Abstract
Diabetes, characterized by hyperglycemia, is a major cause of death and disability worldwide. Peptides, such as insulin and glucagon-like peptide-1 (GLP-1) analogs, have shown promise as treatments for diabetes due to their ability to mimic or enhance insulin's actions in the body. Compared to subcutaneous injection, oral administration of anti-diabetic peptides is a preferred approach. However, biological barriers significantly reduce the efficacy of oral peptide therapeutics. Recent advancements in drug delivery systems and formulation techniques have greatly improved the oral delivery of peptide therapeutics and their efficacy in treating diabetes. This review will highlight (1) the benefits of oral anti-diabetic peptide therapeutics; (2) the biological barriers for oral peptide delivery, including pH and enzyme degradation, intestinal mucosa barrier, and biodistribution barrier; (3) the delivery platforms to overcome these biological barriers. Additionally, the review will discuss the prospects in this field. The information provided in this review will serve as a valuable guide for future developments in oral anti-diabetic peptide therapeutics.
Key words: Oral peptides, Diabetes, Delivery platforms, Insulin, Glucagon-like peptide-1, Biodistribution, Biological barriers, Targeted delivery
Graphical abstract
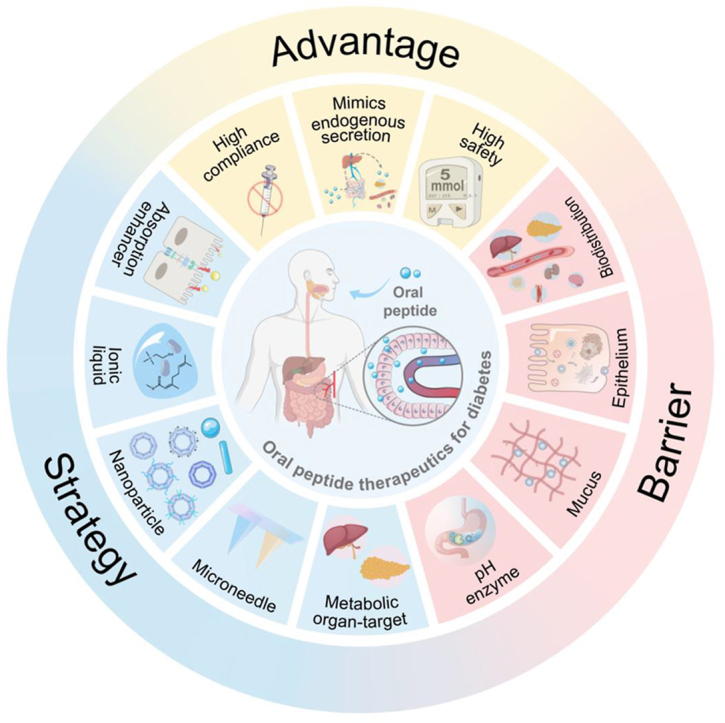
This review assesses physiological advantages, absorption barriers, and delivery strategies for oral administration of anti-diabetic peptides, with a focus on their translational potential from both physiological and commercial perspectives.
1. Introduction
Diabetes is a prevalent chronic disease that affects millions of individuals globally. It is reported that, as of 2021, there are approximately 537 million adults with diabetes worldwide, with a prevalence rate of 10.5%1. Diabetes can be classified into two distinct forms: type 1 and type 2. Type 1 diabetes is caused by self-immune destruction of pancreatic β cells, which leads to an inability to secrete insulin, requiring lifelong insulin injections as the sole means of regulating blood sugar levels2, 3, 4. Type 2 diabetes is characterized by relative insulin deficiency or insulin resistance, causing ineffective utilization of blood sugar and elevated levels due to insufficient sensitivity to insulin in the body. As the disease progresses, the decline in pancreatic β cell function results in insufficient insulin secretion, making insulin the key component of treatment for advanced type 2 diabetes5. Besides, glucagon-like peptide-1 (GLP-1) analogs, including exenatide, liraglutide, dulaglutide, and semaglutide, are also frequently utilized to treat type 2 diabetes by promoting insulin synthesis and secretion. These peptide therapeutics have been shown to be effective and safe in clinical trials and are widely used in clinical practice6,7.
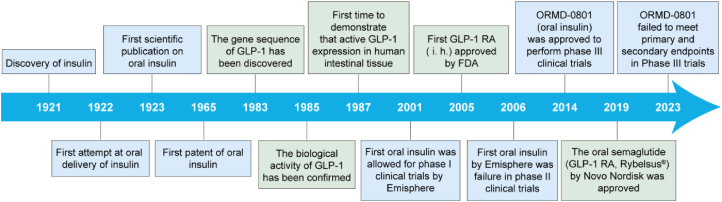
The development of oral peptide therapeutics for diabetes has been a dream for researchers (Fig. 1). In 1922, the second year after the discovery of insulin8, Dr. Joslin attempted to develop oral insulin9. Although the result was a failure, it marked the beginning of a century-long quest for oral insulin10. Since then, various strategies have been developed to facilitate oral insulin delivery. The first patent for oral insulin was issued in 196511. In 2001, an oral insulin preparation developed by Emisphere was approved for clinical trials by FDA12,13. This was the first oral insulin to enter clinical trials. However, the results of phase II clinical trials showed no significant difference compared to the placebo group. In 2014, an oral insulin developed by Oramed (ORMD-0801) was approved for phase III clinical trials14. Unfortunately, ORMD-0801 did not meet both the primary endpoint of improving glycemic control and the second endpoint of achieving a mean change of the fasting plasma glucose from baseline. Despite the extensive efforts on oral insulin, oral GLP-1 analog has also been widely explored for diabetes treatment15,16. One distinctive characteristic of GLP-1, setting it apart from conventional anti-diabetic medications like insulin, is its glucose-dependent mechanism of action17. As a result, the risk of hypoglycemia associated with GLP-1-based treatments is remarkably low18. Since the rapid degradation of native GLP-1 in vivo, the longer-lasting GLP-1 analogs, such as Exenatide and Semaglutide, have been developed in clinical treatments15,19,20. As a significant breakthrough, the world's first oral GLP-1 receptor agonist (GLP-1 RA), Semaglutide tablet, was approved in 201921.
Figure 1.
Evolution and milestones of oral insulin and GLP-1 RA therapeutics for diabetes treatment.
Although oral anti-diabetic peptides are preferred, they encounter two significant challenges: highly individualized pharmacokinetic differences and extremely low bioavailability13,22, which remain unresolved. Variations in patient characteristics, dietary habits, medication routines, and uncertainties in intestinal motility contribute to significant differences in the absorption of oral peptides. Furthermore, the complex physiological barriers in the gastrointestinal tract severely impede the oral absorption of peptides. Fortunately, recent years have witnessed the emergence of numerous new delivery technologies aimed at enhancing the absorption of oral anti-diabetic peptides, instilling hope among diabetes patients. Presently, several oral peptides have either been commercialized or are undergoing clinical trials (Table 1)21,23, 24, 25, 26, 27, 28, 29, 30, and some reviews have summarized the prevailing advancements in oral peptides31, 32, 33. Our work uniquely concentrates on the oral delivery of anti-diabetic peptides, a niche yet crucial area in diabetic treatment. Unlike the reviews on oral peptide delivery, we delve into the advantages and delivery details of anti-diabetic peptides. Another key aspect of our review is the emphasis on the latest delivery platforms specifically designed for targeting organs integral to the metabolism of anti-diabetic peptides, an area not extensively covered in other reviews. Additionally, we provide a comprehensive summary of the challenges in the clinical translation of these peptides in our conclusion, offering insights into potential solutions and future directions in this field.
Table 1.
Oral proteins or peptides currently available in the market or undergoing clinical trials.
| Brand name | Active ingredient | Manufacturer | Technology/principle | Stage | Ref. |
|---|---|---|---|---|---|
| Lupkynis® | Voclosporin | Novartis | Cyclization modification enhances peptide stability | Launched in 2021 | 23 |
| Mycapssa® | Octreotide | Chiasma | Promoting drug absorption through SNAC | Launched in 2020 | 24 |
| Rybelsus® | Semaglutide | Novo Nordisk | Promoting drug absorption through SNAC | Launched in 2019 | 21 |
| – | Insulin | Diasome | Liposomes loaded with insulin possessing liver-targeting functionality | Phase III | 25 |
| – | Insulin | Oramed | POD™: enteric-coated capsules containing enzyme inhibitors and permeation enhancers (EDTA and bile salts) | Phase III | 26, 27, 28 |
| – | Insulin | Bows pharmaceuticals AG | Dextran-based carrier for insulin | Phase II | 29 |
| – | Insulin; Proinsulin; C-peptide |
Oshadi | Inorganic silicon nanoparticles, non-covalently bound complex of polysaccharides and insulin | Phase II | 30 |
‒, not applicable. SNAC, sodium N-[8-(2-hydroxybenzoyl)amino]-caprylate; EDTA, ethylene diamine tetraacetic acid.
2. Advantages of oral administration of anti-diabetic peptide therapeutics
Currently, the primary administration route for anti-diabetic peptides in clinical practice is subcutaneous injection. Although subcutaneous injections offer relatively stable blood glucose control, their long-term and frequent usage can result in significant pain, inconvenience, and adverse reactions, such as hypoglycemia, infection, inflammation, and edema. Studies have reported that approximately 60% of diabetes patients struggle to maintain stable blood glucose levels due to their inability to tolerate prolonged repetitive injections14,34. In comparison, oral administration offers a safer, more effective, and non-invasive treatment strategy, leading to higher patient compliance. Most drugs are administered orally, and the robust regenerative capacity of the gastrointestinal tract ensures that long-term oral administration does not impose any burden. Moreover, oral delivery eliminates the need for additional devices, enhancing convenience for patients35. Considering these advantages, oral delivery of anti-diabetic peptides continues to be a focal point of attention for laboratories and companies.
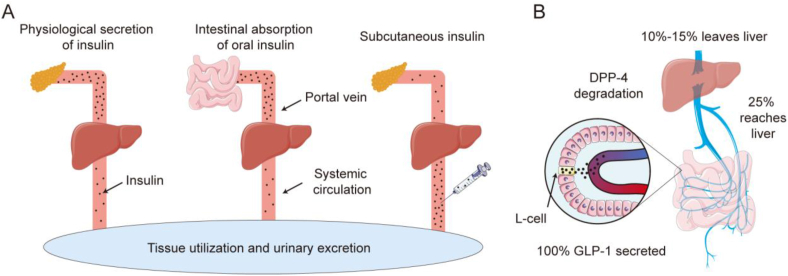
In addition to the above advantages, oral anti-diabetic peptide delivery has unique advantages compared to other administration methods as it mimics the endogenous secretion pathways. Illustrated through the paradigm of insulin, under normal physiological conditions, pancreatic β cells synthesize insulin, which is subsequently conveyed to the liver via the portal vein. Pancreatic insulin secretion exhibits significant fluctuations, but upon reaching the liver through the portal vein, insulin levels gradually stabilize. It is estimated that approximately 50–80% of the insulin is metabolized in the liver, while the remaining insulin enters the peripheral circulation, establishing a liver-peripheral insulin concentration gradient. Thus, the concentration of insulin in the liver is approximately 2–3 times higher than that found in peripheral tissues36, 37, 38. Similarly, when insulin is administered orally, it is absorbed from the small intestine through the intestinal capillaries, enters the portal vein, and is then transported to the liver. This administration route exposes the liver to elevated insulin concentrations while maintaining relatively lower insulin levels in peripheral tissues (Fig. 2A). The liver-peripheral insulin concentration gradient serves as a safeguard against the occurrence of hypoglycemia resulting from excessively high peripheral insulin levels. Additionally, owing to the liver's high sensitivity to insulin, the elevated insulin concentration in the portal vein rapidly stimulates the liver to uptake glucose, store glycogen, and inhibit glycogen breakdown, thereby enhancing blood glucose regulation. In contrast, in non-endogenous insulin secretion processes, the liver's glucose-regulating effects may be delayed, leading to metabolic dysfunction39. Similarly, under normal physiological conditions, GLP-1 is secreted by intestinal L cells40. Due to its rapid metabolism15, approximately 25% of GLP-1 reaches the liver, and ultimately, about 10%–15% of GLP-1 enters the bloodstream (Fig. 2B), establishing a liver-peripheral concentration gradient41. Oral administration of GLP-1 can simulate this physiological process, thereby achieving a safer and more effective therapeutic approach.
Figure 2.
The distribution of insulin and GLP-1 under physiological conditions or after administration. (A) Insulin behavior by physiological secretion, oral, and subcutaneously injectable routes. (B) Secretion and transport of GLP-1.
3. Biological barriers for oral anti-diabetic peptide therapeutics
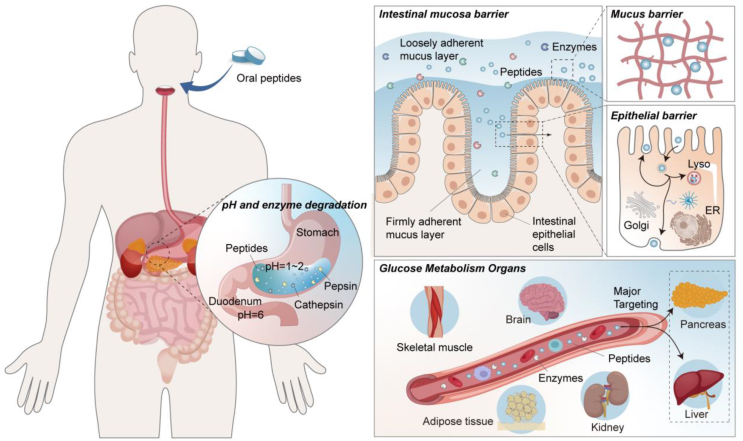
Over the one hundred years, oral peptide formulations have been regarded as the pinnacle of drug delivery in the field of diabetes treatment. This mode of administration holds tremendous promise, but it also presents notable challenges31,42,43. A range of biological barriers within the gastrointestinal tract, encompassing harsh pH conditions, enzymes, and the intestinal mucosa barrier, along with post-intestinal distribution barriers22 within the body after peptides absorption into the bloodstream (Fig. 3), collectively contribute to the significant impediment of oral anti-diabetic peptide absorption, leading to an exceedingly low bioavailability. Existing barriers can be classified into three primary types: pH and enzyme degradation, intestinal mucosa barrier, and biodistribution barrier. The first two barriers are related to absorption, and considerable research has been conducted in this domain. This section offers a summary of commonly employed strategies to overcome absorption barriers, as presented in Table 238,44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83.
Figure 3.
Biological barriers for oral anti-diabetic peptide therapeutics.
Table 2.
