Abstract
The chromosomally encoded TetA(L) protein of Bacillus subtilis is a multifunctional tetracycline-metal/H+ antiporter that also exhibits monovalent cation/H+ antiport activity and a net K+ uptake mode. In this study, B. subtilis mutant strains JC112 and JC112C were found to be representative of two phenotypic types of tetA(L) deletion strains that are generated in the same selection. Both strains exhibited increased sensitivity to low tetracycline concentrations as expected. The mutants also had significantly reduced ability to grow in media containing low concentrations of K+, indicating that the net K+ uptake mode is of physiological consequence; the deficit in JC112 was greater than in JC112C. JC112 also exhibited (i) greater impairment of Na+- or K+-dependent growth at pH 8.3 than JC112C and (ii) a greater degree of Co+2 as well as Na+ sensitivity. Studies were initiated to explore the possibility of two different patterns of compensatory changes in other ion-translocating transporters in these mutants. Increased expression of two loci has thus far been shown. Increased expression of czcD-trkA, a locus with a proposed involvement in K+ uptake, occurred in both mutants. The increase was highest in the presence of Co2+ and was higher in JC112 than in JC112C. Deletion of czcD-trkA resulted in diminished growth of the wild-type and both mutant strains at low [K+], supporting a significant role for this locus in K+ uptake. Expression of yheL, which is a homologue of the Na+/H+ antiporter-encoding nhaC gene from Bacillus firmus OF4, was also increased in both tetA(L) deletion strains, again with higher up-regulation in JC112. The phenotypes resulting from deletion of yheL were consistent with a modest role for YheL in Na+-dependent pH homeostasis in the wild type. No major role for YheL was indicated in the mutants in spite of the overexpression. The studies underscore the multiple physiological functions of TetA(L), including tetracycline, Na+, and alkali resistance and K+ acquisition. The studies also reveal and begin to detail the complexity of the response to mutational loss of these functions.
The chromosomal tetA(L) locus of Bacillus subtilis encodes a protein that confers resistance to low concentrations of tetracycline (Tc) by catalyzing efflux of a Tc-divalent metal ion complex in exchange for protons (Tc-Me+2/H+ antiport) (2, 8). Tc efflux does not occur in the absence of a divalent cation such as Co2+, Mg2+, or Mn2+, with Co2+ being the best. Conversely, the divalent cation does not efflux via TetA(L) without Tc (9, 12, 32). Modest amplification of the gene or changes in the promoter region lead to increased expression and Tc resistance as does expression of the gene from a multicopy plasmid (4, 16). During the past few years, studies in this laboratory have established that TetA(L) is a multifunctional antiporter. In addition to electrogenic Tc-Me+2/H+ antiport, TetA(L) catalyzes the exchange of cytoplasmic Na+ or K+ for a greater number of external H+ or K+ ions (7–9, 12, 13). The monovalent cation/H+ antiporter mode has roles in Na+ resistance and pH homeostasis (8). While not yet demonstrated, the net K+ uptake monovalent cation/K+ antiporter mode could also be of physiological importance.
The enumeration and evaluation of the roles of TetA(L) have been complicated by the variable phenotype of isolates carrying the same tetA(L) deletion. Initial observations on these tetA(L) deletion strains, which focused on the most severe phenotype (e.g., mutant JC112), led to the suggestion that tetA(L) cannot be deleted without compensatory changes that might involve second-site mutation(s) (8). These studies also indicated that the TetA(L) efflux protein had an additional function that accounts for a growth deficit observed in tetA(L) mutants at pH 7 even in the absence of Tc or elevated Na+ (8, 13). Initially, it was hypothesized that there might be an endogenous substrate for TetA(L) whose reduced efflux compromises growth (8). However, the more recent finding of the net K+ uptake mode of TetA(L) (13) raises the possibility that inadequate K+ acquisition is part or all of the basis for the growth defect of tetA(L) mutants at pH 7. The present study was directed toward categorization of the different phenotypes of tetA(L) deletion strains and clarification of the basis for the phenotype at pH 7. We further sought to begin the elucidation of the compensatory changes in tetA(L) deletion strains.
MATERIALS AND METHODS
Plasmids, bacterial strains, and growth conditions.
The plasmids and B. subtilis strains used in this study are described in Table 1. The tetA(L) deletion mutants from the earlier study, including JC112 and JC112C, carried a chloramphenicol resistance (Cmr) cassette which replaced the entire tetA(L) chromosomal coding sequence. The tetA(L) gene had been removed as a 1.8-kb ClaI-NdeI fragment in a previously described plasmid, which was then used to produce the mutants (8). One new tetA(L)-containing plasmid, pTL2, was used in this study. The coding sequence of tetA(L) was excised from pTL1 (8) by BamHI-HindIII digestion and was cloned into the BamHI and HindIII sites downstream of the ermC promoter of a pBK15 derivative named pVEB3 (Table 1). In this plasmid, an internal 750-bp MunI fragment of the Cmr gene of pBK15 was replaced with a 1.2-kb EcoRI fragment of pSP2 containing a spectinomycin resistance (Spr) gene. All new constructs were verified by sequence analysis. The sequencing was performed by the Utah State Biotechnology Center (Logan), using an ABI-100 model 377 sequencer. Construction of bacterial strains carrying deletions and/or gene fusions is described below.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| B. subtilis | ||
| BD99 | hisA1 the-5 trpC2 | Wild type, from A. Garro |
| JC112 | BD99 with tetA(L) deletion | 8 |
| JC112C | BD99 with tetA(L) deletion | 8 |
| BTK15 | BD99 amyE::yheL-lacZ | This study |
