Abstract
The protein, Nuclear factor-E2-related factor 2 (Nrf2), is a transitory protein that acts as a transcription factor and is involved in the regulation of many cytoprotective genes linked to xenobiotic metabolism and antioxidant responses. Based on the existing clinical and experimental data, it can be inferred that neurodegenerative diseases are characterized by an excessive presence of markers of oxidative stress (OS) and a reduced presence of antioxidant defense systems in both the brain and peripheral tissues. The presence of imbalances in the homeostasis between oxidants and antioxidants has been recognized as a substantial factor in the pathogenesis of neurodegenerative disorders. The dysregulations include several cellular processes such as mitochondrial failure, protein misfolding, and neuroinflammation. These dysregulations all contribute to the disruption of proteostasis in neuronal cells, leading to their eventual mortality. A noteworthy component of Nrf2, as shown by recent research undertaken over the last decade, is to its role in the development of resistance to OS. Nrf2 plays a pivotal role in regulating systems that defend against OS. Extant research offers substantiation for the protective and defensive roles of Nrf2 in the context of neurodegenerative diseases. The purpose of this study is to provide a comprehensive analysis of the influence of Nrf2 on OS and its function in regulating antioxidant defense systems within the realm of neurodegenerative diseases. Furthermore, we evaluate the most recent academic inquiries and empirical evidence about the beneficial and potential role of certain Nrf2 activator compounds within the realm of therapeutic interventions.
Keywords: Nrf2, oxidative stress, antioxidant, neurodegenerative disorders, Nrf2 activation
Introduction
The neurotoxic byproducts of the major reactive oxygen species (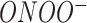 ) degradation are HO●,
) degradation are HO●, 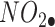 , and
, and  . In the context of treating neurodegenerative diseases, therapeutic interventions may be directed against several phases involved in the production of OS and the disruption of endogenous cellular defense mechanisms in neuronal cells. 1 The transcriptional factor known as Nrf2 has a significant function in cellular defense against OS by facilitating the production of genes that provide cytoprotective effects. Moreover, it has an impact on the maturation process of blood cells and the functionality of drug-metabolizing enzymes.2,3 Proteins such as heme oxygenase-1, SOD1, Catalase (CAT), and enzymes involved in glutathione metabolism show an increase in expression. The protein Nrf2, which is located in the cytoplasm, interacts with the protein Keap1 and has a degradation half-life of 20 min.4,5 The Keap1 protein is accountable for the generation of Nrf2 when exposed to stress. This Nrf2 protein then combines with MAF proteins to create a heterodimeric complex.6 The interaction between Nrf2-MAF and the antioxidant response element (ARE) leads to the synthesis of many cytoprotective genes, including heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1 (NQO1), superoxide dismutase, glutathione cysteine ligase, glutathione S-transferases(GSTs), and CAT. These genes are of paramount importance in safeguarding against oxidative damage.7
. In the context of treating neurodegenerative diseases, therapeutic interventions may be directed against several phases involved in the production of OS and the disruption of endogenous cellular defense mechanisms in neuronal cells. 1 The transcriptional factor known as Nrf2 has a significant function in cellular defense against OS by facilitating the production of genes that provide cytoprotective effects. Moreover, it has an impact on the maturation process of blood cells and the functionality of drug-metabolizing enzymes.2,3 Proteins such as heme oxygenase-1, SOD1, Catalase (CAT), and enzymes involved in glutathione metabolism show an increase in expression. The protein Nrf2, which is located in the cytoplasm, interacts with the protein Keap1 and has a degradation half-life of 20 min.4,5 The Keap1 protein is accountable for the generation of Nrf2 when exposed to stress. This Nrf2 protein then combines with MAF proteins to create a heterodimeric complex.6 The interaction between Nrf2-MAF and the antioxidant response element (ARE) leads to the synthesis of many cytoprotective genes, including heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1 (NQO1), superoxide dismutase, glutathione cysteine ligase, glutathione S-transferases(GSTs), and CAT. These genes are of paramount importance in safeguarding against oxidative damage.7
The following discussion pertains to the substantiation surrounding the regulation of intermediate metabolism and mitochondrial function, as well as the rationale behind considering Nrf2 activation as a potential therapeutic approach for addressing neurodegenerative ailments.8 The Nrf2 pathway is a promising avenue for intervention as a transcriptional antioxidant and cytoprotective mechanism, with the ability to mitigate OS, a recognized pivotal contributor to the pathogenesis of neurodegenerative disorders.9 Novel therapeutic strategies have been identified that effectively target neuroinflammation and oxidative damage. When devising techniques for neuroprotection, it is important to consider the distinct functions of neuronal and non-neuronal cell types in influencing tissue oxidative status, as well as the cell types that have the highest inherent capability for synthesizing antioxidant enzymes.10
Oxidative stress in nerve damage
In order to protect the brain from oxidative stress-related damage, redox homeostasis is essential. Due to its abundance in unsaturated lipids like Fe2+ or Cu+, low antioxidant levels, high OS generation by neurons and microglia, and susceptibility to OS, the brain is a favourable habitat for LP and ferroptosis.11
Iron-dependent, oxidative cell death known as ferroptosis has a severe negative influence on the brain and neurological diseases. It is dependent on excessive iron buildup, a key element in LP.12 Excess iron buildup causes neurodegenerative illnesses by generating OS, impairing mitochondrial function, producing ROS, and damaging DNA. NRF2 and BACH1, which promote or suppress gene expression in the ferroptosis pathways, coordinate ferroptosis.13
The process of ferroptosis, which is an early sign of Alzheimer's disease, makes amyloid peptide and tau clumping worse, which helps Alzheimer's start.14 Recent research has shown that the BACH1/NRF2 ratio has the ability to modulate antioxidant defense mechanisms, potentially leading to the activation of neuroprotective characteristics.15 The triplication of the BACH1 gene is a characteristic of Down syndrome (DS), a genetic disease that may lead to heightened OS if there is impairment in the NRF2 gene.16 Excessive production of BACH1 results in an alteration of the BACH1/NRF2 ratio, hence impeding the activation of genes associated with the antioxidant response and leading to an elevation in oxidative damage. This observation indicates that the triplication of BACH1 in individuals with DS has an impact on the maintenance of redox homeostasis. There exists a correlation between ferroptosis and the occurrence of neurotoxicity as well as traumatic brain injury.17,18
The possible pharmaceutical target for neurotoxicity and brain damage has been discovered via research as ferroptosis. As phospholipid hydroperoxides from polyunsaturated fatty acids (PUFA) rise to deadly amounts during ferroptosis, membrane rupture and damage result. This iron-dependent mechanism may cause dementia, autoimmune disorders, and Pelizaeus-Merzbacher Disease, a rare inherited neurological condition.19
Neurotransmitters such as dopamine, which undergo metabolic processes involving monoamine oxidases (MAO), result in the production of hydrogen peroxide, hence contributing to OS.20 The cerebral cortex, hippocampus, and striatum are considered to be the parts of the brain that exhibit a higher susceptibility to damage or dysfunction. Misfolded proteins and decreased mitochondrial function are the primary factors responsible for the production of ROS in neurodegenerative diseases.21
OS and ROS have a substantial influence on neurodegenerative diseases, as the age-related increase in OS contributes to the development of pathological changes.18,21 In order to maintain redox equilibrium, the antioxidant defense system, referred to as Nrf2, governs both the function and generation of mitochondria. Hence, Nrf2 presents itself as an attractive therapeutic target in both pre-clinical and clinical trials.22 Furthermore, it is worth mentioning that Nrf2 has anti-inflammatory properties, emphasizing the significance of its involvement in neurodegenerative disorders.23 Neuroinflammation pertains to the inflammatory reaction of the central nervous system (CNS) in response to noxious stimuli.24 The inflammasome, a multi-protein complex, is activated by the recognition of several receptors, including nucleotide-binding domain and leucine-rich repeat-containing receptors.25 As a result, the subsequent release of pro-inflammatory cytokines occurs. The redox equilibrium is influenced by neuroinflammation or disrupted mitochondrial function, however the precise mechanism behind the inflammatory response in humans remains unidentified.25,26
Structure of Nrf2
The pleiotropic transcription factor Nrf2, consisting of 605 amino acid residues, has the potential to modulate the expression of related genes and initiate a protective response against oxidative stress and inflammatory harm.27 Nrf2 consists of seven distinct functional domains, namely Neh1 to Neh7, as seen in (Fig. 1).28 Distinct molecular pathways enhance the nuclear translocation of Nrf2 and its subsequent DNA binding. The mechanisms in question are significantly influenced by two key components situated within the Neh1 domain, namely the nuclear localization signal (NLS) and the cap'n'collar basic-region leucine zipper (BZIP) domain.7,29 The Neh2 domain and Keap1 possess two binding sites that engage in interactions, resulting in the formation of a homodimeric structure. The binding between the chromo-ATPase/helicase protein CHD6 and Neh3 takes place through the transactivation domain of CHD6.30 The link between Neh4 and Neh5 mediates the enhancement of Nrf2 transcriptional activation by the activator cyclic adenosine monophosphate (AMP) response element binding protein.5,31 Moreover, previous studies have shown that Neh4 and Neh5 had the capability to bind with the nuclear cofactor RAC3/AIB1/SRC-3, hence enhancing the transcription of genes linked to the ARE that selectively modulate Nrf2. The possible inhibition of Nrf2 activity may occur via the binding of the Neh7 domain to the retinoic X receptor alpha (RXRa).32
Fig. 1.

The structural design of Nrf2's domain. Nrf2 protein has seven Nehl–Neh7 domains. The ETGE and DLG motifs are essential for the direct interaction between the Neh2 domain and the Kelch domain of Keap1.
