Abstract
Expression of the alcohol dehydrogenase gene (ADH) of Arabidopsis is induced during hypoxia. Because many plants increase their ethylene production in response to hypoxic stress, we examined in this report whether ethylene is involved in the hypoxic induction of ADH in Arabidopsis. We found that the hypoxic induction of ADH can be partially inhibited by aminooxy acetic acid, an inhibitor of ethylene biosynthesis. This partial inhibition can be reversed by the addition of 1-aminocyclopropane-1-carboxylic acid, a direct precursor of ethylene. In addition, the hypoxic induction of the ADH gene is also reduced in etr1-1 and ein2-1, two ethylene insensitive mutants in ethylene-signaling pathways, whereas the addition of exogenous ethylene or an increase in cellular ethylene alone does not induce ADH under normoxic conditions. Kinetic analyses of ADH mRNA accumulation indicated that an ethylene signal is required for the induction of ADH during later stages of hypoxia. Therefore, we conclude that ethylene is needed, but not sufficient for, the induction of ADH in Arabidopsis during hypoxia.
To survive prolonged periods of oxygen deficiency, all aerobic organisms have had to evolve mechanisms for sensing oxygen availability and to adjust their cellular metabolism accordingly. Upon transfer from aerobic to hypoxic/anoxic conditions, animal and plant cells switch from aerobic respiration to lactic fermentation (Roberts et al., 1984a, 1984b). Continued lactic fermentation throughout hypoxia leads to the acidification of cytoplasm and rapid cell death in animal tissues. In contrast, after a transient period of lactic fermentation, maize root tip cells will further switch to alcoholic fermentation and allow glycolysis to continue for a longer period (Roberts et al., 1984a, 1984b). Comparative studies of cytoplasmic acidosis indicate that cytoplasmic pH regulation is an important factor in survival under hypoxia (Roberts et al., 1984a, 1984b; Xia and Saglio, 1992).
Anaerobic treatment of maize seedlings causes repression of pre-existing protein synthesis and induces the synthesis of about 20 anaerobic proteins (ANP) after approximately 90 min (Sachs et al., 1980). Most of the ANPs are enzymes involved in glycolysis and fermentation (for review, see Sachs et al., 1996). It was shown recently that most hypoxia-induced proteins in maize root tip cells are also enzymes involved in glycolysis and primary carbohydrate metabolism (Chang et al., 2000). Transcriptional, post-transcriptional, and translational controls have been shown to regulate synthesis of ANPs under low-oxygen stress (Fennoy and Bailey-Serres, 1995; Bailey-Serres and Dawe, 1996; Drew, 1997). Several cis-acting elements and trans-regulatory factors involved in anoxic and hypoxic inductions of the alcohol dehydrogenase (ADH) genes in maize and Arabidopsis have been identified (Ferl and Laughner, 1989; Yang et al., 1993; Dolferus et al., 1994; Kyozuka et al., 1994; Hoeren et al., 1998).
Lysogenic aerenchyma formation, which is characterized by continuous gas spaces in roots and shoots, occurs in the root cortex of several plant species during hypoxia (Campbell and Drew, 1983; Justin and Armstrong, 1987; Drew et al., 2000) and may correlate with tolerance to flooding (Justin and Armstrong, 1987). Lysogenic aerenchyma formation results from the lysis of cells in the cortical tissues of hypoxic-treated plants (He et al., 1994) and is associated with an increased cellulase activity, as well as the induction of a gene encoding a homolog of xyloglucan endo-transglycosylase, a putative cell wall loosening enzyme (He et al., 1994; Saab and Sachs, 1996).
An ethylene signal is required for aerenchyma formation in hypoxic maize roots (for review see, Drew et al., 2000; He et al., 1994, 1996). In contrast, no aerenchyma formation could be observed in maize roots under anoxic conditions in which ethylene biosynthesis is inhibited because the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene by ACC oxidase requires the presence of oxygen (Yang and Hoffman, 1984; Kende, 1993). A series of studies using various signal transduction antagonists showed that an increase in intracellular Ca2+ is involved in the transduction of an ethylene signal, leading to the formation of aerenchyma in roots of maize under hypoxia (He et al., 1996). Ca2+ may also be involved in the signaling pathway leading to the activation of ADH and glycolytic genes. There is a transient increase in cytosolic Ca2+ concentration in the early stage of the flooding of maize roots (Subbaiah et al., 1994a, 1994b). Inhibition of this transient cytosolic Ca2+ increase blocked the induction of the ADH1 gene. A similar anoxic/hypoxic-inducible Ca2+ increase was observed in Arabidopsis (Sedbrook et al., 1996) and Ca2+ signaling is required for the activation of the Arabidopsis ADH gene (Chung and Ferl, 1999).
