Abstract
Objective
Resilience is a complex process of adaptation to new conditions that would permit a positive outcome after adversities, traumas or other sources of stress. However, despite the growing interest in this topic, there is no universally accepted definition and no comprehensive bio-behavioural model. This systematic review aims to provide an overview of the main biological models that have been theorized to date, with a focus on new alternative theories to improve our understanding of the mechanisms underlying the development and strengthening of resilience, with potential implications for the prevention of some psychopathological disorders.
Method
This review was conducted according to PRISMA guidelines and includes 185 studies published in English in PubMed and Embase up to December 2023.
Results
Most studies use the stress-related model, which conceptualizes resilience as the absence of symptoms after the stressful event and mainly deal with the differences between stress-prone and resilient phenotypes in animals exposed to stress. However, the results of this search seem to suggest that resilience might be an independent construct with biological bases rooted in the stress system and the social brain, and widely sculptured by individual and environmental factors, especially early life events and affiliation.
Conclusions
This work contributes to ongoing efforts to understand the intricate mechanisms of resilience, while highlighting the potential of improving social relationships since our birth to promote coping strategies towards stress and traumas, and even a peaceful world.
Keywords: resilience, neurobiology, stress, affiliation
1. Introduction
The term "resilience", originating in the field of technical sciences, refers to the capability of a material to return to its original shape or position after being bent, compressed or stretched (Alexander, 2013; Babić et al., 2020; Shastri, 2013). Recently, it has been translated into psychology and psychopathological domains to denote a sustained positive state and functioning despite the evidence of dangerous or adverse circumstances, as well as to underline a positive outcome after a trauma. As such, it represents an individual process of adaptation to new conditions, adversities, traumas, and any other significant source of stress (Bonanno et al., 2004; Luthar, 2006; Wu et al., 2013).
Resilience is a complex phenomenon that is not a one-dimensional construct, as it encompasses a wide range of characteristics (Horn & Feder, 2018; Murrough & Russo, 2019; Southwick et al., 2014). Although in the past, it was generally conceptualized as an "outcome" according to the classical model of the absence of symptoms after a stressful event, new evidence suggests that resilience is an independent phenomenon and not just the absence of distress or of psychiatric symptoms (Cloninger et al., 2012; van der Werff et al., 2017). The development of resilience, similarly to other individual characteristics and attitudes, may be related to genetics, temperament and cognitive abilities, quality of experiences, and environmental factors (Babić et al., 2020; Cathomas et al., 2019). Therefore, it is not totally innate, but resulting from the interplay of genetic features sculptured by environmental variables and learning, so that it can be improved and strengthened (Babić et al., 2020; Curtis & Cicchetti, 2007; Southwick et al., 2014). This feature is particularly relevant, as the development of resilience is a main goal of preventive strategies in different fields and especially in medical areas. Not surprisingly, a person's psychosocial resources for resilience have been identified as predictors of a better quality of life in different pathological conditions, such as cancer, cardiovascular diseases or diabetes, and mental disorders, up to the point that in the last years resilience-promoting programs have been developed for specific populations (Cal et al., 2015; Lee & Kim, 2017; Harms et al., 2019; Kim et al., 2019; Seiler & Jenewein, 2019; Zizolfi et al., 2019).
Due to recent dramatic events, such as the global pandemic and the increasing number of conflicts and clashes in different parts of the world, the issue of implementing resilience becomes of paramount importance. Nevertheless, neither a unified definition of resilience nor a complete bio-behavioural model has been formulated. Most of the scientific literature assesses resilience “ex post”, as the absence of symptoms and the maintenance of health after trauma, while focusing on the system of fear and the response to stress (Osório et al., 2017; Averill et al., 2018; García-León et al., 2019; Feldman, 2020; Swaminathan et al., 2023). Recently, alternative neurobiological models of resilience have been proposed, based on the notion that an in-depth knowledge of the mechanisms underlying the onset and maintenance of stress-related disorders, as well as those conferring stress resistance, might be useful for the development of novel treatment options (Marazziti et al., 2023; Russo et al., 2012).
The aim of this work was at providing a systematic review of the main biological models proposed for resilience, with a particular attention to latest available data and theories, in order to improve the understanding of the mechanisms underlying the development and strengthening of resilience with a possible impact in the prevention of distress, psychological or psychopathological disturbances.
2. Materials and methods
The present and systematic review was carried out in accordance with the PRISMA guidelines (Page et al., 2021). A combination of controlled vocabulary terms and free text terms, without filters, restrictions, or limits, was used to identify all potentially eligible records by examining the following databases: PubMed and Embase. The basic search string used was: "Resilience" AND “Psychology”, OR "Neurobiology", OR "Pathophysiology", OR "Stress system”, OR “Genetics", OR "Oxytocin", OR "affiliative Brain". All studies up to December 31, 2023, were included in the database search. All authors agreed to consider among the exclusion criteria: studies examining resilience or vulnerability to stress associated with substance and drug use; studies that included subjects with organic diseases; studies carried out in non-mammals; review articles, abstracts, posters and case reports; articles not available in English.
All the authors equally contributed in identifying potential information specific to this topic amongst the titles and abstracts of the publications. Any discrepancy that arose was discussed and consensus was reached. The overall level of agreement between the raters was good.
3. Results
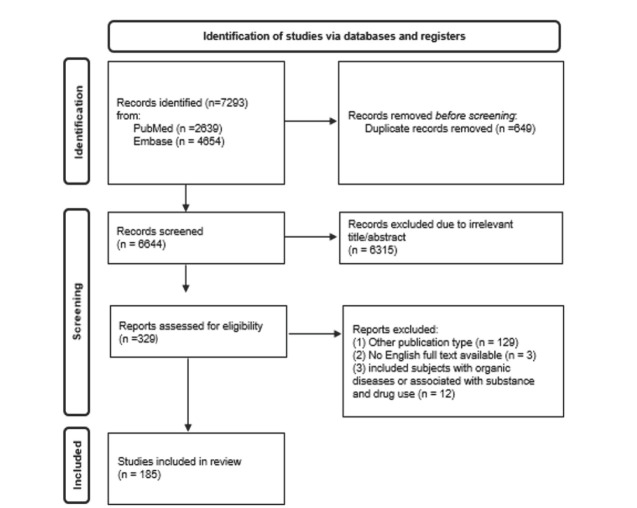
The initial database search produced a total of 7293 records. After that, 6964 articles were removed as duplicates (N=649) or as not relevant (N=6315); other 132 records were excluded because of different publication types (N=129) or because their full text was not available or not in English (N=3). Subsequently, 12 publications were withdrawn for examining resilience or vulnerability to stress associated with substance and drug use or including subjects with organic diseases. In addition, all references cited in the selected studies, including reviews and meta-analysis, were manually screened. However, no suitable articles emerged from this further research. Finally, 185 articles were included in the present review. Inclusion and exclusion decisions are summarized in a flowchart according to PRISMA recommendations (Page et al., 2021) (figure 1).
Figure 1.
Flowchart
4. Discussion
In recent years, the scientific community has become progressively interested in the topic of resilience, that is to say the ability to cope with and to overcome unavoidable stressors. Indeed, recently, the world's populations had and has to face an increasing number of dramatic events, such as the COVID-19 pandemic, the war in Ukraine and in the Middle East, scattered conflicts worldwide, terrorism, economic recession, famine, climate change leading to extreme meteorological events spoiling territories and forcing several individuals to migrate, as well as car crashes and homicides worldwide (World Health Organization, WHO, 2023). However, there is not yet a unified definition of resilience, nor a comprehensive bio-behavioural model of it. At the time of this writing, to our knowledge, this paper seems to be the first systematic review according to PRISMA guidelines (Page et al., 2021) that focuses on both the main and the new alternative biological models of resilience. The psychological features of resilience will be briefly discussed in the next paragraph, as some of them have been related to specific brain processes and/ or parameters.
4.1. Psychological characteristics of resilience
According to psychological research, resilience depends on different factors including temperament, intelligence, cognitive abilities, personality, quality of past experiences, and environmental variables (Leys et al., 2020).
The term “temperament” is used to denote individual differences in behavioural tendencies that are biologically rooted, present early in life, and relatively stable over time, although physical environment and social context may influence it (Dunn & Kendrick, 1982; Josefsson et al., 2013; Zohar et al., 2019). A scholar (Wachs, 2000), while exploring the contribution of temperament to the development of resilience in children, noted how temperament is multifaceted, as it depends on genotype, central nervous system (CNS) structure and processes, and bio-ecological (e.g., nutrition) or environmental factors (e.g., parenting style).
Personality is another fundamental factor contributing to the development of resilience. It can be defined as the whole of individual emotional, cognitive and behavioural characteristics determining the individual approach to life (Craik, 1997; Fleeson & Jayawickreme, 2015; Nieto et al., 2023). According to Cloninger’s theory, the personality can be understood according to a seven-factor model combining four temperament dimensions (harm avoidance, novelty seeking, reward dependence, persistence), each related to major neurotransmitters (serotonin, dopamine and norepinephrine) and three character dimensions (self-directedness, cooperativeness, self-transcendence), measurable by his specific questionnaire named “Temperament and Character Inventory” (TCI) (Cloninger et al., 1993; Cloninger, 1994). While temperament dimensions are considered to be heritable and present early in life, character dimensions fully mature in adulthood. A resilient personality profile seems to be characterized by low harm avoidance, high persistence and high self-directedness (Cloninger, 2004; Granjard et al., 2021). In a sample of 1,102 people, self-directedness resulted to be strongly linked to all the features of wellbeing (Cloninger & Zohar, 2011). An Australian study examined the relationships between resilience, assessed by the Resilience Scale, and personality traits measured by the TCI, in 479 doctors, while reporting that resilience was related to a personality pattern resulting to be responsible, persevering, optimistic, cooperative and mature (Eley et al., 2013). A series of studies, some of which carried out in athletes, cancer patients and subject during the COVID-19 pandemic, confirmed that optimism, positive emotions and extraversion are important for the development of resilience (Cathomas et al., 2019; Fasano et al., 2020; Lee, 2023; Tugade & Fredrickson, 2004; Tutte-Vallarino et al., 2022). On the contrary, low cooperativeness, low self-directedness, high self-transcendence and high harm avoidance resulted to be typical characteristics of survivors who developed post-traumatic stress disorder (PTSD) after the Oklahoma City bombing (North & Cloninger, 2012).
Experimental studies investigating psychological characteristics of resilience are shown in table 1.
Table 1.
