The isolation of Helicobacter pylori from clinical specimens by Marshall and Warren 15 years ago1 launched revolutions in gastroenterology and microbiology.2,3 Like other revolutions in history, the original directions of the H pylori story have changed in response to conflicting ideologies, observation, and practices. Currently, enthusiasts, drug companies, and the lay press are putting pressure on physicians to eliminate H pylori from all patients, symptomatic or not, in whom it is detected. There is little evidence that this is appropriate, and management will continue to change as new knowledge emerges and socioeconomic environments change in ways that are relevant to H pylori and clinical medicine. This article looks at past and current trends to anticipate the future of these common bacteria and the disorders associated with them.
Changing epidemiology of H pylori
H pylori is one of a large family of related bacteria that are well adapted to persist in the stomachs of vertebrates for the life span of their hosts. It is likely that H pylori has colonised our stomachs since well before we became humans.4 Studies of primates and human populations in developing countries suggest that, until the last century, nearly all humans carried H pylori or closely related bacteria in their stomachs. H pylori can thus be regarded as indigenous or “normal” flora, which most humans acquire within the first few years of childhood and then carry for life.
With socioeconomic development, fewer children are acquiring H pylori.5 This is a worldwide phenomenon that seems to have preceded the introduction of antibiotics, although it may have been accelerated by their widespread use. The reasons for the decline in the prevalence of H pylori colonisation are unknown. Improved nutrition and clean water have been proposed. An alternative hypothesis is that most transmission is from child to child and that declining family size reduces the opportunity for transmission and increases the age of acquisition.
In developed countries, for perhaps the first time in the human experience, large numbers of people are now passing their lives without H pylori colonisation. This is a crucial observation with considerable relevance for understanding the changing pattern of gastrointestinal diseases.
Microbiology of H pylori
Genetic studies indicate that H pylori strains are enormously diverse.6 This diversity reflects the bacteria’s ancient ancestry, its niche in varied human populations, its large numbers in colonised hosts (about 108-1010 organisms per stomach), its ability to mutate over decades of colonisation of a single host, and the ease with which it exchanges genes with other H pylori strains.
Possible future developments
Changing epidemiology of upper gastrointestinal diseases (peptic ulceration, oesophageal and gastric adenocarcinoma, gastro-oesophageal reflux disease and sequelae) with changing prevalence of colonisation with Helicobacter pylori
Recognition of host factors that predispose to diseases induced by H pylori
Definition of genetic characteristics of H pylori that are associated with significant variance in risk of disease
Vaccinations against certain diseases based on specific interactions between H pylori and host
Colonisation of some hosts with strains of H pylori with low virulence to reduce risk of particular diseases
The key question is whether any of the diversity is of clinical importance. Recent work suggests a qualified “yes.” Three different genetic loci have been identified—cag, vacA, and iceA—for which people harbouring particular alleles have different risks of disease.7 In Western countries, a person colonised with a cag+, s1a vacA, iceA1 strain is more likely to develop duodenal ulceration than a person harbouring a cag−, s2 vacA, iceA2 strain. These markers are not fully independent of each other and, importantly, are not absolutes but reflect degrees of risk (fig 1). In the future, knowledge of H pylori genotypes could help to identify people at risk of particular diseases.
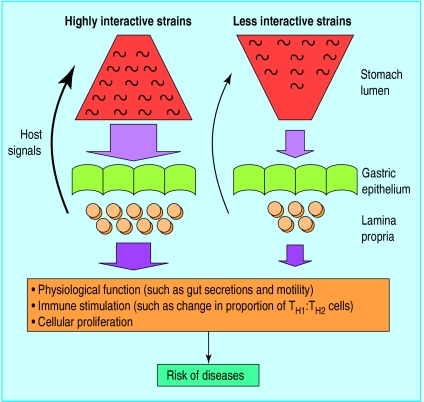
Figure 1.
