Abstract
Background
Clinical decision support systems (CDSS) have been utilized as a low-cost intervention to improve healthcare process measures. Thus, we aim to estimate CDSS efficacy to optimize adherence to oral anticoagulant guidelines in eligible patients with atrial fibrillation (AF).
Methods
A systematic review and meta-analysis of randomized controlled trials (RCTs) retrieved from PubMed, WOS, SCOPUS, EMBASE, and CENTRAL through August 2023. We used RevMan V. 5.4 to pool dichotomous data using risk ratio (RR) with a 95% confidence interval (CI). PROSPERO ID: CRD42023471806.
Results
We included nine RCTs with a total of 25,573 patients. There was no significant difference, with the use of CDSS compared to routine care, in the number of patients prescribed anticoagulants (RR: 1.06, 95% CI [0.98, 1.14], P = 0.16), the number of patients prescribed antiplatelets (RR: 1.01 with 95% CI [0.97, 1.06], P = 0.59), all-cause mortality (RR: 1.19, 95% CI [0.31, 4.50], P = 0.80), major bleeding (RR: 0.84, 95% CI [0.21, 3.45], P = 0.81), and clinically relevant non-major bleeding (RR: 1.05, 95% CI [0.52, 2.16], P = 0.88). However, CDSS was significantly associated with reduced incidence of myocardial infarction (RR: 0.18, 95% CI [0.06, 0.54], P = 0.002) and cerebral or systemic embolic event (RR: 0.11, 95% CI [0.01, 0.83], P = 0.03).
Conclusion
We report no significant difference with the use of CDSS compared to routine care in anticoagulant or antiplatelet prescription in eligible patients with AF. CDSS was associated with a reduced incidence of myocardial infarction and cerebral or systemic embolic events.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12959-024-00614-7.
Keywords: Atrial fibrillation, Oral anticoagulation, Electronic notifications, Electronic alerts.
Introduction
Atrial fibrillation (AF) is the most prevalent arrhythmia worldwide [1–3]. AF increases the risk for stroke up to fivefold, contributing to up to 25% of all strokes [4, 5]. Societal guidelines in the U.S recommend using the CHA2DS2-VASc score to quantify the annual stroke risk and guide oral anticoagulation therapy (OAC) with either direct oral anticoagulants (DOACs) or vitamin K antagonists (VKA). A CHA2DS2-VASc score of one in men and two in women warrants prescribing OAC to reduce the risk of thromboembolic events. However, the CHA2DS2-VASc score is not recommended in AF patients with moderate to severe mitral stenosis or mechanical heart valves, where VKA is warranted [1, 6].
In a meta-analysis including 28,044 patients, prescribing VKA resulted in a 64% relative risk reduction (RRR) of stroke in patients with AF [7]. DOACs, including dabigatran, rivaroxaban, and apixaban, showed at least similar stroke prevention efficacy with a favorable safety profile [8–10]. Despite the significant RRR of stroke by OAC, there has been underutilization of OAC in AF patients [11–16]. In an observational study involving 94,474 patients who had experienced an acute ischemic stroke and had a history of AF, it was found that 84% of them had not been prescribed OAC before the occurrence of the stroke [17].
Clinical decision support systems (CDSS) have been increasingly utilized as a low-cost intervention to improve healthcare process measures; however, their impact on improving clinical outcomes remains controversial [18]. A randomized clinical trial (RCT) showed that an alert system increased the prescription of deep vein thrombosis (DVT) prophylaxis and reduced thromboembolism rates by 41% among hospitalized patients [19]. On the other hand, an alert system did not improve clinical outcomes in hospitalized patients with acute kidney injury [20].
Several RCTs were conducted to study the utility of CDSS and alert systems to improve OAC prescription among AF patients to reduce the risk of stroke and systemic embolism potentially.
We conducted this systematic review and meta-analysis of RCTs to investigate the efficacy of CDSS versus routine care regarding adherence to OAC prescription guidelines and stroke prevention in patients with AF.
Methodology
Protocol Registration
The study’s protocol was registered in PROSPERO with the identification number CRD42023471806, following the Preferred Reporting Items for Systematic Review and Meta-analysis of Interventional Studies (PRISMA) statement [21] and the Cochrane Handbook for Systematic Reviews and Meta-Analysis [22] guidelines.
Data sources & search strategy
PubMed, Web of Science, SCOPUS, EMBASE, and CENTRAL were searched by authors (A.M.A. and M.T.A.) through August 2023 without publication date, language, or geographical area restrictions. The search was done using [all field] with a mention of the usage of “alert” and “anticoagulant” in “Atrial Fibrillation” Patients. More details are in (Table S1).
Eligibility criteria
Randomized controlled trials (RCTs) that met all of our PICO inclusion criteria were selected: population (P): AF patients; intervention (I): CDSS, including email alert, notification alert, and electronic alerts; comparison (C): patients treated with usual care or no intervention; outcomes (O): our primary outcome was OAC prescription, while our secondary outcomes were patients prescribed antiplatelets and patients prescribed VKA. Additionally, we assessed hard outcomes, including mortality, major bleeding, clinically relevant non-major bleeding, myocardial infarction, stroke/transient ischemic attack (TIA), and thromboembolic events. Exclusion criteria were as follows: primary studies other than RCTs, duplicate publications, reviews, and conference abstracts.
Study selection
Four reviewers (M.T., A.E., O.A., and M.A.) initially screened the titles and abstracts independently using the Covidence platform. After erasing the duplicates, they independently screened the full texts in accordance with our previous eligibility criteria.
