Abstract
Introduction
Tff3 peptide exerts important functions in cytoprotection and restitution of the gastrointestinal (GI) tract epithelia. Moreover, its presence in the rodent inner ear and involvement in the hearing process was demonstrated recently. However, its role in the auditory system still remains elusive. Our previous results showed a deterioration of hearing with age in Tff3-deficient animals.
Results
Present detailed analysis of auditory brain stem response (ABR) measurements and immunohistochemical study of selected functional proteins indicated a normal function and phenotype of the cochlea in Tff3 mutants. However, a microarray-based screening of tissue derived from the auditory central nervous system revealed an alteration of securin (Pttg1) and serpina3n expression between wild-type and Tff3 knock-out animals. This was confirmed by qRT-PCR, immunostaining and western blots.
Conclusions
We found highly down-regulated Pttg1 and up-regulated serpina3n expression as a consequence of genetically deleting Tff3 in mice, indicating a potential role of these factors during the development of presbyacusis.
Keywords: Trefoil peptides, Cochlea, Inferior colliculus, Presbyacusis, Expression patterns
Introduction
The trefoil factor family (TFF) in mammals comprises three peptides: TFF1, 2 and 3, predominantly expressed in the gastrointestinal (GI) mucosa but also in several other tissues [1–3]. TFFs play an important physiological role and are upregulated in many pathological conditions of mucosal injury such as inflammatory bowel disease (IBD), gastric and duodenal ulcers [4]. TFF3 maintains the GI integrity [5], promotes intestinal epithelial wound healing [6] and modulates adhesion, migration and survival of epithelial cells [7]. Moreover, Tff3 synthesis was shown in the rat and human hypothalamus [8]. The presence of Tff3 exclusively in oxytocinergic neurons but not in vasopressinergic neurons suggested its role as a neuropeptide acting together with oxytocin along the hypothalamo-pituitary axis [9].
Previously, we demonstrated a new expression pattern of Tff3 in the rodent cochlea and the peptide’s involvement in the hearing process [18]. We showed that Tff3 knock-out mice display a deterioration of hearing with age, a feature characteristic for presbyacusis (age-related hearing loss) [11, 12]. However, in Tff3-deficient animals we did not observe any degeneration of cochlear hair cells and spiral ganglion neurons or atrophy of the stria vascularis (SV) [10].
Presently, we focused our analysis on ABR responses and spatial and temporal expression of particular marker proteins involved in normal hearing comparing Tff3 knock-out to wild-type animals. By studying ABR responses we investigated if the auditory processing pathway is obstructed in Tff3 knock-out animals, whereas examination of different cochlear protein markers may reveal if lack of Tff3 affects expression of particular genes in the cochlea. Lack of striking differences in the cochlea using these assays together with interesting data about functional changes underlying age-related hearing loss in the inferior colliculus (IC) [13, 14] turned our attention to the auditory central nervous system (CNS). We thus performed a microarray-based expression screening of genes differentially expressed in the IC followed by confirmation using quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). Two conspicuous candidate sequences showed significantly altered expression levels. Neither the presence of Pttg1 and serpina3n in the auditory system nor their interplay with trefoil peptides has been noted so far. Therefore, the differential expression pattern of these genes caused by genetic deletion of Tff3 suggests their potential involvement during presbyacusis, which requires additional elucidation of the mechanisms involved.
Materials and methods
Animals
Tff3-deficient animals [5] were obtained from Prof. D.K. Podolsky (Harvard Medical School). These animals were backcrossed to C57BL/6 and to 129/Sv mice, and maintained on a mixed background. Tff3 homozygous sister lines were established representing a Tff3-deficient (Tff3 −/−) and a wild-type genotype (Tff3 +/+), respectively. Animal experiments were approved and complied with all requirements at the University of Tübingen and the German law for use and welfare of laboratory animals (Tierschutzgesetz). Animal studies were also in line with the ‘Ethical principles and guidelines for scientific experiments on animals’ of the Swiss Academy of Medical Sciences. All efforts were made to minimize the number of animals used and their suffering. Animals were killed by decapitation following anesthesia with carbon dioxide, and cochleae were removed according to national ethical guidelines.
ABR responses
A minimum of three wild-type and Tff3 −/− mutants were used for each measurement. All measurements were made under anesthesia. A dose of 50 mg/kg ketamin hydrochloride (Ketamin 50 Curamed, CuraMED Pharma, Germany), 8 mg/kg xylazin hydrochloride (Rompun 290, Bayer Leverkusen, Germany) and 0.25 mg/kg atropin sulfate (Atropinsulfat, Braun, Germany) was used. The mixture was applied intraperitoneally. Depth of anesthesia was checked every 30 min by testing the pedal withdrawal reflex. If necessary, additional injections of about 50% of the initial dose were given. Body temperature was maintained at 37°C using a heating pad and thermo resistor placed under the animals’ body.
ABR recordings were performed in a soundproof chamber (IAC, Niederkrüchten, Germany). For stimulus generation and recording of responses, a multi-function IO-Card (PCI-6259, National Instruments, Austin, TX) was used, housed in an IBM-compatible computer. Sound pressure level was controlled with an attenuator (custom-made) and amplifier. Stimuli were delivered to the ear in a calibrated open system by a loudspeaker (DT911, Beyerdynamics, Heilbronn, Germany) placed 3 cm lateral to the animals’ pinna. Sound pressure was calibrated on-line prior to each measurement with a microphone probe system (Brüel & Kjaer 4191, Bremen, Germany) placed near the animals’ ear. Recorded signals were amplified (100 dB total amplification) and bandpass filtered (0.2–5 kHz). Clicks of 100-μs duration or tone pipes of 3-ms duration (including 1-ms rise and fall time) were presented. To record bioelectrical responses, subdermal silver-wire electrodes were inserted at the vertex (active) and ventro–lateral (reference) to the measured ear. Electrical signals were averaged over 64 repetitions of stimulus pairs with alternating phase. ABRs were measured for clicks or stimulus frequencies between 2.0 and 45.2 kHz and sound pressure levels from 20 to 110 dB SPL in 5-dB steps. To construct the ABR input-output functions, the peak-to-peak amplitudes of the ABR waveforms at the different sound pressure levels were determined. Thresholds were defined as the sound pressure level where a stimulus-correlated response was clearly identified by visual inspection of the averaged signal.
