, the free hydroxyl radical may be free to leave the coordination sphere of the transition metal involved and react independently from it (Eq. 1a). Alternatively, the “crypto” radical, formed in Eq. 1b, oxidizes its substrate within the first or second coordination sphere of the metal. In both of these cases, the electron gap is placed on the oxygen atom. In contrast, a Fe(IV) species (see Eq. 1c) reacts primarily by the charged iron atom, which can lead to different reaction intermediates in comparison to a bound or free hydroxyl radical. The uncertainty of this matter is mainly based on the difficulties in distinguishing between these species. As the redox potentials for free HO· as well as Fe(IV)-oxo species are higher than +1.6 V [35–38], both species will hydroxylate chemical compounds comparably, thus leading to similar or identical results when indirect methods like aromatic hydroxylation are used for hROS detection. Although, in some cases, it seems to be possible to distinguish between these species by using electron-spin resonance (ESR) spectroscopy together with the spin-trapping technique, even this technique does not provide a clear answer to this question [39, 40]. Thus, the term highly reactive oxygen species (hROS) is used in this discussion to cover all these species.
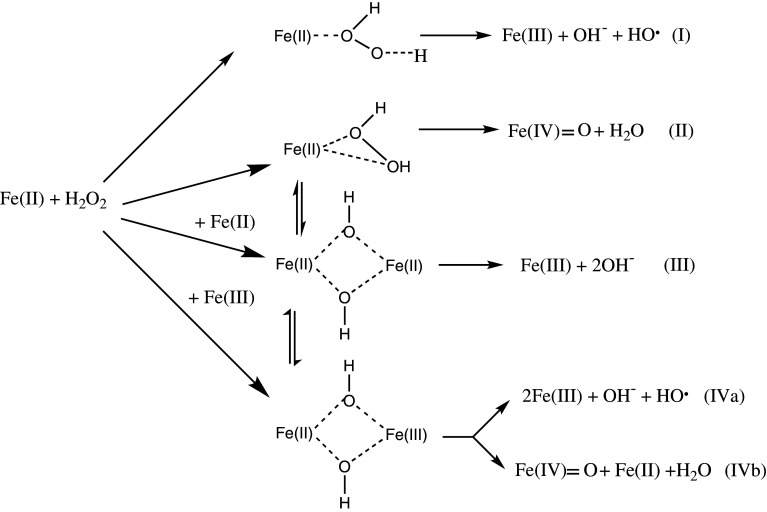
The nature of the oxidizing species produced via a Fenton system has been found to depend on the chemical environment, such as the pH, the ligand(s) of the Fe(II)complex or the presence or absence of O2· [4, 5, 31, 37, 38, 41]. Yamazaki and Piette [42] proposed that this variability may be explained by assuming four competing reaction pathways not all of which lead to hROS formation, as summarized in Scheme 2. Reaction (I) represents the classical Fenton pathway generating a free hydroxyl radical, whereas reaction (II) is an alternative path via a ferryl species. Both of these pathways will lead to a reactive intermediate with a similar oxidation potential, and therefore monitoring hROS formation via aromatic hydroxylation will give the sum of hROS formed via these reaction paths. A key factor in understanding the variance of Fenton activity is reaction (III), as this does not lead to hROS formation, but represents a simple two-electron oxidation of two mol of Fe(II). Reactions (IVa) and (IVb) are only of importance, if the Fe(III) concentration reaches a comparable level to Fe(II). It has been shown [43] that pathway (III) gains importance when Fe(II) is used in excess compared to H2O2 resulting in a lower overall Fenton activity. This effect can be overcome by iron chelation, e.g., with EDTA (ethylenediaminetetraacetic acid), and/or by changing the pH.
Scheme 2.
Lloyd et al. [6] showed by using 18O labeled H2O2 that at neutral pH the oxygen in hydroxylated compounds solely comes from H2O2 and not from water ligands bound to the octahedral iron center, which excludes any electron transfer from the iron centre to the ligands. Our own results in artificial cerebrospinal fluid (aCSF) [28, 43, 44] have confirmed and extended this by showing that the reactive intermediate is not the free hydroxyl radical, but either a crypto radical or a ferryl species; with the evidence supporting the description of the reactive species as a crypto radical.
In addition to arguments about the nature of the oxidizing species, there has been uncertainty about the predominant pathway of its formation. Based on kinetic arguments [25] and in vitro experiments involving leukemia cells [45], several authors have raised doubts about whether the Fenton reaction significantly contributes to oxidative damage. Schafer et al. [45] showed, using ESR spectroscopy, that lipid peroxidation in leukemia cells was enhanced by adding Fe(II), but was significantly reduced when H2O2 was added in the presence of iron. From those results, they concluded that alternative routes involving only iron and oxygen but no H2O2 (Eqs. 2a–c, below) predominate the Fenton pathway. In that work, the perferryl ion (the term in brackets in Eq. 2a) as well as the, already described, ferryl ion (the product in Eq. 2c), were proposed to be the predominant oxidative species initializing lipid peroxidation.
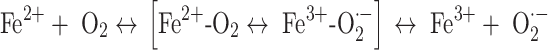 |
2a |
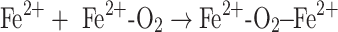 |
2b |
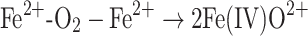 |
2c |
However, the importance of iron redox cycling for producing oxidative damage is generally accepted. Iron chelators, such as deferoxamine (desferal, desferrioxamine B), which are able to eliminate iron redox cycling completely, gave a dramatic decrease of oxidative damage in in vivo experiments related to oxidative stress [46–50]. In this context, it is important to mention that only chelators that strongly bind to all six coordination sites of iron are able to reduce or eliminate iron redox cycling. Other chelators (like EDTA) may have the opposite effect as they do not block oxygen or H2O2 coordination and make the almost insoluble free Fe(III) available for reducing agents like ascorbate [see 28, 30]. However, deferoxamine could only reduce but not eliminate oxidative damage, which leads to the conclusion that also other mechanisms than iron-oxygen chemistry may play an important role in vivo, opening a wide opportunity for future research.
Alternative processes for hROS production
There have been several reports of the production of hydroxyl radicals in enzyme-catalyzed reactions, but these generally appear to be secondary processes, owing to the presence of transition metals. Thus, the reported formation of hydroxyl radicals by the action of xanthine oxidase [50] was shown to be a result of the presence of adventitious iron catalyzing a Fenton process [51].
