Abstract
Self-treatment with vitamin, mineral, and herbal supplements has become increasingly common among patients for the treatment of psychiatric disorders. Magnesium, in particular, is popular on social media for the treatment of anxiety and insomnia. Meanwhile, preclinical studies support associations between magnesium status, sleep quality, and symptoms of anxiety. The extent to which these claims are evidence-based is unclear. Therefore, a systematic review was performed to provide an updated examination of the clinical evidence on the use of magnesium for the treatment of the above conditions given the popularity of such supplements among patients and the public at large.
A thorough search of the PubMed database was performed and results were systematically reviewed using PRISMA guidelines. The search was limited to anxiety disorders and sleep disorders and included interventional trials only. Exclusion criteria included insufficient (<50 mg/12.5% of recommended daily allowance (RDA)) or unknown magnesium dose, >3 other potentially active compounds present in the formulation, and articles in languages other than English. This query returned 860 articles of which 15 met full inclusion criteria. Eight measured sleep-related outcomes, seven measured anxiety-related outcomes, and one examined both. Sleep quality was measured most frequently using the Pittsburg Sleep Quality Index (PSQI). Anxiety measures included self-reported measures such as the Hamilton Anxiety Scale. The majority of included studies demonstrated improvement in at least one sleep- or anxiety-related parameter. Five out of eight sleep-related studies reported improvements in sleep parameters, while two studies reported no improvements, and one reported mixed results. Five out of seven studies measuring anxiety-related outcomes reported improvements in self-reported anxiety.
Firm conclusions were limited by the heterogeneity of the data and the small number of participants involved in most of the studies. The dosages, formulations, and durations of the magnesium interventions used also differed across studies. Furthermore, some studies included additional, potentially active ingredients, further complicating interpretations. Given the generally positive results across studies, the preponderance of preclinical evidence, and minimal side effects, however, supplemental magnesium is likely useful in the treatment of mild anxiety and insomnia, particularly in those with low magnesium status at baseline. Notably, both negative anxiety trials featured populations with underlying endocrine factors likely contributing to their symptoms (patients with premenstrual symptoms and post-partum women). Nonetheless, larger, randomized clinical trials are needed to confirm efficacy and to establish the most effective forms and dosages of magnesium for the treatment of insomnia and anxiety disorders.
Keywords: ocd/anxiety disorders, mineral supplements, micro-nutrient, complementary and alternative medicine (cam), sleep quality & quantity, sleep, insomnia, clinical anxiety, magnesium oxide, magnesium supplements
Introduction and background
Interest in magnesium as a possible over-the-counter remedy for anxiety and sleep disorders is increasing among the lay public. On social media in particular, influencers frequently promote magnesium compounds and products such as anxiolytics and sleep aids. Magnesium-related videos uploaded to TikTok, a popular short-form video-sharing platform available on smartphones, commonly have hundreds of thousands to millions of views. The platform's impact on shaping perceptions and trends among its youthful audience is substantial, highlighting the importance of providing accurate and current information in the literature. Anecdotally, patients in our outpatient psychiatric practice increasingly inquire about magnesium-based products for the treatment of these disorders and may already be supplementing with the mineral upon presentation to our clinic. As such, there is a significant need for an up-to-date review of the evidence on these compounds as they relate to anxiety- and sleep-related symptoms as well as a review of their tolerability and safety.
Magnesium is a ubiquitous element both in the earth's geology and human biology. It is the second most abundant cation in cells, with body stores of about 25 g [1]. It is known to be essential for the function of at least 300 or more enzymes in the body [2]. While frank deficiency is uncommon (and can result in seizures and arrhythmias among other severe adverse effects), it is thought that upward of half of the United States population does not meet the recommended daily allowance (RDA) for magnesium and is at risk for possible insufficiency [3]. The typical serum range for magnesium on lab assays is between roughly 0.75 and 0.95 mmol/L(~1.8 to 2.3 mg/dL) [4]. However, serum magnesium is believed to be an unreliable reflection of magnesium stores, as a significant portion of the body's magnesium is found in bone, muscle, and other tissues [1,3]. Other means of measuring magnesium status, including red blood cell magnesium content and urinary excretion of magnesium after magnesium oral challenge, are thought to possibly reflect body magnesium status more accurately [1].
Clinically, magnesium preparations have been used as prophylaxis in the treatment of migraine, arrhythmias, and acute asthma exacerbations, and have a long history of use in higher doses as a laxative [5]. Magnesium is also used in obstetrics for the treatment of eclampsia and preeclampsia and in neuroprotection for premature infants [5].
Importantly, magnesium exists in the supplement industry as a wide variety of compounds, both inorganic and organic forms, as various salts and amino acid chelates [2,6]. For instance, the mineral often exists on the formularies of hospitals as magnesium oxide (MgO) tablets or as a liquid laxative preparation as hydroxide or citrate (e.g., milk of magnesia). Various other forms of magnesium are also readily available commercially, including magnesium glycinate, taurate, sulfate, chloride, malate, threonate, aspartate, citrate, and orotate among others [6]. It is generally believed that organic forms, such as the amino acid chelates above, are absorbed and assimilated by the body more easily versus inorganic forms (e.g., MgO) [6].
Importantly, it has been discovered that magnesium ions function in the body as NMDA receptor antagonists [7,8]. Mg2+ ions occupy the NMDA receptor pore at typical neuronal membrane potential [7]. As neurons depolarize, the magnesium block is lifted allowing for the movement of Ca2+ and Na+ ions through the receptor pore [8]. Additionally, some research has shown magnesium may also exhibit some level of agonist activity at GABA-A receptors as well [7]. In a broader sense, magnesium often functions to oppose the excitatory action of calcium in the body, such as in the process of muscle contraction or within the central nervous system at NMDA receptors as described above [9].
