Abstract
Enterovirus 70 (EV70), the causative agent of acute hemorrhagic conjunctivitis, exhibits a restricted tropism for conjunctival and corneal cells in vivo but infects a wide spectrum of mammalian cells in culture. Previously, we demonstrated that human CD55 is a receptor for EV70 on HeLa cells but that EV70 also binds to sialic acid-containing receptors on a variety of other human cell lines. Virus recognition of sialic acid attached to underlying glycans by a particular glycosidic linkage may contribute to host range, tissue tropism, and pathogenesis. Therefore, we tested the possibility that EV70 binds to α2,3-linked sialic acid, like other viruses associated with ocular infections. Through the use of linkage-specific sialidases, sialyltransferases, and lectins, we show that EV70 recognizes α2,3-linked sialic acid on human corneal epithelial cells and on U-937 cells. Virus attachment to both cell lines is CD55 independent and sensitive to benzyl N-acetyl-α-d-galactosaminide, an inhibitor of O-linked glycosylation. Virus binding to corneal cells, but not U-937 cells, is inhibited by proteinase K, but not by phosphatidylinositol-specific phospholipase C treatment. These results are consistent with the idea that a major EV70 receptor on corneal epithelial cells is an O-glycosylated, non-glycosyl phosphatidylinositol-anchored membrane glycoprotein containing α2,3-linked sialic acid, while sialylated receptors on U-937 cells are not proteinaceous.
Sialic acids are a ubiquitous family of negatively charged sugar molecules found on the surfaces of mammalian cells, usually at the termini of glycans attached to glycoproteins, glycosphingolipids, and the proteoglycan keratan sulfate (2, 10, 72). Sialic acid is an essential component of cell surface receptors for a variety of microorganisms and microbial toxins (2, 34, 47). Members of at least eight different virus families—Orthomyxoviridae (63), Paramyxoviridae (62), Coronaviridae (40), Reoviridae (3, 6, 20), Picornaviridae (1, 32, 55, 61, 70, 71, 80), Parvoviridae (33, 73, 75), Papovaviridae (8, 12, 25, 43), and Adenoviridae (4, 5)—including enveloped and nonenveloped viruses and RNA and DNA viruses, exploit sialoglycoconjugates for attachment. Sialic acid is normally linked to underlying glycans by α2,3 or α2,6 bonds to a galactose moiety or by α2,8 bonds to an internal sialic acid (2, 72). Combinations of different glycosidic linkages and numerous substitutions that can occur on the pyranoside ring and side chains of sialic acid generate considerable diversity in sialic acid structure. Some viruses bind preferentially to sialic acid attached by a particular glycosidic linkage (4, 29, 33, 43, 49, 51), and this specificity may contribute to virus host range, tissue tropism, and pathogenesis. For example, the preference of human and avian influenza A hemagglutinins (HAs) for α2,6- or α2,3-linked sialic acid, respectively, correlates with the abundance of these linkages on their target cells, respiratory epithelial cells in humans and intestinal cells in birds (16, 44, 45, 49).
Enterovirus 70 (EV70), the causative agent of acute hemorrhagic conjunctivitis, exhibits a restricted in vivo tropism for the human conjunctival and corneal epithelium and has also been associated, in rare instances, with neurological sequelae (69, 77, 78). In vitro, EV70 replicates in cells derived from a wide variety of mammalian species (79). Because virus receptors are recognized as important determinants of host range, tissue tropism, and pathogenesis (23, 54), the identification of EV70 receptors should help to explain the unique properties of this virus. Previously, we demonstrated that the glycosyl phosphatidylinositol (GPI)-linked protein CD55 plays a major role in EV70 binding to, and infection of, HeLa cells (35, 36). However, EV70 attachment to all other human cell lines that we have tested was CD55 independent but required sialic acid, as determined by sensitivity to Vibrio cholerae sialidase, and sialidase treatment of HeLa cells also reduced EV70 binding (1, 28). Like EV70, both adenovirus 37 and some avian influenza A viruses, in particular H7 subtypes, are associated with eye infections (4, 5, 13, 24, 39, 64). Adenovirus 37 (4, 5) and avian influenza viruses (14, 31, 51, 52) recognize receptors containing α2,3-linked sialic acid, suggesting that this specificity contributes to the ocular tropism of these viruses. We hypothesized that EV70 would also recognize α2,3-linked sialic acid. Using a combination of linkage-specific sialidases, sialyltransferases, and lectins, we demonstrate that EV70 exhibits a strong preference for binding to α2,3-linked sialic acid on the surfaces of U-937 cells and human corneal epithelial cells. We also provide evidence that attachment molecules for EV70 on corneal epithelial cells are proteinaceous while the molecules recognized by EV70 on the surfaces of U-937 cells are not.
MATERIALS AND METHODS
Viruses and cell lines.
The EV70 prototype strain J670/71 was obtained from M. Hatch and M. Pallansch (Centers for Disease Control and Prevention, Atlanta, GA). EV70 was propagated and infectious titers were determined in LLC-MK2 rhesus macaque kidney cells as described previously (35). U-937 cells, HeLa T4 cells, and LLC-MK2 cells were maintained in culture as described previously (1, 28). Human corneal epithelial (HCE) cells immortalized with SV40 large T antigen (26) were propagated in keratinocyte serum-free medium (Invitrogen Life Technologies, Burlington, ON, Canada) supplemented with bovine pituitary extract (25 μg/ml; Invitrogen) and recombinant epidermal growth factor (0.2 ng/ml; Invitrogen).
Time course of EV70 replication in U-937 cells.
U-937 cells were counted, washed once, resuspended to a cell density of 3.0 × 107/ml, and infected at a multiplicity of infection (MOI) of 5 PFU/cell in a final volume of 100 μl of OptiMEM (Invitrogen) for 1 h at 32°C. Infected cells were washed three times with 3 ml of medium to remove unbound virus and resuspended in a final volume of 8 ml RPMI 1640 (Sigma). Duplicate 1-ml aliquots were withdrawn immediately and at various times after infection and frozen at −80°C. Following two or three cycles of freezing and thawing, the amount of infectious virus in each sample was determined by plaque assay on monolayers of LLC-MK2 cells (35).
Virus binding assays.
35S-labeled EV70 was harvested from LLC-MK2 cells and purified by sucrose gradient ultracentrifugation as described previously (35). Preparations of radiolabeled EV70 contained approximately 108 PFU/ml. The total amount of protein in virus preparations was determined and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Using these values, we estimated particle/PFU ratios to be ≥2,000:1. For virus binding assays, suspension cells were washed once and resuspended to a cell density of 2.5 × 106/ml in OptiMEM containing 0.1% (wt/vol) sodium azide, and nonsaturating amounts (1 to 4 μl) of radiolabeled EV70 were added per 200-μl sample. Following a 1-h incubation at 32°C, radioactivity in bound and unbound fractions was determined by liquid scintillation counting (35). In these studies, U-937 cells bound 20 to 30% of input radiolabeled EV70. HCE cells were dispersed with 0.05% trypsin-EDTA, washed three times in OptiMEM, and counted before use in binding assays. Typically, HCE cells bound 12 to 18% of input radiolabeled EV70, and there was little or no difference in the extents of EV70 binding to HCE cells dispersed with trypsin-EDTA or with EDTA alone (data not shown). To confirm the specificity of virus attachment, radiolabeled virus was incubated for 1 h with ev-12, an EV70-specific neutralizing monoclonal antibody (76).