Strategies to overcome the absorption barriers.
| Barrier | Strategy employed | Example of agents | Primary function | Ref. |
|---|---|---|---|---|
| pH and enzyme degradation | Colon targeting; Enteric coating; Microcapsules; |
HPMC; Eudragit; Gelatin; α-cyclodextrin |
Preventing denaturation and the low level of proteases | 44, 45, 46, 47 |
| Protease inhibitors | Aprotinin; Na-glycocholate; Bacitracin; Camostat mesylate |
Enzyme inhibition | 48, 49, 50 | |
| Natural degradable polymer shell; | Chitosan; Dextran; Alginate |
Low toxicity and enhanced sustained release | 51, 52, 53 | |
| Nanorobots; | – | Rapid passage through the stomach under propulsive forces; | 54, 55, 56, 57 | |
| Microneedles | Physically piercing tissue barriers to evade the gastrointestinal tract | 58, 59, 60 | ||
| Mucus barrier | Mucus adhesion | Natural/synthetic biodegradable polymer NPs | Facilitate close contact through electrostatic interactions, thereby increasing drug residence time | 60,61 |
| PEGylation | Increase hydrophilicity to enhance adhesive capabilities | 62,63 | ||
| Virus-mimicking | The surface charge is slightly negative, and the outer shell is hydrophilic | 64,65 | ||
| Hydrogels | The swollen hydrogel adheres to the intestinal mucosa | 38,66,67 | ||
| Mucus penetration | NPs with small size | Diffusing faster in the mucus by having a diameter smaller than the average pore size of the mucous mesh structure | 38,68 | |
| Hydrophilic modification | Hydrophilic surface helps reduce hydrophobic interactions and promotes mucus penetration | 69, 70, 71, 72 | ||
| Epithelial barrier | Absorption enhancers | Fatty acids; Surfactants; EDTA |
Perturbed cell membranes, improved cell bypass transport, and selectively opened tight junctions | 73, 74, 75 |
| Hydrogels | Super porous hydrogel; pH-sensitive hydrogels | The expansion of the hydrogel exerts mechanical pressure to open the tight junctions | 38,67,75 | |
| NP carrier systems | Liposomes; Nanospheres; SLN; SNEDDS |
Selectively opening tight junctions | 76, 77, 78 | |
| Modification of specific groups | Ligands (immunoglobulins, transferrin, lectins, biotin, folate, and vitamin B12); | Ligands can be recognized, thereby improving cell internalization | 74,79,80 | |
| CPP (penetratin, transportan, oligoarginine, and TAT) | Endocytosis, cell membrane perturbation and channel generation may be the potential modes of action | 77,81, 82, 83 |
‒, not applicable. HPMC, hydroxypropyl methyl cellulose; NPs, nanoparticles; SLN, solid lipid nanoparticles; SNEDDS, self-nanoemulsifying drug delivery systems; CPP, cell-penetrating peptides; TAT, transactivator of transcription.
3.1. pH and enzyme degradation
Inherent variances in pH levels across distinct segments of the gastrointestinal tract give rise to a multifaceted pH milieu that can induce alterations in the structure, precipitation, or enzymatic breakdown of peptides84, consequently diminishing their efficacy in oral absorption. Initially, the generation of gastric acid by cells in the gastric wall establishes an exceedingly acidic milieu within gastric juice (pH 1–2). Within this setting, protein hydrolytic enzymes, such as pepsin and cathepsin, manifest robust activity, rendering peptides highly susceptible to rapid degradation. Subsequently, researches propose that peptides maintain stability solely within a narrow pH spectrum proximate to their isoelectric point31,85. Upon entry into the duodenum, characterized by a pH of 4–5.586, certain peptides may precipitate due to their proximity to the isoelectric point. Lastly, progression into the small intestine unveils an array of additional protein hydrolytic enzymes, including those secreted by the pancreas, brush-border peptidases expressed by intestinal epithelial cells, cytoplasmic enzymes within intestinal cells87,88, and potentially, enzymes released by local microorganisms73,85, all contributing to peptides degradation. In essence, the inhospitable pH milieu coupled with the presence of diverse protein hydrolytic enzymes throughout the gastrointestinal tract renders peptides highly susceptible to degradation or loss of activity at the beginning of oral administration, constituting the primary obstacle in curtailing the oral absorption of peptides. To enhance the stability of peptides within the gastrointestinal milieu, strategies such as the modification with the natural degradable polymer shell (e.g. chitosan and dextran) or the encapsulation within enteric-coated matrices have been employed, which offer a degree of protection against degradation and denaturation (Table 2).
3.2. Mucus barrier and epithelial barrier
In addition to pH and enzymatic factors, the mucus layer situated within the gastrointestinal tract exerts a substantial influence on the absorption of peptides73,89. Notably, the intestinal mucus layer presents a complex and multifaceted barrier system, encompassing the hydrophobic mesh barrier88, physical-chemical barrier73, and dynamic drug remodeling barrier51.
Intestinal mucus is a gel-like structure composed of mucins lining on the surface of intestinal epithelial cells. Its pore size ranges from approximately 20 to 1800 nm68, facilitating lubrication and protection of intestinal epithelial cells, thereby preventing direct exposure to the intestinal lumen environment. However, when peptides interact with the mucus layer, they encounter the following obstacles: (1) the mucin mesh surface consists of densely packed hydrophilic and hydrophobic regions, leading to hydrophobic interactions between mucin and peptides. This interaction significantly hinders the diffusion of peptides within the mucus, forming a hydrophobic mesh barrier88. (2) Mucus can form various low-affinity interactions with peptides present on the mucus surface, restricting the diffusion of peptides and hindering their upstream transport, creating a physical–chemical interaction barrier73. (3) The mucus can be divided into two layers: the loosely adherent mucus layer and the firmly adherent mucus layer. The loosely adherent mucus layer constantly secretes and sheds mucins, leading to mucus renewal every 4–5 h62,90. Although this rapid clearance of adherent bacteria or pathogenic substances contributes to intestinal health, it also limits the retention time of peptides in the intestines91, forming a physical filtration barrier that significantly reduces the uptake of peptides and establishing a barrier for dynamic peptide reconstruction51, resulting in lower transport efficiency. Currently, several strategies have been employed to overcome the mucus barrier, as summarized in Table 2. Specific natural/synthetic biodegradable polymer nanoparticles (NPs) and hydrogels demonstrate mucus-adhesive properties, thereby augmenting the retention of anti-diabetic peptides and increasing absorption. Besides, the reduction of the carrier's size and the establishment of a hydrophilic surface can also facilitate rapid mucus penetration, ultimately enhancing absorption68.
Situated beneath the intestinal mucus layer lies the compact structure of the intestinal epithelium, composed of a continuous layer of cells. The peptides necessitate transport into the bloodstream via either the transcellular or paracellular pathways. In consideration of the structural features of intestinal epithelial tissue and the lipophilicity of cell membranes92, hydrophilic peptides primarily rely on the actively-mediated transcellular route. However, owing to their substantial molecular weight and the absence of intrinsic active transmembrane transport mechanisms, direct cellular entry becomes challenging93,94. Moreover, in the majority of instances, following their transmembrane ingress into enterocytes, peptides are conveyed to lysosomes, wherein they undergo swift degradation by hydrolases, resulting in their subsequent inactivation95, 96, 97, 98. Furthermore, peptides may also undergo luminal secretion, subsequently re-released onto the membrane surface99, thereby diminishing peptide absorption. Additionally, the composition of the cellular cytoplasm, comprised of myriad proteins, cytoskeletal elements, and organelles, impairs the efficiency of peptide diffusion towards the basal side, thereby influencing their efflux process. At present, the majority of orally administered peptides grapple with the predicament of facile cellular uptake juxtaposed with challenging efflux—a situation aptly characterized as “easy entry, difficult exit”100. Despite existing research efforts, the enhancement of efflux processes for peptides remains an ongoing challenge. Ligand-modified carriers are a favorable choice for enhancing transcellular efficiency and have been extensively employed in peptide delivery101. Besides, additional strategies, such as absorption enhancers, also have the capacity to facilitate the transcellular transport of peptides. The details of these strategies are summarized in Table 2.
3.3. Biodistribution barrier
After successfully navigating through the challenges posed by pH, enzymes, mucus, and epithelial barrier, peptides can be transported adequately into the bloodstream. Nonetheless, the intricate biological distribution of peptides constitutes a long journey, wherein multiple factors hinder peptides access to organs crucial for glucose metabolism, including the liver, pancreas, skeletal muscle, adipose tissue, kidney, and brain.
Upon entering the bloodstream, diverse proteases and peptidases distributed in the blood, tissues, and intercellular spaces promptly initiate the degradation of peptides, leading to a short half-life within the body22,42,102. Specifically, GLP-1 (7–37), one of the active forms of native GLP-1, undergoes rapid breakdown mediated by dipeptidyl peptidase-4 (DPP-4) and is efficiently eliminated by the kidneys within 1–2 min. Additionally, oral peptides must undergo first-pass elimination in the liver. And, due to the high polarity, peptides can easily enter the renal glomerulus, leading to renal excretion. These factors above collectively contribute to an extremely short half-life of peptides within the body. Furthermore, during peptide transport, the immune system participates in their degradation103. The reticuloendothelial system (RES), abundant in macrophages, promptly engulfs drug delivery systems upon entering the circulatory system, leading to the swift clearance of peptides. Additionally, the liver functions as the primary site for insulin and GLP-1 clearance, with Kupffer cells efficiently clearing them to prevent adverse reactions, such as hypoglycemia resulting from peripheral hyperinsulinemia. Hepatocytes emerge as pivotal target cells for the majority of peptides entering the bloodstream via the hepatic portal vein. The phagocytic activity of Kupffer cells poses a significant obstacle to the efficacy of peptides. Hence, evading the phagocytic system and augmenting hepatocyte uptake of oral peptides are imperative for peptide therapy in diabetes treatment. It has been reported that pegylation diminishes the immunogenicity and antigenicity of proteins104,105, thereby preventing clearance by the immune system. RES-blockade strategy can transiently and reversibly impede hepatic clearance, amplifying the accumulation of nanomedicines in tumors and enhancing their antitumor effects, exhibiting favorable biocompatibility106. Furthermore, environmentally responsive biomaterials release peptides on demand at appropriate locations, while targeted formulations release peptides at specific sites, both contributing to the sustained therapeutic effects of peptides107,108. Ultimately, it is worth noting that individual differences may introduce uncertainty in the delivery, absorption, and effectiveness of peptides, making it an important factor that should not be overlooked.
4. Strategies for oral anti-diabetic peptide therapeutics
In the realm of developing oral anti-diabetic peptides, a myriad of strategies have been devised to enhance their absorption. The strategies encompass the utilization of absorption enhancers, ionic liquids (ILs), NPs, and microneedles (MNs)84,109. These innovative techniques have demonstrated their effectiveness in surmounting multiple gastrointestinal barriers, facilitating the efficient delivery of peptides into the bloodstream, thereby enhancing their overall transport efficiency. Furthermore, recent investigations have accentuated a growing emphasis on directing peptides toward organs closely associated with glucose metabolism, particularly the liver and pancreas110, 111, 112. This approach is aimed at optimizing glucose utilization within the body by leveraging a more nuanced understanding of endogenous insulin/GLP-1 secretion patterns and biological distribution107,108. In this section, we present an exhaustive summary of the various strategies employed to enhance the absorption of anti-diabetic peptides. Additionally, we highlight the emerging trend of targeted delivery to the liver and pancreas for maximizing therapeutic effects. These ongoing refinements hold significant promise in augmenting treatment options for individuals afflicted with diabetes.
4.1. Delivery platforms for improved oral absorption
4.1.1. Absorption enhancer
Absorption enhancers represent a class of compounds designed to transiently enhance the permeability of the intestinal epithelial barrier, thus facilitating oral peptide absorption. Notable constituents of this category include fatty acids, fatty acid salts, bioadhesive polymers (such as chitosan and its derivatives), and surfactants113,114.
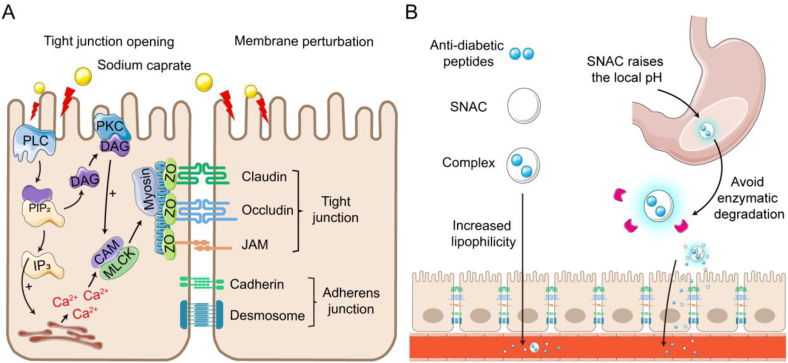
Fatty acids, abundantly generated during the digestion of triglycerides within the gastrointestinal tract, exhibit the capacity to enhance paracellular permeability by modulating the tight junctions between epithelial cells115. Among these, sodium caprate stands out as the most widely employed fatty acid enhancer and holds the distinction of being the first FDA-approved fatty acid absorption enhancer for pharmaceutical use113. Its application in oral insulin 338 (I338) resulted in a bioavailability of 1.5%–2.0%, with no reported toxicity during an eight-week study period116. Mechanistically, sodium caprate activates phospholipase C, instigating an increase in inositol triphosphate levels, subsequently leading to calcium release and elevation of intracellular calcium concentration31,117, 118, 119. This cascade culminates in calmodulin-dependent actin contraction, resulting in the opening of tight junctions and enhanced paracellular transport (Fig. 4A). Sodium caprate has also been observed to facilitate drug absorption through plasma membrane perturbation, as evidenced by ATP leakage from Caco-2 cells117. This perturbation, which is concentration-dependent, remains reversible and mild in the gastrointestinal tract, underscoring its safety as an absorption enhancer.
Figure 4.
The mechanism of action of sodium caprate and SNAC. (A) Sodium caprate enhances the absorption of anti-diabetic peptides by opening tight junctions and causing membrane perturbation. (B) SNAC enhances the absorption of anti-diabetic peptides by forming complexes with anti-diabetic peptides and increasing its lipophilicity (left); by raising the local pH of the anti-diabetic peptides to reduce enzymatic degradation (right).