Tissue preparation
Wild-type and Tff3 knock-out mice were decapitated following anesthesia with carbon dioxide. The bullae were removed, and the cochlear spiral was dissected in Hanks’ Balanced Salt Solution (HBSS: KCl 5.36, MgSO4·7H2O 0.405, MgCl2·5H2O 0.491, NaCl 141, HEPES 9.98, l-glutamin 3.42, CaCl2·2H2O 1.56, d-glucose 6.30; concentration of all components in mMol/l) with a pH of 7.4. Cochleae were fixed by immersion in 2% paraformaldehyde (SIGMA, Munich, Germany; all chemicals from SIGMA, unless indicated otherwise), 125 mM sucrose in 100 mM phosphate-buffered saline (PBS), pH 7.4, for 2 h. Cochleae of animals older than postnatal day 10 were decalcified after fixation for 15 min to 2 h in rapid bone decalcifier (Eurobio, Fischer-Scientific, 61130 Nidderau, Germany). After overnight incubation in 25% sucrose in PBS, pH 7.4, cochleae were embedded in OCT compound (Miles Laboratories, Elkhart, IN), cryosectioned at 10 μm, mounted on SuperFrost*/plus microscope slides, dried for 1 h and stored at −20°C before use. For the detection of securin protein in brain sections, brains were removed and fixed for 48 h in 4% paraformaldehyde, embedded in 4% agarose and stored in PBS + 0.4% paraformaldehyde at 4°C. The tissue was sectioned at 60 μm with the VT 1000S vibrating microtome (Leica, Wetzlar, Germany). Slices were kept in PBS in a 24-well plate.
Fluorescence immunohistochemistry of different markers
For immunohistochemistry, mouse cochlea sections were stained and imaged as described [15, 16]. The following antibodies were used: goat polyclonal anti-KCNQ4 (Santa Cruz Biotechnology, Santa Cruz, CA; sc-9385, lot B161), goat polyclonal anti-megalin (Santa Cruz Biotechnology, lot D0914), rabbit polyclonal anti-KCNQ1 (Santa Cruz Biotechnology, sc-20816, lot B2103), sheep polyclonal anti-neurofilament 200 (NF200, The Binding Site, Heidelberg, Germany, PH510), rabbit polyclonal anti Kir4.1 (Alomone Labs, Jerusalem, Israel, APC-035), rabbit polyclonal anti-otoferlin [17], goat polyclonal anti-synaptophysin (Santa Cruz Biotechnology, sc-7568, lot E2308), rabbit polyclonal anti-securin (Abcam, ab26273), goat polyclonal anti-serpina3n (R&D, AF4709, Lot CBKW01) and rabbit polyclonal anti-Tff3 [18]. To detect prestin, a polyclonal rabbit antibody directed against the C-terminal epitope of rat prestin was used [19]. Primary antisera were detected with fluorescently labeled secondary IgG antibodies (Cy3-conjugated antibodies, Jackson ImmunoResearch Laboratories, West Grove, PA; or Alexa Fluor-488 conjugated antibodies, Molecular Probes, Eugene, OR). Sections were embedded with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI), staining cell nuclei in blue (Vector Laboratories, Burlingame, CA). Specimens were imaged using an Olympus AX-70 microscope equipped with epifluorescence illumination and 40× (numerical aperture 1.0) or 100× oil immersion objectives (numerical aperture 1.35). Images were acquired using a CCD color view 12 camera and imaging system analysis (SIS, Münster, Germany) and additionally processed with Adobe Photoshop 6.0. Immunohistochemical comparison between control and Tff3 knock-out mice were all performed using the same antibody titre for wild-type and mutant mice, and identical exposure times were used when comparative photographs were taken. Immunohistological analyses were performed from cochlea samples taken from three to five Tff3 knock-out and wild-type mice. Representative images were chosen for presentation.
For localization of securin protein in the IC, brain sections of wild-type mice were prepared as described above. For protein detection on these slices, sections were washed briefly in PBS containing 0.05% Tween 20, and endogenous peroxidases were blocked in 3% H2O2. Blocking of streptavidin-biotin was carried out using the streptavidin-biotin blocking kit (Vector Laboratories) according to the manufacturer’s instructions. Sections were incubated over night at 4°C with the primary securin antibody (Abcam, ab26273). Following incubation with the secondary antibody (biotinylated goat anti-rabbit, Vector Labs, Burlingame, CA), the sections were washed in PBS, and the chromogenic detection was carried out (3-amino-9-ethylcarbazole, AEC). Nuclei were counterstained with methyl-green (Vector Laboratories) and viewed using an Olympus AX70 microscope.
Western blot analysis
For Western blot analysis, cochlear tissues of control and Tff3 −/− mice were homogenized and lysed in CelLytic MT Tissue Lysis/Extraction Reagent (Sigma-Aldrich C3228) supplemented with a protease inhibitor cocktail (1:50, Sigma–Aldrich P8340). Nuclei and cell debris were pelleted by low-speed centrifugation at 280g for 5 min at 4°C, and the supernatant was used for Western blotting. Protein contents were determined using the Bradford method. SDS-PAGE and Western blotting were carried out using the XCell II SureLock™ Mini-Cell and XCell II Blot Module from Invitrogen; 35 μg protein lysate per lane of each cochlear or IC sample was loaded on a 4–12% Tris-glycine gel (Invitrogen), resolved and transferred onto PVDF membrane (Invitrogen) according to the manufacturer’s instructions. Blotted proteins were incubated with rabbit polyclonal anti-securin antibody (1:750, Abcam ab26273) together with mouse monoclonal anti-ezrin antibody (0.5 μg/ml, Dianova DLN-10378) as a housekeeping protein, to ensure loading of equal amounts of proteins, followed by ECL™ peroxidase-labeled anti-rabbit or anti-mouse antibodies, respectively (1:2,000, GE Healthcare). Labeled proteins were detected by chemiluminescence using ECL Plus Western blotting detection reagents (GE Healthcare).
Chip hybridization
RNA was isolated from IC of 12-month-old mice using the RNA NOW kit (Biogentex, Seabrook, TX). Two independent RNA samples were obtained from wild-type and Tff3 knock-out mice. RNA preparation, hybridization to oligonucleotide arrays (Set 430A and 430B) and scanning of the arrays were performed as described in the Affymetrix GeneChip expression analysis manual (http://www.affymetrix.com). Scanned files were analyzed with GeneChip (Affymetrix, Santa Clara, CA), and the expression data were saved as an Excel file containing expression values (average difference).