Sialidase treatment of cells.
Streptococcus pneumoniae (Glyko 80020) and Clostridium perfringens (Glyko 80030) sialidases were purchased from Prozyme (San Leandro, CA). Vibrio cholerae sialidase was obtained from Roche (Laval, QC, Canada; no. 1080725). U-937 cells were pelleted, washed, and resuspended to a cell density of 5 × 107/ml in OptiMEM containing 0.1% (wt/vol) sodium azide. The cells were incubated at 37°C for 30 min in the presence or absence of sialidase (10 to 100 mU/ml). Adherent cells were dispersed with trypsin-EDTA, washed three times, and incubated using identical conditions. For V. cholerae sialidase, OptiMEM (adjusted to pH 5.7) was supplemented with 4 mM CaCl2. After sialidase treatment, the cells were washed twice, counted, and used in virus binding assays or for infection. Using similar reaction conditions and 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (Sigma) as a substrate (56), the relative activities of the three sialidases were as follows: C. perfringens sialidase > V. cholerae sialidase > S. pneumoniae sialidase (data not shown). The addition of protease inhibitors during incubation of cells with sialidase did not alter the results (data not shown), indicating that the effects seen were not due to protease contamination of the sialidases.
Sialyltransferase treatment of U-937 cells.
α2,3(N) (ST3GalIII), α2,3(O) (ST3GalII) and α2,6(N) (ST6GalI) sialyltransferases (Calbiochem/Merck products) were purchased from VWR Canlab (Mississauga, ON, Canada). U-937 cells were pelleted, washed twice, and resuspended in OptiMEM (pH 5.7; 4 mM CaCl2) to a final cell density of 5 × 107/ml. The cells were then incubated in the presence or absence (untreated control) of 50 mU/ml V. cholerae sialidase for 30 min at 37°C, washed twice, and reuspended in OptiMEM (pH 7.2) to a final cell density of 4.5 × 107/ml. Sialyltransferase (50 mU/ml) and CMP-β-d-sialic acid (1 mM; VWR Canlab) were added to one aliquot of sialidase-treated cells. All three samples (untreated control cells, sialidase-treated cells, and sialidase- and sialyltransferase-treated cells) were incubated for 4 h at 37°C. The cells were washed twice in OptiMEM and counted, and cell viability was assessed by staining with trypan blue. The cells were pelleted, resuspended to a cell density of 107/ml, and used in binding assays.
Lectin blockade of EV70 binding.
Lectins SNA (Sambucus nigra), MAL I (Maackia amurensis leukoagglutinin), and MAL II (Maackia amurensis hemagglutinin) were purchased from Vector Laboratories (Burlington, ON, Canada). U-937 cells were pelleted, washed, resuspended to a cell density of 3.5 × 106/ml in OptiMEM in the presence or absence of lectin, and incubated for 30 min at room temperature. The cells were then washed once, and virus binding was assessed. HCE cells were dispersed with trypsin-EDTA and washed three times before incubation with lectin.
Antibody blockade of EV70 binding.
The monoclonal antibody EVR1, which is specific for complement control protein repeat 1 of human CD55 (35), was provided by E. Altman (National Research Council of Canada, Ottawa, ON, Canada). Cells (106/ml) were washed once and incubated in the presence or absence of 17.5 μg/ml of monoclonal antibody EVR1 for 1 h at 37°C. This concentration of EVR1 saturates binding sites on several cell lines (data not shown). The cells were then washed and used in virus binding assays. HCE cells were dispersed with trypsin-EDTA and washed three times before incubation with EVR1.
Flow cytometry.
To monitor cell surface CD55, cells were washed once in flow cytometry buffer (PBS containing 2% [wt/vol] bovine serum albumin and 0.1% [wt/vol] sodium azide) and then resuspended (3 × 105 cells/sample) in 150 μl of flow cytometry buffer containing monoclonal antibody EVR1 for 15 min at room temperature. Adherent cells were dispersed with trypsin-EDTA and washed three times before incubation with EVR1. The cells were washed with 4 ml of PBS-azide and resuspended in 150 μl of flow cytometry buffer containing a 1:1,000 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin (Roche) for 15 min at room temperature. The cells were diluted in flow cytometry buffer and analyzed using a Coulter EpicsXL-MCL flow cytometer. A minimum of 5,000 events were counted for each sample in the established gate, and mean fluorescence intensities were determined from single-parameter histograms generated with Epics XL 1.5 software. Controls for autofluorescence and nonspecific secondary antibody binding were included in each analysis. The binding of FITC-conjugated SNA, MAL I, and biotinylated MAL II was used to monitor saturation of lectin binding. The relative levels of MAL I and SNA binding sites on cells were also compared using FITC-tagged lectins (FITC-tagged MAL II was not available). The cells were washed and resuspended (3 × 105 cells/sample) in 150 μl of OptiMEM containing 0.1% (wt/vol) sodium azide and 50 μg/ml of lectin conjugate for 30 min at room temperature. The cells were then washed and resuspended in OptiMEM-azide and analyzed as described above. The cells incubated with biotinylated MAL II were washed and incubated in OptiMEM containing sodium azide and a 1:500 dilution of phycoerythrin-conjugated streptavidin (1-μg/ml stock; Vector Laboratories). Controls for autofluorescence and/or phycoerythrin-streptavidin were included in each analysis.
PiPL-C treatment of cells.
Cells were washed three times with PBS, resuspended to a final density of 5 × 107/ml in 50 μl phosphatidylinositol-specific phospholipase C (PiPL-C) buffer (RPMI 1640, 0.2% bovine serum albumin, 50 μM 2-mercaptoethanol, 10 mM HEPES, 0.1% sodium azide) in the presence or absence of Bacillus cereus PiPL-C (6 U/ml; Sigma), and incubated at 37°C for 90 min. The cells were washed, and virus binding was assessed. A portion of each sample of cells was analyzed by flow cytometry to monitor the extent of enzymatic removal of CD55 from the surfaces of cells. Under these conditions, >70% of CD55 was removed from the surfaces of U-937 (28) and HCE (data not shown) cells.
Inhibition of N- and O-linked glycosylation.
