Abstract
Cyclosporine A (CsA) is an immunosuppressive cyclic peptide that binds with a high affinity to 18 kDa human cyclophilin-A (hCyPA). CsA and its several natural derivatives have some pharmacological potential in treatment of diverse immune disorders. More than 20 paralogues of CyPA are expressed in the human body while expression levels and functions of numerous ORFs encoding cyclophilin-like sequences remain unknown. Certain derivatives of CsA devoid of immunosuppressive activity may have some potential in treatments of Alzheimer diseases, Hepatitis C and HIV infections, amyotrophic lateral sclerosis, congenital muscular dystrophy, asthma and various parasitic infections. Here, we discuss structural and functional aspects of the human cyclophilins and their interaction with various intra-cellular targets that can be under the control of CsA or its complexes with diverse cyclophilins that are selectively expressed in different cellular compartments. Some molecular aspects of the cyclophilins expressed in parasites invading humans and causing diseases were also analyzed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0437-0) contains supplementary material, which is available to authorized users.
Keywords: Cyclophilin, PPIase, Cyclosporine A, Immunophilin, Immunosuppression
Introduction
The archetypal cyclophilin-A has been discovered to be a cyclosporine A (CsA) [1] binding protein [2] with catalytical activity for accelerating cis/trans isomerization of X-pro epitopes in model peptides [3, 4]. Several different groups of proteins exhibit peptidylprolyl cis/trans isomerase (PPIase) activity including the immunosuppressive macrocycle (FK506) binding proteins, known as the FKBPs [3, 4]. In a historical sense, the name ‘cyclophilin’ implied cyclic peptide (CsA) binding protein, but this may now be misleading since some cyclophilin-like domains (CLDs) of diverse proteins do not bind CsA, although they may still have PPIase activity [3, 4]. Cyclophilin-A (CyPA), one of its paralogues residing in the endoplasmic reticulum (cyclophilin-B, CyP-B), and a 40 kDa multidomain cyclophilin (CyP-40) have been isolated in sizable quantities which indicate that some members of the cyclophilin family of proteins are abundantly expressed in different mammalian organs [2, 5, 6]. Multiple other paralogues of the human CyPA have been cloned and some of their physico-chemical properties and biological functions established [3, 4]. Sequencing of the human genome has revealed that it encodes multiple cyclophilin-like open reading frames (ORFs) whose expression profiles and biological functions, if any, remain to be elucidated [7]. Likewise, exact biological functions and interaction networks in which are involved the human paralogues of the archetypal hCyPA remain far from being well established [3, 4]. Although considerable progress has been made in molecular characterization of some cyclophilins expressed in diverse parasites invading humans [8], the knowledge of functional aspects of the differentiated repertoires of the cyclophilins expressed in numerous parasites remains scarce [8–10].
Several members of the cyclophilin family of proteins are high affinity binders of the immunosuppressive drug CsA (thus called immunophilins) [3, 4]. Biochemical and immunological assays aided by analyses of the structural aspects of the hCyPA/CsA complex bound to the calcineurin phosphatase have revealed its plausible physiologically relevant modes of action [11–14]. On the PubMed server at the National Centre of Biotechnology Information (NCBI) (http://ncbi.nlm.nih.gov), one may find nearly 5,000 references on the cyclophilins, whereas about ten times that number, nearly 43,500 on 21 April 2010, concern different aspects of diverse medical applications of CsA and its analogues [7]. For example, CsA and some of its derivatives are often utilized in pharmacological interventions against some immunological disorders and in organ transplantation [1, 3, 4, 12, 15].
The cyclophilin-like domains (CLDs) contain from 145 to 180 amino acid residues (AAs) and have highly diversified sequences, but retain a significant conservation of PPIase activity site [3]. The closed β-barrel folds of the CLDs display a high level of structure conservation [16, 17]. The generic PPIase activity, however, is a property of the multiple paralogues of the archetypal CyPA that could imply that those paralogues fulfill some crucial biological functions other than cis/trans isomerization of X-Pro bonds [3, 4]. Moreover, it has been shown that the surface patches distant to the PPIase cavity can bind some epitopes [18]. Various cyclophilins have been localized to different cellular compartments where they are involved in a multitude of complexes, thereby their structures must comprise some patches having the capacity to bind diverse epitopes of intra-cellular entities [3]. It could be postulated that the spatial arrangement of different structural elements within the nominal cyclophilin ‘fold’ supply some plasticity to the surface patches distant to the PPIase cavity that have the capacity to bind various epitopes. In this review, we present analyses of the sequences and structures of the human cyclophilins and their orthologues expressed in some parasites. Our analyses unraveled some inter-connections between differentiation of their sequences and variations within the intra-molecular interaction networks [19]. Also, some functional aspects of the cyclophilins and their interactions with diverse cellular entities that could be in part under the control of CsA and its derivatives were discussed.
The primary binding site of the CLDs (PPIase cavity)
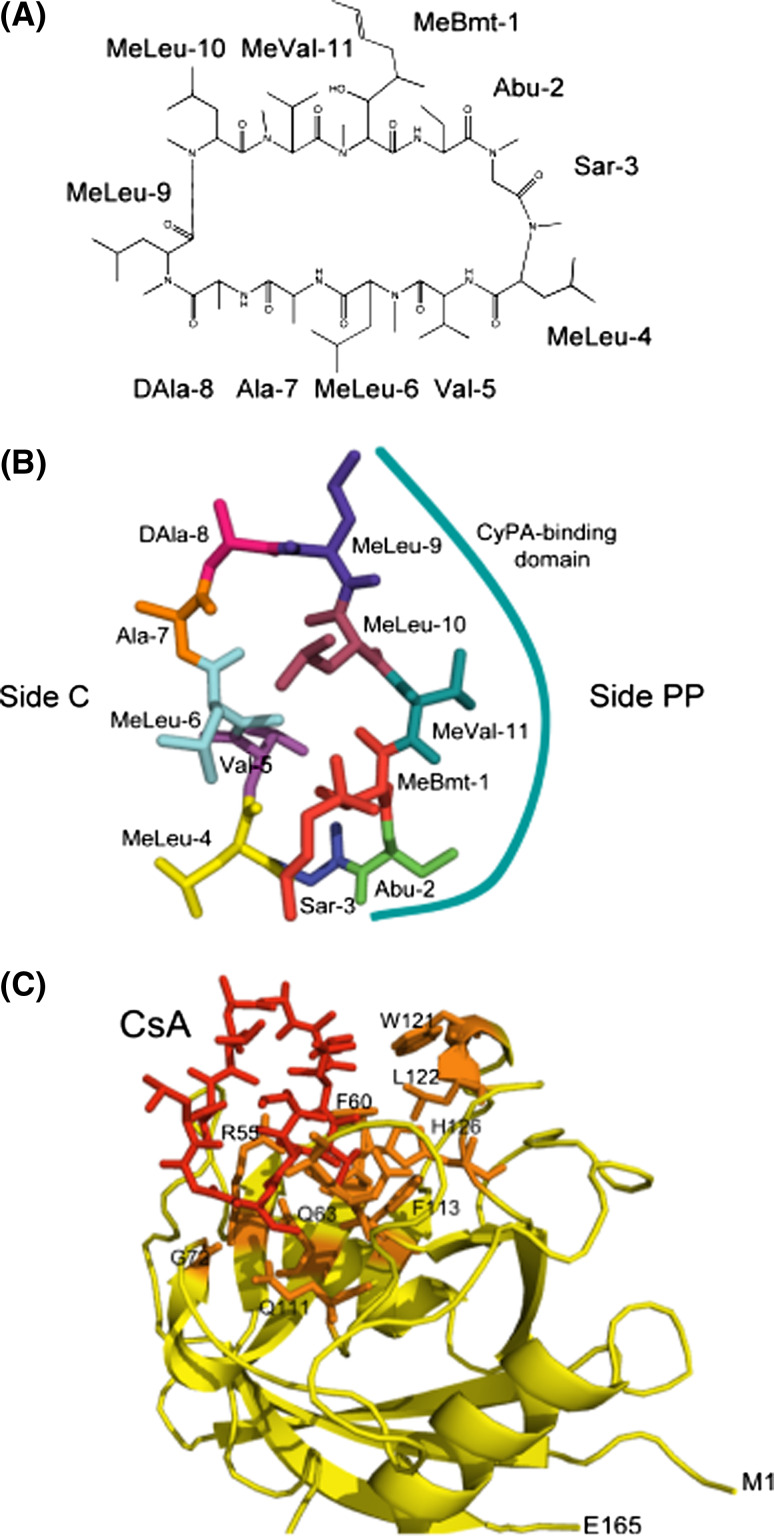
CsA is a hydrophobic cyclic peptide (Fig. 1a) that is soluble in some organic solvents and only sparingly soluble in aqueous solution [3, 4, 20, 21]. Some cyclophilins dissolved in aqueous solution can quickly sequester added CsA with a concomitant loss of their PPIase activity [3, 4]. CsA displays significant conformational polymorphism that is dependent on the chemical composition of the solvent [20–23]. For example, CsA dissolved in chloroform has four internal hydrogen bonds and its conformation is different if bound to hCyPA [23]. The residues of CsA interacting with hCyPA are shown in Fig. 1b and are listed in Table 1A.
Fig. 1.
Cyclosporine-A. a The chemical structure of CsA with the following abbreviations: Abu L-α-aminobutiric acid, MeBmt (4R)-4[(E)-2-butenyl]-4,N-dimethyl-L-threonine, MeLeu N-methylleucine, MeVal N-methylvaline, Sar sarcosine. b CsA structure extracted from its complex with hCyPA (1CWA.pdb); the side interacting with CyPA (side PP) is marked with the solid line and side C with the residues interacting with calcineurin. c Full structure of the hCyPA/CsA complex (1CWA.pdb); CsA is marked as red sticks, the AA residues forming the PPIase cavity are shown as orange sticks while the remaining backbone of the cyclophilin is in yellow. M1 N-terminal methionine, E165 C-terminal glutamic acid
Table 1.
Intermolecular atomic contacts (distance ≤ 4.0 Å) calculated from the X-ray structures of the binary and ternary complexes of some cyclophilins
| A. Complex residue-CsA | (hCyPA/CsA + CnA + CnB) | hCyPA/CsA | BmCyP18/CsA | hCyPF/CsA | ||
|---|---|---|---|---|---|---|
| 1M63.pdb | 1CWA.pdb | 1C5F.pdb | 2Z6W.pdb | |||
| 1 MeBmt1 | 9 | 5 | 7 | 10 | 6 | |
| 2 Abu2 | 21 | 1 | 20 | 21 | 21 | |
| 3 Sar3 | 4 | 3 | 6 | 4 | 5 | |
| 4 MeLeu4 | 13 | 5 | ||||
| 5 Val5 | 2 | 7 | 6 | 3 | 2 | 3 |
| 6 MeLeu6 | 8 | |||||
| 7 Ala7 | 8 | |||||
| 8 DAla8 | 2 | 3 | ||||
| 9 MeLeu9 | 11 | 3 | 16 | 17 | 17 | |
| 10 MeLeu10 | 6 | 7 | 8 | 10 | ||
| 11 MeVal11 | 19 | 20 | 18 | 22 | ||
| Total | 72 | 45 | 16 | 79 | 80 | 84 |
| B. Ligand hCyPA | CsA | CsA | Suc-AGPF-pna | hGag | SfA |
|---|---|---|---|---|---|
| 1M63.pdb | 1CWA.pdb | 1ZKF.pdb | 1M9C.pdb | 1YND.pdb | |
| 1 Arg55 | 7 | 8 | 8 | 10 | 6 |
| 2 Ile57 | 3 | 2 | |||
| 3 Gly59 | 4 | ||||
| 4 Phe60 | 8 | 10 | 8 | 15 | 10 |
| 5 Met61 | 2 | 2 | 2 | ||
| 6 Gln63 | 9 | 6 | 9 | 9 | 9 |
| 7 Asn71 | 2 | ||||
| 8 Gly72 | 8 | 10 | 5 | 7 | 2 |
| 9 Thr73 | 4 | 4 | 5 | ||
| 10 Ala101 | 5 | 4 | 2 | 2 | 7 |
| 11 Asn102 | 13 | 13 | 10 | 8 | 11 |
| 12 Ala103 | 2 | 7 | |||
| 13 Gly104 | 3 | ||||
| 14 Gln111 | 7 | 6 | 2 | 4 | 9 |
| 15 Phe113 | 8 | 9 | 4 | 9 | 5 |
| 16 Trp121 | 5 | 9 | 4 | 8 | 7 |
| 17 Leu122 | 2 | 2 | 3 | 3 | 2 |
| 18 His126 | 3 | 3 | 6 | 6 | 10 |
| 19 Arg148 | 3 | ||||
| Total | 78 | 82 | 68 | 89 | 103 |
CsA and the archetypal hCyPA rapidly form a high affinity complex (1CWA.pdb) that is believed to be the principal vector of therapeutic actions of the immunosuppressive drug [3, 12]. At least 78 inter-molecular atomic distances below 4.0 Å were calculated from the hCyPA/CsA complex; the AAs of hCyPA that are shaded in Table 1B form the hydrophobic PPIase cavity (Fig. 1c) [24]. Some of the atomic contacts below 3 Å give rise to hydrogen bonding between CsA and the atoms in R55, N102 and W121 in hCyPA (Ts1_Dist_all file containing a list of molecular interactions in the complexes listed in Table 1 is in the electronic supplementary material, ESM). CsA bound to hCyPA buries about 50% (Fig. 1c) of its hydrophobic surface [17, 23, 25–27]. We calculated the root-mean-square distance (Rmsd) for the entire cyclophilin structure comprising a free form of hCyPA (2CPL.pdb) [16] and its complex with CsA (1CWA.pdb) [25], and separately for the AAs in its PPIase cavity. Two AAs in the PPIase activity site of hCyPA, namely R55 and Q111, and several other residues such as T5, S21, K31, F67-T68, were affected by the binding of CsA. Different peptides and proteins bind to the CLDs at μM K d [4, 27, 28]. The AAs of hCyPA having inter-molecular atomic contacts (d ≤ 4.0 Å) with a peptide substrate Suc-Ala-Gly-Pro-Phe-pna bound to hCyPA (1ZKF.pdb) [29], and a Gag protein from HIV-1 bound to hCyPA (1M9C.pdb) [30] are shown in Table 1B which illustrates that the CsA-binding site overlaps with the peptide binding site.
Diverse experiments showed that the mammalian CyPA effectively catalyzes only cis/trans isomerization of Pro-X epitopes while its catalytical power is highly diminished if, in place of Pro, there is a six- or four-member ring [3, 4, 31, 32]. Moreover, CyPA does not catalyze isomerization of a secondary amide peptide bond [33, 34]. Both PPIase assays and phage display technique showed that hCyPA has a significant preference for catalyzing and binding Gly-Pro epitopes in linear peptides [35, 36]. Among 400 different dipeptides (diads), only 20 have a Pro in the second position; there are 8,000 triads and 160,000 tetrads that can be formed from 20 natural amino acids [two files containing tabulated data on AA diads (Ts1) and triads (Ts2) computed from the human genomic database are in the ESM]. Analyses of the human genomic database revealed that Gly-Pro is a highly represented diad that is often found in β-turn spatial motifs of many proteins [37]. Proline residue is a particular amino acid that, despite its highly hydrophobic nature, has the highest solubility level in aqueous solution in the series of 20 natural amino acids [38]. Specific affinity of Pro residue to the PPIase cavity may be due to both its hydrophobicity [38] and ring puckering [39], that allow it to well adapt itself to the geometric constraints of the PPIase binding site.
