Abstract
Recent studies revealed that the neuronal network controlling overt rhythms shows striking similarity in various insect orders. The pigment-dispersing factor seems commonly involved in regulating locomotor activity. However, there are considerable variations in the molecular oscillatory mechanism, and input and output pathways among insects. In Drosophila, autoregulatory negative feedback loops that consist of clock genes, such as period and timeless are believed to create 24-h rhythmicity. Although similar clock genes have been found in some insects, the behavior of their product proteins shows considerable differences from that of Drosophila. In other insects, mammalian-type cryptochrome (cry2) seems to work as a transcriptional repressor in the feedback loop. For photic entrainment, Drosophila type cryptochrome (cry1) plays the major role in Drosophila while the compound eyes are the major photoreceptor in others. Further comparative study will be necessary to understand how this variety of clock mechanisms derived from an ancestral one.
Keywords: Insect, Circadian rhythm, Clock genes, Entrainment, Molecular mechanism, Neural network
Introduction
Circadian rhythms of about 24-h periodicity are commonly observed in a variety of physiological functions of organisms from bacteria to humans. The rhythm is governed by a mechanism called the circadian system, generally including a circadian clock that generates a 24-h oscillation, a photoreceptor that is necessary for the clock to synchronize to the light–dark cycles (LD), and an output system that relays the clock information to various tissues to regulate their overt rhythmicity [1, 2].
The rhythm has some common, basic characteristics: it persists for long periods in constant conditions; its period is temperature-compensated and maintains a similar value over a wide range of temperatures; and it synchronizes to daily environmental cycles, using light and temperature as major synchronizing agents or zeitgebers. Insects provide good models for dissecting the circadian system both at the physiological and molecular levels. The circadian clock that regulates the overt behavioral rhythms has been localized in the brain. For example, the optic lobe is the clock locus for crickets, cockroaches, and beetles [3–5], while the central brain is known for moths, flies, and mosquitoes [6–8]. The compound eyes are known as the principal circadian photoreceptor for photic entrainment in most insects, but ocelli and the extraretinal photoreceptors also play a role in others (see [2]).
Recent studies have promoted our understanding of the circadian system at molecular and cellular levels. The circadian oscillatory mechanism has now been uncovered at the molecular level, and the circadian network for generating rhythmicity has been elucidated in detail in fruit flies [9–12]. Molecular and cellular dissection has also been started in other non-drosophilid insects, promoting the comparative and general formulation of the underlying mechanism [13]. This review will focus on our current understanding of the molecular and cellular mechanism of the insect circadian system.
The circadian clock network
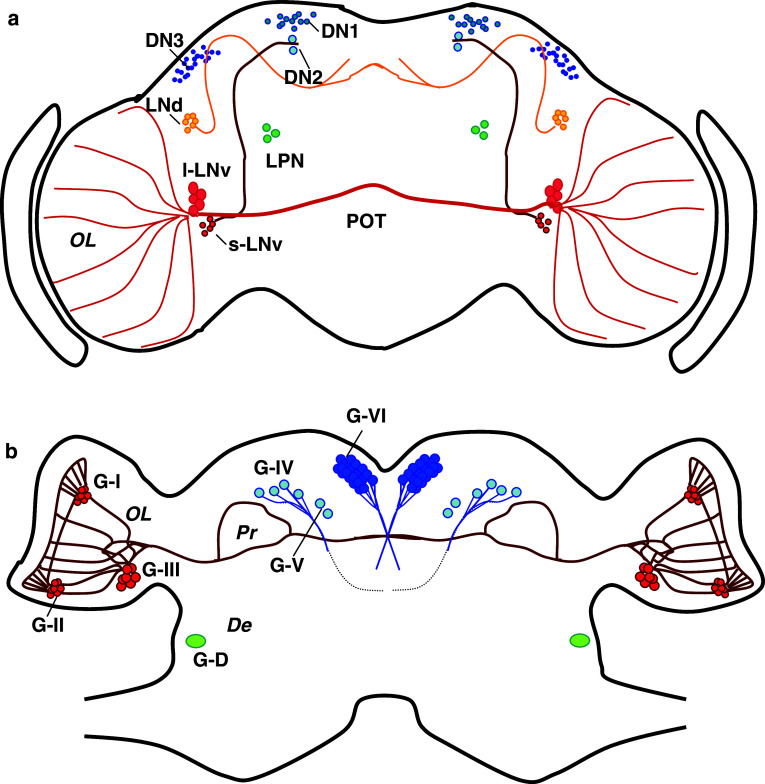
Cellular organization of the clock has been extensively studied in Drosophila and other insects, using molecular probes, such as antibodies against clock gene products and reporter genes driven by clock gene promoter, etc. In Drosophila, there are about 150 cells in the brain that express the clock genes, and they are divided into seven subgroups [10, 14] (Fig. 1a). Three of them are located laterally between the optic lobe and the central brain, and are called lateral neurons (LNs). The ventrally located LNs consist of two subgroups of neurons with larger and smaller cell bodies and are called large-LNv (l-LNv) and small-LNv (s-LNv), respectively. The dorsally located LNs are called LNd. There are three groups of neurons in the dorsal region of the central brain called DN1, DN2, and DN3, respectively. The other group is located on the lateral posterior side of the central brain, and is called lateral posterior neurons (LPNs). Detailed analysis of the role of the cells revealed that s-LNvs drive the morning peak and a part of the evening activity, and the LNds regulate the evening peak [15–17]. Recently, l-LNv cells have been revealed to be involved in light-induced arousal and phase-shifting in the late night [18]. DNs, however, have some roles in locomotor rhythms, since the flies lacking LNs still show a circadian locomotor rhythm for at least a few days in constant conditions [19–21]. In larval brain, DN2 cells have the principal role in temperature entrainment and might regulate the phase of LNs, while under LD, the larval DN2 s are controlled by LNs via PDF signaling [22].
Fig. 1.
