Abstract
The induction of phototropism in etiolated (dark-grown) seedlings exposed to an unidirectional pulse or extended irradiation with low fluence rate blue light (BL) requires the action of the phototropin (nph1) BL receptor. Although cryptochromes and phytochromes are not required for phototropic induction, these photoreceptors do modulate the magnitude of curvature resulting from phototropin activation. Modulatory increases in the magnitude of phototropic curvature have been termed “enhancement.” Here, we show that phototropic enhancement is primarily a phytochrome A (phyA)-dependent red/far-red-reversible low fluence response. This phyA-dependent response is genetically separable from the basal phototropin-dependent response, as demonstrated by its retention under extended irradiation conditions in the nph4 mutant background, which normally lacks the basal BL-induced response. It is interesting that the nph4 mutants fail to exhibit the basal phototropin-dependent and phyA-dependent enhancement responses under limiting light conditions. Given that NPH4 encodes a transcriptional activator, auxin response factor 7 (ARF7), we hypothesize that the ultimate target(s) of phyA action during the phototropic enhancement response is a rate-limiting ARF-containing transcriptional complex in which the constituent ARFs can vary in identity or activity depending upon the irradiation condition.
Recent studies have revealed that complex interactions occur between the multiple photosensory pathways regulating photomorphogenic processes in higher plants (Casal, 2000; Neff et al., 2000). On the surface, phototropism does not appear to suffer from such complexities. For example, under low fluence rate conditions, only phototropin (nph1 holoprotein; Christie et al., 1999) appears to be required for perception of directional blue light (BL) signals (Liscum and Briggs, 1995; Liscum and Stowe-Evans, 2000; Sakai et al., 2000;). However, it is clear that other photoreceptors play secondary roles in phototropism to modulate the magnitude of curvature (Liscum and Stowe-Evans, 2000). For instance, the BL-absorbing cryptochromes appear to influence phototropism through indirect effects on growth and development (Lascève et al., 1999; E.L. Stowe-Evans, J. Casal, and E. Liscum, unpublished data), whereas the red (RL)/far-red light- (FR) absorbing phytochromes appear to directly influence phototropic signal-response processes (Iino, 1990; Poff et al., 1994). Each of these secondary photosensory responses could have appreciable effects on phototropism in the natural environment where light quality and intensity vary spatially and temporally during the establishment of a seedling. Hence, to truly understand phototropism in an ecological and evolutionary context, it will be necessary to understand not only primary signal-response events, but also secondary events that impact those primary events.
It has been known for decades that phytochrome activation leads to enhanced BL-induced phototropism in mono- and dicotyledonous plants (Iino, 1990; Liscum and Stowe-Evans, 2000). Yet only recently have particular phytochromes been associated with the phototropic enhancement response. In Arabidopsis the phytochromes are encoded by a small gene family, PHYA-E (Sharrock and Quail, 1989; Clack et al., 1994), with the phyA and phyB holoproteins playing the dominant roles in most photomorphogenic responses (Casal, 2000; Neff et al., 2000). Studies of pulse-induced phototropism in etiolated (dark-grown) Arabidopsis seedlings suggest that phyA and phyB are also the predominant phytochromes regulating phototropic enhancement (Parks et al., 1996; Janoudi et al., 1997a, 1997b). Although the identification of the phytochrome(s) that regulate phototropic enhancement represents a significant advance, this knowledge is tempered by the fact that nothing is known about the molecular elements operating in the signal-response pathway between phytochrome activation and enhanced phototropic curvature. Results from Arabidopsis and maize, however, suggest that phytochromes may alter the activity and/or abundance of some component downstream of the primary phototropic signaling/response elements that is rate-limiting in the absence of phytochrome action (Liu and Iino, 1996b; Parks et al., 1996; Janoudi et al., 1997a, 1997b).
In recent years several loci-encoding components of the primary phototropic signal-response pathway have been identified through mutational approaches in Arabidopsis (Liscum and Stowe-Evans, 2000). To date, the genes represented by four of these loci have been cloned: NPH1 (NONPHOTOTROPIC HYPOCOTYL1), NPH3, RPT2 (ROOT PHOTOTROPISM2), and NPH4. As mentioned above, the NPH1 gene encodes phototropin, a light-activated Ser/Thr protein kinase that functions as a low fluence rate phototropic receptor (Liscum and Briggs, 1995; Huala et al., 1997; Christie et al., 1998, 1999; ; Sakai et al., 2000; Salamon et al., 2000). NPH3 and RPT2 encode members of a novel family of apparently plant-specific proteins (E. Liscum, unpublished data) and seem to function early in phototropic signaling (Liscum and Briggs, 1996; Motchoulski and Liscum, 1999; Sakai et al., 2000). The NPH4 gene has been found to encode a transcriptional activator, auxin response factor 7 (ARF7; Harper et al., 2000), and appears to act as a regulator of multiple differential growth responses, including phototropism and gravitropism (Liscum and Briggs, 1996; Watahiki and Yamamoto, 1997; Stowe-Evans et al., 1998; Watahiki et al., 1999).