Binding constants for cyclophilin/drug complexes are usually expressed using IC50 (the half maximal inhibitory concentration at which 50% of activity is abolished), K i (the dissociation constant for binding of inhibitor to enzyme) that can be calculated from a series of inhibition curves using one of the available software [3, 4, 40, 41], and K D (binding constant) that can be estimated from binding experiments using, for example, tritiated CsA and a molecular filtration column [2]. Experimental conditions and methods used for the establishment of the inhibition constants usually influence the IC50, K i or K D values. For example, the K is for the hCyPA/CsA complex may vary in certain ranges according to the methods employed for their establishment, namely the K is vary from 30 to 2 nM. Also, chemical modifications of CsA may weaken its binding to CyPA concomitant with the loss of its immunosuppressive activity. Curiously, it was shown, however, that several chemical modifications in both sides of CsA, namely side ‘PP’ which binds to CyPA (Fig. 1b) and side ‘C’ interacting with calcineurin, may enhance its immunosuppressive profile [42, 43].
The CsA/CyPA complex binds with a high affinity to the ternary complex formed with calcineurin-A (CnA), calcineurin-B (CnB) and calmodulin (Cdl) (Table 1A) and inhibits its phosphatase activity [11, 12]. Analyses of the X-ray data of the hCyPA-CsA/(CnA-CnB) complex showed that the CsA residues from 4 to 8 have atomic contacts with CnA and CnB, and thus their modifications may interfere with the tight binding in the ternary complex [13, 14]. Thus, some chemical modifications of residues from 4 to 8 of CsA (side ‘C’, Fig. 1b) may alter its immunosuppressive activity and produce non-immunosuppressive analogues. Moreover, several different natural structural variants of CsA have been characterized, such as CsH and CsG, and many synthetic derivatives of CsA have been assayed for their potentially interesting pharmacological properties in treatment of different diseases [1, 12, 15, 44–47]. Some of those compounds apparently bind to various hydrophobic entities, such as human MDR1 P-glycoprotein ABC transporter or human FPR1 formylpeptide receptor [44, 45]. CyPA also binds several other natural molecules such as sanglifehrin-A (SfA) that was isolated from Streptomyces A [48, 49], cymbimicins isolated from Actinomycetes [50], or cyclolineopeptide A (CLA) isolated from linseed oil [51]. CLA has weaker binding constant to CyPA (K d = 125 nM) that is almost one order of magnitude lower than for the CsA/CyPA complex (K d = from 2 to 30 nM). However, CLA complexed with CyPA binds to CnA/CnB at K d ten times weaker than does the hCyPA/CsA complex, which limits its potential application as an immunosuppressive drug. SfA binds to CyPA with an IC50 = 6.9 nM (Fig. 2) that is lower than IC50 = 20 nM for CsA, but it does not inhibit the CnA/CnB complex and acts as a tolerance inducer in T cells [52, 53].
Fig. 2.
Sanglifehrin A (pink sticks) bound to hCyPA (ribbon structure made from 1YND.pdb [52])
It is worth mentioning, however, that due to the extreme hydrophobicity of CsA and its natural analogues, they may weakly bind to other proteins and diverse hydrophobic moieties [3]. For example, it was shown that interleukin-8 and actin weakly bind at μM ranges to CsA which might suggest that these are rather non-specific interactions [55, 56]. The diversified binding modes and conformational polymorphism displayed by CsA, its natural analogues and other hydrophobic compounds that bind to the various cyclophilins could imply that they might impinge activities of a multitude of cellular entities [3, 4, 12].
Conservation of the primary structure of the human CLDs
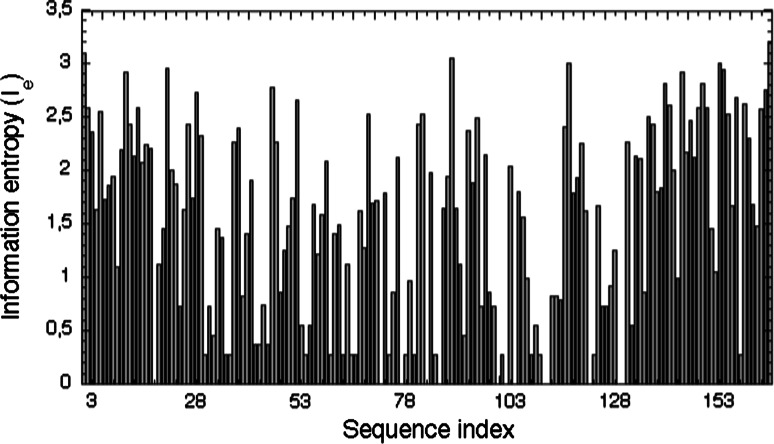
Analyses of several different multiple sequence alignments (MSAs) of the CLDs from different phyla revealed that the AAs, being in the sequence positions equivalent to those of the PPIase activity site of hCyPA, remain well conserved [57]. Fig. Fs1 (in ESM) shows an MSA of the human CLDs with the AAs (in bold face) that are crucial for the PPIase activity whereas in ESM Fig. Fs2 is shown the MSA of 496 sequences comprising various CLDs from diverse organisms. It has been shown that the CLDs from different phyla contain a considerable content of F residues (10–15%) [3]. In consequence, high conservation levels (>0.8) and information entropy I e < 0.9 were calculated for some of the F and Y residues involved in the hydrophobic interaction networks [19]; a plot of I es for the human CLDs is shown in Fig. 3. The low I e values indicate that the given sequence position is well conserved (a low variability in the amino acid composition, AAC), whereas the I e ≥ 1.0 indicate a higher variability of the AAC at the given sequence position. Although the averaged sequence similarity scores (IDs) in the analyzed MSAs of the cyclophilins are below 40%, some sequence patches crucial for the PPIase activity and fold-structuring interaction networks remain highly conserved (I es ≤ 1.0).
Fig. 3.
Information entropy (I e) histogram for the human CLDs (see ESM Fig. Fs5). Low I e values indicate that thye given sequence position is well conserved (a low variability in the AAC) whereas the I e ≥ 1.0 indicate for a higher variability of the AAC at the given sequence position (maximal I e for 20 natural AAs equals to log220 = 4.322)
Analysis of the aligned sequences of the human CLDs (ESM Figs. Fs1 and Fs2) revealed that the AAs crucial for PPIase activity and the ‘aromatic/hydrophobic’ AAs network are well conserved in all the 19 sequences (MSA19) of the human CLDs. For example, the equivalent sequence positions occupied by R55 in hCypA, that is critical for its catalytic activity, are well conserved in the sequences of the human CLDs (MSA19). It has, however, been recently shown that the R55/A55 substitution in hCyPA did not apparently influence the folding rate of carbonic anhydrase, which implies that R55 is crucial for the PPIase activity only if a linear peptide substrate is being used, while the protein itself behaves as a chaperone capable of rescuing misfolded molten globule intermediates [58, 59]. Likewise, the CLD of hCyP58 binds a Gly-Pro epitope of a linear peptide but does not influence its cis/trans equilibrium [54]. R55 was replaced by a P residue in the hCyP18c isoform, whereas in hCyP57 an R residue is positioned two AAs downstream. No data, however, exist to show whether the CLD of hCyP57 has PPIase activity. Q63 (hCyPA) is crucial for PPIase activity in the cyclophilins, and it is conserved in all the aligned sequences with the exception of the hCyP18c isoform, whereas the highly conserved Q111-F112-F113 triad (hCyPA) is also well conserved in the MSA19. W121 (hCyPA), a crucial AA in the PPIase activity site in hCyPA, is replaced in several human CLDs by H or Y residues which may significantly alter the Kd with CsA and its derivatives [3]; see also ESM Fig. Fs3.
Subtle variations within the intra-molecular interaction networks in the CLDs
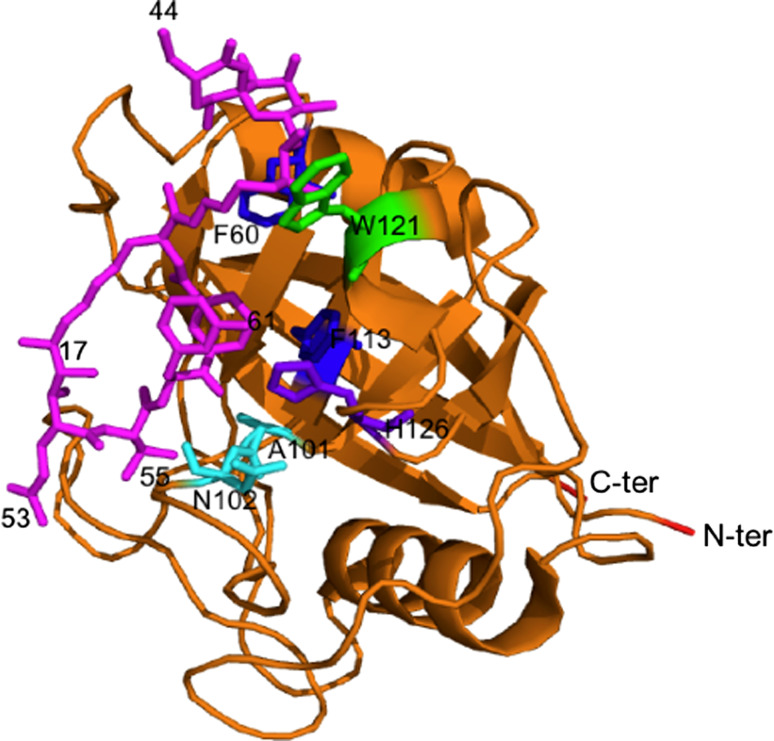
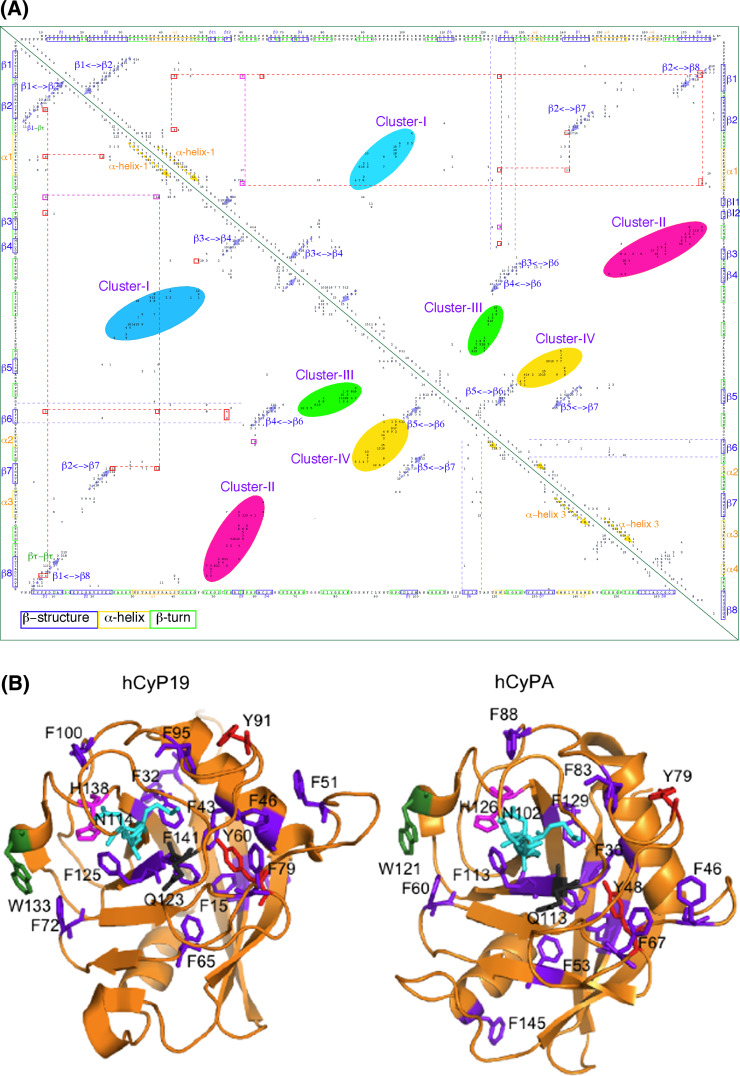
Several X-ray structures of the cyclophilins [60] that are listed in ESM Table Ts3 were analyzed with the Cordan_Pro program, and triangular distance maps were generated for each of the structures [19]. The distance maps computed from the structures of hCyP19 (1QOI.pdb, upper triangle), that is involved in some stages of formation of the spliceosome [18], and the archetypal hCyPA (2CPL.pdb, lower triangle) are shown in Fig. 4a, whereas in Fig. 4b are shown the X-ray structures (ribbon display) with the explicitly indicated spatial positions of some aromatic AAs and several others from the PPIase cavity. The interaction networks of the aromatic residues are shown as red dotted lines; those networks also involve C, V, I and L (violet dotted lines). The aromatic/hydrophobic AAs network seems to have a substantial structuring capacity in the cyclophilin’s fold [3]. These two maps (Fig. 4a) have similar distributions of the intra-molecular interaction clusters to that shown in shown in ESM Table Ts1 which indicate that the tertiary structures of the CLDs are well conserved [17]. Orange, green and blue rectangles in the sequences axe at the top of Fig. 4a (ESM Fig. Fs4a is its high-resolution PDF image) correspond to α-helix, β-turn and β-strand structures, respectively. Interactions between β-strands in anti-parallel β-sheets that are close in the sequence form the perpendicular clusters (blue arrows) close to the diagonal, whereas the interactions between β-strands that are distant in the sequence form the clusters that are distant to the diagonal. Atomic interactions within α-helices are local, and thus their clusters (yellow arrows) are parallel to the diagonal of the 2D map; the remaining near-diagonal elements contain all the interactions between the neighboring AA-residues (close in the sequence). β-sheets and α-helices interact with diverse loops and β-turns and form specific clusters and super-clusters of interactions (color ellipsoids). For example, supercluster-2 (a salmon oval) is made up of three mini-clusters, namely: (1) β-strand 8 interacting with the loop linking β-strands 2 and 3; (2) the β-turns flanking β-strand 3 interacting with the β-turns in front of β-strand 8; and (3) α-helix 3 interacting with the β-turns following β-strand 4. The remaining three super-clusters consist of from 2 to 5 mini-clusters comprising diverse interactions between some elements of the secondary structure with the loop regions. Some β-strands have sparsely distributed networks of interactions, for example β-strand 6 (blue dotted lines).
Fig. 4.
a 2D map of intra-molecular atomic distances calculated in the range 2.7–4.5 Å in the structures of hCyP19 [18] (1QOI.pdb, upper triangle) and hCyPA (2CPL.pdb, lower triangle) [16]. Integer numbers correspond to the sum of the number of inter-residues distances (NIDs) for given pairs of AA residues. At the upper axis and the right side is shown the sequence of hCyP19, whereas the bottom axis has the sequence of hCyPA. b X-ray structures of hCyP19 (1QOI.pdb, left panel), and hCyPA (2CPL.pdb, right panel); the spatial positions of F (violet), Y (red), W (deep green), catalytically crucial MAN motif, E111 (hCyPA) and H126 (hCyPA) are indicated in cyan, black and pink sticks, respectively. The ribbon structures were made with the PyMol program [61]; see Fs4A in ESM
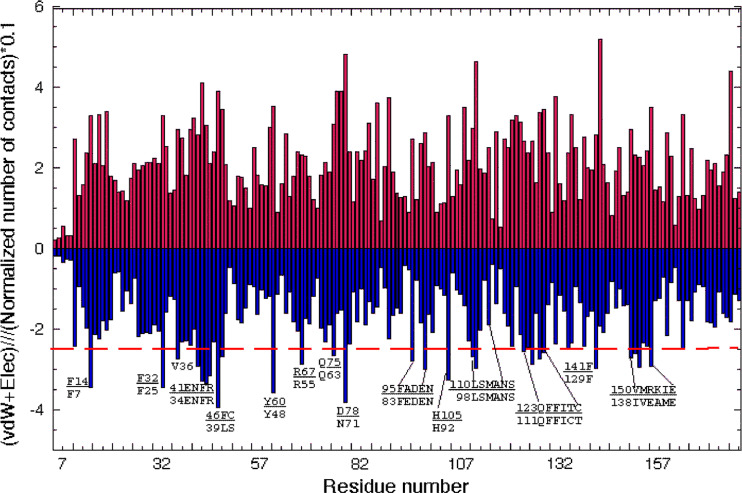
Distribution of the numbers of distances and the sum of the van der Waals (vdW) and Coulombic energy terms calculated per AA residue [19] in hCyP19 (1QOI.pdb) are shown in Fig. 5. The AAs having higher than average energy contributions are indicated in the graph whereas at the bottom of each residue are given their equivalents in the sequence of the archetypal hCyPA. The majority of the AAs indicated in the graph are crucial in the PPIase activity, binding of CsA, and ‘aromatic/hydrophobic’ AA network. For example, R67 corresponds to R55 in hCyPA and is crucial for cis/trans isomerization of X-Pro epitopes in model peptides [4]. It is preceded by a highly conserved Y60 (Y48 in hCyPA) and is followed by Q75 (Q63 in hCyPA) that are a part of the PPIase activity site. As shown in Fig. 5, the AAs participating in the ‘aromatic/hydrophobic’ network and being a part of the PPIase activity site contribute significant stabilization energy terms. The AAs crucial for PPIase activity and structuring aromatic/hydrophobic network have the most significant vdW and Coulombic contributions to stability of the structure.
