Abstract
Statins are thought to have positive effects on migraine but existing data are inconclusive. We aimed to evaluate the causal effect of such drugs on migraines using Mendelian randomization. We used four types of genetic instruments as proxies for HMG-CoA reductase inhibition. We included the expression quantitative trait loci of the HMG-CoA reductase gene and genetic variation within or near the HMG-CoA reductase gene region. Variants were associated with low-density lipoprotein cholesterol, apolipoprotein B, and total cholesterol. Genome-wide association study summary data for the three lipids were obtained from the UK Biobank. Comparable data for migraine were obtained from the International Headache Genetic Consortium and the FinnGen Consortium. Inverse variance weighting method was used for the primary analysis. Additional analyses included pleiotropic robust methods, colocalization, and meta-analysis. Genetically determined high expression of HMG-CoA reductase was associated with an increased risk of migraines (OR = 1.55, 95% CI 1.30–1.84, P = 6.87 × 10−7). Similarly, three genetically determined HMG-CoA reductase-mediated lipids were associated with an increased risk of migraine. These conclusions were consistent across meta-analyses. We found no evidence of bias caused by pleiotropy or genetic confounding factors. These findings support the hypothesis that statins can be used to treat migraine.
Keywords: Statins, HMG-CoA reductase, Migraine, Mendelian randomization
Subject terms: Neuroscience, Neurogenesis
Introduction
Migraine, a common affliction endured by several people in headache clinics, is characterized by recurrent moderate-to-severe throbbing pain1. It affects ~ 12% of the global population and is the second most incapacitating ailment worldwide2. The deleterious impact of migraines on both well-being and quality of life cannot be underestimated because it imposes a substantial burden on a global scale3. Therefore, it is crucial to identify therapeutic targets for migraine to expand the available treatment options.
Observational studies have reported that disorders in the metabolism of lipids, including cholesterol and low-density lipoprotein cholesterol (LDL-C), may increase susceptibility to migraine4,5. Additionally, it has been speculated that certain lipid-lowering drugs such as statins possess migraine-ameliorating properties6–8. However, the available studies on the association between statins and migraine risk disagree, and the causal relationship remains uncertain6–10. Statins are a class of drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR). They have emerged as potent agents for reducing plasma LDL-C levels and play a crucial role in treating atherosclerotic diseases11. In addition to their lipid-lowering effects, statins can bolster the stability of atherosclerotic plaques, improve vascular endothelial function, mitigate oxidative stress and inflammation, and regulate autonomic nervous system function12–14. The efficacy and safety of statins in preventing ischemic stroke are firmly established, and they are widely used in clinical practice11. Accumulating evidence shows that migraine increases the risk of ischemic stroke15,16. Therefore, the discovery of the causal pathways of migraine related to lipids may contribute to improve our understanding of the mechanisms leading to the development of migraine. And it could also help us understand the relationship between migraine and stroke. Importantly, it may benefit the development of personalized treatment strategies, notably for individuals at a high risk of dyslipidemia or with a familial history of stroke.
Observational studies cannot eliminate confounding biases between exposure and outcomes. To circumvent this hurdle, Mendelian randomization (MR) analysis uses genetic variants (single nucleotide polymorphisms, SNPs) as instrumental variables to clarify the potential causal relationships between exposures and outcomes17. MR is comparable to randomized controlled trials in that genetic variations are randomly assigned at conception; therefore, MR can minimize interference by confounding bias18. Interference from reverse causality can also be avoided because genetic variants precede disease onset and are not affected by disease progression18. The expression of protein drug targets may be influenced by variants near the genes that encode them and such variants can therefore be used to predict potential clinical effects19. Drug-target MR uses genetic variants of genes encoding proteins of interest, usually cis-acting quantitative trait loci (cis-QTL), as instrumental variables to clarify the impact of the encoded proteins on disease outcomes20. When the protein of interest is likely to be the target of a drug’s action, MR analysis is referred to as drug-target MR20. If a drug is supported by genetic evidence, it indicates that the medication’s therapeutic impact and mechanism of action are supported by reliable scientific data and that the drug may be an effective treatment for the targeted the disease of interest.
In this study, we aimed to investigate the association between the risk of migraine and statin lipid-lowering agents using a two-sample drug-target MR design.
Methods
We used publicly available genome-wide association studies (GWAS) and expression quantitative trait locus (eQTL) data. Informed consent and ethical approval had been obtained in all the original studies; therefore, no additional ethical approval was required for this study. Two-sample MR was performed according to the STROBE-MR guidelines21.
