Abstract
Exposure to certain chemicals prenatally and in childhood can impact development and may increase risk for attention-deficit/hyperactivity disorder (ADHD). Leveraging a larger set of literature searches conducted to synthesize results from longitudinal studies of potentially modifiable risk factors for childhood ADHD, we present meta-analytic results from 66 studies that examined the associations between early chemical exposures and later ADHD diagnosis or symptoms. Studies were eligible for inclusion if the chemical exposure occurred at least 6 months prior to measurement of ADHD diagnosis or symptomatology. Included papers were published between 1975 and 2019 on exposure to anesthetics (n = 5), cadmium (n = 3), hexachlorobenzene (n = 4), lead (n = 22), mercury (n = 12), organophosphates (n = 7), and polychlorinated biphenyls (n = 13). Analyses are presented for each chemical exposure by type of ADHD outcome reported (categorical vs. continuous), type of ADHD measurement (overall measures of ADHD, ADHD symptoms only, ADHD diagnosis only, inattention only, hyperactivity/impulsivity only), and timing of exposure (prenatal vs. childhood vs. cumulative), whenever at least 3 relevant effect sizes were available. Childhood lead exposure was positively associated with ADHD diagnosis and symptoms in all analyses except for the prenatal analyses (odds ratios (ORs) ranging from 1.60 to 2.62, correlation coefficients (CCs) ranging from 0.14 to 0.16). Other statistically significant associations were limited to organophosphates (CC = 0.11, 95% confidence interval (CI): 0.03–0.19 for continuous measures of ADHD outcomes overall), polychlorinated biphenyls (CC = 0.08, 95% CI: 0.02–0.14 for continuous measures of inattention as the outcome), and both prenatal and childhood mercury exposure (CC = 0.02, 95% CI: 0.00–0.04 for continuous measures of ADHD outcomes overall for either exposure window). Our findings provide further support for negative impacts of prenatal and/or childhood exposure to certain chemicals and raise the possibility that primary prevention and targeted screening could prevent or mitigate ADHD symptomatology. Furthermore, these findings support the need for regular review of regulations as our scientific understanding of the risks posed by these chemicals evolves.
Keywords: Attention deficit/hyperactivity disorder, Meta-analysis, Childhood health, Pediatrics, Chemicals, Anesthesia, Cadmium, Hexachlorobenzene, Lead, Mercury, Organophosphate, Polychlorinated biphenyls
Introduction
Early periods of brain development are critical to later life outcomes and disruptions to normal neurodevelopment can have far-reaching impacts throughout the life course, including impaired functioning and reduced quality of life on the individual level and diminished productivity and high financial costs on the societal level (Gould, 2009; McFarland et al., 2022; Roth, 2012). Even brief exposures to high concentrations of certain environmental toxicants have been shown to adversely affect central nervous system function (Alexander & Delves, 1972; Kanluen & Gottlieb, 1991; Rogan & Gladen, 1992; Ruckart et al., 2004). Frequent low-level exposure to some chemicals may also affect neural development (Axelrad et al., 2007; Perez-Fernandez et al., 2020; Rogan & Gladen, 1992). Regulatory updates for certain chemicals over time reflect the growing body of evidence and increasing attention to the impact of environmentally pervasive chemicals on children’s neurodevelopment. For example, the ban on use of lead paint in 1978 (16 Code of Federal Regulations Part 1303) and the phasing out of the previously widely used organophosphate diazinon beginning in December 2000 (United States Environmental Protection Agency, 2016) were issued with citations of the concern of the harm to children’s health caused by these chemicals. Many of these and other chemicals are still in use today in the USA and could be adversely affecting children’s neurodevelopment (Grandjean & Landrigan, 2014).
Attention-deficit/hyperactivity disorder (ADHD) in particular has been often investigated as a neurodevelopmental outcome of interest in relation to early environmental exposures. ADHD is a neurodevelopmental disorder characterized by six or more symptoms of either inattention (e.g., difficulty with sustained attention, high distractibility) and/or hyperactivity/impulsivity (e.g., restlessness, excessive talking) that are present across multiple settings and are significant enough to interfere with quality of daily functioning (American Psychiatric Association, 2013). During 2016–2019, an estimated 9.8% of children ages 3–17 years in the USA had ever been diagnosed with ADHD based on parent report (Bitsko et al., 2022a) and the disorder is associated with negative long-term outcomes, including poor academic achievement and criminality (Erskine et al., 2016) and higher risk of premature death (Sun et al., 2019).
Many chemical exposures have been investigated as potential risk factors for the development of ADHD; (Froehlich et al., 2011; Goodlad et al., 2013; Nilsen & Tulve, 2020). Lead is one of the more frequently studied chemical exposures in ADHD research. Detrimental neurodevelopmental effects of lead exposure resulting in difficulty learning (Bellinger et al., 1984) and behavioral problems (Needleman et al., 1990) have long been recognized. Multiple studies have also shown positive associations between ADHD and lead exposure, using a variety of study designs (Nilsen & Tulve, 2020). Previous meta-analyses and systematic reviews have identified potential associations between ADHD and exposure to mercury, organophosphates, phthalates, and other environmental contaminants (Froehlich et al., 2011; Nilsen & Tulve, 2020), although these relationships are typically based on fewer studies than are available on lead exposure. Exposures to other chemicals through medical procedures, including anesthesia used during surgery, may also impact development of behavioral disorders such as ADHD (DiMaggio et al., 2011). Timing of chemical exposures has also been of significant interest in ADHD research, as some chemical exposures are thought to be most detrimental to the developing brain in the womb and hypothesized critical exposure windows are associated with increased susceptibility and risk (The American College of Obstetricians and Gynecologists 138, 2021; Grandjean & Landrigan, 2006). Exposure that occurs during childhood can also negatively impact brain development (Grandjean & Landrigan, 2006).
Epidemiologic research on chemical exposures associated with the development of ADHD is numerous; however, studies have employed various methods, and, in some cases, have resulted in seemingly conflicting results. Differences in methodology include how the chemical risk factors are measured, the timing of exposure assessed, and specific outcomes of ADHD (e.g., diagnosis, symptoms). For example, one study (Sioen et al., 2013) found a notably increased risk of ADHD in children exposed to lead prenatally, with exposure assessment based on cord blood lead levels. However, a second study (Forns et al., 2014) showed no association between prenatal lead exposure, measured via maternal urine samples, and ADHD in children. Meta-analysis offers a systematic method of summarizing the available evidence to provide more information than any single study. Although published meta-analytic findings exist for some individual or multiple environmental chemical exposures and their association with ADHD (for example, see Nilsen & Tulve, 2020; Yoshimasu et al., 2014), differences in methods (e.g., inclusion/exclusion criteria, analytic approach) across separately conducted meta-analyses preclude comparisons of results of meta-analyses conducted on different exposures. The purpose of this paper, in addition to providing an updated meta-analysis of current evidence regarding risk factors for ADHD, is to apply identical meta-analytic techniques to the literature on the associations between earlier chemical exposures and later ADHD as part of a series of papers examining other potentially modifiable risk factors including perinatal factors (Bitsko et al., 2022b), parenting and family environment (Claussen et al., 2022), parent mental health (Robinson et al., 2022), parental substance use (Maher et al., this issue), and childhood physical health (So et al., 2022). Together these papers aim to identify potential opportunities for prevention and early intervention.
Methods
Literature Search and Inclusion
The full review protocol of the meta-analyses in which this paper is included can be found elsewhere (Bitsko et al., 2022b). The same analytic methods were followed in each paper, to allow for comparisons of results across as well as within papers.
Search Strategy
Studies for inclusion were identified through a literature search of multiple publication databases using a multi-pronged approach. We searched PubMed, Web of Science (WOS), and EMBASE using structured and comprehensive search strings, originally in January 2014. We employed two strategies to the search: a top-down approach and a bottom-up approach. For the top-down approach, search strings included terms to capture ADHD, including older diagnostic names and related behavioral dimensions, and terms that identify all studies of indicated “risk” factors. For the bottom-up approach, search strings included ADHD or related terms and specific (e.g., organophosphate, lead, mercury) or general terms representing chemical exposure (e.g., pesticide*, insecticid*, organochlorine*). The entire search strings for each database are provided in Supplemental Table 1. Search restrictions and filters (i.e., English language, human subjects) were employed to automatically refine searches to those relevant to our study. In addition, we limited document type in WOS to “Articles, Abstracts, Reviews, or Books” and in EMBASE to “Article, Article in Press, Conference Abstract, Conference Paper, Erratum, Letter, Note, Review, or Short Survey.”
Study Eligibility Determinations
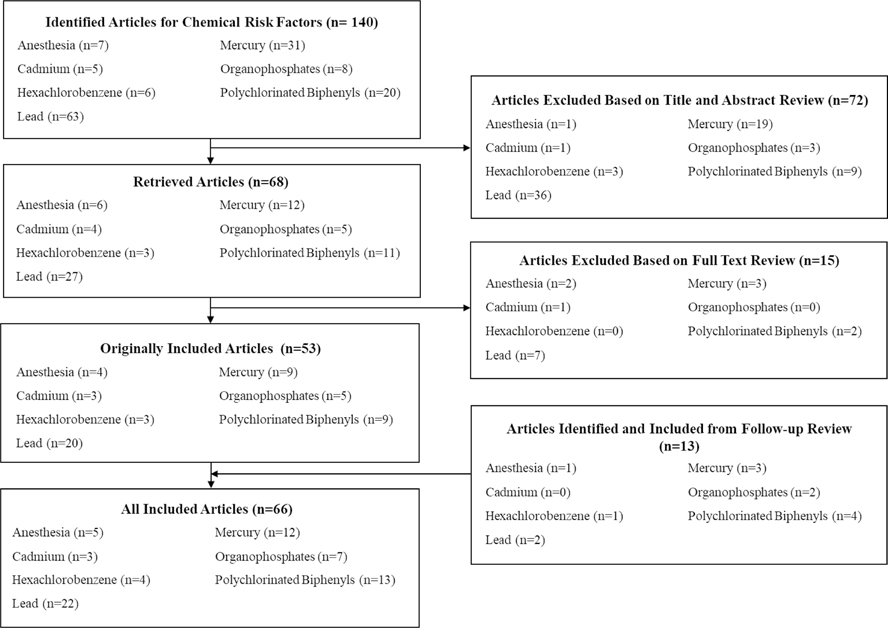
Figure 1 describes the review and exclusion/inclusion process. Coders were trained and 25% of articles were double-coded initially, which was reduced to 15% after demonstrating consistency across coders. Inter-rater reliability was continuously monitored, and coders were retrained if Cohen’s Kappa was less than 0.70. While manually curating articles identified through the comprehensive search, we examined the reference list of each article to identify additional articles that may not have been captured through our initial search. Peer-reviewed studies were included in the meta-analyses if the measurement of the chemical exposure occurred at least 6 months before the measurement of ADHD diagnosis or symptomatology. This includes longitudinal studies in which the chemical exposure was measured before the outcome of ADHD and also cross-sectional studies with retrospective reports of exposure (e.g., records of procedure codes indicative of procedures requiring general anesthesia before age 3 years) or in which the concurrent chemical measurement is an indicator of cumulative previous exposure (e.g., lead level in deciduous teeth).
Fig. 1.
