Abstract
Background
Gene expression is affected by population density. Cell density is a potent negative regulator of cell cycle time during exponential growth. Here, we asked whether SV40 large T antigen (Tag) levels, driven by two different promoters, changed in a predictable and regular manner during exponential growth in clonal astrocyte cell lines, immortalized and dependent on Tag.
Results
Expression and cell cycle phase fractions were measured and correlated using flow cytometry. T antigen levels did not change or increased during exponential growth as a function of the G1 fraction and increasing cell density when Tag was transcribed from the Moloney Murine Leukemia virus (MoMuLV) long terminal repeat (LTR). When an Rb-binding mutant T antigen transcribed from the LTR was tested, levels decreased. When transcribed from the herpes thymidine kinase promoter, Tag levels decreased. The directions of change and the rates of change in Tag expression were unrelated to the average T antigen levels (i.e., the expression potential).
Conclusions
These data show that Tag expression potential in these lines varies depending on the vector and clonal variation, but that the observed level depends on cell density and cell cycle transit time. The hypothetical terms, expression at zero cell density and expression at minimum G1 phase fraction, were introduced to simplify measures of expression potential.
Background
We have been interested in quantitative analysis of gene expression within single cells and the distribution of that expression within populations of cells replicating in culture (e.g.,) [1-3]. Since the level of any specific protein within a cell is dynamic, and since it is difficult to describe cells in tissue culture as "steady state" entities, describing gene expression in cell populations in quantitative terms becomes a complex problem. Here we have explored the relationship of SV40 large T antigen (Tag) expression as a function of cell density and cell cycle duration in clonal populations of Tag-immortalized mouse astrocytes. These cells depend on expression of T antigen for viability.
Expression of Tag in mouse cells under selective conditions that do not require tumorigenic transformation (e.g., transduction by retrovirus and selection for drug resistance) produces immortal cell lines with limited transformation phenotypes [4,5]. However, expression of Tag has profound effects on the cell cycle, significantly reducing cell doubling time and increasing saturation density [4-6]. These direct effects of Tag result from binding and inactivation of the retinoblastoma family proteins (p130, Rb, p107) and the tumor suppressor, p53 [7-9]. Tag is rate limiting for G1 transit, and this effect (as well as saturation density and growth in soft agar) is Tag-dose dependent [3,10-12]. Since the cell lines in this study are capable of entering a quiescent state at saturation density [6], one might expect that the levels of Tag would decrease at saturation and as cells progressively slow down as a function of cell density.
However, we have previously noted that the Tag increased in Tag-transformed NIH 3T3 cells as the population became more dense and the cell cycle time increased during exponential growth [11]. For some cell types, like fibroblasts and lymphocytes, G0 cells have less cell mass than cycling cells, and one might expect that Tag expression would decrease as the G1 phase of the cycle lengthened and cells achieved confluence. Since Tag did not decrease simultaneously with an increasing G1 time, and since Tag was one of the G1 rate-limiting molecules, the activity of Tag must have been decreasing while Tag levels increased. Thus, negative control of the cell cycle as a function of cell density was dominant to the activity of Tag. Though the Tag-transformed NIH-3T3 cells could not maintain a monolayer at confluence (did not enter G0) during the plateau phase of growth, it was expected that expression would eventually plateau or decrease.
The purpose for this study was to explore further the relationship between Tag expression as a function of cell cycle time and cell density. We asked whether Tag expression in immortalized mouse cells always increased as a function of G1 time during exponential growth and whether levels eventually decreased or achieved a steady state level at high cell density. The advantage to using astrocyte lines is that many of these lines can maintain a monolayer in culture for long periods of time [6]. Since Tag levels are significantly determined by the strength of the transcription promoter in this retroviral system [1,11], we examined cell lines immortalized by Tag transcribed from two different promoters. In the analysis of these data, we (1) explored the relationship between expression, G1 phase time, and cell density, (2) asked whether the transcriptional promoter affected those relationships, (3) explored analytical methods for describing expression within the context of these relationships, and (4) asked whether the "intrinsic expression potential" of Tag affected Tag expression at high cell density. The results of this study have practical implications for the use of cell lines as standards in quantitative assays of gene expression [e.g., [3]].
Results
Characterization of tkT lines
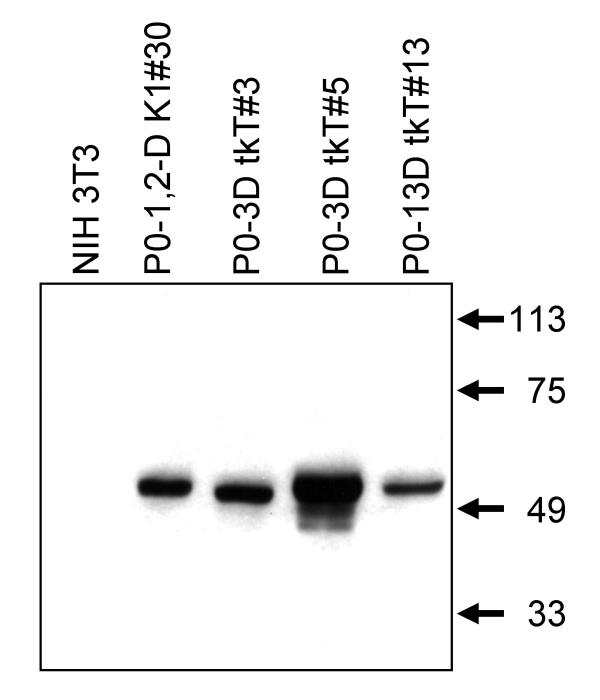
We have previously characterized clonal mouse astrocyte cell lines, immortalized by transduction of SV40 large T antigen expressed from the MoMuLV LTR [6,13]. Here, we compare variation in Tag levels in 3 of these lines and 3 additional lines (termed tkT lines), immortalized with Moloney Sarcoma Virus vectors expressing Tag from an internal herpes thymidine kinase (tk) promoter. The astrocyte lineage of the LTR lines has been published [6,13]. The astrocyte lineage of the tkT lines was confirmed by the presence of the astrocyte-specific intermediate filament protein, GFAP [14] (Figure 1). All of the lines contained GFAP, whereas NIH 3T3 cells treated with dibutyryl cAMP at the same level as the astrocyte lines did not.
Figure 1.
GFAP expression in the Linker tkT cell lines. The tkT cell lines and NIH 3T3 cells were grown in 1 mM dibutyryl cAMP for 3 to 8 days. Intermediate filament extracts from 1 to 6 × 105 cells were loaded in each lane and subjected to SDS PAGE and Western blotting. The membranes were immunostained for GFAP, which is specific for astrocytes. All of the astrocyte cell lines expressed GFAP and the NIH 3T3 cells did not.