Of the phototropic proteins discussed above, only NPH4/ARF7 exhibits properties similar to that expected for a potential target of phytochrome action in phototropism. First, the pleiotropic nature of nph4 mutants indicates that unlike phototropin, NPH3 and RPT2, proteins that have roles in early phototropic signaling, NPH4/ARF7 functions late in the phototropic signal-response pathway. This function appears to occur at or after the convergence point of several stimulus-driven signaling pathways (Liscum and Briggs, 1996; Stowe-Evans et al., 1998; Harper et al., 2000). Second, relative to phototropism, complete loss-of-function nph4 alleles are semidominant, implying that NPH4/ARF7 is limiting (Liscum and Briggs, 1995; Stowe-Evans et al., 1998). Third, whereas nph4 null mutants are completely unresponsive to low fluence rate BL signals alone, they have been reported to exhibit phototropism under irradiation conditions where significant phytochrome photoconversion is likely to have occurred (Liscum and Briggs, 1996). Therefore, whereas NPH4/ARF7 is unlikely to represent the direct target of phytochrome action, the observed phytochrome conditionality may allow us to use nph4 mutants to identify the target(s) and mechanism of phytochrome action in the enhancement of phototropin-induced phototropism. In this report we demonstrate that a majority of the phototropic enhancement occurring in wild-type and nph4 seedlings under long-term irradiation conditions is mediated by phyA, with little contribution from phyB or other phytochromes. This phyA-dependent response occurs within the low fluence range and is R/FR reversible. Our studies with the nph4 mutants suggest that the regulation of phototropic enhancement by phyA under pulse and long-term irradiation conditions is different, but that in both cases the target(s) of phyA action is likely to influence auxin responsiveness.
RESULTS
RL-Dependent Enhancement of Phototropin-Induced Phototropism in Arabidopsis Occurs via a phyA-Dependent Low Fluence Response
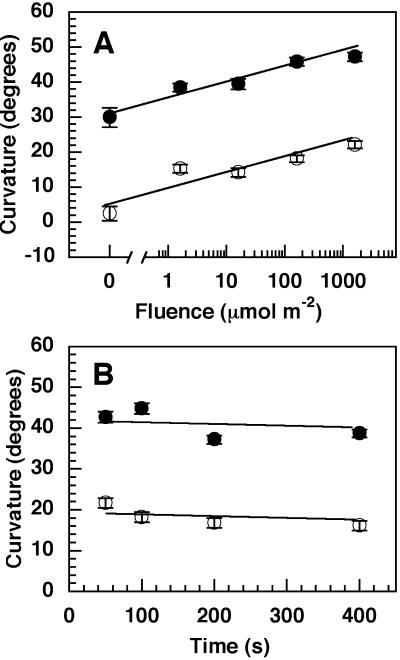
A previous study has shown that etiolated nph4 seedlings, which are unresponsive to unilateral BL alone, recover phototropic responsiveness if pre-irradiated with RL (Liscum and Briggs, 1996). Etiolated wild-type Arabidopsis seedlings exhibit a similar phenotype in that pre-irradiation results in enhanced BL-induced phototropic curvatures (Janoudi and Poff, 1992; Janoudi et al., 1992, 1997a, 1997b; Liscum and Briggs, 1996; Parks et al., 1996). As shown in Figure 1A, wild-type and nph4 mutant seedlings exhibited a BL-dependent phototropic response that increased in magnitude with increasing fluences of RL pre-irradiation. It is interesting that although the basal BL-induced phototropic response of wild-type (approximately 30° ± 3°) and nph4 (approximately 2° ± 2°) seedlings was dramatically different, the slopes of their RL fluence response curves were parallel, suggesting that a similar mechanism mediates recovery of phototropism in nph4 and enhancement of phototropism in wild-type Arabidopsis.
Figure 1.
RL-dependent enhancement of phototropism induced by long-term irradiation with BL in wild-type (●) and nph4 mutant (○) seedlings. A, Fluence response curves for RL-dependent enhancement of phototropism. Irradiation of 64-h-old etiolated seedlings with BL (unilateral; 0.5 μmol m−2 s−1) and RL (actinic from above; 1.6 μmol m−2 s−1) was initiated at the same time. BL was given for a total of 4 h, whereas RL exposure times varied (1–1,000 s) depending upon the desired fluence. At the end of the BL exposure, phototropic curvatures were measured. Data represent the mean response of at least 97 seedlings from three replicate experiments. Vertical bars represent the se values. B, Reciprocity relationships for RL-dependent enhancement of phototropism. Seedlings were handled as described above, except that fluence rates and exposure times of the RL exposure were varied to achieve a single fluence of 160 μmol m−2. Curvatures are plotted relative to the exposure time of the RL irradiation. Data represent the mean response of at least 54 seedlings from three replicate experiments. Vertical error bars represent the se values.
Three sets of data suggest that the recovery/enhancement response may represent a phytochrome-dependent low fluence response (Mancinelli, 1994). First, although a threshold for the RL effect cannot be definitively determined from the data presented in Figure 1A, the recovery/enhancement response exhibited a log-linear relationship over a range of fluences consistent with low fluence responses. Second, at least for irradiation times of 400 s or less, the recovery response in nph4 and the enhancement response in wild type exhibited reciprocity—both responses being dependent only upon the number of incident photons absorbed (Fig. 1B). Reciprocity is a characteristic of low fluence phytochrome responses, but not of very low fluence or high irradiance responses (Mancinelli, 1994; Batschauer, 1999). Third, the recovery/enhancement response was completely R/FR reversible in the wild-type and nph4 backgrounds (Table I). It is important to note that although FR can reverse the effects of RL pre-irradiation, FR pre-irradiation alone has no affect on the phototropin-dependent phototropic response of wild-type or nph4 seedlings.
Table I.
