Abstract
We identified CREB3 as a novel HDAC3-interacting protein in a yeast two-hybrid screen for HDAC3-interacting proteins. Among all class I HDACs, CREB3 specifically interacts with HDAC3, in vitro and in vivo. HDAC3 efficiently inhibited CREB3-enhanced NF-κB activation, whereas the other class I HDACs did not alter NF-κB-dependent promoter activities or the expression of NF-κB target genes. Importantly, both knock-down of CREB3 and overexpression of HDAC3 suppressed the transcriptional activation of the novel CREB3-regulated gene, CXCR4. Furthermore, CREB3 was shown to bind to the CRE element in the CXCR4 promoter and to activate the transcription of the CXCR4 gene by causing dissociation of HDAC3 and subsequently increasing histone acetylation. Importantly, both the depletion of HDAC3 and the overexpression of CREB3 substantially increased the migration of MDA-MB-231 metastatic breast cancer cells. Taken together, these findings suggest that HDAC3 selectively represses CREB3-mediated transcriptional activation and chemotactic signalling in human metastatic breast cancer cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0388-5) contains supplementary material, which is available to authorized users.
Keywords: Histone deacetylase 3, cAMP response element-binding protein 3 (CREB3), Chemokine, CXC motif, Receptor 4 (CXCR4), Migration, Breast cancer cell
Introduction
Histone deacetylase 3 (HDAC3) belongs to the class I HDAC family, which includes HDAC1, HDAC2, HDAC3, and HDAC8 and, similar to HDAC1 and HDAC2 [1], is ubiquitously expressed. Class I HDACs commonly regulate gene expression by modulating the acetylation of histone and nonhistone substrates [2, 3]. However, HDAC3 contains an unusual C terminus and, unlike the predominantly nuclear HDAC1 and HDAC2, localizes to the nucleus, cytoplasm, and plasma membrane, indicating that HDAC3 is functionally distinct from other members of its class [4]. Functional variation of disparate HDACs and HDAC complexes has also been suggested by overexpression/downregulation studies, as well as through the developmental profiling of individual HDACs in Drosophila [5]. Such studies have revealed unique sets of target genes for individual HDACs. Surprisingly, similar numbers of genes have been found to be repressed and activated in each case, perhaps through both direct and indirect effects, indicating that HDACs are not functionally redundant [6].
Biochemical studies have emphasized that the small interference RNA (si-RNA) knock-down of HDAC3 impairs transcriptional repression mediated by unliganded thyroid hormone receptors [7] and antagonist-bound androgen receptor [8, 9]. In contrast, the knock-down of HDAC1 or HDAC2 has little effect. Together, these studies demonstrate the critical role for HDAC3 in repression by unliganded or antagonist-bound nuclear hormone receptors, and point to the conclusion that SMRT and N-CoR are class I HDAC-containing complexes associated primarily with HDAC3. Several studies have shown that HDAC3 also participates in the transcriptional repression mediated by other transcription factors, including c-myc [10], NFATc [11], ZBP-89 [12], and STAT3 [13]. In addition to its crucial role in transcriptional repression, HDAC3 also interacts with and deacetylates a variety of nonhistone substrates [14]. For example, HDAC3 regulates the duration of NF-κB signalling by deacetylating RelA and promoting its interaction with inhibitory-B (IkBα) [15]. On the other hand, HDAC1 is only involved in histone deacetylation during the attenuation of inflammatory signalling [16]. HDAC3 has also been shown to deacetylate the histone acetyltransferases, PCAF and p300/CBP [17]. Therefore, HDAC3 is involved in the modulation of cellular signalling through the deacetylation of target proteins, as well as the modulation of histone codes. It is anticipated that the functions of many proteins are regulated through deacetylation by HDAC3, and that the identification of novel substrates will be necessary to unravel the diverse roles of HDAC3 in cell signalling.
CREB3/LZIP is the primary member of the CREB3 family. Until now, there were four known family members in addition to CREB3, including CREBH/CREB3-like 1 (CREB3L1), BBF2H7/CREB3L2, OASIS/CREB3L3, and CREB4/AIbZIP/Atce1/Tisp40/CREB3L4 [18, 19]. Besides the well-conserved bZIP region, they all share one unique structural motif: a hydrophobic endoplasmic reticulum-transmembrane domain C-terminal to the bZIP region [20]. Under endoplasmic reticulum stress, these CREB3 proteins are thought to be cleaved by the same regulated intramembrane proteolysis mechanism as ATF6, then translocate into the nucleus and activate downstream target genes [21]. CREB3 proteins bind to various enhancer elements commonly found in the promoter regions of UPR-related genes. All CREB3 proteins can activate transcription from CRE and UPRE. CREB3 was originally shown to associate with herpes simplex virus-related host cell factor 1, which is involved in cell proliferation [22]. It has also been reported that CREB3 functions as a positive regulator in leucocyte cell migration induced by chemokines [23]. A previous study has demonstrated that CREB-associated HDAC activity is sensitive to the HDAC inhibitor butyrate, suggesting that CREB interacts with class I or class II HDACs, but not class III HDACs, which are resistant to such inhibitors [24]. Further analysis of class-I HDAC proteins (HDAC1–3) indicated that both HDAC1 and HDAC2 bind to CREB, while HDAC3 does not [25]. Although evidence supporting the diverse functions of class I HDACs in CREB family-mediated cell signalling has accumulated, the functional relevance of HDAC3 to the CREB family is not yet known.
We report here for the first time that CREB3 is a specific HDAC3-associated protein. Based on biochemical analysis, CREB3 selectively interacts with HDAC3, but not other class I HDACs, and specifically represses the expression of the CREB3 target gene, CXCR4. Importantly, we also show that HDAC3 antagonizes the migration of MDA-MB-231 cells through the downregulation of CREB3-mediated CXCR4 expression. This study unravelled the unique role of HDAC3 in CREB3-mediated chemotaxis in metastatic breast cancer cells.
Materials and methods
Cell culture, reagents and plasmids
All cell lines were obtained from the American Type Culture Collection (ATCC). HEK 293, MCF-7, and MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL) supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37°C in an atmosphere containing 5% CO2. Transient transfections were performed using Polyexpress (Excel gene).
The wild-type, full-length CREB3 and HDAC3, CREB3-1, CREB3-2, CREB3-3, CREB3-4, HDAC3-N, and HDAC3-C constructs were generated by PCR and cloned into plasmid vectors, pSG5-KF2M1-FLAG (Sigma) and pGEX4T-1 (Amersham Biosciences). The plasmids for reporter gene assays (NF-κB-Luc/firefly and pSV40/renilla) were purchased from Stratagene and Promega, respectively. All of the plasmid constructs were verified by DNA sequencing.