Sodium N-[8-(2-hydroxybenzoyl)amino]-caprylate (SNAC), a derivative of sodium caprylate, shares similar amphiphilic and surface-active characteristics. SNAC has found widespread use in promoting the absorption of macromolecular drugs. In 2019, Semaglutide tablet (Rybelsus®) was approved for use with SNAC to enhance oral absorption and prevent stomach degradation. Co-administration of 2.5‒40 mg of semaglutide with 300 mg of SNAC led to improved blood glucose control, with the bioavailability of semaglutide increasing over time, reaching 1.4% around 2 h post-administration. Investigations into SNAC's absorption-enhancing mechanism suggest that it can form non-covalent complexes with peptides, augmenting their lipophilicity and promoting transmembrane absorption (Fig. 4B). Previously, we have discovered that SNAC can interact with insulin and form tight complexes, influenced by concentration, ratio, and pH120. As pH increases, SNAC's carboxyl groups (pKa = 5.08) predominantly exist as carboxylates (–COO–), which favor complex formation with insulin's free amino groups. SNAC/insulin complexes are internalized into cells through passive diffusion and remain intact during transport in the cytoplasm. Additionally, the complex accelerates the exocytosis of insulin towards the basolateral side, thereby enhancing its intestinal permeability. Some studies have suggested that high doses of SNAC may affect cell membrane integrity. Brayden et al.121 demonstrated that high SNAC concentrations (50 mg/mL) led to complete loss of trans-epithelial electrical resistance (TEER) and a 36-fold augmentation in [3H]-mannitol permeability in Caco-2 monolayers, while Hess et al.122 found that SNAC (33–66 mmol/L) increased 6-carboxy-fluorescein (6-CF) transport but not [3H]-mannitol, with no decrease in TEER values. However, when SNAC was introduced at a concentration of 165 mmol/L in the jejunal mucus, TEER decreased, concomitant with an increase in the permeability coefficient of [3H]-mannitol, similar to Brayden's findings. Importantly, the loss of membrane integrity does not necessarily indicate tight junction disruption, and direct evidence of SNAC disrupting tight junctions and opening paracellular pathways is lacking122. The increased membrane fluidity of Caco-2 epithelial cells by SNAC aligns with surfactant-induced membrane perturbation. Compared to sodium caprate, SNAC's greater distribution of hydrophilic functional groups in the salicylamide region suggests lower efficiency in domain insertion into phospholipid membranes. This difference may explain why higher SNAC concentrations are required for improved penetration when contrasted with sodium caprate. Notably, SNAC's absorption-enhancing effect may vary depending on the peptides involved, warranting further investigation. Recent work by Novo Nordisk introduced an innovative mechanism of action for SNAC. According to their proposal, SNAC forms a complex around semaglutide within the gastric environment, resulting in a temporary local pH increase that protects semaglutide from pepsin and enhances dissolution (Fig. 4B)123. Moreover, SNAC induces the generation of semaglutide monomers, increases the fluidity of the gastric epithelial membrane without impacting tight junctions, and facilitates semaglutide's entry into the systemic circulation. Regardless of the mechanism at play, SNAC's significant absorption-promoting effect on peptides and its good in vivo safety have captured substantial interest from the industry.
Chitosan (CS), derived from the deacetylation of chitin, is another absorption enhancer with a track record of improving peptide oral absorption124,125. Insulin-loaded core/shell CS-alginate NPs, effectively safeguard insulin from enzymatic degradation, resulting in an insulin bioavailability of 8.11%126. Kim et al.127 conducted optimizations in the ionotropic gelation process involving CS and phytic acid (PA), achieving an impressive encapsulation efficiency of 97.1%, ultimately yielding a relative bioavailability of insulin at 10.6%. The absorption-promoting attributes of CS can be attributed to its charge properties. Possessing positive charges under mildly acidic conditions due to the presence of glucosamine residues, CS adheres robustly to the negatively charged mucus layer via electrostatic interactions, reducing mucus clearance. Additionally, the positive charges on CS can interact with negatively charged serine residues in cell membrane proteins, leading to structural changes and reversible opening of tight junctions, thereby enhancing paracellular transport. Experiments involving CS treatment have demonstrated reduced translocation of zonula occludens-1 (ZO-1) from the membrane fraction, indicating increased paracellular permeability128. Based on these remarkable absorption-enhancing properties, CS has emerged as a promising material for consideration in pharmaceutical formulations.
In conclusion, incorporating absorption enhancers is a common strategy to improve oral peptide absorption. Absorption enhancers with diverse mechanisms of action can be combined and administered at lower doses to synergistically enhance absorption. Ideally, absorption enhancers should be simple, easy to manufacture, and possess a high safety profile to enable oral absorption of peptides, without changing the clinically validated pharmacokinetic profile of the drug. Compared to other strategies with complex preparation processes, the absorption enhancer strategy is more widely used in oral delivery systems for anti-diabetic peptides. Furthermore, the efficacy of absorption enhancers is intricately tied to their localized concentration at the absorption site, with notable variations in their functional performance across distinct sites. Ideally, a delivery system should facilitate the targeted transportation of both the peptides and the absorption enhancer to the precise absorption site. This strategic co-localization is pivotal, as it enables the potentiated effect of the absorption enhancer to manifest prominently, facilitated by their concurrent release at therapeutically efficacious concentrations. Notably, a discernible pattern emerges, with multiple enhancers demonstrating superior absorption-facilitating attributes within the colon and ileum regions compared to the proximal small intestine. This observation underscores the potential advantages of directing co-delivery of peptides and enhancers to the distal small intestine or colon, a strategic approach that may optimize pharmaceutical regimens and dosage formulations.
4.1.2. Ionic liquid
ILs are compounds composed of large organic cations, such as alkyl imidazolium salts, alkyl pyridinium salts, alkyl quaternary ammonium salts, alkyl quaternary salts, heterocyclic aromatic compounds, and derivatives of natural products, combined with organic or inorganic anions. These substances remain in a liquid state at room temperature and typically possess a melting point below 100 °C. Through the pairing of different ions, ILs exhibit a wide range of physical and chemical properties, including viscosity, hydrophobicity, density, and biodegradability. Consequently, they find extensive use in biocatalysis, protein stabilization, penetration enhancement, and solubilization129. In the domain of oral peptide delivery, ILs play a crucial role in enhancing drug absorption by improving drug solubility, stability, and permeability.
One noteworthy class of ILs is choline and geranate (CAGE) ILs, which has emerged as a promising class of biocompatible solvents for both oral peptide delivery. For instance, Banerjee et al.130 developed an efficient oral insulin formulation using CAGE ILs, resulting in a significant reduction of up to 45% in blood glucose levels in non-diabetic rats. In addition to CAGE, other choline-based ILs, such as CGLY2:1 derived from choline and glycollate, have been utilized for the delivery of peptides131. The enhancement of peptide absorption through ILs is predicated on their ability to improve peptide stability and gastrointestinal permeability. ILs effectively prevent peptide aggregation, as demonstrated by Reslan et al.132, who found that the dihydrogen salt ILs (CDHP) remarkably inhibited the decomposition and polymerization of peptides such as Herceptin. At a CDHP content of 53%, it demonstrates strong inhibition of heparin aggregation during the initial stage, resulting in a relative monomer content of up to 90% compared to the control group. Besides, ILs could form a protective shell-like structure around peptides, preserving their structural integrity133. Additionally, ILs enhance peptide stability by inhibiting digestive enzymes. For example, Agatemor et al.134 demonstrated that CAGE ILs effectively curtailed the activity of DPP-4, thus augmenting the stability of GLP-1. ILs can also overcome the gastrointestinal barrier by reducing mucus viscosity, facilitating peptide diffusion within the mucus layer, and improving absorption through the perturbation of tight junctions. Moreover, ILs can overcome the gastrointestinal barrier by diminishing mucus viscosity, facilitating the diffusion of peptides within the mucus layer, and improving absorption through the perturbation of tight junctions. For instance, Banerjee et al.130 developed an acid-resistant capsule composed of CAGE ILs, encapsulating insulin. They found that the anionic constituents within ILs interact with the mucus layer to reduce its viscosity, while cations act on enterocytes, opening tight junctions and promoting insulin uptake through the bypass pathway. However, it's essential to note that the effects of ILs can vary depending on their constituent ions and their relative proportions. The composition of ILs significantly dictates their ultimate impact on absorption, making it a critical consideration in prescription design.
In essence, ILs play a pivotal role in significantly enhancing peptide absorption due to their unique ion compositions. Their advantage lies in their uncomplicated procedures and their ability to concurrently overcome multiple absorption barriers, a feat challenging for other absorption-enhancement methods. Presently, the application of ILs in oral anti-diabetic peptides is constrained by concerns regarding their biocompatibility, particularly the safety of prolonged administration129,135. To address potential toxicity concerns, natural metabolites are incorporated as constituents of ILs. The components, choline, and geranate, integral to GAGE, are FDA-acknowledged as generally regarded as safe (GRAS) ingredients. They elicit a concentration-dependent recovery in the TEER of Caco-2 cells within 24 h130. Similarly, CGLY2:1 demonstrates a favorable safety profile following oral administration131. However, relying solely on these in vivo safety data is insufficient to underpin the clinical translation of ILs. Therefore, further clinical trials are imperative to corroborate therapeutic efficacy and ascertain long-term toxic effects.
4.1.3. Nanoparticles
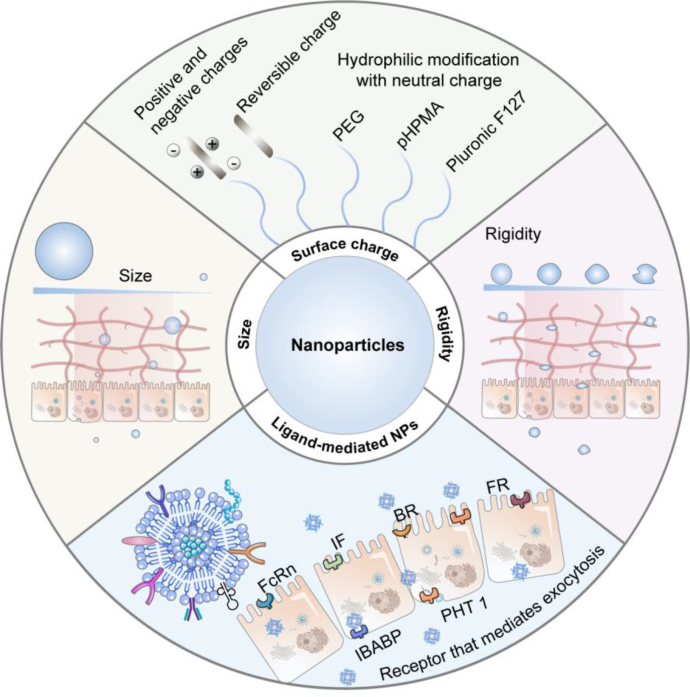
NPs represent a promising avenue for improving the oral absorption of anti-diabetic peptides136,137. Capitalizing on their nanoscale dimensions, NPs possess the capability to effectively surmount the multiple absorption barriers138. Previously, several reviews have comprehensively summarized the different types of NPs in enhancing peptide absorption139, 140, 141. In this review, our focus is on examining the impact of physicochemical properties (e.g. size, surface charge, and rigidity) and ligand modification of NPs for anti-diabetic peptide delivery (Fig. 5). These explorations would contribute to a deeper understanding and improved design of NPs for enhanced peptide delivery.
Figure 5.
Schematic diagram of strategies for enhancing NP absorption: appropriate physicochemical attributes and ligand modifications facilitate NPs in overcoming absorption barriers.
The physicochemical attributes of NPs, including size, surface charge, and rigidity, constitute fundamental determinants of their effectiveness in overcoming mucus and epithelial barriers during absorption68,142. Notably, modulating particle size emerges as a straightforward method to enhance mucus penetration. Smaller NPs exhibit enhanced mucus permeability compared to larger counterparts, owing to the constraints posed by mucus pore size (with an average of approximately 200 nm)143. However, extremely small NPs result in inefficient uptake, given their inadequacy in overcoming membrane tension and deformation resistance. Furthermore, in scenarios where cellular uptake relies on specific interactions like ligand-receptor binding like ligand-receptor binding, these diminutive NPs do not provide an adequate surface area for a substantial ligand-receptor interaction, thereby resulting in diminished absorption efficiency144. Notably, an array of studies substantiates that the optimal range for cellular uptake falls within 30–60 nm, irrespective of core composition or surface charge145. This range may be extended to 500 nm with variations in particle properties and cell models31. Beyond 500 nm, NPs within the intestinal milieu are more susceptible to capture by M cells, consequently fostering heightened transport through lymphatic vessels146.
Surface charge also plays a vital role in particle's absorption. Coating NPs with hydrophilic materials, including Polyethylene glycol (PEG), Pluronic F127, and bovine serum albumin (BSA), can establish a hydrophilic surface endowed with a neutral charge, reducing interactions with mucin and enhancing mucus penetration68. Lai et al.69 noted that densely packed, low molecular weight PEG-modified NPs diffused rapidly in mucus, akin to their diffusion in water. We have observed that the Pluronic F127-modified NPs exhibited more efficient mucus permeability and improved oral insulin delivery efficiency compared to unmodified counterparts, resulting in a 2.5-fold increase in hypoglycemic effect70. Besides, we employed BSA to coat cationic liposomes with a hydrophilic surface and neutral charge. The Mean square displacement (MSD) of neutral charge liposomes increased by 21 times, further yielding an oral insulin bioavailability of 11.9%71. Recently, biomimetic NPs with high-density charges and overall charge neutrality have shown significant mucous permeability72. The MSD was 5-fold and 100-fold greater than PEG NPs and cationic NPs, respectively. Moreover, lyophilized insulin-loaded NP powder within enteric-coated capsules achieved an oral insulin bioavailability of up to 42.6%. Surface characteristics of NPs also assume a pivotal role in traversing epithelial barriers. Typically, NPs with positively charged or hydrophobic surfaces facilitate augmented cell membrane contact probability and duration, thus prompting effective endocytosis147. Recent findings indicate that anionic NPs induce tight junction relaxation and augment intestinal permeability via integrin binding and myosin light chain kinase (MLCK) activation148. In essence, adept utilization of NP size and surface charge effects engenders superior absorption outcomes.
Rigidity characterizes NP deformability and also impacts their absorption. Optimal deformability of drug carriers with moderate rigidity facilitates traversing biological hydrogels and cell membranes. Conversely, rigid NPs cannot deform, while soft ones encounter hindrance from mucin-fibril interactions. Bao et al.149 synthesized short nanotubes (SNTs) and crosslinked short nanotubes (CSNTs), with soft SNTs showcasing rapid permeation compared to stiff CSNTs. Molecular dynamics (MD) simulations disclosed rotational dynamics enhancement within the shear flow and the mucus network. We designed liposomes with varying rigidities by manipulating phospholipid phase transition temperatures. Moderate stiffness liposomes (Young's modulus is approximately 15 MPa) displayed superior mucus penetration and cell uptake efficiency. MD simulations and stimulated emission depletion (STED) microscopy experiments evidenced semi-elastic NPs morphing into ellipsoids, enabling rotation-facilitated swift penetration and elevating insulin oral bioavailability to 13.65%150. Zheng et al.151 noted distinct elastic properties in zwitterionic hydrogel NPs resulting in significantly varying oral insulin absorption in diabetic rats. Among these, the hardest NPs (HNPs) with a Young's modulus around 165.2 MPa exhibited optimal elasticity, affording the most effective hypoglycemic impact and elevating insulin oral bioavailability to approximately 15%. Overall, the advantages of rigidity are noteworthy, becoming more integral in future NP design endeavors.
