Abstract
Global regulation allows bacteria to rapidly modulate the expression of a large variety of unrelated genes in response to environmental changes. Global regulators act at different levels of gene expression. This review focuses on CsrA, a post-transcriptional regulator that affects translation of its gene targets by binding mRNAs. CsrA controls a large variety of physiological processes such as central carbon metabolism, motility and biofilm formation. The activity of CsrA is itself tightly regulated by the CsrB and CsrC small RNAs and the BarA-UvrY two-component system.
Keywords: CsrB, CsrC, Central carbon metabolism, Motility, Biofilm formation
Multiple levels of global regulation in bacteria
Bacteria have evolved complex and efficient mechanisms for responding to their ever-changing lives. As an example, Escherichia coli is able to respond to a sudden depletion in amino acids by synthesizing the ppGpp alarmone which will redirect transcription toward biosynthetic operons and indirectly lead to polyphosphate-dependent degradation of several free ribosomal proteins (for review see [1]). It can also adapt to heat shock by inducing the production of chaperones and proteases that will help to refold and/or get rid of damaged proteins (for review see [2]), it can respond to oxidative stress by inducing expression of antioxidant enzymes (for review see [3]) and it can use the most energetic carbon sources among a mixture of carbon sources (for review see [4]). Examples are multiple and are reviewed in depth in a variety of recent reviews [5–8]. Adaptation to these environmental changes reflects gene expression reprogramming. This is achieved by so-called global regulators that allow bacteria to coordinately control the expression of genes or operons encoding unrelated functions and scattered over the genome (for review see [9]). It has been recognized that such global regulators act at the level of transcription. Recently, global post-transcriptional regulators have been characterized and shown to influence globally gene expression (for review see [10]) (Fig. 1). Global transcriptional regulators are themselves regulated by post-transcriptional regulators. Therefore, global regulation implies a cascade of regulations. The starting point is an extracellular signal that is transduced into the cell, the ultimate point being the phenotypic traits resulting from differential gene expression.
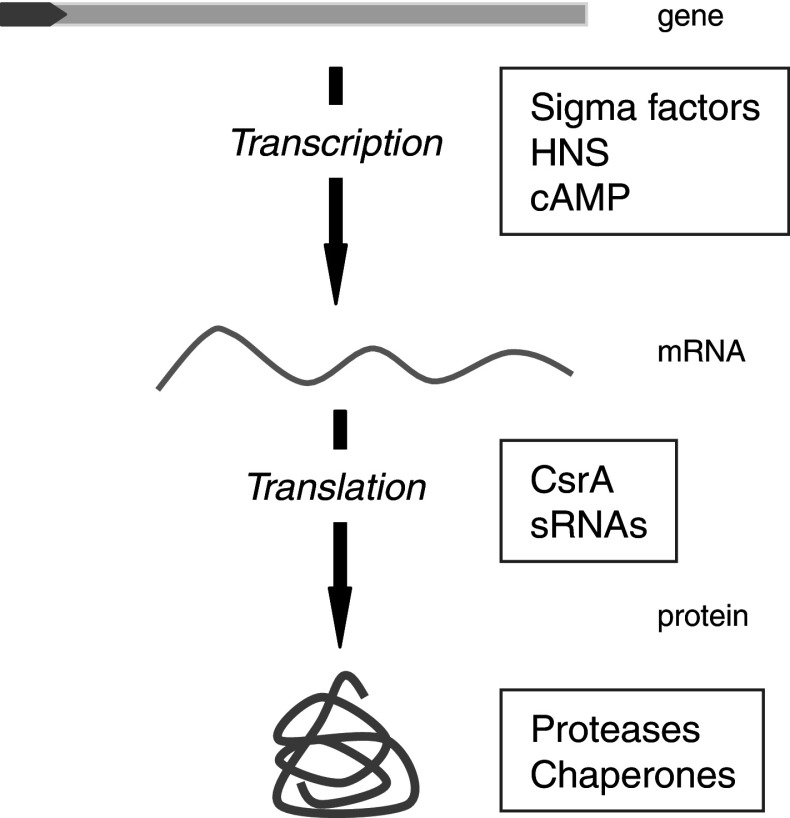
Fig. 1.
Regulation of gene expression by global regulators. Different levels of gene expression regulation by global regulators are represented. Sigma factors, H-NS and cAMP control transcription. CsrA and sRNAs regulate translation by acting on mRNAs at the post-transcriptional level. Proteases and chaperones modulate stability and availability of functional protein at the post-translational level
Transcriptional regulation
Among global transcriptional regulators, sigma (σ) factors are well documented (for reviews see [11, 12]). They allow RNA polymerase to be recruited at specific DNA sequences in gene promoter regions at which they initiate transcription. In addition to the major housekeeping σ70 factor, E. coli contains six other σ factors. Changes in their synthesis and/or degradation rates as well as competition between the different σ factors for the RNA polymerase core will lead to differential gene expression. In species such as Bacillus subtilis that undergo development, the interplay between multiple σ factors controls sporulation (for review see [13]).
H-NS (histone-like nucleoid structuring protein) is another type of global transcriptional regulator found in enterobacteria (for review see [14]). It is a small DNA-associated protein that binds preferentially to curved AT-rich DNA without showing sequence preferences [15–17]. H-NS regulates mostly negatively a variety of physiological pathways such as metabolism [18], fimbriae expression [19, 20], virulence [21], flagellum synthesis and proper function [22–24]. H-NS is thought to downregulate functions needed in the host when bacteria thrive outside it. These functions are positively regulated by environmental conditions.
Other types of global regulators are signalling molecules such as cyclic-AMP (cAMP) and cyclic-di-GMP (see below) [25, 26]. Synthesis of cAMP is negatively regulated by glucose uptake. cAMP binds to CRP (cAMP receptor protein), also known as CAP (catabolite activator protein), which is the effector protein. While CRP alone has no activity, the CRP-cAMP complex forms an active transcription factor that positively regulates gene expression. Recent microarray data highlight the global regulatory activity of cAMP. Directly or indirectly cAMP controls 380 E. coli genes (about 12% of the entire gene pool) [27]. Notably, cAMP regulates carbon source metabolism, flagellum synthesis, biofilm formation, quorum sensing and nitrogen assimilation [27–30].
Post-transcriptional regulation
Small noncoding RNAs (sRNAs) are major players in post-transcriptional global regulation (for review see [31]). A single sRNA can affect multiple targets and drastically modify cell physiology. These sRNAs are involved in stress response regulation as well as pathogenesis and virulence. More than 70 sRNAs have been identified in E. coli. The vast majority belong to the bona fide class of sRNAs that act by base pairing with their mRNA targets. They modify the translation or stability of the targets and most of them act together with the Hfq RNA chaperone. As an example, in E. coli, SgrS is a sRNA induced during the so-called sugar phosphate stress due to cytoplasmic accumulation of hexose sugar phosphate as a consequence of glycolysis inhibition. SgrS binds to the mRNA of ptsG, whose product is involved in glucose uptake, leading to its degradation [32]. In addition to its regulatory role, SgrS encodes a small protein called SgrT which is also involved in glucose uptake inhibition and appears to regulate PtsG activity [33]. This avoids new supply of glucose and allows depletion of the sugar phosphate pool [32]. Furthermore, some sRNAs regulate other global regulators, making them post-transcriptional global regulators according to the definitions proposed above. As an example, the E. coli DsrA sRNA regulates σ38 expression (for review see [34]). The second group of sRNAs binds to proteins. The best-studied is the E. coli CsrB and its homologues in closely related bacteria (for review see [35]). These sRNAs regulate the activity of the CsrA global regulator. This is developed in detail below.
