Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Many medical conditions, including hypertension, diabetes, obesity, sleep apnea, and heart failure (HF), increase the risk for AF. Cardiomyocytes have unique metabolic characteristics to maintain adenosine triphosphate production. Significant changes occur in myocardial metabolism in AF. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been used to control blood glucose fluctuations and weight in the treatment of type 2 diabetes mellitus (T2DM) and obesity. GLP-1RAs have also been shown to reduce oxidative stress, inflammation, autonomic nervous system modulation, and mitochondrial function. This article reviews the changes in metabolic characteristics in cardiomyocytes in AF. Although the clinical trial outcomes are unsatisfactory, the findings demonstrate that GLP-1 RAs can improve myocardial metabolism in the presence of various risk factors, lowering the incidence of AF.
Keywords: Atrial fibrillation, Glucagon-like peptide-1 receptor agonists, Metabolism
Graphical abstract
Highlights
-
•
AF risk is linked to metabolic disorders like diabetes and obesity.
-
•
GLP-1RAs may have a role in improving myocardial metabolism and reducing AF risk.
-
•
More studies are needed to fully understand the effects of GLP-1RAs.
-
•
GLP-1RAs can improve myocardial metabolism, reducing the risk rate of AF.
-
•
GLP-1RAs may offer a promising avenue for AF prevention and treatment.
1. Introduction
Atrial fibrillation (AF) is the most common supraventricular arrhythmia. AF is characterized by disorganized electrical activities and ineffective contraction of the atrium [1]. The lifetime risk of AF for men and women ≥40 years old is approximately 25% [2] and increases with age. Currently, pharmacological therapy for AF is of limited value and has a high recurrence rate [3].
Glucagon-like peptide-1 (GLP-1) is a peptide hormone secreted by pancreatic islets that targets the GLP-1 receptor. It was initially identified for its hypoglycemic effects, stemming from the presence of GLP-1 receptors in pancreatic islet α, β, and δ cells. Moreover, the existence of GLP-1 receptors in myocardial cells highlights its cardiovascular influences. Notably, synthetic GLP-1, compared to its natural form, offers resistance to dipeptidyl peptidase 4 (DPP-4) degradation, which translates to a protracted half-life [4].
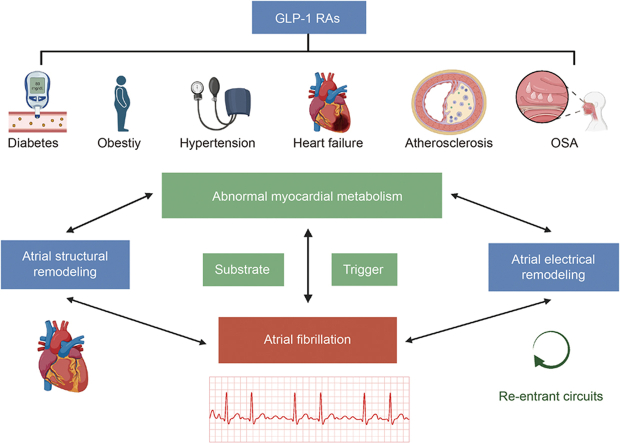
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), including liraglutide, exenatide, dulaglutide, semaglutide, and albiglutide, have been used for blood glucose control in patients with obesity and type II diabetes mellitus (T2DM) [5,6]. Interestingly, GLP-1 RAs can also reduce major adverse cardiovascular events (MACEs), such as cardiovascular death, heart failure (HF), myocardial infarction, and stroke [7]. Recent advances underscore the potential of GLP-1 RAs not only as hypoglycemic agents but also for their therapeutic promise in AF. Emerging data point to GLP-1 RAs being pivotal in moderating atrial electrical and structural remodeling, largely influenced by their action on myocardial metabolism [8]. The core objectives of this study are twofold: first, to unravel the complex interactions between GLP-1 RAs and myocardial metabolism, and second, to investigate their potential implications in the context of AF. Stemming from these objectives, our central hypothesis posits that GLP-1 RAs have the potential to exert protective effects on atrial remodeling, thereby possibly reducing the incidence or severity of AF. Given the emerging evidence, it is imperative to explore the potential of GLP-1 RAs in mediating atrial electrical and structural changes through the modulation of myocardial metabolism. This review endeavors to delve deeply into this potential and critically assess the extent to which GLP-1 RAs can influence the dynamics of AF.
2. Myocardial metabolism and AF
2.1. Energy metabolism in normal myocardium
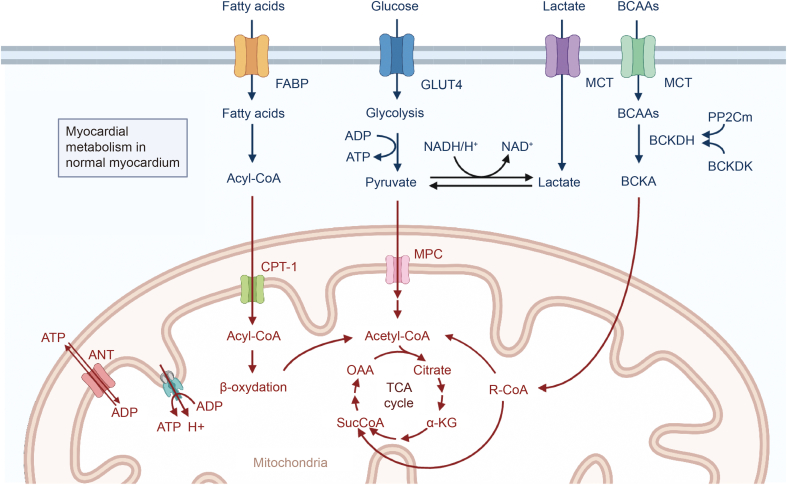
Rhythmic cardiac contractions and relaxations consume large amounts of adenosine triphosphate (ATP). In cardiomyocytes, 95% of ATP is produced by mitochondrial oxidative phosphorylation, and 5% is produced by glycolysis [9]. The myocardium uses fatty acids (FAs), glucose, lactic acid, ketone bodies, and amino acids (AAs) to maintain ATP production, as shown in Fig. 1.
Fig. 1.
Energy metabolism in normal myocardium. In general, the myocardium uses fatty acids (FAs), glucose, lactic acid, ketone bodies, and amino acids (AAs) to maintain adenosine triphosphate (ATP) production. BCAAs: branched chain amino acids; FABP: fatty acid-binding protein; GLUT: glucose transporter; MCT: monocarboxylate transporter; ADP: adenosine diphosphate; NADH: nicotinamide adenine dinucleotide; NAD: nicotinamide adenine dinucleotide; BCKDH: branched chain keto acid dehydrogenase; PP2Cm: protein phosphatase 2C family member; BCKDK: BCKDH kinase; BCKA: branched-chain α-ketoacid; ANT: adenine nucleotide translocator; CPT: carnitine palmitoyl transferase; MPC: mitochondrial pyruvate carrier; OAA: oxaloacetic acid; TCA: tricarboxylic acid cycle; α-KG: α-ketoglutaric acid.
In a healthy heart, FAs provide 40%–60% of the energy needs for normal cardiac activities. FAs are esterified to form acyl-CoA in cardiomyocytes and converted to carnitine by carnitine palmitoyl transferase 1 (CPT-1). Acylated carnitine is then transported to mitochondria, where CoA is converted to fatty acyl-CoA again by transferring the FA group. When acyl-CoA undergoes oxidation, acetyl-CoA, flavin adenine dinucleotide (FADH2), and nicotinamide adenine dinucleotide (NADH) are produced. Acetyl-CoA is an important part of the tricarboxylic acid (TCA) cycle, while FADH2 and NADH enter the electron transport chain and convert adenosine diphosphate (ADP) to ATP. Although FAs can produce the most ATP per two carbon units among all energy substrates, ATP generation from FAs also consumes the most oxygen and is thus not energy efficient in the myocardium [10].
Carbohydrates, including glucose and lactic acid, are also important energy substrates for the heart. Under normal conditions, 2%–8% of ATP comes from glucose, and 10%–15% comes from lactic acid. Glucose can produce ATP through cytoplasmic glycolysis and mitochondrial oxidation of pyruvate, which is considered as the most efficient substrate because glycolysis from glucose to pyruvate produces ATP without oxygen. Glucose enters cardiomyocytes via glucose transporter proteins (GLUT1 and GLUT4) and is phosphorylated by hexokinase to produce glucose-6-phosphate (G6P), which can be utilized in glycolysis [11]. Pyruvate generated during glycolysis can be converted into lactate or transported to mitochondria through the mitochondrial pyruvate carrier (MPC). A large portion of pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase, and acetyl-CoA is further metabolized in the TCA cycle. Additionally, lactic acid enters cardiomyocytes through the unitary anion transporter when the concentration of circulating lactic acid increases and becomes an important energy substrate for the heart. Lactate may be the main source of pyruvate in the heart [12].
Although the main source of ATP is the oxidation of mitochondrial FAs and carbohydrates, the oxidation of ketone bodies and branched chain amino acids (BCAAs) can also contribute to energy production [13]. Acetyl-CoA from the β-oxidation of FA can be the substrate for ketone body formation by ketone body synthase in liver mitochondria. In the heart, β-hydroxybutyric acid is the primary ketone body that can be transformed to acetoacetic acid by β-hydroxybutyrate dehydrogenase 1 and then converted to acetyl-CoA. The oxidation of ketone bodies is more energy efficient than that of FAs and glucose with less oxygen consumption. ATP can also be generated from the oxidation of AAs [14]. BCAAs are first metabolized to branched chain keto acids (BCKAs) in the heart by mitochondrial branched chain aminotransferase (BCATm) with the transfer of α-amino groups to α-ketoglutaric acid, leading to the formation of glutamic acid. BCAAs can be oxidatively decarboxylated by mitochondrial branched chain keto acid dehydrogenase (BCKDH), producing either acetyl-CoA during the TCA cycle or succinyl-CoA during the complement cycle.
2.2. Myocardial metabolism in AF
Abnormalities in myocardial metabolism can set the stage for atrial electrical and structural remodeling, serving as potential catalysts for AF. The findings underscore the central role of myocardial metabolism shifts as potential therapeutic targets in AF. Delving deeper into the therapeutic mechanisms of GLP-1 RAs, it has been uncovered that GLP-1 RAs have multifaceted effects on myocardial metabolism. How GLP-1 RAs could pave the way for innovative AF treatments will be highlighted.
