Abstract
Abnormalities of platelet functions have been linked to reelin-impaired neuronal disorders. However, little attention has been given to understanding the interplay between reelin and platelet. In this study, reelin was found to present in the human platelets and megakaryocyte-like leukemic cells. Reelin-binding assays revealed that extracellular reelin can interact with platelets through the receptor belonging to the low density lipoprotein receptor gene family. The reelin-to-platelet interactions enhance platelet spreading on fibrinogen concomitant with the augmentation of lamellipodia formation and F-actin bundling. In contrast, reelin has no effect on integrin αIIbβ3 activation and agonist-induced platelet aggregation. Molecular analysis revealed that the up-regulation of Rac1 activity and the inhibition of protein kinase C δ-Thr505 phosphorylation are important for reelin-mediated enhancement of platelet spreading on fibrinogen. These findings demonstrate for the first time that reelin is present in platelets and the reelin-to-platelet interactions play a novel role in platelet signaling and functions.
Keywords: Fibrinogen, Platelet spreading, PKCδ, Rac1, Reelin
Introduction
Reelin is an extracellular matrix glycoprotein that plays a pivotal role in the regulation of cell migration and positioning control [1, 2]. The binding of reelin to two membrane receptors, ApoE receptor 2 (ApoER2) and very low-density lipoprotein receptor, results in tyrosine phosphorylation of Disabled-1 (DAB1) and recruitment of SRC family tyrosine kinases (SFKs). The phospho-DAB1 interacts with lissencephaly protein and mediates neuronal cell migration and cortical lamination, whereas SFKs-mediated activation of phosphatidylinositol-3-kinase stabilizes tau protein and increases cytoskeleton plasticity [3–5]. Impaired reelin signaling thereby is linked to the pathogenesis of a number of neurodegeneration disorders including Alzheimer’s disease, frontotemporal dementia, progressive supranuclear palsy, Parkinson’s disease and the psychotic disorders schizophrenia and autism [6–8].
Reelin is also found in the circulating plasma and specific peripheral tissues and cell types of adult mammals [9, 10]. Although circulating reelin may play a role in the extra-central nervous system, the defined function of reelin in the circulation and peripheral tissues is barely characterized. A limited number of studies suggest a possible interplay between reelin and platelet. For instance, platelet aggregation and dense granule secretion stimulated by collagen was altered in schizophrenia patients compared to healthy subjects [11, 12]. The platelet intracellular calcium mobilization after stimulation by serotonin is specifically enhanced in bipolar disorder compared with that in normal controls [13, 14]. Because the pathogenesis of both schizophrenia and bipolar disorder is related to the abnormality of reelin signaling [8], it is likely that reelin has a functional link in modulating platelet functions and signaling.
The aims of this study are to characterize reelin expression in various blood cell types and to analyze the interplay between reelin and platelets. We found that reelin is present in the platelet, megakaryocytic cell lines and circulating plasma. Remarkably, reelin interacts with platelets through the receptor belonging to the low density lipoprotein receptor gene family. The reelin-to-platelet interactions induce platelet lamellipodia formation and F-actin bundling that subsequently enhances full spreading of platelets on fibrinogen. Molecular analysis further unveils that reelin crosstalks with the key regulators of platelet spreading and adhesion, including Rac1 and protein kinase C δ (PKCδ). The functional implications for reelin-modulated platelet signaling and functions are discussed.
Materials and methods
Materials
The anti-reelin monoclonal antibody 142 (mAb142) and rottlerin were purchased from Calbiochem (San Diego, CA). The anti-reelin monoclonal antibody CR-50 (1 mg/ml) was purchased from MBL (Woburn, MA). The anti-Rac1 and the fluorescein isothiocyanate (FITC)-conjugated anti-PAC-1 antibodies were purchased from BD Biosciences (San Jose, CA). The anti-PKCδ and the anti-phospho-Thr505 PKCδ antibodies were purchased from Cell Signaling (Beverly, CA). The anti-platelet factor 4 (PF4) antibody was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Thrombin, fibrinogen, U46619, FITC-conjugated phalloidin and the anti-β-actin antibody were purchased from Sigma (Saint Louis, MO). Collagen type I was purchased from Chronolog (Havertown, PA). Arachidonic acid (AA) was purchased from Alexis Biochemicals (San Diego, CA). The Rac1 inhibitor NSC23766 was purchased from Tocris Bioscience (Ellisville, MO). The murine reelin expression plasmid pCrl was a kind gift of Dr. Tom Curran (University of Pennsylvania). The plasmid (pGST-RAP) for production of recombinant receptor-associated protein (RAP) fused with glutathione-S-transferase (GST) protein was obtained from Dr. Kenji Kadomatsu (Nagoya University) with the permission from Dr. Guojun Bu (Washington University School of Medicine).
Granulocyte, peripheral blood mononuclear cell and platelet isolation
The peripheral blood was drawn from healthy, drug-free volunteers in compliance with the University Ethics Committee guidelines. The granulocyte fraction, which contained more than 95% of neutrophils and the peripheral blood mononuclear cells (PBMC), were isolated by Ficoll gradient centrifugation as described previously [15].
The washed platelets were prepared by mixing whole blood with the anticoagulant solution containing 3.7% sodium citrate and 0.6 μg/ml prostaglandin I2 (PGI2) [16]. The peripheral blood was then centrifuged at 250g for 20 min to obtain platelet-rich plasma (PRP). The PRP was then mixed with PGI2 (0.3 μg/ml) and centrifuged at 900g for 10 min. After several washes with Tyrode’s buffer (137 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM NaH2PO4, 5.5 mM glucose, 5 mM HEPES, 1 mM MgCl2·6H2O, 2 mM CaCl2·2H2O), the washed platelets were resuspended in Tyrode’s buffer without PGI2.
Western blot analysis, RNA isolation and RT-PCR assay
Western blot analysis and RNA isolation were performed as described [17]. For RT-PCR analysis of reelin expression, 500 ng of total RNA was reversed transcribed to cDNA using poly-dT primer. The cDNA was subsequently amplified by PCR using forward primer reelin-F (5′-GCCACAATGGAACAGGTCAT-3′) and reverse primer reelin-R (5′-CAATACTGCCACTGTAACTG-3′). The condition for PCR was 1 cycle of 95°C for 2 min, 40 cycles of 95°C for 30 s, 50°C for 40 s, and 72°C for 1 min, and 1 cycle of 72°C for 5 min.
