Abstract
BACKGROUND:
There is growing evidence that pathogenic mutations do not fully explain hypertrophic (HCM) or dilated (DCM) cardiomyopathy phenotypes. We hypothesized that if a patient’s genetic background was influencing cardiomyopathy this should be detectable as signatures in gene expression. We built a cardiomyopathy biobank resource for interrogating personalized genotype phenotype relationships in human cell lines.
METHODS:
We recruited 308 diseased and control patients for our cardiomyopathy stem cell biobank. We successfully reprogrammed PBMCs (peripheral blood mononuclear cells) into induced pluripotent stem cells (iPSCs) for 300 donors. These iPSCs underwent whole genome sequencing and were differentiated into cardiomyocytes for RNA-seq. In addition to annotating pathogenic variants, mutation burden in a panel of cardiomyopathy genes was assessed for correlation with echocardiogram measurements. Line-specific co-expression networks were inferred to evaluate transcriptomic subtypes. Drug treatment targeted the sarcomere, either by activation with omecamtiv mecarbil or inhibition with mavacamten, to alter contractility.
RESULTS:
We generated an iPSC biobank from 300 donors, which included 101 individuals with HCM and 88 with DCM. Whole genome sequencing of 299 iPSC lines identified 78 unique pathogenic or likely pathogenic mutations in the diseased lines. Notably, only DCM lines lacking a known pathogenic or likely pathogenic mutation replicated a finding in the literature for greater nonsynonymous SNV mutation burden in 102 cardiomyopathy genes to correlate with lower left ventricular ejection fraction in DCM. We analyzed RNA-sequencing data from iPSC-derived cardiomyocytes for 102 donors. Inferred personalized co-expression networks revealed two transcriptional subtypes of HCM. The first subtype exhibited concerted activation of the co-expression network, with the degree of activation reflective of the disease severity of the donor. In contrast, the second HCM subtype and the entire DCM cohort exhibited partial activation of the respective disease network, with the strength of specific gene by gene relationships dependent on the iPSC-derived cardiomyocyte line. ADCY5 was the largest hubnode in both the HCM and DCM networks and partially corrected in response to drug treatment.
CONCLUSIONS:
We have a established a stem cell biobank for studying cardiomyopathy. Our analysis supports the hypothesis the genetic background influences pathologic gene expression programs and support a role for ADCY5 in cardiomyopathy.
Introduction
Hypertrophic cardiomyopathy (HCM) occurs in 1 in 500 individuals, and patient phenotypes range from asymptomatic to serious adverse outcomes such as heart failure or sudden cardiac death.[1] HCM is marked by an enlarged left ventricular muscular wall, with left ventricular ejection fraction typically preserved or increased,[1] whereas dilated cardiomyopathy (DCM) is characterized by reduced ejection fraction.[2] DCM is estimated as the cause of heart failure in ~12.5 percent of patients and has been estimated to affect 1 in 250 individuals,[2] with familial DCM representing a fraction of those cases.[3] Until recently, the accepted inheritance mechanism for HCM and familial DCM was a single or few dominant, rare mutations, most commonly in genes encoding sarcomere proteins (HCM, especially MYH7 and MYBPC3) [1, 3] or across at least nine key cardiac structures and components (DCM, including sarcomere [TTN] and nuclear envelope [LMNA]).[4] However, the full list of genes proposed to harbor HCM or DCM pathogenic variants exceeds 100 and is continually being refined based on increased genome sequencing data and molecular validation studies (Table S4).[5, 6] For sarcomeric genes known to harbor pathogenic mutations for both HCM and DCM, the opposing effect of the specific mutation on tension generation during cardiomyocyte contraction is thought to distinguish between the development of HCM and DCM.[7] However, despite their contrasting phenotypes, there are shared disease processes between HCM, DCM, and common forms of heart disease, and a shared need for tools to dissect genotype-phenotype relationships. We built a biobank of patient-derived induced pluripotent stem cells for studying disease mechanisms of cardiomyopathy, focused on recruitment for the two most common cardiomyopathies, HCM and DCM.
Both HCM and DCM develop gradually with age and are marked by pathogenic mutations with incomplete penetrance and variable expressivity, and subsequent variability in disease manifestation, with just over half of unaffected individuals harboring a pathogenic variant for HCM remaining unaffected for 15 years post-genetic identification, while first degree relatives of a patient with familial DCM have only a 19% risk of developing DCM by age 80.[3] Furthermore, previous cardiomyopathy subtyping efforts have shown minimal correlation to the underlying gene carrying the pathogenic mutation[8] including broad segregation by patients with known sarcomeric pathogenic mutations or not (HCM).[9] Finally, with few, rare exceptions, actionable changes to clinical care do not exist to tailor treatment based on the mutated gene[10, 11]. Potential explanations for the disparate phenotypes of individuals with a common mutated gene include differences in environment and physiology, nuances of the specific mutation within the gene, and a role for modifying mutations to influence disease onset, severity, and symptomology.
Furthermore, a pathogenic or likely pathogenic mutation is identified for only 30–60 percent of HCM patients[3] and ~35 percent of DCM patients[4] who undergo clinical genetic testing. This number has remained recalcitrant to expanded application of whole genome sequencing, and replicated in our study as well (see below). This is likely partially explained by the observation that many mutations are family-specific[12] and therefore lack evidence in the literature to support definitive pathogenicity classification. Increasingly though, there is evidence for a subset of patients to have a different genetic architecture. Oligogenic inheritance, where multiple rare variants drive disease, has been proposed for both DCM[4] and HCM[10], as has polygenic inheritance, and the role of modifying mutations to influence disease manifestation in monogenic cases. Genome-wide association studies for DCM, HCM and cardiac morphological and functional traits have revealed individual loci and polygenic risk scores can partially capture cardiomyopathy inheritance, in both patients with and without known pathogenic mutations and in sporadic cases (non-familial DCM).[4, 13, 14]
We hypothesized that if noncoding variants, or variants outside of traditional cardiomyopathy genes influence cardiomyopathy, that should be detectable as signatures in the gene expression. We sought to use inferred, personalized co-expression networks to test whether transcriptomic subtypes exist for HCM and DCM.
METHODS
The methods are described in the Supplemental Material. This study is in compliance with the Stanford Human Research Protection Program guidelines and approved by the Stanford Institutional Review Board (IRB #30064). In addition, the procedures are in compliance with the International Society of Stem Cell Research guidelines and approved by the Stanford IRB / Stem Cell Research Oversight panel (SCRO #656). The whole genome sequencing and RNA-sequencing data will be made publicly available via dbGaP, with pertinent metadata available in the Supplemental Materials.
RESULTS
We established a cardiomyopathy stem cell biobank
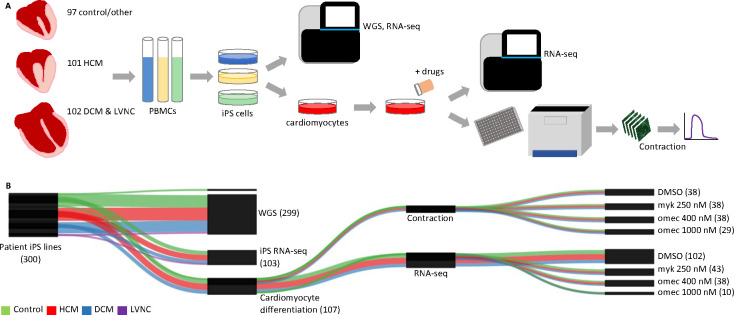
We generated a biobank resource for studying cardiomyopathy. We recruited patients exhibiting either hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), or left ventricular noncompaction (LVNC) or serving as controls (Figure 1A and Tables 1 and S1). We successfully reprogrammed peripheral blood mononuclear cells (PBMCs) into induced pluripotent stem cells (iPSCs) for 300 donors (Figures 1A and S1). This represented 101 HCM donors, 88 DCM donors, 14 LVNC donors, 95 control donors and 2 donors with other cardiac diseases (long QT syndrome [LQT] and Fabry disease). For most of the samples, echocardiogram measurements of the donor were available in the electronic medical record (EMR) for left ventricular ejection fraction (LVEF, 195/205 diseased iPSC lines) and interventricular septum thickness, end diastole (IVSd, 196/205 diseased iPSC lines) (Table S1).