Yeast two-hybrid screen
Bait plasmids for HDAC3 (pGBKT7-HD3DO1 and pGBKT7-HD3DO2) were transformed into yeast strain AH109. Transformants containing each bait plasmid were mated with the pretransformed human HeLa and Testis MATCHMAKER cDNA library (Clontech). Plasmids were harvested and identified by DNA sequencing from positive clones capable of growth in minimal medium lacking tryptophan, leucine, adenosine and histidine, and displaying β-galactosidase expression.
Reporter assays
To measure NF-κB transcriptional activity, HEK 293 cells were transiently cotransfected with reporter construct NF-κB-Luc, pSV40, and various expression plasmids. The Renilla luciferase reporter plasmid was included as an internal control. Cells were harvested and whole-cell extracts were prepared and dual luciferase activity was measured, according to the manufacturer’s instruction (Promega). All reporter activities were normalized relative to Renilla luciferase activities and are presented as the means (±SD) of three independent experiments.
Si-RNA, quantitative RT-PCR, and ChIP analysis
RNA extraction, qRT-PCR, and ChIP assays were performed as described previously [26]. The si-RNAs and primer sequences for HDAC1, HDAC2, HDAC3, HDAC8, CREB3, XIAP, NR4A2, and CXCR4 are presented in Supplementary Table 1. The antibodies against HDAC1, HDAC2, HDAC3, and HDAC8 were purchased from Santa Cruz Biotechnology.
Wound healing assays
MDA-MB-231 cells were transfected with either expression plasmids or si-RNAs against HDAC3 and CREB3 using the Polyexpress transfection reagent. When cell confluence had reached about 90% 48 h after transfection, wounds were created in confluent cells using a 200-μl pipette tip. The cells were then rinsed with medium to remove any free-floating cells and debris. Medium was then added, and the culture plates were incubated at 37°C. Wound healing was observed within the scrape line at different time points, and representative scrape lines for each cell line were photographed. Duplicate wells for each condition were examined for each experiment, and each experiment was repeated three times.
Adhesion assay
A 96-well plate was coated with human fibronectin at 37°C for 1 h, washed with phosphate-buffered saline (PBS), blocked with blocking buffer at 37°C in a CO2 incubator for 45–60 min, washed with PBS, and chilled on ice. Each well was plated with 4 × 105 cells/ml and incubated in a CO2 incubator at 37°C for 30 min. The plate was shaken at 2,000 rpm for 10 s, washed with PBS two or three times, fixed with 4% paraformaldehyde, and incubated at room temperature for 10–15 min. The plate was washed with PBS, stained with 0.1% crystal violet for 10 min, and washed with water. The plate was turned upside down, then 2% SDS was added, the plate was incubated at room temperature 30 min, and the absorbance was read with a microplate reader at 550 nm. The results are presented as average values with error bars representing the standard deviation (±SD) of three independent experiments.
Matrigel invasion assay
In vitro cell invasiveness was reflected by the ability of cells to transmigrate through a layer of extracellular matrix in Biocoat Matrigel invasion chambers (SPL Lifesciences). At 48 h after transfection, cells were trypsinized and seeded at a density of 5.0 × 104 per insert. After 24 h, noninvading cells were removed with cotton swabs. Invading cells were fixed with 100% methanol and stained with 1% toluidine blue (Sigma) before being counted under an inverted microscope. The results presented are the average values with error bars representing the standard deviation (±SD) of three independent experiments.
Statistical analysis
Statistical analyses were done using Student’s t test with the SPSS program. P values <0.05 were considered significant.
Results
HDAC3 specifically interacts with CREB3
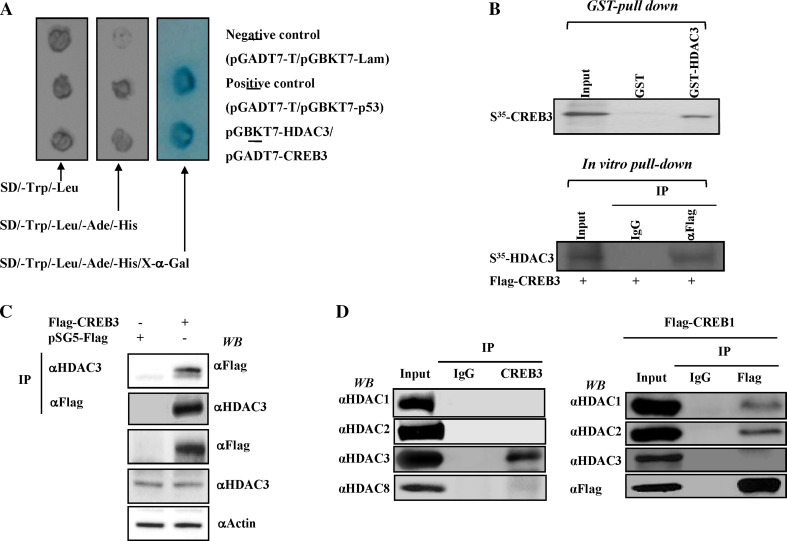
In the yeast two-hybrid screen for HDAC3-interacting proteins, we identified CREB3 as a novel HDAC3-interacting protein. As shown in Fig. 1a, the MAT S.c Y187 (pACT:CREB3) strain was mated with the MATa S.c AH109 bait strain transformed with recombinant pGBKT7 encoding HDAC3 and, as a control, with the unrelated bait, p53, or empty vector, pGBKT7. While each bait strain mated successfully with the prey strain, strains harbouring the empty bait vector failed to show any interaction with CREB3. Conversely, strains encoding CREB3 showed a strong interaction with HDAC3, as assessed by β-galactosidase assays. As a positive control, we confirmed a strong interaction between pGBKT7-p53 and pGADT7-T (Fig. 1a).
Fig. 1.
