Abstract
Cystic fibrosis (CF) lung disease features persistent neutrophil accumulation to the airways from the time of infancy. CF children are frequently exposed to Pseudomonas aeruginosa, and by adulthood, 80% of CF patients are chronically infected. The formation of biofilms is a particularly important phenotypic characteristic of P. aeruginosa that allows for bacterial survival despite aggressive antibiotic therapy and an exuberant immune response. Here, we show that the presence of neutrophils enhances initial P. aeruginosa biofilm development over a period of 72 h through the formation of polymers comprised of actin and DNA. F-actin was found to be a site of attachment for P. aeruginosa. These actin and DNA polymers are present in CF sputum, and disruption of the polymers dispersed the associated P. aeruginosa cells and reduced biofilm development. These findings demonstrate a potential maladaptation of the primary innate response. When the host fails to eradicate the infection, cellular components from necrotic neutrophils can serve as a biological matrix to facilitate P. aeruginosa biofilm formation.
Cystic fibrosis (CF) lung disease features persistent neutrophil accumulation in the airways from the time of infancy (25). In the absence of detectable infection or endotoxin, bronchioalveolar lavage studies have recovered neutrophils ranging from 104 to 106 per ml from the airways of CF children (25, 29, 30). These children are frequently exposed to environmental strains of Pseudomonas aeruginosa, but early infections can be transient or be eradicated by aggressive antibiotic treatment and an exuberant host defense (6, 17, 35). Initial success in eradicating P. aeruginosa acquired from environmental sources likely occurs due to a low density of organisms, a lack of antibiotic resistance, and a generally nonmucoid phenotype. Eventually, persistent P. aeruginosa infection appears inevitable, and by adulthood, 80% of CF patients are chronically infected (7).
Factors that allow P. aeruginosa to become persistent are of particular interest, as chronic P. aeruginosa infection is clearly associated with increased morbidity and mortality in CF patients (14, 32, 37). Persistent P. aeruginosa infection is associated with numerous phenotypic and genetic changes by the bacteria within the CF airway (12, 15, 41), including the formation of biofilms (1, 12, 40). Bacterial biofilms are surface-attached communities of cells encased within a self-produced extracellular polysaccharide matrix. Biofilm development proceeds through a series of programmed steps, including initial surface attachment, the formation of three-dimensional microcolonies, and finally, the development of a mature biofilm (26). The detection of a specific pattern of quorum-sensing signaling molecules in the CF airway suggests that P. aeruginosa in the CF airway exists primarily in the biofilm form (40), and this conclusion is supported by the inability of antibiotics and host defense mechanisms to eradicate the infection (1, 12, 40).
Persistent neutrophil accumulation and necrosis in the CF airways results in sputum highly enriched with DNA, actin, and granule proteins, which are all clearly implicated in the pathogenesis of CF lung disease (2, 25, 28, 33, 36, 38). Based on the concept that early CF lung disease features low numbers of planktonic, environmental strains of P. aeruginosa cells entering a neutrophil-rich airway (6), we tested the effect of neutrophils on the earliest stages of P. aeruginosa biofilm formation using a concentration of neutrophils compatible with the quantity of cells present in the airways of CF children prior to persistent P. aeruginosa infection and concentrations of P. aeruginosa consistent with early infection (8, 29).
MATERIALS AND METHODS
P. aeruginosa and neutrophils.
The P. aeruginosa strain used was PAO1 (a motile, piliated strain) or an isogenic strain of PAO1 carrying the gene encoding the green fluorescent protein (GFP) (31). Human neutrophils were isolated from healthy volunteers, purified by the plasma Percoll method (21), and resuspended in RPMI 1640 (Bio-Whittaker, Walkersville, MD) supplemented with 10 mM HEPES (pH 7.6) and 2% heat-inactivated platelet-poor plasma.
Biofilm assays.
A static biofilm assay was used with polypropylene tubes (10). PA01 was grown overnight under constant rotation to late stationary phase at 37°C in Luria broth. All biofilm studies were initiated with neutrophils (1 × 107 cells/ml) and PAO1 (1 × 106 CFU/ml) in suspension, and cultures were incubated under stationary conditions at 37°C. P. aeruginosa cells adherent to the tube were considered a biofilm, while bacteria not adherent to the surface of the tube were considered planktonic. Viable P. aeruginosa biofilm and planktonic cells were quantified by sonicating the adherent bacteria in Luria broth, followed by serial dilution and plating to determine CFU on Pseudomonas isolation agar (Difco, Sparks, MD). P. aeruginosa biofilm density was quantified by crystal violet (CV) staining (10). The contributions of background staining of neutrophil components, tubes, and reagents were subtracted from depicted values. Neutrophils were lysed with 0.1% Tween 20 (Bio-Rad, Hercules, CA) for 30 min. Neutrophil lysates relatively depleted of F-actin were prepared by precipitating the F-actin using an F-actin/G-actin in vivo Biochem kit (Cytoskeleton, Denver, CO). The total protein present in the whole-cell lysate and the F-actin-depleted lysate was determined by the Bradford protein assay (Bio-Rad, Hercules, CA). Biofilm exopolysaccharide was determined by total carbohydrate assays as previously described (16).
