Abstract
Post-transcriptional mechanisms are key determinants in the modulation of the expression of final gene products. Within this context, fundamental players are RNA-binding proteins (RBPs), and among them ELAV-like proteins. RBPs are able to affect every aspect in the processing of transcripts, from alternative splicing, polyadenylation, and nuclear export to cytoplasmic localization, stability, and translation. Of interest, more than one RBP can bind simultaneously the same mRNA; therefore, since each RBP is endowed with different properties, the balance of these interactions dictates the ultimate fate of the transcript, especially in terms of both stability and rate of translation. Besides RBPs, microRNAs are also important contributors to the post-transcriptional control of gene expression. Within this general context, the present review focuses on ELAV-like proteins describing their roles in the nucleus and in the cytoplasm, also highlighting some examples of interactions with other RBPs and with microRNAs. We also examine the putative role and the observed changes of ELAV-like proteins and of their interactions with other regulatory elements in Alzheimer’s disease, cancer, and inflammation. The changes in the expression of proteins involved in these diseases are examples of how a derangement in the mRNA stabilization process may be associated with disease development and contribute to pathology. Overall, we hope that the topics handled in the present manuscript provide a hint to look at ELAV-like-mediated mRNA stabilization as a mechanism relevant to disease as well as a novel putative drug target.
Keywords: ELAV-like RNA-binding proteins, Post-translational mechanisms, mRNA stability, miRNAs, Alzheimer’s disease, Cancer, Inflammation
Introduction
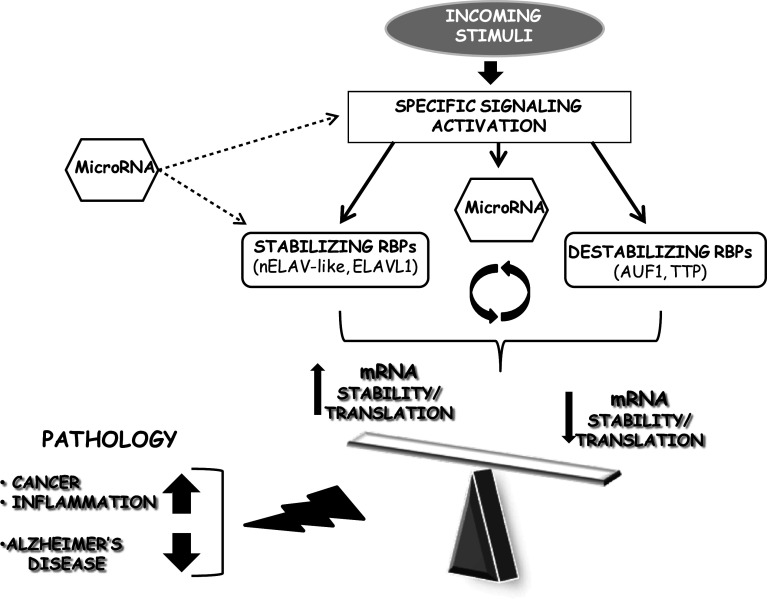
Within the cell, the amount of protein is the result of a multi-step cascade involving gene transcription, post-transcriptional regulation of the transcript itself, translation of the mRNA in the respective protein, and proteolysis. Due to the impact that each step can have on gene expression, this cascade is tightly regulated. Of relevance, post-transcriptional mechanisms are emerging as key determinants in the modulation of gene expression since they allow a rapid adaptation of protein levels to changing environmental conditions [1]. Indeed, post-transcriptional regulation can shape, under different aspects, the fate of a given cell (i.e., whether it will be directed to differentiation, transformation, death, or survival) through the modulation of the expression of selected products. A post-transcriptional control entails some advantages such as the possibility to: (a) affect gene expression within a short time, and (b) allow a localized modification of the protein content in specific subcellular compartments. In addition, alternative splicing of the transcript represents a crucial mechanism that allows the functional proteome to expand. In fact, alternative splicing can generate alternative forms of mRNA that may be endowed with different properties, for example in terms of localization and stability. A less explored mechanism, occurring in the nucleus as well, is alternative polyadenylation, which has also been recognized as an important contributor to gene expression modulation. Considering that mRNA half-life is a parameter able to dramatically influence the extent of the translational processing, modulation of mRNA decay represents another key target of post-transcriptional mechanisms. Consequently, inside the cell, it is possible to identify mRNAs ranging from very stable (many hours) to heavily unstable (a few minutes). Their rates of degradation are controlled by orchestrated interactions between specific cis-acting motifs (present within the sequence of the transcript itself) and trans-acting factors, such as the so-called RNA-binding proteins (RBPs), which bind to the former. Furthermore, RBPs may also modulate the translational process of the transcripts themselves. Therefore, depending on which RBP is associated to a given mRNA, this interaction may result in a higher/lower stability and/or in an enhanced/reduced translation of the transcript itself (Fig. 1). Distinct RBPs can interact with the same mRNA in different binding sites or even compete for the same binding site, thus rendering this entire scenario even more complex. Finally, among the several trans-acting factors binding to the transcript, the involvement of non-coding RNAs that function directly as structural, catalytic, or regulatory RNAs, should also be taken into account. Among these, particular attention has been devoted to microRNAs (miRNAs) [2]. Obviously, any deregulation within all of these processes may determine an alteration in protein expression and hence metabolic changes leading to disease [3] (Fig. 1). This review will especially focus on the involvement of the ELAV (embryonic lethal abnormal vision)-like family of RBPs in dictating the fate of specific mRNAs and on the consequences of alterations of this post-transcriptional regulation in some pathologies, such as Alzheimer’s disease (AD), cancer, and inflammation.
Fig. 1.
Scheme showing the influence of post-transcriptional mechanisms on mRNA stability/translation. Different signaling cascades are triggered upon distinct stimuli, and their activation influences the interplay among stabilizing, destabilizing RBPs, and miRNAs. The resulting effect has consequences on the target mRNA half-life and/or its translation, thus determining an increase or a decrease of the correspondent protein. Moreover, miRNAs may also interfere with the up-stream signaling cascades [179–181] and with the expression of RBPs themselves, as described for ELAVL1 [107–109, 150, 163]. A deregulation of this fine balance may play a role in the development of several pathologies, such as Alzheimer’s disease, cancer, and chronic inflammation (see text for more details)
Regulation of mRNA stability/translation
The ARE signature
Although some general regulators of mRNAs half-life [i.e., 5′-cap structure and the 3′ poly(A) tail] are universally present in all mRNAs, specific cis elements have been identified within the mRNA sequence, which affect the stability and/or the translation of a subset of transcripts. These cis-acting motifs are evolutionarily conserved and a number of them has been found in the three main parts of the transcript: the 5′-untranslated region (5′-UTR), the coding sequence, and the 3′-untranslated region (3′-UTR) (for more details see [4]). However, the vast majority of these cis motifs has been identified in the 3′-UTR, and among them, the most common and well known are the ARE (adenine–uridine-rich elements) sequences, key determinants for turnover and translation processes [5–7]. The ARE signature is constituted by 50–150 nucleotides, rich in adenine and uracil bases, that confer instability to the mRNAs that bear them through the promotion of their deadenylation and, consequently, their degradation [8, 9]. Within this context, in vitro studies have shown that the exosome is required for the degradation of ARE-bearing mRNAs [10]. The first demonstration of an ARE-mediated selective degradation of an mRNA dates back to 1986 when Shaw and Kamen [11] reported that the introduction of a 51-nucleotide AT sequence from a human lymphokine gene, the GM-CSF (granulocyte-macrophage colony-stimulating factor), into the 3′-UTR of the rabbit beta-globin gene caused the otherwise stable beta-globin mRNA to become highly unstable in vivo. Since then, the ARE signature has been identified in at least 5–8% of the human genes [12] and different features have been described. To this regard, many studies have highlighted the presence of the AUUUA pentamer and a certain uridine enrichment as two important traits of an ARE, although they cannot fully explain its destabilizing activity (for a review see [13]). In general, based on the distribution and number of the AUUUA pentamer, ARE sequences have been grouped into three classes [8, 14]: (I) containing up to three copies of the pentamer motif within U-rich regions; (II) presenting at least two overlapping UUAUUUA(U/A)(U/A) nonamers; (III) not possessing any pentamers, but having U-rich stretches. However, it should be said that despite the attempts to elaborate a classification, the canonical ARE structures remain largely unknown.
Functionally, the regulation of mRNA stability via ARE allows a fine-tuning of the response to extra and intracellular stimuli under changing environmental conditions. ARE-bearing mRNAs encode a variety of different structural and regulatory proteins endowing a vast number of actions. ARE motifs have mainly been described in short-living transcripts, such as those coding for early response genes such as cytokines, cell-cycle regulators, oncogenes, and several interleukins [6]. However, mRNAs for proteins devoted to a wide range of other functions have also been recognized as containing AREs [12]. ARE-binding proteins (ARE-BPs) regulate, as mentioned, the stability and translation of specific target mRNAs; to date, at least 14 ARE-BPs have been described [7] and among them a relevant place is certainly taken by ELAV-like.
ELAV-like proteins
In mammals, the ELAV-like protein family includes the ubiquitously expressed ELAVL1 (a.k.a. HuA, HuR), and the neuron-specific members (nELAV-like) named ELAVL2 (a.k.a. HuB), ELAVL3 (a.k.a. HuC), and ELAVL4 (a.k.a. HuD) [15–18]. ELAV-like proteins act post-transcriptionally as regulators of gene expression. In response to intra and extracellular signals, they preferentially bind to ARE sequences present in a subset of mRNAs increasing in many instances their cytoplasmic stability and rate of translation [19–22], although a decrease in the translational process has also been described [23]. Furthermore, it should be mentioned that, besides ARE, other consensus sequences may be implicated in the binding between ELAV-like and target transcripts, as reported in the literature [24–26].
