Abstract
The prevention and treatment of malaria is heavily dependent on antimalarial drugs. However, beginning with the emergence of chloroquine (CQ)-resistant Plasmodium falciparum parasites 50 years ago, efforts to control the disease have been thwarted by failed or failing drugs. Mutations in the parasite’s ‘chloroquine resistance transporter’ (PfCRT) are the primary cause of CQ resistance. Furthermore, changes in PfCRT (and in several other transport proteins) are associated with decreases or increases in the parasite’s susceptibility to a number of other antimalarial drugs. Here, we review recent advances in our understanding of CQ resistance and discuss these in the broader context of the parasite’s susceptibilities to other quinolines and related drugs. We suggest that PfCRT can be viewed both as a ‘multidrug-resistance carrier’ and as a drug target, and that the quinoline-resistance mechanism is a potential ‘Achilles’ heel’ of the parasite. We examine a number of the antimalarial strategies currently undergoing development that are designed to exploit the resistance mechanism, including relatively simple measures, such as alternative CQ dosages, as well as new drugs that either circumvent the resistance mechanism or target it directly.
Keywords: Plasmodium falciparum, Malaria, Drug resistance, Chloroquine, Quinoline, PfCRT
The malaria parasite and the quinoline class of antimalarial drugs
Plasmodium falciparum has persisted as a major cause of human suffering and death despite the deployment of successive classes of potent antimalarial drugs. The parasite has also proven refractory to the vaccine approaches trialled to date. As a result, malaria remains a leading global health problem, currently accounting for approximately 225 million clinical cases and almost 1 million deaths per year [1]. Moreover, the socio-economic burden of the disease is horrendous, particularly in endemic countries where malaria is estimated to cost 1.3 percent of economic growth per year [2, 3], and where human cognitive abilities, education, and productivity are all reduced as a consequence of infection by P. falciparum [4–6].
In the course of its complex life cycle, the parasite invades the erythrocytes of its host, and it is this intra-erythrocytic stage that gives rise to all of the symptoms of malaria and against which the majority of antimalarial drugs act [7]. The first effective antimalarial was an extract prepared from the bark of the South American Cinchona tree. This treatment was introduced to Europe in the seventeenth century, and in 1820 it was shown that the active ingredients were a group of quinoline compounds—including quinine (QN). QN was used extensively (and is still recommended by the WHO as a second-line treatment for both severe and uncomplicated malaria [1]), but wartime shortages of the drug led to the development of synthetic quinoline alternatives. One such synthetic antimalarial—quinacrine (QC)—was heavily used in World War II. However, by the end of the war, it was superseded by a superior synthetic substitute—chloroquine (CQ). CQ proved to be a safer, cheaper and more effective drug, and served as the frontline antimalarial treatment from the mid-1940s to the 1990s, by which time the emergence and spread of CQ-resistant (CQR) parasites had rendered the drug ineffective in most endemic regions. Due to the effectiveness and longevity of CQ and its predecessors, it is estimated that the quinolines have saved more lives than any other class of drug in history [8, 9]. The non-quinoline antimalarials deployed to replace CQ have by comparison suffered short life spans. For example, resistance to Fansidar (sulfadoxine-pyrimethamine) arose within 1 year and rapidly became widespread [10], while artemisinin, the drug recently deployed to treat multi-drug resistant malaria, is beginning to succumb to resistance in Cambodia, Thailand, Burma, and Vietnam [11–15].
Against this backdrop of failed and failing drugs, and in the absence of an effective vaccine, the UN has committed to ending malaria deaths by 2015, and the goals of malaria elimination and eradication have been revived [1]. If these goals are to be met, there is a dire need to expand the arsenal of antimalarial drugs, and to make the most of the weapons at hand. Efforts to understand the mechanisms of drug resistance are vital to extending the longevity and effectiveness of the current set of antimalarials, and could aid the development of the next generation of drugs. Here, we review our current understanding of the mechanism of CQ resistance, and apply these insights to dissect the often perplexing patterns observed in the parasite’s susceptibility to different quinoline drugs. We assess the current antimalarial drug strategies, and provide examples of how inherent weaknesses in the quinoline-resistance mechanisms could be exploited to deliver new, robust antimalarial strategies.
Chloroquine: mechanisms of action and resistance
CQ is a diprotic weak base with the relative proportions of the neutral, mono-protonated and di-protonated species varying with pH. The neutral species enters the parasite and its internal compartments via simple diffusion. On entering the acidic environment of the parasite’s internal ‘digestive vacuole’ (DV; pH ~5; [16–18]), the equilibrium is shifted towards the di-protonated form (CQH2 2+) which, unable to diffuse across the membrane, is trapped and thereby accumulates to high concentrations within this compartment [19, 20]. Here, CQ is thought to bind to the monomeric haem released from the parasite’s digestion of host haemoglobin, preventing its conversion to the inert crystal haemozoin. It is the resulting accumulation of the toxic haem monomers and/or the haem-CQ complex that is thought to kill the parasite [21]. Other related antimalarials, such as QN, QC, amodiaquine (AQ), piperaquine (PIP), and pyronaridine (PN), are also thought to accumulate in the DV and to exert the same ‘anti-haemozoin’ activity [22–27]. However, although the quinoline methanols QN and mefloquine (MQ) have been shown to bind to haem [28] and to inhibit haemozoin formation in vitro, it is probable that these drugs also target other (possibly cyctosolic and/or membrane) processes in the parasite [25]. Likewise, halofantrine [HF; which, along with the related antimalarial lumefantrine (LM), is a synthetic analogue of QN] has been shown to interfere with the crystallisation of haem in vitro, but is also suspected to have other targets within the parasite [29]. The structures of the above aminoquinolines and related antimalarials are shown in Fig. 1a.
Fig. 1.
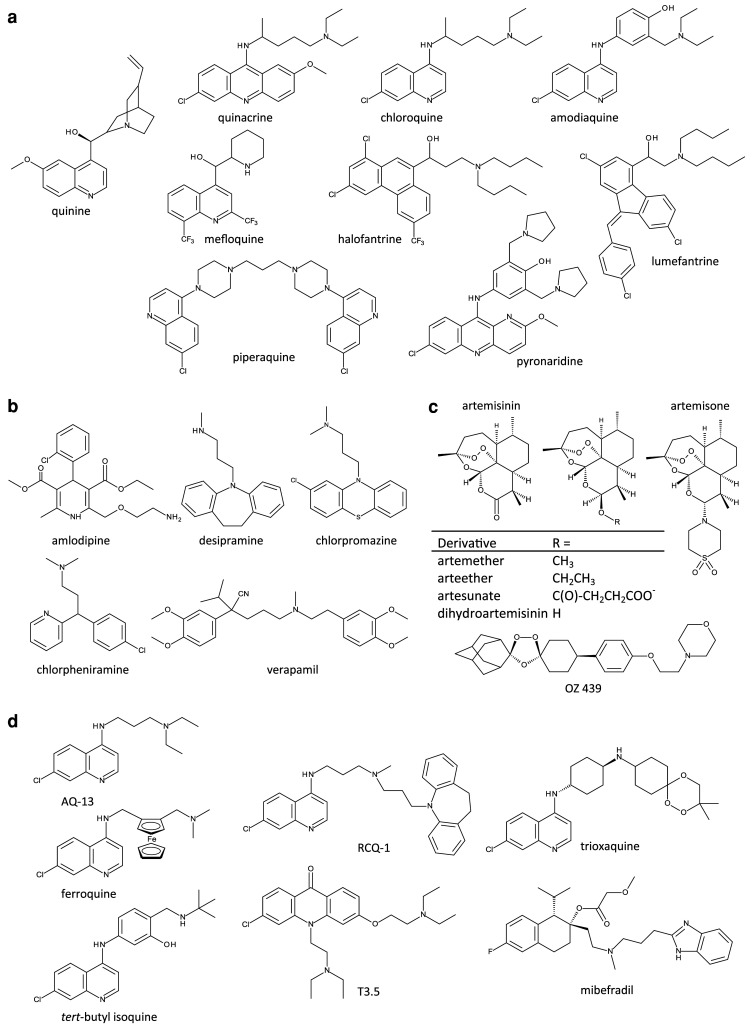
Structure of antimalarial drugs and CQ-resistance reversers. a CQ and related antimalarial compounds. b ‘CQ resistance-reversers’—compounds reported to restore (albeit partially) the sensitivity of CQR parasites to CQ. c The endoperoxide antimalarials currently in use (artemisinin and its derivatives), and two related compounds undergoing development (artemisone and OZ439). d Next-generation 4-aminoquinolines that are active against multi-drug resistant parasites, and two compounds (T3.5 and mibefradil) that possess intrinsic antiplasmodial activity as well as the ability to potentiate the activities of quinolines
CQR parasites accumulate four to ten times less CQ in their DV compared with CQ-sensitive (CQS) parasites [30, 31], and it is this marked decrease in CQ accumulation that underlies the phenomenon of CQ resistance. CQR parasites can be partially re-sensitised to CQ in vitro by a range of weak bases including the calcium channel blocker verapamil (VP) [32]. This ‘resistance reversal’ effect is characterised by both an increase in CQ accumulation and a decrease in the CQ IC50 in CQR parasites [31, 32]. However, the concentration of VP required to reverse CQ-resistance falls outside its therapeutic range [33].
The fact that CQ remained effective over decades (and QN over centuries) of high usage indicates that: (1) the crystallisation of haem into haemozoin is an excellent drug target; and (2) evolution of resistance to anti-haemozoin drugs such as the quinolines is not a feat easily achieved by the parasite. A full understanding of the mechanisms underlying CQ resistance may provide a foundation for the design of new drugs that evade these mechanism(s) and/or the development of strategies by which these mechanisms may be countered and CQ (and/or related compounds) thereby restored as a mainstay of antimalarial chemotherapy.
The malaria parasite’s ‘chloroquine resistance transporter’, PfCRT
Analyses of the haploid progeny arising from a genetic cross between a CQR (Dd2) and a CQS (HB3) strain identified a gene on chromosome 7 that segregated with the VP-reversible CQR phenotype [34–36]. Polymorphisms in this gene, designated the ‘chloroquine resistance transporter’, associate completely with CQ-resistance in parasites from a number of endemic regions [36] and can confer VP-reversible CQ-resistance upon otherwise CQS strains [37].
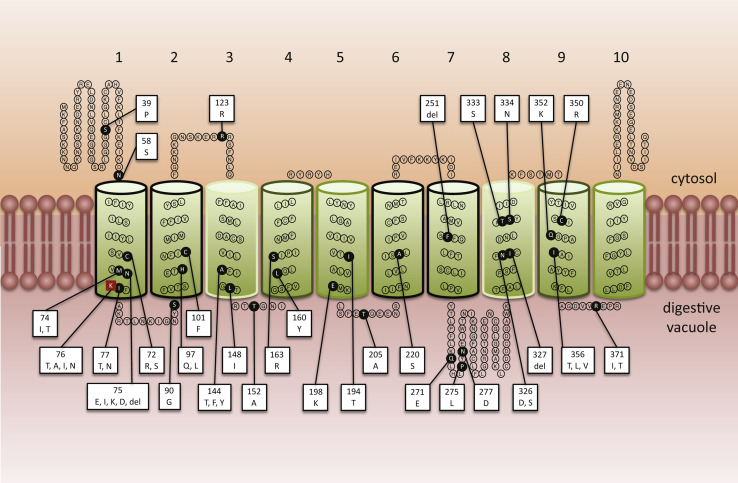
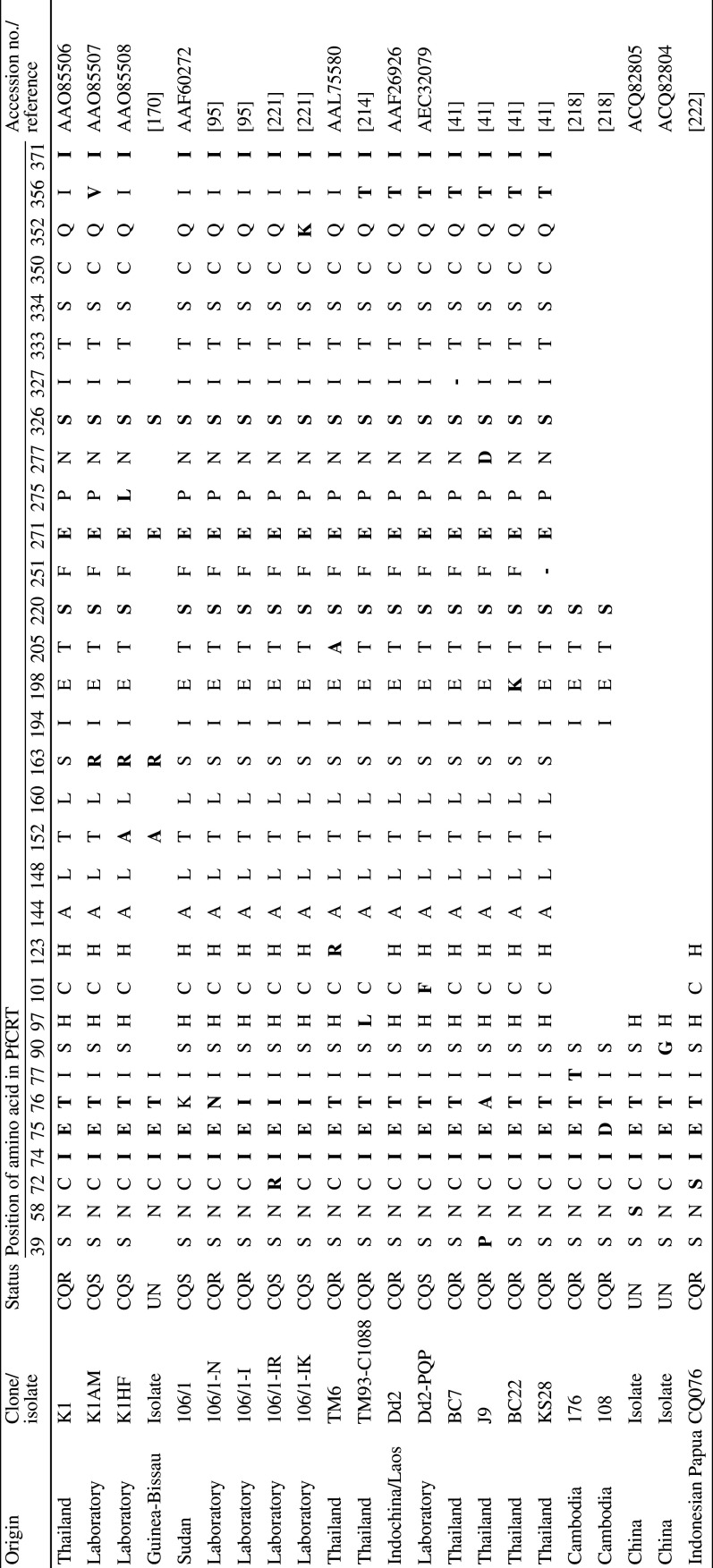
The pfcrt gene encodes a 424 amino acid protein that localises to the DV membrane [36] and which is a member of the Drug/Metabolite Transporter (DMT) superfamily (Transporter Classification (TC) 2.A.7) [38]. PfCRT is predicted to contain 10 transmembrane domains (TMDs) and to be orientated in the DV membrane with the N and C termini extending into the cytosol (Fig. 2; [38]). Trafficking of the protein to the DV membrane is facilitated by phosphorylation of the residues S33, S411 and T416 [39]. CQR parasites arose independently in at least five regions (Columbia, Peru, PNG, the Philippines, and South-east Asia—strains from the latter spread to Africa), and distinct PfCRT haplotypes are associated with each of these regions. A current list of unique PfCRT haplotypes is provided in Table 1. Depending on the strain, PfCRT can contain anywhere between 4 and 10 mutations, with a total of 32 polymorphic residues identified to date. However, one mutation—the substitution of the lysine at position 76 for threonine (K76T)—has been found in almost all CQR field isolates [40], the one exception being a CQR strain which instead contains an alanine at this position (K76A) [41]. Moreover, it has been shown that reversion of this mutation restores CQ sensitivity to CQR strains [40]. The cause(s) of the variation in the number and nature of the mutations which accompany K76T is unclear. These variants of PfCRT may have resulted from different histories of drug use (and therefore different selection pressures) between geographic regions and/or the evolution of alternate sets of PfCRT mutations that confer CQ resistance. It is worth noting that most of the PfCRT mutations found in CQR parasites are located on or towards the vacuolar side of the protein (Fig. 2) and that the key K76T mutation results in the loss of a positive charge from the putative substrate-binding site of the protein [38].
Fig. 2.
Arrangement of known polymorphic residues in PfCRT. PfCRT is predicted to contain 10 α-helical transmembrane domains (TMDs) and to be orientated in the DV membrane with the N- and C-termini extending into the parasite cytosol [38]. The positions of the polymorphic residues are indicated with black circles. The key CQ resistance-associated mutation (K76T) is represented as a red square. The box attached to each polymorphic residue lists the (non-wild-type) amino acid(s) known to occur at that position. The predicted roles of the TMDs are as follows: 4 and 9 (outlined in dark green) are implicated in the binding and translocation of substrates, TMDs 3 and 8 (boxed in light green) are thought to assist in the binding and translocation of the substrate and may also influence the substrate-specificity of the transporter, TMDs 1, 2, 6, and 7 (boxed in black) are involved in recognising and discriminating between substrates, and TMDs 5 and 10 (outlined in mid-green) play a role in the formation of homo-dimers [38]
Table 1.