Expression and purification of recombinant reelin
The expression and purification of reelin protein was performed as described [18]. Briefly, the human embryonic kidney 293T cells were transiently transfected with the pCrl expression plasmid, which contains the full-length mouse reelin cDNA. At 16 h after transfection, the cells were trypsinized and replated onto a 10-cm culture plate for 24 h followed by changing the medium to serum free Opti-MEM. At 60 h after medium replacement, the conditioned medium was collected, cleared by centrifugation (800g) at 4°C for 10 min and purified by Amicon Ultracel (100-kDa molecular weight cutoff) under a centrifugation force of 4,000g at 4°C for 15 min. The concentrated reelin protein was resuspended in 1× phosphate-buffered saline (PBS) and stored at −80°C. Reelin was detected by staining with Coomassie Blue or silver staining. By comparison with a serial dilution of bovine serum albumin (BSA) protein, we estimated the reelin concentration to be approximately 10 μg/ml. The purified recombinant reelin (10 μl) was used throughout the study unless specified in the experiments. In parallel with the preparation of reelin, the conditioned medium from pcDNA3-transfected cells was purified and was used as a control.
Immunofluorescence staining
Immunofluorescence staining of reelin was performed as described previously with some modifications [19]. The washed platelets were cytospun on a glass coverslip, fixed with 3.7% formaldehyde solution at room temperature for 15 min and permeabilized with 0.1% Triton X-100 at 4°C for 10 min. After several washes with 1× PBS, fixed cells were blocked with 5% dry milk and incubated with mAb142 (1:50) at 4°C overnight. Platelets were washed three times with 1× PBS and incubated with Alexa Fluor 488-conjugated donkey anti-mouse secondary antibody at room temperature for 1 h. Finally, the cells were mounted and observed using confocal microscopy. As a negative control, platelets were incubated with the secondary antibody only, and no immunofluorescent signal was detected.
Sucrose-density gradient assay for subcellular fractionation of platelet
Platelets were isolated and fractionated as described previously [19]. The washed platelets (2 × 109) were homogenized in a Thermo French pressure cell (300 psi), and the platelet lysate was centrifuged (2,000g for 10 min at 4°C) to obtain pellets of unhomogenized platelet. The supernatant was laid on top of a linear sucrose-density gradient (30–60% sucrose) and centrifuged at 200,000g for 2 h. Eighteen fractions (700 μl each) were collected from the top of the gradient and subjected to Western blot analysis.
Solid-phase binding assay
The coverslips were pre-coated with purified reelin or the corresponding control for 2 h. After blocking by 1% denatured BSA for 30 min at room temperature, 500 μl of the washed platelets (3 × 108/ml) was added onto the coverslips for 30 min at 37°C. After washing three times with 1× PBS, the platelets were fixed with 3.7% formaldehyde for 10 min, and the coverslips were mounted. Platelet adhesion to the coverslip was observed with a 1,000× magnification in phase contrast microscopy (Zeiss Axiovert 200 M), and the number of platelet adhesions was counted manually for ten different fields. The GST or GST-RAP recombinant protein (40 μg/ml) was included in the binding assay when specified in the experiments.
Soluble-phase binding assay
The binding of reelin to platelet was performed as described previously with some modifications [20]. The platelets (500 μl) at a density of 3 × 108/ml were incubated with purified reelin or the corresponding control at 4°C for 30 min. After washing three times with 1× PBS, the platelets were solubilized in 1× SDS-sample buffer. The platelet lysates were clarified by centrifugation at 13,000g for 10 min at 4°C and were subjected to Western blot analysis with mAb142 to reveal the presence of bound reelin.
Platelet aggregation and platelet spreading assays
Platelet aggregation was performed as described [19]. Briefly, washed platelets were adjusted to a concentration of 3 × 108/ml with Tyrode’s buffer and maintained at 37°C for 1 min while stirring. Platelet aggregation was initiated by addition of agonists, and light transmission was monitored using a platelet aggregometer (Chronolog, Harvertown, PA) connected to the PowerLab data acquisition and recording system (ADInstrument, Castle Hill, NSW, Australia).
For platelet spreading assay, the washed platelets in suspension were pre-incubated with purified reelin or the corresponding control for 15 min. Then the platelets were added onto the coverslip pre-coated with fibrinogen (100 μg/ml) for 30 min at 37°C. After washing three times with 1× PBS, the platelets were fixed with 3.7% formaldehyde for 10 min. The coverslips were then mounted, and the surface area of 100 individual platelets was determined by Image J (National Health Institute). The mean surface area for the platelets in the control group was calculated and designated as MSA. The number of platelets with a surface area larger than MSA was determined and was used as an index for comparing the extent of platelet spreading.
F-actin staining and quantification
F-actin staining was performed by fixing the platelets (3 × 108/ml) with 3.7% formaldehyde at room temperature for 10 min, followed by permeabilization with 0.1% Triton X-100 for 10 min at 4°C. The platelets were then stained with FITC-conjugated phalloidin (50 μg/ml for microscopy analysis and 1 μg/ml for flow cytometry) for 45 min. After several washes with 1× PBS to remove unbound phalloidin, F-actins were observed by fluorescence microscopy or were quantified by flow cytometry.
PAC-1 binding assay
The PAC-1 binding assay was performed as described previously [17]. Briefly, 100 μl of platelets (3 × 108/ml) were incubated with the FITC-conjugated anti-PAC-1 antibody (20 μl) in the dark at room temperature for 30 min. Then the platelets were fixed in 1% paraformaldehyde and stored on ice. Flow cytometry analysis was then performed using the FACScan system with CellQuest software (BD Biosciences).
Rac1 GTPase activity assay
Rac1 activity was determined as described previously [21]. Briefly, 25 μg of GST-PAK1 was incubated with lysates from 5 × 108 platelets for 30 min in the presence of glutathione–Sepharose beads. After three washes with lysis buffer A (100 mM NaCl, 22.5 mM HEPES, pH 7.5, 1% NP-40, 10 mM MgCl2, 1 mM EDTA, 2% glycerol), the active form of Rac1 that bound to GST-PAK1 was eluted with 1× SDS-sample buffer and was detected by Western blot using the anti-Rac1 antibody.
