Introduction
Vanadium is a transition metal that occurs naturally in a variety of minerals and exhibits an exceptional complex chemistry in solution, e.g., several oxidation states ranging from +2 to +5, and formation of vanadium oligomers such as decameric vanadate (+5) species [1–4]. Besides its metallurgical role in steel alloys, vanadium is also an ultra trace element known to participate in many biological processes and considered to be essential for living organisms [5, 6]. It accumulates in a variety of organisms ranging from microbes to vertebrates, where it modulates the activity of an array of key enzymes or participates as a cofactor in the active centre of others [1, 2, 5–9]. In mammals, vanadium compounds can mimic insulin action and may prevent chemical carcinogenesis, most probably through the inhibition of cellular tyrosine phosphatases and subsequent activation of signalling pathways, suggesting their use as pharmacological tools to treat human diabetes mellitus and cancer, respectively [10–14]. Anti-tumoral action of vanadium is, however, controversial as several studies have proposed that vanadium could act as a mitogen, tumor promoter and co-carcinogen (see [15] and references therein). Other studies have reported an osteogenic role for vanadium compounds and suggest that vanadium could also have a therapeutic application in bone-related diseases, such as osteoporosis [16–18]. Decades of research have thus provided evidence for vanadium's physiological and pharmacological properties, supporting the claim that it may represent a promising therapeutic agent for diseases targeting billions of human beings and affecting a wide range of pathological conditions. However, the development of vanadium-based pharmaceuticals will probably take some time since various issues related to vanadium toxicity, speciation and multiple targeting will need to be solved before advancing to clinical trials. Despite being used for decades by researchers as an inhibitor of protein tyrosine phosphatases, it is still not totally clear which vanadium species induce or which signalling pathways transduce physiological and pharmacological effects. Vanadium chemistry is complex, and different species or complexes may induce different pathways [5], affecting different biological processes. This work intends to review what is presently known about the bone-related role of vanadium in mammals and present recent in vitro data on the mineralogenic effect of vanadate in fish, which have become promising model organisms for vertebrate bone-related studies.
Physiological role of vanadium in bone development
The role of vanadium in bone development was first established in studies aiming at demonstrating its essentiality in diets, the primary source of vanadium in animals. Although most dietary vanadium is rapidly excreted in the urine and faeces, some retention occurs in various tissues or organs, principally in the bone, kidney, spleen and liver [19–22]. Studies in sheep, chicken and rodents have shown that bone is the main site of vanadium deposition [20, 23–29], and this is dependent on dietary intake, the chemical form of vanadium and, to some extent, the species [23, 24]. It has been proposed that the retention of dietary vanadium in bone may occur through the substitution of phosphate by vanadate within the hydroxyapatite lattice [19, 30], which could serve either as a detoxification mechanism or to build up bone crystals [30]. Other studies have shown that animals (principally chicken, goat, sheep, mouse and rat) fed a diet enriched or deficient in vanadium exhibited retarded skeletal development and severe bone deformities that could ultimately lead to death [31, 32]. These results suggest a major physiological role for dietary vanadium in bone development, further confirmed by data showing delayed ossification and skeleton malformation in mice exposed in utero to vanadium (see [33] and references therein). On the basis of in vivo data showing that dietary supplements of vanadium can (1) reverse symptoms of osteoporosis in streptozotocin-induced diabetic (STZD) rats [34, 35], (2) increase bone mineral density, mineralisation and formation in diabetic and non-diabetic rats [36], and (3) stimulate the activity of alkaline phosphatase, a marker for bone formation, in weanling rats [37], vanadium has been presented as a promising therapeutic agent to fight impaired bone formation in human diseases such as osteopenia and osteoporosis [38]. Although still under investigation, this bone-related pharmacological role of vanadium has been further evidenced by in vitro studies demonstrating its osteogenic properties (see below).