Post-translational regulation
Another level of post-transcriptional regulation is control of protein stability and folding carried out by ATP-dependent proteases and chaperones [36–38]. Proteases and chaperones act together to regulate the amount of many proteins in the cell, among them global regulators such as σ factors. As an example, the E. coli Lon ATP-dependent proteases regulate flagella expression by degrading the specific σ flagella factor (σ28) [39] as well as the acid shock tolerance regulon by regulating the amount of the GadE master transcriptional regulator [40], gadE expression being itself under the σ38 transcriptional control. As mentioned above, σ38 plays an essential role in the general stress response. Post-translation control is mediated by the ClpXP ATP-dependent protease which degrades σ38 under steady-state conditions. Under certain types of stress or upon entry into the stationary phase, σ38 is no longer degraded and interacts with the RNA polymerase core and transcribes σ38 regulon genes (for review see [41]). Proteolysis relies on intricate interactions between protease subunits and adaptator or antiadaptator proteins depending on the growth conditions [42] (for review see [43]).
Post-transcriptional global regulation by CsrA
In a screen set up to identify trans factors that regulate biosynthesis of glycogen using an E. coli library of transposon mutants, Romeo and coworkers [44] discovered the csrA (carbon storage regulator A) gene. A transposon insertion in csrA (csrA::kan) was isolated and showed pleiotropic phenotypes, which led the authors to propose that CsrA is a global regulator. Cell size and adherence were affected in the csrA insertion mutant as well as glycogenesis and gluconeogenesis (see below). It was later shown that the csrA gene is essential for viability in rich medium and on minimal media supplemented with glycolytic carbon sources [45]. Moreover, the csrA::kan mutation was shown to be leaky, explaining the viability of the csrA::kan on these culture media (see below) [45].
The csrA gene encodes the CsrA protein which is composed of 61 amino acids. It is a post-transcriptional regulator that binds to mRNAs and regulates mRNA stability and translation [46–62]. CsrA acts mostly negatively leading in most cases to the decay of the negatively regulated mRNAs [46–59]. Some cases of positive regulation mediated by CsrA have also been described in the literature [60–62]. CsrA is regulated at the post-translational level by two sRNAs called CsrB and CsrC in E. coli [47, 63–65]. These sRNAs are composed of multiple CsrA binding sites that bind and sequester CsrA, thereby inhibiting its activity (for review see [35]).
CsrA is a global regulator. It regulates multiple unrelated pathways such as central carbon metabolism, motility and biofilm formation, virulence and pathogenesis, quorum sensing and oxidative stress response (Fig. 2), although some subtle differences exist depending on the bacterial species studied [44, 52, 58, 59, 62, 66–81]. Several homologues have been identified in other bacterial species [82]. In related species such as Erwinia carotovora and Pseudomonas aeruginosa, CsrA is called RsmA or RsmE (repressor of secondary metabolites) [83, 84]. Pseudomonas fluorescens contains two homologues of CsrA (RsmA and RsmE). They appear to be redundant since mutation of both is necessary to derepress production of antifungal secondary metabolites and exoenzymes [85]. In Pseudomonas syringae pv. tomato, four CsrA homologues have been identified [86], as in the case of P. fluorescens, the authors proposed that they might have redundant functions.
Fig. 2.
CsrA regulates numerous unrelated biological pathways. CsrA positively regulates glycolysis, acetate metabolism, mobility, pathogenesis, virulence, quorum sensing and oxidative stress response. It negatively controls biofilm formation, glycogenesis, gluconeogenesis and c-di-GMP synthesis, which is involved in the switch between planktonic and sessile life-styles
Molecular mechanisms of CsrA-mediated regulation of gene expression
Structure of CsrA
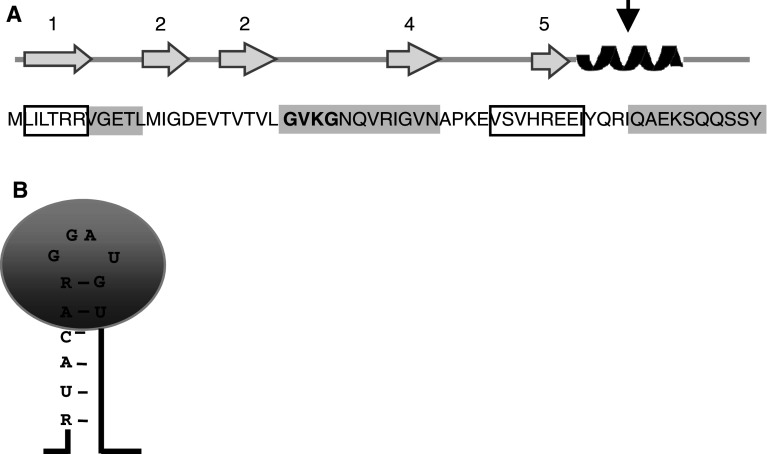
CsrA is a small homodimeric RNA-binding protein (about 7 kDa per monomer) [56, 86, 87]. It contains a degenerated KH (human heterogeneous nuclear ribonucleoprotein, hnRNP, K homology) domain [50]. Two types of structural KH domain exist, one found typically in eukaryotes and the other in prokaryotes (for review see [88]). The proteins containing a KH domain are involved in RNA or ssDNA recognition and contain a typical GxxG loop (where x is preferentially a basic amino acid) (for review see [88]) (Fig. 3a). The structure of CsrA is not similar to that of other proteins containing a KH domain although it does contains the GxxG motif (Fig. 3a) [87]. The three-dimensional structure of CsrA was solved by X-ray radiography (from P. putida CsrA) and by NMR (E. coli and P. fluorescens CsrA), giving very similar structures. CsrA is composed of five β-strands followed by an α-helix (Fig. 3a). The dimer is obtained by interdigitation of five β-strands of each monomer [56, 86, 87]. Binding of CsrA on a mRNA target (glgC, see below) leads to large conformational changes [87]. This was not observed upon binding to the CsrB-negative regulator most likely because of the structure of the CsrB RNA [87]. The loops connecting β-sheets 1 and 2, and 3 and 4 (containing the GxxG motif) as well as β-sheet 4 and the C-terminus are involved in RNA recognition (Fig. 3a, sequence shaded in grey) [87]. Comprehensive alanine-scanning mutagenesis gave different indications and revealed that the two distal regions (in β-strand 1 and in β-strand 5) are involved in RNA recognition [55] (Fig. 3a, sequence boxed in black). The β-strand 1 of one monomer is located near the β-strand 5 of the other monomer, suggesting that they form a functional domain [55]. The csrA::kan mutation is due to transposon insertion at the 51st codon of csrA [89] (Fig. 3a, arrow). This leads to the disruption of the C-terminal α-helix which is involved in RNA recognition as proposed by Mercante and coworkers [55]. Although both RNA recognition domains of CsrA are necessary for proper function [90], this mutant retains partial activity [44, 45].
Fig. 3.
Secondary structure of CsrA and RNA sequence and structure recognition. a CsrA is composed of five β-sheets (grey arrows) followed by an α-helix (black). The amino acid sequence of CsrA from E. coli is indicated. Domains involved in RNA recognition/binding based on the 3-D structure are shaded in grey [87] and on mutagenesis studies are boxed in black [55]. The KH domain is indicated in bold. Point of transposon insertion in the csrA::kan mutant is indicated by an arrow [44]. b The CsrA consensus recognition sequence is 5′-RUACARGGAUGU-3′ that is part of a stem-loop, the GGA motif being in the loop. CsrA is represented in grey
CsrA binding sites
The consensus sequence of the E. coli CsrA binding site has been identified using the SELEX method (systematic evolution of ligands by the exponential enrichment). This method is based on the high-affinity isolation of ligands to a randomized nucleic sequence (for review see [91]). The consensus binding sequence is 5′-RUACARGGAUGU-3′, where R is a purine. Furthermore, the secondary structure of the RNA target is also important for CsrA recognition. Using in silico RNA structure prediction and experimental RNase and Pb2+ digestions, it has been shown that CsrA recognizes this sequence in a hairpin in which the middle GGA motif is located in the loop (Fig. 3b) [46, 47]. The P. fluorescens CsrA (RsmE) recognizes very similar sequences to that recognized by the E. coli CsrA (5′-A/UCANGGANGU/A-3′, where N is any nucleotide). The structure is also similar: the middle ANGGA motif is located in the hairpin loop [56]. The CsrA dimer binds to two adjacent binding sites [55, 90]. It has recently been proposed, based on mRNA shift assay and glgC S30 transcription-translation, that CsrA binds first to a high-affinity site which enables CsrA binding to lower affinity sites [90].
