Abstract
Axonal guidance is key to the formation of neuronal circuitry. Semaphorin 3A (Sema 3A; previously known as semaphorin III, semaphorin D, and collapsin-1), a secreted subtype of the semaphorin family, is an important axonal guidance molecule in vitro and in vivo. The molecular mechanisms of the repellent activity of semaphorins are, however, poorly understood. We have now found that the secreted semaphorins contain a short sequence of high homology to hanatoxin, a tarantula K+ and Ca2+ ion channel blocker. Point mutations in the hanatoxin-like sequence of Sema 3A reduce its capacity to repel embryonic dorsal root ganglion axons. Sema 3A growth cone collapse activity is inhibited by hanatoxin, general Ca2+ channel blockers, a reduction in extracellular or intracellular Ca2+, and a calmodulin inhibitor, but not by K+ channel blockers. Our data support an important role for Ca2+ in mediating the Sema 3A response and suggest that Sema 3A may produce its effects by causing the opening of Ca2+ channels.
The semaphorins are a large family of secreted and membrane-bound proteins that have multiple and diverse functions, including a contribution to axon guidance during development of the nervous system (1). Semaphorin 3A (Sema 3A), a secreted semaphorin expressed in vertebrates, repels sensory neurons by inducing collapse of their axonal growth cones (2, 3). Sema 3A knockout mice, mRNA expression studies, and coculture experiments suggest that its expression results in an exclusion zone for responsive axons and that this guides them in an orderly way to their topographically correct targets (4–9).
The molecular mechanisms by which semaphorins transduce their signal are not well understood. Sema 3A binds to neuropilin 1, a membrane-bound molecule expressed on many neuronal cell types, which is necessary for its repulsion activity (10–12). The cytoplasmic domain of neuropilin 1 is, however, very short, contains no obvious homology to other signaling motifs, and is not necessary for Sema 3A activity (13). Therefore, it has been suggested that the Sema 3A receptor includes another, as of yet unknown, transmembrane signaling component (13, 14). The second messenger systems involved in the mediation of Sema 3A activity are also not well characterized, although recent work has indicated that both the small GTP-binding protein rac 1 and the cGMP pathway may mediate Sema 3A activity (15–17). Activation of the cGMP pathway in rat sensory growth cones inhibits Sema 3A-induced collapse, while Sema 3A-mediated repulsion of Xenopus spinal cord neurons can be converted to attraction by activation of the cGMP pathway (17).
We have now set out to investigate the molecular basis for the growth cone collapse activity of Sema 3A.
Methods
Site-Directed Mutagenesis.
Sema 3A gene was mutated with QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s protocol. The oligonucleotides used to generate the YWD mutant (see Results) were: CTCGCCCGAGACCCTGCCTGTGCTGCGGCTGGTTCTGCATGT and GAACATGCAGAACCAGCCGCAGCACAGGCAGGGTCTCGGGCG. The oligonucleotides used to generate the RD mutant were: CTGAGTGTTGCCTCGCCGCAGCCCCTTACTGTGCTTGGG and CCCAAGCACAGTAAGGGGCTGCGGCGAGGCAACACTCAG.
Generation of Alkaline Phosphatase (AP)-Fusion Protein.
To generate the H-Sema 3A-AP (N terminus) fusion protein expression vector, the human Sema 3A coding sequence was inserted into the BglII and XhoI sites of pAPtag4 to generate a Sema 3A-AP fusion (18). Then, the entire Sema 3A-AP sequence was excised from pAPtag4 vector and inserted into the HindIII and XhoI sites of pCDNA 1.1 Amp. This vector also included myc and His epitope tagged at the C terminus.
Binding Experiments.
pCDNA 1.1Sema 3A-AP or RD-AP were transfected to 293T cells, and the cell supernatants were used for binding experiments with stably transfected PAE-Neuropilin 1 and PAE parental cell lines. Cells were incubated with supernatant containing Sema 3A-AP or RD-AP fusion protein. Values represent means ± SEM. The fusion protein binding experiments were as described (19).
