Abstract
Heterocyclic rings are important structural scaffolds encountered in both natural and synthetic compounds, and their biological activity often depends on these motifs. They are predominantly accessible via cycloaddition reactions, realized by either thermal, photochemical, or catalytic means. Various starting materials are utilized for this purpose, and, among them, diazo compounds are often encountered, especially vinyldiazo compounds that give access to donor-acceptor cyclopropenes which engage in [2+n] cycloaddition reactions. Herein, we describe the development of photochemical processes that produce diverse heterocyclic scaffolds from multisubstituted oximidovinyldiazo compounds. High chemoselectivity, good functional group tolerance, and excellent scalability characterize this methodology, thus predisposing it for broader applications. Experimental and computational studies reveal that under light irradiation these diazo reagents selectively transform into cyclopropenes which engage in cycloaddition reactions with various dipoles, while under thermal conditions the formation of pyrazole from vinyldiazo compounds is favored.
Subject terms: Reaction mechanisms, Synthetic chemistry methodology, Photocatalysis
Heterocycles, highly prized by the pharmaceutical industry, are often constructed from diazo compounds. Here, the authors report that direct photolysis of vinyldiazo compounds followed by [3+2]-cycloaddition gives access to these motifs.
Introduction
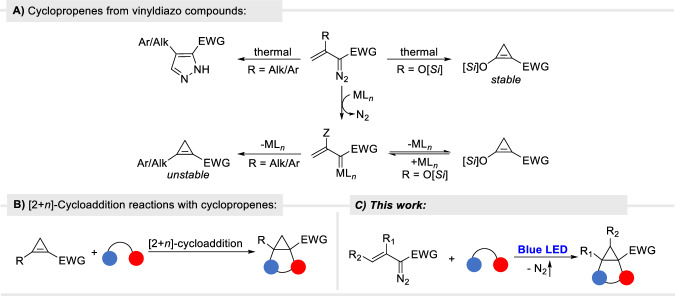
The development of effective, sustainable methodologies giving access to valuable heterocyclic molecules is of paramount importance to modern synthetic chemistry. Among approaches to their synthesis, cycloaddition reactions stand at the forefront, and the use of visible light as the only source of energy for these transformations is highly appealing. Recently, diazo compounds that produce various reactive intermediates have attracted considerable interest1–7. In particular, vinyldiazo compounds have proven to be valued reactants for the synthesis of diverse carbo- and heterocyclic compounds8–10, especially through cycloaddition reactions11,12. Their catalytic applications include highly enantioselective [3 + 3]-, [3 + 2]-, and [3 + 1]-cycloaddition13. Furthermore, silyl-protected enol diazoacetates are known to form stable donor-acceptor cyclopropenes that provide a resting state for incipient metallovinyl carbenes in cycloaddition reactions14. Their photochemically induced dinitrogen extrusion to form vinylcarbene intermediates has been investigated15,16, and the resultant formation of cyclopropene products with alkyl and aryl substituents is well known17–19. Cyclopropenes are highly strained alkenes that are, themselves, highly reactive in cycloaddition reactions20,21; they are activated by strain to undergo “click”-like cycloaddition reactions and although few reports have documented these transformations13,22–25, they have already been employed for site-specific protein conjugation26,27, cell labeling28,29 and other materials development functions30,31.
The linkage between vinyldiazo compounds and cyclopropenes provides versatility in product formation. Donor-acceptor cyclopropenes are produced catalytically from silyl-protected enoldiazo compounds via metallovinyl carbene intermediates32, as well as thermally33 in nearly quantitative yield (Fig. 1A). β-Aryl/alkyl vinyl diazocarbonyl compounds undergo non-reversible catalytic formation of unstable cyclopropenes via metallovinyl carbenes34, but thermally they form 3H-pyrazoles that transform into 1H-pyrazoles by 1,5-H transfer35. In particular, styryldiazoacetates undergo the thermal formation of 1H-pyrazoles, but they do not form cyclopropenes in catalytic reactions36. In catalytic reactions with vinyldiazo compounds, dipolar species can intercept metallo-vinylcarbenes prior to cyclopropene formation but with suitable donor-acceptor substituents; the cyclopropene can be returned to the metallo-vinylcarbene to produce [3+n]-cycloaddition products14. Because of their inherent reactivity, the double bond in cyclopropenes undergoes [2+n]-cycloaddition with viable dipolar species (Fig. 1B)20,21,37,38. However, although vinyldiazo compounds are precursors to cyclopropenes, they have not been used as direct precursors for [2+n]-cycloaddition products that can be achieved with cyclopropenes; instead, the [2+n]-cycloaddition products are formed from cyclopropenes, which, in turn, are separately prepared from vinyldiazo compounds or by alternative methods. We now report a general, efficient methodology for the conversion of vinyldiazo compounds to [2+n]-cycloaddition products through the photochemical production of intermediate cyclopropenes (Fig. 1C).
Fig. 1. Transformations of vinyldiazo compounds.
A Catalytic and thermal formation of cyclopropenes from vinyldiazo compounds; B Cyclopropene cycloaddition reactions; C Tandem cycloaddition reactions of vinyldiazo compounds.
Results and Discussion
Generation of cyclopropenes from vinyldiazo compounds
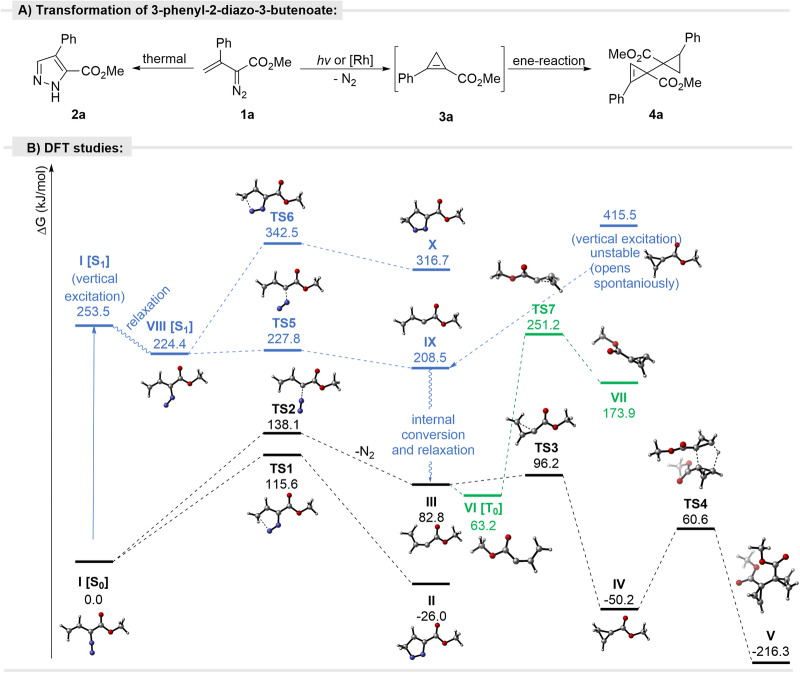
Vinyldiazo compounds form either cyclopropenes (dinitrogen extrusion)33,39 or 1H-pyrazoles (cycloaddition/rearrangement)34,36. This dichotomy is found in the photochemical and thermal reactions of β-aryl-/alkyl-vinyl diazoacetates. The thermal reaction of 3-phenyl-2-diazo-3-butenoate (1a) produces corresponding 1H-pyrazole 2a in high yield, whereas dinitrogen is lost in the photochemical reaction to give cyclopropene dimer 4a resulting from an ene-reaction40 of the unstable cyclopropene intermediate 3a (Fig. 2A). The same 4a is obtained in the rhodium acetate catalyzed reaction of 1a34.