Notably, animal models have repeatedly demonstrated the beneficial effects of magnesium administration in rodents as well as the adverse effects of induced magnesium depletion on anxiety and sleep parameters in rodents [10-15]. Regarding sleep, rodent models have shown magnesium depletion induces significant sleep disorganization, with decreased slow-wave sleep and increased wakefulness [14]. Similarly, multiple preclinical studies have demonstrated that magnesium depletion results in increased anxiety and depressive behaviors in rodents [16]. For example, in a study by Sartori et al. it was shown that magnesium-depleted rodents experienced an upregulated hypothalamic-pituitary-adrenal (HPA) axis, with significant increases in ACTH and CRH, which were then normalized with antidepressant treatment [17].
With the notable increase in interest in magnesium evident on social media platforms and among the general public, coupled with the consistent preclinical evidence, we aimed to systematically examine the existing evidence for the clinical application of magnesium supplements in the treatment of sleep and anxiety disorders in human subjects. This endeavor is intended to contribute valuable insights for future clinical practice.
Review
A systematic review of interventional trials was conducted using the PRISMA 2020 guidelines for new systematic reviews. The Pubmed database was queried up until the date the search was undertaken, 7/22/2023, using the search string: ((magnesium*[Title/Abstract]) OR (Mg*[Title/Abstract])) AND ((anxi*[Title/Abstract]) OR (GAD[Title/Abstract]) OR (Generalized Anxiety Disorder[Title/Abstract]) OR (insomnia[Title/Abstract]) OR (sleep*[Title/Abstract]) OR (panic*[Title/Abstract])).
This search captured various derivatives of the word “anxiety” by the included ‘anxi*’ term and in general was designed to capture a range of anxiety disorders. It was also designed to capture general sleep disorders with the sleep* term, particularly studies relating to insomnia. This search was designed to exclude stress or stress-related disorders, even though magnesium is often promoted as a stress-reducing compound anecdotally and on social media.
Only interventional studies were included in the review (either randomized control trial (RCT) or observational). Inclusion criteria required sufficient reporting detail and inclusion of all relevant variables including magnesium dose, form, and period of administration. Inclusion was not restricted by the age of the participant or the form of magnesium. Included studies featured standardized instruments to measure anxiety and sleep outcomes (or polysomnography in the case of sleep disorders). Only human studies were included (all animal studies were excluded).
Exclusion criteria included an insufficient dose of magnesium, defined as ≤50 mg/12.5% of RDA for purposes of this review, as such amounts were considered unlikely to be therapeutic. Additional exclusion criteria included unknown magnesium dose, the presence of >3 other potentially active compounds present in the formulation, and articles in languages other than English. The limitation on >3 potentially active ingredients was included to minimize the difficulty in attributing positive effects to magnesium that would result from the inclusion of multiple other active ingredients (Table 1).
Table 1. Inclusion and exclusion criteria.
RCT, randomized control trial
| Inclusion criteria |
| · Interventional studies only (either RCT or observational) |
| · Sufficient reporting detail of all relevant variables including magnesium dose, form, and time length of administration |
| · Standardized instruments to measure anxiety or sleep outcomes (or polysomnography in the case of sleep disorders) |
| · Human studies only (all animal studies were excluded) |
| · Inclusion not restricted by age of participant or type of magnesium salt |
| Exclusion criteria |
| · Insufficient dose of magnesium, defined as ≤50 mg/12.5% of RDA for purposes of this review or unknown magnesium dose |
| · Presence of >3 other potentially active compounds in the formulation |
| · Manuscript language other than English |
Following completion of the formal search and exclusion of articles as described in the PRISMA flow chart, article information was extracted including the following variables: study name, authors, year, country, study design, number of participants and specific population studied, inclusion/exclusion criteria of the study, Mg form, dose, presence of additional active ingredients, duration, outcome variables, and description of results.
Results and discussion
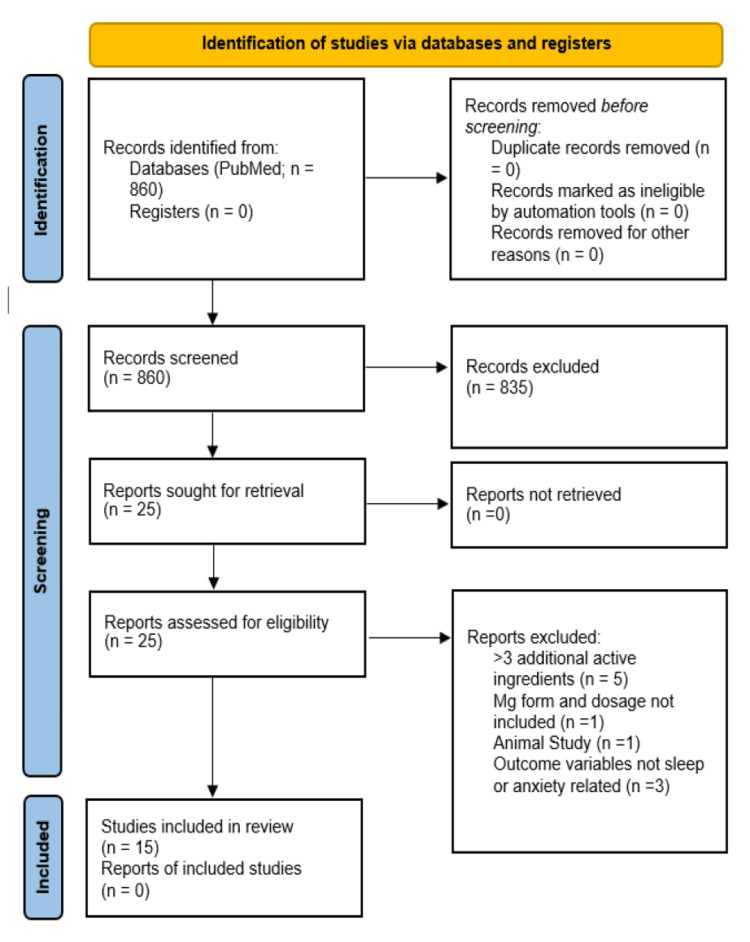
The search revealed a total of 860 articles, of which 835 were excluded using the above criteria after reviewing the title/abstract. The full texts of the remaining articles were obtained from the Jersey Shore University Medical Center (JSUMC) medical library and reviewed against the above criteria. Of these, an additional 10 articles were excluded following full-text review for the following reasons: Five featured >3 potentially active ingredients, one lacked sufficient information detailing the intervention (lack of Mg dose, form, and duration of administration), one was an animal study, and three did not include sleep- or anxiety-related outcome variables. The full PRISMA flowchart is in Figure 1.
