Abstract
The phosphoinositide phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] is a key signaling molecule in animal cells. It can be hydrolyzed to release 1,2-diacyglycerol and inositol 1,4,5-trisphosphate (IP3), which in animal cells lead to protein kinase C activation and cellular calcium mobilization, respectively. In addition to its critical roles in constitutive and regulated secretion of proteins, PtdIns(4,5)P2 binds to proteins that modify cytoskeletal architecture and phospholipid constituents. Herein, we report that Arabidopsis plants grown in liquid media rapidly increase PtdIns(4,5)P2 synthesis in response to treatment with sodium chloride, potassium chloride, and sorbitol. These results demonstrate that when challenged with salinity and osmotic stress, terrestrial plants respond differently than algae, yeasts, and animal cells that accumulate different species of phosphoinositides. We also show data demonstrating that whole-plant IP3 levels increase significantly within 1 min of stress initiation, and that IP3 levels continue to increase for more than 30 min during stress application. Furthermore, using the calcium indicators Fura-2 and Fluo-3 we show that root intracellular calcium concentrations increase in response to stress treatments. Taken together, these results suggest that in response to salt and osmotic stress, Arabidopsis uses a signaling pathway in which a small but significant portion of PtdIns(4,5)P2 is hydrolyzed to IP3. The accumulation of IP3 occurs during a time frame similar to that observed for stress-induced calcium mobilization. These data also suggest that the majority of the PtdIns(4,5)P2 synthesized in response to salt and osmotic stress may be utilized for cellular signaling events distinct from the canonical IP3 signaling pathway.
Phosphoinositides are a class of membrane phospholipids that serve numerous roles in eukaryotic cellular processes. The family of phosphoinositides includes phosphatidylinositol monophosphate species phosphatidylinositol 3-phosphate [PtdIns(3)P] and phosphatidylinositol 4-phosphate [PtdIns(4)P], phosphatidylinositol bisphosphate species phosphatidylinositol 3,4-bisphosphate, phosphatidylinositol 3,5-bisphosphate [PtdIns(3,5)P2], and phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], and the phosphatidylinositol trisphosphate species phosphatidylinositol 3,4,5-trisphosphate. PtdIns(3)P and PtdIns(4)P regulate vesicle-mediated protein transport to the vacuole/lysosome and protein secretion, respectively (Corvera et al., 1999; Hama et al., 1999; Walch-Solimena and Novick, 1999; Odorizzi et al., 2000). Phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate have well-documented roles as second messengers in Tyr kinase and G-protein-coupled receptor signaling pathways in animal cells (Martin, 1998). PtdIns(3,5)P2 has not only been implicated as a signaling molecule during osmotic stress in yeast and plants (Dove et al., 1997; Meijer et al., 1999), but is necessary for maintaining yeast vacuolar morphology and function (Odorizzi et al., 2000). PtdIns(4,5)P2 is involved in signaling via G-protein coupled receptors, regulating vesicle-mediated protein traffic, and actin filament polymerization (for review, see Martin, 1998).
Phosphoinositides have the innate capacity to bind specific proteins, thus altering their cellular localization and/or activity. PtdIns(4,5)P2 is especially interesting because of its multifaceted role within the cell. This membrane lipid can modulate the activity of cytoskeletal-associated proteins (e.g. gelsolin, profilin, and centaurin) and vesicle-trafficking proteins, e.g. phospholipase D, ADP-ribosylation factor (ARF), ARF-GTPase activating protein, and ARF-guanine nucleotide exchange factor, or it can be hydrolyzed into the signaling molecules IP3 and 1,2-diacylglycerol, which trigger calcium release from intracellular stores and activate protein kinase C, respectively (Berridge, 1993).
Compared with animal systems, phosphoinositide-signaling pathways in plant cells are not well characterized (for review, see Drøbak et al., 1999; Stevenson et al., 2000). Various reports suggest that abiotic cues such as salinity, hyper- and hypoosmotic stress, and gravity effects (Einspahr et al., 1988; Perera et al., 1999; Pical et al., 1999), as well as biotic cues such as hormones may activate phosphoinositide-signaling systems (Staxén et al., 1999). It is interesting that IP3-binding channels have been identified in plants (Allen et al., 1995), and delivery of caged IP3 to plant cells has been demonstrated to cause release of calcium from intracellular stores (Alexandre et al., 1990; Franklin-Tong et al., 1996). Altogether, these data support the notion that plants rely on phosphoinositides as second messengers. However, a lack of comprehensive studies linking production of phosphoinositides with downstream effects like calcium signaling has limited our understanding of the mechanisms and universality of these pathways in plants.
In Arabidopsis, components of potential phosphoinositide-signaling pathways have been uncovered. Cloning of genes encoding a phosphatidylinositol 3-kinase (PtdIns 3-kinase; Welters et al., 1994), a phosphatidylinositol 4-kinase (PtdIns 4-kinase; Stevenson et al., 1998; Xue et al., 1999), and a phosphatidylinositol 4-phosphate 5-kinase [PtdIns(4)P 5-kinase; Mikami et al., 1998] suggest that Arabidopsis utilizes phosphoinositides such as PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P2 for signaling. Based on PtdIns 3-kinase antisense experiments, a general role for PtdIns(3)P in plant growth and development has been postulated (Welters et al., 1994). In addition, Hong and Verma (1994) have demonstrated that PtdIns 3-kinase activity is induced during nodule formation and has an undefined role in membrane proliferation in soybean. Moreover, a recent report has demonstrated that PtdIns 3-kinase colocalizes with nuclear transcription sites in soybean, implicating PtdIns(3)P as a potential regulator of transcription (Bunney et al., 2000). Although a well-delineated function has not been postulated for PtdIns(4)P in plants, there is an increasing body of data demonstrating the association of PtdIns 4-kinase activity with intracellular membranes, the cytosol, cytoskeleton, and nucleus (Drøbak et al., 1999). It has also been shown that PtdIns 4-kinase may regulate vesicle-mediated protein traffic in tobacco suspension cells (Matsuoka et al., 1995). It has recently been observed that the pleckstrin homology domain of PtdIns 4-kinase preferentially binds to PtdIns(4)P, suggesting a potential mechanism for regulating cellular PtdIns(4)P pools (Stevenson et al., 1998). The role of PtdIns(4,5)P2 in Arabidopsis signaling pathways is currently unclear. However, a stress-induced PtdIns(4)P 5-kinase (Mikami et al., 1998) and a calcium-dependent phospholipase C (PLC; Hirayama et al., 1995) have been cloned, and these enzymes might participate in the classic PtdIns(4,5)P2-signaling cascade in plants.
