Abstract
Age-related decline in mobility and cognition are associated with cellular senescence and NAD + depletion in dogs and people. A combination of a novel NAD + precursor and senolytic, LY-D6/2, was examined in this randomized controlled trial. Seventy dogs with mild to moderate cognitive impairment were enrolled and allocated into placebo, low or full dose groups. Primary outcomes were change in cognitive impairment measured with the owner-reported Canine Cognitive Dysfunction Rating (CCDR) scale and change in activity measured with physical activity monitors. Fifty-nine dogs completed evaluations at the 3-month primary endpoint, and 51 reached the 6-month secondary endpoint. There was a significant difference in CCDR score across treatment groups from baseline to the primary endpoint (p = 0.02) with the largest decrease in the full dose group. No difference was detected between groups using in house cognitive testing. There were no significant differences between groups in changes in measured activity. The proportion of dogs that improved in frailty and owner-reported activity levels and happiness was higher in the full dose group than other groups, however this difference was not significant. Adverse events occurred equally across groups. All groups showed improvement in cognition, frailty, and activity suggesting placebo effect and benefits of trial participation. We conclude that LY-D6/2 improves owner-assessed cognitive function over a 3-month period and may have broader, but more subtle effects on frailty, activity and happiness as reported by owners.
Keywords: Aging, Senolytic, NAD+, Canine cognitive dysfunction syndrome, Cognitive impairment, Longevity
Subject terms: Clinical trial design, Translational research, Neurology
Introduction
Improved veterinary medical care and migration of dogs from a working role to family member have resulted in extension of the canine lifespan1–3. As a result, dogs, like humans, experience a wide range of age-related morbidities4–9. Indeed, inclusion of dogs in the family household with exposure to the same environmental contaminants and stressors, and adoption of the activity patterns and sometimes nutrition of their owners has resulted in a very similar array of these conditions occurring in both species10,11.
Cognitive function and mobility have been proposed as key hallmarks of functional aging, and their age-associated attrition appears to be linked in both humans12–14 and dogs15,16. Normal age-related cognitive changes include alterations in sleep17, memory18,19, attention20,21 and social interactions22,23. In a high proportion of people and dogs, these develop into Alzheimer’s Disease24 or Canine Cognitive Dysfunction Syndrome (CCDS)25–28 respectively. Similarly, activity and mobility decrease with age in humans29 and dogs30 due to sarcopenia31, decreased motivation32 and common pathologies such as osteoarthritis33. These changes affect the patient’s quality of life, and take a significant toll on the caregiver34–36. Supplements that can reduce cognitive decline and maintain mobility in aging dogs would have enormous impact on dogs and their caregivers, and translational relevance for aging people.
Clinical manifestations of aging have their origin at a molecular level with 12 molecular hallmarks of aging now recognized37. The burgeoning field of geroscience has identified therapeutic targets to mitigate the aging process within these hallmarks and numerous clinical trials are now underway in people38. While the human anti-aging field is flourishing, far fewer studies target age-related decline in dogs. In this randomized, controlled, double-blinded clinical trial we evaluated a combination of supplements that target two important hallmarks of aging, cellular senescence and depletion of cellular nicotinamide adenine dinucleotide (NAD+) concentrations.
Senolytics have emerged as a promising anti-aging therapeutic strategy by targeting and clearing senescent cells39,40. Cellular senescence, a process by which cells arrest and stop replicating but do not proceed through apoptosis, increases with age. While it can be a protective mechanism, it can also cause an unwanted inflammatory response, the Senescence-Associated Secretory Phenotype (SASP) which may exacerbate age-related disease37,41. Many senolytics, such as quercetin and fisetin, are plant derived flavonoids that act through inhibition of anti-apoptotic proteins (such as BCL-2 family proteins)42. Reducing senescence reduces age-related pathology in vitro and extends lifespan and a variety of different functional outcomes in vivo43,44. Senolytics are marketed widely as anti-aging supplements and human clinical trials evaluating senolytics in age-related diseases such as osteoarthritis, heart disease and Alzheimer's disease are underway45, but clinical trials in dogs are lacking.
Supplementation with an NAD+ precursor takes a more broadly targeted approach to aging. NAD+ is involved in regulation of key biological processes, cellular signaling and electron transfer, but it declines with age41,46,47, exacerbating age-related diseases48,49. Precursors such as nicotinamide mononucleotide have been shown to mitigate the effects of aging and age-related pathologies50 and restoring levels of NAD+ improves cellular energy and metabolism, leading to improvements in health and lifespan51,52. Human clinical trials are ongoing53, however there is only one canine study published to date, where muscle function improved following treatment with nicotinamide in dogs with Duchenne’s Muscular Dystrophy54. Senescent cells secrete an NADase (CD38), causing depletion of NAD+. As a result, it has been suggested that combining NAD+ precursors with a senolytic amplifies NAD+ anti-aging properties55.
This study evaluated the effects of a repeated monthly regimen of two consecutive days of the senolytic (LY-D6™) and NAD precursor followed by daily NAD precursor (LY-D2™) on cognition and activity in companion dogs. We hypothesized that supplementation with LY-D6/2 combination would reduce cognitive and activity level decline in aging dogs. Companion dogs aged 10 years or older were randomized to receive either placebo or LY-D6/2 combination at two different doses (low and full) with primary outcomes of change in owner assessed cognition and collar mounted physical activity monitor (PAM) assessed activity levels after 3 months.
Results
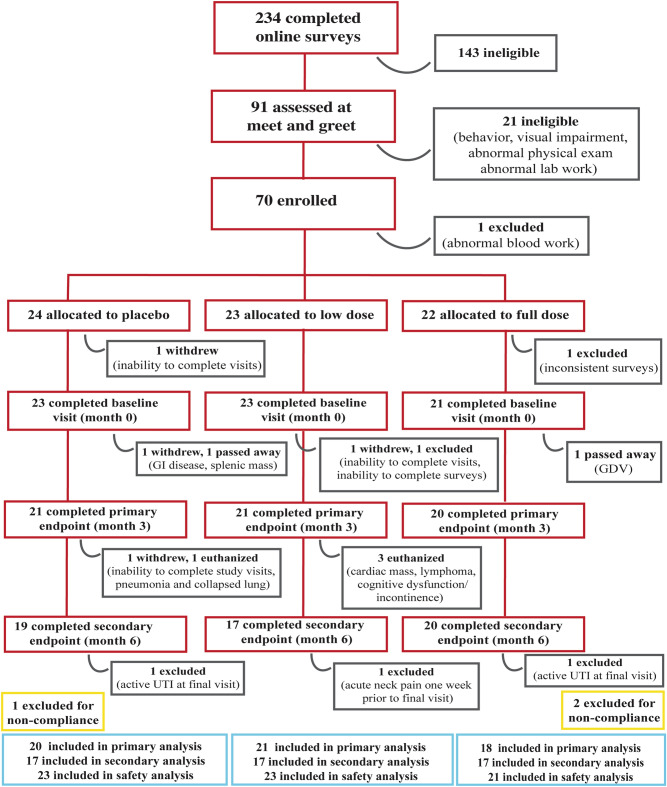
Two hundred and forty-three surveys were completed, 91 dogs were brought in for screening appointments from which 70 dogs were enrolled and 67 completed baseline assessments (Fig. 1). One dog was withdrawn due to pre-existing disease and 66 completed the 1-month assessments. Two dogs died, 1 withdrew and 1 was excluded due to noncompliance, leaving 62 dogs to complete the 3-month (primary endpoint) assessments. Four dogs were euthanized between month 3 and 6 and 2 withdrew due to mobility decline and neck pain. Fifty-six dogs completed all visits. Two dogs had active urinary tract infections (UTIs) at their 6-month visit so their data were excluded from the 6-month analyses. Data from 3 dogs were excluded from 3- and 6-month analyses due to treatment noncompliance (> 20% missed doses).
Figure 1.
Participant flowchart. Diagram featuring patient screening, treatment allocation, attrition, and exclusion throughout the trial. GDV gastric dilation-volvulus, UTI urinary tract infection.
Study population
Demographic details and baseline outcome measures of dogs at time of enrollment are provided in Table 1. The only significant difference between groups in baseline characteristics was age (p = 0.02) with the full dose group being significantly older than the placebo or low dose groups. Owners were asked to maintain consistency in their pet’s routine over the course of the trial, but the number of pets changed in 14 households (addition or euthanasia of a pet). Twenty-eight dogs had a change in medication; we compared the number of household and medication changes from baseline to primary endpoint (month 3) and from primary endpoint to secondary endpoint (month 6) across groups and found no significant differences between groups (Supplementary Table S1).