Haplotypes of PfCRT
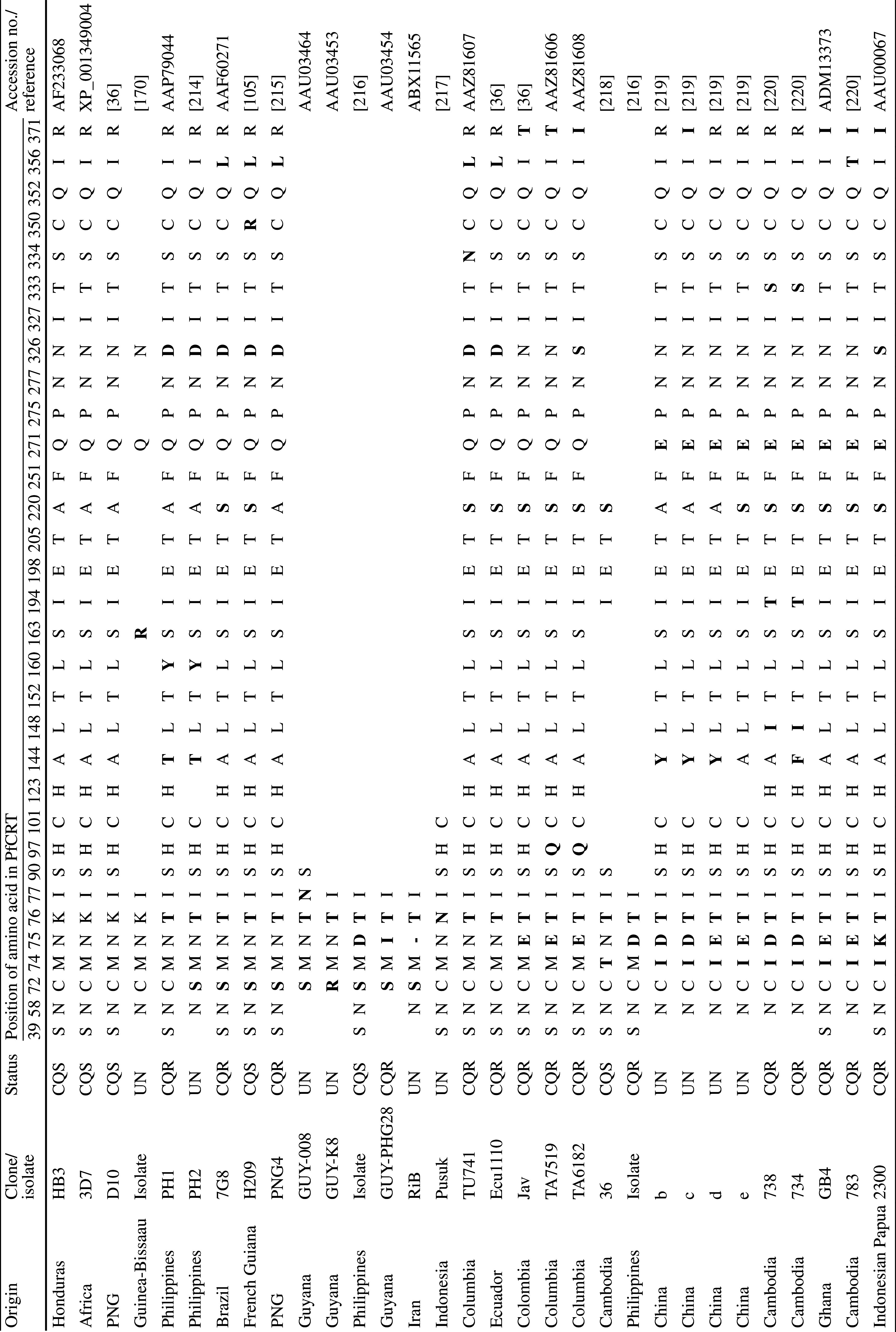
Residues that differ from the wild-type amino acid sequence of PfCRT (i.e., from the CQS strains 3D7, HB3, and D10) are highlighted in bold. Vacant cells indicate regions over which sequence data are unavailable. In those cases where the sequence data has been deposited into the NCBI database, the accession numbers are provided in place of the reference
– Deletion mutation (resulting in the absence of a residue at this position in PfCRT), CQS chloroquine-sensitive, CQR chloroquine-resistant, UN unknown, PNG Papua New Guinea
The mechanism by which mutant PfCRT reduces CQ accumulation within the DV, and thereby confers resistance, has been the subject of much debate (for recent reviews, see [42, 43]). There is now, however, a significant body of data which indicates that the resistance-conferring form of the protein (PfCRTCQR) has the ability to move CQ out of the DV, away from its site of action. For example, PfCRTCQR has been implicated in the transport of radiolabelled CQ in CQR parasites [20, 44, 45] and in a (verapamil-sensitive) CQ-mediated efflux of protons from the DV of CQR parasites [46, 47]. Naude et al [48] also provided indirect evidence of CQ transport via PfCRTCQR using a heterologous expression system; Dictyostelium discoideum transformants expressing PfCRTCQR at endosomal membranes displayed a verapamil-sensitive decrease in CQ accumulation. Finally, a direct demonstration of CQ transport via PfCRTCQR was achieved using the Xenopus oocyte expression system [49]. In this study, PfCRT was expressed in the oocyte plasma membrane where it could be readily assayed for the ability to transport radiolabelled CQ. PfCRTCQR was shown to mediate VP-sensitive CQ transport, whereas the wild-type form of the protein found in CQS parasites (PfCRTCQS) did not exhibit CQ transport activity. The version of PfCRTCQR expressed in oocytes was from the CQR strain Dd2; this protein contains eight mutations not found in the wild-type protein (Table 1).
Transport properties of PfCRT
Functional expression of PfCRT at the oocyte surface has provided a system with which the transport properties of the protein can be investigated in detail. A key finding has been the demonstration that the K76T mutation is necessary but not sufficient for the transport of CQ via PfCRT, consistent with the view that one or more of the other PfCRT mutations act in concert with K76T to confer CQ resistance. In addition to inhibition by VP, CQ transport via PfCRTCQR is inhibited by a number of quinoline antimalarials (including QN, AQ, primaquine, and MQ) as well as the antiviral agent amantadine (which exhibits some antimalarial activity in vitro, particularly against CQR parasites) [50]. By contrast, PIP and artemisinin (both equally effective against CQS and CQR strains) are without significant effect. A strong dependence of PfCRTCQR-mediated CQ uptake on the pH of the medium, together with the observation that uptake was influenced by the membrane potential, indicated that CQ is transported in its charged forms (CQH2 2+ or CQH+). These findings strongly support the mechanistic model for the role of PfCRT in CQ resistance that is presented in Fig. 3a, c. Protonated CQ is unable to interact with PfCRT when the substrate-binding site contains a positive charge (e.g., K76 or R163; Table 1; Fig. 2). Removal of the positive charge alters the substrate specificity of PfCRT to allow the transport of the protonated drug, down its electrochemical gradient, away from its site of action in the parasite’s DV.
Fig. 3.
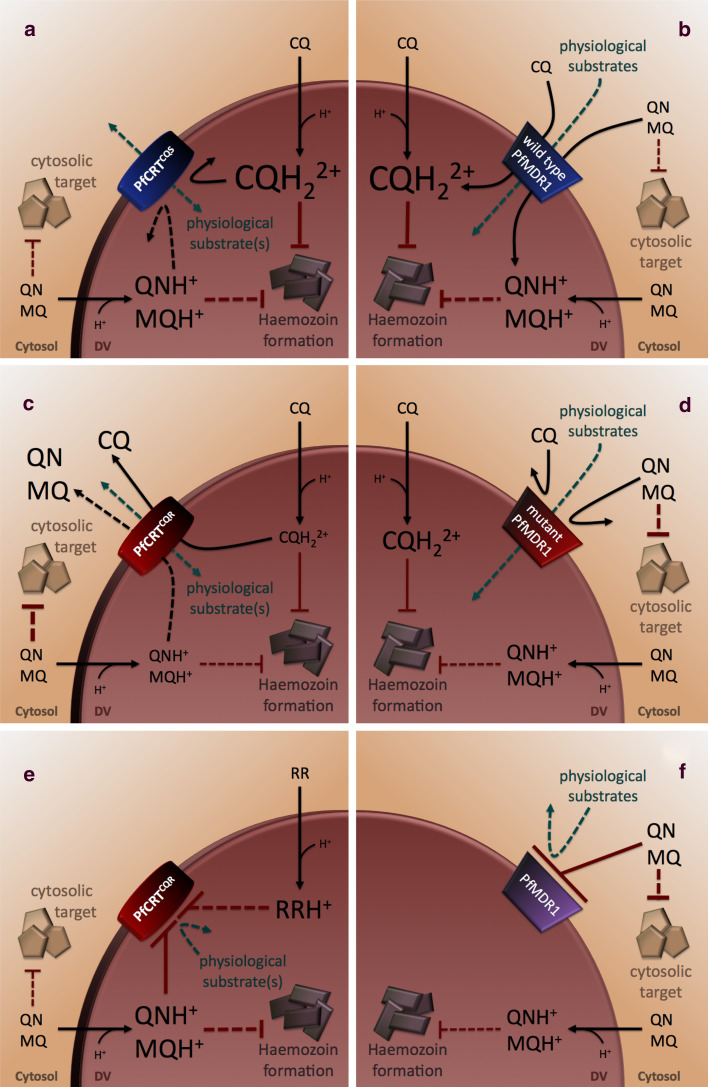
Proposed roles for PfCRT and PfMDR1 in quinoline resistance. a CQ, QN, and MQ are weak bases and therefore accumulate in the acidic environment of the parasite’s digestive vacuole (DV; pH ~5) in their protonated forms (CQH+, CQH2 2+, QNH+, and MQH+, respectively). PfCRTCQS does not interact with CQ [49], nor is it thought to interact with QN or MQ [68], whereas wild-type PfMDR1 (b) imports CQ and QN (and possibly MQ) into the DV [76, 77]. Thus, CQ, QN, and MQ are expected to accumulate in the DV of parasites carrying the native forms of these transporters. When present at high concentrations in the DV, CQ kills the parasite by preventing the conversion of the potentially toxic haem monomers into the inert crystal haemozoin. It is not clear whether QN and MQ share this mechanism of action, or if they instead (or in addition) target other processes in the DV and/or the cytosol [24]. Indeed, amplification of wild-type pfmdr1 has been associated with MQ resistance [82, 87–89, 91–94, 211–213]; it is thought that the resulting overexpression of PfMDR1 causes an increase in MQ accumulation within the DV, which in turn leads to a reduction in the concentration of MQ at its putative cytosolic target. c PfCRTCQR mediates the efflux of CQ (and possibly QN) out of the DV [49, 68]. MQ inhibits transport via PfCRTCQR [49], but does not appear to be a substrate itself [68]. However, direct measurements of MQ transport via PfCRT are required to confirm this finding. d Certain mutations in PfMDR1 abolish the import of CQ and QN (and possibly MQ) via this protein [76, 77]. Hence, the DV concentrations of CQ, MQ and QN are expected to be reduced when the mutant forms of both proteins are present. In the case of CQ, this results in resistance. By contrast, the decreased levels of MQ in the DV (which may lead to an increase in the cytosolic concentration of MQ) might be expected to increase parasite susceptibility to MQ if the primary target of this drug is cytosolic. Likewise, the effect on QN-susceptibility would depend on whether QN exerts its primary mode of action within the DV or cytosol. Alternatively (or in addition), MQ and QN may inhibit PfCRTCQR (e) and/or PfMDR1 (f), and thereby exert part of their antimalarial effect by blocking the physiological functions of these transporters [49, 68, 75, 76, 89]. Several resistance-reversers (RR) have been shown to inhibit PfCRTCQR, and the ability to block this form of the protein may underlie the observed increase in the intrinsic antiplasmodial activities of RRs in CQR versus CQS parasites [56, 65, 69]. Black and green lines indicate transport pathways and red lines denote modes of antimalarial action. Pathways and modes of action that have not been directly demonstrated or characterised are shown as dashed lines
Another important insight gained from the characterisation of PfCRTCQR activity is the observation that the protein behaves as a carrier rather than as a channel (refer to Summers and Martin [43] for a detailed discussion); PfCRTCQR-mediated transport of CQ is saturable, highly temperature-dependent, and its inhibition by a range of different drugs and compounds is concentration-dependent. The saturability of CQ transport (Km of ~245 μM) is of particular relevance since the addition of 100 nM CQ to the extracellular medium is estimated to result in a CQ concentration of ~2 mM in the DV of CQS parasites, and between 200 and 500 μM in the DV of CQR parasites. This finding could have significant implications for the use of CQ against CQR P. falciparum; the resistance mechanism could be overcome simply by increasing the dose of CQ, and thereby the level of CQ in the DV, such that PfCRTCQR can no longer maintain sub-lethal levels of the drug (refer to “Re-examining the CQ dosage regimen” of this review for further discussion of this hypothesis).
Attempts to generate transfectant parasite lines in which pfcrt is knocked-out have been unsuccessful, indicating that PfCRT is essential for parasite viability [40, 51]. Hence, quite apart from its role in mediating CQ resistance, PfCRT appears to fulfill a vital physiological function in the parasite. Indeed, it is thought that some of the mutations that accompany K76T may serve to maintain the normal physiological role of the protein [38, 52]. Within the DMT superfamily, PfCRT bears closest similarity to those proteins known to transport amino acids, weak bases, and divalent organic cations [38]. Given that the only known metabolite transport function of the DV to date is the efflux of peptides and/or amino acids (produced from the digestion of haemoglobin), it was proposed that PfCRT normally functions as an amino acid/peptide exporter [38]. Consistent with this idea, a number of peptides, including several derived from human haemoglobin, were found to cause a marked inhibition of CQ transport via PfCRTCQR, and a radiolabelled peptide was shown to be transported by PfCRTCQR [49]. However, the same peptide was not transported by PfCRTCQS, and at this stage it is unclear whether the interaction of peptides with PfCRTCQR arises from their resemblance to the endogenous substrate or whether it might instead be due to their structural similarity to VP and the quinoline drugs [49, 53].
CQ resistance-reversers
In the 25 years since the CQ-chemosensitising effect of VP in CQR parasites was first described, over 40 resistance-reversers have been identified [54, 55]. Resistance-reversers belong to a wide range of pharmaceutical classes, including calcium channel blockers (e.g., VP and amlodipine), calmodulin inhibitors (e.g., chlorpromazine), antidepressants (e.g., desipramine), antihistamines (e.g., chlorpheniramine, CP), and a number of plant-derived products [53, 56] (Fig. 1b). Reversers of CQ resistance generally exhibit poor antiplasmodial activity, and hence are only effective against the parasite when acting in synergy with CQ (or another quinoline). Small-scale clinical trials have shown that CQ is more effective against CQR parasites when administered in combination with CP [57, 58], but at present, the routine clinical application of resistance-reversers has been prevented by problems with potency and host toxicity.
The CQ resistance-reversing compounds identified to date share several structural features, and a number of studies have demonstrated structure–activity relationships for resistance-reverser activity [59–62]. Bhattacharjee and colleagues [63] developed a 3D pharmacophore model for CQ resistance-reversal using structure–activity profiling. The pharmacophore includes one or two hydrophobic aromatic groups and a protonatable N atom, usually a secondary or a tertiary amine linked by an aliphatic side chain [53, 63]. Using a series of 28 dihydroanthracene derivatives, Alibert and co-workers have expanded on the proposed pharmacophore to describe the properties of a putative binding site for resistance-reversers [59]. This hypothetical binding site features hydrogen bonding between a putative serine OH group and the protonated amine of the resistance-reverser, which is stabilised by an electrostatic interaction with a negatively charged carboxylate group of an aspartate residue [59]. Interestingly, this hypothetical binding site matches the threonine (OH group) and glutamate (COO- group) residues at positions 76 and 75, respectively, in the CVIET haplotype of PfCRTCQR (Table 1), which suggests that resistance-reversers may interact with PfCRTCQR at the same site as CQ [59]. Hence, these compounds may exert their resistance-reversing effect by competing directly with CQ for the substrate-binding site of PfCRTCQR. This hypothesis is supported by a number of genetic studies which suggest that resistance-reversers interact with this region of PfCRTCQR [37, 40, 64–66].
The recent characterisation of PfCRTCQR in the Xenopus oocyte system has confirmed that a number of resistance-reversers (VP, primaquine, and a series of dibemethin-based compounds) interact directly with the protein to inhibit CQ transport in a concentration-dependent manner [49, 67] (Fig. 3e). Findings by Lehane and Kirk [68] suggest that some resistance-reversers are themselves substrates of PfCRTCQR, and that they therefore exert their effect by competing with CQ for transport out of the DV. However, direct measurements of transport are required to confirm that PfCRTCQR possesses the ability to translocate VP (or any other resistance-reverser).
PfCRT as a drug target
The finding that resistance-reversers interact directly with PfCRTCQR to inhibit CQ transport suggests that the normal physiological role of PfCRT may likewise be blocked by these compounds. Since PfCRT is known to be essential to the survival of the parasite, the inhibition of its function by a CQ resistance-reverser could exert an antimalarial effect (Fig. 3e). Indeed, the intrinsic antiplasmodial activities of resistance-reversers from diverse pharmacological classes have been shown to be greater in CQR parasites than in CQS strains [56, 65, 69]. Furthermore, the IC50 values of a range of resistance-reversers were shown to correlate inversely with CQ IC50 values in the offspring of a genetic cross between the Dd2 (CQR) and HB3 (CQS) strains [65]. Genome-wide scans revealed that this effect was directly associated with mutations in pfcrt [65]. Moreover, in high-throughput assays that tested a library of known pharmaceutical compounds in 61 strains of P. falciparum, the antiplasmodial activities of 42 compounds were shown to correlate negatively with that of CQ, and the activities of 17 of these compounds (including several resistance reversers) mapped to the pfcrt loci [70].
Several lines of evidence are therefore consistent with the idea that resistance-reversers inhibit an essential function of PfCRTCQR. In this regard, it is worth noting that the identification of the normal substrate of PfCRT may make it possible to target both mutant and wild-type forms of the transporter; substrate mimics that are potent inhibitors of both PfCRTCQR and PfCRTCQS could be generated using rational drug design strategies. These compounds would be expected to have intrinsic antimalarial activity against both CQR and CQS parasites, as well as acting as CQ resistance-reversers. A paradigm shift towards thinking of PfCRT as a drug target also provides a framework for deciphering the complex patterns that have been observed between the parasite’s resistance to one drug and concomitant changes in its susceptibility to a host of other antimalarials. PfCRT mutations that are associated with drug pressure (or reduced susceptibility) may have arisen in order to mediate the transport of the drug away from a site of action (as is the case with CQ), or, alternatively, to prevent the drug from inhibiting the normal function of PfCRTCQR. That is, PfCRT can be considered as a drug resistance mediator as well as a drug target—a hypothesis which is discussed further in “Dissecting the patterns and underlying mechanisms of quinoline resistance”.
Other proteins involved in CQ resistance
CQ resistance has been linked to polymorphisms in a second protein—the P. falciparum multidrug resistance transporter 1 (PfMDR1) [71, 72]. PfMDR1 is a homologue of the ABC transporters that mediate multi-drug resistance in human cancer cells [72] (TC 3.A.1.201) and is expressed primarily at the parasite’s DV membrane [73]. Its ATP-binding domains are located at the cytosolic face of the membrane [74, 75] and the protein is thought to transport a wide range of substrates, including drugs, from the cytosol into the DV [75–78]. Mutations in PfMDR1 can modulate the level of CQ resistance exhibited by parasites already harbouring PfCRTCQR, but they do not, by themselves, confer CQ resistance [79]. Of the five PfMDR1 polymorphisms initially reported [71], N86Y, S1034C, N1042D and D1246Y all appear to contribute to CQ resistance, whereas Y184F is common to both CQS and CQR strains [71, 79, 80]. It is unclear whether the novel PfMDR1 mutations recently detected in South-east Asia (E130K, V1109I and F1226Y) play a role in CQ resistance [81, 82], but it is thought that the F1226Y mutation is involved in conferring resistance to MQ [82].
