Abstract
Yams are the edible subterranean rhizomes, or tubers, of plants from the genus Dioscorea. There are approximately 600 species of yam plants in the world, with more than 90 of these growing in East Asia. One particular species, Dioscorea opposita Thunb., is highly praised as “the Chinese yam”. This distinction arises from millennia of storied history, both as a nutritional food source and as a principal ingredient in traditional Chinese medicine. Among the many cultivars of Dioscorea opposita Thunb., Huai Shanyao has been widely regarded as the best. This review surveyed the historical background, physiochemical composition, applications as food and medicine, and research prospects for the Chinese yam. Modern science is finally beginning to confirm the remarkable health benefits of this yam plant, long-known to the Chinese people. Chinese yam promises anti-diabetic, anti-oxidative, anti-inflammatory, immunomodulatory, anti-hyperlipidemic, anti-hypertensive, anti-cancer, and combination treatment applications, both as a functional food and as medicine.
Keywords: Dioscorea opposita Thunb., Huai Shanyao, batatasin, polysaccharide, traditional Chinese medicine
1. Introduction
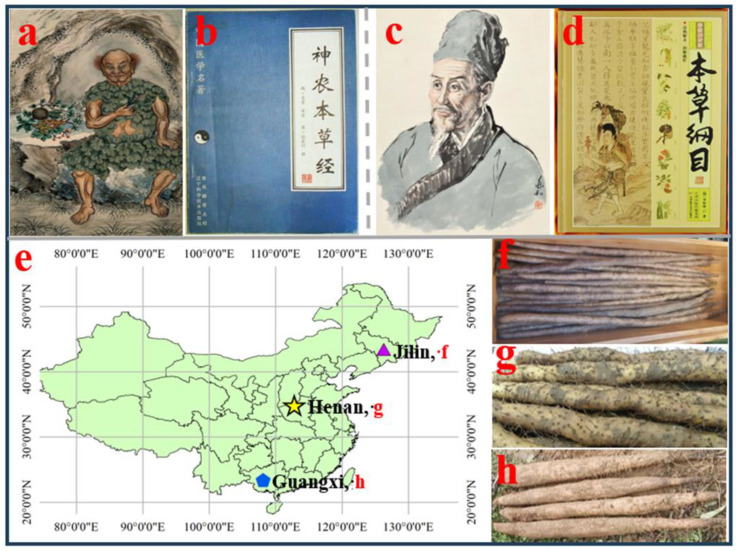
Beginning with the oral tradition of the legendary Chinese figure Shen Nong (circa 2700 BC, Figure 1a), also known as “the Divine Farmer” [1], Dioscorea opposita Thunb. (D. opposita) first appeared in persistent text more than 2000 years later in the earliest Chinese pharmacopoeia, Shen Nong Ben Cao Jing (Figure 1b), which proudly proclaimed it “the Chinese yam” [2,3]. According to Li Shizhen (1518–1593 AD, Figure 1c), the author of Ben Cao Gang Mu (the Ming Dynasty pharmacopoeia, Figure 1d), the Chinese yam tuber was indicated as being effective in alleviating “indigestion, anorexia, diarrhea, and diabetes. It was suggested to have a hypoglycemic effect and promote the health of elderly women” [4].
Figure 1.
History and distribution of the Chinese yam in China. (a) Shen Nong “the Divine Farmer”; (b) Shen Nong Ben Cao Jing “Divine Farmer’s Classic of Materia Medica”; (c) Li Shizhen; (d) Ben Cao Gang Mu “Compendium of Materia Medica”; (e) Chinese yam cultivar distribution; (f) Xichangmao Shanyao in Jilin province; (g) Huai Shanyao in Henan province; (h) Jintian Huai Shanyao in Guangxi province.
Primarily prescribed as an invigorant in traditional Chinese medicine (TCM), Chinese yam has also been reported to improve coughing and dyspnea associated with lung deficiency [5], as well as to promote immune functions [6]. Yams are the edible underground rhizomes, or tubers, of the dioecious monocotyledon plant species of the genus Dioscorea, singular Dioscoraceae. They are a crucial staple food in the diets of many tropical and subtropical countries [7]. They are prized primarily for the carbohydrate nourishment they provide [3]. Globally, an estimated 88.3 million metric tons of yams were produced in 2022. The vast majority of these were cultivated in the “yam belt” of West Africa, with Nigeria, Ghana, and Cote d’Ivoire producing 61.2, 10.7, and 7.6 million metric tons, respectively [8]. There are an estimated 600 species of yam plants in the world, including more than 90 which grow in East Asia [9,10]. D. opposita has been a principal ingredient in the herbal medicine of China for millennia [11,12].
Today, the Chinese yam is a medicinal plant which is extensively cultivated in the People’s Republic of China (PRC). The harvest time is from late October until early December. The starchy tubers are typically blanched, or boiled quickly, to remove irritating oxalate crystals and residual pesticides from their skins, and then carefully cut into slices [13,14]. The next step is to dry them. The crude drug yam discs have been extensively used in “Chinese herbal medicine since ancient times to strengthen the functions of the spleen, kidney, liver, and stomach, to decrease phlegm, and heal fatigue, chronic diarrhea, and diabetes” [15].
In the PRC, Chinese yam is primarily grown and consumed in the Guangxi, Jiangsu, Henan, Shandong, Hebei, Shanxi, and Jilin provinces (Figure 1e). One particular cultivar of D. opposita, Huai Shanyao (HSY), has widely been considered to be the best, both as food and as medicine. HSY can be found in Jiaozuo city, in the Henan province (Figure 1g) [3,12,16]. Xichangmao Shanyao, in the Jilin province, is a cultivar of D. opposita, which was introduced and planted in northern China hundreds of years ago (Figure 1f). The Chinese yam also has another scientific name, Dioscorea batatas Decne., which is used in the southern parts of the PRC (Figure 1h) [17,18,19,20,21]. This name draws attention to the fact that the Chinese yam is the only species known to contain all five batatasins, which regulate the dormancy of temperate plants in the cold winter, instead of during the hot and dry season, as is the case with African yams [18,22].
Various D. opposita cultivars are available from the Wenxian Institute of Agricultural Sciences in Jiaozuo City, Henan Province. There is considerable diversity among different cultivars of Chinese yam. The HSY cultivar is “one of the four famous Huai Chinese traditional medicines with some special traits such as heavy tubers, oily quality, less tendon, sweet soothing, high medicinal effect, and boiling tolerance” [23]. This review surveyed the physiochemical composition, applications as food and medicine, and research prospects of D. opposita. A recent review by Li et al. summarized the nutritional and beneficial functions of Chinese yam [24]. Our current review, however, includes batatasins and excludes diogenin, which was not found by our research group specifically in the Chinese yam [25].
2. Chinese Yam Composition
The Chinese yam contains various chemical components, including starch, non-starch polysaccharides, fiber, protein, allantoin, dopamine, batatasins, phytic acid, choline, and ergosterol (Table 1). The Chinese yam is made up of approximately 13–27% carbohydrates, mostly starch [26]. According to a previous study, “In Chinese market, native starches are considered as health food because they are obtained from green botanical sources with undefiled properties” [27]. Yam tubers are also made up of 1–3% protein, but dried yam is more concentrated, comparable to cereal grains [28]. The major Chinese yam storage protein, comprising 90% of the total protein, is dioscorin [29]. Water makes up most of the remaining mass, approximately 67–84% [26].
Table 1.
Composition of Chinese yam.
| Composition | Content | Description | References |
|---|---|---|---|
| Water | 67−84% | - | [22] |
| Carbohydrates | 13−27% | Mostly starch, including fiber | [10,22] |
| Protein | 1−3% | 90% Dioscorin | [23,26] |
| Minor molecular constituents | Abscisin II, Allantoin, Batatasins, Choline, Dopamine, Glucoprotein, Mannan, Phytic acid, Polysaccharides |
[10] | |
| Minerals | Ca, Cu, Fe, Mg, Na, P, K, Zn | [10] |
Chinese yams hold both water-soluble fiber and insoluble fiber. Chemical studies of the Chinese yam have revealed other minor molecular constituents, such as abscisin II, allantoin, batatasins, choline, dopamine, glucoprotein, mannan, phytic acid, polysaccharides, and minerals such as calcium, copper, iron, magnesium, manganese, phosphorous, potassium, and zinc [10].
2.1. Starch
The Chinese yam starch type is the C-type, having both crystalline A-type and amorphous B-type [5]. Comparatively, potatoes are B-type. Among species of Dioscorea, starch crystallinity decreases with increased amylose content, while starch swelling power decreases with increased amylopectin chain-length, which also increases starch crystallinity. D. opposita has a high amylose to amylopectin ratio and is thus very viscous [30].
Starches undigested after 120 min postprandial are considered to be resistant starches [31]. Boiling Chinese yams can reduce their resistant starch content by 80% [32]. Resistant starches benefit short-chain fatty acid production in the large intestine, inhibiting hepatic cholesterol synthesis and suppressing colon cancer cell proliferation in vitro. “These effects are assumed to prevent the development of coronary heart disease and colon cancer” [32].
2.2. Polysaccharide
Chinese yam mucopolysaccharide (CYMP), or root-tip yam sugar, is composed of approximately 40% polysaccharide and 2% protein [33]. The polysaccharide fraction of CYMP contains mannose, fructose, galactose, xylose, and glucose [34]. The glucose and protein composition of Chinese yam tuber mucilage (CYTM) in dry weight was found to be 11.05% and 13.39%, respectively [35]. CYTM is largely wasted in industrial processes, but it shows tremendous promise as a natural food emulsifier and stabilizer [36,37,38].
