Abstract
The maintenance of mucosal barrier equilibrium in the intestine requires a delicate and dynamic balance between enterocyte loss by apoptosis and the generation of new cells by proliferation from stem cell precursors at the base of the intestinal crypts. When the balance shifts towards either excessive or insufficient apoptosis, a broad range of gastrointestinal diseases can manifest. Recent work from a variety of laboratories has provided evidence in support of a role for receptors of the innate immune system, including Toll-like receptors 2, 4, and 9 as well as the intracellular pathogen recognition receptor NOD2/CARD15, in the initiation of enterocyte apoptosis. The subsequent induction of enterocyte apoptosis in response to the activation of these innate immune receptors plays a key role in the development of various intestinal diseases, including necrotizing enterocolitis, Crohn’s disease, ulcerative colitis, and intestinal cancer. This review will detail the regulatory pathways that govern enterocyte apoptosis, and will explore the role of the innate immune system in the induction of enterocyte apoptosis in gastrointestinal disease.
Keywords: Intestininal inflammation, Necrotizing enterocolitis, Chron’s disease, Ulcerative colitis, TLR4
Introduction
The intestinal barrier must accomplish a seemingly impossible task. On the one hand, it must efficiently mediate the absorption of fluids, nutrients, and minerals from the lumen across the epithelium and into the microcirculation of the microvilli. On the other hand, the barrier must be sufficiently impermeant so as to prevent the transfer of potentially pathogenic microbes and injurious antigens. Under conditions in which the barrier is compromised, the host may show increased susceptibility to intestinal infection, inflammation, and systemic disease. An understanding therefore of the factors that regulate intestinal barrier integrity is critical towards gaining insights into the mechanisms that are required for maintaining the balance between health and disease [1]. Studies from a variety of sources have demonstrated that intestinal barrier integrity is determined in large part by the balance between the proliferation of enterocytes at the base of the crypts and their death along the crypt-villus axis by apoptosis [1, 2]. Moreover, the innate immune system—which plays a central role in the protection of the host from potential pathogens—has been found to play an important role in the induction of enterocyte apoptosis under a defined set of pathological conditions. After defining the differences between apoptosis and necrosis, we will now consider the mechanisms that regulate apoptosis within the intestinal epithelium, the role of the innate immune system in the induction of enterocyte apoptosis, and the consequence of enterocyte apoptosis in the development of disease.
Death and dying in the gut: apoptosis versus necrosis
Apoptosis refers to death in a programmed, well-coordinated manner that has well-defined morphological features and which is followed by the quiescent removal of dying cells [3]. By contrast, cellular necrosis results in cell death that is characterized by the rapid breakdown of membrane integrity and the release of cellular contents into the extracellular space [4]. This release of cellular contents can damage neighboring cells and result in the activation of immune cells, whose release of pro-inflammatory molecules can cause further cellular and tissue damage [4]. Within the gut, activators of enterocyte necrosis by trauma or bacterial toxins leads to membrane degradation, cell swelling, and DNA hydrolysis [1]. Proteins released from these necrotic cells, defined as danger-associated molecular patterns (DAMPs), have been shown to activate pattern-recognition receptors on the innate immune system, which further propagate the inflammatory response leading to further cellular necrosis [5]. For example, high mobility group protein B1 (HMGB1), a DNA-binding protein, is released from damaged enterocytes where it can activate the innate immune receptor Toll-like receptor 4 (TLR4), resulting in an enhanced inflammatory response in neighboring enterocytes [6, 7]. Other DAMPs known to activate the inflammatory cascade contributing to further cellular necrosis include uric acid, heat-shock proteins, and genomic DNA [4, 8]. Despite having the potential to cause additional tissue damage, the ability of the innate immune system to recognize DAMPs may be advantageous as a means of alerting the host to a breach in barrier integrity. While the precise role for enterocyte apoptosis versus necrosis in the maintenance of homeostasis remains incompletely understood, prevailing dogma suggests that while the induction of enterocyte apoptosis is necessary to allow for the turnover of the intestinal epithelium, which is required for maintenance of epithelial integrity, enterocyte necrosis, and the subsequent induction of the inflammatory response is generally associated with pathological barrier disruption. This review will focus on the steps that result in enterocyte death by apoptosis, and will then consider the role of the innate immune system in the induction of enterocyte apoptosis in the steps that lead to the development of intestinal disease.