We now report the direct involvement of a PtdIns(4,5)P2-signaling pathway in Arabidopsis plants in response to salt and osmotic stress, and a correlation between synthesis of PtdIns(4,5)P2, IP3 production and calcium mobilization. For this study, intact plants were labeled with [3H] myo-inositol in liquid culture and were then subjected to stress. The phosphoinositide composition of whole plants was determined using anion-exchange HPLC head-group analysis, enabling determination of phosphoinositide and inositol phosphate levels under different conditions. In addition, we examined the potential role of IP3 as an effector of calcium mobilization using the calcium indicators Fura-2 and Fluo-3. Our results support the hypothesis that phosphoinositide-derived second messengers participate in calcium signaling, which has been demonstrated to alter gene expression in Arabidopsis (Knight et al., 1997). It has been further postulated that calcium signaling and altered gene expression facilitate plant adaptation to salt and osmotic stress (Knight et al., 1997).
RESULTS
PtdIns(4,5)P2 Accumulates in Salt- and Osmotically Stressed Arabidopsis Plants
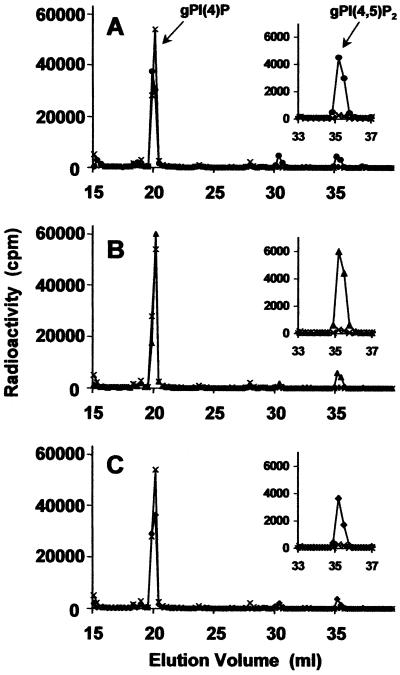
In an effort to determine if higher plant species respond to salt and osmotic stress by producing phosphoinositides, we carried out a study in which 2-week-old Arabidopsis plants grown in liquid media were labeled with [3H] myo-inositol. Plants were treated by immersion in osmotic-adjusting solutions of 0.25 m NaCl (osmotic potential = 1.17 MPa), 0.25 m KCl (osmotic potential = 1.12 MPa), 0.5 m sorbitol (osmotic potential = 1.23 MPa), and 1.0 m sorbitol (osmotic potential = 2.46 MPa). The extracted phosphoinositides were deacylated and their corresponding glycerophosphoinositol head groups were analyzed by HPLC. The relative concentration of glycerophosphoinositols detected using the HPLC analysis accurately represents the relative quantity of phosphoinositides in the plants, and the glycerophosphoinositol phosphates are the deacylated form of the corresponding phosphatidylinositol phosphate species. In non-stressed plants (see control chromatograms in Fig. 1), glycerophosphoinositol 3-phosphate [gPI(3)P] and glycerophosphoinositol 4-phosphate [gPI(4)P] were the two most abundant species, with gPI(4)P levels approximately 40-fold higher than gPI(3)P. In addition, glycerophosphoinositol 4,5-bisphosphate [gPI(4,5)P2] levels were detectable, but very low in non-stressed plants, which is in contrast to findings in yeast, algae, and mammalian cells where basal levels of gPI(4,5)P2 are significantly higher.
Figure 1.
Osmotic stress-induced PtdIns(4,5)P2 production in Arabidopsis plants. Anion-exchange HPLC analysis of deacylated myo-[2-3H]inositol-labeled lipids from plants that were untreated (A, B, and C, ×) or treated with 0.25 m NaCl (A, ●), 0.25 m KCl (B, ▴), and 0.5 m sorbitol (C, ♦) for 30 min. The total counts in each sample were 7 × 105.
When Arabidopsis plants were immersed in a 0.25 m solution of NaCl for 1 h, HPLC analyses revealed that gPI(4,5)P2 increased by approximately 20-fold compared with levels in non-stressed plants (Fig. 1A). This finding provided impetus to determine whether treatment of plants with other osmotic-adjusting chemicals such as potassium chloride and sorbitol would elicit a similar response. When labeled plants were placed in 0.25 m potassium chloride (Fig. 1B) or 0.5 m sorbitol (Fig. 1C), the levels of gPI(4,5)P2 increased dramatically after 30 min, suggesting that osmotic stress also induces PtdIns(4,5)P2 accumulation in Arabidopsis.
In an additional set of experiments, we examined osmotic-induced changes in phosphoinositide levels in Saccharomyces cerevisiae and Chlamydomonas moewusii (data not shown), which corroborated previously published reports indicating that these organisms primarily synthesize PtdIns(3,5)P2 and not PtdIns(4,5)P2 in response to hyperosmotic stress (Dove et al., 1997; Meijer et al., 1999).
Increased Biosynthesis of PtdIns(4,5)P2 Results in Its Rapid Accumulation in Osmotically Stressed Plants
The observed increase in PtdIns(4,5)P2 levels in osmotically stressed Arabidopsis plants could potentially result from a variety of cellular events, including inhibition of PLC activity, increased lipid kinase activity, or the transcriptional/translational up-regulation of PtdIns 4-kinase and PtdIns(4)P 5-kinase expression. Hence, we performed several experiments to address these possibilities.
To assess whether PtdIns(4,5)P2 accumulation occurred via successive activity of the enzymes responsible for its synthesis or as a result of inhibition of its hydrolysis to 1,2-diacylglycerol and IP3, we utilized the lipid kinase inhibitor wortmannin. In plant cells this fungal metabolite has been previously demonstrated to effectively perturb PtdIns 3-kinase and PtdIns 4-kinase activity (Matsuoka et al., 1995). When labeled plants were treated with wortmannin prior to salt stress, both PtdIns(4)P and PtdIns(4,5)P2 were approximately 10-fold lower than in stressed plants not treated with wortmannin (data not shown). These data suggested that inhibition of biosynthesis effectively abolishes PtdIns(4,5)P2 accumulation, and compelled us to examine whether the biosynthetic increase in PtdIns(4,5)P2 levels was due to enhanced protein activity or to up-regulated gene expression.