In the aforementioned examples, it becomes evident that appropriate physical properties primarily aid NPs in overcoming the mucus and epithelial cell barriers during absorption. Nonetheless, these barriers often exhibit contradictory and conflicting requirements for particle design: (1) reducing the size of NPs can enhance their capability to penetrate mucus, yet smaller NPs might encounter challenges in cellular uptake; (2) hydrophilic materials or charge-neutral modifications enhance mucus penetration but prove less effective in cellular internalization. As a result, a range of NPs targeting multiple barriers have been developed, with the application of dissociable coating materials emerging as a strategy to address these multiple challenges. As mentioned in the preceding text, we employed BSA as a coating for cationic liposomes. The resultant liposomes exhibited a neutral surface charge, leading to improved permeability in intestinal mucus71. Wu and colleagues152 devised charge-reversible NPs comprised of cationic peptides and anionic phosphoserine. The overall neutral charge of these NPs facilitates mucus penetration, after which the hydrolysis of phosphoserine exposes the cationic peptides, thereby promoting cellular uptake. Compared to NPs modified with cell-penetrating peptides (CPP), the charge-reversible NPs have increased the oral bioavailability of insulin by 1.9-fold. In another study, Shan and colleagues153 formulated NPs coated with poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA), which disengaged from the particle surface during mucus penetration, subsequently enhancing cellular entry. During osmosis, the protein corona is shed, unveiling the cationic core and facilitating cellular uptake. NPs with a dissociable coating demonstrate a 20-fold increase in absorption within mucus-secreting epithelial cells compared to free insulin. Moreover, the oral bioavailability of insulin delivered via these NPs is 2.1 times higher than that of unmodified NPs. Furthermore, fine-tuning the rigidity of NPs serves as a straightforward and effective approach to overcoming multiple barriers. We contrasted the mucus penetration and cellular uptake capabilities of NPs with varying rigidities, revealing that moderately elastic NPs could adeptly traverse the dual barrier154. Furthermore, in the work mentioned earlier by Bao et al.149, the potential of applying NP rigidity to overcome multiple barriers was similarly observed. To conclude, while contradictions and conflicts exist between the mucus and epithelial cell barriers, the application of dissociable coating materials proves effective in resolving these challenges. Additionally, selecting an appropriate NP rigidity emerges as another efficacious method. As the in vivo fate of NPs is further refined, more optimized strategies are likely to be proposed in the future.
Due to the limited capability of conventional NPs for cellular transcytosis and tight junction opening, ligand-mediated targeted delivery strategies have been widely applied when designing the in vivo fate of NPs155. Currently, receptors or transporters commonly used in the intestines include neonatal Fc receptor (FcRn), transferrin receptor (TfR), intrinsic factor (IF), biotin receptor (BR), and folate receptor (FR), among others156,157. By functionalizing ligands on the surface of carriers that specifically bind to receptors or transporters on the intestinal epithelial cells, the transport efficiency of NPs can be improved. Pridgen et al.158 modified the fragment crystallizable (Fc) fragment of IgG on NPs, enabling them to be taken up by specifically binding to FcRn on the intestinal epithelial cells. Fc-modified NPs show approximately 10 times higher absorption efficiency in vivo compared to unmodified ones. Similarly, Fc-modified exenatide NPs prepared by Shi et al.159 exhibited strong transcellular transport capabilities and sustained hypoglycemic effects (up to 12 h) after oral administration. Ke et al.160 modified VitB12 on the surface of chitosan derivative NPs for insulin delivery. Compared to unmodified NPs, VitB12-modified NPs reduced insulin retention in the intestinal lumen (0.59-fold) and increased its absorption in epithelial tissue (4.8-fold). These studies indicate that ligand modification significantly promotes the endocytic behavior of NPs, enhancing the oral absorption of peptides. However, through in-depth research on the fate of NPs, it has been discovered that targeting apical membrane receptors alone through ligand design leads to limited absorption efficacy, as most NPs cannot effectively escape lysosomes and end up being degraded. Consequently, researchers have developed many NPs with lysosome avoidance and basal lateral release capabilities. NPs modified with the Fc ligand demonstrate certain lysosome escape capability. Under acidic conditions, the Fc ligand exhibits high affinity to FcRn, thus avoiding lysosomal degradation161. Besides lysosome avoidance, another crucial factor for successful exocytosis is targeting the basal lateral membrane. In recent years, studies have found that surface modification of carriers with cholic acid or deoxycholic acid enables efficient endocytosis and exocytosis through different receptors on the membrane. We prepared deoxycholic acid-modified CS NPs (DNPs), which bind to the apical sodium-dependent bile acid transporter (ASBT) on the surface of ileum cells, and are subsequently taken up into the cells. They further disrupt the lysosomal membrane structure to achieve escape. Additionally, the DNPs in the cytoplasm can bind to the ileal bile acid-binding protein (IBABP) and be transported to the basal lateral side of the cells. Through the intestinal bile acid transport pathway, DNPs improve insulin transcytosis efficiency by 12.86 times, ultimately achieving an oral bioavailability of 15.9% for insulin162. Furthermore, we designed a dual ligand-modified nanocarrier (PG-FAPEP) that targets both apical and basal membranes to mediate complete transcytosis of NPs163. In this design, folic acid is used to mediate NP endocytosis by coupling with the core PLGA. Simultaneously, a tripeptide (aspartic acid-phenylalanine-glycine) that specifically binds to the proton-coupled oligopeptide transporter (PHT1) is synthesized to target the basal lateral membrane. PG-FAPEP rapidly penetrates the mucus through the charge effect of the tripeptide and triggers the proton sponge effect in lysosomes to achieve escape. The modified carrier's transcytosis efficiency reaches 19.7%, which is 11.6 times higher than that of unmodified PLGA carriers. After oral administration, the relative bioavailability of insulin in rats reaches as high as 14.3%. These strategies effectively address the exocytosis deficiency observed in single ligand-modified NPs.
Overall, through extensive research on NPs, it has been established that modulating the physical properties and ligand modifications of NPs is an effective strategy to enhance the oral absorption of this class of formulations. Numerous scientific studies have contributed to the gradual design of NPs to optimize their fate in the body, resulting in improved outcomes in overcoming mucus barriers and epithelial cell barriers. However, the current research on the design of NPs with specific physical properties, particularly shape and stiffness control, is still limited. Materials and technical reports in these fields are scarce, such as the shape of NPs, which is one of the essential properties of NPs that we have not mentioned but is very important. The inability to provide safe organic materials limits their application in oral peptide delivery. Future advancements are eagerly anticipated to develop more mature technologies for the controlled design of NPs with diverse physical properties, thereby effectively harnessing their advantages. Regarding ligand-modified NPs, they have demonstrated the ability to partially control the fate of NPs in the body. However, the majority of current research predominantly focuses on the cellular uptake efficiency of these carriers, with limited investigation into their intracellular fate and exocytosis mechanisms. Therefore, further exploration of the intracellular transport processes of drug carriers is necessary to elucidate the correlation between ligand modifications and intracellular transport pathways. This deeper understanding will serve as a guide for the design of drug carriers with highly efficient transcellular activity, ultimately leading to improved oral bioavailability of protein and peptide-based anti-diabetic drugs.
4.1.4. Microneedle-based platforms
MNs are intricately designed needle-like structures created using micro-electromechanical technology, typically characterized by diameters smaller than a few tens of micrometers and lengths ranging from 25 to 2000 μm164. MN technology holds the potential for facilitating the oral delivery of peptides, because it can directly address the challenges encountered by traditional oral formulations, such as biochemical barriers, gastrointestinal mucus barriers, and gastrointestinal cellular barriers, thus effectively enhancing drug absorption.
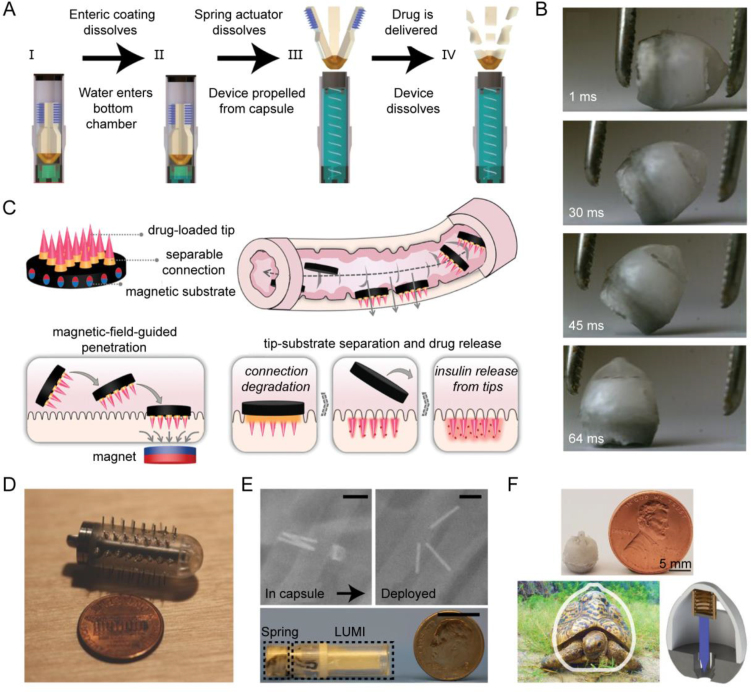
Traverso et al.58 conducted pioneering proof-of-concept experiments in pigs, illustrating the ability of MNs to effectively overcome barriers presented by the gastrointestinal mucus and epithelium, thereby enhancing the oral bioavailability of biomacromolecules. Furthermore, the device demonstrated safety and tolerability during the experimental period. Notwithstanding safety evaluations, concerns persist regarding the potential toxicity associated with metal materials. Consequently, biodegradable or dissolvable MNs have been developed for biomedical purposes. A luminal unfolding MN injector (LUMI) oral capsule has emerged as a result165. Once the LUMI capsule reaches the small intestine, the external capsule degrades, enabling three folded arms inside to spring open and propel a 1 mm-long microneedle (Fig. 6E), loaded with insulin, into the intestinal wall. Upon activation, the capsule splits, and the non-degradable elastomeric core of LUMI is expelled from the gastrointestinal tract (Fig. 6A). LUMI demonstrates a faster onset of action compared to subcutaneously injected insulin, resulting in a systemic absorption efficiency of 10% within 4 h. Currently, this technology is undergoing investigation in clinical trials. However, despite the optimization of forces exerted on the small intestinal wall by LUMI technology to ensure safer functionality, concerns regarding potential intestinal dilation caused by MN persist. Moreover, the thinness of the intestinal wall, ranging from 0.1 to 2 mm, presents a risk of perforation. Given the abundance of intestinal microbes, even minor damage from MNs poses a significant risk of systemic infection. Another limitation is the requirement for LUMI to pass through the stomach, which is subject to a 1–4 h gastric emptying time. This poses challenges for insulin delivery, as timing in relation to food consumption is crucial. Fortunately, the research team has addressed these concerns in their subsequent work. Drawing inspiration from the passive reorientation ability of tortoises, they developed a self-orienting millimeter-scale applicator (SOMA) based on the shell of a leopard tortoise (Fig. 6F)166. SOMA comprises spring-loaded MNs loaded with insulin. The shape and density distribution of SOMA were optimized to ensure the consistent orientation of the MNs upon landing in the stomach, followed by rapid adjustment to an upright position to securely adhere to the stomach wall (Fig. 6B). Subsequently, the MNs penetrate the gastric tissue, releasing insulin into the bloodstream. In vivo results have demonstrated that oral administration of SOMA produces glucose-lowering effects similar to subcutaneous insulin injection, with comparable insulin levels in the bloodstream. Achieving injection in the stomach, SOMA offers improved safety and efficacy. Compared to the intestinal wall, the gastric wall, which is 4–6 mm thick, provides a broader protective layer and more space for drug insertion. Furthermore, gastric tissue exhibits rapid regeneration, and the flowable seal of the mucus barrier temporarily closes any defects in the inner wall. Considering the known variations in gastric emptying, delivering the dose to gastric tissue instead of the small intestine may provide more predictable dosing times and advantages for delivering anti-diabetic drugs.
Figure 6.
Oral microneedle devices and their in vivo localization and injection. (A) LUMI actuation scheme. (B) Self-orients of SOMA. (C) Schematic illustrations of the oral magneto-responsive MN robots. Under the influence of a magnetic field, the MN robot navigates and penetrates the intestinal wall to facilitate drug delivery. (D) Custom-made equipment used for estimating MN safety and transit time. (E) LUMI capsule actuation in vivo in the swine (top) and Encapsulated LUMI (bottom). Scale bars: 1 cm. (F) Comparison of the shell shape between the leopard tortoise (S. pardalis) and SOMA, along with a schematic representation of SOMA's actuation. Reprinted with the permission from Refs. 165, 166, 167, and 58. Copyright © 2019 Springer Nature, 2019 Science, 2021 John Wiley and Sons, and 2015 Elsevier.
MNs need to make targeted contact with the tissue wall to penetrate and deliver drugs, but this process is often hindered by digestive fluids or food in the gastrointestinal tract. To overcome this obstacle, Zhang et al.167 developed a magnetic-responsive MN robot. These MN robots consist of a magnetic substrate, detachable connectors, and a tip component. Encapsulated in commercial enteric capsules, they can be orally administered and released upon entering the small intestine. Guided by a magnetic field, the MN robots traverse the digestive fluids and penetrate the intestinal wall (Fig. 6C). After penetration, the detachable connector degrades, separating the tip from the substrate and leaving the tip inside the small intestine to release the insulin, while the magnetic substrate is expelled from the body. The blood glucose levels of the treated miniature pigs are expected to normalize within approximately 2 h, demonstrating promising therapeutic efficacy. Further clinical research on these products is eagerly awaited. Overall, Oral physical platform devices designed for in-body injection offer several advantages, including easy traversal of multiple absorption barriers, comparable efficacy to subcutaneous injections, and the ability to establish liver-peripheral insulin concentration gradients specific to oral administration, thereby minimizing the risk of hypoglycemic side effects associated with subcutaneous injections. However, the challenge of in-body retention of these physical devices needs to be addressed. While existing research has demonstrated the potential for platform devices to be eliminated from the body, concerns remain regarding their comparative safety when compared to fully biocompatible materials, particularly with regards to gastrointestinal perforation and inflammatory reactions. Furthermore, the effectiveness of oral MNs in achieving in-body injection warrants further investigation, given the potential hindrance posed by digestive fluids and food residues.