Statistical analysis
Statistical comparisons were made with the Student’s t test, and the data were considered significantly different if P < 0.05.
Results
Reelin expression in human peripheral blood cells and hematopoietic cell lines
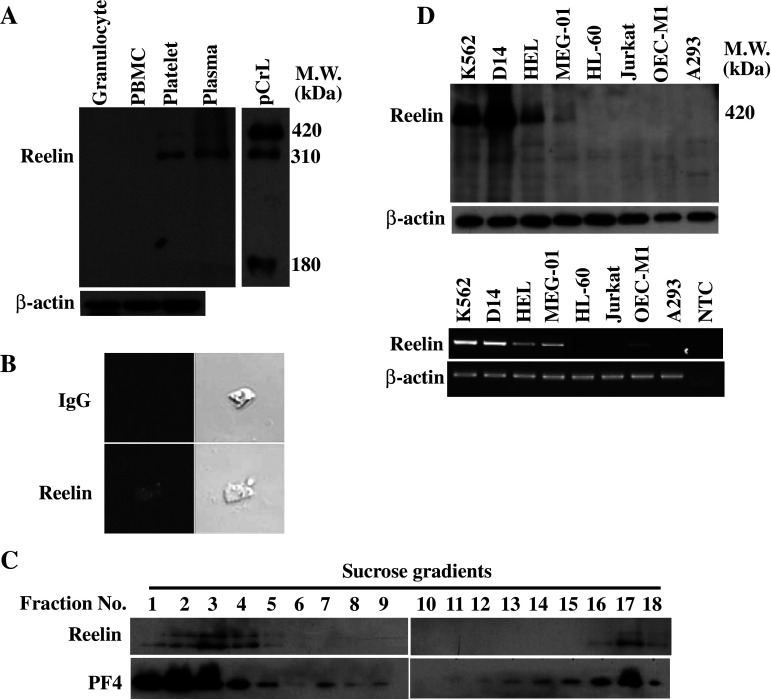
To address whether reelin is present in human peripheral blood cells, the cell lysates of granulocyte, PBMC and platelet from healthy volunteers were subjected to Western blot analysis with the anti-reelin monoclonal antibody mAb142. Reelin was not detectable in the granulocytes and PBMC (Fig. 1a). In contrast, two reelin immunoreactive bands were revealed in the platelet lysates. The molecular mass for the two platelet reelin proteins was determined by comparison with the circulating plasma reelin and the recombinant reelin purified from the conditioned medium of 293T cells transfected with pCrl reelin expression plasmid. For the recombinant reelin, three immunoreactive bands with the molecular mass of 420, 310 and 180 kDa were observed in the Western blot. The 310- and 180-kDa proteins were the proteolytic cleavage products of the 420-kDa full-length reelin, the major form of recombinant reelin [2, 10]. Both 420- and 310-kDa proteins were present in the plasma and platelet with the 310-kDa protein as the major form of reelin. In contrast with the recombinant reelin, the 180-kDa protein was barely detectable in the plasma and platelet lysates.
Fig. 1.
Reelin expression in the peripheral blood cells and various leukemic and cancer cells. a Expression of reelin in peripheral blood cells. The total lysates of granulocyte, PBMC, platelet and plasma were subjected to Western blot analysis with mAb142. The total lysate of 293T cells transfected with reelin expression plasmid pCrl was also included for comparison. b Immunofluorescence staining of reelin in platelets. Immunofluorescence staining with mAb142 or the IgG control was performed with platelets cytospun on a glass slide. The fluorescence signal was observed by confocal microscopy. c Subcellular localization of reelin in human platelets. Platelet homogenates (2 × 109) were separated by sucrose-density-gradient (30–60%) centrifugation. Eighteen fractions (700 μl each) were collected from the top, and aliquots were subjected to Western blot analysis using anti-reelin (mAb142) and anti-PF4 antibodies, respectively. d Expression of reelin in various leukemic and cancer cell lines. Total cell lysates and total RNA from the indicated cell lines were subjected to Western blot analysis using mAb142 (upper panel) and RT-PCR using reelin-specific primers (lower panel), respectively. The expression of β-actin was used as a control for equal protein loading and quality control of total RNA isolation. NTC, no template control
Human platelets were further subjected to immunofluorescence staining with mAb142 to delineate the presence of reelin in platelets. A cluster of positive reelin staining signal was displayed within the platelet in a confocal microscopy analysis (Fig. 1b). No immunofluorescent signal was observed when control IgG was used for staining. To examine the subcellular localization of platelet reelin more closely, we analyzed platelet homogenates by using linear sucrose-density gradient centrifugation to separate different platelet organelles from the bulk of cytosolic and plasma membrane proteins. Western blot analysis of the gradient fractions revealed two distinct pools of reelin (Fig. 1c). Reelin was partly present in the low-density fractions (fractions one to four), which contained most of the cytoplasmic and plasma membrane proteins, and in fractions of higher densities, in which intracellular granules are expected. This distribution was paralleled by PF-4, the marker protein of α-granules. The presence of the α-granule marker in the low-density fractions can be explained by a partial rupture of the granules during homogenization [22]. Reelin is thus considered as a protein present in the platelet with the cellular distribution associated with the α-granule.
To extend our observations in peripheral blood cells, the levels of reelin expression in several human hematopoietic leukemic and cancer cell lines were determined. Western blot analysis revealed that reelin protein was present in the cell lines with megakaryocytic differentiation potential, including K562, D14 (a Disabled-2 protein-deficient K562 subline), HEL and MEG-01 (Fig. 1d, upper panel). One major band with the molecular mass of 420 kDa was observed in these four cell lines. The reelin immunoreactivity of MEG-01 was much weaker than the others. Reelin protein was not detectable in the HL-60 promyelocytic cells, Jurkat T-lymphocytic cells, OECM-1 oral cancer carcinoma and A293 human embryonic kidney cells. Consistent with these results, RT-PCR analysis revealed the presence of a 583-bp reelin PCR product in K562, D14, HEL, MEG-01 and OECM-1, with the band intensity significantly weaker in OECM-1 (Fig. 1d, lower panel). No reelin mRNA was detectable in the other cell lines. Reelin expression is thereby closely linked to the cells in the megakaryocytic differentiation lineage.