| BTK16 | JC112 amyE::yheL-lacZ | This study |
| BTK17 | JC112C amyE::yheL-lacZ | This study |
| BTK18 | BTK16 harboring tetA(L) gene containing plasmid pTL2 | This study |
| BTK19 | BTK17 harboring plasmid pTL2 | This study |
| BTK21 | BD99 yheL::Spr | This study |
| BTK22 | JC112 yheL::Spr | This study |
| BTK23 | JC112C yheL::Spr | This study |
| BTK30 | JC112 harboring plasmid pAG4 | D. Bechhofer |
| BTK32 | JC112C harboring plasmid pAG4 | This study |
| BTK33 | BD99 czcD::Spr | This study |
| BTK34 | JC112 czcD::Spr | This study |
| BTK35 | JC112C czcD::Spr | This study |
| BTK38 | JC112 amyE::tetA(L) | This study |
| BTK39 | JC112C amyE::tetA(L) | This study |
| BTK40 | BD99 containing plasmid pYheL-BK36 | This study |
| BTK24 | BD99 containing pBK36 | This study |
| Plasmids | ||
| pAC7 | Integration vector at amyE locus | 25 |
| pAG4 | tetA(L) gene cloned into pYH56 | 30 |
| pBK15 | pBD142-pBR322 joint replicon with multiple cloning sites downstream of ermC 5′ end | K. Zen |
| pBK36 | pUB110-pBR322 joint replicon with multiple cloning sites downstream of ermC 5′ end | K. Zen |
| pVEB3 | Replace a MunI fragment internal of Cmr in pBK15 with a Spr gene | This study |
| pGEM3zf+ | Cloning vector | Promega |
| pGEM7zf+ | Cloning vector | Promega |
| pAC7-K4L3 | yheL-lacZ fusion plasmid for integration in amyE locus | This study |
| pCZCD2 | 5′ end of czcD gene cloned into pGEM3zf+ | This study |
| pCZCD6 | czcD operon disrupted with Spr gene cloned into pGEM3zf+; for knocking out czcD | This study |
| pNH2 | yheL coding sequence disrupted with Spr gene cloned into pGEM7zf+; for knocking out yheL | This study |
| pYheL-BK36 | yheL coding sequence cloned into pBK36 | This study |
| pTL1 | pBK36 harboring tetA(L) gene | 8 |
| pTL2 | pVEB3 harboring tetA(L) gene from pTL1 | This study |
| pTL6 | tetA(L) cloned into pAC7 for integration in amyE locus | This study |
Except where indicated, growth was conducted at 30°C. Liquid cultures were incubated with shaking. Media TKM and TTM are, respectively, K+-replete and low-K+ Tris-buffered media (both of which have no added Na+ except where noted) (8). For experiments in which growth was measured in the presence of different concentrations of added K+, the inoculum was grown for 15 h in modified TTM supplemented with sodium phosphate instead of potassium phosphate. Two-milliliter cultures of TTM-sodium phosphate with various amounts of added KCl were inoculated with 20 μl of the overnight cultures, and the A600 was measured after 8 h of incubation. SpizKM was used for studying CoCl2 resistance because microprecipitation appeared to occur in the media buffered with Tris. SpizKM contains Spizizen salts (26), 50 mM potassium malate, 0.1% yeast extract, and 50 μg each of l-threonine, l-histidine, and l-tryptophan per ml. CoCl2 was added as indicated. RNA preparation medium (6) was used for growth of cells for some of the Northern analyses.
Determination of MICs of Tc, Co2+, and Na+.
To determine the MIC of Tc, cells were grown in TKM (pH 7.0) with various Tc concentrations. The A600 was measured after 17 h of growth. To avoid precipitation, the MIC for CoCl2 was determined from the A600 of wild-type, JC112, and JC112C cells after 8 h of growth in SpizKM (pH 7.0) containing various CoCl2 concentrations. For determination of the MIC for NaCl, TKM at pH 7.0 or 8.3 was supplemented with various concentrations of NaCl, and the A600 was measured after 15 h of growth. For each of these compounds or ions, the MIC was taken as the lowest concentration at which the A600 after the indicated period of growth was below 0.1.
Integration of a tetA(L) gene into the B. subtilis amyE locus.
The tetA(L)-containing plasmid pAG4 (30) was digested with HindIII and then treated with mung bean nuclease. Subsequent digestion with ClaI released a 2.5-kb fragment containing tetA(L) and its promoter. This fragment was cloned into the SmaI and ClaI sites of pAC7 (25). The resulting plasmid, pTL6, was digested with NruI and used to integrate the tetA(L) gene into the amyE loci of mutant strains JC112 and JC112C. The strains were identified by initial screening for kanamycin resistance (Kmr) followed by identification of starch-negative strains (25). Strains BTK38 and BTK39 were confirmed to be strains of JC112 and JC112C, respectively, which had incorporated the tetA(L) gene and promoter into the amyE locus.
Northern analyses.
Total RNA isolation and Northern analyses were conducted as described previously (6) to assess whether mRNA levels for a variety of genes were elevated. Primers used in PCR to amplify fragments for the probes employed in the analyses are listed in Table 2. A 400-bp PCR fragment (primers yqkI1 and yqkI2) was used as a probe for yqkI; a 400-bp PCR fragment (primers yusP1 and yusP2) was used as a probe for yusP; an internal 270-bp HindIII fragment of the PCR product (primers ycnB1 and ycnB2) was used as a probe for ycnB; a 310-bp NruI fragment of the PCR product (primers yhcA1 and yhcA2) was used as a probe for yhcA; a 700-bp SphI fragment from the mrpA locus was used as a probe for mrpA (14); a 180-bp fragment of pTCC1-25 (5) was used as a probe for yybF. A 780-bp HincII fragment from the yheL coding sequence (Fig. 1A) was used to probe the expression of yheL, an nhaC homologue. A 680-bp PCR fragment (primers Czcd3 and Czcd7) was cloned into pGEM3Zf(+) (Promega) via the HindIII and BamHI sites. The resulting plasmid, pCZCD2, was linearized by HindIII digestion and used as the DNA template for preparation of a riboprobe transcribed in vitro by T7 RNA polymerase. A 410-bp PCR fragment (primers yhaU1 and uhaU2) was used as a probe for yhaU; a 670-bp PCR fragment (primers ykqB1 and ykqB2) was used as a probe for yqkB; an internal 510-kb BclI-ClaI fragment of the PCR product (primers ykrM1 and ykrM2) was used as a probe for ykrM; a ClaI fragment (495 bp) from the 5′ end of a PCR product (primers yuaA2 and yubG2) was used as a probe for yuaA and yubG.