Repulsion Assay.
Mouse embryonic day 13 (E13) dorsal root ganglion (DRG) and Sema 3A-, RD-, or YWD- (pCOS H-Sema 3A-myc) expressing COS-7 cells were cocultured for 40 hr, as described (9), except that collagen was supplemented with 10% growth factor-reduced Matrigel (Collaborative Biomedical Products, Bedford, MA). Paraformaldehyde-fixed cocultures were immunostained with a neurofilament-specific Ab (1:1000; Chemicon) and AP-conjugated secondary Ab (1:400; Roche Molecular Biochemicals).
Collapse Assay.
DRG explants derived from E13 mouse embryos were cultured for 20 hr in F12/N3 medium as described (20), except that the medium contained only 0.5% FBS, 15 mM Hepes (pH 7.5), 0.6 mg/ml hydroxypropylmethyl-cellulose (5600 centipoise; Sigma), and 50 ng/ml nerve growth factor (Roche Molecular Biochemicals) on plates precoated with growth factor-reduced Matrigel (Collaborative Biomedical Products) diluted 1:5 in F12 medium. The collapse assay was performed with Sema 3A-AP-containing medium, essentially as described (2). The DRG were stained with 5 units/ml rhodamine-phalloidin (Molecular Probes) for 20 min, then washed and mounted with Vectashield (Vector Laboratories). Pharmacological agents were added 7 min [CoCl2, CdCl2, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), or hanatoxin] or 60 min (calmidazolium chloride) before the addition of Sema 3A, thrombin, or control conditioned medium. To load BAPTA acetoxymethyl ester (BAPTA-AM), the DRG explants were preincubated at room temperature for 30 min, washed once, and incubated for another 30 min at 37°C before the addition of Sema 3A or control-conditioned medium. CoCl2, CdCl2, and thrombin were obtained from Sigma; BAPTA and BAPTA-AM were obtained from Molecular Probes; calmidazolium chloride was obtained from Calbiochem; hanatoxin, purified from Phrixotrichus spatulata venom, was generously provided by Kenton J. Swartz (Molecular Physiology and Biophysics Unit, National Institutes of Health, Bethesda, MD).
Data Analysis.
Values represent means ± SEM. Paired t test, t test, and one-way ANOVA were utilized to compare different treatment groups as indicated. P ≤ 0.05 was considered significant.
Results
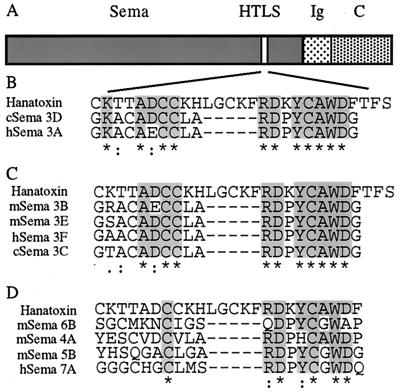
Using primary sequence alignment of the semaphorin genes, we have identified a domain with a sequence homology to hanatoxin, a short peptide tarantula toxin that selectively blocks some voltage-gated K+ and Ca2+ channels (21, 22). The hanatoxin-like sequence (HTLS) is found in the semaphorin domain of all chicken and mammal secreted semaphorins (Fig. 1 B and C). The similarity between semaphorin and hanatoxin lies in two short subdomains, with a small gap between them. Sema 3A and Sema3D show highest homology to hanatoxin (Fig. 1B). Other secreted semaphorins are slightly less homologous (Fig. 1C). All membrane-bound semaphorins are significantly less homologous to hanatoxin, with only one subdomain of the HTLS (Fig. 1D).
Figure 1.
Primary sequence alignment of the semaphorin genes with hanatoxin. (A) Diagram of type III semaphorin genes (the secreted semaphorins). The three major domains, semaphorin domain (Sema), Ig-like domain (Ig), and carboxyl-terminal domain (C), are indicated. The site with sequence homology to hanatoxin (HTLS) is indicated. (B) Primary sequence alignment of the products of the two most homologous semaphorin genes, Sema 3A and Sema 3D, with hanatoxin (amino acids 9–35). (C) Sequence alignment of those secreted semaphorin gene products with less homology to hanatoxin. (D) Primary sequence alignment of four membrane-bound semaphorin. * indicates identity, : indicates high similarity, and . indicates low similarity.