To inhibit N-linked glycosylation, cells were incubated in culture medium containing 0.2 mg/ml tunicamycin (Sigma) for 24 h. To inhibit O-linked glycosylation, cells were incubated in culture medium containing 3 mM benzyl N-acetyl-α-d-galactosaminide (benzyl-GalNAc; Sigma) for 48 h. These conditions have been shown by us (1) and by others (21, 27, 41, 48) to inhibit glycosylation of proteins. The cells were washed twice with PBS, and virus binding was assessed.
Proteinase K treatment of cells.
HCE cells were dispersed with trypsin-EDTA. HCE and U-937 cells were washed twice, counted, resuspended in OptiMEM to a cell density of 4.5 × 107/ml, and then incubated in the presence or absence of proteinase K (200 μg/ml; Roche) for 2 h at 37°C. An equal volume of PBS containing 6% fetal bovine serum, 2 mM phenylmethylsulfonyl fluoride, and 2× concentrated protease inhibitor cocktail (Sigma) was added to inactivate proteinase K. After a 10-min incubation at room temperature, the cells were washed twice and resuspended to a cell density of 3.5 × 107/ml before being used in virus binding or infectious-center or replication assays. For infectious-center assays, virus binding was done at 4°C to reduce virus entry. The cells were then treated a second time with proteinase K at 32°C for 30 min to eliminate surface-bound virus, washed three times, and resuspended in OptiMEM. The cells were counted and diluted, and the number of infected cells was determined by plaque assay on monolayers of LLC-MK2 cells. For replication assays with HCE cells, individual wells in six-well culture dishes were seeded with 3.5 × 105 cells in a volume of 1 ml. Infected HCE cells settled and adhered within 2 h. At various times after infection, the plates were placed at −80°C. Following two or three cycles of freezing and thawing, the amount of infectious virus in each sample was determined by plaque assay on monolayers of LLC-MK2 cells.
RESULTS
EV70 binding is sensitive to sialidase, having specificity for α2,3-linked sialic acid.
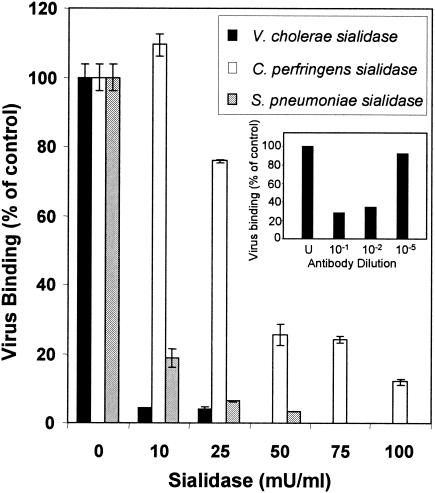
In previous work, we demonstrated that EV70 binding to and infection of several different cell lines were sensitive to prior incubation with V. cholerae sialidase (1, 28). As a first step in determining if EV70 attachment shows specificity for the sialic acid linkage, we tested two other sialidases that exhibit more specificity for the bond linking sialic acid to underlying sugars for their abilities to inhibit attachment of radiolabeled EV70 to U-937 cells. U-937 cells were chosen for these experiments because they are permissive for EV70 and bind substantial amounts of virus. EV70 binding to U-937 cells is also very sensitive to V. cholerae sialidase and independent of CD55. As shown in Fig. 1, pretreatment of U-937 cells with the S. pneumoniae sialidase, which specifically cleaves α2,3-linked sialic acid (Glyko/Prozyme technical data), inhibited radiolabeled EV70 binding by more than 90%, paralleling results obtained with the V. cholerae enzyme. C. perfringens sialidase, which cleaves both α2,3- and α2,6-linked sialic acid (Glyko/Prozyme and reference 15), was less active at removing EV70 binding sites from the surfaces of U-937 cells; however, incubation with higher concentrations of the enzyme reduced virus binding to levels close to those reached with V. cholerae and S. pneumoniae sialidases. The observation that S. pneumoniae sialidase removes EV70 binding sites from U-937 cells almost as readily as V. cholerae sialidase is consistent with the hypothesis that glycans containing α2,3-linked sialic acid constitute the majority of EV70 binding sites on these cells. Preincubation of radiolabeled EV70 for 1 h at 32°C with neutralizing monoclonal antibody ev-12 (76) inhibited virus binding in a dose-dependent manner, confirming the specificity of virus attachment (Fig. 1, inset).
FIG. 1.
EV70 binding to U-937 cells is sensitive to α2,3-specific sialidase. U-937 cells were pelleted, washed, resuspended (5 × 107 cells/ml) in OptiMEM, and incubated in the presence or absence of sialidase (10 to 100 mU/ml, as indicated) at 37°C for 30 min. After sialidase treatment, the cells were washed twice, counted, and used in virus binding assays. The results are presented as mean percentages of virus binding to cells incubated in the presence of sialidase compared to untreated cells ± standard deviation for at least two independent experiments, each performed with triplicate samples. (Inset) Radiolabeled EV70 was incubated at 32°C for 1 h with neutralizing monoclonal antibody ev-12 (76) at the indicated dilution and then used in virus binding assays. The concentration of undiluted antibody was 7.0 μg/ml. U, control samples in which virus was not treated with antibody.
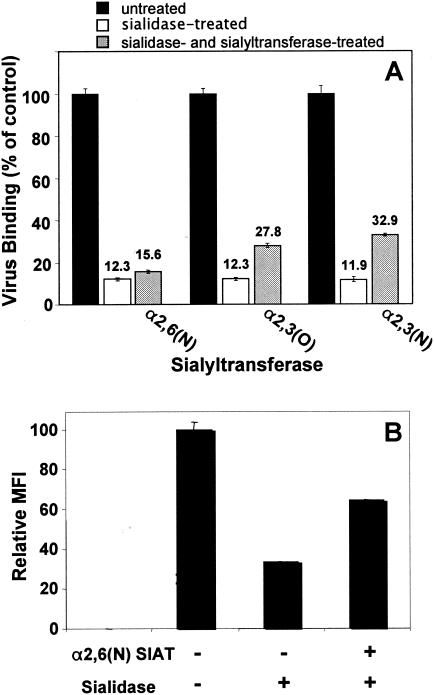
α2,3 sialyltransferase partially restores EV70 binding sites on sialidase-treated cells.