Schematic diagrams showing Drosophila (a) and cockroach (b) cerebral cells expressing PERIOD (redrawn from [23, 125]). a In Drosophila, there are seven main groups of neurons. Three groups (DN1, DN2, and DN3) are located in the dorsal region, and the remaining four (LNd, l-LNv, s-LNv, and LPN) are located laterally. The s-LNv and l-LNv express a neuropeptide, PDF. l-LNvs have their processes in the optic lobe and send their axonal projection to the contralateral optic lobe through the posterior optic tract (POT). s-LNvs have axonal projection to the dorsomedial region of the protocerebrum. b In the cockroach, Blattera germanica, three groups of neurons are located in the optic lobe (G-I, G-II, and G-III) and three groups in the dorsal protocerebrum (G-IV, G-V, and G-VI). There is another group (G-D) in the deutocerebrum. PDF is coexpressed in G-I, -II, -III, -IV and -D cells. OL Optic lobe, Pr protocerebrum, De deutocerebrum
Virtually similar clock structures are reported for other insects, including the blow flies, crickets, and cockroaches [23–25]. In the cockroaches and crickets, some of the PER-immunoreactive neurons are located in the optic lobe (Fig. 1b): These probably correspond to the LNvs in Drosophila. Their importance for overt activity rhythm generation is implicated by various surgical and electrophysiological experiments [4, 26–28]. Like in DNs of Drosophila, the neurons located in the dorsal protocerebrum may also play a role in the control of overt activity rhythms, because some residual rhythms are often observed even after optic lobe removal [29], and temperature or light cycles often induce a rhythm with circadian properties [30–32].
Molecular machinery of the circadian clock
Irrespective of the similarity in the neuronal architecture of the circadian clock system, recent studies have revealed considerable differences in the molecular mechanisms among insects.
Drosophila’s clock machinery
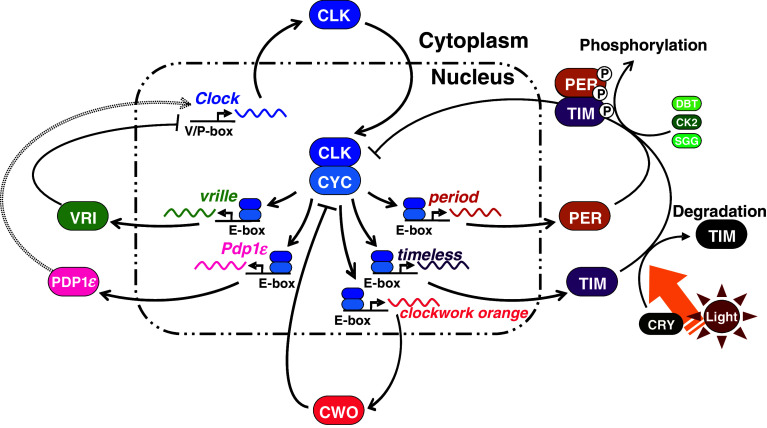
The molecular machinery of the circadian clock has been extensively studied in the fruit fly, Drosophila melanogaster. There are several so-called clock genes involved in the generation of the rhythmicity [9, 13]. At least three interdependent feedback loops are thought to constitute the rhythm-generating machinery (Fig. 2). One major loop is that formed by period (per), timeless (tim), Clock (Clk), and cycle (cyc) (cf. [9, 33]). The transcription of per and tim is activated by product proteins of Clk (CLK) and cyc (CYC). CLK and CYC form a heterodimer and bind to a promoter region, called E-box, of per and tim and promote their transcription during late day to early night. The product proteins, PER and TIM, increase during the night to peak at late night, when their heterodimeric complex enters into the nucleus to repress their own transcription through inhibitory action on CLK-CYC. The repression of per and tim transcription results in reduced level of PER and TIM, which eventually releases CLK-CYC from the repression to reactivate the transcription of per and tim. This reactivation leads the loop to the next round.
Fig. 2.
A scheme illustrating current knowledge about molecular machinery of the Drosophila circadian clock. The circadian clock consists of three feedback loops that are interlocked to oscillate with a period of about 24 h. See text for details
The second loop is for a circadian oscillation of Clk [34, 35]. Besides per and tim, CLK-CYC activates transcription of vrille (vri) and PAR domain protein 1ε (Pdp1ε) during late day to early night. The vri mRNA is soon translated to its product protein VRI, which enters the nucleus and inhibits transcription of CLK by binding to a promoter region, V/P-box. Thus the Clk mRNA is reduced during the night. The translation of Pdp1ε occurs in a rather delayed manner and PDP1ε protein increases during late night to early day. PDP1ε is thought to bind to V/P-box competitively with VRI and activate the transcription of Clk. Thus, the Clk transcripts increase during the day, also leading to an increase of CLK protein during the day. But a recent study revealed that PDP1ε is not essential for the operation of the loop [36].
The third loop includes clockwork orange (cwo), which is a transcriptional repressor belonging to the basic helix-loop-helix ORANGE family [37–40]. cwo is rhythmically expressed to peak under the regulation by CLK-CYC and forms its own negative feedback loop. CWO represses the expression of other clock genes, such as per and tim, through E-box elements. This negative feedback loop contributes to sustaining a high-amplitude circadian oscillation.
For precise temporal control of the molecular oscillation and for function of the molecular oscillator, posttranslational regulation of clock proteins seems crucial [9, 41, 42]. For example, the cyclic activation and inhibition of CLK/CYC is paralleled by cyclic phosphorylation levels [43]. Phosphorylation may regulate the timing of nuclear entry of PER and TIM [44, 45], as well as the degradation of TIM [46]. These posttranslational modifications are suggested to regulate the period, phase, and amplitude of the circadian clock [41].
Recent studies showed that in Drosophila, electrical silencing of clock neurons stops the circadian locomotor rhythm free-running in constant darkness (DD) [47]. Immunohistochemical studies revealed that this results from a cessation of the clock itself caused by absence of nighttime nuclear transport of TIM protein from the cytoplasm [47]. A similar stop of the clock was found in larvae [48]. Thus, the electrical activity is thought to be an essential element of the molecular clock in the cerebral pacemaker neurons. However, the molecular oscillation persists under LD in the flies with electrical silencing of clock neurons, suggesting that there is a light-dependent drive on the molecular clock that at least partially compensates electrical activity in LD [47].
Results in other insects do not fully support the Drosophila hypothesis
The progress of clock study in Drosophila has promoted research on molecular machinery of the clock in other insect species [13, 49]. The studies, however, revealed some significant discrepancies between Drosophila and others. In the silkmoth, Antheraea pernyi, and the cockroach, Blattera germanica, immunohistochemisty with anti-PER and anti-TIM revealed that these proteins always stay in the cytoplasm and never enter into the nucleus [23, 50]. Instead of negative feedback of product proteins, rhythmically expressed per antisense RNA is proposed to produce the rhythmicity in A. pernyi [51]. However, immunohistochemistry may only reveal large amounts of the proteins, and a trace amount may enter into the nucleus and repress transcription [52]. Another explanation may be that, instead of PER-TIM, CRY2, which is a mammalian type CRY [53], enters the nucleus and works as a transcriptional repressor [54, 55]. These still need to be experimentally examined.