Figure 1. PRISMA flow diagram for systematic reviews .
Of the 15 studies included in the final systematic review, eight studies reported sleep-related outcomes and seven reported anxiety-related outcomes. Nearly all studies were conducted in the Middle East or Europe, with only one study conducted in the USA. The two most common countries of origin were France (four studies, one reporting sleep outcomes and three reporting anxiety outcomes) and Iran (five studies, three reporting sleep outcomes and two reporting anxiety outcomes). All but four studies were RCTs. The vast majority of studies used MgO as the specific form of magnesium studied, with the maximum daily dose used in any study being 729 mg of magnesium daily. Adverse events were generally mild and uncommon, with the most commonly reported adverse effect being loose stool.
Sleep-related studies
Eight of the 15 studies included in the review reported on magnesium’s effect on sleep quality. The primary sleep metric used by the majority of studies was the Pittsburgh Sleep Quality Index (PSQI), a self-reported questionnaire validated in clinical populations including patients with fibromyalgia, psychiatric disorders such as major depressive disorder, and patients with sleep disorders such as insomnia [18]. The PSQI consists of 19 self-reported items across seven subcategories, including sleep quality, latency, total sleep time, disturbances of sleep, and dysfunction during the day. Six of the eight sleep-related studies used the PSQI to measure subjective sleep. Four out of the six of these studies featured positive results with improvements in the PSQI. Overall, five out of eight sleep-related studies reported generally positive results on sleep parameters. Two studies reported negative results and one featured mixed results. Six out of eight were RCT study designs, while two were open-label pilot studies.
Five studies used MgO as the intervention, ranging in dosage from 250 mg to 729 mg. Another used magnesium chloride, dosed at 100 mg of a specially formulated, slow-release delivery system. One study used magnesium citrate and one used magnesium L-aspartate. The length of interventions ranged considerably from five days to 10 weeks. Four out of the five studies using MgO reported generally positive results either by self-assessment via PSQI or by EEG measurements. Notably the one negative study featuring MgO as the intervention also used the lowest dose of MgO out of all the studies, 250 mg. Furthermore, the dose was administered early in the day, given with breakfast, where it was likely metabolized and excreted by bedtime. The study using MgCl also reported no significant improvements in sleep-related outcomes. This study, featuring adults with fibromyalgia, dosed MgCl at 100 mg of elemental magnesium, which was the lowest dose of Mg across all sleep-related studies. The investigators used a formulation that reportedly featured higher absorption to counter the lower dose of Mg, though there were no changes in serum or RBC magnesium by the end of the study, in addition to no change in sleep quality as measured by PSQI.
The study by Hornyak et al. (2004) using magnesium L-aspartate featured among the highest daily dose of Mg of any sleep-related study included in the review (729 mg). This study, conducted in primary alcohol-dependent patients during subacute withdrawal from alcohol, described improvements in both subjective sleep quality as measured by PSQI as well as objective EEG measures such as significantly decreased sleep onset latency, from an average of 40.6 minutes pre-treatment to 21.7 minutes (P=0.03). Another study featuring 729 mg of magnesium (as MgO) by Held et al. conducted in a population of healthy elderly subjects aged 61-81 years demonstrated an increase in slow wave sleep by EEG from 10.1 minutes to 16.5 minutes, P< 0.05, along with increases in delta power (a measure of slow wave sleep) and sigma power.
The only study featuring magnesium citrate (Nielsen et al., 2010), conducted in a population of adults >51 years with baseline PSQI >5 (reflecting relatively poor baseline sleep quality), demonstrated significant improvements in sleep quality regardless of allocation to placebo or treatment group. PSQI declined overall from 10.4 to 6.6 (P<0.0001) across both groups. Curiously, RBC magnesium increased significantly in both the treatment group as well as the placebo group, suggesting a possible change in behavior by the participants due to being observed (i.e., the Hawthorne effect). A subset of patients in this study with high baseline inflammatory markers (as demonstrated by high sensitivity CRP >3.0) did demonstrate a decrease in hsCRP with magnesium administration (Table 2).
Table 2. Summaries of magnesium studies reporting sleep-related outcomes.
