Abstract
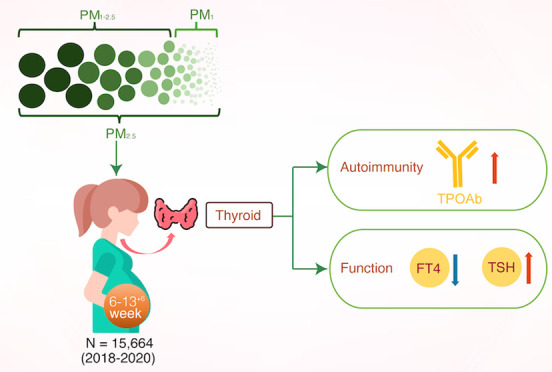
This prospective birth cohort study evaluated the association of exposure to PM2.5 (diameter ≤2.5 μm), PM1–2.5 (1–2.5 μm), and PM1 (≤1 μm) with maternal thyroid autoimmunity and function during early pregnancy. A total of 15,664 pregnant women were included at 6 to 13+6 gestation weeks in China from 2018 to 2020. Single-pollutant models using generalized linear models (GLMs) showed that each 10 μg/m3 increase in PM2.5 and PM1–2.5 was related with 6% (odds ratio [OR] = 1.06, 95% confidence interval [CI]: 1.01, 1.12) and 15% (OR = 1.15, 95% CI: 1.08, 1.22) increases in the risk of thyroid autoimmunity, respectively. The odds of thyroid autoimmunity significantly increased with each interquartile range increase in PM2.5 and PM1–2.5 exposure (P for trend <0.001). PM1 exposure was not significantly associated with thyroid autoimmunity. GLM with natural cubic splines demonstrated that increases in PM2.5 and PM1–2.5 exposure were associated with lower maternal FT4 levels, while a negative association between PM1 and FT4 levels was found when exposure exceeded 32.13 μg/m3. Only PM2.5 exposure was positively associated with thyrotropin (TSH) levels. Our findings suggest that high PM exposure is associated with maternal thyroid disruption during the early pregnancy.
Keywords: PM1, PM2.5, thyroid autoimmunity, TPOAb, FT4, TSH
Short abstract
A limited number of studies have investigated the association of PM exposure with maternal thyroid autoimmunity. This study explored this association based on the China Birth Cohort Study.
1. Introduction
Thyroid autoimmunity refers to the presence of the thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TgAb), or thyroid-stimulating hormone receptor antibody (TRAb) alone or a combination of these three antibodies. Thyroid autoimmunity affects approximately 18% of pregnant women.1 During pregnancy, women suffering from thyroid autoimmunity are at an increased risk of adverse pregnancy outcomes, such as miscarriage and preterm birth.2−4 Pregnant women who have thyroid antibodies are more likely to experience postpartum thyroiditis.5,6 Thyroid autoimmunity in mothers is also associated with impaired neurological development and intrauterine growth retardation in the fetus.7,8 Moreover, the fetus relies completely on thyroid hormones from the mother via placental transfer, particularly during the first half of pregnancy.9 Common biomarkers for maternal thyroid homeostasis, including free thyroxine (FT4), free triiodothyronine (FT3), and thyrotropin (TSH), are essential for fetal growth and development. Therefore, risk factors that cause maternal thyroid disruption must be identified to avoid adverse outcomes during pregnancy and birth. Studies have indicated that both genetic and environmental factors play important roles in maternal thyroid disruption. There is a growing concern about the adverse impact of environmental exposure during pregnancy on maternal and fetal health.10
Nine epidemiological studies have investigated the associations between maternal thyroid function and ambient particulate matter (PM), including PM with a diameter of ≤10 μm (PM10) and a diameter of ≤2.5 μm (PM2.5) and PM2.5 constituents during pregnancy (Table S1). Pooled meta-analysis showed that exposure to PM2.5 during pregnancy disrupts maternal thyroid homeostasis.11 PM with a diameter of ≤1 μm (PM1) was deemed more harmful to health than PM2.5 and PM1–2.5 (with a diameter ranging between 1 and 2.5 μm).12 There have been no reports of maternal thyroid function concerning exposure to PM1 and PM1–2.5 during pregnancy. Moreover, PM2.5 exposure has been linked with an elevated susceptibility to autoimmune diseases such as rheumatoid arthritis, connective tissue diseases, systemic lupus erythematosus, multiple sclerosis, type 1 diabetes mellitus, and inflammatory bowel disease.13,14 Only two cohort studies have described the correlation between prenatal exposure to PM2.5 and maternal thyroid autoimmunity.15,16 Another two studies used TPOAb as a potential modifier to explore the associations between PM2.5 exposure and indicators of thyroid function.17,18 The results of these studies, however, were not consistent. Thus, it is necessary to conduct more investigations to verify the association of PM with maternal thyroid autoimmunity.
The aim of this study was to evaluate the association of PM1, PM1–2.5, and PM2.5 exposure with the development of maternal thyroid autoimmunity during the early pregnancy, based on the China Birth Cohort Study (CBCS).19 In addition, we assessed the impact of PM exposure on the maternal thyroid function.
2. Materials and Methods
2.1. Study Population
This prospective cohort study was based on the CBCS, which has been described elsewhere.19 Participants were recruited from the Beijing Obstetrics and Gynecology Hospital, Capital Medical University, between February 2018 and February 2020. The exclusion criteria included: (1) missing information on thyroid function in the first trimester or request to withdraw; (2) missing the accurate home address; (3) undergoing assisted reproductive technology; (4) multiple births; and (5) a history of thyroid disease (e.g., hypothyroidism, hyperthyroidism, and thyromegaly) or taking medicines that may affect thyroid function before pregnancy (e.g., levothyroxine sodium or propylthiouracil). Participants can withdraw from the study at any stage. This study included 15,664 pregnant women. The Ethics Committee of Beijing Obstetrics and Gynecology Hospital and Capital Medical University approved the protocol. All participants signed an informed consent.
2.2. Assessment of Air Pollution
Ground-monitored data of PM1 and PM2.5 were collected from the China Atmosphere Watch Network (CAWNET) of the China Meteorological Administration. The CAWNET network included 96 stations situated in 73 cities throughout 29 provinces or municipalities within mainland China, with the exception of the Qinghai province and Tianjin. Specifically, the four stations in Beijing were located in Changping, Miyun, Shunyi, and Haidian districts.20 Concentrations of PM at each station were measured by using a GRIMM aerosol spectrometer (Model 1.108, Grimm Aerosol Technik GmbH, Ainring, Germany). The CAWNET provided gravimetric equivalent values of PM1 and PM2.5. Quality controls for PM measurements were performed by two procedures: a “limit check” and a “climatological check”. In the process of the limit check, each valid PM measurement was examined to ensure that it fell within its bounds and remove any outlines. During the climatological check, hourly PM measurements were assessed as the median and the standard deviation (SD) at each site. Any PM values exceeding three SDs from the median PM have been eliminated.