P. aeruginosa actin-binding assay.
Purified G-actin extracted from rabbit skeletal muscle was purchased (Sigma, St. Louis, MO) as a lyophilized powder containing 2 mM Tris (pH 8.0), 0.5 mM beta-mercaptoethanol, 0.2 mM CaCl2, and 0.2 mM ATP and was redissolved in deionized water at a concentration of 3 to 4 mg/ml, which maintains the G-form of actin. G-actin was polymerized to F-actin by incubating it at room temperature for 1 h in the presence of 50 mM KCl and 2 mM MgCl2 in sterile phosphate-buffered saline (PBS). G-actin was also incubated at room temperature for 1 h in the absence of KCl and MgCl2 to prevent polymerization. After 1 h, F-actin and G-actin were plated on a 96-well microtiter plate and incubated overnight at room temperature. All wells were blocked with 1% bovine serum albumin for 1 h before adding the bacteria. P. aeruginosa was labeled with an intracellular fluorescent conjugate carboxy-fluorescein diacetate, succinimidyl ester (CFDA SE), for 45 min (Vybrant CFDA SE Cell Tracer kit; Molecular Probes, Eugene, OR) and then washed with PBS. Labeled P. aeruginosa cells (1 × 106) were added to wells coated with either F- or G-actin or PBS (with or without KCl and MgCl2) controls. The plates were incubated for 4 h at 37°C and then washed carefully with PBS. The fluorescent intensity of bound P. aeruginosa was measured at 492 and 517 nm with a plate reader (Bio-Tek Instruments), and the quantity of P. aeruginosa cells present was determined with a standard curve and expressed as a percentage of the total number of P. aeruginosa cells added to the well. Enhanced F-actin formation on the plates was confirmed by staining with yellow-green fluorescent 7-nitrobenz-2-oxa-1,3-phallacidin (Molecular Probes), with relative quantification read at 465 and 536 nm. Equivalent quantities of total F- and G-actin on the plates were confirmed by exposure of the wells to a mouse anti-pan actin immunoglobulin G (IgG) antibody (NeoMakers, Fremont, CA), followed by secondary binding with an anti-mouse IgG globulin conjugated with horseradish peroxidase which then binds to the antibody-antigen complex. The excess conjugate was removed by washing, followed by the addition of chromogen/substrate tetramethylbenzidine with H2O2 and read at 450 and 570 nm. The total quantities of protein were confirmed to be equivalent for all conditions, as measured by the Bradford assay (Bio-Rad, Hercules, CA).
Purification of granular proteins and DNA.
Genomic DNA was isolated from neutrophils and P. aeruginosa using the DNeasy tissue kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Actin and DNA polymerization was performed as described previously (38). Effects of filament cleavage were tested by initially treating the samples with 90 Kunitz units/ml of DNase I (43) and/or 200 nM gelsolin (Sigma) (42). Granule proteins were isolated from homogenized human neutrophils by differential centrifugation in a discontinuous Percoll-sucrose gradient (34). Heavy and light granules were immediately suspended in RPMI medium and added to P. aeruginosa.
Microscopy.
Samples of sputa from CF patients chronically infected with P. aeruginosa were frozen in liquid nitrogen and stored at −20°C. At the time of analysis, sputa were thawed and resuspended carefully in PBS (1:4 [vol/vol] ratio) containing 107 CFU/ml of P. aeruginosa labeled with the intracellular fluorescent conjugate (CFDA SE) as described in the actin-binding protocol. Samples were incubated for 4 h at 37°C and then dried on Superfrost Plus microscope slides (Fisher Scientific) and stained for 15 min with 10 μl of 0.6 μM Alexa Fluor 546 Phalloidin (Molecular Probes)-10 μl of 0.2 μM 4′,6′-diamidino-2-phenylindole (DAPI)-dilactate (Molecular Probes) to visualize F-actin and DNA. Visualizations of actin (546 and 576 nm), DNA (358 and 461 nm), and P. aeruginosa (492 and 517 nm) were performed sequentially on the same field, followed by an “overlay” view at all three wavelengths. Neutrophil lysates were also combined with GFP-labeled P. aeruginosa for 4 h at 37°C, and staining of F-actin and DNA was conducted as described for CF sputa.
Analysis of P. aeruginosa biofilms by confocal microscopy.
GFP-PAO1 was cultured in eight-chamber polystyrene tissue culture-treated glass slides (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) alone or with neutrophils. At 48 h, biofilms were evaluated using a Zeiss Axiovert 200 M confocal microscope equipped with Slidebook imaging software (Intelligent Imaging, Denver, CO). GFP-PAO1 was excited in the fluorescein isothiocyanate channel at 488 or 500 to 550 nm. Three-dimensional reconstruction of biofilms was formed from images captured at 1-μm intervals, with segmentation and reconstruction using version 3.5 SURFdriver software (Kailua, HI).
Statistical analysis.
Data were analyzed using JMP software (SAS Institute, Cary, NC). Student's unpaired t test (two-tailed) was used to determine the significance of neutrophil and neutrophil lysate enhancement of P. aeruginosa survival and biofilm development (Fig. 1A through C and 4A) and binding of P. aeruginosa to F-actin (Fig. 5D) at individual time points. One-way analysis of variance using Dunnett's method was used to analyze the variance of multiple group means to the control group (P. aeruginosa alone) for biofilm development (Fig. 1D and E, 3A and D, and 4B and C). For all tests, P < 0.05 was considered significant.