Quantitative RT-PCR
For qRT-PCR, 3–4 independent RNA samples were extracted from the adult IC and the cochlea at postnatal day 10 (P10), and at 4 and 14 weeks. The IC was identified in accordance with the mouse atlas of Franklin and Paxinos [21]. For RNA preparation, tissue was dissected with small forceps and immediately frozen in liquid nitrogen and stored at −70°C before use; 2 μg of RNA was reverse transcribed with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Quantitative gene expression analysis was performed on a Lightcycler 480 II (Roche) using SYBR Green. The following primers were used (all sequences from Mus musculus): Gapdh (Genebank accession Nr. NM_008084): forward 5′-TCCTGCACCACCAACTGCTT-3′, reverse 5′-GTGGCAGTGATGGCATGGAC-3′, Rpgrip1 (NM_023879): fw 5′-TGTCACATGCAGAGACCACA-3′, rev 5′-ATGCGGCTGTTCTTGAAGTC-3′, Pttg1 (NM_013917): fw 5′-GGCATCTAAGGATGGGTTGA-3′, rev 5′-CATAGGCTTTTCGGCAACTC-3′, Prl (NM_011164): fw 5′-CCACTTCTTCCCTGGCTACA-3′, rev 5′-GATGTATTCGGGGGCTTCTT, Qk (NM_021881): fw 5′-GCAGCTGATGAACGACAAGA-3′, rev. 5′-CGTCAGGCAATTCTGCACTT-3′, Serpina3n (NM_009252): fw 5′-AGGACATTGATGGTGCTGGT-3′, rev 5′-TAGGGTGTGGTCAGGTCCTC-3′, Kcnj9 (NM_008429): fw 5′-CCTCGAGAGGGACGACTTC-5′, rev 5′-CTCAAAGGTTTCGTGGAAGC-3′, Cap1 (NM_007598): fw 5′-CTGGAAGGCAAGAAATGGAG-3′, rev 5′-ACCAGGCCAAGCTTCTTACA-3′, Tnfrsf12a (NM_013749): fw 5′-TTGGCGCTGGTTTCTAGTTT-3′ and rev 5′-GAATGAATGGACGACGAGTG-3′. The cycling condition comprised 10 min of preincubation at 95°C followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. All primers were validated for their amplification efficiency. Each sample was analyzed in triplicate, and data were analyzed with the 2−ΔΔCt method.
Results
Hearing measurement
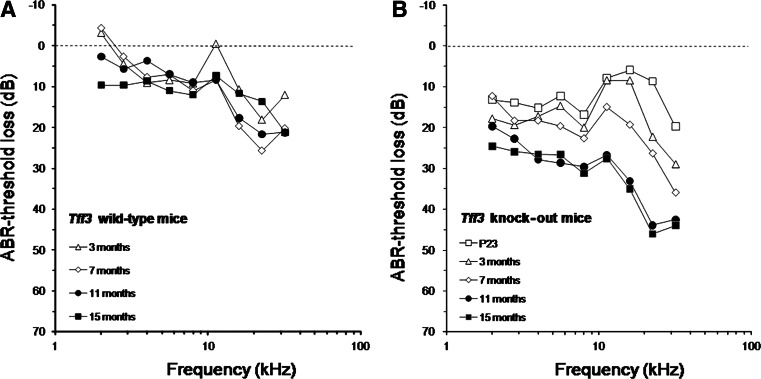
To investigate if auditory processing in Tff3 knock-out animals is obstructed, a detailed study of ABR thresholds and amplitudes was performed by analyzing the animals’ responses to clicks and pure tone bursts. Figure 1 presents averaged ABR-threshold losses of wild-type and Tff3 knock-out mice at different ages; as a reference the average audiogram of P23 wild-type mice was used. In wild-type mice threshold loss was observed at all ages beyond P23, increasing from low to high frequencies at any age. This corresponds to the normal threshold loss pattern seen in mice showing accelerated presbyacusis [22]. In knockout-mice a conspicuous threshold loss in the low-frequency range was observed even in the youngest animals measured. With increasing age the threshold loss increased to values of up to 45–50 dB loss in the frequency range above 20 kHz. Compared to the wild-type mice, at 11 and 15 months the increased pan-cochlear threshold loss of approximately 20 dB in the knockout mice was statistically significant (p < 0.05; t test) at any frequency. The analysis of the ABR waveforms, corrected for elevated thresholds in the knockout mice, showed no alterations in latency or amplitudes (data not shown).
Fig. 1.
Average mouse audiograms at different ages for a wild-type and b Tff3 knockout animals. ABR-threshold curves were measured at the ages indicated in the legend. Threshold loss was calculated relative to the average threshold of the wild-type mice at postnatal day 23. In wild-type mice a threshold loss was observed, increasing from low to high frequencies at any age. In knockout-mice a conspicuous threshold loss in the low-frequency range was observed even in the youngest animals measured. With increasing age the high-frequency threshold loss increased. At 11 and 15 months the difference between knockout and wild-type animals was significant at any frequency
Immunohistochemical analysis of the cochlea of Tff3 mutants
Tff3 knock-out mice developed hearing loss progressing with age. Expression of Tff3 in the organ of Corti, including in hair cells and supporting cells, has been described previously [10, 18]. However, our previous preliminary study revealed no gross morphological abnormalities in the cochlea of Tff3 mutant animals [10]. Therefore, we now performed a detailed study in adult wild-type and Tff3-deficient mice and surveyed the distribution of a series of key proteins related to auditory function.
The Ca2+-binding protein otoferlin, which resides in synaptic vesicle membranes, showed a normal expression pattern in Tff3-deficient animals as well as the neurofilament subunit 200 (NF200), which is a marker for afferent fibers (Fig. 2A). Moreover, outer hair cell-specific markers, such as the voltage-gated potassium channel KCNQ4, was detected at the basal pole of OHCs (Fig. 2b), and the OHC motor protein prestin was present in low-frequency (apical/medial) and high-frequency (midbasal/basal) cochlear turns (Fig. 2c). Expression of KCNJ10, the inwardly rectifying potassium channel, was not changed in the organ of Corti of Tff3 knock-out mice (Fig. 2d). Likewise, a marker for sites of synaptic transmission, synaptophysin, was present throughout efferent boutons in OHCs (Fig. 2e).
Fig. 2.