Figure 1. We built a cardiomyopathy stem cell biobank for 300 donors.
A. We created a biobank of stem cells from 300 donors exhibiting either HCM, DCM, LVNC, or serving as controls. iPS cells were profiled by whole genome sequencing and a subset differentiated into cardiomyocytes for additional profiling via RNA-seq, drug treatment, and microscopy-based contractility assaying. B. Plotted are the datasets which passed quality control filtering. Numbers indicate number of donors for each dataset type. The actual number of datasets is higher due to replicates. Abbreviations: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), left ventricular noncompaction (LVNC), induced pluripotent stem cells (iPS cells), whole genome sequencing (WGS), RNA sequencing (RNA-seq), dimethyl sulfoxide (DMSO), mavacamten (myk), omecamtiv mecarbil (omec).
Table 1.
Demographic and echocardiography metadata.
| Control (n=97) | HCM (n=101) | DCM (n=88) | LVNC (n=14) | |
|---|---|---|---|---|
|
| ||||
| Age | 52.4 ± 18.2 years | 54.4 ± 16.3 years | 50.1 ± 14.6 years | 43.8 ± 17.5 years |
| Male | 54.6 % | 61.4 % | 50 % | 57.1 % |
| Race | ||||
| White | 52.6 % | 71.3 % | 63.6 % | 78.6 % |
| Asian | 27.8 % | 10.9 % | 10.2 % | 7.1 % |
| African American | 7.2 % | 5 % | 10.2 % | 7.1 % |
| Other/Unknown | 12.4 % | 12.9 % | 15.9 % | 7.1 % |
| Hispanic | 11.3 % | 8.9 % | 9.1 % | 0 % |
| LVEF (%) | 61.6 ± 10.7 (n=93) | 40.0 ± 15.4 (n=87) | 51.7 ± 12.5 (n=14) | |
| IVSd (cm) | 1.66 ± 0.49 (n=96) | 0.94 ± 0.18 (n=87) | 0.87 ± 0.20 (n=13) | |
Data are presented as mean ± standard deviation (age/LVEF/IVSd) or as percentage (sex/race/ethnicity). Control includes Healthy Control and Other. Echocardiography data provided where available in electronic medical records. Abbreviations: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), left ventricular noncompaction (LVNC), left ventricular ejection fraction (LVEF), interventricular septum thickness end diastole (IVSd).
We performed whole genome sequencing (WGS) on 299 iPSC lines. We differentiated a subset of iPSC lines into cardiomyocytes and profiled them by RNA-seq. Cardiomyocyte transcriptomic data was generated for 102 lines at baseline after quality control filtering (Figure 1B). This represented 44 HCM, 26 DCM, 31 control, and 1 LQT donors for iPSC-derived cardiomyocyte RNA-seq data. We also performed RNA-seq on 103 iPSC lines as a control dataset. A portion of cardiomyocytes were subjected to cardiac drug treatment followed by RNA-seq and contractility measurements using kinetic image cytometry. This resulted in 11–18 drug-treated lines for each disease condition and each drug (mavacamten and omecamtiv mecarbil) after QC.
Pathogenic variants were annotated via whole genome sequencing
We first sought to identify the pathogenic variants for each iPSC line. Given the open questions around the diversity of the genetic architecture of HCM and DCM, this allowed us to evaluate whether any transcriptomic patterns were evident in both lines with and without known pathogenic mutations. Annotating iPSC lines by their pathogenic variant also enhanced the utility of the biobank as a resource for others.
We performed whole genome sequencing on 299 of the iPSC lines (Tables S2 and S3), and called single nucleotide variants (SNVs) and insertions and deletions (indels). Given the known challenges in identifying pathogenic variants in HCM and DCM our approach was to first filter variants with several different less-stringent criteria and then pool the variants from these different strategies (Figure S2), followed by manual application of the more stringent American Medical College of Genetics (ACMG) guidelines for determining pathogenicity.[15] Identifying pathogenic mutations is dependent on evidence in the literature to support pathogenicity as well as our current understanding of the inheritance model to inform mutation filtering. (We assumed one or several rare dominant mutations in a set of known or potential cardiomyopathy genes (referred to as our “panel genes” [Table S4]) could be pathogenic in any individual). Since both of these may change with time, we provide the specific criteria that we used in Figure S2 and Table S5. Our panel of 235 potential cardiomyopathy genes was purposely broad, encompassing genes from six clinical panels as well as authoritative resources (Table S4, and Supplemental Methods). After applying our initial filters for candidate mutations (pool 1 variants in Figure S2, filtered for frequency <0.001), on average, each iPSC line had only 4–6 rare candidate missense or splicing mutations across the 235 panel genes, and there was no difference between control, HCM, and DCM iPSC lines (Figure 2A), highlighting the challenge of variant classification. Rare candidate truncating, frameshift, or startgain mutations (i.e. mutations potentially altering protein length) were slightly more common in DCM than HCM (0.56 vs 0.33 such mutations per iPSC line).
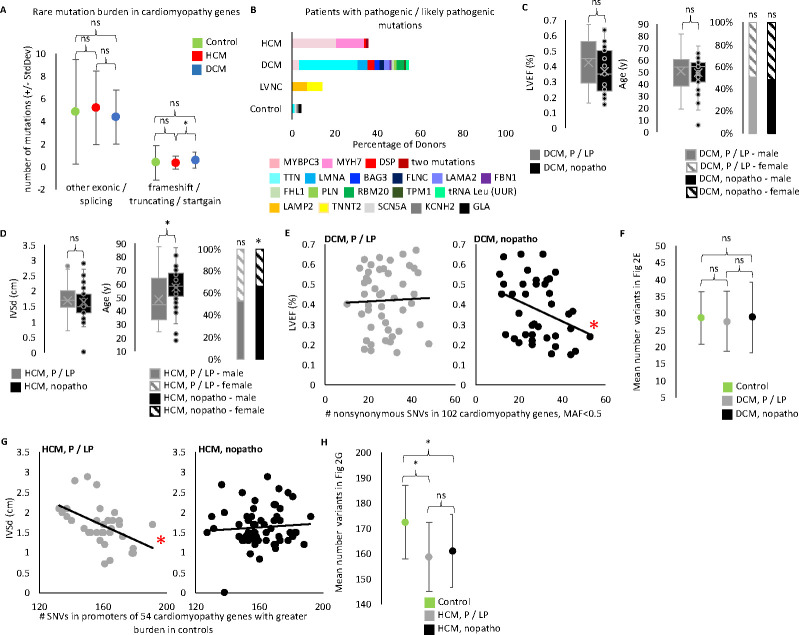
Figure 2. WGS confirms cumulative role of cardiomyopathy variants.
