Abstract
Background and purpose:
Sleep disorders are increasingly implicated as risk factors for stroke, as well as a determinant of stroke outcome. They can also occur secondary to the stroke itself. In this review, we describe the variety of different sleep disorders associated with stroke and analyze their effect on stroke risk and outcome.
Methods:
A search term-based literature review (“sleep,” “insomnia,” “narcolepsy,” “restless legs syndrome,” “periodic limb movements during sleep,” “excessive daytime sleepiness” AND “stroke” OR “cerebrovascular” in PubMed; “stroke” and “sleep” in ClinicalTrials.gov) was performed. English articles from 1990 to March 2023 were considered.
Results:
Increasing evidence suggests that sleep disorders are risk factors for stroke. In addition, sleep disturbance has been reported in half of all stroke sufferers; specifically, an increase is not only sleep-related breathing disorders but also periodic limb movements during sleep, narcolepsy, rapid eye movement (REM) sleep behavior disorder, insomnia, sleep duration, and circadian rhythm sleep–wake disorders. Poststroke sleep disturbance has been associated with worse outcome.
Conclusion:
Sleep disorders are risk factors for stroke and associated with worse stroke outcome. They are also a common consequence of stroke. Recent guidelines suggest screening for sleep disorders after stroke. It is possible that treatment of sleep disorders could both reduce stroke risk and improve stroke outcome, although further data from clinical trials are required.
Keywords: Stroke, sleep, insomnia, narcolepsy, RBD, RLS, PLMS, prevention
Introduction
Cardiovascular disease (CVD) remains the driving factor in all-cause mortality globally and represents the leading cause of long-term morbidity in the Western world. 1 Sleep disorders like sleep-related breathing disorders (SRBD) and insomnia are highly prevalent in modern society and have been increasingly recognized as significant contributors to the development and progression of CVD, including stroke. 2 In this context, SRBD affects 20% of the general population, 50–60% of stroke survivors and increase the risk of incident, recurrent stroke as well as worse functional outcome.3,4 Recent reviews and expert statements have compiled the current evidence and discussed future perspectives of SRBD in cerebrovascular disease.3,4 However, there are several other frequent nonrespiratory sleep disorders which affect stroke risk and can manifest after stroke. The importance of sleep health in the primary stroke prevention has been postulated years ago. Current European guidelines recommend screening and treatment of sleep apnea in the acute phase of stroke; however, stroke care services have not yet implemented these recommendations. 3 Therefore, the goal of the current article is to shed light on the current evidence of an association between sleep disorders and stroke.
SRBD
Obstructive sleep apnea (OSA) is one of the most neglected risk factors for stroke with a prevalence of about 20% and doubled risk of stroke, especially in young/middle-aged adults, if untreated. OSA is also linked to wake-up stroke. Possible pathomechanisms include nondipping blood pressure during sleep, hypoxemia, impaired cerebral hemodynamic and a hypercoagulable state with elevated hematocrit, viscosity, and altered platelet function. Furthermore, OSA is one of the most common reasons for therapy refractive hypertension and has been linked to atrial fibrillation and cerebral microangiopathy.4,5 In addition, it is commonly associated with increased levels of depression and anxiety. 6 On the contrary, as a meta-analysis of five observational studies presented, continuous positive airway pressure (CPAP) therapy is associated with stroke-risk reduction (27%) in primary prevention. Still, other studies (one randomized controlled trial (RCT), 2 health administrative data based) showed equivocal data. In secondary stroke prevention, a meta-analysis of RCTs could not demonstrate a reduction in stroke risk; however, a limitation was the poor CPAP compliance (3.3 h/night). In a post hoc analysis of those using CPAP for more than 4 h/night, treatment was associated with risk reduction. An excellent detailed review on sleep apnea and ischemic stroke by the group of C. Bassetti was recently published. 4
Nonrespiratory sleep disorders as a risk factor for stroke
Restless legs syndrome and periodic limb movements during sleep
Restless legs syndrome (RLS) is frequent but still under-recognized, as emphasized by the population-based Bruneck study (10.8% prevalence of undiagnosed and untreated RLS). 7 It is characterized by unpleasant sensations and an urge to move (mainly the legs) primarily at rest or during inactivity. These sensations are relieved by movement. As the symptoms typically occur in the evening or at night, RLS can cause sleep disturbances, which may be severe.8–10 Most patients with RLS present accompanying periodic limb movements in sleep (PLMS), characterized by limb movement of 15/h in adults according to the American Academy of Sleep Medicine scoring manual. 