When expressed in mammalian CHO cells, PfMDR1 appeared to localise to internal vesicular compartments [77]. Expression of the wild-type form of PfMDR1 caused an increase in the accumulation of CQ and a heightened susceptibility to the drug. Taken together, these findings suggest that wild-type PfMDR1 may increase the CQ susceptibility of CHO cells by mediating import of the drug into internal compartments, where its accumulation appears to exert a toxic effect. It was therefore proposed that wild-type PfMDR1 may perform a similar function in the parasite, that is, to import CQ into the DV (Fig. 3b) [77]. By contrast, expression of a mutant version of PfMDR1—containing the CQ resistance-associated mutations S1034C and N1042D—did not result in an increase in CQ susceptibility. This suggests that certain mutations may reduce or abolish the ability of PfMDR1 to import CQ into the DV (Fig. 3d) [77]. Consistent with this hypothesis, a study by Lanzer and colleagues [76] has revealed that wild-type PfMDR1 mediates CQ transport when expressed in X. laevis oocytes, whereas a number of mutant PfMDR1 haplotypes do not. Thus, CQ resistance-associated mutations in PfCRT and PfMDR1 appear to contribute to the same outcome: a reduction in the concentration of CQ at its site of action in the parasite DV.
Mutations in another putative ABC transporter—the P. falciparum multi-drug resistance-associated protein 1 (PfMRP1; TC 3.A .1.208)—have also been implicated in CQ resistance [83]. Although an association between PfMRP1 mutations and CQ resistance could not be confirmed in studies of freshly isolated field strains [84, 85], the findings from a PfMRP1 knock-down experiment suggest that the transporter may play a role in CQ resistance [86]. When compared to the W2 parent strain, parasites with reduced PfMRP1 expression accumulated more CQ and displayed increased susceptibility to the drug [86]. Given that PfMRP1 is thought to be located at the parasite’s plasma membrane, it was suggested that the protein exports CQ out of the cell, thereby reducing its concentration within the parasite [86]. The W2 strain used in this study was derived from Dd2 parasites, which are known to contain mutations in PfMRP1 that are linked to CQ resistance (Y191H and A437S; [83]). Hence, it remains to be determined whether these mutations enable PfMRP1 to alter CQ accumulation, or if this is an inherent ability of the wild-type protein.
Dissecting the patterns and underlying mechanisms of quinoline resistance
Mefloquine, halofantrine and lumefantrine
Resistance to mefloquine (MQ) is typically associated with an elevated sensitivity to CQ and vice versa [87–92]. Moreover, parasites which display reduced susceptibilities to halofantrine (HF), and lumefantrine (LM) (both of which are structurally related to MQ) usually exhibit cross-resistance to MQ [79, 80, 82, 89, 90]. The major determinant of MQ resistance is the amplification of pfmdr1, and this modification is also linked to reduced susceptibilities to HF and LM [82, 87–89, 91–94]. Following selection for resistance to MQ, former CQR lines were found to have gained additional copies of pfmdr1 and also displayed an increase in sensitivity to CQ [89, 90]. By contrast, selection of high-level CQ resistance is accompanied by an increase in MQ susceptibility and the de-amplification of pfmdr1 [87]. Moreover, the sensitivity of the parasite to MQ can be increased by the introduction of PfMDR1 mutations that are associated with CQ resistance, particularly N86Y [79, 80, 88, 92, 93]. Indeed, it is often the wild-type form of the gene (which contains N86) that is amplified in association with MQ resistance [91–93].
Polymorphisms in PfCRT are not typically associated with differences in MQ susceptibilities between parasites isolated from the field. However, they have been shown to modify MQ responses in vitro [37, 50, 95], and, as is the case with PfMDR1, MQ and CQ, appear to exert opposing selection forces on PfCRT. For example, the parasite’s sensitivity to MQ can be increased by the introduction of CQ resistance-conferring mutations in PfCRT [37]. Furthermore, when the CQR strain K1 was selected for resistance to HF, the resulting ‘K1HF’ line exhibited decreased susceptibility to both HF and MQ, but was simultaneously restored to CQS-status [50]. The K1HF strain was found to contain three novel PfCRT mutations, one of which—S163R—introduces a positive charge to a region of PfCRT which, by homology with related DMT proteins, is implicated in the binding and translocation of substrates [38]. Hence, it was postulated that S163R is a ‘resistance-reversing’ mutation which re-instates the parasite’s sensitivity to CQ by preventing the drug from escaping from the DV via PfCRTCQR [50, 96]. Consistent with this hypothesis, the introduction of S163R into Dd2 PfCRTCQR was found to abolish CQ transport activity in the Xenopus oocyte expression system [49].
Current knowledge of the mechanisms underlying CQ resistance may provide useful insights into the roles played by PfMDR1 and PfCRT in the parasite’s resistance to MQ. Two scenarios that would readily account for the inverse relationship between MQ and CQ resistance are: (1) the primary target of MQ lies outside the DV, and changes in PfMDR1 and/or PfCRT effect MQ susceptibility by altering the distribution of the drug within the parasite (Fig. 3a–d), and (2) MQ targets PfMDR1 and/or PfCRT directly, impairing the physiological function of these transporters (Fig. 3e, f; [76, 89, 97]). In the latter scenario, amplification of pfmdr1 would increase the expression of the transporter, which may alleviate the effects of inhibition by MQ [76]. In the former scenario, changes that reduce the concentration of MQ in the cytoplasm would be expected to increase the parasite’s resistance to this drug. For example, if wild-type PfMDR1 transports MQ into the DV, as has been demonstrated for CQ, then amplification of pfmdr1 could increase the amount of MQ sequestered in this compartment, thereby reducing MQ susceptibility. Conversely, if PfCRTCQR mediates the efflux of MQ from the DV, the concentration of the drug in the cytoplasm would rise, leading to an increase in the parasite’s sensitivity to MQ. It is worth noting that MQ may interact with a broader range of PfMDR1 haplotypes than CQ. For instance, MQ was able to inhibit transport of the substrate ‘fluo-4’ via two mutant haplotypes of PfMDR1, whereas CQ had no effect [75]. Moreover, MQ resistance is not just associated with amplification of wild-type pfmdr1, but is occasionally reported in strains which possess multiple copies of a mutant form of pfmdr1 [89, 98].
Since mechanisms of transport and inhibition often overlap, it is possible that the process of MQ translocation would itself exert an inhibitory effect on the normal functions of PfMDR1 and/or PfCRTCQR. Furthermore, the relative contributions of transport (which would entail MQ translocation) and binding (without the subsequent translocation of MQ) to the inhibitory effect of MQ may differ between the two transporters. Both mechanisms are consistent with the finding that MQ inhibits PfMDR1-mediated transport of the substrate fluo-4 [75]. However, as MQ transport via PfMDR1 has not been directly demonstrated, it is unclear whether MQ competes for transport with fluo-4, or if it instead binds to PfMDR1 but is not translocated. In the case of PfCRT, the decreased MQ sensitivity of CQR parasites harbouring the S163R mutation could be explained in two ways. The re-introduction of a positive charge to the PfCRT substrate binding-site is likely to prevent MQ from interacting with PfCRTCQR, which could either: (1) abolish the ability of the protein to transport MQ, thereby restoring sequestration of the drug within the DV [50, 96], or (2) prevent MQ from binding to and inhibiting PfCRTCQR. However, the first scenario is at odds with a recent finding by Lehane and Kirk [68]. CQ induces a H+ leak from the DV of parasites carrying PfCRTCQR (but not PfCRTCQS) that is thought to represent efflux of the protonated drug. MQ does not induce this leak. While this does not exclude the possibility that MQ has an important cytosolic target, it does indicate that the increased susceptibility of CQR parasites to MQ may not be due to the transport of this drug from the DV into the cytosol. Although MQ does not appear to be a substrate of PfCRTCQR, it is nevertheless an inhibitor of CQ transport via this protein [49], which is consistent with the idea that MQ exerts an antimalarial effect by inhibiting the normal function of PfCRTCQR.
The fact that the S163R mutation also results in the loss of CQ transport activity [49] indicates that CQ and MQ (and quite likely other drugs) interact with the same region of PfCRTCQR, and that PfCRT-mediated decreases in the parasite’s susceptibility to CQ and MQ are mutually exclusive events. This could be a limiting property of the protein, and as such, could be exploited by combination therapies that pair together two drugs that exert opposing selection forces upon PfCRT [97, 99].
Quinine
Although susceptibilities vary, CQR strains often display low-level resistance to quinine (QN) (such that QN remains clinically effective against CQR parasites) [95, 100, 101]. However, QN-resistance phenotypes are complex and can also display cross-resistance to MQ [37, 84, 88]. Reduced susceptibilities to QN have been linked to changes in the same molecular components that are associated with CQ and MQ resistance—PfCRT, PfMDR1 and PfMRP1 [83, 102]. Polymorphisms in the P. falciparum Na+/H+ exchanger (PfNHE; TC 2.A.36) are also thought to contribute to decreases in the parasite’s sensitivity to QN [102]. Unlike resistance to CQ and MQ, reduced susceptibility to QN does not appear to be governed by a single, predominant molecular determinant, but rather by a number of proteins whose contributions vary between strains [102]. This pleitropic response may be a reflection of the counteraction required to combat a drug that has a complex mode of action, and/or the extended period over which the parasite has been afforded the opportunity to develop tolerance to QN. Indeed, it has been suggested that, in addition to haemozoin formation, QN targets one or more other essential processes in the parasite [24, 25]. QN remained highly effective over centuries of use before resistance emerged, and these ‘QN-resistant’ strains typically display only a low level of tolerance. Thus, while the complexities of the QN mode-of-action have proven difficult to unravel, it has also been difficult for the parasite to overcome.
Mutations in PfCRT that confer CQ resistance are often associated with a decrease in the parasite’s susceptibility to QN, which suggests that resistance to this drug is mediated, at least in part, by variants of the transporter [83, 95, 102]. Consistent with these observations, Sanchez and colleagues found that parasites harbouring PfCRTCQR efflux QN at a greater rate than those carrying PfCRTCQS [103]. Furthermore, Lehane and Kirk [68] recently showed that QN—like CQ—causes a H+ leak from the DV of parasites carrying PfCRTCQR, consistent with the mutant (but not wild-type) protein mediating the efflux of the protonated drug. Moreover, direct evidence of an interaction between QN and the mutant protein has come from its ability to inhibit PfCRTCQR-mediated transport in the Xenopus oocyte expression system [49]. Taken together, these data suggest that PfCRTCQR reduces the parasite’s sensitivity to QN by allowing the drug to escape from the DV, away from its putative target (haem; Fig. 3a, c).
Polymorphisms at positions 1034, 1042 and 1246 of PfMDR1 have also been shown to increase QN tolerance [79, 80]. Of these three mutations, N1042D appears to exert the greatest influence upon the parasite’s response to QN [80]. Like CQ, QN is a substrate of wild-type PfMDR1, and this transport activity is similarly abolished by the introduction of certain mutations into PfMDR1 [76]. This suggests that PfMDR1 mutations such as N1042D reduce the parasite’s susceptibility to QN by removing one of the routes by which the drug accesses the DV (Fig. 3b, d). It is worth noting that mutations in PfMDR1 appear to affect QN susceptibility more than they do CQ susceptibility. Indeed, in the absence of PfCRTCQR, the PfMDR1 mutations S1034C, N1042D and D1246Y reduce the parasite’s sensitivity to QN, but have no effect on its response to CQ [79]. This may reflect the fact that QN (pKa values of 4.2 and 8.2–8.5) is likely to accumulate to much lower concentrations than CQ (pKa values of 8.1 and 10.2) within the DV via weak-base trapping [25, 104], such that the relative contribution of PfMDR1 to QN accumulation may be greater than its role in CQ accumulation.
In some instances, the effects of changes in PfCRT and PfMDR1 on the parasite’s susceptibility to QN align more closely with those observed for MQ, consistent with the observation that a reduction in QN sensitivity can be accompanied by cross-resistance to CQ or MQ. For example, when PfCRTCQR was introduced into the CQS strain ‘GC03’ via allelic exchange (resulting in a decrease in CQ-sensitivity), susceptibility to both QN and MQ was increased [37]. Similarly, the parasite’s sensitivity to QN or MQ is often reduced by mutations that re-introduce a positive charge to the putative substrate binding-site of PfCRTCQR and which abolish CQ transport activity (e.g., S163R; [49, 50, 95]). The most recent example of this phenomenon is a field isolate from French Guiana (H209) which contains a novel mutation—C350R—that re-introduces a positive charge to PfCRTCQR. These parasites are CQS, yet they exhibit increased resistance to QN [105]. In addition, reduced susceptibility to QN has been linked to the major determinant of MQ resistance—amplification of pfmdr1 [91, 93].
How to explain the apparent complex interplay between mutations in PfCRT and PfMDR1 and the parasite’s susceptibilities to MQ and QN? One possibility is that MQ and QN both have cytosolic targets. If this were the case, PfMDR1 amplification could serve to increase the sequestration of MQ or QN in the DV and thereby protect the parasite by reducing the concentration of drug at the cytosolic target. Mutations in PfMDR1 that abolish this transport activity would have the opposite effect (Fig. 3b, d). Likewise, PfCRTCQR-mediated efflux of QN from the DV would be expected to increase the concentration of QN at its site of action in the cytosol (unless, of course, a second QN efflux system was present at the parasite plasma membrane; Fig. 3a, c). While this model is easily reconciled with what is understood about MQ resistance, it is a less comfortable fit with the complex range of phenotypes displayed by ‘QN-resistant’ strains. Perhaps the QN-susceptibility of a given strain is the product of a trade-off between the inhibition of haemozoin formation and the inhibition of a cytosolic target. Alternatively, the relationship between QN- and MQ-sensitivity observed within some parasite strains could be due to the ability of these two drugs to target the physiological functions of PfMDR1 and/or PfCRTCQR; MQ is an inhibitor of both PfMDR1 and PfCRTCQR (see above) and QN is both a substrate and inhibitor of PfMDR1 [76] as well as an inhibitor (and possible substrate) of PfCRTCQR (Fig. 3e, f) [49]. It is worth noting that this phenomenon may be the cause of the otherwise confounding instances in which parasites exhibit similar responses to QN and MQ.
As has been reported for CQ resistance, a weak association is thought to exist between a reduction in the parasite’s susceptibility to QN and the PfMRP1 mutations Y191H and A437S [83, 85]. Although, again, this relationship was not apparent in strains isolated from Thailand [84]. When PfMRP1 expression was decreased in the W2 strain by allelic exchange, the resulting parasites accumulated more QN and displayed an increase in their susceptibility to the drug [86]. It was therefore proposed that PfMRP1 also has the ability to transport QN across the plasma membrane, out of the parasite cytosol [86].
Variations in QN susceptibilities between different parasite strains have also been linked to repeat polymorphisms in the microsatellite locus ‘ms4760’ of PfNHE [102]. However, there is a lack of consensus regarding the specific nature of these associations. For instance, while some studies report an association between reduced susceptibility to QN and an increase in the number of ‘DNNND’ repeats in ms4760 [106–108], others could not verify this association [109–111], or found that two DNNND repeats was the optimal number for conferring a reduction in QN-sensitivity [112]. Moreover, amplification of a second ms4760 repeat—‘NHNDNHNNDDD’—has been linked to increases in the parasite’s susceptibility to QN [106–108]. However, the reverse association has also been reported [109], and several studies have failed to confirm either of these findings [110, 111]. It has been suggested that PfNHE repeat polymorphisms may alter the parasite’s susceptibility to QN by effecting a change in the cytosolic pH [113]. However, PfNHE is not thought to play a major role in pH regulation [114], and the cytosolic pH was unaffected when PfNHE expression was reduced by 50% [115]. Moreover, if PfNHE were to alter the parasite’s response to QN by modulating the pH, it would likewise be expected to influence the parasite’s susceptibility to other weak-base antimalarials. Yet the effect of PfNHE knock-down was specific to QN [115]. It also appears that the ability of PfNHE to modulate QN-sensitivity is dependent upon the presence of other resistance-conferring mutations; a reduction in PfNHE expression coincided with an increase in the parasite’s susceptibility to QN, but only in those strains which harboured PfCRTCQR [115].
Amodiaquine
The relationship between CQ and amodiaquine (AQ) resistance is also not straightforward; AQ remains effective against CQR parasites in many parts of Africa [116–118], whereas in South-east Asia, South America, and Papua New Guinea, strains that are moderately resistant to CQ tend to display high levels of AQ resistance [66]. The apparent geographic specificity of AQ resistance may be due to differences between the PfCRTCQR haplotypes found in these locations; it has been suggested that AQ resistance is associated with the ‘SVMNT’ haplotype of PfCRTCQR that is typically carried by CQR South American strains (over the region spanning residues 72–76; see Table 1), rather than the ‘CVIET’ haplotype common to CQR strains of Africa and South-east Asia [66, 119, 120]. Consistent with this hypothesis, in the progeny of a genetic cross between the CQR strains 7G8 (containing SVMNT) and GB4 (containing CVIET), high-level resistance to the major active metabolite of AQ—monodesethyl AQ (MDAQ)—was in part dependant on the presence of 7G8 PfCRTCQR [66]. It is therefore concerning that the SVMNT haplotype, which was previously reported in Africa only once in the 1990s [121], and then at very low levels, is now relatively common in Tanzania [119] and Angola [122]. This has been attributed to an increase in use of AQ on this continent, either alone or in combination with artesunate [119, 123].
Amongst the progeny of the 7G8 × GB4 cross, high-level AQ resistance was also contingent on the presence of the 7G8 PfMDR1 haplotype [66]. Interestingly, this haplotype does not contain the N86Y PfMDR1 mutation that has frequently been linked to AQ resistance, but does contain D1246Y, which is the mutation most often associated with AQ resistance after N86Y [99, 124, 125].
Given the structural similarity between CQ and AQ, it is likely that resistance to these drugs is mediated by a shared mechanism. Thus, certain mutations in PfMDR1 may abolish AQ import into the DV, and mutations in PfCRT may allow the transporter to mediate the efflux of AQ from of the DV. AQ is known to interact directly with at least one of these proteins; when tested in the Xenopus oocyte expression system, AQ inhibited the transport of CQ via PfCRTCQR [49]. It is therefore tempting to speculate that differences in the level of AQ resistance between parasites carrying the SVMNT or CVIET PfCRTCQR haplotypes may be attributable to differences in the affinities of these two transporters for AQ (note that residues 72–76 are located within a region of PfCRT that has been implicated as having a role in the recognition of substrates; Fig. 2). Indeed, an increase in MDAQ resistance appears to be correlated with a decrease in the hydrophobicity of the side chains of residues 72–76 of PfCRTCQR [120]. AQ is a reasonably hydrophobic drug, so perhaps the greater hydrophobicity of the CVIET motif relative to that of SVMNT causes AQ to adhere to the binding site, thereby decreasing its rate of transport or even preventing translocation altogether.