RCT, randomized control trial; PSQI, Pittsburg Sleep Quality Index; PLMS, Periodic Limb Movements in Sleep; RLS, restless leg syndrome; DASS, Depression, Anxiety, Stress Scales; RDA, recommended daily allowance; DHEA, dehydroepiandrosterone; SWS, slow wave sleep
| Authors | Country | Study design | Participants | Inclusion/exclusion criteria | Intervention | Outcome measures | Relevant results | Positive/negative | ||||||
| N | Population | Inclusion | Exclusion | Magnesium form | Dose | Additional therapies | Duration | Description | ||||||
| Gholizadeh‐Moghaddam et al. (2022) [19] | Iran | Double‑blind RCT | 64; control group N=32, tx group N=32 | Females aged 18-45 | Females sex; age 18-45; PCOS dx per Rotterdam criteria | New or change in medication within two weeks; peri- or post-menopausal status; concurrent use of any other vitamin or mineral supplement; pregnancy | MgO | 250 mg | No | 10 weeks | 250 mg MgO taken for 10 weeks after breakfast | Sleep quality as assessed by the PSQI; serum concentration of magnesium, DHEAs, and testosterone; hirsutism as assessed by the Ferriman–Gallwey questionnaire | Sleep quality did not significantly improve after 10 weeks in either the treatment or the control group; no significance between group differences in regard to sleep quality | N |
| Saba et al. (2022) [20] | Iran | Single-blind controlled trial | 60; control group N=30, tx group N=30 | Hospitalized adults undergoing open heart surgery | Age <70 years old; candidate for elective CABG surgery | Hx of atrial fibrillation prior to CABG surgery; hx of liver or renal failure; hx of stroke or recent TIA; postoperative respiratory failure; liver or kidney failure; emergency surgery during the study period; chronic diarrhea; allergy to study drug; hx of sleep disorders; hx of anxiety or depression; parenteral MgSO4 tx | MgO | 500 mg | No | Five days | 500 mg MgO for five days (two pills of 250 mg MgO each) | Sleep quality as assessed by the PSQI | The mean PSQI score was significantly lower in the treatment group versus the control group at the end of the study period; within-group and between-group differences in PSQI score were significant (P=0.001 and P=0.021, respectively) | P |
| Nielsen et al. (2010) [21] | USA | Double‑blind RCT | 96; control group N=47, tx group N=49 | Adults aged >51 years with PSQI >5 | Age >51 years; PSQI >5; normal CBC, liver and kidney function tests | Use of supplements containing >100 mg of magnesium; BMI >40; COPD; use of O2 or CPAP; use of ACE-inhibitors; use of magnesium or potassium retaining drugs | Magnesium citrate | 320 mg | No | Eight weeks | 320 mg of magnesium citrate across five capsules - two taken with AM meal, one taken with noon meal, and two taken with PM meal | Sleep quality as assessed by the PSQI | PSQI declined significantly from 10.4 to 6.6 (P<0.0001); RBC magnesium increased significantly in both the treatment and placebo groups; hsCRP decreased significantly in the subset of pts with hsCRP>3.0 with magnesium treatment but not placebo | Mixed |
| Hornyak et al. (1998) [22] | Germany | Open pilot study | 10 | Patients with RLS or PLMS | Diagnosed RLS or PLMS | Severe RLS or PLMS; abnormal pre-study medical workup (ECG, EEG, CBC); elevated creatinine; use of sedatives or psychiatric medication within four weeks; uremia; chronic bronchitis; Fe deficiency; pregnancy | MgO | 291.6 mg | No | Four to six weeks (average 5.1 weeks) | 291.6 mg MgO QHS | Sleep quality as assessed by the PSQI; sleep EEG; subjective sleep quality measured by SF-A questionnaire; # of periodic limb movements during sleep | PLMS-associated w/ arousals decreased significantly from 17±7 versus 7±7 events per hour of total sleep time (P<0.05); sleep efficiency increased from 75±12% to 85±8% (P<0.01); PLMS w/o arousals trended lower from 33±16 to 21±23 (P=0.07) | P |
| Hornyak et al. (2004) [23] | Germany | Open pilot study | 11 | EtOH-dependent patients in subacute withdrawal | Primary EtOH dependence in subacute withdrawal (two weeks since last drink) | Cognitive impairment; major medical comorbidities (e.g., renal failure, heart failure); OSA, tx with anti-craving medications; magnesium supplementation within two weeks; prolonged EtOH withdrawal; depression; use of psychoactive medication within seven days | Magnesium L-aspartate | 729 mg | No | Four weeks | 243 mg of magnesium aspartate q AM, 486 mg of magnesium Aspartate QHS | Sleep quality measured by PSQI; sleep EEG; subjective sleep quality measured by SF-A; # of periodic limb movements during sleep | Sleep-onset latency decreased significantly from 40.6 to 21.7 minutes (P=0.03); PSQI subjective sleep quality score improved significantly from a mean of 8.1 to 5.8 (P=0.05); total sleep time and slow wave sleep time increased but did not achieve significance | P |
| Held et al. (2002) [24] | Germany | Double-blind, randomized, placebo-controlled crossover trial | 12; control group N=6, tx group N=6 | Healthy elderly subjects aged 61-81 | Healthy status, as measured by bloodwork, EEG, and ECG | Personal or family psychiatric hx; personal or family hx of neurocognitive disorder; hx of substance abuse; hx of transmeridian flight within three months; shift work; sleep‑related movement disorders or respiratory disorders | MgO | 243 mg for three days, 486 mg for three days, 729 mg for 14 days | No | 20 days | 20 days of active tx - 243 mg for three days (dosed in AM), 486 mg for three days (243 mg dosed in AM and noon), 729 mg for 14 days (243 mg dosed in AM, noon and PM) | Sleep EEG; serum ACTH, cortisol, AVP, renin, ATII, aldosterone, magnesium | Significant increase in SWS from 10.1 min to 16.5 min (P<0.05); increase in delta power and sigma power (P<0.05); renin and aldosterone increased and cortisol decreased significantly versus the control (P<0.05) | P |