MR assumptions and study design
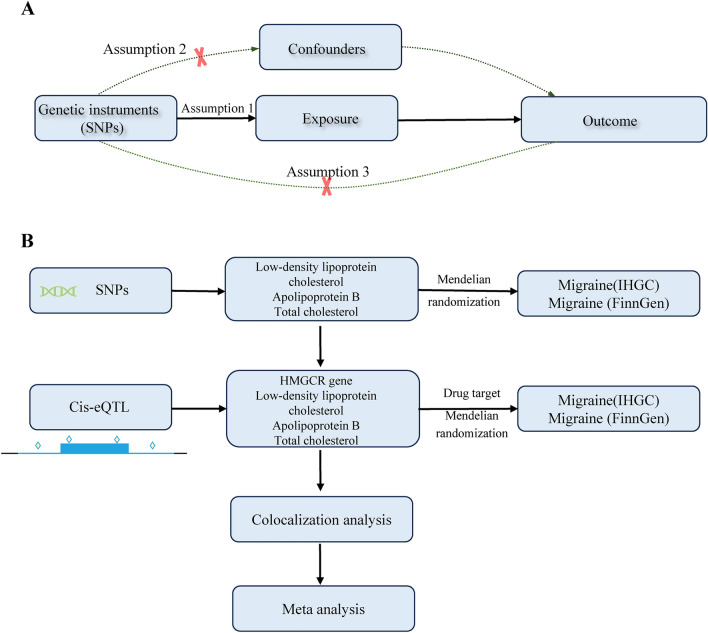
The selection of valid instrumental variables must satisfy three assumptions of MR analysis (Fig. 1)17. Given the observational association between circulating lipids and migraines, we first examined whether genetically predicted circulating lipids (LDL-C; apolipoprotein B, APOB; and total cholesterol, TC) were associated with migraines. Second, drug-targeted MR was performed to determine whether HMGCR expression in the blood affects migraines. Third, a colocalization analysis was performed to determine the presence of common genetic variants. Finally, to verify the observed associations, we assessed whether the levels of HMGCR-regulated LDL-C, APOB, and TC were associated with migraine. An overview of the study design is briefly outlined in Fig. 1.
Figure 1.
Overview of the study design in this study.
Genetic variation for exposure and identifying instrumental variables
Summary-level GWAS data for circulating lipids were obtained from the UK Biobank. The circulating lipids included LDL-C (n = 431,167)22, APOB (n = 439,214)23, and TC (n = 342,508)24. Age, sex, and type of genotyping chip were adjusted as covariates in the GWAS analyses of individuals of European ancestry included in the UK Biobank. The criteria for the selection of instrumental variables were as follows: 1 genome-wide significance (P < 5 e−08); (2) linkage disequilibrium (LD) at R2 < 0.001 within a 10,000-kb window based on the European-based 1000 Genomes Project; (3) palindromic SNPs and SNPs with minor allele frequencies < 0.01 removed; (4) proxies not sought for instrumental variables not available in the outcomes; and (5) F-statistics of instrumental variables calculated using the square of the standard error25, with an F-value > 10 suggesting sufficient instrument strength26.
The available eQTLs for HMGCR (n = 31,684) can serve as genetic proxies for statins, and summary data for eQTLs were obtained from the eQTLGen Consortium27. The instrumental variables used in this study were consistent with those reported by Huang et al.28. The following inclusions were used: 1 genome-wide significant association (P < 5 e−08); (2) defined as cis-eQTLs located within ± 100 kb windows around the coding gene; (3) a minor allele frequency > 1%; and (4) demonstrating independent association (low LD clumping r2 < 0.3).
Complementary analyses were conducted to verify the robustness of the associations obtained using cis-eQTLs as instrumental variables. Originally, we intended to use genetic instrumentation at the circulating HMGCR protein level (i.e., protein quantitative trait loci, pQTL) as a prerequisite for exposure validation. Unfortunately, we found no cis-acting pQTL that met these requirements. Therefore, considering that statins may affect the serum LDL-C, APOB, and TC levels, we selected these three circulating lipids as potential biomarkers11,29,30. We then selected genetic variants located within ± 100 kb of HMGCR (build GRCh37: chromosome 5:74,632,154–74,657,929) showing significant associations with LDL-C, APOB, and TC, respectively, at a genome-wide significance of P < 5 e−08, to serve as surrogates for statin therapy. LD was set to R2 < 0.1 within a 100-kb window, using a European reference panel from the 1000 Genomes Project. This method of selecting instrumental variables was also used in previous studies28,31. Triglycerides and high-density lipoprotein cholesterol were excluded because no instrumental variables that met the above criteria were extracted.
Genetic variation for migraine
For the primary analysis, summary-level GWAS data for migraines were obtained from a meta-analysis conducted by the International Headache Genetics Consortium (IHGC), which included participants of European ancestry. The data were approved by a direct application and material transfer agreement32. The dataset used contained 48,975 migraine cases and 540,381 controls. Migraine cases were identified based on self-reported data or the International Classification of Headache Disorders. The cases included in this meta-analysis were adjusted for sex, age, and ancestry. The remaining details, including demographic characteristics, eligibility criteria, and ethical approval, can be found in the original article32. For replication analysis, summary statistics were obtained from the FinnGen study (nCase = 1,5905, nControl = 264,662, R8 release)33.