Piperaquine
Piperaquine (PIP) was developed simultaneously in China and France to counter widespread CQ resistance, but its extensive use as a monotherapy in China led to the emergence of highly resistant parasites [23]. In recombinant strains carrying different haplotypes of PfCRT, increased susceptibility to PIP was associated with the presence of CQR forms of the protein [126]. However, this finding is at odds with that of another study which found no correlation between PIP resistance and changes in PfCRT, PfMDR1, PfMRP, or PfNHE [85], as well as the observation that PIP remains effective against CQR parasites [127]. Indeed, PIP does not appear to interact with PfCRTCQR; the drug was without significant effect on PfCRTCQR-mediated transport in the Xenopus oocyte expression system [49], and when expressed in D. discoideum, PfCRTCQR did not alter the accumulation of PIP (whereas it did reduce CQ accumulation) [48].
A recent study in which CQR strains were subjected to PIP selection pressure produced parasites that exhibited high-level PIP resistance [128]. These parasites exhibited PIP IC50 values that were approximately 100-fold greater than those of the parental strains, and this drastic decrease in susceptibility to PIP was accompanied by three changes; a novel mutation (C101F) in PfCRTCQR, de-amplification of PfMDR1, and amplification of a 63-kb segment on chromosome five (upstream of PfMDR1). When the PIP-resistant parasites were cultured in the absence of the drug, the loss of high-level PIP resistance coincided with de-amplification of the 63-kb segment of chromosome five, but was not accompanied by reversion of the changes in PfCRT or PfMDR1. This suggests that amplification of a gene within the 63-kb segment is required for high-level PIP resistance. By contrast, changes in PfCRT and PfMDR1 do not appear to be sufficient to confer PIP resistance, although they may contribute to the trait [128].
Pyronaridine
Like PIP, pyronaridine (PN) was developed in China and subsequently underwent extensive use in this country [129]. PN is usually effective against CQR parasites, despite there being evidence of a positive correlation between PN and CQ susceptibilities in vitro [130–133]. While it has been reported that the efficacy of PN in China is decreasing [134], high-level resistance to this drug remains largely undemonstrated. Furthermore, no relationship has been observed between the parasite’s susceptibility to PN and changes in the known molecular determinants of quinoline resistance—PfCRT, PfMDR1, PfMRP and PfNHE [135].
Artemisinin derivatives and combination therapies
Artemisinin-based compounds are now the most effective class of antimalarials available. Extracted from the plant Artemisia annua (sweet wormwood), the endoperoxide artemisinin and its derivatives, artemether, artesunate and dihydroartemisinin (Fig. 1c), are fast acting and highly potent compounds that rapidly reduce the parasite biomass in patients [136, 137]. Due to their short half-life in humans, and the need to delay the onset of drug resistance, the WHO [138] recommends that artemisinins be used in combination with a partner drug which has a different mechanism of action and a longer half-life. These artemisinin combination therapies (ACTs) now form the cornerstone of malaria treatment worldwide. Together with mosquito control measures, ACTs have played a vital role in reducing the burden of malaria in many countries in Sub-Saharan Africa and South-east Asia [1] (a recent review by Maude and colleagues [139] provides a comprehensive history of the use of artemisinins and ACTs).
Despite the success of ACTs over the last decade, there are several drawbacks to the current strategy. The WHO [138] currently recommends five combinations—artemether–LM, artesunate–MQ, artesunate–sulfadoxine–pyrimethamine and dihydroartemisinin–PIP. Of these five partner drugs, only LM had not been used before deployment as an ACT, and the longevity and efficacy of all of the partner drugs has already been compromised by the emergence of resistant strains. This problem is exacerbated by the mismatch in pharmacokinetics between the partner drugs. Artemisinin and its derivatives have short half-lives in the body of between 45 min and 20 h [137, 140], while drugs such as LM and MQ have much longer half-lives of 3–4 [141] and 14–28 days [142], respectively. As a consequence, ACT treatment success rates are highly dependent on the susceptibility of the parasite to the partner drug, and failure rates between 10 and 30% have been reported for all combinations [143] (although in some cases, limitations in bioavailability may also be a factor). In the cases of MQ and PIP, combinations with artesunate and DHA, respectively, were only deployed once the monotherapies started to fail [144, 145]. Furthermore, artemisinin and derivative compounds appear to select for parasites with reduced susceptibilities to the most commonly used partner drugs—MQ and LM [146, 147]. The benefit of combining drugs that have independent modes of action, that elicit different mechanisms of resistance, and which have complementary pharmacokinetics and pharmacodynamics, is well documented, and the potential benefits to antimalarial chemotherapy are substantial [148]. Hence, it is perhaps worth considering how antimalarial combination therapies could be better designed.
Reports of the emergence of artemisinin resistance along the Thai–Cambodia boarder are further cause for concern [143, 149, 150]. Moreover, recent surveys have confirmed that artemisinin-resistant strains have appeared in north-western Thailand, south-eastern Burma, and south-eastern Vietnam [15]. The molecular mechanism(s) underlying the parasite’s resistance to artemisinin remains largely unknown; the findings of several studies suggest that amplification and/or polymorphisms in PfMDR1 may play a role [12, 44]. In addition, parasites that are resistant to MQ also tend to be less susceptible to artemisinin [82, 88]. There is also some evidence for there being a link between mutations in PfCRT and the parasite’s susceptibility to artemisinin. For example, Sidhu et al. [37] observed that expression of the CQR-conferring 106/1-I or 106/1-N haplotype of PfCRT (see Table 1) in CQS strains resulted in a modest increase in susceptibility to artemisinin and dihydroartemisinin, although this effect was not observed in parasites transfected with the Dd2 haplotype of PfCRT. It is also worth noting that an unusual CQS isolate from French Guiana (H209; Table 1) which carries a CQR-like PfCRT haplotype as well as the novel mutation C350R, displays reduced susceptibilities to both artemisinin and QN [105]. Consistent with these findings, Tucker and colleagues [151] recently reported that parasites selected in vitro for transient artemisinin resistance exhibit a heightened susceptibility to CQ, suggesting that artemisinin has an opposing selection force to CQ. However, in vitro selection for stable artemisinin-resistant parasites had no affect on CQ susceptibility [152]. While it is tempting to speculate that the artemisinins interact directly with PfCRTCQR, direct evidence of such an interaction was not detected in the Xenopus oocyte expression system [49]. The one remaining non-artemisinin combination therapy is atovaquone–proguanil, and mass administration of this drug combination is now being considered for the greater Mekong subregion in the hope that this will contain, or even eliminate, artemisinin resistance [153]. However, there is a strong possibility that artemisinin-resistant strains of the parasite will persist or re-emerge.
There is no Plan B
The loss of the artemisinins would be a devastating blow to malaria control and treatment efforts worldwide [12]. There are no new candidate drugs far enough along the development pipeline to replace them if artemisinin-resistant strains spread [154]. Furthermore, the most advanced therapies in the development pipeline are either ACTs (PN-artesunate), other artemisinin derivatives (artemisone; Fig. 1c), or synthetic artemisinin-like compounds (e.g., OZ439; Fig. 1c) [129]. The short-term future of malaria control depends entirely on the continued success of artemisinins and there is no Plan B [154]. Hence, in the short to medium term, there remains a dire need for a readily deployable, cost-effective strategy that is robust to resistance and does not rely on artemisinin derivatives. Even if such a strategy is reserved for the worst-case scenario, there should be a contingency plan in place.
Re-examining the CQ dosage regimen
There is a growing body of evidence which supports the idea that CQ could once again be used in the front line against P. falciparum malaria. CQ was first developed by the US army for the treatment and suppression of P. vivax infections in the South Pacific and Mediterranean during the Second World War [155, 156]. The earliest recorded clinical trial of CQ against P falciparum took place in 1946 with just 18 patients, and using the same regimen recommended for treating vivax malaria [157]. Remarkably little changed between this first treatment regimen and that which was used until CQ was withdrawn at the beginning of this century [i.e., 25 mg of CQ per kg of body weight (mg/kg) over 3 days] [158, 159]. That is, despite the emergence of CQR parasites, few studies investigated the efficacy of alternative dosages of CQ, and attempts to improve the CQ regimen were largely unsuccessful. Table 2 summarises the findings from clinical trials that examined increased dosages of CQ. Despite the relatively low therapeutic index of CQ, no severe adverse events were observed in any of the studies. Although the initial (pre-2002) studies showed that higher doses of CQ improve the short-term parasitological response, none of the final outcomes were considered sufficiently promising to warrant the use of higher CQ dosages against CQR strains. Hence, in 2001, the WHO [159] concluded that “there is no evidence to suggest that increasing the dosage will increase clinical cure rate in such situations and repeated administration of such high doses may produce adverse reactions”.
Table 2.
A summary of published clinical trials that have assessed alternative dosages of CQ for the treatment of P. falciparum malaria
| Country and ref. | Year(s) | CQR prevalencea | Participants | Drug regimenc | Outcomese | Adverse reactionsf | Limitations and comments |
|---|---|---|---|---|---|---|---|
| Indonesia [168] | 1981 | 90% (31) | 11 children (2–9 years) | CQ 15 mg/kg, 4 days (not specified) | 54.5% (11) clear on day 7 | None |
Small sample size. No follow up beyond 7 days. CQ dose-dependent increase in the rate of parasite clearance. |
| 6 | CQ 25 mg/kg, 3 days (10, 10, 5) | 66.7% (6) clear on day 7 | None in patients or 26 volunteers | ||||
| 6 | CQ 37.5 mg/kg, 3 days (15, 15, 7.5) | 83.3% (6) clear on day 7 | None in patients or 23 volunteers | ||||
| Burundi [223] | 1983–84 | 72% (22) | 22 school-children | CQ 35 mg/kg, 5 days (10, 10, 5, 5, 5) | 95.5% (22) day 7; 68% (22) day 14; 50% (22) day 27 | None |
Small sample size. CQ dose-dependent delay in parasite recrudescence. |
| 11 | CQ 40 mg/kg, 4 days (10, 10, 10, 10) | 100% (11) day 7; 91% (11) day 14; 27% (11) day 27 | On day 4, 11 reported ns and sore eyes. No further treatment was administered. | ||||
| 10 | CQ 50 mg/kg, 5 days (10, 10, 10, 10, 10) | 100% (10) day 7; 90% (10) day 14; 70% (10) day 27 | |||||
| Rwanda [224] | 1986 | 59% (27) | 44 children (≤5 years) | CQ 25 mg/kg, 3 days (10, 10, 5) | 45% (44) day 7; 34% (44) day 14 | Mild it (11), di (17), vo (12). | More rapid parasite clearance associated with high-dose CQ, but not significant after 14 days. |
| 48 | CQ 50 mg/kg, 5 days (10, 10, 10, 10, 10) | 71% (48) day 7; 25% (48) day 14 | it (17), di (10), vo (6). | ||||
| Brazil [225] | 1989–91 | Not reported | 58 patients (≥15 years) | CQ 25 mg/kg, 3 days (10, 10, 5) | 47% (55) day 7; 26% (54) day 14; 14% (50) day 30 | it (16), di (7), vo (9) | Migrant population led to reduced follow-up. CQR prevalence not measured. |
| 66 | CQ 50 mg/kg, 3 days (20, 20, 10) | 97% (61) day 7; 82% (57) day 14; 40% (53) day 30 | it (20), di (16), vo (16). | ||||
| Gabon [226] | 1992 | 100% (43) | 32 children (4–15 years) | CQ 25 mg/kg, 3 days (10, 10, 5) | 53% (32) day 7; 34% (32) day 14; 9% (32) day 28 | it (13), ap (3), ns (6). | Ability to compare across studies limited. CQ dose-dependent improvement in clinical outcomes over all time periods. |
| Gabon [227] | 1992–93 | 39 | CQ 35 mg/kg, 3 days (15, 10, 10) | 82% (39) day 7; 15% (39) day 28 | it (41). Rash in 1 patient. | ||
| Gabon [228] | 1993–94 | 41 | CQ 45 mg/kg, 3 days (15, 15, 15) | 93% (41) day 7; 39% (41) day 14; 32% (41) day 28 | it (27%). Severe itching in 1 patient after 30 mg/kg. | ||
| Pakistan [229] | 1998 | 100% (270)b | 83 Afghan refugees | CQ 25 mg/kg, 3 days (10, 10, 5) | 93% (83) day 7; 16% (83) day 28 | None reported | Extended CQ dose reduced the risk of recrudescence. ‘SVMNT’ haplotype in all infections. |
| 80 | CQ 40 mg/kg, 5 days (10, 10, 10, 5, 5) | 93% (80) day 7; 50% (80) day 28 | None reported | ||||
| Guinea-Bissau [230] | 1995–96 | 28% (50)b [163] | 67 children (≤13 years) | CQ 25 mg/kg, 3 days (10, 10, 5) | 84% (62) day 7; 79% (60) day 14; 70% (59) day 28 | it (5), di (5), vo (8) | Low prevalence of CQR strains. High CQ dosage was well-tolerated and delayed recrudescence. |
| 62 | CQ 50 mg/kg, 3 days (2 × 10, 2 × 10, 2 × 5)d | 100% (59) day 7; 100% (57) day 14; 86% (56) day 28 | di (14), vo (14) | ||||
| Guinea-Bissau [231] | 1996–99 | 17–39% [163] | 102 children (≤15 years) | QN 60 mg/kg, 3 days (2 × 10, 2 × 10, 2 × 10)d then CQ 25 mg/kg, 3 days (10,10,5) | 97% (90) day 7; 94% (88) day 14; 70% (78) day 28 | it (2) day 1, vo (16) | Study impacted by civil war in 1998–1999. Standard CQ dosage preceded by QN prevents direct comparisons. |
| 101 | CQ mg/kg 50, 3 days (2 × 10, 2 × 10, 2 × 5)d | 98% (89) day 7; 97% (86) day 14; 85% (71) day 28 | it (6) on day 1, it (3) on day 2, vo (2). | ||||
| Guinea-Bissau [232] | 2001–04 | 23% (478)b [170] | 170 children (≤15 years) | CQ 25 mg/kg, 3 days (10, 10, 5) | 98% (170) day 7; 94% (158) day 14; 76% (132) day 28 | vo (6) | High-dose CQ effective against 78% of CQR infections compared with 38% for the standard dose. |
| 169 | CQ 50 mg/kg, 3 days (2 × 10, 2 × 10, 2 × 5)d | 98% (169) day 7; 97% (159) day 14; 90% (141) day 28 | it (1), vo (10). | ||||
| Guinea-Bissau [164] | 2006–08 | 27% (303)b | 186 children (≤15 years) | Standard artemether-LM 3 day course | 95% (170) day 28; 95% (164) day 42; 89% (154) day 70 | it (5), de (1) | High-dose CQ effective against 87% of CQR infections. |
| 181 | CQ 50 mg/kg, 3 days (2 × 10, 2 × 10, 2 × 5)d | 94% (159) day 28; 91% (152) day 42; 84% (135) day 70 | it (20) |
aThe percentage of patients infected with CQR parasites, as determined by in vitro microtests (unless stated otherwise). The total number of samples that were analysed are shown in parentheses
bDetermined by detection of the K76T mutation in PfCRT
cTotal dosage (mg of drug/kg of body mass) and duration of the treatment. The mg/kg of drug administered in each sequential dose is listed in parentheses (the order is from the first to the last dose). Unless stated otherwise, the doses were given at 24-h intervals
dTwice-daily doses (i.e. a dose in the morning and a second identical dose at night)
eThe percentage of patients clear of parasites on a given day (non-PCR-adjusted). The total number of patients tested is provided in parentheses
fThe percentage of patients that reported reactions during treatment days are given in parentheses. The reactions were itching (it), diarrhoea (di), vomiting (vo), nausea (ns), abdominal pain (ap), and delirium (de)
Despite this recommendation, and in contrast with the earlier studies, high doses of CQ have remained effective and in widespread use for nearly two decades in the West African country of Guinea-Bissau. CQR parasites first appeared in Guinea-Bissau in 1990 [160, 161]. In response to increasing failure rates under the standard regimen, clinicians began prescribing two or three doses of CQ per day (12 or 8 h apart, respectively), resulting in total dosages of 50–75 mg/kg over 3–5 days [162, 163]. A series of clinical trials has confirmed that a twice-daily dose regimen (totalling 50 mg CQ/kg over 3 days) results in parasite clearance rates of 84–90% after 28 days (Table 2). The most recent clinical trial revealed that ‘double-dose’ CQ is just as effective as artesunate–LM in treating P. falciparum infections in Guinea-Bissau [164]. After 28 days, total efficacies of both treatments were greater than 95%. Furthermore, the double-dose CQ regimen cleared 87% of infections by parasites carrying PfCRTCQR [164].
Recent experiments using the Xenopus oocyte expression system have confirmed that PfCRTCQR behaves as a carrier rather than as a channel (see “Transport properties of PfCRT”; [43, 49]). These and other studies [44] indicate that the resistance mechanism is saturable, which raises the possibility that resistance could be overcome if CQ is maintained at sufficiently high concentrations within the DV of CQR parasites. The success of the high-dose regimen in Guinea-Bissau seems to be due to two factors: (1) the increase in the total dosage of CQ, and (2) the increase in the frequency of doses. A higher total dosage that is distributed into doses taken 8–12 h apart appears to sustain concentrations of CQ in the blood that are high enough to kill CQR parasites [165].