R/FR reversibility of phototropic enhancement
| Light Condition | Wild Type | nph4 | nph4 phyA |
|---|---|---|---|
| BL | 33.8° ± 1.2° (59) | 0.9° ± 0.5° (61) | 0.7° ± 0.5° (54) |
| BL + FR | 33.8° ± 1.2° (60) | 1.5° ± 0.8° (67) | 0.6° ± 0.6° (58) |
| BL + RL | 49.7° ± 1.8° (58) | 14.2° ± 1.3° (55) | 0.5° ± 0.6° (63) |
| BL + RL/FR | 29.7° ± 0.9° (52) | 2.6° ± 0.8° (53) | 0.6° ± 0.6° (57) |
| BL + RL/FR/RL | 41.0° ± 1.2° (82) | 11.2° ± 0.8° (87) | 0.8° ± 0.4° (85) |
All seedlings were grown in darkness for 64 h prior to the start of irradiation with unilateral BL (0.5 μmol m−2 s−1). One-minute pulses of RL or FR light (total fluence of 1,600 μmol m−2) were given from above simultaneous with the start of BL irradiation. For reversibility experiments, RL/FR pulses were given in the sequence indicated with no intervening dark period. Data represent the mean responses ±se from two replicate experiments. Nos. of seedlings are shown in the parentheses.
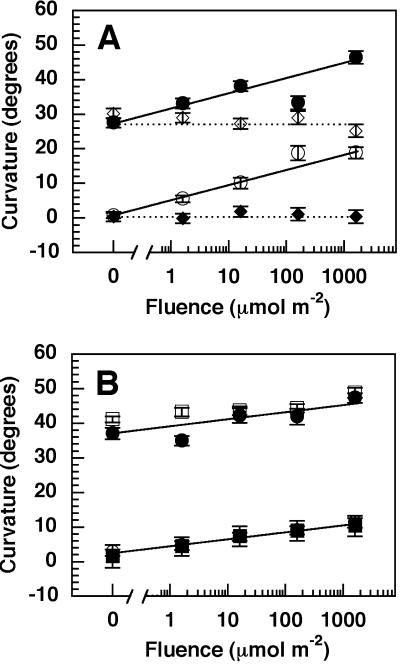
Although low fluence responses are typically thought to reflect phyB activity (Quail, 1998; Whitelam et al., 1998; Batschauer, 1999), the low fluence RL-induced enhancement of pulse-induced phototropism has been shown to occur primarily through the action of phyA (Parks et al., 1996; Janoudi et al., 1997a, 1997b). As shown in Figure 2, this also appears to be the case with the enhancement of phototropic responses induced by long-term BL exposures, since only the phyA mutation affects the RL-induced recovery/enhancement response. The phyA single and nph4 phyA double mutants fail to respond to a RL-pre-irradiation, whereas phyB single and nph4 phyB double mutants were indistinguishable from wild type and nph4, respectively. Thus, it appears that phyA, but not phyB, is necessary and sufficient to mediate the RL-dependent low fluence response leading to enhancement of pulse- (Parks et al., 1996; Janoudi et al., 1997a, 1997b) and long-term BL-induced phototropism.
Figure 2.
Fluence response relationships for RL-dependent enhancement of BL-induced phototropism in nph4 phyA (A) and nph4 phyB (B) double mutant seedlings. Seedlings were handled as described in Figure 1A. Data represent the mean response of at least 63 seedlings from three replicate experiments. Vertical error bars represent the se values. ●, Wild type; ○, nph4; ⋄, phyA; ♦, nph4 phyA; □, phyB; ▪, nph4 phyB.
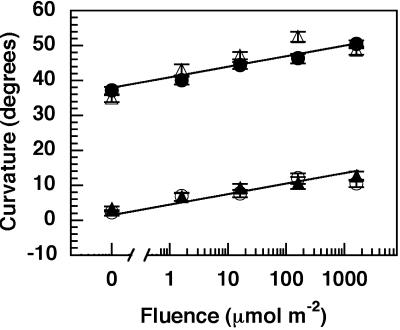
Given the abundant nature of phyA in etiolated Arabidopsis seedlings (Somers and Quail, 1995a, 1995b), one might predict that there is ample phyA to drive the RL-dependent low fluence response leading to phototropic enhancement. The findings that Arabidopsis seedlings overexpressing oat phyA are no more sensitive to RL than wild type or nph4 when carried in either of these backgrounds (Fig. 3) are consistent with this idea. Although we cannot completely rule out the possibility that the oat phyA is non-functional with respect to the phototropic response, it seems unlikely given that Arabidopsis seedlings carrying this transgene exhibit increased light sensitivity for a number of other phyA-dependent processes (Boylan and Quail, 1991; Whitelam et al., 1992).
Figure 3.
RL-dependent enhancement of phototropism induced by long-term irradiation with BL in nph4 seedlings overexpressing phyA. Seedlings were handled as described in Figure 1A. Data represent the mean response of at least 33 seedlings from two replicate experiments. Vertical error bars represent the se values. ●, Wild type; ○, nph4; ▵, phyA overexpressor (AOX); ▴, nph4 AOX.
