Abstract
Cytoadherence of Plasmodium falciparum-infected erythrocytes (PRBC) to endothelial cells causes severe clinical disease, presumably as a of result perfusion failure and tissue hypoxia. Cytoadherence to endothelial cells is increased by endothelial cell activation, which is believed to occur in a paracrine fashion by mediators such as tumor necrosis factor alpha (TNF-α) released from macrophages that initially recognize PRBC. Here we provide evidence that PRBC directly stimulate human endothelial cells in the absence of macrophages, leading to increased expression of adhesion-promoting molecules, such as intercellular adhesion molecule 1. Endothelial cell stimulation by PRBC required direct physical contact for a short time (30 to 60 min) and was correlated with parasitemia. Gene expression profiling of endothelial cells stimulated by PRBC revealed increased expression levels of chemokine and adhesion molecule genes. PRBC-stimulated endothelial cells especially showed increased expression of molecules involved in parasite adhesion but failed to express molecules promoting leukocyte adhesion, such as E-selectin and vascular cell adhesion molecule 1, even after challenge with TNF-α. Collectively, our data suggest that stimulation of endothelial cells by PRBC may have two effects: prevention of parasite clearance through increased cytoadherence and attenuation of leukocyte binding to endothelial cells, thereby preventing deleterious immune reactivity.
Endothelial cells play a key role during the course of infection with the human malarial pathogen Plasmodium falciparum. Erythrocytes (RBC) infected with P. falciparum (PRBC) bind via P. falciparum erythrocyte membrane protein 1 to a number of host endothelial cell receptors, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), platelet endothelial cell adhesion molecule 1 (PECAM-1), P-selectin, E-selectin, CD36, thrombospondin, and chondroitin sulfate A (2, 3, 18, 19, 26). Cytoadherence of PRBC to endothelial cells is considered an escape mechanism for P. falciparum to evade clearance of PRBC by phagocytosing macrophages in secondary lymphoid tissues like the spleen (5). At the same time, binding of PRBC to microvessels in different organs is responsible for microvessel obstruction and subsequent perfusion deficits and may lead to development of organ failure. Binding of PRBC to microvessels bears similarities to the interaction between circulating leukocytes and resident endothelial cells and in part depends on the same key molecules expressed by endothelial cells (10).
Importantly, cytoadherence of PRBC or leukocytes to endothelial cells is believed to increase following activation of endothelial cells by paracrine proinflammatory mediators, and tumor necrosis factor alpha (TNF-α) is the most prominent factor (20). Monocytes/macrophages, as well as dendritic cells, have been shown to release TNF-α after contact with PRBC (31) and thus to contribute through paracrine endothelial cell activation to both immune-mediated pathology and parasite immune escape. However, a recent report demonstrated that P. falciparum parasites impair the immunostimulatory function of monocytes/macrophages, as well as dendritic cells, thus preventing the release of proinflammatory mediators and full activation of the immune response (38). This raises the question of how endothelial cell activation and increased PRBC or leukocyte binding are mediated in the absence of paracrine proinflammatory mediators, as suggested in preliminary experiments by Udeinya et al. (35, 37). Here we provide evidence that P. falciparum-infected erythrocytes directly stimulate primary human endothelial cells. Endothelial cell stimulation required physical contact with PRBC and was correlated with parasite numbers. Contact with PRBC induced increased expression of adhesion molecules, such as ICAM-1 and CD44, and chemokines, such as CCL20 and CCL2. Gene expression profiling of PRBC-stimulated endothelial cells revealed a complex pattern of up-regulated gene expression that is compatible with a proadhesive endothelial phenotype. We concluded that endothelial cells, besides serving as a bystander cell population, are directly involved in pathogenesis during P. falciparum infection.
MATERIALS AND METHODS
Culture of P. falciparum and human cells.
P. falciparum clones were cultured in A+ human erythrocytes and serum as described previously (32). The following P. falciparum clones were used in this study: FCR3 (29), 3D7 (39), ITO4-A4 (27), and ItG-ICAM (18). Human umbilical vein endothelial cells (HUVEC) were purchased from PromoCell (Heidelberg, Germany) and cultured as recommended by the manufacturer. Cells and P. falciparum clones were free of Mycoplasma contamination (Minerva Biolabs, Berlin, Germany).
Endothelial cell activation assays.
Human primary endothelial cells (passages 5 to 7) were grown in 6- or 12-well plates for at least 2 to 4 days before cells were subjected to experiments. Infected erythrocytes were purified by gelatin flotation (12). In brief, 1 volume of RBC was resuspended in 9 volumes of 0.5% gelatin type A from porcine skin (Sigma) in RPMI 1640 and incubated in a water bath at 37°C for 1 h. Activation assays were performed with 5 × 106 gelatin-enriched PRBC/ml in endothelial cell growth medium (without hydrocortisone and amphotericin). FCR3 parasites were used in all experiments, unless specified otherwise. As a negative control, noninfected erythrocytes were grown for at least 1 day in RPMI 1640-10% human serum-20 μg/ml gentamicin and treated with gelatin. TNF-α (10 ng/ml) was used as a positive control for activation of endothelial cells. Endothelial cells and infected erythrocytes were cocultured for 30 min to 22 h, and analysis was performed after 6 to 22 h. Supernatants were collected, centrifuged, and stored at −20°C until analysis by an enzyme-linked immunosorbent assay (ELISA). For flow cytometry, cells were detached by gentle trypsin-EDTA treatment, washed with phosphate-buffered saline (PBS) containing 2% fetal calf serum and 0.1% NaN3 (fluorescence-activated cell sorting [FACS] buffer), and stained with the antibodies described below. For microarray analysis cells were washed with Hanks balanced salt solution, frozen in liquid nitrogen, and stored at −80°C.