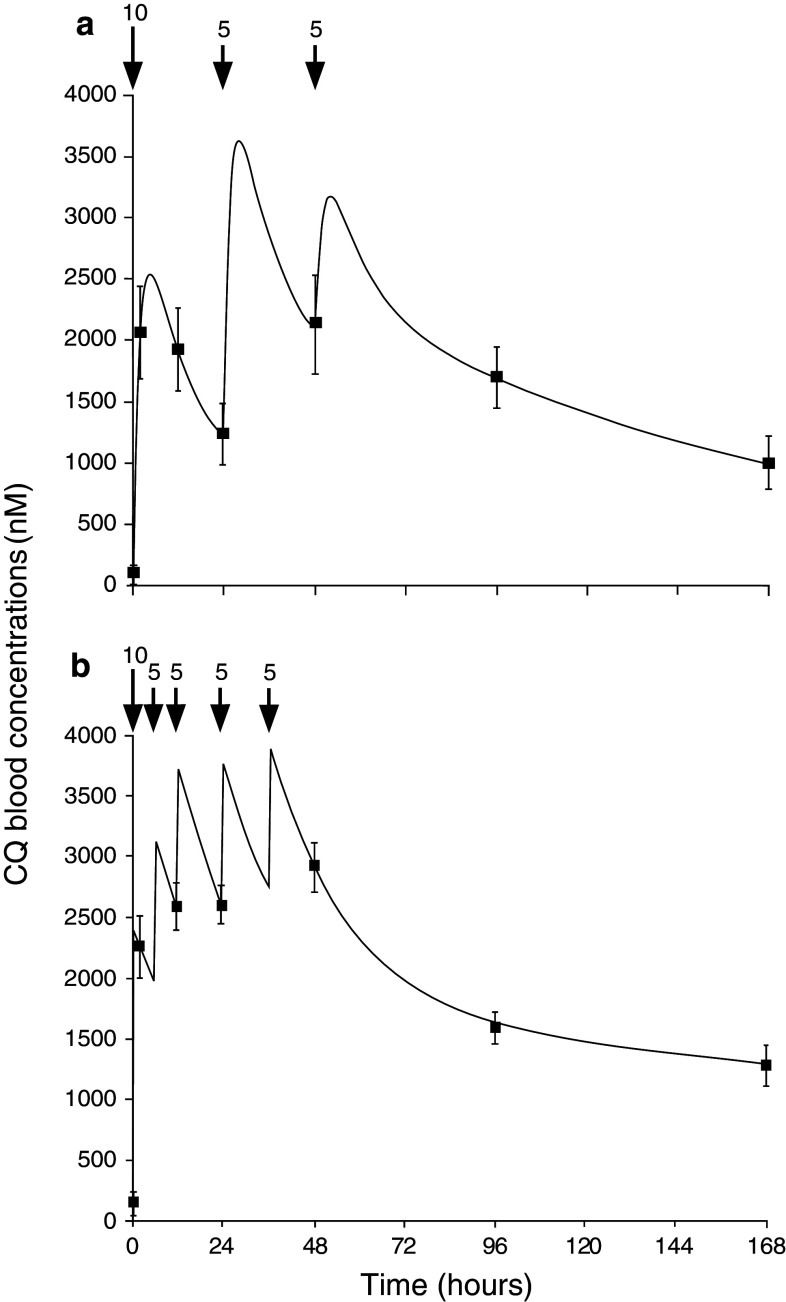
A demonstration of this principle comes from a clinical study of CQS infections in Madagascar [166]. When the dosage of CQ administered in the first 24 h was doubled from 10 to 20 mg/kg, and then split into 4 equally spaced doses, the rate of parasite clearance was double that measured in patients receiving the standard schedule [166]. The blood concentration of CQ in both sets of patients was also monitored over the course of the study (re-produced in Fig. 4). After oral intake, CQ is quickly absorbed into the blood/plasma, reaching peak concentrations within 1–3 h. A very high peak concentration of CQ can result in adverse side effects, but a single oral dose of less than 15 mg CQ/kg peaks below this threshold [167]. The subsequent decrease in the blood concentration of CQ consists of two phases. First, the level of CQ decreases rapidly as the drug distributes into tissues throughout the body. In the second phase, the concentration declines at a reduced rate as CQ is released from these tissues back into the blood stream. In patients receiving the standard regimen, the blood concentration of CQ declined in between doses to levels that were considerably lower than those measured in patients receiving the modified regimen (cf. Fig. 4a, b). Thus, when the time between doses was halved, the difference between the peak and trough concentrations of CQ was reduced dramatically, and the minimum concentration of CQ that parasites were exposed to in the first 2 days of treatment was elevated compared to the standard regimen (Fig. 4b). This ensured that the parasites were exposed to a sustained high concentration of CQ, resulting in significantly improved rates of parasite clearance.
Fig. 4.
The blood concentration of CQ over time during treatment with; a the standard CQ regimen (10 mg/kg at 0 and 24 h, and 5 mg/kg at 48 h), or b under more frequent administration (10 mg/kg at 0, followed by 5 mg/kg doses at 6, 12, 24 and 36 h). Numbers above the arrows indicate CQ doses in mg/kg of body weight. More frequent dosing led to higher blood concentrations of CQ during the first 3 days of treatment. This ensured that parasites were exposed to high CQ concentrations throughout the intraerythrocytic lifecycle and resulted in improved rates of parasite clearance. Reprinted with permission from [166]
Contrary to expectations [168], implementation of the double- or triple-dose CQ regimen has not accelerated the spread of CQR parasites in Guinea-Bissau. Instead, the prevalence of parasites carrying PfCRTCQR has remained relatively low (20% compared with 80% in neighbouring countries) [162, 163]. Several countries have observed the re-emergence of CQS parasites following the withdrawal of CQ—a phenomenon which is thought to be due to a fitness cost borne by parasites carrying the Dd2 form of PfCRTCQR [162]. This indicates that Dd2 PfCRTCQR confers a survival advantage only in the presence of CQ selection pressure. However, in Guinea-Bissau, the resistance-conferring abilities of PfCRTCQR appear to be nullified by the very high CQ selection pressures imposed by high-dose CQ, such that the CQS parasites are again able to outcompete, at least to some extent, their CQR counterparts [162]. It is important to note that the extended application of high CQ dosages in Guinea-Bissau has not resulted in the emergence of strains that are ‘super-resistant’ to CQ. This is consistent with the hypothesis that the resistance mechanism is limited in its capacity to respond to higher concentrations of CQ and/or that the energetic costs of ‘super resistance’ are too great for the parasite to bear [169].
An intriguing feature of the parasite population in Guinea-Bissau is the prevalence of the S163R and T152A mutations in PfCRTCQR. Ursing and colleagues [170] found that, prior to treatment, around one-third of infections consisted of parasites that carried these mutations. However, following standard or double-dose treatments with either AQ or CQ, S163R and T153A were detected in 97% of recrudescent infections. This is a somewhat surprising result given that: (1) these mutations had previously been described in parasite strains that are sensitive to CQ (but resistant to HF and MQ; [50]), and (2) the introduction of S163R into PfCRTCQR is known to abolish CQ transport activity [49]. One possible explanation for this observation is that high-dose CQ exerts a selection pressure on parasites harbouring PfCRTCQR. Indeed, the plasma CQ concentrations that result from treatment with double- or triple-dose CQ are likely to saturate PfCRTCQR. Saturation of the transporter by CQ is in turn likely to inhibit its normal function, and thereby reduce the parasite’s fitness. Thus, under the pressure of high-dose CQ, PfCRTCQR may be a liability rather than an advantage, and mutations that prevent the interaction of CQ with PfCRTCQR (such as S163R) could be the parasite’s response to this dilemma. This would go some way towards explaining several of the unusual features of the P. falciparum population of Guinea-Bissau—the low prevalence of CQR parasites, the high prevalence of the resistance-reversing S163R mutation in those carrying PfCRTCQR, and the absence of super-resistant parasites (despite nearly two decades of widespread use of high-dose CQ).
While the findings from studies performed in Guinea Bissau are promising, a number of issues must first be addressed before high-dose CQ treatments can be considered for deployment elsewhere. Twice-daily doses of 10 mg CQ/kg did not cause severe adverse effects in patients from Guinea-Bissau, but CQ absorption can vary between individuals, and there remains a risk that repeated sub-toxic doses, if taken too closely, could cause adverse effects in a subset of people [171]. Controlled-release formulations of CQ could reduce this risk by providing a relatively uniform blood concentration of CQ with fewer doses, potentially increasing the safety and compliance of a high-dose CQ regimen (see [172] for examples). A greater understanding of the pharmacokinetic and pharmacodynamic properties of high-dose CQ treatment of CQR P. falciparum malaria could guide dosage optimisation, and may provide valuable insights into the limits of the parasite’s CQ-resistance mechanism. A recent study of alternate CQ dosages in a mouse malaria model provides a foundation for this work [173]. However, because the resistance mechanism mediated by PfCRTCQR is unique to P. falciparum, a more relevant model would be one that made use of immuno-compromised mice that carry human blood, as these could be infected with CQR P. falciparum [174]. Furthermore, it is not known whether other variants of PfCRTCQR, such as those carried by the 7G8 or PH1 strains (Table 1), also saturate within physiologically relevant concentrations of CQ. It is worth noting that not all the parasite’s resistance mechanisms appear to be surmounted simply by increasing the dosage of the corresponding drug. In an area of west Cambodia where artemisinin-resistant strains are present, the treatment of malaria patients with high-dose artesunate did not accelerate the clearance of parasites [175], and in any case, the higher dose was found to cause neutropenia [176]. Thus, the saturability of the primary molecular mechanism underpinning CQ-resistance is somewhat unusual and represents a potential Achilles’ heel of the parasite—one which could be exploited by adopting a high-dose CQ regimen similar to the one already commonplace in Guinea-Bissau. The available evidence certainly encourages further clinical trials with double-dose CQ. The WHO-approved increase to the standard dosage of QN [177], and the current development of an azythromycin–CQ combination for intermittent preventative treatment of malaria in pregnancy [158], provide precedence for the reacceptance of CQ and a re-examination of the dose regimen.
MMV to the rescue
While the lack of immediate replacements for artemisinin is a cause for concern, the earlier stages of the antimalarial pipeline are beginning to look more promising [178]. Established as a public–private partnership in 1999, the Medicines for Malaria Venture (MMV) has provided much-needed structure and funding towards the development of new antimalarial drugs [179]. By coordinating between industry and academic groups, the MMV has facilitated the allocation of resources and expertise across an expanding range of drug development projects. An example of the success of this approach has been the application of high-throughput, whole-cell proliferation assays to the testing of large commercial compound libraries for new antimalarial drug leads [70, 180–183]. In recent years, more than 5 million compounds have been tested against P. falciparum-infected erythrocytes, resulting in the identification of more than 20,000 compounds that exhibit antimalarial activity at sub-micromolar concentrations [184]. Most of these compounds were previously undescribed or unpublished, and are predicted to target entirely new aspects of parasite biology [180, 181]. When compared to the traditional approach of drug discovery (whereby compounds are designed and developed to inhibit a known molecular target), the use of whole-cell assays to screen compound libraries has dramatically reduced the time required to obtain viable lead compounds [183, 185]. The progress of the antimalarial drug discovery and development projects currently underway, as well as the challenges faced by the field, are discussed in two recent reviews [129, 186]. A noteworthy point made by Grimberg and Mehlotra [186] is that there is a need not only for the discovery of new antimalarial drugs but also for the redesign of old therapies, as these could be implemented now.
Quinolines are not passé: the next generation of quinoline-based strategies
Given the uncertain life-spans of the existing malaria chemotherapies, and the time and cost required to develop novel drug classes, it is perhaps unwise to abandon the quinolines altogether. Our understanding of the mechanisms underpinning quinoline resistance is steadily deepening, and this knowledge could be applied to the development of a new generation of quinoline-based therapies that are designed to be effective against multidrug-resistant parasites. Here, we discuss a number of avenues that are currently being explored and which could prove fruitful in the search for next-generation quinoline antimalarials.
Overlooked analogues and discarded drugs
Beginning in the early 1960s—in the period when CQR parasites were beginning to emerge and spread—the U.S. Army Antimalarial Drug Development Program tested more than 200,000 new compounds over 10 years in the search for new antimalarial drugs [187]. At this time, however, antimalarial drug screening depended entirely on in vivo experiments using animal models of malaria [188–190]. Given that the antimalarial activity of a compound can vary between different Plasmodium species, and that the PfCRT-based resistance mechanism appears to be unique to P. falciparum, it is possible that these early experiments have overlooked compounds that are in fact active against drug-resistant P. falciparum. Thus, a re-examination of the efficacy of a selection of these analogues using modern in vitro assays could prove fruitful. In addition, drugs that have been withdrawn, or which were only deployed on a small scale, could be redesigned to circumvent tolerability issues. For example, the result of the optimisation of AQ—tert-butyl isoquine—does not generate undesirable products when metabolised ([191]; Fig. 1d), and attempts are underway to design analogues of MQ that do not cross the blood–brain barrier, and which cause fewer adverse side effects [192].
Compounds that evade the resistance mechanism(s)
Analogues of CQ with modified side-chains have been shown to retain activity against CQR parasites. For example, shortening or lengthening the diaminoalkane side chain of CQ improves its activity against CQR parasites [193–195]. One of these short side-chain analogues of CQ, AQ13 (Fig. 1d), is in phase II clinical trials [196]. Moreover, 4-aminoquinolines that contain an aromatic ring in the side-chain (such as AQ, and others [197]), a branched side-chain [198], or a heterocyclic group [199] also tend to retain activity against CQR parasites. In each of these cases, the side-chain modifications are thought to create steric limitations that hinder binding and/or transport by PfCRTCQR. Organometallic 4-aminoquinolines that incorporate a ferrocene moiety in the side-chain, such as ferroquine, have also been shown to be equally active against CQR and CQS strains ([200]; Fig. 1d). How ferroquine overcomes the resistance mechanism(s) is not known, but there are a number of possibilities: (1) it may not be recognised and transported out of the DV by PfCRTCQR, (2) compared with CQ, it may be a more potent inhibitor of haemozoin formation and/or its physicochemical properties may result in higher levels of accumulation within the DV, and (3) the ferrocene moiety may impart one or more additional modes of antimalarial action. All these features would be desirable in a next-generation 4-aminoquinoline [201]. Ferroquine is currently being tested in clinical trials, and a combination therapy (with artesunate as the partner drug) is under development [202]. Further examination of the structure–activity relationships for modified 4-aminoquinolinesis can be found in a review by O’Neill and colleagues [203].
Compounds that target the resistance mechanism(s)
The clinical use of CQ resistance-reversers has been limited due to poor potency, bioavailability, and safety profiles. Many of the resistance-reversing agents identified to date have been approved and optimised for other (unrelated) therapeutic activities, so there is likely to be some scope for improvement of both resistance-reversing activity and safety through chemical modification. In this regard, an understanding of how these compounds interact with and inhibit PfCRTCQR could provide a basis for optimising their potency as resistance-reversers. A drawback of using resistance-reversers in conjunction with CQ is that the combination would, in effect, be a CQ monotherapy. However, two approaches have recently produced resistance-reversers with intrinsic antimalarial activities—one combined an antimalarial pharmacophore with a resistance-reverser [204–208] and the other used whole-cell, high-throughput screening to identify resistance-reversers which possess inherent antimalarial activities [70]. An alternative tactic, possible only once the normal substrate(s) of PfCRT is identified, would be to incorporate substrate-mimicking features into the resistance-reverser structure so as to impart antiplasmodial activity against both CQR and CQS parasites.
Several groups have chemically coupled the antimalarial pharmacophore of CQ (7-chloro-4-aminoquinoline) to a resistance-reversing side-chain [205, 207, 208]. These compounds, known as ‘reversed-CQ’ molecules, have been shown to be highly active against both CQR and CQS parasite strains in vitro, and several are active against mouse malaria in vivo ([204, 205, 207, 208]; Fig. 1d). They also inhibit haemozoin formation in vitro and in vivo and are therefore expected to have a similar mechanism of action to CQ [204]. Kelly and colleagues [206] have used a similar principle to develop a new antimalarial chemotype which superimposes both the resistance-reverser and the anti-haemozoin pharmacophores onto a single tricyclic acridone nucleus. The lead compound—T3.5 (Fig. 1d)—was highly potent against both CQS and CQR parasites in vitro, but also had the ability to chemosensitise CQR parasites to CQ, QN, AQ, and PIP, and to potentiate the activities of QN and PIP in a CQS strain [206]. There are a number of other such ‘dual-function’ compounds that have been designed to combine multiple antimalarial pharmacophores in a single molecule. A notable example, trioxaquine, combines the 4-aminoquinoline nucleus of CQ with an artemisinin-like endoperoxide side-chain and has potent activity against both CQR and CQS parasites ([209] Fig. 1d). A key aim of combination strategies is to delay the emergence of resistance by coupling multiple modes of action in the one therapy. Dual-function drugs progress this concept one step further by synchronising the pharmacokinetics of the two components. Further discussion of hybrid antimalarials can be found in the recent review by Muregi and Ishih [210].
A recent study combined high-throughput antiplasmodial assays with genetic approaches to analyse the range and diversity of responses to antiplasmodial compounds in 61 strains of P. falciparum [70]. Not only did this work identify new antiplasmodial agents with potent activities against most of the strains tested, but Yuan et al. were also able to study correlations in activity between compounds, and to use genomic techniques to determine the elements responsible for these differences. Of the 489 compounds which showed a fivefold or more difference in antiplasmodial activity between strains, more than 200 were associated with mutations in pfcrt [70]. When linkage analysis was used to map the loci associated with the parasite’s response to 49 of these compounds, 96% could be linked directly to mutations in just three genes—pfcrt, pfmdr1, and the gene encoding the P. falciparum dihydrofolate reductase (pfdhfr). Of the 23 compounds linked to pfcrt mutations, the IC50 values of 17 were reduced against the CQR Dd2 parasite strain when combined with low concentrations of CQ [70]. While few of these compounds displayed strong intrinsic antiplasmodial activity, two closely related Ca2+ channel blockers—mibefradil and NNC55-0396 (Fig. 1d)—were found to have IC50 values against Dd2 parasites that were 3–4 times lower than that of VP [70].
Beyond the examination of potential resistance-reversers, the study by Yuan and colleagues identifies some important points relating to the future of antimalarial drug development. The majority of the differential parasite responses were attributable to genes that are already known to be involved in mediating drug resistance [70]. Hence, the more we know about these resistance mechanisms, the better prepared we will be to predict and understand the parasite’s responses to new drugs. When the 492 compounds were grouped according to correlations in antiplasmodial activities, a mere 44 clusters emerged, which suggests that a relatively small number of pathways were targeted. Nevertheless, 1,250 pairs of compounds were identified as having negatively correlated activities [70]. This finding indicates that, although resistance may be a problem for individual compounds, or a class of compounds, it should be possible to identify complementary drugs which exert opposing selection forces upon the parasite. The ability of the parasite to develop resistance to such combinations is likely to be severely constrained by the limitations of protein structure and function.
Towards a ‘Resistance stalemate’
While it is apparent that PfCRT plays a critical role in the susceptibility of the parasite to a multitude of antiplasmodial compounds, and that it is therefore likely to interact with a wide range of structures, the resistance mechanism is nevertheless limited in a number of critical aspects: (1) the saturability of transport via PfCRTCQR means that resistance can be overcome simply by increasing the dosage of CQ, (2) small changes in the structure of the CQ side-chain can restore activity against CQR parasites, (3) a wide range of compounds are able to inhibit, and thereby reverse, the resistance mechanism, and (4) mutations that reduce the parasite’s sensitivity to HF, MQ, and potentially PIP, restore susceptibility to CQ and vice versa, and recent work has identified dozens of additional compounds that have antiplasmodial activities which are negatively correlated with CQ.
An understanding of the structure–function relationships that dictate PfCRT activity, and in particular, the extent of its capacity to undergo mutation while still maintaining its normal physiological function, will facilitate a more fundamental appreciation of the selection forces at play. In this regard, uncovering the normal substrate(s) of PfCRT remains an important goal. Moreover, the observation that one drug can exert a selection force on PfCRT that opposes that of another raises the possibility that the parasite could be trapped in an evolutionary stalemate. Careful design of antimalarial combinations could result in a ‘resistance conundrum’, whereby the mutations required for tolerance to one drug increase the parasite’s sensitivity to the partner drug (and perhaps also impairs the essential function of PfCRT). A coordinated, multidisciplinary effort to understand and combat drug resistance is warranted and could, in time, lead to an evolutionary endgame for PfCRT.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (NHMRC) (grant 1007035) and the L’Oréal Australia For Women in Science programme. R.E.M. was supported by an NHMRC Australian Biomedical Fellowship (fellowship 520320).