Human nELAV-like proteins were first described in patients with certain types of tumors, especially the small cell lung carcinoma, which ectopically express antigenic nELAV-like proteins eliciting the production of autoantibodies able to cross the blood–brain barrier and induce the anti-Hu syndrome, a paraneoplastic neurological disorder characterized by subacute sensory neuropathy, dementia, and/or encephalomyelopathy [27, 28]. The Hu name comes from the first two letters of the last name of the index patient presenting a high titer of autoantibodies in the serum [29]. Following cloning and sequencing, human ELAV-like genes were reported as orthologues of the Drosophila melanogaster elav gene [30, 31], encoding a neuronal nuclear RBP involved in the alternative splicing of some target mRNAs [32, 33]. Notably, the name elav (as already said: embryonic lethal abnormal vision) originates from the observation that, in the fruit fly, the deletion of this gene leads to embryonic lethality in null mutants and to defects in the visual system in hypomorphic alleles [34].
Structurally, the four mammalian ELAV-like proteins present a high degree of sequence homology (70–85%), a molecular weight around 40 kDa, and three ≈90 amino-acid-long RNA-recognizing motifs (RRMs) [35]; the first two being at the N-terminus, separated from the third one at the C-terminus by a hinge region [36]. The N-terminal end and the hinge region are endowed with the highest sequence diversity. Furthermore, the hinge region is functionally involved in the binding to other cellular components and bears the cis-elements necessary for the nucleo-cytoplasmic shuttling of the protein [19, 37]. The first two RRMs, namely RRM1 and RRM2, have been reported to be able to bind ARE sequences by forming a cleft with their β-sheet-containing surfaces in which the mRNA is inserted [38]. The third motif, RRM3, instead, helps to maintain the stability of the RNA–protein complex, and might also bind to poly(A) tails [20, 39]. Notably, the poly(A) tail endows a timing function for the life of each mRNA, since most of the mRNAs are slowly deadenylated before rapid degradation of the body. This mechanism determines a specific lifetime for cytoplasmic translation, which is approximately constant for a given mRNA. Interestingly, the length of the poly(A) tail seems to correlate with the efficiency of the binding of ELAV-like protein to the target mRNA, the affinity being higher when the mRNA bears long versus short tails [40].
Mammalian ELAV-like family proteins have also been shown to form multimers [41–43]. It is likely that multimerization is a common feature shared by ELAV-like proteins, and an important part of the mechanism by which they exert their effects on the bound mRNA. Within this context, it has been documented that, in Drosophila, the major sites for ELAV–ELAV interaction are located in the RRM3 and in a small adjacent region of the hinge [44].
Studies focused on defining the localization of mammalian ELAV-like proteins underline that ELAVL1 is predominantly localized in the nucleus, where it binds target mRNAs and participates in their export to the cytoplasmic compartment protecting them from degradation [45, 46]. Conversely, nELAV-like proteins seem to be preferentially localized in the cytoplasm of cultured mammalian cells, although the cellular distribution may vary among the different cell types [19]. The nuclear export of ELAV-like proteins is triggered by various stimuli activating specific intracellular signaling pathways (reviewed in [6, 47]) such as the mitogen-activated protein kinase (MAPK) [48, 49], AMP-activated kinase [50], and protein kinase C (PKC) [51, 52], thus indicating that the functional role of ELAV-like is also closely linked to their subcellular localization. The nuclear ELAV-like proteins appear not to be associated with large complexes, whereas cytoplasmic ELAV-like proteins bind to microtubules and to the target mRNAs in messenger ribonucleoprotein (mRNPs) complexes which, in turn, associate with polysomes to form a translationally competent complex linked to the cytoskeletal network [53]. It should be highlighted that ELAV-like proteins are not the only RBPs involved in the formation of these complexes. Indeed, mRNPs are assemblies of dozens of different RBPs and target mRNAs functioning as discrete modules of coordinated mRNAs encoding-related proteins. Moreover, considering that, in general, RBPs can bind to more than one mRNA with sequence specificity, such interactions may be implicated in the selective localization, coexpression, and coordination of functionally related subsets of RNAs [54]. In fact, several studies have reported that specific groups of transcripts can coordinately change their stability in response to certain cellular signals. For this reason, it has been proposed that the mRNPs system may replace in eukaryotes the operon-based network found in prokaryotes and guarantee a controlled expression of functionally related genes. Within this context for example, it has been documented that during the induction of neuronal differentiation, ELAV-like proteins bind to subsets of mRNAs within ribonucleoprotein complexes, which then change combinatorially in a dynamic pattern, leading to a continuous remodeling of mRNPs [54, 55].
Nuclear functions of ELAV-like proteins
Alternative splicing
Alternative RNA processing represents a key mechanism that allows to expand the functional proteome from a limited genome size according to different stimuli and conditions. Consistently, a genome-wide bioinformatic study documented that the majority of human pre-mRNAs undergo alternative RNA processing [56, 57]. Specifically, an analysis of high-throughput transcriptome sequencing indicates that 92–94% of the human genes undergo alternative splicing [58, 59], the most extensive occurring in brain tissues [58]. It should be taken into account that these alternative processing events can also influence transcript localization, stability, and transport. Two of the best-characterized classes of splicing regulators are TIA and ELAV-like proteins. Accordingly, in Drosophila, it was reported that the elav protein homologue regulates alternative pre-mRNA processing in neurons [32, 60–62]. However, the possibility that mammalian ELAV-like proteins may also possess this nuclear function remained uncertain until 2006 when Lou’s group [63] found that, in neuron-like cells, ELAV-like proteins regulate the neuron-specific alternative RNA processing of the calcitonin/CGRP pre-mRNA. In particular, ELAV-like proteins act by blocking the activity of the ubiquitously expressed TIA-1/TIAR proteins, known to play an important role in the inclusion of the non-neuronal 3′-terminal exon, exon 4 [64]. From then on, other studies reported the involvement of ELAV-like proteins in the control of alternative splicing of several mRNAs [20, 65, 66]. As previously mentioned, the RRM domains are highly homologous among the various ELAV-like proteins, while the hinge region, which is encoded by the pre-mRNA region that undergoes alternative splicing, manifests the highest variability. In particular, it has been documented that in the mouse only one isoform is generated for ELAVL1, while multiple isoforms are produced for ELAVL2, ELAVL3, and ELAVL4 as a result of the alternative splicing [67]. The function of these different splice variants is poorly understood, although considering that alternatively spliced hinge segments are presumed to be involved in protein–protein interactions, it is possible that the various isoforms interact with the same set of RNAs but with a different set of cellular proteins [68]. Wang et al. recently demonstrated that the ELAVL4 pre-mRNA itself is a potential target of ELAV-like-mediated splicing regulation. They reported the first example where ELAV-like proteins operate as splicing enhancers promoting the inclusion of the ELAVL4 exon 6 by binding to evolutionarily conserved AU-rich intronic sequences [69]. Notably, it has been reported that exon 6 encodes for part of the nuclear export signal located within the hinge region, therefore, isoforms bearing exon 6 may be characterized by a distinct localization with respect to isoforms that are devoid of it [70].
ELAV-like proteins may influence alternative splicing also indirectly by affecting the expression and activity of the neuronal splicing regulator NOVA-1 (neuro-oncological ventral antigen 1) [71]. NOVA-1 is a neuron-specific RBP, which regulates the alternative splicing of a set of transcripts involved in the formation and activity of the synapses [72]. Moreover, its activity has been shown to be necessary for the development of the motor system and the survival of motoneurons [73]. Therefore, a change in ELAV-like protein abundance may dramatically affect the correct processing of NOVA-1 target pre-mRNAs with potential long-term consequences on the overall metabolism and activity of neurons.
Polyadenylation
Almost all eukaryotic mRNAs acquire a poly(A) tail at their 3′ ends in a process called polyadenylation. Polyadenylation represents a key facet of gene expression, since poly(A) tails are able to influence mRNA stability and translation. The polyadenylation process is characterized by two interconnected steps in which the mRNA is first cleaved at a specific site and then adenosine residues are added in a nontemplated fashion [74]. In particular, the polyadenylation signal bears a consensus sequence AAUAAA (or a variant) between 10 and 35 nucleotides upstream of the actual cleavage and polyadenylation site. In addition, some poly(A) sites also contain one or more U-rich upstream sequences surrounding the AAUAAA region [75]. The presence and the consequent use of more than one polyadenylation signal results in alternative polyadenylation. Alternative polyadenylation, although less explored, is an additional mechanism occurring in the nucleus (that may or may not be affected by splicing) that has been recognized as a key contributor to the complexity of gene expression modulation [74]. More than half of the genes in the human genome are alternative polyadenylated in response to developmental or functional needs [76]. One of the consequences of this molecular mechanism is certainly the prospect of generating mRNA products with enhanced or diminished mRNA half-life. In addition, these alternative processes can also influence transcript localization and transport [74], and may have a role in certain disease situations [77]. Within this general context, it has been reported in Xenopus laevis that ElrA, the homolog of ELAVL1, is involved in the polyadenylation and stabilization of the cell-cycle regulator cyclin E1 mRNA [78]. More importantly, Zhu et al., using HeLa cells nuclear extract and recombinant ELAV-like proteins, were first to demonstrate that also mammalian ELAV-like proteins are involved in the regulation of polyadenylation. In fact, they documented that ELAV-like proteins selectively block both cleavage and poly(A) addition at sites containing U-rich sequences, and that this inhibition depends on their binding to the U-rich elements themselves [75]. Furthermore, the authors showed that all three ELAV-like RNA recognition motifs are required for this activity, and that presumably the RRM3 and the hinge region are implicated in the interaction with poly(A) factors. They propose a model in which ELAV-like proteins bind to RNA and poly(A) factors simultaneously in a way that renders the complex non-functional [75]. The ELAV-like genes themselves may code for various alternative polyadenylation variants. Indeed, it has been reported that the ELAVL1 codes for several polyadenylation variants with differential expression, ARE patterns, and stability [79]. Furthermore, the distinct alternative variants present a different response to TTP-induced destabilization [tristetraprolin; a destabilizing RBP that acts promoting the removal of the poly(A) tail] and to ELAVL1-induced stabilization, thus indicating an auto-regulatory function [79].