2.2.1. Substrate metabolism
2.2.1.1. FA metabolism
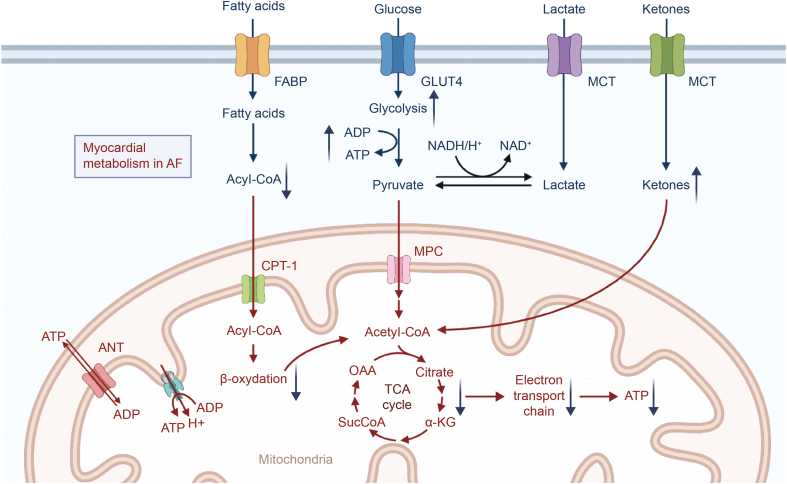
During AF, the efficiency of FA oxidation decreases significantly. The energy metabolism of the myocardium switches to the “embryonic metabolism mode” with glucose as the main energy source to compensate for decreased FA oxidation [15] (Fig. 2). Calcium/calmodulin-dependent protein kinase II (CaMKII) and adenosine monophosphate (AMP)-activated protein kinase (AMPK) are upregulated, and the expression of fatty acid translocase (FAT/CD36) is also increased [16]. FA metabolism imbalance contributes to the progression of AF [17]. A decrease in lipid metabolism in AF may help maintain the periodic response of TCA [18]. Chronic AF patients have increased levels of FA binding protein 3 (FABP3). Four genes (ITGB1, HSP90AA1, CCND1, and HSPA8) are considered to be critically associated with significant changes in lipid biosynthesis in AF [19].
Fig. 2.
Myocardial metabolism in atrial fibrillation (AF). Glucose metabolism was significantly enhanced to compensate for fatty acid (FA) oxidation. Mitochondrial function matters in many pathophysiological processes of AF. FABP: fatty acid-binding protein; GLUT: glucose transporter; MCT: monocarboxylate transporter; ADP: adenosine diphosphate; ATP: adenosine triphosphate; NADH: nicotinamide adenine dinucleotide; NAD: nicotinamide adenine dinucleotide; ANT: adenine nucleotide translocator; CPT: carnitine palmitoyl transferase; MPC: mitochondrial pyruvate carrier; OAA: oxaloacetic acid; TCA: tricarboxylic acid cycle; α-KG: α-ketoglutaric acid.
2.2.1.2. Glucose metabolism
Glucose uptake in atrial muscle decreases, while its metabolism is increased in AF. Metabolic and proteomic analyses have revealed that FA synthesis and oxidation, the TCA cycle, and AA transport and metabolism are decreased, while glycolysis is increased in a nonpersistent atrial pacing sheep model. As a result of irregular pacing, the intracellular Ca2+ concentration during the diastolic phase increases with activation of CaMKII and AMPK, resulting in lipid accumulation, decreased glucose uptake, increased glycogen synthesis, and subsequent enhancement of proapoptotic signaling pathways. Decreases in the expression of peroxisome proliferator-activated receptor (PPAR) α, p-sirtuin1, PPAR coactivator (PGC)-1α, mitochondrial carnitine palmitoyltransferase 1 (mCPT-1), medium-chain acyl-CoA dehydrogenase, and GLUT4 in patients with AF are consistent with a decrease in glucose uptake [20]. Metabolic remodeling with increased glucose metabolism helps maintain the periodic TCA response in AF.
2.2.1.3. Ketone body metabolism
Ketone body metabolism in atrial arrhythmias is poorly understood. The heart tissue of patients with persistent AF has increased levels of β-hydroxybutyric acid (the main substrate of ketone body metabolism), ketogenic AAs, and glycine. 3-Oxotransferase is the key enzyme for ketolytic energy production and is differentially expressed in the ear tissues of human subjects in sinus rhythm and in AF. Acute perfusion of β-hydroxybutyric acid can increase the oxidation rate of myocardial ketone bodies without changing the oxidation rate of glucose or FA, indicating that ketones may be an important source of heart fuel during hunger or on a ketogenic diet or in poorly controlled diabetes [21].
2.2.2. Mitochondrial metabolism
Mitochondria are central to the function of cardiomyocytes. AF is associated with many pathophysiological changes in mitochondria [22]. Data using atrial biopsies in patients with AF have shown mitochondrial dysfunction, as reflected by abnormal ATP levels, upregulation of mitochondrial stress chaperone proteins, and mitochondrial network fragmentation [23].
Reactive oxygen species (ROS) are critically involved in the development and progression of AF [24]. As atrial remodeling occurs during AF, the sources of ROS change from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated metabolism to mitochondrial and endothelin nitric oxide synthase (eNOS)-derived ROS [25], and mitochondrial ROS are associated with cardiac fibrosis [26]. Patients with permanent AF have decreased expression of antioxidant-related genes and increased expression of genes related to ROS production. The expression of ROS-related proteins, including transforming growth factor-β (TGF-β), anti-xanthine oxidase, p22-Phox, and NADPH oxidase (NOX2 and NOX4), is significantly increased [27]. The imbalance between ROS elimination and production in AF leads to morphological and functional changes in human cardiomyocytes, triggering a vicious cycle of oxidation.
2.3. Ways to improve myocardial energy metabolism
Table 1 summarizes the possible strategies for enhancing myocardial energy metabolism.
Table 1.
Measures to improve myocardial energy metabolism.
| FA metabolism regulation | Glucose metabolism promotion | Mitochondrial metabolism regulation |
|---|---|---|
| The activity of AMPK/PGC-1α/PPARα pathway ↑ | PI3K/Akt/eNOS pathway ↑ | Calcium imbalance ↓ |
| The oxidation of defective FAs ↑ | AMPK ↑ | Mfn2 ↓ |
| Autophagy ↑ | The production of ketone bodies ↑ | Oxidative stress ↓ |
| Regulating PPARγ signal pathways | Controlling blood glucose level | The production of intracellular ROS ↓ |
| Propionic acid/FFAR3 signals ↓ | Cardiac sodium channel NaV1.5 ↓ | Apoptosis ↓ |
| Fat secretion of ceramides ↓ | The insulin/glucagon ratio ↓ | RAAS pathway ↓ |
| NF-κB pathway ↓ | PGC-1α/NRF-1/Tfam pathway ↓ | |
| Adipose tissue hypertrophy ↓ | Protein disorders ↓ |
FA: fatty acid; AMPK: adenosine monophosphate (AMP)-activated protein kinase; PGC-1α: peroxisome proliferator-activated receptor (PPAR) coactivator 1α; FFAR: free fatty acid receptor; NF-κB: nuclear factor kappaB; PI3K: phosphatidylinositide 3-kinases; eNOS: endothelin nictric oxide synthase; NRF-1: nuclearrespiratoryfactor1; Tfam: mitochondrial transcription factor A; Mfn: mitochondrial fusion protein; ROS: reactive oxygen species; RAAS: renal-angiotensin-aldosterone system.
2.3.1. Modifications of myocardial FA metabolism
Fenofibrate can significantly restore the activity of the PPAR-α/sirtuin1/PGC-1α pathway, prevent adverse changes in circulating metabolites, reduce adenine nucleotide levels and glycogen and lipid droplet accumulation, reverse atrial effective refractory period shortening, and reduce the risk of AF. Metformin, an AMPK activator, reduces lipid deposition in the left atrium by restoring the activity of the AMPK/PGC-1α/PPARα pathway [28]. l-carnitine can promote the defective oxidation of FAs, thus activating the AMPK signaling pathway and reducing atrial lipid toxicity to reduce AF and structural remodeling due to obesity [29].
Ceramide from fat tissue is an important regulator of the vascular redox state and a potential target to reduce the risk of AF [30]. The accumulation of ceramide promotes adipogenesis and insulin resistance, resulting in low-grade inflammation [31]. In patients with chronic AF, the expression of FABP3 is upregulated along with autophagy-related genes, while there is no change in the level of a toxic FA metabolite, atrial diglyceride (diacylglycerol), suggesting that autophagy may provide cardioprotection against cardiac lipotoxicity in chronic AF. Risk modification of AF can be achieved with agents (such as omega-3 FAs or statins) that reduce oxidative stress and/or prevent atrial fibrosis [[32], [33], [34]]. G-protein signal transduction regulator-4 protects the heart against inflammation and adverse remodeling as well as sympathetic neurolysis by attenuating propionic acid/free fatty acid receptor (FFAR3) signals in cardiomyocytes and sympathetic neurons [35]. DPP-4 inhibitors may delay the development of AF by inhibiting adipose tissue hypertrophy [36].
2.3.2. Promotion of favorable myocardial glucose metabolism
Metformin can reverse the Warburg effect of chronic AF by activating AMPK, thus attenuating atrial remodeling, reducing susceptibility to AF, and maintaining gap junctions during pacing [37]. Sodium-glucose transporter 2 inhibitor (SGLT2i) is a new hypoglycemic drug. SGLT2i treatment increases the clearance of glucose and decreases the insulin/glucagon ratio, thus contributing to lipid mobilization and oxidation in the liver [38] and stimulating the production of ketone bodies [39], leading to favorable energy metabolism, including ketone body oxidation.
2.3.3. Regulation of myocardial mitochondrial metabolism and function
In diabetes, mitochondrial oxidative stress and dysfunction contribute to atrial remodeling and AF. When sodium currents increase persistently in the atrial myocytes of patients with AF, early afterdepolarization, mitochondrial malformations, and atrial fibrosis and enlargement occur. Suppression of mitochondrial oxidative stress preserves mitochondrial function and attenuates atrial fibrosis and remodeling [40]. SGLT2i can protect mitochondria by reducing the production of intracellular ROS in cardiomyocytes, thus potentially decreasing atrial remodeling and the burden of AF for patients with and without diabetes [41]. In addition, other drugs, including DDP-4 inhibitors, ubiquione (coenzyme Q10) [42], metformin, thiazolidinedione, fites, trimetazidine, ranolazine, and Wenxin Keli (an antiarrhythmic traditional Chinese medicine) [43], can inhibit mitochondrial ROS and reduce AF [44]. The renal-angiotensin-aldosterone system (RAAS) is critically involved in blood pressure regulation and contributes to AF development. Aldosterone inhibits myocardial mitochondrial function via ROS production and regulation of NOX2, inhibition of superoxide dismutase 2 expression, and decreased production of myocardial mitochondrial ATP [45]. Therefore, the RAAS pathway could be an important target for the treatment and prevention of AF in patients with hypertension.
2.4. Myocardial metabolism and atrial remodeling
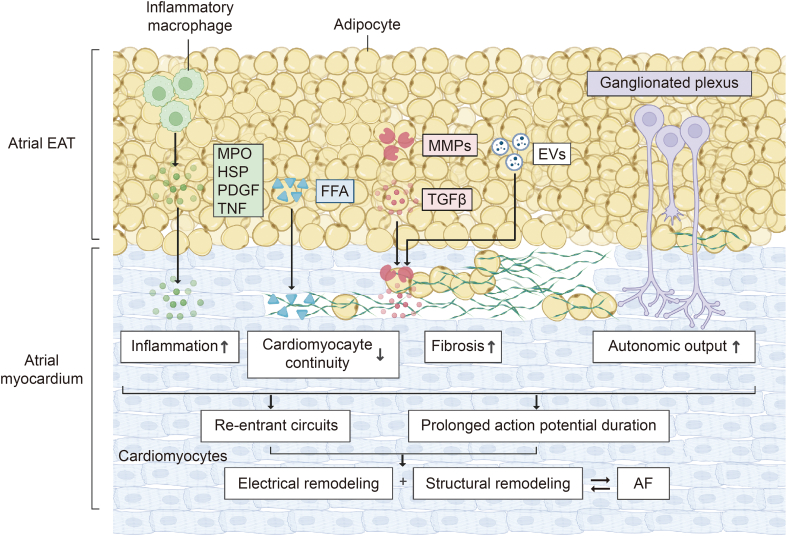
AF is closely related to atrial structural remodeling and electrical remodeling. Changes in myocardial metabolism can promote these atrial remodeling events. Structural remodeling in the atrial tissue with increased fibrosis develops gradually. In addition, it is directly associated with atrial conduction and AF initiation and maintenance [46] (Fig. 3). This section focuses on how abnormal FA and glucose metabolism affect atrial electrical remodeling and structural remodeling.