Although ethylene was shown to be involved in aerenchyma formation, its functional role in the hypoxic induction of ADH remains to be determined. In this report we examined the effect of aminooxy acetic acid (AOA), an inhibitor of ethylene biosynthesis, on the hypoxic induction of ADH in Arabidopsis. In addition, we also examined the hypoxic induction of ADH in mutants that are defective in ethylene responses. Our results suggested that an ethylene signal is required, but not solely responsible, for the induction of ADH during hypoxia.
RESULTS
Effects of AOA on the Hypoxic Induction of ADH::β-glucuronidase (GUS) Transgene
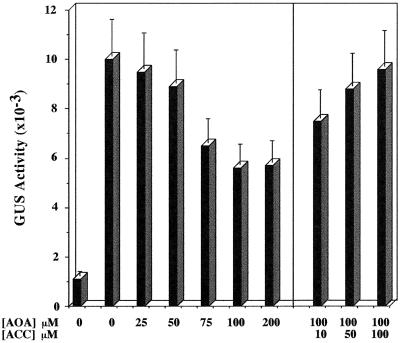
Ethylene is synthesized from S-adenosyl-Met (SAM) via ACC (Adams and Yang, 1979). AOA is a competitive inhibitor of ACC synthase, which catalyzes the conversion of SAM to ACC (Yang and Hoffman, 1984; Abeles et al., 1992). We used the AG2 Arabidopsis transgenic line, which contains an ADH promoter and GUS-coding region fusion (Conley et al., 1999), to examine the effect of AOA on the hypoxic induction of ADH. Arabidopsis plants were subjected to hypoxic treatment for 24 h in the presence of different concentrations of AOA. The data show that there was a dosage-dependent inhibition of hypoxic induction of the ADH::GUS transgene by AOA (Fig. 1). Hypoxic treatment resulted in an 8- to 10-fold increase in GUS activity as compared with the normoxic controls (Fig. 1, columns 1 and 2). The addition of 100 μm AOA resulted in nearly a 50% reduction in the accumulation of GUS activity during hypoxia (Fig. 1). However, further increases in concentrations of AOA resulted in no further reduction in levels of GUS activity. The partial inhibitory effect of AOA could be reversed by the addition of 10 μm ACC (Fig. 1, column 8) and completely reversed by 50 to 100 μm ACC (Fig. 1). These results suggest that an ethylene signal may be involved in the hypoxic induction of ADH and that AOA exerted its effect by blocking the biosynthesis of ethylene.
Figure 1.
Dosage effect of AOA on the hypoxic induction of the ADH::GUS transgene in AG2 plants. AG2 plants were subjected to hypoxic treatment for 28 h in the presence of different concentrations of AOA (columns 2–7 on the left) or of 100 μm AOA plus various concentrations of ACC (right) and were harvested for GUS enzyme activity assays. Column 1 is the GUS activity from AG2 plants grown under normoxic conditions. GUS activity is expressed as pmol of 4-methylumbelliferone (4-MU) min−1 mg−1 protein. The data presented are the average of the determinations from three separate hypoxic treatments done on separate occasions. Plants grown at different times were used for replicated treatments. Bars represent sd.