Fig. 5.
Numbers of interactions (upper panel) and the sum of vdW and Coulombic energy terms [19] per each residue (lower panel) calculated from the X-ray structure of hCyP19 (1QOI.pdb) with written patches of sequences that are crucial for the PPIase cavity and the aromatic/hydrophobic AAs network; red dashed line was arbitrarily placed to indicate the largest energy contributions. The upper parts of the sequence patches come from hCyP19 whereas lower parts were taken from the sequence of hCyPA
Analyses of 2D distance maps of different cyclophilins revealed that the ‘aromatic/hydrophobic’ network consisting of 12 AAs in the archetypal hCyPA (F7, F8, F22, F36, Y48, F53, L98, F112, I114, F129, I158, C161) remains well conserved in the majority of its human paralogues (see ESM Table Ts4) although not all of them exhibit cis/trans isomerization activity with standard peptide substrates; the sequence positions equivalent to the above hydrophobic network of the AAs are well conserved in the different MSAs of the cyclophilins [3, 56]. Larger differences were derived from the comparison of the interaction patterns of the free forms of hCyPA (2CPL.pdb) and hCyP19 (1QOI.pdb) which implies that the AA substitutions caused some subtle changes in the fold. Comparing the two structures yielded Rmsd = 0.344 for the CA atoms of the ‘aromatic/hydrophobic’ AAs network and Rmsd = 0.274 for the CA atoms of the AAs in the PPIase activity site. Despite the ID = 53% for hCyP19/hCyPA, the low values of the Rmsds indicate for a good conservation of the spatial positions of these two groups of crucial AAs.
The I es for the AAs forming the PPIase activity site are small in the MSA containing 496 sequences of the CLDs (MSA496; ESM Fig. Fs5), and in MSA19 (Fig. 3). However, there are multiple subtle AA changes taking place in each functional sub-group of the CLDs [57]. Even if relatively few AA substitutions took place in the triads comprising the AAs implicated in PPIase activity and in the aromatic/hydrophobic AAs network (see ESM Fig. Fs3), the AA triads become highly variable inbetween the conserved patches of AAs reaching the high I es at particular sequence positions. We hypothesize that these highly variable sequence patches are the sites of functional optimization of diverse physicochemical properties of the CLDs, such as PPIase activity levels, structural stability, spatial distribution of charged AAs, hydrophobicity of binding surface patches, and its affinity to diverse epitopes.
Diversified binding sites of the CLDs
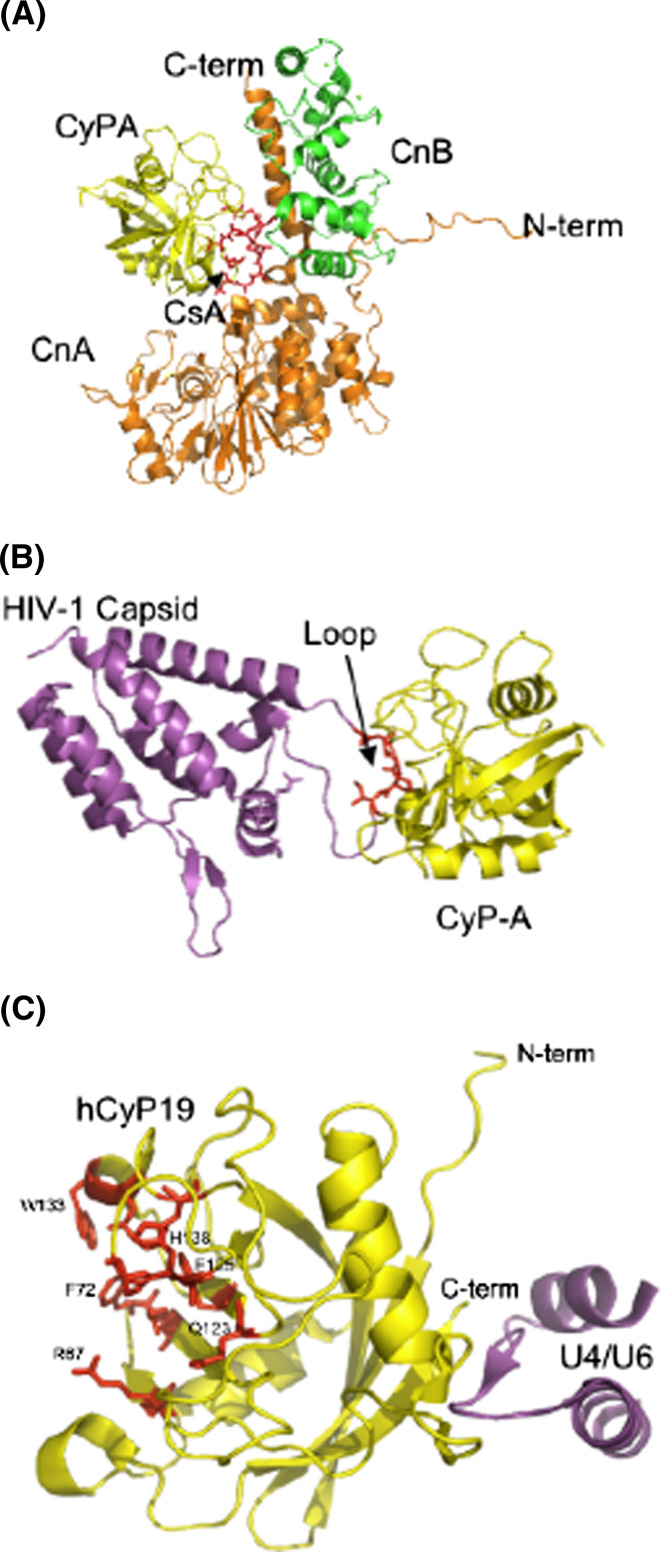
Inter-molecular distances calculated from X-ray structures of several ternary complexes between diverse CLDs and their ligands were summarized in Table 1. hCyPA/CsA binds to CnA/CnB complex (1M63.pdb [13] and 1MF8.pdb [14]) in a somewhat different fashion than is the case for the hFKBP12a/FK506 complex bound to CnA/CnB [62]. Figure 6a shows the structure of the ternary complex (hCyPA/CsA + CnA/CnB) in which CsA is embedded inside its interior (CsA is shown in red sticks marked with an arrow). There are multiple contacts between the residues of CsA and CnA, whereas a smaller number of them exist between CsA and CnB (Ts1_Dist_all file in ESM).
Fig. 6.
Three X-ray structures of the binary and tertiary complexes; a the (hCyPA/CsA + CnA/CnB) complex [13]; CyPA (yellow) binds to CsA (red, indicated with a black arrow) and interacts with CnA (orange ribbons) and CnB (green ribbon) complex. b X-ray structure of HIV-1 capsid protein (violet) bound to the PPIase cavity of hCyPA (yellow ribbon); the loop in red comes from the capsid protein (1M9C.pdb, [30]). c X-ray structure of a short peptide from the U4/U6 snRNP-60K (violet) protein interacting with the hCyP19 (yellow ribbon) with some of the AAs forming its PPIase cavity (red sticks) (1MZW.pdb, [18])
The HIV Gag protein binds to the PPIase cavity of hCyPA with X-Pro bond in a cis conformation as shown in the structure on Fig. 6b (1M9C.pdb) [63, 64]. There are numerous atomic contacts between the peptide and PPIase activity site that are similar to those existing in a complex between cyclophilin and a model peptide Suc-AGPF-pna bound to CyPA (1ZKF.pdb) (Table 1B). The solution [65] and X-ray [18] structures of the complex between a peptide coming from the U4/U6 snRNP-60K protein, which is known to interact with the spliceosomal cyclophilin hCyP19 [18], have revealed that the interaction network between the peptide and hCyP19 involves the AAs that are distant to its PPIase cavity; Fig. 6c shows the X-ray structure of the hCyP19/(the U4/U6 peptide) complex (1MZW.pdb).
Sequence attributes of the CLDs and some biological functions of the multidomain cyclophilins
Table 2 summarizes essential data, such as physicochemical properties, chromosomal localization and some of the biological functions of the human cyclophilins. The PDB codes are for human cyclophilins with two exceptions, namely for Mus musculus (Mm) and Bos taurus (Bt). Only 9 cyclophilins are mono-domain but their sequences are highly diversified. The remaining 11 expressed forms of the human cyclophilins contain distinct domains such as 40-residue Trp-Asp containing β propeller repeat domain (WD40), tetratricopeptide repeat (TPR), RNA-binding (RRM), leucine-rich repeat (LRR), nucleporin domain (Nup), Really-Interesting-Gene-Product (RING) domain, and serine/arginine-rich (SR) domains that are crucial for assembly of spliceosomal complexes. For example, TPR, WD40 and LRR motifs are involved in protein–protein interactions, RING domain is a zinc-finger probably involved in interaction with DNA, RRM interacts with RNAs, and Nup domain is crucial for nucleoporin (hCyP358) forming pores in the nuclear membrane. Known cellular localizations are also indicated, namely nuclear, cytoplasmic, secreted form, membrane associated, and mitochondrial protein. Overall binding capacity of the cyclophilins to CsA are coded as strong for K d or K i ≤ 50 nM, weak for these two values larger than 400 nM, and unknown if no experimental value was established.
Table 2.
The expressed forms of the human cyclophilins and some of their biological functions
| No. | Protein name (alias) | Code | PDB | Chromosome/gene | Naa | Mass (Da) | Entire protein | CLD | Extra- domain |
B_Pra | Cellular localization and biological functionsb |
Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pI | HI | CLD-length | pI; HI | |||||||||||
| 1 | CyP18a (hCyPA) | NP_066953 | 2CPL | 7p13-p11.2/PPIA | 165 | 18013 | 7.8 | 37.6 | Full | St | (C/S) Immunosuppression | [1–4, 11–15] | ||
| Cell-related events | [66, 67] | |||||||||||||
| HIV-1 replication | [68, 69] | |||||||||||||
| HCV replication | [70, 71] | |||||||||||||
| 2 | CyP18b* | NP_839944 | 1q21.1/PPIAL4G | 164 | 18182 | 9.6 | 34.1 | Full | Un | (N) Oncogene | [72] | |||
| 3 | CyP18c (hCyPJ) | NP_115861 | 1XYH | 2q33.1/PPIL3a | 165 | 18627 | 6.6 | 27.3 | Full | Un | (N) Spliceosome | [73] | ||
| 4 | CyP18ci | NP_570981 | 2q33.1/PPIL3b | 161 | 18155 | 6.3 | 27.3 | Full | Un | (N) Spliceosome | [73] | |||
| 5 | CyP18d (CGI-124) | NP_057143 | 1XWN | 6p21.1/PPIL1 | 166 | 18237 | 8.2 | 33.7 | Full | Un | (N) Spliceosome, cancer growth | [73, 74] | ||
| 6 | CyP22b/p (hCyPB) | NP_000933 | 1CYN | 15q21-q22/PPIB | 208 | 22742 | 9.8 | 42.3 | 26-208 | 9.7; 38.3 | St | (ER/S) Protein folding | [5] | |
| TPRV6 channel-associated | [75] | |||||||||||||
| 7 | CyP22C/p (hCyPC) | NP_000934 | 2RMC (Mm) | 5q23.2/PPIC | 212 | 22763 | 8.7 | 52.8 | 28-208 | 9.4; 48.1 | St | (Memb) Unknown | [76] | |
| 8 | CyP22D/p (hCyPF) | NP_005720 | 2BIT,2Z6 W | 10q22-q23/PPIF | 207 | 22040 | 9.8 | 39.1 | 36-207 | 9.3; 35.8 | St | (Mito) Mitochondrial pore | [77] | |
| Mitochondria/Muscular dystrophy | [78] | |||||||||||||
| Hexokinase II/apoptosis | [79] | |||||||||||||
| Mitochondrial function | [80] | |||||||||||||
| 9 | CyP19 (hCyPH) | NP_006338 | 1MZW,1Q0I | 1p34.1/PPIH | 177 | 19208 | 8.1 | 44.1 | Full | St | (N) Spliceosome | [73] | ||
| Pre-RNA splicing | [81] | |||||||||||||
| 10 | CyP33 (hCyPE) | NP_006103 | 1ZCX,1ZMF | 1q32/PPIE | 301 | 33431 | 5.3 | 33.2 | 130-301 | 8.4; 32.0 | RRM | St | (N) RNA-binding/spliceosome | [82] |
| 11 | CyP35 (hCyP35) | NP_775943 | 6q21/PPIL6 | 311 | 35228 | 6.6 | 35.0 | 140-311 | 7.6; 35.5 | RRM | Un | (N) Organism growth | [83] | |
| 12 | CyP40 (hCyPD) | NP_005029 | 1IIP (Bt) | 4q31.3/PPID | 370 | 40764 | 6.8 | 31.6 | 6-192 | 6.8; 38.0 | TPR | Wk | (C) HSP-steroid receptor | [84] |
| 13 | CyP46 | NP_689542 | 14q21.1/PPIL5 | 414 | 46723 | 9.3 | 48.3 | 90-360 | 9.2; 43.5 | LRR | Un | (C/N) Signal transduction | [85] | |
| 14 | CyP54 | NP_005860 | 5q12.3/SDCCAG10 | 472 | 53847 | 5.5 | 16.3 | 1-180 | 5.6; 31.7 | WD40 | Un | (N) Cancer marker/spliceosome | [86] | |
| 15 | CyP57 | NP_624311 | 6q24-q25/PPIL4 | 492 | 57225 | 5.6 | 20.9 | 1-165 | 5.0; 33.9 | RRM | Un | (N) Spliceosome | [87] | |
| 16 | CyP58 (hCyP60) | NP_680480 | 1ZKC | 22q11.21/PPIL2 | 520 | 58824 | 9.0 | 22.5 | 271-446 | 8.6; 22.7 | RING | St | (N) Spliceosome | [73] |
| 17 | CyP73 | NP_056157 | 2A2 N | 5q12.3/PPWD1 | 645 | 73444 | 6.7 | 32.7 | 476-645 | 6.8; 30.0 | WD40 | Un | (N) Spliceosome | [73] |
| 18 | CyP88 (hCyPG) | NP_004783 | 2GW2 | 2q31.1/PPIG | 754 | 88618 | 10.7 | 8.5 | 1-184 | 8.4; 34.8 | SR | St | (N) Spliceosome | [88] |
| 19 | CyP157 | NP_005376 | 2HE9 | 3p23-p11/NKTR | 1403 | 157712 | 10.3 | 13.3 | 51-238 | 8.3; 37.2 | SR | St | (N) Spliceosome | [89] |
| 20 | CyP358 | NP_006258 | 2q11-q13/RANBP2 | 3224 | 358221 | 5.8 | 25.7 | 3024-3224 | 6.7; 35.9 | Nup | Un | (N) Nucleoporin | [90, 91] | |
aOverall binding capacity of the cyclophilins: strong (St) for Kd or Ki ≤ 50 nM, weak (Wk) for these two values larger than 400 nM, and unknown (Un) if no experimental value was established
bKnown cellular localizations: N nuclear, C cytoplasmic, S secreted form, Memb membrane associated, Mito mitochondrial protein
The CLDs have the overall hydrophobicity (HI) ranging from low values, e.g., for the hydrophilic orthologues of hCyP58, to the highly hydrophobic membrane-associated CyPB, CyPC, CyPF and the spliceosome-associated hCyP19 [18, 57]. The overall hydrophobicity (HI) of some cyclophilins is very low which is due to a high content of charged AAs in the SR domains. The cyclophilins are present in different cellular compartments where they are involved in diverse biological functions such as assembly of mRNA splicing supracomplexes [73, 81], mitochondrial pore regulation [77–80], association to different molecular channels [85], and functioning as chaperone and folding co-factors [3].
