Abstract
Purpose: With an increase in the proportion of elderly patients, the global burden of spinal disease is on the rise. This is gradually expected to increase the number of surgical procedures all over the world in the near future. As we know, rehabilitation following spine surgery is critical for optimal recovery. However, the current literature lacks consensus regarding the appropriate post-operative rehabilitation protocol. The purpose of this review is to evaluate the optimal protocol for rehabilitation after lumbar spine surgery in adults. Materials and Methods: The goals of rehabilitation after lumbar spine surgery are to improve physical and psychosocial function and may include multiple modalities such as physical therapy, cognitive behavioral therapy, specialized instruments, and instructions to be followed during activities of daily living. In recent years, not only are a greater number of spine surgeries being performed, but various different techniques of lumbar spine surgery and spinal fusion have also emerged. (1) Our review summarizes post-operative rehabilitation under the following headings—1. Historical aspects, 2. Subjective functional outcomes, and (3) Actual rehabilitation measures, including balance. Results: Physical therapy programs need to be patient-specific and surgery-specific, such that they consider patient-reported outcome measures and take into consideration the technique of spinal fusion used and the muscle groups involved in these surgeries. By doing so, it is possible to assess the level of functional impairment and then specifically target the strengthening of those muscle groups affected by surgery whilst also improving impaired balance and allowing a return to daily activities. Conclusions: Rehabilitation is a multi-faceted journey to restore mobility, function, and quality of life. The current rehabilitation practice focuses on muscle strengthening, but the importance of spinal balance is less elaborated. We thus equally emphasize muscle strengthening and balance improvement post-lumbar spine surgery.
Keywords: lumbar surgery, rehabilitation, physiotherapy, muscle exercise
1. Introduction
Lower back pain is a major cause of morbidity among middle-aged and elderly individuals due to a number of possible etiologies. Even though most of the episodes of low back pain are often self-limiting, the incidence of lifetime recurrence is as high as 85% [1]. Chronic low back pain not only impairs physical and psychological health but also leads to a decline in social responsibilities, including work performance and family life, and is a major cause of increasing health care costs [2]. With advancements in medical care and the increased life expectancy of the aging population, the global burden of spinal disease has increased [3]. With the availability of advanced techniques such as minimally invasive spine surgery, percutaneous pedicle screw fixation, imaging, and navigation, a larger number of spinal surgeries are being performed currently, with some studies documenting the number of spine surgeries to be 2.4 times that of those performed 15 years ago [4,5].
Post-lumbar spinal surgery, post-operative physiotherapy intervention is crucial and recommended for improvement of post-operative functional outcome so that patients can perform their activities of daily living (ADL) at the earliest and return to normal or near normal life in the long term [6,7]. A physiotherapy regimen is supervised or home exercises with proper guidance and instruction given by a physical therapist. Furthermore, active rehabilitation is effective and important for improving short-term and long-term functional status [8]. Rehabilitation includes multiple different modalities based on the requirements of the patients, such as providing instructions, exercise therapy such as stretching and muscle strengthening, manipulation techniques, mobilization techniques, and the use of assistive equipment such as walking aids [9]. When assessing the progress of post-operative patients undergoing rehabilitation, physical therapists and surgeons often have to use disease-specific patient-reported outcome measures and standard physical performance tests. These assessments may provide useful information regarding the progress made by the patients following surgery. With different techniques of lumbar spine surgery and spinal fusion being performed, the physical therapy prescribed should be curated taking into account the technique used and should aim to target strengthening of muscle groups violated during the surgical procedure.
The benefits of physical therapy as per literature in the past have been limited to weak evidence, and the mechanisms of these benefits remain uncertain [10,11]. However, recently there have been several new reports that support the idea that rehabilitation helps to improve clinical outcomes in lumbar fusion surgery [12,13].
This article aims to summarize the historical review of rehabilitation, popular patient-reported outcome assessment methods, contemporary views on post-operative spinal rehabilitation, and ways to introduce rehabilitation after lumbar spine surgery.
2. Historical Review of Rehabilitation (Table 1)
The Roman army probably provided the first rehabilitation services to return wounded soldiers to work. The word “Rehabilitation” was first used in the Oxford English Dictionary in 1533. However, rehabilitation was used extensively in healthcare by 1918. After World War I, society recognized that rehabilitation was a crucial addition to services for injured or disabled patients [14]. Rehabilitation can be considered a planned and systematic societal support process offered to patients after injury or illness. Initially, orthopedic surgeons were mainly involved. The rehabilitation services that did develop in the twentieth century were initially focused on men of working age who were injured in war. Because of the increase in motorcycle accidents and sports injuries, attention has moved to people with spinal cord injuries. Spinal cord injury rehabilitation developed in the 1940s as evidence of rehabilitation’s revolutionary effectiveness [15]. After the World Health Organization (WHO) was established in 1948, they used the biopsychosocial model as a rehabilitation framework in 1980 [16].
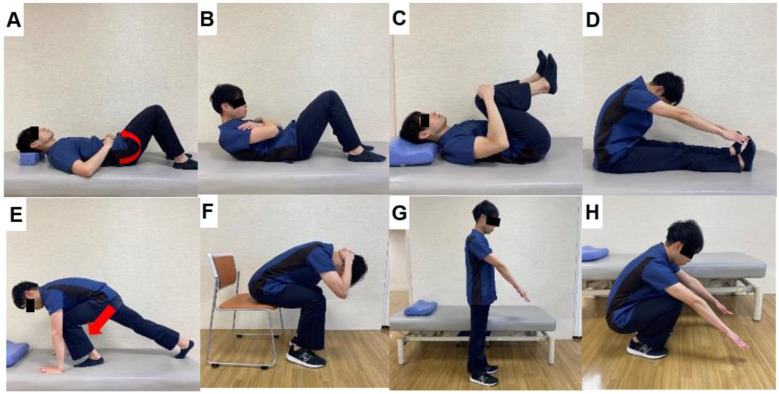
For lower back pain rehabilitation, lumbar stabilization exercises have become popular over the last 40 years. These exercises are focused on strengthening the muscles of truck [17]. Wiliams reported specific exercises known as Williams lumbar flexion exercises in 1937 [18] (Figure 1). These exercises are a series of therapeutic movements and stretches designed to strengthen the abdominal muscles and relax the paraspinal lumbar muscles. In 1955, Kelly addressed the importance of lumbar muscle relaxation with hanging, which is effective for lumbar foraminal enlargement, reducing muscle spasms, and facet joint release [19]. Pleasant developed and mixed Wiliams and Kelly exercises [18]. His methods consisted of three concepts: joint mobilization, soft tissue stretching, and muscle building. Calliet reported that exercise therapy positively improved blood flow and gradually strengthened the ligaments, tendons, and joint capsule, thereby aiding in the recovery of injured regions [20]. He also emphasized that resistance training enhances muscle function by increasing the cross-sectional areas of muscles, thereby preventing injury and mitigating pain further.
Figure 1.
Williams lumbar flexion exercises, (A): Pelvic tilt (B): Sit-up in knee flexion (C): Double knees to chest to stretch the elector spine, (D): Seated reach to toes stretches the hamstrings and elector spine, (E): Forward crouch to stretch iliofemoral ligament (F): Seated flexion (G,H): Strengthening of quadriceps muscles and stretching of gluteus maximus and elector spine.
Table 1.
History of important lumbar exercises.
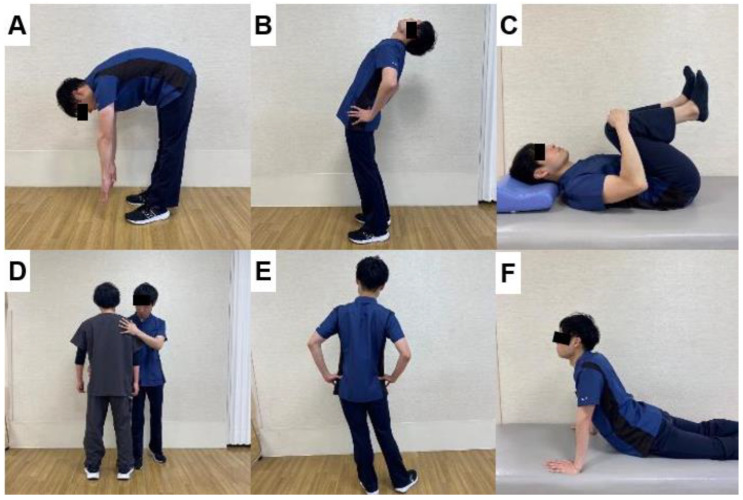
Compared with Williams lumbar flexion exercises, Böhler emphasized the importance of lumbar extensor muscle exercises in 1971 [22]. Then, McKenzie recommended that extension exercises would reduce low backache in certain patients [23]. McKenzie exercises improve spinal mobility and promote good posture (Figure 2). Thus resulting in controlled back pain over a long duration. Recently, motor control stabilization exercises have become popular for patients with chronic, nonspecific lower back pain [24]. These exercises involve voluntary isometric contraction of the core and back muscles in a neutral spine position. WHO has released its first-ever guidelines on managing chronic low back pain in 2023 [25]. According to this guideline, a structured exercise therapy or program and spinal manipulative therapy are suggested for patients with primary chronic lower back pain.
Figure 2.
McKenzie exercises, (A): Flexion in standing (B): Extension in standing (C): Flexion in lying, (D): Therapist-assisted side glide in standing (E): Side glide in standing (F): Extension in lying.
3. Various Kinds of Rehabilitation
Postsurgical rehabilitation is focused on improving function through precise diagnosis, customized treatment protocols, mitigation of complications, and compensating impairment. Furthermore, rehabilitations restore and compensate for loss of functioning and prevent or deterioration in functioning in every area of a patient’s life [26]. Rehabilitation may also comprise assistive modalities, equipment, or products used to maintain, or improve function [26]. Post-surgical rehabilitation can be advised by physical therapists, occupational therapists, chiropractors, general practitioners, and orthopedic surgeons accordingly. Examples of postsurgical rehabilitation interventions are shown in Table 2.
Table 2.