Fig. 2. Photoreactivity of vinyl diazo compounds.
A Transformation of 3-phenyl-2-diazo butanoate. B Calculated reactivity of methyl 2-diazobut-3-enoate in the ground and exited states.
To understand the basis for the divergent outcomes from thermal and photochemical reactions, the chemistry of model methyl 2-diazobut-3-enoate I was investigated computationally on S0 and S1 potential energy surfaces (Fig. 2B)41,42. In the ground state (S0 PES), cyclization of the model vinyl diazoacetate I towards pyrazole II is preferred by 22.5 kJ/mol over extrusion of nitrogen (ΔG‡ = 115.6 and 138.1 kJ/mol, respectively, Fig. 2B), although both are hardly accessible at ambient temperature. The latter of the discussed pathways provides carbene III, which easily (ΔG‡ = 13.4 kJ/mol) cyclizes, yielding cyclopropane IV. The dimerization of IV via an ene-reaction must overcome a barrier of ΔG‡ = 110.8 kJ/mol. A similar reactivity pattern was observed, i.e., preference for cyclization over extrusion of N2 under thermal conditions, for vinyl diazo compounds substituted with simple alkyl and aryl groups (see, SI). Conversely, the calculated mechanistic scenario on the S1 PES is considerably different. The cycloisomerization of methyl 2-diazobut-3-enoate VIII to pyrazole system X not only features a considerable barrier (ΔG‡ = 118.1 kJ/mol) but is also highly endergonic (ΔG = 92.3 kJ/mol). In contrast, the excited vinyl diazoacetate VIII loses dinitrogen in a practically barrierless event (ΔG‡ = 3.4 kJ/mol). However, the subsequent cyclization of resulting carbene IX into a cyclopropene ring cannot occur at S1 PES (high-energy cyclopropene in the excited state spontaneously opens towards the vinyl carbene). Thus, it presumably first undergoes internal conversion to ground state III, at which it then converts into cyclopropene system IV. In contrast, cyclization of carbene VI in the triplet state, which is a ground state15,16, through TS5 is associated with a very high barrier of ΔG‡ = 188.0 kJ/mol, which is not accessible under the reaction conditions.
Cycloaddition reactions of vinyldiazo compounds
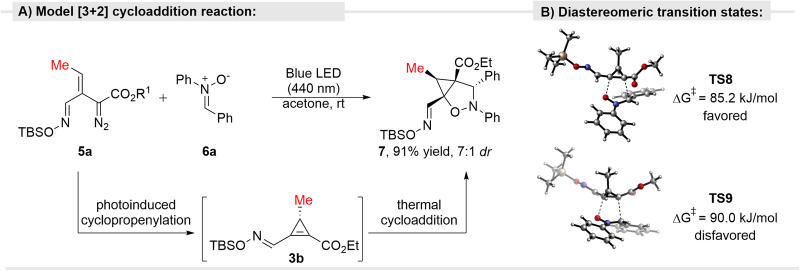
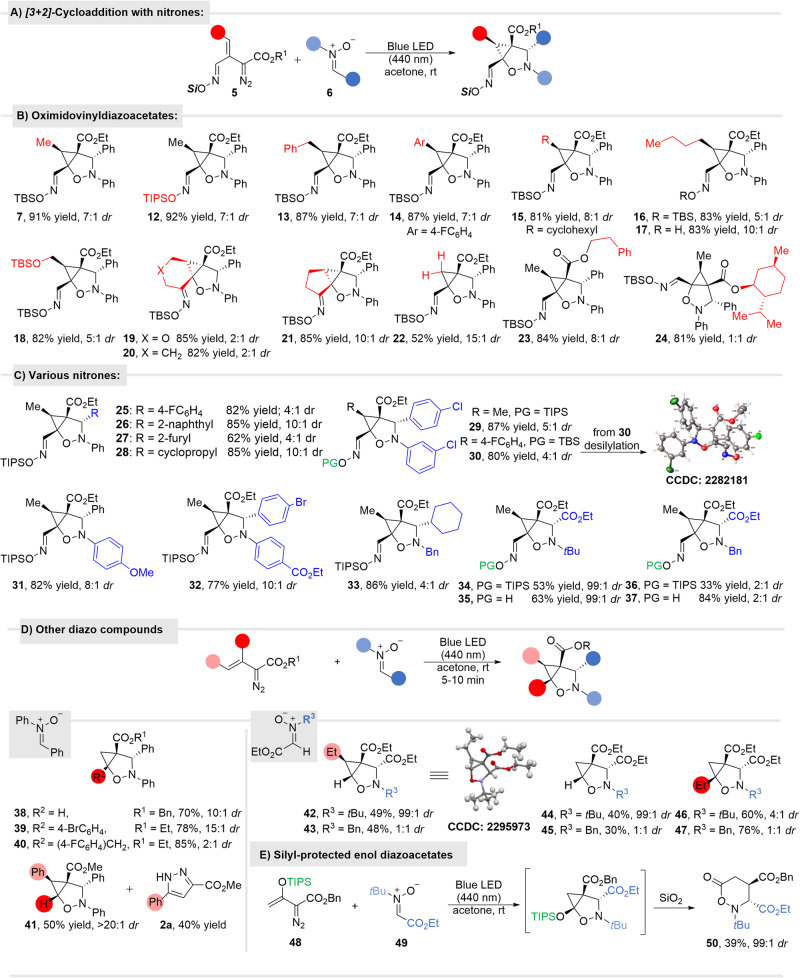
Based on these theoretical studies, we expected that cyclopropenes could be generated from vinyldiazo compounds under light irradiation and, as such, engage in reactions with dipoles. In this regard, we previously observed traces of [3 + 2]-cycloaddition product formed via cyclopropene intermediates, accompanying the intended metallovinylcarbene [3+n]-cycloaddition product, in the Rh-catalyzed reaction of enol diazoacetates with isoquinolinium dicyanomethylides32,34. To evaluate the possibility of developing photochemical tandem reaction, generation of the cyclopropene from vinyldiazo compounds followed by its reaction with dipoles, leading to heterocycles, oximidovinyldiazo acetate 5a was chosen as a model diazo compound (Fig. 3). Such vinyldiazo reagents are conveniently prepared from 1,2,3-triazine 1-oxides43. Since cyclopropene cycloaddition with nitrones is known to occur in reactions involving metallo-vinylcarbene intermediates44–48, we commenced our studies with nitrones 6a as a reliable model dipole11,49. Photolysis with blue light gave the expected isooxazolidine cycloaddition product 7 as a mixture of diastereoisomers (7:1 ratio) in 91% yield. Control experiments revealed that the reaction is photochemical in nature. Under light irradiation cyclopropene forms, as confirmed by the 1H NMR analysis, and after the addition of the nitrone, thermal cycloaddition occurs. Because cyclopropene can undergo rapid dimerization via an ene reaction40, intermediate 3b was not isolated in a pure form. According to DFT calculations, cycloaddition of nitrone 6 to the model cyclopropene (TMS protected) proceeds with a Gibbs free energy of activation of 85.2 and 90.0 kJ/mol for major and minor isomers, respectively, which matches the observed diastereoselectivity for the reaction with cyclopropene 3b. Expectedly, for the reaction of simple acrylate (not a strained analog of cyclopropene) with nitrone 6 a barrier of 109.1 kJ/mol was calculated, corroborating the higher reactivity of strained cyclopropene over acrylate.