Nrf2 signaling pathway
The protein Nrf2 is transient in nature, experiencing ongoing ubiquitination and being specifically marked for destruction by the proteasome.33 Sophisticated methodologies are used to stimulate the activation of the aforementioned entity in response to oxidative or electrophilic assaults. The activity of Nrf2 is modulated by its interactions with Keap1, a negative regulator, along with other proteins. The subcellular distribution and functional significance of Nrf2, together with its participation in the gene network controlled by the ARE, are modulated by several kinases, including those accountable for phosphorylation and ubiquitination.34,35 The Nrf2 protein is of significant importance in mediating the removal of xenobiotics and mitigating oxidative stress inside the human organism. The transcription factor has a fundamental leucine zipper (bZIP) motif, which is a distinguishing feature of the "cap'n'collar" subfamily (CNC), hence enabling its interaction with deoxyribonucleic acid (DNA). The protein under investigation has a modular architecture, with seven Neh 1–7 domains often known as Nrf–ECH homology domains.36 The activation of Nrf2 has been linked to several physiological responses, such as heightened resistance to infections, augmented resistance of tumors to chemotherapy, and, as emphasized in this study, greater protection against neurodegenerative illnesses.37
Under typical circumstances, Keap-1 exerts inhibitory control on Nrf2. The Cul3/Rbx1 E3 ubiquitin ligase complex is recruited by Keap-1 via its interaction with the DLG and ETGE motifs present in the Neh2 domain.38 The aforementioned mechanism results in the ubiquitination of the lysine residues of Nrf2, consequently leading to its destruction inside the proteasome. Nevertheless, when exposed to OS, the cysteine residues of Keap-1 undergo oxidation, resulting in the release of Nrf2 and subsequent increase in the protein's intracellular levels.39 Upon translocating into the nucleus, the unbound Nrf2 protein interacts with sMaf (small muscular aponeurosis fibromatosis) to form a complex that associates with cis-regulatory elements, namely AREs, of select target genes. The aforementioned association takes place only within the nucleotide sequence 50-TGACXXXGC-30. The transcriptional regulation of many enzymes involved in scavenging ROS, including as glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), SOD, and NQO-1, is mediated by the Nrf2-sMaf heterodimer.32,40 Multiple strategies exist for controlling the route, as seen in (Fig. 2).41
Fig. 2.

Regulation of Nrf2. The (1) p62, (2) PI3K/Akt/GSK-3, or (3) RXR pathways may all have an impact on the Nrf2 pathway. Oxidative stress cleaves Nrf2 and Keap-1, preventing Nrf2 from being broken down in the proteasome and enabling it to reach the nucleus, where it forms a heterodimer with sMaf and activates the production of ARE genes, including HO-1, NQO-1, SOD, and GPx.
The protein p62, also known as sequestosome 1, experiences phosphorylation at Ser-351 and plays a role in several physiological mechanisms, including autophagy and the response to oxidative stress. After undergoing phosphorylation, p62 has a significant binding preference for Keap-1, hence hindering the process of ubiquitination and subsequent destruction of Nrf2.41,42
GSK-3, or glycogen synthase kinase-3, is a serine/threonine kinase that is involved in the modulation of Nrf2 activity by the phosphorylation of serine residues located within the Neh6 domain. Consequently, GSK-3 has a suppressive effect on Nrf2 function.43 The E3 adaptor ligase TrCP, sometimes referred to as transducin repeat-containing protein, has a specific binding preference for phosphorylated residue.44 The formation of a complex with Cul3/Rbx results in the ubiquitination of Nrf2 and subsequent destruction. The activation of the PI3K/Akt pathway has the ability to inhibit GSK-3, hence impeding the phosphorylation of Nrf2. In a similar manner, it is noteworthy to acknowledge that the activation of PI3K/Akt may be facilitated by other entities, including ion channels, growth factors, and ligands that bind to G-protein coupled receptors.45
The Retinoid X receptor (RXR) collaborates with the Neh7 domain of Nrf2 to suppress the transcription of genes linked to decreased OS.46
Nrf2–Keap1 pathway
The protein Nrf2, which has 605 amino acids, is sometimes denoted as Neh (N2-erythroid-derived Cap 'n' Collar homology). The entity is distinguished by the existence of seven discrete functional domains.47 The Neh1 domain encompasses the binding area for the leucine zipper motif and the requisite sequence for heterodimerization with tiny Maf transcription co-activators.47,48 The Keap1V homodimer is bound by the Neh2 domain, hence facilitating the ubiquitination of Nrf2 and subsequent destruction mediated by the 26S proteasome. The activation of Neh2 necessitates the existence of the third DIDLID motif, which functions as an attractant for a ubiquitin ligase.49 The protein known as chromodomain helicase DNA-binding domain protein 6 (CHD6) has a strong binding affinity towards the Neh3 domain, which functions as a transcriptional co-activator situated in the C-terminal region.50 The facilitation of the recruitment of the CREB-binding protein (CBP) and/or repressor-associated coactivator (RAC) is achieved by the collective functioning of the Neh4 and Neh5 domains, as seen in (Fig. 1).51 The process of Nrf2 degradation occurs in a manner that is not reliant on Keap1, and is instead assisted by the -transducin repeat-containing protein (-TrCP). This protein forms a complex with the S-phase kinase-associated protein 1 (Skip1)-Cul1-Rbx1 E3 ubiquitin ligase.52 The inhibition of Nrf2 by Retinoid X (RXRs) and retinoic acid (RARs) receptors takes place via the contact with the Neh7 domain, hence impeding the binding of transcription co-activators to the Neh4 and Neh5 domains. The Keap1-Cul3-Rbx1 E3 ubiquitin ligase is the principal mechanism responsible for regulating Nrf2, a transcription factor that is activated by electrophiles or ROS. The precise chemical mechanism behind the evasion of the Keap1 regulatory pathway by Nrf2 has yet to be elucidated.53,54 A notable technique is the modification of a particular cysteine residue inside the Keap1 protein, leading to its separation from Keap1. According to the existing hypothesis, the dissociation mechanism of the Keap1 hinge and latch occurs when Nrf2 binds to the Keap1 homodimer. This process is assisted by the presence of a high-affinity ETGE motif, which functions as the "hinge," and a low-affinity DLG motif, which acts as the "latch". The interaction between ubiquitin and Nrf2 may disrupt the binding location of the weak latch, without causing dissociation of Keap1.30,32 The process of Keap1 ubiquitinylation has the potential to induce activation of Nrf2 by facilitating self-ubiquitination via modification of a cysteine residue in Keap1.55 Cys151 is a pivotal cysteine residue within the Keap1 protein, functioning as a sensor and subject to covalent alteration by interaction with electrophilic ligands or ROS. The amino acids Cys-273 and Cys-288 inside the Keap1 protein play a crucial role in preserving its structural integrity and functional capabilities, hence facilitating the maintenance of its ubiquitin ligase activity.56 The aforementioned alterations induce a structural alteration in Keap1, resulting in a decrease in Nrf2's effectiveness of ubiquitination, inhibition of UPS-mediated degradation, and subsequent elevation of Nrf2 protein levels. The Nrf2 protein, which has been produced lately, has enhanced stoichiometric qualities that enable it to accumulate, translocate to the nucleus, and begin the transcriptional activation of certain target genes.57 The nuclear Keap1 protein plays a role in promoting the export of Nrf2 from the nucleus under conditions when cellular homeostasis is restored. This action successfully puts an end to the process of Keap1-mediated ubiquitinylation and subsequent destruction in the cytoplasm.58
The role of Nrf2 in oxidative stress and neuroinflammation
ROS has the capability to promote several physiological activities when found at low concentrations. Nevertheless, increased concentrations of ROS might potentially result in the initiation of oxidative stress, a complex state closely linked to the emergence and advancement of several pathological mechanisms.59 Neurodegeneration arises due to an imbalance between the production of ROS and the safeguarding mechanisms offered by antioxidant defenses, also known as oxidative stress. The Nrf2 pathway plays a crucial role in the regulation of many anti-oxidation systems, including drug transport, glutathione synthesis, ROS elimination, and drug detoxification.60 Furthermore, it assumes a pivotal function in the activation of cytoprotective genes, hence functioning as a basic component in cellular defense mechanisms against oxidative stress. The existing disparity has the capacity to induce harm to cellular structures and molecular components.61,62 The demise of neurons is triggered by the interplay between ROS and unsaturated fatty acids, namely linoleic acid and arachidonic acid, in the course of oxidative O2 metabolism. The reactive peroxy radicals stated above are specific to certain neurodegenerative diseases (NDDs) and serve as initiators for chain reactions that facilitate the production of ROS.63 The detection of heightened levels of 8-hydroxyguanine and 8-hydroxy2 deoxyguanosine inside the brain tissue of persons afflicted with PD suggests that DNA is susceptible to oxidative harm.64
The formation of crosslinks with cysteine, lysine, and histidine residues by ROS leads to toxicity and DNA alterations. The modifications inflicted upon enzymes and receptors result in their impairment, leading to the cessation of metabolic activities.65 The presence of heightened intracellular calcium levels, protein misfolding and aggregation, compromised functioning of complexes including voltage-dependent calcium channels (VDCC) and N-methyl-D-aspartate (NMDA) receptors, excitotoxicity, and several other adverse effects are also elicited.66 The initiation of oxidative stress promotes the activation of redox-sensitive pathways, resulting in the sustained activation of M1 microglia. Cells have evolved many detoxification strategies to maintain cellular redox equilibrium.67 These processes include the promotion of transcription for phase I enzymes, such as cytochrome P450s, as well as phase II enzymes, which are responsible for detoxification and antioxidant functions.68 As mentioned before, NRF2 plays a crucial role in the regulation of cellular redox homeostasis. The aforementioned process has promising potential in mitigating oxidative or electrophilic stress.69
While inflammation is essential for tissue repair and the body's immunological response to infections and malignancies, it may also have detrimental consequences and substantially contribute to the progression of neurodegenerative illnesses.70 Neuroinflammation is initiated when microglial cells undergo a shift from a dormant state to an activated one via either the M1 or M2 activation pathways. Tissue damage arises as a result of the activation of ROS generation and the secretion of proinflammatory cytokines, including interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), by the proinflammatory M1 phenotype.71,72 The tissue regeneration mechanisms linked to the neuroprotective M2 state facilitate the production of anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13.73
The anti-inflammatory properties of NRF2 activation have been shown by the reduction of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, and nitric oxide synthase (iNOS), in microglia and astrocytes.74 Previous studies have shown that the activation of NRF2 has the capacity to reduce microgliosis, astrogliosis, and the production of proinflammatory cytokines TNF-α and IL-17A in animal models of AD with APP/PSEN1 mutations.75 Moreover, previous studies have shown that patients with HD have reduced inflammatory responses in blood monocytes after pharmaceutical intervention aimed at augmenting NRF2 activity.76
The interplay between antioxidant and anti-inflammatory pathways results in the prioritization of NRF2's antioxidant benefits above its anti-inflammatory and mitochondrial activities.77 The activation of the well-known proinflammatory transcription factor NFkB is triggered by oxidative stress. However, this activation may be inhibited by the NRF2-dependent activation of antioxidant target genes. This process effectively decreases the production of proinflammatory cytokines.78 The NRF2 transcription factor exerts direct regulatory control on the production of many anti-inflammatory mediators, including interleukin-17D, CD36, macrophage receptor with collagenous structure, and G protein-coupled receptor kinase. The NRF2 protein has also been linked to a decrease in the synthesis of proinflammatory cytokines such as TNF-α, IL-6, IL-8, and IL-1β in microglia, macrophages, monocytes, and astrocytes.79,80
NRF2 activators
The activation of endogenous NRF2 may be induced by an elevation in oxidative stress, which can also be caused by external chemicals. A wide range of plant-derived and synthetic compounds have been shown to successfully stimulate the NRF2 pathway.60,81 A list of activators of NRF2 in different phases of clinical development is provided in Table 1.