Flow cytometry.
Flow cytometry of human cells was carried out as previously described (1). Pretreated HUVEC were screened for expression of endothelial cell markers and P. falciparum adhesion receptors by FACS analysis using the following antibodies: anti-ICAM-1, anti-VCAM-1, anti-E-selectin, and anti-CD31/PECAM-1 (all from BD Biosciences, Heidelberg, Germany); anti-CD44 (Biodesign International, England); and anti-CD36 (Immunotech, France). Cells were detached from the surface of 6- or 12-well plates by gentle trypsin-EDTA treatment, washed, and stained for 30 min on ice in 100 μl of the recommended dilution of primary antibody in FACS buffer. Cells were washed once in FACS buffer and then incubated with streptavidin-phycoerythrin (diluted 1:300; BD Biosciences) for 20 min at 4°C. Cells were washed as described above and fixed in PBS containing 2% formaldehyde. FACS analysis of 104 cells was carried out with a FACS Calibur using the CellQuest Pro software (BD Biosciences).
ELISA for determination of cytokine concentrations.
A sandwich ELISA for interleukin-6 (IL-6) in cell culture supernatants was performed according to the standard procedure. Briefly, flat-bottom microtiter plates (Nunc, Wiesbaden, Germany) were coated with antibody specific for IL-6 (clone MQ2-13A5; BD Biosciences) at a concentration of 2 μg/ml at 4°C. After 4 h of incubation, blocking was done with 1% bovine serum albumin-PBS. Three washing steps with PBS-Tween 0.5% were carried out between incubation steps. Cell culture supernatants were assayed in 100-μl (total volume) mixtures and incubated overnight at 4°C. The second specific anti-IL-6 antibody (clone MQ2-29C3, biotinylated; BD Biosciences) was used at a concentration of 2 μg/ml. Detection of bound biotinylated antibody was performed with avidin-horseradish peroxidase (1:1,000; Pierce). Recombinant IL-6 (R&D Systems) was used as a standard to obtain quantitative results. After addition of TMB peroxidase substrate, the optical density at 405 nm was determined with an ELISA reader from Thermo Life Sciences (Egelsbach, Germany). Quantikine ELISA kits for human CCL2 (monocyte chemoattractant protein 1), CCL20 (macrophage inflammatory protein 3α), pro-MMP-1, and CXCL8 (IL-8) were obtained from R&D Systems and used according to the manufacturer's suggestions.
RNA isolation and microarray analysis.
Cells were resuspended in 2 ml TRIZOL reagent (LifeTechnologies, United States), and RNA was prepared according to the manufacturer's instructions. To remove genomic DNA contamination, the remaining RNA was dissolved in diethyl pyrocarbonate-treated sterile water and treated with RNase-free DNase I (Promega, United States) together with the manufacturer-supplied buffer (Promega, United States) for 30 min at 37°C. RNA was extracted sequentially with phenol-chloroform-isoamyl alcohol (25:24:1) and chloroform before RNA was precipitated with ethanol. RNA was collected by centrifugation (4°C, 30 min, 16,000 × g), air dried, and resuspended in diethyl pyrocarbonate-treated sterile water. The RNA content was determined spectrophotometrically, and RNA integrity was evaluated by agarose gel electrophoresis. All RNA samples were isolated using the TRIZOL reagent according to manufacturer's instructions. In addition, the purified RNA was treated with RNase-free DNase I to digest any potentially contaminating DNA. Furthermore, the purity and integrity of the isolated RNA samples were routinely analyzed by means of an Agilent RNA pano or pico chip. Although we occasionally had to discard a sample due to a lack of RNA integrity, we never found any sample that contained genomic DNA.
To screen for the influence of erythrocytes infected with P. falciparum on the mRNA expression pattern of HUVEC, a microarray analysis was performed using a custom-designed oligonucleotide-based “migration array chip.” This chip contained duplicates of 611 genes encoding members of the families containing chemokines and their receptors, adhesion proteins, lysophospholipid receptors, matrix metalloproteinases, TNF and TNF receptors, and endothelial growth factors, as well as housekeeping genes. Dual-channel hybridization was carried out after amplification and labeling of 5 μg of DNase-free RNA of HUVEC cocultivated with RBC infected with P. falciparum or HUVEC cocultivated with noninfected erythrocytes with either UTP-cyanine 3 or UTP-cyanine 5 (1.25 mM; Amersham Pharmacia Biotech) using the cDNA synthesis system (Roche, Germany) together with a MEGAscript T7 kit (Ambion Inc., United States). One hundred nanograms of each cDNA, synthesized according the manufacturer's instructions, was used during the labeling reaction with the MEGAscript T7 kit system, following the manufacturer's instructions but halving the volumes. During preparation of the labeled probes, cDNA and labeled RNA were purified using a High Pure tissue kit (Roche, Germany) according the manufacturer's protocol. Ten micrograms of Cy5- and Cy3-labeled cRNAs obtained from mock-treated HUVEC or HUVEC cocultured with RBC infected with P. falciparum was mixed and hybridized to microarrays in hybridization solution (MWG Biotech) at 42°C overnight and then washed sequentially in 2× SSC-0.1% sodium dodecyl sulfate, 1× SSC, and 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Hybridized arrays were scanned at maximal resolution for both channels using an Affymetrix 428 scanner at variable PMT voltage settings to obtain maximal signal intensities without probe saturation. Using the ImaGene software (version 5.0; MWG-Biotech), the signal intensities for each spot and the corresponding background were estimated. The local background corrected the raw scanning data for each gene. After calculation of the mean of the two replicated values for each gene on the chip at all PMT voltage settings (four to seven) and logarithmic transformation, linear regression analyses were performed to calculate the corrected signal intensity for each gene at the arbitrary PMT voltage setting of 55. To normalize, first nonvariable genes were identified by F-test statistic and equivalence analysis. Based on the mean intensities of these nonvariable genes, each signal on the array was normalized, and subsequently, genes significantly differentially expressed in untreated HUVEC and HUVEC cocultured with RBC infected with P. falciparum were determined by using the “Significance Analysis of Microarray” (SAM) software (34). Furthermore, hierarchical clustering of genes identified by SAM as statistically significantly differently expressed was performed using the Tiger software (version 2.2) with the average linkage clustering method (distance metric, Euclidean) (6, 28).