Fig. 3.
Myocardial metabolism changes and atrial remodeling. Changes in myocardial metabolism during atrial fibrillation (AF) can promote atrial electrical remodeling and structural remodeling, which in turn promote the progression of AF. EAT: epicardial adipose tissue; MPO: myeloperoxidase; HSP: heat shock protein; PDGF: platelet-derived growth factor; TNF: tumor necrosis factor; FFA: free fatty acid; MMP: matrix metalloproteinase; TGF-β: transforming growth factor-β; EV: extracellular vesicle.
2.4.1. FA metabolism and atrial remodeling
FA metabolism can normally produce high ATP levels at the cost of high oxygen consumption in mitochondria [47]. Under pathological conditions, mitochondrial dysfunction reduces oxidative metabolism; thus, FAs are stored in epicardial adipose tissue (EAT) [48]. EAT can cause significant damage to adjacent myocardium through paracrine or vascular secretion of pro-inflammatory and fibrogenic cytokines [49].
Inflammation is a response to harmful stimuli such as pathogens and chemicals or physical damage to tissues or cells [50]. Inflammatory mediators can change atrial electrophysiological features and structural substrates [51]. Inflammation also regulates calcium homeostasis and connexin, triggering abnormal atrial conduction [52]. Myeloperoxidase (MPO), tumor necrosis factor (TNF), and platelet-derived growth factor (PDGF) activate fibroblasts, thus increasing the expression of matrix metallopeptidase (MMP) and TGF-β1 and subsequently increasing collagen synthesis and atrial fibrosis. TNF also induces myolysis and apoptosis of cardiomyocytes [53]. In 2020, Lin et al. [54] revealed the fibrogenic role of osteopontin (OPN) in the atrium by activating the Akt/glycogen synthase kinase 3 beta (GSK-3β)/β-catenin signaling pathway and inhibiting autophagy. Inflammation and inflammatory cytokines contribute to the development of heterogeneous atrial conduction substrates and increase AF susceptibility.
EAT in the left atrium has a highly expressed gene encoding arrhythmogenic factors and releases proinflammatory factors, such as TNF and interleukin-6 (IL-6), leading to atrial myocardial fibrosis and AF [55,56]. FA overload in left atrial EAT can interrupt myocardial continuity, resulting in zigzag conduction and the development of reentrant circuits [57]. As the ganglion plexus becomes more active in EAT, the autonomic impact on atrial cardiomyocytes is increased with a prolonged period of action potential [58]. In general, EAT influences atrial electrical and structural remodeling mainly by promoting inflammation and fibrosis.
2.4.2. Glucose metabolism and atrial remodeling
Advanced glycation end products (AGEs) are generated through nonenzymatic reactions between sugars and proteins and lipids [59]. AGEs can enhance the production of proinflammatory mediators and promote protein cross-linking via interactions with the receptor for AGEs (RAGE), leading to atrial fibrosis [60]. Inhibition of AGE formation can reduce cardiac electromechanical remodeling and susceptibility to tachyarrhythmia in diabetic rats [61]. In addition, renal denervation (RDN) modifies the RAGE/soluble form of RAGE (sRAGE) balance and reduces proinflammatory and fibrogenic RAGE ligands to prevent atrial remodeling in rats with metabolic syndrome [62]. The use of an AGE formation inhibitor or angiotensin II type 1 receptor blocker candesartan can reduce the expression of connective tissue growth factor (CTGF) and significantly prevent atrial fibrosis [63,64]. In 2020, Prasad [65] summarized that changes in the extracellular matrix are one of the mechanisms for AGE-induced AF.
Fluctuations in blood glucose levels lead to structural, electromechanical, electrical, and autonomic nerve remodeling in the myocardium, promoting AF. It has been shown that streptozotocin-induced diabetic rats with blood glucose fluctuations develop AF due to cardiac fibrosis. Upregulation of thioredoxin interacting protein (Txnip) may increase ROS levels, leading to glucose fluctuation-induced fibrosis [66]. High blood glucose variability has been associated with an increased risk of AF in patients with acute coronary syndrome [67]. Blood glucose fluctuations in diabetes are related to inflammation and oxidative stress as well as an increased risk for AF [68]. A significant reduction in the incidence of AF was observed in subjects with improved blood glucose variability [69].
2.5. Analytical methods based on myocardial metabolism
The understanding of myocardial metabolism in AF provides a unique platform to develop and optimize analytical techniques tailored to these specific metabolic changes. This part elaborates more explicitly on the potential analytical methods that could be developed and highlights existing analytical tools and methods currently being utilized to study myocardial metabolism and how they might be optimized or modified.
2.5.1. Potential analytical methods
2.5.1.1. Metabolomic profiling techniques
The heterogeneous nature of myocardial metabolism in AF suggests that a comprehensive metabolomic approach can offer insights into the specific metabolic pathways affected. Techniques such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy can provide high-resolution metabolic profiles, aiding in the identification of novel metabolic markers and therapeutic targets.
2.5.1.2. Imaging modalities
Positron emission tomography (PET) and magnetic resonance imaging (MRI) have shown promise in visualizing myocardial metabolic pathways in real time. Adapting these imaging techniques to target specific metabolic changes in AF can pave the way for improved diagnostic and therapeutic strategies.
2.5.1.3. Biosensor development
The unique metabolic changes observed in AF could be leveraged to design biosensors that detect shifts in myocardial metabolism. Such sensors can serve as real-time monitoring tools for patients at risk or those already diagnosed.
2.5.2. Existing analytical tools
2.5.2.1. Gas chromatography-mass spectrometry (GC-MS)
Widely used for studying myocardial metabolism, GC-MS offers precise identification and quantification of metabolites. There is potential to refine the GC-MS technique to target specific metabolites associated with AF.
2.5.2.2. Fluorescent and bioluminescent imaging
Both are noninvasive tools that provide spatial and temporal information about myocardial metabolic activity. By using specific probes or dyes that respond to the unique metabolic changes in AF, these techniques can be optimized for better resolution and specificity.
2.5.2.3. Microdialysis
This technique allows the continuous monitoring of metabolites in the extracellular fluid of the myocardium. The understanding of AF-induced metabolic changes suggests that microdialysis probes can be modified to capture a broader range of metabolites or to increase the sensitivity toward specific metabolites of interest.
A variety of analytical methods are used in this research. The most common clinical examinations include blood pressure, blood glucose, blood chemistry, electrocardiogram (ECG), and echocardiography. Histological analysis can also be carried out by staining. Molecular biology methods such as Western blotting, enzyme-linked immunosorbent assay (ELISA), real-time quantitative polymerase chain reaction (RT-qPCR), cytokine arrays, and molecular probes were also used. Among them, there is no lack of innovative and unique methods that make a certain contribution to the field of cardiovascular disease (CVD) detection methodology.
In addition, these analytical methods can be widely used in the study of CVD. The use of single-cell RNA sequencing has greatly improved the understanding of cardiovascular system development and the mechanism of CVD [70]. Metabonomics has become a powerful tool for the study of the complex pathophysiology of atherosclerosis and CVD. Metabolites enable us to explore gene-environment interactions and better understand multifactorial diseases such as CVD [71]. Clinical biomarker research is growing rapidly, especially in the cardiovascular field, because of the stringent requirements of providing personalized and accurate medical care [72]. The practical value and versatility of these analytical methods in CVD are fully reflected in this study.
3. GLP-1 RAs and risk factors for AF
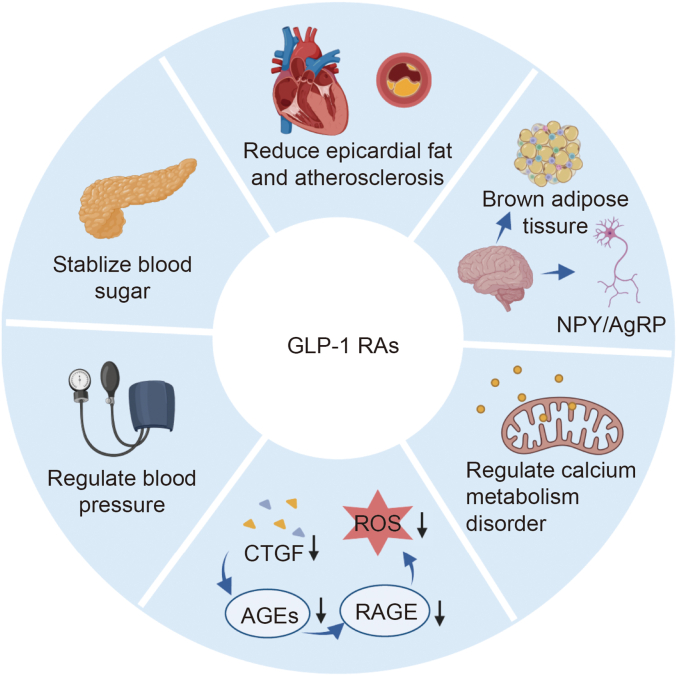
GLP-1 RAs can improve myocardial metabolism via several mechanisms (Fig. 4). GLP-1 RAs can improve myocardial energy metabolism, including modifying myocardial FA metabolism, stabilizing blood glucose levels [73], and regulating myocardial mitochondrial function through RAAS regulation [74]. In addition, they have a close relationship with atrial remodeling, including reducing the accumulation of EAT and inflammatory reactions, lowering the concentration of AGEs [75] and fibrosis factors in the blood, and regulating the level of calcium ions [76].
Fig. 4.
Major mechanisms by which glucagon-like peptide-1 receptor agonists (GLP-1 RAs) regulate atrial fibrillation (AF). Mechanisms mainly include stabilizing blood sugar, reducing epicardial fat, controlling weight, regulating calcium metabolism disorder, inhibiting the production of reactive oxygen species (ROS), and regulating blood pressure. GLP-1 RAs can lower weight by regulating brown adipose tissue thermogenesis and secretion of neuropeptide Y (NPY)/agouti-related peptide (AgRP), and alleviate ROS through lower connective tissue growth factor (CTGF) and inhibition of the advanced glycation end product (AGE)-receptor for advanced glycation end product (RAGE) system.
AF is associated with many medical conditions, including hypertension, hyperglycemia, HF, obstructive sleep apnea (OSA), obesity, valvular heart disease, and coronary artery disease [77]. The specific improvement mechanisms of GLP-1 RAs for different risk factors are described in detail below.
To ensure a thorough and balanced overview of the effects of GLP-1 RAs on myocardial metabolism in AF, we embarked on an extensive literature search across reputable databases such as PubMed, Web of Science, and Scopus. Our focus was on peer-reviewed articles published between 2000 and 2023, ensuring the inclusion of both foundational studies and the most recent advancements. The selection of GLP-1 RAs and the studies involving them were meticulously guided by their relevance to myocardial metabolism and AF, the robustness of the study design, and the quality of the data presented. Furthermore, we critically assessed the analytical techniques employed in these studies, evaluating their sensitivity and reliability in detecting nuanced alterations in myocardial metabolism.
