Abstract
Although platelets are best known as primary mediators of hemostasis, this function intimately associates them with inflammatory processes, and it has been increasingly recognized that platelets play an active role in both innate and adaptive immunity. For example, platelet adhesive interactions with leukocytes and endothelial cells via P-selectin can lead to several pro-inflammatory events, including leukocyte rolling and activation, production of cytokine cascades, and recruitment of the leukocytes to sites of tissue damage. Superimposed on this, platelets express immunologically-related molecules such as CD40L and Toll-like receptors that have been shown to functionally modulate innate immunity. Furthermore, platelets themselves can interact with microorganisms, and several viruses have been shown to cross-react immunologically with platelet antigens. This review discusses the central role that platelets play in inflammation, linking them with varied pathological conditions, such as atherosclerosis, sepsis, and immune thrombocytopenic purpura, and suggests that platelets also act as primary mediators of our innate defences.
Keywords: Platelets, Innate immunity, Toll-like receptors, CD40L, Inflammation
Innate and adaptive immunity
The innate immune system is composed of physical, chemical, and cellular components which act together to mediate the first line of defense against invading microorganisms. These components are intimately linked with inflammatory processes that ultimately lead to the removal of most of the infectious organisms encountered by a host. The innate immune response activates quickly (within seconds) after exposure to foreign infectious agents and is antigen nonspecific in that there is no memory associated with the immunity [1–3]. These characteristics distinguish the innate immune system from the adaptive immune response, that is mediated exclusively by B cells and T cells, is slower to activate, and is exquisitely antigen specific, generating memory with subsequent exposure of the stimulating antigen [1–3]. Table 1 lists the various participants in innate immunity.
Table 1.
Characteristics of innate immunity
| Structure | Characteristic | Function |
|---|---|---|
| External surface barriers (skin and mucosa) |
Keratin Sebum Hydrochloric acid Lysozyme |
Physically excluding infectious agents from entrance Initial chemical attack of infections |
| Internal innate defense (cellular) |
Phagocytes Neutrophils Macrophages Eosinophils NK cells |
Phagocytosis Secretion of inflammatory cytokines Lysis of virally infected cells |
| Internal innate defense (soluble) | Complement | Bacteriocidal |
|
Cytokines Eicosinoids Kinins Histamine Defensins Anti-microbial proteins |
Mediators of inflammation Fever |
Relationship between the innate and adaptive immune systems
For many years, the innate and the adaptive immune systems were regarded as separate entities, and innate immune responses were considered to be of secondary importance in the hierarchy of immune functions [4–6]. Janeway’s seminal experiments showed, however, that the adaptive immune system was essentially in the “off” mode and only responded when it received the appropriate signals from innate immunity [4–6]. He suggested that cells of the innate immune system could distinguish between “non-infectious self” and “infectious non-self” by utilizing groups of germline-encoded receptors (e.g., Toll-like receptors, TLR) that recognize conserved structures present in many different microorganisms [4]. For example, TLR are expressed on antigen presenting cells (APC) such as macrophages and dendritic cells and recognize pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), peptidoglycan, lipoteichoic acids, bacterial RNA, and mucans. This recognition of microbial structures is thought to lead to activation and maturation of the APC to process and present the microbial antigens to host T cells to initiate adaptive immunity [6–8]. Subsequently, Matzinger coined the term “danger” and suggested that the adaptive immune system does not necessarily respond to foreignness but rather to endogenous signals induced by tissue damage and innate immunity [9]. Convincing evidence has since emerged to show that the innate immune system may be the ultimate controller of adaptive immune responses. For example, APC and natural killer (NK) cells of the innate immune system can significantly influence the type of adaptive immune response, by controlling the differentiation of naive T helper (Th0) lymphocytes into effector cells of a particular type (e.g., Th1 or Th2 cells) [10–12]. NK cells also play a key role in bridging innate and adaptive immunity through, for example, the modulation of cytokine networks [10–12]. These cells constitute a critical innate immune response against viruses, parasites, intracellular bacteria, and tumor cells [6, 7]. NK cells have, for example, been shown to enhance the adaptive IgG response against antigenic stimulation by secreting cytokines such as interferon (IFN) [10–12]. Therefore, an understanding of the basis of NK-cell control of adaptive immunity may have a significant impact on immunobiology and medicine.
Recent evidence suggests that platelets themselves may bridge the innate and adaptive immune systems by expressing immunostimulatory molecules such as CD154 and thereby help stimulate adaptive immunity such as antiviral CD8+ T cell induction [13]. In addition, in 2004, it was discovered that platelets themselves express TLR2, 4, and 9 [14, 15], and since then, many reports have confirmed this observation [16–32]. It appears that at least one function of platelet TLR4 expression is to mediate LPS-induced thrombocytopenia and tumor necrosis factor (TNFα) production by leukocytes [19, 20]. These findings suggest that platelets themselves may actively mediate innate immune mechanisms. Perhaps more importantly, they suggest that platelets act as sentinels in the circulation that quickly bind pathogens for presentation and activation of the reticuloendothelial system (RES) [20, 24, 31].
Evolutionary links
The innate immune system is found in all classes of plant and animal life; it is thought to constitute an evolutionary older defense strategy and is the dominant immune system in plants, fungi, invertebrates, and primitive multicellular organisms. For example, arthropod hemolymph contains nucleated circulating cells called hemocytes [33]. Hemocytes look very much like vertebrate macrophages, can mediate phagocytosis, express TLR, and efficiently form a cellular capsule around infectious organisms and secrete antimicrobial peptides [33, 34]. The encapsulation response is a primordial innate immune reaction that occurs against a wide range of pathogens and parasites and ultimately results in the formation of multiple layers of dead melanized hemocytes [35], which isolates the infection from the hemocoel and allows killing by such processes as asphyxiation or cytotoxic compound production [36].
Interestingly, hemocytes are not only responsible for arthropod immunity but are also intimately linked with wound healing and have the ability to aggregate and coagulate hemolymph at sites of tissue damage or exoskeletal breach [34–36]. In this context, hemocytes were termed explosive corpuscles which exocytose several proteins at sites of tissue injury that catalyze coagulogen, the main clotting protein in the hemolymph, to effect coagulation and the formation of a clot. Clotting can occur by three basic processes including adhesion of hemocytes to form a cellular plug, hemocyte agglutination, and coagulation of hemolymph or the rapid coagulation of hemolymph [33]. These hematologically analogous responses may be the evolutionary origins of platelet function, and it is possible that at some point during evolution the immune function and wound healing function of hemocytes diverged into more specialized cells such as vertebrate phagocytes and platelets. This may be a reason why platelets have several functions that appear related to immunity, and perhaps these vestigial functions of platelets are, in fact, still active.
