Abstract
This study investigates the Salmonella effector protein SopA. We show that in Salmonella enterica serovar Dublin-infected cells, SopA1-347 fused to two carboxy-terminal hemagglutinin tags partially colocalized with mitochondria. Transfection of eukaryotic cells with a panel of constructs encoding truncated versions of SopA identified that amino acids 100 to 347 were sufficient to target SopA to the mitochondria.
Type III secretion systems (TTSS) are used by many gram-negative bacteria to secrete and translocate a variety of proteins (effectors) from the bacteria directly into the host cell cytosol. Inside the host cell, these proteins elicit a range of effects which aid bacterial invasion and survival. Salmonella possesses two TTSS, TTSS-1 and TTSS-2, and much effort has been invested in characterizing effector proteins secreted by TTSS. Many Salmonella TTSS-1 effector proteins have been characterized and contribute to invasion of epithelial cells and enteropathogenic responses in the early stages of Salmonella infection. In particular, SopE and SopE2 are guanine nucleotide exchange factors that aid bacterial internalization by inducing actin cytoskeleton rearrangements and membrane ruffling in host cells (1, 8, 9, 24, 27). SopB, an inositol phosphate phosphatase (20), acts on various inositol phosphate signaling pathways in host cells and disrupts ion secretion in the host intestinal epithelium, culminating in fluid secretion into the intestinal lumen (7, 28). An additional role for SopB in providing Salmonella with extra time for replication inside host cells has also been suggested (23).
However, in contrast to these effectors, relatively little is known about SopA. Previous work from our laboratory demonstrated a role for SopA in the Salmonella-induced movement of polymorphonuclear leukocytes (PMNs) across the intestinal epithelium. Insertion and deletion mutations in the sopA gene of Salmonella enterica serovar Dublin resulted in strains with a significantly reduced ability to induce fluid secretion and PMN influx compared to wild-type S. enterica serovar Dublin in the ligated loop model system (26). In addition, in the in vitro T84 epithelial cell model system, the mutation of sopA abrogated the ability of Salmonella to induce the migration of PMNs across the T84 cell monolayer (26). Further work on the role of SopA in inducing enteropathogenic responses showed that SopA acts in concert with other TTSS-1-secreted effector proteins (28). In the bovine ligated loop model, an S. enterica serovar Typhimurium sipA sopABDE2 mutant caused the same low level of fluid accumulation as a sipB mutant, which is unable to secrete any TTSS-1 effector proteins (28). In addition, following oral infection of calves, the sipA sopABDE2 mutant induced only mild diarrhea, indicating that a complement of effectors was required for full enteropathogenic responses (28).
While these data for SopA suggested that the protein was involved in PMN movement and enteropathogenesis, little is known about the target of SopA in host cells and its mechanism of action. SopA has 29% amino acid identity to two proteins from enterohemorrhagic Escherichia coli O157:H7, a putative secreted effector protein (GenBank accession number H90823) and a protein encoded by prophage CP-933N (GenBank accession number F85682); both are proteins of unknown function. We therefore investigated the localization of SopA in host cells to elucidate its mechanism of action.
Strains.
The strains and plasmids used in this study are listed in Table 1. The primers used are listed in Table 2.
TABLE 1.
Bacterial strains and plasmids used throughout this studya
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | recA | Invitrogen |
| XL1-Blue | recA Tetr | 3 |
| S. enterica serovar Dublin | ||
| 2229 | Wild-type virulent field isolate | IAH |
| 2229 SopA1-3472xHA | 2229 carrying pB/S-sopEprom.sopA1-3472xHA | This study |
| Plasmids | ||
| pRK5myc | Ampr eukaryotic expression vector encoding myc tag | 14 |
| pBluescript SK(+/−) | Ampr | Stratagene |
| pRK5myc-sopA1-742 | sopA in pRK5myc | This study |
| pRK5myc-sopA1-347 | Sequence encoding aa 1-347 of SopA in pRK5myc | This study |
| pRK5myc-sopA348-742 | Sequence encoding aa 348-782 of SopA in pRK5myc | This study |
| pRK5myc-sopA1-50 | Sequence encoding aa 1-50 of SopA in pRK5myc | This study |
| pRK5myc-sopA1-200 | Sequence encoding aa 1-200 of SopA in pRK5myc | This study |
| pRK5myc-sopA100-347 | Sequence encoding aa 100-347 of SopA in pRK5myc | This study |
| pRK5myc-sopA200-347 | Sequence encoding aa 200-347 of SopA in pRK5myc | This study |
| pB/S-sopEprom.sopA1-3472xHA | sopE promoter region followed by sopA1-347 and sequence encoding two HA epitope tags | This study |
aa, amino acids; IAH, Institute for Animal Health.
