Abstract
In this review, subtypes of functional α1-adrenoceptor are discussed. These are cell membrane receptors, belonging to the seven-transmembrane-spanning G-protein-linked family of receptors, which respond to the physiological agonist noradrenaline. α1-Adrenoceptors can be divided into α1A-, α1B- and α1D-adrenoceptors, all of which mediate contractile responses involving Gq/11 and inositol phosphate turnover. A fourth α1-adrenoceptor, the α1L-, represents a functional phenotype of the α1A-adrenoceptor. α1-Adrenoceptor subtype knock-out mice have refined our knowledge of the functions of α-adrenoceptor subtypes, particuarly as subtype-selective agonists and antagonists are not available for all subtypes. α1-Adrenoceptors function as stimulatory receptors involved particularly in smooth muscle contraction, especially contraction of vascular smooth muscle, both in local vasoconstriction and in the control of blood pressure and temperature, and contraction of the prostate and bladder neck. Central actions are now being elucidated.
Keywords: α1-Adrenoceptors, α1A-Adrenoceptors, α1B-Adrenoceptors, α1D-Adrenoceptors, Blood pressure, Smooth muscle contraction, Vascular smooth muscle, Benign prostatic hypertrophy
Introduction
Adrenoceptors, or adrenergic receptors, are cell membrane receptors belonging to the seven-transmembrane-spanning G-protein-linked superfamily of receptors. They respond to the sympathetic neurotransmitter noradrenaline and to the hormone adrenaline (and to various exogenous agonists) by producing a response within the cell involving a second messenger or ion channel. Adrenoceptors are classically the receptors involved in the “fight or flight” reaction, the mobilisation of resources caused by activation of the sympathetic nervous system that prepares the body for bouts of severe activity. Sympathetic activation will cause α1-adrenoceptor-mediated vasoconstriction in less vital vascular beds, particularly splanchnic and skin (although the skin vasculature may dilate later to dissipate heat), to divert blood to skeletal muscle in exercise. Sympathetic activation also mobilises blood from the reservoir in the large veins (the capacitance vessels) by veniconstriction, again largely involving α1- (and α2-) adrenoceptors.
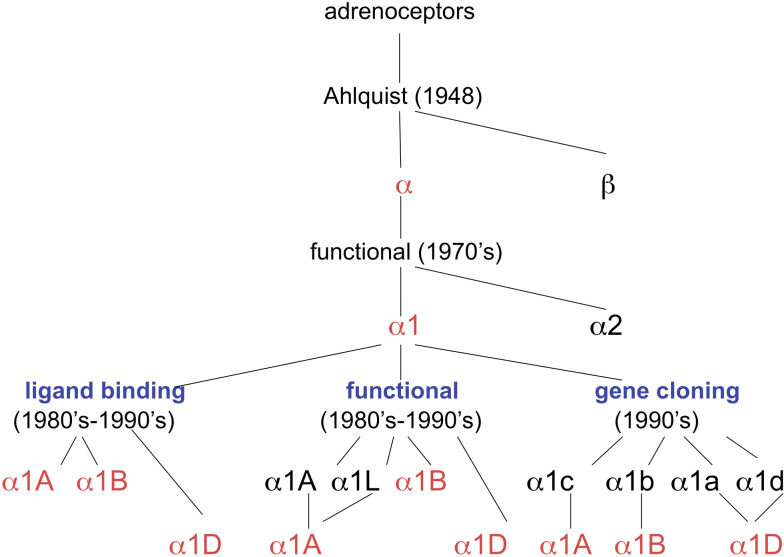
Historically, employing a series of agonists, Ahlquist [1] described two types of adrenoceptor based on the rank order of potency of these agonists. The receptor termed α was mainly excitatory, except in the intestine, and the receptor termed β was mainly inhibitory, except in the heart. In Ahlquist’s classification, α-adrenoceptors were receptors present on smooth muscle, i.e. postjunctional receptors. These were later classified as postjunctional α1-adrenoceptors, when evidence accumulated for prejunctional α2-adrenoceptors [2]. Later, when evidence accumulated for α2-adrenoceptors located postjunctionally, this purely anatomical classification was refined into a pharmacological subclassification, independent of location [3]. Further advances in our understanding of α1-adrenoceptors have come from the development of new pharmacological methodologies for the study of receptors. The first of these was the technique of the radioligand binding assay: α1-adrenoceptors were initially subdivided into α1A and α1B-subtypes, based on the affinities of a series of ligands, especially WB 4101 and prazosin [4], and based on the ability of the alkylating agent chloroethylclonidine to inactivate the α1B but not the α1A subtype [5]. Under this classification, functional receptors mediating contractions of rat vas deferens were α1A-, and those of rat spleen were α1B-adrenoceptors [5] (see Fig. 1).
Fig. 1.
The historical development of the subclassification of α1-adrenoceptors. For details, see text
The study of α-adrenoceptors was revolutionised by molecular biology: cloning techniques revealed initially four subtypes of α1-adrenoceptor. The α1b-adrenceptor subtype (the lower case subscript being used for recombinant receptors and upper case subscript for pharmacologically defined receptor subtypes) was the first to be cloned, from the hamster [6], and this clone expressed a protein with the radioligand binding properties of the α1B-adrenoceptor. Other clones were the rat α1a- [7], the bovine α1c- [8] and rat α1d-adrenoceptor [9]. However, the α1a and α1d clones showed 99.8% homogeneity and appeared to represent the same subtype. It is now clear that the α1a/α1d clone represents a novel subtype of α1-adrenoceptor (α1D), whereas the α1c is now identified with the α1A-ligand binding site. These clones have now been renamed to match the functional receptors: α1A (formerly α1c), α1B (formerly α1b) and α1D (formerly α1a/α1d) (see Fig. 1). Hence, three genes for α1-adrenoceptors have now been identified (α1A, α1B, α1D) [see 10–12].
Figure 1 shows how the subclassification of α1-adrenoceptors has developed since 1948. The α1L-adrenoceptor is dependent on the α1A-adrenoceptor gene and is a phenotype of the α1A-adrenoceptor (see below). α1-Adrenoceptors are predominantly linked to the G-protein Gq/11 and activation of phospholipase C (PLC) (see Table 1). No adrenoceptor belongs to the class of ionotropic receptors, those with an intrinsic ion channel, unlike the situation with another monoamine, 5-hydroxytryptamine.
Table 1.
Summary of α1-adrenoceptor subtype characteristics
| Receptor subtype | α1A | α1B | α1D |
|---|---|---|---|
| Functional responses |
Control of blood pressure; vasoconstriction; smooth muscle contraction |
Regulatory;
minor contractile role |
Control of blood pressure; vasoconstriction; smooth muscle contraction |
| Location (relative to innervation) | Junctional and non-junctional | Junctional (mainly?) | |
| Functional response (model systems) | Rat vas deferens contraction | Rat spleen contraction | Rat aorta contraction |
| Ligand-binding assay (other than transfected) | Rat submandibular gland | Rat spleen | (None) |
| Noradrenaline potency | Moderate | Moderate | High |
| Selective agonists | A61603 | / | / |
| Selective antagonists | RS 100329 | / | BMY 7378 |
| Sensitivity to CECa | + | ++ | + |
| Second messengers systems | Gq/11, PI turnover | Gq/11, PI turnover | Gq/11, PI turnover |
aCEC affects all subtypes
A 61603, (N-[5-(4,5-dihydro-1H-imidazol-2yl)-2-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl]-methanesulfonamide); BMY 7378, (8-[2-(4-(2-methoxyphenyl) piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione); CEC, chloroethylclonidine; Gq/11, G-protein; PI, phosphoinositol
The object of this review is to look at functional subtypes of α1-adrenoceptors and their physiological roles.
Function of α1-adrenoceptors
α1-Adrenoceptors function as stimulatory receptors and are the classical adrenoceptors mediating smooth muscle contraction, and in the vascular system have a major role in the control of blood pressure. A fall in blood pressure due to causes such as haemorrhage will activate the baroreceptor reflex and cause sympathetic activation to vasconstrict less vital vascular beds, especially splanchnic and skin. α1-Adrenoceptor antagonists lower blood pressure in hypertension, but are not widely employed. α1-Adrenoceptor agonist-mediated vasoconstriction can be used to treat hypotension, and these agonists are widely used as over the counter nasal decongestants, acting by reducing blood flow to the nasal musosa. Pseudoephedrine, when used as a nasal decongestant, shows some selectivity for local over cardiovascular actions: it is reported to have nasal actions at 60 mg [13], no cardiovascular actions at up to 120 mg [14] or cardiovascular actions at 120–180 mg [15–17]. The reason for this selectivity is unclear, but pseudoephedrine may show some slight selectivity for α1A-adrenoceptors [18] and moderate potency as a beta-2 adrenoceptor agonist [19].
Ocular effects involve α1-adrenoceptor-mediated dilatation of the pupil by contracting the dilator pupillae muscle, increasing the amount of light reaching the retina. α1-Adrenoceptor agonists also have actions to reduce intraocular pressure, presumably by restricting blood flow. Other actions include bronchoconstriction, constriction of sphincters in the gastrointestinal tract and salivary secretions. α1-Adrenoceptors may be important in the regulation of human lipid metabolism [20] and in the uptake of glucose into adipocytes [21].