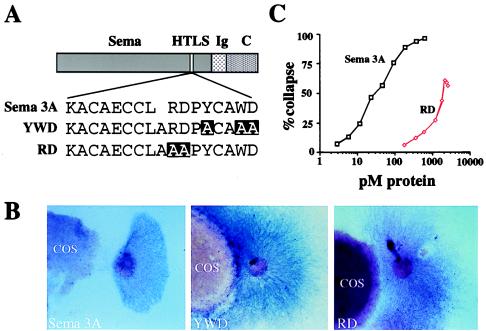
Based on the presence of an HTLS in secreted semaphorins, we hypothesized that the secreted semaphorins may produce repulsion of neuronal growth cones by an interaction with a voltage-gated K+ or Ca2+ channel. To begin testing this hypothesis, we mutated a number of conserved amino acids in one subdomain of the HTLS of Sema 3A to alanine (Fig. 2A). The two mutations generated were RDPYCAWD to AAPYCAWD (RD) and RDPYCAWD to RDPACAAA (YWD). Each of the mutants, as well as wild-type Sema 3A, was transfected into COS-7 cells and tested for its capacity to repel axonal outgrowth by coculturing it for 40 hr with an embryonic (E13.5) mouse explant DRG. DRG axons were repelled from COS-7 cells transfected with wild-type Sema 3A (Fig. 2B). In contrast, COS-7 cells transfected with RD or YWD mutants had only very weak repellent effects on DRG axons, which grew in close proximity to the transfected COS cells (Fig. 2B). The degree of activity retained by the RD mutant was further tested in a growth cone collapse assay, which measures the immediate effects of Sema 3A on growth cone morphology. This mutant was 35.9-fold less potent than the wild-type Sema 3A (EC50 values 0.047 ± 0.003 nM and 1.69 ± 0.54 nM for wild-type and mutant proteins, respectively, n = 4) (Fig. 2C), and never, even at high doses, produced the level of collapse generated by the wild-type protein.
Figure 2.
Repulsion activity of Sema 3A HTLS mutants. (A) Schematic structure of Sema 3A protein showing wild-type and mutant sequences. (B) DRG explants cocultured with COS-7 cells expressing myc-Sema 3A, YWD mutant, or RD mutant were cultured for 40 hr. Note the strong repulsive effect in the wild-type transfected cells in comparison with the mutants. (Magnification, ×40.) (C) Dose–response curve, from one experiment, comparing RD-AP mutant growth cone collapse activity to Sema 3A-AP. Each concentration was repeated with at least 12 explants. EC50 for Sema 3A is 47 pM and for RD is 1466 pM.
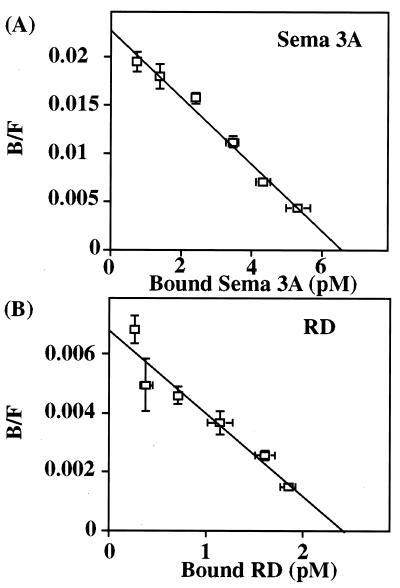
Sema 3A contains two neuropilin 1 binding sites, one in the C domain and the other in the sema domain (amino acids 25–585) (10). A truncated sema domain (amino acids 25–526) has been shown not to exhibit neuropilin 1 binding (10). Because the HTLS is nearby (amino acids 522–538), mutations may impair binding to neuropilin 1 through local or global conformational changes. To test whether a mutation of the HTLS region of Sema 3A altered binding to neuropilin 1, we compared binding of the RD mutant and wild-type Sema 3A to neuropilin 1-expressing cells by using AP tag constructs. Both Sema 3A and the mutant RD, however, did bind to neuropilin 1 with similar affinities (Kd = 218.9 ± 27.9 pM and 418.5 ± 98.3 pM for Sema 3A and RD; n = 8, respectively, P = 0.0673, paired t test) (Fig. 3). We conclude that the HTLS is likely to play a role in the functional activity of Sema 3A, independent of neuropilin 1 binding.