nph4 Mutant Seedlings of Arabidopsis Lack Pulse-Induced BL-Dependent Phototropism Independent of Phytochrome Action
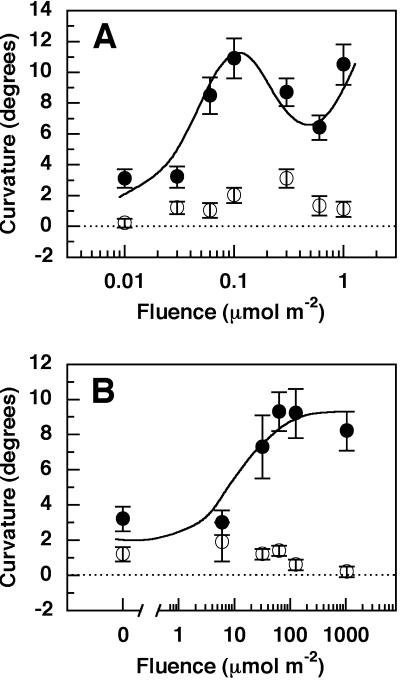
Previous studies have indicated that etiolated seedlings use the same photosensory-response system to achieve phototropic curvatures in response to a pulse of light or a long-term irradiation (Poff et al., 1994). Given this knowledge and the similarities of the phytochrome-dependent recovery of phototropism in the nph4 mutant background and enhancement of phototropism in wild type under long-term irradiations, it seemed probable that nph4 seedlings would also be similar to wild type with respect to phytochrome effects on pulse-induced phototropism. As shown in Figure 4A, nph4 seedlings were aphototropic in response to pulsed BL, as expected from their aphototropic phenotype in extended irradiation conditions (Liscum and Briggs, 1996; Stowe-Evans et al., 1998; Harper et al., 2000). However, unlike what was observed under extended irradiation conditions, nph4 mutant seedlings pre-irradiated with similar fluences of RL remained aphototropic after exposure to pulsed BL (Fig. 4B). The failure of nph4 seedlings to recover pulse-induced phototropism in response to RL pretreatment suggests that a fundamental difference might exist between the phytochrome signal-response pathways leading to phototropic enhancement in wild-type seedlings under pulsed versus long-term irradiation conditions.
Figure 4.
Pulse-induced phototropism in wild-type and nph4 mutant seedlings. A, Fluence response curves for BL pulse-induced phototropism. Seventy-two-h-old etiolated seedlings were irradiated with five pulses of BL at the indicated fluence, and 2 h after the final pulse, curvatures were measured as described in “Materials and Methods.” Data represent the mean response of at least 28 seedlings from five replicate experiments. B, Fluence response curves for RL-dependent enhancement of BL pulse-induced phototropism. Experiments were as described for A, except that 2 h prior to BL irradiation (five pulses at 0.03 μmol m−2 s−1), seedlings were exposed to the indicated fluence of RL. Data represent the mean response from at least 15 seedlings from three replicate experiments. ●, Wild type; ○, nph4.
DISCUSSION
The R/FR-Reversible Low Fluence Response Modulating the Magnitude of Phototropin-Dependent Phototropism Is Regulated Primarily by phyA
In recent years it has become clear that despite its apparent physiological simplicity, phototropism in the natural environment is likely to be modulated by several interacting photosensory-response systems (Iino, 1990; Liscum and Stowe-Evans, 2000). For example, whereas the direction of curvature is determined by the perception and transduction of BL signals via specific BL-absorbing receptors such as phototropin (nph1), the magnitude of curvature appears to be controlled in large part through the action of the primarily R/FR-absorbing phytochromes. This has been best illustrated in the laboratory by two sets of experiments. First, seedlings lacking phototropin are incapable of eliciting phototropic curvatures in response to low fluence rate BL (Liscum and Briggs, 1995; Lascève et al., 1999; Sakai et al., 2000). Second, phyA-deficient seedlings, although capable of establishing a phototropic response, exhibit dramatically reduced curvatures relative to wild type when exposed to sequential RL and BL irradiations (Parks et al., 1996; Janoudi et al., 1997a; Fig. 2).
In contrast to phyA, phyB appears to play a limited role in the modulation of phototropism in etiolated seedlings. As an example, phyB single mutants, in contrast to their phyA-deficient counterparts, show little, if any, change in the magnitude of their phototropic responsiveness (Parks et al., 1996; Janoudi et al., 1997a; Fig. 2). A dominant role for phyA in the modulation of phototropism in etiolated seedlings as they first emerge from the soil would not be unexpected since phyA is very abundant at that stage of development (Batschauer, 1999; Casal, 2000; Neff et al., 2000). Also, as shown previously, phyA does not appear to be limiting for phototropism in etiolated seedlings (Janoudi et al., 1997a; Fig. 3). However, the action of phyB could be significant during or after the deetiolation process when PHYA transcript and protein abundance falls precipitously (Lissemore and Quail, 1988; Vierstra, 1994; Clough et al., 1999) and phyB has more profound effects on photomorphogenesis in general (Casal, 2000; Neff et al., 2000). Janoudi et al. (1997a) reported that an etiolated phyB overexpressing line exhibited increased phototropic curvatures after RL pre-irradiation at fluences ≥100 μmol m−2. Under such conditions, the level of phyA should be intermediate between those of seedlings exposed to a long-term irradiation and those never exposed to light. Under such a condition, the amount of phyA may be sufficient for the modulation of phototropism, but no longer be in excess, such that higher levels of phyB can now exert an effect.