Psychological characteristics of resilience. Experimental studies
| Authors and year | Country | Sample | Examined variables and type of assessment | Findings |
|---|---|---|---|---|
| Cloninger & Zohar, 2011 | Israel | Human sample (1,102 adults) |
|
|
| Eley et al., 2013 | Australia | Human sample (479 family practitioners) |
|
|
| Granjard et al., 2021 | Sweden | Human sample (293 adults) | TCI |
|
| Josefsson et al., 2013 | Finland | Human sample (1,083 volunteers) |
|
|
| Lee et al., 2023 | China | Human sample (182 Indonesian COVID-19 nurse survivors) |
|
|
| Nieto et al., 2023 | Spain | Human sample (439 adults) |
|
|
| North & Cloninger, 2012 | USA | Human sample (151 survivors of the Oklahoma City bombing) |
|
|
| Tugade & Friedrickson, 2004 | USA | Study 1: Human sample (57 adults) |
Study 1:
|
|
| Study 2: Human sample (57 adults) |
Study 2:
|
|
||
| Study 3: Human sample (192 adults) |
Study 3:
|
|
||
| Tutte-Vallarino et al., 2022 | Uruguay | Human sample (121 athletes) |
|
|
| Zohar et al., 2019 | USA | Human sample (752 adolescents) |
|
|
BHS= Beck Hopelessness Scale; CBI= Copenhagen Burnout Inventory; CD-RISC= Connor–Davidson Resilience Scale; DBP= Diastolic Blood Pressure; DIS= Diagnostic Interview Schedule; DS= Disaster Supplemen; ER= Resilience Scale; FPA= Finger Pulse Amplitude; GHQ= subjective health assessment of the General Health Questionnaire; HR= Heart Rate; JTCI= Junior Temperament and Character Inventory; IBD-R= Inventory of Burnout in Athletes Revised; LOT-R= Life Orientation Scale-Revised; NEO-FFI= NEO-Five Factor Inventory; PANAS= Positive and Negative Affect Scale; PES= Psychological Empowerment Scale; PSS= Multidimensional Scale of Perceived Social Support; PTE= Pulse transmission Time to the Ear; PTF= Pulse transmission Times to the Finger; SBP= Systolic Blood Pressure; SWLS= Satisfaction with Life Scale ; SES= Socioeconomical Status; TCI= Temperament and Character Inventory;
4.2. Neurobiology of resilience
One of the main findings emerging from the biological research on resilience is the lack of studies in human samples, as most of the included articles gathered data in animals, mainly rats and mice, exposed to different stressors to analyse their adaptive processes and to assess the differences between stress-proneness and resilient phenotypes. Although animal models have always been used to investigate biological characteristics, this kind of data, albeit useful, cannot be totally overlapping with the human ones. Again, this review confirms the major bias that generally resilience continues to be conceptualized as the absence of symptoms after a trauma, despite the evidence that it is an independent construct (Cloninger et al., 2012; van der Werff et al., 2017). Indeed, resilience is characterized not only by the absence of detrimental psychological outcomes (also defined as "passive resilience"), but also by active adaptive coping mechanisms (i.e. "active resilience") that recognize neural, behavioural, hormonal, molecular and cellular underpinnings (Russo et al., 2012). Interestingly, individual variables seem to be important, as it has been demonstrated that, even when facing highly stressful situations and events, the majority of people do not develop psychological and/or psychopathological consequences (Russo et al., 2012). Generally speaking, in humans research has mainly focused on genetic variables that might be predictive of resilience and almost exclusively on stress response (Cathomas et al., 2019; Niitsu et al., 2019). Regarding the latter, from an evolutionary point of view, stressful stimuli represent an obstacle to the achievement of biological goals. The negative emotive reaction to stressors, which might include anxiety and depression, warns us that our efforts in trying to reach a certain goal are possible to fail. Besides, following evolutionary-based models, goal priorities differ according to sex and age, and are influenced by sociocultural aspects (Troisi, 2018).
In the next paragraphs, we shall review and comment on genetics, stress response, early life events and affiliative processes in relation to resilience.
Experimental studies investigating the neurobiology of resilience are shown in table 2.
Table 2. Neurobiology of resilience. Experimental studies.
2.a. Genetics
| Authors and year | Country | Sample | Materials and methods | Findings |
|---|---|---|---|---|
| Mesquita et al., 2015 | Portugal | Human sample (220 children) |
|
Higher indiscriminate behaviour in s/s homozygotes of 5-HTTLPR, if institutionalized |
| Sweitzer et al., 2013 | USA | Human sample (546 volunteers) |
|
Bidirectional association of DRD4 genotype with temporal discounting, conditioned by participants’ early life circumstances, according to the “differential susceptibility” model |
BDNF= brain-derived neurotrophic factor; DAI= disturbances of attachment interview; DRD4= dopamine D4 receptor; SES= socioeconomic status.
2.b.
Stress response
| Authors and year | Country | Sample | Materials and methods | Findings |
|---|---|---|---|---|
| Anacker et al., 2011 | UK | Human hippocampal progenitor cell line HPC03A/07 | Cells were treated with sertraline for 72 hours during proliferation, and for subsequent 7 days of differentiation | Sertraline increases human hippocampal neurogenesis through a GR-dependent mechanism |
| Anacker et al., 2018 | UK | Animal sample (mice) | In vivo calcium imaging to record neuronal activity from ventral DG cells |
|
| Cole et al., 2022 | USA | Animal sample (mice) |
|
Hippocampal output to the BNST contributes to a net inhibition of the HPA axis |
| Eriksson et al., 1998 | Sweden | Post-mortem human brain tissue (5 adults) | Immunofluorescent labeling for BrdU, NeuN, calbindin, NSE | New neurons are generated from dividing progenitor cells in the DG |
| Licht et al., 1983 | USA | Animal sample (bullfrogs) | Investigation cycles and plasma of seasonal levels gonadal of FSH, LH, estrogen, progesterone, testosterone, 5α-DHT, corticosterone | Gonadotropins and steroids are highly labile, and particularly sensitive to the effects of captivity, especially in males |
| Loprinzi et al., 2011 | USA | Human sample (25 women) | Assessing the effect of a SMART program among women diagnosed with breast cancer through several outcome measures scales, | Significant improvement in resilience, perceived stress, anxiety, and overall quality of life at 12 weeks |
| Moreno-Jiménez et al., 2019 | Spain | Human brain samples | Combining human brain samples using tissue processing methods | Thousands of immature neurons are found in the DG of neurologically healthy individuals up to the 9th decade of life |
| Santarelli et al., 2003 | USA | Animal sample (mice) | Genetic and radiological methods | X-irradiation of the hippocampus prevents the neurogenic and behavioral effects of antidepressants |
| Smith et al., 1995 | USA | Animal sample (rats) |
|
|
| Snyder et al., 2011 | USA | Animal sample (mice) | Inhibition of adult neurogenesis through transgenic or radiation methods | A small pool of DG neurons are critical for hippocampal negative control of the HPA axis, and play a role for adult neurogenesis in depression |
| Somasunda- ram & Devamani, 2016 | India | Human sample (60 adults) | Investigating resilience, perceived social support and hopelessness in cancer patients through assessing scales | Resilience is associated with less hopelessness and higher levels of perceived social support |
| van der Werff et al., 2017 | The Netherlands | Human sample (81 adults) | MRI scanning | Resilient subjects show increased structural connectivity in the corticopontine tract |
| Wang et al., 2013 | The Netherlands | Post-mortem human brain tissue (26 subjects) | GR-immunoreactivity | GRs are prominently expressed in human hippocampus |
5α-DHT= 5α-dihydrotestosterone; BDNF=brain derived neurotrophic factor; BNST= bed nucleus of the stria terminalis; BrdU= bromodeoxyuridine; DG= dentate gyrus; FSH= follicle-stimulating hormone; GR= glucocorticoid receptor; HPA= hypothalamic-pituitary-adrenal; LH= luteinizing hormone; MRI= magnetic resonance imaging; NeuN= hexaribonucleotide binding protein 3; NSE= neuron specific enolase; NT-3= neurotrophin-3; SMART= stress management and resiliency training.
2.c.
Early life stress
| Authors and year | Country | Sample | Materials and methods | Findings |
|---|---|---|---|---|
| Boscarino et al., 1997 | USA | Human sample (,1399 adults) |
|
Direct link between severe military stress exposure and a broad spectrum of human disease (found associations with circulatory, digestive, musculoskeletal, endocrine-nutritional-metabolic, nervous system, respiratory and nonsexually transmitted infectious diseases) |
| Chugani et al., 2001 | USA | Human sample (17 children, 17 adults) |
|
|
| Hodel et al., 2015 | USA | Human sample (172 adolescents) |
|
|
| Luby et al., 2012 | USA | Human sample (92 children) |
|
|
| Lupien et al., 2011 | Canada | Human sample (38 children) |
|
|
| Quirin et al., 2008 | Germany | Human sample (48 women) |
|
-Attachment anxiety is positively related with CRS Attachment anxiety is negatively related with CRA |
| Quirin et al., 2010 | Germany | Human sample (22 young adults) |
|
|
| Tottenham et al., 2010 | USA | Human sample (78 children) |
|
|
| Zuo et al., 2019 | China | Human sample (40 adolescents) |
|
|
ACC= Anterior Cingulate cortex; AIDS= acquired immunodeficiency syndrome; BAI= Becks Anxiety Inventory; CBCL= Child Behavior Checklist; CD-RISC= Connor-Davidson Resilience Scale; CELF-III= Comprehensive Evaluation of Language Functions—Third Edition; CERQ= Cognitive Emotion Regulation Questionnaire; CES-D= Center for Epidemiologic Studies Depression Scale; CRA= Cortisol Response to Awakening; CRS= Cortisol Response to acute Stress; ECR= Experiences in Close Relationships scale; EOWPVT-R= Expressive One-Word Picture Vocabulary Test—Revised; FDG= Fluorodeoxyglucose; GDS= Gordon Diagnostic System; HTQ= Harvard Trauma Questionnaire; IDS= Inventory of Depression Symptomatology; MRI= Magnetic Resonance Imaging; HC= Hippocampus; MADRS=Montgomery-Asberg Depression Rating Scale; MINI= Mini-International Neuropsychiatric Interview; NEO-FFI= NEO Five Factory Inventory; PET= Positron Emission Tomography; PI= Post-Institutionalized; PLES= Police Life Events Schedule; PPVT-3= Peabody Picture, Vocabulary Test—Third Edition; PSS=Perceived Stress Scale; PTSD= Post-traumatic stress disorder; SCARED= Screen for Child Anxiety Related Emotional Disorders; VBM= Voxel-based morphometry; WASI= Wechsler Abbreviated Scale of Intelligence; WISC-III= Weschler Intelligence Scales for Children—Third Edition; WRAML= Wide Range Assessment of Learning and Memory.
2.d.