Figure 3.
Scatchard analysis of Sema 3A and RD mutant. Control PAE cells or PAE-NP-1 cells expressing neuropilin 1 (5 × 104 cells) were treated with varying amounts of media containing Sema 3A-AP or RD-AP mutant. B/F, bound/free. Scatchard plots from one of the experiments for Sema 3A (A) and RD mutant (B) are presented. Kd value for Sema 3A-AP was 305 pM and for RD mutant was 505 pM. Bars indicate SEM for triplicates; where not obvious, the error bars are smaller than the symbols.
Because hanatoxin is a voltage-gated K+ or Ca2+ channel blocker and mutation of the HTLS reduces Sema 3A activity, we addressed the question whether Sema 3A acts through K+ or Ca2+ ion channel blockade or activation. To test this, we investigated whether K+ and Ca2+ ion channel blockers can mimic or inhibit the growth cone collapse caused by Sema 3A. The K+ channel blockers tetraethylammonium (up to 30 mM) and 4-aminopyridine (up to 10 mM) neither induced growth cone collapse by themselves nor modified Sema 3A-induced growth cone collapse. A similar lack of activity was found for a number of specific K+ ion channel toxins [apamin (130 nM), charybdotoxin (100 nM), α-dendrotoxin (100 nM), iberiotoxin (10 μM), kaliotoxin (10 μM), margatoxin (1 μM), stichodactyla toxin (0.5 μM), and tityustoxin Kα (5 μM)].
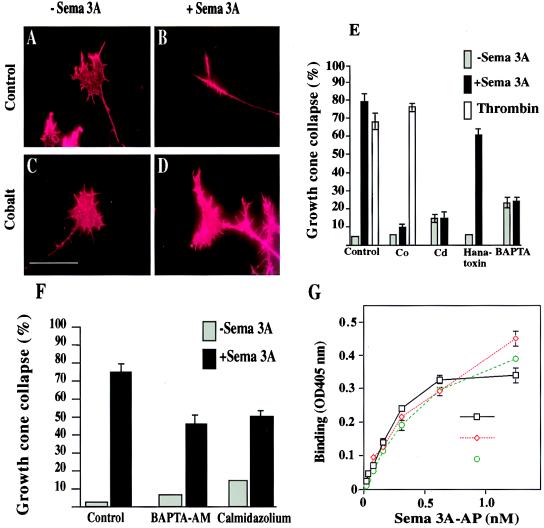
The general Ca2+ channel blocker cobalt (CoCl2, 2 mM) by itself also did not affect DRG growth cones (Fig. 4 C and E). However, when incubated in the presence of Sema 3A, CoCl2 inhibited Sema 3A action on DRG growth cones by 87.8% (n = 12, P < 0.0001 one-way ANOVA; Fig. 4 D and E). Another general Ca2+ channel blocker, cadmium (CdCl2, 200 μM), while having an effect on growth cone morphology (reduction in surface area) by itself, when combined with Sema 3A, inhibited growth cone collapse by 81.2% at concentrations of 200 μM (n = 8, P < 0.0001 one-way ANOVA; Fig. 4E). In addition, we tested several specific Ca2+ channel blockers. The activity of Sema 3A was not blocked by 7 μM nifedipine (L-type Ca2+ channel), 3 μM ω-conotoxin GVIA (N-type Ca2+ channel), or 0.3 μM ω-agatoxin (P-/Q- type Ca2+ channel) (data not shown). Finally, we tested the effects of hanatoxin. Sema 3A activity in the presence of hanatoxin (10 μM) was reduced by 23.2% (n = 12, P < 0.0086 one-way ANOVA; Fig. 4E). At this dose, hanatoxin had no effect on DRG growth cones. The limited availability of hanatoxin prevented any testing of higher concentrations.