Molecular mechanisms of regulation
Negative regulation is mediated by CsrA binding on specific sites which are located nearby or overlapping the Shine-Dalgarno sequence. Binding leads to inhibition of translation initiation and generally to mRNA degradation [46–59]. The RNase(s) involved are still unknown. In the case of the hfq mRNA (a target of CsrA in E. coli), no degradation is observed. Whether it reflects another mode of regulation is unknown [53].
The number of CsrA binding sites depends on the target (Table 1). The number varies between one (hfq mRNA) and six (pgaABCD mRNA) [53, 58]. Whether the number of CsrA binding sites on a target influences the tightness of the regulation is not known. However, deletion of one of the CsrA binding sites in the glgC transcript enhances its translation [90].
Table 1.
Direct targets of CsrA
| Target genes | Function | Regulation | No. of CsrA box | Species | References |
|---|---|---|---|---|---|
| glgCAP | Glycogen metabolism | Negative | 4 | E. coli | [46, 90] |
| pgaABCD | Biofilm attachment phase | Negative | 6 | E. coli | [58] |
| hfq | RNA-binding protein | Negative | 1 | E. coli | [53] |
| cstA | Peptide transport | Negative | 4 | E. coli | [48] |
| ydeH | c-di-GMP metabolism; diguanylate cyclase | Negative | Unknown | E. coli | [54] |
| ycdT | c-di-GMP metabolism; diguanylate cyclase | Negative | Unknown | E. coli | [54] |
| PA0081 | Scaffolding protein | Negative | Unknown | P. aeruginosa | [67] |
| PA0082 | Hypothetical protein | Negative | Unknown | P. aeruginosa | [67] |
| PA4492 | Hypothetical protein | Negative | Unknown | P. aeruginosa | [67] |
| STM1987 | c-di-GMP metabolism; diguanylate cyclase | Negative | Unknown | S. typhimurium | [60] |
| STM3375 (CsrD homologue) | c-di-GMP metabolism; no diguanylate cyclase nor phosphodiesterase activities | Negative | Unknown | S. typhimurium | [60] |
| STM1344 | c-di-GMP metabolism; no diguanylate cyclase nor phosphodiesterase activities | Negative | Unknown | S. typhimurium | [60] |
| STM1697 | c-di-GMP metabolism; probably no phosphodiesterase activity | Negative | Unknown | S. typhimurium | [60] |
| flhDC | Motility regulator | Positive | Unknown | E. coli | [62] |
| sepLespADB | Virulence factors | Positive | Unknown | E. coli | [61] |
| STM3611 | c-di-GMP metabolism; phosphodiesterase activity | Positive | Unknown | S. typhimurium | [60] |
CsrA was shown to bind to the mRNAs indicated, although CsrA binding sites were not systematically experimentally determined.
Regulation of glycogen storage by CsrA in E. coli is well documented (see below). CsrA negatively regulates glycogen accumulation by controlling the expression of the glgCAP operon and glgB of the glgBX operon [46, 50, 52]. While glgBX operon regulation has not been extensively studied, four CsrA binding sites have been identified in the 5′ untranslated region of the glgCAP mRNA, one of them overlapping the Shine-Dalgarno sequence [46, 90]. CsrA binding on these sites induces translation inhibition and rapid mRNA decay [46, 50]. glgCAP transcripts are rapidly degraded in the wild-type strain (half-life of about 4 min) and they are stabilized in the csrA::kan mutant [50]. Positive regulation by CsrA has been less investigated; only three cases have been reported in the literature. CsrA positively regulates expression of flhDC (encoding the master transcriptional regulator of flagellum synthesis in E. coli), sepLespADB (involved in pedestal formation induced by enteropathogenic E. coli) and stm3611 (encoding a phosphodiesterase that degrades c-di-GMP in Salmonella typhimurium) [60–62] (for review see [92]). As for negative regulation, positive regulation mediated by CsrA is based on CsrA binding on the 5′ untranslated region [46, 61, 62], leading to a stabilization of the mRNA [62]. The amount of their cognate mRNA is decreased in the csrA mutant [60, 62].
Physiological roles of CsrA
CsrA regulates central carbon metabolism
Central carbon metabolic pathways comprise glycolysis, the Krebs cycle and the pentose phosphate pathway (Fig. 4) (for review see [93]). These pathways are interconnected and rely on glucose entry by the phosphoenolpyruvate/carbohydrate phosphotransferase system (PTS) and its conversion into glucose-6-phosphate. Glycogenesis represents a fourth metabolic pathway and converts glucose-6-phosphate into glycogen (Fig. 4). In E. coli, the so-called glg genes are involved in glycogen metabolism. The glgA, glgC and glgB genes encode enzymes responsible for glycogen anabolism (for review see [94]). The glgP (also called glgY) (for review see [94]), glgX [95] and glgS gene products are involved in glycogen catabolism [96, 97]. CsrA regulates different steps in central carbon metabolic pathways. It negatively regulates glycogenesis as well as gluconeogenesis, although no data on CsrA binding sites are available for gluconeogenesis genes [52, 80]. As mentioned above, CsrA inhibits translation and induces glgCAP transcript degradation [44, 46, 49, 50]. For the other glg genes (except for glgX, that is not regulated by CsrA), although it has been shown that CsrA regulates their expression, it is not known whether or not CsrA binding sites are present [52]. CsrA also regulates expression of the cstA gene encoding a putative peptide transporter [48]. Expression of this gene is induced during carbon starvation [98].
Fig. 4.
CsrA regulates central carbon metabolism and glycogenesis. The pentose phosphate pathway, gluconeogenesis, glycolysis, glycogenesis and the Krebs cycle are indicated in grey. Genes whose expression is regulated by csrA are indicated (+ positive regulation, − negative regulation). The different steps of glycolysis, gluconeogenesis and glycogenesis are indicated. Since CsrA does not regulate the pentose phosphate pathway or the Krebs cycle, these pathways are not shown in detail (DHAP dihydroxyacetone-3-P, PEP phosphoenolpyruvate). Adapted from reference [80]
Differences are observed between closely related species. In S. typhimurium, CsrA does not appear to regulate glycogen synthesis. However, it regulates other metabolic pathways such as maltose and acetate metabolism [99]. CsrA also positively regulates acetate metabolism in E. coli [81].
Interestingly, CsrA regulates antagonistic pathways in opposite ways; for example, glycolysis (energy consumption) is positively regulated, while gluconeogenesis and glycogenesis (energy storage) are negatively regulated [80]. Thus, inactivation of the csrA gene leads to imbalanced metabolic fluxes towards glycogen accumulation [44, 46, 49, 50, 52]. Due to the positive effect of CsrA on glycolysis, an E. coli ΔcsrA strain is unable to grow on glycolytic carbon sources but is able to grow on gluconeogenic carbon sources such as pyruvate. Deletion of the glgCAP operon restores viability of the ΔcsrA deletion strain on glycolytic carbon sources [45]. This indicates that in the absence of csrA, carbon fluxes are directed towards glycogenesis which impairs glycolysis, even at a basal level [45].
CsrA regulates group behaviour
Bacterial communication
Quorum sensing is a cell-to-cell communication that allows bacteria to sense the size of their population and to coordinate their behaviour (for review see [100]). Quorum sensing relies on intercellular communication using signal molecules and signal transduction systems to regulate gene expression. The nature of signal molecules and of transduction systems varies among species. In gram-negative bacteria, the most common signal molecules are homoserine lactones (AHL) that are synthesized by LuxI-type enzymes. At high cell densities, AHL concentrations exceed a certain threshold, bind to LuxR-type transcriptional regulators and induce a positive feed-back. In several species including Vibrio sp. and Pseudomonas sp., CsrA negatively regulates the production of AHL, although the underlying molecular mechanism is still unknown [79, 101]. In E. carotovora subsp. carotovora, quorum sensing molecules downregulate the expression of RsmA, the CsrA homologue [102].