Statistical analysis
MR analysis
The primary analytical method for MR is random-effects inverse variance-weighted (IVW), which assumes that all SNPs are valid instruments, allows for balanced pleiotropy, and provides the most precise estimates34. Additional sensitivity analyses included the MR–Egger intercept test35, the weighted median test36, the radial MR test37, and the MR pleiotropy residual sum and outliers test (MR-PRESSO)38. The MR–Egger detected horizontal pleiotropy, with P > 0.05 indicating none. The MR-PRESSO and radial MR methods were employed to identify outliers. And visualization methods such as scatter plots and leave-one-out plots are also used to identify outliers. Heterogeneity among the different IVs was evaluated using Cochran’s Q test. Burgess’s online calculator was used to calculate the power of the MR estimates39.
Bayesian colocalization analysis
To avoid the influence of LD or pleiotropy on MR findings, we performed a Bayesian colocalization analysis using the default parameters of the Coloc R package40. Bayesian colocation analysis was employed to assess the probability that the two traits (eQTL and migraine GWAS) shared the same causal variant40,41. We tested the posterior probabilities of five hypotheses: H0, not associated with any trait; H1/H2, associated with only one of the traits; H3, two traits having different causal variants; and H4, both traits having their causal SNPs and sharing the same SNP. We considered a posterior probability of hypothesis 4 (PPH4) > 0.8 (calculated by the Coloc.abf algorithm) as strong evidence for colocalization. For visualization, we used the “locuscomparer” R package42.
The Bonferroni method was employed to adjust the significance threshold for four exposures, requiring P < 1.25 × 10−2. Estimates were considered significant in the MR analysis when at least the IVW method estimates were significant and the three different MR methods were considered consistent in direction. The association results are presented as odds ratios (OR) with 95% confidence intervals (95% CI).
Meta-analysis
We performed a random-effects meta-analysis of the results obtained from the IHGC and FinnGen datasets to produce a comprehensive analysis of causality. The R package “Metafor” was used for this. The significance levels for the heterogeneity tests and the effect values of the meta-analysis results were set to 0.05.
Additional positive control analysis
Given the common and beneficial use of lipid-lowering medications in coronary artery disease, the statin-medicated condition was used as a positive control to evaluate the reliability of the instruments. A total of 122,733 patients and 424,528 controls were included in the GWAS data for coronary artery disease43.
R software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses44. The R package for MR analysis included “TwoSampleMR (version 0.5.6),” “MR-PRESSO (version 1.0),” “RadialMR (version 1.0),” “Coloc (version 1.0),” and “Metafor (version 1.0)”.
Ethical approval and consent to participate
This study used data from published studies. All original studies have been approved by the corresponding ethical review board, and the participants have provided informed consent. In addition, no individual-level data was used in this study. Therefore, separate ethical approval was not required for this study.