Flowchart of triage process for articles identified for meta-analyses of chemical and environmental risk factors associated with attention-deficit/hyperactivity disorder
Meta-analysis Methods
Risk factors were included in the meta-analysis if there were three or more eligible effect sizes for the exposure-ADHD relationship from three independent samples (a single article could have reported on more than one independent sample). Study results were grouped first by chemical exposure and then (within each chemical exposure) by type of ADHD outcome. Summary effect sizes were calculated, and forest plots were constructed, separately for measures from studies reporting continuous and dichotomous outcomes. Correlation coefficients (CCs) with 95% confidence intervals (CIs) were calculated for studies reporting associations with continuous outcome measures; odds ratios (ORs) with 95% CIs were calculated for studies reporting associations with dichotomous outcomes. The chemical exposures with a sufficient number of eligible studies to be included for analyses were anesthesia, cadmium, hexachlorobenzene, lead, mercury, organophosphates, and polychlorinated biphenyls. Within each exposure category we included an “overall ADHD” outcome where we pooled effect sizes with any ADHD outcome measure (separately for continuous and dichotomous outcomes), as well as analyses of subsets of ADHD outcomes, specifically ADHD diagnosis, ADHD symptoms, inattention, and hyperactivity/impulsivity. Of note, all “diagnosis” analyses were dichotomous while analyses of symptoms included both dichotomous and continuous outcome measures. A wide range of ADHD measures were used including clinical assessment, validated scales and tests of symptoms, and diagnosis based on parent report and medical records (see Table 1).
Table 1.
Characteristics of studies included in meta-analyses of chemical and environmental risk factors for attention-deficit/hyperactivity disorder
| Study | Risk factors included | Sample size | Age at outcome measurement (years) | Male (%) | ADHD measurement (included) | Sample (country) | Measurement |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Andrews et al. 2004) | Mercury | 100,572 | 3.7 | n/a | Diagnosis (ICD9 Codes) | Prospective Cohort Study (GPRD; UK) | Number of DTP/DT doses received at 3 months/93 days and 4 months/124 days (from medical birth registry) |
| Bellinger et al. 1994) | Lead | 1782 | 6 | 50.4 | ADHD symptoms (TRP) | Birth cohort (from Lying-In Division of Brigham and Women’s Hospital; USA) | Umbilical cord blood lead level and child dentin lead level |
| Benetou-Marantidou et al. 1988) | Lead | 60 | 6–11 | n/a | ADHD symptoms (Rutter Behavioral Questionnaire) | Prospective Matched Cohort (Greece) | Child blood lead level |
| Boucher et al. 2012) | Polychlorinated biphenyls; mercury; lead | 277–279 | 11.3 | 49.5 | Inattention (TRP) and diagnosis (diagnostic scale completed by parents and teachers) | Prospective cohort (Nunavik Child Development Study; Canada) | Umbilical cord blood levels (previously collected and analyzed at birth in either the Cord Blood Monitoring Program or Environmental Contaminants and Child Development Study) and child blood levels (collected during present study); PCB congener 153 measured |
| Burns et al. 1999) | Lead | 322 | 11–13 | 49.4 | Inattention (CBCL) | Prospective cohort (Port Pirie Cohort Study; Australia) | Umbilical cord blood lead level and child blood lead level |
| Caspersen et al. 2016) | Polychlorinated biphenyls | 1024 | 3.5 | 53 | ADHD symptoms (Preschool Age Psychiatric Assessment Interview) | Prospective cohort (Norway; MoBa) | PCB-153 concentration in food, based on maternal food frequency questionnaire and database of PCB concentrations in Norwegian foods |
| Chandramouli et al. 2009) | Lead | 488 | 7–8 | 56.6 | Inattention (Development and Well-Being Assessment) and hyperactivity (SDQ) | Prospective cohort (subsample of AL-SPAC; UK) | Child blood lead level |
| Ciesielski et al. 2012) | Cadmium | 1097 | 6–15 | 52 | Diagnosis (parent report) | National Survey (NHANES; USA) | Child urinary cadmium concentration |
| Davis et al. 2004) | Lead | 57 | 4–5 | 39 | Inattention (puzzle-based) | School based research study (USA) | Child blood lead levels (obtained from local health department records) |
| Dalsager et al. 2019) | Organophosphates | 948 | 2–4 | 52 | ADHD symptoms (CBCL) | Prospective cohort (Odense Child Cohort; Denmark) | Maternal urine levels (prenatal) of specific metabolite of chlorpyrifos/chlorpyrifos-methyl, TCPY |
| DiMaggio et al. 2011) | Anesthesia | 11,286 | n/a | n/a | Diagnosis (ICD9 Codes) | Retrospective sibling birth cohort (USA) | Any “inpatient or outpatient ICD9 procedure code indicative of exposure to general anesthesia in a child younger than 3 years” |
| Doherty et al. 2019) | Organophosphates | 199 | 3–3.2 | 56 | ADHD symptoms (Behavioral Assessment System for Children) | Birth cohort (subsample of Pregnancy, Infection, and Nutrition Study; USA) | Maternal urine concentration (prenatal) of six metabolites (diphenyl phosphate (DPHP), bis(1,3-dichloro-2-propyl) phosphate (BDCIPP), isopropyl-phenyl phenyl phosphate (ip-PPP), 1-hydroxyl-2-propyl bis(l-chloro-2-propyl) phosphate (BCIPHIPP) |
| Eskenazi et al. 2007) | Organophosphates | 356 | 2 | 49.4 | ADHD symptoms (CBCL; DSM-IV Criteria) and inattention (CBCL) | Birth cohort (Center for the Health Assessment of Mothers and Children of Salinas; USA) | Level of six nonspecific organophosphate dialkylphosphate metabolites (dimethylphosphate, dimethylthiophosphate, dimethyldithiophosphate, diethylphosphate, diethylthiophosphate, and diethyldithiophosphate) in maternal and child urine and metabolites specific to malathion and chlorpyrifos in maternal urine |
| Ethier et al. 2015) | Polychlorinated biphenyls; lead; mercury | 27 | 11.2 | 66.7 | Inattention (modified classic Posner paradigm) | Prospective cohort (subsample of Cord Blood Monitoring Program; Canada) | Umbilical cord blood level and child blood level (PCB 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187 measured as markers for PCBs) |
| Forns et al. 2014) | Cadmium; lead | 385 | 4.1–5.6 | 51.7 | ADHD symptoms (DSM-IV Criteria) | Population-based birth cohort (Environment and Childhood Project; Spain) | Concentration in maternal urine (prenatal) |
| Fortenberry et al. 2014) | Organophosphates | 142 | 6–11 | 48 | ADHD symptoms (Conners Parent Rating Scale) | 3 sequentially enrolled birth cohorts (Early Life Exposures in Mexico to Environmental Toxicants; Mexico) | Chlorpyrifos, chlorpyrifos-methyl, and/or TCPY concentration in maternal urine |
| Geier and Geier (2005) | Mercury | 109,993 | 4.1 | 80 | Diagnosis (ICD9 Codes) | VAERS and Vaccine Safety Datalink VSD (USA) | Cumulative exposure to ethyl mercury calculated based on individual automated vaccination records (from epidemiological database) |
| Geier et al. 2014) | Mercury | 22,069 | 5.7 | 51.9 | Diagnosis (ICD9 Codes) | Case–control (using VSD; USA) | Cumulative total mercury exposure to thimerosal-containing hepatitis B vaccine received within the first six months of life |
| Grandjean and Landrigan (2006) | Organophosphates | 72 | 5.7–8.8 | 47.2 | Digit spans (WISC) | School based research study (Ecuador) | Maternal interview to determine pesticide exposure during pregnancy; erythrocyte acetylcholine esterase activity, measured from child blood; and level of six nonspecific organophosphate dialkylphosphate metabolites (dimethylphosphate, dimethyldithiophosphate, dimethylthiophosphate, diethylphosphate, diethyldithiophosphate, and diethylthiophosphate) in child urine |
| Heron et al. 2004) | Mercury | 7854 | 3.9–6.8 | n/a | Hyperactivity (SDQ) | Prospective Cohort (AL-SPAC; UK) | Number of DTP/DT doses received at 3 months/93 days and 4 months/124 days (from surveillance database) |
| Jacobson et al. 1992) | Polychlorinated biphenyls | 143 | 4.3 | 54.4 | Inattention (Vigilance Paradigm) | Prospective cohort (USA) | Umbilical cord blood PCB level, maternal milk PCB level (and duration of nursing), and child PCB blood level |
| Jacobson and Jacobson (2003) | Polychlorinated biphenyls | 146 | 11 | 60.3 | ADHD symptoms (BSID) | Prospective cohort (USA) | Umbilical cord blood PCB level, maternal blood PCB level, maternal milk PCB level, and child PCB blood level |
| Ji et al. 2018) | Lead | 1479 | 9.6 | 48.1 | Diagnosis (ICD9 Codes) | Birth cohort (Boston Birth Cohort; USA) | Child blood lead levels (obtained from EMRs from routine screening) |
| Ketzer et al. 2012) | Anesthesia | 248 | 6–17 | 50 | Diagnosis (Clinical Assessment) | Matched case–control study (Brazil) | Perinatal anesthesia exposure (dichotomous); information obtained through maternal interview and supplemented with maternal records where possible |
| Ko et al. 2014) | Anesthesia | 14,446 | >3 | 66.3 | Diagnosis (ICD9 Codes) | Retrospective matched cohort (National Health Insurance Research Database; Taiwan) | Procedure code for general anesthesia before 3 years of age |
| Kyriklaki et al. 2016) | Polychlorinated biphenyls | 524 | 4 | n/a | Diagnoses (parent report on diagnostic test), ADHD symptoms (ADHD Test; SDQ) | Prospective, population-based cohort (The Rhea study; Greece) | Maternal blood concentrations (prenatal) of PCB 118, 138, 153, 156, 170, and 180 |
| Landrigan et al. 1975) | Lead | 124 | 3–15 | n/a | Hyperactivity (Parent-Reported) | Prospective matched cohort (USA) | Child blood lead level |
| Lenters et al. 2019) | Hexachlorobenzene; polychlorinated biphenyls | 1199 | 11.1–13.4 | 54.6 | Diagnosis (ICD10 codes) | Birth cohort (Norwegian Human Milk Study, 2002–2009; Norway) | Concentration in breast milk (PCB 74, 99, 105, 114, 118, 138, 153, 156, 167, 170, 180, 189, and 194 measured as markers of PCBs) |
| Leviton et al. 1993) | Lead | 1923 | 8 | 50.6 | Hyperactivity (Boston Teacher Questionnaire) | Birth cohort (USA) | Umbilical cord blood lead level and child dentin lead level |
| Lygre et al. 2018) | Mercury | 15,896 | 3 | 51.1 | ADHD symptoms (6 items from the CBCL and 5 items from the DSM-IV-TR symptom list) | Prospective cohort (MoBa; Norway) | Maternal number of amalgam-filled teeth, and instances of setting and removing of amalgam fillings during pregnancy |
| Minder et al. 1994) | Lead | 43 | 8–12 | 100 | Inattention (Trail Making Test B) | School based research study (The Netherlands) | Child hair lead concentration |
| Myers et al. 2000) | Mercury | 711 | 5.5 | n/a | Inattention (CBCL) | Prospective cohort (Republic of Seychelles) | Child hair mercury concentration and maternal hair mercury concentration |
| Needleman et al. 1979) | Lead | 158 | 7.3–7.6 | 49.5–55.9 | Hyperactivity (Teacher’s Behavioral Scale) | School based research study (USA) | Child dentin lead level |
| Needleman et al. 1996) | Lead | 301 | 7.4 | 100 | Inattention (CBCL) | Retrospective cohort study (subsample of Pittsburgh Youth Study) (USA) | Child bone (tibia) lead concentration |
| Nicolescu et al. 2010) | Mercury; lead | 83 | 8–12 | 51 | ADHD symptoms (FBB-ADHS) | Prospective Cohort (Romania) | Child blood levels |
| Plusquellec et al. 2010) | Lead | 98 | 5.4 | 43.6 | Impulsivity (Infant Behavior Rating Scale) | Prospective cohort (follow-up of the Cord Blood Monitoring Program) (Canada) | Umbilical cord blood level and child blood level |
| Rauh et al. 2006) | Organophosphates | 228 | 3 | 46.5 | ADHD symptoms (CBCL; DSM-IV Criteria) and inattention (CBCL) | Prospective cohort (USA) | Umbilical cord blood and maternal blood levels of chlorpyrifos metabolite |
| Ribas-Fitó et al. 2007) | Hexachlorobenzene | 377 | 4 | 47–59 | ADHD symptoms (DSM-IV Criteria) | Birth cohorts (Spain) | Umbilical cord blood levels of hexachlorobenzene |