Figure 4.
Extracellular Ca2+ influx and a Ca2+ channel are required for the collapse-inducing activity of Sema 3A. (A–E) E13 mouse embryo DRG explant cultures were incubated with or without various calcium channel blocking or depleting agents for 7 min prior to addition of control conditioned medium (A, C, and E) or Sema 3A-AP-conditioned medium (B, D, and E). (A and B) Control conditioned medium only; (C and D) with 2 mM CoCl2. (Scale bar, 50 μm.) (E) The relative responsiveness of DRG growth cones to Sema 3A-AP in the presence of 2 mM CoCl2, 200 μM CdCl2, 10 μM hanatoxin, and 1 mM BAPTA or thrombin (50 units/ml) in the presence or absence of 2 mM CoCl2. The percentage of collapsed growth cones with (black bars) or without (gray bars) Sema 3A-AP or with thrombin (white bars) is shown. (F) E13 mouse embryo DRG explant cultures were incubated with or without 2 μM BAPTA-AM and 0.08 μM calmidazolium chloride for 60 min prior to addition of control conditioned medium or Sema 3A-AP-conditioned media. The percentage of collapsed growth cones with (black bars) or without (gray bars) Sema 3A-AP in the presence of these reagents is presented. (G) Control PAE cells or PAE-NP-1 cells were treated with medium containing Sema 3A-AP with or without 2 mM CoCl2 or 200 μM CdCl2. Bars indicate SEM for triplicates. Where not obvious, the error bars are smaller than the symbols.
To test whether extracellular Ca2+ blockers inhibit DRG growth cone collapse nonspecifically, we tested the effect of cobalt on another molecule capable of inducing growth cone collapse. Thrombin, a G protein-dependent collapsing factor (23), induced collapse activity in E13.5 DRG axons. In contrast to Sema 3A-induced growth cone collapse, however, cobalt did not, at a dose effective for Sema 3A, block the collapse induced by thrombin (n = 8, P = 0.214, t test; Fig. 4E).
The role of Ca2+ on the activity of Sema 3A was further characterized by reducing the extracellular concentration of Ca2+ with the Ca2+ chelator BAPTA (1 mM). The calculated Ca2+ concentration in the medium after BAPTA treatment was about 50 nM, similar to the resting intracellular concentrations in rodent growth cones (24, 25). While BAPTA itself caused a small increase in growth cone collapse (15%), compared with control (Fig. 4E) it completely blocked the growth cone collapsing activity of Sema 3A (Fig. 4E). By using the indicator fluo-3, we were unable to detect consistent change in cytosolic Ca2+ in growth cones on exposure to Sema 3A (data not shown). This result, as with another calcium-dependent axon-guidance cue netrin, and the inositol 1,4,5-trisphosphate (IP3) receptor, is likely to result from technical difficulties related to the low amount of dye in the small volume of the growth cone and the localized nature of Ca2+ flux (26, 27).
Intracellular Ca2+ ([Ca2+]i) manipulations also modified Sema 3A activity. Preloading DRG explants with BAPTA-AM at a dose of 2 μM did not change growth cone morphology, but reduced Sema 3A induced growth cone collapse by 38.8% (n = 9, P < 0.0001, one-way ANOVA; Fig. 4F). Ca2+ influx into growth cones triggered by Sema 3A could activate numerous target proteins such as Ca2+/calmodulin, a major [Ca2+]i receptor abundant in growth cones (28). Pretreatment of DRG explants with the calmodulin inhibitor calmidazolium chloride at 0.08 μM reduced the Sema 3A-induced growth cone collapse by 33.6% (n = 12, P < 0.0001 one-way ANOVA; Fig. 4F). Because higher doses of BAPTA-AM and calmidazolium chloride induced growth cone collapse, an analysis of whether a complete inhibition of Sema 3A’s action could be achieved by a greater [Ca2+]i block could not be performed.