Results
Overall, 344 (LDL-C), 187 (APOB), and 259 (TC) SNPs were included in the analysis of the association between circulating lipids and migraine (Additional file 1: Supplementary Table 1). Two-sample MR analysis revealed no association between migraine and any of the three circulating lipids (Supplementary Tables 2–4). Seven eligible cis-eQTLs were included in the drug-targeted MRI analysis (Supplementary Table 5). From the GWAS summary-level data, 18, 10, and 11 SNPs within or near the HMGCR region were associated with LDL-C, APOB, and TC, respectively (Supplementary Table 5). The F-statistic of all included instrumental variables was greater than 10, indicating the absence of weak instrumental variable bias.
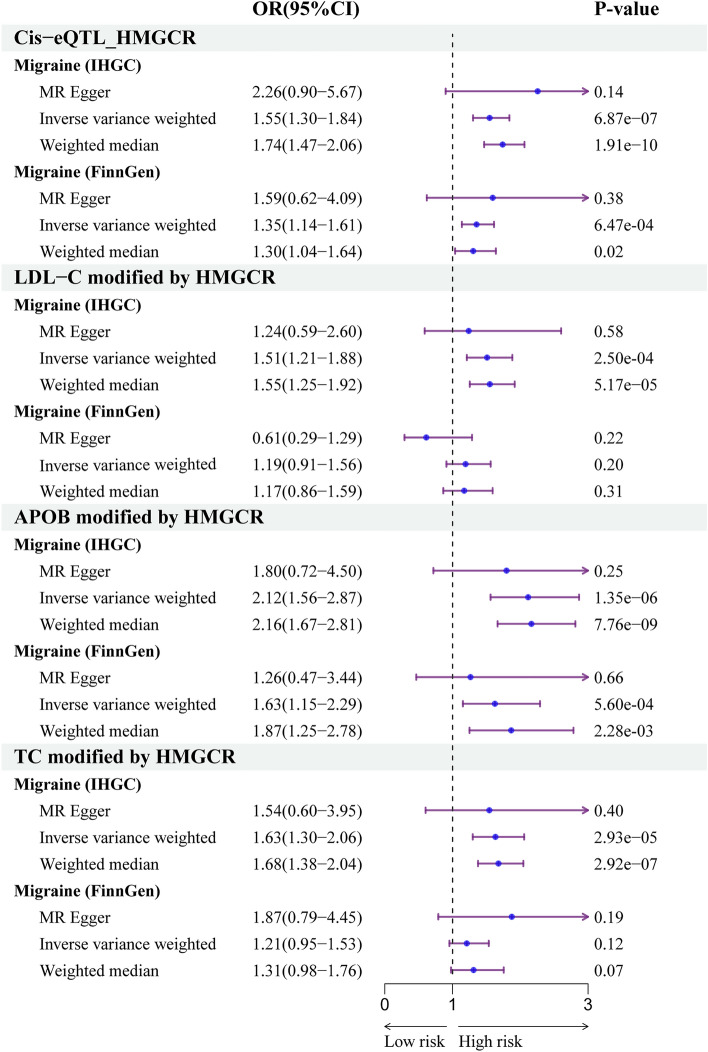
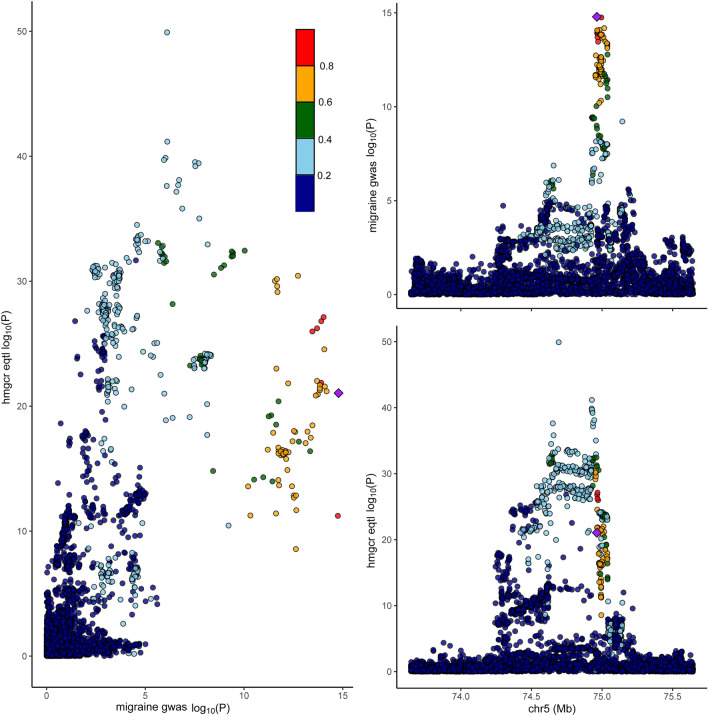
As shown in Fig. 2, the primary analysis of migraine data from the IHGC revealed that genetically predicted expression of HMGCR was associated with increased risk of migraine (OR = 1.55, 95% CI 1.30–1.84, P = 6.87 × 10−7). The replication study using data from FinnGen produced similar results (OR = 1.38, 95% CI 1.14–1.67, P = 7.38 × 10−4). Both sensitivity analyses yielded similar estimates, and in the same direction (Supplementary Table 6). This finding indicated that HMGCR inhibitors may reduce the risk of migraine susceptibility. No statistically significant heterogeneity or horizontal pleiotropy was observed (Supplementary Table 6). When causal variants were present, Bayesian colocalization analysis using data from the IHGC suggested that HMGCR and migraine shared the same variants (Coloc.abf-PPH4 = 0.97, Fig. 3) (Supplementary Table 7).
Figure 2.
Associations linking HMG-CoA reductase gene expression or HMG-CoA reductase mediated low-density lipoprotein cholesterol, apolipoprotein B, and total cholesterol with the risk of migraine.
Figure 3.
The visualization of HMG-CoA reductase gene expression and the colocalization analysis with migraine.
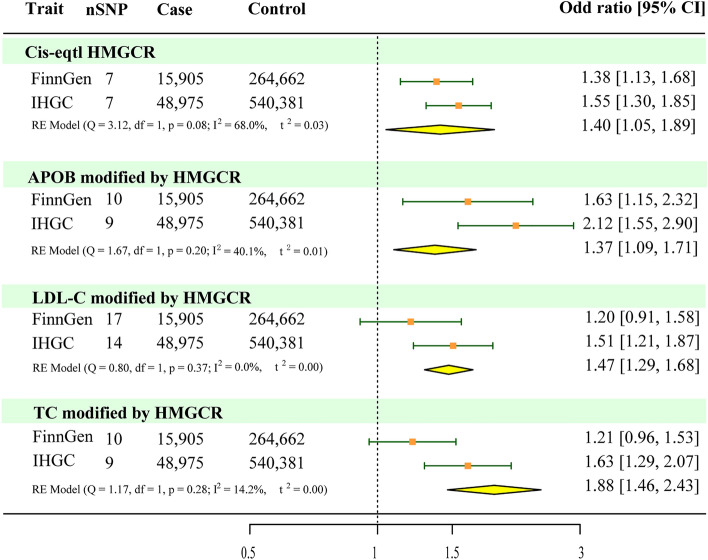
The supplementary analyses suggested that genetically predicted levels of LDL (OR = 1.51, 95% CI 1.21–1.88, P = 2.50 × 10−4), TC (OR = 1.63, 95% CI 1.30–2.06, P = 2.93 × 10−5), and APOB (OR = 2.12, 95% CI 1.56–2.87, P = 1.35 × 10−6) modified by HMGCR were associated with an increased risk of migraine (Fig. 2, Supplementary Table 6). Replication analyses using data from the FinnGen study suggested that APOB (OR = 1.63, 95% CI 1.15–2.29, P = 5.60 × 10−4) modified by HMGCR were associated with an increased risk of migraine, but LDL levels (OR = 1.19, 95% CI 0.91–1.56, P = 0.20) and TC levels (OR = 1.21, 95% CI 0.95–1.53, P = 0.12) modified by HMGCR were not significantly associated with an increased risk of migraine (Fig. 2, Supplementary Table 6). No significant evidence of heterogeneity was observed using the IVW method (Supplementary Table 6). The intercept term of the MR–Egger regression and MR-PRESSO analyses indicated that horizontal pleiotropy was not significant (Supplementary Table 6). The results of the random-effects meta-analysis suggested that LDL, APOB, and TC levels were associated with an increased risk of migraine (Fig. 4). These findings supported the reliability of the MR studies. In summary, these results provide further support for the potential protective effects of HMGCR inhibitors against migraine.