Human platelets interact with immobilized and soluble reelin
Reelin has been shown to play a pivotal role in the central nervous system. However, the defined reelin function in the circulation remains largely unknown. The presence of reelin receptor ApoER2 splicing variants on platelets [23, 24] prompted us to explore whether or not reelin interacts with platelets and modulates platelet functions. To address this, the solid-phase reelin binding assay was performed by applying the washed platelets to a coverslip pre-coated with purified recombinant reelin or the corresponding control purified from the conditioned medium of pcDNA3-transfected cells. As shown in Fig. 2a, platelets tended to adhere on immobilized reelin with a 2.4-fold increase in the number of platelets binding when compared to the control (P < 0.01). In addition, more than 71.3% of the platelets binding to reelin developed filopodia-like protrusions that were significantly higher when compared with the 34.7% of the platelets binding on the control coverslip (P < 0.05).
Fig. 2.
Human platelets interact with soluble and immobilized reelin. a The interaction between platelet and immobilized reelin. The washed platelets (500 μl) at a density of 3 × 108/ml were added to the coverslips pre-coated with purified reelin or the corresponding control for 30 min at 37°C. After washing and fixation, the binding of platelets on immobilized reelin was observed by phase contrast microscopy (upper panel). Note the filopodia-like protrusion for the platelets in the enlarged image. The relative number of platelet adhesions and the percentage of adhered platelets with filopodia-like protrusions were determined (lower panel). The data represent the mean ± SD of three independent experiments. **P < 0.01 and *P < 0.05 when compared with the control-treated coverslip. Length of black bar = 10 μm. C control, R reelin. b GST-RAP attenuates platelet binding on immobilized reelin. Solid phase binding assays were performed as described in the presence of GST or GST-RAP (40 μg/ml). The platelets were observed by phase contrast microscopy, and the relative number of platelets adhered on the control or reelin-coated coverslips was determined. The data represent the mean ± SD of three independent experiments. **P < 0.01 when compared with the platelet binding on immobilized reelin in the presence of GST protein. Length of black bar = 10 μm. c The 420-kDa reelin is involved in reelin-to-platelet interaction. The washed platelets (3 × 108/ml) were incubated with the purified reelin or the corresponding control for 2 h at 4°C. After several washes with 1× PBS, the platelet lysates were subjected to Western blot analysis with mAb142. The protein bands corresponding to the endogenous (endo) and exogenous (exo) reelin were shown. The expression of β-actin was used as a control for equal protein loading
Ligand interactions with the receptors belonging to the members of the LDL receptor gene family have been shown to be antagonized by the presence of a 39-kDa RAP [25]. To determine whether reelin-to-platelet interactions involve the members of LDL receptor gene family such as the platelet ApoER2 splicing variants, the binding assay was performed in the presence of GST-RAP or the control GST protein. An excessive amount of GST protein appeared to reduce the basal levels of platelet binding on the coverslips. Nevertheless, the number of platelets binding on immobilized reelin was still 2.6-fold higher than those binding on the control coverslip (Fig. 2b). Notably, RAP significantly reduced platelet adhesion on immobilized reelin when compared with the control GST protein (P < 0.01). These results indicate that the LDL receptor gene family members, most likely the ApoER2 variants expressing on the platelet surface, act as the receptors for reelin and mediate reelin-to-platelet interactions.
Soluble phase reelin-binding assay was then used to determine which form of reelin is involved in the reelin-to-platelet interactions. In this assay, the washed platelets were mixed with soluble recombinant reelin or the corresponding control. After extensive washes to reduce non-specific binding, the platelet lysates were subjected to Western blot analysis with mAb142. Consistent with the data presented in Fig. 1, a major immunoreactive band (310 kDa) corresponding to the endogenous reelin was detectable in the control and reelin-treated platelets. Incubation of platelets with recombinant reelin did not cause an increase in the 310-kDa band intensity (Fig. 2c). Furthermore, the 420-kDa but not the 180-kDa reelin was present in the lysate of platelets that were incubated with purified reelin. These results imply that the 420 kDa is the major form of reelin involved in the reelin-to-platelet interactions.
Effects of reelin on platelet aggregation and platelet spreading on fibrinogen
To elucidate the functional implication for the interactions between reelin and platelet, the washed platelets or PRP were subjected to platelet aggregation analysis in the presence of purified recombinant reelin or the corresponding control. As shown in Fig. 3a, reelin alone did not induce platelet aggregation. Accordingly, reelin was not sufficient to activate platelet αIIbβ3 as revealed by the binding assays of anti-PAC-1 antibody, which recognizes the active form of αIIbβ3 (Fig. 3b). This was in contrast to the significant PAC-1 binding stimulated by thrombin. Consistent with these findings, reelin had no effect on thrombin-, U46619-, AA- and collagen-stimulating platelet aggregation, even at lower concentrations of agonists (Fig. 3c). These data thereby indicate that reelin did not moderate platelet integrin αIIbβ3 activation and platelet aggregation.
Fig. 3.