Data extraction
Four reviewers (M.A., M.T., A.E., and O.A.) independently extracted data from the eligible studies. M.T.A. and A.M.A. resolved any conflicts. We used an Excel sheet: summary characteristics (study design, country, number of centers, blinding status, registry number, total participants, intervention details, control, participants were on OAC or not, primary outcome, and follow-up duration), baseline characteristics (number of patients in CDSS and control arms, age, gender (male), CHA2DS2VASc score, HAS-BLED score, and patients’ comorbidities (vascular disease, heart disease, diabetes mellitus, hypertension, stroke/transient ischemic attack (TIA), renal disease, liver disease, and prior bleeding). Additionally, the current study outcomes were the number of patients prescribed anticoagulant (OAC), patients prescribed antiplatelets, patients prescribed vitamin K antagonist (VKA), and proportions of why participants were not on OAC. In addition, hard clinical outcomes such as mortality, major bleeding, clinically relevant non-major bleeding, myocardial infarction, stroke/TIA, and thromboembolic events were assessed.
Risk of Bias and Certainty of evidence
Four reviewers (M.A., M.T., A.E., and O.A.) independently used the Cochrane ROB2 tool [23] for quality assessment. The reviewers resolved any conflicts by consensus. We evaluated five domains, assessing the risk of bias due to randomization, deviation from CDSS, missing outcome data, measuring the outcome data, and selecting the reported results.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [24, 25] was used by M.T.A. to evaluate the certainty of evidence for each outcome.
Statistical analysis
RevMan v5.3 was used to run the statistical analysis [26]. To pool the results of dichotomous outcomes, we used the risk ratio (RR), while for the continuous outcomes, we used the mean difference (MD), both with a 95% confidence interval (CI). We performed both the Chi-square and I-square tests to evaluate heterogeneity, where the Chi-square test detects the presence of heterogeneity, and the I-square test evaluates its degree. I-square was interpreted In accordance with the Cochrane Handbook (chapter nine) [22]. as follows: heterogeneity is not significant for 0–40%, moderate for 30–60%, substantial for 50–90%, and considerable for 75–100%. We considered an alpha level below 0.1 for the Chi-square test to detect significant heterogeneity. A leave-one-out sensitivity analysis was employed to resolve the heterogeneity by excluding each study one time from the pooled analyzed studies.
Rstudio (version 4.2.2) was used to conduct a meta-analysis of prevalence using the random effect model with a 95% confidence interval. The I-square test was used to assess for heterogeneity, with I2 > 50% considered to be of significant heterogeneity.
Results
Search results and study selection
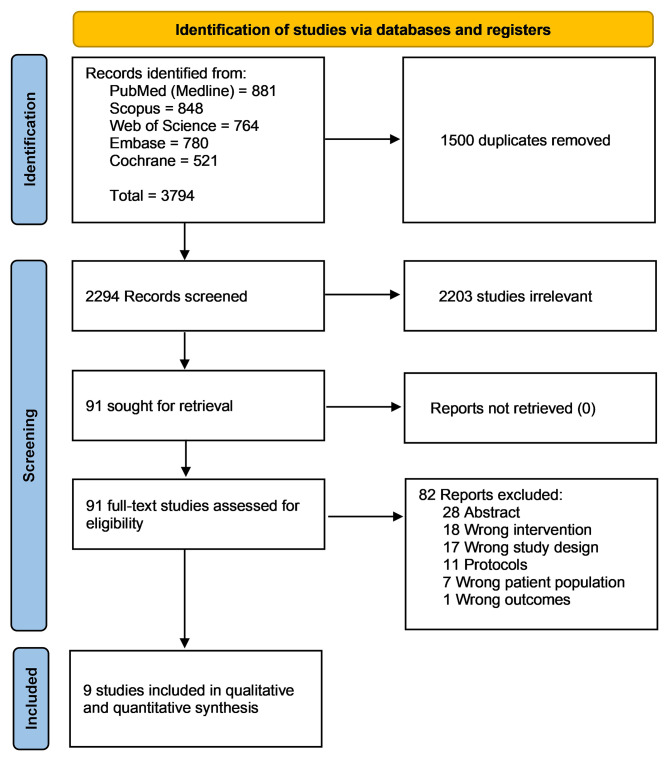
Our literature search retrieved 3,794 unique records. One thousand-five hundred records were removed as duplicates. After title and abstract screening, 91 studies were eligible for full-text screening. Finally, nine studies were included in this systematic review and meta-analysis. The PRISMA flowchart for study selection is shown in (Fig. 1). We have excluded Guo et al. trial [27] due to differences in the intervention compared to our included RCTs’ intervention. Patients could upload reports and pictures of the events, unlike our interventions, which are Electronic Medical Record (EMR) based CDSS.
Fig. 1.
PRISMA flow chart of the screening process
Characteristics of included studies
Nine randomized controlled trials [28–36] were included in the meta-analysis with 25,573 AF patients. All the included studies accessed our primary outcome, the number of patients on OAC. The follow-up duration in those studies ranged from three months to 12 months. These studies were conducted in five countries, mainly in the USA (five trials). The summary and baseline characteristics of the included studies are shown in (Tables 1 and 2). More details about the baseline trials’ participants’ comorbidities and CDSS characteristics are outlined in (Tables S2 and S3).
Table 1.
Summary characteristics of the included RCTs.