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequencea | Corresponding sequence (bp) | Gene and/or GenBank accession no. |
|---|---|---|---|
| yhaU1 | 5′-GGAGAGGGCGTGTGAGCCCGATGG | 60419–60396 | Z99109 BSUB0006 |
| yhaU2 | 5′-CGAGATTGTGATGACGCCTGCG | 60007–60028 | |
| yqkI1 | 5′-GGACTTCTTTTGAAGGATGTAAG | 58183–58161 | yqkI, Z99116 BSUB0013 |
| yqkI2 | 5′-CGACTGTTCCCCATGACGTTC | 57781–57801 | |
| yusP1 | 5′-CCCTTGACGGTACCATTGTCG | 177094–177114 | yusP, Z99120 BSUB0017 |
| yusP2 | 5′-CCAGCCGCCGATTTGCGG | 177491–177474 | |
| ycnB1 | 5′-CCGGATCCTTGAACACAAGTATTGAACAA | 34284–34264 | ycnB, Z99106 BSUB0003 |
| ycnB2 | 5′-TTGGATCCGCTAAATAGCGGGGCTC | 32834–32853 | |
| yhcA1 | 5′-AAGGATCCATGCATCTGTTAAAATTTCTG | 174398–174418 | yhcA, Z99108 BSUB0005 |
| yhcA2 | 5′-AAGGATCCCTGTACAGATTAAGATTCC | 176071–176053 | |
| ykqB1 | 5′-CCGGATCCATGAAAAAAGAATTTGCGG | 125146–125164 | Z99111 BSUB0008 |
| ykqB2 | 5′-CCCCAAGCTTATTTGGCTGCTATTTTG | 125820–125804 | |
| ykrM1 | 5′-CCGGATCCATGAGATTAAAGTTTGG | 20742–20759 | Z99111 BSUB0008 |
| ykrM4 | 5′-CGCCGATGCTTTGAAAACAGG | 21419–21399 | |
| yuaA2 | 5′-GAAGATCTATGGGAAGAATTAAAAATAAG | 189715–189734 | Z99119 BSUB0016 |
| yubG2 | 5′-CTAGATCTCATATCGAATCATAAAAAATCC | 191776–191755 | |
| K1 | 5′-GCGGGTCTGGGCGGGTCGATG | 44687–44707 | yheK, Z99109 BSUB0006 |
| K2 | 5′-CCCATCTCCTCATCAGCCG | (minus strand) 45274–45293 | |
| K4 | 5′-GGGGAATTCCGACCTAAAAAACCGTTC | (minus strand) 45071–45090 | |
| L1 | 5′-GGATATGAGGTTCTACATG | 44118–44146 | yheL, Z99109 BSUB0006 |
| L2 | 5′-GGGGATCCGCTTTTTACAGCGGGG | 42841–42860 | |
| YheL/BF1 | 5′-ATGCGGATCCATGGATTCTCAAAAAAAGCTGACG | 44225–44203 | |
| YheL/HR1 | 5′-AGCAAGCTTATTTTTTAACAAATCCAATC | 42865–42886 | |
| L3 | 5′-GGGGATCCTCATTGTCAGTTTCGAAAG | 43329–43349 | |
| L7 | 5′-TCCATTCAAGCGGAACGCC | 43773–43791 | |
| CzcD1 | 5′-CCGGATCCATGGGTCAACAATCATAATGAAG (containing an extra A) | 124646–124625 | Z99117 BSUB0014 |
| CzcD3 | 5′-CCCAAGCTTGATTGAAGAAAAGG | 124913–124896 | |
| CzcD6 | 5′-CCCCAAGCTTATAGAAGCTATAGGTGG | 124570–124552 | |
| CzcD7 | 5′-CCCGGATCCGCCACTCATCATAATCC | 124230–124249 | |
| TrkA6 | 5′-CCGGTACCTATAAAGTACAATCCTTCTAC | 122676–122699 | |
| TrkA7 | 5′-GCCCAAGCTTATTCACCGTTCATTTGT | 122586–122604 |
Extra nucleotides (underlined) were added for inducing restriction sites.
FIG. 1.
(A) Schematic diagram of the B. subtilis yheKL region. This 2.5-kb fragment represents a reverse strand of nt 42801 to 45300 as reported in the B. subtilis genome project (GenBank accession no. Z99109 BSUB0006). The locations and relative sizes of the yheK and yheL coding sequences, and the oligonucleotides used in this study, are as indicated. The transcription start point mapped in this study is represented by an arrow at nt 44931. (B) Northern analysis of yheL in B. subtilis wild type (Wt) and JC112. A 780-bp HincII fragment from the yheL coding sequence (Fig. 1A) was 32P labeled and used as the probe. The positions of 23S and 16S rRNAs are indicated on the right. (C) Reverse transcription mapping of the yheL transcription start site. The RNA used in the primer extension reactions was isolated from wild type or JC112. Control lanes (labeled A, C, G, and T) are a DNA sequence ladder using the same primer K1 as used in primer extension. The complement of the sequence that contains the mapped transcription start site (+1) and −10 are presented on the right.
Deletion of czcD-trkA from the wild type, JC112 and JC112C.
A fragment containing the putative czcD-trkA operon was amplified by PCR with primers Czd6 and Trka6 (Table 2). Primer Czcd6 contained additional nucleotides creating a HindIII site, and primer Trka6 contained additional nucleotides creating a KpnI site. The PCR product was digested with HindIII and KpnI and ligated into the HindIII and KpnI sites of pGEM3Zf(+) (Promega), resulting in plasmid pCZCD5. A 0.3-kb MunI fragment of pCZCD5, containing the 3′ end of czcD and the ribosome binding site and 5′ end of yheL, was replaced by a PCR-amplified Spr gene, producing plasmid pCZCD6. This plasmid was linearized by HindIII digestion and used to transform the wild type, JC112, and JC112C into Spr strains BTK33, BTK34, and BTK35, respectively. The disruption of the czcD and trkA genes was confirmed by PCR, restriction analyses, and sequencing.
Analysis of the transcriptional start and promoter region of B. subtilis yheL.
The SUPERSCRIPT preamplification system (GIBCO BRL, Life Technologies) and its published standard procedure were applied for mapping the transcription start point. For each reverse transcription reaction, 5 μg of total RNA isolated from the wild type or JC112 and 50 pmol of 5′-end labeled primer K1 or L1 (Fig. 1A and Table 2) were used. Half of each primer extension reaction was resolved on a 5% polyacrylamide-urea denaturing gel, along with a 32P-labeled size marker. A band of about 200 nucleotides (nt) resulted from extension using primer K1, and a band of about 800 nt resulted from primer L1 (data not shown). The other half of the primer extension reaction from primer K1 was resolved on an 8 M urea–6% polyacrylamide gel along side a sequence standard prepared using the same primer, K1, and a template (fragment K2L7) prepared by PCR amplification using primers K2 and L7 (Table 2). Bands of the same size were detected in both the wild type and JC112 (Fig. 1C). Sequencing of promoter region of yheL was conducted in the wild type, JC112, and JC112C. Chromosomal DNA from each strain was used as a template for PCR amplification (primers K2 and L1 [Table 2]) of a segment containing the putative promoter region (Fig. 1A). Each PCR product was gel purified and then sequenced using primers L1 and K1 (Table 2). The sequence of the PCR products was identical in the three strains and corresponded to that in the B. subtilis genome database (GenBank accession no. Z99109 BSUB0006).
Construction of yheL-lacZ fusions in the wild type, JC112, and JC112C.