Pathways involved in vanadium intracellular signalling
In vitro cell systems capable of mineralising their extracellular matrix are suitable models to study the cellular mechanisms affecting mineralisation in a way resembling the in vivo processes. Mammalian and avian in vitro bone-derived cell systems have been used to identify cellular mechanisms and signalling pathways involved in the bone-related effects of vanadium compounds. It was shown, in particular, that vanadate, vanadyl (+4) and various vanadium complexes regulate osteoblast-like cell proliferation and differentiation [16, 39–43]. In general, low concentrations have a stimulatory effect, while higher amounts inhibit cell proliferation and alkaline phosphatase (ALP) activity, a marker commonly used to assess osteoblast differentiation. Vanadate was also shown to stimulate the synthesis of collagen, the main component of osteoblast extracellular matrix [39, 44]. Data on the effects of vanadium on signalling mechanisms in osteoblasts are sparse. A possible mechanism to explain vanadium effects on osteoblast proliferation and differentiation involves the inhibition of protein tyrosine phosphatases, which regulate the phosphorylation state of signalling molecules such as the mitogen-activated protein kinases (MAPK). This inhibition could result from the structural and chemical resemblance between vanadate and phosphate and the consequent effect on phosphate-metabolising enzymes [45–48]. Activation of the MAPK signalling pathway by vanadium compounds and subsequent regulation of osteoblast-specific gene expression by the extracellular signal-regulated kinase (ERK) has been reported in MC3T3-E1 osteoblast-like cells [16, 49], and the phosphatidylinositol-3-kinase (PI-3K) signalling pathway was shown to also partially transduce the vanadium effect on osteoblasts [16]. The activation of both MAPK and PI-3K pathways by vanadium compounds probably explain the insulin-mimetic action of vanadium [41, 43]. Indeed, both insulin and insulin-like growth factors (IGFs) achieve their biological effects through the activation of MAPK and PI-3K signalling pathways [50, 51], in particular the regulation of osteoblast proliferation and differentiation [52–55]. Surprisingly, the characterisation of bone-related effects of vanadium compounds and respective cellular mechanisms is exclusively restricted, in mammals, to the osteoblastic lineage; no in vitro data are available on the vanadium effect in osteoclast (bone resorption) and chondrocyte (endochondral ossification) lineages in mammals.
New in vitro data on bone-related effects of vanadium
Anthropogenic activities have led to an overall enrichment of vanadium in the marine environment (see [56] and references therein), exposing marine organisms to concentrations that could prevent their normal development. Because they might be particularly affected by an increase of vanadium in their environment and because they represent a suitable model to study vertebrate skeletal development, in particular mechanisms associated with bone formation and mineralisation, teleost fish have been recently used, as an alternative to classical model vertebrates, to investigate the biological role of vanadium [57, 58]. Toxicity levels have been determined and shown to largely depend on the mode of administration, concentration, time upon exposure and vanadate species [57, 58]. As reported for other vertebrates, vanadium accumulates upon exposure in various fish organs and tissues, in particular in bone [59], but to the best of our knowledge no in vivo studies demonstrating an effect of vanadium on fish bone development have been published so far. On the contrary, recent in vitro data clearly indicated that in fish, as in mammals, vanadium affects both proliferation and mineralisation of bone-derived cell lines.