A. We performed WGS on 299 of the 300 iPS lines and called SNPs and indels. From a starting pool of ~4.28 million SNPs and indels per donor iPS cell line (mean for biobank, Table S2) we found a mean of only 4–5 missense and splicing mutations per HCM or DCM line and 0.3–0.6 truncating, frameshift, and startgain mutations per HCM or DCM line when we focus on potentially pathogenic variants by filtering for rare variants in 235 cardiomyopathy genes (pool 1 variants in Figure S2, filtered for frequency <0.001). We found control samples show no difference in the number of rare candidate variants in cardiomyopathy genes compared to diseased samples. Rare candidate truncating, frameshift, or startgain mutations (i.e. mutations potentially altering protein length) in cardiomyopathy genes were more common in DCM than HCM. (t-test: DCM vs HCM p-value = 0.0199477. Control vs HCM p-value = 0.9479979. DCM vs Control p-value = 0.2322563). Rare missense and splicing mutations show no difference by disease status. (t-test: Control vs HCM p-value = 0.5390647. Control vs DCM p-value = 0.4026431. HCM vs DCM p-value = 0.05390647). Plotted are the mean number of filtered mutations per line in each disease cohort. Error bars indicated standard deviation. B. We identified a pathogenic or likely pathogenic (P/LP) mutation for 86 out 203 diseased iPS lines. Plotted is the percentage of lines with an identified P/LP mutation by gene for each disease category. The HCM line with two mutations has ALPK3 and MYBPC3 mutations. The control line with a GLA mutation was classified as Other due to the donor’s known condition of Fabry disease (an HCM lookalike syndrome). The other two mutations found in control lines are probably not pathogenic in these donors, however we list their finding here as evidence of the background rate of finding pathogenic mutations when applying our filtering and classification workflow to non-cardiomyopathy donors. C. We compared echocardiogram and demographic data of the DCM donors with a P/LP mutation identified in the iPS line and those without (referred to as nopatho). Neither LVEF, nor age, nor sex differ between P/LP and nopatho in DCM. (LVEF t-test: p-value = 0.09055781 [p-value 0.08214506 when limit to DCM donors with clinical diagnosis, data not shown]. Age t-test: p-value =0.64370666 [p-value 0.569830699 when limit to DCM donors with clinical diagnosis, data not shown]. Sex chi-square: P/LP male vs female p-value = 0.8864 [p-value 0.6617 when limit to DCM donors with clinical diagnosis, data not shown]. nopatho male vs female p-value = 0.8728 [p-value 0.8694 when limit to DCM donors with clinical diagnosis, data not shown].) D. For HCM, P/LP donors are younger (t-test: p-value = 0.00921755), but show no difference in IVSd (t=test: p-value = 0.60639198). Nopatho lines are more commonly male than female, while P/LP lines are equally male and female. (chi-square: nopatho male vs female p-value = 0.0092. P/LP male vs female p-value = 0.7389). E. Pucklewartz et al. defined 102 cardiomyopathy genes whose nonsynonymous SNV mutation burden correlated with LVEF in DCM but not with any HCM echocardiogram metrics tested. We found increased cumulative burden of nonsynonymous variants with a minor allele frequency (MAF) <0.5 in the 102 Puckelwartz genes correlated with worse LVEF only in the nopatho samples (right, linear regression: p-value = 0.03141 [p-value = 0.04568 when limit to DCM donors with clinical diagnosis, data not shown; p-value = 0.05929 when remove MAF filter, data not shown.]) but not P/LP samples (left, linear regression: p-value = 0.8093 [p-value = 0.7954 when limit to DCM donors with clinical diagnosis, data not shown. p-value = 0.7514 when remove MAF filter, data not shown]). F. However the mean number of nonsynonymous variants in the Puckelwartz et al. genes is not different between the nopatho and P/LP cohorts nor between either DCM cohort and control. (t-test: nopatho vs control p-value = 0.984052497. P/LP vs control p-value = 0.469184129. P/LP vs nopatho p-value = 0.600062881.) Bars represent standard deviation. G. We found 54 of the Puckelwartz et al genes had lower mean number of SNVs in the promoter region, in HCM than control. Greater SNVs in this subset of promoters correlated with less enlarged IVSd measurements in the P/LP HCM samples but not the nopatho HCM samples. (Linear regression: P/LP p-value = 0.003773. Nopatho p-value = 0.5669). H. As selected for, control samples had greater SNVs in these 54 promoters. There was no difference in the mean SNV count between P/LP and nopatho HCM samples. (t-test: nopatho vs control p-value = 0.00000497. P/LP vs control p-value = 0.00000491. P/LP vs nopatho p-value = 0.439656222.) Bars represent standard deviation.
We identified a pathogenic or likely pathogenic mutation in 36 percent of HCM lines (36 of 101), most commonly in MYH7 or MYBC3 (13 and 22 iPSC lines, respectively), and in 55 percent of DCM lines (48 of 88), most commonly in TTN (24 iPSC lines; Figure 2B and Table S5). Complementary RNA-seq data from iPSC-derived cardiomyocytes was helpful for evaluating potentially truncating variants, but ultimately did not change our annotation of a mutation from a variant of uncertain significance to pathogenic or likely pathogenic (Figure S3A). 145 of the cardiomyopathy donors had clinical genetic testing results in their EMR. We re-evaluated pathogenicity for any variant listed in the EMR. The rate of identifying a pathogenic or likely pathogenic mutation by whole genome sequencing was only slightly higher than by clinical testing (45.5 percent of lines versus 41.4 percent) (Figure S3B and Table S5). In total, the biobank contains diseased iPSC lines for 21 different mutated genes.
Reduced LVEF is a hallmark of symptomatic DCM and an indicator of poor cardiac function. We saw no difference in the LVEF, age, or sex of DCM donors for which we found a pathogenic or likely pathogenic mutation compared to those without a known pathogenic mutation (Figure 2C). Similarly, we saw no difference in IVSd, one measure of cardiac size, between HCM donors with or without pathogenic and likely pathogenic mutations (Figure 2D). However, we found donors lacking pathogenic mutations were older and more commonly male than female, while HCM donors with a known pathogenic mutation were equally male and female (Figure 2D).
Correlation between mutation burden in cardiomyopathy genes and echocardiogram metrics was distinct between lines with and without known pathogenic mutations
Pucklewartz et al.[16] had previously evaluated mutation burden in 102 cardiomyopathy genes (101 of which are in our “panel genes” list) focusing on nonsynonymous SNVs of any allele frequency and found greater nonsynonymous SNVs correlated with decreased LVEF in DCM but not HCM patients. The authors proposed a role for oligogenic inheritance to contribute to DCM phenotype. By contrast, we found neither our HCM nor DCM cohort displayed this relationship (Figure S4A). However, Pucklewartz et al. specifically enriched for donors without known pathogenic or likely pathogenic mutations in building their cohort. When we distinguished between iPSC lines with a pathogenic or likely pathogenic mutation and lines without, and limited the analysis to variants with an alternate allele frequency <0.5, we found the linear relationship between mutation burden and LVEF is specific to our DCM cohort lacking pathogenic mutations (Figure 2E). Importantly, there was a large range in the mutation burden of nonsynonymous SNVs in the 102 cardiomyopathy genes from 10 to 43 variants across the samples (DCM and control), with no difference between control, DCM with a pathogenic or likely pathogenic mutation, and DCM without a pathogenic or likely pathogenic mutation (Figure 2F). The significance was specifically related to the correlation between the mutation burden and LVEF. This supported the hypothesis in the literature for two different mechanisms of DCM inheritance; the DCM samples with pathogenic mutations exhibiting monogenic inheritance and the DCM samples without known pathogenic mutations potentially exhibiting oligogenic inheritance. Importantly, when we restricted the analysis to a subset of 20 core DCM genes with stronger evidence for pathogenicity (see Supplemental Methods), we no longer saw a correlation between mutation burden and LVEF, further supporting the hypothesis of the Puckelwartz et al. authors for oligogenic inheritance (linear regression: nopatho p-value = 0.06238. P/LP p-value = 0.8751; data not shown). Here we applied the Puckelwartz et al. analysis from 2021 to our cohort, but additional DCM GWAS datasets and machine learning approaches should enable improved selection of loci for which mutation burden could contribute to DCM. Importantly, this analysis does not suggest these particular mutations (coding mutations in 102 cardiomyopathy genes) act as modifier mutations, in that they do not correlate with LVEF in the donors with a known pathogenic mutation.