Genitourinary actions are also important, and α1-adrenoceptors are involved in contraction of the vas deferens and in contracting the neck of the bladder, and are involved in prostate function. α1-Adrenoceptors mediate inhibition of micturition by constriction of the bladder neck, and this may involve mainly α1D-adrenoceptors [22], but the density of alpha1-adrenoceptors in the neck of the bladder is greater in males, suggesting an additional sexual function to prevent retrograde ejaculation into the bladder. α1-Adrenoceptors mediate contraction of the vas deferens and seminal vesicles, and this has an important role in ejaculation. α1A-KO mice, lacking the α1A-adrenoceptor, have a 50% reduction in pregnancy rate, with further reduction with knockout of all three alpha1-adrenoceptors, and this is mainly due to decreased ejaculatory function because of diminished contractile response of the vas deferens [23]. The same is true of mice lacking the purinergic P2X1 receptor [24], suggesting that both α1A- and purinergic responses are required for normal vas deferens function and ejaculation.
A major stimulus to the development of new α1-adrenoceptor antagonist drugs has been drug therapy of benign prostatic hypertrophy, which affects an increasing proportion of men as they age, causing problems with micturition due to outflow obstruction. Outflow obstruction consists of a static component due to compression of the urethra by the enlarged prostate, and a dynamic component due to α1-adrenoceptor-mediated contraction of the bladder neck, prostate and urethra. The dynamic component may contribute nearly 50% of the total urethral obstruction [25], leading to the use of initially non-selective α-adrenoceptor antagonists [26]. New α1-adrenoceptor antagonists were developed for effects in the lower urinary tract (see [27]), and the receptors involved were identifed as α1A-adrenoceptors. Recent evidence suggests that, in addition to α1A-adrenoceptors, α1D-adrenoceptors are also present to a significant extent in human prostate [28]. However, some antagonists that were selective for α1A-adrenoceptors in ligand-binding studies had low potencies in functional studies of the lower urinary tract, e.g. RS 17053 [29]. These studies brought the study of α1A-adrenoceptors into contact with parallel studies of α1L-adrenoceptors (see below).
Selective ligands for alpha1-adrenoceptor subtypes
RS 100329 is a selective α1A-adrenoceptor antagonist [30], and A61603 is an α1A-adrenoceptor selective agonist, reported to be 200 times more potent than noradrenaline at causing contractions of rat vas deferens [31].
Risperidone, AH11110A and cyclazosin have been proposed as selective α1B-adrenoceptor antagonists [32–34], but these selectivities have been questioned in functional studies [35–37]. Chloroethylclonidine has been used to identify subtypes of α1-adrenoceptor because of its reported actions to selectively alkylate α1B-adrenoceptors, but chloroethylclonidine interacts with all subtypes of α1-adrenoceptor [38, 39] and with α2-adrenoceptors [38, 40]. Overall there is currently no useful antagonist for the study of functional α1B-adrenoceptors.
BMY 7378 is a selective α1D-adrenoceptor antagonist [41], but also shows potency as an α2C-adrenoceptor antagonist [42] and is an antagonist/partial agonst at 5-HT1A receptors [43]. Despite this, it had proved to be a very useful selective antagonist in functional studies.
Silodosin (KMD-3213) is reported to be a selective α1A- and α1L-adrenoceptor antagonist, but is marketed as an α1A-adrenoceptor antagonist [44].
In the present author’s opinion, the most reliable and widely studied selective antagonists are RS 100329 (α1A) and BMY 7378 (α1D) when used at appropriate concentrations and taking cogniscence of the pitfalls (see Table 1).
Responses mediated by α1A-adrenoceptors
Contractions are reported to be mediated at least partly by α1A-adrenoceptors in a number of tissues including rat vas deferens [45–48], rat renal artery (also α1D: [49]), rat tail artery [50, 51], rat right atrium (positive inotropic actions [52]), rabbit ear artery [53], pig internal anal sphincter (also α1L: [54]), human vas deferens [55, 56] and human prostate ([57]; also α1B: [58], α1D: [28]; but see below for α1L). In rat vas deferens, α1A-adrenoceptors mediate two types of response: phasic, probably due to release of Ca2+ from ryanodine sensitive stores, and tonic via protein kinase C involving diacylglycerol and influx of Ca2+ via nifedipine-sensitive L-type channels [47] and possibly also T-type channels. Rat submandibular gland has been employed as a model of α1A-adrenoceptor ligand-binding sites (see [59]), but may contain both α1A- and α1B-adrenoceptors [60]. Positive inotropic actions of phenylephrine in mouse involve alpha1A-adrenoceptors [61]. Contractions to noradrenaline were minimal in prostate from the α1A-adrenoceptor KO mice [62].
α1A-Adrenoceptor overexpression increases beta-adrenoceptor-mediated contractility in the heart and improves outcome from myocardiac infarction [63].
α1L-Adrenoceptors: α1A-adrenoceptors
One of the earliest functional subclassifications of α1-adrenoceptors was α1H and α1L, with high and low affinity for prazosin (see [64]), although prazosin has a wide range of affinities for α1-adrenoceptors in functional studies [11, 65]. Muramatsu and coworkers [66] subdivided α1-adrenoceptors into three subtypes, α1H, α1N and α1L, based on their affinities especially for prazosin. α1H-Adrenoceptors had high affinity for prazosin and appeared to match the α1A, α1B, α1D classification [67], whereas α1L (the α1N designation was dropped) had low affinity for prazosin and did not seem to match molecular cloning-based classifications. Under this classification and based on the low potency of prazosin, α1L-adrenoceptors were present in rabbit aorta, mesenteric and carotid arteries [66], guinea-pig aorta [67], rat anococcygeus mucle [68] and rat vas deferens (in addition to α1A: [69]; in longitudinal but not circular muscle: [70]). However, other authors have found that contractions of rat vas deferens to exogenous agonists are mediated by α1A-adrenoceptors as demonstrated by the very significant correlation with α1A-adrenoceptor ligand binding sites [71]. α1L-Adrenoceptors have also been reported in rabbit cutaneous resistance arteries (predominant adrenoceptor is α1B: [72]), rat small mesenteric artery [73], pig prostatic small arteries [74], guinea-pig aorta [75], rabbit iris [76], pig internal anal sphincter [54], rabbit bladder neck [77], in human, rat and dog urethra, dog and mouse prostate, but not in human prostate [58, 78, 79]. In contrast, Muramatsu et al. [67] reported α1L-adrenoceptors in human prostate. α1L-Adrenoceptor-mediated responses in prostate were abolished in α1A-adrenoceptor KO mice [62].
α1-Adrenoceptors displayed properties of the α1A-adrenoceptor in ligand bind studies, but properties of the α1L-adrenoceptor in functional studies [80, 81] or in intact tissue segments [82]. In studies of mRNA levels, α1L-adrenoceptors correlated with tissues expressing predominantly alpha1A-adrenoceptors [83]. Genetic polymorphism of α1A-adrenoceptors does not explain α1L-adrenoceptors, since human α1A-adrenoceptor splice variants [84] and homo- and heterodimers of human α1A variants [85, 86] have been found to have similar pharmacological characteristics. It can be concluded that α1L-adrenoceptors are a functional phenotype of the α1A-adrenoceptor, although as yet it is not clear under what circumstances α1L-adrenoceptor pharmacology is exhibited.
Responses mediated by α1B-adrenoceptors
Studies of α1B-adrenoceptor-mediated function have been hampered by lack of a truly selective antagonist. Contractions are reported to be mediated at least partly by α1B-adrenoceptors in a number of tissues including rat spleen (in addition to α2-adrenoceptors) [45, 46, 48], mouse spleen [87], rat right atrium (positive inotropic, also α1A: [52]), rabbit corpus cavernosum [48], rabbit cutaneous resistance arteries (also α1L: [72]) and human prostate (also α1A: [58], but see also α1L). Rat spleen is employed as a model of α1B-adrenoceptor ligand-binding sites (see [59]). Rat submandibular gland is reported to contain both α1A and α1B-adrenoceptors, but the secretion of saliva may mainly involve α1B-adrenoceptors [60].
The function of α1B-adrenoceptors has been clarified by the use of knockout technology. In aorta from α1B-KO mice there was a small reduction in the potency of noradrenaline or phenylephrine as compared to WT [88], or no significant change in potency [89]. Combined α1B/α1D-KO abolished contractions to noradrenaline and phenylephrine in aorta, having more effect than α1D-KO alone [89]. Daly et al. [90] demonstrated a minor contractile role of α1B-adrenoceptors in mouse arteries, including the aorta and tail artery, using knockout technology. Although α1B-KO mice show some differences in vascular responsiveness, it has been pointed out that if the α1B-adrenoceptor has a regulatory or trophic role or is required for cell surface expression of other subtypes (see [86]), its absence might affect vascular responses involving other α1-adrenoceptors even though it was not directly involved in contraction [90]. Hence, results in studies of KO animals must be considered in the light of information from wild-type animals. Contractions in rat tail artery develop more slowly in α1B-adrenoceptor knock-out mice [91], so that subtle differences can be revealed following receptor knock-out.
α1B-Adrenoceptor overexpression decreases beta-adrenoceptor-mediated contractility in the heart [63] and results in hypertrophy of the cardiac muscle and hypotension [92], and predisposes to heart failure [63]. Pressor responses to phenylephrine in vivo and contractions in the isolated mesenteric artery were unchanged by α1B-adrenoceptor overexpression [92]. Overexpression of α1B-adrenoceptors blunts the positive inotropic actions on phenylephrine in mouse isolated heart because of a reduction in α1A-adrenoceptors, suggesting a regulatory rather than contractile role for this receptor [61].