Fig. 3. [3 + 2]-Cycloaddition reaction of diazo compounds.
A Model reaction of oximidovinyldiazo acetate 5a with nitrone 6. B DFT calculations on the cycloadditon [3 + 2] of model cyclopropene with nitrone 6.
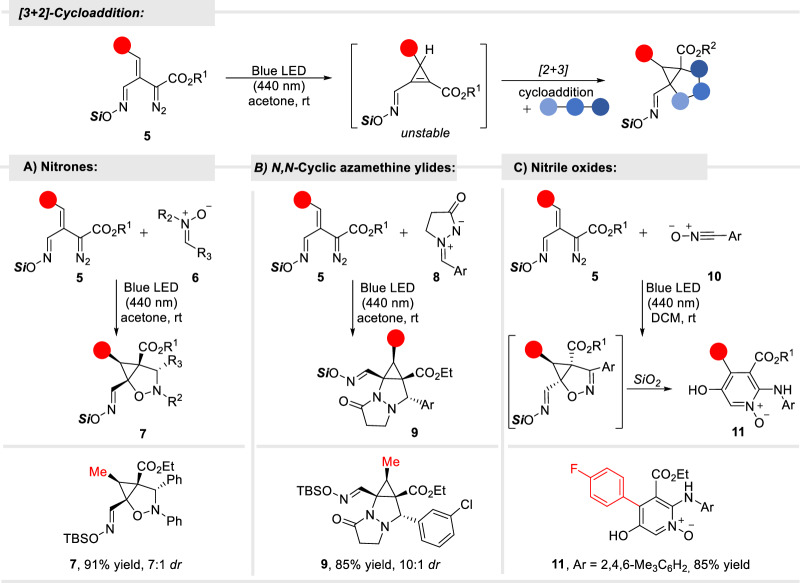
To test the generality of the developed tandem transformation, we also explored azomethine imines and nitrile oxides as other dipole-type substrates. Both reactions gave [3 + 2] products; for azomethine ylide 8 cycloadduct 9 was observed, while an unexpected pyridine N-oxide 11 formed from the cycloadduct produced in reactions of some oximidovinyldiazo esters with 2,4,6-trimethylbenzonitrile oxide (10) (Fig. 4).
Fig. 4. Cycloaddition reactions of oximidovinyldiazo compounds with various dipoles.
A Model reaction of oximidovinyldiazo acetate 5 with nitrone 6. B Model reaction of oximidovinyldiazo acetate 5 with N,N-cyclic azamethine ylide 8. C Model reaction of oximidovinyldiazo acetate 5 with nitrile oxide 10.
Realizing the generality of the proposed strategy and the importance of heterocyclic scaffolds, we next optimized the reaction conditions and evaluated the substrate scope for the newly developed [2 + 3]-cycloaddition reactions.
Cycloaddition of oximidovinyldiazo acetates with nitrones
The model reaction of oximidovinyldiazo acetate 5a with diphenylmethanimine oxide (6) was performed in different solvents, and the highest dr ratio (7:1) and yield (91% isolated) was obtained in acetone (see Table S1 in SI). Reactions in chlorocarbon solvents (dichloromethane, chloroform, 1,2-dichloroethane) also occurred in high yields, but their dr were significantly diminished to only 1:2 to 1:3. Using LED light sources (40 W) at 400 nm produced the cycloaddition product 7 in only 30% yield. The dominant stereoisomer in all cases is the one in which the phenyl group is trans to the carboethoxy group. The stereochemistry of the two diastereomers was determined spectroscopically based on the X-ray structure of the dominant isomer 30 after desilylation (Fig. 5).
Fig. 5. Photocatalytic formal [3 + 2]-cycloaddition of diverse oximidovinyldiazo esters with nitrones.
A The general transformation; B Reaction scope of oximidovinyldiazoacetates; C Reaction scope of nitrones; D Reaction of other types of vinyldiazo compounds with nitrones; E Reaction of silyl-protected enol diazoacetate 48.
The scope of the reaction was determined with a broad spectrum of oximidovinyldiazo compounds 5 and a representative selection of nitrones 6 (Fig. 5).
Reactions with alkyl and aryl substituted oximidovinyldiazo compounds 5 under the optimal conditions gave isooxazolidine products 7, 12–24 in high yields and modest dr values (Fig. 5B). Steric factors appear to play a major role in determining diastereoselectivity. The highest dr was achieved with the vinyldiazo ester without a substituent at the gamma position (22), and the lowest dr intriguingly comes from the reaction of the vinyldiazo ester with a bulky menthyl ester of oximidovinyldiazoacetate 24. In all these reactions, the competing ene reaction of intermediate cyclopropene 3b was only a minor component of the reaction products, amounting to less than 10% yield; the only exception being the reaction of vinyldiazoester with no substituent at the gamma position leading to product 22.
Similarly, the size and the nature of substituents on the nitrone influence the diastereoselectivity of the reaction (Fig. 5C). For Ar and alkyl substituted nitrones, yields remained at the same level (25–33) while for glyoxalic acid derived nitrone reactions were less efficient (34, 36). The yields, however, improved when deprotected oximidovinyldiazoacetate was used as a starting material, suggesting that the free hydroxyl group, by forming hydrogen bonds influences the reaction’s efficacy (products 35, 37). The biggest impact on the stereoselectivity of the reaction had the replacement of N-Bn with bulky tBu group for which reactions produced only one diastereoisomer (compare 34 with 36).
Oximidovinyldiazo compounds 5 are stable reagents in contrast to vinyldiazo and arylvinyldiazo derivatives. However, when tested in the developed method, gratifyingly, desired cycloaddition products 38–47 were also formed from these less stable derivatives, though with slightly diminished yields compared to reactions with oximidovinyldiazoacetates (Fig. 5D). The outcome of the photolytic reaction with aryl-substituted vinyldiazoacetates show contrasting behavior; with β- phenylvinydiazoacetate the yields and dr of isooxazolidine cycloaddition products 38–40 were comparable to those with similarly substituted oximidovinyldiazoacetates, whereas with γ- phenylvinyldiazoacetate (styryldiazoacetate) competition with intramolecular pyrazole 2a formation reduced the yield of the isooxazolidine cycloaddition product 41 which was formed with high diastereocontrol. In general, reactions of vinyldiazo compounds with nitrones derived from glyoxylic acid ester were less efficient. Though diastereoselectivity remained at the same level for N-Bn derivatives, N- tBu analogs proved superior in furnishing products 42 and 44 as single diastereoisomers.
Furthermore, preliminary experiments confirmed that silyl-protected enol diazoacetate 48, which forms a stable donor-acceptor cyclopropene, is also a suitable substrate in the developed tandem transformation (Fig. 5E). In this case, the bicyclic product was, however, not isolated. During the purification, the TIPS protecting group cleaves inducing the subsequent rearrangement giving product 50 in 39% yield.