Effects of reelin on platelet aggregation and integrin αIIbβ3 activation. a Reelin does not induce platelet aggregation. The washed platelets (left panel) and PRP (right panel) were subjected to platelet aggregation analysis in the presence of purified recombinant reelin or the corresponding control. The platelet aggregation curves were obtained by using a platelet aggregometer connected to the PowerLab data acquisition and recording system. b Reelin does not induce integrin αIIbβ3 activation. The platelets with the indicated treatments were incubated with the anti-PAC-1 (20 μl) antibody and subjected to flow cytometry analysis. A total of 10,000 events were determined. Similar results were obtained in three independent experiments. c Reelin does not modulate agonist-induced platelet aggregation. The washed platelets were incubated with purified recombinant reelin or the corresponding control for 1 min, and platelet aggregation was induced by the indicated concentrations of agonists. The platelet aggregation curves were recorded as described in a
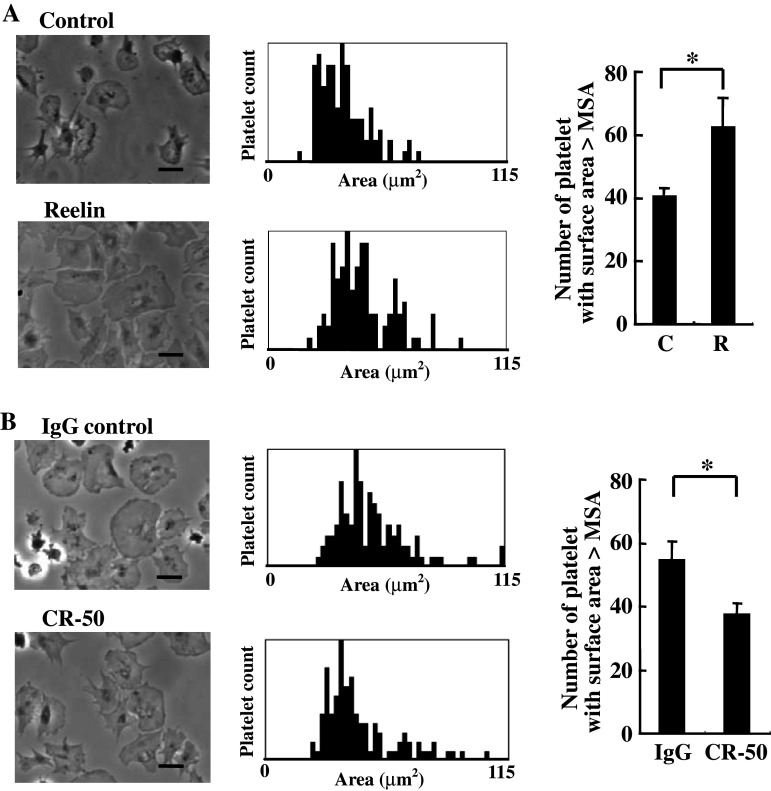
To determine whether reelin-to-platelet interactions modulate platelet spreading on fibrinogen, the washed platelets were pre-incubated with purified recombinant reelin or the corresponding control, and then the washed platelets were applied to a coverslip pre-coated with fibrinogen. In accord with previous study [26], the quiescent, non-stimulating platelets were able to spread on fibrinogen with an increase in platelet surface area (Fig. 4a). The platelets pre-incubated with purified reelin tended to fully spread on fibrinogen and had larger surface areas than the corresponding control (P < 0.05). Similar results were obtained when the experiments were performed using the recombinant reelin that was prepared through gel-filtration chromatography purification (data not shown). To confirm that the enhancement of platelet spreading on fibrinogen is reelin-specific, the platelets were incubated with reelin in the presence of the anti-reelin neutralizing monoclonal antibody CR-50. The CR-50 monoclonal antibody recognizes an epitope located within reelin amino acids 250–407 and has been shown to block reelin function in vitro and in vivo [27–30]. As shown in Fig. 4b, CR-50 impeded reelin function and attenuated reelin-mediated enhancement of platelet spreading on fibrinogen (P < 0.05), suggesting the specific and functional implication of reelin on promoting platelet spreading on fibrinogen.
Fig. 4.
Reelin enhances platelet spreading on fibrinogen. (a and b) The washed platelets (3 × 108/ml) were incubated with the purified reeelin or the corresponding control for 15 min at 37°C (a). Alternatively, the washed platelets were incubated with the purified reelin in the presence of reelin function blocking antibody CR-50 (20 μg/ml) or IgG control antibody for 15 min at 37°C (b). The assays of platelet spreading on fibrinogen were then performed and quantified as described in the “Materials and methods.” Platelet spreading was observed by phase contrast microscopy (left panel). The surface area for a total of 100 platelets in a representative experiment was plotted (middle panel), and the number of platelets with a surface area larger than MSA for the platelets in the control (a) or the IgG control (b) were determined (right panel). The data represent the mean ± SD of three independent experiments. *P < 0.05 when compared with the control (a) or the IgG-control (b). Length of black bar = 10 μm
Reelin mediates its effect through modulation of Rac1 activity and PKCδ phosphorylation
Cytoskeleton reorganization is associated with platelet spreading on fibrinogen [31]. The platelets treated with purified reelin were subjected to F-actin staining with FITC-conjugated phallodin to determine whether reelin modulates actin organization. Quantitative flow cytometry analysis revealed that reelin did not alter the amount of F-actin for both the resting and thrombin-stimulating platelets (Fig. 5a). This was in contrast to the elevation of F-actin levels following thrombin stimulation of resting platelets. However, platelets pre-treated with reelin prompted to elicit distinct F-actin pattern during spreading on fibrinogen (Fig. 5b). The F-actin fibers distributed randomly in the control platelets, whereas reelin caused F-actin fiber bundling and formation of lamellipodia. These data suggest that reelin regulates platelet actin reorganization upon spreading on fibrinogen.
Fig. 5.
Reelin moderates F-actin distribution and up-regulates platelet Rac1 activity. a Reelin does not affect the levels of F-actin for resting and thrombin-stimulating platelets. The washed platelets were pre-treated with purified recombinant reelin or the corresponding control for 30 min. The platelets were either untreated (resting) or were stimulated with thrombin (1 U/ml) for 1 min. F-actin staining with FITC-conjugated phalloidin was then performed and was quantified by flow cytometry. A total of 10,000 events were determined. Similar results were obtained in three independent experiments. b Reelin modifies F-actin distribution. The washed platelets were treated with purified recombinant reelin or the corresponding control for 15 min. The platelets were then spread on a fibrinogen-coated coverslip for 30 min. The distribution of F-actin was observed by staining platelets with FITC-conjugated phalloidin and observed by phase contrast microscopy. c Reelin up-regulates platelet Rac1 activity. The washed platelets were treated with purified recombinant reelin or the corresponding control for the indicated time. Analysis of Rac1 activity was then performed, and the amount of active Rac1 (GTP-Rac1) was analyzed by Western blot with the anti-Rac1 antibody. At the same time, Rac1 expression in the total platelet lysates was analyzed for loading control. Three independent experiments were performed with essentially similar results. d NSC23766 reverses reelin-mediated platelet spreading on fibrinogen. The washed platelets were incubated with purified recombinant reelin in the presence or absence of the Rac1 inhibitor NSC23766 (50 μM). Platelet spreading on fibrinogen was then performed as described in the “Materials and methods.” The patterns of platelet spreading were observed by phase contrast microscopy (left panel), and the surface area for a total of 100 platelets in a representative experiment was plotted (middle panel). The number of platelets with a surface area larger than MSA of the reelin-treated group was determined (right panel). The data represent the mean ± SD of three independent experiments. **P < 0.01 when compared with the reelin-treated group. C control, R reelin, N NSC23766. Length of black bar = 10 μm
Rac1 is involved in the regulation of actin reorganization and formation of lamellipodia [26]. We determined whether reelin modulates platelet Rac1 activity by pulling down the active Rac1 from the platelet lysates using the GST-PAK1 protein as bait (Fig. 5c). In agreement with the results of F-actin staining and the formation of lamellipodia, the platelet Rac1 activity was significantly increased at 15 min after reelin treatment. Consistent with these results, the Rac1 inhibitor NSC23766 reversed reelin-mediated platelet spreading on fibrinogen (P < 0.01, Fig. 5d). Hence, these data imply that reelin signaling activates Rac1, leading to full spreading of platelets on fibrinogen.