HDAC3 specifically interacts with CREB3 among all class I HDACs. a AH109 yeast strains harbouring various plasmids, including pGBKT7-HDAC3, pGBKT7 vector alone, and pGBKT7-p53 were mated to Y187 yeast strains harbouring pGADT2-CREB3, pGADT7 vector alone, and pGADT7-SV40 T antigen. Diploid colonies containing pGBKT7-HDAC3 and pGADT7-CREB3 were viable and turned blue on the high-stringency selection plate. The specificity of the interaction was confirmed by the absence of protein interaction in the negative control. b CREB3 directly binds to HDAC3. In vitro-translated, [35S]-labelled CREB3 and HDAC3 were incubated with glutathione S-transferase (GST) HDAC3 and anti-Flag-conjugated M2 bead (Sigma) and subjected to SDS-PAGE followed by autoradiography. c For the immunoprecipitation analysis, HEK 293 cells were transfected with FLAG-tagged CREB3 plasmid. Cell lysates were immunoprecipitated and subsequently immunoblotted with the respective antibodies, as indicated. Input 10% HEK293 cell lysates were used for immunoprecipitation. d CREB3 specifically interacts with HDAC3 but not other class I HDACs. Endogenous CREB3 was immunoprecipitated using CREB3-specific antibodies and the presence of various HDAC proteins was determined by Western blotting using antibodies as indicated (left panel). HEK 293 cells were transfected with either FLAG-tagged CREB1 (right panel) plasmid. Two days after transfection, the cell lysates were immunoprecipitated and subjected to Western blotting with the respective antibodies, as indicated. Input 10% HEK293 cell lysates were used for immunoprecipitation
To validate the results of the yeast two-hybrid assay, we performed GST pull-down assays to confirm the interaction between HDAC3 and CREB3. In vitro translated 35S-CREB3 bound to GST-HDAC3, but not to the GST control (Fig. 1b). To further confirm the specific interaction between HDAC3 and CREB3, we next performed immunoprecipitation assays in HEK293 cells transfected with Flag-tagged CREB3. As shown in Fig. 1c, Flag-tagged CREB3 specifically interacted with endogenous HDAC3. Strikingly, CREB3 failed to interact with other class I HDACs, including HDAC1, HDAC2, and HDAC8, indicating a selective association of CREB3 with HDAC3 (Fig. 1d, left panel). In accordance with a previous report [25], Flag-tagged CREB1 strongly interacted with both HDAC1 and HDAC2 but not with HDAC3 (Fig. 1d, right panel). These findings collectively demonstrate that HDAC3 specifically interacts with CREB3 among the class I HDACs.
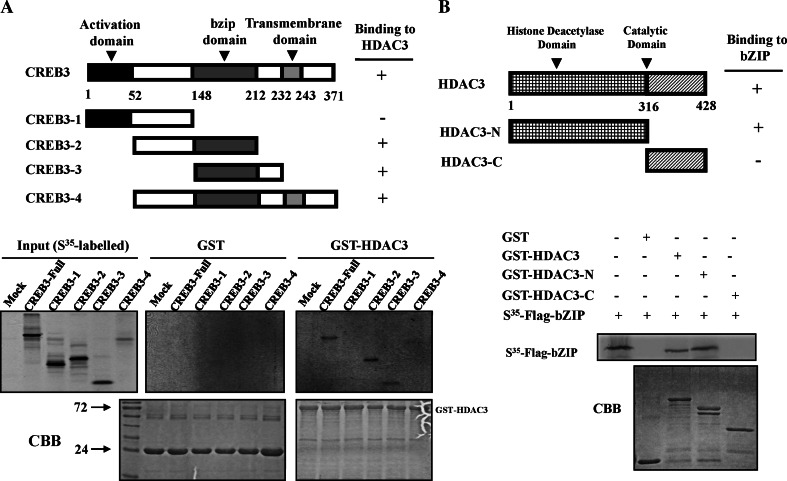
We next sought to elucidate the structure and function of HDAC3 and CREB3 with respect to their interaction domains. For this experiment, the CREB3 protein was divided into four fragments, as shown in Fig. 2a, and the ability of each fragment to interact with HDAC3 was assessed by GST pull-down assays. The bZIP domain of CREB3 directly interacted with GST-HDAC3. In a reciprocal set of experiments, HDAC3 was divided into two fragments (one containing the HDAC domain and the other the catalytic domain) and their ability to bind the bZIP domain of CREB3 was tested in GST pull-down assays. As shown in Fig. 2b, in vitro translated bZIP specifically bound to both GST-HDAC3 and GST-HDAC3-N, but not to GST-HDAC3-C. Taken together, we conclude that the bZIP domain of CREB3 interacts with the HDAC domain of HDAC3.
Fig. 2.
The bZIP domain of CREB3 interacts with the HDAC domain of HDAC3. a Upper panel Schematic diagram of the various domains of CREB3 fused to the FLAG vector used in the mapping studies. Lower panel In vitro-translated, [35S]-labelled CREB3 protein fragments were incubated with GST-HDAC3 and subsequently analysed by SDS-PAGE and autoradiography. b Upper panel Schematic diagram of the various domains of HDAC3 fused to FLAG. Lower panel In vitro-translated, [35S]-labelled bZIP proteins were incubated with various GST-HDAC3 proteins and subjected to SDS-PAGE and autoradiography
HDAC3 selectively inhibits CREB3-enhanced transcription of NF-κB target genes
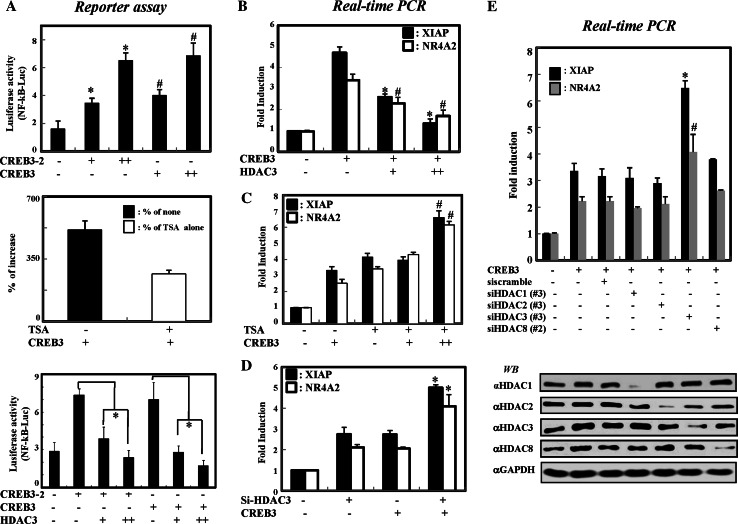
It has already been reported that HDAC3 represses NF-κB-mediated transcription via both epigenetic and nonepigenetic pathways [15]. In addition, CREB3 has also been shown to be involved in NF-κB-mediated transcriptional activation [27]. Based on this accumulated evidence, we first investigated the functional relationship between HDAC3 and CREB3 with a NF-κB-based transcriptional assay system. For this experiment, we first tested whether HDAC3 selectively inhibited CREB3-enhanced NF-κB activation by measuring NF-κB-dependent promoter activity. Expression of either full-length CREB3 or the bZIP domain enhanced NF-κB-mediated gene activation in a dose-dependent manner (Fig. 3a, upper panel). Treatment with trichostatin A (TSA) further increased the NF-κB activation enhanced by CREB3, suggesting an involvement of class I and II HDACs in the repression of CREB3-mediated transcription (Fig. 3a, middle panel). Importantly, the overexpression of HDAC3 repressed CREB3-enhanced NF-κB activity, in a dose-dependent manner (Fig. 3a, lower panel). Thus, these results suggest that HDAC3 is involved in the suppression of CREB3-enhanced NF-κB activation.