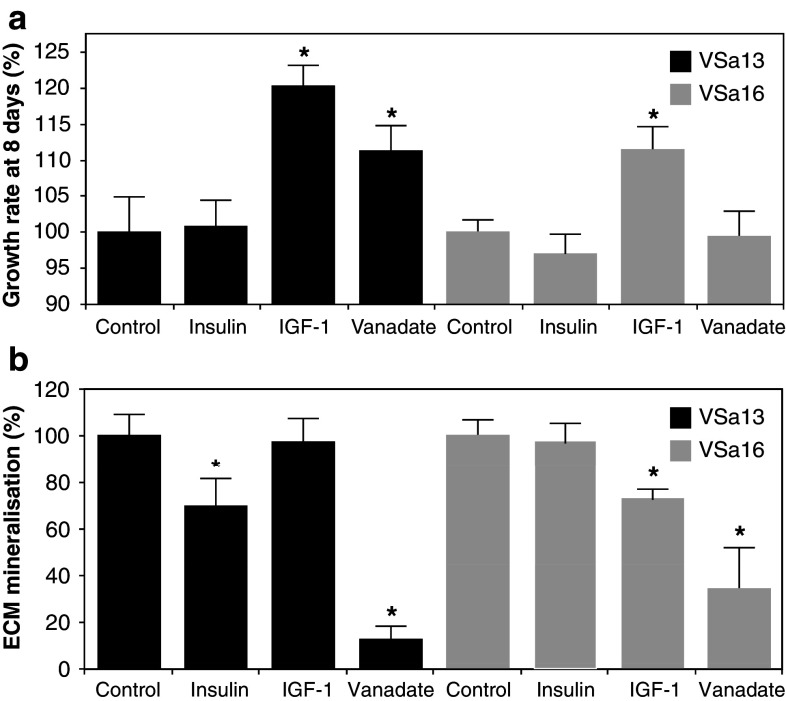
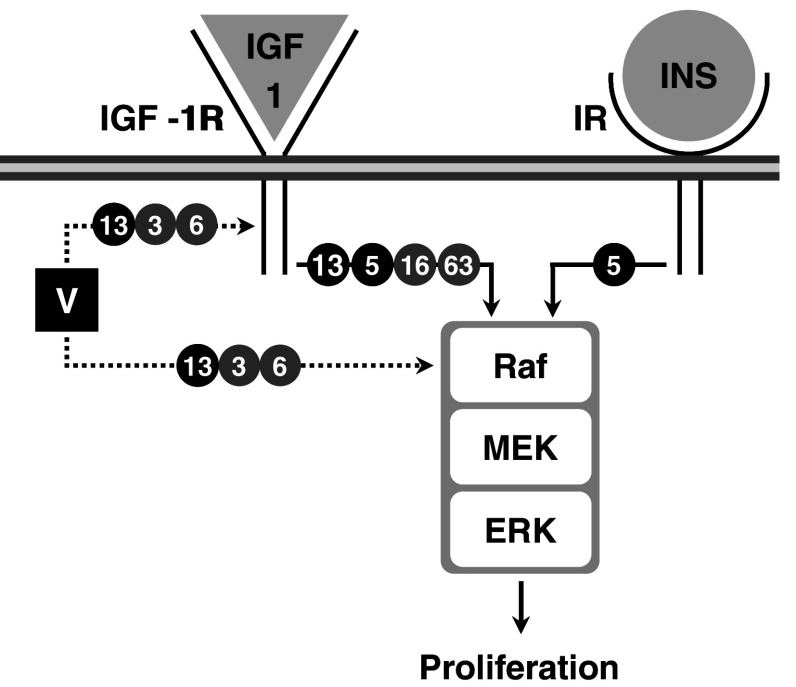
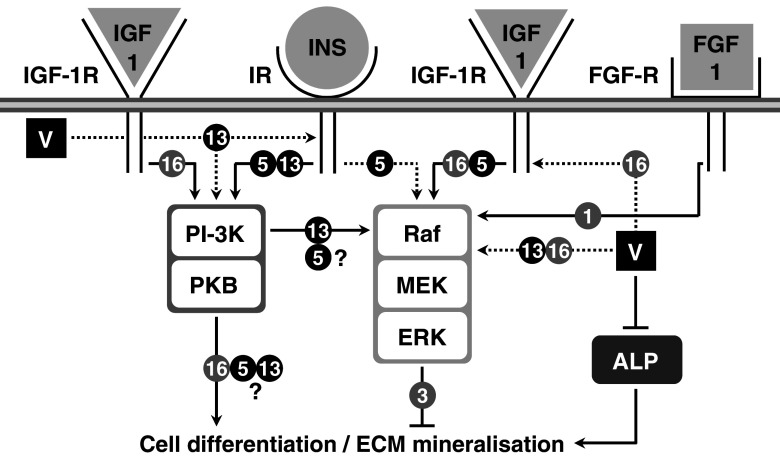
Two continuous cell lines—VSa13 and VSa16—have been recently developed from the calcified vertebra of the gilthead seabream (Sparus aurata L.), an important marine teleost fish for aquaculture and an emergent animal model to study vertebrate skeletal development [60]. Although representing different bone cell types—VSa13 cells have been associated with the chondrocyte lineage, while VSa16 cells would represent pre-osteoblasts—both cell lines are capable of mineralising their extracellular matrix under appropriate culture conditions [60]. These two cell lines, which represent the only bone-derived cell lines of fish origin so far developed, have been used in various studies aiming at unravelling mechanisms of mineralisation in fish, in particular those regulated by vanadium compounds [61–63]. Vanadium was shown to accumulate in fish bone-derived cells upon exposure to micromolar concentrations (5–7.5 µM total vanadium) of monomeric and decameric vanadate species [63] and to affect cellular proliferation and extracellular matrix (ECM) mineralisation [62]. While vanadate only stimulated chondrocyte proliferation, it strongly impaired ECM mineralisation of both chondrocytes and osteoblasts (Fig. 1). Similar proliferative and anti-mineralogenic effects were observed upon exposure to nanomolar concentrations of IGF-1 or insulin (Fig. 1), suggesting that vanadium compounds can mimic insulin-like activity in fish bone-derived cell lines. Similar effects were shown to be promoted by insulin and IGF-1 in osteoblast-like MC3T3-E1 cells [64] and chondrocyte-like ATDC5 cells [65, 66], suggesting that mechanisms associated with insulin-like action on the differentiation of bone-derived cell lines have been conserved throughout evolution and confirm the suitability of VSa13 and VSa16 cell lines to investigate vanadate insulin-like activity in bone. ECM mineralisation was differentially impaired in chondrocyte (~88%) and in osteoblast (~65%) upon vanadate treatment, demonstrating a bone cell type specificity [62]. Cellular mechanisms and signalling pathways involved in proliferative and anti-mineralogenic effects of vanadate in fish chondrocyte and osteoblast cells were investigated using specific inhibitors of the two main pathways previously associated with insulin-like effect in mammalian cell systems: PD98059, a specific inhibitor of the MAPK pathway, and wortmannin, a specific inhibitor of the PI-3K pathway. These results suggest that, under basal culture conditions, PI-3K and MAPK pathways are not activated during proliferation of VSa13 and VSa16 cells, but actively participate in cell differentiation and/or ECM mineralisation. Proliferative effects promoted by IGF-1 and vanadate in VSa13 cells and IGF-1 in VSa16 cells were reverted by PD98059 demonstrating the