Platelets and inflammation
In addition to their critical role in hemostasis and thrombosis, platelets contribute to inflammation, including immune-mediated inflammation and the development of atherosclerosis. Inflammation, which leads to an imbalance between pro-coagulant and anti-coagulant properties of the endothelium, is characterized by multiple interactions between leukocytes, endothelial cells (ECs), and platelets. For example, P-selectin is involved in a number of physiological processes, including platelet aggregation, and platelet–endothelial and platelet–leukocyte interactions. P-selectin is a transmembrane protein of the selectin family of leukocyte adhesion receptors that is synthesized by megakaryocytes and ECs and stored in their α-granules and Weibel–Palade bodies, respectively [37, 38]. Upon platelet and EC stimulation, granule exocytosis translocates P-selectin to the plasma membrane to mediate such processes as leukocyte rolling, one of the first steps in the cell adhesion cascade [39, 40]. Dole et al. [41] have shown that infusion of activated platelets causes Weibel–Palade body release leading to P-selectin-mediated leukocyte rolling, suggesting that platelet P-selectin is also crucial in the process of inflammation. The rolling leukocytes subsequently become activated by chemokines present on the endothelial surface such as monocyte chemoattractant protein I (MCP-I) and RANTES [42, 43]. The importance of this P-selectin-mediated event is apparent when targeted disruption of the P- and E-selectin gene in mice markedly inhibits leukocyte rolling and delays recruitment of monocytes into sites of inflammation, enhancing susceptibility to infection [44–46]. The expressed P-selectin molecules are subsequently proteolytically cleaved off the plasma membrane by currently unknown enzymes called sheddase(s) [41, 47].
Through interaction with sulfatides [48] and the glycoprotein (GP) Ib complex [49], P-selectin may also contribute to the stabilization of platelet aggregates. For example, at the site of vascular injury, particularly at high shear stress, the binding of platelet GPIb complex to von Willebrand factor (VWF) on the injured vessel wall initiates platelet tethering and subsequent adhesion. Platelet aggregation is then mediated by interaction between platelet β3 integrin and fibrinogen (Fg), although Fg-independent platelet aggregation can also occur [50, 51]. While Fg, VWF, and P-selectin are all important molecules supporting hemostasis and thrombosis, and are all involved in inflammation, their mutual impact on these processes remains poorly understood.
Atherosclerosis
Atherosclerosis is a typical example of a chronic inflammatory process [52]. Platelet interactions with leukocytes and ECs represent an important link between inflammation and atherogenesis and include platelet activation, adhesion of platelets to ECs, and platelet release of potent inflammatory and mitogenic substances, which alter the chemotactic, adhesive, and proteolytic properties of ECs, supporting adhesion and migration of monocytes to the sites of inflammation. For example, absence of P-selectin in atherosclerosis-prone LDLR-deficient or apoE-deficient mice significantly delays atherosclerotic lesion formation [53, 54]. Burger and Wagner [55] have shown that platelet P-selectin contributes to atherosclerotic lesion formation by mediating rosetting of monocytes and neutrophils with circulating activated platelets [56], and this increases monocyte adhesion to EC and may facilitate macrophage accumulation in the vessel wall [57]. In addition, leukocyte rolling, adhesion, and transmigration is supported by platelets adherent to the subendothelial matrix through the interaction of P-selectin and leukocyte PSGL-1 [39, 40]. Leukocyte rolling, via platelet activating factor and the leukocyte integrin Mac-1, subsequently mediates firm leukocyte adhesion and binding to fibrinogen bound to platelet αIIbβ3 [58]. During adhesion to endothelium, activated platelets release pro-inflammatory molecules and cytokines, such as interleukin (IL)-1β [59] and CD40L [60], that also stimulate the ECs. Activated platelets also secrete the chemokine CCL5 (RANTES) and platelet factor 4, which are deposited in a P-selectin-dependent manner on microvasculature, aortic endothelium, and monocytes [43]. These pro-inflammatory cytokines activate monocyte integrins and increase monocyte recruitment to the atherosclerotic lesion [61]. Once recruited, platelets contain many molecules that enhance leukocyte chemoattraction [platelet activating factor, macrophage inflammatory protein (MIP)-1α, and cationic protein], stimulate smooth muscle cell and fibroblast proliferation (via TGFβ, platelet-derived growth factor and serotonin), and promote collagen synthesis. The large amount of soluble CD40L shed by activated platelets [62] leads to CD40L/CD40 interaction, which also plays an important role in atherosclerosis. Platelets and their cytokines therefore contribute directly to atherosclerosis progression and maturation [63]. Additionally, CD36, a GP expressed on platelets, has been implicated in hemostasis, thrombosis, inflammation, lipid metabolism, and atherogenesis [64, 65]. There have been several excellent recent reviews, e.g., by Gawaz et al. [66, 67], Davi and Patrono [68], and others [26, 69], of the increasing evidence, using different experimental approaches, supporting the essential role of platelets in the initial stages of atherosclerosis, and showing that thrombosis and inflammation are intricately linked.
Sepsis
The occurrence of thrombocytopenia in critically ill patients has long been known to be associated with increased mortality [70]. Activation of both leukocytes and platelets is commonly observed in sepsis. There is increasing evidence that activation of these cells contributes to the development of disseminated intravascular coagulation (DIC) and multiple organ failure, as blood flow and consequent oxygen delivery is reduced and activation of both pro- and anti-inflammatory cytokine networks are induced [71, 74]. CD62P is increased on platelets from septic versus non-septic patients [75, 76] and activated platelets release platelet microparticles (MP) that express functional surface receptors that allow them to adhere to leukocytes [76]. However, the relative contribution of MP compared with intact platelets in mediating enhanced platelet–neutrophil adhesion in sepsis is not known. Interestingly, Gawaz et al. [72] observed less platelet–neutrophil adhesion in patients with multiorgan failure than in septic patients without organ failure; it may be that in septic shock platelet–neutrophil conjugates are sequestered in the microcirculation, thereby contributing to the development of organ failure [73]. Nonetheless, although both platelets and MP are able to enhance leukocyte adhesion to ECs, the role of platelet–leukocyte adhesion in sepsis and the development of multiorgan failure remains to be clarified.
While activated platelets secrete key components of the coagulation and inflammatory cascades and are involved in the regulation of vascular tone, there are few studies on platelet function in sepsis. It appears that in sepsis aggregation of circulating platelets is reduced, but platelet receptors are present in normal amounts [77]. The effects of bacterial products on platelet function have been inconsistent and appear to vary according to species, timing of the study, and pathogenesis of the sepsis [78]. For example, LPS has been shown to increase platelet aggregation in various animal models [79, 80], yet bacterial products seem to decrease human platelet aggregation in vitro [81, 82]. It has, however, become increasingly apparent that platelets play a complex role in sepsis. When activated, they release many substances capable of modulating not only their own function but also that of cells around them. Interfering with platelet function may prove to be valuable in the treatment of sepsis, but further study is needed to better define the precise mechanisms and effects of platelet activation in sepsis.