As another approach to determine if EV70 binds preferentially to α2,3- or α2,6-linked sialic acid, we stripped EV70 binding sites from the surfaces of U-937 cells with V. cholerae sialidase and then incubated the cells with commercially available sialyltransferases and CMP-β-d-sialic acid, reasoning that resialylation of the U-937 cell surface by one or more of the sialyltransferases would restore EV70 binding. The nomenclature and specificities of the sialyltransferases used in this study are presented in Table 1. As shown in Fig. 2A, resialylation of U-937 cells by α2,3(O) or α2,3(N) sialyltransferase partially restored EV70 binding. Binding of radiolabeled EV70 to U-937 cells resialylated by α2,3(O) or α2,3(N) sialyltransferase was roughly three times higher than for sialidase-treated and α2,6(N) sialyltransferase-treated cells and reached approximately 30% of the level obtained for control untreated cells. The level to which virus binding was restored following resialylation is similar to what has been reported elsewhere for recovery of virus-mediated hemagglutination following resialylation of erythrocytes (33, 50). Binding of EV70 to cells treated with α2,6(N) sialyltransferase only marginally increased EV70 binding above that for sialidase-treated cells. These results support the hypothesis that EV70 binds preferentially to α2,3-linked sialic acid compared to α2,6-linked sialic acid. To confirm that α2,6(N) sialyltransferase was functional, we followed the binding of SNA lectin to sialidase-treated cells and to sialidase- and α2,6(N) sialyltransferase-treated cells by flow cytometry. SNA preferentially binds α2,6-linked sialic acid. Incubation of U-937 cells with V. cholerae sialidase reduced SNA binding by 70 to 75%; subsequent incubation of cells with α2,6(N) sialyltransferase partially restored SNA binding to ∼64% of that of untreated control cells (Fig. 2B). We also examined the binding of MAL I lectin, which preferentially binds to α2,3-linked sialic acid; however, we saw no decrease in MAL I binding to U-937 cells following sialidase treatment (data not shown). This may be explained by the fact that MAL I binds both sialylated and unsialylated glycans under certain experimental conditions, including flow cytometry (11).
TABLE 1.
Nomenclature and template specificities of sialyltransferases used in this study
| Commercial name of sialyltransferase | Approved name (abbreviation; EC no.) | Bond formed and template specificitya |
|---|---|---|
| α2,6(N) sialyltransferase | Sialyltransferase 1; β-galactoside α2,6-sialyltransferase (ST6Gal I; 2.4.99.1) | Siaα2,6Galβ1,4GlcNAc in N-linked oligosaccharides |
| α2,3(N) sialyltransferase | Sialyltransferase 6; N-acetyl-lactosaminide α2,3-sialyltransferase (ST3Gal III; 2.4.99.6) | Siaα2,3Galβ1,3(or 4)GlcNAc in glycoproteins and glycolipids |
| α2,3(O) sialyltransferase | Sialyltransferase 4B; β-galactoside α2,3-sialyltransferase (ST3Gal II; 2.4.99.4) | Siaα2,3Galβ1,3GalNAc in O-linked oligosaccharides of glycoproteins and glycolipids |
Template specificity from reference 53 and Calbiochem/Merck technical data and references therein.
FIG. 2.
EV70 binding sites on sialidase-treated U-937 cells are partially restored by α2,3-specific sialyltransferases. U-937 cells were pelleted, washed twice, resuspended (5 × 107/ml) in OptiMEM (pH 5.7; 4 mM CaCl2), and incubated in the presence (+) or absence (untreated control) of 50 mU/ml V. cholerae sialidase for 30 min at 37°C. The cells were again washed twice and reuspended (5 × 107/ml) in OptiMEM (pH 7.2). Sialyltransferase (50 mU/ml) and CMP-β-d-sialic acid (1 mM) were added to one aliquot of sialidase-treated cells. Untreated control cells, sialidase-treated cells, and cells treated with both sialidase and sialyltransferase were incubated for 4 h at 37°C. The cells were washed twice and used in virus binding assays (A) or for flow cytometry with FITC-conjugated SNA (B). SIAT, sialyltransferase. The error bars represent standard deviations.
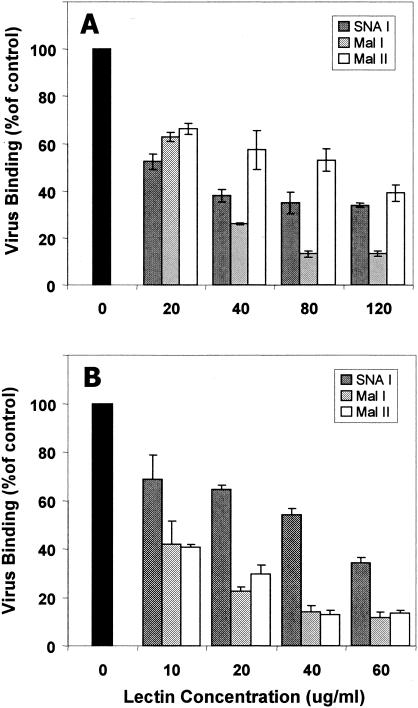
Lectin binding to α2,3-linked sialic acid inhibits EV70 attachment.
As an alternative approach to determine if EV70 binding to sialylated receptors is linkage specific, we tested the abilities of lectins that exhibit specificity for the sialic acid linkage to inhibit radiolabeled EV70 binding to U-937 cells. Lectin specificities are shown in Table 2. All three lectins inhibited EV70 binding to U-937 cells in a dose-dependent manner (Fig. 3A). MAL I, which binds Neu5Acα2,3Gal but not Neu5Acα2,6Gal (11, 30, 37), inhibited EV70 attachment to U-937 cells more efficiently than either SNA or MAL II. At a concentration of 80 μg/ml, MAL I inhibited virus attachment to U-937 cells by almost 90%. Inhibition of EV70 binding by SNA, which binds preferentially to Neu5Acα2,6Gal/GalNAc (57, 58) but has been reported to recognize α2,3-linked sialic acid to a lesser degree (Vector Laboratories technical data), never exceeded 65%, even at higher lectin concentrations. Inhibition by MAL II, which binds with highest affinity to a disialylated structure, Neu5Acα2,3Galβ1,3[Neu5Acα2,6]GalNAc (11, 30, 38), was ≤60%. Therefore, all three lectins could interfere directly with EV70 binding by competing for virus attachment sites containing α2,3-linked sialic acid. These results, in particular those obtained for MAL I, reinforce the conclusion drawn from the sialidase and sialyltransferase experiments that α2,3-linked sialic acid is an important component of EV70 attachment sites on U-937 cells. Lectin binding to oligosaccharides that do not contain α2,3-linked sialic acid may also contribute, to an unknown extent, to the inhibition that was observed by hindering EV70 access to virus attachment sites.
TABLE 2.
Lectin binding specificities
| Lectin | Binding specificitya |
|---|---|
| SNA | Neu5Acα2,6Gal/GalNAc and Neu5Acα2,3Gal |
| MAL I | Neu5Acα2,3Galβ1,4GlcNAc and Galβ1,4GlcNAc |
| MAL II | Neu5Acα2,3Galβ1,3 [Neu5Acα2,6]GalNAc |
FIG. 3.
Lectin inhibition of EV70 binding. HCE cells were dispersed with trypsin-EDTA and washed twice in OptiMEM. U-937 cells (A) and HCE cells (B) were pelleted, washed, resuspended (3.5 × 106 cells/ml) in OptiMEM in the presence or absence of different lectins at the indicated concentrations, and incubated for 30 min at room temperature. The cells were then washed once, and virus binding was assessed. The experiment was repeated three times. The error bars represent standard deviations.