TABLE 2.
Sequences of primers used throughout this study
| Name of primer | Sequence (5′-3′)a |
|---|---|
| SopA5 | CTCGGATCCATGAAGATATCATCAGGCGCAAT |
| SopA3 | CTCTCTAGACTACGCCCAGGACAGTGGCAGGAT |
| SopA350Xba | CTCTCTAGACTAATTTAAAAATCCATCCACAGCGTTCT |
| SopA350Bam | CTCGGATCCCATGAGCACAATAATGGTAAAAGTATT |
| SopA1-50 | CTCTCTAGACTAAAATTTTTCATTTAAAGATGTAT |
| SopA1-200 | CTCTCTAGACTATGCGTTTGAAAAATCGCGATCT |
| SopA100-347 | CTCGGATCCAAAAATATTTCAGCAGAAGAT |
| SopA200-347 | CTCGGATCCGATTTTTCAAACGCAGATTTCCGT |
| SopEprom5 | CTCTCTAGATTGCCCTGCTCCCTTGCCGCTGCA |
| SopEprom3 | CTCGGATCCGCTCCTTTTATATGTACATAACTCAT |
| SopA1-3472xHA | CTCCTCGAGTTACGCATAATCCGGCACATCATACGGATACGCATAATCCGGCACATCATACGGATAGTCGACATTTAAAAATCCATCCACAGCGTT |
Restriction enzyme sites are shown in bold.
HA-tagged SopA1-347 is secreted by Salmonella under TTSS-1-inducing conditions.
Our initial attempts to detect localization of SopA translocated by wild-type Salmonella isolates were unsuccessful. Unlike other effectors such as SopB (7) and SopE (27), translocated SopA was not detectable in host cells by conventional immunocytochemical techniques, probably due to the small amounts of SopA expressed (our unpublished observations). Detection of translocated SopA has been shown previously only by using the Cya fusion system and measurement of intracellular cyclic AMP (26). To alleviate the problem of visualizing translocated SopA, we created a construct encoding the first 347 amino acid residues of SopA fused to two carboxy-terminal hemagglutinin (HA) tags, all under the control of the sopE promoter. This plasmid was introduced into wild-type S. enterica serovar Dublin 2229, generating S. enterica serovar Dublin 2229 SopA1-3472xHA. This approach was chosen since HA epitope tags had been used successfully in studies of other Salmonella effector proteins, particularly those secreted by TTSS-2 (for example, SopD2, SseJ, SifB, and PipB2) (2, 5, 13), and since use of the sopE promoter was expected to boost expression of SopA.
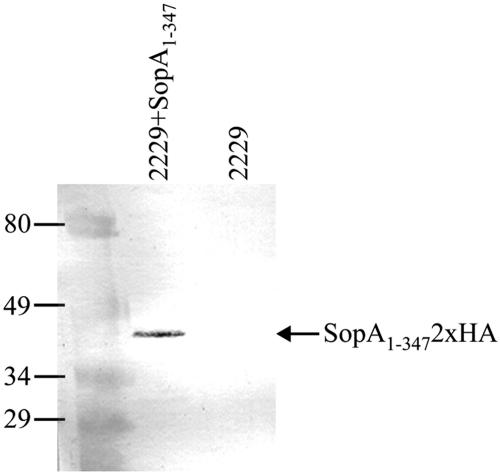
The in vitro expression and purification of TTSS-1-secreted proteins from S. enterica serovar Dublin 2229 SopA1-3472xHA and the S. enterica serovar Dublin 2229 wild type were performed using a temperature shift induction, followed by trichloroacetic acid precipitation as described previously (27), and resulting protein preparations were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). An additional band at approximately 45 kDa (the expected size for SopA1-3472xHA) was visible in the protein profile for S. enterica serovar Dublin 2229 SopA1-3472xHA compared to the secreted protein profile for the S. enterica serovar Dublin 2229 wild type (data not shown). Immunoblotting on a nitrocellulose membrane (Hybond-C Extra; Amersham Biosciences) with an anti-HA monoclonal antibody (anti-HA.11; Covance) diluted 1:1,000 confirmed that this band was HA-tagged SopA1-347 (Fig. 1).