Chinese yam polysaccharide (CYP) is typically separated from dried Chinese yam discs through extraction, and glucose and proteins have been found to comprise 63.25% and 0.21% of CYP, respectively [39]. CYP is a kind of carbohydrate polymer, primarily composed of mannose, glucose, galactose, and glucuronic acid [40]. CYP is regarded as an important bioactive natural product [41]. Once purified, CYP can be useful for several purposes, including anti-diabetic, anti-oxidative, anti-bacterial, immunomodulatory, and anti-cancer applications [42,43].
2.3. Protein
The widespread practice of blanching yams, before cutting them into discs and then drying them, reduces protein content. Drying yam discs at a relatively lower temperature preserves much more of the protein [44].
Found in Chinese yam tubers, but not in the leaves, dioscorins taken from different sources often have different structures and activities [45], but recombinant dioscorin conformational structures are similar to their corresponding native dioscorins [46]. Differences “were closely related to the loss of the glycosylation from the protein” [47]. Dioscorin exhibits carbonic anhydrase activity and weak trypsin inhibitor activity, which can slow protein digestion [48,49]. Dioscorin also shows monodehydroascorbate reductase activity and dehydroascorbate reductase activity [50]. Dioscorin even displays in vitro angiotensin converting enzyme inhibitory activity, and in vivo anti-hypertensive activity [51]. In vitro, dioscorin is a dose- and pH-dependent antioxidant against 1,1-diphenyl-2-picrylhydrazyl, and can also capture hydroxyl free radicals [29].
Other Chinese yam proteins include lectins, a group of proteins or glycoproteins that has carbohydrate binding capability. Chinese yams produce lectins of varying sizes: mannose-binding lectin (DB1) and two maltose-binding lectins [52]. DB1 is insecticidal and helps to preserve these yam tubers against pests [53].
2.4. Batatasins
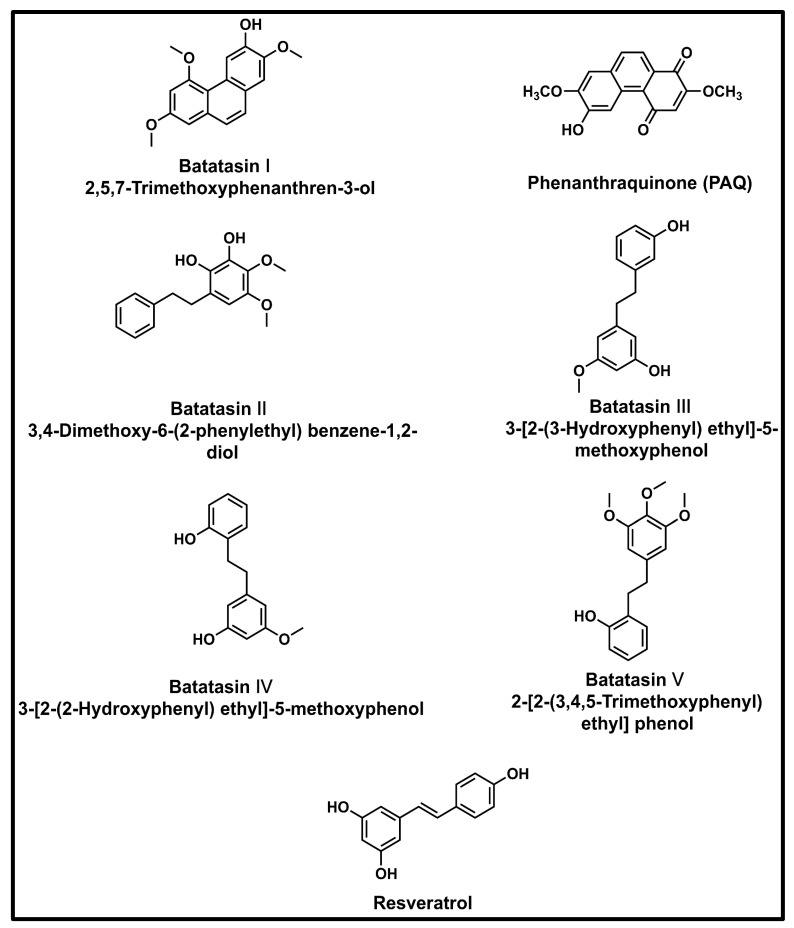
The primary phenolic compounds found in Chinese yams are known as batatasins. Originally, only dormant D. opposita aerial bulbils were thought to contain all five batatasins (Figure 2) [18]. While all five batatasins have not been found in any other species, they have now all been identified within Chinese yam rhizomes [54]. Batatasin I and another structural analogue, 6-hydroxy-2,7-dimethoxy-1,4-phenanthraquinone (PAQ), have been identified in Chinese yam rhizome methanol extract. These compounds are potential therapeutic treatments for allergic-inflammatory conditions [45,55].
Figure 2.
Batatasins I–V and analogues.
The bibenzyl stilbene molecular structures of batatasins II–V are molecular analogues of resveratrol (RV), which is both a powerful natural antioxidant and inhibitor of cyclooxygenase-2 (COX-2) expression. The primary source of RV is grapes, Vitis vinifera [56]. With diverse therapeutic properties, RV has been well-studied as a nutritional supplement, and promises anti-diabetic, anti-oxidative, anti-inflammatory, immunomodulatory, cardio-protective, anti-cancer, and neuro-protective activities [57]. RV derivatives were explored as an adjunct therapy for elderly patients during the COVID-19 epidemic and were thought to offer anti-aging, anti-inflammatory, anti-oxidative, and anti-viral effects [58].
2.5. Other Small Molecular Constituents
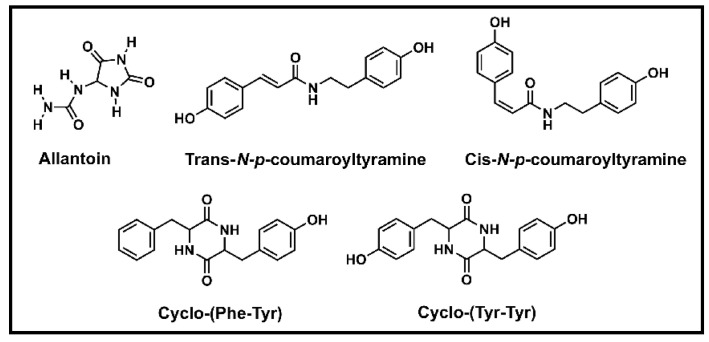
The Chinese yam has a variety of small molecular constituents, such as allantoin, trans-N-p-coumaroyl tyramine (TCT), and the cyclic dipeptides etc. (Figure 3). Allantoin is more concentrated in Chinese yam skin, but is also found in the rhizomes. Allantoin promotes cell proliferation, speeds up healing, and soothes inflammation both internally and externally [34]. Allantoin is an easily synthesized small molecule and has long been used in medicinal and cosmetic preparations [59,60].
Figure 3.
Allantoin, trans-N-p-coumaroyl tyramine, cis-N-p-coumaroyl tyramine, cyclo-(Phe-Tyr), and cyclo-(Tyr-Tyr).
TCT was extracted from the HSY cultivar by our research group and had considerable inhibitory activity against α-glucosidase; TCT transforms into its cis-isomer, cis-N-p-coumaroyl tyramine, with no inhibition of α-glucosidase activity, under ultra-violet light [61]. The concentration of TCT is higher in the yam skin than inside the tuber [62].
Several other plants also contain TCT. For example, TCT from the hemp seeds of Cannabis sativa L. has been shown to exhibit melanogenesis and anti-tyrosinase activity [63,64], while TCT from the stems of Dracaena usambarensis Engl. has been shown to exhibit anti-inflammatory activity [65]. TCT α-glucosidase inhibitory activity, IC50, was 0.42 μM in Tribulus terrestris Linn. and 0.6 μM in Huberantha jenkinsii [66]. One study showed that TCT extract from Tribulus terrestris induced extrinsic, as well as intrinsic, apoptosis pathways in cancer cells [67].
Our research group has also found and isolated the cyclic dipeptides, cyclo-(Phe-Tyr) and cyclo-(Tyr-Tyr), from Chinese yam tubers [68]. Cyclic dipeptides are believed to have multiple important biological functions, such as anti-fungal, anti-bacterial, anti-cancer, immunomodulatory, anti-inflammatory, and anti-viral activities [69].