The observation that the modulatory effects of phyA on phototropism occur via a prototypical R/FR-reversible RL-dependent low fluence response (Figs. 1 and 2; Table I) represents one of the most significant findings of the present work. It has become almost dogmatic to think of phyA as a FR sensor that mediates very low fluence responses and high irradiance responses through non-photoreversible processes, and that phyB (or other light-stable phy species) functions as a R/FR-reversible RL sensor (Batschauer, 1999; Neff et al., 2000). Although the effects of phyA on phototropism in Arabidopsis were previously determined to be induced by RL rather than FR and to occur within the low fluence response range (Parks et al., 1996; Janoudi et al., 1997a, 1997b), R/FR reversibility was less clear (Janoudi and Poff, 1992). Liu and Iino (1996b) demonstrated that RL-dependent enhancement of phototropism in maize coleoptiles is R/FR reversible; however, they were unable to assign a particular phytochrome species to the response. FR-induced hypocotyl growth inhibition in Arabidopsis was recently shown to be a photoreversible phyA-dependent response, but this response is a high-irradiance response and is induced by repeated frequent pulses of FR whose effects are RL reversible (Shinomura et al., 2000). Thus, to our knowledge, the results presented here represent the first unambiguous example of an R/FR-reversible phyA-dependent RL-induced low fluence response.
Complete Loss-of-Function nph4/arf7 Mutants Are Phototropically Unresponsive to BL Alone, But Retain a phyA-Dependent Modulatory Response Indistinguishable from That of Wild-Type under Extended Irradiation Conditions
Previous studies demonstrated that phytochrome modulation of BL-dependent phototropism does not occur through alterations in the sensitivity of the phototropic receptor, e.g. phototropin, (Chon and Briggs, 1966; Janoudi and Poff, 1991; Liu and Iino, 1996a), but rather by altering the abundance and/or activity of some downstream component of the phototropic signal-response pathway that is rate limiting (Iino, 1990; Poff et al., 1994). To date, genetic studies of phototropism have identified only a single locus, NPH4, that has properties consistent with a potential target of phytochrome action. nph4 mutations disrupt multiple differential growth responses, including phototropic and gravitropic responses, suggesting that the NPH4 protein functions as a regulator of growth after the convergence of signal-response pathways initiated by distinct stimuli (Liscum and Briggs, 1996; Stowe-Evans et al., 1998). In addition, complete loss-of-function alleles are semidominant, implying that activity of the NPH4 protein is dose dependent and normally rate limiting (Liscum and Briggs, 1996; Stowe-Evans et al., 1998). The finding that NPH4 encodes the auxin-responsive transcriptional activator ARF7 (Harper et al., 2000), which can function as a homodimer or heterodimer with other ARFs or AUX/IAA proteins (Ulmasov et al., 1999a, 1999b), is consistent with the idea that NPH4 modulates multiple responses.
Etiolated nph4 mutant seedlings fail to exhibit hypocotyl phototropism in response to low fluence BL, given in pulses (Fig. 4) or in an extended irradiation (Liscum and Briggs, 1995, 1996; Stowe-Evans et al., 1998; Harper et al., 2000; Fig. 1). Thus, if NPH4 is a target for phytochrome action, nph4 seedlings should also lack phototropism under light conditions where phototropin and phytochrome are activated. However, results from a previous study suggested this was not the case (Liscum and Briggs, 1996). In the present study we found that etiolated nph4 seedlings, while failing to exhibit any phototropic response under pulse-irradiation conditions, independent of phytochrome status (Fig. 4), in fact retained a phyA-dependent phototropic enhancement response under long-term irradiation conditions that is indistinguishable from wild type (Figs. 1, 2, and 3; Table I). Thus, although NPH4 remains a possible target for phytochrome action under limited light conditions, it is apparently not a target under non-limiting light conditions (e.g. long-term irradiation).
A Potential Mechanism for Phytochrome Modulation of Phototropic Curvatures: phyA-Dependent Activation of a Second ARF System
The biochemical function of NPH4/ARF7 (Ulmasov et al., 1999a, 1999b), together with the phototropic defects of loss-of-function nph4 mutants in BL (Liscum and Briggs, 1996; Stowe-Evans et al., 1998), indicates that changes in auxin-dependent gene expression are necessary for sustained BL-dependent phototropism (Harper et al., 2000). By extension, recovery of phototropic responsiveness in complete loss-of-function nph4 mutants under conditions where phototropin and phytochromes are activated likely also requires changes in gene expression. Given the large number of ARF proteins in Arabidopsis and their apparent overlapping temporal and spatial expression patterns (Kim et al., 1997; Ulmasov et al., 1999b), it is probable that phytochrome photoconversion results in the conditional activation of a second ARF system. This hypothesis is not only consistent with the observed phyA-dependent recovery of phototropism in nph4, but also the enhancement of phototropism in wild-type Arabidopsis.
Two obvious mechanisms exist by which phyA might activate the proposed second ARF system. First, phyA action might alter the metabolism and/or transport of auxin, resulting in local increases in auxin concentration. This increase would result in the partial activation of an ARF with lower, but overlapping, sensitivity to auxin relative to that of NPH4/ARF7. Second, rather than affecting auxin levels, phyA might alter the auxin sensitivity of another ARF complex. In either case, the additional ARF activity would be expected to enhance auxin-induced transcription in the presence of NPH4/ARF7, whereas it would only partially compensate for the loss of NPH4/ARF7. As an example, at least two ARF proteins could be required for changes in gene expression necessary for the development and maintenance of phototropic curvature; NPH4/ARF7 in limited light and another ARF under extended irradiation conditions. Implicit in this hypothesis is the expectation that the activity of the second ARF would increase upon irradiation in a fluence- and time-dependent fashion. These predictions are concordant with the observed affects of phyA action on the magnitude of phototropic curvature in wild-type and nph4 mutant seedlings under extended irradiation conditions (Figs. 1 and 2; Table I).