Temporal Expression Patterns of the ADH Gene during Hypoxia
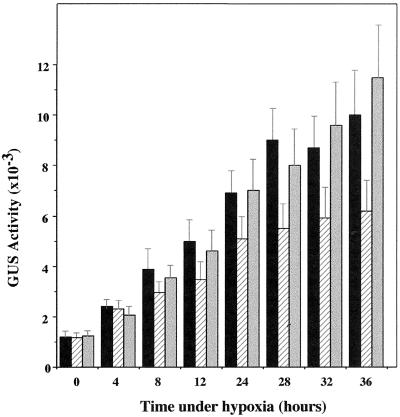
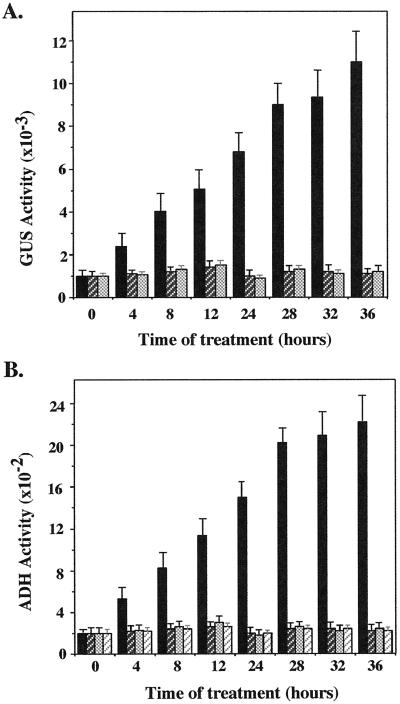
We next examined the effect of AOA on the temporal expression of the ADH::GUS transgene during hypoxia. Figure 2 shows that there was a gradual increase in GUS activity during the hypoxic treatment of AG2 plants, reaching a maximum at 28 h of hypoxic treatment. The addition of 100 μm AOA resulted in a 30% to 50% reduction in the accumulation of GUS activity in later stages of hypoxia. When ACC was included in the medium, the inhibitory effect of AOA was mostly reversed (Fig. 2). However, there was no significance difference in GUS activity between the controls and AOA-treated plants at early stages of hypoxia.
Figure 2.
Effects of AOA and ACC on temporal expression of the ADH::GUS transgene in AG2. AG2 plants were subjected to hypoxic treatment in different media. At different times, samples were harvested and assayed for GUS activity. Bar graphs at each time point (from left to right) represent activities from hypoxic treatment in Murashige and Skoog medium, Murashige and Skoog medium containing 100 μm AOA, or 100 μm AOA plus 100 μm ACC. GUS activity is expressed as pmol 4-MU min−1 mg−1 protein. The data presented are the average of six independent hypoxic treatments. Bars represent sd.
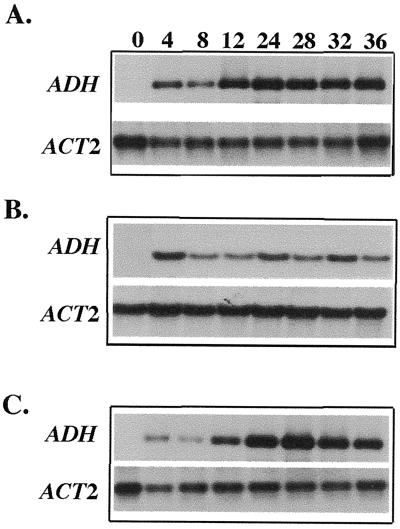
Northern-blot analysis was used to examine the effect of AOA and ACC on the temporal expression pattern of the endogenous ADH gene during hypoxia. The results from one set of representative northern blots are illustrated in Figure 3. The nuclear gene ACT2 that encodes actin from Arabidopsis, the expression of which was not affected by growth conditions (An et al., 1996; M.-C. Shih, unpublished data), was used as an RNA loading standard. Quantification of northern blots indicated that ADH mRNA levels increased gradually during hypoxia, reaching a maximal level after 24 to 28 h of hypoxic treatment (Fig. 3A). This pattern of mRNA accumulation for the endogenous ADH gene during hypoxia is very similar to that of the ADH::GUS transgene shown in Figure 2. As with GUS activity, the addition of AOA and ACC had no apparent effect on ADH mRNA levels during early stages of hypoxia (Fig. 3, B and C). At later stages of hypoxia, the addition of AOA resulted in 30% to 50% reduction in levels of ADH mRNA (Fig. 3B). However, when ACC was added, the inhibitory effect of AOA was mostly reversed (Fig. 3C). Taken together, these results suggest that an ethylene signal contributes to the induction of the ADH gene at later stages during hypoxia.
Figure 3.