Recently, reverse genetic approaches have been applied to those non-model insects. In many of them, cDNAs of canonical clock genes have been cloned and their expressions have been examined. In most of the tested insects, the clock genes, per, tim, and Clk, are shown to cycle in a daily or circadian fashion [50, 55–57]. Functions of some clock genes have been examined in a cultured cell system in the monarch butterfly, Danaus plexippus [55], and CRY2 seems to repress the transcriptional activity of CLK-CYC in vitro [55]. cry belongs to a photolyase family and plays a role in photic entrainment in Drosophila [58, 59]. Molecular evolutionary studies have revealed that gene duplication and loss have resulted in three modes of cry gene expression [54]; in some insects, either cry1 (Drosophila type cry) or cry2 (mammalian type cry) is expressed, and in others, both cry1 and cry2 are expressed (Table 1). In the honey bee, Apis merifella, tim does not exist in the genome, and cry2 is thought to act as a transcriptional repressor like in Danaus [60]. Thus, there seem to be considerable differences in the molecular oscillatory mechanism among insects.
Table 1.
Two types of cryptochrome in insects
| Order | Species | Drosophila type (cry1) | Mammalian type (cry2) |
|---|---|---|---|
| Lepidoptera | Antheraea pernyi | AF333998 | EF117812 |
| Danaus plexippus | AY860425 | DQ184682 | |
| Mamestra brassicae | AY947639 | ||
| Sesamia nonagrioides | DQ243704 | DQ243705 | |
| Spodoptera littoralis | EF364035 | EF396286 | |
| Diptera | Anopheles gambiae | DQ219482 | DQ219483 |
| Culex quinquefasciatus | B0WRR9 | XM_001869421 | |
| Bactrocera tryoni | AY708049 | ||
| Drosophila melanogster | NM_169852 | DNE | |
| Sarcophaga bullata | FJ373353 | ||
| Sarcophaga crassipalpis | AB079536 | ||
| Hymenoptera | Apis mellifera | DNE | EF117814 |
| Bombus impatiens | EF110521 | ||
| Nasonia vitripennis | XM_001606355 | ||
| Coleoptera | Tribolium castaneum | DNE | EF117815 |
| Hemiptera | Acyrthosiphon pisum | XM_001944367 | XM_001950658 |
| Riptortus pedestris | AB379863 | ||
| Orthoptera | Dianemobius nigrofasciatus | AB291231 |
GenBank accession number is indicated. DNE Does not exist
In crickets, the role of clock genes has been examined in a more direct way using RNA interference (RNAi) [61–63]. Like other insects, the expression of cricket’s per gene cycles in a daily fashion in both LD and DD, and peaks at early night. Double stranded RNA (dsRNA) of per knocks down per mRNA levels, and disrupts both its cycling and the locomotor activity rhythm [61]. Functional analysis with RNAi of other clock genes will clarify the molecular oscillatory mechanism in the cricket. However, so far the molecular dissection of the oscillatory mechanism has not been performed in enough species to illustrate its divergence.
Input pathways
Light entrainment
Light is the most important zeitgeber for the circadian clock to synchronize to the environmental cycles. Photoreceptors necessary for the entrainment, or resetting, of the clock have been localized in some insects. In hemimetabolous insects such as crickets and cockroaches, the compound eye is the most important photoreceptor [28, 64], but ocelli also sometimes have minor roles in the photic entrainment [65, 66]. In Drosophila, in addition to these external eyes, H–B eyelet that is a remnant of larval eye, and the blue light receptor molecule CRY1, are known for circadian photoreceptors [66].
In Drosophila, CRY1 is expressed in a circadian fashion in some of the cerebral clock neurons, and shifts the phase of the clock light-dependently [58, 67, 68]. It is known to bind TIM in a light-dependent manner and to lead to TIM’s degradation [68]. This light-dependent degradation of TIM results in a phase shift of the clock in a phase-dependent manner: A reduced level of TIM in the evening leads to a delay of the clock at certain times until TIM increases to a required level, while it causes an advance of the clock in the morning to the time at which the TIM’s level coincides [69, 70]. The phase-dependent phase shifts provide a base of non-parametric entrainment. Danaus CRY1 partially rescued light-induced phase shiftability in Drosophila cry b mutant flies, while CRY2 did not [55]. Experiments using RNA interference revealed that, in DpN1 cells, the light-induced decrease in TIM abundance is mediated through Danaus CRY1 [53]. Thus, CRY1 seems to mediate the light entrainment in the monarch butterfly.
Besides CRY1, rhodopsins (Rhs) are also involved in photic entrainment. In Drosophila, Rh1 and Rh6 were implicated in entrainment to red light [71], and Rh1, Rh5, and Rh6 to green and yellow light [72]. Although histamine is suggested to be an important neurotransmitter for the Rh pathway [73], other neurotransmitters and the intracellular signal pathways have yet to be elucidated. Details of what role photoreceptor molecules play in photic entrainment of other insects and their divergence among insect species deserve to be investigated.
Temperature entrainment
Temperature is also an important time cue to synchronize the clock, especially in dark places, such as eggs laid underground or insects that pupate in darkness. Temperature cycle or temperature steps are known to cause phase shifts of the clock to adjust to the time of environmental cycles [74, 75]. The molecular mechanism for the temperature synchronization is less understood. In Drosophila, the effects of temperature step-up and -down were examined at transcript levels [76]. Under constant light, temperature step-up up-regulates the Clk gene, and step-down down-regulates it. In contrast, per, tim, vri, and Pdp1ε genes are down-regulated and up-regulated by step-up and step-down, respectively. After temperature step-down, all these clock genes showed an oscillation in constant conditions. These temperature responses are virtually eliminated in Clk Jrk mutant flies, suggesting that the Clk gene is the principal component for temperature entrainment [76]. Although the temperature sensing system is still largely unclear, it might involve nocte and PLC, mutation of which disrupts temperature entrainment [77]. PLC was shown to have a role in temperature-dependent per splicing [78, 79]: Under low temperature conditions, splicing of an intron in the 3′ untranslated region of per is enhanced, leading to a rapid accumulation of PER protein and an advance of the phase of the evening activity [80]. In addition, tim mRNA levels are increased earlier at low temperatures through photic stimulation of tim expression [81].
Existence of temperature entrainable oscillators has been reported for other insects including crickets [75], cockroaches [31], and mosquitoes [82]. Unfortunately, no detailed investigation on molecular and cellular mechanisms of temperature entrainment is available for insects other than Drosophila.