References
- 1.WHO (2010) World malaria report 2010
- 2.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 3.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 4.Holding PA, Snow RW. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. Am J Trop Med Hyg. 2001;64(1–2 Suppl):68–75. doi: 10.4269/ajtmh.2001.64.68. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1–2 Suppl):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 6.Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, Binka F, Kilama WL, Ford N, White NJ. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect Dis. 2002;2(9):564–573. doi: 10.1016/S1473-3099(02)00372-9. [DOI] [PubMed] [Google Scholar]
- 7.Kirk K. Membrane transport in the malaria-infected erythrocyte. Physiol Rev. 2001;81(2):495–537. doi: 10.1152/physrev.2001.81.2.495. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood D. The quinine connection. J Antimicrob Chemother. 1992;30(4):417–427. doi: 10.1093/jac/30.4.417. [DOI] [PubMed] [Google Scholar]
- 9.Meshnick SR, Dobson MJ. The history of antimalarial drugs. In: Rosenthal PJ, editor. Antimalarial chemotherapy: mechanisms of action resistance and new directions in drug discovery. Totowa: Humana Press; 2001. pp. 15–25. [Google Scholar]
- 10.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17(12):582–588. doi: 10.1016/S1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 11.Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewska K, Barends M, Brockman A, Anderson T, McGready R, Phaiphun L, Proux S, van Vugt M, Hutagalung R, Lwin KM, Phyo AP, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS ONE. 2009;4(2):e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8(4):272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 13.Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J. 2010;9:273. doi: 10.1186/1475-2875-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2011) Update on artemisinin resistance—September 2011
- 16.Hayward R, Saliba KJ, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J Cell Sci. 2006;119(Pt 6):1016–1025. doi: 10.1242/jcs.02795. [DOI] [PubMed] [Google Scholar]
- 17.Klonis N, Tan O, Jackson K, Goldberg D, Klemba M, Tilley L. Evaluation of pH during cytostomal endocytosis and vacuolar catabolism of haemoglobin in Plasmodium falciparum . Biochem J. 2007;407(3):343–354. doi: 10.1042/BJ20070934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn Y, Rohrbach P, Lanzer M. Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell Microbiol. 2007;9(4):1004–1013. doi: 10.1111/j.1462-5822.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 19.Yayon A. The antimalarial mode of action of chloroquine. Rev Clin Basic Pharm. 1985;5(1–2):99–139. [PubMed] [Google Scholar]
- 20.Bray PG, Mungthin M, Hastings IM, Biagini GA, Saidu DK, Lakshmanan V, Johnson DJ, Hughes RH, Stocks PA, O’Neill PM, Fidock DA, Warhurst DC, Ward SA. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol Microbiol. 2006;62(1):238–251. doi: 10.1111/j.1365-2958.2006.05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egan TJ. Chloroquine and primaquine: combining old drugs as a new weapon against falciparum malaria? Trends Parasitol. 2006;22(6):235–237. doi: 10.1016/j.pt.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob Agents Chemother. 2006;50(6):2197–2200. doi: 10.1128/AAC.00119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis TME, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine—a resurgent antimalarial drug. Drugs. 2005;65(1):75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Fitch CD. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 2004;74(16):1957–1972. doi: 10.1016/j.lfs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79(1):55–87. doi: 10.1016/S0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 26.Hawley SR, Bray PG, Mungthin M, Atkinson JD, O’Neill PM, Ward SA. Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1998;42(3):682–686. doi: 10.1128/aac.42.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mungthin M, Bray PG, Ridley RG, Ward SA. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob Agents Chemother. 1998;42(11):2973–2977. doi: 10.1128/aac.42.11.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou AC, Chevli R, Fitch CD. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- 29.de Villiers KA, Marques HM, Egan TJ. The crystal structure of halofantrine-ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. J Inorg Biochem. 2008;102(8):1660–1667. doi: 10.1016/j.jinorgbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Fitch CD. Chloroquine resistance in malaria: a deficiency of chloroquine binding. Proc Natl Acad Sci USA. 1969;64(4):1181–1187. doi: 10.1073/pnas.64.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- 32.Martin SK, Oduola AM, Milhous WK. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235(4791):899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 33.Watt G, Long GW, Grogl M, Martin SK. Reversal of drug-resistant falciparum malaria by calcium antagonists: potential for host cell toxicity. Trans R Soc Trop Med Hyg. 1990;84(2):187–190. doi: 10.1016/0035-9203(90)90248-D. [DOI] [PubMed] [Google Scholar]
- 34.Wellems TE, Walker-Jonah A, Panton LJ. Genetic mapping of the chloroquine-resistance locus on Plasmodium falciparum chromosome 7. Proc Natl Acad Sci USA. 1991;88(8):3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su X, Kirkman LA, Fujioka H, Wellems TE. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell. 1997;91(5):593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 36.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6(4):861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298(5591):210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin RE, Kirk K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol Biol Evol. 2004;21(10):1938–1949. doi: 10.1093/molbev/msh205. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn Y, Sanchez CP, Ayoub D, Saridaki T, van Dorsselaer A, Lanzer M. Trafficking of the phosphoprotein PfCRT to the digestive vacuolar membrane in Plasmodium falciparum . Traffic. 2010;11(2):236–249. doi: 10.1111/j.1600-0854.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 40.Lakshmanan V, Bray PG, Verdier-Pinard D, Johnson DJ, Horrocks P, Muhle RA, Alakpa GE, Hughes RH, Ward SA, Krogstad DJ, Sidhu AB, Fidock DA. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 2005;24(13):2294–2305. doi: 10.1038/sj.emboj.7600681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaijaroenkul W, Ward SA, Mungthin M, Johnson D, Owen A, Bray PG, Na-Bangchang K. Sequence and gene expression of chloroquine resistance transporter (pfcrt) in the association of in vitro drugs resistance of Plasmodium falciparum . Malar J. 2011;10(1):42. doi: 10.1186/1475-2875-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez CP, Dave A, Stein WD, Lanzer M. Transporters as mediators of drug resistance in Plasmodium falciparum . Int J Parasitol. 2010;40(10):1109–1118. doi: 10.1016/j.ijpara.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Summers RL, Martin RE. Functional characteristics of the malaria parasite’s “chloroquine resistance transporter”: implications for chemotherapy. Virulence. 2010;1(4):304–308. doi: 10.4161/viru.1.4.12012. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez CP, McLean JE, Rohrbach P, Fidock DA, Stein WD, Lanzer M. Evidence for a pfcrt-associated chloroquine efflux system in the human malarial parasite Plasmodium falciparum . Biochemistry. 2005;44(29):9862–9870. doi: 10.1021/bi050061f. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez CP, Rohrbach P, McLean JE, Fidock DA, Stein WD, Lanzer M. Differences in trans-stimulated chloroquine efflux kinetics are linked to PfCRT in Plasmodium falciparum . Mol Microbiol. 2007;64(2):407–420. doi: 10.1111/j.1365-2958.2007.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehane AM, Hayward R, Saliba KJ, Kirk K. A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J Cell Sci. 2008;121:1624–1632. doi: 10.1242/jcs.016758. [DOI] [PubMed] [Google Scholar]
- 47.Lehane AM, Kirk K. Chloroquine resistance-conferring mutations in pfcrt give rise to a chloroquine-associated H + leak from the malaria parasite’s digestive vacuole. Antimicrob Agents Chemother. 2008;52(12):4374–4380. doi: 10.1128/AAC.00666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naude B, Brzostowski JA, Kimmel AR, Wellems TE. Dictyostelium discoideum expresses a malaria chloroquine resistance mechanism upon transfection with mutant, but not wild-type, Plasmodium falciparum transporter PfCRT. J Biol Chem. 2005;280(27):25596–25603. doi: 10.1074/jbc.M503227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science. 2009;325(5948):1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 50.Johnson DJ, Fidock DA, Mungthin M, Lakshmanan V, Sidhu AB, Bray PG, Ward SA. Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol Cell. 2004;15(6):867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waller KL, Muhle RA, Ursos LM, Horrocks P, Verdier-Pinard D, Sidhu AB, Fujioka H, Roepe PD, Fidock DA. Chloroquine resistance modulated in vitro by expression levels of the Plasmodium falciparum chloroquine resistance transporter. J Biol Chem. 2003;278(35):33593–33601. doi: 10.1074/jbc.M302215200. [DOI] [PubMed] [Google Scholar]
- 52.Carlton JM, Fidock DA, Djimde A, Plowe CV, Wellems TE. Conservation of a novel vacuolar transporter in Plasmodium species and its central role in chloroquine resistance of P. falciparum. Curr Opin Microbiol. 2001;4(4):415–420. doi: 10.1016/s1369-5274(00)00228-9. [DOI] [PubMed] [Google Scholar]
- 53.van Schalkwyk DA, Egan TJ. Quinoline-resistance reversing agents for the malaria parasite Plasmodium falciparum . Drug Resist Updat. 2006;9(4–5):211–226. doi: 10.1016/j.drup.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Egan TJ, Kaschula CH. Strategies to reverse drug resistance in malaria. Curr Opin Infect Dis. 2007;20(6):598–604. doi: 10.1097/QCO.0b013e3282f1673a. [DOI] [PubMed] [Google Scholar]
- 55.Guantai E, Chibale K. Chloroquine resistance: proposed mechanisms and countermeasures. Curr Drug Deliv. 2010;7(4):312–323. doi: 10.2174/156720110793360577. [DOI] [PubMed] [Google Scholar]
- 56.Pereira MR, Henrich PP, Sidhu AB, Johnson D, Hardink J, Van Deusen J, Lin J, Gore K, O’Brien C, Wele M, Djimde A, Chandra R, Fidock DA. In vivo and in vitro antimalarial properties of azithromycin-chloroquine combinations that include the resistance reversal agent amlodipine. Antimicrob Agents Chemother. 2011;55(7):3115–3124. doi: 10.1128/AAC.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sowunmi A, Oduola AM, Ogundahunsi OA, Falade CO, Gbotosho GO, Salako LA. Enhanced efficacy of chloroquine-chlorpheniramine combination in acute uncomplicated falciparum malaria in children. Trans R Soc Trop Med Hyg. 1997;91(1):63–67. doi: 10.1016/S0035-9203(97)90399-0. [DOI] [PubMed] [Google Scholar]
- 58.Ogungbamigbe TO, Ojurongbe O, Ogunro PS, Okanlawon BM, Kolawole SO. Chloroquine resistant Plasmodium falciparum malaria in Osogbo Nigeria: efficacy of amodiaquine + sulfadoxine-pyrimethamine and chloroquine + chlorpheniramine for treatment. Mem Inst Oswaldo Cruz. 2008;103(1):79–84. doi: 10.1590/S0074-02762008000100012. [DOI] [PubMed] [Google Scholar]
- 59.Alibert S, Santelli-Rouvier C, Pradines B, Houdoin C, Parzy D, Karolak-Wojciechowska J, Barbe J. Synthesis and effects on chloroquine susceptibility in Plasmodium falciparum of a series of new dihydroanthracene derivatives. J Med Chem. 2002;45(15):3195–3209. doi: 10.1021/jm011046l. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharjee AK, Kyle DE, Vennerstrom JL. Structural analysis of chloroquine resistance reversal by imipramine analogs. Antimicrob Agents Chemother. 2001;45(9):2655–2657. doi: 10.1128/AAC.45.9.2655-2657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan J, Kyle DE, Gerena L, Zhang Q, Milhous WK, Lin AJ. Design, synthesis, and evaluation of new chemosensitizers in multi-drug-resistant Plasmodium falciparum . J Med Chem. 2002;45(13):2741–2748. doi: 10.1021/jm010549o. [DOI] [PubMed] [Google Scholar]
- 62.Kelly JX, Smilkstein MJ, Cooper RA, Lane KD, Johnson RA, Janowsky A, Dodean RA, Hinrichs DJ, Winter R, Riscoe M. Design, synthesis, and evaluation of 10-N-substituted acridones as novel chemosensitizers in Plasmodium falciparum . Antimicrob Agents Chemother. 2007;51(11):4133–4140. doi: 10.1128/AAC.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhattacharjee AK, Kyle DE, Vennerstrom JL, Milhous WK. A 3D QSAR pharmacophore model and quantum chemical structure–activity analysis of chloroquine (CQ)-resistance reversal. J Chem Inf Comput Sci. 2002;42(5):1212–1220. doi: 10.1021/ci0200265. [DOI] [PubMed] [Google Scholar]
- 64.Mehlotra RK, Fujioka H, Roepe PD, Janneh O, Ursos LM, Jacobs-Lorena V, McNamara DT, Bockarie MJ, Kazura JW, Kyle DE, Fidock DA, Zimmerman PA. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc Natl Acad Sci USA. 2001;98(22):12689–12694. doi: 10.1073/pnas.221440898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel JJ, Thacker D, Tan JC, Pleeter P, Checkley L, Gonzales JM, Deng B, Roepe PD, Cooper RA, Ferdig MT. Chloroquine susceptibility and reversibility in a Plasmodium falciparum genetic cross. Mol Microbiol. 2010;78(3):770–787. doi: 10.1111/j.1365-2958.2010.07366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sá JM, Twu O, Hayton K, Reyes S, Fay MP, Ringwald P, Wellems TE. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl Acad Sci USA. 2009;106(45):18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zishiri VK, Hunter R, Smith PJ, Taylor D, Summers R, Kirk K, Martin RE, Egan TJ. A series of structurally simple chloroquine chemosensitizing dibemethin derivatives that inhibit chloroquine transport by PfCRT. Eur J Med Chem. 2011;46(5):1729–1742. doi: 10.1016/j.ejmech.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Lehane AM, Kirk K. Efflux of a range of antimalarial drugs and ‘chloroquine resistance reversers’ from the digestive vacuole in malaria parasites with mutant PfCRT. Mol Microbiol. 2010;77(4):1039–1051. doi: 10.1111/j.1365-2958.2010.07272.x. [DOI] [PubMed] [Google Scholar]
- 69.Peters W, Ekong R, Robinson BL, Warhurst DC, Pan XQ. Antihistaminic drugs that reverse chloroquine resistance in Plasmodium falciparum . Lancet. 1989;2(8658):334–335. doi: 10.1016/S0140-6736(89)90522-9. [DOI] [PubMed] [Google Scholar]
- 70.Yuan J, Cheng KC, Johnson RL, Huang R, Pattaradilokrat S, Liu A, Guha R, Fidock DA, Inglese J, Wellems TE, Austin CP, Su XZ. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science. 2011;333(6043):724–729. doi: 10.1126/science.1205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foote SJ, Kyle DE, Martin RK, Oduola AMJ, Forsyth K, Kemp DJ, Cowman AF. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum . Nature. 1990;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- 72.Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, Wirth DF. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum . Science. 1989;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- 73.Cowman AF, Karcz S, Galatis D, Culvenor JG. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J Cell Biol. 1991;113(5):1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karcz SR, Galatis D, Cowman AF. Nucleotide binding-properties of a P-glycoprotein homolog from Plasmodium falciparum . Mol Biochem Parasitol. 1993;58(2):269–276. doi: 10.1016/0166-6851(93)90048-3. [DOI] [PubMed] [Google Scholar]
- 75.Rohrbach P, Sanchez CP, Hayton K, Friedrich O, Patel J, Sidhu ABS, Ferdig MT, Fidock DA, Lanzer M. Genetic linkage of pfmdr1 with food vacuolar solute import in Plasmodium falciparum . EMBO J. 2006;25(13):3000–3011. doi: 10.1038/sj.emboj.7601203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez CP, Rotmann A, Stein WD, Lanzer M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum . Mol Microbiol. 2008;70(4):786–798. doi: 10.1111/j.1365-2958.2008.06413.x. [DOI] [PubMed] [Google Scholar]
- 77.van Es HHG, Karcz S, Chu F, Cowman AF, Vidal S, Gros P, Schurr E. Expression of the plasmodial pfmdr1 gene in mammalian cells is associated with increased susceptibility to chloroquine. Mol Cell Biol. 1994;14(4):2419–2428. doi: 10.1128/MCB.14.4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volkman SK, Cowman AF, Wirth DF. Functional complementation of the ste6 gene of Saccharomyces cerevisiae with the pfmdr1 gene of Plasmodium falciparum . Proc Natl Acad Sci USA. 1995;92:8921–8925. doi: 10.1073/pnas.92.19.8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum . Nature. 2000;403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 80.Sidhu ABS, Valderramos SG, Fidock DA. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum . Mol Microbiol. 2005;57(4):913–926. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- 81.Khim N, Bouchier C, Ekala MT, Incardona S, Lim P, Legrand E, Jambou R, Doung S, Puijalon OM, Fandeur T. Countrywide survey shows very high prevalence of Plasmodium falciparum multilocus resistance genotypes in Cambodia. Antimicrob Agents Chemother. 2005;49(8):3147–3152. doi: 10.1128/AAC.49.8.3147-3152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veiga MI, Ferreira PE, Jornhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Bjorkman A, Nosten F, Gil JP. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One. 2011;6(5):e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mu JB, Ferdig MT, Feng XR, Joy DA, Duan JH, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol Microbiol. 2003;49(4):977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- 84.Anderson TJC, Nair S, Qin H, Singlam S, Brockman A, Paiphun L, Nosten F. Are transporter genes other than the chloroquine resistance locus (pfcrt) and multidrug resistance gene (pfmdr) associated with antimalarial drug resistance? Antimicrob Agents Chemother. 2005;49(6):2180–2188. doi: 10.1128/AAC.49.6.2180-2188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briolant S, Henry M, Oeuvray C, Amalvict R, Baret E, Didillon E, Rogier C, Pradines B. Absence of association between piperaquine in vitro responses and polymorphisms in the pfcrt, pfmdr1, pfmrp, and pfnhe genes in Plasmodium falciparum . Antimicrob Agents Chemother. 2010;54(9):3537–3544. doi: 10.1128/AAC.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raj DK, Mu JB, Jiang HY, Kabat J, Singh S, Sullivan M, Fay MP, McCutchan TF, Su XZ. Disruption of a Plasmodium falciparum Multidrug Resistance-associated Protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009;284(12):7687–7696. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnes DA, Foote SJ, Galatis D, Kemp DJ, Cowman AF. Selection for high-level chloroquine resistance results in deamplification of the pfmdr1 gene and increased sensitivity to mefloquine in Plasmodium falciparum. EMBO J. 1992;11(8):3067–3075. doi: 10.1002/j.1460-2075.1992.tb05378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaijaroenkul W, Wisedpanichkij R, Na-Bangchang K. Monitoring of in vitro susceptibilities and molecular markers of resistance of Plasmodium falciparum isolates from Thai-Myanmar border to chloroquine, quinine, mefloquine and artesunate. Acta Trop. 2010;113(2):190–194. doi: 10.1016/j.actatropica.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 89.Cowman AF, Galatis D, Thompson JK. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc Natl Acad Sci USA. 1994;91(3):1143–1147. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peel SA, Bright P, Yount B, Handy J, Baric RS. A strong association between mefloquine and halofantrine resistance and amplification, overexpression, and mutation in the P-glycoprotein gene homologue (pfmdr1) of Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1994;51(5):648–658. doi: 10.4269/ajtmh.1994.51.648. [DOI] [PubMed] [Google Scholar]