Fig. 3.
HDAC3 selectively represses CREB3-enhanced transcription of NF-κB target genes. a NF-κB binding promoter-luciferase construct and pSV40 plasmid were cotransfected with Flag-CREB3 or HDAC3, respectively. Two days after cotransfection, HEK 293 cells were harvested and the luciferase activity was determined, according to the manufacturer’s instructions (Promega). The data presented are fold increases relative to the basal activity and are the means (±SD) of three independent experiments. * P < 0.01 versus control plasmid; # P < 0.05 versus control plasmid; * P < 0.01 versus CREB3 or CREB3-2. b HDAC3 represses the transcription level of both XIAP and NR4A2. The levels of mRNAs were determined by RT-PCR. * P < 0.01 versus CREB3; # P < 0.05 versus CREB3. c HDAC activity is required for the repression of CREB3-mediated NF-κB activation. Quantitative real-time PCR was performed, as described in “Materials and methods”. # P < 0.05 for CREB3 + TSA versus TSA. d Depletion of HDAC3 enhances CREB3-mediated transcription. HEK 293 cells were transfected with 100 pmol si-HDAC3. Two days after transfection, HEK 293 cells were harvested, and mRNAs were analysed by qRT-PCR. * P < 0.01 for CREB3 + siHDAC3 versus siHDAC3. e Depletion of HDAC3, but not other HDACs, enhances CREB3-induced XIAP and N4A2 genes expression. HEK 293 cells were cotransfected with 5 μg of FLAG-tagged CREB3 plasmid and 100 pmol of si-RNAs against scrambled (siscramble) or class I HDACs. Two days after transfection, the cells were harvested. The mRNA levels were determined by quantitative real-time PCR (upper panel). GAPDH was used as an internal control. * P < 0.01 for CREB3 + siHDAC3 versus CREB3 + siscramble; # P < 0.01 for CREB3 + siHDAC3 versus CREB3 + siscramble
To confirm these results, we next determined whether HDAC3 specifically inhibits CREB3-enhanced transcription of endogenous NF-κB target genes. For this experiment, we selected two NF-κB target genes, XIAP and NR4A2, since these genes contain well-known NF-κB-responsive element sites, as well as CRE (CREB-responsive element) in their promoter regions (at positions of −139 and −6, respectively). We first examined whether overexpressed CREB3 enhanced the basal level of XIAP and NR4A2 transcription. The results presented in Fig. 3b demonstrate that overexpressed CREB3 efficiently enhanced the basal transcription of both XIAP and NR4A2. In accordance with the results of the reporter assays, the overexpression of HDAC3 inhibited the CREB3-mediated transcription of both genes in a dose-dependent manner. Moreover, as with the overexpression of CREB3, TSA treatment enhanced CREB3-mediated transcription (Fig. 3c). To further confirm the specific involvement of HDAC3, we used si-RNA to knock down HDAC3 in HEK293 cells. As shown in Supplementary Fig. 1a, treatment with si-RNA specific for HDAC3 reduced the level of HDAC3 by more than 90%, as assessed by western blot analysis. Strikingly, the depletion of HDAC3 further enhanced CREB3-mediated NF-κB transcription (Fig. 3d). Thus, these findings suggest that HDAC3 is a transcriptional corepressor of CREB3-mediated NF-κB transcription.
We next investigated whether the inhibition of CREB3-mediated transcription is exclusive to HDAC3 among the class I HDACs, which also include HDAC1, HDAC2 and HDAC8. For this purpose, we designed the three sets of si-RNAs against all the class I HDACs (see “Materials and methods”). Both western blot and RT-PCR analyses clearly showed that each si-RNA treatment reduced the expression of the corresponding target protein (Supplementary Fig. 1b). As a control, we measured the levels of TBL1, a component of the HDAC3 corepressor complex, which showed no significant change after any of the si-RNA treatments. Having established the specific knock-down efficiency of each si-RNA, we next tested the effect of these si-RNAs on the transcription of NF-κB target genes. HEK293 cells were first transfected with each si-RNA as indicated, and the levels of XIAP transcription were determined by quantitative real-time PCR. While the si-RNAs against HDAC1, HDAC2 and HDAC8 had no effect on the CREB3-enhanced transcription of either gene, the knock-down of HDAC3 substantially augmented both XIAP and NR4A2 transcription (Fig. 3e). These findings collectively indicate that among all class I HDACs, HDAC3 is specifically involved in the inhibition of CREB3-mediated transcription.
HDAC3 represses the transcription of novel CREB-regulated gene, CXCR4 in MDA-MB-231 breast cancer cells
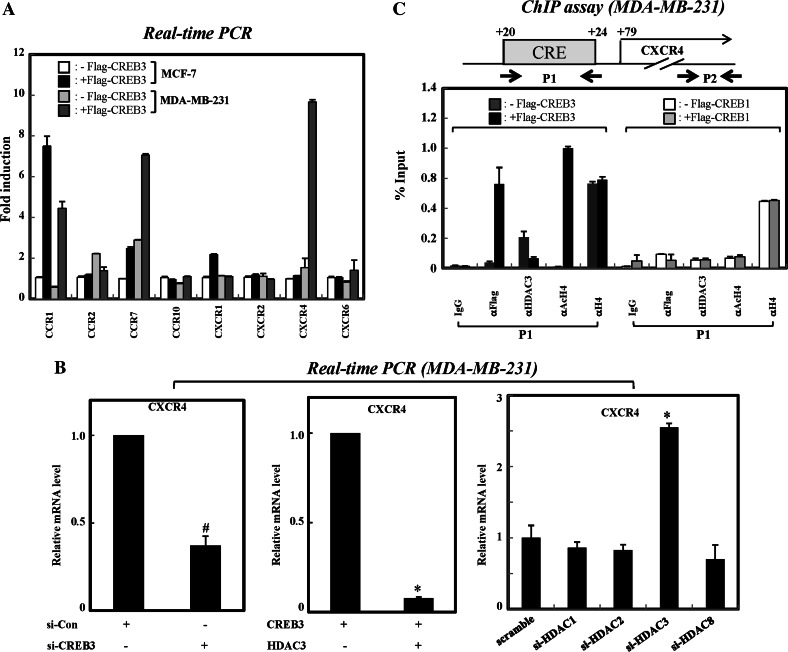
Given the knowledge of the selective repression of CREB3-enhanced NF-κB transcription by HDAC3, we next sought to confirm this result on endogenous CREB3 target gene. It is well established that CREB3 is involved in regulating the expression of chemokine receptors involved with regulating monocyte migration [23, 28]. More recently, HDAC3 has been shown to downregulate the CXCR4 gene in MDA-MB-231 cells treated with hepatocyte growth factor (HGF) [29], suggesting the possible involvement of CREB3 in transcriptional modulation of the CXCR4 gene. To test this possibility, we examined the ability of CREB3 to regulate the genes of chemokine receptors. To this end, we first compared the mRNA levels of each chemokine receptor gene in MCF-7 and MDA-MB-231 cells overexpressing CREB3. As shown in Fig. 4a, overexpression of CREB3 enhanced the transcription of both CCR7 and CXCR4 more dramatically in the MDA-MB-231 cells than in MCF-7 cells. Due to the MDA-MB-231-specific enhancement of CXCR4 expression by CREB3 relative to CCR7, we focused on the functional role of CREB3 in the modulation of CXCR4 expression.