Model of interactions of polar forms of Helicobacter pylori with human gastric mucosa and consequent pathophysiology. With highly interactive strains (such as cag+, s1a vacA, iceA1 genotype), population structure is weighted toward proximity to the mucosa and there is high impact on host tissues, resulting in intense inflammation and host signals (such as changes in gut nutrients, pH, and motility); a regulated dynamic equilibrium between the microbial population and host response has been proposed. Induced changes in host physiology, immune response, and cellular proliferation influence risk of particular diseases. Less interactive strains of H pylori (such as cag−, s2 vacA, iceA2) colonise in lower numbers and with populations preferentially distributed towards the lumen. These populations are also in equilibrium with the host, but with less “gain” and less serious pathophysiological consequences. Hosts may carry both populations simultaneously
Relation of H pylori to disease
Everyone who carries H pylori in their stomach develops a cellular infiltrate in their gastric mucosa, termed chronic gastritis. In most people this causes no symptoms, but H pylori carriers do have an increased risk of developing peptic ulcer disease (about threefold to 10-fold) and adenocarcinoma of the antrum and body of the stomach (twofold to 10-fold). Elimination of H pylori with antimicrobial treatment heals the ulceration and substantially reduces the risk of recurrence.8
Peptic ulcer disease and gastric cancer involving the antrum and body have been declining in the 20th century in precisely those parts of the world in which the prevalence of H pylori colonisation has declined, and much evidence suggests the events are related. Carriage of H pylori also increases the risk (about sixfold) of developing primary non-Hodgkin’s lymphomas of the stomach (MALTomas).9 Elimination of H pylori markedly attenuates the course of low grade MALTomas.10
Non-ulcer dyspepsia occurs roughly as often in H pylori carriers as in non-carriers. Non-ulcer dyspepsia is a heterogeneous group of disorders, and if H pylori plays any role in this it affects only a minority of patients.
While ulcer disease and distal gastric adenocarcinomas have become less common as H pylori is disappearing, a new group of diseases has been increasing rapidly in Western countries—gastro-oesophageal reflux disease and its sequelae, Barrett’s oesophagus, and adenocarcinoma of the (distal) oesophagus. The incidences of adenocarcinoma of the oesophagus and gastric cardia are increasing rapidly in several Western countries,11 and in white American men now exceed the prevalences of squamous cell oesophageal cancers and distal gastric adenocarcinomas respectively. Their rapid rise indicates a strong environmental cause, and there is no evidence that the epidemic is abating.
A key question, therefore, is whether the advent of these diseases is related to the simultaneous fall in carriage of H pylori (fig 2). Evidence links the lack of H pylori with gastro-oesophageal reflux disease, Barrett’s oesophagus, and the risk of adenocarcinoma of the oesophagus and gastric cardia.12–14 In particular, it seems that cag+ strains exert a protective effect whereas cag− strains have essentially no effect.14,15 These preliminary observations need confirmation but, if correct, suggest that clinicians should not eliminate H pylori from everyone, as they will be trading a decreased risk for certain diseases (peptic ulcers, adenocarcinomas of gastric antrum or body) with increased risk for others.
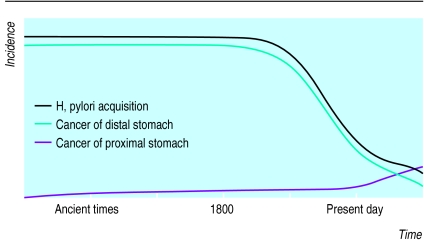
Figure 2.
Changing incidence of acquisition of H pylori and gastric cancers in Western countries. Colonisation with H pylori seems to have been nearly universal among adults before 1800 and then began to diminish until the present, when <20% of children are colonised. Concomitant with the drop in H pylori colonisation, the incidence of adenocarcinoma of the antrum and body (distal stomach) has declined, but adenocarcinomas of the cardia (proximal stomach) and distal oesophagus, once rare, are rising rapidly
The future
Diseases
Because the presence of H pylori in the stomach is related to several different clinical entities, I will predict critical issues for each.
Peptic ulceration
—This is increasingly becoming a disease of the elderly in developed countries, with non-steroidal anti-inflammatory drugs implicated as a major cause. All patients should be investigated to determine whether they are carrying H pylori, which if present should be eliminated. Regardless of the risk of other diseases, removal of H pylori should confer more short term and long term benefit than harm. In developing countries, where the age of acquisition of H pylori is increasing, the incidence of gastric ulcer and distal gastric cancers is likely to fall and that of duodenal ulceration to increase.16
Adenocarcinoma of lower (distal) stomach
—The major challenge is to understand the pathogenesis of this disease so that people at high risk of developing cancer can be identified and the disease prevented or treated at an early stage.17 The dominant form, the “intestinal type,” seems to be related to the development of atrophic gastritis and intestinal metaplasia, and particularly to cag+ strains of H pylori.18 In the future, reliable serological tests to detect abnormal gastric secretory function in conjunction with determining markers for H pylori strains should allow clinicians to identify those at high risk of developing cancer. (Current measurements of serum pepsinogens and gastrin, alone or in combination, are not clinically useful, and new approaches are needed before asymptomatic individuals can be screened.) The less common “diffuse type” of cancer is not associated with atrophic gastritis but has a greater familial tendency and presents earlier. In Japan identification of people with early presymptomatic gastric cancers by routine endoscopy over the age of 50 and the eradication of H pylori seem to have been effective in reducing development of subsequent cancers.19 Thus, even at a late stage in the oncogenic process, relevant pathophysiological steps may be blocked.