8 PLMS are, however, not exclusively present in RLS patients, as they can occur in other sleep disorders and medical conditions or in healthy subjects.11,12 RLS has been linked to an increased risk of diabetes, hypertension, and a higher probability of being obese13,14 while PLMS have been associated with hypertension.15,16 The Wisconsin Sleep Cohort and the Sleep Heart Health Study reported a significant correlation between RLS and CVD including stroke. 17 In a recent systematic review with meta-analysis, RLS patients have a higher risk of stroke and mortality but after adjustment for confounders, only the mortality association remained significant. In large meta-analyses on PLMS patients (five studies, 9823 PMLS patients/9.416 controls), stroke risk was increased by about 25% within 8 years after diagnosis.3,18 Concerning mechanisms connecting RLS/PLMS and CVD, inflammation, 19 sympathetic activation,20,21 metabolic dysregulation, 13 and hypothalamic–pituitary–adrenal system activation 14 seem to play a role, but a definite pathway remains elusive. 22
Central disorders of hypersomnolence (Narcolepsy)
The key subjective complaint of central disorders of hypersomnolence, one of which is narcolepsy, is excessive daytime sleepiness. Patients report daily episodes of irrepressible urges to sleep, and lapses into sleep, which can be objectified in polysomnography and multiple sleep latency testing (MSLT) showing an increased readiness to fall asleep during the day (<8 min). 8 Globally, the estimated prevalence of narcolepsy is 25–50 in 100,000. A distinction is made into (1) narcolepsy type I, with characteristic hypocretin-1 deficiency, increased readiness to fall asleep with sleep-onset rapid eye movement periods (SOREMPs) during polysomnography (PSG)/MSLT and clear cataplexy or (2) narcolepsy type II, with at least two SOREMPs.8,23
Studies on stroke risk in patients with narcolepsy remain scarce. One retrospective analysis (Burden of Narcolepsy Disease (BOND) study) investigated 9312 individuals with type 1 and 2 narcolepsy and revealed an odds ratio (OR) of 2.5 (2.3–2.7) to suffer stroke compared with matched controls. 24 Mechanistically, hypocretin is believed to be involved in autonomic function and control (i.e. hypertension) relating to its plausible causative connection to CVD risk.24,25 Supporting this hypothesis, McAlpine et al. 26 found that in apolipoprotein E knockout mice subjected to sleep deprivation and fragmentation, the hypothalamus produced less hypocretin which increased the likelihood of atherosclerotic lesions. They argued that hypocretin modulates the release of colony-stimulating factor 1 in the bone marrow, which regulates monocyte production, inflammation, and atherosclerosis. 26 Still, further clinical studies are needed to confirm and elucidate mechanisms underlying this link. 27
Rapid eye movement sleep behavior disorder
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by dream enactment behaviors (movement or vocalization) occurring during REM sleep and by the loss of the physiological REM sleep atonia, demonstrated by polysomnography.8,28 It is recognized as a prodromal synuclein-related neurodegenerative disease, as most (>90%) patients with isolated RBD (iRBD) phenoconvert to Parkinson’s disease, dementia with Lewy bodies or, less frequently, multiple system atrophy 15 years or more after onset.8,29–31 One large community-based questionnaire study (n > 12,000) of probable RBD patients reported an increased risk for ischemic (hazard ratio (HR) = 1.93 (1.07–3.46) and hemorrhagic stroke (HR = 6.61 (2.27–19.27) 32 As autonomic nervous system function is frequently impaired in patients with iRBD (e.g. nondipping profile in 24 h blood pressure), the connection to higher risk of CVD is feasible. 33 Still, current evidence is low making future prospective studies in well-characterized cohorts with definite iRBD necessary to establish the potential relationship.
Insomnia, sleep duration, and circadian rhythm sleep–wake disorders
Insomnia, defined by difficulty in sleep initiation/maintenance and early awakening with daytime consequences at least three times per week over a span of at least 3 months, is one of the most frequent sleep disorders with approximately one-third of the general population reporting attributable features.8,34 Insomnia is linked to metabolic syndrome, 35 hypertension 36 and depression. 37 A large meta-analysis of prospective cohort studies concluded that each key feature of insomnia, except for early-morning awakening, is associated with an increased risk of future CVD including stroke. 38 In a recent analysis of the INTERSTROKE cohort, difficulty getting sleep or maintaining sleep was associated with a 30% increased stroke risk. 39 An ancillary study to the CARDIA Study revealed insomnia being associated with a 23% higher fasting glucose level and a 48% higher fasting insulin level. 40 In line, Spiegel et al. 41 found that 1 week of reduced sleep (i.e. <4 h) aggravated the long-term risk profile of otherwise healthy young adults. Furthermore, a recent study of 1413 participants without hypertension or sleep apnea at baseline showed an association between insomnia with objective (polysomnography confirmed <6 h of sleep), but not subjective short-sleep duration and incident hypertension after a median follow-up of 5.1 years. 42