FIG. 1.
SopA1-3472xHA is secreted by Salmonella under TTSS-1-inducing conditions. Secreted proteins were prepared from bacterial cultures of wild-type S. enterica serovar Dublin 2229 (2229) or S. enterica serovar Dublin 2229 carrying a plasmid expressing SopA1-3472xHA under the control of the sopE promoter (2229+SopA1-347). The purified proteins were separated by 12% SDS-PAGE, transferred to a nitrocellulose membrane, and probed by using anti-HA mouse monoclonal and goat anti-mouse alkaline phosphatase secondary antibodies.
HA-tagged SopA1-347 localizes to mitochondria in Salmonella-infected cells.
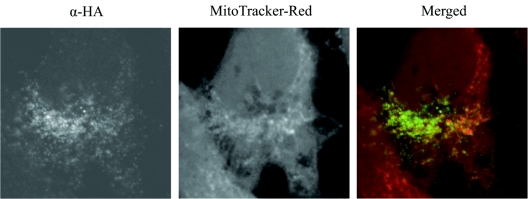
To examine the subcellular localization of SopA, 5 × 104 HeLa cells were seeded on glass coverslips in 24-well plates the day before infection. S. enterica serovar Dublin 2229 SopA1-3472xHA and S. enterica serovar Dublin 2229 were grown overnight in LB medium at 25°C with shaking. Cultures were diluted 1:10 into fresh LB medium and were incubated for 1 h at 37°C with shaking. The HeLa cells were infected at a multiplicity of infection of 20:1. At 4 or 7.5 h postinfection, the cells were fixed in 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained for SopA1-3472xHA with the anti-HA antibody diluted 1:1,000, followed by goat anti-mouse Alexa Fluor 488 (Molecular Probes) diluted 1:500. The cells were costained using organelle markers, including phalloidin-Texas Red (Molecular Probes), which stains the actin cytoskeleton (data not shown), and MitoTracker Red (Molecular Probes), used prior to the fixation step, which stains mitochondria. SopA1-3472xHA staining appeared granular and concentrated in particular areas of the cell, especially the perinuclear region (Fig. 2). We observed partial colocalization of SopA1-3472xHA with mitochondria, as indicated by overlapping of the red and green staining (Fig. 2). These data suggested that HA-tagged SopA1-347 was capable of being translocated by Salmonella into host cells, where it targeted mitochondria. Salmonella effector proteins are known to locate to distinct cellular compartments. For example, SopD2 is targeted to late endosomes/lysosomes (2), and SopB is associated with host cell membranes (15). The location of effector proteins reflects their specific actions in host cells; for example, SopB is an inositol phosphate phosphatase, and the targets of such enzymes are phospholipids, which are located mainly in cell membranes.
FIG. 2.
Bacterially delivered SopA1-3472xHA is localized to the mitochondria of infected cells. Confocal immunofluorescence analysis of HeLa cells infected with S. enterica serovar Dublin 2229 carrying a plasmid expressing SopA1-3472xHA under the control of the sopE promoter. At 7.5 h postinfection, cells were labeled with MitoTracker Red to stain mitochondria (red in merged image) and with anti-HA mouse monoclonal and goat anti-mouse secondary antibodies to detect HA-tagged SopA1-347 (green in merged image). α, anti.
