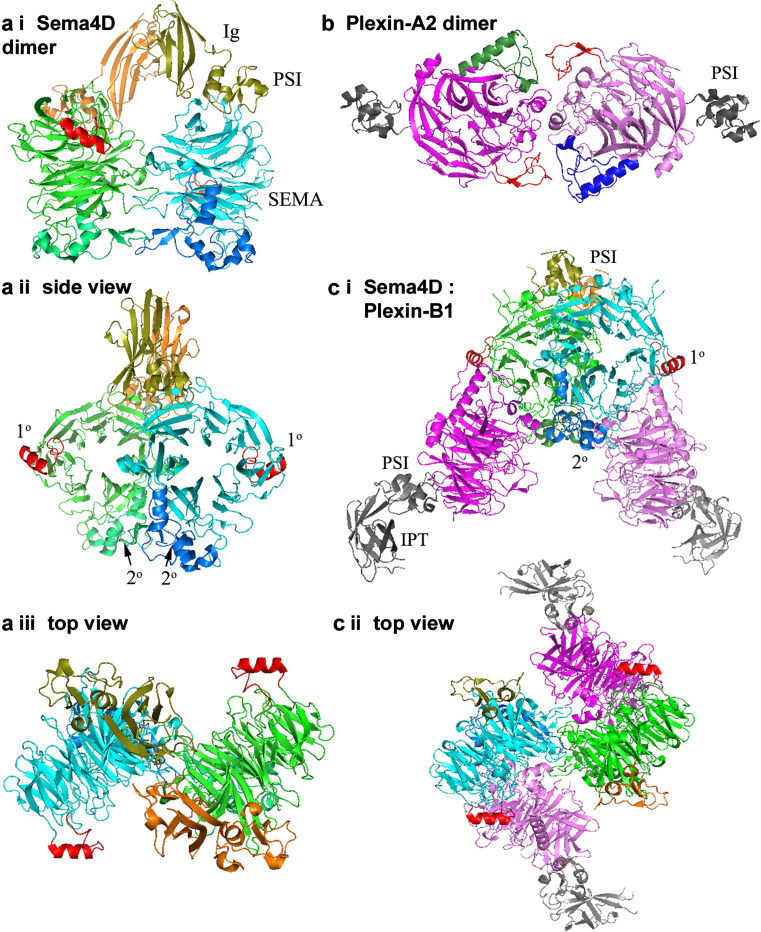
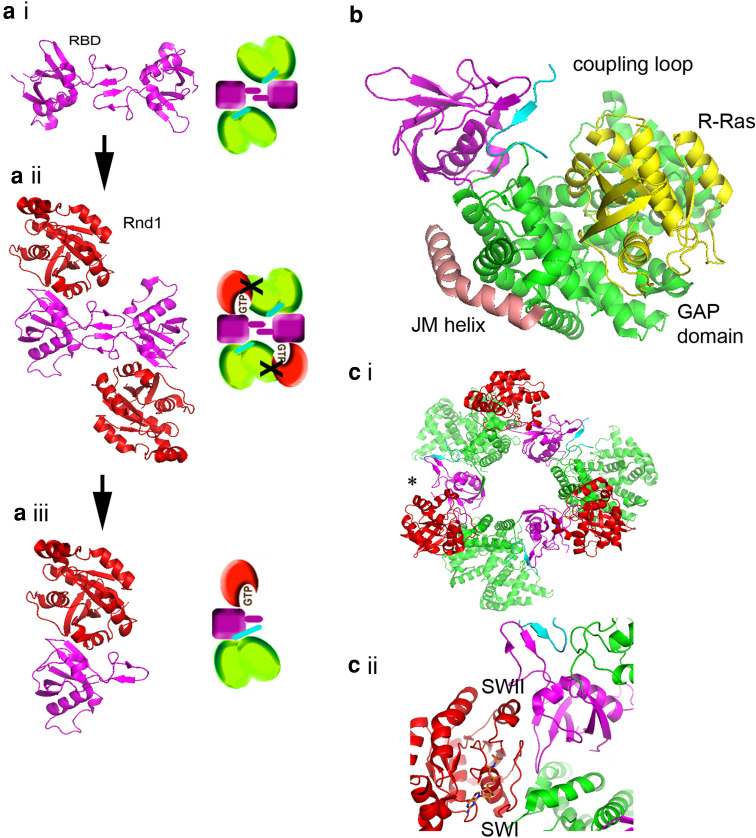
Abstract
Plexin transmembrane receptors and their semaphorin ligands, as well as their co-receptors (Neuropilin, Integrin, VEGFR2, ErbB2, and Met kinase) are emerging as key regulatory proteins in a wide variety of developmental, regenerative, but also pathological processes. The diverse arenas of plexin function are surveyed, including roles in the nervous, cardiovascular, bone and skeletal, and immune systems. Such different settings require considerable specificity among the plexin and semaphorin family members which in turn are accompanied by a variety of cell signaling networks. Underlying the latter are the mechanistic details of the interactions and catalytic events at the molecular level. Very recently, dramatic progress has been made in solving the structures of plexins and of their complexes with associated proteins. This molecular level information is now suggesting detailed mechanisms for the function of both the extracellular as well as the intracellular plexin regions. Specifically, several groups have solved structures for extracellular domains for plexin-A2, -B1, and -C1, many in complex with semaphorin ligands. On the intracellular side, the role of small Rho GTPases has been of particular interest. These directly associate with plexin and stimulate a GTPase activating (GAP) function in the plexin catalytic domain to downregulate Ras GTPases. Structures for the Rho GTPase binding domains have been presented for several plexins, some with Rnd1 bound. The entire intracellular domain structure of plexin-A1, -A3, and -B1 have also been solved alone and in complex with Rac1. However, key aspects of the interplay between GTPases and plexins remain far from clear. The structural information is helping the plexin field to focus on key questions at the protein structural, cellular, as well as organism level that collaboratoria of investigations are likely to answer.
Keywords: Plexin, Semaphorin, Neuropilin, ErbB2, Integrin, L1, VEGFR2, Met, Ron, Receptor tyrosine kinase
Introduction
After briefly summarizing their interaction partners (“Plexin family members and their expression patterns, ligands and co-receptors”), we review the role of plexins in two settings of interest (“Neuronal system” and “Cardiovascular system”). Especially, in the cardiovascular system, plexins have emerged as a key player in cancer through their regulation of angiogenesis but plexins are also involved in cancer metastasis (reviewed in “Cancer metastasis and pathogenic angiogenesis; plexin’s role in metastasis and cancer proliferation”). Plexin-mediated signaling mechanisms are used in a variety of other organs, ranging from the kidney/urinary tract, to lung and the intestine. Plexin’s role in two further systems, are reviewed (“Skeletal system” and “Immune system”). Underlying the multiple functions of plexin receptors are their participation in diverse signaling networks, which are summarized in “Intracellular signaling networks”, with particular emphasis on the role of Rho and Ras GTPases. The section “Outside” provides an overview of recently determined structures of the extracellular domains of plexins, together with ligand and co-receptor structures. The section “Inside” reflects on the structure and function of the intracellular domains of plexins. Finally, “Unanswered questions” addresses several issues that are unresolved and whose investigation is likely to provide important new insights into the plexin system.
Plexin family members and their expression patterns, ligands, and co-receptors
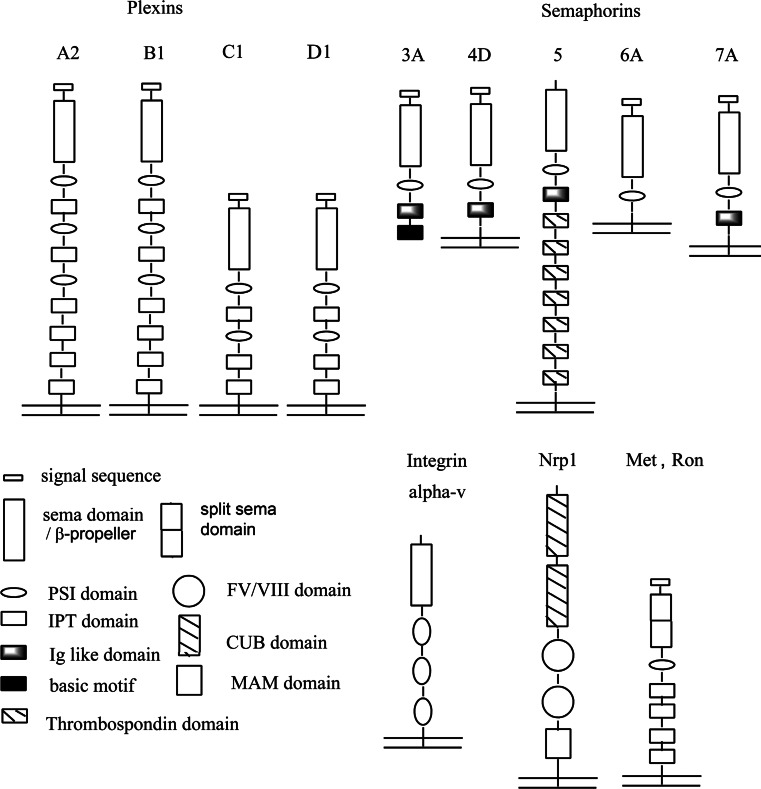
Plexins are transmembrane receptors that regulate the motility and positional maintenance of cells. There are four subfamilies of vertebrate plexins and nine plexins in total: plexin-A1 to A4, -B1 to B3, -C1 and -D [1–3]. These transmembrane receptors have similar domain structures, as discussed in the section on the extracellular structures, “Outside”. L1 [4, 5], Neuropilin-1 and -2 (Nrp1,2) [6] are co-receptors for plexins [7] as well as for VEGFRs [8]. Table 1 summarizes the family of vertebrate plexins, their expression in tissues, and the interaction of their extracellular domains with ligands and co-receptors. As the primary ligand for plexins, semaphorins (Sema) exist in several classes [9]: the soluble (class III), the cell membrane transversing (class IV, V, and VI) and the GPI-linked semaphorins (class VII). Some promiscuity could exist, with certain semaphorins binding to several plexin families (e.g. plexin-D1 binds Sema3E and possibly Sema4A), but might be overcome in a cell type-or tissue-specific manner. Different plexin family members may also bind to one another [10]. Some of the interactions require Neuropilin as a co-receptor, others do not. Several of the interactions that semaphorins (Sema) can make in addition/instead of those with plexins are also indicated in Table 1. However, the receptor tyrosine kinases ErbB2 [11], Met, and Ron [12, 13] are extracellular interaction partners of B-family plexins. Because their interactions could be weak/transitory, it is currently not clear whether higher-order complexes can form (e.g., a plexin:Neuropilin:Sema:VEGFR heterotetramer) and whether plexins and associated proteins can bind to other guidance receptors such as Robo, DCC, or p75NTR [14]. Reports of protein–plexin interactions will continue to increase in the foreseeable future as a consequence of the various “-omics” approaches that are being utilized. These, homology modeling/computational simulations and binding studies will eventually reveal all possible interactions between receptor domains, ligands and co-receptors. Further genetic investigations, also of the cell signaling networks in vivo, will be needed to establish the functional importance of the interactions in specific settings.
Table 1.
Overview of semaphorin and plexin expression in different tissues and their direct extracellular interaction partners
| Tissue expression | Semaphorins | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3A | 3B | 3C | 3D | 3E | 3F | 3G | 4A | 4B | 4C | 4D | 4E | 4F | 4G | 5A | 5B | 5C | 6A | 6B | 6C | 6D | 7A | |
| Cancer | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |||||||||||
| CV | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||||||||||||
| NS | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||
| Bone | ■ | ■ | ■ | ■ | (■) | (■) | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ||||||||
| Immune | ■ | ■ | ■ | ■ | ■ | (■) | (■) | ■ | ■ | |||||||||||||
| Kidney | ■ | ■ | ■ | ■ | (■) | ■ | ■ | ■ | ■ | ■ | ■ | |||||||||||
| Lung | ■ | ■ | ■ | ■ | ■ | ■ | (■) | ■ | ■ | ■ | ■ | ■ | ■ | |||||||||
| Interaction partners |
Nrp-1,2 A1-4 D1 L1 VEGFR2 |
Nrp-1,2 IGFBP-6 Nr-CAM VEGFR2 |
Nrp-1,2 D1 |
Nrp-1 A1-4 Integrins L1 Nr-CAM |
Nrp-1 D1* B2 Ig-CAM VEGFR2 |
Nrp-1, 2 A3 A4 |
Nrp-1, 2 |
Tim-2 B1 B2 B3 (D1) Met ErbB2 |
PSD-95 CLCP-1 |
B1 B2 Met ErbB2 PSD-95 |
B1 B2 C1 CD72 Met ErbB2 PSD-95 |
B2 | PSD-95 | B3 |
Nrp-2 A3 B3 Met HSPGS |
A4 |
A2 A4 |
A2 A4 c-Src |
A1 |
A1 VEGFR2 TREM2** DAP12** |
C1 Integrins | |
| Tissue expression | Plexins | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | B1 | B2 | B3 | C1 | D1 | ||||||||||||||
| Cancer | ■ | ■ | (■) | (■) | ■ | (■) | ■ | ■ | ■ | |||||||||||||
| CV | ■ | ■ | ■ | ■ | ■ | ■ | (■) | ■ | ■ | |||||||||||||
| NS | ■ | ■ | ■ | ■ | ■ | ■ | ||||||||||||||||
| Bone | ■ | ■ | ■ | ■ | ■ | |||||||||||||||||
| Immune | ■ | (■) | ■ | ■ | ■ | |||||||||||||||||
| Kidney | ■ | ■ | ■ | (■) | (■) | |||||||||||||||||
| Lung | ■ | ■ | ■ | ■ | ■ | |||||||||||||||||
| Extracellular interaction partners (except Semas) |
VEGFR2 OTK Syb2 Integrins Nrp1 L1 Nr-CAM |
Nrp2 |
Nrp1 VEGFR2 FGFR1 |
Met ErbB2 PTK-7 |
Met ErbB2 |
Met ErbB2 |
Integrins |
Nrp1 VEGFR2 ErbB2 |
||||||||||||||
As with other macromolecules, the concentration/level of expression of a protein in particular cell types and tissues can provide clues as to its specific function. An overview is complicated because expression patterns need to be further classified by criteria, such as state of the tissue (e.g., developmental stage or disease form) and sub-tissue or subcellular localization. Several research studies have provided such surveys; for example Perälä et al., [15] and others [16] studied the expression of plexins during mouse embryogenesis using in situ hybridization. Other studies investigated the expression of specific plexins in tumor cells [17] and within the nervous and cardiovascular systems, correlating the expression pattern of plexin-B1 and -B2 and their knock-outs with receptor function [18, 19]. Such correlations have also been made between certain plexins, their ligands and co-receptors, e.g., plexin-B2 and Sema4C [20], plexin-C1, and Sema7A [21], and between plexins and neuropilins [22]. Other more broad-based efforts extracted data for plexins from large-scale expression array analyses of cancer cells, some of which have been organized as accessible databases, e.g., [23, 24]. The expression correlations to a large part validate the binding partners (Table 1) that have been classified by coIP and other experiments.
Role in development, disease, and regeneration
Plexins were first reported in 1995 [25], a few years after their ligands, the semaphorins were discovered [26]. Their involvement in many settings is just now becoming clear: plexins function in the development of the neuronal and cardiovascular systems and several family members are associated with seemingly diverse roles such as in higher brain function and in congenital heart disease. Plexin signaling is also implicated in the recovery of the nervous and cardiovascular systems from injury, where plexins appear to be an impediment to regeneration. The role of plexin function in the skeletal, immune, and other systems is also emerging [27]. Finally, the receptors and ligands are involved in cell migration in cancer and in other cancer-related processes (e.g., pathological angiogenesis). The sections below summarize current knowledge of plexin function in development, disease, and regeneration in four of the major systems (important functions of semaphorin ligands as well as VEGFR [28] and Nrp [6] receptors that are independent of plexins are not reviewed here).
Neuronal system
Role of plexins in the development of the nervous system
Plexins, and moreover their ligands and co-receptors, are associated with a wide range of processes in the developing, but also in the mature nervous system (NS). Cell movement and adhesion are the major themes, but so is cell survival and proliferation (see “disease and regeneration” below). Plexin/semaphorin-mediated signaling is employed in the early stages of NS development, specifically in the migration and targeting of neuronal progenitor cells [29]. Once they are positioned, axon guidance is responsible for forming networks between the cells [30–32]. The axons are not just guided but are also branched, bundled, defasciculated, and pruned back [33, 34]. Following correct targeting, plexins are also known to play a role in synaptogenesis and in the formation of dendrites [35]. The cell and tissue types involved are equally diverse and the reader is referred to reports on the expression patterns of plexins, semaphorins, and neuropilins [9, 15, 18, 21, 22, 36] and to reviews of their roles in the nervous system [33, 34, 37–39]. Here we will provide a brief overview of some of the roles of plexins in NS development, leaving details of the cell signaling involved to a latter section (see "Intracellular signaling networks" below). Genetic, knockout or RNAi knockdown studies have established that all four branches of the mammalian plexin family play a role in NS development:
Members of the plexin-A subfamily are widely engaged in setting up boundaries between different parts of the nervous system, e.g., separating the central and peripheral NS [40] or by constraining sympathetic or motor neuron migration [41–43]. Plexin-A4 mediates axon-repulsion in response to both secreted (Sema3A) and transmembrane semaphorins (Sema6A, B) with roles in nerve fiber guidance [44]. Apart from axon guidance, plexin-A3 is also involved in pruning of connections [45] and together with Sema3F in spine distribution [46]. In most cases, the specificity between certain semaphorin ligands and plexin members and the requirement for Nrp1 or Nrp2 co-receptors is remarkable. For example, expression of a dominant-negative plexin-A1 receptor in sensory neurons blocks Sema3A-induced growth cone collapse [47]. Similarly, overexpression of a dominant negative form of plexin-A2 in dorsal root ganglion (DRG) sensory neurons makes the axon unresponsive to the semaphorin [48]. Plexin-A3(−/−) and to a lesser extent plexin-A4(−/−) mice show reduced neuronal apoptosis and an increased number of DRG neurons [49, 50].
Plexin-B family members also play important roles in NS development. Plexin-B1 functions in neurite outgrowth, axonal growth cone collapse, and axonal and dendritic arborization [51]. Plexin-B2 deletion alone resulted in abnormal nervous system development in mice [19, 52, 53], whereas knockout studies of plexin-B1 and also of plexin-B3 did not reveal a neuronally defective or altered phenotype in mice [19, 54], suggesting that other receptors or cell signaling mechanisms may compensate. This contrasts with other settings, where ligands such as Sema5A [55] and associated signaling pathways appear to be unique, if not specific to plexin-B3 [56–58]. Similarly, the GAP activity of plexin-B1 towards M-Ras has only been described in dendrites at present [59].
The role of plexin-C1 is just becoming established, as a receptor mutant was recently reported to result in a partitioning defect between two types of neurons in the hypothalamus [60]. Plexin-D1 is mostly known for its role in cardiovascular development, but also has functions in the central NS, for example, in establishing sensory- and motor-neuron connectivity [61]. Intriguingly, Nrp1 and VERGFR2 co-receptors can switch plexin-D1-mediated axonal repulsion to attraction during brain development [62, 63]. It has recently been reported that Sema3E—plexin-D1 signaling also controls pathway-specific synapse formation [64].
Semaphorin class 4 and 6 members are also transmembrane proteins and their C-terminal cytoplasmic region is known to be regulated by phosphorylation and adaptor protein binding, resulting in a forward as well as reverse signaling mechanism, e.g., [65] of a kind that is similar to that between Eph receptors and their ephrin ligands seen in axon guidance [66]. This makes sense since the cytoplasmic termini of Sema4B, 4C, 4D, and 4F have a PDZ binding motif and interact with the post-synaptic density protein PSD-95. The binding serves to localize the ligand and thus the receptor [67–69]. PSD-95 also interacts with neuroligins (synaptic cell adhesion molecules) and with the synaptic NMDA receptor, responsible for long-term potentiation (learning) [70].
Deletion of the adhesion protein L1 in mice showed cross-talk between Sema3A, plexin-A1, and Nrp1 [71]. The L1 and Nrp1 extracellular domains associate either in cis or in trans, also in a complex with Sema3A and plexin. The association with soluble L1 in trans converts the repulsive guidance response to an attractive one by activation of a NO/cGMP pathway [72] (see also “Unanswered question”). How this pathway is activated is not clear, but both cis and trans interactions with L1 control endocytosis of the L1/Nrp1/Sema3A complex (and also possibly plexin), which is mandatory for Sema3A-mediated cell contraction [73]. The observation that processes such as axon guidance and cell migration are coordinated by cross-talk of several signaling pathways, involving multiple ligands, receptors and molecular mechanisms in transmembrane communication, is expected to become more common in the future, e.g., see recent report that another guidance ligand, Robo1 binds directly to Nrp1 [74].
Plexin and diseases of the nervous system
The involvement of plexins in diseases of the nervous system has been discussed in several reviews [38, 39, 75, 76]. As mentioned in the section above (“Plexin family members and their expression patterns, ligands and co-receptors”), often a role of plexin or semaphorin/neuropilin co-receptors is implied from correlations, e.g., altered gene expression, discovery of mutations and genome-wide association studies. However, a direct functional causative relationship is yet to be established for the majority of the correlations. In a few cases, direct interactions have been discovered: A processed form of Sema3A, of a phosphorylated microtubule associated protein, CRMP-2 (see “Ser/Thr kinases” below) and plexin-A1 and -A2 were found to form a complex in the brain of Alzheimer patients [77, 78]. Detection of these proteins could become an early marker for the disease. Mutations in plexin-A2, but also in its ligand Sema3D and Sema6A may be associated with the development of schizophrenia and heightened anxiety in mice [79–82]. Other mental problems, such as autism and impaired verbal performance, appear to be related to Nrp2 and plexin-B3, respectively [83]. Severe conditions such as Cri-du chat and CHARGE syndrome relate to alterations of the Sema5A and Sema3E genes [84]. Sema5A might also be involved in Parkinson disease [85] and lack of Nrp2 expression causes a predisposition to epileptic seizures [86]. Brain cancers that develop from glial cells (glioblastoma) correlate with altered Sema3B, Nrp1 and plexin-D1 expression [87]. Sema4D and Plexin-B1 play a role in perineural invasion of tumor cells into nerve bundles [88].
Regeneration in the nervous system and therapeutic approaches
Apart from diseases, there are also other types of insults to the NS, including hypoxia and ischemia following a cardiovascular event (e.g., heart attack and stroke), physical injury, and inflammation. As these incidents are of much higher frequency than most of the diseased states, attention has been focused on investigations of NS regeneration (axonal regrowth and targeting) [75, 76, 89–93]. Although in the peripheral NS axons may regenerate successfully, repulsive guidance cues, such as Sema3A [94], are thought to restrict regeneration. Consequently, the expression levels of these ligands need to be appropriate. Damage to the CNS, such as spinal cord injury, is typically permanent as no correct balance between growth inhibitory and promoting factors is established for regeneration [90, 95]. Generally, semaphorins and plexins might be expected to significantly contribute to the maintenance of neuronal network. However, with some exceptions, such as the hippocampus, plexins, and semaphorins are not highly expressed in mature networks, and if they are, they appear to be an impediment to regrowth. A number of complex changes are initiated following injury; for example, class 3 and 4 semaphorins are expressed in the neural and glial scars [96], where Sema3A, in particular, has been associated with cell apoptosis. Sema4D, -6B, and -7A are found in CNS myelin and are strongly upregulated by oligodendrocytes close to the site of injury, likely preventing excessive axon sprouting and migration [97]. Repair of the neuron-coating myelin cells (damaged in multiple sclerosis) is also affected by semaphorins [98, 99]. Cross-talk between the immune and the NS may prevent, but also possibly increase, further damage [100] (see also section entitled “Immune system”, below). Similarly, as they are frequently co-localized, damage often involves both blood vessels and nerves [101]. Some of the plexin ligands, such as Sema3A, have been shown to prevent neuronal as well as vascular regeneration [102]. By contrast, other key players, such as neuropilins (and likely plexin-A family members), appear to be required for regeneration of peripheral nerves [103], in part, possibly, by blocking the apoptotic effect of semaphorins. Downregulation/deletion of one or even multiple ligands and receptors has not yet yielded successful regeneration [104]. However, several promising avenues for regeneration are becoming clear: One is the specific inhibition or stimulation of certain (co-)receptors using small molecule ligands or peptides [105, 106]. This has been reasonably successful in controlling angiogenesis (see “Cancer and pathogenic angiogenesis; plexin’s role in metastasis and cancer proliferation” below), but is still in its infancy for regeneration. Another is to target the direction of axonal regrowth by implantation of neuronal cells and by the localized generation (via expression from viral vectors) of preformed guidance pathways [107].
Cardiovascular system
Common mechanisms in the nervous and cardiovascular systems
Endothelial cells and neurons both form extensive and often structurally and functionally coordinated networks. For instance, sympathetic nerve activity regulates blood pressure by altering peripheral vascular resistance [108]. Thus, it is not unanticipated that the same guidance ligands and receptors are utilized for cardiovascular and neuronal development—a topic of several reviews [101, 109–114].
