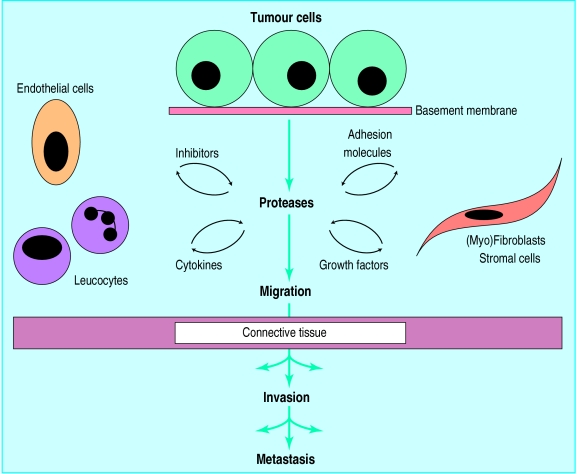
Just how tumours become malignant remains an enigma, despite major advances in our knowledge of genetic susceptibility, cellular derailment processes, and environmental factors. The rapid multiplication of cells in the early phase of a tumour does not usually cause serious disease so long as the growth remains confined to its original tissue boundaries. When, however, cells migrate from their original tissue compartment, invade the normal surrounding tissue, and disseminate throughout the body they have become malignant.
The migration and invasion characteristics of malignant cells requires them to be able to cross extracellular barriers. In the primary organ these predominantly consist of basement membranes and connective tissue, collectively called the extracellular matrix. The extracellular matrix is made up of a dense network of different components including laminin, fibronectin and other glycoproteins, collagens, and proteoglycans. To invade and metastasise, tumours possess a lytic machinery made up of different proteolytic enzymes, the proteases. The main classes of proteases contributing to the lytic processes around tumours are cathepsins, plasminogen activators, and matrix metalloproteinases.1 The first evidence of the active part played by these enzymes in neoplastic disease came from studies showing large amounts of these factors within malignant human tissues. Further evidence came from in vitro and in vivo experiments showing that non-invasive cells became invasive after gene-transfer of the proteolytic enzymes, and—conversely—that invasive cells could be functionally impaired by inhibition of the proteases.
Each class of proteases has natural inhibitors which modulate their activity—for example, the cystatins, which inhibit cathepsins, the plasminogen activator inhibitors, and the tissue inhibitors of matrix metalloproteinases.2 The expression and activity of the proteases is not, however, regulated only by their inhibitors. The proteolytic enzymes are first secreted as inactive proenzymes, and these become activated by proteolytic cleavage, which is thought to evolve as a cascade—cathepsins activate plasminogen activators, which convert plasminogen into plasmin, which in its turn is able to activate pro-matrix metalloproteinases. Other factors involved bidirectionally in the regulation of the proteolytic cascade include leucocyte derived cytokines. For example, tumour necrosis factor alpha induces the synthesis of matrix metalloproteinases, while the intracellular processing of this same tumour necrosis factor is regulated by a matrix metalloproteinase.3,4 Basic fibroblast growth factor, released from the extracellular matrix through plasmin-mediated proteolysis, can induce synthesis of proteolytic factors in tumour and endothelial cells, forming another loop in the proteolytic cascade (see figure).2
Though these processes are strongly implicated in the spread of cancer, similar phenomena take place in (patho)physiological processes such as inflammation, (neo)angiogenesis, ovulation, and wound healing, in all of which cell migration and tissue remodelling occur.5 Matrix metalloproteinases play an important part in the premature aging of skin by sunlight.6
Research into the clinical impact of proteases in human malignancies was boosted in 1988 when Duffy et al reported on the links between the activity of plasminogen activators in breast cancer tissue and the clinical outcome.7 Other groups later confirmed and expanded these observations. Compounds of the plasminogen activation system, cathepsins, and several matrix metalloproteinases were all shown to have a prognostic impact as defined by disease free interval and survival of patients with solid tumours of the breast, stomach, colorectum, cervix, kidney, and lung.7 One of the most consistent observations was the predictive value of the concentration of plasminogen activator inhibitor-1 in extracts of tissue from cancers of the breast, stomach, and lung.8 Recently, a high concentration of tissue inhibitor of matrix metalloproteinase-1 was also found to indicate a poor prognosis in non-small cell lung cancer.9
These findings were initially received with scientific restraint since the inhibitors were supposed to counteract the destructive activity of the proteolytic enzymes. It has, however, become increasingly clear that in most cancers plasminogen activator inhibitor-1 plays an important part in modulating the dynamic process of this kind of proteolysis. The mechanisms include binding to compounds such as vitronectin and adhesion molecules, and clearance of activator-inhibitor complexes via receptors, so regulating focal breakdown of the matrix and cellular adhesion and migration. The cells affected are not only the malignant cells but also myofibroblasts and leucocytes within the tumours.2,8 The study of Nielsen et al in this issue gives an extra dimension to the clinical impact of these proteolytic factors in cancer (p 829).10 They have shown that plasminogen activator inhibitor-1 measured in the circulation (not just in tissue extracts) is associated with the survival of patients with colorectal cancer. Multivariate analysis showed, however, that this relation with prognosis was based on the association with the Dukes stage of the tumours. Previous studies had already indicated that several components of the plasminogen activation system and matrix metalloproteinases were associated with the clinical outcome of subgroups of patients with colorectal cancer,9–14 though the findings were less consistent than those in breast cancer.
