Summary
Background
In ORIENT-15 study, sintilimab plus chemotherapy demonstrated significant improvement on overall survival (OS) versus placebo plus chemotherapy in first-line treatment of advanced esophageal squamous cell carcinoma (ESCC). Here, we report effect of sintilimab plus chemotherapy on health-related quality of life (HRQoL) in patients with advanced ESCC.
Methods
From December 14, 2018 to August 28, 2022, HRQoL was evaluated in all randomized patients using European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 items (QLQ-C30), EORTC Quality of Life Questionnaire Oesophageal Cancer Module 18 items (QLQ-OES18), and visual analogue scale (VAS) of the EuroQol five-dimensional five-level questionnaire (EQ-5D-5L). Mean scores of each scale were described by treatment group through week 60. Least-squares mean (LSM) score change from baseline through week 24 were analyzed using the mixed-model repeated-measures method. Time to the first onset of deterioration (TTD) and OS for each scale were estimated. Clinical Trials Registration: NCT03748134.
Findings
As of August 28, 2022, 689 of 690 enrolled patients were assessed for HRQoL analysis (sintilimab group: 340, placebo group: 349). Median follow-up was 32.2 months. Differences in LSM favored sintilimab over placebo for QLQ-C30 social functioning (LSM difference: 3.06, 95% CI: 0.55 to 5.57; P = 0.0170), pain (−2.24, 95% CI: −4.30 to −0.17; P = 0.0337), fatigue (−2.24, 95% CI: −4.46 to −0.02; P = 0.0479), constipation (−3.27, 95% CI −5.49 to −1.05; P = 0.0039), QLQ-OES18 pain (−1.77, 95% CI −3.11 to −0.43; P = 0.0097), trouble swallowing saliva (−2.09, 95% CI: −3.77 to −0.42; P = 0.0146), and choked when swallowing (−3.23, 95% CI: −5.60 to −0.86; P = 0.0076). TTD favored sintilimab over placebo for QLQ-OES18 dysphagia (Hazard ratio [HR]: 0.76, 95% CI: 0.61–0.94, P = 0.0104), and trouble swallowing saliva (HR: 0.48, 95% CI: 0.35–0.67, P < 0.0001). Improved OS were observed in patients with better performance in several functioning and symptom scales of QLQ-C30 and QLQ-QES18.
Interpretation
The statistically significant differences of several HRQoL scales and improvements in delayed deterioration observed in our study further support the use of sintilimab plus chemotherapy as first-line treatment for advanced ESCC.
Funding
This study was funded by Innovent Biologics and was co-funded by Eli Lilly.
Keywords: Esophageal squamous cell carcinoma, Health-related quality of life, Cancer immunotherapy, Phase 3 clinical trial, Chinese patients
Research in context.
Evidence before this study
Recent clinical trials have reported maintenance or improvements in HRQoL in ESCC patients treated with a PD-(L)1 inhibitor plus chemotherapy versus chemotherapy alone including KEYNOTE-590, CheckMate 648 and ESCORT-1st. However, these trials only reported some domains of HRQoL with a relatively short follow-up. The ORIENT-15 is a randomized, double blind, phase 3 study in which sintilimab plus chemotherapy demonstrated significant improvement on OS versus placebo plus chemotherapy in first-line treatment of ESCC. As one of the exploratory endpoints in ORIENT-15 study, evaluation of HRQoL could provide additional information on clinical benefits of the treatment for patients with advanced ESCC.
Added value of this study
In the present study with median follow up of 32.2 months, numbers of HRQoL scales were improved in patients receiving sintilimab plus chemotherapy including QLQ-C30 social functioning, pain, fatigue, constipation and QLQ-OES18 pain, trouble swallowing saliva, choked when swallowing. Time to the first onset of deterioration (TTD) favored sintilimab over placebo for QLQ-OES18 dysphagia and trouble swallowing saliva. Patients with delayed deterioration of QoLs experienced also improved OS.
Implications of all the available evidence
In addition to previously observed OS benefits and safety profiles, our study observed statistically significant differences of several HRQoL scales and improvements in delayed deterioration which further support the use of sintilimab plus chemotherapy as first-line treatment for advanced ESCC.
Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common histological subtype (accounting for 90%) of esophageal cancer in Asians.1 Patients with advanced ESCC usually experience severe symptom burden at diagnosis, such as dysphagia, eating difficulties, pain and reflux, and associated reductions in their quality of life.2, 3, 4 Patient-reported health-related quality of life (HRQoL) provides additional information on the risk–benefit profile of a given treatment and is reported to be predictive of overall survival (OS) in patients with advanced ESCC.4,5 Numbers of phase 3 trials evaluated anti-PD-1 inhibitor in combination with chemotherapy for patients with advanced ESCC,6, 7, 8, 9, 10 and observed maintained or improved HRQoL in patients receiving PD-1 inhibitor combination therapy compared with those receiving chemotherapy alone.11, 12, 13, 14, 15, 16 However, only limited domains of HRQol were evaluated with relatively short follow-ups.11, 12, 13 A full HRQoL report warrants further investigation to better support clinical decision making.
ORIENT-15 was a randomized, double blind, phase 3 study evaluating sintilimab (a recombinant fully human IgG4 anti-PD-1 monoclonal antibody) in combination with chemotherapy (cisplatin plus paclitaxel or cisplatin plus 5-FU) versus chemotherapy alone for the first-line advanced ESCC.8 At the interim analysis, 659 patients were enrolled from 79 sites in five countries; sintilimab in combination with chemotherapy versus chemotherapy alone demonstrated significant OS improvement versus chemotherapy alone in patients with a PD-L1 combined positive score (CPS) ≥10 (median OS: 17.2 versus 13.6 months; hazard ratio [HR]: 0.64, 95% confidence interval [CI]: 0.48–0.85, P = 0.002) and in all patients (16.7 versus 12.5 months; HR: 0.63, 95% CI: 0.51–0.78, P < 0.001).8 Based on the interim analysis findings from ORIENT-15 study, sintilimab plus chemotherapy was approved by China's National Medical Products Administration as a first-line treatment option for patients with advanced ESCC. At the updated analysis with a median follow-up of 32.2 months, sintilimab plus chemotherapy continued to demonstrate significant OS benefits (HR 0.661, 95% CI: 0.554–0.788, P < 0.001 in all patients; HR 0.635, 95% CI: 0.503–0.803, P < 0.001 in patients with PD-L1 CPS ≥10).17
Patients' HRQoL was prespecified as one of the exploratory endpoints in ORIENT-15 study. Previous results showed that patients receiving sintilimab plus chemotherapy had a decreased risk of deterioration in the global health status (GHS)/quality of life (QoL) domain of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 items (QLQ-C30), and a favorable visual analogue scale (VAS) of the EQ-5D-5L (EuroQol five-dimensional five-level questionnaire) compared with those receiving chemotherapy alone.8 Here, we report a comprehensive evaluation of patients’ HRQoL after an extended follow-up.