Because the Puckelwartz et al. study examined multiple echocardiogram measurements for correlation to mutation burden in HCM and did not find any, we did not pursue this line of investigation, except to confirm the lack of correlation to LVEF as a complement to our DCM analysis. Instead, we performed only one complimentary analysis, which was to compare the IVSd data we had for HCM to mutation burden in the promoters of the Puckelwartz et al. genes. Our hypothesis was that the same genes which may contribute to oligogenic inheritance in DCM could act as modifiers of disease severity in HCM, in which case, non-coding mutations may be sufficient to influence phenotype. While the majority of pathogenic cardiomyopathy mutations influencing protein sequence are harmful, we could not assume whether individual non-coding mutations would be harmful or protective, nor were we powered to determine this statistically. Rather, we distinguished promoters by those with greater mutation burden (SNVs of any allele frequency) in control samples and those with greater mutation burden in HCM samples. Of the 102 promoters, 54 had greater average mutations in control versus HCM lines. Total mutation burden in these 54 promoters was significantly associated with lower IVSd in HCM lines with pathogenic mutations (Figure 2G). The trend remained significant when only analyzing data from white donors (p-value = 0.01004, data not shown). We did not see this relationship in the HCM samples without a pathogenic mutation (Figure 2G). The difference between HCM samples with and without a pathogenic variant was not due to a difference in total mutation burden between these two groups (Figure 2H), but the specific relationship between mutation burden and IVSd. Our limited sample size for selecting and analyzing variants meant these results were not sufficient for making conclusions about the role of these promoters in HCM. Rather, this provided preliminary evidence for our HCM cohort to encompass diverse genetic architecture mechanisms. We thus sought to investigate transcriptomic signatures as a molecular phenotype of cardiomyopathy that may be sensitive to distinct genetic backgrounds. Importantly, we were not attempting to replicate cardiac expression quantitative trait loci (eQTL) studies.[17] Rather, we hypothesized that leveraging a diverse human dataset could uncover important cardiomyopathy disease mechanisms by specifically interrogating shared and personalized transcriptomic features across patients of differing genetic architectures.
Disease co-expression networks identified important cardiomyopathy genes, with ADCY5 as the largest node for both HCM and DCM
iPSC lines were differentiated into cardiomyocytes and profiled via RNA-seq (Table S6) resulting in RNA-seq data from iPSC-derived cardiomyocytes for 102 subjects after QC. We performed traditional differential gene expression analysis using DESeq2 and identified 236 and 62 genes up an down-regulated respectively in HCM and 8 and 21 genes up and downregulated in DCM with gene ontology analysis resulting in few disease pathways (Supplemental Methods, data not shown), likely due to the diverse genetic etiology of the cohort and our limited ability to perform multiple cardiomyocyte differentiations for each of the over 100 iPSC lines. We also expected that for some samples the presence of a pathogenic mutation would not guarantee that the iPSC-derived cardiomyocytes would be mature enough, nor the model stressed enough, to bring out a phenotype for that specific mutation, and we thus lacked a true positive set of samples for building a definition of diseased expression. Nor did we want to build a unique model of cardiomyopathy expression for each sample to accommodate the heterogeneity of symptoms from different pathogenic mutations. Instead, we sought to identify common transcriptional signatures of cardiomyopathy and evaluate each sample by the manifestation of the shared signatures. We focused on gene co-expression relationships based on the supposition that the influence of genetic architecture and noncoding variants may be better captured.
We calculated patient-specific gene co-expression networks using lionessR, an algorithm for linear interpolation to obtain network estimates for single samples.[18, 19] First, for HCM and DCM separately, we built a co-expression network with the 200 most differential gene-gene co-expression relationships calculated between the control and diseased cohort (Figure 3A). A red edge in the HCM network indicated two genes were highly co-expressed (large r2, pearson) with a positive correlation (positive r, both genes up or down expressed similarly across samples) in the HCM cohort compared to the control cohort. By contrast green edges indicated strong, positive co-expression in the control cohort. Separately, we built a DCM network comparing DCM co-expression with control samples. We then used lioness to remove one sample from the cohort, and recalculate the co-expression correlations. The change in the level of co-expression upon sample removal was used to infer the individual contribution of that sample to the network. We thus generated inferred networks for each sample individually. We then asked how the sum of all edges surrounding a gene (node strength) varied across patients (Figure 3B).
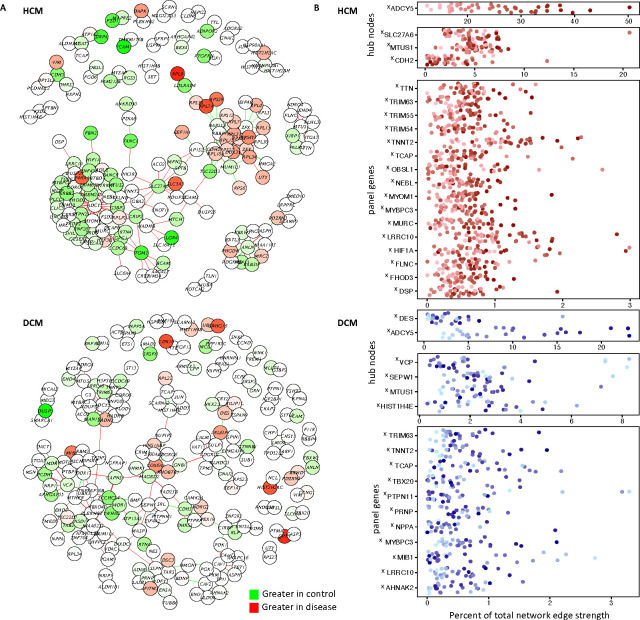
Figure 3. Personalized co-expression networks capture otherwise undetectable genes contributing to the disease signature and reveals line-specific differences in network activation.
A. We calculated an HCM and DCM co-expression network using lionessR, an algorithm for Linear Interpolation to Obtain Network Estimates for Single Samples. (Genes/nodes are represented as circles. Edges are represented as lines. Green indicates stronger edges or greater expression [nodes] in control, while red indicates stronger edges/greater expression in disease.) B. We the inferred personalized co-expression networks for individual lines. For select genes we highlight their contribution to the network (sum of their edge strengths as a percentage of the total edge strengths of the network), plotted for each sample. Samples are colored by their ADCY5 ranking. X indicates genes which only show up in this lines-specific co-expression analysis but are not flagged as significant in traditional DESeq2 analyses for differential expression between control and disease. Panel genes refers to cardiomyopathy gene list used to annotate pathogenic variants.
Of our “panel genes” that we screened for pathogenic variants, 16 were in the HCM network (plus CDH2, which had been shown to be mutated in arrhythmogenic right ventricular cardiomyopathy[20], but not HCM or DCM) and 12 were in the DCM network. Despite not showing up in our traditional differential gene expression analysis as exhibiting a conserved difference in gene expression across the disease cohort, we saw they exhibit disease-specific co-expression. Other genes of interest in the HCM network included SLC27A6 which encodes fatty acid transport protein 6 (FATP6), the primary FATP in the heart.[21] FATPs enable cellular uptake of fatty acid, with fatty acid oxidation being the dominant source of ATP in healthy adult hearts, while classic pathologic transcription remodeling via the “fetal gene program” entails a switch to other substrates.[21] SLC27A6 was previously identified in an exome-wide association study for association with left ventricular internal diastolic dimension in the Hypertension Genetic Epidemiology Network of paired siblings with and without hypertension.[21] MTUS1 was in both the HCM and DCM network. Mtus1A, a MTUS1 splice variant, was shown to be upregulated in a murine model of pressure overload with corresponding increase in cardiac hypertrophy, while overexpression attenuated hypertrophy in response to pressure overload and catecholaminergic stimulation.[22] JUN, also found in both the HCM and DCM networks encodes a transcription factor with a known role in regulating sarcomere gene expression and attenuating cardiac hypertrophy.[23]
In DCM, we found additional examples of genes previously implicated in cardiomyopathy. VCP is a molecular chaperone with roles in mitochondrial maintenance and protein homeostasis whose overexpression or disrupted function in mice can moderate ischemia reperfusion injury and heart failure respectively.[24] HIST1H4E, (encoding Histone H4) was previously identified for differential expression in cardiomyopathy and cardiomyopathy risk factors in microarray datasets.[25] We also identified genes previously understudied in cardiomyopathy, such as SEPW1. Selenium deficient disruption of selenoprotein function has been implicated in heart failure,[26] but little is known of a specific role for SEPW1 in DCM. In total, we saw the personalized co-expression analysis allowed for interrogation of individual genes in a sample-specific manner, as well as capturing otherwise undetectable genes contributing to the disease transcriptome.
