Abstract

The ability to gain spatiotemporal information, and in some cases achieve spatiotemporal control, in the context of drug delivery makes theranostic fluorescent probes an attractive and intensely investigated research topic. This interest is reflected in the steep rise in publications on the topic that have appeared over the past decade. Theranostic fluorescent probes, in their various incarnations, generally comprise a fluorophore linked to a masked drug, in which the drug is released as the result of certain stimuli, with both intrinsic and extrinsic stimuli being reported. This release is then signaled by the emergence of a fluorescent signal. Importantly, the use of appropriate fluorophores has enabled not only this emerging fluorescence as a spatiotemporal marker for drug delivery but also has provided modalities useful in photodynamic, photothermal, and sonodynamic therapeutic applications. In this review we highlight recent work on theranostic fluorescent probes with a particular focus on probes that are activated in tumor microenvironments. We also summarize efforts to develop probes for other applications, such as neurodegenerative diseases and antibacterials. This review celebrates the diversity of designs reported to date, from discrete small-molecule systems to nanomaterials. Our aim is to provide insights into the potential clinical impact of this still-emerging research direction.
1. Introduction
The term cancer is used to refer to a group of diseases caused by the uncontrolled proliferation of cell phenotypes that generate growth signals, but which are insensitive to anti-growth signals. These cells can replicate indefinitely, resist apoptosis, induce angiogenesis, and promote invasion and metastasis.1 With more than 10 million cases being diagnosed each year, cancer-related fatalities are expected to rise in the near future. According to an estimate by the World Health Organization (WHO), over 13.1 million deaths caused by cancer are expected by 2030.2 In the past 10 years, the cancer-related mortality rate has been reduced owing to decreased smoking, a better understanding of tumor biology and improvements in diagnosis, and more effective therapeutic designs. At present, cancer treatment options consist largely of surgical intervention, chemotherapy, radiation therapy, immunotherapy, and various combinations. Conventional chemotherapeutics work largely by disrupting DNA synthesis and mitosis, resulting in the death of rapidly proliferating cancer cells. These agents are non-selective and can harm normal healthy tissues, resulting in significant unanticipated and undesired side effects, such as nausea and appetite loss. Indeed, the deleterious effects of chemotherapies on normal healthy tissues and organs are often dose-limiting and can contribute to poor clinical outcomes. Furthermore, many chemotherapeutics in current use suffer from poor bioavailability and limited uptake in tumors. As a consequence, relatively high doses need to be administrated. This can result in increased toxicity in normal cellular environments and the onset of multiple drug resistance, which is a major limiting factor in controlling metastatic cancer.3 Disease relapse is another major challenge. It is now well-documented that cancer cells develop drug resistance after prolonged exposure to anticancer drugs. The majority of chemotherapeutic drugs are administrated to prolong the patient’s survival and alleviate symptoms, referred to as “palliative chemotherapy”. However, such treatment plans may also have a significant adverse effect on the patient’s mental and physical health, making it difficult to maintain treatment and increase the life expectancy of terminally ill patients. Innovative drug delivery systems with improved targeting abilities offer the promise of addressing these limitations and can thus allow for improvements in cancer therapy.
The limitations of conventional cancer therapy have prompted efforts to understand the real causes of cancerous disease at the molecular and cellular levels, as well as the design of therapeutic agents for treatment. The concept of targeted therapy, developed in the lengthy aftermath of “oncogene addiction” (the reliance of certain tumor cells on a single active oncogenic pathway to retain their malignant characteristics), has spawned the development of several strategies to overcome the primary drawbacks of conventional cancer therapies by targeting disease-related mechanisms and characteristics.4 In principle, an ideal cancer therapy would provide the proper medication at an optimal level to the correct target so as to achieve localized disease control with minimum systemic toxicity. More rigorous diagnostic and therapeutic coordination, better classification of patient characteristics, improved stratification of tumor subpopulations, and the development of therapies customized to individuals are likely needed if this long-standing goal is to be met.
The term “theranostics” refers to systems that combine imaging from non-invasive modalities with a therapeutic component. By definition, the imaging and therapeutic components must be contained within a single construct. Specific functionalization with targeting moieties can allow the direction of the theranostic to cancerous lesions. Optical (absorption, fluorescence, or bioluminescence), nuclear (PET, SPECT) photoacoustic, ultrasound, and MR imaging techniques are widely utilized in theranostics.5−7 They are favored since the expression of receptors corresponding to targets of interest can vary in different tumor types, as well as in distant metastatic locations, which, in turn, makes classic biopsy-based approaches less than ideal.8,9 In contrast, images provided by appropriately designed theranostics can allow the delivery of a cytotoxic agent to a tumor to be confirmed. Imaging data can also be used to monitor therapeutic outcomes. Currently, fluorescence-based optical imaging is receiving considerable attention in the context of theranostic development.10−12 Although such imaging benefits from high sensitivity and resolution, it is currently only employed clinically for superficial regions such as those associated with image-guided surgical resection and ocular imaging.13−15 When applied to deep-seated tumors, auto-fluorescence and scattering are major limitations. These limitations can be partly overcome in a preclinical setting through the use of near-infrared (NIR) fluorophores (excitation in the 650–900 nm range). Translating the promise of NIR-based optical imaging into a clinical setting represents a current challenge in theranostics development to effectively deliver therapeutic payloads.
To deliver cytotoxic payloads effectively, it has proved useful to “mask” them with specific chemical moieties to furnish prodrugs with limited pharmacological activity. To date, the prodrug strategy has been used to optimize the pharmacokinetic or pharmacodynamics properties of drugs that are currently available in the market. Ideally, the constituent therapeutic agents regain their original activity in the presence of cancer-specific biomarkers through a “masked-to-unmasked” conversion thereby providing targeted therapeutic effects with minimal off-target toxicities.16,17 Although prodrugs are generally regarded by the regulatory authorities as being new chemical entities, their enhanced performance in comparison to the parent drug can speed up the drug development process, potentially saving labor, resources and time. Currently, work on theranostic fluorescent probes constitutes a major research area, as underscored by the exponential increase in publications on this topic over the past decade (Figure 1).
Figure 1.
(A) Number of publications per year on “theranostic fluorescent probes” (Web of Science). Search keywords are “theranostic probes and theranostic fluorescent probes”. (B) Schematic illustration of theranostic probes and their application as targeted diagnostics and therapeutics.
In this review, we aim to highlight gaps in our knowledge and address why the field of cancer theranostics has yet to deliver on its promise of improving patient survival. We will provide an overview of our current understanding of tumor biology as it relates to targeted drug delivery systems and rationally designed cancer therapeutics. Particular focus will be placed on fluorogenic theranostic probes that become activated in tumor-specific environments and their application to localization-enhanced chemotherapy, photodynamic therapy (PDT), photothermal therapy (PTT), sonodynamic therapy (SDT), and various mixed modalities (Figure 1). Fluorogenic theranostic probes developed for use in other fields, such as Alzheimer’s, antiaging, and antibacterial applications, will also be covered briefly. For each system, the activation strategy is discussed and the therapeutic potential is compared with the corresponding conventional therapeutic. This treatment, it is hoped, will allow the putative benefits of the theranostic approach to be assessed while guiding further improvements. Barriers to clinical translation will also be discussed. We believe that the present review will promote further research in this field and streamline the development of theranostic agents for clinical use. While nanoparticle-based theranostics are beyond the scope of this review, in certain cases we note the benefits of nanoformulations in the study of specific theranostics. As indicated above, the field has grown rapidly in recent years. As such, the current review is not intended to be comprehensive. Rather, major advances made in the field over the last five years have been highlighted using selected examples. The exclusion of certain papers in this review is not meant to indicate a lack of significance. It simply reflects the vastness of the field, which has necessarily required that a selection be made. We refer the readers to the reviews cited in each section for further information.
2. Theranostic Fluorescence Probes in Cancer Therapy
Tumors are collections of abnormally growing cells that can be benign or malignant. While the difference between benign and malignant tumors is a significant topic in cancer pathology, in broad brushstrokes, benign tumors, such as a skin wart, are largely limited to the parent site, with no appreciable invasion of neighboring normal organs or tissues. Nor, does distant dissemination occur. In contrast, malignant tumors are prone to metastasize or spread throughout the body through the circulatory or lymphatic systems. As a result, malignant tumors are frequently referred to as cancerous, and they are usually more resistant to localized therapy due to their spreading potential. The complex microenvironment of malignant cells, which includes cooperative support via several mechanisms, plays a crucial role in cell survival, progression, and metastatic potential.18−20
Despite considerable progress in cancer-related research, many cancer types can still not be successfully cured. One of the major reasons is the inability to diagnose the oncogenic alterations inside the body during the early stages of cancer development. The liquid biopsy, which relies primarily on screening mutated proteins, DNA, RNA, as well as other elevated markers in patient blood samples, has emerged as a promising strategy in recent years.21−23 More classic tissue biopsies and clinically validated imaging tools are also used widely to detect cancerous diseases at early stages.24−26 However, additional advances are needed. A further limitation to successful patient outcomes is the inability of current therapeutic regimens (e.g., chemotherapy, PDT, PTT, SDT, and immunotherapy) to identify and target malignant cells directly. This lack of specificity represents a major therapeutic constraint reflected in a failure to deliver therapeutic regimens locally to cancerous tissues. Over the past few decades, several novel approaches have been developed, raising hopes for future drug delivery programs. Cancer-targeted therapeutics and their formulations in particular have shown promise in mitigating the drug-mediated toxicities to nearby tissues and organs (Figure 2). This could be helpful in preventing collateral damage, including the one that causes stress and organ failure. At present, these strategies for the most part have only been subject to preclinical testing. There is thus a need for critical clinical studies that might allow a robust assessment of their merit.
Figure 2.
Schematic diagram showing targeted theranostic agents for cancer treatment. (A) Model showing a generalized targeted theranostic agent (chemotherapy/PDT/SDT/PTT/immunotherapy) conjugated with receptor substrate specific to a cancer cell receptor. (B) Targeted cancer therapeutic approach to achieve maximum therapeutic outcomes with reduced side-effects.
2.1. Theranostic Fluorescence Probes in Chemotherapy
The concept of chemotherapy, i.e., destroying cancerous cells through cytotoxic drugs and agents, came into the picture after the first report published in 1947 that mustard gas could harm lymphatic tissues. Later, the results were confirmed in animal models (mice) with nitrogen mustards being shown effective in inhibiting lymphoma tissue.27,28 Chemotherapeutic drugs usually work by mitigating cancer cell growth and ameliorating tumor-related stress. Compared to normal cells, cancer cells are characterized by rapid growth and enhanced proliferation rates. Hence, these drugs typically have a greater effect on cancer cells than normal cells. A plethora of anticancer therapeutic agents have been developed over the years and, not surprisingly, they operate by diverse mechanisms of action. While some agents perturb cellular metabolism, others target crucial cellular enzymes. A majority of reported agents interfere with vital cellular processes, such as DNA damage repair and DNA replication, immune response, apoptosis regulation, etc. (Figure 3).29,30 These modes of action can lead to high activity but also a lack of specificity for tumors over normal tissues. This lack of specificity poses a significant therapeutic constraint in that it can lead to off-target toxicities. One approach to overcoming this limitation involves modifying the structure of the agent in question to create so-called theranostic probes that target selectively cancer cells or the TME and which then release an active payload. In the limit, this strategy, which relies on an appropriate choice of masking/demasking steps can be used to deliver intrinsically nonselective chemotoxins (parent drugs) to cancer cells selectively.31,32 Similar approaches have been used to create activatable theranostic fluorescent probes. In this section, we review various classes of fluorescent theranostic probes organized according to their mode of activation.
Figure 3.

Different classes of chemotherapeutic agents in clinical practices. Go - Resting phase; G1 - growth; S - DNA synthesis and replication; G2 - growth and preparation for mitosis; M - mitosis.
There is a distinction between the microenvironment of normal tissues and that of malignant tumors. Certain specific physiological indicators, such as acidic pH, enhanced reactive oxygen species (ROS), greater intracellular glutathione (GSH) levels, enzyme overexpression, and a reductive or hypoxic microenvironment, distinguish malignant tissues from nearby normal tissues and organs (Figure 4).
Figure 4.
Schematic diagram showing different microenvironments of normal and tumor tissues [pHi = intracellular pH; pHe = extracellular pH, ROS = reactive oxygen species].
We have classified theranostic probes used in various treatment modalities (chemotherapy, PDT, PTT, SDT, immune therapy, etc.) into several subsections in this part to differentiate their mode of activation in the tumor microenvironment.
2.1.1. pH-Responsive Fluorescent Probes
The pH values for normal tissues are typically around 7.4, while those of a tumor microenvironment are typically 0.5–1.0 units lower.33,34 This is due to the vigorous metabolism within tumor cells that promotes rapid glucose uptake and lactic acid production under the hypoxic conditions that characterize most solid tumors. The acidic pH of cancerous lesions plays a significant role in tumor development, recurrence, metastatic spread, and the development of drug resistance. However, not all tumors show a particularly acidic TME. For instance, pHe values of 6.94 ± 0.08 have been reported for soft tissue sarcoma and adenocarcinoma, whereas values of 7.20 ± 0.07 have been noted in malignant melanoma and squamous cell carcinomas.35 Nevertheless, an aberrant pH is regarded as a universal marker of solid tumors, irrespective of tumor type and stage. As a result, pH is often exploited as an endogenous stimulus for cancer-targeting drug delivery systems (DDS). To date, pH-responsive DDS has mainly been reported in MCF-7,36,37 HeLa,38 and BxPC-339 cancer models.
An overarching goal of DDS development is to enhance the therapeutic efficacy of the parent drugs or cytotoxic agents upon which they are based and to minimize deleterious side effects.40,41 To achieve these objectives the system in question must be stable enough to allow delivery and circulation but release the drug efficiently at the target site. In pH-triggered DDS, an increased local proton concentration at the lysosomal, endosomal, cellular, or tumor tissue levels serves as the stimulus to promote drug release. In recent decades, a number of pH-responsive theranostic systems have been reported that take advantage of this strategy. For the most part, the systems in question involve drugs that are attached to acid-labile chemical linkers such as acetals, hydrazines, oximes, and imines. These chemical linkers are attractive because they are relatively stable at physiological pH (7.4), but undergo rapid hydrolysis in acidic endosomes, thus providing for acidic tumor site specificity.25 Unfortunately, this strategy is only applicable to drugs with free aldehyde or ketone groups that can support the formation of this type of labile functionality.
2.1.1.1. Hydrazone/Oxime/Imine-Based Theranostic Probes
The acid-catalyzed hydrolysis of hydrazone linkers has been widely explored in developing pH-sensitive DDS, particularly in the development of small molecule-based theranostics, as well as nanocarriers,42 and polymer-based systems,43 for delivering cytotoxins to tumor sites. Detailed NMR spectral studies conducted in the deuterated buffer by Kalia et al. revealed that in the hydrolysis of hydrazone- and oxime-linked drug molecules, nucleophilic attack of a water molecule on the imine carbon is the rate-determining step.44 This attack is followed by protonation of the imine nitrogen and subsequent hydrolysis to furnish the free drug molecules (Figure 5). Electron-withdrawing substituents reduce the propensity of the nitrogen atom to undergo protonation resulting in a reduced overall hydrolysis rate. Based upon experiments carried out at pH 7.4, the stability order of various linkers was found to be trialkylhydrazonium ≫ oxime ≫ acyl hydrazone > primary-hydrazone ≫ sec-hydrazone > imine. As quaternary ammonium hydrazones are quite stable at pH 5, they are not preferred for acid-sensitive drug delivery applications. On the other hand, acyl hydrazone linkers are inherently attractive due to their high stability at neutral pH and hydrolytic lability in acidic media (pH 5).
Figure 5.
(A) Mode of activation of hydrazone-based pH-responsive theranostic agents. (B) Chemical structures of acid-sensitive theranostic agents (1–3). (C) (i) Cytotoxicity studies performed on U87 cells upon treatment with Apo-M, theranostic agent 2, and free doxorubicin (Dox) at different Dox equivalent concentrations. (ii–v) Confocal images of U87 cells incubated with theranostic agent 2 at a Dox equivalent concentration of 10 μg mL–1 for 42 h. (ii) Hoechst blue, (iii) Dox red, (iv) 5(6)-carboxylfluorescein (FAM) green, and (v) merged (scale bar = 20 μm). Reproduced with permission from ref (53). Copyright 2015 Wiley Intersciences. (D i) Normalized cell viability of theranostic agent 3 (10 μM, black; 5 μM, gray) and amide analogue (white) in nonactivated media, Lipopolysaccharide (LPS)-induced (100 ng mL–1, 18 h) and Interleukin (IL)-4-treated macrophages. (D ii, iii) Fluorescence images of zebrafish treated with 3 (3 μM) without (ii) LPS and (iii) with LPS treatment (100 ng mL–1). Scale bar = 50 μm. (D iv) In vivo macrophage quantification in the regenerated tissue area of zebrafish treated with DMSO, theranostic agent 3 with and without LPS. Errors are ± SD (n 10). n.s. = not significant; * p < 0.05. Reproduced with permission from ref (54). Copyright 2017 American Chemical Society.
The anticancer drug doxorubicin (Dox), an anthracycline drug, used alone or in combination with other agents, constitutes the standard of care for several malignancies, including breast, lung, ovarian, and gastric cancers, multiple myeloma, as well as pediatric cancers, including Hodgkin’s and non-Hodgkin’s lymphomas.45 However, Dox use is often limited by side effects, such as fatigue, hepatotoxicity, and cardiotoxicity.45−47 Several strategies have been proposed to overcome these side-effects. Within this context, considerable effort has been devoted to improving the delivery of Dox to tumor sites. For instance, Dox has been linked through an acid-sensitive hydrazone linker to a cancer-guiding integrin, GRDS-oligopeptide, as well as a coumarin moiety to give the theranostic agent 1 (Figure 5).48 Dox in its free state is intrinsically fluorescent with an emission band at 595 nm (excitation at 470 nm). Hence, it has been widely used in cancer biology-related imaging applications.49−52 Before the release of Dox, the fluorescence of the Dox moiety in 1 is quenched due to a contact-mediated quenching process involving the Dox and coumarin moieties. It was found that about 94% of the Dox originally in 1 was released at pH 5 over 11 h. In contrast, under neutral pH (7.4) only 41% drug release was observed. In integrin-positive human glioblastoma U87cells, a dose-dependent toxicity was observed (IC50 = 0.19 μg mL–1). Further, the fluorescence nature of both free Dox (red) and coumarin (blue) allows prodrug activation and drug localization to be monitored in real-time.
Incorporating cell apoptosis markers in the scaffold design can allow the activation and localization of a cytotoxin to be assessed in a non-invasive manner and the therapeutic dose to be fine-tuned. This strategy is embodied in the dual Forster resonance energy transfer (FRET) agent 2 (Figure 5).53 Here, the anticancer drug (Dox) is linked through an acid-labile hydrazone linker to a potent fluorescence quencher, (4-(dimethylamino azo)benzene-4-carboxylic acid (Dabcyl), and a peptide sequence (Asp-Glu-Val-Asp, DEVD) that is responsive to the apoptosis marker, caspase-3. To achieve the real-time monitoring of drug activation at the cellular level, a 5(6)-carboxylfluorescein (FAM) unit was incorporated into the design. The construct was further tagged with an integrin-specific sequence (Arg-Gly-Asp, RGD) to achieve cancer-selective targeting and uptake. Preliminary solution studies confirmed that agent 2 released 90% of the Dox in active form in acidic environments (pH = 5.0) as compared to a much lower degree of Dox release at pH 7.4 (19%). In U87 cancer cells (integrin positive), agent 2 provided for time-dependent fluorescence enhancement ascribed to DEVD peptide cleavage to furnish a green color fluorescence (corresponding to the free FAM group from its quenched state). The corresponding Dox release was associated with noticeable therapeutic effects (IC50 = 4.3 × 10–6 M) and concomitant caspase-3 activation.
Considerable effort has been devoted to the preparation of constructs that are simpler than 2, and permit both cancer-specific activation and real-time monitoring of drug release at the cellular level. For example, theranostic agent 3 was developed by connecting Dox to a fluorophore, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY), through a hydrazone bond (Figure 5).54 As prepared, construct 3 displayed relatively weak fluorescence at physiological pH. In phagosomes (pH 6.5–4.5), the hydrazone linker is readily cleaved, resulting in an observable fluorescence emission due to the resulting free BODIPY. It was thus tested as a target-based approach for treating immune-related diseases. RAW264.7 macrophages were used as a model system and treated with lipopolysaccharide (LPS) to generate proinflammatory M1 macrophages. This resulted in phagosome acidification. Dose-dependent toxicity was observed in LPS-mediated proinflammatory M1 macrophages treated with 3, along with a fluorescence “Turn-On” response. In contrast, no such benefits were observed in IL-4-mediated anti-inflammatory quiescent or M2 macrophages. A similar pattern was observed in LPS-treated zebra fish, where apoptotic macrophages produced a red fluorescence, corresponding to Dox and a nearby region produced a green fluorescence, corresponding to BODIPY. The theranostic agent 3 was also tested in an in vivo regeneration model (zebra fish) to monitor phagocytic M1 macrophage activity. Treatment with 3 in combination with LPS resulted in improved M1 macrophage polarization, a finding consistent with enhanced tissue regeneration.
Theranostic agent 4 was developed and tested for its ability to improve cancer cell-specific uptake of Dox relative to normal cells (Figure 6).55 Here, Kim et al. utilized the intrinsic Dox fluorescence to monitor prodrug activation and intracellular localization. Specifically, a nitrobenzene moiety was used as a photoinduced electron transfer (PET)-mediated fluorescence quencher and linked to Dox through a hydrazone bond. For cancer-selective targeting, a biotin unit was incorporated into the design. The agent remained largely intact under physiological conditions up to 7 h. However, under acidic conditions, the hydrazone bond underwent hydrolysis, leading to a fluorescence “Turn-On” response (resulting from free Dox). When tested in vitro, agent 4 demonstrated a good selectivity in biotin- positive cancer cells (HepG2) as compared to normal WI38 (biotin-negative) cells.
Figure 6.
(A) Chemical structures of acid-sensitive theranostic agents (4–6). (B) Confocal images of HepG2 and WI-38 cells after treatment with Dox and theranostic agent 4 (5 μM), 1 h, Exc. wavelength 488 nm). Reproduced with permission from ref (55). Copyright 2015 Royal Society of Chemistry. (C) Concentration-dependent cytotoxicity of theranostic agent 5 in A549 (black), CT26 (red) cancer cells and normal fibroblast NIH3T3 (blue) cells. (D) T1-weighted MR images of an A549 cell pellet recorded following treatment with different concentrations of theranostic agent 5. Reproduced with permission from ref (56). Copyright 2016 Royal Society of Chemistry. (E) CLSM images showing cellular uptake and intracellular Dox localization of 6 in HepG2 and A549 cancer cells, as well as HEK293 normal cells at 6 h (1st row) and 2 h (2nd row) post-treatment. Scale bar = 20 μm. Reproduced with permission from ref (57). Copyright 2015 Royal Society of Chemistry.
The same group further developed a multimodal strategy that was designed to assess cellular uptake and activation by means of two different imaging modalities (fluorescence imaging and magnetic resonance imaging (MRI)).56 This strategy, embodied in compound 5 (Figure 6), relies on a hydrazone linkage between two Dox (fluorescent) units and a single paramagnetic motexafin gadolinium (MGd) core, the latter being a specific water-soluble texaphyrin. The diminished fluorescence of intact agent 5 was recovered under acidic conditions. The same phenomenon was seen in cancer cells (A549, CT26). By contrast, no noticeable toxicity was observed in normal fibroblast NIH3T3 cells up to 100 μM concentrations. Construct 5 displayed T1-contrast relaxivities of about 20.1 ± 0.4 mM–1 s–1 (at 60 MHz) and 6.1 ± 0.2 mM–1 s–1 (at 200 MHz) in phosphate buffered saline (PBS) that were greater than those seen for a standard Gd3+ contrast agent. Moreover, at low concentrations (4 μM), cell pellet phantoms treated with 5 reached saturation at a relatively high T1 relaxivity value (cf. Figure 6). Likewise, a tumor-targeting theranostic 6 was developed wherein a targeting sequence AP2H (IHGHHIISVG) was linked through a hydrazone bond to Dox.57 This system, designed to undergo cleavage under mildly acidic conditions, was found to display dose-dependent toxicity in a lysosomal protein transmembrane 4 beta (LAPTM4B) positive lung cancer cell line A549 (IC50 = 1.14 μM) and a liver cancer cell line HepG2 (IC50 = 4.0 μM). In these cell lines, fluorescence studies confirmed that 6 was activated in endosomes and lysosomes with subsequent translocation to the nucleus (Figure 6). In contrast, negligible activation was seen in normal HEK293 cells.
While hydrazone-based linkers have been widely used in small molecule-based systems, some effort has been devoted to the use of oxime-based linkers. However, reports on their use are limited and most of these reports have concerned polymeric systems. In the current review article, we restrict our focus to discrete molecule-based theranostic systems. In a report by Jin et al., a terephthaladehyde moiety was used to form an oxime linker within a PEG-Dox micelle system. Slow hydrolysis was seen at pH 5.0 (t1/2 = 15 h).58 In contrast, a higher drug release rate was observed at neutral pH (20% at 12 h). These results underscore the low stability margin of oxime linkages as compared to acyl hydrazone-based linkers. A dextran-Dox conjugate (7) was developed by Xu et al. as a potential theranostic agent designed to permit acid-sensitive Dox delivery into human hepatoma HepG2 cancer cells (Figure 7). Fluorescence-based drug release experiments revealed that 7 at neutral pH (7.4) showed approximately 25.9% of active Dox release over a period of 72 h. In contrast, under acidic conditions, increased oxime hydrolysis and enhanced Dox release were seen (i.e., pH 6.8 = 40.4%, pH 6.0 = 64.7%, and pH 5.0 = 87%, 72 h).59 A reduced analogue of 7 that was prepared by reduction of the oxime linker was not expected to exhibit acid lability. Endocytosis-mediated cellular uptake of theranostic 7 and further hydrolysis in acidic environments were demonstrated using confocal laser scanning microscopy and HepG2 cancer cells. A cell viability assay conducted on HepG2 cells revealed that treatment with 7 (72 h incubation) led to toxicity (IC50 = 0.73 μg mL–1) comparable to free Dox (IC50 = 0.62 μ g mL–1). Furthermore, tumor inhibition studies conducted on the H22-xenograft murine model revealed that compared to other controls (Dox 45.4%, reduced analog of 7 19.2%), treatment with 7 (intravenous administration) resulted in significant tumor growth inhibition (71.0%) and improved survival rates with decreased side-effects.
Figure 7.
(A) Chemical structure of acid-sensitive theranostic agents (7–11). (B) Cell viability studies of theranostic 8 in KB Folate receptor (FR+) cells and FR-knockdown KB cells. Reproduced with permission from ref (60). Copyright 2014 American Chemical Society. (C) IC50 values of Dox and theranostic 11 in ES-2 cells at different pH. Reproduced with permission from ref (62). Copyright 2017 Royal Society of Chemistry.
The above studies highlight the potential utility of acid-sensitive chemical linkers. However, they underscore a seemingly obvious truism, namely the importance of having a good understanding of the underlying chemistry before choosing a specific type of chemical linker for a particular DDS application.
2.1.1.2. Other Acid Responsive Theranostic Probes
Yang et al. developed the N-ethoxybenzylimidazole (NEBI) moiety as a tunable acid-sensitive chemical linker and used it to achieve the folate-positive cancer cell-selective delivery of the anticancer drug indenoisoquinoline.60 Here, acid-based hydrolysis involves the “aminol” ether functionality. Construct 8 relies on using an imidazole ring nitrogen to form an aminol ether group (Figure 7). In acidic environments, protonation of the imidazole ring facilitated the spontaneous release of the parent drug molecule. The hydrolysis and drug release rate can be tuned by the choice of groups on the phenyl ring. While the addition of an electron-withdrawing group (nitro) resulted in a slower hydrolysis rate (t1/2 = 6900 h, at pH 5.5), substitution with an electron-donating group (methoxy) facilitated a rapid drug release (t1/2 = 0.6 h, at pH 5.5). Further, no matter what type of substituent was employed, the half-life of the prodrug at pH 7.4 was 10-fold higher than at pH 5.5. In vitro studies involving folate-positive KB cells revealed the targeted delivery and release of active drug with an IC50 of 60 μM. In contrast, knockdown of the folate receptor resulted in a decrease in efficacy (IC50 = 655 μM). Further modification of prodrugs with PEG linkers resulted in diminished activity in the folate-positive KB cells (IC50 = 250 μM). Due to the intrinsic fluorescence of the parent drug, prodrug 8 offered the possibility of monitoring its cellular uptake and activation through fluorescence microscopy. This study highlights the potential utility of the NEBI chemical linker to deliver drugs via processes that rely on folate receptor-mediated endocytosis.
Maleic acid-based derivatives have also been used as acid-sensitive linkers for drug delivery applications. In this case, the drug release mechanism is based upon the intramolecular cyclization of the malonyl amine at a pH less than the pKa of the free carboxylic acid moiety. Early research focused on the use of the cis-aconitic anhydride moiety, which allowed the formation of the final conjugate. However, the resulting cis-aconitric acid-based conjugates often suffer from decarboxylation and trans-isomerization.61 An example of this approach is embodied in prodrug 9 that is based on Dox (Figure 7).62 At a pH below the pKa of the carboxylic acid, intermediate 10 is the dominant species. Prodrug 9 could be linked to GSH, resulting in theranostic agent 11. Upon a decrease in pH, an excellent Dox release response was observed with about 70% Dox release being observed at pH 6.0. In contrast, only a 10% Dox release was seen at pH 7.0 over the course of 5 h. Dox, bearing a free amino group (pKa = 8.2), is sensitive to acidic tumor environments in terms of both its uptake and toxicity. In ES-2 (ovarian cancer cells), Dox possesses higher toxicity (IC50 = 1.6 ± 0.1 μM) at extracellular pH 6.7 than at neutral pH 7.4 (IC50 = 0.7 ± 0.1 μM). With maleamic acid-based chemical linkers, this preference is perturbed. Conjugate 10 showed a comparable potency at pH 6.7 (IC50 = 2.1 ± 0.3 μM) and pH 7.4 (IC50 = 3.2 ± 0.4 μM). It is interesting to note that although conjugate 11 was less potent than the parent drug, it mitigates the undesired pH-dependent toxicity profile of Dox. Moreover, the intrinsic fluorescence of Dox offered the possibility of monitoring drug activation and cellular localization, thereby allowing 11 to serve as an acid-sensitive theranostic agent.
In summary, acid-labile chemical linkers continue to be used to develop; small molecule-based drug delivery systems. In acidic environments, ideally tumor microenvironments, the pH-sensitive linker undergoes acid-catalyzed drug release. Depending on the target site, a range of acid-sensitive linkers can be used. For example, for targeted delivery of cytotoxins to solid tumors (pH = 6.0–7.0), maleamic acid-based chemical linkers appear to be an excellent choice as they are sensitive to subtle pH value changes. However, to target lysosomes and endosomes (pH = 4.5–6.0), acyl hydrazone-based linkers appear more suited. In any event, further research devoted to developing chemical linkers appears warranted and could translate into improvements in cancer-targeted drug delivery.
2.1.2. GSH Responsive Fluorescent Probes
A variety of redox processes take place within different intra- and extracellular environments and in tissues. Some of these most common redox couples include nicotinamide adenine dinucleotide phosphate (NADP+/NADPH), oxygen/superoxide (O2/O2•–), thioredoxin (TrxSS/Trx(SH)2), and glutathione (l-γ-glutamyl-l-cysteinyl-glycine) (GSH/GSSG). The glutathione redox couple has garnered considerable attention in the context of theranostic and drug delivery applications owing to the relatively high GSH concentrations inside cells (1–10 mM) and its role in maintaining cellular integrity, as well as mediating cellular differentiation, metabolism, and apoptosis. GSH also plays a key role as an antioxidant by preventing ROS-mediated damage.63−65 In contrast, in the extracellular matrix, blood, and even on cell surfaces, GSH is present in much lower concentrations (2–20 μM). This is due to higher protein concentrations in these regions that are capable of stabilizing disulfides. In contrast, the intracellular microenvironment is kept reductive through the action of nicotinamide adenine dinucleotide phosphate (NADPH), as well as GSH reductase. Thus, within cells, GSH is largely maintained in its reduced form.66,67 It is interesting to note that a significant GSH concentration gradient exists within the intracellular compartments.68 Of significance is that the GSH concentrations are about 4-fold higher in tumor tissues compared to healthy tissues, a finding ascribed to the rapid proliferation of cancerous cells.69−71 On the one hand, these elevated levels of GSH can reduce the efficacy of administered therapeutics in a number of cancers, including ovary, breast, lung, liver, bone marrow, and colon cancer.72 However, the relatively high GSH levels in cancerous lesions can be exploited to create redox-responsive theranostic agents that release active drugs/fluorophores within tumor cells. Since the GSH concentration in the extracellular environment is typically lower, the prodrugs can be tuned in such a way as to possess adequate stability in the extracellular matrix before releasing the cytotoxins once internalized within the cancerous cells. Among the most widely used chemical linkers to achieve this purpose is a disulfide (S–S) bridge, which undergoes thiol–disulfide exchange in the presence of free bithiols.73,74 In fact, under appropriate conditions of design, GSH can serve as a potent bioactivator capable of triggering drug release from S–S containing prodrugs.
The disulfide strategy has been extensively explored in developing prodrugs using a variety of carriers, including antibodies, peptides, and small molecules. In the context of small molecule-based theranostics, several drug candidates and fluorophores, including Dox, camptothecin,75,76 paclitaxel,77,78 gemcitabine,79−81 napthalimide,82 hemicyanine,83 dicyano-methylene-4H-pyran,84−86 and fluorescein,87 have been explored. To achieve the essential disulfide linkage required for GSH-mediated release (and producing a free thiol group), the drug molecules and fluorophores are typically modified before disulfide linker construction. To date three types of strategies have been used predominantly: (i) chemotherapeutic drugs are linked to a fluorophore through a cleavable chemical linker, (ii) chemotherapeutic drugs with intrinsic fluorescence are connected to a targeting ligand through chemical linkers, and (iii) chemotherapeutic drugs are connected to fluorophores and a targeting ligand through a multicomponent strategy (Figure 8).
Figure 8.
(A) Schematic illustration of GSH-responsive activatable molecular theranostic probes. (B) Disulfide cleavage and self-immolative drug release mechanisms. (C) Chemical structures of selected GSH-responsive theranostic probes (12–16).
Under category (i), several strategies have been pursued. For instance, in the case of drugs of fluorophores bearing free hydroxyl and amine groups carbonate or carbamate esters, respectively, may be used to make the connection through a disulfide-containing linker. This approach is illustrated by theranostic agent 12 (Figure 8), which was developed to monitor the GSH-responsive activation of camptothecin (CPT) in an H22 tumor-based mice model.83 Here, the anticancer drug CPT was conjugated to a hemicyanine-based fluorophore through an S–S linkage. Theranostic 12 was found to be weakly fluorescence, presumably because the free amino group of the fluorophore is blocked as a result of linker formation. Upon exposure to GSH, the disulfide bond undergoes reductive cleavage with concomitant fluorescence enhancement (at 702 nm), owing to the formation of the free hemicyanine fluorophore and the simultaneous release of free CPT. Cell-based studies confirmed that theranostic 12 exhibited significant cytotoxicity in the HepG2 cancer cell line, as compared to the HL-7702 normal liver cell line (Figure 9). Further, an intense fluorescence signal (at 702 nm) corresponding to the free hemicyanine fluorophore was observed in the HepG2 cells as compared to a normal liver cell line. This difference was ascribed to the elevated GSH levels in the HepG2 cancer cells. The enhanced emission was also taken as evidence that theranostic 12 provides for preferential tumor localization, as confirmed through an IVIS luminal in vivo imaging (intravenous administration). A similar strategy has been used by numerous groups to monitor the GSH-mediated activation of theranostic agents in various tumor models.79,83−86,88−95
Figure 9.
(A) Cell viability of theranostic probe 12 and parent drug CPT at different concentrations as tested in HepG2 cancer and HL-7702 normal cells. (B) In vivo images of H22-tumor bearing mice after administrating 12 (i.v.) at different time intervals. Reproduced with permission from ref (83). Copyright 2016 American Chemical Society. (C) Drug release profile of theranostic probe 13 after incubation with 0.2 mM and 10 mM GSH at pH 7.0 (mean ± SD, n = 3). Reproduced with permission from ref (95). Copyright 2019 Wiley Intersciences. (D) Colocalization studies of theranostic probe 14 (10 μM) with (i) ER Tracker Red, (ii) Lyso Tracker Red DND-99, and (iii) Mito Tracker Red FM. Excitation 458 nm, 543 nm band-path (505–530 nm), and long-path (>585 nm) emission filters were used. Reproduced with permission from ref (82). Copyright 2014 American Chemical Society. (E) Confocal images of theranostic probes 16 (1.0 μM) treated KB and A549 cells at different time points (excitation = 633 nm, emission = 650–750 nm, scale bar = 20 μm). (F) Colocalization studies showing confocal images of KB cells treated with ER Tracker (excitation = 514, emission = 530–570 nm), theranostic probe 16, and merged image. Scale bar = 20 μm. Reproduced with permission from ref (80). Copyright 2013 American Chemical Society.
Per strategy (ii), anticancer drugs with an intrinsic fluorescence are typically linked to cancer-targeting ligands, through a cleavable disulfide linker. As true for strategy (i), this approach has been used for drug molecules bearing hydroxyl or amine functionalities. An example is the GSH-responsive folate-doxorubicin theranostic 13.95 This system incorporates an α,α-dimethyl-substituted p-thiophenyl urethane-based disulfide, as well as a carbamate ester. The presence of two methyl groups improves the stability of aromatic thiols 2-fold as compared to typical aliphatic disulfide linkers. Further, unlike an aliphatic thiol (pKa 8–9), the low pKa value of aromatic thiols (≈6) was expected to improve the release kinetics. Theranostic probe 13 also incorporates a folate targeting unit. Folate receptors (FR) are known to be upregulated in various cancer types. It was thus expected that conjugate 13 (Figure 8) would be internalized through recycling endosomal pathways.96 Preliminary solution studies confirmed that theranostic 13 exhibits good stability in 0.2 mM GSH in a phosphate-buffered solution, a condition mimicking the redox environment associated with blood circulation. By contrast, upon incubation with 10 mM GSH (mimicking the cancer cell cytosol), a smooth drug release pattern was observed characterized by a t1/2 of about 1.3 h (Figure 9). These results led to the suggestion that 13 might be amenable to clinical translation, where it would be expected to be eliminated from the circulatory system with a t1/2 of about 30 min.97 In addition to 13, several other theranostic agents that embody strategy (ii) have been prepared and tested in various cancer models both in vitro and in vivo.98,99
Strategy (iii) offers the prospect of improving the specificity of the therapeutic payload in a theranostic with a commensurate reduction in systemic toxicity. This is because it involves tagging the drug components with cancer-targeting units. In the case of nonfluorescent drug candidates, this strategy also allows for the visualization of the drug delivery system through additional fluorescence labeling. The potential of this approach was shown by Kim et al., who developed an RGD peptide-decorated naphthalimide pro-camptothecin (pro-CPT) theranostic agent 14 (Figure 8) for image-guided chemotherapy.82 The multifunctional agent 14 consists of an RGD cyclic peptide for cancer targeting, a naphthalimide component as a fluorophore, and the topoisomerase I inhibitor CPT as the anticancer drug, linked through a GSH-responsive disulfide linker. Theranostic 14 was designed to be endocytosed into U87 cancer cells as the result of interactions between the RGD moiety and the avβ3 integrin receptor, which is overexpressed in these cancer cells. The relatively high GSH levels in cancerous cells were then expected to trigger the cleavage of the disulfide bond, resulting in the release of the active cytotoxin CPT and the naphthalimide fluorophore. Incubating U87 cancer cells with 14 gave rise to an intense red-shifted emission output at 535 nm in the endoplasmic reticulum (Figure 9). Meanwhile, the CPT was found to diffuse to the cell nucleus and induce an antiproliferative effect. Further research was conducted to validate the universality of this strategy for otherwise nonfluorescent drug candidates. For example, theranostic agent 15 (Figure 8) was developed to monitor the GSH-selective delivery of gemcitabine, another anticancer drug.81 System 15 contains biotin as the cancer-targeting moiety, coumarin as the fluorophore, and gemcitabine as the model anticancer drug. Tracking the coumarin fluorescence revealed that the theranostic is trapped in the cell lysosomes and that drug activation occurs in the cytoplasm. Thanks to coumarin’s intrinsic two-photon absorption,100 the therapeutic action of 15 could be assessed at the subcellular level.
Other theranostic agents were prepared and tested in the search for improved therapeutic efficacies.101 A representative example is theranostic 16 (Figure 8). This system is a Cy7-gemcitabine drug conjugate linked through a disulfide bond and incorporating cancer-targeting folate at the cyclohexene ring of the fluorophore.80 Cyanine dyes contain two nitrogen atoms linked to a π-conjugated polymethine chain.102 These fluorophores display high molar absorptivity, narrow absorption, and emission bands with tunable spectroscopic profiles in the UV/vis and NIR regions.103−105 Further, they generally display low systematic toxicity and excellent biocompatibility. Cyanines (>650 nm) have been widely used for in vivo applications due to their minimal auto fluorescence, deep tissue penetration, reduced light scattering, and minimal phototoxicity.106−108 Upon incubation with theranostic 16 folate-positive KB cancer cells displayed a prominent fluorescence signal within 20 min. In contrast, no fluorescence signal was observed in folate-negative A549 cancer cells (Figure 9). Based on these results, it is thought that theranostic 16 is preferentially taken up by folate-positive KB cells, through a folate-mediated endocytosis mechanism. Fluorescence-based colocalization studies revealed that the drug is activated in the endoplasmic reticulum and induces apoptosis.
Extensions of the above strategies have been used for the targeted delivery of peptide-based anticancer agents.109−112 There are several peptide-based drug candidates possessing good anticancer activity that are devoid of appreciable side effects; however, their translational into the clinic has been limited due to their low metabolic stability, poor membrane permeability, and low blood circulation residence times.113,114 Peptide-based theranostic agents could overcome some or all of these limitations. With such considerations in mind, Kim et al. developed the theranostic system 17 (Figure 10). This system was designed to provide for the cancer-targeted delivery of the Holliday junction (HJ) inhibitor peptide 2 (KWWCRW), which possesses antimicrobial and anticancer activity. In 17, the cysteine residue on the HJ inhibitor is used to form disulfide linkages with naphthalimide, which was further connected to a biotin moiety.115 Theranostic 17 was selectively taken up in biotin-positive HepG2 cells and displayed a red-shifted fluorescence enhancement at 540 nm. Compared with the HJ inhibitor alone, theranostic 17 showed improved concentration-dependent toxicity in HepG2 cells with the drug being localized in the endoplasmic reticulum. Collectively, these results support the contention that theranostics derived from peptide-based drug candidates may be able to improve the inherent therapeutic efficacy of the parent drug through improved delivery, better therapeutic targeting, and fluorescence-based tracking.
Figure 10.
(A) Chemical structures of GSH-responsive theranostic probes 17–22. (B) Colocalization images of theranostic probe 17 (10 μM) with ER tracker Red, Lyso Tracker Red, and Mito Tracker Red FM in HepG2 cancer cells. Reproduced with permission from ref (115). Copyright 2014 Royal Society of Chemistry. (C) Tumor growth inhibition in HepG2 xenografted mice upon treatment with control, CPT, and theranostic probes 18 and 19. ##p < 0.01 compared to CPT treated group. (D) Tumor weights after treatment per (C). **p < 0.01 compared to the control group and ##p < 0.01 compared to CPT treated group. Reproduced with permission from ref (120). Copyright 2021 American Chemical Society.
In addition to disulfide linkers, other chemical linkers have also been explored for GSH-responsive drug delivery applications, with selenium receiving particular attention. Selenium and sulfur belong to same elemental group and possess similar chemical properties.116 The Se–Se bond exhibits a low redox potential at 172 kJ mol–1, as compared to the C–Se bond (244 kJ mol–1) or S–S bond (268 kJ mol–1).117−119 Therefore, Se–Se linkers typically display higher GSH sensitivity than analogous S–S linked systems. Fang et al. developed two seleno-based CPT theranostic agents, 18 and 19 (Figure 10).120 Incubation with GSH activates both theranostic agents and produces an enhanced fluorescence emission at 430 nm (excitation = 365 nm), as validated by HPLC analyses. In the presence of 1 mM GSH, both theranostic agents demonstrated nearly full drug release in 5 min in a Tris-EDTA buffer. The released selenol intermediates were thought to improve the potency of 18 and 19 through the depletion of intracellular of GSH while providing for increased ROS generation. In a HepG2 xenograft mouse model, treatment with 18 and 19 reduced the tumor weight by around 75%; in contrast, CPT therapy reduced the tumor weight by 53%. These promising results notwithstanding, diselenide-based linkers have been used less frequently than disulfide linkers. This may reflect synthetic barriers, as well as the fact that diselenide linkers display dual sensitivity toward both GSH and ROS, which can cause unexpected activation and unwanted drug release.121
Other biothiols, including cysteine (Cys) and homocysteine (Hcy) also play key roles in maintaining the redox balance of cellular microenvironments.121 Cys deficiency can result in a variety of diseases, including cardiovascular diseases, leukopenia, liver damage, neurological disorders, cancer, and hematopoietic malfunction.122,123 Many GSH-sensitive linkers show sensitivity toward Cys, which can result in undesired drug activation in healthy tissues where both Cys and GSH coexist at certain concentrations. On the other hand, systems that respond to only Cys could allow for selectivity. Recently, 2,4-dinitrobenzenesulfonyl (DBS) has emerged as a Cys-sensitive linker for drug delivery applications. It has seen increased use for use with drug candidates that possess free hydroxyl (OH) or amine (NH2) groups. In separate studies from the groups of Jo and Johansson et al, theranostic agents 20 (based on SN-38, an active metabolite of irinotecan)124 and 21 (using DOX)125 were prepared by connecting the DBS group directly to the respective parent drugs (Figure 10). Functionalization of DBS results in fluorescence quenching in both theranostic agents.
In reductive environments, DBS undergoes aromatic nucleophilic substitution followed by sulfur dioxide (SO2) release, to allow active drug release with concomitant fluorescence enhancement. Introducing self-immolative chemical linkers into DBS linkers can further increase their capacity to support a range of drug candidates and fluorophores. This benefit was demonstrated by Wu et al., who developed theranostic agent 22b. This system contains a thiol-responsive DBS trigger, CPT, and the NIR fluorophore dicyanomethylene-4H-chromene (DCM), connected through a 2,6-bis(hydroxymethyl)-4-cresol linker.126 As prepared, theranostic 22b is nonemissive due to the presence of the DBS and carbamate linkages. In the presence of thiols, a reaction cascade takes place to release both CPT and produce an NIR fluorescence arising from the free DCM. Theranostic agent 22b displayed a dose-dependent cytotoxicity in HeLa cancer cells (IC50 = 5.8 μM) and L929 cells (IC50 = 8.9 μM) that was enhanced compared to a control conjugate without a drug (22a). Intratumoral administration of a liposomal formulation of theranostic 22b demonstrated significant tumor growth inhibition.
2.1.3. Hydrogen Sulfide Responsive Theranostic Probes
Hydrogen sulfide (H2S) is a poisonous gas and is known for its flammability, rotten egg-like smell, and causticity.127 Endogenous H2S, along with carbon monoxide (CO) and nitric oxide (NO) is a primary gasotransmitter.128,129 It plays a vital role in the regulation of several critical physiological processes, including cell differentiation, proliferation, survival/death, and metabolism.130,131 Dysregulation of H2S has been linked to several diseases, including cancer, Alzheimer’s, and diabetes.132 H2S protects neurons from oxidative damage by elevating GSH levels, thus indirectly scavenging ROS.133 The importance of H2S has prompted the development of H2S-responsive theranostic agents as potential cancer treatments. H2S possesses reductive character in addition to being a recognized nucleophilic, particularly in its deprotonated forms. It is capable of selectively reducing azides to amines. The strong electron-donating nature of amines can be further exploited to facilitate fluorophore/drug release. Qian et al. developed the H2S-responsive theranostic agent 23 (Figure 11), containing amonafide, an anticancer drug of interest as a targeted therapy for glioblastoma cancer.134,135 The intrinsic fluorescence of amonafide is quenched due to a PET effect. Incubation with H2S results in the reduction of the azide to an amine, with subsequent self-immolative linker breakdown, thereby furnishing the active drug via a 1,6-elimination reaction. Fluorescence studies confirmed that the drug was localized in the lysosomes in U87MG cells and further translocated to the nucleus, where it induced cytotoxicity through a combination of DNA damage and mitochondrial dysfunction mechanisms. In a U87MG 3D spheroid model, this theranostic agent demonstrated a significant toxicity at a relatively low dose (30 μM, 2 days treatment) and was found to outperform cisplatin (40 μM, 2 days treatment) as inferred from the observed destruction of tumor spheroid integrity. A similar strategy was used to develop theranostic probe 24 (Figure 11) for H2S-mediated drug delivery of SN-38 in colon cancer cells.136
Figure 11.