Hypersomnia (sleep duration >9 h not attributable to other sleep disorders) is independently associated with stroke of microangiopathic origin.43–45 The U-shaped relationship between sleep duration and stroke risk was recently strengthened by Wang et al. 46 reporting that both short- (⩽4 h) and long-sleep duration (⩾9 h) are associated with stroke risk and mortality. Patients with long sleep have a substantially elevated risk of diabetes, atrial fibrillation, inflammation, blood pressure variability, and increased arterial stiffness. The association between long sleep and stroke is independent of confounders, has a dose–response relationship, and is specific for stroke. 47
Circadian rhythm sleep–wake disorders (CRSWD), caused by alteration of the endogenous circadian timing system or misalignment between ones intrinsic rhythm and the required sleep–wake cycle, are known to be associated with CVD. 8 The Nurses’ Health Study identified an increased risk of stroke in shift-working nurses. 48 In line, a meta-analysis by Vyas et al. 49 enveloping more than 2 million people reported shift work being associated with stroke risk (OR = 1.05 (1.01–1.09) even after accounting for confounders. Furthermore, there is a known circadian variation in stroke onset with meta-analyses reporting an excess risk of stroke between morning hours and noon (+50%) and a decrease during night sleep hours, suggesting a protective role of sleep.50–53
Concerning the involved pathomechanisms responsible for the relationship between insomnia/sleep duration/CRSWD and stroke, elevated cortisol levels (reflecting a negative-feedback control of the hypothalamo–pituitary–adrenal axis) result in insulin resistance.54,55 Furthermore, as a systemic review by Irwin et al. 56 revealed elevated CRP and interleukin (IL)-6 levels in such individuals, inflammation, a well-known risk factor for CVD, may play a role. CRSWD and short-sleep duration have also been associated with increased visceral fat potentially resulting in obesity and diabetes mellitus.57,58
Sleep disorders after stroke
A meta-analysis of sleep quality after stroke indicates that poor sleep quality affects 53% of stroke patients. 59 In 2020, a European task force of sleep and stroke researchers emphasized the necessity for clear guidelines to identify, adequately diagnose, and manage sleep disorders in stroke patients, prompting important investigations. 3 The Sleep-disordered breathing in transient ischemic attack (TIA)/Ischemic stroke and continuous positive airway pressure (CPAP) treatment efficacy study conducted by Miano et al. 60 reports severe changes of sleep architecture in individuals with stroke or transient ischemic attack, with sleep efficiency and REM sleep being affected the most. They also demonstrated a loss of cardiac autonomic dynamics during sleep after stroke in addition to diurnal variation of clock gene expression and sleep–wake rhythm biomarkers.61,62 Corresponding changes are also seen in animal models of stroke; in middle-aged C57BL/6 J mice with induced M1 occlusion, compared with sham mice, sleep latency and daytime sleepiness were increased with reduced non-REM sleep duration and disruption in sleep architecture. 63
SRBD
After stroke, the prevalence of SRBD increased to approximately 50–60% with about 30% of patients having severe OSA (apnea–hypopnea index >30/h). The prevalence of OSA did not decrease within the first 2 years after stroke. 64 Even though the occurrence of OSA is unrelated to a specific infarct lesion, poststroke dysphagia and altered sleep position throughout the night (increased supine sleeping in stroke patients) are associated with OSA. On the contrary, poststroke central sleep apnea can be temporary and may manifest after brainstem stroke.
RLS and PLMS
Unilateral, of the paralyzed limb, or bilateral poststroke RLS has been reported in 2.3–15.1% of stroke survivors.65–69 It has been associated with infarcts in the body of caudate nucleus, the lenticulo capsule, corona radiate, and ventral brainstem.69,70 The prevalence of RLS did not change in the acute and chronic phase of stroke patients. 71 Compared with RLS, the evidence on poststroke PLMS is scarce. 3 One prospective polysomnographic study could not find a difference in PLMS frequency in stroke patients compared with controls while a meta-analysis found that periodic limb movement (PLM) index after stroke is increased compared with controls.72,73
Central disorders of hypersomnolence (Narcolepsy)
Even though case reports exist proclaiming that specific ischemia affected areas are associated with clinical symptoms of narcolepsy, the prevalence of poststroke narcolepsy seems to be identical to that of the general population.74–77
RBD
Concerning poststroke RBD, only case reports exist.78,79 Anatomically, both cases described by Reynolds and Roy 78 and Xi and Luning 79 suffered pontine infarction. Whether such associations are causal remains uncertain. One hypothesis states that if brainstem nuclei involved in the control of muscle tone during REM sleep are affected, poststroke RBD may present. However, Tang et al. 80 performed a polysomnographic study of patients with brainstem infarction revealing a reduced time spent in REM with preserved atonia, rendering the anatomic relationship inconclusive.