In addition, platelets are known to mediate phagocytic-like functions and can internalize both bacteria and viruses [83, 84] as well as latex beads [85, 86]. This phenomenon has been interpreted as either a passive passage of the particles through the canalicular system or as a spreading of the platelets over the particle [87]. These characteristics of platelets might suggest a role in the protection and defense of the organism. For example, rabbit platelets contain microbicidal substances such as the bactericidal peptide PMP within their granules [88], and molecules similar to PMP called thrombocidins have also been shown in the granules of human platelets [88]. On the other hand, the binding of infectious agents by platelets may contribute to the transport and spread of the infection.
The engulfment of foreign particles by platelets may be compared to phagocytosis. To do so, like neutrophils, platelets can extend pseudopodia filled with microfilaments and devoid of granules and mitochondria [89]. Even partial digestion of ingested bacteria within platelet lysosomes has been demonstrated [90], although others have argued that engulfed bacteria are within intracellular compartments connected with the extracellular milieu and thus antibacterial pH changes could not occur [87]. Taken together, it appears that platelets do have the ability of engulfing infectious organisms and this may aid in host defense.
Platelet–neutrophil interactions during sepsis
Septicemia is associated with significant activation of neutrophils, primarily within the capillaries of the lungs and the sinusoids of the liver, leading eventually to lung and liver dysfunction and/or failure [91]. Bacterial products such as LPS activate neutrophils, making them structurally more rigid, and it has been suggested that this leads to their trapping in the lungs [92]. This trapping mechanism may be a strategy that infectious agents have developed to inappropriately activate and sequester neutrophils to tissues remote to the infection [24, 31]. On the other hand, it may be that the migration of neutrophils to the lungs during sepsis is an active mechanism of host defense. Subsequent to neutrophil migration to the lungs, platelets also begin to accumulate in these tissues of septic hosts [19, 31]. Experimentally, when neutrophils are depleted prior to the induction of endotoxemia, platelets lose their ability to migrate to lungs and liver suggesting that the neutrophils are essential for the platelet recruitment [19, 31]. The mechanism of this recruitment is not known but could be due to several possibilities such as platelets directly interacting with neutrophils or binding to vascular sites made pro-adhesive by the neutrophils. With respect to the former, it is known that aggregation of platelets with neutrophils can occur in vitro following stimulation with platelet agonists such as thrombin or ADP [56, 93, 94]; related to this, septic patients have increased platelet activation and neutrophil/EC adherence [71, 95], and this has also been observed in murine studies of sepsis [19]. Interestingly, Clark et al. [24] observed that platelets exposed to LPS bound avidly to immobilized neutrophils and the plasma from septic patients also induced significant platelet–neutrophil interaction [24, 31]. Much of the platelet binding to neutrophils could be inhibited by a TLR4 antagonist, suggesting that platelets can be activated by TLR4 ligands in the septic milieu [24, 31].
Platelet activation of neutrophils
Platelets and neutrophils have the potential to trap microbial pathogens independently of each other [83, 96], but together platelet–neutrophil interactions can induce hyperactivation of neutrophils to produce increased pro-inflammatory molecules [96]. Clark et al. [24] recently demonstrated a novel mechanism of platelet–neutrophil interaction that leads to enhanced bacterial trapping. This trapping event was initiated by the activated platelets adhering to immobilized neutrophils and markedly activating them [24]. In addition to neutrophil degranulation, the platelet binding stimulated neutrophils to release their DNA which contributed to the trapping of bacteria. These DNA structures were similar to neutrophil extracellular traps (NETs) [97] and could extend far downstream from the platelet–neutrophil aggregates. The formation of neutrophil NETs was enhanced and occurred rapidly if the platelets were first stimulated with LPS [24]. Elegant in vivo imaging revealed that the liver sinusoids and lung capillaries where platelets and neutrophils bound during sepsis were the primary sites of NETs formation and bacterial trapping [24]. Although NETs are beneficial in enhancing bacterial trapping, NETs may form at the expense of injury to the host. It appears that when LPS-activated neutrophils bind endothelium, little damage occurs, but if the bound neutrophils encounter LPS-bearing platelets, they become significantly activated and release their NETs and reactive oxygen species that damage the underlying endothelium [24]. Depletion of either neutrophils or platelets reduced the endothelial damage, suggesting that the neutrophil is dependent on platelets for activation and NET formation and inadvertent pulmonary damage [24]. These mechanisms are intriguing because they may be related to other pathologies, e.g., transfusion-related acute lung injury (TRALI); bacteria present either in blood products or the recipients of blood products may initiate this lung pathology.
CD40 and CD40L (CD154)
The immune modulator pair, CD40 and CD40L, has been proposed to play a central role in thrombotic diseases. Once expressed, platelet CD40L can interact with membrane bound CD40 on ECs, triggering an inflammatory reaction leading to local or systemic release of ICAM, VCAM, and MCP-1 [42, 60, 98]. Upon platelet activation and thrombus formation, CD40–CD40L interaction among platelets may lead to shedding of CD40L, producing its soluble form, sCD40L. Whether sCD40L is capable of inciting an inflammatory reaction or is inactivated upon cleavage is still a matter of debate [99–103]. It is also not yet fully clarified whether platelet–platelet interaction of CD40 and CD40L abrogates thrombus formation and inflammation by cleavage of CD40L, or whether further thrombus activation is achieved due to pro-thrombogenic effects of CD40 ligation with sCD40L [99–103]. On exposure to CD40-expressing vascular cells (including ECs), sCD40L induces the expression of adhesion molecules, e.g., E-selectin and P-selectin, and the release of inflammatory cytokines, e.g., IL-6 and tissue factor [102–105]. Although the interaction between CD40 and CD40L was originally described in immune cells [106], the majority of sCD40L is derived from activated platelets and may reflect platelet activation [99]. Furthermore, vascular endothelial growth factor (VEGF), which may originate from platelets [107–109], can act as a pro-inflammatory cytokine by inducing the expression of adhesion molecules that bind leukocytes to ECs, as well as increasing tissue factor expression and procoagulant activity. Experimental data link VEGF levels to abnormal angiogenesis and thrombogenesis and suggest a link between the CD40–CD40L system and VEGF-mediated angiogenesis [107, 108]. The role of the CD40–CD40L axis in patients with atherothrombotic diseases has generated great interest, as this molecule may play a pivotal link among platelets, angiogenesis, coagulation, the endothelium, and ultimately thrombosis.
Elzey et al. [13, 110] have pointed out the importance of platelet-mediated modulation of adaptive immunity and indicated that platelet CD40L represents a means of communication between innate and adaptive immune compartments. Such studies extend platelet function to modulation of local inflammatory events through release of chemokines, cytokines, and immunomodulatory ligands, including CD40L, and have shown that platelets, via CD40L, induce dendritic cell (DC) maturation, which is central to development of adaptive immunity to invading pathogens [13]; the DC are also the most potent APC and may thus also be important in immune mediated platelet destruction.