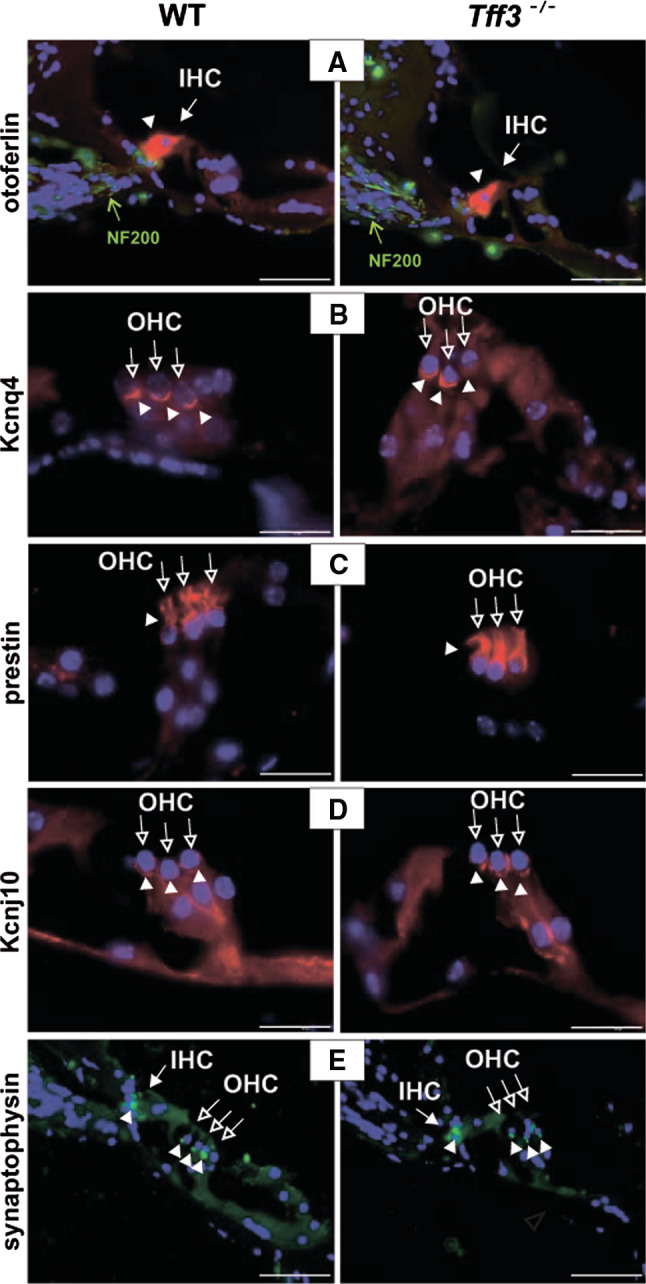
Immunohistochemistry showing expression of otoferlin (red) and neurofilament subunit 200 (NF200, green) a, potassium channel KCNQ4 (red) b, prestin c, potassium channel KCNJ10 (red) d, and e synaptophysin (green) in 12-month-old wild-type and Tff3 knock-out mice. Expression of all proteins was normal in Tff3 knock-out animals in comparison to wild-type mice. Nuclei are stained in blue with DAPI. Open arrows indicate outer hair cells (OHC), filled arrows indicate inner hair cells (IHC), filled arrowheads point out staining with the respective antibodies. Scale bars a, b 50 μm, c–e 20 μm
Next to the already documented expression of Tff3 in the organ of Corti and spiral ganglion [10, 18], we also observed expression in the SV and its absence in Tff3 mouse mutants (Fig. 3a). To examine potential defects caused by loss of Tff3 in the SV, we examined several marker proteins expressed in this tissue. Expression of KCNJ10 was localized in the intermediate cells (Fig. 3b). Strong expression of the voltage-activated potassium channel KCNQ1 and megalin, a protein that is essential for endocytosis of lipoproteins and low molecular weight proteins in absorptive epithelia, was observed in the apical membrane of marginal cells of the SV (Fig. 3c, d respectively). Thus, in summary we found no evidence that loss of Tff3 affects gene expression in the cochlea.
Fig. 3.

Immunohistochemistry showing expression of a Tff3 (red), b potassium channel KCNJ10 (red), c potassium channel KCNQ1 (red), and d megalin (green) in the stria vascularis (SV) of 12-month-old wild-type and Tff3 knock-out mice. In Tff3 mutants and in wild-type mice in the presence of a Tff3 peptide no Tff3 expression was observed (a). Expression of KCNJ10, KCNQ1 and megalin was normal in Tff3 knock-out animals in comparison to wild-type mice. Nuclei are stained in blue with DAPI. Arrows indicate staining in the SV. Scale bars left panels in a and b–d 50 μm, right panels in a 20 μm
A microarray-based screen for Tff3-regulated genes during presbyacusis
Since we observed no evidence for molecular changes in Tff3 mutants in the cochlea we next concentrated on the analysis of the central auditory system. Within the central auditory system the IC, the principal midbrain nucleus of the auditory pathway, has been described to undergo morphological and functional changes underlying age-related hearing loss [13, 14]. To identify potential target genes for Tff3 in the IC we used a microarray-based screen. We employed gene chips covering the expression of 34,000 known mouse genes and expressed sequence tags. In our analysis, we compared the expression profile of the IC from aged Tff3 knock-out mutants to wild-type controls. As a result, a list of genes showing altered expression levels in the absence of Tff3 was generated. We focused our study on a set of selected genes that were highly down- or upregulated as presented in Table 1. These genes corresponded to: retinitis pigmentosa GTPase regulator interacting protein 1 (Rpgrip1; Mouse genome informatics (MGI) reference 1932134), pituitary-transforming gene 1 (Pttg1; (securine), MGI 1353578), prolactin (Prl; MGI 97762), quaking (Qk; MGI 97837), serine (or cysteine) peptidase inhibitor, clade A, member 3N (Serpina3n; MGI 105045), potassium inwardly rectifying channel, subfamily J, member 9 (Kcnj9; MGI 108007), adenylate cyclase-associated protein 1 (Cap1; MGI 88262) and tumor necrosis factor receptor superfamily, member 12a (Tnfrsf12a; MGI 1351484).
Table 1.
Genes decreased or increased in the inferior colliculus of aged Tff3 knockout mutants versus wild-type animals
| Gene | Average fold change | Mouse genome informatics (MGI) |
|---|---|---|
| Retinitis pigmentosa GTPase regulator interacting protein 1 (Rpgrip1) | −13.9 | 1932134 |
| Pituitary-transforming gene 1 (Pttg1, Securin) | −10.5 | 1353578 |
| Prolactin (Prl) | −7.8 | 97762 |
| Quaking (Qk) | −5.7 | 97837 |
| Serine (or cysteine) peptidase inhibitor, clade A, member 3n (Serpine 3n) | +42.8 | 105045 |
| Potassium inwardly-rectifying channel, subfamily J, member 9 (Kcnj9) | +6.6 | 108007 |
| Adenylate cyclase-associated protein (Cap1) | +6.2 | 88262 |
| Tumor necrosis factor receptor superfamily, member 12a (Tnfrsf12a) | +3.5 | 1351484 |
Confirmation of microarray data by qRT-PCR
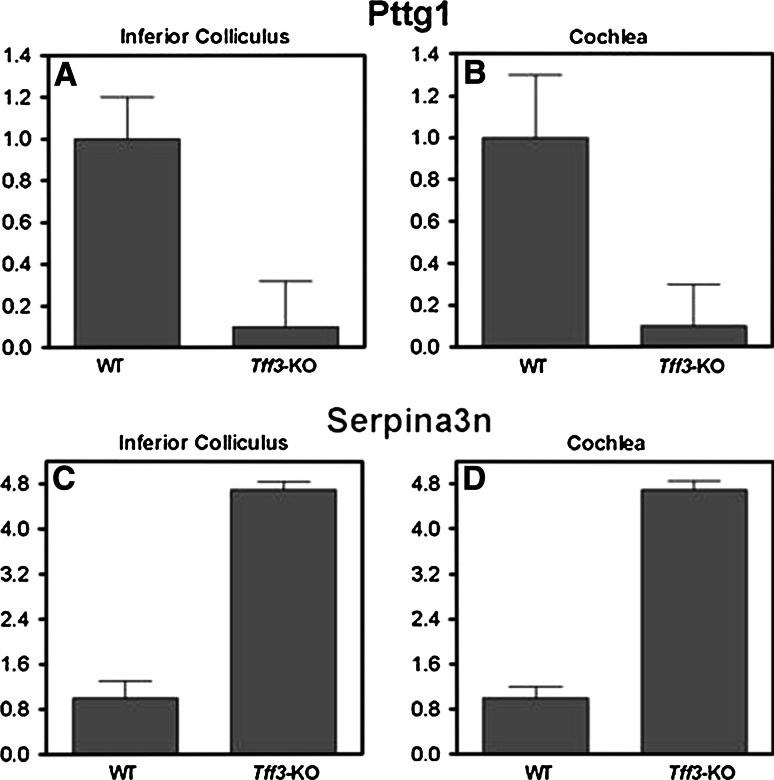
We next employed qRT-PCR to confirm the differential expression of the selected genes independently. Total RNA from the inferior colliculi of adult mice was extracted and served as a template for qRT-PCR with gene-specific primers (see methods). As a control, amplification of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was performed. A strong downregulation of Pttg1 (securin) in the IC of Tff3 knock-out mice (Fig. 4a) was noted, whereas serpina3n was highly upregulated compared to wild-type animals (Fig. 4c), which was consistent with the results from the expression chips (Table 1). In contrast, Prl, Qk, Cap1, Kcnj9 and Tnfrsf12a failed to show a significant difference in mRNA levels between wild-type and Tff3 knock-out animals, and expression of Rpgrip1 was not detected (data now shown).