Output pathways
The pathways through which the clock regulates overt rhythms are not well understood. Pigment-dispersing factor (PDF) is the best characterized output molecule of the circadian clock system. Its involvement was first notified when immunohistochemistry revealed that PDF colocalized with PER in some clock neurons, s-LNv and l-LNv, in the Drosophila brain (Fig.1) [83], and is now thought to be an output neurotransmitter of the circadian clock in a wide variety of insects. It is a member of the pigment dispersing hormone (PDH), an octadeca peptide that was first found in crustacea as a hormone that disperses pigments in the epithelium [84]. pdf gene expression shows no circadian rhythm in Drosophila [85], while the dorsomedial termini of PDF-expressing neurons show daily morphological changes probably controlled by the circadian clock [86]. pdf 01 mutant flies that lack PDF show a rhythm with advanced onset of the evening activity and become arrhythmic within a few days in DD [87]. PDF is required to adjust cycling amplitude, period, and phase in a variety of clock neurons in the brain [88–90] in accordance with the expression of PDF receptors in those neurons [91–93]. Also, like in Drosophila, PDF in cockroaches is both a phase regulator and a locomotor rhythm driver [94, 95]. In crickets, however, since the partial destruction of the optic lobe leads to arrhythmic locomotor activity without elimination of the PDF neurons, PDF may play only minor roles in the behavioral rhythm [96]. Besides regulation of the activity rhythms, PDF plays an important role in the sensory system of crickets. When injected into the optic lobe, PDF is revealed to increase the photoresponsiveness of the optic lobe visual interneurons during early night [97]. In fact, the optic lobe content of PDF increases during the evening [98]. The PDF-induced increase of photoresponsiveness might be associated with the morphological changes reported for flies [99], because the rate of impulse propagation depends on the axonal diameter in unmielinated neurons.
Pdp1ε and takeout (to) have been located downstream of the circadian clock in Drosophila. When the PDP1ε level was kept constantly higher or lower in clock neurons, the clock runs normally but locomotor activity rhythms are disrupted [36]. Thus, PDP1ε seems to drive behavioral rhythms when some specific genes are controlled by its binding to V/P-box. to is expressed in the inner part of cardia and the crop, under the regulation by the circadian clock, in response to starvation. The to expression is arrhythmic in arrhythmic mutants, per01, tim01, ClkJrk and cyc01. to mutant flies show aberrant locomotor activity and die quickly upon starvation. Based on these results, to is concluded to be a clock-controlled output gene controlling feeding behavior [100]. Recently, this link between TO and feeding is suggested to occur through juvenile hormones [101]. Involvement of Pdp1ε and to deserves to be examined in other insects. Besides these, mRNA levels of hundreds of other genes have been shown to oscillate [39, 102]. Some of these genes may be involved in the output pathways of the clock, inducing a structural daily remodeling of the output circuit [86].
Peripheral oscillators
It has been reported many times that circadian oscillators exist in various tissues of insects (see [2]). For example, physiological studies revealed that sperm release from testis to the associated vas deferens, as well as ecdysone secretion from the prothoracic gland, was controlled in a circadian manner by peripheral oscillators in moths (see [103]).
In Drosophila, per-driven luciferase activity in a transgenic reporter strain showed that per is rhythmically expressed in cells of the compound eye, antennae, proboscis, wings, and legs [104]. Basically, similar results were observed in cells of the ring gland and Malpighian tubules (Mts), which are tissues for endocrine and excretory activity in flies, respectively [105, 106]. Rhythmic expression of per in these cells continues in vitro and is able to entrain to the light–dark regime of the culture condition. Transplanted Mts maintain their original phase of oscillation even in the host flies which have been entrained to the reverse light–dark cycle to the donor [107]. Thus, these peripheral oscillators seem to function as a stand-alone pacemaker. The same is true for the olfaction rhythm as an output of the antennal clock [108], and for the circadian synaptic plasticity rhythm, which was recently found as the rhythmic change of size in synaptic boutons [86, 109].
These peripheral tissues are thought to have a similar but not identical molecular machinery of circadian oscillation shown in the central pacemaker described above. One remarkable difference is CRY1’s function. A loss of function mutant of CRY1, cry b, showed intact molecular oscillations of PER and TIM in s-LNv, but lost the molecular oscillation in Mts [110]. In the antennae, the rhythmic electroantennographic (EAG) responses to odorants were also abolished in the mutants [111]. These results indicate that, instead of a photoreceptor in the central pacemaker, CRY1 functions as a core component in these peripheral oscillators [112]. Unlike these, however, another peripheral rhythm, which was recently found in the cuticle deposition, persisted in cry mutant flies [113]. Thus, there are at least two kinds of circadian molecular machinery in peripheral oscillators: one that requires CRY1 as an essential component, and the other that includes it just as a photoreceptor.
The Drosophila antennal clock seems to be one of the best models to investigate how peripheral oscillators control output phenomena at the molecular and behavioral levels. Rhythmic expressions of clock gene products have been observed in the antenna of tephritid fruit flies [114] and moths [115–117]. Thus, the molecular machinery of antennal oscillator seems common principally in insects. Detailed studies in Drosophila have revealed that rhythmic expression of G-protein receptor kinase, regulated by the circadian clock, controls the EAG amplitude in antennae [108, 118, 119]. Although circadian EAG rhythms have also been reported for cockroaches [120] and moths [121, 122], the regulatory mechanism differs substantially from that of Drosophila described above. The EAG rhythms in cockroaches are driven by the central pacemaker, even though individual olfactory receptor neurons exhibit circadian rhythms independent of the central pacemaker [123]. Circadian pheromonal responses in male turnip moths are not controlled at the antennal level [121]. In hawkmoths, octopamine and tyramine rhythmically modulate the sensitivity of olfactory sensilla for pheromone-sensitivity, but have no effect on EAG amplitudes [124]. Thus, it is noteworthy that the scenario illustrated in Drosophila cannot always be applied in other insects.