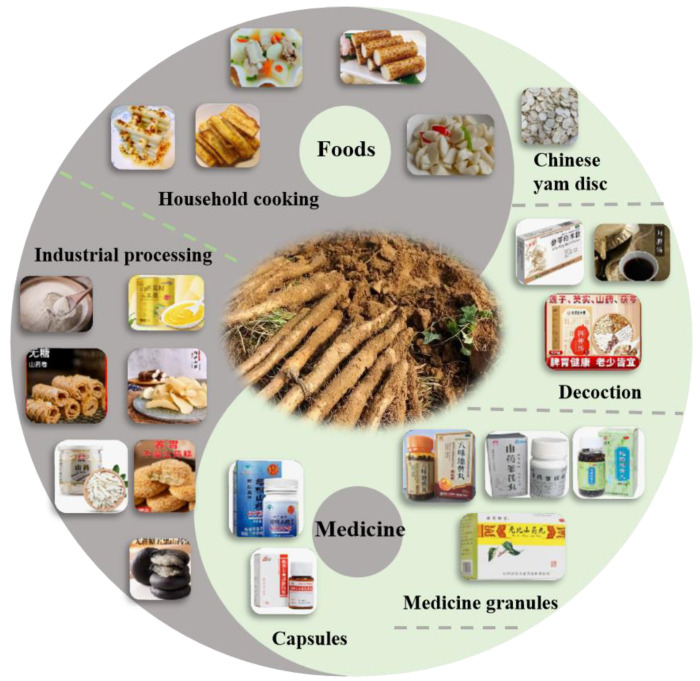
3. Chinese Yam as Food and Medicine
Chinese yam can be served as a nutritious and functional food, and it has several health benefits described in TCM (Figure 4):
… a sweet soothing herb that stimulates the stomach and spleen and has a tonic effect on the lungs and kidneys … The tuber is also anthelmintic and digestive. It is used internally in the treatment of poor appetite, chronic diarrhea, asthma, dry coughs, frequent or uncontrollable urination, diabetes, and emotional instability. It is applied externally to ulcers, boils, and abscesses.
[34]
Figure 4.
Chinese yam as food and medicine. Food: Household cooking and industrial processing. Medicine: Chinese yam discs, decoctions, medicinal granules, and capsules.
3.1. Chinese Yam as Food
Chinese yam tubers can be stored for up to one year after harvesting and may be eaten without cooking. Globally, food yams are usually stored as whole tubers or processed into flour [17]. Freeze-drying yam flour preserves more antioxidant activity than hot air-drying or drum-drying [70,71].
The benefits of wheat flour, yam flour, and boxthorn yam noodles have also been explored, as functional food consumption continues to increase every year. BALB/c mice and humans share a more than 55% homology in the apoA-II amino acid sequence, showing lipid metabolism similarities. Experimental mice fed a yam-boxthorn noodle diet, rather than a strictly wheat flour noodle diet, had significantly lower total cholesterol and triglyceride serum levels [72]. The functional yam-boxthorn noodle could become a staple with hypolipidemic properties. Likely active lipid-lowering Chinese yam components include dietary fiber, mucilage, plant sterols, or a synergism of all these [10].
The addition of 0.2–0.6% weight/volume of D. opposita to yogurt can provide allantoin supplementation without making any modifications to the fermentation and storage process, highlighting one of potential applications of Chinese yam in fermented foods [73]. Microbial hydrolysis by fermentation enhances antioxidant activity in plant-based foods through increased release of phenolic compounds and flavonoids by breaking down cell walls [74]. Raw Chinese yam fermented by Lactobacillus acidophilus has been found to be a superior functional food, healing and preventing gastric lesions in rats [75].
The Japanese cultivar of D. opposita is known as “tsukuneimo” or “iseimo”. Tsukuneimo tororo tuber mucilage hydrolysates can be prepared with the digestive enzymes pepsin, trypsin, and papain. Enzymes are extremely useful tools for improving functional foods [76]. Tsukuneimo tororo soup “should be recognized as one of the health foods to prevent or improve lifestyle-related diseases such as cancer, diabetes, and hypertension” [77].
Yam peel portions are significantly better than the rhizome portions at scavenging reactive oxygen species (ROS) [78]. Recently, yam chips have become highly marketable in China. Yam chip preparation involves a heat pump and far-infrared radiation to achieve “the desired moisture ratio, the smallest shrinkage of dried Chinese yam chips, the highest rehydration percentage, and the smallest changes of color” [79].
CYTM has shown antioxidant activity in vitro [80]. CYTM is usually extracted with solvents, centrifugation, and heat, as it can be difficult to separate CYP and starch from CYTM. Bubble separation has recently been used as an innovative method to purify CYTM, as the process protects antioxidants from chemical damage and heat [81]. Once CYP and starch have been separated from CYTM, they may also serve as potent ingredients. When eaten, CYP promises immunomodulatory benefits [34]. Meanwhile, eating modified resistant yam starch was shown to prevent constipation and significantly improve serum lipid profiles in a murine model [82].
Dioscorin, as the major storage protein in Chinese yam and an antioxidant, may supplement health when eaten as a food additive or consumed in yam tubers [29,83]. It has been shown that consuming dioscorin-containing yam products reduces blood pressure in humans and stimulates the innate immune systems of mice [45].
Eating Chinese yams could also improve several disease conditions by reducing ROS. A Chinese yam-supplemented diet reduced azoxymethane-induced colon carcinogenesis among F344 rats by scavenging ROS, as well as through increased colonic mucosal gene expression of superoxide dismutase, and suppressed colonic mucosal gene expression of inflammatory mediators [84]. Continued Chinese yam food research will facilitate the production of processed foods with more antioxidant and anti-hypertensive activity [85]. Chinese yam has beneficial properties as food, and it also has medicinal benefits, such as anti-oxidative action, serum glucose reduction, and immune improvement [3]. Therefore, it is considered to be a nutraceutical (Table 2).
Table 2.
Nutraceutical properties of Chinese yam.
| Activities | Food Type/Components | Ref. |
|---|---|---|
| As Food | ||
| Anti-diabetic | Tsukuneimo | [77] |
| Anti-oxidative | Yams, Flour, Yogurt, Chips | [70,71,75,77,78,84] |
| Anti-inflammatory | Yams | [84] |
| Anti-hyperlipidemic | Noodles | [72] |
| Anti-hypertensive | Tsukuneimo | [77] |
| Anti-cancer | Tsukuneimo | [77] |
| As Medicine | ||
| Anti-diabetic | Allantoin, Batatasin I, CYP *, TCT * |
[42,43,61,86,87] |
| Anti-oxidative | Dioscorin, Batatasin I, CYP * | [29,40,88] |
| Anti-inflammatory | Batatasin I, TCT *, Cyclo-(Phe-Tyr), Cyclo-(Tyr-Tyr) |
[55,69,89,90] |
| Immunomodulatory | CYP *, Cyclo-(Phe-Tyr), Cyclo-(Tyr-Tyr) |
[69,91,92,93,94] |
| Anti-hyperlipidemic | Allantoin, Starch, Resistant starch | [10,95,96] |
| Anti-hypertensive | Allantoin, Dioscorin | [51,96,97] |
| Anti-cancer | Nagaimo lectin, Resistant starch, CYMP *, CYP *, Cyclo-(Phe-Tyr), Cyclo-(Tyr-Tyr) |
[32,34,42,52,69] |
* Abbreviations: CYP, Chinese yam; TCT, trans-N-p-coumaroyl tyramine; CYMP, Chinese yam mucopolysaccharide.
3.2. Chinese Yam as Medicine
Medicinal Chinese yam is harvested in the winter and processed by washing, peeling, drying, and slicing [10]. There are many pharmacological purposes for medicinal Chinese yam (Table 3).
Table 3.
In vivo bioactivies of Chinese yam.
| Components | Bioactivities | Animals | Dosage | Duration | Ref. |
|---|---|---|---|---|---|
| Allantoin | Anti-diabetic | Streptozotocin-induced diabetic rats | 0.5 mg/kg | 120 min | [86] |
| Anti-hyperlipidemic Anti-hypertensive | High fat diet-induced hyperlipidemic mice | Daily 5 mg/kg |
4 weeks | [96] | |
| TCT * | Anti-inflammatory | Dust mite-induced atopic dermatitis mice | Daily 50 mg/kg |
4 weeks | [90] |
| Dioscorin | Anti-oxidantive | D-galactose-induced oxidative stress mice | Daily 20–80 mg/kg |
5 Weeks | [83] |
| HSY * aqueous Extract |
Anti-hypertensive | 2K1C * renal hypertensive rats | Daily 210 mg/kg |
6 weeks | [98] |
| Starch | Anti-hyperlipidemic | High fat diet-induced hyperlipidemic rats | Daily 6.5 g/kg |
8 weeks | [10] |
| Anti-diabetic | Humans | - | - | [99] | |
| Resistant starch | Anti-hyperlipidemic | Humans | - | - | [95] |
* Abbreviation: TCT, Trans-N-p-coumaroyl tyramine; HSY, Huai Shanyao; 2K1C, Two-kidney one-clip.
3.2.1. Anti-Diabetic
The high amylose to amylopectin ratio in Chinese yam slows digestion, a beneficial anti-diabetic activity. High amylose consumption, as opposed to high amylopectin consumption, significantly lowers serum glucose levels at 30 min and raises levels at 180 min, while lowering postprandial insulin levels at 30 min and 60 min [99].
Streptozotocin-injected rats mimic pre-diabetic status, and injections of Chinese yam extract every day has been shown to contribute to reduced fasting serum glucose levels in these rats [100], leading to improved kidney function [101]. In this same model, allantoin was shown to improve insulin resistance. Allantoin administration increased β-endorphin secretion from the adrenal glands of these rats, activating opioid μ-receptors to increase glucose transporter four expression, which increased skeletal muscle glucose uptake, thereby reducing the serum glucose level [86]. Another study confirmed that allantoin activates imidazoline I3 receptors and enhances insulin secretion, lowering serum glucose levels in the experimental rats [87].