activation of MAPK pathway in both cell lines (Fig. 2; [62] and unpublished results), as previously reported for both factors in mammalian osteoblast [16, 49, 67, 68] and for IGF-1 in chondrocyte [65] lineages, further supporting the idea of conservation of growth-related insulin-like mechanisms throughout evolution and across cell types. Similarly, the MAPK pathway was also involved in anti-mineralogenic effect of insulin and vanadate in VSa13 cells, and IGF-1 and vanadate in VSa16 cells (Fig. 3; [62] and unpublished results), in agreement with recent data showing ERK inhibition of cell differentiation and/or ECM mineralisation in mammalian osteoblast [64] and chondrocyte [65, 66] lineages. Interestingly, the PI-3K pathway was also involved in the anti-mineralogenic effect of insulin and vanadate in VSa13 cells, and the activation of PI-3K\Ras\ERK pathway, as seen in mammalian MC3T3-E1 cells [64], was therefore proposed. This pathway was apparently not involved in the effects promoted by IGF-1 and vanadate in VSa16 cells. Incomplete reversion of the vanadate anti-mineralogenic effect by both PD98059 and wortmannin in fish chondrocytes suggested that other cellular mechanisms might be involved. ALP, by cleaving pyrophosphate, an inhibitor of ECM mineralisation [69], and collagen, an essential structural component of ECM [70], were considered as possible vanadium targets. While involvement of collagen was ruled out, enzymatic activity of ALP was decreased upon vanadate treatment and thus proposed to participate in its anti-mineralogenic effect as previously reported in mammals [8, 16, 17, 71]. Interestingly, none of these effects were promoted by insulin, suggesting that vanadate action was independent of insulin receptor or insulin signalling activation. In light of these results, an alternative mechanism was proposed for vanadate action on VSa13 cells ECM mineralisation, based on specific inhibition of ALP (Fig. 3), probably through substitution of phosphate intermediates during catalysis, as reviewed elsewhere [72].
Fig. 1.
Effect of vanadate, insulin and IGF-1 on VSa13 and VSa16 cell proliferation (a) and ECM mineralisation (b). Cell proliferation was evaluated after 8 days using MTS assay. Cells were seeded in 96-well plates at 1.5 × 103 cell/well then treated either with 10 nM insulin, 10 nM IGF-1, 7.5 µM vanadate or left untreated. Mineral deposition was revealed by von Kossa staining and evaluated by densitometry analysis. Cells were seeded in 24-well plates, grown to confluence then treated for mineralisation [60]. Mineralising cultures were subsequently treated with 10 nM insulin, 10 nM IGF-1, 5 µM vanadate or left untreated. Values are the mean of at least three independent experiments. Asterisks indicate values statistically different from their respective control (P < 0.05; one way ANOVA)
Fig. 2.
Putative mechanisms of action for vanadate proliferative effects in vertebrate bone-derived cell lines. Black circled 5 and 13 indicate pathways related to chondrocyte-like ATDC5 and VSa13 cell lines, respectively. Grey circled 3, 6, 16 and 63 indicate pathways related to osteoblast-like MC3T3-E1, UMR106, VSa16 and MG-63 cell lines, respectively. Black and dashed arrows indicate activated and putatively activated pathways, respectively. V indicates vanadate. Raf, MEK and ERK are intermediates in the MAPK pathway
Fig. 3.