Table 1.
Population demographics and outcome measures of study participants at enrollment by group.
| Placebo (n = 23) | Low dose (n = 23) | Full dose (n = 21) | p value | Fisher’s exact test | |
|---|---|---|---|---|---|
| Age (years) | Mean: 12.94 | Mean: 12.25 | Mean: 13.63 | 0.01 | |
| St Dev: 1.45 | St Dev: 1.42 | St Dev: 1.29 | |||
| Fractional lifespan | Median: 1.10 | Median: 1.03 | Median: 1.12 | 0.19 | |
| Range: 0.76–1.24 | Range: 0.72–1.22 | Range: 0.87–1.23 | |||
| Sex | Female: 10 (43.5%) | Female: 9 (39.1%) | Female: 12 (57.1%) | 0.393 | |
| Male: 13 (56.5%) | Male: 14 (60.9%) | Male: 9 (42.9%) | |||
| Weight (kg) | Median: 24.5 | Median: 27.7 | Median: 22.9 | 0.25 | |
| Range: 7.9–39.5 | Range 6.5–45 | Range: 8.3–33.2 | |||
| Body condition score | 3/9: 1 (4.3%) | 0.15 | 0.19 | ||
| 4/9: 3 (13.0%) | 4/9: 2 (8.7%) | 4/9: 5 (23.8%) | |||
| 5/9: 13 (56.5%) | 5/9: 13 (56.5%) | 5/9: 12 (57.1%) | |||
| 6/9: 3 (13.0%) | 6/9: 8 (34.8%) | 6/9: 2 (9.5%) | |||
| 7/9: 3 (13.0%) | 7/9: 1 (4.8%) | ||||
| 8/9: 1 (4.8%) | |||||
| Muscle condition score | Median: 7 | Median: 6 | Median: 7 | 0.98 | |
| Range: 1–17 | Range: 0–18 | Range: 1–18 | |||
| Frailty status | Non-frail: 15 (65.2%) | Non-frail: 12(52.2%) | Non-frail: 11 (52.4%) | 0.59 | |
| Frail: 8 (34.8%) | Frail: 11 (47.8%) | Frail: 10 (47.6%) | |||
| Frailty score | 0/5: 1 (4.3%) | 0.42 | 0.56 | ||
| 1/5: 6 (26.1%) | 1/5: 5 (21.7%) | 1/5: 3 (14.3%) | |||
| 2/5: 9 (39.1%) | 2/5: 6 (26.1%) | 2/5: 8 (38.1%) | |||
| 3/5: 4 (17.4%) | 3/5: 8 (34.8%) | 3/5: 9 (42.9%) | |||
| 4/5: 3 (13.0%) | 4/5: 1 (4.3%) | 4/5: 1 (4.8%) | |||
| 5/5: 1 (4.3%) | 5/5: 2 (8.7%) | ||||
| Pain score | Median: 7 | Median: 5 | Median: 6 | 0.61 | |
| Range: 0–20 | Range: 1–15 | Range: 2–21 | |||
| CCDR score | Median: 38 | Median: 40 | Median: 39 | 0.28 | |
| Range: 34–46 | Range: 34–57 | Range: 34–58 | |||
| Inhibitory control (cylinder task) | Median: 87.5 | Median: 87.5 | Median: 75 | 0.57 | |
| Range: 0–100 | Range: 0–100 | Range: 0–100 | |||
| Detour | Median: 37.5 | Median: 25 | Median: 12.5 | 0.52 | |
| Range: 0–87.5 | Range: 0–100 | Range:0–100 | |||
| Sustained gaze (s) | Median: 22.78 | Median: 17.74 | Median: 15.09 | 0.22 | |
| Range: 5.92–60 | Range: 4.28–55.56 | Range: 2.12–60 | |||
| Off leash gait speed (m/s) | Median: 1.51 | Median: 1.39 | Median: 1.44 | 0.55 | |
| Range: 0.54–3.56 | Range: 0.59–3.03 | Range: 0.66–3.81 |
Primary outcomes
CCDR
Primary endpoint (3 months)
All dogs entered the trial with mild to moderate cognitive impairment. The CCDR scores did not differ significantly between groups (p = 0.28) (Table 1). Over the first 3 months of the trial, CCDR score decreased (improved) in each treatment group. Individual frailty status at enrollment was significantly associated with change in CCDR and so was incorporated into a repeated measures model comparing CCDR score between groups at baseline, 1 and 3 months. There was a significant difference between treatment groups over the 3-month period (p = 0.02), with the full dose group showing the largest decrease (improvement) in CCDR score (Fig. 2).
Figure 2.
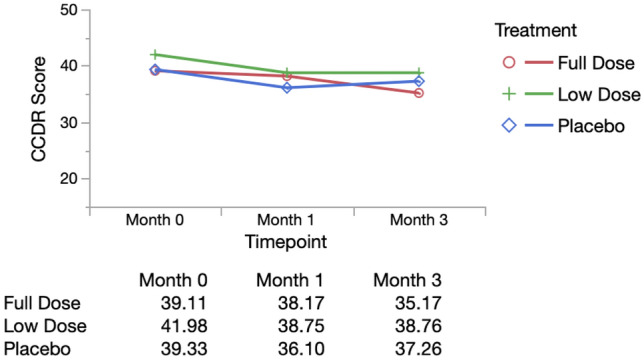
Repeated measures analysis of CCDR Score (adjusted for baseline frailty status). Mean CCDR scores by group adjusted for individual baseline frailty status over time from repeated measures model (MANOVA analysis in JMP). Wilks’ lamda value was evaluated, with p < 0.05 indicating a significant difference between groups. All original outcome measure data are provided in Supplementary Data S1.
Change in CCDR score was also categorized as failure (increase in score representing worsening cognition) or success (static or decreased score) and groups were compared using a chi-square analysis. In the full dose group 16/18 (88.9%) dogs were successes, compared with 15/21 (71.34%) of low dose dogs and 12/20 (60%) of placebo dogs. However, these differences were not statistically significantly (p = 0.11).
Secondary endpoint (6 months)
We next assessed whether individuals maintained their CCDR scores to the final endpoint. Score differences from month 3 to month 6 were calculated and compared across groups. Median change in CCDR was static in all groups (Supplementary Table S2), with no significant difference across groups (p = 0.44).
Activity monitor
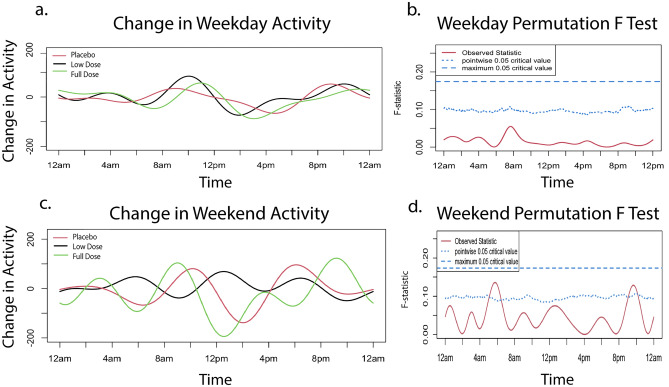
Activity was initially analyzed using functional linear modeling (FLM) to allow patterns of change over the entire 24-h period to be captured. At baseline, activity levels peaked around 6–10 a.m. and 4–9 p.m., reflecting times when owners interact with their dog. There was no significant difference between groups (Fig. 3a,b). Weekend activity did differ between groups around 12 p.m., reaching the global threshold for significance, with the full dose group showing higher activity (Fig. 3c,d).
Figure 3.
Baseline activity. Baseline activity by treatment group prior to starting treatment on weekdays and weekends. The graphs (a,c) on the left illustrate average weekday/weekend activity levels over a 24-h period. The graphs on the right (b,d) indicate the level of significance between groups. When the observed statistic (red line) is above the dashed and dotted lines (blue line), it indicates a global or pointwise significant difference between groups respectively. No significant differences were observed during weekdays. A global threshold for significance is reached around 12 p.m. on weekends. A pointwise significant difference is observed around 12 a.m. on weekends. All original activity data are provided in Supplementary Data S3.
Primary endpoint
When change in activity level from baseline to month 3 was examined on weekdays, groups maintained similar levels of activity compared to baseline. Notably, all groups showed a small increase in morning and evening activity and decrease in afternoon activity with no significant difference between them (Fig. 4a,b). Changes in weekend activity were more variable, reflecting the owners’ less defined weekend schedules (Fig. 4c). The placebo and full dose groups showed larger fluctuations than the low dose group, but the differences between groups did not reach global significance (Fig. 4c,d).