Example of selected interventions for rehabilitation after lumbar surgery.
| Treatment Modality | Details | Example |
|---|---|---|
| Patient education and self-management [9] | Teaching patient’s skills that they can use to manage their health condition | How to deal with pain The importance of physical activity in pain reduction Restrictions and working posture post-operatively (ergonomics) Mitigate pain flare-ups Step-by-step rehabilitation methods for return to routine work |
| Early Mobilization [27] | A subcategory of supervised or unsupervised schematic and structured exercise program (e.g., by a healthcare professional) | Stretching, Muscle strengthening Endurance exercises Neuromuscular closed chain exercises Range of motion exercise |
| Manual therapies [28,29] | Myofascial release: Technique that applies low-impact, prolonged stretching to the fascial complex to alleviate pain and improve function. Neural mobilization: A technique that stretches damaged nerves and improves their glide and extensibility. Manipulation: techniques incorporating a high-velocity low-amplitude impulse or thrust applied at or near the end of a joint’s passive range of motion Mobilization: techniques incorporating a low-velocity and small or large amplitude oscillatory movement, within a joint’s passive range of motion |
Myofascial release Neural mobilization Massage Lumbar manipulation, mobilization |
| Assistive technologies | Any modalities, used to, maintain, or improve the functional capability of the patient and reduce impairment. | Walking aids Socks aids Pants aids Shoehorn Reacher |
4. Patients-Reported Outcome (PRO) Measures Used after Lumbar Surgery
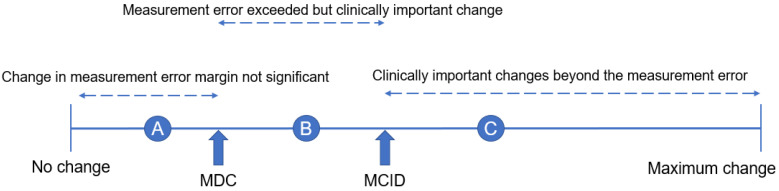
PRO is useful to evaluate the various symptoms of spinal disease separately. It is possible to accurately assess the disability caused by the disease by including the impact of the spinal disease on daily life. It is necessary to use PRO to assess the impact on physical function, ADL, and quality of life. (QOL) These patient-reported outcome questionnaires are frequently used by spine surgeons and physical therapists to assess the functional outcome of patients following spinal surgery. Jaeschke et al. coined the term minimal clinically important difference (MCID) (Figure 3) [30]. The minimal detectable change (MDC) was estimated by means of the standard error of measurement in patients whose self-assessment was unchanged. The MCID describes the smallest clinical difference a patient can perceive in a specific questionnaire of data study.
Figure 3.
Interpretation of changes in post-treatment evaluation results, A: Post-treatment evaluation results are measurement error and clinically not important, B: The post-treatment assessment results showed changes beyond the measurement error, but not clinically important changes, C: The results of the post-treatment evaluation show clinically important changes.
Acknowledging the relevance of such an approach, additional clinically oriented concepts have been introduced that can be used to better interpret PRO measure data. The MCID is relative to the initial symptomatic state before treatment. A helpful concept to rate a cohort’s condition in absolute terms is the patient-acceptable symptom state, defined as the value on a PRO scale beyond which patients with a specific condition consider themselves well or in a satisfactory state [31]. Using all these parameters in the interpretation of evaluation outcomes, a better and patient-oriented description of the obtained success rates in therapeutic approaches can be provided. A systematic review of post-operative MCID for lumbar spine disease has been reported by Issa et al. [32] The reported MCID after surgery for lumbar spine disease is shown (Table 3).
Table 3.
MCID in PRO after surgery for lumbar spine disease.
| Study | PRO | Recommended MCID | Procedure | Diagnosis |
|---|---|---|---|---|
| Parker [33] | ODI | 14.9 | TLIF | Lumber degenerative spondylolisthesis |
| VAS Back | 2.1 | |||
| VAS Leg | 2.8 | |||
| Parker [34] | ODI | 4 | Lumbar fusion | Pseudarthrosis |
| VAS Back | 3 | |||
| Johnsen [35] | ODI | 12.88 | Disk replacement | Degenerative disease |
| Solberg [36] | ODI | 20 | Discectomy | Lumbar disk herniation |
| NRS Back | 2.5 | |||
| NRS Leg | 3.5 | |||
| Yoshida [37] | ODI | 11 | Posterior corrective spinal fusion surgery | Adult spinal deformity |
| Fukushima [38] | ZCQ SSS | 1.0 | Microendoscopic laminectomy | Lumbar spinal stenosis |
| ZCQ PFS | 0.6 |
4.1. Roland-Morris Disability Questionnaire (RMDQ) (Appendix A)
The RMDQ is the most commonly used lumber spine-specific assessment method [39]. Problems with the RMDQ include the lack of questions related to mental health and the fact that it is difficult for patients with only leg pain to answer [40]. The RMDQ Cronbach’s alpha values for lower back pain patients was reported 0.92 [41].
4.2. Oswestry Disability Index (ODI) (Appendix B)
The ODI was initially published by Fairbank to measure disability due to back pain in daily living [42,43]. Score 0–4; No disability, 5–14; Mild disability, 15–24; Moderate disability, 25–34; Severe disability, 35–50; Complete disability. ODI can evaluate ADL impairment due to lower back pain and the influence of lower limb pain, and it is correlated with lower limb pain before and after surgery [44]. ODI is more sensitive to change as compared to other general health measures when tracking the effectiveness of treatments [45]. The ODI Cronbach’s alpha values for ASD patients were reported at 0.87 [46].
4.3. Zurich Claudication Questionnaire (ZCQ)
The ZCQ is a disease-specific assessment of lumbar spinal stenosis (LSS) and is assessed in three domains: symptom severity, functional impairment, and treatment satisfaction [47]. The ZCQ demonstrates reliability and validity in patients with LSS and is recommended as one of the appropriate methods for evaluating LSS treatment outcomes [48]. The ZCQ Cronbach’s alpha values for lumbar canal stenosis patients was reported 0.78 [49].
4.4. Scoliosis Research Society 22-Item Questionnaire (SRS-22)
The SRS-22 is used to assess QOL and surgical outcomes in different types of spinal deformities [50,51]. It consists of 22 questions covering four aspects: (1) pain, (2) functioning, (3) self-image, and (4) satisfaction with the surgery [52,53]. The SRS-22 has been extensively studied and used as a reliable tool suggesting sagittal vertical axis (a marker of sagittal balance), has a significant correlation with all SRS domains, and pelvic tilt, which describes the orientation of the pelvis in relation to the body, has demonstrated correlation with SRS-22, function, and self-image domains [54,55]. The SRS-22 Cronbach’s alpha values for adolescent idiopathic scoliosis patients were reported at 0.82 [56].
4.5. Lumbar Stiffness Disability Index (LSDI)
The LSDI was designed and used as a tool to assess the functional impacts of lumbar spine stiffness on flexibility [57,58,59]. It is particularly used to evaluate patients after spinal fusion surgery, and it has been shown that LSDI worsens and post-operative satisfaction decreases in surgeries that involve a long fusion [60,61]. The SRS-22 Cronbach’s alpha values for lumbar fusion patients were reported at 0.89 [58].
5. Physical Performance Tests
The prevalence of lumbar canal stenosis increases with age and is the most common diagnosis in patients over 65 undergoing spinal surgery [62,63,64,65]. Older patients with lumbar spine disease have locomotive syndrome and reduced physical function [65,66,67]. Therefore, it is important, especially in the rehabilitation field, to assess physical function to aid in the planning of a program of therapeutic interventions. A minimal clinically important difference (MCID) has been reported in physical function assessment as well as in PRO. In general, the following assessments of physical function are used.
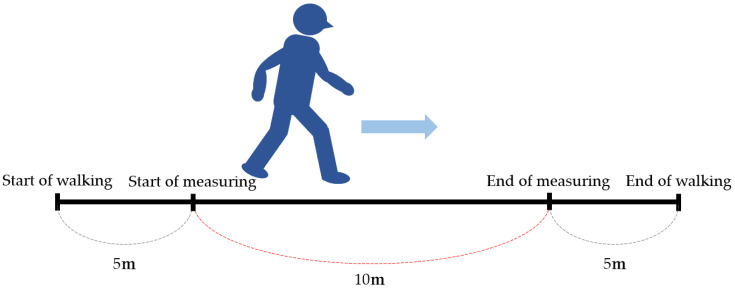
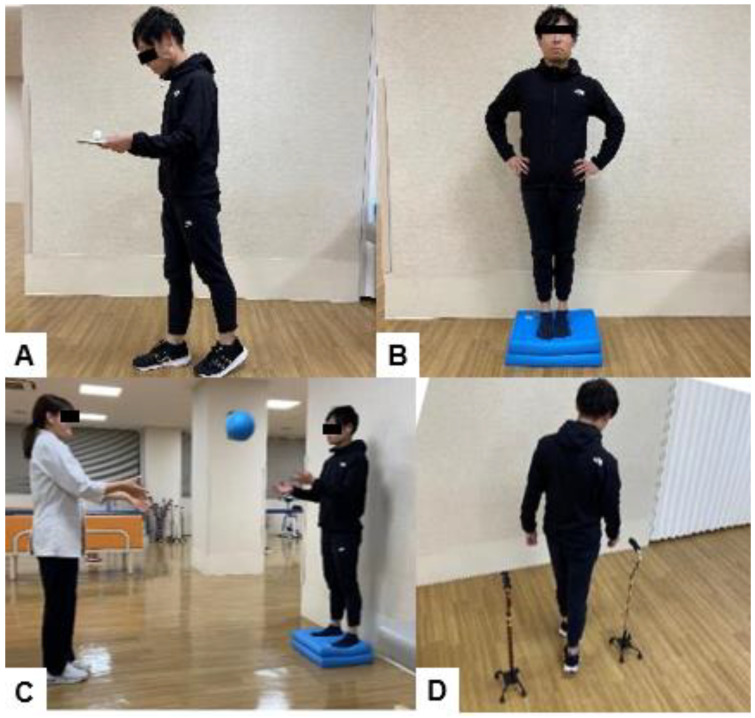
5.1. Walk Velocity (Figure 4)
Walk velocity is used to assess walking speed in meters per second over a short distance [68]. A decrease in walking speed is defined as a walking velocity of 0.8 m/s or less [69]. Changes in post-operative pain after lumbar spine surgery are associated with gait speed. Gait velocity is useful in the assessment of post-operative pain and disability after lumbar spine surgery [70]. The MCID of walk velocity after ASD surgery is 0.1 m/s [71].
Figure 4.
Walk velocity.
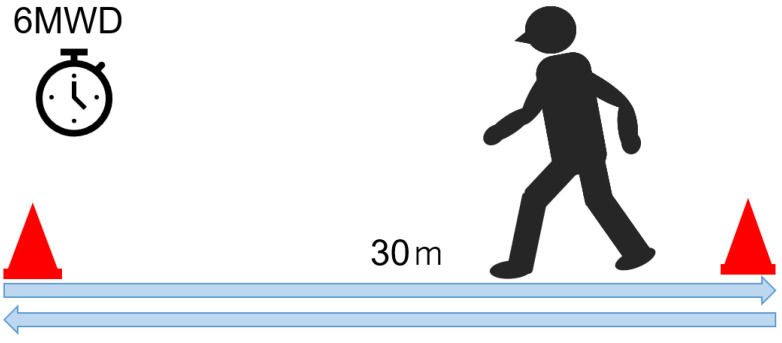
5.2. Six-Minute Walk Test (Figure 5)
The six-minute walk test involves walking for 6 min on a 30 m walking path and measuring the distance [72]. Six-minute walking distance is used to evaluate walking efficiency in patients with neurogenic claudication in LSS and ASD [73,74]. Self-reported walking distance in LSS patients underestimates measured walking distance by 31% and has low validity [75]. Therefore, when comparing the improvement of intermittent claudication after treatment, it is desirable to evaluate the actual walking distance using the 6 min walk test. MCID of 6 min walk distance after LSS surgery is 57.5 m [76].