The molecular mechanisms that regulate apoptosis in enterocytes
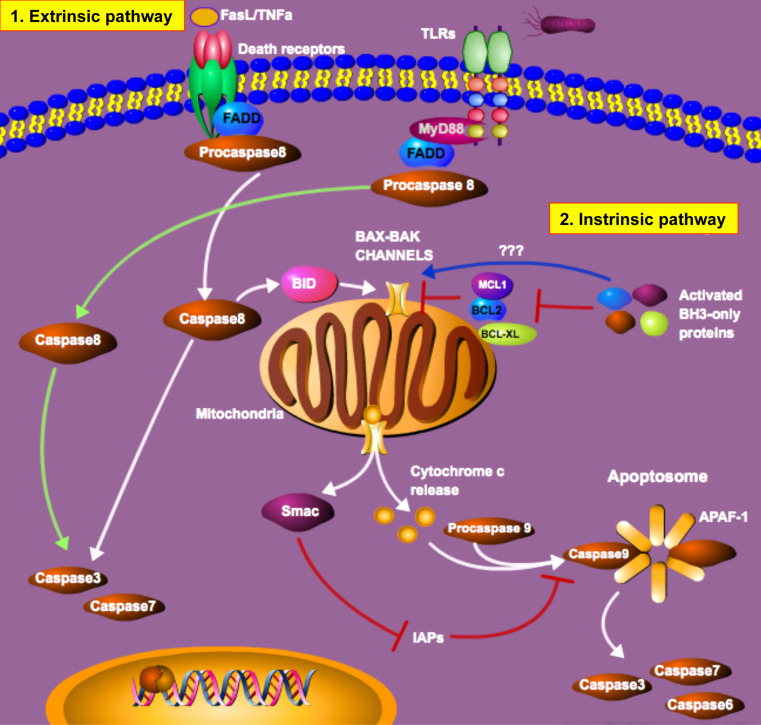
The commitment of a cell to enter a programmed cell death pathway represents a delicate balance between pro- and anti-apoptotic signals (Fig. 1). In mammalian cells, the activation of caspases, which are normally contained in the cells as inactive precursor enzymes, plays a critical role in the commitment to undergo apoptosis. Caspases can be activated through both extrinsic and intrinsic pathways [9]. Activators of the extrinsic pathway of apoptosis include tumor necrosis factor-α (TNF-α), Fas ligand (FasL) and TLR ligands [4, 10–13]. Binding of their cognate receptors sends a rapid pro-apoptotic signal that causes the recruitment of Fas-associated death domain protein (FADD) or Myeloid Differentiation Primary Response gene (88) (MyD88) to the death domain of the receptor, which causes the formation of the Death-Inducing Signaling Complex (DISC) [14]. FADD then complexes with procaspase-8, resulting in the proteolytic activation and release of active caspase-8. Active caspase-8 in turn activates other downstream caspases including caspase-3 and -7, as well as the BH3-interacting domain death agonist (BID), which leads to mitochondrial outer membrane permeabilization and cell death [15]. Pro-apoptotic proteins, including Bcl-2 associated X (BAX) and Bcl-2 antagonist/killer (BAK), remain anchored in the mitochondrial membrane and are required for mitochondrial membrane permeabilization, and upon activation form a pore in the mitochondrial membrane [16]. This causes the release of cytochrome c and the second mitochondrial activator of caspases (Smac) [17, 18]. Cytochrome c, together with apoptotic protease-activating factor-1 (APAF-1) and procaspase-9, form the apoptosome [4, 18], while the release of Smac prevents inhibitors-of-apoptosis proteins (IAP) from blocking apoptosome function [15, 16].
Fig. 1.
Regulation of apoptosis in mammalian cells. As described in the text, apoptosis may be initiated by either extrinsic or intrinsic pathways. The molecular constituents of each of these pathways, and their point of conversion at the mitochondrial membrane, are depicted in this schematic
In comparison to the extrinsic pathway, activation of the intrinsic pathway can be stimulated in response to toxic stress such as DNA damage or high pH [1, 17, 19–21]. Stimuli such as these cause the activation of a subset of pro-apoptotic proteins belonging to the B cell lymphoma-2 (Bcl-2) family. Because these proteins contain only three Bcl-2 homologue (BH) domains, they are referred to as BH3-only proteins [16, 22]. The BH3-only proteins activate BAX and BAK either indirectly by antagonizing the anti-apoptotic proteins Bcl-2, Bcl-2-like protein 1(Bcl-XL) and Induced Myeloid Leukemia Cell Differentiation Protein (MCL1) [19, 22–24] or through direct activation of BAX and BAK [22, 23, 25, 26]. Once BAX and BAK are activated, the cascade of events leading to MOMP and cell death are as previously described.
A link between inflammation and intestinal epithelial apoptosis
The balance between intestinal mucosal injury that is induced in response to enterocyte apoptosis, and mucosal repair that may occur via the combined processes of enterocyte migration and proliferation, is central to the health and function of the gastrointestinal tract. Under normal physiological conditions, apoptosis of enterocytes is thought to serve the purpose of removing senescent, malfunctioning, or potentially dangerous cells [10]. However, if apoptosis occurs at a rate that compromises epithelial barrier integrity, activation of the local and systemic immune system occurs (Fig. 2). While the immune system is critically important for defending and protecting the host from potentially harmful stimuli, we and others have shown that excessive activation of the immune system can also lead to increased rates of intestinal epithelial apoptosis [27–35]. The following section will highlight important regulatory components of innate immunity that are central to inflammation-induced apoptosis.
Fig. 2.
TLR4-induced enterocyte apoptosis leads to a disruption in the intestinal epithelial barrier, bacterial translocation, and systemic sepsis. As is described in the text, TLR4 activation leads to the induction of enterocyte apoptosis, which allows for translocation of enteric bacteria, activation of the immune system, and the development of systemic sepsis and inflammation. In response to the disruption in the enterocyte barrier, enterocytes migrate from healthy regions surrounding the mucosal defect, which serves to re-seal the site of disruption and to restore barrier integrity
NF-κB signaling and apoptosis in the gut
NF-κB is an essential transcription factor required for the proper orchestration of a broad diversity of biological roles, including innate immune signaling and apoptosis. The canonical activation of NF-κB can be initiated by a variety of pro-inflammatory stimuli, which result in the activation of the upstream IκB kinase (IKK) complex [36]. This in turn phosphorylates IκBα, the inhibitor protein preventing NF-κB from translocation to the nucleus [37]. Phosphorylated IκBα then disassociates from NF-κB, and allows for NF-κB to translocate to the nucleus where it influences the transcriptional regulation of genes involved in immune response, cell survival, differentiation and proliferation [38]. Within the intestinal epithelium, NF-κB activation has been shown to be required for homeostatic regulation of enterocyte death and proliferation, as thus to play a key role in the protection from the development of intestinal inflammation [39]. Studies with mice deficient in IKKβ, a protein required for the phosphorylation of IκBα and proper functioning of NF-κB, demonstrated increased rates of intestinal epithelial cell apoptosis and severe intestinal inflammation compared with wild-type mice [40]. Further, mice in which the NF-κB response was ablated showed increased enterocyte apoptosis, and this resulted in an increased susceptibility to ischemia-reperfusion injury [41]. Additionally, mice lacking proper NF-κB signaling in the intestinal epithelial cells of distal small intestine and colon resulted in the increased susceptibility to neonatal intestinal failure and inflammation in a dextran sodium sulphate-induced colitis model, and this susceptibility was mediated partly through the increased rates of epithelial apoptosis [39]. By contrast, as will be discussed below, activation of NF-κB via TLR4 in the newborn small intestine leads to increased rates of enterocyte apoptosis that is not seen in TLR4-deficient mice, nor in adult mice, and which together play a key role in the development of necrotizing enterocolitis [34]. These studies highlight the importance of proper NF-κB function for regulating intestinal homeostasis, in part regulated through the proper control of intestinal apoptosis.