Fig. 4.
HDAC3 specifically represses the expression of novel CREB3 target gene, CXCR4 in MDA-MB-231 cells. a Overexpression of CREB3 induces the expression of CXCR4 in MDA-MB-231 cells. MCF-7 and MDA-MB-231 cells were transfected with empty vector or FLAG-tagged CREB3 plasmid. The mRNA levels were determined by qRT-PCR analysis. b HDAC3 is the main co-repressor of CREB3-mediated CXCR4 expression. MDA-MB-231 cells were transfected with either si-RNAs or plasmids. Two days after transfection, the cells were harvested, and mRNAs were analysed by qRT-PCR. The results presented are the means of two independent experiments performed in triplicate. * P < 0.01; # P < 0.05 for siHDAC3 versus siscramble. c A diagram of the 5′UTR region of CXCR4 showing the position of CRE and primers used for PCR amplification in ChIP assays. MDA-MB-231 cells were transfected with either Flag-CREB1 or Flag-CREB3 construct, and ChIP assays were carried out. The results were analysed by real-time PCR and are shown as percentages in relation to input. The results presented are the means ±SD of three independent experiments
Next, we examined whether CREB3 is required for the transcription of CXCR4 in MDA-MB-231 cells. In this experiment, MDA-MB-231 cells were treated with si-RNA against CREB3. Upon depletion of CREB3, the expression of CXCR4 was greatly reduced. Furthermore, the overexpression of HDAC3 dramatically suppressed CREB3-mediated CXCR4 expression. To test the specificity of HDAC3 in CREB3-mediated CXCR4 transcriptional repression, we next tested the effect of si-RNAs against class I HDACs on CXCR4 expression. The results presented in Fig. 4b show that treatment with si-RNA against HDAC1, HDAC2 or HDAC8 had no apparent effect on the transcription of CXCR4, while a more significant effect was observed with siRNA against HDAC3. Thus, our results reliably support the specific involvement of HDAC3 in repression of CREB3-mediated CXCR4 expression. As specificity controls, the levels of CCR7 gene expression were not affected by either overexpression or depletion of HDAC3 in MDA-MB-231 cells (Supplementary Fig. 2).
Since HDAC3 is known to mainly repress transcription through deacetylation of histone tails [30], we investigated whether HDAC3 is recruited to the CRE region of CXCR4 to subsequently induce the hypoacetylation of the chromatin, and ultimately lead to transcriptional repression. In this experiment, we first identified the CREs in CXCR4. A putative CRE was identified at position +20 (relative to the transcription start site) through sequence mining of CXCR4 (Fig. 4c). To test the ability of CREB3 to bind to this putative CRE, we carried out ChIP experiments with CREB3 using samples derived from MDA-MB-231 cells. Specific PCR primers were designed to amplify sequences (100–150 bp) surrounding the putative CRE (Fig. 4c, P1). As shown in Fig. 4c (left panel), Flag-CREB3 bound to the CRE of CXCR4 at position +20. Under the same experimental conditions, HDAC3 was dissociated from the CRE region of the CXCR4 gene upon overexpression of CREB3. Coincidently, overexpression of CREB3 resulted in an increase in the acetylation of histone H4. As controls, the CREB3 and HDAC3 were not found to associate with the coding region of the CXCR4 gene in the same experiments (data not shown). More importantly, we failed to detect any change in HDAC3 binding and histone acetylation in the Flag-tagged CREB1 expressed MDA-MB-231, suggesting that CREB3 specifically regulates the transcription of CXCR4 gene in highly metastatic breast cancer cells (Fig. 4c, right panel). Collectively, these results show that HDAC3 selectively represses CREB3-mediated CXCR4 transcription at the chromatin level via deacetylation of histone tails.
HDAC3 specifically antagonizes the migration of MDA-MB-231 cells through the downregulation of CREB3-mediated CXCR4 expression
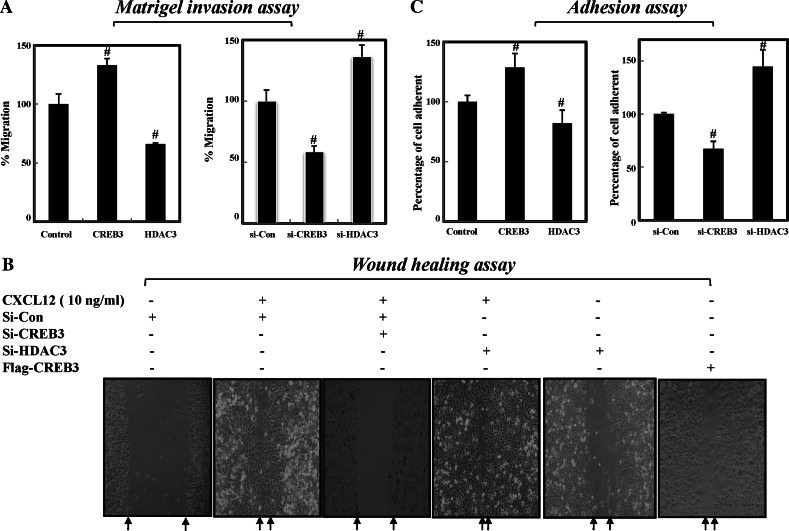
Since recent studies have shown that the inhibition of CXCR4 impairs breast cancer metastasis to regional lymph nodes and lungs [31], we sought to determine whether HDAC3 suppresses the CREB3-dependent migration of MDA-MB-231 cells using invasion, adhesion, and wound healing assays. For the invasion assay, MDA-MB-231 cells with either overexpressed or knocked-down CREB3 and/or HDAC3, were seeded into the upper chamber, and invasiveness was assessed by calculating how many cells passed through the Matrigel-coated membrane or the membrane alone. As shown in Fig. 5a, overexpression of CREB3 enhanced the invasiveness of MDA-MB-231 cells by approximately 30%. Conversely, overexpression of HDAC3 decreased invasiveness of MDA-MB-231 cells by approximately 25%. In accordance with these findings, depletion of CREB3 efficiently suppressed, while knock-down of HDAC3 promoted, migration of MDAMB-231 cells (Fig. 5a, right panel). In accordance with the results of the invasion assays, wound healing analysis showed that depletion of HDAC3 promoted the in vitro mobility of MDA-MB-231 cells, whereas the knock-down of CREB3 decreased the mobility of MDA-MB-231 cells even in the presence of the CXCR4 ligand, CCL12 (Fig. 5b). These results indicate that HDAC3 is a negative regulator of CREB3-dependent chemotaxis in metastatic breast cancer cells.