Figure 5.
6-min walk test.
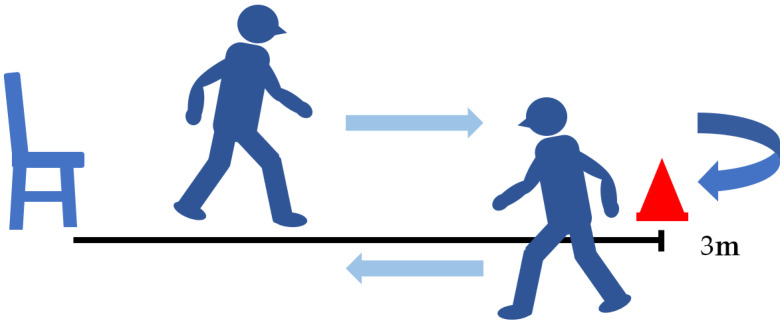
5.3. Timed up and Go Test (TUG) (Figure 6)
The timed up and go test (TUG) is an objective measure of functional disability that can be used to evaluate various activities such as standing, accelerating, walking, decelerating, and turning, which are often limited in patients with lumbar degenerative diseases [77]. TUG can be easily conducted with a chair and a 3 m walking space and does not require special equipment [78]. Previously TUG was used to measure motor impairment in patients with lumbar degenerative diseases, with <11.5 s classified as no impairment, 11.5 to 13.4 s as mild impairment, 13.4 to 18.4 s as moderate impairment, and >18.4 s as severe impairment [77]. TUG is not easily affected by the patient’s mental state, lifestyle, or physique [79,80] and is highly related to factors like lower limb muscle strength, sense of balance, walking ability, and risk of fall [72]. Furthermore, the TUG is used to evaluate motor function in healthy patients as well as with lumbar degenerative diseases [80,81]. Therefore, TUG is useful for evaluating dynamic balance in lumbar spine diseases. The MCID of TUG after ASD surgery and lumbar degenerative disease surgery is reported to be 2.0 s [71] and 2.1 s [82], respectively.
Figure 6.
Timed up and go test.
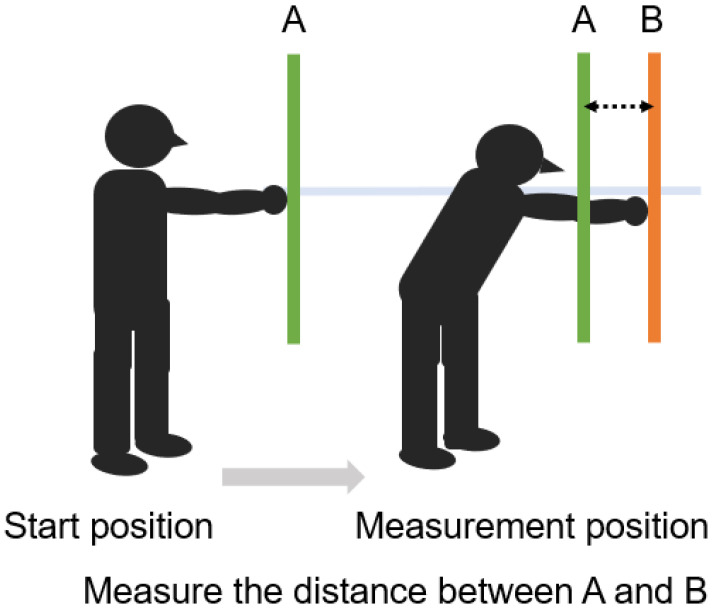
5.4. Functional Reach Test (FRT) (Figure 7)
The FRT quantifies participants’ dynamic in-place standing balance control to reach distance. The distance between the starting and maximal forward reach distance beyond the participant’s arm length represents the reach distance and is recorded in centimeters [83]. Spinal mobility has been shown to significantly impact distance reached [84]. Performance of the FRT involves trunk control and depends on core and back muscle strength [85,86]. Injury to paraspinal muscles and changes in proprioception of paraspinal muscles due to lumbar spine surgery affect trunk muscle strength, leading to decreased trunk control and postural instability [87], so balance assessment using FRT is necessary.
Figure 7.
Functional reach test.
5.5. The Balance Evaluation Systems Test (BESTest) (Table 4)
The BESTest is a functionality scale developed to assess balance and risk of falls in the elderly [88]. It consists of 36 items and is grouped into six subsections, which represent different systems that may constrain balance, namely A: biomechanical constraints, B: stability limits/verticality, C: anticipatory postural adjustments, D: postural responses, E: sensory orientation, and F: stability in gait. Each item is scored on a four-point ordinal scale from 0 (worst performance) to 3 (best performance). Total and subscale scores are translated to a percentage score. The BESTest influences QOL in ASD [89], and the reliability of the BESTest has been reported [90]. BESTest is difficult to use in clinical practice due to its complexity, so a shortened version called Mini-BESTest [91] has been developed (Table 5).
Table 4.
BESTest.
| I. Biomechanical Constraints | II. Stability Limits | III. Anticipatory Postural Adjustments | IV. Postural Responses | V. Sensory Orientation | VI. Stability in Gait |
|---|---|---|---|---|---|
| 1. Base of support | 6. Sitting verticality (left and right) and lateral lean | 9. Sit to stand | 14. In-place response, forward | 19. Sensory integration for balance, Stance on firm surface, | 21. Gait level surface |
| 2. CoM alignment | 7. Functional reach forward | 10. Rise to toes | 15. In-place response, backward | 20. Incline, EC | 22. Change in gait speed |
| 3. Ankle strength and ROM | 8. Functional reach lateral | 11. Stand on one leg | 16. Compensatory stepping correction, forward | 23. Walk with head turns, horizontal | |
| 4. Hip/trunk lateral strength | 12. Alternate stair touching | 17. Compensatory stepping correction, backward | 24. Walk with pivot turns | ||
| 5. Sit on floor and stand up | 13. Standing arm raise | 18. Compensatory stepping correction, lateral | 25. Step over obstacles | ||
| 26. Timed “Get Up and Go” Test | |||||
| 27. Timed “Get Up and Go” Test with dual task |
CoM = center of mass, ROM = range of motion, CTSIB = Clinical Test of Sensory Integration for Balance, EO = eyes open, EC = eyes closed.
Table 5.
Mini Balance Evaluation Systems Test (Mini BESTest).
| Anticipatory Postural Adjustments | Postural Responses | Sensory Orientation | Dynamic Gait |
|---|---|---|---|
| 1. Sit to stand | 4. Compensatory stepping correction, forward | 7. Stance on firm surface, EO | 11. Change in gait speed |
| 2. Rise to toes | 5. Compensatory stepping correction, backward | 9. Stance on foam, EC | 12. Walk with head turns, horizontal |
| 3. Stand on one leg (left and right) | 6. Compensatory stepping correction, lateral (left and right) | 10. Incline, EC | 13. Walk with pivot turns |
| 12. Step over obstacles | |||
| 14. Cognitive Get up and Go |
EO = Eyes Open; EC = Eyes Closed.
5.6. Three-Dimensional Motion Analyzers and Force Plate
Usually, gait analysis is generally performed with 3D motion analyzers [92,93] and force plates [94,95]. These devices can be used to analyze gait patterns, detailed joint movements, and gravity lines [96,97]. However, the disadvantages of these methods are cost-effectiveness, complexity of equipment operation and analysis process.
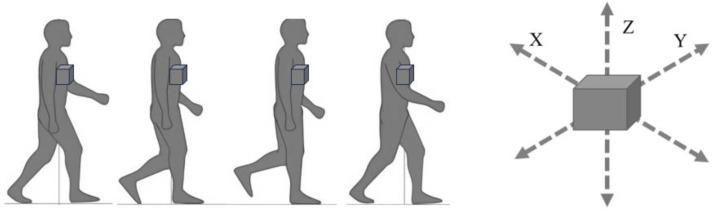
5.7. Triaxial Accelerometer (Figure 8)
Gait sway evaluation using an accelerometer (wearable sensor) has become a popular gait evaluation method due to its cost-effectiveness [98,99,100]. Accelerometers are easy to wear and have no limitations on measurement location, making them simple and practical tools in clinical practice [101]. Root mean square (RMS) of trunk acceleration is an indicator used to study gait sway with accelerometers [102]. RMS represents the degree of amplitude of the waveform, and a larger trunk acceleration RMS during gait indicates a greater gait sway.
Figure 8.
The principle of accelerometer.
6. Physical Therapy after Lumbar Spine Surgery
We believe that post-operative physical therapy after lumbar spine surgery is important to strengthen the affected muscles, improve balance, and facilitate return to ADL. Spinal surgery mainly includes decompression and fusion, with good post-operative results regardless of the surgical technique, and in recent years, multi-level fusion has become increasingly common [64,103]. In a comparison of surgical techniques, fixation as compared to decompression has more blood loss, operative time, and length of hospital stay [104,105].
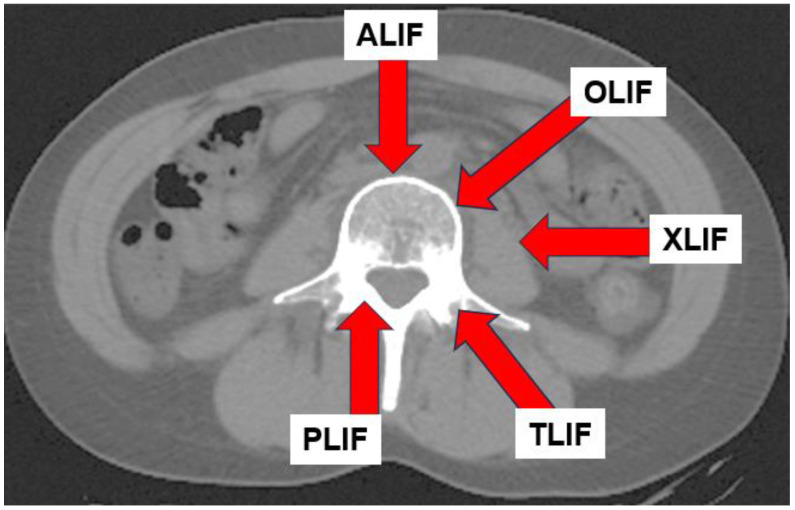
In recent years, lateral lumbar interbody fusion (LLIF) has become more popular, with extreme lateral interbody fusion (XLIF) and oblique lumbar interbody fusion (OLIF) being the most common LLIF techniques. These techniques are less invasive than conventional posterior lumbar interbody fusion (PLIF) [106] and transforaminal lumber interbody fusion (TLIF) [107] and allows for the insertion of a larger cage, which allows for a greater restoration of lumbar lordosis [108,109].