Human TRIM5α is one of more than 70 tripartite motif-containing proteins that may impede HIV-1 replication. It has been shown that the cells of some monkeys living in South America express a fusion protein containing TRIM5α and a CLD that probably is one of the host factors restricting HIV-1 proliferation in the animal [92]. Multiple human ORFs have very similar sequences to those of the CLDs in the TRIM5α-cyclophilin fusion proteins that are expressed in the apes. Perhaps, due to evolutionary pressure, one of the human ORFs encoding a homologous CLD could undergo a gene fusion with the human TRIM5α gene and such a fusion protein would become a host restriction factor against HIV-1 infection [93].
The cyclophilins and diverse human diseases
Numerous reports have appeared on the involvement of CyPA and its diverse paralogues in functioning of different cellular entities [3, 4], some of which are summarized in Table 2. In this section, we discuss just four issues: (1) CsA-induced immunosuppression; (2) extracellular activities of some cyclophilins; (3) hypothetical involvement of CyPA in some stages of diseases caused by HIV-1 and HCV; and (4) cyclophilins and development of cancer cells.
CsA-induced Immunosuppression
The mechanism of CsA-induced suppression of T cell activation by antigen-presenting cells (T cell anergy) was proposed about two decades ago [11, 94]. It was postulated that the hCyPA/CsA complex binds to calcineurin and blocks its phosphatase activity that causes the retention of a phosphorylated form of the transcription factor called nuclear factor for activation of T cells (NF-AT) in the cytosol [11]. Pull-down experiments revealed that the hCyPA/CsA [11] and murine cyclophilin C (mCyPC) complexed to CsA were capable of binding calcineurin [94]. This would suggest that some of the paralogues of the archetypal hCyPA complexed to CsA, such as hCyPB/CsA or hCyPC/CsA, could bind to the phosphatase and block its active site. The membrane-associated mCyPC was not the physiologically relevant target for CsA since only the cytosolic CyPA bound to CsA supposed to be responsible for the inactivation of CnA/CnB in T cells, although no in vivo evidence was supplied to support such a mechanism of action [95]. Moreover, it should be mentioned that CyPA is an abundantly expressed protein whose PPIase activity cannot be entirely abolished in vivo since the concentration of CsA or its analogues would have to reach pharmacologically unacceptable levels [2, 5]. This would imply that the immunosuppressive effect of CsA in T cells could become effective because the expression level of the CnA/CnB phosphatase is low.
Although the PPIA gene is non-essential for mammalian cell viability, its knock-down rendered the mouse resistant to the immunosuppressive effects of CsA [96, 97]. Nothing was, however, established about what other effects could be induced by CsA in the mouse with the PPIA(−/−) gene. For example, it would be relevant to establish if CsA binds to the extracellular pool of mCyPB and if the complex may enter into T cells in the animal. Were some cellular processes controlled by the other paralogues of mCyPA affected by CsA in mouse with the PPIA(−/−) gene?
Extracellular effects of the cyclophilins
CyPB is the major component of the extracellular pool of the cyclophilins, namely a sizeable quantity of CyPB (ca. 20 μM), was found in the plasma [98, 99]. It could be secreted together with certain extracellular proteins and it should be able to sequester some quantity of the in vivo-administered CsA. It was also shown that a modified form of CsA anchored to the surface of T cells could exert its immunomodulatory effects [100], as negatively charged CsA derivatives rest in the extracellular space [101] whereas CsA binds well to the membranes in a temperature-dependent fashion [102]. If CsA binds to the extracellular pool of hCyPB, then such a complex has two crucial attributes for entering into the cell’s interior, namely the large exposed hydrophobic patch of CsA should have some affinity to the hydrophobic environment of the membranes while the positively charged patches of CyPB should induce a substantial driving force for entry into T cells. To what extent the extracellular pool of the hCyPB/CsA complex formed in vivo may enter into T cells and induce immunosuppression remains to be investigated [103].
hCyPB was shown to be involved in cell adhesion [104]. Osteogenesis imperfecta is a disease causing growth deficiency and reduced bone mass. This particular deficiency could be due to a mutation in the PPIB gene encoding hCyPB [105, 106]. Although the level of the extracellular CyPA was too low for detection by an Elisa assay [94], it was claimed that the secreted form of hCyPA is crucial for matrix assembly by hensin that is involved in epithelial differentiation [107]. Large numbers of secreted gene products [7] and extracellular domains (ECDs) of various receptors could interact with the extracellular pool of the small PPIases. But what exact biological effects could be due to those myriads of potential intermolecular interactions? Extracellular matrix re-organization, differentiation of cells, and their growth and proliferation are dependent on in vivo locally secreted gradients of growth factors (morphogens), proteases and other crucial factors [108, 109]. Perhaps the extracellular forms of hCyPA and hCyPB have an overall housekeeping function rather than exerting a profound influence on some crucial biological events. Assessment of functional interactions between the recombinant forms of the cyclophilins and distinct populations of cells under in vitro conditions, in some cases could have been misleading due to sizeable contamination of the former by the bacterial lypopolysaccharide (LPS), a powerful inducer of diverse cellular effects. For example, another abundantly expressed small protein found both in the interior of the cell and in the extracellular space, called macrophage migration inhibitory factor (MIF), had been considered to be a cytokine, although its biological activity was proven to be induced by the associated bacterial LPS [110, 111]. It should be noted, however, that CyPA, CyPB and MIF could be easily isolated from natural sources in a relatively large quantity [2, 5, 112]. Thus, application of CyPA and CyPB isolated from mammalian organs and free of LPS for in vitro assessments of their influence on diverse cells could resolve some doubts caused by the usage of the recombinant LPS-contaminated cyclophilins expressed in E. coli.
CyPA and infections caused by HIV-1 and Hepatitis C viruses (HCV)
Although hCyPA was found to be associated with HIV-1 viruses, probably bound to the Gag (CA) protein, the relevance of CyPA for proliferation of HIV-1 virions have remained enigmatic during the last 15 years [30, 68, 69, 113]. It has been suggested that binding of hCyPA to the CA protein shelters the latter from being sequestered by the cellular factors TRIM5α/Ref that may restrict replication of HIV-1 [114, 115]. The whole-genome siRNA interference experiments have pinpointed numerous intracellular targets that are crucial for different stages of the HIV-1 live cycle such as uncoating of the HIV-1 virus, its intracellular transport and integration with the host DNA, transcription of its 15 genes, assembly of genetic material as its coating, and, finally, budding of newly assembled viral particles from the host cell [115–117]. It is unknown if CyPA or some of its minor isoforms are crucial for any of the multiple candidate proteins established with the siRNA technique [116, 117].
Non-immunosuppressive analogues of CsA, namely Debio-025 or SCY-635, may alter certain replication steps of HCV [70, 71] and as such they could become useful as an additional drug to fight HCV infections in humans. These drugs could bind to the intracellular pool of CyPA and CyPB and interfere with a HCV replication step. However, Debio-025 or SCY-635 could suppress HCV infection due to its interaction with one of the paralogues of CyPA whose expression level is low in the hepatocytes or impair other cellular entities crucial for replication of HCV.
Cyclophilins, intra-cellular signaling and development of cancer
Even if CyPA and some of its paralogues are highly expressed proteins in human cells [2, 5], it has been suggested that they may be involved in some stages of cancer cell development and proliferation [67]. Recent reports show that CyPA interacts with Tp53 and Stat3 which are powerful regulators of gene transcription [118]. CyPA is also a crucial factor for CXCR4-activation transport of the heterogeneous nuclear ribonucleoprotein A2 [66], a spliceosomal RNA-binding protein. The hydrophobic cavity of CyPA and its different paralogues could be associated to various exposed loops of proteins or receptors, but what physiological effects all those interactions may have and to what extent those associations and their possible disruption by CsA could alter some cellular processes still remain unclear [3, 108]. The molecular events leading to formation of primary cancer cells, such as mutations of genes or other chromosomal alterations related to DNA repair or deletion of chromosomal segments, may create diversified populations of cancer cells [119–122]. To what extent the heterogeneous transformations of the genetic material leading to aberrant induction of gradients of morphogens and other auxiliary growth factors and extracellular matrix remodeling enzymes could be controlled by the ubiquitous and abundantly expressed CyPA or its other isoforms still remain unexplored.
The cyclophilins in the protozoan parasites affecting humans
Basic information on the cyclophilins expressed in some protozoan parasites affecting humans is summarized in Table 3. From 11 to 15 different cyclophilins isoforms are encoded in the genomes of diverse parasites [9, 10]. Figure 7 shows overall sequence organization of the cyclophilins encoded in three different genomes that is only to some extent similar to that of the human cyclophilins’ repertoire. The inhibitory constants for the complexes between CsA and diverse cyclophilins are similar to that of the archetypal human CyPA/CsA, which limits the potential therapeutic application of the drug against invading parasites [8, 124, 137].
Table 3.
Overall characteristics of some cyclophilins encoded in the genomes of parasites invading humans
| Parasite | Species (disease) | Cyclophilin | Acc. No. | Observations | Ref. | CsA and its derivatives PPIase activity inhibition | Ref. | CsA and its derivatives Parasiticidal effect | Ref. | Probable CsA mechanism of action |
|---|---|---|---|---|---|---|---|---|---|---|
| Apicomplexa | Plasmodium falciparum (Malaria) | PfCyP19A | XP_001351290 | CyP highly expressed in intra-erythocytic parasites. Mature protein isolated | [124] | CsA K d = 13 nM, K i = 6.9 nM similar K d/K i with 11 CsA-analogs | [124] | [val2]CsA inhibited parasites. PSC-833 EC50 = 0.032 mM CsA = 0.32 mM | [124, 125] | P- glycoprotein as target molecule PfCyP19/CsA can bind to calcineurin |
| PfCyP22 | XP_001351841 | Ibid. ER signal peptide. Mature protein isolated | [126] | CsA IC50 = 10 nM | [126] | |||||
| PfCyP24 | XP_001349469 | CyP highly expressed at the late-ring parasite stage | [127] | Trophozoites more sensitive to CsA due to a high level of PfCyP24 expression | [127] | |||||
| Toxoplasma gondii (Toxoplasmosis) | TgCyP18 | AAA17997 | Mature protein isolated. Secreted. CyP binds to CCR5 receptor. Induces IL-12. Inhibits HIV infectivity by co-receptor antagonism | [128, 129] | CsA IC50 = 32 nM | [128] | T. gondii less susceptible than Plasmodium parasites. SDZ 215-918 EC50 = 0.45 mg/ml with low affinity for T. gondii CyPs | [123] | P- glycoprotein is likely the molecular target | |
| TgCyP20 | XP_002367801 | Mature protein isolated. N-terminal sequence with identity to calcineurin | [128] | CsA IC50 = 5 nM | [128] | |||||
| TgFCBP57.3 | AAX51680 | Dual immunophilin, N-terminal FKBP and C-terminal CLD Essential for parasite survival. | [130] | FK506 IC50 = 70 nM CsA IC50 = 750 nM | [130] | |||||
| Trypanosomatids | Leishmania major (Cutaneous Leishmaniasis) | LmCyP19 | CAA73904 | Cyps of 18, 19, 22 and 40 kDa were CsA affinity isolated. LmCyP19, the most expressed. No affinity for calcineurin | [131, 133] | CsA K i = 5.2 nM | [131] | Extracellular L. major growth inhibition. Relatively insensitive to CsA | [134] | A single AA mutation in LmCyP19 fails to interact with calcineurin |
| Leishmania donovani (Visceral Leishmaniasis) | LdCyP | AAD46565 | CyP of the endoplasmic reticulum. Secreted to culture medium under oxidative stress | [132] | CsA K d = 135 nM | [135] | [135] | Low expression of LdCyP in the cytosol Insensitivity to CsA | ||
| Trypanosoma brucei (Sleeping sickness) | TbCyP19 | AAB07896 | Expressed and differentially transcribed Secreted protein. Cytosolic and flagellum localized | [136] | CsA binds TbCyP19 No inhibition experiments | [136] | ||||
| Trypanosoma cruzi (Chagas´ disease) | TcCyP19 | AAF05985 | TcCyP19, TcCyP22, TcCyP28 and TcCyP40 isolated by CsA affinity. TcCyP19 major cytosolic isoform, present in all parasite stages. Mitochondrial TcCyP21was identified. | [9] | CsA IC50 = 14 nM and H-7-94 analog IC50 = 12 nM | [137, 138] | Trypanocidal activity in vitro and in vivo. H-7-94 CsA analog is the most effective: TcCyP19 IC50 = 12.5 nM, T. cruzi EC50 = 0.8 mM, NIM811 is not effective | [137] | Besides TcCyPs also P-glycoprotein could be a molecular target | |
| Amebae | Entamoeba histolytica (Enteric diseases) | EhCyp | AAB86601 | 60 to 70% sequence identity with the other cyclophilins. Transcribed and expressed. | [139] | PPIase Inhibition with CsA at nM concentration | [139] | CsA EC50 at 1–10 mg/ml on thophozoites | [139, 140] | Interference the activity of calcineurin and P-gP |
| Flagellates | Giardia intestinalis (Enteric diseases) | GiCyP1 | XP_001707838 | Found as a single gene. Similar to EhCyP and hCyP18 | [141] | Total PPIase Inhibition with 500 nM CsA | [141] | |||
| Coccidia | Cryptosporidium parvum (Cryptosporidiosis) | CsA EC50 at 1.5 mM and IC50 of 1 mM for PSC-833 derivative | [142] | CsA inhibits CnA PSC-833 binds to P-glycoprotein | ||||||
| For comparison | Homo sapiens | hCyPA | CAA37039 | Cyclophilin like domain characteristics in the text. | CsA IC50 = 20 nM, NIM811 K i = 2.1 nM, Debio-025 K i = 0.34 nM H-7-94 IC50 = 70 nM PSC-833 does not bind hCyPA | [8, 9, 137, 143] |
Fig. 7.