3.1. GLP-1 RAs and diabetes
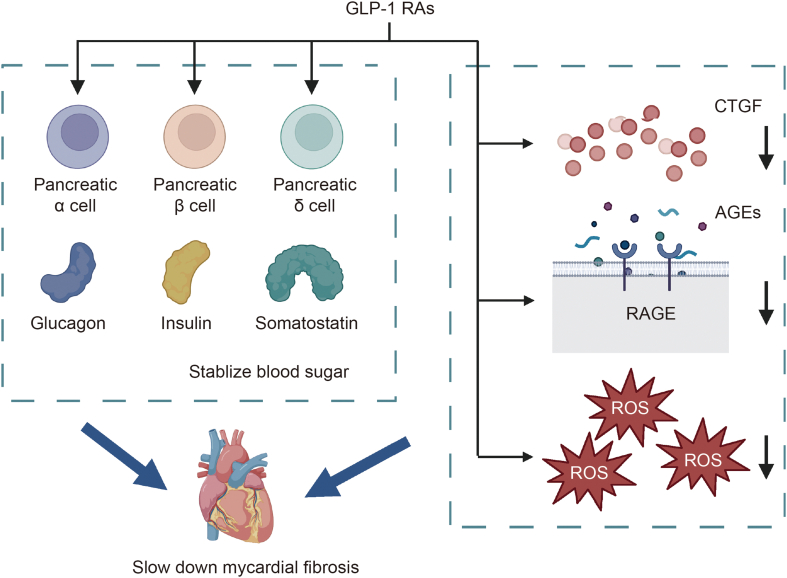
Metabolic syndrome is a condition that includes hyperglycemia, hypertension, and obesity and is a major risk factor for AF in diabetic patients [78]. GLP1 RAs, in addressing metabolic syndrome, exhibit pleiotropic effects on cardiac dysfunction, particularly in mitigating electrical and intracellular Ca2+ anomalies, as well as mitochondrial dysfunction [79]. GLP-1 RAs were first used to treat T2DM and can increase insulin secretion by activating the proliferation and differentiation of pancreatic β-cells and inhibiting their apoptosis. GLP-1 RAs can also promote the secretion of glycogen and increase blood glucose during hypoglycemia, thus stabilizing blood glucose levels. In addition, hyperglycemia can lead to a decrease in tissue GLP-1 receptor (GLP-1R) expression. Studies have shown that early treatment with GLP-1 RAs attenuates the glucotoxicity of β-cells, enhances vasodilation, and reduces the occurrence of AF [80].
AGEs significantly contribute to myocardial fibrosis in diabetic patients. GLP-1 RAs can decrease the level of the upstream regulatory molecule of AGEs, CTGF [81], and inhibit the expression of RAGE [82], thus suppressing AGE-RAGE-induced ROS production and reducing oxidative damage to cardiomyocytes and cardiac fibrosis. Additionally, RDN has been shown to regulate cardiac fibrosis by GLP-1 RAs [83]. GLP-1 RAs hold potential in combating diabetic cardiomyopathy, not only by reducing oxidative stress but also by curbing mitochondrial dysfunction through the inhibition of intercellular lipid accumulation [84,85].
3.2. GLP-1 RAs and hypertension
Hypertension increases the risk of AF through three mechanisms: structural changes in the heart, including cardiac fibrosis and left atrial enlargement, regulation of nerve excitability, and regulation of endocrine function [86]. Recent studies have shown that GLP-1 RAs can reduce AF risk through multiple mechanisms, as shown in Fig. 5. GLP-1 RAs decrease the risk of AF by modulating the RAAS. Furthermore, GLP-1 RAs play a vital role in attenuating myocardial fibrosis and hypertension. This is achieved by suppressing angiotensin II (Ang II)-activated NOX4/NADPH oxidase and the associated mitochondrial dysfunction [87]. GLP-1 attenuates the effect of Ang II and blocks Ang II-induced increases in ROS and activation of inflammatory mediators via the G protein-linked membrane receptor/protein kinase A (PKA) signaling pathway [88]. Regulation of proximal tubule Na+/H+ exchanger isoform 3 (NHE3) by GLP-1 and Ang II has been reported recently [89]. By stimulating cyclic adenosine monophosphate (cAMP)/PKA signaling, GLP-1 inhibits NHE3 and increases NHE3-PS552 levels, whereas Ang II decreases cAMP/PKA-mediated NHE3 phosphorylation at serine 552 in proximal renal tubular cells, enhancing NHE3-mediated transport. By regulating Ang II, GLP-1 alleviates elevated blood pressure, increases the glomerular filtration rate, and induces diuresis and natriuresis. Animal studies have shown that liraglutide also stimulates atrial natriuretic peptide secretion by increasing the levels of Epac2, a downstream target of GLP-1R signaling [90], which in turn reduces cardiac workload.
Fig. 5.
Mechanisms of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) used in patients with hyperglycemia. Mechanisms mainly include regulating reactive oxygen species (ROS) production and islet cells. GLP-1 RAs can stabilize blood sugar mainly by controlling the secretion of glucagon and insulin, and they can also slow the process of fibrosis by reducing reactive oxygen species and fibrogenic cytokines. CTGF: connective tissue growth factor; AGEs: advanced glycation end products; RAGE: receptor for advanced glycation end product.
In addition, TGF-β1 and CTGF can be reduced by GLP-1 RAs to slow the progression of cardiac fibrosis. Studies using spontaneously hypertensive rats have demonstrated that GLP-1 can act on central GLP-1R signaling in solitary tract nucleus (NTS) neurons to reduce hypertensive responses and sympathetic activity [91], attenuate renal self-regulatory feedback, and increase urinary excretion [92]. Stroke-induced AF is more than five times more likely than hypertension-induced AF, and GLP-1 RAs significantly decrease recurrent stroke in patients with hypertension by preventing small vessel occlusion [93].
3.3. GLP-1 RAs and obesity
Obesity also significantly increases the risk of AF due to impaired diastolic function of the heart, inflammation, and increased epicardial fat [94]. GLP-1 RAs have been shown to be effective in weight control in subjects with obesity through different mechanisms. First, GLP-1 RAs can control appetite and reduce weight. GLP-1 RAs appear to effectively decrease appetite by inhibiting the activity of neuropeptide Y (NPY)/agouti-related peptide (AgRP)-producing neurons in the arcuate nucleus [95]. GLP-1 RAs are able to help better control weight in obese patients with or without T2DM who are resistant to dietary restrictions and exercise [96], independent of their gastrointestinal effects [97]. Second, GLP-1 RAs act on GLP-1 R-enriched areas in the dorsomedial hypothalamus to modulate sympathetic nerve activity, which is important for body weight regulation and brown adipose tissue thermogenesis, thereby reducing body weight [98]. In addition, GLP-1 RAs have been shown to lower the risk of AF by reducing the deposition of EAT. Animal studies have revealed that exenatide improves lipid deposition in myocytes by enhancing insulin sensitivity and activating AMPK signaling without reducing body weight [99]. GLP-1 RAs interact with GLP-1 receptors in EAT to reduce local fat production and decrease EAT thickness.
Liraglutide exhibits multifaceted benefits by modulating lipid, glucose, and bile acid metabolism and by decreasing levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglycerides (TGs), and saturated FAs. Moreover, liraglutide has been shown to shield cardiomyocytes from metabolic disturbances and mitochondrial dysfunctions induced by IL-1β [100]. Abnormal accumulation of ceramide may result in myocardial injury. Treatment with liraglutide can decrease the levels of plasma C16:0-ceramide and C16:0-glycosylceramide in patients with CVDs [101].
3.4. GLP-1 RAs and HF
HF is another risk factor for AF in addition to neurohormonal abnormalities, calcium disturbance, conduction abnormalities, and cardiac remodeling [102]. GLP-1 therapy significantly reduces infarct size and improves left ventricular ejection fraction in patients with acute myocardial infarction with percutaneous intervention [103]. Highlighting the potential of GLP-1 RAs, intermittent application of a short-course regimen has been shown to regulate Parkin-dependent mitochondrial turnover, thus limiting adverse cardiac remodeling [104]. GLP-1 RAs attenuate postinfarction remodeling of the myocardium mainly through extracellular matrix modification [105]. Exendin-4 inhibits structural remodeling through the GLP-1 receptor by activating the eNOS/cGMP/protein kinase G (PKG) pathway [106]. In a mouse ischemic HF model, exendin-4 treatment reduces the size of the infarct, prevents ventricular dilation, cardiac hypertrophy and fibrosis, and improves heart function [107]. The beneficial effects of GLP-1 RAs on cardiac hypertrophy and AF may be due to the activation of the AMPK/rapamycin (mammalian target of rapamycin (mTOR)) signaling pathway [108] and reduced production of mitochondrial and intracellular ROS [109]. GLP-1 RAs are involved in regulating Ca2+ levels by decreasing lysine receptor 2 phosphorylation and inhibiting CaMK-II activity. In addition, GLP-1 RAs regulate insulin-like growth factor-1/2 and promote the expression of α-estrogen receptor, which can also decrease drug-induced myocardial injuries and provide cardiac protection in patients on isoprenaline [110]. However, a number of clinical trials in patients with HF have shown that GLP-1 RAs, especially liraglutide, increase heart rate [111], which could increase cardiac workload and increase the risk of AF.
3.5. GLP-1 RAs and coronary atherosclerosis
Coronary atherosclerosis can narrow the coronary arteries, resulting in persistent hypoxia and ischemia of the myocardium, leading to myocardial fibrosis or necrosis, inflammation, and conduction abnormalities, thus increasing the risk of AF.
GLP-1 RAs can slow the progression of atherosclerosis and reduce AF risk through a variety of mechanisms. In addition to reducing fibrogenic factors, GLP-1 RAs can decrease inflammation. Semalutide and liraglutide have been shown to decrease the level of CD163, a marker for plaque instability and inflammation, and reduce the progression of atherosclerosis in low-density lipoprotein receptor (LDLr−/−) deficient mice and apolipoprotein E (ApoE−/−) deficient mice [112]. Abundant GLP-1 R is present in the macrophage-rich regions of atherosclerotic plaques, suggesting that GLP-1 RAs could interact with macrophages to attenuate their activation, recruitment and accumulation in the arterial wall [113,114]. Liraglutide decreases the levels of LDL and inflammatory factors and reduces carotid intima-media thickness [115]. GLP-1 RAs can inhibit the activities of renal sympathetic nerves to increase urinary output and reduce cardiac workload. It can also regulate sympathetic nerve activity to improve brown fat thermogenesis and lipid metabolism. In addition, liraglutide inhibits inflammation by regulating the expression of inflammatory genes in peripheral blood mononuclear cells [116]. OPN is a proinflammatory cytokine and is involved in the recruitment of immune cells. Semaglutide can reduce plasma OPN levels and decrease the expression of OPN in aortic tissue. There is emerging evidence that dulaglutide can prevent atherosclerotic effects of ox-LDL by mediating KLF2 signaling [117]. Simultaneously, these agents show promise in directly safeguarding cardiomyocytes from reperfusion injury by influencing intracellular calcium homeostasis [118].