Table 1.
Selected activators of NRF2.
| Compound | sources | Mechanism of action |
|---|---|---|
| Sulforaphane | Cruciferous vegetables | Stimulate the activation of NRF2 in the hippocampus |
| Lycopene | various plant species including tomatoes, papayas, and watermelons | enhancing antioxidant enzyme activity, reducing inflammation, and alleviating mitochondrial dysfunction in cortical neurons |
| Curcumin | Curcuma longa | NRF2 activation in microglial cells. By preventing NFkB activation and inhibiting KEAP1 expression, it is possible. |
| Green tea | Camellia sinensis | The activation of NRF2 is accompanied by a high concentration of epigallocatechin gallate (EGCG). It is facilitated by the mechanisms of electrophilic disruption and phosphorylation, leading to an increase in its functional capacity. |
| Polyphenol resveratrol | Various fruits | The activation of NRF2. It is linked to the control of mitochondrial biogenesis and the expression of robust anti-inflammatory and antioxidant properties. |
| Alpha-Lipoic acid (ALA) | Several plants such as spinach, broccoli, carrots, and beets | Exhibits NRF2 activation and has neuroprotective properties. It's possible that when KEAP1 and NRF2 interact, lipoyl-cysteinyl mixed disulfides are made. These mess up the binding of NRF2 to its helper protein. |
| Centella asiatica | C. asiatica plants | Possession of NRF2-activating chemicals, including Asiatic acid, madecassic acid, asiaticoside, and madecassoside. These chemicals elicit the activation of NRF2 in several animal models, including both the ageing process and AD. |
| Tertiary butylhydroquinone (tBHQ) | It is a synthetic aromatic organic compound which is a type of phenol. | Disrupts the KEAP1/NRF2 complex, affecting oxidative stress-related physiological processes. |
| Metformin | It is a pharmaceutical agent | Initiate the activation of the NRF2 pathway by stimulating the AMP-activated protein kinase (AMPK). |
Sulforaphane, an isothiocyanate compound that occurs naturally in cruciferous vegetables, has been shown to induce the activation of NRF2 in the hippocampus, resulting in an increase in the synthesis of antioxidant enzymes. The chemical hinders the activation of NFkB induced by TNFa and modifies the behavior of mitochondria via pathways that depend on NRF2, as well as pathways that do not rely on NRF2. The aforementioned effects have been shown to provide neuroprotective advantages in several illnesses, including stroke, traumatic brain injury, AD, PD, HD, and MS.82
Lycopene, a carotenoid found in various plant species including tomatoes, papayas, and watermelons, has demonstrated the ability to augment the function of antioxidant enzymes, alleviate inflammatory reactions, and reduce the concentrations of pro-inflammatory cytokines and mitochondrial dysfunction in cortical neurons.83 Previous studies have shown that lycopene has the ability to alleviate indicators of oxidative stress, neuronal cell death, disruption of the blood–brain barrier (BBB), and neurological impairments in experimental models of subarachnoid hemorrhage.84
Curcumin, a polyphenolic molecule obtained from the botanical source Curcuma longa, has significant anti-inflammatory and antioxidant characteristics.85 The activation of NRF2 is accomplished by the suppression of KEAP1 expression and the prevention of NFkB activation in microglial cells.85,86 Numerous studies have shown that curcumin had the capacity to reduce the expression of proinflammatory genes and alleviate cerebral edema in experimental models of cerebral ischemia and reperfusion. The literature has shown that the activation of NRF2 confers neuroprotective benefits in experimental models of traumatic brain injury (TBI) and intracerebral hemorrhage.87
The activation of NRF2 by green tea, which has a high concentration of epigallocatechin gallate (EGCG), is facilitated by the mechanism of electrophilic disruption and phosphorylation, leading to an increase in its functional capacity.88 The compound known as epigallocatechin gallate (EGCG) has been shown to have the capacity to inhibit the activity of nuclear factor kappa B (NFκB), diminish the production of amyloid-beta (Ab) fibrils, and enhance memory performance in mice.89 The experimental models used to study PD, MS, and traumatic brain injury (TBI) have provided evidence supporting the neuroprotective benefits linked to heightened NRF2 activity, augmented antioxidant activity, and diminished inflammatory responses.90
The activation of the NRF2 pathway via the phosphorylation of p38MAPK has been shown by the presence of the bioactive polyphenol resveratrol in various fruits. The activation of NRF2 is linked to the control of mitochondrial biogenesis, along with the expression of robust anti-inflammatory and antioxidant properties.91 The observed protective benefits in a rotenone model of PD might perhaps be related to the activation of NRF2 and its impact on mitochondrial activity.92 The injection of resveratrol has been shown to attenuate oxidative stress and provide protection against ischemia damage in animal models. The potential of upregulating the pathway to attenuate cognitive deficits caused by traumatic brain injury in mice, as well as relieve cellular and mitochondrial damage in a drosophila model of spinocerebellar ataxia, has been seen.93,94
Alpha-Lipoic acid (ALA), a naturally occurring compound that exhibits NRF2 activation and has neuroprotective properties, may be sourced from several plants such as spinach, broccoli, carrots, and beets.95 The potential outcome of the interaction between KEAP1 and NRF2 is the generation of lipoyl-cysteinyl mixed disulfides, which subsequently impede the binding of NRF2 to its chaperone.96 Prior research has shown the effectiveness of ALA in reducing ROS, enhancing the formation of new mitochondria, restoring ATP levels, and protecting dopaminergic neurons. However, the current work has yet to examine the possible relationship between the anti-inflammatory properties of ALA in mice models of MS and the activation of NRF2.97
Centella asiatica, a botanical species renowned for its medicinal attributes, is recognized for its possession of NRF2-activating chemicals, including Asiatic acid, madecassic acid, asiaticoside, and madecassoside.98 These chemicals have been shown to elicit the activation of NRF2 in several animal models, including both the aging process and AD.99 The plant-derived aqueous extract has shown the ability to cause activation of NRF2 in neuroblastoma cells, primary neurons that have been isolated, and the brains of animals that have undergone therapy. The aforementioned stimulation results in improvements in mitochondrial activity, synaptic density, and cognitive performance.100 Moreover, the plant has antioxidants, anti-inflammatory, and cognitive-enhancing properties in the context of chemically induced neurotoxicity,101 stroke,99 seizure,102 PD100 and hypertension.98 The cognitive advantages seen in healthy aging persons after the administration of C. asiatica's water extract (CAW) are attributed to the activation of NRF2(1).
Tertiary butylhydroquinone (tBHQ) is a chemical compound with electrophilic characteristics, which enables it to disrupt the KEAP1/NRF2 complex, hence influencing the physiological processes associated with the response to oxidative stress.103 The compound exhibits properties of antioxidation and neuroprotection, which contribute to the mitigation of oxidative stress, protection against neuronal damage, and inhibition of amyloid-beta production in NT2N neurons.104 Research conducted on mouse models has shown the effectiveness of therapy in decreasing further injury, improving functional recovery, and ameliorating neurological impairment after intracerebral hemorrhage.105
Metformin, a pharmaceutical agent used for the management of type II diabetes via the reduction of blood glucose levels, has shown the ability to initiate the activation of NRF2 pathway by stimulating the AMP-activated protein kinase (AMPK).106 The activation of NRF2 has been shown to exhibit neuroprotective properties in neurodegenerative models and could provide safeguarding against oxidative stress-induced impairment of BBB.107 Moreover, empirical evidence has shown that this particular compound has an influence on both the function and generation of mitochondria.49,108 Furthermore, it has been shown that in murine models of ischemia injury, this substance has antioxidant and anti-inflammatory properties.109
Modulation of mitochondrial function through Nrf2 (the role of Nrf2 in modulating mitochondrial function)
The transcription factor is a regulatory protein that plays a crucial role in the process of gene expression by binding to certain DNA sequences and influencing the transcription of genetic information into RNA molecules the transcription factor Nrf2 plays a crucial function in cellular defense systems by modulating mitochondrial activity.110 The activation of Nrf2 functions to attenuate the production of ROS inside mitochondria, hence affording defense against toxicants originating from mitochondria. The efficacy of Nrf2 is compromised in pathological conditions related to mitochondrial dysfunction, such as AD, PD, and Friedreich's ataxia.111 Numerous studies have shown that a deficiency in Nrf2 leads to impaired mitochondrial fatty acid oxidation, respiration, and adenosine triphosphate (ATP) production. The use of chemical agents that stimulate the Nrf2 has shown the ability to augment the maintenance of both the structure and functionality of mitochondria.112 This is accomplished by the activation of mitophagy, a targeted mechanism for eliminating impaired mitochondria, or by suppressing the oxidative stress-triggered initiation of the mitochondrial permeability transition pore. Nrf2 protein exerts influence on the process of mitochondrial biogenesis, particularly under conditions of stress. The activation of Nrf2 leads to an increase in proteasomal activity, resulting in the enhanced degradation of the dynamin-related protein 1, a protein involved in mitochondrial fission.113 This pathway eventually facilitates the occurrence of mitochondrial hyperfusion. Previous studies have shown that the activation of Nrf2 leads to a reduction in the levels of Drp1, perhaps offering advantageous effects in the context of AD. The decrease in Drp1 has been linked to a decrease in phosphorylated Tau levels, as well as enhancements in mitochondrial dynamics and synaptic activity in mice expressing the Tau transgene (P301L).114,115
The administration of PNU282987 to primary glial cultures results in an increase in mitochondrial mass and oxygen consumption, even in the absence of oxidative stress.116 The aforementioned modifications were abolished in the absence of Nrf2, suppression of HO-1, or silencing of PGC-1a. The microglia had a significant augmentation in mitochondrial content, while the HO-1 mutant and PGC-1a-deficient strains displayed diminished levels.110 The results of this research suggest that the activation of a7 nicotinic acetylcholine receptors (nAChRs) is associated with an augmentation in the size of glial mitochondria. The aforementioned phenomenon is facilitated by Nrf2, subsequently inducing the upregulation of heme oxygenase-1 (HO-1) and peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1a).117
The findings from transcriptomic and metabolomic analyses of mutant Drosophila lacking the mitochondrial serine/threonine-protein kinase PTEN-induced putative kinase 1 (PINK1), which serves as a model for PD, indicate that PINK1 deficiency induces changes in nucleotide metabolism.110 These results suggest that augmenting nucleotide biosynthetic pathways may offer a potential approach to counteract mitochondrial dysfunction in PD.118 The activation of Nrf2 leads to an elevation in the flow of glucose via the pentose phosphate pathway and influences the metabolic processes of folate and glutamine. This activation, in addition to the upregulation of NADPH and GSH production as previously discussed, ultimately leads to an augmentation in purine biosynthesis.119 Therefore, the activation of Nrf2 may potentially cure mitochondrial dysfunction in cases with PINK1 deficiency by improving nucleotide production. The notion is substantiated by experimental evidence indicating that the pharmacological stimulation of Nrf2 in PINK1- deficient cells reinstates the mitochondrial membrane potential (Δwm) and provides defense against dopamine toxicity.120