Batatasin I and three other bibenzyls, isolated from the HSY tuber crude ethanol extract, have shown considerable α-glucosidase inhibitory activity. These α-glucosidase inhibitory compounds show promise as a potential new class of anti-diabetic drugs [61]. TCT, which our research group has isolated from HSY tubers, also shows considerable inhibitory activity against α-glucosidase (IC50 = 0.40 μM). As a carbohydrate-hydrolase, α-glucosidase releases glucose. Several α-glucosidase inhibitors alleviate postprandial hyperglycemia and have been developed into clinical anti-diabetic agents, including acarbose, miglitol, and voglibose [102]. Diarrhea and hepatotoxicity have been associated with these drugs. The low cost and relative safety of a natural source make them more suitable candidates for α-glucosidase inhibitor screening [59].
High purity CYP, 200 μg/mL, effectively increases the glucose uptake and the expression of glucose transporter two in FL838 cells also treated with tumor necrosis factor-alpha (TNF-α), restoring insulin sensitivity [103].
3.2.2. Anti-Oxidative
Chinese yam autolysate and enzymatic hydrolysates (digested with pepsin, trypsin, and papain) show exceptional anti-oxidative activity [85]. Purified CYP exhibits anti-oxidative ability to scavenge hydroxyl radicals and superoxide anions, and has a reducing power of approximately one-half that of vitamin C. CYP scavenging ability increases with concentration, and purified CYP even shows anti-bacterial activity against Escherichia coli [104].
CYP exhibits a proliferative effect on endometrial epithelial cells in vitro, as a potential natural anti-oxidative therapeutic treatment for female infertility. Endometrial cell proliferation reaches its peak at about 36 h after 100–400 μg/mL CYP administration, due to down regulation of Bax/Bcl-2, or a pro-apoptotic/anti-apoptotic ratio [40].
Chinese yam aerial bulbil methanol extract containing batatasin I and 6,7-dihydroxy-2,4-dimethoxy phenanthrene reduced TNF-α-induced ROS production in vascular smooth muscle cells, and could be useful in preventing atherosclerotic plaque development [88]. Also, dioscorin exhibits powerful anti-oxidative activity, comparable to that of endogenous glutathione at the same concentration [83]. The anti-oxidative properties of dioscorin are greatest under acidic conditions, around pH 5 [71]. In BALB/c mice fed D-galactose to induce oxidative stress, oral administration of dioscorin was shown to attenuate oxidative stress [83].
3.2.3. Anti-Inflauuatory
Chronic inflammatory pathways contribute to the pathogenesis of diabetic complications, but Chinese yam extracts have produced anti-inflammatory effects in multiple models. In pre-diabetic rats, extracts decreased levels of the pro-inflammatory cytokine IL-6 [99], while peel extract protected male Wistar rats from liver damage caused by carbon tetrachloride-induced hepatotoxicity, through its anti-inflammatory capacity [105].
The methanol extract of the Chinese yam rhizome (containing batatasin I and PAQ) exhibits anti-inflammatory activity, inhibiting both eicosanoid generation and degranulation in activated mast cells [55,89]. Both batatasin I and PAQ are dual inhibitors of COX-2 and 5-lipoxygenase (5-LOX). Dual inhibitors of “COX-2 and 5-LOX might synergistically inhibit inflammation and reduce the undesirable side effects that are associated with non-steroidal anti-inflammatory drugs” [55].
Upon oral administration of TCT to atopic dermatitis mice, atopic dermatitis was effectively suppressed through inflammatory regulation, even as the immune response was not significantly affected [90].
3.2.4. Immunomodulatory
In immune regulation, CYP promotes macrophage polarization to the M1 type, thus activating their phagocytic function and secretion of proinflammatory factors [91,92]. CYP also stimulates peripheral lymphocytes, activating both CD4+ and CD8+ T cells (CD3+) for a more effective antigen-specific immune response [34,93,94].
Dioscorin exhibits immunostimulatory activity and reinforces innate immunity, stimulating nitric oxide and cytokine production, as well as enhancing phagocytosis [51]. While consumption of larger amounts can cause inflammation in vivo, smaller amounts of dioscorin may help stimulate “macrophage function and immunomodulatory effect in mucosal-associated lymphocyte tissue” [106].
3.2.5. Anti-Hyperlipidemic
Hyperlipidemia is a major cause of mortality throughout the world. In addition to reducing lipid peroxidation through anti-oxidative effects, a direct effect of Chinese yam on lowering serum lipid levels has been observed. In hyperlipidemic rats fed high-fat diets with Chinese yam starch for 8 weeks, there was a significant reduction in hyperlipidemia when compared to a high-fat diet alone: serum total cholesterol decreased by 33.8%, triglycerides decreased by 46.2%, and low-density lipoprotein-cholesterol decreased by 27.5% [10]. In both rodents and humans, consuming resistant starch has a potent hypolipidemic and prebiotic effect, improving lipid profiles [95]. By weight, raw Chinese yam is more than 33% resistant starch [32].
Allantoin administration to mice fed a high-fat diet improved hepatic steatosis by activating imidazoline I1 receptors. Allantoin activation of imidazoline I1 receptors moderated hyperlipidemia in these mice by increasing LDLR gene expression and reducing ApoC-III and SREBP1c expression [96].
3.2.6. Anti-Hypertensive
Intraperitoneal injections of allantoin reduced systolic blood pressure of mice fed a high-fat diet, without changing their heart rates [96]. Attributed to the activation of imidazoline I1 receptors, intravenous injections of allantoin reduced total peripheral resistance in spontaneously hypertensive rats, reducing blood pressure, heart rate, and cardiac contractility [97].
Anti-hypertensive effects of HSY aqueous extract on 2K1C renal hypertensive rats were significant, reducing mean systolic and diastolic blood pressure and preventing left ventricular hypertrophy. These benefits were attributed to a reduced angiotensin-II level, inhibited endothelin production, and activation of the in vivo antioxidant defense system [98].
3.2.7. Anti-Cancer
The search for alternative medicine continues, as the “majority of chemical compounds, which have been identified as cytotoxic to cancer cells, are also toxic to normal cells” [34]. The D. opposita cultivar Nagaimo lectin shows anti-proliferative activity against several kinds of cancer cells when fully separated and purified. “The inhibitory activity on MCSF7 (breast cancer cells) and HepG2 (hepatoma) cells was more potent than that on CNE2 (nasopharyngeal carcinoma)” [52]. The Nagaimo lectin is especially effective against breast cancer MCSF7 cells, even provoking apoptosis. Additionally, TCT extracts showed prominent cytotoxic activities against cancer cells such as MCSF [107], A431, and HeLa cells [108].
CYMP and CYP have also been shown to have anti-cancer activity. At 10 μg/mL, CYMP treatment significantly increased mouse splenocyte-mediated cytotoxic activity against leukemia cells, and significantly increased lysosomal splenic lymphocyte production of interferon-γ. At 50 μg/mL, CYMP treatment increased lysosomal uptake capacity and phosphatase activity [34]. At 200 μg/mL, CYP significantly inhibited HepG2 and SNU-739 (both hepatoma) cell proliferation [42].
3.2.8. Combination Treatments with Chinese Yam
In TCM, the Chinese yam is always used in combination with other TCM, such as Liuwei dihuang Wan, which is combined with five other TCM ingredients. Modern medicine has clinically confirmed the compatibility of 9–15 g Chinese yam, raw coix seeds, and Poria cocos to treat “puffiness” [109], and has also confirmed that 30 g Chinese yam and astragalus can have beneficial effects on diabetes, diarrhea, and asthma [110], that 12–15 g of Chinese yam and Ophiopogon japonicas can be used to treat a cough [111], and Chinese yam with apricot kernel has demonstrated clinical significance against asthma, cough, phlegm, and constipation [111]. Based on the clinical experience of modern physicians in the applications of Chinese yam, the dosage in decoctions is usually 9–50 g, while 100–200 g or more can be used for acute syndrome, severe syndrome, or rescue. Crude Chinese yam can even reach 500 g in yam congee treatment.
A Chinese yam-epidemium mixture is used to improve dyspnea, exercise ability, and the quality of life in patients with stable, moderate, or severe chronic obstructive pulmonary disease [112]. Natural and relatively inexpensive, Chinese herbal drugs are particularly suited to the long-term management of chronic diseases and should be researched further.
4. Discussion
The drug discovery paradox, “where breakthroughs in technology and an increasing volume of chemical and biological information have been matched by a puzzling decrease in the emergence of new drug entities”, has compelled a return to nature [113]. The main “bright spot” of innovation has involved tools used to study deoxyribonucleic acid (DNA). Previously, little was known about Chinese yam origins and phylogeny. Random amplified polymorphic DNA has been applied to understand the genetic variation of D. opposita cultivars [114]. Remarkably, it has been revealed that HSY is not technically D. opposita, but rather closer to Dioscorea alata L. [115]. Advantages demonstrated in genetic diversity call for “developing long-term strategies, such as modifying breeding programs through increasing utilization of wild species, conserving core germplasm, and establishing gene resource pools” [114].
The most promising of research prospects for future studies of the Chinese yam may focus on its anti-oxidative components and relate them to the control of oxidative stress, which has been implicated as a causative agent in many chronic and degenerative diseases, such as diabetes, cancer, coronary heart disease, Alzheimer’s disease, and even aging itself [29,78]. The Chinese yam has a long and storied history, both as a nutritious food source and as a source of some of the most famous TCM. Modern science is finally confirming the long-known health benefits of the Chinese yam and HSY [116]. Multi-target phytochemicals and TCM demonstrate many advantages [113]. The Chinese yam displays a myriad of distinct physiochemical properties, with exciting potential as both a food and a medicine [78].