Affiliation
| Authors and year | Country | Sample | Materials and methods | Findings |
|---|---|---|---|---|
| Chatzittofis et al., 2014 | Cyprus | Human sample (28 adults) |
|
|
| de Wied et al., 1991 | Netherlands | Animal sample (rats) |
|
Existence of a separate neurohypophyseal hormone receptor complex in the brain affecting memory processes that differs from the peripheral vasopressin receptors and OTXR |
| Domes et al., 2007 | Germany | Human sample (13 adults) | Use of fMRI to assess neural responses to fearful, angry, and happy facial expressions after intranasal application of 24 IU OXT compared with placebo |
|
| Heim et al., 2009 | USA | Human sample (22 adults) | Measurement of OXT concentrations in CSF in women exposed to different degrees of various forms of childhood abuse or neglect |
|
| Heinrichs et al., 2004 | Switzerland | Human sample (38 adults) |
|
Central OXT selectively influences memory performance depending on both the kind of memory test used and the psychobiological relevance of stimuli |
| Kersten et al., 2023 | Germany | Human sample (389 adults) |
|
|
| Kirsch et al., 2005 | Germany | Human sample (15 adults) |
|
|
| Koch et al., 2023a | Netherlands | Human sample (77 adults) |
|
|
| Koch et al., 2023b | Netherlands | Human sample (77 adults) | Effects of OXT administration on subjective anxiety and functional connectivity of BLA and CeM amygdala subregions with prefrontal and salience processing areas |
|
| Levy et al., 2023 | Israel | Human sample (34 mothers and 26 children) |
|
|
| Lucas-Thompson & Holman, 2013 | USA | Human sample (704 adults) |
|
|
| Marazziti et al., 2006 | Italy | Human sample (45 adults) |
|
Attachment anxiety and OXT levels are positively linked to romantic attachment (the higher the OXT levels the higher the score on the anxiety scale of the ECR) |
| Marazziti et al., 2019 | Italy | Human sample (90 adults) |
|
|
| Marazziti et al., 2022 | Italy | Human sample (90 adults) |
|
|
| McCarthy et al., 1996 | USA | Animal sample (mice) |
|
|
| Mirescu et al., 2004 | USA | Animal sample (rats) | Influence of early adverse experience on the regulation of adult neurogenesis in the hippocampus | Early adverse experience inhibits structural plasticity via hypersensitivity to glucocorticoids and reduces the ability of the hippocampus to respond to stress in adulthood |
| Mizuki & Fujiwara, 2015 | Japan | Human sample (80 adults) |
|
|
| Mohiyeddini et al., 2014 | UK | Human sample (90 adults) | Evaluation of early life stress, emotional suppression and plasma OXT levels | Emotional suppression may link early life stress and OXT |
| Pratt et al., 2017 | Israel | Human sample (97 mother and their children) |
|
|
| Ross et al., 2009 | USA | Animal sample (prairie and meadow voles, rats and mice) |
|
|
| Sack et al., 2017 | Germany | Human sample (35 adults) | Evaluation of the patients’ psychic and cardiac response to trauma-script exposure with and without OXT pretreatment | OXT treatment decreases the intensity of provoked PTSD symptoms in female patients |
| Sippel et al., 2017 | USA | Human sample (2,163 adults) | Evaluation of the effects of the OXTR SNP rs53576 and attachment style on the risk for PTSD in U.S. military veterans | Both polymorphisms in the OXTR gene and the attachment style may possibly contribute to vulnerability to PTSD |
| Tollenaar et al., 2017 | Netherlands | Human sample (2,567 adults) |
|
Childhood maltreatment is associated with both lifetime depression and anxiety diagnoses, and with depression and anxiety sensitivity |
| Ulmer-Yaniv et al., 2016 | Israel | Human sample (189 subjects) | Plasma levels of OXT, β-End, and IL-6 | Periods of bond formation are accompanied by increased activity and tighter cross-talk among systems underpinning affiliation, reward, and stress management |
| van Zuiden et al., 2017 | Netherlands | Human sample (120 adults) |
|
|
| Wei et al., 2011 | USA | Animal sample (mice) | Neurogenesis during different developmental phases (juvenile or adult) through its ablation | Juvenile and adult neurogenesis make different contributions to social competence in adult female mice |
| Windle et al., 2004 | UK | Animal sample (rats) |
|
|
β-End= beta endorphin; BLA=basolateral; CAPS= clinician-administered PTSD scale; CeM=centromedial; CGI-S=Clinical Global Impression-severity; CSF=cerebrospinal fluid; dACC= dorsal anterior cingulate cortex; ECR=Experiences in Close Relationships; fMRI=Functional Magnetic Resonance Imaging; HPA= hypothalamic-pituitary-adrenal; HRSD=Hamilton Rating Scale for Depression; IES-R=Impact for Event Scale revised; IL-6= interleukin-6; MRI=magnetic resonance imaging; OCD=Obsessive-compulsive disorder; OXT= oxytocin; OXTR= oxytocin receptor; PTSD= post-traumatic stress disorder; PVN= paraventricular nucleus; SNP= single nucleotide polymorphism; TSST=Trier Social Stress Test; vmPFC=ventromedial prefrontal cortex; Y-BOCS=Yale-Brown Obsessive-compulsive Scale
4.2.1. Genetics
Consistently with the diathesis-stress model, theories and studies have been proposed to assess the role of the combination of genetic factors and stressful events in relation to resilience (Monroe & Simons, 1991; Cicchetti & Blender, 2006; Feder et al., 2009; Kim-Cohen & Gold, 2009). In addition to genetic susceptibility, the concept of "vantage sensitivity" has been used to describe the evidence that some individuals are more sensitive and respond positively to environmental stimuli, and some do not (Pluess & Belsky, 2013; Sweitzer et al., 2012). Some individuals seem to be more vulnerable to the negative effects of an adverse environment but, according to the "differential susceptibility hypothesis", these individuals would also be more responsive to benign environmental conditions. This evolutionary-based concept has been well conveyed by the expression "sensitivity to the environment for better and for worse", and has been opposing to the previous and prevailing diathesis-stress model of psychopathology. More specifically, according to some authors, certain individual characteristics seem to function as "plasticity factors", instead of "vulnerability factors". Conceptualizing plasticity as a gradient, environmental sensitivity/responsiveness is probably represented by a bell-shaped curve (Belsky & Pluess, 2009; Belsky, 2016). Thanks to adaptive developmental plasticity an individual is able to respond to external conditions without undergoing genome modifications. Plasticity is, indeed, sustained by several mechanisms, including epigenetic ones (Troisi, 2018). Nonetheless, a research study conducted in 85 institutionalized and 135 home-reared children, found that s/s homozygotes of the serotonin transporter gene 5-HTTLPR showed higher levels of indiscriminate behaviour if institutionalized, but not if home-reared, in comparison to all the other children. These findings supported the diathesis-stress rather than the differential-susceptibility model (Mesquita et al., 2015).
Several studies have been published aiming to identify genetic factors that facilitate successful adaptation to adverse events, however, they are very heterogeneous and inconclusive (Cahill et al., 2022; Cathomas et al., 2019; Maul et al., 2020; Niitsu et al., 2019). To simplify, resilience and vulnerability can be conceived as located at the opposite extremes along a continuum, with resilience representing the ability of enduring stressful situations, and vulnerability representing the predisposition, based on hereditary and individual factors, to develop an illness, usually in response to environmental stressors. It is important to underline how the individual's resilience/ vulnerability construct is not unidimensional or static, as it may change over time and according to different circumstances. In fact, resilience can be strengthened in vulnerable subjects, but also weakened or lost in strong people when distress overcomes their resources and adaptive skills. This would suggest that resilience is a constant process of adjustment to new conditions, always requiring a growing and broader range of inner resources (Babić et al., 2020).
4.2.2. Stress response
The main systems and processes analysed so far are those of fear and stress response. The hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) play a key role in the response to stress, leading to a "fight-or-flight" response (Goligorsky, 2001; Goldstein, 2010; Chu et al., 2022). Specifically, neurons in the paraventricular nucleus (PVN) of the hypothalamus secrete corticotropin-releasing hormone (CRH) that stimulates the anterior pituitary to produce adrenocorticotropic hormone (ACTH). This, in turn, triggers the release of glucocorticoids by the adrenal cortex, while the activation of the sympathetic system elicits the release of adrenaline from the adrenal medulla. In stressful situations the adrenal cortex also releases dehydroepiandrosterone (DHEA) that exerts antiglucocorticoid effects in the CNS (Jakovljevic, 2019). The glucocorticoid receptors are widely distributed in the CNS, being particularly represented in the hypothalamus, pituitary, cerebellum and hippocampus, and they regulate the HPA axis activity through a negative feedback (McEwen et al., 1968; Wang et al., 2013). Obviously, the stress system is strictly related to neurotransmission, gonadotropins, neurotrophic processes, and neuropeptides, such as oxytocin (OXT) and galanin (Licht et al., 1983; Goldstein, 1990; Smith et al., 1995; Uvnäs-Moberg & Petersson, 2005; Wrenn & Holmes, 2006; Mora et al., 2012; Bath et al., 2013). Regarding the main neurotransmitters, stress may provoke a significant impairment in 5-HT activity and increase in dopamine transmission in the mesolimbic pathway that is in turn modulated by the activation of glutamatergic transmission in the prefrontal cortex (PFC). These stress-induced changes occurring in neurotransmission have been related to the development of some psychiatric disorders, such as depression, anxiety and schizophrenia, and even to vulnerability to addiction (Chaouloff et al., 1999; Moghaddam, 2002; Sinha, 2009). When the stress response is adaptive, it determines the maintenance of homeostasis and is referred to as “allostasis”, when it is not it becomes a so-called “allostatic load” (McEwen, 1998). Chronic stress is considered a vulnerability condition promoting the development not only of stress and related disorders, but also of mood and anxiety disorders, as well as of somatic symptoms (Klengel & Binder, 2015; Davis et al., 2017; Atrooz et al., 2019; Larosa & Wong, 2022). A low resilience has been associated with PTSD, severe major depressive disorder (MDD) and suicide tendencies (Loprinzi et al., 2011; Somasundaram & Devamani, 2016; Osório et al., 2017).
As mentioned above, the hippocampus plays a fundamental role in the stress response, as it regulates the HPA axis, while exerting an inhibitory feedback on the release of glucocorticoids (Snyder et al., 2011; Cole et al., 2022; Larosa & Wong, 2022). The hippocampus is very sensitive to the HPA activity for its large number of glucocorticoid receptors (Joëls, 2018; Leschik et al., 2021). Interestingly, it has been reported that stress and subsequent increased and prolonged glucocorticoid release may lead to a reduction in adult neurogenesis, while diminishing newborn neurons. This might explain why some conditions such as MDD often arise after a long-term stress exposure (Leschik et al., 2021). The relationship between stress and neurogenesis seems to be reciprocal. In fact, while stress influences neurogenesis, it also seems that adult neurogenesis constitutes a protective factor against stress-induced MDD, so that it might be considered a resilience mechanism itself (Leschik et al., 2021). Interestingly, the deletion of a proapoptotic gene has been shown to be associated with increased neurogenesis and to represent a resilience factor in mice (Cathomas et al., 2019). Again, neurogenesis in mice seems to be linked to stress resilience through the inhibition of the activity of mature granule cells in the ventral dentate gyrus (DG) of the hippocampus. More specifically, increased neurogenesis results in a decreased activity of those stress-responsive cells that are activated during anxiogenic situations (Anacker et al., 2018). It is noteworthy that some studies utilizing genetic and radiological methods led to the hypothesis that the effects of antidepressants might be mediated by the increased neurogenesis in the hippocampus (Santarelli et al., 2003; Malberg, 2004; Bremner, 2006; Taupin, 2006; Warner-Schmidt & Duman, 2006; Anacker et al., 2011; Mahar et al., 2014;). Taken altogether, however, data on the relationship between hippocampus and resilience remain inconclusive (van der Werff et al., 2017). It should be also noted that adult neurogenesis has been demonstrated in mammals, mainly through rodent animal models, in two main areas, that is to say the subventricular zone (SVZ) of lateral ventricles, and the hippocampus, specifically the subgranular zone (SGZ) of the DG, while the existence of neurogenesis in adult humans is still debated (Eriksson et al., 1998; Leschik et al., 2021). Adding to this debate is a recent study highlighting the persistence of neurogenesis in the adult hippocampus during aging. Using autopsy samples, Moreno-Jiménez et al. demonstrated the existence of thousands of immature neurons in the DG of neurologically healthy individuals up to the ninth decade of life. This result was obtained by combining human brain samples obtained under strictly controlled conditions using state-of-the-art tissue processing methods (Moreno-Jiménez et al., 2019).