Expression of Tag
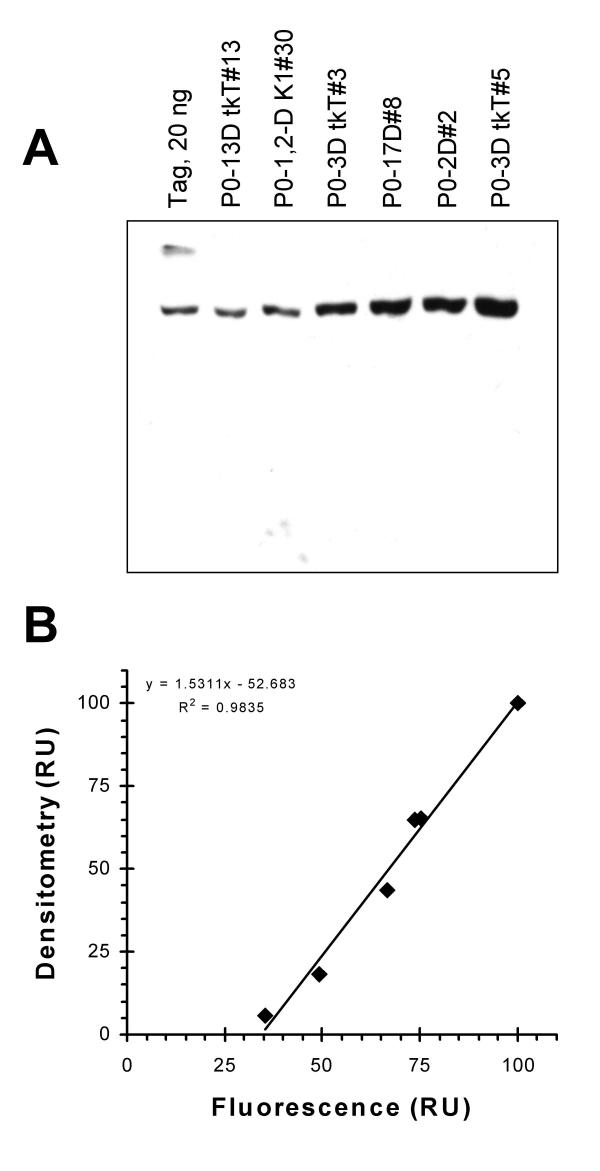
To determine the Tag expression of each cell line at a single density and validate cytometric measurements, Western blotting was performed on all cell lines and compared to purified, recombinant Tag (Figure 2A). Lysates from equal numbers of cells were loaded on the electrophoresis gel with the exception of P0-13D tkT#13, which expressed the lowest levels of Tag. This lysate was loaded at a 2-fold higher cell number. Protein levels of each sample and the recombinant Tag were adjusted to equality by the addition of NIH 3T3 cell extract. The presence of equal protein levels in each lane was confirmed by staining the blotted gel with Coomassie blue and performing densitometry. The protein levels were not significantly different (coefficient of variation (CV) of < 9%). Tag in the cell lines migrated at the correct molecular weight and cross-reacting bands were not detected. When the Tag-specific fluorescence (all cell cycle phase fractions) measured by flow cytometry was compared to the intensity of Western blot bands measured by densitometry, the two measurements showed an essentially linear relationship (Figure 2B). Therefore, immunofluorescence flow cytometry provides an unbiased estimate of the relative levels of Tag expression for the range of measurements presented in this paper.
Figure 2.
Western blot of Tag expressing cell lines. (A) Cell extracts of each line and a purified baculovirus lysate of recombinant Tag were Western blotted (see Materials and Methods). The P0-13D tkT#13 lane contained lysate from 2.5 × 103 cells; for the other lines, lysates from 1.25 × 103 cells were loaded. NIH 3T3 extract was added to the low protein extracts and the authentic Tag so that total protein was equal in each lane. (B) Densitometry of the Western blot was compared to total Tag immunofluorescence determined by cytometry for each cell line.
Effect of cell density related cell cycle control on expression of Tag
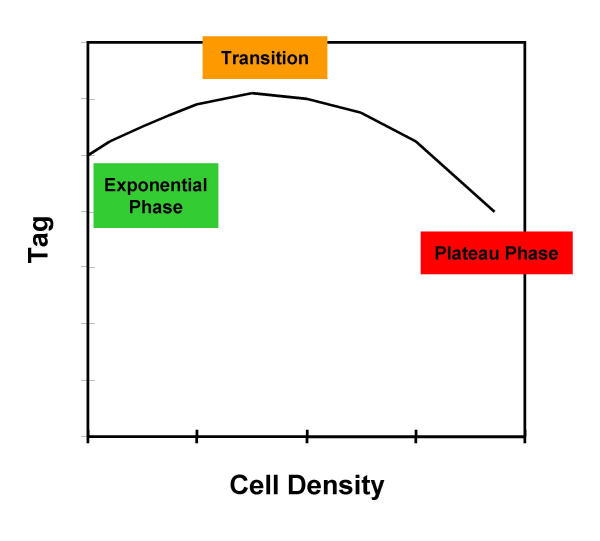
A priori, one might expect that most cell cycle related genes will be less expressed in growth arrested, confluent, or G0 cells relative to proliferating cultures due to a generalized decrease in metabolic activity, cell protein content, and cell size. However, based on unpublished observations and previous work (see Introduction,) [11], we expected that the Tag expression-cell density profiles would look like that in Figure 3. To test this idea, we measured Tag expression by immunofluorescence flow cytometry (see Figure 4) in 6 Tag-immortalized mouse astrocyte lines plated at a range of cell densities and cultured for 2–3 days as previously described [15]. The upper densities ensured in most cases that the cells would be in either the "transition" or "plateau" phase for some of the measurements. Since Tag accumulates throughout the cell cycle, populations with different phase fractions will have different average levels, therefore, we measured Tag from the G1 population to generate measurements independent of the cell cycle phase fraction distribution as previously described [3].
Figure 3.
Expected density-related changes in Tag and G1 phase fraction. Tag increases during exponential growth and then levels off and declines as %G1 stabilizes in the plateau phase of cell growth.
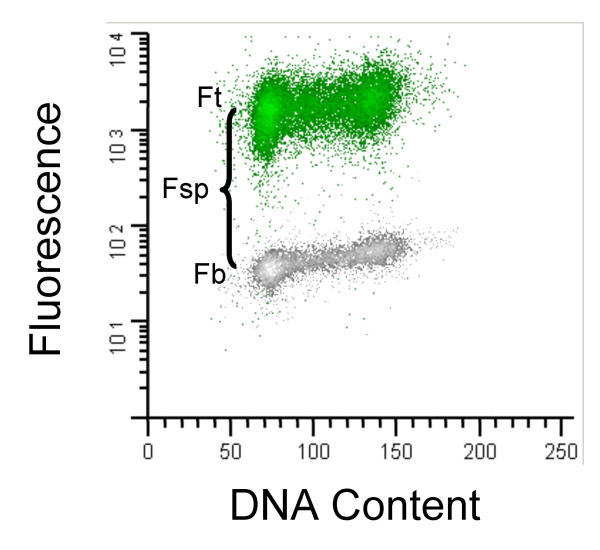
Figure 4.