The use of PNU282987 in primary glial cultures results in an increase in mitochondrial mass and oxygen consumption, even in the absence of oxidative stress. The aforementioned modifications were made ineffective in the absence of Nrf2, suppression of HO-1, or silencing of PGC-1a.121 The microglia had a significant increase in mitochondrial content, but the HO-1 mutant and PGC-1a-deficient strains showed reduced levels. The results of this research suggest that the activation of a7 nicotinic acetylcholine receptors (nAChRs) is associated with an augmentation in the size of glial mitochondria.122 The observed phenomenon is facilitated by the activation of Nrf2, subsequently inducing the upregulation of heme oxygenase-1 (HO-1) and peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1a).117,122
Modulation of Nrf2 signaling pathway by natural products in neuroprotection
The inherent activation of NRF2 occurs in response to elevated levels of oxidative stress, and it may also be induced by exogenous stressors.123 As mentioned earlier, a diverse range of compounds obtained from botanical sources as well as those synthesized in the laboratory have shown notable effectiveness in stimulating NRF2 pathway.123
Quercetin, a naturally occurring compound found in several fruits, vegetables, and tea, has antioxidant and anti-inflammatory properties that have been implicated in potential preventive effects against ailments such as diabetes, cardiovascular diseases, and neurodegenerative disorders.124 The Nrf2 or NF-κB signaling pathways are subject to regulation. The present study aimed to examine the effects of quercetin and isoquercetin, derived from dried leaves of Dendropanax morbifera, on the Nrf2 pathway. The protective effect against the production of ROS induced by glutamate in HT22 cells was seen using the methanolic extract obtained from the leaves.125,126
The compound hydroxytyrosol, which has strong antioxidant properties, has been shown to decrease the aggregation of A and tau proteins in Caenorhabditis elegans mutants.127 This compound is naturally found in the fruits and leaves of olive trees.128 Moreover, the extract prompted the relocation of skinhead-1, a transcription factor that has resemblance to Nrf2, to the nucleus, resulting in increased expression. This discovery suggests that hydroxytyrosol has the potential to function as a therapeutic intervention for neurodegenerative diseases.129
The study revealed that the methanolic extract derived from Korean black bean anthocyanins had the ability to enhance the expression of HO-1, GCLM, and Nrf2. The PI3K/Akt/GSK-3 pathway was shown to be responsible for reducing oxidative stress (OS) and activating Nrf2 in AD.130
Flavonoids are a class of secondary metabolites characterized by their phenolic composition, and they possess a wide range of biological actions that are advantageous for human health.131 The neuroprotective potential of kaempferol, a flavonoid compound, was investigated in primary mouse cortical neurons subjected to dietary stress. The study revealed that it has the capability to stimulate the upregulation of Nrf2, GPx4, and SLC7A1, which is a transporter responsible for the exchange of cysteine and glutamate.132 Nevertheless, with the addition of a Nrf2 inhibitor, the observed impact exhibited a reversal. The research posits that the activation of the kaempferol-induced Nrf2/SLC7A1/GP-4 pathway may be accountable for its observed protective effects.132,133
The compound known as tiliroside, which is a glycoside containing kaempferol, has been shown to increase the expression of nuclear Nrf2, HO-1, and NQO-1 in HT22 cells and BV2 microglia. This therefore leads to an augmentation of their antioxidant capacity.134
The flavonoid isoliquiritigenin, which is present in licorice root, had anti-inflammatory and antioxidant characteristics when tested on microglia BV2 cells that had been stimulated by Aldehyde Oxidase (AO).135 The observed outcome was accomplished by the augmentation of the Nrf2/HO-1 pathway activation and the inhibition of NF-κB. A reduction in the production of nitric oxide and proinflammatory cytokines, known to contribute to the initiation of neuronal damage in persons diagnosed with AD, was noted.136
Pinocembrin-7-methylether (PME), a chemical molecule, demonstrated neuroprotective characteristics in SH-SY5Y cells by successfully attenuating the neurotoxic consequences induced by 6-OHDA. These findings were shown by an observed increase in cellular viability, a decrease in programmed cell death, and an augmentation in the activity of antioxidants.137 The implementation of PME led to a decrease in cytoplasmic Nrf2 concentrations, while concomitantly raising nuclear Nrf2 concentrations. Consequently, the activation of the ARE promoter occurred, resulting in the subsequent augmentation of heme oxygenase-1 (HO-1) and NQO-1 expression. The activation of Nrf2 was positively regulated by the activation of Akt and ERK by PME.138,139
Abelmoschus esculentus, often known as okra, is a botanical species that has been used in traditional Chinese medicine due to its content of bioactive flavonoids.139 The study discovered that an extract derived from A. esculentus, which is rich in flavonoids, had a protective effect against oxidative damage in a condition known as transient cerebral ischemia–reperfusion injury (TCIRI) in Kunming mice. The extract exhibited the ability to scavenge ROS and regulate the Nrf2/HO-1 pathway, indicating its potential as a therapeutic option for treating OS.140
Astaxanthin (ATX), a compound produced from β-carotene sourced from the microalga Haematococcus pluvialis,141 has shown notable effectiveness in the eradication of ROS inside the cellular membrane, including both internal and extracellular environments. The administration of ATX led to increased levels of SOD, Nrf2 and p62.142
Treatment of nerve damage through Nrf2
The reciprocal contact between the brain and immune system gives rise to the manifestation of cerebral inflammation as a result of injury or the existence of neurodegenerative conditions such as AD143 and PD.144,145 Microglia cells play a crucial part in the inflammatory process by releasing proinflammatory cytokines. An association has been seen between cognitive deterioration that occurs with age and increased levels of neuroinflammation and oxidative stress after the activation of microglial cells. The anti-inflammatory properties of Nrf2 signaling have been extensively reported in scientific literature.146 Recent studies have shown that the activation of Nrf2 results in transcriptional repression in several cell types. Sulforaphane, a Nrf2 activator, demonstrated an elevation in Nrf2 DNA-binding activity and stimulated the overexpression of target genes across several cell types. Furthermore, it exhibited a decrease in proinflammatory cytokines.147 There is a notable association observed between the upregulation of NQO1 and the downregulation of iNOS and COX-2 expression in several cell lines and primary mouse peritoneal macrophages when exposed to seven different chemical classes of Nrf2 activators.148 The experimental study demonstrated that the oral administration of kavalactone methysticin resulted in the activation of the Nrf2 pathway in a mouse model of AD harboring the APP/Psen1 mutation.149 The activation of [specific factor/agent] led to a decrease in the occurrence of microgliosis and astrogliosis, as well as the production of TNF-α and IL-17A, along with oxidative damage.150
Numerous studies have shown that n-3 polyunsaturated fatty acids (n-3 PUFA) had advantageous characteristics in ameliorating cognitive deterioration, especially in the first stages before the manifestation of AD.151,152 The benefits indicated above are linked to a decline in the activation of microglial cells, resulting in a reduction in oxidative stress and an enhancement in the capacity to engulf and remove Aβ peptide, a critical component of the Nrf2-dependent antioxidant system.153
The Nrf2 signaling pathway is a crucial therapeutic target in the context of ALS. Significant therapeutic benefits have been shown in ALS mice models when astrocytes, which are the primary providers of glutathione (GSH) to neurons, exhibit elevated levels of Nrf2.154 The Nrf2 signaling pathway plays a crucial role in mitigating neuroinflammation in ALS via its ability to suppress the detrimental impact of activated microglia on the viability of neurons. The administration of small molecule activators, such as cyanoenone triterpenoids, has shown effectiveness in animal models of ALS.155
Conclusion
The activation of Nrf2 has considerable significance in the mitigation of several pathological mechanisms linked to neurodegenerative disorders. These processes include the enhancement of antioxidant defenses, the mitigation of inflammatory reactions, the optimization of mitochondrial efficiency, and the preservation of protein homeostasis. Despite the growing body of academic information on the development and pathogenesis of neurodegenerative diseases, the exact etiology of several ailments remains elusive. Oxidative damage has been recognized as a significant factor in the area of etiology, since studies have shown that exposure to OS leads to cellular damage and neurodegeneration. In instances of extended oxidative stress, the continual activation of signaling pathways occurs due to the presence of ROS and reactive nitrogen species (RNS).
The transcription factor is a regulatory protein that plays a crucial role in the process of gene expression. Nrf2 is a pivotal factor in the modulation of significant physiological processes, including as ferroptosis, inflammasome activation, and autophagy, by exerting its influence on the oxidant system. The aforementioned innovation has generated unique prospects for the progression of pharmaceutical research and development. Numerous research endeavors have been undertaken to elucidate the mechanisms governing the intricate interplay between Nrf2 and Keap1. Consequently, a multitude of compounds have been discovered and comprehensively characterized as agents that activate Nrf2.
The intricate nature of the pathophysiology behind neurodegenerative disorders poses a substantial challenge in terms of their therapeutic treatment. The act of only observing a solitary subject is inadequate in ensuring the efficacy of a therapeutic intervention. The relationship between the activity and physicochemical characteristics of Nrf2/ARE has considerable significance. To achieve optimal permeability of BBB, it is important to effectively diminish the activity of the Keap1-Nrf2 pathway.
The implementation of systematic investigations is crucial for the efficient use of Nrf2 activation, hence facilitating the development of safe, efficient, and controllable strategies for the treatment of neurodegenerative conditions. The investigation of the regulatory systems that govern the functioning of Nrf2 offers a promising opportunity for the identification of new medications that might possibly mitigate, decelerate, or provide therapeutic interventions for diverse neurodegenerative disorders.
Acknowledgments
The authors would like to thank Mohaghegh Ardabili University for concerning this manuscript.
Contributor Information
Arash Abdolmaleki, Department of Biophysics, Faculty of Advanced Technologies, University of Mohaghegh Ardabili, PO Box: 179, Ardabil, 11367-56199, Iran.
Aida Karimian, Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, PO Box: 179, Ardabil, 11367-56199, Iran.
Seyedeh Mahdieh Khoshnazar, Gastroenterology and Hepatology Research Center, Institute of Basic and Clinical Physiology Sciences, Kerman University of Medical Sciences, Imam Khomeini Highway, Mustafa Khomeini Boulevard, Ibn Sina, Kerman, 9986598, Iran.
Asadollah Asadi, Department of Biology, Faculty of Science, University of Mohaghegh Ardabili, PO Box: 179, Ardabil, 11367-56199, Iran.