Biofilm and motility
Bacteria often form sessile multicellular communities associated on a surface that are called biofilms (for review see [103]). This life-style protects them against harsh environmental conditions such as predators and antibiotics. Biofilm formation comprises different steps, one of the earliest being attachment of bacteria to a substrate. In E. coli, numerous bacterial factors are involved in this phase, notably PGA (a linear homopolymer of poly-β-1,6-N-acetyl-d-glucosamine) [104–106]. The pgaABCD operon is involved in PGA production and excretion and CsrA negatively regulates its expression [58]. In an E. coli strain containing csrA under the control of a riboswitch, CsrA depletion leads to autoaggregation in a PGA-dependent manner [107]. A similar phenotype is observed with the ΔglgCAP ΔcsrA strain (Seyll et al., unpublished data). In Vibrio vulnificus and Campylobacter jejuni, CsrA also negatively regulates biofilm formation, although the underlying molecular mechanisms have not been identified [108, 109]. In E. coli, CsrA directly regulates the production of c-di-GMP (bis-(3′-5′)-cyclic-dimeric guanosine monophosphate) [54], a second messenger involved in the control of biofilm formation and motility (for a recent review see [110]). Enzymes responsible for c-di-GMP synthesis and degradation are ubiquitous in the bacterial world, and many species encode a large number of these proteins. Diguanylate cyclases with a conserved GGDEF motif are responsible for c-di-GMP synthesis and phosphodiesterases with a conserved EAL motif are responsible for its degradation. In E. coli, expression of two GGDEF proteins (ycdT and ydeH) is downregulated directly by CsrA [54]. In a recent study, expression of two GGDEF proteins, two GGDEF-EAL proteins and four EAL proteins have been shown to be under CsrA control in S. typhimurium [60].
High levels of c-di-GMP favour the sessile state, whereas low levels promote motility [60]. Thus, CsrA acts at two parallel levels to downregulate biofilm formation: it negatively controls PGA and c-di-GMP production. While being sedentary in a biofilm is certainly advantageous, it is sometimes necessary to be able to quit the biofilm and move to colonize new niches. E. coli is able to swim using flagella. In E. coli, flagella production is controlled by the FlhDC transcriptional activator which is itself positively regulated by CsrA [62]. In S. typhimurium, the flagella class III gene STM3611 is both directly and indirectly regulated by CsrA. STM1344 a regulator of class II genes for flagella synthesis is directly regulated by CsrA [60]. Thus, CsrA appears to be a master regulator involved in the motile/sessile switch, by regulating directly and indirectly expression of behaviour genes.
CsrA regulates pathogenicity and virulence
Virulence and pathogenicity are associated with the ability to adhere to host tissue, to colonize it and to secrete virulence factors. A large amount of evidence that CsrA is involved in virulence control has been obtained in different bacterial species [61, 74, 101, 108, 111–117]. For example, CsrA directly activates expression of genes necessary for pedestal formation in enteropathogenic E. coli [61] and for type III secretion system in P. aeruginosa [77]. In vivo data in mouse models suggest that CsrA is involved in the control of different steps in host colonization and virulence [78, 118].
Regulation of CsrA activity
CsrA is regulated by σ38
In E. carotovora, csrA expression is positively regulated by the homologue of E. coli σ38 but direct regulation has not been shown [119]. In E. coli, σ38 is known to regulate genes during the onset of the stationary phase and in stress conditions (for review see [41]). It has been shown that csrA expression is under the positive control of σ38 when E. coli is grown in synthetic medium containing glucose as the sole carbon source [120]. However, in rich medium, this does not seem to be the case [121, 122].
Regulation by the CsrB and CsrC sRNAs
The activity of CsrA is tightly regulated by the sequestration activity of CsrB and CsrC sRNAs. CsrB sRNA has been found to copurify with CsrA [63]. This 366-nucleotide noncoding RNA is composed of 18 imperfect CsrA binding sites (CAGGAUG). Thus, CsrB sequesters CsrA and therefore inhibits its regulatory activity (Fig. 5). The 245-nucleotide CsrC sRNA regulates CsrA activity in a similar manner, although CsrC contains only nine CsrA-binding sites [65]. Functional homologues of CsrB and CsrC have been identified in other bacterial species. These sRNAs contain variable numbers of CsrA binding sites (from 5 to 30) (for review see [35]). Furthermore, the number of functional homologues of CsrB and CsrC varies between species. Up to four CsrB homologues have been predicted in Photobacterium profundum which has the most in silico predicted CsrB homologues [123].
Fig. 5.
Regulation of CsrA. The BarA-UvrY two-component system is activated by weak acids as well as Krebs cycle intermediates. This two-component system positively regulates expression of the sRNAs CsrB and CsrC. They sequester CsrA, thereby negatively controlling its activity. Expression of the sRNAs is negatively regulated by CsrD. In S. typhimurium and in E. coli, the CsrD homologue is negatively regulated by the CsrA homologue. CsrA also directly or indirectly regulates (via a putative X factor) the BarA-UvrY two-component system. Expression of UvrY is under the positive control of quorum sensing
The CsrB and CsrC sRNAs are unstable due to their degradation by RNaseE [124]. The CsrD membrane-bound protein binds with high affinity to CsrB and CsrC and stimulates their degradation (Fig. 5). Interestingly, CsrD contains characteristic GGDEF and EAL motifs involved in c-di-GMP metabolism (see above), although these motifs are degenerated for key residues. Overproduction of CsrD does not modify c-di-GMP concentrations showing that CsrD is inactive for c-di-GMP synthesis and degradation. However, the EAL domain seems to be necessary for CsrB degradation [124]. Interestingly, in S. typhimurium as well as in E. coli, CsrA negatively regulates expression of the csrD homologue (STM3375) [60, 125]. Although it has been shown that the E. carotovora CsrB is stabilized in presence of CsrA [102], the E. coli CsrB decay is not affected by CsrA [126].
The expression level of CsrB and CsrC depends on the culture medium. In nutrient-poor medium, in the absence of amino acids, CsrB and CsrC are highly expressed, while in the presence of amino acids, their expression is reduced [60]. It is not clear yet what causes the suppression of CsrB and CsrC expression by amino acids. The authors proposed that the expression of these sRNAs is under the control of a signal molecule induced by amino acid starvation, although mutations in the genes necessary for ppGpp synthesis (relA and spoT) had no effect on csrB expression in LB medium [125]. This has still to be tested in nutrient-poor medium.
The BarA-UvrY two-component system
The BarA-UvrY two-component system (TCS) is composed of the BarA sensor kinase and its cognate response regulator UvrY (for review see [127]). Homologues of BarA-UvrY have been found in other bacterial species (called GacS-GacA, SirA or LetS-LetA) (for review see [128]). The BarA-UvrY TCS is triggered by an imbalance in the Krebs cycle intermediates and by weak acids such as formate and acetate [129, 130]. In E. coli, UvrY is regulated by the quorum sensing molecule AI-2 [64]. The ability of E. coli barA and uvrY mutants to grow on different carbon sources has been analysed. Interestingly, the mutants have an advantage on gluconeogenic carbon sources and a disadvantage on glycolytic sources compared to the wild-type strain, showing that the BarA-UvrY system is necessary for the switch between glycolysis and gluconeogenesis [131]. The activation of this TCS leads to the overproduction of CsrB and CsrC (Fig. 5). Furthermore, expression of CsrB and CsrC is upregulated in a UvrY-dependent manner at the onset of the stationary phase [64, 65, 126, 131]. This overproduction leads to sequestration of CsrA, favouring glycogenesis.