Network activation is a measure of the total strength of all edges in the network. High network activation in a diseased sample meant the sample exhibited strong disease-specific gene co-expression. Likewise, an activated hubnode represented a gene with strong co-expression relationships in a sample. In both HCM and DCM, the ADCY5 gene was the largest node (connected to the most other genes) and had the largest contribution to the total strength of the network (Figure 3A). The prominence of ADCY5 in both the HCM and DCM networks indicated ADCY5, despite not being a gene mutated in cardiomyopathy, was co-expressed with multiple cardiomyopathy genes and central to the disease networks. Mouse models have demonstrated the role for ADCY5 perturbation to influence other forms of heart disease,[27] with our network data suggesting ADCY5 may also be important to cardiomyopathy. Importantly, the relative contribution of the ADCY5 hubnode to the level of network activation was highly variable between lines (Figure 3B), prompting us to next examine how differences in network activation related to disease severity.
Personalized networks illuminated distinct relationships between network activation and disease severity
Having confirmed the utility of co-expression analysis for identifying cardiomyopathy genes of interest, we next tested whether the network itself offered disease insights. We defined a hubnode as a gene with at least three edges and asked whether edges around a shared hub node were further co-modulated, signifying the hub node itself was a unit of network activation (Figure 4A). Put simply, if we found in one of our HCM samples that the inferred co-expression relationship between ADCY5 and another gene (for example MYBPC3) was strong, could we expect ADCY5’s co-expression relationship with the other 48 genes it is connected with to also be stronger in that HCM sample as compared to the other HCM samples. This was calculated separately on the two networks (HCM and DCM). Importantly, we calculated this for the control samples separate from the diseased samples, such that we could compare how network activation presented differently for each sample despite having the same disease.
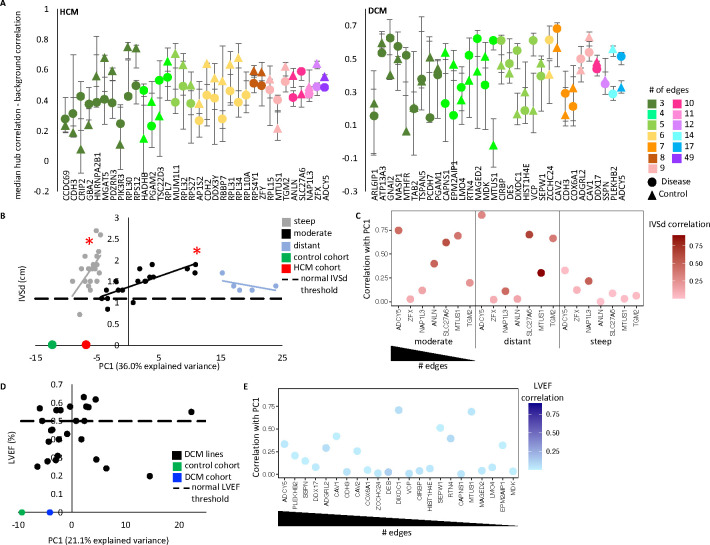
Figure 4. Variable ADCY5 hub node activation corresponds to clinical disease severity in HCM, with two HCM subgroups exhibiting distinct patterns of network activation.
A. Co-modulation of edges around a node was evaluated by comparing the difference in correlation between strength of two randomly selected edges around the node with two randomly selected edges that do not share a node. Correlation was evaluated separately on the diseased and control cohorts. Plotted are the mean and 95% confidence intervals after sampling 10,000 times. B. Principal component analysis of the hCM network was computed on the control-cohort, HCM-cohort, and individual hCM lines. Principal component 1 (PC1) is plotted against IVSd (intraventricular septal thickness end diastole) for the 42 HCM samples with echocardiogram data. For PC1 values −9.02 to −4.4 (gray dots, “steep” samples) and for PC1 −4.3 to 11 (black dots, “moderate” samples), PC1 correlates with IVSd (linear regression: steep p-value = 0.01167, moderate p-value = 0.0001398). The 5 most distant samples (PC1 >15.2, blue dots) show no relationship to IVSd (p-value = 0.3729). C. For hubs with 8 or more edges, we tested how well they served as a proxy for the overall PC1 score. Plotted are the correlation (R2) of the sum of all edge strengths around a hub with the PC1 score for the sample in the hCM cohort (y-axis). Color, indicates the R2 correlation of the sum of edge strengths to IVSd. D. There was no significant relationship between PC1 of the network and LVEF in DCM. E. Plotted are correlation between node strength and PC1 for nodes with 4 or more edges in DCM.
In the HCM network we saw ADCY5 was a unit of network regulation, in that for both the control and HCM lines there was greater co-modulation of ADCY5 edges than background co-modulation of two unconnected edges in the network. Despite many of the individual edges around ADCY5 being stronger in HCM (red edges in Figure 3A top panel), the co-modulation of the ADCY5 edges was lower in HCM (HCM node lower than control node in Figure 4A), suggesting ADCY5 was more activated in HCM, but with individual edges being sporadically activated depending on the sample. In the DCM network, the opposite was true. Like with HCM, ADCY5 had mostly stronger edges in DCM compared to control, however, the co-modulation was also stronger in DCM than control, suggesting the entire ADCY5 hub was upregulated in tandem in DCM samples, to varying degrees. Many additional nodes also behaved as significant units of network activation. Notably, DCM had five nodes with greater co-modulation in diseased samples. MTUS1 showed the largest difference, with co-modulation of the edges around MTUS1 showing no correlation in the healthy cohort. Conversely, HCM had only one such node (SLC27A6), suggesting the level of network activation in HCM samples was not a singular feature, rather the genes being most activated in the network were sample dependent.
For our next analysis of the HCM network, we examined the inferred co-expression values for each HCM sample. We also included the composite values for the HCM cohort as a whole and control cohort as a whole. Principal component analysis was applied and principal component one (PC1) compared to IVSd (Figure 4B). As expected, the PC1 value for the control cohort was the most distant from all the other samples (PC1 = −12.26). Surprisingly, we saw a significant relationship between PC1 and IVSd in the individual HCM samples. For PC1 values closer to control (PC1 −9.02 to −4.4), we saw a linear relationship, where greater distance from control, corresponds to enlarging hearts, with a steep linear trendline. We called these “steep” samples. The linear relationship then reset (PC1 −4.3 to 11) with a moderate linear trendline where greater distance again corresponded to enlargement of the heart. We called these “moderate” samples. The five most distant samples (PC1 >15) showed no relationship to IVSd. This observation provided confidence that the gene expression relationships captured in our network analysis of iPSC-derived cardiomyocytes reflected aspects of the biology of the donor heart and furthermore was measuring critical components of pathologic gene expression remodeling indicative of disease severity. However, while PC1 was useful as a singular indicator value to represent the full disease co-expression network, it was harder to interpret biologically. We next evaluated if individual genes could also be indicators of the network activity.