| Study ID | Study Design | Country | Total Participants | Intervention | Control | Already on OAC | Primary Outcome | Follow-up duration | |
|---|---|---|---|---|---|---|---|---|---|
| Arts et al. 2017 [28] | Single center, RCT | Netherlands | 781 | A real-time CDSS for a single EHR system | Received no messages | BOTH | The effect of the intervention on the proportion of patients with AF treated in accordance with the guideline between the intervention and control groups. | Nine months | |
| Ashburner et al. 2018 [29] | Single center, RCT | USA | 2336 | A physician notification alert and survey | Usual care | NO | the proportion of patients prescribed oral anticoagulants at three months in the intervention group in comparison with the control group | Three months | |
| Bajorek et al. 2016 [30] | Multi-center, RCT | Australia | 393 | computerized antithrombotic risk assessment tool | Usual care | BOTH | Change in anticoagulants and antiplatelets description | 12 months | |
| Chaturvedi et al. 2019 [31] | Multi-center, RCT | USA | 309 | electronic alert (EA) embedded in the electronic health record | Usual care | NO | comparing OAC consumption in active intervention locations to usual care settings | Six months | |
| Kapoor et al. 2020 (SUPPORT-AF II) [32] | Single-center, RCT | USA | 5475 |
electronic profiling/messaging combined with academic detailing |
No intervention | BOTH | Feasibility (how often providers in the intervention group read the emails) and effectiveness (change in anticoagulation status) | Seven months | |
| Karlsson et al. 2018 (CDS-AF) [33] | Multi-center, RCT | Sweden | 14,134 | CDS &alert for physicians | Usual care | BOTH | proportion of patients eligible for stroke prophylaxis who were prescribed anticoagulant therapy 12 months after study initiation. | 12 months | |
| Piazza et al. 2019 (AF-ALERT) [34] | RCT | USA | 458 | Alert-base CDS | No notification | NO | frequency of anticoagulant prescription | Three months | |
| Piazza et al. 2023 (AF-ALERT2) [35] | RCT | USA | 798 | Alert-based CDS | No notification | NO | frequency of anticoagulant prescription | Three months | |
| Silbernagel et al. 2016 [36] | RCT | Switzerland | 889 | computer-based electronic alert system | no alert (usual care) | NO | rate of adequate OAC prescription at hospital discharge | N/A |
RCT: randomized controlled trial; AF: atrial fibrillation; CDSS: clinical decision support system; OAC: oral anticoagulant; N/A.: not available
Table 2.
Baseline characteristics of the participants
| Study ID | Number of patients in each group | Age (Years), Mean (SD) | Gender (Male), N. (%) | CHA2DS2VASC, Mean (SD) | HAS-BLED score, Mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Arts et al. 2017 [28] | 522 | 259 | 72.13 (12.46) | 74.61 (13.63) | N/A | N/A | 3 (1.72) | 3.06 (1.8) | N/A | N/A |
| Ashburner et al. 2018 [29] | 972 | 1364 | 75.7 (11.1) | 76.3 (11.5) | 490(50.4) | 725(53.1) | 4.2 (1.7) | 4.2 (1.6) | N/A | N/A |
| Bajorek et al. 2016 [30] | 206 | 187 | 78.2 (7.1) | 77.7 (7) | 113(54.9) | 101(54) | N/A | N/A | N/A | N/A |
| Chaturvedi et al. 2019 [31] | 164 | 145 | 69.85 (12.53) | 70.57 (11.89) | 93(56.7) | 81(55.9) | 3.78 (1.87) | 3.1 (1.59) | N/A | N/A |
| Kapoor et al. 2020 (SUPPORT-AF II) [32] | 3578 | 1897 | N/A | N/A | 1940(54.2) | 1077(56.8) | N/A | N/A | N/A | N/A |
| Karlsson et al. 2018 (CDS-AF) [33] | 7764 | 6370 | N/A | N/A | 4042(54.4) | 3269(54) | 4(1.48288) | 4(1.4892) | N/A | N/A |
| Piazza et al. 2019 (AF-ALERT) [34] | 248 | 210 | 73.5(11.8) | 73.3(13) | 136(54.8) | 117(55.7) | 4(1.33) | 4(1.166) | 3(1.166) | 3(1.1667) |
| Piazza et al. 2023 (AF-ALERT2) [35] | 395 | 403 | 73.7(11.7) | 72(11.9) | 225(57) | 242(60.1) | 3.66(2.23) | 3.66(2.23) | 3.66(2.23) | 3(1.48) |
| Silbernagel et al. 2016 [36] | 455 | 434 | 74.4(10.9) | 73.3(11.8) | 300(65.9) | 292(67.3) | N/A | N/A | N/A | N/A |
N., number; SD, standard deviation; N/A: not available
Risk of Bias and Certainty of evidence
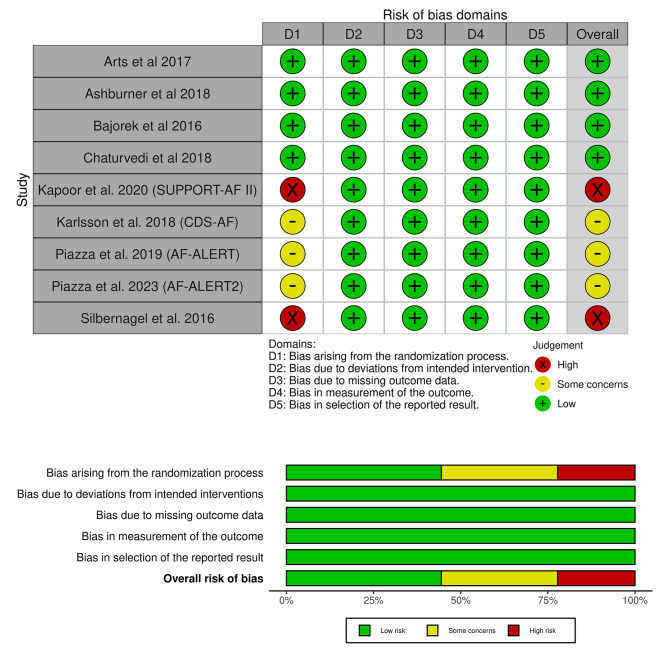
We assessed the quality of included studies according to the Cochrane risk of bias 2 tool, as shown in (Fig. 2). Four included trials had a low risk of randomization process bias (Arts et al. 2017, Ashbumer et al. 2018, Bajorek et al. 2016, and Chaturvedi et al. 2018), three had some concerns (Karlsson et al. 2018, Piazza et al. 2019 and Piazza et al. 2023), and two had a high risk (Kapoor et al. 2020 and Silbemagel et al. 2016). All the included studies had a low risk of deviations from intended intervention bias, missing outcome data bias, measurement of the outcome bias, and selection of the reported result bias. Author judgments are further clarified in (Table S4). Certainty of evidence is demonstrated in a GRADE evidence profile (Table 3).