Flow cytometry measurements of Tag and DNA. This is an example of the cytometric data collected for this study. P0-3D tkT#5 cells were immunofluorescently labeled with PAB 416 (anti-Tag) or IgG2a (isotype control) and FITC-conjugated goat anti-mouse secondary antibody. DNA was stained with propidium diiodide. Mean linear immunofluorescence of the G1 sub-population for the isotype control (Fb) was subtracted from that of the Tag-stained sample (Ft) to generate the measurement of Tag expression (Fs).
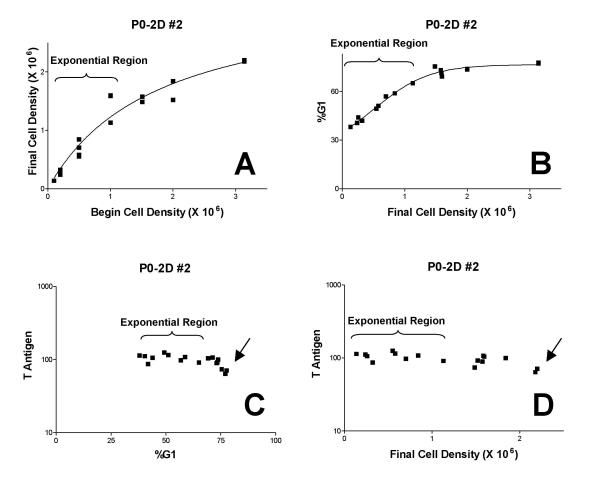
Figure 5A shows the relationship of final cell number to initial cell number for two combined experiments from one cell line. Plots like this were used to determine the samples that represented the exponential portion of a growth curve. The slope of a straight line was calculated and samples were chosen for inclusion when the slope was either maximal or > 2.0 when sufficient data could not be obtained by maximization. The %G1 curves (5B) were used as a secondary guide to selecting the exponential data. Figure 5B shows that the G1 fraction increased as a function of final cell density but that more dense cells arrested with a significant fraction of S, G2, and M cells. The increase in the G1 fraction was expected as we have demonstrated in several cell systems that the G1 cell cycle phase increases in length and frequency during exponential growth as a function of cell density [11,15,16]. This change in G1 can be demonstrated either by successive harvests from cells plated at a single density or by plating cells at different densities and harvesting simultaneously (as in this study). The presence of S phase at confluence is typical of transformed cells. Figure 5 (C, D) shows the expression profiles for Tag versus %G1 (C) and final cell density (D). The exponential region defines the data used for regression measurements. Tag expression at terminal cell density, "Final Tag", was determined by the average of the final two samples (arrow).
Figure 5.
Representative cell cycle and cell density-related changes in Tag. Panel A shows the final cell density versus the initial plating density. Data representing cells in exponential growth are expected to fit an approximate straight line. Data that fall below an extrapolation of that line are at or are approaching density arrest. Panel B shows that the fraction of cells in G1 increases as a function of density during exponential growth and plateaus as cells arrest. Panels C and D show the G1 Tag expression as a function of the fraction of G1 cells (C) or cell density (D). The regions of data used to calculate the rate of change in expression by linear regression are delineated (brackets) and the data used to determine the terminal expression levels are marked (arrows).
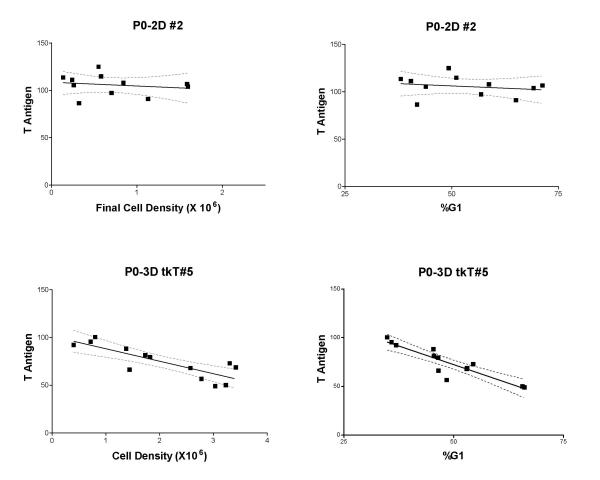
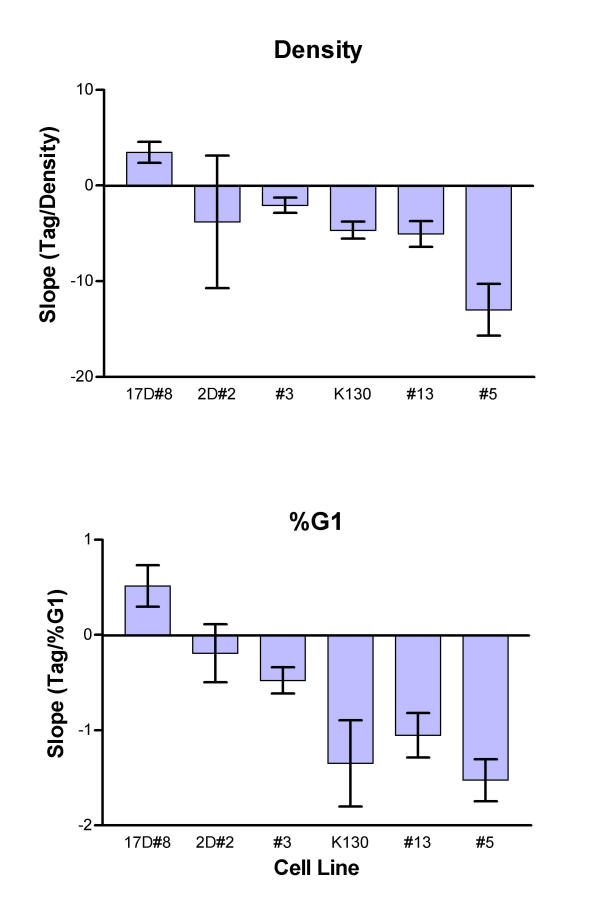
To evaluate expression, Tag measurements for samples in exponential growth (see above criteria) were subjected to linear regression on final cell density to generate an intercept representing the Tag level at a hypothetical zero cell density. This value for each experiment was used to normalize data for each data set to a zero cell density value of 100 and thus correct for differences in fluorescence measurements obtained in multiple experiments, and additionally, scale the data to be independent of cell line specific expression levels. The normalized data were then combined for replicate or triplicate experiments and subjected to linear regression on the fraction of G1 cells and final cell densities. Data for two cell lines are shown in Figure 6. For all cell lines, the slope and SE of the slope were calculated and plotted in Figure 7. In one of the cell lines, the slopes for Tag versus both G1 and cell density were not significantly different from zero. For another line, the slopes had significant positive values, and in the remaining 4 lines both slopes were negative and significant. Figure 6 shows data for the cell line that did not differ from zero and a representative line with negative slopes. The two cell lines that did not display a decrease in Tag during exponential growth were transformed with retroviruses utilizing the retroviral LTR. Three of the cell lines that showed a significant decrease in Tag during exponential growth utilized retroviruses encoding transcription of Tag from the htk promoter, which can be a weak promoter in mouse cells [1,11] but subject to cell line specific effects (see discussion). One of the cell lines showing a significant decrease in Tag expression was K1-30 which encoded an Rb-binding defective mutant Tag transcribed from the retroviral LTR [6].