Cardiovascular development and disease
Among the several developmental processes for the formation of the cardiovascular (CV) system, the de novo formation of the first blood vessels and the growth of blood vessels from pre-existing ones (angiogenesis) stand out. The former processes, referred to as vasculogenesis and cardiac morphogenesis, are considerably less well understood but involve the migration, differentiation, and association of endothelial progenitor cells into blood islands, forming hematopoietic precursors and highly motile angioblast cells. These latter cells proliferate, migrate and associate into tube-like vessels. Such processes form the heart and innervate organs. The main factors that drive and regulate vasculogenesis and cardiac morphogenesis are fibroblast growth factors (FGF), hedgehog signaling, VEGFs and their receptors as well as neuropilins, TGF-β and TGF-β receptors. All of these signaling molecules are thought to be affected by plexins.
Angiogenesis is the formation of new blood vessels by sprouting of endothelial cells from pre-existing vessels. The process is responsible for the formation of the majority of blood vessels in the embryo and for the innervation of organs, especially the brain and the kidney. Regulatory mechanisms encompass Notch, Slit/Netrin, hypoxia-inducible factor (HIF), platelet-derived growth factor (PDGF), Ephrin/Eph and Tie receptor-mediated signaling. Again, plexins, neuropilins, semaphorins and VEGFs/VEGFRs play a part. Plexins influence the processes at multiple levels and the section below focuses on the relationship between plexins/Semas, the vascular endothelial growth factor/receptor (VEGF/VEGFRs) and neuropilins, which are best understood.
Until recently, plexins were understood to be relevant to cardiovascular development through their ligands, the semaphorin and neuropilin guidance cues [115]. Early on, plexin-A2 receptor expression was used in mouse models to test the role of these ligands in neural crest development and congenital heart defects [116, 117]. Sema3C is required for normal development of the aortic arches and for partitioning of the cardiac outflow tract. Defects in the plexin-A2 ligand Sema3C and in Nrp1 null mice are reminiscent of the spectrum of cardiovascular defects seen in the human DiGeorge and Velocardiofacial syndromes [118–120]. Furthermore, VEGF/Nrp1 signaling is critical for the development of partitions in the heart, while Sema/Nrp1 is essential for the development of the atria. An indirect role was envisaged for plexins in that the receptor would function by withdrawing neuropilins from the pool of active free molecules, preventing neuropilin interaction with VEGFs. Antagonistic Sema3E and VEGF signals generate a balanced control of vessel sprouting in some settings such as retinal vascularization during development [121].
More recently, however, plexin has been associated with more direct roles, as most receptors have been found to be expressed in certain epithelial and endothelial cells as well as in the heart [122]. Down-regulation of plexin-A2, for example, causes heart and vasculature defects [116, 117, 120]. Meanwhile detection of plexin expression has become a way to characterize the subpopulations of blood islands before vessel formation [123, 124]. Furthermore, the roles of plexin-A1 came into greater focus as it was revealed that the Sema6D cytoplasmic region also becomes activated for cell signaling upon plexin-A1 receptor binding (a process known as reverse signaling) [125, 126]. Interestingly, plexin-A1 was also shown to form complexes with VEGFR2 and a kinase inactive co-receptor, Off-Track (OTK), in chick cardio-cells [125]. Another family member, plexin-D1 has been found in the vascular cells of developing blood vessels in mice [16, 115, 127] and zebrafish [128]. Its disruption results in congenital heart disease, principally due to neural crest and vascular patterning defects. Significantly, Gu et al. reported that Sema3E—plexin-D1 signaling controls vascular patterning independently of Nrps [127]. This finding overthrows the earlier dogma that neuropilins are essential co-receptors for plexins. VEGF may still have a deep connection to Sema3E-plexin-D1 signaling, since recently it has been shown that VEGF directly controls the expression of plexin-D1 via a Notch signaling pathway [129]. Conversely, plexin-D1 is seen to antagonize VEFR signaling by increasing the concentration of a VEGF decoy receptor, a spliced and soluble variant of Flt1 [130].
Cardiovascular injury and regeneration
Cellular expression of proteins is profoundly affected by tissue-damaging events such as hypoxia (lack of oxygen and nutrients) during stroke or myocardial infarction. Often this results from occlusion of arteries in the brain or the heart, but also from ischemia in other blood vessels. Similarly damaging are events that occur upon tissue reperfusion, as reactive oxygen species are generated. Nrp1 is overexpressed during cerebral ischemia-induced neuronal death [131], and together with Fer-kinase, and the collapsin response mediator (two other key plexin signaling-associated proteins), Nrp1 localizes to membrane rafts under similar conditions [132]. Administration of Sema6A was recently shown to improve functional recovery after stroke in mice [133]. Sema4D is implicated in a platelet response to vascular injury [134] and VEGF-Nrp1/2-VEGFR2 complexes are seen to play a role in early wound healing [135]. However, overall investigations into the role of plexins and their associated proteins in CV injury and regeneration are still in their infancy. By contrast, the investigation of plexin function in angiogenesis and cell migration has attracted a wide range of investigators.
Cancer and pathogenic angiogenesis; plexin’s role in metastasis and cancer proliferation
Cancer is a complex process that includes, among other events, tumor cell proliferation, increased cell survival (avoidance of apoptosis), blood vessel formation to feed tumors (angiogenesis) and cell detachment, migration as well as invasion of the blood stream and other tissues (metastasis) [136]. In this very active and broad field of research, multiple roles for plexins, semaphorins and their co-receptors have been discovered (see [137–144] for recent reviews). A main focus has been on angiogenesis because as seen in cardiovascular development, semaphorins (and by association, plexins) compete for neuropilins with the VEGFR co-receptor, a main player in blood vessel development [7, 28, 145]. Direct interactions with receptor tyrosine kinases such as Met, Ron, and ErbB2 further extend the role of plexins to other processes including cell proliferation and survival.
Plexins and their semaphorin ligands
Although Sema3 family members and neuropilins are implicated in several cancers (see below) the role of plexin-A family receptors in oncogenesis has not yet been extensively investigated. Generally A-family plexins and Sema3 ligands are down regulated, suggesting tumor suppressor roles. Thus, strategies that inhibit tumor angiogenesis by a targeted delivery of Sema3A show an early promise [146]. However, there are exceptions: for instance, plexin-A2 has a higher level of expression in aggressive breast cancer [147] and a gene copy analysis detected amplification and mutations in plexin-A4 in melanomas, some of which could be associated with an inactivation of the receptor [148]. Studies of plexin-A family members and their ligands, their expression levels and cancer associated mutations are in progress [145, 149]. One very recent report suggests that plexin-A1 and VEGFR2 receptors bind to one another and that the presence of Sema6D promotes tyrosine phosphorylation in a plexin-dependent manner. This triggers a pro-survival program that allows anchorage-independent growth of malignant cells [150]. Another study suggests that plexin-A4 associates with VEGFR2 and FGFR1 to promote tumor progression and angiogenesis by activating these latter receptors [151].
Early studies of Comoglio and coworkers linked the association of the receptor tyrosine kinase, Met, with plexin-B1 to Sema4D stimulated invasive cell growth [152] (see below). Plexin-B family members have been a major focus of cancer research, prompted recently also by the observation that plexin-B1 and Sema4D as well as Sema5A expression are lost in several cancers [147, 153–155], but plexin-B1 contributes to the spreading of ovarian cancer [156]. Again, in the former case, plexins are thought to be tumor suppressors (whose loss or inactivation by mutagenesis is then permissive to cancer growth [157]), whereas in the latter case they appear to promote cell migration and invasion [158–160]. Recently, the cell signaling pathways that are involved in both mechanisms are becoming clear [161]. Several mutations in plexin-B1 found in prostate and breast cancer are associated with a loss of binding of Rho GTPases [162]; an interaction that is thought to be important for the activation of some of the receptor’s functions [163]. At first, the role of plexin-B family members in either promoting or inhibiting cancer-associated processes may appear to be contradictory. However, as discussed in “Intracellular signaling networks” below, plexin and the associated co-receptors and ligands are multifunctional, depending on the specific concentrations of the components, their localization, and post-translational modifications. All these variables are likely to be set differently for different types of cells/tumors and at different tumor stages [147]. To give several examples: Plexin-B1 activation in cancer cells has been linked to a change in AKT phosphorylation, a Ser/Thr kinase that is central to cancer development [164], but this can result in different outcomes and, thus, in a different role of plexin as either tumor suppressing or stimulatory [156, 157]. Pro-migratory and angiogenesis effects are thought to be stimulated by the ErbB2 co-receptor, which phosphorylates plexin-B1 and activates RhoA [165, 166]. However, another study showed that purified Sema4D alone was sufficient in promoting angiogenesis via plexin-B1 [167]. Here Sema4D appears to cooperate with VEGF (and is upregulated in case of VEGF inhibition) [168]. Met and Ron co-receptors also phosphorylate plexin but elicit a function through a different pathway discussed below. For plexin-B3, a pathway involving inactivation of Rac1 via interaction with receptor bound RhoGDI has been proposed in glioma cells [56].
Plexin-C1, a receptor for Sema7A, has recently been characterized as a tumor suppressor gene in skin cancer, as receptor expression is lost during melanoma metastasis [169]. Interestingly, again phosphorylation (here via Lim kinase II) plays a role downstream of plexin and links the cell signaling pathway to the actin cytoskeleton [170]. Sema7A also binds to integrin receptors, which play key roles in cancer ([171] and see below).
Plexin-D1 has emerged as a key focus for cancer research because the receptor is found to be overexpressed ubiquitously in tumor and in a wide range of malignant cells, but not in the normal vasculature [17]. Thus, plexin-D1 has become a target for diagnosis (a marker protein) and therapy [172]. As mentioned above, Sema3E—plexin-D1 signaling is independent of the neuropilin co-receptor [127], whereas Sema4A—plexin-D1 signaling was reported to negatively regulate angiogenesis [173]. Such findings indeed point to a complex picture. Knocking down endogenous expression of either Sema3E or plexin-D1 in human metastatic cancer inhibited the metastatic spreading of cells but not their growth. By contrast, overexpression of Sema3E in cancer cells increased their invasiveness (e.g., migration of prostate cancer cells) [174], but inhibited tumor blood vessel formation, resulting in reduced tumor growth in mice. Recently, two distinct cell signaling pathways have been associated with the effects of plexin-D1. In the paracrine manner, Sema3E-plexinD1 can cause endothelial cell repulsion [175], thus reducing blood vessel density and tumor growth, while in Sema3E expressing cells, autocrine signaling, via the plexin-D1-associated ErbB2 oncogenic kinase, is responsible for invasiveness and metastatic spreading [175].
Ligands of neuropilins; semaphorins and VEGF
It is clear from the section above, plexins can be center-stage for angiogenesis- and cancer-associated processes. However, plexins can also be an influence from the sidelines by direct and indirect effects on neuropilins and semaphorins, which are involved with other pathways (principally with VEGF/VEGFR signaling [145]). Similar to plexins, semaphorins often function as tumor as well as angiogenesis suppressors and are down-regulated at the transcriptional level in cancer cells. By contrast, neuropilins collaborate with VEGFR receptors in cell propagation, survival as well as migration and have become markers for cancer cells. Semaphorins and neuropilins have been the subject of many excellent reviews [6, 137–139, 176–178], including some that are very recent [179–181, 144]. This review does not allow such an extensive coverage, but seeks to describe two areas that are of particular interest, also from a structural perspective: first, recent evidence that appears to resolve conflicting results/roles for semaphorin versus VEGF binding to neuropilins. A second related topic are early therapeutic approaches that involve neuropilins in their interactions with semaphorins and VEGF [182].
The extracellular domains of neuropilin-1 and -2 (Nrp1, 2), of several VEGFs [183] and of semaphorins (Sema) (see “Outside”) have been crystallized. However, receptor-ligand complexes are just becoming available. Specifically, VEGF-A as well as VEGF-B homodimers were crystallized with the second domain of VEGFR [184, 185]. The binding interface was used to map results from mutagenesis onto a homology model for VEGFR2, suggesting that several domains of VEGFR are required for tight VEGF binding. Recently, this was confirmed by a structure between VEGF-C and domains 2 and 3 of VEGFR2 [186]. Together with structures of other unbound VEGF-family members, summarized in [183], efforts are under way to understand the binding and functional specificity of the interactions, e.g., [185, 187, 188]. Semaphorin and VEGF interaction sites have been mapped with Nrp domains using extensive mutagenesis and binding experiments. Early studies showed that Sema3A and VEGF competitively interact with Nrp1 by using binding sites that overlap at least slightly. However, recent crystallization of the 4-domain Nrp2 structure has confirmed that the VEGF and Sema3 binding domains are essentially separate. Nrp1 complexes have been solved with antibodies that either block Sema or VEGF-A binding [189, 190] and recently with VEGF-A [191] suggesting the physical basis for the observed functional differences of VEGF-A isoforms. The structure also suggests an organization of a heterohexameric VEGF-A/Nrp1/VEGFR2 complex that would allow binding of heparin. Similarly to plexins, semaphorins may be proteolytically processed [192] and earlier contradictory findings regarding Sema3F binding and function, may be explained by the recent discovery of a cleavage site at its C-terminus [193]. The processed form also binds the VEGF binding site on Nrp1 and strongly inhibits association with VEGF [187]. Another outcome has recently been described for a variant of Sema3E which cannot be cleaved by the furin protease. Although this ligand binds plexin-D1, it does not promote plexin-D1 association with ErbB2 and does not stimulate cell spreading in cancer metastasis [194].
Therapeutic approaches range from the identification of peptides from phage display libraries against VEGFR1 [195, 196], to bevacizumab, a monoclonal antibody against VEGF. The latter is in clinical use as a first-line therapy in a variety of cancers, including colorectal, breast, and lung cancer [197]. Rational design, based on the protein structures mentioned above, has also begun, for example, by taking the C-terminal peptide backbone of VEGF-A, which is known to interact with Nrp1, as a starting point for small molecule design [198]. The intracellular kinase domain of VEGFRs also presents a target and responds well to broad-based receptor tyrosine kinase inhibitors in vitro. However, it has recently become clear that neither the antibody nor small molecular agents against VEGF–VEGFR provide long-term benefits, but instead result in accelerated metastasis and increased invasiveness, illustrating the ability of tumors to survive hypoxic conditions and elicit evasive resistance [199]. Similarly to VEGF/VEGFR, some of the inhibitors to neuropilins have been discovered by library screens and are peptide based. For example, peptides that have sequences from Sema3A [200], or mimic the carboxy-terminal region of VEGF family members, bind to Nrp1 [201]. The latter sequence was then used as a scaffold to design sugar-based peptidomimetic inhibitors [202]. Antibodies that block the VEGF binding domain on Nrp2 are also effective as angiogenesis inhibitors [203]. Interestingly their effect appears to be additive [204] also with administration of a soluble Nrp2 B-domain [205]. It is now accepted that Nrp1 blockade suppresses tumor growth by inhibiting angiogenesis, in addition to directly inhibiting tumor cell proliferation in certain settings [179, 206]. The effects of Nrp inhibition or withdrawal appear to be far-reaching: For instance, a recent report showed that deletion of Nrp1 blocked VEGF’s ability to promote cancer stemness and renewal in skin cell tumors [207]. Another promising strategy is the design of peptides that interfere with the transmembrane region of neuropilin, which is thought to dimerize. The peptide was shown to disrupt the dimerization of the co-receptor and to inhibit glioma growth in vivo [208, 209]. Little is currently known about the short but highly conserved intracellular region of neuropilins, although evidence is recently mounting that this region also has a functional role [210, 211] and may thus become a target for therapeutic approaches. Furthermore, it is becoming apparent that Nrps bind and respond to other receptor tyrosine kinase ligands, such as those for PDGFR (PDGF) and Met (HGF) [211] opening additional avenues for therapy.
Tyrosine kinase co-receptors: Met, Ron, ErbB2 and VEGFR2
A high sequence homology of the extracellular SEMA domain of plexin to that of the Met-hepatocyte growth/scatter factor receptor (HGF/SF) (Met) was noted right at time when plexins were identified [212]. Activation of the Met oncogene is responsible for invasive growth, a complex program that allows cells to dissociate from their neighbors, migrate through the extracellular matrix and colonize new sites [213]. Met kinase activity can be increased by a number of mechanisms, including by germline and somatic mutations in the intracellular catalytic or in the juxtamembrane region [214], but also by interaction with B-family plexins, once the latter are stimulated by Sema4D binding [152, 215, 216]. Upon complex formation, Met is autophosphorylated and plexin is tyrosine phosphorylated. With this, Met appears to switch the normally repulsive signaling function of plexin to an attractive, or at least to a permissive one. Thus, in presence of Met, the tumor suppressor activity of plexin-B1 is typically lost in favor of becoming an accessory for Met’s oncogenic program. However, this effect, again, like Met signaling appears to be cell and cancer type-specific [217, 218]. For example, in melanoma cells the overexpression of plexin-B1, also with the help of Sema4D [219], is thought to downregulate Met signaling and block migration, possibly through an inhibition of RhoA [220].
Met has been a focus of anticancer therapy for some time [218]. Small molecule inhibitors target the intracellular catalytic kinase domain, recently also cancer-specific mutant forms [221, 222]. Intriguingly, Met activity does not depend entirely on the intact kinase, implying that the protein is multifunctional [223]. Sema4D can cooperate with both HGF and MSP, the ligand for Ron (a Met homologous receptor for macrophage stimulating protein, MSP), in activation of the Met and Ron receptor tyrosine kinases. Furthermore, Met and Ron are involved in cross-talk [224] and Met has a large number of other co-receptors (integrins, ErbB2, FAS death receptor, transmembrane protein CD44) [218, 225]. Recently, it has also been suggested that Met interacts with Nrp1, either directly [226] or via HGF/VEGF binding [227]. Similar to VEFR and Nrp receptors, the extracellular domains of Met have become a target for anticancer therapeutic approaches, involving fragments or mimics of the ligands [228], co-receptors or antibodies. Some of these studies are informed by the structural characterization that has been possible for the SEMA-homologous domain of Met and for the HGF-ligands [229, 230]. However, to date, there are no high resolution structures of proteins in complex with Met receptor domains—in particular the interaction with plexin-B family members would be extremely informative, both on the extra- and on the intracellular side. A recent paper by Swiercz and coworkers identified a conserved tyrosine phosphorylation site for Met in the plexin-B family intracellular region, and it could be shown that phosphorylation results in recruitment and activation of p190 RhoGAP [231].
Despite a lack of substantial homology between their extracellular regions, plexins are known to associate with receptor tyrosine kinases VEGFR2 (see above) and ErbB2 [12]. Plexins also interact with cytoplasmic tyrosine kinases FYN, FES, PYK2 and SRC [232]. Amongst these the role of ErbB2 is now becoming well characterized by the groups of Swiercz and Offermanns. ErbB2 activation and phosphorylation of plexin-B1 was shown to be responsible for RhoGEF-mediated RhoA activation and cell collapse [165, 233]—a mechanism that is separate and possibly opposed to the downregulation of RhoA via phosphorylation of plexin-B1 by Met kinase (see above) [231, 234]. Very recently, it was found that the pro-invasive and metastatic activity of Sema3E in tumor cells depends on transactivation of plexin-D1-associated ErbB2 [175]. In addition to tyrosine phosphorylation, a direct Ser/Thr phosphorylation of plexins, regulating the receptor’s function, is highly likely; AKT, for example, is emerging as a key cancer regulatory protein and is also connected to integrins (see “Ser/Thr kinases” below).
Direct and indirect interactions with integrins
Integrins are cell adhesion receptors that regulate a wide group of cellular functions in the initiation, progression and metastasis of tumors [235] as well as in angiogenesis [236]. The GAP activity of all plexins towards R-Ras is suggested to be the cause for down-regulation of integrins (see below). Beyond this, during angiogenesis VEGFR2 forms a complex with Integrin αvβ3 upon VEGF-A binding and attracts Src kinase on the intracellular side which then phosphorylates β3 integrin (PI3K is also activated). Plexin-B1 also elicits a pro-angiogenic phenotype in endothelial cells via RhoA activation, stress-fiber formation and engagement of integrins and the PI3K-AKT signaling pathway [237]. Whether Sema3 or -4 family ligands can bind directly to integrins is not yet clear and the cross-talk is thought to occur at the intracellular level. Recently, however, β4 integrin was shown to form complexes with the Met receptor, mediated by the tetraspanin protein CD151 and amplifying HGF/SF-induced tumor cell growth and survival [238]. As noted, Sema7A does not bind neuropilins but is a ligand for plexin-C1 and also for the integrin β1 receptor. However, upon binding to Sema7A, plexin and integrin receptors have opposite effects on cell spreading and dendrite formation in human melanocytes [239].
Skeletal system
The development and maintenance of the skeletal system shares many similarities with the cardiovascular and nervous systems, particularly in the general manner by which cells migrate, differentiate and are used in patterning. Not only are bones infiltrated with both nerves and blood vessels, but also—as a living tissue—the formation (by osteoblast) and reabsorption of bone (by osteoclast cells) involves the development and guidance of several cells types [240]. Thus, it is of no surprise that plexins and their associated ligands, as well as co-rectors, are involved. Osteoblasts, typically expressing both ligands and receptors, directly interact with osteoclast precursor cells in vitro (osteoclasts mostly express receptors, but not ligands) [241]. Plexin-A1 is expressed in osteoclasts together with co-receptor TREM2 and transmembrane adaptor DAP12. DAP12 is known to activate the kinase Syk and the PI3K-AKT pathway [242]. Also downstream of DAP12 the transcription factor NAFTc1 is activated which is necessary for osteoclastogenesis. The DAP12 and the TREM family of receptors also play significant roles in inflammation and the immune system [243] (see “Immune system” below). Meanwhile, plexin-A2 gene polymorphisms and Sema7A are becoming markers for susceptibility of osteoporosis, fracture risk and bone mineral density [244, 245]. Although plexin-C1 is a binding partner for Sema7A, another one is integrin-β1. It is thought that this integrin-mediated signaling pathway is primarily involved in enhancing osteoclast function/bone cell differentiation. Plexin-D1 is involved in axial skeletogenesis and deficiency in plexin-D1 leads to defects in skeletal patterning [246, 247]. As seen in other tissues, the Sema3 family of plexin ligands is involved in cell death and proliferation decisions. This is also the case for bone formation and maintenance, where a Sema3A knockout causes abnormal bone and cartilage formation [241]. In cartilage Sema3A antagonizes migration of chondrocytes (cartilage forming cells) [248]. Very recently, Sema3A was shown to regulate both osteoblasts and osteoclasts in order to favor bone formation [249]. This may be accomplished by Sema3A—Nrp1 interactions which inhibit osteoclast differentiation by sequestering plexin-A1 away from TREM2. Intravenous Sema3A administration in mice led to increased bone volume and regeneration, making it a very promising therapeutic agent. While Sema3B gene expression levels are regulated by vitamin-D, osteoblast-targeted overexpression of the ligand, by contrast, correlates with reduced bone densities and abnormal skeletal structuring in mice [250]. The study of expression of other Sema ligands in bone cells or their precursors is not yet complete [251]. However, recently, Sema4D and plexin-B1 have been directly implicated in the suppression of bone formation by osteoclasts expressing the ligand. Plexin-B1- and RhoA-mediated signaling was found in osteoblasts [252]. Consistent with this is the observation that Sema4D deficiency leads to a high bone mass phenotype due to decreased bone reabsorption in mature female mice [253]. The search has now begun for agents that inhibit Sema4D and, thereby, stimulate bone formation (e.g., to combat osteoporosis).
Bone formation and patterning requires a supply and balance of nutrients, which are delivered in part through blood vessels. Again, VEGF ligands and the competition between neuropilin and VEGFR receptors play a large role [254, 255]. Issues, related to this topic are bone cancer [256], bone formation or repair after fracture [257, 258], formation of blood cells in the bone marrow [259], leukemia [260, 261] and arthritis [262], all of which also feature plexin related signaling, usually through the VEGF/neuropilin axis that has already been described above.
Immune system
Cell-to-cell contacts and cell migration play critical roles in the development of the immune response. After its encounter with the antigen, the antigen-presenting cells, such as dendritic cells (DC), travel via the lymphatic or vascular system to display the antigen to specific T cells. Moreover, the region of contact between the dendritic and T cells, called the “immunological synapse”, is similar to synapses that connect neurons and thus some of the same receptors and ligands are involved. Semaphorin ligands expressed by DC help to activate and differentiate the T cell to become a T helper cell. The T helper cells in turn stimulate macrophages and promote the inflammatory response. Ligands, including semaphorins that are expressed by the activated T-cell also stimulate the immune response through activation of B- (or antibody producing) cells. Roles of semaphorins, neuropilins and plexins in the immune system have been uncovered over the last decade and many reviews have appeared on the subject e.g., recently [263–266]. As above, the following section summarizes the involvement of plexins, then, briefly of semaphorins and of neuropilins. Alongside we will also mention their emerging roles in immune diseases.