| Macian et al. (2022) [25] | France | Double‑blind RCT | 76; control group N=38, tx group N=38 | Adults with fibromyalgia experiencing moderate to severe stress | Aged >18 years; dx of fibromyalgia (as per ACR 2016 criteria); >18 on the DASS stress subscale | Pregnancy; breastfeeding; women of childbearing age not using contraceptive; diabetes mellitus; kidney disease (CrCl <30); hypermagnesemia (plasma magnesium >1.05 mmol/L); antibiotic treatment; other magnesium-containing medications/supplements | MgCl | 100 mg | No | Four weeks | 100 mg daily of low-dose continuous-release MgCl taken for four weeks | Change in sleep quality as assessed by the PSQI; serum and RBC magnesium measurements | No significant change in sleep quality as measured by PSQI after four weeks; no significant change in serum and RBC magnesium after four weeks | N |
| Abbasi et al. (2012) [26] | Iran | Double‑blind RCT | 46; control group N=23, tx group N=23 | Adults aged 60-75; average age 65±4.6 years | Insomnia as dx by ISI; BMI 25-34.9; magnesium intake <75% of RDA; serum magnesium <0.95 mmL | Tx with loop diuretics, cyclosporine, digoxin, amphotericin, any hormonal therapy; renal disease; acute heart failure; sleep‑related movement disorders or respiratory disorders; any psychiatric disorder; recent stressful life event (e.g., divorce; death or acute illness of a family member); substance/alcohol abuse; transmeridian flight within six weeks | MgO | 500 mg | No | Eight weeks | 500 mg MgO for eight weeks | Sleep time, sleep efficiency, ISI score, sleep onset latency; serum cortisol, renin, and melatonin | Increased sleep time (P=0.002) and sleep efficiency (P=0.03); increased concentration of serum renin (P<0.001) and melatonin (P=0.007); decrease of ISI score (P=0.006), sleep-onset latency (P=0.02), and serum cortisol concentration (P=0.008); early morning awakening (P=0.08), serum magnesium concentration (P=0.06), and total sleep time (P=0.37) did not did not achieve significance | P |
Anxiety-related studies
Seven of the 15 studies included in the systematic review investigated the effects of magnesium supplementation on measures of anxiety. The exact measures used varied across studies with the most common measure being the Hamilton Anxiety Rating Scale (HAM-A), though this was only used in two of the studies [27]. The HAM-A is a commonly used, 14-question assessment of anxiety, with each item rated on a zero to four scale corresponding with "not present" to "very severe." Other anxiety outcome measures used included the Revised Child Anxiety and Depression Scale (RCADS), the Spielberger State-Trait Anxiety Inventory (STAI), the Depression Anxiety Stress Scales (DASS), and the Hospital Anxiety and Depression Scale (HADS) [28-31]. Specific populations studied varied considerably as well and included pediatric migraine patients, women suffering anxiety-related premenstrual symptoms, postpartum women, adults experiencing moderate to severe stress, and hospitalized adults undergoing open heart surgery, as well as patients with generalized anxiety disorder and adjustment disorder with anxiety. Five out of seven studies were RCT study designs while two studies were prospective cohort studies. Intervention length varied significantly from five days to six months, though most trials were between four and eight weeks. Five of the studies used MgO, one used magnesium sulfate, and one used magnesium lactate dihydrate. Importantly, several of the studies included magnesium combined with (three or fewer) other potentially active ingredients in the treatment arms. Three studies included magnesium with vitamin B6 (at doses of 1.4 mg, 30 mg, and 50 mg daily, respectively). Another study used two plant extracts - Crataegus oxyacantha and Eschscholtzia californica - along with 150 mg of elemental magnesium in the form of MgO as the study intervention. Finally, another study combined magnesium and vitamin B6 with 200 mg of fish protein hydrolysate.
Overall, five out of seven studies featuring anxiety-related outcomes reported positive results. The two trials with the greatest reductions in anxiety scores (Noah et al., 2020; Oddoux et al., 2022) used relatively high doses of magnesium (300 mg elemental magnesium each) complexed with other active ingredients (30 mg of pyridoxine and 1.4 mg of pyridoxine plus 200 mg of fish protein hydrolysate). The one study with clear negative results (Edalati Fard et al., 2017) used 320 mg of magnesium sulfate that contained 64.6 mg of elemental magnesium - the lowest amount of magnesium used in any of the 15 studies included in our review. Another study (De Souza et al., 2000) reported decreased self-reported anxiety scores only when magnesium was combined with 50 mg of pyridoxine. However, urinary excretion of magnesium during this trial did not significantly change, leading the authors to question the absorption of the MgO in the trial (Table 3).
Table 3. Summaries of magnesium studies reporting anxiety-related outcomes.