Homologues of the BarA-UvrY TCS system also regulate CsrA activity in other bacterial species via sRNAs [85, 127, 132–135]. Microarray analysis was performed in P. luminescens to identify genes controlled by this TCS [133]. Motility and biofilm formation are respectively under negative and positive control of UvrY. UvrY positively regulates iron uptake as well as quorum sensing, by affecting production of the signal molecule. UvrY is also involved in the regulation of the expression of sugar and peptide transporters as well as that of virulence factors.
Expression of CsrB and CsrC is also positively regulated by CsrA. Because of this feed-back regulation, loss of one of these sRNAs results in an increase of CsrA activity. As a consequence, the remaining sRNA is upregulated [65]. By analysing csrB expression in Csr mutants and by complementation tests, it has been shown that CsrA regulates csrB transcription via UvrY and independently of it (Fig. 4) [64, 126, 127].
Concluding remarks and perspectives
CsrA regulates gene expression by modulating translation and stability of its mRNA targets. It acts together with other global regulators on several pathways such as central carbon metabolism and biofilm formation. This highlights intricate networks of regulation that allow bacteria to adapt to changing environments. Interestingly, CsrA often shows opposite regulation for antagonistic pathways, for example it positively regulates motility and negatively biofilm formation. CsrA regulation is thus involved in a switch between two different modes. Moreover, inside a specific pathway, it regulates several steps, for example for motility, it enhances the synthesis of flagellum components and decreases the synthesis of c-di-GMP and PGA. As we might expect for a global regulator, expression and activity of CsrA is also tightly regulated. It involves several regulatory systems notably the CsrB and CsrC sRNAs that control CsrA activity by sequestration. Expression of these regulators is itself positively controlled by the BarA-UvrY TCS. This TCS appears to be activated by metabolic intermediates, thereby providing a feed-back control of carbon fluxes in the cell.
Although negative regulation mediated by CsrA is well understood, the RNase involved in the degradation of the targeted mRNA is still unknown. What dictates the positive or negative effect of CsrA on translation also remains to be elucidated.
Acknowledgments
Johan Timmermans is supported by a Waleo 3 Program (no. 816876) from the Belgian Wallonia region.
References
- 1.Magnusson LU, Farewell A, Nystrom T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Dougan DA, Mogk A, Bukau B. Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. Cell Mol Life Sci. 2002;59:1607–1616. doi: 10.1007/PL00012487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- 4.Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci Biotechnol Biochem. 2009;73:245–259. doi: 10.1271/bbb.80479. [DOI] [PubMed] [Google Scholar]
- 5.Campbell EA, Greenwell R, Anthony JR, Wang S, Lim L, Das K, Sofia HJ, Donohue TJ, Darst SA. A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Mol Cell. 2007;27:793–805. doi: 10.1016/j.molcel.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuliodori AM, Gualerzi CO, Soto S, Vila J, Tavio MM. Review on bacterial stress topics. Ann N Y Acad Sci. 2007;1113:95–104. doi: 10.1196/annals.1391.008. [DOI] [PubMed] [Google Scholar]
- 7.Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 8.Marles-Wright J, Lewis RJ. Stress responses of bacteria. Curr Opin Struct Biol. 2007;17:755–760. doi: 10.1016/j.sbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira T, Springer M. Post-transcriptional control by global regulators of gene expression in bacteria. Curr Opin Microbiol. 2000;3:154–158. doi: 10.1016/S1369-5274(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 11.Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 12.Potvin E, Sanschagrin F, Levesque RC. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2008;32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroos L. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu Rev Genet. 2007;41:13–39. doi: 10.1146/annurev.genet.41.110306.130400. [DOI] [PubMed] [Google Scholar]
- 14.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 15.Jauregui R, Abreu-Goodger C, Moreno-Hagelsieb G, Collado-Vides J, Merino E. Conservation of DNA curvature signals in regulatory regions of prokaryotic genes. Nucleic Acids Res. 2003;31:6770–6777. doi: 10.1093/nar/gkg882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, Mavathur R, Muskhelishvili G, Pon CL, Rimsky S, Stella S, Babu MM, Travers A. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 2007;35:6330–6337. doi: 10.1093/nar/gkm712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Muramatsu S, Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem. 1990;108:420–425. doi: 10.1093/oxfordjournals.jbchem.a123216. [DOI] [PubMed] [Google Scholar]
- 18.Bertin P, Hommais F, Krin E, Soutourina O, Tendeng C, Derzelle S, Danchin A. H-NS and H-NS-like proteins in Gram-negative bacteria and their multiple role in the regulation of bacterial metabolism. Biochimie. 2001;83:235–241. doi: 10.1016/S0300-9084(01)01247-0. [DOI] [PubMed] [Google Scholar]
- 19.Olsen PB, Schembri MA, Gally DL, Klemm P. Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol Lett. 1998;162:17–23. doi: 10.1111/j.1574-6968.1998.tb12973.x. [DOI] [PubMed] [Google Scholar]
- 20.Schembri MA, Olsen PB, Klemm P. Orientation-dependent enhancement by H-NS of the activity of the type 1 fimbrial phase switch promoter in Escherichia coli. Mol Gen Genet. 1998;259:336–344. doi: 10.1007/s004380050820. [DOI] [PubMed] [Google Scholar]
- 21.Muller CM, Dobrindt U, Nagy G, Emody L, Uhlin BE, Hacker J. Role of histone-like proteins H-NS and StpA in expression of virulence determinants of uropathogenic Escherichia coli. J Bacteriol. 2006;188:5428–5438. doi: 10.1128/JB.01956-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertin P, Terao E, Lee EH, Lejeune P, Colson C, Danchin A, Collatz E. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J Bacteriol. 1994;176:5537–5540. doi: 10.1128/jb.176.17.5537-5540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko M, Park C. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J Mol Biol. 2000;303:371–382. doi: 10.1006/jmbi.2000.4147. [DOI] [PubMed] [Google Scholar]
- 24.Landini P, Zehnder AJ. The global regulatory hns gene negatively affects adhesion to solid surfaces by anaerobically grown Escherichia coli by modulating expression of flagellar genes and lipopolysaccharide production. J Bacteriol. 2002;184:1522–1529. doi: 10.1128/JB.184.6.1522-1529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruckner R, Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett. 2002;209:141–148. doi: 10.1016/S0378-1097(02)00559-1. [DOI] [PubMed] [Google Scholar]
- 26.Pruss BM, Besemann C, Denton A, Wolfe AJ. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J Bacteriol. 2006;188:3731–3739. doi: 10.1128/JB.01780-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez-Rios RM, Freyre-Gonzalez JA, Resendis O, Collado-Vides J, Saier M, Gosset G. Identification of regulatory network topological units coordinating the genome-wide transcriptional response to glucose in Escherichia coli. BMC Microbiol. 2007;7:53. doi: 10.1186/1471-2180-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Lay N, Gottesman S. The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J Bacteriol. 2009;191:461–476. doi: 10.1128/JB.01157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller CM, Aberg A, Straseviciene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5:e1000303. doi: 10.1371/journal.ppat.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng D, Constantinidou C, Hobman JL, Minchin SD. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32:5874–5893. doi: 10.1093/nar/gkh908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottesman S. Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet. 2005;21:399–404. doi: 10.1016/j.tig.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Vanderpool CK, Gottesman S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol. 2004;54:1076–1089. doi: 10.1111/j.1365-2958.2004.04348.x. [DOI] [PubMed] [Google Scholar]