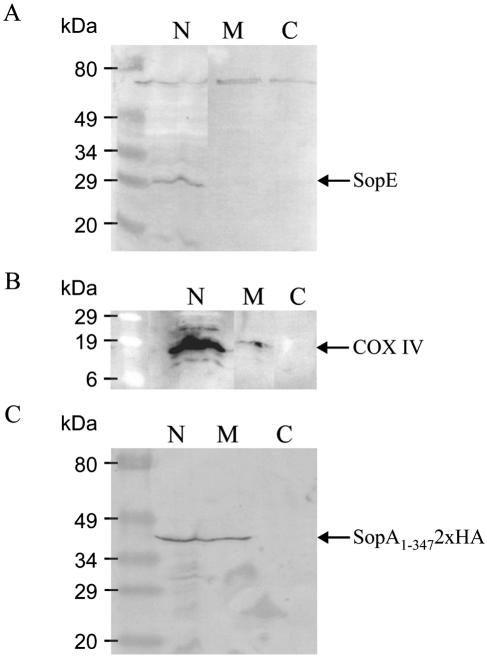
To confirm localization of SopA to mitochondria, we performed subcellular fractionation of Salmonella-infected cells. HeLa cells (8 × 106) were infected with S. enterica serovar Dublin 2229 SopA1-3472xHA or S. enterica serovar Dublin 2229 at a multiplicity of infection of approximately 25:1. Four hours postinfection, cells were harvested and incubated in 250 μl Mito buffer and then disrupted and fractionated by centrifugation as previously described (11). This process resulted in three fractions: nuclei and cell debris, mitochondria, and cytosol. The three fractions were dissolved in SDS-PAGE loading buffer, heated (95°C, 5 min), and subjected to SDS-PAGE and Western blotting on a nitrocellulose membrane (Hybond-C Extra). Probing with antibodies to proteins known to be located in specific subcellular compartments confirmed that fractionation had been successful and that the fractions were not contaminated with proteins from other fractions. For example, SopE, which was detected using anti-SopE 575AA4b monoclonal antibodies (27) at a concentration of 1 μg ml−1, was located in the fraction containing nuclei/cell debris and not in the mitochondria or cytosol fractions (Fig. 3A). This is consistent with recent data suggesting that SopE localizes to plasma membranes in infected host cells (4). Cytochrome c oxidase subunit IV (COX IV), a component of the oxidative phosphorylation system located in the inner mitochondrial membrane, was detected with a monoclonal anti-COX IV antibody (Molecular Probes) at a concentration of 2 μg ml−1 and used as a marker for mitochondria (Fig. 3B). COX IV was also present in the fraction containing nuclei/cell debris, and this is probably due to incomplete lysis and fractionation of the cells. The subcellular fractions were probed with the anti-HA antibody, and a distinct band was observed in the mitochondrial fraction (Fig. 3C), which is consistent with the immunofluorescent data showing that bacterially delivered SopA1-3472xHA localizes to the mitochondria of Salmonella-infected cells.
FIG. 3.
Subcellular fractionation of cells confirms that bacterially delivered SopA1-3472xHA localizes to the mitochondria. HeLa cells infected for 4 h with S. enterica serovar Dublin 2229 expressing SopA1-3472xHA were fractionated into nuclei and cell debris (lanes N), mitochondria (lanes M), and cytosol (lanes C). Fractions were separated by SDS-PAGE, and Western blotting was performed using anti-SopE (A), anti-COX IV (B), or anti-HA (C) monoclonal antibodies and a goat anti-mouse alkaline phosphatase conjugate secondary antibody.
Identification of the region of SopA required for mitochondrial targeting.
Having discovered that bacterially delivered SopA1-347 localized to the mitochondria of infected host cells, we sought to identify the region of SopA involved in mitochondrial targeting. Three constructs comprising an N-terminal myc epitope tag fused to part of the sopA gene were generated. Previously, we used the myc tag in the study of another Salmonella effector protein, TTSS-2-secreted SseJ (22). Three constructs were generated by PCR amplification of the sopA gene and cloning of fragments into the pRK5myc plasmid (14): pRK5myc-SopA1-742 (full length), pRK5myc-SopA1-347 (N terminus), and pRK5myc-SopA348-742 (C terminus), where the numbers refer to the amino acid residues.