Effects of AOA and ACC on the accumulation of ADH mRNA during hypoxia. AG2 plants were subjected to hypoxic treatment in Murashige and Skoog medium (A), Murashige and Skoog medium containing 100 μm AOA (B), or 100 μm AOA plus 100 μm ACC (C). Total RNA (10 μg) samples from these plants were fractionated by agarose gel electrophoresis and were hybridized to the ADH or ACT2 probes. The numbers on top of each lane represent the time (in hours) under hypoxia. Each northern-blot analysis was repeated three times using RNAs prepared from three independent hypoxic treatments.
Production of Ethylene during Hypoxia
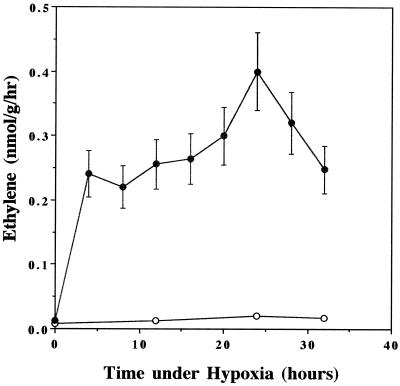
If ethylene is involved in the hypoxic induction of ADH, one would expect an increase in ethylene production in hypoxic-treated Arabidopsis. To investigate this possibility, AG2 plants were subjected to different lengths of hypoxic treatment and were harvested for measurement of ethylene production. The production of ethylene increased rapidly in the first 4 h of hypoxic treatment (Fig. 4). Rates of ethylene production remained roughly constant between 8 and 16 h. Between 20 and 24 h of hypoxia, there was a second increase in the rate of ethylene production. However, ethylene production started to decrease after 28 h of hypoxia. This pattern of hypoxia-induced ethylene production is similar to that of flooded tomato plants (Olson et al., 1995; Shiu, et al., 1998).
Figure 4.
Production of ethylene in AG2 during hypoxia. AG2 plants subjected to hypoxic treatment (●) or normoxic treatment (○) for different time periods were harvested and assayed for ethylene production as described in “Materials and Methods.” The mean of two independent hypoxic treatments is plotted. Bars represent sd.
Ethylene Alone Is Not Sufficient to Induce ADH under Normoxia
Two experiments were performed to determine whether ethylene alone is sufficient to activate ADH gene without a hypoxic signal. First, we investigated whether applying exogenous ethylene can induce ADH gene expression under normoxic conditions. Two- to 3-week-old plants were transferred to liquid Murashige and Skoog media containing 10 μm ethephon, which is an ethylene-generating compound (Abeles et al., 1992), and bubbled continuously with air. The data showed that ethephon alone could not induce the ADH::GUS transgene (Fig. 5A) or the endogenous ADH gene (Fig. 5B) in AG2 plants under normoxia. Second, we found that eto1-1, a mutant that overproduces ethylene in etiolated seedlings (Woeste et al., 1999), also produces a higher level of ethylene under growth conditions used in our laboratory. This ethylene level is comparable with that of AG2 under hypoxia (data not shown). When eto1-1 plants were subjected to normoxic treatment, there was no induction of ADH activity (Fig. 5B). In a similar manner, there was no detectable ADH mRNA level in eto1-1 plants grown under normoxic conditions. In addition, we found that ADH is induced by hypoxia in eto1-1 plants to the same extent as in wild-type plants (data not shown). Taken together, these results show that an addition of exogenous ethylene or an increase in cellular ethylene alone is not enough to activate the transcription of ADH. Therefore, we conclude that ethylene is required, but not sufficient for, the induction of ADH during hypoxia.
Figure 5.
Effects of ethylene on the expression of ADH in plants under hypoxia, normoxia, or normoxia plus ethephon treatment. GUS and ADH activities of AG2 and eto1-1 plants subjected to various treatments were determined. Bar graphs at each time point (from left to right) represent activities for AG2 under hypoxia, AG2 under normoxia, AG2 under normoxia with 10 μm ethephon added, and (B only) eto1-1 under normoxia. GUS activity is expressed as pmol 4-MU min−1 mg−1 protein. A unit of ADH enzyme is defined as an increase in the production of 1 nmol NADH min−1 mg−1 protein. The data presented are the average of three independent experiments done on separate occasions. Error bars indicate sd.
