Abstract
Background
To investigate the effect of perioperative intraocular pressure (IOP) lowering medications on controlling postoperative IOP following uncomplicated phacoemulsification.
Methods
Ovid MEDLINE, EMBASE, and Cochrane CENTRAL databases were searched up until November 2022. Randomised controlled trials (RCTs) that assessed IOP change via applanation tonometry in medicated and control arms following uncomplicated cataract surgery in healthy eyes were included. The primary outcome was the weighted mean difference (WMD) of IOP at 2–8 h, 12–24 h, and 1–7 days postoperatively within each medication class or common fixed-combination formulations. Risk of bias was assessed using the revised risk of bias in randomised trials (RoB-2). Level of evidence was rated using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE)
Results
From 702 screened articles, 30 RCTs involving 2986 eyes were included. There was a statistically significant reduction in IOP favouring treatment arms at 2–8 h (WMD = −3.87 mmHg; 95% CI [−4.75, −3.00]; p < 0.001) and 12–24 h (WMD = −2.69 mmHg; 95% CI [−3.36, −2.02]; p < 0.001), with the effect wearing off beyond 1 day (p = 0.18). Between medication classes, the largest effect at both 2–8 h and 12–24 h was observed with intracameral cholinergics or fixed-combination carbonic anhydrase inhibitor-beta-blocker (FCCB) formulations. Conversely, the smallest effect was observed with prostaglandin analogues, alpha-agonists, and topical carbonic anhydrase inhibitors (CAIs).
Conclusion
Prophylaxis against acute IOP elevations following uncomplicated cataract surgery is effective. FCCB and intracameral cholinergics are the most effective ocular antihypertensive agents, while alpha-agonists, prostaglandin analogues, and topical CAIs were found to be the least effective. These findings may inform future surgical guidelines.
Subject terms: Outcomes research, Glaucoma, Lens diseases
Introduction
Cataract extraction by phacoemulsification with intraocular lens implantation is a highly effective treatment, with more than 90% of patients achieving visual acuity outcomes of 20/40 or better [1–3] Nevertheless, patients may still experience a variety of adverse events. For example, in the early postoperative period, the most common treatable complication is an acute elevation in intraocular pressure (IOP) [4, 5] These IOP spikes can occur as a result of blockage of the trabecular meshwork by surgical or inflammatory debris, or by retained ophthalmic viscoelastic device (OVD) [6–9] Following uncomplicated surgery, IOP can rise between 5–13 mmHg in the early postoperative period, with spikes ≥30 mmHg in up to 70% of patients, depending on the type of OVD used [7, 10] Typically, this peak effect is observed at 1–6 h after surgery [7, 8, 10] A variety of treatment options aimed at preventing IOP spikes have been explored in the perioperative period. However, results have been conflicting, with some studies showing a beneficial effect of medication, and others showing no difference relative to placebo [11–13] The available evidence has been systematically reviewed [14]; however, a more comprehensive meta-analysis is yet to be performed in order to determine the effectiveness of prophylactic medications in this setting. In this study, we sought to address this question in patients undergoing routine uncomplicated cataract surgery.
Methods
Literature search strategy, eligibility criteria, and study selection
A systematic literature search was conducted on Ovid MEDLINE, Ovid EMBASE, and Cochrane CENTRAL from inception to November 2022 (Supplementary Table 1). A minimum of two authors (M.P., and R.K., or A.S.) independently selected studies for inclusion in a two-step screening method. Initially, titles and abstracts were screened, followed by full-text screening. Any uncertainties were resolved through consultation with the senior author (H.S.). Randomised controlled trials (RCT) that reported on efficacy and/or safety parameters for both medication and control arms following uncomplicated phacoemulsification in otherwise healthy eyes were included. Control eyes did not receive prophylactic IOP medications and served as a comparator for analysis. To be included, studies needed to assess IOP via applanation tonometry. Eyes with pre-existing glaucoma or ocular hypertension were excluded. Articles that were non-published, not in English, or that contained duplicate data were excluded. The study protocol was reviewed by the McGill University’s Faculty of Medicine Institutional Review Board and was exempt from ethics review based on article 2.2(b) of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans [15]. This study was conducted according to a pre-specified protocol registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (ID #145904), and the methods followed the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16].
Data collection and outcome measures
The following baseline data were independently extracted from included studies by two authors (M.P., and R.K., or A.S.): study year, number of eyes, gender distribution, age, study design, location, OVD type, dose and concentration of prophylactic antihypertensive medication(s), control intervention, study exclusions, as well as the definition and treatment of IOP spikes. The primary outcome was the weighted mean difference (WMD) of IOP following cataract surgery at postoperative time point ranges of 2–8 h, 12–24 h, and 1–7 days. If a study reported multiple measurements within a single time-point range, all values were pooled and included in the meta-analysis. The overall analysis considered all included prophylactic antihypertensive medications, while a subgroup analysis stratified the results based on medication class (and individual medications if possible) or common fixed-combination formulations. Continuous variables were reported as mean ± standard deviation, while categorical variables were reported as percentages. Data collection was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
Risk of bias assessment
At least two authors (M.P., and R.K., or A.S.) evaluated the risk of bias across included RCTs using the revised Cochrane tool for assessing the risk of bias in randomised trials (RoB 2). The five bias domains examined were randomisation processes, adherence to assigned interventions, missing outcome data, bias of measurement, and bias of reported results [17]. The maximum acceptable loss to follow-up was conservatively set at 10%. Each domain was categorized as low risk of bias, some concerns, or high risk of bias. Studies with high or unclear risk of bias in all categories were excluded. In addition to the formal risk of bias assessment, unique issues to this project were considered, namely, whether there were any protocol violations, post-randomisation exclusions, and whether the unit of analysis was per eye or per patient. The overall certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool [18]. Given that all included studies were RCTs, outcomes were graded as “high quality” evidence, and certainty was downgraded by one or two levels for serious concerns related to risk of bias, inconsistency, indirectness, imprecision, or publication bias.