For production of strains of the wild type, JC112, and JC112C with a yheL-lacZ fusion integrated into the amyE locus, a fragment containing the putative promoter region of the yheL operon, from 160 bp upstream of the mapped transcription start site to the codon for amino acid 299 of the yheL, was amplified by PCR with primers K4 and L3 (Fig. 1A; Table 2). Since there were no sequence differences in the yheL promoter regions among the three strains, only the chromosomal DNA isolated from the wild type was used as a template for the amplification. Primer K4 contains additional nucleotides for an EcoRI site, and primer L3 contains additional nucleotides for a BamHI site. It was thus designed for insertion into plasmid pAC7 (25), which was used to integrate the fusion gene into the amyE locus. The PCR product, K4L3, was digested with EcoRI and BamHI and then ligated into pAC7 at EcoRI and BamHI sites, resulting in an in-frame fusion of K4L3 and the lacZ gene (starting with the first codon of the lacZ coding sequence) in pAC7. Escherichia coli DH5α containing this yheL-lacZ fusion plasmid, pAC7-K4L3, formed blue colonies on Luria-Bertani plates with additions of 0.2 mM isopropylthio-β-d-galactoside (IPTG) and 0.04% (wt/vol) 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal). Plasmid pAC7-K4L3 was digested with restriction enzyme NruI. The linearized pAC7-K4L3 was transformed into the wild type, JC112, and JC112C, and Kmr, starch-negative strains were isolated and verified from each starting strain; BTK15, BTK16, and BTK17 were, respectively, derivatives of the wild type, JC112, and JC112C.
Assays of β-galactosidase activities of strains expressing yheL-lacZ fusions.
Expression of yheL-lacZ fusion protein in strains BTK15, BTK16, and BTK17 and derivatives thereof was studied by analyzing β-galactosidase activity, as described previously (8). β-Galactosidase activity was expressed as nanomoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per A600 (Table 4). Overnight cultures grown in the media specified in Table 4 were used in the β-galactosidase activity assay.
TABLE 4.
β-Galactosidase levels in B. subtilis BTK15, BTK16, and BTK17
| Growth pH | Medium | 0.1 M NaCl | β-Galactosidase activity (nmol/min/A600; mean ± SD)
|
||
|---|---|---|---|---|---|
| BTK15 | BTK16 | BTK17 | |||
| 7.0 | TTM | − | 17.0 ± 1.6 | 125 ± 12.8 | 96.5 ± 8.2 |
| + | 23.7 ± 3.5 | 130 ± 14.8 | 111 ± 4.2 | ||
| TKM | − | 21.9 ± 1.0 | 186 ± 18.3 | 111 ± 9.1 | |
| + | 28.2 ± 3.4 | 145 ± 7.4 | 94.7 ± 12.5 | ||
| 8.0 | TTM | − | 18.6 ± 3.6 | 135 ± 9.5 | 75.3 ± 6.5 |
| + | 22.7 ± 3.5 | 138 ± 12.5 | 91.3 ± 6.9 | ||
| TKM | − | 23.2 ± 2.1 | 213 ± 24.0 | 94.7 ± 4.5 | |
| + | 49.6 ± 11.4 | 307 ± 22.0 | 119 ± 15.2 | ||
| 7.0 | SpizKM | − | 70.6 ± 7.5 | 228 ± 30.9 | 148 ± 11.3 |
| SpizKM + 50 μM CoCl2 | − | 168 ± 20.5 | 224 ± 42.6 | 177 ± 6.6 | |
Deletion of the yheL gene.
First a fragment containing the yheL operon was amplified by PCR with primers K4 and L2 (Table 2; Fig. 1A). Primer K4 contains additional nucleotides for an EcoRI site, and primer L2 contains additional nucleotides for a BamHI site. The PCR product K4L2 was digested with EcoRI and BamHI and ligated into pGEM7Zf(+) (Promega) in the EcoRI and BamHI sites, resulting in plasmid pNH1. The 1-kb HindIII-PstI fragment of pNH1 containing the ribosome binding site and 5′ end of yheL was replaced by a PCR-amplified Spr gene, producing plasmid pNH2. Plasmid pNH2 was digested with XhoI, and the linearized plasmid was then used to transform the wild type, JC112, and JC112C into Spr strains BTK21, BTK22, and BTK23, respectively. The disruption of yheL in the these strains was confirmed by PCR and restriction digestion analyses.
RESULTS
Phenotypes of the tetA(L) deletion strains.
At the start of the study, six tetA(L) deletion strains that had been isolated earlier (8) were compared to the wild-type strain with respect to both MICs for Na+ and Tc and pH profiles for growth. All of the strains are identical in phenotype either to JC112 or to JC112C, which are distinct, stable phenotypic types; both types are often represented in a particular deletion experiment, but the JC112C type predominates and is sometimes the only phenotype formed. JC112 and JC112C were chosen for the studies reported here. First, the MICs for Tc, Na+, and Co2+ were determined. A Tc- and Na+-sensitive phenotype was expected, given the known functions of TetA(L). Both JC112 and JC112C were more sensitive than the wild type to low concentrations of Tc, exhibiting MICs of 0.09 and 0.12 μg/ml, respectively, versus 1.4 μg/ml for the wild type (Table 3). The expected Na+ sensitivity, by contrast, was exhibited by only one of the tetA(L) mutants, JC112, which showed elevated relative sensitivity even at neutral pH, where Na+ is less cytotoxic than at pH 8.3 (Table 3). JC112C did exhibit a modestly compromised ability to use either Na+ or K+ to support growth at pH 8.3, relative to the wild type, but JC112 exhibited a much more pronounced phenotype under such conditions (data not shown). Earlier work showed that this characteristic correlates with Na+(K+)/H+ antiport status in support of pH homeostasis at elevated pH (8).
TABLE 3.
MICs of Tc and CoCl2 for wild-type B. subtilis, JC112, and JC112C and derivatives thereof
| Strain | MIC (mean ± SD)
|
|||
|---|---|---|---|---|
| CoCl2 (μM)a | Tc (μg/ml)b | Na+ (M)c
|
||
| pH 7.0 | pH 8.3 | |||
| BD99 | 425 ± 15 | 1.4 ± 0.13 | 1.5 ± 0.12 | 0.7 ± 0.05 |
| JC112 | 150 ± 10 | 0.09 ± 0.01 | 0.9 ± 0.10 | 0.4 ± 0.06 |
| JC112C | 310 ± 25 | 0.115 ± 0.01 | 1.4 ± 0.09 | 0.7 ± 0.06 |
| BTK38 | 125 ± 10 | 1.4 ± 0.1 | 1.3 ± 0.11 | 0.7 ± 0.04 |
| BTK39 | 350 ± 20 | 1.4 ± 0.1 | NDd | ND |
| BTK33 | 230 ± 15 | 1.8 ± 0.2 | 1.4 ± 0.10 | 0.8 ± 0.06 |
| BTK34 | 140 ± 15 | 0.09 ± 0.01 | 0.9 ± 0.07 | 0.4 ± 0.08 |
| BTK35 | 225 ± 20 | 0.105 ± 0.05 | 1.4 ± 0.08 | 0.8 ± 0.05 |
Minimal concentration at which growth in SpizKM (A600) was below 0.1 after 8 h at 30°C.