Several biochemical processes including the mitochondrial respiratory chain activity [see 52], the Nox family of NAD(P)H oxidases, such as the phagocytic NADPH oxidase [53] and nitric oxide synthase (NOS) when depleted of its substrate arginine or its cofactor 5,6,7,8-tetrahydrobiopterin (BH4) are capable of producing superoxide. This can then form hydroxyl radicals in the presence of a transition metal according to the following Fenton-like process (Eqs. 3a–c) [54–56]:
 |
3a |
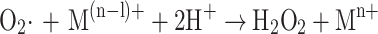 |
3b |
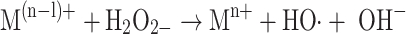 |
3c |
Alternatively, in the presence of nitric oxide, peroxynitrite can be formed, and this may then decompose to give the hydroxyl radical (Eqs. 4–6) [56, 57]:
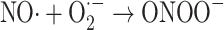 |
4 |
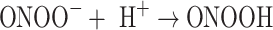 |
5 |
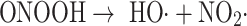 |
6 |
A further process that may result in the formation of hydroxyl radicals is the reaction between superoxide and hypochlorous acid. The hypochlorous acid formation, catalyzed by enzyme myeloperoxidase (Eq. 7), is an important bactericidal response [see 58]:
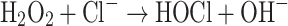 |
7 |
However, superoxide and hypochlorous acid can react to form the hydroxyl radical (Eq. 8), [59–61]:
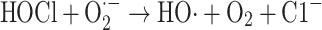 |
8 |
It has also been reported that the hemoprotein peroxidases can also catalyze the reaction (Eq. 9)
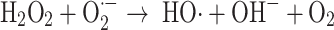 |
9 |
in a process where superoxide converts the heme iron into perferryl form (termed Compound III), in which the heme iron can assume the ferrous state [62]. The relative importance of these systems to the formation of hydroxyl radicals in vivo remains uncertain.
An additional hROS species that should be considered is singlet oxygen (1O2). Although not a true radical, it is a short-lived species (half-life of microseconds), highly reactive species. The possible involvement of singlet oxygen in the Fenton-like process, shown in Eq. (10), was advanced by Kellogg and Fridovich [63]:
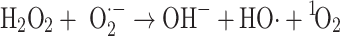 |
10 |
Alternatively, singlet oxygen may be formed by reaction of superoxide and the hydroxyl radical [64]:
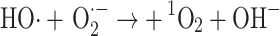 |
11 |
Although it had also been suggested that peroxynitrite might also decompose to form singlet oxygen, this has since been refuted [65]. It has also been suggested that singlet oxygen may be generated by decomposition of lipid peroxides [66]. However, irradiation in the presence of oxygen may be the major source of singlet oxygen. The production of singlet oxygen by plant photopigments is well established [see 67, 68] and light may result in its generation from a number of naturally occurring compounds such as bilirubin, pterins, flavins and vitamin A (retinol) [69, 70], and natural and synthetic dyes, including those found in some cosmetics [see 66]. An intriguing additional source of singlet oxygen is the reaction of ozone with a number of biological compounds including NAD(P)H, uric and ascorbic acids, by a mechanism that is believed to involve epoxide formation. 
The cysteine and methionine amino acid residues in protein could also give rise to singlet oxygen on oxidation by ozone. 
It has been suggested that this may underlie some of the damaging effects of ozone [71].
Specificities and actions of hROS
From a biological viewpoint, the two most important problems related to hROS are (1) to gain information about their direct damaging potential and (2) to examine their role as possible signaling substances under pathological and non-pathological conditions. For (1), the question on the nature of the redox active species is irrelevant, and any hydroxylation reaction that can compete successfully with other biological substances for hROS should give, under the assumption that there is only one hydroxylation product, a reliable representation of the direct damaging potential.
For (2), the question on the redox active species becomes important, since it appears possible that interaction with some specific targets would be a prerequisite for any signaling function. However, a free hydroxyl radical reacts unspecifically, whereas a crypto radical or a ferryl species can react more specifically owing to their charge. In this context the apparently specific role of ‘hydroxyl radicals’ in mobilizing calcium ions [72–74] may prove a valuable area of study. It is, however, necessary to distinguish between a specific signaling function in a biological pathway and a generalized response to non-specific cellular injury [75], but this has often not been demonstrated definitively.
The hydroxyl radical has been variously estimated to have a half-life in the ns range [4–6, 30] under in vivo conditions. This may not in itself be critical for a signaling function but the high reactivity, in that any collisions with other molecules may result in reaction, presents a more important problem. It has been estimated that it would travel only a few Angstrom (1 Å = 10−10 m) before it interacts with another molecule [see 76].
Whereas hydrogen peroxide and superoxide have been reported to have direct tissue damaging effects, high, unphysiological, concentrations have often been necessary [see 77], except in the presence of iron. Thus, it appears that these may be indirect effects associated with hydroxyl radical formation through Fenton-like processes [78, 79]. Similarly, the toxicity of superoxide appears to be secondary to its ability to release iron, in its ferrous form, from proteins, such as ferritin and proteins containing iron–sulfur clusters. This is because of the reduction of bound Fe(III) to Fe(II). For example, in proteins containing 4Fe–4S iron–sulfur clusters the native clusters contain two Fe(II) and two Fe(III) atoms, and the oxidation by superoxide may be written
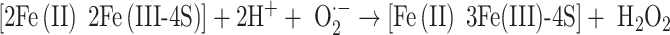 |
The Fe(II), which binds to the protein less tightly than Fe(III) can then dissociate from it
 |
Hydrogen peroxide may modify some sulfur-containing amino acid residues in proteins, oxidizing cysteine, and methionine residues, but the rate of reaction with, for example, methionine is very slow compared to that of HO· or singlet oxygen [81]. Similarly, superoxide is not a powerful oxidant of protein residues, although it can exert deleterious effects by removing iron from, and thereby inactivating, a number of enzymes that are important for normal cellular function [see 82].