Plexin-A1 is expressed in DC and is involved in DC function, particularly in the stimulation of T-cells. When plexin-A1 is knocked down in mice, the activation of T-cells is significantly impaired, possibly because cytoskeletal rearrangements that are required can no longer occur [267, 268]. By contrast to plexin-A1’s typical interaction with Off-Track (OTK) or VEGFR2, in DCs the receptor forms a complex with the co-receptor TREM2 and the adaptor protein DAP12 in response to Sema6C/6D (see above for the role of this complex in osteoclast differentiation for bone formation/homeostasis) [242]. Plexin-A1 is also crucially involved in the entry of DCs into the lymphatic system [269]. A primary site for T cell development is the thymus (a lymphoid tissue), where the expression and interaction of soluble Sema3 family ligands and their family-A plexin receptors as well as neuropilins help to regulate the migration of developing thymocytes [270]. Interestingly, here Plexin-A4 with its binding partners, Sema3A and Nrp, negatively regulates immune responses [271], which is shown by the inhibition of monocyte and T-cell migration and an impairment of T-cell activation. In apparent contrast to these inhibitory functions, plexin-A4 (−/−) innate immune cells exhibited defective inflammatory cytokine production [272].
Plexin-B1 is expressed well in follicular dendritic cells, bone marrow stromal cells and in activated T cells. In conjunction with Sema4D, plexin-B1 stimulates the proliferation of normal and leukemic CD5+ B cells [273]. Sema4 ligands play a large role, however, not through plexin-B family but through immune system-specific receptors (see below). Plexin-C1 is also expressed in activated T cells and DCs, however, in plexin-C1 deficient mice T cell function- but not DC function is reduced [274, 275]. Monocytes and macrophages are activated in the inflammatory response through the Sema7A ligand, but again apparently through a non-plexin pathway (α1β1-integrins). Nevertheless, plexin-C1 may provide competition for the ligand. Plexin-D1 (usually involved in the vascular system) is increased in B cells following their activation, and plexin-D1 (−/−) cells show defects in cytokine stimulated migration towards the memory B-cell producing germinal center [276]. Plexin-D1 may also compete with other receptors for Sema4A ligand binding [173] and is involved in thymocyte migration in response to Sema3E [277].
Sema3A, inhibits human T cell proliferation, cytokine production, the activation of cytotoxic activity and T cell adhesion to tumor cells and might be produced by tumor cells to interfere with a potential anti-neoplastic immune response [278]. In the extreme Sema3A may have an apoptotic function, as seen in other systems. Only class 3 semaphorins are diffusible, whilst classes 4, 6 and 7 are typically membrane attached. However, such attachment can be relieved by proteases (see sections “Plexin family members and their expression patterns, ligands and co-receptors” and “Outside” for details). Members of the Sema4 class, 4A and 4D have been named immune semaphorins, due to their wide expression and importance in this system. Yet, these two semaphorins have different expression patterns and functions in the immune system. Sema4A is highly expressed in DCs, B and T cells and enhances the activation and differentiation of T cells. Knockout mice show defective T cell priming and T helper cell regulation [279]. Sema4A also interacts with its receptor Tim-2 (a member of the family of T-cell immunoglobulin domain and mucin domain containing proteins, highly expressed in activated T-cells), rather than with plexin-B family members [280]. In addition a recombinant soluble Sema4A-Fc enhances T cell proliferation [279]. By contrast, Sema4B negatively regulates Th2 and humoral memory responses [281]. Sema4D (also called CD100) impairs the migration of immature dendritic cells (DC), but not of mature DCs. Already in the early 1990s, it was demonstrated that CD100 induces B cells to aggregate and also improves their survival [282, 283]. In immature DCs the semaphorin receptor, plexin-B1, binds to the soluble CD100 (membrane detached form) and mediates an inhibitory effect on cell migration [284, 285]. Sema4D deficient mice have immune defects, mainly in their B cell response to T cell-dependent antigens [286, 287]. Sema4D is also involved in the immune system through its receptor CD72 [288]. CD72 is expressed well in B cells and by itself negatively regulates B cell activation. Binding of Sema4D, however, induces tyrosine dephosphorylation in CD72, which results in the enhancement in B cell activation [289]. Soluble Sema4D also associates with a protein tyrosine phosphatase CD45 and regulates its activity, which is a critical event in T-cell activation. Sema6D disruption inhibited expression of CD127, which is required during the multiphase antigen presenting cell and T cell interactions [277], as well as T cell proliferation at the late state of activation [290]. Sema7A is preferentially expressed on activated T cells, erythrocytes and on red blood cells. As noted above Sema7 stimulates monocytes macrophages through interaction with α1β1 integrin [291]. Neuropilin-1 is expressed well in human DCs and resting T cells. This Sema/VEGF binding co-receptor is essential for the initiation of the primary immune response by allowing the interactions between DCs and T cells [292].
Intracellular signaling networks
Over the last decade it has become clear that cell signaling processes frequently employ networks rather than pathways [293–295], with many of the molecular players forming higher-order complexes, often more or less transiently [296, 297]. Comprehensive reviews have been published over the years on cell signaling involving plexins, e.g., recently [32, 39, 298, 299]. Below is an account of what has been gleaned thus far from a wide range of genetic and cell biology experiments on the involvement of plexins in a diverse set of intracellular signaling networks (Figure 1). We attempt to be all-inclusive and list the majority of pathways and interactions that have been implicated. However, only in some cases there is evidence for the signaling mechanism in a particular in vivo setting, such as in a specialized cell type, tissue or organism. The reader is referred to the original papers in order to assess which signaling interactions still require further testing. Many ground breaking studies from model organisms, unfortunately, could not be mentioned, since the review is focused on vertebrate plexins.
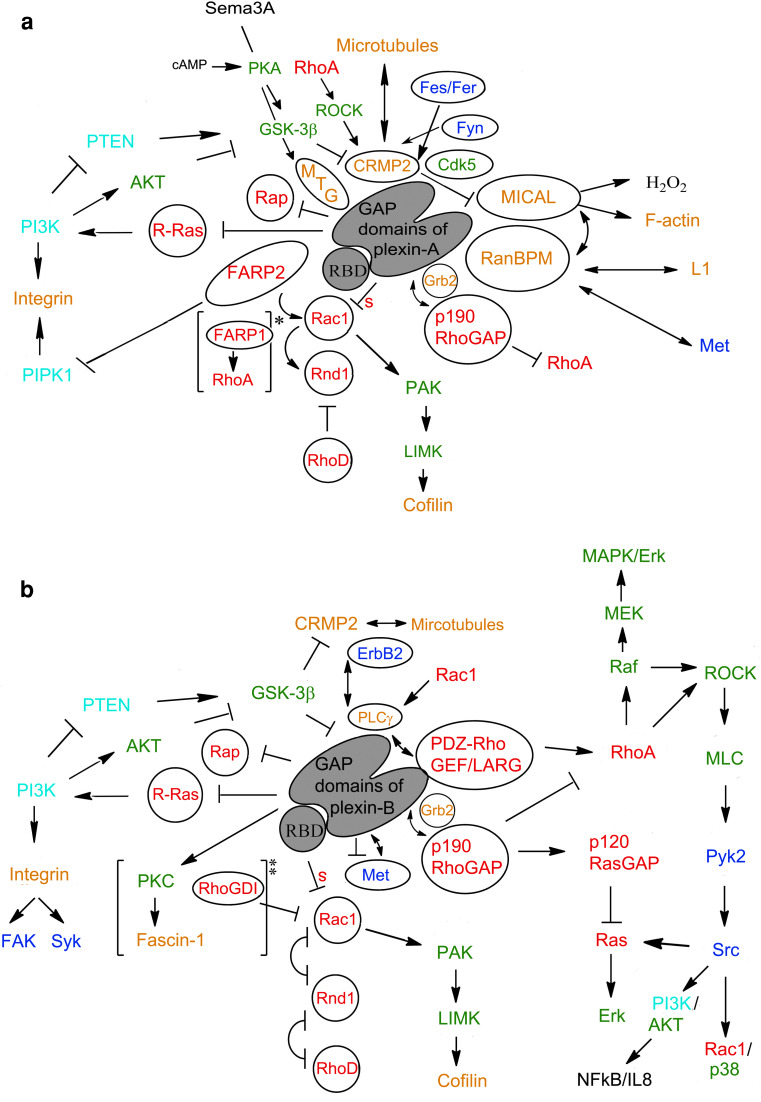
Fig. 1.
a, b Intracellular signaling networks surrounding vertebrate plexin-A and plexin-B function. Proteins that are known to directly interact with plexin-A and/or -B family members are encircled. Color scheme indicates type of protein as follows: red: GTPase or GTPase regulatory protein; blue: tyrosine kinase; green: serine/threonine kinase; light blue: enzyme involved with lipid second messenger; orange: protein functioning as adaptor, scaffold or involved with cytoskeleton [* in plexin-A4, ** in plexin-B3]. Double arrows indicate additional protein–protein interactions that may occur while bound to plexin. Single activating and inhibitory arrows pertain to the functional interaction of the two proteins involved only; e.g. R-Ras stimulates PI3K, even though in plexin signaling PI3K is downregulated because R-Ras is deactivated. Inhibitory arrow with “s” indicates a sequestration mechanism has been suggested. Cell signaling networks are not yet substantially understood for plexin-C1 and -D1. Although some common features are shared with plexin-A and -B family members, many others are likely to be distinct
Involvement of plexin family members with Rho and Ras small GTPases
Although several families of guidance cues and the corresponding receptors have been discovered, plexins are unique. By contrast to other transmembrane proteins the intracellular domains of plexins interact directly with Rho and Ras family small GTPases. Like other GTPases, the Rho and Ras proteins are understood as on/off switches for signaling, depending on the nature of the nucleotide, GTP or GDP, that is bound, e.g., [300, 301]. Active (GTP bound) GTPases associate with effector proteins, typically changing their conformation in order for the signal to be transduced. Alternatively, regulatory proteins bind to the GTPases and effect the rate of nucleotide hydrolysis or exchange as GTPase-activating proteins (GAPs) and guanine exchange factors (GEFs) [300, 302, 303]. In addition to interacting directly with small GTPases, plexins also associate and activate or inactivate a number of GTPase regulatory proteins. These interactions are discussed for the different plexin-subfamilies below.
Rho family small GTPases (e.g., Rac1, Cdc42, RhoA) remodel the actin cytoskeleton and are, therefore, intimately involved with the motility and positional maintenance of cells [304]. These GTPases have roles in a wide range of processes in neuronal development, disease and in regeneration [305–307]. The Rnd subfamily of the Rho-proteins play a special role as GTPase hydrolysis is absent or substantially impaired. Thus they are thought to be constitutively active and their function is controlled to a large part by their level of expression and cellular localization [308]. Nevertheless, the presence of Rnd1 is essential for a wide range of processes, including the disassembly of actin filaments and loss of cell adhesion [309] while it also promotes growth and branching in dendritic neurons and neurites [310, 311]. As an activator of p190 RhoGAP, Rnd1 is known to down-regulate RhoA [310]. Rnd1 may be sequestered away from p190 RhoGAP by a number of proteins such as FRs2b [312], Socius [313], SCG [314], but also regulates their function. Similarly, plexin may function by sequestering Rnd1 and thus temporarily inactivating the GTPase as far as their other interactions and functions are concerned [315]. Rnd1 binding, in turn, could compete for the ability of plexin to associate with other Rho GTPases [316, 317]. Moreover, it has been proposed that plexin sequesters Rac1 (a homologue of Rnd1 that also binds plexin-A and -B family members) away from p21-activated kinase (PAK), an effector protein that stimulates actin polymerization and forward movement of the cell front [315, 318, 319]. However, sequestration models are unlikely to apply to all the Rho GTPases that bind, or to all plexin family members [319]. The signaling must take into account the intracellular status of the cell, allowing the orchestration of a coordinated response. Indeed, there is evidence that signaling through plexins is inside-out as well as outside-in [315, 320], a mechanism that was confirmed at the molecular level and that is partially set up with the help of small GTPases [321]. For this plexins can be considered as GTPase effectors, that is plexins undergo a conformational change upon Rho GTPase binding (see “Inside” below). The precise role of the GTPases is still incompletely understood, however. One question is whether binding of several different Rho GTPases (Rnd1, Rac1 and RhoD to plexin-B1) all have a similar role in the cell signaling mechanism (e.g., by disrupting a common interaction- with plexin as an effector) [321]? Alternatively, binding of different Rho GTPases could have distinct roles in the plexin system. In support of the latter, it is now becoming clear that the different plexin family members have binding preferences for different small Rho GTPases [322–325] but it is not yet clear whether this specificity leads to distinct features in cell signaling.
In the case of vertebrate plexin-A family signaling, Rac1 appears to play a slightly different role than in the plexin-B systems. A Rac1 exchange factor, FARP2 binds to plexin-A1 in a Sema3A stimulated manner and generates active Rac1 GTPase [326]. This, in turn, binds to the RhoGTPase Binding Domain (RBD) of plexin-A1 and creates a conformational change that allows Rnd1 binding and further receptor activation. This sequential model [327] could be unique to members of the plexin-A family, but—if a positive feedback exists—may also explain an earlier report that the amount of Rac1.GTP is increased in presence of plexin-A1 [328]. While presence of active Rac1 enhances signaling, RhoD binding to plexin-A1 antagonizes Rnd1 and plexin signaling by a mechanism that is not yet understood [316].
In vertebrate B-family plexins, Rac1 binds a central region possibly more strongly than in the case of plexin-A1. The RBD region—Rac1 interaction is thought to have an effector function in activating the receptor. Effector binding either causes a conformational change [321] and/or enhances the correct localization of the receptor [315]. Both scenarios are consistent with the finding that Rac1 binding is promoted upon interaction of the Sema4D ligand with plexin-B1 [320].
Early reports suggested that Plexin-C1 and -D1 do not bind Rho-GTPases in vitro. However, Negishi and colleagues have shown a binding of Rnd family proteins in cell extracts [322] and a modest binding of Rnd2 has recently been confirmed to the RBD of plexin in vitro [326]. The interaction of Rnd1 and Rnd2 with plexin-D1 may be similar to that of the -A and -B family in that the GTPase appears to be required for activation of plexin’s GAP function, but the mechanism is not yet known (see “Inside”). In case of plexin-C1 Rnd proteins are unlikely to be involved and how the receptor’s GAP activity is regulated remains to be discovered [322]. Another intriguing and as yet unresolved question is the role of Rac1 in plexin-B1 signaling. It is not yet clear whether Rac1 or other Rho GTPases have the same stimulatory effect that Rnd1 has on plexin-B1 activity.
Ras-GTPases
Ras-family GTPases regulate the transcription of certain genes [329], including for cell growth, and they also influence cell attachment via integrins [330]. An involvement of Ras proteins in plexin signaling was initially inferred from the homology of plexin cytoplasmic regions to the Ras GAP family of regulatory proteins [331]. Later, GAP activity was demonstrated for members of all four human plexin families against R-Ras GTPase (and for plexin-B1 in neurites also against M-Ras) in cellular assays [59, 322, 332], principally by the Negishi laboratory but also confirmed by others [162, 165, 326, 333, 334]. However, it has been difficult to reconstitute the GAP activity for M- or R-Ras with purified protein components in solution [335, 336] and interactions with the plasma membrane are likely needed to stimulate this function. Nevertheless, several Ras GTPases bind the GAP domains of plexin-B1 and the specificity appears to be more selective than other GAPs, such as p120 RasGAP for instance [333, 337–339]. It is not yet known how this binding specificity arises and how the activity is regulated. Recently, cellular settings have been found in which plexin binds R-Ras but does not inactivate it [340, 341], suggesting that a Ras sequestration model may also fulfill the role of withdrawing the GTPase from the integrin system, thus diminishing cell adhesion in cell collapse. However, it is not clear whether sequestration models provide an adequate control of Ras activity [319]. As noted above, in many other cellular experiments the total amount of active R-Ras was shown to be downregulated [322, 326, 332, 342, 343] and Ras-GAP activity of plexin-B1 was confirmed upon stimulation with Rnd1 and receptor clustering in an assay with cell extracts [336]. Intriguingly, the GAP activity appears to be largely separate from the signaling pathway to RhoA via PDZ-RhoGEF/LARG [165] or p190 RhoGAP [231]. Stimulation of GAP activity in both plexin-A1 and -B1 and downregulation of R-Ras inhibits PI3K and results in a dephosphorylation of AKT and GSK-3β in neuronal growth cone collapse [342]. (Note that binary functional interactions are drawn in Fig. 1; thus PI3K and AKT are downregulated following deactivation of R-Ras. This also leads to GSK-3β stimulation, since this latter enzyme is inhibited by AKT and stimulated by PTEN). Furthermore, Sema4D—plexin signaling inhibited PI3K, in turn, causes a downregulation of integrin-β1 affinity for cell adhesion, allowing cell migration [343]. Another common partner for integrins is the focal adhesion kinase (FAK) which in part regulates p120 RasGAP [344], creating the possibility of regulatory feedback loops. Very recently activity against Rap GTPases has been demonstrated in aqueous solution [341], particularly for the plexin-C1 and -D1 intracellular domains, with a more modest activity for plexin-B1. Catalytic activity could be stimulated for plexin-A1 and increased in the other plexins by induced N-terminal dimerization [341]. Using a constitutively active Rap1 protein the cell collapse function of plexin was abolished in Sema3A stimulated cortical neurons, suggesting that these results extend to other cellular settings. Rap1a, b and Rap2 are a subfamily of Ras GTPases and are typically involved in vesicular transport. Rap levels have been associated with axon collapse and outgrowth with other guidance receptors and also implicate loss of cell adhesion via a downregulation of integrins [345]. However the downstream signaling networks are not yet known in the case of plexins [278]. Similar to other GAP proteins [303, 346], plexins appear to be multifunctional and activities against different substrate Ras-family GTPases are likely to be controlled by different mechanisms and environments.
GTPase-regulatory proteins
In early studies an association of plexin-B1 was observed with PDZ-RhoGEF/LARG, an exchange factor for RhoA. This association is promoted by Rnd1 binding [317]. Such behavior may set up to exploit an antagonism between Rnd1 and RhoA signaling that is observed in Rho-mediated regulation of the cytoskeleton in general. Interestingly, several studies [165, 347] suggested that the signaling mechanism of plexin through RhoA is largely independent of Rac1/Rnd1 effects or GAP activity towards Ras proteins. Both LARG and PDZ-RhoGEF bind directly the very C-terminal PDZ binding-motif that is unique to the plexin-B family [337, 348, 349]. The motif should be important for plexin’s ability to activate RhoA, causing stress fiber formation and collapse. The association appears to be constitutive in the case of PDZ-RhoGEF [348] and the intact PDZ binding motif is critical for the localization of the receptor at the plasma membrane [350]. Plexin-D1 has a PDZ-binding motif and studies to identify interaction partners are in progress e.g., [351], while the plexin-A family and plexin-C1 do not have a C-terminal PDZ binding motif. Recently, the work of the Swiercz and Offermanns laboratory, has revealed how PDZ-RhoGEF is regulated by ErbB2. Following phosphorylation of plexin-B1 at Tyr1708 and Tyr1732, SH2 domains of PLCγ bind which in turn recruits PDZ-RhoGEF via an SH3 domain [165]. The mechanism extends to plexin-B2 and likely -B3, as well. Since neither plexin-A family members or plexin-C1 have C-terminal PDZ binding motifs or equivalently positioned tyrosines it has been unclear whether and how RhoA is regulated outside the plexin-B family. Recently it has been proposed that a Rho GTPase exchange factor FARP1 may fulfill a similar role in case of plexin-A4 [352]. Furthermore, a survey of SH3 domains that interact with RhoGAP proteins has recently identified MICAL1 as an interaction partner for ArhGAP26 [353].
In A- and B-family plexins, the receptors intracellular region was shown to interact with the RhoGAP protein p190a, which can locally deactivate RhoA GTPase and is required in endothelial cell chemorepulsion [354, 355]. The regulation of p190 RhoGAP and of its downstream binding partners are thus of interest: The p190a protein has an N-terminal GTPase-like domain and is thought to be phosphorylated, altering activity and substrate specificity; phospholipid and Rnd1 binding are also implicated [356–358]. p190 RhoGAP appears to serve as a convergence point for adhesion regulation by many pathways, including those involving α5β1 integrin, syndecan4 [359], Gα13 [360] and several kinases such as Brk [361], Src, PKC, ABl2/ARG [355]. The p190 RhoGAP activity is in part regulated by accumulation in lipid rafts [362]. Rnd1 and Rnd3, themselves have N-terminal sequences that target them and p190 RhoGAP to lipid rafts where the GAP is activated [363]. Intriguingly, p190a is also able to bind and possibly activate p120 RasGAP, as well as associate with focal adhesion proteins [344, 364]. Thus, is may be possible that the effect of plexin’s own GAP activity is enhanced or is broadened in its specificity by indirect recruitment and activation of p120. Very recently, the mechanism of interaction between plexin and p190 RhoGAP has been elucidated in the context of plexin phosphorylation by the tyrosine kinase Met [231]. Following phosphorylation of plexin-B1 at Tyr2094 by Met, the SH2 domain of Grb2 binds and serves as an adaptor protein. In a mechanism that extends to plexin-A1 (and possibly most plexins, since position 2094 is conserved, except for plexin-B3), Grb2 also recruits p190 RhoGAP by binding to its SH3 domains. p190 then mediates RhoA deactivation [231]. It is not yet clear whether other modes of interaction between plexins and p190 are also possible [354, 355] and whether the Met also participates in p190 activation. The latter is suggested recently for plexin-B2 in the immune system (macrophages), where plexin activation down-regulates Rac1 and Cdc42 GTPases [365], possibly via p190 RhoGAP, by another GAP, or by effecting a Cdc42/Rac1 exchange factor protein. Similarly, it has been reported that plexin-B1 reduces RhoA activity in melanoma cells [366]. Depending on the cellular setting and signaling status, it is possible that competing regulatory mechanisms are engaged, both regulating levels of active RhoA, but in opposite ways. Another example of how plexin may effect a GTPase regulatory protein has been described for plexin-B3. Here, Sema5A promotes Rac1 recruitment to RhoGDIα and reduces its membrane localization in a plexin-B3-dependent manner [59]. This mechanism is similar to the proposed Rac1 sequestration by plexin itself [315]. RhoGDI interacts directly with plexin-B3, but it is not yet known whether this mechanism extends to other plexins.
Recently the Arf family of small GTPases has been implicated in plexin-D1 signaling in response to Sema3E, having an anti- angiogenesis effect [340]. Both R-Ras down-regulation, resulting in rapid disassembly of integrin-mediated adhesive structures, and Arf6 stimulation, resulting in β1-integrin receptor trafficking are involved. The Arf6 exchange factor protein, GEP100/Brag2, is implicated and appears to be activated by phosphatidylinositol 4,5-bisphosphate—lipid signaling molecules—that are generated by PI(4)P-5 kinase, an enzyme that in turn is stimulated by plexin [367]. Involvement of Arf6 (and of other Arf family members) in receptor trafficking is not unprecedented; for example, the Met receptor is recycled by Arf6 signaling. Receptor trafficking [368] and degradation is known to play important regulatory roles in several transmembrane receptors (see also “Unanswered questions”).
Cytosolic tyrosine kinases
The role of receptor tyrosine kinases Met, Ron, VEFGR and ErbB2 have already been mentioned (above and in the section “Cardiovascular Injury and regeneration”). The signaling interactions regarding kinases may be roughly classified into three groups: (1) Phosphorylation of plexin by kinases and the effect this has on plexin signaling, illustrated by ErbB2 and Met above. (2) Phosphorylation of plexin interacting proteins by kinases, including plexin stimulated kinase (auto-)phosphorylation and the effect this has on plexin-mediated signaling; and (3) Downstream effect of plexin signaling on kinases themselves. Although there is no example as of yet, it is likely that kinases may also fit into several of the groups due to feed-back and cross-signaling. Pyk2, Src, FAK, Fer/Fes, Fyn, and Syk are cytoplasmic protein tyrosine kinases which are thought to play roles in the wider plexin-mediated cell signaling network (group 3). Sema4D/plexin-B1 has been reported to induce endothelial cellular migration through a signaling cascade that starts with a sequential integrin-mediated activation of Pyk2 and Src kinase [237, 369]. These kinases then activate PI3K, AKT and Erk. A recent study of the same pathway places the Pyk2 kinase downstream of plexin-B1 via RhoA activation of Rho Kinase (ROCK). Src kinase can also be stimulated via involvement of Met, but in this case a broader cascade of signaling, e.g., via Ras/ERK and Rac1/p38 is implicated. The PI3K/AKT pathway is also involved [370, 371], likely providing a feedback loop to plexin via GSK-3β (see below) Syk kinase activation is greatly reduced in Sema4D knockout mice, resulting in reduced collagen-induced platelet aggregation [372]. The mechanism by which Sema4D stimulates Syk is not entirely clear but is correlated with integrin signaling. As examples of group 2, Fer cytoplasmic tyrosine kinases are thought to interact with the intracellular region of Neuropilin1 in response to Sema3A stimulation and stimulate cell death in cortical neurons [373]. Other roles involve signaling to the collapsing response mediator (CRMP) family of microtubule regulators (which also bind A-family plexins) and to focal adhesions and membrane rafts [132, 374]. Fes/Fps participate similarly in Sema3a/plexin-A1/Nrp1 signaling, possibly by activating plexin-A1 signaling. The kinases are also part of a complex with CRMP2 [375, 376]. Fyn is another Src-family tyrosine kinase that targets members of the CRMP-family of adaptor proteins (see below) [377], but furthermore activates the Ser/Thr kinase Cdk5 in order to regulate the guidance of cortical projections in plexin-A2 mediated signaling [378]. Inhibition of Cdk5 can also reduce the Sema4D-plexin-B1 mediated regulation of RhoA and Rho–Rho-kinase (ROCK) activity [379] via Src activation of p190 RhoGAP [380]. For the cytoplasmic tyrosine kinases, however, there are as yet no examples of group 1 signaling behavior (direct plexin phosphorylation), but these are likely to exist.