Figure 4.
Meta-analysis of mendelian randomization analysis results based on inverse variance weighting.
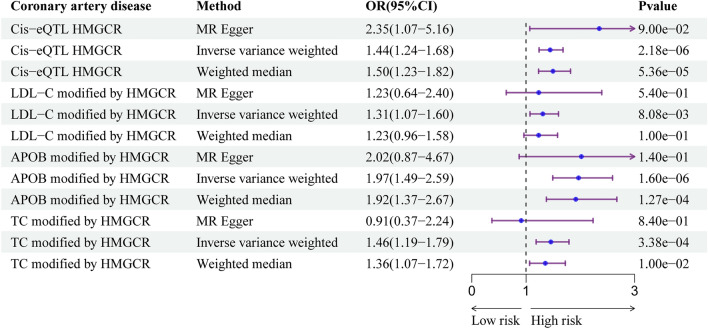
The genetically determined expression of HMGCR and the levels of LDC-C, APOB, and TC modified by HMGCR were associated with an increased risk of coronary artery disease (Fig. 5; Supplementary Table 8). As a reference for the positive control analyses, this result also increased the credibility of the included instrumental variables and confirmed the efficacy of the selected instruments.
Figure 5.
Associations between genetically proxied statins lipid-lowering drugs and the risk of coronary artery disease as a positive control analysis.
Discussion
Consistent results obtained from a rigorous MR analysis indicated that HMGCR expression and the circulating levels of three lipids (LDL-C, APOB, and TC) adjusted by HMGCR were significantly associated with an increased risk of migraine. These findings strongly suggest that HMGCR inhibitors hold promise as potential protective medications against migraines. In line with the prior study by Bi et al. it was found that HMGCR could potentially serve as a therapeutic target for migraines45. The merits of our study include: firstly, the inclusion of a greater number of cis-eQTLs that could influence HMGCR gene expression; secondly, the GWAS studies for migraines encompassed larger sample sizes and a greater number of migraine cases; thirdly, both the discovery and replication analyses indicated that a genetically determined high expression of HMGCR was correlated with an elevated risk of migraines. Nevertheless, this association did not seem to be directly linked to the total circulating levels of LDL-C, APOB, or TC, as we did not identify any significant correlation between these lipid levels and the risk of migraine.
In a recent meta-analysis examining the association between circulating lipids and migraine, individuals with migraine exhibited notably elevated levels of blood cholesterol, triglycerides, and LDL-C compared with healthy controls46. Discerning causality in observational research presents inherent difficulties, but the groundbreaking method of MR analysis has the potential to elucidate hypothetical causal relationships17. Unfortunately, a meticulous MR analysis here failed to reveal any substantial evidence for a causal relationship between the genetically determined levels of three circulating lipids and migraine.
Following the identification of a connection between circulating lipids and migraine in observational studies, researchers became intrigued by the potential therapeutic benefits of lipid-lowering medications in managing migraine. Statins are commonly used as prescription drugs in the field of neurology. Given the neurovascular nature of migraine, its etiology is influenced by inflammation and oxidative stress47,48, both regulated by statins13,14. The multifaceted properties of statins offer promising avenues for the advancement of migraine treatment. Furthermore, compelling evidence from animal studies suggests that statins may possess analgesic properties, further bolstering their potential as pain-relieving agents49. In a recent genetic association study conducted in a female migraine population, an intriguing link was discovered between migraines and specific lipoprotein subfractions, indicating a shared biological mechanism. The results of the colocalization analysis further identified this shared signal as circulating HMGCR, suggesting the potential effectiveness of statin analogs in the treatment of migraines50. Notably, exploring new applications of existing medications requires less time and resources than developing entirely new drugs de novo. In summary, statins have emerged as strong candidates for migraine treatment. Our MR study further supports this by highlighting the potential of HMGCR as a viable target for ameliorating migraine. Recent studies have focused primarily on the associations between statins, pain, and inflammation. For instance, in animal migraine models, atorvastatin suppressed B cell activation in the trigeminal caudal nucleus, demonstrating its ability to alleviate inflammation49. Similarly, in neuropathic pain models, pitavastatin demonstrated inhibitory effects on the JNK/P38/MAPK signaling pathway, resulting in a reduction in the release of inflammatory mediators51.