Is there any experimental support for phytochrome-dependent increases in auxin concentration and/or sensitivity? Although a molecular mechanism remains unknown, increases in polar transport and absolute levels of auxin have been observed in dicot stems under conditions where phytochrome is activated (Eleizer and Morris, 1980; Behringer and Davies, 1992; Shinkle et al., 1998). Direct evidence in support of phytochrome-dependent changes in auxin sensitivity has been more elusive. However, results from several independent studies are suggestive of such a mechanism. For example, mutations in three members of the Aux/IAA gene family of Arabidopsis, SHY2/IAA3, AXR2/IAA7, and AXR3/IAA17, lead to alterations in subsets of auxin and phytochrome responses (Rouse et al., 1998; Soh et al., 1999; Tian and Reed, 1999; Nagpal et al., 2000). Members of the Aux/IAA gene family are transcriptionally induced within minutes of auxin application and encode short-lived nuclear-localized proteins that appear to function as repressors of auxin-induced gene expression via heterodimerization with ARF proteins (Kim et al., 1997; Guilfoyle et al., 1998a, 1998b; Ulmasov et al., 1999b). It is interesting that a recent study by Colón-Carmona et al. (2000) has shown that phyA can interact with and phosphorylate Aux/IAA proteins in vitro. Thus, one possible mechanism by which phytochrome could influence auxin sensitivity of an ARF might be through the modulation of stability and/or activity of an Aux/IAA protein(s) interacting with the second ARF protein. Given that nuclear translocation of phyA occurs within approximately 2 to 10 min of a pulse of low fluence RL (Kircher et al., 1999; Hisada et al., 2000), this is certainly a temporally plausible explanation for phyA-dependent phototropic enhancement, which exhibits a time threshold of approximately 5 to 10 min (Steinitz and Poff, 1986; Janoudi et al., 1992; Liu and Iino, 1996b). We have recently identified several second-site mutations that appear to specifically disrupt the phyA-dependent recovery of phototropism in the nph4-1 null mutant background (E.L. Stowe-Evans and E. Liscum, unpublished data), and expect that molecular analyses of these mutants will allow us to directly address the hypotheses presented here for the action of phyA in phototropic enhancement.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Arabidopsis mutant and transgenic lines used in these studies have been described elsewhere: nph4-1 and nph4-3 (Liscum and Briggs, 1996); phyA-211 (Nagatani et al., 1993); phyB-9 (Reed et al., 1993); AOX (Boylan and Quail, 1991). With the exception of the AOX line, which is in the Nossen ecotype, all lines are carried in the Columbia ecotype.
Double mutants were selected from segregating F2 populations that resulted from a self-pollination of an F1 generated by crossing desired genotypes. Putative nph4-3 phyA-211 double mutants were first selected for the phyA mutation as seedlings failing to exhibit FR-dependent hypocotyl growth inhibition (Nagatani et al., 1993). Next, plants carrying the nph4-3 allele were identified by PCR analysis. DNA from mutant lines were amplified by PCR using primers (5′-TTAGTATCTCTGTATTGCCTTAGT-3′ and 5′-AGTGCCTTTTTGGTTGAC-3′) that flank the insertion/deletion in the nph4-3 allele (Harper et al., 2000). PCR products containing the nph4-3 mutation (191 bp) were easily resolved from wild-type products (246 bp) by separation on 2.0% (w/v) agarose gels. Putative nph4-1 phyB-9 double mutants were first selected for the phyB mutation as seedlings failing to exhibit RL-dependent hypocotyl growth inhibition (Reed et al., 1993). These plants were potted to soil and were allowed to self-fertilize. Plants carrying the nph4-1 mutation were selected in the resultant F3 generation by their failure to exhibit BL-induced hypocotyl phototropism (Stowe-Evans et al., 1998). Putative nph4-1 AOX double mutants were selected as etiolated seedlings lacking BL-dependent phototropism and exhibiting kanamycin resistance (carried on the transgene) when transferred to continuous white light. For all analysis using double mutants, F3 or F4 generation seed was used.
All treatment of Arabidopsis seeds and growth of seedlings were as described previously (Stowe-Evans et al., 1998) with the following exception. For pulse-induced phototropism experiments, seeds were surface sterilized as described previously (Stowe-Evans et al., 1998), air dried on filter paper, and planted in single rows onto agar-solidified (1.0%, w/v) one-half-strength Murashige-Skoog media (approximately 4 mL) on the surface of 75- × 50- × 1-mm microscope slides. Slides were oriented on edge to allow seedlings to grow along the surface of the agar. After cold treatment and induction of germination (Stowe-Evans et al., 1998), seedlings were grown in darkness at 22°C for 64 h (long-exposure experiments) or 72 h (for pulse-induced phototropism experiments) prior to light treatments.
Light Sources
BL for long-exposure experiments was obtained by filtering light from one fluorescent black light bulb (F15T8-BL, General Electric, Fairfield, CT) through one layer of blue acrylic (Rohm and Haas, no. 2424, 3.18 mm thick; Cope Plastics, St. Louis). BL for pulse-induced phototropism experiments was obtained from a single unfiltered blue light-emitting diode (LED). An unfiltered infra-red LED was used as background lighting in pulse-induced phototropism experiments to allow capturing of digital images in the absence of visible light (Hangarter, 1997). With the exception of photoreversibility experiments, RL was obtained by filtering light from gold fluorescent bulbs (F40/GO, Sylvania, Danvers, MA) through two layers of red acrylic (Rohm and Haas, no. 2423, 3.18 mm thick; Cope Plastics). For photoreversibility experiments RL was obtained by filtering light from one halogen lamp (75W Quartzline, General Electric) through 5 cm of 1% (w/v) CuSO4 and one layer of red acrylic. FR was obtained by filtering light from the halogen lamp through 5 cm of water and one layer of FR acrylic (Rohm and Haas, no. FRF 700, 3.18 mm thick; AIN Plastics, San Jose, CA). Fluence rates were controlled by a combination of altering the distance between the source and plant material, and use of varying amounts of cheesecloth as a neutral density filters. Fluence rates of BL and RL sources were measured with a quantum photometer (Li190SA, LI-COR, Lincoln, NE), whereas UV-A and FR sources were measured with a portable spectroradiometer (Li1800, LI-COR).