Pattern-recognition receptors critical for the regulation of apoptosis: TLRs and NODs
The pattern recognition receptors (PRRs) have emerged as the fulcrum between host protection and disease, especially in the gastrointestinal tract. PRRs such as Toll-like receptors (TLR) and nucleotide-binding oligomerization domain protein receptors (NOD) provide a critical function in the detection of extracellular and intracellular microbial patterns including commensal and pathogenic bacteria. Certain PRRs are also known to signal following binding of endogenous ligands [42–48]. While this highly conserved set of receptors provides the potential for protection against a sweeping range of potentially harmful agents, they are highly susceptible to continuous stimulation, particularly when physiological protection such as epithelial barrier integrity is compromised.
TLR4
Toll-like receptor 4 (TLR4) is one of the best characterized pathogen-recognition receptors. It recognizes the bacterial membrane constituent lipopolysaccharide that is common to Gram-negative bacteria [49–52]. TLR4 has a well-characterized cytoprotective role on immune cells where its activation leads to NF-κB activation and cytokine release. Importantly, we and others have shown that TLR4 is expressed in the intestinal epithelia also express TLR4, both on the apical membrane surface [53, 54] and anchored to cytosolic Golgi [55]. The prototypical response of TLR4 following LPS activation is a degree of proinflammatory response characterized by the transcription of several inflammatory cytokines, chemokines, type 1 interferons, and immune response genes [56]. By contrast, on enterocytes, TLR4 activation has a strong role in the induction of mucosal injury in the newborn small intestine via increased enterocyte apoptosis, and an inhibition in mucosal repair, through decreased enterocyte proliferation and migration [35, 57]. These findings are seemingly in contrast to previous findings in adult colon, in which TLR4 signaling is critical for optimal proliferation and protection against apoptosis [58]. Differences in injury model, age of mice and location of injury (colon vs. small intestine) are likely contributing factors to the differences observed between studies, a fact supported by a study from our lab that showed that TLR4 activation did not cause apoptosis in the adult small intestine and colon [34].
What are the potential mechanisms by which TLR4 can regulate enterocyte apoptosis in the newborn small intestine but not in the adult colon? One possible explanation may be found by examining the ontogeny of TLR4 within the developing intestine, in which TLR4 was found to elevate throughout gut development, then to fall shortly after birth, to rise, then to fall again shortly after weaning [59]. It may thus be expected that in the newborn small intestine, upon colonization of the gut with microbes, under conditions in which TLR4 expression remain exaggerated—as may occur in the setting of hypoxia or remote infection, increased TLR4 signaling may ensue, resulting in enterocyte apoptosis and barrier injury. By contrast, in the adult colon, in which TLR4 levels are quite low, minimal signaling would be expected to occur, and TLR4 may play a homeostatic rather than pathological role. Taken together, these studies place the spotlight on TLR4 as an important regulator of apoptosis, and highlight the fact that understanding TLR4 ontogeny within the intestine may provide novel insights into the mechanisms that regulate intestinal homeostasis and the susceptibility to disease.
TLR9
TLR9 is the receptor for microbial DNA and has been shown to play a pivotal role in the regulation of intestinal inflammation. TLR9 is located in the cellular cytosol and is activated by intracellular cytosin-guanosin dinucleotide (CpG) motifs composed of unmethylated CpG dinucleotides, known to be immunostimulatory components of the bacterial DNA [60]. TLR9 activation has been studied extensively in murine antigen-presenting cells including macrophages, dendritic cells, and B lymphocytes [60–64]. Activation of intracellular TLR9 drives the production of numerous proinflammatory cytokines, including tumor necrosis factor (TNF), IL-6 and Il-12 leading to a strong induction of the T-helper 1 immune response [63]. Despite the strong immunostimulatory properties of TLR9 in immune cells, several studies have shown that TLR9 is effective in reducing apoptosis and gastrointestinal inflammatory disease, in part through signaling on enterocytes. In models of experimental colitis, the administration of CpG significantly reduced the pro-inflammatory cytokine expression of interferon (IFN)-γ and IL-6, increased anti-inflammatory IL-10 and reduced disease severity [65]. This finding was corroborated by another study showing that immunostimulatory DNA was able to ameliorate experimental and spontaneous colitis [66]. Several studies from our lab have also shown that CpG oligomers are able to counter the effects of TLR4-dependent inflammation. In a model of necrotizing enterocolitis, administration of CpG was shown to inhibit TLR4 signaling in enterocytes through an IRAK-M-dependant mechanism leading to reduced pro-inflammatory cytokines, reduced apoptosis, and decreased intestinal disease [59]. The ability of TLR9 activation to limit TLR4 signaling in the gut was subsequently validated by a trauma model, in which the administration of CpG-DNA resulted TLR4-dependent mucosal injury of the intestine. DNA administration acting through a TLR9-dependant mechanism reduced the effects of trauma on the small intestine and did so by reducing intestinal apoptosis [6]. However, there is conflicting data to suggest that TLR9 activation can further exacerbate intestinal inflammation and apoptosis. In an experimental model for colitis, CpG administration given following the onset of colitis had a more exacerbated colitis and intestinal breakdown [60, 67]. This highlights the importance of clear delineation of the mechanisms involved in TLR9 activation and careful consideration of when to use immuno-modulatory agents therapeutically.