A generalized additive model and random forest model were developed to estimate the daily PM1 and PM2.5 concentrations at a special resolution of 0.1° × 0.1° from 2018 to 2020.21,22 Results from a 10-fold cross-validation showed R2 values of 57 and 77% for daily and annual predictions for PM1 and 86 and 88% for PM2.5, respectively. The values of root mean squared error were 20.1 and 8.6 μg/m3 for daily and annual predictions for PM1, respectively, and 18.3 and 7.1 μg/m3 for PM2.5, respectively. In this study, the PM1 and PM2.5 average concentration during 0–13+6 gestational weeks for each participant was calculated as the exposure level. The location and relative PM exposure concentration for each participant are shown in Figures S2 and S3. PM1–2.5 was determined by deducting the PM1 concentration from PM2.5.
2.3. Outcome Measurement and Diagnosis
2.3.1. TPOAb, FT4, and TSH
Venous blood samples were collected in the first trimester (6–13+6 gestational weeks) upon enrollment. An automatic chemiluminescence immunoanalyzer (CENTAUR XP, Siemens) was used to determine the levels of TPOAb, FT4, and TSH. If the participants had multiple thyroid function test results, then, only the first determination was used in this study.
2.3.2. Diagnosis of Thyroid Autoimmunity
TPOAb positivity is the most frequently used biomarker of thyroid autoimmunity.23 The diagnostic criterion for TPOAb positivity was ≥60 U/mL.17 Diagnosis was made by certified obstetricians or gynecologists and recorded in the participant’s medical records.
2.4. Covariates and Modifiers
Potential confounding variables for the association between PM and thyroid autoimmunity were identified using a directed acyclic graph (Figure S4) as described elsewhere.17,24,25 The maternal age, prepregnancy body mass index (BMI), annual household income (<200,000 CNY vs ≥200,000 CNY), educational level (<16 years vs ≥16 years), ethnicity (Han vs minority), alcohol consumption (yes vs no), smoking status (yes vs no), gravidity (1 vs ≥2), season of enrollment (spring, summer, autumn, or winter), and gestational week were included in estimates of the effect of PM exposure on thyroid autoimmunity.
2.5. Statistical Analysis
Normality and homogeneity were assessed by Shapiro–Wilk and Bartlett’s test for continuous variables. FT4 and TSH values were ln-transformed for statistical analysis. Mean ± SD or median ± interquartile range (IQR) is used to present continuous variables, while frequency with percentage is used to describe categorical data. To reduce potential bias, baseline characteristics of pregnant women who were included in the study were compared with those who were excluded (Table S2).
A generalized linear regression model with natural cubic splines in single-pollutant models was utilized to assess the associations of PM exposure with TPOAb positivity and biomarkers of thyroid function. Five different knots (2, 3, 4, 5, and 6) were used to calculate the Akaike information criteria (AIC). To keep the results comparable, models with minimal AIC were selected, and the mean of the knots (4, located at the 0th, 33.3th, 66.7th, and 100th) was used in a natural cubic spline model.26 Next, a likelihood ratio test was conducted to compare linear and nonlinear models and evaluate the potential for nonlinearity. The results suggested that linear models could be utilized to estimate the relationship between PM exposure and thyroid autoimmunity with TPOAb positivity; however, nonlinear models were suitable for investigating the associations between PM exposure and biomarkers of thyroid function (FT4 and TSH) (Table S3).
The odds ratio (OR) and 95% confidence interval (CI) were estimated to determine the effect per 10 μg/m3 increase in PM on thyroid autoimmunity with TPOAb positivity. The participants were divided into quartiles by PM exposure and effect estimates were scaled using the IQR. The calculation of P-values for trends was executed. The estimated changes with 95% CI of FT4 and TSH values at the 50th, 75th, and 95th percentiles of PM versus minimum exposure were assessed by using natural cubic splines.
The associations of PM exposure with maternal thyroid autoimmunity (TPOAb positivity) and biomarkers (FT4 and TSH) of thyroid function may differ among subgroups. To investigate these varying associations, stratified analyses were conducted by prepregnancy BMI (<25 kg/m2 vs ≥25 kg/m2), maternal age (<35 years vs ≥35 years), education level (<16 years vs ≥16 years), maternal ethnicity (Han vs minority), annual household income (<200,000 CNY vs ≥200,000 CNY), and gravidity (1 vs ≥2). The interaction between PM1 and PM1–2.5 was also examined by stratified analyses in which low- and high-PM groups were defined according to the median PM levels. Two-sample t-tests were used to calculate the P-values for differences in associations among subgroups of potential modifiers.27
Statistical significance was tested through the use of a two-tailed test, with a significance level set at a P value of <0.05. All analyses were carried out using R software, specifically version 4.2.2 provided by the R Foundation for Statistical Computing.
3. Results
3.1. Participant Characteristics
This study included 15,664 pregnant women who met the inclusion and exclusion criteria (Figure S5). The mean age was 31.78 years. The mean prepregnancy BMI was 21.72 kg/m2. The median concentrations of maternal FT4 and TSH for all participants during the first trimester were 16.03 (IQR 2.53) and 1.36 (IQR 1.31) mIU/L, respectively. TPOAb positivity was identified in 1759 (11.2%) individuals, with a higher likelihood of being older (P = 0.035) and experiencing multigravida (P = 0.038). Significantly lower FT4 and higher TSH levels were found in the TPOAb-positive group (both P < 0.001, Table 1). During the first trimester, the median concentration of PM1, PM1–2.5, and PM2.5 exposure was 33.83 (IQR 11.41) μg/m3, 13.26 (IQR 12.06) μg/m3, and 45.04 (IQR 16.63) μg/m3, respectively (Table 2).