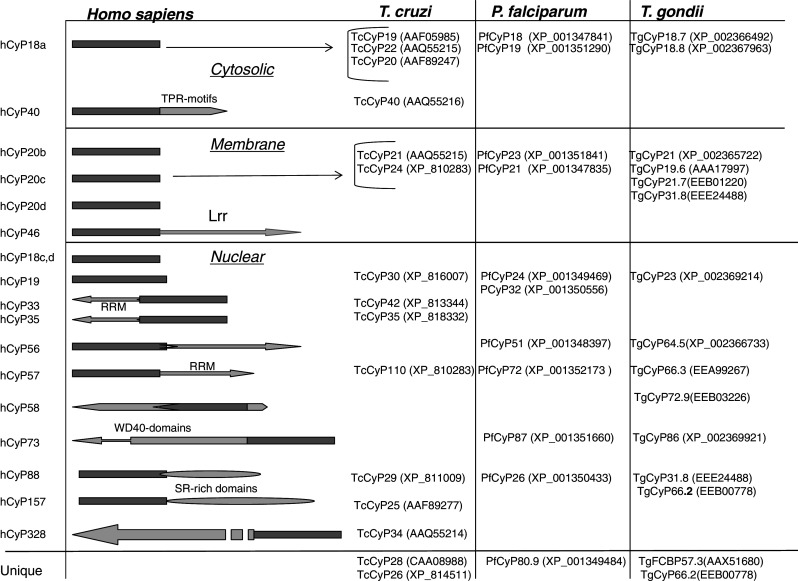
The human cyclophilins and their respective orthologues encoded in the genomes of Trypanosoma cruzi, Plasmodium falciparum and Toxoplasma gondii. The proteins were arranged according to two criteria, namely (1) presence of similar domains, and (2) similar sequence attributes for their respective CLDs in the cases where the large cyclophilins were aligned with their small homologues
It has been shown that the extracellular growth of Leishmania donovani is resistant to the actions of CsA which seems to be due to a low expression level of the archetypal cytosolic CyPA in this parasite [135]. It has also been suggested that the mammalian host cell CyPA seems to be involved in the intracellular parasite’s replication cycle of L. major parasites, as CyPA siRNA interference or CsA reduced the parasite burden [134]. Some of the small cyclophilins from Plasmodium falciparum and Toxoplasma gondii (TgCyP18) have similar inhibitory profiles as those established for the hCyPA (Table 3) [124, 126–129]. Interestingly, TgCyP18 has the capacity to bind to the CXCR5 receptor and block the HIV infectivity on human T cells [129]. T. gondii also expresses an unusual protein (TgFCBP57.3) containing one FKBP-like protein (N-terminus) and one CLD (C-terminus) linked together via three tetratricopeptide (TPR) motifs. Both domains can bind to CsA and FK506, respectively, but only the FKBD/FK506 complex could inhibit the endogenous calcineurin [130]. Knockdown of this dual family gene interfered with the growth of the parasite [130].
In proteomic analyses of Leishmania mexicana (Lm), belonging to Trypanosomatids, LmCyP19, LmCyP22, LmCyP28 and LmCyP40 paralogues were identified [133] that are orthologues to the cyclophilins of T. cruzi (Tc) eluted from CsA-affinity column [9]. The genome of T. cruzi does not encode the fully conserved orthologues of the human cyclophilins containing RNA binding domains probably due to the unusual type of mRNA processing in Trypanosomatids, in which polycistronic transcripts are processed to mature mRNAs through trans-splicing [144]. In contrast, those orthologues are encoded in the genomes of the Apicomplexa parasites [10]. Orthologous genes to that encoding TcCyP40, which is related to the heat shock protein hCyP40, were also found in the genomes of L. mexicana and L. major (Fig. 7) [9].
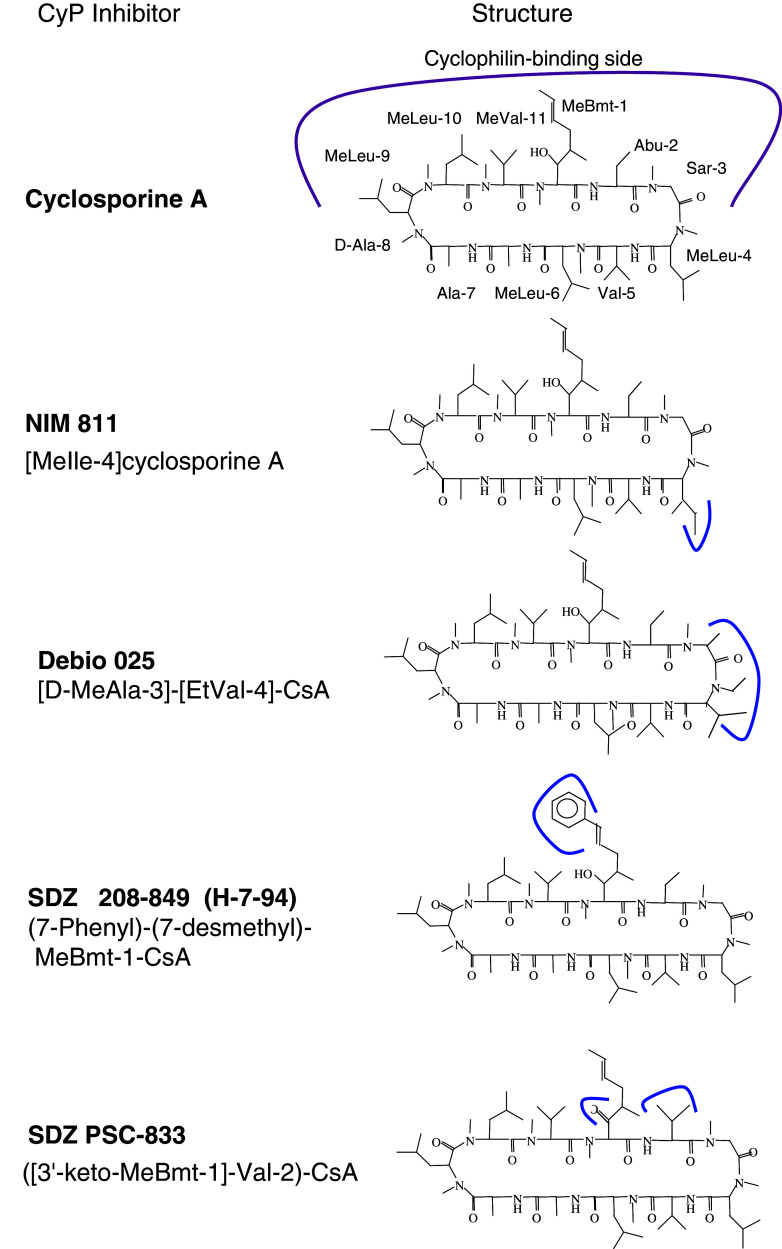
It was demonstrated that CsA has anti-parasitic activity against a wide variety of parasites [8] with the exception of Leishmania that was resistant to the actions of CsA [135]. Figure 8 shows several derivatives of CsA which proved to exhibit anti-parasitic effects on Cryptosporidium parvum, Plasmodium falciparum, Trypanosoma cruzi and Entamoeba histolytica (see Table 3). Curiously, some of these compounds were more effective in inhibiting live functions in the parasites than CsA itself [137], and some of them did not bind the cytosolic cyclophilins [142]. This would imply that CsA and its derivatives could exert their parasiticidal effects that are not necessarily mediated by the archetypal cytosolic CyPA. Moreover, even if the tentative model of immunosuppression in humans involves hCyP18a/CsA inhibition of phosphatase activity of calcineurin in T cells, this model may not be valid in other organisms including the parasites.
Fig. 8.
Chemical structures of derivatives of CsA devoid of immunosuppressive activity in humans. Continuous lines indicate the places where chemical modifications were made while stripped line on CsA indicates the residues that should be hidden inside the PPIase cavity
For example, evidence that the CsA analogs with poor or no immunosuppressive activity were parasiticidal also argues against a mechanism involving calcineurin as the target of various CyPA/(CsA-derivative) complexes. It is possible that anti-parasitic activity of CsA could be due to its binding to other targets such as P-glycoprotein (P-gP), in which the some CsA analogues proved to be potent modifiers of its activity [123, 137, 142]. It was suggested, however, that calcineurin was the target of CsA complexed to the cytosolic cyclophilin expressed in E. histolytica [140]. Some information about the properties of the cylophilins expressed in Helminths is compiled in ESM Table Ts4.
Even if more than three decades have elapsed since the discovery of CsA as an effective parasiticidal drug little is so far known about the mechanisms of its anti-parasitic activity. Moreover, targeting of CsA or its non-immunosuppressive derivatives to the archetypal CyPA of the parasites affecting humans cannot be done selectively since hCyPA and some of its isoforms are highly expressed in diverse organs and tissues and are strong binders of those molecules. Thus, selective targeting of small natural [145] or synthetic molecules to the minor isoforms of the cyclophilins expressed in the parasites could become a method for curing some human diseases (see Table 3).
Perspectives on selective targeting of the multiple isoforms of the cyclophilins
Multiple side effects observed in the long-term usage of CsA in organ transplant recipients, especially the severe nephrotoxicity, remain one of the major obstacles for a broader use of the drug in the treatment of inflammation and other immune disorders [1, 3, 4, 15]. In fact, CyPA is highly expressed in kidneys with a large portion of it tightly associated with the renal brush border membranes [146]. Non-immunosuppressive derivatives of CsA display some potential for being used in treatment of HIV-1 and HCV infections [143]. But are they nephrotoxic? CsA derivatives are cytoprotective (in vitro and ex vivo) when bound to hCyPD [147, 148]. For example, despite the deep differences between apoptosis and necrosis, the mitochondrial permeability transition pore (mPTP) was found to be involved in both types of cell death, but since the mPTP can be blocked by the CyPD/CsA complex, then cells are protected from injury and death [149]. Pharmacological targeting of the cyclophilins could be useful for treatments of Alzheimer [148], Huntington’s disease [150], amyotrophic lateral sclerosis [151], and congenital muscular dystrophy [152], whereas CsA in aerosol form could be useful in the treatment of asthma [153] and airway inflammations [154].
Despite more than twenty-five years of research on the cyclophilins and their ligands still relatively few cyclophilins have been thoroughly investigated and many singular findings have never been independently confirmed or exploited [3, 4]. Among the diverse communications published to date only in a few of them have some uncertain results been addressed. For example, it was proven that the recombinant CyPA does not have nuclease activity [155] and the apparent activity recorded for the recombinant forms of hCyPA, hCyPB and hCyPC must have been due to a nuclease contaminant from E.coli [156]. Involvement of the calcium-modulating cyclophilin ligand (CAML) in restriction of cellular release of the HIV-1 retroviruses seems to be doubtful [157, 158]. Originally, CAML was found to be associated with the endoplasmic reticulum-residing CyPB [159].
If SfA and CsA bound to hCyPA cause different immunological effects, then it would be interesting to know if there are some other natural compounds that could bind to hCyPA and subtly influence different immune processes than those controlled by SfA, CsA and its various derivatives [160, 161]. There are no conclusive findings so far on the selective targeting of the PPIase cavity in diverse cyclophilins that function in various cellular compartments. For example, elucidation of crucial functional aspects of the nine cyclophilins (see Table 2) involved in the supraspliceosome assembly and other nuclear complexes could open some ways for pharmacological intervention in the transcription and splicing of the mRNAs [162–164]. It is still uncertain, however, to what extent the PPIases are associated with different extracellular domains (ECDs) of some receptors [3, 108, 165], as well as which functions of the intracellular proteins [166, 167] and their complexes are dependent on particular members of the cyclophilin family of proteins. Rough estimates have revealed that there are about 7,000 hydrophobic proteins some of which are expressed on the cell surface [38, 168]. Moreover, Pro residues account for about 6.5% of the total amino acid composition of proteins encoded in the human genome, and about 0.2% of the diads Gly-Pro occur in the human sequences (see ESM Ts1) [168]. This could imply that some of those residues in diverse proteins should be accessible for binding to the PPIase cavity of the cyclophilins although it has been shown that linear peptides containing the sequences of proteins that were proven to bind to CyPA do not always bind to the PPIase cavity [3, 4, 36]. It is thus crucial to find out what functional meanings, if any, might those diverse complexes have involving the cyclophilins, and if bound to CsA or the other ligands having high affinity to the cyclophilins [3], which cellular effects could they produce.
Conclusions
The members of the cyclophilin family of proteins are encoded in various genomes ranging from prokaryotes to higher animals [3, 4, 57], including the genome of Mimi virus although the latter cyclophilin lacks any PPIase activity [170]. Diverse paralogues of hCyPA are present in the nuclear compartment where they form complexes with various entities including one of the most sophisticated complexes known as the supraspliceosome entities [162]. Our analyses of the MSAs, 2D distance maps and Rmsds of specific groups of AAs, such as those involved in binding of CsA or being in the ‘aromatic/hydrophobic’ network, revealed that there is a high sequence conservation of the AAs forming the PPIase cavity (ESM Fs3 and Ts4). Moreover, the low I es indicate that some sequence positions remain highly conserved in the cyclophilins from diverse phyla (ESM Fs5) despite multiple AA mutations and gene duplication events that took place during evolution of living species. The highly conserved sequence positions belong to two classes, namely (1) the AAs crucial for maintaining the structural integrity of the CLDs (the extensive interaction networks of the hydrophobic AAs), and (2) functionally-crucial AAs for PPIase activity and specific interactions with diverse cellular targets [3]. The conserved short patches of the AAs are spaced with the AA patches with low levels of sequence conservation that impose the final pI and hydrophobicity of the given CLD.
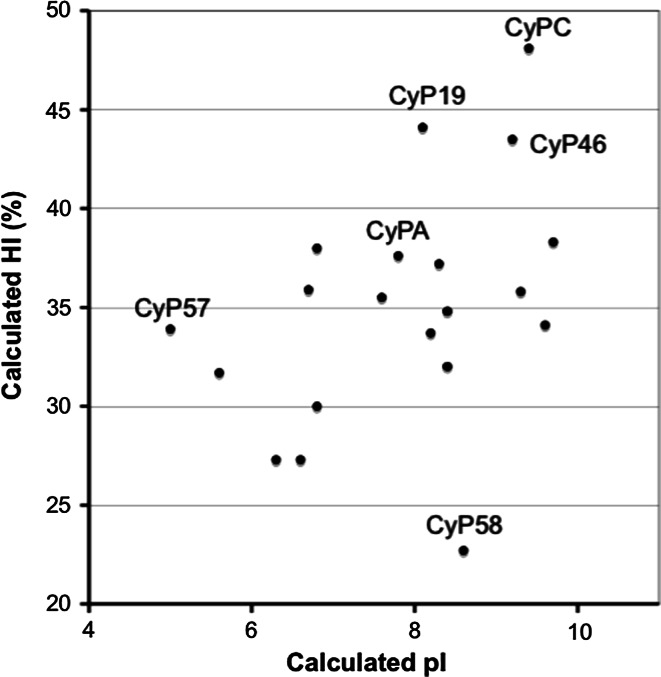
We hypothesize that subtly changed spectra of physicochemical characteristics, such properties as amino acid (AA) hydrophobicity and bulkiness distribution, flexibility of the polypeptide chain and charge distribution, of the AA residues in the sequence patches with low sequence conservation induced ‘functional drift of sequence attributes’ [19]. In consequence, this ‘functional drift’ had to influence the intra-molecular interaction networks in the closed β-barrel fold of the CLDs [3] that adapted their overall properties to: (1) recognition profiles of their specific targets (biological profiles) [3, 18, 36, 172], and (2) the micro-environmental conditions inherent to various intracellular organelles (evolutionary niche). For example, the HIs of the human CLDs (Table 2) vary in a considerable range, namely from 23% for hCyP58 (also known as hCyP60) to 48% for hCyPC (Fig. 9). CyPC, CyPB and CyPF are the hydrophobic proteins associated with the membranes, whereas hCyP40 could interact with the membrane proteins via its hydrophobic TPR motifs [3, 168].
Fig. 9.
Distribution of hydrophobicity versus pI of the human CLDs (see Table 2)
Moreover, it could be theorized that the hydrophilic CLD of hCyP58, the hydrophobic CLD of hCyP73 and the extremely hydrophobic hCyP19 are the cyclophilins that associate to specific spliceosomal entities using their optimized levels of hydrophobic patches (HI) on its surface and specific spatial charge distribution (pI). Our analyses may also indicate that fine alterations in the intra-molecular interaction patterns within the CLD’s fold are crucial for their selective binding to diverse epitopes including CsA and its diverse analogues. Whether subtle functional changes in some cellular entities, such as neurotransmitter-gated ion channels [171] or cellular signalization cascades [3, 12], are solely due to intrinsic temperature-driven cis/trans isomerization of their X-Pro epitopes (conformational switches) is one possibility. Another possibility is that they may be due to a long-distance conformational allostery induced by one of the PPIases [169, 172, 173]. Unraveling the fine structural basis of in vivo specificity of the cyclophilins to their targets, however, would require a sizable number of high-resolution X-ray structures of their complexes with relevant cellular targets functioning in various organelles.