Nevertheless, existing research on the use of statins for migraine treatment presents conflicting results. While some case–control studies support the potential effectiveness of statins in alleviating migraine52, the results of several randomized controlled trials do not align with these results. These discrepancies could be attributed to several factors. First, the types of drugs used and their respective dosages were inconsistent across studies. Second, intervention measures within the control groups varied considerably. Third, the criteria for assessing migraine relief differed among studies. Finally, the durations of the interventions employed in the studies exhibited significant variation. Consequently, owing to the limited number of available studies, conducting a meta-analysis to quantitatively evaluate the efficacy of statins in the treatment of migraines would be premature. For further insight, please refer to Additional file 2, which provides a comprehensive overview of several published studies investigating the use of statins in migraine treatment. Based on current data, we find insufficient evidence to substantiate the use of statins as a definitive treatment for migraines. Further investigation is warranted to clarify the role of statins in migraine treatment across various layers of evidence, including genetic epidemiology.
In this MR investigation, we used genetic variants associated with HMGCR expression and HMGCR-mediated circulating lipid levels as instrumental variables for statins. Primary and supplemental analyses consistently supported HMGCR inhibitors as having the potential to reduce the risk of migraine. Hence, our study has significant implications, warranting case–control and randomized controlled studies to evaluate whether statins prevent migraine. Additionally, it supports a causal connection between HMGCR and migraines. Further investigations should include diverse populations and establish animal models specifically targeting migraine to comprehensively explore the effects and mechanisms of action of statins on migraine management.
Our study had several strengths. First, we employed genetic variants as instrumental variables instead of directly utilizing statins as the exposure, effectively minimizing confounding factors and mitigating the influence of unmeasured biases. Additionally, we incorporated four distinct types of instrumental variable and cross-validated our findings using data from two independent sources to enhance the reliability and robustness of our conclusions. Furthermore, to validate our instrumental variables, a positive control analysis was performed.
This study had some limitations. First, we were unable to identify suitable cis-acting pQTLs pertaining to HMGCR, which prevented us from establishing a clear association at the protein level between blood HMGCR levels and migraine. Second, despite the rigorous implementation of multiple sensitivity analyses to ensure that the MR assumptions were met, the presence of horizontal pleiotropy cannot be completely ruled out, which is an inherent limitation of MR studies. Third, the inclusion of populations of only European ancestry in the GWAS data restricts the generalizability of our findings. Therefore, caution should be exercised when extrapolating these associations to other populations. Fourth, we employed genetic variants in drug targets as proxies for the drug of interest rather than directly measuring drug-target inhibition, making direct comparisons challenging. Fifth, our analysis was limited to summary-level GWAS data, which restricted the depth of analysis of specific migraine subtypes. Finally, although the liver plays a crucial role in lipid metabolism, eQTLs data for this organ were not available.
Conclusion
The findings of this careful MR study imply a causative link between HMGCR inhibition and migraine. These results warrant clinical studies to assess the efficacy of HMGCR inhibition as a therapeutic strategy. Further investigations should elucidate the underlying protective mechanisms associated with this inhibition.
Supplementary Information
Acknowledgements
The authors express their gratitude to the esteemed individuals comprising the International Headache Genetics Consortium, eQTLGen consortium, and FinnGen consortium. They also extend their heartfelt appreciation to the three consortiums for generously contributing pooled data. The dedicated members of the International Headache Genetics Consortium (refer to Supplementary Table 9 for complete details) are acknowledged with utmost respect.
Abbreviations
- GWAS
Genome-wide association study
- MR
Mendelian randomization
- HMGCR
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- LDL-C
Low-density lipoprotein cholesterol
- APOB
Apolipoprotein B
- TC
Total cholesterol
- IHGC
International Headache Genetics Consortium
- SNP
Single nucleotide polymorphism
- eQTL
Expression quantitative trait locus
- pQTL
Protein quantitative trait loci
- cis-QTL
Cis-acting quantitative trait loci
- LD
Linkage disequilibrium
- IVW
Inverse variance weighting
- MR-PRESSO
MR pleiotropy residual sum and outliers test
- OR
Odds ratio
- 95% CI
95% Confidence intervals
Author contributions
K.Q. conceived the study and design and drafted the manuscript. M.X.L., B.H.W. and M.S. analyzed and interpreted the data. M.D. and P.Y. contributed to the critical revision of the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Funding
This work was supported by a grant from the Natural Science Foundation of Jilin Province of China (Grant No. 20200201606JC), Scientific Research Program of Jilin Health and Family Planning Commission (Grant No. 2016J049) and the National Natural Science Foundation of China (Grant No. 31872772) to Ming Dong.
Data availability
Genetic variants of 3 circulating lipids can be obtained through the original studies (https://doi.org/10.2337/db19-1134, https://doi.org/10.1371/journal.pmed.1003062, https://doi.org/10.1038/s41588-020-00757-z). Please visit the highly accessible eQTLGen consortium website at https://www.eqtlgen.org/ to get the GWAS summary data for cis-eQTLs. You can obtain the migraine GWAS summary data from the original study (https://doi.org/10.1038/s41588-021-00990-0) and from FinnGen (www.finngen.fi).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Ming Dong, Email: dongge@jlu.edu.cn.