Fig. 4.
Differential expression of securin (Pttg1) (a, b) and serpina3n (c, d) in adult Tff3 knock-out versus wild-type animals. Results of quantitative RT-PCR (qRT-PCR) experiments performed with RNA samples isolated from the inferior colliculus (a, c) and cochlea (b, d) of wild-type (WT) and Tff3 knock-out (KO) animals. The level of expression in wild-type animals was arbitrarily set to 1. Expression of Gapdh was used as control reference. Differences were tested by a two-tailed Student’s t test, p < 0.01
Due to a strong regulation of expression observed for Pttg1 and serpina3n in the IC, the question arose if these genes were also present and differentially expressed in the peripheral auditory system. Expression of both genes was detected in the cochlea of Tff3 wild-type mice at postnatal day 10 (P10), and at the age of 4 and 14 weeks by qRT-PCR (data not shown). Moreover, in the cochlea of adult Tff3 knock-outs as compared to wild-type animals, Pttg1 and serpina3n were down- or upregulated, respectively, at a similar level as seen in the IC (Fig. 4b, d, respectively).
Expression and downregulation of securin in Tff3 mutants
We next were interested to confirm expression of Pttg1 (securin) and serpina3n at the protein level. Using commercially available antibodies raised against serpina3n, we were unable to observe a signal using immunohistochemistry or Western blotting (data not shown). In contrast polyclonal antibodies raised against securin allowed us to localize immunoreactivity in IC and cochlea. In the adult cochlea securin was strongly expressed in Deiter’s cells, mesenchymal tissue underlying the organ of Corti and spiral ganglion neurons (Fig. 5a). In age-matched Tff3 mutants we observed no immunoreactivity for securin (Fig. 5b). To further confirm this point, we performed Western blots of cochlear protein lysates at P22 and 12 months (Fig. 5c). At P22 Tff3 expression appeared only weakly reduced in cochlear lysates of Tff3 mutants compared to wild-type animals. At 12 months securin expression was found to be downregulated in comparison to P22 in wild-type animals. In cochlear lysates of Tff3 mutants at 12 months of age, no securin expression was observed. Finally, on sections of the IC of adult wild-type mice immunoreactivity for securin was detected at 9 months of age but was strongly downregulated in age-matched Tff3 mutants (Fig. 5d, e). Taken together these data confirm the downregulation of securin mRNA in the cochlea and IC also at the protein level.
Fig. 5.
a Securin expression in the organ of Corti (left panel) and in spiral ganglion neurons (right panel) shown for a 4-month-old Tff3 wild-type mouse. Filled arrowheads point at Deiter’s cells. Filled arrows indicate outer hair cells (OHC); open arrows indicate inner hair cells (IHC). Insets show images where the primary antibody was omitted. b No securin expression could be detected in the spiral ganglion neurons of an age-matched Tff3 −/− mouse. Scale bars left panel in a 50 μm, right panel in a 20 μm, b 20 μm. c Semiquantification of the securin protein level in cochlear tissue of Tff3 wild-type and Tff3 −/− mice at indicated ages was performed using Western blots. Ezrin was co-detected to confirm the loading of approximately equivalent amounts of protein. Securin protein is highly expressed in young animals (P22), both in wild-type as well as in knockout mice, although expression of securin protein in P22 Tff3 −/− mice appears slightly reduced. In adult wild-type mice the level of securin protein in cochlear tissue is downregulated compared to P22. In 12-month-old Tff3 −/− mice, securin protein could not be detected at all. d, e Securin expression in the inferior colliculus shown for a 9-month-old Tff3 wild-type (d) and an age-matched Tff3 −/− mouse (e). Left panels show overview images, and the right panels show a higher magnification. Securin protein is stained in brown (arrows). Inset shows an image where the primary antibody was omitted. Note that securin expression in the inferior colliculus of an age-matched Tff3 −/− mouse is strongly reduced compared to Tff3 wild-type. Scale bars left panel in d, e 200 μm, right panel in d, e 50 μm
Discussion
Earlier studies demonstrated expression of Tff3 in the spiral ganglion and vestibular ganglion neurons. Moreover, in Tff3 knock-out mice a pronounced deterioration of hearing with progressing age was observed. Analysis of middle and inner ear morphology, however, excluded existence of any gross abnormalities, which may affect the hearing process [10].
In the present study, we further analyzed the inner ear phenotype of Tff3 mutants by examining the expression of eight specific marker proteins that are important for normal hearing. When comparing wild-type to Tff3 knock-out animals, no difference in expression patterns of these proteins was noted, excluding their possible contribution to the hearing defect in Tff3 knock-out mice. Furthermore, quantification of spiral ganglion neurons of wild-type and Tff3 knock-out mice at different ages (postnatal day 20 (P20), 4- and 15-month) did not reveal any gross difference in their number or morphology (data not shown). Finally, analysis of data from ABR measurements indicated that the processing of auditory information is not hampered in the Tff3 knock-out mice. All these facts implied that the cause for the auditory defect may be localized at a different level.