Zahra Akhavi Samarein, Department of Counseling, Faculty of Education and Psychology, University of Mohaghegh Ardabili, PO Box: 179, Ardabil, 11367-56199, Iran.
Shukur Wasman Smail, Department of Medical Microbiology, College of Science, Cihan University-Erbil, Kurdistan Region, 1235897, Iraq.
Deepak Bhattacharya, Ph.D., Policy, Nursing, At Fight-Cancer at Home, Medicinal Toxicology & QC, Sri Radha Krishna Raas Mandir, KedarGouri Road, Bhubaneswar, Odisa 751002, India.
Author contributions
A.A. was involved in Conceptualization, designing the study, writing and approved the final version of the manuscript, Methodology, Formal analysis and Software. All authors contributed to the writing and approved the final version of the manuscript.
Funding
This research has not received any funds.
Conflict of interest statement. The authors have no conflicts of interest to declare.
Data availability
All relevant data and materials are provided in the manuscript.
References
- 1. Teleanu DM, Niculescu A-G, Lungu II, Radu CI, Vladâcenco O, Roza E, Costăchescu B, Grumezescu AM, Teleanu RI. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 2022:23(11):5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Boesch-Saadatmandi C, Rimbach G, Jozkowicz A, Dulak J, Loboda A. Cross-talk between micro RNA s, nuclear factor E 2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res. 2013:57(3):504–515. [DOI] [PubMed] [Google Scholar]
- 3. Panieri E, Telkoparan-Akillilar P, Suzen S, Saso L. The NRF2/KEAP1 axis in the regulation of tumor metabolism: mechanisms and therapeutic perspectives. Biomol Ther. 2020:10(5):791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bheereddy P, Yerra VG, Kalvala AK, Sherkhane B, Kumar A. SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell Mol Neurobiol. 2021:41(7):1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020:40(13):e00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katsuoka F, Otsuki A, Takahashi M, Ito S, Yamamoto M. Direct and specific functional evaluation of the Nrf2 and MafG heterodimer by introducing a tethered dimer into small Maf-deficient cells. Mol Cell Biol. 2019:39(20):e00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otsuki A, Yamamoto M. Cis-element architecture of Nrf2–sMaf heterodimer binding sites and its relation to diseases. Arch Pharm Res. 2020:43(3):275–285. [DOI] [PubMed] [Google Scholar]
- 8. Simpson DS, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel). 2020:9(8):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boas SM, Joyce KL, Cowell RM. The NRF2-dependent transcriptional regulation of antioxidant defense pathways: relevance for cell type-specific vulnerability to neurodegeneration and therapeutic intervention. Antioxidants (Basel). 2021:11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzen S, Tucci P, Profumo E, Buttari B, Saso L. A pivotal role of Nrf2 in neurodegenerative disorders: a new way for therapeutic strategies. Pharmaceuticals (Basel). 2022:15(6):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amoroso R, Maccallini C, Bellezza I. Activators of Nrf2 to counteract neurodegenerative diseases. Antioxidants (Basel). 2023:12(3):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brandes MS, Gray NE. NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro. 2020:12:1759091419899782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q, Xing S, Chen Y, Liao Q, Li Q, Liu Y, He S, Feng F, Chen Y, Zhang J, et al. Reasonably activating Nrf2: a long-term, effective and controllable strategy for neurodegenerative diseases. Eur J Med Chem. 2020:185:111862. [DOI] [PubMed] [Google Scholar]
- 14. Bahn G, Jo D-G. Therapeutic approaches to Alzheimer’s disease through modulation of NRF2. NeuroMolecular Med. 2019:21(1):1–11. [DOI] [PubMed] [Google Scholar]
- 15. Perluigi M, Tramutola A, Pagnotta S, Barone E, Butterfield DA. The BACH1/Nrf2 axis in brain in down syndrome and transition to Alzheimer disease-like neuropathology and dementia. Antioxidants (Basel). 2020:9(9):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pagnotta S, Tramutola A, Barone E, di Domenico F, Pittalà V, Salerno L, Folgiero V, Caforio M, Locatelli F, Petrini S, et al. CAPE and its synthetic derivative VP961 restore BACH1/NRF2 axis in down syndrome. Free Radic Biol Med. 2022:183:1–13. [DOI] [PubMed] [Google Scholar]
- 17. Moretti D, Tambone S, Cerretani M, Fezzardi P, Missineo A, Sherman L-T, Munoz-Sajuan I, Harper S, Dominquez C, Pacifici R, et al. NRF2 activation by reversible KEAP1 binding induces the antioxidant response in primary neurons and astrocytes of a Huntington's disease mouse model. Free Radic Biol Med. 2021:162:243–254. [DOI] [PubMed] [Google Scholar]
- 18. Uruno A, Yamamoto M. The KEAP1-NRF2 system and neurodegenerative diseases. Antioxid Redox Signal. 2023:38(13):974–988. [DOI] [PubMed] [Google Scholar]
- 19. Abrescia P, Treppiccione L, Rossi M, Bergamo P. Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog Lipid Res. 2020:80:101066. [DOI] [PubMed] [Google Scholar]
- 20. Duarte P, Michalska P, Crisman E, Cuadrado A, León R. Novel series of dual NRF2 inducers and selective MAO-B inhibitors for the treatment of Parkinson’s disease. Antioxidants (Basel). 2022:11(2):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inose Y, Izumi Y, Takada-Takatori Y, Akaike A, Koyama Y, Kaneko S, Kume T. Protective effects of Nrf2–ARE activator on dopaminergic neuronal loss in Parkinson disease model mice: possible involvement of heme oxygenase-1. Neurosci Lett. 2020:736:135268. [DOI] [PubMed] [Google Scholar]
- 22. Guo J, Cheng M, Liu P, Cao D, Luo J, Wan Y, Fang Y, Jin Y, Xie SS, Liu J. A multi-target directed ligands strategy for the treatment of Alzheimer's disease: dimethyl fumarate plus Tranilast modified Dithiocarbate as AChE inhibitor and Nrf2 activator. Eur J Med Chem. 2022:242:114630. [DOI] [PubMed] [Google Scholar]
- 23. Abdpour S, Jalili-Baleh L, Nadri H, Forootanfar H, Bukhari SNA, Ramazani A, Ebrahimi SES, Foroumadi A, Khoobi M. Chromone derivatives bearing pyridinium moiety as multi-target-directed ligands against Alzheimer’s disease. Bioorg Chem. 2021:110:104750. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Chen S, Guo H, Jiang H, Liu H, Fu H, Wang D. Forsythoside a mitigates alzheimer's-like pathology by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4 axis activation. Int J Biol Sci. 2022:18(5):2075–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saha S, Buttari B, Profumo E, Tucci P, Saso L. A perspective on Nrf2 signaling pathway for neuroinflammation: a potential therapeutic target in Alzheimer's and Parkinson's diseases. Front Cell Neurosci. 2022:15:787258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu J, Wang W-N, Matei N, Li X, Pang JW, Mo J, Chen SP, Tang JP, Yan M, Zhang JH. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxidative Med Cell Longev. 2020:2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018:29(17):1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cores Á, Piquero M, Villacampa M, León R, Menéndez JC. NRF2 regulation processes as a source of potential drug targets against neurodegenerative diseases. Biomol Ther. 2020:10(6):904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang DD, Chapman E. The role of natural products in revealing NRF2 function. Nat Prod Rep. 2020:37(6):797–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madden SK, Itzhaki LS. Structural and mechanistic insights into the Keap1-Nrf2 system as a route to drug discovery. Biochim Biophys Acta, Proteins Proteomics. 2020:1868(7):140405. [DOI] [PubMed] [Google Scholar]
- 31. Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 2022:291:120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horie Y, Suzuki T, Inoue J, Iso T, Wells G, Moore TW, Mizushima T, Dinkova-Kostova AT, Kasai T, Kamei T, et al. Molecular basis for the disruption of Keap1–Nrf2 interaction via Hinge & Latch mechanism. Commun Biol. 2021:4(1):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020:25(22):5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mirzaei S, Mohammadi AT, Gholami MH, Hashemi F, Zarrabi A, Zabolian A, Hushmandi K, Makvandi P, Samec M, Liskova A, et al. Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol Res. 2021:167:105575. [DOI] [PubMed] [Google Scholar]
- 35. Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Azami N, Hamzehlou S, Farahani MV, Hushmandi K, Ashrafizadeh M, et al. Nrf2 signaling pathway in chemoprotection and doxorubicin resistance: potential application in drug discovery. Antioxidants (Basel). 2021:10(3):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jéssica Paloma ÁR, Juan Rafael RE. Activation of the Cap’n’collar C pathway (Nrf2 pathway in vertebrates) signaling in insulin pathway compromised Drosophila melanogaster flies ameliorates the diabetic state upon pro-oxidant conditions. Gen Comp Endocrinol. 2023:335:114229. [DOI] [PubMed] [Google Scholar]
- 37. Chakkittukandiyil A, Sajini DV, Karuppaiah A, Selvaraj D. The principal molecular mechanisms behind the activation of Keap1/Nrf2/ARE pathway leading to neuroprotective action in Parkinson's disease. Neurochem Int. 2022:156:105325. [DOI] [PubMed] [Google Scholar]
- 38. Song M-Y, Lee D-Y, Chun K-S, Kim E-H. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int J Mol Sci. 2021:22(9):4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kopacz A, Rojo AI, Patibandla C, Lastra-Martínez D, Piechota-Polanczyk A, Kloska D, Jozkowicz A, Sutherland C, Cuadrado A, Grochot-Przeczek A. Overlooked and valuable facts to know in the NRF2/KEAP1 field. Free Radic Biol Med. 2022:192:37–49. [DOI] [PubMed] [Google Scholar]