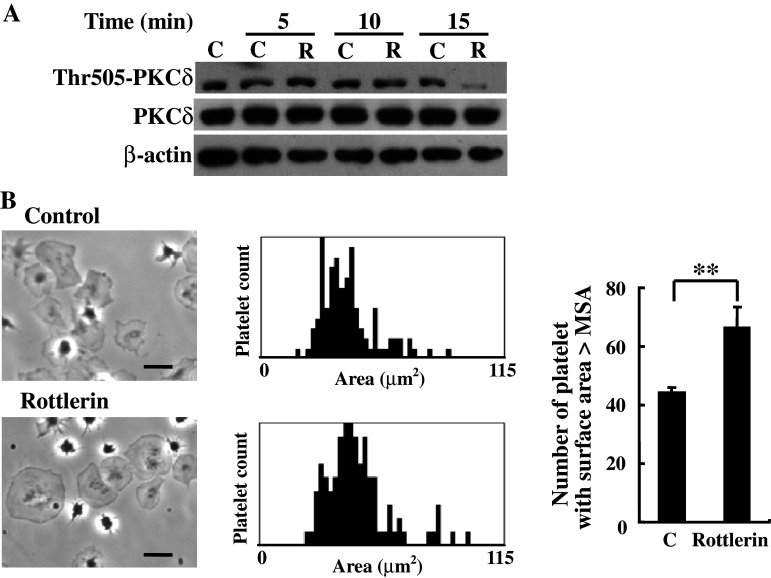
In addition to Rac1, PKCδ phosphorylation at Thr505 has been implicated in the regulation of platelet spreading and adhesion [32]. To further investigate the underlying mechanisms of reelin-induced platelet spreading on fibrinogen, platelets were treated with reelin for the indicated time, and the platelet lysates were isolated for Western blot analysis with the anti-PKCδ Thr505 phospho-specific antibody. As shown in Fig. 6a, reelin decreased PKCδ phosphorylation at Thr505 in a time-dependent manner. Accordingly, platelets pretreated with the PKCδ inhibitor rottlerin enhanced platelet spreading on fibrinogen (P < 0.01, Fig. 6b). These data thereby indicate that reelin-mediated inhibition of PKCδ Thr505 phosphorylation may play a role in the regulation of platelet spreading on fibrinogen.
Fig. 6.
Involvement of PKCδ in reelin signaling and platelet spreading on fibrinogen. a Reelin inhibits PKCδ phosphorylation at Thr505. The washed platelets were treated with recombinant reelin or the corresponding control for the indicated time. The platelet lysates were subjected to Western blot analysis with the anti-phospho-Thr505-PKCδ, anti-PKCδ and anti-β-actin antibodies, respectively. b Inhibition of PKCδ enhances platelet spreading on fibrinogen. Platelet spreading on fibrinogen was performed in the presence or absence of PKCδ inhibitor rottlerin (5 μM). The patterns of platelet spreading were observed by phase contrast microscopy (left panel) and the surface area for a total of 100 platelets in a representative experiment was plotted (middle panel). The number of platelets with a surface area larger than MSA of the control was determined (right panel). The data represent the mean ± SD of three independent experiments. **P < 0.01 when compared with the control-treated group. Length of black bar = 10 μm
Discussion
Several novel aspects of reelin expression and function in platelets are reported in this study. We demonstrate for the first time that reelin is present in human platelets and megakaryocytic cells. Remarkably, extracellular reelin interacts with platelets through the members of the LDL receptor gene family followed by up-regulation of Rac1 activity and inhibition of PKCδ Thr505 phosphorylation. Consequently, reelin modulates F-actin redistribution and augments lamellipodia formation, leading to enhanced spreading of platelets on fibrinogen. This study thereby provides a clue for the functional link between circulating reelin and platelet functions.
Smalheiser et al. [10] reported that reelin was present in the serum and platelet-poor plasma, but not in the platelets of rat, mice and human species. Contrary to their work, we found that at least two reelin immunoreactive proteins with the molecular mass of 420 and 310 kDa are present in the human platelets. The reelin signal we observed in the Western blot of platelet lysates is unlikely the contamination of plasma reelin. This notion is supported by the several pieces of experimental evidence we present in this study. At first, immunofluorescence staining with the anti-reelin antibody unveils positive reelin immunoreactive signal within the platelet. Sucrose density gradient fractionation of platelet organelles further indicates that reelin is co-distributed with the α-granule protein PF-4 in the high-density fractions. Consistent with these observations, we and others found that the reelin transcript is present in the cell lines with megakaryocytic origin as well as in stage 6 of megakaryocytic differentiation during expression analysis of primary mouse megakaryocytic differentiation [33]. This study thereby provides the first piece of evidence showing that reelin is present in human platelets. Because the procedure of platelet preparation and the platelet data were not disclosed in the work of Smalheiser et al., the reasons for the discrepancy between these two studies are not clear. The method of platelet isolation and sample preparation may be critical to preserve reelin protein stability and detection, which may explain the differences in findings among laboratories regarding which peripheral tissues or cell types are positive for reelin [1, 18, 27].