To exclude the possibility that the blocking effects of Sema 3A by heavy metals is simply the result of a dependence of Sema 3A binding to neuropilin 1 on a Ca2+-dependent protein–protein interaction, we tested the effects of these reagents on Sema 3A neuropilin binding. The calculated Kd for Sema 3A-AP binding is 187.0 ± 55.7 pM (n = 6). In the presence of CoCl2 (2 mM) or CdCl2 (200 μM), the calculated Kd for Sema 3A-AP binding is 258.7 ± 80.8 pM (n = 6) and 222.0 ± 88.4 pM (n = 6), respectively. Both CoCl2 and CdCl2 had no significant effect on Sema 3A/neuropilin 1 binding properties (P = 0.438 and 0.563 for CoCl2 and CdCl2, respectively; paired t test; Fig. 4G).
Discussion
Our data suggest that a rise in [Ca2+]i mediated by means of a membrane-bound Ca2+ channel is a key step in producing Sema 3A-induced collapse of mouse sensory neurons growth cones, because a reduction of extracellular Ca2+, a blockade of Ca2+ channels, a reduction of [Ca2+]i, and an inhibition of Ca2+/calmodulin activity all result in a decrease of Sema 3A activity. In contrast to our findings, the repulsion response of Xenopus spinal cord neurons to Sema 3A has been reported to be Ca2+-independent (17). There are several explanations for this apparent contradiction. The first is differences in extracellular Ca2+ levels in the two experiments. We reduced the Ca2+ levels to a degree where no extracellular/intracellular gradient exists across the growth cone membrane. In contrast, in the Xenopus experiments, there was a 10- to 20-fold higher concentration of extracellular than intracellular Ca2+ (1 μM extracellular concentration). Although the actions of a number of guidance molecules (netrin 1, MAG, BDNF) are blocked when Ca2+ concentrations are reduced to 1 μM, that of Sema 3A may not be (26, 29). Second, the response of Xenopus spinal cord neurons to Sema 3A does not involve growth cone collapse, but rather a change in growth direction, which may involve a different signal transduction pathway (17).
The binding of Sema 3A to neuropilin 1 has been shown to be necessary for its collapse activity, but data from multiple experiments suggest that it is not sufficient and that another membrane protein is likely to be involved in the signal transduction triggered by Sema 3A binding (14). The identity of the putative Sema 3A coreceptor is not yet known. Our data suggest that the opening of Ca2+ channels in the membrane is required for the growth-arresting activity of Sema 3A. This may occur indirectly, either through an activation of a second messenger cascade that acts on such Ca2+ channels or secondary to a change in membrane potential mediated by closing K+ or opening Na+ channels. Alternatively, it is possible that Sema 3A interacts directly with an ion channel when it binds to neuropilin, possibly by the binding of its hanatoxin-like region to the channel. The fact that hanatoxin partially blocks the action of Sema 3A rather than mimicking it suggests that hanatoxin has an action opposite to the hanatoxin-like domain in Sema 3A. How could this be? Hanatoxin is a channel blocker that does not block the pore site but binds to the voltage sensor unit of the channel (22). Toxins binding to voltage sensors in voltage-gated ion channels can either block activation (e.g., hanatoxin on K+ and Ca2+ channels) or slow inactivation (e.g., α scorpion toxin on Na+ channels) (22, 30–32). Interestingly, hanatoxin itself can, after point mutations to the hanatoxin-binding site of K+ channels, act as a channel opener rather than blocker (Kenton J. Swartz, personal communication). It is therefore possible that the binding of Sema 3A and hanatoxin to the same site on a channel may result in opposite effects.