Methods
Study design, patients, and treatment
The full information of ORIENT-15 study design has been previously reported.8 Briefly, it was a global multi-regional, double-blind, randomized phase 3 study followed by an open-label single-arm extension phase conducted outside of China. After the interim analysis confirming significant OS benefits with sintilimab plus chemotherapy,8 enrollment for the randomization phase was still ongoing outside of China. This analysis included all randomized patients who were qualified for HRQoL analysis.
Eligible patients with histologically confirmed unresectable, locally advanced, recurrent or metastatic ESCC, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate hematologic and organ function were randomly assigned at 1:1 to receive either sintilimab or placebo, in combination with investigator's choice of chemotherapy (cisplatin + paclitaxel [TP regimen], or cisplatin + fluorourcil [CF regimen]). Sintilimab/Placebo was administered intravenously within 30–60 min at a dose of 3 mg/kg in patients with body weight <60 kg or 200 mg in those with body weight ≥60 kg on day 1 of each 3-week cycle. Cisplatin (75 mg/m2, on day 1 of each 3-week cycle) combined with either paclitaxel (87.5 mg/m2, on days 1 and 8 of the first 3-week cycle; 175 mg/m2, on day 1 from the second 3-week cycle on), or 5-fluorouracil (800 mg/m2 IV, daily on days 1–5 of each 3-week cycle) were administered intravenously. Treatment was continued until disease progression, intolerable toxicity, initiation of new anti-tumor therapy, or any other investigator-determined reasons for treatment discontinuation, whichever occurred first. A maximum of 6 cycles was recommended for TP regimen and CF regimen, and if tolerated by the subject, the duration of chemotherapy was determined by the investigator. Treatment with sintilimab or placebo was infused for a maximum period of 24 months (starting from the first dose). Sintilimab/placebo could be used alone if chemotherapy was intolerable. Chemotherapy was not allowed to switch between TP and CF regimens during the study.
Ethics
This study was approved by the institutional review board or ethics committee at each site (Supplementary Methods: The ethical approval list), and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent before enrollment.
HRQoL assessments
Prespecified HRQoL exploratory objective was to compare the changes in quality of life evaluated by the EuroQoL Five-Dimensions Five-Levels (EQ-5D-5L),18 EORTC QLQ-C30,19 the EORTC Quality of Life Questionnaire Oesophageal Cancer Module 18 items (QLQ-OES18)20 between the sintilimab and placebo groups. The EQ-5D comprises five health state domains (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) and a VAS to measure patient's overall health status.18 The QLQ-C30 questionnaire comprises a GHS/QoL scale, five function scales (physical, role, emotional, cognitive, and social), nine symptom or cancer-specific concern scales (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties).19 The QLQ-OES18 questionnaire comprises four symptom scales (dysphagia, eating, reflux, and pain) and six single-item scales (trouble with swallowing saliva, choking when swallowing, dry mouth, taste abnormality, trouble with coughing, and trouble with talking).20
The officially translated and validated versions of the HRQoL questionnaires21,22 were administered to patients via the paper version at the first dose, once every six weeks for the first 48 weeks, followed by once every 12 weeks, and at the first safety follow-up visit. Responses on all items of the QLQ-C30 and QLQ-OES18 were scored according to the EORTC guidelines. The higher scores represented better outcomes for the GHS/QoL and functioning scales and worse outcomes for the symptom scales. Score change from baseline of ≥10 points was considered a clinically meaningful difference, with deterioration defined as a score decrease of ≥10 in functional scales of QLQ-C30 or a score increase of ≥10 in symptom scales of QLQ-C30 and QLQ-OES18 and improvement defined as a score increase of ≥10 in QLQ-C30 functional scales or a score decrease of ≥10 in symptom scales of QLQ-C30 and QLQ-OES18.
Week 24 was mainly used to evaluate the longitudinal effects of treatment on HRQoL when more than 50% patients remained in the study. Specific HRQoL endpoints were QLQ-C30's scales of GHS/QoL, physical functioning, social functioning, pain, and fatigue symptom, and QLQ-OES18's scales of pain, eating, and dysphagia.
Statistics
HRQoL endpoints were predefined as exploratory objectives in this study and the analyses were not subject to multiplicity adjustment. The HRQoL analysis population included all randomized patients who completed at least one baseline scale in any HRQoL instrument. Compliance and completion rates were summarized by treatment group and visit. Compliance rate was defined by as the proportion of patients who completed the HRQoL instrument at each visit among the patients who were expected to complete the questionnaires (i.e., the patients who had a scheduled visit). Completion rate was defined as the proportion of patients who have completed the HRQoL instrument at each visit among the HRQoL analysis population.
Mean score and change from baseline to week 60 for each QLQ-C30 and QLQ-OES18 scale, and EQ-5D-5L VAS were presented by treatment group at each visit using descriptive analysis. Longitudinal profile of score changes from baseline through week 24 in each HRQoL scale between the two groups were compared using the mixed-model repeated-measures method (MMRM) with patient as the random effect. The treatment group, visit, treatment group∗visit, baseline score and randomization stratification factors were included as the independent variables. Missing values were assumed missing at random and were accounted in the MMRM. Least-squares mean (LSM) of the score change from baseline at each assessment and their between-group differences, along with the 95% CI and P-values (2-sided) were presented. Sensitivity analysis was performed using the control-based Pattern-Mixture Model (PMM).