Table 1. Characteristics of the Study Population (n = 15,664)a.
| variables | total (n = 15,664) | TPOAb negativea (n = 13,905) | TPOAb positive (n = 1759) | P value |
|---|---|---|---|---|
| maternal age, mean (SD), years | 31.78 (3.82) | 31.76 (3.81) | 31.96 (3.90) | 0.035 |
| prepregnancy BMI, mean (SD), kg/m2 | 21.72 (3.17) | 21.71 (3.16) | 21.85 (3.28) | 0.072 |
| annual household income, n (%) | 0.739 | |||
| <200,000 CNY | 5683 (36.3) | 5038 (36.2) | 645 (36.7) | |
| ≥200,000 CNY | 9981 (63.7) | 8867 (63.8) | 1114 (63.3) | |
| ethnicity, n (%) | 0.854 | |||
| Han | 14,449 (92.2) | 12,824 (92.2) | 1625 (92.4) | |
| minority | 1215 (7.8) | 1081 (7.8) | 134 (7.6) | |
| educational levels, years, n (%) | 0.138 | |||
| <16 years | 3448 (22.0) | 3036 (21.8) | 412 (23.4) | |
| ≥16 years | 12,216 (78.0) | 10,869 (78.2) | 1347 (76.6) | |
| smoking status, n (%) | 0.055 | |||
| yes | 492 (3.1) | 423 (3.0) | 69 (3.9) | |
| no | 15,172 (96.9) | 13,482 (97.0) | 1690 (96.1) | |
| alcohol consumption, n (%) | 0.599 | |||
| yes | 720 (4.6) | 644 (4.6) | 76 (4.3) | |
| no | 14,944 (95.4) | 13,261 (95.4) | 1683 (95.7) | |
| gravidity, n (%) | 0.038 | |||
| 1 | 8375 (53.5) | 7476 (53.8) | 899 (51.1) | |
| ≥2 | 7289 (46.5) | 6429 (46.2) | 860 (48.9) | |
| season of enrollment, n (%) | 0.069 | |||
| spring | 4024 (25.7) | 3529 (25.4) | 495 (28.1) | |
| summer | 3582 (22.8) | 3177 (22.8) | 405 (23.1) | |
| autumn | 3883 (24.8) | 3453 (24.9) | 430 (24.4) | |
| winter | 4175 (26.7) | 3746 (26.9) | 429 (24.4) | |
| gestational weeks, mean (SD), week | 8.94 (1.62) | 8.95 (1.61) | 8.72 (1.82) | 0.103 |
| FT4, median (IQR), pmol/L | 16.03 (2.35) | 16.06 (2.52) | 15.74 (2.61) | <0.001 |
| TSH, median (IQR), mIU/L | 1.36 (1.31) | 1.32 (1.25) | 1.76 (1.71) | <0.001 |
TPOAb, thyroid peroxidase antibodies; SD, standard deviation; BMI, body mass index; CNY, Chinese Yuan; FT4, free thyroxine; TSH, thyroid-stimulating hormone; and IQR, interquartile range.
Table 2. Maternal Exposure Levels of PM1, PM2.5, and PM1-2.5 during the First Trimestera.
| variables (μg/m3) | mean | SD | median | Q1 | Q3 | IQR |
|---|---|---|---|---|---|---|
| PM1 | 33.14 | 8.46 | 33.83 | 27.14 | 38.55 | 11.41 |
| PM1–2.5 | 12.91 | 7.03 | 13.26 | 7.17 | 19.23 | 12.06 |
| PM2.5 | 44.62 | 12.33 | 45.04 | 36.12 | 52.75 | 16.63 |
PM1, particulate matter with an aerodynamic diameter less than or equal to 1 μm; PM1–2.5, particulate matter with an aerodynamic diameter between 1 and 2.5 μm; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; SD, standard deviation; Q, quartile; and IQR, interquartile range.
3.2. Association between Exposure to PM and TPOAb Positivity
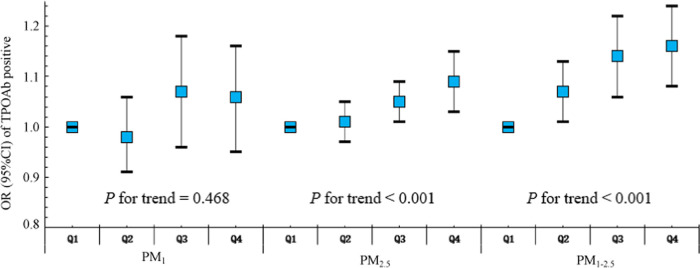
The associations between PM exposure and maternal thyroid autoimmunity with TPOAb positivity were assessed by using a generalized linear regression model (Table 3). Unadjusted analysis with a single-pollutant model showed a positive correlation between PM2.5 and PM1–2.5 exposure and TPOAb positivity during the first trimester. After adjustment for the maternal age, prepregnancy BMI, annual household income, ethnicity, educational level, smoking status, alcohol consumption, gravidity, season of enrollment, and gestational week, a 10 μg/m3 increase in PM2.5 and PM1–2.5 exposure was associated with a higher risk of thyroid autoimmunity with TPOAb positivity in the first trimester (OR for PM2.5: 1.06, 95% CI: 1.01, 1.12; and OR for PM1–2.5: 1.15, 95% CI: 1.08, 1.22). There were no significant associations found between PM1 exposure during the first trimester and maternal thyroid autoimmunity with TPOAb positivity. As expected, the odds of thyroid autoimmunity with TPOAb positivity significantly increased with every IQR escalation in PM2.5 and PM1–2.5 exposure (both P for trend <0.001) (Figure 1).
Table 3. Adjusted Association between PM1, PM2.5, PM1–2.5, and Maternal Thyroid Autoimmunity with TPOAb Positivity in the First Trimester.
| crude
model |
adjusted modela |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| PM1 | 1.06 (0.98, 1.14) | 0.151 | 1.05 (0.97, 1.13) | 0.198 |
| PM1–2.5 | 1.14 (1.08, 1.21) | <0.001 | 1.15 (1.08, 1.22) | <0.001 |
| PM2.5 | 1.06 (1.01, 1.12) | 0.017 | 1.06 (1.01, 1.12) | 0.024 |
Adjusted for the maternal age, prepregnancy BMI, annual household income, ethnicity, educational levels, smoking status, drinking status, gravidity, seasons of enrollment, and gestational weeks. PM1, particulate matter with an aerodynamic diameter less than or equal to 1 μm; PM1–2.5, particulate matter with an aerodynamic diameter between 1 and 2.5 μm; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; TPOAb, thyroid peroxidase antibodies; OR, odds ratio; and 95% CIs, confidence intervals.
Figure 1.
Adjusted odds ratio (95% CI) for positive TPOAb in relation to PM1, PM1–2.5, and PM2.5 quartiles (Q1 = reference) in the first trimester. PM1, particulate matter with an aerodynamic diameter less than or equal to 1 μm; PM1–2.5, particulate matter with an aerodynamic diameter between 1 and 2.5 μm; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; Q: quartile; OR, odds ratio; and 95% CIs, confidence intervals.