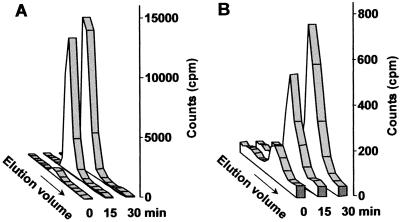
The simplest way to distinguish between these possibilities was to determine how rapidly PtdIns(4,5)P2 accumulates in response to osmotic stress. In accordance with this, labeled plants were submerged in media containing a final concentration of 1 m sorbitol, removed at the indicated times, and were treated with trichloroacetic acid (TCA) to arrest all enzymatic activity. Data from this experiment revealed that although PtdIns(4,5)P2 was quite low (approximately 1,000 cpm) prior to the onset of stress, after 15 and 30 min of treatment with sorbitol, the level of this phosphoinositide specie increased to 27,000 and 35,000 cpm, respectively (Fig. 2A). This trend (also observed in Fig. 3 for 0.25 m NaCl stress) suggests that initial PtdIns(4,5)P2 accumulation is a result of activation of the lipid kinases [PtdIns 4-kinase and PtdIns(4)P 5-kinase] responsible for its synthesis. Moreover, the continued increase in PtdIns(4,5)P2 at later times could feasibly result from an increase in transcription (Mikami et al., 1998) and/or translation of PtdIns 4-kinase and PtdIns(4)P 5-kinase.
Figure 2.
Concomitant increase in osmotic stress-induced PtdIns(4,5)P2 and IP3 levels. Sorbitol (at a final concentration of 1 m) was added to myo-[2-3H]inositol-labeled plants and an equivalent number of plants were withdrawn at 0, 15, and 30 min. A, The elution profile of gPI(4,5)P2 (elution volume of 41–46 mL) is shown. B, The elution profile of inositol 1,4,5-trisphosphate (elution volume of 55–60 mL) is shown. For each HPLC run, the aqueous or organic sample each contained 1 × 106 cpm.
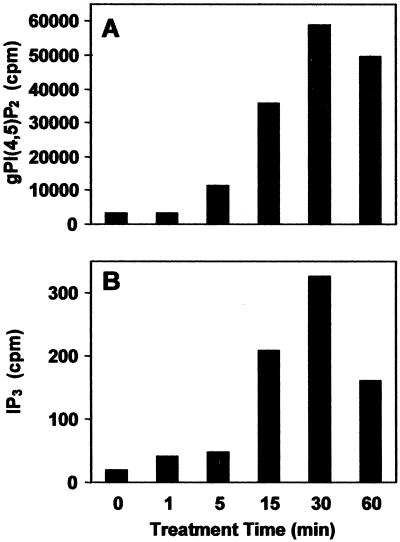
Figure 3.
Time course of salt stress-induced PtdIns(4,5)P2 and IP3 production. NaCl (at a final concentration of 0.25 m) was added to myo-[2-3H]inositol-labeled plants and an equivalent number of plants was withdrawn from solution at 0, 1, 5, 15, 30, and 60 min. A, The extracted and deacylated phosphoinositide head groups (organic) were separated by anion-exchange HPLC and the total number of counts in each gPI(4,5)P2 peak was calculated. B, The aqueous IP3-containing samples were analyzed by anion-exchange HPLC and the total number of counts in each IP3 peak was calculated. For each HPLC run, the aqueous or organic sample each contained 1 × 106 cpm.
PtdIns(4,5)P2 Biosynthesis Results in IP3 Accumulation in Osmotically Stressed Arabidopsis
To help elucidate the role of PtdIns(4,5)P2 in osmotic stress signaling we investigated whether this phosphoinositide could serve as a substrate for Arabidopsis PLC enzymes by measuring not only PtdIns(4,5)P2, but also IP3 levels in stressed (1 m sorbitol) and non-stressed plants (Fig. 2, A and B). It is interesting that IP3 accumulation nearly mirrored the observed trend in PtdIns(4,5)P2 accumulation, increasing roughly 6-fold to over 1,800 cpm in 30 min (Fig. 2B). Because these results seemed to suggest a functional relationship between the production of PtdIns(4,5)P2/IP3 and osmotic stress signaling, we chose to apply a more physiologically acceptable level of stress in subsequent experiments.
Salt Stress Causes Rapid Accumulation of PtdIns(4,5)P2 and IP3
The data presented thus far demonstrates that sorbitol-induced osmotic stress causes the accumulation of PtdIns(4,5)P2 and IP3 in Arabidopsis. We extended this work by subjecting plants to a salt stress (0.25 m NaCl) and examining whether modulation of phosphoinositide and inositol phosphate levels were similar to that observed for sorbitol stress.
To ascertain this information we again measured PtdIns(4,5)P2 and IP3 levels in plants that were immersed in 0.25 m NaCl. Prior to the onset of stress, PtdIns(4,5)P2 and IP3 levels were very low, but detectable (approximately 2,000 and 20 cpm above background, respectively). However, following treatment with NaCl, PtdIns(4,5)P2 increased in a nearly logarithmic fashion for 30 min (to approximately 25-fold above basal levels) before decreasing moderately (Fig. 3A). A similar pattern was observed for IP3 accumulation, in which 1 and 30 min of salt stress induced a 2- and 15-fold increase, respectively (Fig. 3B). This data substantiated the previous changes in phosphoinositide metabolism observed at significantly higher levels of stress (see Fig. 2), and motivated us to examine whether IP3 production is a result of PLC activity in plants challenged with salt stress.
A PLC Inhibitor Blocks IP3 Accumulation during Salt Stress
To establish a connection between IP3 accumulation and PLC activity in salt-stressed plants we utilized the PLC inhibitor U-73122 and its less active analog U-73343. Although U-73122 and U-73343 have been used in animal and plant studies (Bleasdale et al., 1990; Zheng et al., 1997; Staxén et al., 1999; Coursol et al., 2000), in vivo biochemical characterization of these inhibitors is scant. In this study we examined PtdIns(4,5)P2 and IP3 accumulation in salt-stressed (0.25 m NaCl) plants in the presence of U-73122 and U-73343, respectively. Figure 4A demonstrates that plants treated with 1 μm U-73122 prior to a salt stress with 0.25 m NaCl accumulated approximately 8-fold more PtdIns(4,5)P2 than plants treated with NaCl alone. More importantly, we observed that plants treated with 1 μm U-73122 prior to salt stress accumulated 20-fold less IP3 than plants exposed to NaCl alone (Fig. 4B). In addition, treatment of plants with the aforementioned analog U-73343 had no discernible effect on phosphoinositide metabolism (data not shown).
Figure 4.