There has been other recent recognition of the role platelets may play in the immune response; they can facilitate lymphocyte homing [111] and B cell differentiation and antibody class switching due to the large platelet CD40L content [13]. Although the physiological role of large amounts of transforming growth factor (TGF)-β (a potent immunosuppressive factor) in platelets [112] is currently unclear, platelets may also contribute to immune regulation via different granule sorting/release pathways [113, 114]. Furthermore, P-selectin promotes the Th-1-like immune response [115], potentially relevant in immune-related inflammation.
TREM-1
The triggering receptor expressed on myeloid cells (TREM)-1 plays an important role in the innate immune response related to severe infections and sepsis. TREM-1 is a member of the v-type immunoglobulin super family and is expressed on neutrophils and monocytes [116]. The expression of TREM-1 is increased upon stimulation with microbial products and this activates neutrophil effector functions such as respiratory burst, phagocytosis, release of IL-8, and myeloperoxidase in synergy with TLR ligands such as LPS or bacterial lipopeptides [117–120]. Inhibition of TREM-1-associated activation improves the outcome in rodent models for pneumonia and sepsis. The identity of the natural TREM-1 ligands are, however, so far unknown. Recently, however, Haselmayer et al. [121] demonstrated that the natural ligand for TREM-1 is present on human platelets and that recombinant soluble TREM-1 can specifically bind to human platelets. Furthermore, it was demonstrated that co-incubation of neutrophils with platelets in the presence of LPS enhanced the neutrophil respiratory burst and release of IL-8 [121]. These results indicate that, during interactions between neutrophils and platelets during innate immunity, the inflammatory response may be mediated by TREM-1 and is platelet-derived, and thus these interactions may act as targets for the treatment of overwhelming immune responses during sepsis.
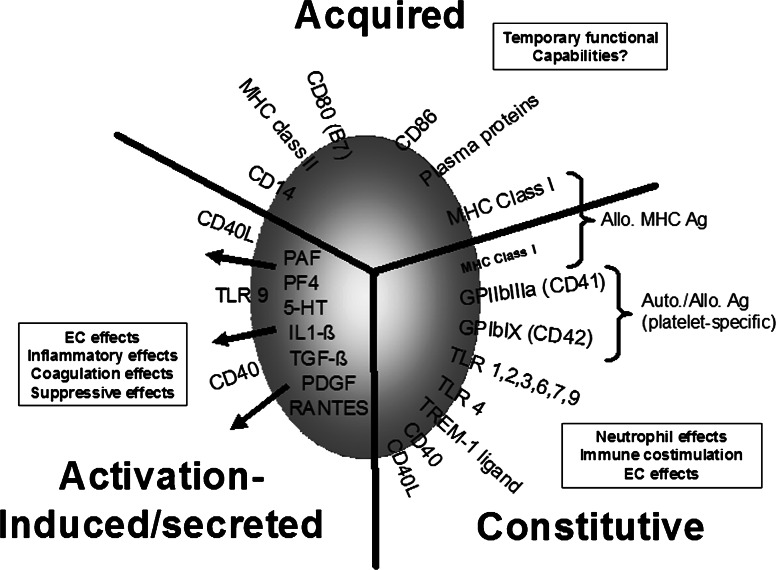
Figure 1 summarizes the pro- and anti-inflammatory molecules and cytokines associated with platelets.
Fig. 1.
Linking platelets to immunity by phenotype. Platelets store/express and secrete many immunomodulatory molecules and that can significantly affect innate immune mechanisms. Some are constitutively expressed whiles others are expressed or secreted upon platelet activation while still others are acquired by either adsorption from the plasma or perhaps by platelet contact with activated leukocytes during inflammation and subsequent membrane redistribution. Also included are MHC class I molecules and the two major platelet-specific glycoproteins, CD41 and CD42, to indicate the major antigenic proteins recognized by host adaptive immunity
Platelet Toll-like receptors (TLR)
Pathogens are first encountered by TLR on professional phagocytes such as neutrophils, macrophages, and dendritic cells [4–8]. TLR are germline-encoded proteins and bind a variety of infectious molecular structures, and are critical for stimulating innate immune mechanisms [4–8]. The ligands of TLR have been extensively studied, and they range from secretory components of pathogens to nucleic acids. Conserved motifs on infectious pathogens that are not found in higher organisms play essential roles in the biology of these pathogens, and these motifs are not subject to mutations; they are termed pathogen-associated molecular patterns (PAMP) and their receptors are called pattern recognition receptors (PRR). Interestingly, TLRs are also displayed by cell types not known to be involved in the recognition of exogenous pathogens such as microbes in the environment. In 2004, it was discovered that both murine and human platelets express TLR 1, 2, 4, and 9 [14, 15]. It was suggested that platelet TLR may be key molecules acting as a bridge between the inflammation resulting from an infectious process and from vascular arteriosclerotic pathologies [14, 15]. Since then, many papers have not only confirmed the existence of TLR on platelets but have also determined that platelet TLR are functional, in that they are responsible for mediating LPS-induced thrombocytopenia and TNFα production in vivo [16–32]. This latter observation suggested that it may be platelets that are primarily responsible for reactivity against bacterial products, and it was speculated that platelets acted as circulating sentinels that bind infectious agents and present them to the RES [20, 24, 31]. It may be this presentation event that is responsible for innate immune system activation. Table 2 summarizes the current literature with respect to platelet TLR and their functions.
Table 2.