4.2. Delivery platforms for major metabolic organs-targeted delivery
Over the past century, research endeavors have predominantly concentrated on enhancing oral anti-diabetic peptides absorptive potential168, 169, 170. In recent years, there has been a shift towards exploring the targeted delivery of anti-diabetic peptides, such as insulin and GLP-1, through oral routes to organs associated with glucose metabolism, particularly the liver and pancreas (Fig. 7).
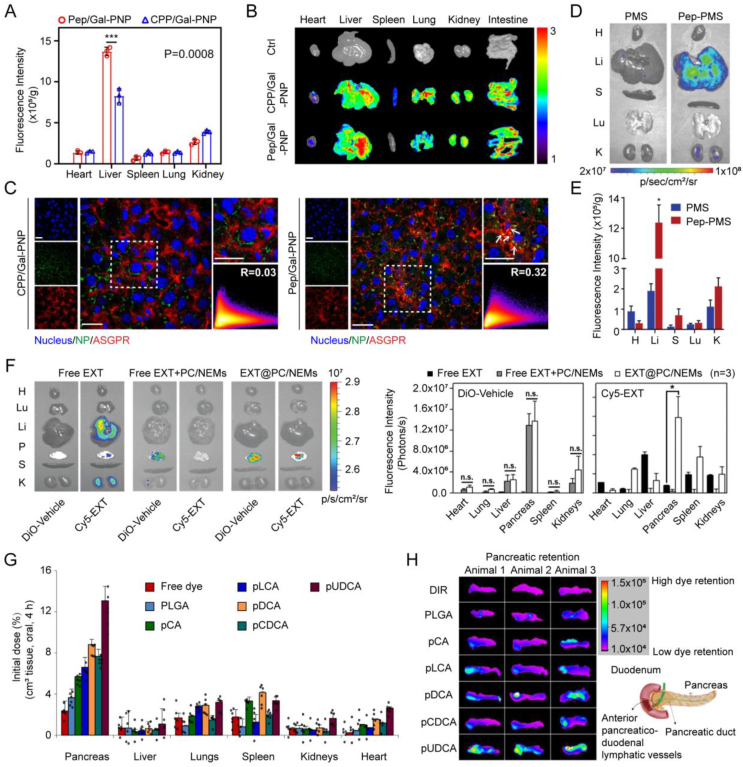
Figure 7.
Delivery Platforms for major metabolic organs-targeted delivery. (A) Fluorescence intensity and (B) accumulation in major organs using the in vivo imaging system (IVIS) and (C) colocalization with ASGPRs in liver sections of rats 4 h after the oral administration of FITC-labeled Pep/Gal-PNP and CPP/Gal-PNP (mean ± SD, n = 3, ∗∗∗P = 0.0008). The color scale represents radiant efficiency × 107 p·s−1·cm−2·sr−1. Scale bars: 20 μm. (D) Organ-level biodistribution measured by IVIS and (E) fluorescence intensity of FITC-labeled PMS and Pep-PMS after oral administration to healthy rats (mean ± SD, n = 3, ∗P < 0.05). (F) Organ-level biodistribution measured by IVIS and corresponding fluorescence intensity within 1 h after SC administration of Cy5-EXT, oral administration of free Cy5-EXT + empty PC/NEMs, or Cy5-EXT@PC/NEMs of rats. (G) Organ-level biodistribution of orally ingested pBA NPs (mean ± SD, n = 6). (H) Accumulation in the pancreas at 4 h after oral gavage. Reprinted with the permission from Refs. 107, 183, and 185. Copyright © 2020 John Wiley and Sons, 2019 John Wiley and Sons, and 2021 Springer Nature.
The liver serves as a crucial target organ for insulin action. Hepatocytes, the primary cell type in the liver responsible for various physiological functions, such as glucose metabolism, storing and releasing glucose, as well as synthesizing and secreting bile171. Moreover, the surface of hepatocytes is characterized by a high density of insulin receptors (IRs), boasting approximately 200,000 receptors per hepatocyte, which makes hepatocytes the main site of insulin action172,173. However, upon entering the liver, oral anti-diabetic peptide formulations first reach the hepatic sinus146, a primary site for substance exchange and metabolic functions. The liver, being a representative RES organ, features Kupffer cells and liver sinusoidal endothelial cells (LSECs) in the hepatic sinus, both capable of engulfing formulations, particularly those with a size exceeding 100 nm146,174,175. This poses a challenge for these formulations to effectively reach hepatocytes. Therefore, achieving targeted delivery to hepatocytes becomes imperative to enable insulin to exert its effects and elicit biological responses effectively. Studies have demonstrated that the utilization of oral insulin delivery strategies, endowed with hepatocytes-targeting attributes, holds the capacity to augment hepatic insulin accumulation while re-establishing the critical liver-peripheral insulin gradient. In this modality, insulin exerts its influence on hepatic insulin receptors, thereby facilitating glucose uptake and glycogen storage, ultimately contributing to the maintenance of euglycemia and the mitigation of hypoglycemic episodes176,177. Toward this objective, we developed two delivery systems with this feature. One is a virus-mimetic multifunctional nano-carrier with a surface ligand-switchable, named Pep/Gal-PNP. This carrier, exhibiting pH-responsive characteristics, efficiently traverses the intestinal mucosa barrier and selectively delivers insulin to the liver by binding to the asialoglycoprotein receptor (ASGPR) on the surface of hepatocytes under physiological blood pH conditions (Fig. 7A–C)108. This selective targeting results in a significantly greater hepatic insulin utilization when compared to peripheral tissues (19.9% vs. ∼7%), culminating in a remarkable 7.2-fold increase in hepatic glycogen production. The other system, termed Pep-PMS, exhibits the capacity to surmount the epithelial barrier and achieve substantial accumulation within the space of Disse (the space between LSECs and hepatocytes), facilitating its interaction with IRs on the cell membrane of hepatocytes (Fig. 7D and E). Remarkably, Pep-PMS releases insulin in a glucose-responsive manner on multiple occasions, thereby ensuring a sustained reduction in blood glucose levels for up to 12 h107. Zhang et al.178 introduced a cholic acid modification group on the surface of oral insulin NPs, enabling precise delivery of encapsulated insulin to the target site, hepatocytes, through the enterohepatic circulation pathway. This strategy successfully increased the bioavailability of insulin to 26.9%. Bao et al.179 integrated cholic acids into the core of oral insulin delivery NPs (CN-DEX) and surface-modified them with dextran. The inclusion of dextran serves a dual purpose: it shields insulin from hydrolysis in the gastrointestinal tract and undergoes gradual degradation as CN-DEX passes through the mucus layer, unveiling cholic acid molecules, which can leverage bile acid transporters to facilitate intestinal absorption and hepatocytes-targeting. Additionally, the oral hepatocytes-targeted liposome technology, developed by the United States-based Diasome Company, has progressed into Phase III clinical trials.
The pancreas, serving as the primary site for insulin secretion, emerges as a pivotal target organ in the realm of diabetes therapy. It is noteworthy that a multitude of ion channels and receptors orchestrating the intricate regulation of insulin secretion are localized on the surface of pancreatic β cells. Among these, the GLP-1 receptors emerge as the preeminent target, prominently featured in contemporary research. The interaction of GLP-1 RA with its receptor triggers the release of insulin, which effect is glucose-dependent, maintaining blood glucose levels in balance without the risk of hypoglycemia and facilitating weight loss.
Due to the widespread distribution of GLP-1 receptors within the pancreas, GLP-1 RA possesses the ability to selectively target and accumulate within pancreatic tissues180. However, GLP-1 RA is susceptible to degradation during transit to the pancreas. Consequently, the majority of research efforts focus on enhancing its stability by amino acid substitution, fatty acid side chain modifications, and PEGylation, among others181. Furthermore, exploiting the targeting capabilities of GLP-1, the conjugation of GLP-1 as a ligand to carrier molecules allows for pancreatic targeting. Notably, Finan et al.182 demonstrated the functional targeting of GLP-1 receptors by conjugating GLP-1 with estrogen. In comparison to the unconjugated GLP-1 control, the synergistic interplay between these two compounds corrected obesity (more than twice the weight loss, P < 0.001), hyperglycemia (P < 0.001), and dyslipidemia (P < 0.05) in mice while mitigating adverse reactions in other tissues. Apart from active targeting, passive pancreatic targeting through the mesenteric lymphatic system has been shown to be advantageous for anti-diabetic peptide therapy. Lin et al.183 engineered phase-transitional nanoemulsions (EXT@PC/NEMs) for oral delivery of Exenatide (EXT). EXT@PC/NEMs are capable of passive targeting to the pancreas through the mesenteric lymphatic system, resulting in a relative pharmacokinetic bioavailability of 23.8 ± 5.0% (Fig. 7F). Additionally, this mode of pancreatic targeting has the potential to enhance blood glucose control. Chuang et al.184 adopted a NP approach composed of CS and poly(γ-glutamic acid) (CS/γPGA NPs) for the oral co-administration of bovine insulin and exendin-4 in a combinatorial therapeutic regimen. CS/γPGA NPs facilitated intestinal bypass penetration and the transport of bovine insulin and exendin-4 to the liver and pancreas, leading to approximately 4-fold and 3-fold increases in glucose absorption in the heart and skeletal muscle compared to control groups. Additionally, it is worth noting that recent research has indicated the potential action of insulin on the pancreas. Lee et al.185 developed oral insulin NPs, named Bile-acid-polymer (pBA) NPs, These NPs preferentially accumulate in the pancreas of mice, exhibiting high affinity with the bile acid receptor TGR5 on pancreatic islet cells (Fig. 7G and H). This interaction activates the secretion of GLP-1 and endogenous insulin, restoring blood glucose levels in mice and pigs with type 1 diabetes.
In summary, the targeted delivery of anti-diabetic peptides to metabolic organs, especially the liver and pancreas, holds the promise of achieving a safer and more effective reduction in blood glucose levels. Additionally, the associated research has illuminated the promise of this therapeutic paradigm. Nonetheless, it is important to note that the current investigations into oral antidiabetic peptide targeting therapy remain relatively limited, with a paucity of studies focusing on other metabolic organs integral to blood glucose regulation, such as white adipose tissue, brown adipose tissue, and skeletal muscles, which have been largely marginalized in the context of diabetes treatment. In light of this, future research should endeavor to expand the purview of therapeutic targets to encompass these metabolic organs, thereby leveraging their critical roles in blood glucose regulation. Such an expansion holds significant promise for the development of a more scientifically rigorous framework for the prevention and treatment of diabetes and its associated complications.
5. Conclusions and outlooks
The oral delivery of anti-diabetic peptides has long been recognized as a crucial goal within the field of diabetes treatment. This approach not only addresses the inconveniences associated with injections but, more importantly, could replicate the biodistribution profile of anti-diabetic peptides under physiological conditions. This emulation holds significant promise in enhancing blood sugar control and reducing the risk of diabetes-related complications. The foundation for achieving stable blood sugar regulation lies in optimizing absorption and ensuring favorable pharmacokinetic parameters. In this review, we present a comprehensive exploration of several exemplary strategies, encompassing a diverse array of approaches aimed at improving absorption and targeting major metabolic organs. These strategies are meticulously designed to surmount absorption barriers and navigate the metabolic pathways of peptides, thus ensuring both drug efficacy and safety. A profound understanding of the underlying mechanisms governing these strategies is pivotal in facilitating their more efficient development and utilization for anti-diabetic peptides.
Regrettably, despite the implementation of these delivery strategies, the conversion rate of oral anti-diabetic peptides remains suboptimal. This issue can be attributed, in part, to technological inadequacies, including challenges such as notably low bioavailability, dietary interference, and significant inter-individual variability in absorption6,186. Furthermore, limitations within the existing delivery strategies contribute to their inability to meet regulatory standards. While some absorption enhancers have facilitated drug marketing, numerous challenges persist in their practical application, encompassing issues such as unknown toxicity, irritation, and drug–excipient interactions, requiring in-depth study. Moreover, the absence of a standardized evaluation system for the absorption-promoting effects of absorption enhancers results in unclear mechanisms of action113. ILs, a more recent entrant for oral delivery of anti-diabetic peptides, have shown improved biocompatibility by incorporating natural metabolites; however, additional clinical data are requisite to verify therapeutic efficacy and ascertain long-term toxic effects129. Nanotechnology, while a relatively mature technology, encounters challenges in large-scale NP preparation, quality assurance, consistency, and stability. Potential immunological responses to NPs, leading to clearance or inflammatory reactions, necessitate further investigation into their biocompatibility187. MNs, capable of traversing multiple absorption barriers with efficacy comparable to subcutaneous injections58, pose challenges due to difficulty in removal, safety concerns related to gastrointestinal perforation, and inflammatory responses. The success rate of in vivo MN injection warrants additional scrutiny, considering potential hindrances posed by digestive juices and food debris. Beyond these challenges, the role of tissue distribution and association concerning anti-diabetic peptides has been somewhat overlooked. Different tissues exhibit varying sensitivities and functionalities in response to anti-diabetic peptides, often interconnected in intricate ways. For example, β cells demonstrate pulsatile insulin secretion, and the portal vein cyclically transports pancreatic insulin to the liver, occurring roughly every 5 min. Subsequently, insulin enters systemic circulation, leading to vasodilation and targeted distribution to vital organs such as the brain, adipose tissue, and kidneys. In this sequential process, the orchestration of tissue-specific effects plays a pivotal role in achieving glycemic homeostasis188, 189, 190. Similarly, GLP-1 receptors are predominantly expressed in pancreatic β cells but are also found in organs like the stomach, small intestine, heart, kidneys, lungs, and brain191, each with varying sensitivities and functions concerning GLP-1. It is essential to maintain concentration gradients to avoid adverse reactions. Therefore, we advocate for the development of targeted formulations for metabolic organs through the oral route, extending beyond liver targeting. These organs, owing to their roles in glucose metabolism, significantly contribute to the broader improvement of systemic glycemic control. However, challenges such as off-target effects, individual variations, and large-scale vehicle production need resolution for the clinical translation of targeted formulations.
The successful examples in this field offer valuable insights. For instance, the case of oral semaglutide tablets, marketed as Rybelsus®, effectively employs Eligen® technology, incorporating SNAC as an absorption enhancer to augment bioavailability21. Notably, while SNAC enhances absorption potential multifacetedly, the oral bioavailability of semaglutide remains within the range of 0.4%‒1%. However, Rybelsus® derives its efficacy not only from SNAC's absorption-enhancing properties but also from semaglutide's extended half-life of 165 h. In the context of a daily dosing regimen, the establishment of a steady-state drug concentration with ten-fold accumulation underscores its impact. Equally important, the pharmacokinetic coefficient of variation (CV) experiences a substantial reduction from an initial 150% to a more controlled range of 70–80% upon reaching a steady state192. This shift implies that sporadic instances of reduced absorption by patients once or twice a week do not result in significant fluctuations in blood sugar levels. Consequently, the diminished CV values, facilitated through careful dietary management and consistent semaglutide administration, effectively compensate for individual variabilities in semaglutide absorption. This approach serves as a notable model for emulation.