Responses mediated by α1D-adrenoceptors
Contractions are reported to be mediated at least partly by α1D-adrenoceptors in a number of tissues including rat aorta, mesenteric artery, iliac artery and pulmonary artery [93, 94], rat renal artery (also α1A: [49]), rat carotid artery, mesenteric artery, aorta [50], rabbit aorta (also possibly α1A: [53]) and rabbit ventricle (also other subtypes: [95]). In contrast, α1D-adrenoceptors are reported not to be involved in caudal, mesenteric or renal arteries [93]. In studies of mouse carotid artery from WT and alpha1D-KO, there was evidence for predominantly α1D-adrenoceptor-mediated contractions with some regulatory role for the α1B-adrenoceptor [96]. Mouse aortic contractions to noradrenaline and phenylephrine were unaffected by α1B-KO [89], markedly reduced by α1D-KO [89, 97], but abolished by combination of α1B/1D-KO [89], suggesting a regulatory or co-operative role for α1B-adrenoceptors. However, overexpression of α1B-adrenoceptors did not affect the α1D-adrenoceptor response of mouse aorta [98] or mesenteric artery [92]. In mouse mesenteric artery, α1B- had no role, α1D-adrenoceptors had a large role in contractions [99], and α1D-adrenoceptors can be revealed in femoral arteries using KO mice [100].
In addition to mediating contractions of vascular smooth muscle, α1-adrenoceptors may induce endothelium-dependent relaxations. It is reported that endothelium-dependent relaxations occur to phenylephrine in the rat mesenteric vascular bed due to α1D-adrenoceptor stimulaton [101], and α1D-adrenoceptor activation has trophic effects on endothelial cells [102].
Adrenoceptors mediating contractions to nerve stimulation are predominantly α1D in both rat [71] and mouse vas deferens (evidence from α1D-KO mice, [103]), although contractions of exogenous noradrenaline are predominantly α1A-adrenoceptor mediated [45]. In rat femoral arteries, contractions to exogenous noradrenaline were mediated by α1A-adrenoceptors, but responses to nerve released noradrenaline involved α1A- and α1D-adrenoceptors [100]. In addition to α1A-adrenoceptors, α1D-adrenoceptors are also expressed to a significant extent in human prostate [28], although their location has not been established.
Sympathectomy has been shown to alter the balance of α1-adrenoceptor subtypes in rat vas deferens. Although ligand-binding studies of normal rat vas deferens demonstrate a single population of α1A-adrenoceptors, tissues from rats sympathectomised with 6-hydroxydopamine demonstrate both α1A- and α1D-adrenoceptors [71]. Results obtained from sympathectomised rats suggests that phasic contractions are mainly α1D-adrenoceptor mediated, whereas tonic contractions are mainly α1A-adrenoceptor mediated, based on the effects of BMY 7378 and the α1A-adrenoceptor antagonist RS 100329. Likewise, it has been reported that α1D-adrenoceptors are involved in reserpine-induced supersensitivity of rat tail artery [104]. These studies suggest that α1D-adrenoceptors are restricted to the junctional region by nerve activity, but if nerves are lost, these receptors spread from the junctional region along the smooth muscle. As a corollary, the rat aorta, which lacks a functional innervation, contains mainly α1D-adrenoceptors on the smooth muscle. How widespread are neuronal α1D-adrenoceptors? Clearly, contractions in a number of tissues are mediated by more than one subtype of α1-adrenoceptor, and currently available subtype-selective antagonists (particularly for α1B-adrenoceptor) are often not selective enough to tease out clearly which receptors are present, requiring the continued use of α1-adrenoceptor KO mice.
Inverse agonists
It has become clear in recent years that antagonists may act as inverse agonists at α1-adrenoceptors. This means that they not only block the actions of agonists at the receptor, they also reduce the constitutive baseline activity of the G-protein coupled receptor in the absence of agonist. Pure antagonists, or neutral antagonists, do not affect baseline activity of the G-protein coupled receptor.
A number of studies have investigated the ability of calcium re-addition to produce contractions in the absence of an α1-adrenoceptor agonist following depletion of calcium stores, particularly in rat aorta, and the ability of α1-adrenoceptor antagonists to inhibit this contraction [105]. This phenomenon occurred in aorta [105] and iliac and proximal mesenteric arteries [106], but not tail artery (see [107]), and was blocked by benoxathian, WB 41001, prazosin, BMY 73778 and 5-methylurapidil [105, 107]. Furthermore, increased potency of BMY 7378 in aorta from SHR suggested an increase in this phenomenon in hypertension (see [107]). It was concluded by these authors that the phenomenon of contraction to calcium re-addition occurred only for α1D-adrenoceptors, suggesting that these are constitutively active. Studies of human aortic smooth muscle cells have confirmed that the α1D-adrenoceptor is coupled to increases in intracellular calcium [108], and other studies of native receptors suggest the α1D is constitutively active [109].
In studies of constitutively active mutations of α1a and α1b adrenoceptors, a number of antagonists exhibited inverse agonism with marked inhibition: 5-methylurapidil, RS 17053 and tamsulosin at the alpha1a, and 5-methylurapidil at the 1b, but prazosin had only minor actions [110].
Receptor dimers and oligomers
G-protein-coupled receptors can also exist as dimers, or oligomers, both homologous and heterologous [111, 112]. Co-expression of the α1D- with α1B [86] or beta2-adrenoceptors [113] is reported to increase the cell surface expression of α1D-adrenoceptors, suggesting that α1D-adrenoceptor expression and function may involve heterodimerization with these other adrenoceptors. How this relates to expression of α1D-adrenoceptors in various smooth muscles is as yet unclear, as α1D-adrenoceptor-mediated actions can be easily investigated in functional studies (see above). Studies of other G-protein coupled receptors have found that the serotonin 5-HT2A and the glutamate mGlu2 receptor form functional dimers with distinct signalling [114].
α1-Adrenoceptor-mediated second messenger systems
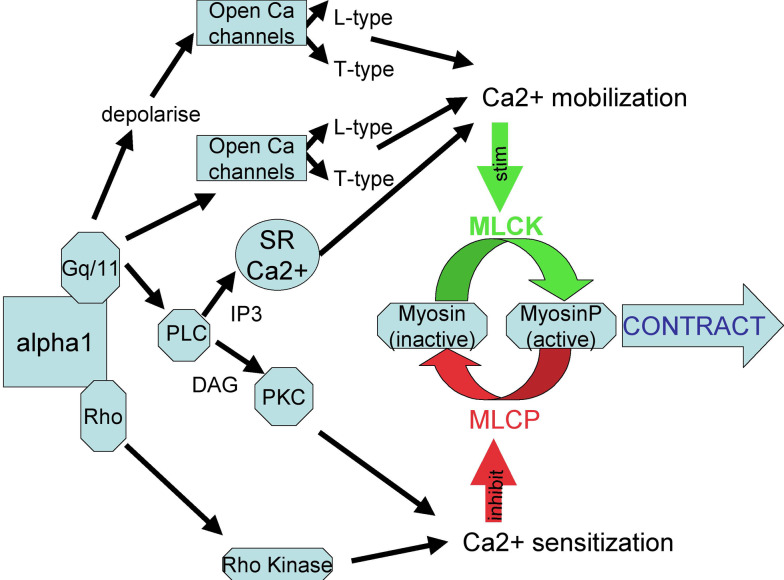
α1-Adrenoceptor agonists can induce smooth muscle contraction and other responses by depolarisation-mediated calcium entry through L-type or T-type calcium channels, by directly activating Ca2+ channels to cause calcium entry, by releasing Ca2+ from intracellular stores or by sensitising the contractile apparatus to Ca2+ [115] (see Fig. 2).
Fig. 2.
A simplified diagram illustrating the possible ways in which α1-adrenoceptor activation can result in contraction, by Ca2+ mobilisation or Ca2+ sensitisation. alpha1 alpha1-adrenoceptor, SR sarcoplasmic reticulum, PLC phospholipase C, IP3 inositol triphosphate, DAG diacylgylcerol, PKC protein kinase C, MLCK myosin light chain kinase, MLCP myosin light chain phosphatase, stim stimulate
α1-Adrenoceptors are coupled to a wide variety of second messenger systems via G-proteins, predominatly by pertussis toxin-insensitive G-proteins of the Gq/11 family to phospholipase C [116, 117]. Activation of all α1-adrenoceptor subtypes results via phospholipase C in formation of inositol triphosphate and diacylglycerol. Diacylglycerol stimulates protein kinase C, and inositol triphosphate acts on the inositol triphosphate receptor in endoplasmic reticulum to release stored calcium: the net result is increased entry of extracellular Ca2+ and/or release from Ca2+ stores [116, 117] (see Fig. 2). α1-Adrenoceptor activation causes phospholipase A2 stimulation and arachidonic acid release in the mammalian COS cell line [118], possibly through Gi/Go [119], causes arachidonic acid release by phospholipase D activation in rat tail artery [120] and can lead to cAMP production [118, 121]. The positive inotropic actions of alpha1-adrenoceptor agonists in rat heart involve Gs and stimulation of cAMP production leading to inhibition of potassium efflux [122].