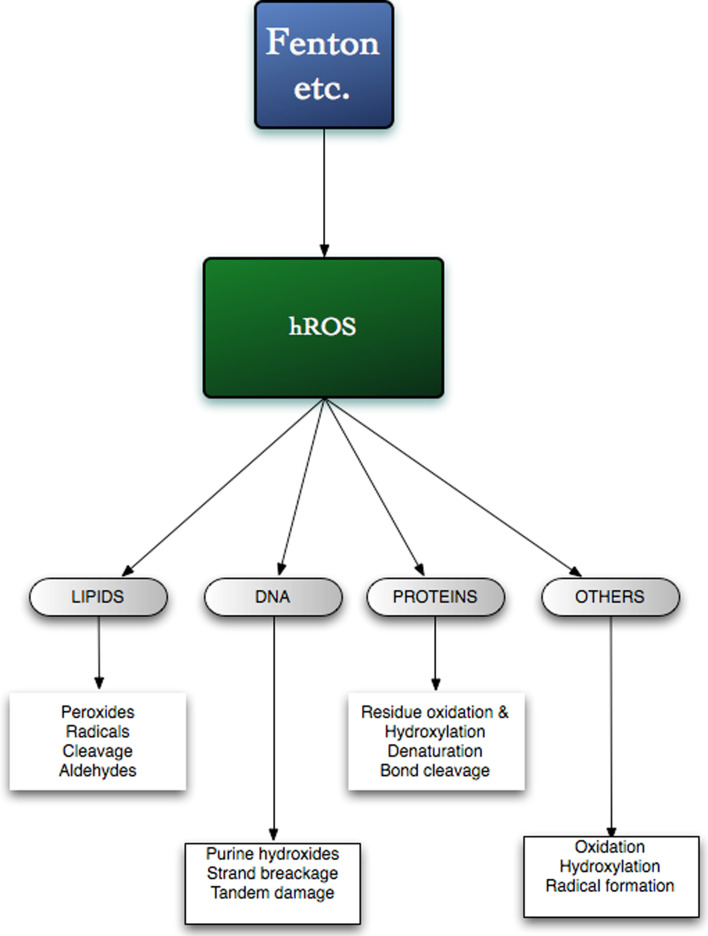
The non-specific reactivity of hROS results in their reaction with a variety of biomolecules (see Fig. 1). They can induce lipid peroxidation, DNA damage and protein modification. Reaction with unsaturated lipids can generate peroxides in a chain-reaction, as shown in Fig. 2. Further reactions of the peroxides can then lead to aldehyde formation. For example, linoleic acid yields 4-hydroxynonenal [see 83], which may serve as a stress-signaling factor [see 84, 85]. Bond cleavage may also occur in diene unsaturated fatty acids and the release of dialdehydes, such as malondialdehyde (MDA), and measurement of MDA has frequently been used as a rough indicator of tissue oxidative stress [see 86, 87]. hROS can react with many different amino acid residues in proteins and result in loss of histidine residues, bityrosine cross links, the introduction of carbonyl groups, and the formation of protein-centered alkyl, alkoxyland alkylperoxyl, ROO·, radicals and peptide bond cleavage [see 88–91]. Hydroxyl radicals and singlet oxygen have been shown to hydroxylate purine residues in DNA forming the 8-oxo-7,8-dihydro-2′-deoxy-derivatives of adenosine and, particularly guanosine [92–94]. It was shown that the this damage by hydroxyl radicals can subsequently result tandem damage that are produced by peroxyl addition on to the C8 of a vicinal purine base and that a proportion of such tandem lesions are resistant to DNA glycohydrolase repair mechanisms [92].
Fig. 1.
Some actions of hROS
Fig. 2.
Hydroxyl radicals and lipid peroxidation
hROS react with many biochemicals and in some cases, such as reaction with ascorbate [95], glutathione [13], bicarbonate [96], melatonin [76, 97], this may be protective. However, the products of such reactions may themselves be toxic. For example, reaction of ascorbate can be converted to the ascorbate radical [95] and furthermore the ability of ascorbate to reduce redox-active metals, such as Fe(III), may stimulate Fenton activity [98]. As discussed below, the ability of hROS to hydroxylate a range of aromatic molecules has formed the basis of a number of detection procedures.
Methods for hROS detection
The extreme reactivity and short lifetimes of hROS make their direct measurement in biological systems practically impossible. All common chemical methods for hROS detection are based on aromatic hydroxylation, as illustrated in Fig. 3. An effective trapping reagent should be biochemically inert and should be specific for hROS. The following sections will summarize the most widely used assays and point out their advantages and disadvantages.
Fig. 3.
Trapping reagents used for hROS detection. DHBA, dihydroxybenzoic acid
Salicylate (SA)
The most commonly used procedure to detect hROS is based on the hydroxylation of salicylic acid, as shown in Fig. 3. Although widely used, this method has several fundamental disadvantages, which have been described in detail elsewhere [see 28, 43, 44]. Essentially the drawbacks of this method are the hydroxylation behavior and bioactivity of SA. The hydroxylation yields mainly 2,5-dihydroxy-benzoic acid (2,5-DHBA) and 1,3-dihydroxy-benzoic acid (2,3-DHBA) plus smaller quantities of catechol. Because 2,5-DHBA can also be produced enzymatically [99, 100], only 2,3-DHBA can be used for hROS determination. The 2,3-DHBA/2,5-DHBA ratio has often been used as a measure of hROS, but the ratio of these two varies dependent on the chemical environment and thus a reliable quantification cannot be performed using this approach [see 28]. In vitro work also demonstrated that even in a simple Fenton system the ratio showed a time dependence, which again raised doubt on its reliability [28, 101–103]. Furthermore, salicylic acid may directly perturb the responses to hROS as it has been shown to affect inflammatory responses [104–107].
Phenylalanine
The hydroxylation reaction of phenylalanine has been proposed as an alternative to salicylate, mainly because the administration of phenylalanine has fewer identified side-effects [108, 109]. However, chemically, this system shows the same drawbacks as the SA method, since reaction of phenylalanine with hROS yields a mixture of the 2-, 3- and 4-hydroxylated products (o-, m- and p-tyrosine), as shown in Fig. 3, and p-tyrosine, as an endogenous compound, which is formed from l-phenylalanine by the enzyme phenylalanine hydroxylase (EC 1.14.16.1), cannot be used for detection of hROS. Since d-phenylalanine is not a substrate for this enzymatic hydroxylation, it has been proposed as a more suitable compound for hROS detection [110], although p-tyrosine still cannot be used for hROS determination unless a detection procedure that can distinguish between the d- and l-enantiomers is used. An additional potential problem is that d-phenylalanine is a substrate for d-amino acid oxidase (EC 1.4.3.3), which is present in brain [111] and produces H2O2 as a product.
Using the same arguments as above it is doubtful whether the sum of o- and m-tyrosine can be taken for an artefact-free hROS quantitation in vivo [see 109, 112, 113], although, in contrast to the situation of salicylate, detailed chemical studies are not available. Nevertheless, it should be mentioned that this method does have the potential advantage that nitration can be detected simultaneously, allowing concurrent monitoring of peroxynitrite formation [113].