ELAV-like proteins and their interplay with other mRNA-binding factors: the example of AUF1 and miRNAs
As previously mentioned, the decision of whether a given mRNA will be finally stabilized or destabilized depends upon the combined action of various mRNA-binding factors acting simultaneously or competitively on different cis-elements spread throughout the mRNA itself. Another aspect is represented by the fact that most of the mRNAs are able to bind more than just one RBP, thus increasing the complexity in their post-transcriptional mode of regulation. This complexity is furthermore underscored by evidence showing that some RBPs, besides modulating the decay, may also exert a translational regulation of the transcript. Finally, the involvement of miRNAs in the regulation of gene expression is also emerging as an important element. For more details on these issues, we refer the reader to more comprehensive reviews [2, 6, 80], hereafter we decided to examine only some examples regarding these possible interactions, namely between ELAVL1 and AUF1, and between ELAVL1 and miRNAs.
ELAVL1 and AUF1 interaction
Recent investigations point to the concept that different RBPs, even when exerting opposite effects, can concurrently bind to common target transcripts (not necessarily to nonoverlapping sites) thus dictating the fate of a given mRNA in a complex way. The most extensive studies have been performed on two ubiquitous RBPs: ELAVL1 and AUF1. AUF1 is a member of the hnRNP protein family, which exerts a key role in DNA repair and signaling, and in the control of transcription and translation. AUF1 is expressed in four isoforms (p37, p40, p42, and p45) that, although some differences have been documented, all basically act enhancing mRNA decay, a process that is closely linked to the ubiquitination and targeting of AUF1 to the proteasome [81, 82]. All AUF1 isoforms can undergo post-translational modifications, such as phosphorylation, methylation, and glycosylation that seem to critically affect their functions [82]. Literature data report that ELAVL1 and AUF1 overlap in their tissue distribution, preferentially localizing in the nucleus, and influence the expression of several common transcripts, strongly suggesting a functional link between them [83–86]. Moreover, the mRNA destabilizing effects operated by AUF1 are often combined with the ELAVL1-dependent stabilizing effects, thus emphasizing the mutual opposing modulation on the stability of a target mRNA operated by these two proteins [80, 87, 88]. It has been suggested that the physical association of ELAVL1 and AUF1 is RNA-dependent and occurs mainly in the nucleus, whereas in the cytoplasm, these two RBPs appear to bind target mRNAs individually. Within this context, Lal et al. [88], using cyclin D1 and p21 (a potent cyclin-dependent kinase inhibitor) as target mRNAs, proposed that ELAVL1 and AUF1 can simultaneously as well as competitively bind to a subset of common transcripts in the nucleus, thus forming a RNP complex that transiently contains both ELAVL1 and AUF1 (and likely additional RBPs) until reaching the cytoplasm. Subsequently, depending on different factors that are still not well defined (i.e., mRNA sequence, relative abundance of the various RBPs, subcellular localization, specific stimuli), either AUF1 is released and ELAVL1 remains bound or, vice versa, ELAVL1 is released and AUF1 remains bound. In the first case, the RNP complex is recruited to polysomes where the target mRNA is translated, and in the second case, the RNP complex is recruited to the exosome system where the target mRNA undergoes degradation ([88]; see also Fig. 2).
Fig. 2.
RBPs interaction in the control of mRNA fate. Stabilizing (SPy) and destabilizing (DPx) RBPs can simultaneously as well as competitively bind to a subset of common transcripts in the nucleus. Subsequently, depending on different factors, either the DPx is released and the SPy remains bound (hence the mRNA is recruited to polysomes and translated) or, vice versa, the SPy is released and the DPx remains bound (hence the mRNA is recruited to the exosome and degraded). Within this context, the best example is proposed by [88] (see text for more details)
Modulation of microRNA-mediated repression by ELAVL1
MiRNAs have been implicated in the control of many fundamental cellular and physiological processes such as tissue development, cellular differentiation and proliferation, metabolic and signaling pathways, and apoptosis [89]. MiRNAs belong to a large family of small ≈21-nucleotide-long noncoding RNAs that play a crucial role in the regulation of gene expression at post-transcriptional level in metazoan animals, plants, and protozoa [2, 90]. Following transcription by RNA polymerase II, primary miRNAs (pri-miRNA) are processed first to precursor miRNAs (pre-miRNAs) and then to mature miRNAs by multiprotein complexes including Drosha and Dicer, respectively, and then incorporated into RISC (RNA-induced silencing complex) (reviewed in [91–93]). MiRNAs inhibit protein synthesis by repressing translation or inducing degradation of the mRNA [91, 94, 95]. In particular, miRNAs act by base-pairing to the bound mRNA and form, at least in the case of the most studied animal miRNAs, imperfect hybrids with sequences located in the 3′-UTR (reviewed in [2]). Usually, multiple sites are required to produce an effective repression, exerted by either the same or by different miRNAs, and when the sites are close to each other, they may act cooperatively [96, 97]. Translation has been shown to be inhibited, at the initiation step, by interference of Argonaute proteins (key components of RISC) with the translation initiation factors eIF6 and eIF4 [91, 98, 99]. More recently, it has been reported that some miRNAs are able to do the opposite, that is, to activate mRNA translation [100–102].
As mentioned, miRNAs act preferentially by binding to the 3′-UTR region of a target mRNA and they are also involved in ARE-mediated mRNA instability [103]. Within this context, some RBPs, such as ELAVL1 [104] or Dnd1 (dead-end 1; [105]), have been shown to modulate miRNA-mediated regulation. Bhattacharyya et al. documented that, in human hepatoma cells, ELAVL1 relieves miR-122-mediated repression of the cationic amino acid transporter 1 (CAT-1) mRNA. In particular, the derepression of CAT-1 mRNA is accompanied by its release from the cytoplasmic processing bodies and its recruitment to polysomes [104]. However, in contrast to the example on CAT-1, ELAVL1 protein enhances, through an interdependent mechanism, the repression induced by let-7 miRNA on c-Myc mRNA (that codes for a protein that is a transcription factor) [106]. The authors suggest that, in this case, ELAVL1 inhibits c-Myc expression by recruiting let-7-loaded RISC to the 3′-UTR of c-Myc. Consistently, the depletion of ELAVL1 counteracts let-7-mediated inhibition of c-Myc [106]. Regarding Dnd1, an RBP that is essential for primordial germ cell survival in zebrafish and mouse, it has been reported that, similarly to the effect of ELAVL1 on CAT-1, it prevents miR-221-induced repression of p27 mRNA in human germline cells [105]. Although Dnd1 and ELAVL1 share a comparable behavior, Dnd1 seems to affect a broader number of target mRNAs in comparison with ELAVL1 that seems to endow a more restricted function [105].
It has been reported that miRNAs may even modulate the expression of ELAV-like proteins themselves. Abdelmohsen et al. have found that miR-519 decreases cell proliferation [107] and tumor growth [108] by reducing ELAVL1 expression, while miR-375 inhibits neurite differentiation by lowering both ELAVL4 mRNA stability and translation, thus recapitulating the effects of ELAVL4 silencing [109].
Involvement of ELAV-like alterations in pathological contexts
Alzheimer’s disease
Learning and memory processes are complex and dynamic mechanisms affecting synaptic strength and the morphology, biochemistry, and physiology of selected regions of the brain, ultimately leading to persistent modifications in the efficacy of cell-to-cell communication. Subcellular, localized changes in gene expression cannot be easily explained by a purely transcriptional control, while the implication of post-transcriptional mechanisms can better offer a molecular basis to justify those long-term changes taking place at neuronal specialized microdomains. We previously demonstrated a physiological role for nELAV-like proteins in controlling, at the post-transcriptional level, gene expression in memory formation [110, 111]. Moreover, in vivo knock-down of one of the nELAV-like impaired learning performance and specifically prevented the up-regulation of GAP-43 (growth-associated protein 43) mRNA whose corresponding protein is involved in synaptic plasticity remodeling [110]. These findings suggest that a derangement in ELAV-like proteins, and hence the stability/translation of the target mRNAs, may have implications in neurodegenerative pathologies characterized by a loss of memory and multiple dysfunctions such as Alzheimer’s disease (AD).
Briefly, AD is clinically characterized by a progressive and inexorable cognitive and memory decline, coupled with a marked neuronal death [112]. It is well known that the beta-amyloid (Aβ) peptide and its aberrant accumulation in senile plaques is, among others, a pathognomonic hallmark of the disease. Aβ peptide formation is regulated by the so-called amyloidogenic pathway, and is the proteolytic product of two proteases, namely β- and γ-secretases, that act by cleaving a transmembrane larger precursor known as APP (amyloid precursor protein). The non-amyloidogenic pathway is, instead, mediated by the intervention of an α-secretase, involving upstream also PKC, that cleaves APP within the Aβ sequence, thus releasing from the cell surface a large ectodomain called sAPPα [113, 114]. Notably, not only α-secretase prevents the formation of Aβ but the released sAPPα is endowed with neuroprotectives effects and plays a positive role in memory mechanisms [115, 116]. In contrast, Aβ alters long-term synaptic plasticity processes inducing memory deficits [117] and it is reported as the primary contributor to neuronal degeneration associated with the disease.
ADAM10 is a member of the ADAMs (a disintegrin and metalloproteinase) family of integral membrane proteins that act as α-secretases. In particular, literature data indicate significantly reduced ADAM10 protein levels in platelets of sporadic AD patients coupled with reduced sAPPα levels in both platelets and cerebrospinal fluid [118]. Complementary to these findings is the observation that α-secretase activity is reduced in temporal cortex homogenates from AD patients [119].
Performing experiments on hippocampal post-mortem samples from subjects with a different degree of AD (CDR 0–5), we demonstrated, for the first time, that nELAV-like protein levels decrease as a function of clinical dementia progression [120]. Additionally, we found that the amount of hippocampal nELAV-like proteins inversely correlates with the total hippocampal content of Aβ1–42. Of interest, we showed that the binding between nELAV-like proteins and the mRNA of ADAM10 is disrupted as a function of Aβ1–42 treatment, thus explaining the significantly diminished levels of ADAM10 protein observed in AD patients [120]. Over all, these findings suggest that nELAV-like might control mRNAs of proteins implicated in AD pathogenesis and raise the possibility that molecules able to bind specific mRNAs and to mimic nELAV-like proteins may help to recover, by acting upon mRNA stabilization, the AD-related deficit in the expression of proteins coded by ARE-bearing mRNAs, as already observed in in vitro experiments for other mRNAs [121].