Figure 4.
Change in activity from month zero to month three. Change in activity from baseline to primary endpoint on weekdays and weekends by treatment group. Activity at baseline was subtracted from activity at month three to obtain the change in activity utilized in the FLM analysis. The graphs on the left (a,c) illustrate change in weekday/weekend activity levels over a 24-h period. The graphs on the right (b,d) indicate the level of significance between groups. When the observed statistic (red line) is above the dashed and dotted blue lines, it indicates a global or pointwise significant difference between groups respectively. There was no significant difference between group activity over the weekdays. On the weekends, pointwise significance was reached at approximately 6 a.m. and 10 p.m. All original activity data are provided in Supplementary Data S3.
Secondary endpoint
Similarly, when evaluating the change in activity from months 3 to 6, activity remained relatively unchanged on weekdays, apart from an increase in all groups in the afternoon to evening, while the weekend activity fluctuated more widely (Fig. 5a,c). There was no significant difference between groups on weekdays but at the weekend, groups differed significantly around 6am with the low dose groups showing a decrease in activity (Fig. 5b,d).
Figure 5.
Change in activity from month three to month six. Change in activity from primary endpoint (month three) to secondary endpoint (month six) on weekdays and weekends by treatment group. Activity at month three was subtracted from activity at month six to obtain the change in activity utilized in the FLM analysis. The graphs on the left illustrate average change in weekday/weekend activity levels over a 24-h period. The graphs on the right indicate the level of significance between groups. When the observed statistic (red line) is above the dashed and dotted lines (blue line), it indicates a global or pointwise significant difference between groups respectively. Pointwise significance is observed to reach threshold level on weekdays around 2 a.m. and 2 p.m. Weekend activity reaches the global significance level at 6am as well as a pointwise significance level around 9 p.m. All original activity data are provided in Supplementary Data S3.
Cumulative activity
To incorporate covariates into the analysis, total cumulative activity for each dog was examined using repeated measures models. Weekday daytime activity was affected by age, and weekday and weekend daytime activity were affected by sex. Neither weekday nor weekend nights required correction for covariates. The repeated measures models for summated activity showed no significant difference over time (from baseline to primary endpoint) between groups during the day (weekday: p = 0.95, weekend: p = 0.71) or during the night (weekday: p = 0.31, weekend: p = 0.65) (Supplementary Fig. S1). When assessing the difference in cumulative activity between 3 and 6 months, we found no significant difference across either time period on weekdays (day: p = 0.89, night: p = 0.10) or weekends (day: p = 0.60, night: p = 0.83) (Supplementary Table S3).
Owner assessed activity
By contrast, when owners were asked to classify activity as static, reduced or increased at the primary endpoint, 8/18 (44%) owners in the full dose group reported static and 7/18 (39%) reported increased activity levels, compared with 13/21 (62%) static and 2/21 (10%) increased in the low dose group and 11/20 (55%) static and 4/20 (20%) increased in the placebo group (Supplementary Data S1). This difference between the groups was not significant (p = 0.29). A majority of dogs across all groups remained static at the final endpoint: 10/17 (59%) of the full dose group, 12/17 (71%) of the low dose group and 11/17 (65%) of the placebo group. The differences were not significant (p = 0.64).
Secondary Outcomes
Primary endpoint
Frailty score
The proportion of dogs that were frail (3 or more of 5 domains classed as impaired) and the number of impaired domains at study outset for each treatment group are provided in Table 1; groups were not significantly different. As expected with a senior pet population, there were changes in frailty over the course of the study which were categorized as success (static or improved) or failure (deteriorated) (Supplementary Data 1). The majority of dogs in full dose (13/18, 72.2%) and low dose (16/21, 76.2%) groups were classified as success after 3 months, as compared with 11/20 dogs (55%) in the placebo group; however groups did not differ significantly (p = 0.32).
Cylinder task (inhibitory control)
Inhibitory control is a test of executive function, similar to impulse control, which decreases with age in both humans and dogs56,57. The full dose group started with a lower mean baseline score (66.1%) than the low dose (78.79%) and placebo group (76.6%); this difference was not significant (p = 0.57) (Table 1). When assessing change from baseline to the primary endpoint, inhibitory control required correction for changes to household and changes to medication. These were therefore incorporated into the repeated measures model, which showed all groups increasing in score (improving their performance in the task) (Fig. 6a). All groups showed a similar trajectory of improvement and there was no significant difference over time across groups (p = 0.95).
Figure 6.
Repeated measures analyses of secondary outcome measures (Cylinder Task, Detour, Sustained Gaze, Off Leash Gait Speed). Mean values (adjusted as necessary) are reported at each timepoint by group. Group scores over time were compared in a repeated measures model (MANOVA analysis in JMP). The Wilks’ lamda value was evaluated with p < 0.05 indicating a significant difference between groups. All original outcome measure data are provided in Supplementary Data S1.
Detour
Detour is a further challenge to the cylinder task, requiring flexibility in problem solving, which becomes more challenging as dogs age56. Dogs had lower baseline scores on detour as compared to the cylinder task. Similar to the cylinder task, the full dose group started out with a lower baseline score (27.3%) compared to both the low dose (34.8%) and placebo group (34.6%); these scores again were not significantly different (p = 0.52) (Table 1). In the repeated measures analysis of change in score over time, detour required correction for fractional lifespan. The groups did not differ significantly across the 3 months (p = 0.85) (Fig. 6b). We saw a small decrease in score (decline in performance) with the full dose group at the primary endpoint.
Sustained gaze
Sustained gaze is a test of focus and attention, with aging dogs shown to decline in the time in which they can maintain gaze for a treat21. At enrollment, there was no significant difference in performance between groups (p = 0.22) (Table 1). No covariates reached significance to be included in the repeated measures analysis. While sustained gaze duration increased in all 3 groups at the primary endpoint, indicating an improvement, there was no significant difference between groups over time (p = 0.59) (Fig. 6c).
Off leash gait speed
Off leash gait speed is both a measure of physical ability and motivation (for a treat). This has been shown to decline with age in both dogs and humans30. There was no significant difference in off leash gait speeds across groups at enrollment (p = 0.55) (Table 1). Gait speed did not require correction for any covariates in the repeated measures analysis. All groups showed little change in speed, with no significant difference between treatment groups over time (p = 0.59) (Fig. 6d).
Secondary endpoint
Similar to the primary outcome measures, we assessed whether individuals were able to maintain their level of frailty, cognitive testing scores and gait speed from months 3 to 6 by comparing change in score across groups. We found the majority of dogs remained static (median change of approximately zero) across all groups from month 3 to month 6 (Supplementary Table S2). There was no significant difference between groups for frailty (p = 0.80), inhibitory control (p = 0.33), detour (p = 0.28), sustained gaze (p = 0.33) or off-leash gait speed (p = 0.64).
Owner assessed happiness
Owners were asked to categorize their dogs’ level of happiness as the same, improved or deteriorated compared to the previous visit at 3 and 6 months. At 3 months, 10/18 (56%) owners with dogs in the full dose group reported static happiness, 8/18 (44%) improved and none reported a deterioration (Supplementary Data 1). While not significant (p = 0.34), this differs from the low dose and placebo groups in which 2/21 (10%) and 3/20 (15%) reported a deterioration. At 6 months, 6/17 (35%) owners with dogs in the full dose group and 8/17 (47%) in the low dose group reported an increased level of happiness. Whereas only 4/17 (24%) of owners in the placebo group reported an increase. These differences were not significant (p = 0.42).
Adverse events
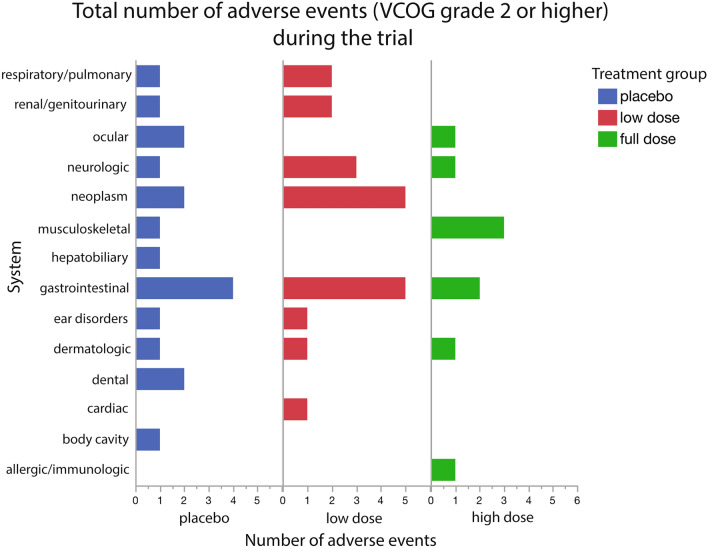
All dogs who received supplements/placebo were included in the adverse events analysis. At each visit, owners completed a checklist regarding possible adverse reactions (Supplementary Form S1). Events were only included if they were newly observable (not present at baseline) during the course of treatment. Of these reported events, 76 were in the placebo group, 102 in the low dose group and 67 in the full dose group (Supplementary Table S4). These events were evenly distributed across the groups.