Putative mechanisms of action for vanadate mineralogenic effects in vertebrate bone-derived cell lines. Black circled 5 and 13 indicate pathways related to chondrocyte-like ATDC5 and VSa13 cell lines, respectively. Grey circled 1, 3, 6, 16 and 63 indicate pathways related to osteoblast-like OB1, MC3T3-E1, UMR106, VSa16 and MG-63 cell lines, respectively. Black and dashed arrows indicate activated and putatively activated pathways, respectively. Intersected lines indicate inhibitory effect. V indicates vanadate. PI-3K and PKB are intermediates in the PI-3K pathway. Raf, MEK and ERK are intermediates in the MAPK pathway
Conclusions/perspectives
Interest in vanadium has been growing because of its significant physiological role in mammals and its promising pharmacological properties, in particular those related to diabetes, cancer and osteoporosis. Research on vanadium, however, is hampered by its complex chemistry and toxic effects at low concentrations. Fish have been recently recognised as a suitable alternative to mammalian systems to study vertebrate skeletal development, in particular for those mechanisms related to bone formation and tissue mineralisation. In addition, given the similarity observed in cellular responses between mammalian osteoblast and chondrocyte cell lines and the recently developed fish bone-derived cell lines VSa13 and VSa16, fish appears to be an adequate model organism for both in vivo and in vitro approaches to study vanadate-related mechanisms of action involved in bone and cartilage formation/maintenance. The recent data summarised in this review provide clear evidence for the evolutionary conservation of mechanisms involved in vanadate-mediated/related pathways between fish and mammals and further support the use of fish to study vanadate-related bone mechanisms.
Acknowledgments
This work was supported by CCMAR plurianual funding. DMT was the recipient of a postdoctoral fellowship (SFRH/BPD/45034/2008) from the Portuguese Science and Technology Foundation.
References
- 1.Chasteen ND. The biochemistry of vanadium. Struct Bond. 1983;53:105–138. doi: 10.1007/BFb0111304. [DOI] [Google Scholar]
- 2.Crans DC. Aqueous chemistry of labile oxovanadate: relevance to biological studies. Comments Inorg Chem. 1994;16:1–33. doi: 10.1080/02603599408035850. [DOI] [Google Scholar]
- 3.Rehder D. Inorganic considerations on the function of vanadium in biological systems. Met Ions Biol Syst. 1995;31:1–43. [PubMed] [Google Scholar]
- 4.Rehder D (ed) (2008) Bioinorganic vanadium chemistry. Inorganic chemistry. Wiley, London, pp 224
- 5.Aureliano M, Crans DC. Decavanadate (V10O28 6−) and oxovanadates: oxometalates with many biological activities. J Inorg Biochem. 2009;103:536–546. doi: 10.1016/j.jinorgbio.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Nechay BR, Nanninga LB, Nechay PSE, Post RL, Grantham JJ, Macara IG, Kubena LF, Phillips TD, Nielsen FH. Role of vanadium in biology. Fed Proc. 1986;45:123–132. [Google Scholar]
- 7.Tromp MGM, Olafsson G, Krenn BE, Wever R. Some structural aspects of vanadium bromoperoxidase from Ascophyllum nodosum . Biochim Biophys Acta. 1990;1040:192–198. doi: 10.1016/0167-4838(90)90075-q. [DOI] [PubMed] [Google Scholar]
- 8.Cortizo AM, Salice VC, Etcheverry SB. Vanadium compounds: their action on alkaline phosphatase activity. Biol Trace Elem Res. 1994;41:331–339. doi: 10.1007/BF02917433. [DOI] [PubMed] [Google Scholar]
- 9.Yoshinaga M, Ueki T, Yamaguchi N, Kamino K, Michibata H. Glutathione transferases with vanadium-binding activity isolated from the vanadium-rich ascidian Ascidia sydneiensis samea . Biochim Biophys Acta. 2006;1760:495–503. doi: 10.1016/j.bbagen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Brichard SM, Henquin J-C. The role of vanadium in the management of diabetes. Trends Pharmacol Sci. 1995;16:265–270. doi: 10.1016/S0165-6147(00)89043-4. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M. Vanadium: an element of atypical biological significance. Toxicol Lett. 2004;150:135–143. doi: 10.1016/j.toxlet.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Evangelou A. Vanadium in cancer treatment. Crit Rev Oncol Hematol. 2002;42:249–265. doi: 10.1016/S1040-8428(01)00221-9. [DOI] [PubMed] [Google Scholar]