Conclusions
Recent physiological and molecular studies on insect circadian rhythms considerably promoted our understanding of the underlying cellular and molecular mechanisms. The cellular network of circadian clock systems shows virtually similar structures among insects, and PDF is commonly involved as an output neuromodulator/neurotransmitter of the system. The molecular oscillatory mechanism is considered to consist of autoregulatory negative feedback loops based mainly on the study of Drosophila. However, with the recent knowledge on other insect species, the structure of the feedback loop should be reconsidered. Although most insects examined possess common canonical clock genes, some have additional genes and some lose certain genes. Perhaps the most striking finding is the involvement of cry2 as a transcriptional repressor [53, 54]. In this context, CRY2 might enter the nucleus in insects where nuclear entry of PER and TIM was not detected by immunocytochemistry, and this deserves to be addressed in future studies. In addition, CRY1 may play a major role in photic entrainment in dipteran and lepidopteran species, but rhodopsins in the external photoreceptors seems to be the major photoreceptor in orthopteran and dyctiopteran species. The molecular mechanisms for photic entrainment through the external photoreceptor pathway remain to be examined. The insect world is estimated to include nearly one million species with highly diverged morphology and physiology to adapt to various environments. Thus, it is likely that the circadian clock system also diverged from an ancestral one, in respect to the core oscillatory mechanism, as well as the photic entrainment pathways. Although information is only available for quite limited orders of the class insecta, future comparative studies should provide an insight for the general understanding of the insect clock mechanism as well as how the variety of clocks have diversified from an ancestral one.
Acknowledgments
We thank Dr. Kouji Yasuyama at Kawasaki Medical School for discussion and reading the earlier version of the manuscript, and Miss Tiffanie Chan for linguistic corrections. We also thank anonymous reviewers for their critical reading of the manuscript.
References
- 1.Dunlap JC, Loros J, DeCoursey PJ. Chronobiology: biological timekeeping. Sunderland: Sinauer; 2004. [Google Scholar]
- 2.Saunders DS. Insect clocks. Amsterdam: Elsevier; 2002. [Google Scholar]
- 3.Page TL. Transplantation of the cockroach circadian pacemaker. Science. 1982;216:73–75. doi: 10.1126/science.216.4541.73. [DOI] [PubMed] [Google Scholar]
- 4.Tomioka K, Chiba Y. Characterization of optic lobe circadian pacemaker by in situ and in vitro recording of neuronal activity in the cricket Gryllus bimaculatus . J Comp Physiol A. 1992;171:1–7. doi: 10.1007/BF00195955. [DOI] [Google Scholar]
- 5.Fleissner G. Isolation of an insect circadian clock. J Comp Physiol. 1982;149:311–316. doi: 10.1007/BF00619146. [DOI] [Google Scholar]
- 6.Truman JW. Physiology of insect rhythms, IV: role of the brain in the regulation of the flight rhythm of the giant silkmoths. J Comp Physiol A. 1974;95:281–296. doi: 10.1007/BF00609702. [DOI] [Google Scholar]
- 7.Helfrich-Förster C. Neurobiology of the fruit fly’s circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 8.Kasai M, Chiba Y. Effects of optic lobe ablation on circadian activity in the mosquito, Culex pipiens pallens . Physiol Entomol. 1987;12:59–65. doi: 10.1111/j.1365-3032.1987.tb00724.x. [DOI] [Google Scholar]
- 9.Hardin PE. Essential and expendable features of the circadian timekeeping mechanism. Curr Opin Neurobiol. 2006;16:686–692. doi: 10.1016/j.conb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich-Förster C. The neural basis of Drosophila’s circadian clock. Sleep Biol Rhythms. 2006;4:224–234. doi: 10.1111/j.1479-8425.2006.00223.x. [DOI] [Google Scholar]
- 11.Sheeba V. The Drosophila melanogaster circadian pacemaker circuit. J Genet. 2008;87:485–493. doi: 10.1007/s12041-008-0071-x. [DOI] [PubMed] [Google Scholar]
- 12.Boothroyd CE, Young MW. The in(put)s and out(put)s of the Drosophila circadian clock. N Y Acad Sci. 2008;1129:350–357. doi: 10.1196/annals.1417.006. [DOI] [PubMed] [Google Scholar]
- 13.Sandrelli F, Costa R, Kyriacou CP, Rosato E. Comparative analysis of circadian clock genes in insects. Insect Mol Biol. 2008;17:447–463. doi: 10.1111/j.1365-2583.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomioka K, Yoshii T. Entrainment of Drosophila circadian rhythms by temperature cycles. Sleep Biol Rhythms. 2006;4:240–247. doi: 10.1111/j.1479-8425.2006.00227.x. [DOI] [Google Scholar]
- 15.Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 16.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila . Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 17.Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster . J Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci USA. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dushay MS, Rosbash M, Hall JC. The disconnected visual system mutations in Drosophila drastically disrupt circadian rhythms. J Biol Rhythms. 1989;4:1–27. doi: 10.1177/074873048900400101. [DOI] [PubMed] [Google Scholar]
- 20.Helfrich-Förster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol A. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 21.Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila . Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picot M, Klarsfeld A, Chélot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci. 2009;29:8312–8320. doi: 10.1523/JNEUROSCI.0279-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen C-J, Lee H-J. Mapping the cellular network of the circadian clock in two cockroach species. Arch Insect Biochem Physiol. 2008;68:215–231. doi: 10.1002/arch.20236. [DOI] [PubMed] [Google Scholar]
- 24.Shiga S, Numata H. Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae . J Exp Biol. 2009;212:867–877. doi: 10.1242/jeb.027003. [DOI] [PubMed] [Google Scholar]
- 25.Lupien M, Marshall S, Leser W, Pollack GS, Honegger H-W. Antibodies against the PER protein of Drosophila label neurons in the optic lobe, central brain, and thoracic ganglia of the crickets Teleogryllus commodus and Teleogryllus oceanics . Cell Tissue Res. 2003;312:377–391. doi: 10.1007/s00441-003-0720-6. [DOI] [PubMed] [Google Scholar]
- 26.Page TL. Interaction between bilaterally paired components of the cockroach circadian system. J Comp Physiol. 1978;124:225–236. doi: 10.1007/BF00657054. [DOI] [Google Scholar]