Sialylated receptors on HCE cells and on U-937 cells are different.
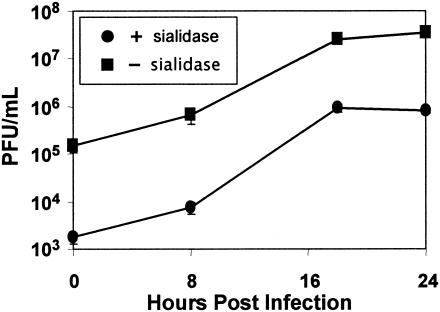
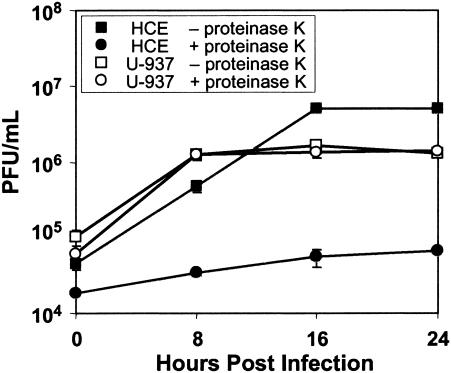
We wished to determine if the characteristics of EV70 binding to a more relevant (HCE) cell line were similar to what we have observed for HeLa cells (sialic acid and CD55 dependent) or for several other human cell lines, including U-937 cells (sialic acid dependent but CD55 independent) (1, 28, 35). Binding of radiolabeled EV70 to HCE cells (Table 3) was sensitive to V. cholerae and S. pneumoniae sialidases but was resistant to PiPL-C and to blockade with a CD55-specific monoclonal antibody. EV70 attachment to HCE cells, therefore, as observed for U-937 cells, requires sialic acid but not CD55. Also, as shown in Fig. 4, the yield of EV70 from sialidase-treated HCE cells was approximately 2 log units less than for untreated cells, indicating that binding to sialylated receptors is an important step in infection of HCE cells. As seen for other cell lines (1, 28), EV70 binding was also significantly inhibited by exposure of HCE cells to an inhibitor of O glycosylation, benzyl-GalNAc, but not by tunicamycin, which inhibits N-linked glycosylation (Table 3). Differences were noted in lectin inhibition of EV70 binding to the two cell lines (Fig. 3 and Table 3). The maximum inhibitions of EV70 binding by MAL I (>80%) and SNA (<60%) were similar for the two cell lines; however, EV70 binding to HCE cells was more sensitive to inhibition by MAL I than was binding to U-937 cells. Also, MAL II blocked EV70 binding to HCE cells as efficiently as did MAL I (>80%) and more efficiently than it blocked binding to U-937 cells (∼50%) (Fig. 3 and Table 3). These differences may reflect differences in the nature or abundance of sialylated EV70 receptors on HCE cells and U-937 cells. To test this possibility, we examined the ability of proteinase K to inhibit EV70 binding to, and infection of, these two cell lines. As indicated in Table 3, proteinase K treatment inhibited EV70 binding to HCE cells by more than 80% but had a much smaller inhibitory effect (<20%) on virus attachment to U-937 cells. As expected, CD55-dependent EV70 binding to HeLa cells was also sensitive to proteinase K. The yield of EV70 at 24 h after infection of proteinase K-treated HCE cells was reduced by almost 2 log units compared to untreated cells, but little difference was seen between virus yields from proteinase K-treated and untreated U-937 cells (Fig. 5). Infectious-center assays also confirmed these results. Proteinase K treatment reduced the number of EV70-infected HCE cells by 90% but had no effect on the number of infected U-937 cells (data not shown). These results strongly suggest that a protein is the major attachment molecule for EV70 on HCE cells, but not on U-937 cells.
TABLE 3.
Characteristics of EV70 binding to HCE, U-937, and HeLa cells
| Cells treated witha: | Virus bindingb (% of control)
|
||
|---|---|---|---|
| HCE cells | U-937 cells | HeLa cells | |
| V. cholerae sialidase (10 mU/ml) | 4.3 | 3.7 | 29.0 |
| S. pneumoniae sialidase (10 mU/ml) | 5.1 | 8.0 | NDc |
| CD55 blockade (17-22 μg/ml EVR1) | 100 | 96.8d | 26.5e |
| PiPL-C (6 U/ml) | 89.3 | 97.9d | 44.0d |
| Benzyl-GalNAc (3 mM) | 36.8 | 42.1f | 22.1f |
| Tunicamycin (0.2 μg/ml) | 100 | 100f | 100f |
| MAL I (40 μg/ml) | 13.8 | 17.1 | ND |
| MAL II (40 μg/ml) | 12.9 | 51.0 | ND |
| SNA (40 μg/ml) | 53.8 | 59.4 | ND |
| Proteinase K (200 μg/ml) | 16.1 | 83.0 | 16.5 |
Details of incubation times and temperatures are described in Materials and Methods.
Values represent virus binding as a percentage of binding to untreated control cells. Each value represents an average of at least two experiments, each done in triplicate.
ND, not done.
See reference 28.
See reference 35.
See reference 1.
FIG. 4.
Sialidase treatment of HCE cells inhibits infection by EV70. HCE cells were incubated in the presence (+) or absence (−) of S. pneumoniae sialidase (50 mU/ml) for 30 min at 37°C, washed twice with OptiMEM, and counted. The cells were infected with EV70 at an MOI of 5 PFU/cell at 32°C for 1 h and then washed three times with OptiMEM. At the indicated times after infection, samples were frozen at −80°C. The cells were subjected to two cycles of freezing and thawing to release virus, and the virus yield was determined by plaque assay on LLC-MK2 cells in duplicate. This experiment was repeated twice. The data in the figure represent one experiment.
FIG. 5.
Proteinase K treatment inhibits EV70 infection of HCE cells but not U-937 cells. HCE cells, dispersed with trypsin-EDTA, and U-937 cells were washed twice, resuspended (4.5 × 107 cells/ml) in OptiMEM, and incubated in the presence (+) or absence (−) of proteinase K (200 μg/ml) for 2 h at 37°C. Protease inhibitors were added for 10 min at room temperature, and the cells were washed twice, resuspended (3.5 × 107 cells/ml) in OptiMEM, and infected with EV70 (MOI = 5). Infected cells were washed five times and used in replication assays. HCE cells were seeded in six-well culture dishes. U-937 cells were maintained in stationary-suspension culture. At the indicated times after infection, samples (plates of HCE cells or aliquots of U-937 cells) were frozen at −80°C. The cells were subjected to two cycles of freezing and thawing to release virus, and the virus yield was determined by plaque assay on LLC-MK2 cells in duplicate. This experiment was repeated twice for each cell line. The data in the figure represent one experiment.