3.6. GLP-1 RAs and obstructive dyspnea
OSA is a chronic disease characterized by intermittent hypoxia. OSA and associated hypoxia can increase sympathetic activities and promote structural and electrical remodeling of the atria, increasing the risk of AF [119]. Some studies have shown that GLP-1 RAs can increase sympathetic excitability and thus accelerate heart rate. In the setting of chronic OSA, GLP-1 RAs are able to lower blood pressure, slow cardiac remodeling, and inhibit the expression of inflammatory factors and the oxidative stress response. GLP-1 RAs have been demonstrated to reduce the risk of AF by lowering basal activities of the carotid body and attenuating chemoreflex-induced blood pressure and sympathetic responses [120]. In the context of chronic intermittent hypoxia, recombinant human GLP-1 has been reported to preclude heart damage. This protection is achieved by promoting early adaptive responses in mitochondrial biogenesis and enhancing myocardial energy metabolism [121]. In addition, GLP-1 RAs can suppress the expression of profibrotic factors such as CTGF and TGF-β1 and inflammatory factors such as C-reactive protein. However, more studies are needed to define the effects of GLP-1 RAs on sleep apnea.
4. Treatment of AF with GLP-1 RAs
4.1. Animal studies
Numerous animal studies have delineated the cardiovascular advantages of GLP-1 RAs. For instance, GLP-1 RAs have been shown to enhance myocardial glucose uptake and ameliorate left ventricular performance in conscious dogs with pacemaker-induced dilated cardiomyopathy, leading to a notable increase in left ventricular stroke output and cardiac output [122]. Moreover, these agents mitigate damage resulting from myocardial ischemia‒reperfusion in rats [123] and offer cardioprotective benefits in mice suffering acute ischemic myocardial injuries [124], characterized by reduced myocardial infarction areas, anti-inflammatory actions, and improved ejection fraction.
Using a dog model of induced AF, it was reported that liraglutide prevents adverse electrophysiological changes, such as the inducibility of AF and a decrease in conduction velocity [125]. GLP-1 RAs can attenuate cardiac fibrosis and suppress electrophysiological and structural cardiac remodeling by regulating the Wnt1/β-catenin signaling pathway [126] and poly ADP-ribose polymerase 1 (PARP1)/nuclear factor--kappaB (NF-κB) axis [127]. Liraglutide blocks Ang II-induced ROS production and collagen expression in cardiac fibroblasts [128]. In addition, GLP-1 RAs can restore the balance of lipid metabolism, decrease inflammation/oxidative stress [129], inhibit mTOR/p70S6K signaling, and enhance autophagy activity [130]. Exendin-4 inhibits structural remodeling and improves Ca2+ homeostasis via the eNOS/cGMP/PKG pathway. More data from animal studies are summarized in Table 2 [87,99,107,[126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137]].
Table 2.
Potential mechanisms of glucagon-like peptide-1 receptor agonists (GLP-1RAs) on atrial fibrillation (AF): animal studies.
| GLP-1 RAs | Year | Models | Outcomes | Refs. |
|---|---|---|---|---|
| Liraglutide | 2022 | Sprague-Dawley rats | Suppressed angiotensin II-activated NOX4/NADPH and mitochondrial issues | [87] |
| Exenatide | 2020 | ob/ob mice and diet-induced obese mice | Reduced intramyocellular lipid deposition without body weight reduction | [99] |
| Exendin-4 | 2017 | Mice with MI | Inhibited structural remodeling and improved Ca2+ homeostasis via eNOS/cGMP/PKG pathway | [107] |
| Exendin-4 | 2020 | Mice with MI | Improved cardiac remodeling via SIRT1-dependent inhibition of PARP1/NF-κB | [126] |
| Exendin-4 | 2021 | Rats with MI | Inhibited Wnt1/β-catenin signaling pathway | [127] |
| Liraglutide | 2021 | C57BL/6J mice with hypertension | Inhibited Ang II-triggered ROS and collagen in cardiac fibroblasts | [128] |
| Exendin-4 | 2013 | Mice induced by high-fat diet (genetic and acquired T2DM) | Balanced lipid metabolism and reduced inflammation/oxidative stress | [129] |
| Liraglutide | 2020 | Mice | Inhibited mTOR/p70S6K signaling and enhanced autophagy activity | [130] |
| Exendin-4 | 2014 | Mice with MI | Improved mitochondrial respiration and function, suppressed the opening of mitochondrial permeability transition pore, and protected mitochondria | [131] |
| Exendin-4 | 2014 | Mice with cardiac-specific monocyte chemoattractant protein-1 overexpression | Enhanced cardiac function and reduced monocyte infiltration, fibrosis, and apoptosis | [132] |
| Exendin-4 | 2016 | Mice with MI | Improved cardiac function and attenuated cardiac remodeling | [133] |
| Liraglutide | 2019 | Sprague-Dawley rats | Reduced cardiac fibrosis and dysfunction via AT1R suppression | [134] |
| Liraglutide | 2020 | Zucker lean (+/+) and obese (fa/fa) rats | Reduced proinflammatory and profibrotic biomarker expressions (NF-κB, CD68, IL-1β, TGF-β1, and osteopontin) | [135] |
| Liraglutide | 2021 | C57BL/6J mice with HFpEF | Reduced cardiac hypertrophy and myocardial fibrosis | [136] |
| Liraglutide | 2022 | Mice with chronic intermittent hypoxia | Ameliorate atrial remodeling by the miR-21/PTEN/PI3K/Akt signaling pathway and modulation of inflammatory responses | [137] |
NOX4: nicotinamide adenine dinucleotide phosphate (NADPH) oxidoreductase 4; MI: myocardial infarction; eNOS: endothelin nictric oxide synthase; cGMP: cyclic guanosine monophosphate; PKG: protein kinase G; SIRT1: silent information regulator 1; PARP1: poly adenosine diphosphate (ADP)-ribose polymerase 1; NF-κB: nuclear factor kappaB; ROS: reactive oxygen species; T2DM: type 2 diabetes mellitus; mTOR: mammalian target of rapamycin; AT1R: angiotensin II type 1 receptor; IL-1β: interleukin-1β; TGF-β1: transforming growth factor-β1; HFpEF: heart failure (HF) with preserved ejection fraction; miR-21: microRNA-21; PTEN: phosphatase and tensin homolog; PI3K: phosphatidylinositide 3-kinase.
4.2. Clinical trials
The data from the available clinical trials on the cardiovascular benefits of GLP-1 RAs are summarized in Table 3 [[138], [139], [140], [141], [142], [143], [144]]. Since 2005, when the first GLP-1 RAs were approved for the treatment of T2DM, accumulating evidence has demonstrated that GLP-1 RAs have beneficial cardiovascular effects in patients with T2DM. Liraglutide, exenatide, dulaglutide [145], albiglutide, and semaglutide have all been shown to reduce MACEs. Accordingly, recent guidelines have recommended the use of GLP-1 RAs for patients with T2DM at high risk of CVD, especially HF and chronic kidney disease [146,147]. However, treatment with GLP-RAs only has minimal benefit in reducing the risk of AF [148].
Table 3.
Clinical experiments on the links between cardiovascular diseases (CVDs) and glucagon-like peptide-1 receptor agonists (GLP-1 RAs).
| GLP-1 RAs | Publish year | Trails | Numbers (experimental/ccontrol) | Follow-up time | Risk of CVDs (experimental/control) | Result | Refs. |
|---|---|---|---|---|---|---|---|
| Semaglutide | 2016 | RCT | 3,297 (1,648/1,649) | 104 weeks | CVD 108/146, P < 0.001 | Semaglutide can significantly lower the risk of CVD. | [138] |
| Liraglutide | 2016 | RCT | 7,598 (3,831/3,767) | 3.8 years | CVD 536/629, P = 0.83 | Liraglutide can slow down the risk of CVD of patients with age ≥50 years and established CVD. | [139] |
| Exenatide | 2017 | RCT | 14,752 (7,356/7,396) | 3.2 years | CVD 839/905, P = 0.06 | The rates of death from cardiovascular causes do not differ significantly between the two groups. | [140] |
| Albiglutide | 2018 | RCT | 9,463 (4,731/4,732) | 1.6 years | CVD 338/428, P = 0.0006 | Albiglutide can reduce the risk of cardiovascular events in patients with T2DM. | [141] |
| GLP-1 RAs | 2020 | Meta-analysis | 63,134 | ≥52 weeks | AF, OR 0.94 (0.84–1.04) | GLP1-RAs did not increase the risk of AF. | [142] |
| Dulaglutide | 2022 | RCT | 9,743 (4,969/4,774) | 5.4 years | Atrial arrhythmias 269/255, P = 0.59 | Dulaglutide was not associated with a reduced incidence of atrial arrhythmia in patients with T2DM. | [143] |
| Liraglutide | 2023 | RCT | 300 (150/150) | 180 days | Arrhythmias 39/21 IRR 1.76 (0.923.37), P = 0.088 | Liraglutide may increase the risk of arrhythmias in HFrEF patients. | [144] |
RCT: randomized controlled trial; T2DM: type 2 diabetes mellitus; AF: atrial fibrillation; OR: odds ratio; IRR: incidence rate ratio; HFrEF: heart failure (HF) with reduced ejection fraction.
However, data from other studies have shown that in stable chronic HF patients with and without diabetes, liraglutide had no effect on left ventricular systolic function compared to placebo while increasing heart rate [149]. Moreover, research indicates that liraglutide might increase heart rate, reduce heart rate variability, and potentially heighten cardiac mortality [150]. Some studies have shown that the most relevant drug for abnormal cardiac conduction is dulaglutide. Furthermore, findings from randomized trials suggest that dulaglutide treatment could manifest symptoms such as sinus tachycardia, prolonged P−R interval, 1st degree atrioventricular block, and increased atrial arrhythmias inducibility [151,152].
The reason for the inconsistency between risk reduction of AF and other beneficial cardiovascular effects of GLP-RAs in patients remains unknown at this point. This may be related to different types of GLP-1 RAs and/or different study populations or study durations. Further studies are needed to clarify the effects of GLP-1 RAs on the cardiovascular system.
5. Conclusion
The metabolic characteristics of cardiomyocytes change significantly in AF and play important roles in atrial remodeling in AF. Studies have shown that GLP-1 RAs improve myocardial metabolism and have cardiovascular protective effects, including atrial electrical and structural remodeling. Both animal and clinical studies have demonstrated that GLP-1 RAs have beneficial effects on a variety of medical conditions, including hypertension, diabetes, obesity, HF, atherosclerosis, and OSA, thus reducing the risk for AF. However, some clinical studies have revealed that treatment with GLP-1 RAs only has minimal benefit in reducing the risk of AF. The reason for the inconsistency between the risk reduction of AF and other beneficial cardiovascular effects of GLP-RAs in patients remains unknown. This may be because the occurrence and development of AF involves multiple mechanisms. GLP-1 RAs reduce the risk of AF mainly by controlling its risk factors, but other potential mechanisms remain to be explored. It may be related to the type of GLP-1 RAs. For instance, liraglutide and dulaglutide are most related to cardiac conduction abnormalities. In addition, populations with different risk factors, such as age and underlying diseases, should be investigated. The duration of clinical trials also has an effect on the observation of trial results.
While our review provides valuable insights, it is imperative to acknowledge its limitations. A notable limitation is the reliance on a limited number of clinical trials specifically investigating the relationship between GLP-1 RAs and AF or arrhythmia. Many studies included in our review did not provide detailed assessments, such as ECG measurements, before and after the administration of GLP-1 RAs, which restricts the depth of our analysis. ECG is an important detection method for the diagnosis of AF. However, according to the papers we have searched, many clinical trial analyses on GLP-1 RAs and AF have not attached corresponding ECG data. Regarding papers on the mechanism of GLP-1 RAs, few researchers have conducted ECG analysis. The lack of ECG data may have led to a lack of accuracy in our review. It is believed that there will be more research on this unexplored area in the future. The scarcity of large-scale, randomized clinical trials and the absence of detailed comparisons between different GLP-1 RAs further constrain our ability to draw definitive conclusions regarding their clinical implications.