In physical therapy, it is necessary to identify the path of entry for spinal fusion and to understand the muscles involved [110] (Figure 9). Muscle atrophy results from denervation due to surgical invasion of the multifidus and erector spinae muscles for posterior approach (PLIF and TLIF) [111,112]. LLIF incises the external oblique, internal oblique, and transversus abdominis muscles, resulting in post-operative muscle weakness (Figure 10). Hence, rehabilitation should be focused according to the procedure performed, as trunk extension and trunk flexion strength strongly correlate with ODI [113].
Figure 9.
The path of entry for different spinal fusion techniques.
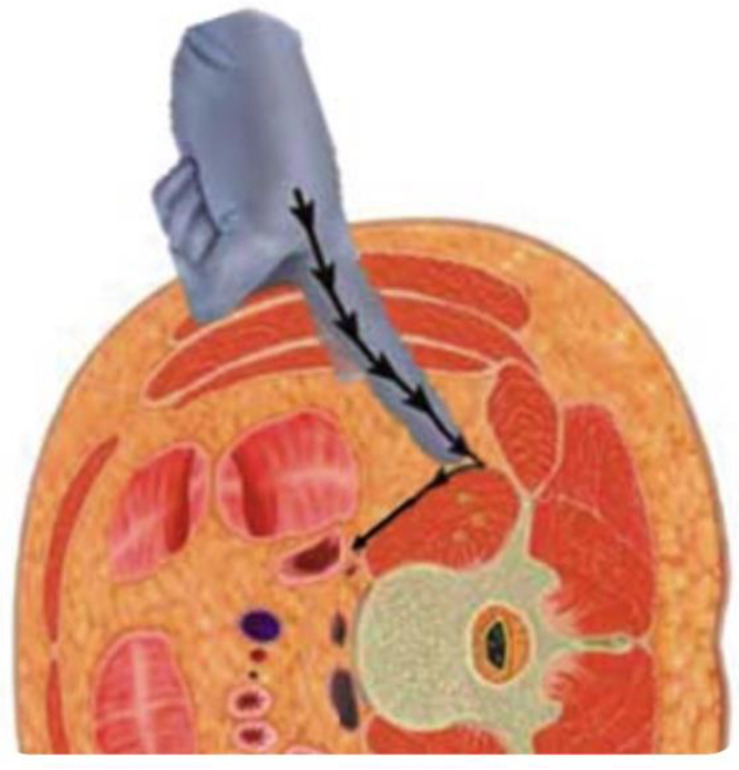
Figure 10.
Oblique lumbar interbody fusion (OLIF) approach.
Rehabilitation can be categorized as follows. Category 1: simple muscle power weakness, Category 2: loss of sustaining power, Category 3: spinal imbalance. The ways to proceed with the rehabilitation program after spinal surgery, considering the above stages of rehabilitation, are as below. Category 1: Exercises focused on weakness of muscles due to a surgical procedure. Category 2: Aerobic, repeated exercises for increasing the sustainability of the core, upper, and lower limbs. Category 3: Dual and multitask balance exercises improve spinal balance for improved daily activity performance.
6.1. Trunk Muscle Strengthening (Figure 11)
After lumbar spine fusion, motion at the level adjacent to the fusion may be altered to compensate for changes caused by the fusion, an occurrence that must also be taken into account when planning post-operative rehabilitation programs. During the early post-operative phase, strengthening exercises should be performed while keeping the lumbar spine in a neutral position to minimize strain on the fused/adjacent segment and thereafter to avoid breakage or pulling out of the implants. In functional neutral spine control exercises (NSCE), a destabilizing force acts on the trunk through loading of the extremities, and therefore proper recruitment of the trunk muscles is required to stabilize the lumbo-pelvic complex [114].
Figure 11.
Functional neutral spine control exercises, (A): Drow-in [115,116], (B): Bird dog exercise, (C): Clam shell exercise, (D): Bilateral shoulder extension, (E): Bilateral shoulder flexion, (F): Hip abduction.
Functional NSCE mimics the trunk muscle activity patterns that occur during activities like lifting, pushing, or pulling movements [117,118]. The NSCE program has two main aims: (i) to improve control of the neutral lumbar spine and (ii) increase trunk and hip muscle coordination and strength [119]. Figure 11 shows the NSCE we have been using since the acute phase.
6.2. Psoas Muscle Strengthening
In XLIF, the disk space is approached through the psoas muscle. XLIF splits the psoas major muscle, resulting in muscle weakness at a rate of 9% to 31% [120]. OLIF avoids splitting of the psoas major muscle but is still associated with a 1.2% incidence of psoas muscle weakness [121]. Corrective spinal fusion for ASD with OLIF has also been shown to decrease psoas major muscle strength [122]. Strength of the psoas major muscle is related to post-operative gait sway after ASD correction [87] and to the rate of bony fusion [123], making post-operative strengthening of the psoas major muscle an important part of physical therapy programs.
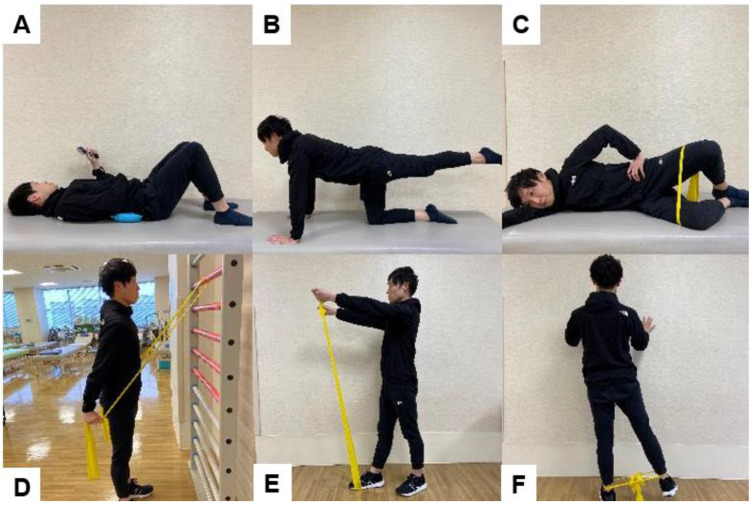
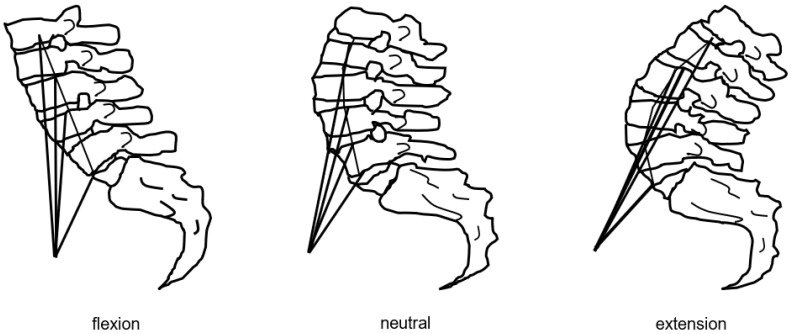
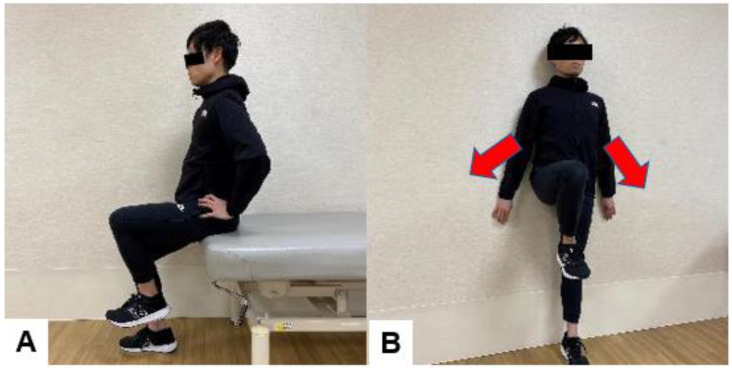
There are some points to keep in mind when strengthening the psoas major muscle after lumbar fusion surgery. The psoas major muscle has a lumbar extension function in lumbar lordosis and a lumbar flexion function in lumbar kyphosis (Figure 12) [124]. Lumbar kyphosis is a factor in the impairment of ADL and adjacent segment diseases [125,126]. Hence, it is necessary to strengthen the psoas major muscles in a posture that can maintain the physiological lordotic position of the lumbar spine. The exercises we perform at our clinic to strengthen the psoas major muscles are shown in Figure 13.
Figure 12.
Effect of iliopsoas muscle in three positions.
Figure 13.
Iliopsoas muscle exercise, (A): Hip flexion exercise in sitting position, (B): Wall standing exercise.
6.3. Exercises to Improve Balance after Spinal Fusion Surgery
Balance dysfunction can occur after spinal surgery, increasing the risk of falls and hip fractures. Patients with long-segment thoracolumbar spine fusions had a significantly higher risk of hip fracture than those with only discectomies [127]. After a spinal fusion, ASD patients exhibit altered proprioception, sensorimotor integration failure, and postural reflex dysfunction [128]. In ASD patients after corrective spinal fusion, dynamic balance capacity improves after 6 months post-operatively [129] and is related to achieving the patient-acceptable symptom state in ODI [130]. In recent years, BESTest has been used to evaluate balance ability in ASD [84,85].
It has been reported that patients with ASD have poorer BESTest results and reduced dynamic balance than healthy elderly people [84]. Halvarsson’s program includes five of the six domains of this model [131] (Figure 14). Training balance during dual-task conditions appears to be necessary to improve balance control in situations with divided attention, as balance training with single-task exercises has been shown to not transfer to dual-task performance [132].
Figure 14.
Dual- and multi-task balance exercise, (A): Walk while trying not to drop the ball on the tray (B): Stand on balance cushions with eyes closed, (C): Catch the ball while standing on the balance cushion, (D): Slalom walking with additional cognitive tasks.
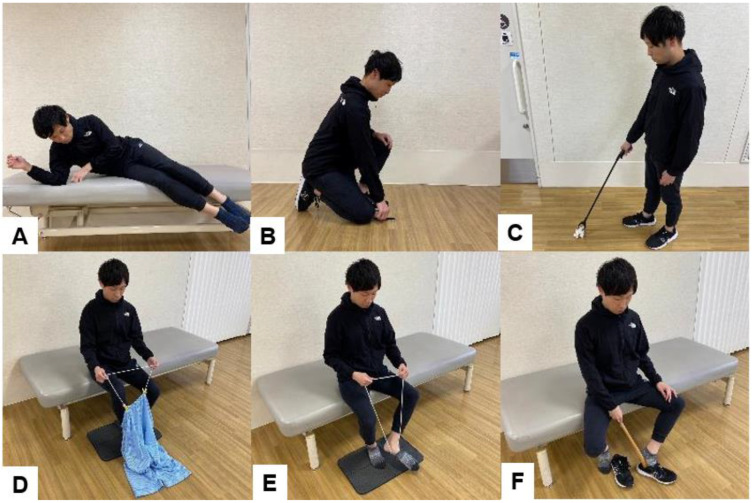
6.4. Guidance on ADL after Spinal Fusion Surgery
Patients who underwent a multilevel fusion, especially more than four levels, reported more limitations because of post-operative lumbar stiffness [133]. Patients with ASD after spinal corrective fusion surgery have difficulty with activities such as picking up objects from the floor, cutting toenails, maintaining personal hygiene, and putting on pants, even 2 years after surgery [134]. Lumbar spinal fusion patients with a fixed pelvis should be taught the use of self-help devices and ADL to prevent implant failure (Figure 15).