4.2.3. The impact of early life stress
Besides the role of chronic stress in the pathogenesis and maintenance of a variety of medical and psychopathological conditions (Boscarino, 1997; Miller & Blackwell, 2006; Salleh, 2008; Wosu et al., 2013; Cattaneo & Riva, 2016; Davis et al., 2017; Liu et al., 2017; Adams et al., 2018), more recently the importance of early life stressful experiences on later life has been increasingly documented (Carr et al., 2013; Juruena et al., 2020; Martins et al., 2011; Nakama et al., 2023; Schiavone et al., 2015; Smith & Pollack, 2020; Targum & Nemeroff, 2019; Ventriglio et al., 2015). The literature on animal models demonstrates that pups experiencing maternal deprivation over a long period of time exhibit increased susceptibility to subsequent stressors in adulthood, hyperactivity of the HPA axis, and altered glucocorticoid responses. However, when stress exposure is less severe, it could have a positive effect on resilience, a process known as “stress inoculation”. Rats exposed to moderate stress early in life show attenuated increases in plasma corticosterone and lower plasma levels of CRH after stress exposure compared with unstressed and severely stressed rats as pups (Cathomas et al., 2019). In humans, studies were conducted mainly using neuroimaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), to examine the effects exerted on the brain by early life stress as occurring in children and adolescents who were institutionalized at a young age as being orphaned or abandoned (Chugani et al., 2001; Hodel et al., 2015; Tottenham et al., 2010). It is well known that institutionalized children show altered daily cortisol patterns (Carlson & Earls, 1997), possibly implying alterations in those brain regions that appear to be more stress-sensitive such as the PFC, the limbic system and the hippocampus (Bremner, 2006; Hodel et al., 2015). Alterations of glucose metabolism have been also reported in the limbic regions, more specifically in the hippocampus and in the amygdala (Chugani et al., 2001) that also resulted, respectively, smaller and larger (Tottenham et al., 2010; Hodel et al., 2015). Again, a correlation was detected between the duration of the early life deprivation and the alterations of hippocampus volume (Hodel et al., 2015). It is noteworthy that disturbances in brain development caused by early deprivation seem to persist years after the stress exposure, as demonstrated by the presence of these modifications in adolescents who had been institutionalized at a very early age, while underlining long-term detrimental effects of early adversities. Moreover, these alterations do not seem to be completely reversible, as they are not mitigated by subsequent ameliorations of life conditions, such as adoption (Hodel et al., 2015). Similarly, a study conducted in children exposed to maternal depressive symptomatology, revealed a larger amygdala volume in comparison to the control group, with no differences in hippocampal volumes. Interestingly, a positive correlation between the mother’s mean depressive score and the amygdala volume was observed (Lupien et al., 2011). Also, acquired immunodeficiency syndrome (AIDS) orphans showed reduced hippocampus and larger anterior cingulate cortex volumes in comparison to the control group, while no differences in amygdala volumes (Zuo et al., 2019). On the contrary, early maternal support seems to be related to larger hippocampal volumes (Luby et al., 2012). Therefore, early stress, through a glucocorticoid dysregulation, results in impaired emotion and stress response persisting in adult life, and sustained by altered hippocampal structure and function (Quirin et al., 2010). Smaller hippocampus and anterior cingulate volumes, and increased amygdala function were also found in patients with PTSD, confirming the lasting changes on structure and function exerted by traumatic stress on the above mentioned brain areas (Bremner, 2006).
According to the “attachment theory” parental care represents another fundamental factor for child development (Bowlby, 1969, 1973, 1980; Ainsworth et al., 1978; Brennan et al., 1998). Indeed, the so-called early attachment, that is the relationship between an infant and its caregiver, might influence even adulthood, not only in terms of response to stressors, but also affecting one’s personality traits and interpersonal relationships. While a supportive caregiver is likely to result in a secure attachment style in the offspring, a poor maternal care is likely to lead to an insecure attachment style that in turn can be distinguished in avoidant and anxious attachments (Perlini et al., 2019; Quirin et al., 2008; 2010). It is noteworthy that different attachment styles have been related to alterations in the amygdala, hippocampus, cingulate, cortex and that insecure attachment style seems to induce a reduced hippocampal volume. More specifically, avoidant and anxious attachment styles were related to, respectively, a bilateral hippocampus reduction and a left hippocampus reduction, with a smaller hippocampal size deriving from diminished cellular volume and density (Quirin et al., 2010).
In the last years, however, new brain areas and circuits have been investigated when exploring the effects of traumas and resilience mechanisms. For example, the importance of the corticopontine tract in stress-related disorders has been highlighted in a study conducted amongst Dutch police officers. In resilient police officers who had been trauma-exposed but showed no psychopathology, an increased structural connectivity was detected in the corticopontine tract. The presence of white matter integrity in this neural pathway was also associated with a copying style of positive reappraisal (van der Werff et al., 2017).
4.2.4. Affiliation
In line with the latest available data, novel biological models have been proposed, such as that based on the neurobiology of affiliation (Bora et al., 2009; Carter et al., 1997; Feldman, 2016; Ulmer-Yaniv et al., 2016) that is closely related to the concept of attachment previously described (Bemporad, 1984; Feldman, 2020; Sheldon & West, 1989). The neurobiology of affiliation is a bio-behavioural model grounded on the concept that mammals are born with an immature brain that grows in the context of variables such as physical proximity and lactation. More specifically, this model relies on the assumption that the neurobiological processes that promote resilience are closely related to the infant's initial dependence on the mother or primary caregiver, as the brain matures in the context of the mother's body and caregiving behaviour. This developmental paradigm is evolutionary- and socially-based, and also survival-related (Carter et al., 1997; Depue et al., 2005; Ross & Young, 2009; Insel, 2010; Stoesz et al., 2013; Feldman, 2017, 2020). From a neurobiological perspective, affiliation is possibly grounded on the social brain, the OXT system, and bio-behavioural synchrony, factors all promoting resilience (Feldman et al., 2016; Feldman, 2020). The affiliative brain is a system that springs from the relationships between the offspring and the caregivers, while allowing the former to develop and maintain all close social relationships along the lifespan. The system supporting affiliation and attachment mainly relies on OXT and on the reward system modulated by dopamine neurotransmission (Insel, 2010; Feldman, 2020; Ross & Young, 2009; Ross et al., 2009). Oxytocin, a nonapeptide primarily synthesized in the paraventricular and supraoptic nuclei of the hypothalamus (Buijs, 1978; Carter, 2017; Carter et al., 2020), is considered a pleiotropic hormone that plays a crucial role in mammals while promoting social behaviours and the formation of social bonds (de Wied et al., 1991; Carter & Keverne, 2009; Carter, 2017; Carter et al., 2020). In addition, it acts as a potent modulator of the immune system and is released during the stress response, while decreasing the magnitude of amygdala responses to aversive stimuli (Carter, 2017; Kirsch et al., 2005; Marazziti et al., 2006; Marazziti & Catena Dell’Osso, 2008; McCarthy et al., 1996; Uvnäs-Moberg & Petersson, 2005). Converging studies showed that the exogenous OXT administration modulated anxiety and fear responses in threatening situations (Landgraf & Neumann, 2004) and mitigated the activation of the HPA axis and sympathetic system, by reducing heart rate, blood pressure and cortisol levels (Windle et al., 2004). Different data in humans showed a significant relationship between reduced endogenous OXT concentrations and traumatic experiences and/or PTSD following early severe and recurrent abuse during childhood. These data suggest that alterations in stress response, including OXT modulation, could explain the increased risk for impaired brain development following severe traumatic experience in children (Chatzittofis et al., 2014; Heim et al., 2009; Mirescu et al., 2004; Mohiyeddini et al., 2014). On the contrary, OXT levels were shown to be increased in children exposed to minor traumas who lived in safe environments (Mizuki & Fujiwara, 2015). Increased OXT levels also seem to be typical of women exposed to traumatic/stressful situations, to perhaps promote pro-social and supporting behaviours (Sack et al., 2017), while highlighting possible gender effects (Marazziti et al., 2019; 2022). Genetic studies showed that some OXT receptor (OXTR) gene polymorphisms might be related to increased risk of developing PTSD and, possibly, reduced resilience (Lucas-Thompson & Holman, 2013; Tollenaar et al., 2017), while other OXTR gene polymorphisms might have a protective function (Cicchetti & Rogosch, 2012). The OXTR rs53576 GG genotype is the most investigated, and it seems to be related to insecure attachments, poor response to social support, emotional dysregulation and decreased resilience (Cicchetti & Rogosch, 2012; Sippel et al., 2017). The administration of exogenous OXT in subjects exposed to trauma experiences or suffering from PTSD led to inconclusive or opposite results (Domes et al. 2007; Heim et al., 2009; Heinrichs et al., 2004; Koch et al., 2016a; 2016b; van Zuiden et al., 2017). More recently, a systematic review concluded that long-term use of OXT might decrease, albeit not at statistical level, the severity of PTSD symptoms (Di Lorenzo et al., 2020).
The behavioural synchrony can be defined as the “coordination of biological and behavioural signals between social partners during moments of social contact” (Feldman, 2012). The behavioural synchrony begins in utero as, for instance, the mother and the infant's heart rhythms and the release of OXT are synchronized, and then continues during parental care and, ultimately, other social interactions (Feldman, 2007, 2012, 2015, 2020; Ulmer-Yaniv et al., 2016; Levy et al., 2017; Pratt et al., 2017; Davis et al., 2018). According to Feldman’s studies, there exist five developmental stages of behavioural synchrony starting from the neonatal period to adulthood, each with distinctive features and interfering factors (Feldman, 2020).
As already mentioned, all the components of the neurobiology of affiliation confer resilience, as they improve stress management, immune functions and emotion regulation, promote reward from affiliation, social brain development, empathy and intimacy, friendship and love relationships, connection with fellows and nature, and they would also enable the generation of abstract ideas (Wei et al., 2011; Kersten et al., 2023; Seyfarth & Cheney, 2013; Walker & McGlone, 2013; Feldman, 2020). According to this notion, resilience seems to derive from plasticity, conceived as flexible adaptation to changes, and applying to all living matter, sociality that can be detected across animal evolution and that increases the chances of survival, and meaning that refers to the human-specific skill of giving a sense to external and internal events (Feldman, 2020). The neurobiological model of affiliation and, thus, the development of resilience can be disrupted by several variables that can depend on the mother (such as post-partum depression), on the child (such as premature birth), and on the context (such as war and poverty) (Feldman, 2020).
What is evident from this review is that some of the crucial factors promoting the correct development of resilience are rooted in child rearing and adequate caregiving. According to some authors, the influence of early childhood development and caregiving providing nurturing environments, might be also related to peacebuilding. They also highlight successful early childhood development initiatives that have been implemented in conflict-affected countries, as well as argue that it is imperative for our next generation of youth to play an active and leading role in peacebuilding initiatives (Leckman, 2019).
In discussing our results, some limitations should be kept in mind. First, we included in this review only English-language articles, so there is a risk of overlooking relevant articles. Another potential limitation of our search is that the available literature encompasses studies that are heterogeneous in terms of study population, endpoints measurements and methodologies used, so it was not possible to perform a meta-analysis or a more structured statistical analysis.