- 33.Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci U S A. 2007;104:20454–20459. doi: 10.1073/pnas.0708102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majdalani N, Vanderpool CK, Gottesman S. Bacterial small RNA regulators. Crit Rev Biochem Mol Biol. 2005;40:93–113. doi: 10.1080/10409230590918702. [DOI] [PubMed] [Google Scholar]
- 35.Babitzke P, Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol. 2007;10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Doyle SM, Wickner S. Hsp104 and ClpB: protein disaggregating machines. Trends Biochem Sci. 2009;34:40–48. doi: 10.1016/j.tibs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Hengge R. Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Res Microbiol. 2009;160:667–676. doi: 10.1016/j.resmic.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Van Melderen L, Aertsen A. Regulation and quality control by Lon-dependent proteolysis. Res Microbiol. 2009;160:645–651. doi: 10.1016/j.resmic.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Barembruch C, Hengge R. Cellular levels and activity of the flagellar sigma factor FliA of Escherichia coli are controlled by FlgM-modulated proteolysis. Mol Microbiol. 2007;65:76–89. doi: 10.1111/j.1365-2958.2007.05770.x. [DOI] [PubMed] [Google Scholar]
- 40.Heuveling J, Possling A, Hengge R. A role for Lon protease in the control of the acid resistance genes of Escherichia coli. Mol Microbiol. 2008;69:534–547. doi: 10.1111/j.1365-2958.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- 41.Klauck E, Typas A, Hengge R. The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli. Sci Prog. 2007;90:103–127. doi: 10.3184/003685007X215922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol Microbiol. 2008;68:298–313. doi: 10.1111/j.1365-2958.2008.06146.x. [DOI] [PubMed] [Google Scholar]
- 43.Gottesman S. Proteolysis in bacterial regulatory circuits. Annu Rev Cell Dev Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- 44.Romeo T, Gong M, Liu MY, Brun-Zinkernagel AM. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J Bacteriol. 1993;175:4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmermans J, Van Melderen L. Conditional essentiality of the csrA gene in Escherichia coli. J Bacteriol. 2009;191:1722–1724. doi: 10.1128/JB.01573-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker CS, Morozov I, Suzuki K, Romeo T, Babitzke P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol Microbiol. 2002;44:1599–1610. doi: 10.1046/j.1365-2958.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 47.Dubey AK, Baker CS, Romeo T, Babitzke P. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA. 2005;11:1579–1587. doi: 10.1261/rna.2990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubey AK, Baker CS, Suzuki K, Jones AD, Pandit P, Romeo T, Babitzke P. CsrA regulates translation of the Escherichia coli carbon starvation gene, cstA, by blocking ribosome access to the cstA transcript. J Bacteriol. 2003;185:4450–4460. doi: 10.1128/JB.185.15.4450-4460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu MY, Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J Bacteriol. 1997;179:4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu MY, Yang H, Romeo T. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on mRNA stability. J Bacteriol. 1995;177:2663–2672. doi: 10.1128/jb.177.10.2663-2672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romeo T. Post-transcriptional regulation of bacterial carbohydrate metabolism: evidence that the gene product CsrA is a global mRNA decay factor. Res Microbiol. 1996;147:505–512. doi: 10.1016/0923-2508(96)84004-6. [DOI] [PubMed] [Google Scholar]
- 52.Yang H, Liu MY, Romeo T. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J Bacteriol. 1996;178:1012–1017. doi: 10.1128/jb.178.4.1012-1017.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker CS, Eory LA, Yakhnin H, Mercante J, Romeo T, Babitzke P. CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence. J Bacteriol. 2007;189:5472–5481. doi: 10.1128/JB.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jonas K, Edwards AN, Simm R, Romeo T, Romling U, Melefors O. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol Microbiol. 2008;70:236–257. doi: 10.1111/j.1365-2958.2008.06411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mercante J, Suzuki K, Cheng X, Babitzke P, Romeo T. Comprehensive alanine-scanning mutagenesis of Escherichia coli CsrA defines two subdomains of critical functional importance. J Biol Chem. 2006;281:31832–31842. doi: 10.1074/jbc.M606057200. [DOI] [PubMed] [Google Scholar]
- 56.Schubert M, Lapouge K, Duss O, Oberstrass FC, Jelesarov I, Haas D, Allain FH. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nat Struct Mol Biol. 2007;14:807–813. doi: 10.1038/nsmb1285. [DOI] [PubMed] [Google Scholar]
- 57.Sorger-Domenigg T, Sonnleitner E, Kaberdin VR, Blasi U. Distinct and overlapping binding sites of Pseudomonas aeruginosa Hfq and RsmA proteins on the non-coding RNA RsmY. Biochem Biophys Res Commun. 2007;352:769–773. doi: 10.1016/j.bbrc.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Dubey AK, Suzuki K, Baker CS, Babitzke P, Romeo T. CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli. Mol Microbiol. 2005;56:1648–1663. doi: 10.1111/j.1365-2958.2005.04648.x. [DOI] [PubMed] [Google Scholar]
- 59.Yakhnin H, Pandit P, Petty TJ, Baker CS, Romeo T, Babitzke P. CsrA of Bacillus subtilis regulates translation initiation of the gene encoding the flagellin protein (hag) by blocking ribosome binding. Mol Microbiol. 2007;64:1605–1620. doi: 10.1111/j.1365-2958.2007.05765.x. [DOI] [PubMed] [Google Scholar]
- 60.Jonas K, Edwards AN, Ahmad I, Romeo T, Romling U, Melefors O. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella typhimurium. Environ Microbiol. 2010;12:524–540. doi: 10.1111/j.1462-2920.2009.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhatt S, Edwards AN, Nguyen HT, Merlin D, Romeo T, Kalman D. The RNA binding protein CsrA is a pleiotropic regulator of the locus of enterocyte effacement pathogenicity island of enteropathogenic Escherichia coli. Infect Immun. 2009;77:3552–3568. doi: 10.1128/IAI.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei BL, Brun-Zinkernagel AM, Simecka JW, Pruss BM, Babitzke P, Romeo T. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol. 2001;40:245–256. doi: 10.1046/j.1365-2958.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- 63.Liu MY, Gui G, Wei B, Preston JF, 3rd, Oakford L, Yuksel U, Giedroc DP, Romeo T. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J Biol Chem. 1997;272:17502–17510. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki K, Wang X, Weilbacher T, Pernestig AK, Melefors O, Georgellis D, Babitzke P, Romeo T. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J Bacteriol. 2002;184:5130–5140. doi: 10.1128/JB.184.18.5130-5140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weilbacher T, Suzuki K, Dubey AK, Wang X, Gudapaty S, Morozov I, Baker CS, Georgellis D, Babitzke P, Romeo T. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol Microbiol. 2003;48:657–670. doi: 10.1046/j.1365-2958.2003.03459.x. [DOI] [PubMed] [Google Scholar]
- 66.Ang S, Horng YT, Shu JC, Soo PC, Liu JH, Yi WC, Lai HC, Luh KT, Ho SW, Swift S. The role of RsmA in the regulation of swarming motility in Serratia marcescens. J Biomed Sci. 2001;8:160–169. doi: 10.1007/BF02256408. [DOI] [PubMed] [Google Scholar]
- 67.Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol. 2009;72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burrowes E, Baysse C, Adams C, O’Gara F. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology. 2006;152:405–418. doi: 10.1099/mic.0.28324-0. [DOI] [PubMed] [Google Scholar]