Insomnia, sleep duration, and CRSWD
Insomnia has been reported in up to 40% of stroke survivors, which is markedly more frequent than in the general population. 81 A recent prospective study delivered robust data on the frequency and evolution of sleep–wake disturbances in poststroke individuals. 82 The authors report excessive daytime sleepiness, fatigue, or insomnia being evident in 14%, 28%, or 28% of cases, respectively, over a follow-up period of 3 years. Over time, insomnia and excessive daytime sleepiness showed improvement while fatigue remained stable. 71 Poststroke insomnia is associated with other symptoms associated with worse outcome in stroke survivors, namely depression. 83 Concerning treatment of insomnia in stroke patients, only few small-scale studies are available. A parallel group randomized controlled trial by Fleming et al. 84 found a favorable effect of digital cognitive behavioral therapy on sleep after stroke but did not report data on its effect on stroke recurrence. Accordingly, antidepressants like mianserin have the potential to improve poststroke sleep but whether this affects stroke risk is unknown. 85 Compared with benzodiazepines, nonbenzodiazepine hypnotics in treatment of insomnia were associated with reduced stroke risk over time in retrospective observational data. 86 Huang et al. 87 described a potential dose–response relationship of benzodiazepine use to stroke risk, favoring lower doses and less frequent intake. Overall, benzodiazepines are not recommended for treatment of sleep disorders in stroke patients as at high dose they have been associated with an increased risk of stroke recurrence, worse cognition, and SRBD. 3
Effect of sleep disorders on poststroke rehabilitation and outcome
Two small studies report less favorable outcome of stroke if RLS is present.88,89 Insomnia and related symptoms were associated with burden on daily living, health-related quality of life, and worse functional recovery in stroke survivors.80,90,91 A recent Mendelian randomization study showed a genetic liability to insomnia with sleep duration further being associated with an increased poststroke functional dependency (modified Rankin Scale (mRS) ⩾ 3). 92 OSA is also related to a high risk of mortality and poor functional outcome after stroke. According to data derived from mouse models, sleep is a major, modifiable factor in regaining cognitive and motor function poststroke. de vivo et al. 93 provided insights on the role of sleep on synaptic plasticity, as sleep, according to the authors, played a role in synaptic strengthening. Zunzunegui et al. 94 reported that extracellular waste removal, an essential mechanism in stroke recovery, is impaired in mice with disrupted sleep. These valuable preclinical data, however, have yet to prompt large-scale clinical studies. Moreover, the impact of increased sleep duration (i.e. hypersomnia), CRSWD/sleep–wake cycle disruption on poststroke outcome have not been investigated yet. The same applies to the effect of treatment of sleep disorders on stroke recovery or stroke recurrence. 82
Take-home messages for clinicians and future perspectives
The link between sleep disorders and stroke is compelling (Figure 1).
Figure 1.
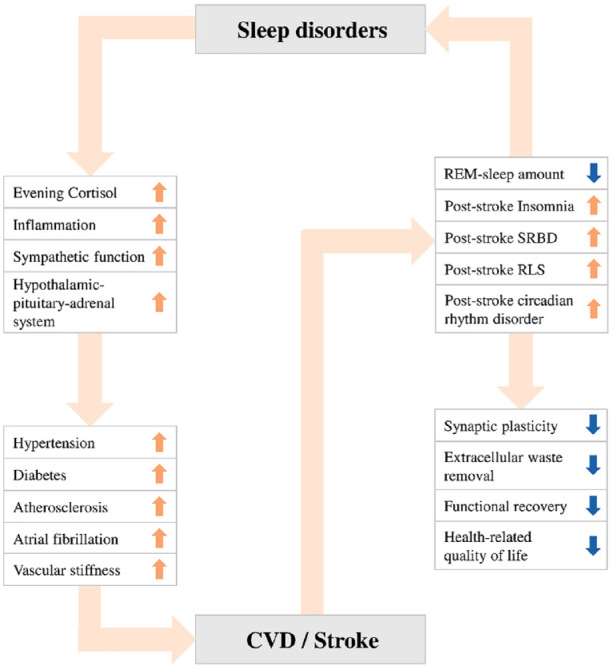
The link between sleep disorders and stroke.
OSA is a relevant risk factor for stroke with its treatment potentially reducing its detrimental effect on poststroke outcome and stroke risk. Still, European but not American guidelines recommend screening and treatment of OSA in the acute phase of stroke.3,4 In a recent review, Baillieul et al. 4 suggest the establishment of systematic screening with easy-to-use techniques in poststroke care with one ongoing study testing whether that should be done in the first days at the stroke unit (NCT03812653). Extending on such investigations and the data presented within this review, the authors support screening for nonrespiratory sleep disorders in stroke patients as well. This may enable the elucidation of pathomechanistic pathways which have to date been hampered through the following factors. First, the relationship between sleep and stroke is complex, particularly because sleep disorders are often co-morbid with other internal, neurological, and psychiatric diseases as well as the potential to co-existing OSA.8,95 Currently, available data mostly stems from observational questionnaire-based studies ensuing a high risk of bias. Second, large-scale studies with adequate screening tools for individual sleep disorders remain scarce. Third, treatment methods of nonrespiratory sleep disorders poststroke and whether current sleep guideline-recommended treatments, including the use of benzodiazepines in insomnia, relate to poststroke outcome are understudied.3,96 A shared statement of the European academy of neurology (EAN)/ the European respiratory society (ERS)/ the European sleep research society (ESRS) and the European stroke organization (ESO) concluded that potential future studies should feature the effect of treating poststroke insomnia on poststroke outcome and risk while taking comorbidities (e.g. depression) and treatment type (i.e. psychotropic drugs) into account. An overview of currently ongoing observational and interventional studies is given in Table 1.
Table 1.