Ca2+ has been shown in many studies to be a major mediator of axonal growth and guidance (33). A reduction of extracellular Ca2+ concentration increases the rate of axonal growth, while an increase in growth cone Ca2+ concentration ([Ca2+]i) leads to growth cone collapse or paralysis (17, 24, 34). A recent in vivo study has shown that [Ca 2+]i in the growth cone has a key role in regulating its motility (35). Transient elevations of growth cone Ca2+ slow rapid axonal growth, while suppressing Ca2+ transients accelerates axon extension (35). Furthermore, growth cone stalling and axon retraction are associated with high frequencies of Ca2+ transients (35). Our data that Ca2+ is required for Sema 3A collapse activity are consistent, therefore, with current findings on the role of Ca2+ in growth cone motility. However, Ca2+ channels are not always necessary for repulsion, as we show for thrombin and as has also been shown for chondroitin sulfate proteoglycan (36). In addition, a role for Ca2+ influx and subsequent calmodulin activation could be consistent with earlier findings that an elevation in cGMP inhibits Sema 3A growth cone collapse (17). An activation of Ca2+/calmodulin-dependent cGMP phosphodiesterase activity on calcium influx will lead to a decrease in cGMP levels, and this may contribute to growth cone collapse.
The type of Ca2+ channel involved in mediating the influx triggered directly or indirectly by Sema 3A is not known. The inability of the specific Ca2+ channel blockers to inhibit the activity of Sema 3A has several different explanations. It is possible that the Ca2+ channel involved in Sema 3A activity is not sensitive to these inhibitors. Interestingly, spontaneous Ca2+ spiking in DRG growth cone is also inhibited by general blockers (i.e., Ni2+, La3+) but not by L-, N-, or P-/Q-type voltage-sensitive Ca2+ channel blockers (24). This may indicate that DRG growth cones express an as of yet unidentified Ca2+ channel. An alternative explanation may be the ability of Sema 3A to act through more than one type of Ca2+ channel. Interestingly, hanatoxin also shows low ion channel specificity, probably as a result of its action on the voltage sensor site (22). If the homology of Sema 3A and hanatoxin reflects a common functional action on voltage sensor, it suggests that Sema 3A may also possess a similar low specificity for Ca2+ channels.
The structure–function relationships of different regions of Sema 3A have been previously analyzed. The sema domain has been shown to be necessary, and when dimerized, it is sufficient for activity (37). This domain has also been shown to bind neuropilin 1, but the sequence responsible for the binding is not yet identified (10). Our results show that amino acids within the HTLS region (amino acids 522–538) are essential for Sema 3A’s repellent activity. Because all secreted semaphorins include this sequence, we propose that the activity of all secreted semaphorins is likely to be mediated through a single common pathway. This activity may be mediated by interaction of the HTLS region with a common membrane-bound protein, while other sequences in the sema domain may enable different semaphorins to interact only with particular subtypes of neurons. In other words, the sema domain may have a common functional region and different specificity regions. Consistent with this possibility is the finding that when a 70-aa (amino acids 166–235) stretch from cSema3D (which normally has no collapse effect on DRG neurons) was replaced by the homologous 70 amino acids from cSema 3A, the chimera protein was able to induce DRG growth cone collapse (37). These amino acids may be responsible for the interaction between different secreted semaphorins and their appropriate targets, whereas the HTLS region may be responsible for the activation of the repellent signal.
Acknowledgments
We thank David E. Clapham, Gary Yellen, and Bruce Bean for helpful comments; Marc Tessier-Lavigne for providing the pCEP4 Sema 3A-AP and pCOS (H-Sema 3A-myc); John G. Flanagan for providing pAPtag-4 plasmids; Shay Soker for providing the PEA and PEA-NP-1 cells; and Kenton J. Swartz for the hanatoxin. This work was supported by the National Institutes of Health Grant NS38253-01, the International Spinal Research Trust, and Medical Research Council Grant G9431792 (to C.J.W.).