Fig. 2.
Quality assessment of risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = green, unclear = yellow, and high = red) for specific types of biases of each study in the review. The lower panel presents risks (low = green, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
Table 3.
GRADE evidence profile
| Certainty assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) Follow-up |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) |
Anticipated absolute effects | ||
| With Usual Care | With CDSS | Risk with Usual Care | Risk difference with CDSS | ||||||||
| number of patients on anticoagulant | |||||||||||
|
24,567 (8 RCTs) |
seriousa | very seriousb | not serious | not serious | none |
⨁◯◯◯ Very low |
6257/10,621 (58.9%) | 9033/13,946 (64.8%) |
RR 1.04 (0.96 to 1.12) |
589 per 1,000 |
24 more per 1,000 (from 24 fewer to 71 more) |
| number of patients on antiplatlets | |||||||||||
|
5183 (6 RCTs) |
seriousa | not serious | not serious | not serious | none |
⨁⨁⨁◯ Moderate |
1651/2743 (60.2%) | 1408/2440 (57.7%) |
RR 1.01 (0.97 to 1.06) |
602 per 1,000 |
6 more per 1,000 (from 18 fewer to 36 more) |
| number of patients on vitamin k antagonist (aka.warfarin) | |||||||||||
|
5027 (6 RCTs) |
seriousa | seriousc | not serious | seriousd | none |
⨁◯◯◯ Very low |
247/2679 (9.2%) | 273/2348 (11.6%) |
RR 1.18 (0.84 to 1.66) |
92 per 1,000 |
17 more per 1,000 (from 15 fewer to 61 more) |
| Adverse - All-cause mortality | |||||||||||
|
1256 (2 RCTs) |
seriousa | seriousc | not serious | very seriousd | none |
⨁◯◯◯ Very low |
34/613 (5.5%) | 33/643 (5.1%) |
RR 1.19 (0.31 to 4.50) |
55 per 1,000 |
11 more per 1,000 (from 38 fewer to 194 more) |
| Adverse - Major bleed | |||||||||||
|
1256 (2 RCTs) |
seriousa | seriousc | not serious | very seriousd | none |
⨁◯◯◯ Very low |
11/613 (1.8%) | 9/643 (1.4%) |
RR 0.84 (0.21 to 3.45) |
18 per 1,000 |
3 fewer per 1,000 (from 14 fewer to 44 more) |
| Adverse - Clinically relevant non-major bleed | |||||||||||
|
1256 (2 RCTs) |
seriousa | not serious | not serious | very seriousd | none |
⨁◯◯◯ Very low |
14/613 (2.3%) | 16/643 (2.5%) |
RR 1.05 (0.52 to 2.16) |
23 per 1,000 |
1 more per 1,000 (from 11 fewer to 26 more) |
| Adverse - Myocardial infarction | |||||||||||
|
1256 (2 RCTs) |
seriousa | not serious | not serious | very seriousd | none |
⨁◯◯◯ Very low |
20/613 (3.3%) | 4/643 (0.6%) |
RR 0.18 (0.06 to 0.54) |
33 per 1,000 |
27 fewer per 1,000 (from 31 fewer to 15 fewer) |
| Adverse - Stroke/TIA or systemic embolic event | |||||||||||
|
16,056 (3 RCTs) |
seriousa | not serious | not serious | very seriousd | none |
⨁◯◯◯ Very low |
8/7121 (0.1%) | 0/8935 (0.0%) |
RR 0.11 (0.01 to 0.83) |
1 per 1,000 |
1 fewer per 1,000 (from 1 fewer to 0 fewer) |
CI: confidence interval; RR: risk ratio
Explanations
a. Karlsson et al. 2018, Piazza et al. 2019 and Piazza et al. 2023 had some concerns of selection biaas, while Kapoor et al. 2020 and Silbemagel et al. 2016 had a high risk of selection bias
b. I-square test > 75%
c. I-square test > 50%
d. Wide confidence interval that does not exclude the risk of appreciable benefit/harm
Primary outcome: number of patients on OAC
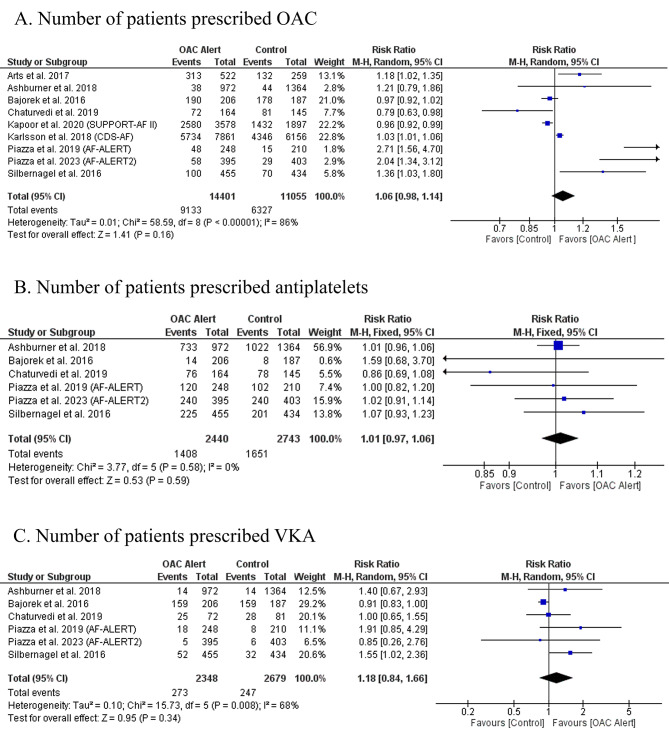
There was no significant difference in the number of patients prescribed OAC between CDSS compared to routine care (RR: 1.06 with 95% CI [0.98, 1.14], P = 0.16) (Fig. 3-A). The pooled studies were heterogeneous (I2 = 87%, P < 0.00001). Heterogeneity was not resolved by leave-one-out sensitivity analysis (Table S5).