In addition to demonstrating the presence of reelin in human platelets, we report in this study that platelets can interact with reelin through the solid-phase and soluble-phase reelin-binding assays. The attenuation of reelin-to-platelet interactions by the presence of functional-blocking protein RAP suggests that the LDL receptor gene family members may act as the receptors for reelin and mediate the reelin-to-platelet interactions. In this regards, three ApoER2 splicing variants have been shown to express on the platelet surface, all of which are involved in signaling [24]. In particular, the ApoER2 splicing variant ApoER2′ lacking the LDL-binding domains 4, 5 and 6 has been shown to bind reelin when the receptor was expressed on the 293T cell surface [23]. We thereby postulate that ApoER2′ is the most likely candidate receptor to mediate reelin-to-platelet interactions and signal transmission.
On the other hand, the binding assay with soluble reelin reveals that the 420-kDa reelin is able to bind to platelets. There is no evidence that the 310- and 180-kDa reelin are involved in the reelin-to-platelet interactions. However, we have noted that the purified recombinant reelin contains all three major forms of reelin (i.e., 420, 310, and 180 kDa) with various levels of expression. Because the protease that is responsible for generating the cleavage products of reelin has not been completely unveiled, it is not yet possible to obtain a “pure” form of recombinant reelin for comparatively analyzing platelet binding affinity between different forms of reelin. Further investigation regarding the nature of reelin-targeting protease is required to generate form-specific reelin to address this issue.
The functional implications of the interactions between reelin and platelets are also addressed in this study. We found that reelin does not modulate αIIbβ3 signaling and platelet activation. Accordingly, reelin has no effect on agonist-induced platelet aggregation. In contrast, reelin modifies platelet F-actin distribution, enhances lamellipodia formation and extends the degree of platelet spreading on fibrinogen. αIIbβ3-mediated platelet spreading to fibrinogen has been implicated in a number of different physiological and pathological processes. Upon vascular injury, fibrinogen binding to the damaged surface may act as one of the proteins to which platelets adhere [34]. Fibrinogen is also present in atherosclerotic plaque with platelet adhesion to fibrinogen contributing to thrombus formation and the atherosclerotic process [35]. Because reelin is abundantly present in the circulation, reelin-mediated full spreading of platelet on fibrinogen can be of physiological and pathological importance.
At the molecular level, we demonstrate that reelin stimulates platelet Rac1 activity and inhibits PKCδ Thr505 phosphorylation. These findings are consistent with previous studies demonstrating the role of Rac1 activation in platelet lamellipodia formation [26] and PKCδ in negative regulation of filopodia through inhibition of VASP-mediated filopodia formation [36]. Based on our current findings, we propose that reelin-to-platelet interactions transmit intracellular singaling leading to the up-regulation of Rac1 activity and inhibition of PKCδ Thr 505 phosphorylation. Consequently, reelin stimulates actin reorganization, augments lamellipodia formation and enhances full spreading of platelets on fibrinogen. Although the underlying mechanisms for reelin-mediated platelet signaling has not yet been completely elucidated, we note from previous studies that the intracellular adaptor proteins such as DAB1 are important mediators for reelin signaling in the central nervous system [37, 38]. We and others have demonstrated that DAB1 [39] and DAB2 [19] proteins are present in platelets. Whether these DAB proteins transmit reelin signaling and contribute to reelin-mediated platelet spreading on fibrinogen is under investigation in our laboratory.
In summary, we provide evidence that reelin is present in platelets. Also, extracellular reelin binds to platelets and positively regulates platelet spreading on fibrinogen. These findings thereby contribute to our understanding of the role of circulating reelin in platelet functions at sites of vascular injury and thrombus formation.
Acknowledgments
This work was supported in part by the grants NHRI-EX98-9612BI, NSC 95-2320-B-182-023-MY3, CMRPD170132, EMRPD180171 and EMRPD180221 to C.P.T; CMU95-335 to J.C.C.
Abbreviations
- AA
Arachidonic acid
- ApoER2
ApoE receptor 2
- BSA
Bovine serum albumin
- DAB
Disabled
- FITC
Fluorescein isothiocyanate
- GST
Glutathione-S-transferase
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate-buffered saline
- PF-4
Platelet factor 4
- PKCδ
Protein kinase C δ
- PGI2
Prostaglandin I2
- PRP
Platelet-rich-plasma
- RAP
Receptor-associated protein
- SFKs
SRC family tyrosine kinases
Contributor Information
Ju-Chien Cheng, Phone: +886-4-22053366, FAX: +886-4-22022073, Email: jccheng@mail.cmu.edu.tw.
Ching-Ping Tseng, Phone: +886-3-2118800, FAX: +886-3-2118355, Email: ctseng@mail.cgu.edu.tw.