Hypoxic Induction of ADH Is Affected in etr1-1 and ein2-1
Several different classes of mutants that fail to display the triple response in the presence of saturating levels of exogenously applied ethylene have been isolated (Guzman and Ecker, 1990; Roman et al., 1995). We chose to examine temporal expression patterns of ADH during hypoxia in two of these mutants, etr1-1 and ein2-1. The ETR1 gene was identified and found to encode a receptor protein with homology to two-component regulators (Chang et al., 1993). Although ETR is present as a small gene family in Arabidopsis, mutations in one of the ETR genes result in a dominant phenotype and cause defects in many ethylene responses. EIN2 was shown to be a bifunctional transducer and may mediate crosstalk between ethylene and stress responses (Alonso et al., 1999).
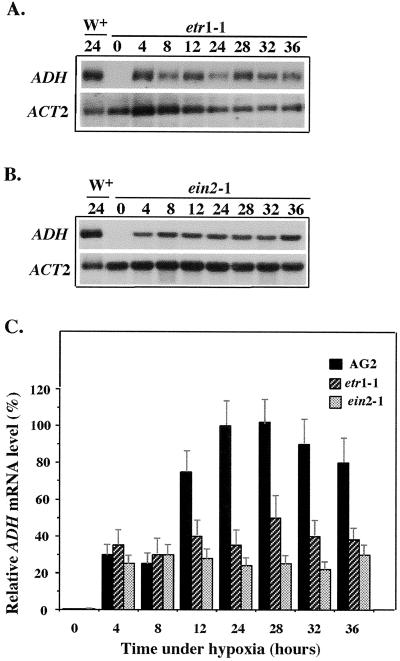
Northern-blot analysis shows that ADH mRNA levels increased during hypoxia in etr1-1 (Fig. 6A) and ein2-1 (Fig. 6B). These blots were quantified using the ADH mRNA level from AG2 plants treated with 24 h of hypoxia (Fig. 6, A and B, lane 1) as 100%. Levels of ADH mRNA were similar among etr1-1, ein2-1, and AG2 in the first 4 to 8 h of hypoxic treatment (Fig. 6C). However, ADH mRNA levels in etr1-1 and ein2-1were about 30% to 50% lower than those of AG2 between 12 and 36 h of hypoxic treatment (Fig. 6C). These results indicated that mutations affecting ethylene responses could also affect the induction of ADH gene at later stages of hypoxia.
Figure 6.
Hypoxic induction of ADH in etr1-1 and ein2-1. RNA samples from etr1-1 (A) and ein2-1 (B) subjected to hypoxic treatment were analyzed by northern-blot analysis. Digitized images of the ADH bands were quantified and normalized to the ACT2 band in each lane using the National Institutes of Health Image Analysis Program 1.62f. The normalized ADH mRNA level from 24-h hypoxic-treated AG2 plants (lane 1) was used as the 100% level. The quantification data presented in C are the average of three independent hypoxic treatments. Bars indicate sd.
DISCUSSION
Two cellular changes are known to occur in plants under oxygen deficiency: switching from aerobic respiration to anaerobic fermentation and the formation of aerenchyma tissues (Drew, 1997). Switching from aerobic respiration to anaerobic fermentation involves the induction of glycolytic and fermentative genes. Although much progress had been made in recent years in the identification of cis- and trans-acting regulatory elements of the hypoxic inducible genes, how the hypoxic signal is transduced in plant cells to trigger these cellular changes remains largely unknown.
Our studies indicated that ethylene, which is known to be involved in various stress responses in different plant species, is involved in the hypoxic induction of the ADH gene in Arabidopsis. We showed that AOA, which is an inhibitor of ACC synthase and hence an inhibitor of ethylene biosynthesis, could reduce the hypoxic induction of ADH::GUS transgene in a dosage-dependent manner (Fig. 1). However, AOA is also known to inhibit other processes such as Gly oxidation (Dry and Wiskich, 1986). The inhibitory effect of AOA on the hypoxic induction of ADH, therefore, could be due to its effect on ethylene production or on other cellular metabolism. If the response is mediated by an ethylene signal, an addition of ACC to the medium should reverse the inhibitory effect of AOA. Our data showed that when 50 to 100 μm of ACC is added, the inhibition of AOA on the induction of the ADH::GUS transgene during hypoxia was mostly reversed (Fig. 1). The amounts of ACC required to reverse the inhibitory effect of AOA on ADH induction is greater than the amounts required to elicit the triple response in etiolated Arabidopsis seedlings. It has been shown that ACC at concentrations between 10 and 100 μm has a saturating effect on the triggering of triple responses in etiolated Arabidopsis seedlings (Luschnig et al., 1998). For most plant species, the conversion of SAM to ACC, which is catalyzed by ACC synthase, is the rate-limiting step during ethylene biosynthesis (Yang and Hoffman, 1984). However, the conversion of ACC to ethylene, which is catalyzed by ACC oxidase, requires oxygen. It is likely that the conversion of ACC to ethylene would become rate limiting under very low oxygen concentration. If this were the case, higher cellular levels of ACC will be needed as a substrate to synthesize sufficient amounts of ethylene in hypoxic-treated plants. It was found that the conversion of ACC to ethylene becomes the rate-limiting step for ethylene synthesis during submergence of Rumex palustris and that higher cellular ACC levels were observed in submerged R. palustris plants (Banga et al., 1996; Vriezen et al., 1999).