Selection of currently recruiting studies investigating the relationship of sleep and stroke (reference clinicaltrials.gov).
| ClinicalTrials.gov identifier | Investigation | N, year completed |
|---|---|---|
| Observational | ||
| NCT03274505 | Prevalence of sleep disorders in transient ischemic attack and stroke. | N = 200, 2023 |
| NCT05242393 | Association of circadian rhythm disorder and outcome 14 days poststroke. | N = 250, 2024 |
| NCT05012605 | Impact of non-OSA sleep disorders on ADL, functional mobility, and recovery of stroke patients. | N = 200, 2025 |
| NCT04312126 | Investigate the role of memory replay during wakeful rest and sleep (naps) in retaining newly learned skills poststroke. | N = 138, 2025 |
| NCT05746260 | Association of sleep disruption and clinical motor outcomes. | N = 150, 2027 |
| Interventional | ||
| NCT03812653 | Whether CPAP treatment for sleep apnea initiated acutely after stroke improves functional outcome and reduces stroke recurrence. | N = 3062, 2023 |
| NCT05867290 | Effect of mindful music listening on subjective and objective insomnia symptoms as well as mood and fatigue poststroke. | N = 6, 2023 |
| NCT04876001 | Effect of nurse-led brief behavior therapy for poststroke insomnia. | N = 60, 2023 |
| NCT05511285 | Efficacy of digital cognitive behavioral therapy in comparison with usual care alone for reducing insomnia symptoms after stroke. | N = 100, 2024 |
| NCT05247125 | Influence of combined blue light exposure and melatonin therapy on molecular biomarkers of circadian rhythms, sleep characteristics, and stroke outcome in acute stroke patients. | N = 80, 2024 |
| NCT05623137 | Effect of transcutaneous electrical nerve stimulation at acupoints on sleep quality, motor function, and cognition in older adult participants with chronic stroke. | N = 70, 2024 |
| NCT02554487 | Effect of early treatment of sleep apnea with adaptive servoventilation on the evolution of stroke lesion volume and clinical outcomes. | N = 201, Unknown |
| NCT05289518 | Effect of remote ischemic conditioning on stroke-related insomnia. | N = 136, Unknown |
| NCT05170386 | Effect of cognitive training on poststroke sleeping disorders. | N = 40, Unknown |
OSA: obstructive sleep apnea; CPAP: continuous positive airway pressure; ADL: activity of daily living.
Large-scale studies based on systematic screening of sleep disorders in stroke patients using stringent inclusion criteria are needed and should go beyond SRBD. To enable such endeavors, the application of new technologies (i.e. wearables/nearables, automated sleep analysis, or artificial intelligence-based analysis methods) and novel biomarkers such as hypoxic stress, 97 odds-ratio-product quantifying sleep quality, 98 or hypnodensity (a neural network generated hypnogram compounding the entire information collected during sleep delivering more detailed analyses of sleep trends compared with sleep stage scoring) may be of particular value. 99
In conclusion, sleep disorders may have a role in stroke prevention and poststroke management. Still, due to the currently limited available data, clear recommendations on screening methods and the effect of treatment of sleep disorders on poststroke outcome and incident stroke risk should be a focus of future research.
Search strategy and selection criteria
Search terms “sleep,” “insomnia,” “narcolepsy,” “restless legs syndrome,” “periodic limb movements during sleep,” “excessive daytime sleepiness” AND “stroke” OR “cerebrovascular” in PubMed. English articles from 1990 to March 2023. Publications recommended by senior authors (MK, BH, AH, and SK) and cited by key articles were added. Furthermore, search terms “stroke” and “sleep” applied to ClinicalTrials.gov database.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by VASCage—Research Centre on Clinical Stroke Research (Austrian Research Promotion Agency/Österreichische Forschungsförderungsgesellschaft; FFG Project number: 898252).
ORCID iDs: Lukas Mayer-Suess  https://orcid.org/0000-0002-2856-0101
https://orcid.org/0000-0002-2856-0101
Stefan Kiechl  https://orcid.org/0000-0002-9836-2514
https://orcid.org/0000-0002-9836-2514
References
- 1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011; 32: 1484–1492. [DOI] [PubMed] [Google Scholar]
- 3. Bassetti CLA, Randerath W, Vignatelli L, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur J Neurol 2020; 27: 1117–1136. [DOI] [PubMed] [Google Scholar]
- 4. Baillieul S, Dekkers M, Brill AK, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol 2022; 21: 78–88. [DOI] [PubMed] [Google Scholar]
- 5. Lau HL, Rundek T, Ramos AR. Sleep and stroke: new updates on epidemiology, pathophysiology, assessment, and treatment. Curr Sleep Med Rep 2019; 5: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Q, Chen L, Xu Q, Xu J, Zhang L, Wang J. Association between obstructive sleep apnea and risk for post-stroke anxiety: a Chinese hospital-based study in noncardiogenic ischemic stroke patients. Sleep Med 2023; 107: 55–63. [DOI] [PubMed] [Google Scholar]
- 7. Wenning GK, Kiechl S, Seppi K, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol 2005; 4: 815–820. [DOI] [PubMed] [Google Scholar]