| Ris et al. 2004) | Lead | 195 | 15–17 | 53.6 | Inattention (CPT) | Prospective cohort (Cincinnati Lead Study) (USA) | Child blood lead levels |
| Ruckart et al. 2004) | Organophosphates | 279 | 2.5–11.5 | 53.4 | Verbal Cancellation (Verbal Cancellation Test) | Prospective cohort (USA) | Environmental wipe samples from residence sprayed with methyl parathion, urine testing for creatine-adjusted para-Nitrophenol (a metabolite of para-Nitrophenol), although no urine testing was done for children with household methyl parathion <25 μg/100 cm2, (both provided by state health departments) |
| Rummo et al. 1979) | Lead | 65 | 4–8 | 50 | Hyperactivity (Werry-Weiss-Peters Activity Scale) | Prospective matched cohort (USA) | Child blood lead levels, accentuated epiphyseal lines (“lead lines”) |
| Sagiv et al. 2010) | Polychlorinated biphenyls | 573 | 7–11 | 51.2 | ADHD symptoms and inattention (CTRS) | Birth cohort (USA) | Umbilical cord blood level (sum of PCB congeners 118, 138, 153, 180) |
| Sagiv et al. 2012) | Mercury | 66 | 8.2 | 50.4 | ADHD symptoms (CTRS) and inattention (Reaction time on Continuous Performance Test) | Birth cohort (USA) | Maternal hair mercury level, prenatal maternal fish consumption |
| Sioen et al. 2013) | Cadmium; hexachlorobenzene; polychlorinated biphenyls; lead | 257–270 | 7–8 | 48.1 | Hyperactivity (SDQ) | Birth cohort (Flemish Environment and Heath Study) (Belgium) | Umbilical cord blood levels (PCB 138, 153, 180, 188, and 170 measured as markers for PCBs) |
| Sprung et al. 2012) | Anesthesia | 4199 | <19 | 52.1 | Diagnosis (school records) | Retrospective birth cohort (USA) | Number of procedures performed with general anesthesia under the age of 2 years (categorized as “none,” “1,” and “≥2”) |
| Strom et al. 2014) | Hexachlorobenzene; polychlorinated biphenyls | 581 | n/a | 52.9 | Diagnosis (medical records) | Prebirth cohort linked to population registry (Danish Fetal Origins 1988 Cohort) (Denmark) | Maternal blood levels during pregnancy (PCB 118, 138, 153, 156, 170, and 180 measured as markers for PCBs) |
| Tsai et al. 2018) | Anesthesia | 4584 | >4 | 70.6 | Diagnosis (ICD9 Codes) | Nationwide retrospective birth cohort (Taiwan) | Anesthesia exposure before 3 years (dichotomous) |
| Tuthill et al. 1996) | Lead | 277 | 6.5–7.5 | 51 | Diagnosis (parent report) and inattention (Abbreviated Boston Teacher’s Rating Scale Score) | Cross-sectional, school-based study (USA) | Child hair lead concentration |
| Verner et al. 2010) | Polychlorinated biphenyls | 153 | 0.9 | 58 | Inattention (BSID) | Prospective cohort (Canada) | Maternal blood level (drawn prenatally), umbilical cord blood level, child blood level in subset of children (simulated PCB-153 profiles generated using physiologically based pharmacokinetic modeling framework) |
| Verstraeten et al. 2003) | Mercury | 13,337–110,833 | 4.2–6 | n/a | Diagnosis (ICD9 Codes) | 2-phased retrospective cohort (VSD; USA) | Cumulative mercury exposure at 1, 3, and 7 months, based on mean mercury content of each vaccine of interest (hepatitis B, DTP, Hib) in multidose vials |
| Vreugdenhil et al. 2004) | Polychlorinated biphenyls | 82 | 9 | 53.6 | Response time (Simple Reaction Time Test) | Prospective cohort (Rotterdam PCB-dioxin cohort) (The Netherlands) | Maternal blood levels (collected prenatally; prenatal exposure to PCBs defined as the sum of PCB 118, 138, 153, and 180 in maternal samples) |
| Wasserman et al. 1997) | Lead | 258 | 6.5–7.5 | 50.2 | Inattention (WlSC-Version III) | Prospective cohort (Kosovo) | Blood lead levels, collected at “mid pregnancy”, delivery, and at 6-month intervals postnatally |
| Young et al. 2008) | Mercury | 278,624 | 4–6 | 51.1 | Diagnosis (medical records) | Birth cohorts (VSD; USA) | Cumulative mercury exposure based on thimerosal-containing vaccines received from birth to 7 months and birth to 13 months (mercury content assumed for vaccines under study were: “Hib = 25 μg (μg) mercury/dose, DTaP/DTaPH = 25 μg mercury/dose, whole-cell DTP/DTPH = 25 μg mercury/dose, and hepatitis B = 12.5 μg mercury/dose”) |
| Yule et al. 1984) | Lead | 166 | 6–12 | n/a | ADHD symptoms (CTRS) | School-based research study (UK) | Child blood lead levels |
ADHD attention-deficit/hyperactivity disorder, AL-SPAC Avon Longitudinal Study of Parents and Children, BSID Bayley Scales of Infant Development, CBCL Child Behavior Checklist, CPT Continuous Performance Test, CTRS Conners Teacher Ratings Scale, DSM-IV Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, DSM-IV-TR Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, DTP/DT diphtheria-tetanus-whole-cell pertussis/diphtheria-tetanus, EMR Electronic Medical Record, FBB-ADHS Fremdbeurteilungsbogen für Aufmerksamkeits-Hyperaktivitäts-Störung’, GPRD General Practice Research Database, Hib Haemophilus influenzae type B vaccine, ICD9 International Classification of Diseases, Ninth Revision, MoBa Norwegian Mother and Child Cohort Study; Norway, NHANES National Health and Nutrition Examination Survey, PCB polychlorinated biphenyls, RBQ Rutter Behavioral Questionnaire, SDQ Strengths and Difficulties Questionnaire, TCPY 3,5,6-trichloro-2-pyridinol, TRP Teacher Report Form, VAERS Vaccine Adverse Event Reporting System, VSD Vaccine Safety Datalink, WISC Wechsler Intelligence Scale for Children
Subsets of studies within a specific exposure were also analyzed separately by a characteristic of exposure (i.e., prenatal/perinatal, childhood, or cumulative exposure). Effect sizes based on cumulative measures of exposure (e.g., lead levels in deciduous teeth) or on measures taken at multiple time points (e.g., cumulative thimerosal exposure from childhood vaccinations) were included in the cumulative exposure analysis. For studies where boys and girls were analyzed separately, the effect sizes were averaged. As exposures experienced earlier in pregnancy may have a more significant impact on neurodevelopmental disorders (Rice & Barone, 2000) than later in pregancy, we selected first trimester exposure for analysis over later exposures if both were presented.
Using identical methodology to the other papers in this supplement, weighted random-effects models were used, with weights based on the inverse of both within-study variation and among-study variation, to provide a more conservative effect size estimate compared to a fixed-effect model (Berlin et al., 1989). For each exposure-ADHD pairing with at least three eligible effect sizes, a single pooled effect size and 95% confidence interval was calculated per exposure category, with each study weighted by its conditional variance (inverse variance method). An alpha level of 0.05 was used to define statistical significance; aggregated effect sizes are also presented. Once the weighted effect size for each group of studies was obtained, the variance across effect sizes within that subgroup was assessed by calculating Cochran’s heterogeneity statistic, Q. The resulting forest plots depict the per-study effect size and confidence interval, in addition to the estimated common pooled effect size and confidence interval (Supplemental Figs. S1–S33).
Upon completion of the original analyses as described above, we conducted an identical process in January 2021 (using the same search terms, triage, and review process) to capture all relevant studies published since 2014. However, we restricted our inclusion criteria to risk factors that were already included in the prior analysis (specified in results below), rather than identifying new risk factors with the updated search (i.e., only those chemicals with at least three effect sizes which were identified and included in the original literature review). No new search terms were included. See Bitsko et al. (2022b), for further details on our meta-analytic approach.
Results
Our comprehensive literature search yielded 66 studies published between 1975 and 2019 (53 from the original search in 2014 and 13 studies from the updated search in 2021) that met all inclusion criteria and provided sufficient statistical information for meta-analysis. The literature search yielded studies of prenatal, cumulative, and overall childhood exposure to anesthesia, cadmium, hexachlorobenzene, lead, mercury, organophosphates, and polychlorinated biphenyls. The characteristics of these studies can be found in Table 1 and the number of studies for each exposure and exposure period is quantified in Table 2. While we also included search terms for studies examining exposure to manganese, other pesticides and insecticides, phthalates, bisphenol A, perfluorinated compounds, polycyclic aromatic hydrocarbon, poly-aromatic hydrocarbon, polynuclear aromatic hydrocarbon, and brominated flame retardants, we were not able to include these exposures in any of our analyses due to an insufficient number of eligible studies.
Table 2.
Meta-analysis results of studies examining selected environmental and chemical risk factors for attention-deficit/hyperactivity disorder
| Risk factor | Outcome type | Overall | Symptoms | Diagnosis only | Inattention | Hyperactivity/impulsivity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Sample size (studies) | ES* (95% CI) | Sample size (studies) | ES** (95% CI) | Sample size (studies) | ES** (95% CI) | Sample size (studies) | ES** (95% CI) | Sample size (studies) | ES** (95% CI) | ||
|
| |||||||||||
| Anesthesia exposure *** | Dichotomous | 34,763 (5) | 1.57 (0.90, 2.76) | ||||||||
| Cadmium exposure | Dichotomous | 1739 (3) | 0.90 (0.49, 1.64) | ||||||||
| Hexachlorobenzene exposure | Dichotomous | 2427 (4) | 1.04 (0.46, 2.31) | ||||||||
| Lead exposure | Dichotomous | 7566 (11) | 1.83 (1.38, 2.41)** | 5322 (8) | 1.79 (1.17, 2.75)** | 3736 (7) | 1.60 (1.01, 2.56)** | 5029 (6) | 2.62 (1.08, 6.39)** + | ||
| Continuous | 1775 (13) | 0.14 (0.09, 0.19)** | 1428 (9) | 0.16 (0.09, 0.23)** | 563 (6) | 0.16 (0.08, 0.24)** | |||||
| Prenatal lead exposure | Dichotomous | 4360 (4) | 1.11 (0.70, 1.75) | 4360 (4) | 1.18 (0.67, 2.07) | ||||||
| Cumulative lead exposure | Dichotomous | 4944 (7) | 2.20 (1.36, 3.55)** | 2863 (5) | 2.00 (1.11, 3.59)** | 4044 (4) | 2.11 (1.14, 3.91)** | ||||
| Mercury exposure | Dichotomous | 659,523 (10) | 1.11 (0.94, 1.32)+ | 635,707 (7) | 1.15 (0.93, 1.44)+ | 8199 (3) | 1.32 (0.78, 2.25) | ||||
| Continuous | 17,062 (6) | 0.02 (0.00, 0.04)** | 887 (4) | 0.03 (−0.04, 0.10) | |||||||
| Prenatal mercury exposure | Continuous | 16,979 (5) | 0.02 (0.00, 0.04)** | ||||||||
| Cumulative mercury exposure | Dichotomous | 643,282 (7) | 1.10 (0.91, 1.32)+ | ||||||||
| Organophosphate exposure | Dichotomous | 1811 (4) | 2.48 (0.76, 8.15)+ | 863 (3) | 4.15 (0.66, 26.14)+ | ||||||
| Continuous | 692 (4) | 0.11 (0.03, 0.19)** | |||||||||
| Polychlorinated biphenyl exposure | Dichotomous | 2050 (3) | 1.24 (0.56, 2.75) | ||||||||
| Continuous | 2940 (9) | 0.05 (−0.01, 0.11)+ | 2940(9) | 0.08 (0.02, 0.14)** + | |||||||
ES = effect size (odds ratio (OR) for dichotomous outcomes and correlation coefficient (CC) for continuous outcomes); 95% CI = 95% confidence interval
Effect size significant at p < 0.05. Plus sign (+) indicates tests of heterogeneity statistically significant at p < 0.05
When not otherwise specified, exposure may include studies from either prenatal period or childhood
The number of studies included, overall sample size, and summary statistics of each random-effects meta-analysis performed are presented in Table 2. Forest plots depicting the per-study effect size and confidence interval, in addition to the estimated common effect size and confidence interval are provided in the Supplemental Materials (Supplemental Figs. S1–S33). Specific findings for each exposure should be interpreted within the context of studies specifically included in the current meta-analyses given our focused inclusion criteria.