U-73122 blocks IP3 accumulation in salt-stressed plants. Myo-[2-3H]inositol-labeled plants were incubated in the absence (control) or presence of the PLC inhibitor (+ U-73122) and were then exposed to 0.25 m NaCl for 0 (□) or 15 (▪) min, respectively. A, The extracted and deacylated phosphoinositide head groups (organic) were separated by anion-exchange HPLC and the total number of counts in each PtdIns(4,5)P2 peak was calculated. B, The aqueous IP3-containing samples were analyzed by anion-exchange HPLC and the total number of counts in each IP3 peak was calculated. The aqueous and organic samples contained 1 × 106 and 3 × 106 cpm, respectively.
Salt Stress Causes Rapid Intracellular Calcium Mobilization in Root Tip Cells
After finding that IP3 levels increased in Arabidopsis plants subjected to osmotic and salt stress, we used microscopy to examine potential calcium fluxes in root tip cells in response to identical treatment. Although whole plants and roots have been used to evaluate calcium mobilization in salt-stressed Arabidopsis (Knight et al., 1997, 1998; Kiegle et al., 2000), we chose to examine intact roots because this tissue is very well characterized (Kiegle et al., 2000). The fluorescent calcium indicator Fluo-3 acetoxymethyl ester (Fluo-3 AM) was used in this study initially because it is excitable by visible wavelengths and capable of moving passively across the cell wall and plasma membrane, eliminating the potential damaging effects of other delivery techniques such as microinjection. In addition, Zhang et al. (1998) successfully demonstrated the utility of passive Fluo-3 AM loading in cellular calcium imaging. Data collection using Fluo-3 was accomplished by employing rationale developed by Malhó and Trewavas (1996) for the use of a single-wavelength calcium indicator dye to approximate root cell calcium concentrations. Data was collected imposing the following criteria. Experiments were conducted only when Fluo-3 was evenly distributed in the cytosol at low but sufficient concentrations to provide measurable pixel intensities. Imaging was done on cortical cells, which reside below the epidermis of Arabidopsis roots. Also, control data was collected to account for photobleaching and was also used in calcium concentration determination. Fluo-3 has weak fluorescence without binding calcium and, therefore, increases in Fluo-3 concentration could be misinterpreted as increases in calcium concentration. When plants were incubated as described above and then imaged without the addition of sorbitol or NaCl, no changes in fluorescence, dye movement, or compartmentalization were observed (data not shown).
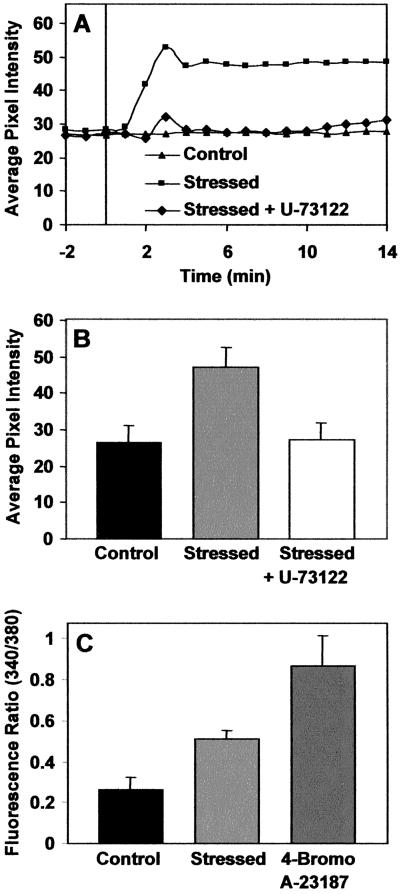
Increases in the fluorescence of the Fluo-3 calcium indicator occurred following 2 min of exposure to 0.25 m NaCl (labeled “stressed” in Fig. 5, A–C), plateaued at 4 min, and then decreased only after 15 min. To determine whether the observed calcium flux correlated with IP3 accumulation we utilized the aforementioned PLC inhibitor U-73122. Figure 5A represents data from a 20-min time course in which fluorescence pixel intensities were collected. Root tips were untreated or treated with U-73122 (at a final concentration of 1 μm), and both groups were then subjected to 0.25 m NaCl and imaged simultaneously. Resultant data indicates that when treated with U-73122 prior to salt stress, root cells mobilize calcium at a level that is comparable with non-stressed plants (Fig. 5, A and B), suggesting that the inhibition of IP3 accumulation (see Fig. 4) interrupts the calcium mobilization measured using this technique. These data suggest that, at least in part, stress-induced calcium mobilization is mediated by inositol trisphosphate accumulation.
Figure 5.
Calcium mobilization in root-tip cells. A, Root tips were loaded with Fluo-3 and were untreated (▴), treated with 0.25 m NaCl (▪), or treated with 0.25 m NaCl in the presence of the PLC inhibitor U-73122 (♦). NaCl was added at t = 0 and data for these experiments was collected simultaneously from plants laying adjacent to each other. For the untreated experiment (▴), 100 μL of buffer was added at t = 0. B, Mean and sd calculations of root tip cell fluorescence (n = 9 for each treatment). Roots were treated as described above, images collected, and relative fluorescence presented; control (black), salt stressed (light gray), and salt stressed plus U-73122 (white). Salt stressed indicates exposure to 0.25 m NaCl. C, Mean and sd calculations of fluorescence ratios using Fura-2-loaded 8-d-old root tips (n = 7 for each treatment). Root tips were untreated (black) or were treated with 0.25 m NaCl (light gray) or 10 μm 4-Bromo A-23187 (dark gray; as a positive control).
To avoid potential misinterpretation of the data using the single-wavelength indicator (Fluo-3), we employed the ratiometric calcium indicator Fura-2, which has been characterized in plants and animals in calcium mobilization studies (Tsien and Poenie, 1986; Helm et al., 1997; Allen et al., 1999). Our method is essentially the same as described previously for Indo-1 (Gilroy 1996; Legué et al., 1997). Ratiometric analysis with Fura-2 revealed that exposure to 0.25 m NaCl caused a 70% increase in the ratio of fluorescence intensity of the cells at the root tip (Fig. 5C). Addition of the calcium ionophore 4-Bromo A-23187 was used as a positive control for the activity of Fura-2 in the root tip cells (Fig. 5C).