Minimal concentration at which growth in TKM (A600) was below 0.1 after 17 h at 30°C.
Minimal concentration at which growth in TKM (A600) was below 0.1 after 17 h at 30°C.
ND, not determined.
Co2+ toxicity was of interest because this is the optimal cation for the Tc-divalent cation complex that effluxes via TetA(L) (12, 31). Thus, if an endogenous substrate, e.g., an antibiotic produced by B. subtilis, normally exits by TetA(L)-mediated efflux in complex with Co2+ just as Tc does, then a tetA(L) deletion strain would be more sensitive than the wild type to growth inhibition by Co+ even when Tc is absent. As shown in Table 3, both JC112 and JC112C were more sensitive than the wild type to inhibition by Co2+. JC112 was significantly more sensitive to Co2+ than JC112C, even though their Tc sensitivities were comparable. While the Co2+ data shown were from experiments conducted in SpizKM, the same relative pattern among the strains was observed in TKM (data not shown). Also, although not shown, when the MICs were determined at 37°C rather than 30°C, all were unchanged except in the case of JC112C, which was consistently found to have an MIC for Co2+ that was higher, at 600 μM, than that of any of the other strains at either temperature. JC112C appears to have a mechanism that counteracts the increased Co2+ sensitivity that accompanies mutational loss of tetA(L). This mechanism appears to minimize the sensitivity relative to JC112 at 30°C and more than compensate at 37°C, e.g., by altered expression of a temperature-dependent regulator of a Co2+ efflux system.
It was of interest to explore whether the Co2+ sensitivity that is exhibited by both JC112 and JC112C at 30°C was a direct consequence of the loss of TetA(L) function. This would be consistent with an involvement of TetA(L) in efflux of a Co2+ endogenous substrate complex. Alternatively, Co2+ sensitivity could be a secondary consequence of the tetA(L) disruption. Reintroduction of an active tetA(L) gene should reverse the Co2+ sensitivity if it is a direct consequence of functional TetA(L) loss and might even reverse sensitivity that was secondary to TetA(L) loss. As anticipated, reintroduction of the tetA(L) gene in single copy, under its own promoters, in the amyE locus restored the Tc resistance of both JC112 (BTK38) and JC112C (BTK39) and the Na+ resistance of JC112 (BTK38) to wild-type levels (Table 3). However, the enhanced Co2+ sensitivity of both JC112 and JC112C remained upon reintroduction of the functional tetA(L) (Table 3) and was even retained when a multicopy plasmid bearing tetA(L) was expressed in JC112 (data not shown). Moreover, JC112 and to a lesser extent JC112C, into which an active tetA(L) was reintroduced, retained some of the characteristic growth deficit at pH 7.0 in the absence of added Na+ and Tc (Fig. 2; see below). Therefore, it is unlikely that the growth deficit at pH 7.0 is related to adverse accumulation of an endogenous substrate such as an antibiotic or that the increased Co2+ sensitivity of tetA(L) deletion mutants relates to a coupling of Co2+ efflux to such an endogenous substrate of TetA(L).
FIG. 2.
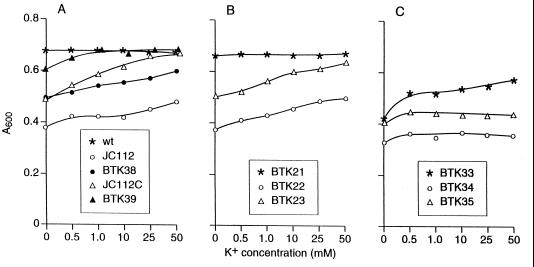
Growth of wild-type (wt) and mutant strains of B. subtilis at various concentrations of added K+. The cells were grown in modified TTM (pH 7.0) and adjusted to the indicated concentrations of K+ added to the medium (see Materials and Methods). The A600 was measured after 15 h of growth at 30°C.
Whatever the basis for the increased Co2+ sensitivity might be, it raises the possibility that the growth defect of tetA(L) deletion mutants at pH 7 in the absence of added Tc or Na+ is attributable to an inhibition of growth by accumulation of toxic divalent cations from normal growth media. Alternatively or in addition, the net K+ uptake mode of TetA(L) may have physiological significance, and the growth defect at pH 7 arises from a diminished capacity for K+ acquisition. As shown in Fig. 2A, both JC112 and JC112C exhibited less growth than the wild type in media containing only contaminating levels of K+. With the addition of increasing [K+] to the media, the growth of JC112C reached that of the wild type at 50 mM added K+. In strain JC112C, into which a single active tetA(L) had been reintroduced (BTK39), there was still a slight deficit in growth at no added K+, but wild-type growth levels were reached at 1 mM added K+. With the more severe phenotype of JC112, growth was enhanced only modestly as [K+] was increased, even to 50 mM. In addition, whereas reintroduction of an active tetA(L) (BTK38) markedly improved the growth profile at increasing [K+], the growth level never reached that of the wild type. Quite possibly, in the more Co2+-sensitive JC112, the growth deficit at pH 7 results from a combination of a major deficit in K+ acquisition, especially at low [K+], and a significant contribution of overaccumulation of toxic divalent cations from the medium. The small residual deficit in growth of JC112C with restored tetA(L) (BTK39), at no added K+, could similarly reflect a contribution, albeit smaller, of toxic divalent cation overaccumulation to the pH 7.0 phenotype.
Preliminary Northern analyses.
From BLAST analyses (1), selection was made of candidate genes that might compensate for different functions of TetA(L). These genes, for which preliminary Northern analyses were conducted, were chosen from three different groups: (i) ycnB, yhcA, yusP, and yybF, four genes whose predicted products have sequence similarity with tetA(L); (ii) mrp (14, 18), a gene locus with known Na+/H+ antiporter activity, and three genes that had sequence similarity to Na+/H+ antiporter-encoding genes from other bacteria, yheL and yqkI, which had similarity to the nhaC gene from alkaliphilic Bacillus firmus OF4 (14, 17), and yhaU, which had sequence similarity to the napA gene from Enterococcus hirae (31); and (iii) a gene locus for which there is a putative K+ uptake function, czcD-trkA (28), and three genes or gene loci that show homology to one or both of the genes of the K+ uptake-encoding ktrAB locus of Vibrio alginolyticus (20), ykqB, ykrM, and yuaA-yubG. Bands of the anticipated size were observed on Northern blots probed for each of these genes or loci (data not shown). The mRNA abundance was clearly elevated for two of them, and for those two loci the increase in mRNA was evident in both JC112 and JC112C. The two loci whose mRNA levels were elevated in JC112 and JC112C were the czcD-trkA locus that had been proposed to be involved in K+ uptake (28) and the nhaC homologue yheL.