Given these limitations, we see a compelling need for future research to explore the underlying mechanisms that connect GLP-1 RAs and AF. This could potentially involve investigating the interaction between GLP-1 RAs and the sympathetic nervous system. We strongly recommend the design and execution of more comprehensive and rigorous clinical trials to elucidate the distinct short-term and long-term impacts of individual GLP-1 RAs on AF progression and treatment.
Our study illuminates the promising role of GLP-1 RAs in addressing the challenges of AF management. The observed regulatory effects of GLP-1 RAs on several AF-associated risk factors suggest the potential for these agents to bring about significant advancements in therapeutic strategies. Beyond their direct cardioprotective effects, which merit further exploration, the ability of GLP-1 RAs, with the notable exception of dulaglutide, to act as potential upstream therapeutic candidates is particularly compelling for patients at elevated risk of AF. The insights gained from this study highlight the possibility of not only managing AF more effectively but also proactively preventing its onset. This represents a potential paradigm shift in the approach to AF patient care, emphasizing the preventive and therapeutic potential of GLP-1 RAs. The novelty and clinical relevance of our findings underscore the need for continued research and exploration in this area.
In conclusion, this review illuminates the intricate and multifaceted roles of GLP-1 RAs in influencing myocardial metabolism and their potential implications in AF. The insights garnered from this study not only open up new avenues for therapeutic interventions but also contribute to a deeper understanding of the complex interplay between metabolic pathways and cardiac arrhythmias. The broader implications of our findings extend beyond the immediate scope of this review, suggesting a promising future for GLP-1 RAs in the advancement of personalized medicine and patient-centric approaches in managing AF.
CRediT author statement
Jiani Zhong and Hang Chen: Writing - Original draft preparation, Reviewing and Editing, Conceptualization; Qiming Liu, Shenghua Zhou, and Zhenguo Liu: Writing - Reviewing and Editing, Supervision; Yichao Xiao: Writing - Reviewing and Editing, Conceptualization, Supervision, Funding acquisition, Resources.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Clinical Medical Technology Innovation Project of Hunan Science and Technology Agency, China (Project No.: 2021SK53519). All figures were created with assistance from Biorender.com.
References
- 1.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC guidelines for the diagnosis and Management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Brundel B.J.J.M., Ai X., Hills M.T., et al. Atrial fibrillation. Nat. Rev. Dis. Primers. 2022;8 doi: 10.1038/s41572-022-00347-9. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S., Heijman J., Zhou L., et al. Molecular basis of atrial fibrillation pathophysiology and therapy: A translational perspective. Circ. Res. 2020;127:51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sfairopoulos D., Liatis S., Tigas S., et al. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones (Athens) 2018;17:333–350. doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- 5.Jastreboff A.M., Kushner R.F. New frontiers in obesity treatment: GLP-1 and nascent nutrient-stimulated hormone-based therapeutics. Annu. Rev. Med. 2023;74:125–139. doi: 10.1146/annurev-med-043021-014919. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis T., Tsapas A., Athanasiadou E., et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2021;174 doi: 10.1016/j.diabres.2021.108737. [DOI] [PubMed] [Google Scholar]
- 7.Davies M.J., Kloecker D.E., Webb D.R., et al. Number needed to treat in cardiovascular outcome trials of glucagon-like peptide-1 receptor agonists: A systematic review with temporal analysis. Diabetes Obes. Metab. 2020;22:1670–1677. doi: 10.1111/dom.14066. [DOI] [PubMed] [Google Scholar]
- 8.Ma X., Liu Z., Ilyas I., et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021;17:2050–2068. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisneski J.A., Stanley W.C., Neese R.A., et al. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J. Clin. Invest. 1990;85:1648–1656. doi: 10.1172/JCI114616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sörensen J., Harms H.J., Aalen J.M., et al. Myocardial efficiency: A fundamental physiological concept on the verge of clinical impact. JACC Cardiovasc. Imaging. 2020;13:1564–1576. doi: 10.1016/j.jcmg.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Jiang M., Xie X., Cao F., et al. Mitochondrial metabolism in myocardial remodeling and mechanical unloading: Implications for ischemic heart disease. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.789267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui S., Ghergurovich J.M., Morscher R.J., et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karwi Q.G., Biswas D., Pulinilkunnil T., et al. Myocardial ketones metabolism in heart failure. J. Card. Fail. 2020;26:998–1005. doi: 10.1016/j.cardfail.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Lopaschuk G.D., Ussher J.R. Evolving concepts of myocardial energy metabolism: More than just fats and carbohydrates. Circ. Res. 2016;119:1173–1176. doi: 10.1161/CIRCRESAHA.116.310078. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J., Sun L., Chen L., et al. Comprehensive metabolomic and proteomic analyses reveal candidate biomarkers and related metabolic networks in atrial fibrillation. Metabolomics. 2019;15:96. doi: 10.1007/s11306-019-1557-7. [DOI] [PubMed] [Google Scholar]
- 16.Lenski M., Schleider G., Kohlhaas M., et al. Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res. Cardiol. 2015;110 doi: 10.1007/s00395-015-0497-2. [DOI] [PubMed] [Google Scholar]
- 17.Shingu Y., Takada S., Yokota T., et al. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS One. 2020;15 doi: 10.1371/journal.pone.0224713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jie Q., Li G., Duan J., et al. Remodeling of myocardial energy and metabolic homeostasis in a sheep model of persistent atrial fibrillation. Biochem. Biophys. Res. Commun. 2019;517:8–14. doi: 10.1016/j.bbrc.2019.05.112. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y., Wu Y., Liu L., et al. The four key genes participated in and maintained atrial fibrillation process via reprogramming lipid metabolism in AF patients. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.821754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G., Hou T., Yuan Y., et al. Fenofibrate inhibits atrial metabolic remodelling in atrial fibrillation through PPAR-α/sirtuin 1/PGC-1α pathway. Br. J. Pharmacol. 2016;173:1095–1109. doi: 10.1111/bph.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho K.L., Karwi Q.G., Wagg C., et al. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc. Res. 2021;117:1178–1187. doi: 10.1093/cvr/cvaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Yang X., Li Y., et al. Mitochondria and the pathophysiological mechanism of atrial fibrillation. Curr. Pharm. Des. 2018;24:3055–3061. doi: 10.2174/1381612824666180903125300. [DOI] [PubMed] [Google Scholar]
- 23.Wiersma M., van Marion D.M.S., Wüst R.C.I., et al. Mitochondrial dysfunction underlies cardiomyocyte remodeling in experimental and clinical atrial fibrillation. Cells. 2019;8 doi: 10.3390/cells8101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason F.E., Pronto J.R.D., Alhussini K., et al. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res. Cardiol. 2020;115 doi: 10.1007/s00395-020-00827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly S.N., Jayaram R., Nahar K., et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: Implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C.F., Yang S., Lo C.H., et al. Role of the ROS-JNK signaling pathway in hypoxia-induced atrial fibrotic responses in HL-1 cardiomyocytes. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22063249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., An N., Zhong C., et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30 doi: 10.1016/j.redox.2020.101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Bai F., Liu N., et al. Metformin improves lipid metabolism and reverses the Warburg effect in a canine model of chronic atrial fibrillation. BMC Cardiovasc. Disord. 2020;20 doi: 10.1186/s12872-020-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Fu Y., Jiang T., et al. Enhancing fatty acids oxidation via L-carnitine attenuates obesity-related atrial fibrillation and structural remodeling by activating AMPK signaling and alleviating cardiac lipotoxicity. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.771940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S.Y., Lu Y.-Y., Lin Y.K., et al. Ceramide modulates electrophysiological characteristics and oxidative stress of pulmonary vein cardiomyocytes. Eur. J. Clin. Invest. 2022;52 doi: 10.1111/eci.13690. [DOI] [PubMed] [Google Scholar]
- 31.Jendle J., Hyötyläinen T., Orešič M., et al. Pharmacometabolomic profiles in type 2 diabetic subjects treated with liraglutide or glimepiride. Cardiovasc. Diabetol. 2021;20 doi: 10.1186/s12933-021-01431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Vecchis R., Paccone A., Di Maio M. Upstream therapy for atrial fibrillation prevention: The role of sacubitril/valsartan. Cardiol. Res. 2020;11:213–218. doi: 10.14740/cr1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z., Yang Y., Wang J., et al. Combined treatment with valsartan and fluvastatin to delay disease progression in nonpermanent atrial fibrillation with hypertension: A clinical trial. Clin. Cardiol. 2020;43:1592–1600. doi: 10.1002/clc.23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu T., Li B., Li X., et al. Dietary ω-3 fatty acids reduced atrial fibrillation vulnerability via attenuating myocardial endoplasmic reticulum stress and inflammation in a canine model of atrial fibrillation. J. Cardiol. 2022;79:194–201. doi: 10.1016/j.jjcc.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Carbone A.M., Borges J.I., Suster M.S., et al. Regulator of G-protein signaling-4 attenuates cardiac adverse remodeling and neuronal norepinephrine release-promoting free fatty acid receptor FFAR3 signaling. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23105803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibasaki I., Nakajima T., Fukuda T., et al. Serum and adipose dipeptidyl peptidase 4 in cardiovascular surgery patients: Influence of dipeptidyl peptidase 4 inhibitors. J. Clin. Med. 2022;11 doi: 10.3390/jcm11154333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Li B., Bai F., et al. Metformin therapy confers cardioprotection against the remodeling of gap junction in tachycardia-induced atrial fibrillation dog model. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117759. [DOI] [PubMed] [Google Scholar]
- 38.Kolesnik E., Scherr D., Rohrer U., et al. SGLT2 inhibitors and their antiarrhythmic properties. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23031678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishinarita R., Niwano S., Niwano H., et al. Canagliflozin suppresses atrial remodeling in a canine atrial fibrillation model. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.119.017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avula U.M.R., Dridi H., Chen B.-X., et al. Attenuating persistent sodium current-induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress. JCI Insight. 2021;6 doi: 10.1172/jci.insight.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng X., Li L., Zhang M., et al. Sodium-glucose cotransporter 2 inhibitors potentially prevent atrial fibrillation by ameliorating ion handling and mitochondrial dysfunction. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martelli A., Testai L., Colletti A., et al. Coenzyme Q10: Clinical applications in cardiovascular diseases. Antioxidants. 2020;9 doi: 10.3390/antiox9040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong M., Yuan M., Meng L., et al. Wenxin Keli regulates mitochondrial oxidative stress and homeostasis and improves atrial remodeling in diabetic rats. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/2468031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pool L., Wijdeveld L.F.J.M., de Groot N.M.S., et al. The role of mitochondrial dysfunction in atrial fibrillation: Translation to druggable target and biomarker discovery. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung C.S., Chang Y.-Y., Tsai C.H., et al. Aldosterone suppresses cardiac mitochondria. Transl. Res. 2022;239:58–70. doi: 10.1016/j.trsl.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Opacic D., van Bragt K.A., Nasrallah H.M., et al. Atrial metabolism and tissue perfusion as determinants of electrical and structural remodelling in atrial fibrillation. Cardiovasc. Res. 2016;109:527–541. doi: 10.1093/cvr/cvw007. [DOI] [PubMed] [Google Scholar]