GAD, generalized anxiety disorder; MHQ, menstrual health questionnaire; HAM-A, Hamilton Anxiety Rating Scale; DASS, Depression, Anxiety, Stress Scales; OCPs, oral contraceptive pills; RCADS, Revised Child Anxiety and Depression Scale; HADS, Hospital Anxiety and Depression Scale; CGI, clinical global impression; STAI, Spielberger State-Trait Anxiety Inventory
| Authors | Country | Study design | Participants | Inclusion/exclusion criteria | Intervention | Outcome measures | Relevant results | Positive/negative | ||||||
| N | Population | Inclusion | Exclusion | Magnesium form | Dose | Additional therapies | Duration | Description | ||||||
| Saba et al. (2022) [20] | Iran | Single-blind controlled trial | 60; control group N=30, tx group N=30 | Hospitalized adults undergoing open heart surgery | Age <70 years old; candidate for elective CABG surgery | Hx of atrial fibrillation prior to CABG surgery; hx of liver or renal failure; hx of stroke or recent TIA; postoperative respiratory failure; liver or kidney failure; emergency surgery during the study period; chronic diarrhea; allergy to study drug; hx of sleep disorders; hx of anxiety or depression; parenteral MgSO4 tx | MgO | 500 mg | No | Five days | 500 mg MgO for five days (two pills of 250 mg MgO each) | Anxiety and depressive sx as assessed by the HADS | The mean HADS score was significantly lower in the treatment group versus the control group (P=0.007) | P |
| Kovacevic et al. (2017) [32] | Serbia | Prospective cohort study | 32 | Pediatric migraine patients without comorbidities | Ages 7-17; dx of migraine at least one month prior; no previous migraine prophylaxis | Presence of any other medical, neurological, or psychiatric disorder | MgO; magnesium glycinate | 4-6 mg/kg/day | NSAIDs for acute migraine attacks during the study period | Six months | MgO or magnesium glycinate, dosed at 4-6 mg/kg/day for six months | Anxiety and depressive sx as assessed by the RCADS | Self-reported anxiety scores decreased significantly between baseline and six months (P=0.001); scores between baseline and three months trended lower but did not achieve significance (P=0.115) | P |
| De Souza et al. (2000) [33] | United Kingdom | Double-blind randomized controlled crossover trial | 44 (crossover trial; each served as own control) | Women with premenstrual anxiety sx | Premenstrual scores >30% higher than post-menstrual sx as measured by the MHQ | Patients already taking any vitamin or mineral supplement; any medication aside from OCPs | MgO | 200 mg | Pyridoxine 50 mg | Five menstrual cycles | Crossover to alternate treatment during each subsequent menstrual period (1) MgO 200 mg, (2) MgO + pyridoxine 50 mg, (3) pyridoxine 50 mg, (4) placebo | Self-reported, subjective anxiety sx reported on a five-point ordinal scale; 24-hour magnesium and creatinine output | Significantly lower anxiety scores during treatment with a combination of magnesium and B6 compared with other treatments (P=0.04); urinary magnesium did not differ significantly between groups | Mixed |
| Oddoux et al. (2022) [34] | France | Prospective cohort study | 93 | Adults aged 18-70 with adjustment disorder with anxiety, at least mild-moderate in severity | Adults aged 18-70 years; HAM-A score >20 | Previous treatment for anxiety within the prior three months (including psychotherapy); the presence of any other mental illness; anxiety lasting >3 months; history of substance abuse; >10 mg/kg of caffeine/day; >1 pack of cigarettes/day; pregnancy; breastfeeding; severe medical comorbidity | MgO and magnesium bisglycinate | 300 mg (270 mg as MgO and 30 mg as magnesium bisglycinate) | 200 mg of fish protein hydrolysate ("Gabolysat®") and 1.4 mg of vitamin B6 (as pyridoxal chlorate) | Four weeks | Four weeks of 300 mg magnesium, 200 mg fish protein hydrolysate, and 1.4 mg B6 combo, taken with breakfast | % of patients with ≥50% decrease in HAM-A score; change in HAM-A score; change in Clinical Global Index Scale | 41.9% (39/93) experienced a ≥50% decrease in HAM-A score (primary endpoint); mean HAM-A score decreased by 12.1 +/- 5.7 points (P<0.001); 75.3% improved significantly or very significantly on CGI scale | P |
| Fard et al. (2017) [35] | Iran | Triple-blind RCT | 99; control group N=33, each tx group N=33 | Postpartum women having given birth within 48 hours | ≥18 years old; living in Tabriz, Iran; low-risk pregnancy | Depression (12 or greater on the Edinburgh Scale); any psychiatric history; complications with childbirth; NICU admission or death in the infant; significant stressful event (divorce, hospitalization, or death of relative); chronic illness; liver or kidney disease; hx of infertility or previous miscarriage | MgSO4 | 320 mg (64.6 elemental magnesium) | None | Eight weeks | Eight weeks of either placebo, 27 mg of ZnSO4, or 320 mg of MgSO4 | STAI; Edinburgh Postnatal Depression Scale | No significant differences were found in depression and anxiety scores between the placebo, zinc, and magnesium groups | N |
| Noah et al. (2020) [36] | France | Single-blind RCT | 264 control group N=132, tx group N=132 | Adults aged 18-50 experiencing moderate-severe stress | Ages 18-50 years old; moderate to severe stress as measured by >18 on the DASS stress subscale; low magnesium status as defined by serum values of 0.66-0.84 mmol/L | Use of levodopa, quinidine, and proton-pump inhibitors within the three months prior to screening; alcohol intake of >3 drinks per day; hx of substance abuse; type 1 and type 2 diabetes mellitus; moderate or severe kidney disease; severe hypomagnesemia (serum magnesium of <0.45 mmol/L) | Magnesium lactate dihydrate | 300 mg elemental magnesium total (across six tablets) | 30 mg of pyridoxine (in tx group; just magnesium lactate alone as control) | Eight weeks | Eight weeks of either magnesium lactate 100 mg AM, 100 mg at noon, and 100 mg PM (control group) or eight weeks of magnesium lactate-B6 100 mg-10 mg AM, 100 mg-10 mg at noon, and 100 mg-10 mg PM (tx group) | DASS depression and anxiety subscales | DASS anxiety subscale scores decreased significantly from baseline in both the magnesium and magnesium+B6 groups (P<0.05); no significant difference between groups was found | P |
| Hanus et al. (2003) [37] | France | Double-blind RCT | 264 control group N=134, tx group N=130 | Patients with mild-moderate GAD | Adults >18 years old with mild-moderate GAD | Other axis 1 disorders; treatment with psychotropic medication, sedatives, or magnesium within one month of study | MgO | 248.7 mg MgO (150 mg elemental magnesium) | Extracts of the plants: Crataegus oxyacantha and Eschscholtzia californica | 90 days | Two tablets of 124.35 mg MgO and plant extracts of Crataegus oxyacantha and Eschscholtzia californica (75 mg elemental magnesium per tablet) before AM and PM meal. 300 mg MgO total daily | Anxiety as assessed by the HAM-A; Patient self-reported anxiety scores (VAS 0-100 scale, 100=very anxious); % responsive (>50% reduction in sx); physician’s CGI | Total HAM-A score decreased significantly more in the tx group versus placebo (-10.6±1.2 versus -8.9±1.2, P=0.005); VAS subjective anxiety score decreased significantly more in tx group versus placebo (-38.5 versus -29.2, P=0.005); significantly greater response rate in tx group versus placebo for both the HAM-A and the VAS scores: 45% versus 32%, P=0.017 and 58% versus 43%, P=0.008, respectively | P |
The two studies in which magnesium was not effective in reducing anxiety scores also featured the two populations with possible significant hormonal perturbations contributing to their symptoms, premenstrual and postpartum women. It remains possible that magnesium is less effective in these populations for the treatment of anxiety.