5. Conclusions
The results of this review suggest a multilevel model of resilience that is independent from whether or not the subject might experience an adverse or traumatic event. This model is characterized by the involvement of multiple biological systems, at both central and peripheral levels, which are intertwined with and influenced by environmental factors, while leading to resilience that, as such, is not merely innate or passive, but constantly changing and, consequently, empowering. However, as shown herein, the data currently available in the literature are inconclusive and do not allow us to establish a clear and certain model for the process of resilience development. However, this systematic review highlights the need for further studies in humans that consider resilience as an independent construct. Understanding the mechanisms underlying this particular aspect of psychological functioning is particularly important, as it could be a potential target for new intervention and prevention strategies. Therefore, it seems necessary to develop longitudinal studies that recruit and follow up subjects from childhood to adulthood in order to possibly identify individual and environmental factors that might promote or decrease resilience. This will be not only beneficial for single individuals, but possibly for the whole population and for a better and peaceful future.
References
- Adams, T. G., Kelmendi, B., Brake, C. A., Gruner, P., Badour, C. L., & Pittenger, C. (2018). The role of stress in the pathogenesis and maintenance of obsessive-compulsive disorder. Chronic Stress (Thousand Oaks, Calif.), 2, 2470547018758043. 10.1177/2470547018758043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, M. D. S., Blehar, M. C., Waters, E., & Wall, S. (1978). Patterns of attachment: A psychological study of the strange situation. Lawrence Erlbaum. [Google Scholar]
- Alexander, D. E. (2013). Resilience and disaster risk reduction: An etymological journey. Natural Hazards and Earth System Sciences, 13(11), 2707–2716. 10.5194/nhess-13-2707-2013 [DOI] [Google Scholar]
- Anacker, C., Zunszain, P. A., Cattaneo, A., Carvalho, L. A., Garabedian, M. J., Thuret, S., Price, J., & Pariante, C. M. (2011). Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Molecular Psychiatry, 16(7), 738–750. 10.1038/mp.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker, C., Luna, V. M., Stevens, G. S., Millette, A., Shores, R., Jimenez, J. C., Chen, B., & Hen, R. (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature, 559(7712), 98–102. 10.1038/s41586-018-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrooz, F., Liu, H., & Salim, S. (2019). Stress, psychiatric disorders, molecular targets, and more. Progress in Molecular Biology and Translational Science, 167, 77–105. 10.1016/bs.pmbts.2019.06.006 [DOI] [PubMed] [Google Scholar]
- Averill, L. A., Averill, C. L., Kelmendi, B., Abdallah, C. G., & Southwick, S. M. (2018). Stress response modulation underlying the psychobiology of resilience. Current Psychiatry Reports, 20(4), 27. 10.1007/s11920-018-0887-x [DOI] [PubMed] [Google Scholar]
- Babić, R., Babić, M., Rastović, P., Ćurlin, M., Šimić, J., Mandić, K., & Pavlović, K. (2020). Resilience in health and illness. Psychiatria Danubina, 32(Suppl 2), 226–232. [PubMed] [Google Scholar]
- Bath, K. G., Schilit, A., & Lee, F. S. (2013). Stress efects on BDNF expression: Effects of age, sex, and form of stress. Neuroscience, 239, 149–156. 10.1016/j.neuroscience.2013.01.074 [DOI] [PubMed] [Google Scholar]
- Belsky, J. (2016). The diferential susceptibility hypothesis: Sensitivity to the environment for better and for worse. JAMA Pediatrics, 170(4), 321–322. 10.1001/jamapediatrics.2015.4263 [DOI] [PubMed] [Google Scholar]
- Belsky, J., & Pluess, M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Bemporad, J. R. (1984). From attachment to afiliation. American Journal of Psychoanalysis, 44(1), 79–97. 10.1007/BF01255422 [DOI] [PubMed] [Google Scholar]
- Bowlby, J. (1969). Attachment. New York: Basic Books. [Google Scholar]
- Bowlby, J. (1973). Attachment and loss: Separation, anxiety and anger (Vol. 2). New York: Basic. [Google Scholar]
- Bowlby, J. (1980). Attachment and loss: Loss, sadness and depression (Vol. 3). New York: Basic. [Google Scholar]
- Bonanno, G. A. (2004). Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? American Psychologist, 59(1), 20–28. 10.1037/0003-066X.59.1.20 [DOI] [PubMed] [Google Scholar]
- Bora, E., Yucel, M., & Allen, N. B. (2009). Neurobiology of human afiliative behaviour: Implications for psychiatric disorders. Current Opinion in Psychiatry, 22(3), 320–325. 10.1097/YCO.0b013e328329e970 [DOI] [PubMed] [Google Scholar]
- Boscarino, J. A. (1997). Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosomatic Medicine, 59(6), 605–614. 10.1097/00006842-199711000-00008 [DOI] [PubMed] [Google Scholar]
- Bremner, J. D. (2006). Stress and brain atrophy. CNS & Neurological Disorders Drug Targets, 5(5), 503–512. 10.2174/187152706778559309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, K. A., Clark, C. L., & Shaver, P. R. (1998). Self-report measurement of adult attachment: An integrative overview. In Simpson J. A. & Rholes W. S. (Eds.), Attachment theory and close relationships (pp. 46–76). The Guilford Press. [Google Scholar]
- Buijs, R. M. (1978). Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell and Tissue Research, 192(3), 423–435. 10.1007/BF00212323 [DOI] [PubMed] [Google Scholar]
- Cahill, S., Chandola, T., & Hager, R. (2022). Genetic variants associated with resilience in human and animal studies. Frontiers in Psychiatry, 13, 840120. 10.3389/fpsyt.2022.840120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal, F. S., Ribero de Sa, L., Glustak, M. E., & Santiago, M. B. (2015). Resilience in chronic diseases: A systematic review. Cogent Psychology, 2(1), 1–9. 1024928. 10.1080/23311908.2015.1024928 [DOI] [Google Scholar]
- Carlson, M., & Earls, F. (1997). Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences, 807, 419–428. 10.1111/j.1749-6632.1997.tb51936.x [DOI] [PubMed] [Google Scholar]
- Carr, C. P., Martins, C. M., Stingel, A. M., Lemgruber, V. B., & Juruena, M. F. (2013). The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. The Journal of Nervous and Mental Disease, 201(12), 1007–1020. 10.1097/NMD.0000000000000049 [DOI] [PubMed] [Google Scholar]
- Carter, C. S. (2017). The oxytocin-vasopressin pathway in the context of love and fear. Frontiers in Endocrinology, 8, 356. 10.3389/fendo.2017.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, C. S., & Keverne, E. B. (2009). The neurobiology of social affiliation and pair bonding. In Pfaf D. W., Arnold A. P., Etgen A. M., Fahrbach S. E., & Rubin R. T. (Eds.), Hormones, brain and behavior (pp. 137–165). Elsevier Academic Press. 10.1016/B978-008088783-8.00004-8 [DOI] [Google Scholar]
- Carter, C. S., Lederhendler, I., & Kirkpatrick, B. (1997). The integrative neurobiology of afiliation. Introduction. Annals of the New York Academy of Sciences, 807, xiii–xviii. 10.1111/j.1749-6632.1997.tb51909.x [DOI] [PubMed] [Google Scholar]
- Carter, C. S., Kenkel, W. M., MacLean, E. L., Wilson, S. R., Perkeybile, A. M., Yee, J. R., Ferris, C. F., Nazarloo, H. P., Porges, S. W., Davis, J. M., Connelly, J. J., & Kingsbury, M. A. (2020). Is oxytocin "nature's medicine"?. Pharmacological Reviews, 72(4), 829–861. 10.1124/pr.120.019398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas, F., Murrough, J. W., Nestler, E. J., Han, M. H., & Russo, S. J. (2019). Neurobiology of resilience: Interface between mind and body. Biological Psychiatry, 86(6), 410–420. 10.1016/j.biopsych.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo, A., & Riva, M. A. (2016). Stress-induced mechanisms in mental illness: A role for glucocorticoid signaling. The Journal of Steroid Biochemistry and Molecular Biology, 160, 169–174. 10.1016/j.jsbmb.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Chaouloff, F., Berton, O., & Mormède, P. (1999). Serotonin and stress. Neuropsychopharmacology, 21(2 Suppl), 28S–32S. 10.1016/S0893-133X(99)00008-1 [DOI] [PubMed] [Google Scholar]
- Chatzittofis, A., Nordström, P., Uvnäs-Moberg, K., Asberg, M., & Jokinen, J. (2014). CSF and plasma oxytocin levels in suicide attempters, the role of childhood trauma and revictimization. Neuro Endocrinology Letters, 35(3), 213–217. [PubMed] [Google Scholar]
- Chu, B., Marwaha, K., Sanvictores, T., & Ayers, D. (2022). Physiology, stress reaction. In StatPearls. StatPearls Publishing. [Google Scholar]
- Chugani, H. T., Behen, M. E., Muzik, O., Juhász, C., Nagy, F., & Chugani, D. C. (2001). Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. NeuroImage, 14(6), 1290–1301. 10.1006/nimg.2001.0917 [DOI] [PubMed] [Google Scholar]
- Cicchetti, D., & Blender, J. A. (2006). A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences, 1094, 248–258. 10.1196/annals.1376.029 [DOI] [PubMed] [Google Scholar]
- Cicchetti, D., & Rogosch, F. A. (2012). Gene × Environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology, 24(2), 411–427. 10.1017/S0954579412000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger., C. R. (1994). Temperament and personality. Current Opinion in Neurobiology, 4(2), 266–273. 10.1016/0959-4388(94)90083-3 [DOI] [PubMed] [Google Scholar]
- Cloninger, C. R. (2004). Feeling good: the science of well being. New York: Oxford University Press. [Google Scholar]
- Cloninger, C. R., Svrakic, D. M., & Przybeck, T. R. (1993). A psychobiological model of temperament and character. Archives of General Psychiatry, 50(12), 975–990. 10.1001/archpsyc.1993.01820240059008 [DOI] [PubMed] [Google Scholar]
- Cloninger, C. R., & Zohar, A. H. (2011). Personality and the perception of health and happiness. Journal of Afective Disorders, 128, 24–32. 10.1016/j.jad.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Cloninger, C. R., Salloum, I. M., & Mezzich, J. E. (2012). The dynamic origins of positive health and wellbeing. International Journal of Person Centered Medicine, 2(2), 179–187. 10.5750/ijpcm.v2i2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, A. B., Montgomery, K., Bale, T. L., & Thompson, S. M. (2022). What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF+ PVN neurons. Neurobiology of Stress, 20, 100473. 10.1016/j.ynstr.2022.100473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik, K. H. (1997). Circumnavigating the personality as a whole: The challenges of integrative methodological pluralism. Journal of Personality, 65(4), 1087–1111. 10.1111/j.1467-6494.1997.tb00545.x [DOI] [PubMed] [Google Scholar]
- Curtis, W. J., & Cicchetti, D. (2007). Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Development and Psychopathology, 19(3), 811–840. 10.1017/S0954579407000405 [DOI] [PubMed] [Google Scholar]
- Davis, M. T., Holmes, S. E., Pietrzak, R. H., & Esterlis, I. (2017). Neurobiology of chronic stress-related psychiatric disorders: Evidence from molecular imaging studies. Chronic Stress (Thousand Oaks, Calif.), 1, 2470547017710916. 10.1177/2470547017710916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M., West, K., Bilms, J., Morelen, D., & Suveg, C. (2018). A systematic review of parent-child synchrony: It is more than skin deep. Developmental Psychobiology, 60(6), 674–691. 10.1002/dev.21743 [DOI] [PubMed] [Google Scholar]