- 69.Chao NX, Wei K, Chen Q, Meng QL, Tang DJ, He YQ, Lu GT, Jiang BL, Liang XX, Feng JX, Chen B, Tang JL. The rsmA-like gene rsmA(Xcc) of Xanthomonas campestris pv. campestris is involved in the control of various cellular processes, including pathogenesis. Mol Plant Microbe Interact. 2008;21:411–423. doi: 10.1094/MPMI-21-4-0411. [DOI] [PubMed] [Google Scholar]
- 70.Gaskell AA, Crack JC, Kelemen GH, Hutchings MI, Le Brun NE. RsmA is an anti-sigma factor that modulates its activity through a [2Fe-2S] cluster cofactor. J Biol Chem. 2007;282:31812–31820. doi: 10.1074/jbc.M705160200. [DOI] [PubMed] [Google Scholar]
- 71.Ge Y, Yang S, Fang Y, Yang R, Mou D, Cui J, Wen L. RpoS as an intermediate in RsmA-dependent regulation of secondary antifungal metabolites biosynthesis in Pseudomonas sp. M18. FEMS Microbiol Lett. 2007;268:81–87. doi: 10.1111/j.1574-6968.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 72.Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, Singer D, Cámara M, Williams P, Haas D. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol. 2004;186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, Romeo T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol. 2002;184:290–301. doi: 10.1128/JB.184.1.290-301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J Med Microbiol. 2003;52:19–28. doi: 10.1099/jmm.0.05024-0. [DOI] [PubMed] [Google Scholar]
- 75.Lucchetti-Miganeh C, Burrowes E, Baysse C, Ermel G. The post-transcriptional regulator CsrA plays a central role in the adaptation of bacterial pathogens to different stages of infection in animal hosts. Microbiology. 2008;154:16–29. doi: 10.1099/mic.0.2007/012286-0. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee A, Cui Y, Liu Y, Dumenyo CK, Chatterjee AK. Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherichia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology. 1996;142(Pt 2):427–434. doi: 10.1099/13500872-142-2-427. [DOI] [PubMed] [Google Scholar]
- 77.Mulcahy H, O’Callaghan J, O’Grady EP, Adams C, O’Gara F. The posttranscriptional regulator RsmA plays a role in the interaction between Pseudomonas aeruginosa and human airway epithelial cells by positively regulating the type III secretion system. Infect Immun. 2006;74:3012–3015. doi: 10.1128/IAI.74.5.3012-3015.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulcahy H, O’Callaghan J, O’Grady EP, Maciá MD, Borrell N, Gómez C, Casey PG, Hill C, Adams C, Gahan CG, Oliver A, O’Gara F. Pseudomonas aeruginosa RsmA plays an important role during murine infection by influencing colonization, virulence, persistence, and pulmonary inflammation. Infect Immun. 2008;76:632–638. doi: 10.1128/IAI.01132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pessi G, Williams F, Hindle Z, Heurlier K, Holden MT, Camara M, Haas D, Williams P. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J Bacteriol. 2001;183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabnis NA, Yang H, Romeo T. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J Biol Chem. 1995;270:29096–29104. doi: 10.1074/jbc.270.49.29096. [DOI] [PubMed] [Google Scholar]
- 81.Wei B, Shin S, LaPorte D, Wolfe AJ, Romeo T. Global regulatory mutations in csrA and rpoS cause severe central carbon stress in Escherichia coli in the presence of acetate. J Bacteriol. 2000;182:1632–1640. doi: 10.1128/JB.182.6.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White D, Hart ME, Romeo T. Phylogenetic distribution of the global regulatory gene csrA among eubacteria. Gene. 1996;182:221–223. doi: 10.1016/S0378-1119(96)00547-1. [DOI] [PubMed] [Google Scholar]
- 83.Blumer C, Heeb S, Pessi G, Haas D. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci U S A. 1999;96:14073–14078. doi: 10.1073/pnas.96.24.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chatterjee A, Cui Y, Liu Y, Dumenyo CK, Chatterjee AK. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl Environ Microbiol. 1995;61:1959–1967. doi: 10.1128/aem.61.5.1959-1967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reimmann C, Valverde C, Kay E, Haas D. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J Bacteriol. 2005;187:276–285. doi: 10.1128/JB.187.1.276-285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rife C, Schwarzenbacher R, McMullan D, Abdubek P, Ambing E, Axelrod H, Biorac T, Canaves JM, Chiu HJ, Deacon AM, DiDonato M, Elsliger MA, Godzik A, Grittini C, Grzechnik SK, Hale J, Hampton E, Han GW, Haugen J, Hornsby M, Jaroszewski L, Klock HE, Koesema E, Kreusch A, Kuhn P, Lesley SA, Miller MD, Moy K, Nigoghossian E, Paulsen J, Quijano K, Reyes R, Sims E, Spraggon G, Stevens RC, van den Bedem H, Velasquez J, Vincent J, White A, Wolf G, Xu Q, Hodgson KO, Wooley J, Wilson IA. Crystal structure of the global regulatory protein CsrA from Pseudomonas putida at 2.05 A resolution reveals a new fold. Proteins. 2005;61:449–453. doi: 10.1002/prot.20502. [DOI] [PubMed] [Google Scholar]
- 87.Gutierrez P, Li Y, Osborne MJ, Pomerantseva E, Liu Q, Gehring K. Solution structure of the carbon storage regulator protein CsrA from Escherichia coli. J Bacteriol. 2005;187:3496–3501. doi: 10.1128/JB.187.10.3496-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valverde R, Edwards L, Regan L. Structure and function of KH domains. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- 89.Romeo T, Gong M. Genetic and physical mapping of the regulatory gene csrA on the Escherichia coli K-12 chromosome. J Bacteriol. 1993;175:5740–5741. doi: 10.1128/jb.175.17.5740-5741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mercante J, Edwards AN, Dubey AK, Babitzke P, Romeo T. Molecular geometry of CsrA (RsmA) binding to RNA and its implications for regulated expression. J Mol Biol. 2009;392:511–528. doi: 10.1016/j.jmb.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoltenburg R, Reinemann C, Strehlitz B. SELEX – a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng. 2007;24:381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Smith TG, Hoover TR. Deciphering bacterial flagellar gene regulatory networks in the genomic era. Adv Appl Microbiol. 2009;67:257–295. doi: 10.1016/S0065-2164(08)01008-3. [DOI] [PubMed] [Google Scholar]
- 93.Holms WH. The central metabolic pathways of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr Top Cell Regul. 1986;28:69–105. doi: 10.1016/b978-0-12-152828-7.50004-4. [DOI] [PubMed] [Google Scholar]
- 94.Preiss J, Yung SG, Baecker PA. Regulation of bacterial glycogen synthesis. Mol Cell Biochem. 1983;57:61–80. doi: 10.1007/BF00223525. [DOI] [PubMed] [Google Scholar]
- 95.Dauvillee D, Kinderf IS, Li Z, Kosar-Hashemi B, Samuel MS, Rampling L, Ball S, Morell MK. Role of the Escherichia coli glgX gene in glycogen metabolism. J Bacteriol. 2005;187:1465–1473. doi: 10.1128/JB.187.4.1465-1473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hengge-Aronis R, Fischer D. Identification and molecular analysis of glgS, a novel growth-phase-regulated and rpoS-dependent gene involved in glycogen synthesis in Escherichia coli. Mol Microbiol. 1992;6:1877–1886. doi: 10.1111/j.1365-2958.1992.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 97.Kozlov G, Elias D, Cygler M, Gehring K. Structure of GlgS from Escherichia coli suggests a role in protein–protein interactions. BMC Biol. 2004;2:10. doi: 10.1186/1741-7007-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultz JE, Matin A. Molecular and functional characterization of a carbon starvation gene of Escherichia coli. J Mol Biol. 1991;218:129–140. doi: 10.1016/0022-2836(91)90879-B. [DOI] [PubMed] [Google Scholar]
- 99.Lawhon SD, Frye JG, Suyemoto M, Porwollik S, McClelland M, Altier C. Global regulation by CsrA in Salmonella typhimurium. Mol Microbiol. 2003;48:1633–1645. doi: 10.1046/j.1365-2958.2003.03535.x. [DOI] [PubMed] [Google Scholar]