Data synthesis and analysis
To account for possible heterogeneity between studies and avoid assumptions about studies estimating a common treatment effect, the meta-analysis was performed using a random effects model. The inverse variance method was employed for continuous data. Throughout, the meta-analysis was represented by WMDs with accompanying 95% confidence intervals (95% CI) for continuous variables. The weighted mean was defined by: while the weighted standard deviation was represented by: . The number of eyes was used throughout as a weighting variable, and a p-value of less than 0.05 was deemed statistically significant. Statistical heterogeneity was investigated in two ways: (1) a chi-square statistic was computed with significance set at p < 0.05, and (2) an I2 measure was computed to investigate the proportion of variance in the meta-analysis attributable to heterogeneity. Review Manager software (version 5.4; Cochrane Collaboration) was used for all statistical analyses, and no protocol amendments were made for this study.
Results
Study inclusions and baseline demographics
The literature search yielded 702 articles (Supplementary Fig. 1). Following title and abstract screening of all articles, 89 full texts were screened, and after exclusions, 30 RCTs reporting on 2986 eyes of 2870 patients were included in the analysis (Supplementary Table 2) [12, 13, 19–46]. Mean age ranged from 54.4 to 78.9 years. Among the studies reporting on gender, female patients comprised 53.6% of the treatment group and 57.2% of the control group. Most studies described a surgical technique wherein a single OVD was used for all steps of the procedure. Of the 30 studies, 11 used one OVD at the start of the case and a different OVD prior to intraocular lens insertion. The most commonly used OVD was sodium chondroitin sulfate 4%-sodium hyaluronate 3%, followed by sodium hyaluronate 1.0% (Supplementary Table 3).
Given the limited data availability, 27 studies were included for the 2–8-hour time point, all 30 studies were included for the 12–24-hour time point, and 10 studies were considered for 1–7 days postoperatively.
Quality assessment
Overall, three studies (10%) were classified as low risk, 20 studies (67%) had some concerns, and seven studies (23%) were classified as high risk for bias (Supplementary Fig. 2). Stratified by the bias domain, only one RCT was high risk for the randomisation process, five were high risk with respect to adherence to assigned interventions, and two were high risk for missing outcome data. No study was found to be high risk with respect to bias of measurement or bias of reported results. A total of seven included studies reported post-randomisation exclusions, four of which were due to extreme IOP spikes. Twenty-four studies included one eye per patient, two articles included two eyes per patient, and the rest had a variable ratio of eyes to patients. According to the GRADE tool, the certainty of the evidence ranged from “low” to “high”, with the majority of analyses achieving a moderate certainty of evidence (Supplementary Table 4).
Overall analysis
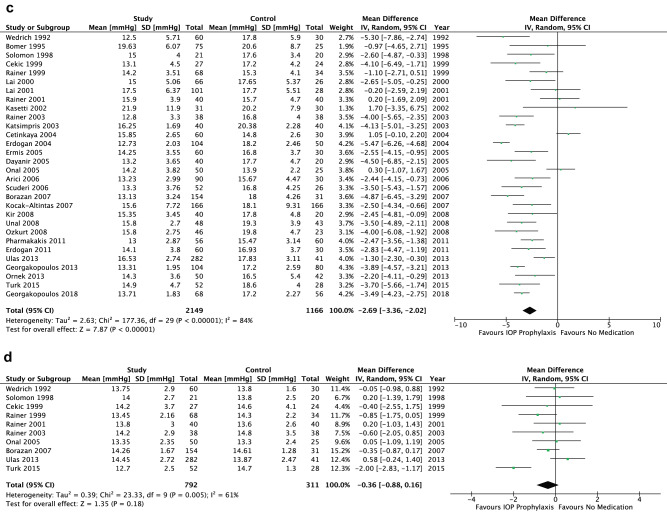
There was no significant difference between the comparator groups for baseline IOP (p = 0.16) (Fig. 2a), and no heterogeneity was evidenced for the meta-analysis at baseline (p = 0.36). The use of prophylactic antihypertensive medications resulted in a significantly lower IOP relative to no prophylaxis up to 24 h postoperatively (2–8 h: WMD = −3.87 mmHg, 95% CI [−4.75, −3.00], p < 0.001; 12–24 h: WMD = −2.69 mmHg, 95% CI [−3.36, −2.02], p < 0.001) (Fig. 1b, c). Significant statistical heterogeneity was found for both endpoints (p < 0.001). Notably, the effect on IOP was not maintained beyond the first postoperative day (p = 0.18, heterogeneity p = 0.005) (Fig. 1d).
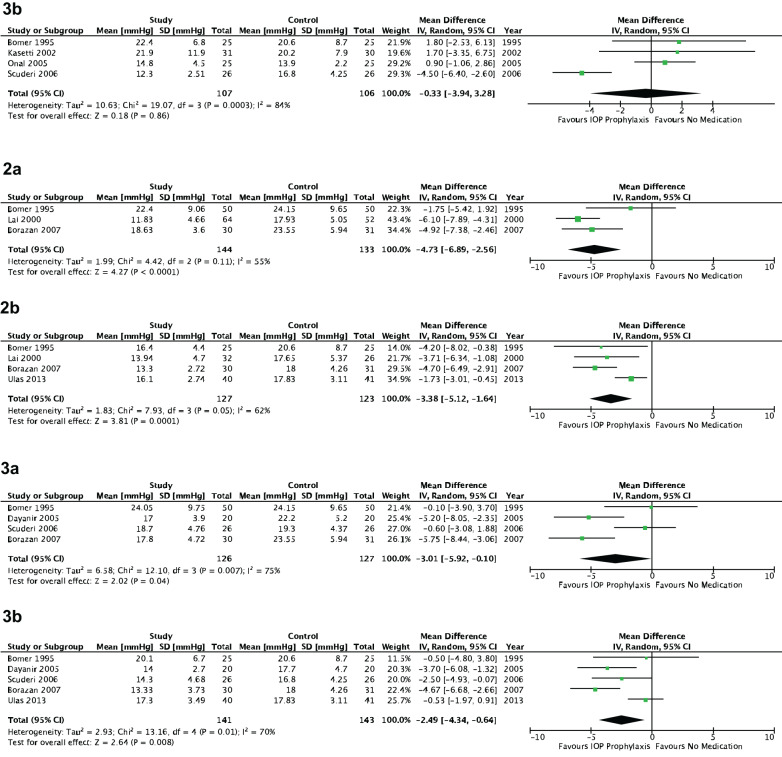
Fig. 2. Intraocular pressure stratified by medication class.