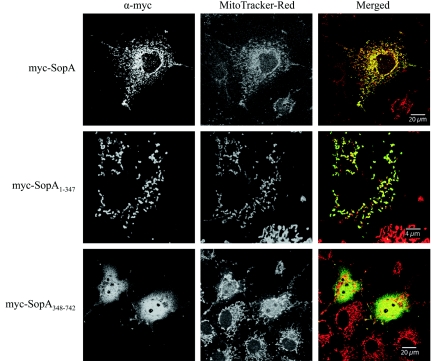
COS-7 cells (5 × 104) were transiently transfected with 0.2 μg plasmid DNA by using Lipofectamine (Invitrogen). COS-7 cells were used for transfections because the cells constitutively express the simian virus 40 large T antigen which binds to the simian virus 40 origin of replication in pRK5myc, allowing COS-7 DNA polymerases to carry out multiple DNA replication cycles and resulting in amplified expression of the gene of interest on the plasmid. Twenty-four hours posttransfection, the cells were incubated with MitoTracker Red, fixed in 3% paraformaldehyde, and permeabilized with 0.1% Triton X-100, and the myc-tagged protein was detected using anti-myc antibody (Invitrogen; 1:1,000), followed by goat anti-mouse Alexa Fluor 488 diluted 1:500. Cells transfected with pRK5myc-SopA1-742 showed variable staining for the myc tag throughout the cell with some overlap with mitochondrial staining (Fig. 4). Transfection with pRK5myc-SopA1-347 resulted in punctate perinuclear staining for the myc tag (Fig. 4). myc-SopA1-347 almost exclusively colocalized with mitochondria (Fig. 4). This indicated that protein produced by host cells from transfected DNA also targeted to host cell mitochondria, like bacterially delivered protein, and that SopA does not require bacterial cofactors for mitochondrial targeting. In contrast, cells transfected with pRK5myc-SopA348-742 showed a diffuse pattern of staining throughout the cytoplasm and nuclei of the cells with no apparent mitochondrial colocalization (Fig. 4). This suggested that the mitochondrial targeting signal was located in the N-terminal region of SopA.
FIG. 4.
The N-terminal half of SopA mediates targeting to mitochondria. Confocal immunofluorescence analysis of COS-7 cells transiently transfected with plasmids expressing myc-SopA1-742, myc-SopA1-347, or myc-SopA348-742. Twenty-four hours posttransfection, cells were labeled with MitoTracker Red to stain mitochondria (red in merged images) and with anti-myc (α-myc) mouse monoclonal and goat anti-mouse secondary antibodies to detect myc-tagged proteins (green in merged images).
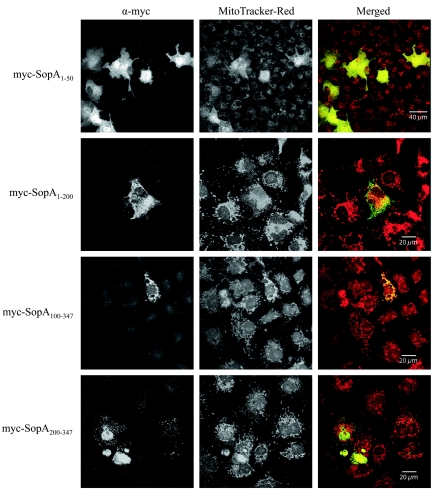
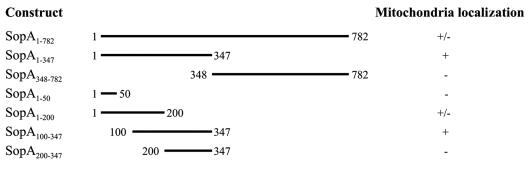
To identify the region of SopA1-347 responsible for mitochondrial targeting, we generated a panel of four additional pRK5myc constructs which encoded the following truncations of SopA1-347: SopA1-50, SopA1-200, SopA100-347, and SopA200-347. COS-7 cells were transiently transfected with these plasmids, and the myc-tagged truncated proteins and mitochondria were labeled using fluorescent antibodies as described above. Only one of the truncated SopA molecules still localized to the mitochondria and showed strong overlap by MitoTracker Red staining. This was myc-SopA100-347 (Fig. 5). myc-SopA1-50, myc-SopA1-200, and myc-SopA200-347 did not appear to localize significantly to mitochondria (Fig. 5). Thus, we concluded that amino acids 100 to 347 are sufficient to direct SopA to the mitochondria of host cells. Interestingly, this region of SopA is not predicted to contain a classic mitochondrial targeting sequence (19) when analyzed using the PSORT II program (http://psort.nibb.ac.jp) (18). The truncated constructs and their mitochondrial localizations are summarized in Fig. 6.