Figure 15.
Self-help devices and coaching of ADL, (A): Getting up from a lateral position, (B): Picking up things from the floor, (C): How to pick up objects from the floor using self-help tools, (D): How to put on pants using a trouser aid, (E): How to put on pants using a trouser aid, (F): How to put on shoes using a shoehorn.
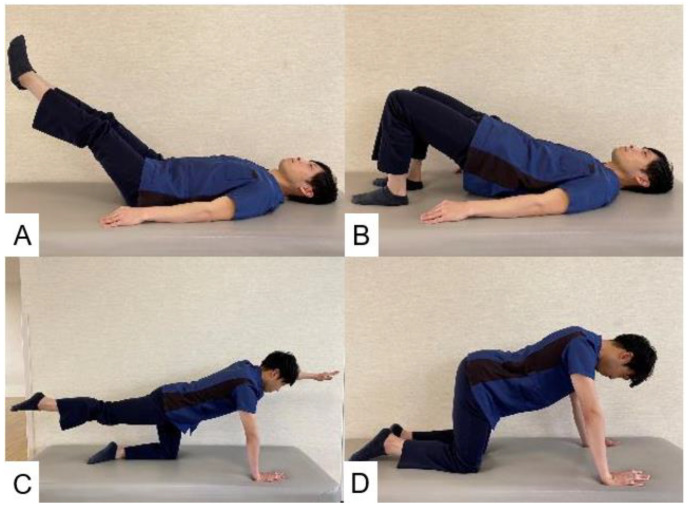
Rohlmann et al. reported movements and exercise therapy that place stress on the spine in patients undergoing lumbar corpectomy [135,136]. Movements that place stress on the spine include bending and lifting weight from the ground, forward elevation of arms with a weight in hands, tying shoes, and forward bending [135]. After lumbar spinal fusion, it is necessary to teach patients to avoid these behaviors. Exercise therapy that places stress on the spine should be avoided until bony fusion. These exercises include lifting both legs in the supine position, lifting the pelvis in the supine position, outstretching one arm with or without simultaneously outstretching contralateral leg in the all-fours position, and arching of the back in the all-fours position (Figure 16) [136]. Some exercises are not available for the elderly or fat patients. The rehabilitation personnel should select appropriate exercises for those patients.
Figure 16.
The restricted exercise, (A): Lifting both legs in the supine position, (B): Lifting of the pelvis in the supine position, (C): Outstretching one arm with or without simultaneously outstretching of the contralateral leg in the all-fours position, (D): Arching of the back in the all-fours position.
7. Conclusions
Rehabilitation is a multi-faceted journey to restore mobility, function, and quality of life. Physical therapy, cognitive-behavioral therapy, and ADL are used to assess and evaluate lumbar spine disease using PROs and physical performance tests. The current rehabilitation practice focuses on muscle strengthening, but the importance of spinal balance is less elaborated. We thus equally emphasize muscle strengthening and balance improvement post-lumbar spine surgery.
Appendix A
Figure A1.
Roland-Morris Disability Questionnaire (RMDQ).
Appendix B
Figure A2.
Oswestry Disability Index (ODI).
Author Contributions
T.S.—writing draft preparation; S.G.—writing and editing; M.T.—conceptualization; T.K. writing and editing; K.L.—data collection; S.J.E.—writing and editing; S.P.P.—writing and editing; K.T.—data collection; Y.Y.—data collection; M.N.—data collection. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional review boards at Okayama Rosai Hospital (approval No. 484, 8 April 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was supported by research funds to promote the hospital functions of the Japan Organization of Occupational Health and Safety (2024-12).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Manchikanti L., Singh V., Falco F.J., Benyamin R.M., Hirsch J.A. Epidemiology of low back pain in adults. Neuromodulation Technol. Neural Interface. 2014;17:3–10. doi: 10.1111/ner.12018. [DOI] [PubMed] [Google Scholar]
- 3.Fehlings M.G., Tetreault L., Nater A., Choma T., Harrop J., Mroz T., Santaguida C., Smith J.S. The aging of the global population: The changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77:S1–S5. doi: 10.1227/NEU.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 4.Sivasubramaniam V., Patel H.C., Ozdemir B.A., Papadopoulos M.C. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: A 15-year time-series study. BMJ Open. 2015;5:e009011. doi: 10.1136/bmjopen-2015-009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi K., Sato K., Kato F., Kanemura T., Yoshihara H., Sakai Y., Shinjo R., Ohara T., Yagi H., Matsubara Y., et al. Trends in the numbers of spine surgeries and spine surgeons over the past 15 years. Nagoya J. Med. Sci. 2022;84:155–162. doi: 10.18999/nagjms.84.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancelliere C., Wong J.J., Yu H., Nordin M., Mior S., Pereira P., Brunton G., Shearer H., Connell G., Verville L., et al. Postsurgical rehabilitation for adults with low back pain with or without radiculopathy who were treated surgically: Protocol for a mixed studies systematic review. BMJ Open. 2020;10:e036817. doi: 10.1136/bmjopen-2020-036817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebenbichler G.R., Inschlag S., Pflüger V., Stemberger R., Wiesinger G., Novak K., Christoph K., Resch K.L. Twelve-year follow-up of a randomized controlled trial of comprehensive physiotherapy following disc herniation operation. Clin. Rehabil. 2015;29:548–560. doi: 10.1177/0269215514552032. Erratum in Clin. Rehabil. 2016, 30, 623. [DOI] [PubMed] [Google Scholar]
- 8.Archer K.R., Devin C.J., Vanston S.W., Koyama T., Phillips S.E., Mathis S.L., George S.Z., McGirt M.J., Spengler D.M., Aaronson O.S., et al. Cognitive-Behavioral-Based Physical Therapy for Patients With Chronic Pain Undergoing Lumbar Spine Surgery: A Randomized Controlled Trial. J. Pain. 2016;17:76–89. doi: 10.1016/j.jpain.2015.09.013. Erratum in J. Pain 2017, 18, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindgreen P., Rolving N., Nielsen C.V., Lomborg K. Interdisciplinary Cognitive-Behavioral Therapy as Part of Lumbar Spinal Fusion Surgery Rehabilitation: Experience of Patients With Chronic Low Back Pain. Orthop. Nurs. 2016;35:238–247. doi: 10.1097/NOR.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rushton A., Eveleigh G., Petherick E.J., Heneghan N., Bennett R., James G., Wright C. Physiotherapy rehabilitation following lumbar spinal fusion: A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2012;2:e000829. doi: 10.1136/bmjopen-2012-000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wibault J., Öberg B., Dedering Å., Löfgren H., Zsigmond P., Peolsson A. Structured postoperative physiotherapy in patients with cervical radiculopathy: 6-month outcomes of a randomized clinical trial. J. Neurosurg. Spine. 2017;28:1–9. doi: 10.3171/2017.5.SPINE16736. [DOI] [PubMed] [Google Scholar]
- 12.Bogaert L., Thys T., Depreitere B., Dankaerts W., Amerijckx C., Van Wambeke P., Jacobs K., Boonen H., Brumagne S., Moke L., et al. Rehabilitation to improve outcomes of lumbar fusion surgery: A systematic review with meta-analysis. Eur. Spine J. 2022;31:1525–1545. doi: 10.1007/s00586-022-07158-2. [DOI] [PubMed] [Google Scholar]
- 13.Manni T., Ferri N., Vanti C., Ferrari S., Cuoghi I., Gaeta C., Sgaravatti I., Pillastrini P. Rehabilitation after lumbar spine surgery in adults: A systematic review with meta-analysis. Arch. Physiother. 2023;13:21. doi: 10.1186/s40945-023-00175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ditunno J.F. Linking spinal cord injury rehabilitation between the World Wars: The R. Tait McKenzie legacy. J. Spinal Cord Med. 2017;40:641–648. doi: 10.1080/10790268.2017.1370522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrosbree R.D. Spinal cord injuries as a result of motorcycle accidents. Paraplegia. 1978;16:102–112. doi: 10.1038/sc.1978.16. [DOI] [PubMed] [Google Scholar]
- 16.Bolton D. Looking forward to a decade of the biopsychosocial model. BJPsych Bull. 2022;46:228–232. doi: 10.1192/bjb.2022.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vásquez-Ríos J.R., Nava-Bringas T.I. Lumbar stabilization exercises. Cir. Cir. 2014;82:306–313. [PubMed] [Google Scholar]
- 18.Dydyk A.M., Sapra A. Williams Back Exercises. Stat Pearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 4 April 2024)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551558/ [Google Scholar]
- 19.Kelly R.P., Johnson J.T. Acute low back pain. J. Am. Med. Assoc. 1955;158:1520–1521. doi: 10.1001/jama.1955.02960170036010b. [DOI] [PubMed] [Google Scholar]
- 20.Pheasant H.C. Practical posture building. Clin. Orthop. 1962;25:83–91. [PubMed] [Google Scholar]
- 21.Cailliet R. Low Back Pain Syndrome. Daves Company; Philadelphia, PA, USA: 1998. pp. 156–178. [Google Scholar]
- 22.Böhler L. Ubungsbehandlung von Wirbelbrüchen [Exercise therapy in vertebral fractures] Hefte Unfallheilkd. 1971;108:60–63. [PubMed] [Google Scholar]
- 23.McKenzie R. Acute low back ache and exercises. N. Z. Med. J. 1994;107:318. [PubMed] [Google Scholar]
- 24.Niederer D., Mueller J. Sustainability effects of motor control stabilisation exercises on pain and function in chronic nonspecific low back pain patients: A systematic review with meta-analysis and meta-regression. PLoS ONE. 2020;15:e0227423. doi: 10.1371/journal.pone.0227423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Guideline for Non-Surgical Management of Chronic Primary Low Back Pain in Adults. [(accessed on 4 April 2024)]. Available online: https://www.who.int/publications/i/item/9789240081789.
- 26.The World Health Organization World Report on Disability: Chapter 4 Rehabilitation. [(accessed on 4 April 2024)]. Available online: https://www.spine.org/Documents/ResearchClinicalCare/Guidelines/LumbarDiscHerniation.pdf.