Time to first deterioration (TTD, defined as the time from randomization to first onset of deterioration) for each scale was estimated using the Kaplan–Meier method. Patients who did not have an observed deterioration was censored at their last available post-baseline assessment. Patients with no post-baseline assessment were censored on the randomization date. The HRs of TTD between the two treatment groups with 95% CIs were estimated using a stratified Cox regression model, where the stratification factors were PD-L1 expression (tumor proportion score <10% versus ≥10%), ECOG performance status (0 versus 1), chemotherapy regimen (TP versus CF), and liver metastasis (presence versus absence). Descriptive analysis was used to summarize the patients whose conditions deteriorated, improved, or remained stable from baseline were summarized at each visit. No imputation for missing data was performed. All statistical analyses were performed using SAS version 9.4.
Role of funding source
This study was funded by Innovent Biologics and was co-funded by Eli Lilly. This trial was designed by the authors in collaboration with Innovent Biologics. The authors and Innovent Biologics were involved in data analysis and interpretation, verification of data accuracy and completeness, writing of the clinical report, and the decision to submit the manuscript for publication.
Results
Patients’ baseline characteristics
Overall, 690 patients were enrolled from the randomization phase, with 341 assigned to the sintilimab group and 349 to the placebo group. Baseline characteristics were well-balanced between the two groups (Table 1), with 57.5% (196/341) patients in the sintilimab group and 57.6% (201/349) in the placebo group having PD-L1 CPS ≥10. Most of the enrolled patients were male (85.5%), Asian (93.3%), and ECOG PS of 1 (75.2%); 90.7% patients received TP regimen. At data cutoff (Aug 28, 2022) for the analysis, median follow-up was 32.2 months (95% CI: 30.78–32.66).
Table 1.
Baseline characteristics for all patients.
| Sintilimab plus chemotherapy (n = 341) | Placebo plus chemotherapy (n = 349) | |
|---|---|---|
| Age, years | ||
| Median (range) | 63 (37–77) | 63 (38–80) |
| ≤65 | 217 (63.6%) | 222 (63.6%) |
| >65 | 124 (36.4%) | 127 (36.4%) |
| Gender | ||
| Male | 290 (85.0%) | 300 (86.0%) |
| Female | 51 (15.0%) | 49 (14.0%) |
| Race | ||
| Asian | 321 (94.1%) | 321 (92.5%) |
| White | 12 (3.5%) | 22 (6.3%) |
| Other | 8 (2.3%) | 4 (1.2%) |
| Country | ||
| China | 319 (93.5%) | 321 (92.0%) |
| Ex-China | 22 (6.5%) | 28 (8.0%) |
| Weight | ||
| <60 kg | 198 (58.1%) | 187 (53.6%) |
| ≥60 kg | 143 (41.9%) | 162 (46.4%) |
| ECOG performance status | ||
| 0 | 83 (24.3%) | 88 (25.2%) |
| 1 | 258 (75.7%) | 261 (74.8%) |
| Disease status at enrollment | ||
| Metastatic | 298 (87.4%) | 301 (86.2%) |
| Local advanced | 43 (12.6%) | 48 (13.8%) |
| Site of metastases | ||
| Liver | 82 (24.0%) | 86 (24.6%) |
| Lung | 118 (34.6%) | 117 (33.5%) |
| Bone | 42 (12.3%) | 48 (13.8%) |
| Chemotherapy regimen | ||
| Cisplatin and paclitaxel | 310 (90.9%) | 316 (90.5%) |
| Cisplatin and 5-fluorouracil | 31 (9.1%) | 33 (9.5%) |
| PD-L1 expression (CPS) | ||
| CPS <10 | 145 (42.5%) | 148 (42.4%) |
| CPS ≥10 | 196 (57.5%) | 201 (57.6%) |
| PD-L1 expression (TPS) | ||
| TPS <10% | 216 (63.3%) | 225 (64.5%) |
| TPS ≥10% | 125 (36.7%) | 124 (35.5%) |
Percentages might not sum to 100% because of data rounding. Abbreviations: ECOG, Eastern Cooperative Oncology Group; CPS, Combined positive score; TPS, Tumor Proportion Score.
Compliance and completion rates
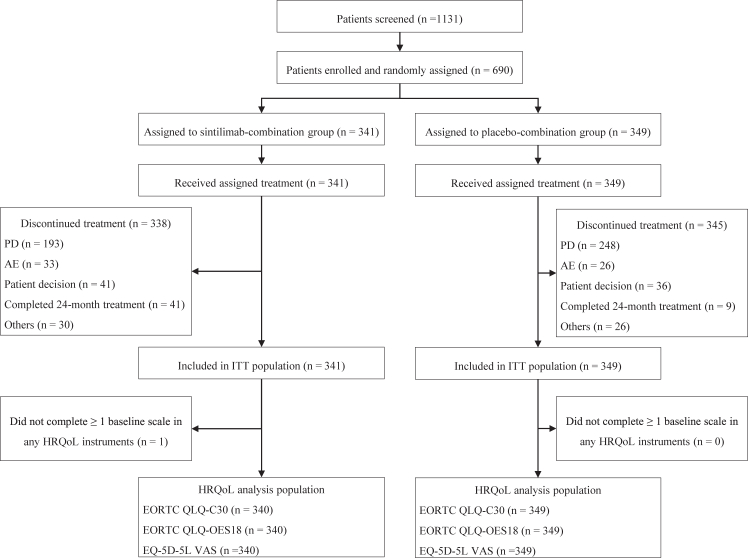
One patient in the sintilimab group did not complete at least one baseline scale of any HRQoL instrument and was excluded from HRQoL analysis. Thus, HRQoL analysis population included 340 patients in the sintilimab group and 349 in the placebo group (Fig. 1). Compliance rates for the EORTC QLQ-C30, EORTC QLQ-OES18, and EQ-5D questionnaires were similar between the sintilimab and placebo groups through 24 weeks post base-line assessment, with rates above 90% (Table 2). Completion rates decreased over time based on treatment discontinuation owing to disease progression or death, but the completion rates for both treatment groups were above 10% at week 60.
Fig. 1.
CONSORT Flow chart of participants. Abbreviations: PD, progressive disease; AE, adverse event; ITT, intention-to-treat.
Table 2.