Diversified cellular effects induced by CsA and its derivatives and especially relevance of their in vivo aspects that are dependent on the formation of various interaction networks involving calcineurin, NF-ATs, TGFβ and other intra- and extracellular proteins are still at the beginning of exploration [174–176]. The large number of the ECDs containing accessible proline epitopes and the multitude of intracellular proteins that can be associated with the cyclophilins imply that there may be myriads of subtle cellular effects induced by CsA and its derivatives. For example, CsA can be used as an immunosuppressive drug [3, 4, 11], its derivative called valspodar (PSC 833) [177, 178] may add enhanced retention of anticancer drugs in the cell by blocking its P-gP activity, whereas some derivatives as NIM811 or Debio-025 could be useful drugs for suppression of viral infections by HIV-1 and HCV provided that they are devoid of profound nephrotoxic activity. In conclusion, in vivo-induced subtle interactions between the cyclic peptide CsA, and its non-immunosuppressive diverse derivatives [179] and diverse targets remain for thorough exploration. Certainly, it is challenging to selectively uncover in vivo essential extracellular and intracellular vectors for CsA and its derivatives and to classify the multiple effects exerted by the drugs on diverse in vivo relevant cellular events.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Multiple sequence alignments of the human CLDs (MSA19) (PDF 47 kb)
MSA of 496 CLDs from various cyclophilins (MSA496) (PDF 490 kb)
MSA496.out containing information on the aligned sequences. (PDF 108 kb)
Mutations in the triads (MSA19) that contain crucial AA residues for PPIase activity and CsA-binding in hCyPA (PDF 21 kb)
A high resolution image of Figure 4A (PDF 1151 kb)
Graph computed from the MSA496 (TIFF 1468 kb)
Statistical distribution of AA-diads computed from the human genomic database. (PDF 50 kb)
Statistical distribution of AA-triads computed from the human genomic database. (PDF 556 kb)
Analyses of the X-ray structures of diverse cyclophilins from vertebrates and some parasites. (PDF 62 kb)
Conservation levels of several different sequence motifs of hCyPA deduced from the MSA496. (PDF 27 kb)
Some of the cyclophilins encoded in Helminths. (PDF 129 kb)
Contains a list of molecular interactions of the complexes shown in Table 1. (PDF 141 kb)
Acknowledgments
We are indebted to SIMOPRO/iBiTec/DSV/CEAEA (Saclay) and INP, ANLIS Malbrán and Grant Number D43TW007888 from the Fogarty International Centre, NIH for financial support.
References
- 1.Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporine A: a new antilymphocytic agent. Agents Actions. 1994;43:179–186. doi: 10.1007/BF01986686. [DOI] [PubMed] [Google Scholar]
- 2.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporine A. Science. 1984;226:544–546. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 3.Galat A, Riviere S. Peptidylprolyl cis/trans isomerases. Oxford: Oxford University Press; 1998. [Google Scholar]
- 4.Edlich F, Fischer G. Pharmacological targeting of catalized protein folding: the example of peptide bond cis/trans isomerases. Hand Exp Pharmacol. 2006;172:359–404. doi: 10.1007/3-540-29717-0_15. [DOI] [PubMed] [Google Scholar]
- 5.Galat A, Bouet F. Cyclophilin B is an abundant protein whose conformation is similar to that of cyclophilin A. FEBS Lett. 1994;347:31–36. doi: 10.1016/0014-5793(94)00501-x. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer LJ, Thalhammer T, Handschumacher RE. Isolation and characterization of a 40-kDa cyclophilin-related protein. J Biol Chem. 1992;267:5503–5507. [PubMed] [Google Scholar]
- 7.Wheeler DL, Church DM, Federhen S, Lash AE, Madden TL, Pontius JU, Schuler GD, Schriml LM, Sequeira E, Tatusova TA, Wagner L. Database resources of National Center for Biotechnology. Nucleic Acids Res. 2003;31:28–33. doi: 10.1093/nar/gkg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell A, Monaghan P, Page AP. Peptidyl-prolyl cis-trans isomerases (immunophilins) and their roles in parasite biochemistry, host-parasite interaction and antiparasitic drug action. Int J Parasitol. 2006;36:261–276. doi: 10.1016/j.ijpara.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Potenza M, Galat A, Minning TA, Ruiz AM, Duran R, Tarleton RL, Marín M, Fichera LE, Bua J. Analysis of the Trypanosoma cruzi cyclophilin gene family and identification of cyclosporine A binding proteins. Parasitology. 2006;132:867–882. doi: 10.1017/S0031182005009558. [DOI] [PubMed] [Google Scholar]
- 10.Krücken J, Greif G, von Samson-Himmelstjerna G. In silico analysis of the cyclophilin repertoire of apicomplexan parasites. Parasit Vectors. 2009;2:27–51. doi: 10.1186/1756-3305-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporine A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 12.Sigal NH, Dumont F. Cyclosporine A, FK506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 13.Huai Q, Kim HY, Liu Y, Zhao Y, Mondragon A, Liu JO, Ke H. Crystal structure of calcineurin-cyclophilin-cyclosporineshows common but distinct recognition of immunophilin-drug complexes. Proc Natl Acad Sci USA. 2002;99:12037–12042. doi: 10.1073/pnas.192206699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin L, Harrison SC. Crystal structure of human calcineurin complexed with cyclosporine A and human cyclophilin. Proc Natl Acad Sci USA. 2002;99:13522–13526. doi: 10.1073/pnas.212504399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahelin HF. The history of cyclosporine A (Sandimmune) revisited: another point of view. Experientia. 1996;52:5–13. doi: 10.1007/BF01922409. [DOI] [PubMed] [Google Scholar]
- 16.Ke H, Zydowsky LD, Liu J, Walsh CT. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 Å resolution. Proc Natl Acad Sci USA. 1991;88:9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke H, Huai Q. Crystal structures of cyclophilin and its partners. Front Biosci. 2004;9:2285–2296. doi: 10.2741/1396. [DOI] [PubMed] [Google Scholar]
- 18.Reidt U, Wahl MC, Fasshauer D, Horowitz DS, Luhrmann R, Ficner R. Crystal structure of a complex between human spliceosomal cyclophilin H and a U4/U6 snRNP-60K peptide. J Mol Biol. 2003;331:45–56. doi: 10.1016/s0022-2836(03)00684-3. [DOI] [PubMed] [Google Scholar]
- 19.Galat A. Functional drift of sequence attributes in the FK506-binding proteins (FKBPs) J Chem Inf Model. 2008;48:1118–1130. doi: 10.1021/ci700429n. [DOI] [PubMed] [Google Scholar]
- 20.Loosli H-R, Kessler H, OshkiNatureH WeberH-P, Petcher TJ, Widmer A. The conformation of cyclosporine A in the crystal and in solution. Helv Chim Acta. 1985;68:682–704. [Google Scholar]
- 21.Kessler H, Kock M, Wein T, Gehrke M. Reinvestigation of the conformation of cyclosporine A in chloroform. Helv Chim Acta. 1990;73:1818–1832. [Google Scholar]
- 22.Altschuh D, Braun W, Kallen J, Mikol V, Spitzfaden C, Thierry C, Vix O, Walkinshaw MD, Wuthrich K. Conformational polymorphism of cyclosporine A. Structure. 1994;2:963–972. doi: 10.1016/s0969-2126(94)00098-0. [DOI] [PubMed] [Google Scholar]
- 23.Wuthrich K, von Freyberg B, Weber C, Wider G, Traber R, Widmer H, Braun W. Receptor-induced conformation change of the immunosuppressant cyclosporine A. Science. 1991;254:953–954. doi: 10.1126/science.1948082. [DOI] [PubMed] [Google Scholar]
- 24.Zydowsky L, Etzkorn FA, Chang HY, Ferguson SB, Stolz LA, Ho SI, Walsh CT. Mutagenesis of human cyclophilin A defines the enzyme active site and separate peptidyl-prolyl isomerase activity from cyclosporin-A binding and calcineurin inhibition. Prot Sci. 1992;1:1092–1099. doi: 10.1002/pro.5560010903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikol V, Kallen J, Pflugl G, Walkinshaw MD. X-ray structure of a monomeric cyclophilin A-cyclosporine A crystal complex at 2.1 Å resolution. J Mol Biol. 1993;234:1119–1130. doi: 10.1006/jmbi.1993.1664. [DOI] [PubMed] [Google Scholar]
- 26.Kallen J, Spitzfaden C, Zurini MGM, Wider G, Widmer H, Wuthrich K, Walkinshaw MD. Structure of human cyclophilin and its binding site for cyclosporine A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 27.Braun W, Kallen J, Mikol V, Walkinshaw MD, Wuthrich K. Three-dimensional structure and actions of immunosuppressants and their immunophilins. FASEB J. 1995;9:63–72. doi: 10.1096/fasebj.9.1.7529736. [DOI] [PubMed] [Google Scholar]
- 28.Kallen J, Walkinshaw MD. The X-ray structure of a tetrapeptide bound to the active site of human cyclophilin A. FEBS Lett. 1992;300:286–290. doi: 10.1016/0014-5793(92)80865-e. [DOI] [PubMed] [Google Scholar]
- 29.Eisenmesser EZ, Thai V, Pozharski E, Kern D. Mechanistic insights of cyclophilin-A from X-ray cyrstallographic and nuclear magnet resonance investigations (1ZKF). http://www.rcsb.org/pdb (accessed October 2007)
- 30.Gamble TR, Vajdos FF, Yoo S, Worthylake DK, Houseweart M, Sundquist WI, Hill CP. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 31.Radzicka A, Acheson SA, Wolfenden R. Cis/trans isomerization at proline: desolvation and its consequences for protein folding. Bioorg Chem. 1992;20:382–386. [Google Scholar]
- 32.Zhao Y, Ke H. Crystal structure implies that cyclophilin predominantly catalyzes the trans to cis isomerization. Biochemistry. 1996;35:7356–7361. doi: 10.1021/bi9602775. [DOI] [PubMed] [Google Scholar]
- 33.Stein RL. Mechanism of enzymatic and nonenzymatic prolyl cis-trans isomerization. Adv Protein Chem. 1993;44:1–24. doi: 10.1016/s0065-3233(08)60562-8. [DOI] [PubMed] [Google Scholar]
- 34.Scholz C, Scherer G, Mayr LM, Schindler T, Fischer G, Schmid FX. Prolyl isomerases do not catalyze isomerization of non-prolyl peptide bonds. Biol Chem. 1998;379:361–365. doi: 10.1515/bchm.1998.379.3.361. [DOI] [PubMed] [Google Scholar]
- 35.Harrison RK, Stein RL. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry. 1990;29:3813–3816. doi: 10.1021/bi00468a001. [DOI] [PubMed] [Google Scholar]
- 36.Piotukh K, Gu W, Kofler M, Labudde D, Helms V, Freund C. Cyclophilin A binds to linear peptide motifs containing a consensus that is present in many human proteins. J Biol Chem. 2005;280:23668–23674. doi: 10.1074/jbc.M503405200. [DOI] [PubMed] [Google Scholar]
- 37.MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol. 1991;218:397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 38.Galat A. On transversal hydrophobicity of some proteins and their modules. J Chem Inf Model. 2009;49:1821–1830. doi: 10.1021/ci9001316. [DOI] [PubMed] [Google Scholar]
- 39.Gibbs PR, Radzicka A, Wolfenden R. The anomalous hydrophilic character of proline. J Am Chem Soc. 1991;113:4714–4715. [Google Scholar]
- 40.Wang XJ, Etzkorn FA. Peptidyl-prolyl isomerase inhibitors. Biopolymers. 2006;84:125–146. doi: 10.1002/bip.20240. [DOI] [PubMed] [Google Scholar]
- 41.Galat A. A large-scale processing of kinetic data files with derivation of the inhibitory constant K i: an application to proline isomerases. Comput Chem. 1996;20:279–281. doi: 10.1016/0097-8485(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 42.Papageorgiou C, Borer X, French RR. Calcineurin has a very tight-binding pocket for the side chain of residue 4 of cyclosporin. Bioorg Med Chem Lett. 1994;4:267–272. [Google Scholar]
- 43.Hu MK, Badger A, Rich DH. Cyclosporine Analogs modified in the 3, 7, 8-positions: substituent effects on peptidylprolyl isomerase inhibition and immunosuppressive activity are nonadditive. J Med Chem. 1995;38:4164–4170. doi: 10.1021/jm00021a005. [DOI] [PubMed] [Google Scholar]
- 44.Loor F, Tiberghien F, Wenandy T, Didier A, Traber R. Cyclosporins: structure-activity relationships for the inhibition of the human MDR1 P-glycoprotein ABC transporter. J Med Chem. 2002;45:4598–4612. doi: 10.1021/jm0109863. [DOI] [PubMed] [Google Scholar]
- 45.Loor F, Tiberghien F, Wenandy T, Didier A, Traber R. Cyclosporins: structure-activity relationships for the inhibition of the human FPR1 formylpeptide receptor. J Med Chem. 2002;45:4613–4628. doi: 10.1021/jm010987v. [DOI] [PubMed] [Google Scholar]
- 46.Wenger R. Cyclosporine and analogues: structural requirements for immunosuppressive activity. Transplant Proc. 1986;18:213–218. [PubMed] [Google Scholar]
- 47.Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporine A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J Virol. 1995;69:2451–2461. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fehr T, Kallen J, Oberer L, Sanglier JJ, Schilling W. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110; Structure elucidation, stereochemistry and physico-chemical properties. J Antibiot (Tokyo) 1999;52:474–479. doi: 10.7164/antibiotics.52.474. [DOI] [PubMed] [Google Scholar]
- 49.Sanglier JJ, Quesniaux V, Fehr T, Hofmann H, Mahnke M, Memmert K, Schuler W, Zenke G, Gschwind L, Maurer C, Schilling W. Sanglifehrins A, B, C and D, novel cyclophilin-binding compounds isolated from Streptomyces sp. A92-308110; Taxonomy, fermentation, isolation and biological activity. J Antibiot (Tokyo) 1999;52:466–473. doi: 10.7164/antibiotics.52.466. [DOI] [PubMed] [Google Scholar]
- 50.Fehr T, Quesniaux VF, Sanglier JJ, Oberer L, Gschwind L, Ponelle M, Schilling W, Wehrli S, Enz A, Zenke G, Schuler W. Cymbimicin A and B, two novel cyclophilin-binding structures isolated from actinomycetes. J Antibiot (Tokyo) 1997;50:893–899. doi: 10.7164/antibiotics.50.893. [DOI] [PubMed] [Google Scholar]
- 51.Gaymes TJ, Cebrat M, Siemion IZ, Kay JE. Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Lett. 1997;418:224–227. doi: 10.1016/s0014-5793(97)01345-8. [DOI] [PubMed] [Google Scholar]
- 52.Kallen J, Sedrani R, Zenke G, Wagner J. Structure of human cyclophilin A in complex with the novel immunosuppressant sanglifehrin A at 1.6 Å resolution. J Biol Chem. 2005;280:21965–21971. doi: 10.1074/jbc.M501623200. [DOI] [PubMed] [Google Scholar]
- 53.Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–4803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 54.Davis TL, Walker JR, Ouyang H, MacKenzie F, Butler-Cole C, Newman EM, Eisenmesser EZ, Dhe-Paganon S. The crystal structure of human WD40 repeat-containing peptidylprolyl isomerase (PPWD1) FEBS J. 2008;275:2283–2295. doi: 10.1111/j.1742-4658.2008.06381.x. [DOI] [PubMed] [Google Scholar]
- 55.Bang H, Brune K, Nager C, Feige U. Interleukin-8 is a cyclosporine A binding protein. Experientia. 1993;49:533–538. doi: 10.1007/BF01955157. [DOI] [PubMed] [Google Scholar]
- 56.Husi H, Zurini MG. Comparative binding studies of cyclophilins to cyclosporine A and derivatives by fluorescence measurements. Anal Biochem. 1994;222:251–255. doi: 10.1006/abio.1994.1481. [DOI] [PubMed] [Google Scholar]
- 57.Galat A. Function-dependent clustering of orthologues and paralogues of cyclophilins. Proteins Struct Funct Bioinform. 2004;56:808–820. doi: 10.1002/prot.20156. [DOI] [PubMed] [Google Scholar]