The further estimates were stratified based on the maternal age, prepregnancy BMI, education level, annual household income, and gravidity (Table S4). The associations between PM2.5 and PM1–2.5 exposure and thyroid autoimmunity with TPOAb positivity were significantly stronger in pregnant women with an annual household income of <200,000 CNY (OR for PM2.5: 1.09, 95% CI: 1.03, 1.17, P = 0.008; and OR for PM1–2.5: 1.12, 95% CI: 1.01, 1.23, P < 0.001). These factors did not affect the association between PM1 exposure and thyroid autoimmunity with TPOAb positivity (all P > 0.05).
3.3. Association between Exposure to PM and Maternal Thyroid Function
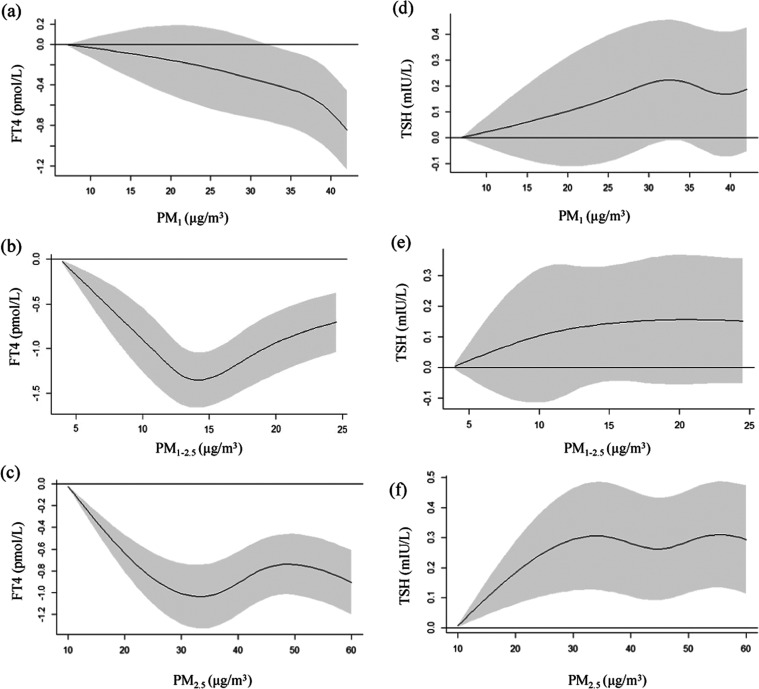
The natural cubic spline analyses of the exposure–response association between PM concentration and thyroid function biomarkers are shown in Figure 2. The increases in PM2.5 and PM1–2.5 exposure were associated with lower maternal FT4 levels. The estimated changes in FT4 at the 50th, 75th, and 95th percentile of PM2.5 against the minimum exposure were −0.77 (95% CI: −1.05, −0.49), −0.76 (95% CI: −1.05, −0.48), and −0.86 (95% CI: −1.15, −0.57) pmol/L, respectively. A similar result was obtained for PM1–2.5, and the estimated changes of FT4 at the 50th, 75th, and 95th percentile of PM1–2.5 against the minimum exposure were −1.31 (95% CI: −1.69, −0.98), −0.95 (95% CI: −1.29, −0.61), and −0.73 (95% CI: −1.09, −0.37) pmol/L, respectively. The spline analyses showed a nonlinear association of PM1 exposure with the maternal FT4 level, and additional analyses suggested a significant negative association at a PM1 exposure of >32.13 μg/m3 (Figure 2 and Table S5).
Figure 2.
Association between PM exposure and maternal thyroid function biomarkers during the first trimester. (a) PM1 exposure and the FT4 level; (b) PM1–2.5 exposure and the FT4 level; (c) PM2.5 exposure and the FT4 level; (d) PM1 exposure and the TSH level; (e) PM1–2.5 exposure and the TSH level; and (f) PM2.5 exposure and the TSH level. PM1, particulate matter with an aerodynamic diameter less than or equal to 1 μm; PM1–2.5, particulate matter with an aerodynamic diameter between 1 and 2.5 μm; PM2.5, particulate matter with an aerodynamic diameter less than or equal to 2.5 μm; FT4, free thyroxine; and TSH, thyroid-stimulating hormone.
An increase in PM2.5 exposure was correlated with elevated TSH levels, and the estimated changes of TSH at the 50th, 75th, and 95th percentiles for PM2.5 compared to the minimum exposure were 0.26 (95% CI: 0.09, 0.43), 0.30 (95% CI: 0.13, 0.48), and 0.30 (95% CI: 0.12, 0.48) mIU/L, respectively. The results demonstrated that there was no link between PM1 and PM1–2.5 exposure and maternal TSH levels (Figure 2 and Table S5).
The stratified analyses showed that the annual household income significantly modified the association between PM exposure and the FT4 concentration. Generally, stronger associations were observed between PM exposure (PM1, PM1–2.5, and PM2.5) and FT4 levels among individuals with low household income than those with high household income (all P < 0.05 except 95th of PM2.5 with P = 0.052). No other factors cause significant modification of the associations between exposure to PM and maternal FT4 levels (all P > 0.05; Tables S6, S7, and S8). Moreover, these potential modifiers did not affect the relationships between PM exposure and maternal TSH levels (Tables S9, S10, and S11).
3.4. Interaction between PM Exposure and Maternal Thyroid Disruption
The interactions between PM1 and PM1–2.5 exposure with thyroid autoimmunity and maternal FT4 and TSH levels were explored by stratified analyses and classified PM levels (Tables S12, S13 and S14). No interaction was found between PM1 and PM1–2.5 in association with positive TPOAb and maternal levels of FT4 and TSH (all P > 0.05).
4. Discussion
This prospective birth cohort study found that PM2.5 and PM1–2.5 exposure was associated with an elevated risk of maternal thyroid autoimmunity with TPOAb positivity during the first trimester. Increases in exposure to PM2.5 and PM1–2.5 were correlated with lower maternal FT4 levels, and a negative association was found between PM1 and FT4 levels when the exposure exceeded 32.13 ug/m3. Only PM2.5 exposure was positively associated with the levels of maternal TSH. These findings provide epidemiological evidence that PM exposure may have adverse effects on maternal thyroid function.