- 27.Colwell CS, Page TL. A circadian rhythm in neural activity can be recorded from the central nervous system of the cockroach. J Comp Physiol A. 1990;166:643–649. doi: 10.1007/BF00240014. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka K, Chiba Y. Effects of nymphal stage optic nerve severance or optic lobe removal on the circadian locomotor rhythm of the cricket, Gryllus bimaculatus . Zool Sci. 1984;1:385–394. [Google Scholar]
- 29.Tomioka K. Residual circadian rhythmicity after bilateral lamina-medulla removal or optic stalk transection in the cricket, Gryllus bimaculatus . J Insect Physiol. 1985;31:653–657. doi: 10.1016/0022-1910(85)90065-4. [DOI] [Google Scholar]
- 30.Rence BG, Loher W. Arrhythmically singing crickets: thermoperiodic reentrainment after bilobectomy. Science. 1975;190:385–387. doi: 10.1126/science.1179217. [DOI] [PubMed] [Google Scholar]
- 31.Page TL. Circadian organization in cockroaches: effects of temperature cycles on locomotor activity. J Insect Physiol. 1985;31:235–243. doi: 10.1016/0022-1910(85)90125-8. [DOI] [Google Scholar]
- 32.Tomioka K, Chiba Y. Photoperiodic entrainment of locomotor activity in crickets (Gryllus bimaculatus) lacking the optic lobe pacemaker. J Insect Physiol. 1989;35:827–835. doi: 10.1016/0022-1910(89)90098-X. [DOI] [Google Scholar]
- 33.Stanewsky R. Clock mechanisms in Drosophila . Cell Tissue Res. 2002;309:11–26. doi: 10.1007/s00441-002-0569-0. [DOI] [PubMed] [Google Scholar]
- 34.Cyran SA, Buchsbaum AM, Reddy KL, Lin M-C, Glossop NRJ, Hardin PE, Young MW, Storti RV, Blau J. vrille, Pdp1 and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/S0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 35.Glossop NR, Houl JH, Zheng H, Ng FS, Dudek SM, Hardin PE. VRILLE feeds back to control circadian transcription of clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. doi: 10.1016/s0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 36.Benito J, Zheng H, Hardin PE. PDP1ε functions downstream of the circadian oscillator to mediate behavioral rhythms. J Neurosci. 2007;27:2539–2547. doi: 10.1523/JNEUROSCI.4870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadener S, Stoleru D, McDonald M, Nawathean P, Rosbash M. Clockwork orange is a transcriptional repressor and a new Drosophila circadian pacemaker component. Genes Dev. 2007;21:1675–1686. doi: 10.1101/gad.1552607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim C, Chung BY, Pitman JL, McGill JJ, Pradhan S, Lee J, Keegan KP, Choe J, Allada R. Clockwork orange encodes a transcriptional repressor important for circadian-clock amplitude in Drosophila. Curr Biol. 2007;17:1082–1089. doi: 10.1016/j.cub.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto A, Ukai-Tadenuma M, Yamada RG, Houl J, Uno KD, Kasukawa T, Dauwalder B, Itoh TQ, Takahashi K, Ueda R, Hardin PE, Tanimura T, Ueda HR. A functional genomics strategy reveals clockwork orange as a transcriptional regulator in the Drosophila circadian clock. Gene Dev. 2007;21:1687–1700. doi: 10.1101/gad.1552207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richier B, Michard-Vanhée C, Lamouroux A, Papin C, Rouyer F. The clockwork orange Drosophila protein functions as both an activator and a repressor of clock gene expression. J Biol Rhythms. 2008;23:103–116. doi: 10.1177/0748730407313817. [DOI] [PubMed] [Google Scholar]
- 41.Bae K, Edery I. Regulating a circadian clock’s period, phase and amplitude by phosphorylation: insights from Drosophila . J Biochem. 2006;140:609–617. doi: 10.1093/jb/mvj198. [DOI] [PubMed] [Google Scholar]
- 42.Weber F. Remodeling the clock: coactivators and signal transduction in the cricadian clockworks. Naturwissenschaften. 2009;96:321–337. doi: 10.1007/s00114-008-0474-9. [DOI] [PubMed] [Google Scholar]
- 43.Weber F, Hung HC, Maurer C, Kay SA. Second messenger and Ras/MAPK signalling pathways regulate CLOCK/CYCLE-dependent transcription. J Neurochem. 2006;98:248–257. doi: 10.1111/j.1471-4159.2006.03865.x. [DOI] [PubMed] [Google Scholar]
- 44.Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR. A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci. 2003;6:251–257. doi: 10.1038/nn1007. [DOI] [PubMed] [Google Scholar]
- 45.Meyer P, Saez L, Young MW. PER-TIM interactions in living Drosophila cells: an interval timer for the circadian clock. Science. 2006;311:226–229. doi: 10.1126/science.1118126. [DOI] [PubMed] [Google Scholar]
- 46.Ashmore LJ, Sehgal A. A fly’s eye view of circadian entrainment. J Biol Rhythms. 2003;18:206–216. doi: 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- 47.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/S0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 48.Nitabach MN, Sheeva V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 49.Zavodska R, Sehadova H, Sauman I, Sehnal F. Light-dependent PER-like proteins in the cephalic ganglia of an apterygote and a pterygote insect species. Histochem Cell Biol. 2005;123:407–418. doi: 10.1007/s00418-004-0728-3. [DOI] [PubMed] [Google Scholar]
- 50.Sauman I, Reppert SM. Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of period protein regulation. Neuron. 1996;17:889–900. doi: 10.1016/S0896-6273(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 51.Sauman I, Reppert SM. Molecular characterization of prothoracicotropic hormone (PTTH) from the giant silkmoth Antheraea pernyi: developmental appearance of PTTH-expressing cells and relationship to circadian clock cells in central brain. Dev Biol. 1996;178:418–429. doi: 10.1006/dbio.1996.0228. [DOI] [PubMed] [Google Scholar]
- 52.Chang DC, McWatters HG, Williams JA, Gotter AL, Levine JD, Reppert SM. Constructing a feedback loop with circadian clock molecules from the silkmoth, Antheraea pernyi . J Biol Chem. 2003;278:38149–38158. doi: 10.1074/jbc.M306937200. [DOI] [PubMed] [Google Scholar]
- 53.Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, Reppert SM. The two CRYs of the butterfly. Curr Biol. 2005;15:R953–R954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 54.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 55.Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:138–155. doi: 10.1371/journal.pbio.0060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goto SG, Denlinger DL. Short-day and long-day expression patterns of genes involved in the flesh fly clock mechanism: period, timeless, cycle and cryptochrome . J Insect Physiol. 2002;48:803–816. doi: 10.1016/S0022-1910(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 57.Miyatake T, Matsumoto A, Matsuyama T, Ueda HR, Toyosato T, Tanimura T. The period gene and allochronic reproductive isolation in Bactrocera cucurbitae . Proc R Soc Lond B. 2002;269:2467–2472. doi: 10.1098/rspb.2002.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cry b mutation identifies cryptochrome as a circadian photoreceptor in Drosophila . Cell. 1998;95:681–692. doi: 10.1016/S0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa T, Matsumoto A, Kato T, Jr, Togashi S, Ryo H, Ikenaga M, Todo T, Ueda R, Tanimura T. DCRY is a Drosophila photoreceptor protein implicated in light entrainment of circadian rhythm. Genes Cells. 1999;4:57–65. doi: 10.1046/j.1365-2443.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 60.Rubin EB, Shemesh Y, Cohen M, Elgavish S, Robertson HM, Bloch G. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 2006;16:1352–1365. doi: 10.1101/gr.5094806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moriyama Y, Sakamoto T, Karpova SG, Matsumoto A, Noji S, Tomioka K. RNA interference of the clock gene period disrupts circadian rhythms in the cricket Gryllus bimaculatus . J Biol Rhythms. 2008;23:308–318. doi: 10.1177/0748730408320486. [DOI] [PubMed] [Google Scholar]