1.1a Topical alpha-agonists—2–8 h postoperatively. 1.2a Topical brimonidine—2–8 h postoperatively. 1.3a Topical apraclonidine—2–8 h postoperatively. 1.1b Topical alpha-agonists—12–24 h postoperatively. 1.2b Topical brimonidine—12–24 h postoperatively. 1.3b Topical apraclonidine—12–24 h postoperatively. 2a Topical beta-blockers—2–8 h postoperatively. 2b Topical beta-blockers—12–24 h postoperatively. 3a Oral carbonic anhydrase inhibitors—2–8 h postoperatively. 3b Oral carbonic anhydrase inhibitors—12–24 h postoperatively.
Fig. 1. Intraocular pressure across all treatments.
a Baseline. b 2–8 H postoperatively. c 12–24 H postoperatively. d 1–7 Days postoperatively.
Subgroup analysis
Alpha-agonists
At 2–8 h postoperatively, prophylactic topical alpha-agonists were associated with a significantly lower IOP relative to no medication (WMD = −2.77 mmHg, 95%CI [−4.27, −1.27], p < 0.001) (Fig. 2.1.1a). When stratified by individual medications, the same result held true for topical brimonidine (WMD = −3.65 mmHg, 95% CI [−5.23, −2.07], p < 0.001) but not for topical apraclonidine (p = 0.44) (Fig. 2.1.2a, 2.1.3a). A similar trend with a smaller effect size was seen at 12–24 h postoperatively, highlighted by a relatively lower IOP for topical alpha-agonists in general (WMD = −1.77 mmHg, 95% CI [−3.47, −0.06], p = 0.04) and brimonidine (WMD = −2.36 mmHg, 95% CI [−4.38, −0.33], p = 0.02), but a statistically insignificant change for apraclonidine (WMD = −0.33 mmHg, 95% CI [−3.94, 3.28], p = 0.86) (Fig. 2.1.1b, 2.1.2b, 2.1.3b). All analyses for alpha-agonists suffered from significant heterogeneity (p < 0.05) except for topical apraclonidine at 2–8 h postoperatively (p = 0.85).
Beta-blockers
There was a significantly lower IOP following prophylaxis with topical beta-blockers relative to no prophylaxis both at 2–8 h (WMD = −4.73 mmHg, 95% CI [−6.89, −2.56], p < 0.001) and 12–24 h postoperatively (WMD = −3.38 mmHg, 95% CI [−5.12, −1.64], p < 0.001) (Fig. 2.2a, b). No significant heterogeneity was found for either endpoint (2–8 h: p = 0.11, 12–24 h: p = 0.05).
Carbonic anhydrase inhibitors (CAI)
Oral CAIs showed a significant reduction in IOP at 2–8 h postoperatively relative to no prophylaxis (WMD = −3.01 mmHg, 95% CI [−5.92, −0.10], p = 0.04), which was sustained at 12–24 h (WMD = −2.49 mmHg, 95% CI [−4.34, −0.64], p = 0.008) (Fig. 2.3a, b). The same conclusion was made for topical CAIs, for which a larger effect size was seen at 2–8 h (WMD = −4.34 mmHg, 95% CI [−6.10, −2.58], p < 0.001) relative to 12–24 h (WMD = −2.03 mmHg, 95% CI [−3.44, −0.62], p = 0.005) (Fig. 3.1a, b). Significant heterogeneity was found for all endpoints in this subgroup analysis (p < 0.05).
Fig. 3. Intraocular pressure stratified by medication class.
1a Topical carbonic anhydrase inhibitors—2–8 h postoperatively. 1b Topical carbonic anhydrase inhibitors—12–24 h postoperatively. 2.1a Intracameral cholinergics—2–8 h postoperatively. 2.2a Intracameral acetylcholine—2–8 h postoperatively. 2.3a Intracameral carbachol—2–8 h postoperatively. 2.1b Intracameral cholinergics—12–24 h postoperatively. 2.2b Intracameral acetylcholine—12–24 h postoperatively. 2.3b Intracameral carbachol—12–24 h postoperatively. 3a Topical prostaglandin analogues—2–8 h postoperatively. 3b Topical prostaglandin analogues—12–24 h postoperatively.
Cholinergics
A significant effect on IOP was seen for prophylactic intracameral cholinergic agents relative to no medication at 2–8 h postoperatively (WMD = −5.94 mmHg, 95% CI [−7.41, −4.47], p < 0.001) (Fig. 3.2.1a). When stratified by individual medications, intracameral carbachol was shown to have a more pronounced effect on IOP compared to no prophylaxis (WMD = −7.18 mmHg, 95% CI [−9.77, −4.59], p < 0.001) relative to acetylcholine (WMD = −5.12 mmHg, 95% CI [−7.12, −3.13], p < 0.001) (Fig. 3.2.2a, 3.2.3a). At 12–24 h postoperatively, prophylactic intracameral cholinergics resulted in a significantly lower IOP relative to no prophylaxis (WMD = −3.51 mmHg, 95% CI [−4.98, −2.05], p < 0.001) (Fig. 3.2.1a). Once again, intracameral carbachol had a greater associated effect size (WMD = −4.58 mmHg, 95% CI [−7.20, −1.96], p < 0.001) compared to intracameral acetylcholine (WMD = −2.95 mmHg, 95% CI [−5.28, −0.62], p = 0.01) at 12–24 h postoperatively (Fig. 3.2.2b, 3.2.3b). Notably, no significant statistical heterogeneity was found for any endpoints in this subgroup analysis other than for carbachol at 12–24 h postoperatively (p = 0.03).