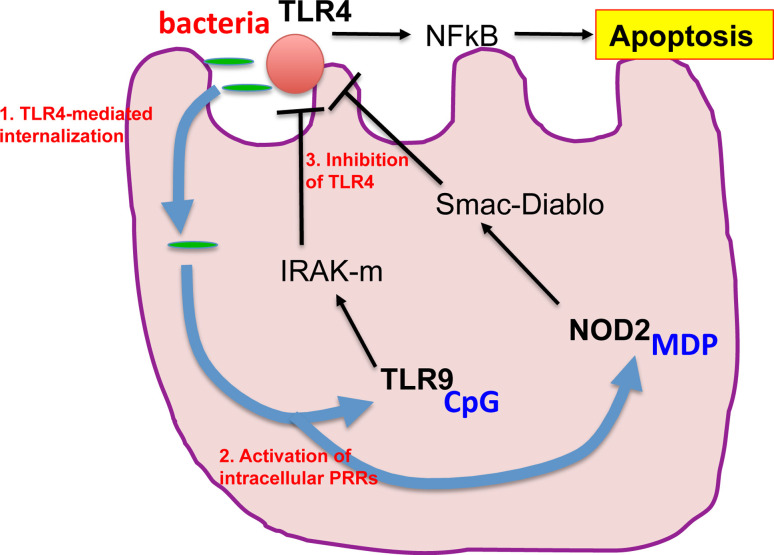
Can an understanding of the relationship between TLR4 and TLR9 signaling within the intestinal epithelium provide insights into the unique susceptibility of the premature infant to necrotizing enterocolitis? To answer this question, we have evaluated the ontogeny of TLR4 and TLR9 expression within the murine developing gut, and have noted that while TLR4 expression rises during development, TLR9 falls, such that the premature mouse is characterized by elevated TLR4 and reduced TLR9 levels. Upon subsequent colonization by bacteria in the immediate postnatal period, the preterm intestine can be thought of as being “primed” for the development of NEC, due to the imbalance between excessive “injurious” TLR4 and reduced “protective” TLR9 levels [59]. Should this pattern of developmental expression also hold true in the human, an understanding of the developmental regulation of TLR4 and TLR9 within the intestine may shed light not only on the factors that link the innate immune system with postnatal gut inflammation, but also may provide the opportunity for therapeutic manipulation of this system in at risk neonates immediately prior to or after delivery (Fig. 3).
Fig. 3.
The interaction between pattern-recognition receptors determines the extent of TLR4-induced enterocyte apoptosis. As is described in the text, TLR4 activation in enterocytes leads to the induction of apoptosis. In parallel, TLR4 activation has been found to play an important role in facilitating the internalization of microbes into enterocytes. These microbes can then activate intracellular pathogen recognition receptors (PRRs) including TLR9 and NOD2/card15 which serve to limit the extent of TLR4 signaling, to reduce the extent of enterocyte apoptosis, and to preserve barrier integrity
TLR2
TLR2 is a transmembrane member of the TLR family that recognizes conserved molecular patterns associated with both Gram-negative and Gram-positive bacteria, including lipoproteins, peptidoglycan (PGN), lipoteichoic acid, and zymosan [68]. Similar to TLR4, TLR2 signals through the NF-κB transcription factor, which when activated produces cytokines and chemokines [69]. Within the intestine, TLR2-mediated pro-inflammatory signaling in the healthy gut is inhibited by factors such as decreased receptor expression, high levels of Tollip, and A20 [68, 70–72]. Within the intestinal epithelium, Cario et al. [73] have shown that TLR2 activation can enhance mucosal integrity through enhanced ZO-1 signaling. In further in vivo studies, they demonstrated that the degree of mucosal inflammation in models of DSS-colitis was significantly increased in TLR2-deficient mice due to increased enterocyte apoptosis [68], while the administration of the TLR2 ligand PCSK significantly suppressed mucosal inflammation and apoptosis by efficiently restoring tight junction-associated integrity of the intestinal epithelium in vivo. TLR2 activation was also shown to induce synthesis of trefoil factor 3 (TFF3), which enhances barrier integrity, providing a further clue as to the reason for the increased mucosal inflammation in the TLR2-deficient mice [30]. These studies clearly point to an important role for TLR2 in the regulation of gut inflammation, in part through effects on enterocyte apoptosis.
NOD2/CARD15
The nucleotide-binding oligomerization domain-containing-2 (NOD2) protein is a member of the NOD-like receptor protein family that functions as an intracellular PRR that recognizes and responds to microbial products that are common among a number of Gram-positive and Gram-negative bacteria [74–77]. One such molecule is the peptidoglycan-derived molecule, muramyl-dipeptide (MDP). Recognition of MDP by NOD2 drives the activation of the pro-inflammatory transcription factor NF-κB and mitogen-activated protein kinase (MAPK) [75, 78]. The importance of NOD2 in intestinal disease is supported by studies that show variants in NOD2 gene to predispose individuals to Crohn’s disease [79–83]. NOD2 was most commonly associated with a role in immune cells such as macrophages, dendritic cells, granulocytes [78, 84, 85], but has also been shown to be expressed in intestinal epithelial cells [34]. A connection between NOD2 activation and inhibition of TLR’s was initially made when NOD2-deficient mice had exacerbated colitis, which was ameliorated in double TLR2/NOD2 knockout mice [86]. Subsequently, NOD2 inhibition of TLR4 was proven in vitro using dendritic cells and in vivo in DSS and TNBS model of experimental colitis [87].
Our laboratory has recently demonstrated that NOD-2 can significantly inhibit TLR4 signaling in the enterocytes of the neonatal small intestine resulting in a marked protection from the induction of apoptosis [34]. Studies using dominant-negative expression of NOD2 and TLR4 in both intestinal epithelial cells (IEC-6) and mice showed that NOD2 activation with MDP was effective in preventing TLR4-dependant apoptosis and to attenuate the severity of it. In seeking to define the mechanisms by which NOD2 activation could attenuate TLR4-mediated enterocyte apoptosis, we further demonstrated a novel link between NOD2 activation and the induction of the protein intermediate SMAC-DIABLO, which stands for second mitochondria-derived activator of caspases/direct inhibitor of apoptosis-binding protein with low PI (SMAC-DIABLO) [34]. Taken together, these findings indicate that by inducing SMAC-DIABLO, a well-known intracellular inhibitor of apoptosis, activation of the innate immune system activation by intracellular pathogens can attenuate the extent of enterocyte apoptosis that may otherwise occur in response to unchecked TLR4 signaling (Fig. 3).