4-Hydroxybenzoic acid (4-HBA)
Detection of hROS with 4-hydroxybenzoic acid (4-HBA) is less complicated, because only one hydroxylation product, 3,4-dihydroxybenzoic acid (3,4-DHBA), is formed in significant amounts (see Fig. 3). Thus, from a chemical viewpoint, this method should provide a much more reliable possibility for hROS quantitation. It has been used in microdialysis experiments, using HPLC separation with electrochemical detection [42, 114, 115]. Although 4-HBA can be hydroxylated by monooxygenases from some microorganisms, this apparently does not occur in mammals, suggesting its suitability as a chemical trap for hROS determination in vivo. As 4-HBA is an endogenous compound that shows little or no apparent toxicity, it has been claimed that it could be also used for human studies [115]. However, it is necessary to use high (mM range) concentrations of 4-HBA and further work is necessary to assess whether any biochemical processes are affected by 4-HBA, or its metabolites, at such levels, particularly in view of it being a substrate for 4-hydroxybenzoate polyprenyltransferase (EC 2.5.1.39), which is involved in the biosynthesis of ubiquinone [115].
Terephthalic acid (TA)
The TA assay does not have the drawbacks of the SA and phenylalanine methods, as the symmetry of the molecule allows only one hydroxylated product to be formed, as shown in Fig. 3. As, in contrast to the essentially non-fluorescent TA2−, 2-hydroxyterephthalic acid (OH-TA) shows a brilliant fluorescence, the method allows the detection down to 0.5 nM OHTA using a commonly available fluorescence detector. This reaction has been used for many years as a dosimeter in radiolysis experiments [see 99, 116], as a radical scavenger [118], and for in vitro studies [119, 120]. Its ease of use and its good performance, especially in the low-dose range [121]. This has led to the suggestion that this system could be used in vivo [117], and Yan et al. [100] reported its application for detecting hROS during brain microdialysis in sheep. In contrast to the other systems described above, the TA is not present endogenously and is poorly metabolized. Moreover, TA has been reported to have low toxicity and be non-accumulative [122, 123].
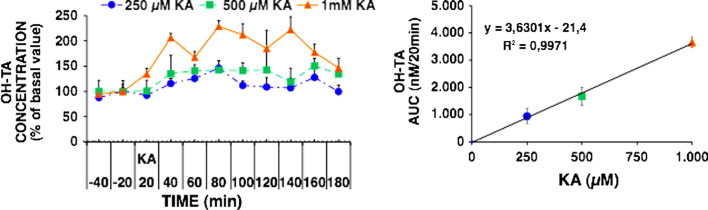
We showed in our previous work that TA does not influence the basal and the evoked levels of the neurotransmitters, glutamate, aspartate, taurine, and GABA (γ-aminobutyrate), in microdialysis experiments using kainate stimulation. Since a significant linear correlation between the formation of OH-TA and kainate concentration was found, it was concluded that it can be used for reliable hROS quantification [43, 44]. TA does not interfere with the use of o-phthalaldehyde (OPA) for derivatizing amino acids, and therefore can be used for the determination of the release of these transmitters and hROS formation in the same microdialysis experiment [43]. Figure 4 illustrates the formation of hROS during the evoked release of amino-acid neurotransmitters. Solutions of TA are difficult to prepare because of its low solubility, but the free acid may be dissolved in NaOH or the easily soluble sodium terephthalate may be used. For quantitative determinations, it is necessary to use 2-hydroxyterephthalate, which may be synthesized as previously described [43].
Fig. 4.
Dependence of 2-hydroxyterephthalic (OH-TA) on kainic acid (KA) concentration. Left panel: OH-TA formation, expressed as percentage of basal value, induced by 250, 500, and 1,000 μM KA. Right panel: the area under the concentration curve (AUCSTIM) of KA-induced OH-TA formation minus the basal AUC (AUCBAS) is plotted against KA concentration (modified from [43])
Determination of H2O2
Measurement of H2O2, has been used in some studies to estimate hROS levels on the assumption that hROS production, quantitatively, follows H2O2 formation. Although this might be true in some cases, it is obvious that it need not always to be the case, as hROS formation could also correlate with, e.g., the release of Fe(II). Several enzyme-catalyzed reactions produce H2O2 without the involvement of hROS and the formation of H2O2 has also been used as a measure of superoxide formation by mitochondria [123, 124]. Furthermore, as discussed above, it is possible for hROS to be formed from superoxide and in other processes that do not involve the participation of H2O2.
In vivo electron-spin resonance (ESR)
Electron spin resonance, also known as electron paramagnetic resonance (EPR) spectroscopy, can be used to detect molecules with unpaired electron spin states, present in radicals, transition metal complexes, like ferryl species, or molecular oxygen. However, the sensitivity is relatively low and high disturbing noise levels have limited its use in vivo. Short-lived species, like hROS, cannot directly be detected but may be transformed to more stable radicals with so-called spin traps.
Among the most widely used substances for that purpose are 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO), α-phenyl-N-tert-butylnitrone (PBN) or α-(4-pyridyl-1-oxide)-N-t-butylnitrone (POBN). As spin traps react specific with reactive oxygen (ROS) or nitrogen (RNS) species, ESR spectroscopy is able to distinguish between certain species of ROS or RNS [see 125–127].
It was not possible to perform measurements in an aqueous medium with the traditionally used X-band ESR, but the application of low-frequency ESR has made this possible, along with the development of methods for in vivo ESR. Besides the opportunity to use ESR for analyzing in vivo samples, e.g., by directly coupling it to a microdialysis facility [128], it allows non-invasive imaging, similar to NMR tomography, showing spatially resolved oxidative processes in the body [125, 129]. As the ability of a biological system to reduce the introduced spin trap is proportional to its antioxidant activity, the disappearance of the ESR signal can be used as a marker for the condition of the antioxidant defense system. Further developments should see wider use of this potentially powerful approach.