Compliance and completion rates for the QLQ-C30, QLQ-OES18, and EQ-5D in HRQoL analysis population.
| Time point | Compliance |
Completion |
||
|---|---|---|---|---|
| Sintilimab plus chemotherapy (N = 340) | Placebo plus chemotherapy (N = 349) | Sintilimab plus chemotherapy (N = 340) | Placebo plus chemotherapy (N = 349) | |
| EORTC QLQ-C30 | ||||
| Baseline | 340 (100%) | 349 (100%) | / | / |
| Week 6 | 305/320 (95.3%) | 318/330 (96.4%) | 305 (89.7%) | 318 (91.1%) |
| Week 12 | 283/300 (94.3%) | 272/284 (95.8%) | 283 (83.2%) | 272 (77.9%) |
| Week 18 | 252/266 (94.7%) | 208/230 (90.4%) | 252 (74.1%) | 208 (59.6%) |
| Week 24 | 220/232 (94.8%) | 178/190 (93.7%) | 220 (64.7%) | 178 (51.0%) |
| Week 30 | 175/188 (93.1%) | 139/149 (93.3%) | 175 (51.5%) | 139 (39.8%) |
| Week 36 | 138/145 (95.2%) | 108/110 (98.2%) | 138 (40.6%) | 108 (30.9%) |
| Week 42 | 117/126 (92.9%) | 72/77 (93.5%) | 117 (34.4%) | 72 (20.6%) |
| Week 48 | 110/117 (94.0%) | 53/54 (98.1%) | 110 (32.4%) | 53 (15.2%) |
| Week 60 | 95/103 (92.2%) | 38/39 (97.4%) | 95 (27.9%) | 38 (10.9%) |
| EORTC QLQ-OES18 | ||||
| Baseline | 340 (100%) | 349 (100%) | / | / |
| Week 6 | 305/320 (95.3%) | 318/330 (96.4%) | 305 (89.7%) | 318 (91.1%) |
| Week 12 | 283/300 (94.3%) | 272/284 (95.8%) | 283 (83.2%) | 272 (77.9%) |
| Week 18 | 253/266 (95.1%) | 208/230 (90.4%) | 253 (74.4%) | 208 (59.6%) |
| Week 24 | 219/232 (94.4%) | 178/190 (93.7%) | 219 (64.4%) | 178 (51.0%) |
| Week 30 | 175/188 (93.1%) | 139/149 (93.3%) | 175 (51.5%) | 139 (39.8%) |
| Week 36 | 138/145 (95.2%) | 107/110 (97.3%) | 138 (40.6%) | 107 (30.7%) |
| Week 42 | 117/126 (92.9%) | 71/77 (92.2%) | 117 (34.4%) | 71 (20.3%) |
| Week 48 | 111/117 (94.9%) | 53/54 (98.1%) | 111 (32.6%) | 53 (15.2%) |
| Week 60 | 95/103 (92.2%) | 37/39 (94.9%) | 95 (27.9%) | 37 (10.6%) |
| EQ-5D-5L | ||||
| Baseline | 340 (100%) | 347 (99.4%) | / | / |
| Week 6 | 305/320 (95.3%) | 317/330 (96.1%) | 305 (89.7%) | 317 (90.8%) |
| Week 12 | 282/300 (94.0%) | 272/284 (95.8%) | 282 (82.9%) | 272 (77.9%) |
| Week 18 | 253/266 (95.1%) | 206/230 (89.6%) | 253 (74.4%) | 206 (59.0%) |
| Week 24 | 220/232 (94.8%) | 177/190 (93.2%) | 220 (64.7%) | 177 (50.7%) |
| Week 30 | 174/188 (92.6%) | 139/149 (93.3%) | 174 (51.2%) | 139 (39.8%) |
| Week 36 | 138/145 (95.2%) | 108/110 (98.2%) | 138 (40.6%) | 108 (30.9%) |
| Week 42 | 117/126 (92.9%) | 71/77 (92.2%) | 117 (34.4%) | 71 (20.3%) |
| Week 48 | 111/117 (94.9%) | 53/54 (98.1%) | 111 (32.6%) | 53 (15.2%) |
| Week 60 | 95/103 (92.2%) | 38/39 (97.4%) | 95 (27.9%) | 38 (10.9%) |
HRQoL score changes from baseline
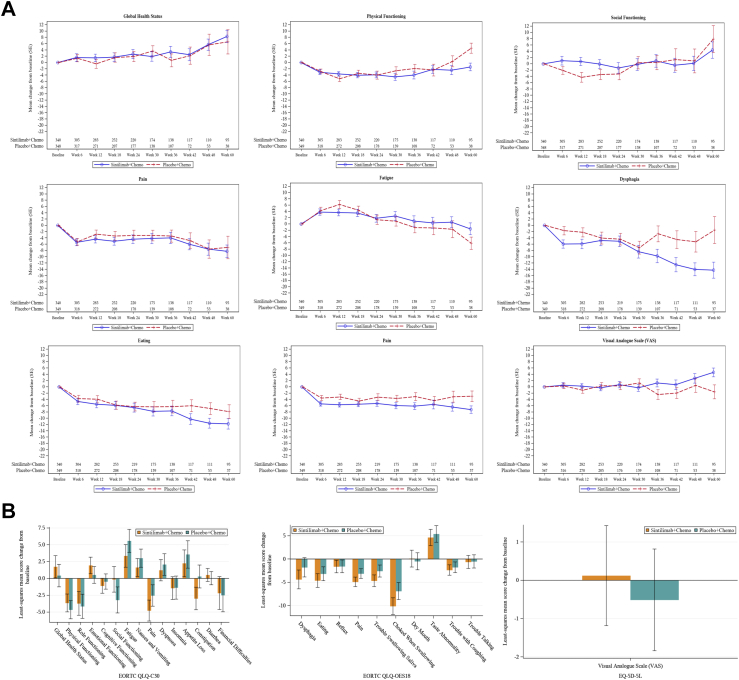
Baseline mean scores for all EORTC QLQ-C30 and QLQ-QES18 functioning and symptom scales and EQ-5D-5L VAS were similar between the sintilimab and placebo groups in the overall HRQoL analysis population (Supplementary Figure S1). For the overall population, mean score changes of QLQ-C30, QLQ-OES18 and EQ-5D-5L scales from baseline to week 60 across the stitilimab group and placebo group were described (Fig. 2A, Supplementary Figure S2). From baseline to week 24, LSM changes of QLQ-C30, QLQ-OES18 and EQ-5D-5L scales in the stitilimab group versus placebo group were compared (Fig. 2B). The HRQoL results for patients with CPS ≥10 were generally consistent with those for the whole population (data not shown).