5. Conclusions
This D. opposita review summarized its chemical compositions, bioactivities, and applications as both food and medicine. The Chinese yam presents various activities, including anti-diabetic, anti-oxidative, anti-inflammatory, immunomodulatory, anti-hyperlipidemic, anti-hypertensive, and anti-cancer functions. For thousands of years, the Chinese yam has been used to treat anorexia, chronic diarrhea, and diabetes. Therefore, the Chinese yam has been deployed in various applications as a functional food and as medicine.
Author Contributions
Conceptualization and supervision, X.L.; writing—original draft preparation and editing, M.K.; writing—review and editing and funding acquisition, F.M.; data curation, W.L.; visualization, L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Henan Provincial Youth Science Foundation of China, grant number 222300420031; Key Scientific and Technological Project of Henan Province, grant number 222102310637; Henan Provincial Science and Technology Research and Development Plan Joint Fund, grant number 232301420099; and the Foundation of Educational Committee of Henan Province, grant number 24A550001.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.The Legend of the Divine Farmer. [(accessed on 24 March 2024)]. Available online: http://publicdomainreview.org/essay/the-legend-of-the-divine-farmer.
- 2.Nagasawa A., Finer J.J. Plant Regeneration from Embryonic Suspension Cultures of Chinese Yam (Dioscorea opposita Thunb.) Plant Sci. 1989;60:263–271. doi: 10.1016/0168-9452(89)90176-3. [DOI] [Google Scholar]
- 3.Lee S.C., Tsai C.C., Chen J.C., Lin J.G., Hu M.L., Lu S. Effects of "Chinese yam" on hepato-nephrotoxicity of acetaminophen in rats. Acta Pharmacol. Sin. 2002;23:503–508. [PubMed] [Google Scholar]
- 4.Fu Y.C., Chen S.H., Huang P.Y., Li Y.J. Application of Bubble Separation for Quantitative Analysis of Choline in Dioscorea (Yam) Tubers. J. Agric. Food Chem. 2005;53:2392–2398. doi: 10.1021/jf048501h. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Yu J., Yu J., Chen H., Pang J., Liu H. Partial characterization of starches from Dioscorea opposita Thunb. cultivars. J. Food Eng. 2008;88:287–293. doi: 10.1016/j.jfoodeng.2006.11.016. [DOI] [Google Scholar]
- 6.Ma C., Wang W., Chen Y.Y., Liu R.N., Wang R.F., Du L.J. Neuroprotective and Antioxidant Activity of Compounds from the Aerial Parts of Dioscorea opposita. J. Nat. Prod. 2005;68:1259–1261. doi: 10.1021/np050021c. [DOI] [PubMed] [Google Scholar]
- 7.Burkhill L.H. The organography and the evolution of Dioscoreaceae, the family of the Yams. Bot. J. Linn. Soc. 1967;56:319–412. doi: 10.1111/J.1095-8339.1960.TB02508.X. [DOI] [Google Scholar]
- 8.The Production of Yams in the World. [(accessed on 24 March 2024)]. Available online: http://knoema.com/data/agriculture-indicators-production+yams.
- 9.Chen H.L., Wang C.H., Chang C.T., Wang T.C. Effects of Taiwanese Yam (Dioscorea japonica Thunb. var. pseudojaponica Yamamoto) on Upper Gut Function and Lipid Metabolism in BALB/c Mice. Nutrition. 2003;19:646–651. doi: 10.1016/s0899-9007(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang S., Yu J., Liu H., Chen W. Characterization and preliminary lipid-lowering evaluation of starch from Chinese yam. Food Chem. 2008;108:176–181. doi: 10.1016/J.FOODCHEM.2007.10.059. [DOI] [Google Scholar]
- 11.Zhang Z., Gao W., Li C., Jiang Q., Xia Y., Wang H., Huang L., Guo L. Effect of different drying methods on the physicochemical and functional properties of Dioscorea opposita Thunb. Starch. Starch. 2013;65:219–226. doi: 10.1002/star.201200009. [DOI] [Google Scholar]
- 12.Luo Z., Wang Y., Jiang L., Xu X. Effect of nano-CaCO3-LDPE packaging on quality and browning of fresh-cut yam. LWT—Food Sci. Technol. 2015;60:1155–1161. doi: 10.1016/j.lwt.2014.09.021. [DOI] [Google Scholar]
- 13.Bhandari M.R., Kawabata J. Cooking effects on oxalate, phytate, trypsin and α-amylase inhibitors of wild yam tubers of Nepal. J. Food Compos. Anal. 2006;19:524–530. doi: 10.1016/j.jfca.2004.09.010. [DOI] [Google Scholar]
- 14.Shi K., Wu X., Ma J., Zhang J., Zhou L., Wang H., Li L. Effects of Planting and Processing Modes on the Degradation of Dithianon and Pyraclostrobin in Chinese Yam (Dioscorea spp.) J. Agric. Food Chem. 2017;65:10439–10444. doi: 10.1021/acs.jafc.7b03916. [DOI] [PubMed] [Google Scholar]
- 15.Hsu Y.J., Weng C.F., Lin K.W., Lin K.C. Suppression of Allergic Reactions in Ovalbumin-Sensitized Mice by Yam Storage Proteins Dioscorins. J. Agric. Food Chem. 2013;61:11460–11467. doi: 10.1021/jf403480s. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y., Zhou C., Yao H., Liu Y., Tu R. Applications of ISSR markers in detection of genetic variation among Chinese yam (Dioscorea opposita Thunb) cultivars. Life Sci. J. 2008;5:6–12. [Google Scholar]
- 17.Coursey D.G. Yam Storage—I: A Review of Yam Storage Practices and of Information on Storage Losses. J. Stored Prod. Res. 1967;2:229–244. doi: 10.1016/0022-474X(67)90070-7. [DOI] [Google Scholar]
- 18.Ireland C.P., Schwabe W.W., Coursey D.G. The Occurrence of Batatasins in the Dioscoreaceae. Phytochemistry. 1981;20:1569–1571. doi: 10.1016/S0031-9422(00)98532-7. [DOI] [Google Scholar]
- 19.Ozo O.N., Caygill J.C., Coursey D.G. Phenolics of Five Yam (Dioscorea) Species. Phytochemistry. 1984;23:329–331. doi: 10.1016/S0031-9422(00)80327-1. [DOI] [Google Scholar]
- 20.Takasugi M., Kawashima S., Monde K., Katsui N., Masamune T., Shirata A. Antifungal Compounds from Dioscorea batatas Inoculated with Pseudomonas cichorii. Phytochemistry. 1987;26:371–375. doi: 10.1016/S0031-9422(00)81417-X. [DOI] [Google Scholar]
- 21.Sautour M., Mitaine-Offer A.C., Miyamoto T., Wagner H., Lacaille-Dubois M.A. A New Phenanthrene Glycoside and Other Constituents from Dioscorea opposita. Chem. Pharm. Bull. 2004;52:1235–1237. doi: 10.1002/chin.200517207. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T., Hasegawa K., Yamaguchi H., Saito M., Ishimoto S. Structure and Synthesis of Batatasins, Dormancy-Inducing Substances of Yam Bulbils. Phytochemistry. 1974;13:2849–2852. doi: 10.1016/0031-9422(74)80255-4. [DOI] [Google Scholar]
- 23.Zhou Y., Su Y.M., Zeng Z.H., Huang X., Zhou P. To Analyse Compatible Mechanism and Clinical Application between Dioscorea opposita and Ophiopogon japonicus. J. Basic Chin. Med. 2012;18:246–247. doi: 10.13422/j.cnki.syfjx.2012.11.075. [DOI] [Google Scholar]
- 24.Li Y., Ji S., Xu T., Zhong Y., Xu M., Liu Y., Li M., Fan B., Wang F., Xiao J., et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023;134:29–40. doi: 10.1016/j.tifs.2023.01.021. [DOI] [Google Scholar]
- 25.Liu X., Bai B. Research on the Difference of Steroidal Constituents from Dioscorea opposita and Related Plants of the Genus Dioscorea L. Henan Univ. Nat. Sci. 2012;42:548–553. doi: 10.15991/j.cnki.411100.2012.05.008. [DOI] [Google Scholar]
- 26.Nagai T., Nagashima T., Suzuki N. Purification and Partial Characterization of Major Viscous Protein from Yam (Dioscorea opposita thunb.) Tuber Mucilage tororo. Int. J. Food Prop. 2007;10:515–526. doi: 10.1080/10942910600923296. [DOI] [Google Scholar]
- 27.Zhou H., Wang J., Fang X., Sun Y., Dou X. Physicochemical properties of new starches isolated from Dioscorea opposita Thunb. bulbils. Starch. 2012;64:290–296. doi: 10.1002/star.201100117. [DOI] [Google Scholar]
- 28.Harvey P.J., Boulter D. Isolation and Characterization of the Storage Protein of Yam Tubers (Dioscorea rotundata) Phytochemistry. 1983;22:1687–1693. doi: 10.1016/S0031-9422(00)80252-6. [DOI] [Google Scholar]