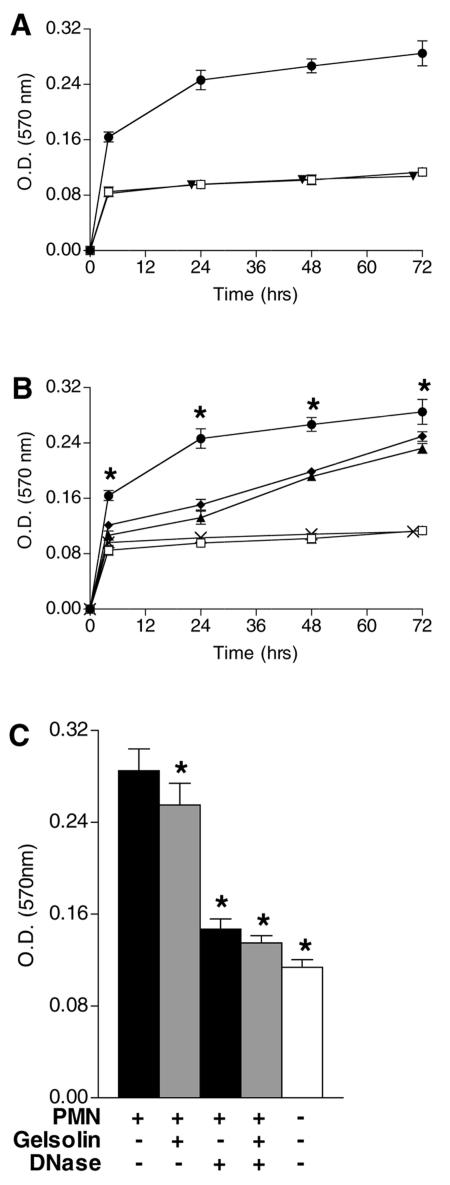
FIG. 1.
Enhancement of P. aeruginosa biofilm formation by human neutrophils. Biofilm development of P. aeruginosa (□) was compared with P. aeruginosa in the presence of neutrophils (•). (A) The presence of neutrophils had little effect on the long-term survival of P. aeruginosa. Viable P. aeruginosa cells from both the biofilm and the planktonic state were quantified in the presence and absence of neutrophils. A significant reduction in the number of viable P. aeruginosa cells was present only after 4 h of incubation. Plot depicts means ± standard deviations (SD) of CFU (n = 4). *, P < 0.05 by Student's t test. Neutrophils (▴) underwent rapid necrosis in the presence of P. aeruginosa. Plot (right axis) depicts the mean percentage of viable neutrophils ± SD (n = 4). (B) The presence of neutrophils resulted in fewer viable P. aeruginosa cells in the planktonic state than of P. aeruginosa cells in the absence of neutrophils. Plot depicts means ± SD of CFU (n = 4). *, P < 0.05 by Student's t test. (C) Neutrophils increased the number of viable P. aeruginosa cells in the biofilm statecompared to P. aeruginosa alone when measured simultaneously with P. aeruginosa in the planktonic state shown in panel B. Plot depicts means ± SD of CFU (n = 4). *, P < 0.05 by Student's t test. (D) Neutrophils increased the biofilm density (assayed by CV staining) compared to P. aeruginosa alone by 4 h. Additional neutrophils introduced at 24 and 48 h (arrows) resulted in further enhancement of the biofilm density (♦) at 48 and 72 h. Plot depicts means ± SD of optical density (O.D.) measurements (n = 21). *, P < 0.05 by Dunnett's t test. (E) Exopolysaccharide staining of biofilm density demonstrated that the presence of neutrophils resulted in a greater quantity of biofilm than did P. aeruginosa in the absence of neutrophils by 4 h. When additional neutrophils were added 24 and 48 h (arrows) after the initiation of the biofilm, further enhancement of the biofilm density (♦) was observed at 48 and 72 h. Plot depicts means ± SD of O.D. measurements (n = 21). *, P < 0.05 by Dunnett's t test.
FIG. 4.
Enhancement of P. aeruginosa biofilm formation by isolated neutrophil components. (A) Isolated neutrophil granule proteins failed to enhance the density of biofilm formation. P. aeruginosa (▾) combined with granule proteins (quantity equivalent to 5 × 106 neutrophils) compared to P. aeruginosa (□) in the absence of granule proteins and P. aeruginosa combined with live neutrophils (•). Plot depicts means ± standard deviations (SD) of optical density (O.D.) crystal violet measurements (n = 4). *, P < 0.05 by Student's t test. (B) Actin and DNA enhance P. aeruginosa biofilm development. While neutrophil-derived DNA (×) (4 μg/ml) alone had little effect, the addition of purified G-actin (▴) (0.4 mg/ml) resulted in enhancement of biofilm development compared to P. aeruginosa (□) in the absence of supplementation. This effect was further augmented by the combination of actin with neutrophil-derived DNA (♦), nearly achieving the extent of biofilm enhancement achieved by P. aeruginosa combined with live neutrophils (•). Plot depicts means ± standard error of the means of O.D. measurements of CV staining (n = 4). Addition of actin or actin with DNA to P. aeruginosa was significantly greater than that to P. aeruginosa alone at times from 4 to 72 h. *, P < 0.05 by Dunnett's t test. (C) Loss of biofilm enhancement by disruption of DNA and actin. DNase, gelsolin, or DNase plus gelsolin, added at the time when neutrophils (polymorphonuclear leukocytes [PMN]) were combined with P. aeruginosa, resulted in significant loss of biofilm enhancement. Plot depicts means ± S.D. of O.D. CV measurements (n = 4). *, P < 0.05 by Dunnett's t test.