Another key enzyme implicated in AD pathogenesis is the β-secretase BACE1 that, as mentioned, participates in the production of Aβ1–42. The expression and enzymatic activity of BACE1 are increased in brains of AD patients [122] and, of interest, BACE1 expression is regulated post-transcriptionally [123, 124]. Although up to now no specific link has been found with ELAV-like proteins, an involvement of some miRNAs, namely miR-298 and miR-328, has been reported in the negative regulation of BACE1 expression and that their levels are decreased in an animal model of AD [125]. Moreover, BACE1 mRNA amount tended to increase as the levels of another miRNA, miR-107, decreased with the progression of AD [126]. These observations may indicate that neurodegenerative diseases like AD tend to reflect a “downward-spiral” effect of multiple processes, where nELAV-like proteins, as well as miRNAs, may play a contributory role.
Besides senile plaques, intracellular neurofibrillary tangles (NT) represent an additional prominent feature of AD. The major component of NT is an abnormally hyperphosphorylated and aggregated form of tau protein, an axonal protein that binds to microtubules and plays an essential role in their assembly and stability [112]. Recently, on the basis of a genome-wide association study in humans coupled with functional screening in a Drosophila tau transgenic model, six candidate loci that influence the accumulation of tau in AD have been identified and, among them, the ortholog of the ELAVL2 gene, namely fne [127]. In fact, the expression of fne strongly increases tau toxicity in the fly eye and, at higher levels, fne caused pupal lethality when co-expressed with tau. Conversely, an fne RNAi line attenuated tau toxicity, thus indicating a causal link between these two genes [127].
Although this paragraph has been focused on AD, it should be mentioned that ELAV-like proteins may have a broader involvement in neurologic and psychiatric diseases, as documented, for example, by data on schizophrenia and Parkinson’s disease (PD). Indeed, a recent genome-wide association study identified ELAVL2 as a susceptibility gene for schizophrenia in both Japanese and Chinese cohorts [128]. Regarding PD, Noureddine et al. [129] demonstrated an allelic association between ELAVL4 and the age-at-onset (AAO) trait of PD. In particular, they show a strong association between the SNP2 ELAVL4 polymorphism with AAO of PD. It is conceivable to speculate that ELAVL4 polymorphisms may influence the interaction of ELAVL4 protein with its targets, thus contributing to disease development. Within this context, as also suggested by the same authors, an important target could be represented by pro-inflammatory cytokines, including TNFα mRNA (see also the following paragraph on inflammation) whose corresponding protein has been reported to be increased in PD [130].
Cancer
Cancer genes are identified by their altered gene expression and/or activity entailing an aberrant phenotype. On the whole, these modifications determine an accelerated cell growth via: (1) increased cell division, (2) resistance to apoptosis, (3) preservation of angiogenesis, (4) tissue invasion and metastasis, and (5) immune response escape [131]. Interestingly, in cancer, deregulations at the post-transcriptional level are increasingly recognized as key contributors to abnormal gene expression in the absence of apparent mutations [7, 132]. As mentioned, a change in RPBs expression, a derangement in their localization or in their ability to bind to ARE sequences may modify the stability and/or the translation of a given mRNA (see also Fig. 1). For brevity, hereinafter we will report only some representative cases of RBPs alterations leading to cancer, mainly focusing our attention on the ELAVL1.
Originally, ELAV-like proteins were identified as specific tumor antigens present in cancers of patients with paraneoplastic neurological disorder [133]. Afterwards, not only among them but also among all RBPs, the most extensively studied protein in cancer has been ELAVL1 [7]. Probably, ELAVL1 involvement in a wide range of tumors depends on some features that render this protein unique such as: (1) it is ubiquitously expressed; (2) it possesses a stabilizing function; (3) it is endowed with less strict requirements for ARE binding in comparison to other RBPs [3]. Tissue array analysis documented that malignant tumors are characterized by a higher content of ELAVL1 in comparison with benign tumors or normal tissues [134–136]. Moreover, subcutaneous injection of ELAVL1-overexpressing RKO colon cancer cells into nude mice caused the appearance of significantly larger tumors with respect to those resulting from control cells; conversely, RKO cells expressing reduced levels of ELAVL1 produced significantly smaller and slower-growing tumors [135]. In several types of cancer, a reduced binding of the destabilizing RBP AUF1 has been shown to be associated with an elevated amount of IL-10 (a cytokine also involved in the inhibition of both tumor rejection and immune surveillance) [7].
Most ARE-BPs, comprising AUF1 and ELAVL1, are preferentially localized in the nucleus and are able to shuttle between the nucleus itself and the cytoplasm. As already mentioned, the cytoplasmic presence of ARE-BPs seems to be strictly related to RBPs ability to affect target mRNAs, therefore changes in their localization may have a role in cancer. Indeed, a cytoplasmic increase of ELAVL1 has been positively correlated to malignancy [135]. Furthermore, an elevated cytoplasmic ELAVL1 expression has been associated with a poor histological differentiation, large tumor size, and poor survival in ductal breast carcinoma [137]. Moreover, lower cytoplasmic levels of the destabilizing AUF1 have been detected in extracts from MNT1 melanoma cells with respect to normal melanocytes [138].
Another aspect to be taken into account is that RBPs may also undergo structural changes, possibly affecting their ability to bind ARE-bearing mRNAs. For example, post-translational modifications have been described for nELAV-like [51] and ELAVL1 [139, 140], although, at least until now, no link with cancer has been documented. However, in hepatocytes, it has been reported that methyl-ELAVL1 may function as a destabilizer of MAT2A (methionine adenosyltransferase) mRNA, potentially influencing their proliferation [141]. MAT catalyzes the synthesis of S-adenosylmethionine, the principal hepatic methyl donor. In mammals, this enzyme is encoded by MAT1A and MAT2A genes: the first one is expressed in the adult liver, whereas MAT2A expression is strongly related to a rapid growth and de-differentiation. A switch between MAT1A/MAT2A mRNA occurs during the de-differentiation of cultured hepatocytes. In these cells, ELAVL1 associates with MAT2A mRNA inducing an enhancement of its levels, while AUF1 interacts with MAT1A mRNA producing a decrease in its abundance [141]. In human hepatocellular carcinoma samples the authors observed an increase of the amount of ELAVL1 and AUF1 proteins coupled with a decrease of methyl-ELAVL1 content. Conversely, in normal human control samples ELAVL1 and AUF1 are expressed at low levels, whereas methyl-ELAVL1 is present at high levels, thus suggesting a role of the ELAVL1/methyl-ELAVL1 and AUF1 system in human hepatocellular carcinoma progression [141]. Concerning tumor progression, a fascinating model proposed by Mazan-Mamczarz et al. supports the role of ELAVL1 as a downstream effector of cancer-related gene expression, emphasizing the previously mentioned concept of the post-transcriptional operon-based network. On the basis of their data in breast carcinoma cells, they demonstrated that global changes in ELAVL1-bound mRNAs are involved in the progression to a more malignant phenotype [142]. Accordingly, they show that, in cells characterized by a less tumorigenic phenotype, ELAVL1 interacts with mRNAs encoding pro-apoptotic proteins, whereas in cells with a more malignant phenotype ELAVL1 switches its mRNA partners and associates, instead, with other mRNAs encoding proteins that favor a more tumorigenic phenotype [142]. A role in cancer onset and/or progression may also be ascribed to alterations in nELAV-like genes, as suggested for ELAVL4 mutations observed in a subset of neuroendocrine lung tumors [143]. Finally, it should be considered that a change in the binding properties of RBPs may also occur as a consequence of genetic alterations within the 3′-UTR itself of target mRNAs, where most of ARE elements are found, thus leading to a deregulation in protein production. Mutations and over-expression of cyclin D1 have been observed in a variety of tumors where they cause an alteration of cell-cycle progression and contribute to tumorigenesis [7]. In agreement with the previous considerations, the 3′-UTR of cyclin D1 is truncated in the human cancer cell line MDA MB-453 or rearranged in patients with mantle cell lymphomas [144, 145]. Moreover, c-myc mRNA has been found to be constitutively stabilized in various tumors, such as in human plasma cell myeloma and in a derivative cell line where a 3′-UTR translocation has been described [146], and in a human T-cell leukemia line missing a 61-nt ARE [147].
To give a complete scenario, it is necessary to address the role of miRNAs in cancer. MiRNAs’ alterations are implicated in the initiation and progression of human cancer, moreover miRNAs-expression profiling of human tumors has recognized signatures correlated to diagnosis, staging, progression, prognosis, and response to treatment [148]. In different types of cancer, a deregulation of miRNAs content is a frequent event; indeed for half of the 217 mammalian miRNAs examined, a general down-regulation in tumors compared to normal tissues has been documented [149]. In addition, within the general context of a crosstalk between miRNAs and RBPs, Xu et al. have recently reported that a defect in the repression of ELAVL1 translation by miR-16 may have a role in human breast carcinoma, thus suggesting that strategies targeting these miRNAs in cancer may represent a more successful approach [150]. The interaction between ELAVL1 and the already-mentioned miR-519 has also been reported to play an important role in cancer. Accordingly, in several human carcinoma cell lines it has been documented that miR-519 induces a repression of ELAVL1 translation without affecting ELAVL1 mRNA abundance, leading to suppressed cell proliferation [107]. Furthermore, lung, kidney, and ovarian cancer specimens showed more elevated ELAVL1 protein content, unchanged ELAVL1 mRNA amount, and markedly reduced levels of miR-519 when compared to healthy tissues [108]. Consistently, cells overexpressing miR-519 formed significantly smaller tumors, whereas cells characterized by reduced miR-519 levels produced substantially larger tumors [109]. Also, the expression of another miRNA, namely miR-125a, has been found to inversely correlate with ELAVL1 expression in various breast carcinoma cell lines [151]. Coherently, re-establishing miR-125a expression in these cells induced a decrease in ELAVL1 protein content and a parallel inhibition of cell growth [151]. Taken together, these data emphasize the concept that miRNAs may function as tumor suppressors, thus representing a novel putative therapeutic tool.