For hub genes with eight or more edges, we tested how well they served as a proxy for the PC1 value (Figure 4C). We found the moderate and distant groups showed a high correlation between ADCY5 node strength and PC1, which was expected as it was the gene with the most co-expression pairs (25%, 49 out of 200 edges) and therefore likely drove the largest variability of network strength. Correspondingly, ADCY5 node strength, like PC1, exhibited significant correlation to IVSd in moderate samples. However, steep samples exhibited a weak correlation between ADCY5 with either PC1 or IVSd. Notably, no other node showed greater correlation to PC1 in steep samples than ADCY5 (even when checking all 34 nodes with a minimum of 3 edges [versus nodes with a minimum of eight edges], data not shown).
From the data in Figures 4B and 4C, we drew the following conclusions. Firstly, we identified two distinct HCM groups based on transcriptional behavior. (We focused on the moderate and steep groups as the distant group was only comprised of five samples.) Secondly, both groups encompassed a spectrum of disease severity (range of IVSd values). Thirdly, they shared a common disease co-expression network, such that for both groups network activation levels corresponded to disease severity of the donor (though notably smaller PC1 values were sufficient to indicate high IVSd values for steep samples). Fourth, the groups were distinguished by the manner with which they activated the disease network (even when comparing samples with similarly severe IVSd measurements). Specifically, in moderate samples, ADCY5 activation was occurring as a unit, such that a moderate HCM sample that exhibited a stronger co-expression relationship between ADCY5 and one of its paired genes was likely to also have stronger co-expression relationships for all of the gene-gene pairs in the network and to have a correspondingly larger heart (IVSd). By contrast, a steep HCM sample with a large heart (IVSd) was expected to also have a high level of network activation relative to other steep samples, but this would be driven by only specific gene-gene pairs exhibiting strong co-expression, with the specific genes depending on the sample. The observation that network activation correlated to disease severity for both HCM-moderate and HCM-steep groups highlighted the importance of the network genes to HCM. The observation that the manner of network activation differed between the groups, even for samples with similar echocardiogram measurements, suggested the difference between steep and moderate samples was not due to differences in disease severity driven by a single pathogenic mutation but could be due to genetic background.
A similar analysis of the DCM network revealed no significant relationship between PC1 and LVEF (Figure 4D). Like what we found for the HCM steep samples, in the DCM samples no single node served as a good proxy for the whole network (Figure 4E).
We looked for experimental features which could explain the segregation of HCM samples into steep and moderate categories. Returning to the principal component analysis of the network, we found PC3 values partially segregated the steep and moderate HCM samples (Figure S5A). While ADCY5 edges were the largest contributor to PC1, ANLN edges represented the edges with the individual greatest relative contribution to PC3 (Figure S5B). We found that HCM lines with pathogenic mutations were more common in the steep group, especially for female lines (Figure S5C), and that ANLN node strength was weaker in steep samples as well as female samples with pathogenic mutations (Figure S5D). ANLN had been shown to turn on in mitotic cardiomyocytes,[28] a process not typical of the adult heart, and thus further testing is needed to determine if ANLN is a feature in the donor hearts, or only in the iPSC-cardiomyocyte model.
The RNA network findings and promoter mutation analyses provided mutual validation, supporting characterization of HCM subtypes
Given that moderate samples showed cohesive activation of the HCM network, we wondered if this signified a partially shared genetic background mechanism. We re-examined our analysis of the Puckelwartz et al. genes. We had preliminarily found that the mutation burden in the promoters of 54 of the Puckelwartz et al. genes was correlated with smaller IVSd in samples with a known pathogenic or likely pathogenic mutation, but not in samples without a known mutation. In fact, we now saw that for moderate samples, both samples with and without pathogenic mutations exhibited this correlation (Figure S5E), while steep samples analyzed on their own did not exhibit this correlation, even amongst those with a pathogenic mutation (Figure S5F). Given that our promoter analysis of the Puckelwartz et al genes was underpowered to draw meaningful conclusions and could represent spurious correlations, we applied a published polygenic risk score for HCM.[29] We saw no difference in the average risk of HCM-steep samples versus control samples. However HCM-moderate samples had significantly higher scores than both control samples and HCM-steep samples (Figure S6G). Further, we found that moderate samples, but not steep samples, exhibited the expected phenomenon whereby the donors with a pathogenic mutation were younger than those without (Figure S6H). Taken together, these data supported our hypothesis that moderate HCM samples represented a subgroup of HCM where shared genetic background mechanisms may be influencing both disease severity and the transcriptional phenotype.
ADCY5 dysregulation was a shared feature of both HCM and DCM and partially corrected with drug treatment
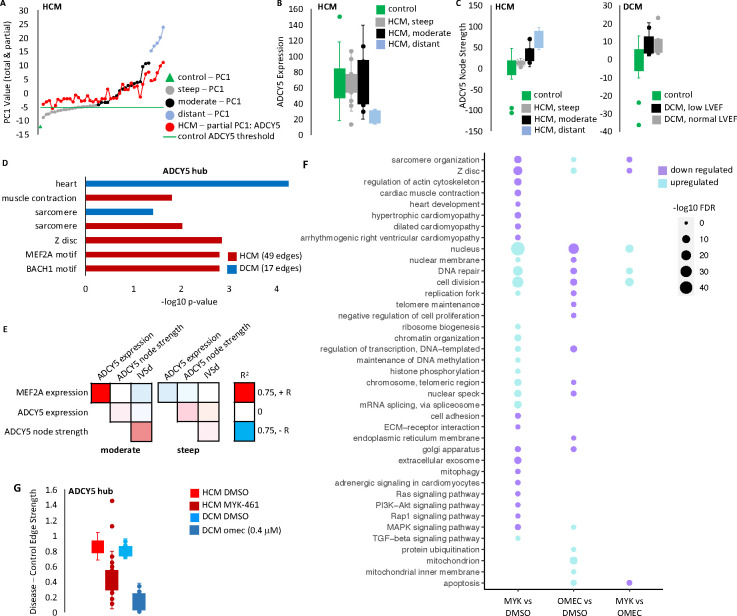
Further investigation of the importance of ADCY5 to the HCM network, revealed ADCY5 node strength explained the vast majority of the network activation in moderate samples and to a lesser extent in distant samples (Figures 5A and 5B), with stronger ADCY5 co-expression relationships in samples with greater network activation. Whereas, ADCY5 was only minimally activated in steep samples with minimal variability between samples as well (Figures 5A and 5B). Importantly, ADCY5 expression showed no difference between the HCM subgroups nor between HCM and control (Figure 5C). This highlighted the value of the network analysis to uncover important pathologic transcriptional remodeling features, but also meant unfortunately investigating future samples could not be done by simply measuring ADCY5 expression in the absence of co-expression analysis. Despite HCM and DCM hearts exhibiting contrasting phenotypes, we found ADCY5 was also important in DCM. Increased ADCY5 node strength compared to control was a shared feature DCM samples, and this was true for both samples coming from donors with normal LVEF (50% or greater) and those with reduced or moderately reduced LVEF (less than 50%) (Figure 5C). Taken together with our previous observation that ADCY5 node activation did not correlate with total network activation in DCM (Figure 4E), this showed that the ADCY5 hub node was being universally activated in the DCM samples. ADCY5 had co-expression relationships with 49 genes in the HCM network and 17 genes in the DCM network. 10 genes were common to both HCM and DCM. Gene ontology analysis of these genes revealed enrichment for the sarcomere (Figure 5D). For HCM specifically, gene ontology analysis also returned 65 significantly enriched transcription factor motifs, the most significant being for MEF2A. MEF2A is a transcription factor with a central role in driving cardiac hypertrophy.[30] We found that in moderate samples but not in steep samples, MEF2A expression and ADCY5 expression is highly correlated (Figure 5E).
Figure 5. Treatment with MYK-461 or omectamtiv mecarbil partially corrects ADCY5.