FIG. 5.
Binding of P. aeruginosa to F-actin in vitro. The relative ability of P. aeruginosa, to associate with neutrophil-derived DNA and purified actin was analyzed. (A) When DNA (blue) was combined with P. aeruginosa (green) for 4 h, the DNA formed small complexes and no association was observed between DNA and P. aeruginosa. (B) Under identical conditions, purified G-actin polymerized into various sizes of F-actin filaments (red) and considerable colocalization of the actin and P. aeruginosa was observed. (C) When combined, neutrophil-derived DNA and F-actin formed well-developed polymers, with nearly all visible P. aeruginosa associating with the filament. Panels A through C are representative of three consecutive experiments. Bar, 20 μm. (D) Actin binding by P. aeruginosa. Planktonic P. aeruginosa was allowed to settle in wells coated with actin (solid bar) or bovine serum albumin-blocked plastic (open bar). After 4 h, significant P. aeruginosa binding to F-actin, but not G-actin, was detected. Plot depicts means ± SD (n = 3). *, P < 0.05 by Student's t test.
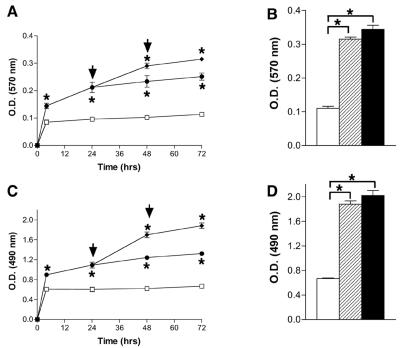
FIG. 3.
Enhancement of P. aeruginosa biofilm formation by lysed neutrophils. Parameters of biofilm development of P. aeruginosa (□) were compared with P. aeruginosa in the presence of lysed neutrophils (•) at time intervals from 0 to 72 h. (A) Crystal violet staining of biofilm density demonstrated that the presence of lysed neutrophils resulted in a greater quantity of biofilm than did P. aeruginosa in the absence of neutrophils by 4 h. When additional neutrophil lysates (♦) were added 24 and 48 h (arrows) after the initiation of the biofilm, further enhancement of the biofilm density was observed at 48 and 72 h. (B) Crystal violet staining of biofilm density demonstrated that by 72 h, lysed neutrophils added at 0, 24, and 48 h (hatched bar) achieved 92% of the biofilm development seen with viable neutrophils added at 0, 24, and 48 h (solid bar). Both conditions resulted in significantly greater biofilm development than did P. aeruginosa in the absence of neutrophils (open bar). (C) Exopolysaccharide staining of biofilm density demonstrated that the presence of lysed neutrophils resulted in a greater quantity of biofilm than did P. aeruginosa in the absence of neutrophils by 4 h. When additional neutrophil lysates (♦) were added 24 and 48 h (arrows) after the initiation of the biofilm, further enhancement of the biofilm density was observed at 48 and 72 h. (D) Exopolysaccharide staining of biofilm density demonstrated that by 72 h, lysed neutrophils added at 0, 24, and 48 h (hatched bar) achieved 94% of the biofilm development seen with viable neutrophils added at 0, 24, and 48 h (solid bar). Both conditions resulted in significantly greater biofilm development than did P. aeruginosa in the absence of neutrophils (open bar). Plots A through D depict means ± SD of O.D. measurements (n = 21). *, P < 0.05 by Dunnett's t test.
RESULTS
Effect of human neutrophils on initial P. aeruginosa biofilm development.
Using a static assay, biofilm development was analyzed for up to 72 h. Neutrophil cytotoxicity equaled 92% after 24 h of exposure to P. aeruginosa (Fig. 1A). In the presence of neutrophils, a reduction in the number of surviving P. aeruginosa cells was detected at 4 h, while at later time points, the bactericidal effects of the neutrophil no longer reached significance (Fig. 1A). When measured simultaneously, the number of viable P. aeruginosa cells in the planktonic state was significantly decreased by the presence of neutrophils while the number of viable P. aeruginosa cells in the biofilm state was significantly increased (Fig. 1B and C). CV staining of P. aeruginosa biofilms formed in the presence of neutrophils demonstrated an increase in biofilm density (Fig. 1D). Enhanced biofilm formation in the presence of neutrophils was also demonstrated by quantifying bacterial exopolysaccharide production (Fig. 1E). For each assay, significant enhancement of biofilm development was observed by 4 h, and by 72 h, the extent of neutrophil-induced biofilm enhancement exceeded 3.5-fold as assessed by viable bacterial colony counts, 2.5-fold as assessed by biofilm density, and twofold as assessed by exopolysaccharide content.