(A) Chemical structures of hydrogen sulfide-responsive theranostic probes 23–25. (B) Changes in 3D U87MG tumor spheroids upon treatment with theranostic probe 23 (30 μM), cisplatin (40 μM), and control at different time points. (C) Confocal images of U87MG tumor spheroids after treatment with 23 (30 μM, 48 h) and staining with acridine orange (AO) (green) ethidium bromide (EB) (red). Scale bar = 100 μm. Reproduced with permission from ref (134). Copyright 2021 Royal Society of Chemistry.
Taking the nucleophilic character of H2S into consideration, Li et al. developed the theranostic agent 25 (Figure 11), which is composed of the anticancer drug SN-38 linked to an electron-withdrawing dinitrophenyl (DNP) ether group.137 As prepared, 25 is non-emissive since possible intramolecular charge transfer (ICT) is blocked. In the presence of H2S, the ether bond is cleaved with a concomitant fluorescence enhancement and active drug release. In vitro, studies confirmed that theranostic agent 25 was preferentially taken up and activated in H2S-elevated HCT116 and 4T1 cancer cells and displayed cytotoxicity. The SN-38 component possesses intrinsic fluorescence and its release allows for the real-time monitoring of drug activation and therapeutic action. Clearly, the use of the nucleophilicity of H2S represents an attractive mode for prodrug activation, although further studies will be needed to study the selectivity of these designs for H2S in the presence of other nucleophiles. Despite this promising report, at present, the authors are unaware of other theranostic agents that exploit this feature of H2S.
2.1.4. Hydrogen Peroxide Responsive Theranostic Probes
The term “ROS” refers to relatively unstable molecules and radicals generated by the reduction of molecular oxygen (O2), including hydrogen peroxide (H2O2), superoxide (O2–), singlet oxygen (1O2), and hydroxyl radicals (•OH).138 Endogenous ROS are primarily generated through mitochondrial metabolism and NADPH-dependent enzyme-catalyzed reactions.139,140 Further, ROS can also be produced exogenously through UV light exposure and xenobiotic agents. When produced in a controlled manner, ROS plays a critical role in cellular homeostasis and acts as a messengers in cellular growth, proliferation, migration, and apoptosis.141 Due to their ability to alter specific protein activities, ROS are also involved in blood vessel modulation, oxygen sensing, immune function, and gene activation.142−144 However, elevated ROS levels may cause oxidative stress, resulting in nucleic acid, lipid, and protein damage. Nonhomeostatic ROS has been correlated with several pathological disorders such as aging,145 cancer,146 cardiovascular diseases,147 diabetes,148 and neurodegenerative diseases.149,150
In mitochondria, H2O2 is produced from superoxide ions through superoxide dismutase (SOD)-mediated processes. Inflamed and cancerous cells generate elevated H2O2 levels (0.5 nmol/104 cells/h) as compared to normal cells (0.050 ± 0.004 nmol/104cells/h).151 H2O2 can act as a precursor for other highly reactive species, such as peroxynitrite, hypochlorite, and hydroxyl radicals. Due to the relatively high stability of H2O2 (t1/2 = 1 ms) in comparison to other ROS (t1/2 < 1 μs in most instances), and the accumulation of H2O2 during oxidative stress, H2O2 has attracted attention as a trigger for ROS-responsive drug delivery systems.152 In the context of cancer-specific theranostic probes, arylboronic esters, thioethers/thioketal, amino acrylates, selenium/tellurium, and polyproline have been explored as H2O2-responsive chemical linkers. In this section, we will summarize theranostic probes activated under oxidative stress (i.e., H2O2).
An example of an H2O2-activated probe was reported by Kim and co-workers who developed a molecular theranostic 26 (Figure 12) composed of two anticancer drug units, 5′-deoxy-5-fluorouridine (5-fluorouracil as the active toxin) linked to a self-immolative linker through two phenyl boronic acid moieties, as well as an ethidium fluorophore (a mitochondrial apoptosis marker).153 In biotin-positive A549 lung cancer cells, theranostic 26 was preferentially taken up and further accumulated into mitochondria due to the positively charged ethidium. The elevated H2O2 levels in these cancer cells served to trigger the release of 5′-deoxy-5-fluorouridine, which was further converted to 5-fluorouridine (5′-FU) by thymidine phosphorylase. The concomitant fluorescence enhancement arising from the free ethidium and subsequent DNA intercalation was used as a fluorescence reporter and allowed the real-time monitoring of cellular apoptosis. Theranostic probe 26 was further evaluated in an A549 xenograft mouse model. Here, tail vein administration of 26 produced a fluorescent signal output corresponding to theranostic probe activation by endogenous H2O2 in tumor tissues. Further, enhanced fluorescence output was observed in the lipopolysaccharide (LPS, 10 μL, directly injected into tumor tissue) treated group, consistent with oxidative stress-mediated theranostic activation. Theranostic probe 26 showed preferential tumor accumulation and promoted increased tumor reduction as compared to other test groups (PBS as control and 5′-FU), respectively.
Figure 12.
(A) Chemical structures of H2O2-responsive theranostic probes (26–29). (B) Confocal images showing mitochondrial localization of theranostic probe 26. (i) 26 (50 μM), (ii) Mito Tracker (iii), and merged image in A549 cancer cells. (C) Tumor inhibition of A549 xenograft model upon treatment with PBS as control, theranostic probe 26, and 5′-FU drug (tail vein administration, n = 8). Reproduced with permission from ref (153). Copyright 2014 American Chemical Society. (D) Severe combined immunodeficiency (SCID) image of (i) U87 MG tumor-bearing mouse after i.v. administration of theranostic probe 27 (1 min post administration) and (ii) images of dissected organs (lungs, heart, kidney, liver, and spleen) from a mouse 5 min post administration (excitation = 595 nm, emission = 700 nm). Reproduced with permission from ref (154). Copyright 2015 Wiley Intersciences. (E) Confocal images showing folate receptor-mediated uptake of nanoreactor F-GOX@NR (folate decorated silica-based glucose oxidase encapsulated nanoreactor. (F) Cell viabilities after treating with 29, nanoreactor F-GOX@NR and controls in the MCF (COX-2 positive, folate positive), Caco-2 (COX-2 negative, folate receptor negative) and MDA-MB-231 (COX-2 positive, Folate receptor negative) cell lines. Reproduced with permission from ref (160). Copyright 2022 Wiley Intersciences.
Shabat and co-workers used a NIR fluorophore (Cy7) to monitor the drug activation and localization with a level of precision commensurate with in vivo applications.154 Toward this end, these researchers created the theranostic agent 27 (Figure 12) composed of boronic ester tagged covalently to a cyanine-based fluorophore and CPT. Incubation with H2O2 resulted in the cleavage of the boronate moiety, followed by a reaction cascade to release the active CPT over approximately 90 min with a concomitant release of free Cy7 and production of an enhanced fluorescence signal with a maximum at 720 nm. In human glioblastoma multiform (GBM) U87 cells, 27 showed good cytotoxicity upon treatment with H2O2 (IC50 = 40 nM), but not so in the absence of H2O2 (IC50 = 250 nM). The authors noted that the theranostic agent exhibited lower toxicity as compared to the parent drug (CPT, IC50 = 20 nM); however, considering the reduced off-target side-effects of theranostic 27, it was deemed of potential benefit in the context of, e.g., personalized therapy. Further animal-based studies confirmed that theranostic probe 27 provided for a distinct fluorescence enhancement (Cy7), which was taken as signaling CPT release, when subject to either intratumoral or intravenous administration. An ostensibly related NIR-based theranostic probe 28 was developed for H2O2-mediated release and monitoring of diclofenac (a nonsteroid anti-inflammatory drug, NSAID) delivery in inflammation-induced macrophages.155
While phenyl boronic ester-based linkers have been widely used for H2O2-mediated theranostic development, their instability under acidic conditions potentially limits their utility.156 This might result in unanticipated drug activation in acidic cellular compartments, such as the lysosomes and endosomes.157 To address this putative stability issue, a thioketal-based linker was developed by Yan Li158 and Nam159 groups. Per the authors’ design expectations, this linker was found to possess better stability in both acidic and basic milieus. Thioketals are oxidatively cleaved to produce the corresponding thiols and ketones and their use in drug delivery systems has already been the subject of several investigations.158,159
As noted above, H2O2 is predominantly produced in the mitochondria. Thus, any theranostic that fails to accumulate effectively in the mitochondria of cancer cells may not produce a robust anticancer response. Landfester and co-workers showed that the direct in situ generation of H2O2 inside cancer cells might be one way to overcome this bottleneck. These researchers developed a multicomponent theranostic probe 29 (Figure 12), composed of celecoxib-tagged SN-38 tethered through an H2O2 responsive thioketal linker. The probe was encapsulated into folate-decorated silica nanoparticles that were further decorated with covalently attached GOX enzymes to give the nanoreactor F-GOX@NR.160 Once internalized into folate-positive cancer cells as the result of receptor-mediated endocytosis, the nanoreactor F-GOX@NR consumes glucose to generate H2O2 via GOX catalysis. Celecoxib tagged SN-38 in its inactive form, binds to COX-2, which facilitates its accumulation within the cytoplasm. The excessive H2O2 produced through nanoreactor F-GOX@NR + 29 triggers cleavage of the thioketal linker and release of active SN-38. Subsequent topoisomerase inhibition then results in cell death. Control experiments revealed that compared with intracellular H2O2-mediated drug activation, the nanoreactor system F-GOX@NR + 29 worked synergistically to release the active drug with a significantly improved potency in folate and COX-2 dual positive MCF-7 cancer cells (IC50 = 0.14 μM, 24 h) as compared to SN-38 (IC50 = 1.2 μM, 24 h) and celecoxib-SN-38 conjugates (IC50 = 2.8 μM, 24 h), respectively. Further, the intrinsic fluorescence of SN-38 provides for a ratiometric response (shift in the maximum from 452 to 560 nm in the presence of H2O2), allowing drug activation and localization to be monitored readily.
2.1.5. Other ROS-Responsive Theranostic Probes
In addition to H2O2, other ROS, including both 1O2 and •OH, have also attracted interest as possible release triggers due to their high reactivity. The hydroxyl radical •OH can react with numerous biomolecules, including amino acids, carbohydrates, lipids, and nucleotides, but typically does so in an indiscriminate manner.1611O2, an excited state of O2, has a higher oxidative potential than that of the •OH radical.162,163 While •OH is produced through H2O2 decomposition in vivo by copper(I) or iron(II)-mediated catalysis, a process also referred to as the Fenton reaction,1641O2 is primarily produced by PSs (PSs) (see Section 2.2 below).165 Both 1O2 and •OH have shorter half-lives than H2O2 in biological environments.
Zhang and co-workers developed theranostic probe 30 (Figure 13) composed of gemcitabine linked to a red light-activatable fluorescent PS (meso-tetraphenylporphyrin; TPP) through a ROS-responsive thioketal linker, which was designed to allow for fluorescence-based image-guided therapy.166 Here, the 5′-OH group of the drug was modified to block its anticancer properties. Since 1O2 has a short half-life and the diffusion rate in aqueous media is very short (≈40 ns and 20–200 nm, respectively), the limited amount of 1O2 present in unilluminated cells fails to elicit appreciable cytotoxicity.167 In contrast, under photoillumination (658 nm, 280 mW cm–2), the TPP acts as a PS and generates 1O2. This results in thioketal bond cleavage followed by cascade-like drug release. The free drug can diffuse to nearby cells and induce toxicity in the unilluminated area as well (so-called bystander effect). Compared to controls (TPP, TPP-UCL-GEM; UCL stands for ROS insensitive alkyl linker), theranostic agent 30 exhibited concentration-dependent toxicity in HeLa cells under illumination (IC50 = 0.25 μM). Tumor inhibition studies conducted using subcutaneous H22-bearing mouse models revealed that a PEG2000-PLA2000-modified version of theranostic 30 (intravenous administration) showed preferential tumor accumulation, and provided for tumor growth inhibition under low-power light illumination. The intrinsic fluorescence of TPP was also exploited to garner information related to the in vivo distribution of this theranostic. Wang and co-workers employed a similar strategy to deliver Dox to cancerous sites in breast cancer mouse models in vivo with minimal side-effects.168
Figure 13.
(A) Chemical structures of ROS-responsive theranostic probes (30 and 31). (B) Cell viability of treated HeLa cells treated with theranostic 30 (TPP-L-GEM) or control (TPP-UCL-GEM) in the presence and absence of light (280 mW/cm2, 1 min). (C) Relative tumor volume in an H22-bearing mouse model after treatment with PBS, GEM, TPP, 30, and TPP-UCL-GEM with and without light (658 nm, 280 mW/cm2, 10 min) (*p < 0.01). Reproduced with permission from ref (166). Copyright 2016 Wiley Intersciences. (D) Cell viabilities of colon 26 cells upon treatment with probes 31a–31e and light illumination (690 nm, 5.6 mW/cm2, 30 min) (±SD n = 3). Reproduced with permission from ref (170). Copyright 2014 American Chemical Society.
Other theranostic agents that rely on 1O2-responsive activation have been explored. Among these are aminoacrylate-based chemical linkers that are used to tether covalently a PS with a parent drug. For hydroxyl-bearing drug candidates, coupling with the carboxylic acid of an aminoacrylate linker offers a synthetically facile drug-loading platform. Conversely, the amino portion of the acrylate linker may be linked to a PS to furnish theranostic agents. Utilizing this strategy, researchers have developed various delivery systems that rely on SN-38,169 combretastine,170,171 paclitaxel,172 and NSAID (ibuprofen and naproxen)173 as the active drug payloads. For example, You et al. reported a series of conjugates 31a–e (Figure 13) consisting of far-red-activatable PSs (silicon phthalocyanine, Pc), covalently connected to an anticancer drug, paclitaxel, via an aminoacrylate chemical linker through the 2′-OH position (a site critical for drug action, tubulin binding).170 The resulting conjugate was further modified with folic acid bearing different PEG spacers (1 kDa, 2 kDa, 3.5 kDa, and 5 kDa) to improve their cancer-targeting ability and solubility. Further experiments led to the conclusion that medium-chain PEGylated conjugates (1k to 3.5k) are optimal for achieving folate receptor-mediated uptake. These systems were also found to provide for greater cytotoxicity than a longer PEG chain (5 kDa) or conjugates lacking the PEG linker. Cell-based experiments conducted on colon-26 cancer cells revealed that one of the theranostic agents (31b) showed folate receptor-mediated uptake and greater toxicity (IC50 = 1.65 nM) upon photoillumination (690 nm) as compared to other conjugates (IC50 = 2.71, 4.03, 4.47, and 4.85 nM) under similar conditions (Figure 13).
2.1.6. Enzyme Responsive Theranostic Probes
Enzymes are mostly proteinaceous biomolecules that accelerate chemical reactions both intra- and extracellularly.174 Their high substrate specificity, robust response, and cell-specific presence make them of interest as potential chemical triggering agents.175 Dysregulation of key enzymes is also a hallmark of pathology across a wide range of diseases, including inflammation,176,177 cancer,178,179 and neurodegenerative disease.180,181 By carefully choosing enzyme-specific substrates it is possible to mask an anticancer drug and create theranostic probes that display target-specific activation, relatively improved stability, and enhanced therapeutic effects. A summary of work along these lines now follows.
2.1.6.1. DT-Diaphorase Responsive Theranostic Probes
Quinone is a common subunit in many natural products, as well as a range of synthetic and semisynthetic molecules, including anticancer agents, antimicrobial drugs, dyes, vitamin K, and enzyme cofactors.182 Quinones play key roles in redox cycling due to their reducible nature. Quinone reductase 1 (NQO1), also referred to as DT-diaphorase, is a two-electron reductase localized primarily in the cell cytosol and at lower levels in the endoplasmic reticulum and the mitochondria.183,184 DT-diaphorase is involved in detoxification processes and is associated with early carcinogenic events with elevated levels being found in several cancer types, including ovarian, thyroid, breast, colon, and pancreatic cancers.185 Hence, the higher level of DT-diaphorase in cancerous tissues over normal healthy tissues have been used as an endogenous trigger for tumor-specific drug delivery applications.
Indolequinone is a recognized substrate for NQO1. Appreciating this, Nishimoto and co-workers prepared the theranostic agent 32a (Figure 14) designed to deliver the cytotoxin SN-38 to cancer cells.186 DT-diaphorase mediated-reduction was then expected to release the active SN-38 drug with concomitant fluorescence changes. To improve the cancer-selective uptake, theranostic agent 32b, composed of an SN-38 moiety linked to indolequinone and an integrin-selective peptide as a cancer-targeting unit was prepared.187 Theranostic agent 32b exhibited preferential uptake in αvβ3 integrin-positive cancer cells with presumed DT-diaphorase-mediated reduction serving to release the active SN-38 and produce 50–70% cancer cell growth inhibition in a human cervical carcinoma (KB) cell line. Studies showed that the alkenyliminium intermediate that is formed after drug release is effective for DNA-alkylation, which was thought to account for the observed cytotoxicity.188
Figure 14.
(A) Chemical structures of DT-diaphorase responsive theranostic probes (32–34). (B) Cell viability of theranostic probe 32b, SN-38, and 32b + DTD in KB cells. Reproduced with permission from ref (187). Copyright 2010 American Chemical Society. (C) Fluorescence images of A549 cells in the absence and presence of theranostic probe 33 (10 μM, 2 h) as recorded at different time points. Reproduced with permission from ref (190). Copyright 2015 Royal Society of Chemistry.
Several researchers have explored quinone-based prodrugs with the goal of achieving better drug release profiles. In this regard, a quinone with a “trialkyl lock” produced by Wang and co-workers has garnered particular attention.189 Under DT-diaphorase-mediated reduction conditions, the quinone is converted to the corresponding hydroquinone, thereby enabling a hydroxyl moiety to form a six-membered ring with a carbonyl moiety through lactonization. This results in the smooth release of the drug payload attached through the carbonyl group of hydroquinone. This strategy is embodied in theranostic agent 33 reported by Wu et al., which is designed to deliver SN-38 to cancer cells.190 The intrinsic fluorescence of SN-38 facilitated the simultaneous monitoring of active drug release and therapeutic action (Figure 14). Later, Kim et al. used an additional functional group to tag biotin as a cancer-targeting unit in the context of theranostic 34,191 a system that displayed preferential cancer cell uptake, as well as an improved therapeutic efficacy following presumed drug activation.
2.1.6.2. Azoreductase Responsive Theranostic Probes
Azoreductases are flavin-dependent enzymes found in eukaryotic and bacterial organisms. They are for the most part cytosolic enzymes that play an important role in homeostasis. They mediate the reduction of substrates in the presence of an electron donor, typically NADH or NADPH. Azoreductases are overexpressed in many cancer types, such as lung,192 breast,193 and pancreatic cancers.194 As a result, efforts have been made to develop theranostic agents based on azoreductases.
Kim et al. developed theranostic agent 35 (Figure 15) for the targeted delivery of a chemotherapeutic drug to the mitochondria of cancer cells.195 System 35 is composed of a rhodamine 123/B analogue conjugated to a N,N′-bis(2-chloroethyl)-1,4-benzenediamine, which serves as a nitrogen mustard analogue, through an azo linkage. Additionally, a lipophilic triphenyl phosphonium moiety is included in the overall construct to provide for mitochondrial targeting. Upon reduction, the azo bond is cleaved to release simultaneously the active drug and a fluorescent reporter, thereby providing a tool to monitor drug activation and localization under hypoxic conditions in cancer cells. However, a complex multistep synthesis and poor solubility resulted in a low translational potential for 35. To address these limitations, Xie developed theranostic 36 (Figure 15), where the positive charge on the fluorophore assured mitochondrial targeting, and the lower apolar surface area enabled a better aqueous solubility.196
Figure 15.
(A) Chemical structure of azoreductase-responsive theranostic probes (35–38). (B) Fluorescence images of theranostic probe 35 under normoxic (21%) and hypoxic (3%) conditions in various cell lines (scale bar = 10 μm, excitation = 555 nm, emission = 585 nm). Reproduced with permission from ref (195). Copyright 2017 Elsevier Ltd. (C) Confocal images of 4T1 cancer cells recorded following treatment with theranostic probe 38 (20 μM) under normoxic and hypoxic conditions after staining with Hoechst 33342 and MitoTracker Green (scale bar = 20 μm). Reproduced with permission from ref (198). Copyright 2018 Royal Society of Chemistry.
Shi and co-workers used a similar strategy to develop theranostic agent 37 (Figure 15). This system relies on Cy as a NIR fluorescent reporter and proved useful for in vivo drug monitoring.197 Likewise, Yu et al. developed a theranostic system 38 (Figure 15) composed of nitrogen mustard and Dox linked through an azo bond.198 In reductive environments, both drugs were released with concomitant fluorescence enhancement being seen that was ascribed to free Dox. This increase in emission intensity was utilized to monitor drug activation and cellular localization. In vitro and in vivo studies involving 4T1 cell-based models served to confirm that theranostic agent 38 has improved cytotoxicity and displays reduced side-effects, as compared to controls (i.e., PBS, free Dox).
2.1.6.3. Nitoreductase Responsive Theranostic Probes
Nitroredutases (NTRs) are flavin mononucleotide (FMN)-containing enzymes that are overexpressed in several tumor types. The levels of NTR expression are closely related to hypoxia in solid tumors.199 Type 1 NTRs are mostly found in bacteria and are oxygen-insensitive enzymes containing FMN as the active center, while type 2 NTRs are oxygen-sensitive, and contain FMN or flavin adenine dinucleotide.200 Hypoxic regions in tumors arise from impaired vascular networks or those insufficient to support rapid growth, resulting in limited blood and oxygen supply. Hypoxia is observed in 50–60% of solid tumors, particularly the inner core of tumors.201 Hypoxia-responsive DDS have been thoroughly investigated and are the topic of a number of published articles.202−205 In this section, we summarize recent advances involving hypoxia-responsive DDS in the area of molecular theranostics.
One of the biggest challenges in hypoxia-responsive therapeutic formulations is tumor angiogenesis, which acts to enhance the tumor oxygen supply, resulting in low therapeutic efficacies. Hence, blocking angiogenetic pathways in combination with hypoxia-responsive drug delivery systems could lead to the production of improved hypoxia-responsive DDS.
Nitroaromatics serve as excellent substrates for both NTRs, resulting in the formation of hydroxylamines and amine derivatives. When presented within a self-immolative system, the subsequent electron redistribution in the aromatic ring results in the release of linked drugs. Appreciating this, Kim et al. developed theranostic agent 39 (Figure 16) composed of an NSAID (indomethacin) linked to SN-38 through a nitrobenzyl alcohol-based linker.206 The incorporation of indomethacin in the theranostic design satisfied two roles; to achieve tumor targeting based on COX-2 overexpression, as well as angiogenesis inhibition mediated by COX-2. Theranostic agent 39 showed concentration-dependent toxicity in COX-2 expressing A549 and HeLa cells under hypoxic conditions (1% O2). Further, theranostic probe 39 exhibited a strong fluorescence corresponding to active SN-38 in multicellular tumor spheroids (A549) of varying sizes (110, 235, 300 μm). This finding was taken as evidence of deep tissue penetration and prodrug activation through indomethacin-mediated antiangiogenesis.
Figure 16.
(A) Chemical structures of nitroreductase-responsive theranostic probes (39 and 40). (B) Cell viabilities of theranostic probe 39 in COX-2 positive (A549, HeLa) and COX-2 negative (WI-38, BJ) cells at different concentrations. (*p < 0.05). (C) Fluorescence images (at day 4) of HeLa cell tumor spheroids under normoxic and hypoxic conditions after treatment with probe 39 (25 μM). Reproduced with permission from ref (206). Copyright 2018 Elsevier Ltd. (D) Fluorescence images of different cell populations recorded after treatment with 40a under normoxic and hypoxic conditions (3% oxygen) (scale bar = 50 μm). (E) Tumor volume vs time plot of CD+133 MDA-MB-231 cells after treatment with DMSO or 40b (5.0 nM, 24 h) and then administered to mice to gauge tumorigenesis. Reproduced with permission from ref (207). Copyright 2021 American Chemical Society.
The same group developed theranostic pair 40a and 40b for imaging and therapy of cancer stem cells (CSCs) (Figure 16).207 Here, the authors used dimethylnitrothiophene as the hypoxia-responsive trigger instead of the p-nitrobenzyl group, owing to its lower reduction potential, which was expected to facilitate drug release. For therapeutic purposes, a curcumin analogue, 3,4-difluorobenzylidene curcumin, was used. Further, active targeting of CSCs was achieved via a well-known carbonic anhydrase (CAIX) inhibitor, acetazolamide.208 For imaging, a naphthylamide fluorophore was employed in lieu of the drug. The main difference within this effective theranostic pair (designed to allow separate imaging and therapy) was the presence of the drug or the phthalimide unit with the targeting unit and hypoxia-responsive trigger entities remaining the same. Because of this similarity, it was assumed that the cellular uptake behavior and activation profile of this theranostic pair would be comparable. Support for this supposition came from cell-based studies using CD+133 MDA-MB-231 breast cancer cells. Both compounds (40a and 40b) demonstrated hypoxia-sensitive activation allowing for imaging and anticancer activity. Treatment with theranostic 40b (tail vein delivery) retarded tumor development in CD+133 MDA-MB-231 xenograft mice compared to the control.
2.1.6.4. Esterase Responsive Theranostic Probes
Carboxylesterases (CESs) are a common class of hydrolases that promote ester, amide, and carbamate bond cleavage.209,210 CESs are key protective enzymes that play a role in detoxifying xenobiotics. Two prominent CEs, namely CES1 and CES2, have been thoroughly studied. Several reports have highlighted the elevated expression of CES2 in pathological tissues as compared to healthy tissues.211,212 Overexpressed CES levels in cancer cells are thought to abet invasion, migration, survival, and tumor growth.213,214 Compared to normal cells (0.17 ± 0.09 U/L male; 0.12 ± 0.07 U/L female), about a 2–4-fold increase in CES activity in malignant colorectal cancer (0.45 ± 0.25 U/L male; 0.45 ± 0.35 U/L female) has been reported.215 Hence, several esterase-responsive theranostic agents have been developed to achieve tumor-specific imaging and improve therapeutic outcomes. For example, Kunimoto and co-workers developed theranostic agent 41 as a CEs-responsive SN-38 analogue (Figure 17).216 The authors used a water-soluble δ-lactone ring linked to the SN-38 drug through a carbamate linker. Incubation with CES resulted in hydrolysis of the carbamate bond to release SN-38.
Figure 17.
(A) Chemical structures of esterase-responsive theranostic probes 41 and 42. (B) Cell viabilities of Dox-sensitive MCF7 and Dox-resistant MCF7/Dox cells after treatment with DMSO, Dox + DCA (1:1), or theranostic probe 42. (C) Representative images of MCF7 and MCF/Dox xenograft tumor models after treatment with DMSO control, Dox + DCA (1:1), or theranostic probe 42, respectively. Reproduced with permission from ref (223). Copyright 2018 Elsevier Ltd.
One of the biggest challenges associated with cancer chemotherapy is the development of multidrug resistance (MDR), a state defined as the ability of cancer cells to survive despite the presence of various chemotherapeutics.217 Statistically, about 90% of cancer mortality is attributed to MDR.218 MDR is predominantly attributed to several factors such as reduced drug uptake, enhanced drug efflux, increased DNA repair, elevated xenobiotic metabolism, and genetic factors (gene amplifications, mutations, and epigenetic alterations).219−222 This results in reduced therapeutic potency of the administrated drugs. To address this limitation, Kim and co-workers developed theranostic agent 42 (Figure 17), composed of a dichloroacetic acid (DCA) moiety linked through an amide linkage to a self-immolative linker, Dox, and a lipophilic TPP mitochondrial targeting unit.223 The rationale behind this design was to sensitize cancerous cells by shifting the aberrant metabolism of cancer cells, which rely largely on glycolysis, back to mitochondrial phosphorylation. It was also appreciated that the delayed release of active Dox would likely lead to improved therapeutic efficacy. DCA is a well-known pyruvate dehydrogenase inhibitor (PDK) used to facilitate the shunting of glycolysis to glucose oxidation. It thus reduces lactate accumulation, reduces intracellular ATP, and mitochondria dysfunction.224 Another aspect of 42 is that it was expected to initially be located within the mitochondria, thus avoiding initial drug efflux through ATP-driven ABC transporters.225 Over time, Dox was expected to translocate to the nucleus to produce the desired anticancer activity. Cell-based studies confirmed that the cytotoxicity was CEs-dependent and that 42 was preferentially taken up by A549 and HepG2 cancerous cells over normal cells (NHDF, IMR90). Theranostic agent 42 outperformed Dox and a coadministered 1:1 combination of DCA and Dox. Moreover, 42 was able to perform its action in both drug-sensitive MCF and drug-resistant MCF/Dox breast cancer cells, as well as in both drug-sensitive and resistant MCF xenograft mouse models.
2.1.6.5. Protease Responsive Theranostic Probes
Proteases are a large family of enzymes that promote the cleavage of peptide bonds.226 They play a vital role in protein metabolism (protein catabolism, protein digestion, and cell signaling), and elevated protease levels are closely associated with several diseases, including inflammatory disease,227 cancer,228,229 cardiovascular,230 and neurodegenerative disease.231 To date, considerable effort has been devoted to the development of protease inhibitors, as well as to protease-responsive diagnostic and therapeutic probes. Caspases are among the proteases involved in inflammation, cancer, and programmed cell death. Specifically, caspase-3, a cysteine-aspartic acid protease is activated through endogenous (programmed cell death) and exogenous (radio/chemotherapy treatment) means.232−234
Byun et al. developed the caspase-3-responsive theranostic agent 43 (Figure 18) composed of a cancer-targeting integrin (Arg-Gly-Asp) tripeptide, a caspase-3-responsive linker (DEVD, Asp-Glu-Val-Asp tetrapeptide), a cellular ester responsive linkage and Dox, as the anticancer drug.235 Dox is known to activate caspase-3, an apoptosis related marker.236 As a result, it was expected that Dox-induced activated caspase-3 would release Dox after ester hydrolysis, resulting in additional caspase-3 activation. This cycle was expected to continue to exert an improved therapeutic efficacy. Per these expectations, caspase-3 was found to induce cleavage of the DEVD linker, followed by ester bond hydrolysis to release the active drug, along with concomitant fluorescence enhancement. In integrin-positive human glioma U-87MG cells, theranostic 43 exhibited preferential uptake ascribed to integrin-mediated endocytosis and demonstrated an improved cytotoxicity as compared to integrin-negative HT-29 colon cancer cells. Cell-based studies confirmed that this self-amplified enzyme apoptosis theranostic agent induced a roughly 154-fold enhancement in caspase-3 activity. In vivo studies conducted using U-87 MF tumor-bearing mice revealed that treatment with 43 produced a near-complete tumor inhibition as compared to various controls.
Figure 18.
(A) Chemical structures of theranostic probes 43 and 44. (B) Confocal images of U-87 MG and HT-29 cancer cells recorded upon treatment with RDEVD-DOX (control) and RGDEVD-DOX (43). The red color is ascribed to doxorubicin fluorescence while the blue color indicates the cell nucleus (scale bar = 50 μm). (C) Tumor inhibition of U-87 MG xenografted mice after treatment with saline, RDEVD-DOX, RGDDEV-DOX, or 43 (RGDEVD-DOX), respectively (n = 6) (±SD * p < 0.05, **p < 0.01, *** p < 0.001 vs control). Reproduced with permission from ref (235). Copyright 2018 Wiley Intersciences Ltd. (D) Cell viability of theranostic probe 44 in MDA-MB-231 and H9C2 cells (*** p < 0.001). (E) Tumor inhibition of MDA-MB-231 tumor-bearing mice after treatment with saline, free Dox, or theranostic probe 44 (* p < 0.05, *** p < 0.001). Reproduced with permission from ref (241). Copyright MDPI.com.
Cathepsins are lysosomal proteolytic enzymes that primarily metabolize proteins and peptides. They play a fundamental role in maintaining tissue homeostasis and are involved in the immune response, as well as cell development, differentiation, and apoptosis.237 Alteration in expression levels of these enzymes is correlated with several pathological disorders, including cancer, and poor prognoses.238,239 Cathepsin B is especially important in terms of promoting proteolysis in the extracellular matrix (ECM), thereby promoting tumor angiogenesis, invasion, and metastasis.240 Special efforts have therefore been made to develop cathepsin B-responsive diagnostic and therapeutic platforms. For example, Kim et al. developed theranostic agent 44 (Figure 18), composed of Dox linked to an albumin-binding maleimide moiety through a cathepsin B-responsive peptide sequence (FRRG).241 Compared with the parent drug (Dox itself; t1/2 = 0.25 h), the maleimide moiety in the scaffold was found to improve the half-life (t1/2 = 3.1 h), presumably as a result of being bound to the plasma albumin. After presumed albumin-mediated passive tumor accumulation, theranostic 44 is activated by cathepsin B to release Dox inside the cancer cells. Cytotoxicity studies revealed that theranostic 44 exhibited enhanced antitumor effects in MDA-MB231 breast cancer cells (IC50 = 7.33 μM) as compared to rat cardiomyocytes H9C2 (IC50 > 200 μM), a result ascribed to the higher cathepsin B activity (24.26 ± 3.08-fold) in the former cells. In vivo studies in MDA-MB231 tumor-bearing mice demonstrated tumor growth inhibition in the theranostic 44-treated group as compared to various controls.
In a separate work, Tian et al. developed theranostic agent 45 (Figure 19) for the cathepsin B-mediated delivery of SN-38 within folate-positive cancer cells.242 Through folate receptor (FR)-mediated endocytosis, the theranostic agent was taken up in FR-positive cancer cells (SK-Hep-1, HeLa, and Siha cells). Further, cathepsin B triggered activation to release the active drug with a concomitant fluorescence enhancement within the nucleus. Cell-based cytotoxicity studies revealed that 45 exhibited significant cytotoxicity in these cancer cells with IC50 values of about 2–3 μM being observed. By contrast, low toxicity was seen in normal cells (16-HBE) and FR-negative A549 lung cancer cells (IC50 = 20 μM). It was thus proposed that the theranostic agent 45 could be used to avoid the off-target side effects seen for the parent drug (SN-38).
Figure 19.
(A) Chemical structures of theranostic probes 45–47. (B) Schematic showing the activation of theranostic probe 46 and drug release. (C) Chemical structure and mode of activation proposed for theranostic probes 47a–c and their preferential accumulation in tumor tissues via active and passive tumor targeting and nucleus accumulation to release the specific drug payload. (D) Time-dependent fluorescence images of theranostic probe 46 in (i) HeLa, (ii) HepG2, (iii) HCT116, (iv) MIA PaCa-2, and (v) Caco-2 cells. Reproduced with permission from ref (248). Copyright 2016 Royal Society of Chemistry. (E) Antimetastatic activity of 47 and controls showing a number of pulmonary metastatic nodules at the experimental end point (n = 5). Reproduced with permission from ref (249). Copyright 2023 Wiley Intersciences.
Histone deacetylases (HDACs) are an important class of epigenetic modulators that regulate the activity and expression of numerous proteins in cellular processes. Several studies have found elevated levels of HDAC in close association with various malignancies, including neuroblastoma,243 gastric,244 ovarian,245 colon,246 and multiple myeloma,247 with poor patient outcomes. Hence, efforts have been made to design HDAC-responsive drug delivery systems. For example, Kim et al. reported theranostic agent 46, consisting of an acetylated lysine residue linked to Dox and indomethacin as a COX-2 positive cancer-targeting unit.248 Preliminary solution studies revealed that 46 on sequential treatment with HDAC and cysteine cathepsin L (CTSL), respectively, resulted in active Dox release with fluorescence enhancement (Figure 19). In cell-based experiments conducted on various cell lines, 46 showed an enhanced fluorescence that was ascribed to the release of Dox. A cell line dependence was seen in the order HepG2, HeLa > HCT116, MIA PaCa-2 > Caco-2 cells that was correlated with their HDAC and CTSL levels, as well as their COX-2 expression levels. Theranostic 46 displayed concentration-dependent cytotoxicity in HeLa cells and preferential tumor localization in COX-2-positive HeLa and HepG2 tumor xenograft mouse models.
In another report involving a dual-mode peptide design strategy, Zheng and co-workers developed a small library of HDAC-responsive theranostic agents 47a–c based on CPT decorated with various peptide sequences for multistage tumor targeting purposes.249 The peptides used by the authors were 1) CRGDK, an RGD sequence for targeting αvβ3 integrins in the tumor extracellular matrix,250 2) CREKA for targeting the tumor vasculature,251 and 3) TAT to target the nucleus252 (Figure 19). Nanoassemblies of these systems were expected to exhibit preferential tumor targeting and HDAC-mediated drug release. Incubation with HDAC1 with these theranostic agents for different time intervals resulted in appreciable CPT release over the course of 1 h (for 47a: 44.1%, 47b: 68.2%, and 47c: 67.7%). Greater than 90% release was seen after 5 h for all three derivatives. These systems exhibited only minimal drug release in the absence of HDAC1, a finding that underscores the high stability and enzyme-selective drug release profile of these systems. All three probes showed significant toxicity in HDAC1-expressing cancer cell lines (47b, IC50 = 22.3 μM in 4T1 cells, IC50 = 0.6 μM in MDA-MB-231 cells; 47a, IC50 = 7.2 μM in 4T1 cells, IC50 = 0.2 μM in MDA-MB-231 cells). Using the 4T1 breast tumor mouse model, the authors were able to show that a dual combo (CPT-Lys(Ac)-RGD/TAT) exerted about 72% tumor growth inhibition while 47b (50%) and 47a (negligible effect) performed significantly more poorly. Likewise, the nanoassemblies also demonstrated potent antitumor activity in the 4T1-luc orthotopic breast cancer mouse model (metastatic cancer model). This was taken as evidence that both vascular and nucleus targeting could be exploited to improve therapeutic efficacy.
Legumain, a lysosomal/vascular asparaginyl endopeptidases (AEP) enzyme, is a cysteine protease that was originally discovered in legumes; however, it is a lysosomal enzyme in mammals.253,254 Legumain is highly expressed in kidney tubulin under physiological conditions and aids renal tubular reabsorption.255 Several studies have shown that legumain is highly expressed in a variety of solid tumors such as prostate,256 breast,257 gastric,258 ovarian,259 and colon cancers260 and is closely associated with risk of malignancy.261 Enhanced activity of the enzyme is observed within the acidic microenvironments of cancer cells. To date, efforts have been made to develop cancer-targeted theranostic agents by incorporating a legumain-selective peptide sequence.262,263 For example, Riu et al. developed a theranostic agent 48 by using a legumain-selective tripeptide sequence, Ala-Ala-Asn, linked to Dox through an amide linker.264 Preliminary activation studies that relied on fluorescence spectroscopy revealed that in the presence of human legumain theranostic 48 (Figure 20) released about 70% of the possible Dox over a 24 h period under acidic conditions (pH 5.5 and 6.5). In contrast, under neutral pH conditions, no drug release was observed. Further confocal studies confirmed that theranostic 48 was taken up by cancer cells through endocytosis and provided for enhanced cytotoxicity and reduced side-effects relative to normal cells. More importantly, this putative theranostic provided for cytotoxicity in both tumor and stromal cells via a “bystander effect”.
Figure 20.
(A) Chemical structures of theranostic probes 48–50. (B) Confocal images of HT-29, HepG2, and HeLa cells after treatment with theranostic probe 49 (10 μM). (C) Tumor growth inhibition of HT-29 xenografts (mouse model) after treatment with Dox and probe 49. Reproduced with permission from ref (273). Copyright 2018 Elsevier Ltd. (D) Cell viability of HepG2, HeLa, and HFF-1 cells upon treatment with theranostic probe 50 at different concentrations. Reproduced with permission from ref (274). Copyright 2022 Royal Society of Chemistry.
Galactosidases are a class of enzymes involved in several vital catabolic processes.265,266 β-Galactosidase (β-gal) is a particularly important lysosomal hydrolase that acts to cleave the terminal galactoside residue from glycoconjugates.267 β-gal is known to be highly expressed in various cancers268−270 and has been used to develop both cancer-targeted imaging and therapeutic agents.271,272 In the context of theranostics, Kim et al. developed the β-gal-responsive agent 49 (Figure 20), consisting of an anticancer drug linked covalently to a galactosidase moiety through a self-immolative linker.273 As prepared, theranostic agent 49 is stable and nonemissive in PBS. However, when treated with β-gal, hydrolysis of the galactosidase moiety occurs, which results in the release of active Dox with a concomitant fluorescence enhancement. Cell-based studies conducted in HT-29 and HepG2 cells revealed that 49 is taken up preferentially through overexpressed asialoglycoprotein (ASGP) receptors. Reduced uptake was seen in HeLa cells that have lower ASGP expression levels. Probe 49 exhibited a concentration-dependent toxicity in the HT-29 and HepG2 cell lines. In vivo tumor inhibition studies conducted using HT-29 cancer xenograft-bearing mice revealed a strong tumor growth inhibition upon treatment with 49, as compared to Dox alone.
Buniya et al. developed the theranostic agent 50 (Figure 20), where a β-gal-responsive galactosidase moiety was linked to a coumarin fluorophore, and further connected to gemcitabine.274 Incubation with β-gal (0.1 U mL–1) served to hydrolyze the galactosidase moiety and trigger an intramolecular electronic rearrangement in the coumarin scaffold thus releasing the active drug and producing a fluorescence enhancement within 30 min. Gemcitabine has a low half-life (t1/2 = 8–94 min) due to rapid metabolism by intracellular enzymes.275 The strategy embodied in 50 was thus expected to enhance drug potency through reductions in the undesired metabolism of gemcitabine. Cytotoxicity studies revealed higher toxicity for theranostic 50 in HepG2 cells (IC50 = 1.6 ± 0.4 μM) as compared to free gemcitabine (IC50 = 3.5 ± 0.4 μM). Theranostic 50 also proved about 12-fold less toxic in normal HFF-1 cells (IC50 = 12.3 ± 0.7 μM).
2.1.7. Dual Stimuli-Responsive Theranostic Probes
As discussed in the above subsections, theranostic probes that are responsive to a single stimulus, such as pH, GSH, H2O2, enzyme, etc., represent a promising approach to increasing the utility of a given drug while reducing side-effects. It is thus perhaps not surprising that to enhance the further effectiveness and tumor specificity in complicated pathological microenvironments, such as the tumor microenvironment, theranostic probes that rely on dual activation strategies have been developed. In principle, it is possible to design systems where the activation mechanisms occur sequentially in distinct environments or concurrently within the same site of action. In this subsection, we review progress made along both of these limiting directions.
Cancerous cells are notoriously heterogeneous. For example, they may constitute reducing environments characterized by elevated levels of intracellular GSH,276,277 or be formally oxidative as the result of overproduction.278−280 These opposed redox conditions (reductive or oxidative) can exist in different tumors or coexist in the same tumor in different areas, or even in a single cancer cell at different time points.281 Recognizing this disparity, Kim and co-workers developed the theranostic agent 51 (Figure 21). This system was designed to enable the redox-responsive delivery of SN-38 under both reductive and oxidative conditions.282 Here, a thioether-based linker was used to connect covalently an SN-38 moiety with a COX-2 inhibitor, indomethacin, incorporated into the structure to provide for cancer targeting as well as a potential immunotherapeutic trigger. Preliminary solution studies confirmed that 51 in the presence of GSH underwent thiol-mediated hydrolysis and drug release, while exposure to H2O2 resulted in sulfone/sulfoxide formation followed by hydrolysis and thus also served to release the active drug. In vitro studies in COX-2-positive LoVo and SW620 cells revealed uptake of 51 and an improvement in the cytotoxicity, relative to COX-2-negative cells (NHDFs, MCF10A). In a colon cancer tumor-bearing mouse model (SW620), treatment with 51 (5 mg/kg/d; intraperitoneal administration) provided for a statistically significant reduction in the tumor burden as compared to controls. Also, as a presumed result of the indomethacin component in 51, a reduction in key pro-inflammatory markers (IL-6, TNF-α, VEGF) was observed. Other studies have provided support for the conclusion that the incorporation of thioether-based redox-responsive linkers can provide an advantage in the design of functional theranostics.283,284
Figure 21.
(A) Chemical structure and proposed mode of activation of theranostic probe 51 under conditions of reductive and oxidative stress. (B) Cell viabilities of probe 51 in normal and cancer cell lines were seen upon incubation with different concentrations. (C) Tumor inhibition provided by DMSO and equal concentrations of SN-38 and probe 51 in a SW620 xenograft mouse model (n = 5, *p < 0.05, **p < 0.01). (D) Anti-inflammatory cytokine mRNA levels seen in the tissues of mice treated with DMSO, SN-38, and 51 (M1, M2, and M3 define three mice). Reproduced with permission from ref (282). Copyright 2019 American Chemical Society.
Light-responsive therapies offer the possibility of illumination-based control over drug release and activation. Unfortunately, phototoxicity to the nearby normal tissues, as well as nonspecific drug release due to exposure to sunlight, can limit the potential applicability of this approach. To overcome this possible limitation, Feng and co-workers developed hypoxia- and photoresponsive theranostic probe 52 (Figure 22). This system incorporates a photoresponsive moiety, o-hydroxyl E-cinnamic acid (CAE), linked to gemcitabine.285 The other end of the CAE group is masked with a hypoxia-responsive 4-nitrobenzyl group. Hence, probe 52 cannot be activated by light under normoxic conditions (i.e., ion cells and tumor environments that lack nitroreductase activity). In contrast, in cancer cells, the prevailing hypoxic conditions allow nitroreductase to reduce the 4-nitrobenzyl group, generating intermediate 52a. This intermediate is sensitive to UV-illumination, generating the active drug through intermediate 52b. Self-immolative cyclizationleads to a fluorescent coumarin derivative characterized by a blue emission. When tested (10 μM) in MCF-7 cells, a strong blue emission was observed under hypoxic conditions (2% oxygen, 6 h), followed by UV irradiation (365 nm, 10 min). Appreciable cytotoxicity was observed. No such emission or cytotoxicity was observed under normoxic conditions (20% oxygen). Thus, this dual-triggered theranostic probe provides more precise drug delivery to the targeted cancer cells, as well as real-time monitoring of the hypoxic status. As importantly, cytotoxin release can be achieved with high spatiotemporal control. However, analogues of 52 incorporating a red-shifted light trigger and a fluorophore that emits further to the red are needed before systems of this general design are likely to see translation into a clinical setting.
Figure 22.
(A) Chemical structure and mode of activation of theranostic probe 52 controlled by hypoxia and sequential light irradiation. Cell viability of MCF-7 cells upon treatment with (B) parent drug GEM and (C) theranostic probe 52 at different concentrations under normoxic and hypoxic conditions in the presence and absence of photoillumination. Reproduced with permission from ref (285). Copyright 2016 Royal Society of Chemistry.
2.2. Theranostic Fluorescence Probes in PDT
2.2.1. pH-Activatable Theranostic Probes in PDT
An acidic tumor microenvironment (TME) is considered a characteristic of cancer and provides an opportunity for the development of activatable PSs. Typically, pH-activated PSs contain components that are responsive to H+ ions, such as pyridine, aniline, piperazine, morpholine, or other tertiary alkyl amine groups. These components can quench the fluorescence and hinder the generation of photoinduced 1O2 by the PSs. These smart PSs are usually inactive under normal physiological pH but can exhibit strong fluorescence emission and efficiently produce ROS under acidic conditions. This offers new possibilities for precise identification and PDT treatment of cancers.
For instance, Siegwart et al. designed water-soluble and pH-activatable near-infrared (NIR) absorbing iodinated BODIPY-based PSs for image-guided PDT against cancer (Figure 23).286 Spectral analysis revealed that compounds 53 and 54 displayed strong NIR absorption and stable NIR emission with absorption maxima at 660 and 690 nm, and emission peaks at 692 and 742 nm, respectively, in acidic media. This makes them ideal for noninvasive imaging of deep tumor tissues. Importantly, the 1O2 quantum yields and fluorescence intensities of 53 and 54b were significantly increased by the acidic pH in the TME. This is due to the protonation of the diethylaminophenyl moieties, which prevents PET quenching effects. Moreover, these BODIPY derivatives selectively accumulated in tumors after intravenous injection, without the need for an additional cancer-targeting agent. This highlights their excellent inherent tumor-targeting properties and their ability to induce significant tumor photoablation under NIR light illumination at low pH.
Figure 23.
(A) Structures of pH-activatable BODIPY-based 53 and 54.286 (B) Mechanism diagram of pH-mediated disaggregation of 55 for activated PDT and PTT.287 (C) Structures of 56–58 featuring pH-reversible ISC and PDT action.288
Based on a similar BODIPY dye parent core, Tang et al. also designed a BODIPY sensitizer with pH-dependent aggregation behavior (55), using bromine as a heavy atom to achieve pH-dependent photodynamic/photothermal effects combined with tumor therapy (Figure 23). This sensitizer exhibited low background toxicity and showed weak fluorescence and 1O2 production capacity at pH 7.4 due to a dual quenching mechanism (a PET effect and the aggregation state).287 In acidic solutions (pH 4.0), the dimethylaminophenyl moiety of 55 became protonated, leading to effective disaggregation of the protonated 55 due to enhanced solubility and charge repulsion. Consequently, both PDT and PTT activity were successfully triggered.
The lysosome, an organelle with a pH of 4.5–5.5, plays a crucial role in the cellular degradation of circulating biomacromolecules and the maintenance of homeostasis. Lysosomal damage can activate various cell death modes, and the lysosome-mediated cell death pathway (LCD) bypasses the classical caspase-dependent apoptotic pathway. Therefore, targeting lysosomes has become an important objective for antitumor therapy. In line with this, Ulrich et al. reported a series of aniline- and iodine-substituted BODIPY derivatives (56–58) as promising lysosome-targeting and pH-activated theranostic PDT agents, exhibiting significant in vitro light-induced cytotoxicity (Figure 23).288
To enhance the effectiveness of PDT against hypoxic tumors, Mou and co-workers developed a proton-driven transformable 1O2-trap nanoparticle system. This system includes pH-reactive dimethylaniline and polymer-encapsulated anthracenyl BODIPY (59) as a smart PDT agent. It is capable of releasing 1O2 in the dark and under hypoxic conditions, as depicted in Figure 24.289 In the acidic endosomal microenvironment, the nanoparticle system achieves efficient endosomal escape through a “proton-sponge” effect. Additionally, it undergoes a transformation from a cubic 1O2 nanotrap (94.1 nm in length) to nanospheres (12.3 nm in diameter). Meanwhile, the protonated form of 59, with diethylamino phenyl groups receiving two protons to form ANBDPH, exhibits stronger fluorescence emission, longer fluorescence lifetime, and higher 1O2 generation capacity compared to the unprotonated form. 59 is conjugated with an anthracenyl group, known to produce endoperoxides, which can prolong the lifespan of 1O2 in dark and hypoxic conditions. In comparison to BODIPY derivatives without anthracene, 59 NPs achieved a 96.7% suppression rate of tumor growth, surpassing NBDP NPs and BDP NPs. This research provides new insights into PDT for hypoxic cancer.
Figure 24.
Preparation of proton-driven transformable 1O2-nanotrap and its mechanism of hypoxic cancer PDT. Reproduced with permission from ref (289). Copyright 2022 Wiley Intersciences.
Due to their easy synthesis and modifications, robust photostability, and high molar extinction coefficients, azo-BODIPY-based structures serve as an excellent platform for advanced PSs. Taking advantage of these characteristics, a family of pH-activated aza-BODIPY-based PSs has been extensively studied. These PSs incorporate anilines or morpholines as proton receptors to BODIPY, resulting in intrinsic BODIPY emission in acidic media. For example, Ju et al. developed a pH-activated nanoprobe consisting of bromophenyl aza-BODIPY (60). This nanoprobe was further encapsulated in a cRGD-functionalized nanomicelle to target integrin-overexpressing tumor cells for near-infrared (NIR) PDT treatment (Figure 25).290 In addition, Dong et al. reported two pH-triggered aza-BODIPY nanoparticles decorated with dimethylaminophenyl units (NAB, 61) or morpholine moieties (MAB, 62), respectively, for photoacoustic (PA) and photothermal imaging-guided simultaneous PTT/PDT (Figure 25).291,292
Figure 25.
Chemical structures of theranostic probes (60–62).
Cyanine dyes are a type of dye that possess excellent optical properties and have been widely used in biosensing, bioimaging, and phototherapy. Specifically, cyanine dye-derived PSs with pH-responsive groups have been developed for the selective recognition and eradication of cancer. In a study by Kamkaew et al., a pH-responsive heptamethine cyanine-based theranostic PDT agent (63) was developed for the treatment of HepG2 cells (Figure 26).293 Probe 63 exhibited a high ΦΔ value and enabled NIR imaging-guided PDT under acidic conditions when the pH-sensitive N-methylpiperazine unit was protonated. The absorption spectra of 63 showed a red shift with decreasing pH values, which was attributed to the inhibition of an intramolecular charge transfer (ICT) process at low pH. Furthermore, theranostic 63 could selectively enter cancer cells and displayed high photocytotoxicity against HepG2 cells under acidic conditions when exposed to 850 nm LED light for 30 min.
Figure 26.
(A) Chemical structure and the pH-activated mechanism of 63.293 (B) Schematic diagram of the design concept of low pH-responsive PS 64.294
Recently, Wang and Zhou et al. used a pyridine unit as a pH-responsive component to construct an efficient pre-PS (64).294Figure 26 shows that cyano units, which have electron-absorbing properties, made 64 exhibit a stronger pH response at lower acidities (pH < 5.0) than the control molecule ZWZ (64a). Moreover, probe 64 demonstrated better cellular uptake and 1O2 generation abilities. It could be activated by intracellular H+ to enhance intramolecular charge transfer (ICT), leading to efficient PDT in the lysosomal environment of cancer cells (such as HepG2, HeLa, and 4T1 cell lines).
Tang and Wang et al. developed a pH-switchable phototheranostic (65) with AIE features for NIR-II FLI-guided type I PDT/PTT in a colorectal cancer model (Figure 27).29565 had higher absorptivity at 808 nm compared to the two control molecules (66, 67). The maximum emission peak of 65 in DMSO was located at 1114 nm within the NIR-II window, enabling FLI-guided phototherapy. Due to strong intramolecular charge transfer, highly efficient intersystem crossing, and sufficient intramolecular motion, compound 65 generated boosted superoxide anions through a type I process and exhibited excellent photothermal performance under 808 nm laser irradiation. Notably, 65 nanoparticles, with a C=N double bond as the pH-responsive site, displayed higher 1O2 production capacity and heat generation capabilities at pH 6.5 than at pH 7.4. These biocompatible 65 nanoparticles showed significantly enhanced type I PDT/PTT in tumors, leading to significant antitumor effects in vitro and in patient-derived colon cancer xenograft models.
Figure 27.

Schematic illustration of 65 NPs as a tumor reversibly pH-responsive theranostic platform. Reproduced with permission from ref (295). Copyright 2023 American Chemical Society.
Huang et al. utilized p-phenylethynylene to design a series of purely organic materials (POMs) 68–72 displaying a pH-responsive reversible switching of ISC for smart PDT (Figure 28).296 The organic structures displayed efficient ISC properties, which were estimated by femtosecond transient absorption (fs-TA) spectroscopy and quantum chemical calculations. The results showed that as the degree of torsion increased, the ISC efficiency increased from 1% to 90%. The 1O2 quantum yield of 72 was calculated to be about 0.48 at pH 6.0 and 0.05 at pH 7.4, respectively, indicating that ISC is enhanced in acidic environments. In vitro, 72 showed outstanding selectivity in tumor cells compared to the commercial PS TMPyP4. This work provides a reversible switching method for adjusting ISC by pH, thereby increasing the accuracy of PDT.
Figure 28.
Chemical structures of 68–71 and schematic diagram of the reversible ISC and PDT effects of 72 at different pH values.296
2.2.2. Biothiol-Activatable Theranostic Probes in PDT
Biothiols, such as GSH, cysteine (Cys), and homocysteine (Hcy), play crucial roles in biological systems, particularly in maintaining the redox balance of living cells.297 Cancer cells, which are under constant oxidative stress, often increase their levels of biothiols as a compensatory mechanism.298 For instance, the concentration of GSH in cancer cells can be 1–10 mM, significantly higher than that in normal cells (more than 5 times higher).299−301 Therefore, abnormal biothiol concentrations can serve as indicators to differentiate cancer cells from normal cells and can also be used to develop biothiol-activatable smart PSs to enhance the effectiveness of PDT against tumors while minimizing damage to healthy tissues.302−305
The sulfhydryl (−SH) group present in cysteine enables GSH or Cys to exhibit strong reductive and nucleophilic properties, allowing them to participate in various chemical and biological reactions. Depending on the reactivity of the −SH group, biothiol-mediated activation strategies or mechanisms can be further categorized into sulfonate ester cleavage, disulfide exchange, nucleophilic substitution reactions, cyclization with aldehydes or cyano groups, GSH-mediated reduction, and Michael additions.
2.2.2.1. Cleavage of Sulfonate Esters
Previous investigations have confirmed that 2,4-dinitrobenzenesulfonyl is a responsive group to thiols. This group possesses a strong electron-withdrawing capability, which has been utilized in the development of GSH-activated PSs. In a recent study by Sun et al., a GSH-triggered near-infrared (NIR) PS (73) was designed by incorporating iodine-substituted hemicyanine (73-OH) with 2,4-dinitrobenzenesulfonate (DNBS) (Figure 29). This PS was intended for use in combined PDT and sulfur dioxide (SO2) therapy.306 SO2, a member of the gasotransmitter family, has traditionally been recognized as an air pollutant. However, recent reports have unveiled its potential in disease treatment, particularly in synergistic cancer therapy to overcome drug resistance. In this study, SO2 was generated by the removal of the DNBS cage group through nucleophilic substitution by intracellular GSH. In addition, the active PS 73-OH was released, enabling the production of cytotoxic 1O2 upon red light irradiation. This PS exhibited a significant decrease in cancer cell viability and inhibited tumor growth, demonstrating its excellent anticancer effect. The structure of 73 consisted of two parts with distinct functions: DNBS served as a GSH recognition site, an effective quencher of the excited state, and an SO2 generator, while hemicyanine, containing a heavy iodine atom, acted as the PS core for light-induced production of cytotoxic ROS. In normal tissues, 73 remained in an “off” state due to the suppression of an intramolecular charge transfer (ICT) process. The excited state energy was mostly released through nonradiative decay. However, upon uptake by tumor cells, the intracellular GSH selectively removed the cage group (DNBS) via nucleophilic substitution, resulting in the generation of SO2. Simultaneously, the active PS 73-OH was released, leading to the production of cytotoxic 1O2 and concomitant red emission for fluorescence imaging-guided PDT. This work introduced a novel approach for designing PSs that combine PDT and SO2 therapy, demonstrating their potential in synergistic anticancer treatments.
Figure 29.
Schematic illustration of 73 for combined PDT and SO2 gas therapy. Reproduced with permission from ref (306). Copyright 2021 Wiley Intersciences.
To minimize nonspecific activation and achieve more precise PDT, Lo et al. designed and synthesized two dual stimuli-activated (cathepsin B and GSH) PSs (74),307 where two or three DNBS-caged ZnPc units were covalently connected with one or two cathepsin B-cleavable peptide linkers (Gly-Phe-LeuGly) through the click reaction (Figure 30). Due to the strong PET effect induced by DNBS moieties and significant self-quenching between ZnPc units, compound 74 (dimeric ZnPc-based PMB 1 and trimeric ZnPc-based PMB 2) was fully quenched in terms of fluorescence emission and 1O2 generation (ΦF = 0.001–0.005, ΦΔ = 0.03–0.07) in contrast to ZnPc (ΦF = 0.28, ΦΔ = 0.56). In the presence of both stimuli conditions (GSH and cathepsin B), or upon internalization into A549 and HepG2 cancer cells, compound 74 was activated to release free phthalocyanine via the cleavage of the peptide linkage and subsequent removal of the DNBS moieties, realizing 80–87% fluorescence recovery and efficient ROS generation, as a result of disaggregation of the photosensitizing units and the disappearance of the PET effect. When A549 and HepG2 were pretreated with 74, followed by light irradiation (λ > 610 nm, 23 mW cm–2, 28 J cm–2), a substantial decrease in viability of both cell lines with low IC50 values of 0.21–0.39 μM was observed. Dual cathepsin B and GSH-responsive PSs offer a promising approach for increasing the specificity of the photodynamic action.
Figure 30.
Schematic illustration of 74 for dual stimuli-activated (cathepsin B and GSH) PDT. Reproduced with permission from ref (307). Copyright 2021 American Chemical Society.
2.2.2.2. Disulfide Exchange
The disulfide bond is a thiol-responsive group that has been widely used as a linker in the fabrication of “smart” therapeutic prodrugs or reduction-sensitive drug delivery systems.308−310 Mitochondria targeting is recognized as an effective strategy to enhance cancer phototherapy, as intracellular mitochondria are more susceptible to cytotoxic 1O2 than other organelles. Taking this into consideration, Zhang et al. developed a GSH-activatable and mitochondria-targeted pro-photosensitizer (75) by covalently linking two meso-amine-substituted cyanine moieties with a disulfide bond (Figure 31). This pro-photosensitizer was then encapsulated within an ultrasensitive pH-responsive polymer to form D75-loaded nanoparticles (76).311 After being endocytosed into cancer cells, 76 dissociated in endosomes, releasing a large quantity of encapsulated D75. The released D75 successfully escaped from endosomes due to the “proton sponge” effect caused by the presence of diisopropyl-substituted tertiary amines. In the cytoplasm, the free D75 was cleaved by abundant intracellular GSH, forming a thiolate-substituted new cyanine. This new compound specifically concentrated in the mitochondria of tumor cells and induced the generation of 1O2 under 808 nm laser irradiation. MTT results showed that 76 exhibited selective phototoxicity against tumor cells compared to normal cells and significantly enhanced PDT efficacy by depleting intracellular GSH. In conclusion, the GSH-activatable and mitochondria-targeted nano PS, based on covalently linked cyanine dyes, offers a new approach for precise tumor phototheranostics.
Figure 31.
Schematic illustration of GSH-activatable photoactivity of D75. Reproduced with permission from ref (311). Copyright 2019 American Chemical Society.
Compared with conventional PSs that often have undesired aggregation-caused quenching (ACQ) effects, the aggregation-induced emission fluorogens (AIEgens) developed by Tang’s group exhibit desirable properties such as a large Stokes shift, high brightness, and good photostability. This opens a new approach for designing bright PSs.312−314 Recognizing these advantages, Kim et al. reported a GSH-activatable PS based on AIE technology. In this design, a ferrocene unit was attached to vinylpyridinium-substituted tetraphenylethylene (77) through a disulfide bond.315 As shown in Figure 32, compound 77 undergoes a photoinduced electron transfer (PET) process between the dye and the ferrocene subunits, resulting in quenching of the excited states and blocking of fluorescence and 1O2 generation (ΦΔ = 2.4%). When the disulfide bond is cleaved by GSH, the PDT-active compound 78 is released, leading to enhanced fluorescence and activated 1O2 generation (ΦΔ = 21.3%). Additionally, 78 exhibits cancer cell-specific imaging and induces apoptosis after irradiation, an effect attributed to the higher concentrations of GSH in cancer cells. This work presents a novel smart PS that combines the characteristics of GSH activation and aggregation-induced emission for imaging-guided PDT.
Figure 32.
Structure of 77 and the proposed mechanism of GSH activation of PDT. Reproduced with permission from ref (315). Copyright 2020 Royal Society of Chemistry.
Numerous studies have shown that dual-mode therapy such as combined chemo- and phototherapy exhibits better anticancer effects than monotherapy, even producing synergistic effects.316−320 However, the simple combination of conventional chemotherapeutics and “always on” PSs often causes strong side effects on normal tissues due to the lack of targeting. To solve the problem, Sun et al. reported a “pro-drug-PS” agent (79), where both the cytotoxicity of the drug (CPT) and the photodynamic activity of MB were blocked by a GSH-activatable disulfide linker (Figure 33).321 Upon being taken up by cancer cells, the enhanced GSH concentration inside cells cleaved the S–S bond to release both CPT and MB, resulting in simultaneous activation of chemical toxicity and photosensitivity. Under a very low laser power density (660 nm, 1 mW cm–2), compound 79 could trigger severe apoptosis and markedly enhanced cytotoxicity toward cancer cells compared to monotherapy, thus revealing a synergetic chemo-photodynamic killing effect. Moreover, in a 4T1-bearing tumor mouse model, 79 exhibited excellent tumor-activatable performance with negligible toxic side effects. This work suggests an intelligent strategy for improving the specificity of targeted PDT and minimizing toxic side effects.
Figure 33.

MB and CPT release mechanism of the activable 79 by GSH. Reproduced with permission from ref (321). Copyright 2022 Elsevier Ltd.
2.2.2.3. Nucleophilic Substitution Reaction
Owning to the strongly reductive power of GSH, electron-deficient pyridine groups are theoretically sensitive to GSH, suggesting that the latter can be used as a recognition site to design GSH-activated PSs. As a proof-of-concept, Zhao et al. designed a class of GSH-activatable phosphorescent iridium(III) complexes bearing various types of benzylpyridiniums (Figure 34).322 By tuning the electron-donating abilities of the pyridinium scaffold, a PET process occurred between the iridium(III) core and the electron-deficient pyridinium, which limited the energy transfer from the excited state of the Ir complex to the ground state of oxygen to generate 1O2, and at the same time facilitated a GSH-induced nucleophilic substitution reaction. In the case of 80, electron transfer from GSH to the positive nitrogen of pyridinium enhanced the susceptibility toward hydroxide nucleophilic attack and promoted the occurrence of a substitution reaction by hydroxide to form 81, thus resulting in irreversible changes of spectral properties, the fluorescence lifetime, and the PDT effect of this PS. For example, after the addition of an aqueous GSH solution (20 μM), the phosphorescence of 80 was gradually blue-shifted (from 627 to 586 nm) accompanied by a 12.5-fold enhancement of emission intensity and an extended emission lifetime (from 84.7 to 690.5 ns). More importantly, the abundant GSH in tumor cells inhibited the intramolecular PET process of 80, achieving GSH-dependent phototoxicity. Hence, this novel Ir complex-based PS can not only selectively distinguish cancer cells by luminescence or lifetime imaging but also amplify PDT effects in cancer cells, providing a new avenue for the design of a smart, responsive theranostic platform. This work demonstrated an effective design strategy for smart GSH-activatable PSs, providing guidance for the exploitation of near-infrared light PSs with a GSH-specific response in the future.
Figure 34.