Prostaglandin analogues
For topical prostaglandin analogues, the meta-analysis showed a small but statistically significant effect on reducing IOP relative to no medication at 2–8 h postoperatively (WMD = −2.12 mmHg, 95% CI [−3.53, −0.72], p = 0.003) (Fig. 2.3a). A similar effect was evidenced at 12–24 h postoperatively (WMD = −2.47 mmHg, 95% CI [−3.90, −1.03], p < 0.001) (Fig. 2.3b). Statistically significant heterogeneity was found for both endpoints (p < 0.05).
Fixed-combination CAI and beta-blocker (FCCB)
Large and statistically significant differences in IOP at 2–8 h postoperatively were seen for topical FCCB agents relative to no prophylaxis (WMD = −5.78 mmHg, 95%CI [−8.61, −2.96], p < 0.001) (Fig. 4.1a). A smaller but still significant effect size was seen at 12–24 h postoperatively (WMD = −3.97 mmHg, 95% CI [−4.53, −3.40], p < 0.001) (Figs. 4.1b and 5). Statistically significant heterogeneity was found for the 2–8-hour time point (p < 0.001), which was not evidenced in the 12–24-h time point (p = 0.44).
Fig. 4. Intraocular pressure stratified by medication class.
1a Topical fixed-combination carbonic anhydrase inhibitor with beta-blocker—2–8 h postoperatively. 1b Topical fixed-combination carbonic anhydrase inhibitor with beta-blocker—12–24 h postoperatively. 2a Topical fixed- combination beta-blocker with alpha-agonist—2–8 h postoperatively. 2b Topical fixed-combination beta-blocker with alpha-agonist—12–24 h postoperatively.
Fig. 5. Comparative effect size for prophylactic antihypertensive medications stratified by class.
AA Alpha-Agonist, BB Beta-Blocker, CAI Carbonic Anhydrase Inhibitor, FCBA Fixed-Combination Beta-Blocker with Alpha-Agonist, FCCB Fixed-Combination Carbonic Anhydrase Inhibitor with Beta-Blocker, PGA Prostaglandin Analogue.
Fixed-combination beta-blocker and alpha-agonist (FCBA)
Compared to prophylaxis, topical FCBA agents led to a significant IOP reduction at both 2–8 h postoperatively (WMD = −5.46 mmHg, 95% CI [−8.90, −2.01], p = 0.002) (Fig. 4.2a) and 12–24 h postoperatively (WMD = −2.50 mmHg, 95% CI [−3.37, −1.62], p < 0.001) (Fig. 4.2b). The heterogeneity was statistically significant for the 2–8-h time point but was insignificant for the 12–24-hour time point (p = 0.86).
Discussion
IOP spikes following cataract surgery are a common phenomenon. Despite the interest in medical prophylaxis, there remains poor agreement in the literature regarding the optimal prophylactic regimen. The current meta-analysis aimed to investigate the comparative efficacy of various ocular antihypertensive medications relative to control arms. It was found that medical prophylaxis against postoperative IOP spikes is effective at both the 2–8-h and 12–24-h postoperative periods (GRADE: moderate; Supplementary Table 4). In the earlier postoperative period, the largest effect size was observed with intracameral cholinergics, topical FCCB, and topical FCBA agents (GRADE: moderate to high; Supplementary Table 4). At both time periods, oral CAIs were found to have a moderate effect size compared to their topical counterparts or other medication classes (GRADE: moderate; Supplementary Table 4). Topical prostaglandin analogues and alpha-agonists were found to be the least effective during the early postoperative period (GRADE: low to moderate; Supplementary Table 4).
The superior performance of topical FCCBs and FCBAs may be attributable to the synergistic effect of simultaneous administration of two topical agents, as compared to monotherapy. CAIs and beta-blockers achieve aqueous suppression through different cellular mechanisms. Both FCCB and FCBA have been shown to have equivalent IOP-lowering effects compared to concomitant administration of their individual agents [47, 48]. Similar comparative studies have yet to be performed following cataract surgery.
In our study, intracameral agents, such as acetylcholine and carbachol, provided excellent IOP prophylaxis. It has been hypothesised that IOP spikes following cataract surgery result from trabecular blockage by surgical debris and retained OVD [7, 49]. Intracameral cholinergics improve conventional outflow via the Schlemm canal, which may explain their beneficial effect in the postoperative period.
Topical alpha-agonists are frequently and effectively used as prophylaxis against IOP spikes following office-based laser procedures, such as laser peripheral iridotomy or laser trabeculoplasty [50]. In this study, we found a relative weaning-off effect from alpha-agonists, showing the smallest effect size beyond 8 h postoperatively across all classes. It is not entirely clear why this may be the case. Pharmacokinetic evaluations have shown that brimonidine has a half-life of approximately 2 h [51]. In clinical studies, maximal IOP-lowering occurs between 2–3 h [52]. Thus, it is possible that the IOP-lowering effect of these agents may wear off by the 12-h mark.
Topical and oral CAIs provided similar effects at both the 2–8-h and 12–24-h time periods. CAIs provide aqueous suppression by inhibiting isoenzymes of carbonic anhydrase present in the non-pigmented ciliary epithelium, the site of aqueous production [53]. In previous cataract surgery studies comparing the additive effects of dorzolamide to acetazolamide in patients already taking topical beta-blockers, both topical and oral CAIs were found to produce similar IOP-lowering effects at 12 weeks [54]. These results suggest no added benefit of oral over topical CAIs after cataract surgery.