Additionally, the case of the oral insulin capsule, ORMD-0801, developed by Oramed through its proprietary POD™ technology, highlights the importance of personalized administration to mitigate the influence of individual differences. Although the phase III clinical trial of ORMD-0801 did not meet primary and secondary endpoints193, it revealed a favorable response within a subgroup of patients stratified based on specific parameters such as BMI. This observation aligns with positive data from the Phase III study conducted in China. The baseline BMI of the subgroup in the United States closely resembled that of subjects in the Chinese clinical trial. In April 2023, the National Medical Products Administration (NMPA) approved the application for listing ORMD-0801 in China, suggesting its anticipated future listing in the country28. Drawing inspiration from the ORMD-0801 experience, it is reasonable to infer that the future prospects of oral insulin may involve a more targeted approach, emphasizing personalized administration to mitigate the influence of individual differences.
In conclusion, oral anti-diabetic peptides represent a rational choice for diabetes treatment, with this approach gaining prominence due to advancements in fundamental research. However, the bioavailability of oral anti-diabetic peptides remains lower than that achieved through injections, highlighting the need for further optimization. The functions and interconnections of metabolic organs in response to anti-diabetic peptides warrant investigation, and the development of targeted formulations for these organs holds promise. Scientifically designed clinical trials, distinct from those for injectable formulations, should be established, guided by the unique pharmacological attributes of oral anti-diabetic peptides. This alignment will enable a robust realization of the clinical translation of oral anti-diabetic peptides, providing diabetic patients with enhanced and convenient therapeutic options.
Author contributions
Bingwen Ding: Writing - original draft, Writing - review & editing. Zhu Zhu: Writing - original draft, Writing - review & editing. Cong Guo: Writing - review. Jiaxin Li: Writing - review. Yong Gan: Writing - review & editing. Miaorong Yu: Writing - original draft, Writing - review & editing.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by the National Science Fund of Distinguished Young Scholars (No. 82025032, China), the National Natural Science Foundation of China (No. 82073773, China), the Key Research Program of Chinese Academy of Sciences (ZDBS-ZRKJZ-TLC005, China), and Young Elite Scientists Sponsorship Program by CAST (No. 2022QNRC001, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yong Gan, Email: ygan@simm.ac.cn.
Miaorong Yu, Email: mryu@simm.ac.cn.
References
- 1.Federation ID. IDF Diabetes Atlas-10th edition[EB/OL]. Available from: https://diabetesatlas.org/.
- 2.Warshauer J.T., Bluestone J.A., Anderson M.S. New frontiers in the treatment of type 1 diabetes. Cell Metab. 2020;31:46–61. doi: 10.1016/j.cmet.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soltani S., Mansouri K., Aleagha M.S.E., Moasefi N., Yavari N., Shakouri S.K., et al. Extracellular vesicle therapy for type 1 diabetes. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.865782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad E., Lim S., Lamptey R., Webb D.R., Davies M.J. Type 2 diabetes. Lancet. 2022;400:1803–1820. doi: 10.1016/S0140-6736(22)01655-5. [DOI] [PubMed] [Google Scholar]
- 6.Nauck M.A., Wefers J., Meier J.J. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endo. 2021;9:525–544. doi: 10.1016/S2213-8587(21)00113-3. [DOI] [PubMed] [Google Scholar]
- 7.Amori R.E., Lau J., Pittas A.G. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Owens D.R., Monnier L., Ceriello A., Bolli G.B. Insulin centennial: milestones influencing the development of insulin preparations since 1922. Diabetes Obes Metab. 2022;24:S27–S42. doi: 10.1111/dom.14587. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann L., Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Technol. 2009;3:568–584. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moroz E., Matoori S., Leroux J.C. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. doi: 10.1016/j.addr.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Jea F, Inventor. Oral blood sugar lowering compositions. US patent US3172814 A1965. 1965 March 9.
- 12.Suarez S., Garcia-Contreras L., Sarubbi D., Flanders E., O'Toole D., Smart J., et al. Facilitation of pulmonary insulin absorption by H-MAP: pharmacokinetics and pharmacodynamics in rats. Pharm Res. 2001;18:1677–1684. doi: 10.1023/a:1013362227548. [DOI] [PubMed] [Google Scholar]
- 13.Zijlstra E., Heinemann L., Plum-Morschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8:458–465. doi: 10.1177/1932296814529988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.Y., Martinez J., Dass C.R. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol. 2016;68:1093–1108. doi: 10.1111/jphp.12607. [DOI] [PubMed] [Google Scholar]
- 15.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 16.Karagiannis T., Avgerinos I., Liakos A., Del Prato S., Matthews D.R., Tsapas A., et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65:1251–1261. doi: 10.1007/s00125-022-05715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drucker D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol Metab. 2022;57 doi: 10.1016/j.molmet.2021.101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorriz J.L., Romera I., Cobo A., O'Brien P.D., Merino-Torres J.F. Glucagon-like peptide-1 receptor agonist use in people living with type 2 diabetes mellitus and chronic kidney disease: a narrative review of the key evidence with practical considerations. Diabetes Ther. 2022;13:389–421. doi: 10.1007/s13300-021-01198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson M.B., Bate G., Kirkpatrick P. Exenatide. Nat Rev Drug Discov. 2005;4:713–714. doi: 10.1038/nrd1828. [DOI] [PubMed] [Google Scholar]
- 20.Lau J., Bloch P., Schaffer L., Pettersson I., Spetzler J., Kofoed J., et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 21.Bucheit J.D., Pamulapati L.G., Carter N., Malloy K., Dixon D.L., Sisson E.M. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol Ther. 2020;22:10–18. doi: 10.1089/dia.2019.0185. [DOI] [PubMed] [Google Scholar]
- 22.Zizzari A.T., Pliatsika D., Gall F.M., Fischer T., Riedl R. New perspectives in oral peptide delivery. Drug Discov Today. 2021;26:1097–1105. doi: 10.1016/j.drudis.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Administration FaD . 2021. Drug trials snapshot: LUPKYNIS. [Google Scholar]
- 24.Brayden D.J., Maher S. Transient Permeation Enhancer(R) (TPE(R)) technology for oral delivery of octreotide: a technological evaluation. Expert Opin Drug Deliv. 2021;18:1501–1512. doi: 10.1080/17425247.2021.1942838. [DOI] [PubMed] [Google Scholar]
- 25.Diasome P. Study of two doses of oral HDV-insulin and placebo with background metformin treatment in patients with type 2 diabetes mellitus. Available from: https://classic.clinicaltrials.gov/show/NCT00814294.
- 26.Oramed L., Integrium A phase 3 study to evaluate the efficacy and safety of ORMD-0801 in subjects with type 2 diabetes mellitus. 2023. https://classic.clinicaltrials.gov/show/NCT04754334 Available from:
- 27.Oramed L., Integrium Study to evaluate the efficacy and safety of ORMD-0801 in subjects with type 2 diabetes mellitus. 2023. https://classic.clinicaltrials.gov/show/NCT04606576 Available from:
- 28.Oramed. Oramed announces that its Chinese partner, HTIT, has successfully completed a phase 3 oral insulin clinical trial and submitted a marketing authorization application in China. 05/15/2023. Available from: https://www.prnewswire.com/news-releases/oramed-announces-that-its-chinese-partner-htit-has-successfully-completed-a-phase-3-oral-insulin-clinical-trial-and-submitted-a-marketing-authorization-application-in-china-301824604.html.
- 29.Bows Pharmaceuticals AG. A two part study of peroral insulin in type 2 diabetes. Available from: https://classic.clinicaltrials.gov/show/NCT00990444.
- 30.Oshadi Drug A. Safety and tolerability of oshadi lcp in patients with type 1 diabetes mellitus―phase Ib clinical study. Available from: https://classic.clinicaltrials.gov/show/NCT01772251.
- 31.Zhu Q.G., Chen Z.J., Paul P.K., Lu Y., Wu W., Qi J.P. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. Acta Pharm Sin B. 2021;11:2416–2448. doi: 10.1016/j.apsb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X.Q., Zhang Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 2012;82:219–229. doi: 10.1016/j.ejpb.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Li X.Y., Yu M.R., Fan W.W., Gan Y., Hovgaard L., Yang M.S. Orally active-targeted drug delivery systems for proteins and peptides. Expert Opin Drug Deliv. 2014;11:1435–1447. doi: 10.1517/17425247.2014.924500. [DOI] [PubMed] [Google Scholar]
- 34.Zambanini A., Newson R.B., Maisey M., Feher M.D. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–246. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 35.Alqahtani M.S., Kazi M., Alsenaidy M.A., Ahmad M.Z. Advances in oral drug delivery. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraghdari Z.B., Imani R., Mohabatpour F. A review on bioengineering approaches to insulin delivery: a pharmaceutical and engineering perspective. Macromol Biosci. 2019;19 doi: 10.1002/mabi.201800458. [DOI] [PubMed] [Google Scholar]
- 37.Shao J.T., Zaro J.L., Shen W.C. Proinsulin-transferrin fusion protein exhibits a prolonged and selective effect on the control of hepatic glucose production in an experimental model of type 1 diabetes. Mol Pharm. 2016;13:2641–2646. doi: 10.1021/acs.molpharmaceut.6b00168. [DOI] [PubMed] [Google Scholar]
- 38.Gedawy A., Martinez J., Al-Salami H., Dass C.R. Oral insulin delivery: existing barriers and current counter-strategies. J Pharm Pharmacol. 2018;70:197–213. doi: 10.1111/jphp.12852. [DOI] [PubMed] [Google Scholar]
- 39.Norton L., Shannon C., Gastaldelli A., DeFronzo R.A. Insulin: the master regulator of glucose metabolism. Metabolism. 2022;129 doi: 10.1016/j.metabol.2022.155142. [DOI] [PubMed] [Google Scholar]
- 40.Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Holst J.J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 42.Muttenthaler M., King G.F., Adams D.J., Alewood P.F. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309–325. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- 43.Han Y., Gao Z.G., Chen L.Q., Kang L., Huang W., Jin M.J., et al. Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins. Acta Pharm Sin B. 2019;9:902–922. doi: 10.1016/j.apsb.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole E.T., Scott R.A., Connor A.L., Wilding I.R., Petereit H.U., Schminke C., et al. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- 45.Lee S.H., Song J.G., Han H.K. Site-selective oral delivery of therapeutic antibodies to the inflamed colon via a folic acid-grafted organic/inorganic hybrid nanocomposite system. Acta Pharm Sin B. 2022;12:4249–4261. doi: 10.1016/j.apsb.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bysell H., Mansson R., Hansson P., Malmsten M. Microgels and microcapsules in peptide and protein drug delivery. Adv Drug Deliv Rev. 2011;63:1172–1185. doi: 10.1016/j.addr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Ichikawa H., Fukumori Y. [Design of nanohydrogel-incorporated microcapsules for appropriate controlled-release of peptide drugs] Yakugaku Zasshi. 2007;127:813–823. doi: 10.1248/yakushi.127.813. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto A., Taniguchi T., Rikyuu K., Tsuji T., Fujita T., Murakami M., et al. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res. 1994;11:1496–1500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto A., Ukai H., Morishita M., Katsumi H. Approaches to improve intestinal and transmucosal absorption of peptide and protein drugs. Pharmacol Ther. 2020;211 doi: 10.1016/j.pharmthera.2020.107537. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto A., Umemori S., Muranishi S. Absorption enhancement of intrapulmonary administered insulin by various absorption enhancers and protease inhibitors in rats. J Pharm Pharmacol. 1994;46:14–18. doi: 10.1111/j.2042-7158.1994.tb03712.x. [DOI] [PubMed] [Google Scholar]
- 51.Murgia X., Loretz B., Hartwig O., Hittinger M., Lehr C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev. 2018;124:82–97. doi: 10.1016/j.addr.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Li Z., Li H.Y., Wang C.F., Xu J.H., Singh V., Chen D.W., et al. Sodium dodecyl sulfate/beta-cyclodextrin vesicles embedded in chitosan gel for insulin delivery with pH-selective release. Acta Pharm Sin B. 2016;6:344–351. doi: 10.1016/j.apsb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen N.H., Kroger C.J., Tisch R.M., Bachelder E.M., Ainslie K.M. Prevention of type 1 diabetes with acetalated dextran microparticles containing rapamycin and pancreatic peptide P31. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800341. [DOI] [PubMed] [Google Scholar]
- 54.Gao W., Dong R.F., Thamphiwatana S., Li J.X., Gao W.W., Zhang L.F., et al. Artificial micromotors in the mouse's stomach: a step toward in vivo use of synthetic motors. ACS Nano. 2015;9:117–123. doi: 10.1021/nn507097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karshalev E., de Avila B.E.F., Beltran-Gastelum M., Angsantikul P., Tang S.S., Mundaca-Uribe R., et al. Micromotor pills as a dynamic oral delivery platform. ACS Nano. 2018;12:8397–8405. doi: 10.1021/acsnano.8b03760. [DOI] [PubMed] [Google Scholar]
- 56.de Ávila B.E.F., Angsantikul P., Li J.X., Gao W., Zhang L.F., Wang J. Micromotors go in vivo: from test tubes to live animals. Adv Funct Mater. 2017;28:1705640. [Google Scholar]
- 57.Li J.X., de Avila B.E.F., Gao W., Zhang L.F., Wang J. Micro/Nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Robot. 2017;2:eaam6431. doi: 10.1126/scirobotics.aam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traverso G., Schoellhammer C.M., Schroeder A., Maa R., Lauwers G.Y., Polat B.E., et al. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015;104:362–367. doi: 10.1002/jps.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S.Y., Bellinger A.M., Glettig D.L., Barman R., Lee Y.A.L., Zhu J.H., et al. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat Mater. 2015;14:1065–1071. doi: 10.1038/nmat4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zahoor I., Singh S., Behl T., Sharma N., Naved T., Subramaniyan V., et al. Emergence of microneedles as a potential therapeutics in diabetes mellitus. Environ Sci Pollut Res Int. 2022;29:3302–3322. doi: 10.1007/s11356-021-17346-0. [DOI] [PubMed] [Google Scholar]