Role of czcD-trkA in the wild-type and tetA(L) deletion strains.
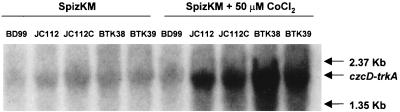
The B. subtilis czcD gene, whose product is 42% identical to that of Ralstonia (previously Alcaligenes) eutrophus (21), is in an apparent operon with a gene that we designated as trkA (28). This locus was identified in our laboratory in an earlier screen of B. subtilis genes that increased K+ acquisition by a K+ uptake-deficient E. coli strain. Although the B. subtilis trkA gene product exhibits only partial sequence similarity to small regions of the TrkA component of an E. coli K+ uptake system, the B. subtilis trkA alone complemented an E. coli carrying a mutation in its own trkA (28). The elevation of czcD-trkA expression in the tetA(L) deletion mutants thus appeared consistent with a compensatory change for purposes of K+ acquisition. Northern analyses (Fig. 3) indicated that (i) the czcD-trkA mRNA abundance is low in the wild type grown on SpizKM at pH 7.0; (ii) this basal level of mRNA abundance is elevated in both JC112 and JC112C, and the increase is not abolished upon reintroduction of a functional tetA(L) gene into the two mutant strains (BTK38 and BTK39, respectively); (iii) all strains had a much higher level of czcD-trkA mRNA when grown in the presence of Co2+, in the rank order BTK38 [JC112 with tetA(L) restored] > BTK39 [JC112C with tetA(L) restored] > JC112 > JC112C ≫ wild type.
FIG. 3.
Northern analysis of czcD mRNA in the wild type, JC112, JC112C, and the two mutant strains upon restoration of an active tetA(L) gene, BTK38 and BTK39, respectively. The cells were grown in SpizKM with or without added CoCl2 as shown.
To assess whether the czcD-trkA locus was related to K+ acquisition or Co2+ sensitivity in vivo, mutants of the wild type, JC112, and JC112C (BTK33, BTK34, and BTK35, respectively) were studied. As shown in Fig. 2C, in comparison with Fig. 2A, deletion of both the czcD and trkA genes from the wild type resulted in a growth deficit at no added K+. Growth of BTK33 at no added K+ was comparable to that exhibited by JC112 carrying no additional mutations. At higher K+ concentrations, BTK33 showed growth somewhat better than that of JC112 but still much less than the wild-type level. The two mutants also showed a more severe phenotype after czcD-trkA disruption. They exhibited reduced growth in medium with no added K+ and responded only slightly to addition of K+. These data support a role for this locus in K+ acquisition and for the hypothesis that czcD-trkA elevation upon tetA(L) deletion provides partial compensation for a reduction in this capacity.
As shown in Table 3, deletion of the czcD and trkA genes increased the Co2+ sensitivity of the wild type and JC112C. This is consistent with the finding that the Ralstonia CzcD mediates exclusion of Co2+ (3). JC112, whose Co2+ sensitivity was very high, did not exhibit a statistically significant increase in sensitivity. These findings are not consistent with overexpressed CzcD-TrkA causing increases in both K+ and Co2+ uptake that underly the increased sensitivity of JC112 and JC112C to Co2+. Some other basis for the enhanced Co2+ sensitivity must exist in the tetA(L) mutants. Neither the Tc nor Na+ sensitivities of the three strains was altered by introduction of the czcD-trkA mutation (Table 3).
Expression and role of yheL in the wild-type and tetA(L) deletion strains.
The putative antiporter-encoding yheL gene is downstream of, and would appear to form a likely operon with, yheK, a gene that exhibits sequence similarity to diverse regulatory genes (Fig. 1A). The size of the RNA detected with a yheL probe was consistent with this expectation, as shown in Fig. 1B, and a transcriptional start was mapped upstream of yheK (Fig. 1A and C). The elevated level of yheL RNA in JC112 relative to the wild type is evident in Fig. 1B. JC112C showed a more variable elevation of yheL mRNA in Northern experiments but had consistently more yheL RNA than the wild type; moreover, the elevated levels of yheL RNA of both mutant strains remained higher than in the wild type upon restoration of a functional tetA(L) either in single copy or on a multicopy plasmid (data not shown).
To better assess possible differences in yheL expression between JC112 and JC112C in the face of variable results of Northern analyses, we undertook experiments using yheL-lacZ fusions that monitored expression from the yheL promoter. The wild-type and mutant promoter regions were first shown, by sequencing, to be identical (see Materials and Methods). The expression of yheL was examined via β-galactosidase activity assay under various conditions. As shown in Table 4, (i) there was a striking increase in β-galactosidase activity in BTK16 (JC112) and BTK17 (JC112C) over BTK15 (wild type) under all conditions, and the activity in BTK16 was consistently and significantly greater than that in BTK17; (ii) in BTK16 (JC112) in particular, K+-replete conditions (TKM or SpizKM versus TTM) favored higher yheL expression; (iii) small effects of added NaCl on yheL expression were observed only in the wild type at elevated pH and on TKM; and (iv) in K+-replete SpizKM, in which Co2+ effects are best studied, yheL expression by BTK15 (wild type) was enhanced in the presence of CoCl2. The major findings confirmed the indications from Northern analyses of greatly elevated yheL expression in JC112C and, even more, in JC112.
To confirm that yheL product functions as a Na+/H+ antiporter of the nhaC type, and to assess whether its elevated expression in JC112 and JC112C was essential for viability of these tetA(L) deletion strains, yheL was deleted from the wild type, JC112, and JC112C as described in Materials and Methods. The successful construction of these strains, BTK21, BTK22, and BTK23, respectively, showed that the two tetA(L) deletion strains were not dependent on yheL. The growth response of these double-deletion strains to various concentrations of K+ was not different from the response of strains prior to yheL deletion (Fig. 2B), and the MICs for Tc, Na+, and Co2+ were also indistinguishable from those of the initial strains (data not shown). The NhaC of B. firmus OF4 is an antiporter with a high Na+ affinity that has little impact on Na+ resistance per se. It does have a modest role in Na+-dependent pH homeostasis (15). Prior studies have indicated that Na+-dependent growth stimulation in low-K+ medium (TTM) at pH 8.3 reflects this function (8, 19). Consistently, the Na+-dependent stimulation of growth yield of the wild type, when challenged by elevated pH, was reproducibly diminished in its yheL derivative (BTK21) (data not shown).