- de Wied, D., Elands, J., & Kovács, G. (1991). Interactive efects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: Mediation by a cerebral neurohypophyseal hormone receptor? Proceedings of the National Academy of Sciences of the United States of America, 88(4), 1494–1498. 10.1073/pnas.88.4.1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue, R. A., & Morrone-Strupinsky, J. V. (2005). A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of afiliation. The Behavioral and Brain Sciences, 28(3), 313–395. 10.1017/S0140525X05000063 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo, G., Longo, L., Jannini, T. B., Niolu, C., Rossi, R., & Siracusano, A. (2020). Oxytocin in the prevention and the treatment of post-traumatic stress disorder: A systematic review of randomized controlled trials. Journal of Psychopathology, 26, 107–118. 10.36148/2284-0249-370 [DOI] [Google Scholar]
- Domes, G., Heinrichs, M., Gläscher, J., Büchel, C., Braus, D. F., & Herpertz, S. C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–1190. 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Dunn, J., & Kendrick, C. (1982). Temperamental differences, family relationships, and young children's response to change within the family. Ciba Foundation Symposium, 89, 87–105. 10.1002/9780470720714.ch6 [DOI] [PubMed] [Google Scholar]
- Eley, D. S., Cloninger, C. R., Walters, L., Laurence, C., Synnott, R., & Wilkinson, D. (2013). The relationship between resilience and personality traits in doctors: Implications for enhancing well being. PeerJ, 1, e216. 10.7717/peerj.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4(11), 1313–1317. 10.1038/3305 [DOI] [PubMed] [Google Scholar]
- Fasano, J., Shao, T., Huang, H. H., Kessler, A. J., Kolodka, O. P., & Shapiro, C. L. (2020). Optimism and coping: Do they influence health outcomes in women with breast cancer? A systemic review and meta-analysis. Breast Cancer Research and Treatment, 183(3), 495–501. 10.1007/s10549-020-05800-5 [DOI] [PubMed] [Google Scholar]
- Feder, A., Nestler, E. J., & Charney, D. S. (2009). Psychobiology and molecular genetics of resilience. Nature Reviews. Neuroscience, 10(6), 446–457. 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R. (2007). Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science, 16(6), 340–345. 10.1111/j.1467-8721.2007.00532.x [DOI] [Google Scholar]
- Feldman, R. (2012). Parent-infant synchrony: A biobehavioral model of mutual influences in the formation of afiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. 10.1111/j.1540-5834.2011.00660.x [DOI] [Google Scholar]
- Feldman, R. (2015). The adaptive human parental brain: Implications for children's social development. Trends in Neurosciences, 38(6), 387–399. 10.1016/j.tins.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Feldman, R. (2016). The neurobiology of mammalian parenting and the biosocial context of human caregiving. Hormones and Behavior, 77, 3–17. 10.1016/j.yhbeh.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Feldman, R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21(2), 80–99. 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Feldman, R. (2020). What is resilience: An afiliative neuroscience approach. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 19(2), 132–150. 10.1002/wps.20729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, R., Monakhov, M., Pratt, M., & Ebstein, R. P. (2016). Oxytocin pathway genes: Evolutionary ancient system impacting on human afiliation, sociality, and psychopathology. Biological Psychiatry, 79(3), 174–184. 10.1016/j.biopsych.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Fleeson, W., & Jayawickreme, E. (2015). Whole trait theory. Journal of Research in Personality, 56, 82–92. 10.1016/j.jrp.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-León, M. Á., Pérez-Mármol, J. M., Gonzalez-Pérez, R., García-Ríos, M. D. C., & Peralta-Ramírez, M. I. (2019). Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiology & Behavior, 202, 87–93. 10.1016/j.physbeh.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Goldstein, D. S. (1990). Neurotransmitters and stress. Biofeedback and Self-regulation, 15(3), 243–271. 10.1007/BF01011108 [DOI] [PubMed] [Google Scholar]
- Goldstein, D. S. (2010). Adrenal responses to stress. Cellular and Molecular Neurobiology, 30(8), 1433–1440. 10.1007/s10571-010-9606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky, M. S. (2001). The concept of cellular "fight-or-flight" reaction to stress. American Journal of Physiology. Renal Physiology, 280(4), F551–F561. 10.1152/ajprenal.2001.280.4.F551 [DOI] [PubMed] [Google Scholar]
- Granjard, A., Garcia, D., Rosenberg, P., Jacobsson, C., Cloninger, K. M., & Cloninger, C. R. (2021). Resilience personality profiles among Swedish long-term unemployed. PsyCh Journal, 10(4), 670–673. 10.1002/pchj.467 [DOI] [PubMed] [Google Scholar]
- Harms, C. A., Cohen, L., Pooley, J. A., Chambers, S. K., Galvão, D. A., & Newton, R. U. (2019). Quality of life and psychological distress in cancer survivors: The role of psycho-social resources for resilience. Psycho-Oncology, 28(2), 271-277. 10.1002/pon.4934 [DOI] [PubMed] [Google Scholar]
- Heim, C., Newport, D. J., Heit, S., Graham, Y. P., Wilcox, M., Bonsall, R., Miller, A. H., & Nemerof, C. B. (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA, 284(5), 592–597. 10.1001/jama.284.5.592 [DOI] [PubMed] [Google Scholar]
- Heim, C., Young, L. J., Newport, D. J., Mletzko, T., Miller, A. H., & Nemeroff, C. B. (2009). Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry, 14(10), 954–958. 10.1038/mp.2008.112 [DOI] [PubMed] [Google Scholar]
- Heinrichs, M., Meinlschmidt, G., Wippich, W., Ehlert, U., & Hellhammer, D. H. (2004). Selective amnesic effects of oxytocin on human memory. Physiology & Behavior, 83(1), 31–38. 10.1016/j.physbeh.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Hodel, A. S., Hunt, R. H., Cowell, R. A., Van Den Heuvel, S. E., Gunnar, M. R., & Thomas, K. M. (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, S. R., & Feder, A. (2018). Understanding resilience and preventing and treating PTSD. Harvard Review of Psychiatry, 26(3), 158–174. 10.1097/HRP.0000000000000194 [DOI] [PubMed] [Google Scholar]
- Insel, T. R. (2010). The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and afiliative behavior. Neuron, 65(6), 768–779. 10.1016/j.neuron.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic, M. (2018). Empathy, sense of coherence and resilience: Bridging personal, public and global mental health and conceptual synthesis. Psychiatria Danubina, 30(4), 380–384. 10.24869/psyd.2018.380 [DOI] [PubMed] [Google Scholar]
- Joëls, M., Karst, H., & Sarabdjitsingh, R. A. (2018). The stressed brain of humans and rodents. Acta Physiologica (Oxford, England), 223(2), e13066. 10.1111/apha.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson, K., Jokela, M., Hintsanen, M., Cloninger, C. R., Pulkki-Råback, L., Merjonen, P., Hutri-Kähönen, N., & Keltikangas-Järvinen, L. (2013). Parental care-giving and home environment predicting ofspring's temperament and character traits after 18 years. Psychiatry Research, 209(3), 643–651. 10.1016/j.psychres.2013.01.007 [DOI] [PubMed] [Google Scholar]
- Juruena, M. F., Eror, F., Cleare, A. J., & Young, A. H. (2020). The role of early life stress in HPA axis and anxiety. Advances in Experimental Medicine and Biology, 1191, 141–153. 10.1007/978-981-32-9705-0_9 [DOI] [PubMed] [Google Scholar]
- Kersten, P., Borschel, E., Neyer, F. J., & Mund, M. (2023). The social side of personality: Do afiliation and intimacy motives moderate associations of personal relationships with wellbeing? Journal of Personality, 91(4), 992–1011. 10.1111/jopy.12746 [DOI] [PubMed] [Google Scholar]
- Kim, G. M., Lim, J. Y., Kim, E. J., & Park, S. M. (2019). Resilience of patients with chronic diseases: A systematic review. Health & Social Care in the Community, 27(4), 797–807. 10.1111/hsc.12620 [DOI] [PubMed] [Google Scholar]
- Kim-Cohen, J., & Gold, A. L. (2009). Measured gene-environment interactions and mechanisms promoting resilient development. Current Directions in Psychological Science, 18(3), 138–142. 10.1111/j.1467-8721.2009.01624.x [DOI] [Google Scholar]
- Kirsch, P., Esslinger, C., Chen, Q., Mier, D., Lis, S., Siddhanti, S., Gruppe, H., Mattay, V. S., Gallhofer, B., & Meyer-Lindenberg, A. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience: The Oficial Journal of the Society for Neuroscience, 25(49), 11489–11493. 10.1523/JNEUROSCI.3984-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel, T., & Binder, E. B. (2015). Epigenetics of stress-related psychiatric disorders and gene × environment interactions. Neuron, 86(6), 1343–1357. 10.1016/j.neuron.2015.05.036 [DOI] [PubMed] [Google Scholar]
- Koch, S. B., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olf, M. (2016a). Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology, 41(6), 1495–1504. 10.1038/npp.2015.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, S. B., van Zuiden, M., Nawijn, L., Frijling, J. L., Veltman, D. J., & Olff, M. (2016b). Intranasal oxytocin normalizes amygdala functional connectivity in post-traumatic stress disorder. Neuropsychopharmacology: Oficial Publication of the American College of Neuropsychopharmacology, 41(8), 2041–2051. 10.1038/npp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf, R., & Neumann, I. D. (2004). Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology, 25(3-4), 150–176. 10.1016/j.yfrne.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Larosa, A., & Wong, T. P. (2022). The hippocampus in stress susceptibility and resilience: Reviewing molecular and functional markers. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 119, 110601. 10.1016/j.pnpbp.2022.110601 [DOI] [PubMed] [Google Scholar]
- Leckman, J. F., Donaldson, C. K., Affolter, F. W., & Ponguta, L. A. (2019). Pathways to wellbeing and a more peaceful and sustainable world: The transformative power of children and families. Japanese Journal of Child and Adolescent Psychiatry, 60(3), 278–298. 10.20615/jscap.60.3_278 [DOI] [Google Scholar]
- Lee, E. H., & Kim, J. H. (2017). Development of resilience index based on flooding damage in urban areas. Water, 9, 428. 10.3390/w9060428 [DOI] [Google Scholar]
- Lee, H. F., Hsu, H. C., Efendi, F., Ramoo, V., & Susanti, I. A. (2023). Burnout, resilience, and empowerment among COVID-19 survivor nurses in Indonesia. PloS One, 18(10), e0291073. 10.1371/journal.pone.0291073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschik, J., Lutz, B., & Gentile, A. (2021). Stress-related dysfunction of adult hippocampal neurogenesis - An Attempt for understanding resilience? International Journal of Molecular Sciences, 22(14), 7339. 10.3390/ijms22147339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, J., Goldstein, A., & Feldman, R. (2017). Perception of social synchrony induces mother-child gamma coupling in the social brain. Social Cognitive and Afective Neuroscience, 12(7), 1036–1046. 10.1093/scan/nsx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys, C., Arnal, C., Wollast, R., Rolin, H., Kotsou, I., & Fossion, P. (2020). Perspectives on resilience: Personality trait or skill? European Journal of Trauma & Dissociation, 4(2), Article 100074. 10.1016/j.ejtd.2018.07.002 [DOI] [Google Scholar]