Inflammation
Cytokines and chemokines shape the way through which the immune system responds to an inflammatory insult. Therefore, a derangement in their expression may lead to altered inflammatory responses, well beyond the original purpose, and to the establishment of chronic inflammation. The majority of cytokines and chemokines, produced during the immune response, are coded by very unstable ARE-bearing mRNAs [6, 12, 13]. This is the case, for example, of the pro-inflammatory cytokines TNFα (tumor necrosis factor alpha), IL-1β (Interleukin-1 beta), IL-6, IL-8, GM-CSF, and of the anti-inflammatory cytokine IL-10, whose synthesis is required to counteract, in the long term, the prolonged effects of pro-inflammatory cytokines. It should be underscored that, since their transcripts are very unstable, a small change in mRNAs’ half-life may determine dramatic modifications in the correspondent protein contents. These considerations emphasize the key importance of RBPs in the regulation of the expression of genes involved in the inflammatory response and their possible role in inflammatory pathologies. Regarding the role of post-transcriptional mechanisms implicated in the regulation of chemokine gene expression, we refer the reader to a comprehensive review by Fan et al. [152]. Hereafter, we will mainly focus on TNFα, the most investigated cytokine involved in inflammation processes.
Collectively, very few data are available on the natural mutations/deletions occurring within the ARE of cytokine transcripts and directly implicated in inflammatory pathologies [3]. However, the experimental deletion of the TNFα ARE in the mouse highlights their importance in vivo. In fact, TNFαΔARE mouse macrophages express a constitutively stable TNFα mRNA leading to a spontaneous production of TNFα protein. Of relevance, mutant mice develop a chronic inflammatory arthritis and a Crohn’s-like inflammatory bowel disease [153]. In addition, mice deficient in TTP (a destabilizing RBP protein) develop symptoms such as cachexia, arthritis, and dermatitis [154] similar to those described in TNFαΔARE mice. These findings are consistent with the observation that, in both cases, the TNFα mRNA becomes more stable: in the first case because of a deletion of its ARE, whereas in the second case because of a defect in the TTP-mediated destabilizing mechanism. Within this context, macrophages lacking in another destabilizing RBP, namely TIA-1, produce significantly more TNFα protein than wild-type controls, being TIA-1 suggested to function as a translational silencer [155]. A key role in the post-transcriptional control of TNFα is certainly played by ELAVL1. Consistently, ELAVL1 has been identified as the major TNFα ARE-binding protein in a mouse macrophage cell line (macrophages are the main TNFα-producing cells) and shown to stabilize TNFα mRNA itself [156]. Furthermore, stable knockdown of ELAVL1 in the same cell line causes, following LPS challenge, a reduced expression of TNFα mRNA due to a marked decrease in its stability [157]. Similarly, chemical inhibitors of the interaction between ELAVL1 and the TNFα mRNA produce decreased levels of TNFα secreted protein in LPS-treated macrophages [158]. Additionally, in a mouse model of spontaneous lupus nephritis, a polymorphism present within the 3′-UTR of TNFα mRNA results in the hindered binding of ELAVL1 to TNFα mRNA itself, leading to a significant reduction in the levels of the corresponding TNFα protein [159].
As mentioned, IL-10 is a potent anti-inflammatory cytokine able to strongly suppress the synthesis of TNFα and other pro-inflammatory cytokines [160, 161]. Consistently, IL-10 knockout mice exhibit increased TNFα levels coupled with an unregulated inflammatory activity [162]. Furthermore, it has been reported that systemic IL-10 treatment of mice, following carotid artery injury (a procedure that induces vascular damage associated with an inflammatory response), blunts the inflammatory cell infiltration and local TNFα expression [163]. A clue to explain the underlying molecular mechanism comes from an in vitro experiment performed in the same work, where the authors show that IL-10 treatment of a human monocytic cell line inhibits the expression of ELAVL1 and represses the binding of ELAVL1 itself to the TNFα ARE sequence, resulting in a greater degradation of the TNFα transcript [163]. The authors suggest the involvement, upstream, of the p38 MAPK, since the activation of LPS-induced p38 MAPK is strongly inhibited by IL-10. However, considering that ELAVL1 contains no putative phosphorylation sites for MAPK, p38 and ELAVL1 may belong to independent pathways finally acting on TNFα mRNA [6], as also suggested in another context [164]. Prolonged inflammation, associated with the production of pro-inflammatory cytokines, is also observed following myocardial infarction (MI) and related to left ventricular dysfunction and adverse remodeling. Notably, IL-10 inhibits this myocardial inflammatory response by suppressing ELAVL1-mediated mRNA stabilization of pro-inflammatory cytokines, thereby also attenuating left ventricular dysfunction and remodeling [165]. Consistently, knockdown of ELAVL1 in the myocardium significantly reduces post-MI inflammatory response and left ventricular dysfunction and remodeling in IL-10 null mice [157].
In line with the implication of ELAVL1 in inflammation, it has also been found that ELAVL1 increases the stability of the mRNA of the Toll-like receptor 4 in vascular smooth muscle cells [166]. The activation of Toll-like receptors results in the NF-kB (nuclear factor-kappa B)-dependent transcription of several cytokines [167]; accordingly, an inhibition of the nuclear activation of NF-kB has been observed in monocytes stimulated with LPS following IL-10 treatment [168]. Furthermore, siRNA knockdown of ELAVL1 inhibits inflammatory responses in endothelial cells, including NF-kB phosphorylation and adhesion of monocytes [169]. However, in contrast with the putative role of ELAVL1 in the hyperactivation of inflammatory mediators, Katsanou et al. [170] demonstrated that, in macrophages, ELAVL1 over-expression induces the translational silencing of specific pro-inflammatory cytokine mRNAs, probably via functional interactions with negative post-transcriptional modulators, such as TIA-1 and TTP. These last findings underscore that the functional effects of ELAVL1 may depend on specific cis-elements present in each target mRNA as well as by the different upstream signaling cascades triggered upon distinct stimuli and cellular contexts.
MiRNAs, such as miR-155 and miR-125b, have also been implicated in the regulation of TNFα production in immune cells [171]. Mice deficient in miR-155 show impaired B cell responses [172, 173] linked to reduced TNFα production [174]. Similarly, transgenic mice over-expressing miR-155 in B cells show higher serum TNFα and elevated susceptibility to endotoxemia [175]. In contrast, miR-125b is down-regulated in LPS-stimulated macrophages and can target the 3′-UTR of TNFα, suggesting a role of miR-125b in post-transcriptional repression of TNFα mRNA [175].
On the whole, the reported data highlight that RBPs targeting cis-elements within the transcript of TNFα, as well as of other mRNAs, combine their functions with the miRNAs machinery towards both activation and suppression, thus strongly supporting the concept that a deregulation of this fine balance may lead to the development of specific pathologies (see also Fig. 1).
Conclusions
The previous paragraphs highlight the crucial role of post-transcriptional processes in the control of gene expression and open a new fascinating route to translate basic mechanisms into clinical medicine. The reported literature data emphasize that a derangement in post-transcriptional mechanisms may represent a common feature of diseases, and indicate that pharmacological interventions may be directed, in some cases, to blunt the excessive function of these networks whereas, in other cases, to potentiate them.
As underscored in this manuscript, ELAV-like proteins are pleiotropic proteins that are important in various contexts, since they can affect the fate of target mRNAs whose corresponding proteins are fundamental for key cellular functions. Within this context, we have recently studied ELAV-like/mRNA interaction from a medicinal chemistry point of view. Particularly, we addressed our efforts to discover ELAV-like-mimicking molecules that, on one hand, may help to better understand mRNA–protein interactions and, on the other, may represent the first step towards a highly innovative pharmacological approach that can open new therapeutic strategies in several pathological contexts. We and our collaborators found that four peptides, corresponding to very conserved motives within the first two RRM domains of ELAV-like, are able to stabilize two target transcripts, namely NOVA-1 (a protein, as mentioned, essential for proper neural development and for synaptic plasticity in the mature brain) and VEGF (vascular endothelial growth factor; a protein that plays a fundamental role in angiogenesis, but also in repairing processes after brain injury), synergizing with phorbol esters [121]. Our findings point out the possibility that molecules able to bind selected mRNAs and to mimic ELAV-like may help to recover the deficit in the expression of proteins, coded by ARE-bearing mRNAs, associated with specific pathologies, such as AD. Conversely, Chae et al. [158] identified some chemicals able to inhibit the interaction between ELAVL1 and the ARE of the TNFα transcript, suggesting that this latter approach can lead to the discovery of new anti-inflammation drugs, where a decrease in the expression of selected key genes, such as TNFα, is more desirable.
Furthermore, considering that miRNA activity is involved in the control of a wide range of biological functions and processes, such as development, differentiation, metabolism, growth, proliferation, and apoptosis [176, 177], innovative strategies targeting miRNAs have also been developed with the purpose of reducing the levels of aberrantly expressed miRNAs or, on the contrary, to elevate the levels of down-regulated miRNAs with beneficial functions [93, 178]. The feasibility of miRNAs as therapeutic targets is supported by the fact that a phase I clinical trial adopting an anti-miRNA was successfully concluded (see: santaris.com), thus pointing that miRNAs inhibitors may become an important novel class of drugs. The inhibition of miRNAs with complementary oligonucleotides leads to the reduction of a specific miRNA-programmed RISC. Of interest, an LNA (locked nucleic acid)-based anti-miRNA targeting a liver-specific miRNA, namely miR-122, is being developed for hepatitis C therapy [93].
Overall, we hope that the topics handled in the present manuscript provide a hint to look at ELAV-like-mediated mRNA stabilization as a mechanism relevant to disease as well as a novel putative drug target.
Acknowledgments
The authors would like to thank Sharon Lynn Carlson for English revision of the text.