International Headache Genetics Consortium:
Aarno Palotie, Alice Pressman, Andrea C. Belin, Anna Bjornsdottir, Arn M. J. M. van den Maagdenberg, Aster V. E. Harder, Bendik S. Winsvold, Bertram Müller-Myhsok, Bru Cormand, Caroline Ran, Carrie Northover, Christian Kubisch, Cornelia van Duijn, Dale R. Nyholt, Daniel I. Chasman, Danielle Posthuma, Davor Lessel, Dorret I. Boomsma, Eija Hämäläinen, Espen S. Kristoffersen, Ester Cuenca-Leon, George Davey-Smith, Gisela M. Terwindt, Gudrun R. Sigurdardottir, Gyda Bjornsdottir, Heidi Hautakangas, Hreinn Stefansson, Irene de Boer, Jaakko Kaprio, Jes Olesen, John-Anker Zwart, Kari Stefansson, Lannie Ligthart, Lenore Launer, Linda M. Pedersen, Lisette J. A. Kogelman, Lyn R. Griffiths, M. Arfan Ikram, Maija Wessman, Mari Kaunisto, Maria G. Hrafnsdottir, Marjo Hiekkala, Marjo-Riitta Järvelin, Martin Dichgans, Matti Pirinen, Mikko Kallela, Mitja Kurki, Mona A. Chalmer, Nancy Pedersen, Olafur A. Sveinsson, Olli Raitakari, Padhraig Gormley, Patricia Pozo-Rosich, Priit Palta, Rainer Malik, Risto Kajanne, Sigrid Børte, Sigurdur H. Magnusson, Terho Lehtimäki, Thomas F. Hansen, Thorgeir E. Thorgeirsson, Tobias Freilinger, Tobias Kurth, Tonu Esko, Verneri Anttila, and Ville Artto
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61628-9.
References
- 1.Dodick DW. Migraine. Lancet (London, England) 2018;391:1315–1330. doi: 10.1016/s0140-6736(18)30478-1. [DOI] [PubMed] [Google Scholar]
- 2.Burch RC, Buse DC, Lipton RB. Migraine: Epidemiology, burden, and comorbidity. Neurol. Clin. 2019;37:631–649. doi: 10.1016/j.ncl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Ashina M, et al. Migraine: Epidemiology and systems of care. Lancet (London, England) 2021;397:1485–1495. doi: 10.1016/s0140-6736(20)32160-7. [DOI] [PubMed] [Google Scholar]
- 4.Gruber HJ, et al. Lipid profile in normal weight migraineurs—Evidence for cardiovascular risk. Eur. J. Neurol. 2010;17:419–425. doi: 10.1111/j.1468-1331.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 5.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: Cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia Int. J. Headache. 2011;31:1459–1465. doi: 10.1177/0333102411421682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buettner C, et al. Simvastatin and vitamin D for migraine prevention: A randomized, controlled trial. Ann. Neurol. 2015;78:970–981. doi: 10.1002/ana.24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherafat A, Sahebnasagh A, Rahmany R, Mohammadi F, Saghafi F. The preventive effect of the combination of atorvastatin and nortriptyline in migraine-type headache: A randomized, triple-blind, placebo-controlled trial. Neurol. Res. 2022;44:311–317. doi: 10.1080/01616412.2021.1981105. [DOI] [PubMed] [Google Scholar]
- 8.Mazdeh M, Mahmudian R, Vafaei SY, Taheri M, Ghafouri-Fard S. Effect of propranolol with and without rosuvastatin on migraine attacks: A triple blind randomized clinical trial. Futur. Neurol. 2020 doi: 10.2217/fnl-2019-0029. [DOI] [Google Scholar]
- 9.Ganji R, et al. Does atorvastatin have augmentative effects with sodium valproate in prevention of migraine with aura attacks? A triple-blind controlled clinical trial. J. Pharm. Health Care Sci. 2021;7:12. doi: 10.1186/s40780-021-00198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesami O, et al. Comparing the effects of atorvastatin with sodium valproate (divalproex) on frequency and intensity of frequent migraine headaches: A double-blind randomized controlled study. Clin. Neuropharmacol. 2018;41:94–97. doi: 10.1097/wnf.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 11.Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018;15:757–769. doi: 10.1038/s41569-018-0098-5. [DOI] [PubMed] [Google Scholar]
- 12.Millar PJ, Floras JS. Statins and the autonomic nervous system. Clin. Sci. (London, England 1979) 2014;126:401–415. doi: 10.1042/cs20130332. [DOI] [PubMed] [Google Scholar]
- 13.Tousoulis D, et al. Innate and adaptive inflammation as a therapeutic target in vascular disease: The emerging role of statins. J. Am. Coll. Cardiol. 2014;63:2491–2502. doi: 10.1016/j.jacc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Liu A, et al. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol. Ther. 2019;195:54–84. doi: 10.1016/j.pharmthera.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Øie LR, Kurth T, Gulati S, Dodick DW. Migraine and risk of stroke. J. Neurol. Neurosurg. Psychiatr. 2020;91:593–604. doi: 10.1136/jnnp-2018-318254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng CYH, et al. Myocardial infarction, stroke and cardiovascular mortality among migraine patients: A systematic review and meta-analysis. J. Neurol. 2022;269:2346–2358. doi: 10.1007/s00415-021-10930-x. [DOI] [PubMed] [Google Scholar]