Conclusions
This systematic review was undertaken to identify and summarize available literature on the use of magnesium in the treatment of anxiety and sleep disorders given the increasing use of the mineral by the lay public for the treatment of these conditions. Overall, most studies were small with significant heterogeneity present among the dosages and treatment periods used and the populations studied, thus making firm conclusions and generalizability of findings difficult. However, higher doses of magnesium appear to be more effective in addressing anxiety and sleep disturbances, as all studies with negative results used low comparative dosages of the mineral in their treatment interventions. Additionally, magnesium may be more effective in combination with other active ingredients, specifically vitamin B6, in the treatment of anxiety disorders. It should be noted that vitamin B6 is heavily involved in neurotransmitter synthesis, which may underlie this possible trend. A determination of the optimal form of magnesium for use in anxiety and sleep disorders was not possible in the present review as the vast majority of studies used MgO. As described previously, inorganic forms of magnesium (e.g., MgO) have been suggested to be less readily absorbed in the gastrointestinal tract. Furthermore, larger studies may likely favor other forms of magnesium over the oxide form in the treatment of these disorders in the future. Adverse events in the included studies were mild if present - most commonly increased bowel movements, a well-known side effect of magnesium administration. Given the mineral’s use as a laxative in high doses, it is likely this adverse effect is dose-dependent.
In general, despite notable heterogeneity, the majority of included trials demonstrated at least modest positive results with regard to sleep quality and anxiety across diverse populations. These findings are consistent with animal-based evidence as well as magnesium’s known receptor activity in the central nervous system. Larger, well-designed trials are needed to further characterize specific forms and doses for routine use of magnesium in clinical practice.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Alexander Rawji, Nitin J. Pothen, Saba Afzal, Kelly Mourtzanakis, Morgan R. Peltier
Acquisition, analysis, or interpretation of data: Alexander Rawji, Samreen Awan, Junaid Rana
Drafting of the manuscript: Alexander Rawji, Kelly Mourtzanakis
Critical review of the manuscript for important intellectual content: Alexander Rawji, Nitin J. Pothen, Saba Afzal, Kelly Mourtzanakis, Samreen Awan, Junaid Rana, Morgan R. Peltier
Supervision: Nitin J. Pothen, Saba Afzal
References
- 1.Magnesium: extracellular, intracellular or total magnesium status? Rotondi S, Mazzaferro S. Nephrol Dial Transplant. 2023;38:1349–1351. doi: 10.1093/ndt/gfad059. [DOI] [PubMed] [Google Scholar]
- 2.Role of cellular magnesium in human diseases. Long S, Romani AM. https://pubmed.ncbi.nlm.nih.gov/25839058/ Austin J Nutr Food Sci. 2014;2:1051. [PMC free article] [PubMed] [Google Scholar]
- 3.Magnesium: are we consuming enough? Razzaque MS. Nutrients. 2018;10:1863. doi: 10.3390/nu10121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnesium Fact Sheet for Health Professionals. 2023. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/ https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- 5.The importance of magnesium in clinical healthcare. Schwalfenberg GK, Genuis SJ. Scientifica (Cairo) 2017;2017:4179326. doi: 10.1155/2017/4179326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bioavailability of magnesium food supplements: a systematic review. Pardo MR, Garicano Vilar E, San Mauro Martín I, Camina Martín MA. Nutrition. 2021;89:111294. doi: 10.1016/j.nut.2021.111294. [DOI] [PubMed] [Google Scholar]
- 7.Papadopol V, Nechifor M, Vink R, Nechifor M. Magnesium in the Central Nervous System. Adelaide (AU): University of Adelaide Press; 2011. Magnesium in neuroses and neuroticism. [PubMed] [Google Scholar]
- 8.Na HS, Ryu JH, Do SH. Magnesium in the Central Nervous System. Adelaide (AU): University of Adelaide Press; 2011. The role of magnesium in pain. [PubMed] [Google Scholar]
- 9.Magnesium. 2001. https://lpi.oregonstate.edu/mic/minerals/magnesium https://lpi.oregonstate.edu/mic/minerals/magnesium
- 10.Antidepressant- and anxiolytic-like activity of magnesium in mice. Poleszak E, Szewczyk B, Kedzierska E, Wlaz P, Pilc A, Nowak G. Pharmacol Biochem Behav. 2004;78:7–12. doi: 10.1016/j.pbb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Anxiolytic effect of chronic intake of supplemental magnesium chloride in rat. Macias-Carballo M, Rosas-Navarro S, Lopez-Meraz ML, Beltran-Parrazal L, Morgado-Valle C. Behav Brain Res. 2021;413:113460–113410. doi: 10.1016/j.bbr.2021.113460. [DOI] [PubMed] [Google Scholar]
- 12.Experimental evidence of a potentiation by alpha, beta magnesium L-aspartate of the anxiolytic effect of diazepam. Four-plate test in mice and qEEG study in primates. Borzeix MG, Akimjak JP, Dupont JM, Cahn R, Cahn J. https://europepmc.org/article/med/1799555. Magnes Res. 1991;4:197–200. [PubMed] [Google Scholar]
- 13.Vigilance states and cerebral monoamine metabolism in experimental magnesium deficiency. Poenaru S, Rouhani S, Durlach J, et al. https://europepmc.org/article/med/6083421. Magnesium. 1984;3:145–151. [PubMed] [Google Scholar]