Conclusions
Unlike hydrogen peroxide and superoxide, which are destroyed by catalase, peroxidases, and the superoxide dismutases, there are no known enzymes that catalyze the removal of hROS. Thus, their formation and removal are rapid but unregulated. Much of the work relating oxidative stress to disease has been based on determining the effects in terms of DNA, lipid and protein damage [13, 86, 87]. However, since these measure the end-products of ROS damage, they will be affected by factors such as the efficiency of local antioxidant defenses and repair mechanisms. These can show considerable inter-individual variability, depending on diet, disease status, age, and genetic factors [130, 131]. In view of the wide-ranging toxicity of hROS, it is difficult to envisage specific regulatory functions, although singlet oxygen has been reported to regulate plastid differentiation in plant seedlings [132]. Further advances in our understanding necessitate the application of sensitive and reliable methods for hROS detection in vivo, such as those discussed in this review. Their use in microdialysis experiments to detect hROS in vivo, in freely moving animals, has considerable potential for research and medicine [see 43, 44]. However, caution and rigor are essential for the correct interpretation of the data obtained. Thus, high basal hROS levels have been reported in some [see, 133, 134], but not all [43] microdialysis studies in the brain and also in studies with isolated cell preparations [135]. It has been reported that hROS formation might be catalyzed by iron leaking from the stainless-steel probes that are used in many microdialysis studies [136]. It has also been suggested that hROS formation might result from cell damage caused by the probe insertion [137]. Clearly this effect would be an interesting field of study, but the use of adequate recovery times after probe insertion should minimize this response. The alternative possibility that the formation and release of hROS under basal conditions is a normal physiological process warrants further investigation.
There are still numerous unsolved questions related to hROS formation and activities in vivo as well as in vitro. In addition to the further development of analytical techniques, a multi-causal and interdisciplinary approach will be necessary for a resolution of many of these questions.
Acknowledgments
We are grateful for financial support from “Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich” (Project 19335-N17), Ente Cassa di Risparmio di Firenze (Firenze, Italy), Università degli Studi di Firenze, Science Foundation Ireland, ERAB: The European Foundation for Alcohol Research (Brussels, Belgium), and also to the EU COST action D34 supporting our international cooperation.
References
- 1.Jameson GNL, Jameson RF, Linert W. New insights into iron release from ferritin: direct observation of the neurotoxin 6-hydroxydopamine entering ferritin and reaching redox equilibrium with the iron core. Org Biomol Chem. 2004;2:2346–2351. doi: 10.1039/B408044K. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 3.Moskovitz J, Yim MB, Chock PB. Free radicals and disease. Arch Biochem Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 4.Rush JD, Koppenol WH. Reactions of iron(II) nitrilotriacetate and iron(II) ethylenediamine-N, N′-diacetate complexes with hydrogen peroxide. J Am Chem Soc. 1988;110:4957–4963. [Google Scholar]
- 5.Walling C. Fenton’s reagent revisited. Acc Chem Res. 1975;8:125–131. [Google Scholar]
- 6.Lloyd RV, Hanna PM, Mason RP. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med. 1997;22:885–888. doi: 10.1016/s0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;8:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 9.Wood PM. The potential diagram for oxygen at pH 7. Biochem J. 1988;253:287–289. doi: 10.1042/bj2530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Ann Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace GW, Leaf CD. The role of oxidative stress in HIV disease. Free Radic Biol Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 12.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes-associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 16.Chinta SJ, Andersen JK. Redox imbalance in Parkinson’s disease. Biochim Biophys Acta. 2008;1780:1362–1367. doi: 10.1016/j.bbagen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin J, Preston TJ, Wiley MJ, Wong AW. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- 18.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1988;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 19.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 20.De la Fuente M. Effects of antioxidants on immune system ageing. Eur J Clin Nutr. 2002;56(Suppl 3):S5–S8. doi: 10.1038/sj.ejcn.1601476. [DOI] [PubMed] [Google Scholar]
- 21.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2004;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 22.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 23.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–778. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 24.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Saran M, Michel C, Stettmaier K, Bors W. Arguments against the significance of the Fenton reaction contributing to signal pathways under in vivo conditions. Free Radic Res. 2000;33:567–579. doi: 10.1080/10715760000301101. [DOI] [PubMed] [Google Scholar]
- 26.De Grey AD. HO2*: the forgotten radical. DNA Cell Biol. 2002;21:251–257. doi: 10.1089/104454902753759672. [DOI] [PubMed] [Google Scholar]
- 27.Linert W, Bridge MH, Huber M, Bjugstad KB, Grossman S, Arendash GW. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson’s and Alzheimer’s diseases. Biochim Biophys Acta. 1999;1454:143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 28.Freinbichler W, Tipton KF, Della Corte L, Linert W. Mechanistic aspects of the Fenton reaction under conditions approximated to the extracellular fluid. J Inorg Biochem. 2009;103:28–34. doi: 10.1016/j.jinorgbio.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Wink DA, Wink CB, Nims RW, Ford PC. Oxidizing intermediates generated in the Fenton reagent: kinetic arguments against the intermediacy of the hydroxyl radical. Environ Health Perspect. 1994;102(Suppl 3):11–15. doi: 10.1289/ehp.94102s311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koppenol WH, Liebman JF. The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2 +) J Phys Chem. 1984;88:99–101. [Google Scholar]