Figure 6.
Exponential growth related Tag expression. The density and %G1 related expression profiles are show for a cell line that does not show a significant density related change (top) and one that does (bottom). P0-2D#2 expresses Tag from the retroviral LTR and P0-3DtkT#5 expresses Tag from the htk promoter. Dashed lines represent the 95% confidence interval about the regression line.
Figure 7.
Density related rate of change in Tag expression. Slopes and SE for each cell line determined as shown in Figures 4 and 5 are plotted. All data are significantly different from zero except 2D#2. See Table 1. Cell line names have been abbreviated: #3 = 3DtkT#3, #13 = 13DtkT#13, #5 = 13DtkT#5.
Serum levels do not affect Tag expression
It is possible that Tag expression could be regulated by factors in serum, and that these factors could be progressively depleted at increasing cell numbers. To test this, the cell line in which Tag control was most sensitive to density, P0-3D tkT#5, was grown for 3 days starting at an intermediate density of 106 cells per 10 cm plate with varying concentrations of serum ranging from 2.5–10%. Tag level as well as %G1 was equivalent at all serum levels (Table 2). Since, there is significantly less serum/cell in 2.5% serum samples, we conclude that factors in serum, at concentrations that maintain exponential growth, do not regulate Tag levels in these cell lines.
Table 2.
Effect of serum on Tag and %G11.
| Serum (%) | Cells2 (×106) | T Antigen | %G1 |
| 2.5 | 3.99 | 60.4 | 71.1 |
| 5 | 4.02 | 57.6 | 73.7 |
| 10 | 3.62 | 57.1 | 71.8 |
1 The P0-3DtkT#5 cell line was inoculated at 106 per 10 cm plate in DMEM containing the indicated amount of serum. Cells were harvested after 3 days and analysed for Tag level and %G1 by cytometry. 2Cell number = final cell number per 10 cm dish.
Characterization of density and growth phase effects on Tag
Tag expression at high cell density
For each cell line, the levels of Tag at high cell density (either transition or plateau phase) were significantly lower than during exponential growth. This level ranged from a 12–63% decrease compared to the hypothetical Tag level of 100 (normalized) at 0 cell density (Table 1).
Table 1.
Tag change during exponential growth (G1) and at high cell density.1
| Cell Line | Tag | Promoter | G1 Slope | p | D Slope | p | FinT | N |
| P0-2D#2 | Wild Type | LTR | -0.190 | 0.54640 | -3.80 | 0.5971 | 88 | 11 |
| P0-17D#8 | Wild Type | LTR | 0.515 | 0.03180 | 3.48 | 0.0059 | 81 | 6 |
| K1-30 | Mutant | LTR | -1.350 | 0.01000 | -4.67 | 0.0001 | 49 | 16 |
| P0-3DtkT#5 | Wild Type | tk | -1.520 | <0.00010 | -12.99 | 0.0006 | 37 | 13 |
| P0-3DtkT#3 | Wild Type | tk | -0.476 | 0.00230 | -2.05 | 0.0166 | 74 | 23 |
| P0-13DtkT#13 | Wild Type | tk | -1.052 | 0.00030 | -5.058 | 0.0017 | 64 | 18 |
1 This is a summary of the data graphed in Figure 7. G1 Slope = slope of Tag versus G1. D Slope = slope of Tag versus cell density. FinT = the Tag level at transition or plateau phase and normalized to a hypothetical value of 100 at 0 cell density. p = p value; N = number of evaluations.
Tag expression at zero and late cell densities
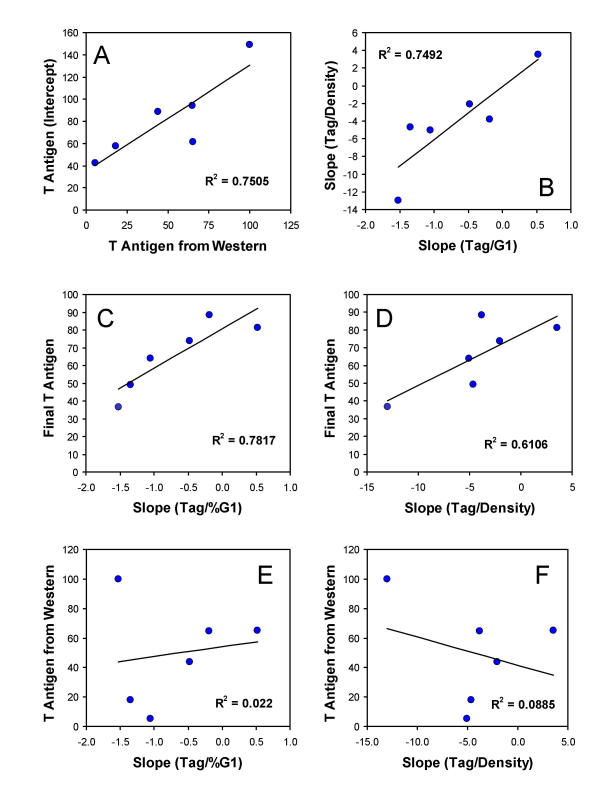
Figure 8A shows the correlation plot for the objective, "expression potential" intercept measurement at zero cell density (defined above) and Tag measured from Western blots in Figure 2. The Western blot data represent a sampling of Tag expression on growing cultures, in late exponential growth, and thus might reflect the average, qualitative experiment. The overall agreement with the intercept measurement supports the use of this objective means to determine expression potential, i.e., expression that is independent of population growth/density effects, in quantitative analysis. The R2 value for the regression is 79%. The single outlier represents P0-17D#8 which is the only cell line that showed an increase in Tag during exponential growth and so would not be expected to fit the regression as well. If P0-17D#8 is removed, the R2 is 97%.