- 91.Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1 . Antimicrob Agents Chemother. 2003;47(8):2418–2423. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, Nosten F, Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43(12):2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364(9432):438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sidhu ABS, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194(4):528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cooper RA, Ferdig MT, Su XZ, Ursos LM, Mu J, Nomura T, Fujioka H, Fidock DA, Roepe PD, Wellems TE. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum . Mol Pharmacol. 2002;61(1):35–42. doi: 10.1124/mol.61.1.35. [DOI] [PubMed] [Google Scholar]
- 96.Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol Microbiol. 2005;56(2):323–333. doi: 10.1111/j.1365-2958.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- 97.Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett. 2011;585(11):1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 98.Uhlemann AC, Ramharter M, Lell B, Kremsner PG, Krishna S. Amplification of Plasmodium falciparum multidrug resistance gene 1 in isolates from Gabon. J Infect Dis. 2005;192(10):1830–1835. doi: 10.1086/497337. [DOI] [PubMed] [Google Scholar]
- 99.Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg. 2006;75(1):162–165. [PubMed] [Google Scholar]
- 100.Huaman MC, Roncal N, Nakazawa S, Long TTA, Gerena L, Garcia C, Solari L, Magill AJ, Kanbara H. Polymorphism of the Plasmodium falciparum multidrug resistance and chloroquine resistance transporter genes and in vitro susceptibility to aminoquinolines in isolates from the Peruvian Amazon. Am J Trop Med Hyg. 2004;70(5):461–466. [PubMed] [Google Scholar]
- 101.Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ. In vitro sensitivities of Plasmodium falciparum to different antimalarial drugs in Uganda. Antimicrob Agents Chemother. 2010;54(3):1200–1206. doi: 10.1128/AAC.01412-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su X, Wellems TE. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol. 2004;52(4):985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 103.Sanchez CP, Stein WD, Lanzer M. Dissecting the components of quinine accumulation in Plasmodium falciparum . Mol Microbiol. 2008;67(5):1081–1093. doi: 10.1111/j.1365-2958.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- 104.Yayon A, Cabantchik ZI, Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984;3(11):2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valderramos SG, Valderramos JC, Musset L, Purcell LA, Mercereau-Puijalon O, Legrand E, Fidock DA. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PloS Pathog. 2010;6(5):e1000887. doi: 10.1371/journal.ppat.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henry M, Briolant S, Zettor A, Pelleau S, Baragatti M, Baret E, Mosnier J, Amalvict R, Fusai T, Rogier C, Pradines B. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob Agents Chemother. 2009;53(5):1926–1930. doi: 10.1128/AAC.01243-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meng H, Zhang RP, Yang HL, Fan Q, Su XZ, Miao J, Cui LW, Yang ZQ. In vitro sensitivity of Plasmodium falciparum clinical isolates from the China-Myanmar border area to quinine and association with polymorphism in the Na(+)/H(+) exchanger. Antimicrob Agents Chemother. 2010;54(10):4306–4313. doi: 10.1128/AAC.00321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sinou V, Le HQ, Pelleau S, Vu NH, Nguyen TH, Le MT, Bertaux L, Desbordes M, Latour C, Lai QL, Nguyen XT, Parzy D. Polymorphism of Plasmodium falciparum Na+/H+ exchanger is indicative of a low in vitro quinine susceptibility in isolates from Viet Nam. Malar J. 2011;10:164. doi: 10.1186/1475-2875-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andriantsoanirina V, Menard D, Rabearimanana S, Hubert V, Bouchier C, Tichit M, Le Bras J, Durand R. Association of microsatellite variations of Plasmodium falciparum Na+/H+ exchanger (Pfnhe-1) gene with reduced in vitro susceptibility to quinine: lack of confirmation in clinical isolates from Africa. Am J Trop Med Hyg. 2010;82(5):782–787. doi: 10.4269/ajtmh.2010.09-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baliraine FN, Nsobya SL, Achan J, Tibenderana JK, Talisuna AO, Greenhouse B, Rosenthal PJ. Limited ability of Plasmodium falciparum pfcrt, pfmdr1, and pfnhe1 polymorphisms to predict quinine in vitro sensitivity or clinical effectiveness in Uganda. Antimicrob Agents Chemother. 2011;55(2):615–622. doi: 10.1128/AAC.00954-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Briolant S, Pelleau S, Bogreau H, Hovette P, Zettor A, Castello J, Baret E, Amalvict R, Rogier C, Pradines B. In vitro susceptibility to quinine and microsatellite variations of the Plasmodium falciparum Na+/H+ exchanger (Pfnhe-1) gene: the absence of association in clinical isolates from the Republic of Congo. Malar J. 2011;10(1):37. doi: 10.1186/1475-2875-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okombo J, Kiara SM, Rono J, Mwai L, Pole L, Ohuma E, Borrmann S, Ochola LI, Nzila A. In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium Isolates from Kenya. Antimicrob Agents Chemother. 2010;54(8):3302–3307. doi: 10.1128/AAC.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bennett TN, Patel J, Ferdig MT, Roepe PD. Plasmodium falciparum Na +/H + exchanger activity and quinine resistance. Mol Biochem Parasitol. 2007;153(1):48–58. doi: 10.1016/j.molbiopara.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spillman NJ, Allen RJW, Kirk K. Acid extrusion from the intraerythrocytic malaria parasite is not via a Na+/H+ exchanger. Mol Biochem Parasitol. 2008;162(1):96–99. doi: 10.1016/j.molbiopara.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 115.Nkrumah LJ, Riegelhaupt PM, Moura P, Johnson DJ, Patel J, Hayton K, Ferdig MT, Wellems TE, Akabas MH, Fidock DA. Probing the multifactorial basis of Plasmodium falciparum quinine resistance: Evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol Biochem Parasitol. 2009;165(2):122–131. doi: 10.1016/j.molbiopara.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molta NB, Oguche S, Paw SD, Omalu ICJ, Afolabi BM, Odujok JB, Amajoh CN, Adeniji B, Wuyep VP, Ekanem OJ. Amodiaquine treatment of uncomplicated malaria in children, in an area of chloroquine-resistant Plasmodium falciparum in north-central Nigeria. Ann Trop Med Parasitol. 2003;97(7):663–669. doi: 10.1179/000349803225002417. [DOI] [PubMed] [Google Scholar]
- 117.Sendagire H, Kaddumukasa M, Ndagire D, Aguttu C, Nassejje M, Pettersson M, Swedberg G, Kironde F. Rapid increase in resistance of Plasmodium falciparum to chloroquine-Fansidar in Uganda and the potential of amodiaquine-Fansidar as a better alternative. Acta Trop. 2005;95(3):172–182. doi: 10.1016/j.actatropica.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Sowunmi A, Ayede AI, Falade AG, Ndikum VN, Sowunmi CO, Adedeji AA, Falade CO, Happi TC, Oduola AMJ. Randomized comparison of chloroquine and amodiaquine in the treatment of acute, uncomplicated, Plasmodium falciparum malaria in children. Ann Trop Med Parasitol. 2001;95(6):549–558. doi: 10.1080/00034980120092507. [DOI] [PubMed] [Google Scholar]
- 119.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen ATR, Enevold A, Ronn AM, Khalil IF, Warhurst DC, Lemnge MM, Theander TG, Bygbjerg IC. Occurrence of the southeast Asian/south American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J Infect Dis. 2006;193(12):1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 120.Warhurst DC. Polymorphism in the Plasmodium falciparum chloroquine-resistance transporter protein links verapamil enhancement of chloroquine sensitivity with the clinical efficacy of amodiaquine. Malar J. 2003;2(1):31. doi: 10.1186/1475-2875-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mehlotra RK, Mattera G, Bockarie MJ, Maguire JD, Baird JK, Sharma YD, Alifrangis M, Dorsey G, Rosenthal PJ, Fryauff DJ, Kazura JW, Stoneking M, Zimmerman A. Discordant patterns of genetic variation at two chloroquine resistance loci in worldwide populations of the malaria parasite Plasmodium falciparum . Antimicrob Agents Chemother. 2008;52(6):2212–2222. doi: 10.1128/AAC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gama BE, Pereira-Carvalho GAL, Kosi F, Oliveira NKA, Fortes F, Rosenthal PJ, Daniel-Ribeiro CT, Ferreira-Da-Cruz MD. Plasmodium falciparum isolates from Angola show the S(tct)VMNT haplotype in the pfcrt gene. Malar J. 2010;9:174. doi: 10.1186/1475-2875-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sá JM, Twu O. Protecting the malaria drug arsenal: halting the rise and spread of amodiaquine resistance by monitoring the PfCRT SVMNT type. Malar J. 2010;9:374. doi: 10.1186/1475-2875-9-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Danquah I, Coulibaly B, Meissner P, Petruschke I, Muller O, Mockenhaupt FP. Selection of pfmdr1 and pfcrt alleles in amodiaquine treatment failure in north-western Burkina Faso. Acta Trop. 2010;114(1):63–66. doi: 10.1016/j.actatropica.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Nsobya SL, Dokomajilar C, Joloba M, Dorsey G, Rosenthal PJ. Resistance-mediating Plasmodium falciparum pfcrt and pfmdr1 alleles after treatment with artesunate-amodiaquine in Uganda. Antimicrob Agents Chemother. 2007;51(8):3023–3025. doi: 10.1128/AAC.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muangnoicharoen S, Johnson DJ, Looareesuwan S, Krudsood S, Ward SA. Role of known molecular markers of resistance in the antimalarial potency of piperaquine and dihydroartemisinin in vitro. Antimicrob Agents Chemother. 2009;53(4):1362–1366. doi: 10.1128/AAC.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Basco LK, Ringwald P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob Agents Chemother. 2003;47(4):1391–1394. doi: 10.1128/AAC.47.4.1391-1394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2011;55(8):3908–3916. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burrows JN, Chibale K, Wells TN. The state of the art in anti-malarial drug discovery and development. Curr Top Med Chem. 2011;11(10):1226–1254. doi: 10.2174/156802611795429194. [DOI] [PubMed] [Google Scholar]
- 130.Childs GE, Boudreau EF, Milhous WK, Wimonwattratee T, Pooyindee N, Pang L, Davidson JDE. A comparison of the in vitro activities of amodiaquine and desethylamodiaquine against isolates of Plasmodium falciparum . Am J Trop Med Hyg. 1989;40(1):7–11. doi: 10.4269/ajtmh.1989.40.7. [DOI] [PubMed] [Google Scholar]
- 131.Elueze EI, Croft SL, Warhurst DC. Activity of pyronaridine and mepacrine against twelve strains of Plasmodium falciparum in vitro. J Antimicrob Chemother. 1996;37(3):511–518. doi: 10.1093/jac/37.3.511. [DOI] [PubMed] [Google Scholar]
- 132.Pradines B, Mabika Mamfoumbi M, Parzy D, Owono Medang M, Lebeau C, Mourou Mbina JR, Doury JC, Kombila M. In vitro susceptibility of African isolates of Plasmodium falciparum from Gabon to pyronaridine. Am J Trop Med Hyg. 1999;60(1):105–108. doi: 10.4269/ajtmh.1999.60.105. [DOI] [PubMed] [Google Scholar]
- 133.Price RN, Marfurt J, Chalfein F, Kenangalem E, Piera KA, Tjitra E, Anstey NM, Russell B. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax . Antimicrob Agents Chemother. 2010;54(12):5146–5150. doi: 10.1128/AAC.00801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang HL, Liu DQ, Yang YM, Huang KG, Dong Y, Yang PF, Liao MZ, Zhang CY. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas. Southeast Asian J Trop Med Public Health. 1997;28(3):460–464. [PubMed] [Google Scholar]
- 135.Pradines B, Briolant S, Henry M, Oeuvray C, Baret E, Amalvict R, Didillon E, Rogier C. Absence of association between pyronaridine in vitro responses and polymorphisms in genes involved in quinoline resistance in Plasmodium falciparum. Malar J. 2010;9:339. doi: 10.1186/1475-2875-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kokwaro G, Mwai L, Nzila A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin Pharmacother. 2007;8(1):75–94. doi: 10.1517/14656566.8.1.75. [DOI] [PubMed] [Google Scholar]
- 137.White NJ, van Vugt M, Ezzet F. Clinical Pharmacokinetics and Pharmacodynamics of Artemether-Lumefantrine. Clin Pharmacokinet. 1999;37(2):105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 138.WHO (2010) Guidelines for the treatment of malaira, 2nd edn
- 139.Maude RJ, Woodrow CJ, White LJ. Artemisinin antimalarials: preserving the “Magic Bullet”. Drug Dev Res. 2010;71(1):12–19. doi: 10.1002/ddr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morris C, Onyamboko M, Capparelli E, Koch M, Atibu J, Lokomba V, Douoguih M, Hemingway-Foday J, Wesche D, Ryder R, Bose C, Wright L, Tshefu A, Meshnick S, Fleckenstein L. Population pharmacokinetics of artesunate and dihydroartemisinin in pregnant and non-pregnant women with malaria. Malar J. 2011;10(1):114. doi: 10.1186/1475-2875-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44(3):697–704. doi: 10.1128/AAC.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Palmer KJ, Holliday SM, Brogden RN. Mefloquine: a review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1993;45(3):430–475. doi: 10.2165/00003495-199345030-00009. [DOI] [PubMed] [Google Scholar]
- 143.WHO (2010) Global report on antimalarial drug efficacy and drug resistance: 2000–2010
- 144.Ashley EA, McGready R, Hutagalung R, Phaiphun L, Slight T, Proux S, Thwai KL, Barends M, Looareesuwan S, White NJ, Nosten F. A randomized, controlled study of a simple, once-daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated, multidrug-resistant falciparum malaria. Clin Infect Dis. 2005;41(4):425–432. doi: 10.1086/432011. [DOI] [PubMed] [Google Scholar]
- 145.Nosten F, Luxemburger C, ter Kuile FO, Woodrow C, Eh JP, Chongsuphajaisiddhi T, White NJ. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J Infect Dis. 1994;170(4):971–977. doi: 10.1093/infdis/170.4.971. [DOI] [PubMed] [Google Scholar]
- 146.Chavchich M, Gerena L, Peters J, Chen NH, Cheng Q, Kyle DE. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum . Antimicrob Agents Chemother. 2010;54(6):2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ngo T, Duraisingh M, Reed MB, Hipgrave D, Biggs B, Cowman AF. Analysis of pfcrt, pfmdr1 dhfr, and dhps mutations and drug sensitivities in Plasmodium falciparum isolates from patients in Vietnam before and after treatment with artemisinin. Am J Trop Med Hyg. 2003;68(3):350–356. [PubMed] [Google Scholar]
- 148.Martinelli A, Moreira R, Ravo PV. Malaria combination therapies: advantages and shortcomings. Mini Rev Med Chem. 2008;8(3):201–212. doi: 10.2174/138955708783744092. [DOI] [PubMed] [Google Scholar]
- 149.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Noedl H, Se Y, Sriwichai S, Schaecher K, Teja-Isavadharm P, Smith B, Rutvisuttinunt W, Bethell D, Surasri S, Fukuda MM, Socheat D, Chan Thap L. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin Infect Dis. 2010;51(11):e82–e89. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 151.Tucker MS, Mutka T, Sparks K, Patel J, Kyle DE. Phenotypic and genotypic analysis of in vitro selected artemisinin-resistant progeny of Plasmodium falciparum. Antimicrob Agents Chemother. 2011;56(1):302–314. doi: 10.1128/AAC.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beez D, Sanchez CP, Stein WD, Lanzer M. Genetic predisposition favors the acquisition of stable artemisinin resistance in malaria parasites. Antimicrob Agents Chemother. 2011;55(1):50–55. doi: 10.1128/AAC.00916-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.WHO (2011) Consideration of mass drug administration for the containment of artemisinin resistant malaria in the Greater Mekong subregion: report of a consensus meeting, 27–28 September 2010, Geneva, Switzerland
- 154.Enserink M. Malaria’s drug miracle in danger. Science. 2010;328(5980):844–846. doi: 10.1126/science.328.5980.844. [DOI] [PubMed] [Google Scholar]
- 155.Loeb MD, Clark WM, Coatney GR LTC, Dieuaide FR, Dochez MD, Hakansson EG, Marshall EK, Jr, Marvel CS, McCoy OR, Sapero JJ, Sebrell WH, Shannon JA, Carden GA., Jr Activity of a new antimalarial agent, chloroquine (SN 7618) J Am Med Assoc. 1946;130:1069. doi: 10.1001/jama.1946.02870160015006. [DOI] [PubMed] [Google Scholar]
- 156.Most H, London IM, et al. Chloroquine for treatment of acute attacks of vivax malaria. J Am Med Assoc. 1946;131:963–967. doi: 10.1001/jama.1946.02870290013005. [DOI] [PubMed] [Google Scholar]
- 157.Most H. Clinical trials of antimalarial drugs. In: Coates JB, editor. Internal medicine in world war II. Washington DC: Office of the Surgeon General, Medical Department, United States Army; 1963. pp. 562–576. [Google Scholar]
- 158.Chico RM, Chandramohan D. Azithromycin plus chloroquine: combination therapy for protection against malaria and sexually transmitted infections in pregnancy. Expert Opin Drug Metab Toxicol. 2011;7(9):1153–1167. doi: 10.1517/17425255.2011.598506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.WHO (2001) The use of antimalarial drugs: report of an informal consultation
- 160.Berger M, Beytout J, Ringwald P, Cambon M, Rey M. A case of Plasmodium falciparum malaria contracted in Guinea-Bissau during chemoprophylaxis with chloroquine. Presse Med. 1990;19(36):1682. [PubMed] [Google Scholar]
- 161.Hellgren U, Johansson I, Dias F, Ericsson O, Stenbeck J, Rombo L. Chloroquine resistant Plasmodium falciparum malaria in Guinea-Bissau. Trans R Soc Trop Med Hyg. 1991;85(1):36. doi: 10.1016/0035-9203(91)90144-N. [DOI] [PubMed] [Google Scholar]