DISCUSSION
Several recent studies have demonstrated conclusively that eukaryotic organisms respond to osmotic stress by synthesizing specific phosphoinositides (Dove et al., 1997; Meijer et al., 1999; Van der Kaay et al., 1999). The yeasts S. cerevisiae and Schizosaccharomyces pombe accumulate significant quantities of PtdIns(3,5)P2 following exposure to osmotic-adjusting chemicals (Dove et al., 1997). A similar, although less dramatic increase in PtdIns(3,5)P2 has been observed in the green algae C. moewusii within min of osmotic stress (Meijer et al., 1999). Likewise, the single-cell lower plant Dunaliella salina accumulates a PtdIns bisphosphate species in response to osmotic shock (Einspahr et al., 1988). In contrast, mammalian cells synthesize phosphatidylinositol 3,4,5-trisphosphate in response to Suc-induced osmotic stress (Van der Kaay et al., 1999), while decreasing PtdIns(3,5)P2 levels (Dove et al., 1997). These observations suggest that different eukaryotic organisms utilize unique phosphoinositide-signaling pathways to elicit the necessary cellular adaptations following a change in the osmotic potential of the surrounding environment.
An amalgam of experimental data support the model that plants respond to salinity and osmotic stress by utilizing phosphoinositides or phos-phoinositide-derived second messengers (Cho et al., 1993; Hirayama et al., 1995; Brearley et al., 1997; Mikami et al., 1998; Munnik et al., 1998; Meijer et al., 1999; Pical et al., 1999) and the aforementioned observation that algal species such as C. moewusii and D. salina respond in a fashion similar to yeast by producing PtdIns(3,5)P2 following osmotic stress would suggest that higher plants might respond similarly. This assumption is not fully accurate because a recent report indicated that Arabidopsis suspension culture cells accumulate primarily PtdIns(4,5)P2 instead of PtdIns(3,5)P2 following osmotic shock (Pical et al., 1999). Our analyses confirm the findings of Pical et al. (1999) and support one potential mechanism for PtdIns(4,5)P2-signaling during stress.
The labeling, extraction, and HPLC procedures used in this study enabled precise comparison of phosphoinositides present in whole Arabidopsis plants. We determined that PtdIns(3)P and PtdIns(4)P were the most abundant species and that their levels correlate with the relatively high respective PtdIns kinase activities extracted from Arabidopsis (Hama et al., 2000). It was additionally noted that the quantity of PtdIns(4,5)P2 in non-stressed plants was very low in comparison with yeast, algae, and mammalian cells. It is interesting that a radiolabeled species elutes where glycerophosphoinositol 5-phosphate would be detected (data not shown), although phosphatidylinositol 5-phosphate has only been reported in animal cells (Rameh et al., 1997). Additional analyses must be performed to determine whether this peak is derived from PtdIns(5)P and if this phosphoinositide is being used as a substrate for phosphoinositide kinases to produce PtdIns(4,5)P2. Furthermore, our analyses uncovered a minor peak that co-elutes with glycerophosphoinositol 3,5-bisphosphate [gPI(3,5)P2; data not shown]; however, concentrations of this species did not change significantly after osmotic or salt stress of Arabidopsis plants.
The most significant findings of this work illustrate that although PtdIns(4,5)P2 and IP3 levels are quite low in non-stressed plants, these species rapidly accumulate following salt stress in a time-dependent and correlated manner (Figs. 2 and 3). The functional relevance of this relationship is underscored not only by the recent cloning of an Arabidopsis PtdIns(4)P 5-kinase (Mikami et al., 1998) and a phosphoinositide-specific PLC (Hirayama et al., 1995), but also by data demonstrating that these enzymes are transcriptionally up-regulated in response to various stresses (Hirayama et al., 1995; Mikami et al., 1998). In addition, we have revealed that root intracellular calcium concentration increases in a time-dependent manner that parallels IP3 production (see Figs. 3 and 5). In concert, these data illustrate that Arabidopsis utilizes a stress-activated phosphoinositide-based signaling system that is similar to the well-documented receptor-mediated signaling pathway in animal cells (Berridge, 1993).
The vacuole and the endoplasmic reticulum are generally accepted as the sources of most internal calcium in plants (for review, see Trewavas, 1999); however, cortical cells in the region examined have very small vacuoles and relatively large nuclei, and it is possible that external sources are in part responsible for the observed increase in root cell cytosolic calcium. Nevertheless, the recent work of Knight and colleagues (Kiegle et al., 2000) and data presented in this study indicate that the cortical cells of Arabidopsis roots respond rapidly and vigorously by mobilizing calcium in response to salt stress. The production of PtdIns(4,5)P2 and IP3, and the eventual mobilization of calcium causes changes in metabolism and gene expression through activation of calmodulin and a variety of protein kinases (for review, see Trewavas and Malhó, 1998; Trewavas, 1999). These changes are likely involved in the molecular mechanisms that allow plants to sense and adapt to salinity.
Although the relationship between PtdIns(4,5)P2, IP3 and calcium has been addressed conceptually (Stevenson et al., 2000), this is the first study to reveal a functional correlation between these phosphoinositide species and calcium mobilization in plants. Furthermore, the data presented herein establish not only the connection between PtdIns(4,5)P2 synthesis and calcium mobilization in plants, but they also suggest that PtdIns(4,5)P2 accumulation under these conditions is fulfilling additional cellular roles. Based on reports elucidating the roles of PtdIns(4,5)P2 in non-plant cells, synthesis of this phosphoinositide could be affecting processes as diverse as signaling via second messengers, cytoskeletal rearrangements, and vesicle-mediated protein trafficking. Our efforts to understand why PtdIns(4,5)P2 was increasing in stressed plants focused on the potential signaling mechanisms via its metabolite IP3. Although PtdIns(4,5)P2 conversion to IP3 with subsequent calcium mobilization is well established in animal cells (Berridge, 1993), less is known about this mechanism of signaling in plant cells (Trewavas, 1999). Prior to this study it was demonstrated that maize root protoplasts and Arabidopsis plants mobilize calcium following salt stress (Lynch et al., 1989; Kiegle et al., 2000) and it has been proposed, but not documented, that this response occurs through phosphoinositide-signaling pathways.