Chemical structures of 80 and 81, and the process of GSH-activatable PDT.
In contrast to single-stimuli-activatable PSs that often encounter nonspecific activation and may even result in false positive signals in complex environments, smart PSs activated by multiple combined stimuli can provide robust and precise therapy. For this purpose, Yang et al. developed a pH and GSH coactivatable supramolecular PS (82-PAE NPs) using the “dual lock-and-key” strategy by encapsulating a GSH-activatable PS 82 in a pH-responsive diblock copolymer poly(ethylene glycol)-poly(β-amino ester) (PEG-PAE) (Figure 35).323 In normal tissues, the aggregation of 82 in polymeric micelles results in quenched excited states. When PEG-PAE was decomposed by the low pH in the TME, compound 82 was released and quickly reacted with GSH to form a water-soluble sensitizer (83) through nucleophilic substitution. The resulting hydrophilic 83 avoided the ACQ issue of conventional BODIPY dyes and possessed a 70 nm redshift in the fluorescence spectrum due to GSH substitution, which allowed for a good spectral overlap with the absorption spectrum of BI, enabling efficient FRET. Thus, an improved light harvesting and 1O2 production ability was achieved simultaneously, and the 1O2 yield of 83 is even better than that of commercial PS Ce6. In vivo results revealed that 82-PAE NPs can be rapidly enriched in cancer cells and can selectively “light up” tumors, further causing irreversible cytotoxicity to tumors without influence on normal tissues. This work represents the first example of a GSH-activated PS based on the mechanism of aromatic nucleophilic substitution.
Figure 35.
Schematic illustration for the fabrication of BIBCl-PAE NPs, and the processes underlying “dual lock-and-key” strategy in a tumor microenvironment to achieve activated and enhanced generation of 1O2. Reproduced with permission from ref (323). Copyright 2020 The Royal Society of Chemistry.
2.2.2.4. Cyclization with Cyano Groups
The CN group can recognize the thiol of GSH to form a thiazole ring via a Michael addition. Based on this reaction, Dong et al. designed and synthesized two kinds of GSH-responsive pyrrolopyrrolidone (DPP) derivatives bearing with different regio-isomers of the CN group, 84 (4-CN groups) and 85 (2-CN groups), which further assembled into corresponding nanoparticles 84 NPs and 85 NPs, via the nanoprecipitation method (Figure 36).324 Both can react with GSH to form a thiazole through the Michael addition, realizing the colorimetric GSH detection without aggregation-induced fluorescence quenching, as well as enhanced PDT/PTT efficacy. Interestingly, 84 NPs showed a higher 1O2 quantum yield (22.3% vs 12.5%) and photothermal conversion efficiency (45.2% vs 34.5%) than 85 NPs. Thus, 84 NPs exhibited a better therapeutic efficacy than 85 NPs even at a low dose (0.2 mg kg–1) without adverse effects.
Figure 36.
Illustration of GSH-responsive DPP derivatives (84, 85) for imaging-guided phototherapy. Reproduced with permission from ref (324). Copyright 2019 The Royal Society of Chemistry.
2.2.2.5. GSH Mediated Reduction
To achieve efficient tumor enrichment and cell uptake PSs that exhibit a switch in polarity from a hydrophobic inactive state to a hydrophilic active state are highly desirable. To this end, Kim et al. reported a GSH-activatable PDT agent (86), where a GSH-sensitive moiety (a ubiquinone analogue) was covalently linked to a near-infrared PS (a meso-ester-2,6-iodinated-BODIPY) via an ester linkage (Figure 37).325 Compound 86 is strongly hydrophobic and easily aggregates to form nanoparticles in aqueous solutions. Not only does this result in quenched photosensitivity and minimal systemic toxicity, but this also favors tumor enrichment through the EPR effect. Upon endocytosis by cancer cells, the ubiquinone moiety was rapidly reduced by GSH to produce the corresponding ubiquinol that underwent a spontaneous elimination reaction to release the anionic 87 and further induced the deaggregation of 86 NPs. The conversion of the aggregated hydrophobic precursor (86) into the photoactive hydrophilic 87 resulted in a “Turn-On” of the fluorescence and 1O2 generation (87, ΦΔ = 0.79), enabling the selective photodynamic killing of cancer cells and the ablation of the tumor guided by fluorescence imaging.
Figure 37.
Chemical structure of 86 conjugate and conversion to 87 under reductive conditions. Reproduced with permission from ref (325). Copyright 2019 The Royal Society of Chemistry.
2.2.2.6. Michael Additions
Like other biothiols, Cys plays critical roles in physiological processes, including redox homeostasis and as an antioxidant. Elevated levels of Cys are directly associated with different pathogenic states, such as cancers and neurodegenerative disorders. Selective imaging of intracellular Cys fluxes by specific fluorescence probes has attracted a lot of attention. However, Cys-activated PSs are quite rare, and to the best of our knowledge, only two cases have been reported so far. Kolemen et al. modified the dicyanomethylene-4H-chromene (DCM) core with a heavy iodine atom at different positions and investigated two PSs (88a, 88b) (Figure 38).326 It was found that the presence of the iodine atom by itself is not enough to obtain a high 1O2 generation yield, but the location of iodine is highly critical. 88a with the iodine atom on the phenolate ring exhibited a much higher 1O2 quantum yield (ΦΔ = 5.2%) than 88b (ΦΔ = 0.6%) where the iodine was placed on the chromone ring of the DCM core. Based on this result, they developed a Cys-activatable PS (88c) by masking the phenol of 88a with a Cys-responsive unit (acrylic ester). Probe 88c could be selectively activated by endogenous Cys, resulting in significant photocytotoxicity to HeLa cells (with high intracellular Cys concentrations) as compared to L920 cells (with low intracellular Cys concentrations), along with a “Turn-On” fluorescence output. In the same year, Kolemen et al. also developed a Cys-responsive pro-PS based on a chlorinated hemicyanine dye.327 Theranostic probe 89 exhibited a significant “Turn-On” NIR fluorescence and enhanced 1O2 generation efficacy (ΦΔ = 1.8%) as well as photothermal conversion (η = 69%) after activated by Cys, triggering significant cancer cell death and causing no harm to normal cells. Overall, the same research group contributed two key cysteine-activated PSs and introduced a new gateway for cancer cell-specific therapeutics triggered by Cys.
Figure 38.
Structures of theranostic probes 88a, 88b, 88c, and 89.
2.2.3. ROS/RNS/H2S-Activatable Theranostic Probes in PDT
Reactive oxygen/nitrogen species (ROS/RNS) are the natural byproducts of metabolism in living organisms.328−330 These chemically reactive substances play critical roles in cellular signal transduction and the maintenance of intracellular redox homeostasis.331−333 Due to the distinctive metabolic capacity between cancerous and normal cells, elevated levels of ROS/RNS have been discovered in cancer cells, as compared to their normal counterparts, suggesting a tight relationship between intracellular ROS/RNS and the occurrence and development of cancers.334−338 Moreover, there is accumulating evidence that the vast production of ROS/RNS inside the cancer cells can also lead to various kinds of cell death.339 Therefore, the exploration of specific ROS/RNS-responsive PSs represents an indispensable direction for the development of innovative cancer-targeting phototheranostics.
2.2.3.1. H2O2-Activatable Theranostic Probes in PDT
The exploration of stimulus-responsive PSs that utilize an oxidative activation strategy has gained increasing research interest.339−341 In particular, regarding the relatively higher chemostability and significantly larger concentration of H2O2 (5 μM to 1.0 mM) in tumorous cells, versus healthy cells,335 a vast number of H2O2-activatable phototheranostics with different types of PSs and diverse responsive mechanisms has been successfully constructed. For example, Takahashi and Toshima et al. studied a natural pigment hypocrellin B-derived pro-PS featuring an arylboronic ester (90, Figure 39) for the specific photodestruction of H2O2-overproducing cancer cells.342 Interestingly, upon modification of the two hydroxy groups of hypocrellin B with two H2O2-cleavable phenylboronic esters, the caged hypocrellin B mainly absorbed in the UV–vis region, and its photosensitizing ability was substantially attenuated. After the specific removal of phenylboronic esters by H2O2, the PS displayed a red-shifted absorption band in the phototherapeutic window (600–900 nm), and its 1O2-generating ability was restored. Additionally, light-initiated protein-destruction assays indicated a more pronounced degradation of BSA by hypocrellin B rather than its boronic ester-caged form, confirming the tunability of the photosensitizing ability. Importantly, photocytotoxicity assays suggested that the caged PS showed a considerably higher photocytotoxicity to H2O2-overexpressing cancerous cells (B16F10) than to normal WI-38 cells under 660 nm-light irradiation conditions, which emphasized the feasibility of designing innovative H2O2-activatable phototheranostics to reduce side effects for cancer-selective PDT.
Figure 39.
Activation of 90 by H2O2. Reproduced with permission from ref (342). Copyright 2022 Royal Society of Chemistry.
To improve tumor-targeting capabilities, Ding and Zhang et al. fabricated a dual-targeting nanophototheranostic consisting of a phenylboronic ester-modified methylene blue-based pro-photosensitizer (91, Figure 40) and biodegradable BSA for tumor-targeted imaging and PDT.343 Upon intravenous injection into a HepG2 cell-bearing tumor mouse model, this nanomedicine displayed specific accumulation in the tumor region via the notable EPR effect, and a selective deboronation reaction triggered by elevated levels of H2O2 in tumorous cells not only switched on the MB fluorescence but also restored its photosensitizing properties. Additionally, the side product, quinone methide, could rapidly and irreversibly deplete intratumoral GSH, leading to amplified oxidative stress, which synergized with the PDT effects. Notably, as MB is a clinically approved PS and the above-mentioned nanomedicine would rapidly degrade to biocompatible small molecules that can be readily excreted, this nanophototheranostic has good potential for future clinical translation.
Figure 40.
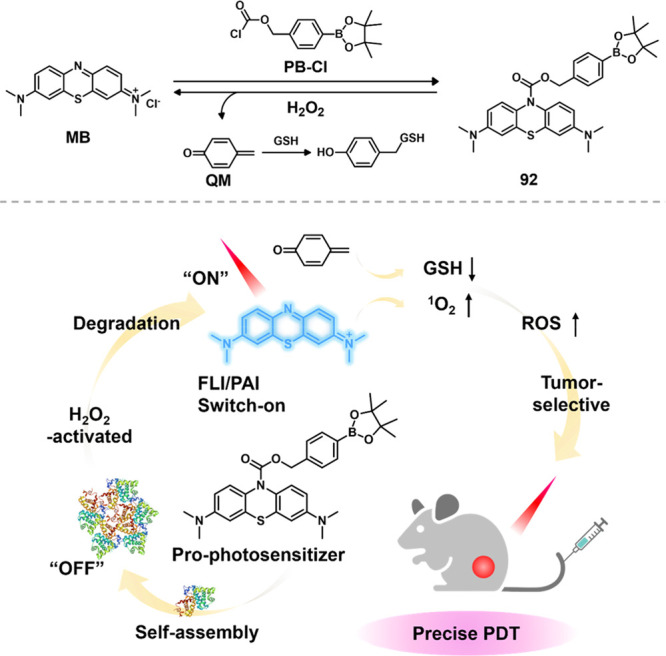
Synthesis of 91 for H2O2-activated bioimaging and amplified PDT via GSH depletion. Reproduced with permission from ref (343). Copyright 2019 Elsevier Ltd.
Mitochondria play indispensable roles in anticipating cellular bioenergetic and metabolic activities. Innovative PDT agents that effectively induce mitochondrial dysfunction are highly attractive for cancer therapy.344 In this context, Feng and Wang et al. reported that the iodinate indolium (92), a common starting material for the synthesis of cyanine dyes, is capable of generating an iodinated trimethine cyanine dye (93, Figure 41) upon reaction with ROS, such as H2O2 and •OH.345 Importantly, as the TME features a higher concentration of ROS, and the cationic nature of 93 allowed its efficient accumulation in mitochondria, the intracellular ROS initiated the in situ generation of a cyanine-based bioimaging reagent and PS in living cancer cells. Moreover, the heavy atom effects rendered 93 a potent PS generating 1O2, resulting in remarkable photodamage of HeLa cells under light irradiation. This intracellular ROS-responsive in situ synthesis of a phototheranostic agent offers an exciting new avenue for preferential cyanine expression and PDT in tumors.
Figure 41.

In situ generation of 93 via reaction with mitochondrial ROS for PDT. Reproduced with permission from ref (345). Copyright 2021 Wiley Intersciences.
Luminescent transition metal complexes are considered promising alternatives to organic phototheranostics due to their competitive photophysical properties and excellent photosensitizing abilities.346 Transition metal complex-based PSs are gaining interest in PDT, with one of them already undergoing clinical trials.347 To take advantage of these benefits, Chao et al. developed a phenylboronic ester-decorated cyclometalated iridium(III) prodrug (94a, Figure 42). This prodrug was then encapsulated into selenium nanoparticles and cancer cell membranes to enhance cancer targeting, membrane permeability, and pharmacokinetics. Selenium nanoparticles were chosen as nanocarriers for their drug-loading capacity and biocompatibility. Inside cancer cells, selenium nanoparticles decompose, releasing selenium, which disrupts mitochondrial function and induces cell apoptosis. In the presence of intracellular H2O2, the nanoformulation rapidly decomposes, resulting in the release of an iridium(III) PS (94b), a GSH scavenger (quinone methide), and a chemotherapeutic agent (selenium nanoparticles). Under two-photon irradiation (730 nm), the generated 1O2 works synergistically with the reduced intracellular GSH and the chemotherapeutic effect of selenium nanoparticles, effectively eradicating a melanoma tumor in a mouse model. This combined PDT and chemotherapy strategy opens new possibilities for designing intelligent phototheranostics for cancer therapy.
Figure 42.
Chemical structures of theranostic probes 94a and 94b.
The emergence of molecular logic has led to active research in developing multiresponsive fluorescent probes that can react to two or more distinct substrates.348 Dual-activatable theranostic agents are typically highly selective toward cancer cells.349 Feng and Wang et al. presented a coactivatable phototheranostic probe (95, Figure 43) that can be activated by both H2O2 and GSH, specifically targeting mitochondria and enabling tumor-specific PDT.350 Notably, probe 95 did not react with GSH alone, even with its high concentration in mitochondria. It required the sequential action of intratumoral H2O2 and GSH to activate, thus forming an efficient PS that avoids nonspecific activation by other redox-active biomolecules. The activated PS demonstrated the simultaneous generation of both 1O2 and superoxide radicals (•O2–) for PDT, helping alleviate the hypoxic microenvironment in solid tumors. This is because type I PSs are significantly less oxygen-dependent. Additionally, the PS could be excited by two-photon irradiation (808 nm), overcoming the limitation of poor penetration depth associated with short excitation wavelengths. This research emphasizes the utilization of the tumor microenvironment as a stimulus for the activation of dual-locked phototheranostics.
Figure 43.
Chemical structure of 95 and an illustration of its activation mechanism.
The limited penetration depth of the excitation light source for PSs is one of the major obstacles to deep tissue PDT. NIR light penetrates deeper but is still limited to only a few centimeters.351 To solve this problem, Xu and An designed a self-illuminating unimolecular nanoparticle for luminescence imaging of inflammation and tumor PDT in vivo via a luminol-modified and PEG-tethered Ce6 PS (96, Figure 44).352 The PS self-assembled from an amphiphilic polymeric conjugate to appropriate-sized nanoparticles. Interestingly, the increased H2O2 concentration and corresponding activating enzymes in the TME initiated luminol bioluminescence, which was subsequently absorbed by the Ce6 unit to trigger the generation of 1O2 through efficient bioluminescence energy transfer, facilitating in vivo imaging and deep tissue PDT, respectively. As this chemiluminescence energy transfer-based phototheranostic system does not require the use of an external light source, it is considered a more favorable therapeutic modality compared to traditional PDT.
Figure 44.
Chemical structure of 96 and an illustration of its activation mechanism. Reproduced with permission from ref (352). Copyright 2019 Science Publishing Group.
2.2.3.2. ClO–/HClO-Activatable Theranostic Probes in PDT
Hypochlorite/Hypochlorous acids (ClO–/HClO), which are enzymatically produced by the intracellular enzyme myeloperoxidase, play essential roles in living organisms and are closely associated with many pathological processes, such as bacterial infection, cardiovascular and neurodegenerative diseases, arthritis and cancer.353 A higher level of ROS including ClO–/HClO facilitates oncogenic transformation and drives the cancer cells to proliferate.328 The basal ClO–/HClO levels in cancerous cells are estimated to reach ca. 10 nM,336 implicating that facile cancer-targeting might be realized by ClO–/HClO-activatable phototheranostic agents. To employ this strategy, Wang and Zhang et al. reported monocomponent nanodots with lysosome-localizing properties to specifically visualize and enable PDT of cervical tumors.354 Interestingly, these Pluronic F127-encapsulated nanodots featuring an AIE-active phenothiazine derivative (97, Figure 45) could respond ratiometrically to elevated ClO–/HClO levels in tumorous tissues, which gave rise to the production of an increased level of 1O2 (nearly 2-fold more efficient than commercial Rose Bengal) and elicited strong photocytotoxicity (IC50 < 7.6 μM). Furthermore, bioimaging studies implicated that these nanodots could be used to visualize the fluctuation of ClO–/HClO in human cervical tissues. This research represents a pioneering ClO–/HClO-responsive nanodrug for the PDT treatment of inflammation-related tumors without causing severe side effects.
Figure 45.
Schematic of synergistic dual-stimuli-regulated 97 by HClO and H+ andfluorescence imaging of cervical cancer tissue and paracancer tissue pretreated with TPE-PTZ-Py and TPE-PTZO-Py, respectively. Red channel: λex = 425 nm, λem = 635–675 nm; yellow channel: λex = 425 nm, λem = 505–545 nm (laser power = 2 mW). Reproduced with permission from ref (354). Copyright 2022 American Chemical Society.
To date, numerous ClO–/HClO-responsive fluorogenic probes have been developed and applied for both in vivo and in vitro imaging studies. However, ClO–/HClO-responsive PSs that integrate both type I and II photosensitizing mechanisms remain rarely explored. In one recent example, Tang and Han et al. reported a mitochondria-targeted and bioimaging-enabled PDT agent by using an AIE-based PS (98, Figure 46) that is specifically activated by intratumoral hypochlorite (ClO–).355 Intriguingly, PS 98 displayed type II photosensitizing features before reacting with ClO–, predominantly generating 1O2 upon irradiation, while upon converting to its oxidized counterpart, bright emission accompanied by type I radical generation was observed. Further studies suggested that PS 98 was able to differentiate tumorous cells from normal cells, rendering this compound a promising phototheranostic agent for tumor-seeking PDT.
Figure 46.
Chemical structure of 98 and activation by ClO–. Reproduced with permission from ref (355). Copyright 2023 Elsevier Ltd.
HClO-activated PDT has also been applied to antibacterial studies. Liu and Tang et al. formulated an HClO-activatable nanomedicine that consists of an AIE-type PS (DTF, 99a) and an HCIO-responsive probe (FFP, 99b, Figure 47) to detect and treat bacterial inflammatory diseases in vivo.356 The nanoformulation (99) was easily prepared by encapsulating 99a and 99b into Pluronic F127. The coexistence of these two substances leads to a quenched photosensitizing ability, owing to the efficient Förster resonance energy transfer process between 99a and 99b. However, upon oxidative destruction of the phenothiazine moiety by endogenous HClO, 99b displayed a dramatic decrease in both absorption and emission intensities, while the emission of 99a remained unchanged, contributing to the disruption of the energy transfer process and the recovery of the photoinduced 1O2 generation. Physicochemical characterization revealed that nanoparticles (99) were highly selective toward HClO over other biological ROS. This nanomedicine has been successfully applied to the tracking of the infection site, which enables in vivo antibacterial PDT. Furthermore, the inhibitory effect of the nanoparticles against S. aureus could last up to 7 days, which was remarkably longer than that of the common antimicrobial drug vancomycin, which only exhibited an antimicrobial effect during 72 h. In conclusion, this HClO-stimulated phototheranostic nanoformulation with tunable luminescence and photosensitizing behavior offers a desirable approach toward infection-origin nanotheranostics.
Figure 47.
Preparation of 99 NPs and their fluorescence and photosensitization activation by HClO at the bacterial inflammatory site for effectively imaging and ablating the bacteria inside invaded phagocytes. Reproduced with permission from ref (356). Copyright 2020 Wiley Intersciences.
2.2.3.3. NO-Activatable Theranostic Probes in PDT
An elevated NO concentration in cancer tissues is usually produced by the enzymatic reaction of intracellular guanidyl-containing substrates and endogenous inducible nitric oxide synthase (iNOS),337,338 which has been utilized as an initiator to activate anticancer phototherapy.357 NO plays crucial roles in many inflammation-associated diseases.337,338,358 Considering these points, Fan et al. developed the first example of a NO-activatable and two-photon excitable fluorogenic pro-PS (100, Figure 48) for bioimaging and PDT applications.359 Upon reaction with NO in cancerous cells, 100 displayed not only a markedly improved emission quantum yield (more than 50-fold higher) and a photoinduced 1O2 generation quantum yield (up to 82%) but also an exponentially increased TPA cross-section (up to 2800 GM), providing proof-of-concept support for bioapplications of NO-triggered two-photon fluorescence imaging and PDT in lipopolysaccharide and interferon-γ coactivated macrophages. Regarding the fact that inflammation predisposes tissues to develop cancer and facilitates the tumorigenic process, the potential of NO-activatable cancer phototheranostics has not been fully exploited, and we anticipate that more intricate NO-activatable PSs will be developed for the treatment of inflammatory cancers in the future.
Figure 48.
Molecular structure and activation of 100 by NO and schematic illustration of the NO-activatable 100 for TP-imaging and TP-PDT in an activated macrophage (in which NO is upregulated). Reproduced with permission from ref (359). Copyright 2018 Royal Society of Chemistry.
2.2.3.4. Peroxynitrite-Activatable Theranostic Probes in PDT
Peroxynitrite (OONO–) refers to a highly oxidative RNS generated by •O2– and NO and is a signal-transducing molecule that plays diverse roles in cellular processes. OONO– has been regarded as a crucial biomarker for many cancers. Consequently, Li et al. explored a proof-of-principle design, based on inhibiting the photosensitizing process, to yield a pro-PS whose photosensitivity could be restored from a suppressed state after reacting with this specific biomarker. A mitochondria-anchoring long-wavelength excitable pro-PS (101, Figure 49) with highly efficient activable photosensitivity for the production of 1O2 was developed.360 Protecting the phenol hydroxy moiety by an OONO–-cleavable functional group offered a simple method for designing smart PSs. A clear activation of the photosensitizing ability of 101 by raised OONO– concentrations in living cancer cells was demonstrated. To further improve the solubility of the PS, several PEG chains with a mitochondria-targeting moiety were introduced. Superior bioimaging performance as well as an outstanding PDT efficiency in tumor-related RAW 264.7 cells was observed.
Figure 49.
Chemical structure of 101 and schematic illustration of the proposed PS activation strategy. Reproduced with permission from ref (360). Copyright 2019 Wiley Intersciences.
Manipulating the molecular packing modes of PSs is a facile and efficient way to optimize their photosensitizing performance. Based on this, Li et al. proposed an effective approach for the preparation of biocompatible J-aggregated nanoparticles by integrating amphiphilic polymeric micelles and an OONO–-activatable iodinated BODIPY dye (102, Figure 50).361 Dye 102 tends to self-assemble into stable plate-like core–shell nanoarchitectures in PEG-PCL-derived polymeric micelles. Interestingly, these nanoassemblies displayed an intrinsic OONO– responsiveness as a result of the phenylboronic ester-modified meso-carboxylate moiety, which could be effectively detached upon the reaction with endogenous OONO–, resulting in reassembly from J-aggregates to amorphous nanospheres, by virtue of the exposure of the generated negative charges. Accordingly, the photoinitiated 1O2 generation of the nanoparticles was turned on, which was further employed for the selective photoeradication of tumor-associated macrophages, expressing elevated OONO– under the influence of LPS and INF-γ. In consideration of the easy access and simple activating principle, this research opens up a new avenue for developing stimuli-responsive phototheranostics that meet clinical needs.
Figure 50.
Activation of 102 by OONO–. Reproduced with permission from ref (361). Copyright 2019 American Chemical Society.
Persistent luminescent materials possess great potential for bioimaging and phototherapeutic applications, owing to their unique ability to retain photon-releasing properties after the removal of incident light exposure or to exploit certain chemical reactions to fuel chemiluminescence. Luminescence can minimize the interference of tissue autofluorescence and thus holds a significantly higher signal-to-noise ratio, which is preferable for biomedical applications. Nevertheless, the clinical application of persistent luminescent materials still suffers from some severe disadvantages, such as undesired cytotoxicity, synthetic difficulties, and unsatisfactory excitation/emission wavelengths. To address these issues, Ding et al. contributed a phototheranostic prodrug nanoassembly (103, Figure 51) that not only exhibited self-reporting drug-release behavior but also prominently stimulated immunogenic cell death processes upon activation of afterglow emissions in the near-infrared region.362 The nanoassemblies are fabricated by sealing an ONOO–-activable afterglow prodrug 103 and an AIE-active PS with NIR emission into DSPE-PEG2000 nanoparticles. To maximize the photocytotoxity, the nanoassemblies were first photoirradiated to produce certain amounts of 1O2, which reacts with the prodrug to afford an afterglow-active 1,2-dioxetane precursor, that in turn is activated in the presence of endogenous ONOO–, triggering the release of the anticancer drug hydroxycamptothecin and initiating the afterglow luminescence. Moreover, the activatable afterglow luminescence continues to excite the AIE-based PS via resonance energy transfer to give NIR emission and large amounts of 1O2, which amplifies the anticancer effects. Additionally, the released anticancer drug hydroxycamptothecin could sensitize the “cold” tumor to photochemotherapy. Further studies revealed that these nanoassemblies can eradicate tumors and prevent tumor recurrence.
Figure 51.

Chemical structure of 103 and schematic showing the AIE/B-AGL-HCPT NPs fabrication and ONOO–-mediated activation of drug release and NIR afterglow luminescence within preirradiated AIE/B-AGL-HCPT NPs. Reproduced with permission from ref (362). Copyright 2022 Wiley Intersciences.
2.2.3.5. H2S-Activatable Theranostic Probes in PDT
Hydrogen sulfide (H2S), a well-known nucleophile and reductant, is noted for its gasotransmitter role that involves numerous biological processes.363 Accumulating evidence points to increased concentrations of H2S (ca. 0.3–3.4 mM) in cancer cells, in particular, colon cancers.364 However, due to a complicated TME and the competitive cross interference by cellular GSH (ca. 0.5 to 10 mM), specific phototheranostics that only show a response to H2S remain a considerable challenge. One example of a selective phototheranostic was described by Yang et al. The authors discovered that self-assembled nanoaggregates with dense molecular packing would only allow small molecules like H2S to diffuse inside and could prevent unwanted interference from GSH, which is too large in size, thus realizing H2S-specific activation of a phototheranostic.365 A nitrophenyl ether-attached BODIPY derivative (104, Figure 52) has been designed and self-assembled in PF127 micelles to form orderly packed J-aggregates with a particle size of approximately 120 nm. Upon treatment with H2S, the blue absorption at around 623 nm gradually diminished together with a bathochromic shift in fluorescent emission, which could be attributed to the nucleophilic attack of the nitrophenyl ether by H2S, resulting in double −SH-substituted BODIPYs. Subsequent bioimaging and photocytotoxic studies have confirmed the effective responsiveness of the nanoassemblies toward endogenous H2S. Under light irradiation, the nanoassemblies exhibited significantly higher cytotoxicity with lower IC50 values against HeLa cells compared to dark controls. This represents the first example of applying J-aggregated pro-PS for H2S to achieve ultrahigh specificity over other biothiols.
Figure 52.
Schematic illustration of the insufficient H2S selectivity of free 104 and self-assembly enhanced selectivity toward H2S of templated self-assembly of 104. Reproduced with permission from ref (365). Copyright 2022 Royal Society of Chemistry.
Supplementing nitrophenyl ethers as H2S-sensitive triggers, Shi et al. reported on a nitrophenyl carboxylic ester modified π-extended heptamethine cyanine derivative (105, Figure 53), which showed an unprecedented NIR-II to NIR-I fluorescent response (F1070 → F720) and photoacoustic response (PA680/PA900) to nucleophilic attack by H2S, providing an exceptional opportunity for imaging the H2S content in various disease models, including tumors in living mice.366 To apply 105 to biomedical applications, it was self-assembled into polymeric nanoparticles using mPEG5000-PCL3000 as a stabilizer and mPEG5000-PCL3000-FA as a cancer-targeting group. The nanoparticles retained a similar H2S responsiveness and demonstrated improved 1O2 photogeneration ability upon H2S activation. In vivo studies revealed an H2S-activatable photocytotoxicity toward colorectal HCT116 cancer cells and subcutaneous tumors, making these nanoassemblies promising for treating cancers with elevated H2S levels.
Figure 53.
Fabrication of ZNNPs@FA and the principle of quantitative visualization of H2S are indicated by 105. Reproduced with permission from ref (366). Copyright 2022 The Author(s).
As noted above, H2S is also a strong reductant, a property that has also been exploited for specific H2S probes. Azido-containing aromatics are among the most investigated H2S-responsive sensory moieties. Based on an azido phenyl ether-functionalized phenoxazine dye, Gunbas et al. developed an H2S-activatable iodinated resorufin pro-PS (106, Figure 54) for the photodynamic treatment of neuroblastoma-derived cancer cells.367 Pro-PS 106 turned out to be an excellent bioimaging reagent and phototherapeutic because it can selectively switch on its red emission at 606 nm and has good photoinduced 1O2 generation ability (ΦΔ = 0.42) upon reaction with endogenous H2S. Surprisingly, 106 displayed markedly high photocytotoxicity (IC50 = ca. 3.29 μM) to H2S-overexpressed human neuroblastoma cells (SH-SY5Y) but was minimally photocytotoxic (IC50 > ca. 3.29 μM) to H2S-lacking noncancerous fibroblast cells (L929). One of the main drawbacks of this study may be the short excitation wavelength (<650 nm), which provides limited penetration depth in tissues. On the other hand, designing NIR-absorbing resorufin derivatives could overcome this issue.
Figure 54.
Activation of theranostic probe 106 by H2S.
2.2.3.6. Bio-orthogonal-Activatable Theranostic Probes in PDT
The past two decades have seen exponentially increasing research interest in the exploration of bio-orthogonal chemistry for bioimaging and/or biomedical applications.304,368−371 Differing from conventional bioimaging reagents, bio-orthogonal theranostics typically involve a two-step labeling process: (1) living cells/organisms are first tagged with unique substrates with high bio-orthogonal reactivity, which can be metabolically anchored on certain parts of cells or organisms; (2) subsequently, the anchored substrates are specifically recognized and covalently integrated with another complementary bio-orthogonal luminogen. This two-step labeling approach not only offers extremely high spatial and temporal resolution but also allows facile visualization of certain subcellular organelles that can hardly be accomplished by traditional dyes. With respect to PDT, bio-orthogonal chemistry has been reported to be able to improve the cancer-targeting specificity and decrease the dark cytotoxicity and undesirable adverse effects of PSs. For example, Vázquez et al. studied the application of a bio-orthogonal activation strategy for PDT to realize tunable organelle-specificity and controllable photocytotoxic effects.372 An iodinated BODIPY featuring a tetrazine unit as a quenching group (107, Figure 55) was optimized to display maximal activation efficiency when reacted with a vinyl-bearing substrate. Notably, the bio-orthogonal activation not only turned the 1O2 generation quantum yields but also triggered a substantial photocytotoxic effect on the subcellular nucleus by metabolically preintegrating 5-vinyl-2′-deoxyuridine into the nuclear DNA. These gratifying results reinforced the feasibility and extended our vision toward various bio-orthogonal reaction pairs. Although the excitation wavelength of 107 is too short, these short wavelength-responsive probes could find applications in preclinical studies.
Figure 55.
Chemical structure of 107 and outline of the DNA-targeted bio-orthogonal strategy for activating the phototoxicity. Reproduced with permission from ref (372). Copyright 2019 Wiley Intersciences.
Instead of using organic dyes as fluorogenic probes and PSs, Lo et al. developed an interesting class of bis-tetrazine functionalized iridium(III) complexes (108, Figure 56) for biomedical applications, including luminogenic bioimaging and activatable PDT.373 Importantly, the tetrazine groups not only show remarkable bio-orthogonal reactivity but also efficiently suppress the luminescence properties of iridium(III) complexes (Φem < 0.09%). Upon reaction with strained alkynes, these complexes exhibited dramatically enhanced emission intensities (up to 2305-fold enhancement) and prolonged emission lifetimes (τ = ∼1.39 μs). Surprisingly, these complexes can also react with overdosed bis-alkynes to afford even larger enhancements in emission (up to 3885-fold brighter than the unreacted iridium(III) tetrazine complexes) and longer excited state lifetimes, which is attributable to the generation of cyclized iridium(III) complexes, as suggested by ESI-MS and RP-HPLC results. The enhanced rigidity of cyclized reaction products might account for the resulting higher emission and longer emission lifetimes, suppressing nonradiative decay and facilitating the emission process. Co-staining studies indicated that these complexes can be efficiently switched on by strained alkyne derivatives to produce an intense lysosomal emission. Remarkably, before activation, all the complexes displayed very low phototoxicity indices, indicative of minimal photocytotoxicity, while after reaction with strained alkynes to restore the photosensitizing ability (ΦΔ = 0.62 to 0.77), low IC50 values (0.45 to 1.6 μM) with enhanced phototoxicity indexes were observed. Other than iridium(III) tetrazine derivatives, Lo’s group also developed an iridium(III) nitrone complex (109, Figure 56) that can similarly interact with an alkyne-tagged protein to activate the photosensitizing process.374
Figure 56.

Chemical structures of theranostic probes 108 and 109.
Most clinically used PDT agents lack sufficient cancer-targeting specificity, which inevitably causes undesired photodamage to surrounding normal tissues.375 To improve tumor-localizing properties, PDT agents are incorporated with various cancer-targeting biomacromolecules like antibodies, peptides, aptamers, and/or small molecules that can bind specifically to overexpressed biomacromolecules in cancers.375 However, unwanted uptake of these PS-ligand hybrids by healthy cells remains unavoidable. To achieve precise targeting, Ng et al. reported a unique dual receptor-involved bio-orthogonal activation strategy to optimize the cancer-targeting specificity of PDT agents. Initially, a biotinylated tetrazine-bearing BODIPY-originated pro-PS (110, Figure 57) and an epidermal growth factor receptor (EGFR)-targeting strained alkyne were delivered into living cancer cells that overproduced both the biotin receptors and EGFR-receptors and the two complementary bio-orthogonal units are accumulated in dual-receptor-overexpressing cells.375 Once these two parts encountered each other, rapid bio-orthogonal reactions occurred simultaneously, resulting in the recovery of the photosensitizing ability. Photocytotoxicity studies against a series of different cells demonstrated that 110 only showed drastic photocytotoxic effects on A549 cancer cells that overexpress both biotin and EGFR receptors and exhibited negligibly cytotoxic effects to other cells that did not express any receptor or express only one receptor, suggesting that this dual-receptor-assisted bio-orthogonal activation approach hold promise for precise PDT in specific cancers.
Figure 57.
Schematic illustration of the dual receptor-mediated endocytosis of two bio-orthogonal partners (110 and its reaction partner) into the target cells, followed by bio-orthogonal activation via the IEDDA coupling. Reproduced with permission from ref (375). Copyright 2022 Wiley Intersciences.
Many bio-orthogonal-activable tumor-targeting methods are considered more effective in theranostic delivery than other targeting modalities since covalent conjugation results in tighter bonds to the tumor site and thus avoids clearance. Liu et al. presented the first bis-cycloalkyne-decorated AIEgen-based “Turn-On” probe (111, Figure 58) for bio-orthogonal tumor labeling and imaging-guided tumor PDT.376 Probe 111 exhibited minimal background emission in dispersed states but displayed intense fluorescence emission when cross-linked with 1,3,5-tris(azidomethyl)benzene. Notably, the burst emission was minimally affected by various surfactants and biomolecules, indicative of negligible nonspecific interactions with potential interference substances. Cellular studies manifested the applicability of probe 111 for targeted imaging of malignant 4T1 cells that were pretreated with AzAcSA, a metabolic precursor for overexpressed sialylated glycans in tumors. After cross-linking with azidoglycans on the cell membrane, the fluorescence of probe 111 was turned on rapidly, which not only allowed exclusive membrane labeling but also motivated the photosensitizing process, achieving a dose-dependent photocytotoxicity with an IC50 value of 10.4 μg mL–1. To further improve the cancer-targeting ability, Liu extended the bio-orthogonal-activation of phototheranostics by using another anionic AIEgen 112 (Figure 59) and a new precusor-cRGD-S-Ac3ManNAz,377 which is specifically bound by overexpressed αvβ3 integrin and internalized selectively in tumor cells. Moreover, before cleavage of the disulfide bond by intratumoral GSH, cRGD-S-Ac3ManNAz was unable to be metabolized on cell membranes, which significantly enhanced the tumor-targeting specificity due to elevated GSH levels in tumor cells. Cellular MTT studies revealed that when the cancerous MDA-MB-231 cells and normal 293T cells were preincubated with Ac3ManNAz with no targeting ability, nondistinguishable photocytotoxicity toward these two cell lines was observed upon bio-orthogonal activation with 112, while when cRGD-S-Ac3ManNAz was applied, a significantly higher photocytotoxicity against cancerous MDA-MB-231 cells (IC50 = 9.5 μM) resulted, compared to the IC50 values for the normal cell lines (IC50 > 9.5 μM), which confirmed the cancer-targeting efficiency. To summarize, specific bio-orthogonal activation in combination with fluorogenic AIEgen and cancer-targeting metabolic precursor provides additional niches for facile accessing of innovative phototheranostics.
Figure 58.

Chemical structure of theranostic probe 111.
Figure 59.
Chemical structure of theranostic probe 112 and confocal images of 112 (10 μM) labeled tumor cells (MDA-MB-231 and MCF-7) and normal 293T cells pretreated with cRGD-S-Ac3ManNAz for 2 h, followed by washing with 1× PBS and incubation in fresh DMEM medium for 2 days. Nucleus is stained with Hoechst 33342, λex = 405 nm, λem = 430–470 nm; 112, λex = 405 nm, λem = >650 nm. Reproduced with permission from ref (377). Copyright 2018 American Chemistry Society.
Apart from bio-orthogonal ligation, the recently developed bio-orthogonal cleavage reaction has also received increasing attention.378 By applying a cleavable nitrobenzyl isonitrile-attached BODIPY PS (113, Figure 60), Ng and Lo et al. employed a dissociative bio-orthogonal reaction for tumor-targeted activation of PSs for specific anticancer PDT applications.379 The 1O2 generation of 113 is not suppressed by the isonitrile moiety but by the nitrobenzyl quenching unit. This strategy allowed controllable activation of the PSs via isonitrile-tetrazine cycloaddition-induced removal of the nitrobenzyl quenching group. After functionalization of tetrazines with a galactose moiety or the GE11 peptide, which targets overexpressed asialoglycoprotein receptors or EGFR receptors in cancerous cells, the specific reaction between 113 and functionalized tetrazines in live cancer cells resulted in drastic fluorescence enhancement and efficient 1O2 generation. Additionally, bio-orthogonal cleavage-activated photocytotoxic effects have also been realized in vivo with satisfactory results.
Figure 60.
Chemical structure of 113 and its bio-orthogonal activation reaction. Reproduced with permission from ref (379). Copyright 2023 Elsevier Ltd.
Finally, the Staudinger reaction, converting azides to primary amines through the formation of an iminophosphorane intermediate, has drawn a lot of attention for biomedical applications owing to its rapidness, biocompatibility, and high reaction yield.380 Considering these merits, Liu prepared a π-extended long wavelength absorbing azide-decorated Se-rhodamine-derived pro-PS (114, Figure 61), which could be bio-orthogonally activated by a Staudinger reaction using triphenylphosphine.381 Interestingly, the azido-rhodamine resulted in the closure of the spiro-ring, with which the NIR emission of Se-rhodamine was completely quenched and the absorption maximum displayed a hypsochromic shift from 616 to 405 nm. Additionally, pro-PS 114 exhibited very limited photocytotoxicity (IC50 = 15.9 μM) before triphenylphosphine activation to HeLa cells but displayed a remarkable photocytotoxic effect (IC50 = 0.218 μM) to the same cell line after activation assisted by the Staudinger reaction.
Figure 61.
Activation of 114 by TPP.
2.2.4. Enzyme-Activatable Theranostic Fluorescent Probes in PDT
Enzymes are critical to many metabolic processes, and elevated enzymatic activities are believed to facilitate several pathological processes, ranging from tumor angiogenesis and cell invasion to metastasis. So far, researchers have detected many overexpressed enzymes in various tumor types, and with the help of biologists, the biological substrates of these enzymes have been disclosed, which can be employed to construct “smart” phototherapeutic agents, whose photoactivity could be switched on by a specific enzyme, thus allowing the distinction of healthy cells from diseased ones and abolishing off-target damage to the skin and neighboring healthy cells.304,305,382
Compared to other tumor markers, such as acidic pH, redox conditions, and elevated ROS levels, most enzyme-catalyzed substrate reactions are fast, mild, and especially highly specific, endowing a high sensitivity and low false positivity rate to enzyme-activated PSs. Therefore, using cancer-associated enzymes to fabricate activable PSs has attracted great attention. In this section, we will discuss several typical examples of enzyme-activatable “smart” PSs, including those activated by nitroreductase (NTR), azoreductase, cathepsin B, β-galactosidase, alkaline phosphatases, tyrosinase, γ-glutamyl transpeptidase, and aminopeptidase.
2.2.4.1. Nitroreductase-Activatable Theranostic Probes in PDT
Nitroreductase is a well-known specific enzyme overexpressed in hypoxic solid tumors due to the reductive stress of low intracellular O2 concentrations, which can effectively reduce nitroaromatics to the corresponding arylamines in the presence of reduced nicotinamide adenine dinucleotide (NADH) as an electron donor. Based on this reduction reaction, many hypoxia-triggered theranostic probes have been designed by installing nitroaromatic groups into dye scaffolds.383−385
2.2.4.1.1. p-Nitrobenzoate Group
Owing to the strong hydrogen-bonding interaction and suitable spatial match with NTR, the p-nitrobenzoate group has been recognized as an ideal NTR substrate with a fast response, high sensitivity, and good selectivity. By introducing this functional group into different PS scaffolds, Peng’s group developed several NTR-activated phototheranostic agents mostly based on PET and ICT mechanisms. For instance, Peng’s group reported a hypoxia-activated D-π-A PS (115-N) by decorating the phenol hydroxyl of an iodine-substituted hemicyanine dye (NIR PS) with 4-nitrobenzyl bromide.386 As shown in Figure 62, the introduction of p-nitrobenzoate restrained the ICT process, leading to weak fluorescence and low 1O2 production. While in hypoxic cancer cells and tumor tissues, 115-N was reduced to 115-OH by intracellular overexpressed NTR. The latter exhibited a markedly increased photosensitivity due to the restoration of ICT after the removal of the caging group. In normal tissues, 115-N was in a fluorescence-off state and exhibited almost no phototoxicity, while hypoxia-induced cell apoptosis and suppression of tumor growth under 660 nm light irradiation, highlighting the PS’s potential for selective tumor hypoxia imaging and PDT. Two additional NTR-activatable theranostic molecules (116 and 117) based on TADF fluorescein derivatives were designed to enable PDT in mildly hypoxic tumors (Figure 63).387 When subjected to screening, 117 performed better than compound 116 in terms of selectivity and response rate to NTR. Importantly, endogenous NTR in tumor cells can catalyze the enzymatic cleavage reaction of 117 to form 118, achieving a high PDT efficiency even under 10% oxygen concentrations.
Figure 62.
Recognition mechanism of 115-N with NTR.
Figure 63.
Molecular structures of 116–118.
2.2.4.1.2. 2-Nitroimidazole Group
Li and co-workers devised a novel NTR-activatable NIR PS (119), which features an intrinsic ER-targeting capability and low oxygen-depletion type I photosensitivity, endowing ERPS with highly efficient phototoxicity against cancer cells under both normoxic and hypoxic conditions.388 As depicted in Figure 64, 119-Im was constructed by caging its hydroxyl group with a 2-nitroimidazole-based triggering group as a recognition site for NTR. To improve the water stability and increase tumor enrichment, 119-Im was further encapsulated within polymeric micelles to yield a spherical 119-Im-NP nanoparticle with favorable colloidal stability. As expected, masking the phenolic hydroxyl group led to the quenching of fluorescence and photosensitizing activity of 119-Im encapsulated in the 119-Im-NP micelle, due to the suppression of the ICT process. After being taken up by cancer cells, the 119-Im cargo was released from 119-Im-NP, and then was specifically converted into active 119 by the overexpressed NTR in hypoxic cancer cells. The converted 119 was mainly localized in the ER with a Pearson’s correlation coefficient as high as 0.97, and 119 efficiently generated O2–• and •OH upon irradiation, which induced severe ER dysfunction and protein misfolding. Overall, benefiting from hypoxia activatability, specific ER-targeting properties, and low oxygen depletion advantages of type I photosensitivity, 119-Im-NP demonstrated a highly selective and efficient PDT-killing effect in hypoxic cells and inhibited solid tumor growth. This work provides a model PS for addressing both off-target effects and hypoxic resistance in tumor therapy.
Figure 64.
Schematic illustration of NTR-activatable PS 119-Im. Figure reproduced with permission from ref (388). Copyright Royal Society of Chemistry.
2.2.4.2. Azoreductase-Activatable Theranostic Probes in PDT
Azoreductase also is one of the typical hypoxia-responsive enzymes, which has been demonstrated to be overexpressed in hypoxic cells, particularly within solid tumors. Its capability to reduce azo groups and generate amino derivatives establishes it as an inherent biomarker for tumor hypoxia. Consequently, the azo moiety can be employed as a caging group to block the photosensitivity of PSs, thereby creating “switch on” PDT molecules specifically designed to fight hypoxic tumors.389−391
Traditional PSs are faced with insufficient light penetration depth, hypoxic sensitivity, and poor tumor targeting, which seriously restricts the efficacy of PDT.392,393 To solve these problems at the same time, Kim et al. designed a hypoxia-responsive, two-photon excitable, type I PS that was further modified by a targeting group. Considering the deep penetration and greater spatial precision of two-photon excitation a compatible type I PS was chosen. To this end, they also developed a series of cyclized-cyanine derivatives (120), shown in Figure 65. These dyes demonstrated impressive fluorescence emission (ΦF up to 60%) and excellent two-photon action cross sections (up to 103 GM).394 Notably, these heavy atom-free small-molecules displayed superior ROS-generating abilities compared with 5-ALA via a type I mechanism. Moreover, the two-photon excitation was demonstrated to be a more efficient approach than one-photon excitation. Azo groups were conjugated to the dye skeleton and two caged PSs (120a and 120b) were obtained that can be activated by hypoxia. When taken up by cancer cells that overexpress azoreductase, 120 showed a rapid response and exhibited excellent cancer cell-killing abilities with negligible dark toxicity. More recently, these authors also linked the PS with a targeting unit (biotin) to realize a highly spatiotemporal-selective two-photon PDT agent for colon cancer (121, Figure 65).
Figure 65.