Fig. 2.
Mean score change from baseline through week 60 in QLQ-C30 scales of GHS, physical functioning, social functioning, pain, and fatigue symptom, QLQ-OES18 scales of dysphagia, eating, and pain, and EQ-5D-5L VAS (A), and least-squares mean differences between treatment groups in all scales of QLQ-C30, QLQ-OES18 and EQ-5D-5L VAS (B).
In QLQ-C30, social functioning was maintained in the sintilimab group (LSM change: −0.14, 95% CI: −2.04 to 1.75) and declined in the placebo group (LSM change: −3.20, 95% CI: −5.14 to −1.27) with significant LSM difference of 3.06 (95% CI: 0.55–5.57; P = 0.0170). Pain symptom showed better improvement in the sintilimab group (LSM change: −4.78, 95% CI: −6.33 to −3.22) than that in the placebo group (LSM change: −2.54, 95% CI: −4.13 to −0.95) with significant LSM difference of −2.24 (95% CI: −4.30 to −0.17; P = 0.0337). Fatigue symptom showed less deterioration in the sintilimab group (LSM change: 3.34, 95% CI: 1.67–5.02) than that in the placebo group (LSM change: 5.58, 95% CI: 3.88–7.29) with significant LSM difference of −2.24 (95% CI: −4.46 to −0.02; P = 0.0479). Constipation was improved in the sintilimab group (LSM change: −2.98, 95% CI: −4.66 to −1.30) and was worsened in the placebo group (LSM change: 0.29, 95% CI: −1.42 to 1.99) with significant LSM difference of −3.27 (95% CI: −5.49 to −1.05; P = 0.0039). For other QLQ-C30 scales including role functioning, cognitive functioning, emotional functioning, dyspnea, insomnia, diarrhea, nausea and vomiting, and appetite loss, and financial difficulties, the mean scores were generally stable through week 24 and LSM changes were similar between the two groups without significant LSM differences (Fig. 2B). GHS was improved in sintilimab group (LSM change: 1.73, 95% CI: 0.08–3.38) and placebo group (LSM change: 0.42, 95% CI: −1.26 to 2.10) without significant LSM difference between treatment groups (1.31, 95% CI: −0.87 to 3.49; P = 0.2376). Physical functioning declined in sintilimab group (LSM change: −3.65, 95% CI: −4.98 to −2.32) and placebo group (LSM change: −4.66, 95% CI: −6.01 to −3.31) without significant LSM difference between treatment groups (1.01, 95% CI: −0.75 to 2.77; P = 0.2614).
In QLQ-OES18, pain symptom showed better improvement in the sintilimab group (LSM change: −4.90, 95% CI: −5.92 to −3.88) versus the placebo group (LSM change: −3.13, 95% CI: −4.16 to −2.10) with significant LSM difference of −1.77 (95% CI: −3.11 to −0.43; P = 0.0097). The sintilimab group also showed favorable changes of trouble swallowing saliva (LSM difference: −2.09, 95% CI: −3.77 to −0.42; P = 0.0146) and choked when swallowing (LSM difference: −3.23, 95% CI: −5.60 to −0.86; P = 0.0076) versus the placebo group (Fig. 2B). Similar score changes were observed between treatment groups without significant LSM differences for other QLQ-OES18 scales, including dysphagia (LSM difference: −2.59, 95% CI: −5.28 to 0.10, P = 0.0588), eating problem (LSM difference: −1.45, 95% CI: −3.39 to 0.50, P = 0.1438), reflux, dry mouth, taste abnormality, trouble with coughing, and trouble talking (Fig. 2B and Supplementary Figure S2).
VAS of the EQ-5D-5L increased slightly from baseline in the sintilimab group while declined in the placebo group, but the LSM difference (0.63, 95% CI: −1.09 to 2.36, P = 0.4720, Fig. 2B) not exceeded significant threshold.
During sensitivity analysis (Supplementary Tables S1–S3), generally consistent results were observed, with slightly differences in fatigue (LSM difference changed from −2.24 to −2.07, P-value changed from 0.0479 to 0.0751) and pain (LSM difference changed from −2.24 to −1.99, P-value changed from 0.0337 to 0.0599) of QLQ-C30.
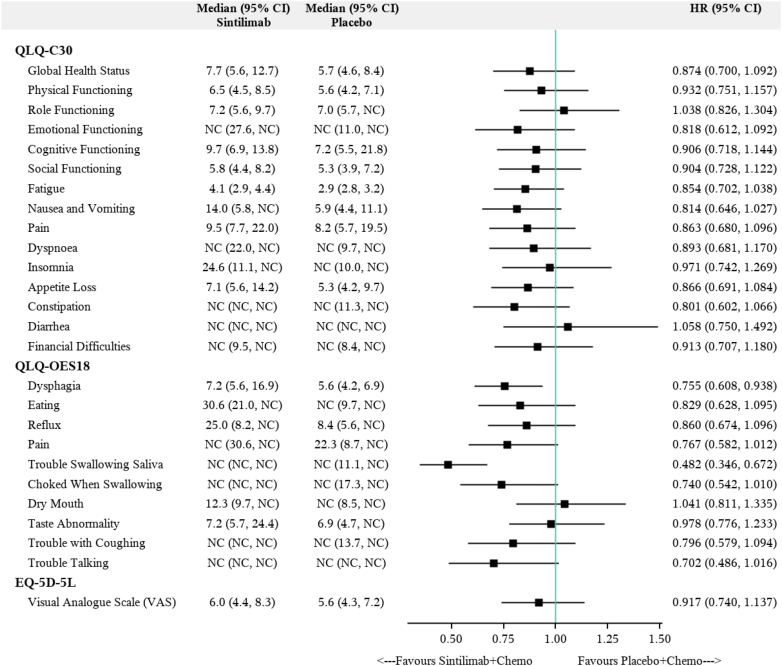
Time to first deterioration
For QLQ-OES18 scales (Fig. 3), patients in the sintilimab group had a significantly decreased risk of deterioration in dysphagia (median TTD: 7.2 months versus 5.6 months; HR: 0.76, 95% CI: 0.61–0.94, P = 0.0104), and trouble swallowing saliva (median TTD: NC [not calculated] versus NC; HR: 0.48, 95% CI: 0.35–0.67, P < 0.0001) compared with patients in the placebo group. TTD to other QLQ-OES18 scales showed no difference between treatment groups, although patients in the sintilimab group showed numerically longer TTD for symptoms including eating problem (median TTD: 30.6 months versus NC months; HR: 0.83, 95% CI: 0.63–1.095, P = 0.1838), pain (median TTD: NC months versus 22.3 months; HR: 0.77, 95% CI: 0.58–1.01, P = 0.0591), reflux (median TTD: 25.0 months versus 8.4 months; HR: 0.86, 95% CI: 0.67–1.10, P = 0.2183).