- 8. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014; 146: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 9. Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord 2011; 26: 114–120. [DOI] [PubMed] [Google Scholar]
- 10. Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. Sleep Med 2003; 4: 101–119. [DOI] [PubMed] [Google Scholar]
- 11. Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev 2006; 10: 169–177. [DOI] [PubMed] [Google Scholar]
- 12. Scofield H, Roth T, Drake C. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep 2008; 31: 1221–1227 [PMC free article] [PubMed] [Google Scholar]
- 13. Innes KE, Selfe TK, Agarwal P. Restless legs syndrome and conditions associated with metabolic dysregulation, sympathoadrenal dysfunction, and cardiovascular disease risk: a systematic review. Sleep Med Rev 2012; 16: 309–339. [DOI] [PubMed] [Google Scholar]
- 14. Schilling C, Schredl M, Strobl P, Deuschle M. Restless legs syndrome: evidence for nocturnal hypothalamic-pituitary-adrenal system activation. Mov Disord 2010; 25: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 15. Alessandria M, Provini F. Periodic limb movements during sleep: a new sleep-related cardiovascular risk factor. Front Neurol 2013; 4: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med 2013; 14: 555–561. [DOI] [PubMed] [Google Scholar]
- 17. Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the sleep heart health study. Neurology 2008; 70: 35–42. [DOI] [PubMed] [Google Scholar]
- 18. Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep 2017; 40: zsx013. [DOI] [PubMed] [Google Scholar]
- 19. Weinstock LB, Walters AS, Paueksakon P. Restless legs syndrome—theoretical roles of inflammatory and immune mechanisms. Sleep Med Rev 2012; 16: 341–354. [DOI] [PubMed] [Google Scholar]
- 20. Cikrikcioglu MA, Hursitoglu M, Erkal H, et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest 2011; 41: 734–742. [DOI] [PubMed] [Google Scholar]
- 21. Baskol G, Korkmaz S, Erdem F, Caniklioglu A, Kocyigit M, Aksu M. Assessment of nitric oxide, advanced oxidation protein products, malondialdehyde, and thiol levels in patients with restless legs syndrome. Sleep Med 2012; 13: 414–418. [DOI] [PubMed] [Google Scholar]
- 22. Chenini S, Barateau L, Rassu AL, et al. Systematic assessment of autonomic symptoms in restless legs syndrome. Sleep Med 2021; 80: 30–38. [DOI] [PubMed] [Google Scholar]
- 23. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 2019; 15: 519–539. [DOI] [PubMed] [Google Scholar]
- 24. Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med 2017; 33: 13–18. [DOI] [PubMed] [Google Scholar]
- 25. Berteotti C, Berteotti C, Silvani A. The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clin Auton Res 2018; 28: 545–555. [DOI] [PubMed] [Google Scholar]
- 26. McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019; 566: 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben-Joseph RH, Saad R, Black J, et al. Cardiovascular Burden of Narcolepsy Disease (CV-BOND): a real-world evidence study. Sleep 2023; 46: zsad161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cesari M, Heidbreder A, St Louis EK, et al. Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: guidelines from the International RBD Study Group. Sleep 2022; 45: zsab257. [DOI] [PubMed] [Google Scholar]
- 29. Dauvilliers Y, Schenck CH, Postuma RB, et al. REM sleep behaviour disorder. Nat Rev Dis Primers 2018; 4: 19. [DOI] [PubMed] [Google Scholar]
- 30. Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009; 72: 1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019; 142: 744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma C, Pavlova M, Liu Y, et al. Probable REM sleep behavior disorder and risk of stroke: a prospective study. Neurology 2017; 88: 1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terzaghi M, Pilati L, Ghiotto N, et al. Twenty-four-hour blood pressure profile in idiopathic REM sleep behavior disorder. Sleep 2022; 45: zsab239. [DOI] [PubMed] [Google Scholar]
- 34. Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep 2004; 27: 1567–1596. [DOI] [PubMed] [Google Scholar]
- 35. Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep 2010; 33: 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res 2013; 36: 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011; 135: 10–19. [DOI] [PubMed] [Google Scholar]
- 38. He Q, Zhang P, Li G, Dai H, Shi J. The association between insomnia symptoms and risk of cardio-cerebral vascular events: a meta-analysis of prospective cohort studies. Eur J Prev Cardiol 2017; 24: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 39. McCarthy CE, Yusuf S, Judge C, et al. Sleep patterns and the risk of acute stroke: results from the INTERSTROKE international case-control study. Neurology 2023; 100: e2191–e2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the coronary artery risk development in young adults (CARDIA) sleep study. Diabetes Care 2011; 34: 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 42. Dai Y, Chen B, Chen L, et al. Insomnia with objective, but not subjective, short sleep duration is associated with increased risk of incident hypertension: the sleep heart health study. J Clin Sleep Med 2023; 19: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leng Y, Cappuccio FP, Wainwright NWJ, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology 2015; 84: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramos AR, Dong C, Rundek T, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res 2014; 23: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwon HS, Kim C, Kim YS, et al. Long sleep duration and dissatisfaction with sleep quality are associated with ischemic stroke in young patients. Cerebrovasc Dis. Epub ahead of print 21 March 2023. DOI: 10.1159/000530003. [DOI] [PubMed] [Google Scholar]