- 40. Bhattacharjee S, Dashwood RH. Epigenetic regulation of NRF2/KEAP1 by phytochemicals. Antioxidants (Basel). 2020:9(9):865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moratilla-Rivera I, Sánchez M, Valdés-González JA, Gómez-Serranillos MP. Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int J Mol Sci. 2023:24(4):3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park H-J, Kim H-N, Kim CY, Seo M-D, Baek S-H. Synergistic protection by isoquercitrin and quercetin against glutamate-induced oxidative cell death in HT22 cells via activating Nrf2 and HO-1 signaling pathway: neuroprotective principles and mechanisms of Dendropanax morbifera leaves. Antioxidants (Basel). 2021:10(4):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Getachew B, Csoka AB, Copeland RL, Manaye KF, Tizabi Y. Dihydromyricetin protects against Salsolinol-induced toxicity in dopaminergic cell line: implication for Parkinson’s disease. Neurotox Res. 2023:41(2):141–148. [DOI] [PubMed] [Google Scholar]
- 44. Pedro N, Cantizani J, Ortiz-López FJ, González-Menéndez V, Cautain B, Rodríguez L, Bills GF, Reyes F, Genilloud O, Vicente F. Protective effects of isolecanoric acid on neurodegenerative in vitro models. Neuropharmacology. 2016:101:538–548. [DOI] [PubMed] [Google Scholar]
- 45. Li Q, Qian J, Huang Q-F, Deng T, Li L-H, Wang H-F, Xu S-Q, Wu X-X, Liu X-R. Advances in molecular mechanism of lung injury induced by paraquat poisoning. J Hainan Med Univ. 2022:28(4):60. [Google Scholar]
- 46. Jiang H, Li R, Zhang Z, Chang C, Liu Y, Liu Z, He Q, Wang Q. Retinoid X receptor α (RXRα)-mediated erythroid-2-related factor-2 (NRF2) inactivation contributes to N, N-dimethylformamide (DMF)-induced oxidative stress in HL-7702 and HuH6 cells. J Appl Toxicol. 2020:40(4):470–482. [DOI] [PubMed] [Google Scholar]
- 47. Tossetta G, Marzioni D. Natural and synthetic compounds in ovarian cancer: a focus on NRF2/KEAP1 pathway. Pharmacol Res. 2022:183:106365. [DOI] [PubMed] [Google Scholar]
- 48. Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264. 7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019:14(5):e0216711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang X, Xing X, Zhang Y, Zhang C, Wu Y, Chen Y, Meng R, Jia H, Cheng Y, Zhang Y, et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf. 2021:207:111231. [DOI] [PubMed] [Google Scholar]
- 50. Bhandari R, Khanna G, Kaushik D, Kuhad A. Divulging the intricacies of crosstalk between NF-kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol Neurobiol. 2021:58(7):3347–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zgorzynska E, Dziedzic B, Walczewska A. An overview of the Nrf2/ARE pathway and its role in neurodegenerative diseases. Int J Mol Sci. 2021:22(17):9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ryter SW. Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. 2021:10(3):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu J, Wu H-h, Zhang Y-c, Zhang J-z, Ma E-b, Zhang X-y. Transcription factors, cap ‘n’collar isoform C regulates the expression of CYP450 genes involving in insecticides susceptibility in Locusta migratoria. Pestic Biochem Physiol. 2023:196:105627. [DOI] [PubMed] [Google Scholar]
- 54. Ruvkun G, Lehrbach N. Regulation and functions of the ER-associated nrf1 transcription factor. Cold Spring Harb Perspect Biol. 2023:15(1):a041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem Pharmacol. 2013:85(6):705–717. [DOI] [PubMed] [Google Scholar]
- 56. Shin JW, Chun K-S, Kim D-H, Kim SJ, Kim SH, Cho NC, Na HK, Surh YJ. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol. 2020:173:113820. [DOI] [PubMed] [Google Scholar]
- 57. Meng X, Waddington JC, Tailor A, Lister A, Hamlett J, Berry N, Park BK, Sporn MB. CDDO-imidazolide targets multiple amino acid residues on the Nrf2 adaptor, Keap1. J Med Chem. 2020:63(17):9965–9976. [DOI] [PubMed] [Google Scholar]
- 58. Zhu D, Xia Y, Li S, Kong M, Chen C, Xue G, Kong L, Luo J. Iso-seco-tanapartholide activates Nrf2 signaling pathway through Keap1 modification and oligomerization to exert anti-inflammatory effects. Free Radic Biol Med. 2022:178:398–412. [DOI] [PubMed] [Google Scholar]
- 59. Iqubal A, Sharma S, Najmi AK, Syed MA, Ali J, Alam MM, Haque SE. Nerolidol ameliorates cyclophosphamide-induced oxidative stress, neuroinflammation and cognitive dysfunction: plausible role of Nrf2 and NF-κB. Life Sci. 2019:236:116867. [DOI] [PubMed] [Google Scholar]
- 60. Jayaram S, Krishnamurthy PT. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson's disease: the therapeutic role of Nrf2 activators. Neurochem Int. 2021:145:105014. [DOI] [PubMed] [Google Scholar]
- 61. Yan T, Mao Q, Zhang X, Wu B, Bi K, He B, Jia Y. Schisandra chinensis protects against dopaminergic neuronal oxidative stress, neuroinflammation and apoptosis via the BDNF/Nrf2/NF-κB pathway in 6-OHDA-induced Parkinson's disease mice. Food Funct. 2021:12(9):4079–4091. [DOI] [PubMed] [Google Scholar]
- 62. Ren P, Chen J, Li B, Zhang M, Yang B, Guo X, Chen Z, Cheng H, Wang P, Wang S, et al. Nrf2 ablation promotes Alzheimer’s disease-like pathology in APP/PS1 transgenic mice: the role of neuroinflammation and oxidative stress. Oxidative Med Cell Longev. 2020:2020:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. AbdElrazek DA, Ibrahim MA, Hassan NH, Hassanen EI, Farroh KY, Abass H. Neuroprotective effect of quercetin and nano-quercetin against cyclophosphamide-induced oxidative stress in the rat brain: role of Nrf2/HO-1/Keap-1 signaling pathway. Neurotoxicology. 2023:98:16–28. [DOI] [PubMed] [Google Scholar]
- 64. Nezu M, Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int J Mol Sci. 2020:21(8):2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Panczyszyn-Trzewik P, Misztak P, Opoka W, Nowak G, Sowa-Kucma M. Oxidative stress responses and their alterations in the Nrf2-NMDA receptor pathway in the brain of suicide victims. J Physiol Pharmacol. 2023:74(3):125–136. 10.26402/jpp.2023.3.08. [DOI] [PubMed] [Google Scholar]
- 66. Azar YO, Badawi GA, Zaki HF, Ibrahim SM. Agmatine-mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF-κB signaling cascade in rotenone lesioned rats. Life Sci. 2022:311(Pt A):121049. [DOI] [PubMed] [Google Scholar]
- 67. Rosini M, Simoni E, Caporaso R, Basagni F, Catanzaro M, Abu IF, Fagiani F, Fusco F, Masuzzo S, Albani D, et al. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer's disease. Eur J Med Chem. 2019:180:111–120. [DOI] [PubMed] [Google Scholar]
- 68. Ren X, Xu Y, Yu Z, Mu C, Liu P, Li J. The role of Nrf2 in mitigating cadmium-induced oxidative stress of Marsupenaeus japonicus. Environ Pollut. 2021:269:116112. [DOI] [PubMed] [Google Scholar]
- 69. Wang X, He Y, Tian J, Muhammad I, Liu M, Wu C, Xu C, Zhang X. Ferulic acid prevents aflatoxin B1-induced liver injury in rats via inhibiting cytochrome P450 enzyme, activating Nrf2/GST pathway and regulating mitochondrial pathway. Ecotoxicol Environ Saf. 2021:224:112624. [DOI] [PubMed] [Google Scholar]
- 70. Ashino T, Yamamoto M, Numazawa S. Nrf2 antioxidative system is involved in cytochrome P450 gene expression and activity: a delay in pentobarbital metabolism in Nrf2-deficient mice. Drug Metab Dispos. 2020:48(8):673–680. [DOI] [PubMed] [Google Scholar]
- 71. Shabani M, Erfani S, Abdolmaleki A, Afzali FE, Khoshnazar SM. Alpha-pinene modulates inflammatory response and protects against brain ischemia via inducible nitric oxide synthase-nuclear factor–kappa B-cyclooxygenase-2 pathway. Mol Biol Rep. 2023:50(8):6505–6516. [DOI] [PubMed] [Google Scholar]
- 72. Abdelzaher WY, Ahmed SM, Welson NN, Alsharif KF, Batiha GE-S, Labib DAA. Dapsone ameliorates isoproterenol-induced myocardial infarction via Nrf2/HO-1; TLR4/TNF-α signaling pathways and the suppression of oxidative stress, inflammation, and apoptosis in rats. Front Pharmacol. 2021:12:669679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peng Y-J, Lu J-W, Lee C-H, Lee HS, Chu YH, Ho YJ, Liu FC, Huang CJ, Wu CC, Wang CC. Cardamonin attenuates inflammation and oxidative stress in interleukin-1β-stimulated osteoarthritis chondrocyte through the Nrf2 pathway. Antioxidants (Basel). 2021:10(6):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Audi SH, Jacobs ER, Taheri P, Ganesh S, Clough AV. Assessment of protection offered by the NRF2 pathway against Hyperoxia-induced acute lung injury in NRF2 knockout rats. Shock. 2022:57(2):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Qu Z, Sun J, Zhang W, Yu J, Zhuang C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic Biol Med. 2020:159:87–102. [DOI] [PubMed] [Google Scholar]
- 76. Eide S, Misztal M, Feng Z-P. Interleukin-6 as a marker of Huntington's disease progression: systematic review and meta-analysis. Brain Behav Immun Health. 2023:30:100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Forcina L, Miano C, Scicchitano BM, Rizzuto E, Berardinelli MG, de Benedetti F, Pelosi L, Musarò A. Increased circulating levels of interleukin-6 affect the redox balance in skeletal muscle. Oxidative Med Cell Longev. 2019:2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020:156:168–175. [DOI] [PubMed] [Google Scholar]
- 79. Lee J, Jang J, Park S-M, Yang S-R. An update on the role of Nrf2 in respiratory disease: molecular mechanisms and therapeutic approaches. Int J Mol Sci. 2021:22(16):8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yen C-H, Hsu C-M, Hsiao SY, Hsiao H-H. Pathogenic mechanisms of myeloma bone disease and possible roles for NRF2. Int J Mol Sci. 2020:21(18):6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee S, Hu L. Nrf2 activation through the inhibition of Keap1–Nrf2 protein–protein interaction. Med Chem Res. 2020:29(5):846–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wei J, Zhao Q, Zhang Y, Shi W, Wang H, Zheng Z, Meng L, Xin Y, Jiang X. Sulforaphane-mediated Nrf2 activation prevents radiation-induced skin injury through inhibiting the oxidative-stress-activated DNA damage and NLRP3 inflammasome. Antioxidants (Basel). 2021:10(11):1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y, Liu Z, Ma J, Xv Q, Gao H, Yin H, Yan G, Jiang X, Yu W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022:57:102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang W, Cao Z, Cui Y, Huo S, Shao B, Song M, Cheng P, Li Y. Lycopene ameliorates aflatoxin B1-induced testicular lesion by attenuating oxidative stress and mitochondrial damage with Nrf2 activation in mice. Ecotoxicol Environ Saf. 2023:256:114846. [DOI] [PubMed] [Google Scholar]