FIG. 5.
Amino acids 100 to 347 of SopA are sufficient to direct targeting to mitochondria. Confocal immunofluorescence analysis of COS-7 cells transiently transfected with plasmids expressing myc-SopA1-50, myc-SopA1-200, myc-SopA100-347, or myc-SopA200-347. Twenty-four hours posttransfection, cells were labeled with MitoTracker Red to stain mitochondria (red in merged images) and with anti-myc (α-myc) mouse monoclonal and goat anti-mouse secondary antibodies to detect myc-tagged proteins (green in merged images).
FIG. 6.
Summary of SopA constructs and mitochondrial targeting. Fragments of sopA were amplified from genomic DNA by using specific primers and were cloned into the eukaryotic expression vector pRK5myc, generating constructs which encoded part of SopA preceded by an N-terminal myc epitope tag. The numbers refer to SopA amino acids. The plasmids were transiently transfected into COS-7 cells, and localization to the mitochondria was investigated using an antibody to the myc tag and the mitochondrial stain MitoTracker Red.
Concluding comments.
The targeting of TTSS-secreted effector proteins to host cell mitochondria has been observed in Salmonella and another intestinal pathogen, enteropathogenic E. coli (EPEC) (10, 12, 21). The Salmonella TTSS-1 effector protein SipB localizes to mitochondria during Salmonella infection of macrophages, and the activation of a caspase-1-independent pathway leading to macrophage cell death was found to be attributable to SipB (10). The authors of this work postulate that the targeting of SipB to mitochondria disrupts the organelle, inducing autophagy and ultimately culminating in cell death (10). The EPEC effector Map (mitochondrial-associated protein) was the first TTSS-secreted protein shown to target mitochondria, where it appears to disrupt the membrane potential (12). Another EPEC effector, EspF, also localizes to mitochondria and induces a decrease in mitochondrial membrane potential and the release of cytochrome c, characteristics which are indicative of mitochondrial membrane permeabilization. Ultimately, these mitochondrial disturbances result in initiation of the mitochondrial death pathway (12, 21).
A number of other bacterial proteins associate with mitochondria and are capable of inducing mitochondrial membrane permeabilization and apoptosis, for example, PorB of Neisseria gonorrhoeae and VacA of Helicobacter pylori (6, 16, 17, 25). Since SopA largely colocalizes with mitochondria, it is tempting to speculate that SopA would also have such a role. However, in the SipB studies (10), an S. enterica serovar Typhimurium “effectorless” mutant, in which all known TTSS-1 effector proteins were disrupted, was indistinguishable from wild-type S. enterica serovar Typhimurium in its ability to induce cell death in macrophages from caspase-1-deficient mice. This finding would suggest that SopA has the same subcellular localization as SipB but is not involved in the caspase-1-independent killing mechanism. Hence, further work is required to elucidate the mechanism of action for SopA and, in particular, how the localization of SopA to mitochondria correlates with its role in virulence.
Acknowledgments
We thank Lowrie Taylor for the construction of pRK5myc- SopA1-782.
This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC), including BBSRC grant 201/D18830 to E.E.G.