- 61.Sheng J.Y., Han L.M., Qin J., Ru G., Li R.X., Wu L.H., et al. N-Trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl Mater Interfaces. 2015;7:15430–15441. doi: 10.1021/acsami.5b03555. [DOI] [PubMed] [Google Scholar]
- 62.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huckaby J.T., Lai S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. doi: 10.1016/j.addr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 64.de Sousa I.P., Moser T., Steiner C., Fichtl B., Bernkop-Schnurch A. Insulin loaded mucus permeating nanoparticles: addressing the surface characteristics as feature to improve mucus permeation. Int J Pharm. 2016;500:236–244. doi: 10.1016/j.ijpharm.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Xiong M.T., Ni X.M., Wang J.R., Rong H.H., Su Y.Q., et al. Virus-mimicking mesoporous silica nanoparticles with an electrically neutral and hydrophilic surface to improve the oral absorption of insulin by breaking through dual barriers of the mucus layer and the intestinal epithelium. ACS Appl Mater Interfaces. 2021;13:18077–18088. doi: 10.1021/acsami.1c00580. [DOI] [PubMed] [Google Scholar]
- 66.Yin L.C., Fei L.K., Cui F.Y., Tang C., Yin C.H. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials. 2007;28:1258–1266. doi: 10.1016/j.biomaterials.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Li X., Fu M., Wu J., Zhang C.Y., Deng X., Dhinakar A., et al. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017;51:294–303. doi: 10.1016/j.actbio.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Liu C., Jiang X.H., Gan Y., Yu M.R. Engineering nanoparticles to overcome the mucus barrier for drug delivery: design, evaluation and state-of-the-art. Med Drug Discovery. 2021;12:100110. [Google Scholar]
- 69.Lai S.K., O'Hanlon D.E., Harrold S., Man S.T., Wang Y.Y., Cone R., et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X.Y., Guo S.Y., Zhu C.L., Zhu Q.L., Gan Y., Rantanen J., et al. Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles. Biomaterials. 2013;34:9678–9687. doi: 10.1016/j.biomaterials.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 71.Wang A.H., Yang T.T., Fan W.W., Yang Y.W., Zhu Q.L., Guo S.Y., et al. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Adv Healthc Mater. 2019;8 doi: 10.1002/adhm.201801123. [DOI] [PubMed] [Google Scholar]
- 72.Han X.F., Lu Y., Xie J.B., Zhang E.S., Zhu H., Du H., et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat Nanotechnol. 2020;15:605–614. doi: 10.1038/s41565-020-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dahlgren D., Olander T., Sjoblom M., Hedeland M., Lennernas H. Effect of paracellular permeation enhancers on intestinal permeability of two peptide drugs, enalaprilat and hexarelin, in rats. Acta Pharm Sin B. 2021;11:1667–1675. doi: 10.1016/j.apsb.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shetty S.S., Halagali P., Johnson A.P., Spandana K.M.A., Gangadharappa H.V. Oral insulin delivery: barriers, strategies, and formulation approaches: a comprehensive review. Int J Biol Macromol. 2023;242 doi: 10.1016/j.ijbiomac.2023.125114. [DOI] [PubMed] [Google Scholar]
- 75.Buckley S.T., Baekdal T.A., Vegge A., Maarbjerg S.J., Pyke C., Ahnfelt-Ronne J., et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10:eaar7047. doi: 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y.N., Zheng Y.X., Wu L., Zhu X., Zhang Z.R., Huang Y. Novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Appl Mater Interfaces. 2018;10:9315–9324. doi: 10.1021/acsami.8b00507. [DOI] [PubMed] [Google Scholar]
- 77.He H.S., Lu Y., Qi J.P., Zhao W.L., Dong X.C., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sin B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goo Y.T., Lee S., Choi J.Y., Kim M.S., Sin G.H., Hong S.H., et al. Enhanced oral absorption of insulin: hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Deliv. 2022;29:2831–2845. doi: 10.1080/10717544.2022.2118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J.Y., Zhang Y.Q., Yu M.R., Wang A.H., Qiu Y., Fan W.W., et al. The upregulated intestinal folate transporters direct the uptake of ligand-modified nanoparticles for enhanced oral insulin delivery. Acta Pharm Sin B. 2022;12:1460–1472. doi: 10.1016/j.apsb.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo G.S., Li Z.B., Lin X., Li X.Y., Chen Y., Xi K., et al. Discovery of an orally active VHL-recruiting PROTAC that achieves robust HMGCR degradation and potent hypolipidemic activity in vivo. Acta Pharm Sin B. 2021;11:1300–1314. doi: 10.1016/j.apsb.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang J.F., Yang V.C. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. 2005;335:734–738. doi: 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- 82.Korivi M., Huang Y.W., Liu B.R. Cell-penetrating peptides as a potential drug delivery system for effective treatment of diabetes. Curr Pharm Des. 2021;27:816–825. doi: 10.2174/1381612826666201019102640. [DOI] [PubMed] [Google Scholar]
- 83.Rehmani S., Dixon J.E. Oral delivery of anti-diabetes therapeutics using cell penetrating and transcytosing peptide strategies. Peptides. 2018;100:24–35. doi: 10.1016/j.peptides.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Brown T.D., Whitehead K.A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat Rev Mater. 2019;5:127–148. [Google Scholar]
- 85.Xiao Y.F., Tang Z.M., Wang J.Q., Liu C., Kong N., Farokhzad O.C., et al. Oral insulin delivery platforms: strategies to address the biological barriers. Angew Chem Int Ed Engl. 2020;59:19787–19795. doi: 10.1002/anie.202008879. [DOI] [PubMed] [Google Scholar]
- 86.Daugherty A.L., Mrsny R.J. Transcellular uptake mechanisms of the intestinal epithelial barrier Part one. Pharm Sci Technol Today. 1999;4:144–151. doi: 10.1016/s1461-5347(99)00142-x. [DOI] [PubMed] [Google Scholar]
- 87.Ren C.J., Zhong D.N., Qi Y.C., Liu C.Y., Liu X.Y., Chen S.H., et al. Bioinspired pH-responsive microalgal hydrogels for oral insulin delivery with both hypoglycemic and insulin sensitizing effects. ACS Nano. 2023;17:14161–14175. doi: 10.1021/acsnano.3c04897. [DOI] [PubMed] [Google Scholar]
- 88.Haddadzadegan S., Dorkoosh F., Bernkop-Schnurch A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Deliv Rev. 2022;182 doi: 10.1016/j.addr.2021.114097. [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Lopez A.L., Gonzalez-Navarro C.J., Aranaz P., Vizmanos J.L., Irache J.M. In vivo testing of mucus-permeating nanoparticles for oral insulin delivery using Caenorhabditis elegans as a model under hyperglycemic conditions. Acta Pharm Sin B. 2021;11:989–1002. doi: 10.1016/j.apsb.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johansson M.E.V., Sjovall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fan W.W., Xia D.N., Zhu Q.L., Hu L., Gan Y. Intracellular transport of nanocarriers across the intestinal epithelium. Drug Discov Today. 2016;21:856–863. doi: 10.1016/j.drudis.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Maher S., Mrsny R.J., Brayden D.J. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319. doi: 10.1016/j.addr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 94.Dey M., Das M., Chowhan A., Giri T.K. Breaking the barricade of oral chemotherapy through polysaccharide nanocarrier. Int J Biol Macromol. 2019;130:34–49. doi: 10.1016/j.ijbiomac.2019.02.094. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen V.H., Thuy V.N., Van T.V., Dao A.H., Lee B.-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano. 2022;8 [Google Scholar]
- 96.Lonn P., Kacsinta A.D., Cui X.S., Hamil A.S., Kaulich M., Gogoi K., et al. Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci Rep. 2016;6 doi: 10.1038/srep32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romero P., Donda A., Hubbell J.A. An optimized antigen-protein fusion. Nat Biomed Eng. 2020;4:583–584. doi: 10.1038/s41551-020-0572-3. [DOI] [PubMed] [Google Scholar]
- 98.Rennick J.J., Johnston A.P.R., Parton R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16:266–276. doi: 10.1038/s41565-021-00858-8. [DOI] [PubMed] [Google Scholar]
- 99.Wang T.R., Luo Y.C. Biological fate of ingested lipid-based nanoparticles: current understanding and future directions. Nanoscale. 2019;11:11048–11063. doi: 10.1039/c9nr03025e. [DOI] [PubMed] [Google Scholar]
- 100.Durnik R., Sindlerova L., Babica P., Jurcek O. Bile acids transporters of enterohepatic circulation for targeted drug delivery. Molecules. 2022;27:2961. doi: 10.3390/molecules27092961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y.Q., Wang Y.Y., Li X., Nie D., Liu C., Gan Y. Ligand-modified nanocarriers for oral drug delivery: challenges, rational design, and applications. J Control Release. 2022;352:813–832. doi: 10.1016/j.jconrel.2022.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Anselmo A.C., Gokarn Y., Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18:19–40. doi: 10.1038/nrd.2018.183. [DOI] [PubMed] [Google Scholar]
- 103.Cai Y.F., Qi J.P., Lu Y., He H.S., Wu W. The in vivo fate of polymeric micelles. Adv Drug Deliv Rev. 2022;188 doi: 10.1016/j.addr.2022.114463. [DOI] [PubMed] [Google Scholar]
- 104.Suk J.S., Xu Q.G., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thi T.T.H., Pilkington E.H., Nguyen D.H., Lee J.S., Park K.D., Truong N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers (Basel) 2020;12:298. doi: 10.3390/polym12020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie J., Shen Q., Huang K.X., Zheng T.Y., Cheng L.T., Zhang Z., et al. Oriented assembly of cell-mimicking nanoparticles via a molecular affinity strategy for targeted drug delivery. ACS Nano. 2019;13:5268–5277. doi: 10.1021/acsnano.8b09681. [DOI] [PubMed] [Google Scholar]
- 107.Wang A.H., Fan W.W., Yang T.T., He S.F., Yang Y.W., Yu M.R., et al. Liver-target and glucose-responsive polymersomes toward mimicking endogenous insulin secretion with improved hepatic glucose utilization. Adv Funct Mater. 2020;30 [Google Scholar]
- 108.Yang T.T., Wang A.H., Nie D., Fan W.W., Jiang X.H., Yu M.R., et al. Ligand-switchable nanoparticles resembling viral surface for sequential drug delivery and improved oral insulin therapy. Nat Commun. 2022;13:6649. doi: 10.1038/s41467-022-34357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ismail R., Csoka I. Novel strategies in the oral delivery of antidiabetic peptide drugs―Insulin, GLP 1 and its analogs. Eur J Pharm Biopharm. 2017;115:257–267. doi: 10.1016/j.ejpb.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 110.Zeynaloo E., Stone L.D., Dikici E., Ricordi C., Deo S.K., Bachas L.G., et al. Delivery of therapeutic agents and cells to pancreatic islets: towards a new era in the treatment of diabetes. Mol Aspects Med. 2022;83 doi: 10.1016/j.mam.2021.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Y.N., De Keersmaecker H., Braeckmans K., De Smedt S., Cani P.D., Preat V., et al. Targeted nanoparticles towards increased L cell stimulation as a strategy to improve oral peptide delivery in incretin-based diabetes treatment. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raval N., Kumawat A., Kalyane D., Kalia K., Tekade R.K. Understanding molecular upsets in diabetic nephropathy to identify novel targets and treatment opportunities. Drug Discov Today. 2020;25:862–878. doi: 10.1016/j.drudis.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Kim J.C., Park E.J., Na D.H. Gastrointestinal permeation enhancers for the development of oral peptide pharmaceuticals. Pharmaceuticals (Basel) 2022;15:1585. doi: 10.3390/ph15121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maher S., Brayden D.J., Casettari L., Illum L. Application of permeation enhancers in oral delivery of macromolecules: an update. Pharmaceutics. 2019;11:41. doi: 10.3390/pharmaceutics11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kondoh M., Yagi K. Progress in absorption enhancers based on tight junction. Expert Opin Drug Deliv. 2007;4:275–286. doi: 10.1517/17425247.4.3.275. [DOI] [PubMed] [Google Scholar]
- 116.Halberg I.B., Lyby K., Wassermann K., Heise T., Zijlstra E., Plum-Morschel L. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endo. 2019;7:179–188. doi: 10.1016/S2213-8587(18)30372-3. [DOI] [PubMed] [Google Scholar]
- 117.Tomita M., Hayashi M., Awazu S. Absorption-enhancing mechanism of sodium caprate and decanoylcarnitine in Caco-2 cells. J Pharmacol Exp Ther. 1995;272:739–743. [PubMed] [Google Scholar]
- 118.Lindmark T., Kimura Y., Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284:362–369. [PubMed] [Google Scholar]
- 119.Feighery L.M., Cochrane S.W., Quinn T., Baird A.W., O'Toole D., Owens S.E., et al. Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro. Pharm Res. 2008;25:1377–1386. doi: 10.1007/s11095-007-9527-6. [DOI] [PubMed] [Google Scholar]
- 120.Weng H.X., Hu L.F., Hu L., Zhou Y.H., Wang A.H., Wang N., et al. The complexation of insulin with sodium N-[8-(2-hydroxybenzoyl)amino]-caprylate for enhanced oral delivery: effects of concentration, ratio, and pH. Chinese Chem Lett. 2022;33:1889–1894. [Google Scholar]
- 121.Brayden D., Creed E., O'Connell A., Leipold H., Agarwal R., Leone-Bay A. Heparin absorption across the intestine: effects of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate in rat in situ intestinal instillations and in Caco-2 monolayers. Pharm Res. 1997;14:1772–1779. doi: 10.1023/a:1012192115828. [DOI] [PubMed] [Google Scholar]
- 122.Hess S., Rotshild V., Hoffman A. Investigation of the enhancing mechanism of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate effect on the intestinal permeability of polar molecules utilizing a voltage clamp method. Eur J Pharm Sci. 2005;25:307–312. doi: 10.1016/j.ejps.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Kane M.P., Triplitt C.L., Solis-Herrera C.D. Management of type 2 diabetes with oral semaglutide: practical guidance for pharmacists. Am J Health Syst Pharm. 2021;78:556–567. doi: 10.1093/ajhp/zxaa413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner A.M., Gran M.P., Peppas N.A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm Sin B. 2018;8:147–164. doi: 10.1016/j.apsb.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barbosa F.C., da Silva M.C., da Silva H.N., Albuquerque D., Gomes A.A.R., Silva S.M.D., et al. Progress in the development of chitosan based insulin delivery systems: a systematic literature review. Polymers (Basel) 2020;12:2499. doi: 10.3390/polym12112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mukhopadhyay P., Chakraborty S., Bhattacharya S., Mishra R., Kundu P.P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol. 2015;72:640–648. doi: 10.1016/j.ijbiomac.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 127.Kim J.U., Shahbaz H.M., Lee H., Kim T., Yang K., Roh Y.H., et al. Optimization of phytic acid-crosslinked chitosan microspheres for oral insulin delivery using response surface methodology. Int J Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119736. [DOI] [PubMed] [Google Scholar]