Structures of theranostic probes 120 and 121.
Hypoxic tumor environments usually provide insufficient oxygen to support the operation of PDT, resulting in limited therapeutic efficacy, especially in the deep inner part of a solid tumor. To overcome this issue, Liu and Kim et al. designed a theranostic molecule (122-Azo) for hypoxia-responsive chemotherapy and phototherapy in solid tumors (Figure 66).395122-Azo was synthesized by linking a rhodol-based NIR fluorophore (122) with nitrogen mustard through an azo bond (−N=N−). Its fluorescence was fully inhibited by intramolecular π–π stacking and a blocked ICT process. Unlike the beforementioned activatable PSs, 122-Azo displayed excellent ROS production (ΦΔ = 0.14) while the active form does 122 not. Thus, the distinctive photosensitivity of 122-Azo, with or without hypoxia stimuli, allowed it to perform via a different anticancer modality. On the surface layer of the tumor, 122-Azo demonstrated an impressive PDT effect, eliminating normoxic cancer cells as the lower expression of azoreductase, presumably due to the fact that this enzyme is unable to cleave the azo bond. However, within the tumor, the reduction of the azo bond triggered the release of 122 and an active nitrogen mustard, enabling the effective killing of hypoxic tumor cells via chemotherapy. Additionally, this process facilitated in situ and real-time monitoring of dosage and kinetics during drug release. Overall, this strategy successfully eradicated cancer cells in both normoxic and hypoxic environments, maximizing therapeutic efficacy for solid tumors.
Figure 66.
Design and chemical structure of the theranostic construct 122-Azo.
2.2.4.3. Cathepsin B-Activatable Theranostic Probes in PDT
Cathepsin B (CTSB) is a cysteine protease present in lysosomes that is highly expressed in a variety of tumors, and when excreted it can degrade extracellular matrix components, such as collagen, laminin, or tenascins, thereby promoting tumor invasion and metastasis during cancer progression. Aberrant expression/activity of CTSB has been employed as a pathological biomarker in the design of theranostic agents for cancer-selective imaging and treatment.305,396
In this regard, Lo et al. devised a cathepsin B-responsive fluorescent probe and PS (123), composed of a zinc(II) phthalocyanine-based PS, a new ferrocenyl BODIPY dark quencher, and a cleavable substrate for cathepsin B (Gly-Phe-Leu-Gly-Lys).397 As depicted in Figure 67, in the absence of cathepsin B, conjugate 123 displayed weak NIR fluorescence and a low 1O2 generation ability because of FRET between the zinc(II) phthalocyanine and the BODIPY unit, followed by a PET process from the ferrocenyl moiety. Upon the addition of cathepsin B, the peptide substrate was cleaved, separating the phthalocyanine and ferrocenyl BODIPY units, thus preventing the FRET-PET effect and restoring the photosensitizing properties of the zinc(II) phthalocyanine. Under light irradiation at 610 nm, 123 exhibited a high photocytotoxicity in HepG2 cells (cathepsin B-positive cells) with IC50 of 0.32 μM, highlighting 123 as a highly efficient cathepsin B-activatable PS.
Figure 67.
Schematic diagram showing the working principle of the cathepsin B-activatable PS 123 based on a FRET-PET process.
In a different approach, Chen et al. developed a novel cathepsin B activated BODIPY-based PS, 125, whose activation mechanisms of photosensitivity rely on the presence of an intramolecular charge transfer (ICT) effect.398 In 125, an orthogonal BODIPY dimer-based PS, 124, was conjugated with an alkynyl group-modified cathepsin B-cleavable peptide (alkyne-Gly-Phe-Leu-Gly) through a p-aminobenzyloxycarbonyl bridge (Figure 68). The cathepsin B-cleavable peptide substrate inhibited the electron-donating ability of the amino group of 124, leading to a blocked ICT process and significant suppression of 1O2 generation. To improve the tumor-targeting ability and antitumor efficacy, 125 was then linked with a cRGD-modified PEG chain (126) to obtain 127, which is used as a nanocarrier to further encapsulate and deliver 10-hydroxycamptothecin (HCPT), a hydrophobic anticancer agent. Benefiting from a tumor cell-targeting peptide (cRGD), the resulting 128 nanoparticles not only exhibit specific cellular uptake in integrin αvβ3-positive 4T1 cells as compared to HeLa cells with a low αvβ3 expression but also promoted tumor penetration in the 4T1 three-dimensional (3D) tumor spheroids models. Notably, after the cleavage of the peptide by intracellular cathepsin B, 128 activated the PDT activity of 124, as the amino group is uncaged, while releasing HCPT, therefore effectively inducing apoptosis of 4T1 cells and shrinking the size of the 4T1 3D tumor spheroids through the combined effects of PDT and chemotherapy. This work reported a novel strategy for constructing cathepsin B-activated PSs and building a promising antitumor platform.
Figure 68.
Schematic illustrations for the synthesis of 125, 126, and 127, the formation of 128 nanoparticles, and the mechanism of the activatable PS.
2.2.4.4. β-Galactosidase-Activatable Theranostic Fluorescent Probes in PDT
β-Galactosidase (β-gal) is an essential glycoside hydrolase located within the lysosomes of cells, which performs a critical function by facilitating the breakdown of glycoside bonds and promoting the conversion of lactose into galactose.399,400 Despite being overexpressed in primary ovarian cancer and gliomas, β-gal is not commonly considered to be a biomarker for cancer. However, recent studies have unveiled its significance as one of the most important hallmarks of senescence, and numerous investigations have focused on developing methods to selectively eliminate senescent cells.
For example, Urano et al. designed an activatable PS 129 to specifically target and eliminate lacZ-positive cells (Figure 69).401 The PS was developed using a Se-substituted Rhodamine compound, where a fluoromethyl group served as an electrophilic site at the 4-position. The pKcycl value of 129 was determined to be 5.4, indicating that, at the physiological pH of 7.4, the compound would predominantly exist in a cyclized, colorless, and nonphototoxic form. By contrast, the reference compound, 130, exhibited a pKcycl of 10.3 and a pKa of 4.8, suggesting that the reference compound would predominantly exist in its open and phototoxic form (ΦΔ = 0.36) at pH 7.4. The hydrolysis of 129 resulted in the activation of both its photosensitizing ability and reactivity to nucleophiles due to the formation of a quinone methide intermediate. As a result, the PS could be trapped inside lacZ-positive cells. Moreover, this mechanism enabled selective killing of β-galactosidase-expressing cells with single-cell resolution. The effectiveness of 129 was confirmed in Drosophila models, demonstrating its promising in vivo applicability.
Figure 69.
Activatable PS (129) targeted to β-galactosidase and time-lapse fluorescence imaging of a coculture of HEK293 and HEK-lacZ(+) cells. HEK-lacZ(+) cells and HEK293 cells were prestained with CellTracker Blue and CellTracker Green, respectively. Reproduced with permission from ref (401). Copyright 2019 American Chemical Society.
Based on the same responsive group, Li et al. developed a photosensitive senolytic prodrug (131) that could be specifically activated in the presence of SA-β-gal (Figure 70).402 To enhance intersystem crossing, ensuring efficient phototherapy efficacy, the authors replaced the oxygen atom in the dicyanomethylene-4H-pyran-based skeleton with selenium. The presence of E. coli β-gal and BSA led to an increased ΦΔ value of 131 from 0.07 to 0.20, while the relative fluorescence quantum yields decreased from 0.33 to 0.08. This modification allowed 131 to exhibit high efficacy and broad-spectrum activity against senescent cells while minimizing side effects on nonirradiated areas. In both a doxorubicin-induced senescence mouse model and naturally aged mice, 131-mediated PDT demonstrated the selective elimination of senescent cells in tissues. This research provides a new perspective on monitoring and selectively removing senescent cells to regulate the aging process.
Figure 70.
Integrated strategy used to design the senotherapeutic probe 131.
2.2.4.5. Alkaline Phosphatase-Activatable Theranostic Probes in PDT
Alkaline phosphatase (ALP) is a type of extracellular plasma membrane-anchored hydrolase that is involved in the dephosphorylation processes during diverse cellular events, including regulation of protein phosphorylation, cell growth, apoptosis, and migration. Elevated activity of ALP is closely related to a range of diseases, such as breast cancer, prostate cancer, kidney tumors, and osteosarcoma; thus, it has been considered a significant biomarker used in clinical diagnosis.403,404 Large numbers of fluorescent probes have been developed for the selective fluorescent visualization of ALP-positive cancer cells, however, reports of activable PSs selectively responsive to ALP are still somewhat limited.
Li and co-workers developed a new type of D-π-A PS scaffold (denoted as 132Se-I), by using a selenium-substituted dicyanomethylene-4H-chromene as the electron acceptor and 2,6-diiodo phenolate as the electron donor (Figure 71).360 In 132Se-I, the introduction of heavy Se atoms contributed to a remarkable redshift of wavelength and significantly amplified the photosensitized 1O2 production, favoring highly efficient PDT in deep tumor tissues. Besides, the two ortho-iodo groups further enhanced the photosensitivity, and more importantly reduced the pKa of the phenolic group, enabling PDT to a typical physiological pH of 7.4. Moreover, by conjugating phosphate (the protecting group and ALP recognition site) to the phenolic hydroxy group of 132Se-I, a representative ALP-activatable PS was prepared (ALP132), whose photosensitivity was completely turned off, due to the suppression of an ICT process induced by the diminished electron-donating ability of the phenolate group in 132Se-I after phosphorylation. Upon the addition of ALP or when incubated with living Hela cells overproducing ALP, ALP132 was quickly converted into 132Se-I, leading to full recovery of the quenched fluorescence and photosensitivity. Live/dead staining images and an MTT assay demonstrated that ALP132 significantly decreased HeLa cell viability. Overall, this work demonstrated an effective strategy for designing ALP-triggered off/on switching of photosensitivity via the caging and uncaging of a phenol.
Figure 71.
Schematic illustration of the design of an ALP-activatable PS (ALP132).
Conventional luminophores feature planar structures and usually suffer from severe aggregation-caused quenching (ACQ) through strong intermolecular π–π donor-acceptor interactions, which limits the concentration that can be applied to biological systems. Recently, aggregation-induced emission luminogens (AIEgens) have emerged as an alternative class of fluorescent materials, which have shown excellent performances in effective ROS generation as well as high fluorescence brightness in the aggregated state, while being highly resistant to photobleaching. Thus, these fluorophores offer unique advantages, such as improved imaging signal-to-noise ratios and phototherapy efficacies.405 So far, AIEgen-based-activatable fluorescent probes have been widely investigated for many biomedical applications.313,314 Very recently, Tang and He et al. developed an ALP-activated AIE PS, 133P, which employs a triphenylamine derivative as the PS core and a phosphate group as an enzyme-labile moiety, respectively (Figure 72), to specifically target and kill the ALP-overexpressed cancer cells.406 Probe 133P was nonemissive in aqueous solutions, while upon the addition of ALP its phosphate group can be efficiently hydrolyzed to obtain 133, which further formed aggregates and emitted a yellow-colored fluorescence (λem = 540 nm). The specific fluorescent response to the presence of the ALP enzyme enabled 133P to discriminate cancer cells from normal cells and to quantify the ALP level in the cells, as a function of the fluorescence intensity. Notably, under light irradiation, neither 133 nor 133P could produce 1O2, but both can induce hydroxyl and superoxide radical generation. The ROS production efficiency of 133 is slightly greater than 133P but higher than Ce6 (a commercially available PS). Given the difference in ALP expression and ALP-specific activation characteristics, the total ROS generation efficiency of 133P aggregates in cancer cells is much lower than those 133P in normal cells, therefore resulting in the selective killing of cancer cells. The present work provided a novel strategy for designing cancer-specific biomarker-responsive AIE PSs.
Figure 72.
Schematic illustration of the hydrolysis of 133P by intracellular ALP in cancer cells.
2.2.4.6. Tyrosinase-Activatable Theranostic Probes in PDT
Melanoma is an aggressive malignancy with rapid growth and early metastasis. Tyrosinase (TYR), a copper-containing oxidase involved in melanin synthesis, which can convert monophenol or catechol to the corresponding o-quinone, is the rate-limiting enzyme for the biosynthesis of melanin from tyrosine. TYR is markedly overexpressed in melanoma cancer cells, thus making it an important biomarker for the diagnosis and treatment of melanoma.407
Sundus Erbas-Cakmak and co-workers developed the first tyrosinase-activatable BODIPY-based PDT agent (Figure 73).408 In this work, an iodine-substituted BODIPY was chosen as the PS scaffold, and 3-hydroxy benzyl was used as a tyrosinase substrate motif. Meanwhile, an acetyl group was introduced to improve cellular internalization and tyrosinase-catalyzed oxidation. Due to a PET effect from the core BODIPY on the electron-poor pyridinium, the photosensitization activity of 134 was completely inhibited. After entering cancer cells, 134 was first rapidly hydrolyzed by esterase to produce intermediate 1 (134a), which was further oxidized into intermediate 2 (134b) by tyrosinase. Once the 3,4-dihydroxy benzyl group was generated, a spontaneous 1,6-elimination took place, to release 135. As the PET acceptor is eliminated, 1O2 could be produced, with quantum yields determined to be 0.02 for 134 and 0.64 for 135, respectively.
Figure 73.
Structure of the enzyme-responsive PS 134 and the proposed mechanism of action. Inactive 134 is converted into active 135 through reactions catalyzed by esterase and tyrosinase.
For fluorescence imaging and PDT of early melanoma, Yoon et al. developed a novel endogenous tyrosinase-activated cyclometalated Pt(II) complex (136-tyro), by connecting a 3-hydroxybenzyloxy moiety (as the tyrosinase recognition unit) to the phenyl pendent of a C∧N ligand of a cyclometalated Pt(II) complex (as the PSs core).409 As depicted in Figure 74, after reacting with tyrosinase, 136-tyro was converted into 136-OH through rapid rearrangement and elimination, leading to an obvious fluorescence off-on response at 530 nm. Moreover, the tyrosinase metabolite 136-OH exhibited a dramatically improved photosensitizing ability relative to 136-tyro, due to the longer-lived triplet excited state of 136-OH. Consequently, 136-tyro can specifically image endogenous tyrosinase in A375 cells (human melanoma cancer cells), and induces a significant growth inhibition toward A375 cells and tumors of A375-bearing Balb/c mice.
Figure 74.
Scheme of 136-tyro for tyrosinase imaging and PDT.
2.2.4.7. GGT-Activatable Theranostic Probes in PDT
γ-Glutamyl transpeptidase (GGT), a well-known cell membrane-associated enzyme, can selectively cleave the γ-glutamyl bond of GSH to convert GSH into cysteinyl-glycine (Cys-Gly), to maintain the cellular homeostasis of GSH and cysteine. It has been shown that GGT expression is upregulated in the cell membranes of various malignant tumors, including glioma as well as liver, lung, and ovarian cancers. The elevated level of GGT has been implicated in promoting tumor progression, invasion, and drug resistance, thus making GGT an important marker in tumor diagnosis and an ideal target for tumor treatment. l-Glutamic acid linked through its side chain has been validated as a specific GGT trigger, to mask a fluorophore and sense GGT enzymes.410,411
Based on this, to overcome the off-target biodistribution of PSs in existing PDT systems and achieve tumor-reporting fluorescence, Han and Li et al. designed and synthesized a trifunctional small-molecule probe, called 137, that contains a type I PS (ENBS), linked to a fluorescence reporter (rhodamine, Rd) via a self-immolative linker, and an l-glutamic acid moiety as a GGT trigger (Figure 75).412 In 137, both the fluorescence and the ROS generation properties of ENBS are almost unaffected by the rhodamine fluorescence, while the fluorescence of Rd is in an “off” state due to the quenching effect between ENBS and Rd. Upon catalytic activation by tumor-associated GGT, 137 releases rhodamine and ENBS, leading to activation of the rhodamine fluorescence. The released rhodamine and ENBS were accumulated in lysosomes, an organelle critical for triggering cell death. Under light irradiation, 137 effectively damaged lysosomes and induced a 2-fold enhancement of cell death in U87 cells (GGT+) over LO2 cells (GGT−). In BALB/c nude mice bearing subcutaneous U87 tumors, 137 exhibited an intense rhodamine fluorescence selectively confined to tumor foci rather than other organs, revealing the potential of 137 in discerning tumors from healthy tissues by a tumor-activated “Turn-On” fluorescent response. Meanwhile, the tumor-specific optical readouts help to direct light irradiation for precise PDT. Overall, this work demonstrated a fluorescently quenched dye–PS pair to yield PS-independent tumor-activatable fluorescence to guide PDT.
Figure 75.
GGT-mediated release of theranostic probes 138 and 139 from 137.
2.2.4.8. Aminopeptidase N-Activatable Theranostic Probes in PDT
Aminopeptidase N (APN/CD13) is a typical membrane-binding zinc-dependent type II metalloproteinase that can specifically cleave an N-terminal leucine amino acid from a protein polypeptide chain. APN plays vital roles in the growth, division, metastasis, and angiogenesis of malignant tumors. Extensive studies have demonstrated that APN is significantly overexpressed on the cell membrane surface in various tumors (∼10-fold than normal tissue), especially in colon, ovarian, breast, and liver tumors. Thus, APN is widely recognized as a distinct marker for cancers and has received attention as a cancer-selective enzyme enabling the design of selective activatable fluorescent probes and theranostic prodrugs. Peng and co-workers reported for the first time an APN-activated near-infrared PS (APN-140) by linking an iodine-substituted hemicyanine and l-alanine through a 4-aminobenzyl alcohol linkage (Figure 76).413 In normal cellular environments, APN-140 is maintained in an “OFF” state, with quenched fluorescence and phototoxicity as a result of an inhibited ICT process. By contrast, overexpressed APN in tumor cells specifically lysed the amide bond between l-alanine and the 4-aminobenzyl alcohol group, which is followed by a spontaneous 1,6-elimination to form 140-OH. 140-OH displayed a greatly enhanced NIR fluorescence signal and 1O2 yield compared to APN-140, and specifically accumulated in the ROS ultrasensitive mitochondria. Under 660 nm light irradiation, APN-140 efficiently induced cancer cell apoptosis by destroying mitochondria and more importantly could distinguish cancer cells from normal cells, leading to the selective killing of cancer cells and a distinct tumor suppression effect.
Figure 76.
Response mechanism of APN-140 with APN.
2.2.4.9. Other Examples
Apart from the aforementioned activation types, researchers have also made significant advances in the development of other types of activatable PSs. Some examples primarily focus on tailored PSs for specific diseases by employing specific markers as activation triggers.
2.2.4.9.1. F– Activation
Fluoride is extensively utilized as an anticaries agent in oral health care, in which fluoride is absorbed by the dental plaque biofilm, thereby acting as a fluoride reservoir in the oral cavity. With this in mind, Yi et al. designed a panel of fluoride-activated PSs (141) for imaging and treatment of human dental plaque (Figure 77).414141 was synthesized by linking leuco-MB to different silyls with different substituents (TBDPS, TIPS, and TBDMS), as silyl ethers are selectively and rapidly cleaved by fluoride. In vitro experiments revealed that 141c possessed the best responsiveness toward fluoride, including a quick desilylation rate and high selectivity as well as a low detection limit. 141c can not only be used to map naturally grown human plaque biofilms by the NIR fluorescence of MB but can also be used to realize fluoride-regulated antimicrobial PDT.
Figure 77.
Fluoride-activated MB releasing platform of 141 and three candidate theranostic probes in this work.
2.2.4.9.2. Photoactivation
Photoswitching molecules can also be used in activation strategies. For example, in the context of fluorescent probes and super-resolution imaging, researchers have explored diverse photo-switches based on different dye skeletons.415,416 Very recently, Tang et al. developed a UV light-activatable PS (142), which integrated tetraphenylethylene (TPE) with a diarylethene (DAE) at the 6-positions of constituent benzothiophenes (Figure 78).417 The open form (OF) of 142 demonstrated short-wavelength absorption/emission and AIE characters; once activated by UV light (365 nm), its closed form (CF) quickly formed, accompanied by a bathochromic-shift towards the visible light range and an enhanced fluorescence intensity (λem = 660 nm) as well as a dramatically strengthened photosensitization capability. 142 was coated with F-127 to form 142 nanoparticles (142-NP) with the aim of improving the water solubility and cellular uptake. Strikingly, 142 retains its photo-switching properties inside the NPs, and the cyclization can be fully finished within 300 s. Finally, a PDT effect of 142-NP was elicited in HeLa cells, in which photoinduced cancer cell death was found to be closely related to apoptosis or necrosis pathways. This research exemplifies the utilization of photo-switches for the development of light-activatable PSs with high spatiotemporal resolution.
Figure 78.
Chemical transformation of theranostic probe 142 (closed form) to 143 (open form) under light irradiation.
2.2.4.9.3. Enzyme-Responsive Double-Locked Molecular Beacon
A photodynamic molecular beacon (PMB) is an inherent self-quenched PS due to intramolecular energy or electron transfer. Generally, PMB molecules are composed of three parts, (i) a PS, (ii) a quencher, and (iii) a linker that can be recognized by specific stimuli (e.g., enzymes). In PMB molecules, utilizing a resonance energy transfer between the PS and quencher is a common strategy, where the excited state energy of the PS is transferred to the quencher after excitation and the resultant dark state of the PS further hinders ROS generation. However, when the linker is cleaved at a specific site (tumor or other diseased cells), the resonance energy transfer loses efficacy, owing to the spatial separation of the PS and the quencher, further switching on the photo-induced ROS generation. Ng and Lo et al.418 constructed a photodynamic molecular beacon molecule 144 by utilizing the classic iodinated-BODIPY scaffold, Black Hole Quencher 3 (BHQ-3) as a quencher, and a cyclic peptide containing PLGVR and GFLG peptide sequences that can be recognized and cleaved by metalloproteinase-2 (MMP-2) and cathepsin B, as shown in Figure 79. In 144, the excited state of the BODIPY scaffold was fully quenched by BHQ-3 and kept 144 in an “inactive” state (ΦF = 0.01, ΦΔ = 0.02), thereby reducing its adverse side effects on normal tissues. Once taken up by MMP-2 and cathepsin B overexpressing cancer cells (e.g., the A549 cell line), cascaded hydrolysis releases the photoactive DSBDP and restores its photodynamic activities. Moreover, the released dye is selectively localized in the lysosomes of cancer cells and causes significant tumor cell death (IC50 = 0.78 ± 0.04 μM for A549) after irradiation (λ > 610 nm, 23 mW cm–2, 28 J cm–2). In an A549 tumor-bearing mice model, 144 effectively suppressed the tumor growth without notable side effects. In particular, 144 does not induce any degree of skin photosensitivity, which is a well-recognized side effect of conventional “always-on” PSs.
Figure 79.
Molecular structure of the double-locked theranostic probe 144. Reproduced with permission from ref (418). Copyright 2023 American Chemical Society.
2.3. Theranostic Fluorescent Probes for PTT
2.3.1. Tumor Microenvironment (TME) Activated Theranostic Probes in PTT
PTT utilizes mild and highly penetrating infrared light to convert external light energy into heat in the presence of a PS, thereby increasing the local temperature. When this photoactivation takes place in a tumor, the in situ generated heat can kill cancer cells without the need for cytotoxic agents being used. PTT has garnered significant research interest due to its non-invasiveness and biosafety due to the use of light to precisely control heat production and the preclusion of drug resistance. TME modulates tumor survival, progression, and metastasis, and has thus become a potential target for activatable tumor imaging and treatment. Considering the inadequate therapeutic efficacy of traditional photothermal agents (PTAs), TME-activatable PTAs have been extensively developed in recent years for targeted cancer therapy. The TMEs exploited include acidic pH, hypoxic conditions, and cancer-specific enzymes.
The TME is characterized by weakly acidic pH conditions resulting from abnormal glycolysis, offering promise for targeted, activatable imaging and therapy of cancer. Shi et al. reported an intelligent near-infrared (NIR) photosensitive probe Cy-1 (145), which can be activated under the synergy of acidic pH and GSH to self-assemble into nanoparticles (145-NPs) in situ in cancer cells (Figure 80).419 When 145 was internalized, it underwent simultaneous citric acid hydrolysis under weakly acidic pH and a disulfide bond cleavage by GSH to produce 145-Core. In tumor cells where a high level of active 145-Core exists, formation of an amphiphilic cyclic dimer (145-Dimer) occurs. Subsequently, π–π stacking between 145 species generated Cy-NPs with enhanced retention in tumors, as well as activated NIR/photoacoustic (PA) imaging modalities and PTT in vivo.
Figure 80.
(A) Schematic diagram of the formation of 145-Dimer mediated by low pH and GSH to in situ generate 145-NPs with recovered photothermal conversion. (B) Schematic illustration of the use of 145-NPs for activated bimodal imaging and PTT of cancer cells. Reproduced with permission from ref (419). Copyright 2020 American Chemical Society.
The expression level of biomarkers varies from individual to individual. To improve the sensitivity of PTAs to a given TME, Liu et al. developed a pH-sensitive nanoprobe (Figure 81).420 The nanoprobe was composed of NIR-IIb quantum dots PbS@CDS, which were conjugated to the surface of a hollow MnO2 nanoshell. A molecular probe IR1061 was then loaded to obtain HvMnO2@Qds-IR1061 (146). The fluorescence signal was absent in normal cell tissues due to the absorption competition-induced emission (ACIE) mechanism. However, in the weakly acidic TME, the HvMnO2 shell was degraded, releasing the encapsulated IR1061 for NIR imaging. Then, treatment using 1064 nm laser irradiation for 5 min increased the local temperature of the tumor from 21 to 72 °C to kill cancer cells, thus achieving imaging-guided PTT, which holds significant promise for surgical applications.
Figure 81.
NIR fluorescence of theranostic probe 146 is activated by low pH to achieve NIR-IIb fluorescence imaging-guided PTT of solid tumors. Reproduced with permission from ref (420). Copyright Elsevier Ltd.
Based on a similar strategy, Park et al. developed a pH-responsive nano-probe that consists of carbonized crosslinked poly(ethylene glycol-g-poly(sulfobetaine methacrylate)) loaded with a photothermal dye IR825 (FNP-I).421 Cai et al. used IR-822 as the PTA to which a proton receptor N1-(pyridine-4-methyl) ethane-1pr 2-diamine (PY) was introduced to form a fluorophore-spacer-receptor molecular probe 147 with quenched fluorescence (Figure 82).422 After being protonated in the acidic TME, the probe allows for NIRF/PA dual-modal imaging-guided PTT applications.
Figure 82.
Chemical structures and schematic illustration of 147 as a pH-responsive theranostic probe for NIRF/PA dual-modal imaging-guided PTT therapy. Reproduced with permission from ref (422). Copyright 2017 Royal Society of Chemistry.
It has been reported that the rough surface of viruses is composed of thorn-like structures that strongly bind to the cell membrane during the process of virus invasion. In 2019, Liu et al. presented a nano-probe that utilizes the biomimetic targeted therapy characteristic.423 The bionic method was used to coordinate the infrared fluorescent IR825 with the chemotherapeutic drug PEM and rare earth metal particles (Nd3+, Nd2+) to design a self-targeting NIR-II nano-sheet. The Nd3+ ion was used as a converter to transform them into virus-like nanoparticles. Tumor acidity-sensitive PEG was used to cover the surface of the virus-like nanodrug, resulting in the creation of virus-core and sphere-shell hierarchical nanostructures under physiological conditions. When triggered by a weakly acidic tumor microenvironment, the “sphere-to-virus” shape reversal occurred, which led to an enhancement in photothermal conversion efficiency, an increase in cell adhesion through a virus-like rough surface, and the activation of folate-receptor-mediated self-targeting realized biomimetic targeted therapy effect of the tumor.
Furthermore, Yan et al. investigated a nanoplatform for simultaneous photothermal and photodynamic (PTT/PDT) therapy with acid activation and when subjected to external radiation, which can be used for accurate tumor-targeting near-infrared (NIR) image-guided therapy.424 The pH-responsive brominated asymmetric cyanine (BAC) was an activable near-infrared PTT/PDT-in-one reagent for accurate tumor-targeted therapy. BAC was connected to persistent luminescence nanoparticles (PLNPs), then biotin (BT) functionalized polyethylene glycol (PEGBT) was introduced into the PLNP to form PTT/PDT-in one nanoplatform (PLNP-BAC-PEGBT), which showed accurate tumor-targeted imaging and strong PTT/PDT effects. In 2022, Yin et al. constructed an organic nanoprobe with acid-activated heptamethine cyanine (Cy-TPA, 148) (Figure 83).425 The nanoprobe can “Turn on” the PTT effect and restore near-infrared fluorescence in a weakly acidic tumor microenvironment. Simultaneously, 148 NPs can accumulate in the tumor site, prolong its retention time, and significantly improve the PTT effect, fluorescence signal activated by the acid environment also provides guidance for PTT therapy. Moreover, Liu et al. developed a series of acid-triggered NIR upconversion NPs (NRhD-PEG-XNPs), which were constructed by rhodamine dyes conjugated with PEG426 and showed good upconversion luminescence and an enhanced photothermal effect in a weak acid environment. NRhD-PEG-XNPs could achieve accurate tumor targeting and PTT without showing side effects, providing a new research strategy for activatable theranostic nanoplatforms.
Figure 83.
Schematic diagram of 148 NPs that rearrange in a weakly acidic environment to “Turn-on” NIRF imaging-guided PTT. Reproduced with permission from ref (425). Copyright 2022 Royal Society of Chemistry.
Human serum albumin with good carrier performance was coated with pH-responsive fluorescent dyes to form a self-assembling complex, when triggered by a weakly acidic tumor microenvironment, the complex forms nanoparticle aggregates at tumor sites, which effectively achieves photodynamic and photothermal treatment effects of tumors. Liu et al. developed albumin-dye nanoparticles (HSA-Croc) by pH-sensitive croconaine (Croc) dye self-assembled with HSA.427 While under acidic conditions, Croc dye has strong absorption at 790 nm and HSA-Croc nanoparticles exhibit accurate tumor-specific photoacoustic imaging and PTT. With the help of similar functions of biological proteins, Yi et al. developed BSA-pH-PTT nanoparticles using pH-sensitive asymmetric cyanine dye (pH-PTT) self-assembled with BSA.428 Under acidic conditions, pH-PTT was converted into a larger conjugated structure with strong absorption at 808 nm, and the self-assembled system formed nanoparticles, which could be concentrated at the tumor site to achieve an effective photothermal therapeutic effect.
2.3.2. Hypoxic Activated Theranostic Fluorescent Probes in PTT
Hypoxia is a common feature in most solid tumors and TME, resulting from uncontrolled proliferation of tumor cells, abnormal blood vessels, and insufficient oxygen supply. However, healthy tissues do not contain hypoxic regions, making hypoxia a specific target for selective cancer treatment. Various mechanisms promote the adaptation of tumor cells to this adverse environment and ultimately enhance drug resistance and survival.429,430 PTT is a promising treatment strategy for hypoxic tumors due to its non-oxygen dependence and high spatiotemporal accuracy.431 PTT uses photothermal agents to trigger local hyperthermia under near-infrared light (NIR) irradiation, with high therapeutic efficiency, leading to irreversible ablation of tumor cells.432,433 To achieve hypoxic reactivity in PTT, hypoxic targeting vectors can be used to deliver photothermal agents or hypoxic-responsive groups can be introduced to activate them.434,435 These strategies are essential for triggering photothermal properties, and the development of novel hypoxia-induced photothermal agents is crucial for controlling the “on/off” state of PTT.436
PTT based on the principle of photothermal conversion has great potential in the effective treatment of cancer, with high tumor ablation efficiency and minimal side effects on normal tissues.437 Cai et al. developed a novel single-molecule probe 149, which was triggered by hypoxia and enzymatic reaction with nitroreductase (NTR). The probe was conjugated with a nitro imidazole group as a specific hypoxia trigger with an IR-1048 dye as a NIR-II/photoacoustic (PA) signal reporter, allowing for high-contrast tumor visualization by NIR-II fluorescence imaging and deep-tissue penetration using 3D PA imaging. Theranostic probe 149 exhibits significant photothermal effects and NIR-II fluorescence when activated at hypoxic tumors, providing more accurate and deeper tissue imaging of tumor location and boundaries for tumor ablation without recurrence. Therefore, the multifunctional 149 probe represents a rapid and sensitive NIR-II fluorescence/photoacoustic imaging probe for hypoxia, that can be used as an activated small molecule PS for PTT (Figure 84).438
Figure 84.
Schematic illustration of the NTR-responsive NIR-II fluorescence/PA probe 149 for visualizing tumors and inducing an NTR-triggered PTT effect.
In order to overcome the hydrophobicity and photodegradation of traditional photothermal reagents. Guo et al. developed a supramolecular PTT system 150 by complexing sulfonated azocalix arene (SAC4A) with organic small molecule photothermal agent IR780, which significantly improved the solubility, photostability, and photothermal conversion rate of the photothermal agent. SAC4A and its reductase reduction products NH2C4A were able to increase the photostability and photothermal conversion of IR780, further enhancing PTT efficacy. This hypoxia-responsive probe-based release of IR780 (fluorescence ON state) from 150 (fluorescence OFF state), enables tumor-selective imaging and supramolecular PTT of 150 in vitro and in vivo (Figure 85).439
Figure 85.
Schematic illustration of supramolecular Photothermal agents 150 constructed using 150a and 150b. And the tumor-selective imaging and PTT application of the supramolecular nanoformulation 150. Adapted with permission from ref (439). Copyright 2022 Ivyspring International Publisher.
Activatable phototheranostics exhibit promise for the precise treatment of cancer. However, most probes in this category only offer PDT or PTT, which may be less effective due to cellular hypoxia and complex TME. To address these issues, Pu et al. have developed a dual-locked activatable phototheranostic probe 151. This probe emits near-infrared fluorescence (NIRF) signals in tumors, triggers PDT in response to a tumor-periphery biomarker, and switches from PDT to PTT when a hypoxic biomarker is detected in the tumor core. This PDT-PTT auto-regulated probe can generate cytotoxic 1O2 around the tumor with a single laser source, producing thermotherapeutic effects in the tumor core that lead to complete tumor ablation. This double-locked probe thus shows promise as a molecular design strategy for precise cancer phototheranostics (Figure 86).440 Moreover, Peng et al. used the smFRET-guided smart molecule design methods incorporating diiododistyrylbodipy (BDP) and croconaine (CR) to construct PS BDP-CR (152), which showed an encouraging “1 + 1 > 2” effect on the combination of PDT and PTT in tumors, due to the synergistic effect of PTT and PDT, broadened the spectral absorption range in the NIR region 600–850 nm and improved the light-harvesting ability. Under normoxic conditions, the absorbed photon energy mainly sensitizes oxygen to generate ROS for PDT and stimulates CR by FRET for PTT. Under severe hypoxic conditions, the photoenergy will mainly kill cancer cells via O2-independent PTT (Figure 87).441 To overcome the low efficacy of PDT in hypoxic tumor environments, Liu et al. developed a novel boron dipyrromethene (BODIPY)-based PS (Ion-BDP), which was regulated by nitroreductase that is overly expressed in the hypoxic tumor microenvironment.436 When Ion-BDP was exposed to near-infrared light, it produced ROS for PDT while also consuming oxygen. In anaerobic microenvironments, nitroreductase cleaves 4-nitrobenzyl from Ion-BDP and converts it into a photothermal agent called BDP for PTT. Studies have shown that Ion-BDP was fluorescent, but this fluorescence was weakened by the PeT effect of activated BDP. These “on–off” PSs provide a model for the development of single-wavelength light sources to stimulate photodynamic and photothermal therapies, simplifying multi-mode phototherapy treatments. Additionally, other dual-function probes with synergistic PDT/PTT therapeutic properties also have been developed, such as 2TPAVDPP, TPATPEVDP, 2TPEVDPP,442 AzoCyS-N NPs,443 and LCT-CyI-TPZ.444
Figure 86.

(A) Schematic illustration for autoregulated PDT-PTT in the tumor. (B) Scheme describing the molecular mechanism of 151 for real-time imaging of tumor and autoregulated PDT-PTT in the presence of GGT and NTR. Reproduced with permission from ref (440). Copyright 2022 Wiley Intersciences.
Figure 87.
Schematic illustration of the smFRET-based combination phototherapy mechanism (152) and light-triggered cancer cell death.
To enhance the photothermal conversion efficiency of supramolecules in tumor hypoxia, Zhang et al. created supramolecular complex 153 by combining a PDI derivative with CB[7].445 It was shown that 153 produced a supramolecular perylene imide radical anion in the hypoxic environment, which could be controlled by restoring oxygen supply to the tumor. Through quenching of the supramolecular PDI radical anions, the “on–off” states could be achieved, significantly improving the photothermal conversion efficiency and inhibiting the expression of HIF-1 (Figure 88).
Figure 88.

Schematic representation of in situ hypoxia-induced supramolecular PDI radical anions in tumors for specific PTT with controlled “on–off” states. Reproduced with permission from ref (445). Copyright 2022 American Chemical Society.
In addition to PDT and PTT synergistic probes, other multi-functional systems based on anaerobic environmental activation have also been developed, such as PTT/bio-imaging and drug/gas synergistic therapy. Chen et al. have designed a dual-function nanomaterial Cy-C-S-NPs (154-NPs) for precision tumor therapy and imaging,446 which consists of a gold nanomaterial with a surface-modified phospholipid layer (C-S-NPs) that is then coated with near-infrared fluorescent dye Cy-DM. The fluorescence of Cy-DM was quenched by C-S-NPs in a non-hypoxic environment. However, the unique hypoxic microenvironment of tumor cells leads to the breaking of the azo bonds, releasing Cy-DM and generating a fluorescence response. Therefore, 154-NPs can achieve dual imaging by fluorescence molecular imaging and Raman imaging, and generate PDT and PTT simultaneously under laser irradiation for efficient synergistic therapy at the tumor site. Moreover, 154-NPs exhibited minimal obvious damage to normal organs such as the heart, liver, spleen, lung, and kidney, indicating its potential application in cancer therapy (Figure 89).
Figure 89.

Mechanism of 154-NPs for the detection of hypoxia and the PDT/PTT of tumors. Reproduced with permission from ref (446). Copyright 2013 Royal Society of Chemistry.
Furthermore, strategies that combine drug and photothermal/photodynamic properties have been developed. Lin et al. developed a multifunctional BAC prodrug that combines the chemotherapeutic drug camptothecin (CPT) and the fluorescent photothermal agent BODIPY through hypoxia-responsive azobenzene linkers.447 This prodrug was then encapsulated in human serum albumin to generate nanoparticles (HSA@BAC) to enhance solubility and tumor accumulation. In hypoxic cancer cells, overexpressed azoreductase reduces the BAC, releasing CPT for effective chemotherapy. When irradiated with a 730 nm laser, the HSA@BAC nanoparticles generate hyperthermia through oxygen-independent PTT, achieving irreversible cancer cell death. Moreover, Zhao et al. developed NP1, a nanovesicle composed of a hypoxia-responsive conjugated polymer (P1), polymetric H2S donor (P2), and near-infrared light-harvesting aza-BODIPY dye (B1) for delivering H2S and synergistic H2S gas therapy/PTT.448 The scaffold of NP1 decomposes in hypoxic environments, triggering the hydrolysis of P2 to continuously release H2S. B1 exhibits high photothermal conversion efficiency and superior photothermal ability under NIR light irradiation, which can inhibit the expression of cytochrome c oxidase (COX IV), cut off the generation of ATP, and inhibit mitochondrial respiration. This enhancement improves the antitumor efficacy of H2S gas therapy/PTT in hypoxic environments.
2.3.3. Tumor-Specific Enzyme and Other Biomolecules Activated Theranostic Probes in PTT
Enzymes as important biomarkers are often used to develop diagnostic and therapeutic tools for tumors. γ-Glutamyl transpeptidase (GGT) is a biomarker that is significantly upregulated in tumor tissues. Liu et al. reported a GGT-responsive near-infrared nanoprobe (NRH-G-NPs) that, through an amide bond couple γ-glutamate (γ-Glu) and cyanine fluorophore (NRH-NH2),449 the nanoprobe (NRH-G-NPs) was converted to NRH-NH2-NPs under the specific reaction with GGT, and the activation product NRH-NH2-NPs exhibited a 180-fold fluorescence signal enhancement and excellent photothermal therapeutic properties. Furthermore, this research found that β-galactosidase was overexpressed in ovarian cancers. In 2018, Pu et al. developed an activatable macrotheranostic probe (CyGal-P, 155) that was composed of a d-galactose-caged NIR hemicyanine dye (CyOH) linked with a long poly(ethylene glycol) (PEG) chain,450 which released its near-infrared fluorescence (NIRF), photoacoustic (PA), and photothermal signals by β-galactosidase enzyme activation, realizing enzyme-activated imaging-guided PTT providing a new direction for the development of β-gal-activated theranostic agents (Figure 90).
Figure 90.
(A) The synthetic steps of 155. (B) Schematic diagram of the activation mechanism of 155 in the tumor. Reproduced with permission from ref (450). Copyright 2018 Wiley Intersciences.
Similar studies have found that alkaline phosphatase (ALP) is overexpressed in metastatic prostate cancer. Yang et al. developed a mitochondria-targeting probe 156 that was activated by ALP and provided NIR FL/PA signals for imaging ALP activity, which undergoes simultaneously in situ self-assembly into a supramolecular structure, enhancing the photothermal therapeutic efficiency toward prostate cancer.451 As such accumulation enzyme activation, fluorescence/photoacoustic imaging synergistic photothermal therapeutic properties provide more scope for practical applications as diagnostic probes (Figure 91).
Figure 91.

Chemical structure and molecular mechanism of probe 156 in tumors. Reproduced with permission from ref (451). Copyright 2019 Royal Society of Chemistry.
In addition to enzymes, many biomolecules are important activating molecules to develop diagnostic tools, e.g., cysteine (Cys), hydrogen sulfide (H2S), and GSH. Clinical studies have shown that high concentrations of Cys exist in tumor cells and play an important role in the balance of redox homeostasis. Kolemen et al. introduced a cysteine (Cys)-activatable chlorinated hemicyanine probe 157 that used chlorinated hemicyanine as the fluorescent core and an acrylate unit attached to the core as a cysteine recognition group.452 Upon reacting with Cys, Cl-Cys turns on its near-infrared fluorescence signal and activates its 1O2 generation as well as photothermal conversion potential. This study is the first report of organic small molecules with integrated photothermal and photodynamic diagnostic properties based on Cys activation. It provides a research direction for the design of biomolecular-activated diagnosis and treatment probes in tumors (Figure 92).
Figure 92.
Molecular mechanism of the interaction between 157 and Cys, which results in NIRF and “Turn-On” PPT/PDT.
Moreover, H2S is a potential pharmacological target for the imaging-guided treatment of colorectal cancer (CRC). Recognizing this, Zhao et al. developed a hydrogen sulfide (H2S)-activatable nanostructured photothermal agent (158) composed of a benzene ring with three PEG chains that was connected to a hemicyanine as a hydrophilic tail and a monochlorinated BODIPY core as an activatable unit.453 under solution conditions, the probe self-assembles to form nanostructured complexes (158 NPs) due to hydrophilic and hydrophobic properties. In the presence of H2S, 158 NPs produced efficient photothermal conversion under 790 nm laser irradiation and successfully achieved effective photothermal ablation of colorectal cancer under image guidance (Figure 93).
Figure 93.
(A) Synthesis of H2S-responsive SSS and self-assembly process of 158. (B) Schematic diagram of 158 NPs activation in tumor for NIR fluorescence-guided PTT. Reproduced with permission from ref (453). Copyright 2018 American Chemical Society.
Similarly, GSH is a characteristic biomolecule found in tumor cells. As such, numerous GSH-triggered “Turn-On” theranostic systems have been developed. In 2018, Jiang et al. developed a self-immolative drug-dye conjugated (DDC) prodrug system that was GSH-responsive,454 which utilized 2-hydroxy-5-methyl-1,3-phenylenedimethanol as its core with carbonate linkages coupling fluorescent dyes and drug groups. In a tumor microenvironment, the disulfide bond was cleaved by high concentrations of GSH and the drug and fluorescent dyes can be released simultaneously. The released fluorescent dyes act as photothermal therapeutic agents to facilitate the penetration of drugs, and thus enhance the therapeutic effect (Figure 94).
Figure 94.

Schematic diagram of the molecular mechanism of drug release, fluorescence activation, and PTT activation strategy in response to GSH.
To increase the retention time of the therapeutic drug at the tumor site, Lan et al. reported an in situ self-assembly strategy aimed at enhancing the PTT of glioblastomas.455 The probe (ICG-PEP-c(RGD)fk) was constructed by GSH-reactive self-assembling polypeptides as the skeleton, coupled to indocyanine green (ICG) as a theranostic agent and cyclic Arg-Gly-Asp [c(RGD)fk] peptides as the targeting groups. In the glioblastomas microenvironment, the disulfide bond was cleaved by GSH, while GSH-reactive self-assembling peptides increased the diagnosis and treatment system retention time and improved the photothermal therapeutic effect. Moreover, multimodal imaging synergistic therapy systems based on GSH-responsive systems have also been developed. Yang et al. reported a novel GSH-responsive theranostic nanoparticle for dual-modal imaging and PTT,456 the nanoparticle consisted of a disulfide-bond-linked hydroxyethyl starch paclitaxel (PTX) conjugate (HES-SS-PTX) and a near-infrared (NIR) cyanine fluorophore DiR. The conjugated PTX and loaded DiR were released when the disulfide bond was cleaved by GSH, and the released PTX could exert its photothermal therapeutic effect.
2.4. Theranostic Fluorescence Probes in SDT
In order to improve the efficacy of cancer treatment and reduce side effects, noninvasive therapy has become an attractive approach for the treatment of malignant tumors.457 SDT is a promising noninvasive cancer treatment, which was first described by Umemura et al. in 1989 to be a substitute of PDT.458 The sonosensitizer was activated through high penetration depth ultrasonic irradiation to generate ROS that can kill cancer cells,459 so as to achieve SDT. It is well-established that the SDT mechanism relies on ultrasonic cavitation and thermal destruction.459,460 Different from traditional cancer therapies, such as chemotherapy,461 radiotherapy,462 and PDT,463,464 SDT has the advantages of deep penetration, low cost, convenient use, and fewer adverse reactions.465 However, the combination of diagnosis with real-time monitoring for the delivery and action of sonosensitizers remains a challenge. In recent years, advanced imaging technology has provided an opportunity to solve this problem. Precision medicine implemented through imaging technology can enable an accurate diagnosis, and monitor the delivery and biodistribution as well as the therapeutic response.466 Generally, theranostic probes with imaging function and SDT properties are loaded on nanocarriers.467 Therapeutic probes are initially silent. When they enter the tumor microenvironment, the therapeutic probes are activated to produce imaging signals with high signal-to-noise ratios, and high sensitivity.468 In order to improve the efficacy of SDT, theranostic fluorescence probes are necessary to enable an accurate clinical judgment by physicians. Small molecule drugs loaded on nanoplatforms can realize the purpose of precise tumor localization. Nanoplatforms can specifically break down/release drugs and therapeutic agents at tumor sites on demand, which not only improves the bioavailability of these drugs but also reduces damage to normal cells or tissues. Therefore, SDT-based nanoplatforms play an indispensable role in the diagnosis and treatment of tumors. In fact, the combination of SDT and imaging technology to prepare theranostic probes can provide a more effective treatment plan for achieving the ultimate goal of tumor eradication. Studies along these lines may be divided into four categories: TME-activated, hypoxia-activated, H2O2-activated , and multifactor synergistically activatable theranostic fluorescent Probestheranostic probes.
2.4.1. TME-Activatable Theranostic Fluorescent Probes in SDT
The tumor microenvironment (TME) provides appropriate conditions for tumor survival and metastasis.469 Tumor areas usually contain antioxidants, specific enzymes,470 lower blood oxygen saturation, and increased acidity,471 which provide directions for tumor treatment. In addition, specific nanocarriers can undergo ligand exchange, decomposition, or aggregation in the TME, resulting in their accumulation within the tumor, with enhanced therapeutic effects.472−477
In general, over-expressed GSH and manganese superoxide dismutase (SOD2) play important roles in tumor cells’ resistance to ROS damage. In this regard, Zhu et al. prepared Fe(III)-porphyrin nanosensitizers (NTP) through the self-assembly of Fe(III) with meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS), followed by siRNA loading to form R-S-NTP (159) (Figure 95).478 The loaded siNTP can effectively down-regulate the expression of manganese superoxide dismutase (SOD2). The porphyrins and Fe(III) in NTP not only endow it with an admirable MR/FL imaging capability but can also effectively increase the ROS production, to improve the SDT efficiency. In addition, Fe(III) induces a cascade of bioreactions within tumor cells, which reduces the level of intracellular GSH and enables a cytotoxic Fenton reaction, leading to cancer cell death. 159 produces more ROS than RNTP, NTP, and TPPS, ascribable to a decrease of cellular antioxidant defense and the occurrence of a Fenton reaction, enhancing the efficiency of SDT. After intravenous injection, fluorescence signals were observed in tumors in the 159 group. The 159 + US group of tumor inhibition efficiency reached 89.96%, suggesting an excellent capability to prevent tumor growth. This work provides a promising approach to overcome the challenges of SDT in clinical settings, due to its excellent efficacy and novel combination of therapeutic modalities.
Figure 95.
(A) Theranostic 159 as a multifunctional sonosensitizer. (B) In vivo FL images. Reproduced with permission from ref (478). Copyright 2019 Wiley Intersciences.
Hyaluronidase (HAase) is an enzyme that is overexpressed in tumors.479 As an endogenous enzyme, hyaluronidase plays a crucial role in the tumor specificity and drug release rate of theranostic molecules. In this regard, Qiu et al. fabricated a nanosystem (DOX@HPNAs, 160) by synthesizing a negatively charged HA-PpIX nano-assembly and then loading the positively charged DOX with a 24.6% load efficiency, to achieve an effective three-mode treatment.480 Under acidic conditions, HAase rapidly degrades HPNA, which leads to the breakdown of nanostructures and the release of drugs. Under light and/or ultrasonic excitation, the nanosystem clustered at the tumor sites and produced fluorescence (Figure 96), and the 1O2 produced by enzymic degradation was 2.5 times that the original HPNA, showing an enhanced photoacoustic sensitization. The results showed that tumor growth was significantly inhibited in the treatment group compared to the fast-growing tumors of the control group. Histological examination of tumor sections showed that the inhibition rate of tumor growth reached 94.4% in the treatment group, indicating an excellent therapeutic effect. This work increased the Dox release efficiency through a trimodal stimulus, including acidity, ultrasonic vibration and enzyme degradation for photo-sono-chemo synergistic therapy, providing meaningful guidance for the future development of novel enzyme-activated nanosystems.
Figure 96.
(A) Scheme illustrating the dynamic effects of endogenous (pH, HAase) and exogenous (NIR, ultrasound, and tissue barrier) factors on activatable polymeric nanosystems (160). (B) Time-dependent fluorescence images and intensities of tumors after the intravenous injection of DOX@HPNAs (160). (C) Relative growth rates of tumors after different treatments. Reproduced with permission from ref (480). Copyright 2022 American Chemical Society.
TME blood oxygen saturation (SaO2) has played a crucial role in the sustained and stable growth and development of cancer cells.481 To reduce SaO2, Yang and co-workers used a self-assembly approach to synthesize a self-targeting therapeutic nanoplatform called TPGS-PEM-ICG (TPI, 161) for fluorescence/photoacoustic (FL/PA) imaging and enhanced chemo-sonodynamic therapy (Figure 97).482 The 161 nanoplatform can selectively identify tumor cells and deliver active targeted drugs on demand through multiple triggers, being the acidity of the TME, lysosomal acidity, esterases, and external ultrasound, combined this reduces the side effects on normal tissues around the tumor. In addition, PEM ligands on the surface of 161 have a strong affinity for overexpressed FA receptors on the surface of HeLa cell membranes, showing prominent tumor enrichment and cellular uptake. The excellent fluorescence signal of FA receptor-overexpressed solid tumors indicated that the nanoplatform can be used to diagnose cancer. PA imaging results showed that the SaO2 signal of 161 dropped sharply after US irradiation, indicating that 161 + US could achieve “starvation therapy” by reducing the SaO2 signal. The killing effect of 161 + US group on tumor cells was more significant than that of other groups, demonstrating a strong antitumor effect. The results of anti-tumor experiments in vivo showed that almost all tumors in the 161 + US group were eliminated, indicating that 161 + US has a spectacular anti-tumor effect. In summary, this strategy, combined with FL/PA imaging, is a good example of real-time monitoring and active targeted tumor SDT, giving guidance to future designs.
Figure 97.
(A) TPGS-PEM prodrug and 161 self-assembly synthesis route. (B) Intensive chemotherapy and sonodynamic therapy with 161-guided by dual imaging. (C) Changes of SaO2 levels in each group. (D) Changes in tumor volume after different treatments within 14 days. (E) HeLa cells stained with calcein AM/PI were incubated with different groups, and 161 (TPI) for 24 h, with or without US irradiation. Reproduced with permission from ref (482). Copyright 2021 Royal Society of Chemistry.
Tumor cells prevent their acidosis by excreting excess lactic acid, causing the low pH state characterstic of the TME.483 On this basis, Li et al. designed a novel phthalocyanine-iron complex 162 as a theranostic nanoreactor for fluorescence/magnetic (FL/MR) resonance dual-mode image-guided SDT/CDT.484 In the acidic TME, 162 reacts with protons to release PcD, forming Fe2+, which is then further oxidized by H2O2 to form Fe3+, finally restoring the fluorescence of PcD (Figure 98). Through this programmable response, the tumor site of 162-treated mice showed a strong fluorescence signal, which was 10.49 times stronger than that of PcD-treated mice, thus demonstrating that 162 causes a pronounced fluorescence imaging (FLI) signal in a tumor. It is worth noting that the magnetic resonance signal of 162 also switched from the “OFF” state to the “ON” state under this programmable regulation by acidity and H2O2. In addition, 162 combined with US irradiation had a significant inhibitory effect on the growth of tumors in mice, with an inhibition rate of 87.15%. In conclusion, 162 achieves specific fluorescence and MR dual-mode image-guided SDT/CDT and represents a promising image-guided tumor therapy approach.
Figure 98.
(A) Preparation and programmable mechanism of 162. (B) The action of 162 in HepG2 hepatoma cells. (C) The mean fluorescence intensity of HepG2 tumor-bearing mice at different time points after intravenous injection of 162 and PcD. (D) Tumor growth curve. Reproduced with permission from ref (484). Copyright 2023 Elsevier Ltd.
2.4.2. Hypoxia-Activatable Theranostic Fluorescent Probes for SDT
SDT can generate ROS to induce oxidative damage to cancer cells, and oxygen is one of the main sources of ROS. However, due to abnormal cell proliferation, vascular system abnormalities, lymphatic system dysfunction, and other reasons,485 solid tumors remain in a state of hypoxia for a long time, which severely limits the efficiency of SDT. Meanwhile, the rapid consumption of oxygen at the tumor sites by SDT process can also lead to the exacerbation of local hypoxia, which further inhibits SDT through a chain reaction, leading to poor therapeutic effects and poor prognosis.486 Hypoxia can also lead to drug resistance for chemotherapy and radiotherapy,487 affecting their therapeutic effectiveness. Therefore, methods to diminish the influence of hypoxia on the efficiency of SDT have become a key research area.
It has been shown that upregulation of O2 content in tumors through tumor-targeted O2 delivery and intratumoral oxygen production strategies are effective ways to alleviate tumor hypoxia. For example, the introduction of Fenton or Fenton-like reactions is a promising method to trigger oxygen production.488−490 Duan et al. developed a nanoplatform 163 involving Fenton-like reactions (Figure 99), which alleviates tumor hypoxia through this oxygen-producing strategy and improves the therapeutic effect of SDT and PDT.491 Among them, porphyrin MOF-525 can not only produce 1O2 for SDT and PDT but also can be used as a two-photon-responsive moiety for NIR photoinduced PDT. Pd nanocubes generate hydroxyl radicals (•OH) and O2 through a Fenton-like reaction, which leads to cell apoptosis and greatly alleviates tumor hypoxia. The surface modified of Pd@MOF-525 by hyaluronic acid (HA) enhances biocompatibility and provides a specific targeting ability toward cancer cells. The cell viability experiments showed that the survival rates of QSG7701 cells treated with 163 were higher than that of HepG2 cells, and the survival rate of HepG2 cells after the addition H2O2 was lower than the control group, indicating that 163 is harmless to normal cells, while •OH can be produced in cells with high H2O2 expression, resulting in decreased cell viability. A cytotoxicity evaluation revealed that the cell survival rate of HepG2 cells after light and ultrasonic irradiation was 10%, which confirms that the nanoplatform exhibits outstanding synergistic therapeutic PDT/SDT effects. This work provides a suitable approach to design novel oxygen-producing nanomaterials that can enhance oxygen-dependent antitumor treatment.
Figure 99.

Preparation of theranostic probe 163, highlighting the process of enhanced photodynamic and sonodynamic therapy. Reproduced with permission from ref (491). Copyright 2022 BioMed Central Ltd.
Since hypoxia within the tumor can only be temporarily alleviated by oxygen production or transport to solid tumors,492 some problems remain such as low oxygen production efficiency or oxygen leakage during these processes.493−497 Therefore, reducing oxygen consumption may represent a feasible solution to replace the methods mentioned above. Since the main function of mitochondria-related oxidative phosphorylation (OXPHOS) is to generate energy by consuming oxygen,498−500 inhibition of OXPHOS activity can be an effective way to reduce oxygen consumption. Therefore, Zhang et al. prepared a pH-responsive drug-carrying liposome (164) to reduce oxygen consumption in tumor areas.501 Metformin molecules in 164 are released in acidic tumor tissues and selectively accumulate in tumors to inhibit the mitochondrial respiratory chain (Figure 100). While the sonosensitizer IR780 was released into the tumor areas to produce ROS for killing the cancer cells under US irradiation. In addition, intravenous 164 could effectively deliver encapsulated drugs to the hypoxic sites of tumors due to its enhanced permeability and retention (EPR) effect. Theranostic 164 exhibits excellent PA/FL imaging capabilities both in vitro and in vivo applications. The results indicated that tumor growth was significantly inhibited in the 164 + US treatment group. Notably, tumor growth was significantly retarded after treatment due to rapid drug release in the acidic microenvironment of the tumor, while rapid tumor growth remained in the control groups. This research has resulted in a nanoplatform able to overcome hypoxia-induced resistance to cancer therapy by interfering with normal energy metabolism processes to reduce oxygen consumption.
Figure 100.