Fig. 5.
HDAC3 antagonizes the CXCR4-dependent migration of metastatic breast cancer cells. a Both depletion of CREB3 and overexpression of HDAC3 suppress cell migration. MDA-MB-231 cells were transfected with either siRNAs or plasmids before seeding into the upper chamber. The data presented are the means ±SD of at least three independent experiments. # P < 0.05 versus control. b HDAC3 antagonizes the CCL12-induced migration of MDA-MB-231 cells. Two days after transfection, the cells were scratched with a 200-μl pipette tip. Floating cells were washed with DMEM. The cells were photographed under a microscope 12 h after scratching. CXCL12 was used as a positive control for invasive latency. The data presented are the means ±SD of at least three independent experiments. # P < 0.05 versus control. c Both the depletion of HDAC3 and overexpression of CREB3 enhance cell adhesion. Adhesion assays were done as described in “Materials and methods”
Adhesion is closely related to cell migration and chemokines induce cell adhesion [32]. Thus, we determined the functional roles of HDAC3 in CREB3-mediated cell adhesion. The results presented in Fig. 5c (left panel) show that overexpression of CREB3 alone induced a rapid increase in cell adhesion to fibronectin; however, concomitant overexpression of HDAC3 in MDAMB-231 cells efficiently blocked cell adhesion. Consequently, the depletion of CREB3 greatly reduced the relative cell adhesion, which correlated with the decrease in CXCR4 expression. Conversely, knock-down of HDAC3 enhanced the adhesion of MDA-MB-231 cells (Fig. 5c, right panel). Collectively, these findings suggest that HDAC3 suppresses the chemotaxis of MDAMB-231 by inhibiting CREB3-dependent CXCR4 gene expression.
Discussion
In the study reported here we identified HDAC3 as a novel corepressor of CREB3-mediated CXCR4 gene expression. The most significant finding of this study is the selective interaction between HDAC3 and CREB3. The coimmunoprecipitation analysis clearly demonstrated that CREB3 exclusively interacts with HDAC3, and not with other class I HDACs. CREB1 has already been shown to interact with both HDAC1 and HDAC2, but not with HDAC3. In accordance with these findings, we also failed to detect an interaction between CREB1 and HDAC3. Thus, this study is the first to show that the class I HDAC isotype, HDAC3, specifically interacts with the CREB family protein, CREB3.
It has been reported that HDAC3 participates in the deacetylation of nonhistone substrates, such as p53 [33], NF-κB [34], and Smad7 [35]. Also, p300/CBP has been shown to acetylate CREB and enhance its transcriptional activity [36]. We thus examined the acetylation of CREB3 using an in vitro acetylation assay. Even though the recombinant p300 efficiently acetylated the GST histone H4, we failed to detect the acetylated form of CREB3 (Supplementary Fig. 3). We postulate that HDAC3 mainly participates in the repression of CREB-mediated transcription via deacetylation of histone tails.
To unravel the functional relationship between HDAC3 and CREB3, we first examined the role of HDAC3 in CREB3-enhanced NF-κB transcription. Since both HDAC3 and CREB3 were already known to participate in NF-κB-mediated transcriptional regulation, we decided to investigate how HDAC3 is selectively involved in CREB3-mediated transcription using NF-κB-based reporter assay and analysis of NF-κB target gene. To minimize the effect of NF-κB itself, CREB3-dependent transcription has been assessed without overexpression of NF-κB or treatment with cytokines such as IL-6 and LPS. The results from transcriptional analysis clearly showed that among all class I HDACs, HDAC3 is selectively involved in the inhibition of CREB3-mediated transcription.
To further confirm the selective involvement of HDAC3 in CREB3-mediated transcription that has already been observed in NF-κB-based reporter systems, we are next sought to verify this result upon endogenous CREB3 target gene. Since CREB3 also participates in the expression of CC chemokine receptors [23, 37], we thus tried to identify CREB3 targets among the chemokine receptor genes. Based on the bioinformatic analysis, we found that the upstream region of several chemokine receptor genes possess CRE sites. We first selected a specific chemokine receptor in a CREB3-dependent manner. Even though overexpressed CREB3 induced the expression of several chemokine receptor genes in both MCF-7 and MDA-MB-231 cells, the induction of MDA-MB-231-specific gene expression by CREB3 was only observed for CXCR4. This is consistent with the recent finding that CXCR4 is highly expressed in primary and metastatic human breast cancer cells, but is undetectable in normal mammary tissue [38]. Furthermore, Oncomine analysis, a microarray database that has a large collection of gene expression experiments on human cancer [39], also showed that CREB3 is upregulated in both lobular and invasive ductal breast carcinoma samples compared to normal breast tissue (Supplementary Fig. 4). Thus, our findings are consistent with the crucial roles of CREB3 in the tumorigenesis and metastasis of breast cancer cells.
In accordance with the results of the NF-κB reporter assay, the depletion of HDAC3 specifically enhanced the expression of CXCR4, but the same was not found for other class I HDACs, suggesting an exclusive role of HDAC3 in CREB3-mediated CXCR4 transcriptional repression. The ChIP assay also showed that overexpressed CREB3 induced histone hyperacetylation and dissociation of HDAC3 from the CRE region of CXCR4, supporting our notion that HDAC3 suppresses CREB3-mediated CXCR4 transcription via a decrease in histone acetylation.