In addition to signalling through heterotrimeric G-proteins, α1-adrenoceptors may mediate responses through other mechanisms. In rat tail artery, α1-adrenoceptor-mediated calcium sensitisation is due mainly to the activation, via the small GTP binding protein RhoA, of Rho kinase [123], which phosphorylates and so inhibits myosin light-chain phosphatase (see [124]) (see Fig. 2).
Control of blood pressure
α1-Adrenoceptors in the vascular system have a major role in the control of blood pressure and in the baroreflex response to falls in blood pressure. Piascik et al. [125] reported that the α1A-adrenoceptor subtype played a role in the tonic maintenance of blood pressure in the conscious rat, whereas the α1B-adrenoceptor (or perhaps more correctly non-alpha1A-adrenoceptor) subtype participates in the response to exogenous agonists. In the pithed rat, both pressor nerve responses and responses to exogenous noradrenaline are reported to involve both α1A- and α1D-adrenoceptors [126], and in the pithed mouse the pressor response to noradrenaline is largely α1A-adrenoceptor mediated [127].
Studies of knock-out mice have given insights into the role of the various subtypes of alpha1-adrenoceptor in blood pressure control. In knock-out mice lacking the α1A-adrenoceptor, there was a significant fall in blood pressure, both in tail cuff meaurement and invasive recording, but the pressor response to phenylephrine was largely unchanged [128] (Table 2). In knock-out mice lacking the α1B-adrenoceptor subtype, there was no effect on basal blood pressure [88, 89], but the pressor responses to phenylephrine were significantly blunted [88] or unchanged [89]. However, in α1A/α1B double knockout mice, there was no significant fall in blood pressure [129]. In mice lacking the alpha1D-adrenoceptor, or both α1D and α1B, there was a significant fall in resting blood pressure both by tail cuff and invasive recording, and a small fall in the pressor response both to phenylephrine and noradrenaline [89, 97]. Of note in Table 2 are several facts. Firstly, tail cuff SBP seems a poor guide to invasive MAP. Secondly, MAP in the WT varied markedly among studies (varying between 116.5 and 138 mmHg). This can be explained partly by differing genetic backgrounds, but perhaps also by surgical preparation. Although all studies were in conscious animals, the animals were allowed to recover for 3–24 h, so that animals would not have fully recovered from the surgical trauma.
Table 2.
Blood pressure responses in WT and α1-adrenoceptor subtype KO mice
| Parameter | WT | α1A-KO | α1B-KO | α1D-KO | Notes | Ref. |
|---|---|---|---|---|---|---|
| Conscious tail cuff SBP (mmHg) | 114 | 104* | / | / | [128] | |
| 99 | / | 99 | 93* | 1B/1D double KO: 92* | [89] | |
| 108.7 | / | / | 99.1* | 1A/1B double KO: 112 | [97] | |
| 111 | / | / | / | [129] | ||
| Conscious invasive MAP (mmHg) | 138 | 121* | / | / | 24 h post surgery | [128] |
| 119.3 | / | 118.5 | / | 3 h post surgery | [88] | |
| 118 | / | 111 | 109* | 1B/1D double KO: 103*; 24 h post surgery | [89] | |
| 116.5 | / | / | 106.9* | 24 h post surgery | [97] | |
| Phe pressor | No change [128] | Decrease [88] | Small decrease [97] | |||
| No change [89] | No change [89] | 1B/1D double KO: decrease [89] | ||||
| NA pressor | Small decrease [88] | Small decrease [97] | ||||
| Small decrease [89] | Decrease [89] | 1B/1D double KO: decrease [89] |
Results taken from [88, 89, 97, 128, 129]
SBP systolic blood pressure (recorded by tail cuff), HR heart rate, MAP mean arterial pressure (recorded by invasive cannula), phe pressor pressor response to phenylephrine relative to WT, NA pressor pressor response to noradrenaline relative to WT
*Significant difference from respective WT
It was found that the pressor responses to the α-adrenoceptor agonists noradrenaline and/or phenylephrine were almost unchanged in α1A-KO mice, but reduced in both α1D- and α1B-KO mice. This is perhaps surprising as one might have expected, from the wealth of published studies, that the α1A and α1D-adrenoceptors would be most important for control of blood pressure. However, there may have been compensatory mechanisms for the loss of α1A-adrenoceptors in particular, and the α1B-adrenoceptor may have a modulatory rather than contractile role (see above). Hence, both α1A- and α1D-adrenoceptors are involved in acute blood pressure control, which would agree with the findings with antagonist drugs.
Temperature control
Another important role of vascular α1-adrenoceptors is temperature control, as vasoconstriction of superficial blood vessels is an important mechanism to conserve heat. Methylenedioxymethamphetamine (MDMA) is a widely used recreational drug of abuse, and toxic effects include a life-threatening hyperthermia that can occur particularly when the drug is used in a “rave” environment. In animal studies, MDMA disrupts thermoregulation, often causing hypothermia at low ambient temperatures and hyperthermia at high ambient temperatures [130, 131]. In the presence of α1-adrenoceptor antagonists, the monophasic hyperthermic response produced by MDMA in mouse became a biphasic response: hypothermia followed by hyperthermia, and this probably involves both α1A- and α1D-adrenoceptors [132]. β3-Adrenoceptors have also been implicated in thermogenesis [133], but β3-adrenoceptor ligands have been associated with α1-adrenoceptor antagonism, and some of the actions may involve alpha1-adrenoceptors [59, 134].
Peripheral effects of MDMA at α1-adrenoceptors can explain a component of the hyperthermia: cutaneous vasoconstriction by MDMA prevents an early hypothermic response to the drug. At low ambient temperatures, cutaneous vasoconstriction is already marked so that MDMA produces little further vasoconstriction and the, presumed central, hypothermic actions of MDMA may predominate. At high ambient temperatures cutaneous dilatation has occurred, allowing a marked vasoconstrictor component to the actions of MDMA, and hyperthermia predominates. Hence, peripheral α1-adrenoceptor-mediated vasoconstrictor actions of MDMA modulate central hypo- and hyperthermic components.
Neuronal α1-adrenoceptors
α1A-Adrenoceptors [135] and α1B-adrenoceptors [136] are involved in a number of actions in neurones and glial cells in the CNS. α1D-Adrenoceptors are also present in the CNS as demonstrated by a significant fall in alpha1-binding in α1D-KO mice [97]. α1B-Adrenoceptor overexpression resulted in apoptotic neurodegeneration with a corresponding multiple system atrophy including a Parkinson-like syndrome and grand mal seizures [137].
α1-Adrenoceptor-mediated inhibition
Although the concept of prejunctional inhibition mediated by α2-adrenoceptors is well established (see [2]), some studies support the contention that inhibitory prejunctional α1-adrenoceptors exist in pithed rat, rat ventricle, rat vas deferens, rat kidney, dog heart, rat atria, rat tail artery, guinea-pig atria and on the cholinergic nerves of rat gastric fundus (for references, see [11]). Prejunctional inhibition in the CNS involving α1-adrenoceptors is also reported in, for instance, the paraventricular nucleus [138]. Other studies suggest transsynaptic inhibition by prostaglandins or purines produced postjunctionally by α1-adrenoceptor stimulation [139, 140], and the possibility of α2-adrenoceptor-mediated actions of some α1-adrenoceptor antagonists must be considered.
α1-Adrenoceptor-mediated facilitation
α1-Adrenoceptor agonists have been reported to facilitate release of acetylcholine in rat heart [141] and cat [142] and rat bladder [143, 144]. These α1-adrenoceptors may be on the soma of bladder parasympathetic neurones and mediate a slow postsynaptic depolarisation [143]. In the CNS, facilitation of rat spinal motoneuron activity [145] and of vasopressin release [146] are reported to be mediated by α1-adrenoceptors. α1-Adrenoceptor activation stimulates inhibitory GABAergic neurotransmission in rat spinal cord [147], rat cerebellum [148], mouse accessory olfactory bulb [149] and mouse hypothalamus [150]. The α1-adrenoceptor-mediated facilitation may involve protein kinase C and increases in intracellular calcium [146, 148, 151].
Concluding remarks
Pharmacological and receptor knockout techniques have greatly increased our understanding of α1-adrenoceptors in terms of location and function of the three subtypes. Areas of particular interest in the next few years will be investigation of the role of α1-adrenoceptor subtypes in the central nervous system, development of ‘missing’ subtype selective agonists and antagonists, further development of drugs for benign prostatic hypertrophy and elucidation of the role of the α1B-adrenoceptor.