Specific Findings
Lead
Childhood lead exposure was significantly associated with ADHD overall for both dichotomous (OR = 1.83, 95% CI: 1.38, 2.41) and continuous (CC = 0.14, 95% CI: 0.09, 0.19) reported outcome measures, as well as with measures of ADHD symptoms only (OR = 1.79, 95% CI: 1.17, 2.75). Childhood lead exposure was found to have statistically significant correlations with inattention (CC = 0.16, 95% CI: 0.09, 0.23) and with hyperactivity/impulsivity (CC = 0.16, 95% CI: 0.08, 0.24) based on analyses of continuous outcomes, as well as dichotomous outcomes (inattention OR: 1.60, 95% CI: 1.01, 2.56; hyperactivity/impulsivity OR: 2.62 95% CI: 1.08; 6.39). However, effect sizes for dichotomous measures of hyperactivity/impulsivity also showed significant heterogeneity (Q5 = 24.08, p = 0.0002). Cumulative lead exposure was significantly associated with ADHD overall (OR = 2.20, 95% CI: 1.36, 3.55), inattention (OR = 2.00, 95% CI: 1.11, 3.59), and hyperactivity/impulsivity (OR = 2.11, 95% CI: 1.14, 3.91). Prenatal lead exposure was not significantly associated with ADHD or any ADHD symptomology.
We also conducted sensitivity analysis for both continuous and dichotomous reported outcome measures of ADHD overall and association with childhood lead exposure, in which we excluded effect sizes that used less precise exposure measurements (e.g., X-ray flourescence spectroscopy of tibia). Sensitivity analysis of ADHD overall with continuous outcomes yielded the same CC and 95% CI and did not alter non-significant results of heterogeneity testing. For the sensitivity analysis of ADHD overall with dichotomous reported outcome measures, the effect size was slightly attenuated, but the association between lead exposure and ADHD remained significant and heterogeneity remained not statistically significant (data not shown). Given the change over time in the suggested cutoffs to define “elevated” lead exposure (Chandran & Cataldo, 2010; United States Centers for Disease Control and Prevention, 2021a), we conducted additional sensitivity tests to understand the influence of that change on presented results. There were five studies of lead that conducted analyses contingent upon a dated blood lead reference value (e.g., comparing ADHD outcomes among children with more or less than 40 mcg/dl blood lead level; Burns et al., 1999; Chandramouli et al., 2009; Davis et al., 2004; Landrigan et al., 1975; Rummo et al., 1979). Of the seven analyses in which these analyses appear (see Supplemental Figs. S4, S5, S6, S7, S11, S15, S16), the pattern of results remained significant for five when removing these five studies. For the remaining two analyses (ADHD overall with dichotomous reported outcome measures and without imprecise measures and dichotomous inattentive symptoms), the lower bound of the 95% CI for the odds ratio included 1.00, so those were no longer considered statistically significant (data not shown).
Mercury
Mercury exposure during childhood was not significantly related to ADHD overall when dichotomous outcomes were considered (and there was significant heterogeneity in these effect sizes; Q(9) = 21.42, p = 0.011), but it was correlated with ADHD overall for continuous outcome measures (CC = 0.02, 95% CI:0.00, 0.04). There was no significant association between mercury exposure and measures of diagnosis only, or between inattention or hyperactivity/impulsivity. As with the overall ADHD analysis with dichotomous outcomes, there was significant heterogeneity in diagnosis-only effect sizes (Q(6) = 19.89, p = 0.0029). Prenatal exposure to mercury was significantly correlated with ADHD overall (CC = 0.02, 95% CI: 0.00, 0.04). Cumulative mercury exposure was not associated with ADHD overall and showed significant heterogeneity in effect sizes (Q(6) = 17.85, p = 0.0066).
Given the retractions of other papers by D. Geier and M. Geier (Kern et al., 2017), we also conducted sensitivity analyses for the analyses of the association between mercury and ADHD that included articles by these authors (ADHD overall with dichotomous reported outcome measures, dichotomous measures of diagnosis, and cumulative mercury and ADHD overall with dichotomous reported outcome measures), for which we excluded three studies by these authors (Geier & Geier, 2005; Geier et al., 2014; Young et al., 2008). These sensitivity analyses still yielded non-statistically significant results (in agreement with the overall findings) and brought the summary ORs closer to the null. Furthermore, the effect sizes from the studies included in these analyses no longer displayed signficant heterogeneity once these three studies were excluded (see Supplemental Figs. S23, S24, S27 compared to S18, S19, S26).
Organophosphates
Organophosphate exposure was not significantly associated with dichotomous measures of ADHD overall or inattention, although it was associated with continuous measures of ADHD overall (CC = 0.11, 95% CI: 0.03, 0.19). The effect sizes based on dichotomous reported outcome measures for associations with ADHD overall and with inattention both showed significant heterogeneity (Q(3) = 8.29, p = 0.040, Q(2) = 7.15, p = 0.028, respectively).
Polychlorinated Biphenyls
Exposure to PCBs was not significantly associated with ADHD overall either for dichotomous or continuous reported outcome measures. However, childhood exposure to PCBs was significantly associated with inattention only (CC = 0.08, 95% CI: 0.02, 0.14). Effect sizes based on continuous outcomes for both ADHD overall and for inattention only showed significant heterogeneity (Q(8) = 17.50, p = 0.0253, Q(8) = 16.96, p = 0.0305, respectively).
Anesthesia, Cadmium, and Hexachlorobenzene
There were no statistically significant findings associated with any of these exposures, nor was there any statistically significant heterogeneity.
Discussion
Our findings suggest that certain chemicals may contribute to the development of ADHD in children. Lead was the chemical exposure with the largest number of publications that met this study’s inclusion criteria, and it was also the exposure with the largest and most consistent associations with the development of ADHD or ADHD symptomatology. Cumulative exposure and exposure to lead during childhood only—but not if exposed during the prenatal period only—was found to be associated with later ADHD and ADHD symptomatology. Other chemical exposures were positively related to ADHD and ADHD symptomatology, though other statistically significant associations were limited to prenatal and childhood mercury exposure and childhood organophosphate and PCB exposure. Although these latter associations were statistically significant, many effect sizes were small and not consistent across all analyses for each exposure. Anesthesia, cadmium, and hexachlorobenzene exposure were all not significantly associated with ADHD and ADHD symptomatology, though analyses for these exposures were based on five or fewer studies. Given limited available evidence on some exposures, non-significant findings cannot be assumed to indicate safety of these exposures.
Potential Mechanisms and Alignment with Prior Findings
Our findings regarding the consistent, statistically significant association between lead exposure and ADHD across most sub-analyses align with other meta-analytic evidence of ADHD risk in children exposed to lead (Nilsen & Tulve, 2020). The most pronounced associations with lead were seen in the studies that measured exposure via blood samples, which is the usual clinical practice for assessing lead exposure. Lead has been found to cause damage to the prefrontal cortex (Sanders et al., 2009), an area of the brain in which poor structure and function of circuitry is associated with ADHD (Arnsten, 2009). Nilsen and Tulve (2020) have also proposed a mechanism in the serotonin pathway related to N-methyl-d-aspartate (NMDA) receptors, an upstream regulator of monoamine oxidase A (MAO-A) (Büsselberg, 1995; Neal & Guilarte, 2010), which has been linked to ADHD (Roohi et al., 2009).
Although mercury exposure has previously been linked to ADHD via meta-analytic findings (Nilsen & Tulve, 2020; Yoshimasu et al., 2014), our study was able to separately analyze prenatal and cumulative exposures. Our findings were mixed. When ADHD is measured continuously, both prenatal mercury exposure and childhood mercury exposure were associated with ADHD overall. Although the other analyses of the association of mercury exposure and ADHD outcomes were not significant, the effect sizes were all greater than one for dichotomous outcomes and all positive for continuous outcomes. In addition, the overall and diagnosis dichotomous outcomes analyses both evidenced significant heterogeneity in effect sizes from included studies, and analyses of inattention and hyperactivity included only four and three studies, respectively. As ADHD has been found to be associated with disrupted dopamine neurotransmission (Klein et al., 2019; Kollins & Adcock, 2014), a potential mechanism by which mercury could exert effects is via decreasing dopamine levels in certain areas of the brain, which has been demonstrated in a mouse model (Bourdineaud et al., 2011). As with lead, a serotonergic mechanism of action relating mercury to interference with NMDA receptors and regulation of MAO-A has also been proposed (Nilsen & Tulve, 2020).
Negative neurodevelopmental impacts of organophosphate exposure have been documented (Muñoz-Quezada et al., 2013) and organophosphates have previously been meta-analytically examined in relation to ADHD in groupings with other organic contaminants, such as PCBs (Nilsen & Tulve, 2020). When assessing effects of organophosphates independently from other compounds, we found an association between exposure to organophosphates and ADHD when examining continuous outcomes. The dichotomous outcome measures were not significant; although these analyses resulted in effect sizes greater than one, the confidence intervals were wide and there was significant heterogeneity in effect sizes of included studies. The most documented mechanism of organophosphate toxicity is via inhibition of acetylcholinesterase (Naughton & Terry, 2018). ADHD has been associated with cholinergic dysregulation (Coccini et al., 2009; English et al., 2009; Johansson et al., 2013). Similar to lead, organophosphates may also impact the serotonergic system (Slotkin & Seidler, 2008).
Our PCB findings also provide meta-analytic evidence of a link between exposure to this class of chemicals and symptoms of inattention. However, the significant association we detected when analyzing continuous outcomes should be interpreted with caution, as the effect sizes included in this analysis evidenced significant heterogeneity. In particular, the effect size from Ethier et al. (2015), which showed the greatest positive association with a CC of 0.60 (95% CI: 0.28, 0.80), appeared to be an outlier in this effect size grouping. Interestingly, Ethier et al. (2015) assessed a greater number of PCB congeners (differentiated by the number and location of attached chlorine atoms) compared to other studies included in this analysis, perhaps better reflecting the exposure mixture people are typically exposed to in the environment (United States Environmental Protection Agency, 2022a). Effect sizes for the association between PCBs and measures of ADHD overall were suggestive of increased odds of ADHD, but not significant. The analytic results for dichotomous measures of ADHD overall were based on three studies and had a wide confidence interval, while the effect sizes for continuous measures of ADHD overall displayed significant heterogeneity. Prior evidence (Bemis & Seegal, 2004) suggests that PCB exposure could also potentially be influencing development of inattention symptomology via dysregulation of dopaminergic activity. However, one major challenge to studying PCBs is the fact that they are a broad class of chemical compounds, with many different congeners and differential toxic effects depending on the congener (Giesy & Kannan, 1998; Wolff et al., 1997).