Indicators affecting thyroid homeostasis can be significantly disrupted by environmental pollutants. Pregnant women with TPOAb positivity are at an increased risk of experiencing subclinical and overt hypothyroidism, which may lead to adverse outcomes for the mother and fetus.1 In this study, 11.2% of the pregnant women were diagnosed as TPOAb-positive, consistent with previous studies of pregnant Chinese women.18,28 We observed a positive association during the first trimester of pregnancy between exposure to PM2.5 and PM1–2.5 and thyroid autoimmunity with TPOAb positivity, while the association of PM1 exposure with maternal thyroid autoimmunity showed a trend similar to that of PM2.5 exposure, although without statistical significance. This finding aligns with a prior study of five combined cohorts that showed that PM exposure during early pregnancy increased the risk of thyroid autoimmunity in one of the five cohorts.15 Wang et al. found positive associations of PM2.5 exposure and its components (organic matter, black carbon, sulfate, and nitrate) with maternal TPOAb levels during pregnancy.16 Zhao et al. observed that the influence of PM2.5 exposure on levels of maternal FT4 was more pronounced in pregnant women who were TPOAb-positive, compared to those who were negative for TPOAb.17 These findings imply that pregnant women with TPOAb-positive TPOAb cells are more susceptible to environmentally driven disruptions in thyroid function. In contrast, limited evidence of effect modification by the TPOAb status was observed in a study examining the associations of PM2.5 and PM2.5-bound metals with indicators of thyroid function.18 Previous studies showed that endocrine disruptors and iodine levels also altered the TPOAb status.29,30 Therefore, future studies should consider environmental endocrine disruptors and the iodine status when exploring the impact of environmental pollutants on thyroid function.
The results from our study of PM2.5–FT4 associations are consistent with a recent meta-analysis of 24,235 pregnant women that demonstrated that during the first trimester, maternal FT4 levels were significantly lower with exposure to PM2.5.11 Previous studies also demonstrated that the significant associations of PM2.5–FT4 were also relevant in the preconception period and the second trimester of pregnancy.17,31 Higher concentrations of PM2.5 components (black carbon and ammonium) and most of the PM2.5-bound metals including beryllium, lead, manganese, nickel, and barium were significantly associated with maternal FT4 levels during pregnancy.16,18 More importantly, this study is the first to show a significant inverse correlation between PM1 exposure and maternal FT4 at exposures of >32.13 μg/m3. These findings must be validated in a replicate cohort.
Our study showed a significant dose–response relationship between PM2.5 exposure during the first trimester and maternal TSH levels. This association was also observed in a prospective cohort study involving 1060 Chinese pregnant women during early pregnancy.32 Ilias et al. showed a positive correlation between log TSH and PM2.5 with 293 Greek women in the second or third trimester.33 Furthermore, Zeng et al. showed that maternal exposure to PM2.5 during pregnancy was positively correlated with abnormal fetal TSH levels.34 A recent meta-analysis revealed that with each 10 μg/m3 rise in the PM2.5 level, the risk of elevated TSH levels (defined as exceeding the 95th percentile) increased 1.452-fold in 357,226 participants, including adults, pregnant women, and newborns. However, no significant association was found in the subgroup analyses involving pregnant women.11
PM of different sizes may have different deleterious effects on health. Some studies have suggested that smaller particles have higher reactivity and a more toxic chemical composition.35 However, our findings suggest that PM1–2.5 may have a greater impact on thyroid disruption than PM1 during the first trimester of pregnancy. A recent study also indicated that PM1–2.5 has a greater impact on lipids compared to other types of PMs (PM1, PM2.5, PM2.5–10, and PM10).36 This might be caused by differences in the composition and deposition fraction of size-fractionated PM.37,38 The total deposition fraction of PM1 in the tracheobronchial and pulmonary airways is significantly lower than PM1–2.5,39 and various PM components might influence diverse health outcomes.40 Meng et al. found that speciated PM2.5 composition is strongly correlated with different adverse birth outcomes.41
The molecular mechanisms by which PM exposure impacts maternal thyroid function remain unclear. Previous population-based studies reported that high levels of PM exposure are linked to a rise in the count of CD4+, CD8+, CD14+, CD16+, immunoglobulin G, and natural killer lymphocytes42,43 and a decreased number of granulocyte–macrophage colony-stimulating factors and interferon-γ.44 White blood cell counts, ferritin levels, and C-reactive protein were also affected by PM exposure.45 These cytokine changes may reflect the role of PM in systemic inflammation and immune dysregulation. Additionally, an animal experimental model confirmed that exposure to PM during pregnancy could activate inflammatory reactions, influence the distribution of T-lymphocyte subsets, and increase oxidative stress levels, leading to dysregulation of maternal immunity.46 Furthermore, Dong et al. suggested that the hypothalamic–pituitary–thyroid (HPT) axis could be activated by increased oxidative stress and inflammation following PM exposure. The HPT axis negatively regulates the biosynthesis and biotransformation of thyroid hormones, causing thyroid dysfunction.47
The study has several strengths. First, maternal exposure to PM1, PM2.5, and PM1–2.5 during the first trimester were analyzed, and to our current understanding, this is the pioneering research to examine the effect of exposure to PM1 and PM1–2.5 on maternal thyroid function during pregnancy. Second, this cohort study included the largest samples used to date to investigate the association of ambient PM exposure with thyroid function during pregnancy, providing adequate statistical power to detect an effect. Third, this is one of the first studies to assess the associations between exposure to PM and maternal thyroid autoimmunity and TPOAb positivity.
This study also has some limitations. First, a previous meta-analysis suggested the intensity of the correlation of PM2.5 and FT4/FT3 varied by the region and pollutant concentration.11 The participants in the current study were mainly from Beijing, which might limit the applicability of our results to regions with varying levels of ambient PM exposure. Second, ambient PM concentration was measured based on the residential address. There was no information available about indoor levels of ambient PM exposure, and the workplace exposure was not calculated. Third, gaseous environmental pollutants, including nitrogen dioxide, ozone, sulfur dioxide, and endocrine organic pollutants, were not considered, although these have been reported to be associated with maternal thyroid function. Finally, maternal blood iodine was not measured, although iodine levels can alter thyroid function during pregnancy.
Acknowledgments
The authors are grateful to all the participants and coordinators in the CBCS for their support in the data collection. We would like to thank Wen-Zhong Huang (Ph.D., Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne VIC, 3004, Australia) for his help with data analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c10191.