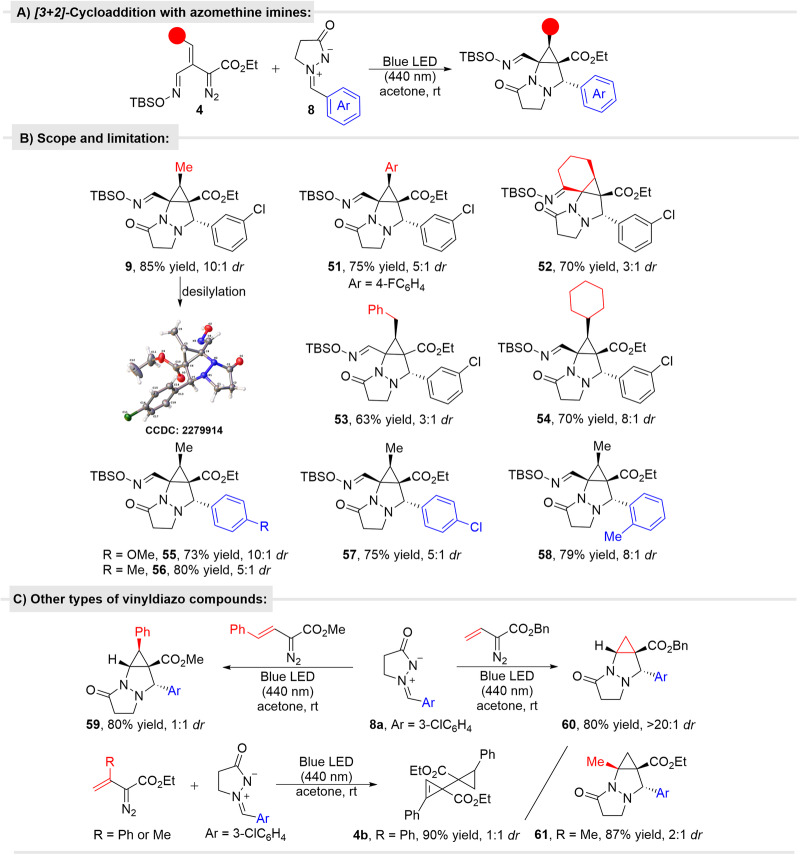
Cycloaddition of oximidovinyldiazo acetates with N,N-cyclic azamethine imines
N,N-Cyclic azamethine imines are also suitable dipoles in cycloaddition reactions that have been reported to undergo [3 + 2]-cycloaddition reactions with propargylic and α,β-unsaturated carbonyl compounds50,51, and cyclic enamines52. With vinyldiazo compounds, these azomethine imines are unreactive unless a transition metal catalyst converts the vinyldiazo compound to a metallovinylcarbene which then undergoes a N-N cleavage reaction to form a diimide with azomethine rather than [3 + 3]-cycloaddition53,54. In our case, photochemical tandem reaction of N,N-cyclic azamethine ylides 8 with vinyldiazo compound 5a gave tricyclic pyrazolone 9 without the use of any catalyst (Fig. 6B).
Fig. 6. Photocatalytic formal [3 + 2]-cycloaddition of diverse oximidovinyldiazo esters with azomethine imines.
A The general transformation; B Reaction scope of oximidovinyldiazoacetates and azomethine imines; C Reaction of other types of vinyldiazo compounds with azomethine imines.
Yields of cycloaddition reactions are comparable to those obtained for nitrones without the need for fine tuning of the reaction conditions. The competing ene dimer 4b was obtained at most in less than 10% yield. Diastereoselectivities are also on a similar level with the trans-R/Ar isomer dominant (configuration certified by X-ray analysis). Interestingly, the reaction with styryldiazoacetate gave the [3 + 2]-cycloaddition product 59 in high yield but with no diastereoselectivity and without the evidence of pyrrole formation (Fig. 6C). In contrast, only one diastereoisomer 60 is produced from the reaction with the unsubstituted ethyl 2-diazo-3-butenoate. With ethyl 3-phenyl-2-diazo-3-butenoate, only the ene dimer 4a was isolated, and there was no evidence for the formation of the [3 + 2]-cycloaddition product, whereas reaction with ethyl 3-methyl-2-diazo-3-butenoate resulted in a high yield of the cycloaddition product 61 although with low diastereocontrol (Fig. 6C).
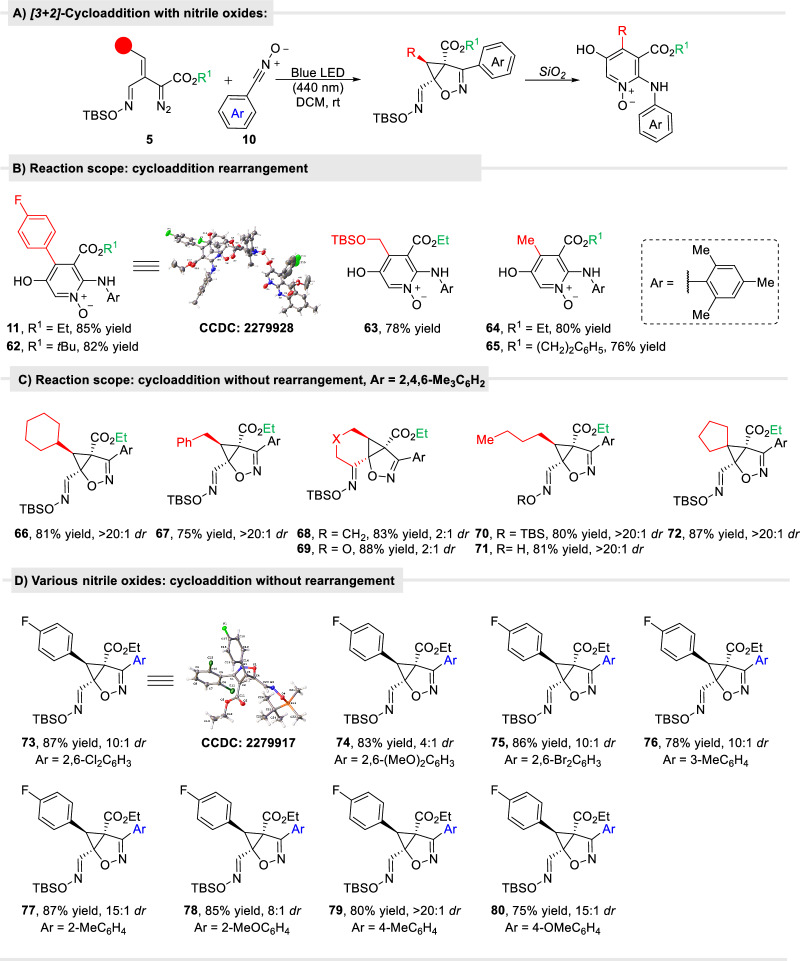
Cycloaddition of oximidovinyldiazo acetates with nitrile oxides
Nitrile oxides are relatively reactive dipolar species that are well known to undergo [3 + 2]-cycloaddition reactions with alkenes and alkynes to form diverse heterocyclic compounds55–57, that have been used in the synthesis of natural products58. We selected the relatively stable aromatic nitrile oxide 10a with the mesityl aromatic ring that does not dimerize and combined this nitrile oxide with vinyldiazoesters. As anticipated, under photolysis with blue light, in the presence of nitrile oxide 10a, cyclopropene intermediate 3a undergoes rapid [3 + 2]-cycloaddition with high diastereoselectivity (Fig. 7).