Abbreviations
- AAO
Age-at-onset
- Aβ
Beta-amyloid
- AD
Alzheimer’s disease
- ADAMs
A disintegrin and metalloproteinase
- APP
Amyloid precursor protein
- ARE
Adenine–uridine-rich elements
- ARE-BPs
ARE-binding proteins
- CAT-1
Cationic amino acid transporter 1
- Dnd1
Dead-end 1
- ELAV
Embryonic lethal abnormal vision
- GAP-43
Growth-associated protein 43
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL-1β
Interleukin-1 beta
- LNA
Locked nucleic acid
- MAPK
Mitogen-activated protein kinase
- MI
Myocardial infarction
- miRNAs
microRNAs
- mRNPs
Messenger ribonucleoprotein particles
- MAT
Methionine adenosyltransferase
- nELAV-like
Neuronal ELAV-like
- NOVA-1
Neuro-oncological ventral antigen-1
- NF
Neurofibrillary tangles
- NF-kB
Nuclear factor-kappa B
- PD
Parkinson’s disease
- PKC
Protein kinase C
- RBPs
RNA-binding proteins
- RISC
RNA-induced silencing complex
- RRMs
RNA-recognizing motifs
- TNFα
Tumor necrosis factor alpha
- TTP
Tristetraprolin
- 5′-UTR
5′-untranslated region
- 3′-UTR
3′-untranslated region
- VEGF
Vascular endothelial growth factor
References
- 1.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 2.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Chi M, Morello D. Aberrant regulation of mRNA 3′ untranslated region in cancers and inflammation. Med Sci (Paris) 2008;24(3):290–296. doi: 10.1051/medsci/2008243290. [DOI] [PubMed] [Google Scholar]
- 4.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265(1–2):11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 5.Ross J. mRNA stability in mammalian cells. Microbiol Rev. 1995;59(3):423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhardt W, Doller A, Akool el S, Pfeilschifter J. Modulation of mRNA stability as a novel therapeutic approach. Pharmacol Ther. 2007;114(1):56–73. doi: 10.1016/j.pharmthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.López de Silanes I, Quesada MP, Esteller M. Aberrant regulation of messenger RNA 3′-untranslated region in human cancer. Cell Oncol. 2007;29(1):1–17. doi: 10.1155/2007/586139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20(11):465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilson GM, Brewer G. The search for trans-acting factors controlling messenger RNA decay. Prog Nucleic Acid Res Mol Biol. 1999;62:257–291. doi: 10.1016/s0079-6603(08)60510-3. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Gao M, O’Connor JP, Raijmakers R, Pruijn G, Lutz CS, Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21(1–2):165–174. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 12.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34(Database issue):D111–D114. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2006;33(22):7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Kruys V, Huez G, Gueydan C. AU-rich element-mediated translational control: complexity and multiple activities of trans-activating factors. Biochem Soc Trans. 2002;30(Pt 6):952–958. doi: 10.1042/bst0300952. [DOI] [PubMed] [Google Scholar]
- 15.Good PJ. A conserved family of ELAV-like genes in vertebrates. Proc Natl Acad Sci USA. 1995;92(10):4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58(2):266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolognani F, Perrone-Bizzozero NI. RNA–protein interactions and control of mRNA stability in neurons. J Neurosci Res. 2008;86(3):481–489. doi: 10.1002/jnr.21473. [DOI] [PubMed] [Google Scholar]
- 18.Pascale A, Amadio M, Quattrone A. Defining a neuron: neuronal ELAV proteins. Cell Mol Life Sci. 2008;65(1):128–140. doi: 10.1007/s00018-007-7017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keene JD. Why is Hu where? Shuttling of early response-gene messenger RNA subsets. Proc Natl Acad Sci USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65(20):3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain RG, Andrews LG, McGowan KM, Pekala PH, Keene JD. Ectopic expression of Hel-N1, an RNA-binding protein, increases glucose transporter (GLUT1) expression in 3T3–L1 adipocytes. Mol Cell Biol. 1997;17(2):954–962. doi: 10.1128/mcb.17.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antic D, Lu N, Keene JD. ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev. 1999;13(4):449–461. doi: 10.1101/gad.13.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullmann M, Göpfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 2002;16(23):3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolognani F, Contente-Cuomo T, Perrone-Bizzozero NI. Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res. 2010;38(1):117–130. doi: 10.1093/nar/gkp863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, Sternjohn JR, Vasdewani J, Karypis G, Reilly CS, Bitterman PB, Bohjanen PR. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol Cell. 2008;29(2):263–270. doi: 10.1016/j.molcel.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wein G, Rössler M, Klug R, Herget T. The 3′-UTR of the mRNA coding for the major protein kinase C substrate MARCKS contains a novel CU-rich element interacting with the mRNA stabilizing factors HuD and HuR. Eur J Biochem. 2003;270(2):350–365. doi: 10.1046/j.1432-1033.2003.03396.x. [DOI] [PubMed] [Google Scholar]
- 27.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to ELAV and Sex-lethal. Cell. 1991;67(2):325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 28.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141(4):881–886. [PMC free article] [PubMed] [Google Scholar]
- 29.Posner JB. The anti-Hu syndrome: a model paraneoplastic disorder. Recent Results Cancer Res. 1994;135:77–90. doi: 10.1007/978-3-642-85039-4_9. [DOI] [PubMed] [Google Scholar]
- 30.Robinow S, Campos AR, Yao KM, White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988;242(4885):1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- 31.Samson ML. Rapid functional diversification in the structurally conserved ELAV family of neuronal RNA-binding proteins. BMC Genomics. 2008;9:392–402. doi: 10.1186/1471-2164-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koushika SP, Lisbin MJ, White K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr Biol. 1996;6(12):1634–1641. doi: 10.1016/s0960-9822(02)70787-2. [DOI] [PubMed] [Google Scholar]
- 33.Koushika SP, Soller M, White K. The neuron-enriched splicing pattern of Drosophila erect wing is dependent on the presence of ELAV protein. Mol Cell Biol. 2000;20(5):1836–1845. doi: 10.1128/mcb.20.5.1836-1845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiménez F, Campos-Ortega JA. Genes in subdivision 1B of the Drosophila melanogaster X chromosome and their influence on neural development. J Neurogenet. 1987;4(4):179–200. [PubMed] [Google Scholar]
- 35.Nagai K, Oubridge C, Ito N, Avis J, Evans P. The RNP domain: a sequence-specific RNA-binding domain involved in processing and transport of RNA. Trends Biochem Sci. 1995;20(6):235–240. doi: 10.1016/s0968-0004(00)89024-6. [DOI] [PubMed] [Google Scholar]
- 36.Deschênes-Furry J, Angus LM, Bélanger G, Mwanjewe J, Jasmin BJ. Role of ELAV-like RNA-binding proteins HuD and HuR in the post-transcriptional regulation of acetylcholinesterase in neurons and skeletal muscle cells. Chem Biol Interact. 2005;157–158:43–49. doi: 10.1016/j.cbi.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Fan XC, Steitz JA. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA. 1998;95(26):15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Tanaka Hall TM. Structural basis for recognition of AU-rich element RNA by the HuD protein. Nat Struct Biol. 2001;8(2):141–145. doi: 10.1038/84131. [DOI] [PubMed] [Google Scholar]
- 39.Ma WJ, Chung S, Furneaux H. The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 1997;25:3564–3569. doi: 10.1093/nar/25.18.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckel-Mitchener AC, Miera A, Keller R, Perrone-Bizzozero NI. Poly(A) tail length-dependent stabilization of GAP-43 mRNA by the RNA-binding protein HuD. J Biol Chem. 2002;277:27996–28002. doi: 10.1074/jbc.M201982200. [DOI] [PubMed] [Google Scholar]
- 41.Gao FB, Keene JD. Hel-N1/Hel-N2 proteins are bound to poly(A) + mRNA in granular RNP structures and are implicated in neuronal differentiation. J Cell Sci. 1996;109(Pt 3):579–589. doi: 10.1242/jcs.109.3.579. [DOI] [PubMed] [Google Scholar]
- 42.Kasashima K, Sakashita E, Saito K, Sakamoto H. Complex formation of the neuron-specific ELAV-like Hu RNA-binding proteins. Nucleic Acids Res. 2002;30:4519–4526. doi: 10.1093/nar/gkf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fialcowitz-White EJ, Brewer BY, Ballin JD, Willis CD, Toth EA, Wilson GM. Specific protein domains mediate cooperative assembly of HuR oligomers on AU-rich mRNA-destabilizing sequences. J Biol Chem. 2007;282:20948–20959. doi: 10.1074/jbc.M701751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toba G, White K. The third RNA recognition motif of Drosophila ELAV protein has a role in multimerization. Nucleic Acids Res. 2008;36(4):1390–1399. doi: 10.1093/nar/gkm1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallouzi IE, Steitz JA. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294(5548):1895–1901. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- 46.Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3(3):195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 47.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20(12):2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Müller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18(18):4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ming XF, Stoecklin G, Lu M, Looser R, Moroni C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21(17):5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Fan J, Yang X, Fürer-Galban S, Lopez de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22(10):3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, Govoni S, Alkon DL, Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha-dependent pathway. Proc Natl Acad Sci USA. 2005;102(34):12065–12070. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doller A, Schlepckow K, Schwalbe H, Pfeilschifter J, Eberhardt W. Tandem phosphorylation of serines 221 and 318 by protein kinase Cdelta coordinates mRNA binding and nucleocytoplasmic shuttling of HuR. Mol Cell Biol. 2010;30(6):1397–1410. doi: 10.1128/MCB.01373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antic D, Keene JD. Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J Cell Sci. 1998;111(Pt 2):183–197. doi: 10.1242/jcs.111.2.183. [DOI] [PubMed] [Google Scholar]
- 54.Keene JD. Minireview: global regulation and dynamics of ribonucleic Acid. Endocrinology. 2010;151(4):1391–1397. doi: 10.1210/en.2009-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell. 2002;9(6):1161–1167. doi: 10.1016/s1097-2765(02)00559-2. [DOI] [PubMed] [Google Scholar]