In the peripheral auditory system both mice and humans undergo similar changes during hearing loss [23]. A common type of presbyacusis occurs at high frequencies and is caused by hair cell degeneration at the basal end of the cochlea [24]. This peripheral hearing loss eliminates evoked activity in high frequency auditory nerve fibers and translates into loss of sensitivity to high frequencies in central auditory nuclei [25]. Loss of high frequency sensitivity at the periphery eliminates neural responses to high frequency simple sounds in the central auditory system. However, it is unclear how age-related hearing loss affects more complex neural responses such as spectral integration, which is important for the analysis of complex sounds [26]. Multiple tuned and combination-sensitive neurons are found in the IC of both mustached bats [26] and mice [27]. Felix and Portfors (2007) found that the majority of combination-sensitive and multiple tuned neurons are sensitive to high frequencies, which led them to suggest that response properties of these types of neurons will be affected by age-related high-frequency hearing loss. They further hypothesized that high frequency hearing loss reduces spectral integration properties in the IC. They based their study on the examination of neural responses in the IC of CB57Bl/6 mice to single and a combination of tones to analyze the extent of spectral integration in the IC after age-related high-frequency hearing loss.
When ABR thresholds in wild-type and Tff3 knock-out mice were assessed, we observed a deterioration of hearing in both groups over age, most pronounced in the high-frequency range [10], a feature quite characteristic for presbyacusis [11]. However, high-frequency hearing loss was more pronounced in the Tff3 knock-out than in the wild-type mice. The Tff3 knockout mouse thus shows the characteristic hallmark of presybacusis and therefore may be a useful model to identify genes involved during this process. Since no degeneration of hair cells, SV or spiral ganglion neurons in the cochlea of Tff3-deficient mice was noted [10] and no changes of molecular markers were observed in the cochlea (present study), we extended our investigation to the auditory CNS and particularly to the IC. To uncover genes related to the effect of presbyacusis, expression microarrays were applied.
A highly significant change in expression of two genes in the IC and cochlea of Tff3 knock-out mice was observed. One of these genes, downregulated in Tff3-deficient animals, is named securin (Pttg1). Pituitary tumor-transforming gene-1 (Pttg1) was isolated from rat pituitary tumor cells in 1997 [28] and subsequently identified as a vertebrate securin, which regulates sister-chromatid separation [29]. Pttg1 is transcriptionally regulated by various growth factors, is highly expressed in most of the tumors and tumor cell lines analyzed to date, and is thus defined as an oncogene (for review see [30]). Securin directly binds to several gene promoters and regulates transcriptional processes by interacting with other proteins such as p53, Sp1, c-Myc, FGF2, cyclin D3, p21 prolactin and MMP2 (for review see [31]). Upregulation of Pttg1 increases cell proliferation, induces cellular transformation and promotes tumor development in nude mice. Conversely, downregulation of Pttg1 in cancer results in suppression of tumor growth and angiogenesis, suggesting that Pttg1 may serve as an important target gene for the treatment of cancer. The molecular mechanism by which Pttg1 mediates its tumorigenic function is still unclear [30]. Pttg1 functions in cell replication [29], cell cycle control [32], DNA damage/repair [33], organ development, metabolism and cell transformation processes [34]. It was shown to be required for human fetal brain development [35] and telencephalic neurogenesis [36].
The second gene, serpina3n, was strongly upregulated in Tff3-deficient animals. Serpins (serin peptidase inhibitors or SPIs) are the largest family of protease inhibitors and extend to all branches of life [37, 38]. Members of this class of protein perform roles in diverse physiological processes such as the blood clotting cascade, apoptosis and chromatin condensation [39]. Gene expression studies suggest that serpina3n (also known as EB22.4 and referred to as muACT-n, murine anti-chymotrypsin) is the closest murine orthologue of huACT (human anti-chymotrypsin). In particular, it is the only member of the serpina3 cluster that is expressed in the murine brain under resting conditions [40], consistent with evidence that huACT plays a role in the inflammatory response in that organ. As with huACT, muACT-n shows a wide tissue distribution: it is found in the liver, brain, testis, lung, thymus and spleen, and to a lesser extent bone marrow, skeletal muscle and kidney [40]. In the rat liver, three members of the SPI family have been cloned and characterized [41]. SPI-1 and SPI-2 genes are expressed in normal rat liver, but SPI-3 is virtually silent in normal rats and only becomes transiently active during acute inflammation in the rat liver [42] and after transient ischemia in the rat brain [43]. Endogenous expression of SPI-3 in response to inflammatory stimulation was also demonstrated in specific cells of ocular tissues (epithelial cells of the iris and ciliary body, and astrocytes in the retina) [44]. Since SPI-3 is expressed only under inflammatory conditions, previous studies speculated that it might have protective effects against inflammatory damage [43, 45]. Many SPIs have been involved in potent protective activities such as wound-healing [46]. SPI-3 may have similar anti-inflammatory properties under local inflammatory conditions. It has been shown that SPI-3 gene is strongly induced during the acute-phase reaction, and it is regulated at the transcriptional level by interleukin 6 (IL-6) [42] and also by interferon gamma (IFNgamma) [47]. IL-6 and IFNgamma are cytokines with pleiotropic activities, each playing an important role in the host defense system. It was demonstrated that transcription factors such as STAT3 and STAT5B are involved in the induction of the SPI-3 promoter, whereas C/EBPbeta reduced the transcriptional activity of the promoter [48]. Intestinal restitution induced by TFF3 is also associated with IL-6/Gp130/STAT signaling [49]. TFF3 activates STAT3 [50], which exerts anti-apoptotic and mitogenic effects [51]. Additionally, unpublished data suggest that optic nerve injury does not induce expression of SPI-3, whereas rat motor nerve transection dramatically induced SPI-3 expression in the injured motor neurons [44]. A human homolog of SPI-3, protein inhibitor 6 (PI-6), was shown to inhibit cathepsin G, which activates a proapoptotic protease, caspase-7 [52, 53]. This may suggest that SPI-3 may prevents caspase-7-mediated apoptosis, which is caused by damage such as nerve injury or inflammatory stimulation. Tff3-deficient mice showed increased sensitivity to intestinal damage with augmented apoptosis, suggesting a protective role of Tff3. Tff3 prevents apoptosis after injuries in a range of cell lines, an effect that requires activation of both the epidermal growth factor (EGF) and phosphoinositide 3-kinase (PI3 K) receptors [49]. Together these data suggest that upregulation of serpina3n plays an important compensatory role when Tff3 protein is missing and may not act as a protector and anti-apoptotic factor. Further studies involving analysis of neurons and synapses of the IC of Tff3 knock-out mice may confirm this postulate.