Interestingly, recent study has revealed that HDAC3 is involved in the transcriptional repression of HGF-induced CXCR4 transcription via c-src signalling [29]. In this study, HDAC3 levels were enhanced by HGF treatment, and thus led to repression of CXCR4 expression, in MDA-MB-231 cells but not in MCF-7 cells. Importantly, this study demonstrated that by enhancing CXCR4 in tumour cells with a low invasive potential, HGF favours their homing to secondary sites, whereas by suppressing CXCR4 in cells with a high invasive potential, HGF might act to retain them in the metastatic sites in an HDAC3-dependent manner. This study thus supports our hypothesis that HDAC3 is a transcriptional corepressor of CXCR4 in metastatic breast cancer cells, which might retain them in metastatic sites. Therefore, HDAC3-targeted inhibitors might be promising candidates for antimetastatic cancer therapy. Even though the relationship between CREB3 and HGF-dependent chemokine signalling is not clear, it is possible that HGF treatment also reduces CREB3-mediated CXCR4 gene by causing the dissociation of CREB3 from the promoter region of CXCR4 and alternatively enhancing the chromatin targeting of HDAC3. Future work will determine the functional role of CREB3 in HGF-dependent cancer metastasis.
There is an increasing amount of evidence from retrospective clinical reports that the aberrant expression of CXCR4 is correlated with metastasis [40]. Importantly, strong CXCR4 expression has been detected using immunohistochemistry in metastatic breast cancer cells [41]. More recently, it has been demonstrated that CXCR4 expression closely correlates with a poor overall survival rate in patients with breast cancer [42]. The finding that HDAC3 selectively represses CREB3-mediated CXCR4 expression led us to examine whether HDAC3 efficiently suppresses CREB3-dependent cancer cell migration. The diverse chemotaxis assays showed that CREB3 is required for CXCR4-dependent chemotaxis of MDA-MB-231 cells and that HDAC3 reverses that effect. These findings collectively unravelled a novel function for HDAC3 in CREB3-dependent CXCR4 gene expression.
The expression of HDAC3 is frequently increased in tumours relative to adjacent normal tissue [43], while the downregulation of HDAC3 results in reduced proliferation and survival of tumour cells. Until now, we did not know whether the decreased level of HDAC3 was inversely correlated with CREB3 in cancer tissues. Extensive clinical studies are currently in progress and will reveal clinical implications of HDAC3 expression in cancer metastasis.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Supplementary Fig. 1 a HEK293 cells were transfected with siRNAs or FLAG-tagged CREB3 plasmid. The mRNA and protein levels were determined by qRT-PCR and western blot analysis. b Confirmation of siRNA efficiencies by western blotting and RT-PCR analysis. si-RNAs were transfected into HEK 293 cells. Two days after transfection, the cells were harvested. mRNAs and cell lysates were analysed by western blotting (upper panel) and RT-PCR (lower panel) (TIFF 606 KB)
Supplementary Fig. 2 Effect of HDAC3 on the expression of the CCR7 gene. MDA-MB-231 cells were transfected with siHDAC3 or FLAG-tagged CREB3 plasmid. The mRNA levels were determined by qRT-PCR (TIFF 225 kb)
Supplementary Fig. 3 In vitro acetylation assays were performed with HeLa nuclear extract as an enzyme source using GST or GST-fused proteins, and subsequently processed for autoradiography (TIFF 1.50 mb)
Supplementary Fig. 4 Oncomine 4.3 analysis for the expression patterns of CREB3 in normal and breast cancer tissues (TIFF 675 mb)
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant from the Korean government (MOST) (R13-2002-054-04002-0 and M1075502001-07N5502-00110), and a grant (code #20070301034007) from the BioGreen 21 program, Rural Development Administration, Republic of Korea.
Footnotes
H.-C. Kim and K.-C. Choi contributed equally to this work.
References
- 1.Emiliani S, Fischle W, Van Lint C, Al-Abed Y, Verdin E. Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci U S A. 1998;95(6):2795–2800. doi: 10.1073/pnas.95.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J Biol Chem. 2007;282(47):33994–34002. doi: 10.1074/jbc.M701847200. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Kadam S, Emerson BM, Bieker JJ. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Kruppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol Cell Biol. 2001;21(7):2413–2422. doi: 10.1128/MCB.21.7.2413-2422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, Seto E. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005;19(7):827–839. doi: 10.1101/gad.1286005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi D, Bergman M, Aihara H, Nibu Y, Mannervik M. Drosophila Ebi mediates snail-dependent transcriptional repression through HDAC3-induced histone deacetylation. EMBO J. 2008;27(6):898–909. doi: 10.1038/emboj.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagianni P, Wong J. HDAC3: taking the SMRT-N-CoRrect road to repression. Oncogene. 2007;26(37):5439–5449. doi: 10.1038/sj.onc.1210612. [DOI] [PubMed] [Google Scholar]
- 7.Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22(6):1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20(5):1048–1060. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, Choi E, Balk SP, Hollenberg AN. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem. 2005;280(8):6511–6519. doi: 10.1074/jbc.M408972200. [DOI] [PubMed] [Google Scholar]
- 10.Sankar N, Baluchamy S, Kadeppagari RK, Singhal G, Weitzman S, Thimmapaya B. p300 provides a corepressor function by cooperating with YY1 and HDAC3 to repress c-Myc. Oncogene. 2008;27(43):5717–5728. doi: 10.1038/onc.2008.181. [DOI] [PubMed] [Google Scholar]
- 11.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009;45(3):579–589. doi: 10.1016/j.bone.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Wang X, Xu L, Pan H, Zhu S, Liang Q, Huang B, Lu J. The transcription factor ZBP-89 suppresses p16 expression through a histone modification mechanism to affect cell senescence. FEBS J. 2009;276(15):4197–4206. doi: 10.1111/j.1742-4658.2009.07128.x. [DOI] [PubMed] [Google Scholar]
- 13.Togi S, Kamitani S, Kawakami S, Ikeda O, Muromoto R, Nanbo A, Matsuda T. HDAC3 influences phosphorylation of STAT3 at serine 727 by interacting with PP2A. Biochem Biophys Res Commun. 2009;379(2):616–620. doi: 10.1016/j.bbrc.2008.12.132. [DOI] [PubMed] [Google Scholar]
- 14.Das C, Kundu TK. Transcriptional regulation by the acetylation of nonhistone proteins in humans—a new target for therapeutics. IUBMB Life. 2005;57(3):137–149. doi: 10.1080/15216540500090629. [DOI] [PubMed] [Google Scholar]
- 15.Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, Piette J, Bours V, Chariot A. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem. 2003;278(47):46541–46548. doi: 10.1074/jbc.M306381200. [DOI] [PubMed] [Google Scholar]