There were 245 adverse events reported and classified into VCOG grades ranging from 1 (mild) to 5 (death): 1 (n = 190), 2 (n = 40), 3 (n = 7), 4 (n = 1), 5 (n = 7)58. Most events observed were VCOG grade 1 or 2. Grade 1 events were considered minimally disruptive by the owner, required no intervention, and resolved quickly on their own. Grade 2 events moderately impacted the patient’s daily life and usually necessitated outpatient veterinary care (antibiotics, pain management, minimally invasive procedure). There were 7 severe (Grade 3) events that required medical intervention and had a significant effect on daily activities but were not immediately life threatening. These events were reported to us and medically managed by each patient’s primary care veterinarian. None of these events could be related to LYD6/2. Only 1 event was graded as 4 (requiring urgent medical attention): this dog presented to their final visit with lethargy, weakness and ascites. Over the course of the trial, 6 dogs developed disease that ultimately led to euthanasia or death (Grade 5); 2 in the placebo group, 3 in the low dose group and 1 in the full dose group. The reasons for these deaths included: severe respiratory disease, neoplasia, poor quality of life (severe cognitive dysfunction and both fecal and urinary incontinence) and surgical complications during a gastric dilation volvulus procedure.
Adverse events occurred across a variety of systems for all groups (Supplementary Table S4 and Fig. 7). Only 2 adverse events occurred in the treatment groups that were not also seen in the placebo group (hypertension and an anaphylactic reaction). The hypertension was pre-existing and well controlled prior to the trial, but over the course of the trial, the dog developed worsening hypertension and was eventually euthanized due to poor quality of life. The anaphylactic reaction was in response to an antibiotic prescribed for a UTI which quickly resolved after discontinuation of the antibiotic. Therefore, it is unlikely that either of these events were a direct result of LY-D6/2.
Figure 7.
Total number of adverse events (VCOG grade 2 or higher) during the trial by group. Events were reported by the owner to be new during the trial (not present during/before baseline) and separated by system. Events were summed based on the system affected. All original adverse event data are provided in Supplementary Table S4.
There were 284 lab work changes over the course of the trial (Supplementary Table S5) and of these, only 2 were graded as VCOG 2 and 1 was graded as VCOG 3. The rest were mild, mostly incidental findings. The most common findings were anemia (n = 21), lymphopenia (n = 21) and hematuria (n = 26). Any observed abnormalities were reported to the owner and pursued by the primary care veterinarian at their own discretion. Each group experienced a similar number of lab work abnormalities; placebo: 95, low dose group:102 and full dose group:87.
Discussion
This randomized controlled blinded trial is one of the first of its kind, evaluating an anti-aging supplement that targets two hallmarks of aging in senior dogs. This clinical trial used a pragmatic approach that included dogs with mild to moderate cognitive impairment who met age, weight and relatively broad health criteria. Dogs were followed to a primary endpoint at 3 months and a secondary endpoint at 6 months. While dogs and people age at different rates at different lifestages59, 3 and 6 months in a dog represent a longer time-span in humans (closer to 1.75 and 3.5 years respectively if using the rule of thumb that 1 year in a dog represents 7 years in a person). There was a significant difference in change in CCDR score across treatment groups from baseline to the primary endpoint at 3 months, with the largest decrease in score in the group of dogs who received the full dose of LY-D6/2. There was no difference between groups in changes in activity level, gait speed or in-house cognitive testing. However, while not significant, a higher percentage of dogs in the placebo group showed a deterioration in frailty (45.0%) compared to both the low and full dose LY-D6/2 groups (23.8% and 27.8%, respectively). The LY-D6/2 supplements were well tolerated; while many health issues occurred in this group of old dogs over the 6-month trial period, there was no difference in prevalence of adverse events between treatment groups and none could be attributed to LY-D6/2.
The rationale of targeting two molecular mechanisms of aging was both to enhance the intervention strategy and to broaden the population of dogs that might benefit from the supplements. Both cellular senescence and depletion of cellular NAD+ are well established molecular hallmarks of aging and manipulation of both events in either direction has been shown to either ameliorate or worsen the aging phenotype in an experimental setting37. The use of a variety of different NAD+ precursors to increase cellular NAD+ concentrations is one of the most popular anti-aging strategies available at this time53. While there are now multiple studies demonstrating the ability to increase blood cell concentrations of NAD+ with oral supplements, and some evidence of a health benefit in middle aged and healthy old people60–62, definitive evidence of an effect on the aging phenotype in people is still lacking53 and the only data in dogs relates to a model of Duchenne's muscular dystrophy54. Cellular NAD+ attrition interfaces with other mechanisms of aging and the possibility of a synergistic effect of NAD+ precursors when combined with another therapy has been proposed55. There has been particular interest in the rather complex interplay between cellular senescence and NAD+ depletion. It is clear that SASP production by senescent cells is both metabolically taxing and reduces NAD+ concentrations by increasing CD38 expression63. In this clinical trial we chose to maximize the potential benefit of the anti-aging intervention by combining a senolytic with an NAD+ precursor.
Given the dearth of published placebo controlled randomized clinical trials examining anti-aging supplements in aged companion dogs, there is insufficient data available on parameters such as anticipated death rates, frequency and impact of comorbidity development, and size of caregiver placebo effect. We powered this clinical trial around detection of change in owner-quantified change in cognitive function. We used preliminary longitudinal data on dogs with mild to moderate cognitive impairment and made the observation that the vast majority of such dogs decline over a 6-month period. This is supported by other published studies64,65 and prevention of this deterioration would represent a meaningful benefit to dogs and their owners. Thus, the clinical trial was designed to detect a 50% reduction in the proportion of dogs that would show a deterioration in owner reported cognitive status. A primary endpoint at 3 months was chosen because of concerns about case attrition due to development of conditions such as cancer in this elderly population of dogs. We chose to perform repeated measures analysis which is a mixed model that incorporates the effects of time and a covariate (i.e. treatment group) on individuals66. This is advantageous in longitudinal trials allowing comparison of individual changes across several time points in addition to group effects. Additional independent covariates that might impact individual performance (such as age, fractional lifespan, body weight, body condition score, sex, or frailty status) can be incorporated into the repeated measures model if that additional covariate reaches a specified threshold (p < 0.1). In this clinical trial, this allowed us to model population and individual variability in a statistically meaningful and relevant way.
Clinical trials can be explanatory or pragmatic, with explanatory trials answering the question of whether an intervention is effective in a very specific patient population and pragmatic trials determining whether a therapy will be effective under normal conditions67,68. In this clinical trial, the study population was intentionally wide and the primary outcomes (owner reported cognitive status and activity detected by a collar mounted PAM) were straightforward to obtain and were directly relevant to a dog’s day-to-day life. The inclusion of simple owner reported assessments of improvement or deterioration in activity levels and happiness allowed identification of meaningful changes by owners blinded to treatment group.
In dogs, cognitive decline is strongly age-associated, with prevalence estimates of mild cognitive impairment in 28% of 11–12 year olds, and up to 68% of 16 year olds69,70. The odds of developing canine CCDS increase by 52% with each additional year of age71. There are several different validated owner questionnaires to quantify canine cognitive decline and the development of CCDS. The initial power analysis and patient recruitment was performed using CADES because this scale identifies mild cognitive changes through an option to choose an event frequency of q6 months. However, owner scores of cognition were solicited at baseline, 1, 3 and 6 months and so the CCDR (the option for lowest frequency of events is q1month, limiting comparisons to within the trial period) was chosen as a more reliable means of sampling cognitive status repeatedly within 6 months. The CCDR and CADES however, are closely correlated64 and detect the same cognitive trends. Using this measure, only 2/18 dogs (11.1%) in the full dose group showed a deterioration in score compared with 6/21 (28.6%) in the low dose group and 8/20 (40%) in the placebo group. Moreover, the repeated measures analysis, using a model that accounted for frailty status at study start, showed a significant difference across groups with the full dose group showing the largest decline in score (clinical improvement). This effect was not maintained through 6 months with CCDR scores plateauing or increasing slightly in all 3 groups. Given this time period represents nearly 3.5 years of human life, this is perhaps not surprising.