Fig. 7. Photocatalytic formal [3 + 2]-cycloaddition of oximidovinyldiazo esters with nitrile oxide compounds.
A The general transformation; B Formation of pyridine-N-oxides from photolytic cyclization/silica induced rearrangement. X-ray structure of 56 is of the hydrogen-bonded dimer; C Reaction scope for [3 + 2]-cycloaddition of oximidovinyldiazoacetates 5 with mesitylnitrile oxide (10); D Reaction scope of oximidovinyldiazo acetate 5a with various arylnitrile oxides.
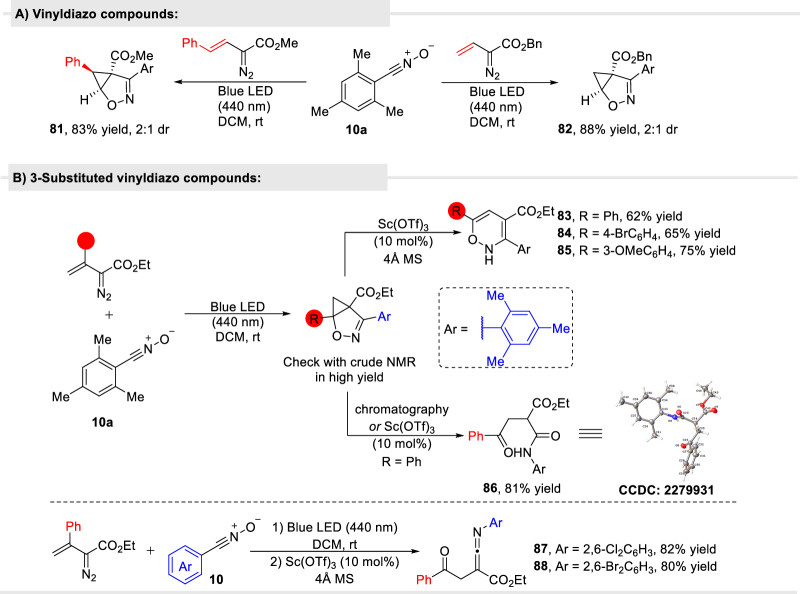
Intriguingly, some of the cycloaddition products converted to new chemical structures (11, 62–65) during chromatography on silica gel, of which one was identified both spectroscopically and by X-ray crystallography to be pyridine-N-oxides 11 (Fig. 7B). However, [3 + 2]-cycloaddition products 66–72 did not undergo rearrangement on silica gel even over long periods of time, or in the presence of Lewis acids (Fig. 7C). Products 73–80, obtained from other aryl nitrile oxides in high yield, were stable and, intriguingly, did not rearrange regardless of the position of substituents and the electronic character of the phenyl ring of nitrile oxide (Fig. 7D). Reactions with vinyldiazo esters other than the oximidovinyldiazo esters 5 also formed stable cycloaddition products (81 and, 82) but with low stereoselectivity (Fig. 8A). As acidic conditions seemed to promote conversion of isoxazole products to pyridine-N-oxides, we wondered whether Sc(OTf)3 treatment of the products from [3 + 2]-cycloaddition of 3-substituted vinyl diazo esters with mesitylnitrile oxide (10a) would lead to pyridine derivatives (Fig. 8B). In this case however, the reaction resulted in ring opening of the cyclopropane ring and formation of 2H-1,2-oxazine 83–85 in the absence of water or afforded cyclic product 86 in the presence of water. On the other hand, ketenimine products (87 and 88) were obtained in the two-step reaction from ethyl 2-diazo-3-phenylbut-3-enoate with arylnitrile oxides (Fig. 8B).
Fig. 8. Reaction of other vinyl diazo compounds with nitrile oxide.
A Cycloaddition of other types of vinyldiazo compounds with arylnitrile oxides. B Photocatalytic formal [3 + 2]-cycloaddition of 3-substituted vinyldiazo compounds with nitrile oxide compounds.
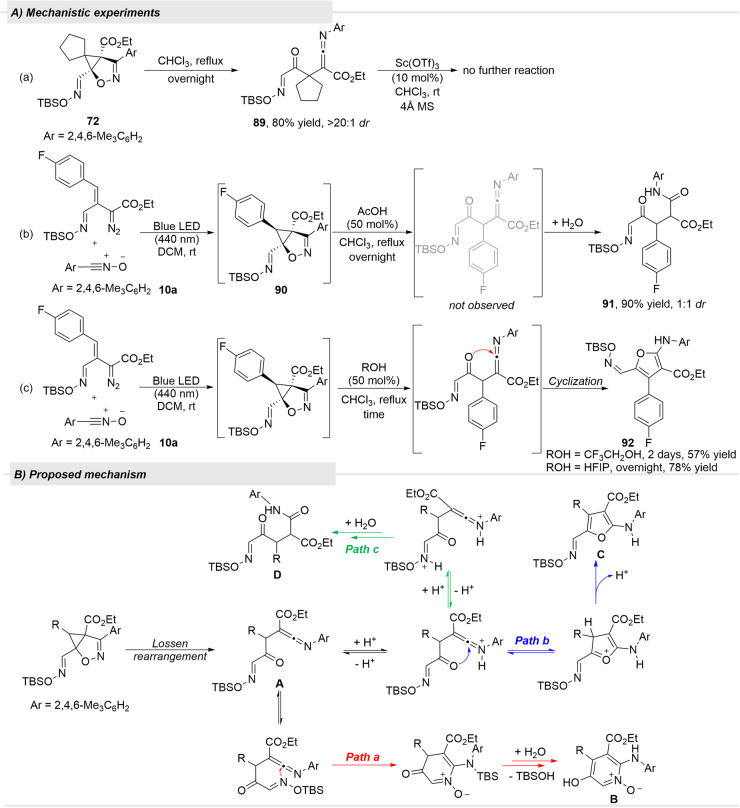
To determine how the pyridine-N-oxide was formed, we subjected several of the [3 + 2]-cycloaddition products to heating at reflux in chloroform. Only spirocyclopentyl derivative 72 underwent reaction and the product of this thermal reaction was ketenimine 89, anticipated to be formed by the Lossen rearrangement, which was previously reported to occur in a dirhodium(II)-catalyzed process involving formation of an intermediate [3 + 2]-cycloaddition product similar to 89 (Fig. 9A, a)59. Indeed, when cycloaddition product 90 from cycloaddition of 4-fluorophenyl oximidovinyldiazoacetates with mesitylnitrile oxide 10a was treated with AcOH in chloroform, the mixed ester/amide 91 as formed in 90% yield, which is similar to the product from the dirhodium(II) catalyzed reaction, thus also pointing to a ketenimine intermediate (Fig. 9A, b). The same treatment of the benzyl and cyclohexyl analogs of 68 produced the corresponding ester/amides in 77% (2:1 dr) and 83% (3:1 dr) yields, respectively. Consistent with this intermediate, treatment of 90 with the less acidic 2,2,2-trifluoroethanol or hexafluoroisopropyl alcohol (HFIP) formed furan 92 in 57% and 78% yield, respectively, that further confirms the ketenimine intermediate (Fig. 9A, c).
Fig. 9. Mechanistic studies for rearrangement of the [3 + 2]-cycloaddition product from ketenime intermediate to pyridine-N-oxide, furan, and ester/amide products.
A Control experiments. B Proposed mechanism.