DISCUSSION
Taken together, the results of the experiments described here indicate that EV70 has a strong preference for binding to O-linked glycans containing terminal sialic acid α2,3 linked to galactose, although the possibility that EV70 has the capacity to interact with glycans containing α2,6-linked sialic acid cannot be completely ruled out. V. cholerae sialidase cleaves terminal sialic acid but not sialic acid from branched internal positions, and S. pneumoniae sialidase, which efficiently stripped cells of EV70 binding sites, exhibits specificity for sialic acid that is α2,3 linked to galactose but is unable to release sialic acid linked to N-acetylgalactosamine in vitro (66). The α2,3-sialyltransferases used in this study sialylate galactose residues (53) (Table 1) and restored EV70 attachment sites to sialidase-treated cells to a much greater degree than did an α2,6-specific sialyltransferase. Because the α2,3-sialyltransferases are capable of adding sialic acid to O-linked glycans (53,72) (Table 1), the sialyltransferase data are also consistent with the observation that benzyl-GalNAc inhibits EV70 binding (1,28) (Table 3), and support the hypothesis that the glycan recognized by EV70 is O linked. Inhibition of EV70 binding by the lectin MAL I, which exhibits specificity for galactose replaced with sialic acid attached by an α2,3 linkage but not an α2,6 linkage, was more pronounced than for lectin SNA, which binds sialic acid attached by α2,6 linkages (57, 58). This also suggests that α-2,3-linked sialic acid is a component of an EV70 receptor. MAL II lectin (which inhibited virus binding to HCE cells but not U-937 cells) also interacts with glycans containing sialic acid linked to galactose by an α2,3 bond (11, 30, 37, 38) (Table 2).
EV70 appears to be able to interact with at least three different cell surface molecules. CD55 is the main binding molecule on HeLa cells, but a second sialylated molecule also serves as a receptor (1, 35). Virus binding to HCE cells is inhibited by proteinase K, sialidase, and benzyl-GalNAc but is PiPL-C insensitive and CD55 independent. The simplest interpretation of these results is that the EV70 receptor on HCE cells is a non-GPI-anchored membrane glycoprotein, although the possibility that two distinct molecules are required for virus binding cannot be ruled out. CD55-independent attachment of EV70 to U-937 cells is also sensitive to sialidase, but not to proteinase K; therefore, the major attachment molecule for EV70 on U-937 cells is likely to be a nonproteinaceous molecule, such as a ganglioside, although a sialylated glycoprotein that is resistant to proteinase K cannot be ruled out. The differential inhibitory effects of Mal I and MAL II on EV70 binding to HCE cells and U-937 cells also support the idea that the EV70 receptors on these two cell lines are different. In any case, the virus appears to recognize sialic acid as part of glycoconjugates containing specific oligosaccharide structures and does not simply interact with all glycoconjugates carrying α2,3-linked sialic acid.
There are several examples where interactions between viruses and sialic acid attached by a particular glycosidic linkage contribute to host range, tissue tropism, and/or pathogenesis. Species specificity and tissue tropism of influenza A viruses correlate with sequence differences in the influenza HA that are associated with the preference of human influenza A viruses for α2,6-linked sialic acid and of avian influenza A viruses for α2,3-linked sialic acid (14, 31, 51, 52, 74). Mutations in HA are critical for the adaptation of influenza A viruses to the human respiratory tract, where α2,6-linked sialic acid is expressed on columnar epithelial cells (9, 16, 44, 45). Sialic acid linkage specificity also correlates with the tropism and pathogenicity of papovaviruses, for which sialic acid is an essential component of receptors. Recently, gangliosides containing terminal α2,3-linked sialic acid were shown to be receptors for mouse polyoma virus (67). It has also been known for some time that highly tumorigenic, large-plaque variants of polyoma virus bind oligosaccharides terminating in Neu5Acα2,3Galβ1,3GalNAc, whereas less tumorigenic, small-plaque variants also recognize a branched oligosaccharide, Neu5Acα2,3Galβ1,3[Neu5Ac2,6]GalNAc (12, 25, 60). Conversely, the receptor for JC virus on glial cells is believed to be an N-linked glycoprotein containing α2,6-linked sialic acid (43), and the expression of α2,6-linked sialic acid, but not α2,3-linked sialic acid, correlates with the susceptibility of cells implicated in JC virus infection and spread, such as B lymphocytes in the tonsil and spleen and oligodendrocytes and astrocytes in the brain (22). Transduction of central nervous system, eye, and lung cells by adeno-associated parvovirus AAV-5 is dependent on α2,3-N-linked sialic acid (75). The recent identification of platelet-derived growth factor receptors α and β as receptors for AAV-5 is consistent with these characteristics, and the in vivo tropism of AAV-5 also correlates with the expression pattern of platelet-derived growth factor receptor α (17, 19, 33). Specific interactions between the σ1 protein of type 1 reovirus and glycoconjugates containing α2,3-linked sialic acid appear to be directly involved in the adherence of type 1 reovirus to the apical surfaces of M cells in the intestinal mucosa (29). A similar interaction may operate in the development of biliary disease in mice following infection by sialic acid binding strains of reovirus type 3 (7). We have identified two binding molecules for EV70, sialic acid and CD55 (1, 28, 35, 36), both of which are found on conjunctival and corneal epithelium or on cells derived from these tissues (18, 42, 46, 65). Our in vitro data suggest that CD55 does not function as a receptor for EV70 on ocular tissue, despite its presence in the conjunctival and corneal epithelia, although this must still be confirmed in other model systems or in vivo. EV70 preferentially binds to α2,3-linked sialic acid, like adenovirus 37 (4, 5), which causes keratoconjunctivitis, and like avian influenza viruses, several of which have been associated with conjunctivitis in humans (13, 24, 39, 64), suggesting that the ocular tropism of these viruses is directly related to their interactions with α2,3-linked sialic acid. The use of sialic acid as a virus attachment molecule should not be considered a requirement for ocular tropism, however, since other viruses that do not use sialic acid as an attachment molecule, for example, herpes simplex virus, also infect the eye (59, 68).
Acknowledgments
The authors thank N. Arnberg, S. Olofsson, and U. Kumlin for sharing their ideas concerning sialic acid and ocular tropism and L. Filion for assistance with flow cytometry.
This work was supported by grant RGPIN-2809-2001 from the Natural Sciences and Engineering Research Council of Canada (NSERC). D.A.A. and A.K. were recipients of NSERC Postgraduate Scholarships.