As neutrophils are recruited continuously to the CF airway, the effect of adding additional viable neutrophils 24 and 48 h after the initiation of biofilm formation was tested. Supplementing neutrophils to P. aeruginosa in the early stages of biofilm development resulted in additional biofilm enhancement (Fig. 1D and E). Confocal microscopy and three-dimensional reconstruction of GFP-labeled P. aeruginosa biofilms in the presence of neutrophils depicted a biofilm thicker and more developed than those depicted in the absence of neutrophils (Fig. 2A and B). Together, data presented in Fig. 1 and 2 demonstrate the potential of the neutrophil to enhance the earliest stages of P. aeruginosa biofilm formation.
FIG. 2.
Confocal microscopy of P. aeruginosa biofilms. PAO1-GFP biofilms at 48 h in the absence and in the presence of neutrophils. (A) Biofilms formed in the presence of neutrophils (right) appeared structurally more intact than control biofilms in the absence of neutrophils (left), which showed blank areas unoccupied by P. aeruginosa. (B) Three-dimensional reconstruction of P. aeruginosa biofilms (left) revealed a biofilm thinner than the biofilm formed in the presence of neutrophils (right). Bar, 25 μm.
Enhancement of P. aeruginosa biofilm formation by lysed neutrophils.
Since neutrophil necrosis is rapid in the presence of P. aeruginosa, the capacity of the cellular content of lysed neutrophils to evoke enhanced P. aeruginosa biofilm development was tested. Combining P. aeruginosa with neutrophil lysates significantly enhanced biofilm formation, as measured by CV staining and exopolysaccharide synthesis, and supplementing the early biofilm with additional quantities of lysed neutrophils at 24 and 48 h further enhanced biofilm production (Fig. 3A and C). Biofilms formed in the presence of lysed neutrophils achieved 92% of the biofilm enhancement of an equivalent number of viable neutrophils when assayed by CV staining and 94% percent when assayed by exopolysaccharide synthesis (Fig. 3B and D). Furthermore, the number of viable surface-attached biofilm cells increased when lysed neutrophils were added while the number of viable planktonic cells decreased (data not shown).
Enhancement of P. aeruginosa biofilm formation by isolated neutrophil components.
The data described above indicate that neutrophil cellular contents are largely responsible for enhanced P. aeruginosa biofilm formation. Analysis of CF sputum demonstrates high concentrations of granule proteins, actin, and DNA released from necrotic neutrophils (18, 28, 38). The capacity of each of these compounds to mediate enhanced early P. aeruginosa biofilm formation was tested. Supplementing planktonic P. aeruginosa with purified granule proteins failed to enhance biofilm production over a range of concentrations (Fig. 4A).
Neutrophil actin and DNA are also abundant in CF sputum and have been observed to bind together, forming polymers that increase the viscosity of CF sputum (38). Supplementing purified globular monomeric actin (G-actin) to P. aeruginosa under conditions known to result in the formation of actin filaments (F-actin) significantly enhanced biofilm formation by 72 h (Fig. 4B). Purified neutrophil DNA alone did not enhance P. aeruginosa biofilm production. Supplementing planktonic P. aeruginosa with both actin and neutrophil DNA achieved an enhancement of biofilm formation equaling 88% of the biofilm developed in the presence of live neutrophils by 72 h. An equivalent effect on biofilm development was observed using DNA isolated from P. aeruginosa cells instead of neutrophils (data not shown); however, DNA found within CF sputa is almost entirely of human origin (28). Neutrophil lysates relatively depleted of F-actin and adjusted to have a protein concentration equal to that of the whole-cell lysates were also found to result in a significant decrease in biofilm enhancement compared to the untreated whole-cell lysates (data not shown).
A recent study reported that extracellular DNA (originating from P. aeruginosa) is required for the initial establishment of P. aeruginosa biofilms and that the addition of DNase strongly inhibits biofilm formation (43). The addition of DNase abolished much of the neutrophil-induced enhancement of biofilm formation (Fig. 4C) without significantly inhibiting bacterial growth or neutrophil survival (data not shown). The addition of gelsolin, a protein that severs noncovalent bonds between the monomers of actin filaments also significantly reduced the neutrophil-induced enhancement of the biofilm, but to a much lesser extent than DNase (Fig. 4C). Although as a single component, the addition of purified actin evoked the greatest biofilm enhancement (Fig. 4C), it must be noted that detectable amounts of DNA (originating from P. aeruginosa) were present in the absence of added DNA or neutrophils (data not shown). Likewise, while DNase evoked the greatest reduction in biofilm enhancement (Fig. 4C), the enzyme can bind to monomeric actin and slowly depolymerize actin filaments (22).
Purified neutrophil DNA (in the absence of actin) forms small fragments with no apparent association with planktonic P. aeruginosa (Fig. 5A). In the absence of exogenous DNA, actin formed filaments of varying size and P. aeruginosa appeared associated with these filaments (Fig. 5B). The combination of purified actin and DNA resulted in robust filament formation, with virtually all visible P. aeruginosa cells attached to the polymer (Fig. 5C). We devised an assay to test for the binding of P. aeruginosa to actin and found that after 4 h of incubation, 44% of P. aeruginosa cells were bound to F-actin, in comparison with 11% of P. aeruginosa cells that bound to G-actin, which was not any different than that for albumin-coated plastic (Fig. 5D).