There are two major classes of ethylene response mutants in Arabidopsis. One involves mutants that display constitutive triple ethylene responses, which result from either ethylene overproduction (eto1, eto2, and eto3) or constitutive activation of the pathway (ctr1), and the other involves mutants that are insensitive to ethylene, which can be due to defects in their ability to perceive (etrt1, etr2, ein4, ers, and other receptor mutants) or respond (ein2, ein3, and ein5) to ethylene (Guzman and Ecker, 1990; Roman et al., 1995). The analysis of these mutants has allowed much progress in elucidating the mechanisms of ethylene perception and signal transduction (for review, see Kieber, 1997; Bleecker et al., 1998; Johnson and Ecker, 1998; Theologis, 1998). Since we found that ethylene may contribute to the signaling pathways leading to the induction of ADH during hypoxia, we expect that hypoxic induction of ADH will be affected by mutations in the ethylene-insensitive class. Our studies showed that ADH mRNA levels in etr1-1 and ein2-1 were about 30% to 50% lower than those of AG2 during hypoxia (Fig. 6). In a similar manner, we found that levels of ADH activity in both mutants were lower than those of AG2 during hypoxia (data not shown). These results provide supporting evidence for the involvement of ethylene in the hypoxic induction of ADH in Arabidopsis.
Our observation that AOA could not completely block the hypoxic induction of ADH (Fig. 1) suggests that an ethylene-independent pathway is also involved. Consistent with this hypothesis, we found that AOA is effective in reducing the expression of ADH::GUS transgene (Fig. 2) and the endogenous ADH (Fig. 3) only in later stages of hypoxia. In contrast, there is no apparent difference in levels of GUS activity and ADH mRNA in early stages of hypoxia between hypoxic-treated AG2 plants in the absence or presence of AOA. In a similar manner, we found that ADH mRNA levels in etr1-1 and ein2-1were reduced in later stages, but not in earlier stages, during hypoxia (Fig. 6). These results can best be interpreted as that two signaling pathways, one ethylene-independent and one ethylene-dependent, are involved in the hypoxic induction of ADH in Arabidopsis and that AOA and mutations in etr1-1 and ein2-1 affect only the ethylene-dependent pathway.
Ethylene is involved in many physiological and developmental processes in plants (Yang and Hoffman, 1984; Kende, 1993). In some instances, ethylene function requires a concomitant contribution of other signaling molecules (Penninckx et al., 1998). In fact, it was shown that an addition of exogenous ethylene could not induce the formation of aerenchyma in anoxic roots in maize, although ethylene is required for the hypoxia-induced aerenchyma formation (He et al., 1994, 1996; Drew, 1997). It was reported that an addition of AOA completely inhibits the induction by flooding of a xyloglucan endo-transglycosylase gene in maize roots (Saab and Sachs, 1996). Under the same condition, the induction of the ADH1 gene decreased slightly. These results suggest that an ethylene-signaling pathway is sufficient for the induction of the xyloglucan endo-transglycosylase gene and that an ethylene-independent pathway is mainly responsible for the induction of ADH1 in flooded maize roots. In Arabidopsis, we found that an application of exogenous ethylene or an increased cellular ethylene in eto1-1 is not capable of inducing the expression of ADH during normoxia. These results suggest that ethylene is necessary, but not solely responsible, for the induction of ADH during hypoxia.