What is the possible teleological explanation for the interplay between NOD2, TLR9, and TLR4 in enterocytes? One possible reason may be found in our observation that TLR4 leads to the internalization of bacteria by enterocytes, a process that may be necessary for antigen sampling and the education of the gut to the presence of various immunologically active peptides [54]. Such intracellular antigens could then conceivably activate TLR9 and NOD2 and thereby restrain TLR4 signaling, effectively preventing further bacterial entry, while also limiting the effects of TLR4-induced intestinal injury. We now propose that under conditions in which TLR4 signaling may be exaggerated—such as in the premature small intestine or in the adult under conditions of stress—the extent of TLR4 signaling in enterocytes may be regulated in large part through the combined interactions of TLR4, NOD2, and other innate immune receptors such as TLR9, and that the extent of these interactions is governed by a dynamic interplay between the microbial flora and the inflammatory microenvironment of the host (Fig. 3).
Enterocyte apoptosis and intestinal disease
As discussed above, while apoptosis is a normal physiological event within the gastrointestinal tract, intestinal disease can result whenever enterocyte apoptosis is increased, or conversely when enterocyte apoptosis is reduced. The following section will describe intestinal diseases that are caused, and exacerbated by, a shift in apoptotic equilibrium within the gastrointestinal tract.
Enterocyte apoptosis and necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a devastating intestinal inflammatory disease and is the leading emergency among preterm infants [31, 88]. NEC is characterized by the acute development of intestinal necrosis, systemic sepsis, intestinal pneumatosis, ischemia reperfusion injury, and multi-organ failure [89]. Factors such as bacterial colonization and endotoxin exposure, hypoxia, ischemia reperfusion, and enteral formula feeding have all been identified as predisposing factors leading to NEC [34, 35, 90–93]. Interestingly, these mentioned factors have also been identified as proapoptotic factors and thus it is not surprising that NEC has been associated with increased rates of intestinal epithelial apoptosis. Early studies identified intestinal apoptosis as one of the early events preceding overt inflammatory necrosis [94]. In this rat model of NEC, formula feeding and hypoxia-induced stress was shown to increase DNA fragmentation and caspases-3 abundance and these biological abnormalities common to apoptosis preceded gross bowel morphological necrosis. Supplementation with a pan-caspase inhibitor reversed these effects by normalizing rates of apoptosis leading to decreased NEC incidence. More recently, activation of the sentinel immune receptor TLR4 has been shown to be directly responsible for increased rates of apoptosis and NEC. Utilization of mice with deficient TLR4 signaling showed that LPS-induced intestinal apoptosis was prevented and NEC incidence reduced [35]. As described above, the TLR4-mediated induction of enterocyte apoptosis in the neonatal intestine could be reduced both by activation of TLR9 [59] as well as by NOD2, through the induction of the endogenous inhibitor of apoptosis SMAC-DIABLO [34]. These studies provide a strong rationale for studying how the regulation of enterocyte apoptosis within the newborn intestine can perhaps provide novel therapeutic insights for preterm infants with NEC.
Enterocyte apoptosis and inflammatory bowel disease
Collectively, ulcerative colitis (UC) and Crohn’s disease (CD) are referred to as inflammatory bowel disease (IBD), a disease of chronic relapsing idiopathic inflammation of the gastrointestinal tract [95]. Current dogma states that IBD results from the interplay of genetic susceptibility, and the exaggerated and inappropriate mucosal immune response to colonizing bacteria. Despite several clinical and pathological differences between UC and CD, apoptosis is a common component to both inflammatory diseases [96]. Several mouse models have been developed to study IBD, introducing genetic susceptibility through the gene-targeted deletions or insertions, or through the utilization of chemical inducers of inflammation [97–102]. Many of these experimental models have studied the role of apoptosis in the development of disease. For instance, in a dextran-sodium sulfate (DSS) model of colitis, mice with the genetic deletion of a known regulator of intestinal barrier integrity, protein kinase C iota (PKCι), experienced impaired recovery for intestinal injury [103], in association with increased mucosal inflammation and apoptosis. It has also been suggested that PKCι signaling leads to improved epithelial restitution through the actions of Trefoil factor 3(TFF3), a molecule released by goblet cells that has previously been shown to inhibit enterocyte apoptosis in the setting of inflammation [30]. Similar studies have shown that TLRs (TLR4 and TLR2) are important regulators of apoptosis that is present in experimental colitis. For instance, in a study by Fukata et al. [58], TLR4-deficient mice showed significantly reduced intestinal epithelial proliferation and increase apoptosis following DSS injury compared to wild-type controls. The authors show that the COX-2 and PGE2, both important mediators of inflammation and epithelial repair, were down-regulated in TLR4-deficient mice, and that supplementation of PGE2 restored proliferation and apoptosis and improved the clinical outcome of TLR4-deficient mice.
Additional studies have assessed the role of tumor necrosis factor (TNF) in the regulation of enterocyte apoptosis in the setting of IBD. TNF is a pro-inflammatory cytokine that has been directly associated with the development of both CD and UC. TNF levels are elevated in the serum, mucosa, and stool of patients with IBD, and infusion of monoclonal anti-TNF antibody is a well-recognized, commonly used, and quite efficacious therapy for IBD in children and adults [104]. Several studies have identified a region on chromosome 6p21, IBD3, as an IBD-susceptibility locus in four independent linkage studies [105–108]. IBD3 encompasses the TNFα gene, a strong positional and functional candidate gene for IBD [104], providing an additional biological explanation for the link between IBD signaling and TNF.