References
- 1.Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 2.Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- 3.Starke K, Langer SZ. A note on the terminology for presynaptic receptors. In: Langer SZ, Starke K, Dubocovich ML, editors. Presynaptic receptors. Oxford: Pergamon Press; 1979. pp. 1–3. [Google Scholar]
- 4.Morrow AL, Creese I. Characterization of alpha1-adrenergic receptor subtypes in rat brain: a re-evaluation of [3H]WB4101 and [3H]prazosin binding. Mol Pharmacol. 1986;29:321–330. [PubMed] [Google Scholar]
- 5.Han C, Abel PW, Minneman KP. Alpha1-adrenoceptor subtypes linked to different mechanisms for increasing intracellular Ca2+ in smooth muscle. Nature. 1987;329:333–335. doi: 10.1038/329333a0. [DOI] [PubMed] [Google Scholar]
- 6.Cotecchia S, Schwinn DA, Randall RR, Lefkowitz RJ, Caron MG, Kobilka BK. Molecular cloning and expression of the cDNA for the hamster alpha1-adrenergic receptor. Proc Natl Acad Sci USA. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomasney JW, Cotecchia S, Lorenz W, Leung W-Y, Schwinn DA, Yang-Feng TL, Brownstein M, Lefkowitz RJ, Caron M. Molecular cloning and expression of the cDNA for the alpha1A-adrenergic receptor. J Biol Chem. 1991;266:6365–6369. [PubMed] [Google Scholar]
- 8.Schwinn DA, Lomasney JW, Lorenz W, Szklut PJ, Fremeau RT, Yang-Feng TL, Caron MG, Lefkowitz RJ, Cotecchia S. Molecular cloning and expression of the cDNA for a novel alpha1-adrenergic receptor subtype. J Biol Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- 9.Perez DM, Piascik MT, Graham RM. Solution-phase library screening for the identification of rare clones: isolation of an alpha1D-adrenergic receptor cDNA. Mol Pharmacol. 1991;40:876–883. [PubMed] [Google Scholar]
- 10.Hieble JP, Bylund DB, Clarke DE, Eikenbur DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR. International Union of Pharmacology. X. Recommendation for nomenclature of alpha1-adrenoceptors: Consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- 11.Docherty JR. Subtypes of functional alphal- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- 12.Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 13.Hodges AN, Lynn BM, Bula JE, Donaldson MG, Dagenais MO, McKenzie DC. Effects of pseudoephedrine on maximal cycling power and submaximal cycling efficiency. Med Sci Sports Exerc. 2003;35:1316–1319. doi: 10.1249/01.MSS.0000078925.30346.F8. [DOI] [PubMed] [Google Scholar]
- 14.Swain RA, Harsha DM, Baenziger J, Saywell RM., Jr Do pseudoephedrine or phenylpropanolamine improve maximum oxygen uptake and time to exhaustion? Clin J Sport Med. 1997;7:168–173. doi: 10.1097/00042752-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Drew CD, Knight GT, Hughes DT, Bush M. Comparison of the effects of d-(−)-ephedrine and l-(+)-pseudoephedrine on the cardiovascular and respiratory systems in man. Br J Clin Pharmacol. 1978;6:221–225. doi: 10.1111/j.1365-2125.1978.tb04588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Empey DW, Young GA, Letley E, John GC, Smith P, McDonnell KA, Bagg LR, Hughes DT. Dose–response study of the nasal decongestant and cardiovascular effects of pseudoephedrine. Br J Clin Pharmacol. 1980;9:351–358. doi: 10.1111/j.1365-2125.1980.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bright TP, Sandage BW, Jr, Fletcher HP. Selected cardiac and metabolic responses to pseudoephedrine with exercise. J Clin Pharmacol. 1981;21:488–492. doi: 10.1002/j.1552-4604.1981.tb05655.x. [DOI] [PubMed] [Google Scholar]
- 18.Ma G, Bavadekar SA, Davis YM, Lalchandani SG, Nagmani R, Schaneberg BT, Khan IA, Feller D. Pharmacological effects of ephedrine alkaloids on human alpha(1)- and alpha(2)-adrenergic receptor subtypes. J Pharmacol Exp Ther. 2007;322:214. doi: 10.1124/jpet.107.120709. [DOI] [PubMed] [Google Scholar]
- 19.Vansal SS, Feller DR. Direct effects of ephedrine isomers on human beta-adrenergic receptor subtypes. Biochem Pharmacol. 1999;58:807–810. doi: 10.1016/s0006-2952(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 20.Ulahannan TJ, Karpe F, Humphreys SM, Matthews DR, Frayn KN. Effects of acute administration of doxazosin on fasting and postprandial haemodynamics and lipid metabolism in healthy subjects. Horm Metab Res. 2002;34:499–503. doi: 10.1055/s-2002-34789. [DOI] [PubMed] [Google Scholar]
- 21.Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH. Sympathetic regulation of glucose uptake by the alpha1-adrenoceptor in human obesity. Obes Res. 2004;12:612–620. doi: 10.1038/oby.2004.70. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Takahashi S, Zhong S, Hosoda C, Zheng HY, Ogushi T, Fujimura T, Ohta N, Tanoue A, Tsujimoto G, Kitamura T. Function of the lower urinary tract in mice lacking alpha1d-adrenoceptor. J Urol. 2005;174:370–374. doi: 10.1097/01.ju.0000161210.17365.cc. [DOI] [PubMed] [Google Scholar]
- 23.Sanbe A, Tanaka Y, Fujiwara Y, Tsumura H, Yamauchi J, Cotecchia S, Koike K, Tsujimoto G, Tanoue A. Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol. 2007;152:332–340. doi: 10.1038/sj.bjp.0707366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 25.Furuya S, Kumamoto Y, Yokoyama E, Tsukamoto T, Izumi T, Abiko Y. Alpha-adrenergic activity and urethral pressure in prostatic zone in benign prostatic hypertrophy. J Urol. 1982;128:836–839. doi: 10.1016/s0022-5347(17)53216-4. [DOI] [PubMed] [Google Scholar]
- 26.Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. J Urol. 1986;136:1–4. doi: 10.1016/s0022-5347(17)44709-4. [DOI] [PubMed] [Google Scholar]
- 27.Nickel JC, Sander S, Moon TD. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract. 2008;62:1547–1559. doi: 10.1111/j.1742-1241.2008.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima Y, Sasaki S, Shinoura H, Hayashi Y, Tsujimoto G, Kohri K. Quantification of alpha1-adrenoceptor subtypes by real-time RT-PCR and correlation with age and prostate volume in benign prostatic hyperplasia patients. Prostate. 2006;66:761–767. doi: 10.1002/pros.20399. [DOI] [PubMed] [Google Scholar]
- 29.Ford APDW, Arredondo NF, Blue DR, Bonhaus DW, Jasper J, Kava MS, Lesnick J, Pister JR, Shieh IM, Vimont RL, Williams TJ, McNea JE, Stamey TA, Clarke DE. RS-17053, a selective alpha1A-adrenoceptor antagonist, displays low affinity for functional alpha1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- 30.Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Gever JR, Kava MS, Morgans D, Padilla F, Tassa S, Vimont RL, Chapple CR, Chess-Williams R, Eglen RM, Clarke DE, Ford AP. In vitro alpha1-adrenoceptor pharmacology of Ro 70-0004 and RS-100329, novel alpha1A-adrenoceptor selective antagonists. Br J Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knepper SM, Buckner SA, Brune ME, DeBernardis JF, Meyer MD, Hancock AA. A-61603, a potent alpha1-adrenergic receptor agonist, selective for the alpha1A receptor subtype. J Pharmacol Exp Ther. 1995;274:97–103. [PubMed] [Google Scholar]
- 32.Sleight AJ, Koek W, Bigg DC. Binding of antipsychotic drugs at alpha1A- and alpha1B-adrenoceptors: risperidone is selective for the alpha 1B-adrenoceptors. Eur J Pharmacol. 1993;238:407–410. doi: 10.1016/0014-2999(93)90876-j. [DOI] [PubMed] [Google Scholar]
- 33.King HK, Goetz AS, Ward SDC, Saussy DL Jr (1994) AH11110A is selective for the α1B subtype of α1-adrenoceptors. Soc Neurosci Abstr 20 p 52
- 34.Marucci G, Angeli P, Buccioni M, Gulini U, Melchiorre C, Sagratini G, Testa R, Giardinà D. (+)-Cyclazosin, a selective alpha1B-adrenoceptor antagonist: functional evaluation in rat and rabbit tissues. Eur J Pharmacol. 2005;522:100–107. doi: 10.1016/j.ejphar.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Eltze M. In functional experiments, risperidone is selective, not for the B, but for the A subtype of alpha-1 adrenoceptor. Eur J Pharmacol. 1996;295:69–73. doi: 10.1016/0014-2999(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 36.Stam WB, Van der Graaf PH, Saxena PR. Functional characterisation of the pharmacological profile of the putative alpha1B-adrenoceptor antagonist, (+)-cyclazosin. Eur J Pharmacol. 1998;361:79–83. doi: 10.1016/s0014-2999(98)00735-3. [DOI] [PubMed] [Google Scholar]
- 37.Eltze M, König H, Ullrich B, Grebe T. Failure of AH11110A to functionally discriminate between alpha(1)-adrenoceptor subtypes A, B and D or between alpha(1)- and alpha(2)-adrenoceptors. Eur J Pharmacol. 2001;415:265–276. doi: 10.1016/s0014-2999(01)00835-4. [DOI] [PubMed] [Google Scholar]
- 38.Michel MC, Kerker J, Branchek TA, Forray C. Selective irreversible binding of chloroethylclonidine at alpha1- and alpha2-adrenoceptor subtypes. Mol Pharmacol. 1993;44:1165–1170. [PubMed] [Google Scholar]
- 39.O’Rourke M, Kearns S, Docherty JR. Investigations of the actions of chloroethylclonidine in rat aorta. Br J Pharmacol. 1995;115:1399–1406. doi: 10.1111/j.1476-5381.1995.tb16630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Rourke M, Gavin K, Docherty JR. Further investigation of the alpha-adrenoceptor-mediated actions of chloroethylclonidine in rat aorta. Eur J Pharmacol. 1997;336:37–42. doi: 10.1016/s0014-2999(97)01257-0. [DOI] [PubMed] [Google Scholar]