REFERENCES
- 1.Bakshi, C. S., V. P. Singh, M. W. Wood, P. W. Jones, T. S. Wallis, and E. E. Galyov. 2000. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol. 182:2341-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brumell, J. H., S. Kujat-Choy, N. F. Brown, B. A. Vallance, L. A. Knodler, and B. B. Finlay. 2003. SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic 4:36-48. [DOI] [PubMed] [Google Scholar]
- 3.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-BLUE: a high efficiency plasmid transforming RecA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5:376-379. [Google Scholar]
- 4.Cain, R. J., R. D. Hayward, and V. Koronakis. 2004. The target cell plasma membrane is a critical interface for Salmonella cell entry effector-host interplay. Mol. Microbiol. 54:887-904. [DOI] [PubMed] [Google Scholar]
- 5.Freeman, J. A., M. E. Ohl, and S. I. Miller. 2003. The Salmonella enterica serovar Typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 71:418-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J.-C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galyov, E. E., M. W. Wood, R. Rosqvist, P. B. Mullan, P. R. Watson, S. Hedges, and T. S. Wallis. 1997. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 25:903-912. [DOI] [PubMed] [Google Scholar]
- 8.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galán. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 9.Hardt, W. D., H. Urlaub, and J. E. Galán. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. USA 95:2574-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez, L. D., M. Pypaert, R. A. Flavell, and J. E. Galán. 2003. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163:1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 13.Knodler, L. A., B. A. Vallance, M. Hensel, D. Jackel, B. B. Finlay, and O. Steele-Mortimer. 2003. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol. Microbiol. 49:685-704. [DOI] [PubMed] [Google Scholar]
- 14.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 15.Marcus, S. L., L. A. Knodler, and B. B. Finlay. 2002. Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell. Microbiol. 4:435-446. [DOI] [PubMed] [Google Scholar]
- 16.Massari, P., Y. Ho, and L. M. Wetzler. 2000. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl. Acad. Sci. USA 97:9070-9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller, A., D. Günther, V. Brinkmann, R. Hurwitz, T. F. Meyer, and T. Rudel. 2000. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19:5332-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakai, K., and P. Horton. 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24:34-36. [DOI] [PubMed] [Google Scholar]
- 19.Neupert, W. 1997. Protein import into mitochondria. Annu. Rev. Biochem. 66:863-917. [DOI] [PubMed] [Google Scholar]
- 20.Norris, F. A., M. P. Wilson, T. S. Wallis, E. E. Galyov, and P. W. Majerus. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 95:14057-14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nougayrède, J.-P., and M. S. Donnenberg. 2004. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6:1097-1111. [DOI] [PubMed] [Google Scholar]
- 22.Ruíz-Albert, J., X.-J. Yu, C. R. Beuzón, A. N. Blakey, E. E. Galyov, and D. W. Holden. 2002. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol. Microbiol. 44:645-661. [DOI] [PubMed] [Google Scholar]
- 23.Steele-Mortimer, O., L. A. Knodler, S. L. Marcus, M. P. Scheid, B. Goh, C. G. Pfeifer, V. Duronio, and B. B. Finlay. 2000. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 275:37718-37724. [DOI] [PubMed] [Google Scholar]
- 24.Stender, S., A. Friebel, S. Linder, M. Rohde, S. Mirold, and W. D. Hardt. 2000. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 36:1206-1221. [DOI] [PubMed] [Google Scholar]
- 25.Willhite, D. C., and S. R. Blanke. 2004. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell. Microbiol. 6:143-154. [DOI] [PubMed] [Google Scholar]
- 26.Wood, M. W., M. A. Jones, P. R. Watson, A. M. Siber, B. A. McCormick, S. Hedges, R. Rosqvist, T. S. Wallis, and E. E. Galyov. 2000. The secreted effector protein of Salmonella dublin, SopA, is translocated into eukaryotic cells and influences the induction of enteritis. Cell. Microbiol. 2:293-303. [DOI] [PubMed] [Google Scholar]
- 27.Wood, M. W., R. Rosqvist, P. B. Mullan, M. H. Edwards, and E. E. Galyov. 1996. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol. 22:327-338. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, S., R. L. Santos, R. M. Tsolis, S. Stender, W.-D. Hardt, A. J. Bäumler, and L. G. Adams. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]