- 58.Moparthi SB, Hammarström P, Carlsson U. A nonessential role for Arg55 in cyclophilin18 for catalysis of proline isomerization during protein folding. Prot Sci. 2009;18:475–479. doi: 10.1002/pro.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moparthi SB, Fristedt R, Mishra R, Almstedt K, Karlsson M, Hammarström P, Carlsson U. Chaperone activity of Cyp18 through hydrophobic condensation that enables rescue of transient misfolded molten globule intermediates. Biochemistry. 2010;49:1137–1145. doi: 10.1021/bi901997q. [DOI] [PubMed] [Google Scholar]
- 60.Berman HM, Henrick K, Nakamura H, Markley JL. The worldwide Protein Data Bank (wwPDB): ensuring a single, uniform archive of PDB data. Nucleic Acids Res. 2007;35:D301–D303. doi: 10.1093/nar/gkl971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeLano WL (2002) The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos. (http://pymol.sourceforge.net/)
- 62.Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon JM, Fleming MA, Caron PR, Hsiao NaviaMA. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 63.Vajdos FF, Yoo S, Houseweart M, Sundquist WI, Hill CP. Crystal structure of cyclophilin A complexed with a binding site peptide from the HIV-1 capsid protein. Prot Sci. 1997;6:2297–2307. doi: 10.1002/pro.5560061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howard BR, Vajdos FF, Li S, Sundquist WI, Hill CP. Structural insights into the catalytic mechanism of cyclophilin A. Nature Struct Biol. 2003;10:475–481. doi: 10.1038/nsb927. [DOI] [PubMed] [Google Scholar]
- 65.Ingelfinger D, Göthel SF, Marahiel MA, Reidt U, Ficner R, Lührmann R, Achsel T. Two protein-protein interaction sites on the spliceosome-associated human cyclophilin CypH. Nucleic Acids Res. 2003;31:4791–4796. doi: 10.1093/nar/gkg660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M, Nyanda AM, Zhang J, Fan GH. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283:623–637. doi: 10.1074/jbc.M704934200. [DOI] [PubMed] [Google Scholar]
- 67.Baum N, Schiene-Fischer C, Frost M, Schumann M, Sabapathy K, Ohlenschlager O, Grosse F, Schlott B. The prolyl cis/trans isomerase cyclophilin 18 interacts with the tumor suppressor p53 and modifies its functions in cell cycle regulation and apoptosis. Oncogene. 2009;28:3915–3925. doi: 10.1038/onc.2009.248. [DOI] [PubMed] [Google Scholar]
- 68.Braaten D, Luban J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001;20:1300–1309. doi: 10.1093/emboj/20.6.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantin R, Méthot S, Tremblay MJ. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J Virol. 2005;79:6577–6587. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandes F, Ansari IU, Striker R. Cyclosporine inhibits a direct interaction between cyclophilins and hepatitis C NS5A. PLoS One. 2010;5(3):e9815. doi: 10.1371/journal.pone.0009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puyang X, Poulin DL, Mathy JE, Anderson LJ, Ma S, Fang Z, Zhu S, Lin K, Fujimoto R, Compton T, Wiedmann B. Mechanism of resistance of hepatitis C virus replicons to structurally distinct cyclophilin inhibitors. Antimicrob Agents Chemother. 2010;54:1981–1987. doi: 10.1128/AAC.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meza-Zepeda LA, Forus A, Lygren B, Dahlberg AB, Godager LH, South AP, Marenholz I, Lioumi M, Florenes VA, Maelandsmo GM, Serra M, Mischke D, Nizetic D, Ragoussis J, Tarkkanen M, Nesland JM, Knuutila S, Myklebost O. Positional cloning identifies a novel cyclophilin as a candidate amplified oncogene in 1q21. Oncogene. 2002;21:2261–2269. doi: 10.1038/sj.onc.1205339. [DOI] [PubMed] [Google Scholar]
- 73.Kuhn AN, van Santen MA, Schwienhorst A, Urlaub H, Lührmann R. Stalling of spliceosome assembly at distinct stages by small-molecule inhibitors of protein acetylation and deacetylation. RNA. 2009;15:153–175. doi: 10.1261/rna.1332609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obama K, Kato T, Hasegawa S, Satoh S, Nakamura Y, Furukawa Y. Overexpression of peptidyl-prolyl isomerase-like 1 is associated with the growth of colon cancer cells. Clin Cancer Res. 2006;12:70–76. doi: 10.1158/1078-0432.CCR-05-0588. [DOI] [PubMed] [Google Scholar]
- 75.Stumpf T, Zhang Q, Hirnet D, Lewandrowski U, Sickmann A, Wissenbach U, Dorr J, Lohr C, Deitmer JW, Fecher-Trost C. The Human TRPV6 channel protein is associated with cyclophilin B in human placenta. J Biol Chem. 2008;283:18086–18098. doi: 10.1074/jbc.M801821200. [DOI] [PubMed] [Google Scholar]
- 76.Schneider H, Charara N, Schmitz R, Wehrli S, Mikol V, Zurini MG, Quesniaux VF, Movva NR. Human cyclophilin C: primary structure, tissue distribution, and determination of binding specificity for cyclosporins. Biochemistry. 1994;33:8218–8224. doi: 10.1021/bi00193a007. [DOI] [PubMed] [Google Scholar]
- 77.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 78.Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J, Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nature Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M, Bernardi P, Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nature Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horowitz DS, Lee E, Mabon SA, Misteli T. A cyclophilin functions in pre-mRNA splicing. EMBO J. 2002;21:470–480. doi: 10.1093/emboj/21.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mi H, Kops O, Zimmermann E, Jaschke A, Tropschug M. A nuclear RNA-binding cyclophilin in human T cells. FEBS Lett. 1996;378:201–205. doi: 10.1016/s0014-5793(96)01248-3. [DOI] [PubMed] [Google Scholar]
- 83.Gudbjartsson DF, Walters GB, Thorleifsson G, Stefansson H, Halldorsson BV, Zusmanovich P, Sulem P, Thorlacius S, Gylfason A, Steinberg S, Helgadottir A, Ingason A, Steinthorsdottir V, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Pedersen O, Aben KK, Witjes JA, Swinkels DW, den Heijer M, Franke B, Verbeek AL, Becker DM, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Gulcher J, Kiemeney LA, Kong A, Thorsteinsdottir U, Stefansson K. Many sequence variants affecting diversity of adult human height. Nature Genet. 2008;40:609–615. doi: 10.1038/ng.122. [DOI] [PubMed] [Google Scholar]
- 84.Carrello A, Allan RK, Morgan SL, Owen BA, Mok D, Ward BK, Minchin RF, Toft DO, Ratajczak T. Interaction of the Hsp90 cochaperone cyclophilin 40 with Hsc70. Cell Stress Chaperones. 2004;9:167–181. doi: 10.1379/CSC-26R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jang LK, Lee ZH, Kim HH, Hill JM, Kim JD, Kwon BS. A novel leucine-rich repeat protein (LRR-1): potential involvement in 4-1BB-mediated signal transduction. Mol Cells. 2001;12:304–312. [PubMed] [Google Scholar]
- 86.Peyrl A, Krapfenbauer K, Slavc I, Yang JW, Strobel T, Lubec G. Protein profiles of medulloblastoma cell lines DAOY and D283: identification of tumor-related proteins and principles. Proteomics. 2003;3:1781–1800. doi: 10.1002/pmic.200300460. [DOI] [PubMed] [Google Scholar]
- 87.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 88.Dubourg B, Kamphausen T, Weiwad M, Jahreis G, Feunteun J, Fischer G, Modjtahedi N. The human nuclear SRcyp is a cell cycle-regulated cyclophilin. J Biol Chem. 2004;279:22322–22330. doi: 10.1074/jbc.M400736200. [DOI] [PubMed] [Google Scholar]
- 89.Sakashita E, Tatsumi S, Werner D, Endo H, Mayeda A. Human RNPS1 and its associated factors: a versatile alternative pre-mRNA splicing regulator in vivo. Mol Cell Biol. 2004;24:1174–1187. doi: 10.1128/MCB.24.3.1174-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rundle NT, Nelson J, Flory MR, Joseph J, Th’ng J, Aebersold R, Dasso M, Andersen RJ, Roberge M. An ent-kaurene that inhibits mitotic chromosome movement and binds the kinetochore protein Ran-binding protein 2. ACS Chem Biol. 2006;1:443–450. doi: 10.1021/cb600196w. [DOI] [PubMed] [Google Scholar]
- 91.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIα. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Li X, Stremlau M, Lee M, Sodroski J. Removal of arginine 332 allows human TRIM5α to bind human immunodeficiency virus capsids and to restrict infection. J Virol. 2006;80:6738–6744. doi: 10.1128/JVI.00270-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grutter C, Martinetti G, Mazzucchelli L, Grutter M, Manz MG, Luban J. Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components. J Clin Invest. 2009;119:3035–3047. doi: 10.1172/JCI39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Friedman J, Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 95.Bram RJ, Hung DT, Martin PK, Schreiber SL, Crabtree GR. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporine A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993;13:4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colgan J, Asmal M, Luban J. Isolation, characterization and targeted disruption of mouse PPIA: cyclophilin A is not essential for mammalian cell viability. Genomics. 2000;68:167–178. doi: 10.1006/geno.2000.6295. [DOI] [PubMed] [Google Scholar]
- 97.Colgan J, Asmal M, Yu B, Luban J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporin. J Immunol. 2005;174:6030–6038. doi: 10.4049/jimmunol.174.10.6030. [DOI] [PubMed] [Google Scholar]
- 98.Allain F, Boutillon C, Mariller C, Spik G. Selective assay for CyPA and CyPB in human blood using highly specific anti-peptide antibodies. J Immunol Methods. 1995;178:113–120. doi: 10.1016/0022-1759(94)00249-v. [DOI] [PubMed] [Google Scholar]
- 99.Yang H, Elmquist WF. The binding of cyclosporine-A to human plasma: an in vitro microdialysis study. Pharm Res. 1996;3:622–627. doi: 10.1023/a:1016066609489. [DOI] [PubMed] [Google Scholar]
- 100.Cacalano NA, Chen BX, Cleveland WL, Erlanger BF. Evidence for a functional receptor for cyclosporine A on the surface of lymphocytes. Proc Natl Acad Sci USA. 1992;89:4353–4357. doi: 10.1073/pnas.89.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malesevic M, Kuhling J, Erdmann F, Balsley MA, Bukrinsky MI, Constant SL, Fischer G. A cyclosporinederivative discriminates between extracellular and intracellular cyclophilins. Angew Chem Int Ed Engl. 2010;49:213–215. doi: 10.1002/anie.200904529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Augustijns PF, Brown SC, Willard DH, Consler TG, Annaert PP, Hendren RW, Bradshaw TP. Hydration changes implicated in the remarkable temperature-dependent membrane permeation of cyclosporine A. Biochemistry. 2000;39:7621–7630. doi: 10.1021/bi9929709. [DOI] [PubMed] [Google Scholar]
- 103.Denys A, Allain F, Masy E, Dessaint JP, Spik G. Enhancing the effect of secreted cyclophilin B on immunosuppressive activity of cyclosporin. Transplantation. 1998;65:1076–1084. doi: 10.1097/00007890-199804270-00012. [DOI] [PubMed] [Google Scholar]
- 104.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci USA. 2002;99:2714–2719. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi JW, Sutor SL, Lindquist L, Evans GL, Madden BJ, Bergen HR, 3rd, Hefferan TE, Yaszemski MJ, Bram RJ. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS Genet. 2009;5:e1000750. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnes AM, Carter EM, Cabral WA, Weis M, Chang W, Makareeva E, Leikin S, Rotimi CN, Eyre DR, Raggio CL, Marini JC. Lack of cyclophilin B in Osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng H, Vijayakumar S, Schiene-Fischer C, Li H, Purkerson JM, Malesevic M, Liebscher J, Al-Awqati Q, Schwartz GJ. Secreted cyclophilin A, a peptidylprolyl cis-trans isomerase, mediates matrix assembly of hensin, a protein implicated in epithelial differentiation. J Biol Chem. 2009;284:6465–6475. doi: 10.1074/jbc.M808964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocrine Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 109.Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007;128:245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Kudrin A, Scott M, Martin S, Chung CW, Donn R, McMaster A, Ellison S, Ray D, Ray K, Binks M. Human macrophage migration inhibitory factor: a proven immunomodulatory cytokine? J Biol Chem. 2006;281:29641–29651. doi: 10.1074/jbc.M601103200. [DOI] [PubMed] [Google Scholar]
- 111.Kudrin A, Ray D. Cunning factor: macrophage migration inhibitory factor as a redox-regulated target. Immunol Cell Biol. 2008;86:232–238. doi: 10.1038/sj.icb.7100133. [DOI] [PubMed] [Google Scholar]
- 112.Galat A, Riviere S, Bouet F, Menez A. A diversified family of 12-kDa proteins with a high amino acid sequence similarity to macrophage migration-inhibitory factor (MIF) Eur J Biochem. 1994;224:417–421. doi: 10.1111/j.1432-1033.1994.00417.x. [DOI] [PubMed] [Google Scholar]
- 113.Franke EK, Yuan HEH, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 114.Chatterji U, Bobardt MD, Stanfield R, Ptak RG, Pallansch LA, Ward PA, Jones MJ, Stoddart CA, Scalfaro P, Dumont JM, Besseghir K, Rosenwirth B, Gallay PA. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J Biol Chem. 2005;280:40293–40300. doi: 10.1074/jbc.M506314200. [DOI] [PubMed] [Google Scholar]
- 115.Strebel K, Luban J, Jeang KT. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009;16(7):48. doi: 10.1186/1741-7015-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 117.Bushman FD, Malani N, Fernandes J, D’Orso I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, Konig R, Bandyopadhyay S, Ideker T, Goff SP, Krogan NJ, Frankel AD, Young JA, Chanda SK. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog. 2009;5:e1000437. doi: 10.1371/journal.ppat.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bauer K, Kretzschmar AK, Cvijic H, Blumert C, Loffler D, Brocke-Heidrich K, Schiene-Fischer C, Fischer G, Sinz A, Clevenger CV, Horn F. Cyclophilins contribute to Stat3 signaling and survival of multiple myeloma cells. Oncogene. 2009;28:2784–2795. doi: 10.1038/onc.2009.142. [DOI] [PubMed] [Google Scholar]
- 119.Torlakovic EE, Keeler V, Wang C, Lim HJ, Lining LA, Laferté S. CyclophilinC-associated protein (CyCAP) knock-out mice spontaneously develop colonic mucosal hyperplasia and exaggerated tumorigenesis after treatment with carcinogen azoxymethane. BMC Cancer. 2009;9:251. doi: 10.1186/1471-2407-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen L, Beare D, Latimer C, Widaa S, Hinton J, Fahey C, Fu B, Swamy S, Dalgliesh GL, Teh BT, Deloukas P, Yang F, Campbell PJ, Futreal PA, Stratton MR. Signatures of mutation and selection in the cancer genome. Nature. 2010;463:893–898. doi: 10.1038/nature08768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stephens PJ, McBride DJ, Lin ML, Varela I, Pleasance ED, Simpson JT, Stebbings LA, Leroy C, Edkins S, Mudie LJ, Greenman CD, Jia M, Latimer C, Teague JW, Lau KW, Burton J, Quail MA, Swerdlow H, Churcher C, Natrajan R, Sieuwerts AM, Martens JW, Silver DP, Langerod A, Russnes HE, Foekens JA, Reis-Filho JS, van ‘t Veer L, Richardson AL, Borresen-Dale AL, Campbell PJ, Futreal PA, Stratton MR. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Teague J, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Forbes S, Jia M, Jones D, Knott H, Kok CY, Lau KW, Leroy C, Lin ML, McBride DJ, Maddison M, Maguire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, O’Meara S, Pleasance E, Rajasingham A, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turrell K, Dykema KJ, Khoo SK, Petillo D, Wondergem B, Anema J, Kahnoski RJ, Teh BT, Stratton MR, Futreal PA. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silverman JA, Hayes ML, Luft BJ, Joiner KA. Characterization of anti-Toxoplasma activity of SDZ 215–918, a cyclosporinederivative lacking immunosuppressive and peptidyl-prolyl isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob Agents Chemother. 1997;41:1859–1866. doi: 10.1128/aac.41.9.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berriman M, Fairlamb AH. Detailed characterization of a cyclophilin from the human malaria parasite Plasmodium falciparum . Biochem J. 1998;334:437–445. doi: 10.1042/bj3340437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar R, Musiyenko A, Barik S. Plasmodium falciparum calcineurin and its association with heat shock protein 90: mechanisms for the antimalarial activity of cyclosporine A and synergism with geldanamycin. Mol Biochem Parasitol. 2005;141:29–37. doi: 10.1016/j.molbiopara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 126.Hirtzlin J, Farber PM, Franklin RM, Bell A. Molecular and biochemical characterization of a Plasmodium falciparum cyclophilin containing a cleavable signal sequence. Eur J Biochm. 1995;232:765–772. [PubMed] [Google Scholar]