Although the correlation between stress-induced PtdIns(4,5)P2 accumulation and calcium mobilization has been demonstrated, the data presented herein raise some currently unresolved questions. First, the quantity of PtdIns(4,5)P2 present in stressed plants was 20- to 50-fold higher than that of IP3. Although it is plausible that the vast difference in the detected concentration of PtdIns(4,5)P2 and IP3 is due to experimental artifacts, this seems unlikely because of the efforts made to block phosphatase and lipase activities by treating plants with TCA prior to lipid extraction. In an alternate manner, the intracellular IP3 pool could be rapidly converted to another inositol phosphate species. In any regard, the substantial accumulation of PtdIns(4,5)P2 implies that the phosphoinositide is being synthesized not only as a precursor for IP3 production, but may also serve other roles in the stress response such as modulating the activity of cytoskeletal-associated effector proteins and/or modifying vesicle-trafficking. Second, the kinetics of IP3 accumulation that we have documented is vastly different than previously published reports (Perera et al., 1999; Staxén et al., 1999). For example, in guard cells IP3 has been implicated in the rapid and transient accumulation of cytosolic calcium that eventually leads to stomatal closure (Staxén et al., 1999), and caged IP3 that has been released by photolysis can induce transient calcium waves in pollen tubes (Franklin-Tong et al., 1996). In contrast to these relatively rapid effects, Arabidopsis plants have elevated IP3 levels for more than 1 h after salt stress. Since the accumulation of IP3 is in part dependent upon the activity of PLC working in opposition to the activities of IP3 phosphatases and kinases that convert IP3 to IP2 and IP4, respectively, the activity of these enzymes may also be altered during the plant stress response. The simplest interpretation of this paradox is that IP3 is involved in signaling processes distinct from its role in calcium mobilization. It is possible that IP3 is converted to inositol phosphate species like IP2, IP4, IP5, or IP6. IP5 and IP6 have recently been implicated in the direct regulation of gene expression (Odom et al., 2000) and mRNA export (York et al., 1999) in yeast. It will be important for future studies to rigorously assess the ultimate role of PtdIns(4,5)P2 and IP3 in mediating plant adaptations to stress.
MATERIALS AND METHODS
Organisms and Media
Arabidopsis ecotype Columbia was grown in a suspension of 0.5× Murashige and Skoog basal salt mixture, pH 5.8 (Murashige and Skoog, 1962) containing B5 vitamins (100 mg/L of myo-inositol, 10 mg/L of thiamine-HCl, and 1 mg/L of each pyridoxine-HCl and nicotinic acid) for phosphoinositide and inositol phosphate analyses. Sterile Erlenmeyer flasks containing the plants were placed on a gyratory shaker at 80 rpm in a growth chamber set at 26°C. Visible radiation (80 μmol m−2 s−1 for 24 h) was provided by fluorescent lamps. In addition, Arabidopsis plants were grown on rafts floated on a 20–10-20 (N-P-K) solution of “Peat-Lite Special” fertilizer (Scotts-Sierra Horticultural Products Company, Marysville, OH) or 0.5× Murashige and Skoog medium containing B5 vitamins in Magenta boxes for the calcium mobilization experiments. These boxes were in a growth chamber set at a 16-h day (24°C)/8-h night (22°C) cycle with a visible radiation level of 100 μmol m−2 s−1 provided by fluorescent lamps. The Chlamydomonas moewusii strain was grown in a standard medium (Meijer et al., 1999) for phosphoinositide analyses. Saccharomyces cerevisiae (W303C) were grown in yeast extract:peptone:Glc medium (1% [w/v] yeast extract:2% [w/v] peptone:2% [w/v] Glc), or synthetic medium (Hama et al., 2000).
Radiolabeling of Cells
Two-week-old Arabidopsis plants were radiolabeled with myo-[2-3H]inositol (Amersham Pharmacia Biotech, Piscataway, NJ) at a final concentration of 50 μCi/mL in 1 to 5 mL of 0.5× Murashige and Skoog medium containing B5 vitamins with reduced myo-inositol (10 μm). Labeling was accomplished for 20 h on a gyratory shaker (80 rpm) at 26°C. For algal phosphoinositide analyses, C. moewusii cells were labeled as described previously (Meijer et al., 1999). Yeast cells were grown in synthetic medium containing 5 μCi/mL of myo-[2-3H]inositol.
Plant Stress Treatment
Plants used for phosphoinositide analysis and calcium mobilization experiments were treated with 0.25 m NaCl or as otherwise indicated. In the experiment using wortmannin (50 μm in dimethyl sulfoxide), labeled plants were treated with 1 m NaCl to ensure a vigorous response. The final concentration of dimethyl sulfoxide was 0.5% (v/v), and a vehicle control elicited no response (data not shown).
Extraction of Phosphoinositides and Inositol Phosphates
Method 1
A detailed description of the lipid extraction from Arabidopsis plants has been published (Hama et al., 2000). In brief, growth of radiolabeled Arabidopsis plants was terminated by addition of TCA (final concentration of 5% [w/v]) followed by incubation on ice for 1 h. Plants were washed five times with 10 mL of water, suspended in 0.5 mL of water in a 5-mL conical Dounce tissue grinder, and homogenized following addition of 0.75 mL of 15:5:1 (v/v), 95% (v/v) ethanol:diethyl ether:pyridine. To maximize the efficiency of lipid extraction, the plant homogenate in ethanol-diethyl ether-pyridine solvent was transferred into 1.6-mL microcentrifuge tubes and incubated at 57°C for 30 min. Cell debris was removed by centrifugation and the supernatant was dried under nitrogen. Lipids from yeast and algae cells were also extracted by this method.
Method 2
For analyses of water-soluble inositol phosphate species and phosphoinositides derived from the same plant sample, a different extraction procedure was carried out. Plants were labeled, treated with TCA, washed with water, and then homogenized in a Dounce tissue grinder in 1.5 mL of hydrochloric acid:chloroform:methanol solution (0.5 1m:1:1, v/v). Samples were centrifuged for 5 min at 5,000g and the bottom (organic) phase was recovered and aqueous phase re-extracted once with chloroform/methanol solvent. Combined organic phases were dried under nitrogen, then deacylated and extracted as described below. The aqueous phase was dried under nitrogen and was counted using a liquid scintillation counter (LS 5801, Beckman Instruments, Fullerton, CA).
Phosphoinositide Deacylation
Lipids derived from the ethanol-diethyl ether-pyridine or chloroform-methanol solvent extraction procedures were deacylated using a previously described method (Serunian et al., 1991) with minor modifications. All the procedures were carried out in 1.6-mL microcentrifuge tubes. Dried lipids were resuspended in 0.5 mL of methylamine reagent (3.75:4:1 [v/v], 25% [w/v] methylamine:methanol:n-butanol) by bath sonication, incubated at 53°C for 50 min, and dried in vacuo. Deacylated lipids were suspended in 0.75 mL of water by sonication and were then extracted with 0.75 mL of n-butanol:petroleum ether:ethyl formate (20:4:1, v/v) four times. The aqueous phase was dried in vacuo, resuspended in water, and the radioactivity was determined using a liquid scintillation counter.