The effect of topical beta-blockers was also found to be substantial. These agents lower IOP through aqueous suppression, and their peak effect tends to occur between 2-3 h after instillation [55]. Topical prostaglandin analogues primarily improve aqueous outflow via the uveoscleral pathway. Their peak effect tends to occur beyond 10 h after administration [56], which may explain why their effect on IOP during the examined time periods was less than that of other classes of medication.
The timing of IOP spikes following cataract surgery has been well studied, though with differing results. Initial studies looking at IOP elevation following extracapsular cataract extraction found that peak spikes occurred between 4–6 h postoperatively [9]. Many studies evaluating phacoemulsification found peak spikes occurring at a similar time period, particularly when cohesive viscoelastic agents were used [7, 25, 28, 57]. However, as most of these studies were designed to initially measure IOP between 4 and 6 h postoperatively, earlier spikes may have been missed. In a study of eyes undergoing phacoemulsification with a dispersive viscoelastic agent, IOP was measured at multiple time points prior to the 4-h mark and peak IOP was found to occur at 1 h postoperatively [7]. Another study by the same group found that peak IOP following the use of cohesive viscoelastic agents occurred between 2 and 8 h [6]. Thus, the ideal agent used in prophylaxis against IOP spikes ought to have a rapid onset and sustained action during the initial 8 h.
Current North American guidelines for cataract surgery do not adequately address the issue of IOP prophylaxis. The American Academy of Ophthalmology Preferred Practice Patterns® acknowledges incomplete evidence, though it does suggest aqueous suppressants or intracameral cholinergics [58]. The Canadian Ophthalmological Society Clinical Practice Guidelines suggest intracameral cholinergics and apraclonidine [59]. National guidelines could thus be updated based on our results. Given the frequency of IOP spikes, we recommend that all eyes undergoing routine phacoemulsification should receive prophylactic treatment, where possible. The most practical treatment of choice would be a single dose of a topical FCCB. Intracameral cholinergic agents are also highly effective, though may be a more expensive option for routine clinical use.
It is important to recognise the strengths and limitations of the current meta-analysis. The study followed the PRISMA recommendations (Supplementary Table 5). The large sample mostly consisted of prospective RCTs of moderate quality that minimised selection bias and confounding by indication. Conclusions were drawn regarding the effect size for various ocular antihypertensive medications at early and intermediate postoperative time points. However, there was some variability in how and when individual studies administered antihypertensive medications, which limits our ability to identify the optimal perioperative treatment time. Additionally, this meta-analysis collected data at the cohort level, thus limiting the interpretation of the results for individual patients. Between-study heterogeneity was commonly found throughout the analysis and was dependent on medication class. The inclusion criteria were limited to English-language RCTs, which may limit the generalisability of the findings. Comparisons made between medication classes regarding effect size should be interpreted with caution due to differences in protocols, patient populations, intervention schedules, and follow-up between trials. Safety endpoints between interventions were inconsistently reported across studies and were thus beyond the scope of the current meta-analysis.
In summary, this meta-analysis demonstrates that medical prophylaxis of IOP spikes following uncomplicated phacoemulsification is effective. The most effective class of medications were topical FCCBs and intracameral cholinergics, while topical alpha-agonists were the least effective. Future research may explore the potential impacts of perioperative IOP prophylaxis, and further head-to-head trials between medication classes would help determine the optimal prophylaxis regimen.
Summary
What was known before
Existing data on the efficacy of intraocular pressure (IOP) lowering medications in controlling IOP after uncomplicated cataract surgery is inconclusive.
A recent systematic review suggested a relatively good IOP-lowering potential for formulations containing timolol, latanoprost, or travoprost.
However, a comprehensive meta-analysis addressing this issue was lacking.
What this study adds
This meta-analysis of 2909 eyes showed that perioperative use of IOP-lowering medications leads to significant IOP reductions following uncomplicated cataract surgery, with the largest effect associated with intracameral cholinergic or fixed-combination carbonic anhydrase inhibitor-beta-blocker (FCCB) formulations.
These findings offer valuable insights that may guide future surgical guidelines.
Based on these results, national guidelines could be revised to recommend the use of intracameral cholinergics or topical FCCBs where appropriate.
Supplementary information
Supplementary Table 2. Characteristics of included studies
Supplementary Table 3: Summary of Ophthalmic Viscoelastic Devices
Supplementary Table 4: Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Summary of Findings Table
Supplementary Figure 1: Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Supplementary Figure 2: Risk of Bias Summary Table Stratified by Study.
Acknowledgements
Financial disclosures: MMP, AS, CH: none. RK: Allergan/Abbvie. IIKA: Alcon, Johnson & Johnson Vision, Bausch Health, Santen, Carl Zeiss AG, Aequus, Akorn, Aquea Health, Inc, ArcScan, Beaver Visitec, Beyeonics, Centricity Vision, Inc, CorNeat Vision, Costum Surgical, ELT Sight, ElutiMed, Equinox, Genentech, Gore, Iantrek, InjectSense, Iridex, iStar, LayerBio, Leica Microsystems, Long Bridge Medical, Inc, MicroOptx, New World Medical, Ocular Instruments, Ocular Therapeutix, Oculo, Omega Ophthalmics, PolyActiva, Radiance Therapeutics, Ripple Therapeutics, Sanoculis, Shifamed, LLC, Sight Sciences, Smartlens, Inc, Stroma, Thea Pharma, ViaLase, Vizzario. HS: Alcon/Novartis, Allergan/Abbvie, Bausch Health, Glaukos, Labtician Thea, Aerie Pharmaceuticals, Johnson & Johnson, Ivantis, Zeiss.