- 31.Merkofer M, Kissner R, Hider RC, Brunk UT, Koppenol WH. Fenton chemistry and iron chelation under physiologically relevant conditions: electrochemistry and kinetics. Chem Res Toxicol. 2006;19:1263–1269. doi: 10.1021/tx060101w. [DOI] [PubMed] [Google Scholar]
- 32.Kremer ML. Is *OH the active Fenton intermediate in the oxidation of ethanol? J Inorg Biochem. 2000;78:255–257. doi: 10.1016/s0162-0134(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 33.Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 34.Jameson GNL, Linert W. The oxidation of 6-hydroxydopamine in aqueous solution. Part 2. Speciation and product distribution with iron(III) as oxidant. J Chem Soc, Perkin Trans. 2001;2:563–568. [Google Scholar]
- 35.Jameson GNL, Kudryavtsev AB, Linert W. The oxidation of 6-hydroxydopamine in aqueous solution. Part 1. The formation of three metastable quinones at low pH. J Chem Soc, Perkin Trans. 2001;2:557–562. [Google Scholar]
- 36.Welch KD, Davis TZ, Aust StD. Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Arch Biochem Biophys. 2002;397:360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- 37.Duesterberg CK, Cooper WJ, Waite T. Fenton-mediated oxidation in the presence and absence of oxygen. Environ Sci Technol. 2005;39:5052–5058. doi: 10.1021/es048378a. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita K, Ozawa T. Recent progress in in vivo ESR spectroscopy. J Radiat Res. 2004;45:373–384. doi: 10.1269/jrr.45.373. [DOI] [PubMed] [Google Scholar]
- 39.Kopáni M, Celec P, Danisovic L, Michalka P, Biró C. Oxidative stress and electron spin resonance. Clin Chim Acta. 2006;364:61–66. doi: 10.1016/j.cca.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Rush JD, Koppenol WH. Oxidizing Intermediates in the reaction of ferrous EDTA with hydrogen peroxide. J Biol Chem. 1986;261:6730–6733. [PubMed] [Google Scholar]
- 41.Di Matteo V, Pieruccia M, Di Giovanni G, Di Santo A, Poggia A, Benigno A, Esposito E. Aspirin protects striatal dopaminergic neurons from neurotoxin-induced degeneration: an in vivo microdialysis study. Brain Res. 2006;1095:167–177. doi: 10.1016/j.brainres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki I, Piette LH. ESR spin-trapping studies on the reaction of Fe2+ ions with H202-reactive species in oxygen toxicity in biology. J Biol Chem. 1990;265:13589–13594. [PubMed] [Google Scholar]
- 43.Freinbichler W, Colivicchi MA, Fattori M, Ballini C, Tipton KF, Linert W, Della Corte L. Validation of a robust and sensitive method for detecting hydroxyl radical formation together with evoked neurotransmitter release in brain microdialysis. J Neurochem. 2008;105:738–749. doi: 10.1111/j.1471-4159.2007.05168.x. [DOI] [PubMed] [Google Scholar]
- 44.Freinbichler W, Bianchi L, Colivicchi MA, Ballini C, Tipton KF, Linert W, Della Corte L. The detection of hydroxyl radicals in vivo. J Inorg Biochem. 2008;102:1329–1333. doi: 10.1016/j.jinorgbio.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Schafer FQ, Qian SY, Buettner GR. Iron and free radical oxidations in cell membranes. Cell Mol Biol (Noisy-le-grand) 2000;46:657–662. [PMC free article] [PubMed] [Google Scholar]
- 46.Agil A, Fuller CJ, Jialal I. Susceptibility of plasma to ferrous iron/hydrogen peroxide-mediated oxidation: demonstration of a possible Fenton reaction. Clin Chem. 1995;41:220–225. [PubMed] [Google Scholar]
- 47.Witte I, Zhu BZ, Lueken A, Magnani D, Stossberg H, Chevion M. Protection by desferrioxamine and other hydroxamic acids against tetrachlorohydroquinone-induced cyto- and genotoxicity in human fibroblasts. Free Radic Biol Med. 2000;28:693–700. doi: 10.1016/s0891-5849(99)00278-6. [DOI] [PubMed] [Google Scholar]
- 48.Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–1018. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- 49.Dikalova AE, Kadiiska MB, Mason RP. An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication–role of the Fenton reaction. Proc Natl Acad Sci USA. 2001;98:13549–13553. doi: 10.1073/pnas.251091098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880–9884. [PubMed] [Google Scholar]
- 51.Britigan BE, Pou S, Rosen GM, Lilleg DM, Buettner GR. Hydroxyl radical is not a product of the reaction of xanthine oxidase and xanthine. The confounding problem of adventitious iron bound to xanthine oxidase. J Biol Chem. 1990;265:17533–17538. [PubMed] [Google Scholar]
- 52.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szasz T, Thakali K, Fink GD, Watts SW. A comparison of arteries and veins in oxidative stress: producers, destroyers, function, and disease. Exp Biol Med (Maywood) 2007;232:27–37. [PubMed] [Google Scholar]
- 54.Cohen MS, Britigan BE, Pou S, Rosen GM. Application of spin trapping to human phagocytic cells: insight into conditions for formation and limitation of hydroxyl radical. Free Radic Res Commun. 1991;12:17–25. doi: 10.3109/10715769109145763. [DOI] [PubMed] [Google Scholar]
- 55.Hogg N, Darley-Usmar VM, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992;281:419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc NatI Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 58.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 59.Long CA, Bielski BH. Rate of reaction of superoxide radical with chloride-containing species. J Phys Chem. 1980;84:555–557. [Google Scholar]
- 60.Ramos CL, Pou S, Britigan BE, Cohen MS, Rosen GM. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992;267:8307–8312. [PubMed] [Google Scholar]
- 61.Candeias LP, Patel KB, Stratford MR, Wardman P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett. 1993;333:151–153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- 62.Chen SX, Schopfer P. Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem. 1999;260:726–735. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 63.Kellogg EW, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975;250:8812–8817. [PubMed] [Google Scholar]
- 64.Khan AU, Kasha M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci USA. 1994;91:12365–12367. doi: 10.1073/pnas.91.26.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez GR, Di Mascio P, Bonini MG, Augusto O, Briviba K, Sies H, Maurer P, Röthlisberger U, Herold S, Koppenol WH. Peroxynitrite does not decompose to singlet oxygen (1DgO2) and nitroxyl (NO−) Proc Natl Acad Sci USA. 2000;97:10307–10312. doi: 10.1073/pnas.190256897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar V, Tripathi MR, Kumar M, Shukla G, Dwivedi S, Sharma V. Studies on production and chemical property of singlet oxygen and superoxide radical by dyestuffs. E-J Chem. 2009;6(S1):S79–S86. [Google Scholar]