- 29.Hou W.C., Lee M.H., Chen H.J., Liang W.L., Han C.H., Liu Y.W., Lin Y.H. Antioxidant Activities of Dioscorin, the Storage Protein of Yam (Dioscorea batatas Decne.) Tuber. J. Agric. Food Chem. 2001;49:4956–4960. doi: 10.1021/jf010606m. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q., Gao W., Li X., Xia Y., Wang H., Wu S., Huang L., Liu C., Xiao P. Characterizations of starches isolated from five different Dioscorea L. species. Food Hydrocoll. 2012;29:35–41. doi: 10.1016/j.foodhyd.2012.01.011. [DOI] [Google Scholar]
- 31.Zou J., Xu M., Wen L., Yang B. Structure and physiochemical properties of native starch and resistant starch in Chinese yam (Dioscorea opposita Thunb.) Carbohydr. Polym. 2020;237:116188. doi: 10.1016/j.carbpol.2020.116188. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura N., Tanabe H., Yamamoto T., Fukushima M. Raw Chinese Yam (Dioscorea opposita) Promotes Cecal Fermentation and Reduces Plasma Non-HDL Cholesterol Concentration in Rats. J. Nutr. Sci. Vitaminol. 2011;57:340–347. doi: 10.3177/jnsv.57.340. [DOI] [PubMed] [Google Scholar]
- 33.Hironaka K., Takada K., Ishibashi K. Chemical Composition of Mucilage of Chinese Yam Dioscorea opposita Thunb. cv. Nagaimo. Nippon Shokuhin Kogyo Gakkaishi. 1990;37:48–51. doi: 10.3136/nskkk1962.37.48. [DOI] [Google Scholar]
- 34.Choi E.M., Koo S.J., Hwang J.K. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas) J. Ethnopharmacol. 2004;91:1–6. doi: 10.1016/j.jep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Ma F., Wang R., Li X., Kang W., Bell A.E., Zhao D., Liu X., Chen W. Physical properties of mucilage polysaccharides from Dioscorea opposita Thunb. Food Chem. 2020;311:126039. doi: 10.1016/j.foodchem.2019.126039. [DOI] [PubMed] [Google Scholar]
- 36.Ma F., Wang D., Zhang Y., Li M., Qing W., Tikkanen-Kaukanen C., Liu X., Bell A.E. Characterisation of the mucilage polysaccharides from Dioscorea opposita Thunb. with enzymatic hydrolysis. Food Chem. 2018;245:13–21. doi: 10.1016/j.foodchem.2017.10.080. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Ren Z., Wang R., Liu L., Zhang J., Ma F., Khan M.Z.H., Zhao D., Liu X. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021;349:129208. doi: 10.1080/09168451.2014.912119. [DOI] [PubMed] [Google Scholar]
- 38.Ren Z., Li X., Ma F., Zhang Y., Hu W., Khan M.Z.H., Liu X. Oil-in-water emulsions prepared using high-pressure homogenisation with Dioscorea opposita mucilage and food-grade polysaccharides: Guar gum, xanthan gum, and pectin. LWT—Food Sci. Technol. 2022;162:113468. doi: 10.1016/j.lwt.2022.113468. [DOI] [Google Scholar]
- 39.Ma F., Zhang Y., Wen Y., Yao Y., Zhu J., Liu X., Bell A., Tikkanen-Kaukanen C. Emulsification properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017;221:919–925. doi: 10.1016/j.foodchem.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 40.Ju Y., Xue Y., Huang J., Zhai Q., Wang X. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014;66:81–85. doi: 10.1016/j.ijbiomac.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L., Wang M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017;95:675–681. doi: 10.1016/j.ijbiomac.2016.11.096. [DOI] [PubMed] [Google Scholar]
- 42.Ma F., Wang R., Zhang Y., Bai J., Fang H., Ma W., Liu W., Li Q., Liu X. Polysaccharides from Dioscorea opposita Thunb.: Isolation, structural characterization, and anti-inflammatory and anti-tumor effects against Hepatocellular carcinoma. Chem. Biol. Technol. Agric. 2023;10:43. doi: 10.1186/s40538-023-00421-8. [DOI] [Google Scholar]
- 43.Wang R., Liu W., Liu L., Ma F., Li Q., Zhao P., Ma W., Cen J., Liu X. Characterization, in vitro digestibility, antioxidant activity and intestinal peristalsis in zebrafish of Dioscorea opposita polysaccharides. Int. J. Biol. Macromol. 2023;250:126155. doi: 10.1016/j.ijbiomac.2023.126155. [DOI] [PubMed] [Google Scholar]
- 44.Chen X.T., Lu J., Li X., Wang Y., Miao J., Mao X.H., Zhao C.C. Effect of blanching and drying temperatures on starch-related physicochemical properties, bioactive components and antioxidant activities of yam flours. LWT—Food Sci. Technol. 2017;82:303–310. doi: 10.1016/j.lwt.2017.04.058. [DOI] [Google Scholar]
- 45.Lu Y.L., Chia C.Y., Liu Y.W., Hou W.C. Biological Activities and Applications of Dioscorins, the Major Tuber Storage Proteins of Yam. J. Tradit. Complement. Med. 2012;2:41–46. doi: 10.1016/s2225-4110(16)30069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jheng Y.J., Tsai W.Y., Chen K.H., Lin K.W., Chyan C.L., Yang C.C., Lin K.C. Recombinant dioscorins of the yam storage protein expressed in Escherichia coli exhibit antioxidant and immunomodulatory activities. Protein Expr. Purif. 2012;85:77–85. doi: 10.1016/j.pep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Xue Y.L., Miyakawa T., Sawano Y., Tanoura M. Cloning of genes and enzymatic characterizations of novel dioscorin isoforms from Dioscorea japonica. Plant Sci. 2012;183:14–19. doi: 10.1016/j.plantsci.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 48.Hou W.C., Liu J.S., Chen H.J., Chen T.E., Chang C.F., Lin Y.H. Dioscorin, the Major Tuber Storage Proteins of Yam (Dioscorea batatas Decne.), with Carbonic Anhydrase and Trypsin Inhibitor Activities. J. Agric. Food Chem. 1999;47:2168–2172. doi: 10.1021/jf980738o. [DOI] [PubMed] [Google Scholar]
- 49.Hou W.C., Chen H.J., Lin Y.H. Dioscorins from different Dioscorea species all exhibit both carbonic anhydrase and trypsin inhibitor activities. Bot. Bull. Acad. Sin. 2000;41:191–196. [Google Scholar]
- 50.Hou W.C., Chen H.J., Lin Y.H. Dioscorins, the major tuber storage proteins of yam (Dioscorea batatas Decne.), with dehydroascorbate reductase and monodehydroascorbate reductase activities. Plant Sci. 1999;149:151–156. doi: 10.1016/S0168-9452(99)00152-1. [DOI] [Google Scholar]
- 51.Liu Y.W., Shang H.F., Wang C.K., Hsu F.L., Hou W.C. Immunomodulatory activity of dioscorin, the storage protein of yam (Dioscorea alata cv. Tainong No. 1) tuber. Food Chem. Toxicol. 2007;45:2312–2318. doi: 10.1016/j.fct.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Chan Y.S., Ng T.B. A Lectin with Highly Potent Inhibitory Activity toward Breast Cancer Cells from Edible Tubers of Dioscorea opposita cv. Nagaimo. PLoS ONE. 2013;8:e54212. doi: 10.1371/journal.pone.0054212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohizumi Y., Gaidamashvili M., Ohwada S., Matsuda K., Kominami J., Nakamura-Tsuruta S., Hirabayashi J., Naganuma T., Ogawa T., Muramoto K. Mannose-Binding Lectin from Yam (Dioscorea batatas) Tubers with Insecticidal Properties against Helicoverpa armigera (Lepidoptera: Noctuidae) J. Agric. Food Chem. 2009;57:2896–2902. doi: 10.1021/jf8040269. [DOI] [PubMed] [Google Scholar]
- 54.Yang M.H., Chin Y.W., Yoon K.D., Kim J.W. Phenolic compounds with pancreatic lipase inhibitory activity from Korean yam (Dioscorea opposita) J. Enzyme Inhib. Med. Chem. 2013;29:1–6. doi: 10.3109/14756366.2012.742517. [DOI] [PubMed] [Google Scholar]
- 55.Jin M.H., Lu Y., Yang J.H., Jo T.H., Park Y.I., Lee C.K., Park S.J., Son K.H., Chang H.W. Anti-inflammatory Activity of 6-Hydroxy-2,7-dimethoxy-1,4-henanthraquinone from Tuberous Roots of Yam (Dioscorea batatas) through Inhibition of Prostaglandin D2 and Leukotriene C4 Production in Mouse Bone Marrow-derived Mast Cells. Arch. Pharm. Res. 2011;34:1495–1501. doi: 10.1007/s12272-011-0911-8. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez-Garza S.L., Laveriano-Santos E.P., Marhuenda-Munoz M., Storniolo C.E., Tresserra-Rimbau A., Vallverdu-Queralt A., Lamuela-Raventos R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients. 2018;10:1892. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shankar S., Singh G., Srivastava R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 58.Liao M.T., Wu C.C., Wu S.F.V., Lee M.C., Hu W.C., Tsai K.W., Yang C.H., Lu C.L., Chiu S.K., Lu K.C. Resveratrol as an Adjunctive Therapy for Excessive Oxidation Stress in Aging COVID-19 Patients. Antioxidants. 2021;10:1440. doi: 10.3390/antiox10091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Y.C., Ferng L.H.A., Huang P.Y. Quantitative analysis of allantoin and allantoic acid in yam tuber, mucilage, skin and bulbil of the Dioscorea species. Food Chem. 2006;94:541–549. doi: 10.1016/j.foodchem.2004.12.006. [DOI] [Google Scholar]