- 62.Moriyama Y, Sakamoto T, Matsumoto A, Noji S, Tomioka K. Functional analysis of the circadian clock gene period by RNA interference in nymphal crickets Gryllus bimaculatus . J Insect Physiol. 2009;55:396–400. doi: 10.1016/j.jinsphys.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Sakamoto T, Uryu O, Tomioka K. The clock gene period plays an essential role in photoperiodic control of nymphal development in the cricket Modicogryllus siamensis . J Biol Rhythms. 2009;24:379–390. doi: 10.1177/0748730409341523. [DOI] [PubMed] [Google Scholar]
- 64.Loher W. Circadian control of stridulation in the cricket Teleogryllus commodus Walker. J Comp Physiol. 1972;79:173–190. doi: 10.1007/BF00697770. [DOI] [PubMed] [Google Scholar]
- 65.Rence BG, Lisy MT, Garves BR, Quilan BJ. The role of ocelli in circadian singing rhythms of crickets. Physiol Entomol. 1988;13:201–212. doi: 10.1111/j.1365-3032.1988.tb00924.x. [DOI] [Google Scholar]
- 66.Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastracture, and putative circadian function. J Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 68.Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Light-dependent sequentation of TIMELESS by CRYPTOCHROME. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 69.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 70.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 71.Hanai S, Hamasaka Y, Ishida N. Circadian entrainment to red light in Drosophila: requirement of Rhodopsin 1 and Rhodopsin 6 . NeuroReport. 2008;19:1441–1444. doi: 10.1097/WNR.0b013e32830e4961. [DOI] [PubMed] [Google Scholar]
- 72.Hanai S, Ishida N. Entrainment of Drosophila circadian clock to green and yellow light by Rh1, Rh5, Rh6 and CRY. NeuroReport. 2009;20:755–758. doi: 10.1097/WNR.0b013e32832a7c4e. [DOI] [PubMed] [Google Scholar]
- 73.Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster . J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 74.Zimmerman WF, Pittendrigh CS, Pavlidis T. Temperature compensation of the circadian oscillation in Drosophila pseudoobscura and its entrainment by temperature cycles. J Insect Physiol. 1968;14:669–684. doi: 10.1016/0022-1910(68)90226-6. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda M, Tomioka K. Temperature dependency of the circadian locomotor rhythm in the cricket Gryllus bimaculatus . Zool Sci. 1993;10:597–604. [Google Scholar]
- 76.Yoshii T, Fujii K, Tomioka K. Induction of Drosophila behavioral and molecular circadian rhythms by temperature steps in constant light. J Biol Rhythms. 2007;22:103–114. doi: 10.1177/0748730406298176. [DOI] [PubMed] [Google Scholar]
- 77.Glaser FT, Stanewsky R. Temperature synchronization of the Drosophila circadian clock. Curr Biol. 2005;15:1352–1363. doi: 10.1016/j.cub.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 78.Collins BH, Rosato E, Kyriacou CP. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc Natl Acad Sci USA. 2004;101:1945–1950. doi: 10.1073/pnas.0308240100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majercak J, Chen W-F, Edery I. Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol Cell Biol. 2004;24:3359–3372. doi: 10.1128/MCB.24.8.3359-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Majercak J, Sidote D, Hardin PE, Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron. 1999;24:219–230. doi: 10.1016/S0896-6273(00)80834-X. [DOI] [PubMed] [Google Scholar]
- 81.Chen W-F, Majercak J, Edery I. Clock-gated photic stimulation of timeless expression at cold temperatures and seasonal adaptation in Drosophila . J Biol Rhythms. 2006;21:256–271. doi: 10.1177/0748730406289306. [DOI] [PubMed] [Google Scholar]
- 82.Chiba Y, Uki M, Kawasaki Y, Matsumoto A, Tomioka K. Entrainability of circadian activity of the mosquito Culex pipiens pallens to 24-h temperature cycles, with special reference to involvement of multiple oscillators. J Biol Rhythms. 1993;8:211–220. doi: 10.1177/074873049300800304. [DOI] [PubMed] [Google Scholar]
- 83.Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster . Proc Natl Acad Sci USA. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rao KR, Riehm JP. Pigment-dispersing hormones: a novel family of neuropeptides from arthropods. Peptides. 1988;9(Suppl.):153–159. doi: 10.1016/0196-9781(88)90239-2. [DOI] [PubMed] [Google Scholar]
- 85.Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila . Proc Natl Acad Sci USA. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernández MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila . Cell. 1999;99:791–802. doi: 10.1016/S0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 88.Yoshii T, Wullbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. J Neurosci. 2009;29:2579–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomioka K, Miyasako Y, Umezaki Y. PDF as a coupling mediator between the light-entrainable and temperature-entrainable clocks in Drosophila melanogaster . Acta Biol Hung. 2008;59(Suppl.):149–155. doi: 10.1556/ABiol.59.2008.Suppl.23. [DOI] [PubMed] [Google Scholar]
- 90.Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- 91.Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, Loof AD, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Hyun S, Lee Y, Hong S-T, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;4:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 93.Lear BC, Merrill CE, Lin J-M, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Petri B, Stengl M. Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae . J Neurosci. 1997;17:4087–4093. doi: 10.1523/JNEUROSCI.17-11-04087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee C-M, Su M-T, Lee H-J. Pigment dispersing factor: an output regulator of the circadian clock in the german cockroach. J Biol Rhythms. 2009;24:35–43. doi: 10.1177/0748730408327909. [DOI] [PubMed] [Google Scholar]