(A) Schematic illustration of self-synthesized 164 (MI-PEOz-lip) and its proposed antitumor mechanism. (B) Tumor growth curves of the five groups after receiving various treatments. Reproduced with permission from ref (501). Copyright 2020 Dove Medical Press Ltd.
Although many methods have been developed to alleviate hypoxia by generating/transporting oxygen502−506 or reducing oxygen consumption within solid tumors, the therapeutic effectiveness may under some conditions be unfavorable and may even have adverse consequences, including barotrauma and hyperoxic seizures.465 Acoustic droplet vaporization effect induces a rapid phase-shift mode for liquid perfluoropentane (PFP) to gas phase upon ultrasonic triggering.507 Microvesicles can improve the ablation effects of high-intensity focused ultrasound (HIFU), reduce the required acoustic energy, and enhance tumor damage. Hence, phase change materials with similar therapeutic behavior are expected to regulate the acoustic environment of hypoxic tumors.508,509 Therefore, the PvP-based cavitation effect is an attractive strategy that can effectively induce anoxic tumor death without the need for oxygen. In addition, CGNKRTR (tLyP-1) is a cell-penetrating peptide used as a ligand for targeting neuroproteinase-1 receptor (np-1) and can effectively penetrate deep into tumor cells through the endocytosis/extracellular transport pathway (CendR pathway).510−512 Based on the above considerations, Luo and co-workers fabricated a functionalized liposome based on tLyP-1, and then loaded porphyrin monomethyl ether gadolinium (H(Gd)) as a sonosensitizer into the phospholipid bilayer to afford PFP@tLyP-1-LIP-H(Gd) (165).513 The liposome can target MDA-MB-231 tumor cells through the specific adhesion of cell-penetrating peptides to specific cells overexpressing NPR-1 and can effectively penetrate hypoxic tumors to facilitate US/NIRF/PA/MR imaging (Figure 101). Under low-intensity focused ultrasound (LIFU) irradiation, the acoustic droplet vaporization effect of perfluoropentane induces rapid “liquid-gas” transition and rapid bubble production to produce hydroxyl radicals (as deep penetrating nano-bombs, DPNB), resulting in cell death in both normoxic and hypoxic microenvironments. 165 exhibits enhanced cytotoxicity after treatment with LIFU. Tumor inhibition rates confirmed that acoustic droplet vaporization combined with SDT inhibited tumor growth. This study was the first to report oxygen-independent SDT based on ultrasonic cavitation effects and “liquid-gas” conversion.
Figure 101.
(A) The generation of ROS and ADV mechanisms for synergistic hypoxia-tolerant SDT against solid hypoxic tumors under multimodal imaging guidance. (B) Relative tumor volume changes of the mice after various treatments. (C) Tumor inhibition rate of the mice after multiple treatments. Reproduced with permission from ref (513). Copyright 2022 Dove Medical Press Ltd.
2.4.3. H2O2-Activatable Theranostic Fluorescent Probes in SDT
ROS include H2O2, ozone (O3), hypochlorous acid/hypochlorite (HOCl/ClO–), hydroxyl radical (•OH), nitric oxide (NO), and peroxynitrite (ONOO–).507 Among them, H2O2 is one of the most important species. It is produced in the mitochondria, mainly through the activation of the nicotinamide adenine phosphate dinucleotide oxidase complex (NADPH),514 which is an important signaling molecule for cell growth, proliferation, and differentiation.515 Increased concentrations of H2O2 at tumor sites are closely related to tumor cell growth, development, and apoptosis. In addition, the intrinsic enhancement of H2O2 levels in tumor cells induces the expression of metastasis-related growth factors, leading to invasion and migration,516,517 which is one of the most important causes of cancer death. Therefore, it has been evaluated as an important target for the design of novel anti-tumor strategies.518 However, currently, no theranostic fluorescent probes activated by H2O2 for SDT have been reported, meaning that more research is urgently needed in this area.
2.4.4. Multifactor Synergistically Activatable Theranostic Fluorescent Probes in SDT
Severe hypoxia, GSH over-expression, and high concentrations of H2O2 at tumor sites restrict the therapeutic effect of ROS in PDT, chemodynamic therapy (CDT), and SDT.518 To address this problem, Wang et al. designed a combination strategy consisting of precision-guided imaging, GSH consumption, targeting, and catalase activity to construct a bio-catalyzed Janus nanocomposite (166) based on an iron-based zirconium porphyrin metal–organic framework [PN-224 (Fe)], mediated by near-infrared (NIR) light and US (Figure 102).519 Fe3+ acts synergically in 166 as a catalase-like nanozyme, which not only catalyzes H2O2 to produce O2, relieving the TME hypoxia but also consumes excess intracellular GSH and promotes ROS generation. Fe2+ reacts with intracellular H2O2 to produce toxic •OH, which improves the CDT performance. 166 exhibits PDT properties when it is excited by 808 nm laser irradiation at high H2O2 concentrations. Synchronous activation using US achieved an additional SDT effect and an enhanced fluorescence signal. The results of cell activity evaluations indicated that the 166+NIR+US group exhibited a high degree of apoptosis in tumor cells. Therefore, using a combination of multiple tumor sites characteristics and imaging guidance provides a promising strategy for improving the therapeutic efficacy of SDT, PDT, and CDT.
Figure 102.
(A) Schematic Illustration of Antitumor Mechanism of 166. (B) The detection of live/dead cells after various treatments. Live and dead cells were stained with calcein-AM (green) and PI (red), respectively. Reproduced with permission from ref (519). Copyright 2021 American Chemical Society.
Later, Liu and co-workers used the acidic TME to develop a multimodal (PA/FL/MR) image-guided multifunctional nanozyme AIMP NPs (167) by encapsulating IR780 and MnO2 within PLGA/Angiopep-2 to enhance SDT (Figure 103).520 Theranostic probe 167 can easily penetrate the blood–brain barrier (BBB) and target gliomas due to Angiopep-2. MnO2 is known to exhibit enzyme-like activity, it can react with high levels of protons, H2O2, and GSH in the cancer TME to generate oxygen and degrade GSH. In addition, Mn2+ is used as an MRI contrast agent, and IR780 has PA/FL imaging capabilities enabling FL/PA/MR imaging-guided cancer therapy. After intravenous injection, fluorescence from Fe-TCPP in 167 was observed at tumor sites, indicating that biotin-modified 167 accumulates in the tumor area through the EPR effect. Cell viability tests indicated that SDT induced apoptosis of tumor cells under LIFU irradiation. Therefore, this work, using multiple mechanisms to maximize the SDT effect in U87MG xenografts, was able to significantly inhibit tumor growth and distal metastasis.
Figure 103.
(A) Schematic illustration of 167 NPs synthesis. (B) Schematic illustration of 167 NPs with BBB and tumor targeting, mitochondrial targeting, deep penetration, enhanced SDT effect, and real-time PA/FL/MR imaging monitoring. (C) CLSM images of live/dead cells after various treatments. Reproduced with permission from ref (520). Copyright 2021 Royal Society of Chemistry.
2.5. Theranostic Fluorescent Probes in Immunotherapy
In 1891, William Coley accidentally discovered that postoperative pyogenic streptococcal infections led to tumor regression in sarcoma patients, which was a prelude to tumor immunotherapy and has been a hotspot for the treatment of tumors ever since.521 Compared with traditional cancer therapies such as chemotherapy and radiotherapy, immunotherapy has the advantages of high selectivity, few side effects, and good efficacy, and has become a promising approach for clinical applications.522−524 Nowadays, cancer immunotherapies mainly include immune checkpoint therapy, adoptive cell therapy, and vaccines. Immune checkpoint blockade therapies based on CTLA-4 and PD-1/PD-L1 have good efficacy.525 Yet nearly 70% of patients do not respond positively to immune checkpoint inhibitors (ICIs). Besides, some adverse drug reactions may even lead to death.526−528 To address those problems and improve immunotherapy effects, researchers are beginning to explor combination therapies.
For example, some theranostic fluorescent probes with the capability of real-time imaging and immunotherapy have been developed by some researchers. These theranostic fluorescent probes with small molecule drugs can generate synergistic effects with checkpoint blockers to improve immunotherapeutic efficacy. In addition, due to the excellent penetration and appropriate half-life,529 theranostic fluorescence probes can provide real-time imaging for clinical diagnostics. Significantly, clinicians can obtain in vivo information rapidly and accurately using small molecule-based probes. As such, small molecule drugs are are appealing for preparing theranostic fluorescence probes for combination strategies.530,531 It has been reported that the metabolic state of the tumor microenvironment (TME) is essential to tumor immunotherapy.532 There are some typical characteristics for immune-related targets of the TME including H2O2 overexpression, aberrant physicochemical properties, and hypoxia.533 Taking advantage of these features, a diversity of theranostic probes have been developed toward the TME based on differences in pH,534 GSH,535 and H2O2536 concentrations as well as hypoxia.537,538 Such highly sensitive probes are suitable for the imaging of tumors, and provide valuable data for advanced analysis. This section summarizes the state-of-the-art of theranostic fluorescent probes based on TME, H2O2, and hypoxia-based activation.
2.5.1. TME-Activatable Theranostic Fluorescent Probes in Immunotherapy
The TME is a complex system that contains tumor cells, immune cells, lymphovascular cells, and various metabolites.539,540 The metabolic state of the TME affects tumor immunity through multiple mechanisms.532 Immune cells can interact with tumor cells to activate an immune response or an immune tolerance.541 When persistent tumor antigen stimulation and immune activation responses are elicited, the relevant effector cells in the microenvironment are depleted or remodeled. As a consequence, the relevant effector cells cannot exert normal function and this can promote the malignant characteristics of tumors. Immunosuppressive TME largely reduces immunotherapy efficacy due to its limitation in recruiting and activating T cells.542 For example, immune checkpoint inhibitor (ICI) immunotherapy exhibits good effects in clinical cancer therapy,543 but relies on an immunosuppressive TME. Therefore, only 30% of patients demonstrate satisfactory ICI treatment outcomes.528,544 As such, mono-modal immunotherapy loses its advantage, and it becomes necessary to improve the immunosuppressive TME and boost immunotherapeutic efficacy. For this reason, immunotherapy has been combined with small molecule-based fluorescent probes to generate synergistic effects, which can not only increase immune cell activity but also induce PTT and PDT for adjuvant therapy.545 Real-time tumor imaging by theranostic probes can help clinicians monitor disease states in vivo and provide valuable treatment information. Therefore, TME-activated theranostic probes represent promising approaches for improved immunotherapy.
Over the past several decades, adaptive immune resistance has been identified as a hallmark of tumor development.546 Programmed cell death receptor 1 (PD-1) and programmed death 1 ligand (PDL1, B7-H1) are two crucial immune checkpoint molecules associated with immune resistance. PD-L1 is significantly expressed in many tumor cells, such as melanoma, breast, and renal cell carcinoma. In cancer cells, PD-L1 can bind with PD-1 of T cells to suppress the immune response and reduce immunotherapeutic efficacy.523 To improve immunotherapy, antibodies have been used to block PD-L1/PD-1 binding, and this strategy has resulted in significantly enhanced cancer treatment efficiencies. Taking low pH-activated drugs as a precursor, Li et al. combined these drugs with immune checkpoint blockade to develop a theranostic probe.547 Li and co-workers synthesized acid-activated multifunctional micelleplexes (168) that consist of the pheophorbide A (PPa) PS, PD-L1-specific siRNA, and the pH-responsive PDPA (Figure 104). These micelles are non-fluorescent and do not exhibit phototoxicity in normal physiological pH environments due to fluorescence resonance energy transfer from PPa. Under acidic TME conditions (pH < 6.2), PPa in these micelles was activated to generate fluorescence signals for real-time imaging. Moreover, the activated PPa promotes ROS production to stimulate an antitumor immune response. Additionally, siRNA was released to block the PD-1/PD-L1 pathway. After intravenous injection, fluorescence signals were observed in the 168 + laser treatment group. In sharp contrast to the control group, the concentration of TNF-α and IFN-γ in the micelles + laser group dramatically increased, suggesting the immune response has been greatly enhanced. In the 168 + laser group, tumors were eliminated, and subcutaneously re-challenging these mice with the same tumor cells failed to result in tumor growth in 75% of these mice. This result indicates that this acidic TME-activated drug platform can lead to excellent anticancer immune efficacy.
Figure 104.
(A) Chemical structure of the acid-activatable 168. (B) Schematic representation of 168-mediated photodynamic cancer immunotherapy. (C) Photographs and (D) H&E staining of the metastatic foci of the B16-F10 tumors. Reproduced with permission from ref (547). Copyright 2016 American Chemical Society.
GSH is a reductive tripeptide with a cellular antioxidative function;488 the conversion between GSH and its oxidated GSSG form can regulate cell redox environments.548 In the TME, GSH over-expression leads to a reductive environment that could be utilized for reduction-activated theranostic probes.549 Yu et al. designed a GSH-activated prodrug 169 that consists of a PS (PPa), an inhibitor (NLG919) of IDO-1 that is activated under reductive conditions, and a PEG corona.550 IDO-1 is an endogenous immune regulator, which can induce apoptosis in T cells, thus triggering the adaptive immune resistance response and reducing the efficacy of immunotherapy. NLG919 could effectively reduce IDO-1 activity to enhance the intratumoral infiltration of CTLs. 169 was silent in normal physiological environments. Since the PEG corona has good cell penetration, bio-compatibility and targeting ability, the prodrug specifically accumulated in tumors. In the reductive TME, 169 was activated by GSH and generated fluorescence signals for real-time imaging. NLG919 was released from 169 and suppressed IDO-1, to eliminate the adaptive immune resistance (Figure 105). In addition, under near-infrared irradiation (NIR 671 nm), PPa promoted ROS production to induce immunogenetic cell death (ICD). In the 169 + laser irradiation treatment group, the flow cytometry results showed that the maturation ratio of DCs was 1.7-fold higher than the control groups, and the intratumor invasion of IFN-γ and Tc was also significantly increased. These observations suggest that the immunotherapy efficacy was largely improved by this self-assembled system.
Figure 105.
(A) Schematic illustration of the MMP-2-sheddable and GSH-activatable prodrug vesicle 169. (B) Tumor growth curves of the 4T1 tumor model and (C) the CT26 tumor model. (D) Survival curves of 4T1-tumor-bearing mice. Reproduced with permission from ref (550). Copyright 2019 American Chemical Society.
To achieve imaging-guided sono-photodynamic immunotherapy, Ye et al. synthesized a tumor-targeting and GSH-activated NIR Zn-chelated pheophorbide probe (Zn-PPA-SH), and then co-assembled it with the IDO1 inhibitor NLG919 and a hydrophilic Gd-DOTA chelate (2-Gd) for MRI to fabricate nanosensitizer 170.551 Zn-PPA-SH can generate ROS under laser irradiation to improve both the efficiency of PDT and immunotherapy. Additionally, Zn-PPA-SH can produce a NIR fluorescence “Turn-On” signal at 672 nm for real-time imaging. After administration into tumor bearing mice, 170 can effectively penetrate tumor sites by cRGD-mediated delivery, which could be tracked through T1-weighted MR imaging. In the TME, a high concentration of GSH can reduce the disulfides of 170. Consequently, Zn-PPA-SH and NLG919 are released (Figure 106). At 8 h post-injection, intense NIR fluorescence signals appear in the tumor areas, which exhibited 6.5-fold higher fluorescence than the control groups. The treatment group that was administered 170 in addition to US and laser irradiation exhibited prolonged survival, which extended to 54 days. In sharp contrast, all mice in the saline-treated group failed to survive more than 32 days. These results imply that GSH-activated 170 can improve the efficacy of cancer therapy.
Figure 106.
(A) Schematic mechanism of 170 for FL and MR bimodal imaging-guided sono-photodynamic immunotherapy of tumors. (B) The chemical structures of released 2-Gd, NLG919, and Zn-PPA-SH. (C) BL images in mice with 170. Reproduced with permission from ref (551). Copyright 2023 Wiley Intersciences.
2.5.2. H2O2-Activatable Theranostic Fluorescent Probes in Immunotherapy
Zhang et al. developed self-illuminating nanoparticles 171 by conjugating a PS Ce6 (an amphiphilic conjugate of chlorin e6) with luminol and PEG (Figure 107).552171 can simultaneously achieve diagnosis and therapy by virtue of chemiluminescence resonance energy transfer (CRET) between luminol and Ce6. In the presence of H2O2, the luminol unit is oxidized by H2O2 to emit blue luminescence. Due to CRET, luminol can generate intense fluorescence signals that correspond linearly to the H2O2 concentration. In addition, 171 can generate 1O2 for PDT and immunotherapy through CRET-mediated in situ excitation of Ce6. Animal experiments were performed to investigate the theranostic effect of 171. After subcutaneous injection, clear luminescent signals appeared in the tumor areas. Moreover, the generation of 1O2 was sustained for approximately 8 ht. 171 was subject to a dose-dependent therapeutic efficacy, with the most effective result achieved at 3.25 mg/kg Ce6.
Figure 107.
Design of a luminescent nanoprobe 171 NPs for imaging and treating tumors expressing high H2O2. Reproduced with permission from ref (552). Copyright 2020 American Chemical Society.
The high concentrations of H2O2 in tumor cells provide new approaches for redox-sensitive theranostic probes. Yu et al. employed an AIE molecule TST with near-infrared luminescence and photothermal function to develop nanoparticles 172 by assembling camptothecin prodrug (CPT-S-PEG) and AZD4635 (an immune checkpoint inhibitor).553172 was silent in a normal physiological environment. However, after entering tumor cells, the nanoparticles exhibit an enhanced fluorescence quantum yield of 15.32%, suggesting these nanoparticles can be used for fluorescence imaging. The redox-sensitive group of the pro-drug (CPT-S-PEG) was degraded by H2O2, and active CPT was released into the TME. On the other hand, TST generated intense near-infrared luminescence suitable for real-time imaging (Figure 108). Moreover, TST promoted ROS production by PTT. Additionally, AZD4635 counteracted the immunosuppression generated by ICD to improve the efficacy of PTT and immunotherapy. After irradiating with a 808 nm laser for 5 min, the temperature of the nanoparticle system increased from 32.7 to 68.8 °C, suggesting that 172 exhibits good photothermal conversion efficiency. By injection into the mice’s tail vein, a clear NIR-II fluorescence image can be observed in the tumor, which was attributed to the EPR effect of the nanoparticles. Besides, the nanoparticles 172 exhibited good antitumor effect and satisfactory safety in vivo. After treatment with nanoparticles 172 for 12 days, the tumor in the mice disappeared. Although H2O2-based cancer diagnosis has been combined with chemotherapy, radiotherapy, PDT, SDT, and PTT518 to diagnose and treat cancer, there is still a long road ahead to fully realize clinical applications for these systems.
Figure 108.
(A) Schematic mechanism of 172 for therapy of tumors. (B) Images of alive small animals after intravenous injection of 172 or controls. Reproduced with permission from ref (553). Copyright 2022 Wiley Intersciences.
Cai et al. prepared core–shell nanoparticles AuNC@MnO2 (AM, 173) by coating gold nanocages with MnO2.554 The nanoparticles 173 as a PS displayed a good PDT effect under NIR irradiation (Figure 109). The MnO2 shell of AM can be decomposed in acidic and H2O2-rich circumstances of solid tumors to in situ produce oxygen and Mn2+, which can improve the oxygenation of TME and endow the nanoparticles 173 with good photoacoustic (PA)/magnetic resonance (MR)/fluorescence (FL) multimodal bioimaging ability. The oxygen can be converted to powerful ROS that can not only kill tumor cells but also elicit an immunogenic cell death (ICD)-mediated antitumor immune response. Under laser irradiation (808 nm), AM can generate enough 1O2 for PDT to induce ICD. For immunotherapy, AM can stimulate, recruit, and activate T cells to improve efficacy. After intravenous injection, the AM + laser treatment group showed the complete elimination of tumors within 15 days, and lung metastatic nodules from this group were also significantly fewer as compared to control groups.
Figure 109.
(A) The therapeutic mechanism of 173 (AuNC@MnO2) for therapy. (B) Tumor growth curves of different treated groups. (C) Mice weight of lung metastasis experiment monitored after PDT treatment. (D) The number of metastatic lesions counted from excised lungs. Reproduced with permission from ref (554). Copyright 2018 Elsevier Ltd.
2.5.3. Hypoxia-Activatable Theranostic Fluorescent Probes in Immunotherapy
Hypoxia is an intrinsic character of the TME.555 To date, the effect of hypoxia on cancer therapy has been extensively studied.556 It has been shown that uncontrolled cell proliferation of tumor cells leads to hypoxia in the TME. This effect is ascribed to the malignant proliferation outgrowing its oxygen supply. On the other hand, this rapid proliferation promotes abnormal growth of new blood vessels, resulting in sluggish blood flow, which also contributes to hypoxia in the TME.557 Generally, the oxygen content in normal physiological environments ranges from 2% to 9%, while it decreases sharply to less than 2% in parts of the TME. Indeed, hypoxia is toxic to both normal and cancer cells. However, tumor cells can survive under hypoxia conditions due to genetic and adaptive changes.558 For immunotherapy, hypoxia plays some important roles: (1) it induces T lymphocyte apoptosis;559 (2) it weakens the effect of natural killer and natural killer T cells;560 (3) it guides programmed dendritic cell death;561 (4) it induces immunosuppressive cells contributing to immune tolerance.562,563 Therefore, hypoxia largely limits therapeutic efficacy. To improve the efficacy of immunotherapy, strategies have been reported to alleviate hypoxia,564 such as the direct delivery of O2 into the tumor, and the in situ O2 generation in the TME. Additionally, hypoxia-responsive targeted drug delivery strategies have been developed. To achieve diagnosis and treatment, hypoxia-activated theranostic probes are the subject of intensive research. Researchers developed hypoxia-activated theranostic probes based on Tirapazamine, TH302, PR104A, AQ4N, etc.
Tirapazamine (TPZ) is a bio-reductively activated drug that can specifically induce cell death under hypoxia conditions. Cao et al. developed a hypoxia-activated theranostic probe 174 by co-loading liposome with the iodinated cyanine dye Cyl and TPZ.444 After intravenous injection, 174 specifically accumulates in the tumor. Under near-infrared laser irradiation (808 nm), Cyl in 174 can generate ROS for PDT and heat for PTT (Figure 110). Furthermore, Cyl emits NIR light, enabling tumor imaging. Moreover, PDT could stimulate the polar immune response, which recruits and activates CD4 helper T cells and CD8 cytotoxic T cells to promote the secretion of IFN-c, TNF-a, GM-CSF, and other cytokines for immunotherapy. Meanwhile, PDT consumes oxygen and this results in exacerbated hypoxia, aiding TPZ activation to selectively kill tumor cells. 1 h post-injection, fluorescence signals appeared at the tumor sites and the fluorescence was maintained for up to 24 h, suggesting LCT has a good imaging capability. After 21 days of treatment, the tumor volume of the 174 + laser treatment group was the smallest, and a good effect on the distal tumor inhibition was observed. This work developed a good combination strategy of PDT/PTT/CT/immunotherapy.
Figure 110.
(A) Scheme of 174 for highly efficient synergistic PDT/PTT/immunotherapy combined with hypoxia-activated chemotherapy. (B) In vivo dynamics of 174 in tumor-bearing mice. Reproduced with permission from ref (444). Copyright 2022 Informa UK Limited, trading as Taylor & Francis Group.
AQ4N can be specifically activated under hypoxic or anoxic environments. By virtue of this feature, AQ4N in combination with PDT and immunotherapy might be a good approach to improve tumor treatment efficacy. Yoon et al. developed a theranostic probe by combining the water-soluble phthalocyanine derivative PcN4 with AQ4N to improve PDT and immunotherapy.565 PcN4 can specifically bind with albumin in vivo to form supramolecular complexes, which endows it with excellent tumor-targeting ability, fluorescence imaging, and PDT antitumor activity. Moreover, PDT induces hypoxia enhancement in a tumor, and AQ4N activation is concomitantly increased in the tumor, which further improves the antitumor activity. After activation under hypoxic conditions, PDT also stimulates ROS production to induce immune checkpoint blockade (ICB) effect. As a consequence, tumor-bearing mice exhibit enhanced immunity under 655 nm laser irradiation. Importantly, the maximum capacity for systemic cancer-specific adaptive immune activation can be achieved by the combination of immune checkpoint blockade therapy with activated PcN4, which enables efficient abscopal responses and enhances anti-metastatic effects.
3. Theranostic Fluorescence Probes in Other Diseases
3.1. Alzheimer’s Disease
Apart from cancers, theranostic fluorescence probes have emerged as a promising tool for the early diagnosis and targeted therapy of other diseases like Alzheimer’s disease (AD). AD is a progressive neurodegenerative disorder that affects the brain’s ability to function properly.566,567 It is the most common form of dementia, accounting for about 60–80% of cases. The disease is characterized by the accumulation of the pathogenic β-amyloid (Aβ) plaques and tau protein tangles in the brain, which disrupts the normal functioning of neurons and eventually leads to their death. This results in cognitive decline and memory loss, as well as other symptoms such as mood changes, disorientation, and difficulty with language and communication. There is currently no cure for AD, and treatment options are limited.568 However, there are medications available that can help alleviate some of the symptoms and slow down the progression of the disease.569 Theranostic fluorescence probes hold great promise for the early diagnosis and targeted therapy of Alzheimer’s disease.
In principle, to obtain an effective theranostic fluorescence probe for AD, several key factors need to be considered, for example (1) the probes should have suitable lipophilicity and molecular weight to facilitate the blood–brain barrier (BBB) permeability; (2) the interactions between probes and the Aβ or Tau fibrillar proteins, e.g., π–π donor-acceptor interactions and hydrogen bonding, should be strong enough to avoid the competitive binding with other unspecific biocomponents; (3) the probes should have sufficient intermolecular interactions with specific amino acid residues (e.g., Aβ42) of Aβ or Tau fibril to interfere the fibril formation; (4) ideal biocompatibility and minimal toxicity are also desired. However, simultaneously integrating such important properties into a single molecule remains a challenge.
In 2022, Tang et al. reported an Aβ-targeted aggregation-induced emission (AIE) theranostic probe for effective attenuation of Aβ-induced neurotoxicity in vivo.570 As depicted in Figure 111, benefiting from the balanced hydrophobicity–hydrophilicity molecular design strategy, probe 175 obtained an ideal lipophilicity (LogP = 1.21), enabling 175 to bypass the BBB and was capable of specifically recognizing and binding to the cavities of Aβ42 fibrils. Theoretical results showed that 175 exerted a strong disruptive effect on Aβ42 species through hydrophobic interaction and π–π stacking interactions. The half maximal inhibitory concentration of 175 on fibril Aβ activity was only 0.19 μM, much lower than that of reported Aβ inhibitors.571 Impressively, due to the merits of the AIE effect, aggregated 175 in the Aβ plaques exhibited intense NIR fluorescence signals, as a consequence allowing for imaging-guided early diagnosis of AD in a high signal-to-background manner. Remarkably, after treating APP/PS1 transgenic mice (i.e., AD mouse model) with 175 6 times via intravenous injection, the AD mice exhibited obvious reduced Aβ plaques in the brain and rescued deficits in learning and memory recovery, indicating that 175 could serve as a theranostic fluorescence probe for real-time NIR imaging of Aβ plaques and AD therapy simultaneously.
Figure 111.
(A) Design and structure of Aβ-targeted NIR aggregation-induced emission (AIE) probe (175). (B) Theranostic mechanism of 175 for in vitro diagnostic imaging and inhibition of the formation of the Aβ protein. Reproduced with permission from ref (570). Copyright 2022 Wiley Intersciences.
As mentioned above, PDT-based theranostics have shown great promise in cancer treatment through the mechanism of ROS-mediated biomolecular photo-oxidation. Actually, this strategy has also been used for Aβ plaque destruction. Upon photoirradiation, PS can generate ROS to oxidize certain amino acid residues of Aβ peptides, e.g., Met and His, leading to Aβ conformational changes and finally decreasing the aggregative propensity of the pathogenic amyloids (Figure 112). However, for photodynamic AD therapy to be used in a multicomponent system such as the brain, the rational design of AD-specific phototheranostics remains challenging.
Figure 112.
Schematic illustration of the light-triggered photo-oxidation of Aβ protein by PS. The oxidation of Met35 residue by PS takes away the ability of Aβ peptides to participate in the redox reactions, as a consequence leading to the aggregative propensity of amyloids decreased. Reproduced with permission from ref (572). Copyright 2018 Elsevier Ltd.
At present, approaches to realize this goal are mainly concentrated on the ligand-assisted direction of PS. As shown in Figure 113, after conjugating a flavin-based PS to an Aβ-binding peptide, the resulting flavin-peptide conjugate (i.e., 176) greatly improved the photo-oxidation selectivity of Aβ proteins in living cells compared to free flavin.573 The intrinsic fluorescence emission properties of PS in principle can be used to indicate the AD area, as such realizing phototheranostic simultaneously. However, this approach suffers from its inherent shortcomings, that is, the targeting efficiency depends highly on the expression level of pathological Aβ protein. If AD is only in its early stages (that is, low Aβ plaque formation), such ligand-assisted targeting strategy may lose its capability and cause off-targeted photodamage to normal brain tissues.
Figure 113.
Fluorescence probe inspired theranostic PS design for selective Aβ photo-oxidation. (A) Aβ-binding flavin-peptide conjugate (176) for photo-oxidation of the Tyr,10 Met,35 His,13 and His14 residues of Aβ1–42 fibrils. Reproduced with permission from ref (573). Copyright 2014 Wiley Intersciences. KGaA, Weinheim. (B) Schematic illustration of a new phototheranostic PS design concept, i.e., TaSCAc, which exhibits “OFF–ON” PDT activities for selective oxidation of Aβ proteins. By conjugating with an Aβ-targeting peptide (i.e., modifying 177 to 178), the treatment selectivity can be further improved. Reproduced with permission from ref (574). Copyright 2016 Springer Nature. (C) Fluorescent Aβ probe (CRANAD-2)-inspired selective phototheranostic 179a and its mechanism of action. Adapted with permission from refs (575, 576). Copyright 2018 Elsevier Ltd. and Copyright 2018 Elsevier Ltd. (D) BBB permeable PS (182) inspired from the probe 180. Reproduced with permission from ref (577). Copyright 2021 AAAS. (E) BAP-1-based phototheranostic (P183) for the clearance of aggregated Aβ in the brain of AD mice. Reproduced with permission from ref (578). Copyright 2021 Oxford University Press. Reproduced with the permission from ref (579). Copyright 2022 Elsevier Ltd.
To address this, a new design concept for AD-targeted phototheranostic, termed targeting sensing catalyst activation (TaSCAc), hass emerged, as depicted in Figure 113.574 In this system, the PDT activity of PS (such as 177) initially remains silent, which means it cannot generate ROS even under the condition of photoirradiation (i.e., “OFF” state); however, once sensing and binding to specific Aβ structures (e.g., the cross-β-sheet quaternary), PS becomes active and is able to photo-oxidize the Aβ proteins (i.e., “ON” state). With this mechanism of action, undesirable off-target and nonspecific photoactivation is therefore inhibited. Aβ-sensing fluorescence probe may emerge as feasible tools for designing such novel PS.
For example, by introducing a bromine atom into the chemical structure of fluorescence Aβ probe CRANAD-2, the resulting compound 179a became a switchable PS (Figure 113).575,576 In 179b, the TICT effect, ascribed to the bond rotation (e.g., bond A and bond B), significantly inhibits the intersystem crossing process, leading to negligible ROS generation. However, when binding to the Aβ fibers, the free bond rotation would be restricted, interfering with the formation of the TICT effect and in turn generating ROS under photoirradiation. Based on this switching mechanism, the further optimized 179b selectively photo-oxidizes the aberrant Aβ aggregates in vitro, in cello, and in AD mice brain lysates via the generated 1O2, even in the presence of multiple off-target substrates. This strategy is also applicable to other TaSCAc systems, such as the BBB permeable compound 182 (inspired by probe 180, Figure 113).577 Moreover, by modifying the BODIPY structure using an iodine atom, an Aβ fluorescence probe BAP-1-derivative 183 was obtained, achieving effective photoclearance of Aβ depositions in the brain of 7-month-old AppNL–G–F/NL–G–F AD mice (Figure 113).579
Overall, these impressive studies shed new light on the development of accurate photodynamic AD treatments. Using such an approach, ROS formation can be confined specifically to the AD regions, thus significantly reducing untoward photodamage of surrounding normal tissues. In particular, if such a TaSCAc system also has a fluorescence emission property, concomitant fluorescent “Turn-On” signals can be further applied for AD diagnosis, thereby affording simultaneous AD diagnosis and therapy. As depicted in Figure 114, a TaSCAc system 184 was designed to acheive this goal.580 The fluorescence emission of 184 was initially silent due to the TICT effect, while the probe exhibited “Turn-On” fluorescence when bonded to Aβ aggregates, which therefore enabled an in situ mapping of Aβ aggregation. Importantly, this process occurred along with enhanced 1O2 generation, which then caused a potent photo-oxidation of Aβ aggregates and reduced their neurotoxicity in PC12 cells.
Figure 114.
NIR phototheranostic 184 for specific fluorescent mapping and photodynamic oxygenation of Aβ aggregates. Reproduced with permission from ref (580). Copyright 2022 American Chemical Society.
Although PDT has been widely adopted as a noninvasive modality with the merit of remote control for disease treatments, the low penetration depth of light in tissues remains an intractable issue.582 To address this barrier, developing NIR PSs within the “phototherapeutic window" (650–900 nm)583 represents a facile avenue, using PSs such as phthalocyanine,584 BODIPY,585,586 cyanine,587 Nile blue derivates,588−591 and upconversion-based small molecules.592,593 As an alternative, designing self-luminescent systems might be another applicable strategy to overcome the light source-caused limited penetration depth in vivo. Very recently, Yan et al. reported a chemiluminescence-excited phototherapeutic nanosystem (185) capable of photo-oxidizing aggregated Aβ without the need for an external excitation source.581 As can be seen in Figure 115, 185 was prepared by loading 187 and 186 in mesoporous silica nanoparticles and then decorated with lactoferrin (Lf). After reaction with the H2O2 in the tumor microenvironment, 186 could generate a high energy intermediate 1,2-dioxa cyclic dione, which was able to produce chemiluminescence to excite 187. Activated 187 subsequently emits fluorescence for imaging and sensitize surrounding oxygen to form 1O2, photo-oxidizing Aβ aggregates and thereby suppressing their neurotoxicity. Based on this study, it is concluded that such an in situ-triggered chemiluminescence might be also applicable to other neurologic diseases.
Figure 115.

(A) Schematic illustration of chemiluminescence-mediated photo-oxidation of Aβ proteins. (B) Mechanism of action of chemiluminescence-triggered PS activation for ROS generation. (C) Chemical structure of 187. Reproduced with the permission from ref (581). Copyright 2022 Elsevier Ltd.
3.2. Senescence
Senescence refers to the biological process of aging in living organisms, which is characterized by a decline in various physiological functions and an increased susceptibility to disease and death.594,595 This process is believed to be influenced by both genetic and environmental factors. In humans and other animals, senescence is associated with a range of age-related diseases such as cancer, neurodegeneration, cardiovascular diseases, and metabolic disorders.596−598 Since pioneering research in 2011 revealed that the genetic removal of P16-positive senescent cells could significantly prolong the lifespan of mice, the development of interventions that can delay or prevent age-related diseases has become a research frontier.599 Nowadays, numerous approaches have been developed, aiming at selectively eliminating senescent cells without causing untoward effects on normal tissues or cells.600,601
In the context of senescence, theranostics can be used to develop diagnostic tools that can detect the presence of senescent cells and evaluate the effectiveness of senolytic therapies.600,602 One potential theranostic approach is the use of molecular imaging techniques, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), to visualize the presence of senescent cells in vivo. These techniques rely on the use of imaging agents that can specifically bind to senescent cells and provide information about their location and quantity.602 Another potential theranostic approach is the use of biomarkers that are overexpressed specifically in senescent cells.595 Biomarkers are measurable indicators that can be used to monitor biological processes or disease states. In the context of senescence, potential biomarkers include markers of cellular senescence, such as p16INK4a and senescence-associated beta-galactosidase (SA-β-gal), as well as markers of inflammation and tissue damage.603−605 By combining these diagnostic tools with senolytic therapies, theranostics can provide a powerful approach for the precise targeting and elimination of senescent cells.
For example, Li et al. developed a senolysis-based fluorescence theranostic prodrug (188, Figure 116) for chronic renal failure.606 After exposure to intracellular overexpressed SA-β-gal, 188 could be specifically activated, sequentially releasing the parent drug gemcitabine to eliminate SA-β-gal-enriched senescent cells and the coumarin fluorophore for fluorescence tracking of the senescent cells. Interestingly, the authors incorporated a novel design that uses the generated coumarin intermediate (i.e., MBP) to bio-orthogonally bind to the nucleophilic residue of cellular proteins, endowing 188 with the ability to recognize senescence with single-cell resolution. In vivo experiments evidenced that in mice with chronic renal failure, abdominal administration of 188 remarkably attenuated the degree of kidney injury and improved kidney functions. This approach could ultimately lead to the development of more effective and personalized therapies for age-related diseases and disorders. However, more research is needed to fully develop and optimize senescence theranostics for clinical use.
Figure 116.
Molecular structure of TSPD and corresponding mechanism of action of SA-β-gal-triggered parent drug release for senescent cell elimination as well as fluorescence recovery for senescence diagnosis. Reproduced with permission from ref (606). Copyright 2022 The Author(s).
Several studies have also investigated the use of PDT-based phototheranostics for the elimination of senescent cells in vitro and in vivo. Recently, Dennis K. P. Ng and co-workers reported an SA-β-gal-activated phototherapeutic system 189a.607 As illustrated in Figure 117, the tailor-made compound 189a consists of an SA-β-gal substrate, a BODIPY-based PS (189b), and a black hole quencher (BHQ-2) connected via an AB2-type self-immolation linker. In the native form, 189b stays closely in proximity to BHQ-2, which favors the formation of a Förster resonance energy transfer (FRET) effect and consequently inhibits the fluorescence emission and PDT activity of 189b. After reacting with SA-β-gal, however, 189a was dissociated, releasing active 189b and showing strong fluorescence emission as well as 1O2 generation. In vitro detection and clearance of senescent cells demonstrated that after being incubated with 189a, bright green fluorescence could be seen in senescent Hela and HT29 cells but not in nonsenescent cells. Meanwhile, under the conditions of light irradiation (λ > 515 nm, 25.5 mW/cm2, 20 min), 189a generated 1O2 and potently killed the senescent HeLa cells. These results demonstrated that the photosensitizing capability of 189a could be selectively activated by β-gal, resulting in specific and efficient photoeradication of senescent cells.
Figure 117.

Molecular structure of 189a for SA-β-gal-triggered photodynamic senescent cell eradication and corresponding self-immolation mechanism for the release of active 189b. Reproduced with permission from ref (607). Copyright 2023 The Royal Society of Chemistry.
Using the same biomarker, Tung et al. reported another SA-β-gal-responsible PDT system (190, Figure 118) based on the FDA-approved PS methylene blue (MB).608190 was prepared by integrating a β-gal recognition domain into the 10-N position of the MB skeleton via a self-immolation linker. With this smart design, the conjugation structure of MB was broken, thus completely blocking the absorption, fluorescence, and PDT activities of MB. In the presence of β-gal, the galactopyranoside was cleaved, followed by a self-immolation reaction via the quinone methide elimination to release the original PS MB. Notably, 190 exhibited an ultrahigh sensitivity in detecting β-gal, with a rapid absorption (60-fold increase in 15 min) and fluorescence recovery (95-fold increase in 15 min) in the presence of β-gal. This sensitivity is also applied to the senescent cell diagnosis. Further, under the condition of photoirradiation (665 nm LED light, 30 mW/cm2, 30 min), 190 effectively killed the β-gal expressing senescent C6/LacZ cells by generating a large amount of 1O2.
Figure 118.
Molecular structure of 190 and corresponding mechanism of action of β-gal-triggered MB release via a self-immolation reaction. Reproduced with permission from ref (608). Copyright 2022 The Royal Society of Chemistry.
These strategies show great promise in terms of phototheranostics for senescence treatments; however, photocontrolled senescence therapy remains in its infancy and few studies have explored their feasibility in vivo. Recently, Li, Guo, and their co-workers proposed a multiplex technology that integrates a SA-β-gal substrate with a fluorescence tag for accurate tracking of senescent cells, a bio-orthogonal receptor triggered by SA-β-gal for targeted-site anchoring of senescent cells with single-cell resolution, and a selenium atom-incorporated PS for achieving selective senescent cell elimination via activatable PDT (Figure 119).402,609 A SA-β-gal probe named 191-O was prepared as a control and a photoactivatable senolytic prodrug named 191-Se was constructed, wherein the Selenium atom-mediated heavy atom effect610 significantly enhanced the intersystem crossing efficiency to promote a high 1O2 quantum yield (ΦΔ(191-Se) = 0.2 vs ΦΔ(191-O) = 0.07). In the native form, 191-Se exhibited poor fluorescence and negligible 1O2 generation. However, after reacting with SA-β-gal, a quinone methide intermediate (i.e., the bio-orthogonal receptor) was generated, which could covalently react with exposed nucleophilic groups on the surfaces of surrounding proteins nearby SA-β-gal, followed by NIR fluorescence signal and PDT “ON”. Importantly, such an “OFF-ON” behavior occurred only after reacting with SA-β-gal. This, therefore, enabled target-site anchoring to and accurate monitoring of senescent cells in a complex system, such as the coculture model of young cells and senescent cells shown in this work. Importantly, in vivo studies using naturally aged mice showed that 191-Se-mediated PDT decreased the expression of senescence-associated genes and markers, successfully countered age-induced losses in liver and renal function as well as inhibited the age-associated physical dysfunction. This unprecedented integration strategy may provide a new method for the treatment of other diseases, including but not limited to senescence.
Figure 119.