- 27.Snowdon M., Peiris C.L. Physiotherapy Commenced Within the First Four Weeks Post-Spinal Surgery Is Safe and Effective: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016;97:292–301. doi: 10.1016/j.apmr.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Elsayyad M.M., Abdel-Aal N.M., Helal M.E. Effect of Adding Neural Mobilization Versus Myofascial Release to Stabilization Exercises after Lumbar Spine Fusion: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021;102:251–260. doi: 10.1016/j.apmr.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Daniels C.J., Cupler Z.A., Gliedt J.A., Walters S., Schielke A.L., Hinkeldey N.A., Golley D.J., Hawk C. Manipulative and manual therapies in the management of patients with prior lumbar surgery: A systematic review. Complement. Ther. Clin. Pract. 2021;42:101261. doi: 10.1016/j.ctcp.2020.101261. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke R., Singer J., Guyatt G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin. Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 31.van Hooff M.L., Mannion A.F., Staub L.P., Ostelo R.W., Fairbank J.C. Determination of the Oswestry Disability Index score equivalent to a “satisfactory symptom state” in patients undergoing surgery for degenerative disorders of the lumbar spine—A Spine Tango registry-based study. Spine J. 2016;16:1221–1230. doi: 10.1016/j.spinee.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Issa T.Z., Lee Y., Henry T.W., Trenchfield D., Schroeder G.D., Vaccaro A.R., Kepler C.K. Values derived from patient reported outcomes in spine surgery: A systematic review of the minimal clinically important difference, substantial clinical benefit, and patient acceptable symptom state. Eur. Spine J. 2023;32:3333–3351. doi: 10.1007/s00586-023-07896-x. [DOI] [PubMed] [Google Scholar]
- 33.Parker S.L., Adogwa O., Paul A.R., Anderson W.N., Aaronson O., Cheng J.S., McGirt M.J. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J. Neurosurg. Spine. 2011;14:598–604. doi: 10.3171/2010.12.SPINE10472. [DOI] [PubMed] [Google Scholar]
- 34.Parker S.L., Adogwa O., Mendenhall S.K., Shau D.N., Anderson W.N., Cheng J.S., Devin C.J., McGirt M.J. Determination of minimum clinically important difference (MCID) in pain, disability, and quality of life after revision fusion for symptomatic pseudoarthrosis. Spine J. 2012;12:1122–1128. doi: 10.1016/j.spinee.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Johnsen L.G., Hellum C., Nygaard O.P., Storheim K., Brox J.I., Rossvoll I., Leivseth G., Grotle M. Comparison of the SF6D, the EQ5D, and the oswestry disability index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet. Disord. 2013;14:148. doi: 10.1186/1471-2474-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solberg T., Johnsen L.G., Nygaard Ø.P., Grotle M. Can we define success criteria for lumbar disc surgery?: Estimates for a substantial amount of improvement in core outcome measures. Acta Orthop. 2013;84:196–201. doi: 10.3109/17453674.2013.786634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida G., Hasegawa T., Yamato Y., Kobayashi S., Shin O., Banno T., Mihara Y., Arima H., Ushirozako H., Yasuda T., et al. Minimum Clinically Important Differences in Oswestry Disability Index Domains and Their Impact on Adult Spinal Deformity Surgery. Asian Spine J. 2019;13:35–44. doi: 10.31616/asj.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukushima M., Oka H., Oshima Y., Yuzawa Y., Matsudaira K., Tanaka S., Inanami H. Evaluation of the Minimum Clinically Important Differences of the Zurich Claudication Questionnaire in Patients With Lumbar Spinal Stenosis. Clin. Spine Surg. 2020;33:E499–E503. doi: 10.1097/BSD.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 39.Roland M., Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara A., Kobayashi N., Saiki K., Kitagawa T., Tamai K., Saotome K. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine. 2003;28:1601–1607. doi: 10.1097/01.BRS.0000077510.95462.39. [DOI] [PubMed] [Google Scholar]
- 41.Kersten R.F.M.R., Fikkers J., Wolterbeek N., Öner F.C., van Gaalen S.M. Are the Roland Morris Disability Questionnaire and Oswestry Disability Index interchangeable in patients after lumbar spinal fusion? J. Back Musculoskelet. Rehabil. 2021;34:605–611. doi: 10.3233/BMR-200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fairbank J.C., Pynsent P.B. The Oswestry Disability Index. Spine. 2000;25:2940–2952; discussion 2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 43.Vianin M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J. Chiropr. Med. 2008;7:161–163. doi: 10.1016/j.jcm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haro H., Maekawa S., Hamada Y. Prospective analysis of clinical evaluation and self-assessment by patients after decompression surgery for degenerative lumbar canal stenosis. Spine J. 2008;8:380–384. doi: 10.1016/j.spinee.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Carreon L.Y., Berven S.H., Djurasovic M., Bratcher K.R., Glassman S.D. The discriminative properties of the SF-6D compared with the SF-36 and ODI. Spine. 2013;38:60–64. doi: 10.1097/BRS.0b013e3182609df6. [DOI] [PubMed] [Google Scholar]
- 46.Koivunen K., Widbom-Kolhanen S., Pernaa K., Arokoski J., Saltychev M. Reliability and validity of Oswestry Disability Index among patients undergoing lumbar spinal surgery. BMC Surg. 2024;24:13. doi: 10.1186/s12893-023-02307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stucki G., Daltroy L., Liang M.H., Lipson S.J., Fossel A.H., Katz J.N. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21:796–803. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 48.North American Spine Society (NASS) Clinical Guidelines for Multidisciplinary Spine Care Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. North American Spine Society (NASS); Burr Ridge, IL, USA: 2007. [Google Scholar]
- 49.Hara N., Matsudaira K., Masuda K., Tohnosu J., Takeshita K., Kobayashi A., Murakami M., Kawamura N., Yamakawa K., Terayama S., et al. Psychometric Assessment of the Japanese Version of the Zurich Claudication Questionnaire (ZCQ): Reliability and Validity. PLoS ONE. 2016;11:e0160183. doi: 10.1371/journal.pone.0160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae J., Theologis A.A., Strom R., Tay B., Burch S., Berven S., Mummaneni P.V., Chou D., Ames C.P., Deviren V. Comparative analysis of 3 surgical strategies for adult spinal deformity with mild to moderate sagittal imbalance. J. Neurosurg. Spine. 2018;28:40–49. doi: 10.3171/2017.5.SPINE161370. [DOI] [PubMed] [Google Scholar]
- 51.Lonner B., Yoo A., Terran J.S., Sponseller P., Samdani A., Betz R., Shuffelbarger H., Shah S.A., Newton P. Effect of spinal deformity on adolescent quality of life: Comparison of operative scheuermann kyphosis, adolescent idiopathic scoliosis, and normal controls. Spine. 2013;38:1049–1055. doi: 10.1097/BRS.0b013e3182893c01. [DOI] [PubMed] [Google Scholar]
- 52.Haher T.R., Gorup J.M., Shin T.M., Homel P., Merola A.A., Grogan D.P., Pugh L., Lowe T.G., Murray M. Results of the Scoliosis Research Society instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine. 1999;24:1435–1440. doi: 10.1097/00007632-199907150-00008. [DOI] [PubMed] [Google Scholar]
- 53.Berven S., Deviren V., Demir-Deviren S., Hu S.S., Bradford D.S. Studies in the modified Scoliosis Research Society Outcomes Instrument in adults: Validation, reliability, and discriminatory capacity. Spine. 2003;28:2164–2169; discussion 2169. doi: 10.1097/01.BRS.0000084666.53553.D6. [DOI] [PubMed] [Google Scholar]
- 54.Yoshihara H., Hasegawa K., Okamoto M., Hatsushikano S., Watanabe K. Relationship between sagittal radiographic parameters and disability in patients with spinal disease using 3D standing analysis. Orthop. Traumatol. Surg. Res. 2018;104:1017–1023. doi: 10.1016/j.otsr.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Smith J.S., Klineberg E., Schwab F., Shaffrey C.I., Moal B., Ames C.P., Hostin R., Fu K.M., Burton D., Akbarnia B., et al. Change in classification grade by the SRS-Schwab Adult Spinal Deformity Classification predicts impact on health-related quality of life measures: Prospective analysis of operative and nonoperative treatment. Spine. 2013;38:1663–1671. doi: 10.1097/BRS.0b013e31829ec563. [DOI] [PubMed] [Google Scholar]
- 56.Doi T., Inoue H., Arai Y., Shirado O., Doi T., Yamazaki K., Uno K., Yanagida H., Takeshita K. Reliability and validity of a novel quality of life questionnaire for female patients with adolescent idiopathic scoliosis: Scoliosis Japanese Questionnaire-27: A multicenter, cross-sectional study. BMC Musculoskelet. Disord. 2018;19:99. doi: 10.1186/s12891-018-2025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hart R.A., Pro S.L., Gundle K.R., Marshall L.M. Lumbar stiffness as a collateral outcome of spinal arthrodesis: A preliminary clinical study. Spine J. 2013;13:150–156. doi: 10.1016/j.spinee.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Hart R.A., Gundle K.R., Pro S.L., Marshall L.M. Lumbar Stiffness Disability Index: Pilot testing of consistency, reliability, and validity. Spine J. 2013;13:157–161. doi: 10.1016/j.spinee.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Furuya H., Ito T., Hirohata K., Mitomo S., Yamasaki K., Igarashi H., Omori K., Hoshino M., Hart R.A. Construct Validity and Reliability of the Japanese Version of the Lumbar Stiffness Disability Index. Spine. 2021;46:E333–E337. doi: 10.1097/BRS.0000000000003772. [DOI] [PubMed] [Google Scholar]
- 60.Durand W.M., Daniels A.H., Hamilton D.K., Passias P.G., Kim H.J., Protopsaltis T., Lafage V., Smith J.S., Shaffrey C., Gupta M., et al. Younger Patients Are Differentially Affected by Stiffness-Related Disability Following Adult Spinal Deformity Surgery. World Neurosurg. 2019;132:e297–e304. doi: 10.1016/j.wneu.2019.08.169. [DOI] [PubMed] [Google Scholar]
- 61.Daniels A.H., Reid D., Durand W., Disilvestro K., Hamilton D.K., Passias P., Kim H.J., Protopsaltis T., LaFage V., Smith J.S., et al. Sexual Dysfunction Secondary to Lumbar Stiffness in Adult Spinal Deformity Patients Before and After Long-Segment Spinal Fusion. World Neurosurg. 2020;139:e474–e479. doi: 10.1016/j.wneu.2020.04.033. [DOI] [PubMed] [Google Scholar]