- 41.Goetz AS, King HK, Ward SDC, True TA, Rimele TJ, Saussy DL. BMY 7378 is a selective antagonist of the D subtype of alpha1-adrenoceptors. Eur J Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- 42.Cleary L, Murad K, Bexis S, Docherty JR. The alpha (1D)-adrenoceptor antagonist BMY 7378 is also an alpha (2C)-adrenoceptor antagonist. Auton Autacoid Pharmacol. 2005;25:135–141. doi: 10.1111/j.1474-8673.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 43.Chaput Y, de Montigny C. Effects of the 5-hydroxytryptamine receptor antagonist, BMY 7378, on 5-hydroxytryptamine neurotransmission: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther. 1988;246:359–370. [PubMed] [Google Scholar]
- 44.Murata S, Taniguchi T, Takahashi M, Okada K, Akiyama K, Muramatsu I. Tissue selectivity of KMD-3213, an alpha(1)-adrenoreceptor antagonist, in human prostate and vasculature. J Urol. 2000;164:578–583. [PubMed] [Google Scholar]
- 45.Aboud RW, Shafii M, Docherty JR. Investigations of the subtypes of alpha1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br J Pharmacol. 1993;109:80–87. doi: 10.1111/j.1476-5381.1993.tb13534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burt RP, Chapple CR, Marshall I. Evidence for a functional alpha1A- (alpha1C-) adrenoceptor mediating contraction of rat epididymal vas deferens and an alpha1B-adrenoceptor mediating contraction of the rat spleen. Br J Pharmacol. 1995;115:467–475. doi: 10.1111/j.1476-5381.1995.tb16356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burt RP, Chapple CR, Marshall I. Alpha1A-Adrenoceptor mediated contraction of rat prostatic vas deferens and the involvement of ryanodine stores and Ca2+ influx stimulated by diacylglycerol and PKC. Br J Pharmacol. 1998;123:317–325. doi: 10.1038/sj.bjp.0701588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noble AJ, Chess-Williams R, Couldwell C, Furukawa K, Uchyiuma T, Korstanje C, Chapple CR. The effects of tamsulosin, a high affinity antagonist at functional alpha1A- and alpha1D-adrenoceptor subtypes. Br J Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villalobos-Molina R, Lopez-Guerrero JJ, Ibarra M. Alpha1D- and alpha1A-adrenoceptors mediate contraction in rat renal artery. Eur J Pharmacol. 1997;322:225–227. doi: 10.1016/s0014-2999(97)00095-2. [DOI] [PubMed] [Google Scholar]
- 50.Villalobos-Molina R, Ibarra M. Alpha1-adrenoceptors mediating contraction in arteries of normotensive and spontaneously hypertensive rats are of the alpha1D or alpha 1A subtypes. Eur J Pharmacol. 1996;298:257–263. doi: 10.1016/0014-2999(95)00781-4. [DOI] [PubMed] [Google Scholar]
- 51.Lachnitt WG, Tran AM, Clarke DE, Ford APDW. Pharmacological characterization of an alpha1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of rat. Br J Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu G-S, Han C. Role of alpha1A- and alpha1B-adrenoceptors in phenylephrine induced positive inotropic response in isolated rat left atrium. J Cardiovasc Pharmacol. 1994;24:745–752. doi: 10.1097/00005344-199424050-00009. [DOI] [PubMed] [Google Scholar]
- 53.Fagura MS, Lyfdford SJ, Douggall IG. Pharmacological classification of alpha1-adrenoceptors mediating contractions of rabbit isolated ear artery: comparison with rat isolated thoracic aorta. Br J Pharmacol. 1997;120:247–258. doi: 10.1038/sj.bjp.0700917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills K, Hausman N, Chess-Williams R. Characterization of the alpha1-adrenoceptor subtype mediating contractions of the pig internal anal sphincter. Br J Pharmacol. 2008;155:110–117. doi: 10.1038/bjp.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furukawa K, Rosario DJ, Smith DJ, Chapple CR, Uchiyama T, Chess-Williams R. Alpha1A-adrenoceptor-mediated contractile responses of the human vas deferens. Br J Pharmacol. 1995;116:1605–1610. doi: 10.1111/j.1476-5381.1995.tb16380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moriyama N, Nasu K, Takeuchi T, Akiyama K, Murata S, Mishimatsu H, Yano J, Tsujimoto G, Kawabe K. Quantification and distribution of alpha1-adrenoceptor subtype mRNAs in human vas deferens: comparison with those of epididymal and pelvic portions. Br J Pharmacol. 1997;122:1009–1014. doi: 10.1038/sj.bjp.0701485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall I, Burt RP, Chapple CR. Noradrenaline contractions of human prostate mediated by alpha1A- (alpha1c-) adrenoceptor subtype. Br J Pharmacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng C-M, Guh J-H, Ko F-N. Functional identification of alpha1-adrenoceptor subtypes in human prostate: comparison with those in rat vas deferens and spleen. Eur J Pharmacol. 1994;265:61–66. doi: 10.1016/0014-2999(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 59.Bexis S, Docherty JR. Role of α1- and β3-adrenoceptor subtypes in the modulation by SR59230A of the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2009;158:259–266. doi: 10.1111/j.1476-5381.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruchas MR, Toews ML, Bockman CS, Abel PW. Characterization of the alpha1-adrenoceptor subtype activating extracellular signal-regulated kinase in submandibular gland acinar cells. Eur J Pharmacol. 2008;578:349–358. doi: 10.1016/j.ejphar.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, Piascik MT, Perez DM. The alpha(1B)-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovasc Res. 2003;60:598–607. doi: 10.1016/j.cardiores.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 62.Gray K, Short J, Ventura S. The alpha1A-adrenoceptor gene is required for the alpha1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate. Br J Pharmacol. 2008;155:103–109. doi: 10.1038/bjp.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodcock EA. Roles of alpha1A- and alpha1B-adrenoceptors in heart: insights from studies of genetically modified mice. Clin Exp Pharmacol Physiol. 2007;34:884–888. doi: 10.1111/j.1440-1681.2007.04707.x. [DOI] [PubMed] [Google Scholar]
- 64.Flavahan NA, Vanhoutte PA. Alpha1-adrenoceptor subclassification in vascular smooth muscle. Trends Pharmacol Sci. 1986;7:347–349. [Google Scholar]
- 65.Docherty JR. The pharmacology of alpha1- and alpha2-adrenoceptors: evidence for and against a further subdivision. Pharmacol Ther. 1989;44:241–284. doi: 10.1016/0163-7258(89)90067-3. [DOI] [PubMed] [Google Scholar]
- 66.Muramatsu I, Ohmura T, Kigoshi S, Hashimoto S, Oshita M. Pharmacological subclassification of alpha1-adrenoceptors in vascular smooth muscle. Br J Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muramatsu I, Oshita M, Ohmura T, Kigoshi S, Akino H, Gobara M, Okada K. Pharmacological characterization of alpha1-adrenoceptor subtypes in the human prostate: functional and binding studies. Br J Urol. 1995;74:572–578. doi: 10.1111/j.1464-410x.1994.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 68.Ford APDW, Berge NV, Clarke DE. Characterisation of alpha1-adrenoceptors in isolated anococcygeus muscle of rat. Br J Pharmacol. 1993;109:112P. [Google Scholar]
- 69.Ohmura T, Oshita M, Kigoshi S, Muramatsu I. Identification of alpha1-adrenoceptor subtypes in the rat vas deferens: binding and functional studies. Br J Pharmacol. 1992;107:698–704. doi: 10.1111/j.1476-5381.1992.tb14509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amobi NI, Guillebaud J, Kaisary AV, Turner E, Smith IC. Discrimination by SZL49 between contractions evoked by noradrenaline in longitudinal and circular muscle of human vas deferens. Br J Pharmacol. 2002;136:127–135. doi: 10.1038/sj.bjp.0704689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cleary L, Slattery J, Bexis S, Docherty JR. Sympathectomy reveals alpha1A- and alpha1D-adrenoceptor components to contractions to noradrenaline in rat vas deferens. Br J Pharmacol. 2004;143:745–752. doi: 10.1038/sj.bjp.0705987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith KM, MacMillan JB, McGrath JC. Investigation of alpha1-adrenoceptor subtypes mediating vasoconstriction in rabbit cutaneous resistance arteries. Br J Pharmacol. 1997;122:825–832. doi: 10.1038/sj.bjp.0701451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van der Graaf PH, Shankley NP, Black JW. Analysis of the effects of alpha1-adrenoceptor antagonists on noradrenaline-mediate contraction of rat small mesenteric artery. Br J Pharmacol. 1996;118:1308–1316. doi: 10.1111/j.1476-5381.1996.tb15538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Recio P, Orensanz LM, Martínez MP, Navarro-Dorado J, Bustamante S, García-Sacristán A, Prieto D, Hernández M. Noradrenergic vasoconstriction of pig prostatic small arteries. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:397–406. doi: 10.1007/s00210-007-0227-x. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto Y, Koike K. Alpha1-adrenoceptors in the guinea pig thoracic aorta. J Smooth Muscle Res. 1999;35:181–192. doi: 10.1540/jsmr.35.181. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura S, Taniguchi T, Suzuki F, Akagi Y, Muramatsu I. Evaluation of alpha1-adrenoceptors in the rabbit iris: pharmacological characterization and expression of mRNA. Br J Pharmacol. 1999;127:1367–1374. doi: 10.1038/sj.bjp.0702675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kava MS, Blue DR, Vimont RL, Clarke DE, Ford APDW. Alpha1L-adrenoceptor mediation of smooth muscle contraction in rabbit bladder neck: a model for lower urinary tract tissues of man. Br J Pharmacol. 1998;123:1359–1366. doi: 10.1038/sj.bjp.0701748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukasawa R, Taniguchi N, Moriyama N, Ukai Y, Yamazaki S, Ueki T, Kameyama S, Kimura K, Kawabe K. The alpha1L-adrenoceptor subtype in the lower urinary tract: a comparison of human urethra and prostate. Br J Urol. 1998;82:733–737. doi: 10.1046/j.1464-410x.1998.00845.x. [DOI] [PubMed] [Google Scholar]