- 67.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J Exp Bot. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 68.Xia Q, Yin JJ, Fu PP, Boudreau MD. Photo-irradiation of Aloe vera by UVA-formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett. 2007;168:165–175. doi: 10.1016/j.toxlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Fu PP, Xia Q, Yin JJ, Cherng SH, Yan J, Mei N, Chen T, Boudreau MD, Howard PC, Wamer WG. Photodecomposition of vitamin A and photobiological implications for the skin. Photochem Photobiol. 2007;83:409–424. doi: 10.1562/2006-10-23-IR-1065. [DOI] [PubMed] [Google Scholar]
- 70.Egorov SY, Krasnovsky AA, Jr, Bashtanov MY, Mironov EA, Ludnikova TA, Kritsky MS. Photosensitization of singlet oxygen formation by pterins and flavins. Time-resolved studies of oxygen phosphorescence under laser excitation. Biochemistry (Mosc) 1999;64:1117–1121. [PubMed] [Google Scholar]
- 71.Kanofsky JR, Sima P. Singlet oxygen production from the reactions of ozone with biological molecules. J Biol Chem. 1991;266:9039–9042. [PubMed] [Google Scholar]
- 72.Simon F, Varela D, Eguiguren AL, Díaz LF, Sala F, Stutzin A. Hydroxyl radical activation of a Ca2+-sensitive non selective cation channel involved in epithelial cell necrosis. Am J Physiol Cell Physiol. 2004;287:963–970. doi: 10.1152/ajpcell.00041.2004. [DOI] [PubMed] [Google Scholar]
- 73.Burlando B, Viarengo A. Ca2+ is mobilized by hydroxyl radical but not by superoxide in RTH-149 cells: the oxidative switching-on of Ca2+ signalling. Cell Calcium. 2005;38:507–513. doi: 10.1016/j.ceca.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol. 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishii M, Shimizu S, Hara Y, Hagiwara T, Miyazaki A, Mori Y, Kiuchi Y. Intracellular-produced hydroxyl radical mediates H2O2-induced Ca2+ influx and cell death in rat b-cell line RIN-5F. Cell Calcium. 2006;39:487–494. doi: 10.1016/j.ceca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 76.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 77.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med. 2007;42:926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCormick ML, Buettner GR, Britigan BE. Endogenous superoxide dismutase levels regulate iron-dependent hydroxyl radical formation in Escherichia coli exposed to hydrogen peroxide. J Bacteriol. 1998;180:622–625. doi: 10.1128/jb.180.3.622-625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liochev SI, Fridovich I. The role of O2·− in the production of: in vitro and in vivo. Free Radic Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 81.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Rouault TA, Tong WH. Iron–sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407. doi: 10.1016/j.tig.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- 85.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(5 Suppl):715S–724S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 87.Rahman I, Biswas SK. Non-invasive biomarkers of oxidative stress: reproducibility and methodological issues. Redox Rep. 2004;9:125–143. doi: 10.1179/135100004225005219. [DOI] [PubMed] [Google Scholar]
- 88.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 89.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Gracanin M, Hawkins CL, Pattison DI, Davies MJ. Singlet-oxygen-mediated amino acid and protein oxidation: formation of tryptophan peroxides and decomposition products. Free Radic Biol Med. 2009;47:92–102. doi: 10.1016/j.freeradbiomed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 91.Zhang X-H. Regulation of protein function by residue oxidation. Proteomics Insights. 2010;3:17–24. [Google Scholar]
- 92.Bergeron F, Auvré F, Radicella JP, Ravanat JL. HO* radicals induce an unexpected high proportion of tandem base lesions refractory to repair by DNA glycosylases. Proc Natl Acad Sci USA. 2010;107:5528–5533. doi: 10.1073/pnas.1000193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cavalcante AK, Martinez GR, Di Mascio P, Menck CF, Agnez-Lima LF. Cytotoxicity and mutagenesis induced by singlet oxygen in wild type and DNA repair deficient Escherichia coli strains. DNA Repair (Amst) 2002;1:1051–1056. doi: 10.1016/s1568-7864(02)00164-7. [DOI] [PubMed] [Google Scholar]
- 94.Mishra PC, Singh AK, Suha S. Interaction of singlet oxygen and superoxide radical anion with guanine and formation of its mutagenic modification 8-oxoguanine. Int J Quantum Chem. 2005;102:282–301. [Google Scholar]
- 95.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen SJ, Csizmadia IG. Hydroxyl radicals piggybacking on hydrogen carbonate. Phys Chem Lett. 2001;431:633–637. [Google Scholar]
- 97.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 98.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 99.Mishin VM, Thomas PE. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem Pharmacol. 2004;68:747–752. doi: 10.1016/j.bcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Yan EB, Unthank JK, Castillo-Melendez M, Miller SL, Langford SJ, Walker DW. A novel Method for in vivo hydroxyl radical measurement by microdialysis in fetal sheep brain, in utero. J Appl Physiol. 2005;98:2304–2310. doi: 10.1152/japplphysiol.00617.2004. [DOI] [PubMed] [Google Scholar]
- 101.Lang K, Wagnerova DM, Brodilova J. The role of hydrogen peroxide in dioxygen induced hydroxylation of salicylic acid. Collect Czechoslov Chem Commun. 1994;59:2447–2453. [Google Scholar]
- 102.Lunak S, Muzart J, Brodilova J. Photochemical hydroxylation of salicylic acid derivatives with hydrogen peroxide, catalyzed with Fe(III) and sensitised with methylene blue. Collect Czechoslov Chem Commun. 1994;59:905–912. [Google Scholar]
- 103.Maskos Z, Rush JD, Koppenol WH. The hydroxylation of the salicylate anion by a Fenton reaction and T-radiolysis: a consideration of the respective mechanisms. Free Radic Biol Med. 1990;8:153–162. doi: 10.1016/0891-5849(90)90088-z. [DOI] [PubMed] [Google Scholar]
- 104.Salzberg-Brenhouse HC, Chen EY, Emerich DF, Baldwin S, Hogeland K, Ranelli S, Lafreniere D, Perdomo B, Novak L, Kladis T, Fu K, Basile AS, Kordower JH, Bartus RT. Inhibitors of cyclooxygenase-2, but not cyclooxygenase-1 provide structural and functional protection against quinolinic acid-induced neurodegeneration. J Pharmacol Exp Ther. 2003;306:218–228. doi: 10.1124/jpet.103.049700. [DOI] [PubMed] [Google Scholar]
- 105.Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong JS. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 106.Catania A, Arnold J, Macaluso A, Hiltz ME, Lipton JM. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc Natl Acad Sci USA. 1991;88:8544–8547. doi: 10.1073/pnas.88.19.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006;20:2009–2016. doi: 10.1096/fj.06-5987com. [DOI] [PubMed] [Google Scholar]