The picture is, then, becoming clearer. Proteases and their inhibitors contribute actively to tumour invasion and metastasis. They are also good indicators of the clinical outcome for patients with many types of cancer. Future research should unravel the complex tumour-associated proteolytic cascades and will identify new participants. Prospective studies will have to establish their value in the clinical management of patients. This might be achieved by selecting patients for further adjuvant therapy on the basis of the proteolytic status of their tumours; but another exciting possibility is that the proteases and their inhibitors might themselves become targets for therapeutic intervention to prevent or inhibit tumour invasion, progression, or recurrence.8,15 The first step along that road has been taken with clinical trials of the new generations of matrix metalloproteinase-inhibitors.16
Figure.
Schematic representation of the cells, regulators, and acellular contributors to protease mediated cancer invasion and metastasis
Papers p 829
References
- 1.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–185. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis; a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Gearing AJH, Beckett P, Christodoulou M, Churchill M, Clements J. Davidson AH, et al. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 4.McGeehan GM, Becherer JD, Bast RC, Jr, Boyer CM, Champion B, Connolly KM, et al. Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature. 1994;370:558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. BMJ. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 6.Opdenakker G, van Damme J. Cytokines and proteases in invasive processes: molecular similarities between inflammation and cancer. Cytokine. 1992;4:251–258. doi: 10.1016/1043-4666(92)90064-x. [DOI] [PubMed] [Google Scholar]
- 7.Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996;2:613–618. [PubMed] [Google Scholar]
- 8.Pappot H, Gårdsvoll H, Rømer J, Pedersen AN, Grøndahl-Hansen J, Pyke C, et al. Plasminogen activator inhibitor type 1 in cancer: therapeutic and prognostic implications. Biol Chem Hoppe-Seyler. 1995;376:259–267. doi: 10.1515/bchm3.1995.376.5.259. [DOI] [PubMed] [Google Scholar]
- 9.Fong KM, Kida Y, Zimmerman PV, Smith PJ. TIMP1 and adverse prognosis in non-small cell lung cancer. Clin Cancer Res. 1996;2:1369–1372. [PubMed] [Google Scholar]
- 10.Nielsen HJ, Pappot H, Christensen IJ, Brünner N, Thorlacius-Ussing O, Moesgaard F, et al. Association between plasma concentrations of plasminogen activator inhibitor-1 and survival in patients with colorectal cancer. BMJ. 1998;316:829–830. doi: 10.1136/bmj.316.7134.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulcahy HE, Duffy MJ, Gibbons D, McCarthy P, Parfrey NA, O’Donoghue DP, et al. Urokinase-type plasminogen activator and outcome in Dukes B colorectal cancer. Lancet. 1994;344:583–584. doi: 10.1016/s0140-6736(94)91968-2. [DOI] [PubMed] [Google Scholar]
- 12.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nature Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 13.Zeng ZS, Huang Y, Cohen AM, Guillem JG. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol. 1996;14:3133–3140. doi: 10.1200/JCO.1996.14.12.3133. [DOI] [PubMed] [Google Scholar]
- 14.Ganesh S, Sier CFM, Heerding MM, van Krieken JHJM, Griffioen G, Welvaart K, et al. Contribution of plasminogen activators and their inhibitors to the survival prognosis of patients with Dukes’ stage B and C colorectal cancer. Br J Cancer. 1997;75:1793–1801. doi: 10.1038/bjc.1997.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt M, Harbeck N, Thomssen C, Wilhelm O, Magdolen V, Reuning U, et al. Clinical impact of the plasminogen activation system in tumour invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost. 1997;78:285–296. [PubMed] [Google Scholar]
- 16.Bramhall SR. The matrix metalloproteinases and their inhibitors in pancreatic cancer. From molecular science to a clinical application. Int J Pancreatol. 1997;21:1–12. doi: 10.1007/BF02785914. [DOI] [PubMed] [Google Scholar]