Molecular structure of control compound 191-O and targeted phototherapeutic agent 191-Se. Reproduced with permission from ref (402). Copyright 2023 The Author(s), under exclusive license to Springer Nature America, Inc.
3.3. Other Diseases
Conventional antimicrobial drugs such as antibiotics, antivirals, antifungals, antiparasitics, etc. have been efficient and successful in many ways in treating microbial infections. However, numerous side-effects and growing resistance to antibiotic drugs are making antimicrobial drugs less effective against microbial infections. To overcome these limitations, new innovative treatment methods are needed, which can effectively fight against infections. Antimicrobial photodynamic therapy (aPDT) is a relatively new treatment approach that uses light and PSs to kill bacteria and other microorganisms. There are several advantages to using an aPDT approach over traditional methods, such as reduced side effects, improved accuracy, and increased efficacy. Smartly designed fluorescent probes can target antibiotic-resistant microbes and selectively kill them upon light irradiation, without harming the noninfected areas. Moreover, fluorescent theragnostic probes can monitor the treatment, by visualizing the infected area until the complete recovery of infection. Recent literature has shown several examples of theranostic fluorescent probes that were successfully applied against microbial infections.341,611−614
Recently, Lopez et al. designed and synthesized a BOPHY–fullerene C60 dyad (BP-C60, 192) by covalently connecting a BOPHY fluorophore with N-methylfulleropyrrolidine (Figure 120).615 In this probe the BOPHY was utilized as an “antenna” to improve the visible light adsorption, resulting in effectively generated O2•– and 1O2, thus enabling both type I and type II PDT. In in vitro experiments, the 192 successfully deactivated Staphylococcus aureus by generating efficient ROS under visible light irradiation. However, dyad 192 was not found to be an efficient aPDT agent against Escherichia coli. Tang and co-workers reported an asymmetrically cationic AIE PS, CN-TPAQ-PF6 (193) (Figure 120) that successfully inactivated drug-resistant bacteria with efficient type I and type II ROS generation under irradiation with mild sunlight.616 They modified a triphenylamine (TPA) moiety with a quinolinium hexafluorophosphate (PF6–) and a nitrile group to afford an asymmetric A-D-A type AIEgen theranostic fluorescence probe. The cationization and nitrile introduction enhanced the hydroxyl radical generation ability of the probe to make it 5.4-fold stronger than crystal violet (CV). The cationic charge on 193 bestows an exceptional bacterial recognition and binding ability, proving an excellent fluorescence image-guided aPDT efficacy, even for methicillin-resistant Staphylococcus aureus “super bacteria”. Although aPDT is a new and developing area, it is anticipated that theranostic fluorescent probes have the potential to make aPDT a more effective and safer treatment against drug-resistant infections.
Figure 120.
(A) Molecular structures of the theranostic fluorescent probe 192 for antimicrobial PDT. (B) Molecular structures of the theranostic fluorescent probe 193. (C) Molecular structures of the theranostic fluorescent probe 194. (D) Structure of the theranostic diad 195 and its response toward H2O2.
Theranostic fluorescent probes can provide a potential solution to several kinds of eye diseases, including diabetic retinopathy, glaucoma, retinoblastoma, and bacterial endophthalmitis. Some of the potential advantages of theranostic probes for eye disease are targeted therapy, selective heat delivery, and a convenient means for monitoring the treatment. Although fluorescent probes are being developed for many eye diseases, theranostic probes are still rare. Here, we will discuss some representative theranostic fluorescence for eye diseases.617
Bacterial endophthalmitis (BE) is a common eye disease that can cause complete blindness. It is a bacterial infection that enters the eye through a corneal break or the bloodstream. Antibiotics are the conventional treatment for BE, but multidrug resistance and retinal toxicity pose challenges to successful therapy. Recently, Tang and colleagues reported a cationic aggregation-induced emission luminogen called triphenylamine thiophene pyridinium (TTPy, 194, Figure 120) for a PDT of BE.618 TTPy (194) can selectively target and kill bacteria without harming normal ocular tissues. In an in vivo experiment on rats infected with Staphylococcus aureus, TTPy (194) not only diagnosed and treated BE but also induced an innate immune response to protect the retina from infection. Moreover, TTPy (194) was found to be a better theranostic probe than Rose Bengal, indicating its excellent potential for clinical applications in treating ocular infections.
Diabetes is a growing public health concern that can lead to life-threatening chronic diseases, including blindness, heart disease, stroke, kidney failure, and certain cancers.619 There are two types of diabetes: type I, which is a genetic condition that appears in early life, and type II, which is often associated with an unhealthy lifestyle. Diabetes is typically diagnosed based on excessive blood glucose levels and the feeling of extreme hunger. However, diabetes symptoms are often underdiagnosed, and significant damage may have already occurred before the confirmation of diabetes, making early diagnosis methods needed for the early stage treatment. While several fluorescence probes have been developed for early diagnosis of diabetes by estimating abnormal levels of chemicals, enzymes, pH labels, and viscosity in cell organelles, the development of theranostic fluorescent probes for early diagnosis and therapy of diabetes is still an evolving field.620,621
Under diabetic conditions, hyperglycemia leads to the production of excess ROS through glucose phosphorylation. H2O2 is one of the major ROS produced in these conditions and is relatively stable. Recent studies have shown that elevated levels of H2O2 in cells can signal diabetes and other related diseases. Therefore, the development of a theranostic fluorescence probe for real-time detection of elevated H2O2 can aid in the early diagnosis and timely management of diabetes. To demonstrate this concept, Yu et al. presented an H2O2-responsive dyad (DX-B-DA, 195, Figure 120) consisting of a near-infrared fluorescent dye (DX) and the type II diabetes drug dapagliflozin (DA), linked through an H2O2-sensitive linker, for the diagnosis and treatment of type II diabetes. In vitro experiments confirmed that the dyad 195 is initially poorly fluorescent and has no therapeutic effect. However, in the presence of H2O2, the drug DA and dye DX are released, resulting in bright fluorescence and the initiation of therapy. The authors also successfully demonstrated that dyad 195 can selectively visualize diabetic liver/kidney damage and treat type II diabetes in mouse models.
4. Challenges in Clinical Translation
Despite several impressive examples of efficient and potent DDS mentioned above, the field of theranostic small-molecule drug delivery systems has not made significant progress beyond the preclinical drug development stages. Various factors contribute to this state of affairs and are outlined below.
First, while theranostic agents offer clear advantages in research settings by enabling the collection of spatiotemporal information on drug delivery and, in some cases, providing spatiotemporal control over therapeutic actions, their benefits in a clinical setting are sometimes less apparent. Even near-infrared light from the NIR-II window only allows penetration of a few centimeters, which limits their applications given the size of the human body.622 However, there are several applications for fluorescent DDS, such as fluorescence-guided surgery and the theranostic treatment of superficial cancerous tissues.
More significant challenges arise from the DMPK (drug metabolism and pharmacokinetics) profile, or rather the lack thereof, of DDS. While most DDS described in this review have demonstrated efficient results in cells and animal tests, they lack comprehensive characterization in terms of pharmacokinetics and metabolism. Small-molecule drugs are typically extensively characterized to determine properties such as solubility, lipophilicity, metabolic stability, plasma protein binding, off-target binding, clearance rate, mechanism, cellular penetration, etc.623 However, most DDS have not undergone such thorough characterization. A significant proportion of DDS is currently administered intravenously and enters cells through active (receptor-mediated) uptake, which eliminates some of the uptake challenges often encountered by small molecules. Additionally, several DDS could be applied topically to treat diseases, reducing the need for compounds with sufficient resistance to hepatic metabolism. However, challenges remain for DDS. In the case of theranostic chemotherapy, the properties of the released small-molecule drug are well-described, but the attachment of a linker potentially alters the profile and kinetics of formed metabolites. As a result, the differing clearance rates and mechanisms between (unactivated) DDS and their small-molecule fragments create a complex pharmacokinetic profile, necessitating further in-depth studies.
Similarly, the fluorescent probes used in DDS designs have often not been characterized from a DMPK perspective and may contribute to additional toxicity. As shown in this review, there is a trend for DDS to exhibit fluorescence with red-shifted wavelengths, which requires large, flat, often polycyclic aromatic structures. The strong π–π donor-acceptor interactions between these hydrophobic molecules severely limit solubility. Furthermore, these structures may undergo hepatic metabolism to form arene-oxides, which can be potentially carcinogenic.624 Therefore, in the design of the next generation of DDS, it is strongly recommended to study also the properties and potential toxicity of the fluorophores and their metabolites. The hepatic metabolism of molecules is often significantly faster for lipophilic compounds, so choosing a more water-soluble fluorophore would likely be beneficial not only for the overall solubility of DDS but also for their metabolic stability and to avoid off-target binding.625,626 As the development of red-shifted biocompatible fluorophores is crucial not only for DDS but for various applications, it is currently a major focus of research, and significant advances in fluorophore design will undoubtedly benefit DDS as well.
Although intravenous administration is most commonly used, DDS amenable to oral uptake could represent a significant breakthrough and facilitate clinical translation. It is evident that the DDS described in this review do not conform to the traditional metrics for small-molecule oral drug design, such as Lipinski’s rule-of-5 (RO5). However, it has become clear that orally available chemical space is not limited to RO5, as evidenced by the emergence of large macrocyclic drugs and proteolysis targeting chimeras (PROTACs). Several new descriptors for the molecular design of larger molecules with a reasonable chance of good oral availability have been described, mainly for macrocycles but with broad applicability. Consensus models have been described, e.g., MW ≤ 1000 Da, −2 ≤ cLogP ≤ 10, HBA ≤ 15, HBD ≤ 6627−629 (with MW: molecular weight, cLogP: the calculated pH-independent lipophilicity, HBA: the number of hydrogen bond acceptors, and HBD: the number of hydrogen bond donors). Other descriptors to improve oral availability have been proposed as well, for example, AbbVie’s AB-MPS score for large molecules, in which molecular design is adjusted to keep this score as small as possible630 (AB-MPS = Abs(cLogD – 3) + #AR + #RB; with cLogD: the calculated pH-dependent lipophilicity, #AR: the number of aromatic rings, and #RB: the number of rotatable bonds). While it may seem a challenging task to redesign DDS with these parameters in mind, which it undoubtedly is, the evolution of our understanding of what allows for good oral availability is still advancing and the clinical translation of DDS will likely benefit from these insights.
The development history of DDS shares similarities with that of PROTACs, which are also large molecules consisting of multiple components connected through a linker.631,632 Initially, PROTAC development faced significant skepticism regarding clinical translation, but the field has made remarkable advances in the past decade, resulting in orally available drugs, some of which can even cross the blood–brain barrier.633 Several PROTAC designs have reached Phase II clinical trials, and a first design recently entered Phase III clinical trials.631,632 Therefore, we believe that with better DMPK characterization of DDS in general, necessary structural optimizations, the use of fluorophores optimized for biocompatibility, and increased focus on oral availability, the DDS field could experience a rapid rise similar to that of the PROTAC field, potentially leading to widespread clinical applications.
5. Conclusion
In this review, we have highlighted recent advances in the design and application of theranostic fluorescent probes. These probes range from small molecules to nanoparticles and have become popular research tools for visualizing and treating tumors. However, their applications are expanding beyond cancer theranostics, with probes being reported in the fields of neurodegenerative diseases, aging, and antibacterials. Therapeutic applications for other indications will also emerge in the future.
The use of theranostic fluorescent probes offers great potential for reducing the systemic toxicity of drugs and providing a means to visualize drug delivery in terms of both spatial and temporal aspects. Some probes are designed to be activated by external stimuli, allowing for precise control over the therapeutic process, such as photodynamic, photothermal, or sonodynamic activation.
Small-molecule tumor-selective theranostic fluorescent probes can be activated in the tumor microenvironment through various mechanisms. These probes are sensitive to factors like low pH, overexpressed (bio)molecules in cancer cells (e.g., GSH), redox imbalances (e.g., H2S, ROS), enzymes activated or enhanced under hypoxia (e.g., DT-diaphorase, azo-reductase, nitro-reductase), as well as other enzymes with increased expression levels in cancerous tissues (e.g., esterases, proteases), and combinations thereof. Similarly, a wide range of design strategies have been reported for nanoparticles and nanovesicles. These designs are based on self-assembled structures of drug conjugates and nanoaggregates with biologically compatible materials such as HSA, biopolymers, and biocompatible synthetic polymers.
While there have been hurdles preventing these theranostic fluorescent probes from reaching their expected translation into the clinic, the potential economic benefits are substantial. With a better understanding of the behavior of large molecules as potential drugs and a growing acceptance of nanoparticles in clinical applications, we anticipate widespread clinical use of these probes in the coming decade(s). We believe that a bright future awaits this research domain.
Acknowledgments
The authors thank the financial support received from the National Research Foundation of Korea (CRI project no. 2018R1A3B1052702, J.S.K,), NSFC (grant no. 21978222, L.Z.). X.P. thanks the National Natural Science Foundation of China (No. 22090011), Fundamental Research Funds for the Central Universities (No. DUT22LAB608), and Shenzhen University 2035 Program for Excellent Research (No. 00000225). M.L. thanks the support of the National Natural Science Foundation of China (No. 22308220) and Shenzhen University Third-Phase Project of Constructing High-Level University (No. 000001032104). X.-P.H. thanks the Natural National Science Foundation of China (Nos. 92253306 and 82130099), the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX03), the Fundamental Research Funds for the Central Universities (222201717003), the Programme of Introducing Talents of Discipline to Universities (B16017), and the Open Funding Project of the State Key Laboratory of Bioreactor Engineering. T.D.J. thanks the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01) for support. A.S. thanks Department of Biotechnology, New Delhi, India, for a prestigious DBT-Ramalingaswami fellowship (BT/RLF/Re-entry/59/2018) and Science & Engineering Research Board, New Delhi (CRG/2021/002476).
Glossary
Abbreviations
- AO
acridine orange
- ACQ
aggregation-caused quenching
- AD
Alzheimer’s disease
- ADV
sound droplet evaporation
- AEP
asparaginyl endopeptidases
- AIEgens
aggregation-induced emission fluorogens
- ALP
alkaline phosphatase
- aPDT
antimicrobial photodynamic therapy
- APN
aminopeptidase N
- ASGP
asialoglycoprotein
- Aβ
β-amyloid
- BAC
brominated asymmetric cyanine
- BBB
blood brain barrier
- BDP
diiododistyrylbodipy
- BE
bacterial endophthalmitis
- BHQ-3
black hole quencher 3
- BL
bioluminescence
- BODIPY
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- BP-C60
BOPHY–fullerene C60 dyad
- CAE
E-cinnamic acid
- CAIX
carbonic anhydrase IX
- CDT
chemodynamic therapy
- CESs
carboxylesterases
- CLSM
confocal laser scanning microscopy
- COX-2
cyclooxygenase-2
- CPT
camptothecin
- CRC
colorectal cancer
- CRET
chemiluminescence resonance energy transfer
- Croc
croconaine
- CTSB
cathepsin B
- CTSL
cysteine cathepsin L
- CV
crystal violet
- CY7
cyanine7
- Cys
cysteine
- Dabcyl
4-(dimethylamino azo)benzene-4-carboxylic acid
- DAE
diarylethene
- DBS
2,4-dinitrobenzenesulfonyl
- DC
dendritic cell
- DCA
dichloroacetic acid
- DCM
dicyanomethylene-4H-chromene
- DDS
drug delivery system
- DMPK
drug metabolism and pharmacokinetics
- DMSO
dimethyl sulfoxide
- DNBS
2,4-dinitrobenzenesulfonate
- DNP
dinitrophenyl
- Dox
doxorubicin
- DPNB
deep penetrating nanombomb
- DPP
pyrrolopyrrolidone
- DSBDP
distyryl boron dipyrromethene
- DSPE
1,2-distearoyl-sn-glycero-3-phosphoethanolamine
- DTD
DT-diaphorase
- EB
ethidium bromide
- ECM
extracellular matrix
- ENBS
5-(ethylamino)-9-diethylamino-benzo[a]phenothiazinium chloride
- EPR
enhanced permeability and retention
- ESI-MS
electrospray ionization mass spectrometry
- FAM
5(6)-carboxylfluorescein
- FL
fluorescence
- FMN
flavin mononucleotide
- FRET
Förster resonance energy transfer
- GEM
gemcitabine
- GGT
γ-glutamyl transpeptidase
- GOx
glucose oxidase
- GSH
glutathione
- HA
hyaluronic acid
- HAase
hyaluronidase
- HAS
human serum albumin
- HCPT
10-hydroxycamptothecin
- Hcy
homocysteine
- HDACs
histone deacetylases
- HES
hydroxyethyl starch
- HIFU
high-intensity focused ultrasound
- HPNA
HA-PpIX nanoassembly
- ICD
immunogenic cell death
- ICG
indocyanine green
- ICI
immune checkpoint inhibitor
- ICT
intramolecular charge transfer
- iNOS
inducible nitric oxide synthase
- LCD
lysosome-mediated cell death
- LIFU
low-intensity focused ultrasound
- LPS
lipopolysaccharide
- MB
methylene blue
- MDR
multidrug resistance
- MMP-2
metalloproteinase-2
- MR
magnetic resonance
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NEBI
N-ethoxybenzylimidazole
- NIR
near-infrared
- NQO1
NAD(P)H quinone oxidoreductase
- NSAID
nonsteroidal anti-inflammatory drug
- NTR
nitroredutases
- OXPHOS
oxidative phosphorylation
- PA
photoacoustic
- PAE
poly β-amino ester
- PBS
phosphate buffered saline
- PD-1
programmed cell death receptor 1
- PDK
pyruvate dehydrogenase inhibitor
- PDL-1
programmed death 1 ligand
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PEM
pemetrexed
- PET
photoinduced electron transfer
- PFP
perfluoropentane
- PLA
polylactic acid
- PLNPs
persistent luminescence nanoparticles
- PMB
photodynamic molecular beacon
- PPa
pheophorbide A
- PROTACs
proteolysis targeting chimeras
- PS
PS
- PTAs
photothermal agents
- PTT
photothermal therapy
- PTX
paclitaxel
- Rd
rhodamine
- RGD
arginylglycylaspartic acid
- RNS
reactive nitrogen species
- RO5
Lipinski’s rule-of-5
- ROS
reactive oxygen species
- RP-HPLC
reverse phase-high performance liquid chromatography
- RT
radiotherapy
- SA-β-gal
senescence-associated beta-galactosidase
- SCID
severe combined immunodeficiency
- SDT
sonodynamic therapy
- SOD
superoxide dismutase
- SPECT
single photon emission computed tomography
- TaSCAc
termed targeting sensing catalyst activation
- TBDMS
tert-butyldimethylsilyl
- TBDPS
tert-butyldiphenylsilyl
- TICT
twisted intramolecular charge transfer
- TIPS
triisopropylsilyl
- TME
tumor microenvironment
- TPA
triphenylamine
- TPE
tetraphenylethylene
- TPGS
d-α-tocopheryl polyethylene glycol 1000 succinate
- TPP
tetraphenylporphyrin
- TPPS
meso-tetrakis(4-sulfonatophenyl) porphyrin
- TPZ
tirapazamine
- TrxSS
thioredoxin
- TYR
tyrosinase
- US
ultrasound
- β-gal
β-galactosidase
Biographies
Amit Sharma is an associate professor at Amity School of Chemical Sciences, Amity University Mohali, Punjab, India. He received his Ph.D. degree from Guru Nanak Dev University, Amritsar in 2010. His research interests include fluorescence, chemiluminescence and PET based cancer diagnostic probes and their bio-applications, molecular theranostic agents for cancer targeted therapeutics, and exploring combination therapeutics.
Peter Verwilst received his Ph.D. from the Department of Chemistry at KU Leuven in 2012. Currently, he is an assistant professor in the Faculty of Pharmaceutical Sciences at the Rega Institute of KU Leuven. His main research interests are the development of small-molecule drugs as antimicrobials, allosteric modulators of chemokine receptors, and chemotherapeutics. He has so far published 63 papers with an h-index of 28.
Mingle Li received his Ph.D. from the Dalian University of Technology in 2019, under the supervision of Prof. Xiaojun Peng. In the same year, he joined as a research professor in Prof. Jong Seung Kim’s team at Korea University. In 2023, he made the transition to Shenzhen University, where he is presently a tenure-track associate professor in the College of Materials Science and Engineering. His research interests include smart dye design, type I PDT, bio-photocatalysis, photobiological tools (e.g., PhotoPyro), and antitumor immunity.
Dandan Ma is a postdoctoral fellow in the State Key Laboratory of Fine Chemicals, College of Material Science and Engineering, Shenzhen University. She graduated from Qingdao University of Science and Technology in 2014 and received her Ph.D. degree in Applied Chemistry from Dalian University of Technology under the supervision of Prof. Xiaojun Peng in 2022. Her research interest focuses on the structure design of novel NIR-I and NIR-II organic dyes and their application in the field of tumor phototheranositcs.
Nem Singh completed his Ph.D. degree under the guidance of Prof. Anil. J. Elias from the Indian Institute of Technology, Delhi, in 2012. Subsequently, he moved to the University of Ulsan, where he was awarded an NRF Korea Fellowship (2013–2017), and later worked as a research scientist at KRICT, Daejeon. Since 2020, he has been serving as a research professor in Prof. Jong Seung Kim’s research group at Korea University, Seoul. His research interests include nanoscale COFs for drug delivery, cell imaging, and phototherapy.
Jiyoung Yoo received her B.S. degree from the Department of Chemistry at Korea University in 2023 and is pursuing her Ph.D. degree in Prof. Jong Seung Kim’s laboratory. Her research interest includes development of novel fluorescent probes in cancer diagnosis.
Yujin Kim received her B.S. degree from the Department of Chemistry at Korea University in 2023 and is pursuing her Ph.D. degree in Prof. Jong Seung Kim’s laboratory. Her research interest includes development of drug delivery system in cancer therapy.
Ying Yang received her B.S. degree from Fujian Agriculture and Forestry University in 2018. She is currently pursuing her M.S. degree in the College of Light and Food Engineering, Guangxi University, under the supervision of Prof. Lintao Zeng. Her current research focuses on fluorescent probes and bio-imaging.
Jing-Hui Zhu received his B.S. (2012), M.S. (2015), and Ph.D. (2022) degrees from Jiangxi University of Science and Technology, Southwest University, and City University of Hong Kong, respectively. He is currently a postdoctoral researcher in the College of Material Science and Engineering at Shenzhen University. His current research interests focus on the development of smart photofunctional theranostic agents for tumor therapy.
Haiqiao Huang obtained his Ph.D. in Applied Chemistry from Dalian University of Technology under the supervision of Prof. Xiaojun Peng in 2022. He is currently a postdoctoral researcher in the College of Material Science and Engineering at Shenzhen University. His research interest is focused on exploiting functional photosensitizers for photodynamic therapy.
Xi-Le Hu received his Ph.D. from the School of Chemistry and Molecular Engineering at East China University of Science and Technology (ECUST) in 2017. Currently, he is a lecturer in the same department at ECUST. His research mainly focuses on developing new chemical agents and materials for the diagnosis and treatment of superbugs. He has so far published over 36 papers with an h-index of 18.
Xiao-Peng He received his Ph.D. from the East China University of Science and Technology (ECUST) in 2011. He is presently a professor of chemical biology at ECUST. His research interests include the development of chemical probes for the study of glycobiology in live cells and animals and the construction of supramolecular glycoclusters for targeted disease therapy. A fellow of the Royal Society of Chemistry, he has published over 200 papers with an h-index of 48.
Lintao Zeng is a professor at Guangxi University. He received his B.S. degree from Hubei Engineering University in 2004. Then, he received M.S. degree in Inorganic Chemistry from Central China Normal University in 2007 and Ph.D. degree from Technical Institute of Physics and Chemistry (CAS) in 2010. Afterward, he worked as a postdoctoral researcher in the group of Prof. Jishan Wu at the National University of Singapore (2010–2012). His research focuses on fluorescent probes and biosensors.
Tony D. James is a professor at the University of Bath and a Fellow of the Royal Society of Chemistry. He received his B.Sc. from the University of East Anglia (1986) and Ph.D. from the University of Victoria (1991). His research interests include many aspects of supramolecular chemistry, including molecular recognition, fluorescent sensor design, fluorescence imaging, chiral recognition, saccharide recognition, anion recognition, and probes for redox imbalance and theranostic systems. He has an h-index of 88 and has been listed by Clarivate as a highly cited researcher (HCR) since 2022.
Xiaojun Peng, a member of the Chinese Academy of Sciences, obtained his Ph.D. from the Dalian University of Technology in 1990. He then worked as a faculty member of Dalian University of Technology. Currently, he serves as a distinguished professor in both the State Key Laboratory of Fine Chemicals at Dalian University of Technology and the College of Materials Science and Engineering at Shenzhen University. His main research interests include smart molecular engineering for application in bioimaging, digital printing, disease diagnosis, and cancer therapy.
Jonathan L. Sessler received his Ph.D. from Stanford University in 1982. He is presently the Doherty-Welch Chair in Chemistry at The University of Texas at Austin. His research interests include drug discovery, sensor design, anion recognition, critical elements, expanded porphyrins, and supramolecular chemistry. He has published more than 900 papers and is an inventor of record on over 90 issued U.S. patents. He is a member of a number of learned societies, including the U.S. National Academy of Sciences.
Jong Seung Kim received his Ph.D. from the Department of Chemistry and Biochemistry at Texas Tech University in 1993. Currently, he is a full professor in the Department of Chemistry at Korea University in Seoul. His main research interests are the application of organic chemistry to drug delivery and diagnosis of various pathologies, including Alzheimer’s disease and malignant neoplasm and their imaging. He has so far published over 600 papers with an h-index of 114. He has been selected as a 0.1% HCR since 2014.
Author Contributions
⬡ A.S., P.V., M.L., D.M. contributed equally to this work. CRediT: Amit Sharma conceptualization, funding acquisition, investigation, supervision, writing-original draft, writing-review & editing; Peter Verwilst writing-original draft, writing-review & editing; Mingle Li investigation, writing-original draft; Dandan Ma investigation, writing-original draft; Nem Singh writing-original draft; Jiyoung Yoo writing-original draft; Yujin Kim writing-original draft; Ying Yang investigation, writing-original draft; Jing-Hui Zhu investigation, writing-original draft; Haiqiao Huang investigation, writing-original draft; Xi-Le Hu investigation, writing-original draft; Xiao-Peng He investigation, writing-original draft, writing-review & editing; Lintao Zeng conceptualization, investigation, writing-original draft, writing-review & editing; Tony D. James writing-review & editing; Xiaojun Peng conceptualization, investigation, writing-original draft, writing-review & editing; Jonathan L. Sessler writing-review & editing; Jong Seung Kim conceptualization, funding acquisition, supervision.
The authors declare no competing financial interest.
Special Issue
Published as part of Chemical Reviewsvirtual special issue “Fluorescent Probes in Biology”.
References
- Lammers T.; Kiessling F.; Hennink W. E.; Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Controlled Release 2012, 161 (2), 175–187. 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- Boyle P.; Levin B.. World cancer report 2008; IARC Press, International Agency for Research on Cancer, 2008. [Google Scholar]
- Vasan N.; Baselga J.; Hyman D. M. A view on drug resistance in cancer. Nature 2019, 575 (7782), 299–309. 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B. Addiction to oncogenes-the Achilles heal of cancer. Science 2002, 297 (5578), 63–64. 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Penet M.-F.; Chen Z.; Kakkad S.; Pomper M. G.; Bhujwalla Z. M. Theranostic imaging of cancer. Eur. J. Radiol. 2012, 81 (1), S124. 10.1016/S0720-048X(12)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Xu C.; Zhang X.; Ning P.; Wang Z.; Tian J.; Chen X. Liposome-based probes for molecular imaging: from basic research to the bedside. Nanoscale 2019, 11 (13), 5822–5838. 10.1039/C9NR00207C. [DOI] [PubMed] [Google Scholar]
- He S.; Song J.; Qu J.; Cheng Z. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47 (12), 4258–4278. 10.1039/C8CS00234G. [DOI] [PubMed] [Google Scholar]
- Antignani A.; Ho E. C. H.; Bilotta M. T.; Qiu R.; Sarnvosky R.; FitzGerald D. J. Targeting receptors on cancer cells with protein toxins. Biomolecules 2020, 10 (9), 1331. 10.3390/biom10091331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowska Z.; Boldanova T.; Adametz D.; Quagliata L.; Vogt J. E.; Dill M. T.; Matter M. S.; Roth V.; Terracciano L.; Heim M. H. Gene expression analysis of biopsy samples reveals critical limitations of transcriptome-based molecular classifications of hepatocellular carcinoma. J. Pathol. Clin. Res. 2016, 2 (2), 80–92. 10.1002/cjp2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Wang Z.; Zhao T.; Li Y.; Huang G.; Sumer B. D.; Gao J. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials 2018, 157, 62–75. 10.1016/j.biomaterials.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioux S.; Choi H. S.; Frangioni J. V. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol. Imaging 2010, 9 (5), 7290. 10.2310/7290.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens E. A.; Henary M.; El Fakhri G.; Choi H. S. Tissue-specific near-infrared fluorescence imaging. Acc. Chem. Res. 2016, 49 (9), 1731–1740. 10.1021/acs.accounts.6b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolrahimzadeh S.; Ciancimino C.; Grassi F.; Sordi E.; Fragiotta S.; Scuderi G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. J. Ophthalmol. 2021, 2021, 5581851. 10.1155/2021/5581851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Hu Z.; Tian J.; Fang C. A narrative review of near-infrared fluorescence imaging in hepatectomy for hepatocellular carcinoma. ANN. TRANSL. MED 2021, 9 (2), 171. 10.21037/atm-20-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrmeijer A. L.; Hutteman M.; Van Der Vorst J. R.; Van De Velde C. J.; Frangioni J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10 (9), 507–518. 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska J. B.; Wojcieszak J.; Olejniczak A. B. Prodrugs: a challenge for the drug development. Pharmacol. Rep. 2013, 65 (1), 1–14. 10.1016/S1734-1140(13)70959-9. [DOI] [PubMed] [Google Scholar]
- Markovic M.; Ben-Shabat S.; Dahan A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12 (11), 1031. 10.3390/pharmaceutics12111031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares J.; Fares M. Y.; Khachfe H. H.; Salhab H. A.; Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5 (1), 28. 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J.; Obenauf A. C. Metastatic colonization by circulating tumour cells. Nature 2016, 529 (7586), 298–306. 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144 (5), 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Alix-Panabieres C.; Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discovery 2016, 6 (5), 479–491. 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- Kalinich M.; Haber D. A. Cancer detection: Seeking signals in blood. Science 2018, 359 (6378), 866–867. 10.1126/science.aas9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U.; Hammond M. Cancer diagnostics based on plasma protein biomarkers: hard times but great expectations. Mol. Oncol. 2021, 15 (6), 1715–1726. 10.1002/1878-0261.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connal S.; Cameron J. M.; Sala A.; Brennan P. M.; Palmer D. S.; Palmer J. D.; Perlow H.; Baker M. J. Liquid biopsies: the future of cancer early detection. J. Transl. Med. 2023, 21 (1), 118. 10.1186/s12967-023-03960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26 (24), 4012–4021. 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun K. A.; Kim J.; Gwak H.; Jung H. I. Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. Analyst 2016, 141 (2), 382–392. 10.1039/C5AN01762A. [DOI] [PubMed] [Google Scholar]
- Needham D. M.; Cohen J. A.; Barrett A. M. The mechanism of damage to the bone marrow in systemic poisoning with mustard gas. Biochem. J. 1947, 41 (4), 631–639. 10.1042/bj0410631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Jia Y.; Song W.; Zhang L. Therapeutic Potential of Nitrogen Mustard Based Hybrid Molecules. Front. Pharmacol. 2018, 9, 1453. 10.3389/fphar.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci M. V.; Arnedos M.; Andre F.; Soria J. C. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discovery 2013, 3 (3), 264–279. 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- Petrova V.; Annicchiarico-Petruzzelli M.; Melino G.; Amelio I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7 (1), 10. 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. A.; Parker H. K. Advances in the Development of Prodrugs as Selective Modulators of Estrogen Receptors. J. Endocr. Soc. 2022, 6 (12), bvac158. 10.1210/jendso/bvac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz F.; Muller I. A.; Ryppa C.; Warnecke A. Prodrug strategies in anticancer chemotherapy. ChemMedChem. 2008, 3 (1), 20–53. 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G.; Cantley L. C.; Thompson C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009, 324 (5930), 1029–1033. 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti M. V.; Locasale J. W. The Warburg Effect: How Does it Benefit Cancer Cells?. Trends Biochem. Sci. 2016, 41 (3), 211–218. 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin K.; Leeper D. B.; Cater J. R.; Thistlethwaite A. J.; Tupchong L.; McFarlane J. D. Extracellular pH distribution in human tumours. Int. J. Hyperthermia 1993, 11 (2), 211–216. 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- Guo X.; Wang L.; Duval K.; Fan J.; Zhou S.; Chen Z. Dimeric Drug Polymeric Micelles with Acid-Active Tumor Targeting and FRET-Traceable Drug Release. Adv. Mater. 2018, 30 (3), 1705436. 10.1002/adma.201705436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B.; Lee S. G.; Yoon H. R.; Lee J. M.; Oh H. J.; Kim H. M.; Jung Y. Four-fold Channel-Nicked Human Ferritin Nanocages for Active Drug Loading and pH-Responsive Drug Release. Angew. Chem., Int. Ed. Engl. 2018, 57 (11), 2909–2913. 10.1002/anie.201800516. [DOI] [PubMed] [Google Scholar]
- Jin S.; Wan J.; Meng L.; Huang X.; Guo J.; Liu L.; Wang C. Biodegradation and Toxicity of Protease/Redox/pH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug delivery. ACS Appl. Mater. Interfaces 2015, 7 (35), 19843–19852. 10.1021/acsami.5b05984. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xu H.; Liang C.; Liu Y.; Li Z.; Yang G.; Cheng L.; Li Y.; Liu Z. Iron oxide@ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7 (8), 6782–6795. 10.1021/nn4017179. [DOI] [PubMed] [Google Scholar]
- Mahato R.; Tai W.; Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Delivery Rev. 2011, 63 (8), 659–670. 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang I.; Boland E. L.; Poon G. M. Prodrug applications for targeted cancer therapy. AAPS J. 2014, 16 (5), 899–913. 10.1208/s12248-014-9638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamala M.; Wilson W. R.; Yang M.; Palmer B. D.; Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- Sonawane S. J.; Kalhapure R. S.; Govender T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. 10.1016/j.ejps.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Kalia J.; Raines R. T. Hydrolytic stability of hydrazones and oximes. Angew. Chem.-Int. Ed. 2008, 47 (39), 7523–7526. 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn C. F.; Oshiro C.; Marsh S.; Hernandez-Boussard T.; McLeod H.; Klein T. E.; Altman R. B. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet. Genomics. 2011, 21 (7), 440–446. 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. B. The anthracyclines: will we ever find a better doxorubicin?. Semin. Oncol. 1992, 19 (2), 670–686. [PubMed] [Google Scholar]
- Cortes-Funes H.; Coronado C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7 (2), 56–60. 10.1007/s12012-007-0015-3. [DOI] [PubMed] [Google Scholar]
- Li S. Y.; Liu L. H.; Jia H. Z.; Qiu W. X.; Rong L.; Cheng H.; Zhang X. Z. A pH-responsive prodrug for real-time drug release monitoring and targeted cancer therapy. Chem. Commun. (Camb.) 2014, 50 (80), 11852–11855. 10.1039/C4CC05008H. [DOI] [PubMed] [Google Scholar]
- Euhus D. M.; Hudd C.; LaRegina M. C.; Johnson F. E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31 (4), 229–234. 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- Lankelma J.; Dekker H.; Fernández Luque R.; Luykx S.; Hoekman K.; Valk P. v. d.; Van Diest P. J.; Pinedo H. M. Doxorubicin gradients in human breast cancer. Clin. Cancer Res. 1999, 5 (7), 1703–1707. [PubMed] [Google Scholar]
- Chen N. T.; Wu C. Y.; Chung C. Y.; Hwu Y.; Cheng S. H.; Mou C. Y.; Lo L. W. Probing the dynamics of doxorubicin-DNA intercalation during the initial activation of apoptosis by fluorescence lifetime imaging microscopy (FLIM). PLoS One 2012, 7 (9), e44947 10.1371/journal.pone.0044947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh N. S.; Parvin P.; Ghasemi F.; Atyabi F. Fluorescence properties of several chemotherapy drugs: doxorubicin, paclitaxel and bleomycin. Biomed. Opt. Express 2016, 7 (6), 2400–2406. 10.1364/BOE.7.002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-Y.; Liu L.-H.; Rong L.; Qiu W.-X.; Jia H.-Z.; Li B.; Li F.; Zhang X.-Z. A Dual-FRET-Based Versatile Prodrug for Real-Time Drug Release Monitoring and In Situ Therapeutic Efficacy Evaluation. Adv. Funct. Mater. 2015, 25 (47), 7317–7326. 10.1002/adfm.201503262. [DOI] [Google Scholar]
- Fernandez A.; Vermeren M.; Humphries D.; Subiros-Funosas R.; Barth N.; Campana L.; MacKinnon A.; Feng Y.; Vendrell M. Chemical Modulation of in Vivo Macrophage Function with Subpopulation-Specific Fluorescent Prodrug Conjugates. ACS Cent. Sci. 2017, 3 (9), 995–1005. 10.1021/acscentsci.7b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.; Kim E.; Kim W. Y.; Kang C.; Kim J. S. Biotin-guided anticancer drug delivery with acidity-triggered drug release. Chem. Commun. (Camb.) 2015, 51 (45), 9343–9345. 10.1039/C5CC03003J. [DOI] [PubMed] [Google Scholar]
- Lee M. H.; Kim E. J.; Lee H.; Park S. Y.; Hong K. S.; Kim J. S.; Sessler J. L. Acid-triggered release of doxorubicin from a hydrazone-linked Gd(3+)-texaphyrin conjugate. Chem. Commun. (Camb.) 2016, 52 (69), 10551–10554. 10.1039/C6CC05673C. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Huang Y.; Yang H.; Liu G.; Zhao R. A peptide-based pH-sensitive drug delivery system for targeted ablation of cancer cells. Chem. Commun. (Camb.) 2015, 51 (77), 14454–14457. 10.1039/C5CC05184C. [DOI] [PubMed] [Google Scholar]
- Jin Y.; Song L.; Su Y.; Zhu L.; Pang Y.; Qiu F.; Tong G.; Yan D.; Zhu B.; Zhu X. Oxime linkage: a robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules 2011, 12 (10), 3460–3468. 10.1021/bm200956u. [DOI] [PubMed] [Google Scholar]
- Xu W.; Ding J.; Xiao C.; Li L.; Zhuang X.; Chen X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. 10.1016/j.biomaterials.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Yang J. Development of a folate receptor (FR)-targeted indenoisoquinoline using a pH-sensitive N-ethoxybenzylimidazole (NEBI) bifunctional cross-linker. Bioconjugate Chem. 2014, 25 (5), 873–878. 10.1021/bc500146p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinand E.; Zloh M.; Brocchini S. Competitive Reactions During Amine Addition to cis-Aconityl Anhydride. Aust. J. Chem. 2002, 55 (7), 467–474. 10.1071/CH02092. [DOI] [Google Scholar]
- Zhang A.; Yao L.; An M. Reversing the undesirable pH-profile of doxorubicin via activation of a di-substituted maleamic acid prodrug at tumor acidity. Chem. Commun. (Camb.) 2017, 53 (95), 12826–12829. 10.1039/C7CC06843C. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27 (9–10), 916–921. 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- Smith C. V.; Jones D. P.; Guenthner T. M.; Lash L. H.; Lauterburg B. H. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 1996, 140 (1), 1–12. 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- Zheng Z.-B.; Zhu G.; Tak H.; Joseph E.; Eiseman J. L.; Creighton D. J. N-(2-hydroxypropyl) methacrylamide copolymers of a glutathione (GSH)-activated glyoxalase i inhibitor and DNA alkylating agent: synthesis, reaction kinetics with GSH, and in vitro antitumor activities. Bioconjugate Chem. 2005, 16 (3), 598–607. 10.1021/bc0499634. [DOI] [PubMed] [Google Scholar]
- Cheng R.; Feng F.; Meng F.; Deng C.; Feijen J.; Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Controlled Release 2011, 152 (1), 2–12. 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Meng F.; Hennink W. E.; Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30 (12), 2180–2198. 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Montero D.; Tachibana C.; Rahr Winther J.; Appenzeller-Herzog C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013, 1 (1), 508–513. 10.1016/j.redox.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito G.; Swanson J. A.; Lee K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Delivery Rev. 2003, 55 (2), 199–215. 10.1016/S0169-409X(02)00179-5. [DOI] [PubMed] [Google Scholar]
- Schafer F. Q.; Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30 (11), 1191–1212. 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Estrela J. M.; Ortega A.; Obrador E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43 (2), 143–181. 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- Parodi P. A role for milk proteins and their peptides in cancer prevention. Curr. Pharm. Des. 2007, 13 (8), 813–828. 10.2174/138161207780363059. [DOI] [PubMed] [Google Scholar]
- Jones L. R.; Goun E. A.; Shinde R.; Rothbard J. B.; Contag C. H.; Wender P. A. Releasable luciferin- transporter conjugates: Tools for the real-time analysis of cellular uptake and release. J. Am. Chem. Soc. 2006, 128 (20), 6526–6527. 10.1021/ja0586283. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Liu B.; Lu J.; Li F.; Li D.; Liang C.; Dang L.; Liu J.; He B.; Badshah S. A.; et al. Progress and Challenges in Developing Aptamer-Functionalized Targeted Drug Delivery Systems. Int. J. Mol. Sci. 2015, 16 (10), 23784–23822. 10.3390/ijms161023784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongbang S.; Jeon H. M.; Lee M. H.; Shin W. S.; Kwon J. K.; Kang C.; Kim J. S. Camptothecin delivery into hepatoma cell line by galactose-appended fluorescent drug delivery system. RSC Adv. 2014, 4 (36), 18744–18748. 10.1039/c4ra02588a. [DOI] [Google Scholar]
- Hou M.; Xue P.; Gao Y. E.; Ma X.; Bai S.; Kang Y.; Xu Z. Gemcitabine-camptothecin conjugates: a hybrid prodrug for controlled drug release and synergistic therapeutics. Biomater. Sci. 2017, 5 (9), 1889–1897. 10.1039/C7BM00382J. [DOI] [PubMed] [Google Scholar]
- Han X.; Chen J.; Jiang M.; Zhang N.; Na K.; Luo C.; Zhang R.; Sun M.; Lin G.; Zhang R.; et al. Paclitaxel-Paclitaxel Prodrug Nanoassembly as a Versatile Nanoplatform for Combinational Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8 (49), 33506–33513. 10.1021/acsami.6b13057. [DOI] [PubMed] [Google Scholar]
- Lim J.; Chouai A.; Lo S.-T.; Liu W.; Sun X.; Simanek E. E. Design, synthesis, characterization, and biological evaluation of triazine dendrimers bearing paclitaxel using ester and ester/disulfide linkages. Bioconjugate Chem. 2009, 20 (11), 2154–2161. 10.1021/bc900324z. [DOI] [PubMed] [Google Scholar]
- Dasari M.; Acharya A. P.; Kim D.; Lee S.; Lee S.; Rhea J.; Molinaro R.; Murthy N. H-Gemcitabine: A new gemcitabine prodrug for treating cancer. Bioconjugate Chem. 2013, 24 (1), 4–8. 10.1021/bc300095m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.; Lee J. H.; Jeon H. M.; Han J. H.; Park N.; He Y.; Lee H.; Hong K. S.; Kang C.; Kim J. S. Folate-based near-infrared fluorescent theranostic gemcitabine delivery. J. Am. Chem. Soc. 2013, 135 (31), 11657–11662. 10.1021/ja405372k. [DOI] [PubMed] [Google Scholar]
- Maiti S.; Park N.; Han J. H.; Jeon H. M.; Lee J. H.; Bhuniya S.; Kang C.; Kim J. S. Gemcitabine-Coumarin-Biotin Conjugates: A target specific theranostic anticancer prodrug. J. Am. Chem. Soc. 2013, 135 (11), 4567–4572. 10.1021/ja401350x. [DOI] [PubMed] [Google Scholar]
- Lee M. H.; Kim J. Y.; Han J. H.; Bhuniya S.; Sessler J. L.; Kang C.; Kim J. S. Direct fluorescence monitoring of the delivery and cellular uptake of a cancer-targeted RGD peptide-appended naphthalimide theragnostic prodrug. J. Am. Chem. Soc. 2012, 134 (30), 12668–12674. 10.1021/ja303998y. [DOI] [PubMed] [Google Scholar]
- Kong F.; Liang Z.; Luan D.; Liu X.; Xu K.; Tang B. A Glutathione (GSH)-Responsive Near-Infrared (NIR) Theranostic Prodrug for Cancer Therapy and Imaging. Anal. Chem. 2016, 88 (12), 6450–6456. 10.1021/acs.analchem.6b01135. [DOI] [PubMed] [Google Scholar]
- Wu X.; Sun X.; Guo Z.; Tang J.; Shen Y.; James T. D.; Tian H.; Zhu W. In vivo and in situ tracking cancer chemotherapy by highly photostable NIR fluorescent theranostic prodrug. J. Am. Chem. Soc. 2014, 136 (9), 3579–3588. 10.1021/ja412380j. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhu S.; Gu K.; Guo Z.; Huang X.; Wang M.; Amin H. M.; Zhu W.; Shi P. GSH-Activated NIR Fluorescent Prodrug for Podophyllotoxin Delivery. ACS Appl. Mater. Interfaces 2017, 9 (35), 29496–29504. 10.1021/acsami.7b07091. [DOI] [PubMed] [Google Scholar]
- Kong Y.; Smith J.; Li K.; Cui J.; Han J.; Hou S.; Brown M. L. Development of a novel near-infrared fluorescent theranostic combretastain A-4 analogue, YK-5–252, to target triple negative breast cancer. Bioorg. Med. Chem. 2017, 25 (7), 2226–2233. 10.1016/j.bmc.2017.02.046. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Dong H.; Cai X.; Wang D.; Li Y. Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Appl. Mater. Interfaces 2012, 4 (6), 3177–3183. 10.1021/am3005225. [DOI] [PubMed] [Google Scholar]
- Tian X.; Li Z.; Ding N.; Zhang J. Near-infrared ratiometric self-assembled theranostic nanoprobe: imaging and tracking cancer chemotherapy. Chem. Commun. (Camb.) 2020, 56 (25), 3629–3632. 10.1039/D0CC00416B. [DOI] [PubMed] [Google Scholar]
- Dutta D.; Alex S. M.; Bobba K. N.; Maiti K. K.; Bhuniya S. New Insight into a Cancer Theranostic Probe: Efficient Cell-Specific Delivery of SN-38 Guided by Biotinylated Poly(vinyl alcohol). ACS Appl. Mater. Interfaces 2016, 8 (49), 33430–33438. 10.1021/acsami.6b10580. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zhu G.; Jacobson O.; Liu Y.; Chen K.; Yu G.; Ni Q.; Fan J.; Yang Z.; Xu F.; et al. Transformative Nanomedicine of an Amphiphilic Camptothecin Prodrug for Long Circulation and High Tumor Uptake in Cancer Therapy. ACS Nano 2017, 11 (9), 8838–8848. 10.1021/acsnano.7b03003. [DOI] [PubMed] [Google Scholar]
- Ye M.; Wang X.; Tang J.; Guo Z.; Shen Y.; Tian H.; Zhu W. H. Dual-channel NIR activatable theranostic prodrug for in vivo spatiotemporal tracking thiol-triggered chemotherapy. Chem. Sci. 2016, 7 (8), 4958–4965. 10.1039/C6SC00970K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Pei Q.; Chen L.; Li Z.; Xie Z. Reduction-responsive fluorescence off-on BODIPY-camptothecin conjugates for self-reporting drug release. J. Mater. Chem. B 2016, 4 (13), 2332–2337. 10.1039/C6TB00009F. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Zeng F. A theranostic prodrug based on FRET for real-time drug release monitoring in response to biothiols. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 77–85. 10.1016/j.msec.2016.11.056. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Fang Z. A novel glutathione-triggered theranostic prodrug for anticancer and imaging in living cells. RSC Adv. 2018, 8 (21), 11419–11423. 10.1039/C8RA00271A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Shen Y.; Meng X.; Wu Y.; Zhao Y.; Wu C. Stabilizing p-Dithiobenzyl Urethane Linkers without Rate-Limiting Self-Immolation for Traceless Drug Release. ChemMedChem. 2019, 14 (12), 1196–1203. 10.1002/cmdc.201900248. [DOI] [PubMed] [Google Scholar]
- Sabharanjak S.; Mayor S. Folate receptor endocytosis and trafficking. Adv. Drug Delivery Rev. 2004, 56 (8), 1099–1109. 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Li J.; Sausville E. A.; Klein P. J.; Morgenstern D.; Leamon C. P.; Messmann R. A.; LoRusso P. Clinical pharmacokinetics and exposure-toxicity relationship of a folate-Vinca alkaloid conjugate EC145 in cancer patients. J. Clin. Pharmacol. 2009, 49 (12), 1467–1476. 10.1177/0091270009339740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S.; Kaittanis C.; Santiesteban O. J.; Perez J. M. Cell-specific, activatable, and theranostic prodrug for dual-targeted cancer imaging and therapy. J. Am. Chem. Soc. 2011, 133 (41), 16680–16688. 10.1021/ja207463b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Jiang Z.; Lovell J. F.; Zhang L.; Zhang Y. Design of a Thiol-Responsive, Traceless Prodrug with Rapid Self-Immolation for Cancer Chemotherapy. ACS Appl. Bio. Mater. 2021, 4 (6), 4982–4989. 10.1021/acsabm.1c00247. [DOI] [PubMed] [Google Scholar]
- Cao D.; Liu Z.; Verwilst P.; Koo S.; Jangjili P.; Kim J. S.; Lin W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119 (18), 10403–10519. 10.1021/acs.chemrev.9b00145. [DOI] [PubMed] [Google Scholar]
- Bhuniya S.; Maiti S.; Kim E. J.; Lee H.; Sessler J. L.; Hong K. S.; Kim J. S. An activatable theranostic for targeted cancer therapy and imaging. Angew. Chem., Int. Ed. 2014, 53 (17), 4469–4474. 10.1002/anie.201311133. [DOI] [PubMed] [Google Scholar]
- Shindy H. A. Basics, Mechanisms and Properties in the Chemistry of Cyanine Dyes: A Review Paper. Mini-Rev. Org. Chem. 2012, 9, 352–360. 10.2174/157019312804699528. [DOI] [Google Scholar]
- Gui L.; Yuan Z.; Kassaye H.; Zheng J.; Yao Y.; Wang F.; He Q.; Shen Y.; Liang L.; Chen H. A tumor-targeting probe based on a mitophagy process for live imaging. Chem. Commun. (Camb.) 2018, 54 (69), 9675–9678. 10.1039/C8CC04246B. [DOI] [PubMed] [Google Scholar]
- Hameed S.; Chen H.; Irfan M.; Bajwa S. Z.; Khan W. S.; Baig S. M.; Dai Z. Fluorescence Guided Sentinel Lymph Node Mapping: From Current Molecular Probes to Future Multimodal Nanoprobes. Bioconjugate Chem. 2019, 30 (1), 13–28. 10.1021/acs.bioconjchem.8b00812. [DOI] [PubMed] [Google Scholar]
- Liu R.; Tang J.; Xu Y.; Dai Z. Bioluminescence Imaging of Inflammation in Vivo Based on Bioluminescence and Fluorescence Resonance Energy Transfer Using Nanobubble Ultrasound Contrast Agent. ACS Nano 2019, 13 (5), 5124–5132. 10.1021/acsnano.8b08359. [DOI] [PubMed] [Google Scholar]
- Luo S.; Zhang E.; Su Y.; Cheng T.; Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32 (29), 7127–7138. 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Choi P. J.; Park T. I. H.; Cooper E.; Dragunow M.; Denny W. A.; Jose J. Heptamethine Cyanine Dye Mediated Drug Delivery: Hype or Hope. Bioconjugate Chem. 2020, 31 (7), 1724–1739. 10.1021/acs.bioconjchem.0c00302. [DOI] [PubMed] [Google Scholar]
- Escobedo J. O.; Rusin O.; Lim S.; Strongin R. M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14 (1), 64–70. 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscano Y.; Onate-Garzon J.; Delgado J. P. Peptides with Dual Antimicrobial-Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25 (18), 4245. 10.3390/molecules25184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison S. R.; Whittaker M.; Harris F.; Phoenix D. A. Anticancer α-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci. 2006, 7 (6), 487–499. 10.2174/138920306779025611. [DOI] [PubMed] [Google Scholar]
- Meng S.; Wang F. Research progresses of antimicrobial peptides with anticancer activities. Chin. J. Biochem. Pharm. 2011, 32, 77–80. [Google Scholar]
- Takeuchi H.; Yamamoto H.; Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv. Drug Delivery Rev. 2001, 47 (1), 39–54. 10.1016/S0169-409X(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Wang L.; Wang N.; Zhang W.; Cheng X.; Yan Z.; Shao G.; Wang X.; Wang R.; Fu C. Therapeutic peptides: current applications and future directions. Signal Transduct. Target. Ther. 2022, 7 (1), 48. 10.1038/s41392-022-00904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boohaker R. J.; Lee M. W.; Vishnubhotla P.; Perez J. L. M.; Khaled A. R. The use of therapeutic peptides to target and to kill cancer cells. Curr. Med. Chem. 2012, 19 (22), 3794–3804. 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.; Jeon H. M.; Le H. T.; Kim T. W.; Kang C.; Kim J. S. A biotin-guided fluorescent-peptide drug delivery system for cancer treatment. Chem. Commun. (Camb.) 2014, 50 (57), 7690–7693. 10.1039/c4cc02878c. [DOI] [PubMed] [Google Scholar]
- Maroney M. J.; Hondal R. J. Selenium versus sulfur: Reversibility of chemical reactions and resistance to permanent oxidation in proteins and nucleic acids. Free Radic. Biol. Med. 2018, 127, 228–237. 10.1016/j.freeradbiomed.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N.; Li Y.; Xu H.; Wang Z.; Zhang X. Dual redox responsive assemblies formed from diselenide block copolymers. J. Am. Chem. Soc. 2010, 132 (2), 442–443. 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- Wei D.; Yu Y.; Zhang X.; Wang Y.; Chen H.; Zhao Y.; Wang F.; Rong G.; Wang W.; Kang X.; et al. Breaking the Intracellular Redox Balance with Diselenium Nanoparticles for Maximizing Chemotherapy Efficacy on Patient-Derived Xenograft Models. ACS Nano 2020, 14 (12), 16984–16996. 10.1021/acsnano.0c06190. [DOI] [PubMed] [Google Scholar]
- Li T.; Pan S.; Gao S.; Xiang W.; Sun C.; Cao W.; Xu H. Diselenide-Pemetrexed assemblies for combined cancer Immuno-, radio-, and chemotherapies. Angew. Chem., Int. Ed. 2020, 59 (7), 2700–2704. 10.1002/anie.201914453. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Wang Z.; Zhong M.; Xu Q.; Li X.; Chang B.; Fang J. Integration of a Diselenide Unit Generates Fluorogenic Camptothecin Prodrugs with Improved Cytotoxicity to Cancer Cells. J. Med. Chem. 2021, 64 (24), 17979–17991. 10.1021/acs.jmedchem.1c01362. [DOI] [PubMed] [Google Scholar]
- Deepagan V. G.; Kwon S.; You D. G.; Nguyen V. Q.; Um W.; Ko H.; Lee H.; Jo D. G.; Kang Y. M.; Park J. H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. 10.1016/j.biomaterials.2016.06.044. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Ong C. N.; Shen H. M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004, 208 (2), 143–153. 10.1016/j.canlet.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Rehman T.; Shabbir M. A.; Inam-Ur-Raheem M.; Manzoor M. F.; Ahmad N.; Liu Z. W.; Ahmad M. H.; Siddeeg A.; Abid M.; Aadil R. M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8 (9), 4696–4707. 10.1002/fsn3.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang C. H.; Yoo E.; Hur S. K.; Kim K. S.; Kim D.; Jo S. A highly GSH-sensitive SN-38 prodrug with an ″OFF-to-ON″ fluorescence switch as a bifunctional anticancer agent. Chem. Commun. (Camb.) 2018, 54 (65), 9031–9034. 10.1039/C8CC05010D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen M. W.; Cebula M.; Zhang J.; Ottosson-Wadlund A.; Dubois L.; Lambin P.; Tew K. D.; Townsend D. M.; Haenen G. R.; Drittij-Reijnders M. J.; et al. Chemical Reactivity Window Determines Prodrug Efficiency toward Glutathione Transferase Overexpressing Cancer Cells. Mol. Pharmaceutics 2016, 13 (6), 2010–2025. 10.1021/acs.molpharmaceut.6b00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Wu H.; Liu P.; Zeng F.; Wu S. A self-immolative prodrug nanosystem capable of releasing a drug and a NIR reporter for in vivo imaging and therapy. Biomaterials 2017, 139, 139–150. 10.1016/j.biomaterials.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Reiffenstein R. J.; Hulbert W. C.; Roth S. H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- Paul B. D.; Snyder S. H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40 (11), 687–700. 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat. Rev. Drug Discovery 2016, 15 (3), 185–203. 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Du J.; Tang C.; Huang Y.; Jin H. H(2)S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429 (4), 543–561. 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L.; Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discovery 2015, 14 (5), 329–345. 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- Zhong H.; Yu H.; Chen J.; Sun J.; Guo L.; Huang P.; Zhong Y. Hydrogen Sulfide and Endoplasmic Reticulum Stress: A Potential Therapeutic Target for Central Nervous System Degeneration Diseases. Front. Pharmacol. 2020, 11, 702. 10.3389/fphar.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C.; Li J.; Liu L.; Liu H. K.; Qian Y. A self-immolated fluorogenic agent triggered by H(2)S exhibiting potential anti-glioblastoma activity. Analyst 2021, 146 (11), 3510–3515. 10.1039/D1AN00457C. [DOI] [PubMed] [Google Scholar]
- Ge C.; Di X.; Han S.; Wang M.; Qian X.; Su Z.; Liu H. K.; Qian Y. Hydrogen sulfide triggered molecular agent for imaging and cancer therapy. Chem. Commun. (Camb.) 2021, 57 (15), 1931–1934. 10.1039/D0CC07982K. [DOI] [PubMed] [Google Scholar]
- Bobba K. N.; Saranya G.; Sujai P. T.; Joseph M. M.; Velusamy N.; Podder A.; Maiti K. K.; Bhuniya S. Endogenous H(2)S-Assisted Cancer-Cell-Specific Activation of Theranostics with Emission Readout. ACS Appl. Bio. Mater. 2019, 2 (3), 1322–1330. 10.1021/acsabm.9b00019. [DOI] [PubMed] [Google Scholar]