Ser/Thr kinases
As an example of a direct downstream interaction of plexin with a Ser/Thr kinase, A recent report suggests that PKC, is transiently stimulated by Sema5A and plexin-B3. Following phosphorylation by PKC, fascin-1 (an actin bundling protein) contributes to the disruption of F-actin stress fibers [57]. However, in terms of downstream signaling (group 3, as defined above) Ser/Thr kinases are often found in signaling networks one or two levels removed from plexins. Furthermore, these signaling proteins frequently receive signals from tyrosine kinases [381]. Such cascades play important roles in many cellular processes. For instance, plexin-B1-induced RhoA activation cooperates with Ras to activate Raf and then the MAPK/Erk pathway [382]. The other common pathway, PI3K-AKT, was already mentioned above (and in “Cancer and pathogenic angiogenesis; plexin’s role in metastasis and cancer proliferation” and “Skeletal system”). Indeed, several pathways may converge and others diverge, and some feed back on B-family plexin signaling. On one hand, the GSK-3β Ser/Thr kinase is inactivated downstream of the RTK-PI3K-AKT pathway, while on the other GSK-3β may be activated though R-Ras GAP-dependent pathways [51]. Recently, it has also been shown that plexin-B1 activates NF-κB and IL-8 through AKT in order to promote a pro-angiogenic response in endothelial cells [383]. In the plexin-A family, activation of GSK-3β can lead to inactivation of CRMP2 for instance [384], while CRMP proteins may also be stimulated by Rho kinases/ROCK [176]. GSK-3β is thought to feed-back on plexin activity either indirectly (via CRMP family of plexin binding proteins) or possibly directly in case of the plexin-B family. However, phosphorylation sites and the signaling mechanisms are not yet known. An example for a group 1 signaling behavior for a Ser/Thr kinase (i.e., direct phosphorylation of plexin) comes from a study of cAMP-dependent protein kinase/protein kinase A (PKA). It was known that Sema3A stimulation elevated cGMP but reduced cAMP levels and PKA activity [385]. A recent study revealed PKA phosphorylation sites in Drosophila plexin-A [333]. Intriguingly, here the identified phosphorylation site is adjacent to the GAP catalytic residues in plexin. The site is relatively well buried in the plexin GAP region and must be made accessible by a large-scale conformational rearrangement. Once phosphorylated, the 14-3-3ε protein was shown to bind, again suggesting a substantial structural change in plexin that would allow interaction with this protein. It could be shown that such structural changes inhibit plexin’s GAP catalytic activity against Ras and interfere with plexin’s function in cell repulsion. The phosphorylation/14-3-3ε binding motif is similar to the sequence in the human plexin-B family and in plexin-C1 and -D1 [333], suggesting a similar mechanism could be utilized to regulate the function of these plexins. Intriguingly, 14-3-3ε proteins are known to inhibit Na+/Ca2+ exchangers [386], further linking this signaling pathway to Ca2+ signaling.
Plexin is able to engage multiple cell signaling pathways independently and can, thus, uncouple cell substrate-adhesion from the cytoskeletal dynamics required for cell migration [387]. The involvement of several GTPase regulatory proteins, tyrosine and serine/threonine kinases suggests that considerable cross-talk and coordination of cell signaling networks are required in order to elicit complex cellular responses that are needed in processes such as cell migration. As part of group 1 and 2 cell signaling behavior (direct effect on plexin by phosphorylation of plexin or on plexin binding proteins) the phosphorylated sites on kinases and other proteins also need to be deactivated by phosphatases. Little insight has been gained on phosphatase signaling so far for the plexin system, except that suppression of PI3K through the Ras-GAP pathway leads to greater dephosphorylation of PTEN and an increase of its activity. PTEN itself decreases the level of phosphatidylinositol (3,4,5)-triphosphate and activates GSK-3β in Sema3A-mediated growth cone collapse [388–390]. GSK-3β controls microtubule architecture and polarized cell movement [391]. Cross-signaling with PI3K and PTEN then defines the front and backsides of migrating cells.
Adaptor and scaffolding proteins
Plexin-B1 interacts with adaptor proteins, such as PLCγ and Grb2 via SH2 domains [165, 231], already described above. Plexin-A from Drosophila was shown to be associated with a larger scaffolding protein, Nervy, that co-localizes and couples the receptor to cAMP—protein-kinase A signaling [392]. Recently interactions were confirmed between a mammalian homologue, Myeloid translocation gene 16b (MTG). MTG was reported to interact with plexin-A1 and -A3 (but not plexin-B1) [393]. It is thought that similar proteins, complexes with the actin based cytoskeleton or with microtubules may link to plexin receptors. Specifically, microtubule binding protein MAP1A was identified as an interaction partner for the RhoGTPase-binding domain of plexin-A1 [394] and recently plexin-B3 (and likely also plexin-A2 and -B1) were found to form complexes with microtubule plus -end binding proteins, EB1, EB2 and EB3 [395]. CRMPs were recently identified as microtubule binding proteins [384], further suggesting that plexin-A family receptors may be associated with the cells cytoskeleton. These interactions may help to localize plexins and -by competition or cooperation for binding sites—regulate cell signaling processes. Another example of this is the RanBPM protein that directly binds to the RhoGTPase binding domain (RBD) of plexin-A1 [394]. Intriguingly RanBPM also interacts with the cytoplasmic regions of integrin-β, the Met and L1 co-receptors [396]. It is thought that the plexin—RanBPM interaction is enhanced by another plexin-A1 binding protein MICAL [394]. This latter multifunctional protein, MICAL, was found to link the receptor to the cytoskeleton by directly binding to F-actin and is linked to actin depolymerization [397].
MICAL
Denoted as molecules interacting with CasL, MICALs, are known to play a key role in the function of plexin-A family members [398, 399]. Firstly, a structural role exists as plexin-A family members interact directly with MICAL and this helps to release MICAL’s autoinhibition in a semaphorin-dependent manner. At the same time the interaction of MICAL with CRMP is increased [400], implying that the two proteins bind to different sites on the intracellular region of plexin. Once activated by conformational change, MICALs are enzymes and function as flavoprotein oxidoreductases. Biochemical studies, using inhibitors and mutagenesis, have shown that the redox activity of MICAL is required for F-actin disassembly and plexin-mediated axon repulsion [397, 401, 402]. The structure of the catalytic region of MICAL has been determined by X-ray crystallography, suggesting a possible site for the interaction with actin [403], but how plexin binding activates MICAL is not yet known.
Structural biology
In this section we introduce the structural knowledge that has been obtained on plexins and the proteins that associate with the receptor on the outside and the inside of the cell. We also discuss the implications these findings have for the mechanisms of plexin-mediated signaling.
Outside
The plexin extracellular region is composed of several domains, which also occur in other proteins, including in proteins that associate with plexins [404]. Figure 2 shows the domain architecture of the plexin family in comparison with representative members of its semaphorin ligand and with Neuropilin-1 (Nrp1) as well as with Met and Ron co-receptors. As an overall theme we ask: What is the function of the domains and how are they linked together? Is the multidomain structure residing in a few well-defined states? Furthermore, how do variations in domain loop and surface features determine domain–domain interactions and binding specificity?
Fig. 2.
Domain organization of the extracellular regions of representatives of the plexin and semaphorin subfamilies. Shown in comparison are some of the proteins that also associate with plexins and/or semaphorins and that have some of the domains in common
SEMA domain
All plexins, semaphorins as well as Met and Ron RTKs contain a highly homologous semaphorin (SEMA) domain, which is known to be the primary site of interaction between plexins and semaphorins. The β-propeller structure of SEMA has 7-blades and contains two extensive inserts (the second is also called “extrusion region”), putting them in a distinct branch of a larger family of β-propeller or SEMA-like domain containing proteins [405]. In fact part of the inserts are known to be responsible for dimerization of the SEMA domain, shown in Fig. 3a. Dimerization is likely for all semaphorins; it has been observed for Sema3A [406], 4D [407], 6A [408, 409] and -7A [410] as well as for a viral semaphorin mimic, A39R [410], that have been crystallized to date. Of the plexins, only plexin-A2 has been crystallized in a dimeric form [408] and this dimer is very weak in solution with a Kd > 300 μM. Nevertheless, the region of the plexin SEMA domain that constitutes the dimerization interface for plexin partially overlaps the region used for binding of semaphorin ligand (shown in Fig. 3b). This rationalizes how plexin-A2 dimers would be disrupted by semaphorin binding, but the overlap also makes the characterization of the plexin-A2 dimer difficult by mutagenesis in vivo. It is not clear at present whether plexins themselves generally dimerize through their SEMA domains. Interestingly, and by contrast to plexin-A2, the SEMA dimerization and plexin binding sites are quite separate in the SEMA domains of semaphorins [407], displayed in Fig. 3a and c. Strong receptor-ligand interactions occur (with Kds as low as nM in cell based assays) while maintaining the ligand dimer, thus leading to a dimerization of the plexin receptor. Using monomerized semaphorins, a strong bivalent effect is seen on binding at high concentrations of plexin receptors. However, even 50 µM monomer SEMA4D ligand does not activate the receptor in cell collapse assays [409]. Very recently three plexin-semaphorin structures have been determined, plexin-C1 bound to Sema7a [410], plexin-B1 in complex with Sema4D [409] and plexin-A2 associated with Sema6A [408, 409].
Fig. 3.
Mainchain folds of a Sema4D homodimer (pdb id 1OLZ), shown in three different orientations (i-iii). The PSI + Ig stalk is shown in brown/orange. The first and second insert regions are colored dark blue/green and red, respectively. b Plexin-A2 homodimer (pdb id 3AL9), color scheme similar as in (a), except plexin SEMA domains are shown in cyan/pink. c The Sema4D:plexin-B1 2:2 heterodimer (pdb id 3OL2) is shown in two orientations. Plexin-PSI/PSI-IPT domains are colored grey
All three complexes share common features, such as the involvement of similar SEMA domain regions in an anti-parallel (trans) interaction [409] (Fig. 3a, b). Most of the contact residues involve the insert/extrusion region of the β-propeller blade 5 (e.g., β4b-β4c or β4c- β4d) (colored dark blue/green in Fig. 3a, c) and a 20-residue insert loop between blades 1 and 2 on the side of the SEMA domain in semaphorin (colored red, Fig. 3a, c). On the side of plexin, involvement of these regions is less prominent. For instance, in the case of the plexin-C1:Sema7A interaction, the extrusion helix-2 region of semaphorin forms surface contacts to plexin, while the extrusion loop 4c-4d inserts into a deep grove created by a bulging of strand 3d in the plexin. In their study, Xiaolin He and coworkers identified the semaphorin loop 4c-4d as the element that is most likely to confer specificity amongst the ligands for binding [410]. However, in the plexin-A2:Sema6A interaction no equivalent deep loop penetration is seen and the conformational changes at the interfaces are minor. Thus, the loop regions that are involved in the interactions have different lengths and conformations in the individual plexin and semaphorin family members. These differences are thought to give rise to binding specificity between plexin and semaphorin family members [409]. Now, that the detailed structures are known, peptide and small molecule inhibitors may be designed (or in silico screened) and will soon deliver new insights into plexin function and possibly therapeutic avenues to combat diseases, including those mentioned above.
Similar to plexins, Met and Ron receptor tyrosine kinases also do not dimerize appreciably through their SEMA domains [411]. Furthermore, it is not yet clear whether plexin-Met-semaphorin interactions primarily involve the SEMA domains. Met and Ron are receptors for scatter-factors (HGF/SF)—ligands, which however do not contain a SEMA domain. A complex between Ron and HGF/SF ligand shows that the latter interacts with different regions of the SEMA domain than are utilized in plexin and semaphorin binding [411, 412], raising the possibility that ternary complex could be formed. However, this contrasts the observation that activation of the Met kinase becomes HGF/SF ligand-independent upon binding to plexin-B1. As mentioned above, the plexin:Met interaction can have profound effects on plexin function. Certain integrins such as αV also contain a 7-blade β-propeller, but these structures do not have an extrusion region [413]. It is not yet known in detail how Sema7A interacts with β-1 integrins, but SEMA:non-SEMA domain interactions are clearly possible. For example, certain plexins interact with neuropilin and with ErbB2, which also do not possess SEMA domains. The interactions with ErbB2 have not yet been characterized in structural detail. In case of neuropilin, inferences regarding the contact regions have been made from cellular studies with receptor and semaphorin fragments [414] as well as from the Sema3A crystal structure [406]: although, Sema3A was crystallized as a dimer, it forms a 1:1 complex with neuropilin. The Sema3A dimerization site and one of the two proposed neuropilin binding sites appear to overlap, consistent with disruption of the Sema3A dimer (structural work on neuropilin itself and strategies to disrupt its interactions are discussed above in section "Ligands of neuropilins"). Importantly, studies with plexin-A1 and neuropilin1 suggested that the plexin may be autoinhibited through interactions of its extracellular domains and that such autoinhibition is overcome by plexin-A1 SEMA domain deletion or by neuropilin binding in presence of Sema3A [414]. The mechanism of autoinhibition is thought to involve the plexin-A1 SEMA domain in interactions with the other extracellular domains, IPT and PSI, discussed next.
PSI, IPT, and Ig domains
Apart from the SEMA domain, the extracellular region of plexins and semaphorin ligands is comprised of several additional domains (Fig. 2). Plexin-semaphorin-integrin (PSI) domains are small, only 50 residues in length and usually contain eight cysteine residues. They occur in extracellular segments of over 500 cell signaling proteins. X-ray and NMR structures have been determined of several PSI domains in isolation [415, 416] and some also in the context of other surrounding domains [417]. In the structures PSI domains are often positioned between SEMA and IPT domains or between IPT domains, forming a wedge between relatively rigid structures and allowing for the correct orientation of the ligand/receptor binding sites. This is seen in plexins, semaphorins and in Met and Ron (Fig. 2), whereas a PSI domain is located near the N-terminus of β-integrins. Binding of antibodies to the PSI domain of β1-integrin causes long range conformational changes that stimulate activity [418], thus illustrating that PSI domains can participate in signaling events. Intriguingly, not all cysteines may be involved in intra-domain disulphide bond formation. In the case of the PSI domain of β3-integrin, a C-terminal cysteine could be regarded as separate from the others and it has been debated whether such cysteines might be involved in interdomain interactions [416, 419].
The name, Immunoglobulin-plexin-transcription (IPT) domains was coined due to their occurrence in plexins and because they have immunoglobulin (Ig) folds as well as a high homology to several transcription factors [404, 420]. IPTs are also found in Met and Ron receptors and in the polycystic kidney/hepatic disease gene1 [421]. While Ig folds are relatively rigid, the loops in them and linkers between them provide a rich repertoire for binding: IPT domains clearly have a functional importance in Met and Ron RTKs, as shown by insertion and deletion studies [422]. In fact, deletion of the first and fourth IPT domain in Ron is thought to cause a conformational change, leading to receptor dimerization and increased activation [423, 424]. Thus, IPT and PSI domains both may play significant roles in the mechanism of receptor activation, if not inhibition. Semaphorins have Ig (immunoglobulin-like) domains which have a different secondary structural topology compared to IPTs.
Models for receptor activation (on the extracellular side)
Nikolov and colleagues suggested a model for auto-inhibition and activation of plexin which is based upon their determination of a dimeric structure of Sema3 and upon a structural/functional homology of plexin to the LDL-receptor and to integrin-αV (two other β-propeller containing proteins). In the proposed auto-inhibited structure of plexin, one or several IPT domains interact with the SEMA domain [406]. These contacts are disrupted when the plexin SEMA domain binds to the SEMA domain of semaphorin. The crystal structure of Sema3A, however, did not contain the full PSI or Igdomains [406]. The structure of Sema4D, determined by another laboratory contained these domains, showing that both Igsegments are close and form a “stalk-like” structure in the dimer [407] (Fig. 3a). The binding of the Ig domains is consistent with a disulphide that is formed in this region prior to entry into the membrane. A similar disulphide also stabilizes the dimeric form of the soluble Sema3A. The recent structures of semaphorin-plexin complexes included further PSI and IPT domains: a PSI + Ig domain for Sema7A and one PSI domain for plexin-C1 [410]. The Sema6A and plexin-B1 structures both included the SEMA proximal PSI domain [408, 409], whereas plexin-A2 [409] included two PSI domains, sandwiching an IPT domain. By contrast to the SEMA4D structure the C-terminal regions of the two plexins emerge in different directions since the N-terminal domains bind (edge-on) to opposite semaphorin units of the SEMA face-to-face dimer (Fig. 3c). Surprisingly, a comparison between the different plexins and semaphorins, as well as between the unbound and plexin-A2 bound structures, revealed only small changes in the orientation of the stalk region [409]. Overall, then, the current crystal structures of plexins do not reveal details how, an autoinhibited structure would be organized (apart from dimerization through its SEMA domain, which is thought to be weak, however, as mentioned above).
Plexin-B1, and likely other plexins, are suggested to dimerize through their GXXXG-like motifs [425] contained in their transmembrane regions. If this is the case, the 1–2 PSI and 5–6 IPT domains (that are missing in the current X-ray structures) may ensure that the receptor, as it emerges from the membrane, can bend sufficiently to bring the SEMA domains together in a head-to-head fashion upon ligand binding. To what extent the C-terminal tails of the extracellular region are close prior to entry into the plasma membrane, however, is a matter of speculation and may vary depending on the state of receptor activation. In fact, variations in this proximity could be a key factor for the transmission of the effect of ligand binding through the membrane to the intracellular region. The study of He and colleagues on plexin-C1 examined whether an autoinhibited structure may exist (along the proposal of Nikolov and coworkers [406] and functional studies). For this the Sema7A ligand binding affinity for the plexin SEMA-PSI construct was measured and compared with the affinity of Sema7A for the full length plexin-C1 extracellular fragment [414]. If the structure were autoinhibited then a markedly reduced binding affinity would be anticipated. However, only a slight decrease was observed, suggesting that a plexin-C1 autoinhibited structure was either not seen or could not be obtained (e.g., the plasma membrane and/or disulphide cross-linking in the stalk region may be necessary). In case of the extracellular region of plexin-A2 (with all SEMA proximal PSI-ITP domains present) a weak dimerization was seen by dynamic light scattering, whereas the plexin-A2 SEMA-PSI-IPT-PSI fragment crystallized as a monomer [409]. No dimerization has yet been reported for extracellular fragments of plexin-B1. Currently, two models have been proposed by Jones and colleagues that involve the polymerization of either autoinhibited or dimeric plexin-B1 extracellular regions into larger arrays upon ligand binding [409]. Studies on PSI-IPT plexin constructs and full length receptors are needed to confirm the models.
Other factors: glycosylation, isoforms, disulfides, and receptor processing
An area that has been difficult to explore is the extent and role of carbohydrate modification for the structure and function of plexin. However, first steps have been made by mutation of putative glycosylation and heparin or chondroitin sulphate proteoglycan sites [426–428]. Furthermore, some of the plexins have been reported to exist in several isoforms due to variations in splicing. For example, a plexin-B1 variant consists of an extracellular fragment and variants of plexin-B3 and -A4 are also reported in PubMed/RefSeq. In addition, several plexins and semaphorins are known to be proteolytically processed [192, 193, 429, 430]. For instance the plexin-B1 dimer mainly exists as a full length protein that is bound (but apparently not disulphide cross-linked) to an extra-cellular plexin-B1 fragment which is truncated just outside the transmembrane region [430]. However, it is unclear how a hetero-truncated dimer can participate in receptor activation, if the latter relies on a bivalent interaction. Finally, cis interactions of receptors and ligands, either expressed by the same cell or available as fragments are beginning to be explored and may give rise to “cis inhibition” signaling in a number of systems, including Notch, Ephrin and Semaphorin [431].
Inside
A starting point of structural studies on the intracellular region of plexins has been the Rho GTPase Binding domain (RBD) as an intriguing insert into the GAP homologous region. More recently investigations have moved onto the entire intracellular region, also with Rho GTPases bound, and the possible role of dimer/trimer formation via a juxtamembrane helix.
RBDs and their interactions with small GTPases
A good homology of intracellular segments of plexins to Ras GTPase-activating proteins was noticed awhile ago [331] and a GTPase-activating function was confirmed in cellular experiments by several groups [326, 332, 333, 334, 336]. The insertion of an approximately ~200 residue segment into the GAP homologous region of a protein is unusual but suggested that this region could comprise a regulatory element for receptor activation. In the plexin-A and -B family the insert region was shown to bind several Rho GTPases [320, 347] (Rnd1/Rac1 to plexin-A1, Rnd1/Rac1 and RhoD to plexin-B1)—thus was named Rho GTPase binding domain (RBD) [321, 432]. Experiments with cell extracts, expressing fragments of the intracellular plexin region, suggested that small Rho GTPase binding would serve to dissociate the two GAP homologous regions, thus activating the receptor [322, 328, 332, 336]. Biophysical studies showed that the plexin-B1 RBD is weakly dimerized [433] and the structure of a monomerized mutant form was determined by solution NMR [163]. The interaction surface with the Rac1, Rnd1 and RhoD GTPases was mapped by NMR onto the RBD domain, whose structure was also determined in the dimeric form by X-ray crystallography [321]. The structure of the RBD is that of an ubiquitin fold. Ubiquitin folds have been utilized to bind small GTPases of the Ras family in one region of the structure [434]. However, relative to this region, the Rho GTPase binding site is located on the opposite side of the structure. Here it is adjacent to, if not partially overlaps with the RBD dimerization loop [321]. Due to this proximity, dimerization and GTPase binding could cooperate or oppose one another. Indeed, a number of biophysical experiments showed that a moderate competition exists between GTPase binding and dimerization [323]. This suggested a model in which the resting inactive form of the intracellular region of plexin is dimerized through the RBD domain. The Rho GTPase binding then serves to disrupt the dimer and causes conformational changes that would lead to receptor activation [321] (Fig. 4a). This model is attractive because it rationalizes functional studies that showed a bi-directional cell signaling mechanism in which Rho GTPase binding inside and ligand binding outside synergize to activate the receptor [315]. The NMR mapping identified the RhoGTPase binding site in the RBD as two sequence, but spatially continuous regions. Sequence homology in the plexin family suggested several Plexin Rho GTPase Association Motifs (named PRAMs) [321, 324]. Almost all plexins, except for plexin-C1 and possibly plexin-A2 were predicted to bind RhoGTPases. In case of plexin-B1 the structure determination of the plexin-B1 RBD was also interesting as at the same time several cancer-associated mutations were mapped to the RBD:GTPase binding interface [162]. It could be shown that the L1815P and L1815F mutant RBD domains of plexin-B1 had lost appreciable affinity for Rac1/Rnd1 [163], thus explaining how the receptor would remain inactive and would no longer cause cell retraction from environments with semaphorin ligand. This would facilitate cell migration in cancer metastasis.
Fig. 4.