- 127.Reddy GR. Cloning and characterization of a Plasmodium falciparum cyclophilin gene that is stage-specifically expressed. Mol Biochem Parasitol. 1995;73:111–121. doi: 10.1016/0166-6851(95)00103-8. [DOI] [PubMed] [Google Scholar]
- 128.High KP, Joiner KA, Handschumacher RE. Isolation, cDNA sequences, and biochemical characterization of the major cyclosporin-binding proteins of Toxoplasma gondii . J Biol Chem. 1994;269:9105–9112. [PubMed] [Google Scholar]
- 129.Golding H, Aliberti J, King LR, Manischewitz J, Andersen J, Valenzuela J, Landau NR, Sher A. Inhibition of HIV-1 infection by a CCR5-binding cyclophilin from Toxoplasma gondii . Blood. 2003;102:3280–3286. doi: 10.1182/blood-2003-04-1096. [DOI] [PubMed] [Google Scholar]
- 130.Adams B, Musiyenko A, Kumar R, Barik S. A novel class of dual-family immunophilins. J Biol Chem. 2005;280:24308–24314. doi: 10.1074/jbc.M500990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rascher C, Pahl A, Pecht A, Brune K, Solbach W, Bang H. Leishmania major parasites express cyclophilin isoforms with an unusual interaction with calcineurin. Biochem J. 1998;334:659–667. doi: 10.1042/bj3340659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sen B, Venugopal V, Chakraborty A, Datta R, Dolai S, Banerjee R, Datta AK. Amino acid residues of Leishmania donovani cyclophilin key to interaction with its adenosine kinase: biological implications. Biochemistry. 2007;46:7832–7843. doi: 10.1021/bi602625h. [DOI] [PubMed] [Google Scholar]
- 133.Paape D, Barrios-Llerena ME, Le Bihan T, Mackay L, Aebischer T. Gel free analysis of the proteome of intracellular Leishmania mexicana . Mol Biochem Parasitol. 2010;169:108–114. doi: 10.1016/j.molbiopara.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 134.Hoerauf A, Rascher C, Bang R, Pahl A, Solbach W, Brune K, Rollinghoff M, Bang H. Host-cell cyclophilin is important for the intracellular replication of Leishmania major . Mol Microbiol. 1997;24:421–429. doi: 10.1046/j.1365-2958.1997.3401716.x. [DOI] [PubMed] [Google Scholar]
- 135.Dutta M, Delhi P, Sinha KM, Banerjee R, Dutta AK. Lack of abundance of cytoplasmic cyclosporine A-binding protein renders free-living Leishmania donovani resistant to cyclosporine A. J Biol Chem. 2001;276:19294–19300. doi: 10.1074/jbc.M009379200. [DOI] [PubMed] [Google Scholar]
- 136.Pelle R, McOdimba F, Chuma F, Wasawo D, Pearson TW, Murphy NB. The African trypanosome cyclophilin A homologue contains unusual conserved central and N-terminal domains and is developmentally regulated. Gene. 2002;290:181–191. doi: 10.1016/s0378-1119(02)00559-0. [DOI] [PubMed] [Google Scholar]
- 137.Bua J, Fichera LE, Fuchs A, Potenza M, Dubin M, Wenger RO, Moretti G, Scabone CM, Ruiz AM. Anti-Trypanosoma cruzi effects of cyclosporine A derivatives: possible role of a P-glycoprotein and parasite cyclophilins. Parasitology. 2008;135:217–228. doi: 10.1017/S003118200700371X. [DOI] [PubMed] [Google Scholar]
- 138.Bua J, Ruiz AM, Potenza M, Fichera LE. In vitro anti-parasitic activity of Cyclosporine A analogs on Trypanosoma cruzi . Bioorg Med Chem Lett. 2004;14:4633–4637. doi: 10.1016/j.bmcl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 139.Ostoa-Saloma P, Cesar Carrero J, Petrossian P, Herion P, Landa A, Pedro Laclette J. Cloning, characterization and functional expression of a cyclophilin of Entamoeba histolytica . Mol Biochem Parasitol. 2000;107:219–225. doi: 10.1016/s0166-6851(00)00190-0. [DOI] [PubMed] [Google Scholar]
- 140.Carrero JC, Lugo H, Perez DG, Ortiz-Martınez C, Laclette JP. Cyclosporine A inhibits calcineurin (phosphatase 2B) and P-glycoprotein activity in Entamoeba histolytica . Int J Parasitol. 2004;34:1091–1097. doi: 10.1016/j.ijpara.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 141.Yu HS, Kong HH, Chung DI. Cloning and characterization of Giardia intestinalis cyclophilin. Korean J Parasitol. 2002;40:131–138. doi: 10.3347/kjp.2002.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perkins ME, Wu TW, Le Blancq SM. Cyclosporine analogs inhibit in vitro growth of Cryptosporidium parvum . Antimicrob Agents Chemother. 1998;42:843–848. doi: 10.1128/aac.42.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gallay PA. Cyclophilin inhibitors. Clin Liver Dis. 2009;13:403–417. doi: 10.1016/j.cld.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 144.Perry K, Agabian N. mRNA processing in the Trypanosomatidae. Experientia. 1991;47:118–128. doi: 10.1007/BF01945412. [DOI] [PubMed] [Google Scholar]
- 145.Pritchard DI. Sourcing a chemical succession for cyclosporinefrom parasites and human pathogens. Drug Discov Today. 2005;10:688–691. doi: 10.1016/S1359-6446(05)03395-7. [DOI] [PubMed] [Google Scholar]
- 146.Demeule M, Laplante A, Sepehr-Araé A, Murphy GM, Wenger RM, Béliveau R. Association of cyclophilin A with renal brush border membranes: redistribution by cyclosporine A. Kidney Int. 2000;57:1590–1598. doi: 10.1046/j.1523-1755.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 147.Malouitre S, Dube H, Selwood D, Crompton M. Mitochondrial targeting of cyclosporine A enables selective inhibition of cyclophilin-D and enhanced cytoprotection after glucose and oxygen deprivation. Biochem J. 2010;425:137–148. doi: 10.1042/BJ20090332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siu WP, Pun PB, Latchoumycandane C, Boelsterli UA. Bax-mediated mitochondrial outer membrane permeabilization (MOMP), distinct from the mitochondrial permeability transition, is a key mechanism in diclofenac-induced hepatocyte injury: multiple protective roles of cyclosporine A. Toxicol Appl Pharmacol. 2008;227:451–461. doi: 10.1016/j.taap.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 149.Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kristal BS, Stavrovskaya IG, Narayanan MV, Krasnikov BF, Brown AM, Beal MF, Friedlander RM. The mitochondrial permeability transition as a target for neuroprotection. J Bioenerg Biomembr. 2004;36:309–312. doi: 10.1023/B:JOBB.0000041759.35731.70. [DOI] [PubMed] [Google Scholar]
- 151.Karlsson J, Fong KS, Hansson MJ, Elmér E, Csiszar K, Keep MF. Life span extension and reduced neuronal death after weekly intraventricular cyclosporineinjections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J Neurosurg. 2004;101:128–137. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- 152.Hicks D, Lampe AK, Laval SH, Allamand V, Jimenez-Mallebrera C, Walter MC, Muntoni F, Quijano-Roy S, Richard P, Straub V, Lochmüller H, Bushby KM. Cyclosporine A treatment for Ullrich congenital muscular dystrophy: a cellular study of mitochondrial dysfunction and its rescue. Brain. 2009;132:147–155. doi: 10.1093/brain/awn289. [DOI] [PubMed] [Google Scholar]
- 153.Lee YC, Kim SH, Seo YB, Roh SS, Lee JC. Inhibitory effects of Actinidia polygama extract and cyclosporine A on OVA-induced eosinophilia and bronchial hyperresponsiveness in a murine model of asthma. Int Immunopharmacol. 2006;6:703–713. doi: 10.1016/j.intimp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 154.Onoue S, Sato H, Kawabata Y, Mizumoto T, Hashimoto N, Yamada S. In vitro and in vivo characterization on amorphous solid dispersion of cyclosporine A for inhalation therapy. J Control Release. 2009;138:16–23. doi: 10.1016/j.jconrel.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 155.Manteca A, Sanchez J. Recombinant cyclophilins lack nuclease activity. J Bacteriol. 2004;186:6325–6326. doi: 10.1128/JB.186.18.6325-6326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montague JW, Gaido ML, Frye C, Cidlowski JA. A calcium-dependent nuclease from apoptotic rat thymocytes is homologous with cyclophilin. Recombinant cyclophilins A, B, and C have nuclease activity. J Biol Chem. 1994;269:18877–18880. [PubMed] [Google Scholar]
- 157.Varthakavi V, Heimann-Nichols E, Smith RM, Sun Y, Bram RJ, Ali S, Rose J, Ding L, Spearman P. Identification of calcium-modulating cyclophilin ligand as a human host restriction to HIV-1 release overcome by Vpu. Nature Med. 2008;14:641–647. doi: 10.1038/nm1778. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Kuhl A, Munch J, Sauter D, Bertram S, Glowacka I, Steffen I, Specht A, Hofmann H, Schneider H, Behrens G, Pohlmann S. Calcium-modulating cyclophilin ligand does not restrict retrovirus release. Nature Med. 2010;16:155–156. doi: 10.1038/nm0210-155. [DOI] [PubMed] [Google Scholar]
- 159.Bram RJ, Crabtree GR. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature. 1994;371:355–358. doi: 10.1038/371355a0. [DOI] [PubMed] [Google Scholar]
- 160.Hackstein H, Steinschulte C, Fiedel S, Eisele A, Rathke V, Stadlbauer T, Taner T, Thomson AW, Tillmanns H, Bein G, Hölschermann H. Sanglifehrin-A blocks key dendritic cell functions in vivo and promotes long-term allograft survival together with low-dose CsA. Am J Transplant. 2007;7:789–798. doi: 10.1111/j.1600-6143.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 161.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 162.Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, Luban J, Kroemer G, Blomgren K. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Le Hir M, Su Q, Weber L, Woerly G, Granelli-Piperno A, Ryffel B. In situ detection of cyclosporine A: evidence for nuclear localization of cyclosporine and cyclophilins. Lab Invest. 1995;73:727–733. [PubMed] [Google Scholar]
- 165.Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Syed F, Rycyzyn MA, Westgate L, Clevenger CV. A novel and functional interaction between cyclophilin A and prolactin receptor. Endocrine. 2003;20:83–90. doi: 10.1385/ENDO:20:1-2:83. [DOI] [PubMed] [Google Scholar]
- 167.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 168.Galat A. Involvement of some large immunophilins and their ligands in the protection and regeneration of neurons: a hypothetical mode of action. Comput Biol Chem. 2006;30:348–359. doi: 10.1016/j.compbiolchem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 169.Jakob RP, Schmid FX. Molecular determinants of a native-state prolyl isomerization. J Mol Biol. 2009;387:1017–1031. doi: 10.1016/j.jmb.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 170.Thai V, Renesto P, Fowler CA, Brown DJ, Davis T, Gu W, Pollock DD, Kern D, Raoult D, Eisenmesser EZ. Structural, biochemical, and in vivo characterization of the first virally encoded cyclophilin from the Mimivirus. J Mol Biol. 2008;378:71–86. doi: 10.1016/j.jmb.2007.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lummis SC, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature. 2005;438:248–252. doi: 10.1038/nature04130. [DOI] [PubMed] [Google Scholar]
- 172.Wang X, Zhang S, Zhang J, Huang X, Xu C, Wang W, Liu Z, Wu J, Shi Y. A large intrinsically disordered region in SKIP and its disorder-order transition induced by PPIL1 binding revealed by NMR. J Biol Chem. 2010;285:4951–4963. doi: 10.1074/jbc.M109.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weininger U, Jakob RP, Eckert B, Schweimer K, Schmid FX, Balbach J. A remote prolyl isomerization controls domain assembly via a hydrogen bonding network. Proc Natl Acad Sci USA. 2009;106:12335–12340. doi: 10.1073/pnas.0902102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W. Molecular mechanisms of TGFβ receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporine A and FK506. J Immunol. 2008;181:2831–2845. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 175.Fehr T, Lucas CL, Kurtz J, Onoe T, Zhao G, Hogan T, Vallot C, Rao A, Sykes M. A CD8 T cell-intrinsic role for the calcineurin-NFAT pathway for tolerance induction in vivo. Blood. 2010;115:1280–1287. doi: 10.1182/blood-2009-07-230680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, Decaudin D, de Thé H, Ghysdael J. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nature Med. 2007;13:736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- 177.Simon N, Dailly E, Combes O, Malaurie E, Lemaire M, Tillement JP, Urien S. Role of lipoproteins in the plasma binding of SDZ PSC 833, a novel multidrug resistance-reversing cyclosporin. Br J Clin Pharmacol. 1998;45:173–175. doi: 10.1046/j.1365-2125.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fischer V, Rodriguez-Gascon A, Heitz F, Tynes R, Hauck C, Cohen D, Vickers AE. The multidrug resistance modulator valspodar (PSC 833) is metabolized by human cytochrome P450 3A; Implications for drug-drug interactions and pharmacological activity of the main metabolite. Drug Metab Dispos. 1998;26:802–811. [PubMed] [Google Scholar]
- 179.Kawai R, Lemaire M, Steimer JL, Bruelisauer A, Niederberger W, Rowland M. Physiologically based pharmacokinetic study on a cyclosporine derivative, SDZ IMM 125. J Pharmacokinet Biopharm. 1994;22:327–365. doi: 10.1007/BF02353860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignments of the human CLDs (MSA19) (PDF 47 kb)
MSA of 496 CLDs from various cyclophilins (MSA496) (PDF 490 kb)
MSA496.out containing information on the aligned sequences. (PDF 108 kb)
Mutations in the triads (MSA19) that contain crucial AA residues for PPIase activity and CsA-binding in hCyPA (PDF 21 kb)
A high resolution image of Figure 4A (PDF 1151 kb)
Graph computed from the MSA496 (TIFF 1468 kb)
Statistical distribution of AA-diads computed from the human genomic database. (PDF 50 kb)
Statistical distribution of AA-triads computed from the human genomic database. (PDF 556 kb)
Analyses of the X-ray structures of diverse cyclophilins from vertebrates and some parasites. (PDF 62 kb)
Conservation levels of several different sequence motifs of hCyPA deduced from the MSA496. (PDF 27 kb)
Some of the cyclophilins encoded in Helminths. (PDF 129 kb)
Contains a list of molecular interactions of the complexes shown in Table 1. (PDF 141 kb)