Exposure Routes and Populations with Greatest Risk of Exposure
Some populations may be at higher risk for exposure to these harmful chemicals. For example, many homes in low-income areas in the USA were built before the 1978 ban on use of lead paint (United States Centers for Disease Control and Prevention, 2021b), and lead-based paint and lead-contaminated dust in older buildings are still common sources of lead exposure in children (United States Centers for Disease Control & Prevention, 2013). This risk is further exacerbated for these communities by the fact that low-income areas are also more likely to have lead-containing faucets, pipes, and plumbing fixtures which can give rise to lead exposure (United States Centers for Disease Control and Prevention, 2021b). The United States Environmental Protection Agency estimates that drinking water typically accounts for 20% of an individual’s total lead exposure but goes up to 40–60% of total exposure from drinking water for infants consuming mostly mixed formula (United States Environmental Protection Agency, 2022b). A well-known example of inequitable community exposure to lead is the water crisis that occurred in Flint, Michigan—a city where over half the population is Black or African American and the poverty rate is almost 40% (World Population Review, 2022)—when the city’s drinking water became contaminated with lead in 2014 (Kennedy et al., 2016).
Mercury is found in air, water, and soil and exists in three forms: elemental/metallic, inorganic, and organic. Routes of exposure to mercury vary depending on the form of mercury. Elemental mercury is used in some thermometers, dental amalgams, fluorescent light bulbs, and in mining and other industrial processes. Children can be exposed by swallowing mercury from a broken thermometer or when they inhale vaporized elemental mercury. Inorganic mercury is formed when mercury combines with other elements such as sulfur, to form salts. This can occur naturally in the environment, but these salts are used for industrial purposes as well (United States Environmental Protection Agency, 2021a). Certain microorganisms in water can also convert other forms of mercury to form an organic mercury compound called methylmercury, which is known to accumulate in aquatic food chains (Mason et al., 1995). The main way that people are exposed to mercury is via consumption of methylmercury-contaminated fish and shellfish (United States Environmental Protection Agency, 2021a). Methylmercury exposure can also occur through maternal consumption of contaminated fish and shellfish, as mercury is able to cross the placenta to affect fetal development (Bose-O’Reilly et al., 2010). Thimerosal is an organic mercury compound that is used in small amounts as a preservative in some medications and vaccines (United States Food & Drug Administration, 2018). Vaccines containing thimerosal were deemed safe for use by the US Food and Drug Administration (Ball et al., 2001) and there is no evidence that low doses of thimerosal in vaccines cause any harm aside from minor reactions such as redness and swelling at the injection site (United States Centers for Disease Control and Prevention, 2020). However, the FDA Modernization Act of 1997 directed the agency to identify drugs and foods that contain intentionally introduced mercury compounds. As a precautionary measure given limited ability to reduce environmental exposures to mercury, the FDA sent a letter to all licensed vaccine manufacturers on July 1st, 1999, soliciting their plan to remove thimerosal from all vaccines licensed in the USA (United States Food & Drug Administration, 2018). Today, all routinely recommended vaccines for children ages 6 years and younger are available in non-thimerosal containing formulations (United States Food & Drug Administration, 2018).
Among our analyses of mercury, statistically significant meta-analytic effect sizes did not arise from analyses which included studies examining thimerosal exposure. In contrast, analyses yielding significant effect sizes did include four studies that had either directly or indirectly (via choosing a study sample known to consume large amounts of marine mammals and fish) examined fish consumption in relation to mercury exposure and development of ADHD (Boucher et al., 2012; Ethier et al., 2015; Myers et al., 2000; Sagiv et al., 2012). These findings are important in highlighting that populations that eat greater quantities of fish than the general population, and therefore are at greater risk of exposure to higher concentrations of methylmercury (United States Environmental Protection Agency, 2021b), may also have greater risk of ADHD development in children. In particular, Native Americans’ and Alaskan Natives’ high subsistence fishing rates compared to other groups may make them more vulnerable to mercury exposure (Gochfeld & Burger, 2011).
Children can be exposed to organophosphate pesticides by dermal absorption, ingestion, or breathing in particles (Eskenazi et al., 1999). As exposure to organophosphates can occur via consumption of contaminated food (Eskenazi et al., 1999), families who cannot afford to buy more costly organic food options may be at increased risk of exposure (Curl et al., 2015; Mie et al., 2017). There is also evidence of different exposure pathways and higher levels of exposure to organophosphates in children living in agricultural regions (Lu et al., 2004; Rohitrattana et al., 2014). The issue of organophosphate use as a pesticide also goes beyond agricultural use. Children in low-income settings can also be at an increased risk of exposure as a result of frequent use of pesticides (including use of restricted pesticides) in and around the home due to severe pest infestations as a result of older, poorly maintained available housing in low-income settings. The often-small living spaces in lower income settings can further exacerbate this issue by putting children in close proximity to the treated areas (Julien et al., 2008).
PCBs are a broad class of commercially produced synthetic chemicals that were manufactured until 1979, when their production was banned in the USA. However, PCBs are present in a variety of products made before the ban, including electrical equipment, cable and thermal insulation, adhesives, and floor finish, and many current issues with PCB exposure relate to the persistence of PCBs in the environment (United States Environmental Protection Agency, 2022). Sources of exposure include eating contaminated food (e.g., fish caught in contaminated bodies of water), breathing air near waste sites, or drinking contaminated water (United States Centers for Disease Control & Prevention, 2014). Given these exposure routes, subsistence fishers who consume larger amounts of locally caught fish may be at risk for high exposure to PCBs and children may be exposed in utero if mothers eat large amounts of contaminated fish during pregnancy. Children may also be exposed to PCBs dermally if older electrical appliances, such as television sets, heat up while operating, and leak small amounts of PCBs. Families that live near incinerators or other PCB-disposal facilities are also at an increased risk of exposure (Agency for Toxic Substances & Disease Registry, 2014). Toxic waste sites are often located near communities with higher minority populations (Hipp & Lakon, 2010; Kramar et al., 2018), putting these groups at an increased risk of experiencing health effects associated with chemicals found in these sites.
ADHD has also been associated with poor socioeconomic status. US national survey data suggests that children from families with a household income below the Federal Poverty Level are more likely to be diagnosed with ADHD than children from families with a household income that is at least twice as high as the Federal Poverty Level (Danielson et al., 2018). Prior countrywide population studies in Denmark have also found associations between ADHD and low social class (Østergaard et al., 2016), and between ADHD and indicators of socioeconomic status, such as low parental education level, parental unemployment, and parental relative poverty (Keilow et al., 2020). Concern for vulnerable populations with increased exposure to these harmful environmental chemicals, as well as higher risk for development of ADHD, further highlights the need for a closer examination of chemical risk factors in the etiology of ADHD. Furthermore, lower income families with children diagnosed with ADHD might face the additional burden of difficulty with access and affordability of treatment.
Policy and Prevention
Given existing awareness of the threat to health posed by these chemicals beyond ADHD risk (Alexander & Delves, 1972; Ratcliffe et al., 1996; Rogan & Gladen, 1992; Steenland, 1996), current policies and regulations are in place to mitigate exposure; for example, lead in paint and leaded gasoline has been discontinued (United States Energy Information Administration, 2020; United States Centers for Disease Control and Prevention, 2022), production of PCBs has been banned (United States Environmental Protection Agency, 2022), and the use of the organophosphate diazinon has been prohibited (United States Environmental Protection Agency, 2016). However, policies and regulations in the USA have continued to change and adapt to the most current science over time, and there is not yet a full understanding of the extent to which these and other chemicals may harm children. For example, PCBs were in use until 1979 and the diazinon ban only went into effect within the past two decades. The findings of this current study suggest a continued value in examining new scientific findings regarding the impact of potentially harmful exposures and in maintaining an openness to modifying regulations as appropriate. Given that numerous sources of lead remain in the environment (United States Centers for Disease Control & Prevention, 2013, United States Centers for Disease Control and Prevention, 2021b, United States Environmental Protection Agency, 2022b) and that continued persistent environmental contamination from disposal of already banned substances is also an ongoing issue (Agency for Toxic Substances & Disease Registry, 2014), our findings highlight the importance of public health prevention programs that address structural factors that contribute to exposure. An example of a currently existing program that aims to prevent children from being exposed to harmful chemicals is the Agency for Toxic Substance and Disease Registry’s development of a list of site activities that should be given special attention when establishing early care and education sites (Agency for Toxic Substances & Disease Registry, 2018). Public health level initiatives such as these can be a valuable tool in mitigating children’s risk of exposure to chemicals in the environment. Furthermore, our findings suggest that early identification of perinatal exposure to certain chemicals could inform future screening efforts for obstetricians and pediatricians, as early identification of risk may allow for improved early referral for intervention services for exposed children, thus improving downstream outcomes for these children (Jones et al., 2008; McGoey et al., 2002; Sonuga-Barke et al., 2011).
Strengths and Limitations
The current meta-analysis has several strengths. First, as mentioned above, all included studies were those in which the exposure began at least 6 months prior to measurement of ADHD, to focus analyses on those exposures that might represent true risk for ADHD based on temporality. Second, we examined not just diagnosed ADHD but also ADHD symptomology, allowing us to assess associations across a spectrum of symptoms, rather than relying on a diagnostic cutoff. Third, our meta-analysis included data from 17 different countries, potentially providing greater generalizability of our findings. Lastly, and importantly, our application of similar meta-analytic methods across analyses of different chemical risk factors, in this paper, and across different non-chemical risk factors, in this group of papers (Bitsko et al., 2022a, 2022b; Claussen et al., 2022; Maher et al., this issue; Robinson et al., 2022; So et al., 2022) allows for comparison across several risk factors and for a more wholistic understanding of ADHD etiology.
However, our study is also subject to several limitations. First, despite the value of the international diversity of our sample (i.e., 17 countries), these results may not be representative of any specific country and may not be generalizable beyond the populations included in these studies. Notably, most studies were from moderate- to high-resource countries, so our sample and respective findings may not be generalizable to lower income countries. Second, to synthesize evidence on a wide range of risk factors, our inclusion criteria allowed for a variety of different exposure and outcome measures which may have influenced the results. For example, measures of ADHD included objective measures of symptoms of ADHD, as well as parent and teacher report measures, and clinical assessment. We observed significant heterogeneity in effect sizes for several risk factors, indicating variability of findings among the individual studies included in those analyses. For example, the analyses examining impact of childhood organophosphate exposure on dichotomized ADHD overall consisted of 4 effect sizes, ranging from 0.86 (Dalsager et al., 2019) to 10.29 (Ruckart et al., 2004) each examining exposure to different organophosphate compounds. Future research could investigate correlates of larger and smaller effects within these and other studies of the same exposures. In addition, due to the wide range of risk factors, we focused on overall effect sizes and could not investigate covariates, effect modifiers, or mediators, including sex, comorbid conditions, gene-environment interactions, or interactions between different risk factors, which could be important future studies for risk factors with significant findings in this meta-analysis. Additionally, we were only able to assess the effect of timing of exposure for lead and mercury, due to a limited number of studies for other risk factors. Future research examining timing of exposure could help to elucidate sensitive periods. Third, although our literature search intended to capture all relevant articles, our findings are limited to published studies and are not comprehensive of all potential chemical risk factors. For example, fewer than three studies on brominated flame retardants and perfluorinated compounds (PFCs/PFAs) were identified for inclusion in our original analysis and therefore, these chemicals were not included in the current study. Given the breadth of risk factors and length of the manuscripts, we were unable to include assessment of potential publication bias. However, we have no reason to suspect different bias risk across the risk factors or the six papers. Finally, although we only included studies where the risk factor was measured before ADHD was measured, we cannot rule out the possibility that ADHD symptoms were present prior to exposure but were just not yet assessed or recognized, and that ADHD-associated behaviors may have increased the risk of exposure. In addition, included studies varied considerably in both the risk factor measurement, and the outcome measurements. Thus, caution is warranted when comparing our findings to meta-analyses that used different inclusion criteria.