- 56.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–19. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 57.Xu Q, Modrek B, Lee C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002;30(17):3754–3766. doi: 10.1093/nar/gkf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40(12):1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 59.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lisbin MJ, Qiu J, White K. The neuron-specific RNA-binding protein ELAV regulates neuroglian alternative splicing in neurons and binds directly to its pre-mRNA. Genes Dev. 2001;15(19):2546–2561. doi: 10.1101/gad.903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soller M, White K. ELAV inhibits 3′-end processing to promote neural splicing of ewg pre-mRNA. Genes Dev. 2003;17(20):2526–2538. doi: 10.1101/gad.1106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soller M, White K. ELAV multimerizes on conserved AU4–6 motifs important for ewg splicing regulation. Mol Cell Biol. 2005;25(17):7580–7591. doi: 10.1128/MCB.25.17.7580-7591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu H, Hasman RA, Barron VA, Luo G, Lou H. A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol Biol Cell. 2006;17(12):5105–5114. doi: 10.1091/mbc.E06-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu H, Hasman RA, Young KM, Kedersha NL, Lou H. U1 snRNP-dependent function of TIAR in the regulation of alternative RNA processing of the human calcitonin/CGRP pre-mRNA. Mol Cell Biol. 2003;23(17):5959–5971. doi: 10.1128/MCB.23.17.5959-5971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellavia D, Mecarozzi M, Campese AF, Grazioli P, Talora C, Frati L, Gulino A, Screpanti I. Notch3 and the Notch3-upregulated RNA-binding protein HuD regulate Ikaros alternative splicing. EMBO J. 2007;26(6):1670–1680. doi: 10.1038/sj.emboj.7601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Izquierdo JM. Cell-specific regulation of Fas exon 6 splicing mediated by Hu antigen R. Biochem Biophys Res Commun. 2010;402(2):324–328. doi: 10.1016/j.bbrc.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 67.Okano HJ, Darnell RB. A hierarchy of Hu RNA-binding proteins in developing and adult neurons. J Neurosci. 1997;17(9):3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antic D, Keene JD. Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am J Hum Genet. 1997;61(2):273–278. doi: 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Molfenter J, Zhu H, Lou H. Promotion of exon 6 inclusion in HuD pre-mRNA by Hu protein family members. Nucleic Acids Res. 2010;38(11):3760–3770. doi: 10.1093/nar/gkq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kasashima K, Terashima K, Yamamoto K, Sakashita E, Sakamoto H. Cytoplasmic localization is required for the mammalian ELAV-like protein HuD to induce neuronal differentiation. Genes Cells. 1999;4(11):667–683. doi: 10.1046/j.1365-2443.1999.00292.x. [DOI] [PubMed] [Google Scholar]
- 71.Ratti A, Fallini C, Colombrita C, Pascale A, Laforenza U, Quattrone A, Silani V. Post-transcriptional regulation of neuro-oncological ventral antigen 1 by the neuronal RNA-binding proteins ELAV. J Biol Chem. 2008;283(12):7531–7541. doi: 10.1074/jbc.M706082200. [DOI] [PubMed] [Google Scholar]
- 72.Ule J, Ule A, Spencer J, Williams A, Hu JS, Cline M, Wang H, Clark T, Fraser C, Ruggiu M, Zeeberg BR, Kane D, Weinstein JN, Blume J, Darnell RB. Nova regulates brain-specific splicing to shape the synapse. Nat Genet. 2005;37(8):844–852. doi: 10.1038/ng1610. [DOI] [PubMed] [Google Scholar]
- 73.Jensen KB, Dredge BK, Stefani G, Zhong R, Buckanovich RJ, Okano HJ, Yang YY, Darnell RB. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron. 2000;25(2):359–371. doi: 10.1016/s0896-6273(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 74.Lutz CS. Alternative polyadenylation: a twist on mRNA 3′ end formation. ACS Chem Biol. 2008;3(10):609–617. doi: 10.1021/cb800138w. [DOI] [PubMed] [Google Scholar]
- 75.Zhu H, Zhou HL, Hasman RA, Lou H. Hu proteins regulate polyadenylation by blocking sites containing U-rich sequences. J Biol Chem. 2007;282(4):2203–2210. doi: 10.1074/jbc.M609349200. [DOI] [PubMed] [Google Scholar]
- 76.Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33(1):201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J Interferon Cytokine Res. 2005;25(1):1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- 78.Slevin MK, Gourronc F, Hartley RS. ElrA binding to the 3′UTR of cyclin E1 mRNA requires polyadenylation elements. Nucleic Acids Res. 2007;35(7):2167–2176. doi: 10.1093/nar/gkm084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Ahmadi W, Al-Ghamdi M, Al-Haj L, Al-Saif M, Khabar KS. Alternative polyadenylation variants of the RNA-binding protein, HuR: abundance, role of AU-rich elements and auto-Regulation. Nucleic Acids Res. 2009;37(11):3612–3624. doi: 10.1093/nar/gkp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A. Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol. 2003;195(3):356–372. doi: 10.1002/jcp.10272. [DOI] [PubMed] [Google Scholar]
- 81.Laroia G, Cuesta R, Brewer G, Schneider RJ. Control of mRNA decay by heat shock-ubiquitin–proteasome pathway. Science. 1999;284(5413):499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]
- 82.Loflin P, Chen CY, Shyu AB. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes Dev. 1999;13(14):1884–1897. doi: 10.1101/gad.13.14.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao FB, Carson CC, Levine T, Keene JD. Selection of a subset of mRNAs from combinatorial 3′ untranslated region libraries using neuronal RNA-binding protein Hel-N1. Proc Natl Acad Sci USA. 1994;91(23):11207–11211. doi: 10.1073/pnas.91.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhattacharya S, Giordano T, Brewer G, Malter JS. Identification of AUF-1 ligands reveals vast diversity of early response gene mRNAs. Nucleic Acids Res. 1999;27(6):1464–1472. doi: 10.1093/nar/27.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen CY, Xu N, Shyu AB. Highly selective actions of HuR in antagonizing AU-rich element-mediated mRNA destabilization. Mol Cell Biol. 2002;22(20):7268–7278. doi: 10.1128/MCB.22.20.7268-7278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu JY, Schneider RJ. Tissue distribution of AU-rich mRNA-binding proteins involved in regulation of mRNA decay. J Biol Chem. 2004;279(13):12974–12979. doi: 10.1074/jbc.M310433200. [DOI] [PubMed] [Google Scholar]
- 87.Blaxall BC, Pende A, Wu SC, Port JD. Correlation between intrinsic mRNA stability and the affinity of AUF1 (hnRNP D) and HuR for A + U-rich mRNAs. Mol Cell Biochem. 2002;232(1–2):1–11. doi: 10.1023/a:1014819016552. [DOI] [PubMed] [Google Scholar]
- 88.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23(15):3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krützfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 92.Kedde M, Agami R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle. 2008;7(7):899–903. doi: 10.4161/cc.7.7.5644. [DOI] [PubMed] [Google Scholar]
- 93.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125(1):92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 95.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–460. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chendrimada TP, Finn KJ, Ji X, Baillat D, Gregory RI, Liebhaber SA, Pasquinelli AE, Shiekhattar R. MicroRNA silencing through RISC recruitment of eIF6. Nature. 2007;447(7146):823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 99.Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z. An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell. 2007;129(6):1141–1151. doi: 10.1016/j.cell.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Jünemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5-UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Vasudevan S, Tong Y, Steitz JA. Cell-cycle control of microRNA-mediated translation regulation. Cell Cycle. 2008;7:1545–1549. doi: 10.4161/cc.7.11.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120(5):623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 104.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 105.Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van Duijse J, Ørom UA, Lund AH, Perrakis A, Raz E, Agami R. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131(7):1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 106.Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23(15):1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdelmohsen K, Srikantan S, Kuwano Y, Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci USA. 2008;105(51):20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, Cabo R, Gorospe M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9(7):1354–1359. doi: 10.4161/cc.9.7.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abdelmohsen K, Hutchison ER, Lee EK, Kuwano Y, Kim MM, Masuda K, Srikantan S, Subaran SS, Marasa BS, Mattson MP, Gorospe M. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol Cell Biol. 2010;30(17):4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quattrone A, Pascale A, Nogues X, Zhao W, Gusev P, Pacini A, Alkon DL. Posttranscriptional regulation of gene expression in learning by the neuronal ELAV-like mRNA-stabilizing proteins. Proc Natl Acad Sci USA. 2001;98(20):11668–11673. doi: 10.1073/pnas.191388398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pascale A, Gusev PA, Amadio M, Dottorini T, Govoni S, Alkon DL, Quattrone A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc Natl Acad Sci USA. 2004;101(5):1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 113.Racchi M, Govoni S. The pharmacology of amyloid precursor protein processing. Exp Gerontol. 2003;38(1–2):145–157. doi: 10.1016/s0531-5565(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 114.Racchi M, Mazzucchelli M, Pascale A, Sironi M, Govoni S. Role of protein kinase C alpha in the regulated secretion of the amyloid precursor protein. Mol Psychiatry. 2003;8(2):209–216. doi: 10.1038/sj.mp.4001204. [DOI] [PubMed] [Google Scholar]
- 115.Mileusnic R, Lancashire CL, Johnston AN, Rose SP. APP is required during an early phase of memory formation. Eur J Neurosci. 2000;12(12):4487–4495. [PubMed] [Google Scholar]
- 116.Marcello E, Epis R, Di Luca M. Amyloid flirting with synaptic failure: towards a comprehensive view of Alzheimer’s disease pathogenesis. Eur J Pharmacol. 2008;585(1):109–118. doi: 10.1016/j.ejphar.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 117.Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res. 2000;60(1):65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 118.Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, Padovani A, Di Luca M. [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol Med. 2002;8(2):67–74. [PMC free article] [PubMed] [Google Scholar]
- 119.Tyler SJ, Dawbarn D, Wilcock GK, Allen SJ. alpha- and beta-secretase: profound changes in Alzheimer’s disease. Biochem Biophys Res Commun. 2002;299(3):373–376. doi: 10.1016/s0006-291x(02)02635-9. [DOI] [PubMed] [Google Scholar]
- 120.Amadio M, Pascale A, Wang J, Ho L, Quattrone A, Gandy S, Haroutunian V, Racchi M, Pasinetti GM. nELAV proteins alteration in Alzheimer’s disease brain: a novel putative target for amyloid-beta reverberating on AbetaPP processing. J Alzheimer’s Dis. 2009;16(2):409–419. doi: 10.3233/JAD-2009-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rossi D, Amadio M, Carnevale Baraglia A, Azzolina O, Ratti A, Govoni S, Pascale A, Collina S. Discovery of small peptides derived from embryonic lethal abnormal vision proteins structure showing RNA-stabilizing properties. J Med Chem. 2009;52(16):5017–5019. doi: 10.1021/jm900741e. [DOI] [PubMed] [Google Scholar]