The formation of ketenimines intermediates from the isoxazole-related products obtained from nitrile oxides explains the formation of pyridine-N-oxides, ester/amides, and furan products. In the proposed mechanism, ketenimine A is formed by the Lossen rearrangement60,61, although the reactivity of the [3 + 2]-cycloaddition products towards this rearrangement is not evident. This multi-substituted intermediate has two basic centers (the imine and oxime nitrogens) whose protonation influences subsequent reactions (Fig. 9B). Although protonation may not be required for the formation of the pyridine-N-oxide B (path a), protonation of the imine nitrogen of intermediate, A activates the central ketenimine carbon for nucleophilic attack by the ketone oxygen to form furan derivative C (path b). If both basic nitrogens are protonated, neither intramolecular reaction occurs, and ketenimine hydrolysis occurs to produce D (path c).
Conclusions
In conclusion, we have found that direct photolysis of vinyldiazo compounds selectively leads to cyclopropenes, which contrasts with the same reactions under thermal conditions that favors the formation of pyrazoles. Once formed, these reactive cyclopropene intermediates undergo [3 + 2]-cycloaddition with diverse dipolar species to yield heterocyclic scaffolds, highly prized by the pharmaceutical industry. Reactions of vinyldiazo compounds with nitrones, N,N-cyclic azamethine ylides, and nitrile oxides afforded heterocyclic products in high yields with mostly good stereoselectivities. The most diastereoselective reactions with nitrones were those derived from N-tBu-glyoxylic acid, for which single isomers were observed. N,N-cyclic azamethine ylides react with the photochemically-generated cyclopropenes in modest to good yields and diastereoselectivities, and the transformation is general for vinyldiazoacetates. Nitrile oxides show similar generality in its cycloaddition reactions with photochemically-generated cyclopropenes, but its bicyclic isoxazole products exhibit a diversity of reactions and reactivities, some of which, especially the formation of pyridine-N-oxides, were unexpected. A great variety of heterocyclic scaffolds accessible by the developed method emphasizes the importance of cyclopropene generated from diazo compounds in a photochemical manner. Further studies of its unique reactivity are currently underway in our laboratory.
Methods
General procedure for [3 + 2]-cycloadditions with nitrones
To a 10-mL oven-dried vial with a magnetic stirring bar, vinyldiazo compound (0.1 mmol) in 1.0 mL acetone was added over 1 min to a solution of nitrones (0.12 mmol, 1.2 equiv.) in the same solvent (1.0 mL) at room temperature with irradiation by 440 nm blue LED (40 W), and the reaction mixture was stirred for 5–15 min under these conditions. When the reaction was complete (monitored by TLC), the reaction mixture was purified by flash column chromatography on silica gel without additional treatment (hexanes: EtOAc = 20:1 to 15:1) to give the pure [3 + 2]-cycloaddition products in good yields.
General procedure for [3 + 2]-cycloaddition with azamethine imines
To a 10-mL oven-dried vial with a magnetic stirring bar, vinyldiazo compound (0.12 mmol, 1.2 equiv.) in 1.0 mL acetone was added to a solution of azamethine imine (0.1 mmol) in the same solvent (1.0 mL) via a syringe pump over 2 h at room temperature with irradiation by 440 nm blue LED (40 W). When the reaction was complete (monitored by TLC), the reaction mixture was purified by flash column chromatography on silica gel without additional treatment (hexanes: EtOAc = 20:1 to 15:1) to give the pure [3 + 2]-cycloaddition products in good yields.
General procedure for [3 + 2]-cycloaddition with nitrile oxides
To a 10-mL oven-dried vial with a magnetic stirring bar, vinyldiazo compound (0.12 mmol, 1.2 equiv.) in 1.0 mL DCM was added to a solution of the nitrile oxide (0.1 mmol) in the same solvent (1.0 mL) via a syringe pump over 1 h at room temperature with irradiation by 440 nm blue LED. When the reaction was complete (monitored by TLC), the reaction mixture was purified by flash column chromatography on silica gel without additional treatment (hexanes: EtOAc = 20:1 to 1:1) to give the pure [3 + 2]-cycloaddition and cycloaddition/rearrangement products in good yields.
Supplementary information
Source data
Acknowledgements
Financial support for this work was supported by the U.S. National Science Foundation (CHE 2054845) for M.P.D. and M. B. and by NCN Poland OPUS UMO-2019/35/B/ST4/03435 for D.G.and K.Ł. Calculations have been carried out using resources provided by Wrocław Center for Networking and Supercomputing (http://wcss.pl), grant No. 518. for W.CH.
Author contributions
M. B. and K. Ł. performed photo-catalytic studies of the vinyldiazo compounds and characterization of products. M. B. and M. Baird prepared the vinyl diazo compounds. M. B., D. G., and M. P. D. conceived and designed the experiments, W. CH. prepared DFT calculations, and M. B., K. Ł., D. G., and M. P. D. prepared the manuscript. All authors contributed to discussions and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks shengbiao tang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The Authors declare that all relevant data generated and analyzed during this study, which include experimental, spectroscopic, crystallographic and computational data, are included in this article and its supplementary information. Should any raw data files be needed in another format, they are available from the corresponding author upon request. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 1) 2282181, 2) 2295973, 3) 2279914, 4) 2279928, 5) 2279917, 6) 2279931. Coordinates of the optimized structures are present as source data. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wojciech Chaładaj, Email: wojciech.chaladaj@icho.edu.pl.
Dorota Gryko, Email: dorota.gryko@icho.edu.pl.
Michael P. Doyle, Email: michael.doyle@utsa.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-48274-5.
References
- 1.Wang, J. & Qiu, D. Recent developments of diazo compounds in organic synthesis. (World Scientific: London, 2020).
- 2.Wang, J., Che, C. M. & Doyle, M. P. (eds) Transition metal-catalyzed carbene transformations. (Wiley-VCH GmbH, Weinheim, Germany, 2022).