- 162.Ursing J, Kofoed PE, Rodrigues A, Bergqvist Y, Rombo L. Chloroquine is grossly overdosed and overused but well tolerated in Guinea-bissau. Antimicrob Agents Chemother. 2009;53(1):180–185. doi: 10.1128/AAC.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ursing J, Schmidt BA, Lebbad M, Kofoed PE, Dias F, Gil JP, Rombo L. Chloroquine resistant P. falciparum prevalence is low, unchanged between 1990 and 2005 in Guinea-Bissau: an effect of high chloroquine dosage? Infect Genet Evol. 2007;7(5):555–561. doi: 10.1016/j.meegid.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 164.Ursing J, Kofoed PE, Rodrigues A, Blessborn D, Thoft-Nielsen R, Bjorkman A, Rombo L. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: a randomized trial. J Infect Dis. 2011;203(1):109–116. doi: 10.1093/infdis/jiq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hand CC, Meshnick SR. Is chloroquine making a comeback? J Infect Dis. 2011;203(1):11–12. doi: 10.1093/infdis/jiq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pussard E, Lepers JP, Clavier F, Raharimalala L, Le Bras J, Frisk-Holmberg M, Bergqvist Y, Verdier F. Efficacy of a loading dose of oral chloroquine in a 36-h treatment schedule for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. 1991;35(3):406–409. doi: 10.1128/aac.35.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.White NJ. Antimalarial pharmacokinetics and treatment regimens. Br J Clin Pharmacol. 1992;34(1):1–10. doi: 10.1111/j.1365-2125.1992.tb04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoffman SL, Masbar S, Hussein PR, Soewarta A, Harun S, Marwoto HA, Campbell JR, Smrkovski L, Purnomo Wiady I. Absence of malaria mortality in villagers with chloroquine-resistant Plasmodium falciparum treated with chloroquine. Trans R Soc Trop Med Hyg. 1984;78(2):175–178. doi: 10.1016/0035-9203(84)90271-2. [DOI] [PubMed] [Google Scholar]
- 169.Ginsburg H. Should chloroquine be laid to rest? Acta Trop. 2005;96(1):16–23. doi: 10.1016/j.actatropica.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 170.Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am J Trop Med Hyg. 2007;76(5):844–848. [PubMed] [Google Scholar]
- 171.AlKadi HO. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53(6):385–391. doi: 10.1159/000109767. [DOI] [PubMed] [Google Scholar]
- 172.Murambiwa P, Masola B, Govender T, Mukaratirwa S, Musabayane CT. Anti-malarial drug formulations and novel delivery systems: a review. Acta Trop. 2011;118(2):71–79. doi: 10.1016/j.actatropica.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 173.Moore BR, Page-Sharp M, Stoney JR, Ilett KF, Jago JD, Batty KT. Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob Agents Chemother. 2011;55(8):3899–3907. doi: 10.1128/AAC.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Angulo-Barturen I, Jimenez-Diaz MB, Mulet T, Rullas J, Herreros E, Ferrer S, Jimenez E, Mendoza A, Regadera J, Rosenthal PJ, Bathurst I, Pompliano DL, Gomez de las Heras F, Gargallo-Viola D. A murine model of falciparum-malaria by in vivo selection of competent strains in non-myelodepleted mice engrafted with human erythrocytes. PLoS One. 2008;3(5):e2252. doi: 10.1371/journal.pone.0002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bethell D, Se Y, Lon C, Tyner S, Saunders D, Sriwichai S, Darapiseth S, Teja-Isavadharm P, Khemawoot P, Schaecher K, Ruttvisutinunt W, Lin J, Kuntawungin W, Gosi P, Timmermans A, Smith B, Socheat D, Fukuda MM. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS One. 2011;6(5):e19283. doi: 10.1371/journal.pone.0019283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bethell D, Se Y, Lon C, Socheat D, Saunders D, Teja-Isavadharm P, Khemawoot P, Darapiseth S, Lin J, Sriwichai S, Kuntawungin W, Surasri S, Lee SJ, Sarim S, Tyner S, Smith B, Fukuda MM. Dose-dependent risk of neutropenia after 7-day courses of artesunate monotherapy in Cambodian patients with acute Plasmodium falciparum malaria. Clin Infect Dis. 2010;51(12):e105–e114. doi: 10.1086/657402. [DOI] [PubMed] [Google Scholar]
- 177.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D’Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fidock DA. Drug discovery: Priming the antimalarial pipeline. Nature. 2010;465(7296):297–298. doi: 10.1038/465297a. [DOI] [PubMed] [Google Scholar]
- 179.Wells TN, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8(11):879–891. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 180.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465(7296):305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 181.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. Chemical genetics of Plasmodium falciparum . Nature. 2010;465(7296):311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci USA. 2008;105(26):9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329(5996):1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wells TN. Microbiology. Is the tide turning for new malaria medicines? Science. 2010;329(5996):1153–1154. doi: 10.1126/science.1194923. [DOI] [PubMed] [Google Scholar]
- 185.Yeung BK, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, Tan J, Wong J, Keller-Maerki S, Fischli C, Goh A, Schmitt EK, Krastel P, Francotte E, Kuhen K, Plouffe D, Henson K, Wagner T, Winzeler EA, Petersen F, Brun R, Dartois V, Diagana TT, Keller TH. Spirotetrahydro beta-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J Med Chem. 2010;53(14):5155–5164. doi: 10.1021/jm100410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grimberg BT, Mehlotra RK. Expanding the antimalarial drug arsenal-now, but how? Pharmaceuticals (Basel) 2011;4(5):681–712. doi: 10.3390/ph4050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Canfield CJ, Rozman RS. Clinical testing of new antimalarial compounds. Bull World Health Organ. 1974;50(3–4):203–212. [PMC free article] [PubMed] [Google Scholar]
- 188.Jacobs RL, Alling DW, Cantrell WF. An Evaluation of Antimalarial Combinations against Plasmodium Berghei in the Mouse. J Parasitol. 1963;49:920–925. doi: 10.2307/3275719. [DOI] [PubMed] [Google Scholar]
- 189.Singh T, Stein RG, Hoops JF, Biel JH, Hoya WK, Cruz DR. Antimalarials. 7-chloro-4-(substituted amino)quinolines. J Med Chem. 1971;14(4):283–286. doi: 10.1021/jm00286a003. [DOI] [PubMed] [Google Scholar]
- 190.Thompson PE, Reinertson JW, Bayles A, Moore AM. The curative action of antimalarial drugs against Plasmodium lophurae in chicks. J Infect Dis. 1953;92(1):40–51. doi: 10.1093/infdis/92.1.40. [DOI] [PubMed] [Google Scholar]
- 191.O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, Biagini GA, Bray PG, Gibbons P, Berry N, Winstanley PA, Mukhtar A, Bonar-Law R, Hindley S, Bambal RB, Davis CB, Bates M, Hart TK, Gresham SL, Lawrence RM, Brigandi RA, Gomez-delas-Heras FM, Gargallo DV, Ward SA. Candidate selection and preclinical evaluation of N-tert-butyl isoquine (GSK369796), an affordable and effective 4-aminoquinoline antimalarial for the 21st century. J Med Chem. 2009;52(5):1408–1415. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 192.Milner E, McCalmont W, Bhonsle J, Caridha D, Cobar J, Gardner S, Gerena L, Goodine D, Lanteri C, Melendez V, Roncal N, Sousa J, Wipf P, Dow GS. Anti-malarial activity of a non-piperidine library of next-generation quinoline methanols. Malar J. 2010;9:51. doi: 10.1186/1475-2875-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.De D, Krogstad FM, Cogswell FB, Krogstad DJ. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1996;55(6):579–583. doi: 10.4269/ajtmh.1996.55.579. [DOI] [PubMed] [Google Scholar]
- 194.Hocart SJ, Liu H, Deng H, De D, Krogstad FM, Krogstad DJ. 4-aminoquinolines active against chloroquine-resistant Plasmodium falciparum: basis of antiparasite activity and quantitative structure-activity relationship analyses. Antimicrob Agents Chemother. 2011;55(5):2233–2244. doi: 10.1128/AAC.00675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A, Masciadri R, Jolidon S, Richter WF, Guenzi A, Girometta MA, Urwyler H, Huber W, Thaithong S, Peters W. 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum . Antimicrob Agents Chemother. 1996;40(8):1846–1854. doi: 10.1128/aac.40.8.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, Chansolme DH, Murphy HA, Melek BH, Tenaglia AN, Mushatt DM, Dreisbach AW, Lertora JJ, Krogstad DJ. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2(1):e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kumar A, Srivastava K, Kumar SR, Puri SK, Chauhan PM. Synthesis of new 4-aminoquinolines and quinoline-acridine hybrids as antimalarial agents. Bioorg Med Chem Lett. 2010;20(23):7059–7063. doi: 10.1016/j.bmcl.2010.09.107. [DOI] [PubMed] [Google Scholar]
- 198.Ray S, Madrid PB, Catz P, LeValley SE, Furniss MJ, Rausch LL, Guy RK, DeRisi JL, Iyer LV, Green CE, Mirsalis JC. Development of a new generation of 4-aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models. J Med Chem. 2010;53(9):3685–3695. doi: 10.1021/jm100057h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Madrid PB, Liou AP, DeRisi JL, Guy RK. Incorporation of an intramolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum. J Med Chem. 2006;49(15):4535–4543. doi: 10.1021/jm0600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Biot C, Glorian G, Maciejewski LA, Brocard JS. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene-chloroquine analogue. J Med Chem. 1997;40(23):3715–3718. doi: 10.1021/jm970401y. [DOI] [PubMed] [Google Scholar]
- 201.Dubar F, Egan TJ, Pradines B, Kuter D, Ncokazi KK, Forge D, Paul JF, Pierrot C, Kalamou H, Khalife J, Buisine E, Rogier C, Vezin H, Forfar I, Slomianny C, Trivelli X, Kapishnikov S, Leiserowitz L, Dive D, Biot C. The antimalarial ferroquine: role of the metal and intramolecular hydrogen bond in activity and resistance. ACS Chem Biol. 2011;6(3):275–287. doi: 10.1021/cb100322v. [DOI] [PubMed] [Google Scholar]
- 202.Mombo-Ngoma G, Supan C, Dal-Bianco MP, Missinou MA, Matsiegui PB, Ospina Salazar CL, Issifou S, Ter-Minassian D, Ramharter M, Kombila M, Kremsner PG, Lell B. Phase I randomized dose-ascending placebo-controlled trials of ferroquine—a candidate anti-malarial drug—in adults with asymptomatic Plasmodium falciparum infection. Malar J. 2011;10:53. doi: 10.1186/1475-2875-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.O’Neill PM, Ward SA, Berry NG, Jeyadevan JP, Biagini GA, Asadollaly E, Park BK, Bray PG. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr Top Med Chem. 2006;6(5):479–507. doi: 10.2174/156802606776743147. [DOI] [PubMed] [Google Scholar]
- 204.Burgess SJ, Kelly JX, Shomloo S, Wittlin S, Brun R, Liebmann K, Peyton DH. Synthesis, structure-activity relationship, and mode-of-action studies of antimalarial reversed chloroquine compounds. J Med Chem. 2010;53(17):6477–6489. doi: 10.1021/jm1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum . J Med Chem. 2006;49(18):5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kelly JX, Smilkstein MJ, Brun R, Wittlin S, Cooper RA, Lane KD, Janowsky A, Johnson RA, Dodean RA, Winter R, Hinrichs DJ, Riscoe MK. Discovery of dual function acridones as a new antimalarial chemotype. Nature. 2009;459(7244):270–273. doi: 10.1038/nature07937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.October N, Watermeyer ND, Yardley V, Egan TJ, Ncokazi K, Chibale K. Reversed chloroquines based on the 3, 4-dihydropyrimidin-2(1H)-one scaffold: synthesis and evaluation for antimalarial, beta-haematin inhibition, and cytotoxic activity. ChemMedChem. 2008;3(11):1649–1653. doi: 10.1002/cmdc.200800172. [DOI] [PubMed] [Google Scholar]
- 208.Zishiri VK, Joshi MC, Hunter R, Chibale K, Smith PJ, Summers RL, Martin RE, Egan TJ. Quinoline Antimalarials Containing a Dibemethin Group are Active against Chloroquine Resistant P. falciparum and Inhibit Chloroquine Transport via the Plasmodium falciparum Chloroquine-Resistance Transporter (PfCRT) J Med Chem. 2011;54(19):6956–6968. doi: 10.1021/jm2009698. [DOI] [PubMed] [Google Scholar]
- 209.Cosledan F, Fraisse L, Pellet A, Guillou F, Mordmuller B, Kremsner PG, Moreno A, Mazier D, Maffrand JP, Meunier B. Selection of a trioxaquine as an antimalarial drug candidate. Proc Natl Acad Sci USA. 2008;105(45):17579–17584. doi: 10.1073/pnas.0804338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Muregi FW, Ishih A. Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug Dev Res. 2010;71(1):20–32. doi: 10.1002/ddr.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nelson AL, Purfield A, McDaniel P, Uthaimongkol N, Buathong N, Sriwichai S, Miller RS, Wongsrichanalai C, Meshnick SR. pfmdr1 genotyping and in vivo mefloquine resistance on the Thai-Myanmar border. Am J Trop Med Hyg. 2005;72(5):586–592. [PubMed] [Google Scholar]
- 212.Picot S, Olliaro P, Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Uhlemann AC, McGready R, Ashley EA, Brockman A, Singhasivanon P, Krishna S, White NJ, Nosten F, Price RN. Intrahost selection of Plasmodium falciparum pfmdr1 alleles after antimalarial treatment on the northwestern border of Thailand. J Infect Dis. 2007;195(1):134–141. doi: 10.1086/509809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen N, Kyle DE, Pasay C, Fowler EV, Baker J, Peters JM, Cheng Q. pfcrt Allelic types with two novel amino acid mutations in chloroquine-resistant Plasmodium falciparum isolates from the Philippines. Antimicrob Agents Chemother. 2003;47(11):3500–3505. doi: 10.1128/AAC.47.11.3500-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cooper RA, Hartwig CL, Ferdig MT. pfcrt is more than the Plasmodium falciparum chloroquine resistance gene: a functional and evolutionary perspective. Acta Trop. 2005;94(3):170–180. doi: 10.1016/j.actatropica.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 216.Hatabu T, Iwagami M, Kawazu S, Taguchi N, Escueta AD, Villacorte EA, Rivera PT, Kano S. Association of molecular markers in Plasmodium falciparum crt and mdr1 with in vitro chloroquine resistance: a Philippine study. Parasitol Int. 2009;58(2):166–170. doi: 10.1016/j.parint.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 217.Huaman MC, Yoshinaga K, Suryanatha A, Suarsana N, Kanbara H. Short report: polymorphisms in the chloroquine resistance transporter gene in Plasmodium falciparum isolates from Lombok, Indonesia. Am J Trop Med Hyg. 2004;71(1):40–42. [PubMed] [Google Scholar]
- 218.Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, Denis MB, Hewitt S, Hoyer S, Socheat D, Merecreau-Puijalon O, Fandeur T. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob Agents Chemother. 2003;47(1):87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang Z, Zhang Z, Sun X, Wan W, Cui L, Zhang X, Zhong D, Yan G. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China. Trop Med Int Health. 2007;12(9):1051–1060. doi: 10.1111/j.1365-3156.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 220.Durrand V, Berry A, Sem R, Glaziou P, Beaudou J, Fandeur T. Variations in the sequence and expression of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance in vitro. Mol Biochem Parasitol. 2004;136(2):273–285. doi: 10.1016/j.molbiopara.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 221.Cooper RA, Lane KD, Deng B, Mu J, Patel JJ, Wellems TE, Su X, Ferdig MT. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol Microbiol. 2007;63(1):270–282. doi: 10.1111/j.1365-2958.2006.05511.x. [DOI] [PubMed] [Google Scholar]
- 222.Nagesha HS, Casey GJ, Rieckmann KH, Fryauff DJ, Laksana BS, Reeder JC, Maguire JD, Baird JK. New haplotypes of the Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene among chloroquine-resistant parasite isolates. Am J Trop Med Hyg. 2003;68(4):398–402. [PubMed] [Google Scholar]
- 223.Coosemans MH, Hendrix L, Barutwanayo M, Butoyi G, Onori E. Drug resistance of Plasmodium falciparum in Burundi. Bull World Health Organ. 1985;63(2):331–338. [PMC free article] [PubMed] [Google Scholar]
- 224.Sexton JD, Deloron P, Bugilimfura L, Ntilivamunda A, Neill M. Parasitologic and clinical efficacy of 25 and 50 mg/kg of chloroquine for treatment of Plasmodium falciparum malaria in Rwandan children. Am J Trop Med Hyg. 1988;38(2):237–243. doi: 10.4269/ajtmh.1988.38.237. [DOI] [PubMed] [Google Scholar]
- 225.de Andrade JG, de Andrade AL, Araujo ES, Oliveira RM, Silva SA, Martelli CM, Zicker F. A randomized clinical trial with high dose of chloroquine for treatment of Plasmodium falciparum malaria in Brazil. Rev Inst Med Trop Sao Paulo. 1992;34(5):467–473. doi: 10.1590/S0036-46651992000500015. [DOI] [PubMed] [Google Scholar]
- 226.Kremsner PG, Winkler S, Brandts C, Neifer S, Bienzle U, Graninger W. Clindamycin in combination with chloroquine or quinine is an effective therapy for uncomplicated Plasmodium falciparum malaria in children from Gabon. J Infect Dis. 1994;169(2):467–470. doi: 10.1093/infdis/169.2.467. [DOI] [PubMed] [Google Scholar]
- 227.Wildling E, Jenne L, Graninger W, Bienzle U, Kremsner PG. High dose chloroquine versus micronized halofantrine in chloroquine-resistant Plasmodium falciparum malaria. J Antimicrob Chemother. 1994;33(4):871–875. doi: 10.1093/jac/33.4.871. [DOI] [PubMed] [Google Scholar]
- 228.Metzger W, Mordmuller B, Graninger W, Bienzle U, Kremsner PG. Sulfadoxine/pyrimethamine or chloroquine/clindamycin treatment of Gabonese school children infected with chloroquine resistant malaria. J Antimicrob Chemother. 1995;36(4):723–728. doi: 10.1093/jac/36.4.723. [DOI] [PubMed] [Google Scholar]
- 229.Howard N, Durrani N, Sanda S, Beshir K, Hallett R, Rowland M. Clinical trial of extended-dose chloroquine for treatment of resistant falciparum malaria among Afghan refugees in Pakistan. Malar J. 2011;10:171. doi: 10.1186/1475-2875-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kofoed PE, Lopez F, Johansson P, Sandstrom A, Hedegaard K, Aaby P, Rombo L. Treatment of children with Plasmodium falciparum malaria with chloroquine in Guinea-Bissau. Am J Trop Med Hyg. 2002;67(1):28–31. doi: 10.4269/ajtmh.2002.67.28. [DOI] [PubMed] [Google Scholar]
- 231.Kofoed PE, Co F, Johansson P, Dias F, Cabral C, Hedegaard K, Aaby P, Rombo L. Treatment of uncomplicated malaria in children in Guinea-Bissau with chloroquine, quinine, and sulfadoxine-pyrimethamine. Trans R Soc Trop Med Hyg. 2002;96(3):304–309. doi: 10.1016/S0035-9203(02)90107-0. [DOI] [PubMed] [Google Scholar]
- 232.Kofoed PE, Ursing J, Poulsen A, Rodrigues A, Bergquist Y, Aaby P, Rombo L. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans R Soc Trop Med Hyg. 2007;101(3):231–238. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]