MALToma
—Lymphoid proliferation is a common feature of H pylori colonisation. Occasionally, monoclonal expansion of gastric B cells results in malignant transformation and the development of high grade lymphoma. It is not clear, however, how often patients who have essentially benign lymphoid proliferation20 are currently being labelled as having malignant disease. Although antimicrobial treatment is relatively safe, a goal for the future will be to differentiate true malignancy from monoclonal lymphoid proliferation.
Oesophageal disease
—Further studies will confirm whether H pylori protects against gastro-oesophageal reflux disease, Barrett’s oesophagus, and adenocarcinomas of the gastric cardia and oesophagus and which bacterial genotypes are important. Assuming that some types of H pylori strains are protective, our whole approach to eradication treatment, which is becoming increasingly indiscriminate, will have to change dramatically. Risks as well as benefits will have to be estimated carefully in each patient for whom treatment is considered.
Proposed temporal relations in upper gastrointestinal diseases
| Time period | Predominant disease | Presumed association with H pylori |
| Ancient | Distal gastric cancer | H pylori acquisition in infancy |
| Pre-modern | Gastric ulcer | H pylori acquisition later |
| Modern | Duodenal ulcer | H pylori acquisition still later |
| Post-modern | Oesophageal disease | H pylori (cag) not acquired |
Non-ulcer dyspepsia
—Despite the absence of firm recommendations, some doctors are now treating large numbers of patients with non-ulcer dyspepsia who are carrying H pylori with eradication treatment. Not surprisingly, results are mixed, which in part may reflect placebo effects. A priority for the future is therefore to stratify risk based on pathophysiological mechanisms or markers to define a subgroup of patients with dyspepsia related to H pylori, who stand to improve with treatment.
Asymptomatic carriage
—Since eradicating H pylori may put people at increased risk of developing oesophageal disease, only in exceptional circumstances should asymptomatic individuals be treated. One group of patients who may benefit are those who have a first degree relative with gastric cancer.
Other clinical problems
—Although an association of H pylori with the development of atherosclerotic heart disease has been reported, the attributable risk is small and could easily be due to unidentified confounding factors. People carrying H pylori may be slightly shorter (and possibly leaner) than their uncolonised counterparts. If true, this may be of epidemiologic interest, but it is unlikely to be clinically relevant.
Improved diagnosis and more rational treatment decisions
Deciding who should be assessed for the presence of H pylori is a contentious issue. However, remembering that (except for research) we should obtain information only if it can be used clinically, then the only people who should be tested at present are those with peptic ulcer disease, MALToma, or a strong family history of gastric cancer. None of the currently available diagnostic tests—biopsy techniques, urea breath tests, or serology—is completely reliable for H pylori because of heterogeneity in the density of bacterial colonisation and in host immune response. The use of two or more independent methods (such as serology and breath test) should bring sensitivity before treatment close to 100%. For evaluating the adequacy of treatment, however, the urea breath test seems to be optimal when done later than one month after the end of treatment. In the future, as we better understand the pathogenesis of the relevant diseases, diagnostic assays to assess differences between H pylori strains and host physiology will become routine.
Resistance to metronidazole and clarithromycin is increasing because of widespread use of these drugs (and related compounds) to treat other clinical conditions, and because failed treatment directed against H pylori leads to the emergence of (secondary) resistance. Current short term (7-10 days) quadruple treatments—including an acid reducing agent, a bismuth salt, and two antimicrobial drugs—are highly effective. After one or sometimes two treatments, it is possible to eliminate H pylori from nearly all patients because multi-drug resistance is still uncommon. The development of new treatments is therefore less crucial than determining which people to treat.