Mainchain folds showing a i–iii that binding of a Rho GTPase (colored red) destabilizes the plexin-B1 RBD dimer (magenta) (pdb ids 2R2O and 2REX). Schematics of the complexes are shown, including the GAP domains (green). A model of the RhoGTPase bound structure of the dimeric plexin structure in a.ii indicates large steric clashes, marked X, between the RhoGTPase and an opposing GAP domain in the entire intracellular region. The dimer is thus predicted to be highly unstable. b Entire intracellular region with R-Ras (yellow) modeled into the binding site of the plexin-B1 GAP region (green) (pdb id 3HM6). The coupling loop (cyan) and juxtamembrane (JM) helix (light brown) are also shown. c.i Trimeric structure of Siebold and coworkers, with the most right unit, shown in the same orientation as b. A closer look is provided in c ii of the putative second Rho GTPase binding site in plexin-B1 (region corresponds to an enlargement of the lower left side of c i, marked *). Binding at this site brings together the RBD (magenta) and switch I region of Rac1 (red) and part of the GAP domain (green) (pdb id 3SUA)
Structures have been solved by crystallography also for isolated RBDs of plexin-A2, -C1 and -D1 [324] and very recently for plexin-A4 and -B2 [Unpublished – pdb ids 4E71 (plexin-B2 RBD) and 4E74 (plexin-A4 RBD)]. The structures and mutagenesis explained why plexin-C1 does not bind RhoGTPases and why plexin-D1 has a preference for Rnd2, rather than for Rnd1 binding. Structures of plexin-A2 and -B1 RBDs were also solved in complex with Rnd1 GTPase The binding region—the PRAMs deduced first with the help of sequence comparison—was slightly refined for interactions with Rnd1 to include a region near the dimerization loop [324]. The binding region is now understood to the extent of being able to make mutations in plexin-C1 and -D1 that generated good binding affinity for Rnd1 in these domains (the wild type domains do not appreciably bind this GTPase). However, the structural changes that are introduced by GTPase binding to the RBD conformation are small and did not immediately suggest a mechanism at the residue level how GTPase binding would destabilize RBD dimers [321, 324]. The absence of large conformational changes in crystal structures, also recently of plexin-A1 in complex with Rac1 were interpreted as evidence for plexin’s role in RhoGTPase sequestration [341]. However, the recent plexin-B1:Rac1 crystal structure shows a more active role of the GTPases upon binding to plexin in that Rac1 destabilizes the intracellular region in an active structure [334] (see below). An NMR study on changes in protein dynamics upon RBD:Rac1 complex formation pointed to changes in flexibility in a region of the Rac1 structure that is far from the RBD binding site [435]. This suggests that such allosteric changes may be rather subtle, and occur at the level of protein flexibility rather than structure [435, 436]. As mentioned above, the specificity of the RBDs for binding to certain Rho GTPases is not yet clear; for example, Rnd1, Rac1 and RhoD belong to distant branches of the Rho GTPase family; why do these GTPases bind and not others? Contrary to results from crystallography, work by solution NMR on the complex of plexin-B1 with Rac1 suggests that the mode of association can be considerably different to Rnd1 binding. The thermodynamics [323] and very likely also the dynamics of RBD:Rac1 and RBD:Rnd1 complex formation are substantially different, a feature that could be exploited in the context of the entire plexin intracellular region and its presence near the plasma membrane.
RBDs and possible activation/inhibition mechanism in context of the entire receptor
Rho GTPase binding and the RBD monomer–dimer equilibrium needs to be considered at the level of the entire intracellular, if not the entire receptor protein. Here two structures published around the same time, considerably advanced the field: The entire intracellular region of plexin-B1 solved by the Buck/Park collaboration [335] and the entire intracellular region plexin-A3 determined by Zhang and colleagues [437] showed nearly identical structures (rmsd of ~ 1Å). Both crystals are of the apoprotein, i.e., without GTPases bound, and both presented monomeric forms, thus questioning the RBD monomer–dimer transition model. However, surprisingly in both structures a well-conserved loop is seen that connects the RBD to the C-terminal GAP homologous region (named coupling loop [335]). This coupling loop is positioned exactly in the same region that would be occupied by the dimerization loop of the second RBD in a dimeric form. The coupling loop is, therefore, an intramolecular element that helps to shift the equilibrium over to the monomeric/active form (Fig. 4a.i-iii). Molecular modeling suggested that a dimeric form of the intracellular region of plexin would not allow Rho GTPase binding, as there would be large clashes between the GTPase and the second Ras GAP homologous protein (Fig. 4a.ii) [335]. Also mutagenesis and functional data support the RBD dimer–monomer model for activation, albeit in an expanded form that includes a pre-stabilized monomeric state. However, such a model may not be generalizable to all plexins, since plexin-C1 and -D1 RBDs are monomeric in solution [324, 335].
Recently, Siebold and coworkers determined the structure of the intracellular region of plexin-B1 bound to Rac1 [334]. Two forms were observed; a monomeric form of an N-terminally truncated plexin and a trimeric form of the full length intracellular plexin. Both forms have the coupling loop in place, preventing RBD dimerization, in presence of bound GTPase. The structures suggest how the plexin intracellular region may contribute to another mode of receptor association. In the complete receptor, dimers may already be preformed due to the transmembrane and/or the extracellular region, but additional clustering could be required for bivalent binding of semaphorin ligands (see "Outside" above). Both the trimeric structure of the intracellular region that is seen in the crystal and the possibility of association of the intracellular regions via a coiled coil trimer formation of the juxtamembrane region would generate a lattice of receptors. This is consistent with the observation that a higher-order clustering is required (in presence of Rnd1) for full receptor activity [332, 336]. Binding of the Rnd1 homologous GTPase, Rac1, as observed in the crystal structure, appears to stabilize the trimeric form of plexin-B1in two ways: firstly, by bridging contacts between the intracellular regions and secondly, by potentially disrupting interactions between the juxtamembrane region and its binding site on the intracellular region (Fig. 4b), [334]. The freed-up juxtamembrane region can then form a coiled–coiled trimer, leading to the formation of a receptor lattice at the membrane. A number of issues await further study.For example, while the juxtamembrane region of plexin-B1 is predicted to form a coiled-coil trimer [334], trimers are less strongly predicted for plexin-B2 and -B3. For plexin-A1-4, -C1 and -D1 dimers or even monomers are suggested to be the most stable species. Thus, a trimeric association may not be the active form for all plexins. Although mutations could be made which disrupt plexin function, it is not yet clear how important direct plexin : GTPase interactions are at the second binding side. The proposed mechanism considers the effects of Rnd1 and Rac1 binding to be equal, whereas it is not clear whether both similarly activate the plexin-B1 receptor in physiological settings. Interestingly, for the plexin-A family, a sequential mechanism has been proposed [326, 327] (see section “Involvement of plexin family members with Rho and Ras small GTPases”), which may in part also be applicable to the plexin-B family.
Mechanism for activating the GAP region
The exact mechanism of plexin inhibition and activation on the intracellular side is not yet clear, although a number of proposals have been made. Zhang and colleagues noticed that several helices which line the Ras binding pocket are slightly tilted inwards in the plexin-A3 as well as in the plexin-B1 crystal structure [437]. A failure to dock a Ras GTPase into the structure led to the suggestion that these structures represent an autoinhibited form of the protein. By contrast Buck and coworkers docked the GTP bound state of R-Ras (homology modeled based on H-Ras.GTP bound to p120 Ras GAP; pdb id 1WQ1) into the plexin-B1 GAP domain structure [335](Fig. 4b). The binding mode and model was predictive in that is showed that H-Ras itself could not bind well [438]. Although the conformation of the intracellular plexin region in the crystal structure and in solution may differ, a reasonable binding affinity was observed for R-Ras, but not for H-Ras by isothermal titration calorimetry [335]. Nevertheless, the proposal that the structure of plexin is partly inhibited has merit as recent studies with mutants in the coupling loop region show that the residue changes that disrupt the coupling loop-RBD-dimerization loop interactions improve the binding affinity of Ras GTPases [438]. The R-Ras GAP function of plexins has been well established by the Negishi group [322, 332, 342, 343, 388] and by other laboratories [162, 165, 326, 333, 334] in cells and in one report using cell lysates [336]. At the same time it has been very difficult to detect GAP function against R-Ras GTPases in vitro using expressed and purified proteins. This suggests that we do not yet understand the mechanism of plexin activation, except that Rnd1 binding and some manner of receptor clustering are required [332, 336]. Crystal structures of the intracellular plexin region are not yet available with Ras GTPase bound, but the structures of the apoprotein indicate that the conformational changes may be rather subtle [335]. Since both Rho and Ras proteins are membrane bound it is likely that coordination of the proteins at the lipid bilayer is an important factor for Ras-GAP activation.
Very recently, (as mentioned in “Involvement of plexin family members with Rho and Ras small GTPases” above) the group of Xuewu Zhang reported that all plexin’s have a GAP activity towards Rap GTPases [341]. From a structural perspective, several GAPs are known that stimulate GTP hydrolysis in both Ras and Rap GTPases and that the activity towards these two substrates can be regulated in different ways [346]. Plexins likely belong to this family of dual specificity GTPases. In the case of plexin’s Rap GAP function, activity appears to be stimulated by dimerization of the intracellular region. This requirement was explored by addition of a protein that dimerizes in a ligand-dependent manner and further by addition of coiled-coils with different linker lengths [341]. Intriguingly, different plexins needed these stabilizers to a different extent. The results are not entirely consistent with the prediction for coiled coil formation in the juxtamembrane helix region of plexins or our current knowledge on the association of the intracellular domains through the RBD [324] or other regions [437]. As mentioned above, the detailed mechanism for activation is not clear from the presently reported structures, also in case of plexin’s Rap GAP activity. Experimental structures of R-Ras, Rap proteins bound to the intracellular region of plexins are needed to shed light on the GAP activation mechanisms at atomic detail.
Apart from the mechanism of GAP activation, structures that show the association with other key proteins would be informative in order to understand the other functions of the plexin receptor. For example, p190 RhoGAP, PDZ-RhoGEF and the ErbB2 as well as Met intracellular regions can be associated with plexin, either directly and/or through adaptor proteins. Regulation of such interactions is likely; for example the juxtamembrane helix region appears to occlude the binding site for PLC [165, 231]. Another interaction of plexin-B1 (and possibly other B-family plexins) is with the PDZ domain of PDZ-RhoGEF and homologous GEF, LARG; Here the PDZ domains interact with the very C-terminal PDZ binding motif in plexin-B1 [337, 350]. The region terminates in a helix in the plexin-B1 crystal structures [334, 335] (though the last six residues are missing) and may be partially occluded from PDZ binding; the structure of the PDZ-RhoGEF PDZ domain bound a peptide encompassing the last eight residues of plexin-B1 has been determined by solution NMR [439] and shows that the PDZ recognition region binds in an extended conformation that is incompatible with a helical conformation.
Unanswered questions
This section outlines a few of the current questions concerning plexin signaling that are of particular interest and whose investigation are likely to provide new significant insights in the future.
What is the precise role and molecular mechanism of dimerization/oligomer formation, clustering and internalization? As discuss in “Structural biology” above, changes in the association within and between plexin receptors are a key to the signaling mechanism. Increasingly well-defined models and mechanisms are emerging from the structures and from biophysical measurements. Comparisons with other systems, such as the EGF receptor [440], integrins [441] and receptor tyrosine phosphatases [442] suggest similarities, but also significant differences. While plexins oligomerize and cluster together, their GAP region needs to be accessible at the domain level [335]. This is most easily achieved by a mechanism that locally disrupts an autoinhibited dimer of the intracellular region, whether this is formed by the RBD or another domain. Formation of dimers and oligomers are known to be involved on the extracellular side, as discussed. The geometry of these assemblies is likely to be critical—for instance, clustering of plexins with antibodies or with ligand appears to be required to activate the receptor in cells. However, clustering on the outside does not necessarily translate into protein–protein interactions in the cell’s cytoplasm. In fact the ligands/antibodies, could in principle dissociate inhibited forms by providing a certain spacing between plexins. Thus, the structure of complexes with known and yet to be characterized binding partners will be extremely informative. For instance it is likely that at least some of the time receptors such as plexin are anchored to the cellular cytoskeleton (actin filaments and microtubules) [443]. Scaffold proteins and a pre-organization of protein complexes are emerging as an important theme in cell signaling and are certainly relevant to plexin-mediated signaling [444].
Control of specific protease cleavage of ligands and of receptor extra- and possibly also of intra-cellular regions adds another level of regulation [430, 445], allowing the possibility of cis- as well as trans-interactions [72, 431, 446]. Receptors or receptor fragments may also migrate to other cell locales [447, 448] and receptor localization/trafficking itself plays a critical role in cell guidance and migration [449–451]. Clustering in lipid domain rafts [452–454] or with other partner proteins that reside there (e.g., upon binding to Rho- and Ras GTPases, which themselves are targeted and sensitive to their environment [455]) are key processes, as is receptor internalization [447]. For instance Rac1 is involved in endocytosis during Ephrin-A2- and Sema3A-induced growth cone collapse [456]. Sema3C induces Nrp1 internalization through lipid-rafts [457]. In the Eph receptor system, which in part has a similar function to plexins, receptors are internalized readily upon ligand binding, and it is possible that the receptors continue to signal in this state [66], even are translocated for functions in the nucleus [448, 458]. Furthermore, also following internalization, receptors are typically polyubiquitinated and degraded (e.g., see [458–460] for Met receptor dynamics). The details of such mechanisms and to what extent they contribute to the regulation of plexin function still need to be explored. Protein transport in vesicular form plays a role in axonal growth and guidance [461, 462], although protein messengers can also be stored at growth cone locations and translated in a regulated fashion [463, 464]. Recently, it has been shown that a guidance receptor, DCC, interacts directly with the ribosome to stimulate the local translation of such proteins [465]. Whether plexins or its co-receptors behave in a similar manner remains to be determined.
What is the extent of GTPase-, phosphorylation- and other cross-signaling? Small Rho and also Ras GTPases are known to cross-signal in several cell signaling networks, often opposing and sometimes enhancing each other’s function [466, 467]. This is true for the direct signaling function of RhoA, Rac1, R-Ras and Rap1 [455, 468, 469]. In the plexin system also regulatory GEF and GAP proteins and receptor binding proteins are implicated, e.g., LARG/RhoGEF and p190 RhoGAP have opposite effects on RhoA activity. These and possible feed-forward/feedback regulatory mechanisms of Ras, RhoA as well as AKT (and other phosphorylation based events), are just beginning to be understood in plexin signaling. It is likely that multiple pathways oppose one another and cooperate in cross-signaling in a time-dependent manner [470–472]. The small GTPase Rac1 is part of the system that generates reactive oxygen species (see below) and small GTPases are sensitive to posttranslational modifications, such as tyrosine phosphorylation of R-Ras and Rnd1 [473, 474]. It is apparent that there are well-defined perimeters of signaling, e.g., ligand to receptor to small GTPase regulatory protein, tyrosine kinase to serine/threonine kinase, as signaling is propagated from transmembrane receptors. Another view holds that these levels are intermixed—the function of the plexin receptor is affected by both tyrosine and serine/threonine phosphorylation [234, 333]. In another example, PLCγ is an adaptor protein for binding PDZ-RhoGEF to plexin-B family members [165], but Rac1, another plexin binder, also participates in PLCγ function [475]; the lipid secondary messengers that are generated by PLCγ and other enzymes may result in changes of receptor localization and/or activity [476–478]. Already several such cross-signaling mechanisms are apparent in plexin signaling, for example with integrins [330] and possibly with TGF-beta [479, 480], a major cytokine associated with many tumors. Cross signaling is likely to be essential for the generation of an orchestrated response to several guidance and cell adhesion/motility signals, and one that results in a coordinated cellular behavior. Structural biology and protein in vitro biophysics will deliver some of the parameters, which then together with imaging studies (measuring protein concentration/localization and activity in cells) can be used to develop system biology-level models of cell migration.
Secondary messengers
RNS/ROS, Ca 2+ , cyclic nucleotides—are they directly involved in plexin signaling? Regulation of cellular signaling by reactive nitrogen (RNS) and oxygen species (ROS) has emerged as a key mechanism for many systems [481, 482]. These species are created especially during cell starvation (hypoxia/ischemia) and during reperfusion; thus they are not only of importance for regular cell signaling but also in cell recovery and tissue regeneration involving Sema3A and -3E e.g., [102, 121]. Plexin co-receptor Met is known to be associated with ROS generation following ligand binding, but is also implicated in cross communication with EGFR and G-protein coupled receptors [483]. These events in turn result in the downstream stimulation of Rac1 GTPase [484]. Similarly VEGF is known to stimulate endothelial Nitrogen oxygenase synthase (eNos) activation in a protein kinase A-dependent manner [485], but has also been reported to generate ROS through Rac1 upregulation [486]. Rac1 is a part of the NADPH oxidase complex that generates H2O2 and plays a role in angiogenesis and also pro-metastatic signaling [487]. ROS are directly involved in regulating Rho activity (and thus the cytoskeleton and cell migration) via inhibition of a tyrosine phosphatase, increasing the phosphorylation and activity of p190 RhoGAP [488], one of the players in plexin signal transduction. Furthermore, a number of members of the plexin signaling network, such as Sema4D and Neuropilin-2 are transcriptionally regulated by the hypoxia-inducible factor-1 (HIF-1) [489, 490]. Intriguingly, Neuropilin-1 (but not -2) is quickly lost during hypoxia and nutrient starvation in cells [491]. A direct effect is also likely since the plexin-A family binds and activates MICAL, a ROS generating complex that is required to be active for these plexins to function in many settings [399, 492] (see sections on "Intracellular signaling networks": above) and regulates actin dynamics through direct oxidation of actin [402]. In addition to actin, ROS/NOS can post-translationally modify and affect the activity of proteins, such as Ras and Rho GTPases [493, 494]—it will be important to establish whether plexins or their associated partner proteins are directly modified by ROS/RNS. One modification that is catalyzed by H2O2 is the oxidation of protein thiol groups to form disulphides. Although the cytoplasm is usually a reducing environment for cysteines, generation of H2O2 can locally lead to the formation of cross-linked protein domains that may have regulatory purposes [495, 496]. While this feature has not been demonstrated for plexin function, we found that cysteine-mediated dimer formation is facile (e.g., a concentration of 20 μM H2O2 is sufficient) for the RBD of plexin-B1, through either one of its cysteines (Cys 1776 is highly conserved in plexins, except for plexin-B3 and -D1) [497]. Again, it will be interesting to see whether disulphide bond breakage or formation plays a functional role in the intra or extracellular domains of plexin system.
Ca2+and cyclic nucleotides
Changing in the intracellular level of calcium (Ca2+) is a key mechanism to signal to cytoplasmic proteins [498], including in cardiovascular and neuronal cells. In neuronal cells guidance of axonal growth cones as well as activity-dependent growth and patterning of dendritic cells is in part determined by intra and extra-cellular calcium levels. Several reviews have been published on the topic: e.g., [499–502]. Recently, it has become clear that Sema3A induces a Ca2+ channel-dependent conversion of axons to dendrites [503]. The experiments involved Ca2+ channel blockers and calmodulin inhibitors. Calmodulin has a potentially a special role as it can integrate Ca2+ and Ras signaling [504], in part by stimulating several kinases, which in turn activate exchange factors, including PDZ-RhoGEF for RhoA [505, 506]. Another protein stimulated by calmodulin is adenylyl cyclase. The cyclic nucleotide, cAMP, generated by this enzyme induces changes in the growth cone direction [507] and stimulates the exchange factor EPAC and the Ras-related proteins, Rap1 and Rap2. These GTPases in turn stimulate long-term potentiation (LTP/learning- memory formation) via the ERK/MAPK-, but also via the PKA pathways. The latter pathway is required for Sema3A stimulated collapse of parasympathetic neurons. Recently, it was reported that Sema3A regulates neuronal polarization by suppressing axon formation and promotion of dendrite grown, an effect mediated by the Sema3A-induced elevation of cytoplasmic cGMP and PDE activity, which in turn caused the reduction of cAMP/PKA activities [385]. In Drosophila, the scaffolding protein (AKAP) Nervy links plexin-A to cAMP and to PKA [392]. It is clear that signals are integrated, for example, cyclic GMP-gated CNF channels function in Sema3A-induced growth cone repulsion and collapse, indicating that plexin-mediated signals can feed back to potentiate Ca2+ cell entry [508] and regulate polarization of the cell membrane [509]. Indeed, it has been proposed that Sema3A contains a sequence similar to a known Ca2+ channel blocker [510]. It is intriguing that early descriptions of plexin signaling were in terms of Ca2+ and cyclic nucleotide sensitivity [25, 511]. Apart from the connection via binding to the PKA-associated protein nervy/MTG (see section “Adaptor and scaffolding proteins”), recently a PKA-induced phosphorylation of Drosophila plexin-A and downregulation of its GAP function has been reported, thus making a connection between cyclic nucleotides and Ras signaling [333]. While the cell signaling pathways discovered so far suggest involvement of Ca2+ or nucleotides up-steam and/or down-stream, a more direct interaction has not yet been excluded as a contributing factor. Further structural and biophysical studies are needed to shed light on this and the other issues raised.
Concluding perspective
The dramatic progress in plexin structural biology over the last several years opens doors to interdisciplinary and multi-level characterization of the receptor, its function and cell signaling mechanisms. Further structures of plexins, especially on the intracellular side in complex with binding partners and GAP substrate proteins are needed. The structures typically only provide snap-shots of the behavior of the protein and are starting points for many investigations. For example, biophysical/biochemical and mutagenesis/functional studies are already required to validate aspects of the structures (e.g., which residue–residue interactions are the most important) and to suggest hypothesis for further investigations at multiple levels. In the foreseeable future, computational tools and experiments will go hand in hand to examine protein dynamics, especially in and near membranes, where traditional structural methods are particularly challenged. Allosteric fluctuations within plexins and their complexes as well as protein processing and localization in cells will also play an important role. At the protein residue level, the insights will aid the understanding of pathological mutations and posttranslational modifications in plexins (e.g., cancer-associated changes). Although plexins are known to be involved in many cell signaling pathways, it is not yet clear which are the most important in certain settings and how these pathways cooperate as networks to effect responses at the cellular level. Together with the molecular level knowledge, these insights are needed to in order to design agents to combat numerous diseases that involve the receptor, or help in neuronal/cardiovascular regeneration. At the super-molecular level, further cell-biological, cell-imaging as well as “-omics”-derived information will be required to model the behavior of signaling networks, also involving plexin in a time-dependent manner. This modeling ultimately must encompass the entire spectrum of “the life of plexins”, from transcription to degradation. Much remains to be done!
Acknowledgments
We apologize to investigators whose important work we omitted to cite due to space restraints and want to thank several colleagues and many members of the plexin/sema/neuropilin community. We are especially grateful to John Basile, Alain Chedotal, Jonathan Duke-Cohen, Silvio Gutkind, Michael Klagsbrun, Alex Kolodkin, Fanny Mann, Hee-Won Park, Andreas Püschel, Jakub Swiercz, Junichi Takagi, Luca Tamagnone, Jonathan Terman, Yufeng Tong and Thomas Worzfeld for their comments on parts of this review, as well as to an anonymous reviewer and several members of the Buck lab., particularly Susmita Borthakur and SoonJeung Kim for their help. Taeku Kim assisted with references. This project is supported by NIH Grant GM073071.