HPLC Analyses
Glycerophosphoinositol head groups [gPI(3)P, gPI(4)P, gPI(3,5)P2, and gPI(4,5)P2] and IP3 were resolved using anion-exchange HPLC with Partisil 10 SAX (4.6 × 250 mm) columns (Whatman, Clifton, NJ) fitted with a SAX guard column (Phenomenex, Torrance, CA). A chromatograph equipped with a UV detector (System Gold, Beckman) and System Gold software (Beckman) was used. All samples contained internal controls of AMP, ADP, and ATP to monitor the column performance. A portion of each sample (0.3–2.5 × 106 cpm as indicated for each experiment) was mixed with approximately 80 nmol each of AMP, ADP, ATP, and was applied to the column. The first gradient (gradient 1) conditions were 5 mL of isocratic 10 mm ammonium phosphate (pH 3.8), followed by a linear gradient from 10 mm to 0.7 m in 40 mL of ammonium phosphate (for glycerophosphoinositol species) at a flow rate of 1 mL/min. The second gradient (gradient 2) conditions were 5 mL of isocratic 10 mm ammonium phosphate (pH 3.8), followed by a linear gradient from 10 mm to 0.8 m in 60 mL (for glycerophosphoinositol and IP3 species) at a flow rate of 1 mL/min. For all runs, fractions were collected every 0.3 min, mixed with EcoLume scintillant (ICN Biomedicals, Irvine, CA), and counted in a liquid scintillation counter.
[32P]gPI(3)P and [32P]gPI(4)P standards were generated by in vitro phosphorylation of phosphatidylinositol with γ-[32P]ATP (Amersham Pharmacia Biotech) followed by deacylation as described previously (Hama et al., 2000). The gPI(3,5)P2 peak was identified as a species in S. cerevisiae that increased 16-fold by osmotic stress (Dove et al., 1997). The gPI(4,5)P2 peak was identified as a species that co-eluted with deacylated [3H]PtdIns(4,5)P2 (Amersham Pharmacia Biotech). The inositol 1,4,5-trisphosphate was identified as a species that co-eluted with myo-[3H]inositol 1,4,5-trisphosphate (Amersham Pharmacia Biotech).
Microscopy
For confocal microscopy, cells were examined using an inverted microscope (Diaphot TE300, Nikon, Tokyo) interfaced with a laser scanning confocal microscope system (MRC 1024, Bio-Rad, Hercules, CA) in the Keller mount position. A krypton/argon laser producing a 488 nm laser line and a bandpass filter (522/32) were used for fluorophore excitation and emission wavelengths isolation, respectively. Images were collected using acquisition software (Laser Sharp, Bio-Rad).
Ratiometric imaging was done on a microscope (Diaphot, Nikon) using a 10× fluorescence objective. Image-1 software (Universal Imaging, West Chester, PA) was used to collect 30 data points for each of the samples. Excitation was alternated between 340 and 380 nm and emission was collected at 510 nm.
Detection of Calcium Mobilization
Arabidopsis plants were grown in a modified hydroponic system using a 20-10-20 (N-P-K) solution of “Peat-Lite Special” fertilizer. In 7- to 10-d-old plants, cortical and/or epidermal cells in the transitional region between the zones of elongation and division were selected for imaging. Their metabolic activity and proximity to the surrounding environment make them viable candidates to sense and respond to osmotic stress. The fluorescent calcium indicator Fluo-3 AM (Molecular Probes, Eugene, OR) was used in this study to determine intracellular calcium concentrations in root tip cells.
For confocal imaging, plants were transferred from their growth media to 0.3 mL of loading solution consisting of 20 μm Fluo-3 AM in 200 μm CaCl2 and were incubated at 4°C for 2 h. Plants were then washed in a 200 μm CaCl2 solution for 2 h at 22°C in the dark (Zhang et al., 1998). For imaging, plants were transferred from the wash solution to 50-mm Petri dishes with 22-mm coverslips mounted over centrally located openings, and were bathed in 0.3 mL of the 200 μm CaCl2 solution. For each data set, 30 images were collected of the same optical section at intervals of 60 s (total time of 30 min). After 3 min (t = 0), 100 μL of 1 m NaCl was added to the bath solution to create a final NaCl concentration of 0.25 m. Data acquisition settings on the confocal microscope were standardized to quantify calcium fluxes within cells using pixel intensity standard curves (data not shown) created utilizing a calcium calibration kit (Molecular Probes) of buffered solutions. Pixel intensities were determined for each concentration of calcium as well as photobleaching rates for Fluo-3 (data not shown) for a 47- × 47-μm area. In addition, the PLC inhibitor U-73122 (Calbiochem, La Jolla, CA) was used to determine whether the observed calcium flux was due to PtdIns(4,5)P2 hydrolysis to IP3. For these experiments, plants were prepared as above with the addition of 1 μm U-73122 for the final 15 min of incubation prior to treatment with NaCl.
For ratiometric imaging, an acid-loading protocol described by Legué et al. (1997) was used. Plants were transferred from the growth media to a Petri dish containing 1 mL of acetate buffer (pH 4.5) containing 25 μm Fura-2 (Molecular Probes) and 200 μm CaCl2 and were incubated in the dark for 60 min at 22°C. Following incubation with the Fura-2, plants were transferred to a Petri dish and were washed twice in the dark in a buffer containing 2 mL of buffer solution containing 10 mm MES [2-(N-morpholino)-ethanesulfonic acid], 50 mm KCl, and 1 mm CaCl2 (pH 6.15) for 30 min at 22°C. For the stressed plants NaCl was added, resulting in a final concentration of 0.25 m for 10 min. Immediately following washing, plants were transferred to a 50-mm Petri dish with a 20-mm hole cut and were covered with a 22-mm coverslip for imaging with an inverted microscope. One hundred microliters of the MES buffer was added and the root was covered with a 15-mm filter paper disc to avoid drying. Plants treated with the calcium ionophore had 10 μm 4-Bromo A-23187 added for 10 min and were then transferred to a Petri dish modified for imaging as described above. Control plants were treated identically to those described above with the exception of NaCl or 4-Bromo A-23187 addition.
Footnotes
This study was supported in part by the U.S. Department of Agriculture (grant no. 1999–01871 to D.B.D. and H.H.), by the American Cancer Society (grant no. RPG–00–126–01–TBE to D.B.D.), by the U.S. National Institutes of Health (grant no. NS29632 to G.D.P.), and by the Utah Agricultural Experiment Station. This is Utah Agricultural Experiment Station paper no. 7,358.