Fig. 3.
Forest plot for time to first deterioration in QLQ-C30, QLQ-QES18, and EQ-5D-5L VAS. Abbreviations: Chemo, chemotherapy; HR, hazard ratio; NC, not calculated.
For QLQ-C30 scales, no significant statistical difference was observed in TTD between the sintilimab and placebo groups. Numerically longer TTD to most QLQ-C30 scales (Fig. 3) were observed in the sintilimab group including global health status (median TTD: 7.7 months versus 5.7 months; HR: 0.87, 95% CI: 0.70–1.09; P = 0.2320), physical functioning (median TTD: 6.5 months versus 5.6 months; HR: 0.93, 95% CI: 0.75–1.16; P = 0.5197), social functioning (median TTD: 5.8 months versus 5.3 months; HR: 0.90, 95% CI: 0.73–1.12; P = 0.3550), pain (median TTD: 9.5 months versus 8.2 months; HR: 0.86, 95% CI: 0.68–1.10, P = 0.2246), fatigue (median TTD: 4.1 months versus 2.9 months; HR: 0.85, 95% CI: 0.70–1.04, P = 0.1074), and nausea and vomiting (median TTD: 14.0 months versus 5.9 months; HR: 0.81, 95% CI: 0.65–1.03, P = 0.0800). Median TTD to EQ-5D-5L VAS was also similar between the sintilimab and placebo groups (Fig. 3).
From week 6 to week 24, proportion of patients with improved QLQ-C30 and QLQ OES-18 status were generally increased or remained in most scales. At week 24, there were small differences in distribution of improved, stable, and deteriorated status on the QLQ-C30 functioning and symptom scales and QLQ OES-18 symptom scales between the two groups (Supplementary Figure S3).
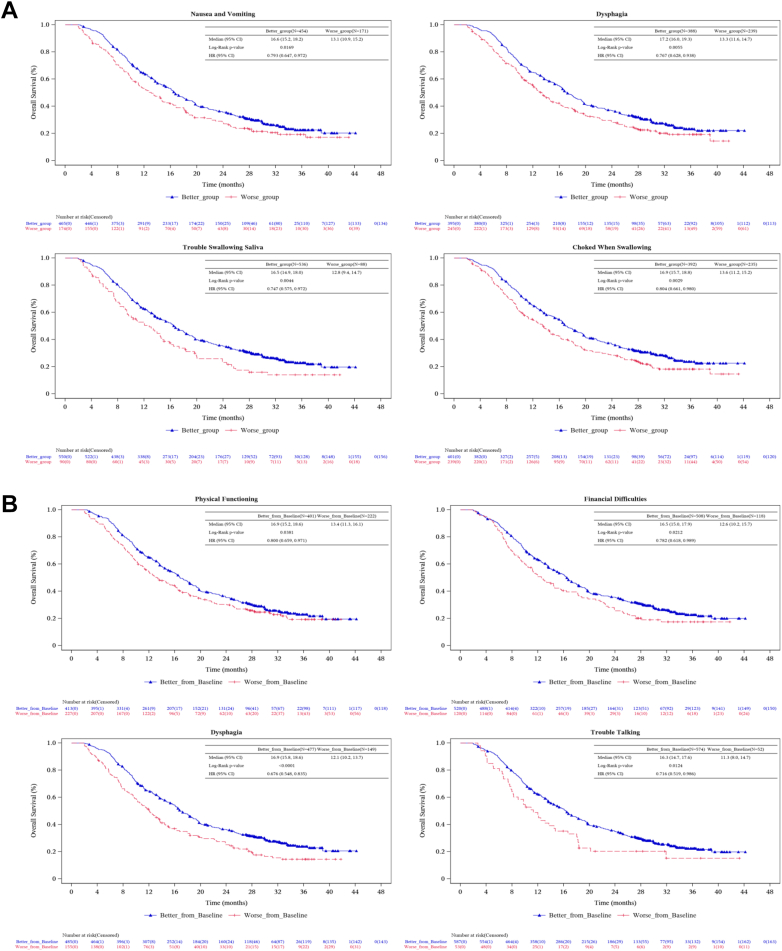
HRQoL results and OS
The OS analysis were performed in patients with different performance of HRQoL (Fig. 4). At week 6, HRQoL scores of each patient were compared with the median value of all patients to define the better group and the worse group. For QLQ-C30 scales, significant improvement of OS was observed in patients with better performance in nausea and vomiting (median OS: 16.6 versus 13.1 months, HR: 0.79, 95% CI: 0.65–0.97, P = 0.0257). For QLQ-OES18 scales, significant improvements of OS were observed in patients with better performance in dysphagia (median OS: 17.2 versus 13.3 months, HR: 0.77, 95% CI: 0.63–0.94, P = 0.0096), trouble swallowing saliva (median OS: 16.5 versus 12.8 months, HR: 0.75, 95% CI: 0.57–0.97, P = 0.0296) and choked when swallowing (median OS: 16.9 versus 13.6 months, HR: 0.80, 95% CI: 0.66–0.98, P = 0.0304).
Fig. 4.
Kaplan–Meier plot of OS in patients with better and worse HRQoL performance at week 6 (A) and with better or worse change of HRQoL performance from baseline to week 6 (B) for QLQ-C30 and QLQ-OES18.
In addition, week 6 HRQoL scores of each patient were compared with baseline to define the better change group and the worse change group. For QLQ-C30 scales, significant improvements of OS were observed in patients with better change in physical functioning (median OS: 16.9 versus 13.4 months, HR: 0.80, 95% CI: 0.66–0.97, P = 0.0241) and financial difficulties (median OS: 16.5 versus 12.6 months, HR: 0.78, 95% CI: 0.62–0.99, P = 0.0402). For QLQ-OES18 scales, significant improvements of OS were observed in patients with better change in dysphagia (median OS: 16.9 versus 12.1 months, HR: 0.68, 95% CI: 0.55–0.83, P = 0.0002) and trouble talking (median OS: 16.3 versus 11.3 months, HR: 0.72, 95% CI: 0.52–0.99, P = 0.0409).