Author contributions
Designing/revising the protocol: RK, MMP, AS, CH, IIKA, HS. - Conducting the search and screening the studies: RK, MMP, AS, HS - Extracting and analysing the data: RK, MMP, AS, HS - Interpreting the results: RK, MMP, AS, CH, IIK, HS - Writing the report and creating figures/tables: RK, MMP, AS, HS - Critically revising the paper: RK, MMP, AS, CH, IIKA, HS.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was reviewed by the McGill University’s Faculty of Medicine Institutional Review Board and was exempt from ethics review based on article 2.2(b) of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. The study adhered to the tenets of the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Raageen Kanjee, Marko M. Popovic.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-024-02940-6.
References
- 1.Lundstrom M, Goh PP, Henry Y, Salowi MA, Barry P, Manning S, et al. The changing pattern of cataract surgery indications: a 5-year study of 2 cataract surgery databases. Ophthalmology. 2015;122:31–8. doi: 10.1016/j.ophtha.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Lundstrom M, Barry P, Henry Y, Rosen P, Stenevi U. Visual outcome of cataract surgery; study from the European Registry of Quality Outcomes for Cataract and Refractive Surgery. J Cataract Refract Surg. 2013;39:673–9. doi: 10.1016/j.jcrs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Day AC, Donachie PH, Sparrow JM, Johnston RL. Royal College of Ophthalmologists’ National Ophthalmology D. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29:552–60. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed II, Kranemann C, Chipman M, Malam F. Revisiting early postoperative follow-up after phacoemulsification. J Cataract Refract Surg. 2002;28:100–8. doi: 10.1016/S0886-3350(01)00994-4. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand GD, Wickremasinghe SS, Tranos PG, Harris ML, Little BC. Efficacy of anterior chamber decompression in controlling early intraocular pressure spikes after uneventful phacoemulsification. J Cataract Refract Surg. 2003;29:1087–92. doi: 10.1016/S0886-3350(02)01891-6. [DOI] [PubMed] [Google Scholar]
- 6.Rainer G, Schmid KE, Findl O, Sacu S, Kiss B, Heinzl H, et al. Natural course of intraocular pressure after cataract surgery with sodium hyaluronate 1% versus hydroxypropylmethylcellulose 2% Ophthalmology. 2007;114:1089–93. doi: 10.1016/j.ophtha.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Rainer G, Menapace R, Schmid KE, Sacu S, Kiss B, Heinze G, et al. Natural course of intraocular pressure after cataract surgery with sodium chondroitin sulfate 4%-sodium hyaluronate 3% (Viscoat) Ophthalmology. 2005;112:1714–8. doi: 10.1016/j.ophtha.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Henry JC, Olander K. Comparison of the effect of four viscoelastic agents on early postoperative intraocular pressure. J Cataract Refract Surg. 1996;22:960–6. doi: 10.1016/S0886-3350(96)80199-4. [DOI] [PubMed] [Google Scholar]
- 9.Passo MS, Ernest JT, Goldstick TK. Hyaluronate increases intraocular pressure when used in cataract extraction. Br J Ophthalmol. 1985;69:572–5. doi: 10.1136/bjo.69.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holzer MP, Tetz MR, Auffarth GU, Welt R, Volcker HE. Effect of Healon5 and 4 other viscoelastic substances on intraocular pressure and endothelium after cataract surgery. J Cataract Refract Surg. 2001;27:213–8. doi: 10.1016/S0886-3350(00)00568-X. [DOI] [PubMed] [Google Scholar]
- 11.Whitehouse G. Brimonidine and postoperative pressure spikes in cataract surgery. Clin Exp Ophthalmol. 2000;28:364–6. doi: 10.1046/j.1442-9071.2000.00340.x. [DOI] [PubMed] [Google Scholar]
- 12.Rainer G, Menapace R, Findl O, Petternel V, Kiss B, Georgopoulos M. Effect of topical brimonidine on intraocular pressure after small incision cataract surgery. J Cataract Refract Surg. 2001;27:1227–31. doi: 10.1016/S0886-3350(01)00790-8. [DOI] [PubMed] [Google Scholar]
- 13.Katsimpris JM, Siganos D, Konstas AG, Kozobolis V, Georgiadis N. Efficacy of brimonidine 0.2% in controlling acute postoperative intraocular pressure elevation after phacoemulsification. J Cataract Refract Surg. 2003;29:2288–94. doi: 10.1016/j.jcrs.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Holm JL, Bach-Holm D, Holm LM, Vestergaard AH. Prophylactic treatment of intraocular pressure elevation after uncomplicated cataract surgery in nonglaucomatous eyes - a systematic review. Acta Ophthalmol. 2019;97:545–57. doi: 10.1111/aos.14092. [DOI] [PubMed] [Google Scholar]
- 15.Secretariat on Responsible Conduct of R, Canadian Institutes of Health R, Natural S, Engineering Research Council C, Social S, Humanities Research Council of C. Tri-council policy statement : ethical conduct for research involving humans. Ottawa, Ontario: Secretariat on Responsible Conduct of Research; 2022. Available from: http://central.bac-lac.gc.ca/.redirect?app=damspub&id=0ab0f8e2-ddb4-4330-8498-8e235944dfc2. https://publications.gc.ca/collections/collection_2023/irsc-cihr/RR4-2-2023-eng.pdf. https://ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2022.html.