Conclusions
Our findings highlight associations between specific chemical exposures in utero and during childhood and the risk of developing ADHD. As previous individual studies on these exposures have yielded inconsistent findings, this meta-analytic evidence addresses gaps in the literature by summarizing across studies where the exposure occurred before ADHD was measured. Furthermore, similarity of methods across examined risk factors allows for comparability and provides a broader view of ADHD etiology. However, given our findings related to exposure to mercury, organophosphates, and PCBs, further research may be helpful to better characterize these relationships. Many of our effect sizes were small, which is consistent with the literature indicating that many genetic and environmental factors contribute to ADHD (Faraone et al., 2021). The inequitable distribution of the exposures examined in this study (Ashrap et al., 2021; Gochfeld & Burger, 2011; Hipp & Lakon, 2010; Julien et al., 2008; Kennedy et al., 2016; Kramar et al., 2018) and new evidence of the modifying effect of psychosocial status on the developmental impact of certain metals and metalloids found in maternal blood (Ashrap et al., 2021) also support further research aimed at elucidating potential psychosocial and demographic moderators of effect sizes in order to develop a more nuanced understanding of how various factors may contribute to the development of ADHD; the body of research in this area has been growing in recent years (Ashrap et al., 2021; Eick et al., 2022; Li et al., 2022; Nilsen & Tulve, 2020) and may be critical in elucidating unknown interactions that can inform prevention (Bellinger, 2000). Furthermore, our findings support existing regulations of certain chemicals, may inform future regulatory decisions, and add to the constantly evolving literature on chemicals that are potentially harmful to children.
Supplementary Material
Funding
This project was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Centers for Disease Control and Prevention. The work presented here was completed through an interagency agreement between the Centers for Disease Control and Prevention and the General Service Administration (13-FED-1303304), under GSA Order Number ID04130157 to Gryphon Scientific, LLC, titled “Identifying Public Health Strategies with Potential for Reducing Risk for Attention Deficit and Hyperactivity Disorder.”
Footnotes
Conflict of Interest The authors declare no competing interests.
Ethical Approval Not applicable. This study includes analyses of data previously published in the literature.
Disclaimer The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This study includes analyses of data previously published in the literature.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11121-023-01601-6.
References
- Agency for Toxic Substances and Disease Registry. (2014). Who is at risk of exposure to PCBs? https://www.atsdr.cdc.gov/csem/polychlorinated-biphenyls/who_risk.html#:~:text=Populations%20with%20increased%20exposure%20to%20PCBs%20include%20Children,nursing%3B%20Farm%20families%20who%20eat%20PCB-contaminated%20food%3B%20and. Accessed 02/09/22.
- Agency for Toxic Substances and Disease Registry. (2018). Choose safe places for early care and education. https://www.atsdr.cdc.gov/safeplacesforECE/cspece_guidance/former_use.html. Accessed 06/23/22.
- Alexander FW, & Delves HT (1972). Deaths from acute lead poisoning. Archives of Disease in Childhood, 47, 3. https://adc.bmj.com/content/archdischild/47/253/446.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). American Psychiatric Publishing Inc. [Google Scholar]
- Andrews N, Miller E, Grant A, Stowe J, Osborne V, & Taylor B (2004). Thimerosal exposure in infants and developmental disorders: A retrospective cohort study in the United kingdom does not support a causal association. Pediatrics, 114(3), 584–591. 10.1542/peds.2003-1177-L [DOI] [PubMed] [Google Scholar]
- Arnsten AFT (2009). The emerging neurobiology of attention deficit hyperactivity disorder: The key role of the prefrontal association cortex. The Journal of Pediatrics, 154(5), I–S43. 10.1016/j.jpeds.2009.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Aker A, Watkins DJ, Mukherjee B, Rosario-Pabón Z, Vélez-Vega CM, et al. (2021). Psychosocial status modifies the effect of maternal blood metal and metalloid concentrations on birth outcomes. Environment International, 149, 106418. 10.1016/j.envint.2021.106418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad DA, Bellinger DC, Ryan LM, & Woodruff TJ (2007). Dose–response relationship of prenatal mercury exposure and IQ: An integrative analysis of epidemiologic data. Environmental Health Perspectives, 115(4), 7. 10.1289/ehp.9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LK, Ball R, & Pratt RD (2001). An assessment of thimerosal use in childhood vaccines. Pediatrics, 107(5), 1147–1154. 10.1542/peds.107.5.1147 [DOI] [PubMed] [Google Scholar]
- Bellinger DC (2000). Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicology and Teratology, 22(1), 133–140. 10.1016/S0892-0362(99)00053-7 [DOI] [PubMed] [Google Scholar]
- Bellinger D, Needleman HL, Bromfield R, & Mintz M (1984). A followup study of the academic attainment and classroom behavior of children with elevated dentine lead levels. Biological Trace Element Research, 6(3), 207–223. 10.1007/BF02917507 [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Allred E, & Rabinowitz M (1994). Pre- and postnatal lead exposure and behavior problems in school-aged children. Environmental Research, 66(1), 12–30. 10.1006/enrs.1994.1041 [DOI] [PubMed] [Google Scholar]
- Bemis JC, & Seegal RF (2004). PCB-Induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicological Sciences, 80(2), 288–295. 10.1093/toxsci/kfh153 [DOI] [PubMed] [Google Scholar]
- Benetou-Marantidou A, Nakou S, & Micheloyannis J (1988). Neurobehavioral estimation of children with life-long increased lead exposure. Archives of Environmental Health, 43(6), 392–395. 10.1080/00039896.1988.9935856 [DOI] [PubMed] [Google Scholar]
- Berlin JA, Laird NM, Sacks HS, & Chalmers TC (1989). A comparison of statistical methods for combining event rates from clinical trials. Statistics in Medicine, 8(2), 141–151. 10.1002/sim.4780080202 [DOI] [PubMed] [Google Scholar]
- Bose-O’Reilly S, McCarty KM, Steckling N, & Lettmeier B (2010). Mercury exposure and children’s health. Current Problems in Pediatric and Adolescent Health Care, 40(8), 186–215. 10.1016/j.cppeds.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. (2022a). Mental health surveillance among children - United States, 2013–2019. Morbidity and Mortality Weekly Report Suppement, 71(2), 1–42. 10.15585/mmwr.su7102a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsko RH, Holbrook JR, O’Masta B, Maher B, Cerles A, Saadeh K, et al. (2022b). A systematic review and meta-analysis of prenatal, birth, and postnatal factors associated with attention-deficit/hyperactivity disorder. Prevention Science. 10.1007/s11121-022-01359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, et al. (2012). Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspectives, 120(10), 1456–1461. 10.1289/ehp.1204976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdineaud J-P, Fujimura M, Laclau M, Sawada M, & Yasutake A (2011). Deleterious effects in mice of fish-associated methylmercury contained in a diet mimicking the Western populations’ average fish consumption. Environment International, 37(2), 303–313. 10.1016/j.envint.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Burns J, Baghurst P, Sawyer M, McMichael A, & Tong S (1999). Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years. The Port Pirie Cohort Study. American Journal of Epidemiology, 149(8), 740–749. 10.1093/oxfordjournals.aje.a009883 [DOI] [PubMed] [Google Scholar]
- Büsselberg D (1995). Calcium channels as target sites of heavy metals. Toxicology Letters, 82–83, 255–261. 10.1016/0378-4274(95)03559-1 [DOI] [PubMed] [Google Scholar]
- Caspersen IH, Aase H, Biele G, Brantsæter AL, Haugen M, Kvalem HE, et al. (2016). The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environment International, 94, 649–660. 10.1016/j.envint.2016.06.033 [DOI] [PubMed] [Google Scholar]
- Chandramouli K, Steer CD, Ellis M, & Emond AM (2009). Effects of early childhood lead exposure on academic performance and behaviour of school age children. Archives of Disease in Childhood, 94(11), 844–848. 10.1136/adc.2008.149955 [DOI] [PubMed] [Google Scholar]
- Chandran L, & Cataldo R (2010). Lead poisoning: Basics and new developments. Pediatrics in Review, 31(10), 399–406. 10.1542/pir.31-10-399 [DOI] [PubMed] [Google Scholar]
- Ciesielski T, Weuve J, Bellinger D, Schwartz J, Lanphear B, & Wright R (2012). Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environmental Health Perspectives, 120(5), 758–763. 10.1289/ehp.1104152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen AH, Holbrook JR, Hutchins HJ, Robinson LR, Bloomfield J, Meng L, et al. (2022). All in the family? A systematic review and meta-analysis of parenting and family environment as risk factors for attention-deficit/hyperactivity disorder (ADHD) in children. Prevention Science. 10.1007/s11121-022-01358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccini T, Crevani A, Rossi G, Assandri F, Balottin U, Nardo RD, et al. (2009). Reduced platelet monoamine oxidase type B activity and lymphocyte muscarinic receptor binding in unmedicated children with attention deficit hyperactivity disorder. Biomarkers, 14(7), 513–522. 10.3109/13547500903144436 [DOI] [PubMed] [Google Scholar]
- Curl CL, Beresford SAA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. (2015). Estimating pesticide exposure from dietary intake and organic food choices: The multi-ethnic study of atherosclerosis (MESA). Environmental Health Perspectives, 123(5), 475–483. 10.1289/ehp.1408197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsager L, Fage-Larsen B, Bilenberg N, Jensen TK, Nielsen F, Kyhl HB, et al. (2019). Maternal urinary concentrations of pyrethroid and chlorpyrifos metabolites and attention deficit hyperactivity disorder (ADHD) symptoms in 2–4-year-old children from the Odense Child Cohort. Environ Research, 176, 108533. 10.1016/j.envres.2019.108533 [DOI] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, & Blumberg SJ (2018). Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. Journal of Clinical Child and Adolescent Psychology, 47(2), 199–212. 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Chang F, Burns B, Robinson J, & Dossett D (2004). Lead exposure and attention regulation in children living in poverty. Developmental Medicine and Child Neurology, 46(12), 825–831. 10.1111/j.1469-8749.2004.tb00448.x [DOI] [PubMed] [Google Scholar]
- DiMaggio C, Sun LS, & Li G (2011). Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesthesia and Analgesia, 113(5), 1143–1151. 10.1213/ANE.0b013e3182147f42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Hoffman K, Keil AP, Engel SM, Stapleton HM, Goldman BD, et al. (2019). Prenatal exposure to organophosphate esters and behavioral development in young children in the Pregnancy, Infection, and Nutrition Study. NeuroToxicology, 73, 150–160. 10.1016/j.neuro.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick SM, Goin DE, Cushing L, DeMicco E, Smith S, Park J-S, et al. (2022). Joint effects of prenatal exposure to per- and poly-fluoroalkyl substances and psychosocial stressors on corticotropin-releasing hormone during pregnancy. Journal of Exposure Science & Environmental Epidemiology, 32(1), 27–36. 10.1038/s41370-021-00322-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English BA, Hahn MK, Gizer IR, Mazei-Robison M, Steele A, Kurnik DM, et al. (2009). Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. Journal of Neurodevelopmental Disorders, 1(4), 252–263. 10.1007/s11689-009-9033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine HE, Norman RE, Ferrari AJ, Chan GCK, Copeland WE, Whiteford HA, et al. (2016). Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 55(10), 841–850. 10.1016/j.jaac.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, & Castorina R (1999). Exposures of children to organophosphate pesticides and their potential adverse health effects. Environmental Health Perspectives, 107(Suppl 3), 409–419. 10.1289/ehp.99107s3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. (2007). Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental Health Perspectives, 115(5), 792–798. 10.1289/ehp.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier AA, Muckle G, Jacobson SW, Ayotte P, Jacobson JL, & Saint-Amour D (2015). Assessing new dimensions of attentional functions in children prenatally exposed to environmental contaminants using an adapted Posner paradigm. Neurotoxicolpgy and Teratology, 51, 27–34. 10.1016/j.ntt.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. (2021). The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neuroscience & Biobehavioral Reviews, 23(7), 519–529. 10.1016/j.neubiorev.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Fort M, Casas M, Caceres A, Guxens M, Gascon M, et al. (2014). Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology, 40, 16–22. [DOI] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sanchez BN, Barr DB, Panuwet P, Bellinger D, et al. (2014). Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: Distribution, temporal variability, and relationship with child attention and hyperactivity. International Journal of Hygiene and Environmental Health, 217(2–3), 405–12. 10.1016/j.ijheh.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, & Gilman RC (2011). Update on environmental risk factors for attention-deficit/hyperactivity disorder. Current Psychiatry Reports, 13(5), 333. 10.1007/s11920-011-0221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, & Geier MR (2005). A two-phased population epidemiological study of the safety of thimerosal-containing vaccines: a follow-up analysis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 11(4), CR160–CR170. http://www.ncbi.nlm.nih.gov/pubmed/15795695 [PubMed] [Google Scholar]