- 14.Effects of a magnesium-deficient diet on sleep organization in rats. Depoortere H, Françon D, Llopis J. Neuropsychobiology. 1993;27:237–245. doi: 10.1159/000118988. [DOI] [PubMed] [Google Scholar]
- 15.Magnesium involvement in sleep: genetic and nutritional models. Chollet D, Franken P, Raffin Y, Henrotte JG, Widmer J, Malafosse A, Tafti M. Behav Genet. 2001;31:413–425. doi: 10.1023/a:1012790321071. [DOI] [PubMed] [Google Scholar]
- 16.The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Boyle NB, Lawton C, Dye L. Nutrients. 2017;9:429. doi: 10.3390/nu9050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Sartori SB, Whittle N, Hetzenauer A, Singewald N. Neuropharmacology. 2012;62:304–312. doi: 10.1016/j.neuropharm.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Effect of magnesium supplementation in improving hyperandrogenism, hirsutism, and sleep quality in women with polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. Gholizadeh-Moghaddam M, Ghasemi-Tehrani H, Askari G, Jaripur M, Clark CC, Rouhani MH. Health Sci Rep. 2023;6:0. doi: 10.1002/hsr2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effect of short-term magnesium supplementation on anxiety, depression and sleep quality in patients after open-heart surgery. Saba S, Faizi F, Sepandi M, Nehrir B. Magnes Res. 2022;35:62–70. doi: 10.1684/mrh.2022.0503. [DOI] [PubMed] [Google Scholar]
- 21.Magnesium supplementation improves indicators of low magnesium status and inflammatory stress in adults older than 51 years with poor quality sleep. Nielsen FH, Johnson LK, Zeng H. Magnes Res. 2010;23:158–168. doi: 10.1684/mrh.2010.0220. [DOI] [PubMed] [Google Scholar]
- 22.Magnesium therapy for periodic leg movements-related insomnia and restless legs syndrome: an open pilot study. Hornyak M, Voderholzer U, Hohagen F, Berger M, Riemann D. Sleep. 1998;21:501–505. doi: 10.1093/sleep/21.5.501. [DOI] [PubMed] [Google Scholar]
- 23.Magnesium treatment of primary alcohol-dependent patients during subacute withdrawal: an open pilot study with polysomnography. Hornyak M, Haas P, Veit J, Gann H, Riemann D. Alcohol Clin Exp Res. 2004;28:1702–1709. doi: 10.1097/01.alc.0000145695.52747.be. [DOI] [PubMed] [Google Scholar]
- 24.Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Held K, Antonijevic IA, Künzel H, et al. Pharmacopsychiatry. 2002;35:135–143. doi: 10.1055/s-2002-33195. [DOI] [PubMed] [Google Scholar]
- 25.Short-term magnesium therapy alleviates moderate stress in patients with fibromyalgia: a randomized double-blind clinical trial. Macian N, Dualé C, Voute M, et al. Nutrients. 2022;14:2088. doi: 10.3390/nu14102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The effect of magnesium supplementation on primary insomnia in elderly: a double-blind placebo-controlled clinical trial. Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3703169/ J Res Med Sci. 2012;17:1161–1169. [PMC free article] [PubMed] [Google Scholar]
- 27.The assessment of anxiety states by rating. Hamilton M. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Assessment of symptoms of DSM-IV anxiety and depression in children: a Revised Child Anxiety and Depression Scale. Chorpita BF, Yim LM, Moffitt CE, Umemoto LA, Francis SE. Behav Res Ther. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 29.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 30.Lovibond SH, Lovibond PF. Sydney, Australia: Psychology Foundation; 1995. Manual for the Depression Anxiety Stress Scales. [Google Scholar]
- 31.The hospital anxiety and depression scale. Zigmond AS, Snaith RP. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.A 6-month follow-up of disability, quality of life, and depressive and anxiety symptoms in pediatric migraine with magnesium prophylaxis. Kovacevic G, Stevanovic D, Bogicevic D, et al. Magnes Res. 2017;30:133–141. doi: 10.1684/mrh.2018.0431. [DOI] [PubMed] [Google Scholar]
- 33.A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: a randomized, double-blind, crossover study. De Souza MC, Walker AF, Robinson PA, Bolland K. J Womens Health Gend Based Med. 2000;9:131–139. doi: 10.1089/152460900318623. [DOI] [PubMed] [Google Scholar]
- 34.Effect of a dietary supplement combining bioactive peptides and magnesium on adjustment disorder with anxiety: a clinical trial in general practice. Oddoux S, Violette P, Cornet J, Akkoyun-Farinez J, Besnier M, Noël A, Rouillon F. Nutrients. 2022;14:2425. doi: 10.3390/nu14122425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effects of zinc and magnesium supplements on postpartum depression and anxiety: a randomized controlled clinical trial. Fard FE, Mirghafourvand M, Mohammad-Alizadeh Charandabi S, Farshbaf-Khalili A, Javadzadeh Y, Asgharian H. Women Health. 2017;57:1115–1128. doi: 10.1080/03630242.2016.1235074. [DOI] [PubMed] [Google Scholar]
- 36.Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Noah L, Pickering G, Mazur A, Dubray C, Hitier S, Dualé C, Pouteau E. Magnes Res. 2020;33:45–57. doi: 10.1684/mrh.2020.0468. [DOI] [PubMed] [Google Scholar]
- 37.Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Hanus M, Lafon J, Mathieu M. Curr Med Res Opin. 2004;20:63–71. doi: 10.1185/030079903125002603. [DOI] [PubMed] [Google Scholar]