The complex response of B. subtilis to tetA(L) deletion apparently involves increased expression of multiple membrane-associated proteins that is not reversed upon reintroduction of tetA(L). This suggested the possibility that the increased Co2+ sensitivity of such mutants might be a secondary by-product of this complex response in which the large increase in aggregate or some specific membrane proteins leads to a compromise in the membrane barrier to Co2+. As an initial assessment of such a hypothesis, wild-type cells were transformed with the vector pBK36 (BTK24) or with pBK36 expressing yheL (BTK40). Whereas the control vector did not change the MIC for Co2+ of the wild-type strain, the expression of yheL resulted in a markedly increased sensitivity such that BTK40 was sensitive to as little as 100 μM CoCl2 (data not shown).
DISCUSSION
A major finding of this study is that the net K+ uptake mode (13) of the chromosomally encoded TetA(L) protein plays a significant physiological role in K+ acquisition by B. subtilis. The growth deficit of tetA(L) deletion strains, in the absence of a challenge by alkali, Na+ and Tc, correlates qualitatively with their [K+]-related growth profile. A small growth deficit in JC112C, and larger component in JC112, is not abolished when a functional tetA(L) is restored to these mutants. This irreversible component of the mutants' phenotype is probably an indirect result of a pattern of changes in the expression of other genes. Complex patterns of response to tetA(L) deletion are the other major finding of these studies. The complexity may relate to the diverse functions of TetA(L).
This study showed that tetA(L) deletion strains exhibit two, partially distinct patterns of change in the expression of other genes. Two genes whose expression is significantly elevated have been identified in both phenotypic types of tetA(L) deletion mutants, but the catalogue is almost certainly incomplete. We hypothesize that there may be a regulon whose member genes encode ion-translocating transporters with roles in monovalent cation, pH, and perhaps divalent cation homeostasis. The two gene loci found here to be up-regulated in JC112 and JC112C, i.e., czcD-trkA and yheL, would be members of this putative regulon. Another member is probably tetA(L) itself. It was earlier noted that the basal level of expression of a tetA(L)-lacZ fusion was markedly increased in JC112 (8). A unifying hypothesis would be that the putative regulon has one or more master control genes and it is the function of such a gene that is altered upon tetA(L) deletion. The experiments here show that the increased expression of czcD-trkA and yheL is not abolished by restoration of active tetA(L). This suggests that there has been a mutation or some kind of irreversible change. If one or more second-site mutations are in fact involved, the mutation(s) may not obligatorily or immediately accompany tetA(L) deletion. Preliminary data (J. Jin and D. H. Bechhofer, unpublished data) indicate that the frequency of tetA(L) deletion is just as high as that of a clearly nonessential gene in the same protocol. Thus, TetA(L) functions may not be essential for viability. However, their loss may create selective pressure that leads to the patterns of change that we have begun to characterize here. Further investigation will be required to clarify the timing and nature of the emergence of the irreversible adaptations to tetA(L) deletion. It will also be of interest to determine the full panoply of genes that are up-regulated when tetA(L) is deleted, e.g., by a DNA array technology. It should be noted that some compensatory adaptations to tetA(L) deletion might involve genes that show little or no transcriptional regulation. Even tetA(L) itself is predominantly regulated by posttranscriptional mechanisms (27).
The phenotypic type of mutant represented by JC112C presumably exhibits up-regulation of one or more additional genes beyond those genes that are up-regulated in both JC112 and JC112C. In addition to the probable temperature-sensitive Co2+ efflux system, JC112C must have an elevated compensatory transport activity(ies) that accounts for its lack of Na+ sensitivity and only modest alkali sensitivity relative to JC112.
One of the two loci whose expression is markedly elevated in both JC112 and JC112C is the czcD-trkA locus. The best-studied homologue, the first czcD gene reported, was initially described as having roles in sensing the substrates of the czcCBA locus of R. eutrophus and in regulating expression of the locus (21, 29). CzcCBA is a toxic divalent cation efflux antiporter whose substrates are cadmium, zinc, and cobalt; the divalent cation is exchanged for external H+ (22). CzcD is a member of a family of membrane proteins called cation diffusion facilitators (22, 24). Some of these proteins, including CzcD, have most recently been shown to exclude their substrates when expressed in Ralstonia (3). The B. subtilis CzcD is not as closely related to the Ralstonia homologue as many other homologues, including some B. subtilis gene products. Paulsen and Saier (24) noted that functions of such less related proteins may not involve or may not solely involve transport of toxic divalent cations. In view of the findings here, it will be of interest to examine the actual transport properties of CzcD and CzcD-TrkA in vitro and, in particular, to determine whether the complex catalyzes divalent cation/K+ antiport. Such an activity would account for its apparent in vivo contributions to K+ acquisition and Co2+ resistance.
The YheL protein, like its homologue in alkaliphilic B. firmus OF4 (15), appears to have a modest role in Na+-dependent pH homeostasis but makes no detectable contribution to Na+ resistance. We propose that yheL be designated nhaC. It is striking that despite the significant up-regulation of yheL in both tetA(L) deletion mutants, deletion of yheL has little effect on these mutants. This is consistent with yheL being part of a regulon containing other genes that are more important compensatory genes for TetA(L) functions, e.g., czcD and trkA. Thus, the strong up-regulation of yheL would be a by-product of an effect on the regulon as a whole. In fact, a plausible but tentative hypothesis for the basis of the Co2+ sensitivity of JC112 and JC112C (albeit more modest) is an adverse by-product of the elevation of YheL and other proteins. The barrier function of the membrane may be compromised with respect to divalent cation exclusion when one or more of the membrane proteins is elevated as much as YheL is in JC112. The plausibility of this possibility is supported by the marked decrease in the MIC for Co2+ in wild-type B. subtilis cells transformed with a multicopy plasmid expressing yheL. Expression of the gram-negative TetA(C) gene has been correlated with an increase in cadmium sensitivity, and the mechanism is not yet understood (10, 11).
Finally, apart from the enhanced Co2+ sensitivity of tetA(L) mutants, the demonstrated effect of Co2+ on expression of both czcD-trkA and yheL is notable. It is reasonable to hypothesize that just as Na+ stress is exacerbated at elevated pH and K+ insufficiency (23), there may be an intersection among stresses related to TetA(L) function and the stress caused by inhibitory concentrations of toxic divalent cations. For example, Co2+ toxicity may be strongly dependent on pH, K+ status, or Na+ levels. Any such intersection may be clarified when all genes whose expression is significantly altered in tetA(L) deletion mutants have been catalogued.