Phototropic Assays
For long-exposure experiments, seedlings were exposed to 8 h of unilateral irradiation with BL at a fluence rate of 0.5 μmol m−2 s−1. For enhancement experiments, RL was provided from above and began concurrently with the unilateral BL irradiation (a total of 4 h of exposure). With the exception of reciprocity experiments, the fluence rate of RL was 1.6 μmol m−2 s−1, and the length of the RL exposure varied depending upon the desired final fluence. For reciprocity experiments the fluence rate and exposure time were varied to achieve a fluence of 160 μmol m−2. Phototropic curvatures were determined at the end of the unilateral BL or UV-A exposure as described previously (Liscum and Briggs, 1995).
Pulse-induced phototropism was induced with five pulses of BL at a fluence rate of 0.002 μmol m−2 s−1, each separated by a 20-min dark period (Steinitz and Poff, 1986). For RL-dependent enhancement of pulse-induced phototropism, RL at a fluence rate of 1.6 μmol m−2 s−1 was given 2 h prior to the first BL pulse. Different fluences of BL and RL were obtained by varying the irradiation time. Photographs of seedlings were taken before the first BL pulse (T0) and 2 h after the final pulse (T210) with a digital camera (QuickCam, Connectix, San Mateo, CA) modified to allow image capturing under ambient infra-red light (Hangarter, 1997). Digital images were then printed and curvatures were measured with a protractor. Final curvatures for each seedling were determined by subtracting curvatures at T0 from those observed at T210.
ACKNOWLEDGMENTS
We thank Drs. Tobias Baskin and Karen Cone for critical reading of the manuscript, and the Liscum laboratory for many helpful discussions during the course of these studies. We also thank Drs. Jason Reed and Peter Quail for donating phytochrome mutant/transgenic seed. Last, we would like to thank Dr. Roger Hangarter for helpful discussions about the QuickCam system.
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–9723124 to E.L.) and by the University of Missouri Research Board (grant no. RB96–055 to E.L). E.L.S.-E. was supported by a predoctoral fellowship from the University of Missouri Maize Biology Training Program, a unit of the U.S. Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Research in Plant Biology Program. D.R.L. was supported by a University of Missouri Undergraduate Arts and Sciences Fellowship.
LITERATURE CITED
- Batschauer A. Light perception in higher plants. Cell Mol Life Sci. 1999;55:153–165. doi: 10.1007/s000180050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer FJ, Davies PJ. Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark- and light-grown Pisum seedlings. Planta. 1992;188:85–92. doi: 10.1007/BF00198943. [DOI] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA. 1991;88:10806–10810. doi: 10.1073/pnas.88.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chon HP, Briggs WR. Effect of red light on the phototropic sensitivity of corn coleoptiles. Plant Physiol. 1966;41:1715–1724. doi: 10.1104/pp.41.10.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR. Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA. 1999;96:8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Clough RC, Jordan-Beebe ET, Lohman KN, Marita JM, Walker JM, Gatz C, Vierstra RD. Sequences within both the N- and C-terminal domains of phytochrome a are required for PFR ubiquitination and degradation. Plant J. 1999;17:155–167. doi: 10.1046/j.1365-313x.1999.00360.x. [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A, Chen DL, Yeh K-C, Abel S. Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 2000;124:1739–1751. doi: 10.1104/pp.124.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleizer J, Morris DA. Cell length, light and 14C-labeled indol-3yl-acetic acid transport in Pisum sativum L. and Phaseolus vulgaris L. Planta. 1980;149:327–331. doi: 10.1007/BF00571165. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G, Ulmasov T, Murfett J. How does auxin turn on genes? Plant Physiol. 1998a;118:341–347. doi: 10.1104/pp.118.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ, Ulmasov T, Hagen G. The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell Mol Life Sci. 1998b;54:619–627. doi: 10.1007/s000180050190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP. Gravity, light and plant form. Plant Cell Environ. 1997;20:796–800. doi: 10.1046/j.1365-3040.1997.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissues. Plant Cell. 2000;12:757–770. doi: 10.1105/tpc.12.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada A, Hanzawa H, Weller JL, Nagatani A, Reid JB, Furuya M. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell. 2000;12:1063–1078. doi: 10.1105/tpc.12.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han I-S, Larsen E, Briggs WR. Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- Iino M. Phototropism: mechanisms and ecological implications. Plant Cell Environ. 1990;13:633–650. [Google Scholar]
- Janoudi A-K, Gordon WR, Wagner D, Quail P, Poff KL. Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis thaliana. Plant Physiol. 1997a;113:975–979. doi: 10.1104/pp.113.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Konjevic R, Poff KL. Time threshold for second positive phototropism is decreased by a pre-irradiation with red light. Plant Physiol. 1992;99:1422–1425. doi: 10.1104/pp.99.4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Konjevic R, Whitelam G, Gordon W, Poff KL. Both phyA and phyB are required for normal expression of phototropism in Arabidopsis thaliana seedlings. Physiol Plant. 1997b;101:278–282. [Google Scholar]
- Janoudi A-K, Poff KL. Characterization of adaptation in phototropism of Arabidopsis thaliana. Plant Physiol. 1991;95:517–521. doi: 10.1104/pp.95.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Poff KL. Action spectrum for enhancement of phototropism by Arabidopsis thaliana seedlings. Photochem Photobiol. 1992;56:655–659. [Google Scholar]
- Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Kozma-Bognar L, Kim L, Adam E, Harter K, Schäfer, Nagy F. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR. Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol. 1999;120:606–614. doi: 10.1104/pp.120.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR. Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol. 1996;112:291–296. doi: 10.1104/pp.112.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Stowe-Evans EL. Phototropism: a “simple” physiological response mediated by multiple interacting photosensory-response pathways. Photochem Photobiol. 2000;72:273–282. doi: 10.1562/0031-8655(2000)072<0273:pasprm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lissemore JL, Quail PH. Rapid transcriptional regulation by phytochrome of the genes for phytochrome and chlorophyll a/b-binding protein in Avena sativa. Mol Cell Biol. 1988;8:4840–4850. doi: 10.1128/mcb.8.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Iino M. Effect of red light on the fluence-response relationship for pulse-induced phototropism of maize coleoptiles. Plant Cell Environ. 1996a;19:609–614. doi: 10.1111/j.1365-3040.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Iino M. Phytochrome is required for the occurrence of time-dependent phototropism in maize coleoptiles. Plant Cell Environ. 1996b;19:1379–1388. doi: 10.1111/j.1365-3040.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–269. [Google Scholar]
- Motchoulski A, Liscum E. Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286:961–964. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123:563–573. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M, Fankhauser C, Chory J. Light: an indicator of time and place. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP. Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol. 1996;110:155–162. doi: 10.1104/pp.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff KL, Janoudi A-K, Rosen ES, Orbovi V, Konjevic R, Fortin M-C, Scott TK. The physiology of tropisms. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 639–664. [Google Scholar]
- Quail PH. The phytochrome family: dissection of functional roles and signaling pathways among family members. Phil Trans R Soc Lond B. 1998;353:1399–1403. doi: 10.1098/rstb.1998.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. Changes in auxin response from mutations in an Aux/IAA gene. Science. 1998;279:1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell. 2000;12:225–236. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon M, Christie JM, Knieb E, Lempert U, Briggs WR. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry. 2000;39:9401–9410. doi: 10.1021/bi000585+. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinkle J, Kadakia R, Jones A. Dim-red-light-induced increase in polar-auxin transport in cucumber seedlings. Plant Physiol. 1998;116:1505–1513. doi: 10.1104/pp.116.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Uchida K, Furuya M. Elementary processes of photoperception by phytochrome A for high-irradiance response of hypocotyl elongation in Arabidopsis. Plant Physiol. 2000;122:147–156. doi: 10.1104/pp.122.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh MS, Hong SH, Kim BC, Vizir I, Park PH, Choi G, Hong MY, Chung Y-Y, Furuya M, Nam HG. Regulation of both light- and auxin-mediated development by the Arabidopsis IAA3/SHY2 gene. J Plant Biol. 1999;42:239–246. [Google Scholar]
- Somers DE, Quail PH. Phytochrome-mediated light regulation of PHYA- and PHYB-GUS transgenes in Arabidopsis thaliana seedlings. Plant Physiol. 1995a;107:523–534. doi: 10.1104/pp.107.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Quail PH. Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J. 1995b;7:413–427. doi: 10.1046/j.1365-313x.1995.7030413.x. [DOI] [PubMed] [Google Scholar]
- Steinitz B, Poff KL. A single positive phototropic response induced with pulsed light in hypocotyls of Arabidopsis thaliana seedlings. Planta. 1986;168:305–315. doi: 10.1007/BF00392354. [DOI] [PubMed] [Google Scholar]
- Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E. NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 1998;118:1265–1275. doi: 10.1104/pp.118.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. Activation and repression of transcription by auxin response factors. Proc Natl Acad Sci USA. 1999a;96:5844–5849. doi: 10.1073/pnas.96.10.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. Dimerization and DNA binding of auxin response factors. Plant J. 1999b;19:1–11. doi: 10.1046/j.1365-313x.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- Vierstra RD. Phytochrome degradation. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 141–162. [Google Scholar]
- Watahiki MK, Tatematsu K, Fujihira K, Yamamoto M, Yamamoto KT. The MSG1 and AXR1 genes of Arabidopsis are likely to act independently in growth-curvature responses of hypocotyl. Planta. 1999;207:362–369. doi: 10.1007/s004250050493. [DOI] [PubMed] [Google Scholar]
- Watahiki MK, Yamamoto KT. The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol. 1997;115:419–426. doi: 10.1104/pp.115.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, McCormac AC, Boylan MT, Quail PH. Photoresponses of Arabidopsis seedlings expressing an introduced oat phyA cDNA: persistence of etiolated plant type responses in light-grown plants. Photochem Photobiol. 1992;56:617–621. [Google Scholar]
- Whitelam GC, Patel S, Devlin PF. Phytochrome and photomorphogenesis in Arabidopsis. Phil Trans R Soc Lond B. 1998;353:1445–1453. doi: 10.1098/rstb.1998.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]