RESULTS
P. falciparum-infected erythrocytes induce ICAM-1 and IL-6 gene expression in human endothelial cells.
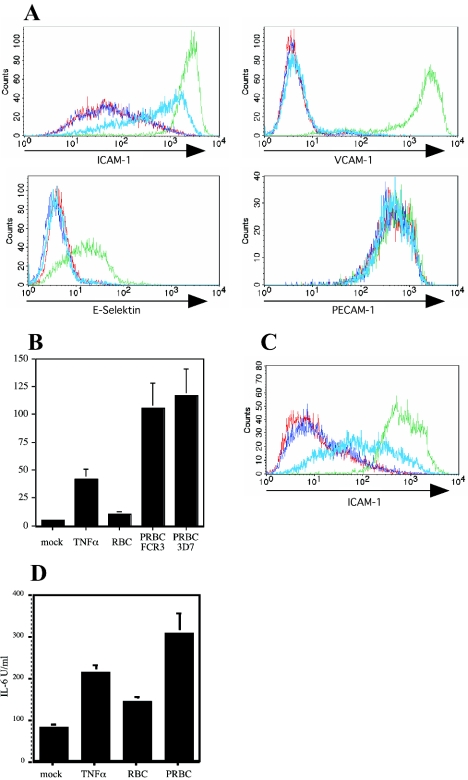
Human erythrocytes infected with parasites of the well-characterized P. falciparum clonal line FCR3 were incubated with HUVEC. Figure 1A demonstrates that erythrocytes infected with FCR3 parasites (PRBC) induced increased endothelial surface expression levels of ICAM-1 but not of VCAM-1 or E-selectin, whereas TNF-α stimulation of HUVEC led to increased expression of all these molecules. In cultures of endothelial cells incubated with PRBC no TNF-α was detected (data not shown), eliminating the possibility of contamination of cell preparations with macrophages. Erythrocytes alone did not alter the endothelial expression levels of any of these molecules, and constitutive PECAM-1 expression was not modified by soluble TNF-α or PRBC (Fig. 1A). TNF-α-mediated endothelial cell activation led to high and homogeneous expression levels of ICAM-1, whereas the ICAM-1 surface expression induced by PRBC was lower and more variable (Fig. 1A). As a further marker for endothelial cell activation, we detected large concentrations of IL-6 in cell culture supernatants of HUVEC incubated with PRBC (Fig. 1B). Interestingly, HUVEC released large amounts of IL-6 in response to PRBC (Fig. 1B). Similarly, P. falciparum clonal line 3D7 mediated comparable levels of endothelial cell activation (Fig. 1B). Human lung endothelial cells showed a similar reaction pattern with PRBC (Fig. 1C and D), demonstrating that PRBC-induced activation was not restricted to HUVEC.
FIG. 1.
P. falciparum-infected erythrocytes induce endothelial cell activation. (A) Cell surface expression levels of ICAM-1, VCAM-1, E-selectin, and PECAM-1 were determined by indirect immunostaining on HUVEC after incubation for 20 h with medium alone (red line), RBC alone (dark blue line), TNF-α (10 ng/ml) (green line), or PRBC infected with P. falciparum clone FCR3 (light blue line). (B) HUVEC were cultured in the presence of medium, RBC, TNF-α, or PRBC. Cell culture supernatants were collected after 20 h, and the IL-6 concentration was measured by the ELISA. IL-6 secretion by HUVEC treated with TNF-α (P < 0.001, compared with the medium control; n = 8) and with P. falciparum-infected erythrocytes (P < 0.001, compared with the RBC control; n = 8) was significantly increased. (C and D) Surface expression of ICAM-1 on human lung endothelial cells (C) and cell culture supernatant concentration of IL-6 (D). Human lung endothelial cells were treated as described above for panel A.