ACKNOWLEDGMENTS
This work was supported by research grants GM52837 from the National Institute of General Medical Sciences to T.A.K. and from the Inoue Enryo Memorial Foundation for Promoting Science to M.I.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amano H, Ives C L, Bott K F, Shishido K. A limited number of Bacillus subtilis strains carry a tetracycline-resistance determinant at a site close to the origin of replication. Biochim Biophys Acta. 1991;1088:251–258. doi: 10.1016/0167-4781(91)90061-p. [DOI] [PubMed] [Google Scholar]
- 3.Anton A, Grosse C, Reissmann J, Pribyl T, Nies D H. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J Bacteriol. 1999;181:6876–6881. doi: 10.1128/jb.181.22.6876-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechhofer D H, Stasinopoulos S J. tetA(L) mutants of a tetracycline-sensitive strain of Bacillus subtilis with the polynucleotide phosphorylase gene deleted. J Bacteriol. 1998;180:3470–3473. doi: 10.1128/jb.180.13.3470-3473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechhofer D H, Wang W. Decay of ermC mRNA in a polynucleotide phosphorylase mutant of Bacillus subtilis. J Bacteriol. 1998;180:5968–5977. doi: 10.1128/jb.180.22.5968-5977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMari J F, Bechhofer D H. Initiation of mRNA decay in Bacillus subtilis. Mol Microbiol. 1993;7:705–717. doi: 10.1111/j.1365-2958.1993.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Guffanti A A, Krulwich T A. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J Biol Chem. 1994;269:27365–27371. [PubMed] [Google Scholar]
- 8.Cheng J, Guffanti A A, Wang W, Krulwich T A, Bechhofer D H. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J Bacteriol. 1996;178:2853–2860. doi: 10.1128/jb.178.10.2853-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Hicks D B, Krulwich T A. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+/H+ exchange. Proc Natl Acad Sci USA. 1996;93:14446–14451. doi: 10.1073/pnas.93.25.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith J K, Buckingham J M, Hanners J L, Hildebrand C E, Walters R A. Plasmid-conferred tetracycline resistance confers collateral cadmium sensitivity to E. coli cells. Plasmid. 1982;8:86–88. doi: 10.1016/0147-619x(82)90044-0. [DOI] [PubMed] [Google Scholar]
- 11.Griffith J K, Cuellar D H, Fordyce C A, Hutchings K C, Mondragon A A. Structure and function of the class C tetracycline/H+ antiporter: three independent groups of phenotypes are conferred by TetA(C) Mol Membr Biol. 1994;11:271–277. doi: 10.3109/09687689409160437. [DOI] [PubMed] [Google Scholar]
- 12.Guffanti A A, Krulwich T A. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by the Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J Bacteriol. 1995;177:4557–4561. doi: 10.1128/jb.177.15.4557-4561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guffanti A A, Cheng J, Krulwich T A. Electrogenic antiport activities of the gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J Biol Chem. 1998;273:26447–26454. doi: 10.1074/jbc.273.41.26447. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Guffanti A A, Oudega B, Krulwich T A. mrpA: a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+, and in pH homeostasis. J Bacteriol. 1999;181:2394–2402. doi: 10.1128/jb.181.8.2394-2402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Guffanti A A, Zemsky J, Ivey D M, Krulwich T A. Role of the nhaC-encoded Na+/H+ antiporter of alkaliphilic Bacillus firmus OF4. J Bacteriol. 1997;179:3851–3857. doi: 10.1128/jb.179.12.3851-3857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ives C L, Bott K F. Cloned Bacillus subtilis chromosomal DNA mediates tetracycline resistance when present in multiple copies. J Bacteriol. 1989;171:1801–1810. doi: 10.1128/jb.171.4.1801-1810.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivey D M, Guffanti A A, Bossewitch J S, Padan E, Krulwich T A. Molecular cloning and sequencing of a gene from alkaliphilic Bacillus firmus OF4 that functionally complements an Escherichia coli strain carrying a deletion in the nhaA Na+/H+ antiporter gene. J Biol Chem. 1991;266:23483–23489. [PubMed] [Google Scholar]
- 18.Kosono S, Morotomi S, Kitada M, Kudo T. Analyses of a Bacillus subtilis homologue of the Na+/H+ antiporter gene which is important for pH homeostasis of alkaliphilic Bacillus sp. C-125. Biochim Biophys Acta. 1999;1409:171–175. doi: 10.1016/s0005-2728(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 19.Krulwich T A, Cheng J, Guffanti A A. The role of monovalent cation/proton antiporters in Na+-resistance and pH homeostasis in Bacillus: an alkaliphile versus a neutralophile. J Exp Biol. 1994;196:457–470. doi: 10.1242/jeb.196.1.457. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Yuda R, Unemoto T, Bakker E. KtrAB, a new type of bacterial K+ uptake system from Vibrio alginolyticus. J Bacteriol. 1998;180:3491–3494. doi: 10.1128/jb.180.13.3491-3494.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nies D H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J Bacteriol. 1992;174:8102–8110. doi: 10.1128/jb.174.24.8102-8110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nies D H, Silver S. Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol. 1995;14:186–199. doi: 10.1007/BF01569902. [DOI] [PubMed] [Google Scholar]
- 23.Padan E, Krulwich T A. Sodium stress. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 117–130. [Google Scholar]
- 24.Paulsen I T, Saier M H., Jr A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol. 1997;156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- 25.Shimotsu H, Henner D J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43:85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 26.Spizizen J, Prestidge L. Conditions for competence in the Bacillus licheniformis transformation system. J Bacteriol. 1969;99:70–77. doi: 10.1128/jb.99.1.70-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stasinopoulos S J, Farr G A, Bechhofer D H. Bacillus subtilis tetA(L) gene expression: evidence for regulation by translational reinitiation. Mol Microbiol. 1998;30:923–932. doi: 10.1046/j.1365-2958.1998.01119.x. [DOI] [PubMed] [Google Scholar]
- 28.Sturr M G, Ablooglu A J, Krulwich T A. A Bacillus subtilis locus encoding several gene products affecting transport of cations. Gene. 1997;188:91–94. doi: 10.1016/s0378-1119(96)00784-6. [DOI] [PubMed] [Google Scholar]
- 29.Van der Leslie D, Scwuchhow T, Schwidestzky U, Wuertz S, Baeyens W, Mergeay M, Nies D H. Two-component regulatory system involved in transcriptional control of heavy-metal homeostasis in Alcaligenes eutrophus. Mol Microbiol. 1997;23:493–503. doi: 10.1046/j.1365-2958.1997.d01-1866.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Bechhofer D H. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J Bacteriol. 1996;178:2375–2382. doi: 10.1128/jb.178.8.2375-2382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waser M, Hess-Bienz D, Davies K, Solioz M. Cloning and disruption of a putative Na/H-antiporter gene of Enterococcus hirae. J Biol Chem. 1992;267:5396–5400. [PubMed] [Google Scholar]
- 32.Yamaguchi A, Udagawa T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]