Summary of recent phenotypic and/or functional studies and reviews related to TLR, bacterial LPS and platelets
| TLR | Speciesa | Study typeb | Functional effect | Reference | |||
|---|---|---|---|---|---|---|---|
| H | M | B | C | ||||
| 1,6 | Y | P | Platelet TLR expression | [14] | |||
| 2 | Y | P | Platelet, megakaryocyte, coronary thrombi expression | [15] | |||
| 5 | Y | P | Platelet proteomic analysis | [16] | |||
| 2,4,9 | Y | P | Platelet intracellular and membrane expression | [17] | |||
| 4 | Y | P/F | Platelet TLR2/4 agonists do not modulate platelet activation | [18] | |||
| 2,4 | Y | P/F | Platelet TLR4-LPS-induced thrombocytopenia via lung sequestration | [19] | |||
| 2,4,9 | Y | Y | P/F | Platelet TLR4-LPS-induced thrombocytopenia and TNF-α production | [20] | ||
| 4 | Y | F | Platelet TLR4 polymorphism associated with reduced TXA2 synthesis | [21] | |||
| 2,4 | Y | F | Platelet TLR4-LPS-medated release of soluble CD40L/cytokines | [151–153] | |||
| 4 | Y | Y | P/F | Platelet TLR4-LPS leads to platelet consumption in HUS | [22] | ||
| 4 | Y | F | Platelet production affected by LPS via megakaryocyte TLR4 | [23] | |||
| 4 | Y | Y | F | Platelet TLR4-LPS activates lung neutrophils to secrete NETS | [24] | ||
| 4 | Y | F | Platelet LPS/autoantibody synergism of phagocytosis | [25] | |||
| 4 | Y | F | Platelet LPS/antibody enhances thrombocytopenia in vivo | [28] | |||
| 4 | Y | P/F | Platelet TLR4 signals through MAP kinase and NF-kB | [29] | |||
| 4 | Y | F | Platelets binding gram –ve bacteria directly induce apoptosis/activation of EC | [31, 32] | |||
| 2 | Y | F | Platelets TLR2-induced thromboinflammatory effect via Phosphoinositide 3-Kinase | [154] | |||
| 4 | Y | P/F | Lipopolysaccharide stimulates platelet activation via TLR4/MyD88 signalling | [155] | |||
| 4 | Y | F | TLR4 ligands induce platelet secretion of immunomodulatory molecules | [153] | |||
| Reviews | [26, 27, 30, 155–157] | ||||||
aH Human, M murine, B bovine, C chicken
bP Phenotypic, F functional
Platelets, infections and the initiation of autoimmunity
Immune thrombocytopenic purpura (ITP) is a bleeding disorder caused by autoantibodies that opsonize platelets and enhance their destruction by FcR-mediated phagocytosis within the spleen [122, 123]. Although both acute and chronic forms can be distinguished, acute ITP primarily affects children and often occurs after a viral or bacterial infection [122, 123]. Furthermore, viral-specific antibodies with cross-reactivity against platelets have been identified in children with acute ITP [124, 125] and in patients with HIV-related ITP [125, 126] suggesting infections may play a role in ITP pathogenesis. On the other hand, in some patients with chronic ITP, infections are associated with an exacerbation of thrombocytopenia, and this has also been demonstrated in a mouse model [127]. Alternatively, eradication of the gram negative bacterium H. pylori in patients with ITP increases platelet counts, although this has not been observed in all studies [128–131]. It is possible that, in susceptible individuals, infectious agents in the presence of anti-platelet antibodies affect platelet–monocyte interactions and alter platelet destruction. Related to this, Semple et al. [25] recently demonstrated that if autoantibody-opsonized platelets were exposed to LPS, there was a significant and synergistic enhancement of Fc-dependent platelet phagocytosis by human monocytes. Thus, LPS in conjunction with IgG antiplatelet autoantibodies from patients with ITP can significantly enhance platelet phagocytosis [25]. The mechanism of how platelet-bound LPS together with autoantibody opsonization synergizes to enhance platelet phagocytosis is unknown, but since the increase was Fc-dependent, it may suggest that the interaction of TLR- and FcR-mediated signaling pathways could be responsible. It may be that the combination of LPS and autoantibody presented by platelets to the RES utilize shared components that synergistically increase signaling events and maximally stimulate macrophage phagocytosis. Taken together, these results suggest infectious agents in combination with antiplatelet antibodies could affect platelet destruction in vivo and may be at least one explanation of why thrombocytopenia worsens in some patients with ITP during infections and, alternatively, resolves in other patients with ITP who are treated with bacterial eradication therapy.
Platelet alloimmunity
In addition to modulating innate immunity, evidence is mounting that the alloimmune response against transfused platelets is also critically linked to innate immune stimulation. It appears that transfused platelets stimulate an early innate NK cell-derived IFNγ response that activates inducible nitric oxide synthase (iNOS) within recipient macrophages and generates NO, which is essential for the subsequent production of anti-donor platelet antibodies [132–136]. In addition, how NO is metabolized within the APC determines whether allogeneic platelet-derived antigens will stimulate IgG anti-donor immunity or suppress it. For example, if NO is converted to peroxynitrite (ONOO-), platelet protein antigens can be primarily nitrated, particularly on tyrosine residues (tyr-NO2), or S-nitrosylated, on cysteine residues (cys-SNO) [137]. Platelet nitration does not destroy B cell (antibody)-recognizing epitopes but prevents the platelets from stimulating an IgG anti-donor response when transfused into allogeneic recipients [137]. This lack of immunity to platelets appears to be due to an inability of recipient APC to engulf nitrated platelets (whether they are IgG opsonized or not). This suggests that if platelets are first phagocytosed by APC and nitrated within the phagolysosomes, their mobility within the cell will be severely limited and thus they may “starve” immune-stimulatory antigen processing pathways [137]. On the other hand, NO is essential for platelet MHC antigens to become immunogenic [132–134], but the mechanisms relating to how NO can also stimulate immunity are at present unknown. Interestingly, protein nitration and nitrosylation has been shown to regulate several enzyme systems and signaling cascades related to membrane movements that could ultimately affect antigen processing and presentation [138–143], and it may be that these types of reactions are responsible for stimulating platelet immunity. Thus, it appears that the ability to respond immunologically to platelets lies in the balance of ONOO-production (suppression) and other NO-dependent (stimulation) processes within the APC. What is striking, however, is that, like Janeway’s tenets, platelet MHC adaptive antibody production is dependent on early (24–72 h post-transfusion) innate immune activation. Thus, in order to respond immunologically to allogeneic platelets, stimulation of the host’s innate immune system is an absolute requirement.
Transfusion-related acute lung injury (TRALI)
TRALI denotes non-cardiogenic pulmonary edema in conjunction with transfusion of plasma-containing blood products [144, 145]. TRALI is potentially life-threatening and has recently been ranked as the leading cause of transfusion-related fatalities [146–149]. The recent discovery of TLR on platelets, as well as the capacity of platelets to liberate sCD40L, have led to investigation of a relationship between platelet immunomodulatory molecules and their ability to mediate (TRALI). Recently, Khan et al. [150] have demonstrated that CD40L released from platelets during storage may be responsible for modulating TRALI. In addition, it appears that the binding of platelet TLR can modulate the release of sCD40L [151–153]. It is possible that there is a relationship between platelets, their ability to bind bacteria and the release of sCD40L in mediating TRALI but more research is required to confirm this.