References
- 1.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 2.The Semaphorin Nomenclature Committee Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- 3.Yu HH, Kolodkin AL. Semaphorin signaling: a little less per-plexin. Neuron. 1999;22:11–14. doi: 10.1016/s0896-6273(00)80672-8. [DOI] [PubMed] [Google Scholar]
- 4.Gavert N, Ben-Shmuel A, Raveh S, Ben-Ze’ev A. L1-CAM in cancerous tissues. Expert Opin Biol Ther. 2008;8:1749–1757. doi: 10.1517/14712598.8.11.1749. [DOI] [PubMed] [Google Scholar]
- 5.Schäfer MK, Frotscher M. Role of L1CAM for axon sprouting and branching. Cell Tissue Res. 2012 Feb 28. [Epub ahead of print] [DOI] [PubMed]
- 6.Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 7.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 9.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usui H, Taniguchi M, Yokomizo T, Shimizu T. Plexin-A1 and plexin-B1 specifically interact at their cytoplasmic domains. Biochem Biophys Res Commun. 2003;300:927–931. doi: 10.1016/s0006-291x(02)02966-2. [DOI] [PubMed] [Google Scholar]
- 11.Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annu Rev Cell Dev Biol. 2010;26:639–665. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- 12.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 13.Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol. 2007;213:316–325. doi: 10.1002/jcp.21183. [DOI] [PubMed] [Google Scholar]
- 14.Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Perälä NM, Immonen T, Sariola H. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5:355–362. doi: 10.1016/j.modgep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.van der Zwaag B, Hellemons AJ, Leenders WP, Burbach JP, Brunner HG, Padberg GW, Van Bokhoven H. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- 17.Roodink I, Verrijp K, Raats J, Leenders WP. Plexin D1 is ubiquitously expressed on tumor vessels and tumor cells in solid malignancies. BMC Cancer. 2009;9:297. doi: 10.1186/1471-2407-9-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worzfeld T, Püschel AW, Offermanns S, Kuner R. Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development. Eur J Neurosci. 2004;19:2622–2632. doi: 10.1111/j.0953-816X.2004.03401.x. [DOI] [PubMed] [Google Scholar]
- 19.Deng S, Hirschberg A, Worzfeld T, Penachioni JY, Korostylev A, Swiercz JM, Vodrazka P, Mauti O, Stoeckli ET, Tamagnone L, Offermanns S, Kuner R. Plexin-B2, but not Plexin-B1, critically modulates neuronal migration and patterning of the developing nervous system in vivo. J Neurosci. 2007;27:6333–6347. doi: 10.1523/JNEUROSCI.5381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zielonka M, Xia J, Friedel RH, Offermanns S, Worzfeld T. A systematic expression analysis implicates Plexin-B2 and its ligand Sema4C in the regulation of the vascular and endocrine system. Exp Cell Res. 2010;316:2477–2486. doi: 10.1016/j.yexcr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Pasterkamp RJ, Kolk SM, Hellemons AJ, Kolodkin AL. Expression patterns of Semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol. 2007;7:98. doi: 10.1186/1471-213X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauti O, Sadhu R, Gemayel J, Gesemann M, Stoeckli ET. Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev Biol. 2006;6:32. doi: 10.1186/1471-213X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 24.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, Tanaka H, Fujisawa H. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14:1189–1199. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 26.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 27.Perälä N, Sariola H, Immonen T. More than nervous: the emerging roles of plexins. Differentiation. 2012;83:77–91. doi: 10.1016/j.diff.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 29.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Derijck AA, Van Erp S, Pasterkamp RJ. Semaphorin signaling: molecular switches at the midline. Trends Cell Biol. 2010;20:568–576. doi: 10.1016/j.tcb.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Chédotal A. Further tales of the midline. Curr Opin Neurobiol. 2011;21:68–75. doi: 10.1016/j.conb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3. (pii: a001727) [DOI] [PMC free article] [PubMed]
- 33.Waimey KE, Cheng HJ. Axon pruning and synaptic development: how are they per-plexin? Neuroscientist. 2006;12:398–409. doi: 10.1177/1073858406292631. [DOI] [PubMed] [Google Scholar]
- 34.Bilimoria PM, Bonni A (2011) Molecular regulation of axon branching. Neuroscientist. Dec 15. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 35.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59:24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- 38.Gay CM, Zygmunt T, Torres-Vázquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev Biol. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waimey KE, Huang PH, Chen M, Cheng HJ. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315:448–458. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renaud J, Kerjan G, Sumita I, Zagar Y, Georget V, Kim D, Fouquet C, Suda K, Sanbo M, Suto F, Ackerman SL, Mitchell KJ, Fujisawa H, Chédotal A. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci. 2008;11:440–449. doi: 10.1038/nn2064. [DOI] [PubMed] [Google Scholar]
- 44.Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25:3628–3637. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 46.Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 48.Rohm B, Ottemeyer A, Lohrum M, Püschel AW. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2010;93:95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Zvi A, Manor O, Schachner M, Yaron A, Tessier-Lavigne M, Behar O. The Semaphorin receptor PlexinA3 mediates neuronal apoptosis during dorsal root ganglia development. J Neurosci. 2008;28:12427–12432. doi: 10.1523/JNEUROSCI.3573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45:513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Vodrazka P, Korostylev A, Hirschberg A, Swiercz JM, Worzfeld T, Deng S, Fazzari P, Tamagnone L, Offermanns S, Kuner R. The semaphorin 4D-plexin-B signalling complex regulates dendritic and axonal complexity in developing neurons via diverse pathways. Eur J Neurosci. 2009;30:1193–1208. doi: 10.1111/j.1460-9568.2009.06934.x. [DOI] [PubMed] [Google Scholar]
- 52.Hirschberg A, Deng S, Korostylev A, Paldy E, Costa MR, Worzfeld T, Vodrazka P, Wizenmann A, Götz M, Offermanns S, Kuner R. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30:764–780. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedel RH, Kerjan G, Rayburn H, Schüller U, Sotelo C, Tessier-Lavigne M, Chédotal A. Plexin-B2 controls the development of cerebellar granule cells. J Neurosci. 2007;27:3921–3932. doi: 10.1523/JNEUROSCI.4710-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worzfeld T, Rauch P, Karram K, Trotter J, Kuner R, Offermanns S. Mice lacking Plexin-B3 display normal CNS morphology and behaviour. Mol Cell Neurosci. 2009;42:372–381. doi: 10.1016/j.mcn.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. J Biol Chem. 2010;285:32436–32445. doi: 10.1074/jbc.M110.120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Law JW, Lee AY. (2011) Semaphorin 5A and plexin-B3 regulate human glioma cell motility and morphology through Rac1 and the actin cytoskeleton. Oncogene. Jun 27. doi: 10.1038/onc.2011.256 [DOI] [PubMed]
- 58.Hartwig C, Veske A, Krejcova S, Rosenberger G, Finckh U. Plexin B3 promotes neurite outgrowth, interacts homophilically, and interacts with Rin. BMC Neurosci. 2005;6:53. doi: 10.1186/1471-2202-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase-activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep. 2009;10:614–621. doi: 10.1038/embor.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu C, Fan CM. Allocation of paraventricular and supraoptic neurons requires Sim1 function: a role for a Sim1 downstream gene PlexinC1. Mol Endocrinol. 2007;21:1234–1245. doi: 10.1210/me.2007-0034. [DOI] [PubMed] [Google Scholar]
- 61.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66(2):205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Ding JB, Oh WJ, Sabatini BL, Gu C (2011) Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat Neurosci. Dec 18. doi: 10.1038/nn.3003. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 65.Comoglio PM, Tamagnone L, Giordano S. Invasive growth: a two-way street for semaphorin signalling. Nat Cell Biol. 2004;6:1155–1157. doi: 10.1038/ncb1204-1155. [DOI] [PubMed] [Google Scholar]
- 66.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Schultze W, Eulenburg V, Lessmann V, Herrmann L, Dittmar T, Gundelfinger ED, Heumann R, Erdmann KS. Semaphorin4F interacts with the synapse-associated protein SAP90/PSD-95. J Neurochem. 2001;78:482–489. doi: 10.1046/j.1471-4159.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 68.Burkhardt C, Müller M, Badde A, Garner CC, Gundelfinger ED, Püschel AW. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS Lett. 2005;579:3821–3828. doi: 10.1016/j.febslet.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 69.Wang LH, Kalb RG, Strittmatter SM. A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem. 1999;274:14137–14146. doi: 10.1074/jbc.274.20.14137. [DOI] [PubMed] [Google Scholar]
- 70.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 72.Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Hernández-Miranda LR, Cariboni A, Faux C, Ruhrberg C, Cho JH, Cloutier JF, Eickholt BJ, Parnavelas JG, Andrews WD. Robo1 regulates semaphorin signaling to guide the migration of cortical interneurons through the ventral forebrain. J Neurosci. 2011;31:6174–6187. doi: 10.1523/JNEUROSCI.5464-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: implication for neurological diseases. Prog Neurobiol. 2007;82:57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Yaron A, Zheng B. Navigating their way to the clinic: emerging roles for axon guidance molecules in neurological disorders and injury. Dev Neurobiol. 2007;67:1216–1231. doi: 10.1002/dneu.20512. [DOI] [PubMed] [Google Scholar]
- 77.Good PF, Alapat D, Hsu A, Chu C, Perl D, Wen X, Burstein DE, Kohtz DS. A role for semaphorin 3A signaling in the degeneration of hippocampal neurons during Alzheimer’s disease. J Neurochem. 2004;91:716–736. doi: 10.1111/j.1471-4159.2004.02766.x. [DOI] [PubMed] [Google Scholar]
- 78.Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 79.Mah S, Nelson MR, Delisi LE, Reneland RH, Markward N, James MR, Nyholt DR, Hayward N, Handoko H, Mowry B, Kammerer S, Braun A. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Mol Psychiatry. 2006;11:471–478. doi: 10.1038/sj.mp.4001785. [DOI] [PubMed] [Google Scholar]
- 80.Wray NR, James MR, Mah SP, Nelson M, Andrews G, Sullivan PF, Montgomery GW, Birley AJ, Braun A, Martin NG. Anxiety and comorbid measures associated with PLXNA2. Arch Gen Psychiatry. 2007;64:318–326. doi: 10.1001/archpsyc.64.3.318. [DOI] [PubMed] [Google Scholar]
- 81.Fujii T, Uchiyama H, Yamamoto N, Hori H, Tatsumi M, Ishikawa M, Arima K, Higuchi T, Kunugi H. Possible association of the semaphorin 3D gene (SEMA3D) with schizophrenia. J Psychiatr Res. 2011;45:47–53. doi: 10.1016/j.jpsychires.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Rünker AE, O’Tuathaigh C, Dunleavy M, Morris DW, Little GE, Corvin AP, Gill M, Henshall DC, Waddington JL, Mitchell KJ. Mutation of Semaphorin-6A disrupts limbic and cortical connectivity and models neurodevelopmental psychopathology. PLoS ONE. 2011;6:e26488. doi: 10.1371/journal.pone.0026488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rujescu D, Meisenzahl EM, Krejcova S, Giegling I, Zetzsche T, Reiser M, Born CM, Möller HJ, Veske A, Gal A, Finckh U. Plexin B3 is genetically associated with verbal performance and white matter volume in human brain. Mol Psychiatry. 2007;12:190–194. doi: 10.1038/sj.mp.4001903. [DOI] [PubMed] [Google Scholar]
- 84.Lalani SR, Safiullah AM, Molinari LM, Fernbach SD, Martin DM, Belmont JW. SEMA3E mutation in a patient with CHARGE syndrome. J Med Genet. 2004;41:e94. doi: 10.1136/jmg.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarimon J, Scholz S, Fung HC, Hardy J, Eerola J, Hellstrom O, Chen CM, Wu YR, Tienari PJ, Singleton A. Conflicting results regarding the semaphorin gene (SEMA5A) and the risk for Parkinson disease. Am J Hum Genet. 2006;78:1082–1084. doi: 10.1086/504727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gant JC, Thibault O, Blalock EM, Yang J, Bachstetter A, Kotick J, Schauwecker PE, Hauser KF, Smith GM, Mervis R, Li Y, Barnes GN. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–645. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Formolo CA, Williams R, Gordish-Dressman H, MacDonald TJ, Lee NH, Hathout Y. Secretome signature of invasive glioblastoma multiforme. J Proteome Res. 2011;10:3149–3159. doi: 10.1021/pr200210w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Binmadi NO, Yang YH, Zhou H, Proia P, Lin YL, Batista De Paula AM, Sena Guimarães AL, Poswar FO, Sundararajan D, Basile JR (2012) Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. Am J Pathol. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Retracted]
- 89.de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71:249–267. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 91.Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361:1499–1511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCormick AM, Leipzig ND (2012) Neural regenerative strategies incorporating biomolecular axon guidance signals. Ann Biomed Eng. [Epub ahead of print] [DOI] [PubMed]
- 94.Syed YA, Hand E, Möbius W, Zhao C, Hofer M, Nave KA, Kotter MR. Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci. 2011;31:3719–3728. doi: 10.1523/JNEUROSCI.4930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Winter F, Holtmaat AJ, Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv Exp Med Biol. 2002;515:115–139. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- 96.Shifman MI, Selzer ME. Differential expression of class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration. J Comp Neurol. 2007;501:631–646. doi: 10.1002/cne.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chédotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams A, Piaton G, Aigrot MS, Belhadi A, Théaudin M, Petermann F, Thomas JL, Zalc B, Lubetzki C. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain. 2007;130:2554–2565. doi: 10.1093/brain/awm202. [DOI] [PubMed] [Google Scholar]
- 99.Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease–can we wrap it up? Brain. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 100.Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett. 2011;585:3829–3835. doi: 10.1016/j.febslet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 101.Segura I, de Smet F, Hohensinner PJ, Ruiz de Almodovar C, Carmeliet P. The neurovascular link in health and disease: an update. Trends Mol Med. 2009;15:439–451. doi: 10.1016/j.molmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honoré JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beauséjour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bannerman P, Ara J, Hahn A, Hong L, McCauley E, Friesen K, Pleasure D. Peripheral nerve regeneration is delayed in neuropilin 2-deficient mice. J Neurosci Res. 2008;86:3163–3169. doi: 10.1002/jnr.21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JK, Chow R, Xie F, Chow SY, Tolentino KE, Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J Neurosci. 2010;30:10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams G, Eickholt BJ, Maison P, Prinjha R, Walsh FS, Doherty P. A complementary peptide approach applied to the design of novel semaphorin/neuropilin antagonists. J Neurochem. 2005;92:1180–1190. doi: 10.1111/j.1471-4159.2004.02950.x. [DOI] [PubMed] [Google Scholar]
- 106.Montolio M, Messeguer J, Masip I, Guijarro P, Gavin R, Antonio Del Río J, Messeguer A, Soriano E. A semaphorin 3A inhibitor blocks axonal chemorepulsion and enhances axon regeneration. Chem Biol. 2009;16:691–701. doi: 10.1016/j.chembiol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Long JB, Jay SM, Segal SS, Madri JA. VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol. 2009;334:119–132. doi: 10.1016/j.ydbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Autiero M, Smet FD, Claes F, Carmeliet P. Role of neural guidance signals in blood vessel navigation. Cardiovasc Res. 2005;65:629–638. doi: 10.1016/j.cardiores.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 110.Carmeliet P, Tessier-Lavigene M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 111.Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113:607–626. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- 112.Gelfand MV, Hong SGuC. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trend Cell Biol. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 114.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gitler AD, Lu MM, Epstein JA. PlexinD1 and Semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- 117.Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci USA. 2009;106:13933–13938. doi: 10.1073/pnas.0904744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldmuntz E, Emanuel BS. Genetic disorders of cardiac morphogenesis. The DiGeorge and velocardiofacial syndromes. Circ Res. 1997;80:437–443. doi: 10.1161/01.res.80.4.437. [DOI] [PubMed] [Google Scholar]
- 119.Kochilas L, Merscher-Gomez S, Lu MM, Potluri V, Liao J, Kucherlapati R, Morrow B, Epstein JA. The role of neural crest during cardiac development in a mouse model of DiGeorge syndrome. Dev Biol. 2002;251:157–166. doi: 10.1006/dbio.2002.0819. [DOI] [PubMed] [Google Scholar]
- 120.Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, Kumanogoh A, Kikutani H. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321:251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 121.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toyofuku T, Kikutani H. Semaphorins: receptor and intracellular signaling mechanisms, Chapter 9, Semaphorin signaling during cardiac development. Adv Exp Med Biol. 2007;600:109–117. doi: 10.1007/978-0-387-70956-7_9. [DOI] [PubMed] [Google Scholar]
- 123.Herzog Y, Guttmann-Raviv N, Neufeld G. Segregation of arterial and venous markers in subpopulations of blood islands before vessel formation. Dev Dyn. 2005;232:1047–1055. doi: 10.1002/dvdy.20257. [DOI] [PubMed] [Google Scholar]
- 124.Lamont RE, Lamont EJ, Childs SJ. Antagonistic interactions among plexins regulate the timing of intersegmental vessel formation. Dev Biol. 2009;331:199–209. doi: 10.1016/j.ydbio.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 125.Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, PlexinA1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204–1211. doi: 10.1038/ncb1193. [DOI] [PubMed] [Google Scholar]
- 127.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 128.Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Pham VN, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-Plexin signaling guides pattering of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 129.Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vázquez J. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang SX, Sheldrick M, Desbois A, Slinn J, Hou ST. Neuropilin-1 is a direct target of the transcription factor E2F1 during cerebral ischemia-induced neuronal death in vivo. Mol Cell Biol. 2007;27:1696–1705. doi: 10.1128/MCB.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whitehead SN, Gangaraju S, Slinn J, Hou ST. Transient and bilateral increase in Neuropilin-1, Fer kinase and collapsin response mediator proteins within membrane rafts following unilateral occlusion of the middle cerebral artery in mouse. Brain Res. 2010;1344:209–216. doi: 10.1016/j.brainres.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 133.Rogalewski A, Dittgen T, Klugmann M, Kirsch F, Kruger C, Pitzer C, Minnerup J, Schabitz WR, Schneider A. Semaphorin 6A improves functional recovery in conjunction with motor training after cerebral ischemia. PLoS ONE. 2010;5:e10737. doi: 10.1371/journal.pone.0010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg. 2009;96:1484–1491. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- 136.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 137.Neufeld G, Lange T, Varshavsky A, Kessler O. Semaphorin signaling in vascular and tumor biology. Adv Exp Med Biol. 2007;600:118–131. doi: 10.1007/978-0-387-70956-7_10. [DOI] [PubMed] [Google Scholar]
- 138.Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 2005;12:1044–1056. doi: 10.1038/sj.cdd.4401707. [DOI] [PubMed] [Google Scholar]
- 139.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment–two sides of a coin. J Cell Sci. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 140.Staton CA. Class 3 semaphorins and their receptors in physiological and pathological angiogenesis. Biochem Soc Trans. 2011;39:1565–1570. doi: 10.1042/BST20110654. [DOI] [PubMed] [Google Scholar]
- 141.Micucci C, Orciari S, Catalano A. Semaphorins and their receptors in stem and cancer cells. Curr Med Chem. 2010;17:3462–3475. doi: 10.2174/092986710792927796. [DOI] [PubMed] [Google Scholar]
- 142.Sakurai A, Doci C, Gutkind JS. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 2012;22:23–32. doi: 10.1038/cr.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med. 2012;2(1):a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Muratori C, Tamagnone L. Semaphorin signals tweaking the tumor microenvironment. Adv Cancer Res. 2012;114:59–85. doi: 10.1016/B978-0-12-386503-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 145.Stuttfeld E, Ballmer-Hofer K. Structure and function of VEGF receptors. IUBMB Life. 2009;61:915–922. doi: 10.1002/iub.234. [DOI] [PubMed] [Google Scholar]
- 146.Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, Squadrito ML, Venneri MA, Mazzone M, Larsson E, Carmeliet P, De Palma M, Naldini L, Tamagnone L, Rolny C. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol. 2011;31:741–749. doi: 10.1161/ATVBAHA.110.211920. [DOI] [PubMed] [Google Scholar]
- 147.Gabrovska PN, Smith RA, Tiang T, Weinstein SR, Haupt LM, Griffiths LR. Semaphorin-plexin signalling genes associated with human breast tumourigenesis. Gene. 2011;489:63–69. doi: 10.1016/j.gene.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 148.Balakrishnan A, Penachioni JY, Lamba S, Bleeker FE, Zanon C, Rodolfo M, Vallacchi V, Scarpa A, Felicioni L, Buck M, Marchetti A, Comoglio PM, Bardelli A, Tamagnone L. Molecular profiling of the “plexinome” in melanoma and pancreatic cancer. Hum Mutat. 2009;30:1167–1174. doi: 10.1002/humu.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staton CA, Shaw LA, Valluru M, Hoh L, Koay I, Cross SS, Reed MW, Brown NJ. Expression of class 3 semaphorins and their receptors in human breast neoplasia. Histopathology. 2011;59:274–282. doi: 10.1111/j.1365-2559.2011.03922.x. [DOI] [PubMed] [Google Scholar]
- 150.Catalano A, Lazzarini R, Di Nuzzo S, Orciari S, Procopio A. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res. 2009;69:1485–1493. doi: 10.1158/0008-5472.CAN-08-3659. [DOI] [PubMed] [Google Scholar]
- 151.Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood. 2011;118:4285–4296. doi: 10.1182/blood-2011-03-341388. [DOI] [PubMed] [Google Scholar]
- 152.Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- 153.Rody A, Holtrich U, Gaetje R, Gehrmann M, Engels K, von Minckwitz G, Loibl S, Diallo-Danebrock R, Ruckhäberle E, Metzler D, Ahr A, Solbach C, Karn T, Kaufmann M. Poor outcome in estrogen receptor-positive breast cancers predicted by loss of plexin B1. Clin Cancer Res. 2007;13:1115–1122. doi: 10.1158/1078-0432.CCR-06-2433. [DOI] [PubMed] [Google Scholar]
- 154.Rody A, Karn T, Ruckhäberle E, Hanker L, Metzler D, Müller V, Solbach C, Ahr A, Gätje R, Holtrich U, Kaufmann M. Loss of Plexin B1 is highly prognostic in low proliferating ER positive breast cancers—results of a large-scale microarray analysis. Eur J Cancer. 2009;45:405–413. doi: 10.1016/j.ejca.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 155.Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC, Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, Chuang EY. Identification of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers Prev. 2010;19:2590–2597. doi: 10.1158/1055-9965.EPI-10-0332. [DOI] [PubMed] [Google Scholar]
- 156.Ye S, Hao X, Zhou T, Wu M, Wei J, Wang Y, Zhou L, Jiang X, Ji L, Chen Y, You L, Zhang Y, Xu G, Zhou J, Ma D, Wang S. Plexin-B1 silencing inhibits ovarian cancer cell migration and invasion. BMC Cancer. 2010;10:611. doi: 10.1186/1471-2407-10-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, Ahn NG. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–2709. doi: 10.1038/onc.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017–9022. doi: 10.1073/pnas.0508825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42:379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- 160.Kato S, Kubota K, Shimamura T, Shinohara Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F, Ishiguro H, Nakaigawa N, Nagashima Y, Taguri M, Kubota Y, Goshima Y, Morita S, Endo I, Maeda S, Nakajima A, Nakagama H. Semaphorin 4D, a lymphocyte semaphorin, enhances tumor cell motility through binding its receptor, plexinB1, in pancreatic cancer. Cancer Sci. 2011;102:2029–2037. doi: 10.1111/j.1349-7006.2011.02053.x. [DOI] [PubMed] [Google Scholar]
- 161.Ch’ng ES, Kumanogoh A. Roles of Sema4D and Plexin-B1 in tumor progression. Mol Cancer. 2010;9:251. doi: 10.1186/1476-4598-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wong OG, Nitkunan T, Oinuma I, Zhou C, Blanc V, Brown RS, Bott SR, Nariculam J, Box G, Munson P, Constantinou J, Feneley MR, Klocker H, Eccles SA, Negishi M, Freeman A, Masters JR, Williamson M. Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci USA. 2007;104:19040–19045. doi: 10.1073/pnas.0702544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tong Y, Hota PK, Hamaneh MB, Buck M. Insights into oncogenic mutations of plexin-B1 based on the solution structure of the Rho GTPase binding domain. Structure. 2008;16:246–258. doi: 10.1016/j.str.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swiercz JM, Worzfeld T, Offermanns S. Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex. Mol Cell Biol. 2009;29:6321–6334. doi: 10.1128/MCB.00103-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest. 2012;122(4):1296–1305. doi: 10.1172/JCI60568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 168.Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR (2012) Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis Apr 3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Retracted]
- 169.Lazova R, Gould Rothberg BE, Rimm D, Scott G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol. 2009;31:177–181. doi: 10.1097/DAD.0b013e318196672d. [DOI] [PubMed] [Google Scholar]
- 170.Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol. 2009;129:954–963. doi: 10.1038/jid.2008.329. [DOI] [PubMed] [Google Scholar]
- 171.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RM, Leenders WP. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 173.Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blanc V, Nariculam J, Munson P, Freeman A, Klocker H, Masters J, Williamson M. A role for class 3 semaphorins in prostate cancer. Prostate. 2011;71:649–658. doi: 10.1002/pros.21281. [DOI] [PubMed] [Google Scholar]
- 175.Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goshima Y, Ito T, Sasaki Y, Nakamura F. Semaphorins as signals for cell repulsion and invasion. J Clin Invest. 2002;109:993–998. doi: 10.1172/JCI15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–760. doi: 10.2741/1569. [DOI] [PubMed] [Google Scholar]
- 178.Koch S. Neuropilin signalling in angiogenesis. Biochem Soc Trans. 2012;40:20–25. doi: 10.1042/BST20110689. [DOI] [PubMed] [Google Scholar]
- 179.Rizzolio S, Tamagnone L. Multifaceted role of neuropilins in cancer. Curr Med Chem. 2011;18:3563–3575. doi: 10.2174/092986711796642544. [DOI] [PubMed] [Google Scholar]
- 180.Serini G, Maione F, Giraudo E, Bussolino F. Semaphorins and tumor angiogenesis. Angiogenesis. 2009;12:187–193. doi: 10.1007/s10456-009-9138-4. [DOI] [PubMed] [Google Scholar]