Presently there are no known patients with hearing impairment linked to both new candidate genes. SERPINA3 is localized on 14q32.1, and there is no known gene related to hearing nearby. PTTG1 is on 5q35.1. Somewhat in the vicinity, in 5q31, there are two genes, DIAPH1 and POU4F3, and unknown genes for DFNA42 and DFNA54, but on the molecular genetic level this distance excludes SERPINA3 and PTTG1 as being involved at the present time. Therefore, the expression changes observed in both proteins may be unrelated to hearing loss. However, loss of Pttg1 has been related to senescence in the pancreas and pituitary, suggesting a general defect in various organs during aging [54, 55]. In the case of serpina3n it is worthwhile mentioning that a member of the serpin gene family, SERPINB6, has recently been associated with hearing loss in human patients [56]. Therefore, further studies directed at the function of Pttg1 and serpina3n in the Tff3 knockout mouse model may provide important insights on the roles of these genes during the development of age-related hearing loss.
Acknowledgments
This work was supported by Ciberned, MiCINN and Red de Terapia Célular de Castilla y León. We would like to thank I. López-Hernández and Katja Gutsche for testing serpina3n antibodies.
Abbreviations
- ABR
Auditory brain stem responses
- GI
Gastrointestinal
- huACT
Human antychymotrypsin
- muACT
Murine antychymotrypsin
- IC
Inferior colliculus
- IBD
Inflammatory bowel disease
- IHC
Inner hair cell
- OHC
Outer hair cell
- PI3K
Phosphoinositide 3-kinases
- PI-6
Protein inhibitor 6
- SPIs
Serin peptidase inhibitors
- SV
Stria vascularis
- TFF
Trefoil factor family
Contributor Information
N. Blin, Phone: +49-7071-295233, FAX: +49-70712-972192, Email: blin@uni-tuebingen.de
T. Schimmang, Phone: +34-983184818, FAX: +34-983184800, Email: schimman@ibgm.uva.es
References
- 1.Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997;53:888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319–334. doi: 10.14670/HH-16.319. [DOI] [PubMed] [Google Scholar]
- 3.Madsen J, Nielsen O, Tornoe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505–513. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 4.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 5.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 6.Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou JA, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright NA, Bodmer WF, Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Probst JC, Zetzsche T, Weber M, Theilemann P, Skutella T, Landgraf R, Jirikowski GF. Human intestinal trefoil factor is expressed in human hypothalamus and pituitary: evidence for a novel neuropeptide. FASEB J. 1996;10:1518–1523. doi: 10.1096/fasebj.10.13.8940297. [DOI] [PubMed] [Google Scholar]
- 9.Jagla W, Wiede A, Dietzmann K, Rutkowski K, Hoffmann W. Co-localization of TFF3 peptide and oxytocin in the human hypothalamus. FASEB J. 2000;14:1126–1131. doi: 10.1096/fasebj.14.9.1126. [DOI] [PubMed] [Google Scholar]
- 10.Lubka M, Muller M, Baus-Loncar M, Hinz M, Blaschke K, Hoffmann W, Pfister M, Lowenheim H, Pusch CM, Knipper M, Blin N. Lack of Tff3 peptide results in hearing impairment and accelerated presbyacusis. Cell Physiol Biochem. 2008;21:437–444. doi: 10.1159/000129636. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson R, Rosenhall U, Gause-Nilsson I, Steen B. Auditory function in 70- and 75-year-old of four age cohorts, A cross-sectional and time-lag study of presbyacusis. Scand Audiol. 1998;27:81–93. doi: 10.1080/010503998420324. [DOI] [PubMed] [Google Scholar]
- 12.Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res. 1995;91:19–32. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 13.Kazee AM, Han LY, Spongr VP, Walton JP, Salvi RJ, Flood DG. Synaptic loss in the central nucleus of the inferior colliculus correlates with sensorineural hearing loss in the C57BL/6 mouse model of presbycusis. Hear Res. 1995;89:109–120. doi: 10.1016/0378-5955(95)00128-6. [DOI] [PubMed] [Google Scholar]
- 14.Felix RA, 2nd, Portfors CV. Excitatory, inhibitory and facilitatory frequency response areas in the inferior colliculus of hearing impaired mice. Hear Res. 2007;228:212–229. doi: 10.1016/j.heares.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- 16.Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U. Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol. 2000;83:3101–3112. doi: 10.1152/jn.2000.83.5.3101. [DOI] [PubMed] [Google Scholar]
- 17.Schug N, Braig C, Zimmermann U, Engel J, Winter H, Ruth P, Blin N, Pfister M, Kalbacher H, Knipper M. Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur J Neurosci. 2006;24:3372–3380. doi: 10.1111/j.1460-9568.2006.05225.x. [DOI] [PubMed] [Google Scholar]
- 18.Lubka M, Pusch C, Blin N, Baus-Loncar M. Novel Expression Patterns for Trefoil Peptides: presence of Tff2 and Tff3 in Rodent Cochlea. Croat Chem Acta. 2008;81:113–117. [Google Scholar]
- 19.Weber T, Zimmermann U, Winter H, Mack A, Kopschall I, Rohbock K, Zenner HP, Knipper M. Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci USA. 2002;99:2901–2906. doi: 10.1073/pnas.052609899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panford-Walsh R, Singer W, Ruttiger L, Hadjab S, Tan J, Geisler H-S, Zimmermann U, Kopschall I, Rohbock K, Vieljans A, Oestreicher E, Knipper M. Midazolam reverses salicylate-induced changes in brain-derived neurotrophic factor and arg3.1 expression: implications for tinnitus perception and auditory plasticity. Mol Pharmacol. 2008;74:595–604. doi: 10.1124/mol.108.046375. [DOI] [PubMed] [Google Scholar]
- 21.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2008. [Google Scholar]
- 22.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/S0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry KR. Ageing and audition. In: Willot JF, editor. The auditory psychobiology of the mouse. Springfield: Charles C. Thomas; 1983. pp. 470–493. [Google Scholar]
- 24.Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Int J Audiol. 2003;42(Suppl 2):2S11–2S16. [PubMed] [Google Scholar]
- 25.Moore B. Frequency analysis and pitch perception. In: Yost WPA, Fay R, editors. Human psychophysics. New York: Springer; 1999. pp. 56–115. [Google Scholar]
- 26.Portfors CV, Wenstrup JJ. Excitatory and facilitatory frequency response areas in the inferior colliculus of the mustached bat. Hear Res. 2002;168:131–138. doi: 10.1016/S0378-5955(02)00376-3. [DOI] [PubMed] [Google Scholar]