Abbreviations
- Sema 3A
Semaphorin 3A
- DRG
dorsal root ganglion
- HTLS
hanatoxin-like sequence
- AP
alkaline phosphatase
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BAPTA-AM
BAPTA acetoxymethyl ester
- [Ca2+]i
intracellular calcium
- En
embryonic day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mark M D, Lohrum M, Puschel A W. Cell Tissue Res. 1997;290:299–306. doi: 10.1007/s004410050934. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Raible D, Raper J A. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 3.Kolodkin A L, Matthes D J, Goodman C S. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 4.Behar O, Golden J A, Mashimo H, Schoen F J, Fishman M C. Nature (London) 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 6.Ulupinar E, Datwani A, Behar O, Fujisawa H, Erzurumlu R. Mol Cell Neurosci. 1999;13:281–292. doi: 10.1006/mcne.1999.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giger R J, Wolfer D P, De Wit G M, Verhaagen J. J Comp Neurol. 1996;375:378–392. doi: 10.1002/(SICI)1096-9861(19961118)375:3<378::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Wright D E, White F A, Gerfen R W, Silos-Santiago I, Snider W D. J Comp Neurol. 1995;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- 9.Messersmith E K, Leonardo E D, Shatz C J, Tessier-Lavigne M, Goodman C S, Kolodkin A L. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Tessier-Lavigne M. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 11.Kolodkin A L, Levengood D V, Rowe E G, Tai Y T, Giger R J, Ginty D D. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 12.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura F, Tanaka M, Takahashi T, Kalb R G, Strittmatter S M. Neuron. 1998;21:1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 14.Yu H H, Kolodkin A L. Neuron. 1999;22:11–14. doi: 10.1016/s0896-6273(00)80672-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn T B, Brown M D, Wilcox C L, Raper J A, Bamburg J R. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin Z, Strittmatter S M. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song H, Ming G, He Z, Lehmann M, Tessier-Lavigne M, Poo M. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan J G, Leder P. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H J, Flanagan J G. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 20.Tessier-Lavigne M, Placzek M, Lumsden A G, Dodd J, Jessell T M. Nature (London) 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 21.Swartz K J, MacKinnon R. Neuron. 1995;15:941–949. doi: 10.1016/0896-6273(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 22.Li-Smerin Y, Swartz K J. Proc Natl Acad Sci USA. 1998;95:8585–8589. doi: 10.1073/pnas.95.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalink K, Moolenaar W H. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez T M, Snow D M, Letourneau P C. Neuron. 1995;14:1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn T B, Williams C V, Dou P, Kater S B. J Neurosci. 1998;18:184–194. doi: 10.1523/JNEUROSCI.18-01-00184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ming G L, Song H J, Berninger B, Holt C E, Tessier-Lavigne M, Poo M M. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 27.Takei K, Shin R M, Inoue T, Kato K, Mikoshiba K. Science. 1998;282:1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 28.Letourneau P C, Snow D M, Gomez T M. Prog Brain Res. 1994;102:35–48. doi: 10.1016/S0079-6123(08)60530-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Zheng J Q. J Neurosci. 1998;18:4973–4984. doi: 10.1523/JNEUROSCI.18-13-04973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catterall W A. J Biol Chem. 1977;252:8660–8668. [PubMed] [Google Scholar]
- 31.Catterall W A, Beress L. J Biol Chem. 1978;253:7393–7396. [PubMed] [Google Scholar]
- 32.Catterall W A. J Gen Physiol. 1979;74:375–391. doi: 10.1085/jgp.74.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg D J, Grabham P W. Neuron. 1999;22:423–425. doi: 10.1016/s0896-6273(00)80697-2. [DOI] [PubMed] [Google Scholar]
- 34.Bandtlow C E, Schmidt M F, Hassinger T D, Schwab M E, Kater S B. Science. 1993;259:80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- 35.Gomez T M, Spitzer N C. Nature (London) 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 36.Snow D M, Atkinson P B, Hassinger T D, Letourneau P C, Kater S B. Dev Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- 37.Koppel A M, Feiner L, Kobayashi H, Raper J A. Neuron. 1997;19:531–537. doi: 10.1016/s0896-6273(00)80369-4. [DOI] [PubMed] [Google Scholar]