- 85. Shahcheraghi SH, Salemi F, Peirovi N, Ayatollahi J, Alam W, Khan H, Saso L. Nrf2 regulation by curcumin: molecular aspects for therapeutic prospects. Molecules. 2021:27(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rahban M, Habibi-Rezaei M, Mazaheri M, Saso L, Moosavi-Movahedi AA. Anti-viral potential and modulation of Nrf2 by curcumin: pharmacological implications. Antioxidants (Basel). 2020:9(12):1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu X-F, Wang Q, Zheng J-F, Chai Z-H, Dai F, Jin X-J, Zhou B. Developing dietary curcumin mono-carbonyl piperidinone analogs as Nrf2-dependent cytoprotectors against oxidative damage: structure-activity relationship and mechanisms. Free Radic Biol Med. 2022:186:66–75. [DOI] [PubMed] [Google Scholar]
- 88. Talebi M, Talebi M, Farkhondeh T, Mishra G, Ilgün S, Samarghandian S. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications. Phytother Res. 2021:35(6):3078–3112. [DOI] [PubMed] [Google Scholar]
- 89. Tang Y, Chen Q, Chen J, Mo Z, Li H, Peng L, Ke Y, Liang B, Li R, Zhu H. Green tea polyphenols cause apoptosis and autophagy in HPV-16 subgene-immortalized human cervical epithelial cells via the activation of the Nrf2 pathway. Nutr Cancer. 2022:74(10):3769–3778. [DOI] [PubMed] [Google Scholar]
- 90. Thorley J, Thomas C, Thon N, Nuttall H, Martin NRW, Bishop N, Bailey SJ, Clifford T. Combined effects of green tea supplementation and eccentric exercise on nuclear factor erythroid 2-related factor 2 activity. Eur J App Physiol. 2023:124(1):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yamagishi N, Yamamoto Y, Nishi T, Ito T, Kanai Y. Lansoprazole protects hepatic cells against cisplatin-induced oxidative stress through the p38 MAPK/ARE/Nrf2 pathway. PLoS One. 2023:18(6):e0287788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Deramaudt TB, Chehaitly A, Charrière T, Arnaud J, Bonay M. High-frequency repetitive magnetic stimulation activates bactericidal activity of macrophages via modulation of p62/Keap1/Nrf2 and p38 MAPK pathways. Antioxidants (Basel). 2023:12(9):1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Azouz AA, Omar HA, Hersi F, Ali FE, Hussein Elkelawy AMM. Impact of the ACE2 activator xanthenone on tacrolimus nephrotoxicity: modulation of uric acid/ERK/p38 MAPK and Nrf2/SOD3/GCLC signaling pathways. Life Sci. 2022:288:120154. [DOI] [PubMed] [Google Scholar]
- 94. Khalaf M, Hassan S, Sayed A, Abo-Youssef A. Carvacrol mitigates vancomycin-induced nephrotoxicity via regulation of IkBα/p38MAPK and Keap1/Nrf2 signaling pathways: an experimental study with in silico evidence. Eur Rev Med Pharmacol Sci. 2022:26(23):8738–8755. [DOI] [PubMed] [Google Scholar]
- 95. Zheng Q, Ma P, Yang P, Zhai S, He M, Zhang X, Tu Q, Jiao L, Ye L, Feng Z, et al. Alpha lipoic acid ameliorates motor deficits by inhibiting ferroptosis in Parkinson’s disease. Neurosci Lett. 2023:810:137346. [DOI] [PubMed] [Google Scholar]
- 96. Fasipe B, Faria A, Laher I. Potential for novel therapeutic uses of alpha Lipoic acid. Curr Med Chem. 2023:30(35):3942–3954. [DOI] [PubMed] [Google Scholar]
- 97. Li M, Fang Q, Xiu L, Yu L, Peng S, Wu X, Chen X, Niu X, Wang G, Kong Y. The molecular mechanisms of alpha-lipoic acid on ameliorating aflatoxin B1-induced liver toxicity and physiological dysfunction in northern snakehead (Channa argus). Aquat Toxicol. 2023:257:106466. [DOI] [PubMed] [Google Scholar]
- 98. Tripathy S, Verma DK, Thakur M, Chakravorty N, Singh S, Srivastav PP. Recent trends in extraction, identification and quantification methods of Centella asiatica phytochemicals with potential applications in food industry and therapeutic relevance: a review. Food Biosci. 2022:49:101864. [Google Scholar]
- 99. Wright KM, Bollen M, David J, Speers AB, Brandes MS, Gray NE, Alcázar Magaña A, McClure C, Stevens JF, Maier CS, et al. Pharmacokinetics and pharmacodynamics of key components of a standardized Centella asiatica product in cognitively impaired older adults: a phase 1, double-blind, randomized clinical trial. Antioxidants (Basel). 2022:11(2):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. He Z, Hu Y, Niu Z, Zhong K, Liu T, Yang M, Ji L, Hu W. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb. J Ethnopharmacol. 2022:302(Pt A):115865. [DOI] [PubMed] [Google Scholar]
- 101. Shamsuddin N, Mat Zain M, Adenan MI, Ahmad Noorden MS. Ethanol extract of Centella asiatica improved methamphetamine-induced neurotoxicity on mouse model via stimulating superoxide dismutase II and microRNA-34A expression. Sains Malays. 2023:52(1):233–244. [Google Scholar]
- 102. Ding L, Liu T, Ma J. Neuroprotective mechanisms of Asiatic acid. Heliyon. 2023:9(5):e15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen Y, Cao H, He W, Zhang X, Xu R. Tert-Butylhydroquinone-induced formation of high-molecular-weight p62: a novel mechanism in the activation of Nrf2-Keap1. Cell Biol Int. 2022:46(9):1345–1354. [DOI] [PubMed] [Google Scholar]
- 104. Khezerlou A, Akhlaghi AP, Alizadeh AM, Dehghan P, Maleki P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: a narrative review. Toxicol Rep. 2022:9:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu X, Yang L, Zhang G, Ling J. Neuroprotective effects of phenolic antioxidant Tert-butylhydroquinone (tBHQ) in brain diseases. Mol Neurobiol. 2023:60(9):4909–4923. [DOI] [PubMed] [Google Scholar]
- 106. Chen B, He Q, Yang J, Pan Z, Xiao J, Chen W, Chi W, Li M, Li S, Zeng J, et al. Metformin suppresses oxidative stress induced by high glucose via activation of the Nrf2/HO-1 signaling pathway in type 2 diabetic osteoporosis. Life Sci. 2023:312:121092. [DOI] [PubMed] [Google Scholar]
- 107. Sharma S, Zhang Y, Sifat AE, Akter KA, Archie SR, Nozohouri S, Abbruscato T. Evaluation of influx and efflux transporters of metformin in an In vitro blood-brain barrier model during normoxic and ischemic conditions. Pharmaceutics. 2023;15(5):1357. 10.3390/pharmaceutics15051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang T, Wang Y, Yao W, Chen Y, Zhang D, Gao Y, Jin S, Li L, Yang S, Wu Y. Metformin antagonizes nickel-refining fumes-induced cell pyroptosis via Nrf2/GOLPH3 pathway in vitro and in vivo. Ecotoxicol Environ Saf. 2022:247:114233. [DOI] [PubMed] [Google Scholar]
- 109. Li J, Zhu Z, Ye L, Wang Z, Xiang G, Li S, Yue L. Metformin exerts anti-neoplastic effects via the reactive oxygen species-dependent apoptosis and inhibition of the AMPK/mTOR/Nrf2 pathway in papillary thyroid cancer. J Biomed Nanotech. 2023:19(5):852–863. [Google Scholar]
- 110. Esteras N, Abramov AY. Nrf2 as a regulator of mitochondrial function: energy metabolism and beyond. Free Radic Biol Med. 2022:189:136–153. [DOI] [PubMed] [Google Scholar]
- 111. Cuciniello R, Luongo D, Ferramosca A, Lunetti P, Rotondi-Aufiero V, Crispi S, Zara V, Maurano F, Filosa S, Bergamo P. Conjugated linoleic acid downregulates Alzheimer's hallmarks in aluminum mouse model through an Nrf2-mediated adaptive response and increases brain glucose transporter levels. Free Radic Biol Med. 2022:191:48–58. [DOI] [PubMed] [Google Scholar]
- 112. Kaur K, Narang R, Singh S. Role of Nrf2 in oxidative stress, Neuroinflammation and autophagy in Alzheimer's disease: regulation of Nrf2 by different Signaling pathways. Curr Mol Med. 2023:95:153–167. 10.2174/1566524023666230726145447. [DOI] [PubMed] [Google Scholar]
- 113. Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022:179:133–143. [DOI] [PubMed] [Google Scholar]
- 114. Wang X-L, Zhu Q-Q, Simayi A, Xu G-P. Nrf2 protects against myocardial ischemia-reperfusion injury in diabetic rats by inhibiting Drp1-mediated mitochondrial fission. Open Med. 2023:18(1):20230711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brackhan M, Arribas-Blazquez M, Lastres-Becker I. Aging, NRF2, and TAU: a perfect match for neurodegeneration? Antioxidants (Basel). 2023:12(8):1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mugayar AA, Silva Guimarães G, Oliveira PHT, Miranda RL, dos Santos AA. Apoptosis in the neuroprotective effect of α7 nicotinic receptor in neurodegenerative models. J Neurosci Res. 2023:101(12):1795–1802. [DOI] [PubMed] [Google Scholar]
- 117. Zhou J, Shen R, Makale EC, Zhong W, Chen Z, Huang Q. SS31 confers cerebral protection by reversing mitochondrial dysfunction in early brain injury following subarachnoid Hemorrhage, via the Nrf2-and PGC-1α-dependent pathways. Neurochem Res. 2023:48(5):1580–1595. [DOI] [PubMed] [Google Scholar]
- 118. Rajan S, Tryphena KP, Khan S, Vora L, Srivastava S, Singh SB, Khatri DK. Understanding the involvement of innate immunity and the Nrf2-NLRP3 axis on mitochondrial health in Parkinson's disease. Ageing Res Rev. 2023:87:101915. [DOI] [PubMed] [Google Scholar]