- 181.Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geretti E, Klagsbrun M. Neuropilins: novel targets for anti-angiogenesis therapies. Cell Adh Migr. 2007;1:56–61. doi: 10.4161/cam.1.2.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grünewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta. 2010;1804:567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 184.Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res. 2009;15:1860–1864. doi: 10.1158/1078-0432.CCR-08-0563. [DOI] [PubMed] [Google Scholar]
- 185.Iyer S, Darley PI, Acharya KR. Structural insights into the binding of vascular endothelial growth factor-B by VEGFR-1(D2): recognition and specificity. J Biol Chem. 2010;285:23779–23789. doi: 10.1074/jbc.M110.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leppänen VM, Prota AE, Jeltsch M, Anisimov A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K, Alitalo K. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc Natl Acad Sci USA. 2010;107:2425–2430. doi: 10.1073/pnas.0914318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leppänen VM, Jeltsch M, Anisimov A, Tvorogov D, Aho K, Kalkkinen N, Toivanen P, Ylä-Herttuala S, Ballmer-Hofer K, Alitalo K. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood. 2011;117:1507–1515. doi: 10.1182/blood-2010-08-301549. [DOI] [PubMed] [Google Scholar]
- 188.Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766–2776. doi: 10.1091/mbc.E09-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vander Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci USA. 2007;104:6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Parker MW, Xu P, Li X, Vander Kooi CW (2012) Structural basis for the selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 192.Adams RH, Lohrum M, Klostermann A, Betz H, Püschel AW. The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 1997;16:6077–6086. doi: 10.1093/emboj/16.20.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW. Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry. 2010;49:4068–4075. doi: 10.1021/bi100327r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol Med. 2012;4(3):234–250. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Giordano RJ, Anobom CD, Cardó-Vila M, Kalil J, Valente AP, Pasqualini R, Almeida FC, Arap W. Structural basis for the interaction of a vascular endothelial growth factor mimic peptide motif and its corresponding receptors. Chem Biol. 2005;12:1075–1083. doi: 10.1016/j.chembiol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 196.Giordano RJ, Cardó-Vila M, Salameh A, Anobom CD, Zeitlin BD, Hawke DH, Valente AP, Almeida FC, Nör JE, Sidman RL, Pasqualini R, Arap W. From combinatorial peptide selection to drug prototype (I): targeting the vascular endothelial growth factor receptor pathway. Proc Natl Acad Sci USA. 2010;107:5112–5117. doi: 10.1073/pnas.0915141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grothey A, Ellis LM. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer J. 2008;14:170–177. doi: 10.1097/PPO.0b013e318178d9de. [DOI] [PubMed] [Google Scholar]
- 198.Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N, Ellard K, Aqil R, Lynch R, Chapman C, Hartzoulakis B, Nally J, Stewart M, Cheng L, Menon M, Tickner M, Djordjevic S, Driscoll PC, Zachary I, Selwood DL. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J Med Chem. 2010;53:2215–2226. doi: 10.1021/jm901755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 200.Ueyama H, Horibe T, Nakajima O, Ohara K, Kohno M, Kawakami K. Semaphorin 3A lytic hybrid peptide binding to neuropilin-1 as a novel anti-cancer agent in pancreatic cancer. Biochem Biophys Res Commun. 2011;414:60–66. doi: 10.1016/j.bbrc.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 201.Cébe-Suarez S, Grünewald FS, Jaussi R, Li X, Claesson-Welsh L, Spillmann D, Mercer AA, Prota AE, Ballmer-Hofer K. Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J. 2008;22:3078–3086. doi: 10.1096/fj.08-107219. [DOI] [PubMed] [Google Scholar]
- 202.Novoa A, Pellegrini-Moïse N, Bechet D, Barberi-Heyob M, Chapleur Y. Sugar-based peptidomimetics as potential inhibitors of the vascular endothelium growth factor binding to neuropilin-1. Bioorg Med Chem. 2010;18:3285–3298. doi: 10.1016/j.bmc.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 203.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, Berry L, Kasman I, Zlot C, Cheng Z, Le Couter J, Filvaroff EH, Plowman G, Peale F, French D, Carano R, Koch AW, Wu Y, Watts RJ, Tessier-Lavigne M, Bagri A. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 204.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti- VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 205.Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res. 2010;8:1063–1073. doi: 10.1158/1541-7786.MCR-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jubb AM, Strickland LA, Liu SD, Mak J, Schmidt M, Koeppen H. Neuropilin-1 expression in cancer and development. J Pathol. 2011;226(1):50–60. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 207.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, Blanpain C. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 208.Roth L, Nasarre C, Dirrig-Grosch S, Aunis D, Crémel G, Hubert P, Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol Biol Cell. 2008;19:646–654. doi: 10.1091/mbc.E07-06-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nasarre C, Roth M, Jacob L, Roth L, Koncina E, Thien A, Labourdette G, Poulet P, Hubert P, Crémel G, Roussel G, Aunis D, Bagnard D. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene. 2010;29:2381–2392. doi: 10.1038/onc.2010.9. [DOI] [PubMed] [Google Scholar]
- 210.Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development. 2011;138:4185–4191. doi: 10.1242/dev.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans. 2011;39:1583–1591. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 212.Maestrini E, Tamagnone L, Longati P, Cremona O, Gulisano M, Bione S, Tamanini F, Neel BG, Toniolo D, Comoglio PM. A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor. Proc Natl Acad Sci USA. 1996;93:674–678. doi: 10.1073/pnas.93.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 215.Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 216.Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- 217.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 218.Corso S, Comoglio PM, Giordano S. Cancer therapy: can the challenge be MET? Trends Mol Med. 2005;11:284–292. doi: 10.1016/j.molmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 219.Soong J, Chen Y, Shustef EM, Scott GA. Sema4D, the Ligand for Plexin B1, Suppresses c- Met Activation and Migration and Promotes Melanocyte Survival and Growth. J Invest Dermatol. 2012;132(4):1230–1238. doi: 10.1038/jid.2011.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stevens L, McClelland L, Fricke A, Williamson M, Kuo I, Scott G. Plexin B1 suppresses c-Met in melanoma: a role for plexin B1 as a tumor-suppressor protein through regulation of c-Met. J Invest Dermatol. 2010;130:1636–1645. doi: 10.1038/jid.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bellon SF, Kaplan-Lefko P, Yang Y, Zhang Y, Moriguchi J, Rex K, Johnson CW, Rose PE, Long AM, O’Connor AB, Gu Y, Coxon A, Kim TS, Tasker A, Burgess TL, Dussault I. c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem. 2008;283:2675–2683. doi: 10.1074/jbc.M705774200. [DOI] [PubMed] [Google Scholar]
- 222.Ma PC, Kijima T, Maulik G, Fox EA, Sattler M, Griffin JD, Johnson BE, Salgia R. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–6281. [PubMed] [Google Scholar]
- 223.Bertotti A, Burbridge MF, Gastaldi S, Galimi F, Torti D, Medico E, Giordano S, Corso S, Rolland-Valognes G, Lockhart BP, Hickman JA, Comoglio PM, Trusolino L. Only a subset of Met-activated pathways are required to sustain oncogene addiction. Sci Signal. 2009;2:ra80. doi: 10.1126/scisignal.2000643. [DOI] [PubMed] [Google Scholar]
- 224.Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–3049. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 225.Bertotti A, Comoglio PM. Tyrosine kinase signal specificity: lessons from the HGF receptor. Trends Biochem Sci. 2003;28:527–533. doi: 10.1016/j.tibs.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 226.Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall FF, Chung LW, Wu D. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sulpice E, Plouët J, Bergé M, Allanic D, Tobelem G, Merkulova-Rainon T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood. 2008;111:2036–2045. doi: 10.1182/blood-2007-04-084269. [DOI] [PubMed] [Google Scholar]
- 228.Tolbert WD, Daugherty J, Gao C, Xie Q, Miranti C, Gherardi E, Woude GV, Xu HE. A mechanistic basis for converting a receptor tyrosine kinase agonist to an antagonist. Proc Natl Acad Sci USA. 2007;104:14592–14597. doi: 10.1073/pnas.0704290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Holmes O, Pillozzi S, Deakin JA, Carafoli F, Kemp L, Butler PJ, Lyon M, Gherardi E. Insights into the structure/function of hepatocyte growth factor/scatter factor from studies with individual domains. J Mol Biol. 2007;367:395–408. doi: 10.1016/j.jmb.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 230.Gherardi E, Sandin S, Petoukhov MV, Finch J, Youles ME, Ofverstedt LG, Miguel RN, Blundell TL, Vande Woude GF, Skoglund U, Svergun DI. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci USA. 2006;103:4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sun T, Krishnan R, Swiercz JM (2012) Grb2 mediates Semaphorin4D-dependent RhoA inactivation. J Cell Sci. (in press) [DOI] [PubMed]
- 232.Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Swiercz JM, Worzfeld T, Offermanns S. ErbB-2 and met reciprocally regulate cellular signaling via plexin-B1. J Biol Chem. 2008;283:1893–1901. doi: 10.1074/jbc.M706822200. [DOI] [PubMed] [Google Scholar]
- 235.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 236.Serini G, Napione L, Arese M, Bussolino F. Besides adhesion: new perspectives of integrin functions in angiogenesis. Cardiovasc Res. 2008;78:213–222. doi: 10.1093/cvr/cvn045. [DOI] [PubMed] [Google Scholar]
- 237.Basile JR, Gavard J, Gutkind JS. Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem. 2007;282:34888–34895. doi: 10.1074/jbc.M705467200. [DOI] [PubMed] [Google Scholar]
- 238.Franco M, Muratori C, Corso S, Tenaglia E, Bertotti A, Capparuccia L, Trusolino L, Comoglio PM, Tamagnone L. The tetraspanin CD151 is required for Met-dependent signaling and tumor cell growth. J Biol Chem. 2010;285:38756–38764. doi: 10.1074/jbc.M110.145417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Scott GA, McClelland LA, Fricke AF. Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. J Invest Dermatol. 2008;128:151–161. doi: 10.1038/sj.jid.5700974. [DOI] [PubMed] [Google Scholar]
- 240.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 241.Gomez C, Burt-Pichat B, Mallein-Gerin F, Merle B, Delmas PD, Skerry TM, Vico L, Malaval L, Chenu C. Expression of Semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev Dyn. 2005;234:393–403. doi: 10.1002/dvdy.20512. [DOI] [PubMed] [Google Scholar]
- 242.Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–622. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 243.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hwang JY, Lee JY, Park MH, Kim KS, Kim KK, Ryu HJ, Lee JK, Han BG, Kim JW, Oh B, Kimm K, Park BL, Shin HD, Kim TH, Hong JM, Park EK, Kim DJ, Koh JM, Kim GS, Kim SY. Association of PLXNA2 polymorphisms with vertebral fracture risk and bone mineral density in postmenopausal Korean population. Osteoporos Int. 2006;17:1592–1601. doi: 10.1007/s00198-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 245.Delorme G, Saltel F, Bonnelye E, Jurdic P, Machuca-Gayet I. Expression and function of semaphorin 7A in bone cells. Biol Cell. 2005;97:589–597. doi: 10.1042/BC20040103. [DOI] [PubMed] [Google Scholar]
- 246.Kanda T, Yoshida Y, Izu Y, Nifuji A, Ezura Y, Nakashima K, Noda M. PlexinD1 deficiency induces defects in axial skeletal morphogenesis. J Cell Biochem. 2007;101:1329–1337. doi: 10.1002/jcb.21306. [DOI] [PubMed] [Google Scholar]
- 247.Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev Biol. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Okubo M, Kimura T, Fujita Y, Mochizuki S, Niki Y, Enomoto H, Suda Y, Toyama Y, Okada Y. Semaphorin 3A is expressed in human osteoarthritic cartilage and antagonizes vascular endothelial growth factor 165-promoted chondrocyte migration: an implication for chondrocyte cloning. Arthritis Rheum. 2011;63:3000–3009. doi: 10.1002/art.30482. [DOI] [PubMed] [Google Scholar]
- 249.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H- (2012) Osteoprotection by semaphorin 3A. Nature. Apr 18. doi: 10.1038/nature11000 [DOI] [PubMed]
- 250.Sutton AL, Zhang X, Dowd DR, Kharode YP, Komm BS, Macdonald PN. Semaphorin 3B is a 1,25-Dihydroxyvitamin D3-induced gene in osteoblasts that promotes osteoclastogenesis and induces osteopenia in mice. Mol Endocrinol. 2008;22:1370–1381. doi: 10.1210/me.2007-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lallier TE. Semaphorin profiling of periodontal fibroblasts and osteoblasts. J Dent Res. 2004;83:677–682. doi: 10.1177/154405910408300904. [DOI] [PubMed] [Google Scholar]
- 252.Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 253.Dacquin R, Domenget C, Kumanogoh A, Kikutani H, Jurdic P, Machuca-Gayet I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS ONE. 2011;6:e26627. doi: 10.1371/journal.pone.0026627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Takimoto A, Nishizaki Y, Hiraki Y, Shukunami C. Differential actions of VEGF-A isoforms on perichondrial angiogenesis during endochondral bone formation. Dev Biol. 2009;332:196–211. doi: 10.1016/j.ydbio.2009.05.552. [DOI] [PubMed] [Google Scholar]
- 255.Thi MM, Suadicani SO, Spray DC. Fluid flow-induced soluble vascular endothelial growth factor isoforms regulate actin adaptation in osteoblasts. J Biol Chem. 2010;285:30931–30941. doi: 10.1074/jbc.M110.114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Handa A, Tokunaga T, Tsuchida T, Lee YH, Kijima H, Yamazaki H, Ueyama Y, Fukuda H, Nakamura M. Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. Int J Oncol. 2000;17:291–295. doi: 10.3892/ijo.17.2.291. [DOI] [PubMed] [Google Scholar]
- 257.Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 259.Belaid-Choucair Z, Lepelletier Y, Poncin G, Thiry A, Humblet C, Maachi M, Beaulieu A, Schneider E, Briquet A, Mineur P, Lambert C, Mendes-Da-Cruz D, Ahui ML, Asnafi V, Dy M, Boniver J, Nusgens BV, Hermine O, Defresne MP. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells. 2008;26:1556–1564. doi: 10.1634/stemcells.2008-0068. [DOI] [PubMed] [Google Scholar]
- 260.Karjalainen K, Jaalouk DE, Bueso-Ramos CE, Zurita AJ, Kuniyasu A, Eckhardt BL, Marini FC, Lichtiger B, O’Brien S, Kantarjian HM, Cortes JE, Koivunen E, Arap W, Pasqualini R. Targeting neuropilin-1 in human leukemia and lymphoma. Blood. 2011;117:920–927. doi: 10.1182/blood-2010-05-282921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Schuch G, Machluf M, Bartsch G, Jr, Nomi M, Richard H, Atala A, Soker S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100:4622–4628. doi: 10.1182/blood.V100.13.4622. [DOI] [PubMed] [Google Scholar]
- 262.Kong JS, Yoo SA, Kim JW, Yang SP, Chae CB, Tarallo V, De Falco S, Ryu SH, Cho CS, Kim WU. Anti-neuropilin-1 peptide inhibition of synoviocyte survival, angiogenesis, and experimental arthritis. Arthr Rheum. 2010;62:179–190. doi: 10.1002/art.27243. [DOI] [PubMed] [Google Scholar]
- 263.Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol. 2010;7:83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mizui M, Kumanogoh A, Kikutani H. Immune semaphorins: novel features of neural guidance molecules. J Clin Immunol. 2009;29:1–11. doi: 10.1007/s10875-008-9263-7. [DOI] [PubMed] [Google Scholar]
- 265.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 266.Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immunesystem. Trends Immunol. 2012;33(3):127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 267.Tran-Van H, Avota E, Börtlein C, Mueller N, Schneider-Schaulies S. Measles virus modulates dendritic cell/T-cell communication at the level of plexinA1/neuropilin-1 recruitment and activity. Eur J Immunol. 2011;41:151–163. doi: 10.1002/eji.201040847. [DOI] [PubMed] [Google Scholar]
- 268.O’Connor BP, Ting JP. The evolving role of semaphorins and plexins in the immune system: Plexin-A1 regulation of dendritic cell function. Immunol Res. 2008;41:217–222. doi: 10.1007/s12026-008-8026-0. [DOI] [PubMed] [Google Scholar]
- 269.Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, Mizui M, Kang S, Nojima S, Tsujimura T, Nakatsuji Y, Katayama I, Toyofuku T, Kikutani H, Kumanogoh A. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by neuropilin-1/semaphorin-3A-mediated interactions. Proc Natl Acad Sci USA. 2007;104:5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yamamoto M, Suzuki K, Okuno T, Ogata T, Takegahara N, Takamatsu H, Mizui M, Taniguchi M, Chédotal A, Suto F, Fujisawa H, Kumanogoh A, Kikutani H. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20:413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- 272.Wen H, Lei Y, Eun SY, Ting JP. Plexin-A4-semaphorin 3A signaling is required for Toll-like receptor- and sepsis-induced cytokine storm. J Exp Med. 2010;207:2943–2957. doi: 10.1084/jem.20101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5 + B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- 274.Chabbert-de Ponnat I, Marie-Cardine A, Pasterkamp RJ, Schiavon V, Tamagnone L, Thomasset N, Bensussan A, Boumsell L. Soluble CD100 functions on human monocytes and immature dendritic cells require plexin C1 and plexin B1, respectively. Int Immunol. 2005;17:439–447. doi: 10.1093/intimm/dxh224. [DOI] [PubMed] [Google Scholar]
- 275.Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174:51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- 276.Holl EK, O’Connor BP, Holl TM, Roney KE, Zimmermann AG, Jha S, Kelsoe G, Ting JP. Plexin-D1 is a novel regulator of germinal centers and humoral immune responses. J Immunol. 2011;186:5603–5611. doi: 10.4049/jimmunol.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29(6):888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–3329. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 279.Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, Takegahara N, Marukawa S, Ishida I, Morishita H, Prasad DV, Tamura M, Mizui M, Toyofuku T, Akira S, Takeda K, Okabe M, Kikutani H. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 280.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch’ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 281.Nakagawa Y, Takamatsu H, Okuno T, Kang S, Nojima S, Kimura T, Kataoka TR, Ikawa M, Toyofuku T, Katayama I, Kumanogoh A. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J Immunol. 2011;186:2881–2888. doi: 10.4049/jimmunol.1003485. [DOI] [PubMed] [Google Scholar]
- 282.Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA. 1996;93:11780–11785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Herold C, Elhabazi A, Bismuth G, Bensussan A, Boumsell L. CD100 is associated with CD45 at the surface of human T lymphocytes. Role in T cell homotypic adhesion. J Immunol. 1996;157:5262–5268. [PubMed] [Google Scholar]
- 284.Delaire S, Billard C, Tordjman R, Chédotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- 285.Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood. 2001;97:3498–3504. doi: 10.1182/blood.v97.11.3498. [DOI] [PubMed] [Google Scholar]
- 286.Shi W, Kumanogoh A, Watanabe C, Uchida J, Wang X, Yasui T, Yukawa K, Ikawa M, Okabe M, Parnes JR, Yoshida K, Kikutani H. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 287.Okuno T, Nakatsuji Y, Moriya M, Takamatsu H, Nojima S, Takegahara N, Toyofuku T, Nakagawa Y, Kang S, Friedel RH, Sakoda S, Kikutani H, Kumanogoh A. Roles of Sema4D-plexin-B1 interactions in the central nervous system for pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:1499–1506. doi: 10.4049/jimmunol.0903302. [DOI] [PubMed] [Google Scholar]
- 288.Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int Immunol. 2003;15:1027–1034. doi: 10.1093/intimm/dxg098. [DOI] [PubMed] [Google Scholar]
- 289.Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, Matsumoto M, Yoshida K, Yakura H, Pan C, Parnes JR, Kikutani H. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 290.O’Connor BP, Eun SY, Ye Z, Zozulya AL, Lich JD, Moore CB, Iocca HA, Roney KE, Holl EK, Wu QP, van Deventer HW, Fabry Z, Ting JP. Semaphorin 6D regulates the late phase of CD4 + T cell primary immune responses. Proc Natl Acad Sci USA. 2008;105:13015–13020. doi: 10.1073/pnas.0803386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 292.Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Roméo PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- 293.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332:680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kiel C, Yus E, Serrano L. Engineering signal transduction pathways. Cell. 2010;140:33–47. doi: 10.1016/j.cell.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 295.Jordan JD, Landau EM, Iyengar R. Signaling networks: the origins of cellular multitasking. Cell. 2000;103:193–200. doi: 10.1016/s0092-8674(00)00112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 298.Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol. 2007;600:90–108. doi: 10.1007/978-0-387-70956-7_8. [DOI] [PubMed] [Google Scholar]
- 299.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 300.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 301.Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 303.van Dam TJ, Bos JL, Snel B. Evolution of the Ras-like small GTPases and their regulators. Small GTPases. 2011;2:4–16. doi: 10.4161/sgtp.2.1.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 305.Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 2010;68:340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- 307.Benarroch EE. Rho GTPases: role in dendrite and axonal growth, mental retardation, and axonal regeneration. Neurology. 2007;68:1315. doi: 10.1212/01.wnl.0000259588.97409.8f. [DOI] [PubMed] [Google Scholar]
- 308.Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- 309.Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ishikawa Y, Katoh H, Negishi M. Small GTPase Rnd1 is involved in neuronal activity dependentdendritic development in hippocampal neurons. Neurosci Lett. 2006;400:218–223. doi: 10.1016/j.neulet.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 311.Ishikawa Y, Katoh H, Negishi M. A role of Rnd1 GTPase in dendritic spine formation in hippocampal neurons. J Neurosci. 2003;23:11065–11072. doi: 10.1523/JNEUROSCI.23-35-11065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Harada A, Katoh H, Negishi M. Direct interaction of Rnd1 with FRS2 beta regulates Rnd1-induced down-regulation of RhoA activity and is involved in fibroblast growth factor-induced neurite outgrowth in PC12 cells. J Biol Chem. 2005;280:18418–18424. doi: 10.1074/jbc.M411356200. [DOI] [PubMed] [Google Scholar]
- 313.Katoh H, Harada A, Mori K, Negishi M. Socius is a novel Rnd GTPase-interacting protein involved in disassembly of actin stress fibers. Mol Cell Biol. 2002;22:2952–2964. doi: 10.1128/MCB.22.9.2952-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li YH, Ghavampur S, Bondallaz P, Will L, Grenningloh G, Püschel AW. Rnd1 regulates axon extension by enhancing the microtubule destabilizing activity of SCG10. J Biol Chem. 2009;284:363–371. doi: 10.1074/jbc.A808126200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zanata SM, Hovatta I, Rohm B, Püschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Oinuma I, Katoh H, Harada A, Negishi M. Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells. J Biol Chem. 2003;278:25671–25677. doi: 10.1074/jbc.M303047200. [DOI] [PubMed] [Google Scholar]
- 318.Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 319.Negishi M, Buck M (2012) “Ras/Rap GAP function and GTPase sequestration in plexin-mediated cell signaling mechanisms” Commentary/E-letter in Science Signaling http://stke.sciencemag.org/cgi/eletters/5/207/ra6
- 320.Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc Natl Acad Sci USA. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, Buck M. Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem. 2007;282:37215–37224. doi: 10.1074/jbc.M703800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Uesugi K, Oinuma I, Katoh H, Negishi M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J Biol Chem. 2009;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hota PK, Buck M. Thermodynamic characterization of two homologous protein complexes: associations of the semaphorin receptor plexin-B1 RhoGTPase binding domain with Rnd1 and active Rac1. Protein Sci. 2009;18:1060–1071. doi: 10.1002/pro.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wang H, Hota PK, Tong Y, Li B, Shen L, Nedyalkova L, Borthakur S, Kim S, Tempel W, Buck M, Park HW. Structural basis of Rnd1 binding to plexin Rho GTPase binding domains (RBDs) J Biol Chem. 2011;286:26093–26106. doi: 10.1074/jbc.M110.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhou C, Wong OG, Masters JR, Williamson M. Effect of cancer-associated mutations in the PlexinB1 gene. Mol Cancer. 2012;11:11. doi: 10.1186/1476-4598-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- 327.Püschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- 328.Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J Biol Chem. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- 329.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 330.Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–776. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- 331.Rohm B, Rahim B, Kleiber B, Hovatta I, Püschel AW. The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett. 2000;486:68–72. doi: 10.1016/s0014-5793(00)02240-7. [DOI] [PubMed] [Google Scholar]
- 332.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase-activating protein for R-Ras. Science. 2004;305:862–865. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 333.Yang T, Terman JR. 14-3-3epsilon couples protein kinase A to semaphoring signaling and silences plexin Ras GAP-mediated axonal repulsion. Neuron. 2012;74:108–121. doi: 10.1016/j.neuron.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bell CH, Aricescu AR, Jones EY, Siebold C. A dual binding mode for RhoGTPases in plexin signalling. PLoS Biol. 2011;9:e1001134. doi: 10.1371/journal.pbio.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Tong Y, Hota PK, Penachioni JY, Hamaneh MB, Kim S, Alviani RS, Shen L, He H, Tempel W, Tamagnone L, Park HW, Buck M. Structure and function of the intracellular region of the plexin-b1 transmembrane receptor. J Biol Chem. 2009;284:35962–35972. doi: 10.1074/jbc.M109.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Oinuma I, Katoh H, Negishi M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci. 2004;24:11473–11480. doi: 10.1523/JNEUROSCI.3257-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Perrot V, Vazquez-Prado J, Gutkind JS. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem. 2002;277:43115–43120. doi: 10.1074/jbc.M206005200. [DOI] [PubMed] [Google Scholar]
- 338.Ohba Y, Mochizuki N, Yamashita S, Chan AM, Schrader JW, Hattori S, Nagashima K, Matsuda M. Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J Biol Chem. 2000;275:20020–20026. doi: 10.1074/jbc.M000981200. [DOI] [PubMed] [Google Scholar]
- 339.Quilliam LA, Castro AF, Rogers-Graham KS, Martin CB, Der CJ, Bi C. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J Biol Chem. 1999;274:23850–23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- 340.Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, Cowan CW, Zhang X. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M. Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7:704–709. doi: 10.1038/sj.embor.7400737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta (1) integrin activity. J Cell Biol. 2006;173:601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci. 2007;27:14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Dai Y, Walker SA, de Vet E, Cook S, Welch HC, Lockyer PJ. Ca2+-dependent monomer and dimer formation switches CAPRI Protein between Ras GTPase-activating protein (GAP) and RapGAP activities. J Biol Chem. 2011;286:19905–19916. doi: 10.1074/jbc.M110.201301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11:339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 348.Driessens MH, Olivo C, Nagata K, Inagaki M, Collard JG. B plexins activate Rho through PDZ-RhoGEF. FEBS Lett. 2002;529:168–172. doi: 10.1016/s0014-5793(02)03323-9. [DOI] [PubMed] [Google Scholar]