- 47.Anthony S.R., Guarnieri A.R., Gozdiff A., et al. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. (Lond.) 2019;133:2329–2344. doi: 10.1042/CS20190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022;19:593–606. doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe I., Teshima Y., Kondo H., et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018;15:1717–1727. doi: 10.1016/j.hrthm.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 50.Weiss U. Inflammation. Nature. 2008;454 doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- 51.Boos C.J. Infection and atrial fibrillation: Inflammation begets AF. Eur. Heart J. 2020;41:1120–1122. doi: 10.1093/eurheartj/ehz953. [DOI] [PubMed] [Google Scholar]
- 52.Heijman J., Muna A.P., Veleva T., et al. Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ. Res. 2020;127:1036–1055. doi: 10.1161/CIRCRESAHA.120.316710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren M., Li X., Hao L., et al. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: A novel potential therapeutic target? Ann. Med. 2015;47:316–324. doi: 10.3109/07853890.2015.1042030. [DOI] [PubMed] [Google Scholar]
- 54.Lin R., Wu S., Zhu D., et al. Osteopontin induces atrial fibrosis by activating Akt/GSK-3β/β-catenin pathway and suppressing autophagy. Life Sci. 2020;245 doi: 10.1016/j.lfs.2020.117328. [DOI] [PubMed] [Google Scholar]
- 55.Cabaro S., Conte M., Moschetta D., et al. Epicardial adipose tissue-derived IL-1β triggers postoperative atrial fibrillation. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.893729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu T., Li G. Periatrial epicardial fat, local pro- and anti-inflammatory balance, and atrial fibrillation. J. Am. Coll. Cardiol. 2011;57:1249. doi: 10.1016/j.jacc.2010.09.068. author reply 1249. [DOI] [PubMed] [Google Scholar]
- 57.O’Connell R.P., Musa H., Gomez M.S., et al. Free fatty acid effects on the atrial myocardium: Membrane ionic currents are remodeled by the disruption of T-tubular architecture. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao J., Zhang Y., Yin Z., et al. Impact of proinflammatory epicardial adipose tissue and differentially enhanced autonomic remodeling on human atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2023;165:e158–e174. doi: 10.1016/j.jtcvs.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Li Y.M., Mitsuhashi T., Wojciechowicz D., et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: Relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc. Natl. Acad. Sci. U S A. 1996;93:11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bi X., Song Y., Song Y., et al. Collagen cross-linking is associated with cardiac remodeling in hypertrophic obstructive cardiomyopathy. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang G.J., Yeh Y.H., Chen W.J., et al. Inhibition of advanced glycation end products formation attenuates cardiac electrical and mechanical remodeling and vulnerability to tachyarrhythmias in diabetic rats. J. Pharmacol. Exp. Ther. 2019;368:66–78. doi: 10.1124/jpet.118.252080. [DOI] [PubMed] [Google Scholar]
- 62.Selejan S.R., Linz D., Mauz M., et al. Renal denervation reduces atrial remodeling in hypertensive rats with metabolic syndrome. Basic Res. Cardiol. 2022;117 doi: 10.1007/s00395-022-00943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato T., Yamashita T., Sekiguchi A., et al. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J. Cardiovasc. Electrophysiol. 2008;19:415–420. doi: 10.1111/j.1540-8167.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 64.Kato T., Yamashita T., Sekiguchi A., et al. Angiotensin II type 1 receptor blocker attenuates diabetes-induced atrial structural remodeling. J. Cardiol. 2011;58:131–136. doi: 10.1016/j.jjcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Prasad K. AGE-RAGE stress in the pathophysiology of atrial fibrillation and its treatment. Int. J. Angiol. 2020;29:72–80. doi: 10.1055/s-0039-3400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito S., Teshima Y., Fukui A., et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 2014;104:5–14. doi: 10.1093/cvr/cvu176. [DOI] [PubMed] [Google Scholar]
- 67.Xia J., Xu J., Li B., et al. Association between glycemic variability and major adverse cardiovascular and cerebrovascular events (MACCE) in patients with acute coronary syndrome during 30-day follow-up. Clin. Chim. Acta. 2017;466:162–166. doi: 10.1016/j.cca.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 68.Guckel D., Sohns C., Sommer P. Rhythm and metabolic control. Herz. 2022;47:410–418. doi: 10.1007/s00059-022-05128-4. [DOI] [PubMed] [Google Scholar]
- 69.Cai Y., Zhang H., Li Q., et al. Correlation between blood glucose variability and early therapeutic effects after intravenous thrombolysis with alteplase and levels of serum inflammatory factors in patients with acute ischemic stroke. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.806013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paik D.T., Cho S., Tian L., et al. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol. 2020;17:457–473. doi: 10.1038/s41569-020-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iida M., Harada S., Takebayashi T. Application of metabolomics to epidemiological studies of atherosclerosis and cardiovascular disease. J. Atheroscler. Thromb. 2019;26:747–757. doi: 10.5551/jat.RV17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Revuelta-López E., Barallat J., Cserkóová A., et al. Pre-analytical considerations in biomarker research: Focus on cardiovascular disease. Clin. Chem. Lab. Med. 2021;59:1747–1760. doi: 10.1515/cclm-2021-0377. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Parajuli K.R., Fava G.E., et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68:34–44. doi: 10.2337/db18-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puglisi S., Rossini A., Poli R., et al. Effects of SGLT2 inhibitors and GLP-1 receptor agonists on renin-angiotensin-aldosterone system. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.738848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Y., Liu P., Xiang Q., et al. Glucagon-like peptide-1 attenuates diabetes-associated osteoporosis in ZDF rat, possibly through the RAGE pathway. BMC Musculoskelet. Disord. 2022;23 doi: 10.1186/s12891-022-05396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J., Xu S., Zhou W., et al. Exendin-4 reduces ventricular arrhythmia activity and calcium sparks-mediated sarcoplasmic reticulum Ca leak in rats with heart failure. Int. Heart J. 2020;61:145–152. doi: 10.1536/ihj.19-327. [DOI] [PubMed] [Google Scholar]
- 77.Lau D.H., Nattel S., Kalman J.M., et al. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163. [DOI] [PubMed] [Google Scholar]
- 78.Bell D.S.H., Goncalves E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of anti-diabetic therapies, Diabetes Obes. Metab. 2019;21:210–217. doi: 10.1111/dom.13512. [DOI] [PubMed] [Google Scholar]
- 79.Durak A., Akkus E., Canpolat A.G., et al. Glucagon-like peptide-1 receptor agonist treatment of high carbohydrate intake-induced metabolic syndrome provides pleiotropic effects on cardiac dysfunction through alleviations in electrical and intracellular Ca2+ abnormalities and mitochondrial dysfunction. Clin. Exp. Pharmacol. Physiol. 2022;49:46–59. doi: 10.1111/1440-1681.13590. [DOI] [PubMed] [Google Scholar]
- 80.Kaneto H., Kimura T., Shimoda M., et al. Favorable effects of GLP-1 receptor agonist against pancreatic β-cell glucose toxicity and the development of arteriosclerosis: “the earlier, the better” in therapy with incretin-based medicine. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22157917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W., Cui M., Wei Y., et al. Inhibition of the expression of TGF-β1 and CTGF in human mesangial cells by exendin-4, a glucagon-like peptide-1 receptor agonist. Cell. Physiol. Biochem. 2012;30:749–757. doi: 10.1159/000341454. [DOI] [PubMed] [Google Scholar]
- 82.Chen S., Yin L., Xu Z., et al. Inhibiting receptor for advanced glycation end product (AGE) and oxidative stress involved in the protective effect mediated by glucagon-like peptide-1 receptor on AGE induced neuronal apoptosis. Neurosci. Lett. 2016;612:193–198. doi: 10.1016/j.neulet.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Patel K.P., Zheng H. Role of renal sympathetic nerves in GLP-1 (glucagon-like peptide-1) receptor agonist exendin-4-mediated diuresis and natriuresis in diet-induced obese rats. J. Am. Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.022542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durak A., Turan B. Liraglutide provides cardioprotection through the recovery of mitochondrial dysfunction and oxidative stress in aging hearts. J. Physiol. Biochem. 2023;79:297–311. doi: 10.1007/s13105-022-00939-9. [DOI] [PubMed] [Google Scholar]
- 85.Qian P., Tian H., Wang Y., et al. A novel oral glucagon-like peptide 1 receptor agonist protects against diabetic cardiomyopathy via alleviating cardiac lipotoxicity induced mitochondria dysfunction. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114209. [DOI] [PubMed] [Google Scholar]
- 86.Gumprecht J., Domek M., Lip G.Y.H., et al. Invited review: Hypertension and atrial fibrillation: Epidemiology, pathophysiology, and implications for management. J. Hum. Hypertens. 2019;33:824–836. doi: 10.1038/s41371-019-0279-7. [DOI] [PubMed] [Google Scholar]
- 87.Banks T.E., Rajapaksha M., Zhang L., et al. Suppression of angiotensin II-activated NOX4/NADPH oxidase and mitochondrial dysfunction by preserving glucagon-like peptide-1 attenuates myocardial fibrosis and hypertension. Eur. J. Pharmacol. 2022;927 doi: 10.1016/j.ejphar.2022.175048. [DOI] [PubMed] [Google Scholar]
- 88.Skov J. Effects of GLP-1 in the kidney. Rev. Endocr. Metab. Disord. 2014;15:197–207. doi: 10.1007/s11154-014-9287-7. [DOI] [PubMed] [Google Scholar]
- 89.Martins F.L., Bailey M.A., Girardi A.C.C. Endogenous activation of glucagon-like peptide-1 receptor contributes to blood pressure control: Role of proximal tubule Na+/H+ exchanger isoform 3, renal angiotensin II, and insulin sensitivity. Hypertension. 2020;76:839–848. doi: 10.1161/HYPERTENSIONAHA.120.14868. [DOI] [PubMed] [Google Scholar]
- 90.Kim M., Platt M.J., Shibasaki T., et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 91.Katsurada K., Nakata M., Saito T., et al. Central glucagon-like peptide-1 receptor signaling via brainstem catecholamine neurons counteracts hypertension in spontaneously hypertensive rats. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen E.P., Møller S., Hviid A.V., et al. GLP-1-induced renal vasodilation in rodents depends exclusively on the known GLP-1 receptor and is lost in prehypertensive rats. Am. J. Physiol. Renal Physiol. 2020;318:F1409–F1417. doi: 10.1152/ajprenal.00579.2019. [DOI] [PubMed] [Google Scholar]
- 93.Strain W.D., Frenkel O., James M.A., et al. Effects of semaglutide on stroke subtypes in type 2 diabetes: Post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke. 2022;53:2749–2757. doi: 10.1161/STROKEAHA.121.037775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nalliah C.J., Sanders P., Kottkamp H., et al. The role of obesity in atrial fibrillation. Eur. Heart J. 2016;37:1565–1572. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]
- 95.Nauck M.A., Quast D.R., Wefers J., et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol. Metab. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frias J.P., Choi J., Rosenstock J., et al. Efficacy and safety of once-weekly efpeglenatide monotherapy versus placebo in type 2 diabetes: The AMPLITUDE-M randomized controlled trial. Diabetes Care. 2022;45:1592–1600. doi: 10.2337/dc21-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quast D.R., Nauck M.A., Schenker N., et al. Macronutrient intake, appetite, food preferences and exocrine pancreas function after treatment with short- and long-acting glucagon-like peptide-1 receptor agonists in type 2 diabetes. Diabetes Obes. Metab. 2021;23:2344–2353. doi: 10.1111/dom.14477. [DOI] [PubMed] [Google Scholar]