Conclusions
Platelets, primary actors in hemostasis and thrombin generation, like leukocytes, maintain multiple functions of innate defense mechanisms. These cellular fragments express and secrete many pro-inflammatory molecules that serve to initiate and modulate innate immune functions. Exciting recent studies on platelet TLRs and their functions have opened new understanding of the role of platelets in infectious processes and stimuated recent investigations in platelet alloimmunity and autoimmunity.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–6. [Google Scholar]
- 5.Janeway CA., Jr The immune system evolved to discriminate infectious non-self from non-infectious self. Immunol Today. 1992;13:11–15. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 6.Medzitov R, Janeway CA., Jr Innate immunity. N Engl J Med. 2000;343:338–346. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 7.Medzitov R, Janeway CA Jr (2003) The innate immune system. In: Paul WE (ed) Fundamental immunology, 5th edn. Lippincott/Williams and Wilkins, Philadelphia
- 8.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/S0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger P. Tolerance, danger and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 10.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–214. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 11.Raulet DH (2003) Natural killer cells. In: Paul WE (ed) Fundamental immunology, 5th edn. Lippincott/Williams and Wilkins, Philadelphia
- 12.Le Page C, Génin P, Baines MG, Hiscott J. Interferon activation and innate immunity. Rev Immunogenet. 2000;2:374–392. [PubMed] [Google Scholar]
- 13.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/S1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Aslam R, Freedman J, Semple JW. Murine platelets express Toll-like receptor 2: a potential regulator of innate and adaptive immunity. Platelets. 2004;15:267. [Google Scholar]
- 15.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y, Ejiri J, Kobayashi S, Hirata K, Kawashima S, Yokoyama M. Expression of Toll-like receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 17.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;88:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 18.Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, Dower SK, Buttle DJ, Sabroe I. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–838. [PubMed] [Google Scholar]
- 19.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 20.Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 21.Patrignani P, Di Febbo C, Tacconelli S, Moretta V, Baccante G, Sciulli MG, Ricciotti E, Capone ML, Antonucci I, Guglielmi MD, Stuppia L, Porreca E. Reduced thromboxane biosynthesis in carriers of Toll-Like receptor 4 polymorphisms in vivo. Blood. 2006;107:3572–3574. doi: 10.1182/blood-2005-12-4811. [DOI] [PubMed] [Google Scholar]
- 22.Ståhl AL, Svensson M, Mörgelin M, Svanborg C, Tarr PI, Mooney JC, Watkins SL, Johnson R, Karpman D. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets via TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayachandran M, Brunn GJ, Karnicki K, Miller RS, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: implications for thrombotic risk. J Appl Physiol. 2007;102:429–433. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 24.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 25.Semple JW, Aslam R, Kim M, Speck ER, Freedman J. Platelet-bound lipopolysaccharide enhances Fc receptor-mediated phagocytosis of IgG opsonized platelets. Blood. 2007;109:4803–4805. doi: 10.1182/blood-2006-12-062695. [DOI] [PubMed] [Google Scholar]
- 26.von Hundelshausen P, Weber C. Platelets as immune cells. Bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 27.Cognasse F, Semple JW, Garraud O. Platelets as potential immunomodulators: is there a role for platelet toll like receptors? Curr Immunol Rev. 2007;3:109–115. doi: 10.2174/157339507780655522. [DOI] [Google Scholar]
- 28.Tremblay T, Aubin E, Lemieux R, Bazin R. Picogram doses of lipopolysaccharide exacerbate antibody-mediated thrombocytopenia and reduce the therapeutic efficacy of intravenous immunoglobulin in mice. Br J Haematol. 2007;139:297–302. doi: 10.1111/j.1365-2141.2007.06777.x. [DOI] [PubMed] [Google Scholar]
- 29.Scott T, Owens MD. Thrombocytes respond to lipopolysaccharide through Toll-like receptor 4, MAP kinase and NFK beta. Mol Immunol. 2007;45:1001–1008. doi: 10.1016/j.molimm.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuckleburg CJ, Tiwari R, Czuprynski CJ. Endothelial cell apoptosis induced by bacteria-activated platelets requires caspase-8 and -9 and generation of reactive oxygen species. Thromb Haemost. 2008;99:363–372. doi: 10.1160/TH07-07-0474. [DOI] [PubMed] [Google Scholar]
- 32.Kuckleburg CJ, McClenahan DJ, Czuprynski CJ. Platelet activation by Histophilus somni and its lipooligosaccharide induces endothelial cell proinflammatory responses and platelet internalization. Shock. 2008;29:189–196. [PMC free article] [PubMed] [Google Scholar]
- 33.Hose JE, Martin GG, Gerard AS. A decapod hemocyte classification scheme integrating morphology, cytochemistry, and function. Biol Bull. 1990;178:33–45. doi: 10.2307/1541535. [DOI] [PubMed] [Google Scholar]
- 34.Götz P, Boman HG. Insect immunity. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology. Biochemistry and pharmacology. Oxford: Pergamon; 1985. pp. 453–485. [Google Scholar]
- 35.Gillespie JP, Kanost MR, Trenczek T. Biological mediators of insect immunity. Annu Rev Entomol. 1997;42:611–643. doi: 10.1146/annurev.ento.42.1.611. [DOI] [PubMed] [Google Scholar]
- 36.Nappi AJ, Vass E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993;6:117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 37.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel–Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 39.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 40.Kuijper PH, Gallardo Torres HI, van der Linden JA, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet-dependent primary hemostasis promotes selectin- and integrin-mediated neutrophil adhesion to damaged endothelium under flow conditions. Blood. 1996;87:3271–3281. [PubMed] [Google Scholar]
- 41.Dole VS, Bergmeier W, Patten IS, Hirahashi J, Mayadas TN, Wagner DD. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thromb Haemost. 2007;98:806–812. [PubMed] [Google Scholar]
- 42.Gawaz M, Neumann FJ, Dickfeld T, Koch W, Laugwitz KL, Adelsberger H, Langenbrink K, Page S, Neumeier D, Schomig A, Brand K. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98:1164–1171. doi: 10.1161/01.cir.98.12.1164. [DOI] [PubMed] [Google Scholar]
- 43.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 44.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/S0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 45.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med. 1996;5:2329–2336. doi: 10.1084/jem.183.5.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-J. [DOI] [PubMed] [Google Scholar]
- 47.Berndt MC, Karunakaran D, Gardiner EE, Andrews RK. Programmed autologous cleavage of platelet receptors. J Thromb Haemost. 2007;5(Suppl 1):212–219. doi: 10.1111/j.1538-7836.2007.02484.x. [DOI] [PubMed] [Google Scholar]
- 48.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102:1931–1936. doi: 10.1161/01.cir.102.16.1931. [DOI] [PubMed] [Google Scholar]
- 49.Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, Lopez JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–814. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H, Reheman A, Chen P, Zhu G, Hynes RO, Freedman J, Wagner DD, Ni H. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost. 2006;4:2230–2237. doi: 10.1111/j.1538-7836.2006.02116.x. [DOI] [PubMed] [Google Scholar]