- Huang K.; Xie S.; Wang W.; Wu Z.-S.; Wu J.; Jiang L.; Chen J.; Li J. A hydrogen sulfide-responsive prodrug for monitoring real-time release and improving therapeutic effects of anticancer drug SN-38. Sens. Actuators B Chem. 2022, 373, 132750. 10.1016/j.snb.2022.132750. [DOI] [Google Scholar]
- Bayir H. Reactive oxygen species. Crit. Care Med. 2005, 33 (12 Suppl), S498–S501. 10.1097/01.CCM.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- Circu M. L.; Aw T. Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48 (6), 749–762. 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B.; San Martin A.; Griendling K. K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110 (10), 1364–1390. 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U.; Das D.; Banerjee R. K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77 (5), 658–666. [Google Scholar]
- Alfadda A. A.; Sallam R. M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo S.; Reed T. T.; Venditti P.; Victor V. M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourova J.; Kottova M.; Voprsalova M.; Pour M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol. 2010, 198 (1), 15–35. 10.1111/j.1748-1716.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- Haigis M. C.; Yankner B. A. The aging stress response. Mol. Cell 2010, 40 (2), 333–344. 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D.; Alexandre J.; Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?. Nat. Rev. Drug Discovery 2009, 8 (7), 579–591. 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Moris D.; Spartalis M.; Spartalis E.; Karachaliou G. S.; Karaolanis G. I.; Tsourouflis G.; Tsilimigras D. I.; Tzatzaki E.; Theocharis S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. ANN. TRANSL. MED 2017, 5 (16), 326. 10.21037/atm.2017.06.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. H.; Griffiths H. R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. 10.1016/j.freeradbiomed.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Singh A.; Kukreti R.; Saso L.; Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24 (8), 1583. 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J.; Trigo-Damas I.; Quiroga-Varela A.; Jackson-Lewis V. R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Zhao J.; Zhang L.; Wei F.; Lian Y.; Wu Y.; Gong Z.; Zhang S.; Zhou J.; Cao K.; Li X.; Xiong W.; Li G.; Zeng Z.; Guo C. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8 (5), 761. 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N.; Xiao W.; Song X.; Wang W.; Dong X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nanomicro Lett. 2020, 12 (1), 15. 10.1007/s40820-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Han J.; Lim H. J.; Ren W. X.; Lim J. Y.; Kim J. H.; Kim J. S. Mitochondrial induced and self-monitored intrinsic apoptosis by antitumor theranostic prodrug: in vivo imaging and precise cancer treatment. J. Am. Chem. Soc. 2014, 136 (51), 17836–17843. 10.1021/ja510421q. [DOI] [PubMed] [Google Scholar]
- Redy-Keisar O.; Ferber S.; Satchi-Fainaro R.; Shabat D. NIR Fluorogenic Dye as a Modular Platform for Prodrug Assembly: Real-Time in vivo Monitoring of Drug Release. ChemMedChem. 2015, 10 (6), 999–1007. 10.1002/cmdc.201500060. [DOI] [PubMed] [Google Scholar]
- Sufian A.; Bhattacherjee D.; Barman P.; Srivastava A.; Thummer R. P.; Bhabak K. P. Stimuli-responsive prodrug of non-steroidal anti-inflammatory drug diclofenac: self-immolative drug release with turn-on near-infrared fluorescence. Chem. Commun. (Camb.) 2022, 58 (56), 7833–7836. 10.1039/D2CC02132C. [DOI] [PubMed] [Google Scholar]
- Negri G. E.; Deming T. J. Protein Complexation and pH Dependent Release Using Boronic Acid Containing PEG-Polypeptide Copolymers. Macromol. Biosci. 2017, 17 (1), 1600136. 10.1002/mabi.201600136. [DOI] [PubMed] [Google Scholar]
- Hu Y. B.; Dammer E. B.; Ren R. J.; Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N.; Xie D.; Zhang Z.; Kuang J.; Zheng Y.; Wang Q.; Li Y. Thioketal-crosslinked: ROS-degradable polycations for enhanced in vitro and in vivo gene delivery with self-diminished cytotoxicity. J. Biomater. Appl. 2019, 34 (3), 326–338. 10.1177/0885328219845081. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Jo S. D.; Seah G. L.; Kim I.; Nam Y. S. ROS-induced biodegradable polythioketal nanoparticles for intracellular delivery of anti-cancer therapeutics. J. Ind. Eng. Chem. 2015, 21, 1137–1142. 10.1016/j.jiec.2014.05.026. [DOI] [Google Scholar]
- Jo S. M.; Kim H. S.; Won M.; Champanhac C.; Kim J. S.; Wurm F. R.; Landfester K.. Dual-Targeted Nanoreactors and Prodrugs: Hydrogen Peroxide Triggers Oxidative Damage and Prodrug Activation for Synergistic Elimination of Cancer Cells. Adv. Funct. Mater. 2022, 32 ( (26), ). 10.1002/adfm.202200791. [DOI] [Google Scholar]
- Gutteridge J. M. C. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxy sugars, nucleosides and benzoate. Biochem. J. 1984, 224 (3), 761–767. 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now?. J. Neurochem. 2006, 97 (6), 1634–1658. 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Barrios B.; Mohrhardt B.; Doskey P. V.; Minakata D. Mechanistic Insight into the Reactivities of Aqueous-Phase Singlet Oxygen with Organic Compounds. Environ. Sci. Technol. 2021, 55 (12), 8054–8067. 10.1021/acs.est.1c01712. [DOI] [PubMed] [Google Scholar]
- Lyngsie G.; Krumina L.; Tunlid A.; Persson P. Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 2018, 8 (1), 10834. 10.1038/s41598-018-29075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Lin L.; Lin H.; Wilson B. C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9 (11–12), 1314–1325. 10.1002/jbio.201600055. [DOI] [PubMed] [Google Scholar]
- Liu L.-H.; Qiu W.-X.; Li B.; Zhang C.; Sun L.-F.; Wan S.-S.; Rong L.; Zhang X.-Z. A Red Light Activatable Multifunctional Prodrug for Image-Guided Photodynamic Therapy and Cascaded Chemotherapy. Adv. Funct. Mater. 2016, 26 (34), 6257–6269. 10.1002/adfm.201602541. [DOI] [Google Scholar]
- Sugimoto W.; Iwata H.; Yokoshima K.; Murakami Y.; Takasu Y. Proton and Electron Conductivity in Hydrous Ruthenium Oxides Evaluated by Electrochemical Impedance Spectroscopy: The Origin of Large Capacitance. J. Phys. Chem. B 2005, 109 (15), 7330–7338. 10.1021/jp044252o. [DOI] [PubMed] [Google Scholar]
- Pei P.; Sun C.; Tao W.; Li J.; Yang X.; Wang J. ROS-sensitive thioketal-linked polyphosphoester-doxorubicin conjugate for precise phototriggered locoregional chemotherapy. Biomaterials 2019, 188, 74–82. 10.1016/j.biomaterials.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Hossion A. M.; Bio M.; Nkepang G.; Awuah S. G.; You Y. Visible Light Controlled Release of Anticancer Drug through Double Activation of Prodrug. ACS Med. Chem. Lett. 2013, 4 (1), 124–127. 10.1021/ml3003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkepang G.; Bio M.; Rajaputra P.; Awuah S. G.; You Y. Folate receptor-mediated enhanced and specific delivery of far-red light-activatable prodrugs of combretastatin A-4 to FR-positive tumor. Bioconjugate Chem. 2014, 25 (12), 2175–2188. 10.1021/bc500376j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P.; Li M.; Karki R.; Bio M.; Rajaputra P.; Nkepang G.; Woo S.; You Y. Folate-PEG Conjugates of a Far-Red Light-Activatable Paclitaxel Prodrug to Improve Selectivity toward Folate Receptor-Positive Cancer Cells. ACS Omega 2017, 2 (10), 6349–6360. 10.1021/acsomega.7b01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P.; Li M.; Bio M.; Rajaputra P.; Nkepang G.; Sun Y.; Woo S.; You Y. Far-Red Light-Activatable Prodrug of Paclitaxel for the Combined Effects of Photodynamic Therapy and Site-Specific Paclitaxel Chemotherapy. J. Med. Chem. 2016, 59 (7), 3204–3214. 10.1021/acs.jmedchem.5b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M. Y.; Dolphin D. Site-Specific Prodrug Release Using Visible Light. J. Am. Chem. Soc. 2008, 130 (13), 4236–4237. 10.1021/ja800140g. [DOI] [PubMed] [Google Scholar]
- Robinson P. K. Enzymes: principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. 10.1042/bse0590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung-wen Liu T. B.Comprehensive Natural Products III; Elsevier, 2020. [Google Scholar]
- Zhao H.; Wu L.; Yan G.; Chen Y.; Zhou M.; Wu Y.; Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6 (1), 263. 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariaule V.; Kriaa A.; Soussou S.; Rhimi S.; Boudaya H.; Hernandez J.; Maguin E.; Lesner A.; Rhimi M. Digestive Inflammation: Role of Proteolytic Dysregulation. Int. J. Mol. Sci. 2021, 22 (6), 2817. 10.3390/ijms22062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S. Molecular functions of SIRPalpha and its role in cancer. Biomed. Rep. 2018, 9 (1), 3–7. 10.3892/br.2018.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. I.; Cozzo A.; Ji X.; Roberts L. S.; Louie S. M.; Mulvihill M. M.; Luo K.; Nomura D. K. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (37), 14912–14917. 10.1073/pnas.1310894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones M. M.; Gallegos A. M.; Lin F. V.; Heffner K. Dysregulation of inflammation, neurobiology, and cognitive function in PTSD: an integrative review. Cogn. Affect. Behav. Neurosci. 2020, 20 (3), 455–480. 10.3758/s13415-020-00782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland N. R. W.; Al-Juboori S. I.; Dobrinskikh E.; Bruce K. D. Altered substrate metabolism in neurodegenerative disease: new insights from metabolic imaging. J. Neuroinflammation 2021, 18 (1), 248. 10.1186/s12974-021-02305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D.; Siegel D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. 10.1016/j.redox.2021.101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefeli R. H.; Erb M.; Gemperli A. C.; Robay D.; Courdier Fruh I.; Anklin C.; Dallmann R.; Gueven N. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS One 2011, 6 (3), e17963 10.1371/journal.pone.0017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D.; Kepa J. K.; Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) localizes to the mitotic spindle in human cells. PLoS One 2012, 7 (9), e44861 10.1371/journal.pone.0044861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.; Li L.; Yan G.; Meng K.; Lin Z.; Nan Y.; Jin G.; Li C. High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer 2015, 15, 244. 10.1186/s12885-015-1271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Tanabe K.; Hatta H.; Nishimoto S. Bioreduction activated prodrugs of camptothecin: molecular design, synthesis, activation mechanism and hypoxia selective cytotoxicity. Org. Biomol. Chem. 2005, 3 (10), 1905–1910. 10.1039/b502813b. [DOI] [PubMed] [Google Scholar]
- Huang B.; Desai A.; Tang S.; Thomas T. P.; Baker J. R. Jr The Synthesis of a c(RGDyK) Targeted SN38 Prodrug with an Indolequinone Structure for Bioreductive Drug Release. Org. Lett. 2010, 12 (7), 1384–1387. 10.1021/ol1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K.; Harada H.; Narazaki M.; Tanaka K.; Inafuku K.; Komatsu H.; Ito T.; Yamada H.; Chujo Y.; Matsuda T.; et al. Monitoring of Biological One-Electron Reduction by 19F NMR Using Hypoxia Selective Activation of an 19F-Labeled Indolequinone Derivative. J. Am. Chem. Soc. 2009, 131 (44), 15982–15983. 10.1021/ja904953b. [DOI] [PubMed] [Google Scholar]
- Wang B.; Nicolaou M. G.; liu S.; Borchardt R. T. Structural analysis of a facile lactonization system facilitated by a ″trimethyl lock″. Bioorg. Chem. 1996, 24, 39–49. 10.1006/bioo.1996.0005. [DOI] [Google Scholar]
- Liu P.; Xu J.; Yan D.; Zhang P.; Zeng F.; Li B.; Wu S. A DT-diaphorase responsive theranostic prodrug for diagnosis, drug release monitoring and therapy. Chem. Commun. (Camb.) 2015, 51 (46), 9567–9570. 10.1039/C5CC02149A. [DOI] [PubMed] [Google Scholar]
- Shin W. S.; Han J.; Verwilst P.; Kumar R.; Kim J. H.; Kim J. S. Cancer Targeted Enzymatic Theranostic Prodrug: Precise Diagnosis and Chemotherapy. Bioconjugate Chem. 2016, 27 (5), 1419–1426. 10.1021/acs.bioconjchem.6b00184. [DOI] [PubMed] [Google Scholar]
- Li Z.; Zhang Y.; Jin T.; Men J.; Lin Z.; Qi P.; Piao Y.; Yan G. NQO1 protein expression predicts poor prognosis of non-small cell lung cancers. BMC Cancer 2015, 15, 207. 10.1186/s12885-015-1227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Zhang Y.; Wu Q.; Cui X.; Lin Z.; Liu S.; Chen L. Clinical implications of high NQO1 expression in breast cancers. J. Exp. Clin. Cancer Res. 2014, 33 (1), 14. 10.1186/1756-9966-33-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M.; Ough M.; Hinkhouse M. M.; Tsao M. S.; Oberley L. W.; Cullen J. J. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol. Carcinog. 2005, 43 (4), 215–224. 10.1002/mc.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwilst P.; Han J.; Lee J.; Mun S.; Kang H. G.; Kim J. S. Reconsidering azobenzene as a component of small-molecule hypoxia-mediated cancer drugs: A theranostic case study. Biomaterials 2017, 115, 104–114. 10.1016/j.biomaterials.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Hu M.; Yang C.; Luo Y.; Chen F.; Yang F.; Yang S.; Chen H.; Cheng Z.; Li K.; Xie Y. A hypoxia-specific and mitochondria-targeted anticancer theranostic agent with high selectivity for cancer cells. J. Mater. Chem. B 2018, 6 (16), 2413–2416. 10.1039/C8TB00546J. [DOI] [PubMed] [Google Scholar]
- Zhao X. B.; Ha W.; Gao K.; Shi Y. P. Precisely Traceable Drug Delivery of Azoreductase-Responsive Prodrug for Colon Targeting via Multimodal Imaging. Anal. Chem. 2020, 92 (13), 9039–9047. 10.1021/acs.analchem.0c01220. [DOI] [PubMed] [Google Scholar]
- Li S.; Jiang X.; Zheng R.; Zuo S.; Zhao L.; Fan G.; Fan J.; Liao Y.; Yu X.; Cheng H. An azobenzene-based heteromeric prodrug for hypoxia-activated chemotherapy by regulating subcellular localization. Chem. Commun. (Camb.) 2018, 54 (57), 7983–7986. 10.1039/C8CC03430C. [DOI] [PubMed] [Google Scholar]
- Bonnet D.; Dick J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3 (7), 730–737. 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. R.; Meyer D. J.; Taylor M. C.; Bromley E. V.; Miles M. A.; Kelly J. M. The Trypanosoma cruzi enzyme TcGPXI is a glycosomal peroxidase and can be linked to trypanothione reduction by glutathione or tryparedoxin. J. Biol. Chem. 2002, 277 (19), 17062–17071. 10.1074/jbc.M111126200. [DOI] [PubMed] [Google Scholar]
- Vaupel P.; Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26 (2), 225–239. 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zeng W.; Huang P.; Zeng X.; Mei L. Smart materials for drug delivery and cancer therapy. VIEW 2021, 2 (2), 20200042. 10.1002/VIW.20200042. [DOI] [Google Scholar]
- Sharma A.; Arambula J. F.; Koo S.; Kumar R.; Singh H.; Sessler J. L.; Kim J. S. Hypoxia-targeted drug delivery. Chem. Soc. Rev. 2019, 48 (3), 771–813. 10.1039/C8CS00304A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R.; Sunil D.; Ningthoujam R. S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Controlled Release 2020, 319, 135–156. 10.1016/j.jconrel.2019.12.041. [DOI] [PubMed] [Google Scholar]
- Thambi T.; Park J. H.; Lee D. S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: recent approaches and future perspectives. Chem. Commun. (Camb.) 2016, 52 (55), 8492–8500. 10.1039/C6CC02972H. [DOI] [PubMed] [Google Scholar]
- Kim H. S.; Sharma A.; Ren W. X.; Han J.; Kim J. S. COX-2 Inhibition mediated anti-angiogenic activatable prodrug potentiates cancer therapy in preclinical models. Biomaterials 2018, 185, 63–72. 10.1016/j.biomaterials.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Verwilst P.; Won M.; Lee J.; Sessler J. L.; Han J.; Kim J. S. A Small Molecule Strategy for Targeting Cancer Stem Cells in Hypoxic Microenvironments and Preventing Tumorigenesis. J. Am. Chem. Soc. 2021, 143 (35), 14115–14124. 10.1021/jacs.1c03875. [DOI] [PubMed] [Google Scholar]
- Lock F. E.; McDonald P. C.; Lou Y.; Serrano I.; Chafe S. C.; Ostlund C.; Aparicio S.; Winum J. Y.; Supuran C. T.; Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013, 32 (44), 5210–5219. 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- Fukami T.; Yokoi T. The emerging role of human esterases. Drug Metab. Pharmacokinet. 2012, 27 (5), 466–477. 10.2133/dmpk.DMPK-12-RV-042. [DOI] [PubMed] [Google Scholar]
- Kohnz R. A.; Mulvihill M. M.; Chang J. W.; Hsu K. L.; Sorrentino A.; Cravatt B. F.; Bandyopadhyay S.; Goga A.; Nomura D. K. Activity-Based Protein Profiling of Oncogene-Driven Changes in Metabolism Reveals Broad Dysregulation of PAFAH1B2 and 1B3 in Cancer. ACS Chem. Biol. 2015, 10 (7), 1624–1630. 10.1021/acschembio.5b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A.; Grandis J. R. Phospholipase C-γ1 in tumor progression. Clin. Exp. Metastasis 2003, 20 (4), 285–290. 10.1023/A:1024088922957. [DOI] [PubMed] [Google Scholar]
- Aranda J.; Cerqueira N. M.; Fernandes P. A.; Roca M.; Tunon I.; Ramos M. J. The catalytic mechanism of carboxylesterases: a computational study. Biochemistry 2014, 53 (36), 5820–5829. 10.1021/bi500934j. [DOI] [PubMed] [Google Scholar]
- Nomura D. K.; Long J. Z.; Niessen S.; Hoover H. S.; Ng S. W.; Cravatt B. F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 2010, 140 (1), 49–61. 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H.; Ruan Z. H. The role of monoacylglycerol lipase (MAGL) in the cancer progress. Cell Biochem. Biophys. 2014, 70 (1), 33–36. 10.1007/s12013-014-9899-2. [DOI] [PubMed] [Google Scholar]
- Niu R.; Jing H.; Chen Z.; Xu J.; Dai J.; Yan Z. Differentiating malignant colorectal tumor patients from benign colorectal tumor patients by assaying morning urinary arylsulfatase activity. Asia-Pac. J. Clin. Oncol. 2012, 8 (4), 362–367. 10.1111/j.1743-7563.2012.01545.x. [DOI] [PubMed] [Google Scholar]
- Kunimoto T.; Nitta K.; Tanaka T.; Uehara N.; Baba H.; Takeuchi M.; Yokokura T.; Sawada S.; Miyasaka T.; Mutai M. Antitumor Activity of 7-Ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin, a Novel Water-soluble Derivative of Camptothecin, against Murine Tumors. Cancer Res. 1987, 47 (22), 5944–5947. [PubMed] [Google Scholar]
- Zahreddine H.; Borden K. L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski K.; Kciuk M.; Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21 (9), 3233. 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani Y. A. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14 (Suppl 1), 35–48. 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- Wang J.; Seebacher N.; Shi H.; Kan Q.; Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget 2017, 8 (48), 84559. 10.18632/oncotarget.19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Yang Z.; Nie Y.; Shi Y.; Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014, 347 (2), 159–166. 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Dallavalle S.; Dobricic V.; Lazzarato L.; Gazzano E.; Machuqueiro M.; Pajeva I.; Tsakovska I.; Zidar N.; Fruttero R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682. 10.1016/j.drup.2020.100682. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Lee M.-G.; Shi H.; Won M.; Arambula J. F.; Sessler J. L.; Lee J. Y.; Chi S.-G.; Kim J. S. Overcoming Drug Resistance by Targeting Cancer Bioenergetics with an Activatable Prodrug. Chem. 2018, 4 (10), 2370–2383. 10.1016/j.chempr.2018.08.002. [DOI] [Google Scholar]
- Nayak M. K.; Dhanesha N.; Doddapattar P.; Rodriguez O.; Sonkar V. K.; Dayal S.; Chauhan A. K. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018, 2 (15), 2029–2038. 10.1182/bloodadvances.2018022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. H.; Yu A. M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20 (5), 793–807. 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C.; Bond J. S. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283 (45), 30433–30437. 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S.; Bond J. S. Prointerleukin-18 Is Activated by Meprin β in Vitro and in Vivo in Intestinal Inflammation. J. Biol. Chem. 2008, 283 (46), 31371–31377. 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D.; Ma S.; Shan L.; Wang Y.; Wang Y.; Cao C.; Liu B.; Yang C.; Wang L.; Tian S.; et al. Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J. Clin. Invest. 2018, 128 (10), 4280–4296. 10.1172/JCI120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matters G. L.; Manni A.; Bond J. S. Inhibitors of polyamine biosynthesis decrease the expression of the metalloproteases meprin alpha and MMP-7 in hormone-independent human breast cancer cells. Clin. Exp. Metastasis 2005, 22 (4), 331–339. 10.1007/s10585-005-0660-5. [DOI] [PubMed] [Google Scholar]
- Hua Y.; Nair S. Proteases in cardiometabolic diseases: Pathophysiology, molecular mechanisms and clinical applications. Biochim. Biophys. Acta 2015, 1852 (2), 195–208. 10.1016/j.bbadis.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.; Park M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell. Mol. Life Sci. 2020, 77 (14), 2659–2680. 10.1007/s00018-019-03428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.; Li F.; Liu X.; Li W.; Shi W.; Liu F. F.; O’Sullivan B.; He Z.; Peng Y.; Tan A. C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17 (7), 860–866. 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E.; Egashira J.; Urase K.; Kuida K.; Momoi T. Caspase-9 processing by caspase-3 via a feedback amplification loop in vivo. Cell Death Differ. 2001, 8 (4), 335–344. 10.1038/sj.cdd.4400824. [DOI] [PubMed] [Google Scholar]
- Duclos C.; Lavoie C.; Denault J. B. Caspases rule the intracellular trafficking cartel. FEBS J. 2017, 284 (10), 1394–1420. 10.1111/febs.14071. [DOI] [PubMed] [Google Scholar]
- Chung S. W.; Choi J. U.; Cho Y. S.; Kim H. R.; Won T. H.; Dimitrion P.; Jeon O. C.; Kim S. W.; Kim I. S.; Kim S. Y.; et al. Self-Triggered Apoptosis Enzyme Prodrug Therapy (STAEPT): Enhancing Targeted Therapies via Recurrent Bystander Killing Effect by Exploiting Caspase-Cleavable Linker. Adv. Sci. (Weinh.) 2018, 5 (7), 1800368. 10.1002/advs.201800368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M.; Kakinuma Y.; Yuhki K.; Murakoshi N.; Iemitsu M.; Miyauchi T.; Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J. Pharmacol. Sci. 2006, 101 (2), 151–158. 10.1254/jphs.FP0050980. [DOI] [PubMed] [Google Scholar]
- Reiser J.; Adair B.; Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Invest. 2010, 120 (10), 3421–3431. 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V.; Joyce J. A. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 2007, 6 (1), 60–64. 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- Chen S.; Dong H.; Yang S.; Guo H. Cathepsins in digestive cancers. Oncotarget 2017, 8 (25), 41690–41700. 10.18632/oncotarget.16677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G. J.; Peng Z. K.; Lu J. P.; Tang F. Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013, 4 (4), 91–101. 10.4331/wjbc.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.; Shim M. K.; Yang S.; Song S.; Moon Y.; Kim J.; Byun Y.; Ahn C. H.; Kim K. Cathepsin B-Overexpressed Tumor Cell Activatable Albumin-Binding Doxorubicin Prodrug for Cancer-Targeted Therapy. Pharmaceutics 2022, 14 (1), 83. 10.3390/pharmaceutics14010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X.; Zhang J.; Jin X.; Liu L.; Tian X. Folate Receptor Targeting and Cathepsin B-Sensitive Drug Delivery System for Selective Cancer Cell Death and Imaging. ACS Med. Chem. Lett. 2020, 11 (8), 1514–1520. 10.1021/acsmedchemlett.0c00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehme I.; Deubzer H. E.; Wegener D.; Pickert D.; Linke J. P.; Hero B.; Kopp-Schneider A.; Westermann F.; Ulrich S. M.; von Deimling A.; et al. Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin. Cancer Res. 2009, 15 (1), 91–99. 10.1158/1078-0432.CCR-08-0684. [DOI] [PubMed] [Google Scholar]
- Sudo T.; Mimori K.; Nishida N.; Kogo R.; Iwaya T.; Tanaka F.; Shibata K.; Fujita H.; Shirouzu K.; Mori M. Histone deacetylase 1 expression in gastric cancer. Oncol. Rep. 2011, 26 (4), 777–782. 10.3892/or.2011.1361. [DOI] [PubMed] [Google Scholar]
- Weichert W.; Denkert C.; Noske A.; Darb-Esfahani S.; Dietel M.; Kalloger S. E.; Huntsman D. G.; Kobel M. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia 2008, 10 (9), 1021–1027. 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.; Nian H.; Rajendran P.; Kim E.; Dashwood W. M.; Pinto J. T.; Boardman L. A.; Thibodeau S. N.; Limburg P. J.; Lohr C. V.; et al. HDAC8 and STAT3 repress BMF gene activity in colon cancer cells. Cell Death Differ. 2014, 5 (10), e1476 10.1038/cddis.2014.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithraprabhu S.; Kalff A.; Chow A.; Khong T.; Spencer A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics 2014, 9 (11), 1511–1520. 10.4161/15592294.2014.983367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J. H.; Lee H.; Sharma A.; Lee S. M.; Lee T. H.; Kang C.; Kim J. S. Indomethacin-guided cancer selective prodrug conjugate activated by histone deacetylase and tumour-associated protease. Chem. Commun. (Camb.) 2016, 52 (64), 9965–9968. 10.1039/C6CC04255D. [DOI] [PubMed] [Google Scholar]
- Bai H.; Wang H.; Zhou Z.; Piao Y.; Liu X.; Tang J.; Shen X.; Shen Y.; Zhou Z.. Histone Deacetylase-Triggered Self-Immolative Peptide-Cytotoxins for Cancer-Selective Drug Delivery. Adv. Funct. Mater. 2023, 33 ( (16), ). 10.1002/adfm.202214025. [DOI] [Google Scholar]
- Liu Z.; Yu L.; Wang X.; Zhang X.; Liu M.; Zeng W. Integrin (αvβ3) targeted RGD peptide based probe for cancer optical imaging. Curr. Protein Pept. Sci. 2016, 17 (6), 570–581. 10.2174/1389203717666160101124015. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Qutaish M.; Han Z.; Schur R. M.; Liu Y.; Wilson D. L.; Lu Z. R. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat. Commun. 2015, 6, 7984. 10.1038/ncomms8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.; He Q.; Liu J.; Chen Y.; Ma M.; Zhang L.; Shi J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134 (13), 5722–5725. 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- Xiang R.; Luo Y.; Niethammer A. G.; Reisfeld R. A. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol. Rev. 2008, 222, 117–128. 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- Song M. The Asparaginyl Endopeptidase Legumain: An Emerging Therapeutic Target and Potential Biomarker for Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23 (18), 10223. 10.3390/ijms231810223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.; Matthews S. P.; Reinheckel T.; Fleming S.; Watts C. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J. 2011, 25 (5), 1606–1617. 10.1096/fj.10-172312. [DOI] [PubMed] [Google Scholar]
- Ohno Y.; Nakashima J.; Izumi M.; Ohori M.; Hashimoto T.; Tachibana M. Association of legumain expression pattern with prostate cancer invasiveness and aggressiveness. World J. Urol. 2013, 31 (2), 359–364. 10.1007/s00345-012-0977-z. [DOI] [PubMed] [Google Scholar]
- D’Costa Z. C.; Higgins C.; Ong C. W.; Irwin G. W.; Boyle D.; McArt D. G.; McCloskey K.; Buckley N. E.; Crawford N. T.; Thiagarajan L.; et al. TBX2 represses CST6 resulting in uncontrolled legumain activity to sustain breast cancer proliferation: a novel cancer-selective target pathway with therapeutic opportunities. Oncotarget 2014, 5 (6), 1609–1620. 10.18632/oncotarget.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P.; Zhu Z.; Sun Z.; Wang Z.; Zheng X.; Xu H. Expression of legumain correlates with prognosis and metastasis in gastric carcinoma. PLoS One 2013, 8 (9), e73090 10.1371/journal.pone.0073090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Chen S.; Zhang M.; Li N.; Chen Y.; Su W.; Liu Y.; Lu D.; Li S.; Yang Y.; et al. Legumain: a biomarker for diagnosis and prognosis of human ovarian cancer. J. Cell Biochem. 2012, 113 (8), 2679–2686. 10.1002/jcb.24143. [DOI] [PubMed] [Google Scholar]
- Haugen M. H.; Boye K.; Nesland J. M.; Pettersen S. J.; Egeland E. V.; Tamhane T.; Brix K.; Maelandsmo G. M.; Flatmark K. High expression of the cysteine proteinase legumain in colorectal cancer - implications for therapeutic targeting. Eur. J. Cancer 2015, 51 (1), 9–17. 10.1016/j.ejca.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Li N.; Liu Q.; Su Q.; Wei C.; Lan B.; Wang J.; Bao G.; Yan F.; Yu Y.; Peng B.; et al. Effects of legumain as a potential prognostic factor on gastric cancers. Med. Oncol. 2013, 30, 1–12. 10.1007/s12032-013-0621-9. [DOI] [PubMed] [Google Scholar]
- Liu C.; Sun C.; Huang H.; Janda K.; Edgington T. Overexpression of Legumain in Tumors Is Significant for Invasion/Metastasis and a Candidate Enzymatic Target for Prodrug Therapy1. Cancer Res. 2003, 63 (11), 2957–2964. [PubMed] [Google Scholar]
- Wu W.; Luo Y.; Sun C.; Liu Y.; Kuo P.; Varga J.; Xiang R.; Reisfeld R.; Janda K. D.; Edgington T. S.; et al. Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Cancer Res. 2006, 66 (2), 970–980. 10.1158/0008-5472.CAN-05-2591. [DOI] [PubMed] [Google Scholar]
- Chen H.; Liu X.; Clayman E. S.; Shao F.; Xiao M.; Tian X.; Fu W.; Zhang C.; Ruan B.; Zhou P.; et al. Synthesis and Evaluation of a CBZ-AAN-Dox Prodrug and its in vitro Effects on SiHa Cervical Cancer Cells Under Hypoxic Conditions. Chem. Biol. Drug. Des. 2015, 86 (4), 589–598. 10.1111/cbdd.12525. [DOI] [PubMed] [Google Scholar]
- Juers D. H.; Matthews B. W.; Huber R. E. LacZ beta-galactosidase: structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012, 21 (12), 1792–1807. 10.1002/pro.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma D.; Sakabe M.; Kamiya M.; Yamamoto K.; Hiratake J.; Ogawa M.; Kosaka N.; Choyke P. L.; Nagano T.; Kobayashi H.; et al. Sensitive beta-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo. Nat. Commun. 2015, 6, 6463. 10.1038/ncomms7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna D. R.; Sperker B.; Fritz P.; Klotz U. Does pH 6 beta-galactosidase activity indicate cell senescence?. Mech. Ageing Dev. 1999, 109 (2), 113–123. 10.1016/S0047-6374(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. K.; Bhattacharya M.; Barlow J. J. Glycosyltransferase and glycosidase activities in ovarian cancer patients. Cancer Res. 1979, 39 (6 Pt 1), 1943–1951. [PubMed] [Google Scholar]
- Wagner J.; Damaschke N.; Yang B.; Truong M.; Guenther C.; McCormick J.; Huang W.; Jarrard D. Overexpression of the novel senescence marker beta-galactosidase (GLB1) in prostate cancer predicts reduced PSA recurrence. PLoS One 2015, 10 (4), e0124366 10.1371/journal.pone.0124366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H.; Murayama Y.; Ogawa S.; Matsumoto T.; Yubakami M.; Ohashi T.; Kubota T.; Okamoto K.; Kamiya M.; Urano Y.; et al. beta-Galactosidase is a target enzyme for detecting peritoneal metastasis of gastric cancer. Sci. Rep. 2021, 11 (1), 10664. 10.1038/s41598-021-88982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe M.; Asanuma D.; Kamiya M.; Iwatate R. J.; Hanaoka K.; Terai T.; Nagano T.; Urano Y. Rational design of highly sensitive fluorescence probes for protease and glycosidase based on precisely controlled spirocyclization. J. Am. Chem. Soc. 2013, 135 (1), 409–414. 10.1021/ja309688m. [DOI] [PubMed] [Google Scholar]
- Li X.; Pan Y.; Chen H.; Duan Y.; Zhou S.; Wu W.; Wang S.; Liu B. Specific Near-Infrared Probe for Ultrafast Imaging of Lysosomal beta-Galactosidase in Ovarian Cancer Cells. Anal. Chem. 2020, 92 (8), 5772–5779. 10.1021/acs.analchem.9b05121. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Kim E. J.; Shi H.; Lee J. Y.; Chung B. G.; Kim J. S. Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomaterials 2018, 155, 145–151. 10.1016/j.biomaterials.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Maiti M.; Kikuchi K.; Athul K. K.; Kaur A.; Bhuniya S. beta-Galactosidase-activated theranostic for hepatic carcinoma therapy and imaging. Chem. Commun. (Camb.) 2022, 58 (44), 6413–6416. 10.1039/D2CC01825J. [DOI] [PubMed] [Google Scholar]
- Noble S.; Goa K. L. Gemcitabine. Drugs 1997, 54 (3), 447–472. 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- Lee F. Y.; Vessey A.; Rofstad E.; Siemann D. W.; Sutherland R. M. Heterogeneity of glutathione content in human ovarian cancer. Cancer Res. 1989, 49 (19), 5244–5248. [PubMed] [Google Scholar]
- Zhang L.; Tew K. D. Reductive stress in cancer. Adv. Cancer Res. 2021, 152, 383–413. 10.1016/bs.acr.2021.03.009. [DOI] [PubMed] [Google Scholar]
- Konstantinov A. A.; Peskin A. V.; Popova E. Y.; Khomutov G. B.; Ruuge E. K. Superoxide generation by the respiratory chain of tumor mitochondria. Biochim Biophys Acta Bioenerg 1987, 894 (1), 1–10. 10.1016/0005-2728(87)90206-4. [DOI] [PubMed] [Google Scholar]
- Arfin S.; Jha N. K.; Jha S. K.; Kesari K. K.; Ruokolainen J.; Roychoudhury S.; Rathi B.; Kumar D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10 (5), 642. 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B.; Di Donato M.; Pezone A.; Di Zazzo E.; Giovannelli P.; Galasso G.; Castoria G.; Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 2020, 52 (2), 192–203. 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grek C. L.; Tew K. D. Redox metabolism and malignancy. Curr. Opin. Pharmacol. 2010, 10 (4), 362–368. 10.1016/j.coph.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; Lee M. G.; Won M.; Koo S.; Arambula J. F.; Sessler J. L.; Chi S. G.; Kim J. S. Targeting Heterogeneous Tumors Using a Multifunctional Molecular Prodrug. J. Am. Chem. Soc. 2019, 141 (39), 15611–15618. 10.1021/jacs.9b07171. [DOI] [PubMed] [Google Scholar]
- Wang J.; Sun X.; Mao W.; Sun W.; Tang J.; Sui M.; Shen Y.; Gu Z. Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Adv. Mater. 2013, 25 (27), 3670–3676. 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- Luo C.; Sun J.; Liu D.; Sun B.; Miao L.; Musetti S.; Li J.; Han X.; Du Y.; Li L.; et al. Self-Assembled Redox Dual-Responsive Prodrug-Nanosystem Formed by Single Thioether-Bridged Paclitaxel-Fatty Acid Conjugate for Cancer Chemotherapy. Nano Lett. 2016, 16 (9), 5401–5408. 10.1021/acs.nanolett.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.; Gao C.; Liu W.; Ren H.; Wang C.; Ge K.; Li S.; Zhou G.; Li H.; Wang S.; et al. A novel anticancer theranostic pro-prodrug based on hypoxia and photo sequential control. Chem. Commun. (Camb.) 2016, 52 (60), 9434–9437. 10.1039/C6CC02932A. [DOI] [PubMed] [Google Scholar]
- Xiong H.; Zhou K.; Yan Y.; Miller J. B.; Siegwart D. J. Tumor-Activated Water-Soluble PSs for Near-Infrared Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10 (19), 16335–16343. 10.1021/acsami.8b04710. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Xu C.; Teng L.; Liu H.-W.; Ren T.-B.; Xu S.; Lou X.; Guo H.; Yuan L.; Zhang X.-B. pH stimulus-disaggregated BODIPY: an activated photodynamic/photothermal sensitizer applicable to tumor ablation. Chem. Commun. 2020, 56 (13), 1956–1959. 10.1039/C9CC09790B. [DOI] [PubMed] [Google Scholar]
- Figliola C.; Anton H.; Sutter C.; Chériaux C.; Sutter A.; Mazan V.; Elhabiri M.; Didier P.; Jacquemin D.; Ulrich G.. Lysosomes targeting pH activable imaging-guided photodynamic agents. ChemBioChem. 2023. 10.1002/cbic.202300139. [DOI] [PubMed] [Google Scholar]
- Chen D.; Dai H.; Wang W.; Cai Y.; Mou X.; Zou J.; Shao J.; Mao Z.; Zhong L.; Dong X.; et al. Proton-Driven Transformable1 O2 -Nanotrap for Dark and Hypoxia Tolerant Photodynamic Therapy. Adv. Sci. 2022, 9 (17), 2200128. 10.1002/advs.202200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Zhou J.; Shen Z.; Ding L.; Yu J.-S.; Ju H. A pH-activatable and aniline-substituted PS for near-infrared cancer theranostics. Chem. Sci. 2015, 6 (10), 5969–5977. 10.1039/C5SC01721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Xue L.; Yu Q.; Chen D.; Cheng Z.; Wang W.; Shao J.; Dong X. Smart Aza-BODIPY PS for Tumor Microenvironment-Enhanced Cancer Phototherapy. ACS Appl. Bio Mater. 2019, 2 (12), 5888–5897. 10.1021/acsabm.9b00836. [DOI] [PubMed] [Google Scholar]
- Tang Q.; Xiao W.; Huang C.; Si W.; Shao J.; Huang W.; Chen P.; Zhang Q.; Dong X. pH-Triggered and Enhanced Simultaneous Photodynamic and Photothermal Therapy Guided by Photoacoustic and Photothermal Imaging. Chem. Mater. 2017, 29 (12), 5216–5224. 10.1021/acs.chemmater.7b01075. [DOI] [Google Scholar]
- Siriwibool S.; Kaekratoke N.; Chansaenpak K.; Siwawannapong K.; Panajapo P.; Sagarik K.; Noisa P.; Lai R. Y.; Kamkaew A. Near-Infrared Fluorescent pH Responsive Probe for Targeted Photodynamic Cancer Therapy. Sci. Rep. 2020, 10 (1), 1283. 10.1038/s41598-020-58239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Bu Y.; Lu X.; Chen D.; Yu Z.; Zhang J.; Wang L.; Zhou H. Molecular engineering to construct specific cancer cell lysosome targeting PS by adjusting the proton binding ability. Sens. Actuators B Chem. 2022, 371, 132546. 10.1016/j.snb.2022.132546. [DOI] [Google Scholar]
- Xiao P.; Xie W.; Zhang J.; Wu Q.; Shen Z.; Guo C.; Wu Y.; Wang F.; Tang B. Z.; Wang D. De Novo Design of Reversibly pH-Switchable NIR-II Aggregation-Induced Emission Luminogens for Efficient Phototheranostics of Patient-Derived Tumor Xenografts. J. Am. Chem. Soc. 2023, 145 (1), 334–344. 10.1021/jacs.2c10076. [DOI] [PubMed] [Google Scholar]
- Hu W.; He T.; Zhao H.; Tao H.; Chen R.; Jin L.; Li J.; Fan Q.; Huang W.; Baev A.; et al. Stimuli-Responsive Reversible Switching of Intersystem Crossing in Pure Organic Material for Smart Photodynamic Therapy. Angew. Chem.-Int. Ed. 2019, 58 (32), 11105–11111. 10.1002/anie.201905129. [DOI] [PubMed] [Google Scholar]
- Jung H. S.; Chen X.; Kim J. S.; Yoon J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem. Soc. Rev. 2013, 42 (14), 6019–6031. 10.1039/c3cs60024f. [DOI] [PubMed] [Google Scholar]
- Niu L. Y.; Chen Y. Z.; Zheng H. R.; Wu L. Z.; Tung C. H.; Yang Q. Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44 (17), 6143–6160. 10.1039/C5CS00152H. [DOI] [PubMed] [Google Scholar]
- Lee S.; Li J.; Zhou X.; Yin J.; Yoon J. Recent progress on the development of glutathione (GSH) selective fluorescent and colorimetric probes. Coord. Chem. Rev. 2018, 366, 29–68. 10.1016/j.ccr.2018.03.021. [DOI] [Google Scholar]
- Gamcsik M. P.; Kasibhatla M. S.; Teeter S. D.; Colvin O. M. Glutathione levels in human tumors. Biomarkers 2012, 17 (8), 671–691. 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J.; Du K.; Sun J.; Yang X.; Wang X.; Zhang X.; Song G.; Feng F. Photocatalytic Generation of Hydrogen Radical (H·) with GSH for Photodynamic Therapy. Angew. Chem., Int. Ed. 2023, 62 (9), e202214991 10.1002/anie.202214991. [DOI] [PubMed] [Google Scholar]
- Lucero M. Y.; Chan J. Photoacoustic imaging of elevated glutathione in models of lung cancer for companion diagnostic applications. Nat. Chem. 2021, 13 (12), 1248–1256. 10.1038/s41557-021-00804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H. H.; Wang H. M.; Jangili P.; Li M.; Wu L.; Zang Y.; Sedgwick A. C.; Li J.; He X. P.; James T. D.; et al. The design of small-molecule prodrugs and activatable phototherapeutics for cancer therapy. Chem. Soc. Rev. 2023, 52 (3), 879–920. 10.1039/D2CS00673A. [DOI] [PubMed] [Google Scholar]
- Pham T. C.; Nguyen V. N.; Choi Y.; Lee S.; Yoon J. Recent Strategies to Develop Innovative PSs for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121 (21), 13454–13619. 10.1021/acs.chemrev.1c00381. [DOI] [PubMed] [Google Scholar]
- Li H.; Kim Y.; Jung H.; Hyun J. Y.; Shin I. Near-infrared (NIR) fluorescence-emitting small organic molecules for cancer imaging and therapy. Chem. Soc. Rev. 2022, 51 (21), 8957–9008. 10.1039/D2CS00722C. [DOI] [PubMed] [Google Scholar]
- Wang R.; Xia X.; Yang Y.; Rong X.; Liu T.; Su Z.; Zeng X.; Du J.; Fan J.; Sun W.; et al. A Glutathione Activatable PS for Combined Photodynamic and Gas Therapy under Red Light Irradiation. Adv. Healthc. Mater. 2022, 11 (4), e2102017 10.1002/adhm.202102017. [DOI] [PubMed] [Google Scholar]
- Tam L. K. B.; Yu L.; Wong R. C. H.; Fong W. P.; Ng D. K. P.; Lo P. C. Dual Cathepsin B and Glutathione-Activated Dimeric and Trimeric Phthalocyanine-Based Photodynamic Molecular Beacons for Targeted Photodynamic Therapy. J. Med. Chem. 2021, 64 (23), 17455–17467. 10.1021/acs.jmedchem.1c01634. [DOI] [PubMed] [Google Scholar]
- Xiao Y.; Zhang T.; Ma X.; Yang Q. C.; Yang L. L.; Yang S. C.; Liang M.; Xu Z.; Sun Z. J. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. (Weinh.) 2021, 8 (24), e2101840 10.1002/advs.202101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Su W.; Ye M.; Gao Y.; Qiu W.; Liang M.; Xue P.; Kang Y.; Sun Z.-J.; Xu Z. Endogenous/exogenous stimulies inspired polyprodrug nano-inducer switches pyroptosis path for promoting antitumor immunity. Nano Today 2023, 48, 101727. 10.1016/j.nantod.2022.101727. [DOI] [Google Scholar]
- An R.; Cheng X.; Wei S.; Hu Y.; Sun Y.; Huang Z.; Chen H. Y.; Ye D. Smart Magnetic and Fluorogenic PS Nanoassemblies Enable Redox-Driven Disassembly for Photodynamic Therapy. Angew. Chem., Int. Ed. Engl. 2020, 59 (46), 20636–20644. 10.1002/anie.202009141. [DOI] [PubMed] [Google Scholar]
- Yang G.; Chen C.; Zhu Y.; Liu Z.; Xue Y.; Zhong S.; Wang C.; Gao Y.; Zhang W. GSH-Activatable NIR Nanoplatform with Mitochondria Targeting for Enhancing Tumor-Specific Therapy. ACS Appl. Mater. Interfaces 2019, 11 (48), 44961–44969. 10.1021/acsami.9b15996. [DOI] [PubMed] [Google Scholar]
- Liu S.; Feng G.; Tang B. Z.; Liu B. Recent advances of AIE light-up probes for photodynamic therapy. Chem. Sci. 2021, 12 (19), 6488–6506. 10.1039/D1SC00045D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Chen X.; Liu X.; Guan W.; Lu C. Aggregation-induced emission-active micelles: synthesis, characterization, and applications. Chem. Soc. Rev. 2023, 52 (4), 1456–1490. 10.1039/D2CS01021F. [DOI] [PubMed] [Google Scholar]
- Ouyang J.; Sun L.; Zeng F.; Wu S. Biomarker-activatable probes based on smart AIEgens for fluorescence and optoacoustic imaging. Coord. Chem. Rev. 2022, 458, 214438. 10.1016/j.ccr.2022.214438. [DOI] [Google Scholar]
- Zhang Y. H.; Li X.; Huang L.; Kim H. S.; An J.; Lan M.; Cao Q. Y.; Kim J. S. AIE based GSH activatable PS for imaging-guided photodynamic therapy. Chem. Commun. (Camb.) 2020, 56 (71), 10317–10320. 10.1039/D0CC02045A. [DOI] [PubMed] [Google Scholar]
- Luo D.; Carter K. A.; Miranda D.; Lovell J. F. Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Adv. Sci. (Weinh.) 2017, 4 (1), 1600106. 10.1002/advs.201600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.; Fan T.; An J.; Choi W.; Duo Y.; Ge Y.; Zhang B.; Nie G.; Xie N.; Zheng T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49 (22), 8065–8087. 10.1039/D0CS00215A. [DOI] [PubMed] [Google Scholar]
- Seung Lee J.; Kim J.; Ye Y. S.; Kim T. I. Materials and device design for advanced phototherapy systems. Adv. Drug Delivery Rev. 2022, 186, 114339. 10.1016/j.addr.2022.114339. [DOI] [PubMed] [Google Scholar]
- Bian H.; Ma D.; Pan F.; Zhang X.; Xin K.; Zhang X.; Yang Y.; Peng X.; Xiao Y. Cardiolipin-Targeted NIR-II Fluorophore Causes ″Avalanche Effects″ for Re-Engaging Cancer Apoptosis and Inhibiting Metastasis. J. Am. Chem. Soc. 2022, 144 (49), 22562–22573. 10.1021/jacs.2c08602. [DOI] [PubMed] [Google Scholar]
- Bian H.; Ma D.; Zhang X.; Xin K.; Yang Y.; Peng X.; Xiao Y. Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 nm Laser toward Deep-Seated Tumors. Small 2021, 17 (21), e2100398 10.1002/smll.202100398. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang Y.; Wang R.; Rong X.; Liu T.; Xia X.; Fan J.; Sun W.; Peng X. A glutathione activatable pro-drug-PS for combined chemotherapy and photodynamic therapy. Chin. Chem. Lett. 2022, 33 (10), 4583–4586. 10.1016/j.cclet.2022.03.040. [DOI] [Google Scholar]
- Huang T.; Yu Q.; Liu S.; Zhang K. Y.; Huang W.; Zhao Q. Rational Design of Phosphorescent Iridium(III) Complexes for Selective Glutathione Sensing and Amplified Photodynamic Therapy. Chembiochem 2019, 20 (4), 576–586. 10.1002/cbic.201800507. [DOI] [PubMed] [Google Scholar]
- Teng K. X.; Niu L. Y.; Kang Y. F.; Yang Q. Z. Rational design of a ″dual lock-and-key″ supramolecular PS based on aromatic nucleophilic substitution for specific and enhanced photodynamic therapy. Chem. Sci. 2020, 11 (35), 9703–9711. 10.1039/D0SC01122C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J.; Xue L.; Yang N.; Ren Y.; Fan Z.; Wang W.; Si W.; Zhang Y.; Huang W.; Dong X. A glutathione responsive pyrrolopyrrolidone nanotheranostic agent for turn-on fluorescence imaging guided photothermal/photodynamic cancer therapy. Mater. Chem. Front. 2019, 3 (10), 2143–2150. 10.1039/C9QM00471H. [DOI] [Google Scholar]
- Hwang B.; Kim T. I.; Kim H.; Jeon S.; Choi Y.; Kim Y. Ubiquinone-BODIPY nanoparticles for tumor redox-responsive fluorescence imaging and photodynamic activity. J. Mater. Chem. B 2021, 9 (3), 824–831. 10.1039/D0TB02529A. [DOI] [PubMed] [Google Scholar]
- Kilic E.; Elmazoglu Z.; Almammadov T.; Kepil D.; Etienne T.; Marion A.; Gunbas G.; Kolemen S. Activity-Based PSs with Optimized Triplet State Characteristics Toward Cancer Cell Selective and Image Guided Photodynamic Therapy. ACS Appl. Bio. Mater. 2022, 5 (6), 2754–2767. 10.1021/acsabm.2c00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savani S.; Onbasli K.; Gunduz H.; Aydindogan E.; Erkisa M.; Muti A.; Khan M.; Sennaroglu A.; Ulukaya E.; Yagci Acar H.; et al. Development of a cysteine responsive chlorinated hemicyanine for image-guided dual phototherapy. Bioorg. Chem. 2022, 122, 105725. 10.1016/j.bioorg.2022.105725. [DOI] [PubMed] [Google Scholar]
- Cheung E. C.; Vousden K. H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22 (5), 280–297. 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Beckman J. S.; Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87 (1), 315–424. 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K.; Takenaga K.; Akimoto M.; Koshikawa N.; Yamaguchi A.; Imanishi H.; Nakada K.; Honma Y.; Hayashi J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 2008, 320 (5876), 661–664. 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Fang F. C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2004, 2 (10), 820–832. 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194 (1), 7–15. 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P. H. G. M.; Rossignol R.; Dieteren C. E. J.; Murphy M. P.; Koopman W. J. H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22 (2), 207–218. 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Han J.; Lim H.-J.; Ren W. X.; Lim J.-Y.; Kim J.-H.; Kim J. S. Mitochondrial Induced and Self-Monitored Intrinsic Apoptosis by Antitumor Theranostic Prodrug: In Vivo Imaging and Precise Cancer Treatment. J. Am. Chem. Soc. 2014, 136 (51), 17836–17843. 10.1021/ja510421q. [DOI] [PubMed] [Google Scholar]
- Hagen H.; Marzenell P.; Jentzsch E.; Wenz F.; Veldwijk M. R.; Mokhir A. Aminoferrocene-based prodrugs activated by reactive oxygen species. J. Med. Chem. 2012, 55 (2), 924–934. 10.1021/jm2014937. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Fan J.; Wang J.; Mu H.; Peng X. An “Enhanced PET”-Based Fluorescent Probe with Ultrasensitivity for Imaging Basal and Elesclomol-Induced HClO in Cancer Cells. J. Am. Chem. Soc. 2014, 136 (37), 12820–12823. 10.1021/ja505988g. [DOI] [PubMed] [Google Scholar]
- Li T.; Liu Z.; Hu J.; Chen L.; Chen T.; Tang Q.; Yu B.; Zhao B.; Mao C.; Wan M. A Universal Chemotactic Targeted Delivery Strategy for Inflammatory Diseases. Adv. Mater. 2022, 34 (47), 2206654. 10.1002/adma.202206654. [DOI] [PubMed] [Google Scholar]
- Chen H.; Li T.; Liu Z.; Tang S.; Tong J.; Tao Y.; Zhao Z.; Li N.; Mao C.; Shen J.; et al. A nitric-oxide driven chemotactic nanomotor for enhanced immunotherapy of glioblastoma. Nat. Commun. 2023, 14 (1), 941. 10.1038/s41467-022-35709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Sun W.; Du J.; Fan J.; Peng X. Immuno-photodynamic Therapy (IPDT): Organic PSs and Their Application in Cancer Ablation. JACS Au 2023, 3 (3), 682–699. 10.1021/jacsau.2c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H.; Liu W.; Li H.; Sun W.; Du J.; Fan J.; Peng X. 2,1,3-Benzothiadiazole derivative AIEgens for smart phototheranostics. Coord. Chem. Rev. 2022, 473, 214803. 10.1016/j.ccr.2022.214803. [DOI] [Google Scholar]
- Nguyen V.-N.; Zhao Z.; Tang B. Z.; Yoon J. Organic PSs for antimicrobial phototherapy. Chem. Soc. Rev. 2022, 51 (9), 3324–3340. 10.1039/D1CS00647A. [DOI] [PubMed] [Google Scholar]
- Kitamura T.; Nakata H.; Takahashi D.; Toshima K. Hypocrellin B-based activatable PSs for specific photodynamic effects against high H2O2-expressing cancer cells. Chem. Commun. 2021, 58 (2), 242–245. 10.1039/D1CC05823A. [DOI] [PubMed] [Google Scholar]
- Zeng Q.; Zhang R.; Zhang T.; Xing D. H2O2-responsive biodegradable nanomedicine for cancer-selective dual-modal imaging guided precise photodynamic therapy. Biomaterials 2019, 207, 39–48. 10.1016/j.biomaterials.2019.03.042. [DOI] [PubMed] [Google Scholar]
- Guo X.; Yang N.; Ji W.; Zhang H.; Dong X.; Zhou Z.; Li L.; Shen H. M.; Yao S. Q.; Huang W. Mito-Bomb: Targeting Mitochondria for Cancer Therapy. Adv. Mater. 2021, 33 (43), 2007778. 10.1002/adma.202170340. [DOI] [PubMed] [Google Scholar]
- Heng H.; Song G.; Cai X.; Sun J.; Du K.; Zhang X.; Wang X.; Feng F.; Wang S. Intrinsic Mitochondrial Reactive Oxygen Species (ROS) Activate the In Situ Synthesis of Trimethine Cyanines in Cancer Cells. Angew. Chem., Int. Ed. 2022, 61 (38), e202203444 10.1002/anie.202203444. [DOI] [PubMed] [Google Scholar]
- Lee L. C.-C.; Lo K. K.-W. Luminescent and Photofunctional Transition Metal Complexes: From Molecular Design to Diagnostic and Therapeutic Applications. J. Am. Chem. Soc. 2022, 144 (32), 14420–14440. 10.1021/jacs.2c03437. [DOI] [PubMed] [Google Scholar]
- Monro S.; Colón K. L.; Yin H.; Roque J.; Konda P.; Gujar S.; Thummel R. P.; Lilge L.; Cameron C. G.; McFarland S. A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119 (2), 797–828. 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbas-Cakmak S.; Kolemen S.; Sedgwick A. C.; Gunnlaugsson T.; James T. D.; Yoon J.; Akkaya E. U. Molecular logic gates: the past, present and future. Chem. Soc. Rev. 2018, 47 (7), 2228–2248. 10.1039/C7CS00491E. [DOI] [PubMed] [Google Scholar]
- Wu L.; Huang J.; Pu K.; James T. D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 2021, 5 (6), 406–421. 10.1038/s41570-021-00277-2. [DOI] [PubMed] [Google Scholar]
- Heng H.; Song G.; Cai X.; Sun J.; Du K.; Zhang X.; Wang X.; Feng F.; Wang S. Intrinsic Mitochondrial Reactive Oxygen Species (ROS) Activate the In Situ Synthesis of Trimethine Cyanines in Cancer Cells. Angew. Chem., Int. Ed. 2022, 61 (38), e202203444 10.1002/anie.202203444. [DOI] [PubMed] [Google Scholar]
- Xin Q.; Ma H.; Wang H.; Zhang X. D. Tracking tumor heterogeneity and progression with near-infrared II fluorophores. Exploration 2023, 3 (2), 20220011. 10.1002/EXP.20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; An H.; Zhang D.; Tao H.; Dou Y.; Li X.; Huang J.; Zhang J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci. Adv. 2019, 5 (1), eaat2953 10.1126/sciadv.aat2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Li Y.; Yu L.; Yang Z.; Ding J.; Wang K. N.; Zhang Y. Monocomponent Nanodots with Dichromatic Output Regulated by Synergistic Dual-Stimuli for Cervical Cancer Tissue Imaging and Photodynamic Tumor Therapy. Anal. Chem. 2022, 94 (2), 811–819. 10.1021/acs.analchem.1c03488. [DOI] [PubMed] [Google Scholar]
- Huang T.; Ji H.; Yan S.; Zuo Y.; Li J.; Lam J. W. Y.; Han C.; Tang B. Z. A hypochlorite-activated strategy for realizing fluorescence turn-on, type I and type II ROS-combined photodynamic tumor ablation. Biomaterials 2023, 297, 122108. 10.1016/j.biomaterials.2023.122108. [DOI] [PubMed] [Google Scholar]
- Wu M.; Wu W.; Duan Y.; Liu X.; Wang M.; Phan C. U.; Qi G.; Tang G.; Liu B. HClO-Activated Fluorescence and Photosensitization from an AIE Nanoprobe for Image-Guided Bacterial Ablation in Phagocytes. Adv. Mater. 2020, 32 (47), e2005222 10.1002/adma.202005222. [DOI] [PubMed] [Google Scholar]
- Teng L.; Song G.; Liu Y.; Han X.; Li Z.; Wang Y.; Huan S.; Zhang X. B.; Tan W. Nitric Oxide-Activated ″Dual-Key-One-Lock″ Nanoprobe for in Vivo Molecular Imaging and High-Specificity Cancer Therapy. J. Am. Chem. Soc. 2019, 141 (34), 13572–13581. 10.1021/jacs.9b05901. [DOI] [PubMed] [Google Scholar]
- Greten F. R.; Grivennikov S. I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51 (1), 27–41. 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W.; Xie M.; Zhao H.; Tang Y.; Yao S.; He T.; Ye C.; Wang Q.; Lu X.; Huang W.; et al. Nitric oxide activatable PS accompanying extremely elevated two-photon absorption for efficient fluorescence imaging and photodynamic therapy. Chem. Sci. 2018, 9 (4), 999–1005. 10.1039/C7SC04044J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai W.; Zhang Y.; Liu M.; Zhang H.; Zhang J.; Li C. Universal Scaffold for an Activatable PS with Completely Inhibited Photosensitivity. Angew. Chem., Int. Ed. Engl. 2019, 58 (46), 16601–16609. 10.1002/anie.201907510. [DOI] [PubMed] [Google Scholar]
- Su M.; Li S.; Zhang H.; Zhang J.; Chen H.; Li C. Nano-Assemblies from J-Aggregated Dyes: A Stimuli-Responsive Tool Applicable To Living Systems. J. Am. Chem. Soc. 2019, 141 (1), 402–413. 10.1021/jacs.8b10396. [DOI] [PubMed] [Google Scholar]
- Gao Z.; Jia S.; Ou H.; Hong Y.; Shan K.; Kong X.; Wang Z.; Feng G.; Ding D. An Activatable Near-Infrared Afterglow Theranostic Prodrug with Self-Sustainable Magnification Effect of Immunogenic Cell Death. Angew. Chem., Int. Ed. 2022, 61 (40), e202209793 10.1002/anie.202209793. [DOI] [PubMed] [Google Scholar]
- Yang L.; Zhu Y.; Liang L.; Wang C.; Ning X.; Feng X. Self-Assembly of Intelligent Nanoplatform for Endogenous H(2)S-Triggered Multimodal Cascade Therapy of Colon Cancer. Nano Lett. 2022, 22 (10), 4207–4214. 10.1021/acs.nanolett.2c01131. [DOI] [PubMed] [Google Scholar]
- An L.; Wang X.; Rui X.; Lin J.; Yang H.; Tian Q.; Tao C.; Yang S. The In Situ Sulfidation of Cu(2) O by Endogenous H(2) S for Colon Cancer Theranostics. Angew. Chem., Int. Ed. Engl. 2018, 57 (48), 15782–15786. 10.1002/anie.201810082. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Xiong M. C.; Niu L. Y.; Yang Q. Z. Nano-assemblies from J-aggregated dyes to improve the selectivity of a H2S-activatable PS. Chem. Commun. (Camb.) 2022, 58 (72), 10060–10063. 10.1039/D2CC04191J. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Fang J.; Ye S.; Zhao Y.; Wang A.; Mao Q.; Cui C.; Feng Y.; Li J.; Li S.; et al. A hydrogen sulphide-responsive and depleting nanoplatform for cancer photodynamic therapy. Nat. Commun. 2022, 13 (1), 1685. 10.1038/s41467-022-29284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirak M.; Kepil D.; Almammadov T.; Elmazoglu Z.; Cetin S.; Ozogul N.; Gunbas G.; Kolemen S. Selective monitoring and treatment of neuroblastoma cells with hydrogen sulfide activatable phototheranostic agent. Dyes Pigment. 2023, 210, 111011. 10.1016/j.dyepig.2022.111011. [DOI] [Google Scholar]
- Bertozzi C. R. A decade of bioorthogonal chemistry. Acc. Chem. Res. 2011, 44 (9), 651–653. 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj N. K. The Future of Bioorthogonal Chemistry. ACS Cent. Sci. 2018, 4 (8), 952–959. 10.1021/acscentsci.8b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K. K. Molecular Design of Bioorthogonal Probes and Imaging Reagents Derived from Photofunctional Transition Metal Complexes. Acc. Chem. Res. 2020, 53 (1), 32–44. 10.1021/acs.accounts.9b00416. [DOI] [PubMed] [Google Scholar]
- Liu L.; Zhang D.; Johnson M.; Devaraj N. K. Light-activated tetrazines enable precision live-cell bioorthogonal chemistry. Nat. Chem. 2022, 14 (9), 1078–1085. 10.1038/s41557-022-00963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden G.; Zhang L.; Pieck F.; Linne U.; Kosenkov D.; Tonner R.; Vazquez O. Conditional Singlet Oxygen Generation through a Bioorthogonal DNA-targeted Tetrazine Reaction. Angew. Chem., Int. Ed. Engl. 2019, 58 (37), 12868–12873. 10.1002/anie.201907093. [DOI] [PubMed] [Google Scholar]
- Yip A. M.; Lai C. K.; Yiu K. S.; Lo K. K. Phosphorogenic Iridium(III) bis-Tetrazine Complexes for Bioorthogonal Peptide Stapling, Bioimaging, Photocytotoxic Applications, and the Construction of Nanosized Hydrogels. Angew. Chem., Int. Ed. 2022, 61 (16), e202116078 10.1002/anie.202116078. [DOI] [PubMed] [Google Scholar]
- Leung P. K.; Lo K. K. Modulation of emission and singlet oxygen photosensitisation in live cells utilising bioorthogonal phosphorogenic probes and protein tag technology. Chem. Commun. (Camb.) 2020, 56 (45), 6074–6077. 10.1039/D0CC02056G. [DOI] [PubMed] [Google Scholar]
- Chu J. C. H.; Wong C. T. T.; Ng D. K. P. Toward Precise Antitumoral Photodynamic Therapy Using a Dual Receptor-Mediated Bioorthogonal Activation Approach. Angew. Chem., Int. Ed. 2023, 62 (2), e202214473 10.1002/anie.202214473. [DOI] [PubMed] [Google Scholar]
- Hu F.; Mao D.; Kenry; Cai X.; Wu W.; Kong D.; Liu B. A Light-Up Probe with Aggregation-Induced Emission for Real-Time Bio-orthogonal Tumor Labeling and Image-Guided Photodynamic Therapy. Angew. Chem., Int. Ed. Engl. 2018, 57 (32), 10182–10186. 10.1002/anie.201805446. [DOI] [PubMed] [Google Scholar]
- Hu F.; Yuan Y.; Wu W.; Mao D.; Liu B. Dual-Responsive Metabolic Precursor and Light-Up AIEgen for Cancer Cell Bio-orthogonal Labeling and Precise Ablation. Anal. Chem. 2018, 90 (11), 6718–6724. 10.1021/acs.analchem.8b00547. [DOI] [PubMed] [Google Scholar]
- Scinto S. L.; Bilodeau D. A.; Hincapie R.; Lee W.; Nguyen S. S.; Xu M.; am Ende C. W.; Finn M. G.; Lang K.; Lin Q., et al. Bioorthogonal chemistry. Nat. Rev. Method. Prim., 2021, 1 ( (1), ). 10.1038/s43586-021-00028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.; Xue E. Y.; Wu Q.; Lo P. C.; Ng D. K. P. A tetrazine-responsive isonitrile-caged photosensitiser for site-specific photodynamic therapy. J. Controlled Release 2023, 353, 663–674. 10.1016/j.jconrel.2022.12.015. [DOI] [PubMed] [Google Scholar]
- Heiss T. K.; Dorn R. S.; Prescher J. A. Bioorthogonal Reactions of Triarylphosphines and Related Analogues. Chem. Rev. 2021, 121 (12), 6802–6849. 10.1021/acs.chemrev.1c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv W.; Chi S.; Feng W.; Liang T.; Song D.; Liu Z. Development of a red absorbing Se-rhodamine PS and its application for bio-orthogonally activatable photodynamic therapy. Chem. Commun. (Camb.) 2019, 55 (49), 7037–7040. 10.1039/C9CC03018B. [DOI] [PubMed] [Google Scholar]
- Lu S. B.; Wei L.; He W.; Bi Z. Y.; Qian Y.; Wang J.; Lei H.; Li K. Recent Advances in the Enzyme-Activatable Organic Fluorescent Probes for Tumor Imaging and Therapy. ChemistryOpen 2022, 11 (10), e202200137 10.1002/open.202200137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Long S.; Li M.; Cao J.; Li Y.; Guo L.; Sun W.; Du J.; Fan J.; Peng X. Oxygen-Dependent Regulation of Excited-State Deactivation Process of Rational PS for Smart Phototherapy. J. Am. Chem. Soc. 2020, 142 (3), 1510–1517. 10.1021/jacs.9b11800. [DOI] [PubMed] [Google Scholar]
- Li M.; Gebremedhin K. H.; Ma D.; Pu Z.; Xiong T.; Xu Y.; Kim J. S.; Peng X. Conditionally Activatable Photoredox Catalysis in Living Systems. J. Am. Chem. Soc. 2022, 144 (1), 163–173. 10.1021/jacs.1c07372. [DOI] [PubMed] [Google Scholar]
- Li X.; Yang M.; Cao J.; Gu H.; Liu W.; Xia T.; Sun W.; Fan J.; Peng X. H-Aggregates of Prodrug-Hemicyanine Conjugate for Enhanced Photothermal Therapy and Sequential Hypoxia-Activated Chemotherapy. ACS Mater. Lett. 2022, 4 (4), 724–732. 10.1021/acsmaterialslett.2c00015. [DOI] [Google Scholar]
- Xu F.; Li H.; Yao Q.; Ge H.; Fan J.; Sun W.; Wang J.; Peng X. Hypoxia-activated NIR PS anchoring in the mitochondria for photodynamic therapy. Chem. Sci. 2019, 10 (45), 10586–10594. 10.1039/C9SC03355F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Song F.; Shi W.; Gurzadyan G.; Yin H.; Song B.; Liang R.; Peng X. Nitroreductase-Activatable Theranostic Molecules with High PDT Efficiency under Mild Hypoxia Based on a TADF Fluorescein Derivative. ACS Appl. Mater. Interfaces 2019, 11 (17), 15426–15435. 10.1021/acsami.9b04488. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhang Y.; Zhang H.; Zhai W.; Shi X.; Li C. A hypoxia-activatable theranostic agent with intrinsic endoplasmic reticulum affinity and type-I photosensitivity. J. Mater. Chem. B 2023, 11 (18), 4102–4110. 10.1039/D3TB00328K. [DOI] [PubMed] [Google Scholar]