- 98.Lee S.J., Sanchez-Watts G., Krieger J.P., et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol. Metab. 2018;11:33–46. doi: 10.1016/j.molmet.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu F., Cao H., Chen Z., et al. Short-term GLP-1 receptor agonist exenatide ameliorates intramyocellular lipid deposition without weight loss in ob/ob mice. Int. J. Obes. (Lond.) 2020;44:937–947. doi: 10.1038/s41366-019-0513-y. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L., Tian J., Diao S., et al. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem. Biol. Interact. 2020;332 doi: 10.1016/j.cbi.2020.109252. [DOI] [PubMed] [Google Scholar]
- 101.Akawi N., Checa A., Antonopoulos A.S., et al. Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J. Am. Coll. Cardiol. 2021;77:2494–2513. doi: 10.1016/j.jacc.2021.03.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sugumar H., Nanayakkara S., Prabhu S., et al. Pathophysiology of atrial fibrillation and heart failure: Dangerous interactions. Cardiol. Clin. 2019;37:131–138. doi: 10.1016/j.ccl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Huang M., Wei R., Wang Y., et al. Protective effect of glucagon-like peptide-1 agents on reperfusion injury for acute myocardial infarction: A meta-analysis of randomized controlled trials. Ann. Med. 2017;49:552–561. doi: 10.1080/07853890.2017.1306653. [DOI] [PubMed] [Google Scholar]
- 104.Germano J.F., Huang C., Sin J., et al. Intermittent use of a short-course glucagon-like peptide-1 receptor agonist therapy limits adverse cardiac remodeling via Parkin-dependent mitochondrial turnover. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-64924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robinson E., Cassidy R.S., Tate M., et al. Exendin-4 protects against post-myocardial infarction remodelling via specific actions on inflammation and the extracellular matrix. Basic Res. Cardiol. 2015;110 doi: 10.1007/s00395-015-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li P.C., Liu L., Jou M.J., et al. The GLP-1 receptor agonists exendin-4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neurosci. 2016;17 doi: 10.1186/s12868-016-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J., Wang D., Wang F., et al. Exendin-4 inhibits structural remodeling and improves Ca2+ homeostasis in rats with heart failure via the GLP-1 receptor through the eNOS/cGMP/PKG pathway. Peptides. 2017;90:69–77. doi: 10.1016/j.peptides.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Y., He X., Chen Y., et al. Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem. Biophys. Res. Commun. 2015;468:394–399. doi: 10.1016/j.bbrc.2015.09.179. [DOI] [PubMed] [Google Scholar]
- 109.Nuamnaichati N., Mangmool S., Chattipakorn N., et al. Stimulation of GLP-1 receptor inhibits methylglyoxal-induced mitochondrial dysfunctions in H9c2 cardiomyoblasts: Potential role of epac/PI3K/Akt pathway. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darwesh A.M., El-Azab M.F., Abo-Gresha N.M., et al. Cardioprotective mechanisms of exenatide in isoprenaline-induced myocardial infarction: Novel effects on myocardial α-estrogen receptor expression and IGF-1/IGF-2 system. J. Cardiovasc. Pharmacol. 2018;71:160–173. doi: 10.1097/FJC.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 111.Baggio L.L., Ussher J.R., McLean B.A., et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 2017;6:1339–1349. doi: 10.1016/j.molmet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rakipovski G., Rolin B., Nøhr J., et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 2018;3:844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Parlevliet E.T., Geerling J.J., et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharmacol. 2014;171:723–734. doi: 10.1111/bph.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arakawa M., Mita T., Azuma K., et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nikolic D., Giglio R.V., Rizvi A.A., et al. Liraglutide reduces carotid intima-media thickness by reducing small dense low-density lipoproteins in a real-world setting of patients with type 2 diabetes: A novel anti-atherogenic effect. Diabetes Ther. 2021;12:261–274. doi: 10.1007/s13300-020-00962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zobel E.H., Ripa R.S., von Scholten B.J., et al. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-97967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang W., Zhu F., Zheng H., et al. Glucagon-like peptide-1 receptor agonist dulaglutide prevents ox-LDL-induced adhesion of monocytes to human endothelial cells: An implication in the treatment of atherosclerosis. Mol. Immunol. 2019;116:73–79. doi: 10.1016/j.molimm.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 118.Hu S., Zhang Y., Zhu P., et al. Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. J. Geriatr. Cardiol. 2017;14:57–66. doi: 10.11909/j.issn.1671-5411.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang B., Liu H., Scherlag B.J., et al. Atrial fibrillation in obstructive sleep apnea: Neural mechanisms and emerging therapies, Trends Cardiovasc. Med. 2021;31:127–132. doi: 10.1016/j.tcm.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Pauza A.G., Thakkar P., Tasic T., et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ. Res. 2022;130:694–707. doi: 10.1161/CIRCRESAHA.121.319874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tao L., Wang L., Yang X., et al. Recombinant human glucagon-like peptide-1 protects against chronic intermittent hypoxia by improving myocardial energy metabolism and mitochondrial biogenesis. Mol. Cell. Endocrinol. 2019;481:95–103. doi: 10.1016/j.mce.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Nikolaidis L.A., Elahi D., Hentosz T., et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 123.Zhao T., Parikh P., Bhashyam S., et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 124.McLean B.A., Wong C.K., Kabir M.G., et al. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol. Metab. 2022;66 doi: 10.1016/j.molmet.2022.101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakamura H., Niwano S., Niwano H., et al. Liraglutide suppresses atrial electrophysiological changes. Heart Vessels. 2019;34:1389–1393. doi: 10.1007/s00380-018-01327-4. [DOI] [PubMed] [Google Scholar]
- 126.Eid R.A., Alharbi S.A., El-Kott A.F., et al. Exendin-4 ameliorates cardiac remodeling in experimentally induced myocardial infarction in rats by inhibiting PARP1/NF-κB axis in A SIRT1-dependent mechanism. Cardiovasc. Toxicol. 2020;20:401–418. doi: 10.1007/s12012-020-09567-5. [DOI] [PubMed] [Google Scholar]
- 127.Eid R.A., Khalil M.A., Alkhateeb M.A., et al. Exendin-4 attenuates remodeling in the remote myocardium of rats after an acute myocardial infarction by activating β-arrestin-2, protein phosphatase 2A, and glycogen synthase kinase-3 and inhibiting β-catenin. Cardiovasc. Drugs Ther. 2021;35:1095–1110. doi: 10.1007/s10557-020-07006-9. [DOI] [PubMed] [Google Scholar]
- 128.Chen P., Yang F., Wang W., et al. Liraglutide attenuates myocardial fibrosis via inhibition of AT1R-mediated ROS production in hypertensive mice. J. Cardiovasc. Pharmacol. Ther. 2021;26:179–188. doi: 10.1177/1074248420942007. [DOI] [PubMed] [Google Scholar]
- 129.Monji A., Mitsui T., Bando Y.K., et al. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H295–H304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 130.Zheng R., Zhang W., Ji Y., et al. Exogenous supplement of glucagon like peptide-1 protects the heart against aortic banding induced myocardial fibrosis and dysfunction through inhibiting mTOR/p70S6K signaling and promoting autophagy. Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173318. [DOI] [PubMed] [Google Scholar]
- 131.DeNicola M., Du J., Wang Z., et al. Stimulation of glucagon-like peptide-1 receptor through exendin-4 preserves myocardial performance and prevents cardiac remodeling in infarcted myocardium. Am. J. Physiol. Endocrinol. Metab. 2014;307:E630–E643. doi: 10.1152/ajpendo.00109.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Younce C.W., Niu J., Ayala J., et al. Exendin-4 improves cardiac function in mice overexpressing monocyte chemoattractant protein-1 in cardiomyocytes. J. Mol. Cell. Cardiol. 2014;76:172–176. doi: 10.1016/j.yjmcc.2014.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Du J., Zhang L., Wang Z., et al. Exendin-4 induces myocardial protection through MKK3 and Akt-1 in infarcted hearts. Am. J. Physiol. Cell Physiol. 2016;310:C270–C283. doi: 10.1152/ajpcell.00194.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zheng R.-H., Bai X.-J., Zhang W.-W., et al. Liraglutide attenuates cardiac remodeling and improves heart function after abdominal aortic constriction through blocking angiotensin II type 1 receptor in rats. Drug Des. Devel. Ther. 2019;13:2745–2757. doi: 10.2147/DDDT.S213910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sukumaran V., Tsuchimochi H., Sonobe T., et al. Liraglutide treatment improves the coronary microcirculation in insulin resistant Zucker obese rats on a high salt diet. Cardiovasc. Diabetol. 2020;19:24. doi: 10.1186/s12933-020-01000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Withaar C., Meems L.M.G., Markousis-Mavrogenis G., et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc. Res. 2021;117:2108–2124. doi: 10.1093/cvr/cvaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J., Guo R., Ma X., et al. Liraglutide inhibits AngII-induced cardiac fibroblast proliferation and ECM deposition through regulating miR-21/PTEN/PI3K pathway. Cell Tissue Bank. 2023;24:125–137. doi: 10.1007/s10561-022-10021-9. [DOI] [PubMed] [Google Scholar]
- 138.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 139.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holman R.R., Bethel M.A., Mentz R.J., et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hernandez A.F., Green J.B., Janmohamed S., et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 142.Nreu B., Dicembrini I., Tinti F., et al. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: An updated meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020;30:1106–1114. doi: 10.1016/j.numecd.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Raubenheimer P.J., Cushman W.C., Avezum A., et al. Dulaglutide and incident atrial fibrillation or flutter in patients with type 2 diabetes: A post hoc analysis from the REWIND randomized trial. Diabetes Obes. Metab. 2022;24:704–712. doi: 10.1111/dom.14634. [DOI] [PubMed] [Google Scholar]
- 144.Neves J.S., Vasques-Nóvoa F., Borges-Canha M., et al. Risk of adverse events with liraglutide in heart failure with reduced ejection fraction: A post hoc analysis of the FIGHT trial. Diabetes Obes. Metab. 2023;25:189–197. doi: 10.1111/dom.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 146.Li S., Vandvik P.O., Lytvyn L., et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: A clinical practice guideline. BMJ. 2021;373 doi: 10.1136/bmj.n1091. [DOI] [PubMed] [Google Scholar]
- 147.Thotamgari S.R., Grewal U.S., Sheth A.R., et al. Can glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors help in mitigating the risk of atrial fibrillation in patients with diabetes? Cardiovasc. Endocrinol. Metab. 2022;11 doi: 10.1097/XCE.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu J., Yu X., Zheng Y., et al. Association of glucose-lowering medications with cardiovascular outcomes: An umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8:192–205. doi: 10.1016/S2213-8587(19)30422-X. [DOI] [PubMed] [Google Scholar]
- 149.Jorsal A., Kistorp C., Holmager P., et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 150.Kumarathurai P., Anholm C., Larsen B.S., et al. Effects of liraglutide on heart rate and heart rate variability: A randomized, double-blind, placebo-controlled crossover study. Diabetes Care. 2017;40:117–124. doi: 10.2337/dc16-1580. [DOI] [PubMed] [Google Scholar]
- 151.Lee J., Umana I.E., Nguyen J. Exacerbation of atrial fibrillation related to dulaglutide use. Clin. Case Rep. 2021;9 doi: 10.1002/ccr3.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Husain M., Bain S.C., Holst A.G., et al. Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: Combined post hoc analysis of the SUSTAIN and PIONEER trials. Cardiovasc. Diabetol. 2020;19 doi: 10.1186/s12933-020-01106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]