References
- 1.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 2.Quattrocchi CC, Wannenes F, Persico AM, Ciafre SA, D’Arcangelo G, Farace MG, Keller F. Reelin is a serine protease of the extracellular matrix. J Biol Chem. 2002;277:303–309. doi: 10.1074/jbc.M106996200. [DOI] [PubMed] [Google Scholar]
- 3.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- 4.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/S0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 5.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/S0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 6.Botella-Lopez A, Burgaya F, Gavin R, Garcia-Ayllon MS, Gomez-Tortosa E, Pena-Casanova J, Urena JM, Del Rio JA, Blesa R, Soriano E, Saez-Valero J. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutsch SI, Rosse RB, Lakshman RM. Dysregulation of tau phosphorylation is a hypothesized point of convergence in the pathogenesis of alzheimer’s disease, frontotemporal dementia and schizophrenia with therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1369–1380. doi: 10.1016/j.pnpbp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Fatemi SH, Kroll JL, Stary JM. Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. Neuroreport. 2001;12:3209–3215. doi: 10.1097/00001756-200110290-00014. [DOI] [PubMed] [Google Scholar]
- 9.Botella-Lopez A, de Madaria E, Jover R, Bataller R, Sancho-Bru P, Candela A, Compañ A, Pérez-Mateo M, Martinez S, Sáez-Valero J. Reelin is overexpressed in the liver and plasma of bile duct ligated rats and its levels and glycosylation are altered in plasma of humans with cirrhosis. Int J Biochem Cell Biol. 2008;40:766–775. doi: 10.1016/j.biocel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Smalheiser NR, Costa E, Guidotti A, Impagnatiello F, Auta J, Lacor P, Kriho V, Pappas GD. Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc Natl Acad Sci USA. 2000;97:1281–1286. doi: 10.1073/pnas.97.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich-Muszalska A, Olas B. The changes of aggregability of blood platelets in schizophrenia. World J Biol Psychiatry. 2007;14:1–6. doi: 10.1080/15622970701557993. [DOI] [PubMed] [Google Scholar]
- 12.Yao JK, van Kammen DP, Gurklis J, Peters JL. Platelet aggregation and dense granule secretion in schizophrenia. Psychiatry Res. 1994;54:13–24. doi: 10.1016/0165-1781(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Kusumi I, Akimoto T, Sasaki Y, Koyama T. Altered 5-HT-induced calcium response in the presence of staurosporine in blood platelets from bipolar disorder patients. Neuropsychopharmacology. 2003;28:1210–1214. doi: 10.1038/sj.npp.1300159. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Kusumi I, Sasaki Y, Koyama T. Serotonin-induced platelet intracellular calcium mobilization in various psychiatric disorders: is it specific to bipolar disorder? J Affect Disord. 2001;64:291–296. doi: 10.1016/S0165-0327(00)00221-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang YJ, Chen IS, Tseng CP, Day YJ, Lin YC, Liao CH. (2R, 3R)-2-(3′, 4′-dihydroxybenzyl)-3-(3″, 4″-dimethoxybenzyl)butyrolactone suppresses fMLP-induced superoxide production by inhibiting fMLP-receptor binding in human neutrophils. Biochem Pharmacol. 2008;75:688–697. doi: 10.1016/j.bcp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Medina C, Jurasz P, Santos-Martinez MJ, Jeong SS, Mitsky T, Chen R, Radomski MW. Platelet aggregation-induced by caco-2 cells: regulation by matrix metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp Ther. 2006;317:739–745. doi: 10.1124/jpet.105.098384. [DOI] [PubMed] [Google Scholar]
- 17.Huang CL, Cheng JC, Liao CH, Stern A, Hsieh JT, Wang CH, Hsu HL, Tseng CP. Disabled-2 is a negative regulator of integrin αIIbβ3-mediated fibrinogen adhesion and cell signaling. J Biol Chem. 2004;279:42279–42289. doi: 10.1074/jbc.M402540200. [DOI] [PubMed] [Google Scholar]
- 18.Lugli G, Krueger JM, Davis JM, Persico AM, Keller F, Smalheiser NR. Methodological factors influencing measurement and processing of plasma reelin in human. BMC Biochem. 2003;4:9. doi: 10.1186/1471-2091-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang CL, Cheng JC, Stern A, Hsieh JT, Liao CH, Tseng CP. Disabled-2 is a novel αIIb-integrin-binding protein that negatively regulates platelet-fibrinogen interactions and platelet aggregation. J Cell Sci. 2006;119:4420–4429. doi: 10.1242/jcs.03195. [DOI] [PubMed] [Google Scholar]
- 20.D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/S0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 21.Huang CH, Cheng JC, Chen JC, Tseng CP. Evaluation of the role of Disabled-2 in nerve growth factor-mediated neurite outgrowth and cellular signalling. Cell Signal. 2007;19:1339–1347. doi: 10.1016/j.cellsig.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Mairhofer M, Steiner M, Mosgoeller W, Prohaska R, Salzer U. Stomatin is a major lipid-raft component of platelet α-granules. Blood. 2002;100:897–904. doi: 10.1182/blood.V100.3.897. [DOI] [PubMed] [Google Scholar]
- 23.Brandes C, Kahr L, Stockinger W, Hiesberger T, Schneider WJ, Nimpf J. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J Biol Chem. 2001;276:22160–22169. doi: 10.1074/jbc.M102662200. [DOI] [PubMed] [Google Scholar]
- 24.Pennings MT, Derksen RH, Urbanus RT, Tekelenburg WL, Hemrika W, de Groot PG. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 25.Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr Opin Lipidol. 1998;9:149–155. doi: 10.1097/00041433-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 26.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 28.Miyata T, Nakajima K, Mikoshiba K, Ogawa M. Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J Neurosci. 1997;17:3599–3609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Froyscher M, Soriano F. A role of Cajai-Retzius cells and reelin in the development of hippocampal connections. Nautre. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- 31.Hartwig JH, Barkalow K, Azim A, Italiano J. The elegant platelet: signals controlling actin assembly. Thromb Haemost. 1999;82:392–398. [PubMed] [Google Scholar]
- 32.Harper MT, Poole AW. Isoform-specific functions of protein kinase C: the platelet paradigm. Biochem Soc Trans. 2007;35:1005–1008. doi: 10.1042/BST0351005. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Hu M, Shivdasani A. Expression analysis of primary mouse megakaryocyte differentiation and its application in identifying stage-specific molecular markers and a novel transcriptional target of NF-E2. Blood. 2007;109:1451–1459. doi: 10.1182/blood-2006-08-038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jirousková M, Jaiswal JK, Coller BS. Ligand density dramatically affects integrin αIIbβ3-mediated platelet signaling and spreading. Blood. 2007;109:5260–5269. doi: 10.1182/blood-2006-10-054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 36.Pula G, Schuh K, Nakayama K, Nakayama KI, Walter U, Poole AW. PKCdelta regulates collagen-induced platelet aggregation through inhibition of VASP-mediated filopodia formation. Blood. 2006;108:4035–4044. doi: 10.1182/blood-2006-05-023739. [DOI] [PubMed] [Google Scholar]
- 37.Morimura T, Hattori M, Ogawa M, Mikoshiba K. Disabled 1 regulates the intracellular trafficking of reelin receptors. J Biol Chem. 2005;280:16901–16908. doi: 10.1074/jbc.M409048200. [DOI] [PubMed] [Google Scholar]
- 38.Cuitino L, Matute R, Retamal C, Bu G, Inestrosa NC, Marzolo MP. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its Rafts’ association. Traffic. 2005;6:820–838. doi: 10.1111/j.1600-0854.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 39.Urbanus RT, Pennings MTT, Derksen RHWM, De Groot PG. Platelet activation by dimeric β2-glycoprotein I requires signaling via both glycoprotein Iba and apolipoprotein E receptor 2’. J Thromb Haemost. 2008;6:1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]