- 13.Molinuevo MS, Barrio DA, Cortizo AM, Etcheverry SB. Antitumoral properties of two new vanadyl(IV) complexes in osteoblasts in culture: role of apoptosis and oxidative stress. Cancer Chemother Pharmacol. 2004;53:163–172. doi: 10.1007/s00280-003-0708-7. [DOI] [PubMed] [Google Scholar]
- 14.Thompson KH. Vanadium and diabetes. Biofactors. 1999;10:43–51. doi: 10.1002/biof.5520100105. [DOI] [PubMed] [Google Scholar]
- 15.Hulley P, Davison A. Regulation of tyrosine phosphorylation cascades by phosphatases: what the actions of vanadium teach us. J Trace Elem Exp Med. 2003;16:281–290. doi: 10.1002/jtra.10040. [DOI] [Google Scholar]
- 16.Cortizo AM, Molinuevo MS, Barrio DA, Bruzzone L. Osteogenic activity of vanadyl(IV)-ascorbate complex: evaluation of its mechanism of action. Int J Biochem Cell Biol. 2006;38:1171–1180. doi: 10.1016/j.biocel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Barrio DA, Etcheverry SB. Vanadium and bone development: putative signaling pathways. Can J Physiol Pharmacol. 2006;84:677–686. doi: 10.1139/Y06-022. [DOI] [PubMed] [Google Scholar]
- 18.Barrio DA, Cattaneo ER, Apezteguia MC, Etcheverry SB. Vanadyl(IV) complexes with saccharides. Bioactivity in osteoblast-like cells in culture. Can J Physiol Pharmacol. 2006;84:765–775. doi: 10.1139/Y06-021. [DOI] [PubMed] [Google Scholar]
- 19.Talvitie NA, Wagner WD. Studies in vanadium toxicology. Distribution and excretion of vanadium in animals. Arch Ind Hyg Occup Med. 1954;9:414–422. [PubMed] [Google Scholar]
- 20.Setyawati IA, Thompson KH, Yuen VG, Sun Y, Battell M, Lyster DM, Vo C, Ruth TJ, Zeisler S, McNeill JH, Orvig C. Kinetic analysis and comparison of uptake, distribution, and excretion of 48V-labeled compounds in rats. J Appl Physiol. 1998;84:569–575. doi: 10.1152/jappl.1998.84.2.569. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SQ, Zhong XY, Lu WL, Zheng L, Zhang X, Sun F, Fu GY, Zhang Q. Pharmacodynamics and pharmacokinetics of the insulin-mimetic agent vanadyl acetylacetonate in non-diabetic and diabetic rats. J Inorg Biochem. 2005;99:1064–1075. doi: 10.1016/j.jinorgbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Hathcock JN, Hill CH, Matrone G. Vanadium toxicity and distribution in chicks and rats. J Nutr. 1964;82:106–110. doi: 10.1093/jn/82.1.106. [DOI] [PubMed] [Google Scholar]
- 23.Berg LR, Lawrence WW. Cottonseed meal, dehydrated grass and ascorbic acid as dietary factors preventing toxicity of vanadium for chick. Poult Sci. 1971;50:1399–1404. doi: 10.3382/ps.0501399. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins LL, Mohr HE. Effect of vanadium deficiency on plasma cholesterol of chicks. Fed Proc. 1971;30:462. [Google Scholar]
- 25.Hansard SL, Ammerman CB, Fick KR, Miller SM. Performance and vanadium content of tissues in sheep as influenced by dietary vanadium. J Anim Sci. 1978;46:1091–1095. doi: 10.2527/jas1978.4641091x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma RP, Oberg SG, Parker RDR. Vanadium retention in rat-tissues following acute exposures to different dose levels. J Toxicol Environ Health. 1980;6:45–54. doi: 10.1080/15287398009529829. [DOI] [PubMed] [Google Scholar]
- 27.Parker RDR, Sharma RP, Oberg SG. Distribution and accumulation of vanadium in mice tissues. Arch Environ Contam Toxicol. 1980;9:393–403. doi: 10.1007/BF01055291. [DOI] [PubMed] [Google Scholar]
- 28.Mongold JJ, Cros GH, Vian L, Tep A, Ramanadham S, Siou G, Diaz J, McNeill JH, Serrano JJ. Toxicological aspects of vanadyl sulfate on diabetic rats––effects on vanadium levels and pancreatic B-cell morphology. Pharmacol Toxicol. 1990;67:192–198. doi: 10.1111/j.1600-0773.1990.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramanadham S, Heyliger C, Gresser MJ, Tracey AS, McNeill JH. The distribution and half-life for retention of vanadium in the organs of normal and diabetic rats orally fed vanadium(IV) and vanadium(V) Biol Trace Elem Res. 1991;30:119–125. doi: 10.1007/BF02990348. [DOI] [PubMed] [Google Scholar]