Figure 8.
Relationships between Tag and density or growth phase effects. (see text)
Relationship of Tag expression as a function of cell density and G1 phase time
An increase in the fraction of G1 cells as a linear function of cell density is a common observation in this laboratory [e.g., Figure 4B, [11,15,16]]. Figure 8B shows that there is a correlated relationship between the slopes, i.e., changes in Tag concentration as a function of G1 phase time is correlated with changes in Tag as a function of cell density.
Relationship between final Tag and the expression profile for G1
Figure 8C shows that there is a significant correlation between the rate of change of T antigen as a function of cell cycle time and the end-point expression level determined at or near a plateau phase in these experiments. Therefore, the levels at confluence or plateau can be predicted by the direction and rate of change during exponential growth. Figure 8D shows that the same relationship holds true for cell density, as would be expected from Figure 8B.
Control of Tag expression during exponential growth is independent of the intrinsic expression potential
Figures 8E and 8F show the regression of average Tag expression (Western blot in Figure 2) on the G1 and cell density slopes. Regression of data for the intercept calculations (e.g., as in Figure 8A) gave a similar plot. There appears to be no relationship between the intrinsic expression "strength" or potential and change in Tag expression as a function of G1 phase fraction (time) or cell density, i.e., growth related control of Tag expression.
Discussion
There is good evidence that quantitative changes in gene expression are important for some regulatory genes. For example, periodic quantitative changes in the levels of cyclin proteins that activate cyclin dependent serine/threonine kinases (cdk's) appear to be the "core" cell cycle machinery in mammalian cells. In this case, cyclin molecular concentration is directly related to cdk activity and activity is directly related to cell cycle transition rates [17]. When regulatory proteins affect cell processes involved in disease, measurement of molecular concentration takes on added importance. For example, the expression of a number of oncogenes, proto-oncogenes, and reduced or absence of expression of recessive oncogenes has been postulated to provide diagnostic and/or prognostic information in human cancers [e.g., [18-20]]. Currently, cancer gene expression research is seldom done in a rigorously quantitative manner, which may contribute to the sometimes conflicting results [21]. Much evidence leads to the conclusion that the tumorigenic phenotype of mammalian cells is markedly affected by quantitative changes in expression of regulatory genes. In experimental systems, small shifts in expression can have profound consequences. For instance, in NIH 3T3 cells, expression of SV40 large T antigen (Tag) at high levels (~2 × 106 molecules per G1 cell) decreases the G1 phase time by ~18% [3,11]. However, ~43% of this effect can be accounted for with the initial expression of ~ 6000–14,000 molecules per G1 cell and ~87% can be accounted for by expression of ~ 103,000–133,000 molecules [3,11].
Previously, we measured the relative dose response for G1 transit for SV40 Large T antigen expressed in NIH 3T3 cells. In this work, the levels of Tag in G1 increased 18% during exponential population growth as the length of G1 was increasing [11]. Since Tag is rate-limiting for G1 transit in these cells, the increase in transit time as a function of G1 Tag concentration represented an apparent paradox that is resolved (in the simplest and obvious manner) by stating that additional rate-limiting entities operate to set the G1 transit time. One easily measured and pervasive entity that regulates G1 phase time is cell density, which is taken as a surrogate for cell-cell contact. This effect can be separated analytically from growth factor concentration [15,16] and therefore, appears to measure independent cell cycle regulatory mechanisms. Our work [6,11] and most other work indicates that the cell density dependent mechanisms that negatively regulate cell proliferation are dominant to Tag. Indeed this may be universal for oncogenes, since we are not aware of instances when anchorage dependent cells fail to enter a plateau phase in tissue culture.
The study presented in this paper represents further investigation into the effect of cell density on gene expression. Cell lines were created by incorporation of retroviral vectors into the genome and expression of Tag from either the retroviral LTR (considered to be a strong promoter) or the herpes virus thymidine kinase promoter, internal to the viral LTR [1]. In our established NIH 3T3 cell line, Tag is expressed from the htk promoter at considerably lower levels than from the LTR for a considerable time after transduction [unpublished observations, and [11]]. However, when a large number of htk-Tag astrocyte clones were checked 100 days after creation, the expression levels displayed a very large range, and thus we expect that this weak promoter is subject to positional effects on transcription or mutational events that affect gene expression (unpublished data). Polyclonal populations produced by transduction of htk-Tag vectors that were passaged without selection, displayed expression levels equivalent to or higher than LTR-Tag cell lines (Frisa and Jacobberger, unpublished data). This selection for increased Tag expression makes sense given the low growth rate of primary mouse astrocytes and the ability of Tag to decrease the cell cycle time [11] and increase saturation density [e.g., [4,6]]. In this study, the expression potential of cell lines was not related to proliferation related changes in expression of Tag, emphasizing that clonal variation for whatever reason (which includes promoter strength) accounts for the large range of htk related expression.
Making sense of a quantitative description of the role of Tag and other regulatory proteins in cell cycle regulation and cell transformation requires either that all the parameters affecting expression are measured at the same time so that differences can be accounted for, or an ability to compute some base line or "expression potential" statistic with which to evaluate differences (e.g., between cell lines). A practical application of this knowledge is the ability to create biological standards from cell lines that express varying levels of a given protein and then use these in quantitative and/or longitudinal experiments. Clearly, in cases where gene expression varies significantly with cell density (e.g., P0-3DtkT#5), use of these cells as a standard in a longitudinal study would require that the standard was measured at the same cell density for each experimental sampling, or that a derived statistic (Expression/Density or Expression/G1 intercepts) is used to normalize the data. In this regard, the good agreement between the intercepts and non-density corrected Western blot data for cell lines varying over a 20-fold range is supportive.