- 128.Smith J.M., Dornish M., Wood E.J. Involvement of protein kinase C in chitosan glutamate-mediated tight junction disruption. Biomaterials. 2005;26:3269–3276. doi: 10.1016/j.biomaterials.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 129.Curreri A.M., Mitragotri S., Tanner E.E.L. Recent advances in ionic liquids in biomedicine. Adv Sci (Weinh) 2021;8 doi: 10.1002/advs.202004819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Banerjee A., Ibsen K., Brown T., Chen R.W., Agatemor C., Mitragotri S. Ionic liquids for oral insulin delivery. Proc Natl Acad Sci U S A. 2018;115:7296–7301. doi: 10.1073/pnas.1722338115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Angsantikul P., Peng K.V., Curreri A.M., Chua Y., Chen K.V.Z., Ehondor J., et al. Ionic liquids and deep eutectic solvents for enhanced delivery of antibodies in the gastrointestinal tract. Adv Funct Mater. 2020;31:2002912. [Google Scholar]
- 132.Reslan M., Ranganathan V., Macfarlane D.R., Kayser V. Choline ionic liquid enhances the stability of Herceptin(R) (trastuzumab) Chem Commun (Camb) 2018;54:10622–10625. doi: 10.1039/c8cc06397d. [DOI] [PubMed] [Google Scholar]
- 133.Palanisamy K., Prakash M. The molecular mechanism behind the stabilization of insulin by choline and geranate (CAGE) ionic liquids - computational insights into oral insulin drug formulation. Phys Chem Chem Phys. 2021;23:25298–25307. doi: 10.1039/d1cp03349b. [DOI] [PubMed] [Google Scholar]
- 134.Agatemor C., Brown T.D., Gao Y.S., Ohmori N., Ibsen K.N., Mitragotri S. Choline-geranate deep eutectic solvent improves stability and half-life of glucagon-like peptide-1. Adv Thera. 2020;4 [Google Scholar]
- 135.Jiang L.X., Sun Y., Lu A., Wang X.Y., Shi Y.J. Ionic liquids: promising approach for oral drug delivery. Pharm Res. 2022;39:2353–2365. doi: 10.1007/s11095-022-03260-8. [DOI] [PubMed] [Google Scholar]
- 136.Li Y., Zhang W., Zhao R.C., Zhang X. Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact Mater. 2022;15:392–408. doi: 10.1016/j.bioactmat.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zaiki Y., Lim L.Y., Wong T.W. Critical material designs for mucus- and mucosa-penetrating oral insulin nanoparticle development. Int Mater Rev. 2022;68:121–139. [Google Scholar]
- 138.Lundquist P., Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 139.Chen M.C., Sonaje K., Chen K.J., Sung H.W. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–9838. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 140.McClements D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: a review. Adv Colloid Interface Sci. 2018;253:1–22. doi: 10.1016/j.cis.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 141.Souto E.B., Souto S.B., Campos J.R., Severino P., Pashirova T.N., Zakharova L.Y., et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules. 2019;24:4209. doi: 10.3390/molecules24234209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Banerjee A., Qi J.P., Gogoi R., Wong J., Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu M.R., Wang J.L., Yang Y.W., Zhu C.L., Su Q., Guo S.Y., et al. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett. 2016;16:7176–7182. doi: 10.1021/acs.nanolett.6b03515. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S.L., Gao H.J., Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoshyar N., Gray S., Han H.B., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond) 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu M.Z., Qi Y.M., Liu G.S., Song Y.Q., Jiang X.Y., Du B.J. Size-dependent in vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS Nano. 2023;17:20825–20849. doi: 10.1021/acsnano.3c05853. [DOI] [PubMed] [Google Scholar]
- 147.Ma N.N., Ma C., Li C.Y., Wang T., Tang Y.J., Wang H.Y., et al. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J Nanosci Nanotechnol. 2013;13:6485–6498. doi: 10.1166/jnn.2013.7525. [DOI] [PubMed] [Google Scholar]
- 148.Lamson N.G., Berger A., Fein K.C., Whitehead K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020;4:84–96. doi: 10.1038/s41551-019-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bao C., Liu B., Li B., Chai J.J., Zhang L.W., Jiao L.L., et al. Enhanced transport of shape and rigidity-tuned alpha-lactalbumin nanotubes across intestinal mucus and cellular barriers. Nano Lett. 2020;20:1352–1361. doi: 10.1021/acs.nanolett.9b04841. [DOI] [PubMed] [Google Scholar]
- 150.Yu M.R., Song W.Y., Tian F.L., Dai Z., Zhu Q.L., Ahmad E., et al. Temperature- and rigidity-mediated rapid transport of lipid nanovesicles in hydrogels. Proc Natl Acad Sci U S A. 2019;116:5362–5369. doi: 10.1073/pnas.1818924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng Y.X., Xing L.Y., Chen L.Q., Zhou R., Wu J.W., Zhu X., et al. Tailored elasticity combined with biomimetic surface promotes nanoparticle transcytosis to overcome mucosal epithelial barrier. Biomaterials. 2020;262 doi: 10.1016/j.biomaterials.2020.120323. [DOI] [PubMed] [Google Scholar]
- 152.Wu J.W., Zheng Y.X., Liu M., Shan W., Zhang Z.R., Huang Y. Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl Mater Interfaces. 2018;10:9916–9928. doi: 10.1021/acsami.7b16524. [DOI] [PubMed] [Google Scholar]
- 153.Shan W., Zhu X., Liu M., Li L., Zhong J.J., Sun W., et al. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano. 2015;9:2345–2356. doi: 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- 154.Yu M.R., Xu L., Tian F.L., Su Q., Zheng N., Yang Y.W., et al. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nat Commun. 2018;9:2607. doi: 10.1038/s41467-018-05061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rieux A.D., Pourcelle V., Cani P.D., Marchand-Brynaert J., Preat V. Targeted nanoparticles with novel non-peptidic ligands for oral delivery. Adv Drug Deliv Rev. 2013;65:833–844. doi: 10.1016/j.addr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 156.Kaklotar D., Agrawal P., Abdulla A., Singh R.P., Mehata A.K., Singh S., et al. Transition from passive to active targeting of oral insulin nanomedicines: enhancement in bioavailability and glycemic control in diabetes. Nanomedicine (Lond) 2016;11:1465–1486. doi: 10.2217/nnm.16.43. [DOI] [PubMed] [Google Scholar]
- 157.Yang D., Liu D.C., Deng H.L., Zhang J., Qin M.M., Yuan L., et al. Transferrin functionization elevates transcytosis of nanogranules across epithelium by triggering polarity-associated transport flow and positive cellular feedback loop. ACS Nano. 2019;13:5058–5076. doi: 10.1021/acsnano.8b07231. [DOI] [PubMed] [Google Scholar]
- 158.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R., Blumberg R.S., et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi Y.A., Sun X.F., Zhang L.P., Sun K.X., Li K.K., Li Y.X., et al. Fc-modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci Rep. 2018;8:726. doi: 10.1038/s41598-018-19170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ke Z.Y., Guo H., Zhu X., Jin Y., Huang Y. Efficient peroral delivery of insulin via vitamin B12 modified trimethyl chitosan nanoparticles. J Pharm Pharm Sci. 2015;18:155–170. doi: 10.18433/j3j88q. [DOI] [PubMed] [Google Scholar]
- 161.Dickinson B.L., Claypool S.M., D'Angelo J.A., Aiken M.L., Venu N., Yen E.H., et al. Ca2+-dependent calmodulin binding to FcRn affects immunoglobulin G transport in the transcytotic pathway. Mol Biol Cell. 2008;19:414–423. doi: 10.1091/mbc.E07-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fan W.W., Xia D.N., Zhu Q.L., Li X.Y., He S.F., Zhu C.L., et al. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials. 2018;151:13–23. doi: 10.1016/j.biomaterials.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 163.Xi Z.Y., Ahmad E., Zhang W., Li J.Y., Wang A.H., Faridoon, et al. Dual-modified nanoparticles overcome sequential absorption barriers for oral insulin delivery. J Control Release. 2022;342:1–13. doi: 10.1016/j.jconrel.2021.11.045. [DOI] [PubMed] [Google Scholar]
- 164.Lyu S., Dong Z.F., Xu X.X., Bei H.P., Yuen H.Y., Cheung C.W.J., et al. Going below and beyond the surface: microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioact Mater. 2023;27:303–326. doi: 10.1016/j.bioactmat.2023.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abramson A., Caffarel-Salvador E., Soares V., Minahan D., Tian R.Y., Lu X.Y., et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat Med. 2019;25:1512–1518. doi: 10.1038/s41591-019-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abramson A., Caffarel-Salvador E., Khang M., Dellal D., Silverstein D., Gao Y., et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363:611–615. doi: 10.1126/science.aau2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X.X., Chen G.P., Fu X., Wang Y.T., Zhao Y.J. Magneto-responsive microneedle robots for intestinal macromolecule delivery. Adv Mater. 2021;33 doi: 10.1002/adma.202104932. [DOI] [PubMed] [Google Scholar]
- 168.Kremsmayr T., Aljnabi A., Blanco-Canosa J.B., Tran H.N.T., Emidio N.B., Muttenthaler M. On the utility of chemical strategies to improve peptide gut stability. J Med Chem. 2022;65:6191–6206. doi: 10.1021/acs.jmedchem.2c00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luo Z., Paunovic N., Leroux J.C. Physical methods for enhancing drug absorption from the gastrointestinal tract. Adv Drug Deliv Rev. 2021;175 doi: 10.1016/j.addr.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 170.Yang Y.X., Zhou R.Y., Wang Y.F., Zhang Y.Q., Yu J.C., Gu Z. Recent advances in oral and transdermal protein delivery systems. Angew Chem Int Ed Engl. 2023;62 doi: 10.1002/anie.202214795. [DOI] [PubMed] [Google Scholar]
- 171.Titchenell P.M., Lazar M.A., Birnbaum M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28:497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Edgerton D.S., Scott M., Farmer B., Williams P.E., Madsen P., Kjeldsen T., et al. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight. 2019;5 doi: 10.1172/jci.insight.126974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li H.C., Jin K., Luo M., Wang X.J., Zhu X.W., Liu X.P., et al. Size Dependency of circulation and biodistribution of biomimetic nanoparticles: red blood cell membrane-coated nanoparticles. Cells. 2019;8:881. doi: 10.3390/cells8080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.He C.B., Hu Y.P., Yin L.C., Tang C., Yin C.H. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 176.Kurtzhals P., Ostergaard S., Nishimura E., Kjeldsen T. Derivatization with fatty acids in peptide and protein drug discovery. Nat Rev Drug Discov. 2023;22:59–80. doi: 10.1038/s41573-022-00529-w. [DOI] [PubMed] [Google Scholar]
- 177.Holt R.I.G., Mitchell A.J. Diabetes mellitus and severe mental illness: mechanisms and clinical implications. Nat Rev Endocrinol. 2015;11:79–89. doi: 10.1038/nrendo.2014.203. [DOI] [PubMed] [Google Scholar]
- 178.Zhang Z., Li H.X., Xu G.R., Yao P. Liver-targeted delivery of insulin-loaded nanoparticles via enterohepatic circulation of bile acids. Drug Deliv. 2018;25:1224–1233. doi: 10.1080/10717544.2018.1469685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bao X.Y., Qian K., Yao P. Insulin- and cholic acid-loaded zein/casein-dextran nanoparticles enhance the oral absorption and hypoglycemic effect of insulin. J Mater Chem B. 2021;9:6234–6245. doi: 10.1039/d1tb00806d. [DOI] [PubMed] [Google Scholar]
- 180.Knerr L., Prakash T.P., Lee R., Drury W.J., Nikan M., Fu W.X., et al. Glucagon like peptide 1 receptor agonists for targeted delivery of antisense oligonucleotides to pancreatic beta cell. J Am Chem Soc. 2021;143:3416–3429. doi: 10.1021/jacs.0c12043. [DOI] [PubMed] [Google Scholar]
- 181.Alavi S.E., Cabot P.J., Moyle P.M. Glucagon-like peptide-1 receptor agonists and strategies to improve their efficiency. Mol Pharm. 2019;16:2278–2295. doi: 10.1021/acs.molpharmaceut.9b00308. [DOI] [PubMed] [Google Scholar]
- 182.Finan B., Yang B., Ottaway N., Stemmer K., Muller T.D., Yi C.X., et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lin P.Y., Chen K.H., Miao Y.B., Chen H.L., Lin K.J., Chen C.T., et al. Phase-changeable nanoemulsions for oral delivery of a therapeutic peptide: toward targeting the pancreas for antidiabetic treatments using lymphatic transport. Adv Funct Mater. 2019;29 [Google Scholar]
- 184.Chuang E.Y., Nguyen G.T.H., Su F.Y., Lin K.J., Chen C.T., Mi F.L., et al. Combination therapy via oral co-administration of insulin- and exendin-4-loaded nanoparticles to treat type 2 diabetic rats undergoing OGTT. Biomaterials. 2013;34:7994–8001. doi: 10.1016/j.biomaterials.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 185.Lee J.S., Han P., Chaudhury R., Khan S., Bickerton S., McHugh M.D., et al. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat Biomed Eng. 2021;5:983–997. doi: 10.1038/s41551-021-00791-0. [DOI] [PubMed] [Google Scholar]
- 186.Mathieu C., Martens P.J., Vangoitsenhoven R. One hundred years of insulin therapy. Nat Rev Endocrinol. 2021;17:715–725. doi: 10.1038/s41574-021-00542-w. [DOI] [PubMed] [Google Scholar]
- 187.Wong C.Y., Al-Salami H., Dass C.R. Current status and applications of animal models in pre-clinical development of orally administered insulin-loaded nanoparticles. J Drug Target. 2020;28:882–903. doi: 10.1080/1061186X.2020.1759078. [DOI] [PubMed] [Google Scholar]
- 188.Moen J. The reponic classification of insulin. Can J Diabetes. 2023;47:680–681. doi: 10.1016/j.jcjd.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 189.Heni M., Kullmann S., Preissl H., Fritsche A., Haring H.U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol. 2015;11:701–711. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 190.Dominiczak M.H. Medical Biochemistry. 4th ed. Elsevier; Amsterdam: 2014. Glucose homeostasis and fuel metabolism: diabetes mellitus; pp. 264–290. Available from: https://www.evolve.elsevier.com/cs/product/9781455745821. [Google Scholar]
- 191.Zhao X., Wang M.H., Wen Z.T., Lu Z.H., Cui L.J., Fu C., et al. GLP-1 receptor agonists: beyond their pancreatic effects. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.721135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Administration USFaD. Rybelsus clinical pharmacology review(s). Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213182Orig1s000ClinPharmR.pdf.
- 193.FDAnews. Oramed's ORMD-0801 fails in type 2 diabetes study. Available from: https://www.fdanews.com/articles/210827-orameds-ormd-0801-fails-in-type-2-diabetes-study.