- 30.Etcheverry SB, Apella MC, Baran EJ. A model study of the incorporation of vanadium in bone. J Inorg Biochem. 1984;20:269–274. doi: 10.1016/0162-0134(84)85025-4. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen FH, Ollerich DA. Studies on a vanadium deficiency in chicks. Fed Proc. 1973;32:929. [Google Scholar]
- 32.Anke M. Vanadium––an element both essential and toxic to plants, animals and humans? Anal Real Acad Nac Farm. 2004;70:961–999. [Google Scholar]
- 33.Domingo JL. Vanadium: a review of the reproductive and developmental toxicity. Reprod Toxicol. 1996;10:175–182. doi: 10.1016/0890-6238(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa S. Insulin mimetic effects of vanadyl sulfate on bone status in streptozotocin-induced diabetic rats. Aichi Gakuin J Dent Sci. 1999;37:113–124. [Google Scholar]
- 35.Arai M. Effects of vanadyl sulfate on osteopenia in streptozotocin-induced diabetic (STZD) rats. Comparison with those of insulin. Nippon Yakurigaku Zasshi. 1992;100:401–414. doi: 10.1254/fpj.100.401. [DOI] [PubMed] [Google Scholar]
- 36.Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD. The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone. 2006;38:368–377. doi: 10.1016/j.bone.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi M, Oishi H, Suketa Y. Effect of vanadium on bone metabolism in weanling rats: zinc prevents the toxic effect of vanadium. Res Exp Med. 1988;189:47–53. doi: 10.1007/BF01856029. [DOI] [PubMed] [Google Scholar]
- 38.Cullinan GJ (1996) Vanadium compounds for inhibiting bone loss. Patent: EP0743065, USA
- 39.Lau KH, Tanimoto H, Baylink DJ. Vanadate stimulates bone cell proliferation and bone collagen synthesis in vitro. Endocrinology. 1988;123:2858–2867. doi: 10.1210/endo-123-6-2858. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RB, Henderson JS. Enhancement by sodium orthovanadate of the formation and mineralization of bone nodules by chick osteoblasts in vitro. Arch Oral Biol. 1997;42:271–276. doi: 10.1016/S0003-9969(97)00009-5. [DOI] [PubMed] [Google Scholar]
- 41.Cortizo AM, Etcheverry SB. Vanadium derivatives act as growth factor-mimetic compounds upon differentiation and proliferation of osteoblast-like UMR106 cells. Mol Cell Biochem. 1995;145:97–102. doi: 10.1007/BF00935481. [DOI] [PubMed] [Google Scholar]
- 42.Barrio DA, Braziunas MD, Etcheverry SB, Cortizo AM. Maltol complexes of vanadium (IV) and (V) regulate in vitro alkaline phosphatase activity and osteoblast-like cell growth. J Trace Elem Med Biol. 1997;11:110–115. doi: 10.1016/S0946-672X(97)80035-1. [DOI] [PubMed] [Google Scholar]
- 43.Etcheverry SB, Crans DC, Keramidas AD, Cortizo AM. Insulin-mimetic action of vanadium compounds on osteoblast-like cells in culture. Arch Biochem Biophys. 1997;338:7–14. doi: 10.1006/abbi.1996.9778. [DOI] [PubMed] [Google Scholar]
- 44.Mahanti HS, Barnes RM. Determination of major, minor and trace elements in bone by inductively-coupled plasma emission spectrometry. Anal Chim Acta. 1983;151:409–417. doi: 10.1016/S0003-2670(00)80103-8. [DOI] [Google Scholar]
- 45.Stankiewicz P, Tracey A, Crans D. Inhibition of phosphate-metabolizing enzymes by oxovanadium(V) complexes. Met Ions Biol Syst. 1995;31:287–324. [PubMed] [Google Scholar]
- 46.Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, Fantus IG, Ng JB, Hall DA, Lum BS. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- 47.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 48.Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982;107:1104–1109. doi: 10.1016/0006-291X(82)90635-0. [DOI] [PubMed] [Google Scholar]
- 49.Barrio DA, Williams PAM, Cortizo AM, Etcheverry SB. Synthesis of a new vanadyl(IV) complex with trehalose (TreVO): insulin-mimetic activities in osteoblast-like cells in culture. J Biol Inorg Chem. 2003;8:459–468. doi: 10.1007/s00775-002-0438-z. [DOI] [PubMed] [Google Scholar]
- 50.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeRoith D. Insulin-like growth factor I receptor signalling––overlapping or redundant pathways? Endocrinology. 2000;141:1287–1288. doi: 10.1210/en.141.4.1287. [DOI] [PubMed] [Google Scholar]