- 46. Wang H, Sun J, Sun M, Liu N, Wang M. Relationship of sleep duration with the risk of stroke incidence and stroke mortality: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Sleep Med 2022; 90: 267–278. [DOI] [PubMed] [Google Scholar]
- 47. Cai C, Atanasov S. Long sleep duration and stroke-highly linked, poorly understood. Neurol Int 2023; 15: 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brown DL, Feskanich D, Sánchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol 2009; 169: 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012; 345: e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Windt C, van Gijn J. Cerebral infarction does not occur typically at night. J Neurol Neurosurg Psychiatry 1988; 51: 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke 1998; 29: 992–996. [DOI] [PubMed] [Google Scholar]
- 52. Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology 2011; 76: 1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pariona-Vargas F, Mun KT, Lo EH, et al. Circadian variation in stroke onset: differences between ischemic and hemorrhagic stroke and weekdays versus weekends. J Stroke Cerebrovasc Dis 2023; 32: 107106. [DOI] [PubMed] [Google Scholar]
- 54. Dallman MF, Strack AM, Akana SF, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 1993; 14: 303–347. [DOI] [PubMed] [Google Scholar]
- 55. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338: 171–179. [DOI] [PubMed] [Google Scholar]
- 56. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016; 80(1): 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Phua CS, Jayaram L, Wijeratne T. Relationship between sleep duration and risk factors for stroke. Front Neurol 2017; 8: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab 2002; 87: 5587–5593. [DOI] [PubMed] [Google Scholar]
- 59. Luo Y, Yu G, Liu Y, Zhuge C, Zhu Y. Sleep quality after stroke: a systematic review and meta-analysis. Medicine 2023; 102: e33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miano S, Fanfulla F, Nobili L, et al. SAS CARE 1: sleep architecture changes in a cohort of patients with Ischemic Stroke/TIA. Sleep Med 2022; 98: 106–113. [DOI] [PubMed] [Google Scholar]
- 61. Tobaldini E, Proserpio P, Oppo V, et al. Cardiac autonomic dynamics during sleep are lost in patients with TIA and stroke. J Sleep Res 2020; 29: e12878. [DOI] [PubMed] [Google Scholar]
- 62. Pajediene E, Paulekas E, Salteniene V, et al. Diurnal variation of clock genes expression and other sleep-wake rhythm biomarkers among acute ischemic stroke patients. Sleep Med 2022; 99: 1–10. [DOI] [PubMed] [Google Scholar]
- 63. Sharma R, Chischolm A, Parikh M, Qureshi AI, Sahota P, Thakkar MM. Ischemic stroke disrupts sleep homeostasis in middle-aged mice. Cells 2022; 11: 2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lisabeth LD, Zhang G, Chervin RD, et al. Longitudinal assessment of sleep Apnea in the year after stroke in a population-based study. Stroke 2023; 54: 2356–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol 2010; 6: 337–346. [DOI] [PubMed] [Google Scholar]
- 66. Winter AC, Schürks M, Glynn RJ, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med 2013; 126: 220–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee SJ, Kim JS, Song IU, An JY, Kim YI, Lee KS. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord 2009; 24: 77–84. [DOI] [PubMed] [Google Scholar]
- 68. Gupta A, Shukla G, Sharma G, Roy A, Afsar M, Bhargava B. Restless legs syndrome/Willis-Ekbom disease among patients with resistant hypertension versus stroke patients–a prospective study. Sleep Breath 2022; 26: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 69. Wang XX, Feng Y, Tan EK, Ondo WG, Wu YC. Stroke-related restless legs syndrome: epidemiology, clinical characteristics, and pathophysiology. Sleep Med 2022; 90: 238–248. [DOI] [PubMed] [Google Scholar]
- 70. Wu X, Xu J, Lu B. Acute post-stroke restless legs syndrome: the body of caudate nucleus considerations. Sleep Med 2020; 70: 66–70. [DOI] [PubMed] [Google Scholar]
- 71. Duss SB, Bauer-Gambelli SA, Bernasconi C, et al. Frequency and evolution of sleep-wake disturbances after ischemic stroke: a 2-year prospective study of 437 patients. Sleep Med 2023; 101: 244–251. [DOI] [PubMed] [Google Scholar]
- 72. Manconi M, Fanfulla F, Ferri R, et al. Periodic limb movements during sleep in stroke/TIA: prevalence, course, and cardiovascular burden. Neurology 2018; 90: e1663–e1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin TC, Zeng BY, Chen YW, et al. Cerebrovascular accident risk in a population with periodic limb movements of sleep: a preliminary meta-analysis. Cerebrovasc Dis 2018; 46: 1–9. [DOI] [PubMed] [Google Scholar]