- 51.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 53.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 54.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 56.Larsen E, Celi A, Gilbert GE, Furie BC, Erban JK, Bonfanti R, Wagner DD, Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59:305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- 57.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of P-selectin- and VCAM-1 dependent mono-nuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res. 1998;84:1237–1244. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 58.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Invest. 1997;100:2085–2093. doi: 10.1172/JCI119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 61.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 62.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.CIR.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 63.Ross R. Platelets, platelet-derived growth factor, growth control, their interactions with the vascular wall. J Cardiovasc Pharmacol. 1985;7(Suppl 3):S186–S190. doi: 10.1097/00005344-198500073-00022. [DOI] [PubMed] [Google Scholar]
- 64.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 65.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–486. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- 68.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 69.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 70.Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993;104:1243–1247. doi: 10.1378/chest.104.4.1243. [DOI] [PubMed] [Google Scholar]
- 71.Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med. 1997;23:379–385. doi: 10.1007/s001340050344. [DOI] [PubMed] [Google Scholar]
- 72.Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Lösche W. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17:263–268. doi: 10.1097/00024382-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leukocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25:843–851. doi: 10.1111/j.1365-2362.1995.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 74.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Severity of multiple organ failure (MOF) but not sepsis correlates with irreversible platelet degranulation. Infection. 1995;23:16–23. doi: 10.1007/BF01710051. [DOI] [PubMed] [Google Scholar]
- 75.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51:639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801–809. doi: 10.1097/00005373-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Vincent J-L, Yagushi A, Pradier O. Platelet function in sepsis. Crit Care Med. 2002;30(Suppl 5):S313–S317. doi: 10.1097/00003246-200205001-00022. [DOI] [PubMed] [Google Scholar]
- 78.Boldt J, Menges T, Wöllbruck M, Sonneborn S, Hempelmann G. Platelet function in critically ill patients. Chest. 1994;106:899–903. doi: 10.1378/chest.106.3.899. [DOI] [PubMed] [Google Scholar]
- 79.Matera C, Falzarano C, Berrino L, Rossi F. Effects of tetanus toxin, Salmonella typhimurium porin, and bacterial lipopolysaccharides on platelet aggregation. J Med. 1992;23:327–338. [PubMed] [Google Scholar]
- 80.Isogai E, Kitagawa H, Isogai H, Matsuzawa T, Shimizu T, Yanagihara Y, Kitami K. Effect of leptospiral lipopolysaccharide on rabbit platelets. Int J Med Microbiol. 1992;271:186–196. doi: 10.1016/s0934-8840(89)80072-6. [DOI] [PubMed] [Google Scholar]
- 81.Saba HI, Saba SR, Morelli G, Hartmann RC. Endotoxin-mediated inhibition of human platelet aggregation. Thromb Res. 1984;34:19–33. doi: 10.1016/0049-3848(84)90103-8. [DOI] [PubMed] [Google Scholar]
- 82.Sheu JR, Hsiao G, Lee C, Chang W, Lee L, Su C, Lin C. Antiplatelet activity of Staphylococcus aureus lipoteichoic acid is mediated through a cyclic AMP pathway. Thromb Res. 2000;99:249–258. doi: 10.1016/S0049-3848(00)00244-9. [DOI] [PubMed] [Google Scholar]
- 83.Youssefian T, Drouin A, Masse JM, Guichard J, Cramer EM. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 2002;99:4021–4029. doi: 10.1182/blood-2001-12-0191. [DOI] [PubMed] [Google Scholar]
- 84.Zucker-Franklin D, Seremetis S, Zheng ZY. Internalization of human immunodef-iciency virus type I and other retroviruses by megakaryocytes and platelets. Blood. 1990;75:1920–1923. [PubMed] [Google Scholar]
- 85.White JG, Clawson CC. Effects of large latex particle uptake of the surface connected canalicular system of blood platelets: a freeze-fracture and cytochemical study. Ultrastruct Pathol. 1981;2:277–287. doi: 10.3109/01913128109048311. [DOI] [PubMed] [Google Scholar]
- 86.White JG, Clawson CC. Effects of small latex particle uptake on the surface connected canalicular system of blood platelets: a freeze-fracture and cytochemical study. Diagn Histopathol. 1982;2:3–10. [PubMed] [Google Scholar]
- 87.White JG. Platelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular system. Platelets. 2005;16:121–131. doi: 10.1080/09537100400007390. [DOI] [PubMed] [Google Scholar]
- 88.Koo SP, Bayer AS, Sahl HG, Proctor RA, Yeaman MR. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klinger MH, Wilhelm D, Bubel S, Sticherling M, Schroder JM, Kuhnel W. Immunocytochemical localization of the chemokines RANTES and MIP-1 alpha within human platelets and their release during storage. Int Arch Allergy Immunol. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 90.Lewis JC, Maldonado JE, Mann KG. Phagocytosis in human platelets: localization of acid phosphatase-positive phagosomes following latex uptake. Blood. 1946;47:833–840. [PubMed] [Google Scholar]
- 91.Welbourn CR, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- 92.McClenahan DJ, Evanson OA, Walcheck BK, Weiss DJ. Association among filamentous actin content, CD11b expression, and membrane deformability in stimulated and unstimulated bovine neutrophils. Am J Vet Res. 2000;61:380–386. doi: 10.2460/ajvr.2000.61.380. [DOI] [PubMed] [Google Scholar]
- 93.Zarbock A, Polanowska-Grabowska R, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Peters MJ, Heyderman RS, Hatch DJ, Klein NJ. Investigation of plateletneutrophil interactions in whole blood by flow cytometry. J Immunol Methods. 1997;209:125–135. doi: 10.1016/S0022-1759(97)00139-7. [DOI] [PubMed] [Google Scholar]
- 95.Mavrommatis AC, Theodoridis T, Orfanidou A, Roussos C, Christopoulou-Kokkinou V, Zakynthinos S. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med. 2000;28:451–457. doi: 10.1097/00003246-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 96.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 97.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 98.Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182:32–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.V98.4.1047. [DOI] [PubMed] [Google Scholar]
- 100.Prasad KSS, André P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces β3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003;92:1041–1048. doi: 10.1161/01.RES.0000070111.98158.6C. [DOI] [PubMed] [Google Scholar]
- 102.Hammwöhner M, Ittenson A, Dierkes J, Bukowska A, Klein HU, Lendeckel U, Goette A. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med. 2007;232:581–589. [PubMed] [Google Scholar]
- 103.Anand SX, Viles-Gonzalez JF, Badimon JJ. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost. 2003;90:377–384. doi: 10.1160/TH03-05-0268. [DOI] [PubMed] [Google Scholar]
- 104.Slupsky JR, Kalbas M, Willuweit A. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 105.Zhou L, Stordeur P, de Lavareille A. CD40 engagement on endothelial cells promotes tissue factor-dependent proco-agulant activity. Thromb Haemost. 1998;79:1025–1028. [PubMed] [Google Scholar]
- 106.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 107.Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, Orpana A, Ristimaki A, Heikinheimo M, Joensuu H. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80:171–175. [PubMed] [Google Scholar]
- 108.Webb NJ, Bottomley MJ, Watson CJ. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci. 1998;94:395–404. doi: 10.1042/cs0940395. [DOI] [PubMed] [Google Scholar]