P. aeruginosa association with actin-DNA polymers from neutrophils and in CF sputa.
Immunofluorescence of necrotic neutrophils stained for actin (red) and DNA (blue) revealed colocalization of both components (Fig. 6) and confirmed the formation of actin-DNA filaments in CF sputa, as previously reported (38). In both neutrophil lysates and CF sputa, P. aeruginosa (green) localized primarily to actin-DNA filaments after 4 h of incubation (Fig. 6), supporting the concept that these fibers provide a matrix for initial P. aeruginosa attachment and biofilm establishment. Treatment of both the neutrophil lysates and CF sputum with DNase I resulted in a near-complete disruption of the actin-DNA filaments and dispersion of the P. aeruginosa cells, with a greater number of planktonic bacterial cells that were visually observable (Fig. 6).
FIG. 6.
Localization of P. aeruginosa with actin-DNA polymers from neutrophil lysates and CF sputum. Lysed neutrophils were incubated with GFP-labeled P. aeruginosa and stained for DNA and actin (left column). When DNA (blue) was overlaid with actin (red), the colocalization of the components into polymers was observed. Under 488-nm excitation, the GFP-labeled P. aeruginosa cells were found to be localized predominantly on the actin-DNA fibers. Addition of DNase resulted in the near-complete disruption of the actin-DNA polymers (by 4 h), with dispersion of the GFP-labeled P. aeruginosa. Sputa from CF patients were combined with P. aeruginosa cells labeled with an intracellular fluorescent conjugate for 4 h and then stained for DNA and actin (right column). When DNA (blue) was overlaid with actin (red), colocalization of the components into polymers was observed. Staining for P. aeruginosa (green) demonstrated the association of the exogenous bacteria on these polymers. Addition of DNase resulted in the rapid and near-complete disruption of the actin-DNA polymers, with dispersion of the P. aeruginosa cells. Bar, 20 μm. Actin and DNA staining of CF sputa was representative of six samples.
DISCUSSION
Herein, we report that human neutrophils can serve to enhance the initial development of P. aeruginosa biofilms. The biofilm enhancement coincides with a significant reduction of P. aeruginosa in the planktonic phase, resulting in little decrease in the overall number of viable bacteria after the first 4 h of incubation. The mechanism of neutrophil biofilm enhancement was identified as polymers comprised of actin and DNA. The bacteria bind to F-actin, and disruption of the polymers with DNase results in dispersion of the bacteria and a reduction in biofilm development. The presence of these actin-DNA polymers, with colocalization of P. aeruginosa, was confirmed in both neutrophil lysates and CF sputum. The introduction of additional neutrophils after 24 and 48 h further enhanced P. aeruginosa biofilm development, while exposure to fewer neutrophils resulted in a lesser degree of biofilm enhancement (data not shown).
While no in vitro model can recreate the complexities of the airway, we believe that the static model of biofilm development employed in this study has relevance to CF lung disease. P. aeruginosa biofilm formation in the CF airways appears to occur in the context of stagnant mucous plugs which are lodged in the airway lumen and are largely composed of dead and dying neutrophils (45, 46). The short life span of the neutrophil in our study is consistent with neutrophil survival in the bloodstream and in other in vitro systems where typical survival is 6 to 18 h (13, 27). When neutrophils and P. aeruginosa cells are combined in vitro, neutrophil killing of planktonic P. aeruginosa cells is maximal at about 50 min (20), but subsequent necrosis of the leukocyte occurs rapidly and over time, the ability of the remaining viable neutrophils to ingest and kill P. aeruginosa is overshadowed by bacterial multiplication. In the presence of infection or proinflammatory stimuli, apoptosis is prevented or delayed and cells may be viable up to 36 h (13, 27). The concentration of neutrophils (107/ml) used in this analysis was based on bronchoalveolar lavage sampling of the airways of CF infants prior to persistent P. aeruginosa infection where neutrophil recovery ranged from 104 to 106 per ml (25, 29, 30), with an estimated recovery rate of approximately 1 to 2% (11). An even broader range of the quantity of P. aeruginosa cells has been isolated from CF children during early infection (7, 29), and we selected 106 CFU/ml as a representative concentration.
In clinical settings that do not feature massive accumulation of neutrophils, various host products have been found in association with bacterial biofilms. Heterogeneous salivary films contain secretory IgA and α-amylase, which represent binding sites for Streptococcus sanguis in the formation of dental plaques (19). Nearly all types of in-dwelling medical devices can become coated with host proteins, electrolytes, and organic materials which appear to contribute to the presence of persistent infection (9). Uropathogenic strains of Escherichia coli can form organized biofilm-like colonies within the cytoplasms of bladder cells during a phase of urinary tract infection (24), and clots comprised of fibrin and platelets facilitate Streptococcus viridans survival in endocarditis (23). However, the potential of an immune cell, integral to host defense, to increase the formation of a bacterial biofilm has never been reported.
As virtually every eukaryotic cell contains significant quantities of actin, it is certainly possible that other necrotic cells, in addition to neutrophils, could enhance P. aeruginosa biofilm development. This report focused on the neutrophil, as vast numbers of necrotic neutrophils are a predominant feature of the CF airway. Based on our results, it seems plausible that in other clinical situations, different types of necrotic cells could serve to enhance P. aeruginosa biofilm formation. For example, in a severe skin burn, P. aeruginosa could conceivably utilize actin and DNA from necrotic epithelial cells.