- 122.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51(6):783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 123.Marambaud P, Chevallier N, Ancolio K, Checler F. Post-transcriptional contribution of a cAMP-dependent pathway to the formation of alpha- and beta/gamma-secretases-derived products of beta APP maturation in human cells expressing wild-type and Swedish mutated beta APP. Mol Med. 1998;4(11):715–723. [PMC free article] [PubMed] [Google Scholar]
- 124.Rossner S, Sastre M, Bourne K, Lichtenthaler SF. Transcriptional and translational regulation of BACE1 expression–implications for Alzheimer’s disease. Prog Neurobiol. 2006;79(2):95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Boissonneault V, Plante I, Rivest S, Provost P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J Biol Chem. 2009;284(4):1971–1981. doi: 10.1074/jbc.M807530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shulman JM, Chipendo P, Chibnik LB, Aubin C, Tran D, Keenan BT, Kramer PL, Schneider JA, Bennett DA, Feany MB, De Jager PL. Functional screening of Alzheimer pathology genome-wide association signals in Drosophila . Am J Hum Genet. 2011;88(2):232–238. doi: 10.1016/j.ajhg.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamada K, Iwayama Y, Hattori E, Iwamoto K, Toyota T, Ohnishi T, Ohba H, Maekawa M, Kato T, Yoshikawa T. Genome-wide association study of schizophrenia in Japanese population. PLoS One. 2011;6(6):e20468. doi: 10.1371/journal.pone.0020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noureddine MA, Qin XJ, Oliveira SA, Skelly TJ, van der Walt J, Hauser MA, Pericak-Vance MA, Vance JM, Li YJ. Association between the neuron-specific RNA-binding protein ELAVL4 and Parkinson disease. Hum Genet. 2005;117(1):27–33. doi: 10.1007/s00439-005-1259-2. [DOI] [PubMed] [Google Scholar]
- 130.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172(1-2):151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 131.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 132.Audic Y, Hartley RS. Post-transcriptional regulation in cancer. Biol Cell. 2004;96(7):479–498. doi: 10.1016/j.biolcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer–a quantitative western blot analysis. Ann Neurol. 1990;27(5):544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 134.Blaxall BC, Dwyer-Nield LD, Bauer AK, Bohlmeyer TJ, Malkinson AM, Port JD. Differential expression and localization of the mRNA-binding proteins, AU-rich element mRNA-binding protein (AUF1) and Hu antigen R (HuR), in neoplastic lung tissue. Mol Carcinog. 2000;28(2):76–83. [PubMed] [Google Scholar]
- 135.López de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22(46):7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 136.Denkert C, Weichert W, Winzer KJ, Müller BM, Noske A, Niesporek S, Kristiansen G, Guski H, Dietel M, Hauptmann S. Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clin Cancer Res. 2004;10(16):5580–5586. doi: 10.1158/1078-0432.CCR-04-0070. [DOI] [PubMed] [Google Scholar]
- 137.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, Furneaux H, Hla T, Haglund C, Ristimäki A. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65(6):2157–2161. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 138.Brewer G, Saccani S, Sarkar S, Lewis A, Pestka S. Increased interleukin-10 mRNA stability in melanoma cells is associated with decreased levels of A + U-rich element-binding factor AUF1. J Interferon Cytokine Res. 2003;23(10):553–564. doi: 10.1089/107999003322485053. [DOI] [PubMed] [Google Scholar]
- 139.Amadio M, Scapagnini G, Laforenza U, Intrieri M, Romeo L, Govoni S, Pascale A. Post-transcriptional regulation of HSP70 expression following oxidative stress in SH-SY5Y cells: the potential involvement of the RNA-binding protein HuR. Curr Pharm Des. 2008;14:2651–2658. doi: 10.2174/138161208786264052. [DOI] [PubMed] [Google Scholar]
- 140.Amadio M, Bucolo C, Leggio GM, Drago F, Govoni S, Pascale A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem Pharmacol. 2010;80(8):1230–1237. doi: 10.1016/j.bcp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 141.Vázquez-Chantada M, Fernández-Ramos D, Embade N, Martínez-Lopez N, Varela-Rey M, Woodhoo A, Luka Z, Wagner C, Anglim PP, Finnell RH, Caballería J, Laird-Offringa IA, Gorospe M, Lu SC, Mato JM, Martínez-Chantar ML. HuR/methyl-HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology. 2010;138(5):1943–1953. doi: 10.1053/j.gastro.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mazan-Mamczarz K, Hagner PR, Corl S, Srikantan S, Wood WH, Becker KG, Gorospe M, Keene JD, Levenson AS, Gartenhaus RB. Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene. 2008;27(47):6151–6163. doi: 10.1038/onc.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D’Alessandro V, Muscarella LA, la Torre A, Bisceglia M, Parrella P, Scaramuzzi G, Storlazzi CT, Trombetta D, Kok K, De Cata A, Sperandeo M, Zelante L, Carella M, Vendemiale G. Molecular analysis of the gene in neuroendocrine lung cancers. Lung Cancer. 2010;67(1):69–75. doi: 10.1016/j.lungcan.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 144.Lebwohl DE, Muise-Helmericks R, Sepp-Lorenzino L, Serve S, Timaul M, Bol R, Borgen P, Rosen N. A truncated cyclin D1 gene encodes a stable mRNA in a human breast cancer cell line. Oncogene. 1994;9(7):1925–1929. [PubMed] [Google Scholar]
- 145.Rimokh R, Berger F, Bastard C, Klein B, French M, Archimbaud E, Rouault JP, Santa Lucia B, Duret L, Vuillaume M, Coiffier B, Bryon P-A, Magaud JP. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood. 1994;83(12):3689–3696. [PubMed] [Google Scholar]
- 146.Hollis GF, Gazdar AF, Bertness V, Kirsch IR. Complex translocation disrupts c-myc regulation in a human plasma cell myeloma. Mol Cell Biol. 1988;8(1):124–129. doi: 10.1128/mcb.8.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aghib DF, Bishop JM, Ottolenghi S, Guerrasio A, Serra A, Saglio G. A 3′ truncation of MYC caused by chromosomal translocation in a human T-cell leukemia increases mRNA stability. Oncogene. 1990;5(5):707–711. [PubMed] [Google Scholar]
- 148.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 149.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 150.Xu F, Zhang X, Lei Y, Liu X, Liu Z, Tong T, Wang W. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. J Cell Biochem. 2010;111(3):727–734. doi: 10.1002/jcb.22762. [DOI] [PubMed] [Google Scholar]
- 151.Guo X, Wu Y, Hartley RS. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6(5):575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J. 2005;26(5):933–947. doi: 10.1183/09031936.05.00120204. [DOI] [PubMed] [Google Scholar]
- 153.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 154.Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4(5):445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- 155.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19(15):4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol. 2001;21(3):721–730. doi: 10.1128/MCB.21.3.721-730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. FASEB J. 2010;24(7):2484–2494. doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chae MJ, Sung HY, Kim EH, Lee M, Kwak H, Chae CH, Kim S, Park WY. Chemical inhibitors destabilize HuR binding to the AU-rich element of TNF-alpha mRNA. Exp Mol Med. 2009;41(11):824–831. doi: 10.3858/emm.2009.41.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Di Marco S, Hel Z, Lachance C, Furneaux H, Radzioch D. Polymorphism in the 3′-untranslated region of TNFalpha mRNA impairs binding of the post-transcriptional regulatory protein HuR to TNFalpha mRNA. Nucleic Acids Res. 2001;29(4):863–871. doi: 10.1093/nar/29.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 161.Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge: clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162(5):2457–2461. [PubMed] [Google Scholar]
- 162.Rennick D, Davidson N, Berg D. Interleukin-10 gene knock-out mice: a model of chronic inflammation. Clin Immunol Immunopathol. 1995;76(3 Pt 2):S174–S178. doi: 10.1016/s0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 163.Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 2006;20(12):2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 164.Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol Cell Biol. 2004;24(11):4835–4847. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104(2):e9–e18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin FY, Chen YH, Lin YW, Tsai JS, Chen JW, Wang HJ, Chen YL, Li CY, Lin SJ. The role of human antigen R, an RNA-binding protein, in mediating the stabilization of toll-like receptor 4 mRNA induced by endotoxin: a novel mechanism involved in vascular inflammation. Arterioscler Thromb Vasc Biol. 2006;26(12):2622–2629. doi: 10.1161/01.ATV.0000246779.78003.cf. [DOI] [PubMed] [Google Scholar]
- 167.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 168.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270(16):9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 169.Rhee WJ, Ni CW, Zheng Z, Chang K, Jo H, Bao G. HuR regulates the expression of stress-sensitive genes and mediates inflammatory response in human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2010;107(15):6858–6863. doi: 10.1073/pnas.1000444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, Kontoyiannis DL. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005;19(6):777–789. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 171.Stamou P, Kontoyiannis DL. Posttranscriptional regulation of TNF mRNA: a paradigm of signal-dependent mRNA utilization and its relevance to pathology. Curr Dir Autoimmun. 2010;11:61–79. doi: 10.1159/000289197. [DOI] [PubMed] [Google Scholar]
- 172.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27(6):847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316(5824):604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 175.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 176.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 177.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 178.Yang M, Mattes J. Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs. Pharmacol Ther. 2008;117(1):94–104. doi: 10.1016/j.pharmthera.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 179.Kwak PB, Iwasaki S, Tomari Y. The microRNA pathway and cancer. Cancer Sci. 2010;101(11):2309–2315. doi: 10.1111/j.1349-7006.2010.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Law PT, Wong N. Emerging roles of microRNA in the intracellular signaling networks of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(3):437–449. doi: 10.1111/j.1440-1746.2010.06512.x. [DOI] [PubMed] [Google Scholar]
- 181.Zhou S, Yu B, Qian T, Yao D, Wang Y, Ding F, Gu X (2011) Early changes of microRNAs expression in the dorsal root ganglia following rat sciatic nerve transection. Neurosci Lett. doi:10.1016/j.neulet.2011.02.064 [DOI] [PubMed]