- 3.Ford A, et al. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015;115:9981–10080. doi: 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- 4.Jurgberg ID, Davies HML. Blue light-promoted photolysis of aryldiazoacetates. Chem. Sci. 2018;9:5112–5118. doi: 10.1039/C8SC01165F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Wang S, George V, Galder L, König B. Photo-induced homologation of carbonyl compounds for iterative syntheses. Angew. Chem. Int. Ed. 2022;61:e202211578. doi: 10.1002/anie.202211578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durka J, Turkowska J, Gryko D. Lightening diazo compounds? ACS Sustain. Chem. Eng. 2021;9:8895–8918. doi: 10.1021/acssuschemeng.1c01976. [DOI] [Google Scholar]
- 7.Yang Z, Stivanin ML, Jurberg ID, Koenigs RM. Chem. Soc. Rev. 2020;49:6833–6847. doi: 10.1039/D0CS00224K. [DOI] [PubMed] [Google Scholar]
- 8.López E, González-Pelayo S, López LA. Recent developments in coinage metal catalyzed transformations of stabilized vinyldiazo compounds: beyond carbenic pathways. Chem. Rec. 2017;17:312–325. doi: 10.1002/tcr.201600099. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Q-Q, Yu Y, Yedoyan J, Doyle MP. Vinyldiazo reagents and metal catalysts: a versatile toolkit for heterocycle and carbocycle construction. ChemCatChem. 2018;10:488–496. doi: 10.1002/cctc.201701346. [DOI] [Google Scholar]
- 10.López E, Bernardo O, López LA. Coinage metal-catalyzed carbo- and heterocyclizations involving alkenyl carbene intermediates as C3 synthons. Tetrahedron Lett. 2022;109:154156. doi: 10.1016/j.tetlet.2022.154156. [DOI] [Google Scholar]
- 11.Marichev KO, Doyle MP. Catalytic asymmetric cycloaddition reactions of enoldiazo compounds. Org. Biomol. Chem. 2019;17:4183–4195. doi: 10.1039/C9OB00478E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Zhang J. Gold-catalyzed transformations of α-diazocarbonyl compounds: Selectivity and diversity. Chem. Soc. Rev. 2016;45:506–516. doi: 10.1039/C5CS00821B. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Q-Q, Deng Y, Lankelma M, Doyle MP. Cycloaddition reactions of enoldiazo compounds. Chem. Soc. Rev. 2017;46:5425–5443. doi: 10.1039/C7CS00324B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong K, Marichev KO, Doyle MP. The role of donor-acceptor cyclopropenes in metal carbene reactions. conversion of E-substituted enoldiazoacetates to Z-substituted metallo-enolcarbenes. Organometallics. 2019;38:4043–4050. doi: 10.1021/acs.organomet.9b00427. [DOI] [Google Scholar]
- 15.Ciszewski LW, Rybicka-Jasinska K, Gryko D. Recent developments in photochemical reactions of diazo compounds. Org. Biomol. Chem. 2019;17:432–448. doi: 10.1039/C8OB02703J. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Kubicvki J, Platz MS. Ultrafast UV-visible and infrared spectroscopic observation of a singlet vinylcarbene and the intramolecular cyclopropenation reaction. J. Am. Chem. Soc. 2009;131:13602–13603. doi: 10.1021/ja905992p. [DOI] [PubMed] [Google Scholar]
- 17.Closs GL, Closs LE. Alkenylcarbenes as precursors of cyclopropenes. J. Am. Chem. Soc. 1961;83:2015–2016. doi: 10.1021/ja01469a057. [DOI] [Google Scholar]
- 18.Padwa A, Blacklock TJ, Getman D, Hatanaka N, Loza R. On the problem of regioselectivity in the photochemical ring-opening reaction of 3-phenyl- and 3-vinyl-substituted cyclopropenes to indenes and 1,3-cyclopentadienes. J. Org. Chem. 1978;43:1481–1492. doi: 10.1021/jo00402a002. [DOI] [Google Scholar]
- 19.Zhu Z-B, Wei Y, Shi M. Recent developments of cyclopropene chemistry. Chem. Soc. Rev. 2011;40:5534–5563. doi: 10.1039/c1cs15074j. [DOI] [PubMed] [Google Scholar]
- 20.Molchanov AP, Efremova MM, Kuznetsov MA. Cyclopropenes and methylenecyclopropanes in 1,3-dipolar cycloaddition reactions. Russ. Chem. Bull. 2022;71:620–650. doi: 10.1007/s11172-022-3460-z. [DOI] [Google Scholar]
- 21.Ravasco JMJ, Monteiro CM, Trindade AF. Cyclopropenes: a new tool for the study of biological systems. Org. Chem. Front. 2017;4:1167–1198. doi: 10.1039/C7QO00054E. [DOI] [Google Scholar]
- 22.Gahtory D, Sen R, Kuzmyn AR, Escorihuela J, Zuilhof H. Strain-promoted cycloaddition of cyclopropenes with o-quinones: a rapid click reaction. Angew. Chem. Int. Ed. 2018;57:10118–10122. doi: 10.1002/anie.201800937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Liang Y, Šečkutė J, Houk KN, Devaraj NK. Synthesis and reactivity comparisons of 1-methyl-3-substituted cyclopropene mini-tags for tetrazine bioorthogonal reactions. Chem. Eur. J. 2014;20:3365–3375. doi: 10.1002/chem.201304225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oblak EZ, VanHeyst MD, Li J, Weimer AJ, Wright DL. Cyclopropene cycloadditions with annulated furans: Total synthesis of (+)- and (−)-frondosin B and (+)-frondosin A. J. Am. Chem. Soc. 2014;136:4309–4315. doi: 10.1021/ja413106t. [DOI] [PubMed] [Google Scholar]
- 25.Kumar GS, Lin Q. Light-triggered click chemistry. Chem. Rev. 2021;121:6991–7031. doi: 10.1021/acs.chemrev.0c00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrmann, A. et al. Bioconjugate Chem. 26, 257–261 (2015). [DOI] [PubMed]
- 27.Bruins JJ, et al. Inducible, site-specific protein labeling by tyrosine oxidation-strain-promoted (4 + 2) cycloaddition. Bioconjugate Chem. 2017;28:1189–1193. doi: 10.1021/acs.bioconjchem.7b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George A, et al. Accelerated strain-promoted and oxidation-controlled cyclooctyne-quinone cycloaddition for cell labeling. ChemistrySelect. 2017;2:7117–7122. doi: 10.1002/slct.201700581. [DOI] [Google Scholar]
- 29.Patterson DM, Nazarova LA, Xie B, Kamber DN, Prescher JA. Functionalized cyclopropenes as bioorthogonal chemical reporters. J. Am. Chem. Soc. 2012;134:18638–18643. doi: 10.1021/ja3060436. [DOI] [PubMed] [Google Scholar]
- 30.Jonker AM, et al. A fast and activatable cross-linking strategy for hydrogel formation. Adv. Mater. 2015;27:1235–1240. doi: 10.1002/adma.201404448. [DOI] [PubMed] [Google Scholar]
- 31.Kaur G, Singh G, Singh J. Photochemical tuning of materials: a click chemistry perspective. Mater. Today Chem. 2018;8:56–84. doi: 10.1016/j.mtchem.2018.03.002. [DOI] [Google Scholar]
- 32.Xu X, Zavalij PY, Doyle MP. Catalytic asymmetric syntheses of quinolizidines by dirhodium-catalyzed dearomatization of isoquinolinium/pyridinium methylides-the role of catalyst and carbene source. J. Am. Chem. Soc. 2013;135:12439–12447. doi: 10.1021/ja406482q. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Jing C, Doyle MP. Dinitrogen extrusion from enoldiazo compounds under thermal conditions: synthesis of donor-acceptor cyclopropenes. Chem. Commun. 2015;51:12924–12927. doi: 10.1039/C5CC05006E. [DOI] [PubMed] [Google Scholar]
- 34.Zheng H, Faghihi I, Doyle MP. Copper(I)-catalyzed highly enantioselective [3+3]-cycloaddition of β-aryl/Alkyl vinyl diazoacetates with nitrones. Helv. Chim. Acta. 2021;104:e2100081. doi: 10.1002/hlca.202100081. [DOI] [Google Scholar]
- 35.Kardile RD, Liu RS. Gold(I)-catalyzed reactions between 2-(1-alkynyl)-2-alken-1-ones and vinyldiazo ketones for divergent synthesis of nonsymmetric heteroaryl-substituted triarylmethanes: N- versus C-attack paths. Org. Lett. 2020;22:8229–8233. doi: 10.1021/acs.orglett.0c02765. [DOI] [PubMed] [Google Scholar]
- 36.Padwa A, Kulkarni YS, Zhang Z. Reaction of carbonyl compounds with ethyl lithio-diazoacetate. studies dealing with the rhodium(II)-catalyzed behavior of the resulting adducts. J. Org. Chem. 1990;55:4144–4153. doi: 10.1021/jo00300a035. [DOI] [Google Scholar]
- 37.Liu F, Liang Y, Houk KN. Bioorthogonal cycloadditions: computational analysis with the distortion/interaction model and predictions of reactivities. Acc. Chem. Res. 2017;50:2297–2308. doi: 10.1021/acs.accounts.7b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincente R. Recent progresses towards the strengthening of cyclopropene chemistry. Synthesis. 2016;48:2343–2360. doi: 10.1055/s-0035-1561644. [DOI] [Google Scholar]
- 39.Marichev KO, et al. Rhodium(II)-catalysed generation of cycloprop-1-en-1-yl ketones and their rearrangement to 5-aryl-2-siloxyfurans. Chem. Commun. 2018;54:9513–9516. doi: 10.1039/C8CC05623D. [DOI] [PubMed] [Google Scholar]
- 40.Deng Q, Thomas BE, Houk KN, Dowd P. Transition structures of the ene reactions of cyclopropene. J. Am. Chem. Soc. 1997;119:6902–6908. doi: 10.1021/ja963248q. [DOI] [PubMed] [Google Scholar]
- 41.Frisch, M. J. et al. Gaussian 16, Revision C.01, (Gaussian, Inc., Wallingford CT, 2019).