- 60.Mollica J.Q., Cara D.C., D’Auriol M., Oliveira V.B., Cesar I.C., Brandao M.G. Anti-inflammatory activity of American yam Dioscorea trifida L.f. in food allergy induced by ovalbumin in mice. J. Funct. Foods. 2013;5:1975–1984. doi: 10.1016/j.jff.2013.09.020. [DOI] [Google Scholar]
- 61.Zhang L., Bai B., Liu X.H., Wang Y., Li M.J., Zhao D.B. α-Glucosidase inhibitors from Chinese Yam (Dioscorea opposita Thunb.) Food Chem. 2011;126:203–206. doi: 10.1016/j.foodchem.2010.10.100. [DOI] [Google Scholar]
- 62.Li H., Chen K., Wang Y., Wang Y., Liu X. HPLC determination of trans-N-p-coumaroyltyramine in Dioscorea opposita Thunb. Chem. Res. 2018;29:241–244. doi: 10.14002/j.hxya.2018.03.003. [DOI] [Google Scholar]
- 63.Kim J.K., Heo H., Park S., Kim H., Oh J.J., Sohn E., Jung S., Lee K. Characterization of Phenethyl Cinnamide Compounds from Hemp Seed and Determination of their Melanogenesis Inhibitory Activity. ACS Omega. 2021;6:31945–31954. doi: 10.1021/acsomega.1c04727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rea J., Garcia-Gimenez M.D., Santiago M., De La Peurta R., Fernandez-Arche M.A. Hydroxycinnamic acid derivatives isolated from hempseed and their effects on central nervous system enzymes. Int. J. Food Sci. Nutr. 2021;72:184–195. doi: 10.1080/09637486.2020.1793305. [DOI] [PubMed] [Google Scholar]
- 65.Nchiozem-Ngnitedem V., Omosa L., Bedane K., Derese S., Brieger L., Strohmann C., Spiteller M. Anti-inflammatory steroidal sapogenins and a conjugated chalcon-stilbene from Dracaena usambarensis Engl. Fitoterapia. 2020;146:104717. doi: 10.1016/j.fitote.2020.104717. [DOI] [PubMed] [Google Scholar]
- 66.San H., Chaowasku T., Mekboonsonglarp W., Rodsiri R., Sritularak B., Buraphaka H., Putalun W., Likhitwitayawuid K. Constituents of Huberantha jenkinsii and Their Biological Activities. Molecules. 2020;25:3533. doi: 10.3390/molecules25153533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Y.H., Kim D.W., Curtis-Long M.J., Park C., Son M., Kim J.Y., Yuk H.J., Lee K.W., Park K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem. 2016;114:201–208. doi: 10.1016/j.ejmech.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Shi H., Li M., Zhao Y., Liu X. Determination of polyphenolics in Dioscorea opposita Thunb. and D. alata L. J. Henan Univ. Nat. Sci. 2005;35:41–44. doi: 10.15991/j.cnki.411100.2005.04.010. [DOI] [Google Scholar]
- 69.Belin P., Moutiez M., Lautru S. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat. Prod. Rep. 2012;29:961–979. doi: 10.1039/c2np20010d. [DOI] [PubMed] [Google Scholar]
- 70.Hsu C.L., Chen W., Weng Y.M., Tseng C.Y. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003;83:85–92. doi: 10.1016/S0308-8146(03)00053-0. [DOI] [Google Scholar]
- 71.Chen Y.T., Kao W.T., Lin K.W. Effects of pH on the total phenolic compound, antioxidative ability and the stability of dioscorin of various yam cultivars. Food Chem. 2008;107:250–257. doi: 10.1016/j.foodchem.2007.08.017. [DOI] [Google Scholar]
- 72.Lin J.Y., Lu S., Liou Y.L., Liou H.L. Antioxidant and hypolipidaemic effects of a novel yam-boxthorn noodle in an in vivo murine model. Food Chem. 2006;94:377–384. doi: 10.1016/j.foodchem.2004.11.028. [DOI] [Google Scholar]
- 73.Kim S.H., Lee S.Y., Palanivel G., Kwak H.S. Effect of Dioscorea opposita Thunb. (yam) supplementation on physicochemical and sensory characteristics of yogurt. J. Dairy Sci. 2011;94:1705–1712. doi: 10.3168/jds.2010-3918. [DOI] [PubMed] [Google Scholar]
- 74.Hur S.J., Lee S.Y., Kim Y.C., Choi I., Kim G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- 75.Lee S.Y., Ganesan P., Ahn J., Kwak H.S. Lactobacillus acidophilus Fermented Yam (Dioscorea opposita thunb.) and Its Preventative Effects on Gastric Lesion. Food Sci. Biotechnol. 2011;20:927–932. doi: 10.1007/s10068-011-0128-0. [DOI] [Google Scholar]
- 76.Nagai T., Suzuki N., Kai N., Tanoue Y. Functional properties of autolysate and enzymatic hydrolysates from yam tsukuneimo (Dioscorea opposita Thunb.) tuber mucilage tororo: Antioxidative activity and antihypertensive activity. J. Food Sci. Technol. 2012;51:3838–3845. doi: 10.1007/s13197-012-0910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagai T., Nagashima T. Functional Properties of Dioscorin, a Soluble Viscous Protein from Japanese Yam (Dioscorea opposita Thunb.) Tuber Mucilage Tororo. Z. Naturforsch C. J. Biosci. 2006;61:792–798. doi: 10.1515/znc-2006-11-1204. [DOI] [PubMed] [Google Scholar]
- 78.Chen P.Y., Tu Y.X., Wu C.T., Jong T.T., Chang C.M.J. Continuous Hot Pressurized Solvent Extraction of 1,1-Diphenyl-2-picrylhydrazyl Free Radical Scavenging Compounds from Taiwan Yams (Dioscorea alata) J. Agric. Food Chem. 2004;52:1945–1949. doi: 10.1021/jf0351826. [DOI] [PubMed] [Google Scholar]
- 79.Song X., Hu H., Zhang B. Drying characteristics of Chinese Yam (Dioscorea opposita Thunb.) by far-infrared radiation and heat pump. J. Saudi Soc. Agric. Sci. 2018;17:290–296. doi: 10.1016/j.jssas.2016.05.008. [DOI] [Google Scholar]
- 80.Chang S.J., Lee Y.C., Liu S.Y., Chang T.W. Chinese Yam (Dioscorea alata cv. Tainung No. 2) Feeding Exhibited Antioxidative Effects in Hyperhomocysteinemia Rats. J. Agric. Food Chem. 2004;52:1720–1725. doi: 10.1021/jf0345954. [DOI] [PubMed] [Google Scholar]
- 81.Fu Y.C., Huang P.Y., Chu C.J. Use of continuous bubble separation process for separating and recovering starch and mucilage from yam (Dioscorea pseudojaponica Yamamoto) LWT—Food Sci. Technol. 2005;38:735–744. doi: 10.1016/j.lwt.2004.09.008. [DOI] [Google Scholar]
- 82.Huang H., Jiang Q., Chen Y., Li X., Mao X., Chen X., Huang L., Gao W. Preparation, physico-chemical characterization and biological activities of two modified starches from yam (Dioscorea opposita Thunb.) Food Hydrocoll. 2016;55:244–253. doi: 10.1016/j.foodhyd.2015.11.016. [DOI] [Google Scholar]
- 83.Han C.H., Lin Y.F., Lin Y.S., Lee T.L., Huang W.J., Lin S.Y., Hou W.C. Effects of yam tuber protein, dioscorin, on attenuating oxidative status and learning dysfunction in D-galactose-induced BALB/c mice. Food Chem. Toxicol. 2014;65:356–363. doi: 10.1016/j.fct.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Son I.S., Lee J.S., Lee J.Y., Kwon C.S. Antioxidant and Anti-inflammatory Effects of Yam (Dioscorea batatas Decne.) on Azoxymethane-induced Colonic Aberrant Crypt Foci in F344 Rats. Prev. Nutr. Food Sci. 2014;19:82–86. doi: 10.3746/pnf.2014.19.2.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagai T., Suzuki N., Tanoue Y., Kai N., Nagashima T. Antioxidant and antihypertensive activities of autolysate and enzymatic hydrolysates from yam (Dioscorea opposita Thunb.) ichyoimo tubers. J. Food Agric. Environ. 2007;5:64–68. doi: 10.3168/jds.2006-793. [DOI] [PubMed] [Google Scholar]
- 86.Niu C.S., Chen W., Wu H.T., Cheng K.C., Wen Y.J., Lin K.C., Cheng J.T. Decrease of Plasma Glucose by Allantoin, an Active Principle of Yam (Dioscorea spp.), in Streptozotocin-induced Diabetic Rats. J. Agric. Food Chem. 2010;58:12031–12035. doi: 10.1021/jf103234d. [DOI] [PubMed] [Google Scholar]
- 87.Tsai C.C., Chen L.J., Niu H.S., Chung K.M., Cheng J.T., Lin K.C. Allantoin activates imidazoline I-3 receptors to enhance insulin secretion in pancreatic β-cells. Nutr. Metab. 2014;11:41. doi: 10.1186/1743-7075-11-41. [DOI] [Google Scholar]