A. Plotted is the principal component data from Figure 4B. PC1 for each HCM sample is shown (gray, black, blue, by HCM subgroup). PC1 is a sum of values for each edge. Also plotted is the sum of the scores for all ADCY5 edges specifically. HCM-moderate samples show ADCY5 scores increasing with PC1, while ADCY5 scores are similar across HCM-steep samples. B. ADCY5 expression is similar between control, moderate and steep samples, but ADCY5 node strength (C) is increased in HCM and DCM. D. Gene ontology analysis of genes sharing edges with ADCY5. E. Pearson correlation values between MEF2A expression and ADCY5. F. Cardiomyocytes were treated with the small molecule sarcomere activator (omecamtiv mecarbil) or inhibitor (mavacamten) at 0 hours and 24 hours, with RNA harvested at 48 hours for RNA-seq analysis. Gene ontology analysis revealed many shared drug targets. G. For the ADCY5 node, mean edge strength in the DMSO-treated control cohort was compared with the mean strength in the DMSO-treated or drug-treated disease cohort. Plotted are the difference for each edge around the node in the respective comparisons. In both cases, we see a partial correction (smaller difference vs control) with drug treatment.
Finally, mavacamten (known commercially as Camzyos), is a small molecule inhibitor of MHY7 for treating patients with obstructive HCM that.[31] We treated cardiomyocytes with both mavacamten as well as an MYH7 inhibitor, omecamtiv mecabril[32] for 48 hours and then performed RNA-seq. Kinetic image cytometry was used to visually measure cellular deformation over time and confirm the treatment strategy successfully altered contractility. As expected, mavacamten reduced contractility, while omecamtiv mecarbil increased contractility (Figures S6A and S6B). Gene ontology analysis of RNA-seq after drug treatment revealed many shared drug targets between mavacamten and omecamtiv mecarbil, such as an opposing effect on expression of Z disc components (Figure 5F). For each edge around ADCY5, we compared the mean edge strength in the diseased cohort to the control cohort before and after drug treatment and found drug treatment partially corrected the ADCY5 node for both HCM and DCM (Figure 5G).
DISCUSSION
We identified ADCY5 as a central hub node in both the HCM and DCM diseased networks. Adenylyl cyclases catalyze ATP to cAMP conversion, with ADCY5 and ADCY6 being the major isoforms in the heart.[27] ADCY5 is sensitive to and able to influence contractile regulation. Beta-adrenergic stimulation and PKC activate ADCY5 which in turn catalyzes cAMP formation, driving PKA signaling. PKA phosphorylation and local calcium levels inhibit ADCY5.[33] Previous studies support a role for ADCY5 in heart disease. Adenylyl cyclases drive the increased inotropy and lusitropy induced by beta-adrenergic agonist stimulation of the heart by producing cAMP which activates downstream pathways of protein kinase A.[34] In mice, ADCY5 overexpression increases oxidative stress and worsens cardiomyopathy outcome under chronic stress conditions, while ADCY5 knockout is protective in chronic stress conditions and a high fat diet model of diabetic cardiomyopathy.[27] Furthermore ADCY5 knockout mice have increased lifespan, and blunted aging-associated left ventricular hypertrophy and cardiomyopathy.[27] In mice and rabbits, pharmaceutical inhibition of ADCY5 shortly after coronary artery reperfusion reduced myocardial infarct size.[35] Alternately, Gαq overexpression-induced cardiomyopathy mice have decreased ADCY5, and further ADCY5 knockout is not protective.[27] In silico analysis of HCM and DCM identified ADCY5 as a potential drug target for modulating other disease processes.[36] We found ADCY5 activation was a universal feature of DCM lines, while serving as a biomarker of network activation and donor disease severity for a subgroup of HCM. Importantly, only 10 edges were shared between the ADCY5 node in HCM (49 edges) and DCM (17 edges). These included contractile genes MYBPC3, TNNT2, TRIM63, and regulators of excitation and excitation-contraction coupling RFN207 and LRRC10[37, 38], with the nodes as a whole enriched for sarcomere constituents (Figure 5D). Here we demonstrated increased ADCY5 activation in multiple genetic backgrounds from both HCM and DCM, and in the context of disparate pathogenic mutations in a human cell-line model. We further show ADCY5 node activation is sensitive to contractility modulation through drug treatment and posit it may be sensitive to pathogenic mutations in contractile proteins. In turn, we propose ADCY5 represents a shared molecular phenotype that can influence molecular remodeling downstream of contractile dysfunction, and that targeting ADCY5 may be able to influence contractile dysfunction stemming from multiple etiologies.
Additionally, we confirmed and expanded on the Puckelwartz et al observation of cumulative mutation burden in cardiomyopathy genes to correlate with DCM severity finding in our cohort the relationship is specific to samples without known pathogenic mutations. This supports the hypothesis for distinct DCM inheritance mechanisms and highlights the need for further studies which can properly delineate the risk loci responsible, as it is understood many of the cardiomyopathy gene variants used in this analysis likely do not contribute.
Finally, we characterized individual samples by RNA signatures. For DCM we found individual hub genes represented units of diseased network activation (Figure 4A). However, the relative degree of activation of separate hub genes varied by sample (Figure 4E). Thus the network constituents are important indicators of disease biology and may represent conserved candidates for therapeutic intervention (including ADCY5, Figure 5C), but additional RNA signatures are needed to explain disease severity. In HCM, we defined a single diseased transcriptional network with applicability to distinct HCM subgroups, in that for all subgroups, network activation corresponded to more severe echocardiogram measurements of the donor. We interpret the differences in the moderate and steep RNA subtypes as indicative of distinct genetic backgrounds. These data represent preliminary evidence for genetic background to influence molecular phenotype in cardiomyopathy.
Supplementary Material
Acknowledgments
We wish to thank the patients who contributed to our cardiomyopathy biobank, making this research possible.
Sources of Funding
This work was supported in part by the California Institute for Regenerative Medicine (GC1R-06673-A: CIP#1) as well as NIH P01 HL141084, R01 HL141371, R01 HL126527, 75N92020D00019 (JCW).
Nonstandard Abbreviations and Acronyms
- ADCY5
adenylyl cyclase type 5
- DCM
dilated cardiomyopathy
- DMSO
dimethyl sulfoxide
- EMR
electronic medical record
- HCM
hypertrophic cardiomyopathy
- indels
insertion and deletions
- iPSC
induced pluripotent stem cell
- IVSd
interventricular septum thickness, end diastole
- lioness
linear interpolation to obtain network estimates for single samples
- LVEF
left ventricular ejection fraction
- LVNC
left ventricular noncompaction
- MAF
minor allele frequency
- myk
mavacamten / MYK-461 *this is an unconventional abbreviation created for labeling figures
- nopatho
lines without a known pathogenic or likely pathogenic mutation *this is an unconventional abbreviation created for labeling figures
- omec
omecamtiv mecarbil *this is an unconventional abbreviation created for labeling figures
- P/LP
pathogenic or likely pathogenic mutation
- PBMC
peripheral blood mononuclear cell
- PC
principal component
- SNV
single nucleotide variant
- WGS
whole genome sequencing
Footnotes
Disclosures
M.P.S. is a co-founder and the scientific advisory board member of Personalis, Qbio, January, SensOmics, Filtricine, Akna, Protos, Mirvie, NiMo, Onza, Oralome, Marble Therapeutics and Iollo. He is also on the scientific advisory board of Danaher, Genapsys, and Jupiter.