Discussion
Given the heavy disease burden of ESCC in the Asian population and worsening HRQoL in this patient population, treatment regimens that prolong survival while maintain or improve HRQoL are in high demand for patients with advanced ESCC. In the ORIENT-15 study, patients with advanced ESCC receiving first-line sintilimab plus chemotherapy generally experienced stable or improved HRQoL outcomes and lower risk of deterioration, in addition to an improved overall survival benefit,8 compared with those receiving chemotherapy alone.
Generally, the present HRQoL findings favored the sintilimab group versus the placebo group as evidenced by better scores or trends across most EORTC QLQ-C30 and OES-18 scales in sintilimab-chemotherapy-treated patients versus placebo-chemotherapy-treated patients based on a between-group LSM difference at week 24. Particularly, the sintilimab group had significantly better QLQ-C30 functioning and symptom scores for social functioning, fatigue, pain, and constipation and esophageal cancer-specific symptom scores for pain, trouble swallowing saliva, and choked when swallowing versus the placebo group. These improvements are clinically meaningful considering the worsening of these domains would greatly affect the quality of life of patients with ESCC. Additionally, the decreased risk of TTD for almost all EORTC scales (except for role functioning, diarrhea, and dry mouth) observed in the sintilimab group versus the placebo group supported a trend of a delay in deterioration in the sintilimab group, although the upper 95% CI of the HR for TTD in most of these scales crossed 1. These HRQoL results were internally consistent, as supported by the scale analysis across the QLQ-C30 and QLQ-OES-18 questionnaires and the analysis for the same scale in two different ways. In ESCC patients, nutritional status and quality of life were known to closely associated with OS.4,5 To further evaluate clinical benefits of HRQoL, we performed OS analysis in patients with better and worse performance in HRQoL. Patients with better performance in several functioning and symptom scales of QLQ-C30 and QLQ-QES18 showed improved OS compared with the worse group. Notably, QLQ-OES18 trouble swallowing saliva was consistently significant during LSM, TTD and OS analysis. Meanwhile, QLQ-OES18 choked when swallowing was consistently significant during LSM and OS analysis, and QLQ-OES18 dysphagia was consistently significant during TTD and OS analysis. Taken those results together, sintilimab plus chemotherapy could provide not only a longer overall survival8 but also maintained/improved HRQoL compared with placebo plus chemotherapy for patients with advanced ESCC.
The present HRQoL results showed consistency with the anti-tumor efficacy and safety profile of the treatment regimens on the study population. A decreased risk of deterioration in dysphagia was observed for sintilimab versus placebo, corresponding to the higher objective tumor response rate observed with the sintilimab group.8 Fatigue was a common symptom for target patient population. The sintilimab group showed better symptom score for fatigue compared with the placebo group, which presumably had association with the lower incidence of grade ≥3 adverse event of decreased white cell count observed in the sintilimab group (17.4% versus 22.3%).8
Recent clinical trials have reported maintenance or improvements in HRQoL in advanced ESCC patients treated with a PD-(L)1 inhibitor plus chemotherapy versus chemotherapy alone. In the double-blind, phase 3 KEYNOTE-590 study of patients with advanced esophageal cancer or esophagogastric junction adenocarcinoma, HRQoL was stable and similar in QLQ-C30 GHS, QLQ-OES18 reflux and dysphagia, and EQ-5D-5L VAS and was better in QLQ-OES18 pain over 18 weeks in the pembrolizumab plus chemotherapy group versus the chemotherapy alone group.13 In the open-label, phase 3 CheckMate 648 study, which assessed HRQoL using Functional Assessment of Cancer Therapy-Esophageal (FACT-E) and EQ-5D-3L, it showed trends towards better HRQoL and decreased risk of deterioration with nivolumab plus chemotherapy versus chemotherapy alone.11 The double-blind, China-only, phase 3 ESCORT-1st study reported HRQoL assessment up to 36 weeks after initial treatment and the results favored the camrelizumab-chemotherapy group for QLQ-C30 GHS and pain, and QLQ-OES18 symptoms of eating, trouble swallowing saliva, and choked when swallowing.12 However these trials only reported some domains of HRQoL with a relatively short follow-up. Considering the between-group difference in some HRQoL domains may not be captured with a shorter-term follow-up, a complete HRQoL evaluation with a longer-term follow-up would better inform clinical decision making. The ORIENT-15 study enrolled patients from China and other regions and provided a full report on HRQoL after a median of 32 months follow-up. The HRQoL benefit observed with the sintilimab group versus the placebo group provided solid evidence on the effect of first-line PD-1 inhibitor in combination with chemotherapy on HRQoL in patients with advanced ESCC.
To the best of our knowledge, this is the first study of HRQoL exclusively in patients with advanced ESCC in a double-blind, phase 3 study evaluating the first-line PD-1 inhibitor plus chemotherapy. The randomized and double-blind design reduced bias in patient-reported outcomes compared with an open-label design which may introduce bias due to knowledge of their assigned treatment. Additionally, this study gave a full report on all C30, ES18 and EQ-5D-5L scales after a long-term follow-up. Although no formal statistical analysis was performed, the general trends in HRQoL observed from baseline to week 24 remained consistent through week 60, especially with continuous improvement of esophageal cancer-specific symptoms, indicating stable HRQoL over time.
Some limitations of our study should be noted when interpreting the results. Given the protocol and statistical analysis plan did not prespecify specific HRQoL endpoints, all HRQoL outcomes were not subject to statistical testing hierarchy, thus the statistical analysis results should be considered as descriptive although nominal P values for certain comparison were provided. In addition, symptoms of HRQoL scales were reported by patients through a semiquantitative approach while most symptoms may not require additional clinical interventions. Despite significant differences were observed at statistical level in our study, their clinical significance should be interpreted with caution. A study suggested cutoffs of LSM differences ranging from 9 to 19 for different QLQ-C30 scales to define their clinical effects.23 Further clinical investigations are warranted to evaluate HRQoL in clinical perspectives.