- 27.Portfors CV, Felix RA., 2nd Spectral integration in the inferior colliculus of the CBA/CaJ mouse. Neuroscience. 2005;136:1159–1170. doi: 10.1016/j.neuroscience.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Mol Endocrinol. 1997;11:433–441. doi: 10.1210/me.11.4.433. [DOI] [PubMed] [Google Scholar]
- 29.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 30.Panguluri SK, Yeakel C, Kakar SS. PTTG: an important target gene for ovarian cancer therapy. J Ovarian Res. 2008;1:6. doi: 10.1186/1757-2215-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Y, Eigler T. Transcriptional targets for pituitary tumor-transforming gene-1. J Mol Endocrinol. 2009;43:179–185. doi: 10.1677/JME-08-0176. [DOI] [PubMed] [Google Scholar]
- 32.Tong Y, Ben-Shlomo A, Zhou C, Wawrowsky K, Melmed S. Pituitary tumor transforming gene 1 regulates Aurora kinase A activity. Oncogene. 2008;27:6385–6395. doi: 10.1038/onc.2008.234. [DOI] [PubMed] [Google Scholar]
- 33.Romero F, Multon MC, Ramos-Morales F, Dominguez A, Bernal JA, Pintor-Toro JA, Tortolero M. Human securin, hPTTG, is associated with Ku heterodimer, the regulatory subunit of the DNA-dependent protein kinase. Nucleic Acids Res. 2001;29:1300–1307. doi: 10.1093/nar/29.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Moro E, Kovacs K, Yu R, Melmed S. Pituitary tumor transforming gene-null male mice exhibit impaired pancreatic beta cell proliferation and diabetes. Proc Natl Acad Sci USA. 2003;100:3428–3432. doi: 10.1073/pnas.0638052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelaert K, McCabe CJ, Tannahill LA, Gittoes NJ, Holder RL, Watkinson JC, Bradwell AR, Sheppard MC, Franklyn JA. Pituitary tumor transforming gene and fibroblast growth factor-2 expression: potential prognostic indicators in differentiated thyroid cancer. J Clin Endocrinol Metab. 2003;88:2341–2347. doi: 10.1210/jc.2002-021113. [DOI] [PubMed] [Google Scholar]
- 36.Tarabykin V, Britanova O, Fradkov A, Voss A, Katz LS, Lukyanov S, Gruss P. Expression of PTTG and prc1 genes during telencephalic neurogenesis. Mech Dev. 2000;92:301–304. doi: 10.1016/S0925-4773(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 37.Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.GR-1478R. [DOI] [PubMed] [Google Scholar]
- 38.Irving JA, Steenbakkers PJ, Lesk AM, Op den Camp HJ, Pike RN, Whisstock JC. Serpins in prokaryotes. Mol Biol Evol. 2002;19:1881–1890. doi: 10.1093/oxfordjournals.molbev.a004012. [DOI] [PubMed] [Google Scholar]
- 39.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O’Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 40.Horvath AJ, Forsyth SL, Coughlin PB. Expression patterns of murine antichymotrypsin-like genes reflect evolutionary divergence at the Serpina3 locus. J Mol Evol. 2004;59:488–497. doi: 10.1007/s00239-004-2640-9. [DOI] [PubMed] [Google Scholar]
- 41.Pages G, Rouayrenc JF, Le Cam G, Mariller M, Le Cam A. Molecular characterization of three rat liver serine-protease inhibitors affected by inflammation and hypophysectomy. Protein and mRNA analysis and cDNA cloning. Eur J Biochem. 1990;190:385–391. doi: 10.1111/j.1432-1033.1990.tb15587.x. [DOI] [PubMed] [Google Scholar]
- 42.Kordula T, Bugno M, Lason W, Przewlocki R, Koj A. Rat contrapsins are the type II acute phase proteins: regulation by interleukin 6 on the mRNA level. Biochem Biophys Res Commun. 1994;201:222–227. doi: 10.1006/bbrc.1994.1692. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda M, Kitagawa K, Imaizumi K, Wanaka A, Tohyama M, Takagi T. Induction of SPI-3 mRNA, encoding a serine protease inhibitor, in gerbil hippocampus after transient forebrain ischemia. Brain Res Mol Brain Res. 1996;35:314–318. doi: 10.1016/0169-328X(95)00211-A. [DOI] [PubMed] [Google Scholar]
- 44.Takamiya A, Takeda M, Yoshida A, Kiyama H. Expression of serine protease inhibitor 3 in ocular tissues in endotoxin-induced uveitis in rat. Invest Ophthalmol Vis Sci. 2001;42:2427–2433. [PubMed] [Google Scholar]
- 45.Chen MC, Schuit F, Pipeleers DG, Eizirik DL. IL-1beta induces serine protease inhibitor 3 (SPI-3) gene expression in rat pancreatic beta-cells. Detection by differential display of messenger RNA. Cytokine. 1999;11:856–862. doi: 10.1006/cyto.1999.0525. [DOI] [PubMed] [Google Scholar]
- 46.Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–1153. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- 47.Kordula T, Travis J. Activation of the rat serine proteinase inhibitor 3 gene by interferon gamma via the interleukin 6-responsive element. Biochem J. 1995;309(Pt 1):63–67. doi: 10.1042/bj3090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kordula T, Ripperger J, Morella KM, Travis J, Baumann H. Two separate signal transducer and activator of transcription proteins regulate transcription of the serine proteinase inhibitor-3 gene in hepatic cells. J Biol Chem. 1996;271:6752–6757. doi: 10.1074/jbc.271.12.6752. [DOI] [PubMed] [Google Scholar]
- 49.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivat C, Rodrigues S, Bruyneel E, Pietu G, Robert A, Redeuilh G, Bracke M, Gespach C, Attoub S. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3)–and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res. 2005;65:195–202. [PubMed] [Google Scholar]
- 51.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Q, Salvesen GS. Activation of pro-caspase-7 by serine proteases includes a non-canonical specificity. Biochem J. 1997;324(Pt 2):361–364. doi: 10.1042/bj3240361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott FL, Hirst CE, Sun J, Bird CH, Bottomley SP, Bird PI. The intracellular serpin proteinase inhibitor 6 is expressed in monocytes and granulocytes and is a potent inhibitor of the azurophilic granule protease, cathepsin G. Blood. 1999;93:2089–2097. [PubMed] [Google Scholar]
- 54.Chesnokova V, Wong C, Zonis S, Gruszka A, Wawrowsky K, Ren S-G, Benshlomo A, Yu R. Diminished pancreatic beta-cell mass in securin-null mice is caused by beta-cell apoptosis and senescence. Endocrinology. 2009;150:2603–2610. doi: 10.1210/en.2008-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chesnokova V, Melmed S. Pituitary senescence: the evolving role of Pttg. Mol Cell Endocrinol. 2010;326:55–59. doi: 10.1016/j.mce.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sirmaci A, Erbek S, Price J, Huang M, Duman D, Cengiz FB, Bademci G, Tokgoz-Yilmaz S, Hismi B, Ozdag H, Ozturk B, Kulaksizoglu S, Yildirim E, Kokotas H, Grigoriadou M, Petersen MB, Shahin H, Kanaan M, King M-C, Chen Z-Y, Blanton SH, Liu XZ, Zuchner S, Akar N, Tekin M. A truncating mutation in SERPINB6 is associated with autosomal-recessive nonsyndromic sensorineural hearing loss. Am J Hum Genet. 2010;86:797–804. doi: 10.1016/j.ajhg.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]