REFERENCES
- 1.Marian A.J., Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circ Res, 2021. 128(10): p. 1533–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershberger R.E., Hedges D.J., and Morales A., Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol, 2013. 10(9): p. 531–47. [DOI] [PubMed] [Google Scholar]
- 3.Tsao C.W., et al. , Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation, 2023. 147(8): p. e93–e621. [DOI] [PubMed] [Google Scholar]
- 4.Hershberger R.E., et al. , The Complex and Diverse Genetic Architecture of Dilated Cardiomyopathy. Circ Res, 2021. 128(10): p. 1514–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh R., et al. , Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med, 2017. 19(2): p. 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh R., et al. , Defining the genetic architecture of hypertrophic cardiomyopathy: re-valuating the role of non-sarcomeric genes. Eur Heart J, 2017. 38(46): p. 3461–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis J., et al. , A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy. Cell, 2016. 165(5): p. 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tayal U., et al. , Precision Phenotyping of Dilated Cardiomyopathy Using Multidimensional Data. J Am Coll Cardiol, 2022. 79(22): p. 2219–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neubauer S., et al. , Distinct Subgroups in Hypertrophic Cardiomyopathy in the NHLBI HCM Registry. J Am Coll Cardiol, 2019. 74(19): p. 2333–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzarotto F., et al. , Contemporary Insights Into the Genetics of Hypertrophic Cardiomyopathy: Toward a New Era in Clinical Testing? J Am Heart Assoc, 2020. 9(8): p. e015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdonschot J.A.J., et al. , Role of Targeted Therapy in Dilated Cardiomyopathy: The Challenging Road Toward a Personalized Approach. J Am Heart Assoc, 2019. 8(11): p. e012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maron B.J., Maron M.S., and Semsarian C., Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol, 2012. 60(8): p. 705–15. [DOI] [PubMed] [Google Scholar]
- 13.Pirruccello J.P., et al. , Analysis of cardiac magnetic resonance imaging in 36,000 individuals yields genetic insights into dilated cardiomyopathy. Nat Commun, 2020. 11(1): p. 2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esslinger U., et al. , Correction: Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy. PLoS One, 2020. 15(2): p. e0229472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards S., et al. , Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 2015. 17(5): p. 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puckelwartz M.J., et al. , Genomic Context Differs Between Human Dilated Cardiomyopathy and Hypertrophic Cardiomyopathy. J Am Heart Assoc, 2021. 10(7): p. e019944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koopmann T.T., et al. , Genome-wide identification of expression quantitative trait loci (eQTLs) in human heart. PLoS One, 2014. 9(5): p. e97380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes-Ramos C.M., et al. , Sex Differences in Gene Expression and Regulatory Networks across 29 Human Tissues. Cell Rep, 2020. 31(12): p. 107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuijjer M.L., et al. , Estimating Sample-Specific Regulatory Networks. iScience, 2019. 14: p. 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayosi B.M., et al. , Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ Cardiovasc Genet, 2017. 10(2). [DOI] [PubMed] [Google Scholar]
- 21.Irvin M.R., et al. , Whole-Exome Sequencing and hiPSC Cardiomyocyte Models Identify MYRIP, TRAPPC11, and SLC27A6 of Potential Importance to Left Ventricular Hypertrophy in an African Ancestry Population. Front Genet, 2021. 12: p. 588452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito S., et al. , Identification of the Mtus1 Splice Variant as a Novel Inhibitory Factor Against Cardiac Hypertrophy. J Am Heart Assoc, 2016. 5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windak R., et al. , The AP-1 transcription factor c-Jun prevents stress-imposed maladaptive remodeling of the heart. PLoS One, 2013. 8(9): p. e73294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu H., et al. , Emerging role of VCP/p97 in cardiovascular diseases: novel insights and therapeutic opportunities. Biochem Soc Trans, 2021. 49(1): p. 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidar M.N., et al. , Network-based computational approach to identify genetic links between cardiomyopathy and its risk factors. IET Syst Biol, 2020. 14(2): p. 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Mubarak A.A., van der Meer P., and Bomer N., Selenium, Selenoproteins, and Heart Failure: Current Knowledge and Future Perspective. Curr Heart Fail Rep, 2021. 18(3): p. 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vatner S.F., et al. , Adenylyl cyclase type 5 in cardiac disease, metabolism, and aging. Am J Physiol Heart Circ Physiol, 2013. 305(1): p. H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouleisa R.R.E., et al. , Transient Cell Cycle Induction in Cardiomyocytes to Treat Subacute Ischemic Heart Failure. Circulation, 2022. 145(17): p. 1339–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harper A.R., et al. , Common genetic variants and modifiable risk factors underpin hypertrophic cardiomyopathy susceptibility and expressivity. Nat Genet, 2021. 53(2): p. 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akazawa H. and Komuro I., Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res, 2003. 92(10): p. 1079–88. [DOI] [PubMed] [Google Scholar]
- 31.Debbia E., Pesce A., and Schito G.C., In vitro interactions between teicoplanin and other antibiotics against enterococci and staphylococci. J Hosp Infect, 1986. 7 Suppl A: p. 73–7. [DOI] [PubMed] [Google Scholar]
- 32.Lehman S.J., Crocini C., and Leinwand L.A., Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol, 2022. 19(6): p. 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa Y. and Homcy C.J., The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res, 1997. 80(3): p. 297–304. [DOI] [PubMed] [Google Scholar]
- 34.Feldman A.M., Adenylyl cyclase: a new target for heart failure therapeutics. Circulation, 2002. 105(16): p. 1876–8. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., et al. , A novel adenylyl cyclase type 5 inhibitor that reduces myocardial infarct size even when administered after coronary artery reperfusion. J Mol Cell Cardiol, 2018. 121: p. 13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theodoris C.V., et al. , Transfer learning enables predictions in network biology. Nature, 2023. 618(7965): p. 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiamvimonvat N. and Song L.S., LRRC10 (Leucine-Rich Repeat Containing Protein 10) and REEP5 (Receptor Accessory Protein 5) as Novel Regulators of Cardiac Excitation-Contraction Coupling Structure and Function. J Am Heart Assoc, 2018. 7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roder K., et al. , RING finger protein RNF207, a novel regulator of cardiac excitation. J Biol Chem, 2014. 289(49): p. 33730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruber J.J., et al. , Chromatin Remodeling in Response to BRCA2-Crisis. Cell Rep, 2019. 28(8): p. 2182–2193 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streeter I., et al. , The human-induced pluripotent stem cell initiative-data resources for cellular genetics. Nucleic Acids Res, 2017. 45(D1): p. D691–D697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panopoulos A.D., et al. , iPSCORE: A Resource of 222 iPSC Lines Enabling Functional Characterization of Genetic Variation across a Variety of Cell Types. Stem Cell Reports, 2017. 8(4): p. 1086–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burridge P.W., et al. , Chemically defined generation of human cardiomyocytes. Nat Methods, 2014. 11(8): p. 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendig K.I., et al. , Sentieon DNASeq Variant Calling Workflow Demonstrates Strong Computational Performance and Accuracy. Front Genet, 2019. 10: p. 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H. and Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 2009. 25(14): p. 1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DePristo M.A., et al. , A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet, 2011. 43(5): p. 491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hershberger R.E., et al. , Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med, 2018. 20(9): p. 899–909. [DOI] [PubMed] [Google Scholar]
- 47.Wang K., Li M., and Hakonarson H., ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res, 2010. 38(16): p. e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiffin N., et al. , CardioClassifier: disease- and gene-specific computational decision support for clinical genome interpretation. Genet Med, 2018. 20(10): p. 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherman B.T., et al. , DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res, 2022. 50(W1): p. W216–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKeithan W.L., et al. , An Automated Plalorm for Assessment of Congenital and Drug-Induced Arrhythmia with hiPSC-Derived Cardiomyocytes. Front Physiol, 2017. 8: p. 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perea-Gil I., et al. , Serine biosynthesis as a novel therapeutic target for dilated cardiomyopathy. Eur Heart J, 2022. 43(36): p. 3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma A., et al. , Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc, 2018. 13(12): p. 3018–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.