Technical terms
cagA—The cytotoxin-association gene, which encodes a high molecular weight protein of unknown function, is a marker for the 38 kilobase cag island, which is present in H pylori strains that are more interactive with their hosts
Urea breath test—Urea labelled with 14C or 13C is fed to a subject, and the carbon isotope is detected in exhaled CO2. High values signify the presence of gastric urease activity, which is nearly always due to the presence of H pylori
vacA—The gene encoding the vacuolating cytotoxin is present in all H pylori strains but is polymorphic. In Western populations particular alleles (such as s1a) are associated with increased risk of disease
Disease prevention
If a suitable vaccine were available (research projects are ongoing) to prevent H pylori colonisation, would we increase or decrease the world’s disease burden? There may not be a single answer to this question; answers may vary with country, host genotype, and dietary factors. For example, a vaccination may be appropriate in parts of China where gastric cancer rates are high but not in the United States, where rates are low and the incidence of gastro-oesophageal reflux disease is increasing. In any case, with continuing socioeconomic development, H pylori colonisation is becoming less common. Monitoring trends in colonisation rates and upper gastrointestinal disease in differing populations should provide clues. Vaccination directed at preventing particular diseases rather than H pylori colonisation per se may be the way forward. The recent identification of the complete genomic sequence of one strain of H pylori21 will undoubtably provide much information relevant to treatment, prevention, and diagnostics. Comparison of genetic sequences with those of “low virulence” strains should allow identification of the bacterial genetic bases of pathogenicity.
Are there individuals whom we should inoculate with H pylori to counter disease? And if so, which natural or attenuated strain, or combination of strains, should be used? We cannot answer these questions yet, but in the future it is conceivable that we will assess each infant and, based on factors such as its genotype, anticipated diet, and prevalence of particular diseases in its community, prescribe a strain (or strains) of H pylori to colonise that infant’s stomach to maximise its chances of health. This may seem far-fetched, but it is in effect exactly what nature has done for us over millions of years; we are not randomly colonised, there has been selection for our normal flora. Particular organisms that increased the risk of diseases before the end of reproductive life have been selected against, while useful interactions have been selected for. The diseases we now associate with H pylori (or its absence) occur nearly exclusively after the reproductive period, so there is little natural selection for or against colonisation. Nevertheless, to best understand our future association with H pylori, we must be thinking in biological terms rather than merely in terms of patients’ symptoms or of the hyperbole in drug advertisements and the lay press.
Acknowledgments
I thank Drs John Atherton, Ernst Kuipers, Emad El-Omar, Richard Peek, and Hans-Peter Wirth for their criticisms.
Footnotes
Funding: This work was supported by grants R01 DK50837 and R01 DK53707 from the National Institutes of Health, and by the Veterans Affairs Medical Research Service.
Conflict of interest: MJB holds patents relating to H pylori genotypes, serology, and vaccines.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 3.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;60:177–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ. Not all Helicobacter pylori strains are created equal: should all be eliminated? Lancet. 1997;349:1020–1022. doi: 10.1016/S0140-6736(96)09133-7. [DOI] [PubMed] [Google Scholar]
- 5.Parsonnet J. Incidence of H pylori infection. Aliment Pharmacol Ther. 1995;9(suppl 2):45–51. [PubMed] [Google Scholar]
- 6.Go MF, Kapur V, Graham DY, Musser JM. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton JC, Cao P, Peek RM, Tummuru MKR, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 8.Hentschel E, Brandstatter G, Dragoisics B, Hirschl AM, Nemec H, Schütze K, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308–312. doi: 10.1056/NEJM199302043280503. [DOI] [PubMed] [Google Scholar]
- 9.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 10.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 11.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 12.Werdmuller BFM, Loffeld RJLF. Helicobacter pylori infection has no role in the pathogenesis of reflux esophagitis. Dig Dis Sci. 1997;42:103–105. doi: 10.1023/a:1018841222856. [DOI] [PubMed] [Google Scholar]
- 13.Labenz J, Blum AL, Bayerdorffer E, Meining A, Stolte M, Borsch G. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442–1447. doi: 10.1016/s0016-5085(97)70024-6. [DOI] [PubMed] [Google Scholar]
- 14.Chow W-H, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 15.Vicari JJ, Peek RM, Falk GW, Goldblum JR, Easley KA, Schnell J, et al. The seroprevalence of cagA positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology (in press). [DOI] [PubMed]
- 16.Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562–565. [PubMed] [Google Scholar]
- 17.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 18.Blaser MJ, Pérez-Pérez GI, Kleanthous H, Cover TH, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 19.Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 20.Collins RD. Is clonality equivalent to malignancy: specifically, is immunoglobulin gene rearrangement diagnostic of malignant lymphoma? Hum Pathol. 1997;28:757–759. doi: 10.1016/s0046-8177(97)90145-3. [DOI] [PubMed] [Google Scholar]
- 21.Tomb J-F, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]