Enterocyte apoptosis and intestinal cancer
While this review has so far focused on the role of exaggerated enterocyte apoptosis in the setting of mucosal inflammation through increased barrier disruption, a widening body of literature has indicated that deficiencies in apoptotic programs within the intestine can lead to the development of malignancy [109]. In general terms, when proliferation of malignant tumors is offset by similar rates of apoptosis, the tendency to develop a malignancy is reduced, while an impairment in the degree of apoptosis and a corresponding increase in proliferation favors the development of intestinal malignancy [110, 111]. In a recent study, Voisin et al. [112] showed that re-establishing the balance between apoptosis and proliferation within tumors through the stimulation of OX1R, a proapoptotic transmembrane receptor, was sufficient to slow tumor growth and prevent new tumor formation. Furthermore, several studies have found that deficiencies in the apoptotic mechanism can also result from abnormalities or mutations in important homeostatic proteins such as p53, K-ras, APC, EGF-R, and TLRs [1, 113–115], and some of these mutations may serve to be informative biomarkers of disease. Another key contributor to the development of cancer within the intestine is chronic inflammation, and this field of focus has expanded significantly in recent years with the observation that anti-inflammatory strategies that may reduce the risk of malignant change [116–118]. In particular, inflammatory mediators such as IL-1, TNF-α, IL-8, nitric oxide, or prostaglandin-2 derivatives may each act as mediators connecting inflammation and cancer development [119]. In a study with germ-free rats, it was shown that the continual inflammatory stimulation imposed by colonizing bacteria is also important in the development of colorectal carcinomas as germ-free rats developed fewer and smaller tumors than conventionally reared animals [120]. The exact mechanism regarding the inflammation-dependant growth of gastrointestinal malignancies still remains incompletely understood, but some evidence suggests that constitutively high levels of NF-κB activation as well as TLR signaling may be partially responsible for malignancy progression [121–123]. Interestingly, due to the influence of colonizing bacteria on chronic inflammation and cancer development, therapeutic strategies involving the administration of beneficial bacteria (probiotics) for the prevention of intestinal inflammatory diseases are starting to emerge [120]. These findings raise the exciting possibility that future strategies based upon the manipulation of the extent of enterocyte apoptosis may emerge as novel approaches to both the treatment and prevention of cancer in the gut.
Conclusions and future perspectives
This review highlights the importance of the innate immune system in the regulation of enterocyte apoptosis in the steps that lead to the development of intestinal inflammation—including necrotizing enterocolitis and inflammatory bowel disease—and also examined the role of enterocyte apoptosis in the pathogenesis of intestinal malignancy. A recurrent theme raised by each of these studies is the importance of the intestinal flora in disease development, which provide ligands for the innate immune receptors found on the intestinal epithelium. Thus, further delineation and understanding of the role of the intestinal microbiome in the regulatory pathways of gastrointestinal apoptosis is likely to be critical for our ability to therapeutically manipulate the role of apoptosis and prevention of intestinal disease. This line of thinking has lead to the emergence of probiotics for both the prophylactic prevention and treatment of gastrointestinal inflammatory diseases. In an extremely exciting line of investigation, evidence suggests that transgenic probiotic bacteria may serve as a method of reducing manipulating apoptosis and inflammation [124, 125]. While this a promising therapeutic approach, there may be some apprehension towards the delivery of transgenic bacteria to compromised individuals. Further avenues of research will include the incorporation of high-throughput screens—which may be performed in silico as well as in cells and tissue—to identify agents that can bridge the innate immune system with critical components of the apoptosis pathways in the gut, and can thus serve as novel anti- or pro-apoptotic drugs. Those agents with pharmacologic properties that enhance their gastrointestinal selectivity may be particular exciting in this regard, and thus offer novel strategies for the prevention and treatment of necrotizing enterocolitis, inflammatory bowel disease, and intestinal cancers.
References
- 1.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 2.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 3.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10:221. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 5.Piccinini AM, Midwood KS (2010) DAMPening inflammation by modulating TLR signaling. Mediators Inflamm 2010:672395 [DOI] [PMC free article] [PubMed]
- 6.Sodhi C, Levy RM, Gill R, Neal M, Richardson W, Branca M, Russo A, Prindle T, Billiar TR, Hackam DJ. DNA Attenuates Enterocyte Toll-like Receptor 4-mediated Intestinal Mucosal Injury after Remote Trauma. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G862–873. doi: 10.1152/ajpgi.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai S, Sodhi C, Cetin S, Richardson W, Branca M, Neal MD, Prindle T, Ma C, Shapiro RA, Li B, Wang JH, Hackam DJ. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of Toll-like receptor-4 and increased cell-matrix adhesiveness. J Biol Chem. 2010;285:4995. doi: 10.1074/jbc.M109.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 9.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185. doi: 10.1016/S1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 10.Hausmann M. How bacteria-induced apoptosis of intestinal epithelial cells contributes to mucosal inflammation. Int J Inflamm. 2010;2010:574568. doi: 10.4061/2010/574568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen WH, Wang J, Wu J, Zhurkin VB, Yin Y. Mitogen-activated protein kinase phosphatase 2: a novel transcription target of p53 in apoptosis. Cancer Res. 2006;66:6033. doi: 10.1158/0008-5472.CAN-05-3878. [DOI] [PubMed] [Google Scholar]
- 12.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 13.Salaun B, Romero P, Lebecque S. Toll-like receptors' two-edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37:3311. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi C, Kirchhoff S, Krammer PH, Peter ME. Apoptosis signaling in lymphocytes. Curr Opin Immunol. 1999;11:277. doi: 10.1016/S0952-7915(99)80045-4. [DOI] [PubMed] [Google Scholar]
- 15.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 16.Oh KJ, Singh P, Lee K, Foss K, Lee S, Park M, Aluvila S, Kim RS, Symersky J, Walters DE. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J Biol Chem. 2010;285:28924. doi: 10.1074/jbc.M110.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens TW, Valentijn AJ, Upton JP, Keeble J, Zhang L, Lindsay J, Zouq NK, Gilmore AP. Apoptosis commitment and activation of mitochondrial Bax during anoikis is regulated by p38MAPK. Cell Death Differ. 2009;16:1551. doi: 10.1038/cdd.2009.102. [DOI] [PubMed] [Google Scholar]