MATERIALS AND METHODS
Growth Conditions and Stress Treatment
Seeds of Arabidopsis AG2 were surface sterilized and treated with 15 μm gibberellin at 4°C overnight. Seeds were sown onto plates with Murashige and Skoog medium containing 1% (w/v) Suc and 0.8% (w/v) agar and were grown at 20°C with 16-h light/8-h dark cycles. After 1 week, seedlings were transferred to fresh Murashige and Skoog plates containing 2% (w/v) agar and were grown for additional 7 to 10 d with plates in vertical positions. For hypoxic treatments, plants were submerged in liquid Murashige and Skoog medium through which gas containing 4.5% to 5% (w/v) oxygen and balanced with nitrogen was bubbled continuously.
GUS and ADH Enzymatic Assays
GUS enzyme activity assays were performed essentially as described by Jefferson et al. (1987). Fluorescence of the 4-methylumbelliferyl product was quantified using a minifluorometer (model TKO-100, Hoefer Scientific Instruments, San Francisco). ADH activity assay was performed according to the procedures described in Xie and Wu (1989). The assay uses ethanol as the substrate and measures the production of NADH. Measurement of NADH formation was performed in a spectrophotometer (DU 64, Beckman Instruments, Fullerton, CA). A unit of ADH is defined as the production of 1 nmol of NADH min−1 mg−1 protein.
RNA Isolation and Northern-Blot Analysis
Total RNA was isolated by an acidic phenol protocol adapted from the procedures described by Chomczynski and Sacchi (1987). RNA samples (10 μg) were denatured in 6.5% (w/v) formaldehyde/50% (w/v) formamide at 65°C and electrophoresed through 1.2% (w/v) agarose gels with 6.5% (w/v) formaldehyde/1× MOPS [3-(N-morpholino)-propanesulfonic acid] buffer as described in Sambrook et al. (1989). RNA was transferred to a Magnacharge 0.45-μm nylon membrane (Micron Separations, Westborough, MA) overnight in 10× SSC and 0.1% (w/v) SDS. Filters were hybridized with random primer-labeled cDNA probes (Feinberg and Vogelstein, 1983). Final post-hybridization washings were performed at 65°C in 0.1× SSC/0.1% (w/v) SDS. The hybridization probes were as follows: ADH, a 525-bp cDNA fragment generated from reverse transcriptase-PCR based on the sequence from Chang and Meyerowitz (1986); and ACT2, a 800-bp cDNA fragment for Arabidopsis Actin2 gene generated by reverse transcriptase-PCR based on the sequence from An et al. (1996). Membranes were exposed to film (XAR-5, Eastman-Kodak, Rochester, NY) with intensifying screens at −70°C. Quantification was performed by scanning autoradiograms and analyzing the images using the National Institutes of Health Image Analysis Program 1.62f.
Measurement of Ethylene Production
AG2 plants were grown and subjected to hypoxic treatment exactly as described in prior sections. At different time intervals, 10 plants were collected in a 13 × 100-mm test tube and capped for 1 h at room temperature. The amounts of ethylene produced were measured as described by Jackson and Campbell (1976).
Chemicals and Seeds
AOA, gibberellin A3, antibiotics, and other chemicals were purchased from Sigma Chemical (St. Louis). 5-Bromo-4-chloro-3-indolyl-β-d-GlcUA cyclohexylammonium salt was purchased from Gold Biotechnology (St. Louis). Restriction and modification enzymes were from New England BioLabs (Boston) and Promega (Madison, WI). Seed stocks for etr1-1 and ein2-1 were obtained from the Arabidopsis Biological Resources Center at the Ohio State University.
ACKNOWLEDGMENTS
We thank Drs. Richard Sjölund and Wei-Yeh Wang for comments on the manuscript. We also thank Su-Jen Chou for her assistance in the measurement of ethylene production.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant nos. 9900647 and 2000–00665 to M.-C.S.).