P. falciparum-mediated activation of endothelial cells requires direct and short physical contact.
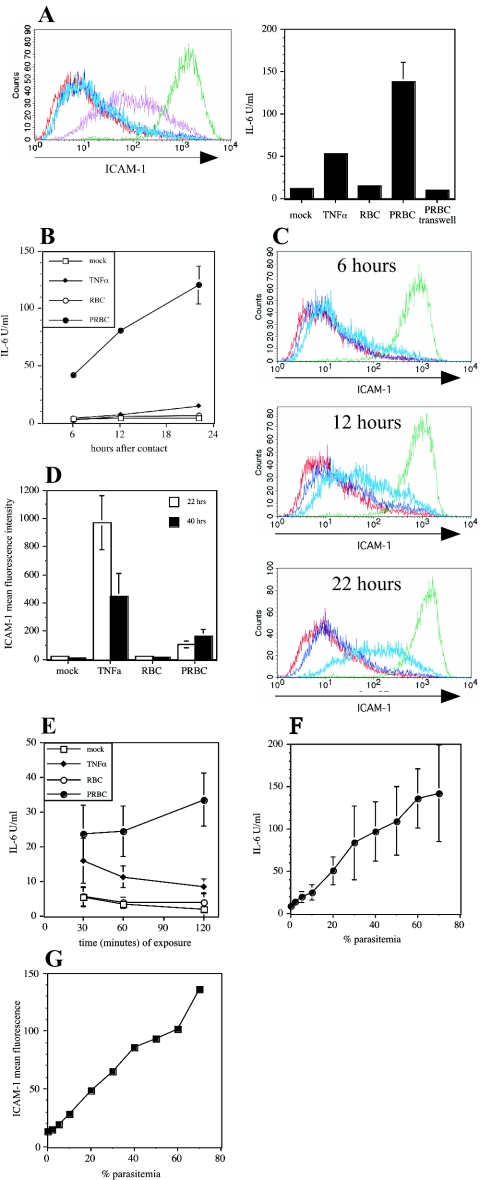
In order to characterize whether soluble factors released from PRBC or direct physical contact was required for endothelial cell activation, we performed transwell experiments. HUVEC were cultured in the bottom chamber, and PRBC were loaded into the top chamber. Figure 2A clearly demonstrates that direct physical contact with PRBC is required for increased endothelial expression of ICAM-1 and IL-6. There was no activation of endothelial cells by PRBC at all if parasites were incubated in the top chamber lacking contact with HUVEC (Fig. 2A). However, soluble TNF-α induced endothelial cell activation perfectly well (Fig. 2A). This further demonstrates that no proinflammatory mediators were contaminating the PRBC preparations. Trypsin treatment of PRBC prevented activation of HUVEC (data not shown), demonstrating that parasite proteins localized at the surface of infected erythrocytes were involved in stimulation of the endothelial cells.
FIG. 2.
Characteristics of PRBC-mediated activation of HUVEC. (A) HUVEC were cocultured with RBC or PRBC either in direct physical contact or separated by a transwell system (pore size, 0.4 μm). Cell surface expression levels of ICAM-1 on HUVEC were detected by flow cytometry following incubation with medium (red line), RBC alone (dark blue line), TNF-α (10 ng/ml) (green line), PRBC in the transwell (light blue line), or PRBC in direct physical contact (purpleline). The IL-6 concentration was determined in cell culture supernatants by the ELISA. (B) Expression of IL-6 by HUVEC was determined after 6, 12, and 22 h of continuous incubation with medium, TNF-α, RBC, or PRBC. (C) HUVEC were incubated as described in the legend to Fig. 1A for 6, 12, and 22 h and were then analyzed for ICAM-1 surface expression levels. (D) HUVEC were incubated for 22 h as indicated in the legend to Fig. 1A. Cells were either analyzed for ICAM-1 surface expression levels or incubated in the absence of stimulators for another 18 h, after which ICAM-1 surface expression levels were determined. (E) HUVEC were incubated for different times (30 min, 60 min, and 120 min) with medium, TNF-α, RBC, or PRBC, and the IL-6 concentrations in cell culture supernatants were determined after a total incubation time of 21 h. (F and G) To study the correlation between parasite number and endothelial cell activation, HUVEC were cultured with 7 × 106 RBC/ml containing PRBC at the percentages indicated, and the concentrations of IL-6 in cell culture supernatants (F) or the cell surface expression levels of ICAM-1 (G) were determined as described above.
Endothelial cell activation through PRBC is a rapid phenomenon. Six hours after the initial contact with PRBC, significant concentrations of IL-6 were found in the supernatant of endothelial cells, and the concentrations continued to increase at 12 and 24 h after activation (Fig. 2B). The increase in endothelial ICAM-1 surface levels occurred with slower kinetics after contact with PRBC (Fig. 2C). Incubation with TNF-α, however, led to a fast increase in ICAM-1 surface levels in endothelial cells, which reached a maximum after 6 h (Fig. 2C). However, the ICAM-1 surface levels induced by PRBC remained elevated for prolonged periods, whereas TNF-α-induced ICAM-1 expression quickly subsided after the stimulus was removed (Fig. 2D). Prolonged expression of ICAM-1 on PRBC-stimulated HUVEC may support cytoadhesion of subsequent parasite generations. The endothelial cell reaction to PRBC not only was fast but also required only short physical contact. HUVEC were incubated with PRBC for 30, 60, and 120 min, the parasites were then removed by extensive washing, and IL-6 release was measured after 21 h (total time) of incubation. Figure 2E reveals that even 30 min of incubation with PRBC was sufficient to trigger endothelial cell activation.
To determine the sensitivity of endothelial cell activation, we diluted PRBC with uninfected RBC and incubated HUVEC with 7.2 × 106 RBC/ml. There was a direct correlation between the percentage of PRBC in the total RBC suspension and increased endothelial expression levels of ICAM-1 and IL-6. A clear increase in ICAM-1 and IL-6 expression was observed with parasitemia greater than 10% (Fig. 2F and G).