Fig. 3.
Forest plot of the primary outcome (prescription of OAC) with the secondary outcome (prescription of antiplatelet and VKA), RR: risk ratio, CI: confidence interval
Secondary outcomes
Efficacy outcomes
There was no significant difference whether using CDSS or not in the number of patients prescribed antiplatelets (RR: 1.01 with 95% CI [0.97, 1.06], P = 0.59) (Fig. 3-B) and the number of patients prescribed VKA (RR: 1.18 with 95% CI [0.84, 1.66], P = 0.34) (Fig. 3-C).
The pooled studies were homogenous in number of patients prescribed antiplatelets (I2 = 0%, P = 0.58). However, pooled studies were heterogeneous in number of patients prescribed VKA (I2 = 68%, P = 0.008). Regarding the number of patients prescribed VKA, heterogeneity was best resolved by excluding Bajorek et al. 2016 and Silbernagel et al. 2016 (I2 = 0%, P = 0.48), (I2 = 36%, P = 0.18), respectively (Table S5).
Reasons why participants were not on OAC
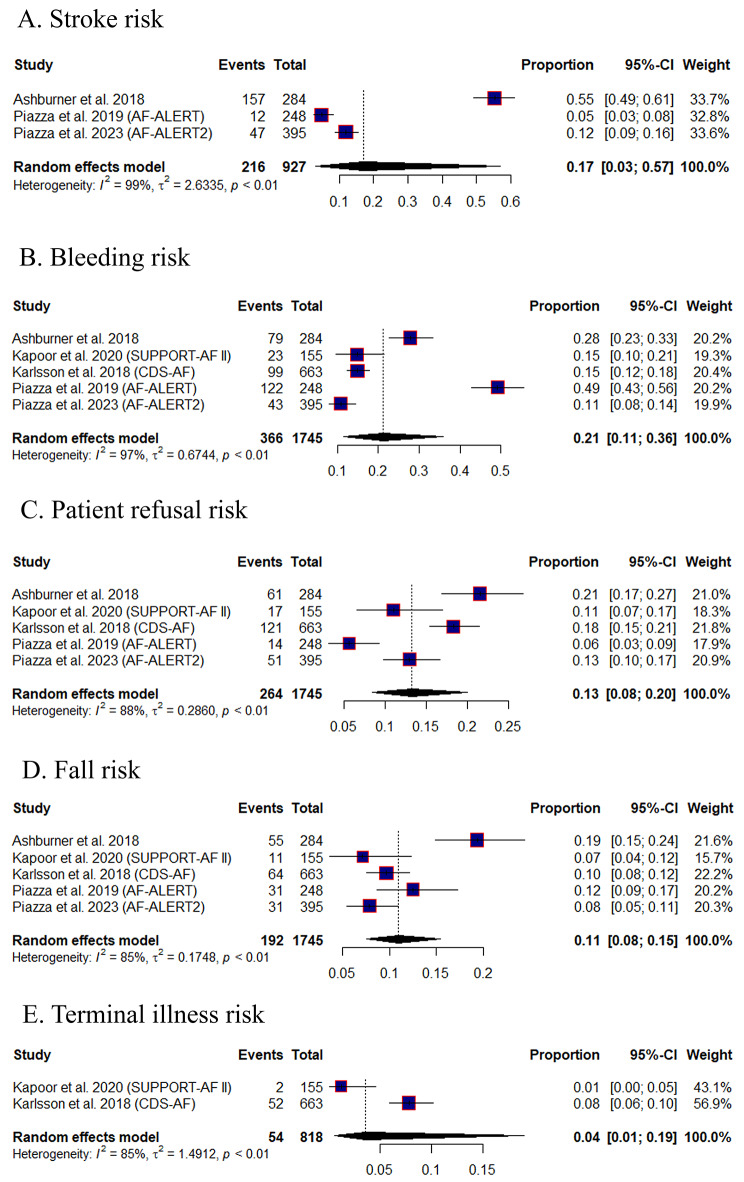
The pooled prevalence of stroke risk, from three studies (n = 927), was 17% (95% CI [0.03, 0.57], I2 = 99%) (Fig. 4-A), bleeding risk, from five studies (n = 1745), was 21% (95% CI [0.11, 0.36], I2 = 97%) (Fig. 4-B), patient refusal, from five studies (n = 1745), was 13% (95% CI [0.08, 0.20], I2 = 88%) (Fig. 4-C), fall risk, from five studies (n = 1745), was 11% (95% CI [0.08, 0.15], I2 = 85%) (Fig. 4-D), and terminal illness or hospice, from two studies (n = 818), was 4% (95% CI [0.01, 0.19], I2 = 85%) (Fig. 4-E).
Fig. 4.