Owners of 60% of dogs in the placebo group documented an improvement in cognition over a 3-month period suggesting that there was a sizable and long-lasting caregiver placebo effect72. This caregiver placebo effect could be the result of optimism on the part of the owner; in addition, many owners participating in a longitudinal study of aging with our research group report that the interactions their dogs have when they visit the research site improve their attitude and engagement. Thus, this trial might be capturing the effect of increased social interaction and problem solving in elderly dogs through study participation. This is supported by studies in purpose bred dogs in which behavioral enrichment was associated with preservation of learning73,74, through elevation of brain levels of BDNF expression75.
The results of the secondary cognitive tests of attention and executive control performed at the research site did not differ between groups, with most groups showing a slight improvement. These cognitive tests have not been used in a longitudinal clinical trial previously, and it is possible that these dogs learned how to perform the tests better with each visit. In addition, we might again be capturing the positive effect of trial participation.
Assessing activity levels in senior and geriatric dogs is challenging because they tend to lead quite sedentary lives and, as for all companion dogs, their activity is strongly influenced by their owner’s activity76,77. We used collar mounted activity monitors to collect data over a 2-week period at each evaluation point, and evaluated weekdays and weekends separately to allow for owner schedules differing over the weekend78. The baseline data showed the typical peaks in activity when the household rises in the morning and comes home from a day of work during the week. We first performed an FLM analysis as this captures and smooths data allowing a per-minute comparison between groups over a 24-h period. Given that multiple comparisons are made when performing an analysis in this way, only group differences that reach the threshold of global significance are compelling, while those that reach pointwise significance highlight areas of interest. However, this analysis did not reveal any pattern of consistent change between groups. It was interesting to note that morning and evening activity on weekdays did increase in all 3 groups, again suggesting either placebo or a true beneficial effect of trial participation. Given these elderly dogs are not very active, but were reported to demonstrate small bursts of energy by owners, we also compared the sum of the total daytime and nighttime activity counts for each dog between groups. Unlike the FLM, which cannot be performed with covariates, this allowed us to build a model taking covariates into account, but no differences were noted between groups. Finally, we compared the owner reports of activity changes and more full dose group owners noted an improvement than the other groups. This raises the question of how best to capture activity changes in elderly dogs.
Frailty is a well described syndrome in aging people but descriptions of frailty are few in dogs79–81. We have developed a rapid screening tool for frailty that combines owner responses to questions around the 5 key frailty domains, and assessment of body and muscle condition score by the research group82. While there was no significant difference between groups in percentage of frail individuals at study start, approximately 35% of the placebo group were frail compared with 48% in both treatment groups. One would anticipate that aged dogs would become frailer over time, but in our study, the majority of dogs in all three groups improved in their frailty status over the first 3 months with a lower percentage of dogs in the treatment groups deteriorating than in the placebo group. Currently there is a dearth of data following frailty in elderly dogs longitudinally and so it is difficult to comment on whether this is unusual, but once again, it does suggest either a placebo effect, or a positive effect of trial participation.
This study has some weaknesses, first, this was a pragmatic clinical trial with no attempt to target dogs who exhibited higher levels of senescence or lower levels of NAD; a more targeted trial might be better able to capture changes due to alterations in these systems. Further, biomarkers of senescence and NAD+ levels were not measured. Future studies may benefit from incorporating these biological markers as outcomes. In addition, our study included dogs of varying breeds, weights and diets in order to recapitulate the variability observed in a clinical aging population and increase the ecological validity of our findings. Our inclusion of cognitive tests was designed to capture changes in performance measures (as compared to owner-rated changes in signs) however these tests have not been used longitudinally, and while correlations have been seen between some of these measures and owner-questionnaires83, their responsiveness as outcome measures was unknown. Dogs may learn to perform these tasks with practice, and additional longitudinal data will be needed to understand how performance on these tasks changes with time. Given the possibility that occult UTIs could affect overall health and behavior, it would have been ideal to obtain urine cultures at every assessment regardless of clinical signs and UA findings. However, cost limitations precluded this. Finally, the full dose group was significantly older than the other groups, although fractional lifespan did not differ. Cognitive decline is associated with age rather than fractional lifespan, and so this could have influenced the outcomes negatively for this group. However, age was examined in the univariate analysis of CCDR change and was not associated with the outcome.
We conclude that LY-D6/2 can be used safely in senior dogs with mild-moderate cognitive impairment over a period of 6 months and might have broader effects on dog health manifesting as improved happiness and reduced frailty. However, these results are preliminary and additional investigation into LY-D6/2 and age-related clinical outcomes is recommended. More research is needed to understand the clinical impact of interventions on canine aging. For example, minimal clinically important differences have not been determined for any canine cognitive rating scale currently in use, including CCDR. This trial highlights the viability of targeting hallmarks of aging to impact the health of aging dogs and will inform future senior dog clinical trial design and execution. The pragmatic design means the results are applicable to a clinically relevant aging dog population and are potentially translatable to the comparably variable geriatric human population. Further, clinical trial participation appears to be beneficial in an aging dog population.
Methods
This blinded, randomized, controlled (RCT) clinical trial was conducted and reported according to the CONSORT and ARRIVE Guidelines with the approval of the North Carolina State University Institutional Animal Care and Use Committee under protocol # 21-376-O. All procedures were performed in accordance with these approved protocols and institutional guidelines. Owners of the dogs who participated in these studies reviewed and signed an informed consent form. IRB approval was not sought because all collected data pertained to dogs and as such the work was categorized as “Non-Human Subject Research”.
Study design
This 3-arm RCT was designed to evaluate the effect of LY-D6/2 on cognitive function and activity in aged dogs over a 6-month period. The primary endpoint of the study was 3 months and the secondary endpoint was 6 months. The primary outcomes were change in owner assessed cognitive function (via Canine Cognitive Dysfunction Rating (CCDR) scale)84 and activity level (via a collar mounted physical activity monitor (PAM))85. Secondary outcomes included changes in frailty phenotype, attention (sustained gaze), inhibitory control (cylinder task), cognitive flexibility (detour task) and off-leash gait speed. Preliminary data from 6 mild to moderately cognitively affected dogs (based on owner assessment using the Canine Dementia Scale (CADES)64 were used to perform the power analysis. Five of these 6 dogs showed a deterioration in owner reported cognitive function over a 6-month period. It was determined that 20 dogs per group would detect a 50% reduction in the number of dogs who show cognitive deterioration with a power of 80%. Ten additional dogs were included in order to account for attrition. Dogs were randomized in blocks of 9 (3 per group) by the NC State pharmacy using a random number generator. Investigators and owners were blinded to treatment identity until data analysis was complete. The timeline of participation is provided in Supplementary Fig. S2.
Inclusion and exclusion criteria
In order to participate, dogs had to be greater than or equal to 10 years of age (senior status), weigh more than 6 kg (dictated by available capsule size), and be able to walk independently with sufficient hearing and vision to be able to perform cognitive tests. They had to be treat-motivated, willing to engage with the investigators, and show signs of mild to moderate cognitive impairment (based on CADES score). Owners had to be able to complete online questionnaires, administer the supplements, keep an activity monitor on their dogs for two-week periods and attend scheduled appointments.
Dogs with comorbidities likely to significantly adversely affect health over the course of the clinical trial were excluded. Examples include metastatic neoplasia, hyperadrenocorticism, diabetes mellitus, congestive heart failure, and refractory epilepsy. Aggressive dogs were excluded as they could not safely perform the cognitive tests. Dogs with evidence of an active urinary tract infection (presence of clinical signs, bacteriuria and pyuria)86 were excluded until treated with resolution of clinical signs and an inactive urine sediment.
Intervention
The investigational veterinary product (IVP), LY-D6/2 was a proprietary combination of a senolytic and an NAD+ precursor. Dogs were administered placebo, low or full dose LY-D6/2 starting the day following Day 0 assessment. The doses were determined in pharmacokinetic and safety studies performed by Animal Bioscience. Owners were instructed to administer the NAD+ precursor (or placebo) capsule(s) once daily and the senolytic (or placebo) capsule(s) on two consecutive days each month. Owners were given customized calendars to record administration of the capsules throughout the study. Both these calendars and capsule counts (checked at each visit) were utilized to determine compliance.