Previous studies of associations of PM with thyroid autoimmunity or thyroid function biomarkers during pregnancy, comparison of characteristics among pregnant women included and excluded in this study, likelihood ratio test between the nonlinear model and linear model, adjusted associations between PM1, PM1–2.5, PM2.5, and thyroid autoimmunity with TPOAb positivity, stratified by potential modifiers, estimated changes and 95% CIs of thyroid function biomarkers at the 50th, 75th, and 95th percentiles of PM exposure against the minimal percentile of PM in natural cubic splines, association between PM1, PM1–2.5, and PM2.5 exposure and maternal thyroid function biomarkers, stratified by potential modifiers, interaction between PM1 and PM1–2.5 in associations with maternal TPOAb positivity and FT4 and TSH levels, location of study populations and PM1 and PM2.5 concentrations, DAG for the association between PM exposure and thyroid autoimmunity during pregnancy using DAGitty, and flowchart of study enrollment (PDF)
Author Contributions
○ E.Z., Z.Z., G.C., and Y.T.Z contributed equally and share the first authorship. All authors reviewed and approved the final version of the article. The contributions of all authors to this article were as follows. C.Y., R.L., and G.H.D.: supervision, conceptualization, interpretation, and editing; E.Z., Z.Z., G.C., and Y.T.Z.: design, methodology, formal analysis, drafting the article, and review; S.X., J.L., Y.Z., and Y.C.: collecting clinical data; B.Y.Y. and Y.G.: generating data of PM1 and PM2.5; S.S. and G.S.,: methodology and statistical analysis; W.Y. and Q.W.: validation and editing; and C.Y., G.H.D., and E.Z.: funding.
This research was funded by the National Key Research and Development Program of China (Nos. 2016YFC1000100, 2018YFE0106900), the National Natural Science Foundation of China (No. 82304088), the Beijing Hospitals Authority Youth Programme (No. QML20231407), the Leading Talents in the Construction Project of High Level Public Health Technical Talents in Beijing (2022–1–003), and the Monash Early Career Postdoctoral Fellowship and NHMRC Centre for Safe Air Postdoctoral Research Fellowship.
The authors declare no competing financial interest.
Supplementary Material
References
- De Leo S.; Pearce E. N. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018, 6 (7), 575–586. 10.1016/S2213-8587(17)30402-3. [DOI] [PubMed] [Google Scholar]
- Thangaratinam S.; Tan A.; Knox E.; Kilby M. D.; Franklyn J.; Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011, 342, d2616 10.1136/bmj.d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard E.; Vissenberg R.; Land J. A.; van Wely M.; van der Post J. A.; Goddijn M.; Bisschop P. H. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum. Reprod. Update 2011, 17 (5), 605–619. 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- He X.; Wang P.; Wang Z.; He X.; Xu D.; Wang B. Thyroid antibodies and risk of preterm delivery: a meta-analysis of prospective cohort studies. Eur. J. Endocrinol. 2012, 167 (4), 455–464. 10.1530/EJE-12-0379. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A.; Schwartz A.; Gismondi R.; Tinelli A.; Mangieri T.; Negro R. High rate of persistent hypothyroidism in a large-scale prospective study of postpartum thyroiditis in southern Italy. J. Clin. Endocrinol. Metab. 2011, 96 (3), 652–657. 10.1210/jc.2010-1980. [DOI] [PubMed] [Google Scholar]
- Nicholson W. K.; Robinson K. A.; Smallridge R. C.; Ladenson P. W.; Powe N. R. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid 2006, 16 (6), 573–582. 10.1089/thy.2006.16.573. [DOI] [PubMed] [Google Scholar]
- Pop V. J.; de Vries E.; van Baar A. L.; Waelkens J. J.; de Rooy H. A.; Horsten M.; Donkers M. M.; Komproe I. H.; van Son M. M.; Vader H. L. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development?. J. Clin. Endocrinol. Metab. 1995, 80 (12), 3561–3566. 10.1210/jcem.80.12.8530599. [DOI] [PubMed] [Google Scholar]
- Mannisto T.; Vaarasmaki M.; Pouta A.; Hartikainen A. L.; Ruokonen A.; Surcel H. M.; Bloigu A.; Jarvelin M. R.; Suvanto-Luukkonen E. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J. Clin. Endocrinol. Metab. 2009, 94 (3), 772–779. 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- Delitala A. P.; Capobianco G.; Cherchi P. L.; Dessole S.; Delitala G. Thyroid function and thyroid disorders during pregnancy: a review and care pathway. Arch. Gynecol. Obstet. 2019, 299 (2), 327–338. 10.1007/s00404-018-5018-8. [DOI] [PubMed] [Google Scholar]
- Ju L.; Hua L.; Xu H.; Li C.; Sun S.; Zhang Q.; Cao J.; Ding R. Maternal atmospheric particulate matter exposure and risk of adverse pregnancy outcomes: A meta-analysis of cohort studies. Environ. Pollut. 2023, 317, 120704 10.1016/j.envpol.2022.120704. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhao K.; Qian T.; Li X.; Yi W.; Pan R.; Huang Y.; Ji Y.; Su H. Association between ambient air pollution and thyroid hormones levels: A systematic review and meta-analysis. Sci. Total Environ. 2023, 904, 166780 10.1016/j.scitotenv.2023.166780. [DOI] [PubMed] [Google Scholar]