- 18.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 19.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 20.Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J Biol Chem. 2001;276:6453. doi: 10.1074/jbc.M006391200. [DOI] [PubMed] [Google Scholar]
- 21.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005;102:17975. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331. doi: 10.1016/S0092-8674(02)01036-X. [DOI] [PubMed] [Google Scholar]
- 26.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060. [PMC free article] [PubMed] [Google Scholar]
- 27.Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-Like Receptor 5 Stimulation Protects Mice from Acute Clostridium difficile Colitis. Infect Immun. 2011;79:1498. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA, Lenaerts K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 29.O’Gorman A, Colleran A, Ryan A, Mann J, Egan LJ. Regulation of NF-kappaB responses by epigenetic suppression of IkappaBalpha expression in HCT116 intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G96. doi: 10.1152/ajpgi.00460.2009. [DOI] [PubMed] [Google Scholar]
- 30.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 32.Le Mandat Schultz A, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS One. 2007;2:e1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 34.Richardson WM, Sodhi CP, Russo A, Siggers RH, Afrazi A, Gribar SC, Neal MD, Dai S, Prindle T, Jr, Branca M, Ma C, Ozolek J, Hackam DJ. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 36.Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29:469. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 40.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 41.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 42.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 43.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 44.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 45.Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2010;10:98. doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Lee KM, Seong SY. Partial role of TLR4 as a receptor responding to damage-associated molecular pattern. Immunol Lett. 2009;125:31. doi: 10.1016/j.imlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res. 2009;10:31. doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, Vallabhaneni R, Vodovotz Y, Billiar TR. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov R, Janeway CA., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12. doi: 10.1016/S0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 50.Medzhitov R, Janeway CA., Jr Self-defense: the fruit fly style. Proc Natl Acad Sci USA. 1998;95:429. doi: 10.1073/pnas.95.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 52.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 53.Cetin S, Ford HR, Sysko LR, Agarwal C, Wang J, Neal MD, Baty C, Apodaca G, Hackam DJ. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004;279:24592. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- 54.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 55.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torchinsky MB, Garaude J, Blander JM. Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 2010;22:55. doi: 10.1016/j.coi.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obermeier F, Dunger N, Strauch UG, Hofmann C, Bleich A, Grunwald N, Hedrich HJ, Aschenbrenner E, Schlegelberger B, Rogler G, Scholmerich J, Falk W. CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology. 2005;129:913. doi: 10.1053/j.gastro.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 61.Obermeier F, Strauch UG, Dunger N, Grunwald N, Rath HC, Herfarth H, Scholmerich J, Falk W. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut. 2005;54:1428. doi: 10.1136/gut.2004.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Askew D, Chu RS, Krieg AM, Harding CV. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J Immunol. 2000;165:6889. doi: 10.4049/jimmunol.165.12.6889. [DOI] [PubMed] [Google Scholar]
- 63.Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19:618. doi: 10.1016/S0264-410X(00)00249-8. [DOI] [PubMed] [Google Scholar]
- 64.Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, Weiner GJ. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J Immunol. 2000;165:6244. doi: 10.4049/jimmunol.165.11.6244. [DOI] [PubMed] [Google Scholar]
- 65.Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Scholmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 67.Obermeier F, Dunger N, Deml L, Herfarth H, Scholmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol. 2002;32:2084. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 68.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430:218. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 70.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 72.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. Gastroenterology. 2004;127:224. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Lamkanfi M, Amer A, Kanneganti TD, Munoz-Planillo R, Chen G, Vandenabeele P, Fortier A, Gros P, Nunez G. The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol. 2007;178:8022. doi: 10.4049/jimmunol.178.12.8022. [DOI] [PubMed] [Google Scholar]
- 77.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 78.Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology. 2011;140:231. doi: 10.1053/j.gastro.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fritz T, Niederreiter L, Adolph T, Blumberg RS, Kaser A. Crohn's disease: NOD2, autophagy and ER stress converge. Gut. 2011;60(11):1580–1588. doi: 10.1136/gut.2009.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zilbauer M, Jenke A, Wenzel G, Goedde D, Postberg J, Phillips AD, Lucas M, Noble-Jamieson G, Torrente F, Salvestrini C, Heuschkel R, Wirth S. Intestinal alpha-defensin expression in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2010;17(10):2076–2086. doi: 10.1002/ibd.21577. [DOI] [PubMed] [Google Scholar]
- 81.Dassopoulos T, Nguyen GC, Talor MV, Datta LW, Isaacs KL, Lewis JD, Gold MS, Valentine JF, Smoot DT, Harris ML, Oliva-Hemker M, Bayless TM, Burek CL, Brant SR. Intestinal alpha-defensin expression in pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105:378. doi: 10.1038/ajg.2009.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsumoto S, Hara T, Nagaoka M, Mike A, Mitsuyama K, Sako T, Yamamoto M, Kado S, Takada T. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009;128:e170. doi: 10.1111/j.1365-2567.2008.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maconi G, Colombo E, Sampietro GM, Lamboglia F, D’Inca R, Daperno M, Cassinotti A, Sturniolo GC, Ardizzone S, Duca P, Porro GB, Annese V. CARD15 gene variants and risk of reoperation in Crohn's disease patients. Am J Gastroenterol. 2009;104:2483. doi: 10.1038/ajg.2009.413. [DOI] [PubMed] [Google Scholar]
- 84.Qiu F, Maniar A, Quevedo Diaz M, Chapoval AI, Medvedev AE. Activation of cytokine-producing and antitumor activities of natural killer cells and macrophages by engagement of Toll-like and NOD-like receptors. Innate Immun. 2010;17(4):375–387. doi: 10.1177/1753425910372000. [DOI] [PubMed] [Google Scholar]