Recruitment and eligibility
Dogs were recruited from the local community through postings on the NC State CVM clinical trials website and social media from January 2022 through June 2023. Dogs were initially screened via an online survey platform (Qualtrics) and those meeting age ( 10 years), weight ( 6 kg) and cognitive status (CADES category mild or moderate) requirements proceeded to a telephone interview to confirm cognitive changes were age-related and that the owner understood the clinical trial design and commitments. Eligible participants proceeded to an in-person screening at the NC State College of Veterinary Medicine. Owners reviewed and signed informed consent forms at this time. The screening visit included physical examination, lab work (CBC and serum biochemistry panel) and behavioral assessment to confirm eligibility prior to enrollment. A PAM (WGT3X-BT monitor, Actigraph, Pensacola, FL) was placed on the dog’s collar and the owners were provided with a log to record times when the collar/PAM was removed and/or changes from routine activity. The appointment for the first day of the trial was scheduled 2 weeks after the screening appointment in order to allow for adequate PAM data collection prior to beginning treatment (Supplementary Fig. S2). A urinalysis (UA) was performed at the baseline appointment on voided urine. If a UTI was suspected, dogs were directed to their primary veterinarian for urine culture and treatment. Dogs were required to complete an appropriate course of antibiotics (as determined by their veterinarian) and have resolution of their signs of UTI prior to re-entering the clinical trial, at which point another UA was performed in order to confirm resolution of clinical signs, bacteriuria and pyuria.
Data collection
Study data were collected and managed using REDCap electronic data capture tools hosted at NC State University87,88.
Questionnaires were distributed to owners via REDCAP( https://www.project-redcap.org/) 7 days prior to each appointment. Basic signalment and medical history were obtained. Owners were also instructed to indicate any changes in pet health, medication, environment and attitude. RedCap questionnaires included CCDR and frailty phenotype. The CCDR Questionnaire consists of 13 behavioral items related to orientation, memory, apathy, olfaction and locomotion. (Supplementary Form S2). Scores from each item are summed (16–80), where a score of 50 or greater indicates CCDS84. The frailty phenotype screening tool assesses age-related deficits in mobility, exhaustion, social activity, muscle condition, and nutritional status and predicts 6-month mortality82. Criteria have been established to classify an individual as impaired in each of these 5 domains and the individual was classified as frail if criteria are met for 3 or more of these domains. (Supplementary Form S3) These data were expressed both as frail, yes or no and as scores from 0 to 5 (number of impaired domains). In addition to chronological age, fractional lifespan was also calculated for each dog using an equation that incorporates dog height and weight89. In order to evaluate the owner's perception of efficacy, each was asked to categorize their dog’s mobility and their level of happiness as improved, the same or worse at months 3 and 6.
Dogs underwent a physical, neurological and orthopedic examination (Supplementary Forms S4, 5 and 6). Joint and spinal pain was quantified using an established scale, where scores are summated to give a score from 0 to 7690,91. Muscle condition score was quantified using an established scale, where temporal, epaxial and all 4 limb scores were summed to give a score of 0–1892. Off leash gait speed over a 5-meter distance was measured in triplicate and the mean value was calculated30. Cognitive testing included the sustained gaze task and 2 tests of executive function (cylinder and detour tasks). In the sustained gaze task, dogs are asked to focus on a treat held by the handler’s face and the time they maintain the gaze is recorded, performed in triplicate for calculation of the mean value in seconds21. The cylinder and detour tasks both generate outcomes as a percentage of correct choices in a set of trials (n = 8). All tasks are described in detail elsewhere83. All findings from examinations, mobility tests and cognitive tests were recorded in the REDCap database.
These assessments were performed at time 0, and at 1, 3 and 6 months. Blood work and urinalysis was repeated at 1 and 6 months, and a urine dipstick test was performed at 3 months. All lab work performed during the study are provided in Supplementary Data S2. The PAM device was placed for 2 weeks at the screening appointment and again at months 1, 3 and 6. It was removed after 2 weeks and returned to the research group along with the owner log that recorded collar removal or unusual activity. Data were downloaded for analysis after each time period using ActiLife software (version 6.13.3; Actigraph, Pensacola, FL) All raw activity data are provided in Supplementary Data S3.
Changes in the dog’s environment (e.g. address change, addition of another pet or other household changes) were categorized as present or absent at 1, 3 and 6 months as were changes in medications the dogs were receiving. Adverse events (changes in blood work and/or new clinical signs) were categorized as grade 1 through 5 according to the VCOG-CTCAEv2 guidelines58. Those classified as grade 2 or above were reviewed by the safety monitor and the treatment mask broken if considered appropriate.
Statistical analysis
All statistical analyses were performed with patient and group identity masked. Analyses were performed using JMP Pro 16 (SAS Institute, Cary, NC). Summary statistics were generated for dog demographics in each group at time of study entry. Continuous data were reported as mean and standard deviation (SD) or median and range depending on data distribution; categorical and ordinal data were reported as population proportion. Data were examined for normality based on inspection on Q–Q plots, histogram and Shapiro–Wilks test. Baseline demographic differences between groups were assessed using one-way ANOVA or Wilcoxon Rank Sum test (based on data normality) for continuous data. For ordinal/categorical data (Sex, BCS, Frailty Status [Y/N], Frailty Score) contingency tables were constructed and a chi-square test was performed with Fisher Exact test performed if needed. Changes in household and changes in medication were assessed in a similar manner, utilizing a chi-square test to assess significance (p < 0.05).
Changes in owner-based assessments (CCDR and frailty phenotype) from time zero to the primary endpoint (month three) were categorized as success (unchanged or decreased score) or failure (increased score). These categorical changes in CCDR and frailty were compared between groups by constructing contingency tables and performing a chi-square test.
Changes in CCDR score, cognitive testing performance and off-leash gait speed over the study were compared between groups with repeated measures ANOVA. Repeated measures ANOVA requires approximately normal distribution, so the residuals of these data within the model were assessed for distribution and outliers based on inspection of Q–Q plots and histogram. To account for covariates that might influence outcomes in senior dogs, univariate analyses of age, fractional lifespan, weight, sex (M or F), BCS, baseline frailty status (frail in 3 domains, yes or no), change in household and change in medication was performed for each of the outcome measures. Those covariates that reached significance of p < 0.1 were incorporated into the repeated measures ANOVA model for that outcome in addition to treatment effect. Overall differences between groups over time were assessed via Wilk’s lambda using a threshold of p < 0.05.
In order to assess whether any treatment effect was maintained from month 3 to month 6, changes in individual CCDR, Frailty, cognitive testing and off leash gait speed were calculated (month 6–month 3) and compared across groups. Changes were assessed via a Wilcoxon test, with p < 0.05 indicating significance. Owner’s perception of mobility and happiness level (categorized as improved, static or worse) were evaluated at months 3 and 6 via a chi-square test.
Activity data for weekdays and weekends were analyzed separately given the impact of owner schedule on dogs’ activity16,78. Data underwent quality control to identify the first 5 weekdays and 2 weekend days with complete data. Data from days that owners reported removal of the collar or unusual activity and days that included more than 3 consecutive hours with no (zero) activity detected were excluded. The mean activity per minute was calculated from 5 weekdays and from 2 weekend days and the change in mean activity/minute was calculated for each individual dog between study start and the primary end point at month 3. The change between 3 and 6 months was also calculated. Baseline and change in activity were analyzed using functional linear modeling (FLM) in order to facilitate modeling of activity data over time, thus avoiding the data loss that occurs when periods of time are averaged16,85. This was performed using RStudio 2021 (PBC, Boston, Ma) package “Actigraphy” version 1.4.0, which uses a Fourier expansion model to transform raw data into smoothed activity curves. Differences between groups were evaluated using a non-parametric permutation F-test with 1000 permutations performed. This generates a curve of the F-statistic over time. Point-wise (a curve with the F permutation proportion at each time point) and global (single number referring to the proportion of maximized F values from each permutation) critical F values are calculated to set thresholds for statistical significance93.
The FLM analysis only allows the incorporation of a single covariate (treatment group) and therefore cannot account for other potential confounding influences that we predict exist in the senior pet population. For this reason, we also analyzed total day time (5:00 a.m.–10:59 p.m.) and night-time (11:00 p.m.–4:59 a.m.) activity in a repeated measures analysis (allowing incorporation of covariates, similar to the rest of the outcome measures). Covariates (age, fractional lifespan, weight, sex, BCS, frailty status, pain, change in household and change in medication) were incorporated if they reached significance (p < 0.1) in a univariate analysis. As with the FLM analysis, weekday and weekend data were assessed separately and wilks lambda values were assessed for significance (p < 0.05). Change in cumulative activity during days and nights on weekdays and weekends across groups was assessed via a Wilcoxon test with p < 0.05 representing significance.