Recent reports have identified new mechanisms by which neutrophils successfully kill bacteria. Neutrophils actively generate “neutrophil extracellular traps” (NETs) that bind to Staphylococcus aureus and other bacteria (4). Viable neutrophils secrete NETs within minutes, which appear to trap bacteria and augment killing by retaining the microorganism in close proximity to a variety of antimicrobial granule proteins (4). These delicate NETs are composed primarily of DNA, as well as histones and granule proteins, but do not contain actin. In distinct contrast, the polymers described in this report were comprised of both actin and DNA, did not require the action of granule proteins, were a product of the necrotic neutrophil, and increased the number of surviving P. aeruginosa cells (Fig. 1A) over a period of days. Fragmentation of the NETs by DNase increased bacterial survival, while disruption of the actin-DNA polymers by DNase reduced biofilm formation. Thus, construction of NETs represents an elegant mechanism of successful bacterial killing by the live neutrophil, while actin-DNA enhancement of biofilm formation may represent a maladaptive response to years of relentless accumulation and neutrophil death in the CF airway.
Although the neutrophil contains a number of proteins with significant antimicrobial potential, it appears that successful bacterial killing by granule proteins is highly dependent on the extracellular milieu. Recently, purified lactoferrin, a major component of the secondary neutrophil granules, was found to prevent P. aeruginosa biofilm development (39). Although lactoferrin is relatively abundant in CF sputum, it is only one of at least 50 proteins contained within neutrophil granules (3) and its inhibitory effect was not evident when the total content of neutrophil granules was combined with P. aeruginosa (Fig. 4A). It is likely that during Pseudomonas-induced neutrophil necrosis, lactoferrin (and other potential beneficial proteins) is degraded by neutrophil- and Pseudomonas-derived proteases (5, 44).
The unique environment of the CF airway exerts selective pressures, which can result in profound genetic alterations within the bacteria. P. aeruginosa strains isolated at the time of initial infection resemble environmental strains which are motile, are nonmucoid, lack antibiotic resistance, and have a smooth-type penta-acylated lipopolysaccharide. After years of infection, CF strains of P. aeruginosa emerge with an extensive array of altered phenotypes (6), including a mucoid, nonmotile phenotype; extensive resistance to antibiotics; and a rough-type, arabinomannan-modified, hexa/hepta-acylated lipopolysaccharide (12, 15). The PAO1 strain used in these studies clearly resembles the environmental strains of early infection. We believe it is of importance that the enhancement of biofilm formation described in this report is achieved with a nonmucoid, non-CF strain of P. aeruginosa, as this may represent a mechanism that allows environmental strains to initially persist in the CF airway. Once present in the biofilm form, environmental P. aeruginosa strains would have the opportunity to adapt to the intense inflammatory conditions and antibiotic treatment over decades without eradication.
Acknowledgments
Portions of this work were supported by a CF Foundation Genome Analysis Program Grant (JAN), the Max and Yetta Karasik Foundation (JAN), National Institutes of Health HL061407 (GSW) and HL068743 (JAN), American Heart Association 0275035N (MBF), and National Institute of Allergy and Infectious Diseases AI15950 (MLV).
Editor: J. N. Weiser
REFERENCES
- 1.Aaron, S. D., W. Ferris, K. Ramotar, K. Vandemheen, F. Chan, and R. Saginur. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J. Clin. Microbiol. 40:4172-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balfour-Lynn, I. M. 1999. The protease-antiprotease battle in the cystic fibrosis lung. J. R. Soc. Med. 92(Suppl. 37):23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 4.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D. S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 5.Britigan, B. E., and B. L. Edeker. 1991. Pseudomonas and neutrophil products modifiy transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J. Clin. Investig. 88:1092-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183:444-452. [DOI] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Foundation. 2004. Cystic Fibrosis Foundation: patient registry annual data report 2003. Cystic Fibrosis Foundation, Bethesda, Md.