- 16.Gao Z, Chiao P, Zhang X, Lazar MA, Seto E, Young HA, Ye J. Coactivators and corepressors of NF-kappaB in IkappaB alpha gene promoter. J Biol Chem. 2005;280(22):21091–21098. doi: 10.1074/jbc.M500754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco-Garcia N, Asensio-Juan E, de la Cruz X, Martinez-Balbas MA. Autoacetylation regulates P/CAF nuclear localization. J Biol Chem. 2009;284(3):1343–1352. doi: 10.1074/jbc.M806075200. [DOI] [PubMed] [Google Scholar]
- 18.Freiman RN, Herr W. Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev. 1997;11(23):3122–3127. doi: 10.1101/gad.11.23.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbelo PD, Kozak CA. Mapping of the murine LZIP gene (Creb3) to chromosome 4. Genomics. 1998;54(2):357–358. doi: 10.1006/geno.1998.5574. [DOI] [PubMed] [Google Scholar]
- 20.Luciano RL, Wilson AC. N-terminal transcriptional activation domain of LZIP comprises two LxxLL motifs and the host cell factor-1 binding motif. Proc Natl Acad Sci U S A. 2000;97(20):10757–10762. doi: 10.1073/pnas.190062797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Audas TE, Li Y, Liang G, Lu R. A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol. 2008;28(12):3952–3966. doi: 10.1128/MCB.01439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luciano RL, Wilson AC. An activation domain in the C-terminal subunit of HCF-1 is important for transactivation by VP16 and LZIP. Proc Natl Acad Sci U S A. 2002;99(21):13403–13408. doi: 10.1073/pnas.202200399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung HJ, Kim YS, Kang H, Ko J. Human LZIP induces monocyte CC chemokine receptor 2 expression leading to enhancement of monocyte chemoattractant protein 1/CCL2-induced cell migration. Exp Mol Med. 2008;40(3):332–338. doi: 10.3858/emm.2008.40.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Shinohara F, Sato K, Taniguchi T, Takada H, Rikiishi H. Interleukin-1beta converting enzyme subfamily inhibitors prevent induction of CD86 molecules by butyrate through a CREB-dependent mechanism in HL60 cells. Immunology. 2003;108(3):375–383. doi: 10.1046/j.1365-2567.2003.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canettieri G, Morantte I, Guzman E, Asahara H, Herzig S, Anderson SD, Yates JR, 3rd, Montminy M. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol. 2003;10(3):175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HG, Choi Y, Cole PA, Wong J. Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol Cell Biol. 2005;25(1):324–335. doi: 10.1128/MCB.25.1.324-335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang SW, Kim YS, Lee YH, Ko J. Role of human LZIP in differential activation of the NF-kappaB pathway that is induced by CCR1-dependent chemokines. J Cell Physiol. 2007;211(3):630–637. doi: 10.1002/jcp.20968. [DOI] [PubMed] [Google Scholar]
- 28.Jang SW, Kim YS, Kim YR, Sung HJ, Ko J. Regulation of human LZIP expression by NF-kappaB and its involvement in monocyte cell migration induced by Lkn-1. J Biol Chem. 2007;282(15):11092–11100. doi: 10.1074/jbc.M607962200. [DOI] [PubMed] [Google Scholar]
- 29.Matteucci E, Ridolfi E, Maroni P, Bendinelli P, Desiderio MA. c-Src/histone deacetylase 3 interaction is crucial for hepatocyte growth factor dependent decrease of CXCR4 expression in highly invasive breast tumor cells. Mol Cancer Res. 2007;5(8):833–845. doi: 10.1158/1541-7786.MCR-07-0054. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas E, Ait-Si-Ali S, Trouche D. The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein. Nucleic Acids Res. 2001;29(15):3131–3136. doi: 10.1093/nar/29.15.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Z, Wu H, Reddy S, Zhu A, Wang S, Blevins D, Yoon Y, Zhang Y, Shim H. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363(3):542–546. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, Zhang N. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005;65(4):1433–1441. doi: 10.1158/0008-5472.CAN-04-1163. [DOI] [PubMed] [Google Scholar]
- 33.Monte M, Simonatto M, Peche LY, Bublik DR, Gobessi S, Pierotti MA, Rodolfo M, Schneider C. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci U S A. 2006;103(30):11160–11165. doi: 10.1073/pnas.0510834103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, Lee JM, Yoon HG. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69(2):583–592. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 35.Tabata T, Kokura K, Ten Dijke P, Ishii S. Ski co-repressor complexes maintain the basal repressed state of the TGF-beta target gene, SMAD7, via HDAC3 and PRMT5. Genes Cells. 2009;14(1):17–28. doi: 10.1111/j.1365-2443.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 36.Yuan LW, Gambee JE. Histone acetylation by p300 is involved in CREB-mediated transcription on chromatin. Biochim Biophys Acta. 2001;1541(3):161–169. doi: 10.1016/S0167-4889(01)00141-0. [DOI] [PubMed] [Google Scholar]
- 37.Ko J, Jang SW, Kim YS, Kim IS, Sung HJ, Kim HH, Park JY, Lee YH, Kim J, Na DS. Human LZIP binds to CCR1 and differentially affects the chemotactic activities of CCR1-dependent chemokines. FASEB J. 2004;18(7):890–892. doi: 10.1096/fj.03-0867fje. [DOI] [PubMed] [Google Scholar]
- 38.Su YC, Wu MT, Huang CJ, Hou MF, Yang SF, Chai CY. Expression of CXCR4 is associated with axillary lymph node status in patients with early breast cancer. Breast. 2006;15(4):533–539. doi: 10.1016/j.breast.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 41.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64(23):8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 42.Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Kodama R, Sanke T, Nakamura Y. Cytoplasmic CXCR4 expression in breast cancer: induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariadason JM. Dissecting HDAC3-mediated tumor progression. Cancer Biol Ther. 2008;7(10):1581–1583. doi: 10.4161/cbt.7.10.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 a HEK293 cells were transfected with siRNAs or FLAG-tagged CREB3 plasmid. The mRNA and protein levels were determined by qRT-PCR and western blot analysis. b Confirmation of siRNA efficiencies by western blotting and RT-PCR analysis. si-RNAs were transfected into HEK 293 cells. Two days after transfection, the cells were harvested. mRNAs and cell lysates were analysed by western blotting (upper panel) and RT-PCR (lower panel) (TIFF 606 KB)
Supplementary Fig. 2 Effect of HDAC3 on the expression of the CCR7 gene. MDA-MB-231 cells were transfected with siHDAC3 or FLAG-tagged CREB3 plasmid. The mRNA levels were determined by qRT-PCR (TIFF 225 kb)
Supplementary Fig. 3 In vitro acetylation assays were performed with HeLa nuclear extract as an enzyme source using GST or GST-fused proteins, and subsequently processed for autoradiography (TIFF 1.50 mb)
Supplementary Fig. 4 Oncomine 4.3 analysis for the expression patterns of CREB3 in normal and breast cancer tissues (TIFF 675 mb)