- Piao W.; Hanaoka K.; Fujisawa T.; Takeuchi S.; Komatsu T.; Ueno T.; Terai T.; Tahara T.; Nagano T.; Urano Y. Development of an Azo-Based PS Activated under Mild Hypoxia for Photodynamic Therapy. J. Am. Chem. Soc. 2017, 139 (39), 13713–13719. 10.1021/jacs.7b05019. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Wang X.; Zhu G.; Liu Z.; Chen X. M.; Bisoyi H. K.; Chen X.; Chen X.; Xu Y.; Li J.; et al. Hypoxia-Responsive PS Targeting Dual Organelles for Photodynamic Therapy of Tumors. Small 2023, 19 (1), e2205440 10.1002/smll.202205440. [DOI] [PubMed] [Google Scholar]
- Hao B.; Wang J.; Wang C.; Xue K.; Xiao M.; Lv S.; Zhu C. Bridging D-A type PSs with the azo group to boost intersystem crossing for efficient photodynamic therapy. Chem. Sci. 2022, 13 (14), 4139–4149. 10.1039/D2SC00381C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Kwon N.; Guo T.; Liu Z.; Yoon J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem., Int. Ed. Engl. 2018, 57 (36), 11522–11531. 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- Fan W.; Huang P.; Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45 (23), 6488–6519. 10.1039/C6CS00616G. [DOI] [PubMed] [Google Scholar]
- Juvekar V.; Lim C. S.; Lee D. J.; Park S. J.; Song G. O.; Kang H.; Kim H. M. An azo dye for photodynamic therapy that is activated selectively by two-photon excitation. Chem. Sci. 2021, 12 (1), 427–434. 10.1039/D0SC05686C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.; Wang P.; Son S.; Zhong C.; Zhang F.; Mao Z.; Liu Z.; Kim J. S. Engineering a theranostic platform for synergistic hypoxia-responsive photodynamic therapy and chemotherapy. Matter 2022, 5 (5), 1502–1519. 10.1016/j.matt.2022.02.019. [DOI] [Google Scholar]
- Wang Y.; Jiang L.; Zhang Y.; Lu Y.; Li J.; Wang H.; Yao D.; Wang D. Fibronectin-Targeting and Cathepsin B-Activatable Theranostic Nanoprobe for MR/Fluorescence Imaging and Enhanced Photodynamic Therapy for Triple Negative Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12 (30), 33564–33574. 10.1021/acsami.0c10397. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Yu L.; Wong R. C. H.; Lo P. C. Construction of cathepsin B-responsive fluorescent probe and PS using a ferrocenyl boron dipyrromethene dark quencher. Eur. J. Med. Chem. 2019, 179, 828–836. 10.1016/j.ejmech.2019.06.082. [DOI] [PubMed] [Google Scholar]
- Lu S.; Lei X.; Ren H.; Zheng S.; Qiang J.; Zhang Z.; Chen Y.; Wei T.; Wang F.; Chen X. PEGylated Dimeric BODIPY PSs as Nanocarriers for Combined Chemotherapy and Cathepsin B-Activated Photodynamic Therapy in 3D Tumor Spheroids. ACS Appl. Bio. Mater. 2020, 3 (6), 3835–3845. 10.1021/acsabm.0c00394. [DOI] [PubMed] [Google Scholar]
- Xiong J.; Chu J. C. H.; Fong W. P.; Wong C. T. T.; Ng D. K. P. Specific Activation of PS with Extrinsic Enzyme for Precisive Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144 (23), 10647–10658. 10.1021/jacs.2c04017. [DOI] [PubMed] [Google Scholar]
- Zhen X.; Zhang J.; Huang J.; Xie C.; Miao Q.; Pu K. Macrotheranostic Probe with Disease-Activated Near-Infrared Fluorescence, Photoacoustic, and Photothermal Signals for Imaging-Guided Therapy. Angew. Chem., Int. Ed. Engl. 2018, 57 (26), 7804–7808. 10.1002/anie.201803321. [DOI] [PubMed] [Google Scholar]
- Chiba M.; Kamiya M.; Tsuda-Sakurai K.; Fujisawa Y.; Kosakamoto H.; Kojima R.; Miura M.; Urano Y. Activatable PS for Targeted Ablation of lacZ-Positive Cells with Single-Cell Resolution. ACS Cent. Sci. 2019, 5 (10), 1676–1681. 10.1021/acscentsci.9b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.; Liu W.; Gao Y.; Li X.; Huang Y.; Li X.; James T. D.; Guo Y.; Li J. Photoactivatable senolysis with single-cell resolution delays aging. Nature Aging 2023, 3 (3), 297–312. 10.1038/s43587-023-00360-x. [DOI] [PubMed] [Google Scholar]
- Li Y.; Song H.; Xue C.; Fang Z.; Xiong L.; Xie H. A self-immobilizing near-infrared fluorogenic probe for sensitive imaging of extracellular enzyme activity in vivo. Chem. Sci. 2020, 11 (23), 5889–5894. 10.1039/D0SC01273D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.; Gao H.; Mu W.; Ni X.; Yi X.; Shen J.; Liu Q.; Bao P.; Ding D. Enzyme-instructed self-assembly leads to the activation of optical properties for selective fluorescence detection and photodynamic ablation of cancer cells. J. Mater. Chem. B 2018, 6 (17), 2566–2573. 10.1039/C7TB02685D. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhang Y.; Wang P.; Yu L.; An J.; Deng G.; Sun Y.; Seung Kim J. Reactive oxygen species, thiols and enzymes activable AIEgens from single fluorescence imaging to multifunctional theranostics. Coord. Chem. Rev. 2021, 427, 213559. 10.1016/j.ccr.2020.213559. [DOI] [Google Scholar]
- Lam K. W. K.; Chau J. H. C.; Yu E. Y.; Sun F.; Lam J. W. Y.; Ding D.; Kwok R. T. K.; Sun J.; He X.; Tang B. Z. An Alkaline Phosphatase-Responsive Aggregation-Induced Emission PS for Selective Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano 2023, 17 (8), 7145–7156. 10.1021/acsnano.2c08855. [DOI] [PubMed] [Google Scholar]
- Li Z.; Wang Y. F.; Zeng C.; Hu L.; Liang X. J. Ultrasensitive Tyrosinase-Activated Turn-On Near-Infrared Fluorescent Probe with a Rationally Designed Urea Bond for Selective Imaging and Photodamage to Melanoma Cells. Anal. Chem. 2018, 90 (6), 3666–3669. 10.1021/acs.analchem.7b05369. [DOI] [PubMed] [Google Scholar]
- Verirsen I.; Uyar B.; Ozsamur N. G.; Demirok N.; Erbas-Cakmak S. Enzyme activatable photodynamic therapy agents targeting melanoma. Org. Biomol. Chem. 2022, 20 (45), 8864–8868. 10.1039/D2OB01937J. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhao H.; Li L.; Yang B.; Yue Y.; Li M.; Shi X.; Zhang B.; Wang L.; Qi C.; et al. A tyrosinase-activated Pt(II) complex for melanoma photodynamic therapy and fluorescence imaging. Sens. Actuators B Chem. 2023, 374, 132836. 10.1016/j.snb.2022.132836. [DOI] [Google Scholar]
- Chiba M.; Ichikawa Y.; Kamiya M.; Komatsu T.; Ueno T.; Hanaoka K.; Nagano T.; Lange N.; Urano Y. An Activatable PS Targeted to gamma-Glutamyltranspeptidase. Angew. Chem., Int. Ed. Engl. 2017, 56 (35), 10418–10422. 10.1002/anie.201704793. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhao X.; Xiong T.; Du J.; Sun W.; Fan J.; Peng X. NIR PSs activated by γ-glutamyl transpeptidase for precise tumor fluorescence imaging and photodynamic therapy. Sci. China Chem. 2021, 64 (5), 808–816. 10.1007/s11426-020-9947-4. [DOI] [Google Scholar]
- Li J.; Wang T.; Jiang F.; Hong Z.; Su X.; Li S.; Han S. A fluorescence-activatable tumor-reporting probe for precise photodynamic therapy. J. Mater. Chem. B 2021, 9 (29), 5829–5836. 10.1039/D1TB00704A. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Li H.; Shi C.; Xu F.; Zhang Z.; Yao Q.; Ma H.; Sun W.; Shao K.; Du J.; et al. An APN-activated NIR PS for cancer photodynamic therapy and fluorescence imaging. Biomaterials 2020, 253, 120089. 10.1016/j.biomaterials.2020.120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P.; Xue F.; Shi Y.; Strand R.; Chen H.; Yi T. A fluoride activated methylene blue releasing platform for imaging and antimicrobial photodynamic therapy of human dental plaque. Chem. Commun. (Camb.) 2018, 54 (93), 13115–13118. 10.1039/C8CC07410K. [DOI] [PubMed] [Google Scholar]
- Roubinet B.; Weber M.; Shojaei H.; Bates M.; Bossi M. L.; Belov V. N.; Irie M.; Hell S. W. Fluorescent Photoswitchable Diarylethenes for Biolabeling and Single-Molecule Localization Microscopies with Optical Superresolution. J. Am. Chem. Soc. 2017, 139 (19), 6611–6620. 10.1021/jacs.7b00274. [DOI] [PubMed] [Google Scholar]
- Roubinet B.; Bossi M. L.; Alt P.; Leutenegger M.; Shojaei H.; Schnorrenberg S.; Nizamov S.; Irie M.; Belov V. N.; Hell S. W. Carboxylated Photoswitchable Diarylethenes for Biolabeling and Super-Resolution RESOLFT Microscopy. Angew. Chem., Int. Ed. Engl. 2016, 55 (49), 15429–15433. 10.1002/anie.201607940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhang R.; Liu K.; Li Y.; Wang X.; Xie X.; Jiao X.; Tang B. A light-activatable PS for photodynamic therapy based on a diarylethene derivative. Chem. Commun. (Camb.) 2021, 57 (67), 8320–8323. 10.1039/D1CC02102H. [DOI] [PubMed] [Google Scholar]
- Tam L. K. B.; Chu J. C. H.; He L.; Yang C.; Han K. C.; Cheung P. C. K.; Ng D. K. P.; Lo P. C. Enzyme-Responsive Double-Locked Photodynamic Molecular Beacon for Targeted Photodynamic Anticancer Therapy. J. Am. Chem. Soc. 2023, 145 (13), 7361–7375. 10.1021/jacs.2c13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.; Mao Q.; Zhao M.; Ye S.; Fang J.; Cui C.; Zhao Y.; Zhang Y.; Zhang Y.; Zhou F. pH/reduction dual stimuli-triggered self-assembly of NIR theranostic probes for enhanced dual-modal imaging and photothermal therapy of tumors. Anal. Chem. 2020, 92 (24), 16113–16121. 10.1021/acs.analchem.0c03800. [DOI] [PubMed] [Google Scholar]
- Wang P.; Li J.; Wei M.; Yang R.; Lou K.; Dang Y.; Sun W.; Xue F.; Liu X. Tumor-microenvironment triggered signal-to-noise boosting nanoprobes for NIR-IIb fluorescence imaging guided tumor surgery and NIR-II photothermal therapy. Biomaterials 2022, 287, 121636. 10.1016/j.biomaterials.2022.121636. [DOI] [PubMed] [Google Scholar]
- Kang E. B.; Lee J. E.; Mazrad Z. A. I.; In I.; Jeong J. H.; Park S. Y. pH-Responsible fluorescent carbon nanoparticles for tumor selective theranostics via pH-turn on/off fluorescence and photothermal effect in vivo and in vitro. Nanoscale 2018, 10 (5), 2512–2523. 10.1039/C7NR07900A. [DOI] [PubMed] [Google Scholar]
- Meng X.; Li W.; Sun Z.; Zhang J.; Zhou L.; Deng G.; Gong P.; Cai L. Tumor-targeted small molecule for dual-modal imaging-guided phototherapy upon near-infrared excitation. J. Mater. Chem. B 2017, 5 (47), 9405–9411. 10.1039/C7TB02496G. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lin J.; Wang P.; Luo Q.; Lin H.; Zhang Y.; Hou Z.; Liu J.; Liu X. Tumor microenvironment responsive shape-reversal self-targeting virus-inspired nanodrug for imaging-guided near-infrared-II photothermal chemotherapy. ACS Nano 2019, 13 (11), 12912–12928. 10.1021/acsnano.9b05425. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Zhao K.-C.; Chen L.-J.; Liu Y.-S.; Liu J.-L.; Yan X.-P. A pH reversibly activatable NIR photothermal/photodynamic-in-one agent integrated with renewable nanoimplants for image-guided precision phototherapy. Chem. Sci. 2021, 12 (1), 442–452. 10.1039/D0SC04408C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Wei K.; Ma G.; Ji C.; Yin M. A heptamethine cyanine with meso-N-induced rearrangement for acid-activated tumour imaging and photothermal therapy. Biomater. Sci. 2022, 10 (11), 2964–2971. 10.1039/D2BM00413E. [DOI] [PubMed] [Google Scholar]
- Tan B.; Zhao C.; Wang J.; Tiemuer A.; Zhang Y.; Yu H.; Liu Y. Rational design of pH-activated upconversion luminescent nanoprobes for bioimaging of tumor acidic microenvironment and the enhancement of photothermal therapy. Acta Biomater. 2023, 155, 554–563. 10.1016/j.actbio.2022.08.078. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Liu X.; Zeng J.; Cheng Z.; Liu Z. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials 2016, 98, 23–30. 10.1016/j.biomaterials.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Xue F.; Wen Y.; Wei P.; Gao Y.; Zhou Z.; Xiao S.; Yi T. A smart drug: a pH-responsive photothermal ablation agent for Golgi apparatus activated cancer therapy. Chem. Commun. 2017, 53 (48), 6424–6427. 10.1039/C7CC03168H. [DOI] [PubMed] [Google Scholar]
- Wei X.; Chen Y.; Jiang X.; Peng M.; Liu Y.; Mo Y.; Ren D.; Hua Y.; Yu B.; Zhou Y.; et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 2021, 20 (1), 1–18. 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Han F.; Du Y.; Shi H.; Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8 (1), 70. 10.1038/s41392-023-01332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Lovell J. F.; Yoon J.; Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17 (11), 657–674. 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- Wang S.-B.; Zhang C.; Ye J.-J.; Zou M.-Z.; Liu C.-J.; Zhang X.-Z. Near-infrared light responsive nanoreactor for simultaneous tumor photothermal therapy and carbon monoxide-mediated anti-inflammation. ACS Cent. Sci. 2020, 6 (4), 555–565. 10.1021/acscentsci.9b01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S.; Morimoto Y.; Tsujimoto H.; Harada M.; Saitoh D.; Hara I.; Ozeki E.; Satoh A.; Takayama E.; Hase K. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using indocyanine green lactosome. bioRxiv 2019, 659334. 10.1101/659334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X.; Shao Z.; Zhao Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv. Sci. 2021, 8 (3), 2002504. 10.1002/advs.202002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Zhang W.; Ji W.; Wang J.; Wang N.; Wu W.; Wu Q.; Hou X.; Hu W.; Li L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9 (38), 7909–7926. 10.1039/D1TB01310F. [DOI] [PubMed] [Google Scholar]
- Lin H.-H.; Yang X.-Z.; Yu M.-Y.; Liu J.-Y. A switchable phototherapeutic strategy based on a hypoxia-responsive PS for heterogeneous tumor ablation. Dyes Pigment. 2022, 201, 110217. 10.1016/j.dyepig.2022.110217. [DOI] [Google Scholar]
- Yang J.; Choi J.; Bang D.; Kim E.; Lim E.-K.; Park H.; Suh J.-S.; Lee K.; Yoo K.-H.; Kim E.-K.; et al. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells. Angew. Chem., Int. Ed. 2011, 50 (2), 441–444. 10.1002/anie.201005075. [DOI] [PubMed] [Google Scholar]
- Meng X.; Zhang J.; Sun Z.; Zhou L.; Deng G.; Li S.; Li W.; Gong P.; Cai L. Hypoxia-triggered single molecule probe for high-contrast NIR II/PA tumor imaging and robust photothermal therapy. Theranostics 2018, 8 (21), 6025. 10.7150/thno.26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.-X.; Hou X.; Kong Y.; Yang F.; Yue Y.-X.; Shah M. R.; Li H.-B.; Huang F.; Liu J.; Guo D.-S. A hypoxia-responsive supramolecular formulation for imaging-guided photothermal therapy. Theranostics 2022, 12 (1), 396. 10.7150/thno.67036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.; Zhang C.; He S.; Huang J.; Huang J.; Liew S. S.; Zeng Z.; Pu K. A Dual-Locked Activatable Phototheranostic Probe for Biomarker-Regulated Photodynamic and Photothermal Cancer Therapy. Angew. Chem., Int. Ed. 2022, 61 (26), e202202966 10.1002/anie.202202966. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Long S.; Xiong T.; Zhao X.; Sun W.; Du J.; Fan J.; Peng X. Single-molecule forster resonance energy transfer-based PS for synergistic photodynamic/photothermal therapy. ACS Cent. Sci. 2021, 7 (2), 327–334. 10.1021/acscentsci.0c01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.; Li C.; Liu L.; Wang Z.; Chen Z.; Yu J.; Ji W.; Jiang G.; Zhang P.; Wang J.; et al. Acceptor planarization and donor rotation: A facile strategy for realizing synergistic cancer phototherapy via type I PDT and PTT. ACS Nano 2022, 16 (3), 4162–4174. 10.1021/acsnano.1c10019. [DOI] [PubMed] [Google Scholar]
- Yao S.; Chen Y.; Xu H.; Qi F.; Zhang Y.; Yang T.; Wu Y.; Fang H.; He W.; Guo Z. Hypoxia-responsive near infrared thioxanthene-hemicyanine nanoparticle for multimodal imaging-guided photothermal/photodynamic therapy. Dyes Pigment. 2022, 206, 110583. 10.1016/j.dyepig.2022.110583. [DOI] [Google Scholar]
- Dong Y.; Zhou L.; Shen Z.; Ma Q.; Zhao Y.; Sun Y.; Cao J. Iodinated cyanine dye-based nanosystem for synergistic phototherapy and hypoxia-activated bioreductive therapy. Drug Delivery 2022, 29 (1), 238–253. 10.1080/10717544.2021.2023701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Xue K.-F.; Yang Y.; Hu H.; Xu J.-F.; Zhang X. In situ hypoxia-induced supramolecular perylene diimide radical anions in tumors for photothermal therapy with improved specificity. J. Am. Chem. Soc. 2022, 144 (5), 2360–2367. 10.1021/jacs.1c13067. [DOI] [PubMed] [Google Scholar]
- Fu L.; Huang Y.; Hou J.; Sun M.; Wang L.; Wang X.; Chen L. A Raman/fluorescence dual-modal imaging guided synergistic photothermal and photodynamic therapy nanoplatform for precision cancer theranostics. J. Mater. Chem. B 2022, 10 (41), 8432–8442. 10.1039/D2TB01696F. [DOI] [PubMed] [Google Scholar]
- Yang D.-C.; Wen L.-F.; Du L.; Luo C.-M.; Lu Z.-Y.; Liu J.-Y.; Lin Z. A Hypoxia-Activated Prodrug Conjugated with a BODIPY-Based Photothermal Agent for Imaging-Guided Chemo-Photothermal Combination Therapy. ACS Appl. Mater. Interfaces 2022, 14 (36), 40546–40558. 10.1021/acsami.2c09071. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Tu L.; Zhu T.; Zhu H.; Liu S.; Sun Y.; Zhao Q. Hypoxia-Activatable Nanovesicles as In Situ Bombers for Combined Hydrogen-Sulfide-Mediated Respiration Inhibition and Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14 (45), 50637–50648. 10.1021/acsami.2c15844. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Yang S.; Zhao C.; Liu W.; Yao X.; Yu H.; Sun X.; Liu Y. γ-Glutamyl transpeptidase-activatable near-infrared nanoassembly for tumor fluorescence imaging-guided photothermal therapy. Theranostics 2021, 11 (14), 7045. 10.7150/thno.60586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X.; Zhang J.; Huang J.; Xie C.; Miao Q.; Pu K. Macrotheranostic probe with disease-activated near-infrared fluorescence, photoacoustic, and photothermal signals for imaging-guided therapy. Angew. Chem. 2018, 130 (26), 7930–7934. 10.1002/ange.201803321. [DOI] [PubMed] [Google Scholar]
- Yao D.; Yang S.; Wang Y.; Bian K.; Yang W.; Wang D.; Zhang B. An ALP-activatable and mitochondria-targeted probe for prostate cancer-specific bimodal imaging and aggregation-enhanced photothermal therapy. Nanoscale 2019, 11 (13), 6307–6314. 10.1039/C9NR00913B. [DOI] [PubMed] [Google Scholar]
- Pan Z.; Wang Y.; Chen N.; Cao G.; Zeng Y.; Dong J.; Liu M.; Ye Z.; Li Y.; Huang S.; et al. Aggregation-Induced Emission PS with Lysosomal Response for Photodynamic Therapy Against Cancer. Bioorg. Chem. 2023, 132, 106349. 10.1016/j.bioorg.2023.106349. [DOI] [PubMed] [Google Scholar]
- Shi B.; Yan Q.; Tang J.; Xin K.; Zhang J.; Zhu Y.; Xu G.; Wang R.; Chen J.; Gao W.; et al. Hydrogen Sulfide-Activatable Second Near-Infrared Fluorescent Nanoassemblies for Targeted Photothermal Cancer Therapy. Nano Lett. 2018, 18 (10), 6411–6416. 10.1021/acs.nanolett.8b02767. [DOI] [PubMed] [Google Scholar]
- Sun T.; Zhang G.; Wang Q.; Chen Q.; Chen X.; Lu Y.; Liu L.; Zhang Y.; He X.; Ruan C.; et al. A targeting theranostics nanomedicine as an alternative approach for hyperthermia perfusion. Biomaterials 2018, 183, 268–279. 10.1016/j.biomaterials.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Song W.; Zhang X.; Song Y.; Fan K.; Shao F.; Long Y.; Gao Y.; Cai W.; Lan X. Enhancing Photothermal Therapy Efficacy by In Situ Self-Assembly in Glioma. ACS Appl. Mater. Interfaces 2023, 15 (1), 57–66. 10.1021/acsami.2c14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Wu Y.; Chen J.; Wan J.; Xiao C.; Guan J.; Song X.; Li S.; Zhang M.; Cui H.; et al. A Simple Glutathione-Responsive Turn-On Theranostic Nanoparticle for Dual-Modal Imaging and Chemo-Photothermal Combination Therapy. Nano Lett. 2019, 19 (8), 5806–5817. 10.1021/acs.nanolett.9b02769. [DOI] [PubMed] [Google Scholar]
- Watkin N. A.; Morris S. B.; Rivens I. H.; Woodhouse C. R. J. A feasibility study for the non-invasive treatment of superficial bladder tumours with focused ultrasound. Br. J. Urol. 1996, 78 (5), 715–721. 10.1046/j.1464-410X.1996.02189.x. [DOI] [PubMed] [Google Scholar]
- Zeng W.; Xu Y.; Yang W.; Liu K.; Bian K.; Zhang B. An Ultrasound-Excitable Aggregation-Induced Emission Dye for Enhanced Sonodynamic Therapy of Tumors. Adv. Healthc. Mater. 2020, 9 (17), 2000560. 10.1002/adhm.202000560. [DOI] [PubMed] [Google Scholar]
- McHale A. P.; Callan J. F.; Nomikou N.; Fowley C.; Callan B.. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. In Therapeutic Ultrasound; Escoffre J.-M., Bouakaz A., Eds.; Springer International Publishing, 2016; pp 429–450. [DOI] [PubMed] [Google Scholar]
- Rengeng L.; Qianyu Z.; Yuehong L.; Zhongzhong P.; Libo L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 19, 159–166. 10.1016/j.pdpdt.2017.06.003. [DOI] [PubMed] [Google Scholar]
- DeVita V. T. Jr; Chu E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68 (21), 8643–8653. 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Using DNA, radiation therapy gets personal. Science 2016, 353 (6306), 1348–1349. 10.1126/science.353.6306.1348. [DOI] [PubMed] [Google Scholar]
- Yang X.; Yang Y.; Gao F.; Wei J.-J.; Qian C.-G.; Sun M.-J. Biomimetic Hybrid Nanozymes with Self-Supplied H+ and Accelerated O2 Generation for Enhanced Starvation and Photodynamic Therapy against Hypoxic Tumors. Nano Lett. 2019, 19 (7), 4334–4342. 10.1021/acs.nanolett.9b00934. [DOI] [PubMed] [Google Scholar]
- Xu J.; Shi R.; Chen G.; Dong S.; Yang P.; Zhang Z.; Niu N.; Gai S.; He F.; Fu Y.; et al. All-in-One Theranostic Nanomedicine with Ultrabright Second Near-Infrared Emission for Tumor-Modulated Bioimaging and Chemodynamic/Photodynamic Therapy. ACS Nano 2020, 14 (8), 9613–9625. 10.1021/acsnano.0c00082. [DOI] [PubMed] [Google Scholar]
- Bao Y.; Chen J.; Qiu H.; Zhang C.; Huang P.; Mao Z.; Tong W. Erythrocyte Membrane-Camouflaged PCN-224 Nanocarriers Integrated with Platinum Nanoparticles and Glucose Oxidase for Enhanced Tumor Sonodynamic Therapy and Synergistic Starvation Therapy. ACS Appl. Mater. Interfaces 2021, 13 (21), 24532–24542. 10.1021/acsami.1c05644. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang X.; Yang H.; Yu L.; Xu Y.; Sharma A.; Yin P.; Li X.; Kim J. S.; Sun Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021, 50 (20), 11227–11248. 10.1039/D1CS00403D. [DOI] [PubMed] [Google Scholar]
- Xie J.; Lee S.; Chen X. Nanoparticle-based theranostic agents. Adv. Drug Delivery Rev. 2010, 62 (11), 1064–1079. 10.1016/j.addr.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F.; Li F.; Ren L.; Wang Q.; Jiang C.; Zhang Y.; Li M.; Song X.; Zhang S. Acoustic-Based Theranostic Probes Activated by Tumor Microenvironment for Accurate Tumor Diagnosis and Assisted Tumor Therapy. ACS Sens. 2022, 7 (12), 3611–3633. 10.1021/acssensors.2c02129. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27 (45), 5904–5912. 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo R.; Gu Z. Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 2016, 19 (5), 274–283. 10.1016/j.mattod.2015.11.025. [DOI] [Google Scholar]
- Gatenby R. A.; Gillies R. J. Why do cancers have high aerobic glycolysis?. Nat. Rev. Cancer 2004, 4 (11), 891–899. 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Fleige E.; Quadir M. A.; Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Delivery Rev. 2012, 64 (9), 866–884. 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Lehner R.; Wang X.; Wolf M.; Hunziker P. Designing switchable nanosystems for medical application. J. Controlled Release 2012, 161 (2), 307–316. 10.1016/j.jconrel.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Du J.-Z.; Mao C.-Q.; Yuan Y.-Y.; Yang X.-Z.; Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014, 32 (4), 789–803. 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Liu J.; Huang Y.; Kumar A.; Tan A.; Jin S.; Mozhi A.; Liang X.-J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32 (4), 693–710. 10.1016/j.biotechadv.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Tian L.; Bae Y. H. Cancer nanomedicines targeting tumor extracellular pH. Colloid Surf. B-Biointerfaces 2012, 99, 116–126. 10.1016/j.colsurfb.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.; Meng F.; Deng C.; Klok H.-A.; Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34 (14), 3647–3657. 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Chu C.; Li D.; Pang X.; Zheng H.; Wang J.; Shi Y.; Zhang Y.; Cheng Y.; Ren E.; et al. Fe(III)-Porphyrin Sonotheranostics: A Green Triple-Regulated ROS Generation Nanoplatform for Enhanced Cancer Imaging and Therapy. Adv. Funct. Mater. 2019, 29 (36), 1904056. 10.1002/adfm.201904056. [DOI] [Google Scholar]
- Chao K. L.; Muthukumar L.; Herzberg O. Structure of Human Hyaluronidase-1, a Hyaluronan Hydrolyzing Enzyme Involved in Tumor Growth and Angiogenesis. Biochemistry 2007, 46 (23), 6911–6920. 10.1021/bi700382g. [DOI] [PubMed] [Google Scholar]
- Qiu P.; Huang M.; Wu S.; Wen M.; Yu N.; Chen Z. Dynamic Effects of Endo-Exogenous Stimulations on Enzyme-Activatable Polymeric Nanosystems with Photo-Sono-Chemo Synergy. ACS Appl. Mater. Interfaces 2022, 14 (26), 29537–29549. 10.1021/acsami.2c05276. [DOI] [PubMed] [Google Scholar]
- Xia J.; Yao J.; Wang L. V. Photoacoustic tomography: principles and advances. Electromagn. Waves (Camb.) 2014, 147, 1–22. 10.2528/PIER14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Fan Z.; Zheng K.; Shi D.; Su G.; Ge D.; Zhao Q.; Fu X.; Hou Z. A novel self-targeting theranostic nanoplatform for photoacoustic imaging-monitored and enhanced chemo-sonodynamic therapy. J. Mater. Chem. B 2021, 9 (27), 5547–5559. 10.1039/D1TB01025E. [DOI] [PubMed] [Google Scholar]
- Chen J.; Zhu Y.; Wu C.; Shi J. Engineering lactate-modulating nanomedicines for cancer therapy. Chem. Soc. Rev. 2023, 52 (3), 973–1000. 10.1039/D2CS00479H. [DOI] [PubMed] [Google Scholar]
- Li D.; Pan J.; Xu S.; Cheng B.; Wu S.; Dai Q.; Ke M.-R.; Zheng B.-Y.; Chu C.; Liu C.; et al. Programmable phthalocyanine-iron-based nanoreactor for fluorescence/magnetic resonance dual-modality imaging-guided sono/chemodynamic therapies. Chem. Eng. J. 2023, 452, 139330. 10.1016/j.cej.2022.139330. [DOI] [Google Scholar]
- Cheng L.; Xiao-Quan Y.; An J.; Cheng K.; Xiao-Lin H.; Xiao-Shuai Z.; Yong-Guo H.; Liu B.; Yuan-Di Z. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy. Theranostics 2020, 10 (2), 867–879. 10.7150/thno.37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; Feng L.; Liang C.; Yang K.; Liu Z. Ultrasound Triggered Tumor Oxygenation with Oxygen-Shuttle Nanoperfluorocarbon to Overcome Hypoxia-Associated Resistance in Cancer Therapies. Nano Lett. 2016, 16 (10), 6145–6153. 10.1021/acs.nanolett.6b02365. [DOI] [PubMed] [Google Scholar]
- Gao M.; Liang C.; Song X.; Chen Q.; Jin Q.; Wang C.; Liu Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29 (35), 1701429. 10.1002/adma.201701429. [DOI] [PubMed] [Google Scholar]
- Cai X.; Xie Z.; Ding B.; Shao S.; Liang S.; Pang M.; Lin J. Monodispersed Copper(I)-Based Nano Metal-Organic Framework as a Biodegradable Drug Carrier with Enhanced Photodynamic Therapy Efficacy. Adv. Sci. 2019, 6 (15), 1900848. 10.1002/advs.201900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Xu H.; Xu C.; Tong Z.; Zhang S.; Bai Y.; Chen Y.; Xu Q.; Zhou L.; Ding H.; et al. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal-Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. 2020, 7 (17), 2001060. 10.1002/advs.202001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X.; Song L.; Pan W.; Zhong H.; Li N.; Tang B. Tumor-Targeted Cascade Nanoreactor Based on Metal-Organic Frameworks for Synergistic Ferroptosis-Starvation Anticancer Therapy. ACS Nano 2020, 14 (9), 11017–11028. 10.1021/acsnano.9b07789. [DOI] [PubMed] [Google Scholar]
- Duan W.; Li B.; Zhang W.; Li J.; Yao X.; Tian Y.; Zheng J.; Li D. Two-photon responsive porphyrinic metal-organic framework involving Fenton-like reaction for enhanced photodynamic and sonodynamic therapy. J. Nanobiotechnol. 2022, 20 (1), 217. 10.1186/s12951-022-01436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix S. M.; Borden M. A.; Dayton P. A. Therapeutic gas delivery via microbubbles and liposomes. J. Controlled Release 2015, 209, 139–149. 10.1016/j.jconrel.2015.04.027. [DOI] [PubMed] [Google Scholar]
- Tang X.; Cheng Y.; Huang S.; Zhi F.; Yuan A.; Hu Y.; Wu J. Overcome the limitation of hypoxia against photodynamic therapy to treat cancer cells by using perfluorocarbon nanodroplet for PS delivery. Biochem. Biophys. Res. Commun. 2017, 487 (3), 483–487. 10.1016/j.bbrc.2017.03.142. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Cheng H.; Jiang C.; Qiu X.; Wang K.; Huan W.; Yuan A.; Wu J.; Hu Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6 (1), 8785. 10.1038/ncomms9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T.; Cheng L.; Gong F.; Xu J.; Li X.; Han G.; Liu Z. Upconversion Composite Nanoparticles for Tumor Hypoxia Modulation and Enhanced Near-Infrared-Triggered Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10 (18), 15494–15503. 10.1021/acsami.8b03238. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Dong Z.; Fu T.; Liu J.; Chen Q.; Li Y.; Zhu R.; Xu L.; Liu Z. Modulation of Hypoxia in Solid Tumor Microenvironment with MnO2 Nanoparticles to Enhance Photodynamic Therapy. Adv. Funct. Mater. 2016, 26 (30), 5490–5498. 10.1002/adfm.201600676. [DOI] [Google Scholar]
- Zheng D.-W.; Li B.; Li C.-X.; Fan J.-X.; Lei Q.; Li C.; Xu Z.; Zhang X.-Z. Carbon-Dot-Decorated Carbon Nitride Nanoparticles for Enhanced Photodynamic Therapy against Hypoxic Tumor via Water Splitting. ACS Nano 2016, 10 (9), 8715–8722. 10.1021/acsnano.6b04156. [DOI] [PubMed] [Google Scholar]
- Song X.; Feng L.; Liang C.; Gao M.; Song G.; Liu Z. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 2017, 10 (4), 1200–1212. 10.1007/s12274-016-1274-8. [DOI] [Google Scholar]
- Benej M.; Hong X.; Vibhute S.; Scott S.; Wu J.; Graves E.; Le Q.-T.; Koong A. C.; Giaccia A. J.; Yu B.; et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (42), 10756–10761. 10.1073/pnas.1808945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J. R.; Sun Y.; Protopopova M.; Gera S.; Bandi M.; Bristow C.; McAfoos T.; Morlacchi P.; Ackroyd J.; Agip A.-N. A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24 (7), 1036–1046. 10.1038/s41591-018-0052-4. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Tan Y.; Yan L.; Zhang C.; Xu M.; Guo H.; Zhuang B.; Zhou L.; Xie X. Modulation of Tumor Hypoxia by pH-Responsive Liposomes to Inhibit Mitochondrial Respiration for Enhancing Sonodynamic Therapy. Int. J. Nanomed. 2020, 15, 5687–5700. 10.2147/IJN.S256038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Li T.; Zhu Y.; Hao Y. Ultrasound-Activated Oxygen and ROS Generation Nanosystem Systematically Modulates Tumor Microenvironment and Sensitizes Sonodynamic Therapy for Hypoxic Solid Tumors. Adv. Funct. Mater. 2019, 29 (51), 1906195. 10.1002/adfm.201906195. [DOI] [Google Scholar]
- Cabral H.; Matsumoto Y.; Mizuno K.; Chen Q.; Murakami M.; Kimura M.; Terada Y.; Kano M. R.; Miyazono K.; Uesaka M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6 (12), 815–823. 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- Chen J.; Luo H.; Liu Y.; Zhang W.; Li H.; Luo T.; Zhang K.; Zhao Y.; Liu J. Oxygen-Self-Produced Nanoplatform for Relieving Hypoxia and Breaking Resistance to Sonodynamic Treatment of Pancreatic Cancer. ACS Nano 2017, 11 (12), 12849–12862. 10.1021/acsnano.7b08225. [DOI] [PubMed] [Google Scholar]
- Yang K.; Yue L.; Yu G.; Rao L.; Tian R.; Wei J.; Yang Z.; Sun C.; Zhang X.; Xu M.; et al. A hypoxia responsive nanoassembly for tumor specific oxygenation and enhanced sonodynamic therapy. Biomaterials 2021, 275, 120822. 10.1016/j.biomaterials.2021.120822. [DOI] [PubMed] [Google Scholar]
- Weaver L. K.; Hopkins R. O.; Chan K. J.; Churchill S.; Elliott C. G.; Clemmer T. P.; Orme J. F.; Thomas F. O.; Morris A. H. Hyperbaric Oxygen for Acute Carbon Monoxide Poisoning. N. Engl. J. Med. 2002, 347 (14), 1057–1067. 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- Lou Z.; Li P.; Han K. Redox-responsive fluorescent probes with different design strategies. Acc. Chem. Res. 2015, 48 (5), 1358–1368. 10.1021/acs.accounts.5b00009. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Cai X.; Gao W.; Wang R.; Xu C.; Yao Y.; Hao L.; Sheng D.; Chen H.; Wang Z.; et al. A Multifunctional Theranostic Nanoagent for Dual-Mode Image-Guided HIFU/Chemo- Synergistic Cancer Therapy. Theranostics 2016, 6 (3), 404–417. 10.7150/thno.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Zhang N.; Wang Z.; Wu M.; Chen Y.; Ma M.; Chen H.; Shi J. Endogenous Catalytic Generation of O2 Bubbles for In Situ Ultrasound-Guided High Intensity Focused Ultrasound Ablation. ACS Nano 2017, 11 (9), 9093–9102. 10.1021/acsnano.7b03772. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I.; Tannock I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6 (8), 583–592. 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Tan X.; Huang J.; Wang Y.; He S.; Jia L.; Zhu Y.; Pu K.; Zhang Y.; Yang X. Transformable Nanosensitizer with Tumor Microenvironment-Activated Sonodynamic Process and Calcium Release for Enhanced Cancer Immunotherapy. Angew. Chem., Int. Ed. 2021, 60 (25), 14051–14059. 10.1002/anie.202102703. [DOI] [PubMed] [Google Scholar]
- Pang H.-B.; Braun G. B.; Friman T.; Aza-Blanc P.; Ruidiaz M. E.; Sugahara K. N.; Teesalu T.; Ruoslahti E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5 (1), 4904. 10.1038/ncomms5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Qiao B.; Yang C.; Zhang P.; Xie Z.; Cao J.; Yang A.; Xiang Q.; Ran H.; Wang Z.; et al. Low Intensity Focused Ultrasound Ignited “Deep-Penetration Nanobomb” (DPNB) for Tetramodal Imaging Guided Hypoxia-Tolerant Sonodynamic Therapy Against Hypoxic Tumors. Int. J. Nanomed. 2022, 17, 4547–4565. 10.2147/IJN.S361648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B.; Cheng G.; Hardy M.; Ouari O.; Bennett B.; Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018, 15, 347–362. 10.1016/j.redox.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; Bai S.; He N.; Wang R.; Xing Y.; Lv C.; Yu F. Real-Time Evaluation of Hydrogen Peroxide Injuries in Pulmonary Fibrosis Mice Models with a Mitochondria-Targeted Near-Infrared Fluorescent Probe. ACS Sens. 2021, 6 (3), 1228–1239. 10.1021/acssensors.0c02519. [DOI] [PubMed] [Google Scholar]
- Hempel N.; Ye H.; Abessi B.; Mian B.; Melendez J. A. Altered redox status accompanies progression to metastatic human bladder cancer. Free Radic. Biol. Med. 2009, 46 (1), 42–50. 10.1016/j.freeradbiomed.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel N.; Bartling T. R.; Mian B.; Melendez J. A. Acquisition of the Metastatic Phenotype Is Accompanied by H2O2-Dependent Activation of the p130Cas Signaling Complex. Mol. Cancer Res. 2013, 11 (3), 303–312. 10.1158/1541-7786.MCR-12-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zhang J.; Jia Q.; Ge J.; Wang P. Innovative strategies of hydrogen peroxide-involving tumor therapeutics. Mater. Chem. Front. 2021, 5 (12), 4474–4501. 10.1039/D1QM00134E. [DOI] [Google Scholar]
- Wang Z.; Liu B.; Sun Q.; Feng L.; He F.; Yang P.; Gai S.; Quan Z.; Lin J. Upconverted Metal-Organic Framework Janus Architecture for Near-Infrared and Ultrasound Co-Enhanced High Performance Tumor Therapy. ACS Nano 2021, 15 (7), 12342–12357. 10.1021/acsnano.1c04280. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zhang W.; Chen Q.; Hou J.; Wang J.; Zhong Y.; Wang X.; Jiang W.; Ran H.; Guo D. Multifunctional nanozyme for multimodal imaging-guided enhanced sonodynamic therapy by regulating the tumor microenvironment. Nanoscale 2021, 13 (33), 14049–14066. 10.1039/D1NR01449H. [DOI] [PubMed] [Google Scholar]
- McCarthy E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [PMC free article] [PubMed] [Google Scholar]
- Mellman I.; Coukos G.; Dranoff G. Cancer immunotherapy comes of age. Nature 2011, 480 (7378), 480–489. 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12 (4), 252–264. 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P.; Allison J. P. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 2015, 161 (2), 205–214. 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella E.; Zullo L.; Marconi S.; Rossi G.; Coco S.; Dellepiane C.; Alama A.; Rozeboom L.; Bennicelli E.; Parisi F.; et al. Immunotherapy-chemotherapy combinations for non-small cell lung cancer: current trends and future perspectives. Expert Opin. Biol. Ther. 2022, 22 (10), 1259–1273. 10.1080/14712598.2022.2116273. [DOI] [PubMed] [Google Scholar]
- Hu J.-R.; Florido R.; Lipson E. J.; Naidoo J.; Ardehali R.; Tocchetti C. G.; Lyon A. R.; Padera R. F.; Johnson D. B.; Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115 (5), 854–868. 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. B.; Chandra S.; Sosman J. A. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA 2018, 320 (16), 1702–1703. 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- Wang D. Y.; Salem J.-E.; Cohen J. V.; Chandra S.; Menzer C.; Ye F.; Zhao S.; Das S.; Beckermann K. E.; Ha L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4 (12), 1721–1728. 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B.; Yuan W.-E.; Su J.; Liu Y.; Chen J. Recent advances in small molecule based cancer immunotherapy. Eur. J. Med. Chem. 2018, 157, 582–598. 10.1016/j.ejmech.2018.08.028. [DOI] [PubMed] [Google Scholar]
- Imai K.; Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6 (9), 714–727. 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- Adams J. L.; Smothers J.; Srinivasan R.; Hoos A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discovery 2015, 14 (9), 603–622. 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- Morad G.; Helmink B. A.; Sharma P.; Wargo J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184 (21), 5309–5337. 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.; Wang M.; Wang M.; Shu M.; Ding B.; Li C.; Pang M.; Cui S.; Hou Z.; Lin J. A Multifunctional Cascade Bioreactor Based on Hollow-Structured Cu2MoS4 for Synergetic Cancer Chemo-Dynamic Therapy/Starvation Therapy/Phototherapy/Immunotherapy with Remarkably Enhanced Efficacy. Adv. Mater. 2019, 31 (51), 1905271. 10.1002/adma.201905271. [DOI] [PubMed] [Google Scholar]
- Yang X.; Grailer J. J.; Rowland I. J.; Javadi A.; Hurley S. A.; Matson V. Z.; Steeber D. A.; Gong S. Multifunctional Stable and pH-Responsive Polymer Vesicles Formed by Heterofunctional Triblock Copolymer for Targeted Anticancer Drug Delivery and Ultrasensitive MR Imaging. ACS Nano 2010, 4 (11), 6805–6817. 10.1021/nn101670k. [DOI] [PubMed] [Google Scholar]
- Kong F.; Liang Z.; Luan D.; Liu X.; Xu K.; Tang B. A Glutathione (GSH)-Responsive Near-Infrared (NIR) Theranostic Prodrug for Cancer Therapy and Imaging. Anal. Chem. 2016, 88 (12), 6450–6456. 10.1021/acs.analchem.6b01135. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Liang C.; Sun X.; Chen J.; Yang Z.; Zhao H.; Feng L.; Liu Z. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (21), 5343–5348. 10.1073/pnas.1701976114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyose K.; Hanaoka K.; Oushiki D.; Nakamura T.; Kajimura M.; Suematsu M.; Nishimatsu H.; Yamane T.; Terai T.; Hirata Y.; et al. Hypoxia-Sensitive Fluorescent Probes for in Vivo Real-Time Fluorescence Imaging of Acute Ischemia. J. Am. Chem. Soc. 2010, 132 (45), 15846–15848. 10.1021/ja105937q. [DOI] [PubMed] [Google Scholar]
- Zschaeck S.; Haase R.; Abolmaali N.; Perrin R.; Stützer K.; Appold S.; Steinbach J.; Kotzerke J.; Zips D.; Richter C.; et al. Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its correlation to pattern of failure. Acta Oncol. 2015, 54 (9), 1355–1363. 10.3109/0284186X.2015.1074720. [DOI] [PubMed] [Google Scholar]
- Mayer A. T.; Gambhir S. S. The Immunoimaging Toolbox. J. Nucl. Med. 2018, 59 (8), 1174–1182. 10.2967/jnumed.116.185967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay D. S.; Ryan E. P.; Pawelec G.; Talib W. H.; Stagg J.; Elkord E.; Lichtor T.; Decker W. K.; Whelan R. L.; Kumara H. M. C. S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Spano D.; Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin. Exp. Metastasis 2012, 29 (4), 381–395. 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- She P.; Liu Y.; Luo Z.; Chen L.; Zhou L.; Hussain Z.; Wu Y. PA2146 Gene Knockout Is Associated With Pseudomonas aeruginosa Pathogenicity in Macrophage and Host Immune Response. Front. Cell. Infect. Microbiol. 2020, 10, 1–10. 10.3389/fcimb.2020.559803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottoli M.; Carrega P.; Zullo L.; Dellepiane C.; Rossi G.; Parisi F.; Barletta G.; Zinoli L.; Coco S.; Alama A.; et al. Immune Checkpoint Blockade: A Strategy to Unleash the Potential of Natural Killer Cells in the Anti-Cancer Therapy. Cancers 2022, 14 (20), 5046. 10.3390/cancers14205046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C.; Ribas A.; Wolchok J. D.; Hodi F. S.; Hamid O.; Kefford R.; Weber J. S.; Joshua A. M.; Hwu W.-J.; Gangadhar T. C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384 (9948), 1109–1117. 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- Li J.; Van Valkenburgh J.; Hong X.; Conti P. S.; Zhang X.; Chen K. Small molecules as theranostic agents in cancer immunology. Theranostics 2019, 9 (25), 7849–7871. 10.7150/thno.37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. The Hallmarks of Cancer. Cell 2000, 100 (1), 57–70. 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wang T.; Liu J.; Yu H.; Jiao S.; Feng B.; Zhou F.; Fu Y.; Yin Q.; Zhang P.; et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16 (9), 5503–5513. 10.1021/acs.nanolett.6b01994. [DOI] [PubMed] [Google Scholar]
- Schafer F. Q.; Buettner G. R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30 (11), 1191–1212. 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Kim A.; Miyata K.; Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Delivery Rev. 2016, 104, 61–77. 10.1016/j.addr.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Gao A.; Chen B.; Gao J.; Zhou F.; Saeed M.; Hou B.; Li Y.; Yu H. Sheddable Prodrug Vesicles Combating Adaptive Immune Resistance for Improved Photodynamic Immunotherapy of Cancer. Nano Lett. 2020, 20 (1), 353–362. 10.1021/acs.nanolett.9b04012. [DOI] [PubMed] [Google Scholar]
- Liu L.; Zhang J.; An R.; Xue Q.; Cheng X.; Hu Y.; Huang Z.; Wu L.; Zeng W.; Miao Y.; et al. Smart Nanosensitizers for Activatable Sono-Photodynamic Immunotherapy of Tumors by Redox-Controlled Disassembly. Angew. Chem., Int. Ed. 2023, 62 (10), e202217055 10.1002/anie.202217055. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang F.; Hyun J. Y.; Wei T.; Qiang J.; Ren X.; Shin I.; Yoon J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45 (10), 2976–3016. 10.1039/C6CS00192K. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Yu L.; Wang Y.; Wang C.; Mu Q.; Liu X.; Yu M.; Wang K.-N.; Yao G.; Yu Z. Dynamic Adjust of Non-Radiative and Radiative Attenuation of AIE Molecules Reinforces NIR-II Imaging Mediated Photothermal Therapy and Immunotherapy. Adv. Sci. 2022, 9 (8), 2104793. 10.1002/advs.202104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R.; Liu L.; He H.; Chen Z.; Han Z.; Luo Z.; Wu Z.; Zheng M.; Ma Y.; Cai L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Jing X.; Yang F.; Shao C.; Wei K.; Xie M.; Shen H.; Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 1–15. 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis M. I.; Giatromanolaki A.; Skarlatos J.; Corti L.; Blandamura S.; Piazza M.; Gatter K. C.; Harris A. L. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001, 61 (5), 1830–1832. [PubMed] [Google Scholar]
- Brown J. M.; Giaccia A. J. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998, 58 (7), 1408–1416. [PubMed] [Google Scholar]
- Roma-Rodrigues C.; Mendes R.; Baptista P. V.; Fernandes A. R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20 (4), 840. 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpekris F.; Voutouri C.; Baish J. W.; Duda D. G.; Munn L. L.; Stylianopoulos T.; Jain R. K. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (7), 3728–3737. 10.1073/pnas.1919764117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Khouzam R.; Goutham H. V.; Zaarour R. F.; Chamseddine A. N.; Francis A.; Buart S.; Terry S.; Chouaib S.. Integrating tumor hypoxic stress in novel and more adaptable strategies for cancer immunotherapy. In Semin. Cancer Biol.; Elsevier, 2020; Vol. 65, pp 140–154. [DOI] [PubMed] [Google Scholar]
- Daniel S.; Sullivan K.; Labadie K.; Pillarisetty V. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin. Transl. Med. 2019, 8 (1), 1–17. 10.1186/s40169-019-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Saxena S.; Singh R. K. Neutrophils in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. 10.1007/978-3-030-35723-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I.; Manic G.; Coussens L. M.; Kroemer G.; Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019, 30 (1), 36–50. 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Sahu A.; Kwon I.; Tae G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials 2020, 228, 119578. 10.1016/j.biomaterials.2019.119578. [DOI] [PubMed] [Google Scholar]
- Li X.; Jeon Y.-H.; Kwon N.; Park J.-G.; Guo T.; Kim H.-R.; Huang J.-D.; Lee D.-S.; Yoon J. In Vivo-assembled phthalocyanine/albumin supramolecular complexes combined with a hypoxia-activated prodrug for enhanced photodynamic immunotherapy of cancer. Biomaterials 2021, 266, 120430. 10.1016/j.biomaterials.2020.120430. [DOI] [PubMed] [Google Scholar]
- Scheltens P.; De Strooper B.; Kivipelto M.; Holstege H.; Chételat G.; Teunissen C. E.; Cummings J.; van der Flier W. M. Alzheimer’s disease. Lancet 2021, 397 (10284), 1577–1590. 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.; Langerman H. Alzheimer’s Disease - Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. 10.2147/DNND.S228939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsteinsson A. P.; Isaacson R. S.; Knox S.; Sabbagh M. N.; Rubino I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer's Dis. 2021, 8 (3), 371–386. 10.14283/jpad.2021.23. [DOI] [PubMed] [Google Scholar]
- Mintun M. A.; Lo A. C.; Duggan Evans C.; Wessels A. M.; Ardayfio P. A.; Andersen S. W.; Shcherbinin S.; Sparks J.; Sims J. R.; Brys M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384 (18), 1691–1704. 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Chen X.; Yuan C.; Pang X.; Shangguan P.; Liu Y.; Han L.; Sun J.; Lam J. W. Y.; Liu Y.; et al. Near-Infrared Aggregation-Induced Emission Luminogens for In Vivo Theranostics of Alzheimer’s Disease. Angew. Chem., Int. Ed. 2023, 62 (2), e202211550 10.1002/anie.202211550. [DOI] [PubMed] [Google Scholar]
- Gestwicki J. E.; Crabtree G. R.; Graef I. A. Harnessing Chaperones to Generate Small-Molecule Inhibitors of Amyloid ß Aggregation. Science 2004, 306 (5697), 865–869. 10.1126/science.1101262. [DOI] [PubMed] [Google Scholar]
- Lee B. I.; Chung Y. J.; Park C. B. Photosensitizing materials and platforms for light-triggered modulation of Alzheimer’s β-amyloid self-assembly. Biomaterials 2019, 190–191, 121–132. 10.1016/j.biomaterials.2018.10.043. [DOI] [PubMed] [Google Scholar]
- Taniguchi A.; Sasaki D.; Shiohara A.; Iwatsubo T.; Tomita T.; Sohma Y.; Kanai M. Attenuation of the aggregation and neurotoxicity of amyloid-beta peptides by catalytic photooxygenation. Angew. Chem., Int. Ed. Engl. 2014, 53 (5), 1382–1385. 10.1002/anie.201308001. [DOI] [PubMed] [Google Scholar]
- Taniguchi A.; Shimizu Y.; Oisaki K.; Sohma Y.; Kanai M. Switchable photooxygenation catalysts that sense higher-order amyloid structures. Nat. Chem. 2016, 8 (10), 974–982. 10.1038/nchem.2550. [DOI] [PubMed] [Google Scholar]
- Ni J.; Taniguchi A.; Ozawa S.; Hori Y.; Kuninobu Y.; Saito T.; Saido T. C.; Tomita T.; Sohma Y.; Kanai M. Near-Infrared Photoactivatable Oxygenation Catalysts of Amyloid Peptide. Chem. 2018, 4 (4), 807–820. 10.1016/j.chempr.2018.02.008. [DOI] [Google Scholar]
- Lebrun V.; Faller P. Burning Amyloid-β with a Near-Infrared PS. Chem. 2018, 4 (4), 663–665. 10.1016/j.chempr.2018.03.019. [DOI] [Google Scholar]
- Nagashima N.; Ozawa S.; Furuta M.; Oi M.; Hori Y.; Tomita T.; Sohma Y.; Kanai M. Catalytic photooxygenation degrades brain Abeta in vivo. Sci. Adv. 2021, 7 (13), eabc9750 10.1126/sciadv.abc9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S.; Hori Y.; Shimizu Y.; Taniguchi A.; Suzuki T.; Wang W.; Chiu Y. W.; Koike R.; Yokoshima S.; Fukuyama T.; et al. Photo-oxygenation by a biocompatible catalyst reduces amyloid-beta levels in Alzheimer’s disease mice. Brain 2021, 144 (6), 1884–1897. 10.1093/brain/awab058. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Xiong H.; Zhang B.; Lee I.; Xie J.; Li M.; Zhang H.; Seung Kim J. Photodynamic Alzheimer’s disease therapy: From molecular catalysis to photo-nanomedicine. Coord. Chem. Rev. 2022, 470, 214726. 10.1016/j.ccr.2022.214726. [DOI] [Google Scholar]
- Yang J.; Wang X.; Liu J.; Chi W.; Zhang L.; Xiao L.; Yan J.-w. Near-Infrared Photooxygenation Theranostics Used for the Specific Mapping and Modulating of Amyloid-β Aggregation. Anal. Chem. 2022, 94 (45), 15902–15907. 10.1021/acs.analchem.2c04042. [DOI] [PubMed] [Google Scholar]
- Yang J.; Chi W.; Shi W.-J.; Zhang L.; Yan J.-W. An in situ-triggered and chemi-excited photooxygenation system for Aβ aggregates. Chem. Eng. J. 2023, 456, 140998. 10.1016/j.cej.2022.140998. [DOI] [Google Scholar]
- Li M.; Xiong T.; Du J.; Tian R.; Xiao M.; Guo L.; Long S.; Fan J.; Sun W.; Shao K.; et al. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141 (6), 2695–2702. 10.1021/jacs.8b13141. [DOI] [PubMed] [Google Scholar]
- Li M.; Tian R.; Fan J.; Du J.; Long S.; Peng X. A lysosome-targeted BODIPY as potential NIR PS for photodynamic therapy. Dyes Pigment. 2017, 147, 99–105. 10.1016/j.dyepig.2017.07.048. [DOI] [Google Scholar]
- Zhao Y.-Y.; Zhang L.; Chen Z.; Zheng B.-Y.; Ke M.; Li X.; Huang J.-D. Nanostructured Phthalocyanine Assemblies with Efficient Synergistic Effect of Type I Photoreaction and Photothermal Action to Overcome Tumor Hypoxia in Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143 (34), 13980–13989. 10.1021/jacs.1c07479. [DOI] [PubMed] [Google Scholar]
- Li M.; Long S.; Kang Y.; Guo L.; Wang J.; Fan J.; Du J.; Peng X. De Novo Design of Phototheranostic Sensitizers Based on Structure-Inherent Targeting for Enhanced Cancer Ablation. J. Am. Chem. Soc. 2018, 140 (46), 15820–15826. 10.1021/jacs.8b09117. [DOI] [PubMed] [Google Scholar]
- Li M.; Xu Y.; Pu Z.; Xiong T.; Huang H.; Long S.; Son S.; Yu L.; Singh N.; Tong Y.; et al. Photoredox catalysis may be a general mechanism in photodynamic therapy. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (34), e2210504119 10.1073/pnas.2210504119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Long S.; Li M.; Cao J.; Li Y.; Guo L.; Sun W.; Du J.; Fan J.; Peng X. Oxygen-Dependent Regulation of Excited-State Deactivation Process of Rational PS for Smart Phototherapy. J. Am. Chem. Soc. 2020, 142 (3), 1510–1517. 10.1021/jacs.9b11800. [DOI] [PubMed] [Google Scholar]
- Li M.; Xia J.; Tian R.; Wang J.; Fan J.; Du J.; Long S.; Song X.; Foley J. W.; Peng X. Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors. J. Am. Chem. Soc. 2018, 140 (44), 14851–14859. 10.1021/jacs.8b08658. [DOI] [PubMed] [Google Scholar]
- Li M.; Shao Y.; Kim J. H.; Pu Z.; Zhao X.; Huang H.; Xiong T.; Kang Y.; Li G.; Shao K.; et al. Unimolecular Photodynamic O2-Economizer To Overcome Hypoxia Resistance in Phototherapeutics. J. Am. Chem. Soc. 2020, 142 (11), 5380–5388. 10.1021/jacs.0c00734. [DOI] [PubMed] [Google Scholar]
- Koo S.; Lee M.-G.; Sharma A.; Li M.; Zhang X.; Pu K.; Chi S.-G.; Kim J. S. Harnessing GLUT1-Targeted Pro-oxidant Ascorbate for Synergistic Phototherapeutics. Angew. Chem., Int. Ed. 2022, 61 (17), e202110832 10.1002/anie.202110832. [DOI] [PubMed] [Google Scholar]
- Xiong T.; Peng Q.; Chen Y.; Li M.; Du J.; Fan J.; Jia L.; Peng X. A Novel Nanobody-PS Conjugate for Hypoxia Resistant Photoimmunotherapy. Adv. Funct. Mater. 2021, 31 (37), 2103629. 10.1002/adfm.202103629. [DOI] [Google Scholar]
- Ma D.; Bian H.; Long S.; Zhou P.; Tian R.; Wu Y.; Ge H.; Li M.; Du J.; Fan J.; et al. Se-sensitized NIR hot band absorption PS for anti-Stokes excitation deep photodynamic therapy. Sci. China Chem. 2022, 65 (3), 563–573. 10.1007/s11426-021-1179-7. [DOI] [Google Scholar]
- Tian R.; Sun W.; Li M.; Long S.; Li M.; Fan J.; Guo L.; Peng X. Development of a novel anti-tumor theranostic platform: a near-infrared molecular upconversion sensitizer for deep-seated cancer photodynamic therapy. Chem. Sci. 2019, 10 (43), 10106–10112. 10.1039/C9SC04034J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Torres B.; Estepa-Fernández A.; Rovira M.; Orzáez M.; Serrano M.; Martínez-Máñez R.; Sancenón F. The chemistry of senescence. Nat. Rev. Chem. 2019, 3 (7), 426–441. 10.1038/s41570-019-0108-0. [DOI] [Google Scholar]
- Hernandez-Segura A.; Nehme J.; Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28 (6), 436–453. 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- He S.; Sharpless N. E. Senescence in Health and Disease. Cell 2017, 169 (6), 1000–1011. 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S.; Farr J. N.; Tchkonia T.; Kirkland J. L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16 (5), 263–275. 10.1038/s41574-020-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B. G.; Durik M.; Baker D. J.; van Deursen J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 2015, 21 (12), 1424–1435. 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J.; Wijshake T.; Tchkonia T.; LeBrasseur N. K.; Childs B. G.; van de Sluis B.; Kirkland J. L.; van Deursen J. M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479 (7372), 232–236. 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Kishimoto Y.; Grammatikakis I.; Gottimukkala K.; Cutler R. G.; Zhang S.; Abdelmohsen K.; Bohr V. A.; Misra Sen J.; Gorospe M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22 (5), 719–728. 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J. M. Senolytic therapies for healthy longevity. Science 2019, 364 (6441), 636–637. 10.1126/science.aaw1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Bi Z.; Wang L.; Xia Y.; Xie Y.; Liu Y. Recent Advances in Strategies for Imaging Detection and Intervention of Cellular Senescence. ChemBioChem. 2023, 24 (1), e202200364 10.1002/cbic.202200364. [DOI] [PubMed] [Google Scholar]
- Wang A. S.; Dreesen O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. 10.3389/fgene.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlova N.; De Sanctis J. B.; Hajduch M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. 10.3390/ijms23084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.; Won M.; Li H.; Kim W. Y.; Li M.; Yan C.; Sharma A.; Guo Z.; Zhu W.-H.; Sessler J. L.; et al. Harnessing α-l-fucosidase for in vivo cellular senescence imaging. Chem. Sci. 2021, 12 (29), 10054–10062. 10.1039/D1SC02259H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.; Li X.; Shi D.; Sun T.; Liu W.; Li X.; Qiao S.; Chen X.; Guo Y.; Li J. A senolysis-based theragnostic prodrug strategy towards chronic renal failure. Chem. Sci. 2022, 13 (40), 11738–11745. 10.1039/D2SC03525A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.; Cheung Y.-K.; Fong W.-P.; Wong C. T. T.; Ng D. K. P. Selective photodynamic eradication of senescent cells with a β-galactosidase-activated photosensitiser. Chem. Commun. 2023, 59 (23), 3471–3474. 10.1039/D2CC06661K. [DOI] [PubMed] [Google Scholar]
- Lee S. K.; Shen Z.; Han M. S.; Tung C.-H. Developing a far-red fluorogenic beta-galactosidase probe for senescent cell imaging and photoablation. RSC Adv. 2022, 12 (8), 4543–4549. 10.1039/D2RA00377E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Kim J. S.; Li M. Illuminating anti-ageing. Nat. Chem. 2023, 15 (4), 451–452. 10.1038/s41557-023-01164-7. [DOI] [PubMed] [Google Scholar]
- Li M.; Gebremedhin K. H.; Ma D.; Pu Z.; Xiong T.; Xu Y.; Kim J. S.; Peng X. Conditionally Activatable Photoredox Catalysis in Living Systems. J. Am. Chem. Soc. 2022, 144 (1), 163–173. 10.1021/jacs.1c07372. [DOI] [PubMed] [Google Scholar]
- Alves E.; Faustino M. A.; Neves M. G.; Cunha A.; Tome J.; Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6 (2), 141–164. 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- Durantini A. M.; Heredia D. A.; Durantini J. E.; Durantini E. N. BODIPYs to the rescue: Potential applications in photodynamic inactivation. Eur. J. Med. Chem. 2018, 144, 651–661. 10.1016/j.ejmech.2017.12.068. [DOI] [PubMed] [Google Scholar]
- Sobotta L.; Skupin-Mrugalska P.; Piskorz J.; Mielcarek J. Porphyrinoid PSs mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. 10.1016/j.ejmech.2019.04.057. [DOI] [PubMed] [Google Scholar]
- Sobotta L.; Skupin-Mrugalska P.; Piskorz J.; Mielcarek J. Non-porphyrinoid PSs mediated photodynamic inactivation against bacteria. Dyes Pigment. 2019, 163, 337–355. 10.1016/j.dyepig.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez Lopez E. J.; Sarotti A. M.; Martínez S. R.; Macor L. P.; Durantini J. E.; Renfige M.; Gervaldo M. A.; Otero L. A.; Durantini A. M.; Durantini E. N.; et al. BOPHY-Fullerene C60 Dyad as a PS for Antimicrobial Photodynamic Therapy. Chem. Eur. J. 2022, 28 (5), e202103884 10.1002/chem.202103884. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Liu Y.; Chen Y.; Chen J.; Feng G.; Tang B. Z. Cationic AIE-active PSs for highly efficient photodynamic eradication of drug-resistant bacteria. Mater. Chem. Front. 2022, 7 (1), 96–105. 10.1039/D2QM01043G. [DOI] [Google Scholar]
- Livingston E. T.; Mursalin M. H.; Callegan M. C. A pyrrhic victory: The PMN response to ocular bacterial infections. Microorganisms 2019, 7 (11), 537. 10.3390/microorganisms7110537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Wu Y.; Cai W.; Wang D.; Ren C.; Shen T.; Yu D.; Qiang S.; Hu C.; Zhao Z.; et al. Vision defense: Efficient antibacterial AIEgens induced early immune response for bacterial endophthalmitis. Adv. Sci. 2022, 9 (25), 2202485. 10.1002/advs.202202485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic D.; Shaw J. E.; Magliano D. J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18 (9), 525–539. 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep B. O.; Thomaidou S.; van Tienhoven R.; Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17 (3), 150–161. 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Ley S. H.; Hu F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14 (2), 88–98. 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Xue L.; Zhu Q.; Feng Y.; Wu M. Recent advances in second near-infrared region (NIR-II) fluorophores and biomedical applications. Front. Chem. 2021, 9, 852. 10.3389/fchem.2021.750404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H. What ADME tests should be conducted for preclinical studies?. ADMET and DMPK 2013, 1 (3), 19–28. 10.5599/admet.1.3.9. [DOI] [Google Scholar]
- Daly J.; Jerina D.; Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia 1972, 28, 1129–1149. 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Lewis D. F.; Dickins M. Baseline lipophilicity relationships in human cytochromes P450 associated with drug metabolism. Drug Metab. Rev. 2003, 35 (1), 1–18. 10.1081/DMR-120018245. [DOI] [PubMed] [Google Scholar]
- Tarcsay A.; Keserű G. r. M. Contributions of molecular properties to drug promiscuity: miniperspective. J. Med. Chem. 2013, 56 (5), 1789–1795. 10.1021/jm301514n. [DOI] [PubMed] [Google Scholar]
- Ren C.; Morohashi K.; Plotnikov A. N.; Jakoncic J.; Smith S. G.; Li J.; Zeng L.; Rodriguez Y.; Stojanoff V.; Walsh M.; et al. Small-molecule modulators of methyl-lysine binding for the CBX7 chromodomain. Chem. Biol. 2015, 22 (2), 161–168. 10.1016/j.chembiol.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordanetto F.; Kihlberg J. Macrocyclic drugs and clinical candidates: what can medicinal chemists learn from their properties?. J. Med. Chem. 2014, 57 (2), 278–295. 10.1021/jm400887j. [DOI] [PubMed] [Google Scholar]
- Doak B. C.; Zheng J.; Dobritzsch D.; Kihlberg J. How beyond rule of 5 drugs and clinical candidates bind to their targets. J. Med. Chem. 2016, 59 (6), 2312–2327. 10.1021/acs.jmedchem.5b01286. [DOI] [PubMed] [Google Scholar]
- DeGoey D. A.; Chen H.-J.; Cox P. B.; Wendt M. D. Beyond the rule of 5: lessons learned from AbbVie’s drugs and compound collection: miniperspective. J. Med. Chem. 2018, 61 (7), 2636–2651. 10.1021/acs.jmedchem.7b00717. [DOI] [PubMed] [Google Scholar]
- Békés M.; Langley D. R.; Crews C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discovery 2022, 21 (3), 181–200. 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Zhi Y.; Liu B.; Yao Q. Advancing strategies for proteolysis-targeting chimera design. J. Med. Chem. 2023, 66 (4), 2308–2329. 10.1021/acs.jmedchem.2c01555. [DOI] [PubMed] [Google Scholar]
- Liu X.; Kalogeropulou A. F.; Domingos S.; Makukhin N.; Nirujogi R. S.; Singh F.; Shpiro N.; Saalfrank A.; Sammler E.; Ganley I. G.; et al. Discovery of XL01126: A Potent, Fast, Cooperative, Selective, Orally Bioavailable, and Blood-Brain Barrier Penetrant PROTAC Degrader of Leucine-Rich Repeat Kinase 2. J. Am. Chem. Soc. 2022, 144 (37), 16930–16952. 10.1021/jacs.2c05499. [DOI] [PMC free article] [PubMed] [Google Scholar]