Gene expression profiling of HUVEC cultured together with PRBC revealed a complex pattern of activation.
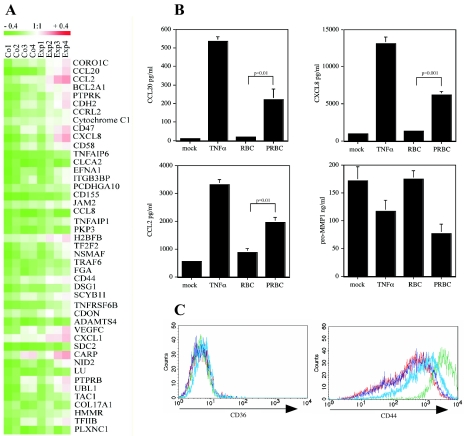
To characterize the changes in PRBC-stimulated endothelial cells in more detail, we performed gene expression profiling. Total RNA from HUVEC that were either mock treated or incubated with PRBC was analyzed twice in cross-checking microarray analyses. By applying the SAM software after normalization with a default Δ value of 2.17371, 45 genes were determined as genes that were significantly differentially expressed in PRBC-treated HUVEC compared to mock-treated controls (Fig. 3A). Among the most strongly expressed genes were genes for chemokines (CXCL8, CCL20, CXCL1, and CCL2) and for CD44 (Fig. 3A).
FIG. 3.
Gene expression analysis of HUVEC after contact with PRBC. (A) RNA was isolated from RBC or PRBC-treated HUVEC and used for hybridization as described in Materials and Methods. The corresponding FDR value was 0.19417. Hierarchical clustering of genes identified by SAM as being statistically significantly differently expressed was performed using the Tiger software (version 2.2) with the average linkage clustering method (distance metric, Euclidean). (B) Results of gene expression profiling were validated at the protein level. Cell culture supernatants from HUVEC treated as described in the text were assayed for CCL20, CCL2, CXCL8, and pro-MMP-1 by specific ELISA. (C) Cell surface expression levels of CD44 and CD36 on HUVEC were determined by immunofluorescent staining and flow cytometric analysis.
We confirmed the results of gene expression profiling at the protein level. PRBC induced increased endothelial release of CCL20, CXCL8, and CCL2 from cell culture supernatant, as determined by ELISA (Fig. 3B). As a negative control we included pro-MMP-1, which did not show increased gene expression levels. Similarly, increased CD44 surface expression was found on HUVEC following contact with PRBC (Fig. 3C); CD36 served as a negative control. Taken together, these results demonstrate that PRBC induced extensive activation of endothelial cells that resulted in a proadhesive phenotype. Interestingly, we found that PRBC-mediated activation led to up-regulation of antiapoptotic genes (such as bclx), which may explain why endothelial cells survived the contact with PRBC in vitro even though this contact has been reported to lead to endothelial cell apoptosis (22).
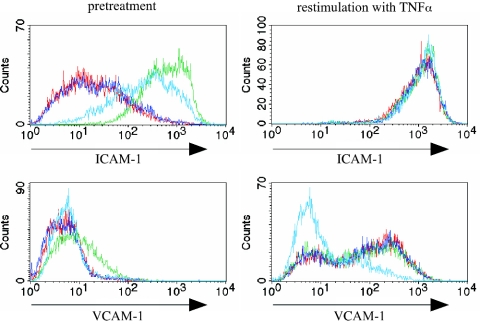
PRBC-stimulated HUVEC show a unique phenotype upon restimulation.
Given the reported influence of PRBC on the immune function of macrophages and dendritic cells, we wondered whether activation of HUVEC by incubation with PRBC subsequently altered endothelial cell function. To study this, we exposed endothelial cells previously stimulated with PRBC to a strong proinflammatory stimulus, such as TNF-α. (Pre)treatment of endothelial cells for 20 h with PRBC led to increased expression levels of ICAM-1 but not VCAM-1, as shown in Fig. 1. Exposure of mock-treated endothelial cells to TNF-α led to strong up-regulation of expression levels for ICAM-1 and VCAM-1 within 6 h (Fig. 4). However, endothelial cells previously exposed to PRBC failed to show TNF-α-triggered increased expression levels for VCAM-1, whereas the TNF-α-induced expression levels of ICAM-1 were similar for RBC- and PRBC-treated endothelial cells (Fig. 4). These results support the notion that PRBC stimulation leads to functional alteration in endothelial cells characterized by the failure to express molecules relevant for leukocyte recruitment upon contact with TNF-α.
FIG. 4.
Phenotype of PRBC-stimulated HUVEC after contact with TNF-α. HUVEC were incubated for 20 h with medium (red line), RBC alone (dark blue line), TNF-α (10 ng/ml) (green line), or PRBC (light blue line), and cells were washed three times with medium and cultivated for an additional 6 h with medium or with TNF-α (10 ng/ml). Cells were analyzed by flow cytometry after indirect immunostaining with antibodies recognizing ICAM-1, VCAM-1, and E-selectin. The histograms show the expression levels of the surface molecules.