- 74. Ohayon MM, Priest RG, Zulley J, Smirne S, Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 2002; 58: 1826–1833. [DOI] [PubMed] [Google Scholar]
- 75. Pasic Z, Smajlovic D, Dostovic Z, Kojic B, Selmanovic S. Incidence and types of sleep disorders in patients with stroke. Med Arh 2011; 65: 225–227. [DOI] [PubMed] [Google Scholar]
- 76. Scammell TE, Nishino S, Mignot E, Saper CB. Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology 2001; 56: 1751–1753. [DOI] [PubMed] [Google Scholar]
- 77. Rivera VM, Meyer JS, Hata T, Ishikawa Y, Imai A. Narcolepsy following cerebral hypoxic ischemia. Ann Neurol 1986; 19: 505–508. [DOI] [PubMed] [Google Scholar]
- 78. Reynolds TQ, Roy A. Isolated cataplexy and REM sleep behavior disorder after pontine stroke. J Clin Sleep Med 2011; 7: 211–213. [PMC free article] [PubMed] [Google Scholar]
- 79. Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med 2009; 10: 143–146. [DOI] [PubMed] [Google Scholar]
- 80. Tang WK, Grace Lau C, Mok V, Ungvari GS, Wong KS. Insomnia and health-related quality of life in stroke. Top Stroke Rehabil 2015; 22: 201–207. [DOI] [PubMed] [Google Scholar]
- 81. Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev 2020; 49: 101222. [DOI] [PubMed] [Google Scholar]
- 82. Duss SB, Brill AK, Bargiotas P, et al. Sleep-wake disorders in stroke-increased stroke risk and deteriorated recovery? An evaluation on the necessity for prevention and treatment. Curr Neurol Neurosci Rep 2018; 18: 72. [DOI] [PubMed] [Google Scholar]
- 83. Chen YK, Lu JY, Mok VC, et al. Clinical and radiologic correlates of insomnia symptoms in ischemic stroke patients. Int J Geriatr Psychiatry 2011; 26: 451–457. [DOI] [PubMed] [Google Scholar]
- 84. Fleming MK, Smejka T, Macey E, et al. Improving sleep after stroke: a randomised controlled trial of digital cognitive behavioural therapy for insomnia. J Sleep Res 2023: e13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Palomäki H, Berg A, Meririnne E, et al. Complaints of poststroke insomnia and its treatment with mianserin. Cerebrovasc Dis 2003; 15: 56–62. [DOI] [PubMed] [Google Scholar]
- 86. Zhu J, Jiang J, Zhang Y, Qi J, Fan T. Non-benzodiazepine hypnotic drug is correlated with decreased risk of ischemic stroke. Int J Clin Exp Med 2016; 9: 23777–23780. [Google Scholar]
- 87. Huang WS, Muo CH, Chang SN, Chang YJ, Tsai CH, Kao CH. Benzodiazepine use and risk of stroke: a retrospective population-based cohort study. Psychiatry Clin Neurosci 2014; 68: 255–262. [DOI] [PubMed] [Google Scholar]
- 88. Boulos MI, Wan A, Black SE, Lim AS, Swartz RH, Murray BJ. Restless legs syndrome after high-risk TIA and minor stroke: association with reduced quality of life. Sleep Med 2017; 37: 135–140. [DOI] [PubMed] [Google Scholar]
- 89. Medeiros CA, de Bruin PF, Paiva TR, Coutinho WM, Ponte RP, de Bruin VM. Clinical outcome after acute ischaemic stroke: the influence of restless legs syndrome. Eur J Neurol 2011; 18: 144–149. [DOI] [PubMed] [Google Scholar]
- 90. Glozier N, Moullaali TJ, Sivertsen B, et al. The course and impact of poststroke insomnia in stroke survivors aged 18 to 65 years: results from the psychosocial outcomes in StrokE (POISE) study. Cerebrovasc Dis Extra 2017; 7: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kim KT, Moon HJ, Yang JG, Sohn SI, Hong JH, Cho YW. The prevalence and clinical significance of sleep disorders in acute ischemic stroke patients-a questionnaire study. Sleep Breath 2017; 21: 759–765. [DOI] [PubMed] [Google Scholar]
- 92. Zhang Z, Wang M, Gill D, Zhu W, Liu X. Genetically predicted sleep traits and functional outcome after ischemic stroke: a mendelian randomization study. Neurology 2023; 100: e1159–e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Vivo L, Bellesi M, Marshall W, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017; 355: 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zunzunegui C, Gao B, Cam E, Hodor A, Bassetti CL. Sleep disturbance impairs stroke recovery in the rat. Sleep 2011; 34: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bassetti CL, Ferini-Strambi L, Brown S, et al. Neurology and psychiatry: waking up to opportunities of sleep: state of the art and clinical/research priorities for the next decade. Eur J Neurol 2015; 22: 1337–1354. [DOI] [PubMed] [Google Scholar]
- 96. Hale E, Gottlieb E, Usseglio J, Shechter A. Post-stroke sleep disturbance and recurrent cardiovascular and cerebrovascular events: a systematic review and meta-analysis. Sleep Med 2023; 104: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Blanchard M, Gervès-Pinquié C, Feuilloy M, et al. Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur Respir J 2021; 57: 2004022. [DOI] [PubMed] [Google Scholar]
- 98. Younes M, Ostrowski M, Soiferman M, et al. Odds ratio product of sleep EEG as a continuous measure of sleep state. Sleep 2015; 38: 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stephansen JB, Olesen AN, Olsen M, et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat Commun 2018; 9: 5229. [DOI] [PMC free article] [PubMed] [Google Scholar]