- Wu C.; Zhang Y.; Wei J.; Zhao Z.; Norback D.; Zhang X.; Lu C.; Yu W.; Wang T.; Zheng X.; Zhang L. Associations of Early-Life Exposure to Submicron Particulate Matter With Childhood Asthma and Wheeze in China. JAMA Network Open 2022, 5 (10), e2236003 10.1001/jamanetworkopen.2022.36003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C. N.; Xu Z.; Wu G. C.; Mao Y. M.; Liu L. N.; Qian W.; Dan Y. L.; Tao S. S.; Zhang Q.; Sam N. B.; Fan Y. G.; Zou Y. F.; Ye D. Q.; Pan H. F. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019, 18 (6), 607–614. 10.1016/j.autrev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Adami G.; Pontalti M.; Cattani G.; Rossini M.; Viapiana O.; Orsolini G.; Benini C.; Bertoldo E.; Fracassi E.; Gatti D.; Fassio A. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open 2022, 8 (1), e002055 10.1136/rmdopen-2021-002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A.; Pierotti L.; Basterrechea M.; Chatzi L.; Estarlich M.; Fernandez-Somoano A.; Fleisch A. F.; Gold D. R.; Julvez J.; Karakosta P.; Lertxundi A.; Lopez-Espinosa M. J.; Mulder T. A.; Korevaar T. I. M.; Oken E.; Peeters R. P.; Rifas-Shiman S.; Stephanou E.; Tardon A.; Tiemeier H.; Vrijheid M.; Vrijkotte T. G. M.; Sunyer J.; Guxens M. Association of Exposure to Ambient Air Pollution With Thyroid Function During Pregnancy. JAMA Network Open 2019, 2 (10), e1912902 10.1001/jamanetworkopen.2019.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Liu C.; Zhang M.; Han Y.; Aase H.; Villanger G. D.; Myhre O.; Donkelaar A. V.; Martin R. V.; Baines E. A.; Chen R.; Kan H.; Xia Y. Evaluation of Maternal Exposure to PM(2.5) and Its Components on Maternal and Neonatal Thyroid Function and Birth Weight: A Cohort Study. Thyroid 2019, 29 (8), 1147–1157. 10.1089/thy.2018.0780. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Cao Z.; Li H.; Su X.; Yang Y.; Liu C.; Hua J. Air pollution exposure in association with maternal thyroid function during early pregnancy. J. Hazard. Mater. 2019, 367, 188–193. 10.1016/j.jhazmat.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Qiu L.; Shen W.; Ye C.; Wu J.; Zheng S.; Lou B.; Chen Z.; Xu P.; Xu D.; Wang X.; Feng B. Association of exposure to PM(2.5)-bound metals with maternal thyroid function in early pregnancy. Sci. Total Environ. 2022, 810, 151167 10.1016/j.scitotenv.2021.151167. [DOI] [PubMed] [Google Scholar]
- Yue W.; Zhang E.; Liu R.; Zhang Y.; Wang C.; Gao S.; Su S.; Gao X.; Wu Q.; Yang X.; Papageorghiou A. T.; Yin C. The China birth cohort study (CBCS). Eur. J. Epidemiol. 2022, 37 (3), 295–304. 10.1007/s10654-021-00831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Morawska L.; Zhang W.; Li S.; Cao W.; Ren H.; Wang B.; Wang H.; Knibbs L. D.; Williams G.; Guo J.; Guo Y. Spatiotemporal variation of PM1 pollution in China. Atmos. Environ. 2018, 178, 198–205. 10.1016/j.atmosenv.2018.01.053. [DOI] [Google Scholar]
- Chen G.; Knibbs L. D.; Zhang W.; Li S.; Cao W.; Guo J.; Ren H.; Wang B.; Wang H.; Williams G.; Hamm N. A. S.; Guo Y. Estimating spatiotemporal distribution of PM(1) concentrations in China with satellite remote sensing, meteorology, and land use information.. Environ. Pollut. 2018, 233, 1086–1094. 10.1016/j.envpol.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Chen G.; Li S.; Knibbs L. D.; Hamm N. A. S.; Cao W.; Li T.; Guo J.; Ren H.; Abramson M. J.; Guo Y. A machine learning method to estimate PM(2.5) concentrations across China with remote sensing, meteorological and land use information.. Sci. Total Environ. 2018, 636, 52–60. 10.1016/j.scitotenv.2018.04.251. [DOI] [PubMed] [Google Scholar]
- Korevaar T. I. M.; Derakhshan A.; Taylor P. N.; Meima M.; Chen L.; Bliddal S.; Carty D. M.; Meems M.; Vaidya B.; Shields B.; Ghafoor F.; Popova P. V.; Mosso L.; Oken E.; Suvanto E.; Hisada A.; Yoshinaga J.; Brown S. J.; Bassols J.; Auvinen J.; Bramer W. M.; Lopez-Bermejo A.; Dayan C.; Boucai L.; Vafeiadi M.; Grineva E. N.; Tkachuck A. S.; Pop V. J. M.; Vrijkotte T. G.; Guxens M.; Chatzi L.; Sunyer J.; Jimenez-Zabala A.; Riano I.; Murcia M.; Lu X.; Mukhtar S.; Delles C.; Feldt-Rasmussen U.; Nelson S. M.; Alexander E. K.; Chaker L.; Mannisto T.; Walsh J. P.; Pearce E. N.; Steegers E. A. P.; Peeters R. P.; Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-analysis. JAMA 2019, 322 (7), 632–641. 10.1001/jama.2019.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. N.; Albrecht D.; Scholz A.; Gutierrez-Buey G.; Lazarus J. H.; Dayan C. M.; Okosieme O. E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14 (5), 301–316. 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- Ragusa F.; Fallahi P.; Elia G.; Gonnella D.; Paparo S. R.; Giusti C.; Churilov L. P.; Ferrari S. M.; Antonelli A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res., Clin. Endocrinol. Metab. 2019, 33 (6), 101367 10.1016/j.beem.2019.101367. [DOI] [PubMed] [Google Scholar]
- Zhang Y. T.; Zeeshan M.; Su F.; Qian Z. M.; Dee Geiger S.; Edward McMillin S.; Wang Z. B.; Dong P. X.; Ou Y. Q.; Xiong S. M.; Shen X. B.; Zhou P. E.; Yang B. Y.; Chu C.; Li Q. Q.; Zeng X. W.; Feng W. R.; Zhou Y. Z.; Dong G. H. Associations between both legacy and alternative per- and polyfluoroalkyl substances and glucose-homeostasis: The Isomers of C8 health project in China. Environ. Int. 2022, 158, 106913 10.1016/j.envint.2021.106913. [DOI] [PubMed] [Google Scholar]
- Di Q.; Dai L.; Wang Y.; Zanobetti A.; Choirat C.; Schwartz J. D.; Dominici F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA 2017, 318 (24), 2446–2456. 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Teng D.; Li C.; Peng S.; Mao J.; Wang W.; Xie X.; Fan C.; Li C.; Meng T.; Zhang S.; Du J.; Gao Z.; Shan Z.; Teng W. Association between iodine intake and thyroid autoantibodies: a cross-sectional study of 7073 early pregnant women in an iodine-adequate region. J. Endocrinol. Invest. 2020, 43 (1), 43–51. 10.1007/s40618-019-01070-1. [DOI] [PubMed] [Google Scholar]