- 42.Marenich AV, Cramer CJ, Truhlar DG. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B. 2009;113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 43.Bao, M. et al. Expanded access to vinyldiazo compounds and their catalytic enantioselective application. Chem Catal. 3, (in press 2023).
- 44.Wang X, Xu X, Zavalij PY, Doyle MP. Asymmeric formal [3 + 3]-cycloaddition reactions of nitrones with electrophilic vinylcarbene intermediates. J. Am. Chem. Soc. 2011;133:16402–16405. doi: 10.1021/ja207664r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Abrahams QM, Zavalij PY, Doyle MP. Highly regio- and stereoselective dirhodium vinylcarbene inducedNitrone cycloaddition with subsequent cascade carbenoid aromatic cycloaddition/N-O cleavage and rearrangement. Angew. Chem. Int. Ed. 2012;51:5907–5910. doi: 10.1002/anie.201201917. [DOI] [PubMed] [Google Scholar]
- 46.Qian Y, et al. Rhodium(II)- and copper(II)-catalyzed reactions of enol diazoacetates with nitrones: metal carbene versus Lewis acid directed pathways. Angew. Chem. Int. Ed. 2012;51:5900–5903. doi: 10.1002/anie.201202525. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Q-Q, Yedoyan J, Arman H, Doyle MP. Copper-catalyzed divergent addition reactions of enoldiazoacetamides with nitrones. J. Am. Chem. Soc. 2016;138:44–47. doi: 10.1021/jacs.5b10860. [DOI] [PubMed] [Google Scholar]
- 48.Adly FG, Marichev KO, Jensen JA, Arman H, Doyle MP. Enoldiazosulfones for highly enantioselective [3 + 3]-cycloadditionwith nitrones catalyzed by copper(I) with chiral BOX ligands. Org. Lett. 2019;21:40–44. doi: 10.1021/acs.orglett.8b03421. [DOI] [PubMed] [Google Scholar]
- 49.Diev VV, et al. Nitrone cycloadditions to 1,2-diphenylcyclopropenes and subsequent transformations of the isoxazolidine cycloadducts. J. Org. Chem. 2008;73:2396–2399. doi: 10.1021/jo702379d. [DOI] [PubMed] [Google Scholar]
- 50.Deepthi A, Thomas NV, Sruthi SL. An overview of the reactions involving azomethine imines over half a decade. N. J. Chem. 2021;45:8847–8873. doi: 10.1039/D1NJ01090E. [DOI] [Google Scholar]
- 51.Borah B, Chowhan LR. Recent updates on the stereoselective synthesis of structurally functionalized spiro-oxindoles mediated by isatin N, N´-cyclic azomethine imine 1, 3-dipoles. Tetrahedron Lett. 2022;104:154014. doi: 10.1016/j.tetlet.2022.154014. [DOI] [Google Scholar]
- 52.Cao VD, Kim K, Kwak J, Joung S. [3 + 2] Cycloaddition reaction of the endocyclic N-silyl enamine and N,N′-cyclic azomethine imine. Org. Lett. 2022;24:1974–1978. doi: 10.1021/acs.orglett.2c00366. [DOI] [PubMed] [Google Scholar]
- 53.Qian Y, Zavalij PY, Hu W, Doyle MP. Bicyclic pyrazolidinone derivatives from diastereoselective catalytic [3+3]-cycloaddition reactions of enoldiazoacetates with azomethine imines. Org. Lett. 2013;15:1564–1567. doi: 10.1021/ol400339c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filatov AS, et al. Stereo- and regioselective 1,3-dipolar cycloaddition of the stable ninhydrin-derived azomethine ylide to cyclopropenes: Trapping of unstable cyclopropene dipolarophiles. J. Org. Chem. 2019;84:7017–7036. doi: 10.1021/acs.joc.9b00753. [DOI] [PubMed] [Google Scholar]
- 55.Rane D, Sibi M. Recent advances in nitrile oxide cycloadditions. synthesis of isoxazolines. Curr. Org. Synth. 2011;8:616–627. doi: 10.2174/157017911796957320. [DOI] [Google Scholar]
- 56.Easton CJ, Hughes CMM, Savage GP, Simpson GW. Cycloaddition reactions of nitrile oxides with alkenes. Adv. Heterocycl. Chem. 1994;60:261–327. doi: 10.1016/S0065-2725(08)60184-1. [DOI] [Google Scholar]
- 57.Heaney F. Nitrile oxide/alkyne cycloadditions-a credible platform for synthesis of bioinspired molecules by metal-free molecular clicking. Eur. J. Org. Chem. 2012;16:3043–3048. doi: 10.1002/ejoc.201101823. [DOI] [Google Scholar]
- 58.Bode JW, Carreira EM. Stereoselective syntheses of epothilones A and B via nitrile oxide cycloadditions and related studies. J. Org. Chem. 2001;66:6410–6424. doi: 10.1021/jo015791h. [DOI] [PubMed] [Google Scholar]
- 59.Xu X, Shabashov D, Zavalij D, Doyle MP. Unexpected catalytic reactions of silyl-protected enol diazoacetates with nitrile oxides that form 5-arylaminofuran-2(3H)-one-4-carboxylates. Org. Lett. 2012;14:800–803. doi: 10.1021/ol203331r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui Y, Zhang G, Ding C. Recent advances in Lossen rearrangement. Chin. J. Org. Chem. 2022;42:2015–2017. doi: 10.6023/cjoc202202030. [DOI] [Google Scholar]
- 61.Thomas M, et al. The lossen rearrangement from free hydroxamic acids. Org. Biomol. Chem. 2019;17:5420–5427. doi: 10.1039/C9OB00789J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Authors declare that all relevant data generated and analyzed during this study, which include experimental, spectroscopic, crystallographic and computational data, are included in this article and its supplementary information. Should any raw data files be needed in another format, they are available from the corresponding author upon request. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 1) 2282181, 2) 2295973, 3) 2279914, 4) 2279928, 5) 2279917, 6) 2279931. Coordinates of the optimized structures are present as source data. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided in this paper.