- 85.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73:7967. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 89.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Jiang P, Sangild PT, Siggers RH, Sit WH, Lee CL, Wan JM. Bacterial Colonization Affects the Intestinal Proteome of Preterm Pigs Susceptible to Necrotizing Enterocolitis. Neonatology. 2010;99:280. doi: 10.1159/000317807. [DOI] [PubMed] [Google Scholar]
- 91.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 92.Caplan MS, Kelly A, Hsueh W. Endotoxin and hypoxia-induced intestinal necrosis in rats: the role of platelet activating factor. Pediatr Res. 1992;31:428. doi: 10.1203/00006450-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 93.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161:583. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 94.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 95.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 96.Chen L, Park SM, Turner JR, Peter ME. Cell death in the colonic epithelium during inflammatory bowel diseases: CD95/Fas and beyond. Inflamm Bowel Dis. 2010;16:1071. doi: 10.1002/ibd.21191. [DOI] [PubMed] [Google Scholar]
- 97.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 98.Chaniotou Z, Giannogonas P, Theoharis S, Teli T, Gay J, Savidge T, Koutmani Y, Brugni J, Kokkotou E, Pothoulakis C, Karalis KP. Corticotropin-releasing factor regulates TLR4 expression in the colon and protects mice from colitis. Gastroenterology. 2010;139:2083. doi: 10.1053/j.gastro.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N, Li JS. Corticotropin-releasing factor regulates TLR4 expression in the colon and protects mice from colitis. Mol Immunol. 2010;47:2467. doi: 10.1016/j.molimm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 100.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 101.Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16:1017. doi: 10.1002/ibd.21151. [DOI] [PubMed] [Google Scholar]
- 102.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calcagno SR, Li S, Shahid MW, Wallace MB, Leitges M, Fields AP, Murray NR. Protein kinase C iota in the intestinal epithelium protects against dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2010;17(8):1685–1697. doi: 10.1002/ibd.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Heel DA, Udalova IA, De Silva AP, McGovern DP, Kinouchi Y, Hull J, Lench NJ, Cardon LR, Carey AH, Jewell DP, Kwiatkowski D. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factors. Hum Mol Genet. 2002;11:1281. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- 105.Yang H, Ohmen JD, Ma Y, Bentley LG, Targan SR, Fischel-Ghodsian N, Rotter JI. Additional evidence of linkage between Crohn's disease and a putative locus on chromosome 12. Genet Med. 1999;1:194. doi: 10.1097/00125817-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Hampe J, Schreiber S, Shaw SH, Lau KF, Bridger S, Macpherson AJ, Cardon LR, Sakul H, Harris TJ, Buckler A, Hall J, Stokkers P, van Deventer SJ, Nurnberg P, Mirza MM, Lee JC, Lennard-Jones JE, Mathew CG, Curran ME. A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet. 1999;64:808. doi: 10.1086/302294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hampe J, Shaw SH, Saiz R, Leysens N, Lantermann A, Mascheretti S, Lynch NJ, MacPherson AJ, Bridger S, van Deventer S, Stokkers P, Morin P, Mirza MM, Forbes A, Lennard-Jones JE, Mathew CG, Curran ME, Schreiber S. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet. 1999;65:1647. doi: 10.1086/302677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB, Bitton A, Williams CN, Greenberg GR, Cohen Z, Lander ES, Hudson TJ, Siminovitch KA. Am J Hum Genet. 2000;66:1863. doi: 10.1086/302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 110.Yu HG, Yu LL, Yang Y, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Increased expression of RelA/nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology. 2003;65:37. doi: 10.1159/000071203. [DOI] [PubMed] [Google Scholar]
- 111.Sinicrope FA, Roddey G, McDonnell TJ, Shen Y, Cleary KR, Stephens LC. Increased apoptosis accompanies neoplastic development in the human colorectum. Clin Cancer Res. 1996;2:1999. [PubMed] [Google Scholar]
- 112.Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, Paradis V, Bedossa P, Henin D, Lehy T, Laburthe M. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases : an openable gate to apoptosis. Cancer Res. 2011;17(9):375–387. doi: 10.1158/0008-5472.CAN-10-3473. [DOI] [PubMed] [Google Scholar]
- 113.Rao B, Gao Y, Huang J, Gao X, Fu X, Huang M, Yao J, Wang J, Li W, Zhang J, Liu H, Wang L. Mutations of p53 and K-ras correlate TF expression in human colorectal carcinomas: TF downregulation as a marker of poor prognosis. Int J Colorectal Dis. 2011;26(5):593–601. doi: 10.1007/s00384-011-1164-1. [DOI] [PubMed] [Google Scholar]
- 114.Dempke WC, Heinemann V. Resistance to EGF-R (erbB-1) and VEGF-R modulating agents. Eur J Cancer. 2009;45:1117. doi: 10.1016/j.ejca.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 115.Zhu W, Fang C, Gramatikoff K, Niemeyer CC, Smith JW. Proteins and an Inflammatory Network Expressed in Colon Tumors. J Proteome Res. 2011;10(5):2129–2139. doi: 10.1021/pr101190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, Bronner MP, Al-Lahham B, Kowdley KV, Lindor KD, Rabinovitch PS, Brentnall TA. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71:1669. doi: 10.1158/0008-5472.CAN-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 118.Cheng Y, Desreumaux P. 5-aminosalicylic acid is an attractive candidate agent for chemoprevention of colon cancer in patients with inflammatory bowel disease. World J Gastroenterol. 2005;11:309. doi: 10.3748/wjg.v11.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, Rossmann P, Hrncir T, Kverka M, Zakostelska Z, Klimesova K, Pribylova J, Bartova J, Sanchez D, Fundova P, Borovska D, Srutkova D, Zidek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vannucci L, Stepankova R, Kozakova H, Fiserova A, Rossmann P, Tlaskalova-Hogenova H. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int J Oncol. 2008;32:609. [PubMed] [Google Scholar]
- 121.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukata M, Shang L, Santaolalla R, Sotolongo J, Pastorini C, Espana C, Ungaro R, Harpaz N, Cooper HS, Elson G, Kosco-Vilbois M, Zaias J, Perez MT, Mayer L, Vamadevan AS, Lira SA, Abreu MT. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2010;17(7):1464–1473. doi: 10.1002/ibd.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, Herdman S, Varki N, Corr M, Lee J, Raz E. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palffy R, Gardlik R, Behuliak M, Jani P, Balakova D, Kadasi L, Turna J, Celec P. Salmonella-mediated gene therapy in experimental colitis in mice. Exp Biol Med (Maywood) 2011;236:177. doi: 10.1258/ebm.2010.010277. [DOI] [PubMed] [Google Scholar]
- 125.Gardlik R, Behuliak M, Palffy R, Celec P, Li CJ. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy. Gene Ther. 2011;18(5):425–431. doi: 10.1038/gt.2010.176. [DOI] [PubMed] [Google Scholar]