- 17.Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 18.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. JASN. 2016;27:3253–3265. doi: 10.1681/asn.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill D, et al. Mendelian randomization for studying the effects of perturbing drug targets. Wellcome Open Res. 2021;6:16. doi: 10.12688/wellcomeopenres.16544.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt AF, et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 2020;11:3255. doi: 10.1038/s41467-020-16969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrivankova VW, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. Jama. 2021;326:1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 22.Klimentidis YC, et al. Phenotypic and genetic characterization of lower LDL cholesterol and increased type 2 diabetes risk in the UK Biobank. Diabetes. 2020;69:2194–2205. doi: 10.2337/db19-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson TG, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinnott-Armstrong N, et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021;53:185–194. doi: 10.1038/s41588-020-00757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li BB, Martin EB. An approximation to the F distribution using the chi-square distribution. Comput. Stat. Data Anal. 2002;40:21–26. doi: 10.1016/S0167-9473(01)00097-4. [DOI] [Google Scholar]
- 26.Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 27.Võsa U, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 2021;53:1300–1310. doi: 10.1038/s41588-021-00913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang W, Xiao J, Ji J, Chen L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. elife. 2021 doi: 10.7554/eLife.73873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams SP, Tsang M, Wright JM. Lipid-lowering efficacy of atorvastatin. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD008226.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behbodikhah J, et al. Apolipoprotein B and cardiovascular disease: Biomarker and potential therapeutic target. Metabolites. 2021;11:690. doi: 10.3390/metabo11100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao SS, Yiu ZZN, Barton A, Bowes J. Association of lipid-lowering drugs with risk of psoriasis: A Mendelian randomization study. JAMA Dermatol. 2023;159:275–280. doi: 10.1001/jamadermatol.2022.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hautakangas H, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022;54:152–160. doi: 10.1038/s41588-021-00990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurki MI, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–518. doi: 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowden J, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int. J. Epidemiol. 2018;47:1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 2014;43:922–929. doi: 10.1093/ije/dyu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giambartolomei C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuber V, et al. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am. J. Hum. Genet. 2022;109:767–782. doi: 10.1016/j.ajhg.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Gloudemans MJ, Rao AS, Ingelsson E, Montgomery SB. Abundant associations with gene expression complicate GWAS follow-up. Nat. Genet. 2019;51:768–769. doi: 10.1038/s41588-019-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikpay M, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing (2020).
- 45.Bi Y, Zhu Y, Tang S, Huang Y. Lipids, lipid-modifying drug target genes and migraine: A Mendelian randomization study. J. Headache Pain. 2023;24:112. doi: 10.1186/s10194-023-01633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liampas I, et al. Serum lipid abnormalities in migraine: A meta-analysis of observational studies. Headache. 2021;61:44–59. doi: 10.1111/head.14039. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018;40:301–314. doi: 10.1007/s00281-018-0676-y. [DOI] [PubMed] [Google Scholar]
- 48.Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J. The metabolic face of migraine—from pathophysiology to treatment. Nat. Rev. Neurol. 2019;15:627–643. doi: 10.1038/s41582-019-0255-4. [DOI] [PubMed] [Google Scholar]
- 49.Yin Z, et al. Atorvastatin attenuates NF-kappaB activation in trigeminal nucleus caudalis in a rat model of migraine. Neurosci. Lett. 2009;465:61–65. doi: 10.1016/j.neulet.2009.08.081. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y, et al. Phenotypic and genotypic associations between migraine and lipoprotein subfractions. Neurology. 2021;97:e2223–e2235. doi: 10.1212/wnl.0000000000012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goel R, Kumar N, Kumar Saxena P, Pratap Singh A, Bana S. Pitavastatin attenuates neuropathic pain induced by partial sciatic nerve in Wistar rats. J. Pharm. Pharmacol. 2023;75:66–75. doi: 10.1093/jpp/rgac079. [DOI] [PubMed] [Google Scholar]
- 52.Medeiros FL, Medeiros PL, Valença MM, Dodick D. Simvastatin for migraine prevention. Headache. 2007;47:855–856. doi: 10.1111/j.1526-4610.2007.00824.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genetic variants of 3 circulating lipids can be obtained through the original studies (https://doi.org/10.2337/db19-1134, https://doi.org/10.1371/journal.pmed.1003062, https://doi.org/10.1038/s41588-020-00757-z). Please visit the highly accessible eQTLGen consortium website at https://www.eqtlgen.org/ to get the GWAS summary data for cis-eQTLs. You can obtain the migraine GWAS summary data from the original study (https://doi.org/10.1038/s41588-021-00990-0) and from FinnGen (www.finngen.fi).