- 349.Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- 351.Shalaby MA, Hampson L, Oliver A, Hampson I. Identification of PlexinD1 and AHDC1 as a putative interactors for Tip-1 protein. Genes & Genomics. 2011;33:399–405. [Google Scholar]
- 352.Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron. 2009;61:359–372. doi: 10.1016/j.neuron.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Okada H, Uezu A, Mason FM, Soderblom EJ, Moseley MA, 3rd, Soderling SH. SH3 domain-based phototrapping in living cells reveals Rho family GAP signaling complexes. Sci Signal. 2011;4:rs13. doi: 10.1126/scisignal.2002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase-activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 355.Shimizu A, Mammoto A, Italiano JE, Jr, Pravda E, Dudley AC, Ingber DE, Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Tatsis N, Lannigan DA, Macara IG. The function of the p190 Rho GTPase-activating protein is controlled by its N-terminal GTP binding domain. J Biol Chem. 1998;273:34631–34638. doi: 10.1074/jbc.273.51.34631. [DOI] [PubMed] [Google Scholar]
- 357.Lévay M, Settleman J, Ligeti E. Regulation of the substrate preference of p190RhoGAP by protein kinase C-mediated phosphorylation of a phospholipid binding site. Biochemistry. 2009;48:8615–8623. doi: 10.1021/bi900667y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mori K, Amano M, Takefuji M, Kato K, Morita Y, Nishioka T, Matsuura Y, Murohara T, Kaibuchi K. Rho-kinase contributes to sustained RhoA activation through phosphorylation of p190A RhoGAP. J Biol Chem. 2009;284:5067–5076. doi: 10.1074/jbc.M806853200. [DOI] [PubMed] [Google Scholar]
- 359.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–1026. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bartolomé RA, Wright N, Molina-Ortiz I, Sánchez-Luque FJ, Teixidó J. Activated G(alpha)13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008;68:8221–8230. doi: 10.1158/0008-5472.CAN-08-0561. [DOI] [PubMed] [Google Scholar]
- 361.Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML, Settleman J, Chen RH. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 362.Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J Cell Sci. 2007;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- 363.Oinuma I, Kawada K, Tsukagoshi K, Negishi M. Rnd1 and Rnd3 targeting to lipid raft is required for p190 RhoGAP activation. Mol Biol Cell. 2012;23:1593–1604. doi: 10.1091/mbc.E11-11-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bradley WD, Hernández SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Roney KE, O’Connor BP, Wen H, Holl EK, Guthrie EH, Davis BK, Jones SW, Jha S, Sharek L, Garcia-Mata R, Bear JE, Ting JP. Plexin-B2 negatively regulates macrophage motility, Rac, and Cdc42 activation. PLoS ONE. 2011;6:e24795. doi: 10.1371/journal.pone.0024795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.McClelland L, Chen Y, Soong J, Kuo I, Scott G. Plexin B1 inhibits integrin-dependent pp125FAK and Rho activity in melanoma. Pigment Cell Melanoma Res. 2011;24:165–174. doi: 10.1111/j.1755-148X.2010.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sakurai A, Jian X, Lee CJ, Manavski Y, Chavakis E, Donaldson J, Randazzo PA, Gutkind JS. Phosphatidylinositol-4-phosphate 5-kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-plexin-D1 through Arf6 protein. J Biol Chem. 2011;286:34335–34345. doi: 10.1074/jbc.M111.259499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Parachoniak CA, Luo Y, Abella JV, Keen JH, Park M. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev Cell. 2011;20:751–763. doi: 10.1016/j.devcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;16:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Fan S, Meng Q, Laterra JJ, Rosen EM. Role of Src signal transduction pathways in scatter factor-mediated cellular protection. J Biol Chem. 2009;284:7561–7577. doi: 10.1074/jbc.M807497200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Segarra J, Balenci L, Drenth T, Maina F, Lamballe F. Combined signaling through ERK, PI3 K/AKT, and RAC1/p38 is required for met-triggered cortical neuron migration. J Biol Chem. 2006;281:4771–4778. doi: 10.1074/jbc.M508298200. [DOI] [PubMed] [Google Scholar]
- 372.Wannemacher KM, Zhu L, Jiang H, Fong KP, Stalker TJ, Lee D, Tran AN, Neeves KB, Maloney S, Kumanogoh A, Kikutani H, Hammer DA, Diamond SL, Brass LF. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood. 2010;116:5707–5715. doi: 10.1182/blood-2010-04-279943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Jiang SX, Whitehead S, Aylsworth A, Slinn J, Zurakowski B, Chan K, Li J, Hou ST. Neuropilin 1 directly interacts with Fer kinase to mediate semaphorin 3A-induced death of cortical neurons. J Biol Chem. 2010;285:9908–9918. doi: 10.1074/jbc.M109.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shapovalova Z, Tabunshchyk K, Greer PA. The Fer tyrosine kinase regulates an axon retraction response to Semaphorin 3A in dorsal root ganglion neurons. BMC Dev Biol. 2007;7:133. doi: 10.1186/1471-213X-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Mitsui N, Inatome R, Takahashi S, Goshima Y, Yamamura H, Yanagi S. Involvement of Fes/Fps tyrosine kinase in semaphorin3A signaling. EMBO J. 2002;21:3274–3285. doi: 10.1093/emboj/cdf328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Deo RC, Schmidt EF, Elhabazi A, Togashi H, Burley SK, Strittmatter SM. Structural bases for CRMP function in plexin-dependent semaphorin3A signaling. EMBO J. 2004;23:9–22. doi: 10.1038/sj.emboj.7600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Buel GR, Rush J, Ballif BA. Fyn promotes phosphorylation of collapsin response mediator protein 1 at tyrosine 504, a novel, isoform-specific regulatory site. J Cell Biochem. 2010;111:20–28. doi: 10.1002/jcb.22659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, Ohno S, Nakamura F, Goshima Y. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002;35:907–920. doi: 10.1016/s0896-6273(02)00857-7. [DOI] [PubMed] [Google Scholar]
- 379.Lin X, Ogiya M, Takahara M, Yamaguchi W, Furuyama T, Tanaka H, Tohyama M, Inagaki S. Sema4D-plexin-B1 implicated in regulation of dendritic spine density through RhoA/ROCK pathway. Neurosci Lett. 2007;428:1–6. doi: 10.1016/j.neulet.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 380.Tripathi BK, Zelenka PS. Cdk5-dependent regulation of Rho activity, cytoskeletal contraction, and epithelial cell migration via suppression of Src and p190RhoGAP. Mol Cell Biol. 2009;29:6488–6499. doi: 10.1128/MCB.01098-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ahmed A, Eickholt BJ. Intracellular kinases in semaphorin signaling. Adv Exp Med Biol. 2007;600:24–37. doi: 10.1007/978-0-387-70956-7_3. [DOI] [PubMed] [Google Scholar]
- 382.Aurandt J, Li W, Guan KL. Semaphorin 4D activates the MAPK pathway downstream of plexin-B1. Biochem J. 2006;394:459–464. doi: 10.1042/BJ20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yang YH, Zhou H, Binmadi NO, Proia P, Basile JR. Plexin-B1 activates NF-κB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE. 2011;6:e25826. doi: 10.1371/journal.pone.0025826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 384.Lin PC, Chan PM, Hall C, Manser E. Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J Biol Chem. 2011;286:41466–41478. doi: 10.1074/jbc.M111.283580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, Poo MM. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011;71:433–446. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pulina MV, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the plasma membrane Na+/Ca2+ exchangers with the 14-3-3 proteins. J Biol Chem. 2006;281:19645–19654. doi: 10.1074/jbc.M602033200. [DOI] [PubMed] [Google Scholar]
- 387.Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 2004;18:592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- 388.Oinuma I, Ito Y, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem. 2010;285:28200–28209. doi: 10.1074/jbc.M110.147546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci. 2006;119:951–957. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- 390.Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol. 2002;157:211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yucel G, Oro AE. Cell migration: GSK3β steers the cytoskeleton’s tip. Cell. 2011;144:319–321. doi: 10.1016/j.cell.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Terman JR, Kolodkin AL. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- 393.Fiedler SE, Schillace RV, Daniels CJ, Andrews SF, Carr DW. Myeloid translocation gene 16b is a dual A-kinase anchoring protein that interacts selectively with plexins in a phospho-regulated manner. FEBS Lett. 2010;584:873–877. doi: 10.1016/j.febslet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 394.Togashi H, Schmidt EF, Strittmatter SM. RanBPM contributes to Semaphorin3A signaling through plexin-A receptors. J Neurosci. 2006;26:4961–4969. doi: 10.1523/JNEUROSCI.0704-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Laht P, Pill K, Haller E, Veske A. Plexin-B3 interacts with EB-family proteins through a conserved motif. Biochim Biophys Acta. 2012 Feb 21 [DOI] [PubMed]
- 396.Cheng L, Lemmon S, Lemmon V. RanBPM is an L1-interacting protein that regulates L1-mediated mitogen-activated protein kinase activation. J Neurochem. 2005;94:1102–1110. doi: 10.1111/j.1471-4159.2005.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. Mical links semaphorins to F-actin disassembly. Nature. 2010;463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109:887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 399.Hung RJ, Terman JR. Extracellular inhibitors, repellents, and semaphorin/plexin/MICAL-mediated actin filament disassembly. Cytoskeleton (Hoboken) 2011;68:415–433. doi: 10.1002/cm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Schmidt EF, Shim SO, Strittmatter SM. Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein. J Neurosci. 2008;28:2287–2297. doi: 10.1523/JNEUROSCI.5646-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Nadella M, Bianchet MA, Gabelli SB, Barrila J, Amzel LM. Structure and activity of the axon guidance protein MICAL. Proc Natl Acad Sci USA. 2005;102:16830–16835. doi: 10.1073/pnas.0504838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334:1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Siebold C, Berrow N, Walter TS, Harlos K, Owens RJ, Stuart DI, Terman JR, Kolodkin AL, Pasterkamp RJ, Jones EY. High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc Natl Acad Sci USA. 2005;102:16836–16841. doi: 10.1073/pnas.0504997102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Bork P, Doerks T, Springer TA, Snel B. Domains in plexins: links to integrins and transcription factors. Trends Biochem Sci. 1999;24:261–263. doi: 10.1016/s0968-0004(99)01416-4. [DOI] [PubMed] [Google Scholar]
- 405.Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669–678. doi: 10.1016/j.sbi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 406.Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, Rajashankar KR, Lu M, Hoemme C, Püschel AW, Nikolov DB. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 407.Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 408.Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, Takagi J. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- 409.Janssen BJ, Robinson RA, Pérez-Brangulí F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Niemann HH. Structural insights into Met receptor activation. Eur J Cell Biol. 2011;90:972–981. doi: 10.1016/j.ejcb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 412.Stamos J, Lazarus RA, Yao X, Kirchhofer D, Wiesmann C. Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. EMBO J. 2004;23:2325–2335. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Takahashi T, Strittmatter SM. Plexin-A1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 415.Kozlov G, Perreault A, Schrag JD, Park M, Cygler M, Gehring K, Ekiel I. Insights into function of PSI domains from structure of the Met receptor PSI domain. Biochem Biophys Res Commun. 2004;321:234–240. doi: 10.1016/j.bbrc.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 416.Xiong JP, Stehle T, Goodman SL, Arnaout MA. A novel adaptation of the integrin PSI domain revealed from its crystal structure. J Biol Chem. 2004;279:40252–40254. doi: 10.1074/jbc.C400362200. [DOI] [PubMed] [Google Scholar]
- 417.Shi M, Sundramurthy K, Liu B, Tan SM, Law SK, Lescar J. The crystal structure of the plexin-semaphorin-integrin domain/hybrid domain/I-EGF1 segment from the human integrin beta2 subunit at 1.8-A resolution. J Biol Chem. 2005;280:30586–30593. doi: 10.1074/jbc.M502525200. [DOI] [PubMed] [Google Scholar]
- 418.Mould AP, Travis MA, Barton SJ, Hamilton JA, Askari JA, Craig SE, Macdonald PR, Kammerer RA, Buckley PA, Humphries MJ. Evidence that monoclonal antibodies directed against the integrin beta subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J Biol Chem. 2005;280:4238–4246. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Daburon V, Mella S, Plouhinec JL, Mazan S, Crozatier M, Vincent A. The metazoan history of the COE transcription factors. Selection of a variant HLH motif by mandatory inclusion of a duplicated exon in vertebrates. BMC Evol Biol. 2008;8:131. doi: 10.1186/1471-2148-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schöneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ma Q, Zhang K, Guin S, Zhou YQ, Wang MH. Deletion or insertion in the first immunoglobulin-plexin-transcription (IPT) domain differentially regulates expression and tumorigenic activities of RON receptor Tyrosine Kinase. Mol Cancer. 2010;9:307. doi: 10.1186/1476-4598-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zhou YQ, He C, Chen YQ, Wang D, Wang MH. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene. 2003;22:186–197. doi: 10.1038/sj.onc.1206075. [DOI] [PubMed] [Google Scholar]
- 424.Zhang K, Zhou YQ, Yao HP, Wang MH. Alterations in a defined extracellular region of the RON receptor tyrosine kinase promote RON-mediated motile and invasive phenotypes in epithelial cells. Int J Oncol. 2010;36:255–264. [PubMed] [Google Scholar]
- 425.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix–helix association. J Mol Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 426.de Wit J, Verhaagen J. Proteoglycans as modulators of axon guidance cue function. Adv Exp Med Biol. 2007;600:73–89. doi: 10.1007/978-0-387-70956-7_7. [DOI] [PubMed] [Google Scholar]
- 427.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 428.Bondeva T, Wojciech S, Wolf G. Advanced glycation end products inhibit adhesion ability of differentiated podocytes in a neuropilin-1-dependent manner. Am J Physiol Renal Physiol. 2011;301:F852–F870. doi: 10.1152/ajprenal.00575.2010. [DOI] [PubMed] [Google Scholar]
- 429.Esselens C, Malapeira J, Colomé N, Casal C, Rodríguez-Manzaneque JC, Canals F, Arribas J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J Biol Chem. 2010;285:2463–2473. doi: 10.1074/jbc.M109.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Artigiani S, Barberis D, Fazzari P, Longati P, Angelini P, van de Loo JW, Comoglio PM, Tamagnone L. Functional regulation of semaphorin receptors by proprotein convertases. J Biol Chem. 2003;278:10094–10101. doi: 10.1074/jbc.M210156200. [DOI] [PubMed] [Google Scholar]
- 431.Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Curr Opin Cell Biol. 2007;19:543–550. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 432.Tong Y, Buck M. 1H, 15 N and 13C Resonance assignments and secondary structure determination reveal that the minimal Rac1 GTPase binding domain of plexin-B1 has a ubiquitin fold. J Biomol NMR. 2005;31:369–370. doi: 10.1007/s10858-005-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tong Y, Hughes D, Placanica L, Buck M. When monomers are preferred: a strategy for the identification and disruption of weakly oligomerized proteins. Structure. 2005;13:7–15. doi: 10.1016/j.str.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Kiel C, Wohlgemuth S, Rousseau F, Schymkowitz J, Ferkinghoff-Borg J, Wittinghofer F, Serrano L. Recognizing and defining true Ras binding domains II: in silico prediction based on homology modelling and energy calculations. J Mol Biol. 2005;348:759–775. doi: 10.1016/j.jmb.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 435.Bouguet-Bonnet S, Buck M. Compensatory and long-range changes in picosecond-nanosecond main-chain dynamics upon complex formation: 15 N relaxation analysis of the free and bound states of the ubiquitin-like domain of human plexin-B1 and the small GTPase Rac1. J Mol Biol. 2008;377:1474–1487. doi: 10.1016/j.jmb.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Zhang L, Bouguet-Bonnet S, Buck M (2011) “Combining NMR and Molecular Dynamics Studies for Insights into the Allostery of Small GTPase-Protein Interactions”—book chapter for Methods in Molecular Biology: Allostery.Methods Proto (Humana Press) 796:235-56 [DOI] [PMC free article] [PubMed]
- 437.He H, Yang T, Terman JR, Zhang X. Crystal structure of the plexin A3 intracellular region reveals an autoinhibited conformation through active site sequestration. Proc Natl Acad Sci USA. 2009;106:15610–15615. doi: 10.1073/pnas.0906923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Kim, S, Hota, PK, Zhang, L, Borthakur, Buck, M (2012) in advanced preparation
- 439.Unpublished—solution structure pdb id 2OS6
- 440.Ward CW, Lawrence MC, Streltsov VA, Adams TE, McKern NM. The insulin and EGF receptor structures: new insights into ligand-induced receptor activation. Trends Biochem Sci. 2007;32:129–137. doi: 10.1016/j.tibs.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 441.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin JA. Structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–597. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 442.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Müller S, Knapp S. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 444.Marinissen MJ, Gutkind JS. Scaffold proteins dictate Rho GTPase-signaling specificity. Trends Biochem Sci. 2005;30:423–426. doi: 10.1016/j.tibs.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 445.Murai T, Maruyama Y, Mio K, Nishiyama H, Suga M, Sato C. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Haklai-Topper L, Mlechkovich G, Savariego D, Gokhman I, Yaron A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J. 2010;29:2635–2645. doi: 10.1038/emboj.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem. 2010;285:5361–5368. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Mann F, Rougon G. Mechanisms of axon guidance: membrane dynamics and axonal transport in semaphorin signalling. J Neurochem. 2007;102:316–323. doi: 10.1111/j.1471-4159.2007.04578.x. [DOI] [PubMed] [Google Scholar]
- 450.Zylbersztejn K, Petkovic M, Burgo A, Deck M, Garel S, Marcos S, Bloch-Gallego E, Nothias F, Serini G, Bagnard D, Binz T, Galli T. The vesicular SNARE Synaptobrevin is required for Semaphorin 3A axonal repulsion. J Cell Biol. 2012;196:37–46. doi: 10.1083/jcb.201106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Casaletto JB, McClatchey AI. Spatial regulation of receptor tyrosine kinases in development and cancer. Nat Rev Cancer. 2012;12(6):387–400. doi: 10.1038/nrc3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J Cell Biol. 2002;159:1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290–2299. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 454.Lu YC, Chen HC. Involvement of lipid rafts in adhesion-induced activation of Met and EGFR. J Biomed Sci. 2011;18:78. doi: 10.1186/1423-0127-18-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Ellis S, Mellor H. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 2000;10:85–88. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- 456.Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, Mukhopadhyay D, Horowitz A. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103:e71–e79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Li N, Lorinczi M, Ireton K, Elferink LA. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the cMet-tyrosine kinase. J Biol Chem. 2007;282:16764–16775. doi: 10.1074/jbc.M610835200. [DOI] [PubMed] [Google Scholar]
- 460.Carter S, Urbé S, Clague MJ. The met receptor degradation pathway: requirement for Lys48-linked polyubiquitin independent of proteasome activity. J Biol Chem. 2004;279:52835–52839. doi: 10.1074/jbc.M407769200. [DOI] [PubMed] [Google Scholar]
- 461.Sabo SL, McAllister AK. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6:1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- 462.Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- 463.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 464.Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 467.Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 468.Govek EE, Hatten ME, Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol. 2011;71:528–553. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Shang X, Cancelas JA, Li L, Guo F, Liu W, Johnson JF, Ficker A, Daria D, Geiger H, Ratner N, Zheng Y. R-Ras and Rac GTPase Cross-talk Regulates Hematopoietic Progenitor Cell Migration, Homing, and Mobilization. J Biol Chem. 2011;286:24068–24078. doi: 10.1074/jbc.M111.226951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Goryachev AB, Pokhilko AV. Computational model explains high activity and rapid cycling of Rho GTPases within protein complexes. PLoS Comput Biol. 2006;2:e172. doi: 10.1371/journal.pcbi.0020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Lipshtat A, Jayaraman G, He JC, Iyengar R. Design of versatile biochemical switches that respond to amplitude, duration, and spatial cues. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1247–1252. doi: 10.1073/pnas.0908647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Tsyganov MA, Kolch W, Kholodenko BN. The topology design principles that determine the spatiotemporal dynamics of G-protein cascades. Mol BioSyst. 2012;8:730–743. doi: 10.1039/c2mb05375f. [DOI] [PubMed] [Google Scholar]
- 473.Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci U S A. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Vayssière B, Zalcman G, Mahé Y, Mirey G, Ligensa T, Weidner KM, Chardin P, Camonis J. Interaction of the Grb7 adapter protein with Rnd1, a new member of the Rho family. FEBS Lett. 2000;467:91–96. doi: 10.1016/s0014-5793(99)01530-6. [DOI] [PubMed] [Google Scholar]
- 475.Bunney TD, Opaleye O, Roe SM, Vatter P, Baxendale RW, Walliser C, Everett KL, Josephs MB, Christow C, Rodrigues-Lima F, Gierschik P, Pearl LH, Katan M. Structural insights into formation of an active signaling complex between Rac and phospholipase C gamma 2. Mol Cell. 2009;34:223–233. doi: 10.1016/j.molcel.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 476.Binmadi NO, Proia P, Zhou H, Yang YH, Basile JR. Rho-mediated activation of PI(4)P5 K and lipid second messengers is necessary for promotion of angiogenesis by Semaphorin 4D. Angiogenesis. 2011;14:309–319. doi: 10.1007/s10456-011-9214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 478.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 479.Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis. 2011;32:613–621. doi: 10.1093/carcin/bgq281. [DOI] [PubMed] [Google Scholar]
- 480.Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 482.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 483.Fischer OM, Giordano S, Comoglio PM, Ullrich A. Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J Biol Chem. 2004;279:28970–28978. doi: 10.1074/jbc.M402508200. [DOI] [PubMed] [Google Scholar]
- 484.Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, Giordano S, Pani G, Galeotti T. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS) Oncogene. 2006;25:3689–3698. doi: 10.1038/sj.onc.1209409. [DOI] [PubMed] [Google Scholar]
- 485.Lu Y, Xiong Y, Huo Y, Han J, Yang X, Zhang R, Zhu DS, Klein-Hessling S, Li J, Zhang X, Han X, Li Y, Shen B, He Y, Shibuya M, Feng GS, Luo J. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci USA. 2011;108:2957–2962. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284:25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 488.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 489.Sun Q, Zhou H, Binmadi NO, Basile JR. Hypoxia-inducible factor-1-mediated regulation of semaphorin 4D affects tumor growth and vascularity. J Biol Chem. 2009;284:32066–32074. doi: 10.1074/jbc.M109.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Coma S, Shimizu A, Klagsbrun M. Hypoxia induces tumor and endothelial cell migration in a Semaphorin 3F- and VEGF-dependent manner via transcriptional repression of their common receptor Neuropilin 2. Cell Adh Migr. 2011;5:266–275. doi: 10.4161/cam.5.3.16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Bae D, Lu S, Taglienti CA, Mercurio AM. Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. J Biol Chem. 2008;283:28074–28080. doi: 10.1074/jbc.M804203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Morinaka A, Yamada M, Itofusa R, Funato Y, Yoshimura Y, Nakamura F, Yoshimura T, Kaibuchi K, Goshima Y, Hoshino M, Kamiguchi H, Miki H. (2011) Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci Signal. 4:ra26 [DOI] [PubMed]
- 493.Heo J, Campbell SL. Ras regulation by reactive oxygen and nitrogen species. Biochemistry. 2006;45:2200–2210. doi: 10.1021/bi051872m. [DOI] [PubMed] [Google Scholar]
- 494.Heo J, Raines KW, Mocanu V, Campbell SL. Redox regulation of RhoA. Biochemistry. 2006;45:14481–14489. doi: 10.1021/bi0610101. [DOI] [PubMed] [Google Scholar]
- 495.Day AM, Veal EA. Hydrogen peroxide-sensitive cysteines in the Sty1 MAPK regulate the transcriptional response to oxidative stress. J Biol Chem. 2010;285:7505–7516. doi: 10.1074/jbc.M109.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol. 2004;14:679–686. doi: 10.1016/j.sbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 497.Gatherwright, Hota PK, and Buck M. unpublished data
- 498.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 499.Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44:174–183. [PubMed] [Google Scholar]
- 500.Yoshida T, Uchida S, Mishina M. Regulation of synaptic vesicle accumulation and axon terminal remodeling during synapse formation by distinct Ca signaling. J Neurochem. 2009;111:160–170. doi: 10.1111/j.1471-4159.2009.06309.x. [DOI] [PubMed] [Google Scholar]
- 501.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 502.Henley J, Poo MM. Guiding neuronal growth cones using Ca2 + signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Nishiyama M, Togashi K, von Schimmelmann MJ, Lim CS, Maeda S, Yamashita N, Goshima Y, Ishii S, Hong K. Semaphorin 3A induces Ca(V)2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol. 2011;13:677–686. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 505.Ying Z, Giachini FR, Tostes RC, Webb RC. PYK2/PDZ-RhoGEF links Ca2 + signaling to RhoA. Arterioscler Thromb Vasc Biol. 2009;29:1657–1663. doi: 10.1161/ATVBAHA.109.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca(2 +) sensitization pathway in smooth muscle. Structure. 2004;12:1955–1965. doi: 10.1016/j.str.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 507.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 508.Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, Molday RS, Goshima Y, Hong K. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 509.Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- 510.Behar O, Mizuno K, Badminton M, Woolf CJ. Semaphorin 3A growth cone collapse requires a sequence homologous to tarantula hanatoxin. Proc Natl Acad Sci U S A. 1999;96:13501–13505. doi: 10.1073/pnas.96.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]