Originally, we pursued a strategy of reduced Tag expression from the htk promoter as a means of producing immortalized, differentiated cells that may have a reduced transformation phenotype. We noticed however, that initially slow growing cell lines produced with htk driven gene expression needed to be cloned early to prevent overgrowth of more rapidly growing cells that expressed high levels of Tag. This was not true for clonal or polyclonal transductants that expressed Tag from the MoMuLV LTR, which supports a high level of expression (unpublished observations). In the context of this study, we wished to explore density dependent expression as a function of these two types of transformants. The data presented here show that LTR driven Tag expressing cell lines either increase Tag during exponential growth despite a progressively increasing G1 phase and contact inhibition (density) or the levels to not change significantly, whereas those created with the htk promoter show varying degrees of reduced expression, that is consistent in magnitude between experiments, as the cell cycle lengthens. This suggests that the LTR is less density regulated than the htk promoter, which is subject to down regulation as the cell cycle lengthens during contact inhibition of growth. The single exception is the pattern of expression observed for an LTR driven line expressing a form of Tag that is mutant in binding the Rb family proteins [e.g., [22]]. This density-dependent reduction inTag expression was somewhat surprising. We do not know the half life of this mutant T antigen (K1). Since it is expressed at levels that are 1–2 fold that of wild-type Tag in NIH 3T3 cells [23], we would not expect that the half life was significantly lower than that of wild type. Alternatively, the cell line used here is a low expressing cell line (<2 fold above the lowest expressing cell line). Thus, in this cell line, for unknown reasons the protein could be less stable. If the idea that transcription strength mainly drives Tag expression is kept as the working hypothesis, then there are at least three possibilities to explain the K1 data: 1) the K1 protein has a mildly shorter half-life and the small difference results in a different phenotype vis-à-vis the density related expression; 2) failure to bind Rb family members significantly affects the half-life during exponential growth; 3) the LTR vectors can occasionally display integration position effects that in this special case was subject to density dependent control that is different from most LTR driven cell populations.
Both the promoters as well as the protein investigated here are viral. The cells are dependent on Tag expression, and so there is significant selective pressure against extinction of Tag expression. Effects of viral promoters may reflect the evolution of the viruses and their hosts. We have additional evidence that the same type of density effects (i.e., changes in gene expression detected continuously during exponential growth) may operate on native proteins. Tag immortalized human tracheal epithelial (HTE) cells grown at different densities and assayed for cytokeratins 6 and 18 showed a density dependent decrease in cytokeratin immunoreactivity and a concomitant increase in %G1 both in normal growth medium and in differentiation medium (Frisa & Jacobberger, unpublished). Additionally, the expression levels of cyclin B1 are lower in replicating Hela cells at high cell density (Soni & Jacobberger, unpublished results). It is conceivable that other proteins that are at high levels in density-arrested cells may be positively regulated by density during exponential growth. There are many papers that describe genes that are up or down regulated at confluence. Several recent papers identify genes that are up-regulated by cell density. These include TEM1/endosialin, IGF-1, IGF-1R, IGFBP-2, Bak, drp, SP1, p27Kip1[24-28]. However, cell context differences are apparent [e.g. IGFR, [29]], and one study used several cell lines and demonstrated distinct effects of the cell genetic background [25]. Down-regulated genes include bFGF, FGF-2, Topoisomerase II-α, EGFR,, human HDAC1, as well as cell cycle regulatory genes among others [30-37]. Regions in the bFGF promoter have been identified as associated with density-dependent down-regulation of bFGF [31]. The IGF-II P3 promoter is down-regulated as a function of cell density, and a 7-base pair sequence has been identified that plays a critical role in this down-regulation [32]. Neither the MoMuLV LTR or the htk promoters encode this sequence, although both encode a 10-base pair sequence that is identical to the P3 sequence with an insertion of 3 base pairs at position -1081. Most studies focus on transcription and/or protein levels. Fewer studies have addressed translation, however, iso-forms of c-myc and FGF-2 are translationally regulated by cell density [34]. For many of these genes, down regulation at confluence is not surprising (e.g., cell proliferation associated genes), and upregulation of differentiation specific genes at confluence is equally unsurprising. However, a glance at the literature does not suggest that any quick classification or generalizations should be made.
Regulation of protein levels is multi-factorial. Transcription, translation, protein stability and degradation are involved. Tag levels in rapidly growing polyclonal cell lines appear to be set primarily by transcription [11,12]. However, as discussed above, Tag levels in clonal cell lines produced with the weak htk promoter and generated from slowly growing primary cells are related to clonality. SV40 Tag is a long-lived protein with a half-life variously measured at 48–90 hrs. The long half-life of Tag would not normally implicate protein stability, however, the magnitude of changes that we discuss here, although biologically relevant, are within two fold and might be affected by small differences in protein stability. Given this line of reasoning, the data presented here can be interpreted as evidence for a cell contact (density) / cell cycle length related effect on transcription that does not affect the retroviral LTR to the extent that it does the htk promoter. This effect is predictive of the plateau growth phase levels of Tag and thus, ultimately should affect, in the case of Tag or similar oncogenes, the fate of clones in polyclonal populations. For example if an LTR Tag line and htk Tag line with equal expression potential were co-cultured, over time, one would expect the LTR driven line to dominate.
Finally, the htk lines here varied significantly in terms of Tag expression potential, and in terms of the rate at which expression decreased as a function of cell density. There was no correlation in the rate of change in density related expression and the density independent expression (here referred to as intrinsic expression potential). This supports the idea that the density independent expression in the htk lines is set by the clone specific effects (e.g., integration position) and the density/cell cycle related expression is set in these cases by the promoter.
Conclusions
These data show quantitatively that the Tag expression potential of introduced Tag varies over a wide range depending on the vector used and clonal variation. The expression level is a complex measurement that can be partially simplified by accounting for the cell cycle transit time and cell density.
The hypothetical terms, expression at zero cell density and expression at minimum G1 phase fraction, were introduced to simplify measures of expression potential.
Accounting for both cell cycle transit time and cell density should increase the precision and accuracy with which gene expression can be measured. This is important for the use of cell lines as quantitative standards for gene expression studies, for relating expression to phenotype, and for an understanding of gene expression in general, and affects ideas on cell engineering.
Materials and Methods
Generation of astrocyte cell lines
Cortical astrocytes were harvested from neonatal C57BL/6 mice and infected with a replication-defective retrovirus encoding Tag and neomycin resistance as previously described [13]. The transferred viruses were SV40-6 [5], in which wild type Tag is expressed from the M-MuLV LTR promoter; SV(X)TK1, which is constructed the same as SV40-6 except that the Tag is mutant [38] and defective for binding the retinoblastoma protein family [39]; Linker-tkT, in which the cDNA for Tag is expressed from the herpes simplex 1 tk promoter [11]. The differentiation and transformation phenotypes of the cell lines utilizing the M-MuLV LTR promoter have been described [6,13]. The Linker-tkT lines (tkT) were either isolated as clones after G418 selection (as previously described) [6] or frozen immediately after selection (pooled clones) and clonally selected at a later time. Typically, it took 40 days after infection to obtain colonies and 60 more days to generate a rapidly growing line that could be serially cultured (split 1:80 at weekly intervals). The lines termed early passage in this paper were 140–200 days post infection, and all others were 340–860 days post infection. The names of the cell lines designate the number of days the primary neonatal astrocytes were cultured prior to infection. For example, P0-17D#8 is the eighth clone derived from cortical astrocytes isolated on the day of birth and infected 17 days later. Immortalized cells were maintained in Dulbecco's modified Eagle medium (DMEM, Sigma, St. Louis, MO) with 5% fetal bovine serum (FBS) and 5% calf serum in 5% CO2 at 37°C.