- 79.Gray KT, Ventura S. Alpha1L-adrenoceptors mediate contractions of the isolated mouse prostate. Eur J Pharmacol. 2006;540:155–161. doi: 10.1016/j.ejphar.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 80.Ford APDW, Daniels D, Chang DJ, Gever JR, Jasper JR, Lesnick JD, Clarke DE. Pharmacological pleiotropism of the human recombinant alpha1A-adrenoceptor: implications for alpha1-adrenoceptor classification. Br J Pharmacol. 1997;121:137–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daniels DV, Gever JR, Jasper JR, Kava MS, Lesnick JD, Meloy TD, Stepan G, Williams TJ, Clarke DE, Chang DJ, Ford AP. Human cloned alpha1A-adrenoceptor isoforms display alpha1L-adrenoceptor pharmacology in functional studies. Eur J Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 82.Morishima S, Suzuki F, Yoshiki H, Md Anisuzzaman AS, Sathi ZS, Tanaka T, Muramatsu I. Identification of the alpha1L-adrenoceptor in rat cerebral cortex and possible relationship between alpha1L- and alpha1A-adrenoceptors. Br J Pharmacol. 2008;153:1485–1494. doi: 10.1038/sj.bjp.0707679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martí D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, Moreno L, Villagrasa V, Barettino D, D’Ocon P. Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 2005;289:H1923–H1932. doi: 10.1152/ajpheart.00288.2005. [DOI] [PubMed] [Google Scholar]
- 84.Shibata K, Hirasawa A, Moriyama N, Kawabe K, Ogawa S, Tsujimoto G. Alpha1a-adrenoceptor polymorphism: pharmacological characterization and association with benign prostatic hypertrophy. Br J Pharmacol. 1996;118:1403–1408. doi: 10.1111/j.1476-5381.1996.tb15552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramsay D, Carr IC, Pediani J, Lopez-Gimenez JF, Thurlow R, Fidock M, Milligan G. High-affinity interactions between human alpha1A-adrenoceptor C-terminal splice variants produce homo- and heterodimers but do not generate the alpha1L-adrenoceptor. Mol Pharmacol. 2004;66:228–239. doi: 10.1124/mol.66.2.228. [DOI] [PubMed] [Google Scholar]
- 86.Uberti MA, Hall RA, Minneman KP. Subtype-specific dimerization of alpha1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- 87.Eltze M. Functional evidence for an alpha1B-adrenoceptor mediating contraction of the mouse spleen. Eur J Pharmacol. 1996;311:187–198. doi: 10.1016/0014-2999(96)00430-x. [DOI] [PubMed] [Google Scholar]
- 88.Cavalli A, Lattion A, Hummler E, Nenninger M, Pedrazzini T. Decreased blood pressure response in mice deficient of the alpha 1b-adrenergic receptor. Proc Nat Acad Sci USA. 1997;94:11589–11595. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hosoda C, Koshimizu TA, Tanoue A, Nasa Y, Oikawa R, Tomabechi T, Fukuda S, Shinoura H, Oshikawa S, Takeo S, Kitamura T, Cotecchia S, Tsujimoto G. Two alpha1-adrenergic receptor subtypes regulating the vasopressor response have differential roles in blood pressure regulation. Mol Pharmacol. 2005;67:912–922. doi: 10.1124/mol.104.007500. [DOI] [PubMed] [Google Scholar]
- 90.Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, McGrath JC. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- 91.Daly CJ, Cotecchia S, McGrath JC. Low frequency electrical field stimulation elicits responses in segments of mouse tail artery which are slower in alpha1B-knockout mice than in control mice. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:R600. [Google Scholar]
- 92.Zuscik MJ, Chalothorn D, Hellard D, Deighan C, McGee A, Daly CJ, Waugh DJ, Ross SA, Gaivin RJ, Morehead AJ, Thomas JD, Plow EF, McGrath JC, Piascik MT, Perez DM. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the alpha 1B-adrenergic receptor. J Biol Chem. 2001;276:13738–13743. doi: 10.1074/jbc.M008693200. [DOI] [PubMed] [Google Scholar]
- 93.Piascik MT, Guarino RD, Smith MS, Soltis EE, Saussy DL, Perez DM. The specific contribution of the novel alpha-1D adrenoceptor to the contraction of vascular smooth muscle. J Pharmacol Exp Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- 94.Hussain M, Marshall I. Characterization of alpha1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang H-T, Endoh M. Pharmacological evidence for alpha1D-adrenoceptors in the rabbit ventricular myocardium: analysis with BMY 7378. Br J Pharmacol. 1997;122:1541–1550. doi: 10.1038/sj.bjp.0701506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deighan C, Methven L, Naghadeh MM, Wokoma A, Macmillan J, Daly CJ, Tanoue A, Tsujimoto G, McGrath JC. Insights into the functional roles of alpha(1)-adrenoceptor subtypes in mouse carotid arteries using knockout mice. Br J Pharmacol. 2005;144:558–565. doi: 10.1038/sj.bjp.0706089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chalothorn D, McCune DF, Edelmann SE, Tobita K, Keller BB, Lasley RD, Perez DM, Tanoue A, Tsujimoto G, Post GR, Piascik MT. Differential cardiovascular regulatory activities of the alpha1B- and alpha1D-adrenoceptor subtypes. J Pharmacol Exp Ther. 2003;305:1045–1053. doi: 10.1124/jpet.102.048553. [DOI] [PubMed] [Google Scholar]
- 99.Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, Cotecchia S, Kitamura T, Tsujimoto G, Koike K. Correlation between vasoconstrictor roles and mRNA expression of alpha1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol. 2005;146:456–466. doi: 10.1038/sj.bjp.0706325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zacharia J, Hillier C, Tanoue A, Tsujimoto G, Daly CJ, McGrath JC, MacDonald A. Evidence for involvement of alpha1D-adrenoceptors in contraction of femoral resistance arteries using knockout mice. Br J Pharmacol. 2005;146:942–951. doi: 10.1038/sj.bjp.0706395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Filippi S, Parenti A, Donnini S, Granger HJ, Fazzini A, Ledda F. Alpha(1D)-adrenoceptors cause endothelium-dependent vasodilatation in the rat mesenteric vascular bed. J Pharmacol Exp Ther. 2001;296:869–875. [PubMed] [Google Scholar]
- 102.Vinci MC, Bellik L, Filippi S, Ledda F, Parenti A. Trophic effects induced by alpha1D-adrenoceptors on endothelial cells are potentiated by hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H2140–H2147. doi: 10.1152/ajpheart.00390.2007. [DOI] [PubMed] [Google Scholar]
- 103.Bexis S, Cleary L, McGrath JC, Tanoue A, Tsujimoto G, Docherty JR. Alpha(1D)-adrenoceptors mediate nerve and agonist-evoked contractions in mouse vas deferens: evidence obtained from knockout technology. Auton Autacoid Pharmacol. 2008;28:81–85. doi: 10.1111/j.1474-8673.2008.00420.x. [DOI] [PubMed] [Google Scholar]
- 104.Taki N, Tanaka T, Zhang L, Suzuki F, Israilova M, Taniguchi T, Hiraizumi-Hiraoka Y, Shinozuka K, Kunitomo M, Muramatsu I. Alpha-1D adrenoceptors are involved in reserpine-induced supersensitivity of rat tail artery. Br J Pharmacol. 2004;142:647–656. doi: 10.1038/sj.bjp.0705817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noguera MA, Ivorra MD, D’Ocon P. Functional evidence of inverse agonism in vascular smooth muscle. Br J Pharmacol. 1996;119:158–164. doi: 10.1111/j.1476-5381.1996.tb15689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ziani K, Gisbert R, Noguera MA, Ivorra MD, D’Ocon P. Modulatory role of a constitutively active population of alpha(1D)-adrenoceptors in conductance arteries. Am J Physiol Heart Circ Physiol. 2002;282:H475–H481. doi: 10.1152/ajpheart.00411.2001. [DOI] [PubMed] [Google Scholar]
- 107.Gisbert R, Pérez-Vizcaino F, Cogolludo AL, Noguera MA, Ivorra MD, Tamargo J, D’Ocon P. Cytosolic Ca2+ and phosphoinositide hydrolysis linked to constitutively active alpha1D-adrenoceptors in vascular smooth muscle. J Pharmacol Exp Ther. 2003;305:1006–1014. doi: 10.1124/jpet.102.046169. [DOI] [PubMed] [Google Scholar]