LITERATURE CITED
- Alexandre J, Lassalles JP, Kado RT. Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5 trisphosphate. Nature. 1990;343:567–570. [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Brearley CA, Parmar PN, Hanke DE. Metabolic evidence for PtdIns(4,5)P2-directed phospholipase C in permeabilized plant protoplasts. Biochem J. 1997;324:123–131. doi: 10.1042/bj3240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, Watkins PAC, Beven AF, Shaw PJ, Hernandez LE, Lomonossoff GP, Shanks M, Peart J, Drøbak BK. Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell. 2000;12:1679–1687. doi: 10.1105/tpc.12.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MH, Shears SB, Boss WF. Changes in phosphatidylinositol metabolism in response to hyperosmotic stress in Daucus carota L. cells grown in suspension culture. Plant Physiol. 1993;103:637–647. doi: 10.1104/pp.103.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, D'Arrigo A, Stenmark H. Phosphoinositides in membrane traffic. Curr Opin Cell Biol. 1999;11:460–465. doi: 10.1016/S0955-0674(99)80066-0. [DOI] [PubMed] [Google Scholar]
- Coursol S, Giglioli-Guivarc' HN, Vidal J, Pierre JN. An increase in phosphoinositide-specific phospholipase C activity precedes induction of C4 phosphoenolpyruvate carboxylase phosphorylation in illuminated and NH4Cl-treated protoplasts from Digitaria sanguinalis. Plant J. 2000;23:497–506. doi: 10.1046/j.1365-313x.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- Drøbak BK, Dewey RE, Boss WF. Phosphoinositide kinases and the synthesis of polyphosphoinositides in higher plant cells. Int Rev Cytol. 1999;189:95–130. doi: 10.1016/s0074-7696(08)61386-8. [DOI] [PubMed] [Google Scholar]
- Einspahr KJ, Peeler TC, Thompson GA., Jr Rapid changes in polyphosphoinositide metabolism associated with the response of Dunaliella salina to hypoosmotic shock. J Biol Chem. 1988;263:5775–5779. [PubMed] [Google Scholar]
- Franklin-Tong VE, Drøbak BK, Allan AC, Watkins PAC, Trewavas AJ. Growth of pollen tubes of Papaver rheoas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-triphosphate. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S. Signal transduction in barley aleurone protoplasts is calcium dependent and independent. Plant Cell. 1996;8:2193–2209. doi: 10.1105/tpc.8.12.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Hama H, Takemoto JY, DeWald DB. Analysis of phosphoinositides in protein trafficking. Methods. 2000;20:465–473. doi: 10.1006/meth.2000.0959. [DOI] [PubMed] [Google Scholar]
- Helm PJ, Patwardhan A, Manders EMM. A study of the precision of confocal, ratiometric, Fura-2-based [Ca2+] measurements. Cell Calcium. 1997;22:287–298. doi: 10.1016/s0143-4160(97)90067-1. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. A gene encoding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Verma DPS. A phosphatidylinositol 3-kinase is induced during soybean nodule organogenesis and is associated with membrane proliferation. Proc Natl Acad Sci USA. 1994;91:9617–9621. doi: 10.1073/pnas.91.20.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 2000;23:267–278. doi: 10.1046/j.1365-313x.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- Knight H, Brandt S, Knight MR. A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J. 1998;16:681–687. doi: 10.1046/j.1365-313x.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Polito VS, Läuchli A. Salinity stress increases cytoplasmic Ca activity in maize root protoplasts. Plant Physiol. 1989;90:1271–1274. doi: 10.1104/pp.90.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhó R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/tpc.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TFJ. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer HJG, Divecha N, van den Ende H, Musgrave A, Munnik T. Hyperosmotic stress induces rapid synthesis of phosphatidyl-D-inositol 3,5-bisphosphate in plant cells. Planta. 1999;208:294–298. [Google Scholar]
- Mikami K, Katagiri T, Iuchi S, Yamaguchi-Shinozaki K, Shinozaki K. A gene encoding phosphatidylinositol-4-phosphate 5-kinase is induced by water stress and abscisic acid in Arabidopsis thaliana. Plant J. 1998;15:563–568. doi: 10.1046/j.1365-313x.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signalling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Odom AR, Stahlberg A, Wente SR, York JD. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci. 2000;25:229–235. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pical C, Westergren T, Dove SK, Larsson C, Sommarin M. Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J Biol Chem. 1999;274:38232–38240. doi: 10.1074/jbc.274.53.38232. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- Serunian LA, Auger KR, Cantley LC. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]
- Staxén I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci U S A. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Boss WF. A phosphatidylinositol 4-kinase pleckstrin homology domain that binds phosphatidylinositol 4-monophosphate. J Biol Chem. 1998;273:22761–22767. doi: 10.1074/jbc.273.35.22761. [DOI] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF. Inositol signaling and plant growth. Trends Plant Sci. 2000;5:357–363. doi: 10.1016/s1360-1385(00)01739-8. [DOI] [PubMed] [Google Scholar]
- Trewavas AJ. Le calcium, c'est la vie: calcium makes waves. Plant Physiol. 1999;120:1–6. doi: 10.1104/pp.120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Malhó R. Ca2+ signalling in plant cells: the big network! Curr Opin Plant Biol. 1998;1:428–433. doi: 10.1016/s1369-5266(98)80268-9. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Poenie M. Fluorescence ratio imaging: a new window into intracellular ionic signaling. Trends Biochem Sci. 1986;11:450–455. [Google Scholar]
- Van der Kaay J, Beck M, Gray A, Downes CP. Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J Biol Chem. 1999;274:35963–35968. doi: 10.1074/jbc.274.50.35963. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Welters P, Takegawa K, Emr SD, Chrispeels MJ. AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc Natl Acad Sci USA. 1994;91:11398–11402. doi: 10.1073/pnas.91.24.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue HW, Pical C, Brearley C, Elge S, Müller-Röber B. A plant 126-kDa phosphatidylinositol 4-kinase with a novel repeat structure: cloning and functional expression in baculovirus-infected insect cells. J Biol Chem. 1999;274:5738–5745. doi: 10.1074/jbc.274.9.5738. [DOI] [PubMed] [Google Scholar]
- York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Rengel Z, Kuo J. Determination of intracellular Ca+2 in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo-3 under low temperature. Plant J. 1998;15:147–151. [Google Scholar]
- Zheng L, Krsmanovic LZ, Vergara LA, Catt KJ, Stojilkovic SS. Dependence of intracellular signaling and neurosecretion on phospholipase D activation in immortalized gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA. 1997;94:1573–1578. doi: 10.1073/pnas.94.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]