Cell density experiments
To test density effects, cells were plated in duplicate at initial numbers of 1, 2, 5, 10, 20 and 40 × 105 per 100 mm cell culture dish with DMEM containing 5% FBS and 5% calf serum. After 2 to 3 days of culture, cells were harvested with trypsin and counted on a Coulter Counter (Coulter Electronics, Hialeah, FL), then fixed for cytometry in 0.5% formaldehyde at 37°C followed by 95% methanol at -20°C.
Cytometry
Fixed samples were immunostained with anti-Tag, PAb416 (Ab2, Oncogene Sciences, Mineola, NY), and fluorescein isothiocyanate (FITC) conjugated secondary antibody followed by RNase treatment and propidium staining at 50 μg/ml, as previously described [13]. Cells were excited with the 488 nm line of the argon laser of a Coulter ESP Elite Cytometer (Coulter Electronics, Miami, FL). Final filters were 525 nm bandpass (FITC) and 640 nm long pass (propidium). A sample from a large batch of fixed cells was used to standardize assays in time. This sample was stained concurrently in each experiment and used to adjust the PMT's to the same FITC and PI fluorescences in all experiments. This corrected for differences in staining and cytometer set up.
Analysis
Mean FITC-fluorescence of the G1 cell cycle phase population was measured by gating to generate a statistic that would be independent of changes in cell cycle fractions. Mean G1 fluorescence of samples stained with the anti-Tag isotype, IgG2a, and FITC-conjugated secondary antibody served as a control for non-specific fluorescence and was subtracted from the G1 fluorescence of corresponding Tag-stained samples (Figure 4). The result of the subtraction is the Tag specific fluorescence and corresponds to the amount of Tag in the average G1 cell for a specific population. Forward and right angle light scatter were used to gate debris. Modeling software (ModFit, Verity Software House, Topsham, ME) was used to determine the phase fractions from analysis of single parameter DNA data. Linear regression, using GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego California USA, http://www.graphpad.com), was used to determine the slope, standard error (SE), and p-value for Tag expression as a function of cell density or the frequency of G1 cells.
Electrophoresis and western blotting
Cell extracts were prepared in 10% SDS lysis buffer (0.137 M NaCl, 2% Nonidet P-40, 10% SDS, 1% sodium deoxycholate, 20 mM Tris, pH 8.0, 2 mM PMSF, 10 μl/ml protease inhibitor cocktail), which solubilizes cytoskeletal and nuclear matrices. Samples of known numbers of cells from the cell lines and recombinant, purified Tag prepared from a baculovirus lysate [40] were loaded on 10% polyacrylamide discontinuous mini-gels (Bio-Rad, Hercules, CA) and electrophoresed conventionally [41]. Gels were then electrophoretically blotted [42]onto PVDF membrane (Bio-Rad) without methanol for 15 min at 100 V. Tag was visualized by immunostaining with PAb416 and alkaline phosphatase conjugated secondary antibody (Sigma) using chemiluminescent detection with CDP-Star substrate according to the manufacturers directions (Tropix, Bedford, MA). Images were developed on X-ray film (Kodak, X-Omat, Rochester, NY). Quantification was performed by densitometry on the Sci Scan 5000 automated scanning system (US Biochemicals, Cleveland, OH).
To test for astrocyte lineage, early passage cells were grown in dibutyryl cAMP (Sigma) to enhance GFAP expression. Intermediate filament extracts of the cell lines [43,44] were electrophoretically blotted and detected with polyclonal anti GFAP (DAKO, Carpenteria, CA) and secondary reagents as above.
Acknowledgments
Acknowledgements
This work was supported by grants from NIH, RO1-CA-73413 and P30-CA-43703.
Contributor Information
Phyllis S Frisa, Email: jwj@po.cwru.edu.
James W Jacobberger, Email: psf@po.cwru.edu.
References
- Sladek TL, Jacobberger JW. Flow cytometric titration of retroviral expression vectors: comparison of methods for analysis of immunofluorescence histograms derived from cells expressing low antigen levels. Cytometry. 1993;14:23–31. doi: 10.1002/cyto.990140106. [DOI] [PubMed] [Google Scholar]
- Jacobberger JW, Sramkoski RM, Wormsley SB, Bolton WE. Estimation of kinetic cell-cycle-related gene expression in G1 and G2 phases from immunofluorescence flow cytometry data. Cytometry. 1999;35:284–289. doi: 10.1002/(SICI)1097-0320(19990301)35:3<284::AID-CYTO12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Frisa PS, Lanford RE, Jacobberger JW. Molecular quantification of cell cycle-related gene expression at the protein level. Cytometry. 2000;39:79–89. doi: 10.1002/(SICI)1097-0320(20000101)39:1<79::AID-CYTO11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Risser R, Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974;59:477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisa PS, Walter EI, Ling L, Kung HJ, Jacobberger JW. Stepwise transformation of astrocytes by simian virus 40 large T antigen and epidermal growth factor receptor overexpression. Cell Growth Differ. 1996;7:223–233. [PubMed] [Google Scholar]
- Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- Pipas JM, Levine AJ. Role of T antigen interactions with p53 in tumorigenesis. Semin Cancer Biol. 2001;11:23–30. doi: 10.1006/scbi.2000.0343. [DOI] [PubMed] [Google Scholar]
- Saenz-Robles MT, Sullivan CS, Pipas JM. Transforming functions of Simian Virus 40. Oncogene. 2001;20:7899–7907. doi: 10.1038/sj/onc/1204936. [DOI] [PubMed] [Google Scholar]
- Sladek TL, Jacobberger JW. Simian virus 40 large T-antigen expression decreases the G1 and increases the G2 + M cell cycle phase durations in exponentially growing cells. J Virol. 1992;66:1059–1065. doi: 10.1128/jvi.66.2.1059-1065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek TL, Jacobberger JW. Dependence of SV40 large T-antigen cell cycle regulation on T-antigen expression levels. Oncogene. 1992;7:1305–1313. [PubMed] [Google Scholar]
- Sladek TL, Fisher SD, Rubenstein BN. Dose-response of 2 cellular proliferation phenotypes produced by simian-virus-40 large T-antigen. Cell Prolif. 1994;27:711–721. [Google Scholar]
- Frisa PS, Goodman MN, Smith GM, Silver J, Jacobberger JW. Immortalization of immature and mature mouse astrocytes with SV40 T antigen. J Neurosci Res. 1994;39:47–56. doi: 10.1002/jnr.490390107. [DOI] [PubMed] [Google Scholar]