Forest plots of the meta proportion of why participants were not on OAC, CI: confidence interval
Hard clinical outcomes
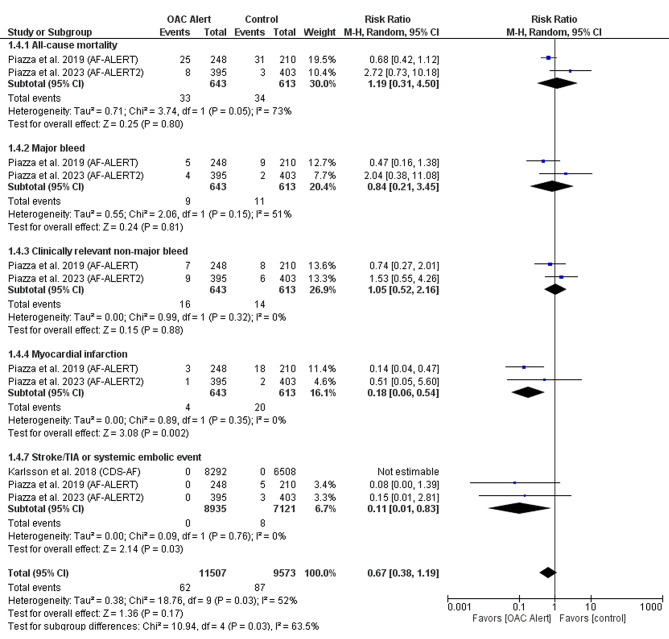
CDSS was significantly associated with a reduced incidence of myocardial infarction (RR: 0.18 with 95% CI [0.06, 0.54], P = 0.002) and reduced incidence of stroke/TIA or systemic embolic event (RR: 0.11 with 95% CI [0.01, 0.83], P = 0.03). However, there was no significant difference between CDSS compared to routine care in the incidence of all-cause mortality (RR: 1.19 with 95% CI [0.31, 4.50], P = 0.80), the incidence of major bleeding (RR: 0.84 with 95% CI [0.21, 3.45], P = 0.81), and the incidence of clinically relevant non-major bleeding (RR: 1.05 with 95% CI [0.52, 2.16], P = 0.88) (Fig. 5).
Fig. 5.
Forest plot of the clinical hard outcomes, RR: risk ratio, CI: confidence interval
The pooled studies were homogenous in clinically non-relevant major bleed (I2 = 0%, P = 0.32), myocardial infarction (I2 = 0%, P = 0.35), and stroke/TIA or thromboembolic event (I2 = 0%, P = 0.76). However, pooled studies were heterogeneous for all-cause mortality (I2 = 73%, P = 0.05) and major bleeding (I2 = 51%, P = 0.15).
Discussion
In this systematic review and meta-analysis of nine RCTs involving 25,573 AF patients, we investigated the efficacy of CDSS in oral anticoagulant prescriptions for eligible patients with AF. Key findings include: (1) CDSS was not associated with a significant difference in OAC and antiplatelet prescription rates between CDSS and routine care. (2) CDSS use was associated with significantly reduced rates of myocardial infarction and cerebral or systemic embolic events (3) There was no significant difference in all-cause mortality, major bleeding, and clinically relevant non-major bleeding between CDSS use and routine care.
The Atrial Fibrillation Better Care (ABC) pathway was developed for integrated care for AF patients. It includes a simple approach (avoid stroke, better symptom management, and cardiovascular and comorbidity risk reduction) that guides clinicians through decision-making. In the ABC pathway, prescribing an oral anticoagulant is only one piece of the integrated care approach [37]. The ABC pathway has been shown to improve outcomes in patients with AF [38, 39]. The above approach aligns with AF guidelines, which recommend a patient-centered, holistic approach, necessitating the involvement of multiple stakeholders in AF management decisions. Therefore, CDSS development and application contribute to a more holistic approach to caring for patients with AF, ensuring proper OACs management [40].
Multiple provider-directed interventions have been studied to improve anticoagulation rates among AF patients. For example, email notification to the provider was not associated with increased prescription rates [29]. In addition, the Support-AF trial found no benefit to email and inbox notifications [41]. Subsequently, electronic health record (EHR)-based CDSS alerts were developed to improve adherence to guidelines and increase anticoagulation rates in eligible AF patients.
Provider-directed EHR CDSS alerts were introduced as a cost-effective intervention to enhance work efficiency and clinical outcomes in inpatient and ambulatory settings. Kawamoto et al. described four essential features of CDSS, including “(a) provide decision support automatically as part of clinician workflow, (b) deliver decision support at the time and location of decision making, (c) provide actionable recommendations, and (d) use a computer to generate the decision support.” [42].
CDSS were studied in different clinical conditions with variable efficacy in improving clinical outcomes. Kucker et al. demonstrated increased use of DVT prophylaxis and reduced DVT and pulmonary embolism incidence with CDSS alerts (HR = 0.59, P = 0.001) [19]. Van Wyk et al. showed improved dyslipidemia screening and treatment with CDSS alerts [43]. On the other hand, Wilson et al. found no improvement in hospitalized patients with acute kidney injury [20]. Bright et al. conducted a large systematic review, including 148 trials assessing the efficacy of CDSS. Results demonstrated that process measures were often used as study endpoints rather than patient-related outcomes. 128/148 studies assessed healthcare process measures, while only 29/148 assessed clinical outcomes. There was a significant improvement in healthcare process measures, but evidence for clinical outcomes was sparse [18].
We report no significant difference in rates of anticoagulation prescription; however, this finding should be interpreted with caution due to significant heterogeneity among the included studies. An observational study by Osterland et al. reported no significant change in the trend of anticoagulant use before and after implementation of best practice advisory in eligible ambulatory AF patients [44]. Our results suggested a significant reduction in the incidence of myocardial infarction and cerebral or systemic emboli events. These results align with previous research on CDSS use across different diseases on improving clinical outcomes in other disease states, such as DVT and dyslipidemia [19, 43]. Additionally, there was no significant increase in bleeding complications. The efficacy and safety outcomes with the use of CDSS were variable. This is likely due to the limited duration of follow-up. The duration of follow-up of 3–12 months in the included studies may be too short to assess the impact on stroke or systemic embolism.
Barriers to CDSS tools include alert fatigue, increased number of clicks, time constraints, and clinician burnout [45, 46]. Arts et al. studied the physicians’ perspective of the CDSS; perceived barriers included workflow interruption, increased number of recommendations, and irrelevant recommendations [47]. Context-aware CDSS models could help address some of these barriers, possibly by limiting recommendations to a specific encounter [48].