- 96.Okamoto A, Mori H, Tomioka K. The role of optic lobe in generation of circadian rhythms with special reference to the PDH immunoreactive neurons. J Insect Physiol. 2001;47:889–895. doi: 10.1016/S0022-1910(01)00061-0. [DOI] [Google Scholar]
- 97.Saifullah ASM, Tomioka K. Pigment-dispersing factor sets the night state of the medulla bilateral neurons in the optic lobe of the cricket, Gryllus bimaculatus . J Insect Physiol. 2003;49:231–239. doi: 10.1016/S0022-1910(02)00270-6. [DOI] [PubMed] [Google Scholar]
- 98.Abdelsalam S, Uemura H, Umezaki Y, Saifullah ASM, Shimohigashi M, Tomioka K. Characterization of PDF-immunoreactive neurons in the optic lobe and cerebral lobe of the cricket, Gryllus bimaculatus . J Insect Physiol. 2008;54:1205–1212. doi: 10.1016/j.jinsphys.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Pyza E, Meinertzhagen IA. Neurotransmitters regulate rhythmic size changes amongst cells in the fly’s optic lobe. J Comp Physiol A. 1996;178:33–45. doi: 10.1007/BF00189588. [DOI] [PubMed] [Google Scholar]
- 100.Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythm and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/S0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 101.Meunier N, Belgacem YH, Martin J-R. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila . J Exp Biol. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto A. Genome-wide screenings for circadian clock genes in Drosophila . Sleep Biol Rhythms. 2006;4:248–254. doi: 10.1111/j.1479-8425.2006.00226.x. [DOI] [Google Scholar]
- 103.Giebultowicz JM. Insect circadian clocks: is it all in their heads? J Insect Physiol. 1999;45:791–800. doi: 10.1016/S0022-1910(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 104.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila . Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 105.Emery IF, Noveral JM, Jamison CF, Siwickii KK. Rhythms of Drosophila period gene in culture. Proc Natl Acad Sci USA. 1997;94:4092–4096. doi: 10.1073/pnas.94.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giebultowicz JM, Hege DM. Circadian clock in Malphigian tubules. Nature. 1997;386:664. doi: 10.1038/386664a0. [DOI] [PubMed] [Google Scholar]
- 107.Giebultowicz JW, Stanewsky R, Hall JC, Hege DM. Transplanted Drosophila excretory tubules maintain circadian clock cycling out of phase with the host. Curr Biol. 2000;10:107–110. doi: 10.1016/S0960-9822(00)00299-2. [DOI] [PubMed] [Google Scholar]
- 108.Tanoue S, Krishnan P, Chatterjee A, Hardin PE. G protein-coupled receptor kinase 2 is required for rhythmic olfactory responses in Drosophila . Curr Biol. 2008;18:787–794. doi: 10.1016/j.cub.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mehnert KI, Cantera R. A peripheral pacemaker drives the circadian rhythm of synaptic boutons in Drosophila independently of synaptic activity. Cell Tiss Res. 2008;334:103–109. doi: 10.1007/s00441-008-0670-0. [DOI] [PubMed] [Google Scholar]
- 110.Ivanchenko M, Stanewsky R, Giebultowicz JM. Circadian photoreception in Drosophila: functions of cryptochrome in periopheral and central clocks. J Biol Rhythms. 2001;16:205–215. doi: 10.1177/074873040101600303. [DOI] [PubMed] [Google Scholar]
- 111.Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- 112.Emery P, Stanewsky R, Hall JC, Rosbash M. Drosophila cryptochromes: a unique circadian-rhythm photoreceptor. Nature. 2000;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- 113.Ito C, Goto SG, Shiga S, Tomioka K, Numata H. Peripheral circadian clock for the cuticle deposition rhythm in Drosophila melanogaster . Proc Natl Acad Sci USA. 2008;105:8446–8451. doi: 10.1073/pnas.0800145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.An X, Tebo M, Song S, Frommer M, Raphael KA. The cryptochrome (cry) gene and a mating isolation mechanism in tephritid fruit flies. Genetics. 2004;168:2025–2036. doi: 10.1534/genetics.104.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merlin C, François MC, Queguiner I, Maïbèche-Coisné M, Jacquin-Joly E. Evidence for a putative antennal clock in Mamestra brassicae: Molecular cloning and characterization of two clock genes—period and cryptochrome—in antennae. Insect Mol Biol. 2006;15:137–145. doi: 10.1111/j.1365-2583.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 116.Schuckel J, Siwicki KK, Stengl M. Putative circadian pacemaker cells in the antenna of the hawkmoth Manuca sexta . Cell Tiss Res. 2007;330:271–278. doi: 10.1007/s00441-007-0471-x. [DOI] [PubMed] [Google Scholar]
- 117.Merlin C, Lucas P, Rochat D, François MC, Maïbèche-Coisné M, Jacquin-Joly E. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis . J Biol Rhythms. 2007;22:502–514. doi: 10.1177/0748730407307737. [DOI] [PubMed] [Google Scholar]
- 118.Krishnan P, Dryer SE, Hardin PE. Measuring circadian rhythms in olfaction using electroantennograms. Meth Enzymol. 2005;393:495–508. doi: 10.1016/S0076-6879(05)93025-5. [DOI] [PubMed] [Google Scholar]
- 119.Krishnan P, Chatterjee A, Tanoue S, Hardin PE. Spike amplitude of single-unit responses in antennal sensillae is controlled by the Drosophila circadian clock. Curr Biol. 2008;18:803–807. doi: 10.1016/j.cub.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Page TL, Koelling E. Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J Insect Physiol. 2003;49:697–707. doi: 10.1016/S0022-1910(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 121.Rosén WQ, Han GB, Lofstedt C. The circadian rhythm of the sex-pheromone-mediated behavioral response in the tunip moth, Agrotis segetum, is not controlled at the peripheral level. J Biol Rhythms. 2003;18:402–408. doi: 10.1177/0748730403256869. [DOI] [PubMed] [Google Scholar]
- 122.Flecke C, Dolzer J, Krannich S, Stengl M. Perfusion with cGMP analogue adapts the action potential response of pheromone-sensitive sensilla trichoidea of the hawkmoth Manduca sexta in a daytime-dependent manner. J Exp Biol. 2006;209:3898–3912. doi: 10.1242/jeb.02432. [DOI] [PubMed] [Google Scholar]
- 123.Saifullah ASM, Page TL. Circadian regulation of olfactory receptor neurons in the cockroach antenna. J Biol Rhythms. 2009;24:144–152. doi: 10.1177/0748730408331166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flecke C, Stengl M. Octopamine and tyramine modulate pheromone-sensitive olfactory sensilla of the hawkmoth Manduca sexta in a time-dependent manner. J Comp Physiol A. 2009;195:529–545. doi: 10.1007/s00359-009-0429-4. [DOI] [PubMed] [Google Scholar]
- 125.Helfrich-Förster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology. 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]