- Geier DA, Hooker BS, Kern JK, King PG, Sykes LK, & Geier MR (2014). A dose-response relationship between organic mercury exposure from thimerosal-containing vaccines and neurodevelopmental disorders. International Journal of Environmental Research and Public Health, 11(9), 9156–9170. 10.3390/ijerph110909156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, & Kannan K (1998). Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): Implications for risk assessment. Critical Reviews in Toxicology, 28(6), 511–569. 10.1080/10408449891344263 [DOI] [PubMed] [Google Scholar]
- Gochfeld M, & Burger J (2011). Disproportionate exposures in environmental justice and other populations: The importance of outliers. American Journal of Public Health, 101(Suppl 1), S53–S63. 10.2105/AJPH.2011.300121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad JK, Marcus DK, & Fulton JJ (2013). Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clinical Psychology Review, 33(3), 417–425. 10.1016/j.cpr.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Gould E (2009). Childhood lead poisoning: Conservative estimates of the social and economic benefits of lead hazard control. Environmental Health Perspectives, 117(7), 1162–1167. 10.1289/ehp.0800408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2006). Developmental neurotoxicity of industrial chemicals. The Lancet, 368(9553), 2167–2178. 10.1016/S0140-6736(06)69665-7 [DOI] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2014). Neurobehavioural effects of developmental toxicity. The Lancet Neurology, 13(3), 330–338. 10.1016/S1474-4422(13)70278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron J, Golding J, & the ALAPAC Study Team. (2004). Thimerosal exposure in infants and developmental disorders: A prospective cohort study in the United Kingdom does not support a causal association. Pediatrics, 114(3), 577–583. 10.1542/peds.2003-1176-L [DOI] [PubMed] [Google Scholar]
- Hipp JR, & Lakon CM (2010). Social disparities in health: Disproportionate toxicity proximity in minority communities over a decade. Health & Place, 16(4), 674–683. 10.1016/j.healthplace.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Jacobson JL, & Jacobson SW (2003). Prenatal exposure to polychlorinated biphenyls and attention at school age. The Journal of Pediatrics, 143(6), 780–788. 10.1067/S0022-3476(03)00577-8 [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Padgett RJ, Brumitt GA, & Billings RL (1992). Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Developmental Psychology, 28(2), 297–306. 10.1037/0012-1649.28.2.297 [DOI] [Google Scholar]
- Ji Y, Hong X, Wang G, Chatterjee N, Riley AW, Lee LC, et al. (2018). A prospective birth cohort study on early childhood lead levels and attention deficit hyperactivity disorder: New insight on sex differences. Journal of Pediatrics, 199, 124–131. e8. 10.1016/j.jpeds.2018.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J, Landgren M, Fernell E, Lewander T, & Venizelos N (2013). Decreased binding capacity (B max) of muscarinic acetylcholine receptors in fibroblasts from boys with attention-deficit/hyperactivity disorder (ADHD). ADHD Attention Deficit and Hyperactivity Disorders, 5(3), 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Daley D, Hutchings J, Bywater T, & Eames C (2008). Efficacy of the incredible years programme as an early intervention for children with conduct problems and ADHD: Long-term follow-up. Child: Care, Health and Development, 34(3), 380–390. 10.1111/j.1365-2214.2008.00817.x [DOI] [PubMed] [Google Scholar]
- Julien R, Adamkiewicz G, Levy JI, Bennett D, Nishioka M, & Spengler JD (2008). Pesticide loadings of select organophosphate and pyrethroid pesticides in urban public housing. Journal of Exposure Science & Environmental Epidemiology, 18(2), 167–174. 10.1038/sj.jes.7500576 [DOI] [PubMed] [Google Scholar]
- Kanluen S, & Gottlieb CA (1991). A clinical pathologic study of four adult cases of acute mercury inhalation toxicity. Archives of Pathology and Laboratory medicine, 115(1), 56–60. http://europepmc.org/abstract/MED/1987914 [PubMed] [Google Scholar]
- Keilow M, Wu C, & Obel C (2020). Cumulative social disadvantage and risk of attention deficit hyperactivity disorder: Results from a nationwide cohort study. SSM - Population Health, 10, 100548. 10.1016/j.ssmph.2020.100548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Yard E, Dignam T, Buchanan S, Condon S, Brown MJ, et al. (2016). Blood lead levels among children aged < 6 years — Flint, Michigan, 2013–2016. Morbidity and Mortality Weekly Report, 65(25), 650–654. https://www.jstor.org/stable/24858150 [DOI] [PubMed] [Google Scholar]
- Kern JK, Geier DA, Deth RC, Sykes LK, Hooker BS, Love JM, et al. (2017). RETRACTED ARTICLE: Systematic assessment of research on autism spectrum disorder and mercury reveals conflicts of interest and the need for transparency in autism research. Science and Engineering Ethics, 23(6), 1689–1690. 10.1007/s11948-015-9713-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketzer CR, Gallois C, Martinez AL, Ronde LA, & Schmitz M (2012). Is there an association between perinatal complications and attention-deficit/hyperactivity disorder-inattentive type in children and adolescents? Brazilian Journal of Psychiatry, 34(3), 321–328. 10.1016/j.rbp.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, & Correa RG (2019). Dopamine: Functions, signaling, and association with neurological diseases. Cellular and Molecular Neurobiology, 39(1), 31–59. 10.1007/s10571-018-0632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko WR, Liaw YP, Huang JY, Zhao DH, Chang HC, Ko PC, et al. (2014). Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: A nationwide, retrospective matched-cohort study. Paediatric Anaesthesia, 24(7), 741–8. 10.1111/pan.12371 [DOI] [PubMed] [Google Scholar]
- Kollins SH, & Adcock RA (2014). ADHD, Altered dopamine neurotransmission, and disrupted reinforcement processes: Implications for smoking and nicotine dependence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 52, 70–78. 10.1016/j.pnpbp.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar DE, Anderson A, Hilfer H, Branden K, & Gutrich JJ (2018). A spatially informed analysis of environmental justice: analyzing the effects of gerrymandering and the proximity of minority populations to U.S. superfund sites. Environmental Justice, 11(1), 29–39. 10.1089/env.2017.0031 [DOI] [Google Scholar]
- Kyriklaki A, Vafeiadi M, Kampouri M, Koutra K, Roumeliotaki T, Chalkiadaki G, et al. (2016). Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4 years of age: The Rhea mother-child cohort, Crete, Greece. Environment International, 97, 204–211. 10.1016/j.envint.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Landrigan P, Whitworth R, Baloh R, Staehling N, Barthel W, & Rosenblum B (1975). Neuropsychological dysfunction in children with chronic low-level lead absorption. The Lancet, 1(7909), 708–712. 10.1016/S0140-6736(75)91627-X [DOI] [PubMed] [Google Scholar]
- Lenters V, Iszatt N, Forns J, Čechová E, Kočan A, Legler J, et al. (2019). Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environment International, 125, 33–42. 10.1016/j.envint.2019.01.020 [DOI] [PubMed] [Google Scholar]
- Leviton A, Bellinger D, Allred E, Rabinowitz M, Needleman H, & Schoenbaum S (1993). Pre- and postnatal low-level lead exposure and children’s dysfunction in school. Environmental Research, 60(1), 30–43. 10.1006/enrs.1993.1003 [DOI] [PubMed] [Google Scholar]
- Li Z, Christensen GM, Lah JJ, Marcus M, Russell AG, Ebelt S, et al. (2022). Neighborhood characteristics as confounders and effect modifiers for the association between air pollution exposure and subjective cognitive functioning. Environmental Research, 212, 113221. 10.1016/j.envres.2022.113221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Kedan G, Fisker-Andersen J, Kissel JC, & Fenske RA (2004). Multipathway organophosphorus pesticide exposures of preschool children living in agricultural and nonagricultural communities. Environmental Research, 96(3), 283–289. 10.1016/j.envres.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Lygre GB, Aase H, Haug K, Lie SA, & Björkman L (2018). Prenatal exposure to dental amalgam and risk of symptoms of attention-deficit and hyperactivity disorder (ADHD). Community Dentistry and Oral Epidemiology, 46(5), 472–481. 10.1111/cdoe.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher B, Kaminski J, O’Masta B, Cerles A, Holbrook JR, Mahmooth Z et al. (this issue). A systematic meta-analysis of the relationship between exposure to parental substance use and attention-deficit/hyperactivity disorder. Prevention Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Reinfelder JR, & Morel FMM (1995). Bioaccumulation of mercury and methylmercury. Water, Air, and Soil Pollution, 80(1), 915–921. 10.1007/BF01189744 [DOI] [Google Scholar]
- McFarland MJ, Hauer ME, & Reuben A (2022). Half of US population exposed to adverse lead levels in early childhood. Proceedings of the National Academy of Sciences, 119(11), e2118631119. 10.1073/pnas.2118631119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoey KE, Eckert TL, & Dupaul GJ (2002). Early intervention for preschool-age children with ADHD: A literature review. Journal of Emotional and Behavioral Disorders, 10(1), 14–28. 10.1177/106342660201000103 [DOI] [Google Scholar]
- Mie A, Andersen HR, Gunnarsson S, Kahl J, Kesse-Guyot E, Rembiałkowska E, et al. (2017). Human health implications of organic food and organic agriculture: A comprehensive review. Environmental Health, 16(1), 111. 10.1186/s12940-017-0315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minder B, Das-Smaal E, Brand E, & Orlebeke J (1994). Exposure to lead and specific attentional problems in school children. Journal of Learning Disabilities, 27(6), 393–399. 10.1177/0022219494027006 [DOI] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. (2013). Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology, 39, 158–168. 10.1016/j.neuro.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Palumbo D, Shamlaye C, Cox C, Cernichiari E, et al. (2000). Secondary analysis from the Seychelles Child Development Study: The child behavior checklist. Environmental Research, 84(1), 12–19. 10.1006/enrs.2000.4085 [DOI] [PubMed] [Google Scholar]
- Naughton SX, & Terry AV Jr. (2018). Neurotoxicity in acute and repeated organophosphate exposure. Toxicology, 408, 101–112. 10.1016/j.tox.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, & Guilarte TR (2010). Molecular neurobiology of lead (Pb2+): Effects on synaptic function. Molecular Neurobiology, 42(3), 151–160. 10.1007/s12035-010-8146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, & Barrett P (1979). Deficits in psychologic and classroom performance of children with elevated dentine lead levels. The New England Journal of Medicine, 300(13), 689–695. 10.1056/NEJM197903293001301 [DOI] [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, & Allred EN (1990). The long-term effects of exposure to low doses of lead in childhood. New England Journal of Medicine, 322(2), 83–88. 10.1056/nejm199001113220203 [DOI] [PubMed] [Google Scholar]
- Needleman H, Riess J, Tobin M, Biesecker G, & Greenhouse J (1996). Bone lead levels and delinquent behavior. Journal of the American Medical Association, 275(5), 363–369. 10.1001/jama.1996.03530290033034 [DOI] [PubMed] [Google Scholar]