Supplementary Information
Acknowledgements
This clinical trial was funded by Animal Bioscience, Boston MA. Animal Bioscience played no role in data acquisition, analysis or presentation. We would like to acknowledge all the dogs and their owners and Amanda Valentino and Katerina Slaughter for assistance with dog handling.
Author contributions
K.E.S., N.J.O., M.E.G. and K.R. were responsible for the design, analysis, and primary writing of the manuscript for this study. K.E.S., A.M., K.R., B.C., C.W., Z.A., C.Y. all participated in the data acquisition; all authors participated in editing and review of the manuscript.
Data availability
Data are provided in full in the supplementary data files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Margaret E. Gruen and Natasha J. Olby.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-63031-w.
References
- 1.Selman C, Nussey DH, Monaghan P. Ageing: It's a dog's life. Curr. Biol. 2013;23:451–453. doi: 10.1016/j.cub.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Lewis TW, Wiles BM, Llewellyn-Zaidi AM, Evans KM, O'Neill DG. Longevity and mortality in Kennel Club registered dog breeds in the UK in 2014. Canine Genet. Epidemiol. 2018;5:1–17. doi: 10.1186/s40575-018-0066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarver AL, Makielski KM, DePauw TA, Schulte AJ, Modiano JF. Increased risk of cancer in dogs and humans: A consequence of recent extension of lifespan beyond evolutionarily determined limitations? Aging Cancer. 2022;3:3–19. doi: 10.1002/aac2.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creevy KE, Austad SN, Hoffman JM, O'Neill DG, Promislow DE. The companion dog as a model for the longevity dividend. Cold Spring Harb. Perspect. Med. 2016;6:a026633. doi: 10.1101/cshperspect.a026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin K, Hoffman JM, Creevy KE, O'Neill DG, Promislow DE. Multiple morbidities in companion dogs: A novel model for investigating age-related disease. Pathobiol. Aging Age Relat. Dis. 2016;6:33276. doi: 10.3402/pba.v6.33276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzatenta A, Carluccio A, Robbe D, Giulio CD, Cellerino A. The companion dog as a unique translational model for aging. Semin. Cell Dev. Biol. 2017;70:141–153. doi: 10.1016/j.semcdb.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie BA, Chen FL, Gruen ME, Olby NJ. Canine geriatric syndrome: A framework for advancing research in veterinary geroscience. Front. Vet. Sci. 2022;9:853743. doi: 10.3389/fvets.2022.853743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruple A, MacLean E, Snyder-Mackler N, Creevy KE, Promislow D. Dog models of aging. Annu. Rev. Anim. Biosci. 2022;10:419–439. doi: 10.1146/annurev-animal-051021-080937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salt C, Saito EK, O'Flynn C, Allaway D. Stratification of companion animal life stages from electronic medical record diagnosis data. J. Gerontol. Ser. A. 2023;78:579–586. doi: 10.1093/gerona/glac220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman JM, Creevy KE, Franks A, O'Neill DG, Promislow DEL. The companion dog as a model for human aging and mortality. Aging Cell. 2018;17:e12737. doi: 10.1111/acel.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias-Pereira P. Morbidity and mortality in elderly dogs—A model for human aging. BMC Vet. Res. 2022;18:457. doi: 10.1186/s12917-022-03518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: Perspectives from life course epidemiology and geroscience. J. Gerontol. Ser. A. 2016;71:1184–1194. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demnitz N, et al. Cognition and mobility show a global association in middle- and late-adulthood: Analyses from the Canadian Longitudinal Study on Aging. Gait posture. 2018;64:238–243. doi: 10.1016/j.gaitpost.2018.06.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray EE, et al. Associations between physical activity and cognitive dysfunction in older companion dogs: Results from the Dog Aging Project. GeroScience. 2023;45:645–661. doi: 10.1007/s11357-022-00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondino A, et al. Activity patterns are associated with fractional lifespan, memory, and gait speed in aged dogs. Sci. Rep. 2023;13:2588. doi: 10.1038/s41598-023-29181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casagrande M, Forte G, Favieri F, Corbo I. Sleep quality and aging: A systematic review on healthy older people, mild cognitive impairment and Alzheimer's disease. Int. J. Environ. Res. Public Health. 2022;19:8457. doi: 10.3390/ijerph19148457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tapp PD, et al. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J. Neurosci. 2004;24:8205–8213. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. BioMed Res. Int. 2015;2015:748212. doi: 10.1155/2015/748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Commodari E, Guarnera M. Attention and aging. Aging Clin. Exp. Res. 2008;20:578–584. doi: 10.1007/BF03324887. [DOI] [PubMed] [Google Scholar]
- 21.Hoel JA, et al. Sustained gaze is a reliable in-home test of attention for aging pet dogs. Front. Vet. Sci. 2021;8:819135. doi: 10.3389/fvets.2021.819135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piolatto M, Bianchi F, Rota M, Marengoni A, Akbaritabar A, Squazzoni F. The effect of social relationships on cognitive decline in older adults: An updated systematic review and meta-analysis of longitudinal cohort studies. BMC Public Health. 2022;22:278. doi: 10.1186/s12889-022-12567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapagain D, Wallis LJ, Range F, Affenzeller N, Serra J, Virányi Z. Behavioural and cognitive changes in aged pet dogs: No effects of an enriched diet and lifelong training. PLoS One. 2020;15:e0238517. doi: 10.1371/journal.pone.0238517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia X, Jiang Q, McDermott J, Han JJ. Aging and Alzheimer's disease: Comparison and associations from molecular to system level. Aging Cell. 2018;17:e12802. doi: 10.1111/acel.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiol. Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 26.Cotman CW, Head E. The canine (dog) model of human aging and disease: Dietary, environmental and immunotherapy approaches. J. Alzheimer's Dis. 2008;15:685–707. doi: 10.3233/JAD-2008-15413. [DOI] [PubMed] [Google Scholar]
- 27.Chapagain D, Range F, Huber L, Virányi Z. Cognitive aging in dogs. Gerontology. 2018;64:165–171. doi: 10.1159/000481621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrenzweig J, Hunter RP. Canine cognitive decline and Alzheimer disease: Clinical insights to solve a shared one-health problem. J. Am. Vet. Med. Assoc. 2023;261:1–8. doi: 10.2460/javma.23.02.0095. [DOI] [PubMed] [Google Scholar]
- 29.Studenski S, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondino A, et al. Winning the race with aging: Age-related changes in gait speed and its association with cognitive performance in dogs. Front. Vet. Sci. 2023;10:1150590. doi: 10.3389/fvets.2023.1150590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dao T, et al. Sarcopenia and muscle aging: A brief overview. Endocrinol. Metab. 2020;35:716–732. doi: 10.3803/EnM.2020.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarmohammadi S, Mozafar Saadati H, Ghaffari M, Ramezankhani A. A systematic review of barriers and motivators to physical activity in elderly adults in Iran and worldwide. Epidemiol. Health. 2019;41:e2019049. doi: 10.4178/epih.e2019049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12:412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge L, Mordiffi SZ. Factors associated with higher caregiver burden among family caregivers of elderly cancer patients: A systematic review. Cancer Nurs. 2017;40:471–478. doi: 10.1097/NCC.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie BA, Chen F, LaCroix-Fralish ML. The phenotype of aging in the dog: How aging impacts the health and well-being of dogs and their caregivers. J. Am. Vet. Med. Assoc. 2022;260:963–970. doi: 10.2460/javma.22.02.0088. [DOI] [PubMed] [Google Scholar]
- 36.Spitznagel MB, et al. Relationships among owner consideration of euthanasia, caregiver burden, and treatment satisfaction in canine osteoarthritis. Vet. J. 2022;286:105868. doi: 10.1016/j.tvjl.2022.105868. [DOI] [PubMed] [Google Scholar]
- 37.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, et al. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology. 2021;22:165–187. doi: 10.1007/s10522-021-09910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Micco, R., Krizhanovsky, V., Baker, D., & d'Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021). [DOI] [PMC free article] [PubMed]
- 40.Zhang L, Pitcher LE, Prahalad V, Niedernhofer LJ, Robbins PD. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023;290:1362–1383. doi: 10.1111/febs.16350. [DOI] [PubMed] [Google Scholar]