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. Ed 2. New York: Academic Press; 1992. Chapter 7; pp. 264–285. [Google Scholar]
- Adams DO, Yang SF. Ethylene biosynthesis: identification of 1-aminocyclopropane 1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 1996;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Dawe RK. Both 5′ and 3′ sequences of maize adh1 mRNA are required for enhanced translation under low-oxygen conditions. Plant Physiol. 1996;112:685–695. doi: 10.1104/pp.112.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga M, Slaa EJ, Blom CWPM, Voesenek LACJ. Ethylene biosynthesis and accumulation under drained and submerged conditions. Plant Physiol. 1996;112:229–237. doi: 10.1104/pp.112.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Esch JJ, Hall AE, Rodriguez FI, Binder BM. The ethylene-receptor family from Arabidopsis: structure and function. Philos Trans Roy Soc Lond B. 1998;353:1405–1412. doi: 10.1098/rstb.1998.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, Drew MC. Electron microscopy of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to oxygen shortage. Planta. 1983;157:350–357. doi: 10.1007/BF00397407. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz E. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chang C, Meyerowitz E. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci USA. 1986;83:1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Hunag L, Shen M, Webster C, Burlingame A, Roberts JKM. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000;122:295–318. doi: 10.1104/pp.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–160. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung H-J, Ferl RJ. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 1999;121:429–436. doi: 10.1104/pp.121.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley TR, Peng H-P, Shih M-C. Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis. Plant Physiol. 1999;119:599–607. doi: 10.1104/pp.119.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, Jacobs M, Peacock W, Dennis E. Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol. 1994;105:1075–1087. doi: 10.1104/pp.105.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Drew MC, He C-J, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–127. doi: 10.1016/s1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- Dry I, Wiskich JT. Comparative aspects of aminooxyacetate inhibition of glycin oxidation and aminotransferase activity of pea leaf mitochondria. Plant Sci. 1986;44:33–28. [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J. Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Ferl R, Laughner B. In vivo detection of the regulatory factor binding sites of Arabidopsis thaliana Adh. Plant Mol Biol. 1989;12:357–366. doi: 10.1007/BF00017576. [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C-J, Drew MC, Page WM. Induction of enzymes associated with lysogenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen-starvation. Plant Physiol. 1994;105:861–865. doi: 10.1104/pp.105.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C-J, Page WM, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Campbell DJ. Production of ethylene by excised segments of plant tissue prior to the effect of wounding. Planta. 1976;129:273–274. doi: 10.1007/BF00398271. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Justin SHF, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 1987;106:465–495. [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, Olive M, Peacock W, Dennis E, Shimamoto K. Promoter elements required for developmental expression of the maize Adh1 gene in transgenic rice. Plant Cell. 1994;6:799–810. doi: 10.1105/tpc.6.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Gene Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DC, Oetiker JH, Yang SF. Analysis of LE-ACS3, a 1-aminocyclopropane-1-carboxylic acid synthase gene expressed during flooding in the roots of tomato plants. J Biol Chem. 1995;270:14056–14061. doi: 10.1074/jbc.270.23.14056. [DOI] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JK, Callis J, Jardetsky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci USA. 1984b;81:6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JK, Callis J, Wemmer D, Walbot V, Jardetsky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci USA. 1984a;81:6029–6033. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab I, Sachs MM. A flooding-induced xyloglucan endo-transglycosylase homolog in maize is responsive to ethylene and associated with aerenchyma. Plant Physiol. 1996;112:385–391. doi: 10.1104/pp.112.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Sabb IN. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T, editors. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH. Transgenic aequorin reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996;111:243–257. doi: 10.1104/pp.111.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. Plant Cell. 1994a;6:1747–1762. doi: 10.1105/tpc.6.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM. Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol. 1994b;105:369–376. doi: 10.1104/pp.105.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A. Ethylene signaling: redundant receptors all have their say. Curr Biol. 1998;8:R875–R878. doi: 10.1016/s0960-9822(07)00549-0. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ. 1-aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiol. 1999;121:189–196. doi: 10.1104/pp.121.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber J. Two Arabidopsis mutants that overproduce ethylene are affected in the post-transcriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–529. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wu R. Rice alcohol dehydrogenase genes: anaerobic induction, organ-specific expression and characterization of cDNA clones. Plant Mol Biol. 1989;13:53–56. doi: 10.1007/BF00027335. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yang Y, Kwon HB, Peng H-P, Shih M-C. Stress responses and metabolic regulation of glyceraldehyde-3-phosphate dehydrogenase genes in Arabidopsis. Plant Physiol. 1993;101:209–216. doi: 10.1104/pp.101.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]