- 100.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 101.Cui Y, Chatterjee A, Liu Y, Dumenyo CK, Chatterjee AK. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chatterjee A, Cui Y, Chatterjee AK. RsmA and the quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone, control the levels of rsmB RNA in Erwinia carotovora subsp. carotovora by affecting its stability. J Bacteriol. 2002;184:4089–4095. doi: 10.1128/JB.184.15.4089-4095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moons P, Michiels CW, Aertsen A. Bacterial interactions in biofilms. Crit Rev Microbiol. 2009;35:157–168. doi: 10.1080/10408410902809431. [DOI] [PubMed] [Google Scholar]
- 104.Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, Meisner J, Beveridge TJ, Preston JF, 3rd, Romeo T. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-beta-1,6-N-acetyl-d-glucosamine. J Bacteriol. 2008;190:3670–3680. doi: 10.1128/JB.01920-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Izano EA, Sadovskaya I, Wang H, Vinogradov E, Ragunath C, Ramasubbu N, Jabbouri S, Perry MB, Kaplan JB. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathog. 2008;44:52–60. doi: 10.1016/j.micpath.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin Y, Watt RM, Danchin A, Huang JD. Use of a riboswitch-controlled conditional hypomorphic mutation to uncover a role for the essential csrA gene in bacterial autoaggregation. J Biol Chem. 2009;284:28738–28745. doi: 10.1074/jbc.M109.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fields JA, Thompson SA. Campylobacter jejuni CsrA mediates oxidative stress responses, biofilm formation, and host cell invasion. J Bacteriol. 2008;190:3411–3416. doi: 10.1128/JB.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones MK, Warner EB, Oliver JD. csrA inhibits the formation of biofilms by Vibrio vulnificus. Appl Environ Microbiol. 2008;74:7064–7066. doi: 10.1128/AEM.01810-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 111.Altier C, Suyemoto M, Ruiz AI, Burnham KD, Maurer R. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol Microbiol. 2000;35:635–646. doi: 10.1046/j.1365-2958.2000.01734.x. [DOI] [PubMed] [Google Scholar]
- 112.Barnard FM, Loughlin MF, Fainberg HP, Messenger MP, Ussery DW, Williams P, Jenks PJ. Global regulation of virulence and the stress response by CsrA in the highly adapted human gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;51:15–32. doi: 10.1046/j.1365-2958.2003.03788.x. [DOI] [PubMed] [Google Scholar]
- 113.Forsbach-Birk V, McNealy T, Shi C, Lynch D, Marre R. Reduced expression of the global regulator protein CsrA in Legionella pneumophila affects virulence-associated regulators and growth in Acanthamoeba castellanii. Int J Med Microbiol. 2004;294:15–25. doi: 10.1016/j.ijmm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Kerrinnes T, Zelas ZB, Streckel W, Faber F, Tietze E, Tschape H, Yaron S. CsrA and CsrB are required for the post-transcriptional control of the virulence-associated effector protein AvrA of Salmonella enterica. Int J Med Microbiol. 2009;299:333–341. doi: 10.1016/j.ijmm.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Rasis M, Segal G. The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol Microbiol. 2009;72:995–1010. doi: 10.1111/j.1365-2958.2009.06705.x. [DOI] [PubMed] [Google Scholar]
- 116.Sahr T, Bruggemann H, Jules M, Lomma M, Albert-Weissenberger C, Cazalet C, Buchrieser C. Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol Microbiol. 2010;72:741–762. doi: 10.1111/j.1365-2958.2009.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teplitski M, Goodier RI, Ahmer BM. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J Bacteriol. 2003;185:7257–7265. doi: 10.1128/JB.185.24.7257-7265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molofsky AB, Swanson MS. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol Microbiol. 2003;50:445–461. doi: 10.1046/j.1365-2958.2003.03706.x. [DOI] [PubMed] [Google Scholar]
- 119.Mukherjee A, Cui Y, Ma W, Liu Y, Ishihama A, Eisenstark A, Chatterjee AK. RpoS (sigma-S) controls expression of rsmA, a global regulator of secondary metabolites, harpin, and extracellular proteins in Erwinia carotovora. J Bacteriol. 1998;180:3629–3634. doi: 10.1128/jb.180.14.3629-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dong T, Schellhorn HE. Control of RpoS in global gene expression of Escherichia coli in minimal media. Mol Genet Genomics. 2009;281:19–33. doi: 10.1007/s00438-008-0389-3. [DOI] [PubMed] [Google Scholar]
- 121.Patten CL, Kirchhof MG, Schertzberg MR, Morton RA, Schellhorn HE. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol Genet Genomics. 2004;272:580–591. doi: 10.1007/s00438-004-1089-2. [DOI] [PubMed] [Google Scholar]
- 122.Rahman M, Hasan MR, Oba T, Shimizu K. Effect of rpoS gene knockout on the metabolism of Escherichia coli during exponential growth phase and early stationary phase based on gene expressions, enzyme activities and intracellular metabolite concentrations. Biotechnol Bioeng. 2006;94:585–595. doi: 10.1002/bit.20858. [DOI] [PubMed] [Google Scholar]
- 123.Kulkarni PR, Cui X, Williams JW, Stevens AM, Kulkarni RV. Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri. Nucleic Acids Res. 2006;34:3361–3369. doi: 10.1093/nar/gkl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suzuki K, Babitzke P, Kushner SR, Romeo T. Identification of a novel regulatory protein (CsrD) that targets the global regulatory RNAs CsrB and CsrC for degradation by RNase E. Genes Dev. 2006;20:2605–2617. doi: 10.1101/gad.1461606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jonas K, Tomenius H, Romling U, Georgellis D, Melefors O. Identification of YhdA as a regulator of the Escherichia coli carbon storage regulation system. FEMS Microbiol Lett. 2006;264:232–237. doi: 10.1111/j.1574-6968.2006.00457.x. [DOI] [PubMed] [Google Scholar]
- 126.Gudapaty S, Suzuki K, Wang X, Babitzke P, Romeo T. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J Bacteriol. 2001;183:6017–6027. doi: 10.1128/JB.183.20.6017-6027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jonas K, Melefors O. The Escherichia coli CsrB and CsrC small RNAs are strongly induced during growth in nutrient-poor medium. FEMS Microbiol Lett. 2009;297:80–86. doi: 10.1111/j.1574-6968.2009.01661.x. [DOI] [PubMed] [Google Scholar]
- 128.Heeb S, Blumer C, Haas D. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J Bacteriol. 2002;184:1046–1056. doi: 10.1128/jb.184.4.1046-1056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lapouge K, Schubert M, Allain FH, Haas D. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol Microbiol. 2008;67:241–253. doi: 10.1111/j.1365-2958.2007.06042.x. [DOI] [PubMed] [Google Scholar]
- 130.Chavez RG, Alvarez AF, Romeo T, Georgellis D. The physiological stimulus for the BarA sensor kinase. J Bacteriol. 2010;192:2009–2012. doi: 10.1128/JB.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takeuchi K, Kiefer P, Reimmann C, Keel C, Dubuis C, Rolli J, Vorholt JA, Haas D. Small RNA-dependent expression of secondary metabolism is controlled by Krebs cycle function in Pseudomonas fluorescens. J Biol Chem. 2009;284:34976–34985. doi: 10.1074/jbc.M109.052571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pernestig AK, Georgellis D, Romeo T, Suzuki K, Tomenius H, Normark S, Melefors O. The Escherichia coli BarA-UvrY two-component system is needed for efficient switching between glycolytic and gluconeogenic carbon sources. J Bacteriol. 2003;185:843–853. doi: 10.1128/JB.185.3.843-853.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hyytiainen H, Montesano M, Palva ET. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme gene expression through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact. 2001;14:931–938. doi: 10.1094/MPMI.2001.14.8.931. [DOI] [PubMed] [Google Scholar]
- 134.Krin E, Derzelle S, Bedard K, Adib-Conquy M, Turlin E, Lenormand P, Hullo MF, Bonne I, Chakroun N, Lacroix C, Danchin A. Regulatory role of UvrY in adaptation of Photorhabdus luminescens growth inside the insect. Environ Microbiol. 2008;10:1118–1134. doi: 10.1111/j.1462-2920.2007.01528.x. [DOI] [PubMed] [Google Scholar]
- 135.Valverde C, Heeb S, Keel C, Haas D. RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol Microbiol. 2003;50:1361–1379. doi: 10.1046/j.1365-2958.2003.03774.x. [DOI] [PubMed] [Google Scholar]