- Webster G. M.; Rauch S. A.; Marie N. S.; Mattman A.; Lanphear B. P.; Venners S. A. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007–2008). Environ. Health Perspect. 2016, 124 (7), 935–942. 10.1289/ehp.1409589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G. M.; Venners S. A.; Mattman A.; Martin J. W. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ. Res. 2014, 133, 338–347. 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Li J.; Liao J.; Hu C.; Bao S.; Mahai G.; Cao Z.; Lin C.; Xia W.; Xu S.; Li Y. Preconceptional and the first trimester exposure to PM(2.5) and offspring neurodevelopment at 24 months of age: Examining mediation by maternal thyroid hormones in a birth cohort study. Environ. Pollut. 2021, 284, 117133 10.1016/j.envpol.2021.117133. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Zhu Q.; Wang P.; Li J.; Luo R.; Zhao W.; Zhang L.; Shi H.; Zhang Y. Early pregnancy PM(2.5) exposure and its inorganic constituents affect fetal growth by interrupting maternal thyroid function. Environ. Pollut. 2022, 307, 119481 10.1016/j.envpol.2022.119481. [DOI] [PubMed] [Google Scholar]
- Ilias I.; Kakoulidis I.; Togias S.; Stergiotis S.; Michou A.; Lekkou A.; Mastrodimou V.; Pappa A.; Venaki E.; Koukkou E. Atmospheric Pollution and Thyroid Function of Pregnant Women in Athens, Greece: A Pilot Study. Med. Sci. 2020, 8 (2), 19 10.3390/medsci8020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.; Xu X.; Wang Q.; Zhang Z.; Meng P.; Huo X. Maternal exposure to atmospheric PM(2.5) and fetal brain development: Associations with BAI1 methylation and thyroid hormones. Environ. Pollut. 2022, 308, 119665 10.1016/j.envpol.2022.119665. [DOI] [PubMed] [Google Scholar]
- Samek L.; Furman L.; Mikrut M.; Regiel-Futyra A.; Macyk W.; Stochel G.; van Eldik R. Chemical composition of submicron and fine particulate matter collected in Krakow, Poland. Consequences for the APARIC project. Chemosphere 2017, 187, 430–439. 10.1016/j.chemosphere.2017.08.090. [DOI] [PubMed] [Google Scholar]
- Hu M.; Wei J.; Hu Y.; Guo X.; Li Z.; Liu Y.; Li S.; Xue Y.; Li Y.; Liu M.; Wang L.; Liu X. Long-term effect of submicronic particulate matter (PM(1)) and intermodal particulate matter (PM(1–2.5)) on incident dyslipidemia in China: A nationwide 5-year cohort study. Environ. Res. 2023, 217, 114860 10.1016/j.envres.2022.114860. [DOI] [PubMed] [Google Scholar]
- Cho S. H.; Tong H.; McGee J. K.; Baldauf R. W.; Krantz Q. T.; Gilmour M. I. Comparative toxicity of size-fractionated airborne particulate matter collected at different distances from an urban highway. Environ. Health Perspect. 2009, 117 (11), 1682–1689. 10.1289/ehp.0900730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Guo B.; Yang X.; Li J.; Baima Y.; Yin J.; Yu J.; Xu H.; Zeng C.; Feng S.; Wei J.; Hong F.; Zhao X. Role of Liver Enzymes in the Relationship Between Particulate Matter Exposure and Diabetes Risk: A Longitudinal Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107 (10), e4086–e4097. 10.1210/clinem/dgac438. [DOI] [PubMed] [Google Scholar]
- Wang H.; Yin P.; Fan W.; Wang Y.; Dong Z.; Deng Q.; Zhou M. Mortality Risk Associated with Short-Term Exposure to Particulate Matter in China: Estimating Error and Implication. Environ. Sci. Technol. 2021, 55 (2), 1110–1121. 10.1021/acs.est.0c05095. [DOI] [PubMed] [Google Scholar]
- Nunez Y.; Boehme A. K.; Goldsmith J.; Li M.; van Donkelaar A.; Weisskopf M. G.; Re D. B.; Martin R. V.; Kioumourtzoglou M. A. PM(2.5) composition and disease aggravation in amyotrophic lateral sclerosis: An analysis of long-term exposure to components of fine particulate matter in New York State. Environ. Epidemiol. 2022, 6 (2), e204 10.1097/EE9.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.; Liu J.; Shen J.; Del Rosario I.; Lakey P. S. J.; Shiraiwa M.; Su J.; Weichenthal S.; Zhu Y.; Oroumiyeh F.; Paulson S. E.; Jerrett M.; Ritz B. Fine Particulate Matter Metal Composition, Oxidative Potential, and Adverse Birth Outcomes in Los Angeles. Environ. Health Perspect. 2023, 131 (10), 107012 10.1289/EHP12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi G. S.; Houthuijs D.; Steerenberg P. A.; Fletcher T.; Armstrong B.; Antova T.; Lochman I.; Lochmanova A.; Rudnai P.; Erdei E.; Musial J.; Jazwiec-Kanyion B.; Niciu E. M.; Durbaca S.; Fabianova E.; Koppova K.; Lebret E.; Brunekreef B.; van Loveren H. Immune biomarkers in relation to exposure to particulate matter: a cross-sectional survey in 17 cities of Central Europe. Inhalation Toxicol. 2000, 12 (4), 1–14. 10.1080/08958370050164833. [DOI] [PubMed] [Google Scholar]
- Pope C. A. 3rd; Bhatnagar A.; McCracken J. P.; Abplanalp W.; Conklin D. J.; O’Toole T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119 (11), 1204–1214. 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L.; Macias-Parra M.; Hoffmann H. J.; Valencia-Salazar G.; Henriquez-Roldan C.; Osnaya N.; Monte O. C.; Barragan-Mejia G.; Villarreal-Calderon R.; Romero L.; Granada-Macias M.; Torres-Jardon R.; Medina-Cortina H.; Maronpot R. R. Immunotoxicity and environment: immunodysregulation and systemic inflammation in children. Toxicol. Pathol. 2009, 37 (2), 161–169. 10.1177/0192623308329340. [DOI] [PubMed] [Google Scholar]
- Lee H.; Myung W.; Jeong B. H.; Choi H.; Jhun B. W.; Kim H. Short- and long-term exposure to ambient air pollution and circulating biomarkers of inflammation in non-smokers: A hospital-based cohort study in South Korea. Environ. Int. 2018, 119, 264–273. 10.1016/j.envint.2018.06.041. [DOI] [PubMed] [Google Scholar]
- Liu W.; Zhang M.; Feng J.; Fan A.; Zhou Y.; Xu Y. The Influence of Quercetin on Maternal Immunity, Oxidative Stress, and Inflammation in Mice with Exposure of Fine Particulate Matter during Gestation. Int. J. Environ. Res. Public Health 2017, 14 (6), 592 10.3390/ijerph14060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.; Wu W.; Yao S.; Li H.; Li Z.; Zhang L.; Jiang J.; Xu J.; Zhang F. PM(2.5) disrupts thyroid hormone homeostasis through activation of the hypothalamic-pituitary-thyroid (HPT) axis and induction of hepatic transthyretin in female rats 2.5. Ecotoxicol. Environ. Saf. 2021, 208, 111720 10.1016/j.ecoenv.2020.111720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.