- 108.García-Cazarín ML, Smith JL, Olszewski KA, McCune DF, Simmerman LA, Hadley RW, Kraner SD, Michael T, Piascik MT. The α1D-adrenergic receptor is expressed intracellularly and coupled to increases in intracellular calcium and reactive oxygen species in human aortic smooth muscle cells. J Mol Signal. 2008;3:6. doi: 10.1186/1750-2187-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCune DF, Edelmann SE, Olges JR, Post GR, Waldrop BA, Waugh DJ, Perez DM, Piascik MT. Regulation of the cellular localization and signaling properties of the alpha1B- and alpha1D-adrenoreceptors by agonists and inverse antagonists. Mol Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- 110.Rossier O, Abuin L, Fanelli F, Leonardi A, Cotecchia S. Inverse agonism and neutral antagonism at alpha1A- and alpha1B-adrenergic receptor subtypes. Mol Pharmacol. 1999;56:858–866. doi: 10.1124/mol.56.5.858. [DOI] [PubMed] [Google Scholar]
- 111.Minneman KP. Heterodimerization and surface localization of G protein coupled receptors. Biochem Pharmacol. 2007;73:1043–1050. doi: 10.1016/j.bcp.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dalrymple MB, Pfleger KD, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-adrenergic receptors. J Pharmacol Exp Ther. 2005;313:16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]
- 114.González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:9. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen XL, Rembold CM. Phenylephrine contracts rat tail artery by one electromechanical and three pharmacomechanical mechanisms. Am J Physiol. 1995;268:H74–H81. doi: 10.1152/ajpheart.1995.268.1.H74. [DOI] [PubMed] [Google Scholar]
- 116.Minneman KP. Alpha1-adrenergic receptor subtypes, inositol phosphates and sources of cell Ca2+ . Pharmacol Rev. 1988;40:87–119. [PubMed] [Google Scholar]
- 117.Wu D, Katz A, Lee C, Simon MI. Activation of phospholipase C by alpha1-adrenergic receptors in mediated by the alpha subunits of Gq family. J Biol Chem. 1992;267:25798–25802. [PubMed] [Google Scholar]
- 118.Perez DM, DeYoung MP, Graham RM. Coupling of expressed alpha1B- and alpha1D-adrenergic receptors to multiple signaling pathways is both G-protein and cell type specific. Mol Pharmacol. 1993;44:784–795. [PubMed] [Google Scholar]
- 119.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 120.Gu H, Trajkovic S, LaBelle EF. Norepinephrine-induced phosphatidylcholine hydrolysis by phospholipases D and C in rat tail artery. Am J Physiol. 1992;262:C1376–C1383. doi: 10.1152/ajpcell.1992.262.6.C1376. [DOI] [PubMed] [Google Scholar]
- 121.Ruan Y, Kan H, Parmentier J-H, Fatima S, Allen LF, Malik KU. Alpha-1A adrenergic receptor stimulation with phenylephrine promotes arachidonic acid release by activation of phospholipase D in rat-1 fibroblasts: inhibition by protein kinase A. J Pharmacol Exp Ther. 1998;284:575–585. [PubMed] [Google Scholar]
- 122.Gallego M, Setién R, Puebla L, Boyano-Adánez Mdel C, Arilla E, Casis O. Alpha1-adrenoceptors stimulate a Galphas protein and reduce the transient outward K+ current via a cAMP/PKA-mediated pathway in the rat heart. Am J Physiol Cell Physiol. 2005;288:C577–C585. doi: 10.1152/ajpcell.00124.2004. [DOI] [PubMed] [Google Scholar]
- 123.Mueed I, Bains P, Zhang L, MacLeod KM. Differential participation of protein kinase C and Rho kinase in α1-adrenoceptor mediated contraction in rat arteries. Can J Physiol Pharmacol. 2004;82:895–902. doi: 10.1139/y04-086. [DOI] [PubMed] [Google Scholar]
- 124.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and non muscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 125.Piascik MT, Kusiak JW, Barron KW. Alpha1-adrenoceptor subtypes and the regulation of peripheral hemodynamics in the conscious rat. Eur J Pharmacol. 1990;186:273–278. doi: 10.1016/0014-2999(90)90443-a. [DOI] [PubMed] [Google Scholar]
- 126.Castillo EF, López RM, Rodríguez-Silverio J, Bobadilla RA, Castillo C. Alpha 1D-adrenoceptors contribute to the neurogenic vasopressor response in pithed rats. Fundam Clin Pharmacol. 1998;12:584–589. doi: 10.1111/j.1472-8206.1998.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 127.López-Guerrero JJ, Ibarra M, Villalobos-Molina R. Postjunctional alpha1-adrenoceptors in the vasculature of the pithed mouse are of the alpha1A-subtype. Auton Autacoid Pharmacol. 2005;25:101–103. doi: 10.1111/j.1474-8673.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 128.Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3, 4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Green AR, O’Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bexis S, Docherty JR. Role of alpha(1)-adrenoceptor subtypes in the effects of methylenedioxy methamphetamine (MDMA) on body temperature in the mouse. Br J Pharmacol. 2008;153:591–597. doi: 10.1038/sj.bjp.0707590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE. Attenuation of 3, 4-methylenedioxymethamphatamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoceptor antagonists. Br J Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brahmadevara N, Shaw AM, MacDonald A. Alpha1-adrenoceptor antagonist properties of CGP 12177A and other beta-adrenoceptor ligands: evidence against beta(3)- or atypical beta-adrenoceptors in rat aorta. Br J Pharmacol. 2004;142:781–787. doi: 10.1038/sj.bjp.0705840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM. Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: alpha1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol. 2006;497:209–222. doi: 10.1002/cne.20992. [DOI] [PubMed] [Google Scholar]
- 136.Papay R, Gaivin R, McCune DF, Rorabaugh BR, Macklin WB, McGrath JC, Perez DM. Mouse alpha1B-adrenergic receptor is expressed in neurons and NG2 oligodendrocytes. J Comp Neurol. 2004;478:1–10. doi: 10.1002/cne.20215. [DOI] [PubMed] [Google Scholar]
- 137.Zuscik MJ, Sands S, Ross SA, Waugh DJ, Gaivin RJ, Morilak D, Perez DM. Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy. Nat Med. 2000;6:1388–1394. doi: 10.1038/82207. [DOI] [PubMed] [Google Scholar]
- 138.Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther. 2006;316:733–742. doi: 10.1124/jpet.105.094797. [DOI] [PubMed] [Google Scholar]
- 139.Rump LC, Majewski H. Modulation of noradrenaline release through alpha1- and alpha2-adrenoceptors in rat isolated kidney. J Cardiovasc Pharmacol. 1987;9:500–507. doi: 10.1097/00005344-198704000-00016. [DOI] [PubMed] [Google Scholar]
- 140.Shinozuka K, Kunitomo M, Bjur RA, Westfall DP, Hattori K. Effect of methoxamine on noradrenaline release in the caudal artery of hypertensive rats. Clin Exp Pharmacol Physiol. 1995;22:S88–S90. doi: 10.1111/j.1440-1681.1995.tb02982.x. [DOI] [PubMed] [Google Scholar]
- 141.Bognar TI, Baretti R, Fischer S, Veldet C, Fuder H. Alpha-adrenoceptor mediated facilitation of acetylcholine release in rat perfused heart. J Pharmacol Exp Ther. 1990;254:702–710. [PubMed] [Google Scholar]
- 142.Keast JR, Kawatani M, De Groat WC. Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by alpha1- and alpha2-adrenoceptors. Am J Physiol. 1990;258:R44–R50. doi: 10.1152/ajpregu.1990.258.1.R44. [DOI] [PubMed] [Google Scholar]
- 143.Yoshimura N, de Groat WC. Patch clamp analysis of afferent and efferent neurons that innervate the urinary bladder of the rat. Soc Neurosci Abstr. 1992;18:126. [Google Scholar]
- 144.Somogyi GT, Tanowitz M, de Groat WC. Prejunctional facilitatory alpha-1 adrenoceptors in the rat urinary bladder. Br J Pharmacol. 1995;114:1710–1716. doi: 10.1111/j.1476-5381.1995.tb14961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wada T, Hasegawa Y, Ono H. Characterization of alpha1-adrenoceptor subtypes in facilitation of rat spinal motoneuron activity. Eur J Pharmacol. 1997;340:45–52. doi: 10.1016/s0014-2999(97)01406-4. [DOI] [PubMed] [Google Scholar]
- 146.Sladek CD, Song Z. Regulation of vasopressin release by co-released neurotransmitters: mechanisms of purinergic and adrenergic synergism. Prog Brain Res. 2008;170:93–107. doi: 10.1016/S0079-6123(08)00409-3. [DOI] [PubMed] [Google Scholar]
- 147.Yuan WX, Chen SR, Chen H, Pan HL. Stimulation of alpha(1)-adrenoceptors reduces glutamatergic synaptic input from primary afferents through GABA(A) receptors and T-type Ca(2+) channels. Neuroscience. 2009;158:1616–1624. doi: 10.1016/j.neuroscience.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herold S, Hecker C, Deitmer JW, Brockhaus J. Alpha1-adrenergic modulation of synaptic input to Purkinje neurons in rat cerebellar brain slices. J Neurosci Res. 2005;82:571–579. doi: 10.1002/jnr.20660. [DOI] [PubMed] [Google Scholar]
- 149.Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci. 2006;26:3292–3298. doi: 10.1523/JNEUROSCI.4768-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Y, van den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Somogyi GT, Tanowitz M, Zernova G, de Groat WC. M1 muscarinic receptor-induced facilitation of ACh and noradrenaline release in the rat bladder is mediated by protein kinase C. J Physiol. 1996;496:245–254. doi: 10.1113/jphysiol.1996.sp021681. [DOI] [PMC free article] [PubMed] [Google Scholar]