REFERENCES
- 1.Alexander, D. A., and K. Dimock. 2002. Sialic acid functions in enterovirus 70 binding and infection. J. Virol. 76:11265-11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angata, T., and A. Varki. 2002. Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102:439-469. [DOI] [PubMed] [Google Scholar]
- 3.Arias, C. F., P. Isa, C. A. Guerrero, E. Mendez, S. Zarate, T. Lopez, R. Espinosa, P. Romero, and S. Lopez. 2002. Molecular biology of rotavirus cell entry. Arch. Med. Res. 33:356-361. [DOI] [PubMed] [Google Scholar]
- 4.Arnberg, N., P. Pring-Akerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76:8834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 6.Barton, E. S., J. L. Connelly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 7.Barton, E. S., B. E. Youree, D. H. Ebert, J. C. Forrest, J. L. Connolly, T. Valyi-Nagy, K. Washington, J. D. Wetzel, and T. S. Dermody. 2003. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 111:1823-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, P. H., C. Cui, T. Stehle, S. C. Harrison, J. A. DeCaprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 73:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum, L. G., and J. C. Paulson. 1990. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 40:35-38. [PubMed] [Google Scholar]
- 10.Bhavanandan, V. P. 1992. Proteoglycans: structure, synthesis, function, p. 167-202. In H. J. Allen and E. C. Kisailus (ed.), Glycoconjugates: composition, structure, and function. Marcel Dekker, New York, N.Y.
- 11.Brinkman-Van der Linden, E. C., J. L. Sonnenburg, and A. Varki. 2002. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal. Biochem. 303:98-104. [DOI] [PubMed] [Google Scholar]
- 12.Cahan, L. D., R. Singh, and J. C. Paulson. 1983. Sialyloligosaccharide receptors of binding variants of polyoma virus. Virology 130:281-289. [DOI] [PubMed] [Google Scholar]
- 13.Choi, S., and T. Tsang. 1998. An update on influenza A H5N1 in Hong Kong. Public Health Epidemiol. Bull. 7:1-8. [Google Scholar]
- 14.Connor, R. J., Y. Kawaoka, R. G. Webster, and J. C. Paulson. 1994. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205:17-23. [DOI] [PubMed] [Google Scholar]
- 15.Corfield, A. P., H. Higa, J. C. Paulson, and R. Schauer. 1983. The specificity of viral and bacterial sialidases for α(2-3)- and α(2-6)-linked sialic acids in glycoproteins. Biochim. Biophys. Acta 744:121-126. [DOI] [PubMed] [Google Scholar]
- 16.Couceiro, J. N., J. C. Paulson, and L. G. Baum. 1993. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of host cell in selection of hemagglutinin receptor specificity. Virus Res. 29:155-165. [DOI] [PubMed] [Google Scholar]
- 17.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diebold, Y., M. Calonge, A. E. de Salamanca, S. Callejo, R. M. Corrales, V. Saez, K. F. Siemasko, and M. E. Stern. 2003. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 44:4263-4274. [DOI] [PubMed] [Google Scholar]
- 19.Di Pasquale, G., B. L. Davidson, C. S. Stein, I. Martins, D. Scudiero, A. Monks, and J. A. Chiorini. 2003. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9:1306-1312. [DOI] [PubMed] [Google Scholar]
- 20.Dormitzer, P. R., Z. Y. Sun, G. Wagner, and S. C. Harrison. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galactin fold with a novel carbohydrate binding site. EMBO J. 21:885-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duksin, D., and W. C. Mahoney. 1982. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J. Biol. Chem. 257:3105-3109. [PubMed] [Google Scholar]
- 22.Eash, S., R. Tavares, E. G. Stopa, S. H. Robbins, L. Brossay, and W. J. Atwood. 2004. Differential distribution of the JC virus receptor-type sialic acid in normal human tissues. Am. J. Pathol. 164:419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 24.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried, H., L. D. Cahan, and J. C. Paulson. 1981. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology 109:188-192. [DOI] [PubMed] [Google Scholar]
- 26.Griffith, M., R. Osborne, R. Munger, X. Xiong, C. J. Doillon, N. L. Laycock, M. Hakim, Y. Song, and M. A. Watsky. 1999. Functional human corneal equivalents constructed from cell lines. Science 286:2169-2172. [DOI] [PubMed] [Google Scholar]
- 27.Guan, E., S. L. Robinson, E. B. Goodman, and A. J. Tenner. 1994. Cell-surface protein identified on phagocytic cells modulates the C1q-mediated enhancement of phagocytosis. J. Immunol. 152:4005-4016. [PubMed] [Google Scholar]
- 28.Haddad, A., M. R. Nokhbeh, D. A. Alexander, S. J. Dawe, C. Grise, N. Gulzar, and K. Dimock. 2004. Binding to decay-accelerating factor is not required for infection of human leukocyte cell lines by enterovirus 70. J. Virol. 78:2674-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helander, A., K. J. Silvey, N. J. Mantis, A. B. Hutchings, K. Chandran, W. T. Lucas, M. L. Nibert, and M. R. Neutra. 2003. The viral sigma1 protein and glycoconjugates containing α2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 77:7964-7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imberty, A., C. Gautier, J. Lescar, S. Perez, L. Wyns, and R. Loris. 2000. An unusual carbohydrate binding site revealed by structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J. Biol. Chem. 275:17541-17548. [DOI] [PubMed] [Google Scholar]
- 31.Ito, T., Y. Suzuki, L. Mitnaul, A. Vines, H. Kida, and Y. Kawaoka. 1997. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 227:493-499. [DOI] [PubMed] [Google Scholar]
- 32.Jin, Y. M., I. U. Pardoe, A. T. Burness, and T. I. Michalak,. 1994. Identification and characterization of the cell surface 70-kilodalton sialoglycoprotein(s) as a candidate receptor for encephalomyocarditis virus on human nucleated cells. J. Virol. 68:7308-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson, K.-A. 1998. Meaning and therapeutic potential of microbial recognition of host glycoconjugates. Mol. Microbiol. 29:1-11. [DOI] [PubMed] [Google Scholar]
- 35.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 266:83-88. [PubMed] [Google Scholar]
- 38.Konami, Y., K. Yamamoto, T. Osawa, and T. Irimura. 1994. Strong affinity of Maackia amurensis hemagglutinin (MAH) for sialic acid-containing Ser/Thr-linked carbohydrate chains of N-terminal octapeptides from human glycophorin A. FEBS Lett. 342:334-338. [DOI] [PubMed] [Google Scholar]
- 39.Koopmans, M., B. Bilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R. Fouchier, A. Osterhaus, and A. Bosman. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587-593. [DOI] [PubMed] [Google Scholar]
- 40.Krempl, C., M. L. Ballesteros, G. Zimmer, L. Enjuanes, H. D. Klenk, and G. Herrler. 2000. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 81:489-496. [DOI] [PubMed] [Google Scholar]
- 41.Kuan, S. F., J. C. Byrd, C. Basbaum, and Y. S. Kim. 1989. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J. Biol. Chem. 264:19271-19277. [PubMed] [Google Scholar]
- 42.Lass, J. H., E. I. Walter, T. E. Burris, H. E. Grossniklaus, M. I. Roat, D. L. Skelnik, L. Needham, M. Singer, and M. E. Medof. 1990. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Investig. Ophthalmol. Vis. Sci. 31:1136-1148. [PubMed] [Google Scholar]
- 43.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC virus is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J. Virol. 72:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matrosovich, M. N., S. Krauss, and R. G. Webster. 2001. H9N2 influenza A virus from poultry in Asia have human virus-like receptor specificity. Virology 281:156-162. [DOI] [PubMed] [Google Scholar]
- 45.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medof, M. E., E. I. Walter, J. L. Rutgers, D. M. Knowles, and V. Nussenzweig. 1987. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J. Exp. Med. 165:848-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mouricout, M. 1997. Interactions between the enteric pathogen and the host. An assortment of bacterial lectins and a set of glycoconjugate receptors. Adv. Exp. Med. Biol. 412:109-123. [PubMed] [Google Scholar]
- 48.Nepomuceno, R. R., S. Ruiz, M. Park, and A. J. Tenner. 1999. C1qRp is a heavily O-glycosylated cell surface protein involved in the regulation of phagocytic activity. J. Immunol. 162:3583-3589. [PubMed] [Google Scholar]
- 49.Paulson, J. C. 1985. Interactions of animal viruses with cell surface receptors, p. 131-219. In M. Conn (ed.), The receptors. Academic Press, Orlando, Fla.