In our study, reasons for not prescribing an OAC included bleeding risk (21%), patient refusal (13%), fall risk (11%), and terminal illness (4%). Incorporation of bleeding and thromboembolism risk scoring tools might be helpful to support clinical decision-making in high bleeding risk patients. Given the high rates of patient refusal, data from the IMPACT-AF trial suggests that patient-directed educational interventions could also lead to a significant increase in anticoagulation rates [49]. Patient refusal can be attributed to anticoagulation cost, repeated falls, concerns about bleeding, advanced age, and occupational implications [50].
Limitations
Our review has the following limitations. Firstly, variations in baseline characteristics were noted among different study populations. Secondly, there was notable heterogeneity among studies in the effect size of various outcomes, including the number of patients on anticoagulants, all-cause mortality, and major bleeding. Thirdly, there was notable heterogeneity in CDSS interventions among different studies, which presents a valid concern when interpreting pooled meta-analysis results. The variation in CDSS interventions could explain some conflicting study results well. Fourthly, there was a considerable difference in study weights, which may significantly influence the contribution of certain studies to the pooled results. Given the aforementioned limitations, our study provides a systematic review to accurately interpret the results of individual studies. Moreover, true heterogeneity is expected in prevalence estimates due to differences in the time and place where the included studies were conducted. I2 statistics may not be discriminative and should be interpreted with caution in this case. In case of substantial heterogeneity, planned sensitivity analysis can help elucidate the factors associated with the variability among estimates [51]. Additionally, hard clinical outcomes were exclusively assessed by the same research group, Piazza et al., in AF-ALERT and AF-ALERT2, with the analysis involving a smaller patient cohort (n = 643). Moreover, challenges in CDSS implementation include a lack of medical informatics expertise in certain centers.
Implications on Future Research
Future trials are required to investigate the impact of CDSS on clinical patient outcomes, particularly all-cause mortality and Major Adverse Cardiovascular Events (MACE). Additional research is warranted to define the optimal characteristics of CDSS, including the potential integration of artificial intelligence and machine learning to enhance its effectiveness. Future research should also explore the physician perspective, with attention to potential issues such as alarm fatigue impacting CDSS usage and effectiveness in real-world settings.
Conclusion
Our meta-analysis underscores CDSS’s potential to reduce the incidence of myocardial infarction and cerebral or systemic embolic events in patients with AF. However, we report no significant difference in the rate of prescribing OAC and antiplatelets, all-cause mortality, major bleeding, or clinically relevant non-major bleeding. These insights can guide clinicians in optimizing CDSS use in AF management.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
M.T.A. conceived the idea. A.M.A. and M.T.A. designed the research workflow. A.M.A. and M.A. searched the databases. M.T., O.A., A.E., and M.A. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and B.A. resolved the conflicts. A.M.A. and A.A.I. performed the analysis. A.M.A., R.G., and M.T.A. wrote the final manuscript. B.A. supervised the project. All authors have read and agreed to the final version of the manuscript.
Funding
We received no funding for this study.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation [Internet]. 2014;130:e199–267. 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of Disease 2010 study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin AM, Ghaly R, Ibrahim AA, Ali MA, Almaadawy O, Elzahaby A et al. Efficacy and safety of high-power short-duration ablation for atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. J Interv Card Electrophysiol [Internet]. 2024; 10.1007/s10840-024-01782-2. [DOI] [PMC free article] [PubMed]
- 4.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–66. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JCJ, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the heart. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 11.Bassand J-P, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KAA, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–9. doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arts DL, Visscher S, Opstelten W, Korevaar JC, Abu-Hanna A, van Weert HCPM. Frequency and risk factors for under- and over-treatment in stroke prevention for patients with non-valvular atrial fibrillation in general practice. PLoS ONE. 2013;8:e67806. doi: 10.1371/journal.pone.0067806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinh T, Nieuwlaat R, Tieleman RG, Büller HR, van Charante NAM, Prins MH, et al. Antithrombotic drug prescription in atrial fibrillation and its rationale among general practitioners, internists and cardiologists in the Netherlands–The EXAMINE-AF study. A questionnaire survey. Int J Clin Pract. 2007;61:24–31. doi: 10.1111/j.1742-1241.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 14.Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study. Stroke. 2013;44:3103–8. doi: 10.1161/STROKEAHA.113.002329. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar AK, Mueller I, Bassand J-P, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE. 2013;8:e63479. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GYH. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–e6454. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Xian Y, O’Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of Preceding Antithrombotic Treatment with Acute ischemic stroke severity and In-Hospital outcomes among patients with Atrial Fibrillation. JAMA. 2017;317:1057–67. doi: 10.1001/jama.2017.1371. [DOI] [PubMed] [Google Scholar]
- 18.Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157:29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 19.Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–77. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 20.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet (London England) 2015;385:1966–74. doi: 10.1016/S0140-6736(15)60266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev [Internet]. 2021;10:89. 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed]
- 22.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ WV, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2023.
- 23.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is quality of evidence and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RevMan. | Cochrane Training [Internet]. [cited 2021 Aug 3]. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revmanNo Title.