- 8.Dakin, C. J., A. H. Numa, H. Wang, J. R. Morton, C. C. Vertzyas, and R. L. Henry. 2002. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165:904-910. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche, R. O. 2001. Device-associated infections: a macroproblem that starts with microadherence. Clin. Infect. Dis. 33:1567-1572. [DOI] [PubMed] [Google Scholar]
- 10.Déziel, E., Y. Comeau, and R. Villemur. 2001. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 183:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downey, G. P., G. S. Worthen, P. M. Henson, and D. M. Hyde. 1993. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am. Rev. Respir. Dis. 147:168-176. [DOI] [PubMed] [Google Scholar]
- 12.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, S. W., D. A. Moulding, M. Derouet, and R. J. Moots. 2003. Regulation of neutrophil apoptosis, p. 204-224. In M. A. Cassatella (ed.), The neutrophil, vol. 83. S. Karger, Basel, Switzerland. [DOI] [PubMed]
- 14.Emerson, J., M. Rosenfeld, S. McNamara, B. Ramsey, and R. L. Gibson. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 34:91-100. [DOI] [PubMed] [Google Scholar]
- 15.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 16.Favre-Bonte, S., T. Kohler, and C. Van Delden. 2003. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 52:598-604. [DOI] [PubMed] [Google Scholar]
- 17.Frederiksen, B., S. Lanng, C. Koch, and N. Hoiby. 1996. Improved survival in the Danish center-treated cystic fibrosis patients: results of aggressive treatment. Pediatr. Pulmonol. 21:153-158. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, W., and G. Doring. 1986. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am. Rev. Respir. Dis. 134:49-56. [DOI] [PubMed] [Google Scholar]
- 19.Gong, K., L. Mailloux, and M. C. Herzberg. 2000. Salivary film expresses a complex, macromolecular binding site for Streptococcus sanguis. J. Biol. Chem. 275:8970-8974. [DOI] [PubMed] [Google Scholar]
- 20.Hammer, M. C., A. L. Baltch, N. T. Sutphen, R. P. Smith, and J. V. Conroy. 1981. P. aeruginosa: quantitation of maximum phagocytosis and bactericidal capabilities of normal human granulocytes. J. Lab. Clin. Med. 98:938-948. [PubMed] [Google Scholar]
- 21.Haslett, C., L. A. Guthrie, M. M. Kopaniak, R. B. Johnston, Jr., and P. M. Henson. 1985. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 119:101-110. [PMC free article] [PubMed] [Google Scholar]
- 22.Hitchcock, S. E., L. Carisson, and U. Lindberg. 1976. Depolymerization of F-actin by deoxyribonuclease I. Cell 7:531-542. [DOI] [PubMed] [Google Scholar]
- 23.Hook, E. W., III, and M. A. Sande. 1974. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect. Immun. 10:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan, T. Z., J. S. Wagener, T. Bost, J. Martinez, F. J. Accurso, and D. W. H. Riches. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151:1075-1082. [DOI] [PubMed] [Google Scholar]
- 26.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 27.Lee, A., M. K. B. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 28.Lethem, M. I., S. L. James, C. Marriott, and J. F. Burke. 1990. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur. Respir. J. 3:19-23. [PubMed] [Google Scholar]
- 29.Muhlebach, M. S., and T. L. Noah. 2002. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165:911-915. [DOI] [PubMed] [Google Scholar]
- 30.Muhlebach, M. S., P. W. Stewart, M. W. Leigh, and T. L. Noah. 1999. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am. J. Respir. Crit. Care Med. 160:186-191. [DOI] [PubMed] [Google Scholar]
- 31.Ochsner, U. A., A. Snyder, A. I. Vasil, and M. L. Vasil. 2002. Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc. Natl. Acad. Sci. USA 99:8312-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parad, R. B., C. J. Gerard, D. Zurakowski, D. P. Nichols, and G. B. Pier. 1999. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect. Immun. 67:4744-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perks, B., and J. K. Shute. 2000. DNA and actin bind and inhibit interleukin-8 function in cystic fibrosis sputa: in vitro effects of mucolytics. Am. J. Respir. Crit. Care Med. 162:1767-1772. [DOI] [PubMed] [Google Scholar]
- 34.Riches, D. W., S. K. Young, J. F. Seccombe, J. E. Henson, K. L. Clay, and P. M. Henson. 1990. The subcellular distribution of platelet-activating factor in stimulated human neutrophils. J. Immunol. 145:3062-3070. [PubMed] [Google Scholar]
- 35.Rosenfeld, M., R. L. Gibson, S. McNamara, J. Emerson, J. L. Burns, R. Castile, P. Hiatt, K. McCoy, C. B. Wilson, A. Inglis, A. Smith, T. R. Martin, and B. W. Ramsey. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 32:356-366. [DOI] [PubMed] [Google Scholar]
- 36.Roum, J. H., R. Buhl, N. G. McElvaney, Z. Borok, and R. G. Crystal. 1993. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 75:2419-2424. [DOI] [PubMed] [Google Scholar]
- 37.Schaedel, C., I. de Monestrol, L. Hjelte, M. Johannesson, R. Kornfalt, A. Lindblad, B. Strandvik, L. Wahlgren, and L. Holmberg. 2002. Predictors of deterioration of lung function in cystic fibrosis. Pediatr. Pulmonol. 33:483-491. [DOI] [PubMed] [Google Scholar]
- 38.Sheils, C. A., J. Kas, W. Travassos, P. G. Allen, P. A. Janmey, M. E. Wohl, and T. P. Stossel. 1996. Actin filaments mediate DNA fiber formation in chronic inflammatory airway disease. Am. J. Pathol. 148:919-927. [PMC free article] [PubMed] [Google Scholar]
- 39.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 40.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 41.Spencer, D. H., A. Kas, E. E. Smith, C. K. Raymond, E. H. Sims, M. Hastings, J. L. Burns, R. Kaul, and M. V. Olson. 2003. Whole-genome sequence variation among multiple isolates of Pseudomonas aeruginosa. J. Bacteriol. 185:1316-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasconcellos, C. A., P. G. Allen, M. E. Wohl, J. M. Drazen, P. A. Janmey, and T. P. Stossel. 1994. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science 263:969-971. [DOI] [PubMed] [Google Scholar]
- 43.Whitchurch, C. B., T. Tolker-Nielsen, P. C. Ragas, and J. S. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 44.Wilderman, P. J., A. I. Vasil, Z. Johnson, M. J. Wilson, H. E. Cunliffe, I. L. Lamont, and M. L. Vasil. 2001. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 69:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]