DISCUSSION
Cytoadherence of P. falciparum-infected erythrocytes to endothelial cells is one of the key events in malaria pathophysiology leading to sequestration and microvessel obstruction. So far, endothelial cells have been regarded as bystander cells, which require activation by soluble mediators in order to serve as an adhesive platform for PRBC. In earlier experiments a direct interaction between PRBC and endothelial cells was observed (36, 37), and it was suggested that this interaction increased adhesiveness of endothelial cells (35). In the present study, we demonstrated that P. falciparum-infected RBC directly activate endothelial cells of various organs not requiring paracrine stimulation by proinflammatory mediators released from PRBC-stimulated immune cells, and we characterized the phenotype of PRBC-stimulated endothelial cells.
The encounter between PRBC and cells of the immune system, such as monocytes, macrophages, and dendritic cells, leads to activation and release of the prototypical proinflammatory mediator TNF-α, which exerts potent local and widespread stimulatory effects on cells in the vicinity and on vascular endothelial cells, respectively (9, 33). Binding of PRBC to previously activated endothelial cells with a proadhesive phenotype is believed to represent an immune escape mechanism that prevents parasite elimination in the spleen through sequestration of parasites in the peripheral circulation. At the same time, parasite sequestration represents a central pathophysiological event that is responsible for deficits in blood perfusion leading to tissue hypoxia and organ failure. Along this line, excessive release of TNF-α has been linked to severe malaria and a poor clinical outcome (17). Presumably, coevolution of the host and parasite has occurred over time to ensure parasite survival in the presence of a sufficient host immune response to control parasite expansion but not to drive immune pathology. Consistent with this notion, P. falciparum is known to employ a number of escape strategies to avoid activation of the immune system. In the absence of opsonizing antibodies, CD36-mediated binding and uptake of parasites do not lead to induction of TNF-α expression by monocytes/macrophages (16, 30). Moreover, PRBC modulate the maturation of dendritic cells and thereby hamper the development of a parasite-specific immune response (38). This prompted us to determine whether endothelial cells themselves are a target for PRBC and are subsequently modulated in their functional properties.
We show here that erythrocytes infected with different P. falciparum clones directly activate endothelial cells to express increased levels of ICAM-1. We did not observe significant and strong cytoadherence of PRBC to naïve endothelial cells in vitro (data not shown), which may be explained by the absence of suitable adhesion receptors, such as CD36, on the endothelial cells used in our experiments (HUVEC). The P. falciparum clonal lines used for this study have a polymorphic adhesion phenotype, with predominantly CD36. Similar results were obtained using Plasmodium strains ITO4-A4 and ItG-ICAM with an ICAM-1 binding phenotype (data not shown). However, endothelial activation by PRBC clearly required physical contact, as shown in transwell experiments (Fig. 2A), a finding similar to that of Udeinya et al. (35), who showed increased adhesiveness to an unidentified receptor by direct contact of PRBC (and TNF). The binding phenotype of the PRBC used in the study of Udeinya et al. was not known, although it appeared to be CD36 independent. Cytoadherence of PRBC to endothelial cells in vitro is known to occur under flow conditions typically found in postcapillary venules (41). Moreover, using human skin grafts on immunodeficient mice, the interaction between microvascular endothelial cells and PRBC was directly visualized, revealing that cytoadherence of PRBC is a multistep process similar to adhesion of leukocytes (10). Although experiments presented here were performed under static conditions, we found that physical contact of PRBC for 30 min was sufficient to elicit strong endothelial cell activation in vitro (Fig. 2). Further preliminary experiments using flow conditions revealed that PRBC stimulated endothelial cells, leading to increased cytoadhesion of PRBC infected with FCR3-ICAM-1 (data not shown). These data indicate that short-lived physical contact with PRBC may be sufficient to induce endothelial activation in vivo.
Activation of endothelial cells was directly correlated with the extent of parasitemia and led to increased endothelial expression levels of ICAM-1 (Fig. 1), which is known to be involved in cytoadherence of PRBC to endothelial cells under physiological shear stress (5). Even though we detected increased CD44 expression levels on HUVEC after contact with PRBC, we could not determine whether it is a chondroitin sulfate A-containing isoform of CD44 that was shown to be an adhesion receptor for PRBC (23). The glycosaminoglycan chondroitin sulfate A detected on HUVEC used in this study could be attached to CD44 and/or thrombomodulin expressed by HUVEC (8, 21; data not shown).
A detailed analysis of endothelial gene expression profiles after contact with PRBC revealed the induction of a proadhesive endothelial cell phenotype (Fig. 3A). Interestingly, a number of chemokine genes (the CCL2, CXCL,1 and CCL20 genes, as well as the CXCL8 gene) were strongly expressed in PRBC-exposed endothelial cells. Not only are chemokines expressed by activated endothelial cells relevant for attraction and binding of leukocytes (15), but CXCL8 in particular has been demonstrated to be involved in RBC invasion (11). Moreover, activation of endothelial cells leads to increased chemokine-dependent platelet binding (4), and endothelium-bound platelets promote, via expression of CD36, strong cytoadherence of PRBC to CD36-negative endothelial cells (40). Taken together, our data demonstrate that endothelial cells are sensitive to stimulation by PRBC, leading to increased expression of molecules, which may be involved in parasite adhesion.