- 62.Watters W.C., 3rd, Baisden J., Gilbert T.J., Kreiner S., Resnick D.K., Bono C.M., Ghiselli G., Heggeness M.H., Mazanec D.J., O’Neill C., et al. Degenerative lumbar spinal stenosis: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8:305–310. doi: 10.1016/j.spinee.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 63.Ishimoto Y., Yoshimura N., Muraki S., Yamada H., Nagata K., Hashizume H., Takiguchi N., Minamide A., Oka H., Kawaguchi H., et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012;20:1103–1108. doi: 10.1016/j.joca.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Deyo R.A., Mirza S.K., Martin B.I., Kreuter W., Goodman D.C., Jarvik J.G. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagai S., Kawabata S., Michikawa T., Ito K., Takeda H., Ikeda D., Kaneko S., Fujita N. Association between frailty and locomotive syndrome in elderly patients with lumbar spinal stenosis: A retrospective longitudinal analysis. Geriatr. Gerontol. Int. 2024;24:116–122. doi: 10.1111/ggi.14785. [DOI] [PubMed] [Google Scholar]
- 66.Kato S., Demura S., Kabata T., Matsubara H., Kurokawa Y., Okamoto Y., Kuroda K., Kajino Y., Yokogawa N., Inoue D., et al. Risk factors that hinder locomotive syndrome improvement following surgery for musculoskeletal diseases in older patients: A multicentre prospective study. Mod. Rheumatol. 2023;33:836–842. doi: 10.1093/mr/roac082. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi T., Morimoto T., Otani K., Mawatari M. Locomotive Syndrome and Lumbar Spine Disease: A Systematic Review. J. Clin. Med. 2022;11:1304. doi: 10.3390/jcm11051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M., Brach J., Chandler J., Cawthon P., Connor E.B., et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. Erratum in Age Ageing 2019, 48, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wada T., Tanishima S., Kitsuda Y., Osaki M., Nagashima H., Noma H., Hagino H. Walking speed is associated with postoperative pain catastrophizing in patients with lumbar spinal stenosis: A prospective observational study. BMC Musculoskelet. Disord. 2022;23:1108. doi: 10.1186/s12891-022-06086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakaguchi T., Meena U., Tanaka M., Xiang H., Fujiwara Y., Arataki S., Taoka T., Takamatsu K., Yasuda Y., Nakagawa M., et al. Minimal Clinically Important Differences in Gait and Balance Ability in Patients Who Underwent Corrective Long Spinal Fusion for Adult Spinal Deformity. J. Clin. Med. 2023;12:6500. doi: 10.3390/jcm12206500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh S.J., Puhan M.A., Andrianopoulos V., Hernandes N.A., Mitchell K.E., Hill C.J., Lee A.L., Camillo C.A., Troosters T., Spruit M.A., et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014;44:1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 73.Takenaka H., Kamiya M., Sugiura H., Nishihama K., Ito A., Suzuki J., Hanamura S. Responsiveness and Minimal Clinically Important Difference of the 6-minute Walk Distance in Patients Undergoing Lumbar Spinal Canal Stenosis Surgery. Clin. Spine Surg. 2022;35:E345–E350. doi: 10.1097/BSD.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 74.Kondo R., Yamato Y., Nagafusa T., Mizushima T., Hasegawa T., Kobayashi S., Togawa D., Oe S., Kurosu K., Matsuyama Y. Effect of corrective long spinal fusion to the ilium on physical function in patients with adult spinal deformity. Eur. Spine J. 2017;26:2138–2145. doi: 10.1007/s00586-017-4987-9. [DOI] [PubMed] [Google Scholar]
- 75.Tomkins-Lane C.C., Battié M.C. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine. 2010;35:2097–2102. doi: 10.1097/BRS.0b013e3181f5e13b. [DOI] [PubMed] [Google Scholar]
- 76.Takenaka H., Kamiya M., Sugiura H., Nishihama K., Suzuki J., Hanamura S. Minimal Clinically Important Difference of the 6-Minute Walk Distance in Patients Undergoing Lumbar Spinal Canal Stenosis Surgery: 12 Months Follow-Up. Spine. 2023;48:559–566. doi: 10.1097/BRS.0000000000004566. [DOI] [PubMed] [Google Scholar]
- 77.Gautschi O.P., Smoll N.R., Corniola M.V., Joswig H., Chau I., Hildebrandt G., Schaller K., Stienen M.N. Validity and Reliability of a Measurement of Objective Functional Impairment in Lumbar Degenerative Disc Disease: The Timed Up and Go (TUG) Test. Neurosurgery. 2016;79:270–278. doi: 10.1227/NEU.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 78.Lin S.I., Lin R.M. Disability and walking capacity in patients with lumbar spinal stenosis: Association with sensorimotor function, balance, and functional performance. J. Orthop. Sports Phys. Ther. 2005;35:220–226. doi: 10.2519/jospt.2005.35.4.220. [DOI] [PubMed] [Google Scholar]
- 79.Joswig H., Stienen M.N., Smoll N.R., Corniola M.V., Chau I., Schaller K., Hildebrandt G., Gautschi O.P. Effects of Smoking on Subjective and Objective Measures of Pain Intensity, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disk Disease. World Neurosurg. 2017;99:6–13. doi: 10.1016/j.wneu.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 80.Stienen M.N., Joswig H., Smoll N.R., Corniola M.V., Schaller K., Hildebrandt G., Gautschi O.P. Influence of Body Mass Index on Subjective and Objective Measures of Pain, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disc Disease. World Neurosurg. 2016;96:570–577.e1. doi: 10.1016/j.wneu.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 81.Podsiadlo D., Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 82.Maldaner N., Sosnova M., Ziga M., Zeitlberger A.M., Bozinov O., Gautschi O.P., Weyerbrock A., Regli L., Stienen M.N. External Validation of the Minimum Clinically Important Difference in the Timed-up-and-go Test After Surgery for Lumbar Degenerative Disc Disease. Spine. 2022;47:337–342. doi: 10.1097/BRS.0000000000004128. [DOI] [PubMed] [Google Scholar]
- 83.Duncan P.W., Weiner D.K., Chandler J., Studenski S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990;45:M192–M197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- 84.Schenkman M., Morey M., Kuchibhatla M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:M441–M445. doi: 10.1093/gerona/55.8.m441. [DOI] [PubMed] [Google Scholar]
- 85.de Waroquier-Leroy L., Bleuse S., Serafi R., Watelain E., Pardessus V., Tiffreau A.V., Thevenon A. The Functional Reach Test: Strategies, performance and the influence of age. Ann. Phys. Rehabil. Med. 2014;57:452–464. doi: 10.1016/j.rehab.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Biering-Sørensen F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine. 1984;9:106–119. doi: 10.1097/00007632-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Song J., Araghi K., Dupont M.M., Shahi P., Bovonratwet P., Shinn D., Dalal S.S., Melissaridou D., Virk S.S., Iyer S., et al. Association between muscle health and patient-reported outcomes after lumbar microdiscectomy: Early results. Spine J. 2022;22:1677–1686. doi: 10.1016/j.spinee.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horak F.B., Wrisley D.M., Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009;89:484–498. doi: 10.2522/ptj.20080071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moke L., Severijns P., Schelfaut S., Van de Loock K., Hermans L., Molenaers G., Jonkers I., Scheys L. Performance on Balance Evaluation Systems Test (BESTest) Impacts Health-Related Quality of Life in Adult Spinal Deformity Patients. Spine. 2018;43:637–646. doi: 10.1097/BRS.0000000000002390. [DOI] [PubMed] [Google Scholar]
- 90.Severijns P., Overbergh T., Scheys L., Moke L., Desloovere K. Reliability of the balance evaluation systems test and trunk control measurement scale in adult spinal deformity. PLoS ONE. 2019;14:e0221489. doi: 10.1371/journal.pone.0221489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franchignoni F., Horak F., Godi M., Nardone A., Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miura K., Kadone H., Koda M., Abe T., Funayama T., Noguchi H., Mataki K., Nagashima K., Kumagai H., Shibao Y., et al. Thoracic kyphosis and pelvic anteversion in patients with adult spinal deformity increase while walking: Analyses of dynamic alignment change using a three-dimensional gait motion analysis system. Eur. Spine J. 2020;29:840–848. doi: 10.1007/s00586-020-06312-y. [DOI] [PubMed] [Google Scholar]
- 93.Asada T., Miura K., Koda M., Kadone H., Funayama T., Takahashi H., Noguchi H., Shibao Y., Sato K., Eto F., et al. Can Proximal Junctional Kyphosis after Surgery for Adult Spinal Deformity Be Predicted by Preoperative Dynamic Sagittal Alignment Change with 3D Gait Analysis? A Case-Control Study. J. Clin. Med. 2022;11:5871. doi: 10.3390/jcm11195871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haddas R., Wood A., Lieberman I., Derman P.B. Assessing the cone of economy in patients with spinal disease using only a force plate: An observational retrospective cohort study. Eur. Spine J. 2021;30:2504–2513. doi: 10.1007/s00586-021-06836-x. [DOI] [PubMed] [Google Scholar]
- 95.Godzik J., Frames C.W., Smith Hussain V., Olson M.C., Kakarla U.K., Uribe J.S., Lockhart T.E., Turner J.D. Postural Stability and Dynamic Balance in Adult Spinal Deformity: Prospective Pilot Study. World Neurosurg. 2020;141:e783–e791. doi: 10.1016/j.wneu.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 96.Yagi M., Ohne H., Konomi T., Fujiyoshi K., Kaneko S., Takemitsu M., Machida M., Yato Y., Asazuma T. Walking balance and compensatory gait mechanisms in surgically treated patients with adult spinal deformity. Spine J. 2017;17:409–417. doi: 10.1016/j.spinee.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 97.Huysmans S.M.D., Senden R., Jacobs E., Willems P.J.B., Marcellis R.G.J., Boogaart M.V.D., Meijer K., Willems P.C. Gait alterations in patients with adult spinal deformity. N. Am. Spine Soc. J. 2023;17:100306. doi: 10.1016/j.xnsj.2023.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong R., Rau P.P. Are cost-effective technologies feasible to measure gait in older adults? A systematic review of evidence-based literature. Arch. Gerontol. Geriatr. 2020;87:103970. doi: 10.1016/j.archger.2019.103970. [DOI] [PubMed] [Google Scholar]