- 109.Choudhury A, Freeston B, Patel J, Lip GYH. Relationship of soluble CD40 ligand to vascular endothelial growth factor, angiopoietins, and tissue factor in atrial fibrillation. Chest. 2007;132:1913–1919. doi: 10.1378/chest.07-1565. [DOI] [PubMed] [Google Scholar]
- 110.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111:5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 112.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-β in human platelets: identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 113.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White GC, Rompietti R. Platelet secretion: indiscriminately spewed forth or highly orchestrated? J Thromb Haemost. 2007;5:2006–2008. doi: 10.1111/j.1538-7836.2007.02731.x. [DOI] [PubMed] [Google Scholar]
- 115.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 116.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 117.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 118.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 119.Fortin CF, Lesur O, Fulop T., Jr Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007;19:41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- 120.Radsak MP, Salih HR, Rammensee HG, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 121.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 122.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 123.Blanchette V. Childhood chronic immune thrombocytopenic purpura (ITP) Blood Rev. 2002;16:23–26. doi: 10.1054/blre.2001.0176. [DOI] [PubMed] [Google Scholar]
- 124.Wright JF, Blanchette V, Wang H, Arya N, Petric M, Semple JW, Freedman J. Characterization of platelet-reactive antibodies in children with varicella-associated acute immune thrombocytopenic purpura (ITP) Br J Haematol. 1996;95:145–152. doi: 10.1046/j.1365-2141.1996.d01-1872.x. [DOI] [PubMed] [Google Scholar]
- 125.Chia WK, Blanchette VS, Mody M, Wright JF, Freedman J. Characterization of HIV-1-specific antibodies and HIV-1-crossreactive antibodies to platelets in HIV-1-infected haemophiliac patients Br. J Haematol. 1998;103:1365–2141. doi: 10.1046/j.1365-2141.1998.01116.x. [DOI] [PubMed] [Google Scholar]
- 126.Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1–related immunologic thrombocytopenia. Blood. 2005;106:572–576. doi: 10.1182/blood-2005-01-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Musaji A, Cormont F, Thirion G, Cambiaso CL, Coutelier J-P. Exacerbation of autoantibody-mediated thrombocytopenic purpura by infection with mouse viruses. Blood. 2004;104:2102–2106. doi: 10.1182/blood-2004-01-0310. [DOI] [PubMed] [Google Scholar]
- 128.Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812–814. doi: 10.1182/blood.V97.3.812. [DOI] [PubMed] [Google Scholar]
- 129.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878–879. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 130.Stasi R, Rossi Z, Stipa E, Amadori S, Newland AC, Provan D. Helicobacter pylori eradication in the management of patients with idiopathic thrombocytopenic purpura. Am J Med. 2005;118:420–421. doi: 10.1016/j.amjmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 131.Jarque I, Andreu R, Llopis I, De la Rubia J, Gomis F, Senent L, Jiménez C, Martín G, Martínez JA, Sanz GF, Ponce J, Sanz MA. Absence of platelet response after eradication of Helicobacter pylori infection in patients with chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2001;115:1002–1003. doi: 10.1046/j.1365-2141.2001.03194.x. [DOI] [PubMed] [Google Scholar]
- 132.Bang A, Speck ER, Blanchette VS, Freedman J, Semple JW. Recipient humoral immunity against allogeneic leukoreduced platelets is inhibited by aminoguanidine, a selective inhibitor of inducible nitric oxide synthase (iNOS) Blood. 1996;88:2959–2966. [PubMed] [Google Scholar]
- 133.Bang KWA, Speck ER, Blanchette VS, Freedman J, Semple JW. Unique processing pathways within recipient antigen presenting cells determine IgG immune responsiveness against donor platelet MHC antigens. Blood. 2000;95:1735–1742. [PubMed] [Google Scholar]
- 134.Semple JW, Freedman J. Recipient antigen processing pathways of allogeneic platelet antigens: essential mediators of immunity. Transfusion. 2002;42:958–961. doi: 10.1046/j.1537-2995.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- 135.Sayeh E, Sterling K, Speck ER, Freedman J, Semple JW. IgG anti-platelet immunity is dependent on an early innate natural killer cell-derived interferon-γ response that is regulated by CD8 + T cells. Blood. 2004;103:2705–2709. doi: 10.1182/blood-2003-10-3552. [DOI] [PubMed] [Google Scholar]
- 136.Sayeh E, Aslam R, Speck ER, Letien H, Lazarus AH, Freedman J, Semple JW. Immune responsiveness against allogeneic platelet transfusions is determined by the recipient’s MHC class II phenotype. Transfusion. 2004;44:1572–1578. doi: 10.1111/j.1537-2995.2004.04197.x. [DOI] [PubMed] [Google Scholar]
- 137.Semple JW, Speck ER, Fabron A, Jr, Aslam R, Kim M, Freedman J. A novel immunosuppressive pathway involving peroxynitrate-mediated nitration of platelet antigens within antigen presenting cells. Transfusion. 2008;48:1917–1924. doi: 10.1111/j.1537-2995.2008.01793.x. [DOI] [PubMed] [Google Scholar]
- 138.Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine nitration induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 139.Eiserich JP, Estévez AG, Bamberg TV. Microtubule dysfunction by posttranslational nitrotyrosination of α-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Nat Acad Sci USA. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O’Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/S0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 142.Bogdan C. The multiplex function of nitric oxide in (auto)immunity. J Exp Med. 1998;187:1361–1365. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 144.Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis. 1983;128:185–189. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- 145.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 146.Jawa RS, Anillo S, Kulaylat MN. Transfusion-related acute lung injury. J Intensive Care Med. 2008;23:109–121. doi: 10.1177/0885066607312994. [DOI] [PubMed] [Google Scholar]
- 147.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004;126:249–258. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 148.Toy P, Gajic O. Transfusion-related acute lung injury. Anesth Analg. 2004;99:1623–1624. doi: 10.1213/01.ANE.0000138033.24633.4E. [DOI] [PubMed] [Google Scholar]
- 149.Bux J. Transfusion-related acute lung injury: a neglected but life-threatening transfusion reaction. Infusionsther Transfusionsmed. 2004;29:271–276. [Google Scholar]
- 150.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CS. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40L release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. 2007;33:382–384. doi: 10.1007/s00134-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 152.Damås JK, Jensenius M, Ueland T, Otterdal K, Yndestad A, Frøland SS, Rolain JM, Myrvang B, Raoult D, Aukrust P. Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J Immunol. 2006;177:2699–2706. doi: 10.4049/jimmunol.177.4.2699. [DOI] [PubMed] [Google Scholar]
- 153.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 154.Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang G, Han J, Welch EJm, Yem RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cognasse F, Hamzeh-Cognasse H, Garraud O. Platelets “Toll-like receptor” engagement stimulates the release of immunomodulating molecules. Transfusion Clin Biol. 2008;15:139–147. doi: 10.1016/j.tracli.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 157.Miller VM, Jayachandran M, Hashimoto K, Heit JA, Owen WG. Estrogen, inflammation, and platelet phenotype. Gend Med. 2008;5(Suppl A):S91–S102. doi: 10.1016/j.genm.2008.03.009. [DOI] [PubMed] [Google Scholar]