Stimulation of endothelial cells by PRBC, however, does not mirror activation by the paracrine TNF-α. The ICAM-1 levels determined after contact with PRBC were considerably lower than those after activation with TNF-α. Molecules necessary to trigger leukocyte adhesion to endothelial cells under shear stress, such as E-selectin or VCAM-1, were not induced at all in endothelial cells by incubation with PRBC (Fig. 1), in contrast to stimulation with TNF-α. Moreover, PRBC-activated endothelial cells failed to up-regulate E-selectin and VCAM-1 expression following subsequent exposure to TNF-α (Fig. 4). Exposure to PRBC apparently induces in endothelial cells a particular refractory state to the action of the paracrine TNF-α. These results support the notion that PRBC-mediated stimulation of endothelial cells is incomplete and promotes parasite sequestration in the peripheral vascular bed rather than triggering local inflammation, which requires leukocyte adhesion to activated endothelial cells. Endothelial responsiveness to TNF-α has been shown to be a determinant of pathology during infections with a number of pathogens (7, 13, 14, 24, 25). It is intriguing to speculate that development of partial anergy towards stimulation by the paracrine TNF-α as a result of continuous exposure to PRBC contributes to the absence of immune pathology and clinical disease during chronic malaria infection, which is observed in individuals living in areas with a high prevalence of infected mosquitoes who have a high burden of circulating parasites. Temporary clearance or absence of PRBC from the circulation (e.g., due to seasonal variations in the number of infectious mosquito bites or long-term sojourn outside areas where malaria is endemic) may render host endothelial cells again susceptible to the paracrine TNF-α secreted from monocytes/macrophages during reexposure to P. falciparum and may explain preferential development of clinical disease in freshly infected individuals.
Taken together, our data demonstrate that human endothelial cells, like monocytes/macrophages and dendritic cells, serve as targets for P. falciparum and that contact with parasites modulates endothelial cell function. This interaction may provide mutual benefits for the parasite and the host: immune escape on the part of the parasite and prevention of immune pathology in the host.
Acknowledgments
We thank Alister Craig for critical discussions and Nicole Klatt for excellent technical assistance.
This work was supported by grants from the European Commission (QLRT-PL-1999-30109) to P.A.K., from the SFB 544 “Kontrolle tropischer Infektionskrankheiten” to M.L., and from the Boehringer Ingelheim Fonds to N.K.V. and by NGFN grants 01GS0102 and 01GS0438 to R.F.
Editor: J. L. Flynn
REFERENCES
- 1.Andrews, K. T., N. K. Viebig, F. Wissing, N. Klatt, N. Oster, H. Wickert, P. Knolle, and M. Lanzer. 2003. A human schwannoma cell line supports the in vitro adhesion of Plasmodium falciparum infected erythrocytes to chondroitin-4-sulfate. Parasitol. Res. 89:188-193. [DOI] [PubMed] [Google Scholar]
- 2.Barnwell, J. W., A. S. Asch, R. L. Nachman, M. Yamaya, M. Aikawa, and P. Ingravallo. 1989. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J. Clin. Investig. 84:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berendt, A. R., D. L. Simmons, J. Tansey, C. I. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 4.Clemetson, K. J., J. M. Clemetson, A. E. Proudfoot, C. A. Power, M. Baggiolini, and T. N. Wells. 2000. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 96:4046-4054. [PubMed] [Google Scholar]
- 5.Craig, A., and A. Scherf. 2001. Molecules on the surface of the Plasmodium falciparum infected erythrocyte and their role in malaria pathogenesis and immune evasion. Mol. Biochem. Parasitol. 115:129-143. [DOI] [PubMed] [Google Scholar]
- 6.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer, P. B., P. Chaturvedi, R. E. Fine, A. J. Ritchie, J. S. Pober, T. G. Cleary, and D. S. Newburg. 2001. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect. Immun. 69:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusai, T., D. Parzy, D. Spillmann, F. Eustacchio, B. Pouvelle, C. Lepolard, A. Scherf, and J. Gysin. 2000. Characterisation of the chondroitin sulphate of Saimiri brain microvascular endothelial cells involved in Plasmodium falciparum cytoadhesion. Mol. Biochem. Parasitol. 108:25-37. [DOI] [PubMed] [Google Scholar]
- 9.Grau, G. E., F. Tacchini-Cottier, C. Vesin, G. Milon, J. N. Lou, P. F. Piguet, and P. Juillard. 1993. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur. Cytokine Netw. 4:415-419. [PubMed] [Google Scholar]
- 10.Ho, M., M. J. Hickey, A. G. Murray, G. Andonegui, and P. Kubes. 2000. Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J. Exp. Med. 192:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horuk, R., C. E. Chitnis, W. C. Darbonne, T. J. Colby, A. Rybicki, T. J. Hadley, and L. H. Miller. 1993. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 261:1182-1184. [DOI] [PubMed] [Google Scholar]
- 12.Jensen, J. B. 1978. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 27:1274-1276. [DOI] [PubMed] [Google Scholar]