- 108.Themann C, Teismann P, Kuschinsky K, Ferger B. Comparison of two independent aromatic hydroxylation assays in combination with intracerebral microdialysis to determine hydroxyl free radicals. J Neurosci Methods. 2001;108:57–64. doi: 10.1016/s0165-0270(01)00370-3. [DOI] [PubMed] [Google Scholar]
- 109.Kaur H, Halliwell B. Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals. Anal Biochem. 1994;220:11–15. doi: 10.1006/abio.1994.1291. [DOI] [PubMed] [Google Scholar]
- 110.Moreno S, Nardacci R, Cimini A, Ceru MP. Immunocytochemical localization of d-amino acid oxidase in rat brain. J Neurocytol. 1999;28:169–185. doi: 10.1023/a:1007064504007. [DOI] [PubMed] [Google Scholar]
- 111.Reddy S, Halliwel B, Jones AD, Longhurst JC. The use of phenylalanine to detect hydroxyl radical production in vivo: a cautionary note. Free Radic Biol Med. 1999;27:1465. doi: 10.1016/s0891-5849(99)00165-3. [DOI] [PubMed] [Google Scholar]
- 112.Ferger B, Themann C, Rose S, Halliwell B, Jenner P. 6-Hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. J Neurochem. 2001;78:509–514. doi: 10.1046/j.1471-4159.2001.00429.x. [DOI] [PubMed] [Google Scholar]
- 113.Liu M, Liu S, Peterson SL, Miyake M, Liu KJ. On the application of 4-hydroxybenzoic acid as a trapping agent to study hydroxyl radical generation during cerebral ischemia and reperfusion Source. Mol Cell Biochem. 2002;234:379–385. [PubMed] [Google Scholar]
- 114.Marklund N, Clausen F, Lewander T, Hillered L. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J Neurotrauma. 2001;18:1217–1227. doi: 10.1089/089771501317095250. [DOI] [PubMed] [Google Scholar]
- 115.Kalén A, Appelkvist E-L, Chojnacki T, Dallner G. Nonaprenyl-4-hydroxybenzoate transferase, an enzyme involved in ubiquinone biosynthesis, in the endoplasmic reticulum–Golgi system of rat liver. J Biol Chem. 1990;265:1158–1164. [PubMed] [Google Scholar]
- 116.Matthews RW. The radiation chemistry of the terephthalate dosimeter. Radiat Res. 1980;83:27–41. [PubMed] [Google Scholar]
- 117.Schimmel M, Bauer G. Proapoptotic and redox state-related signaling of reactive oxygen species generated by transformed fibroblasts. Oncogene. 2002;21:5886–5896. doi: 10.1038/sj.onc.1205740. [DOI] [PubMed] [Google Scholar]
- 118.Barreto JC, Smith GS, Strobel NH, McQuillin PA, Miller TA. Terephthalic acid: a dosimeter for the detection of hydroxyl radicals in vitro. Life Sci. 1995;56:PL89–PL96. doi: 10.1016/0024-3205(94)00925-2. [DOI] [PubMed] [Google Scholar]
- 119.Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeira A, Muñoz-Patiño AM, Labandeira-Garcia JL. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. J Neurochem. 2000;74:1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- 120.Saran M, Summer KH. Assaying for hydroxyl radicals: hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic Res. 1999;31:429–436. doi: 10.1080/10715769900300991. [DOI] [PubMed] [Google Scholar]
- 121.Dai GD, Cui LB, Song L, Zhao RZ, Chen JF, Wang YB, Chang HC, Wang XR. Metabolism of terephthalic acid and its effects on CYP4B1 induction. Biomed Environ Sci. 2006;19:8–14. [PubMed] [Google Scholar]
- 122.OECD SIDS (2001) Terephthalic acid (TPA): initial assessment report. For 12th SIAM (Paris, France June 2001), United Nations Environment Programme (UNEP) Publications, Nairobi
- 123.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Togashi H, Shinzawa H, Matsuo T, Takeda Y, Takahashi T, Aoyama M, Oikawa K, Kamada H. Analysis of hepatitic oxidative stress status by electron spin resonance spectroscopy and imaging. Free Radic Biol Med. 2000;28:846–853. doi: 10.1016/s0891-5849(99)00280-4. [DOI] [PubMed] [Google Scholar]
- 125.Kopáni M, Celec P, Danisovic L, Michalka P, Biró C. Oxidative stress and electron spin resonance. Clin Chim Acta. 2006;364:61–66. doi: 10.1016/j.cca.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Swartz HM, Khan N, Khramtsov VV. Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid Redox Signal. 2007;9:1757–1771. doi: 10.1089/ars.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ueda Y, Doi T, Nagatomo K, Nakajim A. In vivo activation of N-methyl-d-aspartate receptors generates free radicals and reduces antioxidant ability in the rat hippocampus: experimental protocol of in vivo ESR spectroscopy and microdialysis for redox status evaluation. Brain Res. 2007;1178:20–27. doi: 10.1016/j.brainres.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 128.Gallez B, Swartz HM. In vivo EPR: when, how and why? NMR Biomed. 2004;17:223–225. doi: 10.1002/nbm.913. [DOI] [PubMed] [Google Scholar]
- 129.Utsumi H, Yasukawa K, Soeda T, Yamada KI, Shigemi R, Yao T, Tsuneyoshi M. Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats. J Pharmacol Exp Ther. 2006;317:228–235. doi: 10.1124/jpet.105.095166. [DOI] [PubMed] [Google Scholar]
- 130.Halliwell B. Antioxidant defence mechanisms: from the beginning to the end of the beginning. Free Radic Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 131.Kohen P, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 132.Kim C, Lee KP, Baruah A, Nater M, Göbel C, Feussner I, Apel K. 1O2-mediated retrograde signaling during late embryogenesis predetermines plastid differentiation in seedlings by recruiting abscisic acid. Proc Natl Acad Sci USA. 2009;106:9920–9924. doi: 10.1073/pnas.0901315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Obata T, Inada T, Yamanaka Y. Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation by antidepressant drugs in the rat. Neurosci Res Commun. 1997;21:223–229. [Google Scholar]
- 134.Camarero J, Sanchez V, O’Shea E, Green AR, Colada MI. Studies, using in vivo microdialysis, on the effect of the dopamine uptake inhibitor GBR 12909 on 3, 4-methylenedioxymethamphetamine (‘ecstasy’)-induced dopamine release and free radical formation in the mouse striatum. J Neurochem. 2002;81:961–972. doi: 10.1046/j.1471-4159.2002.00879.x. [DOI] [PubMed] [Google Scholar]
- 135.Mason RB, Pluta RM, Walbridge S, Wink DA, Oldfield EH, Boock RJ. Production of reactive oxygen species after reperfusion in vitro and in vivo: protective effect of nitric oxide. J Neurosurg. 2000;93:99–107. doi: 10.3171/jns.2000.93.1.0099. [DOI] [PubMed] [Google Scholar]
- 136.Montgomery J, Ste-Marie L, Boismenu D, Vachon L. Hydroxylation of aromatic compounds as indices of hydroxyl radical production: a cautionary note revisited. Free Radic Biol Med. 1995;19:927–933. doi: 10.1016/0891-5849(95)02004-t. [DOI] [PubMed] [Google Scholar]
- 137.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]