- 41.Covarrubias AJ, et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2020;2:1265–1283. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkland JL, Tchkonia T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicin. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022;28:1556–1568. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat. Metab. 2020;2:9–31. doi: 10.1038/s42255-019-0161-5. [DOI] [PubMed] [Google Scholar]
- 47.Chu X, Raju RP. Regulation of NAD+ metabolism in aging and disease. Metab. Clin. Exp. 2022;126:154923. doi: 10.1016/j.metabol.2021.154923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie N, et al. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadeeshani H, Li J, Ying T, Zhang B, Lu J. Nicotinamide mononucleotide (NMN) as an anti-aging health product—Promises and safety concerns. J. Adv. Res. 2021;37:267–278. doi: 10.1016/j.jare.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Q, Zhou X, Xu K, Liu S, Zhu X, Yang J. The safety and antiaging effects of nicotinamide mononucleotide in human clinical trials: An update. Adv. Nutr. 2023;14:1416–1435. doi: 10.1016/j.advnut.2023.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardoso D, Barthélémy I, Blot S, Muchir A. Replenishing NAD+ content reduces aspects of striated muscle disease in a dog model of Duchenne muscular dystrophy. Skelet. Muscle. 2023;13:20. doi: 10.1186/s13395-023-00328-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma A, Chabloz S, Lapides RA, Roider E, Ewald CY. Potential synergistic supplementation of NAD+ promoting compounds as a strategy for increasing healthspan. Nutrients. 2023;15:445. doi: 10.3390/nu15020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tapp PD, et al. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn. Mem. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guarino A, Forte G, Giovannoli J, Casagrande M. Executive functions in the elderly with mild cognitive impairment: A systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health. 2020;24:1028–1045. doi: 10.1080/13607863.2019.1584785. [DOI] [PubMed] [Google Scholar]
- 58.LeBlanc AK, et al. Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021;19:311–352. doi: 10.1111/vco.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, et al. Quantitative translation of dog-to-human aging by conserved remodeling of the DNA methylome. Cell Syst. 2020;11:176–185. doi: 10.1016/j.cels.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolopikou CF, et al. Acute nicotinamide riboside supplementation improves redox homeostasis and exercise performance in old individuals: A double-blind cross-over study. Eur. J. Nutr. 2020;59:505–515. doi: 10.1007/s00394-019-01919-4. [DOI] [PubMed] [Google Scholar]
- 61.Niu KM, et al. The impacts of short-term NMN supplementation on serum metabolism, fecal microbiota, and telomere length in pre-aging phase. Front. Nutr. 2021;8:756243. doi: 10.3389/fnut.2021.756243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang H. A Multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Front. Aging. 2022;3:851698. doi: 10.3389/fragi.2022.851698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chini CCS, Cordeiro HS, Tran NLK, Chini EN. NAD metabolism: Role in senescence regulation and aging. Aging Cell. 2024;23:e13920. doi: 10.1111/acel.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madari A, et al. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES) Appl. Anim. Behav. Sci. 2015;171:138–145. doi: 10.1016/j.applanim.2015.08.034. [DOI] [Google Scholar]
- 65.Schütt T, Toft N, Berendt M. Cognitive function, progression of age-related behavioral changes, biomarkers, and survival in dogs more than 8 years old. J. Vet. Intern. Med. 2015;29:1569–1577. doi: 10.1111/jvim.13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan LM. Repeated measures. Circulation. 2008;117:1238–1243. doi: 10.1161/CIRCULATIONAHA.107.654350. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J. Chronic Dis. 1967;20:637–648. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- 68.Ford I, Norrie J. Pragmatic trials. N. Engl. J. Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 69.Neilson JC, Hart BL, Cliff KD, Ruehl WW. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001;218:1787–1791. doi: 10.2460/javma.2001.218.1787. [DOI] [PubMed] [Google Scholar]
- 70.Azkona G, García-Belenguer S, Chacón G, Rosado B, León M, Palacio J. Prevalence and risk factors of behavioural changes associated with age-related cognitive impairment in geriatric dogs. J. Small Anim. Pract. 2009;50:87–91. doi: 10.1111/j.1748-5827.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 71.Yarborough S, Fitzpatrick A, Schwartz SM, Dog Aging Project Consortium Evaluation of cognitive function in the Dog Aging Project: Associations with baseline canine characteristics. Sci. Rep. 2022;12:13316. doi: 10.1038/s41598-022-15837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conzemius MG, Evans RB. Caregiver placebo effect for dogs with lameness from osteoarthritis. J. Am. Vet. Med. Assoc. 2012;241:1314–1319. doi: 10.2460/javma.241.10.1314. [DOI] [PubMed] [Google Scholar]
- 73.Milgram NW, et al. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: A two-year longitudinal study. Neurobiol. Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Pop V, et al. Synergistic effects of long-term antioxidant diet and behavioral enrichment on beta-amyloid load and non-amyloidogenic processing in aged canines. J. Neurosci. 2010;30:9831–9839. doi: 10.1523/JNEUROSCI.6194-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fahnestock M, et al. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol. Aging. 2012;33:546–554. doi: 10.1016/j.neurobiolaging.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Degeling C, Burton L, McCormack GR. An investigation of the association between socio-demographic factors, dog-exercise requirements, and the amount of walking dogs receive. Can. J. Vet. Res. 2012;76:235–240. [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HS, Song JG, Lee JY. Influences of dog attachment and dog walking on reducing loneliness during the COVID-19 pandemic in Korea. Animals. 2022;12:483. doi: 10.3390/ani12040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dow C, Michel KE, Love M, Brown DC. Evaluation of optimal sampling interval for activity monitoring in companion dogs. Am. J. Vet. Res. 2009;70:444–448. doi: 10.2460/ajvr.70.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hua J, et al. Assessment of frailty in aged dogs. Am. J. Vet. Res. 2016;77:1357–1365. doi: 10.2460/ajvr.77.12.1357. [DOI] [PubMed] [Google Scholar]
- 80.Banzato T, et al. A Frailty Index based on clinical data to quantify mortality risk in dogs. Sci. Rep. 2019;9:16749. doi: 10.1038/s41598-019-52585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melvin RL, Ruple A, Pearson EB, Olby NJ, Fitzpatrick AL, Creevy KE. A review of frailty instruments in human medicine and proposal of a frailty instrument for dogs. Front. Vet. Sci. 2023;10:1139308. doi: 10.3389/fvets.2023.1139308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russell, K. Establishing a Frailty Phenotype for Aging Dogs. Oral Presentation. ACVIM Forum. (2022).
- 83., Fefer, G. et al. Use of cognitive testing, questionnaires, and plasma biomarkers to quantify cognitive impairment in an aging pet dog population. J. Alzheimer's Dis.87, 1367–1378 (2022). [DOI] [PMC free article] [PubMed]
- 84.Salvin HE, McGreevy PD, Sachdev PS, Valenzuela MJ. The canine cognitive dysfunction rating scale (CCDR): A data-driven and ecologically relevant assessment tool. Vet. J. 2011;188:331–336. doi: 10.1016/j.tvjl.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Woods HJ, Li MF, Patel UA, Lascelles BDX, Samson DR, Gruen ME. A functional linear modeling approach to sleep–wake cycles in dogs. Sci. Rep. 2020;10:22233. doi: 10.1038/s41598-020-79274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weese JS, Blondeau J, Boothe D, Guardabassi LG, Gumley N, Papich M, Jessen LR, Lappin M, Rankin S, Westropp JL, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019;247:8–25. doi: 10.1016/j.tvjl.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harris PA, et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res. Vet. Sci. 2007;82:208–214. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 90.Knazovicky D, Helgeson ES, Case B, Gruen ME, Maixner W, Lascelles BDX. Widespread somatosensory sensitivity in naturally occurring canine model of osteoarthritis. Pain. 2016;157:1325–1332. doi: 10.1097/j.pain.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mondino A, et al. Static posturography as a novel measure of the effects of aging on postural control in dogs. PLoS One. 2022;17:e0268390. doi: 10.1371/journal.pone.0268390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cline MG, et al. 2021 AAHA nutrition and weight management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2021;57:153–178. doi: 10.5326/JAAHA-MS-7232. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, et al. Measuring the impact of apnea and obesity on circadian activity patterns using functional linear modeling of actigraphy data. J. Circadian Rhythms. 2011;9:11. doi: 10.1186/1740-3391-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided in full in the supplementary data files.