- 88.Choi K.W., Um S.H., Kwak J.H., Park H.J., Kim K.H., Moon E.Y., Kwon S.T., Pyo S.P. Suppression of adhesion molecule expression by phenanthrene-containing extract of bulbils of Chinese Yam in vascular smooth muscle cells through inhibition of MAPK, Akt, and NF-κB. Food Chem. Toxicol. 2012;50:2792–2804. doi: 10.1016/j.fct.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Lu Y., Jin M.H., Park S.J., Son K.H., Son J.K., Chang H.W. Batatasin I, a Naturally Occurring Phenanthrene Derivative, Isolated from Tuberous Roots of Dioscorea batatas Suppresses Eicosanoids Generation and Degranulation in Bone Marrow Derived-Mast Cells. Biol. Pharm. Bull. 2011;34:1021–1025. doi: 10.1248/bpb.34.1021. [DOI] [PubMed] [Google Scholar]
- 90.Choi E.J., Ryu Y., Tang Y., Kim B., Lee W., Debnath T., Fan M., Kim E.K., Lee H.S. Effect of cinnamamides on atopic dermatitis through regulation of IL-4 in CD4+ cells. Enzyme Inhib. Med. Chem. 2019;34:410–419. doi: 10.1080/14756366.2019.1569647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hao L.X., Zhao X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016;27:667–677. doi: 10.1080/09540105.2016.1148666. [DOI] [Google Scholar]
- 92.Li M., Chen L.X., Chen S.R., Deng Y., Zhao J., Wang Y., Li S.P. Non-starch polysaccharide from Chinese yam activated RAW 264.7 macrophages through the Toll-like receptor 4 (TLR4)-NF-κB signaling pathway. J. Funct. Foods. 2017;37:491–500. doi: 10.1016/j.jff.2017.08.025. [DOI] [Google Scholar]
- 93.Zhao G., Kan J., Li Z., Chen Z. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr. Polym. 2005;61:125–131. doi: 10.1016/j.carbpol.2005.04.020. [DOI] [Google Scholar]
- 94.Luo L., Qin T., Huang Y., Zheng S., Bo R., Liu Z., Xing J., Hu Y., Liu J., Wang D. Exploring the immunopotentiation of Chinese yam polysaccharide poly(Lactic-co-glycolic acid) nanoparticles in an ovalbumin vaccine formulation in vivo. Drug Deliv. 2017;24:1099–1111. doi: 10.1080/10717544.2017.1359861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo J., Tan L., Kong L. Multiple levels of health benefits from resistant starch. J. Agric. Food Res. 2022;10:100380. doi: 10.1016/j.jafr.2022.100380. [DOI] [Google Scholar]
- 96.Yang T.T., Chiu N.H., Chung H.H., Hsu C.T., Lee W.J., Cheng J.T. Stimulatory Effect of Allantoin on Imidazoline I1 Receptors in Animal and Cell Line. Horm. Metab. Res. 2012;44:879–884. doi: 10.1055/s-0032-1312624. [DOI] [PubMed] [Google Scholar]
- 97.Chen M.F., Tsai J.T., Chen L.J., Wu T.P., Yang J.J., Yin L.T., Yang Y.L., Chiang T.A., Lu H.L., Wu M.C. Antihypertensive Action of Allantoin in Animals. BioMed. Res. Int. 2014;2014:690135. doi: 10.1155/2014/690135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amat N., Amat R., Abdureyim S., Hoxur P., Osman Z., Mamut D., Kijjoa A. Aqueous extract of Dioscorea opposita Thunb. normalizes the hypertension in 2K1C hypertensive rats. BMC Complement. Altern. Med. 2014;14:36. doi: 10.1186/1472-6882-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Behall K.M., Scholfield D.J., Yuhaniak I., Canary J. Diets containing high amylose vs amylopectin starch: Effects on metabolic variables in human subjects. Am. J. Clin. Nutr. 1989;49:337–344. doi: 10.1093/ajcn/49.2.337. [DOI] [PubMed] [Google Scholar]
- 100.Lee H.C., Cheng W.Y., Huang B.E.G., Hsu Y.H., Huang S.Y. Anti-inflammatory and hypoglycemic efficacy of Poria cocos and Dioscorea opposita in prediabetes mellitus rats. RSC Adv. 2014;4:55649–55657. doi: 10.1039/C4RA10539G. [DOI] [Google Scholar]
- 101.Yu J., Zhou M.N., Lu Q.J. Effects of Yam Polysaccharides on P-Selectin Expression and Macrophage Infiltration in Diabetic Nephropathy Model Rats. Clin. Exp. Med. Sci. 2015;3:11–21. doi: 10.12988/cems.2015.521. [DOI] [Google Scholar]
- 102.Liu M., Yin H., Liu G., Dong J.J., Qian Z.H., Mina J.L. Xanthohumol, a Prenylated Chalcone from Beer Hops, Acts as an α-Glucosidase Inhibitor in vitro. J. Agric. Food Chem. 2014;62:5548–5554. doi: 10.1021/jf500426z. [DOI] [PubMed] [Google Scholar]
- 103.Lee B.H., Hsu W.H., Pan T.M. Inhibitory Effects of Dioscorea Polysaccharide on TNF-α-induced Insulin Resistance in Mouse FL83B Cells. J. Agric. Food Chem. 2011;59:5279–5285. doi: 10.1021/jf200651c. [DOI] [PubMed] [Google Scholar]
- 104.Yang W., Wang Y., Li X., Yu P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015;117:1021–1027. doi: 10.1016/j.carbpol.2014.09.082. [DOI] [PubMed] [Google Scholar]
- 105.Yeh Y.H., Hsieh Y.L., Lee Y.T. Effects of Yam Peel Extract against Carbon Tetrachloride-Induced Hepatotoxicity in Rats. J. Agric. Food Chem. 2013;61:7387–7396. doi: 10.1021/jf401864y. [DOI] [PubMed] [Google Scholar]
- 106.Lin P.L., Lin K.W., Weng C.F., Lin K.C. Yam Storage Protein Dioscorins from Dioscorea alata and Dioscorea japonica Exhibit Immunomodulatory Activities in Mice. J. Agric. Food Chem. 2009;57:4606–4613. doi: 10.1021/jf8038499. [DOI] [PubMed] [Google Scholar]
- 107.Song Y., Mei T., Liu Y., Kong S., Zhang J., Xie M. Metabolites Identification of Chemical Constituents from the Eggplant (Solanum melongene L.) Calyx in Rats by UPLC/ESI/qTOF-MS Analysis and Their Cytotoxic Activities. Front. Pharmacol. 2021;12:655008. doi: 10.3389/fphar.2021.655008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masi M., Koirala M., Delicato A., Leece D., Merindol N., Ka S., Seck M., Tuzi A., Desgagne-Penix I., Calabro V., et al. Isolation and Biological Characterization of Homoisoflavanoids and the Alkylamide N-p-Coumaroyltyramine from Crinum biflorum Rottb., an Amaryllidaceae Species Collected in Senegal. Biomolecules. 2021;11:1298. doi: 10.3390/biom11091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gu C., Wang H., Piao C. Experience in the Treatment of Puffiness with Coix Seed, Poria, and Common Yam Rhizome—Three Prescription by Professor TONG, Xiaolin. Jilin J. Chin. Med. 2020;40:712–714. [Google Scholar]
- 110.Zhou Y., Shu C.Q., Tang X., Zeng Z.H., Yang J.X. To Analysis the Significance of the Clinical Compatability Application between Shanyao and Huangqi. J. Basic Chin. Med. 2017;23:1332–1333. doi: 10.19945/j.cnki.issn.1006-3250.2017.09.048. [DOI] [Google Scholar]
- 111.Zhou Y., Shu C.Q., Tang X., Zeng Z.H., Zhou P. Analysis and Clinical Compatibility Application between Shanyao and Xingren. J. Basic Chin. Med. 2017;23:1003–1004. doi: 10.19945/j.cnki.issn.1006-3250.2017.07.040. [DOI] [Google Scholar]
- 112.Zhao Y.L., Song H.R., Fei J.X., Liang Y., Zhang B.H., Liu Q.P., Wang J., Hu P. The effects of Chinese Yam-Epidemium mixture on respiratory function and quality of life in patients with chronic obstructive pulmonary disease. J. Tradit. Chin. Med. 2012;32:203–207. doi: 10.1016/s0254-6272(13)60012-6. [DOI] [PubMed] [Google Scholar]
- 113.Ehrman T.M., Barlow D.J., Hylands P.J. In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorg. Med. Chem. 2010;18:2204–2218. doi: 10.1016/j.bmc.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 114.Wen G.Q., Li J., Liu X.H., Zhang Y.S., Wen S.S. Extraction of total DNA and optimization of the RAPD reaction system in Dioscorea opposita Thunb. Genet. Mol. Res. 2014;13:1339–1347. doi: 10.4238/2014.February.28.6. [DOI] [PubMed] [Google Scholar]
- 115.Wu Z.G., Li X.X., Lin X.C., Jiang W., Tao Z.M., Mantri N., Fan C.Y., Bao X.Q. Genetic diversity analysis of yams (Dioscorea spp.) cultivated in China using ISSR and SRAP markers. Genet. Resour. Crop Evol. 2014;61:639–650. doi: 10.1007/s10722-013-0065-2. [DOI] [Google Scholar]
- 116.Li M., Li J., Liu W., Liu L., Lu J., Niu J., Liu X., Yang Q. A protocol for in vitro production of microtubers in Chinese yam (Dioscorea opposita) Biosci. Biotechnol. Biochem. 2014;78:1005–1009. doi: 10.1080/09168451.2014.912119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.