- 13.Kita, E., Y. Yunou, T. Kurioka, H. Harada, S. Yoshikawa, K. Mikasa, and N. Higashi. 2000. Pathogenic mechanism of mouse brain damage caused by oral infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 68:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou, J., Y. Gasche, L. Zheng, B. Critico, C. Monso-Hinard, P. Juillard, P. Morel, W. A. Buurman, and G. E. Grau. 1998. Differential reactivity of brain microvascular endothelial cells to TNF reflects the genetic susceptibility to cerebral malaria. Eur. J. Immunol. 28:3989-4000. [DOI] [PubMed] [Google Scholar]
- 15.Mackay, C. R. 2001. Chemokines: immunology's high impact factors. Nat. Immunol. 2:95-101. [DOI] [PubMed] [Google Scholar]
- 16.McGilvray, I. D., L. Serghides, A. Kapus, O. D. Rotstein, and K. C. Kain. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96:3231-3240. [PubMed] [Google Scholar]
- 17.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 18.Ockenhouse, C. F., R. Betageri, T. A. Springer, and D. E. Staunton. 1992. Plasmodium falciparum-infected erythrocytes bind ICAM-1 at a site distinct from LFA-1, Mac-1, and human rhinovirus. Cell 68:63-69. [DOI] [PubMed] [Google Scholar]
- 19.Ockenhouse, C. F., N. N. Tandon, C. Magowan, G. A. Jamieson, and J. D. Chulay. 1989. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science 243:1469-1471. [DOI] [PubMed] [Google Scholar]
- 20.Ockenhouse, C. F., T. Tegoshi, Y. Maeno, C. Benjamin, M. Ho, K. E. Kan, Y. Thway, K. Win, M. Aikawa, and R. R. Lobb. 1992. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J. Exp. Med. 176:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parzy, D., T. Fusai, B. Pouvelle, M. Torrentino, F. Eustacchio, C. Lepolard, A. Scherf, and J. Gysin. 2000. Recombinant human thrombomodulin (csa+): a tool for analyzing Plasmodium falciparum adhesion to chondroitin-4-sulfate. Microbes Infect. 2:779-788. [DOI] [PubMed] [Google Scholar]
- 22.Pino, P., I. Vouldoukis, J. P. Kolb, N. Mahmoudi, I. Desportes-Livage, F. Bricaire, M. Danis, B. Dugas, and D. Mazier. 2003. Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J. Infect. Dis. 187:1283-1290. [DOI] [PubMed] [Google Scholar]
- 23.Pouvelle, B., P. Meyer, C. Robert, L. Bardel, and J. Gysin. 1997. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum infected erythrocytes. Mol. Med. 3:508-518. [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi, M. H., J. Cook-Mills, D. E. Doherty, and B. A. Garvy. 2003. TNF-alpha-dependent ICAM-1- and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J. Immunol. 171:4700-4707. [DOI] [PubMed] [Google Scholar]
- 25.Ramegowda, B., J. E. Samuel, and V. L. Tesh. 1999. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J. Infect. Dis. 180:1205-1213. [DOI] [PubMed] [Google Scholar]
- 26.Robert, C., B. Pouvelle, P. Meyer, K. Muanza, H. Fujioka, M. Aikawa, A. Scherf, and J. Gysin. 1995. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res. Immunol. 146:383-393. [DOI] [PubMed] [Google Scholar]
- 27.Roberts, D. J., A. G. Craig, A. R. Berendt, R. Pinches, G. Nash, K. Marsh, and C. I. Newbold. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed, A. I., V. Sharov, J. White, J. Li, W. Liang, N. Bhagabati, J. Braisted, M. Klapa, T. Currier, M. Thiagarajan, A. Sturn, M. Snuffin, A. Rezantsev, D. Popov, A. Ryltsov, E. Kostukovich, I. Borisovsky, Z. Liu, A. Vinsavich, V. Trush, and J. Quackenbush. 2003. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34:374-378. [DOI] [PubMed] [Google Scholar]
- 29.Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, and M. Lanzer. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 17:5418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serghides, L., and K. C. Kain. 2001. Peroxisome proliferator-activated receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-alpha secretion by monocytes/macrophages. J. Immunol. 166:6742-6748. [DOI] [PubMed] [Google Scholar]
- 31.Taverne, J., C. A. Bate, D. Kwiatkowski, P. H. Jakobsen, and J. H. Playfair. 1990. Two soluble antigens of Plasmodium falciparum induce tumor necrosis factor release from macrophages. Infect. Immun. 58:2923-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 33.Turner, G. D., V. C. Ly, T. H. Nguyen, T. H. Tran, H. P. Nguyen, D. Bethell, S. Wyllie, K. Louwrier, S. B. Fox, K. C. Gatter, N. P. Day, N. J. White, and A. R. Berendt. 1998. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am. J. Pathol. 152:1477-1487. [PMC free article] [PubMed] [Google Scholar]
- 34.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udeinya, I. J., and C. O. Akogyeram. 1993. Induction of adhesiveness in human endothelial cells by Plasmodium falciparum-infected erythrocytes. Am. J. Trop. Med. Hyg. 48:488-495. [DOI] [PubMed] [Google Scholar]
- 36.Udeinya, I. J., C. Magowan, and J. D. Chulay. 1989. Long-term cultured human vascular endothelial cells (EC-FP5) bind Plasmodium falciparum infected erythrocytes. Am. J. Trop. Med. Hyg. 41:400-405. [DOI] [PubMed] [Google Scholar]
- 37.Udeinya, I. J., J. A. Schmidt, M. Aikawa, L. H. Miller, and I. Green. 1981. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science 213:555-557. [DOI] [PubMed] [Google Scholar]
- 38.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 39.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]
- 40.Wassmer, S. C., C. Lepolard, B. Traore, B. Pouvelle, J. Gysin, and G. E. Grau. 2004. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J. Infect. Dis. 189:180-189. [DOI] [PubMed] [Google Scholar]
- 41.Wick, T. M., and V. Louis. 1991. Cytoadherence of Plasmodium falciparum-infected erythrocytes to human umbilical vein and human dermal microvascular endothelial cells under shear conditions. Am. J. Trop. Med. Hyg. 45:578-586. [DOI] [PubMed] [Google Scholar]