- 99.Sekine M., Tamura T., Yoshida M., Suda Y., Kimura Y., Miyoshi H., Kijima Y., Higashi Y., Fujimoto T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013;10:118. doi: 10.1186/1743-0003-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakaguchi T., Sake N., Tanaka M., Fujiwara Y., Arataki S., Taoka T., Kodama Y., Takamatsu K., Yasuda Y., Nakagawa M., et al. Use of a Triaxial Accelerometer to Measure Changes in Gait Sway and Related Motor Function after Corrective Spinal Fusion Surgery for Adult Spinal Deformity. J. Clin. Med. 2024;13:1923. doi: 10.3390/jcm13071923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hulleck A.A., Menoth Mohan D., Abdallah N., El Rich M., Khalaf K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022;4:901331. doi: 10.3389/fmedt.2022.901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menz H.B., Lord S.R., Fitzpatrick R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. 2003;18:35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 103.Amundsen T., Weber H., Nordal H.J., Magnaes B., Abdelnoor M., Lilleâs F. Lumbar spinal stenosis: Conservative or surgical management?: A prospective 10-year study. Spine. 2000;25:1424–1435; discussion 1435–1436. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 104.Liang H.F., Liu S.H., Chen Z.X., Fei Q.M. Decompression plus fusion versus decompression alone for degenerative lumbar spondylolisthesis: A systematic review and meta-analysis. Eur. Spine J. 2017;26:3084–3095. doi: 10.1007/s00586-017-5200-x. [DOI] [PubMed] [Google Scholar]
- 105.Chen Z., Xie P., Feng F., Chhantyal K., Yang Y., Rong L. Decompression Alone Versus Decompression and Fusion for Lumbar Degenerative Spondylolisthesis: A Meta-Analysis. World Neurosurg. 2018;111:e165–e177. doi: 10.1016/j.wneu.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 106.Briggs H., Milligan P.R. Chip fusion of the low back following exploration of the spinal canal. J. Bone Jt. Surg. 1944;26:125–130. [Google Scholar]
- 107.Hammad A., Wirries A., Ardeshiri A., Nikiforov O., Geiger F. Open versus minimally invasive TLIF: Literature review and meta-analysis. J. Orthop. Surg. Res. 2019;14:229. doi: 10.1186/s13018-019-1266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan M.W.P., Sayampanathan A.A., Jiang L., Guo C.M. Comparison of Outcomes Between Single-level Lateral Lumbar Interbody Fusion and Transforaminal Lumbar Interbody Fusion: A Meta-analysis and Systematic Review. Clin. Spine Surg. 2021;34:395–405. doi: 10.1097/BSD.0000000000001107. [DOI] [PubMed] [Google Scholar]
- 109.Hsieh P.C., Koski T.R., O’Shaughnessy B.A., Sugrue P., Salehi S., Ondra S., Liu J.C. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: Implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J. Neurosurg. Spine. 2007;7:379–386. doi: 10.3171/SPI-07/10/379. [DOI] [PubMed] [Google Scholar]
- 110.Mobbs R.J., Phan K., Malham G., Seex K., Rao P.J. Lumbar interbody fusion: Techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J. Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Waschke A., Hartmann C., Walter J., Dünisch P., Wahnschaff F., Kalff R., Ewald C. Denervation and atrophy of paraspinal muscles after open lumbar interbody fusion is associated with clinical outcome--electromyographic and CT-volumetric investigation of 30 patients. Acta Neurochir. 2014;156:235–244. doi: 10.1007/s00701-013-1981-9. [DOI] [PubMed] [Google Scholar]
- 112.Cho S.M., Kim S.H., Ha S.K., Kim S.D., Lim D.J., Cha J., Kim B.J. Paraspinal muscle changes after single-level posterior lumbar fusion: Volumetric analyses and literature review. BMC Musculoskelet. Disord. 2020;21:73. doi: 10.1186/s12891-020-3104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarnanen S., Neva M.H., Kautiainen H., Ylinen J., Pekkanen L., Kaistila T., Vuorenmaa M., Häkkinen A. The early changes in trunk muscle strength and disability following lumbar spine fusion. Disabil. Rehabil. 2013;35:134–139. doi: 10.3109/09638288.2012.690496. [DOI] [PubMed] [Google Scholar]
- 114.Tarnanen S.P., Neva M.H., Häkkinen K., Kankaanpää M., Ylinen J., Kraemer W.J., Newton R.U., Häkkinen A. Neutral spine control exercises in rehabilitation after lumbar spine fusion. J. Strength Cond. Res. 2014;28:2018–2025. doi: 10.1519/JSC.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 115.Hagins M., Adler K., Cash M., Daugherty J., Mitrani G. Effects of practice on the ability to perform lumbar stabilization exercises. J. Orthop. Sports Phys. Ther. 1999;29:546–555. doi: 10.2519/jospt.1999.29.9.546. [DOI] [PubMed] [Google Scholar]
- 116.Grooms D.R., Grindstaff T.L., Croy T., Hart J.M., Saliba S.A. Clinimetric analysis of pressure biofeedback and transversus abdominis function in individuals with stabilization classification low back pain. J. Orthop. Sports Phys. Ther. 2013;43:184–193. doi: 10.2519/jospt.2013.4397. [DOI] [PubMed] [Google Scholar]
- 117.McGill S.M., Karpowicz A., Fenwick C.M., Brown S.H. Exercises for the torso performed in a standing posture: Spine and hip motion and motor patterns and spine load. J. Strength. Cond. Res. 2009;23:455–464. doi: 10.1519/JSC.0b013e3181a0227e. [DOI] [PubMed] [Google Scholar]
- 118.Voight M.L., Hoogenboom B.J., Cook G. The chop and lift reconsidered: Integrating neuromuscular principles into orthopedic and sports rehabilitation. N. Am. J. Sports Phys. Ther. 2008;3:151–159. [PMC free article] [PubMed] [Google Scholar]
- 119.Tarnanen S., Neva M.H., Dekker J., Häkkinen K., Vihtonen K., Pekkanen L., Häkkinen A. Randomized controlled trial of postoperative exercise rehabilitation program after lumbar spine fusion: Study protocol. BMC Musculoskelet. Disord. 2012;13:123. doi: 10.1186/1471-2474-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Epstein N.E. Review of Risks and Complications of Extreme Lateral Interbody Fusion (XLIF) Surg. Neurol. Int. 2019;10:237. doi: 10.25259/SNI_559_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li J.X., Phan K., Mobbs R. Oblique Lumbar Interbody Fusion: Technical Aspects, Operative Outcomes, and Complications. World Neurosurg. 2017;98:113–123. doi: 10.1016/j.wneu.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 122.Yagi M., Fujita N., Hasegawa T., Inoue G., Kotani Y., Ohtori S., Orita S., Oshima Y., Sakai D., Sakai T., et al. Nationwide Survey of the Surgical Complications Associated with Lateral Lumbar Interbody Fusion in 2015-2020. Spine Surg. Relat. Res. 2022;7:249–256. doi: 10.22603/ssrr.2022-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi M.K., Kim S.B., Park C.K., Malla H.P., Kim S.M. Cross-Sectional Area of the Lumbar Spine Trunk Muscle and Posterior Lumbar Interbody Fusion Rate: A Retrospective Study. Clin. Spine Surg. 2017;30:E798–E803. doi: 10.1097/BSD.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 124.Santaguida P.L., McGill S.M. The psoas major muscle: A three-dimensional geometric study. J. Biomech. 1995;28:339–345. doi: 10.1016/0021-9290(94)00064-b. [DOI] [PubMed] [Google Scholar]
- 125.Blondel B., Schwab F., Ungar B., Smith J., Bridwell K., Glassman S., Shaffrey C., Farcy J.P., Lafage V. Impact of magnitude and percentage of global sagittal plane correction on health-related quality of life at 2-years follow-up. Neurosurgery. 2012;71:341–348; discussion 348. doi: 10.1227/NEU.0b013e31825d20c0. [DOI] [PubMed] [Google Scholar]
- 126.Phan K., Nazareth A., Hussain A.K., Dmytriw A.A., Nambiar M., Nguyen D., Kerferd J., Phan S., Sutterlin C., 3rd, Cho S.K., et al. Relationship between sagittal balance and adjacent segment disease in surgical treatment of degenerative lumbar spine disease: Meta-analysis and implications for choice of fusion technique. Eur. Spine J. 2018;27:1981–1991. doi: 10.1007/s00586-018-5629-6. Erratum in Eur. Spine J. 2021, 30, 3774. [DOI] [PubMed] [Google Scholar]
- 127.Li C.Y., Chang C.L., Tai T.W. Incidence and risk factors for hip fracture in elderly patients undergoing lumbar spine surgery: A nationwide database study with 11-year follow-up. Osteoporos. Int. 2018;29:2717–2723. doi: 10.1007/s00198-018-4734-z. [DOI] [PubMed] [Google Scholar]
- 128.Yagi M., Ohne H., Kaneko S., Machida M., Yato Y., Asazuma T. Does corrective spine surgery improve the standing balance in patients with adult spinal deformity? Spine J. 2018;18:36–43. doi: 10.1016/j.spinee.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 129.Sakaguchi T., Tanaka M., Suthar H., Fujiwara Y., Uotani K., Arataki S., Yamauchi T., Sugyo A., Takamatsu K., Yasuda Y., et al. Chronological Evaluation of Gait Ability and Posture Balance after Adult Spinal Deformity Surgery. Appl. Sci. 2022;12:4285. doi: 10.3390/app12094285. [DOI] [Google Scholar]
- 130.Sakaguchi T., Tanaka M., Sake N., Latka K., Fujiwara Y., Arataki S., Yamauchi T., Takamatsu K., Yasuda Y., Nakagawa M., et al. The Most Significant Factor Affecting Gait and Postural Balance in Patients’ Activities of Daily Living Following Corrective Surgery for Deformity of the Adult Spine. Medicina. 2022;58:1118. doi: 10.3390/medicina58081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Halvarsson A., Dohrn I.M., Ståhle A. Taking balance training for older adults one step further: The rationale for and a description of a proven balance training programme. Clin. Rehabil. 2015;29:417–425. doi: 10.1177/0269215514546770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agmon M., Belza B., Nguyen H.Q., Logsdon R.G., Kelly V.E. A systematic review of interventions conducted in clinical or community settings to improve dual-task postural control in older adults. Clin. Interv. Aging. 2014;9:477–492. doi: 10.2147/CIA.S54978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kimura H., Fujibayashi S., Otsuki B., Takahashi Y., Nakayama T., Matsuda S. Effects of Lumbar Stiffness After Lumbar Fusion Surgery on Activities of Daily Living. Spine. 2016;41:719–727. doi: 10.1097/BRS.0000000000001300. [DOI] [PubMed] [Google Scholar]
- 134.Togawa D., Hasegawa T., Yamato Y., Yoshida G., Kobayashi S., Yasuda T., Oe S., Banno T., Arima H., Mihara Y., et al. Postoperative Disability After Long Corrective Fusion to the Pelvis in Elderly Patients With Spinal Deformity. Spine. 2018;43:E804–E812. doi: 10.1097/BRS.0000000000002540. [DOI] [PubMed] [Google Scholar]
- 135.Rohlmann A., Pohl D., Bender A., Graichen F., Dymke J., Schmidt H., Bergmann G. Activities of everyday life with high spinal loads. PLoS ONE. 2014;9:e98510. doi: 10.1371/journal.pone.0098510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rohlmann A., Schwachmeyer V., Graichen F., Bergmann G. Spinal loads during post-operative physiotherapeutic exercises. PLoS ONE. 2014;9:e102005. doi: 10.1371/journal.pone.0102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.