- 27.Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W et al. Mobile Health Technology to Improve Care for Patients With Atrial Fibrillation. J Am Coll Cardiol [Internet]. 2020;75:1523–34. https://www.sciencedirect.com/science/article/pii/S0735109720305325. [DOI] [PubMed]
- 28.Arts DL, Abu-Hanna A, Medlock SK, van Weert HCPM. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: a cluster randomized controlled trial. PLoS ONE. 2017;12:e0170974. doi: 10.1371/journal.pone.0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashburner JM, Atlas SJ, Khurshid S, Weng L-C, Hulme OL, Chang Y, et al. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. J Gen Intern Med. 2018;33:2070–7. doi: 10.1007/s11606-018-4612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajorek BV, Magin PJ, Hilmer SN, Krass I. Optimizing Stroke Prevention in patients with Atrial Fibrillation: a cluster-randomized controlled trial of a Computerized Antithrombotic Risk Assessment Tool in Australian General Practice, 2012–2013. Prev Chronic Dis. 2016;13:E90. doi: 10.5888/pcd13.160078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi S, Kelly AG, Prabhakaran S, Saposnik G, Lee L, Malik A, et al. Electronic decision support for Improvement of Contemporary Therapy for Stroke Prevention. J Stroke Cerebrovasc Dis off J Natl Stroke Assoc. 2019;28:569–73. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Kapoor A, Amroze A, Vakil F, Crawford S, Der J, Mathew J, et al. SUPPORT-AF II: supporting Use of anticoagulants through Provider profiling of oral anticoagulant therapy for Atrial Fibrillation: a cluster-randomized study of electronic profiling and messaging combined with academic detailing for providers making Decis. Circ Cardiovasc Qual Outcomes. 2020;13:e005871. doi: 10.1161/CIRCOUTCOMES.119.005871. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson LO, Nilsson S, Bång M, Nilsson L, Charitakis E, Janzon M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: a cluster-randomized trial in a Swedish primary care setting (the CDS-AF study) PLoS Med. 2018;15:e1002528. doi: 10.1371/journal.pmed.1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piazza G, Hurwitz S, Campia U, Bikdeli B, Lou J, Khairani CD, et al. Electronic alerts for ambulatory patients with atrial fibrillation not prescribed anticoagulation: a randomized, controlled trial (AF-ALERT2) Thromb Res. 2023;227:1–7. doi: 10.1016/j.thromres.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Piazza G, Hurwitz S, Galvin CE, Harrigan L, Baklla S, Hohlfelder B, et al. Alert-based computerized decision support for high-risk hospitalized patients with atrial fibrillation not prescribed anticoagulation: a randomized, controlled trial (AF-ALERT) Eur Heart J. 2020;41:1086–96. doi: 10.1093/eurheartj/ehz385. [DOI] [PubMed] [Google Scholar]
- 36.Silbernagel G, Spirk D, Hager A, Baumgartner I, Kucher N. Electronic Alert System for improving Stroke Prevention among hospitalized oral-Anticoagulation-Naïve patients with Atrial Fibrillation: a Randomized Trial. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed]
- 37.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–8. doi: 10.1038/nrcardio.2017.153. [DOI] [PubMed] [Google Scholar]
- 38.Romiti GF, Proietti M, Bonini N, Ding WY, Boriani G, Huisman MV, et al. Adherence to the Atrial Fibrillation Better Care (ABC) pathway and the risk of major outcomes in patients with atrial fibrillation: a post-hoc analysis from the prospective GLORIA-AF Registry. EClinicalMedicine. 2023;55:101757. doi: 10.1016/j.eclinm.2022.101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive Management with the ABC (Atrial Fibrillation Better Care) pathway in clinically complex patients with Atrial Fibrillation: a Post Hoc Ancillary Analysis from the AFFIRM Trial. J Am Heart Assoc. 2020;9:e014932. doi: 10.1161/JAHA.119.014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation [Internet]. 2024;149:e1–156. 10.1161/CIR.0000000000001193. [DOI] [PMC free article] [PubMed]
- 41.Kapoor A, Amroze A, Golden J, Crawford S, O’Day K, Elhag R, et al. SUPPORT-AF: piloting a multi-faceted, Electronic Medical Record-based intervention to improve prescription of Anticoagulation. J Am Heart Assoc. 2018;7:e009946. doi: 10.1161/JAHA.118.009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wyk JT, van Wijk MAM, Sturkenboom MCJM, Mosseveld M, Moorman PW, van der Lei J. Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation. 2008;117:371–8. doi: 10.1161/CIRCULATIONAHA.107.697201. [DOI] [PubMed] [Google Scholar]
- 44.Osterland AJ, Yasuda M, Widmer RJ, Colavecchia AC, Gums T, Emir B, et al. An interrupted time series study of electronic health record clinical decision support for providers caring for patients with atrial fibrillation at increased stroke risk. Am J Heal Pharm AJHP off J Am Soc Heal Pharm. 2023;80:1830–9. doi: 10.1093/ajhp/zxad188. [DOI] [PubMed] [Google Scholar]
- 45.Sennesael A-L, Krug B, Sneyers B, Spinewine A. Do computerized clinical decision support systems improve the prescribing of oral anticoagulants? A systematic review. Thromb Res. 2020;187:79–87. doi: 10.1016/j.thromres.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Piazza G, Goldhaber SZ. Computerized decision support for the cardiovascular clinician: applications for venous thromboembolism prevention and beyond. Circulation. 2009;120:1133–7. doi: 10.1161/CIRCULATIONAHA.109.884031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arts DL, Medlock SK, van Weert HCPM, Wyatt JC, Abu-Hanna A. Acceptance and barriers pertaining to a general practice decision support system for multiple clinical conditions: a mixed methods evaluation. PLoS ONE. 2018;13:e0193187. doi: 10.1371/journal.pone.0193187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duke JD, Bolchini D. A successful model and visual design for creating context-aware drug-drug interaction alerts. AMIA. Annu Symp proceedings AMIA Symp. 2011;2011:339–48. [PMC free article] [PubMed]
- 49.Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet (London England) 2017;390:1737–46. doi: 10.1016/S0140-6736(17)32165-7. [DOI] [PubMed] [Google Scholar]
- 50.Gebreyohannes EA, Salter S, Chalmers L, Bereznicki L, Lee K. Non-adherence to Thromboprophylaxis Guidelines in Atrial Fibrillation: a narrative review of the extent of and factors in Guideline non-adherence. Am J Cardiovasc Drugs Drugs Devices Other Interv. 2021;21:419–33. doi: 10.1007/s40256-020-00457-3. [DOI] [PubMed] [Google Scholar]
- 51.Migliavaca CB, Stein C, Colpani V, Barker TH, Ziegelmann PK, Munn Z, et al. Meta-analysis of prevalence: I(2) statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13:363–7. doi: 10.1002/jrsm.1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.