- 52.Hatakeyama N, Kojima T, Iba K, Murata M, Thi MM, Spray DC, Osanai M, Chiba H, Ishiai S, Yamashita T, Sawada N. IGF-I regulates tight-junction protein claudin-1 during differentiation of osteoblast-like MC3T3-E1 cells via a MAP-kinase pathway. Cell Tissue Res. 2008;334:243–254. doi: 10.1007/s00441-008-0690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol. 2008;215:442–451. doi: 10.1002/jcp.21323. [DOI] [PubMed] [Google Scholar]
- 54.Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122:254–260. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 55.Li S-H, Guo D-Z, Li B, Yin H-B, Li J-K, Xiang J-M, Deng G-Z. The stimulatory effect of insulin-like growth factor-1 on the proliferation, differentiation, and mineralisation of osteoblastic cells from Holstein cattle. Vet J. 2009;179:430–436. doi: 10.1016/j.tvjl.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Hope BK (2008) A dynamic model for the global cycling of anthropogenic vanadium. Global Biogeochem Cycles 22, GB4021
- 57.Soares SS, Martins H, Gutierrez-Merino C, Aureliano M. Vanadium and cadmium in vivo effects in teleost cardiac muscle: metal accumulation and oxidative stress markers. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:168–178. doi: 10.1016/j.cbpc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Soares SS, Martins H, Duarte RO, Moura JJG, Coucelo J, Gutierrez-Merino C, Aureliano M. Vanadium distribution, lipid peroxidation and oxidative stress markers upon decavanadate in vivo administration. J Inorg Biochem. 2007;101:80–88. doi: 10.1016/j.jinorgbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Söremark R. Vanadium in some biological specimens. J Nutr. 1967;92:183–190. doi: 10.1093/jn/92.2.183. [DOI] [PubMed] [Google Scholar]
- 60.Pombinho AR, Laizé V, Molha DM, Marques SMP, Cancela ML. Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004;315:393–406. doi: 10.1007/s00441-003-0830-1. [DOI] [PubMed] [Google Scholar]
- 61.Tiago DM, Laizé V, Aureliano M, Cancela ML. Impairment of mineralization by metavanadate and decavanadate solutions in a fish bone-derived cell line. In: Aureliano M, editor. Vanadium biochemistry. Kerala: Research Signpost; 2007. pp. 269–283. [DOI] [PubMed] [Google Scholar]
- 62.Tiago DM, Cancela ML, Aureliano M, Laizé V. Vanadate proliferative and anti-mineralogenic effects are mediated by MAPK and PI-3K/Ras/Erk pathways in a fish chondrocyte cell line. FEBS Lett. 2008;582:1381–1385. doi: 10.1016/j.febslet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 63.Tiago DM, Laizé V, Cancela ML, Aureliano M. Impairment of mineralization by metavanadate and decavanadate solutions in a fish bone-derived cell line. Cell Biol Toxicol. 2008;24:253–263. doi: 10.1007/s10565-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 64.Kono SJ, Oshima Y, Hoshi K, Bonewald LF, Oda H, Nakamura K, Kawaguchi H, Tanaka S. Erk pathways negatively regulate matrix mineralization. Bone. 2007;40:68–74. doi: 10.1016/j.bone.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Phornphutkul C, Wu K-Y, Yang X, Chen Q, Gruppuso PA. Insulin-like growth factor-I signaling is modified during chondrocyte differentiation. J Endocrinol. 2004;183:477–486. doi: 10.1677/joe.1.05873. [DOI] [PubMed] [Google Scholar]
- 66.Phornphutkul C, Wu KY, Gruppuso PA. The role of insulin in chondrogenesis. Mol Cell Endocrinol. 2006;249:107–115. doi: 10.1016/j.mce.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Zhang WY, Lee JC, Kumar S, Gowen M. ERK pathway mediates the activation of Cdk2 in IGF-1-induced proliferation of human osteosarcoma MG-63 cells. J Bone Miner Res. 1999;14:528–535. doi: 10.1359/jbmr.1999.14.4.528. [DOI] [PubMed] [Google Scholar]
- 68.Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–4893. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]
- 69.Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. Biochem J. 2002;364:329–341. doi: 10.1042/BJ20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canalis E. Effect of growth factors on bone cell replication and differentiation. Clin Orthop Rel Res. 1985;193:246–263. [PubMed] [Google Scholar]
- 72.Crans DC, Smee JJ, Gaidamauskas E, Yang LQ. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev. 2004;104:849–902. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]