- Licht, P., McCreery, B. R., Barnes, R., & Pang, R. (1983). Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. General and Comparative Endocrinology, 50(1), 124–145. 10.1016/0016-6480(83)90249-6 [DOI] [PubMed] [Google Scholar]
- Liu, Y. Z., Wang, Y. X., & Jiang, C. L. (2017). Inflammation: The common pathway of stress-related diseases. Frontiers in Human Neuroscience, 11, 316. 10.3389/fnhum.2017.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loprinzi, C. E., Prasad, K., Schroeder, D. R., & Sood, A. (2011). Stress Management and Resilience Training (SMART) program to decrease stress and enhance resilience among breast cancer survivors: A pilot randomized clinical trial. Clinical Breast Cancer, 11(6), 364–368. 10.1016/j.clbc.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Luby, J. L., Barch, D. M., Belden, A., Gaffrey, M. S., Tillman, R., Babb, C., Nishino, T., Suzuki, H., & Botteron, K. N. (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proceedings of the National Academy of Sciences of the United States of America, 109(8), 2854–2859. 10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Thompson, R. G., & Holman, E. A. (2013). Environmental stress, oxytocin receptor gene (OXTR) polymorphism, and mental health following collective stress. Hormones and Behavior, 63(4), 615–624. 10.1016/j.yhbeh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Lupien, S. J., Parent, S., Evans, A. C., Tremblay, R. E., Zelazo, P. D., Corbo, V., Pruessner, J. C., & Séguin, J. R. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America, 108(34), 14324–14329. 10.1073/pnas.1105371108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar, S. S. (2006). Resilience in development: A synthesis of research across five decades. In Cicchetti D. & Cohen D. J. (Eds.), Developmental psychopathology: Risk, disorder, and adaptation (pp. 739–795). John Wiley & Sons, Inc. [Google Scholar]
- Mahar, I., Bambico, F. R., Mechawar, N., & Nobrega, J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant efects. Neuroscience and Biobehavioral Reviews, 38, 173–192. 10.1016/j.neubiorev.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Malberg, J. E. (2004). Implications of adult hippocampal neurogenesis in antidepressant action. Journal of Psychiatry & Neuroscience: JPN, 29(3), 196–205. [PMC free article] [PubMed] [Google Scholar]
- Marazziti, D., & Catena Dell'Osso, M. (2008). The role of oxytocin in neuropsychiatric disorders. Current Medicinal Chemistry, 15(7), 698–704. 10.2174/092986708783885291 [DOI] [PubMed] [Google Scholar]
- Marazziti, D., Dell'Osso, B., Baroni, S., Mungai, F., Catena, M., Rucci, P., Albanese, F., Giannaccini, G., Betti, L., Fabbrini, L., Italiani, P., Del Debbio, A., Lucacchini, A., & Dell'Osso, L. (2006). A relationship between oxytocin and anxiety of romantic attachment. Clinical Practice and Epidemiology in Mental Health, 2, 28. 10.1186/1745-0179-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti, D., Baroni, S., Mucci, F., Piccinni, A., Moroni, I., Giannaccini, G., Carmassi, C., Massimetti, E., & Dell'Osso, L. (2019). Sex-related differences in plasma oxytocin levels in humans. Clinical Practice and Epidemiology in Mental Health, 15, 58–63. 10.2174/1745017901915010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti, D., Carter, C. S., Carmassi, C., Della Vecchia, A., Mucci, F., Pagni, G., Carbone, M. G., Baroni, S., Giannaccini, G., Palego, L., & Dell'Osso, L. (2022). Sex matters: The impact of oxytocin on healthy conditions and psychiatric disorders. Comprehensive Psychoneuroendocrinology, 13, 100165. 10.1016/j.cpnec.2022.100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti, D., Carmassi, C., Cappellato, G., Chiarantini, I., Massoni, L., Mucci, F., Arone, A., Violi, M., Palermo, S., De Iorio, G., & Dell'Osso, L. (2023). Novel pharmacological targets of post-traumatic stress disorders. Life, 13(8), 1731. 10.3390/life13081731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, C. M. S., de Carvalho Tofoli, S. M., Von Werne Baes, C., & Juruena, M. (2011). Analysis of the occurrence of early life stress in adult psychiatric patients: A systematic review. Psychology & Neuroscience, 4(2), 219–227. 10.3922/j.psns.2011.2.007 [DOI] [Google Scholar]
- Maul, S., Giegling, I., Fabbri, C., Corponi, F., Serretti, A., & Rujescu, D. (2020). Genetics of resilience: Implications from genome-wide association studies and candidate genes of the stress response system in post-traumatic stress disorder and depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 183(2), 77–94. 10.1002/ajmg.b.32763 [DOI] [PubMed] [Google Scholar]
- McCarthy, M. M., McDonald, C. H., Brooks, P. J., & Goldman, D. (1996). An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiology & Behavior, 60(5), 1209–1215. 10.1016/s0031-9384(96)00212-0 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen, B. S., Weiss, J. M., & Schwartz, L. S. (1968). Selective retention of corticosterone by limbic structures in rat brain. Nature, 220(5170), 911–912. 10.1038/220911a0 [DOI] [PubMed] [Google Scholar]
- Mesquita, A. R., Belsky, J., Li, Z., Baptista, J., Carvalho-Correia, E., Maciel, P., & Soares, I. (2015). Institutionalization and indiscriminate social behavior: Diferential-susceptibility versus diathesis-stress models for the 5-HTTLPR and BDNF genotypes. Physiology & Behavior, 152(Pt A), 85–91. 10.1016/j.physbeh.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Miller, G. E., & Blackwell, E. (2006). Turning up the heat: Inflammation as a mechanism linking chronic stress, depression, and heart disease. Current Directions in Psychological Science, 15(6), 269–272. 10.1111/j.1467-8721.2006.00450.x [DOI] [Google Scholar]
- Mirescu, C., Peters, J. D., & Gould, E. (2004). Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience, 7(8), 841–846. 10.1038/nn1290 [DOI] [PubMed] [Google Scholar]
- Mizuki, R., & Fujiwara, T. (2015). Association of oxytocin level and less severe forms of childhood maltreatment history among healthy Japanese adults involved with child care. Frontiers in Behavioral Neuroscience, 9, 138. 10.3389/fnbeh.2015.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam, B. (2002). Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biological Psychiatry, 51(10), 775–787. 10.1016/s0006-3223(01)01362-2 [DOI] [PubMed] [Google Scholar]
- Mohiyeddini, C., Opacka-Juffry, J., & Gross, J. J. (2014). Emotional suppression explains the link between early life stress and plasma oxytocin. Anxiety, Stress, and Coping, 27(4), 466–475. 10.1080/10615806.2014.887696 [DOI] [PubMed] [Google Scholar]
- Monroe, S. M., & Simons, A. D. (1991). Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin, 110(3), 406–425. 10.1037/0033-2909.110.3.406 [DOI] [PubMed] [Google Scholar]
- Mora, F., Segovia, G., Del Arco, A., de Blas, M., & Garrido, P. (2012). Stress, neurotransmitters, corticosterone and body-brain integration. Brain Research, 1476, 71–85. 10.1016/j.brainres.2011.12.049 [DOI] [PubMed] [Google Scholar]
- Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., & Llorens-Martín, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nature Medicine, 25(4), 554–560. 10.1038/s41591-019-0375-9 [DOI] [PubMed] [Google Scholar]
- Murrough, J. W., & Russo, S. J. (2019). The neurobiology of resilience: Complexity and hope. Biological Psychiatry, 86(6), 406–409. 10.1016/j.biopsych.2019.07.016 [DOI] [PubMed] [Google Scholar]
- Nakama, N., Usui, N., Doi, M., & Shimada, S. (2023). Early life stress impairs brain and mental development during childhood increasing the risk of developing psychiatric disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 126, 110783. 10.1016/j.pnpbp.2023.110783 [DOI] [PubMed] [Google Scholar]
- Nieto, M., Visier, M. E., Silvestre, I. N., Navarro, B., Serrano, J. P., & Martínez-Vizcaíno, V. (2023). Relation between resilience and personality traits: The role of hopelessness and age. Scandinavian Journal of Psychology, 64(1), 53–59. 10.1111/sjop.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu, K., Rice, M. J., Houfek, J. F., Stoltenberg, S. F., Kupzyk, K. A., & Barron, C. R. (2019). A systematic review of genetic influence on psychological resilience. Biological Research for Nursing, 21(1), 61–71. 10.1177/1099800418800396 [DOI] [PubMed] [Google Scholar]
- North, C. S., & Cloninger, C. R. (2012). Personality and major depression among directly exposed survivors of the Oklahoma City bombing. Depression Research and Treatment, 2012, 204741. 10.1155/2012/204741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osório, C., Probert, T., Jones, E., Young, A. H., & Robbins, I. (2017). Adapting to stress: Understanding the neurobiology of resilience. Behavioral Medicine (Washington, D.C.), 43(4), 307–322. 10.1080/08964289.2016.1170661 [DOI] [PubMed] [Google Scholar]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaf, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlini, C., Bellani, M., Rossetti, M. G., Zovetti, N., Rossin, G., Bressi, C., & Brambilla, P. (2019). Disentangle the neural correlates of attachment style in healthy individuals. Epidemiology and Psychiatric Sciences, 28(4), 371–375. 10.1017/S2045796019000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess, M., & Belsky, J. (2013). Vantage sensitivity: Individual diferences in response to positive experiences. Psychological Bulletin, 139(4), 901–916. 10.1037/a0030196 [DOI] [PubMed] [Google Scholar]
- Pratt, M., Apter-Levi, Y., Vakart, A., Kanat-Maymon, Y., Zagoory-Sharon, O., & Feldman, R. (2017). Mother-child adrenocortical synchrony; Moderation by dyadic relational behavior. Hormones and Behavior, 89, 167–175. 10.1016/j.yhbeh.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Quirin, M., Pruessner, J. C., & Kuhl, J. (2008). HPA system regulation and adult attachment anxiety: Individual diferences in reactive and awakening cortisol. Psychoneuroendocrinology, 33(5), 581–590. 10.1016/j.psyneuen.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Quirin, M., Gillath, O., Pruessner, J. C., & Eggert, L. D. (2010). Adult attachment insecurity and hippocampal cell density. Social Cognitive and Affective Neuroscience, 5(1), 39–47. 10.1093/scan/nsp042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, H. E., & Young, L. J. (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology, 30(4), 534–547. 10.1016/j.yfrne.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, H. E., Cole, C. D., Smith, Y., Neumann, I. D., Landgraf, R., Murphy, A. Z., & Young, L. J. (2009). Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience, 162(4), 892–903. 10.1016/j.neuroscience.2009.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, S. J., Murrough, J. W., Han, M. H., Charney, D. S., & Nestler, E. J. (2012). Neurobiology of resilience. Nature Neuroscience, 15(11), 1475–1484. 10.1038/nn.3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, M., Spieler, D., Wizelman, L., Epple, G., Stich, J., Zaba, M., & Schmidt, U. (2017). Intranasal oxytocin reduces provoked symptoms in female patients with post-traumatic stress disorder despite exerting sympathomimetic and positive chronotropic efects in a randomized controlled trial. BMC Medicine, 15(1), 40. 10.1186/s12916-017-0801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleh, M. R. (2008). Life event, stress and illness. The Malaysian Journal of Medical Sciences: MJMS, 15(4), 9–18. [PMC free article] [PubMed] [Google Scholar]
- Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., Belzung, C., & Hen, R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, 301(5634), 805–809. 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Schiavone, S., Colaianna, M., & Curtis, L. (2015). Impact of early life stress on the pathogenesis of mental disorders: Relation to brain oxidative stress. Current Pharmaceutical Design, 21(11), 1404–1412. 10.2174/1381612821666150105143358 [DOI] [PubMed] [Google Scholar]
- Seiler, A., & Jenewein, J. (2019). Resilience in cancer patients. Frontiers in Psychiatry, 10, 208. 10.3389/fpsyt.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth, R. M., & Cheney, D. L. (2013). Afiliation, empathy, and the origins of theory of mind. Proceedings of the National Academy of Sciences of the United States of America, 110 Suppl 2(Suppl 2), 10349–10356. 10.1073/pnas.1301223110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri, P. C. (2013). Resilience: Building immunity in psychiatry. Indian Journal of Psychiatry, 55(3), 224–234. 10.4103/0019-5545.117134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, A. E., & West, M. (1989). The functional discrimination of attachment and afiliation: Theory and empirical demonstration. The British Journal of Psychiatry, 155, 18–23. 10.1192/bjp.155.1.18 [DOI] [PubMed] [Google Scholar]
- Sinha, R. (2009). Stress and addiction: A dynamic interplay of genes, environment, and drug intake. Biological Psychiatry, 66(2), 100–101. 10.1016/j.biopsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel, L. M., Han, S., Watkins, L. E., Harpaz-Rotem, I., Southwick, S. M., Krystal, J. H., Olff, M., Sherva, R., Farrer, L. A., Kranzler, H. R., Gelernter, J., & Pietrzak, R. H. (2017). Oxytocin receptor gene polymorphisms, attachment, and PTSD: Results from the National Health and Resilience in Veterans Study. Journal of Psychiatric Research, 94, 139–147. 10.1016/j.jpsychires.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. E., & Pollak, S. D. (2020). Early life stress and development: Potential mechanisms for adverse outcomes. Journal of Neurodevelopmental Disorders, 12(1), 34. 10.1186/s11689-020-09337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. A., Makino, S., Kvetnansky, R., & Post, R. M. (1995). Stress and glucocorticoids afect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of Neuroscience, 15(3 Pt 1), 1768–1777. 10.1523/JNEUROSCI.15-03-01768.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, J. S., Soumier, A., Brewer, M., Pickel, J., & Cameron, H. A. (2011). Adult hippocampal neurogenesis bufers stress responses and depressive behaviour. Nature, 476(7361), 458–461. 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram, R. O., & Devamani, K. A. (2016). A comparative study on resilience, perceived social support and hopelessness among cancer patients treated with curative and palliative care. Indian Journal of Palliative Care, 22(2), 135–140. 10.4103/0973-1075.179606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick, S. M., Bonanno, G. A., Masten, A. S., Panter-Brick, C., & Yehuda, R. (2014). Resilience definitions, theory, and challenges: Interdisciplinary perspectives. European Journal of Psychotraumatology, 5, 10.3402/ejpt.v5.25338. 10.3402/ejpt.v5.25338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoesz, B. M., Hare, J. F., & Snow, W. M. (2013). Neurophysiological mechanisms underlying affiliative social behavior: Insights from comparative research. Neuroscience and Biobehavioral Reviews, 37(2), 123–132. 10.1016/j.neubiorev.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Swaminathan, A., Gliksberg, M., Anbalagan, S., Wigoda, N., & Levkowitz, G. (2023). Stress resilience is established during development and is regulated by complement factors. Cell Reports, 42(1), 111973. 10.1016/j.celrep.2022.111973 [DOI] [PubMed] [Google Scholar]
- Sweitzer, M. M., Halder, I., Flory, J. D., Craig, A. E., Gianaros, P. J., Ferrell, R. E., & Manuck, S. B. (2013). Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: Evidence for diferential susceptibility. Social Cognitive and Affective Neuroscience, 8(5), 499–508. 10.1093/scan/nss020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum, S. D., & Nemeroff, C. B. (2019). The efect of early life stress on adult psychiatric disorders. Innovations in Clinical Neuroscience, 16(1-2), 35–37. [PMC free article] [PubMed] [Google Scholar]
- Taupin, P. (2006). Neurogenesis and the efect of antidepressants. Drug Target Insights, 1, 13–17. [PMC free article] [PubMed] [Google Scholar]
- Tollenaar, M. S., Molendijk, M. L., Penninx, B. W. J. H., Milaneschi, Y., & Antypa, N. (2017). The association of childhood maltreatment with depression and anxiety is not moderated by the oxytocin receptor gene. European Archives of Psychiatry and Clinical Neuroscience, 267(6), 517–526. 10.1007/s00406-017-0784-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., Millner, A., Galvan, A., Davidson, M. C., Eigsti, I. M., Thomas, K. M., Freed, P. J., Booma, E. S., Gunnar, M. R., Altemus, M., Aronson, J., & Casey, B. J. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and dificulties in emotion regulation. Developmental Science, 13(1), 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi, A. (2018). Psychotraumatology: What researchers and clinicians can learn from an evolutionary perspective. Seminars in Cell & Developmental Biology, 77, 153–160. 10.1016/j.semcdb.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Tugade, M. M., & Fredrickson, B. L. (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86(2), 320–333. 10.1037/0022-3514.86.2.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutte-Vallarino, V., Malán-Ernst, E., Reyes-Bossio, M., Peinado-Portero, A., de Álvaro, J. I., Ortín Montero, F. J., & Garcés de Los Fayos Ruiz, E. J. (2022). Relationship between resilience, optimism, and burnout in Pan-American athletes. Frontiers in Psychology, 13, 1048033. 10.3389/fpsyg.2022.1048033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer-Yaniv, A., Avitsur, R., Kanat-Maymon, Y., Schneiderman, I., Zagoory-Sharon, O., & Feldman, R. (2016). Affiliation, reward, and immune biomarkers coalesce to support social synchrony during periods of bond formation in humans. Brain, Behavior, and Immunity, 56, 130–139. 10.1016/j.bbi.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg, K., & Petersson, M. (2005). Oxytocin, ein vermittler von antistress, wohlbefinden, sozialer Interaktion, wachstum und heilung [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing]. Zeitschrift fur Psychosomatische Medizin und Psychotherapie, 51(1), 57–80. 10.13109/zptm.2005.51.1.57 [DOI] [PubMed] [Google Scholar]
- van der Werf, S. J. A., Elzinga, B. M., Smit, A. S., & van der Wee, N. J. A. (2017). Structural brain correlates of resilience to traumatic stress in Dutch police oficers. Psychoneuroendocrinology, 85, 172–178. 10.1016/j.psyneuen.2017.08.019 [DOI] [PubMed] [Google Scholar]
- van Zuiden, M., Frijling, J. L., Nawijn, L., Koch, S. B. J., Goslings, J. C., Luitse, J. S., Biesheuvel, T. H., Honig, A., Veltman, D. J., & Olf, M. (2017). Intranasal oxytocin to prevent post-traumatic stress disorder symptoms: A randomized controlled trial in emergency department patients. Biological Psychiatry, 81(12), 1030–1040. 10.1016/j.biopsych.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Ventriglio, A., Gentile, A., Baldessarini, R. J., & Bellomo, A. (2015). Early-life stress and psychiatric disorders: Epidemiology, neurobiology and innovative pharmacological targets. Current Pharmaceutical Design, 21(11), 1379–1387. 10.2174/1381612821666150105121244 [DOI] [PubMed] [Google Scholar]
- Wachs, T. D. (2000). Linking nutrition and temperament. In Molfese V. J. & Molfese D. L. (Eds.), Temperament and personality development across the life span (pp. 57–84). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Walker, S. C., & McGlone, F. P. (2013). The social brain: Neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides, 47(6), 379–393. 10.1016/j.npep.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Wang, Q., Van Heerikhuize, J., Aronica, E., Kawata, M., Seress, L., Joels, M., Swaab, D. F., & Lucassen, P. J. (2013). Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiology of Aging, 34(6), 1662–1673. 10.1016/j.neurobiolaging.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt, J. L., & Duman, R. S. (2006). Hippocampal neurogenesis: Opposing efects of stress and antidepressant treatment. Hippocampus, 16(3), 239–249. 10.1002/hipo.20156 [DOI] [PubMed] [Google Scholar]
- Wei, L., Meaney, M. J., Duman, R. S., & Kafman, A. (2011). Afiliative behavior requires juvenile, but not adult neurogenesis. The Journal of Neuroscience, 31(40), 14335–14345. 10.1523/JNEUROSCI.1333-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle, R. J., Kershaw, Y. M., Shanks, N., Wood, S. A., Lightman, S. L., & Ingram, C. D. (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. The Journal of Neuroscience, 24(12), 2974–2982. 10.1523/JNEUROSCI.3432-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2023). Emergencies. https://www.who.int/emergencies/situations [Google Scholar]
- Wosu, A. C., Valdimarsdóttir, U., Shields, A. E., Williams, D. R., & Williams, M. A. (2013). Correlates of cortisol in human hair: Implications for epidemiologic studies on health efects of chronic stress. Annals of Epidemiology, 23(12), 797–811. e2. 10.1016/j.annepidem.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn, C. C., & Holmes, A. (2006). The role of galanin in modulating stress-related neural pathways. Drug News & Perspectives, 19(8), 461–467. 10.1358/dnp.2006.19.8.1043963 [DOI] [PubMed] [Google Scholar]
- Wu, G., Feder, A., Cohen, H., Kim, J. J., Calderon, S., Charney, D. S., & Mathé, A. A. (2013). Understanding resilience. Frontiers in Behavioral Neuroscience, 7, 10. 10.3389/fnbeh.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizolfi, D., Poloni, N., Caselli, I., Ielmini, M., Lucca, G., Diurni, M., Cavallini, G., & Callegari, C. (2019). Resilience and recovery style: A retrospective study on associations among personal resources, symptoms, neurocognition, quality of life and psychosocial functioning in psychotic patients. Psychology Research and Behavior Management, 12, 385–395. 10.2147/PRBM.S205424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar, A. H., Zwir, I., Wang, J., Cloninger, C. R., & Anokhin, A. P. (2019). The development of temperament and character during adolescence: The processes and phases of change. Development and Psychopathology, 31(2), 601–617. 10.1017/S0954579418000159 [DOI] [PubMed] [Google Scholar]
- Zuo, P., Wang, Y., Liu, J., Hu, S., Zhao, G., Huang, L., & Lin, D. (2019). Efects of early adversity on the brain: Larger-volume anterior cingulate cortex in AIDS orphans. PloS One, 14(1), e0210489. 10.1371/journal.pone.0210489 [DOI] [PMC free article] [PubMed] [Google Scholar]