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedrich A, Menapace R. Intraocular pressure following small-incision cataract surgery and polyHEMA posterior chamber lens implantation. A comparison between acetylcholine and carbachol. J Cataract Refract Surg. 1992;18:500–5. doi: 10.1016/S0886-3350(13)80106-X. [DOI] [PubMed] [Google Scholar]
- 20.Unal M, Yucel I. Effect of bimatoprost on intraocular pressure after cataract surgery. Can J Ophthalmol. 2008;43:712–6. doi: 10.3129/i08-150. [DOI] [PubMed] [Google Scholar]
- 21.Ulas F, Balbaba M, Celebi S. Effect of prophylactic intraocular pressure-lowering medication on pain during cataract surgery. J Ocul Pharm Ther. 2013;29:658–62. doi: 10.1089/jop.2012.0244. [DOI] [PubMed] [Google Scholar]
- 22.Turk A, Ceylan OM, Gokce G, Borazan M, Kola M. Comparison of brimonidine-timolol and dorzolamide-timolol in the management of intraocular pressure increase after phacoemulsi fi cation. Int J Ophthalmol. 2015;8:945–9. doi: 10.3980/j.issn.2222-3959.2015.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon KD, Stewart WC, Hunt HH, Stewart JA, Cate EA. Intraoperative intracameral carbachol in phacoemulsification and posterior chamber lens implantation. Am J Ophthalmol. 1998;125:36–43. doi: 10.1016/S0002-9394(99)80232-3. [DOI] [PubMed] [Google Scholar]
- 24.Scuderi G, Regine F, Perdicchi A, Mannino G, Recupero SM. Comparative efficacy of acetazolamide and apraclonidine in the control of intraocular pressure following phacoemulsification. Ophthalmologica. 2006;220:356–60. doi: 10.1159/000095860. [DOI] [PubMed] [Google Scholar]
- 25.Rainer G, Menapace R, Schmetterer K, Findl O, Georgopoulos M, Vass C. Effect of dorzolamide and latanoprost on intraocular pressure after small incision cataract surgery. J Cataract Refract Surg. 1999;25:1624–9. doi: 10.1016/S0886-3350(99)00260-6. [DOI] [PubMed] [Google Scholar]
- 26.Rainer G, Menapace R, Findl O, Sacu S, Schmid K, Petternel V, et al. Effect of a fixed dorzolamide-timolol combination on intraocular pressure after small-incision cataract surgery with Viscoat. J Cataract Refract Surg. 2003;29:1748–52. doi: 10.1016/S0886-3350(02)01981-8. [DOI] [PubMed] [Google Scholar]
- 27.Pharmakakis N, Giannopoulos K, Stasinos S, Makri OE, Georgakopoulos CD. Effect of a fixed brimonidine-timolol combination on intraocular pressure after phacoemulsification. J Cataract Refract Surg. 2011;37:279–83. doi: 10.1016/j.jcrs.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 28.Ozkurt Y, Oral Y, Karacan O, Comez A, Dogan OK. Comparison of the effects of dorzolamide-timolol fixed combination and brimonidine on intraocular pressure after phacoemulsification surgery. Eye Contact Lens. 2008;34:21–3. doi: 10.1097/ICL.0b013e3180587e0a. [DOI] [PubMed] [Google Scholar]
- 29.Ornek K, Buyuktortop N, Ornek N, Ogurel R, Erbahceci IE, Onaran Z. Effect of 1% brinzolamide and 0.5% timolol fixed combination on intraocular pressure after cataract surgery with phacoemulsification. Int J Ophthalmol. 2013;6:851–4. doi: 10.3980/j.issn.2222-3959.2013.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onal S, Gozum N, Gucukoglu A. Effect of apraclonidine versus dorzolamide on intraocular pressure after phacoemulsification. Ophthalmic Surg Lasers Imaging. 2005;36:457–62. doi: 10.3928/1542-8877-20051101-05. [DOI] [PubMed] [Google Scholar]
- 31.Lai JS, Chua JK, Loo A, Ho SY, Lam DS. Effect of intracameral acetylcholine on latanoprost in preventing ocular hypertension after phacoemulsification and intraocular lens implantation. J Cataract Refract Surg. 2001;27:700–5. doi: 10.1016/S0886-3350(00)00729-X. [DOI] [PubMed] [Google Scholar]
- 32.Lai JS, Chua JK, Leung AT, Lam DS. Latanoprost versus timolol gel to prevent ocular hypertension after phacoemulsification and intraocular lens implantation. J Cataract Refract Surg. 2000;26:386–91. doi: 10.1016/S0886-3350(99)00364-8. [DOI] [PubMed] [Google Scholar]
- 33.Kocak-Altintas AG, Anayol MA, Cakmak HB, Simsek S. Effects of topical dorzolamide on IOP after phacoemulsification with different types of ophthalmic viscosurgical devices. Eur J Ophthalmol. 2007;17:38–44. doi: 10.1177/112067210701700106. [DOI] [PubMed] [Google Scholar]
- 34.Kir E, Cakmak H, Dayanir V. Medical control of intraocular pressure with brinzolamide 1% after phacoemulsification. Can J Ophthalmol. 2008;43:559–62. doi: 10.3129/i08-130. [DOI] [PubMed] [Google Scholar]
- 35.Kasetti SR, Desai SP, Sivakumar S, Sunderraj P. Preventing intraocular pressure increase after phacoemulsification and the role of perioperative apraclonidine. J Cataract Refract Surg. 2002;28:2177–80. doi: 10.1016/S0886-3350(02)01454-2. [DOI] [PubMed] [Google Scholar]
- 36.Georgakopoulos CD, Makri OE, Plotas P, Pharmakakis N. Brinzolamide-timolol fixed combination for the prevention of intraocular pressure elevation after phacoemulsification. Clin Exp Ophthalmol. 2013;41:662–7. doi: 10.1111/ceo.12092. [DOI] [PubMed] [Google Scholar]
- 37.Georgakopoulos CD, Kagkelaris K, Pagoulatos D, Plotas P, Makri OE. Brinzolamide-brimonidine fixed combination for the prevention of intraocular pressure elevation after phacoemulsification. Eur J Ophthalmol. 2020;30:293–8. doi: 10.1177/1120672118817997. [DOI] [PubMed] [Google Scholar]
- 38.Ermis SS, Ozturk F, Inan UU. Comparing the effects of travoprost and brinzolamide on intraocular pressure after phacoemulsification. Eye. 2005;19:303–7. doi: 10.1038/sj.eye.6701470. [DOI] [PubMed] [Google Scholar]