- 119. Kong J, Xiang Q, Shi G, Xu Z, Ma X, Wang Y, Xuan Z, Xu F. Licorice protects against ulcerative colitis via the Nrf2/PINK1-mediated mitochondrial autophagy. Immun Inflamm Dis. 2023:11(1):e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen Z, Wang H, Hu B, Chen X, Zheng M, Liang L, Lyu J, Zeng Q. Transcription factor nuclear factor erythroid 2 p45-related factor 2 (NRF2) ameliorates sepsis-associated acute kidney injury by maintaining mitochondrial homeostasis and improving the mitochondrial function. Eur J Histochem. 2022:66(3):99–113. 10.4081/ejh.2022.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Takata K, Kimura H, Yanagisawa D, Harada K, Nishimura K, Kitamura Y, Shimohama S, Tooyama I. Nicotinic acetylcholine receptors and microglia as therapeutic and imaging targets in Alzheimer’s disease. Molecules. 2022:27(9):2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bye LJ, Finol-Urdaneta RK, Tae H-S, Adams DJ. Nicotinic acetylcholine receptors: key targets for attenuating neurodegenerative diseases. Int J Biochem Cell Biol. 2023:157:106387. [DOI] [PubMed] [Google Scholar]
- 123. Scuto M, Modafferi S, Rampulla F, Zimbone V, Tomasello M, Spano’ S, Ontario ML, Palmeri A, Trovato Salinaro A, Siracusa R, et al. Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: from molecular basis to therapy. Mech Ageing Dev. 2022:205:111686. [DOI] [PubMed] [Google Scholar]
- 124. Bayazid AB, Lim BO. Quercetin is an active agent in berries against neurodegenerative diseases progression through modulation of Nrf2/HO1. Nutrients. 2022:14(23):5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Srivastava A, Kumari A, Jagdale P, Ayanur A, Pant AB, Khanna VK. Potential of quercetin to protect cadmium induced cognitive deficits in rats by modulating NMDA-R mediated downstream Signaling and PI3K/AKT—Nrf2/ARE Signaling pathways in hippocampus. NeuroMolecular Med. 2023:25(3):426–440. [DOI] [PubMed] [Google Scholar]
- 126. Zhou Y, Qian C, Tang Y, Song M, Zhang T, Dong G, Zheng W, Yang C, Zhong C, Wang A, et al. Advance in the pharmacological effects of quercetin in modulating oxidative stress and inflammation related disorders. Phytother Res. 2023:37(11):4999–5016. [DOI] [PubMed] [Google Scholar]
- 127. Han H, Zhong R, Zhang S, Wang M, Wen X, Yi B, Zhao Y, Chen L, Zhang H. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J Nutr Biochem. 2023:113:109256. [DOI] [PubMed] [Google Scholar]
- 128. Ewees MGE-D, Orfali R, Rateb EE, Hassan HM, Hozzein WN, Alkhalfah DHM, Sree HTA, Abdel Rahman FEZS, Rateb ME, Mahmoud NI. Modulation of mi-RNA25/ox-LDL/NOX4 signaling pathway by polyphenolic compound Hydroxytyrosol as a new avenue to alleviate cisplatin-induced acute kidney injury, a mechanistic study in rats. Environ Toxicol Pharmacol. 2023:103:104262. [DOI] [PubMed] [Google Scholar]
- 129. Wang Q, Wang C, Abdullah, Tian W, Qiu Z, Song M, Cao Y, Xiao J. Hydroxytyrosol alleviates dextran sulfate sodium-induced colitis by modulating inflammatory responses, intestinal barrier, and microbiome. J Agric Food Chem. 2022:70(7):2241–2252. [DOI] [PubMed] [Google Scholar]
- 130. Sidiropoulou GA, Metaxas A, Kourti M. Natural antioxidants that act against Alzheimer’s disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action. Front Endocrinol. 2023:14:154–167. 10.3389/fendo.2023.1217730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xu T, Hu S, Liu Y, Sun K, Luo L, Zeng L. Hawk tea flavonoids as natural hepatoprotective agents alleviate acute liver damage by reshaping the intestinal microbiota and modulating the Nrf2 and NF-κB signaling pathways. Nutrients. 2022:14(17):3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hussain Y, Khan H, Alsharif KF, Hayat Khan A, Aschner M, Saso L. The therapeutic potential of Kaemferol and other naturally occurring polyphenols might Be modulated by Nrf2-ARE Signaling pathway: current status and future direction. Molecules. 2022:27(13):4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Almatroudi A, Allemailem KS, Alwanian WM, Alharbi BF, Alrumaihi F, Khan AA, Almatroodi SA, Rahmani AH. Effects and mechanisms of Kaempferol in the Management of Cancers through modulation of inflammation and signal transduction pathways. Int J Mol Sci. 2023:24(10):8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hu C, Zhao J-F, Wang Y-M, Wu X-l, Ye L. Tiliroside induces ferroptosis to repress the development of triple-negative breast cancer cells. Tissue Cell. 2023:83:102116. [DOI] [PubMed] [Google Scholar]
- 135. Liu J-Q, Zhao X-T, Qin F-Y, Zhou JW, Ding F, Zhou G, Zhang XS, Zhang ZH, Li ZB. Isoliquiritigenin mitigates oxidative damage after subarachnoid hemorrhage in vivo and in vitro by regulating Nrf2-dependent Signaling pathway via targeting of SIRT1. Phytomedicine. 2022:105:154262. [DOI] [PubMed] [Google Scholar]
- 136. Ding M-R, Qu Y-J, Hu B, An H-M. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed Pharmacother. 2022:152:113208. [DOI] [PubMed] [Google Scholar]
- 137. Chen J, Xu J, Huang P, Luo Y, Shi Y, Ma P. The potential applications of traditional Chinese medicine in Parkinson's disease: a new opportunity. Biomed Pharmacother. 2022:149:112866. [DOI] [PubMed] [Google Scholar]
- 138. Muhammad F, Liu Y, Zhou Y, Yang H, Li H. Antioxidative role of traditional Chinese medicine in Parkinson's disease. J Ethnopharmacol. 2022:285:114821. [DOI] [PubMed] [Google Scholar]
- 139. Zou Z-C, Fu J-J, Dang Y-Y, Zhang Q, Wang X-F, Chen H-B, Jia XJ, Lee SMY, Li CW. Pinocembrin-7-methylether protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity via modulating Nrf2 induction through AKT and ERK pathways. Neurotox Res. 2021:39(4):1323–1337. [DOI] [PubMed] [Google Scholar]
- 140. Huang X, Li X, Deng Y, Zhou T, Chen T, Wu S, Xia R, Kang Y, Yin W. The flavonoids extract from okra flowers protects against DSS-induced colitis via regulating NF-κB signaling pathway and gut microbiota. J Funct Foods. 2022:99:105335. [Google Scholar]
- 141. Karimian A, Mir Mohammadrezaei F, Hajizadeh Moghadam A, Bahadori MH, Ghorbani-Anarkooli M, Asadi A, Abdolmaleki A. Effect of astaxanthin and melatonin on cell viability and DNA damage in human breast cancer cell lines. Acta Histochem. 2022:124(1):151832. [DOI] [PubMed] [Google Scholar]
- 142. Davinelli S, Saso L, D’Angeli F, Calabrese V, Intrieri M, Scapagnini G. Astaxanthin as a modulator of Nrf2, NF-κB, and their crosstalk: molecular mechanisms and possible clinical applications. Molecules. 2022:27(2):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Qin X, Hua J, Lin S-j, Zheng HT, Wang JJ, Li W, Ke JJ, Cai HB. Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway. Biochem Biophys Res Commun. 2020:531(3):431–437. [DOI] [PubMed] [Google Scholar]
- 144. Jiang T, Cheng H, Su J, Wang X, Wang Q, Chu J, Li Q. Gastrodin protects against glutamate-induced ferroptosis in HT-22 cells through Nrf2/HO-1 signaling pathway. Toxicol in Vitro. 2020:62:104715. [DOI] [PubMed] [Google Scholar]
- 145. Zhan J, Li X, Luo D, Yan W, Hou Y, Hou Y, Chen S, Luan J, Zhang Q, Lin D. Polydatin attenuates OGD/R-induced neuronal injury and spinal cord ischemia/reperfusion injury by protecting mitochondrial function via Nrf2/ARE signaling pathway. Oxidative Med Cell Longev. 2021:2021:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Song C, Heping H, Shen Y, Jin S, Li D, Zhang A, Ren X, Wang K, Zhang L, Wang J, et al. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020:244:125495. [DOI] [PubMed] [Google Scholar]
- 147. Shah A, Varma M, Bhandari R. Exploring sulforaphane as neurotherapeutic: targeting Nrf2-Keap & Nf-kb pathway crosstalk in ASD. Metab Brain Dis. 2023:39(3):373–385. [DOI] [PubMed] [Google Scholar]
- 148. Xiao-Lei S, Tian-Shuang X, Yi-Ping J, Na-Ni W, Ling-Chuan X, Ting H, Hai-Liang X. Humulus lupulus L. extract and its active constituent xanthohumol attenuate oxidative stress and nerve injury induced by iron overload via activating AKT/GSK3 β and Nrf2/NQO1 pathways. J Nat Med. 2023:77(1):12–27. [DOI] [PubMed] [Google Scholar]
- 149. Sardone R, Lampignano L, Guerra V, Zupo R, Donghia R, Castellana F, Battista P, Bortone I, Procino F, Castellana M, et al. Relationship between inflammatory food consumption and age-related hearing loss in a prospective observational cohort: results from the Salus in Apulia study. Nutrients. 2020:12(2):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Parcha V, Bhandari P, Chhetri S, Kumar U, Kumar D. 12 A comprehensive review. In: Medicinal roots and tubers for pharmaceutical and commercial applications. United States, Boca: CRC Press, 2023. p. 140. 10.1201/b22924. [DOI] [Google Scholar]
- 151. Xu Q, Zhang Z, Tang M, Xing C, Chen H, Zheng K, Zhao Z, Zhou S, Zhao AZ, Li F, et al. Endogenous production of ω-3 polyunsaturated fatty acids mitigates cisplatin-induced myelosuppression by regulating NRF2-MDM2-p53 signaling pathway. Free Radic Biol Med. 2023:201:14–25. [DOI] [PubMed] [Google Scholar]
- 152. Zailani H, Satyanarayanan SK, Liao W-C, Liao H-F, Huang S-Y, Gałecki P, Su KP, Chang J. Omega-3 polyunsaturated fatty acids in managing comorbid mood disorders in chronic obstructive pulmonary disease (COPD): a review. J Clin Med. 2023:12(7):2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kouhihabibidehkordi G, Ghanavatinejad F, Taheri K, Soleimannejad M, Lorigooini Z, Amini-Khoei H, Mobini G. The protective effects of lavender officinalis extract against impairment of antioxidant–detoxification system induced by glucose deprivation through Nrf2 expression. Proc Natl Acad Sci India Sect B Biol Sci. 2023:94(1):135–143. [Google Scholar]
- 154. Yang B, Pan J, Zhang X-N, Wang H, He L, Rong X, Li X, Peng Y. NRF2 activation suppresses motor neuron ferroptosis induced by the SOD1G93A mutation and exerts neuroprotection in amyotrophic lateral sclerosis. Neurobiol Dis. 2023:184:106210. [DOI] [PubMed] [Google Scholar]
- 155. Wang Q, Lin D, Liu X-F, Dai F, Jin X-J, Zhou B. Engineering piperlongumine-inspired analogs as Nrf2-dependent neuroprotectors against oxidative damage by an electrophilicity-based strategy. Free Radic Biol Med. 2023:194:298–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data and materials are provided in the manuscript.