- 50.Paulson, J. C., and G. N. Rogers. 1987. Resialylated erythrocytes for assessment of the specificity of sialyloligosaccharide binding proteins. Methods Enzymol. 138:162-168. [DOI] [PubMed] [Google Scholar]
- 51.Rogers, G. N., and J. C. Paulson. 1983. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127:361-373. [DOI] [PubMed] [Google Scholar]
- 52.Rogers, G. N., J. C. Paulson, R. S. Daniels, J. J. Skehel, I. A. Wilson, and D. C. Wiley. 1983. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature 304:76-78. [DOI] [PubMed] [Google Scholar]
- 53.Sasaki, K. 1996. Molecular cloning and characterization of sialyltransferases. Trends Glycosci. Glycotechnol. 8:195-215. [Google Scholar]
- 54.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413-1429. [DOI] [PubMed] [Google Scholar]
- 55.Shah, A. H., and H. L. Lipton. 2002. Low-neurovirulence Theiler's viruses use sialic acid moieties on N-linked oligosaccharide structures for attachment. Virology 304:443-450. [DOI] [PubMed] [Google Scholar]
- 56.Shibuta, H., A. Nozawa, T. Shioda, and T. Kanda. 1983. Neuraminidase activity and syncytial formation in variants of parainfluenza 3 virus. Infect. Immun. 41:780-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2,6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596-1601. [PubMed] [Google Scholar]
- 58.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. Fractionation of sialylated oligosaccharides, glycopeptides, and glycoproteins on immobilized elderberry (Sambucus nigra L.) bark lectin. Arch. Biochem. Biophys. 254:1-8. [DOI] [PubMed] [Google Scholar]
- 59.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 60.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 61.Stoner, G. D., B. Williams, A. Kniazeff, and M. B. Shimkin. 1973. Effect of neuraminidase pretreatment on the susceptibility of normal and transformed mammalian cells to bovine enterovirus 261. Nature 245:319-320. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki, T., A. Portner, R. A. Scroggs, M. Uchikawa, N. Koyama, K. Matsuo, Y. Suzuki, and T. Takimoto. 2001. Receptor specificities of human respiroviruses. J. Virol. 75:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki, Y., T. Ito, T. Suzuki, R. E. Holland, Jr., T. M. Chambers, M. Kiso, H. Ishida, and Y. Kawaoka. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825-11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam, J. S. 2002. Influenza A (H5N1) in Hong Kong: an overview. Vaccine 20:77-81. [DOI] [PubMed] [Google Scholar]
- 65.Terraciano, A. J., N. Wang, J. S. Schuman, G. Haffner, N. Panjwani, Z. Zhao, and Z. Yang. 1999. Sialyl Lewis X, Lewis X, and N-acetyllactosamine expression on normal and glaucomatous eyes. Curr. Eye Res. 18:73-78. [DOI] [PubMed] [Google Scholar]
- 66.Toivonen, S., O. Aitio, and O. Renkonen. 2001. α2,3-sialylation of terminal GalNAcβ1-3Gal determinants by ST3Gal II reveals the multifunctionality of the enzyme. J. Biol. Chem. 276:37141-37148. [DOI] [PubMed] [Google Scholar]
- 67.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tullo, A. 2003. Pathogenesis and management of herpes simplex virus keratitis. Eye 17:919-922. [DOI] [PubMed] [Google Scholar]
- 69.Uchida, Y. 1989. Clinical features of acute hemorrhagic conjunctivitis due to enterovirus 70, p. 213-223. In Y. Uchida, K. Ishii, K. Miyamura, and S. Yamazaki (ed.), Acute hemorrhagic conjunctivitis. Etiology, epidemiology and clinical manifestations. S. Karger AG, New York, N.Y.
- 70.Uncapher, C. R., C. M. Dewitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 71.Utagawa, E. T., K. Miyamura, A. Mukoyama, and R. Kono. 1982. Sialidase-sensitive erythrocyte receptor for enterovirus type 70. J. Gen. Virol. 63:141-148. [DOI] [PubMed] [Google Scholar]
- 72.Varki, A. 1999. Sialic acids, p. 195-209. In A. Varki, R. Cummings, J. Esko, H. Freeze, G. Hart, and J. Marth (ed.), Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 73.Vihinen-Ranta, M., S. Suikkanen, and C. R. Parrish. 2004. Pathways of cell infection by parvoviruses and adeno-associated viruses. J. Virol. 78:6709-6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vines, A., K. Wells, M. Matrosovich, M. R. Castrucci, T. Ito, and Y. Kawaoka. 1998. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J. Virol. 72:7626-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walters, R. W., S. M. P. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 76.Wiley, J. A., J. Hamel, and B. R. Brodeur. 1992. Monoclonal anti-idiotypes induce neutralizing antibodies to enterovirus 70 conformational epitopes. J. Virol. 66:5744-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright, P. W., G. H. Strauss, and M. P. Langford. 1992. Acute hemorrhagic conjunctivitis. Am. Fam. Physician 45:173-178. [PubMed] [Google Scholar]
- 78.Yamazaki, S., and K. Miyamura. 1989. General characteristics of enterovirus 70, p. 345-357. In Y. Uchida, K. Ishii, K. Miyamura, and S. Yamazaki (ed.), Acute hemorrhagic conjunctivitis. Etiology, epidemiology and clinical manifestations. S. Karger AG, New York, N.Y.
- 79.Yoshii, T., K. Natori, and R. Kono. 1977. Replication of enterovirus 70 in nonprimate cell cultures. J. Gen. Virol. 36:377-384. [DOI] [PubMed] [Google Scholar]
- 80.Zhou, L., Y. Luo, Y. Wu, J. Tsao, and M. Luo. 2000. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J. Virol. 74:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]