To conclude, HRQoL data from ORIENT-15 study added to the limited body of evidence on QoL in patients with advanced ESCC receiving first-line PD-1 inhibitor in combination with chemotherapy. This study showed that compared with chemotherapy alone, patients with sintilimab plus chemotherapy had statistically significant differences of several HRQoL scales and improvements in delayed deterioration. Together with the observed OS benefit and safety profile with sintilimab plus chemotherapy, the findings in this study supported this combination regimen as first-line treatment for patients with advanced ESCC.
Contributors
Zlu and LS contributed to study supervision. ZLu, LK, BW and LS contributed to study design. LK and BW contributed equally. ZLu, LK, BW, JW, LL, YS, LY, GS, GC, YJ, TC, HLiu, WQ, NL, GLi, HLuo, XH, YZ, WY, LX, ZLiu, YP, SG, XW, ZP, SZ, GLin, YX, KG, TR, WL, TL, SF, WH, YF, JL and BX contributed to data collection. ZLu, LZ and SW contributed to data analysis. ZLu, LK, BW, LZ, SW and LS contributed to data interpretation. ZLu, LK and BW contributed to manuscript drafting. All authors contributed to manuscript revision. ZLu, LK, BW, LZ, SW and LS has directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
Reasonable requests for data sharing should be made to the corresponding author and will be handled in line with the data access and sharing policy of Human Genetic Resource Administration of China.
Declaration of interests
All authors reports receiving support from Innovent Biologics and Eli Lilly for the submitted work. LS reports receiving research funding from Innovent Biologics, Beijing Xiantong Biomedical Technology, Qilu Pharmaceutical, ZaiLab Pharmaceutical, Beihai Kangcheng (Beijing) Medical Technology, and Jacobio Pharmaceuticals; consultant fees from MSD, Merck, Mingji Biopharmaceutical, Haichuang Pharmaceutical, Herbour Biomed, and BI; honoraria from Hutchison Whampoa, Hengrui, ZaiLab, and CSTONE pharmaceutical; and serving as a consultant for Rongchang Pharmaceutical, ZaiLab, CSTONE Pharmaceutical, and BMS.
Acknowledgements
The authors thank all the patients who participated in this trial. Medical writing assistance was provided by Xiaoyun Wang and Yuxi Zhou from Innovent Biologics, Inc.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102623.
Appendix A. Supplementary data
References
- 1.Arnold M., Ferlay J., van Berge Henegouwen M.I., et al. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 2.Smyth E.C., Lagergren J., Fitzgerald R.C., et al. Oesophageal cancer. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunde B., Lindblad M., Malmström M., et al. Health-related quality of life one year after the diagnosis of oesophageal cancer: a population-based study from the Swedish National Registry for Oesophageal and Gastric Cancer. BMC Cancer. 2021;21:1277. doi: 10.1186/s12885-021-09007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kleef J.J., Dijksterhuis W.P.M., van den Boorn H.G., et al. Prognostic value of patient-reported quality of life for survival in oesophagogastric cancer: analysis from the population-based POCOP study. Gastric Cancer. 2021;24:1203–1212. doi: 10.1007/s10120-021-01209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ter Veer E., van Kleef J.J., Schokker S., et al. Prognostic and predictive factors for overall survival in metastatic oesophagogastric cancer: a systematic review and meta-analysis. Eur J Cancer. 2018;103:214–226. doi: 10.1016/j.ejca.2018.07.132. [DOI] [PubMed] [Google Scholar]
- 6.Shen L., Kato K., Kim S.-B., et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol. 2022;40:3065–3076. doi: 10.1200/JCO.21.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doki Y., Ajani J.A., Kato K., et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 8.Lu Z., Wang J., Shu Y., et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377 doi: 10.1136/bmj-2021-068714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J.-M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.-X., Cui C., Yao J., et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40:277–288.e3. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Bridgewater J.A., Chau I., Gricar J., et al. Health-related quality of life (HRQoL) in patients (pts) with advanced esophageal squamous cell carcinoma (ESCC) treated with nivolumab (N) plus chemotherapy (CT) or nivo plus ipilimumab (I) versus chemo: results from CheckMate 648. J Clin Oncol. 2022;40:262. [Google Scholar]
- 12.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansoor W., Kulkarni A.S., Kato K., et al. Health-related quality of life (HRQoL) of pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase III KEYNOTE-590 study. J Clin Oncol. 2021;39:168. [Google Scholar]
- 14.Van Cutsem E., Kato K., Ajani J., et al. Tislelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): impact on health-related quality of life. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adenis A., Kulkarni A.S., Girotto G.C., et al. Impact of pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer on health-related quality of life in KEYNOTE-181. J Clin Oncol. 2022;40:382–391. doi: 10.1200/JCO.21.00601. [DOI] [PubMed] [Google Scholar]
- 16.Moehler M., Xiao H., Blum S.I., et al. Health-related quality of life with nivolumab plus chemotherapy versus chemotherapy in patients with advanced gastric/gastroesophageal junction cancer or esophageal adenocarcinoma from CheckMate 649. J Clin Oncol. 2023;41:5388–5399. doi: 10.1200/JCO.23.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Z., Wang J., Shu Y., et al. Abstract CT075: updated overall survival outcomes from a randomized, double-blind phase III study of sintilimab versus placebo in combination with chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma (ORIENT-15) Cancer Res. 2023;83:CT075. [Google Scholar]
- 18.Pickard A.S., De Leon M.C., Kohlmann T., et al. Psychometric comparison of the standard EQ-5D to a 5 level version in cancer patients. Med Care. 2007;45:259–263. doi: 10.1097/01.mlr.0000254515.63841.81. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 20.Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 1990. 2003;39:1384–1394. doi: 10.1016/s0959-8049(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 21.Quality of life of cancer patients. EORTC – Quality of Life; 2017. https://qol.eortc.org/questionnaire/eortc-qlq-c30/ [cited 2024 Feb 20] Available from: [Google Scholar]
- 22.Oesophageal. EORTC – Quality of Life; 2018. https://qol.eortc.org/questionnaire/qlq-oes18/ [cited 2024 Feb 20] Available from: [Google Scholar]
- 23.Cocks K., King M.T., Velikova G., et al. Evidence-based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire Core 30. J Clin Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.