- 39.Erdogan H, Ozec AV, Caner C, Toker MI, Arici MK, Topalkara A. Effect of latanoprost/timolol and dorzolamide/tiomolol on intraocular pressure after phacoemulsification surgery. Int J Ophthalmol. 2011;4:190–4. doi: 10.3980/j.issn.2222-3959.2011.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayanir V, Ozcura F, Kir E, Topaloglu A, Ozkan SB, Aktunc T. Medical control of intraocular pressure after phacoemulsification. J Cataract Refract Surg. 2005;31:484–8. doi: 10.1016/j.jcrs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Cetinkaya A, Akman A, Akova YA. Effect of topical brinzolamide 1% and brimonidine 0.2% on intraocular pressure after phacoemulsification. J Cataract Refract Surg. 2004;30:1736–41. doi: 10.1016/j.jcrs.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Cekic O, Batman C. Effect of intracameral carbachol on intraocular pressure following clear corneal phacoemulsification. Eye. 1999;13:209–11. doi: 10.1038/eye.1999.52. [DOI] [PubMed] [Google Scholar]
- 43.Borazan M, Karalezli A, Akman A, Akova YA. Effect of antiglaucoma agents on postoperative intraocular pressure after cataract surgery with Viscoat. J Cataract Refract Surg. 2007;33:1941–5. doi: 10.1016/j.jcrs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 44.Bomer TG, Lagreze WD, Funk J. Intraocular pressure rise after phacoemulsification with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon’s experience. Br J Ophthalmol. 1995;79:809–13. doi: 10.1136/bjo.79.9.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arici MK, Erdogan H, Toker I, Vural A, Topalkara A. The effect of latanoprost, bimatoprost, and travoprost on intraocular pressure after cataract surgery. J Ocul Pharm Ther. 2006;22:34–40. doi: 10.1089/jop.2006.22.34. [DOI] [PubMed] [Google Scholar]
- 46.Erdogan H, Toker MI, Arici MK, Topalkara A. Effect of latanoprost 0.005% and brimonidine 0.2% on intraocular pressure after phacoemulsification and intraocular lens implantation surgery. Jpn J Ophthalmol. 2004;48:600–1. doi: 10.1007/s10384-004-0116-5. [DOI] [PubMed] [Google Scholar]
- 47.Hutzelmann J, Owens S, Shedden A, Adamsons I, Vargas E. Comparison of the safety and efficacy of the fixed combination of dorzolamide/timolol and the concomitant administration of dorzolamide and timolol: a clinical equivalence study. International Clinical Equivalence Study Group. Br J Ophthalmol. 1998;82:1249–53. doi: 10.1136/bjo.82.11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goni FJ, Brimonidine/Timolol Fixed Combination Study G. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005;15:581–90. doi: 10.1177/112067210501500508. [DOI] [PubMed] [Google Scholar]
- 49.Berson FG, Patterson MM, Epstein DL. Obstruction of aqueous outflow by sodium hyaluronate in enucleated human eyes. Am J Ophthalmol. 1983;95:668–72. doi: 10.1016/0002-9394(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg LF, Krupin T, Ruderman J, McDaniel DL, Siegfried C, Karalekas DP, et al. Apraclonidine and anterior segment laser surgery. Comparison of 0.5% versus 1.0% apraclonidine for prevention of postoperative intraocular pressure rise. Ophthalmology. 1995;102:1312–8. doi: 10.1016/S0161-6420(95)30869-X. [DOI] [PubMed] [Google Scholar]
- 51.Lee AJ, McCluskey P. Fixed combination of topical brimonidine 0.2% and timolol 0.5% for glaucoma and uncontrolled intraocular pressure. Clin Ophthalmol. 2008;2:545–55. doi: 10.2147/OPTH.S3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costagliola C, dell’Omo R, Romano MR, Rinaldi M, Zeppa L, Parmeggiani F. Pharmacotherapy of intraocular pressure: part I. Parasympathomimetic, sympathomimetic and sympatholytics. Expert Opin Pharmacother. 2009;10:2663–77. doi: 10.1517/14656560903300103. [DOI] [PubMed] [Google Scholar]
- 53.Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78:625–31. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 54.Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res. 2000;19:87–112. doi: 10.1016/S1350-9462(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 55.Nieminen T, Lehtimaki T, Maenpaa J, Ropo A, Uusitalo H, Kahonen M. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67:237–45. doi: 10.1080/00365510601034736. [DOI] [PubMed] [Google Scholar]
- 56.Orzalesi N, Rossetti L, Bottoli A, Fogagnolo P. Comparison of the effects of latanoprost, travoprost, and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology. 2006;113:239–46. doi: 10.1016/j.ophtha.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 57.Schwenn O, Xia N, Krummenauer F, Dick HB. [Prevention of early postoperative increase in intraocular pressure after phacoemulsification. Comparison of different antiglaucoma drugs] Ophthalmologe. 2001;98:934–43. doi: 10.1007/s003470170040. [DOI] [PubMed] [Google Scholar]
- 58.Olson RJ, Braga-Mele R, Chen SH, Miller KM, Pineda R, 2nd, Tweeten JP, et al. Cataract in the Adult Eye Preferred Practice Pattern(R) Ophthalmology. 2017;124:P1–P119. doi: 10.1016/j.ophtha.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 59.Canadian Ophthalmological Society Cataract Surgery Clinical Practice Guideline Expert C. Canadian Ophthalmological Society evidence-based clinical practice guidelines for cataract surgery in the adult eye. Can J Ophthalmol. 2008;43:S7–57. doi: 10.3129/i08-133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 2. Characteristics of included studies
Supplementary Table 3: Summary of Ophthalmic Viscoelastic Devices
Supplementary Table 4: Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Summary of Findings Table
Supplementary Figure 1: Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Supplementary Figure 2: Risk of Bias Summary Table Stratified by Study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.