- Chiu FC, Norton WT, Fields KL. The cytoskeleton of primary astrocytes in culture contains actin, glial fibrillary acidic protein, and the fibroblast-type filament protein, vimentin. J Neurochem. 1981;37:147–155. doi: 10.1111/j.1471-4159.1981.tb05302.x. [DOI] [PubMed] [Google Scholar]
- DiSalvo CV, Zhang D, Jacobberger JW. Regulation of NIH-3T3 cell G1 phase transit by serum during exponential growth. Cell Prolif. 1995;28:511–524. doi: 10.1111/j.1365-2184.1995.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Jacobberger JW. TGF-beta 1 perturbation of the fibroblast cell cycle during exponential growth: switching between negative and positive regulation. Cell Prolif. 1996;29:289–307. doi: 10.1111/j.1365-2184.1996.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol. 1999;287:821–828. doi: 10.1006/jmbi.1999.2640. [DOI] [PubMed] [Google Scholar]
- Barcus ME, Ferreira-Gonzalez A, Buller AM, Wilkinson DS, Garrett CT. Genetic changes in solid tumors. Semin Surg Oncol. 2000;18:358–370. doi: 10.1002/(SICI)1098-2388(200006)18:4<358::AID-SSU11>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Sheinfeld J, Dalbagni G. Genetic studies and molecular markers of bladder cancer. Semin Surg Oncol. 1997;13:319–327. doi: 10.1002/(SICI)1098-2388(199709/10)13:5<319::AID-SSU5>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Katso RM, Manek S, O'Byrne K, Playford MP, Le Meuth V, Ganesan TS. Molecular approaches to diagnosis and management of ovarian cancer. Cancer Metastasis Rev. 1997;16:81–107. doi: 10.1023/A:1005796307286. [DOI] [PubMed] [Google Scholar]
- Slaton JW, Benedict WF, Dinney CP. P53 in bladder cancer: mechanism of action, prognostic value, and target for therapy. Urology. 2001;57:852–859. doi: 10.1016/S0090-4295(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Gjoerup O, Chao H, DeCaprio JA, Roberts TM. pRB-dependent, J domain-independent function of simian virus 40 large T antigen in override of p53 growth suppression. J Virol. 2000;74:864–874. doi: 10.1128/JVI.74.2.864-874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobberger JW. Flow Cytometric Analysis of Intracellular Protein Epitopes. In: Stewart C, Nicholson JKA, editor. Immunophenotyping. New York: Wiley-Liss, Inc.; 2000. [Google Scholar]
- Opavsky R, Haviernik P, Jurkovicova D, Garin MT, Copeland NG, Gilbert DJ, Jenkins NA, Bies J, Garfield S, Pastorekova S, Oue A, Wolff L. Molecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal cells in vivo. J Biol Chem. 2001;276:38795–38807. doi: 10.1074/jbc.M105241200. [DOI] [PubMed] [Google Scholar]
- Wang L, Adamo ML. Cell density influences insulin-like growth factor I gene expression in a cell type-specific manner. Endocrinology. 2000;141:2481–2489. doi: 10.1210/endo.141.7.7577. [DOI] [PubMed] [Google Scholar]
- Kutoh E, Margot J, Schwander J. Cell-density (cycle) dependent silencer of the rat insulin-like growth factor binding protein-2 (IGFBP-2) gene. Growth Factors. 1999;16:217–223. doi: 10.3109/08977199909002131. [DOI] [PubMed] [Google Scholar]
- Leung LK, Do L, Wang TT. Regulation of death promoter Bak expression by cell density and 17 beta-estradiol in MCF-7 cells. Cancer Lett. 1998;124:47–52. doi: 10.1016/S0304-3835(97)00430-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wharton W, Donovan M, Coppola D, Croxton R, Cress WD, Pledger WJ. Density-dependent growth inhibition of fibroblasts ectopically expressing p27(kip1). Mol Biol Cell. 2000;11:2117–2130. doi: 10.1091/mbc.11.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen RL, Shaw LE, Larsen C, Corcoran D, Darbre PD. Insulin-like growth factor receptor levels are regulated by cell density and by long-term estrogen deprivation in MCF7 human breast cancer cells. J Biol Chem. 2001;276:40080–40086. doi: 10.1074/jbc.M105892200. [DOI] [PubMed] [Google Scholar]
- Singh RK, Llansa N, Bucana CD, Sanchez R, Koura A, Fidler IJ. Cell density-dependent regulation of basic fibroblast growth factor expression in human renal cell carcinoma cells. Cell Growth Differ. 1996;7:397–404. [PubMed] [Google Scholar]
- Moffett J, Kratz E, Myers J, Stachowiak EK, Florkiewicz RZ, Stachowiak MK. Transcriptional regulation of fibroblast growth factor-2 expression in human astrocytes: implications for cell plasticity. Mol Biol Cell. 1998;9:2269–2285. doi: 10.1091/mbc.9.8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B, Wu H, Holthuizen E, Singh P. Identification of a novel cis element required for cell density-dependent down-regulation of insulin-like growth factor-2 P3 promoter activity in Caco2 cells. J Biol Chem. 2001;276:6937–6944. doi: 10.1074/jbc.M007789200. [DOI] [PubMed] [Google Scholar]
- Valkov NI, Gump JL, Engel R, Sullivan DM. Cell density-dependent VP-16 sensitivity of leukaemic cells is accompanied by the translocation of topoisomerase IIalpha from the nucleus to the cytoplasm. Br J Haematol. 2000;108:331–345. doi: 10.1046/j.1365-2141.2000.01832.x. [DOI] [PubMed] [Google Scholar]
- Galy B, Maret A, Prats AC, Prats H. Cell transformation results in the loss of the density-dependent translational regulation of the expression of fibroblast growth factor 2 isoforms. Cancer Res. 1999;59:165–171. [PubMed] [Google Scholar]
- Hamburger AW, Mehta D, Pinnamaneni G, Chen LC, Reid Y. Density-dependent regulation of epidermal growth factor receptor expression. Pathobiology. 1991;59:329–334. doi: 10.1159/000163672. [DOI] [PubMed] [Google Scholar]
- Gray SG, Ekstrom TJ. Effects of cell density and trichostatin A on the expression of HDAC1 and p57Kip2 in Hep 3B cells. Biochem Biophys Res Commun. 1998;245:423–427. doi: 10.1006/bbrc.1998.8449. [DOI] [PubMed] [Google Scholar]
- Afrakhte M, Heldin NE, Westermark B. Inhibition of G1 cyclin-dependent kinase activity in cell density-dependent growth arrest in human fibroblasts. Cell Growth Differ. 1998;9:983–988. [PubMed] [Google Scholar]
- Kalderon D, Smith AE. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Lanford RE. Expression of simian virus 40 T antigen in insect cells using a baculovirus expression vector. Virology. 1988;167:72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Bell PB, Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979;76:3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger JM, Brown WE, Goldman AE, Goldman RD. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978;78:93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]