- Nicolescu R, Petcu C, Cordeanu A, Fabritius K, Schlumpf M, Krebs R, et al. (2010). Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: Performance and questionnaire data. Environmental Research, 110(5), 476–483. 10.1016/j.envres.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Nilsen FM, & Tulve NS (2020). A systematic review and meta-analysis examining the interrelationships between chemical and non-chemical stressors and inherent characteristics in children with ADHD. Environmental Research, 180, 108884. 10.1016/j.envres.2019.108884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard SD, Larsen JT, Dalsgaard S, Wilens TE, Mortensen PB, Agerbo E, et al. (2016). Predicting ADHD by assessment of Rutter’s indicators of adversity in infancy. PLoS ONE, 11(6), e0157352. 10.1371/journal.pone.0157352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fernandez C, Morales-Navas M, Guardia-Escote L, Garrido-Cárdenas JA, Colomina MT, Giménez E, et al. (2020). Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: A locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food and Chemical Toxicology, 135, 16. [DOI] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, et al. (2010). The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology, 31(1), 17–25. 10.1016/j.neuro.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Ratcliffe HE, Swanson GM, & Fischer LJ (1996). Human exposure to mercury: A critical assessment of the evidence of adverse health effects. Journal of Toxicology and Environmental Health, 49(3), 221–270. 10.1080/00984108.1996.11667600 [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. (2006). Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics, 118(6), e1845–e1859. 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fitó N, Torrent M, Carrizo D, Júlvez J, Grimalt JO, & Sunyer J (2007). Exposure to hexachlorobenzene during pregnancy and children’s social behavior at 4 years of age. Environmental Health Perspectives, 115(3), 447–450. 10.1289/ehp.9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, & Barone S (2000). Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives, 108(suppl 3), 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris M, Dietrich K, Succop P, Berger O, & Bornschein R (2004). Early exposure to lead and neuropsychological outcome in adolescence. Journal of the International Neuropsychological Society, 10(2), 261–270. 10.1017/S1355617704102154 [DOI] [PubMed] [Google Scholar]
- Robinson LR, Bitsko RH, O’Masta B, Holbrook JR, Ko J, Barry CM, et al. (2022). A systematic review and meta-analysis of parental depression, antidepressant usage, antisocial personality disorder, and stress and anxiety as risk factors for attention-deficit/hyperactivity disorder (ADHD) in Children. Prevention Science. 10.1007/s11121-022-01383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan WJ, & Gladen BC (1992). Neurotoxicology of PCBs and related compounds. Neurotoxicology, 13(1), 27–35. http://europepmc.org/abstract/MED/1508429 [PubMed] [Google Scholar]
- Rohitrattana J, Siriwong W, Tunsaringkarn T, Panuwet P, Ryan PB, Barr DB, et al. (2014). Organophosphate pesticide exposure in school-aged children living in rice and aquacultural farming regions of Thailand. Journal of Agromedicine, 19(4), 406–416. 10.1080/1059924X.2014.947457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohi J, DeVincent CJ, Hatchwell E, & Gadow KD (2009). Association of a monoamine oxidase-a gene promoter polymorphism with ADHD and anxiety in boys with autism spectrum disorder. Journal of Autism and Developmental Disorders, 39(1), 67–74. 10.1007/s10803-008-0600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL (2012). Epigenetics of neurobiology and behavior during development and adulthood. Developmental Psychobiology, 54(6), 590–597. 10.1002/dev.20550 [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Kakolewski K, Bove FJ, & Kaye WE (2004). Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environmental Health Perspectives, 112(1), 46–51. 10.1289/ehp.6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummo JH, Routh DK, Rummo NJ, & Brown JF (1979). Behavioral and neurological effects of symptomatic and asymptomatic lead exposure in children. Archives of Environmental Health, 34(2), 120–124. http://www.ncbi.nlm.nih.gov/pubmed/434933 [DOI] [PubMed] [Google Scholar]
- Sagiv S, Thurston S, Bellinger D, Amarasiriwardena C, & Korrick S (2012). Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Archives of Pediatrics & Adolescent Medicine, 166(12), 1123–1131. 10.1001/archpediatrics.2012.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, & Korrick SA (2010). Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. American Journal of Epidemiology, 171(5), 593–601. 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, & Tchounwou PB (2009). Neurotoxic effects and biomarkers of lead exposure: A review. Reviews on Environmental Health, 24(1), 15–45. 10.1515/reveh.2009.24.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N et al. (2013). Prenatal exposure to environmental contaminants and behavioural problems at age 7–8years. Environment International, 59, 225–231. http://ac.els-cdn.com/S0160412013001323/1-s2.0-S0160412013001323-main.pdf?_tid=82f1b568-c4eb-11e3-8c0c-00000aacb35e&acdnat=1397600334_1c82bc12d238834eaae94b7dacec00cf [DOI] [PubMed] [Google Scholar]
- Slotkin TA, & Seidler FJ (2008). Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicology and Applied Pharmacology, 233(2), 211–219. 10.1016/j.taap.2008.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M, Dziuban EJ, Pedati C, Holbrook JR, O’Masta B, Maher B et al. (2022). Attention-deficit/hyperactivity disorder and childhood physical health: A meta-analysis. Prevention Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Koerting J, Smith E, McCann DC, & Thompson M (2011). Early detection and intervention for attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics, 11(4), 557–563. 10.1586/ern.11.39 [DOI] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanić K, et al. (2012). Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clinic Proceedings, 87(2), 120–129. 10.1016/j.mayocp.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K (1996). Chronic neurological effects of organophosphate pesticides. BMJ, 312(7042), 1312. 10.1136/bmj.312.7042.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, & Halldorsson TI (2014). Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes–a prospective study with long-term follow-up. Environment International, 68, 41–8. 10.1016/j.envint.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Sun S, Kuja-Halkola R, Faraone SV, D’Onofrio BM, Dalsgaard S, Chang Z, & Larsson H (2019). Association of psychiatric comorbidity with the risk of premature death among children and adults with attention-deficit/hyperactivity disorder. JAMA Psychiatry, 76(11), 1141–1149. 10.1001/jamapsychiatry.2019.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists 138. (2021). ‘Reducing prenatal exposure to toxic environmental agents’ C. o. O. Practice The American College of Obstetricians and Gynecologists Opinion. 07/2021. Obstetrics and Gynecology, p. 15. Available at: https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2021/07/reducing-prenatal-exposure-to-toxic-environmental-agents.pdf [Google Scholar]
- Tsai C-J, Lee CT-C, Liang SH-Y, Tsai P-J, Chen VC-H, & Gossop M (2018). Risk of ADHD after multiple exposures to general anesthesia: A nationwide retrospective cohort study. Journal of Attention Disorders, 22(3), 229–239. 10.1177/1087054715587094 [DOI] [PubMed] [Google Scholar]
- Tuthill R (1996). Hair lead levels related to children’s classroom attention-deficit behavior. Archives of Environmental Health, 51(3), 214–220. 10.1080/00039896.1996.9936018 [DOI] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention. (2013). Factsheet: Lead. https://www.cdc.gov/biomonitoring/Lead_FactSheet.html. Accessed November 9 2021.
- United States Centers for Disease Control and Prevention. (2014). “Polychlorinated biphenyls-ToxFAQs’. 07/2014.https://www.atsdr.cdc.gov/toxfaqs/tfacts17.pdf#:~:text=Polychlorinated%20biphenyls%20%28PCBs%29%20are%20a%20mixture%20of%20individual,sites%20identified%20by%20the%20Environmental%20Protection%20Agency%20%28EPA%29. Accessed: February 9 2022.
- United States Centers for Disease Control and Prevention. (2020). Thimerosal and vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed July 25, 2022.
- United States Centers for Disease Control and Prevention. (2021a). Blood lead reference value. https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm. Accessed June 6th, 2022 2022.
- United States Centers for Disease Control and Prevention. (2021b). Populations at higher risk. https://www.cdc.gov/nceh/lead/prevention/populations.htm#:~:text=For%20example%2C%20older%20houses%20and%20houses%20in%20low-income,are%20at%20the%20greatest%20risk%20of%20lead%20exposure. Accessed January 25 2022.
- United States Centers for Disease Control and Prevention. (2022). Lead in paint. https://www.cdc.gov/nceh/lead/prevention/sources/paint.htm. Accessed May 5 2022.
- United States Energy Information Administration. (2020). Gasoline and the environment. Updated 11/19/20. https://www.eia.gov/energyexplained/gasoline/gasoline-and-the-environment-leaded-gasoline.php. Accessed November 9 2021.
- United States Environmental Protection Agency. (2016). Diazinon IRED facts https://archive.epa.gov/pesticides/reregistration/web/html/diazinon_ired_fs.html. Accessed November 8 2021.
- United States Environmental Protection Agency. (2021a). Basic information about mercury. https://www.epa.gov/mercury/basic-information-about-mercury#:~:text=It%20exists%20in%20several%20forms%3A%201%20Elemental%20%28metallic%29,mercury%20compounds%203%20Methylmercury%20and%20other%20organic%20compounds. Accessed February 8 2022.
- United States Environmental Protection Agency. (2021b). How people are exposed to mercury. https://www.epa.gov/mercury/how-people-are-exposed-mercury. Accessed February 8 2022.
- United States Environmental Protection Agency. (2022a). Learn about polychlorinated biphenyls (PCBs). https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls-pcbs#commercial. Accessed April 7 2022.
- United States Environmental Protection Agency. (2022b). Basic information about lead in drinking water. https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water#:~:text=If%20the%20level%20of%20lead%20in%20a%20child%27s,more%20of%20a%20person%E2%80%99s%20total%20exposure%20to%20lead. Accessed July 25 2022.
- United States Food and Drug Administration. (2018). Thimerosal and vaccines. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines#action. Accessed February 8 2022.
- Verner MA, Plusquellec P, Muckle G, Ayotte P, Dewailly E, Jacobson SW, et al. (2010). Alteration of infant attention and activity by polychlorinated biphenyls: Unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology, 31(5), 424–431. 10.1016/j.neuro.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, et al. (2003). Safety of thimerosal-containing vaccines: A two-phased study of computerized health maintenance organization databases. Pediatrics, 112(5), 1039–1048. [PubMed] [Google Scholar]
- Vreugdenhil HJ, Mulder PG, Emmen HH, & Weisglas-Kuperus N (2004). Effects of perinatal exposure to PCBs on neuropsychological functions in the Rotterdam cohort at 9 years of age. Neuropsychology, 18(1), 185–93. 10.1037/0894-4105.18.1.185 [DOI] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, & Stellman SD (1997). Proposed PCB congener groupings for epidemiological studies. Environmental Health Perspectives, 105(1), 13–14. 10.1289/ehp.9710513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman G, Liu X, Lolacono N, Factor-Litvak P, Kline J, Popovac D, et al. (1997). Lead exposure and intelligence in 7-year-old children: The Yugoslavia Prospective Study. Environmental Health Perspectives, 105(9), 956–962. 10.1289/ehp.97105956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Population Review. (2022). Flint, Michigan population 2022. https://worldpopulationreview.com/us-cities/flint-mi-population. Accessed February 7 2022. [Google Scholar]
- Yoshimasu K, Kiyohara C, Takemura S, & Nakai K (2014). A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicology, 44, 121–131. 10.1016/j.neuro.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Young H, Geier D, & Geier M (2008). Thimerosal exposure in infants and neurodevelopmental disorders: An assessment of computerized medical records in the Vaccine Safety Datalink. Journal of the Neurological Sciences, 271(1–2), 110–118. 10.1016/j.jns.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Yule W, Urbanowicz M-A, Lansdown R, & Millar I (1984). Teachers’ ratings of children’s behaviour in relation to blood lead levels. British Journal of Developmental Psychology, 2(4), 295–305. 10.1111/j.2044-835X.1984.tb00937.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.